Abstract
Cytochrome P450 1A1 (CYP1A1), like many monooxygenases, can produce reactive oxygen species during its catalytic cycle. Apart from the well-characterized xenobiotic-elicited induction, the regulatory mechanisms involved in the control of the steady-state activity of CYP1A1 have not been elucidated. We show here that reactive oxygen species generated from the activity of CYP1A1 limit the levels of induced CYP1A1 mRNAs. The mechanism involves the repression of the CYP1A1 gene promoter activity in a negative-feedback autoregulatory loop. Indeed, increasing the CYP1A1 activity by transfecting CYP1A1 expression vectors into hepatoma cells elicited an oxidative stress and led to the repression of a reporter gene driven by the CYP1A1 gene promoter. This negative autoregulation is abolished by ellipticine (an inhibitor of CYP1A1) and by catalase (which catalyzes H2O2 catabolism), thus implying that H2O2 is an intermediate. Down-regulation is also abolished by the mutation of the proximal nuclear factor I (NFI) site in the promoter. The transactivating domain of NFI/CTF was found to act in synergy with the arylhydrocarbon receptor pathway during the induction of CYP1A1 by 2,3,7,8-tetrachloro-p-dibenzodioxin. Using an NFI/CTF-Gal4 fusion, we show that NFI/CTF transactivating function is decreased by a high activity of CYP1A1. This regulation is also abolished by catalase or ellipticine. Consistently, the transactivating function of NFI/CTF is repressed in cells treated with H2O2, a novel finding indicating that the transactivating domain of a transcription factor can be targeted by oxidative stress. In conclusion, an autoregulatory loop leads to the fine tuning of the CYP1A1 gene expression through the down-regulation of NFI activity by CYP1A1-based H2O2 production. This mechanism allows a limitation of the potentially toxic CYP1A1 activity within the cell.
Cytochrome P450 monooxygenases are drug-metabolizing enzymes that play a major role in the detoxification and elimination of hydrophobic xenobiotics. Paradoxically, these enzymes also generate reactive metabolites which can form DNA adducts and lead to mutations (26). CYP1A1 is a ubiquitous member of the P450 superfamily which is among the products of the aryl hydrocarbon (Ah)-inducible gene battery (reference 50 and references therein). It is highly inducible by the persistent environmental contaminant 2,3,7,8-tetrachloro-p-dibenzodioxin (TCDD) and by planar aromatic hydrocarbons, such as 3-methylcholanthrene or benzo(a)pyrene (BP) (24, 44, 59). CYP1A1 is known to play a critical role in the activation of BP into a metabolite that can form DNA adducts (7, 32, 47). High-CYP1A1-inducibility phenotypes and the MspI polymorphism are suspected to be correlated with increased lung cancer frequency, at least in some populations (28, 34).
The Ah receptor (AhR), once activated by ligands such as TCDD or planar aromatic hydrocarbons, translocates from the cytosol into the nucleus, heterodimerizes with Arnt (AhR nuclear translocator), and binds to a class of promoter DNA sequences called xenobiotic-responsive elements (XRE) (references 17 and 48 and references therein). This mechanism of induction has been widely studied at several laboratories (references 21, 30, 38, and 59; for a recent extensive review, see reference 57). Studies on the murine cyp1A1 gene have shown that its regulation involves cross talk between enhancer and promoter sequences and concomitant changes in the chromatin structure (30). The 5′ upstream region of the CYP1A1 gene contains several XRE that mediate the Ah response, a negative regulatory element (6, 42, 56), and a proximal promoter. In the latter, a short sequence, also referred to as the basic transcriptional element (BTE), has been shown to be critical for both basal and induced activities (24, 59). This complex sequence contains overlapping binding sites for Sp1 and nuclear factor I (NFI) and can bind several proteins (23, 60).
In addition to the well-known positive regulation of CYP1A1, the expression of this gene is repressed by several agents and conditions. Inflammatory cytokines as well as oxidative stress have been shown to down-regulate the CYP1A1 gene expression and the expression of other cytochromes P450 (1, 3, 4, 37, 40). The mechanisms involved are transcriptional, and in the case of tumor necrosis factor alpha (TNF-α), H2O2 addition, or glutathione depletion, we have shown that the integrity of the NFI site in the BTE was critical. The binding of NFI/CTF to its consensus DNA site is blunted by treatment of cells with either addition at millimolar concentrations of H2O2 or glutathione depletion (37). Yet, an additional mechanism is likely to be involved since NFI-driven promoter activities (including that of CYP1A1) were repressed by addition of submillimolar concentrations of H2O2 in an NFI-depletion manner (37). Whether the transcriptional activating domain (TAD) of NFI is targeted by the H2O2 treatment is still an unresolved issue.
The down-regulation of CYP1A1 by H2O2 raised an important question. Indeed, in vitro experiments showed that some cytochromes P450 can release reactive oxygen species (ROS) like H2O2 during their catalytic cycles, particularly with uncoupled substrate (5, 41). Within the cellular context, CYP2E1 overexpression was shown to produce ROS (reference 8 and references within). In this study, we assayed the ROS release due to the catalytic activity of CYP1A1 in intact cells and hypothesized that it could influence the expression of the CYP1A1 gene, leading to a feedback loop controlling the steady-state level of the enzyme. Previous studies have suggested that such an autoregulatory loop should be functional. Indeed, it has been observed that endogenous CYP1A1 mRNA levels were higher in a mouse hepatoma mutant cell line expressing a nonfunctional CYP1A1 enzyme than in the wild-type cell line (14, 45). Another study also suggested that overexpression of CYP1A1 decreased its own expression (25). However, the regulatory mechanism for such an autoregulation process has remained unclear.
The mechanisms of autoregulation of several genes have already been described. They mostly concern genes coding for transcription factors whose promoters contain cognate DNA sequences for the encoded transcription factors themselves. Positive feedback and negative feedback have been reported (22, 36, 55). To our knowledge, the mechanisms of autoregulation of a gene resulting from the activity of the encoded enzyme itself have been rarely described in mammalian cells. Protein kinase C has been shown to up-regulate its own expression through the phosphorylation of some transduction proteins (18), yet this mechanism is similar to the autoactivation of genes coding for transcription factors and seems to be designed to activate more efficiently a response to a signal. In the yeast, the activity of a metallothionein protein has been reported to repress the expression of its own gene (58).
In the present study, we provide a mechanism for the autoregulation of CYP1A1 gene expression by the CYP1A1 enzymatic activity. We show that the release of H2O2 during the catalytic cycle targets the NFI/CTF transcription factor and thus represses the promoter activity. We also show that the TAD of NFI/CTF is involved in this regulation and that it specifically cooperates with the signaling mediated by the AhR.
MATERIALS AND METHODS
Chemicals.
H2O2 was from a 30% stock (Merck). TCDD was obtained from Promochem (Strasbourg, France). 2′,7′-Dichlorodihydrofluorescein-diacetate (H2DCF-DA) was purchased from Interchim (Asnière, France). BP and other chemicals were obtained from Sigma (L’Ile d’Abeau, France), and oligonucleotides were obtained from Genset (Paris, France).
Cell culture.
The human hepatoma cell line HepG2 (29) was maintained as described elsewhere (12). These cells were used because the endogenous CYP1A1 gene is regulated by H2O2 (37) and because of their excellent transfection efficiency.
Intracellular H2O2 generation assay.
The oxidation-sensitive probe H2DCF-DA is a nonpolar compound that readily diffuses into cells, where it is hydrolyzed by endogenous esterases (46). The resulting compound is not fluorescent but yields the fluorescent compound 2′,7′-dichlorofluorescein (DCF) when oxidized. Cells were cultured in 6-well plates (Costar, Corning, N.Y.). H2DCF-DA (200 μM) was added directly to the culture medium, and cells were cultured under standard conditions for 1 h. The fluorescence of DCF was then measured with a Bio-Tek FL-600 fluorimeter (Fisher, Elancourt, France) by using 485 and 530 nm as the excitation and emission wavelengths, respectively. In each well (diameter, 3.5 cm), 109 measurements were made with a 3-mm-diameter optic so as to cover the whole well surface. The result given for each well was expressed as the sum of the 109 values obtained.
