Abstract
Simple Summary
The invasive brown marmorated stink bug (BMSB), Halyomorpha halys, is a polyphagous species and a serious pest worldwide. Classical biological control (CBC), i.e., the introduction of a natural enemy from the native area of the pest, is considered as the preferential solution for BMSB regulation. Adventive populations of exotic egg parasitoids of BSMB, Trissolcus japonicus and T. mitsukurii, have been reported worldwide. With the aim to characterize the French diversity of egg parasitoids associated to various stink bugs including BMSB, field surveys were conducted between 2018 and 2020. Surprisingly, morphological and molecular analyses unambiguously revealed 12 specimens of T. mitsukurii, an Asian egg parasitoid of BSMB. Although its permanent establishment has to be confirmed, this first record in France could actually facilitate CBC initiatives, T. mitsukurii being considered with T. japonicus as the two most promising biological control agents against BMSB.
Abstract
We report the first detection of Trissolcus mitsukurii in France. More than 1860 sentinel egg masses of Halyomorpha halys (BMSB) were exposed in the field during the 2018–2020 period, and 12 specimens of T. mitsukurii emerged from one egg mass. Their taxonomic identification was confirmed both by morphological and molecular analysis. Trissolcus mitsukurii, similar to T. japonicus, is an egg parasitoid of BMSB in its area of origin in Asia, and both species are considered to be candidates for a classical biological control strategy against BMSB. Trissolcus mitsukurii was previously recorded in Italy where it is well established and widespread, and this may be the source of the French population. Possible permanent establishment and dispersion of T. mitsukurii in France should be monitored with emphasis on its potential effect on BMSB populations.
Keywords: biological control, tramp species, DNA barcoding, exotic species, brown marmorated stink bug
1. Introduction
Numerous studies document the impact of biological invasions on biodiversity, human health, and economies [1]. Arthropods are among the most important invasive species [2,3,4,5], and the establishment of new agricultural and economic pests has increased drastically in the last few years [6,7], mainly due to the growth of trade and globalization.
Classical biological control (CBC) was developed to restore the ecosystem balance disrupted by newly arrived pests which are usually lacking natural enemies. Consequently, CBC can reduce the volume of chemical treatments required for their control. However, the development of CBC programmes to deliberately import and introduce exotic natural enemies requires regulatory approvals in many countries [8,9]. Prior to importation and release, researchers have to assess the risks and benefits of the potential introductions depending on each candidate species and concerned country. As explained by Mason et al. [10], the unintentional introductions of natural enemies are probably more common than expected. This phenomenon of “tramp” species was documented in Platygastroidea both for Platygastridae and Scelionidae [11,12].
In particular, the invasions of some emerging pests in Pentatomidae and Plataspidae (Hemiptera) in recent years have led to new CBC projects: Halyomorpha halys (Stål) [13,14], Bagrada hilaris (Burmeister) [14,15], and Megacopta cribaria (F.) [16,17]. Preliminary studies resulted in the detection of fortuitous populations of various exotic egg parasitoids in different countries, e.g., Trissolcus japonicus (Ashmead) in the USA [18,19], Canada [20], Switzerland, Italy [21,22], and Germany [23]; Trissolcus mitsukurii (Ashmead) in Italy [21,24] and Slovenia [25] against H. halys; Trissolcus hyalinipennis Rajmohana & Narendran in the USA [26] against B. hilaris; and Paratelenomus saccharalis (Dodd) in the USA [27] against M. cribaria.
France has not been spared by pests belonging to Pentatomidae: mainly, Palomena prasina (Linnaeus) (indigenous), the southern green stink bug, Nezara viridula (Linnaeus), and H. halys. Halyomorpha halys were firstly identified in France in April 2013, and the French Agency for Food, Environmental, and Occupational Health & Safety (ANSES) concluded that this invasive pest represents an important risk for many French crops [28]. In other countries, H. halys has become one of the major pests of apple orchards, causing more than 37 million dollars in losses in the Mid-Atlantic region of the USA in 2010 [29]. In France, since 2015, average bug damage on French hazelnut trees increased continuously from 0.2% in 2015 to up to 2% in 2018, with peaks that reached more than 14%. These data are in accordance with damage reported in hazelnut orchards in Italy and Georgia where H. halys is now established [30].
Among the natural enemies of H. halys in its native range, T. japonicus is regarded as the most promising candidate for CBC [31,32,33]. Numerous studies have been conducted to assess the parasitoid’s biosafety as part of the requirements to seek approval from the corresponding regulatory agencies for its legal introduction in the USA [34], Canada [20], New Zealand [35], and Europe [36]. However, in many cases, adventive populations of T. japonicus have also been reported [18,19,20,21,22,23,37,38,39]. Trissolcus mitsukurii was also recently found in northern Italy and western Slovenia [21,25]. This egg parasitoid is also known as a natural enemy of H. halys in its area of origin [21,32].
