Abstract
Kidney disease is one of the most common health problems and kidney failure can be fatal. It is one of the health disorders associated with extreme pain and discomfort in patients. In developing countries, such as Morocco where socioeconomic and sanitary conditions are precarious, medicinal plants are considered the primary source of medication. In the present work an ethnobotanical survey was conducted in a remote area of North-Eastern Morocco and we focused on (1) establishing a record of medicinal plants used traditionally by local people to treat kidney diseases and (2) correlate the obtained ethnomedical use with well-studied pharmacological evidence. From February 2018 to January2020, information was gathered from 488 informants using semi-structured questionnaires. The data were analyzed using three quantitative indices: The use value (UV), family use value (FUV), and informant consensus factor (ICF). A total of 121 plant species belonging to 57 botanical families were identified to treat kidney diseases. The families most represented were Asteraceae (14 species), followed by Lamiaceae (12 species) and Apiaceae (10 species). The most commonly used plant parts were leaves, followed by the whole plant and they were most commonly prepared by decoction and infusion. The highest value of the (UV) index was attributed to Herniaria hirsuta L. (UV = 0.16), and the highest family use value (FUV) was assigned to Caryophyllaceae with (FUV = 0.163). Regarding the informant consensus factor (ICF), this index’s highest values were recorded for kidney stones (ICF = 0.72). The use of 45% of the selected plants were validated based on literature review. This study helped document and preserve crucial traditional plant knowledge of 121 plant species used to treat kidney problems that can be used in the search for new biologically active compounds through more upcoming pharmacological studies.
Keywords: Ethnobotany, ethnopharmacology, traditional medicine, renal diseases, folk medicine, traditional knowledge, kidney problems, lithiasis, calculus, diuretic
1. Introduction
Nowadays, kidneys and their problems have gained increasing interest concomitant with life changes, industrialization and malnutrition. Plants have always played a significant role in traditional medicine in underdeveloped countries and have also been an integral part of local communities’ history and cultural practices [1]. Medicinal plants have been recognized for centuries as a rich source of medicinal agents for preventing and treating a variety of ailments in Morocco [2]. Several researches conducted in different regions of Morocco indicated that people excessively use medicinal plants to meet their healthcare needs in Morocco (at least 75% of the population) [3,4] and it is due to several factors, such as the high cost of conventional medicines, the lack of adequate sanitary facilities, and frangible socioeconomic conditions of users, especially those living in poor, remote areas and also their safety and low incidence of adverse effects [5].
As other regions of Morocco, people living in North-Eastern Morocco have a common cultural past that dates back to the Arab civilization in the seventh century. The original cumulative culture has maintained a well-developed traditional knowledge of medicinal plants’ uses that form the basis of the traditional medical system existing until now [6,7]. Unfortunately, this local cultural and natural heritage is threatened with extinction. The decrease of these phyto-therapeutic practices and the degradation of phyto-genetic resources are due to several factors, mainly the lack of documentary databases related to traditional medical practices and the scarcity of ethnobotanical information archives aggravate this natural and cultural heritage loss.
Regarding, these ancestral medical practices in this country, we found that there are many investigations carried out in different regions of Morocco that deal with traditional use of medicinal plants for the treatment of renal diseases. In fact, an ethnobotanical survey conducted in the Fez-Meknes region was able to document traditional knowledge related to the 69 plant species belonging to 38 families, used as traditional remedies for the treatment of kidney diseases in this region [8]. In the Boulemane region of Morocco, Jouad et al. (2001) conducted an ethnobotanical survey to document traditional medicinal practices related to medicinal plants used for the treatment of diabetes and kidney disease, among which they identify 33 medicinal plants used specifically to treat kidney problems [9]. In addition, a study led by Khouchlaa et al. (2016) in the Rabat region provided a catalogue of 35 medicinal plants with information on therapeutic practices for treating urinary lithiasis [10]. All these ethnobotanical fieldworks cited are practically concentrated on a part of the Moroccan territory, which shows that the ancestral medical practices in this country have remained to be preserved with regard to kidney diseases.
To the best of our knowledge, no ethnobotanical survey on the use of medicinal plants used in treating kidney diseases has been conducted in North-Eastern Morocco, appealing this study to be conducted with the objective of (1) record, evaluate, and document medicinal plants and know-how related used by local people, in the control and healing of renal disorders, in six provinces of the North-Eastern region of Morocco, (2) make a quantitative analysis of traditional knowledge assigned to species inventoried, (3) provide pharmacological and toxicological data of the plant species listed in the present paper.
2. Results and Discussion
2.1. Socio-Demographic Data
2.1.1. Global Data
In Table 1, we regrouped the information on the participants’ sociodemographic characteristics in this study. The variable including age, gender, education level, income, and attitude toward medication. These data showed that 488 local informants were interviewed, including 476 non-specialists and 12 health herbalists (care professionals). The use of medicinal plants in the area of study is widespread in all age groups. As indicated in Table 1, participants in the age group [46–65 years] have more knowledge of medicinal plants than other age groups, with a frequency of use 53%, followed by the group [25–45 years] with 27%, age group [over 65 years] with 11% and the last group [under 25 years], with a percentage 9%. These results confirm the data indicated previously in other ethnobotanical studies conducted in other areas in Morocco [11,12]. The high proportion of participants was dominated by women, with 58% followed by men with 42%. The high possession of the traditional phytotherapy knowledge, detained by women, could be explained by the nature of women’s behaviors within their families; in fact, the women were frequently sitting at home and are responsible for the care of their children and to maintain the health of their families in the most effective and economic ways [13]. So, we can say that women were more connected to traditional practices than men. These results are consistent with other national work results [6,14,15,16]. Regarding the level of education, the results showed that 59% of the respondents were illiterate, followed by secondary and primary education categories, with percentages, respectively, of 17% and 14%. However, people with a university-level education represented a low percentage of 9%. These observations showed that traditional remedies used by people living in this region of study to treat renal diseases are affected by the educational level and age of participants. Our findings resonate with other results observed in other ethnobotanical fieldworks conducted in other Morocco regions [17,18,19].
Table 1.
Socio-demographic characteristics of the informants in North-Eastern Morocco.
| Distribution of Informants | Categories | Number of Informants | Percentage of Informants (%) |
|---|---|---|---|
| By sex | Men | 207 | 42 |
| Women | 281 | 58 | |
| By age range | Less than 25 years | 42 | 9 |
| 25–45 | 132 | 27 | |
| 46–65 | 260 | 53 | |
| More than 65 years | 54 | 11 | |
| By education level | Illiterate | 290 | 59 |
| Primary education | 69 | 14 | |
| Secondary education | 85 | 17 | |
| University education | 44 | 9 | |
| By income/month | Unemployed | 311 | 64 |
| 500–2000 DH * | 108 | 22 | |
| 2000–6000 DH * | 53 | 11 | |
| >6000 DH * | 16 | 3 | |
| By choice of medicine | Herbal medicine | 264 | 54 |
| Both conventional and herbal medicine | 161 | 33 | |
| Modern medicine | 63 | 13 |
* 1 MAD (Moroccan Dirham) = 0.11 USD (United States Dollar).
2.1.2. Attitude of the Population toward Pattern of Uses
In this part of the text and for convenience, we divided medical practices, adopted by the population in North-Eastern Morocco, for treating renal diseases into three categories: those using only medicinal plants for these purposes, those using conventional medicine, and those using both traditional and conventional medicine. As indicated in Table 1, the population’s attitude in this region toward the treatment of renal diseases is variable. These data highlight the great diversity in patterns of use. The majority of interviewers cited traditional healthcare as their first-choice treatment option when they felt sick, with a percentage equaling to 54%, followed by the second choice, corresponding to the use of both conventional and herbal medicine, with a percentage of 33%, and in the third choice relative to the persons using exclusively modern medicine, with a percentage of 13%. Within the context of a dual health care system (traditional and western), the most significant determinants behind the participants’ attitude towards traditional medicine were the socio-economic factors and the residence of the users. Several factors were behind the driving force leading the majority of the interviewers toward traditional medicine. The results regrouped in Table 1 and Table 2 showed that the total people interviewed were living in remote areas and had a low socio-economic level. In addition to the lacking money and the high cost of modern medical treatment of renal diseases, the travel to cities, where the patients could have access to health facilities, constitutes a barrier to reach modern medicine and pull factors that attract people into seeking traditional treatments in the local area of study. This is congruent with studies conducted among populations in other Morocco regions [20].
Table 2.
Number of informants for each station.
| Provinces | Stations | Number of Informants | |
|---|---|---|---|
| Population | Herbalist | ||
| Guercif | Ras Laksar | 22 | 0 |
| Saka | 43 | 1 | |
| Jal | 25 | 0 | |
| Taourirt | Gteter | 37 | 1 |
| Debdou | 21 | 1 | |
| Jerada | Ain Benimathar | 17 | 1 |
| Guenfouda | 48 | 2 | |
| Jerada | 25 | 0 | |
| Berkane | Naima | 18 | 0 |
| Tafoughalt | 15 | 2 | |
| Ahfir | 14 | 0 | |
| Chouihia | 21 | 1 | |
| Nador | Tiztoutine | 29 | 1 |
| Bouarg | 22 | 1 | |
| Bni Sidel Jbel | 18 | 0 | |
| Afsou | 20 | 0 | |
| Oujda-Angad | Bni Drar | 69 | 1 |
| Sidi Moussa Lemhaya | 12 | 0 | |
| Total | 18 stations | 476 | 12 |
Based on the information mentioned above, we deduced that informal health care approaches “traditional medication” have been reported to be shared among people living in this region, especially for renal diseases. Despite the population in this region’s lack of trust in the modern healthcare system, our findings confirm that patients still consider traditional medical practices a better option than conventional healthcare approaches.
So, according to these observations, we can say that the socio-economic conditions, patients’ residence, culture, and tradition influenced the user’s decision to use traditional healthcare approaches.
2.1.3. Source of Information
Among participants who chose informal healthcare as their first-choice treatment option, their subsequent decision to use standard healthcare options depended on their experiences or their initial interaction with the older and herbalists, when that exists. According to our results, most parts of ethnobotanical information generated from this inquiry were given by people living in remote areas. Based on this inquiry’s ethnobotanical information, we deduced that the accumulated experiences with age are the primary source of information at the local level. The highest age respondents provide more reliable information because they hold much of the oral tradition’s ancestral knowledge. However, the young generation detained less information related to traditional knowledge because they were influenced by modernization and exotic culture and the tendency to disinterest and the gradual mistrust of this herbal medicine. So, the present-day, the substantial holder of traditional knowledge, which is becoming very old, and the lack of interest among the younger generation as well as their tendency to migrate to cities to ensure their basic needs, could harm the transmission of the traditional know-how on medicinal plants of the elderly to the young people.
Consequently, the traditional indigenous knowledge that has been transferred orally, which is fast disappearing, is in danger, and there is a possibility of losing this wealth of knowledge shortly. Indeed, this traditional knowledge on phytotherapy, which is transmitted from one generation to the next, is on the verge of extinction if no effort is made to save it [21].
2.2. Diversity of Plants Species Used to Treat Kidney Diseases
In the present study, 121 species of medicinal plants belonging to 57 families were used to treat kidney diseases. Ethnobotanical information related to these plants’ use was documented, including vernacular names, traditional uses, parts used, method of preparation, and route of administration (Table 3).
Table 3.
List of medicinal plants species used by local people for the treatment of kidney diseases.
| Scientific Name (Voucher Number) | Local/English Name | Therapeutic Uses | Part Used/Mode of Preparation/Mode of Administration | Common Traditional Dosages | UR | UV | FUV |
|---|---|---|---|---|---|---|---|
| ALLIACEAE Allium cepa L. (HUMPOM628) |
البصل/Onion |
Renal insufficiency, renal colic, kidney stones, diuretic | bu, st, fr/jui, dec/oral | - |
6 |
0.014 |
0.017 |
| Allium sativum L. (HUMPOM631) | الثوم/Garlic | Renal insufficiency, kidney stones, kidney inflammation, pyelonephritis, polycystic kidney disease | bu/dec | - | 2 | 0.005 | |
| ALOACEAE Aloe vera (L.) Burm.f (HUMPOM632) |
الالوفيرا/Aloe v. |
Renal insufficiency, polycystic kidney disease |
wp, ap/jui, dec/oral |
Spoon, glass |
2 |
0.005 |
0.007 |
| Aloe succotrina Lam. (HUMPOM629) | الصبار/Fynbos aloe | Renal insufficiency | wp, ap/jui/oral | Spoon, glass | 2 | 0.005 | |
| AMARANTHACEAE Anabasis aretioides Moq. and Coss. ex Bunge * (HUMPOM692) |
أكنود/Anabasis |
Diuretic, polycystic kidney disease |
lf/dec/oral |
Teapot |
1 |
0.002 |
0.010 |
| Beta vulgaris subsp. adanensis (Pamukç.) Ford-Lloyd and J.T. Williams (HUMPOM630) | باربة/beetroot | Diuretic | rt/mac/oral | Handful | 1 | 0.002 | |
| Dysphania ambrosioides (L.) Mosyakin and Clemants (HUMPOM693) | مخينزة/Mexican tea | Diuretic, kidney stones | lf/inf, dec/oral | Handful, Teapot | 3 | 0.007 | |
| ANACARDIACEAE Pistacia atlantica Desf. * (HUMPOM694) |
لبطم/Atlas mastic tree |
Diuretic |
cortex/dec/oral |
Spoon |
1 |
0.002 |
0.008 |
| Pistacia lentiscus L. * (HUMPOM632) | المسكةالحرة, ذرو/Mastic tree | Diuretic, renal insufficiency, kidney stones | lf/dec, inf/oral | Spoon, handful | 3 | 0.007 | |
| APIACEAE Daucus carota L. * (HUMPOM696) |
زرودية, خيزو/Wild carrot |
Renal pain, diuretic, pyelonephritis |
rh/inf/oral |
Glass |
1 |
0.002 |
0.010 |
| Foeniculum vulgare Mill. * (HUMPOM697) | النافع/Fennel | Kidney stones, renal colic, renal detoxification | se, lf/inf, tis, dec/oral | Handful, spoon, teapot | 8 | 0.019 | |
| Petroselinum crispum (Mill.) Fuss * (HUMPOM695) | المعدنوس, البقدونس/Parsley | Kidney stones, renal colic, renal detoxification Renal pain, diuretic, kidney inflammation, polycystic kidney disease |
wp, lf, ap, st, se, rt/inf, mac, dec, oil, jui /oral | Teapot, pinch handful, | 48 | 0.114 | |
| Ammi visnaga (L.) Lam. * (HUMPOM698) | البشنيخة/Toothpick-plant | Kidney stones, renal pain, renal colic, polycystic kidney disease | se, fr, lf/dec, inf, mac/oral | Spoon, glass | 7 | 0.017 | |
| Ammodaucus leucotrichus Coss. * (HUMPOM699) | الكمون الصوفي/- | Renal colic, polycystic kidney disease | lf/dec/oral | Handful | 1 | 0.002 | |
| Apium graveolens L. * (HUMPOM633) | الكرافس/Celery | Improved kidney performance, kidney swelling, kidney stones, renal detoxification, renal pain, diuretic, renal colic, polycystic kidney disease | rt, tw, ap, lf/inf, dec/oral | Glass, teapot | 17 | 0.040 | |
| Coriandrum sativum L. * (HUMPOM700) | قصبور/Coriander | Kidney stones, diuretic | wp, ap, lf/inf, dec/oral | Glass, teapot | 5 | 0.012 | |
| Cuminum cyminum L. (HUMPOM701) | الكمون/Cumin | Diuretic, kidney stones | lf/inf, dec/oral | Spoon | 2 | 0.005 | |
| Daucus crinitus Desf. * (HUMPOM702) | بوزفور/Common carrot | Detoxification of the kidneys | rt/dec/oral | teapot | 1 | 0.002 | |
| Pimpinella anisum L. (HUMPOM703) | حبة حلاوة/Aniseed | Diuretic; kidney stones | fr, lf/dec/oral | Spoon | 1 | 0.002 | |
| ASCLEPIADACEAE Caralluma europaea (Guss.) N.E.Br. * (HUMPOM634) |
الدغموس/Caralluma |
Urine retention, kidney stones, polycystic kidney disease |
wp, ap/inf/oral |
Spoon |
2 |
0.005 |
0.005 |
| ASPHODELACEAE Asphodelus microcarpus Salzm. and Viv. (HUMPOM745) |
البروغ/Common asphodel |
Diuretic |
rt/dec/oral |
Handful |
1 |
0.002 |
0.002 |
| ARALIACEAE Panax bipinnatifidus var. angustifolius (Burkill) J.Wen (HUMPOM635) |
جينسخ/Panax |
Diuretic |
rh/tis/oral |
Spoon |
1 |
0.002 |
0.002 |
| ASTERACEAE Echinops spinosissimus Turra * (HUMPOM704) |
تسكرة/Spiny globe thistle |
Diuretic, kidney stones, polycystic kidney disease |
ap, rt/inf, dec/oral |
