Abstract
Zinc (Zn2+) plays an essential role in epithelial physiology. Among its many effects, most prominent is its action to accelerate cell proliferation, thereby modulating wound healing. It also mediates affects in the gastrointestinal system, in the testes, and in secretory organs, including the pancreas, salivary, and prostate glands. On the cellular level, Zn2+ is involved in protein folding, DNA, and RNA synthesis, and in the function of numerous enzymes. In the mammary gland, Zn2+ accumulation in maternal milk is essential for supporting infant growth during the neonatal period. Importantly, Zn2+ signaling also has direct roles in controlling mammary gland development or, alternatively, involution. During breast cancer progression, accumulation or redistribution of Zn2+ occurs in the mammary gland, with aberrant Zn2+ signaling observed in the malignant cells. Here, we review the current understanding of the role of in Zn2+ the mammary gland, and the proteins controlling cellular Zn2+ homeostasis and signaling, including Zn2+ transporters and the Gq-coupled Zn2+ sensing receptor, ZnR/GPR39. Significant advances in our understanding of Zn2+ signaling in the normal mammary gland as well as in the context of breast cancer provides new avenues for identification of specific targets for breast cancer therapy.
Keywords: zinc, zinc signaling, Zn2+ transporters, ZnR/GPR39, mammary gland, breast cancer
1. Zinc, an Essential Micronutrient
Zinc is a vital trace element present in all body tissues and organs. There are differences in recommendation of expert groups regarding the daily allowance of dietary zinc, with suggested intake of 10–20 mg/day, depending on age and gender [1]. Insufficient nutritional zinc intake increases the risk of zinc deficiency [2,3], and low plasma zinc levels have been recorded in as many as 80% of children in developing countries [4,5]. However, zinc deficiency is not unique in low income countries, as it is frequently found in developed countries as well [2,6], with an estimated 17% of the world’s population at risk of zinc deficiency [5]. Insufficient zinc contributes to the etiology of a wide range of pathologies, including immune system failure, digestive system diseases, wound healing, and cognitive impairment [6,7]. The most severe consequence of zinc deficiency is seen in the rare genetic disorder, acrodermatitis enteropathica (AE), a genetic disorder in which impaired intestinal zinc absorption produces an acute, potentially fatal zinc deficiency [8,9]. Importantly, symptoms of zinc deficiency can typically be reversed by zinc supplementation [10,11]. In neonates and young infants, zinc deficiency is associated with skin lesions, growth retardation and impaired development [12,13]. Surprisingly, symptoms of severe zinc deficiency have been observed in exclusively breast-fed babies [14,15]. These infants exhibited a failure to thrive, which was later determined to be related to defective zinc secretion into milk [16,17,18]. Thus, regulation of zinc homeostasis in the mammary gland is crucial during lactation. Conditioned zinc deficiencies are also known to occur in malabsorption syndromes, chronic liver or renal disease, excessive intake of alcohol, and in certain instances of neoplastic malignancies [19]. Indeed, epidemiological studies have linked dietary zinc deficiency to increased risk of cancer [20,21]. However, a complicated picture of both abnormally high and low serum zinc levels in malignant cells, suggests that zinc may be involved in various aspects of cancer progression.
Zinc ions (Zn2+) act as structural components of many enzymes and transcription factors [22]. This important structural function may account for a well-established role of Zn2+ in proliferation, and, indeed, Zn2+ deficiency is associated with attenuation of cell growth [23,24,25]. Moreover, DNA damage was demonstrated in Zn2+-depleted cells, while its supplementation enhanced genome stability even following radiation treatment [26]. Nevertheless, Zn2+ has another important role: acting as a signaling molecule. This conclusion is supported by the large number of proteins involved in compartmentalization of the ion, allowing transient local changes in Zn2+ concentrations, as required for signaling [27,28,29]. Transient changes in cytoplasmic Zn2+ levels and its interaction with cellular kinases or receptors induce what is termed Zn2+ signaling. An example of this is the interaction of Zn2+ with the growth hormone receptor, and its modulation of the insulin-like growth factor-I (IGF-1) pathway [25]. More recent studies have identified Zn2+ as a first messenger, regulating the ZnR/GPR39 G-protein coupled receptor, or as a second messenger, activating major pathways associated with proliferation [30,31,32].
Under physiological conditions, cellular Zn2+ is mostly bound to proteins, and “free” Zn2+ concentrations are in the picomolar to nanomolar range [33]. Yet, “free” Zn2+ ions do occur, and are typically sequestered in cellular vesicles and in organelles, including the Golgi, endoplasmic reticulum, and mitochondria, thereby serving as Zn2+ reservoirs [34,35,36]. Regulation of free Zn2+ in the cytosol is largely achieved either by binding to metallothioneins (MT) or by Zn2+ transporters [27,37,38]. Two protein families are known to mediate Zn2+ transport across cellular membranes, as Zrt- and Irt-related proteins (ZIP) mediate influx into the cytoplasm and Zn2+ transporters (ZnT) move Zn2+ out of the cytoplasm [37,39]. Zn2+ homeostasis is tightly regulated by these proteins under normal circumstances. Intriguingly, Zn2+ levels are dysregulated in the microenvironment surrounding cancer tissue [29]. This finding correlates with changes in the expression profile of various Zn2+ transporters related to the presence of cytokines and growth factors in the tumor’s microenvironment [40].
Zn2+ is emerging as an important player in mammary gland function under both physiological and neoplastic conditions. We will discuss molecular mechanisms mediating the activities of Zn2+ in this tissue.
2. Zn2+ as a Signaling Molecule
For Zn2+ to act as a signaling molecule, both its intracellular and extracellular levels must transiently change. In addition, there must be protein targets that detect these changes and subsequently trigger downstream effects. On a fast scale, intracellular Zn2+ transients involve a Zn2+ release from intracellular stores, e.g., endoplasmic reticulum or Zn2+-binding metallothioneins, in response to stimuli [32,41,42]. For example, antigen binding in mast cells induces extracellular Ca2+ influx that upregulates mitogen-activated kinase (MAPK). This, in turn, activates the release of sequestered Zn2+, thereby raising the intracellular Zn2+ concentration [32]. Similar rapid changes in intracellular Zn2+ levels are produced by its release from stores following epidermal growth factor (EGF) stimulation, which induces casein kinase 2 phosphorylation of a Zn2+ transporter, ZIP7 [43]. In addition, activation of the signal transducers and activators of transcription 3, STAT3 pathway functionally modulates Zn2+ transporter activity [27,43]. Late Zn2+ signaling events, occurring several hours after cell stimulation, and are dependent upon changes in expression of Zn2+ transporters that modulate the transfer of Zn2+ from one compartment to another [27,37]. Both fast and late types of cytoplasmic Zn2+ rises place this ion in the category of classical second messenger, directly regulating major signaling pathways. Among those pathways activated by Zn2+ are EGFR [44], the IGFR-1 [45], and MAPK [46]. Changes in cytosolic Zn2+ levels can also modulate signaling via inhibition of phosphatases activity [47].
Extracellular Zn2+ levels can also transiently increase, as many cell types secrete Zn2+ from intracellular vesicles to the extracellular region. Such release is documented in neurons, pituitary cells, prostate epithelial cells, mast cells, granulocytes, Paneth cells in the intestines, pancreatic cells, and mammary gland epithelia [48,49,50,51,52]. The secreted Zn2+ can then interact with cell surface proteins that have Zn2+-binding modulatory sites, including ion channels, neurotransmitter transporters, and G protein-coupled receptors [53,54,55]. For example, changes in extracellular Zn2+ induce transactivation of EGFR in airway epithelial cells [56], as well as activation of the MAPK pathway [57,58]. Thus, extracellular Zn2+, acting as a first messenger in mouse embryonic stem cells (ES), induces proliferation through upregulation of PI3K, MAPK, and mTOR signaling pathways [59]. Significantly, a Gq protein-coupled receptor (GPCR), initially called ZnR, is a specific target that distinctly senses extracellular Zn2+ and triggers metabotropic calcium (Ca2+) release [60]. Later, it was demonstrated that Zn2+ is the ligand of the orphan receptor GPR39 [61], and we showed that it mediates metabotropic ZnR activity, which is now termed ZnR/GPR39 [30,62].
3. Zn2+ and Its Transporters in the Physiology of Mammary Gland
The importance of Zn2+ to mammary gland development is manifested by the severe consequences of Zn2+ deficiency. Under these conditions, extreme defects in morphology and function, including oxidative stress and inflammation, are described in the non-lactating [63,64] as well as in the lactating gland [65]. During puberty, growth and differentiation of the epithelial glandular structures within the fat pad are driven by the growth hormones, EGF and IGF-1, as well as by the estrogen receptor [66]. Interestingly, all of these pathways are modulated by Zn2+, as described above [67]. During pregnancy and lactation, progesterone and prolactin are responsible for growth of the alveolar epithelial structures, and milk production and secretion [68]. At this stage, Zn2+ is suggested to regulate proliferation and differentiation of the epithelial cells via its interaction with kinases downstream to the prolactin receptor. Mammary gland involution, following lactation, is also highly dependent on Zn2+ that acts as a regulator of apoptosis via mitochondrial or lysosomal pathways [69,70]. The Zn2+ transporter, ZnT2, is essential for accumulation of Zn2+ in lysosomes and assembly of the vacuolar ATPase for initiation of mammary gland involution [70], via a mechanism only partially understood.
