Abstract
Growth regulatory factors (GRF) are plant-specific transcription factors that play an important role in plant resistance to stress. This gene family in strawberry has not been investigated previously. In this study, 10 GRF genes were identified in the genome of the diploid woodland strawberry (Fragaria vesca). Chromosome analysis showed that the 10 FvGRF genes were unevenly distributed on five chromosomes. Phylogenetic analysis resolved the FvGRF proteins into five groups. Genes of similar structure were placed in the same group, which was indicative of functional redundance. Whole-genome duplication/segmental duplication and dispersed duplication events effectively promoted expansion of the strawberry GRF gene family. Quantitative reverse transcription-PCR analysis suggested that FvGRF genes played potential roles in the growth and development of vegetative organs. Expression profile analysis revealed that FvGRF3, FvGRF5, and FvGRF7 were up-regulated under low-temperature stress, FvGRF4 and FvGRF9 were up-regulated under high-temperature stress, FvGRF6 and FvGRF8 were up-regulated under drought stress, FvGRF3, FvGRF6, and FvGRF8 were up-regulated under salt stress, FvGRF2, FvGRF7, and FvGRF9 were up-regulated under salicylic acid treatment, and FvGRF3, FvGRF7, FvGRF9, and FvGRF10 were up-regulated under abscisic acid treatment. Promoter analysis indicated that FvGRF genes were involved in plant growth and development and stress response. These results provide a theoretical and empirical foundation for the elucidation of the mechanisms of abiotic stress responses in strawberry.
Keywords: GRF, gene family, strawberry, abiotic stress, expression pattern, cis-acting element
1. Introduction
Strawberry (Fragaria × ananassa) is cultivated worldwide on account of its ornamental value and nutritional fruit. Strawberry is widely grown under a protected environment and China is among the largest strawberry producers in the world. Strawberry is a suitable model plant [1,2] for fruit research because of its small fruit size, short growth cycle, and high efficiency for genetic transformation. Therefore, strawberry can potentially make an important contribution to commercial production and scientific research in the fruit and vegetable industry. However, the cultivated hybrid strawberry is octaploid [3]; thus, its genetic background is highly complex. The diploid ancestral species, woodland strawberry (Fragaria vesca), is more amenable to genetic analysis. In addition, published strawberry genome sequences enable identification of the genetic basis of desirable agronomic traits and stress-resistance genes in strawberry at the genome level.
Given the environmental variability experienced during growth, plants are vulnerable to diverse abiotic stresses, such as low or high temperature, drought, and high salinity, which may adversely affect growth and development [4,5]. In response to exposure to adverse external variables, transcription factors (TFs) play an important role in the regulation of functional gene expression in response to growth and development and signal transduction under stress [6,7]. Growth-regulating factors (GRFs) are plant-specific proteins [8] that regulate growth and development and stress responses. GRF proteins contain two conserved domains (QLQ and WRC domains) in the N-terminal region [9,10]. The QLQ domain is similar to the N-terminus of SWI2/SNF2 in yeast and can bind with SNF11 to form a chromatin remodeling complex [11]. In addition, the QLQ domain can interact with the conserved structure of the GIF protein SNH to perform the function of transcriptional activation. The WRC domain contains a nuclear localization signal motif and a zinc finger motif that plays a role in DNA binding, which can regulate the expression of downstream genes [12] by binding to the cis-acting region of the target gene.
The GRF transcription factor family has been identified and analyzed at the genome level in many plant species; for example, nine GRF family members have been identified in Arabidopsis [13], 12 in rice [14], 35 in rapeseed [15], 17 in Chinese cabbage [16], 25 in tobacco [17], and 30 in wheat [18]. GRF genes play an important role in the plant response to abiotic stress [19,20]. The overexpression of AtGRF7 results in up-regulation of the dehydrogenation response element binding protein 2A (DREB2A), which confers increased tolerance to salt and drought stress [21]. Under abiotic stress, AtGRF7 directly binds to the cis-acting regulatory element TGTCAGG in the promoter of DREB2A to inhibit the expression of wild-type DREB2A, thus maintaining the growth rate [22,23]. Gene expression profile analysis of the atgrf7-1 T-DNA insertion mutant showed that a large proportion of the up-regulated genes are associated with responses to stress and abscisic acid (ABA) [22]. The downstream targets of AtGRF1 and AtGRF3 are involved in defense response and disease resistance, which indicates that AtGRF1 and AtGRF3 play primary roles in the coordination of plant growth and defense signals [20]. Expression patterns of GhGRF1A, GhGRF1D, and GhGRF17D in cotton change in response to salt stress [24]. Under shade stress, all GmGRF genes are significantly down-regulated in soybean [25]. Tomato SlGRF1, SlGRF4, SlGRF5, SlGRF7, SlGRF10, SlGRF11, and SlGRF12 each play an important role in ABA signal transduction [26].
In addition, GRF transcription factors are involved in plant growth and development, including root development [27], flowering [28,29], and leaf size and longevity [30]. The overexpression in Arabidopsis of AtGRF1 and AtGRF2 leads to cotyledon and leaf enlargement, and AtGRF8 is involved in Arabidopsis flower development. The overexpression of BnGRF2 increases seed weight and oil content in rapeseed by regulating the cell number and photosynthesis. Although the GRF family has been well characterized in model plants and other plant species, information on the function and evolutionary characteristics of the GRF gene family in strawberry remains limited.
Therefore, we studied the role of the GRF gene family in abiotic stress responses in strawberry. A reference genome for woodland strawberry (F. vesca, 2 n = 2 x = 14) has been published [31]. The genome comprises approximately 240 MB and presents an opportunity for the genome-wide mining of GRF transcription factors. Although the GRF gene family has been previously characterized in many plant species, little is known about the family in F. vesca. In this study, we conducted a genome-wide search and identified 10 members of the GRF gene family in the woodland strawberry genome. The functions of FvGRFs in response to abiotic stresses (low temperature, high temperature, drought, and salinity) and hormone treatment (salicylic acid and abscisic acid) were explored. In addition, bioinformatics analyses were conducted to provide insights into the structure and function, as well as the evolution, of the FvGRF genes. The results provide a foundation for further investigation of the functions of strawberry GRF genes in response to abiotic stresses.
2. Results
2.1. Phylogenetic Analysis of the GRF Family
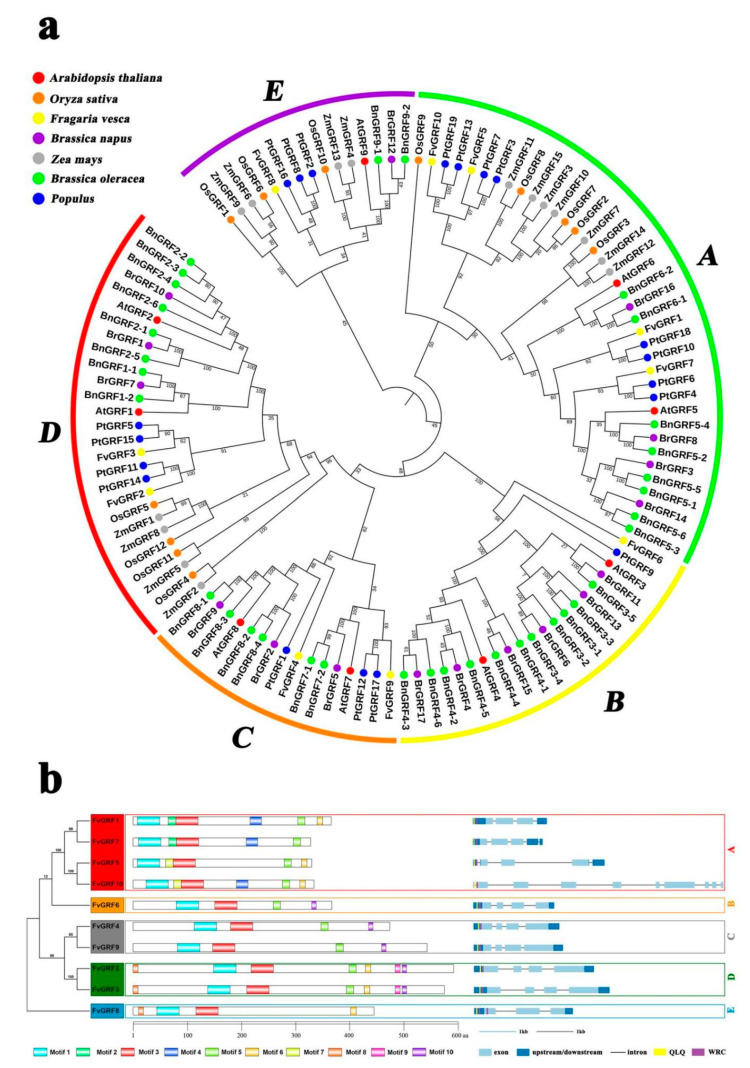
To identify the GRF gene sequence of strawberry, GRF candidate genes were searched from the Fragaria vesca genome according to two strategies: Hidden Markov Model search (HMM search) using the HMM profiles PF08880 (QLQ domain) and PF08879 (WRC domain); BLASTP search using GRF proteins from Arabidopsis as queries. As a result, ten members of the FvGRF gene family were identified from the diploid woodland strawberry genome (V4a) (Supplementary materials Table S1). The genes were designated FvGRF1 to FvGRF10 according to their homologous relationship with Arabidopsis thaliana genes. To explore the functions of the FvGRF family members, a phylogenetic analysis of GRF protein sequences from Arabidopsis, rice, woodland strawberry, poplar, maize, Chinese cabbage, and rapeseed was performed. The strawberry GRF proteins were resolved into five clades, herein designated subfamilies A to E, of which FvGRF1, FvGRF5, FvGRF7, and FvGRF10 belonged to subfamily A, FvGRF6 belonged to subfamily B, FvGRF4 and FvGRF9 belonged to subfamily C, FvGRF2 and FvGRF3 belonged to subfamily D, and FvGRF8 belonged to subfamily E (Figure 1a).
