Abstract
Salt stress harms the growth and development of plants, and the degree of soil salinization in North China is becoming increasingly severe. Ectomycorrhiza (ECM) is a symbiotic system formed by fungi and plants that can improve the growth and salt tolerance of plants. No studies to date have examined the salt tolerance of Quercus mongolica, a typical ectomycorrhizal tree species of temperate forests in the northern hemisphere. Here, we inoculated Q. mongolica with two ectomycorrhizal fungi (Gomphidius viscidus; Suillus luteus) under NaCl stress to characterize the effects of ECM. The results showed that the symbiotic relationship of Q. mongolica with G. viscidus was more stable than that with S. luteus. The cross-sectional area of roots increased after inoculation with the two types of ectomycorrhizal fungi. Compared with the control group, plant height, soluble sugar content, and soluble protein content of leaves were 1.62, 2.41, and 2.04 times higher in the G. viscidus group, respectively. Chlorophyll (Chl) content, stomatal conductance (Gs), and intracellular CO2 concentration (Ci) were significantly higher in Q. mongolica inoculated with ectomycorrhizal fungi than in the control, but differences in the net photosynthetic rate (Pn), transpiration rate (Tr), and photosystem II maximum photochemical efficiency (Fv/Fm) were lower. The relative conductivity of Q. mongolica inoculated with the two ectomycorrhizal fungi was consistently lower than that of non-mycorrhizal seedlings, with the effect of G. viscidus more pronounced than that of S. luteus. The malondialdehyde (MDA) content showed a similar pattern. Peroxidase (POD) and catylase (CAT) levels in mycorrhizal seedlings were generally higher than those of non-mycorrhizal seedlings under normal conditions, and were significantly higher than those of non-mycorrhizal seedlings on the 36th and 48th day after salt treatment, respectively. Overall, the results indicated that the salt tolerance of Q. mongolica seedlings was improved by ectomycorrhizal inoculation.
Keywords: Quercus mongolica, ectomycorrhiza, NaCl, stress
1. Introduction
Salinity causes a series of cellular, metabolic, and physiological changes. Excessive salt in the soil is harmful to the growth and development of plants, as salt stress has both osmotic effects on cells and can lead to ion toxicity. The secondary effects of salt stress are also complex and include: oxidative stress; damage to cell membrane lipids, proteins, nucleic acids, and other cellular components; metabolic disorders [1]. Salt stress can also result in the competitive absorption of Na+ and K+ by plant roots, thereby affecting normal cellular function [2]. Plants reduce the absorption of Na+ and, thus, the upward transport of Na+ by regulating the gene expression and protein function of membrane transporters. Plants also limit transpiration by closing stomata and regulating root water absorption, which is mainly driven by the strict regulation of aquaporins [3,4]. To prevent oxidative stress in cells, plants also accumulate compatible osmolytes or osmoprotectants in response to salt stress [5]. Chaves et.al. [6] found that salinity led to a reduction in CO2 availability. One of the first responses of plants to salt stress is stomatal closure, which impairs CO2 uptake [6]. In addition, a de-activation of the carboxylating enzyme Rubisco by low intercellular CO2 has been observed [7]. The supply of CO2 to Rubisco is therefore impaired. At the cellular level, salt stress can cause osmotic stress and ion deficiency, damage to cell membranes, decreased chlorophyll content, decreased enzyme activity, and, ultimately, disruption of photosynthesis [6,8]. However, with several previous studies reporting that these responses may not be sufficient for combating prolonged salt stress, further physiological, ecological, and evolutionary adaptations are required.
Many approaches have been used to enhance the salt tolerance of plants. Ectomycorrhizal inoculation has been shown to be a particularly effective approach [9], as several previous studies have reported that ectomycorrhizal associations can protect plants against salt stress [10,11,12,13,14,15,16]. Recently, Zwiazek et al. [17] found that urban ectomycorrhizal fungi could improve the tolerance of Pinus contorta seedlings to salt stress. Several strategies have been proposed to enhance the tolerance of plants to salt stress, including restricting the entry of Na+ into plant tissues helping to maintain mineral nutrition and water balance [9]. In addition, Otgonsuren et al. [18] found that ectomycorrhiza (ECM) enhanced the chlorophyll content of Populus nigra and maintained normal levels of root respiration under saline conditions. Sa et al. [15] demonstrated that ECM contributes to enhanced salt tolerance in Populus canescens hybrid lines by helping to maintain NO3- levels in plants during salt stress. Moreover, the stress response pathway (metabolites, hormones) activated by ectomycorrhizal colonization can help alleviate salt stress [14]. However, the exact mechanism by which ectomycorrhizal fungi contribute to alleviating the effects of salinity on their host plants remains unclear [9].
With more than 300 species, the genus Quercus is one of the most diverse and widely distributed groups of forest tree species in the world. In China, Quercus accounts for 13% of the total area of natural forest, making it the most widely distributed tree species in the country. Thus, Quercus plants play an important role in the stability of forest ecosystems. Ectomycorrhizal fungi are important symbionts of the roots of Fagaceae species in boreal and temperate forests, including Quercus [19,20]. Previous studies have shown that ectomycorrhizal fungi can improve the growth of Quercus plants. Inoculation with Tuber melanosporum enhances the net assimilation rate, stomatal conductance, and root conductance per unit leaf surface of Q. ilex seedlings [21]. Q. rubra seedlings colonized by ectomycorrhizal fungus show enhanced productivity in several respects compared with uncolonized seedlings, including the number of leaves produced, leaf area, and basal stem diameter; colonized seedlings also supported lower caterpillar growth and experienced lower consumption rates [22]. Inoculation with Gomphidius viscidus and Russula foetens significantly promotes the growth of Q. liaotungensis seedlings [23]. Mycorrhization has also been shown to improve seedling growth, total phosphorus absorption, and water uptake of the seedlings during summer drought [24]. However, the effect of ectomycorrhizal fungus on the tolerance of Quercus plants to other stress conditions, especially salt stress, remains unknown.
Q. mongolica is a tree species, distributed in forests of North China, which has high ecological and ornamental value. Q. mongolica is often used in garden landscaping, as its leaves are unique in shape and turn red in autumn. The ability of Q. mongolica to tolerate salt stress and its underlying mechanism, especially the role of ECM, have not been studied to date. Here, we tested the hypothesis that inoculation of Q. mongolica seedlings by ECM affects their morphology, photosynthesis, and physiology. We inoculated Q. mongolica seedlings with either G. viscidus or S. luteus, grew them under salt-free conditions or in the presence of 120 mM NaCl, and compared the morphology, photosynthesis characteristics, and physiology of these inoculated plants with uninoculated control plants.
2. Results
2.1. External Morphology and Anatomical Structure of ECM
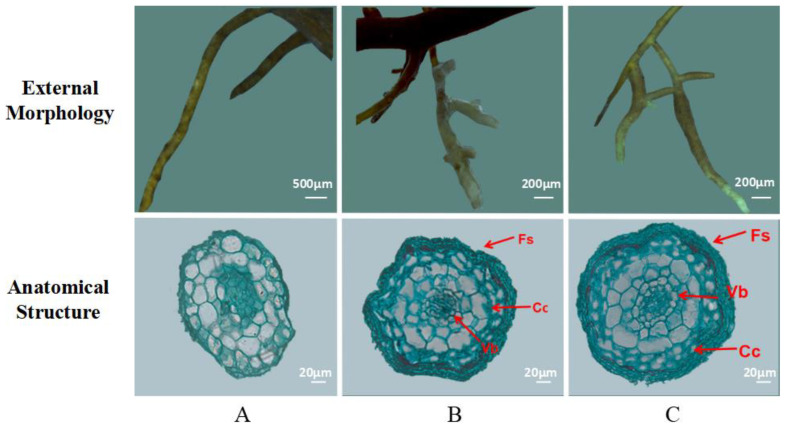
Figure 1 shows the morphology and anatomical structure of the roots of Q. mongolica seedlings after inoculation with two ectomycorrhizal fungi. The typical structural characteristics of the ECM were observed. ECM was significantly shorter and thicker than the normal root system; the mycelial mantle, which is white and tawny, is formed by mycelium around the root system. The ECM included the constricting ring and cortical cells. The cross-sectional area of roots in different fungal inoculation groups varied; the cross-sectional area was highest for the S. luteus group, followed by the G. viscidus group and the control group. As the inoculation time extended, the root cortical cells appeared to occur deeper in the Hartig net. Cortical cells changed in diverse ways under the different fungal inoculations. After inoculation, the cortical cells in G. viscidus seedlings began to shrink before those in S. luteus seedlings, and the degree of shrinkage was more severe in G. viscidus seedlings.