A developing offspring requires relatively large amounts of Zn2+ to support its rapid growth and development. Mammary cells are tasked with providing this important nutrient, which they do at a rate of about 1 mg Zn2+ per day during lactation [12,14,71]. Failure of this process results in severe Zn2+ deficiency to the infant, leading to, among other things, growth retardation and skin lesions [18,72]. Even under conditions of moderate Zn2+ deficiency, diminished cognitive development is observed [73,74]. Indeed, Zn2+ is found in secretory vesicles in the mammary gland and its loss is associated with a condition termed neonatal Zn2+ deficiency [15]. During lactation, import of Zn2+ from the circulation into the mammary cells is therefore crucial, and its concentration in milk is maintained over a wide range of maternal dietary Zn2+ intake [75,76,77,78]. In agreement with the strict requirements for Zn2+ in the ingested milk, several Zn2+ transporters are present in the mammary tissue (Figure 1), providing the means to adjust the levels of this ion and its distribution into the appropriate cellular compartments [71,79,80]. Most prominent is ZnT2, which is responsible for the transport of Zn2+ into the secretory vesicles in mammary gland epithelia during lactation [81]. A point mutation in ZnT2 is associated with a dramatic and injurious decrease in levels of Zn2+ in the milk [82]. Additional studies have identified other mutations in the gene encoding ZnT2 which result in severely Zn2+-deficient breast-fed infants [17,82,83]. ZnT2 has also been linked to the function of mammary epithelial cells during all developmental stages [70,81,84]. During pregnancy, in preparation for lactation, ZnT2 is essential for development of alveolar structures and is linked to regulation of cell polarity and formation of acidic secretory vesicles through recruitment of the vacuolar ATPase [84]. Subsequently, during lactation, expression of ZnT2 on the secretory vesicles is responsible for transport of Zn2+ into these milk-containing vesicles [81]. Lactogenic hormones, prolactin and glucocorticoids, regulate ZnT2 expression via activation of kinase signaling cascades [85]. Studies in pancreatic cells, however, indicate that Zn2+ itself can transcriptionally activate ZnT2 expression via activation of the metal transcription factor, MTF-1 [85,86]. Following cessation of lactation, the mammary gland undergoes involution, which is also associated with Zn2+ transporters. An initial stage in this process is relocation of ZnT2 from late endosomes into lysosomes [70,87,88]. Subsequent interaction of ZnT2 with the vacuolar ATPases plays an important role in lysosomal-induced cell death [84,88]. Interestingly, Zn2+ is also required by matrix metalloproteinases (MMPs), which degrade extracellular matrix components and are essential for remodeling of the tissue during pregnancy and involution [28]. The Zn2+ transporters, ZnT5 and ZnT6, are expressed on the Golgi apparatus and play a prominent role in activation of Zn2+-binding enzymes, such as the tissue non-specific alkaline phosphatase (TNAP) [89,90]. Interestingly, these transporters are also associated with Zn2+-deficient maternal milk, as well as low TNAP activity in mothers of severely symptomatic neonates [91].
Figure 1.
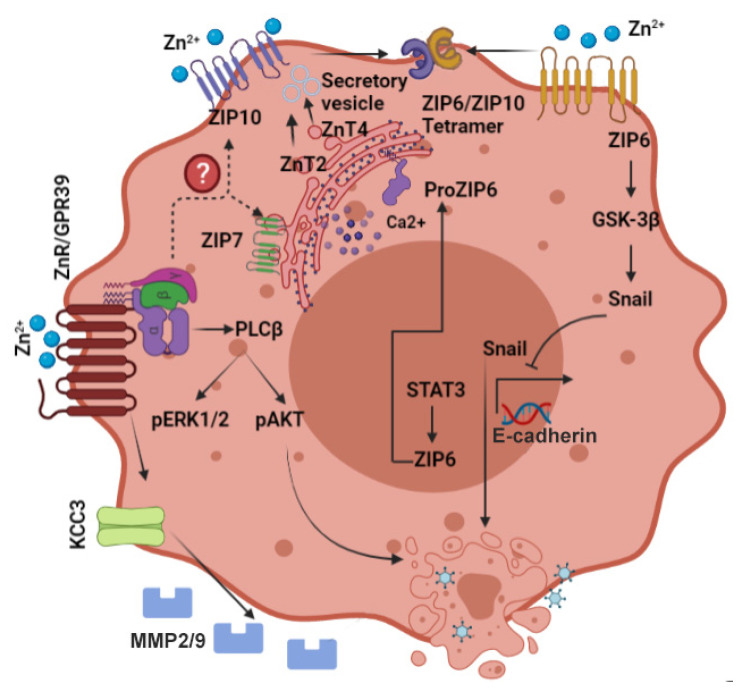
Scheme describing the main signaling pathways activated by Zn2+ in the normal mammary gland cells. The Zn2+ transporters that play a role in mammary gland physiology and their localization are described in the figure. In particular, note ZnT2, that plays a role in vesicular Zn2+ accumulation during lactation and involution, and ZIP3/ZIP5, that were suggested to mediate Zn2+ influx. Arrows depict established pathways, while dotted lines and question marks are putative pathways that remain to be explored.
To accumulate cytoplasmic Zn2+, several Zn2+ importers from the ZIP family have been identified in the mammary epithelium (Figure 1) [67]. Expression of ZIP5 on the surface of epithelial mammary cells suggested that this transporter is involved in Zn2+ uptake [67]. Surprisingly, ZIP3 mediates Zn2+ reuptake from the secreted milk within the alveolar lumen [79]. Release of Zn2+ from intracellular stores and regulation of proliferation are associated with phosphorylation of the Golgi Zn2+ transporter ZIP7, following activation of EGF cascade [43]. It remains to be seen how other ZnTs and ZIPs that are expressed on mammary epithelial cells function to maintain Zn2+ homeostasis and normal function of the mammary gland.
4. Zn2+ Homeostasis and Zn2+ Transporters in Breast Cancer
Considering the well-established roles for Zn2+ in cell proliferation and survival, it is not surprising that this ion also participates in cancer progression [25,92]. However, because Zn2+ modulates immune responses, it may also be considered as an anti-cancer agent, for example via regulation of anti-tumor activity by T-lymphocytes [93,94,95]. Loss of intracellular Zn2+ in prostate tumor epithelial cells is associated with downregulation of the transporter ZIP1 and tumor progression via enhanced cell proliferation [96,97]. Thus, it would appear likely that correcting Zn2+ deficiency will attenuate prostate cancer progression. However, treatment of cancerous prostate tumors by elevating dietary or serum Zn2+, which does not directly affect the “free” Zn2+ content of prostate cells, proved controversial, and more specific tools were sought [98,99]. In breast cancer patients, measurement of total Zn2+ in the serum or within the malignant cells demonstrated abnormal concentrations, suggesting the involvement of Zn2+ dysregulation in progression of this malignancy as well [100,101]. While serum Zn2+ levels are reduced in most cancers, breast and lung tumor tissues have elevated levels of “free” Zn2+ when compared to the normal tissue [102,103]. Tissue Zn2+ accumulation, moreover, is dependent on breast cancer subtype, suggesting this could serve to define the molecular subtype of the cancer and predict its response to therapy [103,104]. Analyses of isotopic composition of Zn2+ in breast tumors, relative to normal mammary tissue, determined that Zn2+ is mostly bound to sulfur-rich proteins, likely metallothioneins in the malignant tissue [105,106]. Such analyses suggest a novel approach for using Zn2+ as a biomarker to identify the tumor subtype and prognosis [107].
Changes in cytoplasmic and extracellular “free” Zn2+ can affect tumor progression via multiple pathways. Cytoplasmic “free” Zn2+ may rise in the context of oxidation/reduction reactions that liberate Zn2+ from metallothioneins [38,108,109] and are very common in tumor environments. This Zn2+ can then modulate phosphatases and kinases associated with signaling pathways involved in cell proliferation and metastasis [47,90]. In addition, increased cytoplasmic “free” Zn2+ can alter the expression of various Zn2+ transporters that are regulated, either directly via glycosylation [110] or via the Zn2+ sensing metal transcription factor MTF-1 [111]. Changes in expression of Zn2+ transporters may, in turn, increase efflux of Zn2+ [112] or affect the vesicular concentration of this ion which is available for release by exocytosis. Moreover, hypoxic conditions and tumor necrosis can also trigger Zn2+ release from the injured cells [113]. Such changes in extracellular “free” Zn2+ levels can regulate matrix metalloproteinases [114,115], which further degrade the extracellular matrix and allow migration (metastasis) of tumor cells. Changes in extracellular Zn2+ have also been shown to activate the G-protein-coupled receptor, ZnR/GPR39, and to promote signaling leading to epithelial repair [30,113,116,117]. Only some of these pathways have been studied in breast cancer tissue, and further analysis may provide new and discrete therapeutic targets.
5. ZnT Family and Breast Cancer
A comprehensive analysis of the distribution of Zn2+ transporters in breast cancer tissue and cell lines revealed that transporter protein levels are aberrant [103]. Significant differences in expression of ZnT5, ZnT6, ZnT8, and ZnT9, between basal-estrogen receptor negative (ER-) and luminal-ER positive (ER+) subtypes of breast cancer were demonstrated [103], though how they affect cellular signaling or breast cancer progression remains unclear (Figure 2).
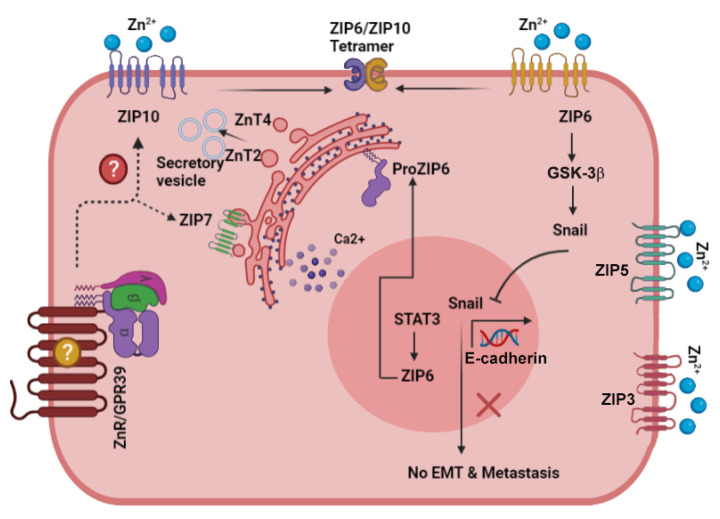
Figure 2.
Scheme describing the activation of signaling pathways involved in breast cancer progression and metastasis. Zn2+ dyshomeostasis results in activation of multiple pathways that affect cell morphology, migration, and proliferation. Note specifically ZIP10 and ZIP6 that interact to regulate epithelial mesenchymal transition, and ZnR/GPR39 that induces membrane protrusions and MMP2/9 release. Arrows depict established pathways, while dotted lines and question marks are putative pathways for regulation and interaction.
ZnT2
Studies intended to show how Zn2+ accumulation is regulated in tumor cells have largely focused on ZnT2, which has a well-described role in the normal mammary gland (see Section 3). In one such study, ZnT2 was overexpressed in an ER+ breast cancer cell line (T47D) where it appeared to enhance vesicular Zn2+ levels in these cells [118]. Knockdown of this transporter increased cytosolic, i.e., “free” Zn2+, as well as autophagic cell death, suggesting that, by accumulating Zn2+ in vesicles/reducing cytosolic Zn2+, ZnT2 reduces cell death and enhances survival of the malignant cells [118]. In addition, increased ZnT2 expression levels in an ER breast cancer cell model induced lysosomal Zn2+ accumulation and reduced MMP-2 activity, leading to a decrease in invasive properties of the cells [119]. When breast cancer tumor biopsies were studied, ZnT2 overexpression was demonstrated in luminal (ER+) breast tumors compared to its level in basal (ER-) tumors, and corresponding cell line models [103]. Indeed, in MDA-MB-231 cells, representing basal tumors, overexpression of ZnT2 increased vesicular Zn2+ accumulation and decreased their invasiveness [103]. It will be interesting to monitor whether the well-described mutations in ZnT2, which are associated with low levels of Zn2+ secretion during lactation, are also linked to specific breast cancer subtypes. In addition, dimerization of ZnT transporters was suggested to provide anther pathway for their localization and functional regulation [120]. Changes in the ZnT2 expression pattern in breast cancer cells may provide a key to modulating Zn2+ homeostasis in this tumor. However, it should be noted that such changes may affect expression patterns of other ZnT family members, and this should be carefully addressed.
