Abstract
The producers of essential oils from the Republic of Moldova care about the quality of their products and at the same time, try to capitalize on the waste from processing. The purpose of the present study was to analyze the chemical composition of lavender (Lavanda angustifolia L.) essential oil and some by-products derived from its production (residual water, residual herbs), as well as to assess their “in vitro” antimicrobial activity. The gas chromatography-mass spectrometry analysis of essential oils produced by seven industrial manufacturers led to the identification of 41 constituents that meant 96.80–99.79% of the total. The main constituents are monoterpenes (84.08–92.55%), followed by sesquiterpenes (3.30–13.45%), and some aliphatic compounds (1.42–3.90%). The high-performance liquid chromatography analysis allowed the quantification of known triterpenes, ursolic, and oleanolic acids, in freshly dried lavender plants and in the residual by-products after hydrodistillation of the essential oil. The lavender essential oil showed good antibacterial activity against Bacillus subtilis, Pseudomonas fluorescens, Xanthomonas campestris, Erwinia carotovora at 300 μg/mL concentration, and Erwinia amylovora, Candida utilis at 150 μg/mL concentration, respectively. Lavender plant material but also the residual water and ethanolic extracts from the solid waste residue showed high antimicrobial activity against Aspergillus niger, Alternaria alternata, Penicillium chrysogenum, Bacillus sp., and Pseudomonas aeroginosa strains, at 0.75–6.0 μg/mL, 0.08–0.125 μg/mL, and 0.05–4.0 μg/mL, respectively.
Keywords: Lavandula angustifolia L., essential oil, by-products, terpenic compounds, chromatographic analyses, antimicrobial activity, statistical data analysis
1. Introduction
Lavandula angustifolia Mill. (syn. Lavandula vera DC, syn. Lavandula officinalis Chaix ex Vill., syn. Lavandula spica L.) is a perennial evergreen shrub of the family Lamiaceae, native to the Mediterranean region. Nowadays, this species is naturalized almost all over Europe, North Africa, United States, and Australia [1]. L.angustifolia (Lavander) is one of the most valuable medicinal and aromatic plants traditionally used to treat pain, parasitic infections, burns, insect bites, cramps, and muscle spasms [2]. In addition to its application in herbal treatment, lavender is also cultivated for the essential oils used in aromatherapy and the cosmetic, food, and flavour industries [3,4,5].
This is possible due to the presence of a set of biologically active substances, especially in essential oil, which possesses a multidirectional therapeutic activity being used in the treatment of gastrointestinal, cardiovascular, respiratory, and urinary infections [6]. Scientific studies reported anti-inflammatory [7], antioxidant [8,9], sedative [10], cytotoxic [11,12], analgesic [7], antimicrobial [6,13,14], and anticonvulsive [15] properties of L. angustifolia essential oil. Literature data reveal a huge variation in terms of L. angustifolia essential oil content, with values ranging between 0.5 and 6.25% in the case of essential oil obtained from fresh and dry inflorescences [16]. The main constituents of L. angustifolia essential oil are linalool, linalyl acetate, 1,8-cineole, borneol, camphor, lavandulyl acetate, β-caryophyllene, β-ocimene, α-fenchone, terpinen-4-ol, caryophyllene oxide, limonene, pinenes, geranyl acetate, β-farnesene, santalene, lavandulol, camphene, geraniol, and α-terpineol [8,11,13,14,17,18,19,20,21,22,23,24,25,26]. The content of oxygenated monoterpenes prevails in L. angustifolia essential oil and varies between 36.33 and 92.90% [16].
The therapeutic effects of L. angustifolia are also determined by secondary metabolites such as oleanolic and ursolic acids, together with other pentacyclic triterpenes. [27,28]. It has been proven experimentally that both compounds in pure forms, as well as their synthetic derivatives, show multiple biological activities [29,30,31,32,33,34,35,36,37,38].
