Abstract
Background
It was shown that immunocompromised patients have significantly reduced immunologic responses to COVID-19 vaccines. The immunogenicity of COVID-19 vaccine/infection in patients with solid tumors is reduced. We evaluated the immunologic response to COVID-19 and/or the BNT162b2 mRNA COVID-19 vaccine among cancer patients on active treatments and reviewed previous literature to identify subgroups that may require third vaccination.
Patients and methods
Anti-SARS-CoV-2 S1/S2 antibodies were measured in a cohort of 202 cancer patients on active treatment with chemotherapy (96), immunologic (52), biologic (46), and hormonal (12) treatments for early (n = 66, 32.7%) or metastatic disease (n = 136, 67.3%). Of those, 172 had received two vaccine doses, and 30 had COVID-19 infection (20/30 also received one dose of vaccine). Specific anti-S receptor-binding domain antibodies were further measured in patients with equivocal anti-S1/S2 results.
Results
Among cancer patients, the SARS-CoV-2 antibody response rate was 89.1% (180/202) after COVID-19 vaccination or infection and 87.2% (150/172) in patients after vaccination without a history of COVID-19, compared with 100% positive serologic tests in a control group of 30 health care workers (P < 0.001). Chemotherapy treatment was independently associated with significantly reduced humoral response to infection or vaccination, with an 81.3% response rate, compared with 96.2% in patients on other treatments (P = 0.001). In vaccinated patients on chemotherapy, the positive response rate was 77.5%. In a multiple regression model, a neutralizing antibody titer (>60 AU/ml) was more likely with immunotherapy (odds ratio 2.44) and less likely with chemotherapy (odds ratio 0.39).
Conclusions
Overall, both COVID-19 vaccine and natural infection are highly immunogenic among cancer patients. Our study, however, identifies those under chemotherapy as significantly less responsive, and with lower antibody levels. These findings justify close virological and serological surveillance along with consideration of these patients for booster (third dose) vaccine prioritization, as new highly spreading SARS-CoV-2 variants emerge.
Key words: COVID-19, cancer, solid tumors, vaccine, serologic response, chemotherapy
Highlights
-
•
Solid tumor patients might be in a relatively immunocompromised state.
-
•
Patients on chemotherapy are less responsive, and with lower antibody levels following COVID-19 vaccination or infection.
-
•
Patients on chemotherapy should be considered for booster vaccine prioritization.
Introduction
COVID-19, caused by SARS-CoV-2, was declared as a global pandemic by the World Health Organization in March 2020. Cancer patients under active therapy might be at significant risk for worse outcomes.1, 2, 3, 4, 5, 6
Following massive worldwide efforts, the United States Food and Drug Administration (FDA) authorized emergency use of several SARS-CoV-2 vaccines, including the BNT162b2 mRNA vaccine by Pfizer. In the randomized phase III trial, the BNT162b2 vaccine was administered in two doses, 21 days apart, and was 95% effective in preventing COVID-19 [95% confidence interval (CI) 90.3% to 97.6%], with an excellent safety profile.7 A recent study of nationwide mass vaccination in Israel confirmed similar efficacy as in the randomized trials.8 Notably, slightly lower efficacy was reported among patients with comorbidities.8
The immune response to infection or vaccination includes an immediate humoral response to the viral proteins, as assessed by serologic tests for antibodies to the spike and the nucleocapsid proteins among infected individuals. Memory B cells and T cells are essential for long-term immunity, with robust boosting after a single vaccine inoculation in COVID-19 recovered individuals.9 The reported seropositivity is 98%-100% in vaccinated healthy control groups in various studies.10, 11, 12 Yet, several studies found a reduced serologic response among immunocompromised patients.10, 11, 12, 13 In hematologic patients, several studies confirmed lower seropositivity in chronic lymphocytic leukemia,10 and myeloma,11 especially in patients on immunosuppressive treatment such as Bruton tyrosine kinase (BTK) inhibitors, anti-CD20, and after bone marrow transplantation.12,13 The efficacy in subgroups of solid tumor patients, however, is less clear. Overall, the reported seroconversion rate in patients with solid tumors is in the range of 90%.12, 13, 14, 15, 16, 17 The small number of patients and the inclusion of patients with hematologic malignancies in a few studies, however, limited the evaluation of the effect of chemotherapy, immunotherapy, and biologic treatments in patients with solid tumors.
