Abstract
Knowledge on the presence of Cytauxzoon sp. and Hepatozoon spp. in Italy is scant and mostly limited to a few areas of Northern and Southern regions, respectively. The present study updated the current epidemiological scenario by investigating the occurrence of these protozoa in domestic cats from three broad regions of North-Eastern Italy. Blood samples from cats at risk of vector-borne diseases were processed by PCR to detect Cytauxzoon and Hepatozoon DNA. Blood smears were observed for haemoparasite inclusions. The influence of cat individual data (e.g., provenance, management, indoor/outdoor lifestyle) on the prevalence of haemoprotozoan infections was statistically evaluated. Among 158 cats, Cytauxzoon and Hepatozoon DNA were detected in 6 (3.8%) and 26 (16.5%) animals, respectively. No Hepatozoon gamonts were detected in blood smears, whereas all Cytauxzoon PCR-positive samples were microscopically positive, though with low levels of parasitaemia. Two species of Hepatozoon were identified, Hepatozoon felis (n = 10) and Hepatozoon silvestris (n = 16). Hepatozoon silvestris prevalence values were significantly (p < 0.05) higher in the region Friuli Venezia Giulia and in stray cats. Cytauxzoon sp. was detected in 6/39 (15.4%) stray cats from Friuli Venezia Giulia (Trieste province). These data add new information on the occurrence of these neglected protozoa in domestic cats’ populations.
Keywords: Cytauxzoon sp., Hepatozoon felis, Hepatozoon silvestris, cat, Italy
1. Introduction
Cytauxzoon sp. and Hepatozoon spp. are two apicomplexan protozoa belonging to Orders Piroplasmida and Eucoccidiorida, respectively [1]. The genus Cytauxzoon was reported for the first time in a domestic cat (Felis silvestris catus) in 1976 in the US, and the species was named Cytauxzoon felis [2]. Then, reports of Cytauxzoon in cats were described only in some US regions [3,4], until the 2000s, when cases were also reported in Europe. More recently, cats positive for Cytauxzoon have been recorded in Spain [5,6], France [7,8], Portugal [9], Switzerland [10], and Germany [11]. In Italy, cases were limited to an area in the North-Eastern region of Friuli Venezia Giulia, where an endemic focus was described in the city of Trieste with a prevalence rate of 23% among owned and stray cats [12]. Subsequently, clinical cases were then recorded in other Italian regions, i.e., Veneto, Tuscany, and Latium [13]. Molecular analyses showed that isolates of Cytauxzoon in Europe are different from C. felis affecting felid populations in the USA. Indeed, Cytauxzoon is a monophyletic group, characterised by different isolates grouped in separate species (i.e., C. felis, Cytauxzoon manul) [14]. In addition, among the isolates from European wild felids, three genotypes of Cytauxzoon (i.e., major-EU1, minor-EU2, rare-EU3), defined as three new species, were recently detected [15].
Hepatozoon spp. was reported in domestic cats in India at the beginning of the 1900s [16], then only a few reports were published until 1973, when schizonts of Hepatozoon-like protozoa were described in the myocardium of a domestic cat in Israel [17]. Since then, Hepatozoon has been described worldwide, including in Africa [18,19,20], the US and South America [21,22], and Europe [6,7,23,24,25,26,27,28]. In Italy, hepatozoonosis was described in the Emilia Romagna region [29] and in Southern regions, i.e., Apulia and Basilicata [30] and the Aeolian Islands [31]. Three species of Hepatozoon infect cats (i.e., Hepatozoon felis, Hepatozoon silvestris, and Hepatozoon canis) [27,30].
Bridging parasite infections between wild felids and domestic cats occur frequently in areas of sympatry with relevant clinical and epizootiological impacts, as recently described for nematodes [32,33,34,35]. Different species of wild felids are reservoirs for Cytauxzoon sp. and Hepatozoon spp.: bobcat (Lynx rufus) in North America [36], Pallas’ cat (Otocolobus manul) in Asia [37], and Iberian lynx (Lynx pardinus) [38], Eurasian lynx (Lynx lynx), and European wildcat (Felis silvestris silvestris) [39] in Europe. In particular, both Cytauxzoon sp. and Hepatozoon spp. occur frequently in European wildcats [15,33,40,41,42]. The recent rise of reports of cytauxzoonosis and hepatozoonosis in domestic cats of Europe [6,8,10,15,28] indicates the merit to further investigate the presence of these protozoa in populations of domestic cats at risk of infection for the occurrence of arthropod vectors and/or local presence of wild reservoirs.
Due to the merit in improving knowledge on the occurrence of cat cytauxzoonosis and hepatozoonosis in populations of domestic cats, the aim of this work was to investigate the presence and distribution of Cytauxzoon sp. and Hepatozoon spp. in domestic cats in North-Eastern Italy, aiming towards an update of the current epidemiological scenario.
2. Results
2.1. Feline Population
Overall, 158 domestic cats were included in the study, both owned (n = 103, 65.2%) and stray cats (n = 55, 34.8%), living in Veneto—Site 1 (n = 99, 62.7%), Friuli Venezia Giulia—Site 2 (n = 39, 24.7%), and Trentino Alto Adige—Site 3 (n = 20, 12.7%) regions. Regarding their habits, recruited cats had mostly an outdoor lifestyle (n = 112, 70.9%). Descriptions of individual data regarding the region of provenance (Sites 1, 2, and 3), sex, age classes (<12 months, 12–35 months, ≥ 36 months), management (owned, stray cats), lifestyle (indoor, outdoor), immunosuppressive infections (FIV, FeLV), clinical signs, and ectoparasites infestations are reported in Table 1.