Northern blotting.
RNA preparation and Northern blotting were performed as already described (11). Probes were synthesized from cDNAs with the Megaprime DNA labeling kit (Amersham) according to the manufacturer’s instructions. Quantifications were performed by using a Phosphorimager and the ImageQuant software (Molecular Dynamics, Inc).
Plasmids.
The plasmid p1A1-FL, containing the 5′ region of the human CYP1A1 gene (positions −1566 to +73) upstream of the firefly luciferase coding sequence, and the plasmid pαglob-RL, which expresses Renilla luciferase and was used as the control plasmid in transfection experiments, were described previously (37).
The plasmid p50mut1A1-FL is identical to p1A1-FL except for a double mutation at the −50 position in the NFI site. The NFI half-site sequence GCCA was converted to CGCA. This mutation was obtained by site-directed mutagenesis on p1A1-FL, using a mutated oligonucleotide and the GeneEdit kit (Promega) according to the manufacturer’s instructions.
A DNA sequence containing five Gal4 binding sites and a TATA box was excised from the plasmid pG5BCAT (described in reference 2) and inserted between the SacI and XmaI sites of the pGL3-FL vector (Promega), to give pG5-FL. A double-stranded oligonucleotide (5′ CCTTCTCACGCAACGCCGCGGCGCACGCAAGCTCTTCTCACGCGAG 3′) containing three XRE sequences (underlined) located around the −980, −900, and −500 positions in the human CYP1A1 gene promoter was inserted between the SacI and EcoRI sites of pG5-FL, upstream of the Gal4 sites, to give pXRE3G5-FL.
A 1,560-bp cDNA of the human CYP1A1 gene, a generous gift of I. de Waziers (INSERM, Paris, France) was inserted between the NotI and XbaI sites into the pOPRSVI/MCS vector (Stratagene) (also named pRSV/MCS in this study), which contains two procaryotic lac operons, to give pRSV-1A1. pLacI (from the Inducible Mammalian Expression System kit; Stratagene) expresses the LacI protein under the control of the cytomegalovirus (CMV) promoter. The smaller BamHI-NotI fragment from pRSV-1A1 was subcloned into the BamHI and NotI sites of pcDNA 1.1 AmpR (Invitrogen) (also named pCMV/MCS in this study) to give pCMV-1A1, which thus expresses the human CYP1A1 protein under the control of the CMV promoter.
pRSV.Gal.CTF, pRSV.Gal.Sp1, and pRSV.Gal.Oct are derived from the plasmids pGal(399–499), pGalOct2, and pGalSp1 (described in reference 2), in which the simian virus 40 promoters have been replaced by Rous sarcoma virus (RSV) promoters. They express fusion proteins containing the Gal4 DNA binding domain linked to the TAD of the human transcription factors NFI/CTF, Sp1 and Oct, respectively (2).
Transfection experiments.
Transfection experiments were performed in HepG2 cells by a standard calcium phosphate coprecipitation technique as previously described (37). The transfection efficiency was assayed with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining of intact cells transfected with a β-galactosidase expression vector according to the protocol of the p-Hook kit (Invitrogen). The metabolism of this compound by the enzyme β-galactosidase yields a blue staining of cells. Briefly, approximately 0.5 × 106 cells were trypsinized 30 h after transfection and resuspended in 100 μl of X-Gal reagent (a phosphate-buffered saline solution [pH 7.4] containing the following: X-Gal, 1 mg/ml in dimethylformamide; K3Fe(CN)6, 4 mM; K4Fe(CN)6, 4 mM; MgCl2, 2 mM). After overnight incubation at room temperature on a rocker, blue cells were counted under a microscope. The transfection efficiency was expressed as the quotient of the number of blue cells and the total number of cells multiplied by 100.
Dual luciferase assay (firefly and Renilla) was performed with a Promega kit. Renilla luciferase activity was used to normalize the transfection efficiency in all culture dishes. Blanks were obtained by assaying luciferase activity in mock-transfected cells.
Statistics.
Student’s two-tailed t-tests were performed by using Statview software (Abacus Concepts, Inc.).
RESULTS
CYP1A1-mediated intracellular H2O2 production.
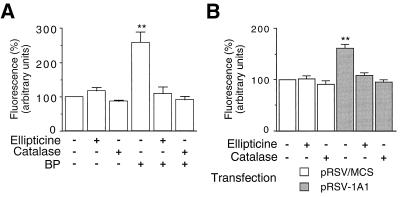
The production of ROS by CYP1A1 in cultured HepG2 cells was assayed as described in the Materials and Methods section, using H2DCF-DA as a probe. This compound yields DCF, a fluorescent compound, when oxidized within the cell by ROS, especially H2O2 (46). The induction of the endogenous CYP1A1 gene by BP caused a two- to threefold increase in DCF fluorescence (Fig. 1A). A similar increase was observed when cells were treated with dioxin (data not shown). The addition of ellipticine (a CYP1A1 inhibitor [53]) or catalase (an H2O2 scavenger) to the culture medium abolished the increase in DCF fluorescence. The compounds used to treat cell cultures (BP, ellipticine, and catalase) did not affect cell growth or viability (data not shown). These results suggest that the activity of CYP1A1 leads to an intracellular production of ROS, essentially H2O2.
FIG. 1.
Intracellular H2O2 generation by CYP1A1 activity. H2O2 levels within HepG2 cells were assayed as described in the Materials and Methods section. Cells were cultured for 30 h with or without addition of ellipticine (10 μM) or catalase (200 U/ml). Plates were then read in a fluorometer, and fluorescence was expressed in arbitrary units. Results were expressed as means ± standard errors of the means (n = 6), normalized to 100% for control cells. Statistical differences from values for the control are marked with double asterisks (P < 0.01). (A) Assay of the H2O2 produced following the induction of the endogenous CYP1A1 by BP (2.5 μM). (B) Assay of the H2O2 produced following the transfection of a CYP1A1 expression vector (pRSV-1A1). Control cells were transfected with pRSV/MCS.
The transfection of a plasmid expressing the human CYP1A1 isoform also led to a 60% increase in DCF fluorescence (Fig. 1B). As in the previous experiment, the increase was abolished by ellipticine or catalase. In our experiments, the transfection efficiency in HepG2 cells (see the Materials and Methods section) was about 20% (19.8% ± 4.4%, n = 3; data not shown). Thus, the level of intracellular oxidative stress obtained with the induction of the endogenous CYP1A1 gene and that obtained with the transfection of a CYP1A1 expression vector are within the same range.
The production of H2O2 limits CYP1A1 mRNA induction.
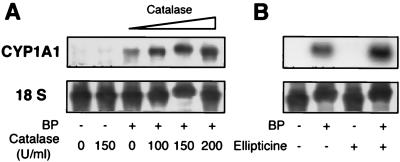
The effect of H2O2 (i.e., H2O2 released by CYP1A1 activity) on CYP1A1 induction was studied in cells of the human hepatoma cell line HepG2, where the regulation of this gene by Ah ligands, oxidative stress, and cytokines was demonstrated previously (37). The induction of the endogenous CYP1A1 gene was achieved by BP treatment of cell cultures and assayed by Northern blotting experiments. As shown in Fig. 2A, the induction was greatly enhanced when catalase was added together with the BP, whereas catalase alone did not increase the basal (i.e., noninduced) levels of CYP1A1 mRNAs. Ellipticine also enhanced the levels of CYP1A1 mRNAs induced by BP (Fig. 2B). The mean values for fold enhancement of the BP inducing effect by catalase (200 U/ml) and ellipticine (10 μM) were 4.2 ± 0.7 and 4.4 ± 1.3, respectively (n = 6). These experiments show that the induction of the endogenous CYP1A1 gene by BP alone is not maximal and is limited by a repressive mechanism. This limitation is, at least partially, abolished when the activity of CYP1A1 is inhibited or when the H2O2 release resulting from this activity is blunted. These data suggest that the expression of the endogenous CYP1A1 gene could be regulated in a negative-feedback loop by the activity of the enzyme itself.
