Abstract
Many signaling pathways are dysregulated in cancer cells and the host tumor microenvironment. Aberrant receptor tyrosine kinase (RTK) pathways promote cancer development, progression, and metastasis. Hence, numerous therapeutic interventions targeting RTKs have been actively pursued. Axl is an RTK that belongs to the Tyro3, Axl, MerTK (TAM) subfamily. Axl binds to a high affinity ligand growth arrest specific 6 (Gas6) that belongs to the vitamin K-dependent family of proteins. The Gas6/Axl signaling pathway has been implicated to promote progression, metastasis, immune evasion, and therapeutic resistance in many cancer types. Therapeutic agents targeting Gas6 and Axl have been developed, and promising results have been observed in both preclinical and clinical settings when such agents are used alone or in combination therapy. This review examines the current state of therapeutics targeting the Gas6/Axl pathway in cancer and discusses Gas6- and Axl-targeting agents that have been evaluated preclinically and clinically.
Keywords: receptor tyrosine kinase inhibitors, Gas6/Axl pathway, cancer therapeutics, small molecule inhibitors, therapeutic resistance
1. Introduction
Receptor tyrosine kinases (RTKs) are cell surface receptors that mediate a number of physiological responses and homeostasis. However, gene amplification, overexpression, and activating mutations of RTKs are often associated with cancer development, progression, and metastasis and have served as pharmacological targets in cancer treatment. Axl belongs to the TAM (Tyro3, Axl, MerTK) subfamily of the RTKs. Physiologically, the Gas6/Axl pathway plays an important role in platelet aggregation and vessel integrity [1,2,3]. Axl knockout in germ cells does not result in embryonic lethality [4]. The growth arrest specific 6 (Gas6) protein belongs to the vitamin K-dependent family of proteins and is a high affinity ligand for Axl. Overexpression and activation of Axl are widely observed in various cancer types and have been implicated in multiple steps of cancer pathogenesis. In addition, high Axl expression and activation are associated with poor prognosis, outcome, and resistance to therapy in cancer patients [5,6,7,8,9,10,11]. As such, the Gas6/Axl pathway has gained attention as a promising therapeutic target for drug development in multiple tumor types. In this review, we present an overview of the Gas6/Axl signaling pathway and highlight the Gas6 and Axl inhibitors that are being investigated preclinically and clinically.
2. Gas6/Axl Pathway in Cancer
Axl was first isolated from chronic myelogenous leukemia cells in 1988 [12]. Structurally, Axl contains two immunoglobulin-like (IgL) domains, two fibronectin III (FNIII) domains, a transmembrane domain, and an intracellular kinase domain [13]. The Axl pathway can be activated by its ligand Gas6, which belongs to the vitamin K-dependent family of proteins. Gas6 contains the γ-carboxyglutamic acid (Gla) domain, four epidermal growth factor (EGF)-like domains, and two laminin G-like (LG) domains [14,15]. The N-terminus Gla domain mediates binding to phosphatidylserine expressed on cell membranes, apoptotic cells, and debris [16,17]. Gas6 and Axl interact via the first LG-like domain of Gas6 and the two IgL domains of the Axl ectodomain [18]. Gas6 and Axl bind at a one-to-one receptor-to-ligand ratio and then dimerize with another Gas6–Axl complex to initiate a downstream signaling cascade.
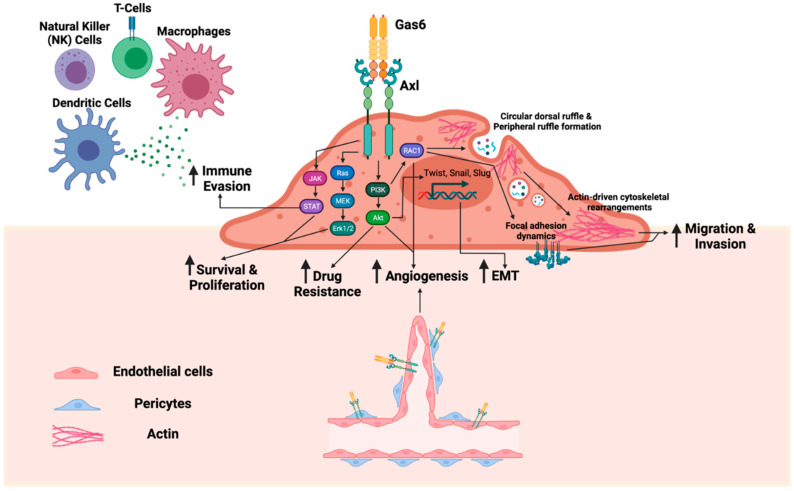
The role of Gas6 and Axl in cancer has been reviewed previously [19,20,21,22]. Briefly, Axl is overexpressed in many cancer types and is associated with therapeutic resistance, poor clinical prognosis, and worse outcome [23,24,25,26]. Axl also mediates key components of the metastatic cascade, including, but not limited to, epithelial-to-mesenchymal transition, migration and invasion, proliferation, survival, stemness, angiogenesis, and immune evasion [19,20,22,27] (Figure 1). A study by Zdzalik-Bielecka and colleagues demonstrated that the Gas6/Axl pathway mediates actin cytoskeleton remodeling for cell migration and invasion via formations of peripheral ruffles and circular dorsal ruffles [28]. Such Gas6-induced Axl activation contributes to focal adhesion turnover, cell spreading, and elongation through the activation of PI3K and RAC1 [28,29]. Another study similarly demonstrated that Axl mediates cell invasion through the regulation of lysosome peripheral distribution [30].
Figure 1.
The Gas6/Axl pathway mediates multiple steps of the metastatic cascade. Upon Axl binding to its ligand growth arrest specific 6 (Gas6) protein, Axl dimerizes and autophosphorylates its tyrosine residues in the kinase domain. Axl activation regulates downstream signaling, including the JAK/STAT pathway, Ras/MEK/Erk1/2 pathway, and PI3K/Akt pathway. In turn, the Gas6/Axl pathway upregulates pro-tumorigenic functions, such as immune evasion, survival, proliferation, drug resistance, angiogenesis, epithelial-to-mesenchymal transition (EMT), migration, and invasion. Gas6 and Axl are also expressed by stromal cells, including endothelial cells, pericytes, and subsets of immune cells, to promote tumor progression and metastasis.
In the tumor immune microenvironment, Axl signaling in tumor cells polarizes tumor-associated macrophages toward the M2 phenotype via the Axl/Pi3K/Akt/NF-kB pathway [31]. Axl inhibition elicits a favorable immune response, characterized by a decreased infiltration of CD206+ macrophages and neutrophils and an increased infiltration of CD45+ immune cells, CD8+ T cells, CD4+ T cells, and I-A/I-E+ macrophages [32].
Additionally, it is becoming increasingly clear that the Gas6/Axl signaling axis also impacts non-neoplastic cell populations, which may be of particular relevance when viewed in the context of the tumor microenvironment. For example, pericyte FAK expression is a negative regulator of tumor angiogenesis and tumor growth [33]. In FAK-depleted pericytes, activated Pyk2 increases Gas6 transcript levels and signals via the Gas6/Axl/Akt/Cyr61 pathway to promote tumor cell proliferation and angiogenesis [34,35]. Axl is also expressed by bone marrow-derived cells, dendritic cells, monocytes, natural killer cells, and platelets [36,37,38,39,40,41,42]. A study by Tirado-Gonzalez and colleagues also demonstrated that Axl-expressing leukemia-associated macrophages contribute to immune suppression and impair the functions of NK- and T-cell-mediated tumor cell killing [43].
3. Therapeutic Targeting of Gas6
3.1. Warfarin
Warfarin is a vitamin K antagonist that inhibits the enzyme vitamin K epoxide reductase to recycle inactive vitamin K epoxide to its active form. Warfarin blocks vitamin K-dependent gamma-carboxylation of the γ-carboxyglutamic acid-rich (Gla) domain of Gas6 [44], which prevents the Gas6 interaction with externalized phosphatidylserine on the surface of apoptotic cells and debris (Table 1). Preclinically, the ability of warfarin to impair Gas6 function has been investigated in pancreatic cancer, lung cancer, melanoma, and breast cancer models [42,45,46,47]. Zweemer and colleagues demonstrated that warfarin disrupts the phosphatidyl serine (PS)–Gas6 interaction and consequently impairs tumor cell migration [47]. In addition, Paolino and colleagues demonstrated that warfarin inhibits Gas6-mediated activation of TAM receptors on NK cells and exerts anti-metastatic activity [42]. While an early Phase I study to evaluate the effect of warfarin on markers of the Axl pathway was initiated in 2019, the study was withdrawn in 2021 due to lack of accrual (Clinical Trial Identification #: NCT03536208).
Table 1.
Gas6 and Select Class of Axl Inhibitors.
| Inhibitor/Developer | Target | Type | Indications | Phase | Strategy | Status |
|---|---|---|---|---|---|---|
| Warfarin | Gas6 | Vitamin K agonist | Pancreatic cancer, lung cancer, melanoma, breast cancer | Preclinical/ Phase I |
Monotherapy | Withdrawn |
| MYD1/MYD1-72 Stanford University |
Gas6 | Soluble receptor | Ovarian cancer, pancreatic cancer | Preclinical | Monotherapy/ Combination |
- |
| AVB-500 Aravive/Stanford University |
Gas6 | Soluble receptor | Ovarian, renal cell carcinoma, pancreatic adenocarcinoma | Phase I/II | Monotherapy/ Combination |
Active, not recruiting/ Recruiting |
| 20G7-D9 Oribase Pharma/French Research Agency of Health/University of Montpellier/Regional Clinical Cancer Center |
Axl | Monoclonal antibody | Pancreatic cancer, breast cancer | Preclinical | Monotherapy | - |
| Axl-107-MMAE Genmab/Oncode Institute, The Netherlands Cancer Institute |
Axl | Antibody drug conjugate | Cervical cancer, lung cancer, melanoma | Phase I/II | Monotherapy | Active, not recruiting |
| BA3011 BioAtla |
Axl | Antibody drug conjugate | Lung cancer, prostate cancer, ovarian cancer, pancreatic cancer | Phase I/II | Monotherapy/ Combination |
Not yet recruiting/ Recruiting |
| YW327.6S2 Genentech |
Axl | Monoclonal antibody | NSCLC and breast cancer | Preclinical | Combination | - |
| Mab173 University of Southern California |
Axl | Monoclonal antibody | Kaposi sarcoma | Preclinical | Monotherapy | - |
| Axl-CAR-T Beijing Institute of Radiation Medicine |
Axl | CAR-T cells | Triple-negative breast cancer | Preclinical | Monotherapy | - |
| Axl-CAR.C7R XinJiang Medical University |
Axl | CAR-T cells | Triple-negative breast cancer | Preclinical | Monotherapy | - |
| CCT301 CAR-T PerHum Therapeutics |
Axl | CAR-T cells | Renal cell carcinoma | Phase I/II | Monotherapy | Active, not recruiting |
| Axl-CAR-T Hunan Zhaotai Yongren Medical Innovation/Guangdong Zhaotai InVivo Biomedicine |
Axl | CAR-T cells | Lung cancer | Phase I | Monotherapy | Recruiting |
| Viscum album | Axl | Natural product | NSCLC | Preclinical | Monotherapy | - |
| Celastrol | Axl | Natural product | NSCLC | Preclinical | Combination | - |
| Yuanhuadine | Axl | Natural product | NSCLC | Preclinical | Combination | - |
3.2. Soluble Receptors
3.2.1. MYD1/MYD1-72
MYD1 is an Axl decoy receptor with a high affinity for Gas6 (Table 1) [48]. MYD1 contains the major site of Axl Ig1 with four mutations to improve binding to Gas6 [48]. MYD1 inhibited Gas6-mediated Axl-, Akt-, and Erk-phosphorylation and decreased the number of peritoneal metastases in an ovarian cancer model [48].
MYD1-72 is a second-generation Axl decoy receptor that has a higher binding affinity than that of MYD1 (KD: 420 fM vs. 93 fM, respectively). In preclinical studies, MYD1-72 decreased Axl phosphorylation and impaired tumor growth and metastatic burden in vivo. A combination of MYD1-72 with gemcitabine significantly improved the survival of mice with pancreatic cancer to 57 days compared to 17 days in the control and MYD1-72 alone groups and 35 days in the gemcitabine group. A clinical evaluation of this compound has not been reported.
3.2.2. AVB-500
AVB-500 is an Axl decoy receptor with an affinity of 150 fM for Gas6 (Table 1). AVB-500 has been preclinically investigated in renal cell carcinoma and ovarian cancer. The results showed that treatment with AVB-500 decreases Gas6-induced Axl and Src phosphorylation, tumor vessel density, tumor growth, and metastatic burden [49,50,51].
AVB-500 is undergoing Phase I/II clinical trials for patients with platinum-resistant or recurrent ovarian, fallopian tube, or peritoneal cancers as a combination therapy (Clinical Trial Identification #s: NCT03639246, NCT04019288). Three other studies are currently recruiting patients for ovarian cancer, renal cell carcinoma, and pancreatic adenocarcinoma to evaluate its efficacy as a combination therapy with paclitaxel, cabozantinib, and nab-paclitaxel/gemcitabine, respectively (Clinical Trial Identification #s: NCT04729608, NCT04300140, NCT04983407).
4. Therapeutic Targeting of Axl
4.1. Type I Kinase Inhibitors
Small molecular kinase inhibitors can be classified based on the structure of the protein–inhibitor complex [52]. The N-terminal Asp-Phe-Gly (DFG) motif of the activation loop is conserved and regulates kinase activity. Type I protein kinase inhibitors bind to the active protein kinase conformation or DFG-in conformation.