This study evaluated the serologic response to COVID-19 infection and/or BNT162b2 mRNA vaccine among patients with solid tumors on various active treatments to identify subgroups that show altered seropositivity due to disease stage or specific treatments. In addition, we also compare our findings with previous studies.
Methods
To assess the immunologic response of cancer patients to COVID-19-related exposures, we investigated the serologic response to the BTN162b2 mRNA COVID-19 vaccine, COVID-19 infection, or both in cancer patients under active treatment. During April and May 2021, cancer patients treated at the outpatient oncology clinics at Hadassah Hebrew University Medical Center were offered the chance to participate in the study. Patients’ demographics, disease status (local, metastatic), type of therapy [chemotherapy, biological agents, immunotherapy (solely checkpoint inhibitors), hormonal], and date of infection/vaccination were reported by the patients and obtained from medical files.
A blood sample was collected from patients at a median of 77 days after the second vaccine (range 21-97) and 121 days after infection (range 44-271). Sera were analyzed at our virology laboratory for antibodies against the spike protein (S) (Liaison SARS-CoV-2 S1/S2 IgG, DiaSorin, Saluggia, Italy) with a range of 3.8 to >400 AU/ml. The upper antibody titer limit was capped at >400 AU/ml; thus, the titer differences may be higher than those recorded. Results of <12 are considered negative, 12-19 equivocal, and >19 AU/ml are defined as positive serology. In addition, we considered a cutoff titer >60 IU/ml as potentially neutralizing, as this was found to be the median antibody level in a group of patients with positive virus neutralizing assay test results.18 Specific anti-S receptor-binding domain (RBD) antibodies (SARS-CoV-2 IgG II Quant, Abbott Park, IL) were further measured in seven patients with equivocal anti S1/S2 results.
Patients’ results are compared with 30 serological tests carried out in a control group of health care workers at our institution,19 matched with regards to the post-vaccination sample timing (median 77.5 days, range 6-118 days, after the second vaccine inoculation).
All patients and controls signed an informed consent approved by the institutional ethics committee.
Statistical analyses
For analysis of the study populations’ characteristics, continuous variables were compared using the Student’s t-test, and categorical variables were compared with the chi-square test. The data were analyzed using Software Package for Statistics and Simulation (IBM SPSS version 27, Armonk, NY) and R (R Foundation for Statistical Computing, Vienna, Austria) (also used for generating figures). The dispersion of anti-S1/S2 antibody titers by patient group was plotted as linked dot, box, and violin plots using the ‘ggplot2’ package and selected pairwise t-test results were added using the ‘ggsignif’ package. A logistic general linear model was fitted with titer at or above 60 IU/ml as the response variable and chemotherapy, immunotherapy, and biological therapy as independent variables, with resulting adjusted odds ratios (OR) reported along with 95% CIs. Correlations were reported using Spearman’s test with the corresponding P value.
