Abstract
In the current scenario of changing climatic conditions and the rising global population, there is an urgent need to explore novel, efficient, and economical natural products for the benefit of humankind. Biosurfactants are one of the latest explored microbial synthesized biomolecules that have been used in numerous fields, including agriculture, pharmaceuticals, cosmetics, food processing, and environment-cleaning industries, as a source of raw materials, for the lubrication, wetting, foaming, emulsions formulations, and as stabilizing dispersions. The amphiphilic nature of biosurfactants have shown to be a great advantage, distributing themselves into two immiscible surfaces by reducing the interfacial surface tension and increasing the solubility of hydrophobic compounds. Furthermore, their eco-friendly nature, low or even no toxic nature, durability at higher temperatures, and ability to withstand a wide range of pH fluctuations make microbial surfactants preferable compared to their chemical counterparts. Additionally, biosurfactants can obviate the oxidation flow by eliciting antioxidant properties, antimicrobial and anticancer activities, and drug delivery systems, further broadening their applicability in the food and pharmaceutical industries. Nowadays, biosurfactants have been broadly utilized to improve the soil quality by improving the concentration of trace elements and have either been mixed with pesticides or applied singly on the plant surfaces for plant disease management. In the present review, we summarize the latest research on microbial synthesized biosurfactant compounds, the limiting factors of biosurfactant production, their application in improving soil quality and plant disease management, and their use as antioxidant or antimicrobial compounds in the pharmaceutical industries.
Keywords: biosurfactants, critical micelle concentration (C.M.C.), antioxidant, microorganism, soil quality, plant disease management
1. Introduction
The rapid industrialization and rising global population excavate the challenges of food security and environmental management. Moreover, the changing climatic conditions, such as rising temperature, irregular rainfall, and biotic and abiotic stress factors adversely affect agricultural productivity. In addition, an eruption of new pests, pathogens, or plant diseases are some primary concerns for the agronomist, researchers, and scientific community. Indeed, a larger population of developed and developing countries rely on chemical pesticides or agrochemicals for pathogen control or plant disease management. Nevertheless, the undistributed and continuous use of agrochemicals results in the deposition of toxic chemical residue in the food, low nutrient quality, and the emergence of pesticide-resistant pathogens.
Additionally, the deposition of agrochemicals adversely affects the texture, nutrient quality, or the native microflora of the soil and also leads to environmental challenges via polluting soil and water ecosystems [1]. However, to mitigate these challenges, in the last two decades, microbes and their products have been frequently utilized to enhance agricultural productivity and crop yield or mitigate toxic and hazardous environmental contaminants. Moreover, the ubiquitous nature of microbes, easy cultivation methods, cost-effectiveness, and low or even no toxic effect on the surrounding environment makes them most preferable in the various fields for sustainable growth and production.
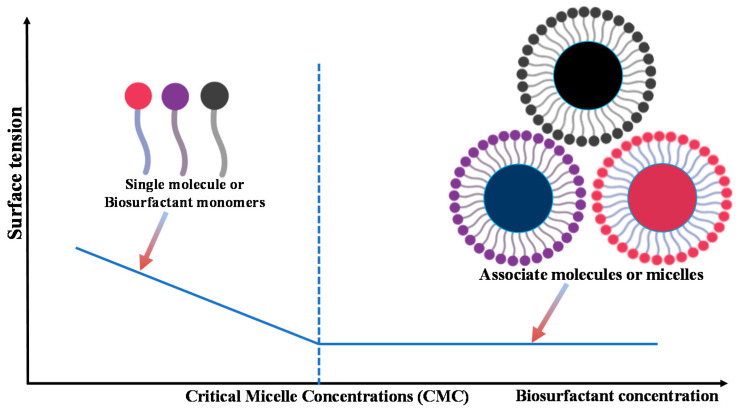
Biosurfactants are one of the latest explored microbial produced/synthesized biomolecules, and are frequently utilized in various agricultural, waste management, or pharmaceutical industries as raw materials, for the lubrication, wetting, foaming, emulsions formulations, or stabilizing dispersions [2,3]. The term biosurfactant has been referred to as the surface-acting agents that can improve surface–surface interactions through forming micelles produced by the natural source of origin, such as plants, microbes, and animals [4,5]. In addition, biosurfactants have been used during the applications to reduce the interfacial surface tension between solution and the surface, or air/water or oil/water interfaces [6]. In other aspects, the addition of surfactants into an oil/water or water/air system causes a reduction in the surface tension up to a point at which surfactants form structures, such as micelles, vesicles, and bilayers; usually, this critical point is known as critical micelle concentration (C.M.C.) (Figure 1).
Figure 1.
Critical micelle concentration (CMC) and micelle formation of biosurfactant monomers.
However, in a combined mixture or after the addition of surfactant in the water and oil mixture, the surfactant resides at the oil/water interface and forms emulsions, which confer excellent emulsifying, foaming, and dispersing capacities. This makes surfactants one of the most versatile chemicals for industrial processes [7]. The most commonly used surfactants are of chemical origin, but their toxic nature, low degradation rate, and high persistence power limit their frequent use in the food, cosmetics, and pharmaceutical industries [8]. Surfactants of microbial origins have several advantages over synthetic or chemical surfactants: higher temperature tolerance, stability in pH variation, high salinity tolerance, higher degradation rate, less toxicity, and better selectivity [9,10].
Biosurfactants are usually composed of amphipathic molecules that have both hydrophilic and hydrophobic constituents. The hydrophilic compounds generally consist of positive, negative, or amphoteric charged ions, whereas the hydrophobic compounds are made up of a long chain of fatty acids [11]. Irrespective of their chemical counterparts, biosurfactants are generally classified based on molecular weight (low or high), critical micelle concentration (C.M.C.), microorganism produced, and their mode of action. Glycolipids, phospholipids, and lipopeptides are the most commonly reported low molecular weight; however, high molecular weight biosurfactants are composed of polysaccharides, lipopolysaccharides, and a complex mixture of biopolymers. A detailed classification and some common examples are illustrated in Table 1 and Figure 2.
Table 1.
Biosurfactant classification and some common examples.
| Types of Biosurfactants | Common Examples | |
|---|---|---|
| Low molecular mass | Glycolipids | Rhamnolipids |
| Sophorolipids | ||
| Trehalose lipids | ||
| Phospholipids | Phospholipids | |
| Corinomiocolic acid | ||
| Fatty acids | ||
| Lipopeptides | Surfactin | |
| Wisconsin | ||
| Gramicidin | ||
| Subtilisin | ||
| Peptide lipid | ||
| Lichenysin | ||
| High molecular mass | Polymeric | Liposan |
| Emulsan | ||
| Biodispersion | ||
| Mannan-lipid protein | ||
| Carbohydrate lipid-protein | ||
| Particulate | Vesicles |
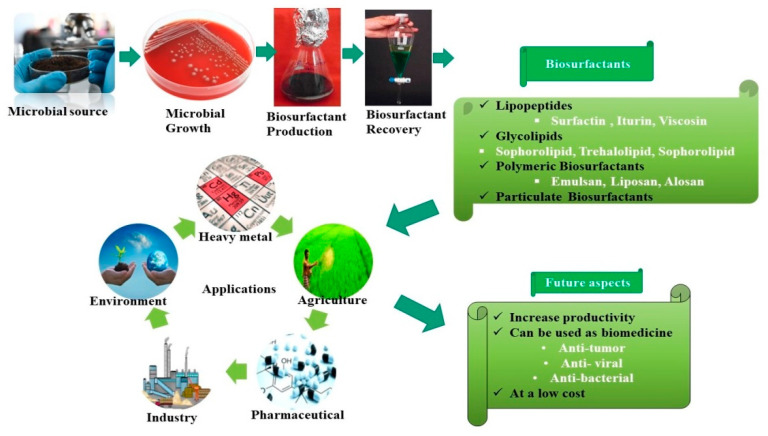
Figure 2.
Schematic representation of biosurfactant production utilizing microbial resources and their potential applications.
The low molecular weight of microbial and synthesized biosurfactants offer excellent capability for reducing surface tension; however, high molecular weight is associated with the ability to make a stable emulsion [7,12,13]. The eco-friendly and multifunctional attributes of biosurfactants are considered the surfactant of the next generation and are frequently utilized in various industries worldwide. According to a published report, the market size of biosurfactants is expected to increase by 0.8% of the compound annual growth rate (CAGR) in the forecast period of 2020 to 2025. It will be expected to reach about USD 1446.5 million by 2025 from the USD 1403.1 million reported in 2019 (Global Biosurfactant Market 2020 by Manufacturers, Regions, Type, and Application, Forecast to 2025).
For sustainable agricultural practices, biosurfactants have been used to improve soil quality by degrading toxic and hazardous contaminants or making trace elements available in the soil, and are frequently utilized as antagonistic molecules against pests/pathogens or plant diseases. Surfactants produced by microbial strain possess antimicrobial properties, which effectively inhibit pathogen growth. In several cases, it protects the plant from pathogen infection via stimulating the plant immune system [14]. Furthermore, utilization of biosurfactants showed additional benefit in the plant through enhancing growth promotion. Additionally, the native microflora of the soil or plant system uses these biosurfactant molecules as a source of energy for regulating plants’ physiological parameters and maintaining the plant system’s health and quality.
Moreover, nowadays, in pharmaceutical industries, biosurfactant molecules are broadly used as antioxidants, antimicrobial and anticancerous agents, raw materials, or emulsifying or dispersing agents. Thus, the application of biosurfactants in treating human ailments is cost-effective and safe from toxic side effects. In the present review, we summarize the latest aspect of biosurfactant synthesis from microbial sources, limiting factors of biosurfactant production. In addition, it also discussed the potential application of biosurfactants in sustainable agriculture, specifically their role in improving soil quality or plant pathogen management and pharmaceutical industries as an antioxidant or antimicrobial molecule.
2. Microorganisms and Biosurfactants
Currently, numerous microbial strains of bacteria, fungi, and yeasts have been reported for the efficient production of biosurfactants. However, the quality and quantity of biosurfactants depend on several factors, including the type of microorganism, media supplements, nature of the substrate, and different intrinsic and extrinsic factors at the time of microbial culture growth [15,16]. The selection of microbial strain is the primary step of biosurfactant production. However, biosurfactant synthesis in the microbial strain is carried out either intracellularly or extracellularly during the exponential or stationary phase of growth, when the nutrient conditions are limiting [17]. The nature of biosurfactants also depends on microorganisms’ source and isolation strategies; for instance, a strain isolated from a contaminated site is considered a suitable choice for the degradation of that particular contaminant. The probable reason for this concept is that the isolated microorganism can use that contaminant as a source of energy or substrate, where other microorganisms or non-surfactant-producing microorganisms cannot survive [18].
Furthermore, biosurfactants play a physiologic role in increasing the bioavailability of hydrophobic molecules involved in cellular signaling or differentiation processes and facilitate the consumption of carbon sources present in the soil [19]. Indeed, the physiological aspect of biosurfactant production in the contaminated site is not clearly understood but considered for enhancing the nutrient uptake from the hydrophobic substrate, biofilm formation, and cellular motility by reducing the surface tension at the phase boundary [7]. The development of rapid and reliable methods for the isolation and screening of microbial strains and further evaluation of role in emulsification, reducing interfacial or surface tension are critical factors during the exploration of biosurfactant molecules [20]. In early 1941, Bushnell and Hass [21] reported biosurfactants produced by the bacterial strain Corynebacterium simplex and Pseudomonas grown in the minimal media containing kerosene, mineral oil, or paraffin [22]. Then, numerous microbial strains, including bacteria, fungi, and yeast, have been reported for efficient biosurfactant production. Details of microbial strains and their synthesized biosurfactants are illustrated in Table 2A–C.
Table 2.
(A). Different types of biosurfactants produced from bacterial strains. (B). Different types of biosurfactants produced from fungal strains. (C). Different types of biosurfactants produced from yeast strains.
