Abstract
Simple Summary
The one-humped camel (Camelus dromedarius) is an important livestock species and is present in more than 46 national entities, with 80% of the camel population inhabiting Africa. In these regions, the role of camels in the livestock economy is highly valuable and a part of this camel herd is valorized on national or international markets for meat production, live animal export or milk production. Even if camels are the species that is most adapted to the harsh conditions of arid/semi-arid rangelands, they can be susceptible to a high number of pathogens, including S. aureus. This latter is often associated with asymptomatic carriage but can also be responsible for several diseases, therefore causing considerable economical losses. Continued monitoring and control assume particular importance in containing the spread of the bacterium since it constitutes an important zoonotic disease.
Abstract
A total of 318 nasal and rectal swabs were collected from 159 apparently healthy camels (Camelus dromedarius) randomly selected from five regions in southern and central Tunisia and screened for Staphylococcus aureus carriage. Staphylococcus spp. were recovered from 152 of 159 camels studied (95.6%) and in total 258 swabs (81%) were positive. Among these isolates, 16 were coagulase positive Staphylococcus (CoPS) (6.2%) and were characterized by biochemical and molecular tests as S. aureus. These were isolated from 14 camels (8.8%) with co-carriage in nasal and rectal mucosa by two camels. All S. aureus isolates recovered were methicillin-susceptible Staphylococcus aureus (MSSA) and were characterized by spa typing and PFGE. Three different spa types were recovered: t729, t4013 and a spa type newly registered as t19687, which was the most common. PFGE analysis revealed seven different patterns and these were characterized by MLST, which revealed five different sequence types (ST6, ST88, ST3583 and two new sequences, ST6504 and ST6506). All isolates harbored different virulence genes, including hld, encoding delta hemolysin; lukE–lukD, encoding bicomponent leukotoxin LukE–LukD; the clfB gene, encoding clumping factor B; the laminin gene, encoding laminin-binding protein; and cap8, encoding capsule type 8. Fifteen isolates harbored hemolysin beta (hlb) and fourteen encoded hemolysin alpha (hla) and hemolysin G2 (hlgv). Adhesin factors, including clfA and fnbB, were detected in five and four isolates respectively. Binding proteins, including collagen (cbp) and elastin-binding protein (ebp), were detected in two S. aureus isolates while fibrinogen-binding protein (fib) was identified in four isolates. This study provides the first set of genotyping data on the population structure and presence of toxin genes of S. aureus strains in Tunisian camels.
Keywords: Camelus dromedarius, Tunisia, Staphylococcus aureus, virulence, typing, antimicrobial resistance
1. Introduction
According to the most recent data available in 2020, the global population of dromedary camels (Camelus dromedarius, one-humped camel) is around 35 million, with a distribution limited to the African and Middle Eastern countries [1]. Cameline livestock in Tunisia, the number of which is estimated to comprise 80,000 female units, are mainly located in the southern and central parts of the country [2]. Tunisian camels are commonly kept under traditional pastoral production systems and they make the best use of this production system thanks to their morphological and physiological particularities. Camels are indeed able to adapt not only to meteorological constraints (aridification of the environment), but also to the zootechnical changes brought by more intensive farming systems emerging from increasing urbanization [3]. The ability of camels to survive in arid and semiarid areas, with high potential to convert the scant resources of the desert into milk and meat, makes them a valuable livestock species, providing a significant percentage of the population with animal protein and milk [4].
Camels were formerly thought not to be affected by most of the diseases commonly impacting livestock. However, recent data have confirmed their susceptibility to a high number of pathogens [5]. Moreover, the increased consumption of and contact with camel meat and milk represent a serious source for zoonotic disease transmission to humans.
Staphylococci in general, and S. aureus in particular, are listed among the most important camel zoonotic diseases and infections reported worldwide [6]. S. aureus is a common and widespread bacterium usually associated with asymptomatic carriage, which colonizes the skin, nares and other mucosa of various animal species [7]. However, when a decrease in immunitary efficiency is noticed, it may behave as an opportunistic pathogen, causing a wide variety of diseases ranging from skin and soft tissue infections to sepsis and toxic shock [8].
In ruminants and pseudoruminants, such as dromedaries, S. aureus is usually associated with mastitis, skin infections (including skin abscesses and necrosis), respiratory tract diseases (pneumonia) and endometritis; it therefore impacts the livestock productivity, resulting in considerable economical losses [9,10,11,12,13].
Isolation of the bacterium from both healthy and diseased camels has been reported by a number of researchers worldwide. However, the lack of information about the carriage of Staphylococcus spp. in dromedaries in Tunisia launched the present investigation of this animal species.
The objectives of this study were to determine the nasal and rectal carriage of Staphylococcus spp., especially S. aureus, in healthy dromedaries in Tunisia, to carry out the molecular typing of the recovered isolates and to determine their antimicrobial resistance profile and virulence genes.
2. Results
2.1. Global Carriage of Staphylococcus spp. and S. aureus in Camels
Staphylococcus spp. isolates were recovered from 152 (95.6%) of the 159 apparently healthy camels studied. A total of 258 swabs from the 318 swabs collected (81%) were positive for Staphylococcus spp., among which 139 (53%) were obtained from nasal cavities and 119 (46%) from rectal samples. Of these isolates, 242 were found to be coagulase-negative (93.7%), while only 16 were coagulase-positive (6.2%).
All CoPS isolates were subject to further testing for formal species identification, using both conventional and species-specific PCR methods. All 16 isolates were confirmed as belonging to the species S. aureus and only 4 among them (25%) were obtained from rectal samples, while the remaining isolates (12) had a nasal origin (75%). From the total population of camels (159), S. aureus was detected in 14 camels (8.8%), since co-carriage of S. aureus in the nasal and rectal mucosa was observed in two animals.
2.2. Resistance Genes and Virulence Markers among S. aureus Isolates
All S. aureus isolates were phenotypically susceptible to the tested antimicrobial agents. After further investigation, the mecA gene defining MRSA was not detected, but we could discern the common virulence markers (Table 1), since all MSSA isolates carried the hld gene encoding delta hemolysin, the lukE–lukD gene encoding the bicomponent leukotoxin LukE–LukD, the clfB gene encoding the clumping factor B, the laminin gene encoding the laminin-binding protein and cap8 encoding the type 8 capsule. The other virulence genes carried by MSSA isolates were hlb (15 isolates, 93.7%), hla/hlgv (14 isolates, 87.5%), ebp (10 isolates, 62%), clfA (5 isolates, 31%), fnbB/fib (4 isolates, 25%) and cbp (2 isolates, 12.5%). On the other hand, no genes encoding for the staphylococcal enterotoxins were retrieved in any of the S. aureus isolates, nor were the nine virulence genes siet, eta, etb, fnbA, PVL S and F, lukM, hlg, bsp and cap5.
Table 1.