Figure 1.
The external morphology and anatomical structure of the mycorrhiza of Q. mongolica. Note: (A) control group (non-mycorrhizal roots, no salt stress); (B) G. viscidus group; (C) S. luteus group. Fs: fungus sheath; Cc: cortical cells; Vb: vascular bundle.
As the inoculation time extended, the root radius, middle column radius, and cortex thickness of the ECM in Q. mongolica increased (Table 1). There was no significant correlation between the thickness of the constricting ring with the time of inoculation and with fungal species. The radius of the middle column and the roots in the control group and S. luteus seedlings increased continuously, whereas those in G. viscidus seedlings decreased significantly. On the 30th day of inoculation, the ratio of the column radius to the root radius in G. viscidus seedlings was 0.337, and that of S. luteus seedlings was 0.378, which was the highest for the middle column in ECM.
Table 1.
The thickness, radius, and ratio of each part of the anatomical structure in the ECM formation period of Q. mongolica.
| Treatment | Root Radius (μm) | Vascular Bundle Radius (μm) | Vascular Bundl Radius/Root Radius |
Cortical Cells Thickness (μm) | Fungus Sheath Thickness (μm) | |
|---|---|---|---|---|---|---|
| Control group | 10 d | 42.46 e | 22.96 f | 0.54 b | ─ | ─ |
| 20 d | 44.74 e | 24.08 e | 0.54 b | ─ | ─ | |
| 30 d | 52.20 d | 40.19 a | 0.77 a | ─ | ─ | |
| G. viscidus group | 10 d | 70.34 c | 24.91 e | 0.35 c | 47.58 c | 16.43 a |
| 20 d | 75.98 b | 26.56 d | 0.35 c | 50.52 b | 16.98 a | |
| 30 d | 81.27 a | 27.42 cd | 0.34 d | 54.48 a | 17.17 a | |
| S. luteus group | 10 d | 77.37 b | 26.37 d | 0.34 cd | 50.94 b | 14.31 b |
| 20 d | 79.99 ab | 28.41 c | 0.36 c | 51.62 b | 17.81 a | |
| 30 d | 81.88 a | 30.93 b | 0.38 c | 51.23 b | 17.70 a | |
Note: Different lowercase letters within columns indicate significant differences at p < 0.05.
2.2. Effect of NaCl Treatment on Growth
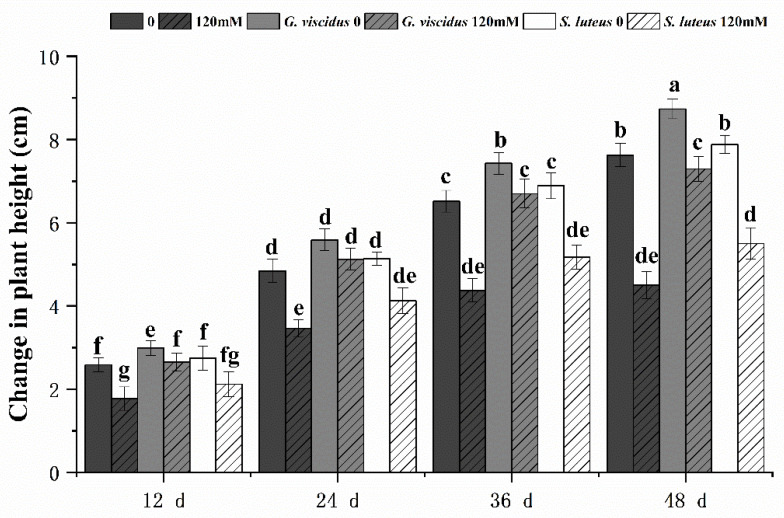
Salt had a negative effect on seedling growth; the degree of growth inhibition (i.e., plant height) was lower in inoculated Q. mongolica than in the control. Under the NaCl treatment, the growth of seedlings inoculated with ectomycorrhizal fungi was inhibited to different degrees; inoculation with G. viscidus alleviated this negative effect, whereas inoculation with S. luteus had virtually no effect (Figure 2). The growth of G. viscidus and S. luteus seedlings was higher than that of control group seedlings under NaCl stress; the growth of G. viscidus seedlings on day 48 under 120 mM stress was 1.52 times higher than that of control group seedlings, whereas that of S. luteus seedlings was only 1.15 times higher. The difference in the growth of S. luteus seedlings relative to the control group seedlings decreased gradually throughout the experimental period.
Figure 2.
Changes in plant height in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
2.3. Effect of NaCl Treatment on Photosynthesis
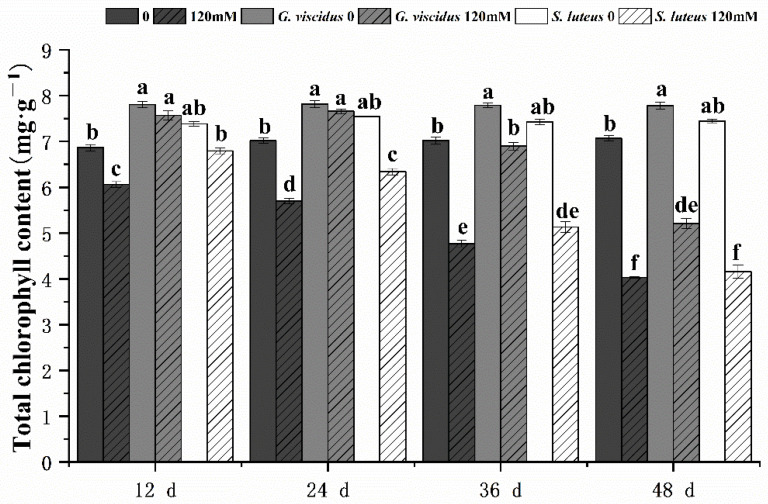
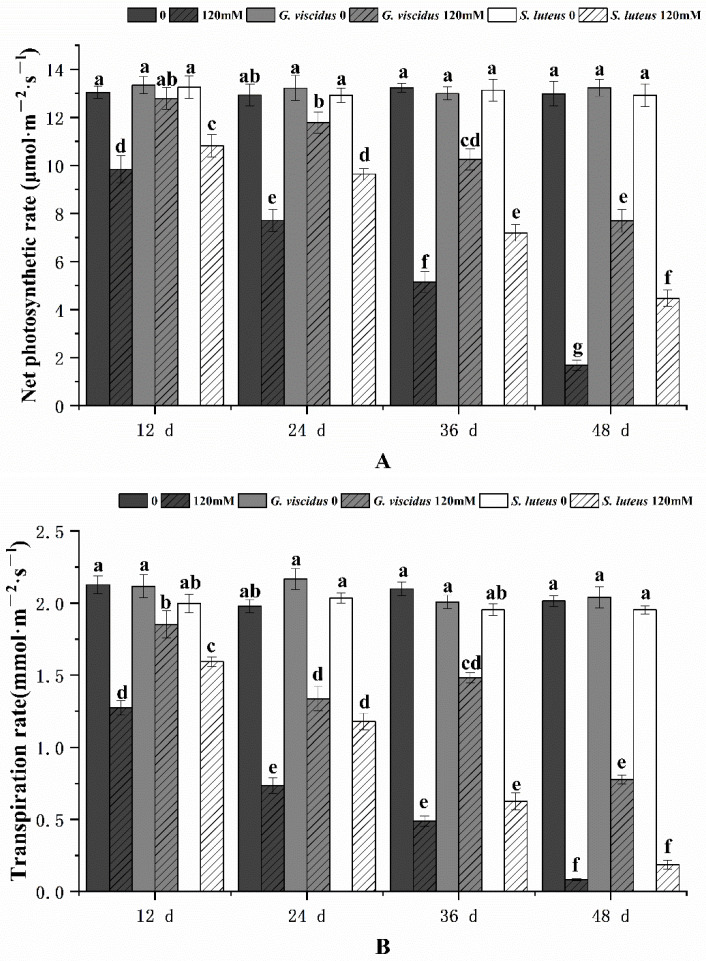
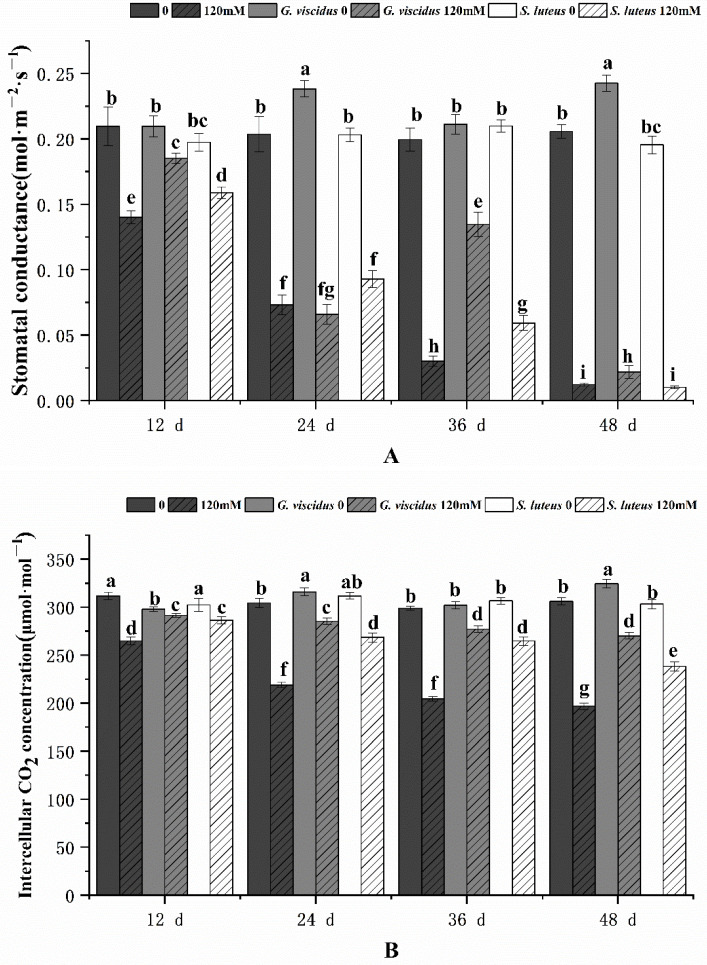