| (A). Different Types of Biosurfactants Produced from Bacterial Strains | ||||
| Bacterial Strains | Biosurfactants | Properties | Isolation Source | References |
| Pontibacter korlensis strain SBK-47 | Pontifactin | Surface-active, antimicrobial, and antibiofilm activities | Coastal waters of Karaikal, Puducherry, India | [23] |
| Bacillus licheniformis | Lipopeptides | Heat resistance and capacity to emulsify oils used in cosmetics | Deception Island (Antarctica) | [24] |
| Paracoccus sp. MJ9 | Rhamnolipid | Enhance solubility of hydrophobic compounds | Jiaozhou Bay in Qingdao, Shandong Province | [25] |
| Pseudomonas aeruginosa UCP0992 | Rhamnolipids | High emulsifying activities against different oils, capacity to remove hydrophobic contaminants, and did not show toxicity |
Centre of Research in Environmental Sciences, Catholic University of Pernambuco, Brazil |
[26] |
| Pseudomonas aeruginosa PA1 | Rhamnolipid | Capacity to use as carbon sources | Oil production wastewater in the northeast of Brazil | [27] |
| Pseudomonas desmolyticum NCIM 2112 | Rhamnolipid | Degradation of textile dye |
National Center for Industrial Microorganisms (NCIM), Pune, India | [28] |
| Serratia marcescens SS-1 | Serrawettins | Produces lipopeptide surfactants, having the capability to reduce surface tension |
Taiwan | [29] |
| Bacillus subtilis | Cyclic lipopeptides |
A significant reduction in the activities of acetylcholinesterase, a-carboxylesterase, and acid phosphatases | Namakkal and Tirunelveli district, Tamil Nadu, India | [30] |
| Bacillus subtilis | Pumilacidin | Antiviral activity against Herpes simplex virus 1 (HSV-1) | Tree trunk near lake Yamanaka, Japan | [31] |
| Pseudomonas aeruginosa S5 | Glycolipid | Removal of polycyclic aromatic hydrocarbons | Supelco (Bellefonte, PA, USA) | [32] |
| Pseudomonas protegens F6 | Orfamide A | Insecticidal against Myzus persicae | Soil from previously reported diesel oil-contaminated site | [33] |
| Pseudomonas aeruginosa DS9 | Rhamnolipid | Antifungal agents against F. sacchari in pokkah boeng Disease |
Lakota oil-field of Sivsagar district, Assam, India |
[34] |
| Pseudomonas fluorescens BD5 | Pseudofactin II | Antiadhesive activity and disinfectant | Freshwater from the Arctic Archipelago of Svalbard | [35] |
| Bacillus sp. BS3 | Lipopeptide | Anticancer activity and antiviral properties |
Solar salt works in Tamilnadu, India |
[36] |
| Pseudomonas aeruginosa | Rhamnolipid | Enhanced oil recovery through anaerobic production of Rhamnolipid | Daqing oilfield-produced water | [37] |
| Bacillus subtilis A21 | Lipopeptide | Removal of petroleum hydrocarbons, heavy metals | Adityapur Industrial Area, Jharkhand | [38] |
| Rhodotorula bogoriensis | Sophorolipid | Antimicrobial property against Propionibacterium acnes | American Type Culture Collection | [39] |
| (B). Different Types of Biosurfactants Produced from Fungal Strains | ||||
| Fungi | Biosurfactants | Properties | Isolation Source | References |
| Candida utilis | Emulsifiers | Emulsifiers | Culture collection from the Department of Antibiotics of the Universidade Federal de, Pernambuco, Brazil | [40] |
| Candida lipolytica UCP 0988 | Lipopeptide | Not toxic against different vegetable seed | Culture collection of Nucleus of Research in Environmental Sciences, Catholic University of Pernambuco, Recife-PE, Brazil | [41] |
| Penicillium chrysogenum SNP5 | Lipopeptide | Role in pharmaceuticals as well as in the petroleum and oil industry | Soil-contaminated grease waste | [42] |
| Cunninghamella echinulata | Complex Carbohydrate/protein/lipid | Reduce and increase the viscosity of hydrophobic substrates and their molecules |
Caatinga soil of Pernambuco, Northeast of Brazil |
[43] |
| Candida Antarctica | Mixtures of 4 mannosylerythritol lipids | Produced the lipids from different vegetable oils | Centraalbureau voor Schimmelcultures, the Netherlands | [44] |
| Microsphaeropsis sp. | Eremophilane derivative | Antimicrobial properties | Waters around the Caribbean Island of Dominica | [45] |
|
Yarrowia lipolytica NCIM 3589 |
Bioemulsifier | Increased the hydrophobicity of the cells during the growth phase | Seawater near Mumbai, India |
[46] |
| Yarrowia lipolytica IMUFRJ50682 | Carbohydrate protein complex |
Capable of stabilizing oil-in-water emulsions | Guanabara Bay in Rio de Janeiro | [47] |
| Ustilago maydis | Cellobiose lipids | Secreted cellobiose lipid having antifungal activity | - | [48] |
| Torulopsis bombicola | Sophorose lipid | Sophorose lipid fermentation | American Type Culture Collection | [49] |
| Aspergillus ustus | Glycolipoprote | Antimicrobial activity | Peninsular coast of India | [50] |
| Candida bombicola ATCC 22214 | Sophorolipid | Used in low-end consumer products and household application | American Type Culture Collection | [51] |
| Ustilago maydis FBD12 | Glycolipids | Antimicrobial activity | American Type Culture Collection | [52] |
| (C). Different Types of Biosurfactants Produced from Yeast Strains | ||||
| Yeast | Biosurfactants | Properties | Isolation source | References |
| Starmerella bombicola | Sophorolipids | Cytotoxic effect on MDA-MB-321 breast cancer cell line | Fungal Biodiversity Centre |
[53] |
|
Torulopsis Petrophilum ATCC 20225 |
Glycolipids | Protein emulsifier | American Type Culture Collection | [54] |
| Kluyveromyces marxianus FII 510700 | Mannanoprotein | Source of emulsifier in the food industry | Culture Collection of the University of New South Wales, UNSW | [55] |
| Pseudozyma aphids, DSM 70725 and DSM 14930 | Mannosylerythritol lipids | Foam formation | Deutsche Stammsammlung von Mikroorganismen und Zellkulturen (DSMZ), Braunschweig, Germany | [56] |
| Pseudozyma tsukubaensis | Glycolipid | Producing diastereomer MEL-B from vegetable oils | Leaves of Perilla frutescens on Ibaraki in Japan | [57] |
| Saccharomyces cerevisiae URM 6670 | Glycolipid | Antioxidant activity and cytotoxic potential | Culture Collection of the Department of Antibiotics of the Federal University of Pernambuco (Brazil) | [58] |
| Trichosporon asahii | Sophorolipid | Efficient degrader of diesel oil, higher hydrophobicity, emulsification activity, and surface tension reduction | Petroleum hydrocarbon-contaminated soil in India | [59] |
| Meyerozyma guilliermondii YK32 | Sophorolipid | Emulsification properties | Soil samples collected from hydrocarbon-polluted locations of Hisar, Haryana | [60] |
| Rhodotorula babjevae YS3 | Sophorolipid | Antimicrobial activity | Agricultural field in Assam, Northeast India | [61] |
| Pichia caribbica | Xylolipid | Reduced the surface the tension of distilled water |
Microbial type culture collection, India | [62] |
| Candida ishiwadae Y12 | Monoacylglycerols: Glycolipid | Exhibited high surfactant activities | Plant material in Thailand | [63] |
3. Factors Affecting Biosurfactant Production
Traditionally, most biosurfactant-producing bacterial strains have been isolated from petroleum/oil-contaminated soil or fermented food, but nowadays, microbial isolates are screened from various sources. The production of biosurfactants started with the growth, identification, and characterization of microbial strains. The growth conditions of the cultures should be maintained according to the sample sites. However, the methodology, substrate, and purification process should be cost-effective for biosurfactant production at a commercial or industrial scale. According to a published report, 10–30% of the total cost accounted for raw materials during biosurfactant production [64], while up to 60% of the total cost has been spent on the downstream or purification processes [64,65]. The media components of the microorganism play an essential role in biosurfactant production and significantly impact the production cost. Carbon (C) and nitrogen (N) sources in the media are an essential requirement for microbial growth [66]. The type, amount, and ratio of carbon and nitrogen in the media directly affect microbial growth and biosurfactant production in both laboratories and large-scale industrial fermenters [67]. In most studies, glucose, sucrose, and glycerol are used as carbon and yeast extract, while NaNO3, urea, and soya broth have been used as a nitrogen source in the media [68,69]. For instance, an abundance of carbon sources and limiting nitrogen conditions are preferred for optimum biosurfactant production. For example, the ratio of C: N ≈ 20 has been found most favorable for Pseudomonas sp. [70]. In a study, Onwosi and Odibo [71] evaluated the role of carbon and nitrogen source, on the rhamnolipid production by the strain Pseudomonas nitroreductase and recovered 5.28 and 4.38 gL−1 of rhamnolipid using glucose as a carbon and sodium nitrate as a nitrogen source respectively.
Furthermore, the highest yield of 5.46 gL−1 was observed when the ratio of C: N (glucose/sodium nitrate) was 22. Thus, the selection of media sources has a significant impact on biosurfactant production. A detailed survey on the utilization of carbon and nitrogen sources and their implications for biosurfactant recovery has been described in Table 3.
Table 3.
The common substrates used in biosurfactant production and their yields.
| Substrate | Conc. (gL−1) | Microorganisms | Yield (gL−1) | References |
|---|---|---|---|---|
| Glucose | 40 | P. aeruginosa | 0.3 | [72] |
| 40 | B. subtilis | 3.6 | [73] | |
| 40 | B. subtilis | 0.72 | [74] | |
| 30 | B. pumilus | 0.72 | [75] | |
| 20 | P. aeruginosa | 3.88 | [76] | |
| 10 | B. subtilis | 0.16 | [77] | |
| - | Pseudomonas sp. | 0.35 | [78] | |
| Sucrose | 20 | P. putida | 1.30 | [79] |
| Glucose and fructose | 16.55 | B. subtilis | 0.93 | [80] |
| Glucose + Yeast extract | 1:3 | Bacillus sp. | 2.56 | [81] |
| Glycerol + yeast extract | 30:5 | P. aeruginosa | 2.7 | [82] |
| Yeast extract | 1 | P. taiwanensis | 1.12 | [83] |
| Yeast extract | 2 | Bacillus sp. | 2.5 | [84] |
| NaNO3 | 0.2 M | P. aeruginosa | 2.73 | [85] |
| NaNO3 | 5 | B. subtilis | 1.12 | [86] |
| Peptone | 4 | Serratia marcescens | 1.2 | [81] |
| NH4NO3 | 1 | P. fluorescens | 2 | [87] |
The production cost of biosurfactants largely depends on the media source, primarily the carbon and nitrogen sources. Therefore, in the recent past, a range of new and novel resources, such as residual waste products of the food industry, e.g., frying oil, distillery, molasses, and vegetable- and plant-derived oil, has been trailed in the media as a carbon and nitrogen source as single or together with the stabilized resource. The utilization of these products can cut or reduce the cost of biosurfactant production [88,89,90]. The use of vegetable oil and hydrocarbon-based substrates appear as economical and profitable substrates for large-scale biosurfactant production, especially from Pseudomonas, Bacillus, and Candida sp. [91].
There are numerous reports available in biosurfactant production using different nutritional sources and limiting environmental factors. For example, Agarwal and Sharma [92] utilized different C sources, such as glycerol, molasses, rice water, cheese whey, potato peels, and glucose, to evaluate their impact on biosurfactant production. They observed similar biosurfactant activity, using molasses and glycerol sources, and biosurfactants were produced using a glucose source. In addition, the utilization of NH4Cl, NH4NO3, and NaNO3 as a nitrogen source yielded good results. Similarly, Al-Bahry et al. [93] recovered 2.29 ± 0.38 gL−1 of biosurfactant, using date molasses as a carbon source from the strain Bacillus subtilis B20, which had the capability to reduce surface tension and interfacial tension from 60 to 25 mN m−1 and 27 to 5.02 mN m−1, respectively. In addition, biosurfactants showed stability against a wide range of temperatures, pH variations, and salt concentrations. Hentati et al. [94] reported 50 mgL−1 of biosurfactant production by the strain Bacillus stratosphericus FLU5 using residual frying oil as a carbon source. At this concentration, the surface tension of the water was reduced from 72 to 28 mN m−1. Similarly, Souza et al. [95] reported biosurfactant production by the strain Wickerhamomyces anomalus CCMA. Under optimized culture conditions, various amounts of biosurfactant has been recovered from the yeast strain using different energy resources, such as yeast extract (4.64 gL−1), ammonium sulfate (4.22 gL−1), glucose (1.39 gL−1), and olive oil (10 gL−1). However, the highest yield of biosurfactant was recovered from the 24-hour-old culture. Additionally, the biosurfactant remained stable even at a higher temperature of 121 °C, NaCl concentrations of 300 gL−1, and pH ranges of 6–12. A brief survey of biosurfactant production using alternative carbon and nitrogen sources and their impact on yield oand properties has been described in Table 4.
Table 4.
The common alternative substrates and their impact on biosurfactant yield.
| Microorganism | Alternative Media Source | Yield and Properties | References |
|---|---|---|---|
| Bacillus subtilis ATCC 6051 | Brewery waste (trub) | The product yield of 100.76 mgL−1 | [96] |
| Bacillus subtilis PC | Sugar cane vinasse | Able to reduce surface tension 32 mN m−1 and the E24 to 51.10%. | [97] |
| Bacillus subtilis | Corn steep liquor | Biosurfactant yields 1.3 gL−1; the different yields increased (up to 4.1, 4.4, and 3.5 g/L for iron, manganese, and magnesium supplements, respectively). However, at the optimum concentration, the yield of these three metals increased up to 4.8 gL−1. | [98] |
| Bacillus subtilis MTCC 2423 | Rice mill polishing residue | Surfactin yield 4.17 g kg−1 residue | [99] |
| Bacillus licheniformis AL1.1 | Molasses | Lichenysin yield of 3·2 gL−1 | [100] |
| Bacillus pseudomycoides | Soybean oil waste | C.M.C. of lipopeptide 56 mgL−1 and able to reduce the surface tension of water from 71.6 mN m−1 to 30.2 mN m−1 | [101] |
| Bacillus subtilis DSM 3256 | Two-phase olive mill waste | Surfactin yields 0.068 g g−1, and the surface tension of the culture medium is reduced to 30.1 ± 0.9 mN m−1. | [102] |
| Bacillus subtilis | Rapeseed cake | Surfactin analogues | [103] |
| Bacillus amyloliquefaciens | Distillers’ grains | Surfactin yield 1.04 gL−1 | [104] |
| Bacillus nealsonii S2MT | Glycerol 2% (v/v) and NH4NO3 0.1% (w/v) | The maximum biosurfactant yield was 1300 mg/L and reduced the surface tension (34.15 ± 0.6 mN/m). Additionally, highly stable at environmental factors such as salinity, pH and temperature variations. |
[105] |
| Staphylococcus sp. | Residual frying oil, expired milk | The C.M.C. of the purified lipopeptides was 65–750 mg/L, depending upon carbon source. Additionally, it was stable within a broad range of pH, temperature, and salinity values. | [106] |
| Halomonas venusta PHKT | Glycerol | Surfactin, Pumilacidin, and Bios-PHKT have a critical micelle concentration (C.M.C.) of 125 mgL−1 and showed a high steadiness against a broad spectrum of salinity (0–120 gL−1 NaCl), temperature (4–121 °C), and pH values (2–12). | [107] |
| Rhodotorula sp. | Olive oil mills | Potent biosurfactant producer with E24 = 69% and a significant reduction in S.T. from 72 to 35 mN m−1. In addition, it showed stability over a wide range of pH (2–12), temperature (4–100 °C), and salinity values (1–10%). | [108] |
| Volvariella volvacea | Edible paddy straw mushroom | Biosurfactant effectively showed a reduction in the surface tension, emulsification index, and oil spreading activity of 35.15 dyne/cm, 80%, and 11 cm, respectively. |
[109] |
Besides nutrient sources, the production of biosurfactants depends on several factors, such as incubation time, incubation temperature, pH of growth culture, and the speed of rotation rate of shaking incubator, which directly affect the microbial growth and biosurfactant production. In one study, Achim et al. [110] evaluated the biosurfactant production potential of Azotobacter chrococcum under controlled nutritional and environmental conditions. The highest 68% of surface tension and emulsification index (EC24) was observed at pH 7. Sunflower oil and heavy oil 150 had shown the best response among different carbon sources and accounted for 76.6% and 74.1% of E.C. 24, respectively. However, higher EC24 was recorded after supplementing yeast extract (83.3%) and (NH4)2SO4 (80%) among different nitrogen sources. The optimum recovery of biosurfactant was achieved from 4 days old culture incubated at 30 °C, in a shaking incubator at 150 rpm.
Similarly, Joaad and Hassan [111] evaluated the biosurfactant potential of yeast strain Candida guilliermondii using the VITTEK2 compact system under controlled environmental and nutritional conditions. The maximum EC24 was 70% observed at pH 4 and 75% at 30 °C. However, sesame oil and heavy oil 150 were shown to give the best response when used as a carbon, along with addition of NaNO3 as nitrogen source. Additionally, the shaking incubator at 150 pm resulted in higher emulsifier production on the 7th day of culture growth.