Table 1.
Description of individual data of the feline population distributed among the three investigated sites.
| Site 1 n (%) |
Site 2 n (%) |
Site 3 n (%) |
Total n (%) |
||
|---|---|---|---|---|---|
| Sex | M | 49 (49.5) | 13 (33.3) | 12 (60.0) | 74 (46.8) |
| F | 50 (50,5) | 26 (66.7) | 8 (40.0) | 84 (53.2) | |
| Age classes | <12 months | 38 (38.4) | 9 (23.1) | 5 (25.0) | 52 (32.9) |
| 12–35 months | 23 (23.2) | 15 (38.5) | 8 (40.0) | 46 (29.1) | |
| ≥36 months | 37 (37.4) | 13 (33.3) | 7 (35.0) | 57 (36.1) | |
| NR a | 1 (1.0) | 2 (5.1) | 0 (0.0) | 3 (1.9) | |
| Management | Owned cats | 64 (64.6) | 19 (48.7) | 20 (100.0) | 103 (65.2) |
| Stray cats | 35 (35.4) | 20 (51.3) | 0 (0.0) | 55 (34.8) | |
| Lifestyle | Indoor | 28 (28.3) | 11 (28.2) | 7 (35.0) | 46 (29.1) |
| Outdoor | 71 (71.7) | 28 (71.8) | 13 (65.0) | 112 (70.9) | |
| Immunosuppressive infections (FIV and/or FeLV) | Positive | 15 (15.2) | 9 (23.1) | 1 (5.0) | 25 (15.8) |
| Negative | 84 (84.8) | 30 (76.9) | 19 (95.0) | 133 (84.2) | |
| Clinical signs (gastro-intestinal and respiratory signs) | Presence | 10 (10.1) | 1 (2.6) | 1 (5.0) | 12 (7.6) |
| Absence | 89 (89.9) | 38 (97.4) | 19 (95.0) | 146 (92.4) | |
| Ectoparasites infestations | Presence | 15 (15.2) | 11 (28.2) | 3 (15.0) | 29 (18.4) |
| Absence | 84 (84.8) | 28 (71.8) | 17 (85.0) | 129 (81.6) | |
| Total | 99 | 39 | 20 | 158 |
a Age not reported.
A total of 29 cats (18.4%) were reported to be infested with ectoparasites (21 with only fleas, 2 with only ticks, 5 with fleas and ticks, and 1 with fleas and lice).
2.2. Laboratory Analysis and Geographical Distribution
From the microscope observation, 6/158 blood smears evidenced mild parasitaemia (1–5 erythrocytes with parasitic inclusions) attributable to Cytauxzoon, whereas no samples showed circulating Hepatozoon gamonts.
Out of 158 sera, 25 (15.8%) were positive for the immunosuppressive infections FIV and/or FeLV (8 of which for FIV, 14 for FeLV, and 3 co-infected).
PCR amplified Cytauxzoon sp. and Hepatozoon spp. DNA in 6/158 (3.8%) and 26/158 (16.5%) blood samples, respectively. Among Hepatozoon-positive samples, H. felis (10/26, 38.5%) and H. silvestris (16/26, 61.5%) were identified, comparing the obtained nucleotide sequences to those deposited in GenBank® using BLAST software (https://blast.ncbi.nlm.nih.gov/Blast) (accessed date: 2 August 2021).
All Cytauxzoon blood smear samples were also positive using the molecular assay.
All sequences of Cytauxzoon sp. (from MZ227613 to MZ227618), H. felis (from MZ227585 to MZ227594), and H. silvestris (from MZ227596 to MZ22611) were deposited in GenBank.
The BLAST analysis retrieved 99.68–100% homology with sequences depositedas Cytauxzoon sp. isolated from domestic cats in France [8], in Portugal [9], in Switzerland [10], and in Germany [11], together with isolates from the European wildcat in Romania and Bosnia and Herzegovina [39,42].
Regarding H. felis, the same analysis retrieved 97.92–100% identity from domestic cats in Southern Italy [30], Spain [43,44], and Israel [45]. For H. silvestris, BLAST analysis retrieved 96.28–97.71% identity from domestic cats in Southern Italy [30] and in Switzerland [26], and in addition, from European wildcat in Bosnia and Herzegovina [41,42].
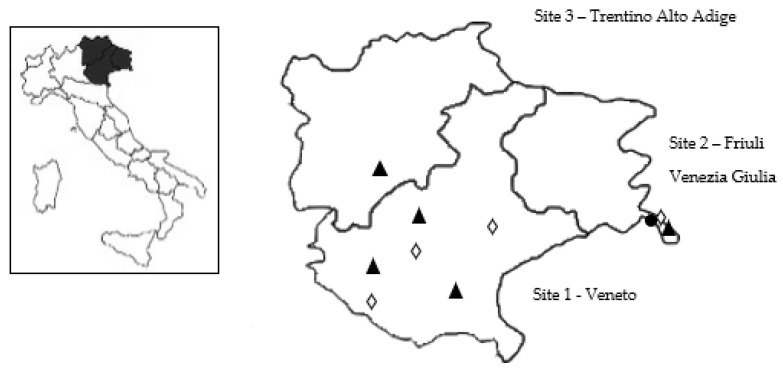
Regarding geographical distribution, Cytauxzoon sp. was found only in Site 2, in particular in one province (Trieste). Contrariwise, Hepatozoon spp. was distributed in all investigated regions (Figure 1).