4.1.1. Bemcentinib (BGB324, R428)
Bemcentinib is a highly specific and selective Axl inhibitor with an IC50 of 14 nM in cellular assays (Table 2) [53,54]. This agent has been widely studied in the laboratory in a variety of cancer models, including breast, prostate, lung, pancreatic, and ovarian cancer, and has been shown to decrease tumor cell migration, invasion, and colony formation in vitro and impair primary tumor growth, immune cell infiltration, and metastasis in vivo [55,56,57,58,59,60,61,62]. Axl inhibition alters the expression patterns of EMT markers, upregulating epithelial markers, such as E-cadherin, and downregulating mesenchymal markers, such as N-cadherin, ZEB1, Snail, Slug, Twist, and MMP9 [63,64,65]. Furthermore, several studies have demonstrated that Axl inhibition reverses the therapeutic resistance of certain chemotherapies. Axl has also been associated with immune evasion, and the systematic treatment of tumor-bearing mice with bemcentinib has led to a reduction in tumor-infiltrating host cells, most notably cells of the myeloid lineage [66].
Table 2.
Type I kinase inhibitors against Axl.
| Inhibitor/Developer | Target(s) | IC50 for Axl | Indications Approved by the FDA | ||||
|---|---|---|---|---|---|---|---|
| Crizotinib (PF-02341066) Pfizer |
Axl, Alk, c-Met, Ron |
in vitro: 294 nM |
Approved for patients with ALK- or ROS1-positive NSCLC and pediatric ALK-positive anaplastic large-cell lymphoma | ||||
| Bosutinib (SKI-606) Pfizer |
Axl, Src kinases, Abl, TGF, BMP | in vitro: 0.56 μM cells: 1.65 μM |
Approved for patients with chronic, accelerated, or blast phase Philadelphia chromosome chronic myelogenous leukemia | ||||
| Sunitinib (SU11248) Pfizer |
Axl, Kit, Flt3, PDGFR, VEGFR2 | in vitro: 9 nM | Approved for renal cell carcinoma and imatinib-resistant gastrointestinal stromal tumor and metastatic pancreatic neuroendocrine tumors | ||||
| Inhibitor/Developer | Target(s) | IC50 for Axl | Clinical Trial # | Phase | Strategy | Status | Indications |
| Bemcentinib (BGB324, R428) Rigel Pharmaceuticals/BerGen BIO |
Axl | in vitro: 14 nM cells: <30 nM |
NCT03965494 | I | Monotherapy | Recruiting | Glioblastoma |
| NCT02922777 | I | Combination | Recruiting | NSCLC | |||
| NCT03649321 | I/II | Combination | Recruiting | Pancreatic adenocarcinoma | |||
| NCT02872259 | I/II | Combination | Recruiting | Melanoma | |||
| NCT03824080 | II | Monotherapy | Active, not recruiting | Acute myeloid leukemia (AML), Myelodysplastic syndrome | |||
| NCT03654833 | II | Combination | Recruiting | Mesothelioma | |||
| NCT03184558 | II | Combination | Completed | TNBC, TN-inflammatory breast cancer | |||
| NCT03184571 | II | Combination | Recruiting | NSCLC | |||
| Amuvatinib (MP-470) Astex Pharmaceuticals |
c-Kit, Axl, c-Met, PDGFRα, FLT3, c-Ret, RAD51 | cells: <1 μM | NCT00881166 | I | Combination | Completed | Invasive malignancy except non-melanoma skin cancers or cervical carcinoma in situ |
| NCT00894894 | I | Monotherapy | Completed | Unresectable or metastatic solid tumor | |||
| NCT01357395 | II | Monotherapy | Completed | Small-cell lung cancer (SCLC) | |||
| Dubermatinib (TP-0903) Tolero Pharmaceuticals |
Axl, Aurora A and B, JAK2, Alk, Abl, Mer | in vitro: 27 nM cells: 222 nM |
NCT02729298 | I | Monotherapy | Active, not recruiting | Advanced metastatic or progressive solid tumor (Phase 1b: EGFR positive NSCLC; BRAF-, KRAS-, or NRAS-mutated colorectal carcinoma; recurrent ovarian carcinoma; BRAF-mutated melanoma) |
| NCT03013998 | I/II | Monotherapy/ Combination |
Recruiting | AML | |||
| NCT04518345 | I/II | Monotherapy | Recruiting | AML | |||
| NCT03572634 | I/II | Combination | Terminated | Chronic lymphocytic leukemia, small lymphocytic lymphoma | |||
|
S49076 Servier |
Met mutants, Axl, Mer, FGFRs | in vitro: 7 nM cells: 56 nM |
ISRCTN00759419 | I | Monotherapy | Completed | Advanced solid tumors |
| ISRCTN11619481 | I/II | Combination | Completed | GBM | |||
| SNS-314 Sunesis Pharmaceuticals |
Aurora-A, -B, -C, Trk A/B, Flt4, Axl, c-Raf | in vitro: 84 nM |
NCT00519662 | I | Monotherapy | Completed | Advanced solid tumors |
| NA80x1 Wyeth-Ayerst Research/ Max Planck Institute of Biochemistry |
Axl | in vitro: 12.8 μM cells: 4.11 μM |
Preclinical | - | Monotherapy | - | - |
| SGI-7079 Northwestern University |
Axl, Met, Mer, Yes, Ret, Flt3 | in vitro: 58 nM cells: <1 μM |
Preclinical | - | Monotherapy/ Combination |
- | - |
| DP-3975 Deciphera Pharamaceuticals |
Axl | cells: 0.1 μM | Preclinical | - | Monotherapy | - | - |
| UNC569 University of North Carolina |
Mer, Tyro3, Axl | in vitro: 37 nM |
Preclinical | - | Monotherapy | - | - |
| UNC2025 University of North Carolina |
Mer, Flt3, Axl, Tyro3 | in vitro: 1.6 nM |
Preclinical | - | Monotherapy | - | - |
Bold: preferred target.
Bemcentinib entered clinical trials as the first Axl-specific inhibitor and is undergoing Phase I/II clinical trials for melanoma, non-small-cell lung cancer (NSCLC), mesothelioma, acute myeloid leukemia, glioblastoma, and pancreatic adenocarcinoma (Clinical Trial Identification Numbers: NCT02872259; NCT02922777; NCT03184571; NCT03654833; NCT03824080; NCT03965494; NCT03649321). A Phase II clinical trial of bemcentinib in combination with pembrolizumab for patients with triple-negative breast cancer has been completed, but the results are not yet available (Clinical Trial Identification #: NCT03184558).
4.1.2. Amuvatinib (MP-470)
Amuvatinib is a c-Kit/Axl kinase inhibitor (Table 2), with higher selectivity against c-Kit than against Axl (IC50 < 1 μM in cellular assays). Amuvatinib has been studied in models of gastrointestinal stromal tumors, lung cancer, and prostate cancer in the context of overcoming therapeutic resistance [60,67,68]. In the gastrointestinal stromal tumor (GIST) model, Mahadevan and colleagues showed that an imatinib-resistant GIST cell line overexpressed Axl compared to its parental cell line [68]. Treatment with amuvatinib showed synergistic, cytotoxic effects with docetaxel [68]. In an erlotinib-resistant lung cancer model, Axl inhibition by amuvatinib restored sensitivity to erlotinib [67].
Clinically, amuvatinib is undergoing Phase I/II trials for small-cell lung cancer (SCLC) and other unresectable or metastatic solid tumors (Clinical Trial Identification Numbers: NCT01357395; NCT00894894; NCT00881166). In a Phase I, dose-escalation study of patients with advanced solid tumors, amuvatinib was well tolerated up to 1500 mg/day with no report of dose-limiting toxicities; indeed, the study did not reach the maximum tolerated dose [69]. In a Phase Ib study of amuvatinib in combination with standard of care therapies for adults with advanced solid tumors, 12% demonstrated partial response to amuvatinib combined with paclitaxel/carboplatin or carboplatin/etoposide in neuroendocrine, NSCLC, and SCLC tumors [70].
4.1.3. Dubermatinib (TP-0903)
Dubermatinib is an Axl inhibitor that has additional activity against Aurora-A and -B and Janus kinase 2 (JAK2). Dubermatinib has been studied in breast cancer, acute myeloid leukemia, neuroblastoma, and chronic lymphocytic leukemia models (Table 2) [71,72,73,74]. Dubermatinib impairs tumor cell migration, invasion, survival, proliferation, and colony formation and can affect chemotherapeutic agent sensitivity [75,76,77,78]. At the molecular level, it inhibits Axl signaling and Aurora B activation to induce G2/M cell cycle arrest [77]. In CLL B cells, dubermatinib induced apoptosis through the downregulation of Mcl-1, Bcl-2, and XIAP and the upregulation of BIM [72]. In a neuroblastoma model, a phospho-kinase array of dubermatinib-treated cells showed greater than 50% increases in p53 S392 and Chk-2 T68 phosphorylation, in line with a corresponding increase in histone H2A.X phosphorylation [73].
Dubermatinib has been studied as monotherapy and combination therapy. Preclinically, combination strategies with all-trans retinoic acid (ATRA), cisplatin, and VP16 demonstrated synergistic, cytotoxic effects [73]. Aveic and colleagues showed that a dubermatinib/ATRA combination in neuroblastoma cell lines was particularly effective against CD133+ cells, a marker associated with chemo- and radio-resistance [79,80,81]. Phase I/II studies are ongoing for patients with advanced metastatic or progressive solid tumors and AML to determine the dose, safety, pharmacokinetics, and pharmacodynamics of dubermatinib (Clinical Trial Identification Numbers: NCT02729298, NCT04518345, NCT03013998). A Phase I/II clinical trial investigating dubermatinib monotherapy and combination therapy with ibrutinib for patients with chronic lymphocytic leukemia was terminated (Clinical Trial Identification #: NCT03572634).
4.1.4. Crizotinib (XALKORI®, PF-02341066)
Crizotinib is a multitargeted, small molecule tyrosine kinase inhibitor that inhibits c-Met, ALK, Ron, and Axl (IC50-Axl = 294 nM in cell-free assay) (Table 2). Crizotinib has been studied in a variety of cancer types, including NSCLC, gastric carcinoma, glioblastoma, anaplastic large-cell lymphoma, and leiomyosarcoma [82,83,84,85]. With respect to the role of crizotinib on the Axl signaling pathway, Dantas-Barbosa and colleagues showed that crizotinib impaired Axl phosphorylation, cell growth, cell cycle, and colony formation in leiomyosarcoma cells [82].
Crizotinib has been approved by the FDA to treat patients with ALK- or ROS1-positive NSCLC and pediatric patients with relapsed or refractory ALK-positive systemic anaplastic large-cell lymphoma (ALCL) [86,87]. There are 151 registered studies associated with crizotinib in the ClinicalTrials.gov database, and 44.4% (67 studies) are currently recruiting, enrolling by invitation, or active/not recruiting for monotherapy and combination therapy strategies. Acquisition of crizotinib resistance is common [88,89], typically developing within a few years for anaplastic lymphoma kinase-rearranged NSCLC [88]. Crizotinib-resistant NSCLC cells showed overexpression of Axl, epithelial-to-mesenchymal transition, and the acquisition of cancer stem cell-like properties [88,90,91].
4.1.5. Bosutinib (BOSULIF®, SKI-606)
Bosutinib is a second-generation, multitargeted, small molecule tyrosine kinase inhibitor that targets Src, Abl, TGFβ, and Axl (Table 2). Bosutinib has been studied in BCR-ABL-dependent diseases, breast cancer, colorectal cancer, and NSCLC [92]. Bosutinib decreases Gas6-mediated Axl phosphorylation, migration, and invasion [93,94,95]. An immunoblot of bosutinib-treated cells showed decreased phosphorylation of Src pY419, FAK Y576/577/925, Pyk2 Y580, and p130Cas Y410 [93]; expression of vimentin and Slug; and increased expression of E-cadherin [96]. In a preclinical thyroid cancer model using ThrbPV/PPten+/- mice, bosutinib treatment significantly prolonged survival and impaired the development of lung metastases [96].
Bosutinib was approved by the FDA to treat patients with chronic, accelerated, or blast-phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia [97]. Bosutinib monotherapy and combination therapy with chemotherapy and other small molecule inhibitors are also being investigated in patients with breast cancer, glioblastoma, and other advanced solid cancers (Clinical Trial identification #s: NCT00319254, NCT01331291, NCT01001936).
4.1.6. S49076
S49076 is a potent inhibitor of c-Met, as well as Axl, MerTK, and FGFR (Table 2). S49076 has been studied preclinically in gastric, lung, and liver cancer models [98,99] and has been shown to decrease the phosphorylation of Axl in a dose-dependent manner. S49076 impairs tumor cell viability, migration, and colony formation in vitro and tumor growth in vivo [98]. Clémenson and colleagues demonstrated that the mechanism of action of S49076 is dependent upon c-Met signaling dependency [100,101,102], where S49076 targets Aurora B kinase in c-Met-independent cells [99].
In a Phase I open-label study in patients with advanced solid tumors, a recommended once-daily dose of 600 mg was defined [103]. While 81.4% of patients had drug-related adverse events, the majority of the adverse events were grade I–II [103]. In a Phase I/II study in combination with bevacizumab in patients with recurrent glioblastoma, the combination did not improve patient survival outcomes [104]. The recommended Phase II dose was confirmed at 600 mg once daily, and no additional dose-limiting toxicity was observed [105,106].