Results
A total of 238 patients underwent serologic anti-SARS-CoV-S1/S2 testing. Of them, 36 were excluded from analyses due to serologic testing after one vaccine dose (n = 7) or testing within 21 days from the second vaccination (n = 6). For 19 patients, data regarding infection or vaccination dates were unavailable and four were neither vaccinated nor infected with COVID-19; therefore, the analyses were carried out on 202 patients. The demography and clinical characteristics of the patients are presented in Table 1. The median age of the patients was 62.1 ± 14.1 years (range 23-91). One hundred and thirty-six patients (67.3%) had metastatic disease. The cancer diagnosis included 66 breast cancers, 38 lung cancers, 36 gastrointestinal cancers, 22 genitourinary cancers, 10 gynecological cancers, and 30 patients with other cancers. The treatments were chemotherapy in 96 (47.5%), checkpoint inhibitors in 52 (25.7%), in 17 of them in combination with chemotherapy, biologic treatments in 46 (22.8%), and hormonal treatments in 12 patients [as single therapy (5) or combined with cyclin-dependent kinase (CDK) 4/6 inhibitors (6) or everolimus (1)]. A total of 42 patients were not on treatment at the time of vaccination, 37 started treatment post-vaccination, and 5 were on best supportive care.
Table 1.
Patients’ baseline demographics and disease characteristics
| Variable | Value |
|---|---|
| Age | |
| Mean ± SD | 62.1 ± 14.1 |
| >65 years old, % (n) | 52.0 (105) |
| Males, % (n) | 44.1 (89) |
| Cancer site, % (n) | |
| Breast | 32.7 (66) |
| Genitourinary | 10.9 (22) |
| Lung | 18.8 (38) |
| Gynecological | 5.0 (10) |
| Gastrointestinal | 17.8 (36) |
| Other | 14.9 (30) |
| Metastatic disease | 67.3 (136) |
| Treatment, % (n) | |
| No treatmenta | 18.8 (37) |
| Chemotherapy | 47.5 (96) |
| Biology | 22.8 (46) |
| Hormonal | 5.9 (12) |
| Immunotherapy | 25.7 (52) |
| Best supportive care | 2.5 (5) |
| Past COVID illness | 14.9 (30) |
| Days after the second vaccine | 83.7 ± 42.0 |
| 1st Quartile | 22-60 |
| 2nd Quartile | 60-80 |
| 3rd Quartile | 80-89 |
| 4th Quartile | 90-315 |
SD, standard deviation.
These patients started treatment after vaccination.
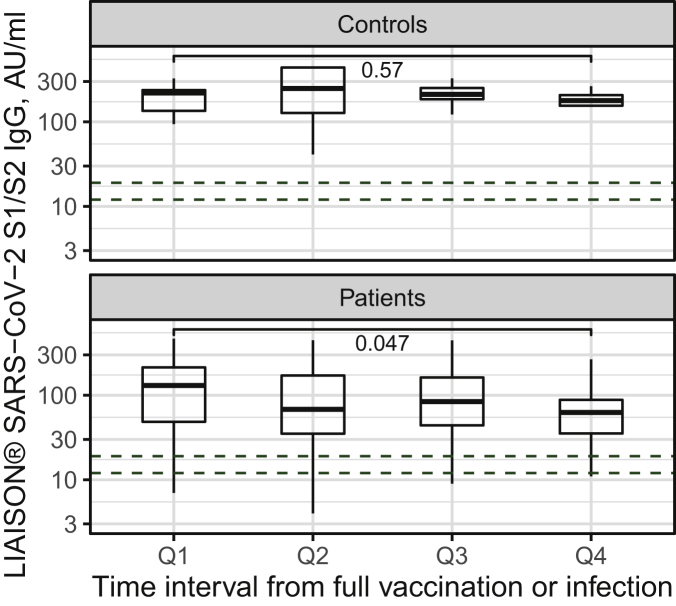
Among nine patients with equivocal anti-S1/S2 antibody levels, specific anti-S RBD antibodies were negative in two and positive in seven. Thus, anti-SARS-CoV-2 S1/S2 or specific anti-S RBD antibodies were positive in 89.1% (180/202) patients after vaccine administration or infection and in 87.2% (150/172) patients after vaccination alone without a history of COVID-19 (uninfected). In a control group of 30 vaccinated uninfected healthcare workers, 100% of serologic tests were positive (P < 0.001).19 Figure 1 shows quantitative antibody titer dynamics in patients and controls. While in control participants antibody levels do not differ across quartiles of time interval from full vaccination, in cancer patients a mild yet significant drop is observed between the first and fourth interval quartiles. At all time intervals, respective antibody levels were higher in controls (all pairwise t-test P values < 0.05).