In France, neither T. japonicus nor T. mitsukurii has been detected until now. Therefore, since 2017, field monitoring was conducted to (i) clarify the French diversity of pentatomid egg parasitoids, (ii) identify the best potential candidate against the various stink bugs under consideration, and (iii) initiate preliminary studies in a context of CBC against H. halys prior to the exotic candidate introduction request.
2. Material and Methods
2.1. Field Surveys
Two main sampling methodologies were used to assess the diversity of the French egg parasitoids of stink bugs: exposure of sentinel egg masses and collection of field-laid egg masses (including H. halys and other Pentatomidae). The field surveys took place from 2017 to 2020 and covered different geographical areas (Table 1). Samples included field-collected egg masses and exposed sentinel egg masses as described below and egg masses occasionally provided by citizen scientist volunteers.
Table 1.
Summary of the different main sampling areas.
| Period | Area | Type of Sampling | Number of Egg Masses | Number of Parasitized Egg Masses | % of Parasitized Egg Masses |
|---|---|---|---|---|---|
| 2018–2019 | National-focus PACA | Sentinel-frozen | 1020 | 223 | 21.82 |
| Sentinel-fresh | 582 | 10 | 17.18 | ||
| 2020 | National-focus PACA | Natural | 129 | 60 | 46.51 |
| May–September 2020 | southwestern France | Sentinel-frozen | 234 | 13 | 5.55 |
| Sentinel-fresh | 15 | 2 | 13.33 | ||
| May–September 2020 | southwestern France | Natural | 289 | 79 | 27.33 |
Sentinel egg masses
Regular exposure was performed in the Alpes Maritimes (southeastern France), close to the Italian border from 2018 to 2019, and in nut orchards in southwestern France in 2020. Occasional surveys were performed in other regions of France (Auvergne Rhône-Alpes, Bourgogne, Bretagne, Corse, Occitanie, and Pays de la Loire) during 2019.
Field-collected adults and nymphs of H. halys were transferred to the laboratory of INRAE Sophia Antipolis in 2016 and to the ANPN laboratory in 2020. They were reared in net cages (40 cm × 40 cm × 40 cm) (Bugdorm®, MegaView Science Co., Ltd., Taichung, Taiwan) containing fresh beans, complemented with fresh fruits, peanuts, or shelled hazelnuts. Mass rearing was performed at 25 ± 1 °C and RH 60 ± 10%, with an L:D of 16:8 h.
Halyomorpha halys egg masses were collected daily from the laboratory rearing (INRAE Sophia-Antipolis and ANPN) and directly exposed in the field or were frozen at −18 °C for at least 48 h. The use of fresh or frozen egg masses of H. halys depended on the preference of the owner of each experimental site.
Egg masses were glued to thin paper pieces (1 cm × 3 cm) and stapled to the undersides of the leaves of a wide variety of host plants, mostly woody trees (e.g., Prunus spp., Acer spp.) and shrubs (e.g., Corylus avellana L.). Sentinel egg masses were retrieved from the field three days after deployment.
Natural egg masses
Field-laid egg masses from H. halys and from other stink bug species were collected throughout France in 2019, and a collection in nut orchards in southwestern France was performed in 2020. During the surveys, egg masses of stink bug species were visually located on various host plants and collected.
Emergence of parasitoids
All egg masses, sentinel and field-laid, were reared at INRAE Sophia-Antipolis or ANPN until emergence of bug nymphs or parasitoid adults. Egg masses were individually placed in a glass tube (3 cm × 1 cm) together with a fine drop of honey and sealed with a cotton plug. Egg masses were kept under controlled conditions at 22 ± 1 °C and RH 60 ± 5%, with an L:D of 16:8 h. Each egg mass was checked daily for emergence, and the emerged parasitoid adults were stored in 96% ethanol at −18 °C.
2.2. Species Identification
DNA barcoding
DNA extraction was performed with 30 µL of buffer using the DNA extraction kit LUCIGEN (MA150E, QuickExtract™ DNA Extraction Solution, Middleton, WI, USA) (following company specifications. This method allows a non-destructive extraction of the DNA, so that the voucher (exoskeleton) remains intact for morphological identification.
PCR amplifications were performed on a portion of the Cytochrome Oxydase I subunit (COI) locus using the LCO-HCO primer: HCO2198 (5′-TAAA CTT CAG GGT GAC CAA AAA ATC A-3′), LCO1490(5′-GGTC AAC AAA TCA TAA AGA TAT TGG-3′) [40], allowing amplification of an approximately 600–700 bp portion of DNA on this locus. Product was sent to Beckman Coulter Genomics Genewiz (Leipzig, Germany, Essex, UK) for a double single way sequencing with HCO2198 primer. All residual DNA is archived at INRAE Sophia-Antipolis (France).