Figure 1.
Map depicting Site 1, Site 2, and Site 3, showing the areas resulted positive to Cytauxzoon sp. (●), Hepatozoon felis (▲), and Hepatozoon silvestris (◊).
Individual data of cats positive for Cytauxzoon sp. and Hepatozoon spp. are reported in Table 2.
Table 2.
Distribution of positivity according to individual factors in investigated cats.
| Haemoparasite | ||||||||
|---|---|---|---|---|---|---|---|---|
| Factors | Variables | Tested | Cytauxzoon sp. n (%) |
Hepatozoon spp. n (%) |
Hepatozoon felis n (%) |
Hepatozoon silvestris n (%) |
||
| Sex | M | 74 | 1 (1.4) | 13 (17.6) | 5 (6.8) | 8 (10.8) | ||
| F | 84 | 5 (6.0) | 13 (15.5) | 5 (6.0) | 8 (9.5) | |||
| Age Class | <12 months | 52 | 0 (0.0) | 9 (17.3) | 5 (9.6) | 4 (7.7) | ||
| 12–35 months | 46 | 1 (2.2) | 7 (15.2) | 0 (0.0) | 7 (15.2) | |||
| ≥36 months | 57 | 4 (7.0) | 9 (15.8) | 5 (8.8) | 4 (7.0) | |||
| NR a | 3 | 1 (33.3) | 1 (33.3) | 0 (0.0) | 1 (33.3) | |||
| Region | Site 1 | 99 | 0 (0.0) | * | 12 (12.1) | 5 (5.1) | 7 (7.1) | * |
| Site 2 | 39 | 6 (15.4) | 11 (28.2) | 2 (5.1) | 9 (23.1) | |||
| Site 3 | 20 | 0 (0.0) | 3 (15.0) | 3 (15.0) | 0 (0.0) | |||
| Management | Owned cats | 103 | 0 (0.0) | * | 12 (11.7) | 9 (8.7) | 3 (2.9) | * |
| Stray cats | 55 | 6 (10.9) | 14 (25.5) | 1 (1.8) | 13 (23.6) | |||
| Lifestyle | Indoor | 46 | 0 (0.0) | 5 (10.9) | 4 (8.7) | 1 (2.2) | ||
| Outdoor | 112 | 6 (5.4) | 21 (18.8) | 6 (5.4) | 15 (13.4) | |||
| Immunosuppressive infections (FIV and/or FeLV) | Positive | 25 | 3 (12.0) | 5 (20.0) | 2 (8.0) | 3 (12.0) | ||
| Negative | 133 | 3 (2.3) | 21 (15.8) | 8 (6.0) | 13 (9.8) | |||
| Clinical signs (gastro-intestinal and respiratory signs) | Presence | 12 | 0 (0.0) | 1 (3.8) | 0 (0.0) | 1 (8.3) | ||
| Absence | 148 | 6 (4.1) | 25 (16.9) | 10 (6.8) | 15 (10.1) | |||
| Ectoparasites infestation | Presence | 29 | 2 (6.9) | 8 (27.6) | 2 (6.9) | 6 (20.7) | ||
| Absence | 129 | 4 (3.1) | 18 (14.0) | 8 (6.2) | 10 (7.8) | |||
| Total | 158 | 6 (3.8) | 26 (16.5) | 10 (6.3) | 16 (10.1) | |||
Note: significant differences (p < 0.05) based on the Pearson Chi-Square test or the Fisher exact test are evidenced by *. a Age not reported.
2.3. Statistical Evaluation
Differences in the infection rate among sub-groups of animals were found by the Pearson Chi-Square test for Cytauxzoon sp. and H. silvestris for two factors: a significantly higher prevalence (p < 0.05) was found in stray cats compared to owned animals, and in the cats living in Trieste province (Site 2) compared to the other two sites. Moreover, cats infected with immunosuppressive viruses seem to be at higher risk of positivity of Cytauxzoon sp. (p = 0.051), and cats with ectoparasites had a higher prevalence of H. silvestris (p = 0.080). However, in both cases, the Fisher exact test showed a p-value slightly higher than the 0.05 threshold. No significant differences were observed for H. felis, nor for the other factors in general.
3. Discussion
To date, cytauxzoonosis and hepatozoonosis are neglected diseases in feline populations. Data on the Cytauxzoon species circulating among European cats are still limited [6,46,47] and information on Hepatozoon spp. in felids is also poor [48,49].
The present study confirmed that Trieste (Site 2) is an endemic site for the presence of Cytauxzoon sp. in domestic cats. As in a previous study, these results are supported both by blood smear examinations and molecular analysis, with a prevalence value similar to that reported (23%) almost ten years ago in 2012 [12].