Some by-products, e.g., pomace or solid residues, that resulted after hydrodistillation of essential oil-producing plants could be considered as a source of biologically active compounds such as ursolic and oleanolic acids. In addition, residual distillation waters have various applications due to their aromatic and antimicrobial properties [39,40,41,42,43].
Antibiotic resistance is becoming one of the main problems of modern medicine since it substantially reduces the effectiveness of antibacterial treatments and is linked to increased patient mortality. As a result, known antibacterial preparations cease to be safe and effective against infections caused by resistant bacteria, leading to increasingly serious cases, including hospital-acquired complications. This requires the discovery of new classes of antibiotics or optimization and a combination of known compounds. However, microorganisms will likely evolve resistance in time and further research and development may be hard to sustain by the pharmaceutical companies. For this reason, studies are being conducted to identify effective remedies against multidrug-resistant strains. Preference is given to natural products among which are the essential oils [44], including lavender [45], or their combination with antibiotics [46]. Still, information about the antimicrobial activity of residual water and ethanolic extracts is very scanty and is mainly related to Lavander hydrosol, which is produced synthetically [47].
The aim of this study was to (i) evaluate the chemical composition of lavender essential oil and some of the waste by-products produced industrially in the Republic of Moldova using different chromatographic techniques; (ii) assess the in vitro antimicrobial activity of extracted compounds; and (iii) distinguish, using statistical analysis, between different lavender oils produced in different regions of the Republic of Moldova (Northern, Central, and Southern), based on the terpenic and aliphatic compounds.
2. Results
2.1. GC-MS Analysis Results
A total of 41 constituents of lavender essential oil were identified by means of gas chromatography-mass spectrometry (GC-MS) analysis (Table 1).
Table 1.
Phytochemical (terpenic and aliphatic compounds) composition of lavender essential oil of Moldovan origin.
| No. | RT* (min) |
Component | Producer, Content (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| P1 | P2 | P3 | P4 | P5 | P6 | P7 | |||
| 1 | 4.416 | α-Pinene | 0.36 | 0.57 | 0.36 | 0.18 | 0.09 | 0.26 | 0.57 |
| 2 | 4.710 | Camphene | 0.34 | 0.47 | 0.30 | 0.09 | 0.09 | 0.26 | 0.63 |
| 3 | 5.179 | Sabinene | 0.14 | - | - | - | - | - | - |
| 4 | 5.240 | 1-Octen-3-ol | 0.83 | - | - | - | 0.15 | 0.27 | |
| 5 | 5.263 | β-Pinene | 0.51 | - | 0.34 | 0.39 | 0.38 | 0.22 | 0.57 |
| 6 | 5.398 | Octan-3-one | 0.28 | 0.51 | 0.31 | 0.25 | 0.39 | 0.12 | 0.21 |