Virulence-associated genes of the 16 MSSA isolated from the healthy camels.
| CODE | Hemolysins | Leucocidins | Adhesin Factor | Binding Proteins | Capsular Type |
|---|---|---|---|---|---|
| SA1 | hla, hlb, hld | lukDE | clfA, clfB | ebp, lamin | 8 |
| SA2 | hld, hlg2 | lukDE | clfB | ebp, lamin | 8 |
| SA3 | hla, hlb, hld, hlg2 | lukDE | clfB | fib, ebp, lamin | 8 |
| SA4 | hlb, hld, hlg2 | lukDE | clfA, clfB | fib, ebp, lamin | 8 |
| SA5 | hla, hlb, hld, hlg2 | lukDE | clfB | fib, lamin | 8 |
| SA6 | hla, hlb, hld, hlg2 | lukDE | clfB | fib, ebp, lamin | 8 |
| SA7 | hla, hlb, hld, hlg2 | lukDE | clfB | cbp, lamin, ebp | 8 |
| SA8 | hla, hlb, hld, hlg2 | lukDE | clfB, fnbB | cbp, lamin, ebp | 8 |
| SA9 | hla, hlb, hld, hlg2 | lukDE | clfA, clfB, fnbB | lamin | 8 |
| SA10 | hla, hlb, hld, hlg2 | lukDE | clfB | lamin | 8 |
| SA11 | hla, hlb, hld, hlg2 | lukDE | clfA, clfB | ebp, lamin | 8 |
| SA12 | hla, hlb, hld, hlg2 | lukDE | clfB | ebp, lamin | 8 |
| SA13 | hla, hlb, hld, hlg2 | lukDE | clfB, fnbB | ebp, lamin | 8 |
| SA14 | hla, hlb, hld | lukDE | clfB | lamin | 8 |
| SA15 | hla, hlb, hld, hlg2 | lukDE | clfB, fnbB | lamin | 8 |
| SA16 | hla, hlb, hld, hlg2 | lukDE | clfA, clfB | lamin | 8 |
2.3. Characteristics of MSSA Detected in This Study
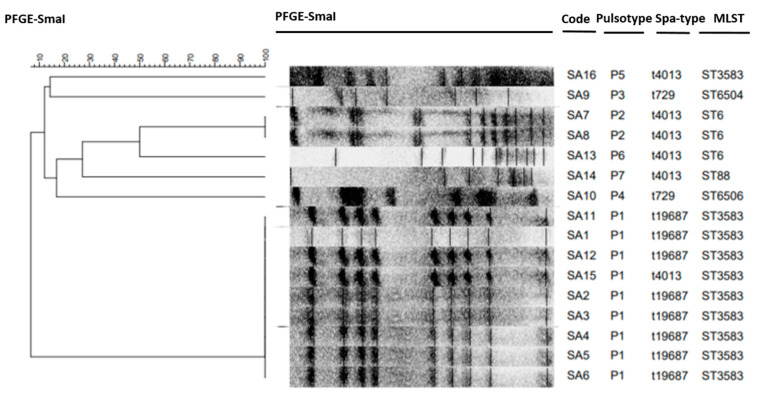
The results of bacterial typing of the 16 MSSA isolates individualized in this study are detailed in Figure 1. All of these isolates were submitted to spa typing, which allowed the detection of three different spa types: t729, t4013 and a newly registered spa type, t19687, the last being the most common. The analysis of their SmaI macro-restriction profiles revealed seven different PFGE patterns. MLST was conducted for seven representative MSSA (per PFGE profile) isolates showing five different STs: ST6, ST88, ST3583 and two STs newly registered as ST6504 and ST6506. Table 2 summarizes the characteristics of the 16 S. aureus isolates recovered from 14 healthy camels in this study.
Figure 1.
Dendrogram of pulsed-field gel electrophoresis SmaI patterns among 16 Staphylococcus aureus isolates recovered in this study, generated by GelCompar II software using the unweighted pair group method with arithmetic mean (UPGMA) algorithm and the Dice similarity coefficients.
Table 2.
Characteristics of the 16 S. aureus isolates recovered from 14 healthy camels.
| Code | Animal | Sites | Geographic Origin | Spa Type | Pulsotype | ST | Virulence Genes |
|---|---|---|---|---|---|---|---|
| SA1 | 1 | Nasal | Hamam Sousse | t19687 | P1 | 3583 | clfA, clfB, lukDE, hla, hlb, hld, lamin, ebp, cap8 |
| SA2 | 2 | Nasal | Hamam Sousse | t19687 | P1 | 3583 | clfB, lukDE, hld, hlg2, lamin, ebp, cap8 |
| SA3 | 3 | Nasal | Hamam Sousse | t19687 | P1 | 3583 | clfB, lukDE, hla, hlb, hld, hlg2, fib, lamin, ebp, cap8 |
| SA4 | 4 | Rectal | Hamam Sousse | t19687 | P1 | 3583 | clfA, clfB, lukDE, hlb, hld, hlg2, fib, lamin, ebp, cap8 |
| SA5 | 5 | Nasal | Hamam Sousse | t19687 | P1 | 3583 | clfB, lukDE, hla, hlb, hld, hlg2, fib, lamin, cap8 |
| SA6 | 5 | Rectal | Hamam Sousse | t19687 | P1 | 3583 | clfB, lukDE, hla, hlb, hld, hlg2, fib, lamin, ebp, cap8 |
| SA7 | 6 | Nasal | Hamam Sousse | t4013 | P2 | 6 | clfB, lukDE, hla, hlb, hld, hlg2, cbp, lamin, ebp, cap8 |
| SA8 | 7 | Nasal | Hamam Sousse | t4013 | P2 | 6 | clfB, lukDE, hla, hlb, hld, hlg2, fnbB, cbp, lamin, ebp, cap8 |
| SA9 | 8 | Nasal | Hamam Sousse | t729 | P3 | 6504 | clfA, clfB, lukDE, hla, hlb, hld, hlg2, fnbB, lamin, cap8 |
| SA10 | 9 | Nasal | Hamam Sousse | t729 | P4 | 6506 | clfB, lukDE, hla, hlb, hld, hlg2, lamin, cap8 |
| SA11 | 10 | Nasal | Hamam Sousse | t19687 | P1 | 3583 | clfA, clfB, lukDE, hla, hlb, hld, hlg2, lamin, ebp, cap8 |
| SA12 | 10 | Rectal | Hamam Sousse | t19687 | P1 | 36551 | clfB, lukDE, hla, hlb, hld, hlg2, lamin, ebp, cap8 |
| SA13 | 11 | Rectal | Hamam Sousse | t4013 | P6 | 6 | clfB, lukDE, hla, hlb, hld, hlg2, fnbB, lamin, ebp, cap8 |
| SA14 | 12 | Nasal | Bouficha | t4013 | P7 | 88 | clfB, lukDE, hla, hlb, hld, lamin, cap8 |
| SA15 | 13 | Nasal | Bouficha | t4013 | P1 | 3583 | clfB, lukDE, hla, hlb, hld, hlg2, fnbB, lamin, cap8 |
| SA16 | 14 | Nasal | Bouficha | t4013 | P5 | 3583 | clfA, clfB, lukDE, hla, hlb, hld, hlg2, lamin, cap8 |
3. Discussion
Although S. aureus is a well-known bacterial pathogen incriminated in human and animal infections, little information is currently available about its occurrence, antibiotic resistance and toxinogenic potential in dromedaries.