Correction, annotation, and alignment were performed manually using BioEdit Geneious R10 software. The comparison of nucleotide sequences with sequences available in the NCBI database (GenBank) was performed using Blastn [41] with standard settings. Analysis of sequence data was done with the MegaX software [42], using the neighbour joining (NJ) method [43], with Bootstrap values based on 100 replications. Nucleotide distances in NJ trees were estimated by the Kimura’s two-parameters method [44].
Morphological identification
Morphological identifications of Trissolcus specimens were performed using the specific taxonomic keys on Trissolcus [45,46]. Ethanol-stored and DNA-extracted specimens were mounted as recommended by Talamas et al. [46] and dried and glued on card points. Stereomicroscopes Leica M205C (INRAE) and Leitz large-field TS (DISAFA) were used for morphological diagnosis. Voucher specimens are located at INRAE UMR ISA in Sophia-Antipolis (southern France).
3. Results
Various species of native egg parasitoids belonging to Scelionidae (Trissolcus spp. and Telenomus spp.), Eupelmidae (Anastatus bifasciatus (Geoffroy)), and Encyrtidae (Ooencyrtus spp.) (unpublished data) emerged from egg masses of native Pentatomidae and H. halys collected during our surveys. All of the parasitoids collected are known to be parasitoids of stink bug eggs.
Trissolcus mitsukurii
Among the fresh sentinel egg masses of H. halys exposed at Bergerac (France—Nouvelle-Aquitaine), one exposed on Prunus laurocerasus L. in mid-August 2020 was parasitized (Table 2) and produced 12 specimens of T. mitsukurii.
Table 2.
GenBank accession number and sample information for COI sequences presented in this study.
| Species | Collection Code | Department Country | Year of Collection | GPS Coordinates (DMS) | Host Species | GenBank Accession Number |
|---|---|---|---|---|---|---|
| Trissolcus mitsukurii | 42314_HCO | Lot, France | 2020 | 44°50′8.21″, 0°29′48.534″ | Halyomorpha halys | MZ343334 |
| Trissolcus mitsukurii | 42315_HCO | Lot, France | 2020 | 44°50′8.21″, 0°29′48.534 | Halyomorpha halys | MZ343335 |
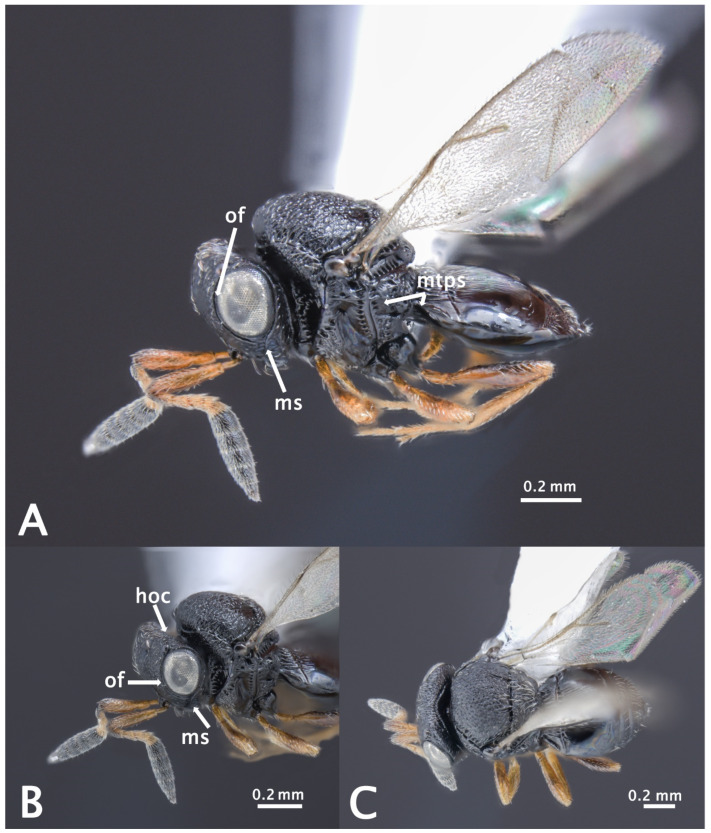
The specimens matched perfectly with the diagnosis provided in Talamas et al. [46] and Sabbatini-Peverieri et al. [21]. Females had an antennal clava of five clavomeres distinctly larger and darker than preceding flagellomeres. Other diagnostic features were the presence of an expanded orbital furrow at the intersection with the malar sulcus, the presence of a hyperoccipital carina posterior to the lateral ocelli and absent on the vertex, the absence of setae below the metapleural sulcus, rugose macrosculpture throughout the surface of the mesoscutellum, and the presence of a setal patch on the first laterotergite of the metasoma (Figure 1).