Figure 1.
(a). Phylogenetic analysis of GRF proteins of Arabidopsis, rice, woodland strawberry, poplar, maize, Chinese cabbage, and rapeseed. The full-length amino acid sequences of GRF proteins from Arabidopsis (AtGRF), rice (OsGRF), woodland strawberry (FvGRF), poplar (PtGRF), maize (ZmGRF), Chinese cabbage (BrGRF), and rapeseed (BnGRF) were aligned using ClustalX. The phylogenetic tree was constructed using the maximum likelihood method with 1000 bootstrap replicates using MEGA6.0. The branches are colored to indicate GRF subfamilies. (b). Structural analysis of strawberry GRF protein. The protein domains of the strawberry GRF genes are shown on the left and are denoted by rectangles with different colors. The exon–intron organization is shown on the right, with exons and introns represented by light blue rectangles and black lines, respectively; UTRs are indicated by dark blue rectangles. The sequence of yellow boxes and purple boxes code the QLQ domain and WRC domain, respectively. The red, orange, gray, green, and blue rectangles are used to cluster the genes into the A, B, C, D, and E subfamilies, respectively.
Multiple sequence alignment and conserved domain analysis of the amino acid sequences of the FvGRF family members revealed that all FvGRF proteins contained QLQ and WRC domains. FvGRF4 and FvGRF8 also contained a second WRC domain downstream of the first WRC domain. In addition, the zinc finger motif (CCCH) [32] was observed in the WRC domain of all identified FvGRF proteins (Supplementary Materials Figure S1).
Analysis of the gene structure and conserved motifs supported the phylogenetic reconstruction of the GRF gene family. FvGRF1, FvGRF5, and FvGRF7 in subfamily A contained three introns, whereas FvGRF10 contained nine introns, the members of subfamilies B, C, and D contained four introns, and the members of subfamily E contained two introns (Supplementary Materials Table S7). To identify potential conserved motifs, the MEME tool was used to analyze the sequences of the 10 FvGRF genes. Ten conserved motifs were detected, which were designated motif1 to motif10. Among these motifs, each gene contained not only the QLQ and WRC motifs, but also 2–5 additional conserved motifs. Interestingly, motif2, motif4, and motif7 were observed only in subfamily A members, motif8 only in subfamily D and E members, and motif9 only in subfamily D members. These specific motifs may contribute to the functional diversity of GRF genes from different subfamilies (Figure 1b).
2.2. Chromosomal Location, Gene Structure, and Conserved Motif Analysis
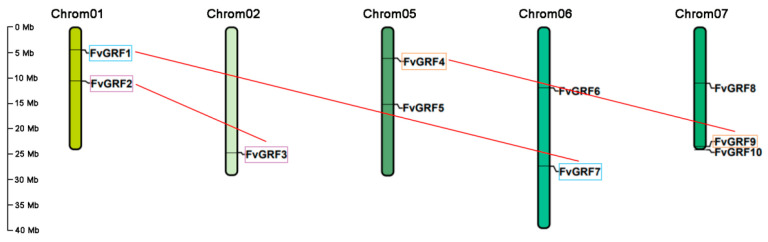
The FvGRF genes were not evenly distributed on all chromosomes. The 10 FvGRF genes were distributed on five chromosomes. FvGRF1 and FvGRF2 were located on Chrom01, FvGRF3 was located on Chrom02, FvGRF4 and FvGRF5 were located on Chrom05, FvGRF6 and FvGRF7 were located on Chrom06, and FvGRF8, FvGRF9, and FvGRF10 were located on Chrom07. Thus, Chrom03 and Chrom04 did not carry FvGRF genes (Figure 2).
Figure 2.
Chromosomal location of FvGRF genes of woodland strawberry. The chromosomal location of each FvGRF gene was determined in accordance with the woodland strawberry genome assembly v4.0.a1. The number of each chromosome is shown above the chromosome. Replicated fragments are represented by a colored box connected by a red line. Note: FvGRF1 (FvH4_1g08440.1), FvGRF2 (FvH4_1g18220.1), FvGRF3 (FvH4_2g32670.1), FvGRF4 (FvH4_5g10750.1), FvGRF5 (FvH4_5g23900.1), FvGRF6 (FvH4_6g18130.1), FvGRF7 (FvH4_6g34730.1), FvGRF8 (FvH4_7g12130.1), FvGRF9 (FvH4_7g32780.1), and FvGRF10 (FvH4_7g34250.1).
2.3. Different Duplication Events Control the Expansion of GRF Genes in Strawberries and Arabidopsis Thaliana
To gain further insight into the evolution of strawberry GRF genes, we analyzed the duplication events of GRF genes in Arabidopsis thaliana and strawberry. Dispersed duplication was the primary mode of GRF gene replication in Arabidopsis, accounting for 55.56% (five of nine), singleton genes accounted for 11.11% (one of nine), and 33.33% of the genes involved WGD/segmental duplication (three of nine) (Figure 3). In contrast, WGD/segmental duplication was the primary mode of replication of strawberry GRF genes, accounting for 60% (six out of ten), and genes involved in dispersed duplication events and singleton genes accounted for 20% each (two out of ten) (Supplementary materials Table S5). Proximal duplication and tandem duplication events were not detected in the Arabidopsis and strawberry GRF families. These results suggested that different gene duplication events controlled expansion of the GRF family in Arabidopsis and strawberry (Supplementary file Figure S2).
Figure 3.