We thus undertook the present study in order to investigate the presence of Staphylococcus spp. in camels’ nostrils and rectum, as well as to assess the prevalence of S. aureus among the confirmed isolates, which allowed an initial insight into population characterization, and the relative abundances of virulence-associated genes of S. aureus in camels.
To our knowledge, this is the first study regarding the carriage of staphylococci in camels performed in Tunisia. Previously, we conducted studies in other species, including farm animals and pets [14], sheep [15], goats [16] and donkeys [17]. In our study, a relatively high prevalence of carriage of Staphylococcus spp. in the nasal and rectal mucosa (95.6%) was observed in healthy camels, particularly in nasal swabs. This observation can be explained by the fact that commensal staphylococci are mainly found in the upper respiratory tract of animals, and they play a pathogenic role when the general resistance of the host decreases [18].
However, lower respiratory carriage rates of Staphylococcus spp. in healthy camels have been reported in previous studies carried out in Africa and the Middle East, including Kenya, Iran, Sudan and Ethiopia (34%, 29%, 30.4% and 12%, respectively) [19,20,21]. In Sudan and Ethiopia, camels with pneumonic lesions were also positive for Staphylococcus spp. (30.4% and 26.5%, respectively) [18,21]. Very few studies have focused on the carriage of Staphylococcus spp. in rectal mucosa; Al-Thani et al. (2012) could not report any isolates in the rectums of healthy camels [22].
Coagulase production by Staphylococcus species is considered a virulence factor that enables the pathogen to evade the host’s immune system and it is frequently used to identify S. aureus [23]. Indeed, compared to coagulase-negative staphylococci, the coagulase-positive species group is commonly associated with severe infections [24]. In our study, a relatively low recovery rate of CoPS was noticed in the nasal and rectal samples collected from healthy camels, and all were identified as S. aureus. This latter is acknowledged to be a commensal colonizer of the skin, nose and mucous membranes of healthy humans and animals, but also to be an opportunistic pathogen in several infectious diseases [25]. In fact, S. aureus is the most important CoPS species due to its combination of toxin-mediated virulence, its invasiveness and its antibiotic resistance, and it is a major causative agent of several pathologies in animals [26]. It is mostly associated with mastitis, suppurative dermatitis, abscesses, arthritis, endometritis and respiratory infections in camels [27].
In our study, S. aureus was more commonly retrieved in intensive farming units located in Hamam Sousse and Bouficha, which are touristic areas. This observation can be explained by the intensification of breeding in the region of Sahel for purely touristic purposes and the closer proximity of individuals that this generates, especially when associated with poor hygiene conditions. In fact, in these farms, the high density of animals and their use in densely populated urban areas favor the colonization of nasal cavities by coagulase-positive staphylococci, with possible zoonotic transmission, particularly when close contact between humans and camels occurs, as it can do on various occasions during watering, riding, grooming and milking [28]. Human-to-animal transmission of Staphylococcus can been suggested, since human-related clonal lineages have been identified in Staphylococcus strains from non-human primates, goats, sheep, poultry and pets [25].
The isolation rate of S. aureus in dromedaries in our study was relatively low (6.2%) compared to the other species in Tunisia, such as in domestic carnivores (6.5%), goats (16.5%), sheep and ewes (44.8% and 26.9%) and donkeys (50%) [14,15,16,29,30]. Moreover, S. aureus was predominantly recovered from the nasal samples (75%) rather than the rectal samples (25%). These different carriage rates could be at least partially explained by self-care behavior, such as nose licking, and/or by different kinds of farm management. In humans, anterior nares are the main ecological niche for S. aureus, which persistently or intermittently colonizes the nares of 30 to 50% of healthy adults [31]. The study of the human nasal microbiota has shown that there is competition between S. aureus and other bacteria, like coagulase-negative staphylococci, through the production of antimicrobial molecules, and the same phenomenon could probably explain the relatively low rate of S. aureus carriage in camels, given the high carriage rate of coagulase-negative staphylococci [32].
Currently, the scientific literature shows that the prevalence of S. aureus nasal carriage in camels varies between countries. Previous work carried out on healthy camels in some African and Middle Eastern countries showed higher nasal carriage of S. aureus, including in Algeria (53%), Jordan (13.7%), Qatar (43.9%), Saudi Arabia (56.3% and 89.1%) and Nigeria (14%) [6,22,33,34,35,36,37]. A prevalence of 19.2% was registered in Sudan, the samples being nasal swabs collected from camels with clinical signs of pneumonia [38]. The range of the carriage rates reported in these different countries is large, and these discrepancies may partially originate from the differences in the sampling quality and in the bacterial culture techniques used in these studies.
All the currently available data concur that S. aureus resides as a normal inhabitant of the upper respiratory tract, and several studies show that the nares are the most consistent area from which this organism can be isolated. The carriage of S. aureus in the nasal cavities appears to play a key role in the epidemiology and pathogenesis of pulmonary infection, very likely causing secondary pneumonia in immunocompromised animals [21,39].
Over the past decade, the problem of antimicrobial resistance in the African continent has attracted a special interest [40]; however, little is known about the real extent of the problem, especially in camels, since few studies have focused on this species. In the present study, resistance genes were absent in S. aureus isolates, which revealed their susceptibility to all the tested antimicrobials. Furthermore, MRSA was not detected among the tested isolates, no were any conspicuous resistance markers, such as van genes (glycopeptide resistance). In fact, it was demonstrated that MRSA isolates generally have resistance to other non-beta-lactam agents in addition to methicillin resistance, while MSSA isolates show susceptibility to most tested antimicrobials [25]. This high susceptibility among S. aureus isolates recovered from camels is remarkable, and it is likely explained by the fact that camels are sturdy animals and are therefore exceptionally treated. This finding is in contrast with the high frequencies of resistance reported in other species.
Staphylococcus aureus produces a wide variety of exoproteins that contribute to its ability to colonize and cause disease in mammalian hosts. The pathogenesis of this organism relies on the production of an arsenal of virulence factors [41]. To our knowledge, the prevalence of virulence genes among camels’ isolates has never been investigated in Tunisia, and only scarcely in other countries, which makes it difficult to compare our results with previous data. Isolates’ virulence profiles were very similar, and hemolysin genes, the clumping factor, the capsule gene and leukocidin DE were very common and harbored by all isolates.
The four classes of hemolysins (alpha, beta, delta and gamma) were detected in our isolates; it is known that these toxins are produced by most S. aureus strains [42]. hld was identified in all isolates, which was consistent with Dinges et al. (2000) and with studies conducted in Algeria and Saudi Arabia that reported higher than 97% positivity in camel nasal and meat isolates [36,43]. Furthermore, hlb has been described as having a human specificity, suggesting a human origin and an adaptation to its animal host [43,44]. The combination hla–hlab–hld–hlgv was the most prevalent among our strains (75%), and these genes might be either located in the same or associated genetic elements.
