Abstract
Simple Summary
Isolated hepatic perfusion is one of the available treatment options for patients with liver metastases from uveal melanoma. This is an open surgical procedure where the liver is isolated from the circulation and perfused with a chemotherapeutic agent. A modern development is the minimally invasive percutaneous hepatic perfusion, where the liver is endovascularly isolated and then perfused with a chemotherapeutic agent through a catheter in the arterial system. Within this systematic review and meta-analysis, we aim to compare these modalities in terms of overall survival, progression-free survival, complications and response.
Abstract
Background: Uveal melanoma is the most commonly occurring primary intraocular malignancy in adults, and patients have a high risk of developing metastatic disease, mostly in the liver. Isolated hepatic perfusion (IHP) with melphalan is a liver-directed therapy for patients with liver metastases. Percutaneous hepatic perfusion (PHP), a minimally invasive technique, is available as well. PHP benefits from the fact that the procedure can be repeated and therefore possibly offers better survival. We conducted a systematic review and meta-analysis comparing both techniques. Methods: A systematic literature search was performed using the electronic databases of Scopus, MEDLINE, Web of Science, PubMed and Cochrane CENTRAL. A total of nine articles reporting on eight studies were included in the analysis. Individual survival data were extracted from each study. Results: The median overall survival (OS) was 17.1 months for IHP and 17.3 months for PHP. The median progression-free survival (PFS) was 7.2 months for IHP and 9.6 months for PHP. The median hepatic progression-free survival was 10 months for IHP and 9.5 months for PHP. The complication rate and 30-day mortality rate were 39.1% and 5.5% for IHP and 23.8% and 1.8% for PHP. Conclusion: There was no difference in OS or PFS between IHP and PHP for patients with uveal melanoma liver metastases, but patients have significantly less of a risk for complications and mortality following PHP.
Keywords: uveal melanoma, liver metastases, isolated hepatic perfusion, percutaneous hepatic perfusion
1. Introduction
Uveal melanoma is the most commonly occurring primary intraocular malignancy in adults [1]. It is very different from cutaneous melanoma, considering clinical behavior, response to treatment and known mutations [2,3]. In the United States, uveal melanoma accounts for approximately 5% of all new melanoma patients [2,4,5]. In 80–85% of the patients with uveal melanoma, the tumor arises from melanocytes in the choroid region, while the remaining tumors arise from the iris and ciliary body [1,6,7]. Uveal melanoma has a poor prognosis, as 25–31% of the patients will develop metastases within 5 years, 34–45% within 10–15 years and 49% within 25 years [8,9]. When looking at subgroups of baseline tumor dimensions (longest basal diameter and apical height), patients with smaller tumor dimensions have a lower risk of metastasizing than those with increased dimensions [9]. The liver is the most common site for metastases, and it is involved in up to 90% of cases for patients with metastatic disease. Other common metastatic sites are the lungs and skeleton [1,7,9,10,11,12].
Liver-directed therapies are being used in an attempt to cure or stabilize liver metastases in patients with the disease. This meta-analysis focuses on hepatic perfusion, but other liver-directed therapies include liver surgery, intra-arterial therapies (e.g., hepatic artery infusion/HAI, trans-arterial chemoembolization/TACE, selective internal radiotherapy/SIRT, immunoembolization/IE) and hyperthermic therapies (e.g., radio frequent ablation/RFA and laser-induced interstitial thermotherapy/LITT). Systematic reviews and meta-analyses of liver-directed therapies other than hepatic perfusion have shown that they do not provide a significantly higher survival rate as a benefit when compared to the best alternative care [13,14,15,16,17].
Isolated hepatic perfusion (IHP) was first applied 60 years ago to treat liver metastases [18]. First, the vena cava circulation is bypassed through a veno-venous shunt from the femoral vein to the external jugular vein. Then, using laparotomy, the liver is isolated from the systemic circulation. This involves extensive dissection, after which a catheter is positioned in the proper hepatic artery, while another catheter is positioned in the infrahepatic caval vein. The catheters are connected to a heart–lung machine and the liver is perfused with a high dose of chemotherapy under hyperthermia [19].
The first reported use of the minimally invasive technique to perform a percutaneous hepatic perfusion (PHP) was in 1994 [20]. In this procedure, a catheter is placed percutaneously in the proper hepatic artery for the infusion of chemotherapy. A second, double-balloon catheter is placed in the inferior caval vein to prevent leakage to the systemic circulation. The catheter is fenestrated between these balloons to aspirate the blood coming from the hepatic veins, that is then run through an extra-corporeal filtration system before returning to the patient through a third catheter in the jugular vein [21]. This minimally invasive technique was developed to reduce the morbidity and mortality related to the open procedure and to reduce the length of the procedure. Another benefit is that the percutaneous procedure can be repeated, which could potentially have a positive effect on response and survival.
Today, both IHP and PHP are used for the treatment of liver metastases from uveal melanoma, but a comparison of the two methods has never been reported in the literature. The aim of this study was to perform a systematic review and meta-analysis comparing these techniques in terms of overall survival, progression-free survival, response and complications.
2. Materials and Methods
This study was conducted in accordance with the Meta-analysis of Observational Studies (MOOSE) reporting guidelines [22]. The study protocol was designed prospectively and registered online with PROSPERO, the international prospective register of systematic reviews (PROSPERO ID CRD42021255132) [23].
2.1. Database Search Methodology
A systematic literature search was conducted across the Scopus, Ovid MEDLINE, PubMed, Web of Science and Cochrane CENTRAL electronic databases. The primary outcomes were overall and progression-free survival, with the risks of complications, mortality and response as secondary outcomes.
The search strategy carried out in the Scopus database was as follows: (uveal melanoma OR choroidal melanoma OR ciliary body melanoma OR ciliochoroidal melanoma OR iridociliary melanoma OR iris melanoma OR intraocular melanoma OR ocular melanoma) AND (metast *) AND (perfusion). For Ovid MEDLINE: (uveal melanoma OR choroidal melanoma OR ciliary body melanoma OR ciliochoroidal melanoma OR iridociliary melanoma OR iris melanoma OR intraocular melanoma OR ocular melanoma) AND (perfusion). For PubMed and Web of Science: (uveal melanoma OR choroidal melanoma OR ciliary body melanoma OR ciliochoroidal melanoma OR iridociliary melanoma OR iris melanoma OR intraocular melanoma OR ocular melanoma) AND (metast * OR stage IV) AND (perfusion). Additionally, for Cochrane CENTRAL: “(uveal neoplasms):ti,ab,kw AND (melanoma):ti,ab,kw AND (neoplasm metastasis):ti,ab,kw” (word variations have been searched). The database search was conducted on 26 May 2021.
2.2. Inclusion Criteria
The inclusion criteria were: original research studies on hepatic perfusion for uveal melanoma metastases written in English, containing search text words, basic patient characteristics and a table or Kaplan–Meier curve for survival data. Because of the evolution of liver perfusion techniques, the publication search range was set between 1 January 2000 to the present date (26 May 2021). Duplicate records were excluded. Two authors (M.S.B., R.O.B.) reviewed the remaining titles to confirm that the subject was the treatment of uveal melanoma metastases in the liver with either IHP or PHP. Review articles were excluded. Articles on animal models, laboratory investigations, imaging, primary or locally recurrent tumors, prognosis, staging and quality of life were excluded. Articles that included primary cutaneous or mucosal melanoma were excluded unless patients with uveal melanoma were reported separately. If it was uncertain whether patients in any articles from the same study group overlapped, we excluded the article(s) with fewer patients. Finally, we compared reference lists with our search and archives to identify additional articles.