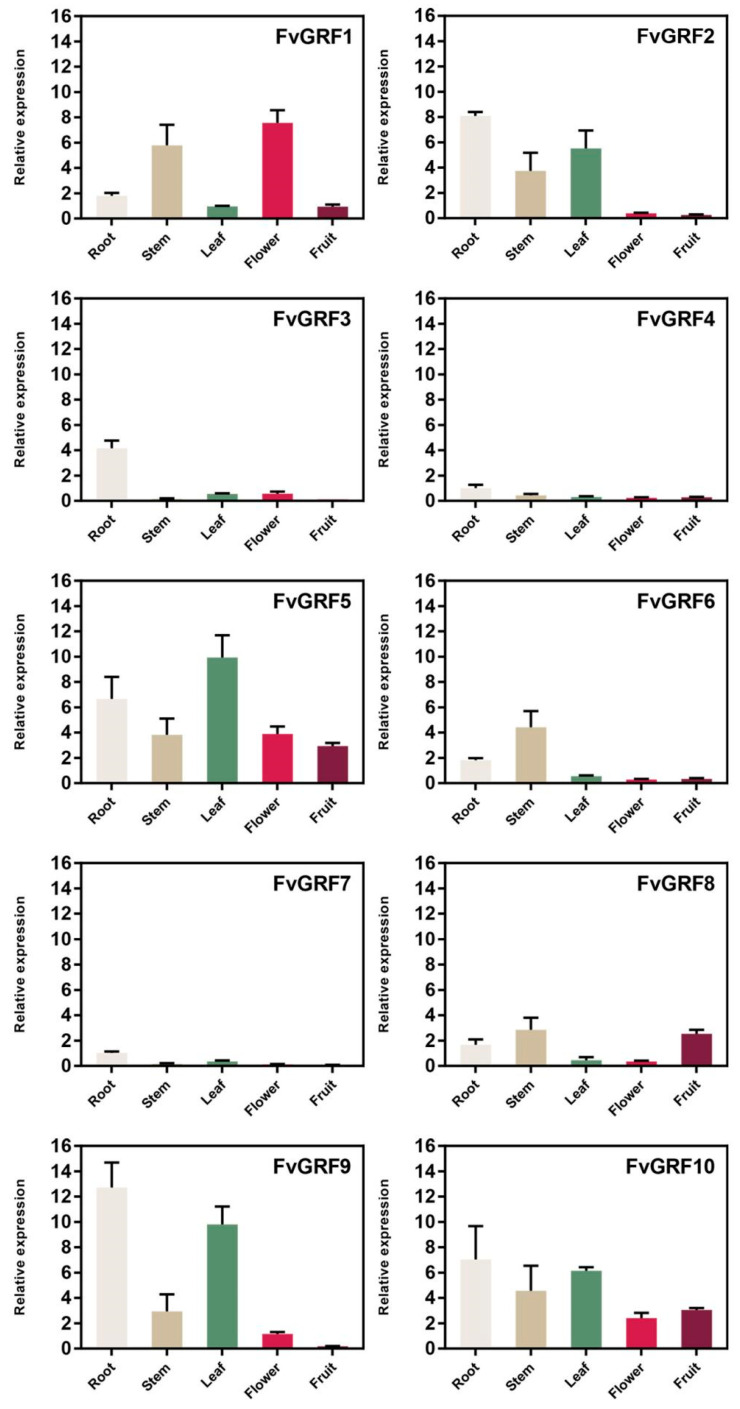
Quantitative RT-PCR analysis of 10 FvGRF genes in different organs of woodland strawberry. Roots, stems, and leaves are sampled during the period of vigorous growth, 42 days after the flowers are sampled, and the fruits are sampled 26 days after the flowers. Expression levels were normalized to that of FvPDB. The experiment was repeated three times.
2.4. Expression Patterns of FvGRF Genes in Different Organs
To study the expression patterns of GRF genes in different organs of diploid woodland strawberry, qRT-PCR was used to quantify the expression level of FvGRF genes in the root, stem, leaf, flower, and fruit. Certain FvGRF genes were highly expressed in specific organs, whereas other FvGRF genes showed similar expression patterns in different organs (Figure 3), which may be indicative of functional differences of the genes in strawberry growth and development. For example, FvGRF3, FvGRF4, and FvGRF7 were expressed at relatively high levels in the root, whereas FvGRF2 and FvGRF9 were mainly expressed in vegetative organs (Figure 4). FvGRF5 and FvGRF10 were highly expressed in different tissues. FvGRF1 was more highly expressed in the stem and flower. The expression level of FvGRF8 was higher in the root, stem, and fruit. Except for FvGRF1, the expression level of FvGRF genes in vegetative organs was generally higher than that in reproductive organs, which indicated that FvGRF genes may play important roles in the growth and development of vegetative organs.
Figure 4.
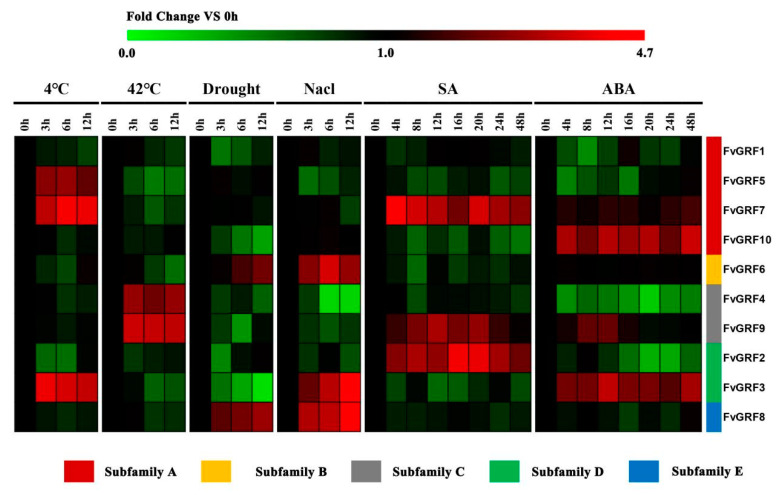
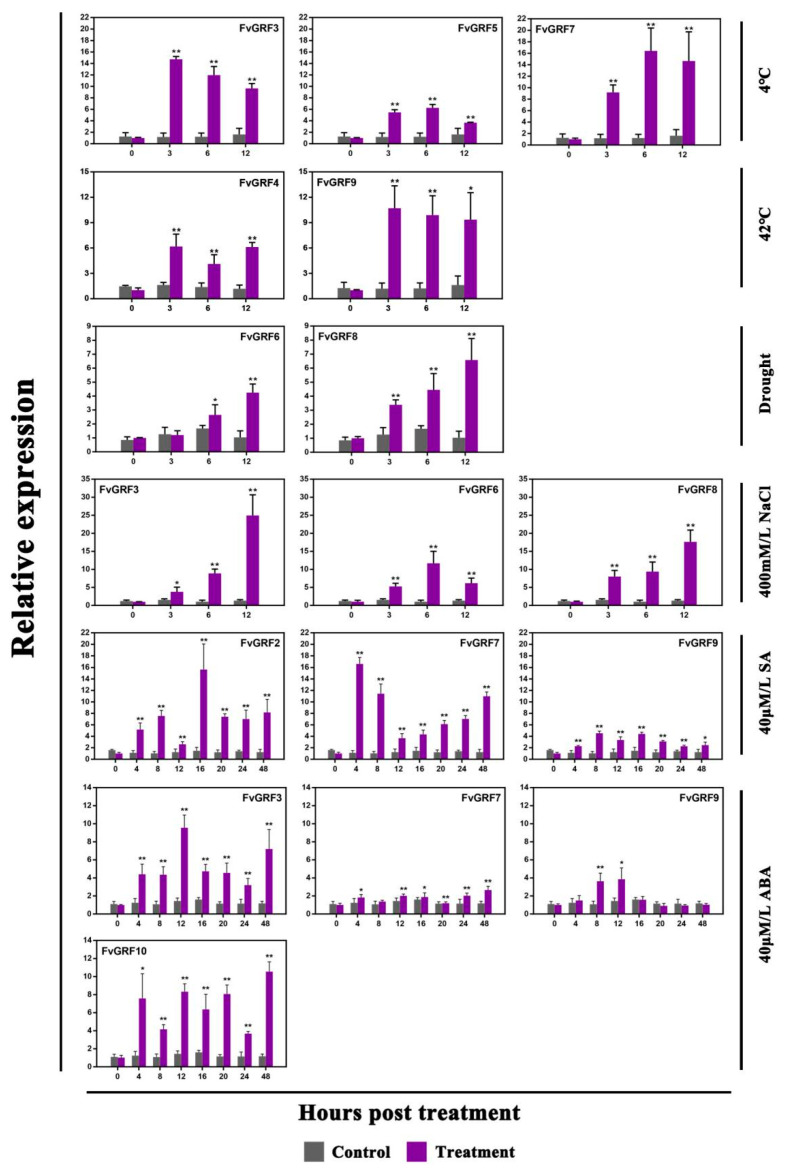
Expression accumulation profiles of 10 woodland strawberry GRF genes in response to low temperature (4 °C), high temperature (42 °C), drought, NaCl, salicylic acid (SA), and abscisic acid (ABA) treatments as determined by quantitative RT-PCR analysis and displayed in the form of heat maps. The color scale indicates the change in expression level, where red indicates an increase and green indicates a decrease, relative to that at 0 h. The experiment was repeated three times and the results were consistent.