Site 2 is the only region in which different wild felids acting as reservoirs for cytauxzoonosis are endemic, i.e., the Eurasian lynx [50] and the European wildcat [39,51]. Moreover, Site 2 is a border region, and wildlife movements from the nearby Slovenia are extensively described [52].
The significant difference in the prevalence between the type of management (owned vs. stray cats) highlights how stray cats that live mostly outdoors are more exposed to cytauxzoonosis than owned cats (10.9% vs. 0.0%). This is probably due to the sharing of the same environment with wild felids and the presence of infected vectors. Indeed, the continuous reduction of wildlife habitat due to anthropization favours the sympatric occurrence of wild and domestic cats in many areas [53], and this has the implication of sharing parasites with high pathogenic potential, as recently investigated for nematodes affecting the cardio-respiratory system [32,33,34,35,54].
Two species of Hepatozoon spp. have been found in North-Eastern Italy (i.e., H. felis and H. silvestris). The finding of H. silvestris in Northern Italy is especially noteworthy. Indeed, this species has been reported mainly in wild felids in Europe, and rarely in domestic cats. Nevertheless, it was recently described in a domestic cat in Southern Italy during an epidemiological survey [30] and in another one in Switzerland associated with a fatal myocarditis [26].
In agreement with Baneth et al. [49], who described an extremely low level of parasitaemia in felids, no sample showed Hepatozoon gamonts in blood smear examinations.
Positive cats were mostly sub-clinically infected, in apparently good physical condition, and only in one case were diarrhoea and rhinitis present (Table 2), thus evoking the infections as well-tolerated in most cases. No correlation between Hepatozoon positivity and potentially immunosuppressive infectious diseases (i.e., FIV and FeLV) was statistically found, as already reported by Baneth et al. [45]. Instead, cats positive for immunosuppressive viruses showed a higher prevalence of Cytauxzoon sp., indicating a tendency of being more at risk to becoming infected with haemoprotozoa, as previously suggested [8,12].
This result underlines the importance of investigating subclinical infections, and in parallel highlights the diagnostic limitations posed by stand-alone cytology. Differently, all Cytauxzoon-positive cats presented mild parasitaemia. Although few parasitised erythrocytes per monolayer were observed, the positivity suggests a potential epidemiological role of clinically healthy animals as carriers and sources of infection for potential vectors.
No significant differences between individual variables (i.e., provenance, management, and lifestyle) and H. felis prevalence were found. However, the high prevalence value obtained in indoor and owned cats, that are commonly less exposed to vectors’ activity due to their lifestyle, suggests that alternative ways of transmission are possible, as already predicted (e.g., vertical transmission, predation of infected preys) [45]. Indeed, H. canis may also be spread through intra-uterine transmission from the mother to the offspring, and Hepatozoon americanum may be transmitted by ingestion of infected preys [49].
On the contrary, H. silvestris showed a significant difference in its distribution between regions, especially in Site 2, achieving a prevalence rate of 23.1%, most likely for a habitat/vector sharing between domestic and wild felids, as previously mentioned for Cytauxzoon sp.
The presence of H. silvestris in Site 1, where the Eurasian lynx and the European wildcats are absent, indicates that the role of wildlife as reservoirs could be unnecessary. This supports new considerations, as the possibility that H. silvestris might have another route of transmission related to the predatory instinct of cats and the carnivorism of potential paratenic hosts such as small rodents could be supported, as already described for H. americanum in the US [49]. As H. silvestris was found mainly in stray cats, this hypothesis is even more appropriate due to their predatory and hunting activities.
In conclusion, this study demonstrated that Cytauxzoon sp. and Hepatozoon spp. circulate in the feline population of North-Eastern Italy involving both owned and stray cats, focusing on the risk of exposure that some individual attitude or lifestyle factors might encourage.
Information about these haemoprotozoa is still lacking, and further studies are needed to obtain important data about their lifecycles with the evaluation of their pathogenicity and their impact on cat health as well as the potential ways of transmission, including wildlife as possible reservoirs and the involved arthropod vector, to carry out adequate control measures.
4. Materials and Methods
4.1. Blood Collection, Blood Analysis, and DNA Extraction
K3EDTA blood and blood smears were collected in collaboration with veterinary practitioners working in the investigated regions of North-Eastern Italy, during routine clinical examinations, from cats of all ages, exposed to at least one season at risk of arthropod vectors’ activity, preferably without any regular treatments against ectoparasites.
For each sampled cat region of provenance (i.e., Veneto—Site 1, Friuli Venezia Giulia—Site 2, and Trentino Alto Adige—Site 3, Figure 1), sex, age classes (<12 months, 12–35 months, ≥36 months), management (owned, stray cats), lifestyle (indoor, outdoor), clinical signs, and ectoparasite infestations were reported. Moreover, all the involved owners or veterinary health authorities for colony/stray cats signed an informed consent form for participating in the study. Recruited animals were submitted to routine veterinary procedures not depending on this research project.
Blood smears were stained using Hemacolor® (Merck KGaA, Darmstadt, Germany) and then observed by microscope at 100× magnification with immersion oil to evaluate the presence of Hepatozoon gamonts and Cytauxzoon merozoites according to an existing key [12,40,48]. The parasitaemia level for Cytauxzoon sp. was graded as reported by Carli et al. [12], observing how many erythrocytes presented parasitic inclusions per the entire monolayer and defining based on the following scale: mild (n ≤ 5 parasitised red blood cells), moderate (n ≤ 20), marked (n ≤ 50), and very marked (n > 50). Serum obtained from each blood sample was also analysed by the SNAP® Combo Plus FeLV Ag/FIV Ab test (IDEXX Laboratories Inc., Westbrook, ME, USA) following the manufacturer’s instructions.