6. ZIP Family and Breast Cancer
The LIV-1 subfamily of ZIP proteins was initially characterized as estrogen-sensitive proteins in breast cancer cells (Figure 2), and specifically associated with ER+ breast cancer, and with metastatic spread of breast cancer tumors [121,122]. Members of the LIV-1 family include ZIP4–8 and ZIP10, all of whom contain a histidine-rich sequence on transmembrane domain 5 that is associated with their Zn2+ transport activity [123,124].
6.1. ZIP6
The ZIP6 protein, suggested to mediate cytoplasmic Zn2+ uptake, was first identified in ER+ breast tumors and is considered as an indicator of this type of cancer [125,126,127]. Expression of ZIP6 is observed primarily in metastases to lymph nodes [128]. Interestingly, ZIP6 is a downstream target of the transcription factor, i.e., signal transducer and activator of transcription 3 (STAT3), which is involved in epithelial to mesenchymal transition (EMT) during development of zebrafish embryos [129]. In breast cancer cells, activation of ZIP6 occurs by its N-terminal cleavage, which induces its translocation to the plasma membrane, triggering cytoplasmic Zn2+ influx. This “free” Zn2+ rise then activates nuclear localization of the transcription factor, Snail, likely by inhibition of glycogen synthase kinase, GSK-3β, and by reduced expression of the junctional protein, E-cadherin [130]. Repression of E-cadherin expression reduces cell adhesion and induces EMT, a hallmark of invasive cancer. Indeed, silencing ZIP6 in HeLa cervical cancer cells led to reduced cell invasion and dysregulation of the Snail pathway [131].
6.2. ZIP7
ZIP7 is localized to the endoplasmic reticulum and is responsible for Zn2+ release from intracellular stores [132]. This transporter is regulated by phosphorylation of casein kinase 2 which induces an increase in cellular Zn2+ and tyrosine kinase activation [133]. Aberrant signaling triggered by ZIP7 in hormone-resistant breast cancer cells and changes in its expression level in breast tumor tissues have strongly linked this transporter to breast cancer development [134,135]. ZIP7 induces a cytoplasmic Zn2+ rise that activates major signaling cascades such as c-SRC, EGFR, and MAPK, and thereby enhances invasion of these cells [135]. Numerous therapeutic approaches are aimed to attenuate tyrosine kinase pathways that play a major role in cancer progression [136,137]. In this way, ZIP7 can serve as a target for regulation of tyrosine kinase pathways. Moreover, protein kinase B/AKT that is constitutively activated in breast cancer cells [138] is also directly activated by ZIP7-mediated Zn2+ release in hormone-resistant cells [139]. In a tamoxifen-resistant MCF-7-derived breast cancer cell model (TamR), increased expression of ZIP7 leads to a more aggressive phenotype than the original MCF-7 cells [135]. Removal of ZIP7 from these TamR cells inhibits EGFR and IGF-1R [135], both of which are known to trigger growth of these cells [140]. Significantly, analysis of breast cancer biopsies showed that ZIP7 was positively linked with the proliferation marker Ki67, and was increased in breast cancer samples with metastases to lymph nodes [122,132].
6.3. ZIP10
Expression of ZIP10 mRNA is increased in breast cancer cell lines, while its reduction (by knockdown) attenuated migration of these cells [141]. Corresponding elevated levels of ZIP10 mRNA were detected in lymph node metastases of breast cancer biopsies [141]. Several Zn2+ transporters have been shown to act as heterodimers, and their interaction is associated with synergistic function. Among these, most prominent is the interaction between ZIP10 and ZIP6 [142]. Heterodimerization of ZIP6 and ZIP10 synergistically enhanced glucose-dependent breast cancer cell migration [143], which could be associated with increased breast cancer mortality in diabetic patients [144]. Knockdown of ZIP10 results in morphological changes in breast cancer cells, which are followed with loss of adhesion and enhanced proliferation of the cells [142]. An elegant recent study has shown that surface expression of ZIP10 and ZIP6 heterodimers is upregulated during mitosis [145]. The Zn2+ influx mediated by these transporters drives serine phosphorylation of STAT3 by an unknown mechanism, which then mediates microtubule re-organization and mitosis [145].
7. The Zinc Sensing Receptor ZnR/GPR39 and Cancer
The selective Zn2+-sensing Gq-protein-coupled receptor (ZnR/GPR39) acts as a link between extracellular Zn2+ and intracellular pathways that regulate cell proliferation and survival [30,146]. The ZnR/GPR39 is a member of the rhodopsin-like family, positioned on chromosome 2, q21.2 that encodes two splice variants: GPR39-1a, a full-length receptor of 435 amino acids via two exons 52 kilo Dalton (kDa) in size, and GPR39-1b, which is encoded by the first exon alone (1-285), resulting in a 32 kDa protein that is non-functional [147]. The first exon contains five transmembrane domains (TM 1–5) and the second contains two TM domains (TM 6–7). It was shown that the full-length protein is activated by extracellular Zn2+ and mediates metabotropic Ca2+ signaling via the IP3 pathway in colonocytes, keratinocytes, and neurons [62,113,116,148,149]. Extracellular Zn2+ interacts with ZnR/GPR39 and initiates cell signaling events that trigger release of intracellular Ca2+ [60,61,150].
Downstream to the Ca2+ signaling, ZnR/GPR39 activates MAPK via extracellular signal-regulated kinase (ERK1/2), and the phosphoinositide-3 (PI3)-kinase pathway [148], both closely linked to cell growth and proliferation [151]. Indeed, cell proliferation and migration, mediated by ZnR/GPR39 in a Zn2+-dependent manner, accelerated wound closure in HaCaT keratinocytes [113]. A similar effect on colon epithelial cells was observed in a model of colitis, where the recovery of colon tissue in mice lacking ZnR/GPR39 was impaired [152]. However, BrdU staining indicated that baseline proliferation rates were not significantly different between wildtype and ZnR/GPR39 knockout colon epithelial cells. In prostate cancer cells, ZnR/GPR39 was also associated with upregulation of ERK1/2 and PI3K phosphorylation, and enhanced cell growth [149]. Interestingly, cytoplasmic Zn2+ levels in the prostate are high [97], and expression of the ZnR/GPR39 in non-cancer cells was very low, likely due to desensitization of the receptor. In prostate cancer, however, when the Zn2+ concentration is decreased [96,97], ZnR/GPR39 may be re-sensitized, such that extracellular Zn2+ binding to it will activate Ca2+ signaling, leading to proliferation. Further evidence for the role of ZnR/GPR39 in cancer comes from a study of esophageal squamous cell carcinoma (ESCC) shows that overexpression of GPR39 is associated with lymph node metastasis [153]. In contrast, silencing of GPR39 significantly reduced mobility of the ESCC cells. Recently, our lab has determined that ZnR/GPR39 is present in breast cancer cells, and upon activation by extracellular Zn2+, increases cell proliferation [154]. Furthermore, consistent with a role for ZnR/GPR39 in breast cancer progression, we demonstrated an increase in expression of the receptor in biopsies from higher grade mammary tumors [154].
More evidence of a role for ZnR/GPR39 activation in cancer progression comes from work showing that it regulates several important ion transporters thought to play a role in cell survival and proliferation. In neurons and colonocytes, activation of ZnR/GPR39 enhanced H+ transport by the Na+/H+ exchanger (NHE) [116,155]. ZnR/GPR39 upregulation of NHE was also seen in colon epithelial cells from wild type, but not ZnR/GPR39 knockout mice [156]. Furthermore, inhibition of PI3K pathways in HT29 colonocytes, which exhibit ZnR/GPR39 signaling, resulted in reversal of the effect of Zn2+ on NHE activity and attenuation of the recovery from acidification [116]. In keratinocytes, upregulation of NHE by the ZnR/GPR39 signaling cascade resulted in more rapid epithelial wound repair [113]. Upregulation of NHE activity via ZnR/GPR39 signaling was also observed in mouse hippocampal neurons, leading to recovery of intercellular pH [155], which helps to promote neuronal survival [157]. It has been hypothesized that upregulation of NHE activity may lead to tumor cell proliferation by enhancing aerobic glycolysis [158]. Moreover, elevated NHE activity is correlated with increased cellular pH and decreased extracellular pH. Lowering extracellular pH in tumors is a known trigger for metastasis [159]. In addition to NHE1 stimulation of Na+/H+ exchange, it also acts as a scaffold protein by recruiting cytoskeletal linker proteins, PI3K and AKT, thereby triggering cell growth [160,161]. It is thought that this may actually be the mechanism mediating the effects of Zn2+ and ZnR/GPR39 in cell proliferation and migration.
In hippocampal CA3 neurons, ZnR/GPR39 increases surface expression and activation of K+/Cl− -cotransport (KCC) isoform 2 [162,163]. KCC2 has a role in regulating the Cl− gradient and, thereby, GABAA currents in neurons [164]. Similar ZnR/GPR39-dependent upregulation of KCC1 was demonstrated in colonocytes [165] and resulted in enhanced Cl− absorption and decreased water loss in a diarrhea model. Other KCC family members are essential for regulating epithelial cell volume, a function linked to morphological changes underlying formation of plasma membrane protrusions involved in metastasis as well as cell proliferation [166]. Studies in breast cancer cells show that KCC3 is upregulated by ZnR/GPR39 in the ER+ cell lines [167]. A recent study reports that ZnR/GPR39 activation in breast cancer cells leads to activation of K+/Cl- cotransporter KCC3, which may locally affect cell volume resulting in formation of protrusions [53]. In addition, ZnR/GPR39 triggers release of matrix metalloproteases, MMP-2 and MMP-9 [53], which degrade the extracellular matrix and facilitate migration. This pathway is essential for enhancing invasion of breast cancer cells through Matrigel and scratch closure. These results indicate that ZnR/GPR39 may be a novel therapeutic target for controlling breast tumor growth and progression.