Figure 1.
Trissolcus mitsukurii, voucher N° ISA_42315; (A) habitus in lateral view, metapleuron without setae below metapleural sulcus (mtps), (B) head with orbital furrow (of) and hyperoccipital carina (hoc), (C) habitus in dorsal view. Scale bars in millimetres.
This identification was further confirmed by result of the partial sequencing of the COI gene from three specimens resulting in two available sequences but one unique COI haplotype of 600 bp. This haplotype does not have a stop codon in the predicted partial protein. These two sequences were deposited in GenBank (Table 2).
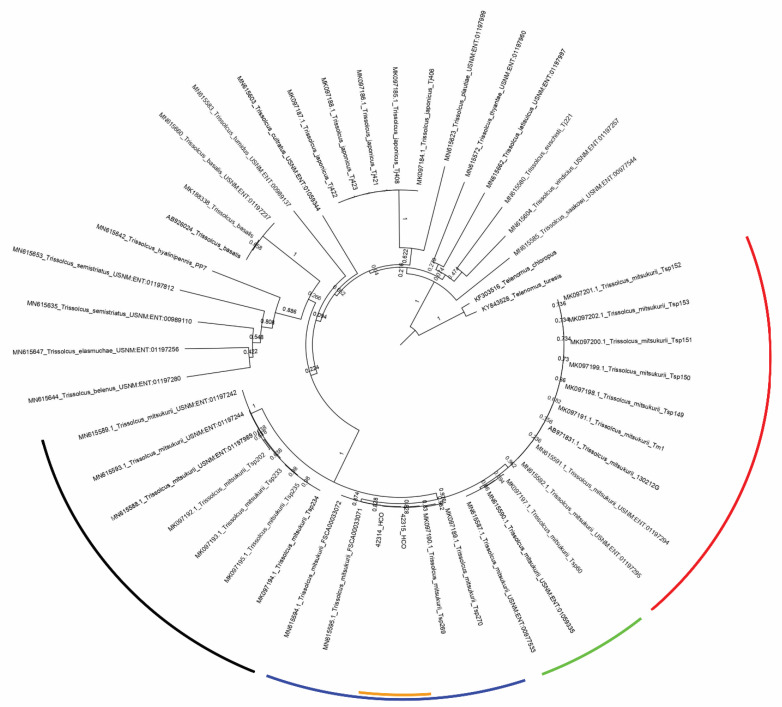
The best NCBI matches (100% identity) were obtained with the sequences gb |MK097190.1|, |MK097189.1|, |MN615594.1|, and |MN615595.1|, which correspond to T. mitsukurii specimens from Italy. The nucleotide sequences were then used to build a neighbour joining tree confirming the morphological identification (Figure 2). The geographical origins of the different T. mitsukurii sequences were indicated. The two sequences of T. mitsukurii from France were a retrieved cluster with the Italian specimens, well separated by a South Korean cluster and a cluster of Chinese and Japanese specimens.
Figure 2.
Molecular clustering of 48 sequences including the Trissolcus mitsukurii French individuals. All sequences of T. mitsukurii available in GenBank were used as some reference sequences of other Trissolcus species. Telenomus turesis Walker and Te. chloropus (Thomson) were used as out-group. Origins of T. mitsukurii are highlighted by colours: Japan in red, China in green, South Korea in black, Italy in blue, and French in orange.
4. Discussion and Conclusions
The sampling effort on French egg parasitoids of stink bugs allowed us to collect various species of Scelionidae, Eupelmidae, and Encyrtidae. This method is effective for the detection of Trissolcus species, including exotic ones [18,19,20,21,22,23,37,38,39].
Moreover, regarding the geographic range of our sampling, we consider it likely that we detected the first introduction of T. mitsukurii in France. A migration of the two exotic species T. japonicus and T. mitsukurii in southern France has been expected as (i) they are highly present and largely widespread in Italy and Switzerland, and (ii) the relative high density (but still lower than that observed in Italy) of H. halys provides an invasion opportunity for its parasitoids [47]. However, neither T. japonicus nor T. mitsukurii was collected in the Alpes-Maritimes close to Italy or close to Switzerland. Thus, the presence of T. mitsukurii in Bergerac, without populations along a gradient from East to West, appears to be the result of human activities, not natural dispersion. Moreover, the sampling point was not far from a plant nursery, which could be an indication that the parasitoids were transported with plant material. Regarding the COI clustering of the French population of T. mitsukurii with the Italian population, we consider that importation from Italy is more likely than direct importation from Asia.