DNA extraction was performed starting from 200 µL of k3EDTA blood by the NucleoSpin® Tissue kit (Macherey-Nagel, Düren, Germany), in accordance with the manufacturer’s protocol.
4.2. Molecular Analysis and Sequencing
DNA was processed by conventional PCR targeting the 18S-rRNA gene using the Piroplasmid primers pair 5′-CCAGCAGCCGCGGTAATTC-3′ and 5′-CTTTCGCAGTAGTTYGTCTTTAACAAATCT-3′, as already described by Tabar et al. [55]. Positive (i.e., DNA of sequenced field sample) and negative (no DNA added) controls were included in each PCR reaction. Amplicons were sequenced following Sanger technology (Macrogen Spain, Madrid, Spain) and the obtained nucleotide sequences were compared to those deposited in GenBank® using BLAST software (https://blast.ncbi.nlm.nih.gov/Blast) (accessed date: 2 August 2021)..
4.3. Data Analysis
In order to evaluate the presence of differences in infection rates among subgroups of the investigated cat population, a statistical evaluation was performed by means of the Pearson Chi-Square test or the Fisher exact test, if appropriate, using SPSS for Windows, version 27.0. The factors taken into consideration were: sex (i.e., males, females), age classes (i.e., <12 months, 12–35 months, ≥36 months), region of provenance (i.e., Site 1, Site 2, Site 3), lifestyle (i.e., indoor, outdoor), management (i.e., owned, stray cat), infection with immunosuppressive virus (i.e., positive for FIV and/or FeLV, or negative), presence of clinical signs (i.e., gastro-intestinal and respiratory signs), and ectoparasite infestation.
Acknowledgments
The authors are grateful to the veterinary practitioners involved in the sample collection, especially to Francesca Fiorio and to the staff of “Clinica Veterinaria Airone”, Fulvia Ada Rossi and to the staff of “Clinica Veterinaria Tergeste”, Jesus Catalan Pradas and all the staff of “il Gattile” association, Francesco Marta and the staff of “Clinica Veterinaria delle Dolomiti”, Silvia Rossi, Adriano Monino, Daniela Zago, Erica Bagatella, Carmelo Furnari, and Viviana Genna, and the staff of the sanitary kennel of Verona AULSS 9, Roberto Guadagnini and the staff of “Zoolife” veterinary clinic, and Michele Berlanda and Gaia Pagani of the Veterinary Teaching Hospital of the University of Padua.
Author Contributions
Conceptualisation, M.G., G.S. and A.F.d.R.; Data curation, M.G.; Formal analysis, M.G. and R.C.; Funding acquisition, G.S.; Investigation, M.G., G.S. and E.M.; Methodology, M.G., C.T. and G.D.; Project administration, G.S.; Resources, G.S.; Software, R.C.; Supervision, D.T. and A.F.d.R.; Validation, C.T. and G.D.; Writing—original draft, M.G. and D.T.; Writing—review and editing, G.S. and A.F.d.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Department of Animal Medicine, Production and Health of University of Padua (BIRD193835, 2019).
Institutional Review Board Statement
Ethical review and approval were waived for this study, due to the involved animals being submitted to routine veterinary procedures not depending on this research project.
Informed Consent Statement
Informed consent for participating to the study was obtained from all the involved owners or veterinary health authorities for colony cats.
Data Availability Statement
The authors declare that data are available upon request to the corresponding author, by email.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Taylor M.A., Coop R.L., Wall R.L. Veterinary Parasitology. 3rd ed. Blackwell Publishing; Oxford, UK: 2007. p. 874. [Google Scholar]
- 2.Wagner J.E. A fatal cytauxzoonosis-like disease in cats. J. Am. Vet. Med. Assoc. 1976;168:585–588. [PubMed] [Google Scholar]
- 3.Miller J., Davis C.D. Increasing frequency of feline cytauxzoonosis cases diagnosed in western Kentucky from 2001 to 2011. Vet. Parasitol. 2013;198:205–208. doi: 10.1016/j.vetpar.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tarigo J.L., Scholl E.H., McK Bird D., Brown C.C., Cohn L.A., Dean G.A., Levy M.G., Doolan D.L., Trieu A., Nordone S.K., et al. A novel candidate vaccine for cytauxzoonosis inferred from comparative apicomplexan genomics. PLoS ONE. 2013;8:e71233. doi: 10.1371/annotation/943b121e-343b-4df1-a06b-7f8a205a057d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Criado-Fornelio A., Gónzalez-del-Río M.A., Buling-Saraña A., Barba-Carretero J.C. The “expanding universe” of piroplasms. Vet. Parasitol. 2004;119:337–345. doi: 10.1016/j.vetpar.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 6.Díaz-Regañón D., Villaescusa A., Ayllón T., Rodríguez-Franco F., Baneth G., Calleja-Bueno L., García-Sancho M., Agulla B., Sainz Á. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasites Vectors. 