2.3. Data Extraction
Data were extracted from the included articles and transferred to a standard sheet including the title, first author, year of publication, characteristics of study design and study participants as well as overall survival (OS), hepatic progression-free survival (hPFS), overall progression-free survival (PFS), response and reported morbidity and mortality. The survival times were defined as the time elapsed from treatment with either IHP or PHP to censorship or the event of interest: death for OS, tumor progression in the liver for hPFS and tumor progression in the whole body for PFS. Responses were compared between articles that reported responses according to the Response Evaluation Criteria in Solid Tumors (RECIST) and revised RECIST guideline (RECIST 1.1) [24,25]. Morbidity was compared considering Clavien–Dindo grade III or IV complications [26] (not including hematotoxic complications related to the chemotherapeutic agent used) and mortality was compared using reported 30-day mortality rates.
A database combining all available individual patient data from the different articles on OS, hPFS and PFS was created. If there were no numerical individual patient data presented in the text or a table, we used WebPlotDigitizer version 4.4 (a web-based plot digitizing tool available to users free of charge) to extract survival data from the available Kaplan–Meier plots or other graphs [27]. After launching the digitizer, users upload a screenshot of an XY chart and are then prompted to calibrate the axes. After calibrating, the user manually clicks each data point within the data series and then downloads the extracted coordinates as a spreadsheet. Studies have shown high levels of intercoder reliability and validity [28,29]. By using this application, survival times for each step for deaths, and a tick for each censored event, were obtained. Where censored events were not displayed, but a numbers-at-risk table was available, the at-risk reduction minus deaths was taken to be the number of censored events during each interval, which was then assigned to its midpoint [30]. Finally, a new Kaplan–Meier curve was created with the extracted data using SPSS, Version 27.0.1.0 (IBM SPSS Statistics for Macintosh, Armonk, NY, USA: IBM Corp.), then copied to Affinity Photo, version 1.9.3 (Serif (Europe) Ltd., Nottingham, UK), and layered with the original graph to verify that a matching curve was obtained.
2.4. Statistical Analysis
Kaplan–Meier estimations were used to assess OS, hPFS and overall PFS. OS data were censored if patients were stated as alive in descriptive text or table. The log-rank test was used to compare curves. For descriptive statistics, a Chi-square test was used to compare outcomes. p values of <0.05 were considered statistically significant. Analyses were performed using SPSS, Version 27.0.1.0 (IBM SPSS Statistics for Macintosh, Armonk, NY, USA: IBM Corp.).
3. Results
3.1. Study and Patient Characteristics
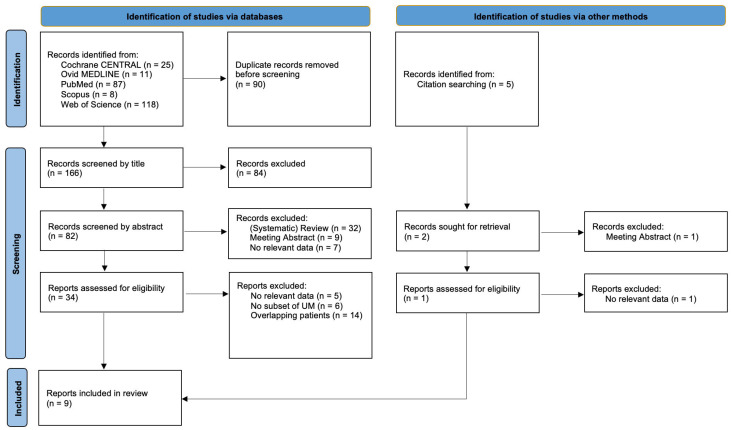
The search strategies generated 256 identified records (Scopus n = 15; Ovid MEDLINE n = 11; PubMed n = 87; Web of Science n = 118; Cochrane CENTRAL n = 25), where 90 records were excluded due to duplications. The remaining 166 titles were screened, which led to the exclusion of another 84 records. The abstracts of the remaining 82 articles were extracted and screened and another 48 articles were excluded. A citation search of the remaining articles was performed, and this identified five additional records. Of these, three were excluded based on their title. After screening the other two abstracts, only one additional full-text article was included. Finally, all 35 selected full-text articles were screened and assessed for eligibility. Five articles were excluded due to a lack of relevant data [31,32,33,34,35], seven articles were excluded due to not reporting a separate subset of patients with liver metastases of uveal melanoma [36,37,38,39,40,41,42] and fourteen articles were excluded due to an overlap in the reporting of the included patient series with other articles from the same study groups [43,44,45,46,47,48,49,50,51,52,53,54,55,56]. A more detailed description of the decisions made in the exclusion process is provided in Supplement file 1. In total, nine articles describing eight different patient series were included in the systematic review (Figure 1).
Figure 1.
Search flow diagram. UM: uveal melanoma.
The study design differed between the eight reported patient series: one combined reporting of a phase I + phase II clinical trial, one phase II clinical trial and six retrospective cohorts. There were six single-center reports and two multicenter studies. The mean number of participants in the included studies was 37 (range 19–68). Patients were treated with IHP in three and with PHP in five of the reported series (Table 1). In total, 292 patients were included in the meta-analysis, of which 128 patients underwent IHP and 164 patients PHP. The total number of PHP procedures was 367 with a median of two procedures per patient (range 1–6). Further baseline characteristics of the patients in the eight included series are provided in Table 2.
Table 1.
Patient series characteristics.
| 1st Author | Year of Publication |
Study Design | No. of Centers (Country) | Number of Patients |
Technique | Years of Inclusion |
|---|---|---|---|---|---|---|
| Alexander [57] | 2003 | Phase I + II | 1 (USA) | 29 | IHP | 1997–2002 |
| Ben-Shabat [58] | 2016 | Retrospective | 1 (SWE) | 68 | IHP | 1989–2013 |
| Brüning [59] | 2020 | Retrospective | 1 (DEU) | 19 | PHP | 2014–2019 |
| Dewald [60] | 2021 | Retrospective | 1 (DEU) | 30 | PHP | 2014–2019 |
| Estler [61] | 2021 | Retrospective | 1 (DEU) | 29 | PHP | 2015–2020 |
| Karydis [62] | 2018 | Retrospective | 2 (GBR and USA) | 51 | PHP | 2008–2016 |
| de Leede [63] | 2016 | Retrospective | 2 (NLD) | 31 | IHP | 1999–2009 |
| Meijer [64,65] | 2019 + 2021 | Phase II | 1 (NLD) | 35 | PHP | 2014–2017 |
USA: United States of America, SWE: Sweden, DEU: Germany, GBR: United Kingdom, NLD: the Netherlands.
Table 2.