FIG. 2.
Limitation of level of CYP1A1 mRNAs induced by CYP1A1 activity. Northern blots were prepared by using 20 μg of HepG2 cell mRNAs in each lane and probed with a labeled human CYP1A1 cDNA. Cells were treated for 72 h with the compounds mentioned below. After 36 h, the culture medium was changed and the treatment was resumed. BP (2.5 μM) was used in order to induce CYP1A1 mRNAs. (A) In addition to BP, the indicated concentrations of catalase were added to the culture medium. (B) In addition to BP, ellipticine (10 μM) was added, or not, to the culture medium.
Direct assessment of the autoregulation of CYP1A1.
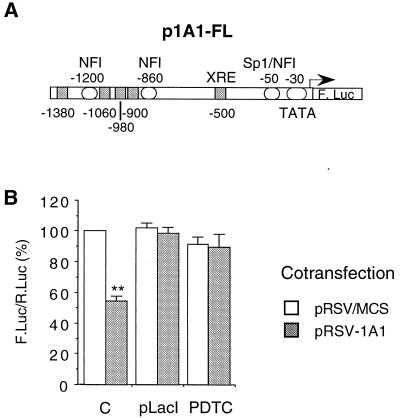
We next designed an experimental approach to directly assess the effect of increased CYP1A1 activity on CYP1A1 gene expression. Indeed, the induction of the endogenous enzyme by AhR ligands interferes with the promoter activity and may not be appropriate for studying the repression. Thus, the increase in CYP1A1 activity was achieved by transfection of a CYP1A1-expressing vector (pRSV-1A1). As shown above (Fig. 1), this procedure leads to a modification of the intracellular redox status that is similar to the one produced by the endogenous enzyme induction (Fig. 1). Cotransfection of a luciferase reporter gene driven by the CYP1A1 gene promoter (Fig. 3A) allowed us to evaluate the effect of increased CYP1A1 activity on its own gene promoter activity. When cells were cotransfected with the pRSV-1A1 vector expressing the human CYP1A1 protein, the activity of the CYP1A1 gene promoter decreased by half (Fig. 3B). The pRSV-1A1 vector contains two lac operons located in the immediate vicinity of the RSV promoter. This allows the repression of the latter promoter by the LacI protein. When the pLacI vector, expressing the LacI protein, was cotransfected with pRSV-1A1, the inhibitory effect on the CYP1A1 promoter observed was abolished (Fig. 3B). Thus, this effect was indeed mediated by the transfected CYP1A1 gene expression. Treatment of cells with pyrrolidine dithiocarbamate, a redox-active compound containing two thiol moieties, prevented the effect of the CYP1A1 protein expression, suggesting a contribution of ROS in this regulation (Fig. 3B).
FIG. 3.
Autoregulation of CYP1A1 (basal activity). HepG2 cells were transfected with the p1A1-FL reporter plasmid (2 μg). (A) Structure of the 1.6-kb-long human CYP1A1 promoter used in transfection experiments (p1A1-FL vector). (B) Effect of the expression of CYP1A1 on the basal activity of the CYP1A1 promoter. In order to express the CYP1A1 protein, cells were cotransfected with 4 μg of the pRSV-1A1 expression vector (gray bars). In control cells (open bars), the same plasmid lacking the CYP1A1 cDNA (pRSV/MCS) was transfected in order to have an equivalent amount of total transfected DNA. All the cells were cotransfected with pαglob-RL (1 μg) as an internal control. Results were expressed as the means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n = 10), normalized to 100% for control cells transfected with pRSV/MCS. A statistical difference between pRSV/MCS and pRSV-1A1 for the same condition is marked with a double asterisk (P < 0.0001).
The experiments described above were performed in the absence of an exogenous substrate of CYP1A1. It is thus likely that overexpressed CYP1A1 metabolized endogenous substrates.
Autoregulation of the induced CYP1A1 in the presence of BP.
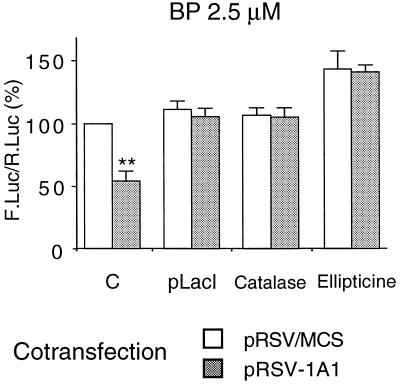
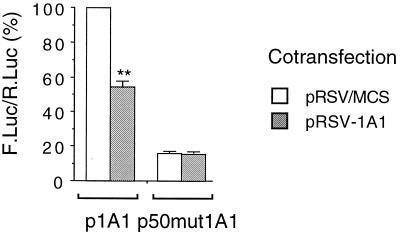
BP, which is a known ligand of the AhR, strongly induced the transcription driven by the CYP1A1 promoter (2.5 μM BP for 24 h led to a 35 ± 9 fold induction; data not shown). It is also a substrate of CYP1A1 that can be converted into an epoxide by this enzyme (reference 27 and references within). In the presence of BP, the cotransfection of pRSV-1A1 led to a decrease by half of the induced CYP1A1 gene promoter activity (Fig. 4). As observed with the basal activity, this effect was the result of the transfected CYP1A1 cDNA expression since it was abolished by the cotransfection of the pLacI vector. In cells treated with catalase (an H2O2-scavenging enzyme) or ellipticine, the BP-induced activity of the CYP1A1 promoter was no longer sensitive to CYP1A1 protein expression. Thus, both basal and induced CYP1A1 promoter activities are down-regulated by the activity of CYP1A1, in an H2O2-dependent manner.
FIG. 4.
Autoregulation of CYP1A1 (BP-induced activity). HepG2 cells were transfected with the p1A1-FL plasmid (2 μg). In order to express the CYP1A1 protein, cells were cotransfected with 4 μg of the pRSV-1A1 expression vector (gray bars). In control cells (open bars), the same plasmid lacking the CYP1A1 cDNA (pRSV/MCS) was transfected in order to have an equivalent amount of total transfected DNA. All cells were cotransfected with pαglob-RL (1 μg) as an internal control. Results were expressed as the means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n = 10), normalized to 100% for control cells transfected with pRSV/MCS. A statistical difference between pRSV/MCS and pRSV-1A1 for the same condition is marked with a double asterisk (P < 0.0001). All cells were treated by BP (2.5 μM) for 24 h before harvest. C, control cells; pLacI, cells cotransfected with the pLacI plasmid (1 μg); Catalase, cells treated with catalase (100 U/ml) for 24 h; and Ellipticine, cells treated with ellipticine (10 μM) for 24 h.
A mutation in the proximal NFI site abolishes the autoregulation.
We have shown previously that NFI-driven promoters are sensitive to oxidative stress (37). We thus tested whether the mutation of the proximal NFI site in the CYP1A1 gene promoter affected the autoregulation. The p50mut1A1-FL vector is identical to the p1A1-FL vector except for a mutation in the NFI site located at the −50 position in the proximal promoter of the CYP1A1 gene. This mutation decreases the basal activity about fivefold (Fig. 5), but this activity remains well above background levels (37). We have shown previously that this mutation abolished the repression of the promoter by oxidative stress (37). The results shown in Fig. 5 indicate that the expression of the CYP1A1 protein had no effect on the activity of p50mut1A1-FL, suggesting a role for the NFI site in the autoregulatory process (Fig. 5).