2017;10:112. doi: 10.1186/s13071-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Criado-Fornelio A., Buling A., Pingret J.L., Etievant M., Boucraut-Baralon C., Alongi A., Agnone A., Torina A. Hemoprotozoa of domestic animals in France: Prevalence and molecular characterization. Vet. Parasitol. 2009;159:73–76. doi: 10.1016/j.vetpar.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 8.Legroux J.P., Halos L., René-Martellet M., Servonnet M., Pingret J.L., Bourdoiseau G., Baneth G., Chabanne L. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Vet. Res. 2017;13:81. doi: 10.1186/s12917-017-1009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alho A.M., Silva J., Fonseca M.J., Santos F., Nunes C., De Carvalho L.M., Rodrigues M., Cardoso L. First report of Cytauxzoon sp. infection in a domestic cat from Portugal. Parasites Vectors. 2016;9:220. doi: 10.1186/s13071-016-1506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nentwig A., Meli M.L., Schrack J., Reichler I.M., Riond B., Gloor C., Howard J., Hofmann-Lehmann R., Willi B. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: Natural and transfusion-transmitted infections. Parasites Vectors. 2018;11:292. doi: 10.1186/s13071-018-2728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panait L.C., Stock G., Globokar M., Balzer J., Groth B., Mihalca A.D., Pantchev N. First report of Cytauxzoon sp. infection in Germany: Organism description and molecular confirmation in a domestic cat. Parasitol. Res. 2020;119:3005–3011. doi: 10.1007/s00436-020-06811-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carli E., Trotta M., Chinelli R., Drigo M., Sinigoi L., Tosolini P., Furlanello T., Millotti A., Caldin M., Solano-Gallego L. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet. Parasitol. 2012;183:343–352. doi: 10.1016/j.vetpar.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Carli E., Trotta M., Bianchi E., Furlanello T., Caldin M., Pietrobelli M., Solano-Gallego L. Cytauxzoon sp. infection in two free ranging young cats: Clinicopathological findings, therapy and follow up. Türkiye Parazitolojii Derg. 2014;38:185–189. doi: 10.5152/tpd.2014.3540. [DOI] [PubMed] [Google Scholar]
- 14.Jalovecka M., Sojka D., Ascencio M., Schnittger L. Babesia life cycle—When phylogeny meets biology. Trends Parasitol. 2019;35:356–368. doi: 10.1016/j.pt.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Panait L.C., Mihalca A.D., Modrý D., Juránková J., Ionică A.M., Deak G., Gherman C.M., Heddergott M., Hodžić A., Veronesi F., et al. Three new species of Cytauxzoon in European wild felids. Vet. Parasitol. 2021;290:109344. doi: 10.1016/j.vetpar.2021.109344. [DOI] [PubMed] [Google Scholar]
- 16.Patton W.S. The haemogregarines of mammals and reptiles. Parasitology. 1908;1:318–321. doi: 10.1017/S0031182000003620. [DOI] [Google Scholar]
- 17.Klopfer U., Nobel T.A., Neumann F. Hepatozoon-like parasite (schizonts) in the myocardium of the domestic cat. Vet. Pathol. 1973;10:185–190. doi: 10.1177/030098587301000301. [DOI] [PubMed] [Google Scholar]
- 18.Leeflang P., Ilemobade A.A. Tick-borne disease of domestic animals in northern Nigeria. II. Research summary, 1966 to 1976. Trop. Anim. Health Prod. 1977;9:211–218. doi: 10.1007/BF02240342. [DOI] [PubMed] [Google Scholar]
- 19.Van Amstel S. Hepatozoonose i’n kat. J. S. Afr. Vet. Med. Assoc. 1979;50:215–216. [PubMed] [Google Scholar]
- 20.Pereira C., Maia J.P., Marcos R., Luzzago C., Puente-Payo P., Dall’Ara P., Faustino A., Lauzi S. Molecular detection of Hepatozoon felis in cats from Maio Island, Republic of Cape Verde and global distribution of feline hepatozoonosis. Parasites Vectors. 2019;12:294. doi: 10.1186/s13071-019-3551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ewing G.O. Granulomatous cholangiohepatitis in a cat due to a protozoan parasite resembling Hepatozoon canis. Feline Pract. 1977;7:37–40. [Google Scholar]
- 22.Perez R.R., Rubini A.S., O’Dwyer L.H. The first report of Hepatozoon spp. (Apicomplexa, Hepatozoidae) in domestic cats from São Paulo state, Brazil. Parasitol. Res. 2004;94:83–85. doi: 10.1007/s00436-004-1167-8. [DOI] [PubMed] [Google Scholar]
- 23.Beaufils J.P., Martin-Granel J., Jumelle P. Hepatozoon spp. parasitemia and feline leukemia virus infection in two cats. Feline Pract. 1998;26:10–13. [Google Scholar]