Spoon, teapot |
5 |
0.019 |
0.083 |
| Helianthus annuus L. (HUMPOM636) | نوار الشمس/Sunflower | Renal pain, kidney inflammation | se, fl/dec, inf, mac/oral | Spoon | 14 | 0.033 | |
| Lactuca sativa L. (HUMPOM637) | خس/Lettuce | Kidney inflammation, polycystic kidney disease | lf/mac/oral | Spoon | 1 | 0.002 | |
| Artemisia arborescens (Vaill.) L. (HUMPOM638) | الشيبة/Tree wormwood | Kidney stones, renal colic, renal detoxification, diuretic, renal colic, pyelonephritis, polycystic kidney disease | lf/dec, inf, mac/oral | Teapot, glass | 6 | 0.014 | |
| Artemisia campestris L. (HUMPOM705) | ألاال/Wormwood sagewort | Kidney stones | lf/dec/oral | Spoon | 1 | 0.002 | |
| Brocchia cinerea (Delile) Vis. (HUMPOM706) | قرطوفة/- | Kidney stones | lf/mac/oral | Spoon | 1 | 0.002 | |
| Cichorium intybus L. * (HUMPOM707) | بوعكاد/Common chicory | Diuretic | rt/dec/oral | Spoon | 1 | 0.002 | |
| Cynara cardunculus L. (HUMPOM709) | الخرشف/Cardoon | Pyelonephritis | rt/pow/oral | Handful | 1 | 0.002 | |
| Dittrichia viscosa (L.) Greuter * (HUMPOM708) | مكرمان/False yellowhead | Kidney stones, pyelonephritis | wp/dec/oral | Handful | 1 | 0.002 | |
| Glebionis coronaria (L.) Cass. ex Spach * (HUMPOM710) | رجل لفلوس/Garland chrysanthemum | Kidney stones | wp/inf/oral | Handful | 1 | 0.002 | |
| Rhaponticum acaule (L.) DC. (HUMPOM712) | التابغة/Maral root | Renal detoxification, renal pain | rt/dec/oral | Glass | 1 | 0.002 | |
| Scorzonera undulata Vahl (HUMPOM711) | التالمة/Viper’s grass | Renal detoxification | rt/dec/oral | Spoon | 1 | 0.002 | |
| Taraxacum campylodes G.E.Haglund * (HUMPOM639) | الهندباء/Common dandelion | Renal detoxification, kidney stones, kidney inflammation, pyelonephritis, diuretic | wp, lf, se/inf, dec/oral | Spoon, teapot, glass | 13 | 0.030 | |
| Tanacetum cinerariifolium (Trevir.) Sch.Bip (HUMPOM713) | عود العطاس/Pyrethrum | Kidney stones | st/inf/oral | Spoon | 1 | 0.002 | |
| BERBERIDACEAE Berberis vulgaris subsp. australis (Boiss.) Heywood * (HUMPOM714) |
إرغيس/Common barberry |
Kidney stones |
st/pow/oral |
Spoon |
1 |
0.002 |
0.002 |
| BORAGINACEAE Borago officinalis L * (HUMPOM715) |
الحريشة/Burrage |
Diuretic |
lf/dec/oral |
Spoon |
1 |
0.002 |
0.002 |
| BRASSICACEAE Brassica napus L. (HUMPOM640) |
الفت/Annual rape |
Diuretic |
ap/dec/oral |
Spoon |
1 |
0.002 |
0.006 |
| Brassica oleracea L. (HUMPOM641) | لكروم/Wild cabbage | Renal pain | lf/dec/oral | Handful | 1 | 0.002 | |
| Lepidium sativum L. (HUMPOM642) | حب الرشاد/Common cress | Urine retention | se/dec/oral | Spoon | 1 | 0.002 | |
| BURSERACEAE Boswellia ameero Balf.f. (HUMPOM716) |
لبان ذكر/ Socotra Frankincense Tree |
Pyelonephritis |
se/dec/oral |
Spoon |
1 |
0.0024 |
0.0024 |
| CACTACEAE Opuntia ficus-indica (L.) Mill. (HUMPOM717) |
الهندية/ Prickly Pear |
Diuretic, kidney stones |
fl, lf, fr/dec, mac/oral |
Spoon |
3 |
0.007 |
0.007 |
| CAESALPINIACEAE Ceratonia siliqua L. (HUMPOM118) |
الخروب, تسلغو/ Carob |
Renal insufficiency, renal colic, kidney stones |
fr/dec, pow/oral |
Spoon, handful |
3 |
0.007 |
0.007 |
| CARYOPHYLLACEAE Corrigiola litoralis subsp. telephiifolia (Pourr.) Briq. * (HUMPOM719) |
سرغينة/Strapwort |
Diuretic |
wp/dec/oral |
Handful |
1 |
0.002 |
0.162 |
|
Herniaria hirsuta L. * (HUMPOM730) |
هراسة لحجر/Hairy rupturewort |
Kidney stones, renal colic, pyelonephritis, renal pain, diuretic, renal detoxification, polycystic kidney disease |
wp, ap, st, lf/inf, dec/oral |
Handful, Spoon, teapot, glass |
68 |
0.161 |
|
| CONVOLVULACEAE Convolvulus althaeoides L. * (HUMPOM720) |
اللواية/Mallow bindweed |
Kidney stones, polycystic kidney disease |
se/pow/oral |
Handful |
2 |
0.005 |
0.005 |
| CUCURBITACEAE Citrullus lanatus (Thunb.) Matsum. and Nakai (HUMPOM643) |
الدلاح, الدليع/Watermelon |
Urine retention, renal colic, renal detoxification, renal insufficiency, polycystic kidney disease |
ba, fr/inf, jui, dec/oral |
Spoon, glass |
6 |
0.014 |
0.034 |
| Bryonia cretica subsp. dioica (Jacq.) Tutin (HUMPOM644) | عنب الديب/Bryony | Kidney inflammation | fr/dec/oral | Glass | 1 | 0.002 | |
| Cucumis melo L. (HUMPOM645) | بتيخ/Honeydew | Renal pain | fr/eat/oral | - | 1 | 0.002 | |
| Cucumis sativus L. (HUMPOM646) | خيار/Cucumber | Renal pain | fr/jui/oral | Glass | 1 | 0.002 | |
| Cucurbita pepo L. (HUMPOM647) | الكارعة/Pumpkin | Kidney stones, urine retention, renal pain, diuretic | se, lf/inf, dec/oral | Spoon | 6 | 0.014 | |
| CUPRESSACEAE Juniperus oxycedrus L. * (HUMPOM721) |
تيغا/Prickly juniper |
Kidney stones, renal colic |
lf/dec/oral |
Spoon |
5 |
0.012 |
0.015 |
| Tetraclinis articulata (Vahl) Mast. * (HUMPOM722) | العرعار/Arar tree | Renal colic, kidney stones, diuretic | lf/dec/oral | Spoon, handful | 3 | 0.007 | |
| EQUISETACEAE Equisetum arvense L. (HUMPOM746) |
عشبةذيلالحصان/Field horsetail |
Renal colic, kidney stones |
ap/dec, inf/oral |
Spoon |
3 |
0.007 |
0.005 |
| ERICACEAE Vaccinium macrocarpon Aiton (HUMPOM747) |
التوت البري/Cranberry |
Kidney stones, renal insufficiency, diuretic |
fr/mac, dec/oral |
Glass |
5 |
0.012 |
0.024 |
| Arbutus unedo L. * (HUMPOM748) | ساسنو/Strawberry tree | Renal pain, diuretic, renal colic, polycystic kidney disease | rt, lf/dec/oral | Spoon, handful | 5 | 0.012 | |
| EUPHORBIACEAE Euphorbia retusa Forssk (HUMPOM723) |
تنورا/Spurge |
Kidney stones |
lf/inf/oral |
Handful |
1 |
0.002 |
0.002 |
| FABACEAE Anagyris foetida L. * (HUMPOM648) |
فول الكلب/Stinking bean trefoil |
Kidney stones |
se/inf/oral |
Handful |
1 |
0.002 |
0.022 |
| Arachis hypogaea L. (HUMPOM649) | القاوقاو/Peanut | Urine retention | ba, se/dec, mec/oral | Handful | 2 | 0.005 | |
| Glycyrrhiza glabra L. (HUMPOM650) | عرق السوس/Lecorice | Renal colic, diuretic, renal pain | rt, st/inf, dec, mac/oral | Teapot | 7 | 0.017 | |
| Trigonella foenum-graecum L. (HUMPOM725) | الحلبة/Spice fenugreek | Improved kidney performance, renal pain, diuretic | se/inf, dec, mac/oral | Spoon | 4 | 0.010 | |
| Vicia faba L. (HUMPOM724) | الفول/Broad bean | Renal pain | se/dec/oral | Handful | 1 | 0.002 | |
| FAGACEAE Quercus suber L. * (HUMPOM651) |
الدباغ/Cork oak |
Kidney stones |
lf, ba/dec/oral |
Spoon |
1 |
0.002 |
0.002 |
| GENTIANACEAE Centaurium erythraea Rafn * (HUMPOM726) |
كوزة الحية/Common centaury |
Renal pain |
ap/dec/oral |
Handful |
1 |
0.002 |
0.002 |
| GLOBULARIACEAE Globularia alypum L. * (HUMPOM728) |
تسلغا/Alypo globe daisy |
Kidney stones, pyelonephritis |
lf/dec/oral |
Spoon, handful |
4 |
0.010 |
0.010 |
| HYACINTHACEAE Drimia maritima (L.) Stearn * (HUMPOM729) |
بصلة لخلا/Maritime squill |
Diuretic, kidney stones, urine retention |
bu/inf, dec, mac/oral |
Glass |
4 |
0.010 |
0.010 |
| IRIDACEAE Crocus sativus L. (HUMPOM652) |
الزعفران/Saffron |
Kidney stones, diuretic, renal colic, kidney inflammation, polycystic kidney disease |
ap, sta/pow, dec, inf/oral |
Pinch |
6 |
0.014 |
0.014 |
| JUNCACEAE Juncus acutus L. * (HUMPOM731) |
سمار، أزلاف/Spiny rush |
Diuretic |
wp/inf/oral |
Spoon |
1 |
0.002 |
0.002 |
| LAMIACEAE Ajuga iva (L.) Schreb. * (HUMPOM653) |
شندكورة/Southern bugle |
Renal detoxification, kidney stones |
ap/dec, inf/oral |
Handful |
2 |
0.005 |
0.107 |
| Clinopodium nepetasubsp. glandulosum (Req.) Govaerts * (HUMPOM654) | مانتة/Lesser calamint | Renal colic, diuretic | lf, st, ap/oin, mac, dec, inf/mas, oral | Handful, teapot | 7 | 0.017 | |
| Lavandula dentata L. * (HUMPOM655) | الخزامة/French lavender | Kidney swelling, urine retention, renal detoxification | lf, wp, fl/dec, inf/oral | Spoon | 7 | 0.017 | |
| Mentha pulegium L. * (HUMPOM656) | فليو/Pennyroyal | Renal colic, kidney stones | wp, ap/dec, inf/oral | Teapot | 2 | 0.005 | |
| Mentha spicata L. (HUMPOM657) | نعناع/Mint | Renal pain | lf/dec/oral | Teapot | 1 | 0.002 | |
| Mentha suaveolens Ehrh. * (HUMPOM658) | تمرصاد/Bigleaf mint | Kidney inflammation | lf/pow/oral | Spoon | 1 | 0.002 | |
| Ocimum basilicum L. (HUMPOM659) | لحبق/Sweet basil | Renal pain | lf/inf/oral | Spoon | 1 | 0.002 | |
| Origanum compactum Benth. * (HUMPOM660) | الزعتر/Oregano | Renal colic, kidney swelling, urine retention, pyelonephritis, renal pain, polycystic kidney disease | lf, ap/inf, dec, tis/oral | Spoon, teapot | 5 | 0.012 | |
| Origanummajorana L. (HUMPOM661) | البرددوش/Sweet marjoram | Renal colic, renal pain, urine retention, pyelonephritis | lf/inf, dec/oral | Handful | 2 | 0.005 | |
| Rosmarinus officinalis L. * (HUMPOM662) | أزير/Rosemary | Kidney stones, kidney inflammation, renal detoxification, urine retention, renal colic, diuretic, renal pain, polycystic kidney disease | lf, ap/inf, dec/oral | Teapot | 10 | 0.024 | |
| Salvia officinalis L. (HUMPOM663) | السالمية/Sage | Kidney stones, diuretic, renal colic | ap, lf/inf, dec/oral | Handful, spoon | 7 | 0.017 | |
| Thymus saturejoides Coss. * (HUMPOM664) | أزوكني/Thyme | Kidney inflammation | lf/inf, dec/oral | Handful | 1 | 0.002 | |
| LAURACEAE Laurus nobilis L. * (HUMPOM732) |
ورقسيدناموسى/Bay tree |
Renal colic, kidney stones |
lf/dec, inf/oral |
Handful |
2 |
0.005 |
0.010 |
| Cinnamomum cassia (L.) J.Presl (HUMPOM733) | القرفة/Chinese cassia | Kidney stones | ba/pow/oral | Spoon | 3 | 0.007 | |
| LINACEAE Linum usitatissimum L. (HUMPOM734) |
زريعة الكتان/Flaxseed |
Renal diseases, diuretic |
se/dec/oral |
Spoon |
1 |
0.002 |
0.002 |
| LYTHRACEAE Lawsonia inermis L. (HUMPOM665) |
الحنى/Mignonette tree |
Kidney stone |
lf/dec/oral |
Spoon |
1 |
0.002 |
0.002 |
| MORACEAE Morus alba L. (HUMPOM735) |
التوت/White mulberry |
Renal colic, diuretic, renal detoxification |
lf, fr/mac, dec, inf/oral |
Glass |
4 |
0.010 |
0.010 |
| MYRTACEAE Myrtus communis L. (HUMPOM666) |
الريحان/Common myrtle |
Renal detoxification, pyelonephritis |
lf/dec/oral |
Spoon |
1 |
0.002 |
0.020 |
| Eucalyptus globulus Labill. (HUMPOM667) | الكاليتوس/Tasmanian blue gum | Renal colic | lf/dec/oral | Handful, teapot | 4 | 0.010 | |
| Syzygium aromaticum (L.) Merr. and L.M.Perry (HUMPOM668) | القرنفل/Clove | Renal insufficiency, renal colic, renal pain | ap, lf/dec, inf/oral | Spoon | 4 | 0.010 | |
|
OLEACEAE Fraxinus excelsior L. (HUMPOM737) |
لسان طير/Common ash |
Kidney stones, pyelonephritis, polycystic kidney disease |
lf/inf/oral |
Handful, spoon |
4 |
0.010 |
0.007 |
| Olea europaea L. (HUMPOM736) | الزيتون/Olive | Renal detoxification, kidney stones, diuretic | lf, fr/dec, oil/oral | Spoon (oil), handful | 3 | 0.007 | |
| PAPAVERACEAE Papaver rhoeas L. * (HUMPOM669) |
بنعمان/Common poppy |
Kidney stones, kidney inflammation, pyelonephritis |
se/pow/oral |
Handful |
1 |
0.002 |
0.002 |
| PIPERACEAE Piper cubeba L. f. (HUMPOM670) |
كبابة/Cubeb pepper |
Pyelonephritis, polycystic kidney disease |
fr/inf/oral |
Handful |
1 |
0.002 |
0.002 |
| PLANTAGINACEAE Globularia repens Lam. (HUMPOM671) |
عين لرنب/Creeping globe daisy |
Renal insufficiency, kidney stones, urine retention |
lf/pow/oral |
Spoon |
3 |
0.007 |
0.007 |
| POACEAE Pennisetum glaucum (L.) R.Br. (HUMPOM738) |
إيلان/Yellow bristlegrass |
Renal pain, polycystic kidney disease, pyelonephritis |
se/pow/oral |
Spoon |
1 |
0.002 |
0.074 |
| Avena sativa L. (HUMPOM741) | الخرطال/Common oat | Diuretic, renal pain | se/dec/oral | Handful | 1 | 0.002 | |
| Cynodon dactylon (L.) Pers. * (HUMPOM740) | عروق النجم/Bermuda grass | Kidney stones, renal pain, diuretic | rt, lf/dec, mac, inf/oral | Handful | 3 | 0.007 | |
| Hordeum vulgare L. (HUMPOM739) | شعير/Barley | Diuretic, kidney stones | fr, se/dec, mac/oral | Handful | 2 | 0.005 | |
| Zea mays L. (HUMPOM742) | الذرة/Maize | Kidney stones, kidney swelling, renal insufficiency, renal pain, diuretic, kidney inflammation, polycystic kidney disease | fr, fl/dec, inf/oral | Handful | 22 | 0.052 | |
| POLYGONACEAE Rumex vesicarius L. (HUMPOM672) |
حميضة/Ruby dock |
Renal detoxification, kidney stones |
ap, lf/dec, inf/oral |
Handful |
2 |
0.005 |
0.007 |
| PUNICACEAE Punica granatum L. (HUMPOM743) |
الرمان/Pomegranate |
Renal detoxification, renal colic, kidney stones, renal pain, pyelonephritis |
ba, fr/dec, pow/oral |
Glass |
5 |
0.012 |
0.012 |
| RANUNCULACEAE Nigella sativa L. (HUMPOM744) |
السانوج, حبةالكحلة/Black caraway |
Detoxification of the kidneys, diuretic |
se/dec, inf, oil/oral |
Pinch |
3 |
0.007 |
0.007 |
| RHAMNACEAE Ziziphus jujuba Mill. (HUMPOM673) |
زفيزف/Lotus jujube |
Renal detoxification, pyelonephritis |
fr/inf/oral |
Handful |
1 |
0.002 |
0.084 |
| Ziziphus lotus (L.) Lam. (HUMPOM674) | السدرة، النبق/Lotus tree | Urine retention, diuretic, renal colic, kidney stones, pyelonephritis, polycystic kidney disease | rt, fr, lf/dec, inf, pow/oral | Handful, spoon | 35 | 0.083 | |
| ROSACEAE Malus sylvestris (L.) Mill. (HUMPOM675) |
التفاح/Common apple |
Kidney swelling, renal colic, kidney stones |
fr/inf, eat/oral |
- |
5 |
0.012 |
0.020 |
| Prunus cerasus L. (HUMPOM677) | حب لملوك/Sour cherry | Renal colic, renal pain, diuretic, kidney stones | tw, fr/dec/oral | Handful | 3 | 0.007 | |
| Rosa canina L. * (HUMPOM676) | الورد البلدي/Common briar | Diuretic, pyelonephritis | lf/mac/oral | Handful | 1 | 0.002 | |
| RUBIACEAE Rubia peregrina L. (HUMPOM678) |
الفوة/Common wild madder |
Renal pain, kidney stones, diuretic |
lf, ap/dec, inf/oral |
Handful |
4 |
0.010 |
0.010 |
| RUTACEAE Citrus × aurantium L. (HUMPOM690) |
الرنج/Lime |
Kidney stones, renal pain |
fr/jui/oral |
Glass |
2 |
0.005 |
0.044 |
| Citrus limon (L.) Osbeck (HUMPOM691) | اليم/Lemon | Kidney stones, renal insufficiency, renal detoxification, | bu, fr/jui, inf/oral | Glass | 6 | 0.014 | |
| Citrus saliaefolius L (HUMPOM688) | وظمي/Sage-leaved rock-rose | Renal detoxification | rt/dec/oral | Handful | 1 | 0.002 | |
| Citrus sinensis (L.) Osbeck (HUMPOM689) | الليمون/Sweet orange | Renal colic, renal insufficiency, kidney stones | fr/jui, inf/oral | Glass | 6 | 0.014 | |
| Ruta montana (L.) L. * (HUMPOM687) | أورمي/Rue | Kidney stones, polycystic kidney disease | lf/inf/oral | Spoon | 1 | 0.002 | |
| SOLANACEAE Capsicum annuum L. (HUMPOM679) |
الحار/Capsicum pepper |
Diuretic |
fr/dec, pow/oral |
Pinch |
2 |
0.005 |
0.005 |
| THYMELAEACEAE Thymelaea microphylla Meisn. * (HUMPOM680) |
المتنان/Sparrow-worts |
Diuretic, renal colic, kidney stones, pyelonephritis |
lf, ap/dec, mac/oral |
Handful, teapot |
14 |
0.005 |
0.036 |
| TILIACEAE Tilia sylvestris Desf (HUMPOM681) |
زيزفون/Small-leaved linden |
Kidney stones, renal detoxification |
ap, lf/dec/oral |
Glass |
1 |
0.002 |
0.010 |
| URTICACEAE Urtica dioica L. * (HUMPOM682) |
الحريكة الملساء/Common nettle |
Urine retention, kidney stones, diuretic, renal insufficiency, renal pain, kidney swelling, pyelonephritis, renal colic, kidney inflammation |
st, ap, wp, lf/dec, pow, inf/oral |
Handful |
15 |
0.036 |
0.036 |
| VERBENACEAE Aloysia citriodora Palau (HUMPOM683) |
اللويزة/Lemon verbena |
Diuretic, pyelonephritis |
lf/dec, inf/oral |
Handful |
3 |
0.007 |
0.007 |
| VITACEAE Vitis vinifera L. (HUMPOM684) |
الدالية/Wine grape |
Renal detoxification, diuretic, pyelonephritis |
lf/dec |
Glass |
3 |
0.007 |
0.007 |
| ZINGIBERACEAE Curcuma longa L. (HUMPOM685) |
الخرقوم/Turmeric |
Renal detoxification, renal colic, kidney stones |
rh/pow, dec, inf/oral |
Spoon |
8 |
0.019 |
0.060 |
| Zingiber officinale Roscoe (HUMPOM686) | الزنجبيل,سكينجبير/Ginger | Kidney swelling, kidney stones, renal detoxification, detoxification of the kidneys, kidney inflammation, renal pain, diuretic, polycystic kidney disease | rh, rt/pow, dec, inf/oral | Spoon, pinch | 19 | 0.045 |
Abbreviation: parts used: bu: bulb; st: stem; fr: fruit; wp: whole plant; ap: aerial part; lf: leaf; rt: root; rh: rhizome; se: seeds; tw: twigs; fl: flowers; ba: bark; sta: stamen. Mode of preparation: juice: jui; decoction: dec; infusion: inf; maceration: mac; powder: pow; tisane: tis; ointment: oin; massage: mas; UV: Use Value. FUV: Family Use Value. UR: Use reports. *: Endemic.
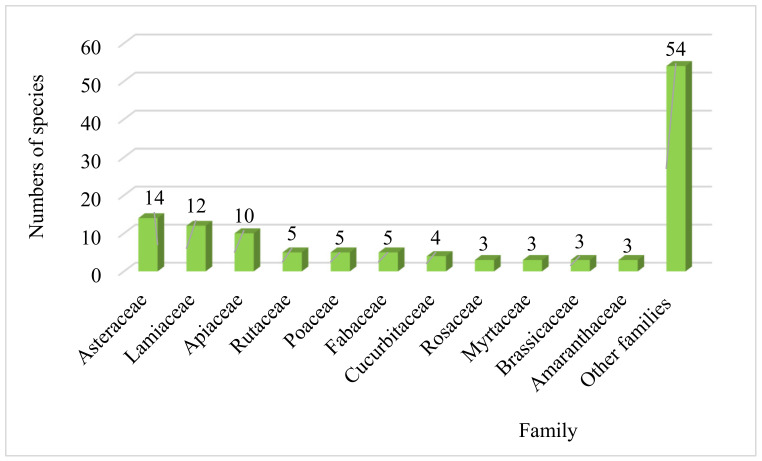
The dominated families that have been used to treat and relieve renal disorders were the Asteraceae (14 species), followed by the Lamiaceae (12 species), the Apiaceae (10 species), Rutaceae, Poaceae and Fabaceae (5 species) each, Cucurbitaceae with (4 species), Rosaceae, Myrtaceae, Brassicaceae and Amaranthaceae with (3 species for each), while the other families represent less than three species (Figure 1). The predominance of Asteraceae, Lamiaceae, and Apiaceae, has already been proven in several ethnobotanical studies carried out in other Moroccan regions [14,22,23,24], as well as in other countries such as Turkey [25] and Italy [26]. Furthermore, the predominance of these plant families has already been confirmed in the results of specific ethnobotanical work for kidney disorders conducted in the Moroccan territory [17,22,27]. On the other hand, these botanical families dominate the Moroccan flora and are also almost omnipresent in the Moroccan territory [28].
Figure 1.
Dominant botanical families.
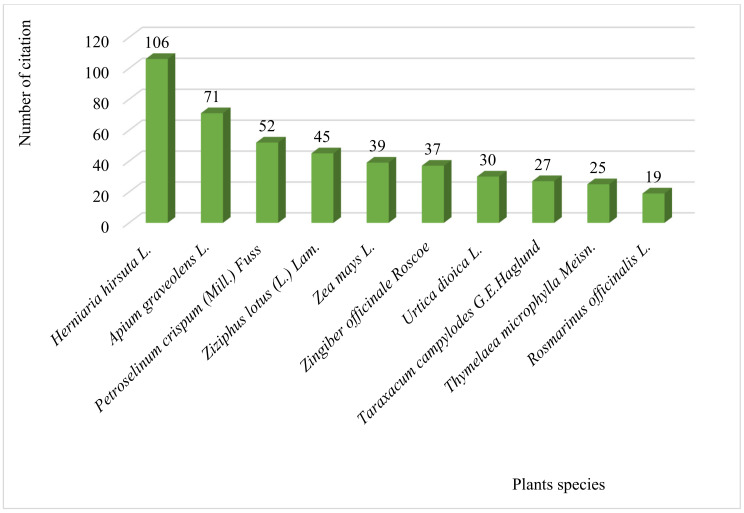
As shown in Figure 2, the most preferred plant species used to treat kidney diseases in remote areas of North-Eastern Moroccan folk medicine were H. hirsuta with (106 use reports; 14.29% of total use reports), followed by A. graveolens (71 use reports; 9.57% of total use reports), P. crispum (52 use reports; 7.00% of total use reports), and Z. lotus (45 use reports; 6.06% of total use reports), Z. mays (39 use reports; 5.26% of total use reports), Z. officinale (37 use reports; 4.99% of total use reports), U. dioica (30 use reports; 4.04% of total use reports), T. campylodes (27 use reports; 3.64% of total use reports), T. microphylla (25 use reports; 3.37% of total use reports), and R. officinalis (19 use reports; 2.57% of total use reports). These ten species accounted for 60.78% of total use reports, and the remaining 101 species represent only 39.22% of total use reports. The frequent use of H. hirsuta, P. crispum, Z. lotus, and Z. mays against kidney pain are already confirmed in the results of a study conducted in the Fes-Meknes region of Morocco [19]. These four medicinal plants are widely used in Moroccan folk medicine to manage various diseases [4].