FIG. 5.
Effect of CYP1A1 expression on the NFI-mutated CYP1A1 promoter. Cells were transfected with the p1A1-FL or the p50mut1A1-FL reporter plasmid and cotransfected with either the CYP1A1-expressing vector pRSV-1A1 (4 μg) (gray bars) or the vector pRSV/MCS (4 μg) (open bars) in order to transfect similar amounts of DNA. All cells were cotransfected with pαglob-RL (1 μg) as an internal control. Results were expressed as means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n = 8), normalized to 100% for cells transfected with p1A1-FL and pRSV/MCS. A statistical difference between pRSV/MCS and pRSV-1A1 for the same condition is marked with a double asterisk (P < 0.0001).
The transactivating function of NFI/CTF is sensitive to CYP1A1 activity.
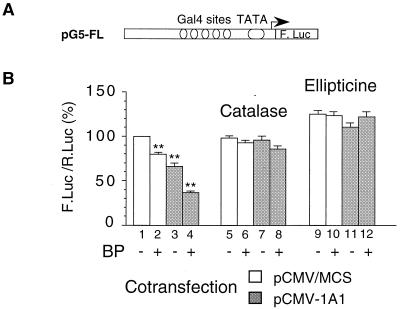
To study the role of the transcription factor NFI in the activation of the CYP1A1 gene promoter and its down-regulation by the activity of the CYP1A1 protein, we focused on its TAD. Indeed, our previous studies suggested that the transactivating function of NFI/CTF was repressed at lower concentrations of H2O2 than its DNA binding activity. Fusion proteins containing the DNA binding domain of the bacterial Gal4 protein coupled to the TADs of various human transcription factors were cotransfected with the pG5-FL reporter vector containing five Gal4 binding sites (Fig. 6A). The cotransfection of the vector expressing the NFI/CTF-Gal4 fusion protein activated the transcription of pG5-FL about fourfold (3.6 ± 0.2 fold, data not shown; see also Fig. 8). In the experiments whose results are shown in Fig. 6 and 7, we used pCMV-1A1 as a CYP1A1 expression vector (this vector was used instead of pRSV-1A1 because we noticed a poor expression of pG5-FL upon cotransfection with pRSV-1A1, possibly due to a decreased expression of NFI/CTF, which is also driven by an RSV promoter).
FIG. 6.
The transactivating function of NFI/CTF is altered by CYP1A1 activity. (A) Structure of the promoter driving the reporter gene in the pG5-FL plasmid. (B) Activity driven by the TAD of NFI/CTF. Cells were cotransfected with the pG5-FL (1.75 μg) reporter vector and 2.5 μg of the pRSV.Gal.CTF vector expressing a Gal-TAD fusion protein (see the Materials and Methods section). Cells were cotransfected with 3 μg of either the CYP1A1 expression vector pCMV-1A1 (gray bars) or the empty pCMV/MCS vector (open bars). All cells were cotransfected with pαglob-RL (0.75 μg) as an internal control. Cells were untreated or treated with BP (2.5 μM) for 40 h. In addition, cell were treated with catalase (100 U/ml) (lanes 5 to 8) or ellipticine (10 μM) (lanes 9 to 12) for 40 h. Dimethyl sulfoxide vehicle (0.5% [vol/vol] final concentration) did not significantly change the expression of the reporter gene. Results were expressed as means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n > 8), normalized to 100% for control cells transfected with pCMV/MCS. For each group of results (lanes 1 to 4, 5 to 8, and 9 to 12), values were compared to that for the corresponding pCMV/MCS-transfected control (lane 1, 5, and 9, respectively), and only differences that are statistically significant are marked with double asterisks (P < 0.001).
FIG. 8.
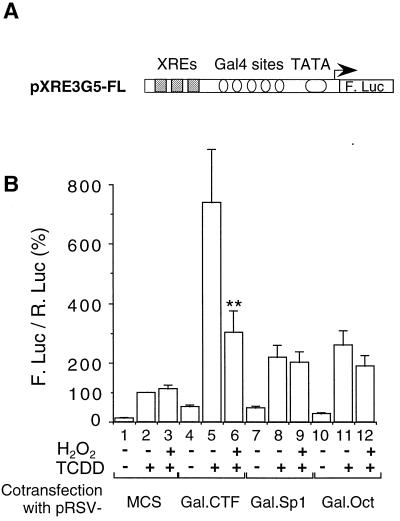
Synergistic effect of TCDD signaling and NFI/CTF. (A) Structure of the promoter driving the reporter gene in the pXRE3G5-FL plasmid. (B) Cells were transfected with the pXRE3G5-FL reporter vector (2.5 μg) and with 3 μg of either pRSV.Gal.CTF, pRSV.Gal.Sp1, pRSV.Gal.Oct (see the Materials and Methods section), or pRSV/MCS. All cells were cotransfected with pαglob-RL (1 μg) as an internal control. Cells were left untreated or treated with TCDD (3 nM, and/or H2O2 (50 μM) for 16 h. Results were expressed as means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n = 9), normalized to 100% for cells transfected with pRSV/MCS and treated with TCDD. For each Gal fusion, statistical differences between TCDD-treated cells treated with H2O2 (50 μM) and cells not treated are marked with a double asterisk (P < 0.0001).
FIG. 7.
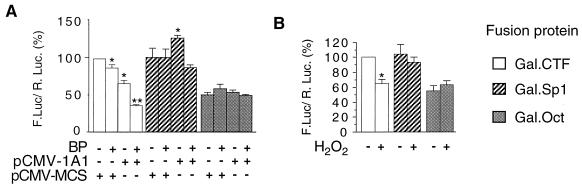
Comparison of the sensitivities of the TADs of NFI/CTF, Sp1, and Oct. Cells were cotransfected with the pG5-FL (1.75 μg) reporter vector and 2.5 μg of either pRSV.Gal.CTF, pRSV.Gal.Sp1, or pRSV.Gal.Oct (see the Materials and Methods section). All cells were cotransfected with pαglob-RL (0.75 μg) as an internal control. Results were expressed as means ± standard errors of the means for the quotient of firefly luciferase activity and Renilla luciferase activity (n > 8), normalized to 100% for control cells transfected with pRSV.Gal.CTF and pCMV/MCS. For each Gal-TAD fusion, statistical differences from the untreated pRSV/MCS-transfected control cells are marked with an asterisk (P < 0.01) or a double asterisk (P < 0.0001). (A) Cells were cotransfected with either the CYP1A1-expressing vector pCMV-1A1 (3 μg) or the pCMV/MCS (3 μg) vector in order to transfect similar amounts of DNA and were treated or not with BP (2.5 μM) for 40 h. (B) Effect of oxidative stress on the TADs of NFI/CTF, Sp1, and Oct. Cells were treated or not with H2O2 (17.5 μM) for 16 h.
As can be seen in Fig. 6B, the transactivating function of NFI/CTF was decreased upon cotransfection of pCMV-1A1 (compare lanes 1 and 3). The addition of BP to the culture medium slightly (but significantly) decreased the transactivating function of NFI/CTF, even in the absence of pCMV-1A1 (20% inhibition; compare lanes 1 and 2). This is probably due to the induction of the endogenous CYP1A1. It is noticeable that the addition of BP did not lead to a significant decrease when cells were treated with catalase or ellipticine (compare lanes 5 and 6 and lanes 9 and 10).