4.1.7. Sunitinib (SU111248)
Sunitinib is an oral, multitargeted, small molecule receptor tyrosine kinase inhibitor that targets VEGFR2, PDGFRβ, c-Kit, and Axl (Table 2). Sunitinib is approved by the FDA to treat patients with gastrointestinal stromal cancer, advanced RCC, and progressive, well-differentiated pancreatic neuroendocrine tumors [107,108]. However, Axl overexpression is associated with sunitinib resistance in renal cell carcinoma cells [109,110]. In patients with renal cell carcinoma, elevated Axl expression is associated with shorter overall survival [109]. Genetic and pharmacologic Axl inhibition in sunitinib-resistant cell lines demonstrate decreased tumor cell migration, invasion, EMT, and angiogenesis [109].
4.1.8. SNS-314
SNS-314 is an aurora kinase inhibitor that also targets Axl, with an IC50 of 84 nM in a cellular assay (Table 2). SNS-314 impairs colony formation, histone H3 phosphorylation, and tumor growth in vivo [111,112]. In 2007, SNS-314 entered a Phase I study for patients with advanced solid tumors to evaluate safety and tolerability (Clinical Trial Identification Number: NCT00519662). The maximum tolerated dose was not established, and no responses were observed [113].
4.1.9. Other Type I Axl Kinase Inhibitors Undergoing Preclinical Evaluation
NA80x1 is a 3-quinolinecarbonitrile that inhibits Axl phosphorylation, tumor cell migration, and invasion [94]. The IC50 of NA80x1 against Axl is 4.11 μM in a cellular assay and demonstrates inhibitory activity against Src kinase [94,114]. SGI-7079 is a selective Axl inhibitor that reduces Gas6-induced Axl phosphorylation and impairs the growth of mesenchymal NSCLC xenograft tumors [115]. In addition, Axl inhibitor DP-3975 [116,117], a TAM kinase inhibitor UNC569 [118], and MerTK/Flt3 dual inhibitor UNC2025 [119] are also type I Axl inhibitors with promising preclinical results.
4.2. Type II Kinase Inhibitors
Unlike type I protein kinase inhibitors, type II protein kinase inhibitors prefer to bind the inactive, DFG-out conformation.
4.2.1. Cabozantinib (BMS-907351)
Cabozantinib is an oral, multitargeted, small molecule tyrosine kinase inhibitor that targets VEGFR, c-Met, and Axl (Table 3). Cabozantinib has been studied in RCC, esophageal squamous cell carcinoma, and lung cancer. Preclinically, cabozantinib treatment decreased Axl phosphorylation and TGF-β-induced E-cadherin expression, cell viability, migration, and tumor growth [120,121].
Table 3.
Type II kinase inhibitors against Axl.
| Inhibitor/ Developer |
Target(s) | IC50 for Axl | Indications Approved by the FDA | ||||
|---|---|---|---|---|---|---|---|
| Cabozantinib (BMS-907351) Exelixis |
VEGFR2, c-Met, Ret, Tie2, c-Kit, Axl | in vitro: 7 nM cells: 42 nM |
Approved for patients with advanced renal cell carcinoma, sorafenib-resistant hepatocellular carcinoma, and combination therapy with nivolumab for advanced renal cell carcinoma | ||||
|
Inhibitor/
Developer |
Target(s) | IC50 for Axl | Clinical Trial # | Phase | Strategy | Status | Indications |
| Foretinib (GSK1363089) GlaxoSmithKline |
c-Met, VEGFR2, Ron, Axl | in vitro: 11 nM cells: <100 nM |
NCT01138384 | I/II | Combination | Completed | Invasive, HER2-positive breast cancer |
| NCT01147484 | II | Monotherapy | Completed | Recurrent triple-negative breast cancer | |||
| NCT00726323 | II | Monotherapy | Completed | Renal cell carcinoma | |||
| NCT00725712 | II | Monotherapy | Completed | Metastatic gastric carcinoma | |||
| Sitravatinib (MGCD516) Mirati Therapeutics |
c-Kit, PDGFRα/β, Axl, c-Met | in vitro: 1.5 nM cells: 250–800 nM |
NCT04123704 | II | Monotherapy | Recruiting | Metastatic TNBC |
| NCT03680521 | II | Combination | Active, not recruiting |
ccRCC | |||
| Glesatinib (MGCD265) Mirati Therapeutics |
c-Met, RON, Axl, VEGFR | Ν/A | NCT02954991 | II | Combination | Active, not recruiting |
NSCLC |
| Rebastinib (DCC-2036) Deciphera Pharmaceuticals |
Bcr-Abl, Axl, Flt3, VEGFR2, Src | in vitro: 42 nM |
NCT02824575 | I | Combination | Recruiting | HER2-negative breast cancer |
| NCT03717415 | I/II | Combination | Recruiting | Advanced or metastatic solid tumor | |||
| NCT03601897 | I/II | Combination | Recruiting | Locally advanced or metastatic solid tumor | |||
| NCT00827138 | I | Monotherapy | Completed | CML | |||
| Merestinib (LY2801653) Eli Lilly and Company |
c-Met, MST1R, Axl, ROS1, Flt3 | in vitro: 11 nM cells: 2 nM |
NCT02711553 | II | Combination | Active, not recruiting |
Advanced or metastatic biliary tract cancer |
| NCT02920996 | II | Monotherapy | Active, not recruiting |
NSCLC | |||
| NCT03125239 | I | Combination | Completed | Relapsed or refractory AML | |||
| BMS-777607 Aslan Pharmaceuticals |
Axl, Ron, c-Met, Tyro3 | in vitro: 1.1 nM |
NCT01721148 | I | Monotherapy | Completed | Advanced or metastatic solid tumor |
| NCT00605618 | I/II | Monotherapy | Completed | Advanced or metastatic solid tumor | |||
| RXDX-106 (CEP-40783) Ignyta, Inc. |
Axl, Tyro3, Mer, c-Met | in vitro: 7 nM | NCT03454243 | I | Monotherapy | Terminated | Locally advanced or metastatic solid tumor |
| NPS-1034 Neopharm |
c-Met, Axl | in vitro: 10 nM |
Preclinical | - | Combination | - | - |
| LDC1267 Lead Discovery Center |
Axl, Tyro3, Mer, c-Met, Src | in vitro: 29 nM |
Preclinical | - | Monotherapy/ Combination |
- | - |
Bold: preferred target.
Cabozantinib has been approved by the FDA for patients with advanced renal cell carcinoma and sorafenib-resistant hepatocellular carcinoma. In January 2021, combination therapy of cabozantinib with nivolumab was approved as first-line treatment for patients with advanced renal cell carcinoma. The efficacy of the combination therapy versus sunitinib alone was evaluated in CHECKMATE-9ER, a randomized, open-label trial in patients with previously untreated advanced renal cell carcinoma [122] (Clinical Trial Identification: NCT03141177). The trial demonstrated significant improvement in progression-free survival, overall survival, and confirmed overall response rate for patients in the combination therapy arm compared with those who received sunitinib monotherapy [122].
4.2.2. Foretinib (GSK1363089)
Foretinib is an oral, small molecule kinase inhibitor of c-Met, VEGFR, and Axl. Foretinib has been studied in esophageal squamous cell carcinoma, breast cancer, leiomyosarcoma, head and neck cancer, and gastric cancer (Table 3) [11,82,123,124,125]. Foretinib decreases Axl and Akt phosphorylation and impairs tumor cell viability and xenograft tumor growth [11]. In a lapatinib-resistant, HER2-positive, ER-positive breast cancer model, the foretinib-mediated downregulation of Axl restored lapatinib sensitivity [126].
Clinically, foretinib has been studied as a monotherapy and a combination therapy. In a Phase I dose-escalation study in patients with advanced solid tumors, the recommended maximum tolerated dose of foretinib was identified [127] (Clinical Trial Identification #: NCT00742131). Stable disease and partial responses were identified in 22 and 3 cases, respectively [127]. In a Phase I study of foretinib in combination with lapatinib in patients with HER2-positive metastatic breast cancer, no responses or improvement in progression-free survival were observed [123].
4.2.3. Sitravatinib (MGCD516)
Sitravatinib is a multitargeted, small molecule receptor tyrosine kinase inhibitor that inhibits c-Kit, PDGFRα/β, c-Met, and Axl (Table 3). Sitravatinib has been shown to decrease Axl phosphorylation, colony formation, angiogenesis, and proliferation in sunitinib- or axitinib-resistant cells, as well as impairing tumor growth inhibition in vivo [128,129,130]. Du and colleagues demonstrated that sitravatinib alters the immune landscape of tumors by reducing the number of tumor-associated immunosuppressive myeloid cells and increasing the number of CD4+ T cells and proliferating CD8+ T cells [130]. Combination treatment of sitravatinib and PD-1 blockade demonstrated complete remission in 2 out of 14 mice with E0771 tumors [130].
In the clinic, sitravatinib is being investigated for use as monotherapy and combination therapy. A Phase I study is currently ongoing for patients with advanced cancer to evaluate its safety, pharmacokinetic, metabolism, pharmacodynamic, and clinical activity profiles (Clinical Trial Identification #: NCT02219711). In addition, several Phase I/II studies are underway to evaluate sitravatinib in combination with immune checkpoint inhibitors (Clinical Trial Identification #s: NCT03015740, NCT04734262, NCT03666143, NCT02954991).
4.2.4. Glesatinib (MGCD265)
Glesatinib is an oral, multitargeted tyrosine kinase inhibitor that targets c-Met, VEGFR, Ron, Tie-2, and Axl (Table 3). Glesatinib impairs tumor angiogenesis and upregulates the expression of PD-L1 [130]. Du and colleagues demonstrated that combination therapy of glesatinib with anti-PD1 therapy delayed tumor growth [130]. As such, a Phase II study of glesatinib in combination with nivolumab in patients with non-small-cell lung cancer is ongoing (Clinical Trial Identification #: NCT02954991).
4.2.5. Rebastinib (DCC-2036)
Rebastinib is a Bcr-Abl inhibitor that also impairs Src family proteins, c-Met, Tie-2, and Axl (Table 3). In a preclinical study utilizing breast cancer models, rebastinib impaired Axl phosphorylation, colony formation, cell cycle distribution, migration, invasion, and tumor growth and pulmonary metastasis in vivo [131]. Phase I/II studies are ongoing for patients with advanced metastatic or progressive solid tumors, breast cancer, and chronic myeloid leukemia to evaluate its safety, pharmacokinetics, and pharmacodynamics as monotherapy (Clinical Trial Identification Number: NCT00827138) or combination therapy with paclitaxel (NCT03601897, NCT02824575), eribulin (NCT02824575), and carboplatin (NCT03717415).
4.2.6. Merestinib (LY2801653)
Merestinib is an oral, multitarget kinase inhibitor that impairs c-Met, Axl, Ron, and Flt3 (Table 3) [132]. Merestinib impairs cell proliferation, angiogenesis, and tumor growth in vivo [132,133]. Merestinib is being investigated clinically in multiple indications, including advanced or metastatic solid tumor, biliary tract cancer, non-small-cell lung cancer, and chronic myeloid leukemia, as a monotherapy or combination therapy (Clinical Trial Identification #s: NCT02711553, NCT02920996, NCT03125239). A Phase I study of merestinib with or without cetuximab, cisplatin, or gemcitabine identified the recommended dose at 120 mg once daily [134] (Clinical Trial Identification #: NCT01285037). One complete response and three partial responses were observed in patients with cholangiosarcoma [134].
4.2.7. BMS-777607
BMS-777607 is a small molecule kinase inhibitor designed against c-Met, but it also has activity against Axl, Ron, and Tyro3 (Table 3). BMS-777607 has been studied in multiple indications, including prostate cancer, breast cancer, brain cancer (glioma/GBM), and fibrosarcoma [135,136,137,138,139,140]. BMS-777607 inhibits HGF-induced Met-autophosphorylation, colony formation, migration, and invasion [137,138]. In vivo, BMS-777607 impaired KHT sarcoma pulmonary metastases [135]; however, this was not the case for DU-145 and MDA-MB-231-4175-LM2 models [140]. Phase I/II clinical trials have been completed for patients with advanced or metastatic solid tumors, but the results are not yet available (Clinical Trial Identification #s: NCT01721148, NCT00605618).
4.2.8. RXDX-106
RXDX-106 is an oral, small molecule inhibitor targeting the TAM receptors and c-Met (Table 3). RXDX-106 has been characterized in colorectal and breast cancer models and has been shown to impair Axl, Tyro3, MerTK, Akt, and Erk phosphorylation; tumor growth in vivo; and infiltration of leukocytes, macrophages, NK cells, and CD8+ T cells [141]. While an early Phase I study to evaluate RXDX-106 safety, tolerability, pharmacokinetics, pharmacodynamics, and clinical activity was planned, the study was terminated in 2018 (Clinical Trial Identification #: NCT03454243).
4.2.9. Other Type II Axl Kinase Inhibitors Undergoing Preclinical Evaluation
LDC1267 is a selective TAM kinase inhibitor that provides anti-metastatic potential by enhancing NK cell activity in vivo [42]. In addition, NPS-1034 is a c-Met/Axl inhibitor that is efficacious against EGFR-TKI resistant non-small-cell lung cancer cells with c-Met and Axl activation [142].