The photosynthetic characteristics of mycorrhizal and non-mycorrhizal Q. mongolica seedlings were also measured after NaCl treatment. Under salt stress, the Chl content, Pn, Tr, Gs, and Ci in seedlings inoculated with ectomycorrhizal fungi were significantly increased, and these parameters were higher for G. viscidus inoculated seedlings than for S. luteus inoculated seedlings. Under long-term salt stress, all the photosynthetic indexes of mycorrhizal seedlings decreased, with the exception of Gs and Tr of G. viscidus seedlings, which increased on day 36. All indicators for the G. viscidus and S. luteus seedlings differed the most from control group seedlings at day 36 or 48 under salt stress. The Chl (Figure 3), Pn (Figure 4A), Tr (Figure 4B), Gs (Figure 5A), and Ci (Figure 5B) contents on day 48 of 120 mM stress were 1.29, 4.59, 9.38, 1.80, and 1.37 times higher in G. viscidus seedlings than in control group seedlings but 1.03, 2.67, 2.24, 0.83, and 1.21 times higher in S. luteus seedlings than in control group seedlings, respectively.
Figure 3.
Changes in the total chlorophyll content in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
Figure 4.
Changes in the net photosynthetic rate (A) and transpiration rate (B) in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
Figure 5.
Changes in the stomatal conductance (A) and intercellular CO2 concentration (B) in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
2.4. Effect of NaCl Treatment on Chlorophyll Fluorescence Parameters
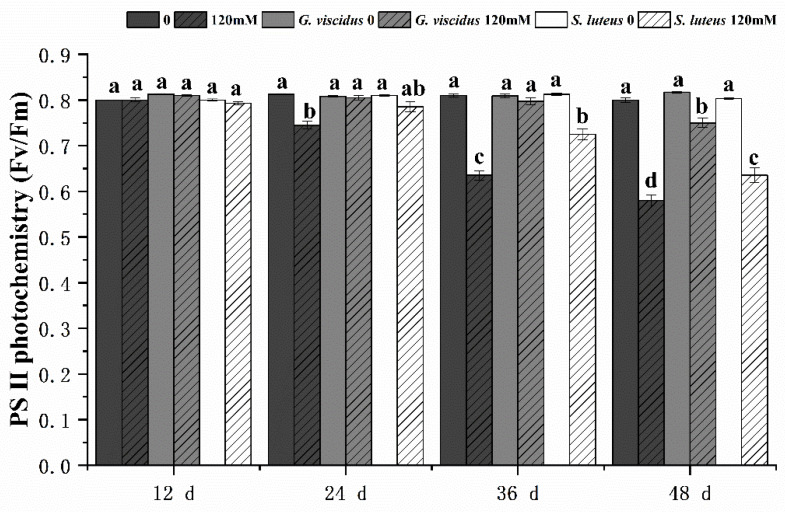
Under pre-salt stress, Fv/Fm (Figure 6) showed little change, but at day 36 and 48, Fv/Fm had changed significantly in G. viscidus and S. luteus seedlings compared with control group seedlings. In addition, Fv/Fm was significantly higher in G. viscidus seedlings than in S. luteus seedlings. Fv/Fm on day 48 of 120 mM stress was 1.29 times and 1.09 times higher in G. viscidus and S. luteus seedlings than in control group seedlings, respectively.
Figure 6.
Changes in PSⅡ photochemistry (Fv/Fm) in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
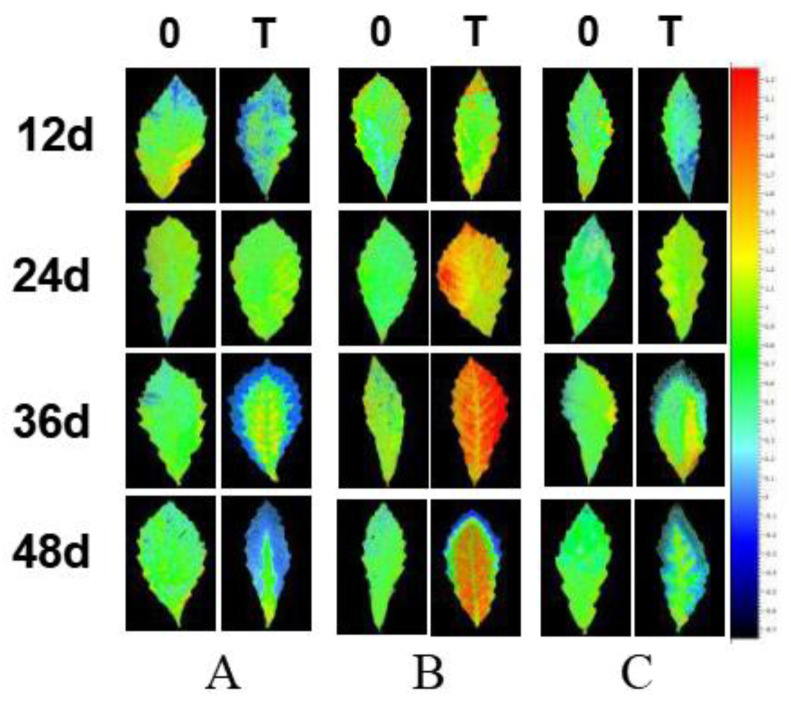
Under salt stress, changes in the NPQ in different inoculation groups differed. The yellow on the leaf margins of the seedlings became more pronounced, and the NPQ of the necrotic leaf edge was reduced to 0, which is blue in Figure 7. Overall, the NPQ of the leaf margin of each inoculation group decreased, and the blue area gradually increased. G. viscidus seedlings showed the smallest change, and the blue area was the largest in control group seedlings, followed by S. luteus and G. viscidus seedlings. Healthy mesophyll in S. luteus seedlings were generally yellow/green, and there was no significant difference in the color compared with control group seedlings. In G. viscidus seedlings, the internal leaves turned orange/yellow to red; the NPQ was greater than 1.5 and increased continuously as the stress increased to approximately 2.1, which was significantly higher compared with control group seedlings. NPQ could protect the photosystem from the damage of superfluous luminous energy via heat dissipation. Chloroplasts in leaves became injured under salt stress, the ability of chloroplasts to regulate and protect PSII was weakened, and NPQ decreased. G. viscidus might protect chlorophyll and increase the NPQ in the leaves of Q. mongolica to a certain extent. Under salt stress, Q. mongolica inoculated with G. viscidus might improve the NPQ of healthy leaves to make up the NPQ lost in damaged leaves and thus protect the photosystem.