This detection is insufficient to make a conclusion about the establishment of T. mitsukurii in France or its future dispersal. Nonetheless, the shared distribution of T. mitsukurii and T. japonicus in Asia, as well as their presence in Italy [47], could imply that their ecological preferences would be similar. According to Avila and Charles [48], French climatic conditions, as those of Europe, are favourable for widespread establishment of T. japonicus following the presence of its host, H. halys. The situation in Italy seems to indicate that the spread of T. mitsukurii is important, especially in comparison with T. japonicus which benefits from an artificial releasing programme [47]. This study also highlights the predicted spread of both T. japonicus and T. mitsukurii in all types of habitats where H. halys is present. Given that the climate of southern France is similar to northern Italy, we consider it likely that T. mitsukurii will become established. This is supported by the climate suitability model (CLIMEX Ecoclimatic Index) for T. mitsukurii performed by Yonow et al. [49]. Sampling in 2021 will be essential to determine if this is the case.
In a recent study aimed at defining the physiological host range of T. mitsukurii in Europe, T. mitsukurii displayed less host specialization in no-choice laboratory tests, similar to T. japonicus [50]. However, in choice tests, T. mitsukurii showed a similar host preference for more than one pentatomid species rather than its coevolved host H. halys [50], as opposed to T. japonicus [36]. Nonetheless, the parasitism of native species was rarely observed in the field [47].
At this point we cannot predict the ability of T. mitsukurii to control H. halys in France. Further studies are needed to determine if it is established and spreading in France and to assess non-target risks and potential side effects before complementary and augmentative releases are recommended.
Acknowledgments
The authors acknowledge Felise Gutierrez for the first English revision of the manuscript. Many thanks to Lily Cesari, Nicolas Bonetti, Claire Caravel, and Melina Archinard for their technical assistance in field and laboratory work. Special acknowledgment to all volunteers who participated in punctual exposition or sending sampled egg masses. The authors thank the different reviewers of this manuscript for their valuable comments and English improvement. Additionally, we acknowledge the Plant-BioS Microscopy Facility of the Institute Sophia AgroBiotech UMR INRA 1355-CNRS7254—Université Côte d’Azur for access to instruments and technical advice.
Author Contributions
Conceptualization, A.B., L.T., M.T. and R.H.; methodology, A.B., M.T. and R.H.; Investigation, A.B., M.T. and R.H.; Morphological identification, A.B. and F.T.; Molecular analysis, A.B. and S.W.; Data curation A.B., F.T. and R.H.; Writing—Original draft preparation, A.B. and M.T.; Writing—Review and Editing, A.B., F.T., L.T., M.T., R.H. and S.W.; Visualization, A.B.; Supervision, A.B., M.T. and R.H.; Funding acquisition A.B., M.T. and R.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the project REPLIK (2019–2022) (N°7838010) which is supported by funds of the Region-FEDER and benefits mainly from the support of the INRAE Scientific Department of Plant Health and Environment (SPE) and the Scientific Interest Group “GIS Fruit”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vilà M., Hulme P.E. Impact of Biological Invasions on Ecosystem Services. Springer; New York, NY, USA: 2017. [DOI] [Google Scholar]
- 2.Kenis M., Auger-Rozenberg M.-A., Roques A., Timms L., Péré C., Cock M., Settele J., Augustin S., Lopez-Vaamonde C. Ecological effects of invasive alien insects. Biol. Invasions. 2008;11:21–45. doi: 10.1007/s10530-008-9318-y. [DOI] [Google Scholar]
- 3.Kenis M., Branco M. Chapter 5: Impact of alien terrestrial arthropods in Europe. BioRisk. 2010;4:51–71. doi: 10.3897/biorisk.4.42. [DOI] [Google Scholar]
- 4.Vaes-Petitgnat S., Nentwig W. Environmental and economic impact of alien terrestrial arthropods in Europe. NeoBiota. 2014;22:23–42. [Google Scholar]