8. Emerging Targets for Breast Cancer Treatment
Development of the normal breast is mostly regulated by estrogen acting through the ER [168], thereby controlling a variety of functions, including cell proliferation, angiogenesis, and apoptosis [66]. The ER signaling is used by breast cancer cells and, in the initial stages of the disease, serves as a major, estrogen-driven, survival pathway [168,169,170]. Therefore, expression of ER is used as a biomarker to guide therapy in breast cancer [171]. As a first line, ER-expressing (positive) breast cancer patients are generally treated with antihormones such as tamoxifen [169,172]. Electron microscope analysis showed that tamoxifen treatment of breast cancer cells induced apoptosis or autophagy, with some cells displaying signs of both [173,174]. While this treatment is initially highly effective in attenuating growth, resistance of tumors to tamoxifen gradually develops usually in all patients, leading to intermittence and re-appearance of the disease [175,176]. Tamoxifen resistance may occur through modification of different signaling pathways involving growth factors such as EGF, IGF receptors, and the HER2 tyrosine kinase receptor [77,170,172]. Even though detection and treatment of metastatic breast cancer have advanced in recent years, the mortality caused by this disease remains high, due principally to such therapy-resistant breast cancer cells [169,177,178]. Therapy-resistant breast cancer can be intrinsic, which accounts for approximately 15–20% of ER-positive breast cancer, or acquired, which accounts for an additional 25–30%, and involves the loss of the hormonal effects [179,180]. A more aggressive type of breast cancer is characterized by the absence of all hormone receptors and is referred to as triple negative breast cancer, or TNBC. This type of tumor is defined by negative expression of ER, progesterone receptor (PR), and human epidermal growth factor receptor-2 (HER2) proteins [181]. TNBC has a higher rate of frequency and shorter survival in the metastatic setting compared to other subtypes of breast cancer. The TNBC cells show a large heterogeneity, and have been divided into several subtypes, presenting unique molecular signatures. Many of these involve mutations in P53 or BRCA genes [171]. Recent and novel therapeutic approaches are aimed at specific checkpoints in the cell cycle, but a lack of biomarkers in TNBC means that chemotherapy remains the first line of treatment. The emergence of immune therapy has led to the targeting of the highly expressed programmed death-ligand 1 (PD-L1) checkpoint protein and to the large numbers of lymphocytes in a subset of TNBC tumors [182]. Identification of novel targets on TNBC tumor cells that can be modulated to reduce proliferation and invasiveness is an as-yet-unmet clinical need. Previous reports suggested specific proteins, such as ER, P53, or of more general targets including GPCRs, which are overexpressed in cancer cells compared to normal tissue [183,184]. As such, ZnR/GPR39, which was shown previously to be overexpressed in breast cancer tissue [154] may prove to be a convenient and effective target. In ER breast cancer cell lines, ZnR/GPR39 activation increased MAPK and PI3K phosphorylation [154]. Thus, ZnR/GPR39 may provide an alternative trigger to MAPK and PI3K/mTOR pathway in ER cells, thereby increasing their cell growth. In agreement with this, increased expression of ZnR/GPR39 was associated with more aggressive phenotypes in breast cancer biopsies [154]. Considering the well-known ability of the pharmaceutical industry to identify modulators of GPCRs, future identification of specific agonists or antagonists that interact with ZnR/GPR39 seems assured, and may well prove an important therapy in and of itself, or in combination with immunotherapy. Such molecular modulators are likely to be at least useful as adjunct therapies given the sheer number of proteins modulated by Zn2+, among them are the Zn2+ transporters discussed above. Thus, a modulator of ZnR/GPR39 that will not trigger other Zn2+-dependent pathways is of clear importance.
9. Summary and Conclusions
Zinc is an essential ion, required for cell function and proliferation. Less appreciated is its role in promoting oncogenesis and in metastasis. The important role of this ion in itself and of the transporters that are involved in maintaining cellular Zn2+ homeostasis suggest that they may provide novel targets for cancer therapies. In addition, our work suggests that ZnR/GPR39 can activate critical compensatory pathways following the loss of hormone regulation of mammary cell growth. Thus, ZnR/GPR39 represents a highly viable target for new molecular or immune approaches to hormone-resistant cancers and TNBC.
Acknowledgments
We thank Ze’ev Silverman for fruitful discussions and thoughtful editing of the manuscript.
Author Contributions
M.C. Conceptualization, writing; M.H. Conceptualization, writing and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Israeli Science Foundation (ISF # 812/20 to M.H.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gibson R.S., King J.C., Lowe N. A Review of Dietary Zinc Recommendations. Food Nutr. Bull. 2016;37:443–460. doi: 10.1177/0379572116652252. [DOI] [PubMed] [Google Scholar]
- 2.Sandstead H.H., Freeland-Graves J.H. Dietary phytate, zinc and hidden zinc deficiency. J. Trace Elem. Med. Biol. 2014;28:414–417. doi: 10.1016/j.jtemb.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 3.King J.C., Brown K.H., Gibson R.S., Krebs N.F., Lowe N.M., Siekmann J.H., Raiten D.J. Biomarkers of Nutrition for Development (BOND)-Zinc Review. J. Nutr. 2015;146:858S–885S. doi: 10.3945/jn.115.220079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wessells K.R., Brown K.H. Estimating the global prevalence of zinc deficiency: Results based on zinc availability in national food supplies and the prevalence of stunting. PLoS ONE. 2012;7:e50568. doi: 10.1371/journal.pone.0050568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hesse S. Zinc Deficiency. In: de Pee S., Taren D., Bloem M., editors. Nutrition and Health in a Developing World. 3rd ed. Humana Press; Cham, Switzerland: 2017. pp. 265–285. [Google Scholar]
- 6.Prasad A.S. Discovery of human zinc deficiency: 50 years later. J. Trace Elem. Med. Biol. 2012;26:66–69. doi: 10.1016/j.jtemb.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Prasad A.S. Clinical manifestations of zinc deficiency. Annu. Rev. Nutr. 1985;5:341–363. doi: 10.1146/annurev.nu.05.070185.002013. [DOI] [PubMed] [Google Scholar]
- 8.Dufner-Beattie J., Weaver B.P., Geiser J., Bilgen M., Larson M., Xu W., Andrews G.K. The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum. Mol. Genet. 2007;16:1391–1399. doi: 10.1093/hmg/ddm088. [DOI] [PubMed] [Google Scholar]
- 9.Neldner K.H., Hambidge K.M., Walravens P.A. Acrodermatitis enteropathica. Int. J. Derm. 1978;17:380–387. doi: 10.1111/ijd.1978.17.5.380. [DOI] [PubMed] [Google Scholar]
- 10.Neldner K.H., Hambidge K.M. Zinc therapy of acrodermatitis enteropathica. N. Engl. J. Med. 1975;292:879–882. doi: 10.1056/NEJM197504242921702. [DOI] [PubMed] [Google Scholar]
- 11.Brown K.H., Peerson J.M., Rivera J., Allen L.H. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am. J. Clin. Nutr. 2002;75:1062–1071. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 12.Ackland M.L., Michalczyk A.A. Zinc and infant nutrition. Arch. Biochem. Biophys. 2016;611:51–57. doi: 10.1016/j.abb.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Prasad A.S. Zinc deficiency. Bmj. 2003;326:409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krebs N.F., Hambidge K.M. Zinc requirements and zinc intakes of breast-fed infants. Am. J. Clin. Nutr. 1986;43:288–292. doi: 10.1093/ajcn/43.2.288. [DOI] [PubMed] [Google Scholar]
- 15.Murphy J.F., Gray O.P., Rendall J.R., Hann S. Zinc deficiency: A problem with preterm breast milk. Early. Hum. Dev. 1985;10:303–307. doi: 10.1016/0378-3782(85)90062-3. [DOI] [PubMed] [Google Scholar]
- 16.Kuramoto Y., Igarashi Y., Tagami H. Acquired zinc deficiency in breast-fed infants. Semin. Derm. 1991;10:309–312. [PubMed] [Google Scholar]
- 17.Lasry I., Seo Y.A., Ityel H., Shalva N., Pode-Shakked B., Glaser F., Berman B., Berezovsky I., Goncearenco A., Klar A., et al. A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J. Biol. Chem. 2012;287:29348–29361. doi: 10.1074/jbc.M112.368159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman A.W., Hambidge K.M., Lepow M.L., Greenberg R.D., Stover M.L., Casey C.E. Acrodermatitis in breast-fed premature infants: Evidence for a defect of mammary zinc secretion. Pediatrics. 1982;69:176–183. [PubMed] [Google Scholar]
- 19.Prasad A.S. Zinc deficiency in human subjects. Prog. Clin. Biol. Res. 1983;129:1–33. [PubMed] [Google Scholar]
- 20.Leone N., Courbon D., Ducimetiere P., Zureik M. Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology. 2006;17:308–314. doi: 10.1097/01.ede.0000209454.41466.b7. [DOI] [PubMed] [Google Scholar]
- 21.Levenson C.W., Somers R.C. Nutritionally regulated biomarkers for breast cancer. Nutr. Rev. 2008;66:163–166. doi: 10.1111/j.1753-4887.2008.00020.x. [DOI] [PubMed] [Google Scholar]
- 22.Vallee B.L., Falchuk K.H. The biochemical basis of zinc physiology. Physiol. Rev. 1993;73:79–118. doi: 10.1152/physrev.1993.73.1.79. [DOI] [PubMed] [Google Scholar]
- 23.Corniola R.S., Tassabehji N.M., Hare J., Sharma G., Levenson C.W. Zinc deficiency impairs neuronal precursor cell proliferation and induces apoptosis via p53-mediated mechanisms. Brain Res. 2008;1237:52–61. doi: 10.1016/j.brainres.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 24.Wong S.H., Zhao Y., Schoene N.W., Han C.T., Shih R.S., Lei K.Y. Zinc deficiency depresses p21 gene expression: Inhibition of cell cycle progression is independent of the decrease in p21 protein level in HepG2 cells. Am. J. Physiol. Cell Physiol. 2007;292:C2175–C2184. doi: 10.1152/ajpcell.00256.2006. [DOI] [PubMed] [Google Scholar]
- 25.MacDonald R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000;130:1500S–1508S. doi: 10.1093/jn/130.5.1500S. [DOI] [PubMed] [Google Scholar]