Regarding its pathogenicity, S. aureus produce up to five different leukocidins: lukD–lukE, in particular, is one of the only leucocidins to exhibit broad activity in a wide variety of cell types from various species, and it was harbored by 100% of our strains. In fact, it has been reported that this protein possesses a substantial similarity to lukSF-I and lukPQ, encoded respectively by S. pseudintermedius, associated with infections in dogs, and S. aureus, associated with infections in horses [45]. In Algeria, Saudi Arabia and the United Arab Emirates, lukE–lukD was reported in 43.4%, 100% and 55% of camels’ nasal and meat isolates, respectively [9,36,43].
Africa is considered endemic for Panton–Valentine leukocidin (PVL)-positive MSSA isolates, especially in humans. Nevertheless, PVL was not reported in our study: indeed, it is commonly admitted that this protein is produced by 2 to 3% of strains and it is known to be rare in animals [46]. However, Agabou et al. (2017) and Raji et al. (2016) reported the existence of PVL in MSSA and MRSA camel isolates and stated that the presence of this cytotoxic virulence factor in animals must be taken into consideration by public health professionals, given its high pathogenicity in humans and animals [36,43]. The same studies reported the presence of lukM in 4.5% of the studied camels’ strains, whereas its absence in our isolates was noticed. This latter has been predominantly found in S. aureus incriminated in bovine infections, as a phage-borne lukPV/lukM has been proven to encode a bi-component leukotoxin highly active against bovine neutrophiles [45,47].
The staphylococcal capsular polysaccharides increase the resistance of bacteria to phagocytosis by polymorphonuclear leucocytes by expressing either the cap5 or cap8 genes [48], and all our isolates harbored the cap8 gene. Similar results were found in Algeria and Saudi Arabia with, respectively, 78.2% and 100% [36,43]. In Algeria, cap5, which is predominantly harbored by human isolates and is rare in animal’s strains, has been reported with a low recovery rate (21.7%) [36], but it was not detected in our study. Shuiep et al. (2009) reported that 85% of S. aureus isolates from healthy camels’ raw milk in Sudan possessed the cap5 gene [49].
In addition, S. aureus expresses up to 25 different cell wall-anchored (CWA) proteins. ClfA, ClfB and FnbB are among the most important bacterial adhesins and contribute to initiating infection [26]. It has been shown in previous research that ClfB facilitates the colonization of the nasal cavities through its high-affinity interactions with the cornified envelope in the anterior nares, and that this gene is carried by most strains of S. aureus [50,51]. Our results are in concordance with those divulged by Agabou et al. (2017), who found that 100% of camel nasal strains carried the clfB gene [36]. Our study allowed us to demonstrate that this binding protein may also promote rectal colonization. Others binding proteins were recovered at a low rate, including ClfA, FnbB, Fib and EBP.
Furthermore, none of the isolates found in our study were positive for the remaining toxin genes tested, including enterotoxins and exfoliative toxins. In other studies, enterotoxins were isolated with low recovery in camels, and only sec was recently reported in three S. aureus isolates from pasteurized camel milk, while seg and seh were retrieved in camel nasal samples at frequencies of 4.3% and 17.4%, respectively [36,52]. The occurrence of multiple toxinogenic genes in S. aureus is considered to be rare, which may explain the absence of many of these genes.
The molecular typing of our S. aureus isolates by MLST, PFGE and spa typing exhibited little variability among our strains. The analysis of the SmaI macrorestriction profiles of the 16 MSSA isolates revealed seven different PFGE patterns that we classified in seven categories from S1 to S7, each defining a clone, and the highest detection rate was registered for the pulsotype S1 (56.3% of isolates, nine isolates). Furthermore, the PFGE analysis showed high clonality among the strains with the same spa types. Furthermore, MLST genotyping, widely used to investigate the dynamic nature of S. aureus by providing a highly efficient molecular characterization, allowed us to identify five sequence types (ST6, ST88, ST3583 and two newly registered types, ST6504 and ST6506) among the MSSA isolated from camel nasal and rectal swabs. Our work thus described for the first time the presence of these STs in isolates originating from healthy camels. Few studies have focused on the molecular characterization of MSSA isolates in camels, and when they have the presence of other STs was reported, including ST152 and ST1278 in cameline nasal swabs in Algeria [36]; ST1156 in camel meat from Saudi Arabia [43]; ST15, ST1153 and ST130 from Egyptian camels’ milk [53]; and ST1755 in the Emirates [9].
It is useful to note that the ST6 and ST88 spa types described in our study have also been identified in MSSA and MRSA isolates from other animal species and humans. ST88 is known as a typical African clone and has been well described in several Sub-Saharan African countries as a major circulating clone within both hospital and community settings [54]. This clone has also been identified at a lower frequency in Asia and in other parts of the world. In livestock, it has been reported only in Africa, with MRSA ST88 isolated in one pig in Senegal and in healthy sheep in the Ivory Coast [55,56]. More recently, MRSA ST88 was also reported in pigs and pig farm workers in Nigeria [57]. In China, MRSA ST88 has been detected in foodstuff of animal origin and in livestock workers [58,59].
On the other hand, ST6 has been described in MSSA from domestic carnivore nasal swabs (dogs and cats) in Tunisia but also in MSSA nasal swabs from domestic animals (cats, dogs and sheep) and wild animals (monkeys) in many African countries [14,56]. ST6 was also detected in bovine milk in Tunisia [30]. Furthermore, in this context, this clone is believed to be the most dominant sequence type in MSSA associated with food poisoning in China, including milk, meat, etc. [60], suggesting that livestock can be a reservoir of pathogenic bacteria with the ability to cross the host species barrier through their zoonotic capacities, hence becoming a serious public health threat. Movement of camels via trade and contact with farmworkers, veterinarians and tourists should be therefore assessed.
4. Conclusions
This study provides interesting initial genotyping data regarding the nasal and rectal carriage of S. aureus in Tunisian dromedaries. Our study highlights that camels’ nostrils and feces should be considered as probable sources of human staphylococcal infections and environmental spread, given their zoonotic capacity. We also underline that the isolates recovered in this study harbored many virulence factors, thus representing a potential threat. In future investigations, it would therefore be interesting to genotype isolates (i) from diseased and healthy animals, including camels from other regions and other domestic animal species in contact with camels, and (ii) from diseased and healthy humans, with a special focus on herdsmen. This could help in understanding the host specificity and assessing the zoonotic potential of S. aureus.
5. Material and Methods
5.1. Study Area and Sampling
The CoPS carriage study was carried out from April 2015 to June 2017 on 159 dromedaries from four Tunisian governorates and five regions (Figure 2): one governorate from Central Tunisia, represented by Kairouan (n = 10; semi-arid bioclimatic area; 35°40′41″ N/10°05′46″ E; altitude = 65 m); Sousse, from the coast of Tunisia, including two regions—Hammam Sousse (n = 40; semi-arid bioclimatic area, 35°51′39″ N/10°36′11″ E; altitude = 10 m) and Bouficha (n = 23; semi-arid bioclimatic area, 36°17′49″ N/10°27′21″ E; altitude = 8 m); and two governorates from Southern Tunisia, represented by Tatatouine (n = 32; Saharan bioclimatic area: 32°55′46″ N/10°27′06″ E; altitude = 238 m) and Douz (n = 54, 33°275′8″ N/9°01′13″ E; altitude = 62 m). The choice of the sampling area was dictated by the fact that the four selected locations harbor more than 50% of the national cameline population [61].