- 5.Schindler S., Staska B., Adam M., Rabitsch W., Essl F. Alien species and public health impacts in Eu-rope: A literature review. NeoBiota. 2015;27:1–23. doi: 10.3897/neobiota.27.5007. [DOI] [Google Scholar]
- 6.Roy H.E., Bacher S., Essl F., Adriaens T., Aldridge D.C., Bishop J.D.D., Blackburn T.M., Branquart E., Brodie J., Carboneras C., et al. Developing a list of invasive alien species likely to threaten biodiversity and ecosystems in the European Union. Glob. Chang. Biol. 2018;25:1032–1048. doi: 10.1111/gcb.14527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roques A., Shi J., Auger-Rozenberg M.-A., Ren L., Augustin S., Luo Y.-Q. Are Invasive Patterns of Non-native Insects Related to Woody Plants Differing Between Europe and China? Front. For. Glob. Chang. 2020;2 doi: 10.3389/ffgc.2019.00091. [DOI] [Google Scholar]
- 8.Bigler F., Babendreier D., Kuhlmann U., editors. Environmental Impact of Invertebrates for Biological Control of Arthropods: Methods and Risk Assessment. CABI Publishers; Walling-ford, UK: 2006. [DOI] [Google Scholar]
- 9.Heimpel G.E., Cock M. Shifting paradigms in the history of classical biological control. BioControl. 2017;63:27–37. doi: 10.1007/s10526-017-9841-9. [DOI] [Google Scholar]
- 10.Mason P.G., Olfert O.O., Haye T., Gariepy T.D., Abram P.K., Gillespie D.R. Risks and benefits of accidental introductions of biological control agents in Canada; Proceedings of the 5th International Symposium on Biological Control of Arthropods; Langkawi, Malaysia. 11–15 September 2017; [DOI] [Google Scholar]
- 11.Masner L., Johnson N.F., Musetti L. Calliscelio elegans (Perkins), a tramp species, and a review of the status of the genus Caenoteleia Kieffer (Hymenoptera: Platygastridae) Zootaxa. 2009;2237:59–66. doi: 10.11646/zootaxa.2237.1.4. [DOI] [Google Scholar]
- 12.Popovici O.A., Masner L., Viciriuc M., Pintilioaie A., Notton D.G., Talamas E. New distribution data for some charismatic tramp species of Platygastroidea (Hymenoptera) Zootaxa. 2018;4370:1–22. doi: 10.11646/zootaxa.4370.1.1. [DOI] [PubMed] [Google Scholar]
- 13.Leskey T.C., Nielsen A.L. Impact of the Invasive Brown Marmorated Stink Bug in North America and Europe: History, Biology, Ecology, and Management. Annu. Rev. Èntomol. 2018;63:599–618. doi: 10.1146/annurev-ento-020117-043226. [DOI] [PubMed] [Google Scholar]
- 14.Conti E., Avila G., Barratt B., Cingolani F., Colazza S., Guarino S., Hoelmer K., Laumann R.A., Maistrello L., Martel G., et al. Biological control of invasive stink bugs: Review of global state and future prospects. Entomol. Exp. Appl. 2021;169:28–51. doi: 10.1111/eea.12967. [DOI] [Google Scholar]
- 15.Taylor M.E., Bundy C.S., McPherson J.E. Life history and laboratory rearing of Bagrada hilaris (He-miptera: Heteroptera: Pentatomidae) with descriptions of immature stages. Ann. Entomol. Soc. Am. 2015;108:536–551. doi: 10.1093/aesa/sav048. [DOI] [Google Scholar]
- 16.Eger J.E., Ames L.M., Suiter D.R., Jenkins T.M., Rider D.A., Halbert S.E. Occurrence of the Old World bug Megacopta cribaria (Fabricius) (Heteroptera: Plastaspididae) in Georgia: A serious home invader and potentil legume pest. Insecta Mundi. 2010;121:1–11. [Google Scholar]
- 17.Gardner W.A., Peeler H.B., Laforest J., Roberts P.M., Sparks A.N., Greene J.K., Reisig D., Suiter D.R., Bacheler J.S., Kidd K., et al. Confirmed Distribution and Occurrence of Megacopta cribraria (F.) (Hemiptera: Heteroptera: Plataspidae) in the Southeastern United States. J. Èntomol. Sci. 2013;48:118–127. doi: 10.18474/0749-8004-48.2.118. [DOI] [Google Scholar]
- 18.Talamas E.J., Herlihy M.V., Dieckhoff C., Hoelmer K.A., Buffington M., Bon M.C., Weber D.C. Tris-solcus japonicus (Ashmead) (Hymenoptera, Scelionidae) emerges in North America. J. Hymenopt. Res. 2015;43:119–128. doi: 10.3897/JHR.43.4661. [DOI] [Google Scholar]
- 19.Milnes J.M., Wiman N.G., Talamas E.J., Brunner J.F., Hoelmer K.A., Buffington M.L., Beers E.H. Discovery of an Exotic Egg Parasitoid of the Brown Marmorated Stink Bug, Halyomorpha halys (Stål) in the Pacific Northwest. Proc. Entomol. Soc. Wash. 2016;118:466–470. doi: 10.4289/0013-8797.118.3.466. [DOI] [Google Scholar]