- 26.Sharif R., Thomas P., Zalewski P., Graham R.D., Fenech M. The effect of zinc sulphate and zinc carnosine on genome stability and cytotoxicity in the WIL2-NS human lymphoblastoid cell line. Mutat. Res. 2011;720:22–33. doi: 10.1016/j.mrgentox.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Fukada T., Yamasaki S., Nishida K., Murakami M., Hirano T. Zinc homeostasis and signaling in health and diseases: Zinc signaling. J. Biol. Inorg. Chem. 2011;16:1123–1134. doi: 10.1007/s00775-011-0797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelleher S.L., McCormick N.H., Velasquez V., Lopez V. Zinc in specialized secretory tissues: Roles in the pancreas, prostate, and mammary gland. Adv. Nutr. 2011;2:101–111. doi: 10.3945/an.110.000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murakami M., Hirano T. Intracellular zinc homeostasis and zinc signaling. Cancer Sci. 2008;99:1515–1522. doi: 10.1111/j.1349-7006.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hershfinkel M. The Zinc Sensing Receptor, ZnR/GPR39, in Health and Disease. Int. J. Mol. Sci. 2018;19:439. doi: 10.3390/ijms19020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maret W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017;18:2285. doi: 10.3390/ijms18112285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamasaki S., Sakata-Sogawa K., Hasegawa A., Suzuki T., Kabu K., Sato E., Kurosaki T., Yamashita S., Tokunaga M., Nishida K., et al. Zinc is a novel intracellular second messenger. J. Cell Biol. 2007;177:637–645. doi: 10.1083/jcb.200702081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maret W., Krezel A. Cellular zinc and redox buffering capacity of metallothionein/thionein in health and disease. Mol. Med. 2007;13:371–375. doi: 10.2119/2007-00036.Maret. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kashiv Y., Austin J.R., Lai B., Rose V., Vogt S., El-Muayed M. Imaging trace element distributions in single organelles and subcellular features. Sci. Rep. 2016;6:21437. doi: 10.1038/srep21437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Q., Haragopal H., Slepchenko K.G., Stork C., Li Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharm. 2016;8:35–43. [PMC free article] [PubMed] [Google Scholar]
- 36.Sensi S.L., Ton-That D., Weiss J.H., Rothe A., Gee K.R. A new mitochondrial fluorescent zinc sensor. Cell Calcium. 2003;34:281–284. doi: 10.1016/S0143-4160(03)00122-2. [DOI] [PubMed] [Google Scholar]
- 37.Kambe T., Taylor K.M., Fu D. Zinc transporters and their functional integration in mammalian cells. J. Biol. Chem. 2021;296:100320. doi: 10.1016/j.jbc.2021.100320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maret W. Metallothionein redox biology in the cytoprotective and cytotoxic functions of zinc. Exp. Gerontol. 2008;43:363–369. doi: 10.1016/j.exger.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Hara T., Takeda T.A., Takagishi T., Fukue K., Kambe T., Fukada T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017;67:283–301. doi: 10.1007/s12576-017-0521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alam S., Kelleher S.L. Cellular mechanisms of zinc dysregulation: A perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4:875–903. doi: 10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Knoch M.E., Hartnett K.A., Hara H., Kandler K., Aizenman E. Microglia induce neurotoxicity via intraneuronal Zn2+ release and a K+ current surge. Glia. 2008;56:89–96. doi: 10.1002/glia.20592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McCord M.C., Aizenman E. Convergent Ca2+ and Zn2+ signaling regulates apoptotic Kv2.1 K+ currents. Proc. Natl. Acad. Sci. USA. 2013;110:13988–13993. doi: 10.1073/pnas.1306238110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor K.M., Hiscox S., Nicholson R.I., Hogstrand C., Kille P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 2012;5:ra11. doi: 10.1126/scisignal.2002585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samet J.M., Dewar B.J., Wu W., Graves L.M. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol. Appl. Pharm. 2003;191:86–93. doi: 10.1016/S0041-008X(03)00219-9. [DOI] [PubMed] [Google Scholar]
- 45.Haase H., Maret W. Intracellular zinc fluctuations modulate protein tyrosine phosphatase activity in insulin/insulin-like growth factor-1 signaling. Exp. Cell Res. 2003;291:289–298. doi: 10.1016/S0014-4827(03)00406-3. [DOI] [PubMed] [Google Scholar]
- 46.Kim Y.M., Reed W., Wu W., Bromberg P.A., Graves L.M., Samet J.M. Zn2+-induced IL-8 expression involves AP-1, JNK, and ERK activities in human airway epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2006;290:L1028–L1035. doi: 10.1152/ajplung.00479.2005. [DOI] [PubMed] [Google Scholar]
- 47.Bellomo E., Birla Singh K., Massarotti A., Hogstrand C., Maret W. The metal face of protein tyrosine phosphatase 1B. Coord. Chem. Rev. 2016;327–328:70–83. doi: 10.1016/j.ccr.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Podany A.B., Wright J., Lamendella R., Soybel D.I., Kelleher S.L. ZnT2-Mediated Zinc Import Into Paneth Cell Granules Is Necessary for Coordinated Secretion and Paneth Cell Function in Mice. Cell Mol. Gastroenterol. Hepatol. 2016;2:369–383. doi: 10.1016/j.jcmgh.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hennigar S.R., Kelleher S.L. Zinc networks: The cell-specific compartmentalization of zinc for specialized functions. Biol. Chem. 2012;393:565–578. doi: 10.1515/hsz-2012-0128. [DOI] [PubMed] [Google Scholar]
- 50.Cole T.B., Wenzel H.J., Kafer K.E., Schwartzkroin P.A., Palmiter R.D. Elimination of zinc from synaptic vesicles in the intact mouse brain by disruption of the ZnT3 gene. Proc. Natl. Acad. Sci. USA. 1999;96:1716–1721. doi: 10.1073/pnas.96.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakashima-Kaneda K., Matsuda A., Mizuguchi H., Sasaki-Sakamoto T., Saito H., Ra C., Okayama Y. Regulation of IgE-dependent zinc release from human mast cells. Int. Arch. Allergy Immunol. 2013;161((Suppl. S2)):44–51. doi: 10.1159/000350359. [DOI] [PubMed] [Google Scholar]
- 52.Gee K.R., Zhou Z.L., Qian W.J., Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J. Am. Chem. Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- 53.Chakraborty M., Asraf H., Sekler I., Hershfinkel M. ZnR/GPR39 controls cell migration by orchestrating recruitment of KCC3 into protrusions, re-organization of actin and activation of MMP. Cell Calcium. 2021;94:102330. doi: 10.1016/j.ceca.2020.102330. [DOI] [PubMed] [Google Scholar]
- 54.Gore A., Moran A., Hershfinkel M., Sekler I. Inhibitory mechanism of store-operated Ca2+ channels by zinc. J. Biol. Chem. 2004;279:11106–11111. doi: 10.1074/jbc.M400005200. [DOI] [PubMed] [Google Scholar]
- 55.Krall R.F., Tzounopoulos T., Aizenman E. The Function and Regulation of Zinc in the Brain. Neuroscience. 2021;457:235–258. doi: 10.1016/j.neuroscience.2021.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wu W., Silbajoris R.A., Whang Y.E., Graves L.M., Bromberg P.A., Samet J.M. p38 and EGF receptor kinase-mediated activation of the phosphatidylinositol 3-kinase/Akt pathway is required for Zn2+-induced cyclooxygenase-2 expression. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L883–L889. doi: 10.1152/ajplung.00197.2005. [DOI] [PubMed] [Google Scholar]
- 57.Samet J.M., Graves L.M., Quay J., Dailey L.A., Devlin R.B., Ghio A.J., Wu W., Bromberg P.A., Reed W. Activation of MAPKs in human bronchial epithelial cells exposed to metals. Am. J. Physiol. 1998;275:L551–L558. doi: 10.1152/ajplung.1998.275.3.L551. [DOI] [PubMed] [Google Scholar]
- 58.Hansson A. Extracellular zinc ions induces mitogen-activated protein kinase activity and protein tyrosine phosphorylation in bombesin-sensitive Swiss 3T3 fibroblasts. Arch. Biochem. Biophys. 1996;328:233–238. doi: 10.1006/abbi.1996.0168. [DOI] [PubMed] [Google Scholar]
- 59.Ryu J.M., Lee M.Y., Yun S.P., Han H.J. Zinc chloride stimulates DNA synthesis of mouse embryonic stem cells: Involvement of PI3K/Akt, MAPKs, and mTOR. J. Cell Physiol. 2009;218:558–567. doi: 10.1002/jcp.21628. [DOI] [PubMed] [Google Scholar]
- 60.Hershfinkel M., Moran A., Grossman N., Sekler I. A zinc-sensing receptor triggers the release of intracellular Ca2+ and regulates ion transport. Proc. Natl. Acad. Sci. USA. 2001;98:11749–11754. doi: 10.1073/pnas.201193398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yasuda S., Miyazaki T., Munechika K., Yamashita M., Ikeda Y., Kamizono A. Isolation of Zn2+ as an endogenous agonist of GPR39 from fetal bovine serum. J. Recept. Signal. Transduct. Res. 2007;27:235–246. doi: 10.1080/10799890701506147. [DOI] [PubMed] [Google Scholar]
- 62.Besser L., Chorin E., Sekler I., Silverman W.F., Atkin S., Russell J.T., Hershfinkel M. Synaptically released zinc triggers metabotropic signaling via a zinc-sensing receptor in the hippocampus. J. Neurosci. 2009;29:2890–2901. doi: 10.1523/JNEUROSCI.5093-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bostanci Z., Mack Jr R.P., Lee S., Soybel D.I., Kelleher S.L. Paradoxical zinc toxicity and oxidative stress in the mammary gland during marginal dietary zinc deficiency. Reprod. Toxicol. 2015;54:84–92. doi: 10.1016/j.reprotox.2014.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mutch P.B., Hurley L.S. Mammary gland function and development: Effect of zinc deficiency in rat. Am. J. Physiol. 1980;238:E26–E31. doi: 10.1152/ajpendo.1980.238.1.E26. [DOI] [PubMed] [Google Scholar]
- 65.Dempsey C., McCormick N.H., Croxford T.P., Seo Y.A., Grider A., Kelleher S.L. Marginal maternal zinc deficiency in lactating mice reduces secretory capacity and alters milk composition. J. Nutr. 2012;142:655–660. doi: 10.3945/jn.111.150623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rusidzé M., Adlanmérini M., Chantalat E., Raymond-Letron I., Cayre S., Arnal J.F., Deugnier M.A., Lenfant F. Estrogen receptor-α signaling in post-natal mammary development and breast cancers. Cell Mol. Life Sci. 2021;78:5681–5705. doi: 10.1007/s00018-021-03860-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McCormick N.H., Hennigar S.R., Kiselyov K., Kelleher S.L. The biology of zinc transport in mammary epithelial cells: Implications for mammary gland development, lactation, and involution. J. Mammary. Gland. Biol. Neoplasia. 2014;19:59–71. doi: 10.1007/s10911-013-9314-4. [DOI] [PubMed] [Google Scholar]