Figure 7.
Changes in the non-photochemical quenching (NPQ) in ectomycorrhizal seedlings of Q. mongolica under NaCl stress (0: 0 mM; T: 120 mM; (A) control group; (B) G. viscidus group; (C) S. luteus group).
2.5. Effect of NaCl Treatment on Cell Membrane Permeability
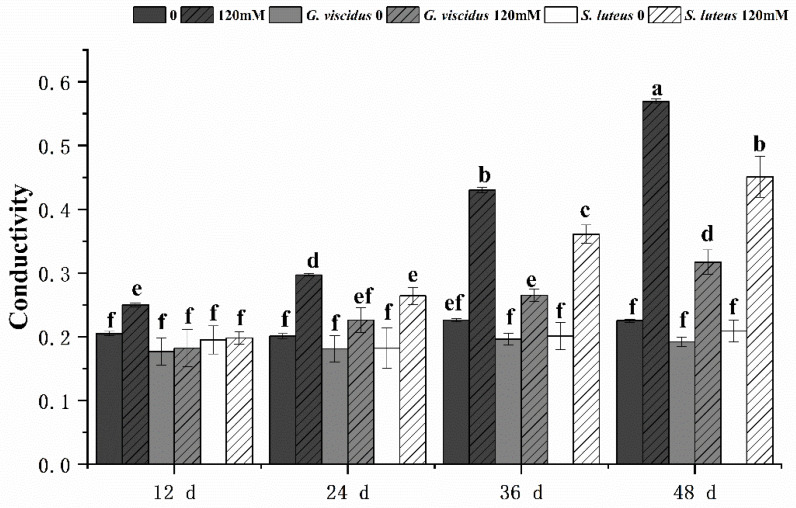
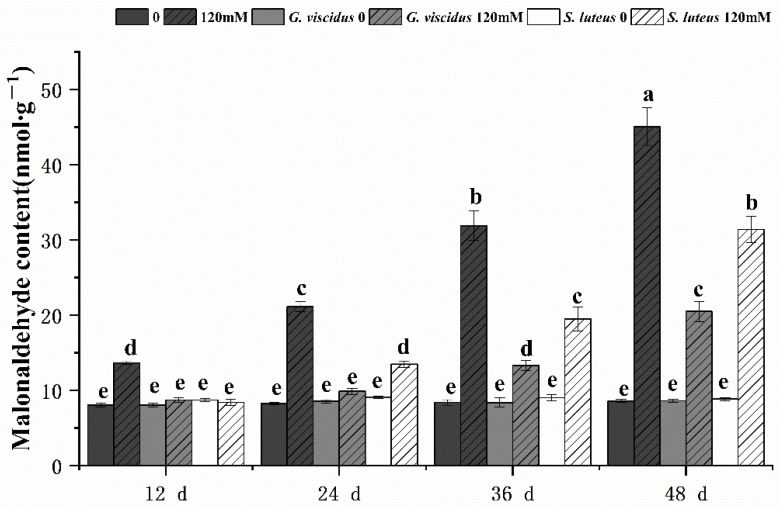
Under NaCl stress, the cell membrane permeability in the leaves of G. viscidus, S. luteus, and control group seedlings was compared by measuring the relative conductivity (Figure 8) and MDA content (Figure 9). Both indicators increased continuously as the stress duration increased. Compared with the control group, both indexes of the mycorrhizal seedlings were lower at the same time points, and their rates of increase were lower. The patterns of change in the indexes of the seedlings inoculated with different ectomycorrhizal fungi varied; specifically, the relative conductivity and MDA content were significantly lower for G. viscidus seedlings than for control group seedlings at all time points, and the relative conductivity and MDA content were 0.56 and 0.46 times higher than those in control group seedlings at day 48 of 120 mM stress, respectively. The two indexes were significantly increased at day 48 of stress in S. luteus seedlings compared with control group seedlings, and the relative conductivity was 1.25 times higher in S. luteus seedlings than in control group seedlings at day 36.
Figure 8.
Changes in the relative conductivity in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
Figure 9.
Changes in the malonaldehyde content in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
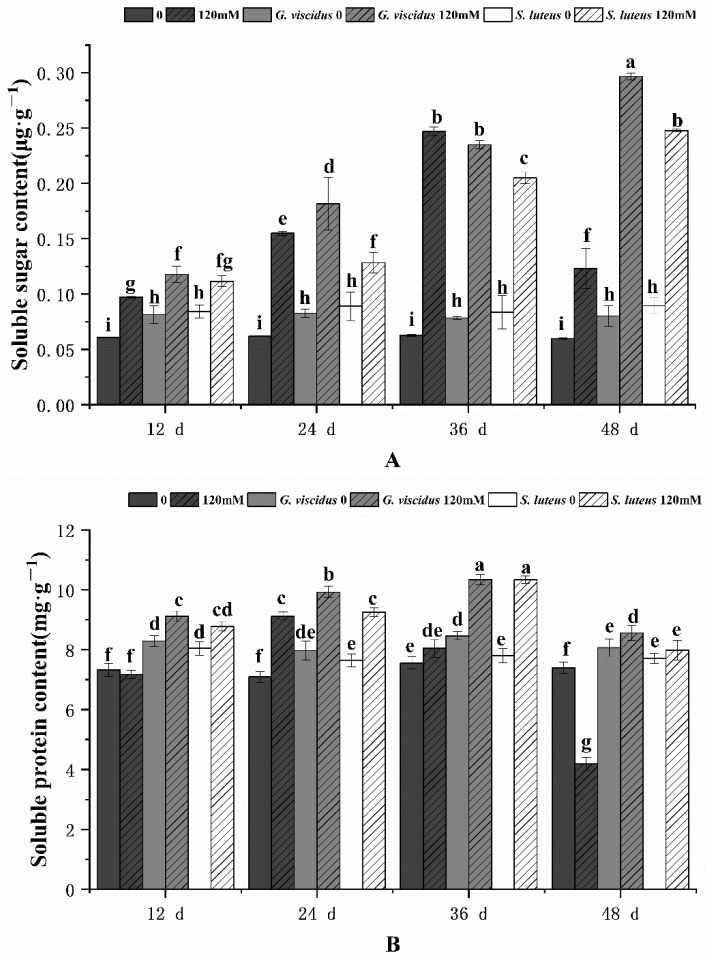
2.6. Effect of NaCl Treatment on Osmotic Adjustment Substances
Figure 10 shows the effect of NaCl treatment on sugar content and soluble protein. As the stress duration increased, the soluble sugar content and soluble protein content in G. viscidus, S. luteus, and control group seedlings initially increased but decreased when the stress was a continuous increase; the exception was for the soluble sugar content in G. viscidus and S. luteus seedlings, which continually increased and never decreased. ECM enhanced the increase in soluble sugar and soluble protein in seedlings in the pre-stress period, and the greatest increase was observed in G. viscidus seedlings. The soluble sugar content was 2.41 and 2.01 times higher in G. viscidus and S. luteus seedlings than in control group seedlings at day 48 of 120 mM stress, respectively. The soluble protein content was 2.04 and 1.91 times higher in G. viscidus and S. luteus seedlings than in control group seedlings at day 48, respectively.
Figure 10.