Figure 2.
Map of Tunisia showing the visited governorates.
Samples were randomly collected from apparently healthy animals. From each dromedary, both nasal and rectal mucous membranes were sampled. Commercial sterile cotton-tipped swabs were used for the swabbing, and we proceeded by rubbing the swab against the mucosal surface for approximately 5–10 s at a depth of 5–10 cm into the anterior nares and the rectum. Data concerning herds (zootechnic usage of camels, feeding, herd size) as well as information about dromedaries (gender, age, health status) were checked.
5.2. Bacterial Isolation and Identification
We proceeded, using the sampled swabs, to a direct inoculation for enrichment in a brain–heart infusion broth with 6.5% sodium chloride and 10% mannitol concentrations, and the incubation, at 37 °C, lasted 18–24 h. A loopful of each broth inoculum was streaked on the mannitol salt agar (Biokar) selective medium, and the culture plates were incubated at 37 °C for a supplementary duration of 24 h. Positive cultures for Staphylococcus spp. were initially identified by conventional methods, including Gram staining and a catalase test. Coagulase-positive and -negative Staphylococcus were then respectively determined by whether they had the ability to coagulate rabbit plasma (Biokar).
Among the CoPS, standard biochemical tests were first used to identify Staphylococcus aureus with the application of conventional methods [62]. S. aureus identification and methicillin resistance/susceptibility were then confirmed through the amplification of the specific genus gene (sta), the species-specific thermonuclease gene (nuc) and the mecA gene by multiplex PCR [24].
5.3. Antimicrobial Susceptibility Testing
S. aureus isolates were subjected to an in vitro antimicrobial testing method on Mueller-Hinton agar, employing fresh nutrient agar culture and antibiotic discs, according to the performance standards of the Antibiogram Committee of the French Society of Microbiology (CA-SFM 2019). The organisms were left to grow overnight in nutrient agar and adjusted to the 0.5 MacFarland standard, then spread on Mueller-Hinton agar plates and incubated at 37 °C for 18–24 h. A panel of fourteen (14) antimicrobial discs was tested, including penicillin (6 μg), oxacillin (5 μg), cefoxitin (30 μg), ertapenem (10 μg), chloramphenicol (30 μg), tetracycline (30 μg), gentamicin (10 μg), streptomycin (10 μg), vancomycin (5 μg), teicoplanin (30 μg), erythromycin (15 μg), clindamycin (2 μg), sulfamethoxazole (25 μg) and marbofloxacin (5 μg). Moreover, a double-disk diffusion test (D-test) with erythromycin and clindamycin was implemented in all isolates to detect inducible clindamycin resistance. The zones of inhibition around the discs were measured and interpreted as sensitive, intermediate and resistant, according to the interpretation chart of the CA-SFM 2019.
5.4. Detection of Staphylococcal Virulence Genes
All isolates were subject to PCR in order to detect one (or more) of the 18 genes coding for staphylococcal enterotoxins (sea, seb, sec, sed, see, seg, seh, sei, sej, sek, sel, sem, sen, seo, sep, seq, ser and seu) [63]. We also screened the isolates to ascertain the presence of genes encoding for any exfoliative toxins (siet, eta, etb), hemolysins (hla, hlb, hld, hlg and hlgv), the tst gene encoding for the TSST, the lukS–lukF genes encoding for PVL leukocidin, the lukE–lukD genes encoding the bicomponent leukotoxin LukE–LukD and the lukM gene encoding leukocidin M. Adhesin factors, including clumping factors (clfA, clfB) and fibronectin-binding protein (fnbA, fnbB); binding proteins, including fibrinogen-binding protein (fib), collagen-binding protein (cbp), bone sialoprotein-binding protein (bsp), laminin-binding protein (lamin), elastin-binding protein (ebp); and genes encoding capsules (cap5, cap8) were also tested (Supplementary Table S1) [64,65].
5.5. Typing of S. aureus Isolates
5.5.1. Spa Typing
S. aureus protein A (spa) typing was performed in all S. aureus isolates as described by Harmsen et al. (2003) [66]. The polymorphic X region of the spa gene was amplified by PCR, and the sequences were analyzed using Ridom Staph-Type software, v.1.5.21 (Ridom GmbH), which automatically detects spa repeats and assigns a spa type in concordance with the specifications of http://spaserver.ridom.de/ (accessed on 10 May 2021).
5.5.2. Pulsed-Field Gel-Electrophoresis (PFGE)
All S. aureus isolates were characterized by pulsed-field gel-electrophoresis (PFGE) employing SmaI restriction enzyme digestion, as described by Bouzaiane et al. (2008) [67]. Samples were run on a 1% agarose gel in 0.5 Tris-borate-EDTA (TBE) buffer kept at 14 °C, with the support of a CHEF DR-II PFGE system, using switching times ranging from 5 to 40 s over 20 h at 6 V/cm. The DNA fingerprints generated by PFGE were visually and digitally analyzed according to the Tenover criteria [68] and by GelCompar II 6.6 software, respectively.
5.5.3. Multi-Locus Sequence Typing (MLST)
One representative strain per detected PFGE profile was characterized by multilocus sequence typing (MLST). The allelic profile of each isolate was obtained by sequencinginternal fragments of seven unlinked housekeeping genes (arcC, aroE, glpF, gmk, pta, tpi and yqiL) [69], in order to determine the sequence type (ST) and clonal complex (CC) assigned by MLST and BURST (Based Upon Related Sequence Types) analyses (www.pubmlst.org, accessed on 15 May 2021).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ani11092754/s1, Table S1: Primers, amplicon size, and amplification conditions of staphylococcal virulence genes.
Author Contributions
F.B.C. conceptualized the study. R.S. collected the samples and data. F.B.C., H.G. and W.T. performed the experiments. F.B.C. wrote the original draft. L.M., S.K., K.B.S. and M.D. participated in the reviewing and editing. L.M. supervised the work and was responsible for the funding acquisition and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the USAID-funded research project PEER 7-349 “Monitoring of antimicrobial resistance of bacteria for a better health of animals in Tunisia”.
Institutional Review Board Statement
The study was approved by the Ethics Committee CEEA of the National School of Veterinary Medecine of Sidi Thabet (ENMV) (33/21, 15 July 2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are openly available at www.pubmlst.org (accessed on 30 April 2021).
Conflicts of Interest
The authors declare that they have no potential conflicts of interest with respect to the research, authorship and publication of this article.
Ethics Approval
All technical procedures of animal restraint and sampling were in accordance with the European legislation regarding ethics and animal welfare. Nasal and rectal swabs were professionally collected by veterinarians from restrained camels during their monitoring to avoid animal suffering. Informed verbal consent was obtained from the camel owners participating in this study.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAOSTAT. 2020. [(accessed on 12 April 2020)]. Available online: http://www.fao.org/faostat/en/#data/QA.