Figure 2.
Plant species commonly used traditionally by local people to treatkidney disease.
2.3. Ethnic Medicinal Characteristics
Used Plant Parts and Method of Preparation
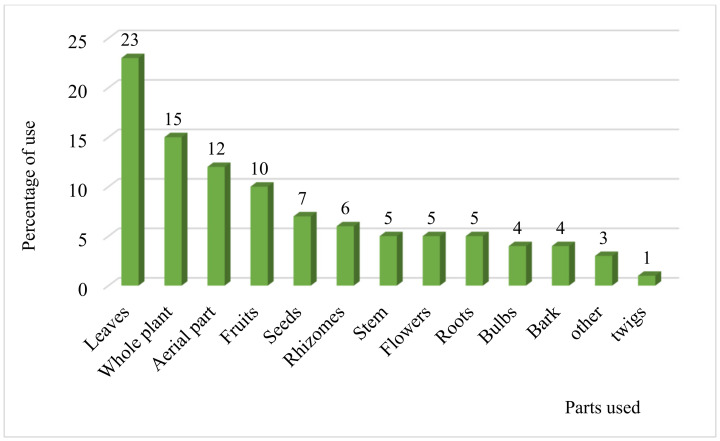
In this survey, several parts of plant species are used as medicine (Figure 3). The most widely used medicinal plant part was the leaves with a frequency of 23%, followed by the whole plant with a percentage of 15%, aerial parts (12%), fruits (10%), seeds (7%), rhizomes (6%) and the other parts (stems, flowers, roots, bulbs, bark, and twig) are represented by a rate lower than 6%. Likewise, several communities in other regions of Morocco and other countries use leaves to prepare herbal medicines [29,30,31]. The frequent use of one part over another in herbal medicine depends on its active ingredient content. The leaves are the most exploited plant parts. This could be explained by the fact that they are both sites of photosynthesis and reservoirs of secondary metabolites that have [32,33]. The rapidity and ease of leaf harvesting also explain their predominance over other plant parts [29]. Besides, harvesting these organs is a relatively sustainable practice compared to other plant parts, such as roots and stem. The harvesting of the roots could contribute to the extermination and disappearance of the plants.
Figure 3.
Percentage of the different parts used.
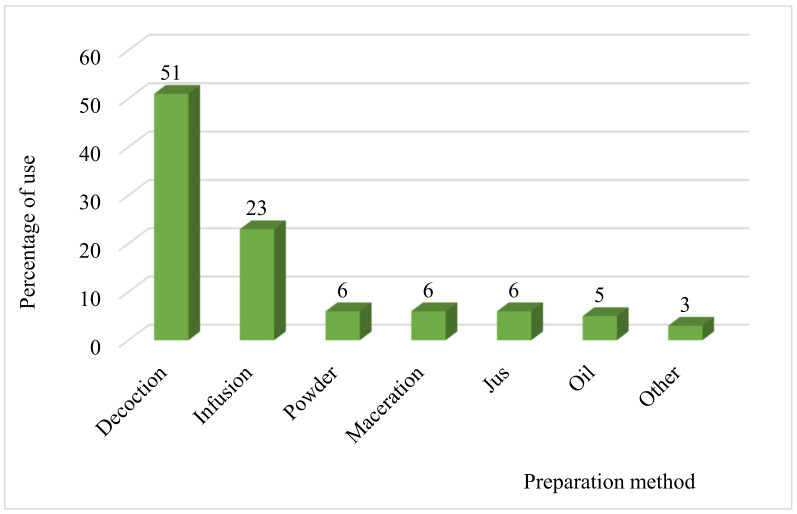
As shown in Figure 4, the preparation method most used by the population of North-Eastern Morocco for the treatment of kidney disorders is decoction with a frequency of 51%, followed by infusion (23%), powder, maceration, and juice with a percentage of 6% for each, oil (5%), and other methods of preparation represent only 3%. This high percentage of decoction shows that the local population grows at this mode of preparation and finds it suitable for warming the body and disinfecting the plant [34]. On the other hand, the decoction makes it possible to collect the most active ingredients and attenuates or cancels specific recipes’ toxic effects [35].
Figure 4.
Percentage of different mode of preparation.
2.4. Commonly Treated Kidney Diseases and Noteworthy Plants
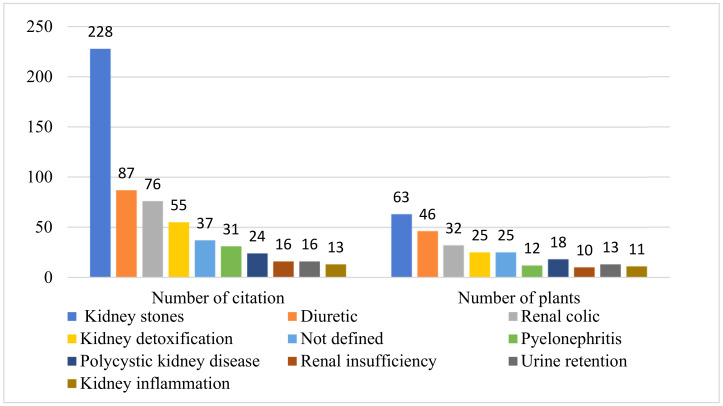
Traditionally, the local population uses the species inventoried in this survey to treat a wide range of kidney symptoms. Nevertheless, it should be noted that the most mentioned kidneys symptoms (Figure 5) are kidney stones (228 citations, 63 plants), followed by diuretic (87 citations, 46 plants), renal colic (76 citations, 32 plants), kidney detoxification (55 citations, 25 plants) and Pyelonephritis (31 citations, 12 plants). Some species such as H. hirsuta (106 use reports), A. graveolens (71 use reports), and P. crispum (52 use reports) were the most commonly used species for the treatment of kidney symptoms. The aerial parts of H. hirsuta, in decoction, are used against kidney stones, the infused leaves are used against Pyelonephritis and renal colic, the whole plant, in decoction, is used to relieve pain in the kidneys, and as well as for detoxifying the kidneys. The aerial part of A. graveolens, in decoction, is used against swelling of the kidneys, decocted roots are used to improve the kidneys’ performance, and the infusion of the aerial part against renal colic and kidney stones.
Figure 5.
Distribution of plants used traditionally to treat various kidney syndromes.
2.5. Quantitative Analysis
2.5.1. The Use Value (UV)
The local population’s choice to use certain medicinal species more than others to treat different kidney symptoms is confirmed by the use-value index (UV). The high score of this index reflects the importance of the plant in the study area population. The use-value (UV) results were presented in Table 3, with limited values between 0.16 and 0.0024. According to our results, H. hirsuta is the most used by the local population to treat renal disorders with high use value (UV = 0.161), followed by P. crispum (UV = 0.114), Z. lotus (UV = 0.083), Z. mays (UV = 0.052), Z. officinale (UV = 0.050), A. graveolens (UV = 0.040), U. dioica (UV = 0.036), T. microphylla (UV = 0.0355), H. annuus (UV = 0.034), T. campylodes (UV = 0.031), R. officinalis (UV = 0.024) and C. longa (UV = 0.021). The intensive use of these medicinal species by the population of North-Eastern Morocco is also mentioned with high percentages for the treatment of kidney diseases in the ethnobotanical study conducted in the Northcentral region of Morocco [9], and in other led in the region of Rabat on kidney stones [36].
2.5.2. Botanical Family Use Value (FUV)
As shown in Table 3, the distribution of botanical families of medicinal species in the study area fluctuated between a minimum importance value of 0.0023 and a maximum value of 0.161. Regarding the family use value of the plants recorded in this paper, the results show the high score for Caryophyllaceae (FUV = 0.163), followed by Lamiaceae (FUV = 0.106), Apiaceae (FUV = 0.099), Rhamnaceae (FUV = 0.084), Asteraceae (FUV = 0.083) Poaceae (FUV = 0.074), Asteraceae (FUV = 0.071), Zingiberaceae (FUV = 0.060), Rutaceae (FUV = 0.044), Thymelaeaceae and Urticaceae (FUV = 0.036) for each, Cucurbitaceae (FUV = 0.034) and Ericaceae (FUV = 0.024). The other families have the use value less than 0.024.
2.5.3. Informant Consensus Factor (ICF)
The ICF was calculated for each category of renal symptoms, and the index values range from a maximum significance value of 0.72 to a minimum value of 0.16 (Figure 6). Based on these results, we noted that the highest values of this index (ICF) were recorded for kidney stones (ICF = 0.72) with 63 plant species, followed by Pyelonephritis (ICF = 0.63) with 12 plant species, renal colic (ICF = 0.58), kidney poisoning (ICF = 0.56) and diuretic (ICF = 0.47). High values (close to 1) of this index for kidney stones and pyelonephritis indicate that few species were used by a large proportion of informants for each of these two disease categories. For kidney inflammation and urinary retention, the index values were ICF = 0.16 and ICF = 0.22, which means that the number of citations is almost equal to the number of plants used by informants to treat these symptoms. High ICF values for kidney stones may be due to their high incidence of occurrence in the study area [37].
Figure 6.
Informant consensus factor (ICF).
2.6. Pharmacological Validation from Literature
Our ethnobotanical fieldwork indicated that people living in North-Eastern Morocco have important knowledge regarding the use of medicinal plants for the treatment of renal diseases. These ethnobotanical data, which described a wide variety of quantitative indicators, were very interesting for bioprospection purposes. It could be interesting to screen in the literature these plants for their pharmacological activities.
According to the studied literature, among 121 medicinal species inventoried during this survey, 54 plants were studied for their pharmacological properties against kidney disorders, which seems that traditional medicine could be an excellent classical basis for the selection of plant species against kidney problems. The grouped pharmacological data (the plant’s scientific name, the part extracted from the plant; the type of extracts; the experimental model used; the dose used, and the pharmacological effect) of these 54 plants were summarized in Table 4.
Table 4.
Pharmacological data of the medicinal species cited by local people to treat kidney diseases.
| Scientific Name | Used Parts | Used Extracts | Experimental Model | Pharmacological Uses | Therapeutic Doses | References |
|---|---|---|---|---|---|---|
| Ajuga iva (L.) Schreb. | Whole plant | Aqueous extract | Rats | Beneficial for correcting the hyperglycemia and preventing diabetic complications in liver, pancreas and kidneys | 50 mg/kg of body weight daily for 3 weeks | [38] |
| Allium sativum L. | Bulbs | Aqueous extract | Rats | Modulatory effects on renal oxidative stress and nitric oxide production in streptozotocin-induced diabetic nephropathy in rats | 200–400 mg/kg of body weight for 30 consecutive days | [39] |
| Bulbs | Aqueous extract | Rats | Modulates the expression of angiotensin II AT2 receptor in adrenal and renal tissues of streptozotocin-induced diabetic rats | 500 mg/kg of body weight 8 weeks after diabetes induction | [40] | |
| Bulbs | Aqueous extract | Rats | Protects hepatic and renal toxicity of alloxan in rats | 100–200 mg/kg of body weight/day; given by oral gavage for 21 days | [41] | |
| Bulbs | Ethanol extract | Rats | Ameliorative effects on renal parenchyma of gentamicin-induced nephropathic rats | 200 mg/kg of body weight for 10 days | [42] | |
| Aloe vera (L.) Burm.f. | Leaves | Leaf pulp extract | Rats | Protective effect on mild damage caused by type II diabetic on kidney tissue | 500 mg/kg of body weight | [43] |
| Leaves | Ethanol extract | Rats | Protective role on liver and kidney of streptozotocin-induced diabetic rats | 300 mg/kg of body weight for 30 days | [44] | |
| Leaves | Ethanol extract | Rats | Antinephropathy effect on PKC-β level of rat kidney in diabetes mellitus | 30–120 mg/kg of body weight | [45] | |
| Ammi visnaga (L.) Lam. | Fruits | Aqueous extract | LLC-PK1and Madin-Darby-canine kidney (MDCK) cells |
Prevent cell damage caused by oxalate in renal epithelial cells | (100 µg/mL) | [46] |
| Fruits | Aqueous extract | Rats | Prevention of renal crystal deposition | 125–500 mg/kg of body weight for 14 days | [47] | |
| Apium graveolens L. | Aerial parts | Fresh celery | Rabbits | Accentuates urinary Ca+2 excretions in experimental model of nephrocalcinosis |
8 g/kg added to the animal food |
[48] |
| Stem, leaves | Ethanolic extract | Rats | Protective effect on kidney damage in ischemia/reperfusion injury rats model | 250–1000 mg/kg of body weight for 14 days | [49] | |
| Fruits | Essential oil | Dogs | Diuretic effect | 0.004–0.008 mL/kg of body weight | [50] | |
| Arachis hypogaea L. | Peanuts pods | Methanol and aqueous extracts | Mice | Nephroprotective effect on CCl4 induced kidney damage in mice |
50–100 mg/kg of body weight | [51] |
| Arbutus unedo L. | Leaves | Aqueous extracts | Rats | Prevent cardiovascular and renal hemodynamic effects in L-NAME-induced hypertensive rats | 250 mg/kg of body weight/day | [52] |
| Artemisia arborescens (Vaill.) L. | Leaves | Hydroalcoholic extract | Rats | Nephroprotective effects against oestroprogestative-induced kidney damages in rats | 200 mg/kg body weight during 6 weeks | [53] |
| Artemisia campestris L. | Aerial parts | Essential oil | Rats | Protective effect on Deltamethrin induced oxidative stress in kidney and brain of rats | 200 mg/kg of body weight for two weeks | [54] |
| Avena sativa L. | Seeds | Powder | Human | Beneficial effect on serum albumin and serum potassium in patients with CKD | 50 g of oat flour per day for 8 weeks | [55] |
| Seeds | Seeds prepared with food pellets and distilled water to get a cohesive paste | Mice | Protective effects of against oxidative stress-induced kidney damage resulting from an estrogen deficiency in ovariectomized swiss mice model | 200 mg/kg of body weight | [56] | |
| Berberis vulgaris subsp. australis (Boiss.) Heywood | Bark | Ethanolic extract | Rats | Ameliorative effects on lipid profile, kidney and liver function in experimental dyslipidemia | 300–500 mg/kg of body weight for eight weeks | [57] |
| Brassica oleracea L. | Broccoli sprouts | Juice | Rats | Protective effects toward renal damage in high-salt-fed SHRSP: role of AMPK/PPARa/UCP2 axis | 340 mL/120 mg in diet | [58] |
| Ceratonia siliqua L. | Pulp and seeds | Aqueous extract | Rats | Protective effect against a dextran sulfate sodium-induced alteration in liver and kidney in rat | 50 and 100 mg/kg of body weight for 21 days | [59] |
| Leaves | Ethyl acetate fraction | Rats | Ameliorative effects against CCl4 induced hepatic oxidative damage and renal failure in rats | 250 mg/kg of body weight for 8 days | [60] | |
| Cichorium intybus L. | Seeds | Aqueous extract | Rats | Improving effect on renal parameters in experimentally induced early and late diabetes type 2 in rats | 125 mg/kg of body weight for 21 days | [61] |
| Aerial parts | Ethanol extract | Rats | Against cisplatin induced renal toxicity | 500 mg/kg of body weight for 10 consecutive days | [62] | |
| Flowers | Aqueous extract | Rats | Preventive effects on ethylene glycol-induced renal calculi in rats | 50–200 mg/kg of body weight for 30 days | [63] | |
| Roots | Aqueous extract | Rats | Improving effects on serum oxidative stress, liver and kidney volume, and cyclin B1 and Bcl-2 levels in the brains of rats with ethanol induced damage | 200 mg/kg of body weight for 18 days | [64] | |
| Roots | Unspecified | Rats | Ameliorates hydroxyapatite nanoparticles induced kidney damage in rats | 20 and 300 mg/kg of body weight for 4 weeks | [65] | |
| Cinnamomum cassia (L.) J.Presl | Bark | Methanol extract | Rats | Ameliorative effect against Ni-NPs-induced liver and kidney damage in male Sprague Dawley rats | 175–225 mg/kg of body weight | [66] |
| Citrus sinensis (L.) Osbeck | Leaves | Essential oil | Rats | Ameliorative effect on some liver and kidney function indices of diabetic rats | 110 mg/Kg of body weight for 15 days | [67] |
| Stems | Aqueous and methanolic extracts |
Human Embryonic Kidney Carcinoma (HEK) cell line | Anti-proliferative or cytopathic potential effects against human embryonic kidney carcinoma cell line | IC50 at 32-fold dilution of the extract | [68] | |
| Coriandrum sativum L. | Seeds | Aqueous and ethanol extracts | Mices | Protective role against lead nitrate induced oxidative stress and tissue damage in the liver and kidney in mal mice | WE (300 and 600 mg/kg of body weight), EtOH (250 and 500 mg/kg of body weight) | [69] |
| Crocus sativus L. | Unspecified | Aqueous extract | Cats | Increase the glomerular filtration rate and shortened the emptying half-time of radiopharmaceutical | 90 mg/kg body weight | [70] |
| Saffron threads | Aqueous extract | Rats | Protect the kidney and liver of diabetic rats against damage caused by hyperglycemia-induced inflammation, due to its anti-inflammatory potential | 200 mg/kg of body weight | [71] | |
| Petals | Hydroalcoholic extract | Rats | Beneficial for the kidneys | 200–600 mg/kg of body weight/day | [72] | |
| Petals | Hydroalcoholic extract | Rats | Protects the kidney | 167.5 and 335 mg/kg of body weight/day | [73] | |
| Cucumis melo L | Seeds | Ethanolic extract | Mice | Renoprotective effects in gentamicin-induced renal damage | 250–500 mg/kg of body weight for 8 days | [74] |
| Leaves | Ethanol extract | Rats | Potential and effective role in inhibiting inflammation and oxidative stress in the kidney of diabetic rats | 30–120 mg/kg of body weight for 30 consecutive days | [75] | |
| Cucumis sativus L. | Pulp | Ethanol extract | Rats | Ameliorative effect on alloxan-induced kidney toxicity in male adult Wistar rats | 100–500 mg/kg of body weight for 28 days | [76] |
| Cucurbita pepo L. | Seeds | Methanol extract | Rats | Antiurolithic against sodium oxalate-induced renal calculi | In vivo (250–1000 mg/kg of body weight), in vitro (20–80 mg/mL) | [77] |
| Curcuma longa L. | Rhizomes | Ethanol extract | Rats | Effect on antioxidant enzymes in kidney of alloxan induced type-1 diabetic male rats | 250 mg/kg of body weight | [78] |
| Rhizomes | Hydro-alcoholic extract | Rats | Protective effect on adriamycin-induced oxidative stress in kidney rat | 1000 mg/kg of body weight | [79] | |
| Rhizomes | Ethanol extract | Chickens | Effect on biochemical and pathological parameters of liver and kidney in chicken aflatoxicosis | 5 mg mixed with 1 kg of diet | [80] | |
| Rhizomes | Polyphenol extract | Rats | Effect on doxorubicin-induced kidney injury in rats | 5 mg mixed with 1 g of died | [81] | |
| Cynodon dactylon (L.) Pers. | Whole plant | Aqueous extract | Rats | Against kidney stones | 12.5, 50 and 200 mg/kg of body weight | [82] |
| Daucus carota L. | Seeds | Methanol extract | Rats | Antihyperlipidemic properties et protective effect on liver and kidney function in diabetic rats | 100–300 mg/kg of body weight for 6 days using gavage) | [83] |
| Roots | Petroleum ether and methanol extract | Rats | Protective and curative potential on renal ischemia reperfusion injury in rats | 250–500 mg/kg of body weight for 14 days | [84] | |
| Carrot tuber | Aqueous extract | Rats | Hepatoprotective, hepatocurative and nephro-curative properties and could be explored in nutrition and health | 300 mg/kg of body weight for 6 weeks | [85] | |
| Eucalyptus globulus Labill. | Leaves | Methanol extract | Mice | Hepato–renal protective potential againstCyclophosphamide induced toxicity in mice | 50–100 mg/kg of body weight for 15 days | [86] |
| Leaves | Aqueous-ethanol extracts | Rats | Protective effect against acetaminophen-induced kidney damages in male rat | 130 mg /kg of body weight/day; for 42 days | [87] | |
| Foeniculum vulgare Mill. | Fruits | Aqueous extract | Rats | Inhibition of calcium oxalate renal crystals formation in rats | 4 mL/100 g body weight for 4 weeks | [88] |
| Seeds | Aqueous extract | Rats | Protect liver, kidney and gonadal functionsagainst cadmium intoxication | 150 mg/kg diet | [89] | |
| Seeds | Aqueous extract | Rats | Effect on the kidney in experimental polycystic ovary syndrome female rats | 150 mg/kg body weight for 4 weeks | [90] | |