4.3. Monoclonal Antibodies
4.3.1. 20G7-D9
20G7-D9 has been studied in pancreatic cancer and triple-negative breast cancer models (Table 1) [143,144]. In pancreatic cancer cell lines Panc-1 and BxPC-3, 20G7-D9 reduced Gas6-induced Axl and Akt phosphorylation and impaired tumor cell migration, viability, and tumor growth in vivo [143]. Similarly, in a triple-negative breast cancer model, 20G7-D9 decreased tumor growth in vivo and silenced the Gas6-induced upregulation of EMT markers, including Snail, Slug, Twist, and Zeb1/2 [144].
4.3.2. Axl-107-MMAE
Axl-107-MMAE is an antibody drug conjugate, containing human Axl antibody linked to the microtubule-disrupting agent monomethyl auristatin E (Table 1) [145]. Axl-107-MMAE induces cytotoxicity in vitro and impairs tumor growth in cervical cancer, lung cancer, and melanoma models [145]. In addition, combination treatment with MAPK pathway inhibitors cooperatively inhibited melanoma patient-derived xenograft growth [145]. The clinical safety and efficacy of Axl-107-MMAE are currently being evaluated in a Phase I/II clinical study for patients with advanced solid tumors (Clinical Trial Identification #: NCT02988817).
4.3.3. BA3011
BA3011 is an anti-Axl humanized monoclonal antibody conjugated to monomethyl auristatin E using a cleavable linker (CAB-Axl-ADC) (Table 1). BA3011 is capable of reversibly binding to recombinant Axl and Axl-expressing cells in the tumor microenvironment but not in normal tissues. Preclinical studies demonstrate that BA3011 induces cytotoxicity in vitro and impairs tumor growth in lung, prostate, and pancreatic xenograft models [146]. The clinical safety and efficacy of BA3011 are currently being evaluated in Phase I/II and II clinical studies for patients with solid tumors, NSCLC, and ovarian cancers (Clinical Trial Identification #s: NCT03425279, NCT04681131, NCT04918186).
4.3.4. Other Monoclonal Axl Antibodies Undergoing Preclinical Evaluation
YW327.6S2 is a phage-derived monoclonal antibody that recognizes both human and murine Axl [147]. In NSCLC and breast cancer models, YW327.6S2 impaired xenograft tumor growth, reduced breast cancer metastasis, and enhanced the efficacy of erlotinib as well as chemotherapy when used in combination [147]. Mab173 has been demonstrated to induce Axl degradation and impair tumor cell migration, invasion, apoptosis, and xenograft tumor growth in a Kaposi sarcoma model [148].
4.4. Axl-Specific Chimeric Antigen Receptor (CAR) T Cells
T cells with a chimeric antigen receptor (CAR) consisting of a single-chain variable fragment against Axl have been developed and evaluated preclinically. In a triple-negative breast cancer model, Axl-CAR-T cells inhibited tumor growth and showed association with increased secretion of antitumor cytokines, including TNFα, IFNγ, IL-2, IL-6, and IL-17A (Table 1) [149,150]. Axl-CAR.C7R (Axl-targeting CAR-T cells) that co-express constitutively activated the IL-7 receptor (C7R), showed enhanced activation of Axl-CAR-T cells in vitro, and impaired xenograft tumor growth (Table 1) [151].
In the clinical landscape, CCT301 CAR-T is undergoing a Phase I/II study for patients with renal cell carcinoma to assess safety, tolerability, and anti-tumor activity (Table 1) (Clinical Trial Identification #: NCT03393936). A Phase I study for Axl-CAR-T cell therapy for patients with lung cancer is also being initiated to evaluate safety, tolerability, and preliminary efficacy (Clinical Trial Identification #: NCT03198052).
4.5. Natural Products
4.5.1. Viscum album
Mistletoe extract prepared from Viscum album extract (VAE) has demonstrated anti-Axl activities (Table 1). VAE treatment decreased Axl expression and phosphorylation and impaired colony formation and tumor cell proliferation of naïve cells as well as cisplatin- and erlotinib-resistant cells [152].
4.5.2. Celastrol
Celastrol is a pentacyclic triterpenoid extracted from Tripterygium wilfordii Hook F and Celastrus regelii, L., which has anti-Axl activities in non-small-cell lung cancer cells (Table 1). Celastrol reduces Axl expression and impairs tumor cell migration and proliferation in both naïve and gefitinib-resistant non-small-cell lung cancer cells [153].
4.5.3. Yuanhuadine
Yuanhuadine is a daphnane diterpenoid from the flowers of Daphne genkwa, which has previously shown anti-proliferative effects in human lung cancer cells (Table 1) [154]. Yuanhuadine decreases Axl expression in both naïve and gefitinib- and osimertinib-resistant non-small-cell lung cancer cells [155]. Yuanhuadine suppresses tumor growth in vivo [155] and synergistically inhibits the growth of EGFR-TKI-resistant cells in vitro and in vivo [156].
5. Conclusions
Receptor tyrosine kinases have served as pharmacological targets in cancer treatment due to their frequent dysregulations, including gene amplifications, overexpression, and activating mutations, which promote tumor development, progression, and metastasis. The Gas6/Axl signaling pathway has gained significant attention as a therapeutic target due to its implications in cancer progression, metastasis, and therapeutic resistance [19,20,21]. In addition, studies demonstrate that the Gas6/Axl signaling pathway also modulates the tumor microenvironment [22]. Gas6 is secreted by both cancer and stromal cells [76,157,158,159,160,161,162,163], and studies demonstrate that Axl is also expressed by cancer and stromal cells [22]. Therefore, targeting the Gas6/Axl pathway offers an attractive and promising approach to impair tumor progression and dissemination.
In addition, Axl overexpression is associated with resistance to conventional anticancer treatments, such as chemotherapy and radiotherapy, as well as targeted therapies [60,67,77,109,110,115,126,155,164,165,166,167,168,169,170]. Studies demonstrate that the Axl targeting of resistant tumors can restore sensitivity. Hence, combination therapy with Axl inhibitors offers a rational treatment strategy to overcome therapeutic resistant tumors. Furthermore, Axl inhibition has been shown to modulate the tumor immune microenvironment by multiple mechanisms [22]. Chimeric antigen receptor T cells against Axl have been developed and studied preclinically [149,150,151], and a number of clinical studies are ongoing to evaluate the combination therapy of small molecule Axl inhibitors with immune checkpoint inhibitors. Since Axl is involved in multiple facets of the hallmarks of cancer, results from the ongoing clinical trials will highlight its implications in therapeutic resistance, immune evasion, and metastasis while also demonstrating its safety, tolerability, and efficacy.
Overall, a number of strategies have been employed to target Gas6 and Axl, including small molecule inhibitors, soluble receptors, monoclonal antibodies, CAR-T cell therapy, and natural compounds. These inhibitors have been tested as a monotherapy as well as combination therapies. While targeting the Gas6/Axl pathway has shown promising preclinical and clinical efficacy, targeting Gas6 or Axl as a monotherapy raises a concern about a possible narrow therapeutic index due to its expression and regulation of tumor growth in various cell types of the tumor. The Gas6/Axl pathway has been implicated in drug resistance and immune evasion, and preclinical and clinical studies have demonstrated that combination therapy may exhibit synergistic antitumor activity and drug selectivity. Hence, rational combination approaches and the selection of an appropriate patient population remain a necessity.
Although Axl overexpression is associated with poor clinical prognosis and outcome in multiple cancer types [20], the histological confirmation of Axl expression or activation is often absent in clinical study inclusion criteria. Furthermore, as Gas6 mRNA expression may not predict breast cancer outcomes [171], the assessment of Gas6 protein expression or serum levels, as a potential biomarker for patient response and outcome, is also warranted. Since small molecule tyrosine kinase inhibitors often have multiple targets, results from clinical trials utilizing a monoclonal antibody against Axl (i.e., Axl-107-MMAE and BA301), a chimeric antigen receptor T (CAR-T) cell against Axl (CCT301 CAR-T), and a soluble receptor against Gas6 (ABV-500) are highly anticipated and will offer efficacy and toxicity profiles and guidance for the future development of treatment strategies. Still, preclinical and clinical findings for various Gas6 and Axl inhibitors are favorable and make targeting this axis an attractive and promising approach to impair tumor progression and dissemination.
Author Contributions
Conceptualization, M.T.; investigation, M.T.; resources, D.W.S.; data curation, M.T.; writing—original draft preparation, M.T.; writing—review and editing, D.W.S.; supervision, D.W.S.; funding acquisition, D.W.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was in part funded by a grant from the National Institutes of Health (1R01CA197477).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Burstyn-Cohen T., Heeb M.J., Lemke G. Lack of protein S in mice causes embryonic lethal coagulopathy and vascular dysgenesis. J. Clin. Investig. 2009;119:2942–2953. doi: 10.1172/JCI39325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelillo-Scherrer A., de Frutos P., Aparicio C., Melis E., Savi P., Lupu F., Arnout J., Dewerchin M., Hoylaerts M., Herbert J., et al. Deficiency or inhibition of Gas6 causes platelet dysfunction and protects mice against thrombosis. Nat. Med. 2001;7:215–221. doi: 10.1038/84667. [DOI] [PubMed] [Google Scholar]
- 3.Angelillo-Scherrer A., Burnier L., Flores N., Savi P., DeMol M., Schaeffer P., Herbert J.M., Lemke G., Goff S.P., Matsushima G.K., et al. Role of Gas6 receptors in platelet signaling during thrombus stabilization and implications for antithrombotic therapy. J. Clin. Investig. 2005;115:237–246. doi: 10.1172/JCI22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu Q., Gore M., Zhang Q., Camenisch T., Boast S., Casagranda F., Lai C., Skinner M.K., Klein R., Matsushima G.K., et al. Tyro-3 family receptors are essential regulators of mammalian spermatogenesis. Nature. 1999;398:723–728. doi: 10.1038/19554. [DOI] [PubMed] [Google Scholar]
- 5.Shikawa M., Sonobe M., Nakayama E., Kobayashi M., Kikuchi R., Kitamura J., Imamura N., Date H. Higher expression of receptor tyrosine kinase Axl, and differential expression of its ligand, Gas6, predict poor survival in lung adenocarcinoma patients. Ann. Surg. Oncol. 2013;20((Suppl. S3)):S467–S476. doi: 10.1245/s10434-012-2795-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka K., Tokunaga E., Inoue Y., Yamashita N., Saeki H., Okano S., Kitao H., Oki E., Oda Y., Maehara Y. Impact of Expression of Vimentin and Axl in Breast Cancer. Clin. Breast Cancer. 2016;16:520–526.e2. doi: 10.1016/j.clbc.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 7.Lozneanu L., Pinciroli P., Ciobanu D.A., Carcangiu M.L., Canevari S., Tomassetti A., Caruntu I.D. Computational and Immunohistochemical Analyses Highlight AXL as a Potential Prognostic Marker for Ovarian Cancer Patients. Anticancer Res. 2016;36:4155–4163. [PubMed] [Google Scholar]