2.5. Expression Pattern of FvGRF Genes under Stress Treatments
To explore the potential role of FvGRF genes in plant responses to various environmental stresses, the expression level of the 10 FvGRF genes under low temperature, high temperature, drought, and salt stress was determined by qRT-PCR analysis. Generally, the FvGRF genes differed in the degree of response to low temperature, high temperature, drought, and salt stress. Compared with the high temperature and drought treatments, the FvGRF genes were more highly responsive to low temperature and salt stress (Figure 4). The expression of FvGRF3, FvGRF5, and FvGRF7 was up-regulated under low-temperature stress, FvGRF4 and FvGRF9 were up-regulated under high-temperature stress, FvGRF6 and FvGRF8 were up-regulated under drought stress, FvGRF3, FvGRF6, and FvGRF8 were up-regulated under salt stress, and the other genes were down-regulated to varying degrees. The degree of response of FvGRF genes to low-temperature stress was similar to that under salt stress.
The plant hormones SA and ABA play important roles in plant stress signal response and in plant growth and development. To evaluate if FvGRF gene expression was induced in response to plant hormone treatment, the expression patterns of the FvGRF genes in the leaf in response to exogenous SA and ABA treatment were analyzed by qRT-PCR. In response to SA treatment, the expression levels of FvGRF2, FvGRF7, and FvGRF9 were significantly increased to a high level from 4 h after treatment, and were maintained at a high expression level during the entire experimental period, whereas the other genes were up-regulated or down-regulated in certain periods (Figure 5). Under ABA treatment, FvGRF3, FvGRF7, FvGRF9, and FvGRF10 were up-regulated to varying degrees, whereas all other FvGRF genes were down-regulated or almost unchanged. It is notable that FvGRF7 and FvGRF9 were up-regulated in response to treatment with SA and ABA (Figure 4).
Figure 5.
Quantitative RT-PCR analysis of woodland strawberry GRF gene expression in response to low temperature, high temperature, drought, NaCl, salicylic acid (SA), and abscisic acid (ABA) treatments. The expression levels of the FvGRF genes revealed the epigenetic patterns in response to the SA and ABA treatments. The expression levels were normalized to that of FvPDB. The experiment was repeated three times and consistent results were obtained. The mean and SD were calculated from three biological and three technical replicate samples. Asterisks indicate that the gene was significantly up-regulated or down-regulated after treatment (* p < 0.05, ** p < 0.01; Student’s t-test).
2.6. Identification of Cis-Acting Regulatory Elements in the Promoter of FvGRF Genes
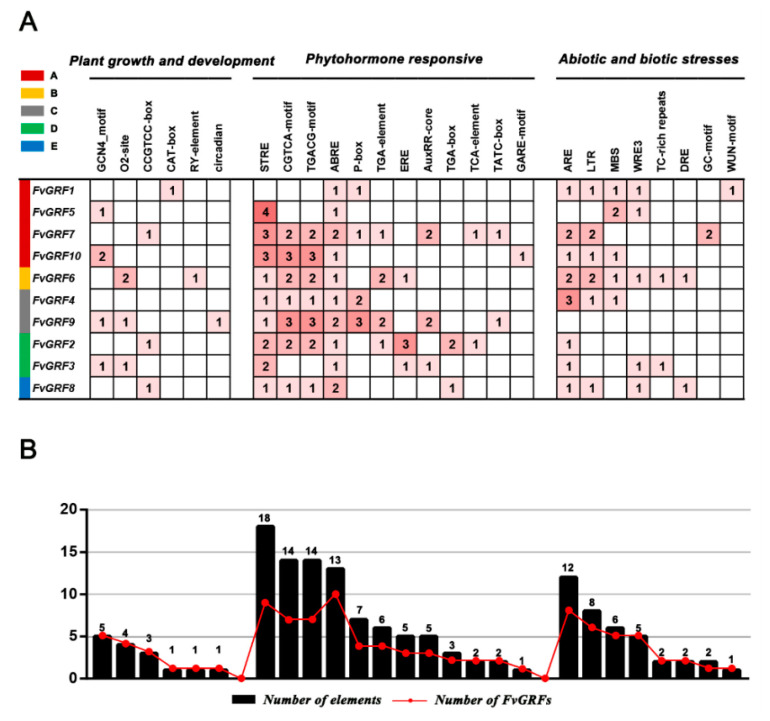
To explore the regulation of strawberry GRF family members, analysis of the cis-acting regulatory elements in the 1.5 kb region upstream of the initiation codon of all FvGRF family members was conducted using the PlantCARE online portal. The GCN4 motif [33], a cis-acting regulatory element associated with endosperm expression, was detected in FvGRF3, FvGRF5, FvGRF9, and FvGRF10 (Figure 6). It was noteworthy that the element involved in the regulation of circadian rhythms [34] was detected in FvGRF9, and only FvGRF1 contained an element involved in mechanical injury response (WUN motif). In addition, the gliadin metabolic regulatory element (O2-site), meristem expression and specific activation elements (CAT-box and CCGTCC-box), and seed-specific regulatory element (RY-element) [35] were identified in the promoter of FvGRF genes. With regard to hormone-related cis-acting elements, the SA response element (TCA-element) [36], methyl jasmonate response elements (CGTCA-motif and TGACG-motif) [37], and ABA response element (ABRE) [38] were identified in the promoters of two, seven, and 10 FvGRF genes, respectively. Gibberellin response elements (GARE-motif and P-box) [39] and auxin response elements (TGA-element and AuxRR-core) [40] were observed in five and three FvGRF genes, respectively. A large number of elements associated with hormone response were identified in the FvGRF promoter sequence, which indicated that plant hormones may play a crucial role in regulating the functions of FvGRF genes in plant growth and development (Figure 6B). In addition, several cis-acting elements associated with response to stresses (such as drought, extreme temperature, and salinity) were observed in the promoter region of FvGRF genes (Supplementary Materials Table S6).
Figure 6.
Analysis of cis-acting regulatory elements in the promoter of woodland strawberry GRF genes. (A) Number of each cis-acting element in the promoter region (1.5 kb upstream of the translation start site) of FvGRF genes. (B) Statistics for the total number of FvGRF genes, including the corresponding cis-acting element (red dot) and the total number of cis-acting elements in the FvGRF gene family (black box). On the basis of the functional annotation, the cis-acting elements were classified into three major classes: plant growth and development, phytohormone response, and abiotic and biotic stress response.
3. Discussion
Growth regulatory factors are plant-specific transcription factors. Previous studies have shown that GRFs play an important role in coordinating growth under stress. The present study aimed to identify candidate genes involved in stress regulation among members of the strawberry GRF family. Using the diploid woodland strawberry reference genome (V4a version), 10 members of the FvGRF gene family were identified in this study. Whole-genome duplication/segmental duplication was the primary driving force for expansion of the strawberry GRF family. Strawberry GRF genes were predominantly expressed in vegetative organs and expression levels changed to varying degrees in response to low temperature, high temperature, drought, and salinity stress, and to exogenous SA and ABA treatment. These results indicated that FvGRF genes showed potential regulatory functions in abiotic stress responses.
Gene duplication plays an important role in gene family evolution and is the primary mechanism for the generation of novel evolutionary innovations, such as tandem duplication and WGD/segmental duplication [41]. The present results indicated that dispersed duplication and WGD/segmental duplication effectively promoted expansion of the GRF gene family in Arabidopsis and strawberry, but tandem duplication events were not detected in either gene family. This phenomenon has previously been reported [8]. A gene family may show a common nonrandom origin pattern and a conserved duplication pattern in different species [42]. However, in the present study, decentralized duplication was the predominant driving force in Arabidopsis, whereas WGD/segmental duplication was the primary driving force in strawberry. These results indicated that the main duplication patterns of the Arabidopsis and strawberry GRF gene families were not always strictly conserved and that nonrandom patterns from different sources were common.