Patient characteristics.
| Study | Age (Years) (Range) |
Female: Male (No. of Patients) |
Tumor Load (No. of Patients) |
Extra-Hepatic Disease (No. of Patients, %) |
|
|---|---|---|---|---|---|
| Alexander [57] | 49 (26–73) |
15:14 | <25% 25–50% >50% |
20 8 1 |
Not Included |
| Ben-Shabat [58] | 61 (18–77) |
40:28 | 1–4 met 5–10 met 11–100 met >100 met |
11 24 13 4 |
Not Included |
| Brüning [59] | 58 (range not specified) |
8:11 | Not specified | Included Number of patients not specified |
|
| Dewald [60] | 57 (52–66) |
21:9 | ≤30% >30% |
21 8 |
5 (16.7%) |
| Estler [61] | 69.7 (30–81) |
18:11 | <25% >25% |
22 7 |
12 (41.4%) |
| Karydis [62] | 57.9 (27.9–77.1) |
28:23 | 1–3 met 4–10 met >10 met |
12 23 16 |
8 (15.7%) |
| de Leede [63] | 57 (27–68) |
19:12 | <50% | 31 | Not Included |
| Meijer [64,65] | 59 (42–71) |
19:16 | 1–5 met 6–9 met ≥10 met |
9 8 18 |
Not Included |
Met: metastases.
3.2. Overall Survival
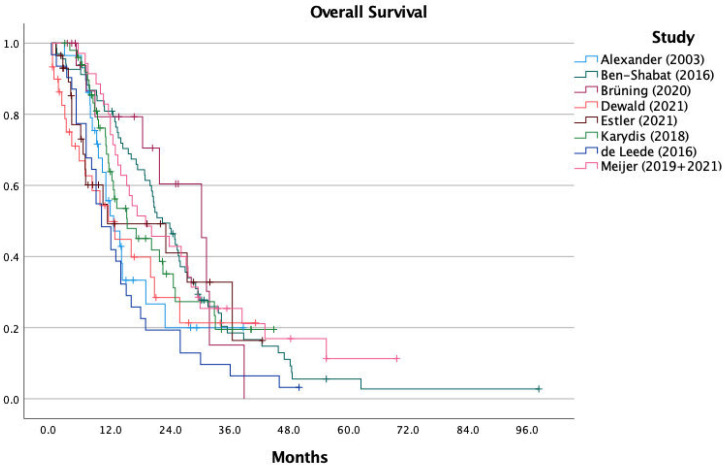
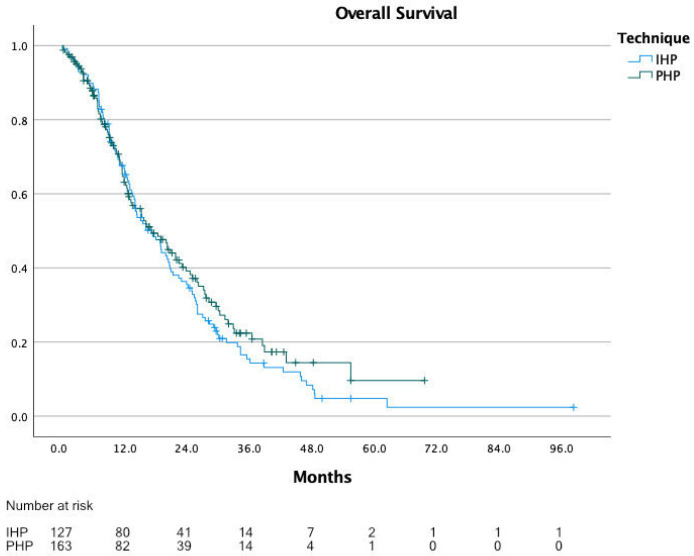
The range of the reported median OS in the eight included studies was 10.0 to 22.4 months, with a calculated median OS of 14.1 months for all patients combined. For IHP, the median OS was 12.1 months (range 10.0 to 22.4 months) and for PHP, 15.3 months (range 12.0 to 19.1 months). Figure 2 shows the KM curves of all included studies combined in one graph. For OS, individual patient data were extracted for all 292 patients, with a calculated median OS of 17.1 months for all patients, 17.1 months for IHP and 17.3 months for PHP (p = 0.366) (Figure 3).
Figure 2.
Overall survival per study.
Figure 3.
Overall survival comparing IHP vs. PHP.
3.3. Progression-Free Survival
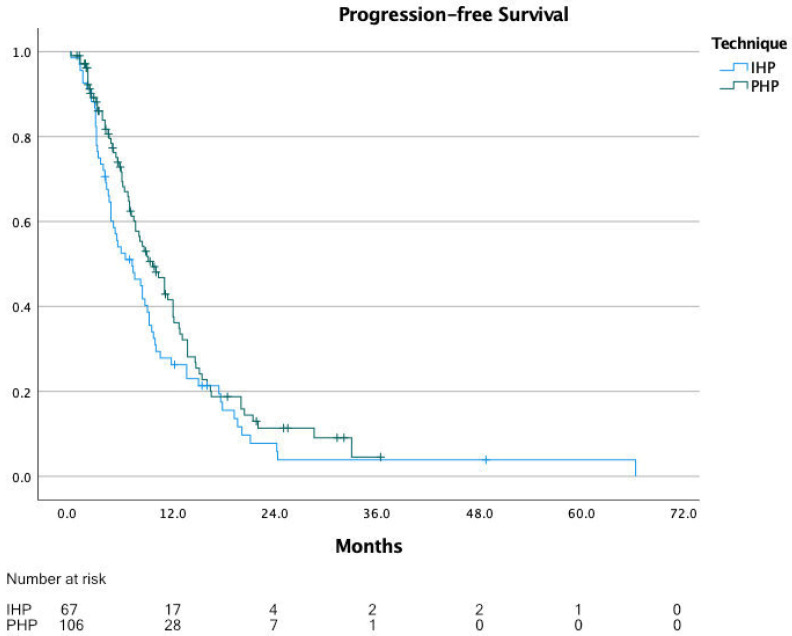
The range of the reported median PFS was 6.0 to 14.3 months, with a calculated median PFS of 7.8 months for IHP and PHP patients combined. For IHP, the median PFS was 8.0 months (range 6.0 to 13.9 months) and for PHP, 7.6 months (range 6.0 to 14.3 months). For PFS, individual patient data were extracted for 175 patients, of which 68 underwent IHP and 107 underwent PHP. The calculated median PFS from these data was 8.4 months for all patients, 7.2 months for IHP and 9.6 months for PHP (p = 0.094) (Figure 4).
Figure 4.
Progression-free survival.