- 8.Cardone C., Blauensteiner B., Moreno-Viedma V., Martini G., Simeon V., Vitiello P.P., Ciardiello D., Belli V., Matrone N., Troiani T., et al. AXL is a predictor of poor survival and of resistance to anti-EGFR therapy in RAS wild-type metastatic colorectal cancer. Eur. J. Cancer. 2020;138:1–10. doi: 10.1016/j.ejca.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Liu J., Wang K., Yan Z., Xia Y., Li J., Shi L., Zou Q., Wan X., Jiao B., Wang H., et al. Axl Expression Stratifies Patients with Poor Prognosis after Hepatectomy for Hepatocellular Carcinoma. PLoS ONE. 2016;11:e0154767. doi: 10.1371/journal.pone.0154767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bottai G., Raschioni C., Szekely B., Di Tommaso L., Szasz A.M., Losurdo A., Gyorffy B., Acs B., Torrisi R., Karachaliou N., et al. AXL-associated tumor inflammation as a poor prognostic signature in chemotherapy-treated triple-negative breast cancer patients. NPJ Breast Cancer. 2016;2:16033. doi: 10.1038/npjbcancer.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh M.S., Yang P.W., Wong L.F., Lee J.M. The AXL receptor tyrosine kinase is associated with adverse prognosis and distant metastasis in esophageal squamous cell carcinoma. Oncotarget. 2016;7:36956–36970. doi: 10.18632/oncotarget.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu E., Hjelle B., Bishop J.M. Transforming genes in chronic myelogenous leukemia. Proc. Natl. Acad. Sci. USA. 1988;85:1952–1956. doi: 10.1073/pnas.85.6.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Bryan J.P., Frye R.A., Cogswell P.C., Neubauer A., Kitch B., Prokop C., Espinosa R., 3rd, Le Beau M.M., Earp H.S., Liu E.T. Axl, a transforming gene isolated from primary human myeloid leukemia cells, encodes a novel receptor tyrosine kinase. Mol. Cell. Biol. 1991;11:5016–5031. doi: 10.1128/MCB.11.10.5016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellido-Martin L., de Frutos P.G. Vitamin K-dependent actions of Gas6. Vitam. Horm. 2008;78:185–209. doi: 10.1016/S0083-6729(07)00009-X. [DOI] [PubMed] [Google Scholar]
- 15.Manfioletti G., Brancolini C., Avanzi G., Schneider C. The protein encoded by a growth arrest-specific gene (gas6) is a new member of the vitamin K-dependent proteins related to protein S, a negative coregulator in the blood coagulation cascade. Mol. Cell. Biol. 1993;13:4976–4985. doi: 10.1128/MCB.13.8.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang M., Rigby A.C., Morelli X., Grant M.A., Huang G., Furie B., Seaton B., Furie B.C. Structural basis of membrane binding by Gla domains of vitamin K-dependent proteins. Nat. Struct. Biol. 2003;10:751–756. doi: 10.1038/nsb971. [DOI] [PubMed] [Google Scholar]
- 17.Mark M.R., Chen J., Hammonds R.G., Sadick M., Godowsk P.J. Characterization of Gas6, a member of the superfamily of G domain-containing proteins, as a ligand for Rse and Axl. J. Biol. Chem. 1996;271:9785–9789. doi: 10.1074/jbc.271.16.9785. [DOI] [PubMed] [Google Scholar]
- 18.Sasaki T., Knyazev P.G., Clout N.J., Cheburkin Y., Gohring W., Ullrich A., Timpl R., Hohenester E. Structural basis for Gas6-Axl signalling. EMBO J. 2006;25:80–87. doi: 10.1038/sj.emboj.7600912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Axelrod H., Pienta K.J. Axl as a mediator of cellular growth and survival. Oncotarget. 2014;5:8818–8852. doi: 10.18632/oncotarget.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rankin E.B., Giaccia A.J. The Receptor Tyrosine Kinase AXL in Cancer Progression. Cancers. 2016;8:103. doi: 10.3390/cancers8110103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gay C.M., Balaji K., Byers L.A. Giving AXL the axe: Targeting AXL in human malignancy. Br. J. Cancer. 2017;116:415–423. doi: 10.1038/bjc.2016.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka M., Siemann D.W. Gas6/Axl Signaling Pathway in the Tumor Immune Microenvironment. Cancers. 2020;12:1850. doi: 10.3390/cancers12071850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutterer M., Knyazev P., Abate A., Reschke M., Maier H., Stefanova N., Knyazeva T., Barbieri V., Reindl M., Muigg A., et al. Axl and growth arrest-specific gene 6 are frequently overexpressed in human gliomas and predict poor prognosis in patients with glioblastoma multiforme. Clin. Cancer Res. 2008;14:130–138. doi: 10.1158/1078-0432.CCR-07-0862. [DOI] [PubMed] [Google Scholar]
- 24.Shinh Y.-S., Lai C.-Y., Kao Y.-R., Shiah S.-G., Chu Y.-W., Lee H.-S., Wu C.-W. Expression of Axl in Lung Adenocarcinoma and Correlation with Tumor Progression. Neoplasia. 2005;7:1058–1064. doi: 10.1593/neo.05640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gjerdrum C., Tiron C., Høiby T., Stefansson I., Haugen H., Sandal T., Collett K., Li S., McCormack E., Gjertsen B.T., et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl. Acad. Sci. USA. 2010;107:1124–1129. doi: 10.1073/pnas.0909333107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gustafsson A., Martuszewska D., Johansson M., Ekman C., Hafizi S., Ljungberg B., Dahlbäck B. Differential expression of Axl and Gas6 in renal cell carcinoma reflecting tumor advancement and survival. Clin. Cancer Res. 2009;15:4742–4749. doi: 10.1158/1078-0432.CCR-08-2514. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka M., Siemann D.W. Axl signaling is an important mediator of tumor angiogenesis. Oncotarget. 2019;10:2887–2898. doi: 10.18632/oncotarget.26882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zdzalik-Bielecka D., Poswiata A., Kozik K., Jastrzebski K., Schink K.O., Brewinska-Olchowik M., Piwocka K., Stenmark H., Miaczynska M. The GAS6-AXL signaling pathway triggers actin remodeling that drives membrane ruffling, macropinocytosis, and cancer-cell invasion. Proc. Natl. Acad. Sci. USA. 2021;118:e2024596118. doi: 10.1073/pnas.2024596118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abu-Thuraia A., Goyette M.A., Boulais J., Delliaux C., Apcher C., Schott C., Chidiac R., Bagci H., Thibault M.P., Davidson D., et al. AXL confers cell migration and invasion by hijacking a PEAK1-regulated focal adhesion protein network. Nat. Commun. 2020;11:3586. doi: 10.1038/s41467-020-17415-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maacha S., Hong J., von Lersner A., Zijlstra A., Belkhiri A. AXL Mediates Esophageal Adenocarcinoma Cell Invasion through Regulation of Extracellular Acidification and Lysosome Trafficking. Neoplasia. 2018;20:1008–1022. doi: 10.1016/j.neo.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiu K.C., Lee C.H., Liu S.Y., Chou Y.T., Huang R.Y., Huang S.M., Shieh Y.S. Polarization of tumor-associated macrophages and Gas6/Axl signaling in oral squamous cell carcinoma. Oral Oncol. 2015;51:683–689. doi: 10.1016/j.oraloncology.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Goyette M.A., Elkholi I.E., Apcher C., Kuasne H., Rothlin C.V., Muller W.J., Richard D.E., Park M., Gratton J.P., Cote J.F. Targeting Axl favors an antitumorigenic microenvironment that enhances immunotherapy responses by decreasing Hif-1alpha levels. Proc. Natl. Acad. Sci. USA. 2021;118:e2023868118. doi: 10.1073/pnas.2023868118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen Z., Xu X.H., Hu J. Role of pericytes in angiogenesis: Focus on cancer angiogenesis and anti-angiogenic therapy. Neoplasma. 2016;63:173–182. doi: 10.4149/201_150704N369. [DOI] [PubMed] [Google Scholar]
- 34.Lechertier T., Reynolds L.E., Kim H., Pedrosa A.R., Gomez-Escudero J., Munoz-Felix J.M., Batista S., Dukinfield M., Demircioglu F., Wong P.P., et al. Pericyte FAK negatively regulates Gas6/Axl signalling to suppress tumour angiogenesis and tumour growth. Nat. Commun. 2020;11:2810. doi: 10.1038/s41467-020-16618-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collett G., Wood A., Alexander M.Y., Varnum B.C., Boot-Handford R.P., Ohanian V., Ohanian J., Fridell Y.W., Canfield A.E. Receptor tyrosine kinase Axl modulates the osteogenic differentiation of pericytes. Circ. Res. 2003;92:1123–1129. doi: 10.1161/01.RES.0000074881.56564.46. [DOI] [PubMed] [Google Scholar]
- 36.Kasikara C., Davra V., Calianese D., Geng K., Spires T.E., Quigley M., Wichroski M., Sriram G., Suarez-Lopez L., Yaffe M.B., et al. Pan-TAM Tyrosine Kinase Inhibitor BMS-777607 Enhances Anti-PD-1 mAb Efficacy in a Murine Model of Triple-Negative Breast Cancer. Cancer Res. 2019;79:2669–2683. doi: 10.1158/0008-5472.CAN-18-2614. [DOI] [PubMed] [Google Scholar]
- 37.Satomura K., Derubeis A.R., Fedarko N.S., Ibaraki-O’Connor K., Kuznetsov S.A., Rowe D.W., Young M.F., Gehron Robey P. Receptor tyrosine kinase expression in human bone marrow stromal cells. J. Cell. Physiol. 1998;177:426–438. doi: 10.1002/(SICI)1097-4652(199812)177:3<426::AID-JCP6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Seitz H.M., Camenisch T.D., Lemke G., Earp H.S., Matsushima G.K. Macrophages and dendritic cells use different Axl/Mertk/Tyro3 receptors in clearance of apoptotic cells. J. Immunol. 2007;178:5635–5642. doi: 10.4049/jimmunol.178.9.5635. [DOI] [PubMed] [Google Scholar]
- 39.Subramanian M., Hayes C.D., Thome J.J., Thorp E., Matsushima G.K., Herz J., Farber D.L., Liu K., Lakshmana M., Tabas I. An AXL/LRP-1/RANBP9 complex mediates DC efferocytosis and antigen cross-presentation in vivo. J. Clin. Investig. 2014;124:1296–1308. doi: 10.1172/JCI72051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng T., Zhang Y., Chen Q., Yan K., Han D. Toll-like receptor-mediated inhibition of Gas6 and ProS expression facilitates inflammatory cytokine production in mouse macrophages. Immunology. 2012;135:40–50. doi: 10.1111/j.1365-2567.2011.03511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neubauer A., Fiebeler A., Graham D.K., O’Bryan J.P., Schmidt C.A., Barckow P., Serke S., Siegert W., Snodgrass H.R., Huhn D., et al. Expression of axl, a transforming receptor tyrosine kinase, in normal and malignant hematopoiesis. Blood. 1994;84:1931–1941. doi: 10.1182/blood.V84.6.1931.1931. [DOI] [PubMed] [Google Scholar]
- 42.Paolino M., Choidas A., Wallner S., Pranjic B., Uribesalgo I., Loeser S., Jamieson A.M., Langdon W.Y., Ikeda F., Fededa J.P., et al. The E3 ligase Cbl-b and TAM receptors regulate cancer metastasis via natural killer cells. Nature. 2014;507:508–512. doi: 10.1038/nature12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tirado-Gonzalez I., Descot A., Soetopo D., Nevmerzhitskaya A., Schaffer A., Kur I.M., Czlonka E., Wachtel C., Tsoukala I., Muller L., et al. AXL inhibition in macrophages stimulates host-versus-leukemia immunity and eradicates naive and treatment resistant leukemia. Cancer Discov. 2021 doi: 10.1158/2159-8290.CD-20-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lew E.D., Oh J., Burrola P.G., Lax I., Zagorska A., Traves P.G., Schlessinger J., Lemke G. Differential TAM receptor-ligand-phospholipid interactions delimit differential TAM bioactivities. Elife. 2014;3:e03385. doi: 10.7554/eLife.03385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirane A., Ludwig K.F., Sorrelle N., Haaland G., Sandal T., Ranaweera R., Toombs J.E., Wang M., Dineen S.P., Micklem D., et al. Warfarin Blocks Gas6-Mediated Axl Activation Required for Pancreatic Cancer Epithelial Plasticity and Metastasis. Cancer Res. 2015;75:3699–3705. doi: 10.1158/0008-5472.CAN-14-2887-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tsou W.I., Nguyen K.Q., Calarese D.A., Garforth S.J., Antes A.L., Smirnov S.V., Almo S.C., Birge R.B., Kotenko S.V. Receptor tyrosine kinases, TYRO3, AXL, and MER, demonstrate distinct patterns and complex regulation of ligand-induced activation. J. Biol. Chem. 2014;289:25750–25763. doi: 10.1074/jbc.M114.569020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zweemer A.J.M., French C.B., Mesfin J., Gordonov S., Meyer A.S., Lauffenburger D.A. Apoptotic Bodies Elicit Gas6-Mediated Migration of AXL-Expressing Tumor Cells. Mol. Cancer Res. 2017;15:1656–1666. doi: 10.1158/1541-7786.MCR-17-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kariolis M.S., Miao Y.R., Jones D.S., 2nd, Kapur S., Mathews I.I., Giaccia A.J., Cochran J.R. An engineered Axl ‘decoy receptor’ effectively silences the Gas6-Axl signaling axis. Nat. Chem. Biol. 2014;10:977–983. doi: 10.1038/nchembio.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rankin E.B., Fuh K.C., Taylor T.E., Krieg A.J., Musser M., Yuan J., Wei K., Kuo C.J., Longacre T.A., Giaccia A.J. AXL is an essential factor and therapeutic target for metastatic ovarian cancer. Cancer Res. 2010;70:7570–7579. doi: 10.1158/0008-5472.CAN-10-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rankin E.B., Fuh K.C., Castellini L., Viswanathan K., Finger E.C., Diep A.N., LaGory E.L., Kariolis M.S., Chan A., Lindgren D., et al. Direct regulation of GAS6/AXL signaling by HIF promotes renal metastasis through SRC and MET. Proc. Natl. Acad. Sci. USA. 2014;111:13373–13378. doi: 10.1073/pnas.1404848111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xiao Y., Zhao H., Tian L., Nolley R., Diep A.N., Ernst A., Fuh K.C., Miao Y.R., von Eyben R., Leppert J.T., et al. S100A10 Is a Critical Mediator of GAS6/AXL-Induced Angiogenesis in Renal Cell Carcinoma. Cancer Res. 2019;79:5758–5768. doi: 10.1158/0008-5472.CAN-19-1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roskoski R., Jr. Classification of small molecule protein kinase inhibitors based upon the structures of their drug-enzyme complexes. Pharmacol. Res. 2016;103:26–48. doi: 10.1016/j.phrs.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 53.Holland S.J., Pan A., Franci C., Hu Y., Chang B., Li W., Duan M., Torneros A., Yu J., Heckrodt T.J., et al. R428, a selective small molecule inhibitor of Axl kinase, blocks tumor spread and prolongs survival in models of metastatic breast cancer. Cancer Res. 2010;70:1544–1554. doi: 10.1158/0008-5472.CAN-09-2997. [DOI] [PubMed] [Google Scholar]