The expression patterns of the FvGRF genes were analyzed to evaluate their potential roles in different organs of strawberry. Six FvGRF genes were most highly expressed in the roots. Previous studies have shown that GRF proteins may play an important role in plant root development or physiological processes [27]. In Arabidopsis, AtGRF1 and AtGRF3 are highly expressed in the roots [20]. In the present study, FvGRF2 and FvGRF3 were shown to be homologous with AtGRF1 and were highly expressed in the roots, suggesting that members of this subfamily perform a potentially conserved function in the roots. In rice, OsGRF10 is highly expressed in the leaves [14]. In the present study, we observed that FvGRF5 and FvGRF10 were homologous with OsGRF10 and were also highly expressed in the leaves. Thus, members of this subfamily may show a potentially conserved function in the leaves. GRF genes are usually more highly expressed in actively growing tissues than in mature tissues [43]. Overall, the expression level of FvGRF genes in vegetative organs was higher than that in reproductive organs, which suggested that FvGRF genes may play important roles in the growth and development of vegetative organs.
The ABA signaling pathway is considered to be a central regulator of abiotic stress in plants and mediates the expression of stress-resistance genes. The ABRE is the main cis-acting element involved in ABA-responsive gene expression. Among strawberry GFR genes, FvGRF3 attained the highest expression level at 12 h after ABA treatment, FvGRF7 showed the highest level at 48 h, FvGRF9 attained the highest level at 12 h, and FvGRF10 was the most highly expressed gene at 48 h and was up-regulated by 10.56-fold. All FvGRF genes contained an ABRE in the promoter. The presence of multiple ABRE copies can provide ABA with the ability to respond to the promoters, whereas a single-copy ABRE is not responsive to ABA [44]. In the present study, we observed that FvGRF7 and FvGRF9 contained two ABRE-acting elements, which may be the reason for their up-regulation in response to exogenous ABA. However, only one ABRE is present in the promoter of rd29A and there is no known coupling element, but exogenous ABA strongly induces rd29A expression, which indicates that a potential cis-acting regulatory element may act as a coupling element in the ABA response [45]. We identified only one ABRE in the promoters of FvGRF3 and FvGRF10. It is speculated that other motifs may replace the function of the ABRE or coupling element, thereby resulting in the up-regulation of FvGRF3 and FvGRF10 in response to exogenous ABA.
Similarly, SA plays an important role in the regulation of plant responses to abiotic stress. Under SA treatment, FvGRF2, FvGRF7, and FvGRF9 were up-regulated. Among these genes, FvGRF2 attained the highest expression level at 4 h after SA treatment, which represented up-regulation by almost 16-fold, whereas FvGRF7 showed the highest expression level at 4 h after SA treatment, which represented up-regulation by almost 17-fold. Promoter sequence analysis predicted the presence of the SA-responsive TCA-element in FvGRF2 and FvGRF7 but not in FvGRF9. Previous studies have shown that SA can induce RLK gene expression because a TTGCA sequence is present upstream of the RLK gene, which plays an important role in inducing the expression of many plant defense-related genes [46]. We observed the TTGCA sequence in the upstream region of FvGRF9, which may be an important reason for the up-regulation of FvGRF9 expression in response to SA treatment. However, additional experimental evidence is needed to elucidate the transcriptional regulation of FvGRF genes in strawberry.
Drought and high salt stress strongly impact on plant growth and productivity. These stresses induce the expression of many genes in different plants. Various cis-acting elements in the stress response promoter play an important role in plant adaptation to environmental stress. A dehydration response element (DRE; TACCGACAT) responsible for dehydration and high salt-induced gene expression is present in the promoter region of rd29A (Narusaka Y et al., 2003). In the present study, expressions of FvGRF6 and FvGRF8 were up-regulated under drought and salt stress, and a DRE was observed in the promoter sequence of each gene. It is speculated that the DRE may be the reason for the up-regulated expression of FvGRF6 and FvGRF8 in response to salt and drought stress. Previous studies have shown that GT-1 components directly control the up-regulation of OsRAV2 in response to high salinity [47]. Two salt stress response elements for FvGRF3 were identified upstream of the GT-1 (GAAAAA) promoter. It is speculated that this element may account for the up-regulation of FvGRF3 expression under salt stress.
In addition, plants are vulnerable to temperature fluctuations. High- and low-temperature stresses lead to plant stunting and reduce crop yields. Therefore, it is important to improve the ability of plants to resist low- and high-temperature stress. In Arabidopsis, AtGRF7 can be used as a transcriptional activator of DREB2A and other stress-responsive genes to increase tolerance to high-temperature stress [22]. In the current study, FvGRF4 and FvGRF9 were up-regulated under high-temperature stress. Phylogenetic analysis resolved AtGRF7, FvGRF4, and FvGRF9 in subfamily C, which suggested that this subfamily may perform a potentially conserved function under high-temperature stress. In the promoter of the AtHsp90-1 gene, interaction of the CCAAT-box and stress response elements (STREs) increase AtHsp90-1 expression in response to heat shock [48]. In the present study, the upstream region of the FvGRF4 promoter was observed to contain CCAAT-box and STRE elements. We speculate that these elements may account for the up-regulation of FvGRF4 expression in response to high-temperature stress. Analysis of FvGRF expression under low-temperature stress revealed that FvGRF3, FvGRF5, and FvGRF7 were up-regulated in response to low-temperature treatment. The barley blt4.9 gene promoter contains the CCGAAA sequence. Mutation analysis showed that deletion of the CCGAAA motif reduces the basic level of response to low temperature, which indicates that the CCGAAA motif plays an important role in the low-temperature stress response in barley [49]. In the present study, CCGAAA-motif elements were observed in the FvGRF7 promoter and may explain the up-regulation of FvGRF7 under low-temperature stress. In addition, FvGRF3 and FvGRF5 were up-regulated in response to low-temperature treatment, but no known cold-responsive regulatory elements were identified in the promoter of either gene. We speculate that the upregulation of FvGRF3 and FvGRF5 expression may be due to the presence of novel motifs in the promoter that may be the crucial elements in their response to low-temperature stress.
Different environmental factors affect gene expression, and gene expression also requires the coordination of inducible cis-acting regulatory elements and transcription factors in the promoter of environment-responsive genes. The type, number, and location of these elements may affect the level of gene expression. Therefore, further research is needed to gain an improved understanding of transcriptional regulatory mechanisms in strawberry, including transcription factors and their specific cis-acting regulatory elements.
4. Materials and Methods
4.1. Plant Materials, Growth Conditions, and Stress Treatments
Seeds of wild diploid woodland strawberry were obtained from the strawberry germplasm resource nursery of the College of Horticulture, Fujian Agriculture and Forestry University (26°10′ N, 119°23′ E), Fuzhou, China. The seeds were sown on Murashige and Skoog medium and then transferred to soil for subsequent growth after germination. The growth environment was 22 °C, relative humidity 75%, and a photoperiod of 13 h/11 h (day/night). As materials for gene expression analysis in different organs of woodland strawberry plants, samples of the roots, stems, leaves, flowers (at anthesis), and fruits (at the red fruit stage) were collected during reproductive growth. Each sample contains 3 replicates, with each replicate including 3 plants. All of the collected samples were snap-frozen in liquid nitrogen and kept at −80 °C until further use.
Four-month-old seedlings of uniform growth were selected for abiotic stress treatment. A solution of 400 mM/L of NaCl was sprayed onto potted plants to induce salt stress for 12 h. Potted plants were transferred to a growth room maintained at either 4 °C or 42 °C for 12 h for treatment with low-temperature and high-temperature stress, respectively. Plants were prepared by withholding water for 12 h of drought treatment. In addition, leaves were evenly sprayed with 40 μmol/L of ABA or 40 μmol/L of SA solution. The leaves of treated plants were sampled at 0, 3, 6, and 12 h after initiation of low temperature, high temperature, drought, and salt stress. The leaves of plants treated with SA or ABA were sampled at 0, 4, 8, 12, 16, 20, 24, and 48 h after treatment. Six leaves from three individual seedlings were selected and combined as one sample. All treatments were evaluated in triplicate. All collected samples were immediately wrapped in tin foil, frozen in liquid nitrogen, and stored at −80 °C until use.