Figure 1.
Anti-S1/S2 titers in controls (top panel) and patients (bottom panel) at the specified time interval quartiles after full vaccination or disease.
Interval Q1 ≤ 58 days, interval Q2 59 to 80 days, interval Q3 81 to 93 days, and interval Q4 ≥ 94 days.
Table 2 presents the associations of antibody seropositivity with patients’ characteristics, in those who were uninfected and received two vaccinations with the BNT162b2 mRNA COVID-19 vaccine (n = 172). In univariate analyses, only chemotherapy treatment was significantly associated with a reduced humoral response rate to vaccination; 77.5% (62/80) among patients receiving chemotherapy compared with 95.7% (88/92) in patients not receiving chemotherapy (OR 6.39, 95% CI 2.06-19.79, P < 0.001). The corresponding rates (Table 3) among all 202 patients including 30 patients after COVID-19 infection were 81.3% in patients receiving chemotherapy and 96.2% in others (OR 5.89, 95% CI 1.91-18.09, P = 0.001). Age, disease status, metastatic versus local disease, and treatment with immunotherapy, hormonal, or biologic agents were not associated with antibody response. Seropositivity was 100% in 30 patients after COVID-19 infection, 20 of whom also received a single vaccine dose, compared with 87.2% in patients with no history of COVID-19 (150/172, P = 0.038, Table 3).
Table 2.
Univariate analysis of antibody response rate in cancer patients after two BNT162b2 vaccinations (n = 172)
| Variable | Category | Serological response, n (%) |
P value | Odds ratio | |
|---|---|---|---|---|---|
| Positive | Negative | ||||
| Age | |||||
| <65 years | 74 (87.1) | 11 (12.9) | 1.0 | 1.03 (0.42-2.5) | |
| ≥65 years | 76 (87.4) | 11 (12.9) | |||
| Sex | |||||
| Male | 62 (83.8) | 12 (16.2) | 0.26 | 1.70 (0.69-4.21) | |
| Female | 88 (89.8) | 10 (10.2) | |||
| Time from vaccination | |||||
| <4 weeks | 2 (66.7) | 1 (33.3) | 0.28 | 3.52 (0.30-40.6) | |
| ≥4 weeks | 148 (87.6) | 21 (12.4) | |||
| Cancer status | |||||
| Early | 47 (82.5) | 10 (17.5) | 0.20 | 0.55 (0.20-1.36) | |
| Metastatic | 103 (89.6) | 12 (10.4) | |||
| Treatment | |||||
| Any treatment | |||||
| Yes | 119 (85.6) | 20 (14.4) | 0.20 | 0.38 (0.90-1.73) | |
| No | 31 (93.9) | 2 (6.1) | |||
| Chemotherapy | |||||
| Yes | 62 (77.5) | 18 (22.5) | <0.001 | 6.39 (2.06-19.8) | |
| No | 88 (95.7) | 4 (4.3) | |||
| Biological | |||||
| Yes | 34 (87.2) | 5 (12.8) | 1.0 | 1.03 (0.35-2.92) | |
| No | 116 (87.2) | 17 (12.8) | |||
| Hormonal | |||||
| Yes | 9 (90.0) | 1 (10.0) | 0.78 | 0.75 (0.09-6.19) | |
| No | 141 (87.0) | 21 (13.0) | |||
| Immunotherapy | |||||
| Yes | 42 (91.3) | 4 (8.7) | 0.33 | 0.57 (0.18-1.80) | |
| No | 108 (85.7) | 18 (14.3) | |||
| Best supportive care | |||||
| Yes | 3 (75.0) | 1 (25.5) | 0.46 | 2.33 (0.23-23.5) | |
| No | 147 (87.5) | 21 (12.5) | |||
Table 3.