- 54.Vouri M., An Q., Birt M., Pilkington G.J., Hafizi S. Small molecule inhibition of Axl receptor tyrosine kinase potently suppresses multiple malignant properties of glioma cells. Oncotarget. 2015;6:16183–16197. doi: 10.18632/oncotarget.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goyette M.A., Duhamel S., Aubert L., Pelletier A., Savage P., Thibault M.P., Johnson R.M., Carmeliet P., Basik M., Gaboury L., et al. The Receptor Tyrosine Kinase AXL Is Required at Multiple Steps of the Metastatic Cascade during HER2-Positive Breast Cancer Progression. Cell Rep. 2018;23:1476–1490. doi: 10.1016/j.celrep.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 56.Wang C., Jin H., Wang N., Fan S., Wang Y., Zhang Y., Wei L., Tao X., Gu D., Zhao F., et al. Gas6/Axl Axis Contributes to Chemoresistance and Metastasis in Breast Cancer through Akt/GSK-3beta/beta-catenin Signaling. Theranostics. 2016;6:1205–1219. doi: 10.7150/thno.15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ludwig K.F., Du W., Sorrelle N.B., Wnuk-Lipinska K., Topalovski M., Toombs J.E., Cruz V.H., Yabuuchi S., Rajeshkumar N.V., Maitra A., et al. Small-Molecule Inhibition of Axl Targets Tumor Immune Suppression and Enhances Chemotherapy in Pancreatic Cancer. Cancer Res. 2018;78:246–255. doi: 10.1158/0008-5472.CAN-17-1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.D’Errico G., Alonso-Nocelo M., Vallespinos M., Hermann P.C., Alcala S., Garcia C.P., Martin-Hijano L., Valle S., Earl J., Cassiano C., et al. Tumor-associated macrophage-secreted 14-3-3zeta signals via AXL to promote pancreatic cancer chemoresistance. Oncogene. 2019;38:5469–5485. doi: 10.1038/s41388-019-0803-9. [DOI] [PubMed] [Google Scholar]
- 59.Antony J., Zanini E., Kelly Z., Tan T.Z., Karali E., Alomary M., Jung Y., Nixon K., Cunnea P., Fotopoulou C., et al. The tumour suppressor OPCML promotes AXL inactivation by the phosphatase PTPRG in ovarian cancer. EMBO Rep. 2018;19:e45670. doi: 10.15252/embr.201745670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lin J.Z., Wang Z.J., De W., Zheng M., Xu W.Z., Wu H.F., Armstrong A., Zhu J.G. Targeting AXL overcomes resistance to docetaxel therapy in advanced prostate cancer. Oncotarget. 2017;8:41064–41077. doi: 10.18632/oncotarget.17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Axelrod H.D., Valkenburg K.C., Amend S.R., Hicks J.L., Parsana P., Torga G., DeMarzo A.M., Pienta K.J. AXL Is a Putative Tumor Suppressor and Dormancy Regulator in Prostate Cancer. Mol. Cancer Res. 2019;17:356–369. doi: 10.1158/1541-7786.MCR-18-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tanaka M., Dykes S.S., Siemann D.W. Inhibition of the Axl pathway impairs breast and prostate cancer metastasis to the bones and bone remodeling. Clin. Exp. Metastasis. 2021;38:321–335. doi: 10.1007/s10585-021-10093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Asiedu M.K., Beauchamp-Perez F.D., Ingle J.N., Behrens M.D., Radisky D.C., Knutson K.L. AXL induces epithelial-to-mesenchymal transition and regulates the function of breast cancer stem cells. Oncogene. 2014;33:1316–1324. doi: 10.1038/onc.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cichon M.A., Szentpetery Z., Caley M.P., Papadakis E.S., Mackenzie I.C., Brennan C.H., O’Toole E.A. The receptor tyrosine kinase Axl regulates cell-cell adhesion and stemness in cutaneous squamous cell carcinoma. Oncogene. 2014;33:4185–4192. doi: 10.1038/onc.2013.388. [DOI] [PubMed] [Google Scholar]
- 65.Koorstra J.B., Karikari C.A., Feldmann G., Bisht S., Rojas P.L., Offerhaus G.J., Alvarez H., Maitra A. The Axl receptor tyrosine kinase confers an adverse prognostic influence in pancreatic cancer and represents a new therapeutic target. Cancer Biol. Ther. 2009;8:618–626. doi: 10.4161/cbt.8.7.7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guo Z., Li Y., Zhang D., Ma J. Axl inhibition induces the antitumor immune response which can be further potentiated by PD-1 blockade in the mouse cancer models. Oncotarget. 2017;8:89761–89774. doi: 10.18632/oncotarget.21125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Z., Lee J.C., Lin L., Olivas V., Au V., LaFramboise T., Abdel-Rahman M., Wang X., Levine A.D., Rho J.K., et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nat. Genet. 2012;44:852–860. doi: 10.1038/ng.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mahadevan D., Cooke L., Riley C., Swart R., Simons B., Della Croce K., Wisner L., Iorio M., Shakalya K., Garewal H., et al. A novel tyrosine kinase switch is a mechanism of imatinib resistance in gastrointestinal stromal tumors. Oncogene. 2007;26:3909–3919. doi: 10.1038/sj.onc.1210173. [DOI] [PubMed] [Google Scholar]
- 69.Tibes R., Fine G., Choy G., Redkar S., Taverna P., Oganesian A., Sahai A., Azab M., Tolcher A.W. A phase I, first-in-human dose-escalation study of amuvatinib, a multi-targeted tyrosine kinase inhibitor, in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2013;71:463–471. doi: 10.1007/s00280-012-2019-3. [DOI] [PubMed] [Google Scholar]
- 70.Mita M., Gordon M., Rosen L., Kapoor N., Choy G., Redkar S., Taverna P., Oganesian A., Sahai A., Azab M., et al. Phase 1B study of amuvatinib in combination with five standard cancer therapies in adults with advanced solid tumors. Cancer Chemother. Pharmacol. 2014;74:195–204. doi: 10.1007/s00280-014-2481-1. [DOI] [PubMed] [Google Scholar]
- 71.Patel V., Keating M.J., Wierda W.G., Gandhi V. Preclinical combination of TP-0903, an AXL inhibitor and B-PAC-1, a procaspase-activating compound with ibrutinib in chronic lymphocytic leukemia. Leuk. Lymphoma. 2016;57:1494–1497. doi: 10.3109/10428194.2015.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinha S., Boysen J., Nelson M., Secreto C., Warner S.L., Bearss D.J., Lesnick C., Shanafelt T.D., Kay N.E., Ghosh A.K. Targeted Axl Inhibition Primes Chronic Lymphocytic Leukemia B Cells to Apoptosis and Shows Synergistic/Additive Effects in Combination with BTK Inhibitors. Clin. Cancer Res. 2015;21:2115–2126. doi: 10.1158/1078-0432.CCR-14-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aveic S., Corallo D., Porcu E., Pantile M., Boso D., Zanon C., Viola G., Sidarovich V., Mariotto E., Quattrone A., et al. TP-0903 inhibits neuroblastoma cell growth and enhances the sensitivity to conventional chemotherapy. Eur. J. Pharmacol. 2018;818:435–448. doi: 10.1016/j.ejphar.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 74.Jeon J.Y., Buelow D.R., Garrison D.A., Niu M., Eisenmann E.D., Huang K.M., Zavorka Thomas M.E., Weber R.H., Whatcott C.J., Warner S.L., et al. TP-0903 is active in models of drug-resistant acute myeloid leukemia. JCI Insight. 2020;5:e140169. doi: 10.1172/jci.insight.140169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sen T., Tong P., Diao L., Li L., Fan Y., Hoff J., Heymach J.V., Wang J., Byers L.A. Targeting AXL and mTOR Pathway Overcomes Primary and Acquired Resistance to WEE1 Inhibition in Small-Cell Lung Cancer. Clin. Cancer Res. 2017;23:6239–6253. doi: 10.1158/1078-0432.CCR-17-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kanzaki R., Naito H., Kise K., Takara K., Eino D., Minami M., Shintani Y., Funaki S., Kawamura T., Kimura T., et al. Gas6 derived from cancer-associated fibroblasts promotes migration of Axl-expressing lung cancer cells during chemotherapy. Sci. Rep. 2017;7:10613. doi: 10.1038/s41598-017-10873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Balaji K., Vijayaraghavan S., Diao L., Tong P., Fan Y., Carey J.P., Bui T.N., Warner S., Heymach J.V., Hunt K.K., et al. AXL Inhibition Suppresses the DNA Damage Response and Sensitizes Cells to PARP Inhibition in Multiple Cancers. Mol. Cancer Res. 2017;15:45–58. doi: 10.1158/1541-7786.MCR-16-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Park I.K., Mundy-Bosse B., Whitman S.P., Zhang X., Warner S.L., Bearss D.J., Blum W., Marcucci G., Caligiuri M.A. Receptor tyrosine kinase Axl is required for resistance of leukemic cells to FLT3-targeted therapy in acute myeloid leukemia. Leukemia. 2015;29:2382–2389. doi: 10.1038/leu.2015.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen K.H., Hsu C.C., Song W.S., Huang C.S., Tsai C.C., Kuo C.D., Hsu H.S., Tsai T.H., Tsai C.Y., Woung L.C., et al. Celecoxib enhances radiosensitivity in medulloblastoma-derived CD133-positive cells. Childs Nerv. Syst. 2010;26:1605–1612. doi: 10.1007/s00381-010-1190-2. [DOI] [PubMed] [Google Scholar]
- 80.Blazek E.R., Foutch J.L., Maki G. Daoy medulloblastoma cells that express CD133 are radioresistant relative to CD133- cells, and the CD133+ sector is enlarged by hypoxia. Int. J. Radiat. Oncol. Biol. Phys. 2007;67:1–5. doi: 10.1016/j.ijrobp.2006.09.037. [DOI] [PubMed] [Google Scholar]
- 81.Lin J., Zhang X.M., Yang J.C., Ye Y.B., Luo S.Q. gamma-secretase inhibitor-I enhances radiosensitivity of glioblastoma cell lines by depleting CD133+ tumor cells. Arch. Med. Res. 2010;41:519–529. doi: 10.1016/j.arcmed.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 82.Dantas-Barbosa C., Lesluyes T., Loarer F.L., Chibon F., Treilleux I., Coindre J.M., Meeus P., Brahmi M., Bally O., Ray-Coquard I., et al. Expression and role of TYRO3 and AXL as potential therapeutical targets in leiomyosarcoma. Br. J. Cancer. 2017;117:1787–1797. doi: 10.1038/bjc.2017.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Christensen J.G., Zou H.Y., Arango M.E., Li Q., Lee J.H., McDonnell S.R., Yamazaki S., Alton G.R., Mroczkowski B., Los G. Cytoreductive antitumor activity of PF-2341066, a novel inhibitor of anaplastic lymphoma kinase and c-Met, in experimental models of anaplastic large-cell lymphoma. Mol. Cancer Ther. 2007;6:3314–3322. doi: 10.1158/1535-7163.MCT-07-0365. [DOI] [PubMed] [Google Scholar]
- 84.Cullinane C., Dorow D.S., Jackson S., Solomon B., Bogatyreva E., Binns D., Young R., Arango M.E., Christensen J.G., McArthur G.A., et al. Differential (18)F-FDG and 3’-deoxy-3’-(18)F-fluorothymidine PET responses to pharmacologic inhibition of the c-MET receptor in preclinical tumor models. J. Nucl. Med. 2011;52:1261–1267. doi: 10.2967/jnumed.110.086967. [DOI] [PubMed] [Google Scholar]
- 85.Zou H.Y., Li Q., Lee J.H., Arango M.E., McDonnell S.R., Yamazaki S., Koudriakova T.B., Alton G., Cui J.J., Kung P.P., et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67:4408–4417. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 86.FDA Approves Crizotinib for Children and Young Adults with Relapsed or Refractory, Systemic Anaplastic Large Cell Lymphoma. [(accessed on 18 August 2021)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-crizotinib-children-and-young-adults-relapsed-or-refractory-systemic-anaplastic-large.
- 87.Highlights of Prescribing Information: XALKORI. [(accessed on 18 August 2021)]; Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/202570s021lbl.pdf.