| 7 | 5.489 | β-Myrcene | 1.06 | 1.50 | 0.96 | 0.80 | 0.89 | 0.62 | 0.89 |
| 8 | 5.577 | Octan-3-ol | 0.13 | 0.18 | 0.20 | 0.17 | 0.20 | - | - |
| 9 | 5.962 | n-Hexyl acetate | 0.59 | 1.27 | 0.31 | 0.42 | 0.55 | 0.52 | 1.17 |
| 10 | 6.284 | p-Cymene | 0.22 | 0.46 | 0.22 | 0.14 | 0.10 | 0.24 | 0.39 |
| 11 | 6.400 | Limonene | 0.52 | 1.79 | 0.79 | 0.45 | 0.55 | 1.17 | 1.93 |
| 12 | 6.455 | 1,8-Cineol (eucalyptol) | 5.00 | 3.81 | 2.22 | 3.73 | 4.44 | 3.83 | 9.29 |
| 13 | 6.574 | (E)-Ocimene | 8.06 | 5.87 | 6.85 | 7.86 | 4.37 | 5.25 | 7.15 |
| 14 | 6.807 | (Z)-Ocimene | 3.74 | 3.45 | 2.59 | 2.55 | 1.86 | 1.76 | 2.16 |
| 15 | 7.087 | γ-Terpinene | 0.10 | 0.29 | 0.29 | 0.06 | 0.07 | 0.07 | 0.15 |
| 16 | 7.443 | Linalool oxide | - | 0.17 | 0.07 | 0.11 | 0.12 | - | - |
| 17 | 7.824 | δ-Terpinene | 0.27 | - | - | - | - | - | - |
| 18 | 7.825 | α-Terpinolene | - | 0.58 | 0.25 | 0.21 | 0.32 | 0.16 | 0.23 |
| 19 | 8.238 | Linalool | 23.54 | 27.98 | 29.06 | 25.57 | 40.68 | 33.29 | 26.19 |
| 20 | 8.392 | Oct-1-en-3-yl acetate | 0.56 | 0.82 | 0.60 | 0.71 | 0.63 | 0.39 | 0.58 |
| 21 | 9.308 | Camphor | 0.47 | 0.47 | 0.37 | 0.36 | 0.30 | 0.32 | 0.61 |
| 22 | 9.847 | Borneol | 1.92 | 2.15 | 1.68 | 1.28 | 1.65 | 1.41 | 2.40 |
| 23 | 10.00 | (3E,5Z)-Undeca-1,3,5-triene | 0.17 | - | - | - | - | - | - |
| 24 | 10.15 | Terpin-1-en-4-ol | 1.30 | 4.65 | 5.98 | 0.94 | 1.67 | 1.41 | 1.03 |
| 25 | 10.41 | Cryptone | 0.29 | 0.29 | - | - | - | 0.30 | 0.33 |
| 26 | 10.50 | α-Terpineol | 2.42 | 3.31 | 2.02 | 1.42 | 7.95 | 1.49 | 1.61 |
| 27 | 11.49 | Nerol | 0.38 | 0.46 | 0.23 | 0.14 | 1.14 | - | - |
| 28 | 11.84 | p-Cumic aldehyde | 0.13 | 0.15 | - | - | - | 0.18 | - |
| 29 | 12.32 | Linalyl acetate | 26.55 | 20.26 | 28.65 | 32.25 | 16.68 | 33.30 | 28.10 |
| 30 | 13.01 | Bornyl acetate | 0.32 | 0.25 | 0.24 | 0.27 | 0.19 | 0.17 | 0.24 |
| 31 | 13.11 | Lavandulyl acetate | 4.88 | 2.84 | 2.36 | 4.83 | 4.78 | 2.56 | 3.07 |
| 32 | 14.98 | Neryl acetate | 0.78 | 0.91 | 0.39 | 0.33 | 1.53 | 0.31 | 0.37 |
| 33 | 15.47 | Geranyl acetate | 1.31 | 1.69 | 0.79 | 0.73 | 2.70 | 0.59 | 0.67 |
| 34 | 15.66 | α-Zingiberene | 0.15 | - | - | - | - | - | - |
| 35 | 16.47 | β-Caryophyllene | 6.25 | 5.33 | 4.62 | 5.44 | 1.64 | 4.93 | 4.32 |
| 36 | 16.80 | α-Bergamotene | 0.27 | 0.28 | 0.19 | 0.20 | 0.05 | 0.16 | - |
| 37 | 17.31 | (E)-β-Farnesene | 4.86 | 2.59 | 3.65 | 3.94 | 1.23 | 2.46 | 2.45 |
| 38 | 17.97 | β-Cubebene | 1.12 | 0.82 | 1.03 | 0.69 | 0.17 | - | - |
| 39 | 18.75 | γ-Cadinene | 0.18 | 0.53 | - | - | - | 0.68 | 0.65 |
| 40 | 20.39 | Caryophyllene oxide | 0.45 | 0.69 | 0.19 | 0.29 | 0.21 | 0.35 | 0.28 |
| 41 | 21.69 | Cadinol | 0.17 | 0.57 | - | - | - | 0.18 | - |
| Total content, (%) | 99.80 | 98.79 | 98.17 | 96.80 | 97.62 | 99.11 | 98.51 | ||
*RT: Retention time; P 1–7: Producers.