3.4. Hepatic Progression-Free Survival
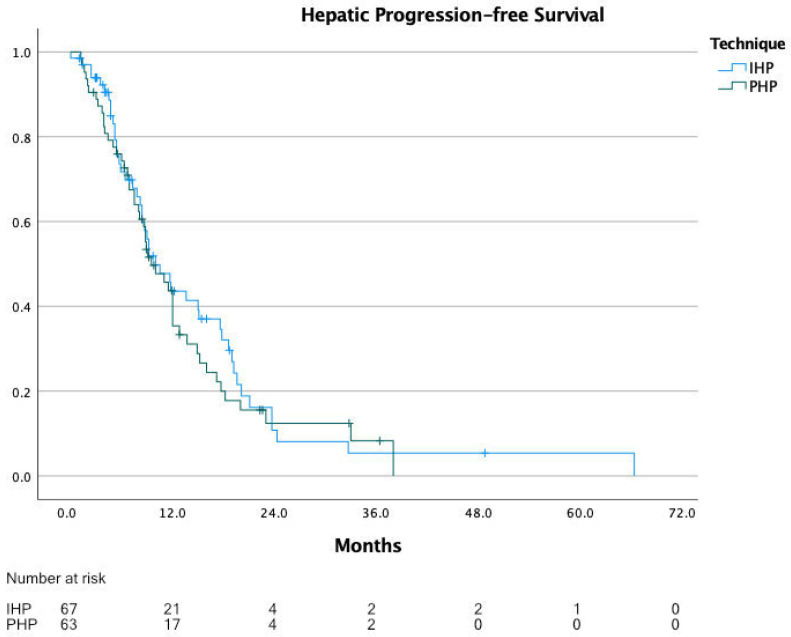
The range of reported median hPFS was 6.0 to 12.0 months, with a calculated median progression-free survival of 10.0 months for IHP and PHP patients combined. It is to be noted that three studies did not report hPFS. For IHP, the median hPFS was 11.0 months (range 10.0 to 12.0 months), and for PHP, 9.1 months (range 6.0 to 11.2 months). For hPFS, individual patient data were extracted for 131 patients, of which 68 underwent IHP and 63 underwent PHP. The calculated median hPFS from these data was 10.0 months for all patients, 10.0 months for IHP and 9.5 months for PHP (p = 0.544) (Figure 5).
Figure 5.
Hepatic progression-free survival.
3.5. Complications and Mortality
The three studies on IHP reported a total of 50 Clavien–Dindo Grade III or IV complications in a total of 128 patients (39.1%) and the five studies on PHP reported a total of 39 complications in a total of 164 patients (23.8%) (p = 0.005). The reported 30-day mortality for IHP was seven patients (5.5%) and for PHP, three patients (1.8%) (p = 0.09). When calculated in relation to the number of performed PHP procedures (367 in total), and compared to IHP, the Clavien–Dindo Grade III or IV complication rate was 10.8% (p < 0.001) and the 30-day mortality rate was 0.8% (p = 0.001).
3.6. Response
Two studies on IHP reported response rates, including 15 complete responses (15.5%, p < 0.001) and 44 partial responses (45.4%) in a total of 97 patients. The five studies on PHP reported 4 complete responses (2.4%) and 76 partial responses (46.3%) in a total of 164 patients.
4. Discussion
The calculated OS data show that there is no difference between IHP and PHP for patients with liver metastases from uveal melanoma in the long term, but that patients have a significantly lower risk of complications and mortality following PHP.
All three studies reporting on IHP excluded patients with extra-hepatic disease, whereas four out of the five studies on PHP included patients with extra-hepatic disease if it was either “limited” or the patients had a liver-dominant metastasizing pattern. The study that included most patients with extra-hepatic disease (12 out of 29 patients) found no difference in OS between patients with or without these extra-hepatic metastases at the time of PHP [61]. One study on IHP excluded patients with a tumor burden of more than 50% of the liver volume [63]. In another study, these patients were excluded in the last of three periods, because tumor burden >50% was associated with higher mortality for the patients included in the first two periods [58]. For PHP, only one study mentions a maximum tumor burden of 60% [64,65]. Of all patients included in the IHP studies, the majority did not receive any prior treatment for their liver metastases. In at least two out of the five articles on PHP, most of the patients had prior treatment for their liver metastases. All the previously mentioned exclusion criteria, or characteristics of the included patients, could imply a selection bias from which survival rates after IHP could benefit.
Overall survival data could be extracted for all reported patients in the eight included studies. The difference in outcome, when comparing the results of the reported median survival data or from the combined extracted data, shows that the extraction process is of value. Longer OS for PHP was to be expected as repeated procedures are possible and were performed in most patients, but this was not shown by the data. It might be that the normothermic perfusion during PHP is less effective than the hyperthermic perfusion during IHP [66]. This hypothesis can be supported by the fact that significantly more patients have a complete response after IHP, although this does not lead to a significantly longer hPFS for IHP. The latter may be caused by higher mortality and complication rates after IHP, which may have also limited the duration of response. Survival might also have been influenced by the available systemic treatments over the included years, as the patients treated with IHP were included between 1989 and 2013 and those treated with PHP between 2008 and2020.
PHP proves to be safer than IHP in this meta-analysis. The higher mortality in the IHP group is possibly partially due to the period in which the patients were included. The study on IHP that reports the highest mortality rate (5/68 patients) used different chemotherapeutic strategies, higher temperatures, and included patients with tumor burden over 50% in the earlier years of their cohort. TNF-alpha was not used after the first period in this study due to higher mortality. The dose of melphalan was lowered after the second period in this study, in combination with excluding patients with tumor burden over 50%, due to higher mortality [37,58]. After these changes, the mortality rate was only 1/59 patients (2.5%) [58], comparable to the 1.8% mortality (3/164 patients) reported for PHP. Higher occurrence of Clavien–Dindo grade III and IV complications after IHP can probably be related to major abdominal surgery and to the inclusion of patients with a high tumor burden as well. These complications might even have an influence on the duration of response and/or survival.
To evaluate response, all studies reporting on PHP used the RECIST 1.1 guidelines. In summary, this means that partial response (PR) was defined as a decrease in volume of 30% or more from baseline measurements, and progressive disease (PD) as an increase in volume of 20% or more or one or more new lesions. All the studies reporting on IHP used different definitions. Ben-Shabat et al. used a decrease in volume of 30% or more for PR as well, but used any increase in volume or new lesions as the definition for PD [58]. Alexander et al. used a decrease in volume of 50% or more for PR and an increase in volume of 25% or more as definition for PD [57]. De Leede et al. provide no definition for PR and used an increase in volume of 25% or more as a definition for PD [63]. These differences in definitions could possibly have led to lower partial response rates and longer hepatic progression-free survival times in the IHP group.
Differences in the format of the results presented and methodologies used made it challenging to pool the data from the original publications. For example, some articles did not report definitions on survival times. Others did not supply censored events or at-risk tables along with Kaplan-–Meier curves, so we used estimations for the unreported data to recreate the curves. After combining multiple publications, which increased the number of deaths and censored events, and by adding the extra step of checking that the original and recreated curves overlap, the error margin of the estimated data probably falls within a couple of weeks of the actual survival. Regarding conflicts of interest or possible publication bias, two studies were fully or partially funded by industry, with authors reporting a conflict of interest, or both. One study was externally funded but not by the industry. In four studies, neither the industry nor any external funding was involved and for one study, such information was not available.