- 88.Dagogo-Jack I., Shaw A.T. Crizotinib resistance: Implications for therapeutic strategies. Ann. Oncol. 2016;27((Suppl. S3)):iii42–iii50. doi: 10.1093/annonc/mdw305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Casaluce F., Sgambato A., Sacco P.C., Palazzolo G., Maione P., Rossi A., Ciardiello F., Gridelli C. Resistance to Crizotinib in Advanced Non-Small Cell Lung Cancer (NSCLC) with ALK Rearrangement: Mechanisms, Treatment Strategies and New Targeted Therapies. Curr. Clin. Pharmacol. 2016;11:77–87. doi: 10.2174/1574884711666160502124134. [DOI] [PubMed] [Google Scholar]
- 90.Nakamichi S., Seike M., Miyanaga A., Chiba M., Zou F., Takahashi A., Ishikawa A., Kunugi S., Noro R., Kubota K., et al. Overcoming drug-tolerant cancer cell subpopulations showing AXL activation and epithelial-mesenchymal transition is critical in conquering ALK-positive lung cancer. Oncotarget. 2018;9:27242–27255. doi: 10.18632/oncotarget.25531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kato Y., Ninomiya K., Ohashi K., Tomida S., Makimoto G., Watanabe H., Kudo K., Matsumoto S., Umemura S., Goto K., et al. Combined effect of cabozantinib and gefitinib in crizotinib-resistant lung tumors harboring ROS1 fusions. Cancer Sci. 2018;109:3149–3158. doi: 10.1111/cas.13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Isfort S., Keller-v Amsberg G., Schafhausen P., Koschmieder S., Brummendorf T.H. Bosutinib: A novel second-generation tyrosine kinase inhibitor. Recent Results Cancer Res. 2014;201:81–97. doi: 10.1007/978-3-642-54490-3_4. [DOI] [PubMed] [Google Scholar]
- 93.Vultur A., Buettner R., Kowolik C., Liang W., Smith D., Boschelli F., Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Y.X., Knyazev P.G., Cheburkin Y.V., Sharma K., Knyazev Y.P., Orfi L., Szabadkai I., Daub H., Keri G., Ullrich A. AXL is a potential target for therapeutic intervention in breast cancer progression. Cancer Res. 2008;68:1905–1915. doi: 10.1158/0008-5472.CAN-07-2661. [DOI] [PubMed] [Google Scholar]
- 95.Tan D.S., Haaland B., Gan J.M., Tham S.C., Sinha I., Tan E.H., Lim K.H., Takano A., Krisna S.S., Thu M.M., et al. Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol. Cancer. 2014;13:13. doi: 10.1186/1476-4598-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim W.G., Guigon C.J., Fozzatti L., Park J.W., Lu C., Willingham M.C., Cheng S.Y. SKI-606, an Src inhibitor, reduces tumor growth, invasion, and distant metastasis in a mouse model of thyroid cancer. Clin. Cancer Res. 2012;18:1281–1290. doi: 10.1158/1078-0432.CCR-11-2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hochhaus A., Gambacorti-Passerini C., Abboud C., Gjertsen B.T., Brummendorf T.H., Smith B.D., Ernst T., Giraldo-Castellano P., Olsson-Stromberg U., Saussele S., et al. Bosutinib for pretreated patients with chronic phase chronic myeloid leukemia: Primary results of the phase 4 BYOND study. Leukemia. 2020;34:2125–2137. doi: 10.1038/s41375-020-0915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Burbridge M.F., Bossard C.J., Saunier C., Fejes I., Bruno A., Leonce S., Ferry G., Da Violante G., Bouzom F., Cattan V., et al. S49076 is a novel kinase inhibitor of MET, AXL, and FGFR with strong preclinical activity alone and in association with bevacizumab. Mol. Cancer Ther. 2013;12:1749–1762. doi: 10.1158/1535-7163.MCT-13-0075. [DOI] [PubMed] [Google Scholar]
- 99.Clemenson C., Chargari C., Liu W., Mondini M., Ferte C., Burbridge M.F., Cattan V., Jacquet-Bescond A., Deutsch E. The MET/AXL/FGFR Inhibitor S49076 Impairs Aurora B Activity and Improves the Antitumor Efficacy of Radiotherapy. Mol. Cancer Ther. 2017;16:2107–2119. doi: 10.1158/1535-7163.MCT-17-0112. [DOI] [PubMed] [Google Scholar]
- 100.Pennacchietti S., Cazzanti M., Bertotti A., Rideout W.M., 3rd, Han M., Gyuris J., Perera T., Comoglio P.M., Trusolino L., Michieli P. Microenvironment-derived HGF overcomes genetically determined sensitivity to anti-MET drugs. Cancer Res. 2014;74:6598–6609. doi: 10.1158/0008-5472.CAN-14-0761. [DOI] [PubMed] [Google Scholar]
- 101.Zhang Y., Xia M., Jin K., Wang S., Wei H., Fan C., Wu Y., Li X., Li X., Li G., et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer. 2018;17:45. doi: 10.1186/s12943-018-0796-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma S.V., Settleman J. Oncogene addiction: Setting the stage for molecularly targeted cancer therapy. Genes Dev. 2007;21:3214–3231. doi: 10.1101/gad.1609907. [DOI] [PubMed] [Google Scholar]
- 103.Rodon J., Postel-Vinay S., Hollebecque A., Nuciforo P., Azaro A., Cattan V., Marfai L., Sudey I., Brendel K., Delmas A., et al. First-in-human phase I study of oral S49076, a unique MET/AXL/FGFR inhibitor, in advanced solid tumours. Eur. J. Cancer. 2017;81:142–150. doi: 10.1016/j.ejca.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 104.Hoang-Xuan K., Hottinger A., Royer-Perron L., Alentorn A., Savatovsky J., Micheli R.D., Homicsko K., Banquet S., Pauly J., Sudey I., et al. Phase I/II study of S49076, a multi-target inhibitor of c-MET, AXL, FGFR in combination with bevacizumab in patients with recurrent glioblastoma. J. Clin. Oncol. 2016;34:2033. doi: 10.1200/JCO.2016.34.15_suppl.2033. [DOI] [Google Scholar]
- 105.Anonymized Synopsis for CL1-49076-002. [(accessed on 12 August 2021)]. Available online: https://clinicaltrials.servier.com/wp-content/uploads/CL1-49076-002-anonymised-synopsis.pdf.
- 106.Study That Tested the Combination of 2 Medicinesfor People with Glioblastoma, a Kind of Brain Cancer: A New Drug Called S49076 with a Marketed Drug Called Bevacizumab. [(accessed on 12 August 2021)]. Available online: https://clinicaltrials.servier.com/wp-content/uploads/CL1-49076-002-lay-summary.pdf.
- 107.Blumenthal G.M., Cortazar P., Zhang J.J., Tang S., Sridhara R., Murgo A., Justice R., Pazdur R. FDA approval summary: Sunitinib for the treatment of progressive well-differentiated locally advanced or metastatic pancreatic neuroendocrine tumors. Oncologist. 2012;17:1108–1113. doi: 10.1634/theoncologist.2012-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gyawali B., Goldstein D.A. The US Food and Drug Administration’s Approval of Adjuvant Sunitinib for Renal Cell Cancer: A Case of Regulatory Capture? JAMA Oncol. 2018;4:623–624. doi: 10.1001/jamaoncol.2017.5697. [DOI] [PubMed] [Google Scholar]
- 109.Zhou L., Liu X.D., Sun M., Zhang X., German P., Bai S., Ding Z., Tannir N., Wood C.G., Matin S.F., et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35:2687–2697. doi: 10.1038/onc.2015.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van der Mijn J.C., Broxterman H.J., Knol J.C., Piersma S.R., De Haas R.R., Dekker H., Pham T.V., Van Beusechem V.W., Halmos B., Mier J.W., et al. Sunitinib activates Axl signaling in renal cell cancer. Int. J. Cancer. 2016;138:3002–3010. doi: 10.1002/ijc.30022. [DOI] [PubMed] [Google Scholar]
- 111.Oslob J.D., Romanowski M.J., Allen D.A., Baskaran S., Bui M., Elling R.A., Flanagan W.M., Fung A.D., Hanan E.J., Harris S., et al. Discovery of a potent and selective aurora kinase inhibitor. Bioorg. Med. Chem. Lett. 2008;18:4880–4884. doi: 10.1016/j.bmcl.2008.07.073. [DOI] [PubMed] [Google Scholar]
- 112.Arbitrario J.P., Belmont B.J., Evanchik M.J., Flanagan W.M., Fucini R.V., Hansen S.K., Harris S.O., Hashash A., Hoch U., Hogan J.N., et al. SNS-314, a pan-Aurora kinase inhibitor, shows potent anti-tumor activity and dosing flexibility in vivo. Cancer Chemother. Pharmacol. 2010;65:707–717. doi: 10.1007/s00280-009-1076-8. [DOI] [PubMed] [Google Scholar]
- 113.Viracta Therapeutics, Inc. FORM 10-K. 31 March 2010. [(accessed on 14 August 2021)]. Available online: http://www.getfilings.com/sec-filings/100331/SUNESIS-PHARMACEUTICALS-INC_10-K/#ixzz74TL3YKdu.
- 114.Boschelli D.H., Wang Y.D., Ye F., Wu B., Zhang N., Dutia M., Powell D.W., Wissner A., Arndt K., Weber J.M., et al. Synthesis and Src kinase inhibitory activity of a series of 4-phenylamino-3-quinolinecarbonitriles. J. Med. Chem. 2001;44:822–833. doi: 10.1021/jm000420z. [DOI] [PubMed] [Google Scholar]
- 115.Byers L.A., Diao L., Wang J., Saintigny P., Girard L., Peyton M., Shen L., Fan Y., Giri U., Tumula P.K., et al. An epithelial-mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 2013;19:279–290. doi: 10.1158/1078-0432.CCR-12-1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ou W.B., Hubert C., Corson J.M., Bueno R., Flynn D.L., Sugarbaker D.J., Fletcher J.A. Targeted inhibition of multiple receptor tyrosine kinases in mesothelioma. Neoplasia. 2011;13:12–22. doi: 10.1593/neo.101156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ou W.B., Corson J.M., Flynn D.L., Lu W.P., Wise S.C., Bueno R., Sugarbaker D.J., Fletcher J.A. AXL regulates mesothelioma proliferation and invasiveness. Oncogene. 2011;30:1643–1652. doi: 10.1038/onc.2010.555. [DOI] [PubMed] [Google Scholar]
- 118.Christoph S., Deryckere D., Schlegel J., Frazer J.K., Batchelor L.A., Trakhimets A.Y., Sather S., Hunter D.M., Cummings C.T., Liu J., et al. UNC569, a novel small-molecule mer inhibitor with efficacy against acute lymphoblastic leukemia in vitro and in vivo. Mol. Cancer Ther. 2013;12:2367–2377. doi: 10.1158/1535-7163.MCT-13-0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang W., DeRyckere D., Hunter D., Liu J., Stashko M.A., Minson K.A., Cummings C.T., Lee M., Glaros T.G., Newton D.L., et al. UNC2025, a potent and orally bioavailable MER/FLT3 dual inhibitor. J. Med. Chem. 2014;57:7031–7041. doi: 10.1021/jm500749d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhao Z., Zhu X., Cui K., Mancuso J., Federley R., Fischer K., Teng G., Mittal V., Gao D., Zhao H., et al. In Vivo Visualization and Characterization of Epithelial-Mesenchymal Transition in Breast Tumors. Cancer Res. 2016;76:2094–2104. doi: 10.1158/0008-5472.CAN-15-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yang P.W., Liu Y.C., Chang Y.H., Lin C.C., Huang P.M., Hua K.T., Lee J.M., Hsieh M.S. Cabozantinib (XL184) and R428 (BGB324) Inhibit the Growth of Esophageal Squamous Cell Carcinoma (ESCC) Front. Oncol. 2019;9:1138. doi: 10.3389/fonc.2019.01138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Choueiri T.K., Powles T., Burotto M., Escudier B., Bourlon M.T., Zurawski B., Oyervides Juarez V.M., Hsieh J.J., Basso U., Shah A.Y., et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021;384:829–841. doi: 10.1056/NEJMoa2026982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chia S.K., Ellard S.L., Mates M., Welch S., Mihalcioiu C., Miller W.H., Jr., Gelmon K., Lohrisch C., Kumar V., Taylor S., et al. A phase-I study of lapatinib in combination with foretinib, a c-MET, AXL and vascular endothelial growth factor receptor inhibitor, in human epidermal growth factor receptor 2 (HER-2)-positive metastatic breast cancer. Breast Cancer Res. 2017;19:54. doi: 10.1186/s13058-017-0836-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seiwert T., Sarantopoulos J., Kallender H., McCallum S., Keer H.N., Blumenschein G., Jr. Phase II trial of single-agent foretinib (GSK1363089) in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Investig. New Drugs. 2013;31:417–424. doi: 10.1007/s10637-012-9861-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kataoka Y., Mukohara T., Tomioka H., Funakoshi Y., Kiyota N., Fujiwara Y., Yashiro M., Hirakawa K., Hirai M., Minami H. Foretinib (GSK1363089), a multi-kinase inhibitor of MET and VEGFRs, inhibits growth of gastric cancer cell lines by blocking inter-receptor tyrosine kinase networks. Investig. New Drugs. 2012;30:1352–1360. doi: 10.1007/s10637-011-9699-0. [DOI] [PubMed] [Google Scholar]
- 126.Liu L., Greger J., Shi H., Liu Y., Greshock J., Annan R., Halsey W., Sathe G.M., Martin A.M., Gilmer T.M. Novel mechanism of lapatinib resistance in HER2-positive breast tumor cells: Activation of AXL. Cancer Res. 2009;69:6871–6878. doi: 10.1158/0008-5472.CAN-08-4490. [DOI] [PubMed] [Google Scholar]
- 127.Eder J.P., Shapiro G.I., Appleman L.J., Zhu A.X., Miles D., Keer H., Cancilla B., Chu F., Hitchcock-Bryan S., Sherman L., et al. A phase I study of foretinib, a multi-targeted inhibitor of c-Met and vascular endothelial growth factor receptor 2. Clin. Cancer Res. 2010;16:3507–3516. doi: 10.1158/1078-0432.CCR-10-0574. [DOI] [PubMed] [Google Scholar]