Upon cotransfection of the pCMV-1A1 vector, BP strongly inhibited the transactivating function of NFI/CTF, by almost 70% (compare lanes 1 and 4). The addition of catalase or ellipticine abolished the inhibitory effect observed with pCMV-1A1, in the absence of BP (compare lanes 5 and 7 and lanes 9 and 11) or in the presence of BP (compare lanes 5 and 8 and lanes 9 and 12). These data suggest that the TAD of NFI/CTF is a likely target of CYP1A1 activity and mediates the autoregulation of the CYP1A1 gene expression.
Specificity of NFI/CTF TAD regulation.
In order to assess the specificity of the NFI/CTF role in CYP1A1 autoregulation, the TADs of the ubiquitous transcription factors Sp1 and Oct were also tested. The Sp1 transcription factor has been shown to play an important role in the activation of the CYP1A1 gene promoter (31). In this study, the TAD of Oct was used as a negative control. In contrast to NFI/CTF, the transactivating functions of Sp1 and Oct were decreased neither by the expression of the CYP1A1 protein nor by BP treatment (Fig. 7A). It should be noted that NFI/CTF and Sp1 had a similar efficiency in activating transcription, whereas Oct was about 60% as efficient as NFI/CTF (Fig. 7A). We then assessed the sensitivity of these TADs to direct oxidative stress (Fig. 7B). Upon H2O2 treatment, the NFI transactivating function was significantly decreased whereas that of either Sp1 or Oct was not. These data highlight the particular sensitivity of NFI to oxidative stress.
Reconstitution of the cooperation between NFI and XRE sites.
The data presented above suggested a critical role of the TAD of NFI/CTF in the autoregulation of the CYP1A1 gene and its regulation by oxidative stress. Since the major transcription factor controlling the induction of the CYP1A1 gene is the Ah receptor, we next asked whether we could provide evidence for a putative synergy between the TAD of NFI/CTF and the AhR. We thus attempted to reconstitute an AhR-sensitive system using the pXRE3G5-FL reporter vector (Fig. 8A). Three XRE present in the CYP1A1 promoter (see the Materials and Methods section) were inserted upstream of the Gal4 binding sites in the pG5-FL vector. Upon cotransfection with vectors expressing the above-mentioned fusion proteins and TCDD induction, this vector allowed us to assess the role that the TAD of a transcription factor can play in the Ah response mediated by the XRE sequences. As can be seen in Fig. 8B, TCDD induced almost eightfold the expression of the luciferase gene in the pXRE3G5-FL vector (compare lanes 1 and 2). This expression was also activated upon cotransfection of the vectors expressing the fusion proteins Gal.CTF, Gal.Sp1, and Gal.Oct in the absence of TCDD treatment (compare lane 1 to lanes 4, 7, and 10, respectively). The inducing effect of the Gal.CTF fusion protein on the expression of the reporter gene was similar to that of the Gal.Sp1 fusion (about fivefold) and approximately twice that of Gal.Oct, in agreement with the results shown in Fig. 7. Thus, the neighboring XRE and Gal4 binding sites are responsive to their respective specific stimuli.
When the cells were cotransfected with the Gal.CTF expression vector and treated with TCDD, the reporter gene expression was increased almost 60-fold, revealing a synergistic effect of the two stimuli (compare lane 5 to lanes 2 and 4). Cooperation with the TCDD treatment was much less potent in the case of the Gal.Sp1 and Gal.Oct fusions. Indeed, in the presence of Gal.CTF, TCDD elicited a 14-fold increase in the reporter gene activity (compare lanes 4 and 5), whereas in the case of Gal.Sp1, TCDD elicited only a fourfold increase (compare lanes 7 and 8). Thus, the TAD of NFI/CTF displays a particularly efficient synergy with the AhR signaling, at least with the target promoter and the experimental conditions used in this study.
We then tested the effect of oxidative stress on the activation of the XRE-Gal4 promoter (pXRE3G5-FL reporter vector). H2O2 did not affect the TCDD response in the absence of cotransfected fusion protein (compare lanes 2 and 3). This suggests that, under our experimental conditions, the AhR activity itself is not sensitive to oxidative stress. Furthermore, H2O2 treatment did not significantly affect the induction of the promoter by a combination of TCDD treatment and either Gal.Sp1 or Gal.Oct cotransfection. In contrast, under the same experimental conditions, H2O2 decreased by more than 60% the promoter activity elicited by a combination of TCDD treatment and Gal.CTF cotransfection.
In conclusion, the activity of the TAD of NFI/CTF is specifically repressed by oxidative stress in two different promoter contexts, i.e., when it is the major activator of transcription (cf. Fig. 7B) and when it acts in synergy with the AhR (cf. Fig. 8B).
DISCUSSION
The cellular steady state of a protein results from a balance among several processes: the positive and negative regulation of mRNA transcription, mRNA stability, protein stability, and transport. It has been shown that the fine tuning of these processes sometimes requires feedback loops and autoregulatory mechanisms. This is the case in particular for several transcription factors and nuclear receptors which regulate their own expressions (as discussed in the introduction). Yet, autoregulatory mechanisms have been rarely described for other proteins.
Autoregulation requirements are particularly important when the gene is highly inducible because several steady states are reached under different conditions. CYP1A1 is a typical case of a highly inducible gene. Furthermore, the precise determination of the levels of cytochromes P450 is all the more important that these enzymes can produce ROS during their catalytic cycles (8, 41), leading to cell toxicity (8, 9, 39). It is therefore critical to control the activity of an enzyme such as CYP1A1, in particular by regulating its expression level.
In this study, we present data supporting the following model for the autoregulation of CYP1A1 expression (Fig. 9): (i) BP activates CYP1A1 expression via the AhR pathway; (ii) the subsequent increase in CYP1A1 activity leads to H2O2 production; (iii) NFI transactivating function is altered by H2O2, which in turn represses CYP1A1 expression. This mechanism should limit the expression of the CYP1A1 gene and thus prevent an excessively large accumulation of the protein and of ROS.
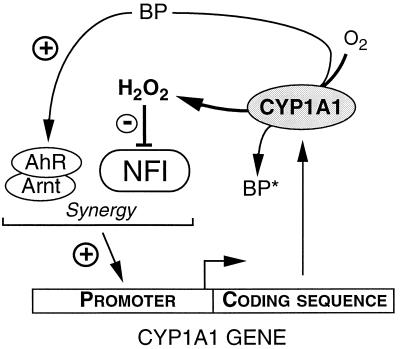
FIG. 9.
Autoregulation of CYP1A1 gene expression. This scheme summarizes the autoregulatory mechanism proposed in this study. The Ah receptor (AhR), after activation by a ligand such as BP, dimerization with Arnt, and interaction with transcription factors, stimulates the CYP1A1 gene promoter. The interaction with NFI leads to a synergistic effect on transcription. This leads to the synthesis of the enzyme. CYP1A1 enzymatic activity generates H2O2, especially in the presence of uncoupled substrates such as BP (which can be converted into a metabolite [BP*]). H2O2 then alters NFI function, mainly at the level of its TAD. In consequence, NFI loses its ability to activate the CYP1A1 gene basal promoter activity and to act in synergy with the AhR signaling, which limits the expression of the CYP1A1 enzyme.