Changes in soluble sugar (A), soluble protein (B) in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars keyed with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
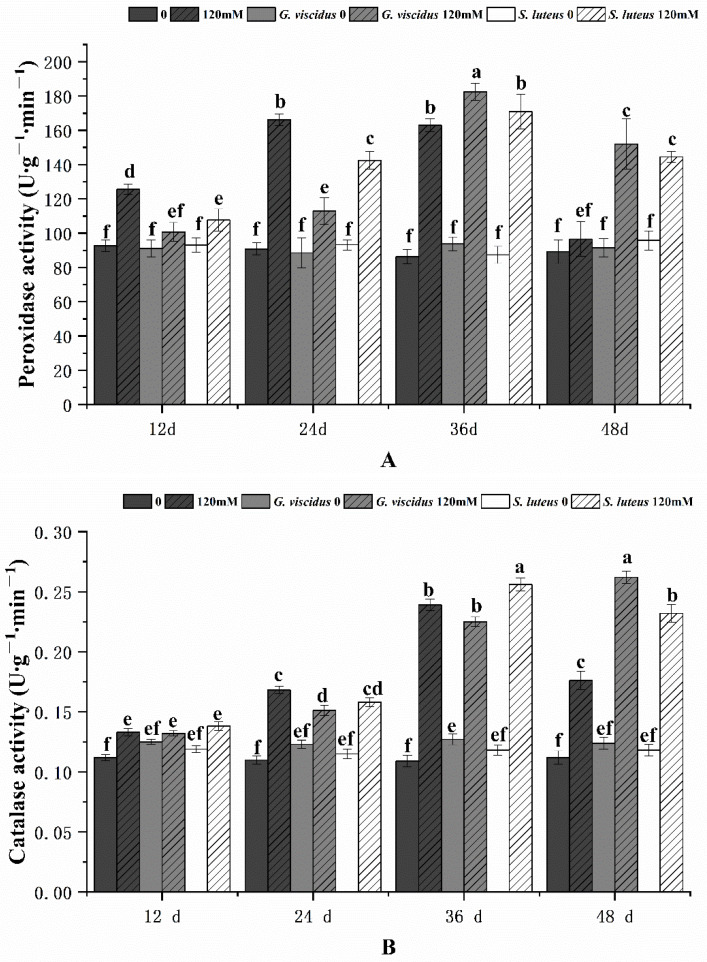
2.7. Effect of NaCl Treatment on the Antioxidant Enzyme System
As the stress duration increased, the activities of POD and CAT in G. viscidus, S. luteus, and control group seedlings first increased and then decreased; the exception was for CAT activity in G. viscidus seedlings, which continually increased (Figure 11). The POD activities of G. viscidus and S. luteus seedlings were highest on day 36, which were 1.12 times and 1.04 times higher compared with control group seedlings, respectively. The CAT activities in G. viscidus and S. luteus seedlings were highest at day 48 and 36, which were 1.49 times and 1.07 times higher compared with control group seedlings, respectively.
Figure 11.
Changes in the POD (A), CAT (B) activity in ectomycorrhizal seedlings of Q. mongolica under NaCl stress. Bars represent means of five replicates (±SD). For each parameter, bars with different letters indicate significant differences among treatments, p ≤ 0.05, following one-way ANOVA.
3. Discussion
Inoculation with ectomycorrhizal fungi is reported to be an effective approach for enhancing plant salt tolerance [9,11,14,15,17]. Castanea mollissima and Q. mongolica are Fagaceae plants. Previous studies have shown that most of the ectomycorrhizal symbioses species of C. mollissima belong to Basidiomycota [25], which includes G. viscidus and S. luteus. S. luteus has a wide distribution and has been studied in many Pinaceae plants [26,27,28]; less research has been conducted on G. viscidus. However, neither of these two types of ectomycorrhizal fungi have been thoroughly studied in Quercus. In Q. mongolica, an important tree species in the forests of North China, the role of ECM in salt tolerance has not been studied. In this study, we inoculated Q. mongolica seedlings with two ectomycorrhizal fungi, G. viscidus and S. luteus, and studied their salt tolerance relative to a control inoculated with sterile solid inoculant.
The affinity of the root system of the same host can differ among different strains, which indicates that the host has different degrees of mycorrhizal dependence on different strains [29]. G. viscidus can invade the cortical cells of the roots of seedlings more rapidly and form a symbiotic structure. This indicates that G. viscidus can more effectively promote the mycorrhization of seedlings. The Hartig net is considered an important indicator of ECM formation [30]. Krause et al. [31] found that indole-3-acetic acid of fungal origin acts as a diffusible signal that increases Hartig net formation in ECM. The two ectomycorrhizal fungi-inoculated seedlings of Q. mongolica invaded the two layers of cortical cells with a Hartig net, with no significant difference in the degree of Hartig net formation. This may be the result of the comprehensive effect of host plant age, soil fungus diversity, and effector secreted by the fungus [32].
Salt stress inhibits plant growth [33], and inoculation with ectomycorrhizal fungi can alleviate the adverse effects of various abiotic stresses on plant growth [9]. The ECM can improve the growth of host plants in high-salt environments [34]. Wen et al. [35] found that ectomycorrhizal fungi inoculations improved the salt tolerance of Pinus thunbergii to different degrees. The effect of Laccaria gomezii was stronger than that of Cenococcum geophilum. In this study, we found that ectomycorrhizal fungi significantly promoted the growth of Q. mongolica. G. viscidus seedlings had the largest increase in plant growth under salt stress; there are several possible explanations for this observation. Some mycorrhizal symbionts mainly regulate soil pH value by secreting hydrogen ions and organic acids to reduce the damage of weak bases to plants [36]. Mycorrhiza may effectively increase the absorption of water [37]. Mycorrhizal extraradical mycelium can alter the surrounding soil to enhance the acquisition of water and nutrients [38]. The hyphal contribution to water absorption in mycorrhizal plants may be affected by the fungal species and water status [37]. Additionally, ECM had greater antioxidant enzyme activity relative to non-mycorrhizal roots, an effect that reduced cellular damage and increased plant growth by preventing ROS formation and ROS detoxification [39].
Several studies have shown that salt stress can lead to complex physiological and biochemical reactions in plants, including osmotic stress, ion toxicity, and oxidation-reduction reactions, which can damage the chloroplasts of leaves [40,41]. Under salt stress, plant chloroplasts tend to disintegrate, and the synthesis of chlorophyll and carotenoids is inhibited. Previous studies have shown that ectomycorrhizal fungi can increase the Chl content of P. thunbergii seedlings under salt stress [42]. In our study, the Chl content was higher in mycorrhizal Q. mongolica seedlings than in control group seedlings under NaCl treatment, and the decrease in the Chl content became less pronounced as the duration of stress increased, indicating that the chloroplasts of the mycorrhizal seedlings were less damaged. This is similar to the change in Chl content observed in the needles of P. thunbergii inoculated with C. geophilum under salt stress [42].
As the stress duration increased, Pn and Gs of Q. mongolica decreased significantly, and the decrease in Pn became more pronounced, which may be caused by multiple factors. Previous studies have shown that under mild salt stress, the decrease in Pn in plants is related to the Chl content; under severe stress, the decrease in Pn stems from the combined effect of Chl content, Gs, and Ci [43]. The decrease in Pn in seedlings may be related to the change in Chl and Gs. Under salt stress, Gs declined, and stomatal closure was aggravated, which ultimately led to a substantial decrease in Pn under severe salt stress. This pattern was similar to that observed in cotton plants [44]. As the stress duration in Q. mongolica increased, Gs decreased, and the degree of stomatal closure increased. Under salt stress, ectomycorrhizal fungi alleviated the stomatal restriction of Zelkova serrata and enhanced transpiration, and the decrease in Pn was less pronounced [45]. Both ectomycorrhizal fungi significantly increased the Pn and Gs of seedlings with respect to the control, which was consistent with results obtained for Z. serrata [45].