- 128.Dolan M., Mastri M., Tracz A., Christensen J.G., Chatta G., Ebos J.M.L. Enhanced efficacy of sitravatinib in metastatic models of antiangiogenic therapy resistance. PLoS ONE. 2019;14:e0220101. doi: 10.1371/journal.pone.0220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Patwardhan P.P., Ivy K.S., Musi E., de Stanchina E., Schwartz G.K. Significant blockade of multiple receptor tyrosine kinases by MGCD516 (Sitravatinib), a novel small molecule inhibitor, shows potent anti-tumor activity in preclinical models of sarcoma. Oncotarget. 2016;7:4093–4109. doi: 10.18632/oncotarget.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Du W., Huang H., Sorrelle N., Brekken R.A. Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight. 2018;3:e124184. doi: 10.1172/jci.insight.124184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shen Y., Zhang W., Liu J., He J., Cao R., Chen X., Peng X., Xu H., Zhao Q., Zhong J., et al. Therapeutic activity of DCC-2036, a novel tyrosine kinase inhibitor, against triple-negative breast cancer patient-derived xenografts by targeting AXL/MET. Int. J. Cancer. 2019;144:651–664. doi: 10.1002/ijc.31915. [DOI] [PubMed] [Google Scholar]
- 132.Yan S.B., Peek V.L., Ajamie R., Buchanan S.G., Graff J.R., Heidler S.A., Hui Y.H., Huss K.L., Konicek B.W., Manro J.R., et al. LY2801653 is an orally bioavailable multi-kinase inhibitor with potent activity against MET, MST1R, and other oncoproteins, and displays anti-tumor activities in mouse xenograft models. Investig. New Drugs. 2013;31:833–844. doi: 10.1007/s10637-012-9912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barat S., Bozko P., Chen X., Scholta T., Hanert F., Gotze J., Malek N.P., Wilkens L., Plentz R.R. Targeting c-MET by LY2801653 for treatment of cholangiocarcinoma. Mol. Carcinog. 2016;55:2037–2050. doi: 10.1002/mc.22449. [DOI] [PubMed] [Google Scholar]
- 134.He A.R., Cohen R.B., Denlinger C.S., Sama A., Birnbaum A., Hwang J., Sato T., Lewis N., Mynderse M., Niland M., et al. First-in-Human Phase I Study of Merestinib, an Oral Multikinase Inhibitor, in Patients with Advanced Cancer. Oncologist. 2019;24:e930–e942. doi: 10.1634/theoncologist.2018-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dai Y., Bae K., Pampo C., Siemann D.W. Impact of the small molecule Met inhibitor BMS-777607 on the metastatic process in a rodent tumor model with constitutive c-Met activation. Clin. Exp. Metastasis. 2012;29:253–261. doi: 10.1007/s10585-011-9447-z. [DOI] [PubMed] [Google Scholar]
- 136.Schroeder G.M., An Y., Cai Z.W., Chen X.T., Clark C., Cornelius L.A., Dai J., Gullo-Brown J., Gupta A., Henley B., et al. Discovery of N-(4-(2-amino-3-chloropyridin-4-yloxy)-3-fluorophenyl)-4-ethoxy-1-(4-fluorophenyl)-2-oxo-1,2-dihydropyridine-3-carboxamide (BMS-777607), a selective and orally efficacious inhibitor of the Met kinase superfamily. J. Med. Chem. 2009;52:1251–1254. doi: 10.1021/jm801586s. [DOI] [PubMed] [Google Scholar]
- 137.Onken J., Torka R., Korsing S., Radke J., Krementeskaia I., Nieminen M., Bai X., Ullrich A., Heppner F., Vajkoczy P. Inhibiting receptor tyrosine kinase AXL with small molecule inhibitor BMS-777607 reduces glioblastoma growth, migration, and invasion in vitro and in vivo. Oncotarget. 2016;7:9876–9889. doi: 10.18632/oncotarget.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dai Y., Siemann D.W. BMS-777607, a small-molecule met kinase inhibitor, suppresses hepatocyte growth factor-stimulated prostate cancer metastatic phenotype in vitro. Mol. Cancer Ther. 2010;9:1554–1561. doi: 10.1158/1535-7163.MCT-10-0359. [DOI] [PubMed] [Google Scholar]
- 139.Sharma S., Zeng J.Y., Zhuang C.M., Zhou Y.Q., Yao H.P., Hu X., Zhang R., Wang M.H. Small-molecule inhibitor BMS-777607 induces breast cancer cell polyploidy with increased resistance to cytotoxic chemotherapy agents. Mol. Cancer Ther. 2013;12:725–736. doi: 10.1158/1535-7163.MCT-12-1079. [DOI] [PubMed] [Google Scholar]
- 140.Hughes V.S., Siemann D.W. Failures in preclinical and clinical trials of c-Met inhibitors: Evaluation of pathway activity as a promising selection criterion. Oncotarget. 2019;10:184–197. doi: 10.18632/oncotarget.26546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yokoyama Y., Lew E.D., Seelige R., Tindall E.A., Walsh C., Fagan P.C., Lee J.Y., Nevarez R., Oh J., Tucker K.D., et al. Immuno-oncological Efficacy of RXDX-106, a Novel TAM (TYRO3, AXL, MER) Family Small-Molecule Kinase Inhibitor. Cancer Res. 2019;79:1996–2008. doi: 10.1158/0008-5472.CAN-18-2022. [DOI] [PubMed] [Google Scholar]
- 142.Rho J.K., Choi Y.J., Kim S.Y., Kim T.W., Choi E.K., Yoon S.J., Park B.M., Park E., Bae J.H., Choi C.M., et al. MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation. Cancer Res. 2014;74:253–262. doi: 10.1158/0008-5472.CAN-13-1103. [DOI] [PubMed] [Google Scholar]
- 143.Leconet W., Larbouret C., Chardes T., Thomas G., Neiveyans M., Busson M., Jarlier M., Radosevic-Robin N., Pugniere M., Bernex F., et al. Preclinical validation of AXL receptor as a target for antibody-based pancreatic cancer immunotherapy. Oncogene. 2014;33:5405–5414. doi: 10.1038/onc.2013.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Leconet W., Chentouf M., du Manoir S., Chevalier C., Sirvent A., Ait-Arsa I., Busson M., Jarlier M., Radosevic-Robin N., Theillet C., et al. Therapeutic Activity of Anti-AXL Antibody against Triple-Negative Breast Cancer Patient-Derived Xenografts and Metastasis. Clin. Cancer Res. 2017;23:2806–2816. doi: 10.1158/1078-0432.CCR-16-1316. [DOI] [PubMed] [Google Scholar]
- 145.Boshuizen J., Koopman L.A., Krijgsman O., Shahrabi A., van den Heuvel E.G., Ligtenberg M.A., Vredevoogd D.W., Kemper K., Kuilman T., Song J.Y., et al. Cooperative targeting of melanoma heterogeneity with an AXL antibody-drug conjugate and BRAF/MEK inhibitors. Nat. Med. 2018;24:203–212. doi: 10.1038/nm.4472. [DOI] [PubMed] [Google Scholar]
- 146.Sharp L.L., Chang C., Frey G., Wang J., Liu H., Xing C., Yalcin S., Walls M., Ben Y., Boyle W.J., et al. Abstract 827: Anti-tumor efficacy of BA3011, a novel Conditionally Active Biologic (CAB) anti-AXL-ADC. Cancer Res. 2018;78:827. doi: 10.1158/1538-7445.AM2018-827. [DOI] [Google Scholar]
- 147.Ye X., Li Y., Stawicki S., Couto S., Eastham-Anderson J., Kallop D., Weimer R., Wu Y., Pei L. An anti-Axl monoclonal antibody attenuates xenograft tumor growth and enhances the effect of multiple anticancer therapies. Oncogene. 2010;29:5254–5264. doi: 10.1038/onc.2010.268. [DOI] [PubMed] [Google Scholar]
- 148.Liu R., Gong M., Li X., Zhou Y., Gao W., Tulpule A., Chaudhary P.M., Jung J., Gill P.S. Induction, regulation, and biologic function of Axl receptor tyrosine kinase in Kaposi sarcoma. Blood. 2010;116:297–305. doi: 10.1182/blood-2009-12-257154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reppert S., Koch S., Finotto S. IL-17A is a central regulator of lung tumor growth. Oncoimmunology. 2012;1:783–785. doi: 10.4161/onci.19735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei J., Sun H., Zhang A., Wu X., Li Y., Liu J., Duan Y., Xiao F., Wang H., Lv M., et al. A novel AXL chimeric antigen receptor endows T cells with anti-tumor effects against triple negative breast cancers. Cell. Immunol. 2018;331:49–58. doi: 10.1016/j.cellimm.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 151.Zhao Z., Li Y., Liu W., Li X. Engineered IL-7 Receptor Enhances the Therapeutic Effect of AXL-CAR-T Cells on Triple-Negative Breast Cancer. Biomed. Res. Int. 2020;2020:4795171. doi: 10.1155/2020/4795171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kim S., Kim K.C., Lee C. Mistletoe (Viscum album) extract targets Axl to suppress cell proliferation and overcome cisplatin- and erlotinib-resistance in non-small cell lung cancer cells. Phytomedicine. 2017;36:183–193. doi: 10.1016/j.phymed.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 153.Lee Y.J., Kim S.Y., Lee C. Axl is a novel target of celastrol that inhibits cell proliferation and migration, and increases the cytotoxicity of gefitinib in EGFR mutant nonsmall cell lung cancer cells. Mol. Med. Rep. 2019;19:3230–3236. doi: 10.3892/mmr.2019.9957. [DOI] [PubMed] [Google Scholar]
- 154.Hong J.Y., Chung H.J., Lee H.J., Park H.J., Lee S.K. Growth inhibition of human lung cancer cells via down-regulation of epidermal growth factor receptor signaling by yuanhuadine, a daphnane diterpene from Daphne genkwa. J. Nat. Prod. 2011;74:2102–2108. doi: 10.1021/np2003512. [DOI] [PubMed] [Google Scholar]
- 155.Bae S.Y., Hong J.Y., Lee H.J., Park H.J., Lee S.K. Targeting the degradation of AXL receptor tyrosine kinase to overcome resistance in gefitinib-resistant non-small cell lung cancer. Oncotarget. 2015;6:10146–10160. doi: 10.18632/oncotarget.3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kim D., Bach D.H., Fan Y.H., Luu T.T., Hong J.Y., Park H.J., Lee S.K. AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells. Cell Death Dis. 2019;10:361. doi: 10.1038/s41419-019-1601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mills K.L., Gomes A.M., Standlee C.R., Rojo M.D., Carmeliet P., Lin Z., Machado H.L. Gas6 is dispensable for pubertal mammary gland development. PLoS ONE. 2018;13:e0208550. doi: 10.1371/journal.pone.0208550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Shiozawa Y., Pedersen E.A., Patel L.R., Ziegler A.M., Havens A.M., Jung Y., Wang J., Zalucha S., Loberg R.D., Pienta K.J., et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia. 2010;12:116–127. doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Shiozawa Y., Pedersen E.A., Taichman R.S. GAS6/Mer axis regulates the homing and survival of the E2A/PBX1-positive B-cell precursor acute lymphoblastic leukemia in the bone marrow niche. Exp. Hematol. 2010;38:132–140. doi: 10.1016/j.exphem.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Khoo W.H., Ledergor G., Weiner A., Roden D.L., Terry R.L., McDonald M.M., Chai R.C., De Veirman K., Owen K.L., Opperman K.S., et al. A niche-dependent myeloid transcriptome signature defines dormant myeloma cells. Blood. 2019;134:30–43. doi: 10.1182/blood.2018880930. [DOI] [PubMed] [Google Scholar]
- 161.Bae C.A., Ham I.H., Oh H.J., Lee D., Woo J., Son S.Y., Yoon J.H., Lorens J.B., Brekken R.A., Kim T.M., et al. Inhibiting the GAS6/AXL axis suppresses tumor progression by blocking the interaction between cancer-associated fibroblasts and cancer cells in gastric carcinoma. Gastric Cancer. 2020;23:824–836. doi: 10.1007/s10120-020-01066-4. [DOI] [PubMed] [Google Scholar]
- 162.Gomes A.M., Carron E.C., Mills K.L., Dow A.M., Gray Z., Fecca C.R., Lakey M.A., Carmeliet P., Kittrell F., Medina D., et al. Stromal Gas6 promotes the progression of premalignant mammary cells. Oncogene. 2019;38:2437–2450. doi: 10.1038/s41388-018-0593-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Loges S., Schmidt T., Tjwa M., van Geyte K., Lievens D., Lutgens E., Vanhoutte D., Borgel D., Plaisance S., Hoylaerts M., et al. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 164.Skinner H.D., Giri U., Yang L.P., Kumar M., Liu Y., Story M.D., Pickering C.R., Byers L.A., Williams M.D., Wang J., et al. Integrative Analysis Identifies a Novel AXL–PI3 Kinase–PD-L1 Signaling Axis Associated with Radiation Resistance in Head and Neck Cancer. Clin. Cancer Res. 2017;23:2713–2722. doi: 10.1158/1078-0432.CCR-16-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brand T.M., Iida M., Stein A.P., Corrigan K.L., Braverman C.M., Coan J.P., Pearson H.E., Bahrar H., Fowler T.L., Bednarz B.P., et al. AXL Is a Logical Molecular Target in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2015;21:2601–2612. doi: 10.1158/1078-0432.CCR-14-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Aguilera T.A., Rafat M., Castellini L., Shehade H., Kariolis M.S., Hui A.B., Stehr H., von Eyben R., Jiang D., Ellies L.G., et al. Reprogramming the immunological microenvironment through radiation and targeting Axl. Nat. Commun. 2016;7:13898. doi: 10.1038/ncomms13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hong C.C., Lay J.D., Huang J.S., Cheng A.L., Tang J.L., Lin M.T., Lai G.M., Chuang S.E. Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett. 2008;268:314–324. doi: 10.1016/j.canlet.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 168.Kurokawa M., Ise N., Omi K., Goishi K., Higashiyama S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 2013;104:904–911. doi: 10.1111/cas.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dufies M., Jacquel A., Belhacene N., Robert G., Cluzeau T., Luciano F., Cassuto J.P., Raynaud S., Auberger P. Mechanisms of AXL overexpression and function in Imatinib-resistant chronic myeloid leukemia cells. Oncotarget. 2011;2:874–885. doi: 10.18632/oncotarget.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Meyer A.S., Miller M.A., Gertler F.B., Lauffenburger D.A. The receptor AXL diversifies EGFR signaling and limits the response to EGFR-targeted inhibitors in triple-negative breast cancer cells. Sci. Signal. 2013;6:ra66. doi: 10.1126/scisignal.2004155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ibrahim T., Mercatali L., Amadori D. A new emergency in oncology: Bone metastases in breast cancer patients (Review) Oncol. Lett. 2013;6:306–310. doi: 10.3892/ol.2013.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]