- 68.Lee S., Kelleher S.L. Molecular regulation of lactation: The complex and requisite roles for zinc. Arch. Biochem. Biophys. 2016;611:86–92. doi: 10.1016/j.abb.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 69.McCormick N.H., Lee S., Hennigar S.R., Kelleher S.L. ZnT4 (SLC30A4)-null (“lethal milk”) mice have defects in mammary gland secretion and hallmarks of precocious involution during lactation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016;310:R33–R40. doi: 10.1152/ajpregu.00315.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hennigar S.R., Seo Y.A., Sharma S., Soybel D.I., Kelleher S.L. ZnT2 is a critical mediator of lysosomal-mediated cell death during early mammary gland involution. Sci. Rep. 2015;5:8033. doi: 10.1038/srep08033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelleher S.L., Seo Y.A., Lopez V. Mammary gland zinc metabolism: Regulation and dysregulation. Genes. Nutr. 2009;4:83–94. doi: 10.1007/s12263-009-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Golan Y., Kambe T., Assaraf Y.G. The role of the zinc transporter SLC30A2/ZnT2 in transient neonatal zinc deficiency. Metallomics. 2017;9:1352–1366. doi: 10.1039/C7MT00162B. [DOI] [PubMed] [Google Scholar]
- 73.Bhatnagar S., Taneja S. Zinc and cognitive development. Br. J. Nutr. 2001;85((Suppl. S2)):S139–S145. doi: 10.1079/BJN2000306. [DOI] [PubMed] [Google Scholar]
- 74.Krebs N.F., Miller L.V., Hambidge K.M. Zinc deficiency in infants and children: A review of its complex and synergistic interactions. Paediatr. Int. Child. Health. 2014;34:279–288. doi: 10.1179/2046905514Y.0000000151. [DOI] [PubMed] [Google Scholar]
- 75.Sian L., Mingyan X., Miller L.V., Tong L., Krebs N.F., Hambidge K.M. Zinc absorption and intestinal losses of endogenous zinc in young Chinese women with marginal zinc intakes. Am. J. Clin. Nutr. 1996;63:348–353. doi: 10.1093/ajcn/63.3.348. [DOI] [PubMed] [Google Scholar]
- 76.Krebs N.F. Zinc transfer to the breastfed infant. J. Mammary. Gland. Biol. Neoplasia. 1999;4:259–268. doi: 10.1023/A:1018797829351. [DOI] [PubMed] [Google Scholar]
- 77.Ortega R.M., Andrés P., Martínez R.M., López-Sobaler A.M., Quintas M.E. Zinc levels in maternal milk: The influence of nutritional status with respect to zinc during the third trimester of pregnancy. Eur. J. Clin. Nutr. 1997;51:253–258. doi: 10.1038/sj.ejcn.1600393. [DOI] [PubMed] [Google Scholar]
- 78.Dufner-Beattie J., Huang Z.L., Geiser J., Xu W., Andrews G.K. Mouse ZIP1 and ZIP3 genes together are essential for adaptation to dietary zinc deficiency during pregnancy. Genesis. 2006;44:239–251. doi: 10.1002/dvg.20211. [DOI] [PubMed] [Google Scholar]
- 79.Kelleher S.L., Lopez V., Lönnerdal B., Dufner-Beattie J., Andrews G.K. Zip3 (Slc39a3) functions in zinc reuptake from the alveolar lumen in lactating mammary gland. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;297:R194–R201. doi: 10.1152/ajpregu.00162.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kelleher S.L., Velasquez V., Croxford T.P., McCormick N.H., Lopez V., MacDavid J. Mapping the zinc-transporting system in mammary cells: Molecular analysis reveals a phenotype-dependent zinc-transporting network during lactation. J. Cell Physiol. 2012;227:1761–1770. doi: 10.1002/jcp.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S., Hennigar S.R., Alam S., Nishida K., Kelleher S.L. Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation. J. Biol. Chem. 2015;290:13064–13078. doi: 10.1074/jbc.M115.637439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chowanadisai W., Lönnerdal B., Kelleher S.L. Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J. Biol. Chem. 2006;281:39699–39707. doi: 10.1074/jbc.M605821200. [DOI] [PubMed] [Google Scholar]
- 83.Itsumura N., Kibihara Y., Fukue K., Miyata A., Fukushima K., Tamagawa-Mineoka R., Katoh N., Nishito Y., Ishida R., Narita H., et al. Novel mutations in SLC30A2 involved in the pathogenesis of transient neonatal zinc deficiency. Pediatr. Res. 2016;80:586–594. doi: 10.1038/pr.2016.108. [DOI] [PubMed] [Google Scholar]
- 84.Lee S., Rivera O.C., Kelleher S.L. Zinc transporter 2 interacts with vacuolar ATPase and is required for polarization, vesicle acidification, and secretion in mammary epithelial cells. J. Biol. Chem. 2017;292:21598–21613. doi: 10.1074/jbc.M117.794461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Qian L., Lopez V., Seo Y.A., Kelleher S.L. Prolactin regulates ZNT2 expression through the JAK2/STAT5 signaling pathway in mammary cells. Am. J. Physiol. Cell Physiol. 2009;297:C369–C377. doi: 10.1152/ajpcell.00589.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo L., Lichten L.A., Ryu M.S., Liuzzi J.P., Wang F., Cousins R.J. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc. Natl. Acad. Sci. USA. 2010;107:2818–2823. doi: 10.1073/pnas.0914941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hennigar S.R., Kelleher S.L. TNFα Post-Translationally Targets ZnT2 to Accumulate Zinc in Lysosomes. J. Cell Physiol. 2015;230:2345–2350. doi: 10.1002/jcp.24992. [DOI] [PubMed] [Google Scholar]
- 88.Rivera O.C., Hennigar S.R., Kelleher S.L. ZnT2 is critical for lysosome acidification and biogenesis during mammary gland involution. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018;315:R323–R335. doi: 10.1152/ajpregu.00444.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fukunaka A., Suzuki T., Kurokawa Y., Yamazaki T., Fujiwara N., Ishihara K., Migaki H., Okumura K., Masuda S., Yamaguchi-Iwai Y., et al. Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J. Biol. Chem. 2009;284:30798–30806. doi: 10.1074/jbc.M109.026435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukunaka A., Kurokawa Y., Teranishi F., Sekler I., Oda K., Ackland M.L., Faundez V., Hiromura M., Masuda S., Nagao M., et al. Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J. Biol. Chem. 2011;286:16363–16373. doi: 10.1074/jbc.M111.227173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumar L., Michalczyk A., McKay J., Ford D., Kambe T., Hudek L., Varigios G., Taylor P.E., Ackland M.L. Altered expression of two zinc transporters, SLC30A5 and SLC30A6, underlies a mammary gland disorder of reduced zinc secretion into milk. Genes. Nutr. 2015;10:487. doi: 10.1007/s12263-015-0487-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prasad A.S. Zinc: An overview. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 93.Maares M., Haase H. Zinc and immunity: An essential interrelation. Arch. Biochem. Biophys. 2016;611:58–65. doi: 10.1016/j.abb.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 94.Kim B., Lee W.W. Regulatory Role of Zinc in Immune Cell Signaling. Mol. Cells. 2021;44:335–341. doi: 10.14348/molcells.2021.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maywald M., Wang F., Rink L. Zinc supplementation plays a crucial role in T helper 9 differentiation in allogeneic immune reactions and non-activated T cells. J. Trace Elem. Med. Biol. 2018;50:482–488. doi: 10.1016/j.jtemb.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 96.Huang L., Kirschke C.P., Zhang Y. Decreased intracellular zinc in human tumorigenic prostate epithelial cells: A possible role in prostate cancer progression. Cancer Cell Int. 2006;6:10. doi: 10.1186/1475-2867-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Costello L.C., Franklin R.B. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch. Biochem. Biophys. 2016;611:100–112. doi: 10.1016/j.abb.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leitzmann M.F., Stampfer M.J., Wu K., Colditz G.A., Willett W.C., Giovannucci E.L. Zinc supplement use and risk of prostate cancer. J. Natl. Cancer Inst. 2003;95:1004–1007. doi: 10.1093/jnci/95.13.1004. [DOI] [PubMed] [Google Scholar]
- 99.Gonzalez A., Peters U., Lampe J.W., White E. Zinc intake from supplements and diet and prostate cancer. Nutr. Cancer. 2009;61:206–215. doi: 10.1080/01635580802419749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gumulec J., Masarik M., Adam V., Eckschlager T., Provaznik I., Kizek R. Serum and tissue zinc in epithelial malignancies: A meta-analysis. PLoS ONE. 2014;9:e99790. doi: 10.1371/journal.pone.0099790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lossow K., Schwarz M., Kipp A.P. Are trace element concentrations suitable biomarkers for the diagnosis of cancer? Redox. Biol. 2021;42:101900. doi: 10.1016/j.redox.2021.101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pavithra V., Sathisha T.G., Kasturi K., Mallika D.S., Amos S.J., Ragunatha S. Serum levels of metal ions in female patients with breast cancer. J. Clin. Diagn. Res. 2015;9:BC25–BC27. doi: 10.7860/JCDR/2015/11627.5476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chandler P., Kochupurakkal B.S., Alam S., Richardson A.L., Soybel D.I., Kelleher S.L. Subtype-specific accumulation of intracellular zinc pools is associated with the malignant phenotype in breast cancer. Mol. Cancer. 2016;15:2. doi: 10.1186/s12943-015-0486-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Farquharson M.J., Al-Ebraheem A., Geraki K., Leek R., Jubb A., Harris A.L. Zinc presence in invasive ductal carcinoma of the breast and its correlation with oestrogen receptor status. Phys. Med. Biol. 2009;54:4213–4223. doi: 10.1088/0031-9155/54/13/016. [DOI] [PubMed] [Google Scholar]
- 105.Sullivan K.V., Moore R.E.T., Capper M.S., Schilling K., Goddard K., Ion C., Layton-Matthews D., Leybourne M.I., Coles B., Kreissig K., et al. Zinc stable isotope analysis reveals Zn dyshomeostasis in benign tumours, breast cancer, and adjacent histologically normal tissue. Metallomics. 2021;13:mfab027. doi: 10.1093/mtomcs/mfab027. [DOI] [PubMed] [Google Scholar]
- 106.Larner F., Woodley L.N., Shousha S., Moyes A., Humphreys-Williams E., Strekopytov S., Halliday A.N., Rehkämper M., Coombes R.C. Zinc isotopic compositions of breast cancer tissue. Metallomics. 2015;7:112–117. doi: 10.1039/C4MT00260A. [DOI] [PubMed] [Google Scholar]
- 107.Larner F., Shousha S., Coombes R.C. Zinc isotopes: A novel approach to biomarkers of breast cancer? Biomark. Med. 2015;9:379–382. doi: 10.2217/bmm.15.8. [DOI] [PubMed] [Google Scholar]