4.2. Genome-Wide Identification of GRF Genes
The genome sequence, coding sequence, and amino acid sequence of the diploid strawberry (Fragaria vesca) genome assembly v4.0.a1 were downloaded from the Genome Database for Rosaceae (https://www.rosaceae.org (accessed on 16 March 2021)). The hidden Markov models of the characteristic GRF protein domains QLQ (PF08880) and WRC (PF08879) were downloaded from the Pfam database [50] (http://pfam.xfam.org/ (accessed on 16 March 2021)). Members of the GRF family in the complete strawberry genome were identified using HMMER software under the condition of E-value < 1 × 10−10. The amino acid sequences of Arabidopsis GRF family members were compared with those in the strawberry genome database using the BLASTP tool. The CD-Search tool (https://www.ncbi.nlm.nih.gov (accessed on 16 March 2021)) was used to verify the protein domains (Supplementary Materials Table S4). The ExPASy ProtParam tool (https://web.expasy.org/protparam/ (accessed on 17 March 2021)) was used to predict the deduced protein sequence length, molecular weight, isoelectric point, and instability coefficient of strawberry GRF family members.
4.3. Phylogenetic Analysis
The GRF protein sequences of Arabidopsis thaliana were downloaded from The Arabidopsis Information Resource (https://www.arabidopsis.org (accessed on 16 March 2021)), those of rice from the Rice Genome Annotation Project (http://rice.plantbiology.msu.edu/analyses_search_locus.shtml (accessed on 16 March 2021)), those of Chinese cabbage from the Brassica database (http://brassicadb.org/brad/ (accessed on 16 March 2021)), those of rapeseed from BrassicaDB (http://brassicadb.org/brad/searchGene.php (accessed on 16 March 2021)), those of poplar and maize from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html (accessed on 16 March 2021)), and those of strawberry from the Genome Database for Rosaceae (https://www.rosaceae.org (accessed on 16 March 2021)). A multiple sequence alignment of GRF protein sequences of Arabidopsis, rice, rapeseed, poplar, maize, and strawberry was generated using ClustalX software [51] (Supplementary Materials Table S3). A phylogenetic tree was constructed using the maximum likelihood method with MEGA6.0 software [40]. Support for the topology was assessed by performing a bootstrap analysis with 1000 replicates. The phylogenetic tree was visualized and edited using the iTOL online tool [52] (https://itol.embl.de/itol.cgi (accessed on 18 March 2021)).
4.4. Chromosomal Location, Gene Structure, and Conserved Sequence Analysis
The gff3 annotation file for the woodland strawberry genome was downloaded from the Genome Database for Rosaceae and the chromosomal locations of the FvGRF genes were extracted. TBtools [53] was used to visualize the chromosomal location of the FvGRF genes. The GSDS 2.0 online server [54] (https://gsds,cbi.pku.edu.cn/ (accessed on 20 March 2021)) was used to visualize the exon and intron structure of each gene. The amino acid sequence of each GRF protein of strawberry was submitted to the MEME online analysis website [55] (http://meme-suite.org/tools/meme (accessed on 20 March 2021)) to identify conserved protein motifs. The optimized MEME parameters were as follows: minimum pattern width 6; maximum pattern width 100; and use of the maximum number of programs, including up to 10. The conserved sequences were visualized using Adobe Illustrator 2020 software.
4.5. Synteny Analysis
Based on the method used in the Plant Genome Duplication Database [56], we used an improved method for synteny analysis. First, a BLASTP search was performed on the entire genome to identify candidate homologous gene pairs (E-value < 1 × 10−5, the first five matches). The candidate genes were analyzed with MCScanX [57] for the detection of syntenic blocks using the default parameters. MCScanX was used to distinguish singleton genes, whole-genome duplication (WGD)/segmental duplication, dispersed duplication, proximal duplication, and tandem duplication events in the strawberry GRF family.
4.6. RNA Extraction and Gene Expression Analysis
To evaluate the expression levels of GRF genes in strawberry, total RNA was extracted from collected plant samples or treated leaves using the RNAprep Pure Plant Plus Kit (Tiangen Biotech, Beijing, China) in accordance with the manufacturer’s recommendations. Before reverse transcription, the RNA was treated with DNase I (Tiangen Biotech) to remove residual DNA contamination. According to the RNA concentration of the sample extract, 1–7 μg of total RNA was reverse-transcribed into the first-strand cDNA using the FastKing gDNA Dispelling RT SuperMix. The reaction mixture contained 4 μL of 2 × FastKing-RT SuperMix, 4 μL of total RNA, and 12 μL of RNase-free ddH2O in a total reaction volume of 20 μL. The DNA polymerization temperature was 42 °C for 15 min and reverse transcriptase was deactivated at 95 °C for 3 min. Primer 5 software was used to design gene-specific primers for each FvGRF gene.
qRT-PCR was performed on a CFX ConnectTM real-time system (BIO-RAD) using SuperReal PreMix Plus (SYBR Green). Quantitative reverse transcription-PCR (qRT-PCR) reactions were performed in a 10 μL volume containing 5 μL of 2 × Super Real Pre Mix Plus, 0.3 μL of forward primers, 0.3 μL of reverse primers, 1 μL of cDNA template, and 3.4 μL of RNase-free ddH2O. Each amplification was conducted under the following conditions: pre-denaturation of 1 cycle for 15 min at 95 °C, followed by 40 cycles of denaturation for 10 s at 95 °C and annealing/extension for 32 s at 60 °C. The temperature was gradually increased by 0.5 °C every 10 s to analyze the melting curve. FvPDB [58] was used as an internal reference gene to normalize expression data. For each sample, the determination comprised three technical replicates and three biological replicates. Each replication included the internal reference gene. The relative expression level of each gene was calculated using the 2−ΔΔCt method, and the standard deviation was calculated from the three biological replicates and three biological replicates [59]. Sequences of primers used for qRT-PCR are listed in Supplementary materials Table S2.
4.7. Analysis of Cis-Acting Regulatory Elements in the Promoter of FvGRFs
Using the woodland strawberry reference genome, the sequence 1500 bp upstream of the start codon for each FvGRF gene was selected for analysis of cis-acting elements in the promoter using TBtools software, and the cis-acting elements were predicted using PlantCARE [60] (http://bionformatics.psb.ugent.be/webtools/plantcare/html (accessed on 23 March 2021)). The motifs that may be involved in plant growth and development, plant hormone responses, and abiotic and biotic stress responses were summarized.
4.8. Statistical Analysis
Statistical significance was determined using Student’s t-test as implemented in Graphpadprism7.0 software. The average ± standard deviation of at least three repeated samples was calculated. The significance of differences compared with the control were expressed as * p < 0.05 and ** p < 0.01.
5. Conclusions
We identified 10 FvGRF family members in the genome of woodland strawberry. Whole-genome duplication/segmental duplication is indicated to have been the primary driving force for expansion of the strawberry GRF family. The FvGRF genes were differentially expressed in vegetative and reproductive organs of strawberry, but were especially expressed in the roots. The genes showed varying degrees of response to low temperature, high temperature, drought, and salinity stress, and to exogenous SA and ABA treatment. The present results provide a basis for investigation of the transcriptional regulatory mechanisms of growth and development, and the functional identification of stress-resistance genes in strawberry.
Acknowledgments
The authors would like to thank anonymous reviewers for comments on this manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/plants10091916/s1. Supporting data come from additional documents and supplementary documents, Figure S1: Sequence alignment of FvGRF (Fragaria vesca GRF) proteins and the QLQ and WRC domains are indicated upside. Identical amino acids are indicated by the background color, Figure S2: Structural analysis of strawberry GRF protein. The protein domains of the strawberry GRF genes are shown on the left and are denoted by rectangles with different colors. The exon–intron organization is shown on the right, with exons and introns represented by green round-corner rectangles and red shrunken lines, respectively; UTRs are indicated by gray rectangles. The red, orange, gray, green, and blue rectangles are used to cluster the genes into the A, B, C, D, and E subfamilies, respectively, Table S1: Molecular properties, chromosomal location, and subfamilial classification of the encoded protein for woodland strawberry FvGRF family members, Table S2: Primers used for this study, Table S3: GRF genes were identified in Arabidopsis thaliana, Fragaria vesca, Oryza sativa L., Populus L., Zea mays L., and Brassica napus L., Table S4: Conserved domains were predicted by CDD, Table S5: Gene duplication modes of GRF genes in Arabidopsis thaliana and Fragaria vesca, Table S6: Cis-elements were predicted by PlantCARE in promoter sequences of FvGRF genes, Table S7: Gene structure of FvGRF genes.