The formation of H2O2 by microsomal cytochromes P450 has been consistently observed, particularly with uncoupled substrates (19, 33) including BP (20). Recently, in vitro experiments studying electron transport during the catalytic cycle of human CYP1A1 showed that this enzyme could produce H2O2 (43). Within the cellular context, 8-oxo-guanine formation in DNA was observed after CYP1A1 induction (39), showing that CYP1A1 can generate oxidative stress in vivo and have a nuclear impact. Consistently, a recent study showed that TCDD treatment of mice led to a sustained oxidation of hepatic glutathione and 8-oxo-guanine formation (51). In this study, we show that the activity of CYP1A1 leads to an intracellular H2O2 production in HepG2 cells. Our data suggest that the H2O2 released by CYP1A1 is required for the autoregulatory process since antioxidants, in particular catalase, disrupt this effect. The activity of CYP1A1 was required for gene repression since ellipticine could prevent this effect. The next step in our model, i.e., the repression of the TAD of NFI/CTF by oxidative stress, is supported by data from this study. We have previously shown that NFI binding to DNA was blunted by millimolar amounts of H2O2 or by glutathione depletion (37). Here we show that the TAD of NFI is repressed by much smaller amounts (in the range from 10 to 100 μM H2O2). Thus, this function of NFI is the likely relevant target in vivo. Furthermore, we show that the expression of CYP1A1 represses the TAD of NFI as efficiently as exogenous H2O2 addition. The question of whether the conformation of the TAD is modified by oxidative stress needs further investigation. In this respect, we are presently investigating the amino acid targeted by H2O2 in the TAD and have found that a critical cysteine seems to be involved. These molecular mechanisms are novel since most studies (in eukaryotic cells) on the oxidative modulation of transcription factors have focused on their DNA binding activities without a specific assessment of their transactivating functions. It appears to be a likely mechanism in vivo since it involves limited H2O2 concentrations (such as those generated by CYP1A1 activity within the cell). Since NFI is a ubiquitous transcription factor, its oxidative repression could modulate the expression of several genes (depending on the relative importance of NFI function compared to that of other transcription factors for the activation of the promoter).
The last step in our model is the contribution of NFI to the expression and regulation of the CYP1A1 gene promoter. We have previously shown that the mutation of the NFI site located in the proximal promoter (BTE) decreased both basal and induced promoter activities, in agreement with other studies (24). This mutation prevented the negative regulation by H2O2 (37). We also observed that other, more conserved NFI sites within the enhancer regions also contribute to the induction of the promoter but not to its basal activity (data not shown). We have reconstituted a system evaluating the synergy between the AhR pathway and transcription factor transactivating domains and have shown that the TAD of NFI is more efficient than other TADs in cooperating with the Ah activation of a XRE-driven promoter. These data do not preclude the contribution of other transcription factors that bind to the promoter, such as Sp1 (31), but they highlight the important contribution of NFI to the activation of CYP1A1 by AhR ligands and its crucial role in the repression of this activation by H2O2. Indeed, in our experiments, the induction of the XRE-driven reporter gene by TCDD, in the absence of the TAD of NFI, was not altered by H2O2, underscoring again the role of NFI in the down-regulation (e.g., in the autoregulation) of CYP1A1 by oxidative stress. It would be interesting to assess whether H2O2 alters directly a putative NFI-AhR interaction or if the mechanism involves an interaction with a common coactivator.
The autoregulatory loop shown here may not be the only one operating to control the level of CYP1A1 gene expression. TCDD, which strongly induces CYP1A1 expression, also stimulates the expression of the cytokines TNF-α and interleukin-1 (16). These cytokines can in turn down-regulate the CYP1A1 gene expression (1, 4, 40). In the case of TNF-α, we have shown that NFI and ROS were involved in the signaling mechanism (37). In an interesting study, Fujii-Kuriyama et al. have suggested that the AhR, stimulated by TCDD, could induce the expression of a specific repressor (AhRR), which then limits the AhR effect (35). Indeed, the autoregulatory mechanism does not imply a decrease in AhR or Arnt production, since the expression of CYP1A1 does not affect the levels of their mRNAs (25). Moreover, it was reported recently that the transcription factor NF-κB (which is induced by H2O2 and TNF-α [13, 49]) and the AhR displayed mutual functional repression (54). Thus, more than one mechanism could be involved in the fine tuning of the CYP1A1 gene expression. The existence of several such mechanisms is likely to be due to the deleterious effect of the oxidative stress generated by a high activity of the CYP1A1 enzyme (39, 51, 52) as well as the activation of carcinogenic compounds (references 26 and 47 and references therein). The autoregulation of CYP1A1 gene expression and its down-regulation by H2O2 and inflammatory cytokines are part of a general cellular response to exogenous or endogenous oxidative stress. This response involves the well-known activation of antioxidant enzymes (10). However, this may not be sufficient, and it is important to prevent the intracellular production of ROS by repressing ROS-producing metabolic pathways, such as those catalyzed by monooxygenases (e.g., CYP1A1). In this respect, we are presently studying the redox regulation of other CYP isoforms. Owing to an historical focus on gene inductions, transcriptional repression by oxidative stress has been less studied than its activating capacity and few molecular mechanisms have been demonstrated. The repression of the transactivating function of NFI/CTF appears to be one such mechanism.
In addition to oxidative stress, CYP1A1 is also repressed under hypoxic conditions. The group of Poellinger has shown that, in the case of hypoxia, the hypoxia-inducible factor HIF1α was stabilized and could recruit Arnt, its heterodimerization partner (15). A decrease in the available Arnt protein could, in turn, alter the AhR response and thus limit CYP1A1 expression. This contributes to the limitation of O2 waste in monooxygenase-based metabolism under hypoxic conditions. In conclusion, the CYP1A1 gene appears to undergo several repressive regulations at the transcriptional level, owing to its high inducibility and to the possible subsequent toxic effect within the cell.
ACKNOWLEDGMENTS
This work was supported by INSERM, Université Paris V-René Descartes, Fondation pour la Recherche Médicale (grant 1000031401), and Région Ile-de-France. N.M. is supported by grants from the Swiss NSF and the Etat de Vaud.
We especially thank M. Kobr for his kind and useful help with Gal fusions experiments. We thank P. Beaune and M. Aggerbeck for critical reading of the manuscript and I. de Waziers and P. Maurel for the generous gifts of CYP1A1 cDNA and gene promoter, respectively.