Univariate analysis of antibody response rate in cancer patients after COVID-19 infection and/or vaccination (n = 202)
| Variable | Serological response, n (%) | P value | Odds ratio | |
|---|---|---|---|---|
| COVID-19 infection | ||||
| Yes | 30 (100.0) | 0 (0.0) | 0.038 | |
| No | 150 (87.2) | 22 (12.8) | ||
| Chemotherapy | ||||
| Yes | 78 (81.3) | 18 (18.8) | 0.001 | 5.89 (1.91-18.09) |
| No | 102 (96.2) | 4 (3.8) | ||
To better elucidate the relationship with specific treatment combinations (Table 4), we further report serologic response rate and antibody levels in several treatment subgroups. Serologic response rates among 172 patients after vaccination were 77.5%, 83.3%, and 94.1% among patients on chemotherapy, chemotherapy and immunotherapy, and immunotherapy ± biologic treatments, respectively. Figure 2 compares anti-SARS-CoV-2 S1/S2 levels distribution among all 202 patients receiving chemotherapy combinations, immunotherapy combinations, both immunotherapy and chemotherapy, or neither treatments, with correspondent P values. In Supplementary Figure S1, available at https://doi.org/10.1016/j.esmoop.2021.100283, patients are further subdivided to show those patients treated solely with biologics or hormonal therapy. Chemotherapy is associated with lower mean levels compared with patients receiving neither chemotherapy nor immunotherapy (P = 0.00067). Mean antibody levels in patients on immunotherapy were significantly higher than in those on chemotherapy (P = 0.0017), but not when compared with patients receiving neither treatment (Figure 2). In a multiple logistic regression model, a putatively protective antibody level (>60 AU/ml)18 was more likely with immunotherapy (OR 2.44, P < 0.05) and less likely with chemotherapy (OR 0.39, P < 0.05).
Table 4.
Summary of previously reported SARS-CoV-2 antibody response rate among patients with solid tumors receiving various treatments
| Publication, Therapy subgroups | Positive/all (n) | Serologic RR (%) Rate (%) |
|---|---|---|
| Studies including solid tumor patients only | ||
| Massarweh et al.15 | ||
| All | 92/102 | 90 |
| All chemo combinations | 55/64 | 85.8 |
| All immuno combinations | 36/41 | 87.8 |
| IC only (22) or +biologic (5) | 26/27 | 96.2 |
| Immuno + chemo | 10/14 | 71.4 |
| Goshen-Lago et al.16 | ||
| All | 187/218 | 85.8 |
| All chemo combinations | 102/125 | 81.6 |
| Biologic | 70/77 | 90.9 |
| All immuno combinations | 8/79 | 89.9 |
| Barriere et al.14 | ||
| All | 42 | 95.2 |
| Grinshpun, Rottenberg et al., this study | ||
| All | 150/172 | 87.2 |
| All chemo combinations | 63/80 | 77.5 |
| All immuno | 4/46 | 91.3 |
| Immuno only | 32/34 | 94.1 |
| Immuno + chemo | 10/12 | 83.3 |
| Studies that included hematologic patients (solid tumor patients are extracted, but specific treatment groups include hematologic patients) | ||
| Thakkar et al.12 | ||
| All solid tumor patients | 136 | 98 |
| Chemotherapy | 112 | 93 |
| Immunotherapy | 31 | 97 |
| Other | 47 | 100 |
| Addeo et al.13 | ||
| All solid tumor patients | 101 | 98 |
| Cytotoxic | 30 | 93 |
| Immunotherapy | 14 | 92.8 |
| Other | 63 | 98.4 |
| Iacono et al.17 (>age 80) | ||
| All solid tumor patients | 26 | 96 |
Chemo, chemotherapy; IC, immunotherapy; immune, immunotherapy.
Figure 2.