Under salt stress, the NPQ of Q. mongolica decreased in the leaf margin and remained unchanged in the central part of the blade. The edge of the leaf was scorched and withered, which reduced heat consumption. Moms alba can increase the heat dissipation of energy by increasing NPQ to protect the photosynthetic system from damage [46]. Our study showed that G. viscidus and S. luteus can protect the photosynthetic system of Q. mongolica under stress, and G. viscidus had a stronger effect. This result was similar to that observed in Populus cathayana [47]. Under salt stress, ectomycorrhizal fungi can increase plant chlorophyll fluorescence efficiency and alleviate the inhibition of plant photosynthesis by salt stress [11]. The inoculation of Q. mongolica with G. viscidus may facilitate salt adaptation. However, the heat dissipation of S. luteus seedlings remained unchanged, which could still protect the photosynthetic system of seedlings to a certain extent under stress.
The relative conductivity and MDA content can reflect the degree of plant cell membrane damage. As the duration of salt stress increased, the relative conductivity and MDA content of Q. mongolica increased, indicating greater damage to the cell membrane. In this study, the MDA content was lower in Q. mongolica seedlings inoculated with two types of ectomycorrhizal fungi than in non-mycorrhizal seedlings under salt stress. The ECM significantly reduced root hydraulic conductance in Ulmus americana but did not affect leaf hydraulic conductance and net photosynthesis under NaCl stress [13]. This indicates that inoculation with ectomycorrhizal fungi can inhibit the production of membrane lipid peroxides and reduce the electrolyte extravasation of seedlings under salt stress. A relative conductivity of more than 50% is a sign of lethal damage to plants. Compared with non-ectomycorrhizal seedlings, the relative conductivity of ectomycorrhizal seedlings does not exceed 50%, which protects plants from damage.
The accumulation of osmotic adjustment substances is positively correlated with the degree of salt tolerance in plants [48]. Under salt stress, Malus halliana accumulates soluble sugar to alleviate salt damage and improve salt tolerance [49]. Under long-term stress, the cell membrane was seriously damaged, and the osmotic balance was gradually disrupted. Sharifi et al. [50] found that the proline content in soybean was significantly increased after mycorrhizal fungi inoculation. We found that two types of ectomycorrhizal fungi promoted the accumulation of soluble sugar and soluble protein in Q. mongolica pre-stress and enhanced the salt tolerance of seedlings by delaying the onset of osmotic imbalance under long-term stress.
Plants can remove free radicals produced under salt stress to enhance protection from oxidative stress by increasing the activity of POD, CAT, and SOD [48]. As salt stress increases, the antioxidant enzymes of Q. dentata first increase and then decrease [51]. This pattern is consistent with our results. Ghorhanli et al. [52] found that mycorrhizal plants have higher SOD and POD activity, and that mycorrhizal plants treated with salt have higher SOD and POD activity than those not treated with salt. This may stem from the fact that ectomycorrhizal fungi can enhance the salt tolerance of Q. mongolica by increasing antioxidant activity and delaying enzyme inactivation to adapt to long-term stress.
4. Materials and Methods
4.1. Plant Material
The seeds of Q. mongolica were collected from Beijing Pinggu Glass Valley Forest Farm, soaked with 0.5% KMnO4 for 1 h, washed with clean water, and germinated in sand in a greenhouse. The sand substrate was river sand sterilized by high-pressure steam (121 °C, 20 min). After the radicle broke through the seed coat and measured 5 cm, the axial root of the seed was retained and the lateral root was cut to promote germination. The soil matrix ratio was peat–perlite–vermiculite–organic fertilizer = 4:2:1:1.
4.2. Preparation of Fungal Inoculant and Fungal Inoculation
Two types of fungi purchased from Chinese strain storage, G. viscidus and S. luteus, were cultured with potato dextrose agar medium (PDA). Small fruiting bodies were selected and inoculated into a conical bottle containing PDA. After oscillating at a constant temperature for 1 week, the culture fluid was filtered and rinsed with distilled water, which finally gave the solid inoculant.
Fungi inoculation and ectomycorrhizal synthesis were carried out on the seedlings when the seedlings had two leaves. For each pot of seedlings, inoculations were conducted at three points equidistant to the root system, and the three points were connected to form an equilateral triangle. Each inoculation point was inoculated with 20 g of solid inoculant, and the inoculation depth was 5–10 cm. The control group was inoculated in the same way but with sterilized solid inoculant (121 °C, 20 min). First-stage lateral root mycorrhizal segments were then collected from healthy seedlings with uniform growth to characterize the morphology and anatomical structure of the ECM.
4.3. Salinity Treatment
Mycorrhizal seedlings with the same growth trend were selected for NaCl treatment. A concentration of 120 mM NaCl was applied to seedlings in the experiment. The seedlings were irrigated with 500 mL of NaCl solution prepared with deionized water every 12 days, and the control group was irrigated with an equal amount of deionized water. There were four NaCl applications over 48 days. When a small number of seedlings died, the experiment was stopped.
4.4. Morphological and Anatomical Structure Measurement
The second-stage lateral roots were observed under a ZEISS Axioskop 40 Stereomicroscope. The representative first-stage lateral root segments were selected for photography and then fixed in formaldehyde–acetic acid–ethanol fixative. Paraffin sections were used to observe the anatomical structure of the ECM. The ECM was observed and recorded with a ZEISS Axiocam 506 color optical microscope after dehydration (soaked in 70%, 80%, 90%, and 100% alcohol for 30 min each), development of transparency (soaked in alcohol–xylene (1:1) and twice in xylene for 30 min each), embedding, sectioning, rehydration (soaked in 100%, 95%, 85%, 75%, 50%, and 30% alcohol for 30 min each), and staining. The anatomical structure of five root segments was studied in each inoculation group at different times after inoculation.
4.5. Physiological and Biochemical Indicators Measurement
The MDA and soluble sugar content in leaves was determined by the thiobarbituric acid method [53]. Peroxidase and Catalase activity were assayed by guaiacol colorimetric method and ultraviolet absorption method [54]. Soluble protein content [53] was measured by Coomassie brilliant blue G-250.
On days 12, 24, 36, and 48 after the first NaCl application, the leaves were collected. Two g of leaves were placed in a mortar, and small amounts of CaCO3, quartz sand, and 3 mL of 95% ethanol were added. After grinding into a fine powder, 10 mL of 95% ethanol was added, and the sample was further ground until the leaves turned white. After 30 min, the leaves were filtered into a 100 mL volumetric flask. The absorbance value of the extract was determined by a UV-2100 ultraviolet-visible spectrophotometer at the wavelengths of 470 nm, 649 nm, and 665 nm, with 95% ethanol used as a blank control. The total chlorophyll (Chl) content was calculated using the formula of the standard curve.
Live leaves were rinsed 3 times with deionized water; the leaves were then wiped to remove surface moisture, avoiding the leaf vein, and punched into 10 pieces. The sample was then placed in a centrifuge tube; after adding 10 mL of deionized water, the sample was placed in a vacuum pump until the blade sank to the bottom. The sample was then removed and left to stand for 4 h. A DDSJ-308A conductivity meter was used to measure the conductivity value S0 of the deionized water and the initial conductivity value S1. The sample was heated in a boiling water bath for 10 min, rinsed with cold water for 10 min; the final conductivity value S2 was then measured. The relative conductivity was calculated according to the formula in a previous study [53].
4.6. Photosynthesis Index Measurements
On days 12, 24, 36, and 48 after the first NaCl application, healthy mature functional leaves with the same size and light exposure were selected for instantaneous measurements of photosynthetic parameters using a LI6400 photosynthesis meter. A light level of 1000 µmol photons m−2 s−1 was supplied by a red–blue LED. The net photosynthetic rate (Pn), transpiration rate (Tr), stomatal conductance (Gs), and intercellular CO2 concentration (Ci) of the leaves were determined. The following set of parameters were used during measurements: stomatal flow rate, 500 mL·min−1; leaf temperature, 30 ± 1 °C; relative air humidity, 60%; and concentration of CO2 in leaf chamber, 400 μmol·mol−1.
4.7. Chlorophyll Fluorescence Parameter Measurements
On days 12, 24, 36, and 48 after the first NaCl application, healthy mature functional leaves with the same size and light exposure were selected for measurements of chlorophyll fluorescence parameters. After 15 min of dark adaptation, photosystem II maximum photochemical efficiency (Fv/Fm) and the coefficient of photochemical quenching (NPQ) were measured using a FluorCam 800 MF.