| Globularia alypum L. | Aerial parts | Aqueous extract | Rats | Decreases hypertriglyceridemia and ameliorates oxidative status of the muscle, kidney, and heart in rats fed a high-fructose diet | 0.5% in diet | [91] |
| Whole plant | Chloroform, ethyl acetate and aqueous extracts | Mice | Protective effect against oxonate-induced hyperuricemia and renal dysfunction in mice | 100 mg/kg of body weight | [92] | |
| Glycyrrhiza glabra L. | Roots | Powder | Rats | Metabolic effects on lipid distribution pattern, liver and renal functions of albino rats | 5–10% of Powder in diet | [93] |
| Roots | Aqueous extract | Rats | Effect of licorice on adrenal-kidney pituitary axis in rats | 100–500 mg/kg of body weight for 15 consecutive days | [94] | |
| Helianthus annuus L. | Roots | Petroleum ether extract | Rats | Ameliorative potential on hepatoprotective and some kidney function indices of alloxan induced diabetic rats | 100–300 mg/kg of body weight for three weeks | [95] |
| Herniaria hirsuta L. | Aerial parts | Hydro-ethanolic and aqueous extracts | Oxalo-calcic and cystine stones of patients | Dissolution of oxalo-calcic and cystine stones | 0.5% of plant extracts in physiological solution (9 g of NaCl /L) |
[96] |
| Aerial parts | Aqueous extract | Human urine samples | Promoted the precipitation of calcium oxalate particles in urine | 0.0625–1 mg/mL | [97] | |
| Aerial parts | Aqueous extract | Rats | Against calcium oxalate stones induced by ethylene glycol and ammonium chloride | Final concentration was 50 mg/mL (rats received 1 mL/day of extract for 14 days) | [98] | |
| Aerial parts | Aqueous extract | Rats | Against calcium oxalate urolithiasis risk in rats | The water supply was replaced with an infusion of 4 g/L of plant for 7 days | [99] | |
| Aerial parts | Aqueous extract | Renal epithelial cells of the Madin Darby canine kidney (MDCK) line | Against adhesion of calcium oxalate monohydrate crystals to cultured renal cells | 200 to 800 µg/mL | [100] | |
| Aerial parts | Aqueous extract | patients | Against cystine stones in different patients with congenital cystinuria | Placing calculations and fragments of calculations cystine in the presence of 20 mL of extract plant for 8 weeks | [101] | |
| Hordeum vulgare L. | Seeds | Aqueous and alcoholic seed extracts | Rats | Against ethylene glycol and ammonium chloride-induced urolithasis in rats | 200–300 mg/kg of body weight for 35 days | [102] |
| Lactuca sativa L. | Aerial parts | Essential oil | Rabbits | Beneficial effect for the functions and histology of the kidneys | 0.1–0.2 mL/kg orally for 17 days |
[103] |
| Lawsonia inermis L. | Leaves | Ethanol extract | Rats | Decreased blood glucose level and was able to restore the kidney destruction of alloxan-induced diabetic rats | 400–600 mg/kg of body weight for 28 days | [104] |
| Lepidium sativum L. | Seeds | Aqueous extract | Rats | Protective effect against aluminum-induced liver and kidney effects in albino rat | 20 mg/kg of body weight for 8 weeks | [105] |
| Seeds | Aqueous extract | Rats | Effect on renal glucose reabsorption and urinary TGF-β1 levels in diabetic rats | 20 mg/kg of body weight | [106] | |
| Linum usitatissimum L. | Seeds | Ethanolic extract | Rats | Renoprotective effect through hemodynamic changes and conservation of antioxidant enzymes in renal ischemia/reperfusion injury in rats | 200 mg/kg and 400 mg/kg for 4 weeks |
[107] |
| Seeds | Aqueous and methanolic extract | Rats | Increased serum estradiol, progesterone, total proteins, total cholesterol, ALT and AST activity, and decreased ovarian cholesterol levels, while it had no effect on kidney function in immature female rats | 500 mg/kg daily for 14 days | [108] | |
| Unspecified | Essential oil | Rats | Ameliorative effects on roundup-induced biochemical and histopathological changes in the liver and kidney of rats | 0.5 g/kg of body weight | [109] | |
| Morus alba L. | Leaves | Methanol extract | Mice | Antioxidant effect on kidney, testes, spleen and intestine of mice | 200–800 mg/kg of body weight for 10 days | [110] |
| Leaves | Aqueous extract | Rats | Ameliorative effect against diabetes-induced changes in kidney | 1 g/100 g of diet | [111] | |
| Leaves | Acetone extract | Rats | Ameliorative effect on urine creatinine levels and histology of diabetic rat kidney | 90–150 mg/Kg of body weight for 14 days | [112] | |
| Leaves | Methanol extract | Mice | Ameliorative effect against Schistosoma mansoni-induced renal and testicular injuries in mice | 200–800 mg/kg of body weight/day for 10 days | [113] | |
| Nigella sativa L. | Whole plant | Essential oil | Rabbits | Against oxytetracycline-induced hepato-renal toxicity in rabbits | 2 mL/kg of body weight | [114] |
| Seeds | Aqueous and ethanol extracts | Rats | Protective effect on renal ischemia-reperfusion-induced oxidative damage in rats | 0.7, 1 and 1.6 g/kg of body weight | [115] | |
| Seeds | Ethanol extract | Rats | Nephroprotective effect in cisplatin-induced renal injury | 50 mg/kg of body weight | [116] | |
| Seeds | Aqueous extract | Rats | Significantly prevented renal ischemia/reperfusion induced functional and histological injuries | 1 g/kg of body weight | [117] | |
| Seeds | Ethanol extract | Rats | Protective effect against cisplatin-induced renal toxicity and oxidative stress in wistar rats | 100–200 mg/kg of body weight for 5 days | [118] | |
| Ocimum basilicum L. | Aerial parts | Hydroalcoholic extract | Rats | Against cisplatin models of acute renal failure | 100–500 mg/kg of body weight | [119] |
| Aerial parts | Essential oils | Rats | Renoprotective effect against diabetes induced renal affection in albino rats | 500 mg/kg of body weight/day; given to rats through gastric tube for six weeks) | [120] | |
| Leaves | Ethanolic extract | Rats | Hepato-renal protective against paracetamol toxicity in rat model | 200–400 mg/kg of body weight; once daily for 30 consecutive days) | [121] | |
| Aerial parts | Hydroalcoholic extract | Rats | Decreased cell injury and apoptosis and preventive effect in kidney tissue damages produced by exposure to electromagnetic field in rats | 1.5g/kg of body weight for 40 consequence day | [122] | |
| Olea europaea L. | Leaves | Ethanol extract (oleuropein) | Rats | Improvement of blood pressure and cardiac performances, but tends to retain elevated vascular resistance, therefore, reducing the inflow of blood into the brain and kidneys of the spontaneously hypertensive rats | 25–50 mg/kg of body weight | [123] |
| Leaves | Ethanol extract | Human rhabdmyosarcom cells (RD) (line CCL-136) | Antitumoral activity and the cytotoxicity on renal cells | IC50 (75.6 μg/mL) | [124] | |
| Leaves | Unspecified | Rats | Protective effect against oxidative stress injury generated with renal ischemia reperfusion | 100–200 mg/kg of body weight for 15 days | [125] | |
| Leaves | Ethanol extract | Rats | Up-regulates Nrf2/ARE/HO-1 signaling and attenuates cyclophosphamide-induced oxidative stress, inflammation and apoptosis in rat kidney | 100–200 mg/kg of body weight for 15 days | [126] | |
| Leaves | Essential oil | Rats | Benificial effects on the adrenal-kidney-pituitary axis in rats | 100–500 μg/kg of body weight for 14 consecutive days | [127] | |
| Opuntia ficus-indica (L.) Mill. | Cladodes | Aqueous extract | Rats | Diuretic effect on rats, and the lyophilized extract has a diuretic and hypotensive effect on normotensive rabbits without deterioration in renal function test | 100 mg/kg of body weight | [128] |
| Cladodes | Aqueous extract | Rats | Nephroprotective effect on sodium dichromate-induced kidney injury in rats | 100 mg/kg of body weight for 40 days | [129] | |
| Fruits | Prickly pear juice | Rats | Alleviates ethanol-induced kidney injury in rats | 20 and 40 mL/kg of body weight | [130] | |
| Origanum majorana L. | Whole plant | Essential oil | Rats | Protective effect on hepatic and renal toxicities induced by nickel chloride in male albino rats | 0.5 mL/kg of body weight for 4 weeks | [131] |
| Petroselinum crispum (Mill.) Fuss | Fruits | Fresh celery | Women and men urine | Effect on urinary apigenin excretion in human subjects | 20 g parsley/10 mL/days | [132] |
| Leaves and stems | Ethanolic extract | Rats | Protective effects on ischemia/reperfusion-induced acute kidney injury | 100–200 mg/kg of body weight | [133] | |
| Seeds | Ethanolic extract | Rats | Protective effect on histopathological changes in kidney induced by sodium valproate in male rats | 200 mg/kg of body weight/day for 7 weeks | [134] | |
| Leaves | Parsley juice | Mice | Improving effect against cadmium induces changes in lipid profile, lipid peroxidation and catalase activity in kidneys of male albino mice | 0.1 mL of parsley juice/days | [135] | |
| Leaves | Aqueous extract | Rats | Attenuates serum uric acid level and improves liver and kidney structures in oxonate-induced hyperuricemia rats | 3.5–10.5 g/kg of body weight/day | [136] | |
| Pimpinella anisum L. | Unspecified | Essential oil | Rats | Decreased the toxicity of aspartame-induced hepatorenal toxicity | 0.5 mL/kg of body weight/day; for 2 months | [137] |
| Pistacia lentiscus L. | Fruits | Essential oil | Rabbits | Safe with no adverse effect on liver functions and renal functions with possible anti-glycogenesis activity | 1 mL/kg of body weight for 6 consecutive weeks | [138] |
| Punica granatum L. | Fruits | Juice and methanol extract | Rats | Antioxidant properties of pomegranate in hepatic and renal tissues of rats | Juice (3 mL/kg body weight; for 21 days), MtOH (200 mg/kg; body weight; for 21 days) | [139] |
| Fruits | Juice | Rats | Reduces lead-induced cell damage in kidney, liver and heart tissue | 30–60 μL/days for 5 weeks | [140] | |
| Seeds | Juice | Rats | Improving effect on diabetes-induced changes in kidney | 7.5% of pomegranate seeds in an AIN-76 diet, for a period of two months. | [111] | |
| Seeds, fruits and peel | Peel MtOH, SOE, fruit juices | Rats | Effects on apoptosis in rat kidney induced by diethylnitrosamine and phenobarbital | Peel MtOH (250 mg/kg; body weight), Fruits juice (250 mg/kg; body weight), SOE (2 mL/kg; body weight) | [141] | |
| Fruits | Pomegranate juice and methanolic extract of peel | Mice | Improving effect on steroid induced proximal and distal tubular dilatation in mice kidney | Juice (3mL/kg of body weight, for 8 weeks), MtOH peel extract (200 mg/kg of body weight, for 8 weeks) | [142] | |
| flowers | Hydroalcoholic extract | Rats | Against glycerol-induced acute renal failure in rats | 125 and 250 mg/kg of body weight twice daily for 3 days | [143] | |
| Rosa canina L. | Fruit | Ethanolic extract | Rats | Protective effects on renal disturbances induced by reperfusion injury in rats | 2700 mg/kg of body weight in 3 mL volume through gavage for 7 days | [144] |
| Rosmarinus officinalis L. | unspecified | Tosemary extract containing 40% carnosic acid | rats | Protective effect against etoposide-induced changes in liver and kidney functions, and DNA damage in rats | 220 mg/kg of body weight /twice weekly | [145] |
| Leaves | Essential oil | Mice | Ameliorants effect on histology and biological parameters of liver and kidney | 100–400 mL | [146] | |
| Leaves | Aqueous extract | Rats | Improving effect on kidney and liver of diabetic rats | 0.2 mg/mL/day for 30 days | [147] | |
| Salvia officinalis L. | Leaves | Aqueous extract | Mice | Effects on development of mice embryos kidney and some hormonal effect of treated mothers | 83.9, 167.8 mg/kg; body weight for 6 weeks | [148] (p. 42) |
| Leaves | Essential oil | Mice | Protective effects against hyperlipidemia, liver, and kidney injuries in mice submitted to a high-fat diet | 4 mg/kg body weight for 8 weeks | [149] | |
| Leaves | Ethanol extract | Rats | Preventive effects on chlorpyrifos-and methomyl-induced renal toxicity and oxidative stress in albino rats | 50 mg/kg body weight for 4 weeks | [150] | |
| Leaves | Essential oil | Mice | Protective role against carbon tetrachloride-induced liver and kidney damage in mice | 0.1, 0.2, and 0.4 mL/kg body weight for 2 weeks | [151] | |
| Syzygium aromaticum (L.) Merr. and L.M.Perry | Clove | Clove oil | Rats | Protective role against acrylamide induced oxidative damage and impairment of liver, kidney, and testicular functions in albino rats | 100 and 200 mg/kg of body weight for 21 consecutive days | [152] |
| Trigonella foenum-graecum L. | Seeds | Aqueous extract | Rats | Protective effect on kidney function and morphology in diabetic rats via its antioxidant activity | 440–1740 mg/kg of body weight for 6 weeks | [153] |
| Seeds | Ethanol extract | Rats | Protective effect against carbon tetrachloride-induced toxicity in liver and kidney of male rat | 10% in pellet rat feed for 7 weeks |
[154] | |
| Seeds | Powder | Rats | Against ethylene glycol-induced kidney stone in rats | 10 g of fenugreek in 100 mL of water and 10 g in 100 g of standard diet |
[155] | |
| Seeds | Aqueous extract | Rats | Attenuated radiation-induced oxidative stress in liver and kidney tissues | 1 g/kg of body weight during 7 days before irradiation | [156] | |
| Urtica dioica L. | Leaves | Aqueous extract | Rats | Effects on the expression level of cyclooxygenase-2 and caspase-3 in the liver and kidney of streptozotocin-induced diabetic rats | 100 mg/kg of body weight/daily | [157] |
| Leaves | Methanolic extract | Rats | Ameliorative effect on acute kidney injury induced by gentamicin in rats | 200 mg/kg of body weight/day | [158] | |
| Leaves | Aqueous extract | Rats | Effects on some blood and urine parameters, and liver and kidney histology in diabetic rats |
0.5% infusion of the leaves | [159] | |
| Vitis vinifera L. | Grape seeds | Aqueous extract | Mice | Protective role in some biochemical parameters and histological changes in methionine for liver, kidney and heart in mice (Mus musculus) | 10–30% mg/kg of body weight during 30 days | [160] |
| Zea mays L. | Stigmata | Aqueous extract | Rats | Antilithiatic effects | The water supply was replaced with an infusion of 2g/L of plant for 7 days | [161] |
| Corn silk, leaves | Ethanolic extract | Rats | Improved kidney failure in rat model induced by gentamicin | 75 mg/kg of body weight for 4 weeks | [162] | |
| Corn silk | Aqueous extract | Human urine samples | Solubility of calcium in kidney stones and diuretic effect | 2–10% of infuse solution | [163] | |
| Zingiber officinale Roscoe | Fresh ginger | Powder | Rats | Protective effect against kidney damage in rats | 2.5–5.0% powder of ginger | [164] |
| Fresh ginger | Aqueous extract | Mice | Protective effect against injury in the kidney of mice treated with CCL4 | 500 mg/kg of body weight | [165] | |
| Fresh ginger | Hydro-alcoholic extract | Rats | Effects on treating lead-poisoned kidney of neonatal rats. |
2 g/kg of body weight | [166] | |
| Rhizomes | Ethanol extract | Mice | Protective effect on acute renal failure induced by cisplatin and liver of rats exposed to carbendazim | 250 mg/kg of body weight | [167] | |
| Rhizomes | Powder | Rats | Effects on some physiological parameters and kidney structure in rats | Rats fed with diet contain 5% ZOR Roscoe |
[168] | |
| Rhizomes | Aqueous extract | Rats | Alleviate liver and kidney dysfunctions and oxidative stress induced by mercuric chloride in male rats | 125 mg/kg of body weight | [169] | |
| Rhizomes | Aqueous extract | Rats | Ameliorative effect on the cadmium-induced liver and kidney injury in females’ rats | 2 g/L for 40 days | [170] |
Among 121 medicinal plants listed in our survey, three plant species, H. hirsuta, A. graveolens, and P. crispum have been the most cited by North-Eastern Morocco people to treat or prevent the traditionally multiple forms of kidneys. In the following paragraphs, we will discuss the potential of these three plants to validate their activity against kidney disorders:
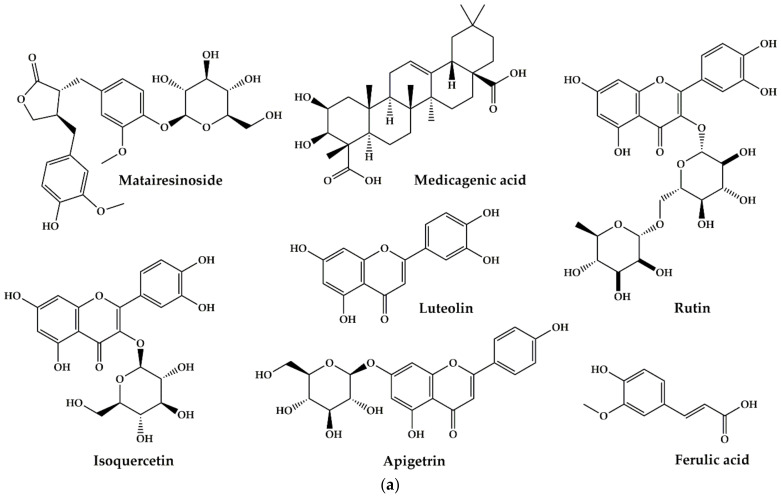
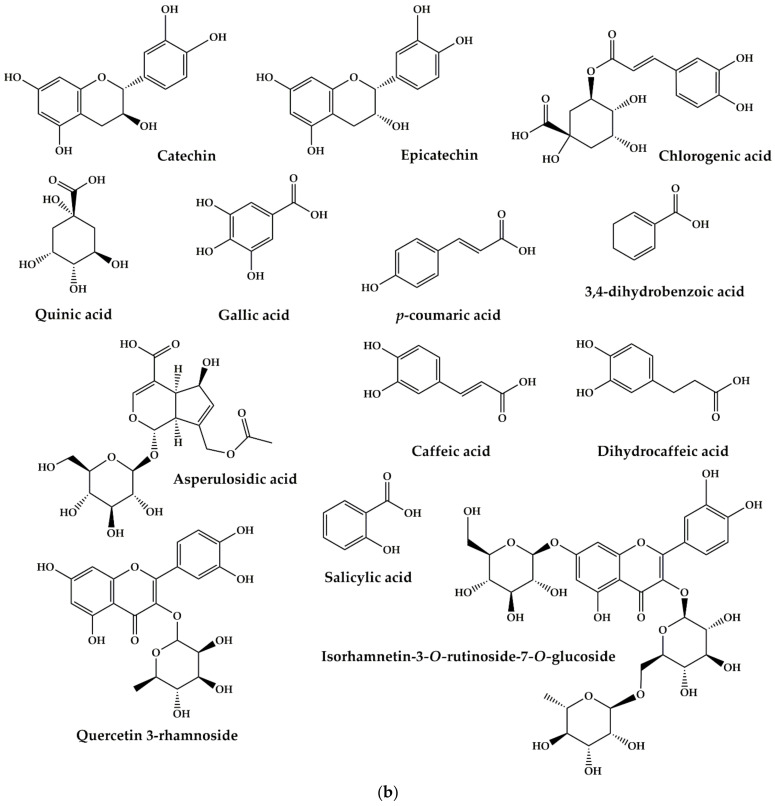
H. hirsuta is ranked first as the most cited plant (14.29% of total use reports). According to the traditional knowledge of the North-Eastern Moroccan population, this plant is considered a powerful and common medicinal herb that has shown significant results in treating kidney stones; renal colic; pyelonephritis; kidney pain; diuretic; detoxification of the kidneys; and polycystic kidney disease. From a pharmacological point of view, the aqueous extract of the aerial part of this plant has an inhibitory effect on the crystallization of calcium oxalate in vitro at doses of 0.0625 mg/mL and 0.5% of plant extracts in physiological solution (9 g of NaCl /L) [96,97], and in vivo at a concentration of 50 mg/mL [98], also has an effect on cystine stones in different patients with congenital cystinuria at a dose of 20 g/L [101]. Phytochemical studies have reported and identified some components of H. hirsuta include flavonoids, coumarin, tannins and saponins [100,171,172,173]. The active component in the prevention of lithiasis has not yet been identified. However, the literature suggests that the antilithiatic potential of H. hirsuta is attributed to saponins with a high probability [171,174]. Recently, a phytochemical study conducted to identify the bioactive constituents of H. hirsuta has shown that the aerial part of this plant is rich in phenolic compounds (Figure 7a,b) [171]. According to the literature, these compounds are well known for various pharmacological effects [175,176,177,178,179]. Therefore, the antilithiasic activity of H. hirsuta may be due to the presence of these compounds.