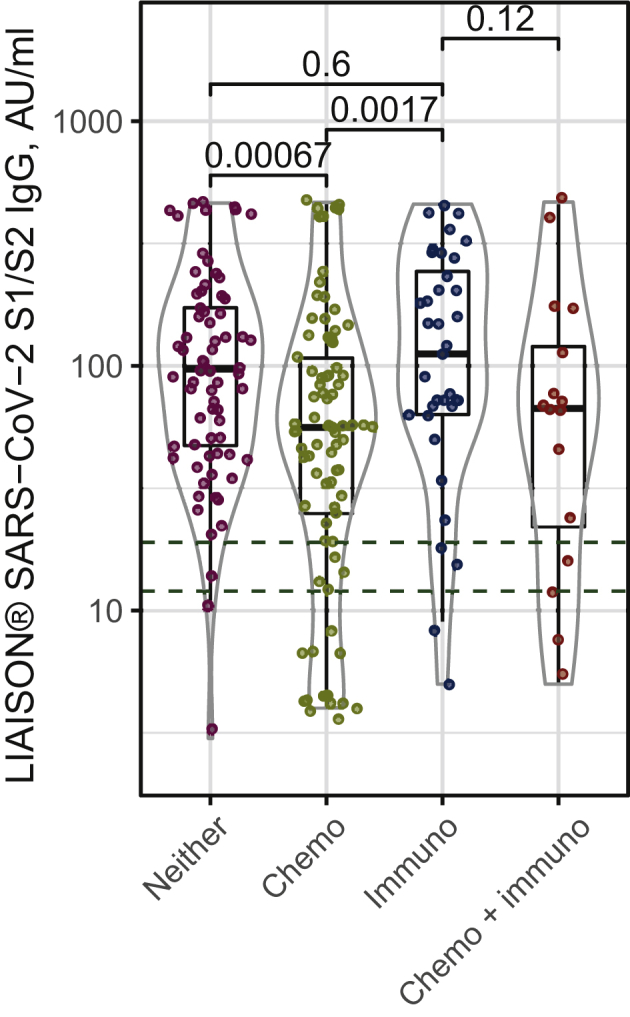
Anti-SARS-CoV-2 S1/S2 levels distribution among cancer patients with solid tumors treated with chemotherapy, immunotherapy, neither, or both.
Chemo, chemotherapy; immune, immunotherapy.
Two patients in our cohort had COVID-19 after the first vaccine and a third patient was recently diagnosed, with the rapid increase in delta variant COVID-19 cases in Israel. She had metastatic breast cancer, was treated with immunotherapy and chemotherapy at the time of vaccination, and had a level of 62 AU/ml 3 months before she had symptomatic COVID-19, for which she required intensive care.
Discussion
Our findings confirm an overall excellent immunogenicity as manifested by antibody response to BTN162b2 mRNA COVID-19 vaccination or COVID-19 infection, with approximately 90% seroconversion in actively treated patients with solid tumors. Although this rate is significantly lower than the 99%-100% found in our control group of health care workers18 and reported control groups in the literature,10, 11, 12 it is markedly higher than the frequency in other immunocompromised patients such as chronic lymphocytic lymphoma patients10 (39%) and liver20 (52.5%) and kidney (44%) transplanted patients.19
Chemotherapy treatment was independently associated with a significantly reduced rate of humoral response to infection or vaccination with 77.5% seropositivity among patients receiving chemotherapy compared with >95.7% in patients receiving other treatments. Seroconversion in patients receiving chemotherapy after COVID-19 infection was 100%, however, possibly reflecting a selection bias. Serologic response in patients on checkpoint inhibitors alone or in combinations did not differ significantly from other groups; 94.1% (32/34) for immunotherapy without chemotherapy and 83.3% (10/12) for immunotherapy and chemotherapy. The antibody levels in patients on chemotherapy were significantly lower in comparison to all other groups (i.e. immunotherapy, biologic, no treatment). Interestingly, immunotherapy had an opposite effect, with an independent (namely, chemo- and biological therapy-adjusted) OR of 2.44 for >60 AU/ml antibody levels, whereas chemotherapy had an OR of 0.39, raising the possibility that immunotherapy boosts an antibody response, when present, to potentially higher protective levels, a possibility that has been previously suggested in established vaccination programs.21,22
Table 4 summarizes the previously reported serologic responses to two anti-SARS-CoV-2 vaccinations in patients with solid tumors. A study from Israel by Massarweh et al.15 included only solid tumor patients and reported 90% positive serology among 102 patients receiving active treatments. The rate was 85.8% in patients on chemotherapy only or in combination, 96.2% in patients on immunotherapy with or without biologic agents, and 71.4% in those on immunotherapy combined with chemotherapy (10/14). Only the combination of immunotherapy and chemotherapy was significantly associated with reduced antibody titer levels.