- 24.Vilhena H., Martinez-Díaz V.L., Cardoso L., Vieira L., Altet L., Francino O., Pastor J., Silvestre-Ferreira A.C. Feline vector-borne pathogens in the north and center of Portugal. Parasites Vectors. 2013;6:99. doi: 10.1186/1756-3305-6-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Attipa C., Papasouliotis K., Solano-Gallego L., Baneth G., Nachum-Biala Y., Sarvani E., Knowles T.G., Mengi S., Morris D., Helps C., et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasites Vectors. 2017;10:130. doi: 10.1186/s13071-017-2063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kegler K., Nufer U., Alic A., Posthaus H., Olias P., Basso W. Fatal infection with emerging apicomplexan parasite Hepatozoon silvestris in a domestic cat. Parasites Vectors. 2018;11:428. doi: 10.1186/s13071-018-2992-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Basso W., Görnerb D., Globokarc M., Keidelc A., Pantchevc N. First autochthonous case of clinical Hepatozoon felis infection in a domestic cat in Central Europe. Parasitol. Int. 2019;72:101945. doi: 10.1016/j.parint.2019.101945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morelli S., Diakou A., Traversa D., Di Gennaro E., Simonato G., Colombo M., Dimzas D., Grillini M., Frangipane di Regalbono A., Beugnet F., et al. First record of Hepatozoon spp. in domestic cats in Greece. Ticks Tick Borne Dis. 2021;12:101580. doi: 10.1016/j.ttbdis.2020.101580. [DOI] [PubMed] [Google Scholar]
- 29.Ebani V.V., Guardone L., Marra F., Altomonte I., Nardoni S., Mancianti F. Arthropod-borne pathogens in stray cats from Northern Italy: A serological and molecular survey. Animals. 2020;10:2334. doi: 10.3390/ani10122334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Giannelli A., Latrofa M.S., Nachum-Biala Y., Hodžić A., Greco G., Attanasi A., Annoscia G., Otranto D., Baneth G. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick Borne Dis. 2017;8:721–724. doi: 10.1016/j.ttbdis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Otranto D., Napoli E., Latrofa M.S., Annoscia G., Tarallo V.D., Greco G., Lorusso E., Gulotta L., Falsone L., Basano F.S., et al. Feline and canine leishmaniosis and other vector-borne diseases in the Aeolian Islands: Pathogen and vector circulation in a confined environment. Vet. Parasitol. 2017;236:144–151. doi: 10.1016/j.vetpar.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Stevanović O., Diakou A., Morelli S., Paraš S., Trbojević I., Nedić D., Sladojević Ž., Kasagić D., Di Cesare A. Severe verminous pneumonia caused by natural mixed infection with Aelurostrongylus abstrusus and Angiostrongylus chabaudi in a European wildcat from Western Balkan area. Acta Parasitol. 2019;64:411–417. doi: 10.2478/s11686-019-00029-9. [DOI] [PubMed] [Google Scholar]
- 33.Diakou A., Dimzas D., Astaras C., Savvas I., Di Cesare A., Morelli S., Neofitos Κ., Migli D., Traversa D. Clinical investigations and treatment outcome in a European wildcat (Felis silvestris silvestris) infected by cardio-pulmonary nematodes. Vet. Parasitol. Reg. Stud. Rep. 2020;19:100357. doi: 10.1016/j.vprsr.2019.100357. [DOI] [PubMed] [Google Scholar]
- 34.Di Cesare A., Morelli S., Colombo M., Simonato G., Veronesi F., Marcer F., Diakou A., D’Angelosante R., Pantchev N., Psaralexi E., et al. Is angiostrongylosis a realistic threat for domestic cats? Front. Vet. Sci. 2020;7:195. doi: 10.3389/fvets.2020.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Traversa D., Morelli S., Di Cesare A., Diakou A. Felid cardiopulmonary nematodes: Dilemmas solved and new questions posed. Pathogens. 2021;10:30. doi: 10.3390/pathogens10010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kocan A.A., Blouin E.F., Glenn B.L. Hematologic and serum chemical values for free-ranging bobcats, Felis rufus (Schreber), with reference to animals with natural infections of Cytauxzoon felis Kier, 1979. J. Wildl. Dis. 1985;21:190–192. doi: 10.7589/0090-3558-21.2.190. [DOI] [PubMed] [Google Scholar]
- 37.Mason V.R., Van Den Bussche R.A., Meinkoth J.H., Hoovert J.P., Kokan A.A. A new species of Cytauxzoon from Pallas’ cats caught in Mongolia and comments on the systematics and taxonomy of piroplasmids. J. Parasitol. 2005;91:420–426. doi: 10.1645/GE-384R. [DOI] [PubMed] [Google Scholar]
- 38.Millán J., Naranjo V., Rodríguez A., De la Lastra J.M., Mangold A.J., De la Fuente J. Prevalence of infection and 18S rRNA gene sequences of Cytauxzoon species in Iberian lynx (Lynx pardinus) in Spain. Parasitology. 2007;134:995–1001. doi: 10.1017/S003118200700248X. [DOI] [PubMed] [Google Scholar]
- 39.Gallusová M., Jirsová D., Mihalca A.D., Gherman C.M., D’Amico G., Qablan M.A., Modrý D. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic space: Further evidence for a different Cytauxzoon species in European felids. J. Parasitol. 2016;102:377–380. doi: 10.1645/15-881. [DOI] [PubMed] [Google Scholar]
- 40.Veronesi F., Ravagnan S., Cerquetella M., Carli E., Olivieri E., Santoro A., Pesaro S., Berardi S., Rossi G., Ragni B., et al. First detection of Cytauxzoon spp. infection in European wildcats (Felis silvestris silvestris) of Italy. Ticks Tick Borne Dis. 2016;7:853–858. doi: 10.1016/j.ttbdis.2016.04.003. [DOI] [PubMed] [Google Scholar]