4. Biosurfactant Applications in Improving the Soil Quality
The growth and productivity of the crop ecosystem rely on the availability and presence of an optimum concentration of micro- or macronutrients in the soil. Trace elements present in the soil directly influence the physiological processes of the plant. Indeed, deficiency or excess availability of these elements led to various diseases and poor quality of plant growth. The ongoing changing climatic condition, rising global temperature, variation in soil pH, increase in salinity, or deposition of environmental contaminants adversely affect the efficacy of trace elements in the soil, resulting in poor availability to the plants, which ultimately results in lower crop production and poor food quality [112].
The addition of biosurfactants in the soil significantly enhances the availability of micronutrients in the mineral deficient soil through various processes. The addition of surfactant makes a complex with the metal ion, which, through biochemical processes such as oxidation reduction, adsorption, and deadsorption, increases their bioavailability or concentration in the soil [113]. In detail, an anionic charge of surfactant binds with the cationic charge of the metal and forms a complex; through this way, it acts as a sequestering agent and performs desorption of the soil [114]. However, in contaminated water, the flushing of water through soil can remove metal surfactant complex from the soil because of the strong electrostatic interaction between the opposite charge ion of the metal and surfactant, resulting in metal mobilization in the water [115,116]. The application of biosurfactants can also help mitigate the challenge of soil alkalinity, which is considered one of the paramount factors of micronutrient deficiency in the soil. The addition of biosurfactants makes the metal–biosurfactant complex available by removing or unbinding the metal from the soil complex [117]. During this interaction, the bond strength of the metal–biosurfactant interaction is much higher than the metal–soil interaction, which further desorbed the metal–biosurfactant complex from the soil matrix to the soil solution, due to a lowering of the interfacial tension, resulting in the availability of trace elements to the plant roots [115,118]. The addition of surfactant reduces the interfacial tension between the metal and soil, forms micelles, and transfers them to the root zone interface.
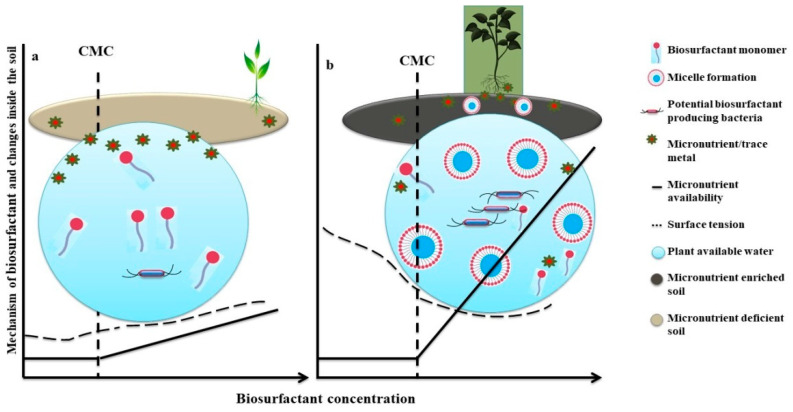
The use of biosurfactants in the agricultural field to improve or enhance the availability of micronutrients to the soil is the new approach and is, nowadays, broadly practiced in different parts of the world [115]. The amphipathic nature of biosurfactants can reduce the interfacial tension between two immiscible liquids and enhance the solubility of organic and inorganic components [119,120]. In the agricultural process, different biosurfactants are reported to decrease the interfacial surface tension between the solid surfaces and the trace metal cations, resulting in increased solubility and mobility of trace elements [121] (Figure 3).
Figure 3.
Impact of micronutrient deficiency on plant growth and soil quality on a micronutrient-deficient soil. (a) Mechanisms of biosurfactant application enhancing micronutrient availability in micronutrient-deficient soil to the plant, soil quality, and related water quality through increasing nutrient solubility at a fixed concentration of biosurfactant molecule. (b) C.M.C. at which there is a sudden increase in metal solubility in the system. Figures are adapted and modified from Mulligan [13] and Singh et al. [114].
For instance, Sheng et al. [122] reported that the strain Bacillus sp. J119 has biosurfactant capability, which significantly enhances the uptake of trace elements and promotes the growth potential of canola maize, sudangrass, and tomato. Furthermore, Stacey et al. [123] reported the formation and plant uptake of lipophilic metal-rhamnolipid complexes that facilitate the Cu, Mn, and Zn uptake and movement in Brassica napus and Triticum durum roots.
In addition, the application of biosurfactants, as they have a microbial origin, significantly modulates plant growth via synthesizing phytohormones and inducing resistance. Therefore, the efficiency and availability of micronutrients in the soil to the plants might be increased, either due to bioaugmentation of biosurfactant-producing bacteria [124]. The application of biosurfactants also influences the native microflora of the plants or soil, directly or indirectly responsible for growth promotion, mitigating biotic and abiotic stresses, and removing contaminants from the soil or plant roots. In one study, Liao et al. [125] used the pot experiment with maize to examine the effect of biosurfactant (rhamnolipid and lecithin) and chemical surfactant (Tween 80). After application in the crude oil-contaminated soil, it did not significantly affect the maize biomass, while rhamnolipid and lecithin application enhanced the microbial population, resulting in increased petroleum hydrocarbons from the contaminated soil. March-Mikołajczyk et al. [126] reported on the Glycolipid produced by endophytic bacterial strain Bacillus pumilus 2A, which after application significantly improves the growth of bean, radish, and beetroot. Chopra et al. [127] evaluated different rhizobacterial strains of tea, in which one of the strains, Pseudomonas aeruginosa RTE4, produced di-rhamnolipid biosurfactant and showed multiple growth-promoting traits as well as fungicidal activity. Similarly, Alsohim et al. [128] reported that the viscosin produced by Pseudomonas fluorescens S.B.W. 25 helps with spreading the motility, which facilitates the colonization efficacy of microbial strain and showed growth promotion potential.
5. Biosurfactant Application in Plant Disease Management
Plant disease causes a significant reduction in agricultural commodities during pre-or post-harvest conditions and is considered a severe threat to food security for the rising global population. It has been estimated that approximately 30% of the total agricultural production is destroyed due to various plant diseases and pathogen attacks, either during pre- or post-harvest storage conditions [129]. However, to manage phytopathogen and plant diseases, farmers most often rely on chemical pesticides. Nevertheless, the undistributed and continuous use of chemical pesticides during crop production led to various adverse consequences, such as poor food quality, soil and water pollution, pest resistance, effects on natural microbiota, and severe health issues to consumers. Moreover, various microbial biocontrol agents, including bacteria, fungi, and yeasts, have been frequently utilized to manage plant diseases. They showed an effective response against phytopathogen growth, fruit quality maintenance, or storage life enhancement.
Agrochemicals are preferred more frequently than other crop protection or plant disease management resources because of their easy availability and quick response. Nevertheless, traditional formulation and low dispersion capacity on the target site, either the plant surface or the pathogen, led to lower efficacy and environmental pollution. According to the report, it has been estimated that only about 0.1% of the total applied pesticides reach the target organisms, and the remaining bulk contaminates the surrounding [130,131].
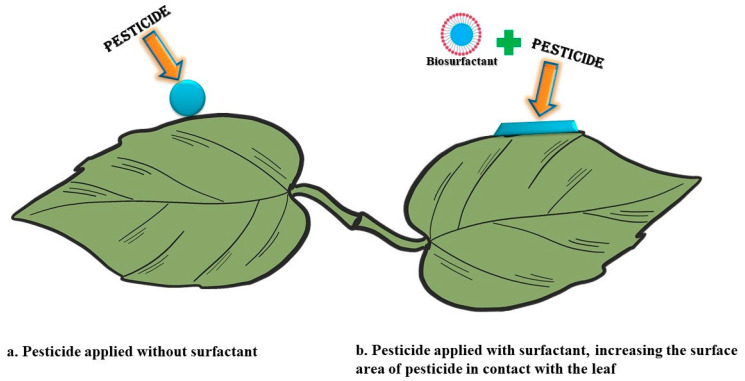
In common practice, a pesticide is either sprayed directly on the plant and surfaces or the plant is sometimes dipped into the pesticide solutions. Still, drift does not reach the target site and shows poor efficacy against disease management [132]. However, nowadays, to improve the effectiveness of pesticides applications, the delivery mechanism has been upgraded via adding surfactants, nano-based formulations, and improved spraying technology [133,134]. In general, during pesticide application, surfactants have been used as an additive or adjuvants and mixed with pesticides that help in dispersion, emulsification, better spreading, or increasing the contact area with the plant surface, which enables the pesticides to better reach the target pests or target organisms [135] (Figure 4).
Figure 4.
Depiction of the effect of surfactant on leaf surfaces. (a) Pesticide applied without surfactant; (b) pesticide applied with a surfactant, increasing the surface area of the pesticide in contact with the leaf. Figure adapted from Jibrin et al. [138].
However, after mixing and applying biosurfactants with pesticides, care should be taken, because surfactant application may harm the non-target phytobiome and plant physiological process [136]. Moreover, the enhanced permeability of pesticides may lead to increased residue levels in plant tissue and fruits [137]. Therefore, selection and the concentration of surfactants are prime factors that need to be considered for better disease management strategies.
Currently, a range of chemically synthesized surfactants, such as Triton X-100, Cohere, Agral 90, Silwet L-77, and Tween 20, are some of the most common synthetic surfactants used for plant disease management, and they have displayed improved insecticidal potential during in vitro and in vivo applications [138,139]. However, due to its chemical and toxic nature, direct application on the plant surface was avoided. Unlike synthetic surfactants, which are usually used as adjuvants, most biosurfactants have been directly applied on the plant surface for disease management [140,141], and nowadays, continuously new biosurfactant-producing microorganisms have been screened and explored for their optimum recovery and applied as antagonistic agents against a range of pest and plant pathogens. The utilization of microbial antagonistic bacteria, fungi, and yeast strains to manage plant disease or growth of phytopathogen during pre- or post-harvest management has been well elucidated [142,143]. Indeed, the addition of biosurfactants modulates the action mechanism, such as antibiosis, induced systemic resistance, competition, and parasitism of biocontrol agents [139].
Pseudomonas and Bacillus are the most common bacterial genera used for biosurfactant production. There are numerous reports available that showed their potency in biosurfactant production and their implication in successful phytopathogen management [138]. Varnier et al. [144] reported on Rhamnolipid, produced by the Pseudomonas aeruginosa, which enhanced the immune response of grapevine against the Botrytis cinerea after application. In addition, the application of surfactants inhibits the spore germination and mycelium growth of the pathogen. Kruijt et al. [145] reported the surfactants produced by Pseudomonas putida, which, after application, impede the growth of pathogen Phytophthora capsici in cucumber through zoospores lysis. Nielsen and Sorensen [146] reported that the surfactant cyclic lipopeptides produced by Pseudomonas fluoresecens have antifungal properties. Pernell et al. [147] evaluated the combined application of phenazines and rhamnolipid surfactant produced by Pseudomonas aeruginosa PNA1 strain. The application of both the metabolites showed a synergistic effect against the pathogen Pythium splendens of bean and Pythium myriotylum of cocoyam. In addition, substantial vacuolization and disintegration of Pythium hyphae were observed during microscopic analysis. Similarly, D’aes et al. [148] reported that phenazine and cyclic lipopeptide produced strain Pseudomonas CMR12a, which showed effective biocontrol potential against Rhizoctonia root rot on bean. Velho et al. [149] reported on the lipopeptide surfactant produced by Bacillus, having strong antagonistic activity against the pathogens Aspergillus sp., Fusarium sp., and Biopolaris sorokiniana.
Similarly, in another study, Akladious et al. [150] evaluated the biosurfactant produced by strain Bacillus licheniformis, which after application significantly controls the pathogen Rhizoctonia solani, the causal agent of root rot in faba beans. Hussain et al. [151] investigated biosurfactants produced by Bacillus subtilis, having bio-nematicidal activities against the pathogen Meloidogyne incognita, which is the causal agent of Root gall. Shalini et al. [152] investigated a glycolipid surfactant produced by the Acinetobacter sp., which showed antagonistic activity against Xanthomonas oryzae P.V. Oryzae XAV24. Haddad et al. [153] investigated surfactin biosurfactants produced by Brevibacillus brevis, having antibacterial and antifungal properties. Similarly, the endophytic strain Burkholderia sp. produced Glycolipid. The biosurfactant showed broad-spectrum antibacterial activity against the pathogens Pseudomonas aeruginosa, E. coli, and Salmonella paratyphi [154]. A detailed summary of the biosurfactants used in plant disease management has been described in Table 5.
Table 5.
The common biosurfactants used in plant disease management.
| Microorganism | Biosurfactant | Properties | Reference |
|---|---|---|---|
| Pseudomonas sp. EP-3 | Rhamnolipid | Insecticidal activity | [155] |
| Pseudomonasaeruginosa PAO1 | Rhamnolipid | Biofilm formation | [156] |
| Pseudomonas aeruginosa | Rhamnolipids | Control of Phytophthora cryptogea | [157] |
| Pseudomonas aeruginosa | Rhamnolipids | Resistance to Botrytis cinerea in grapevine | [144] |
| Pseudomonas putida | Biosurfactants | Zoospores of the oomycete pathogen Phytophthora capsici | [145] |
| Pseudomonas koreensis | Biosurfactant | Late blight on potato | [158] |
| Acinetobacter sp. ACMS25 | Glycolipid | Biocontrol of Xanthomonas oryzae | [152] |
| Burkholderia sp. WYAT7 | Glycolipid | Antibacterial and ant- biofilm potentials | [154] |
| Bacillus licheniformis | Biosurfactant | Biocontrol of Rhizoctonia solani causing root rot in faba bean | [150] |
| Pseudomonas CMR12a | Lipopeptides | Biological control of Rhizoctonia root rot on bean | [148] |
| Brevibacillus brevis | Lipopeptides | Antibacterial and Antifungal properties | [153] |
| Bacillus sp. | Lipopeptides | Growth inhibition of Fusarium spp., Aspergillus spp., and Biopolaris sorokiniana | [149] |
| Bacillus subtilis R14 | Lipopeptide | Antimicrobial activity | [159] |
| Bacillus subtilis | Lipopeptides Iturin A, fengycin, and surfactin | Colletotrichum gloeosporioides, the causative agent for anthracnose on papaya leaves | [160] |
The application of surfactants acts differently during pathogen management. For example, Edosa et al. [161] investigated the action mechanism of some biosurfactants for insect pest management. The biosurfactant acts on the cell wall of the pests and causes significant damage due to dehydration. In a study, Yun et al. [162] investigated surfactin produced by Bacillus amyloliquefaciens, which affects the aphid cuticle after application, resulting in dehydration from the cuticle membrane, leading to dehydration and death. Similarly, Khedher et al. [163] observed vacuolization, necrosis, and basement membrane disintegration in the larval midgut of Spodoptera littoralis after histopathological examination of biosurfactant treatment. These reported biosurfactants and their application in plant disease management showed an excellent alternative to chemical pesticides, which are currently utilized in different parts of the world. However, the additional benefit of using microbial surfactant is the enhancement in plant growth and the providing nutrient source and favorable conditions for the native microflora that are essential for the plants to mitigate them from various biotic and abiotic stresses and for the degradation of toxic and hazardous environmental contaminants.