After the approval of checkpoint inhibitors for metastasized uveal melanoma, patients more recently diagnosed with progression could probably have a prolonged survival which is not due to their liver-directed treatment. Immunotherapy with nivolumab, pembrolizumab and/or ipilimumab has shown to only have a limited effect for patients with uveal melanoma [13,14,15,16,67,68,69,70,71]. A recent phase II trial combining epigenetic therapy using the HDAC inhibitor entinostat with the PD-1 inhibitor pembrolizumab resulted in durable responses in a subset of patients [72]. Even more promising results were obtained in a phase III study using tebentafusp, a bispecific fusion protein linking melanoma cells with T-cells. This is the first phase III trial to show a significant improvement in OS (22 months vs. 16 months); however, the treatment is only available for patients with an HLA-A*02 serotype, and the increase in survival is still modest compared to the effects of immunotherapy in cutaneous melanoma [73].
The last question, that this meta-analysis cannot answer, is whether hepatic perfusion in any form gives patients a survival benefit over the “best alternative care”. There are currently two ongoing randomized controlled trials on this topic. The SCANDIUM trial is a phase III randomized controlled multicenter trial evaluating whether IHP increases overall survival in comparison with the best alternative care in patients with isolated liver metastases from uveal melanoma [74]. The inclusion for the SCANDIUM trial has just been completed. The FOCUS trial is a phase III randomized controlled multicenter trial comparing PHP with the best alternative care in patients with liver-dominant metastases from uveal melanoma [75]. The preliminary results from the FOCUS trial were recently presented at the 2021 ASCO Annual Meeting, showing a progression-free survival of 9.03 months following PHP compared to 3.06 months with the best alternative care [76]. We eagerly await the survival outcomes of these two trials.
5. Conclusions
Overall survival data show that there is no difference between IHP and PHP for patients with liver metastases from uveal melanoma in the long term, but patients could potentially have a lower risk of complications and mortality following PHP.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/cancers13184726/s1, Supplement file 1: full-text article selection process.
Author Contributions
Conceptualization, M.S.B. and R.O.B.; methodology, M.S.B. and R.O.B.; validation, D.K.; formal analysis, M.S.B. and R.O.B.; investigation, M.S.B. and R.O.B.; resources, R.O.B.; data curation, M.S.B.; writing—original draft preparation, M.S.B.; writing—review and editing, D.K. and R.O.B.; visualization, M.S.B. and R.O.B.; supervision, R.O.B.; project administration, R.O.B.; funding acquisition, R.O.B. All authors have read and agreed to the published version of the manuscript.
Funding
Knut and Alice Wallenberg Foundation: 2021, Wallenberg Centre for Molecular and Translational Medicine, University of Gothenburg, Sweden.
Conflicts of Interest
R.O.B. has received institutional research grants from Bristol-Myers Squibb (BMS) and SkyLineDx, speaker honorarium from Roche and Pfizer and has served on advisory boards for Amgen, BD/BARD, Bristol-Myers Squibb (BMS), Merck Sharp and Dohme (MSD), Novartis, Roche and Sanofi Genzyme. The other two authors (M.S.B. and D.K.) declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jovanovic P., Mihajlovic M., Djordjevic-Jocic J., Vlajkovic S., Cekic S., Stefanovic V. Ocular melanoma: An overview of the current status. Int. J. Clin. Exp. Pathol. 2013;6:1230–1244. [PMC free article] [PubMed] [Google Scholar]
- 2.Amaro A., Gangemi R., Piaggio F., Angelini G., Barisione G., Ferrini S., Pfeffer U. The biology of uveal melanoma. Cancer Metastasis Rev. 2017;36:109–140. doi: 10.1007/s10555-017-9663-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vidwans S.J., Flaherty K.T., Fisher D.E., Tenenbaum J.M., Travers M.D., Shrager J. A melanoma molecular disease model. PLoS ONE. 2011;6:e18257. doi: 10.1371/journal.pone.0018257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jager M.J., Shields C.L., Cebulla C.M., Abdel-Rahman M.H., Grossniklaus H.E., Stern M.H., Carvajal R.D., Belfort R.N., Jia R., Shields J.A., et al. Uveal melanoma. Nat. Rev. Dis. Primers. 2020;6:24. doi: 10.1038/s41572-020-0158-0. [DOI] [PubMed] [Google Scholar]
- 5.Shoushtari A.N., Carvajal R.D. Treatment of Uveal Melanoma. Cancer Treat. Res. 2016;167:281–293. doi: 10.1007/978-3-319-22539-5_12. [DOI] [PubMed] [Google Scholar]
- 6.Bronkhorst I.H., Jager M.J. Inflammation in uveal melanoma. Eye. 2013;27:217–223. doi: 10.1038/eye.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isager P., Engholm G., Overgaard J., Storm H. Uveal and conjunctival malignant melanoma in Denmark 1943–1997: Observed and relative survival of patients followed through 2002. Ophthalmic Epidemiol. 2006;13:85–96. doi: 10.1080/09286580600553330. [DOI] [PubMed] [Google Scholar]
- 8.Kujala E., Makitie T., Kivela T. Very long-term prognosis of patients with malignant uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2003;44:4651–4659. doi: 10.1167/iovs.03-0538. [DOI] [PubMed] [Google Scholar]
- 9.Diener-West M., Reynolds S.M., Agugliaro D.J., Caldwell R., Cumming K., Earle J.D., Hawkins B.S., Hayman J.A., Jaiyesimi I., Jampol L.M., et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch. Ophthalmol. 2005;123:1639–1643. doi: 10.1001/archopht.123.12.1639. [DOI] [PubMed] [Google Scholar]
- 10.Carvajal R.D., Schwartz G.K., Tezel T., Marr B., Francis J.H., Nathan P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017;101:38–44. doi: 10.1136/bjophthalmol-2016-309034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spagnolo F., Caltabiano G., Queirolo P. Uveal melanoma. Cancer Treat. Rev. 2012;38:549–553. doi: 10.1016/j.ctrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Singh A.D., Turell M.E., Topham A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology. 2011;118:1881–1885. doi: 10.1016/j.ophtha.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Agarwala S.S., Eggermont A.M., O’Day S., Zager J.S. Metastatic melanoma to the liver: A contemporary and comprehensive review of surgical, systemic, and regional therapeutic options. Cancer. 2014;120:781–789. doi: 10.1002/cncr.28480. [DOI] [PubMed] [Google Scholar]