- 41.Hodžić A., Alić A., Prašović S., Otranto D., Baneth G., Duscher G.G. Hepatozoon silvestris sp. nov.: Morphological and molecular characterization of a new species of Hepatozoon (Adeleorina: Hepatozoidae) from the European wild cat (Felis silvestris silvestris) Parasitology. 2017;144:650–661. doi: 10.1017/S0031182016002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hodžić A., Alić A., Duscher G.G. High diversity of blood-associated parasites and bacteria in European wild cats in Bosnia and Herzegovina: A molecular study. Ticks Tick Borne Dis. 2018;9:589–593. doi: 10.1016/j.ttbdis.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 43.Ortuño M., Nachum-Biala Y., García-Bocanegra I., Resa M., Berriatua E., Baneth G. An epidemiological study in wild carnivores from Spanish Mediterranean ecosystems reveals association between Leishmania infantum, Babesia spp. and Hepatozoon spp. infection and new hosts for Hepatozoon martis, Hepatozoon canis and Sarcocystis spp. Transbound. Emerg. Dis. 2021:1–16. doi: 10.1111/tbed.14199. [DOI] [PubMed] [Google Scholar]
- 44.Criado-Fornelio A., Ruas J.L., Casado N., Farias N.A., Soares M.P., Müller G., Brumt J.G., Berne M.E., Buling-Saraña A., Barba-Carretero J.C. New molecular data on mammalian Hepatozoon species (Apicomplexa: Adeleorina) from Brazil and Spain. J. Parasitol. 2006;92:93–99. doi: 10.1645/GE-464R.1. [DOI] [PubMed] [Google Scholar]
- 45.Baneth G., Sheiner A., Eyal O., Hahn S., Beaufils J.P., Anug Y., Talmi-Frank D. Redescription of Hepatozoon felis (Apicomplexa: Hepatozoidae) based on phylogenetic analysis, tissue and blood form morphology, and possible transplacental transmission. Parasites Vectors. 2013;6:102. doi: 10.1186/1756-3305-6-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morganti G., Veronesi F., Stefanetti V., Di Muccio T., Fiorentino E., Diaferia M., Santoro A., Passamonti F., Gramiccia M. Emerging feline vector-borne pathogens in Italy. Parasites Vectors. 2019;12:193. doi: 10.1186/s13071-019-3409-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spada E., Proverbio D., Galluzzo P., Perego R., Bagnagatti De Giorgi G., Roggero N., Caracappa S. Frequency of piroplasms Babesia microti and Cytauxzoon felis in stray cats from northern Italy. Biomed. Res. Int. 2014;2014:943754. doi: 10.1155/2014/943754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Latrofa M.S., Iatta R., Toniolo F., Furlanello T., Ravagnan S., Capelli G., Schunack B., Chomel B., Zatelli A., Mendoza-Roldan J., et al. A molecular survey of vector-borne pathogens and haemoplasmas in owned cats across Italy. Parasites Vectors. 2020;13:116. doi: 10.1186/s13071-020-3990-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baneth G. Perspectives on canine and feline hepatozoonosis. Vet. Parasitol. 2011;181:3–11. doi: 10.1016/j.vetpar.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Fattori U., Rucli A., Zanetti M. Grandi Carnivori ed Ungulati Nell’area Confinaria Italo-Slovena. Stato di Conservazione. 2nd ed. Regione Autonoma Friuli Venezia Giulia; Udine, Italy: 2010. pp. 1–80. [Google Scholar]
- 51.Mattucci F., Oliveira R., Bizzarri L., Vercillo F., Anile S., Ragni B., Lapini L., Sforzi A., Alves P.C., Lyons L.A., et al. Genentic structure of wildcat (Felis silvestris) populations in Italy. Ecol. Evol. 2013;3:2443–2458. doi: 10.1002/ece3.569. [DOI] [Google Scholar]
- 52.Genovesi P., Angelini P., Bianchi E., Dupré E., Ercole S., Giacanelli V., Ronchi F., Stoch F. Specie e Habitat di Interesse Comunitario in Italia: Distribuzione, Stato di conservazione e Trend. ISPRA Serie Rapporti; ISPRA-Settore Editoria; Roma, Italy: 2014. p. 194. [Google Scholar]
- 53.Anile S., Devillard S., Ragni B., Rovero F., Mattucci F., Lo Valvo M. Habitat fragmentation and anthropogenic factors affect wildcat Felis silvestris silvestris occupancy and detectability on Mt Etna. Wildl. Biol. 2019;1:1–13. doi: 10.2981/wlb.00561. [DOI] [Google Scholar]
- 54.Traversa D., Morelli S., Cassini R., Crisi P.E., Russi I., Grillotti E., Manzocchi S., Simonato G., Beraldo P., Viglietti A., et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019;193:227–235. doi: 10.1016/j.actatropica.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 55.Tabar M.D., Altet L., Francino O., Sánchez A., Ferrer L., Roura X. Vector-borne infections in cats: Molecular study in Barcelona area (Spain) Vet. Parasitol. 2008;151:332–336. doi: 10.1016/j.vetpar.2007.10.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that data are available upon request to the corresponding author, by email.