Another study from Israel, by Goshen-Lago et al.,16 reported serologic responses of 81.6% (102/125) in patients receiving chemotherapy and 89.9% (8/79) in patients receiving immunotherapy. The report did not separate the immunotherapy group according to combination with chemotherapy.
Three additional studies12, 13, 14 included also patients with hematologic malignancies and reported 96%-98% positive serologic response rates in patients with solid tumors. These studies included a large group of solid tumor patients on hormonal treatments or surveillance and reported 98%-100% seropositivity in the latter, which confirms that serologic response is similar to the general population in solid tumor patients on treatments other than chemotherapy, regardless of disease stage. Both studies reported seropositivity of 93% in patients receiving chemotherapy. Thakkar et al.12 found 97% seropositivity in 31 patients receiving immunotherapy while Addeo et al.13 reported 93% (13/14 patients). Interestingly, Thakkar et al.12 found 100% seropositivity but significantly lower antibody titers in five patients on CDK4/6 inhibitors. We also had five patients on CDK4/6 inhibitors who, on the contrary, had high antibody levels, reinforcing the need for larger patient numbers for these analyses.
The specific subgroups in these studies are in part small, and the differences were not necessarily significant. Taken together with our results, however, there was overall high seropositivity in patients with solid tumors on hormonal and biologic treatments or surveillance, and lower seropositivity of approximately 80% among patients on chemotherapy.
A potential limitation of the current study is a lack of neutralization titer measurements among study participants. Yet, IgG antibody levels have been shown to serve as a good correlative to neutralization.7,20,23
As for immunotherapy using checkpoint inhibitors, the possible heightened immunogenicity of the COVID-19 vaccine, as reflected by the higher OR for antibody levels of >60 AU/ml in those receiving immunotherapy, has been previously suggested in established influenza vaccination programs,21, 22 and should be further studied in larger series of cancer patients on immunotherapy.
Conclusion
Our study suggests a high overall antibody response rate, of >90%, to the COVID-19 mRNA BNT162b2 vaccine or infection among cancer patients receiving active treatment. We also extend previous studies showing that chemotherapy treatment is associated with a reduced serologic response rate of approximately 80% in solid tumor patients regardless of disease status or combination. In contrast, patients on other treatments have a response rate similar to the general population. Thus, close virological and serological surveillance is warranted in cancer patients receiving chemotherapy to ensure early diagnosis and proper management. In addition, this group of patients should be considered for booster (third dose) vaccine prioritization, as new highly spreading SARS-CoV-2 variants emerge.
Acknowledgments
Funding
None declared.
Disclosure
The authors have declared no conflicts of interest.