- 20.Abram P.K., Talamas E.J., Acheampong S., Mason P.G., Gariepy T.D. First detection of the samurai wasp, Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae), in Canada. J. Hymenopt. Res. 2019;68:29–36. doi: 10.3897/jhr.68.32203. [DOI] [Google Scholar]
- 21.Sabbatini-Peverieri G., Talamas E., Bon M.C., Marianelli L., Bernardinelli I., Malossini G., Benvenuto L., Roversi P.F., Hoelmer K. Two Asian egg parasitoids of Halyomorpha halys (Stål) (Hemiptera, Penta-tomidae) emerge in northern Italy: Trissolcus mitsukurii (Ashmead) and Trissolcus japonicus (Ashmead) (Hymenoptera, Scelionidae) J. Hymenopt. Res. 2018;67:37–53. doi: 10.3897/jhr.67.30883. [DOI] [Google Scholar]
- 22.Stahl J., Tortorici F., Pontini M., Bon M.C., Hoelmer K., Marazzi C., Haye T. First discovery of ad-ventive populations of Trissolcus japonicus in Europe. J. Pest Sci. 2019;92:371–379. doi: 10.1007/s10340-018-1061-2. [DOI] [Google Scholar]
- 23.Dieckhoff C., Wenz S., Renninger M., Reißig A., Rauleder H., Zebitz C., Reetz J., Zimmermann O. Add Germany to the List—Adventive Population of Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) Emerges in Germany. Insects. 2021;12:414. doi: 10.3390/insects12050414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scaccini D., Falagiarda M., Tortorici F., Martinez-Sañudo I., Tirello P., Reyes-Domínguez Y., Gallmetzer A., Tavella L., Zandigiacomo P., Duso C., et al. An insight into the role of Trissolcus mitsuku-rii as biological control agent of Halyomorpha halys in Northeastern Italy. Insects. 2020;11:306. doi: 10.3390/insects11050306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rot M., Maistrello L., Costi E., Bernardinelli I., Malossini G., Benvenuto L., Trdan S. Native and Non-Native Egg Parasitoids Associated with Brown Marmorated Stink Bug (Halyomorpha halys [Stål, 1855]; Hemiptera: Pentatomidae) in Western Slovenia. Insects. 2021;12:505. doi: 10.3390/insects12060505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganjisaffar F., Talamas E.J., Bon M.-C., Brown B.V., Gonzalez L., Perring T.M. Trissolcus hyalinipennis Rajmohana & Narendran (Hymenoptera, Scelionidae), a parasitoid of Bagrada hilaris (Burmeister) (Hemiptera, Pentatomidae), emerges in North America. J. Hymenopt. Res. 2018;65:111–130. doi: 10.3897/jhr.65.25620. [DOI] [Google Scholar]
- 27.Gardner W.A., Blount J.L., Golec J.R., Jones W.A., Hu X.P., Talamas E., Evans R.M., Dong X., Ray C.H., Buntin G.D., et al. Discovery of Paratelenomus saccharalis (Dodd) (Hymenoptera: Platygastridae), an Egg Parasitoid of Megacopta cribraria F. (Hemiptera: Plataspidae) in its Expanded North American Range. J. Èntomol. Sci. 2013;48:355–359. doi: 10.18474/0749-8004-48.4.355. [DOI] [Google Scholar]
- 28.Haye T., Hoelmer K.A., Rossi J.P., Streito J.C., et Tassus X. Avis de l’Anses, Rapport D’expertise Collective. 2014. [(accessed on 11 March 2021)]. Analyse de risque phytosanitaire express Halyomorpha halys—la punaise diabolique. Edition Scientifique, Maisons Alfort. Available online: http://www.anses.fr/sites/default/files/documents/SVEG2013sa0093Ra.pdf. [Google Scholar]
- 29.Leskey T.C., Short B.D., Butler B.R., Wright S.E. Impact of the Invasive Brown Marmorated Stink Bug, Halyomorpha halys(Stål), in Mid-Atlantic Tree Fruit Orchards in the United States: Case Studies of Commercial Management. Psyche A J. Èntomol. 2012;2012:1–14. doi: 10.1155/2012/535062. [DOI] [Google Scholar]
- 30.Bosco L., Moraglio S.T., Tavella L. Halyomorpha halys, a serious threat for hazelnut in newly invaded areas. J. Pest Sci. 2017;91:661–670. doi: 10.1007/s10340-017-0937-x. [DOI] [Google Scholar]
- 31.Qiu L., Yang Z., Tao W. Biology and Population Dynamics of Trissolcus halyomorphae. Sci. Silvae Sin. 2007;43:62–65. doi: 10.11707/j.1001-7488.20071111. [DOI] [Google Scholar]
- 32.Yang Z.-Q., Yao Y.-X., Qiu L.-F., Li Z.-X. A New Species of Trissolcus (Hymenoptera: Scelionidae) Parasitizing Eggs of Halyomorpha halys (Heteroptera: Pentatomidae) in China with Comments on Its Biol-ogy. Annu. Entomol. Soc. Am. 2009;102:39–47. doi: 10.1603/008.102.0104. [DOI] [Google Scholar]