Figure 7.
(a) Bioactive compounds found in Herniaria hirsuta L. extracts; (b) Bioactive compounds found in Herniaria hirsuta L. extracts.
Apium graveolens L. is ranked second, with a percentage of citations of (9.57% of total use reports). It is commonly used to treat several kidney problems: improved kidney performance, kidney swelling, kidney stones, kidney detoxification, kidney pain, diuretic, renal colic, and renal polycystic. The aerial part of Apium graveolens L. accentuates urinary excretion of Ca2+ in an experimental model of nephron-calcinosis in rabbits at an amount of 8 g/kg added to the animal feed [48]. The ethanolic extract from the stem and leaves of Apium graveolens L. demonstrated in vivo a protective effect on kidney damage in the model of rats with ischemia/reperfusion at a dose of 1000 mg/kg body weight [49]. The ethanolic extract and essential oils of fruits of Apium graveolens L. have a diuretic effect in vivo in dogs at doses (25 mg/kg; b.w) for the ethanolic extract and (0.004 mL/kg; b.w) for essential oils [50]. The presence of phenolic compounds in the parts of Apium graveolens L. is the reason why celery is the plant most used in traditional medicine [180,181]. Previously published photochemical studies have shown that extracts of Apium graveolens L. are rich in bioactive compounds such as polyphenols and flavonoids [182,183] (Figure 8). It is well known that these secondary compounds present in Apium graveolens L. have considerable pharmacological activities, suggesting that the activities mentioned below may be due to these secondary metabolites.
Figure 8.
Bioactive compounds from Apium g. extracts.
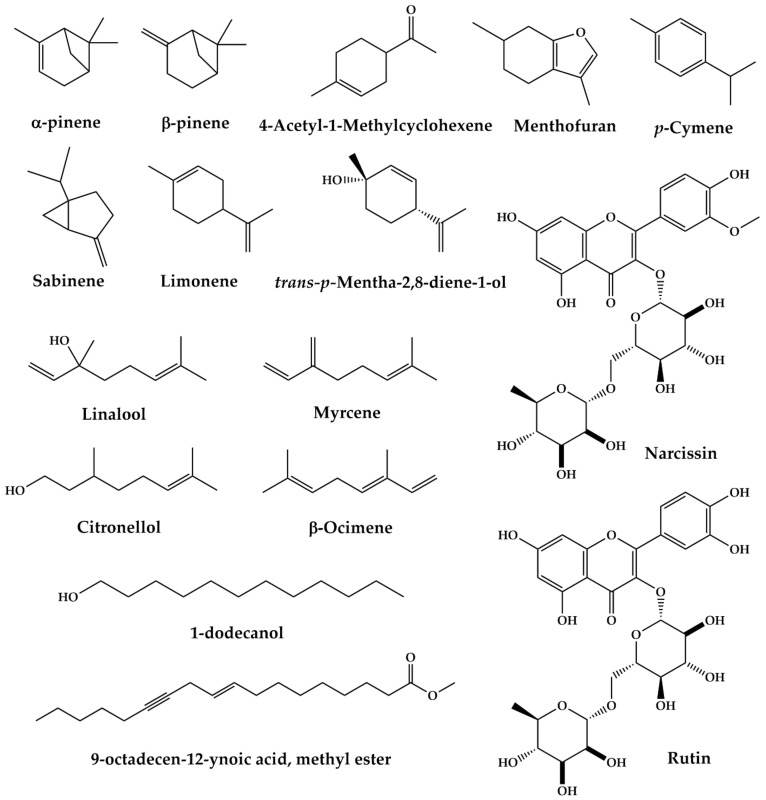
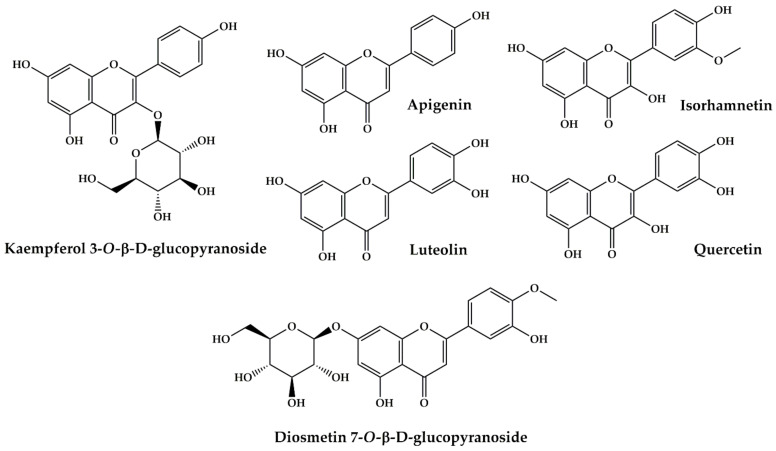
P. crispum is ranked third as the most cited plant with 7.00% of total use reports. The North-Eastern people of Morocco use this plant against kidney stones, renal colic, and kidney inflammation. The ethanolic extract from the leaves and stem of P. crispum has protective effects on acute renal damage induced by ischemia/reperfusion in vivo in rats at doses 100, 150, and 200 mg/kg body weight [133]. At a 200 mg/kg bodyweight concentration, the seeds ethanolic extract showed a protective effect on histopathological changes in the kidneys induced by sodium valproate in male rats [134]. The juice of P. crispum has an ameliorative effect against cadmium-induced changes in lipid profile, lipid peroxidation, and catalase activity in the kidneys of albino male mice [135]. The aqueous extract from these plant leaves attenuates serum uric acid levels and improves liver and kidney structures in oxolane-induced hyperuricemia rats at doses 3.5, 7.0, 10.5 g/kg of the body weight [136]. Indeed, the pharmacological properties of P. crispum are mainly discussed by a wide range of active biomolecules present in this plant. Phytochemical constituents of P. crispum were isolated from seeds, roots, leaves or petioles through different separation methods [184]. These phytochemical constituents can be grouped into flavonoids, carbohydrates, coumarins, essential oils and other various compounds. A literature review conducted by Agyare et al. (2017) shows that flavonoids are the most dominant compounds of P. crispum such as isorhamnetin, apigenin, quercetin, luteolin, diosmetin 7-O—D-Glucopyranoside, kaempferol 3-O-β-d-glucopyranoside (Figure 9) [184]. These phytochemicals may be at the origin of the pharmacological activities of P. crispum against the kidney disorders mentioned above.
Figure 9.
Bioactive compounds from Petroselinum crispum (Mill.) Fuss extracts.
2.7. Constraints of Medicinal Plant’ Uses
Adherence to traditional medical practices by people living in remotes areas of North-Eastern Morocco should be taken with great care. Skepticism, which is, in most cases, based on personal or peer experience regarding the use of medicinal plants, especially the safety and efficacy of the herbal treatment of renal disease. Some medicinal plants’ toxicity, which some users often overlook because of the incorrectly held belief that herbal medications are innocuous, remained critical. The use of medicinal plants faces many problems related to these herbs’ safety that could harm health. This conception was identified as a barrier to some participants that they felt distraught. We know that folk medicine, especially herbal medications, lack the required essential standards of consistency in the pharmacologically active principles of second metabolites containing in these herbs. Besides, the incorrect identification leading to substitution of an innocuous herb, the process of extraction, the adulteration, and the standardization of the use of these herbs contribute to the dangerousness of these herbs.
Several assumptions confirm these. Our team reported that some common plants used as medicine by people in North-eastern Morocco, such as A. baetica and B. dioica, have evidence of significant concern nephrotoxicity [185]. According to these authors, this toxicity’s causal factors are threefold; the substitution, the misidentification, and the toxic compounds containing in these two species (Aristolochic acid in A. baetica and cucurbitacin in B. dioica). Another work, published recently by our team reviewed toxic plants in the region, indicated that out of 287 medicinal plants used by local people, 87 plant species had been identified as toxic [186]. In the current work, we found that out of 121 medicinal plant species used traditionally to treat renal diseases, only seven plant species have been identified as toxic by the respondents. The information reported during our interview showed that E. spinosissimus and B. cretica subsp. Dioica, used separately, could have toxic effects targeting the nervous system leading to excitement and convulsive effects; the consumption of L. usitatissimum could have some physiological turbulence such as colic, numbness, and/or respiratory acceleration; A. succotrina, when ingested, could have intense organic congestion, eczematous dermatitis, the bulbs of D. maritima, in decoction, causes digestive disorders with vomiting, the use of C. litoralis subsp. telephiifolia. at high dose could have intense diarrhea, and prolonged treatment with the aerial part of G. glabra can lead to the digestive system’s neuronal toxicity and disorders. The literature review revealed that among the seven species cited as toxic by the local population, three species were studied for their toxic effects on the laboratory. Hydroethanolic extract of the roots of C. litoralis subsp. telephiifolia. Showed toxicity in mice with the oral mean lethal dose (LD50) value of 14,000 mg/kg body weight [187]. D. maritima showed a cytotoxic effect against cancer cells of different lines as in the cell line of non-small cell lung cancer A549 (NSCLC) with IC50 = 0.02 µg/mL and in human cervical cancer cell lines Siha and Hela, hosting HPV16 and HPV 18, respectively [188,189]. The aqueous extract of the roots of B. cretica subsp. Dioica. showed cytotoxic and apoptogenic activity in Burkitt BL41 lymphoma cell lines at a dose of 125 g/mL [187].
From these observations, we can deduce that although these plants were used traditionally by local people for the treatment of renal diseases and are considered to be safe, for some respondents, they may cause damage due to their unwanted side effects. Therefore, studying medicinal plants’ side effects would have an influential role in identifying and diagnosing the herbs’ safety profile. So, medicinal plants’ consumption without studies of efficacy and safety can result in several side effects that may affect people’s health.
3. Materials and Methods
3.1. Study Area
The study was conducted in North-Eastern Morocco (Figure 10). This region is limited in the North by the Mediterranean Sea (200 km of coastline), in the East by Moroccan-Algeria fronter, in the south by part of the desert (Figuig province), and in the west by a part of middle Atlas (Taza province). The region includes Benisnassen, Rif, and Horst’s mountainous area, culminating respectively to 1800 m and 1500 m. These geographical features provide the region with a Mediterranean climatic zone that is characterized by hot and dry summers while winters are more cool and wet with average rainfall between 100 mm per year in the South (Saharan bioclimatic zone) and 400 mm per year in the North (Influenced by the Mediterranean Sea). Additionally, the region encompasses several Sites of Biological and Ecological Interest (SBEI) and protected areas such as Benisnassen, Jbel Gorougou. Indeed, these sites had already been identified for their original flora as well as for their biological and ecological qualities [4]. According to the national census conducted in 2014, the region’s total area is 90,130 km2, representing 12% of the national territory. Historically, North-Eastern Morocco people have a shared cultural past dating back to the Arab civilization in the seventh century. The cumulative traditional culture, related to ethnobotanical knowledge, has been maintained until now and constitutes the basis for the region’s traditional medical system [6,7].
Figure 10.
Geographical location of the study area.
3.2. Ethnobotanical Survey
In order to collect the traditional knowledge about medicinal plants used by people living in the study area, an ethnobotanical survey was conducted from February 2018 to January 2020 in thirteen rural communes of the North-Eastern region of Morocco (Table 2) spread over six provinces (Guercif, Taourirt, Jerada, Berkane, Nador, Oujda-Angad). The ethnobotanical data were randomly selected at thirteen sites visited by conducting semi-structured interviews with 476 respondents from the local population and 12 traditional herbalists. The application of simple random sampling achieved the selection of informants. This sampling technique has the main advantage of ensuring the representativeness of the population. Informants who do not live in the study area are excluded from this study. The questionnaire used consists of two parts: the first one focused on the demographic characteristics of the participants (age, gender, level of education, ethnomedicinal knowledge sources and income of participants…), and the second one focuses on the plant species used in popular medicine for the treatment of kidney disease (vernacular name, parts used, methods of preparation and route of administration).
3.3. Identification of Medicinal Plant Species
All local names of plants collected during this study were translated into botanical names, based on the following references [7,190]. For the authentication and the accuracy of plant names listed in this paper of scientific names, we consulted documents specializing in the taxonomy of Moroccan flora (Then, the identification was performed by using standard floras available in Morocco [191,192,193,194,195,196]. For the accuracy and authentication of the scientific nomenclature, the plants recorded were checked against database available online: Catalogue of Life: 2019 Annual Checklist (https://www.catalogueoflife.org/col/) (accessed on 13 April 2020), the Plant List (http://www.theplantlist.org/) (accessed on 13 April 2020) and African Plant Database (http://www.ville-ge.ch/musinfo/bd/cjb/africa/recherche.php) (accessed on 15 April 2020). Only the plant names accepted in these databases were retained. Following the Angiosperm Phylogeny Group IV (2016), the plant families listed in this paper were checked with database APG-IV 2016 [197].
Once the name of each plant species selected was identified correctly, the whole or a part of the picked plants were pressed with a plant press and dried properly. A voucher number was attributed to each specimen and deposited in the Herbarium (HUMPOM), at Mohammed first University, Oujda, Morocco.
3.4. Quantitative Data Analysis
3.4.1. Medicinal Use Value (UV)
To give the relative importance of each plant species known locally to be used in popular medicine, we calculated the use-value (UV) for each species. This index was calculated using the following formula [198]:
| (1) |
where UV = use value of species, U = number of quotations per species, N = number of informants.
The value of UV will be higher if there are many reports of use for a plant, which implies that the plant is important, while they will be close to zero if there are few reports related to its use.
3.4.2. Botanical Family Use Value (FUV)
In order to assess the relationship between botanical families and users of species belonging to these families, we used the index called Family Use Value (FUV) which is equal to the average total use value for each species in the family [199].
| (2) |
where FUV = family use value, which equals the average total use value for each species in the family, UV = use value of the species belonging to the family, N = number of species in the family.
3.4.3. Informant Consensus Factor (ICF)
To know about informants’ agreement and consensus, we calculated Index Consensus Factor (ICF) by using the following formula [200]:
| (3) |
where Nur is the number of use-reports for a particular ailment category, Nt refers to the number of taxa used for a particular ailment category by all informants.
The ICF values’ margin varies between 0 and 1, where values close to 0 show that the plants are randomly selected or that there is no exchange of information on their use among the informants. Values close to 1 are obtained when there is a well-defined selection criterion within the given community and/or if the information is exchanged between informants.
3.5. Bibliographic Review
A review of the available literature on the plants’ biological activities identified against kidney disease was undertaken using the following electronic databases: PubMed, Science Direct, Google Scholar, Scopus, and Web of Science using the following keywords “kidney disease”, “renal disease”, “renal insufficiency”, “nephropathy”, combined with the scientific name of the plant. Chemical structures of plant compounds were performed by Chem Draw 18.1 software.
4. Conclusions
This survey showed that people living in North-Eastern Morocco’s remote areas still use medicinal plants to treat ailments, especially renal diseases. The choice of these people was based on their socio-economic and cultural conditions. This preference offered the best chance for them to manage renal sequelae. The people in the study region found that traditional uses of medicinal plants possess suitable healing properties. The results demonstrate the promising role of medicinal plants in managing this particular health problem for these users. However, this preference should be taken with great care. To confirm their therapeutic uses, more investigations are needed to approve the safety and efficacy of their bioactive compounds. Additionally, in predicting the traditionally believed effects of these herbs, researchers need to find out the actuality of their clinical effectiveness and active substances. Once the positive effects of these herbs were proved to be accurate, it is possible to produce drugs useful in the treatment of renal disorders.