- 2.OIE. World Organization for Animal Health Animal Health Situation, Country: Tunisia, Year: 2018. World Animal Health Information Database (WAHIS Interface)—Version 1. 2018. [(accessed on 30 December 2018)]. Available online: http://weboieint/wahis.
- 3.Salmi C., Jaouad M., Faye B., Haouat F. Typologie des éleveurs camelin au sud-est tunisien en vue de leurs performances économiques. Revue Régions Arides. 2018;44:209–214. [Google Scholar]
- 4.Abdurahman O.A.S. Livestock Research for Rural Development. Volume 18. Swedish University of Agricultural Sciences; Uppsala, Sweden: 2006. Udder health and milk quality among camels in the Errer valley of eastern Ethiopia; p. 8. [Google Scholar]
- 5.El Harrak M., Faye B., Bengoumi M. Main Pathologies of Camels, Breeding of Camels, Constraints, Benefits and Perspectives. Conf. OIE. 2011. [(accessed on 17 August 2021)]. Available online: https://www.oie.int/doc/ged/D12812.PDF.
- 6.Jaradat Z., Al Aboudi A., Shatnawi M., Ababneh Q. Staphylococcus aureus isolates from camels differ in coagulase production, genotype and methicillin resistance gene profiles. J. Microbiol. Biotechnol. Food Sci. 2013;2:2455–2461. [Google Scholar]
- 7.Iverson S.A., Brazil A.M., Ferguson J.M., Nelson K., Lautenbach E., Rankin S.C., Morris D.O., Davis M.F. Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin-resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI) Vet. Microbiol. 2015;176:202–208. doi: 10.1016/j.vetmic.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Weese J.S., van Duijkeren E. Methicillin-resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Vet. Microbiol. 2010;140:418–429. doi: 10.1016/j.vetmic.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 9.Monecke S., Ehricht R., Slickers P., Wernery R., Johnson B., Jose S., Wernery U. Microarray-based genotyping of Staphylococcus aureus isolates from camels. Vet. Microbiol. 2011;150:309–314. doi: 10.1016/j.vetmic.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Fitzgerald J.R. Livestock-associated Staphylococcus aureus: Origin, evolution and public health threat. Trends Microbiol. 2012;20:192–198. doi: 10.1016/j.tim.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Zidan K.H., Mazloum K., Saran M.A., Hatem M.E. Abscesses in dromedary camels, sheep and goats etiology and pathology; Proceedings of the 1st International Scientific Conference of Pathology Department, Faculty of Veterinary Medicine; Giza, Egypt. 25–27 April 2013; pp. 47–59. [Google Scholar]
- 12.Ali A., Derar R., Al-Sobayil F., Al-Hawas A., Hassanein K. A retrospective study on clinical findings of 7300 cases (2007–2014) of barren female dromedaries. Theriogenology. 2015;84:452–456. doi: 10.1016/j.theriogenology.2015.03.039. [DOI] [PubMed] [Google Scholar]
- 13.Bani Ismail Z. Pneumonia in Dromedary Camels (Camelus dromedarius): A Review of Clinico-Pathological and Etiological Characteristics. J. Camel Pract. Res. 2017;24:49–54. doi: 10.5958/2277-8934.2017.00007.8. [DOI] [Google Scholar]
- 14.Gharsa H., Ben Slama K., Gomez-Sanz E., Lozano C., Zarazaga M., Messadi L., Boudabous A., Torres C. Molecular characterization of Staphylococcus aureus from nasal samples of healthy farm animals and pets in Tunisia. Vector-Borne Zoonotic Dis. 2015;15:109–115. doi: 10.1089/vbz.2014.1655. [DOI] [PubMed] [Google Scholar]
- 15.Gharsa H., Ben Slama K., Lozano C., Gomez-Sanz E., Klibi N., Ben Sallem R., Gomez P., Zarazaga M., Boudabous A., Torres C. Prevalence, antibiotic resistance, virulence traits and genetic lineages of Staphylococcus aureus in healthy sheep in Tunisia. Vet. Microbiol. 2012;156:367–373. doi: 10.1016/j.vetmic.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Daaloul-Jedidi M., Soudani A., Messadi L. Nasal and rectal carriage of coagulase positive Staphylococcus in healthy goats. J. New Sci. Agric. Biotechnol. 2016;33:1910–1913. [Google Scholar]
- 17.Gharsa H., Slama K.B., Gómez-Sanz E., Gómez P., Klibi N., Zarazaga M., Boudabous A., Torres C. Characterisation of nasal Staphylococcus delphini and Staphylococcus pseudintermedius isolates from healthy donkeys in Tunisia. Equine Vet. J. 2015;47:463–466. doi: 10.1111/evj.12305. [DOI] [PubMed] [Google Scholar]
- 18.Muna E., Tibin M., Mohammed A.E. Bacteria Associated with Pneumonia in Camels (Camelus Dromedarius) in the Sudan and Sensitivity of Some Isolates to Antibiotics using Vitek 2 Compact. Glob. J. Sci. Front. Res. C Biol. Sci. 2015;15:11–20. [Google Scholar]
- 19.Azizollah E., Bentol-hoda M., Raziah K. The aerobic bacterial population of the respiratory passageway of healthy Dromedariusin Najaf-Abbad abattoir central Iran. J. Camelid Sci. 2009;2:26–29. [Google Scholar]
- 20.Mutua J.M., Gitao C.G., Bebora L.C., Mutua F.K. Antimicrobial Resistance Profiles of Bacteria Isolated from the Nasal Cavity of Camels in Samburu, Nakuru, and Isiolo Counties of Kenya. J. Vet. Med. 2017;2017:1216283. doi: 10.1155/2017/1216283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gebru M., Tefera G., Dawo F., Tessema T.S. Aerobic bacteriological studies on the respiratory tracts of apparently healthy and pneumonic camels (Camelus dromedaries) in selected districts of Afar Region, Ethiopia. Trop. Anim. Health Prod. 2018;50:603–611. doi: 10.1007/s11250-017-1476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Thani R.F., Al-Ali F. Incidences and antimicrobial susceptibility profile of Staphylococcus species isolated from animals in different Qatari farms. Afr. J. Microbiol. 2012;6:7454–7458. [Google Scholar]