REFERENCES
- 1.Abdel-Razzak Z, Corcos L, Fautrel A, Campion J-P, Guillouzo A. Transforming growth factor-β1 down-regulates basal and polycyclic aromatic hydrocarbon-induced cytochromes P-450 1A1 and 1A2 in adult human hepatocytes in primary culture. Mol Pharmacol. 1994;46:1100–1110. [PubMed] [Google Scholar]
- 2.Alevizopoulos A, Dusserre Y, Tsai-Pflugfelder M, von der Weid T, Wahli W, Mermod N. A proline-rich TGF-β-responsive transcriptional activator interacts with histone H3. Genes Dev. 1995;9:3051–3066. doi: 10.1101/gad.9.24.3051. [DOI] [PubMed] [Google Scholar]
- 3.Barker C W, Fagan J B, Pasco D S. Down-regulation of P450 1A1 and 1A2 mRNA expression in isolated hepatocytes by oxidative stress. J Biol Chem. 1994;269:3985–3990. [PubMed] [Google Scholar]
- 4.Barker C W, Fagan J B, Pasco D S. Interleukin-1β suppresses the induction of P4501A1 and P4501A2 mRNAs in isolated hepatocytes. J Biol Chem. 1992;267:8050–8055. [PubMed] [Google Scholar]
- 5.Bondy S C, Naderi S. Contribution of hepatic cytochrome P450 systems to the generation of reactive oxygen species. Biochem Pharmacol. 1994;48:155–159. doi: 10.1016/0006-2952(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 6.Boucher P D, Piechocki M P, Hines R N. Partial characterization of the human CYP1A1 negatively acting transcription factor and mutational analysis of its cognate DNA recognition sequence. Mol Cell Biol. 1995;15:5144–5151. doi: 10.1128/mcb.15.9.5144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butkiewicz D, Grzybowska E, Hemminki K, Ovrebo S, Haugen A, Motykiewicz G, Chorazy M. Modulation of DNA adduct levels in human mononuclear white blood cells and granulocytes by CYP1A1, CYP2D6 and GSTM1 genetic polymorphisms. Mutat Res. 1998;415:97–108. doi: 10.1016/s1383-5718(98)00064-3. [DOI] [PubMed] [Google Scholar]
- 8.Chen Q, Cederbaum A I. Cytotoxicity and apoptosis produced by cytochrome P450 2E1 in Hep G2 cells. Mol Pharmacol. 1998;53:638–648. doi: 10.1124/mol.53.4.638. [DOI] [PubMed] [Google Scholar]
- 9.Chen Q, Galleano M, Cederbaum A I. Cytotoxicity and apoptosis produced by arachidonic acid in Hep G2 cells overexpressing human cytochrome P4502E1. J Biol Chem. 1997;272:14532–14541. doi: 10.1074/jbc.272.23.14532. [DOI] [PubMed] [Google Scholar]
- 10.Crawford D R, Davies K J. Adaptive response and oxidative stress. Environ Health Perspect. 1994;102(Suppl. 10):25–28. doi: 10.1289/ehp.94102s1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feilleux-Duché S, Garlatti M, Aggerbeck M, Bouguet J, Hanoune J, Barouki R. Phorbol esters inhibit the glucocorticoid-mediated stimulation of cytosolic aspartate aminotransferase gene transcription. Biochem J. 1994;297:497–502. doi: 10.1042/bj2970497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garlatti M, Aggerbeck M, Bouguet J, Barouki R. Contribution of nuclear factor I binding site to the glucocorticoid regulation of cytosolic aspartate aminotransferase gene promoter. J Biol Chem. 1996;271:32629–32634. doi: 10.1074/jbc.271.51.32629. [DOI] [PubMed] [Google Scholar]
- 13.Ginn-Pease M E, Whisler R L. Redox signals and NF-κB activation in T cells. Free Radic Biol Med. 1998;25:346–361. doi: 10.1016/s0891-5849(98)00067-7. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez F J, Nebert D W. Autoregulation plus upstream positive and negative control regions associated with transcriptional activation of the mouse P1(450) gene. Nucleic Acids Res. 1985;13:7269–7288. doi: 10.1093/nar/13.20.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gradin K, McGuire J, Wenger R H, Kvietikova I, Whitelaw M L, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grantley D C, Shiverick K T. 2,3,7,8-Tetrachloro-p-dioxin increases mRNAs levels for interleukin-1β, urokinase plasminogen activator and tumor necrosis factor alpha in human uterine endometrial adenocarcinoma RL95-2 cells. Biochem Biophys Res Commun. 1997;238:338–342. doi: 10.1006/bbrc.1997.7291. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson O. The aryl hydrocarbon receptor complex. Annu Rev Pharmacol Toxicol. 1995;35:307–340. doi: 10.1146/annurev.pa.35.040195.001515. [DOI] [PubMed] [Google Scholar]
- 18.Harris D, Reiss N, Naor Z. Differential activation of protein kinase C δ and ɛ gene expression by gonadotropin-releasing hormone in alphaT3-1 cells. Autoregulation by protein kinase C. J Biol Chem. 1997;272:13534–13540. doi: 10.1074/jbc.272.21.13534. [DOI] [PubMed] [Google Scholar]
- 19.Heinemeyer G, Hildebrandt A G, Roots I, Lehne L, Nigam S. Demonstration of drug-ethanol interactions by changes in activity of hepatic microsomal oxidase/oxygenase cytochrome P-450 function. Arch Toxicol Suppl. 1979;2:491–496. doi: 10.1007/978-3-642-67265-1_62. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrandt A G, Bergs C, Heinemeyer G, Schlede E, Roots I, Abbas-Ali B, Schmoldt A. Studies on the mechanism of stimulation of microsomal H2O2 formation and benzo(a)pyrene hydroxylation by substrates and flavone. Adv Exp Med Biol. 1981;136A:179–198. doi: 10.1007/978-1-4757-0674-1_11. [DOI] [PubMed] [Google Scholar]
- 21.Hines R N, Mathis J M, Jacob C S. Identification of multiple regulatory elements on the human cytochrome P450IA1 gene. Carcinogenesis. 1988;9:1599–1605. doi: 10.1093/carcin/9.9.1599. [DOI] [PubMed] [Google Scholar]
- 22.Ichiba M, Nakajima K, Yamanaka Y, Kiuchi N, Hirano T. Autoregulation of the Stat3 gene through cooperation with a cAMP-responsive element-binding protein. J Biol Chem. 1998;273:6132–6138. doi: 10.1074/jbc.273.11.6132. [DOI] [PubMed] [Google Scholar]
- 23.Imataka H, Sogawa K, Yasumoto K, Kikuchi Y, Sasano K, Kobayashi A, Hayami M, Fujii-Kuriyama Y. Two regulatory proteins that bind to the basic transcription element (BTE), a GC box sequence in the promoter region of the rat P-4501A1 gene. EMBO J. 1992;11:3663–3671. doi: 10.1002/j.1460-2075.1992.tb05451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jones K W, Whitlock J P., Jr Functional analysis of the transcriptional promoter for the CYP1A1 gene. Mol Cell Biol. 1990;10:5098–5105. doi: 10.1128/mcb.10.10.5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jorgensen E C, Autrup H. Autoregulation of human CYP1A1 gene promoter activity in HepG2 and MCF-7 cells. Carcinogenesis. 1996;17:435–441. doi: 10.1093/carcin/17.3.435. [DOI] [PubMed] [Google Scholar]
- 26.Kadlubar F, Hammons G. The role of cytochrome P450 in the metabolism of chemical carcinogens. In: Guengerich F P, editor. Mammalian cytochromes P-450. Boca Raton, Fla: CRC Press; 1987. pp. 81–130. [Google Scholar]
- 27.Kim J, Stansbury K, Walker N, Trush M, Strickland P, Sutter T. Metabolism of benzo(a)pyrene and benzoapyrene-7,8-diol by human cytochrome P450 1B1. Carcinogenesis. 1998;19:1847–1853. doi: 10.1093/carcin/19.10.1847. [DOI] [PubMed] [Google Scholar]
- 28.Kiyohara C, Nakanishi Y, Inutsuka S, Takayama K, Hara N, Motohiro A, Tanaka K, Kono S, Hirohata T. The relationship between CYP1A1 aryl hydrocarbon hydroxylase activity and lung cancer in a Japanese population. Pharmacogenetics. 1998;8:315–323. doi: 10.1097/00008571-199808000-00005. [DOI] [PubMed] [Google Scholar]
- 29.Knowles B B, Howe C D, Aden D P. Human hepatocellular carcinoma cell lines secrete the major plasma proteins and hepatitis B surface antigen. Science. 1980;209:497–499. doi: 10.1126/science.6248960. [DOI] [PubMed] [Google Scholar]