6. Biosurfactant Application in Pharmaceutical Industries
6.1. Antioxidant Properties of Biosurfactants
Nowadays, microbial surfactants have been used in the food and pharmaceutical industries as antioxidant agents. The antioxidants are the compounds need to neutralize the free radicals generated in the body during various physiological processes. The highly reactive nature of free radicals led to severe damage, known as oxidative stress or oxidative damage [164].The microbial origin source of biosurfactants can alter the physicochemical properties of surfaces. Thus, they can obviate the binding of other bacterial adhesions on the surface [165].
Similarly, they can also block the oxidative chain reaction flow by rendering the antioxidant activities [165]. Considering the biosurfactant characteristics, such as low toxicity and biodegradable, antimicrobial, and antioxidant properties, they gained significant industrial attention and are now preferred over the usage of synthetic antioxidants [166]. To overcome the toxic effects of synthetic surfactants and subside their side effects upon consumption, it is a prerequisite for finding natural and non-toxic bio-based products with potential antioxidant products [167]. Natural biosurfactants are one such natural product that is also reportedly capable of blocking the oxidative chain reaction flow by rendering the antioxidant activities. Hence, they also can effectively impede the elevation of reactive oxygen species (ROS) and reactive nitrogen species (RNS); hence, they could be highly useful for therapeutic purposes against cancer and the cure of heart-related diseases and neurodegenerative diseases [168]. Likewise, they were also highly instrumental in manufacturing probiotics, bio preservatives, and food ingredients [169].
Recently, several research groups explored various biosurfactants bestowed with excellent potential antioxidant properties from diverse sources. In addition to their potential antioxidant activity, some of the biosurfactants also displayed antimicrobial and antiproliferative activities [170,171,172]. In line with these findings, biosurfactant MB15, isolated from the non-pathogenic marine Marinobacter litoralis bacteria [171], was found to have no cytotoxic effect, but had a potent antioxidant and antimicrobial activity. Another report by Giri et al. assessed the antioxidant, antibiotic, and antiadhesive properties of the biosurfactant compounds isolated from Bacillus subtilis VSG4 and Bacillus licheniformis VS16. Their study revealed that the Bacillus subtilis VSG4 displayed better antioxidant activity than Bacillus licheniformis VS16 [173]. Meghna et al. also characterized a biosurfactant BS-LBL from Lactobacillus casei, and their experiment enlightened the efficient antioxidant, antimicrobial, and antiproliferative properties upon testing [172]. Likewise, Ohadi et al. [174] examined a biosurfactant obtained from Acinetobacter junii. They confirmed that the lipopeptide biosurfactant (LBS) from A. junii bestowed high antioxidant capacity with excellent wound healing ability in the mouse. Similar findings were also reported by other studies [170,175,176]. Collectively, the utilization and application of biosurfactants with antioxidant, antimicrobial, and antiproliferative substances will be a great addition to the products to safeguard consumer health benefits.
A few more reports have also consolidated the potent antioxidant and antimicrobial activities of biosurfactants lately. For example, Mouafao et al. identified and characterized a biosurfactant from Lactobacillus casei subsp. casei TM1B, which also confers efficient antioxidant and broad-spectrum antimicrobial activities convoyed with good emulsification and surface activities [177]. The biosurfactant MB588 from Halobacillus karajiensis showed a comparable antioxidant capacity as a positive control among all the isolates. It also showed higher antimicrobial activity; together, this suggests that the bacteria from extreme halophilic soils can also be helpful for the isolation of novel biosurfactants [178].
Similarly, another study by Abdollahi et al. compared two biosurfactants derived from two autochthonous strains for their antioxidant ability. Their study revealed that Bacillus amyloliquefaciens NS6-derived surfactin-natured biosurfactant displayed a more robust antioxidant capacity than Pseudomonas aeruginosa MN1-derived rhamnolipid-structured biosurfactant. However, they found that the rhamnolipid treated surfaces displayed higher antiadhesive and antibiofilm activities than surfactin-treated surfaces [165]. More examples of biosurfactants that possess antioxidant and antimicrobial activities [178,179] are listed in Table 6.
Table 6.
Antioxidant properties of biosurfactants.
| Source | Chemical Nature of Biosurfactant | Antioxidant Activity Assessment | Antioxidant | Antibacterial | Antiproliferative | Reference |
|---|---|---|---|---|---|---|
| Lactobacillus casei subsp. casei TM1B | Rhamnolipid-like biosurfactant | DPPH (1-diphenyl-2-picrylhydrazyl) assay, ABTS (2.2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) assay | yes | yes | not tested | [177] |
| Pseudomonas aeruginosa MN1 | Rhamnolipid | FRAP and DPPH assay | yes | yes | not tested | [165] |
| Bacillus amyloliquefaciens NS6 | Surfactin | Ferric reducing antioxidant power (FRAP) and DPPH assay | yes | yes | not tested | [165] |
| Marinobacter litoralis MB15 | Rhamnolipid | DPPH assay | yes | yes | yes | [171] |
| Halomonas elongata, Halobacillus karajiensis and Alkalibacillus almallahensis | Glycolipid | DPPH assay | yes | yes | not tested | [178] |
| Bacillus subtilis VSG4 | Lipopeptide | DPPH assay | yes | yes | not tested | [173] |
| Bacillus licheniformis VS16 | Phospholipopeptide | DPPH assay | yes | yes | not tested | [173] |
| Lactobacillus casei (BS-LBl) | Not mentioned | DPPH assay | yes | yes | yes | [172] |
| Acinetobacter junii B6 | Lipopeptide | DPPH and FRAP assay | yes | yes | not tested | [174] |
| Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315 | Exo polysaccharides | DPPH assay and superoxide and hydroxy radical estimation | yes | yes | not tested | [180] |
| Bacillus methylotrophicus DCS1 | Lipopeptide | DPPH assay | yes | yes | not tested | [179] |
| Pseudozyma hubeiensis | Mannosylerythritol lipids | DPPH assay | yes | not tested | yes | [175] |
| Bacillus subtilis SPB1 | Lipopeptide | DPPH assay | yes | not tested | yes | [176] |
| Bacillus cereus MMIC | Lipopeptide | DPPH assay | yes | yes | yes | [170] |
Few studies have explored the efficacy of natural biosurfactants that originated from a cost-effective substrate and compared their total antioxidant capacity (TAC) with a synthetic surfactant. Amaro da Silva et al. assed the TAC of a biosurfactant isolated from a low-cost substrate by Candida bombicola URM 3718 and compared it with a commonly used synthetic surfactant called Guar gum for its emulsification and total antioxidant capacity. Their study revealed that the biosurfactant predominantly displayed better antioxidant and emulsification ability than guar gum [166,174]. Other studies with different objectives also approved the need for biosurfactants to overcome the shortcomings of synthetic surfactants [180,181,182]. In summary, it is important to consider the numerous promising attributes of biosurfactants and their strong potential to elicit antioxidant activity on the detrimental reactive oxygen species and free oxygen radicals (H2O2, O2_, OH* and 1O2) from diverse sources. Because of natural origin, at the certain extent, biosurfactants also conferred antimicrobial activity and offer attractive opportunities to replace synthetic surfactants in the pharmaceutical, probiotic, and cosmetic industries. Thus, there is an urgent need for comprehensive characterization of each type of biosurfactant to be established to harness the best benefits and efficient application process.
6.2. Antimicrobial Properties of Biosurfactants
Multidrug resistance (MDR) is an emerging challenge for the growing world, especially in developing countries. However, in the recent past, antibiotic resistance has opened the door to search for alternative antimicrobial medicine to treat human ailments [183]. In this context, bacteriostatic and bactericidal, and biofilm disruption potential of biosurfcatants, make them ideal as an antimicrobial agent [184]. Numerous reports are available that showed the effectiveness of biosurfactants against different pathogens. For example, Foschi and others [185] reported antimicrobial effects against Neisseria gonorrhoeae. Similarly, Morais and others [186] observed against Candida albicans, Dusane and others [187] reported biofilm degradative behavior of rhamnolipid surfactant against Bacillus pumilus.
However, biosurfactants produced by microbial strain act differentially during pathogen inhibition. For instance, Rhamnolipids possess activity through a permeabilizing effect, which leads to the disruption of the bacterial cell plasma membrane. The amphipathic nature of rhamnolipids binds with the charges of the bacterial cell membrane and changes their hydrophobicity. This prevents biofilm formation and makes the pathogen highly susceptible to the antimicrobial agent [188]. Several studies have suggested that rhamnolipids may act more effectively against Gram-positive bacteria than Gram-negative bacteria due to the absence of an outer membrane. The presence of the outer layer may exclude biosurfactant molecules [189]. However, the lipopolysaccharides biosurfactants attribute antimicrobial property via penetrating or damaging the lipid. The charge imbalance led to pore formation in the cell membrane lipids, which ultimately caused damage to or death of the pathogens, especially of Gram-negative bacteria [190].
In recent years, biosurfactants, such as lipopolysaccharides and glycolipids produced by microbial strains, have been used directly or indirectly as anticancer agents. Biosurfactants’ structural diversity and physio-chemical nature showed a broad-spectrum application during chemotherapy or drug delivery formulations. Currently, various reports show the effectiveness of glycolipids and lipopolysaccharides in controlling the proliferation of cancer cells and disrupting cell membranes through apoptosis pathways [191]. Zhao and others [192] reported the antitumor activity of lipopolysaccharides, composed of peptides and fatty acid chains. Dey and others [193] reported that Iturin synthesized by Bacillus strains inhibits the proliferation of MDA-MB-231 cancer cells.
7. Future Directions and Concluding Remarks
Biosurfactants are considered to be the multifunctional biomolecules of the 21st century, due to their broad application, ranging from daily life to industrial purposes. Currently, numerous microbial strains have been identified and screened for biosurfactant ability, and each day, some novel biosurfactant molecules have been identified and recovered. However, the biosurfactant’s fragile nature, lower stability, and high production cost appear as a critical barrier for frequent use in the industries. In the recent past, to reduce the production cost, various alternative sources of carbon or nitrogen, which are the essential requirements for microbial growth, have been utilized, and up to a certain extent, researchers have been successful. Nevertheless, the lower yield of biosurfactants, using alternative sources, is still a limiting factor. Therefore, there is a need for extensive study of certain factors, including biosynthesis pattern, growth, environmental conditions, and media composition for the large-scale production of biosurfactant molecules for the industrial uses and economic standpoint.
Currently, rapid industrialization and anthropogenic behavior is leading to the deposition of toxic and hazardous contaminants in the soil, affecting environmental conditions and limiting agricultural production. Nowadays, biosurfactants have been broadly utilized to degrade the toxic, hazardous, and hydrophobic environmental contaminants and to improve the soil quality by maintaining the concentration of trace elements. The most commonly used surfactants are of chemical origin, and their uses in the agricultural fields can lead to food toxicity and can also adversely affect the natural microflora. However, the surfactants with a microbial origin have no such impact, and can even accelerate the growth of plants and microflora, which are required to degrade environmental contaminants. The selection of biosurfactants according to soil contaminants can enhance the soil quality better and in less time. For the sustainable growth of the rising global population, management of pre- and post-harvest losses of agricultural products is an immediate need. Currently, surfactants are mainly used as adjuvants or sometimes directly to the plant surface for phytopathogen management. However, there is still a need to explore the director adjuvants’ use of biosurfactants and their impact on the natural phytomicrobiome, residual level in fruits, and their impact on the physiological aspect of plants.
Moreover, there is also a need for the extensive investigation of biosurfactant molecules to explore novel antimicrobial agents, antioxidant molecules, and antiproliferative agents. This is not only cost-effective, but also protects the body from its toxic side effects. However for the treatment chronic diseases, cancer therapy and drug delivery using biosurfactants needs an extensive research. In addition, after the advancements in technology and resource materials, the high production cost, and the low yield of biosurfactants are still challenging tasks that need to be overcome.
Acknowledgments
The authors are thankful to the Department of Life Science, Dongguk University and Agriculture Research Organization, Volcanic entre, Israel, for providing lab facilities.
Author Contributions
A.K. and M.K. designed study; M.K., A.K. and S.K.S. wrote the manuscript; M.K. acquired funding; M.K., A.K. and S.D. supervised the study; C.K., H.V., D.K., P.P.S., A.M., M.S.K., H.A., S.K.B., G.D.S., R.G.S. and S.-M.C. provided valuable feedback to this study. All authors have read and agreed to the published version of the manuscript.