4.8. Statistical Data Analysis
All data were analyzed by one-way ANOVA using SPSS 13.0. p < 0.05 was the threshold for statistical significance. There were five biological replicates for each treatment.
5. Conclusions
Inoculation of G. viscidus and S. luteus could alleviate the growth inhibition of Q. mongolica seedlings under salt stress and relieve the symptoms of salt injury. The inoculation of the two fungi also promoted an increase in the cross-sectional area of the root system, Chl content, Pn, osmotic adjustment substances content, and antioxidant enzyme activity in seedlings. The effects of G. viscidus were stronger than those of S. luteus under salt stress. Generally, the results of our study underscore the need to screen for suitable ectomycorrhizal fungal species to inoculate Q. mongolica to enhance its salt resistance and, thus, ensure normal growth under salt stress.
Acknowledgments
We thank the Beijing Municipal Education Commission for their financial support through the Innovative Transdisciplinary Program of Ecological Restoration Engineering.
Author Contributions
Study design: P.-S.L. and H.H.; H.H. and X.-N.B. performed the work and conceived the experiments; H.H. performed the analysis of the data; X.-N.B. wrote the manuscript with the help of Z.-H.H. and P.-S.L. All the authors contributed to the discussion, review, and approval of the final manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Scientific Research Project of Beijing Educational Committee [number SQKM20181002015].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tavakkoli E., Rengasamy P., McDonald G.K. High concentrations of Na+ and Cl− ions in soil solution have simultaneous detrimental effects on growth of faba bean under salinity stress. J. Exp. Bot. 2010;61:4449–4459. doi: 10.1093/jxb/erq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Afzal Z., Howton T.C., Sun Y., Mukhtar M.S. The roles of aquaporins in plant stress responses. J. Dev. Biol. 2016;4:9. doi: 10.3390/jdb4010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdoucq L., Maurel C. Plant Aquaporins. Elsevier; Amsterdam, The Netherlands: 2018. pp. 25–56. [Google Scholar]
- 5.Parihar P., Singh S., Singh R., Singh V.P., Prasad S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. Int. 2015;22:4056–4075. doi: 10.1007/s11356-014-3739-1. [DOI] [PubMed] [Google Scholar]
- 6.Chaves M.M., Flexas J., Pinheiro C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009;103:551–560. doi: 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meyer S., Genty B. Mapping intercellular CO2 mole fraction (Ci) in rosa rubiginosa leaves fed with abscisic acid by using chlorophyll fluorescence imaging. Significance of ci estimated from leaf gas exchange. Plant Physiol. 1998;116:947–957. doi: 10.1104/pp.116.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Negrão S., Schmöckel S.M., Tester M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2016;119:1–11. doi: 10.1093/aob/mcw191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerrero-Galan C., Calvo-Polanco M., Zimmermann S.D. Ectomycorrhizal symbiosis helps plants to challenge salt stress conditions. Mycorrhiza. 2019;29:291–301. doi: 10.1007/s00572-019-00894-2. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen H., Polanco M.C., Zwiazek J.J. Gas exchange and growth responses of ectomycorrhizal Picea mariana, Picea glauca, and Pinus banksiana seedlings to NaCl and Na2SO4. Plant Biol. 2006;8:646–652. doi: 10.1055/s-2006-924106. [DOI] [PubMed] [Google Scholar]
- 11.Bois G., Bigras F.J., Bertrand A., Piché Y., Fung M.Y.P., Khasa D.P. Ectomycorrhizal fungi affect the physiological responses of Picea glauca and Pinus banksiana seedlings exposed to an NaCl gradient. Tree Physiol. 2006;26:1185–1196. doi: 10.1093/treephys/26.9.1185. [DOI] [PubMed] [Google Scholar]
- 12.Polanco M.C., Zwiazek J.J., Jones M.D., MacKinnon M.D. Responses of mycorrhizal jack pine (Pinus banksiana) seedlings to NaCl and boron. Trees. 2008;22:825–834. doi: 10.1007/s00468-008-0243-6. [DOI] [Google Scholar]
- 13.Polanco M.C., Zwiazek J.J., Voicu M.C. Responses of ectomycorrhizal American elm (Ulmus americana) seedlings to salinity and soil compaction. Plant Soil. 2008;308:189–200. doi: 10.1007/s11104-008-9619-z. [DOI] [Google Scholar]
- 14.Ma X., Sun M., Sa G., Zhang Y., Li J., Sun J., Shen X., Polle A., Chen S. Ion fluxes in Paxillus involutus-inoculated roots of Populus × canescens under saline stress. Environ. Exp. Bot. 2014;108:99–108. doi: 10.1016/j.envexpbot.2013.11.016. [DOI] [Google Scholar]
- 15.Sa G., Yao J., Deng C., Liu J., Zhang Y., Zhu Z., Zhang Y., Ma X., Zhao R., Lin S., et al. Amelioration of nitrate uptake under salt stress by ectomycorrhiza with and without a Hartig net. New Phytol. 2019;222:1951–1964. doi: 10.1111/nph.15740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhsin T.M., Zwiazek J.J. Colonization with Hebeloma crustuliniforme increases water conductance and limits shoot sodium uptake in white spruce (Picea glauca) seedlings. Plant Soil. 2002;238:217–225. doi: 10.1023/A:1014435407735. [DOI] [Google Scholar]
- 17.Zwiazek J.J., Equiza M.A., Karst J., Senorans J., Wartenbe M., Calvo-Polanco M. Role of urban ectomycorrhizal fungi in improving the tolerance of lodgepole pine (Pinus contorta) seedlings to salt stress. Mycorrhiza. 2019;29:303–312. doi: 10.1007/s00572-019-00893-3. [DOI] [PubMed] [Google Scholar]
- 18.Otgonsuren B., Rewald B., Godbold D.L., Göransson H. Ectomycorrhizal inoculation of Populus nigra modifies the response of absorptive root respiration and root surface enzyme activity to salinity stress. Flora. 2016;224:123–129. doi: 10.1016/j.flora.2016.07.016. [DOI] [Google Scholar]
- 19.Smith M.E., Pfister D.H. Tuberculate ectomycorrhizae of angiosperms: The interaction between Boletus rubropunctus (Boletaceae) and Quercus species (Fagaceae) in the United States and Mexico. Am. J. Bot. 2009;96:1665–1675. doi: 10.3732/ajb.0900058. [DOI] [PubMed] [Google Scholar]
- 20.Jumpponen A., Jones K.L., Mattox D.J., Yaege C. Massively parallel 454-sequencing of fungal communities in Quercus spp. ectomycorrhizas indicates seasonal dynamics in urban and rural sites. Mol. Ecol. 2010;19((Suppl. S1)):41–53. doi: 10.1111/j.1365-294X.2009.04483.x. [DOI] [PubMed] [Google Scholar]
- 21.Nardinia A., Salleoa S., Tyreeb M.T., Vertoveca M. Influence of the ectomycorrhizas formed by Tuber melanosporum Vitt. on hydraulic conductance and water relations of Quercus ilex L. seedlings. Ann. For. Sci. 2000;57:305–312. doi: 10.1051/forest:2000121. [DOI] [Google Scholar]
- 22.Rieske L.K. Influence of symbiotic fungal colonization on oak seedling growth and suitability for insect herbivory. Environ. Entomol. 2001;30:849–854. doi: 10.1603/0046-225X-30.5.849. [DOI] [Google Scholar]
- 23.Yan X., Wang Q. Effects of ectomycorrhizal inoculation on the seedling growth of Quercus liaotungensis. Acta Phytoecol. Sin. 2002;26:701–707. [Google Scholar]
- 24.Núñez J.A.D., Serrano J.S., Barreal J.A.R., González J.A.S.d.O. The influence of mycorrhization with Tuber melanosporum in the afforestation of a Mediterranean site with Quercus ilex and Quercus faginea. For. Ecol. Manag. 2006;231:226–233. doi: 10.1016/j.foreco.2006.05.052. [DOI] [Google Scholar]