Supplementary data
References
- 1.Brar G., Pinheiro L.C., Shusterman M. COVID-19 Severity and outcomes in patients with cancer: a matched cohort study. J Clin Oncol. 2020;38(33):3914–3924. doi: 10.1200/JCO.20.01580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang W., Guan W., Chen R. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai M., Liu D., Liu M. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuderer N.M., Choueiri T.K., Shah D.P. Clinical impact of COVID-19 on patients with cancer (CCC19): A cohort study. Lancet. 2020;395(10241):1907–1918. doi: 10.1016/S0140-6736(20)31187-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aschele C., Negru M.E., Pastorino A. Incidence of SARS-CoV-2 infection among patients undergoing active antitumor treatment in Italy. JAMA Oncol. 2020;7(2):304–306. doi: 10.1001/jamaoncol.2020.6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee L.Y.W., Phil D., Cazier J. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet. 2020;395:1919–1926. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagan N., Barda N., Kepten E. BNT162b2 mRNA COVID19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel R.R., Apostilidis S.A., Painter M.M. Distinct antibody and memory B cell responses in SARS-CoV-19 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6(58):eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herishanu Y., Avivi I., Aharon A. Efficacy of the BNT162b2 mRNA vaccine in patients with chronic lymphcytic leukemia. Blood. 2021;137(23):3165–3173. doi: 10.1182/blood.2021011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Oekelen O., Gleason C.R., Agte S. Highly variable SARS-CoV-2 spike antibody responses to two doses of COVID-19 RAN vaccination in patients with multiple myeloma. Cancer Cell. 2021;39(8):1028–1030. doi: 10.1016/j.ccell.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakkar A., Gonzales-Lugo J.D., Goradia N. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell. 2021;39(8):1081–1090.e2. doi: 10.1016/j.ccell.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Addeo A., Shah P.K., Bordry N. Immunogenicity of SARS-CoV-2 messenger RNA vaccine in patients with cancer. Cancer Cell. 2021;39:1–8. doi: 10.1016/j.ccell.2021.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barriere J., Chamorey E., Adjtoutah Z. Impaired immunogenicity of BNT162b2 anti-SARS-CoV-2 vaccine in patients treated with solid tumors. Ann oncol. 2021;32(8):1053–1055. doi: 10.1016/j.annonc.2021.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Massarweh A., Eliakim-Raaz N., StemmerA Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goshen-Lago T., Waldhorn I., Holland R. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol. 2021:e212675. doi: 10.1001/2021.2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iacono D., Cerbone L., Palombi L. Serological response to COVID-19 vaccination in patients with cancer older than 80 years. J Geriatr Oncol. 2021 doi: 10.1016/j.jgo.2021.06.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonelli F., Sarasini A., Zeirold C. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58(9) doi: 10.1128/JCM.01224-20. e01224-e01220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ben-Dov I.Z., Oster Y., Tzukert K. The 5-months impact of tozinameran (BNT162b2) mRNA vaccine on kidney transplant and chronic dialysis patients. medRxiv. 2021 doi: 10.1007/s40620-021-01210-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rabinowich L., Geupper A., Baruch R. Low immunogenicity to SARS-CoV-2 vaccination among liver transplan recipient. J Hepatol. 2021;75(2):435–438. doi: 10.1016/j.jhep.2021.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keam B., Kang C.K., Jun K.I. Immunogenicity of influenza vaccination in patients with cancer receiving immune checkpoint inhibitors. Clin Infect Dis. 2020;71(2):422–425. doi: 10.1093/cid/ciz1092. [DOI] [PubMed] [Google Scholar]
- 22.Elefhteiadis T., Pissas G., Liakopoulos V., Stefanidis I. Anti-PD1 immunotherapy for metastatic renal cancer boosted humoral immunity in a hemodialysis patient. J Immunother. 2021;44(4):164–166. doi: 10.1097/CJI.0000000000000365. [DOI] [PubMed] [Google Scholar]
- 23.Lustig Y., Sapir E., Regev-Yochay G. BNT162b2 COVID-19 vaccine and correlates of humoral immune responses and dynamics: a prospective, single-centre, longitudinal cohort study in health-care workers. Lancet Respir Med. 2021;9(9):999–1009. doi: 10.1016/S2213-2600(21)00220-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.