Author Contributions
Experiments were performed by Z.L. and J.Y.; Z.L. and Q.X. analyzed the data; Z.L. drafted the manuscript; J.C. and Q.C. supervised the experiments and finalized the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project of Germplasm Innovation and industrialization of characteristic Horticultural plants in Fuzhou, Fujian Agriculture and Forestry University (Grant No. KHF200005).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All data in the present study are available in the public database as referred in the Materials and Methods section.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kumar R., Khurana A., Sharma A.K. Role of plant hormones and their interplay in development and ripening of fleshy fruits. J. Exp. Bot. 2014;65:4561–4575. doi: 10.1093/jxb/eru277. [DOI] [PubMed] [Google Scholar]
- 2.Symons G.M., Chua Y.J., Ross J.J., Quittenden L.J., Davies N.W., Reid J.B. Hormonal changes during non-climacteric ripening in strawberry. J. Exp. Bot. 2012;63:4741–4750. doi: 10.1093/jxb/ers147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edger P.P., Poorten T.J., VanBuren R., Hardigan M.A., Colle M., McKain M.R., Smith R.D., Teresi S.J., Nelson A.D.L., Wai C.M., et al. Origin and evolution of the octoploid strawberry genome. Nat. Genet. 2019;51:541–547. doi: 10.1038/s41588-019-0356-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bari R., Jones J.D. Role of plant hormones in plant defence responses. Plant. Mol. Biol. 2009;69:473–488. doi: 10.1007/s11103-008-9435-0. [DOI] [PubMed] [Google Scholar]
- 5.Cui L.G., Shan J.X., Shi M., Gao J.P., Lin H.X. The miR156-SPL9-DFR pathway coordinates the relationship between development and abiotic stress tolerance in plants. Plant J. 2014;80:1108–1117. doi: 10.1111/tpj.12712. [DOI] [PubMed] [Google Scholar]
- 6.Li J., Besseau S., Törönen P., Sipari N., Kollist H., Holm L., Palva E.T. Defense-related transcription factors WRKY70 and WRKY54 modulate osmotic stress tolerance by regulating stomatal aperture in Arabidopsis. New Phytol. 2013;200:457–472. doi: 10.1111/nph.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh K., Foley R.C., Oñate-Sánchez L. Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 2002;5:430–436. doi: 10.1016/S1369-5266(02)00289-3. [DOI] [PubMed] [Google Scholar]
- 8.Omidbakhshfard M.A., Proost S., Fujikura U., Mueller-Roeber B. Growth-Regulating Factors (GRFs): A Small Transcription Factor Family with Important Functions in Plant Biology. Mol. Plant. 2015;8:998–1010. doi: 10.1016/j.molp.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D.F., Li B., Jia G.Q., Zhang T.F., Dai J.R., Li J.S., Wang S.C. Isolation and characterization of genes encoding GRF transcription factors and GIF transcriptional coactivators in Maize (Zea mays L.) Plant Sci. 2008;175:809–817. doi: 10.1016/j.plantsci.2008.08.002. [DOI] [Google Scholar]
- 10.van der Knaap E., Kim J.H., Kende H. A Novel Gibberellin-Induced Gene from Rice and Its Potential Regulatory Role in Stem Growth. Plant Physiol. 2000;122:695–704. doi: 10.1104/pp.122.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treich I., Cairns B.R., de los Santos T., Brewster E., Carlson M. SNF11, a new component of the yeast SNF-SWI complex that interacts with a conserved region of SNF2. Mol. Cell. Biol. 1995;15:4240–4248. doi: 10.1128/MCB.15.8.4240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim J.H., Kende H. A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2004;101:13374–13379. doi: 10.1073/pnas.0405450101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J.H., Choi D., Kende H. The AtGRF family of putative transcription factors is involved in leaf and cotyledon growth in Arabidopsis. Plant J. 2003;36:94–104. doi: 10.1046/j.1365-313X.2003.01862.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi D., Kim J.H., Kende H. Whole genome analysis of the OsGRF gene family encoding plant-specific putative transcription activators in rice (Oryza sativa L.) Plant Cell Physiol. 2004;45:897–904. doi: 10.1093/pcp/pch098. [DOI] [PubMed] [Google Scholar]
- 15.Ma J.Q., Jian H.J., Yang B., Lu K., Zhang A.X., Liu P., Li J.N. Genome-wide analysis and expression profiling of the GRF gene family in oilseed rape (Brassica napus L.) Gene. 2017;620:36–45. doi: 10.1016/j.gene.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 16.Wang F., Qiu N., Ding Q., Li J., Zhang Y., Li H., Gao J. Genome-wide identification and analysis of the growth-regulating factor family in Chinese cabbage (Brassica rapa L. ssp. pekinensis) BMC Genom. 2014;15:807. doi: 10.1186/1471-2164-15-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J., Li Z., Jin J., Xie X., Zhang H., Chen Q., Luo Z., Yang J. Genome-wide identification and analysis of the growth-regulating factor family in tobacco (Nicotiana tabacum) Gene. 2018;639:117–127. doi: 10.1016/j.gene.2017.09.070. [DOI] [PubMed] [Google Scholar]
- 18.Huang W., He Y., Yang L., Lu C., Zhu Y., Sun C., Ma D., Yin J. Genome-wide analysis of growth-regulating factors (GRFs) in Triticum aestivum. PeerJ. 2021;9:e10701. doi: 10.7717/peerj.10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Casadevall R., Rodriguez R.E., Debernardi J.M., Palatnik J.F., Casati P. Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell. 2013;25:3570–3583. doi: 10.1105/tpc.113.117473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hewezi T., Maier T.R., Nettleton D., Baum T.J. The Arabidopsis microRNA396-GRF1/GRF3 regulatory module acts as a developmental regulator in the reprogramming of root cells during cyst nematode infection. Plant Physiol. 2012;159:321–335. doi: 10.1104/pp.112.193649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakuma Y., Maruyama K., Osakabe Y., Qin F., Seki M., Shinozaki K., Yamaguchi-Shinozaki K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell. 2006;18:1292–1309. doi: 10.1105/tpc.105.035881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J.S., Mizoi J., Kidokoro S., Maruyama K., Nakajima J., Nakashima K., Mitsuda N., Takiguchi Y., Ohme-Takagi M., Kondou Y., et al. Arabidopsis growth-regulating factor7 functions as a transcriptional repressor of abscisic acid- and osmotic stress-responsive genes, including DREB2A. Plant Cell. 2012;24:3393–3405. doi: 10.1105/tpc.112.100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q., Kasuga M., Sakuma Y., Abe H., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Two transcription factors, DREB1 and DREB2, with an EREBP/AP2 DNA binding domain separate two cellular signal transduction pathways in drought- and low-temperature-responsive gene expression, respectively, in Arabidopsis. Plant Cell. 1998;10:1391–1406. doi: 10.1105/tpc.10.8.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao J.F., Huang J.Q., Liu X., Huang C.C., Zheng Z.S., Zhang X.F., Shangguan X.X., Wang L.J., Zhang Y.G., Wendel J.F., et al. Genome-wide characterization of the GRF family and their roles in response to salt stress in Gossypium. BMC Genom. 2020;21:575. doi: 10.1186/s12864-020-06986-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen F., Yang Y., Luo X., Zhou W., Dai Y., Zheng C., Liu W., Yang W., Shu K. Genome-wide identification of GRF transcription factors in soybean and expression analysis of GmGRF family under shade stress. BMC Plant Biol. 2019;19:269. doi: 10.1186/s12870-019-1861-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ai G., Zhang D., Huang R., Zhang S., Li W., Ahiakpa J.K., Zhang J. Genome-Wide Identification and Molecular Characterization of the Growth-Regulating Factors-Interacting Factor Gene Family in Tomato. Genes. 2020;11:1435. doi: 10.3390/genes11121435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bao M., Bian H., Zha Y., Li F., Sun Y., Bai B., Chen Z., Wang J., Zhu M., Han N. miR396a-Mediated basic helix-loop-helix transcription factor bHLH74 repression acts as a regulator for root growth in Arabidopsis seedlings. Plant Cell Physiol. 2014;55:1343–1353. doi: 10.1093/pcp/pcu058. [DOI] [PubMed] [Google Scholar]