- 23.McAdow M., Missiakas D.M., Schneewind O. Staphylococcus aureus Secretes Coagulase and von Willebrand Factor Binding Protein to Modify the Coagulation Cascade and Establish Host Infections. J. Innate Immun. 2012;4:141–148. doi: 10.1159/000333447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki T., Tsubakishita S., Tanaka Y., Sakusabe A., Ohtsuka M., Hirotaki S., Kawakami T., Fukata T., Hiramatsu K. Multiplex-PCR method for species identification of coagulase-positive staphylococci. J. Clin. Microbiol. 2010;48:765–769. doi: 10.1128/JCM.01232-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lozano C., Gharsa H., Ben Slama K., Zarazaga M., Torres C. Staphylococcus aureus in Animals and Food: Methicillin Resistance, Prevalence and Population Structure. A Review in the African Continent. Microorganisms. 2016;4:12. doi: 10.3390/microorganisms4010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foster T.J., Geoghegan J.A., Ganesh V.K., Höök M. Adhesion, invasion and evasion: The many functions of the surface proteins of Staphylococcus aureus. Nat. Rev. Microbiol. 2014;12:49–62. doi: 10.1038/nrmicro3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moroni P., Pisoni G., Vimercati C., Rinaldi M., Castiglioni B., Cremonesi P., Boettcher P. Characterization of Staphylococcus aureus isolated from chronically infected dairy goats. J. Dairy Sci. 2005;88:3500–3509. doi: 10.3168/jds.S0022-0302(05)73035-6. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadpour R., Champour M., Tuteja F., Mostafavi E. Zoonotic implications of camel diseases in Iran. Vet. Med. Sci. 2020;6:359–381. doi: 10.1002/vms3.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharsa H., Ben Sallem R., Ben Slama K., Gómez-Sanz E., Lozano C., Jouini A., Klibi N., Zarazaga M., Boudabous A., Torres C. High diversity of genetic lineages and virulence genes in nasal Staphylococcus aureus isolates from donkeys destined to food consumption in Tunisia with predominance of the ruminant associated CC133 lineage. BMC Vet. Res. 2012;8:203. doi: 10.1186/1746-6148-8-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ben Said M., Abbassi M.S., Gómez P., Ruiz-Ripa L., Sghaier S., El Fekih O., Hassen A., Torres C. Genetic characterization of Staphylococcus aureus isolated from nasal samples of healthy ewes in Tunisia. High prevalence of CC130 and CC522 lineages. Comp. Immunol. Microbiol. Infect. Dis. 2017;51:37–40. doi: 10.1016/j.cimid.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Laux C., Peschel A., Krismer B. Staphylococcus aureus Colonization of the Human Nose and Interaction with Other Microbiome Members. Microbiol. Spectr. 2019;7:2–7. doi: 10.1128/microbiolspec.GPP3-0029-2018. [DOI] [PubMed] [Google Scholar]
- 32.Krismer B., Weidenmaier C., Zipperer A., Peschel A. The commensal lifestyle of Staphylococcus aureus and its interactions with the nasal microbiota. Nat. Rev. Microbiol. 2017;15:675–687. doi: 10.1038/nrmicro.2017.104. [DOI] [PubMed] [Google Scholar]
- 33.Abdulsalam A., Alhendi B. Nasal microflora of camels (Camelus dromedarius) under two different conditions. Pak. Vet. J. 1999;19:164–167. [Google Scholar]
- 34.Alzohairy M.A. Colonization and antibiotic susceptibility pattern of methicillin resistance Staphylococcus aureus (MRSA) among farm animals in Saudi Arabia. Afr. J. Bacteriol. Res. 2011;3:63–68. [Google Scholar]
- 35.Mai-siyama I., Okon O., Adamu N., Askira M., Isyaka T., Adamu G., Mohammed A. Methicllin-resistant Staphylococcus aureus (MRSA) colonization rate among ruminant animals slaughtered for human consumption and contact persons in Maiduguri, Nigeria. Afr. J. Microbiol. Res. 2014;8:2643–2649. doi: 10.5897/AJMR2014.6855. [DOI] [Google Scholar]
- 36.Agabou A., Ouchenane Z., Ngba Essebe C., Khemissi S., Chehboub M.T.E., Chehboub I.B., Sotto A., Dunyach-Remy C., Lavigne J.P. Emergence of Nasal Carriage of ST80 and ST152 PVL+ Staphylococcus aureus Isolates from Livestock in Algeria. Toxins. 2017;9:303. doi: 10.3390/toxins9100303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yusuf S.T., Kwaga J.K.P., Okolocha E.C., Bello M. Phenotypic occurrence of methicillin-resistant Staphylococcus aureus in camels slaughtered at Kano abattoir, Kano, Nigeria. Sokoto J. Vet. Sci. 2017;15:29–35. doi: 10.4314/sokjvs.v15i2.4. [DOI] [Google Scholar]
- 38.Al-Doughaym A.M., Mustafa K.M., Mohammad G.E. Aetiological Study on Pneumonia in Camel (Camelus dromedarius) and in vitro Antibacterial Sensitivity Pattern of the Isolates. Pak. J. Biol. Sci. 1999;2:1102–1105. doi: 10.3923/pjbs.1999.1102.1105. [DOI] [Google Scholar]
- 39.Kluytmans J., van Belkum A., Verbrugh H. Nasal carriage of Staphylococcus aureus: Epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 1997;10:505–520. doi: 10.1128/CMR.10.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tadesse B.T., Ashley E.A., Ongarello S., Havumaki J., Wijegoonewardena M., González I.J., Dittrich S. Antimicrobial resistance in Africa: A systematic review. BMC Infect. Dis. 2017;17:616. doi: 10.1186/s12879-017-2713-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes-Robles T., Alonzo F., Kozhaya L., Lacy D.B., Unutmaz D., Torres V.J. Staphylococcus aureus Leukotoxin ED Targets the Chemokine Receptors CXCR1 and CXCR2 to Kill Leukocytes and Promote Infection. Cell Host Microbe. 2013;14:453–459. doi: 10.1016/j.chom.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dinges M.M., Orwin P.M., Schlievert P.M. Exotoxins of Staphylococcus aureus. Clin. Microbiol. Rev. 2000;13:16–34. doi: 10.1128/CMR.13.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raji M.A., Garaween G., Ehricht R., Monecke S., Shibl A.M., Senok A. Genetic Characterization of Staphylococcus aureus Isolated from Retail Meat in Riyadh, Saudi Arabia. Front. Microbiol. 2016;7:911. doi: 10.3389/fmicb.2016.00911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sung J.M., Lloyd D.H., Lindsay J.A. Staphylococcus aureus host specificity: Comparative genomics of human versus animal isolates by multi-strain microarray. Microbiology. 2008;154:1949–1959. doi: 10.1099/mic.0.2007/015289-0. [DOI] [PubMed] [Google Scholar]
- 45.Koop G., Vrieling M., Storisteanu D.M.L., Lok L.S.C., Monie T., van Wigcheren G., Raisen C., Ba X., Gleadall N., Hadjirin N., et al. Identification of LukPQ, a novel, equid-adapted leukocidin of Staphylococcus aureus. Sci. Rep. 2017;7:40660. doi: 10.1038/srep40660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alonzo F., Torres V.J. The Bicomponent Pore-Forming Leucocidins of Staphylococcus aureus. Microbiol. Mol. Biol. Rev. MMBR. 2014;78:199–230. doi: 10.1128/MMBR.00055-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spaan A.N., van Strijp J.A., Torres V.J. Leukocidins: Staphylococcal bi-component pore-forming toxins find their receptors. Nat. Rev. Microbiol. 2017;15:435–447. doi: 10.1038/nrmicro.2017.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sutra L., Rainard P., Poutrel B. Phagocytosis of mastitis isolates of Staphylococcus aureus and expression of type 5 capsular polysaccharide are influenced by growth in the presence of milk. J. Clin. Microbiol. 