Author Contributions
Conceptualization, N.B. and M.E.; methodology, N.B. and A.E.; software, L.K., C.H., M.A., H.O. and M.B.; validation, B.E. and I.A.M.; investigation, N.B. and A.E. data curation, H.M. and I.E.-s.; writing—original draft preparation, N.B.; supervision, M.E. All authors have read and agreed to the published version of the manuscript.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data are included in the present study.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajalakshmi S., Vijayakumar S., Arulmozhi P. Ethnobotanical Survey of Medicinal Plants in Thanjavur and Its Surrounding (Tamil Nadu-India) Acta Ecol. Sin. 2019;39:380–397. doi: 10.1016/j.chnaes.2018.09.010. [DOI] [Google Scholar]
- 2.Bencheikh N., Bouhrim M., Merrouni I.A., Boutahiri S., Kharchoufa L., Addi M., Tungmunnithum D., Hano C., Eto B., Legssyer A., et al. Antihyperlipidemic and Antioxidant Activities of Flavonoid-Rich Extract of Ziziphus Lotus (L.) Lam. Fruits. Appl. Sci. 2021;11:7788. doi: 10.3390/app11177788. [DOI] [Google Scholar]
- 3.Alami Merrouni I., Elachouri M. Anticancer Medicinal Plants Used by Moroccan People: Ethnobotanical, Preclinical, Phytochemical and Clinical Evidence. J. Ethnopharmacol. 2021;266:113435. doi: 10.1016/j.jep.2020.113435. [DOI] [PubMed] [Google Scholar]
- 4.Fakchich J., Elachouri M. An Overview on Ethnobotanico-Pharmacological Studies Carried out in Morocco, from 1991 to 2015: Systematic Review (Part 1) J. Ethnopharmacol. 2020;267:113–200. doi: 10.1016/j.jep.2020.113200. [DOI] [PubMed] [Google Scholar]
- 5.Ahmed M.J., Akhtar T. Indigenous Knowledge of the Use of Medicinal Plants in Bheri, Muzaffarabad, Azad Kashmir, Pakistan. Eur. J. Integr. Med. 2016;8:560–569. doi: 10.1016/j.eujim.2016.01.006. [DOI] [Google Scholar]
- 6.Ziyyat A., Legssyer A., Mekhfi H., Dassouli A., Serhrouchni M., Benjelloun W. Phytotherapy of Hypertension and Diabetes in Oriental Morocco. J. Ethnopharmacol. 1997;58:45–54. doi: 10.1016/S0378-8741(97)00077-9. [DOI] [PubMed] [Google Scholar]
- 7.Fakchich J., Elachouri M. Ethnobotanical Survey of Medicinal Plants Used by People in Oriental Morocco to Manage Various Ailments. J. Ethnopharmacol. 2014;154:76–87. doi: 10.1016/j.jep.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 8.Ammor K., Mahjoubi F., Bousta D., Chaqroune A. Ethnobotanical Survey of Medicinal Plants Used in the Treatment of Kidney Stones in Region of Fez-Meknes, Morocco. Ethnobot. Res. Appl. 2020;19:1–12. doi: 10.32859/era.19.50.1-12. [DOI] [Google Scholar]
- 9.Jouad H., Haloui M., Rhiouani H., El Hilaly J., Eddouks M. Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes, Cardiac and Renal Diseases in the North Centre Region of Morocco (Fez–Boulemane) J. Ethnopharmacol. 2001;77:175–182. doi: 10.1016/S0378-8741(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 10.Khouchlaa A., Tijane M., Chebat A., Hseini S., Kahouadji A. Enquête ethnopharmacologique des plantes utilisées dans le traitement de la lithiase urinaire au Maroc. Phytothérapie. 2017;15:274–287. doi: 10.1007/s10298-016-1073-4. [DOI] [Google Scholar]
- 11.Es-Safi I., Mechchate H., Amaghnouje A., Jawhari F.Z., Bari A., Cerruti P., Avella M., Andriy A., Andriy D. Medicinal Plants Used to Treat Acute Digestive System Problems in the Region of Fez-Meknes in Morocco: An Ethnopharmacological Survey. Ethnobot. Res. Appl. 2020;20:1–14. doi: 10.32859/era.20.25.1-14. [DOI] [Google Scholar]
- 12.Kharchoufa L., Bouhrim M., Bencheikh N., Addi M., Hano C., Mechchate H., Elachouri M. Potential Toxicity of Medicinal Plants Inventoried in Northeastern Morocco: An Ethnobotanical Approach. Plants. 2021;10:1108. doi: 10.3390/plants10061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laadim M., Ouahidi M., Zidane L., Hessni A., Ouichou A., Mesfioui A. Ethnopharmacological Survey of Plants Used for the Treatment of Diabetes in the Town of Sidi Slimane (Morocco) J. Pharmacogn. Phytother. 2017;9:101–110. doi: 10.5897/jpp2016.0437. [DOI] [Google Scholar]
- 14.Tahraoui A., El-Hilaly J., Israili Z.H., Lyoussi B. Ethnopharmacological Survey of Plants Used in the Traditional Treatment of Hypertension and Diabetes in South-Eastern Morocco (Errachidia Province) J. Ethnopharmacol. 2007;110:105–117. doi: 10.1016/j.jep.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Benkhnigue O., Zidane L., Fadli M., Elyacoubi H., Rochdi A., Douira A. Etude Ethnobotanique Des Plantes Médicinales Dans La Région de Mechraâ Bel Ksiri (Région Du Gharb Du Maroc) Acta Bot. Barcinonensia. 2010;53:191–216. [Google Scholar]
- 16.Chaachouay N., Benkhnigue O., Fadli M., El Ibaoui H., Zidane L. Ethnobotanical and Ethnopharmacological Studies of Medicinal and Aromatic Plants Used in the Treatment of Metabolic Diseases in the Moroccan Rif. Heliyon. 2019;5:e02191. doi: 10.1016/j.heliyon.2019.e02191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benkhnigue O., Hachi M., Fadli M., Douira A., Zidan L. Catalogue of the Medicinal Plants Used in the Treatment of Urinary Infections in the Area of Al-Haouz Rhamna (Central Morocco) Eur. J. Bot. Plant Sci. Phytol. 2016;3:1–49. [Google Scholar]
- 18.Ghourri M., Zidane L., Douira A. La Phytothérapie et Les Infections Urinaires (La Pyélonéphrite et La Cystite) Au Sahara Marocain. J. Anim. Plant Sci. 2014;20:3171–3193. [Google Scholar]
- 19.Chebaibi M., Bousta D., Iken I., Hoummani H., Ech-Choayeby A., Najdi A., Houssaini T.S., Achour S. Ethnopharmacological Survey of Medicinal Plants Used in Traditional Treatment of Kidney Diseases in Fez–Meknes Region, Morocco. Phytothérapie. 2020;18:99–114. doi: 10.3166/phyto-2019-0189. [DOI] [Google Scholar]
- 20.El Hassani M., Douri E.M., Bammi J., Zidane L., Badoc A., Douira A. Plantes Médicinales de la Moyenne Moulouya (Nord-Est Du Maroc) Ethnopharmacologia. 2013;50:39–53. [Google Scholar]
- 21.Sargin S.A., Selvi S., Büyükcengiz M. Ethnomedicinal Plants of Aydincik District of Mersin, Turkey. J. Ethnopharmacol. 2015;174:200–216. doi: 10.1016/j.jep.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Bencheikh N., Bouhrim M., Kharchoufa L., Al Kamaly O.M., Mechchate H., Es-safi I., Dahmani A., Ouahhoud S., El Assri S., Eto B., et al. The Nephroprotective Effect of Zizyphus lotus L. (Desf.) Fruits in a Gentamicin-Induced Acute Kidney Injury Model in Rats: A Biochemical and Histopathological Investigation. Molecules. 2021;26:4806. doi: 10.3390/molecules26164806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eddouks M., Ajebli M., Hebi M. Ethnopharmacological Survey of Medicinal Plants Used in Daraa-Tafilalet Region (Province of Errachidia), Morocco. J. Ethnopharmacol. 2017;198:516–530. doi: 10.1016/j.jep.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Merzouki A., Ed-derfoufi F., Molero Mesa J. Contribution to the Knowledge of Rifian Traditional Medicine. II: Folk Medicine in Ksar Lakbir District (NW Morocco) Fitoterapia. 2000;71:278–307. doi: 10.1016/S0367-326X(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 25.Güzel Y., Güzelşemme M., Miski M. Ethnobotany of Medicinal Plants Used in Antakya: A Multicultural District in Hatay Province of Turkey. J. Ethnopharmacol. 2015;174:118–152. doi: 10.1016/j.jep.2015.07.042. [DOI] [PubMed] [Google Scholar]
- 26.Leto C., Tuttolomondo T., La Bella S., Licata M. Ethnobotanical Study in the Madonie Regional Park (Central Sicily, Italy)—Medicinal Use of Wild Shrub and Herbaceous Plant Species. J. Ethnopharmacol. 2013;146:90–112. doi: 10.1016/j.jep.2012.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Khouchlaa A., Talbaoui A., El Yahyaoui El Idrissi A., Bouyahya A., Ait Lahsen S., Kahouadji A., Tijane M. Détermination Des Composés Phénoliques et Évaluation de l’activité Litholytique in Vitro Sur La Lithiase Urinaire d’extrait de Zizyphus Lotus L. d’origine Marocaine. Phytotherapie. 2017:1–6. doi: 10.1007/s10298-017-1106-3. [DOI] [Google Scholar]
- 28.Fennane M., Tattou M.I. Observations sur la flore vasculaire endémique, rare ou menacée du Maroc. Flora Mediterr. 1999;9:113–124. [Google Scholar]
- 29.Yemele M.D., Telefo P.B., Lienou L.L., Tagne S.R., Fodouop C.S.P., Goka C.S., Lemfack M.C., Moundipa F.P. Ethnobotanical Survey of Medicinal Plants Used for Pregnant Womens Health Conditions in Menoua Division-West Cameroon. J. Ethnopharmacol. 2015;160:14–31. doi: 10.1016/j.jep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 30.Mrabti H.N., Jaradat N., Kachmar M.R., Ed-Dra A., Ouahbi A., Cherrah Y., El Abbes Faouzi M. Integrative Herbal Treatments of Diabetes in Beni Mellal Region of Morocco. J. Integr. Med. 2019;17:93–99. doi: 10.1016/j.joim.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 31.Yebouk C., Redouan F.Z., Benítez G., Bouhbal M., Kadiri M., Boumediana A.I., Molero-Mesa J., Merzouki A. Ethnobotanical Study of Medicinal Plants in the Adrar Province, Mauritania. J. Ethnopharmacol. 2020;246:112217. doi: 10.1016/j.jep.2019.112217. [DOI] [PubMed] [Google Scholar]
- 32.Didi O., Ould El Hadj M., Hadj-Mahammed M., Zabeirou H. Place Des Plantes Spontanees Dans La Medicine Traditionnelle de La Region de Ouargla (Sahara Septentrional Est) Courr. Savoir. 2003;3:47–51. [Google Scholar]
- 33.Benlamdini N., Elhafian M., Rochdi A., Zidane L. Étude Floristique et Ethnobotanique de La Flore Médicinale Du Haut Atlas Oriental (Haute Moulouya) J. Appl. Biosci. 2014;78:6771. doi: 10.4314/jab.v78i1.17. [DOI] [Google Scholar]
- 34.Aguiar N., Meira D., Raquel S. Study on the Efficacy of the Portuguese Cooperative Taxation. REVESCO Rev. Estudios Coop. 2015;121:7–32. doi: 10.5209/rev_REVE.2016.v121.51306. [DOI] [Google Scholar]
- 35.Tahri N., Basti A.E.L., Zidane L., Rochdi A., Douira A. Etude Ethnobotanique Des Plantes Medicinales Dans La Province De Settat (Maroc) Kastamonu Univ. J. For. Fac. 2014;2:192–208. [Google Scholar]
- 36.Khouchlaa A., Tijane M., Chebat A., Hseini S., Kahouadji A. Ethnopharmacology Study of Medicinal Plants Used in the Treatment of Urolithiasis (Morocco) Phytotherapie. 2017;15:274–287. doi: 10.1007/s10298-016-1073-4. [DOI] [Google Scholar]
- 37.El Guerrouj B., Bouhrim M., Bentata Y., Daudon M., Melhaoui M., Kharchoufa L., Bencheikh N., Bekkouch O. Kidney Stone Disease (Urolithiasis): Epidemiological Study in the Eastern Region of Morocco. Eur. J. Sci. Res. 2019;155:40–57. [Google Scholar]
- 38.Hamden K., Ayadi F., Jamoussi K., Masmoudi H., Elfeki A. Therapeutic Effect of Phytoecdysteroids Rich Extract from Ajuga Iva on Alloxan Induced Diabetic Rats Liver, Kidney and Pancreas. BioFactors. 2009;33:165–175. doi: 10.3233/BIO-2009-1040. [DOI] [PubMed] [Google Scholar]
- 39.Mariee A.D., Abd-allah G.M., El-yamany M.F. Renal Oxidative Stress and Nitric Oxide Production in Streptozotocin-Induced Diabetic Nephropathy in Rats: The Possible Modulatory Effects of Garlic (Allium Sativum L.) Biotechnol. Appl. Biochem. 2009;232:227–232. doi: 10.1042/BA20080086. [DOI] [PubMed] [Google Scholar]
- 40.Mansour M.H., Al-qattan K., Thomson M., Ali M. Garlic (Allium sativum) Modulates the Expression of Angiotensin II AT 2 Receptor in Adrenal and Renal Tissues of Streptozotocin-Induced Diabetic Rats. Adv. Biol. Chem. 2011;1:93–102. doi: 10.4236/abc.2011.13011. [DOI] [PubMed] [Google Scholar]
- 41.Aprioku J.S., Amah-Tariah F.S. Garlic (Allium sativum L.) Protects Hepatic and Renal Toxicity of Alloxan in Rats. J. Pharm. Res. Int. 2017;17:1–7. doi: 10.9734/JPRI/2017/34909. [DOI] [Google Scholar]
- 42.Omotoso D.R., Olajumoke J.M. Ameliorative Effects of Ascorbic Acid and Allium sativum (Garlic) Ethanol Extract on Renal Parenchyma of Gentamicin-Induced Nephropathic Rats. J. Complement. Altern. Med. Res. 2020;9:1–8. doi: 10.9734/jocamr/2020/v9i430146. [DOI] [Google Scholar]
- 43.Bolkent S., Akev N., Ozsol N., $engezer-Inceli M., Can A., Okyar A., Yanardag R. Effect of Aloe vera (L.) Burm. Fil. Leaf Gel and Pulp Extracts on Kidney in Type-II Diabetic Rat Models. Ind. J. Exp. Biol. 2004;42:48–52. [PubMed] [Google Scholar]
- 44.Ramachandraiahgari R.M.Y., Somesula S.R., Adi P.J., Shaik I.M., Enamala M., Matcha B. Protective Role of Ethanolic Extract of Aloe vera Antioxidant Properties on Liver and Kidney of Streptozotocin-Induced Diabetic Rats. Dig. J. Nanomater. Biostruct. 2012;7:175–184. [Google Scholar]
- 45.Utami J.P., Lestari U., Wulandari N., Susanto H., Handaya Y. Antinephropathie Effect of Aloe vera Gel to PKC-β Level on Wistar Rat Kidney in Diabetes Mellitus. KnE Life Sci. 2015;2:45–50. doi: 10.18502/kls.v2i1.115. [DOI] [Google Scholar]
- 46.Vanachayangkul P., Byer K., Khan S., Butterweck V. An Aqueous Extract of Ammi visnaga Fruits and Its Constituents Khellin and Visnagin Prevent Cell Damage Caused by Oxalate in Renal Epithelial Cells. Phytomedicine. 2010;17:653–658. doi: 10.1016/j.phymed.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanachayangkul P., Chow N., Khan S.R., Butterweck E. Prevention of Renal Crystal Deposition by an Extract of Ammi visnaga L. and Its Constituents Khellin and Visnagin in Hyperoxaluric Rats. Urol. Res. 2011;39:189–195. doi: 10.1007/s00240-010-0333-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Al Jawad F.H., Al Razzuqi R.A.M., Al Jeboori A.A. Apium Graveolens Accentuates Urinary Ca +2 Excretions in Experimental Model of Nephrocalcinosis. Int. J. Green Pharm. 2011;5:100–102. doi: 10.4103/0973-8258.85160. [DOI] [Google Scholar]
- 49.Afifah A., Muflikhah K., Ati V.R.B., Tsani R.M., Khasanah D., Maulana W. Protective Effect of Ethanol Extract of Celery (Apium Graveolens L.) on Kidney Damage in Ischemia/Reperfusion Injury Rats Model. Molekul. 2019;14:11–17. doi: 10.20884/1.jm.2019.14.1.448. [DOI] [Google Scholar]
- 50.Mahran G.H., Kadry H.A., Isaac Z.G., Thabett C.K. Investigation of Diuretic Drug Plants. 1. Phytochemical Screening and Pharmacological Evaluation of Anethum graveolens L., Apium graveolens L., Daucus carota L. and Eruca sativa Mill. Phytother. Res. 1991;5:169–172. doi: 10.1002/ptr.2650050406. [DOI] [Google Scholar]
- 51.Ibrahim A.K., AL-Azawi A.H. Nephro-Protective Effect of (Arachis hypogaea L.) Peanut Skin Extracts on CCl 4 Induced Kidney Damage in Mice. Int. J. Nat. Eng. Sci. 2019;13:7–14. [Google Scholar]
- 52.Afkir S., Nguelefack T.B., Aziz M., Zoheir J., Cuisinaud G., Bnouham M., Mekhfi H., Legssyer A., Lahlou S., Ziyyat A. Arbutus Unedo Prevents Cardiovascular and Morphological Alterations in L-NAME-Induced Hypertensive Rats. Part I: Cardiovascular and Renal Hemodynamic Effects of Arbutus Unedo in L-NAME-Induced Hypertensive Rats. J. Ethnopharmacol. 2008;116:288–295. doi: 10.1016/j.jep.2007.11.029. [DOI] [PubMed] [Google Scholar]
- 53.Rifqiyati N., Wahyuni A. Fennel (Foeniculum vulgare) Leaf Infusion Effect on Mammary Gland Activity and Kidney Function of Lactating Rats. Nusant. Biosci. 2019;11:101–105. doi: 10.13057/nusbiosci/n110117. [DOI] [Google Scholar]
- 54.Saoudi M., Badraoui R., Bouhajja H., Ncir M., Rahmouni F., Grati M., Jamoussi K., Feki A. El Deltamethrin Induced Oxidative Stress in Kidney and Brain of Rats: Protective Effect of Artemisia Campestris Essential Oil. Biomed. Pharmacother. 2017;94:955–963. doi: 10.1016/j.biopha.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 55.Rouhani M.H., Najafabadi M.M., Surkan P.J., Esmaillzadeh A., Feizi A., Azadbakht L. The Impact of Oat (Avena sativa) Consumption on Biomarkers of Renal Function in Patients with Chronic Kidney Disease: A Parallel Randomized Clinical Trial. Clin. Nutr. 2016:1–21. doi: 10.1016/j.clnu.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 56.Ltaif M., Gargouri M., Magné C., El Feki A., Soussi A. Protective Effects of Avena sativa against Oxidative Stress-Induced Kidney Damage Resulting from an Estrogen Deficiency in Ovariectomized Swiss Mice Model. Food Biochem. 2020;44:1–13. doi: 10.1111/jfbc.13205. [DOI] [PubMed] [Google Scholar]
- 57.Neag M.A., Bocsan I.C., Catinean A., Vesa S.C., Balan G.G., Parvu M., Muntean D.M., Vlase L., Melincovici C.S., Pop R., et al. Effects of Berberis Vulgaris Extract on Lipid Profile, Kidney and Liver Function in Experimental Dyslipidemia. Rev. Chim. 2019;70:614–618. doi: 10.37358/RC.19.2.6968. [DOI] [Google Scholar]
- 58.Rubattu S., Castro S.D., Cotugno M., Bianchi F., Mattioli R., Baima S., Stanzione R., Madonna M., Bozzao C., Marchitti S., et al. Protective Effects of Brassica Oleracea Sprouts Extract toward Renal Damage in High-Salt-Fed SHRSP: Role of AMPK/PPAR a /UCP2 Axis. J. Hypertens. 2015;33:1–15. doi: 10.1097/HJH.0000000000000562. [DOI] [PubMed] [Google Scholar]
- 59.Rtibi K., Selmi S., Jabri M.-A., El-Benna J., Amri M., Marzouki L., Sebai H. Protective Effect of Ceratonia siliqua L. Against a Dextran Sulfate Sodium-Induced Alterations in Liver and Kidney in Rat. J. Med. Food. 2016;19:882–889. doi: 10.1089/jmf.2016.0020. [DOI] [PubMed] [Google Scholar]
- 60.Hsouna A.B., Saoudi M., Trigui M., Jamoussi K., Boudawara T., Jaoua S., Feki A. El Characterization of Bioactive Compounds and Ameliorative Effects of Ceratonia siliqua Leaf Extract against CCl 4 Induced Hepatic Oxidative Damage and Renal Failure in Rats. Food Chem. Toxicol. 2011;49:3183–3191. doi: 10.1016/j.fct.2011.09.034. [DOI] [PubMed] [Google Scholar]
- 61.Pourfarjam Y., Rezagholizadeh L., Nowrouzi A., Meysamie A., Ghaseminejad S., Ziamajidi N., Norouzi D. Effect of Cichorium intybus L. Seed Extract on Renal Parameters in Experimentally Induced Early and Late Diabetes Type 2 in Rats. Ren. Fail. 2016;39:1–11. doi: 10.1080/0886022X.2016.1256317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noori S., Mahboob T. Role of Electrolytes Disturbances and Na + -K + -ATPase in Cisplatin—Induced Renal Toxicity and Effects of Ethanolic Extract of Cichorium intybus. Pak. J. Pharm. Sci. 1985;25:857–862. [PubMed] [Google Scholar]
- 63.Emamiyan M.Z., Vaezi G., Tehranipour M., Shahrohkabadi K., Shahrohkabadi K. Preventive Effects of the Aqueous Extract of Cichorium intybus L. Flower on Ethylene Glycol-Induced Renal Calculi in Rats. Avicenna J. Phytomed. 2018;8:170–178. [PMC free article] [PubMed] [Google Scholar]
- 64.Erkec O.E., Arihan O., Colcimen N., Kara M., Karatas E., Demir H., Ragbetli M.C. Effects of Cichorium intybus on Serum Oxidative Stress, Liver and Kidney Volume, and Cyclin B1 and Bcl-2 Levels in the Brains of Rats with Ethanol Induced Damage. Cell. Mol. Biol. 2018;64:30–35. doi: 10.14715/cmb/2018.64.7.6. [DOI] [PubMed] [Google Scholar]
- 65.El-masry T.A., Altwaijry N., Alotaibi B., Tousson E., Alboghdadly A., Saleh A. Chicory (Cichorium intybus L.) Extract Ameliorates Hydroxyapatite Nanoparticles Induced Kidney Damage in Rats Chicory (Cichorium intybus L.) Extract Ameliorates Hydroxyapatite Nanoparticles Induced Kidney Damage in Rats. Pak. J. Pharm. Sci. 2020;33:1251–1260. doi: 10.36721/PJPS.2020.33.3.SUP.1251-1260.1. [DOI] [Google Scholar]