- 14.Khoja L., Atenafu E.G., Suciu S., Leyvraz S., Sato T., Marshall E., Keilholz U., Zimmer L., Patel S.P., Piperno-Neumann S., et al. Meta-analysis in metastatic uveal melanoma to determine progression free and overall survival benchmarks: An international rare cancers initiative (IRCI) ocular melanoma study. Ann. Oncol. 2019;30:1370–1380. doi: 10.1093/annonc/mdz176. [DOI] [PubMed] [Google Scholar]
- 15.Rantala E.S., Hernberg M., Kivela T.T. Overall survival after treatment for metastatic uveal melanoma: A systematic review and meta-analysis. Melanoma Res. 2019;29:561–568. doi: 10.1097/CMR.0000000000000575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodriguez-Vidal C., Fernandez-Diaz D., Fernandez-Marta B., Lago-Baameiro N., Pardo M., Silva P., Paniagua L., Blanco-Teijeiro M.J., Pineiro A., Bande M. Treatment of Metastatic Uveal Melanoma: Systematic Review. Cancers. 2020;12:2557. doi: 10.3390/cancers12092557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowcroft A., Loveday B.P.T., Thomson B.N.J., Banting S., Knowles B. Systematic review of liver directed therapy for uveal melanoma hepatic metastases. HPB. 2020;22:497–505. doi: 10.1016/j.hpb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 18.Ausman R.K. Development of a technic for isolated perfusion of the liver. N. Y. State J. Med. 1961;61:3993–3997. [PubMed] [Google Scholar]
- 19.Ben-Shabat I., Hansson C., Sternby Eilard M., Cahlin C., Rizell M., Lindner P., Mattsson J., Olofsson Bagge R. Isolated hepatic perfusion as a treatment for liver metastases of uveal melanoma. J. Vis. Exp. 2015:52490. doi: 10.3791/52490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ravikumar T.S., Pizzorno G., Bodden W., Marsh J., Strair R., Pollack J., Hendler R., Hanna J., D’Andrea E. Percutaneous hepatic vein isolation and high-dose hepatic arterial infusion chemotherapy for unresectable liver tumors. J. Clin. Oncol. 1994;12:2723–2736. doi: 10.1200/JCO.1994.12.12.2723. [DOI] [PubMed] [Google Scholar]
- 21.De Leede E.M., Burgmans M.C., Martini C.H., Tijl F.G., van Erkel A.R., Vuyk J., Kapiteijn E., Verhoef C., van de Velde C.J., Vahrmeijer A.L. Percutaneous Hepatic Perfusion (PHP) with Melphalan as a Treatment for Unresectable Metastases Confined to the Liver. J. Vis. Exp. 2016 doi: 10.3791/53795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brooke B.S., Schwartz T.A., Pawlik T.M. MOOSE Reporting Guidelines for Meta-analyses of Observational Studies. JAMA Surg. 2021;156:787–788. doi: 10.1001/jamasurg.2021.0522. [DOI] [PubMed] [Google Scholar]
- 23.PROSPERO International Prospective Register of Systematic Reviews. [(accessed on 21 June 2021)]. Available online: https://www.crd.york.ac.uk/PROSPERO/
- 24.Therasse P., Arbuck S.G., Eisenhauer E.A., Wanders J., Kaplan R.S., Rubinstein L., Verweij J., Van Glabbeke M., van Oosterom A.T., Christian M.C., et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 25.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rohatgi A. WebPlotDigitizer (Version 4.4) 2020. [(accessed on 11 May 2021)]. Available online: https://automeris.io/WebPlotDigitizer.
- 28.Drevon D., Fursa S.R., Malcolm A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017;41:323–339. doi: 10.1177/0145445516673998. [DOI] [PubMed] [Google Scholar]
- 29.Moeyaert M., Maggin D., Verkuilen J. Reliability, Validity, and Usability of Data Extraction Programs for Single-Case Research Designs. Behav. Modif. 2016;40:874–900. doi: 10.1177/0145445516645763. [DOI] [PubMed] [Google Scholar]
- 30.Earle C.C., Pham B., Wells G.A. An assessment of methods to combine published survival curves. Med. Decis. Mak. 2000;20:104–111. doi: 10.1177/0272989X0002000113. [DOI] [PubMed] [Google Scholar]
- 31.Pingpank J.F., Libutti S.K., Chang R., Wood B.J., Neeman Z., Kam A.W., Figg W.D., Zhai S., Beresneva T., Seidel G.D., et al. Phase I study of hepatic arterial melphalan infusion and hepatic venous hemofiltration using percutaneously placed catheters in patients with unresectable hepatic malignancies. J. Clin. Oncol. 2005;23:3465–3474. doi: 10.1200/JCO.2005.00.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heusner T.A., Antoch G., Wittkowski-Sterczewski A., Ladd S.C., Forsting M., Verhagen R., Scheulen M. Transarterial hepatic chemoperfusion of uveal melanoma metastases: Survival and response to treatment. Fortschr Röntgenstr. 2011;183:1151–1160. doi: 10.1055/s-0031-1281743. [DOI] [PubMed] [Google Scholar]
- 33.Buzzacco D.M., Abdel-Rahman M.H., Park S., Davidorf F., Olencki T., Cebulla C.M. Long-term survivors with metastatic uveal melanoma. Open Ophthalmol. J. 2012;6:49–53. doi: 10.2174/1874364101206010049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ludwig J., Haubold J., Heusner T.A., Bauer S., Siveke J.T., Richly H., Wetter A., Umutlu L., Theysohn J.M. Lactate Dehydrogenase Prior to Transarterial Hepatic Chemoperfusion Predicts Survival and Time to Progression in Patients with Uveal Melanoma Liver Metastases. Fortschr Röntgenstr. 2021;193:683–691. doi: 10.1055/a-1299-1627. [DOI] [PubMed] [Google Scholar]
- 35.Teal L., Yorio J. Fulminant Hepatic Failure after Chemosaturation with Percutaneous Hepatic Perfusion and Nivolumab in a Patient with Metastatic Uveal Melanoma. Case Rep. Oncol. Med. 2021;2021:8870334. doi: 10.1155/2021/8870334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libutti S.K., Barlett D.L., Fraker D.L., Alexander H.R. Technique and results of hyperthermic isolated hepatic perfusion with tumor necrosis factor and melphalan for the treatment of unresectable hepatic malignancies. J. Am. Coll. Surg. 2000;191:519–530. doi: 10.1016/S1072-7515(00)00733-X. [DOI] [PubMed] [Google Scholar]
- 37.Rizell M., Mattson J., Cahlin C., Hafstrom L., Lindner P., Olausson M. Isolated hepatic perfusion for liver metastases of malignant melanoma. Melanoma Res. 2008;18:120–126. doi: 10.1097/CMR.0b013e3282f8e3c9. [DOI] [PubMed] [Google Scholar]
- 38.Magge D., Choudry H.A., Zeh H.J., 3rd, Cunningham D.E., Steel J., Holtzman M.P., Jones H.L., Pingpank J.F., Bartlett D.L., Zureikat A.H. Outcome analysis of a decade-long experience of isolated hepatic perfusion for unresectable liver metastases at a single institution. Ann. Surg. 2014;259:953–959. doi: 10.1097/SLA.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 39.Vogl T.J., Zangos S., Scholtz J.E., Schmitt F., Paetzold S., Trojan J., Orsi F., Lotz G., Ferrucci P. Chemosaturation with percutaneous hepatic perfusions of melphalan for hepatic metastases: Experience from two European centers. Fortschr Röntgenstr. 2014;186:937–944. doi: 10.1055/s-0034-1366081. [DOI] [PubMed] [Google Scholar]