Funding
M.K. would like to thank Dongguk University 2020 for funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popp J., Pető K., Nagy J. Pesticide productivity and food security: A review. Agron. Sustain. Dev. 2013;33:243–255. doi: 10.1007/s13593-012-0105-x. [DOI] [Google Scholar]
- 2.Muthusamy K., Gopalakrishnan S., Ravi T.K., Sivachidambaram P. Biosurfactants: Properties, commercial production and application. Curr. Sci. 2008;94:736–747. [Google Scholar]
- 3.Singh S.K., Singh M.K., Verma H., Singh P.P., Singh A.V., Rashmi K., Kumar A. Microbe Mediated Remediation of Environmental Contaminants. Woodhead Publishing; Sawston, UK: 2021. Biosurfactant producing microbes for clean-up of soil contaminants; pp. 89–93. [Google Scholar]
- 4.Rahman P.K., Gakpe E. Production, characterisation and applications of biosurfactants-Review. Biotechnology. 2008;7:360–370. doi: 10.3923/biotech.2008.360.370. [DOI] [Google Scholar]
- 5.Varjani S.J., Upasani V.N. Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant. Bioresour. Technol. 2017;232:389–397. doi: 10.1016/j.biortech.2017.02.047. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya S., Ganguli A.K. Microemulsion Methods for Synthesis of Nanostructured Materials. Elsevier; Amsterdam, The Netherlands: 2019. [Google Scholar]
- 7.Sáenz-Marta C.I., de Lourdes Ballinas-Casarrubias M., Rivera-Chavira B.E., Nevárez-Moorillón G.V. Advances in Bioremediation of Wastewater and Polluted Soil. Volume 5 InTechopen; London, UK: 2015. Biosurfactants as useful tools in bioremediation. [Google Scholar]
- 8.Mohanty S.S., Koul Y., Varjani S., Pandey A., Ngo H.H., Chang J.S., Wong J.W., Bui X.T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Fact. 2021;20:1–13. doi: 10.1186/s12934-021-01613-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuyukina M.S., Ivshina I.B., Makarov S.O., Litvinenko L.V., Cunningham C.J., Philp J.C. Effect of biosurfactants on crude oil desorption and mobilization in a soil system. Environ. Int. 2005;31:155–161. doi: 10.1016/j.envint.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Shekhar S., Sundaramanickam A., Balasubramanian T. Biosurfactant producing microbes and their potential applications: A review. Crit. Rev. Environ. Sci. Technol. 2015;45:1522–1554. doi: 10.1080/10643389.2014.955631. [DOI] [Google Scholar]
- 11.Bhatt P., Verma A., Gangola S., Bhandari G., Chen S. Microbial glycoconjugates in organic pollutant bioremediation: Recent advances and applications. Microb. Cell Fact. 2021;20:1–18. doi: 10.1186/s12934-021-01556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mukherjee A.K., Das K. Microbial Surfactants and Their Potential Applications: An Overview. In: Sen R., editor. Biosurfactants. Volume 672. Springer; New York, NY, USA: 2010. [DOI] [PubMed] [Google Scholar]
- 13.Mulligan C.N. Environmental applications for biosurfactants. Environ. Pollut. 2005;133:183–198. doi: 10.1016/j.envpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Vatsa P., Sanchez L., Clement C., Baillieul F., Dorey S. Rhamnolipid biosurfactants as new players in animal and plant defense against microbes. Int. J. Mol. Sci. 2010;11:5095–5108. doi: 10.3390/ijms11125095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tahir Z., Nazir M.S., Aslam A.A., Bano S., Ali Z., Akhtar M.N., Azam K., Abdullah M.A. Green Sustainable Process for Chemical and Environmental Engineering and Science. Elsevier; Amsterdam, The Netherlands: 2021. Active metabolites and biosurfactants for utilization in environmental remediation and eco-restoration of polluted soils; pp. 31–51. [Google Scholar]
- 16.Prasad N.K., Jayakumar R., Wagdevi P., Ramesh S. Green Sustainable Process for Chemical and Environmental Engineering and Science. Elsevier; Alpharetta, GA, USA: 2021. Application of biosurfactants as bioabsorption agents of heavily contaminated soil and water; pp. 21–30. [Google Scholar]
- 17.Santos D.K.F., Rufino R.D., Luna J.M., Santos V.A., Sarubbo L.A. Biosurfactants: Multifunctional biomolecules of the 21st century. Int. J. Mol. Sci. 2016;17:401. doi: 10.3390/ijms17030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patowary K., Patowary R., Kalita M.C., Deka S. Characterization of biosurfactant produced during degradation of hydrocarbons using crude oil as sole source of carbon. Front. Microbiol. 2017;8:279. doi: 10.3389/fmicb.2017.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saharan B.S., Sahu R.K., Sharma D. A review on biosurfactants: Fermentation, current developments and perspectives. Genet. Eng. Biotechnol. J. 2011;1:1–14. [Google Scholar]
- 20.Chen C.Y., Baker S.C., Darton R.C. The application of a high throughput analysis method for the screening of potential biosurfactants from natural sources. J. Microbiol. Methods. 2007;70:503–510. doi: 10.1016/j.mimet.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Bushnell L.D., Haas H.F. The utilization of certain hydrocarbons by microorganisms. J. Bacteriol. 1941;41:653–673. doi: 10.1128/jb.41.5.653-673.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ilori M.O., Adebusoye S.A., Ojo A.C. Isolation and characterization of hydrocarbon-degrading and biosurfactant-producing yeast strains obtained from a polluted lagoon water. World J. Microbiol. Biotechnol. 2008;24:2539–2545. doi: 10.1007/s11274-008-9778-3. [DOI] [Google Scholar]
- 23.Balan S.S., Kumar C.G., Jayalakshmi S. Pontifactin, a new lipopeptide biosurfactant produced by a marine Pontibacter korlensis strain SBK-47: Purification, characterization and its biological evaluation. Process Biochem. 2016;51:2198–2207. doi: 10.1016/j.procbio.2016.09.009. [DOI] [Google Scholar]
- 24.Coronel-León J., de Grau G., Grau-Campistany A., Farfan M., Rabanal F., Manresa A., Marqués A.M. Biosurfactant production by AL 1.1, a Bacillus licheniformis strain isolated from Antarctica: Production, chemical characterization and properties. Ann. Microbiol. 2015;65:2065–2078. doi: 10.1007/s13213-015-1045-x. [DOI] [Google Scholar]
- 25.Xu M., Fu X., Gao Y., Duan L., Xu C., Sun W., Li Y., Meng X., Xiao X. Characterization of a biosurfactant-producing bacteria isolated from Marine environment: Surface activity, chemical characterization and biodegradation. J. Environ. Chem. Eng. 2020;8:104277. doi: 10.1016/j.jece.2020.104277. [DOI] [Google Scholar]
- 26.Silva S.N., Farias C.B., Rufino R.D., Luna J.M., Sarubbo L.A. Glycerol as substrate for the production of biosurfactant by Pseudomonas aeruginosa UCP0992. Colloids Surf. B Biointerfaces. 2010;1:174–183. doi: 10.1016/j.colsurfb.2010.03.050. [DOI] [PubMed] [Google Scholar]
- 27.Santa Anna L.M., Sebastian G.V., Menezes E.P., Alves T.L.M., Santos A.S., Pereira N., Jr., Freire D.M.G. Production of biosurfactants from Pseudomonas aeruginosa PA 1 isolated in oil environments. Braz. J. Chem. Eng. 2002;19:159–166. doi: 10.1590/S0104-66322002000200011. [DOI] [Google Scholar]
- 28.Jadhav M., Kalme S., Tamboli D., Govindwar S. Rhamnolipid from Pseudomonas desmolyticum NCIM-2112 and its role in the degradation of Brown 3REL. J. Basic Microbiol. 2011;51:385–396. doi: 10.1002/jobm.201000364. [DOI] [PubMed] [Google Scholar]
- 29.Wei Y.H., Lai H.C., Chen S.Y., Yeh M.S., Chang J.S. Biosurfactant production by Serratia marcescens SS-1 and its isogenic strain SMΔR defective in SpnR, a quorum-sensing LuxR family protein. Biotechnol. Lett. 2004;26:799–802. doi: 10.1023/B:BILE.0000025881.95596.23. [DOI] [PubMed] [Google Scholar]
- 30.Revathi K., Chandrasekaran R., Thanigaivel A., Kirubakaran S.A., Sathish-Narayanan S., Senthil-Nathan S. Effects of Bacillus subtilis metabolites on larval Aedes aegypti L. Pestic. Biochem. Physiol. 2013;107:369–376. doi: 10.1016/j.pestbp.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 31.Naruse N., Tenmyo O., Kobaru S., Kamei H., Miyaki T., Konishi M., Oki T. Pumilacidin, a complex of new antiviral antibiotics: Production, isolation, chemical properties, structure and biological activity. J. Antibiot. 1990;43:267–280. doi: 10.7164/antibiotics.43.267. [DOI] [PubMed] [Google Scholar]
- 32.Sun S., Wang Y., Zang T., Wei J., Wu H., Wei C., Qiu G., Li F. A biosurfactant-producing Pseudomonas aeruginosa S5 isolated from coking wastewater and its application for bioremediation of polycyclic aromatic hydrocarbons. Bioresour. Technol. 2019;281:421–428. doi: 10.1016/j.biortech.2019.02.087. [DOI] [PubMed] [Google Scholar]
- 33.Jang J.Y., Yang S.Y., Kim Y.C., Lee C.W., Park M.S., Kim J.C., Kim I.S. Identification of orfamide A as an insecticidal metabolite produced by Pseudomonas protegens F6. J. Agric. Food Chem. 2013;61:6786–6791. doi: 10.1021/jf401218w. [DOI] [PubMed] [Google Scholar]
- 34.Goswami D., Handique P.J., Deka S. Rhamnolipid biosurfactant against Fusarium sacchari the causal organism of pokkah boeng disease of sugarcane. J. Basic Microbiol. 2014;54:548–555. doi: 10.1002/jobm.201200801. [DOI] [PubMed] [Google Scholar]
- 35.Janek T., Lukaszewicz M., Krasowska A. Antiadhesive activity of the biosurfactant pseudofactin II secreted by the Arctic bacterium Pseudomonas fluorescens BD5. BMC Microbiol. 2012;12:1–9. doi: 10.1186/1471-2180-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donio M.B., Ronica S.F., Viji V.T., Velmurugan S., Jenifer J.A., Michaelbabu M., Citarasu T. Isolation and characterization of halophilic Bacillus sp. BS3 able to produce pharmacologically important biosurfactants. Asian Pac. J. Trop. Med. 2013;6:876–883. doi: 10.1016/S1995-7645(13)60156-X. [DOI] [PubMed] [Google Scholar]
- 37.Zhao F., Shi R., Zhao J., Li G., Bai X., Han S., Zhang Y. Heterologous production of Pseudomonas aeruginosa rhamnolipid under anaerobic conditions for microbial enhanced oil recovery. J. Appl. Microbiol. 2015;118:379–389. doi: 10.1111/jam.12698. [DOI] [PubMed] [Google Scholar]
- 38.Singh A.K., Cameotra S.S. Efficiency of lipopeptide biosurfactants in removal of petroleum hydrocarbons and heavy metals from contaminated soil. Environ. Sci. Pollut. Res. 2013;20:7367–7376. doi: 10.1007/s11356-013-1752-4. [DOI] [PubMed] [Google Scholar]
- 39.Solaiman D.K., Ashby R.D., Crocker N.V. High-titer production and strong antimicrobial activity of sophorolipids from Rhodotorula bogoriensis. Biotechnol. Prog. 2015;31:867–874. doi: 10.1002/btpr.2101. [DOI] [PubMed] [Google Scholar]
- 40.Campos J.M., Stamford T.L., Sarubbo L.A. Production of a bioemulsifier with potential application in the food industry. Appl. Biochem. Biotechnol. 2014;172:3234–3252. doi: 10.1007/s12010-014-0761-1. [DOI] [PubMed] [Google Scholar]
- 41.Rufino R.D., De Luna J.M., De Campos Takaki G.M., Sarubbo L.S. Characterization and properties of the biosurfactant produced by Candida lipolytica UCP 0988. Electron. J. Biotechnol. 2014;17:6. doi: 10.1016/j.ejbt.2013.12.006. [DOI] [Google Scholar]
- 42.Gautam G., Mishra V., Verma P., Pandey A.K., Negi S.A. Cost Effective Strategy for Production of Bio-surfactant from Locally Isolated Penicillium chrysogenum SNP5 and its Applications. J. Bioprocess. Biotechnol. 2014;4:1. doi: 10.4172/2155-9821.1000177. [DOI] [Google Scholar]
- 43.Andrade S., Luna M.A., Santiago A.L., Franco L.O., Silva G.K., de Souza P.M., Okada K., Albuquerque C.D., da Silva C.A., Campos-Takaki G.M. Biosurfactant-and-bioemulsifier produced by a promising Cunninghamella echinulata isolated from Caatinga soil in the northeast of Brazil. Int. J. Mol. Sci. 2014;15:15377–15395. doi: 10.3390/ijms150915377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kitamoto D., Haneishi K., Nakahara T., Tabuchi T. Production of mannosylerythritol lipids by Candida antarctica from vegetable oils. Agric. Biol. Chem. 1990;54:37–40. doi: 10.1271/bbb1961.54.37. [DOI] [Google Scholar]
- 45.Zinjarde S., Pant A. Emulsifier from tropical marine yeast, Yarrowia lipolytica NCIM 3589. J. Basic Microbiol. 2002;42:67–73. doi: 10.1002/1521-4028(200203)42:1<67::AID-JOBM67>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 46.Holler U., Konig G.M., Wright A. Three new metabolites from marine derived fungi of the genera Coniothyrium and Microsphaeropsis. J. Nat. Prod. 1999;62:114–118. doi: 10.1021/np980341e. [DOI] [PubMed] [Google Scholar]
- 47.Amaral P.F.F., Da Silva J.M., Lehocky B.M., Barros-Timmons A.M.V., Coelho M.A.Z., Marrucho I.M., Coutinho J.A.P. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochem. 2006;41:1894–1898. doi: 10.1016/j.procbio.2006.03.029. [DOI] [Google Scholar]
- 48.Teichmann B., Linne U., Hewald S., Marahiel M.A., Bölker M. A biosynthetic gene cluster for a secreted cellobiose lipid with antifungal activity from Ustilago maydis. Mol. Microbiol. 2007;66:525–533. doi: 10.1111/j.1365-2958.2007.05941.x. [DOI] [PubMed] [Google Scholar]
- 49.Kim S.Y., Oh D.K., Lee K.H., Kim J.H. Effect of soybean oil and glucose on sophorose lipid fermentation by Torulopsis bombicola in continuous culture. Appl. Microbiol. Biotechnol. 1997;48:23–26. doi: 10.1007/s002530051009. [DOI] [PubMed] [Google Scholar]