- 66.Iqbal S., Jabeen F., Peng C., Ijaz M.U., Chaudhry A.S. Cinnamomum Cassia Ameliorates Ni-NPs-Induced Liver and Kidney Damage in Male Sprague Dawley Rats. Hum. Exp. Toxicol. 2020;39:1565–1581. doi: 10.1177/0960327120930125. [DOI] [PubMed] [Google Scholar]
- 67.Soji-Omoniwa O., Muhammad N.O., Usman L.A., Omoniwa B.P. Effect of Leaf Essential Oil of Citrus Sinensis at Different Harvest Time on Some Liver and Kidney Function Indices of Diabetic Rats. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014;8:484–488. [Google Scholar]
- 68.Mahmood N., Manzoor F., Khaled A., Javed M., Qureshi Z. Anti-Proliferative or Cytopathic Potential of Thapsia Garganica, Citrus Sinensis, Citrus Limon and Vinca Rosea Extracts Against Human Embryonic Kidney Carcinoma Cell Line. West Indian Med. J. 2016:1–16. doi: 10.7727/wimj.2015.325. [DOI] [Google Scholar]
- 69.Kansal L., Sharma V., Sharma A., Lodi S., Sharma S.H. Protective Role of Coriandrum Sativum (Coriander) Extracts against Lead Nitrate Induced Oxidative Stress and Tissue Damage in the Liver and Kidney in Male Mice. Int. J. Appl. Biol. Pharm. Technol. 2011;2:65–83. [Google Scholar]
- 70.Vosough D., Hooshyar S.H., Moini E. Effect of Saffron (Crocus sativus) Administration on Kidney Function in Normal Cats as Determined by Use of 99m Tc-DTPA Renal Scintigraphy. Iran. J. Vet. Surg. 2014;9:45–50. [Google Scholar]
- 71.Ashrafi M., Afsar Z., Erjaee H., Nazifi S. The Effects of Saffron (Crocus sativus) Aqueous Extract on TNF-α Levels in Liver, Kidney, and Lens Tissues of Diabetic Rats. Turk. J. Endocrinol. Metab. 2018;22:217–224. doi: 10.25179/tjem.2018-59710. [DOI] [Google Scholar]
- 72.Azizi M., Ahmadi M.R.H., Mohamadpour M., Daemi A., Asadi S., Shirzadpour E., Amraei M. Investigating the Histopathological Effects of Saffron Petal (Crocus sativus L.) Hydroalcoholic Extract on Kidney and Liver Functional Parameters in Rats. Biomed. Res. Ther. 2020;7:3727–3738. doi: 10.15419/bmrat.v7i4.600. [DOI] [Google Scholar]
- 73.Azizi M., Abbasi N., Mohamadpour M., Bakhtiyari S., Asadi S., Shirzadpour E., Aidy A., Mohamadpour M., Amraei M. Investigating the Effect of Crocus sativus L. Petal Hydroalcoholic Extract on Inflammatory and Enzymatic Indices Resulting from Alcohol Use in Kidney and Liver of Male Rats. J. Inflamm. Res. 2019;12:269–283. doi: 10.2147/JIR.S216125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saleem M., Javed F., Asif M., Baig M.K., Arif M. HPLC Analysis and In Vivo Renoprotective Evaluation of Hydroalcoholic Extract of Cucumis melo Seeds in Gentamicin-Induced Renal Damage. Medicina. 2019;55:107. doi: 10.3390/medicina55040107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.El-Maksoud M.A.E.A. Effect of Cucumis melo Var. Flexuosus Leaves Extract on Renal Oxidative Injury and Inflamamation in Diabetic Male Albino Rats. Egypt. J. Zool. 2019;71:13–20. doi: 10.21608/ejz.2019.10515.1007. [DOI] [Google Scholar]
- 76.Ofoego U.C., Nweke E.O., Nzube O.M. Ameliorative Effect of Ethanolic Extract of Cucumis Sativus (Cucumber) Pulp on Alloxan Induced Kidney Toxicity in Male Adult Wistar Rats Ameliorative Effect of Ethanolic Extract of Cucumis Sativus (Cucumber) Pulp on Alloxan Induced Kidney Toxicity. J. Nat. Sci. Res. 2020;9:12–22. doi: 10.7176/JNSR. [DOI] [Google Scholar]
- 77.Shehzad A., Saleem U., Muhammad A.S., Cruz C.V.L., Khan A.H., Ahmad B. Antiurolithic Evaluation of Cucurbita Pepo Seeds Extract against Sodium Oxalate-Induced Renal Calculi. Pharmacogn. Mag. 2019;15:S38–S46. doi: 10.4103/pm.pm. [DOI] [Google Scholar]
- 78.Sekhar M.G., Basha S.S., Madhavi Y.R.R., Krichna S.R., Ismail S.M., Bhaskar M. The Effects of Curcuma longa and Trigonella foenum Graecum on Antioxidant Enzymes in Kidney of Alloxan Induced Type-1 Diabetic Male Rats. Adv. Pharmacol. Toxicol. 2010;11:95–105. [Google Scholar]
- 79.Mohebbati R., Shafei M.N., Soukhtanloo M., Mohammadian Roshan N., Khajavi Rad A., Anaeigoudari A., Hosseinian S., Karimi S., Beheshti F. Adriamycin-Induced Oxidative Stress Is Prevented by Mixed Hydro-Alcoholic Extract of Nigella sativa and Curcuma longa in Rat Kidney. Avicenna J. Phytomed. 2016;6:86–94. doi: 10.22038/ajp.2016.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gholami-Ahangaran M., Rangsaz N., Azizi S. Evaluation of Turmeric (Curcuma longa) Effect on Biochemical and Pathological Parameters of Liver and Kidney in Chicken Aflatoxicosis. Pharm. Biol. 2016;54:780–787. doi: 10.3109/13880209.2015.1080731. [DOI] [PubMed] [Google Scholar]
- 81.Russo E.R., Facincani I., Nakazato K.C., Coimbra T.M., Crevelin E.J., Pereira A.M.S., Carmona F. Oral Administration of Powdered Dried Rhizomes of Curcuma longa L. (Turmeric, Zingiberaceae) Is Effective in the Treatment of Doxorubicin-Induced Kidney Injury in Rats. Phytother. Res. 2018;32:2408–2416. doi: 10.1002/ptr.6176. [DOI] [PubMed] [Google Scholar]
- 82.Golshan A., Hayatdavoudi P., Hadjzadeh M.A., Khajavi Rad A., Mohamadian Roshan N., Abbasnezhad A., Mousavi S.M., Pakdel R., Zarei B., Aghaee A. Kidney Stone Formation and Antioxidant Effects of Cynodon Dactylon Decoction in Male Wistar Rats. Avicenna J. Phytomed. 2017;7:180–190. doi: 10.22038/ajp.2016.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pouraboli I., Ranjbar B. The Effect of Daucus Carota Seeds Extract on Lipid Profile, LFT and Kidney Function Indicators in Streptozocin-Induced Diabetic Rats. Int. J. Plant Sci. Ecol. 2015;1:84–87. [Google Scholar]
- 84.Afzal M., Kazmi I., Kaur R., Ahmad A., Anwar F. Comparison of Protective and Curative Potential of Daucus Carota Root Extract on Renal Ischemia Reperfusion Injury in Rats. Pharm. Biol. 2013;51:856–862. doi: 10.3109/13880209.2013.767840. [DOI] [PubMed] [Google Scholar]
- 85.Nwaichi E.O. Influence of Daucus Carota on the Hepatic and Renal Biomarkers of Dichlorvos-Exposed Albino Rats. EC Pharmacol. Toxicol. 2019;10:1067–1075. [Google Scholar]
- 86.Ghareeb M.A., Sobeh M., El-Maadawy W.H., Mohammed H.S., Khalil H., Botros S., Wink M. Chemical Profiling of Polyphenolics in Eucalyptus globulus and Evaluation of Its Hepato–Renal Protective Potential against Cyclophosphamide Induced Toxicity in Mice. Antioxidants. 2019;8:415. doi: 10.3390/antiox8090415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dhibi S., Mbarki S., Elfeki A., Hfaiedh N. Eucalyptus globulus Extract Protects upon Acetaminophen-Induced Kidney Damages in Male Rat. Bosn. J. Basic Med. Sci. 2014;14:99–104. doi: 10.17305/bjbms.2014.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ibrahim F.Y., El-Khateeb A.Y. Effect of Herbal Beverages of Foeniculum vulgare and Cymbopogon proximus on Inhibition of Calcium Oxalate Renal Crystals Formation in Rats. Ann. Agric. Sci. 2013;58:221–229. doi: 10.1016/j.aoas.2013.07.006. [DOI] [Google Scholar]
- 89.Abdel-Wahab A., Hashem Abdel-Razik A.R., Abdel Aziz R.L. Rescue Effects of Aqueous Seed Extracts of Foeniculum vulgare and Carum carvi against Cadmium-Induced Hepatic, Renal and Gonadal Damage in Female Albino Rats. Asian Pac. J. Trop. Med. 2017;10:1123–1133. doi: 10.1016/j.apjtm.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 90.Sadrefozalayi S., Farokhi F. Effect of the Aqueous Extract of Foeniculum vulgare (Fennel) on the Kidney in Experimental PCOS Female Rats. Avicenna J. Phytomed. 2014;4:110–117. doi: 10.22038/ajp.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Taleb-dida N., Krouf D., Bouchenak M. Globularia Alypum Aqueous Extract Decreases Hypertriglyceridemia and Ameliorates Oxidative Status of the Muscle, Kidney, and Heart in Rats Fed a High-Fructose Diet. Nutr. Res. 2011;31:488–495. doi: 10.1016/j.nutres.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 92.Naouel B., Hayat T., Iman K., Lekhmissi A., Abderrahmane B. Kinetics of Inhibition of Xanthine Oxidase by Globularia Alypum and Its Protective Effect against Oxonate-Induced Hyperuricemia and Renal Dysfunction in Mice. J. Appl. Pharm. Sci. 2016;6:159–164. doi: 10.7324/JAPS.2016.60422. [DOI] [Google Scholar]
- 93.Sitohy M.Z., El-Massry R.A., El-Saadany S.S., Labib S.M. Metabolic Effects of Licorice Roots (Glycyrrhiza glabra) on Lipid Distribution Pattern, Liver and Renal Functions of Albino Rats. Nahrung. 1991;35:799–806. doi: 10.1002/food.19910350803. [DOI] [PubMed] [Google Scholar]
- 94.Al-Qarawi A.A., Abdel-Rahman H.A., Ali B.H., El Mougy S.A. Liquorice (Glycyrrhiza glabra) and the Adrenal-Kidney-Pituitary Axis in Rats. Food Chem. Toxicol. 2002;40:1525–1527. doi: 10.1016/S0278-6915(02)00080-7. [DOI] [PubMed] [Google Scholar]
- 95.Ojo A.B., Adanlawo I.G., Ojo O.A. Ameliorative Potentials of Saponins from Helianthus Annuus Roots on Hepatoprotective and Some Kidney Function Indices of Alloxan-Induced Diabetic Rats. Pharmacol. Online. 2016;3:73–79. [Google Scholar]
- 96.Ammor K., Mahjoubi F., Bousta D., Elhabbani R., Chaqroune A. In Vitro Litholytic Activity of Extracts and Phenolic Fractions of Some Medicinal Plants on Urinary Stones. Mediterr. J. Chem. 2020;9:468–477. doi: 10.13171/mjc9602001101135ka. [DOI] [Google Scholar]
- 97.Atmani F., Khan S.R. Effects of an Extract from Herniaria Hirsuta on Calcium Oxalate Crystallization in Vitro. BJU Int. 2000;85:621–625. doi: 10.1046/j.1464-410x.2000.00485.x. [DOI] [PubMed] [Google Scholar]
- 98.Atmani F., Slimani Y., Mimouni M., Hacht B. Prophylaxis of Calcium Oxalate Stones by Herniaria Hirsuta on Experimentally Induced Nephrolithiasis in Rats. BJU Int. 2003;92:137–140. doi: 10.1046/j.1464-410X.2003.04289.x. [DOI] [PubMed] [Google Scholar]
- 99.Grases F., Ramis M., Costa-Bauzá A., March J.G. Effect of Herniaria Hirsuta and Agropyron Repens on Calcium Oxalate Urolithiasis Risk in Rats. J. Ethnopharmacol. 1995;45:211–214. doi: 10.1016/0378-8741(94)01218-O. [DOI] [PubMed] [Google Scholar]
- 100.Atmani F., Farell G., Lieske J.C. Extract from Herniaria Hirsuta Coats Calcium Oxalate Monohydrate Crystals and Blocks Their Adhesion to Renal Epithelial Cells. J. Urol. 2004;172:1510–1514. doi: 10.1097/01.ju.0000131004.03795.c5. [DOI] [PubMed] [Google Scholar]
- 101.Meiouet F., El Kabbaj S., Daudon M. Étude in Vitro de l’activité Litholytique de Quatre Plantes Médicinales Vis-à-Vis Des Calculs Urinaires de Cystine. Prog. Urol. 2011;21:40–47. doi: 10.1016/j.purol.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 102.Patel M., Raval S.K., Modi R.J. Effect of Seed Extracts of Vigna Unguiculta and Hordeum Vulgare on Kidney Homogenate and Rat Kidney Injury Molecule-1 in Ethylene Glycol and Ammonium Chloride Induced Urolithasis in Rats. Ind. J. Vet. Sci. Biotechnol. 2020;15:1–4. [Google Scholar]
- 103.Khudhiar A.S., Dakheel M.H., Twuej M.A.S., Faris J.K. Effect of Use of (Lactuca sativa) on the Function and Histological Structure of Kidney in Locale Male Rabbits. Plant Arch. 2020;20:2742–2746. [Google Scholar]
- 104.Sari M.I., Antika M.A., Anggraini D.R. Blood Glucose Levels and the Microscopic Structure of Kidney Wistar Rat Diabetes Mellitus under the Effect of Lawsonia inermis (Linn.) Leaves Ethanolic Extract. Asian J. Pharm. Clin. Res. 2018;11:257–261. doi: 10.22159/ajpcr.2018.v11i4.23502. [DOI] [Google Scholar]
- 105.Balgoon M.J. Assessment of the Protective Effect of Lepidium sativum against Aluminum-Induced Liver and Kidney Effects in Albino Rat. BioMed Res. Int. 2019;2019:4516730. doi: 10.1155/2019/4516730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Eddouks M., Mhamed M. Effect of Lepidium sativum L. on Renal Glucose Reabsorption and Urinary TGF-Β1 Levels in Diabetic Rats. Phytother. Res. 2008;22:1–5. doi: 10.1002/ptr.2101. [DOI] [PubMed] [Google Scholar]
- 107.Ghule A.E., Jadhav S.S., Bodhankar S.L. Renoprotective Effect of Linum usitatissimum Seeds through Haemodynamic Changes and Conservation of Antioxidant Enzymes in Renal Ischaemia-Reperfusion Injury in Rats. Arab J. Urol. 2019;9:215–221. doi: 10.1016/j.aju.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ahmad N., Zia-ur-Rahman, Akhtar N., Ali S. Effects of Aqueous Methanolic Extract of Flax Seeds (Linum usitatissimum) on Serum Estradiol, Progesterone, Kidney and Liver Functions and Some Serum Biochemical Metabolites in Immature Female Rats. Pak. Vet. J. 2011;8318:2074–7764. [Google Scholar]
- 109.Djaber N., Ounaceur L.S., Moubine B.N., Khaldi T., Rouag M., Berrouague S., Amara H., Taibi F., Boumendjel M., Boumendjel A., et al. Roundup-Induced Biochemical and Histopathological Changes in the Liver and Kidney of Rats: The Ameliorative Effects of Linum usitatissimum Oil. Acta Biochim. Pol. 2020;67:53. doi: 10.18388/abp.2020_2898. [DOI] [PubMed] [Google Scholar]
- 110.Dkhil M.A., Bauomy A.A., Diab M.S.M., Al-Quraishy S. The Antioxidant Effect of Morus alba Leaves Extract on Kidney, Testes, Spleen and Intestine of Mice. Pak. J. Zool. 2015;47:393–397. [Google Scholar]
- 111.Gurukar M.S.A., Nandini C.D., Mahadevamma S., Salimath P. V Ocimum Sanctum and Morus alba Leaves and Punica Granatum Seeds in Diet Ameliorate Diabetes-Induced Changes in Kidney. J. Pharm. Res. 2012;5:4729–4733. [Google Scholar]
- 112.Sartono N., Novianto D., Ulfa E., Susanto A.B., Puspitaningrum R. Crude Extract Mulberry (Morus alba L.) Leaves Chlorophyll Improves Urine Creatinine Levels and Histology of Diabetic Rat Kidney. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015;17:451–459. [Google Scholar]
- 113.Diab M.S.M., Bauomy A.A., Dkhil M.A., Amer O.S.O., Al-Quraishy S. Role of Morus alba in Ameliorating Schistosoma Mansoni-Induced Renal and Testicular Injuries in Mice. Pak. J. Zool. 2013;45:1367–1375. [Google Scholar]
- 114.Abdel-Daim M.M., Ghazy E.W. Effects of Nigella sativa Oil and Ascorbic Acid against Oxytetracycline-Induced Hepato-Renal Toxicity in Rabbits. Iran J. Basic Med. Sci. 2015;18:221–227. doi: 10.22038/ijbms.2015.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hosseinzadeh H., Montahaei R. Protective Effect of Nigella sativa L. Extracts and Thymoquinone, Its Active Constituent, on Renal Ischemia-Reperfusion-Induced Oxidative Damage in Rats. Pharm. Online. 2007;1:176–189. [Google Scholar]
- 116.Salama R.H.M., Abd-El-Hameed N.A.-M., Abd-El-Ghaffar S.K., Mohammed Z.T., Ghandour N.M.A. Nephroprotective Effect of Nigella sativa and Matricaria Chamomilla in Cisplatin Induced Renal Injury. Int. J. Clin. Med. 2011;2:185–195. doi: 10.4236/ijcm.2011.23031. [DOI] [Google Scholar]
- 117.Mousavi G. Study on the Effect of Black Cumin (Nigella sativa Linn.) on Experimental Renal Ischemia-Reperfusion Injury in Rats. Acta Cir. Bras. 2015;30:542–550. doi: 10.1590/S0102-865020150080000005. [DOI] [PubMed] [Google Scholar]
- 118.Busari A.A., Adejare A.A., Shodipe A.F., Oduniyi O.A., Ismail-Badmus K.B., Oreagba I.A. Protective but Non-Synergistic Effects of Nigella sativa and Vitamin E against Cisplatin-Induced Renal Toxicity and Oxidative Stress in Wistar Rats. Drug Res. 2018;68:696–703. doi: 10.1055/a-0626-7003. [DOI] [PubMed] [Google Scholar]
- 119.Zaveri M., Desai N., Movaliya V. Effect of Ocimum Basilicum on Cisplatin Models of Acute Renal Failure. Adv. Res. Pharm. 2011;1:91–100. [Google Scholar]
- 120.Almalki D.A. Renoprotective Effect of Ocimum Basilicum (Basil) Against Diabetes-Induced Renal Affection in Albino Rats. Mater. Socio Med. 2019;31:236. doi: 10.5455/msm.2019.31.236-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Soliman A.M., Rizk H.A., Shalaby M.A., Elkomy A.A. Mechanisms of Hepato-Renal Protective Activity of Ocimum Basilicum Leaf Extract against Paracetamol Toxicity in Rat Model. Adv. Anim. Vet. Sci. 2020;8:385–391. doi: 10.17582/journal.aavs/2020/8.4.385.391. [DOI] [Google Scholar]
- 122.Khaki A.A., Azad F.F., Khaki A. Treatment Effects of Ocimum Basilicum on Kidney Cells Apoptosis Produced by Exposure to Electromagnetic Field (EMF) in Rats. Adv. Environ. Biol. 2011;5:2019–2023. [Google Scholar]
- 123.Ivanov M., Vajic U., Mihailovic-Stanojevic N., Miloradovic Z., Jovovic D., Grujic-Milanovic J., Karanovic D., Dekanski D. Highly Potent Antioxidant Olea europaea L. Leaf Extract Affects Carotid and Renal Haemodynamics in Experimental Hypertention: The Role of Oleurpein. EXCLI J. 2018;17:29–44. doi: 10.17179/excli2017-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Elamrani A. The Antitumoral Activity and the Cytotoxicity on Renal Cells of Ethanolic Extracts from the Leaves of Four Varieties of Olea europaea L. Grown in Morocco. Anal. Chem. Lett. 2015;7928:63–69. doi: 10.1080/22297928.2011.10648205. [DOI] [Google Scholar]
- 125.Senturk H., Yıldız F. Protective Effects of Olea Europiaea. (Olive) Leaf Extract against Oxidative Stress Injury Generated with Renal Ischemia Reperfusion. J. Anim. Plant Sci. 2018;28:1027–1033. [Google Scholar]
- 126.Alhaithloul H.A.S., Alotaibi M.F., Bin-jumah M., Elgebaly H., Mahmoud A.M. Olea europaea Leaf Extract Up-Regulates Nrf2/ARE/HO-1 Signaling and Attenuates Cyclophosphamide-Induced Oxidative Stress, in Fl Ammation and Apoptosis in Rat Kidney. Biomed. Pharmacother. 2019;111:676–685. doi: 10.1016/j.biopha.2018.12.112. [DOI] [PubMed] [Google Scholar]
- 127.ElMougy S.A., Al-Qarawi A.A., Bazaid S.A. The Effect of an Aqueous Extract of Olive (Olea europaea) Leaves on the Adrenal-Kidney-Pituitary Axis in Rats. J. Herbs Spices Med. Plants. 2015;15:37–41. doi: 10.1080/10496470903507932. [DOI] [Google Scholar]
- 128.Bakour M., Al-Waili N., El-Haskoury R., El-Menyiy N., Al-Waili T., AL-Waili A., Lyoussi B. Comparison of Hypotensive, Diuretic and Renal Effects between Cladodes of Opuntia ficus-Indica and Furosemide. Asian Pac. J. Trop. Med. 2017;10:900–906. doi: 10.1016/j.apjtm.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 129.Mbarkaa H., Dalel B., Nizar Z.M., Lazhar Z. Phytochemical Analysis and Nephroprotective Effect of Cactus (Opuntia ficus-Indica) Cladodes on Sodium Dichromate-Induced Kidney Injury in Rats. Appl. Physiol. Nutr. Metabol. 2018;44:1–34. doi: 10.1139/apnm-2018-0184. [DOI] [PubMed] [Google Scholar]
- 130.Alimi H., Bouoni Z., Feriani A., Hfaeidh N., Sakly M., Rhouma K. Ben Opuntia ficus Indica f. Inermis Fruit Juice Alleviates Ethanol-Induced Kidney Injury in Rats. Asian J. Biomed. Pharm. Sci. 2017;4:116–123. [Google Scholar]
- 131.Hashem M.A., Ismail H.T.H., Hassan E.H.M. Protective Effect of Angelica sinensis Extract and Origanum majorana Oil on Hepatic and Renal Toxicities Induced by Nickel Chloride in Male Albino Rats. J. Zagazig Vet. 2019;47:306–316. doi: 10.21608/zvjz.2019.13886.1050. [DOI] [Google Scholar]