- 40.Hughes M.S., Zager J., Faries M., Alexander H.R., Royal R.E., Wood B., Choi J., McCluskey K., Whitman E., Agarwala S., et al. Results of a Randomized Controlled Multicenter Phase III Trial of Percutaneous Hepatic Perfusion Compared with Best Available Care for Patients with Melanoma Liver Metastases. Ann. Surg. Oncol. 2016;23:1309–1319. doi: 10.1245/s10434-015-4968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbott A.M., Doepker M.P., Kim Y., Perez M.C., Gandle C., Thomas K.L., Choi J., Shridhar R., Zager J.S. Hepatic Progression-free and Overall Survival After Regional Therapy to the Liver for Metastatic Melanoma. Am. J. Clin. Oncol. 2018;41:747–753. doi: 10.1097/COC.0000000000000356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dewald C.L.A., Becker L.S., Maschke S.K., Meine T.C., Alten T.A., Kirstein M.M., Vogel A., Wacker F.K., Meyer B.C., Hinrichs J.B. Percutaneous isolated hepatic perfusion (chemosaturation) with melphalan following right hemihepatectomy in patients with cholangiocarcinoma and metastatic uveal melanoma: Peri- and post-interventional adverse events and therapy response compared to a matched group without prior liver surgery. Clin. Exp. Metastasis. 2020;37:683–692. doi: 10.1007/s10585-020-10057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noter S.L., Rothbarth J., Pijl M.E., Keunen J.E., Hartgrink H.H., Tijl F.G., Kuppen P.J., van de Velde C.J., Tollenaar R.A. Isolated hepatic perfusion with high-dose melphalan for the treatment of uveal melanoma metastases confined to the liver. Melanoma Res. 2004;14:67–72. doi: 10.1097/00008390-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 44.Van Iersel L.B., Hoekman E.J., Gelderblom H., Vahrmeijer A.L., van Persijn van Meerten E.L., Tijl F.G., Hartgrink H.H., Kuppen P.J., Nortier J.W., Tollenaar R.A., et al. Isolated hepatic perfusion with 200 mg melphalan for advanced noncolorectal liver metastases. Ann. Surg. Oncol. 2008;15:1891–1898. doi: 10.1245/s10434-008-9881-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Iersel L.B., de Leede E.M., Vahrmeijer A.L., Tijl F.G., den Hartigh J., Kuppen P.J., Hartgrink H.H., Gelderblom H., Nortier J.W., Tollenaar R.A., et al. Isolated hepatic perfusion with oxaliplatin combined with 100 mg melphalan in patients with metastases confined to the liver: A phase I study. Eur. J. Surg. Oncol. 2014;40:1557–1563. doi: 10.1016/j.ejso.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 46.Van Etten B., Brunstein F., van I.M.G., Marinelli A.W., Verhoef C., van der Sijp J.R., Guetens G., de Boeck G., de Bruijn E.A., de Wilt J.H., et al. Isolated hypoxic hepatic perfusion with orthograde or retrograde flow in patients with irresectable liver metastases using percutaneous balloon catheter techniques: A phase I and II study. Ann. Surg. Oncol. 2004;11:598–605. doi: 10.1245/ASO.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 47.Verhoef C., de Wilt J.H., Brunstein F., Marinelli A.W., van Etten B., Vermaas M., Guetens G., de Boeck G., de Bruijn E.A., Eggermont A.M. Isolated hypoxic hepatic perfusion with retrograde outflow in patients with irresectable liver metastases; a new simplified technique in isolated hepatic perfusion. Ann. Surg. Oncol. 2008;15:1367–1374. doi: 10.1245/s10434-007-9714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Etten B., de Wilt J.H., Brunstein F., Eggermont A.M., Verhoef C. Isolated hypoxic hepatic perfusion with melphalan in patients with irresectable ocular melanoma metastases. Eur. J. Surg. Oncol. 2009;35:539–545. doi: 10.1016/j.ejso.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Kirstein M.M., Marquardt S., Jedicke N., Marhenke S., Koppert W., Manns M.P., Wacker F., Vogel A. Safety and efficacy of chemosaturation in patients with primary and secondary liver tumors. J. Cancer Res. Clin. Oncol. 2017;143:2113–2121. doi: 10.1007/s00432-017-2461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogl T.J., Koch S.A., Lotz G., Gebauer B., Willinek W., Engelke C., Bruning R., Zeile M., Wacker F., Vogel A., et al. Percutaneous Isolated Hepatic Perfusion as a Treatment for Isolated Hepatic Metastases of Uveal Melanoma: Patient Outcome and Safety in a Multi-centre Study. Cardiovasc. Interv. Radiol. 2017;40:864–872. doi: 10.1007/s00270-017-1588-2. [DOI] [PubMed] [Google Scholar]
- 51.Schonfeld L., Hinrichs J.B., Marquardt S., Voigtlander T., Dewald C., Koppert W., Manns M.P., Wacker F., Vogel A., Kirstein M.M. Chemosaturation with percutaneous hepatic perfusion is effective in patients with ocular melanoma and cholangiocarcinoma. J. Cancer Res. Clin. Oncol. 2020;146:3003–3012. doi: 10.1007/s00432-020-03289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Artzner C., Mossakowski O., Hefferman G., Grosse U., Hoffmann R., Forschner A., Eigentler T., Syha R., Grozinger G. Chemosaturation with percutaneous hepatic perfusion of melphalan for liver-dominant metastatic uveal melanoma: A single center experience. Cancer Imaging. 2019;19:31. doi: 10.1186/s40644-019-0218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Olofsson R., Cahlin C., All-Ericsson C., Hashimi F., Mattsson J., Rizell M., Lindner P. Isolated hepatic perfusion for ocular melanoma metastasis: Registry data suggests a survival benefit. Ann. Surg. Oncol. 2014;21:466–472. doi: 10.1245/s10434-013-3304-z. [DOI] [PubMed] [Google Scholar]
- 54.Forster M.R., Rashid O.M., Perez M.C., Choi J., Chaudhry T., Zager J.S. Chemosaturation with percutaneous hepatic perfusion for unresectable metastatic melanoma or sarcoma to the liver: A single institution experience. J. Surg. Oncol. 2014;109:434–439. doi: 10.1002/jso.23501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alexander H.R., Libutti S.K., Bartlett D.L., Puhlmann M., Fraker D.L., Bachenheimer L.C. A phase I-II study of isolated hepatic perfusion using melphalan with or without tumor necrosis factor for patients with ocular melanoma metastatic to liver. Clin. Cancer Res. 2000;6:3062–3070. [PubMed] [Google Scholar]
- 56.Varghese S., Xu H., Bartlett D., Hughes M., Pingpank J.F., Beresnev T., Alexander H.R., Jr. Isolated hepatic perfusion with high-dose melphalan results in immediate alterations in tumor gene expression in patients with metastatic ocular melanoma. Ann. Surg. Oncol. 2010;17:1870–1877. doi: 10.1245/s10434-010-0998-z. [DOI] [PubMed] [Google Scholar]
- 57.Alexander H.R., Jr., Libutti S.K., Pingpank J.F., Steinberg S.M., Bartlett D.L., Helsabeck C., Beresneva T. Hyperthermic isolated hepatic perfusion using melphalan for patients with ocular melanoma metastatic to liver. Clin. Cancer Res. 2003;9:6343–6349. doi: 10.1016/j.ajo.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 58.Ben-Shabat I., Belgrano V., Ny L., Nilsson J., Lindner P., Olofsson Bagge R. Long-Term Follow-Up Evaluation of 68 Patients with Uveal Melanoma Liver Metastases Treated with Isolated Hepatic Perfusion. Ann. Surg. Oncol. 2016;23:1327–1334. doi: 10.1245/s10434-015-4982-5. [DOI] [PubMed] [Google Scholar]