- 50.Kiran G.S., Hema T.A., Gandhimathi R., Selvin J., Thomas T.A., Ravji T.R., Natarajaseenivasan K. Optimization and production of a biosurfactant from the sponge-associated marine fungus Aspergillus ustus MSF3. Colloids Surf. B Biointerfaces. 2009;73:250–256. doi: 10.1016/j.colsurfb.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 51.Felse P.A., Shah V., Chan J., Rao K.J., Gross R.A. Sophorolipid biosynthesis by Candida bombicola from industrial fatty acid residues. Enzyme Microb. Technol. 2007;40:316–323. doi: 10.1016/j.enzmictec.2006.04.013. [DOI] [Google Scholar]
- 52.Alej C.S., Humberto H.S., Maria J.F. Production of glycolipids with antimicrobial activity by Ustilago maydis FBD12 in submerged culture. Afr. J. Microbiol. Res. 2011;5:2512–2523. [Google Scholar]
- 53.Ribeiro I.A., Faustino C.M., Guerreiro P.S., Frade R.F., Bronze M.R., Castro M.F., Ribeiro M.H. Development of novel sophorolipids with improved cytotoxic activity toward MDA-MB-231 breast cancer cells. J. Mol. Recognit. 2015;28:155–165. doi: 10.1002/jmr.2403. [DOI] [PubMed] [Google Scholar]
- 54.Cooper D.G., Paddock D.A. Torulopsis petrophilum and surface activity. Appl. Environ. Microbiol. 1983;46:1426–1429. doi: 10.1128/aem.46.6.1426-1429.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lukondeh T., Ashbolt N.J., Rogers P.L. Evaluation of Kluyveromyces marxianus FII 510700 grown on a lactose-based medium as a source of a natural bioemulsifier. J. Ind. Microbiol. Biotechnol. 2003;30:715–720. doi: 10.1007/s10295-003-0105-6. [DOI] [PubMed] [Google Scholar]
- 56.Rau U., Nguyen L.A., Roeper H., Koch H., Lang S. Fed-batch bioreactor production of mannosylerythritol lipids secreted by Pseudozyma aphidis. Appl. Microbiol. Biotechnol. 2005;68:607–613. doi: 10.1007/s00253-005-1906-5. [DOI] [PubMed] [Google Scholar]
- 57.Morita T., Takashima M., Fukuoka T., Konishi M., Imura T., Kitamoto D. Isolation of basidiomycetous yeast Pseudozyma tsukubaensis and production of glycolipid biosurfactant, a diastereomer type of mannosylerythritol lipid-B. Appl. Microbiol. Biotechnol. 2010;88:679–688. doi: 10.1007/s00253-010-2762-5. [DOI] [PubMed] [Google Scholar]
- 58.Ribeiro B.G., Guerra J.M.C., Sarubbo L.A. Potential food application of a biosurfactant produced by Saccharomyces cerevisiae URM 6670. Front. Bioeng. Biotechnol. 2020;8:434. doi: 10.3389/fbioe.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chandran P.R., Das N.I. Biosurfactant production and diesel oil degradation by yeast species Trichosporon asahii isolated from petroleum hydrocarbon contaminated soil. Int. J. Eng. Sci. Technol. 2010;2:6942–6953. [Google Scholar]
- 60.Sharma P., Sangwan S., Kaur H. Process parameters for biosurfactant production using yeast Meyerozyma guilliermondii YK32. Environ. Monit. Assess. 2019;191:531. doi: 10.1007/s10661-019-7665-z. [DOI] [PubMed] [Google Scholar]
- 61.Sen S., Borah S.N., Bora A., Deka S. Production, characterization, and antifungal activity of a biosurfactant produced by Rhodotorula babjevae YS3. Microb. Cell Fact. 2017;16:95. doi: 10.1186/s12934-017-0711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Joshi-Navare K., Singh P.K., Prabhune A.A. New yeast isolate Pichia caribbica synthesizes xylolipid biosurfactant with enhanced functionality. Eur. J. Lipid Sci. Technol. 2014;116:1070–1079. doi: 10.1002/ejlt.201300363. [DOI] [Google Scholar]
- 63.Thanomsub B., Watcharachaipong T., Chotelersak K., Arunrattiyakorn P., Nitoda T., Kanzaki H. Monoacylglycerols: Glycolipid biosurfactants produced by a thermotolerant yeast, Candida ishiwadae. J. Appl. Microbiol. 2004;96:588–592. doi: 10.1111/j.1365-2672.2004.02202.x. [DOI] [PubMed] [Google Scholar]
- 64.Mukherjee S., Das P., Sen R. Towards commercial production of microbial surfactants. Trends Biotechnol. 2006;24:509–515. doi: 10.1016/j.tibtech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 65.McInerney M.J., Duncan K.E., Youssef N., Fincher T., Maudgalya S.K., Folmsbee M.J., Knapp R., Simpson R.R., Ravi N., Nagle D. Development of Microorganisms with Improved Transport and Biosurfactant Activity for Enhanced oil Recovery. University of Oklahoma; Norman, OK, USA: 2005. [Google Scholar]
- 66.Sheppard J.D., Mulligan C.N. The production of surfactin by Bacillus subtilis grown on peat hydrolysate. Appl. Microbiol. Biotechnol. 1987;27:110–116. doi: 10.1007/BF00251931. [DOI] [Google Scholar]
- 67.Singh P., Patil Y., Rale V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2019;126:2–13. doi: 10.1111/jam.14057. [DOI] [PubMed] [Google Scholar]
- 68.Wu T., Xu J., Xie W., Yao Z., Yang H., Sun C., Li X. Pseudomonas aeruginosa L10: A hydrocarbon-degrading, biosurfactant-producing, and plant-growth-promoting endophytic bacterium isolated from a reed (Phragmites australis) Front. Microbiol. 2018;9:1087. doi: 10.3389/fmicb.2018.01087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pansiripat S., Pornsunthorntawee O., Rujiravanit R., Kitiyanan B., Somboonthanate P., Chavadej S. Biosurfactant production by Pseudomonas aeruginosa SP4 using sequencing batch reactors: Effect of oil-to-glucose ratio. Biochem. Eng. J. 2010;49:185–191. doi: 10.1016/j.bej.2009.12.011. [DOI] [Google Scholar]
- 70.Hassan M., Essam T., Yassin A.S., Salama A. Optimization of rhamnolipid production by biodegrading bacterial isolates using Plackett-Burman design. Int. J. Biol. Macromol. 2016;8:573–579. doi: 10.1016/j.ijbiomac.2015.09.057. [DOI] [PubMed] [Google Scholar]
- 71.Onwosi C.O., Odibo F.J.C. Effects of carbon and nitrogen sources on rhamnolipid biosurfactant production by Pseudomonas nitroreducens isolated from soil. World J. Microbiol. Biotechnol. 2012;28:937–942. doi: 10.1007/s11274-011-0891-3. [DOI] [PubMed] [Google Scholar]
- 72.Moussa T.A.A., Mohamed M.S., Samak N. Production and characterization of di-rhamnolipid produced by Pseudomonas aeruginosa TMN. Braz. J. Chem. Eng. 2014;31:867–880. doi: 10.1590/0104-6632.20140314s00002473. [DOI] [Google Scholar]
- 73.Yeh M., Wei Y., Chang J. Enhanced Production of Surfactin from Bacillus subtilis by addition of solid carriers. Biotechnol. Prog. 2005;21:1329–1334. doi: 10.1021/bp050040c. [DOI] [PubMed] [Google Scholar]
- 74.Ghribi D., Ellouze-Chaabouni S. Enhancement of Bacillus subtilis lipopeptide biosurfactants production through optimization of medium composition and adequate control of aeration. Biotechnol. Res. Int. 2011;2011:653654. doi: 10.4061/2011/653654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fooladi T., Bin A., Hamid A., Mohtar W., Yusoff W. Production of Biosurfactant by Indigenous Isolated Bacteria in Fermentation System. AIP Conf. Proc. 2013;197:197–201. [Google Scholar]
- 76.Tomar G.S., Srinikethan G. Studies on production of biosurfactant from Pseudomonas aeruginosa (MTCC7815) & its application in microbial enhanced oil recovery. Res. J. Chem. Environ. Sci. 2016;4:84–91. [Google Scholar]
- 77.Willenbacher J., Rau J.T., Rogalla J., Syldatk C., Hausmann R. Foam-free production of Surfactin via anaerobic fermentation of Bacillus subtilis DSM 10T. AMB Express. 2015;5:1–9. doi: 10.1186/s13568-015-0107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ejike Ogbonna K., Victor Agu C., Okonkwo C.C., Tochukwu Ughamba K., Akor J., Njoku O.U. Use of Spondias Mombin fruit pulp as a substrate for biosurfactant production. Bioengineered. 2021;12:1–12. doi: 10.1080/21655979.2020.1853391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kanna R., Gummadi S.N., Kumar G.S. Production and characterization of biosurfactant by Pseudomonas putida MTCC 2467. J. Biol. Sci. 2014;14:436–445. doi: 10.3923/jbs.2014.436.445. [DOI] [Google Scholar]
- 80.de França Í.W.L., de Oliveira D.W.F., Giro M.E.A., Melo V.M.M., Gonçalves L.R.B. Production of surfactin by Bacillus subtilis LAMI005 and evaluation of its potential as tensoactive and emulsifier. Can. J. Chem. Eng. 2021 doi: 10.1002/cjce.24240. [DOI] [Google Scholar]
- 81.Agarry S.E., Salam K.K., Arinkoola A., Aremu M.O. Biosurfactant production by indigeneous Pseudomonas and Bacillus species isolated from auto-mechanic soil environment toward microbial enhanced oil recovery. Eur. J. Eng. Technol. 2015;3:27–39. [Google Scholar]
- 82.Cherif N., Tifrit A., Larbi Daouadji K., Mezouari S., Chama Z., Abbouni B. Effect of carbon and nitrogen source on the microbial production of biosurfactants by Pseudomonas aeruginosa. Pharm. Lett. 2015;7:42–48. [Google Scholar]
- 83.Abouseoud M., Maachi R., Amrane A., Boudergua S., Nabi A. Evaluation of different carbon and nitrogen sources in production of biosurfactant by Pseudomonas fluorescens. Desalination. 2008;223:143–151. doi: 10.1016/j.desal.2007.01.198. [DOI] [Google Scholar]
- 84.Abdel-Mawgoud A.M., Aboulwafa M.M., Hassouna N.A.H. Optimization of surfactin production by Bacillus subtilis isolate BS5. Appl. Biochem. Biotechnol. 2008;150:305–325. doi: 10.1007/s12010-008-8155-x. [DOI] [PubMed] [Google Scholar]
- 85.Liu C., You Y., Zhao R., Sun D., Zhang P., Jiang J., Zhu A., Liu W. Biosurfactant production from Pseudomonas taiwanensis L1011 and its application in accelerating the chemical and biological decolorization of azo dyes. Ecotoxicol. Environ. Saf. 2017;145:8–15. doi: 10.1016/j.ecoenv.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 86.Baskaran S.M., Zakaria M.R., Sabri A.S.M.A., Mohamed M.S., Wasoh H., Toshinari M., Hassan M.A., Banat I.M. Valorization of biodiesel side stream waste glycerol for rhamnolipids production by Pseudomonas aeruginosa RS6. Environ. Pollut. 2021;276:116742. doi: 10.1016/j.envpol.2021.116742. [DOI] [PubMed] [Google Scholar]
- 87.Almansoory A.F., Hasan H.A., Idris M., Abdullah S.R.S., Anuar N. Biosurfactant production by the hydrocarbon-degrading bacteria (HDB) Serratia marcescens: Optimization using central composite design (CCD) J. Ind. Eng. Chem. 2017;47:272–280. doi: 10.1016/j.jiec.2016.11.043. [DOI] [Google Scholar]
- 88.Patel P., Modi A., Minipara D., Kumar A. Sustainable Environmental Clean-Up. Elsevier; Amsterdam, The Nederland: 2021. Microbial biosurfactants in management of organic waste; pp. 211–230. [Google Scholar]
- 89.Almeida P.F., Moreira R.S., Almeida R.C.C., Guimardes A.K., Carvalho A.S., Quintella C. Selection and application of microorganisms to improve oil recovery. Eng. Life Sci. 2004;4:319–324. doi: 10.1002/elsc.200420033. [DOI] [Google Scholar]
- 90.De Lima C.J.B., Ribeiro E.J., Servulo E.F.C., Resende M.M., Cardoso V.L. Biosurfactant production by Pseudomonas aeruginosa grown in residual soybean oil. Appl. Biochem. Biotechnol. 2009;152:156–168. doi: 10.1007/s12010-008-8188-1. [DOI] [PubMed] [Google Scholar]
- 91.Sivapathasekaran C., Sen R. Origin, properties, production and purification of microbial surfactants as molecules with immense commercial potential. Tenside Surfactants Deterg. 2017;54:92–107. [Google Scholar]
- 92.Agarwal P., Sharma D.K. Studies on the production of biosurfactant for the microbial enhanced oil recovery by using bacteria isolated from oil contaminated wet soil. Pet. Sci. Technol. 2009;27:1880–1893. doi: 10.1080/10916460802686640. [DOI] [Google Scholar]
- 93.Al-Bahry S.N., Al-Wahaibi Y.M., Elshafie A.E., Al-Bemani A.S., Joshi S.J., Al-Makhmari H.S., Al-Sulaimani H.S. Biosurfactant production by Bacillus subtilis B20 using date molasses and its possible application in enhanced oil recovery. Int. Biodeterior. Biodegrad. 2013;81:141–146. doi: 10.1016/j.ibiod.2012.01.006. [DOI] [Google Scholar]
- 94.Hentati D., Chebbi A., Hadrich F., Frikha I., Rabanal F., Sayadi S., Manresa A., Chamkha M. Production, characterization and biotechnological potential of lipopeptide biosurfactants from a novel marine Bacillus stratosphericus strain FLU5. Ecotoxicol. Environ. Saf. 2019;167:441–449. doi: 10.1016/j.ecoenv.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 95.Souza K.S.T., Gudiña E.J., Schwan R.F., Rodrigues L.R., Dias D.R., Teixeira J.A. Improvement of biosurfactant production by Wickerhamomyces anomalus CCMA 0358 and its potential application in bioremediation. J. Hazard. Mater. 2018;346:152–158. doi: 10.1016/j.jhazmat.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 96.Nazareth T.C., Zanutto C.P., Maass D., de Souza A.A.U., Ulson S.M.D.A.G. A low-cost brewery waste as a carbon source in bio-surfactant production. Bioprocess Biosyst. Eng. 2021:1–8. doi: 10.1007/s00449-021-02602-x. [DOI] [PubMed] [Google Scholar]
- 97.De Lima A.M., De Souzaa R.R. Use of sugar cane vinasse as substrate for biosurfactant production using Bacillus subtilis PC. Chem. Eng. 2014;37:673–678. [Google Scholar]