- 108.Kiedrowski L. Proton-dependent zinc release from intracellular ligands. J. Neurochem. 2014;130:87–96. doi: 10.1111/jnc.12712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hara H., Aizenman E. A molecular technique for detecting the liberation of intracellular zinc in cultured neurons. J. Neurosci. Methods. 2004;137:175–180. doi: 10.1016/j.jneumeth.2004.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nishito Y., Kambe T. Zinc transporter 1 (ZNT1) expression on the cell surface is elaborately controlled by cellular zinc levels. J. Biol. Chem. 2019;294:15686–15697. doi: 10.1074/jbc.RA119.010227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bird A.J., McCall K., Kramer M., Blankman E., Winge D.R., Eide D.J. Zinc fingers can act as Zn2+ sensors to regulate transcriptional activation domain function. Embo. J. 2003;22:5137–5146. doi: 10.1093/emboj/cdg484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ho L.H., Ruffin R.E., Murgia C., Li L., Krilis S.A., Zalewski P.D. Labile zinc and zinc transporter ZnT4 in mast cell granules: Role in regulation of caspase activation and NF-kappaB translocation. J. Immunol. 2004;172:7750–7760. doi: 10.4049/jimmunol.172.12.7750. [DOI] [PubMed] [Google Scholar]
- 113.Sharir H., Zinger A., Nevo A., Sekler I., Hershfinkel M. Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J. Biol. Chem. 2010;285:26097–26106. doi: 10.1074/jbc.M110.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Singer C.F., Kronsteiner N., Marton E., Kubista M., Cullen K.J., Hirtenlehner K., Seifert M., Kubista E. MMP-2 and MMP-9 expression in breast cancer-derived human fibroblasts is differentially regulated by stromal-epithelial interactions. Breast. Cancer Res. Treat. 2002;72:69–77. doi: 10.1023/A:1014918512569. [DOI] [PubMed] [Google Scholar]
- 115.Yin X.F., Jiang L.B., Ma Y.Q., Xu J., Gu H.J., Wu X.H., Li X.L., Dong J. Decreased Zn2+ Influx Underlies the Protective Role of Hypoxia in Rat Nucleus Pulposus Cells. Biol. Trace Elem. Res. 2015;168:196–205. doi: 10.1007/s12011-015-0335-2. [DOI] [PubMed] [Google Scholar]
- 116.Azriel-Tamir H., Sharir H., Schwartz B., Hershfinkel M. Extracellular zinc triggers ERK-dependent activation of Na+/H+ exchange in colonocytes mediated by the zinc-sensing receptor. J. Biol. Chem. 2004;279:51804–51816. doi: 10.1074/jbc.M406581200. [DOI] [PubMed] [Google Scholar]
- 117.Cohen L., Sekler I., Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, controls proliferation and differentiation of colonocytes and thereby tight junction formation in the colon. Cell Death Dis. 2014;5:e1307. doi: 10.1038/cddis.2014.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lopez V., Foolad F., Kelleher S.L. ZnT2-overexpression represses the cytotoxic effects of zinc hyper-accumulation in malignant metallothionein-null T47D breast tumor cells. Cancer Lett. 2011;304:41–51. doi: 10.1016/j.canlet.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 119.Bostanci Z., Alam S., Soybel D.I., Kelleher S.L. Prolactin receptor attenuation induces zinc pool redistribution through ZnT2 and decreases invasion in MDA-MB-453 breast cancer cells. Exp. Cell Res. 2014;321:190–200. doi: 10.1016/j.yexcr.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 120.Golan Y., Berman B., Assaraf Y.G. Heterodimerization, altered subcellular localization, and function of multiple zinc transporters in viable cells using bimolecular fluorescence complementation. J. Biol. Chem. 2015;290:9050–9063. doi: 10.1074/jbc.M114.617332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Taylor K.M., Morgan H.E., Johnson A., Hadley L.J., Nicholson R.I. Structure-function analysis of LIV-1, the breast cancer-associated protein that belongs to a new subfamily of zinc transporters. Biochem. J. 2003;375:51–59. doi: 10.1042/bj20030478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Taylor K.M., Morgan H.E., Smart K., Zahari N.M., Pumford S., Ellis I.O., Robertson J.F., Nicholson R.I. The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol. Med. 2007;13:396–406. doi: 10.2119/2007-00040.Taylor. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Taylor K.M., Nicholson R.I. The LZT proteins; the LIV-1 subfamily of zinc transporters. Biochim. Biophys Acta. 2003;1611:16–30. doi: 10.1016/S0005-2736(03)00048-8. [DOI] [PubMed] [Google Scholar]
- 124.Nimmanon T., Taylor K.M. Posttranslational Mechanisms of Zinc Signaling. In: Collins J., editor. Molecular, Genetic, and Nutritional Aspects of Major and Trace Minerals. 1st ed. Academic Press; Amsterdam, Netherlands: 2017. pp. 273–281. [Google Scholar]
- 125.Manning D.L., McClelland R.A., Gee J.M., Chan C.M., Green C.D., Blamey R.W., Nicholson R.I. The role of four oestrogen-responsive genes, pLIV1, pS2, pSYD3 and pSYD8, in predicting responsiveness to endocrine therapy in primary breast cancer. Eur. J. Cancer. 1993;29A:1462–1468. doi: 10.1016/0959-8049(93)90021-7. [DOI] [PubMed] [Google Scholar]
- 126.Tozlu S., Girault I., Vacher S., Vendrell J., Andrieu C., Spyratos F., Cohen P., Lidereau R., Bieche I. Identification of novel genes that co-cluster with estrogen receptor alpha in breast tumor biopsy specimens, using a large-scale real-time reverse transcription-PCR approach. Endocr. Relat. Cancer. 2006;13:1109–1120. doi: 10.1677/erc.1.01120. [DOI] [PubMed] [Google Scholar]
- 127.Schneider J., Ruschhaupt M., Buness A., Asslaber M., Regitnig P., Zatloukal K., Schippinger W., Ploner F., Poustka A., Sültmann H. Identification and meta-analysis of a small gene expression signature for the diagnosis of estrogen receptor status in invasive ductal breast cancer. Int. J. Cancer. 2006;119:2974–2979. doi: 10.1002/ijc.22234. [DOI] [PubMed] [Google Scholar]
- 128.Manning D.L., Robertson J.F., Ellis I.O., Elston C.W., McClelland R.A., Gee J.M., Jones R.J., Green C.D., Cannon P., Blamey R.W., et al. Oestrogen-regulated genes in breast cancer: Association of pLIV1 with lymph node involvement. Eur. J. Cancer. 1994;30A:675–678. doi: 10.1016/0959-8049(94)90543-6. [DOI] [PubMed] [Google Scholar]
- 129.Yamashita S., Miyagi C., Fukada T., Kagara N., Che Y.S., Hirano T. Zinc transporter LIVI controls epithelial-mesenchymal transition in zebrafish gastrula organizer. Nature. 2004;429:298–302. doi: 10.1038/nature02545. [DOI] [PubMed] [Google Scholar]
- 130.Hogstrand C., Kille P., Ackland M.L., Hiscox S., Taylor K.M. A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3) Biochem. J. 2013;455:229–237. doi: 10.1042/BJ20130483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zhao L., Chen W., Taylor K.M., Cai B., Li X. LIV-1 suppression inhibits HeLa cell invasion by targeting ERK1/2-Snail/Slug pathway. Biochem. Biophys. Res. Commun. 2007;363:82–88. doi: 10.1016/j.bbrc.2007.08.127. [DOI] [PubMed] [Google Scholar]
- 132.Taylor K.M., Morgan H.E., Johnson A., Nicholson R.I. Structure-function analysis of HKE4, a member of the new LIV-1 subfamily of zinc transporters. Biochem. J. 2004;377:131–139. doi: 10.1042/bj20031183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hogstrand C., Kille P., Nicholson R.I., Taylor K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends. Mol. Med. 2009;15:101–111. doi: 10.1016/j.molmed.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 134.Ziliotto S., Gee J.M.W., Ellis I.O., Green A.R., Finlay P., Gobbato A., Taylor K.M. Activated zinc transporter ZIP7 as an indicator of anti-hormone resistance in breast cancer. Metallomics. 2019;11:1579–1592. doi: 10.1039/C9MT00136K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Taylor K.M., Vichova P., Jordan N., Hiscox S., Hendley R., Nicholson R.I. ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology. 2008;149:4912–4920. doi: 10.1210/en.2008-0351. [DOI] [PubMed] [Google Scholar]
- 136.Xuhong J.C., Qi X.W., Zhang Y., Jiang J. Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am. J. Cancer Res. 2019;9:2103–2119. [PMC free article] [PubMed] [Google Scholar]
- 137.Schlam I., Swain S.M. HER2-positive breast cancer and tyrosine kinase inhibitors: The time is now. NPJ Breast Cancer. 2021;7:56. doi: 10.1038/s41523-021-00265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chang F., Lee J.T., Navolanic P.M., Steelman L.S., Shelton J.G., Blalock W.L., Franklin R.A., McCubrey J.A. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: A target for cancer chemotherapy. Leukemia. 2003;17:590–603. doi: 10.1038/sj.leu.2402824. [DOI] [PubMed] [Google Scholar]
- 139.Nimmanon T., Ziliotto S., Morris S., Flanagan L., Taylor K.M. Phosphorylation of zinc channel ZIP7 drives MAPK, PI3K and mTOR growth and proliferation signalling. Metallomics. 2017;9:471–481. doi: 10.1039/C6MT00286B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Knowlden J.M., Hutcheson I.R., Barrow D., Gee J.M., Nicholson R.I. Insulin-like growth factor-I receptor signaling in tamoxifen-resistant breast cancer: A supporting role to the epidermal growth factor receptor. Endocrinology. 2005;146:4609–4618. doi: 10.1210/en.2005-0247. [DOI] [PubMed] [Google Scholar]
- 141.Kagara N., Tanaka N., Noguchi S., Hirano T. Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci. 2007;98:692–697. doi: 10.1111/j.1349-7006.2007.00446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Taylor K.M., Muraina I.A., Brethour D., Schmitt-Ulms G., Nimmanon T., Ziliotto S., Kille P., Hogstrand C. Zinc transporter ZIP10 forms a heteromer with ZIP6 which regulates embryonic development and cell migration. Biochem. J. 2016;473:2531–2544. doi: 10.1042/BCJ20160388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takatani-Nakase T., Matsui C., Maeda S., Kawahara S., Takahashi K. High glucose level promotes migration behavior of breast cancer cells through zinc and its transporters. PLoS ONE. 2014;9:e90136. doi: 10.1371/journal.pone.0090136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Peairs K.S., Barone B.B., Snyder C.F., Yeh H.C., Stein K.B., Derr R.L., Brancati F.L., Wolff A.C. Diabetes mellitus and breast cancer outcomes: A systematic review and meta-analysis. J. Clin. Oncol. 2011;29:40–46. doi: 10.1200/JCO.2009.27.3011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nimmanon T., Ziliotto S., Ogle O., Burt A., Gee J.M.W., Andrews G.K., Kille P., Hogstrand C., Maret W., Taylor K.M. The ZIP6/ZIP10 heteromer is essential for the zinc-mediated trigger of mitosis. Cell Mol. Life Sci. 2021;78:1781–1798. doi: 10.1007/s00018-020-03616-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sunuwar L., Gilad D., Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, in health and disease. Front. Biosci. 2017;22:1469–1492. doi: 10.2741/4554. [DOI] [PubMed] [Google Scholar]