- 59.Bruning R., Tiede M., Schneider M., Wohlmuth P., Weilert H., Oldhafer K., Stang A. Unresectable Hepatic Metastasis of Uveal Melanoma: Hepatic Chemosaturation with High-Dose Melphalan-Long-Term Overall Survival Negatively Correlates with Tumor Burden. Radiol. Res. Pract. 2020;2020:5672048. doi: 10.1155/2020/5672048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dewald C.L.A., Hinrichs J.B., Becker L.S., Maschke S., Meine T.C., Saborowski A., Schonfeld L.J., Vogel A., Kirstein M.M., Wacker F.K. Chemosaturation with Percutaneous Hepatic Perfusion: Outcome and Safety in Patients with Metastasized Uveal Melanoma. Fortschr Röntgenstr. 2021;193:928–936. doi: 10.1055/a-1348-1932. [DOI] [PubMed] [Google Scholar]
- 61.Estler A., Artzner C., Bitzer M., Nikolaou K., Hoffmann R., Hepp T., Hagen F., Eigentler T., Forschner A., Grozinger G. Efficacy and tolerability of chemosaturation in patients with hepatic metastases from uveal melanoma. Acta Radiol. 2021:2841851211019808. doi: 10.1177/02841851211019808. [DOI] [PubMed] [Google Scholar]
- 62.Karydis I., Gangi A., Wheater M.J., Choi J., Wilson I., Thomas K., Pearce N., Takhar A., Gupta S., Hardman D., et al. Percutaneous hepatic perfusion with melphalan in uveal melanoma: A safe and effective treatment modality in an orphan disease. J. Surg. Oncol. 2018;117:1170–1178. doi: 10.1002/jso.24956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Leede E.M., Burgmans M.C., Kapiteijn E., Luyten G.P., Jager M.J., Tijl F.G., Hartgrink H.H., Grunhagen D.J., Rothbarth J., van de Velde C.J., et al. Isolated (hypoxic) hepatic perfusion with high-dose chemotherapy in patients with unresectable liver metastases of uveal melanoma: Results from two experienced centres. Melanoma Res. 2016;26:588–594. doi: 10.1097/CMR.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 64.Meijer T.S., Burgmans M.C., Fiocco M., de Geus-Oei L.F., Kapiteijn E., de Leede E.M., Martini C.H., van der Meer R.W., Tijl F.G.J., Vahrmeijer A.L. Safety of Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Liver Metastases from Ocular Melanoma Using the Delcath Systems’ Second-Generation Hemofiltration System: A Prospective Non-randomized Phase II Trial. Cardiovasc. Interv. Radiol. 2019;42:841–852. doi: 10.1007/s00270-019-02177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meijer T.S., Burgmans M.C., de Leede E.M., de Geus-Oei L.F., Boekestijn B., Handgraaf H.J.M., Hilling D.E., Lutjeboer J., Vuijk J., Martini C.H., et al. Percutaneous Hepatic Perfusion with Melphalan in Patients with Unresectable Ocular Melanoma Metastases Confined to the Liver: A Prospective Phase II Study. Ann. Surg. Oncol. 2021;28:1130–1141. doi: 10.1245/s10434-020-08741-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Quadri H.S., Payabyab E.C., Chen D.J., Figg W., Hughes M.S. Percutaneous hepatic perfusion with melphalan for unresectable liver metastasis. Hepatoma Res. 2016;2:197–202. doi: 10.20517/2394-5079.2016.24. [DOI] [Google Scholar]
- 67.Heppt M.V., Amaral T., Kahler K.C., Heinzerling L., Hassel J.C., Meissner M., Kreuzberg N., Loquai C., Reinhardt L., Utikal J., et al. Combined immune checkpoint blockade for metastatic uveal melanoma: A retrospective, multi-center study. J. Immunother. Cancer. 2019;7:299. doi: 10.1186/s40425-019-0800-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Heppt M.V., Heinzerling L., Kahler K.C., Forschner A., Kirchberger M.C., Loquai C., Meissner M., Meier F., Terheyden P., Schell B., et al. Prognostic factors and outcomes in metastatic uveal melanoma treated with programmed cell death-1 or combined PD-1/cytotoxic T-lymphocyte antigen-4 inhibition. Eur. J. Cancer. 2017;82:56–65. doi: 10.1016/j.ejca.2017.05.038. [DOI] [PubMed] [Google Scholar]
- 69.Algazi A.P., Tsai K.K., Shoushtari A.N., Munhoz R.R., Eroglu Z., Piulats J.M., Ott P.A., Johnson D.B., Hwang J., Daud A.I., et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122:3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zimmer L., Vaubel J., Mohr P., Hauschild A., Utikal J., Simon J., Garbe C., Herbst R., Enk A., Kampgen E., et al. Phase II DeCOG-study of ipilimumab in pretreated and treatment-naive patients with metastatic uveal melanoma. PLoS ONE. 2015;10:e0118564. doi: 10.1371/journal.pone.0118564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mignard C., Deschamps Huvier A., Gillibert A., Duval Modeste A.B., Dutriaux C., Khammari A., Avril M.F., Kramkimel N., Mortier L., Marcant P., et al. Efficacy of Immunotherapy in Patients with Metastatic Mucosal or Uveal Melanoma. J. Oncol. 2018;2018:1908065. doi: 10.1155/2018/1908065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ny L., Jespersen H., Karlsson J., Alsen S., Filges S., All-Eriksson C., Andersson B., Carneiro A., Helgadottir H., Levin M., et al. The PEMDAC phase 2 study of pembrolizumab and entinostat in patients with metastatic uveal melanoma. Nat. Commun. 2021;12:5155. doi: 10.1038/s41467-021-25332-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piperno-Neumann S., Hassel J.C., Rutkowski P., Baurain J., Butler M.O., Schlaak M., Sullivan R.J., Ochsenreither S., Dummer R., Kirkwood J.M., et al. Phase 3 randomized trial comparing tebentafusp with investigator’s choice in first line metastatic uveal melanoma; Proceedings of the Proceedings of the 112th Annual Meeting of the American Association for Cancer Research; Philadelphia, PA, USA. 10–15 April 2021; 17–21 May 2021.. [Google Scholar]
- 74.Olofsson R., Ny L., Eilard M.S., Rizell M., 2021Cahlin C., Stierner U., Lonn U., Hansson J., Ljuslinder I., Lundgren L., et al. Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): Study protocol for a randomized controlled trial. Trials. 2014;15:317. doi: 10.1186/1745-6215-15-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. NCT02678572, Percutaneous Hepatic Perfusion vs. Best Alternative Care in Patients with Hepatic-dominant Ocular Melanoma. [(accessed on 10 June 2021)];2016 Available online: https://clinicaltrials.gov/show/NCT02678572.
- 76.Zager J.S., Orloff M., Ferrucci P.F., Glazer E.S., Ejaz A., Richtig E., Ochsenreither S., Lowe M.C., Reddy S.A., Beasley G., et al. Percutaneous hepatic perfusion (PHP) with melphalan for patients with ocular melanoma liver metastases: Preliminary results of FOCUS (PHP-OCM-301/301A) phase III trial. J. Clin. Oncol. 2021;39:9510. doi: 10.1200/JCO.2021.39.15_suppl.9510. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.