- 28.Liu H., Guo S., Xu Y., Li C., Zhang Z., Zhang D., Xu S., Zhang C., Chong K. OsmiR396d-regulated OsGRFs function in floral organogenesis in rice through binding to their targets OsJMJ706 and OsCR4. Plant Physiol. 2014;165:160–174. doi: 10.1104/pp.114.235564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang G., He H., Li Y., Wang F., Yu D. Molecular mechanism of microRNA396 mediating pistil development in Arabidopsis. Plant Physiol. 2014;164:249–258. doi: 10.1104/pp.113.225144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Debernardi J.M., Mecchia M.A., Vercruyssen L., Smaczniak C., Kaufmann K., Inze D., Rodriguez R.E., Palatnik J.F. Post-transcriptional control of GRF transcription factors by microRNA miR396 and GIF co-activator affects leaf size and longevity. Plant J. 2014;79:413–426. doi: 10.1111/tpj.12567. [DOI] [PubMed] [Google Scholar]
- 31.Edger P.P., VanBuren R., Colle M., Poorten T.J., Wai C.M., Niederhuth C.E., Alger E.I., Ou S., Acharya C.B., Wang J., et al. Single-molecule sequencing and optical mapping yields an improved genome of woodland strawberry (Fragaria vesca) with chromosome-scale contiguity. Gigascience. 2018;7:1–7. doi: 10.1093/gigascience/gix124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rushton P.J., Macdonald H., Huttly A.K., Lazarus C.M., Hooley R. Members of a new family of DNA-binding proteins bind to a conserved cis-element in the promoters of alpha-Amy2 genes. Plant Mol. Biol. 1995;29:691–702. doi: 10.1007/BF00041160. [DOI] [PubMed] [Google Scholar]
- 33.Washida H., Wu C.Y., Suzuki A., Yamanouchi U., Akihama T., Harada K., Takaiwa F. Identification of cis-regulatory elements required for endosperm expression of the rice storage protein glutelin gene GluB-1. Plant Mol. Biol. 1999;40:1–12. doi: 10.1023/A:1026459229671. [DOI] [PubMed] [Google Scholar]
- 34.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bobb A.J., Chern M.S., Bustos M.M. Conserved RY-repeats mediate transactivation of seed-specific promoters by the developmental regulator PvALF. Nucleic Acids Res. 1997;25:641–647. doi: 10.1093/nar/25.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldsbrough A.P., Albrecht H., Stratford R. Salicylic acid-inducible binding of a tobacco nuclear protein to a 10 bp sequence which is highly conserved amongst stress-inducible genes. Plant J. 1993;3:563–571. doi: 10.1046/j.1365-313X.1993.03040563.x. [DOI] [PubMed] [Google Scholar]
- 37.Rouster J., Leah R., Mundy J., Cameron-Mills V. Identification of a methyl jasmonate-responsive region in the promoter of a lipoxygenase 1 gene expressed in barley grain. Plant J. 1997;11:513–523. doi: 10.1046/j.1365-313X.1997.11030513.x. [DOI] [PubMed] [Google Scholar]
- 38.Shen Q., Ho T.H. Functional dissection of an abscisic acid (ABA)-inducible gene reveals two independent ABA-responsive complexes each containing a G-box and a novel cis-acting element. Plant Cell. 1995;7:295–307. doi: 10.1105/tpc.7.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim J.K., Cao J., Wu R. Regulation and interaction of multiple protein factors with the proximal promoter regions of a rice high pI alpha-amylase gene. Mol. Gen. Genet. 1992;232:383–393. doi: 10.1007/BF00266241. [DOI] [PubMed] [Google Scholar]
- 40.Ulmasov T., Murfett J., Hagen G., Guilfoyle T.J. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiao Y., Wickett N.J., Ayyampalayam S., Chanderbali A.S., Landherr L., Ralph P.E., Tomsho L.P., Hu Y., Liang H., Soltis P.S., et al. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- 42.Innan H., Kondrashov F. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010;11:97–108. doi: 10.1038/nrg2689. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez R.E., Mecchia M.A., Debernardi J.M., Schommer C., Weigel D., Palatnik J.F. Control of cell proliferation in Arabidopsis thaliana by microRNA miR396. Development. 2010;137:103–112. doi: 10.1242/dev.043067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skriver K., Olsen F.L., Rogers J.C., Mundy J. cis-acting DNA elements responsive to gibberellin and its antagonist abscisic acid. Proc. Natl. Acad. Sci. USA. 1991;88:7266–7270. doi: 10.1073/pnas.88.16.7266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narusaka Y., Nakashima K., Shinwari Z.K., Sakuma Y., Furihata T., Abe H., Narusaka M., Shinozaki K., Yamaguchi-Shinozaki K. Interaction between two cis-acting elements, ABRE and DRE, in ABA-dependent expression of Arabidopsis rd29A gene in response to dehydration and high-salinity stresses. Plant J. 2003;34:137–148. doi: 10.1046/j.1365-313X.2003.01708.x. [DOI] [PubMed] [Google Scholar]
- 46.Ohtake Y., Takahashi T., Komeda Y. Salicylic acid induces the expression of a number of receptor-like kinase genes in Arabidopsis thaliana. Plant Cell Physiol. 2000;41:1038–1044. doi: 10.1093/pcp/pcd028. [DOI] [PubMed] [Google Scholar]
- 47.Duan Y.B., Li J., Qin R.Y., Xu R.F., Li H., Yang Y.C., Ma H., Li L., Wei P.C., Yang J.B. Identification of a regulatory element responsible for salt induction of rice OsRAV2 through ex situ and in situ promoter analysis. Plant Mol. Biol. 2016;90:49–62. doi: 10.1007/s11103-015-0393-z. [DOI] [PubMed] [Google Scholar]
- 48.Haralampidis K., Milioni D., Rigas S., Hatzopoulos P. Combinatorial interaction of cis elements specifies the expression of the Arabidopsis AtHsp90-1 gene. Plant Physiol. 2002;129:1138–1149. doi: 10.1104/pp.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dunn M.A., White A.J., Vural S., Hughes M.A. Identification of promoter elements in a low-temperature-responsive gene (blt4.9) from barley (Hordeum vulgare L.) Plant Mol. Biol. 1998;38:551–564. doi: 10.1023/A:1006098132352. [DOI] [PubMed] [Google Scholar]
- 50.Finn R.D., Coggill P., Eberhardt R.Y., Eddy S.R., Mistry J., Mitchell A.L., Potter S.C., Punta M., Qureshi M., Sangrador-Vegas A., et al. The Pfam protein families database: Towards a more sustainable future. Nucleic Acids Res. 2016;44:D279–D285. doi: 10.1093/nar/gkv1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Larkin M.A., Blackshields G., Brown N.P., Chenna R., McGettigan P.A., McWilliam H., Valentin F., Wallace I.M., Wilm A., Lopez R., et al. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 52.Letunic I., Bork P. Interactive tree of life (iTOL) v3: An online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res. 2016;44:W242–W245. doi: 10.1093/nar/gkw290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Hu B., Jin J., Guo A.Y., Zhang H., Luo J., Gao G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics. 2015;31:1296–1297. doi: 10.1093/bioinformatics/btu817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bailey T.L., Johnson J., Grant C.E., Noble W.S. The MEME Suite. Nucleic Acids Res. 2015;43:W39–W49. doi: 10.1093/nar/gkv416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee T.H., Tang H., Wang X., Paterson A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2013;41:D1152–D1158. doi: 10.1093/nar/gks1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang Y., Tang H., Debarry J.D., Tan X., Li J., Wang X., Lee T.H., Jin H., Marler B., Guo H., et al. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012;40:e49. doi: 10.1093/nar/gkr1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann T., Kalinowski G., Schwab W. RNAi-induced silencing of gene expression in strawberry fruit (Fragaria × ananassa) by agroinfiltration: A rapid assay for gene function analysis. Plant J. 2006;48:818–826. doi: 10.1111/j.1365-313X.2006.02913.x. [DOI] [PubMed] [Google Scholar]
- 59.Schmittgen T.D., Livak K.J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 60.Lescot M., Déhais P., Thijs G., Marchal K., Moreau Y., Van de Peer Y., Rouzé P., Rombauts S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–327. doi: 10.1093/nar/30.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data in the present study are available in the public database as referred in the Materials and Methods section.