- 30.Ko H P, Okino S T, Ma Q, Whitlock J P., Jr Dioxin-induced CYP1A1 transcription in vivo: the aromatic hydrocarbon receptor mediates transactivation, enhancer-promoter communication, and changes in chromatin structure. Mol Cell Biol. 1996;16:430–436. doi: 10.1128/mcb.16.1.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kobayashi A, Sogawa K, Fujii-Kuriyama Y. Cooperative interaction between AhR.Arnt and Sp1 for the drug-inducible expression of CYP1A1 gene. J Biol Chem. 1996;271:12310–12316. doi: 10.1074/jbc.271.21.12310. [DOI] [PubMed] [Google Scholar]
- 32.Koch K S, Fletcher R G, Grond M P, Inyang A I, Lu X P, Brenner D A, Leffert H L. Inactivation of plasmid reporter gene expression by one benzo(a)pyrene-diol-epoxide DNA adduct in adult rate hepatocytes. Cancer Res. 1993;53:2279–2286. [PubMed] [Google Scholar]
- 33.Kuthan H, Ullrich V. Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur J Biochem. 1982;126:583–588. doi: 10.1111/j.1432-1033.1982.tb06820.x. [DOI] [PubMed] [Google Scholar]
- 34.Le Marchand L, Sivaraman L, Pierce L, Seifried A, Lum A, Milkens L, Lau A. Associations of CYP1A1, GSTM1 and CYP2E1 polymorphisms with lung cancer suggest cell type specificities to tobacco carcinogens. Cancer Res. 1998;58:4858–4863. [PubMed] [Google Scholar]
- 35.Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Molina C A, Foulkes N S, Lalli E, Sassone-Corsi P. Inducibility and negative autoregulation of CREM: an alternative promoter directs the expression of ICER, an early response repressor. Cell. 1993;75:875–886. doi: 10.1016/0092-8674(93)90532-u. [DOI] [PubMed] [Google Scholar]
- 37.Morel Y, Barouki R. Down-regulation of cytochrome P450 1A1 gene promoter by oxidative stress: critical contribution of NFI. J Biol Chem. 1998;273:26969–26976. doi: 10.1074/jbc.273.41.26969. [DOI] [PubMed] [Google Scholar]
- 38.Neuhold L A, Shirayoshi Y, Ozato K, Jones J E, Nebert D W. Regulation of mouse CYP1A1 gene expression by dioxin: requirement of two cis-acting elements during induction. Mol Cell Biol. 1989;9:2378–2386. doi: 10.1128/mcb.9.6.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Park J Y, Shigena M K, Ames B N. Induction of cytochrome P4501A1 by 2,3,7,8-tetrachlorodibenzo-p-dioxin or indolo(3,2-b)carbazole is associated with oxidative DNA damage. Proc Natl Acad Sci USA. 1996;93:2322–2327. doi: 10.1073/pnas.93.6.2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Paton T E, Renton K W. Cytokine-mediated down-regulation of CYP1A1 in Hepa1 cells. Biochem Pharmacol. 1998;55:1791–1796. doi: 10.1016/s0006-2952(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 41.Perret A, Pompon D. Electron shuttle between membrane-bound cytochrome P450 3A4 and b5 rules uncoupling mechanisms. Biochemistry. 1998;37:11412–11424. doi: 10.1021/bi980908q. [DOI] [PubMed] [Google Scholar]
- 42.Piechocki M P, Hines R N. Functional characterization of the human CYP1A1 negative regulatory element: modulation of Ah receptor mediated transcriptional activity. Carcinogenesis. 1998;19:771–780. doi: 10.1093/carcin/19.5.771. [DOI] [PubMed] [Google Scholar]
- 43.Pompon D, Perret A, Laine R, Urban P. Abstracts of the 1st International Workshop on Free Radicals in Liver Metabolism and Disease, 1997, Dinard, France. Rennes, France: Université de Rennes I; 1997. Molecular mechanism of modulation of the oxidative stress generated by uncoupling of the P450-dependant monooxygenases and related enzymes: controlling role of the global chemical environment, abstr. 3; p. 3. [Google Scholar]
- 44.Postlind H, Vu T P, Tukey R H, Quattrochi L C. Response of human CYP1-luciferase plasmids to 2,3,7,8-tetrachlorodibenzo-p-dioxin and polycyclic aromatic hydrocarbons. Toxicol Appl Pharmacol. 1993;118:255–262. doi: 10.1006/taap.1993.1031. [DOI] [PubMed] [Google Scholar]
- 45.RayChaudhuri B, Nebert D W, Puga A. The murine Cyp1a-1 gene negatively regulates its own transcription and that of other members of the aromatic hydrocarbon-responsive [Ah] gene battery. Mol Endocrinol. 1990;4:1773–1781. doi: 10.1210/mend-4-12-1773. [DOI] [PubMed] [Google Scholar]
- 46.Royall J A, Ischiropoulos H. Evaluation of 2′,7′-dichlorofluorescin and dihydrorhodamine 123 as fluorescent probes for intracellular H2O2 in cultured endothelial cells. Arch Biochem Biophys. 1993;302:348–355. doi: 10.1006/abbi.1993.1222. [DOI] [PubMed] [Google Scholar]
- 47.Schmalix W A, Maser H, Kiefer F, Reen R, Wiebel F J, Gonzalez F, Seidel A, Glatt H, Greim H, Doehmer J. Stable expression of human cytochrome P450 1A1 cDNA in V79 Chinese hamster cells and metabolic activation of benzo[a]pyrene. Eur J Pharmacol. 1993;248:251–261. doi: 10.1016/0926-6917(93)90052-r. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt J V, Bradfield C A. Ah receptor signaling pathways. Annu Rev Cell Dev Biol. 1996;12:55–89. doi: 10.1146/annurev.cellbio.12.1.55. [DOI] [PubMed] [Google Scholar]
- 49.Schmidt K, Amstad P, Cerruti P, Baeuerle P. The role of hydrogen peroxide and superoxide as messengers in the activation of nuclear factor NF-κB. Chem Biol. 1995;2:13–22. doi: 10.1016/1074-5521(95)90076-4. [DOI] [PubMed] [Google Scholar]
- 50.Schrenk D. Impact of dioxin-type induction of drug-metabolizing enzymes on the metabolism of endo- and xenobiotics. Biochem Pharmacol. 1998;55:1155–1162. doi: 10.1016/s0006-2952(97)00591-1. [DOI] [PubMed] [Google Scholar]
- 51.Shertzer H G, Nebert D W, Puga A, Ary M, Sonntag D, Dixon K, Robinson L J, Cianciolo E, Dalton T P. Dioxin causes a sustained oxidative stress response in the mouse. Biochem Biophys Res Commun. 1998;253:44–48. doi: 10.1006/bbrc.1998.9753. [DOI] [PubMed] [Google Scholar]
- 52.Stohs S J. Oxidative stress induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Free Radic Biol Med. 1990;9:79–90. doi: 10.1016/0891-5849(90)90052-k. [DOI] [PubMed] [Google Scholar]
- 53.Tassaneeyakul W, Birkett D J, Veronese M E, McManus M E, Tukey R H, Quattrochi L C, Gelboin H V, Miners J O. Specificity of substrate and inhibitor probes for human cytochromes P450 1A1 and 1A2. J Pharmacol Exp Ther. 1993;265:401–407. [PubMed] [Google Scholar]
- 54.Tian Y, Ke S, Denison M S, Rabson A B, Gallo M A. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- 55.Timchenko N, Wilson D R, Taylor L R, Abdelsayed S, Wilde M, Sawadogo M, Darlington G J. Autoregulation of the human C/EBPα gene by stimulation of upstream stimulatory factor binding. Mol Cell Biol. 1995;15:1192–1202. doi: 10.1128/mcb.15.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Walsh A A, Tullis K, Rice R H, Denison M S. Identification of a novel cis-acting negative regulatory element affecting expression of the CYP1A1 gene in rat epidermal cells. J Biol Chem. 1996;271:22746–22753. doi: 10.1074/jbc.271.37.22746. [DOI] [PubMed] [Google Scholar]
- 57.Whitlock J J. Induction of cytochrome P450 1A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- 58.Wright C F, Hamer D H, McKenney K. Autoregulation of the yeast copper metallothionein gene depends on metal binding. J Biol Chem. 1988;263:1570–1574. [PubMed] [Google Scholar]
- 59.Yanagida A, Sogawa K, Yasumoto K I, Fujii-Kuriyama Y. A novel cis-acting DNA element required for a high level of inducible expression of the rat P-450c gene. Mol Cell Biol. 1990;10:1470–1475. doi: 10.1128/mcb.10.4.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang W, Shields J M, Sogawa K, Fujii-Kuriyama Y, Yang V W. The gut-enriched Kruppel-like factor suppresses the activity of the CYP1A1 promoter in an Sp1-dependent fashion. J Biol Chem. 1998;273:17917–17925. doi: 10.1074/jbc.273.28.17917. [DOI] [PMC free article] [PubMed] [Google Scholar]