- 147.Popovics P., Stewart A.J. GPR39: A Zn2+-activated G protein-coupled receptor that regulates pancreatic, gastrointestinal and neuronal functions. Cell Mol. Life Sci. 2011;68:85–95. doi: 10.1007/s00018-010-0517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Asraf H., Salomon S., Nevo A., Sekler I., Mayer D., Hershfinkel M. The ZnR/GPR39 interacts with the CaSR to enhance signaling in prostate and salivary epithelia. J. Cell Physiol. 2014;229:868–877. doi: 10.1002/jcp.24514. [DOI] [PubMed] [Google Scholar]
- 149.Dubi N., Gheber L., Fishman D., Sekler I., Hershfinkel M. Extracellular zinc and zinc-citrate, acting through a putative zinc-sensing receptor, regulate growth and survival of prostate cancer cells. Carcinogenesis. 2008;29:1692–1700. doi: 10.1093/carcin/bgn027. [DOI] [PubMed] [Google Scholar]
- 150.Storjohann L., Holst B., Schwartz T.W. Molecular mechanism of Zn2+ agonism in the extracellular domain of GPR39. FEBS Lett. 2008;582:2583–2588. doi: 10.1016/j.febslet.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 151.Mut M., Lule S., Demir O., Kurnaz I.A., Vural I. Both mitogen-activated protein kinase (MAPK)/extracellular-signal-regulated kinases (ERK) 1/2 and phosphatidylinositide-3-OH kinase (PI3K)/Akt pathways regulate activation of E-twenty-six (ETS)-like transcription factor 1 (Elk-1) in U138 glioblastoma cells. Int. J. Biochem. Cell Biol. 2012;44:302–310. doi: 10.1016/j.biocel.2011.10.025. [DOI] [PubMed] [Google Scholar]
- 152.Sunuwar L., Medini M., Cohen L., Sekler I., Hershfinkel M. The zinc sensing receptor, ZnR/GPR39, triggers metabotropic calcium signalling in colonocytes and regulates occludin recovery in experimental colitis. Philos. Trans. R Soc. Lond B Biol. Sci. 2016;371 doi: 10.1098/rstb.2015.0420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Xie F., Liu H., Zhu Y.H., Qin Y.R., Dai Y., Zeng T., Chen L., Nie C., Tang H., Li Y., et al. Overexpression of GPR39 contributes to malignant development of human esophageal squamous cell carcinoma. BMC Cancer. 2011;11:86. doi: 10.1186/1471-2407-11-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ventura-Bixenshpaner H., Asraf H., Chakraborty M., Elkabets M., Sekler I., Taylor K.M., Hershfinkel M. Enhanced ZnR/GPR39 Activity in Breast Cancer, an Alternative Trigger of Signaling Leading to Cell Growth. Sci. Rep. 2018;8:8119. doi: 10.1038/s41598-018-26459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ganay T., Asraf H., Aizenman E., Bogdanovic M., Sekler I., Hershfinkel M. Regulation of neuronal pH by the metabotropic Zn2+-sensing Gq-coupled receptor, mZnR/GPR39. J. Neurochem. 2015;135:897–907. doi: 10.1111/jnc.13367. [DOI] [PubMed] [Google Scholar]
- 156.Cohen L., Azriel-Tamir H., Arotsker N., Sekler I., Hershfinkel M. Zinc sensing receptor signaling, mediated by GPR39, reduces butyrate-induced cell death in HT29 colonocytes via upregulation of clusterin. PLoS ONE. 2012;7:e35482. doi: 10.1371/journal.pone.0035482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schneider D., Gerhardt E., Bock J., Müller M.M., Wolburg H., Lang F., Schulz J.B. Intracellular acidification by inhibition of the Na+/H+-exchanger leads to caspase-independent death of cerebellar granule neurons resembling paraptosis. Cell Death Differ. 2004;11:760–770. doi: 10.1038/sj.cdd.4401377. [DOI] [PubMed] [Google Scholar]
- 158.Birkeland E.S., Koch L.M., Dechant R. Another Consequence of the Warburg Effect? Metabolic Regulation of Na+/H+ Exchangers May Link Aerobic Glycolysis to Cell Growth. Front. Oncol. 2020;10:1561. doi: 10.3389/fonc.2020.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harguindey S., Arranz J.L., Polo Orozco J.D., Rauch C., Fais S., Cardone R.A., Reshkin S.J. Cariporide and other new and powerful NHE1 inhibitors as potentially selective anticancer drugs—An integral molecular/biochemical/metabolic/clinical approach after one hundred years of cancer research. J. Transl. Med. 2013;11:282. doi: 10.1186/1479-5876-11-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Wu K.L., Khan S., Lakhe-Reddy S., Jarad G., Mukherjee A., Obejero-Paz C.A., Konieczkowski M., Sedor J.R., Schelling J.R. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J. Biol. Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- 161.Baumgartner M., Patel H., Barber D.L. Na+/H+ exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes. Am. J. Physiol. Cell Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- 162.Chorin E., Vinograd O., Fleidervish I., Gilad D., Herrmann S., Sekler I., Aizenman E., Hershfinkel M. Upregulation of KCC2 activity by zinc-mediated neurotransmission via the mZnR/GPR39 receptor. J. Neurosci. 2011;31:12916–12926. doi: 10.1523/JNEUROSCI.2205-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gilad D., Shorer S., Ketzef M., Friedman A., Sekler I., Aizenman E., Hershfinkel M. Homeostatic regulation of KCC2 activity by the zinc receptor mZnR/GPR39 during seizures. Neurobiol. Dis. 2015;81:4–13. doi: 10.1016/j.nbd.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rivera C., Voipio J., Payne J.A., Ruusuvuori E., Lahtinen H., Lamsa K., Pirvola U., Saarma M., Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- 165.Sunuwar L., Asraf H., Donowitz M., Sekler I., Hershfinkel M. The Zn2+-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl− absorption, via basolateral KCC1, and reduces fluid loss. Biochim. Biophys. Acta. Mol. Basis. Dis. 2017;1863:947–960. doi: 10.1016/j.bbadis.2017.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Pedersen S.F., Hoffmann E.K., Novak I. Cell volume regulation in epithelial physiology and cancer. Front. Physiol. 2013;4:233. doi: 10.3389/fphys.2013.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Mero M., Asraf H., Sekler I., Taylor K.M., Hershfinkel M. ZnR/GPR39 upregulation of K(+)/Cl−-cotransporter 3 in tamoxifen resistant breast cancer cells. Cell Calcium. 2019;81:12–20. doi: 10.1016/j.ceca.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 168.Ali S., Coombes R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer. 2002;2:101–112. doi: 10.1038/nrc721. [DOI] [PubMed] [Google Scholar]
- 169.Liu Y., Ma H., Yao J. ERα, A Key Target for Cancer Therapy: A Review. Onco. Targets. 2020;13:2183–2191. doi: 10.2147/OTT.S236532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Luque-Bolivar A., Pérez-Mora E., Villegas V.E., Rondón-Lagos M. Resistance and Overcoming Resistance in Breast Cancer. Breast. Cancer. 2020;12:211–229. doi: 10.2147/BCTT.S270799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.da Silva J.L., Cardoso Nunes N.C., Izetti P., de Mesquita G.G., de Melo A.C. Triple negative breast cancer: A thorough review of biomarkers. Crit. Rev. Oncol. Hematol. 2020;145:102855. doi: 10.1016/j.critrevonc.2019.102855. [DOI] [PubMed] [Google Scholar]
- 172.Gee J.M., Robertson J.F., Gutteridge E., Ellis I.O., Pinder S.E., Rubini M., Nicholson R.I. Epidermal growth factor receptor/HER2/insulin-like growth factor receptor signalling and oestrogen receptor activity in clinical breast cancer. Endocr. Relat. Cancer. 2005;12((Suppl. S1)):S99–S111. doi: 10.1677/erc.1.01005. [DOI] [PubMed] [Google Scholar]
- 173.Perry R.R., Kang Y., Greaves B. Effects of tamoxifen on growth and apoptosis of estrogen-dependent and -independent human breast cancer cells. Ann. Surg. Oncol. 1995;2:238–245. doi: 10.1007/BF02307030. [DOI] [PubMed] [Google Scholar]
- 174.Bursch W., Hochegger K., Torok L., Marian B., Ellinger A., Hermann R.S. Autophagic and apoptotic types of programmed cell death exhibit different fates of cytoskeletal filaments. J. Cell.Sci. 2000;113:1189–1198. doi: 10.1242/jcs.113.7.1189. [DOI] [PubMed] [Google Scholar]
- 175.Nardone A., De Angelis C., Trivedi M.V., Osborne C.K., Schiff R. The changing role of ER in endocrine resistance. Breast. 2015;24((Suppl. S2)):S60–S66. doi: 10.1016/j.breast.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Viedma-Rodríguez R., Baiza-Gutman L., Salamanca-Gómez F., Diaz-Zaragoza M., Martínez-Hernández G., Ruiz Esparza-Garrido R., Velázquez-Flores M.A., Arenas-Aranda D. Mechanisms associated with resistance to tamoxifen in estrogen receptor-positive breast cancer (review) Oncol. Rep. 2014;32:3–15. doi: 10.3892/or.2014.3190. [DOI] [PubMed] [Google Scholar]
- 177.Hasson S.P., Rubinek T., Ryvo L., Wolf I. Endocrine resistance in breast cancer: Focus on the phosphatidylinositol 3-kinase/akt/mammalian target of rapamycin signaling pathway. Breast Care. 2013;8:248–255. doi: 10.1159/000354757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Anurag M., Ellis M.J., Haricharan S. DNA damage repair defects as a new class of endocrine treatment resistance driver. Oncotarget. 2018;9:36252–36253. doi: 10.18632/oncotarget.26363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hanker A.B., Sudhan D.R., Arteaga C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell. 2020;37:496–513. doi: 10.1016/j.ccell.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dent R., Trudeau M., Pritchard K.I., Hanna W.M., Kahn H.K., Sawka C.A., Lickley L.A., Rawlinson E., Sun P., Narod S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 182.Mediratta K., El-Sahli S., D’Costa V., Wang L. Current Progresses and Challenges of Immunotherapy in Triple-Negative Breast Cancer. Cancers. 2020;12:3529. doi: 10.3390/cancers12123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Sørlie T., Perou C.M., Tibshirani R., Aas T., Geisler S., Johnsen H., Hastie T., Eisen M.B., van de Rijn M., Jeffrey S.S., et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kübler E., Albrecht H. Large set data mining reveals overexpressed GPCRs in prostate and breast cancer: Potential for active targeting with engineered anti-cancer nanomedicines. Oncotarget. 2018;9:24882–24897. doi: 10.18632/oncotarget.25427. [DOI] [PMC free article] [PubMed] [Google Scholar]