1990;28:2253–2258. doi: 10.1128/jcm.28.10.2253-2258.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shuiep E.S., Kanbar T., Eissa N., Alber J., Lammler C., Zschock M., El Zubeir I.E., Weiss R. Phenotypic and genotypic characterization of Staphylococcus aureus isolated from raw camel milk samples. Res. Vet. Sci. 2009;86:211–215. doi: 10.1016/j.rvsc.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 50.McCarthy A.J., Lindsay J.A. Genetic variation in Staphylococcus aureus surface and immune evasion genes is lineage associated: Implications for vaccine design and host-pathogen interactions. BMC Microbiol. 2010;10:173. doi: 10.1186/1471-2180-10-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lacey K.A., Mulcahy M.E., Towell A.M., Geoghegan J.A., McLoughlin R.M. Clumping factor B is an important virulence factor during Staphylococcus aureus skin infection and a promising vaccine target. PLoS Pathog. 2019;15:e1007713. doi: 10.1371/journal.ppat.1007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yehia H.M., Ismail E.A., Hassan Z.K., Al-masoud A.H., Al-Dagal M.M. Heat resistance and presence of genes encoding staphylococcal enterotoxins evaluated by multiplex-PCR of Staphylococcus aureus isolated from pasteurized camel milk. Biosci. Rep. 2019;39:BSR20191225. doi: 10.1042/BSR20191225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ali A.O., Hyah M. Epidemiological studies based on multi-locus sequence typing genotype of methicillin susceptible Staphylococcus aureus isolated from camel’s milk. Onderstepoort J. Vet. Res. 2017;84:e1–e5. doi: 10.4102/ojvr.v84i1.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kpeli G., Buultjens A.H., Giulieri S., Owusu-Mireku E., Aboagye S.Y., Baines S.L., Seemann T., Bulach D., da Silva A.G., Monk I.R., et al. Genomic analysis of ST88 community-acquired methicillin resistant Staphylococcus aureus in Ghana. PeerJ. 2017;5:e3047. doi: 10.7717/peerj.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fall C., Seck A., Richard V., Ndour M., Sembene M., Laurent F. Epidemiology of Staphylococcus aureus in pigs and farmers in the largest farm in Dakar, Senegal. Foodborne Pathog. Dis. 2012;9:962–965. doi: 10.1089/fpd.2012.1197. [DOI] [PubMed] [Google Scholar]
- 56.Schaumburg F., Pauly M., Anoh E., Mossoun A., Wiersma L., Schubert G., Flammen A., Alabi A., Muyembe-Tamfum J.J., Grobusch M.P., et al. Staphylococcus aureus complex from animals and humans in three remote African regions. Clin. Microbiol. Infect. 2014;21:345.e1–345.e8. doi: 10.1016/j.cmi.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Otalu O.J., Kwaga J.K.P., Okolocha E.C., Islam M.Z., Moodley A. High Genetic Similarity of MRSA ST88 Isolated from Pigs and Humans in Kogi State, Nigeria. Front. Microbiol. 2018;9:3098. doi: 10.3389/fmicb.2018.03098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X., Li G., Xia X., Yang B., Xi M., Meng J. Antimicrobial susceptibility and molecular typing of methicillin-resistant staphylococcus aureus in retail foods in Shaanxi, China. Foodborne Pathog. Dis. 2014;11:281–286. doi: 10.1089/fpd.2013.1643. [DOI] [PubMed] [Google Scholar]
- 59.Ye X., Wang X., Fan Y., Peng Y., Li L., Li S., Huang J., Yao Z., Chen S. Genotypic and Phenotypic Markers of Livestock-Associated Methicillin-Resistant Staphylococcus aureus CC9 in Humans. Appl. Environ. Microbiol. 2016;82:3892–3899. doi: 10.1128/AEM.00091-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen Q., Xie S. Genotypes, Enterotoxin Gene Profiles, and Antimicrobial Resistance of Staphylococcus aureus Associated with Foodborne Outbreaks in Hangzhou, China. Toxins. 2019;11:307. doi: 10.3390/toxins11060307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.OEP Office D’elevage et de Paturage. Données Sectorielles, Effectif du Cheptel, Tunisie 2018. [(accessed on 20 April 2021)]. Available online: https://www.oep.nat.tn.
- 62.Brown D.F., Edwards D.I., Hawkey P.M., Morrison D., Ridgway G.L., Towner K.J., Wren M.W. Guidelines for the laboratory diagnosis and susceptibility testing of methicillin-resistant Staphylococcus aureus (MRSA) J. Antimicrob. Chemother. 2005;56:1000–1018. doi: 10.1093/jac/dki372. [DOI] [PubMed] [Google Scholar]
- 63.Hwang S.Y., Kim S.H., Jang E.J., Kwon N.H., Park Y.K., Koo H.C., Jung W.K., Kim J.M., Park Y.H. Novel multiplex PCR for the detection of the Staphylococcus aureus superantigen and its application to raw meat isolates in Korea. Int. J. Food Microbiol. 2007;117:99–105. doi: 10.1016/j.ijfoodmicro.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 64.Thompson T.A., Brown P.D. Association between the agr locus and the presence of virulence genes and pathogenesis in Staphylococcus aureus using a Caenorhabditis elegans model. Int. J. Infect. Dis. 2017;54:72–76. doi: 10.1016/j.ijid.2016.11.411. [DOI] [PubMed] [Google Scholar]
- 65.Rossato A.M., Reiter K.C., d’Azevedo P.A. Coexistence of virulence genes in methicillin-resistant Staphylococcus aureus clinical isolates. Rev. Soc. Bras. Med. Trop. 2018;51:361–363. doi: 10.1590/0037-8682-0339-2017. [DOI] [PubMed] [Google Scholar]
- 66.Harmsen D., Claus H., Witte W., Rothgänger J., Claus H., Turnwald D., Vogel U. Typing of methicillin-resistant Staphylococcus aureus in a university hospital setting by using novel software for spa repeat determination and database management. J. Clin. Microbiol. 2003;41:5442–5448. doi: 10.1128/JCM.41.12.5442-5448.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bouzaiane O., Abbassi M., Gtari M., Belhaj O., Jedidi N., Ben Hassen A., Hassen A. Molecular typing of staphylococcal communities isolated during municipal solid waste composting process. Ann. Microbiol. 2008;58:387–394. doi: 10.1007/BF03175533. [DOI] [Google Scholar]
- 68.Tenover F.C., Arbeit R.D., Goering R.V., Mickelsen P.A., Murray B.E., Persing D.H., Swaminathan B. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: Criteria for bacterial strain typing. J. Clin. Microbiol. 1995;33:2233–2239. doi: 10.1128/jcm.33.9.2233-2239.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Enright M.C., Day N.P., Davies C.E., Peacock S.J., Spratt B.G. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 2000;38:1008–1015. doi: 10.1128/JCM.38.3.1008-1015.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available at www.pubmlst.org (accessed on 30 April 2021).