- 98.Gudiña E.J., Fernandes E.C., Rodrigues A.I., Teixeira J.A., Rodrigues L.R. Biosurfactant production by Bacillus subtilis using corn steep liquor as culture medium. Front. Microbiol. 2015;6:59. doi: 10.3389/fmicb.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gurjar J., Sengupta B. Production of surfactin from rice mill polishing residue by submerged fermentation using Bacillus subtilis MTCC 2423. Bioresour. Technol. 2015;189:243–249. doi: 10.1016/j.biortech.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 100.Coronel-León J., Marqués A.M., Bastida J., Manresa A. Optimizing the production of the biosurfactant lichenysin and its application in biofilm control. J. Appl. Microbiol. 2016;120:99–111. doi: 10.1111/jam.12992. [DOI] [PubMed] [Google Scholar]
- 101.Li J., Deng M., Wang Y., Chen W. Production and characteristics of biosurfactant produced by Bacillus pseudomycoides BS6 utilizing soybean oil waste. Int. Biodeterior. Biodegrad. 2016;112:72–79. doi: 10.1016/j.ibiod.2016.05.002. [DOI] [Google Scholar]
- 102.Maass D., Moya Ramirez I., Garcia Roman M., Jurado Alameda E., Ulson de Souza A.A., Borges Valle J.A., Altmajer Vaz D. Two-phase olive mill waste (alpeorujo) as carbon source for biosurfactant production. J. Chem. Technol. Biotechnol. 2016;91:1990–1997. doi: 10.1002/jctb.4790. [DOI] [Google Scholar]
- 103.Jajor P., Piłakowska-Pietras D., Krasowska A., Łukaszewicz M. Surfactin analogues produced by Bacillus subtilis strains grown on rapeseed cake. J. Mol. Struct. 2016;1126:141–146. doi: 10.1016/j.molstruc.2016.02.014. [DOI] [Google Scholar]
- 104.Zhi Y., Wu Q., Xu Y. Production of surfactin from waste distillers’ grains by co-culture fermentation of two Bacillus amyloliquefaciens strains. Bioresour. Technol. 2017;235:96–103. doi: 10.1016/j.biortech.2017.03.090. [DOI] [PubMed] [Google Scholar]
- 105.Phulpoto I.A., Yu Z., Hu B., Wang Y., Ndayisenga F., Li J., Liang H., Qazi M.A. Production and characterization of surfactin-like biosurfactant produced by novel strain Bacillus nealsonii S2MT and it’s potential for oil contaminated soil remediation. Microb. Cell Fact. 2020;19:1–12. doi: 10.1186/s12934-020-01402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hentati D., Cheffi M., Hadrich F., Makhloufi N., Rabanal F., Manresa A., Sayadi S., Chamkha M. Investigation of halotolerant marine Staphylococcus sp. CO100, as a promising hydrocarbon-degrading and biosurfactant-producing bacterium, under saline conditions. J. Environ. Manag. 2021;277:111480. doi: 10.1016/j.jenvman.2020.111480. [DOI] [PubMed] [Google Scholar]
- 107.Cheffi M., Maalej A., Mahmoudi A., Hentati D., Marques A.M., Sayadi S., Chamkha M. Lipopeptides production by a newly Halomonas venusta strain: Characterization and biotechnological properties. Bioorg. Chem. 2021;109:104724. doi: 10.1016/j.bioorg.2021.104724. [DOI] [PubMed] [Google Scholar]
- 108.Derguine-Mecheri L., Kebbouche-Gana S., Djenane D. Biosurfactant production from newly isolated Rhodotorula sp. YBR and its great potential in enhanced removal of hydrocarbons from contaminated soils. World J. Microbiol. Biotechnol. 2021;37:1–8. doi: 10.1007/s11274-020-02983-3. [DOI] [PubMed] [Google Scholar]
- 109.Ahmadi N., Shafaati M., Mirgeloybayat M., Lavasani P.S., Khaledi M., Afkhami H. Molecular identification and primary evaluation of bio-surfactant production in edible paddy straw mushroom. Gene Rep. 2021;24:101258. doi: 10.1016/j.genrep.2021.101258. [DOI] [Google Scholar]
- 110.Auhim H.S., Mohamed A.I. Effect of different environmental and nutritional factors on biosurfactant production from Azotobacter chroococcum. Int. J. Adv. Pharm. Biol. Chem. 2013;2:477–481. [Google Scholar]
- 111.Joaad A.I., Hassan S.S. Effect of Different Environmental and Nutritional Factors on Biosurfactant Production from Candida guilliermondii. Iraqi J. Sci. 2015;56:329–336. [Google Scholar]
- 112.Robinson B.H., Bañuelos G., Conesa H.M., Evangelou M.W., Schulin R. The phytomanagement of trace elements in soil. Crit. Rev. Plant Sci. 2009;28:240–266. doi: 10.1080/07352680903035424. [DOI] [Google Scholar]
- 113.Roy A. Review on the biosurfactants: Properties, types and its applications. J. Fundam. Renew. Energy Appl. 2017;8:1–14. doi: 10.4172/2090-4541.1000248. [DOI] [Google Scholar]
- 114.Singh R., Glick B.R., Rathore D. Biosurfactants as a biological tool to increase micronutrient availability in soil: A review. Pedosphere. 2018;28:170–189. doi: 10.1016/S1002-0160(18)60018-9. [DOI] [Google Scholar]
- 115.Pacwa-Płociniczak M., Płaza G.A., Piotrowska-Seget Z., Cameotra S.S. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011;12:633–654. doi: 10.3390/ijms12010633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mnif I., Ghribi D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Pept. Sci. 2015;104:129–147. doi: 10.1002/bip.22630. [DOI] [PubMed] [Google Scholar]
- 117.Rufino R.D., Luna J.M., Sarubbo L.A., Rodrigues L.R.M., Teixeira J.A.C., Campos-Takaki G.M. Antimicrobial and anti-adhesive potential of a biosurfactant Rufisan produced by Candida lipolytica UCP 0988. Colloids Surf. B Biointerfaces. 2011;84:1–5. doi: 10.1016/j.colsurfb.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 118.Singh P., Cameotra S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004;22:142–146. doi: 10.1016/j.tibtech.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 119.Olaniran A.O., Balgobind A., Pillay B. Bioavailability of heavy metals in soil: Impact on microbial biodegradation of organic compounds and possible improvement strategies. Int. J. Mol. Sci. 2013;14:10197–10228. doi: 10.3390/ijms140510197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bustamante M., Duran N., Diez M.C. Biosurfactants are useful tools for the bioremediation of contaminated soil: A review. J. Soil Sci. Plant Nutr. 2012;12:667–687. doi: 10.4067/S0718-95162012005000024. [DOI] [Google Scholar]
- 121.Wang S., Mulligan C.N. An evaluation of surfactant foam technology in remediation of contaminated soil. Chemosphere. 2004;57:1079–1089. doi: 10.1016/j.chemosphere.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 122.Sheng X., He L., Wang Q., Ye H., Jiang C. Effects of inoculation of biosurfactant-producing Bacillus sp. J119 on plant growth and cadmium uptake in a cadmium-amended soil. J. Hazard. Mater. 2008;155:17–22. doi: 10.1016/j.jhazmat.2007.10.107. [DOI] [PubMed] [Google Scholar]
- 123.Stacey S.P., McLaughlin M.J., Çakmak I., Hettiarachchi G.M., Scheckel K.G., Karkkainen M. Root uptake of lipophilic zinc- rhamnolipid complexes. J. Agric. Food Chem. 2008;56:2112–2117. doi: 10.1021/jf0729311. [DOI] [PubMed] [Google Scholar]
- 124.Singha L.P., Pandey P. Rhizosphere assisted bioengineering approaches for the mitigation of petroleum hydrocarbons contamination in soil. Crit. Rev. Biotechnol. 2021;41:1–26. doi: 10.1080/07388551.2021.1888066. [DOI] [PubMed] [Google Scholar]
- 125.Liao C., Xu W., Lu G., Deng F., Liang X., Guo C., Dang Z. Biosurfactant-enhanced phytoremediation of soils contaminated by crude oil using maize (Zea mays. L) Ecol. Eng. 2016;92:10–17. doi: 10.1016/j.ecoleng.2016.03.041. [DOI] [Google Scholar]
- 126.Marchut-Mikołajczyk O., Drożdżyński P., Polewczyk A., Smułek W., Antczak T. Biosurfactant from endophytic Bacillus pumilus 2A: Physicochemical characterization, production and optimization and potential for plant growth promotion. Microb. Cell Factories. 2021;20:1–11. doi: 10.1186/s12934-021-01533-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Chopra A., Bobate S., Rahi P., Banpurkar A., Mazumder P.B., Satpute S. Pseudomonas aeruginosa RTE4: A Tea Rhizobacterium With Potential for Plant Growth Promotion and Biosurfactant Production. Front. Bioeng. Biotechnol. 2020;8:861. doi: 10.3389/fbioe.2020.00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Alsohim A.S., Taylor T.B., Barrett G.A., Gallie J., Zhang X.X., Altamirano-Junqueira A.E., Johnson L.J., Rainey P.B., Jackson R.W. The biosurfactant viscosin produced by Pseudomonas fluorescens SBW 25 aids spreading motility and plant growth promotion. Environ. Microbiol. 2014;16:2267–2281. doi: 10.1111/1462-2920.12469. [DOI] [PubMed] [Google Scholar]
- 129.Droby S., editor. Food Security and Plant Disease Management. Elsevier Science & Technology; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 130.Gill H.K., Garg H. Pesticide: Environmental impacts and management strategies. Pestic. Toxic Asp. 2014;8:187. [Google Scholar]
- 131.Mostafalou S., Abdollahi M. Pesticides and human chronic diseases: Evidences, mechanisms, and perspectives. Toxicol. Appl. Pharmacol. 2013;268:157–177. doi: 10.1016/j.taap.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 132.Preininger C., Sauer U., Bejarano A., Berninger T. Concepts and applications of foliar spray for microbial inoculants. Appl. Microbiol. Biotechnol. 2018;102:7265–7282. doi: 10.1007/s00253-018-9173-4. [DOI] [PubMed] [Google Scholar]
- 133.Huang H., Yu Q., Ren H., Geng J., Xu K., Zhang Y., Ding L. Towards physicochemical and biological effects on detachment and activity recovery of aging biofilm by enzyme and surfactant treatments. Biores. Technol. 2018;247:319–326. doi: 10.1016/j.biortech.2017.09.082. [DOI] [PubMed] [Google Scholar]
- 134.Palma-Bautista C., Tataridas A., Kanatas P., Travlos I.S., Bastida F., Domínguez-Valenzuela J.A., De Prado R. Can Control of Glyphosate Susceptible and Resistant Conyza sumatrensis Populations Be Dependent on the Herbicide Formulation or Adjuvants? Agronomy. 2020;10:1599. doi: 10.3390/agronomy10101599. [DOI] [Google Scholar]
- 135.Buffington E.J., McDonald S.K. Adjuvants & Surfactants. Volume 301 Colorado Environmental Pesticide Education Program; Fort Collins, CO, USA: 2006. [Google Scholar]
- 136.Rebello S., Asok A.K., Mundayoor S., Jisha M.S. Surfactants: Chemistry, Toxicity and Remediation. In: Lichtfouse E., Schwarzbauer J., Robert D., editors. Pollutant Diseases, Remediation and Recycling. Volume 4 Springer; Cham, Switzerland: 2013. [Google Scholar]
- 137.Ryckaert B., Spanoghe P., Haesaert G., Heremans B., Isebaert S., Steurbaut W. Quantitative determination of the influence of adjuvants on foliar fungicide residues. Crop Prot. 2007;26:1589–1594. doi: 10.1016/j.cropro.2007.02.011. [DOI] [Google Scholar]
- 138.Jibrin M.O., Liu Q., Jones J.B., Zhang S. Surfactants in plant disease management: A brief review and case studies. Plant Pathol. 2021;70:495–510. doi: 10.1111/ppa.13318. [DOI] [Google Scholar]
- 139.Sachdev D.P., Cameotra S.S. Biosurfactants in agriculture. Appl. Microbiol. Biotechnol. 2013;97:1005–1016. doi: 10.1007/s00253-012-4641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mizumoto S., Shoda M. Medium optimization of antifungal lipopeptide, iturin A, production by Bacillus subtilis in solid-state fermentation by response surface methodology. Appl. Microbiol. Biotechnol. 2007;76:101–108. doi: 10.1007/s00253-007-0994-9. [DOI] [PubMed] [Google Scholar]
- 141.Crouzet J., Arguelles-Arias A., Dhondt-Cordelier S., Cordelier S., Pršić J., Hoff G., Mazeyrat-Gourbeyre F., Baillieul F., Clément C., Ongena M., et al. Biosurfactants in plant protection against diseases: Rhamnolipids and lipopeptides case study. Front. Bioeng. Biotechnol. 2020;8:8. doi: 10.3389/fbioe.2020.01014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kumar A., Zhimo Y., Biasi A., Salim S., Feygenberg O., Wisniewski M., Droby S. Endophytic Microbiome in the Carposphere and Its Importance in Fruit Physiology and Pathology. In: Spadaro D., Droby S., Gullino M.L., editors. Postharvest Pathology. Volume 11 Springer; Cham, Switzerland: 2021. [Google Scholar]
- 143.Singh V.K., Singh A.K., Kumar A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech. 2017;7:255. doi: 10.1007/s13205-017-0896-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Varnier A.L., Sanchez L., Vatsa P., Boudesocque L., Garcia-Brugger A.N.G.E.L.A., Rabenoelina F., Sorokin A., Renault J.H., Kauffmann S., Pugin A., et al. Bacterial rhamnolipids are novel MAMPs conferring resistance to Botrytis cinerea in grapevine. Plant Cell Environ. 2009;32:178–193. doi: 10.1111/j.1365-3040.2008.01911.x. [DOI] [PubMed] [Google Scholar]
- 145.Kruijt M., Tran H., Raaijmakers J.M. Functional, genetic and chemical characterization of biosurfactants produced by plant growth-promoting Pseudomonas putida 267. J. Appl. Microbiol. 2009;107:546–556. doi: 10.1111/j.1365-2672.2009.04244.x. [DOI] [PubMed] [Google Scholar]
- 146.Nielsen T.H., Sørensen J. Production of cyclic lipopeptides by Pseudomonas fluorescens strains in bulk soil and in the sugar beet rhizosphere. Appl. Environ. Microbiol. 2003;69:861–868. doi: 10.1128/AEM.69.2.861-868.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Perneel M., D’hondt L., De Maeyer K., Adiobo A., Rabaey K., Höfte M. Phenazines and biosurfactants interact in the biological control of soil-borne diseases caused by Pythium spp. Environ. Microbiol. 2008;10:778–788. doi: 10.1111/j.1462-2920.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 148.D’aes J., Hua G.K.H., De Maeyer K., Pannecoucque J., Forrez I., Ongena M., Dietrich L.E., Thomashow L.S., Mavrodi D.V., Höfte M. Biological control of Rhizoctonia root rot on bean by phenazine-and cyclic lipopeptide-producing Pseudomonas CMR12a. Phytopathology. 2011;101:996–1004. doi: 10.1094/PHYTO-11-10-0315. [DOI] [PubMed] [Google Scholar]