- 132.Nielsen S.E., Young J.F., Daneshvar B., Lauridsen S.T., Knuthsen P., Sandstrom B., Dragsted L.O. Effect of Parsley (Petroselinum crispum) Intake on Urinary Apigenin Excretion, Blood Antioxidant Enzymes and Biomarkers for Oxidative Stress in Human Subjects. Br. J. Nutr. 1999;81:447–455. doi: 10.1017/S000711459900080X. [DOI] [PubMed] [Google Scholar]
- 133.Roshankhah S., Jalili C., Salahshoor M.R. Protective Effects of Petroselinum crispum on Ischemia/Reperfusion—Induced Acute Kidney Injury in Rats. Physiol. Pharmacol. 2019;23:129–139. [Google Scholar]
- 134.Jassim A.M. Protective Effect of Petroselinum crispum (Parsley) Extract on Histopathological Changes in Liver, Kidney and Pancreas Induced by Sodium Valproate—In Male Rats. Kufa J. Vet. Med. Sci. 2013;81:20–27. [Google Scholar]
- 135.Yousuf H.A., Al-zubaidi F.S., Yousif W.H. Study of the Interaction Effect between Parsley Petroselinum crispum and Cadmium on Lipid Profile, Lipid Peroxidation and Catalase Activity of Albino Mice Males′ Liver and Kidney. Iraqi J. Sci. 2014;55:711–721. [Google Scholar]
- 136.Rahmat A., Ahmad N.S.S., Ramli N.S. Parsley (Petroselinum crispum) Supplementation Attenuates Serum Uric Acid Level and Improves Liver and Kidney Structures in Oxonate-Induced Hyperuricemic Rats. Orient. Pharm. Exp. Med. 2019;19:393–401. doi: 10.1007/s13596-018-0353-7. [DOI] [Google Scholar]
- 137.El Haliem N.G.A., Mohamed D.S. The Effect of Aspartame on the Histological Structure of the Liver and Renal Cortex of Adult Male Albino Rat and the Possible Protective Effect of Pimpinella Anisum Oil. Egypt. J. Histol. 2011;34:715–726. doi: 10.1097/01.EHX.0000406589.05585.8d. [DOI] [Google Scholar]
- 138.Djerrou Z., Hamdi-Pacha Y., Belkhiri A.M., Djaalab H., Riachi F., Serakta M., Boukeloua A., Maameri Z. Evaluation of Pistacia Lentiscus Fatty Oil Effects on Glycemic Index, Liver Functions and Kidney Functions of New Zealand Rabbits. Afr. J. Tradit. Complement. Altern. Med. 2011;8:214–219. doi: 10.4314/ajtcam.v8i5S.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Moneim A.E.A., Dkhil M.A., Al-Quraishy S. Studies on the Effect of Pomegranate (Punica granatum) Juice and Peel on Liver and Kidney in Adult Male Rats. J. Med. Plant Res. 2011;5:5083–5088. [Google Scholar]
- 140.Aksu D.S., Sağlam Y.S., Yildirim S., Aksu T. Effect of Pomegranate (Punica granatum L.) Juice on Kidney, Liver, Heart and Testis Histopathological Changes, and the Tissues Lipid Peroxidation and Antioxidant Status in Lead Acetate-Treated Rats. Cell. Mol. Biol. 2017;6:33–43. doi: 10.14715/cmb/2017.63.10.5. [DOI] [PubMed] [Google Scholar]
- 141.Asmaa F.H., Shaban N.Z. Short and Long Term Effects of Pomegranate (Punica granatum) Extracts on Apoptosis in Rat Kidney Induced by Diethylnitrosamine and Phenobarbital. J. Pharm. Pharmacol. 2016;4:52–63. doi: 10.17265/2328-2150/2016.02.002. [DOI] [Google Scholar]
- 142.Ali H., Shadab A.B., Qamar K. Ameliorative Effect of Punica granatum on Steroid Iduced Proximal and Distal Tubular Dilatation in Mice Kidney. Pak. Armed Forces Med. J. 2018;68:333–338. [Google Scholar]
- 143.Singh A.P., Singh A.J., Singh N. Pharmacological Investigations of Punica granatum in Glycerol-Induced Acute Renal Failure in Rats. Indian J. Pharmacol. 2011;43:551–556. doi: 10.4103/0253-7613.84971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ashtiyani S.C., Najafi H., Jalalvandi S., Hosseinei F. Protective Effects of Rosa canina L. Fruit Extracts on Renal Disturbances Induced by Reperfusion Injury in Rats. Iran. J. Kidney Dis. 2013;7:290–298. [PubMed] [Google Scholar]
- 145.Almakhatreh M., Hafez E., Tousson E., Masoud A. Biochemical and Molecular Studies on the Role of Rosemary (Rosmarinus officinalis) Extract in Reducing Liver and Kidney Toxicity Due to Etoposide in Male Rats. Asian J. Res. Med. Pharm. Sci. 2019;7:1–11. doi: 10.9734/ajrimps/2019/v7i430126. [DOI] [Google Scholar]
- 146.Qadori Y.T., Rashid K.I., Madhloom I.I., Al-Shaikh M.N. Histological Study for the Biological Effect of Rosemary Rosmarinus officinalis L. Essential Oil on Liver and Kidney Tissues. J. Biotechnol. Res. Cent. 2013;7:48–53. [Google Scholar]
- 147.Al-badry F.A.M. Effect of Aqueous Extract of Rosmarinus officinalis on Kidney and Liver of Male Rats Experimentally Infected With Diabetic. J. Coll. Educ. Pure Sci. 2017;7:171–186. [Google Scholar]
- 148.Al-Ani B.T., Al Saadi R.R., Reshan R.G. Investigating Effects of Salvia officinalis (Sage) on Development of Mice Embryos Kidney and Some Hormonal Effect of Treated Mothers. Indian J. Forensic Med. Toxicol. 2020;14:649–654. doi: 10.37506/v14/i1/2020/ijfmt/192975. [DOI] [Google Scholar]
- 149.Koubaa-Ghorbel F., Chaâbane M., Turki M., Makni-Ayadi F., El Feki A. The Protective Effects of Salvia officinalis Essential Oil Compared to Simvastatin against Hyperlipidemia, Liver, and Kidney Injuries in Mice Submitted to a High-Fat Diet. J. Food Biochem. 2020;44:1–14. doi: 10.1111/jfbc.13160. [DOI] [PubMed] [Google Scholar]
- 150.Ashour M.B., Ahmed O.M., Asran A.E.M.A., Ali M.A. Assessment of the Preventive Effects of Salvia officinalis and Ruta graveolens Ethanolic Leaf Extracts on Chlorpyrifos- and Methomyl-Induced Renal Toxicity and Oxidative Stress in Albino Rats. Int. J. Prev. Treat. 2017;6:34–44. doi: 10.5923/j.ijpt.20170602.03. [DOI] [Google Scholar]
- 151.Fahmy M.A., Diab K.A., Abdel-Samie N.S., Omara E.A., Hassan Z.M. Carbon Tetrachloride Induced Hepato/Renal Toxicity in Experimental Mice: Antioxidant Potential of Egyptian Salvia officinalis L. Essential Oil. Environ. Sci. Pollut. Res. 2018;25:27858–27876. doi: 10.1007/s11356-018-2820-6. [DOI] [PubMed] [Google Scholar]
- 152.Elkomy A., Aboubakr M., Ibrahim S., Abdelhamid Y. Protective Effects of Syzygium Aromaticum Oil (Clove) against Acrylamide Induced Hepatic, Renal, and Testicular Toxicity in Rats. Int. J. Pharmacol. Toxicol. 2018;6:12. doi: 10.14419/ijpt.v6i1.10972. [DOI] [Google Scholar]
- 153.Xue W., Lei J., Li X., Zhang R. Trigonella foenum Graecum Seed Extract Protects Kidney Function and Morphology in Diabetic Rats via Its Antioxidant Activity. Nutr. Res. 2011;31:555–562. doi: 10.1016/j.nutres.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 154.Mbarki S., Alimi H., Bouzenna H., Elfeki A., Hfaiedh N. Phytochemical Study and Protective Effect of Trigonella foenum Graecum (Fenugreek Seeds) against Carbon Tetrachloride-Induced Toxicity in Liver and Kidney of Male Rat. Biomed. Pharmacother. 2017;88:19–26. doi: 10.1016/j.biopha.2016.12.078. [DOI] [PubMed] [Google Scholar]
- 155.Shekha M.S., Qadir A.B., Ali H.H., Selim X.E. Effect of Fenugreek (Trigonella foenum-Graecum) on Ethylene Glycol Induced Kidney Stone in Rats. Jordan J. Biol. Sci. 2014;7:257–260. doi: 10.12816/0008248. [DOI] [Google Scholar]
- 156.El-Tawil G.A. Effect of Fenugreek (Trigonella foenum-Graecum) Supplementation on Radiation-Induced Oxidative Stress in Liver and Kidney of Rats. J. Radiat. Res. Appl. Sci. 2009;2:19–30. [Google Scholar]
- 157.Khanaki K., Abedinzade M., Hamidi M. The Effects of Urtica dioica and Lamium Album Extracts on the Expression Level of Cyclooxygenase-2 and Caspase-3 in the Liver and Kidney of Streptozotocin-Induced Diabetic Rats. Pharm. Sci. 2019;25:37–43. doi: 10.15171/PS.2019.6. [DOI] [Google Scholar]
- 158.Hajihashemi S., Ahmadi M., Chehrei A., Ghanbari F. Ameliorative Effect of Cotreatment with the Methanolicleafextract of Urtica dioica on Acute Kidney Injury Induced by Gentamicin in Rats. Avicenna J. Phytomed. 2020;10:273–286. [PMC free article] [PubMed] [Google Scholar]
- 159.Güneş H.V., Değirmenci İ., Aydin M., Bozan B., Aral E., Tunalier Z., Üstüner C., Erçakir M., Başer K.H.C., Başaran A. The Effects of Rumex patientia L. and Urtica dioica L. on Some Blood and Urine Parameters, and Liver and Kidney Histology in Diabetic Rats. Turk. J. Med. Sci. 1999;29:227–232. [Google Scholar]
- 160.Ahmed A.H., Alabbasy R.H., Khaleel Z.I. The Protective Role of Aqueous Extract of Grape Seeds Vitis vinifera in Some Biochemical Parameters and Histological Changes in Methionine for Liver, Kidney and Heart in Mice (Mus musculus) AIP Conf. Proc. 2020;2213:020304 [Google Scholar]
- 161.Grases F., March J.G., Ramis M., Costa-Bauzá A. The Influence of Zea mays on Urinary Risk Factors for Kidney Stones in Rats. Phytother. Res. 1993;7:146–149. doi: 10.1002/ptr.2650070210. [DOI] [Google Scholar]
- 162.Sukandar E.Y., Sigit J.I., Adiwibowo L.F. Study of Kidney Repair Mechanisms of Corn Silk (Zea mays L. Hair)-Binahong (Anredera Cordifolia (Ten.) Steenis) Leaves Combination in Rat Model of Kidney Failure. Int. J. Pharmacol. 2013;9:12–23. doi: 10.3923/ijp.2013.12.23. [DOI] [Google Scholar]
- 163.Pardede T.R., Bachri M. Analysis on Calcium Solubility in Kidney Stones (In Vitro) and Diuretic Effect (In Vivo) Using Corn Silk (Zea mays L.) Infuse. Asian J. Pharm. Clin. Res. 2018;11:80–83. doi: 10.22159/ajpcr.2018.v11s1.26573. [DOI] [Google Scholar]
- 164.Al Shammari A.M.N. Protective Effect of Ginger (Zingiber officinale) Consumption Against Kidney Damage in Rats. Life Sci. J. 2018;15:80–85. doi: 10.7537/marslsj150118.14. [DOI] [Google Scholar]
- 165.Abdulhameed I.S., Al-Mohamadamin D.F.H., Abed A.B., Abid W.B. The Effect of Ginger Plant (Zingiber officinale) Aqueous Extract on Function The Effect of Ginger Plant (Zingiber officinale) Aqueous Extract on Function and Histological Structure of Kidney in Mice Treated with Carbon Tetrachloride. Int. J. Chem. Tech. Res. 2017;10:208–219. [Google Scholar]
- 166.Johari H., Delirnasab F., Sharifi E., Hemayat-Khah V., Pourdanesh M., Kargar H., Nikpour M., Yazdani M. The Effects of Hydro-Alcoholic Extract of Zingiber officinale on Prevention from Plumbism in Kidney Tissue of Neonatal Rats. Zahedan J. Res. Med. Sci. J. 2012;15:13–17. [Google Scholar]
- 167.Ajith T.A., Nivitha V., Usha S. Zingiber officinale Roscoe Alone and in Combination with α-Tocopherol Protect the Kidney against Cisplatin-Induced Acute Renal Failure. Food Chem. Toxicol. 2007;45:921–927. doi: 10.1016/j.fct.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 168.Abd El Hamid A.A. Effects of Zingiber officinale and Ambrosia maritima on Some Physiological Parameters and Kidney Structure in Rats. J. Anim. Poult. Prod. 2019;10:351–355. doi: 10.21608/jappmu.2019.67871. [DOI] [Google Scholar]
- 169.Joshi D., Kumar S., Belemkar S., Dixit V.A. Zingiber of Fi Cinale and 6-Gingerol Alleviate Liver and Kidney Dysfunctions and Oxidative Stress Induced by Mercuric Chloride in Male Rats: A Protective Approach. Biomed. Pharmacother. 2017;91:645–655. doi: 10.1016/j.biopha.2017.04.108. [DOI] [PubMed] [Google Scholar]
- 170.Mohammad S.I., Abdulqader I.A.M., Shang Z.A. Ameliorative Effect of the Aqueous Extract of Zingiber officinale on the Cadmium-Induced Liver and Kidney Injury in Females Rats. Jordan J. Biol. Sci. 2013;6:231–234. doi: 10.12816/0001539. [DOI] [Google Scholar]
- 171.Peeters L., Van der Auwera A., Beirnaert C., Bijttebier S., Laukens K., Pieters L., Hermans N., Foubert K. Compound Characterization and Metabolic Profile Elucidation after In Vitro Gastrointestinal and Hepatic Biotransformation of an Herniaria Hirsuta Extract Using Unbiased Dynamic Metabolomic Data Analysis. Metabolites. 2020;10:111. doi: 10.3390/metabo10030111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ammor K., Bousta D., Jennan S., Bennani B., Chaqroune A., Mahjoubi F. Phytochemical Screening, Polyphenols Content, Antioxidant Power, and Antibacterial Activity of Herniaria hirsuta from Morocco. Sci. World J. 2018;2018:7470384. doi: 10.1155/2018/7470384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Uddin G., Ali J., Feroz S. Antimicrobial, Antioxidant and Phytochemical Analysis of Herniaria Hirsuta. Univ. Swabi J. 2017;1:84–93. [Google Scholar]
- 174.Van Dooren I., Foubert K., Bijttebier S., Theunis M., Velichkova S., Claeys M., Pieters L., Exarchou V., Apers S. Saponins and Flavonoids from an Infusion of Herniaria Hirsuta. Planta Med. 2016;82:1576–1583. doi: 10.1055/s-0042-118710. [DOI] [PubMed] [Google Scholar]
- 175.Sharma P., Tyagi A., Bhansali P., Pareek S., Singh V., Ilyas A., Mishra R., Poddar N.K. Saponins: Extraction, Bio-Medicinal Properties and Way Forward to Anti-Viral Representatives. Food Chem. Toxicol. 2021;150:112075. doi: 10.1016/j.fct.2021.112075. [DOI] [PubMed] [Google Scholar]
- 176.Cibulski S., Teixeira T.F., Varela A.P.M., de Lima M.F., Casanova G., Nascimento Y.M., Fechine Tavares J., da Silva M.S., Sesterheim P., Souza D.O., et al. IMXQB-80: A Quillaja Brasiliensis Saponin-Based Nanoadjuvant Enhances Zika Virus Specific Immune Responses in Mice. Vaccine. 2021;39:571–579. doi: 10.1016/j.vaccine.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 177.Navarro Del Hierro J., Casado-Hidalgo G., Reglero G., Martin D. The Hydrolysis of Saponin-Rich Extracts from Fenugreek and Quinoa Improves Their Pancreatic Lipase Inhibitory Activity and Hypocholesterolemic Effect. Food Chem. 2021;338:128113. doi: 10.1016/j.foodchem.2020.128113. [DOI] [PubMed] [Google Scholar]
- 178.Mechchate H., Es-safi I., Mohamed Al kamaly O., Bousta D. Insight into Gentisic Acid Antidiabetic Potential Using In Vitro and In Silico Approaches. Molecules. 2021;26:1932. doi: 10.3390/molecules26071932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Amaghnouje A., Mechchate H., Es-Safi I., Boukhira S., S Aliqahtani A., Noman M.O., Nasr A.F., Conte R., Calarco A., Bousta D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum majorana L. Polyphenols in Swiss Albino Mice. Molecules. 2020;25:5653. doi: 10.3390/molecules25235653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kooti W., Daraei N. A Review of the Antioxidant Activity of Celery (Apium Graveolens L.) J. Evid. Based Complement. Altern. Med. 2017;22:1029–1034. doi: 10.1177/2156587217717415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kooti W., Ali-Akbari S., Asadi-Samani M., Ghadery H., Ashtary-Larky D. A Review on Medicinal Plant of Apium Graveolens. Adv. Herb. Med. 2015;1:48–59. [Google Scholar]
- 182.Misic D., Tadic V., Korzeniowska M., Nisavic J., Aksentijevic K., Kuzmanovic J., Zizovic I. Supercritical Fluid Extraction of Celery and Parsley Fruit-Chemical Composition and Antibacterial Activity. Molecules. 2020;25:3163. doi: 10.3390/molecules25143163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Khalid K.A., Hussein M.S. Effect of Cattle and Liquid Manures on Essential Oil and Antioxidant Activities of Celery (Apium graveolens L.) Fruits. J. Essent. Oil Bear. Plants. 2012;15:97–107. doi: 10.1080/0972060X.2012.10644025. [DOI] [Google Scholar]
- 184.Agyare C., Appiah T., Boakye Y.D., Apenteng J.A. Petroselinum crispum: A Review. Med. Spices Veg. Afr. 2017:527–547. doi: 10.1016/B978-0-12-809286-6.00025-X. [DOI] [Google Scholar]
- 185.Yamani A., Bunel V., Antoine M.H., Husson C., Stévigny C., Duez P., Elachouri M., Nortier J. Substitution between Aristolochia and Bryonia Genus in North-Eastern Morocco: Toxicological Implications. J. Ethnopharmacol. 2015;166:250–260. doi: 10.1016/j.jep.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 186.Kharchoufa L., Merrouni I.A., Yamani A., Elachouri M. Profile on Medicinal Plants Used by the People of North Eastern Morocco: Toxicity Concerns. Toxicon. 2018;154:90–113. doi: 10.1016/j.toxicon.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 187.Lakmichi H., Bakhtaoui F.Z., Gadhi C.A., Ezoubeiri A., Jahiri Y.E., Mansouri A.E., Zrara I., Loutfi K. Toxicity Profile of the Aqueous Ethanol Root Extract of Corrigiola Telephiifolia Pourr. (Caryophyllaceae) in Rodents. Evid. Based Complement. Altern. Med. 2011;2011:317090. doi: 10.1155/2011/317090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Bozcuk H., Ozdogan M., Aykurt O., Topçuoğlu F., Öztürk H., Ekinci D., Karadeniz A., Mutlu A., Burgucu D. Urginea mariti-Ma (L.) Baker (Liliaceae) Extract Induces More Cytotoxicitythan Standard Chemotherapeutics in the A549 Non-Smallcell Lung Cancer (NSCLC) Cell Line. Turk. J. Med. Sci. 2011;41:101–108. [Google Scholar]
- 189.Merghoub N., Benbacer L., Amzazi S., Morjani H., Mohamed E.M. Cytotoxic Effect of Some Moroccan Medicinal Plantextracts on Human Cervical Cell Lines. J. Med. Plants. 2009;12:1045–1050. [Google Scholar]
- 190.Bellakhdar J., Claisse R., Fleurentin J., Younos C. Repertory of Standard Herbal Drugs in the Moroccan Pharmacopoea. J. Ethnopharmacol. 1991;35:123–143. doi: 10.1016/0378-8741(91)90064-K. [DOI] [PubMed] [Google Scholar]
- 191.Jahandiez E., Maire R. Catalogue Des Plantes Du Maroc. Volume 1 Minerva, Alger et Lechevalier; Paris, France: 1931. [Google Scholar]
- 192.Jahandiez E., Maire R. Catalogue Des Plantes Du Maroc. Volume 2 Minerva, Alger et Lechevalier; Paris, France: 1932. [Google Scholar]
- 193.Jahandiez E., Maire R. Catalogue Des Plantes Du Maroc. Volume 3 Minerva, Alger et Lechevalier; Paris, France: 1934. [Google Scholar]
- 194.Fennane M., Ibn Tattou M., Mathez J., Ouyahya A., El Oualidi J. Flore Pratique Du Maroc: Pteridophyta, Gymnospermae, Angiospermae (LauraceaeNeuradaceae): Manuel de Détermination Des Plantes Vasculaires, Série Botanique N°36. Volume 1 Travaux de l’Institut Scientifique; Rabat, Morocco: 1999. [Google Scholar]
- 195.Fennane M., Ibn Tattou M., Ouyahya A., El Oualidi J. Volume 2 Travaux de l’Institut Scientifique; Rabat, Morocco: 2007. Flore Pratique Du Maroc: Angiospermae (Leguuminosae—Lentibulariaceae): Manuel de Détermination Des Plantes Vasculaires, Série Botanique N°38. [Google Scholar]
- 196.Fennane M., Ibn Tattou M., Ouyahya A., El Oualidi J. Flore Pratique Du Maroc: Dicotyledones (P.P), Monocotyledones: Manuel de Détermination Des Plantes Vasculaires, Série Botanique N°40. Volume 3 Travaux de l’Institut Scientifique; Rabat, Morocco: 2014. [Google Scholar]
- 197.Angiosperm Phylogeny Group An Update of the Angiosperm Phylogeny Group Classification for the Orders and Families of Flowering Plants: APG III. Bot. J. Linn. Soc. 2009;161:105–121. doi: 10.1111/j.1095-8339.2009.00996.x. [DOI] [Google Scholar]
- 198.Tabuti J.R.S., Dhillion S.S., Lye K.A. Traditional Medicine in Bulamogi County, Uganda: Its Practitioners, Users and Viability. J. Ethnopharmacol. 2003;85:119–129. doi: 10.1016/S0378-8741(02)00378-1. [DOI] [PubMed] [Google Scholar]
- 199.Hoffman B., Gallaher T. Importance Indices in Ethnobotany. Ethnobot. Res. Appl. 2007;5:201–218. doi: 10.17348/era.5.0.201-218. [DOI] [Google Scholar]
- 200.Kayani S., Ahmad M., Sultana S., Khan Shinwari Z., Zafar M., Yaseen G., Hussain M., Bibi T. Ethnobotany of Medicinal Plants among the Communities of Alpine and Sub-Alpine Regions of Pakistan. J. Ethnopharmacol. 2015;164:186–202. doi: 10.1016/j.jep.2015.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data are included in the present study.