- 25.Ling Q. Research on symbiotical fungi species and ectomycorrhizae occurrence of Chestnut (Castanea mollissima BL.) J. Beijing Agric. Coll. 1995;10:71–75. [Google Scholar]
- 26.Liu H., Chen H., Ding G., Li K., Wang Y. Proteomic insight into the symbiotic relationship of Pinus massoniana Lamb and Suillus luteus towards developing Al-stress resistance. Life. 2021;11:177. doi: 10.3390/life11020177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin D., Deng X., Chet I., Song R. Physiological responses of Pinus sylvestris var. Mongolica seedlings to the interaction between Suillus luteus and Trichoderma virens. China Acad. J. Electron. Publ. House. 2014;69:334–342. doi: 10.1007/s00284-014-0589-5. [DOI] [PubMed] [Google Scholar]
- 28.Korhonen A., Lehto T., Heinonen J., Repo T. Whole-plant frost hardiness of mycorrhizal (Hebeloma sp. or Suillus luteus) and non-mycorrhizal Scots pine seedlings. Tree Physiol. 2019;39:526–535. doi: 10.1093/treephys/tpy105. [DOI] [PubMed] [Google Scholar]
- 29.Dalong M., Luhe W., Guoting Y., Liqiang M., Chun L. Growth response of Pinus densiflora seedlings inoculated with three indigenous ectomycorrhizal fungi in combination. Braz. J. Microbiol. 2011;42:1197–1203. doi: 10.1590/S1517-83822011000300045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henke C., Jung E.-M., Kothe E. Hartig’ net formation of Tricholoma vaccinum-spruce ectomycorrhiza in hydroponic cultures. Environ. Sci. Pollut. Res. 2015;22:19394–19399. doi: 10.1007/s11356-015-4354-5. [DOI] [PubMed] [Google Scholar]
- 31.Krause K., Henke C., Asiimwe T., Ulbricht A., Klemmer S., Schachtschabel D., Boland W., Kothe E. Biosynthesis and secretion of indole-3-acetic acid and its morphological effects on Tricholoma vaccinum-spruce ectomycorrhiza. Appl. Environ. Microbiol. 2015;81:7003–7011. doi: 10.1128/AEM.01991-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Navarro-RoDenas A., Xu H., Kemppainen M., Pardo A.G., Zwiazek J.J. Laccaria bicolor aquaporin LbAQP1 is required for Hartig net development in trembling aspen (Populus tremuloides) Plant Cell Environ. 2015;38:2475–2486. doi: 10.1111/pce.12552. [DOI] [PubMed] [Google Scholar]
- 33.Van Zelm E., Zhang Y., Testerink C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020;71:403–433. doi: 10.1146/annurev-arplant-050718-100005. [DOI] [PubMed] [Google Scholar]
- 34.Kohler J., Caravaca F., Roldán A. An AM fungus and a PGPR intensify the adverse effects of salinity on the stability of rhizosphere soil aggregates of Lactuca sativa. Soil Biol. Biochem. 2010;42:429–434. doi: 10.1016/j.soilbio.2009.11.021. [DOI] [Google Scholar]
- 35.Wen Z., Zhu X., Liu C. Effects of two ectomycorrhizal fungi on growth of Pinus thunbergii seedlings planted in saline soil. China Acad. J. Electron. Publ. House. 2019;39:22–27. doi: 10.14067/j.cnki.1673-923x.2019.04.005. [DOI] [Google Scholar]
- 36.Shi L., Xue J., Liu B., Dong P., Wen Z., Shen Z., Chen Y. Hydrogen ions and organic acids secreted by ectomycorrhizal fungi, Pisolithus sp1, are involved in the efficient removal of hexavalent chromium from waste water. Ecotoxicol. Environ. Saf. 2018;161:430–436. doi: 10.1016/j.ecoenv.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Ruiz-Lozano J.M., Azcon R. Hyphal contribution to water uptake in mycorrhizal plants as affected by the fungal species and water status. Physiol. Plant. 1995;95:472–478. doi: 10.1111/j.1399-3054.1995.tb00865.x. [DOI] [Google Scholar]
- 38.Zou Y.N., Srivastava A.K., Ni Q.D., Wu Q.S. Disruption of mycorrhizal extraradical mycelium and changes in leaf water status and soil aggregate stability in rootbox-grown trifoliate orange. Front. Microbiol. 2015;6:203. doi: 10.3389/fmicb.2015.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alvarez M., Huygens D., Fernandez C., Gacitua Y., Olivares E., Saavedra I., Alberdi M., Valenzuela E. Effect of ectomycorrhizal colonization and drought on reactive oxygen species metabolism of Nothofagus dombeyi roots. Tree Physiol. 2009;29:1047–1057. doi: 10.1093/treephys/tpp038. [DOI] [PubMed] [Google Scholar]
- 40.Gong X., Chao L., Zhou M., Hong M., Luo L., Wang L., Ying W., Cai J., Songjie G., Hong F. Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil. 2011;340:443–452. doi: 10.1007/s11104-010-0616-7. [DOI] [Google Scholar]
- 41.Munns R. Genes and salt tolerance: Bringing them together. New Phytol. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 42.Shi L., Wang J., Liu B., Nara K., Lian C., Shen Z., Xia Y., Chen Y. Ectomycorrhizal fungi reduce the light compensation point and promote carbon fixation of Pinus thunbergii seedlings to adapt to shade environments. Mycorrhiza. 2017;27:823–830. doi: 10.1007/s00572-017-0795-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang B., Yu M.-K., Sun H.-J., Cheng X.-R. Photosynthetic characters of Quercus acutissima from different provenances under effects of salt stress. Chin. J. Appl. Ecol. 2009;20:1817–1824. [PubMed] [Google Scholar]
- 44.Chen W., Feng C., Guo W., Shi D., Yang C. Comparative effects of osmotic-, salt- and alkali stress on growth, photosynthesis, and osmotic adjustment of cotton plants. Photosynthetica. 2011;49:417–425. doi: 10.1007/s11099-011-0050-y. [DOI] [Google Scholar]
- 45.Zhu L., Fu Z., Zhang J., Wang J. Effects of mycorrhizal fungi on photosynthetic characteristics of Zelkova serrata Thunb. China Acad. J. Electron. Publ. House. 2018;42:121–127. [Google Scholar]
- 46.Ke Y., Zhou J., Lu N., Zhang X. Effects of salinity on photosynthetic physiology and chlorophyll fluorescence characteristics of mulberry (Moms alba) seedlings. For. Res. 2009;22:200–206. doi: 10.1007/978-1-4020-9623-5_5. [DOI] [Google Scholar]
- 47.Wu N., Li Z., Liu H., Tang M. Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol. Plant. 2015;37:183. doi: 10.1007/s11738-015-1932-6. [DOI] [PubMed] [Google Scholar]
- 48.Koca H., Bor M., Özdemir F., Türkan İ. The effect of salt stress on lipid peroxidation, antioxidative enzymes and proline content of sesame cultivars. Environ. Exp. Bot. 2007;60:344–351. doi: 10.1016/j.envexpbot.2006.12.005. [DOI] [Google Scholar]
- 49.Jia X.M., Zhu Y.F., Wang H. Study on physiological response of Malus halliana to saline-alkali stress. Acta Ecol. Sin. 2019;39:6349–6361. [Google Scholar]
- 50.Sharifi M., Ghorbanli M., Ebrahimzadeh H. Improved growth of salinity-stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. J. Plant Physiol. 2007;164:1144–1151. doi: 10.1016/j.jplph.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 51.HAO H., Cao L., Chen W. Effects of salt stress on the ion balance and physiological-biochemical characteristics of Quercus dentata seedlings. Acta Ecol. Sin. 2020;40:6897–6904. [Google Scholar]
- 52.Ghorbanli M., Ebrahimzadeh H., Sharifi M. Effects of NaCl and mycorrhizal fungi on antioxidative enzymes in soybean. Biol. Plant. 2004;48:575–581. doi: 10.1023/B:BIOP.0000047157.49910.69. [DOI] [Google Scholar]
- 53.Lu W., Li Y. Experimental Course of Plant Physiology. Volume 1 China Forestry Publishing House; Beijing, China: 2012. [Google Scholar]
- 54.Shi S., Ya Y., Wei L. Experimental Guidance of Plant Physiology. Volume 4 China Forestry Publishing House; Beijing, China: 2011. [Google Scholar]