- 149.Velho R.V., Medina L.F.C., Segalin J., Brandelli A. Production of lipopeptides among Bacillus strains showing growth inhibition of phytopathogenic fungi. Folia Microbiol. 2011;56:297. doi: 10.1007/s12223-011-0056-7. [DOI] [PubMed] [Google Scholar]
- 150.Akladious S.A., Gomaa E.Z., El-Mahdy O.M. Efficiency of bacterial biosurfactant for biocontrol of Rhizoctonia solani (AG-4) causing root rot in faba bean (Vicia faba) plants. Eur. J. Plant Pathol. 2019;153:15–35. doi: 10.1007/s10658-018-01639-1. [DOI] [Google Scholar]
- 151.Hussain T., Haris M., Shakeel A., Ahmad G., Khan A.A., Khan M.A. Bio-nematicidal activities by culture filtrate of Bacillus subtilis HussainT-AMU: New promising biosurfactant bioagent for the management of Root Galling caused by Meloidogyne incognita. Vegetos. 2020;33:229–238. doi: 10.1007/s42535-020-00099-5. [DOI] [Google Scholar]
- 152.Shalini D., Benson A., Gomathi R., Henry A.J., Jerritta S., Joe M.M. Isolation, characterization of glycolipid type biosurfactant from endophytic Acinetobacter sp. ACMS25 and evaluation of its biocontrol efficiency against Xanthomonas oryzae. Biocatal. Agric. Biotechnol. 2017;11:252–258. doi: 10.1016/j.bcab.2017.07.013. [DOI] [Google Scholar]
- 153.Haddad N.I., Wang J., Mu B. Isolation and characterization of a biosurfactant producing strain, Brevibacilis brevis HOB1. J. Ind. Microbiol. Biotechnol. 2008;35:1597–1604. doi: 10.1007/s10295-008-0403-0. [DOI] [PubMed] [Google Scholar]
- 154.Radhakrishnan E.K., Mathew J. Characterization of biosurfactant produced by the endophyte Burkholderia sp. WYAT7 and evaluation of its antibacterial and antibiofilm potentials. J. Biotechnol. 2020;313:1–10. doi: 10.1016/j.jbiotec.2020.03.005. [DOI] [PubMed] [Google Scholar]
- 155.Kim S.K., Kim Y.C., Lee S., Kim J.C., Yun M.Y., Kim I.S. Insecticidal activity of rhamnolipid isolated from Pseudomonas sp. EP-3 against green peach aphid (Myzus persicae) J. Agric. Food. Chem. 2011;59:934–938. doi: 10.1021/jf104027x. [DOI] [PubMed] [Google Scholar]
- 156.Davey M.E., Caiazza N.C., O’Toole G.A. Rhamnolipid surfactant production affects biofilm architecture in Pseudomonas aeruginosa PAO1. J. Bacteriol. 2003;185:1027–1036. doi: 10.1128/JB.185.3.1027-1036.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.De Jonghe K., De Dobbelaere I., Sarrazyn R., Höfte M. Control of Phytophthora cryptogea in the hydroponic forcing of witlo of chicory with the rhamnolipid-based biosurfactant formulation PRO1. Plant Pathol. 2005;54:219–222. doi: 10.1111/j.1365-3059.2005.01140.x. [DOI] [Google Scholar]
- 158.Hultberg M., Bengtsson T., Liljeroth E. Late blight on potato is suppressed by the biosurfactant-producing strain Pseudomonas koreensis 2.74 and its biosurfactant. BioControl. 2010;55:543–550. doi: 10.1007/s10526-010-9289-7. [DOI] [Google Scholar]
- 159.Fernandes P.A., Arruda I.R., Santos A.F., Araújo A.A., Maior A.M., Ximenes E.A. Antimicrobial activity of surfactants produced by Bacillus subtilis R14 against multidrug-resistant bacteria. Braz. J. Microbiol. 2007;38:704–709. doi: 10.1590/S1517-83822007000400022. [DOI] [Google Scholar]
- 160.Kim P.I., Ryu J., Kim Y.H., Chi Y.T. Production of biosurfactant lipopeptides Iturin A, fengycin and surfactin A from Bacillus subtilis CMB32 for control of Colletotrichum gloeosporioides. J. Microbiol. Biotechnol. 2010;20:138–145. doi: 10.4014/jmb.0905.05007. [DOI] [PubMed] [Google Scholar]
- 161.Edosa T.T., Hun Jo Y., Keshavarz M., Soo Han Y. Biosurfactants: Production and potential application in insect pest management. Trends Entomol. 2018;14:79–87. doi: 10.31300/TENT.14.2018.79-87. [DOI] [Google Scholar]
- 162.Yun D.C., Yang S.Y., Kim Y.C., Kim I.S., Kim Y.H. Identification of surfactin as an aphicidal metabolite produced by Bacillus amyloliquefaciens G1. J. Korean Soc. Appl. Biol. Chem. 2013;56:751–753. doi: 10.1007/s13765-013-3238-y. [DOI] [Google Scholar]
- 163.Khedher S.B., Boukedi H., Dammak M., Kilani-Feki O., Sellami-Boudawara T., Abdelkefi-Mesrati L., Tounsi S. Combinatorial effect of Bacillus amyloliquefaciens AG1 biosurfactant and Bacillus thuringiensis Vip3Aa16 toxin on Spodoptera littoralis larvae. J. Invertebr. Pathol. 2017;144:11–17. doi: 10.1016/j.jip.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 164.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 165.Abdollahi S., Tofighi Z., Babaee T., Shamsi M., Rahimzadeh G., Rezvanifar H., Saeidi E., Mohajeri Amiri M., Saffari Ashtiani Y., Samadi N. Evaluation of Antioxidant and Anti-biofilm Activities of Biogenic Surfactants Derived from Bacillus amyloliquefaciens and Pseudomonas aeruginosa. Iran. J. Pharm. Res. 2020;19:115–126. doi: 10.22037/IJPR.2020.1101033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Amaro da Silva I., Gercyane Oliveira Bezerra K., Batista Durval I.J., Bronzo Barbosa Farias C., Galdino Junior C.J., Mendes Da Silva Santos E., Moura De Luna J., Asfora Sarubbo L. Evaluation of the Emulsifying and Antioxidant Capacity of the Biosurfactant Produced by Candida Bombicola Urm 3718. Chem. Eng. 2020;79:67–72. [Google Scholar]
- 167.Palanisamy S., Vinosha M., Marudhupandi T., Rajasekar P., Prabhu N.M. Isolation of fucoidan from Sargassum polycystum brown algae: Structural characterization, in vitro antioxidant and anticancer activity. Int. J. Biol. Macromol. 2017;102:405–412. doi: 10.1016/j.ijbiomac.2017.03.182. [DOI] [PubMed] [Google Scholar]
- 168.Markande A.R., Patel D., Varjani S. A review on biosurfactants: Properties, applications and current developments. Biores. Technol. 2021;330:124963. doi: 10.1016/j.biortech.2021.124963. [DOI] [PubMed] [Google Scholar]
- 169.Sharma D., Singh Saharan B. Simultaneous Production of Biosurfactants and Bacteriocins by Probiotic Lactobacillus casei MRTL3. Int. J. Microbiol. 2014;2014:698713. doi: 10.1155/2014/698713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Basit M., Rasool M.H., Naqvi S.A.R., Waseem M., Aslam B. Biosurfactants production potential of native strains of Bacillus cereus and their antimicrobial, cytotoxic and antioxidant activities. Pak. J. Pharm. Sci. 2018;31:251–256. [PubMed] [Google Scholar]
- 171.Ekramul H., Kayalvizhi K., Saqib H. Biocompatibility, Antioxidant and Anti-Infective Effect of Biosurfactant Produced by Marinobacter litoralis MB15. Int. J. Pharm. Investig. 2020;10:173–178. [Google Scholar]
- 172.Merghni A., Dallel I., Noumi E., Kadmi Y., Hentati H., Tobji S., Ben Amor A., Mastouri M. Antioxidant and antiproliferative potential of biosurfactants isolated from Lactobacillus casei and their anti-biofilm effect in oral Staphylococcus aureus strains. Microb. Pathog. 2017;104:84–89. doi: 10.1016/j.micpath.2017.01.017. [DOI] [PubMed] [Google Scholar]
- 173.Giri S.S., Ryu E.C., Sukumaran V., Park S.C. Antioxidant, antibacterial, and anti-adhesive activities of biosurfactants isolated from Bacillus strains. Microb. Pathog. 2019;132:66–72. doi: 10.1016/j.micpath.2019.04.035. [DOI] [PubMed] [Google Scholar]
- 174.Ohadi M., Forootanfar H., Rahimi H.R., Jafari E., Shakibaie M., Eslaminejad T., Dehghannoudeh G. Antioxidant Potential and Wound Healing Activity of Biosurfactant Produced by Acinetobacter junii B6. Curr. Pharm. Biotechnol. 2017;18:900–908. doi: 10.2174/1389201018666171122121350. [DOI] [PubMed] [Google Scholar]
- 175.Takahashi M., Morita T., Fukuoka T., Imura T., Kitamoto D. Glycolipid biosurfactants, mannosylerythritol lipids, show antioxidant and protective effects against H2O2-induced oxidative stress in cultured human skin fibroblasts. J. Oleo Sci. 2012;61:457–464. doi: 10.5650/jos.61.457. [DOI] [PubMed] [Google Scholar]
- 176.Zouari R., Moalla-Rekik D., Sahnoun Z., Rebai T., Ellouze-Chaabouni S., Ghribi-Aydi D. Evaluation of dermal wound healing and in vitro antioxidant efficiency of Bacillus subtilis SPB1 biosurfactant. Biomed. Pharmacother. 2016;84:878–891. doi: 10.1016/j.biopha.2016.09.084. [DOI] [PubMed] [Google Scholar]
- 177.Mouafo H.T., Mbawala A., Somashekar D., Tchougang H.M., Harohally N.V., Ndjouenkeu R. Biological properties and structural characterization of a novel rhamnolipid like-biosurfactants produced by Lactobacillus casei subsp. casei TM1B. Biotechnol. Appl. Biochem. 2021;68:585–596. doi: 10.1002/bab.1966. [DOI] [PubMed] [Google Scholar]
- 178.Li S., Huang R., Shah N.P., Tao X., Xiong Y., Wei H. Antioxidant and antibacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus Plantarum R315. J. Dairy Sci. 2014;97:7334–7343. doi: 10.3168/jds.2014-7912. [DOI] [PubMed] [Google Scholar]
- 179.Jemil N., Ben Ayed H., Manresa A., Nasri M., Hmidet N. Antioxidant properties, antimicrobial and anti-adhesive activities of DCS1 lipopeptides from Bacillus methylotrophicus DCS1. BMC Microbiol. 2017;17:144. doi: 10.1186/s12866-017-1050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ceresa C., Fracchia L., Fedeli E., Porta C., Banat I.M. Recent Advances in Biomedical, Therapeutic and Pharmaceutical Applications of Microbial Surfactants. Pharmaceutics. 2021;13:466. doi: 10.3390/pharmaceutics13040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fariq A., Yasmin A. Production, characterization and bioactivities of biosurfactants from newly isolated strictly halophilic bacteria. Process. Biochem. 2020;98:1–10. doi: 10.1016/j.procbio.2020.07.011. [DOI] [Google Scholar]
- 182.Kharat M., McClements D.J. Fabrication and characterization of nanostructured lipid carriers (NLC) using a plant-based emulsifier: Quillaja saponin. Food Res. Int. 2019;126:108601. doi: 10.1016/j.foodres.2019.108601. [DOI] [PubMed] [Google Scholar]
- 183.DOH and DEFRA UK Five-Year Antimicrobial Resistance Strategy 2013 to 2018. [(accessed on 12 February 2019)];2013 Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/244058/20130902_UK_5_year_AMR_strategy.pdf.
- 184.Dıaz De Rienzo M.A., Banat I.M., Dolman B., Winterburn J., Martin P.J. Sophorolipid biosurfactants: Possible uses as antibacterial and antibiofilm agent. New Biotechnol. 2015;32:720–726. doi: 10.1016/j.nbt.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 185.Foschi C., Salvo M., Cevenini R., Parolin C., Vitali B., Marangoni A. Vaginal Lactobacilli reduce Neisseria gonorrhoeae viability through multiple strategies: An in vitro study. Front. Cell. Infect. Microbiol. 2017;7:502. doi: 10.3389/fcimb.2017.00502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Morais I.M.C., Cordeiro A.L., Teixeira G.S., Domingues V.S., Nardi R.M.D., Monteiro A.S., Alves R.J., Siqueira E.P., Santos V.L. Biological and physicochemical properties of biosurfactants produced by Lactobacillus jensenii P 6A and Lactobacillus gasseri P 65. Microb. Cell Fact. 2017;16:1–15. doi: 10.1186/s12934-017-0769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Dusane D.H., Nancharaiah Y.V., Zinjarde S.S., Venugopalan V.P. Rhamnolipid mediated disruption of marine Bacillus pumilus biofilms. Colloids Surf. B Biointerfaces. 2010;81:242–248. doi: 10.1016/j.colsurfb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 188.Kaczorek E. Effect of external addition of rhamnolipids biosurfactant on the modification of gram positive and gram negative bacteria cell surfaces during biodegradation of hydrocarbon fuel contamination. Pol. J. Environ. Stud. 2012;21:901–909. [Google Scholar]
- 189.Bharali P., Konwar B.K. Production and physicochemical characterization of abiosurfactant produced by Pseudomonas aeruginosa OBP1 isolated from petroleum sludge. Appl. Biochem. Biotechnol. 2011;164:1444–1460. doi: 10.1007/s12010-011-9225-z. [DOI] [PubMed] [Google Scholar]
- 190.Naughton P.J., Marchant R., Naughton V., Banat I.M. Microbial biosurfactants: Current trends and applications in agricultural and biomedical industries. J. Appl. Microbiol. 2019;127:12–28. doi: 10.1111/jam.14243. [DOI] [PubMed] [Google Scholar]
- 191.Gudina E.J., Rangarajan V., Sen R., Rodrigues L.R. Potential therapeutic applications of biosurfactants. Trends Pharmacol. Sci. 2013;34:667–675. doi: 10.1016/j.tips.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 192.Zhao H., Yan L., Xu X., Jiang C., Shi J., Zhang Y., Liu L., Lei S., Shao D., Huang Q. Potential of Bacillus subtilis lipopeptides in anti-cancer I: Induction of apoptosis and paraptosis and inhibition of autophagy in K562 cells. AMB Express. 2018;8:1–16. doi: 10.1186/s13568-018-0606-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dey G., Bharti R., Dhanarajan G., Das S., Dey K.K., Kumar B.N., Sen R., Mandal M. Marine lipopeptide Iturin A inhibits Akt mediated GSK3b and FoxO3asignalling and triggers apoptosis in breast cancer. Sci. Rep. 2015;5:10316. doi: 10.1038/srep10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is available within the article.