- 33.Zhang J., Zhang F., Gariepy T., Mason P., Gillespie D., Talamas E., Haye T. Seasonal parasitism and host specificity of Trissolcus japonicus in northern China. J. Pest Sci. 2017;90:1127–1141. doi: 10.1007/s10340-017-0863-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hedstrom C., Lowenstein D., Andrews H., Bai B., Wiman N. Pentatomid host suitability and the dis-covery of introduced populations of Trissolcus japonicus in Oregon. J. Pest Sci. 2017;90:1169–1179. doi: 10.1007/s10340-017-0892-6. [DOI] [Google Scholar]
- 35.Charles J.G., Avila G.A., Hoelmer K.A., Hunt S., Gardner-Gee R., MacDonald F., Davis V. Experi-mental assessment of the biosafety of Trissolcus japonicus in New Zealand, prior to the anticipated arrival of the invasive pest Halyomorpha halys. Biocontrol. 2019;64:367–379. doi: 10.1007/s10526-019-09949-x. [DOI] [Google Scholar]
- 36.Haye T., Moraglio S.T., Stahl J., Visentin S., Gregorio T., Tavella L. Fundamental host range of Tris-solcus japonicus in Europe. J. Pest Sci. 2020;93:171–182. doi: 10.1007/s10340-019-01127-3. [DOI] [Google Scholar]
- 37.Jarrett B.J.M., Pote J., Talamas E., Gut L., Szucs M. The Discovery of Trissolcus japonicus (Hymenop-tera: Scelionidae) in Michigan. Great Lakes Entomol. 2019;52:8. [Google Scholar]
- 38.Holthouse M.C., Schumm Z.R., Talamas E.J., Spears L.R., Alston D.G. Surveys in northern Utah for egg parasitoids of Halyomorpha halys (Stål) (Hemiptera: Pentatomidae) detect Trissolcus japonicus (Ashmead) (Hymenoptera: Scelionidae) Biodivers. Data J. 2020;8:e53363. doi: 10.3897/BDJ.8.e53363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moraglio S.T., Tortorici F., Pansa M.G., Castelli G., Pontini M., Scovero S., Visentin S., Tavella L. A 3-year survey on parasitism of Halyomorpha halys by egg parasitoids in northern Italy. J. Pest Sci. 2019;93:183–194. doi: 10.1007/s10340-019-01136-2. [DOI] [Google Scholar]
- 40.Folmer O., Black M., Hoeh W., Lutz R., Vrijenhoek R. DNA primers for amplification of mitochon-drial cytochrome c oxydase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994;3:294–299. [PubMed] [Google Scholar]
- 41.Altschul S.F., Gish W., Miller W., Myers E.W., Lipman D.J. Basic local alignment search tool. J. Mol. Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 42.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 44.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 45.Talamas E.J., Johnson N.F., Buffington M. Key to Nearctic species of Trissolcus Ashmead (Hymenoptera, Scelionidae), natural enemies of native and invasive stink bugs (Hemiptera, Pentatomidae) J. Hymenopt. Res. 2015;43:45–110. doi: 10.3897/JHR.43.8560. [DOI] [Google Scholar]
- 46.Talamas E., Buffington M., Hoelmer K. Revision of Palearctic Trissolcus Ashmead (Hymenoptera, Scelionidae) J. Hymenopt. Res. 2017;56:79–261. doi: 10.3897/jhr.56.10158. [DOI] [Google Scholar]
- 47.Zapponi L., Tortorici F., Anfora G., Bardella S., Bariselli M., Benvenuto L., Bernardinelli I., Butturini A., Caruso S., Colla R., et al. Assessing the Distribution of Exotic Egg Parasitoids of Halyomorpha halys in Europe with a Large-Scale Monitoring Program. Insects. 2021;12:316. doi: 10.3390/insects12040316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Avila G.A., Charles J.G. Modelling the potential geographic distribution of Trissolcus japonicus: A biologi-cal control agent of the brown marmorated stink bug, Halyomorpha halys. Biocontrol. 2018;63:505–518. doi: 10.1007/s10526-018-9866-8. [DOI] [Google Scholar]
- 49.Yonow T., Kriticos D., Ota N., Avila G., Hoelmer K., Chen H., Caron V. Modelling the Potential Geographic Distribution of Two Trissolcus Species for the Brown Marmorated Stink Bug, Halyomorpha halys. Insects. 2021;12:491. doi: 10.3390/insects12060491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giovannini L., Sabbatini-Peverieri G., Marianelli L., Rondoni G., Conti E., Roversi P.F. Physiological host range of Trissolcus mitsukurii, a candidate biological control agent of Halyomorpha halys in Europe. J. Pest Sci. 2021;102:1–14. doi: 10.1007/s10340-021-01415-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.