It must be mentioned that the essential oil with the richest content was made by producer P1, which is the largest and operates a stationary modern factory. By contrast, producers P2 to P7 use mobile installations and process raw plant material directly in the field, in modernized or artisanal installations, and this may influence the chemical composition of essential oils and resulting by-products.
According to the GC-MS data, the chemical composition of lavender essential oil produced in Moldova consisted mainly of terpenic and aliphatic compounds and their content varied within the limits indicated in Table 2.
Table 2.
Chemical composition of lavender essential oil.
| Class | Subclass | Content, (%) |
|---|---|---|
| Terpenic compounds | 94.89–97.77 | |
| Monoterpenes | 84.08–92.55 | |
| Monoterpene hydrocarbons | 8.72–15.32 | |
| Oxygenated monoterpenes | 69.00–83.83 | |
| Sesquiterpenes | 3.30–13.45 | |
| Sesquiterpene hydrocarbons | 3.09–12.83 | |
| Oxygenated sesquiterpenes | 0.19–1.26 | |
| Aliphatic compounds | 1.42–3.90 | |
| Hydrocarbons | 0.17 | |
| Alcohols | 0.13–1.01 | |
| Ketones | 0.25–0.80 | |
| Esters | 0.91–2.09 | |
| Total | 96.80–99.79 |
The GC-MS analysis of extracts from residual waters (RW) showed that they contained only several hydrophilic components (see Section 3.3) and represented about 0.3–0.5% of the volume.
2.2. RP-HPLC Analysis Results
The content of triterpenic oleanolic acid (OA) and ursolic acid (UA) was established in freshly dried lavender plants and in dried solid residues (after hydrodistillation) via RP-HPLC analysis.
The results were expressed as mg/g for extracts and mg/100 g for the ratio plant material/solid residue (Table 3 and Table 4). It was observed that fresh plants had a much higher content of OA and UA.
Table 3.
The OA and UA content of lavender plant material (DW).
| Lavender Plant Material | Extract Yield (%) | Concentration (mg/g Extract) |
Concentration (mg/100 g Lavender Plant Material, DW) |
||
|---|---|---|---|---|---|
| OA | UA | OA | UA | ||
| LPM 1 | 9.94 | 16.19 | 37.46 | 160.95 | 372.36 |
| LPM 2 | 8.83 | 19.09 | 60.82 | 168.57 | 537.00 |
| LPM 3 | 9.91 | 13.43 | 33.28 | 133.11 | 329.83 |
Table 4.
The OA and UA content of lavender by-product (solid waste residue), (DW).
| Lavender by-Product (Solid Residue, SR) | Extract Yield (%) | Concentration (mg/g Extract) |
Concentration (mg/100 g Dry Solid Residue) |
||
|---|---|---|---|---|---|
| OA | UA | OA | UA | ||
| SR 1 | 3.88 | 29.21 | 80.82 | 113.47 | 313.95 |
| SR 2 | 3.68 | 39.37 | 135.56 | 144.98 | 499.15 |
| SR 3 | 4.15 | 27.48 | 87.90 | 114.07 | 364.89 |
The lower content of OA and UA in solid residues can be explained by their loss and derivatization/degradation during hydrodistillation in an aqueous medium at elevated temperatures (Table 4). The latter seemed more relevant since neither OA nor UA was found in residual water extracts (see Section 3.3).
2.3. Microbial Inhibition Assessment Results
The microbial activity assessment of lavender essential oil extracts from lavender plant material (LPM), lavender by-products (residual water (RW), and solid waste residue (SR)) was performed by serial dilution methods against several non-pathogenic Gram-positive and Gram-negative bacteria strains and fungi species, including phytopathogenic ones (e.g., Xanthomonas campestris, Erwinia amylovora, and Erwinia carotovora).
The results of the lavender essential oil antibacterial and antifungal activity tests are presented in Table 5.
Table 5.
The antimicrobial activity of lavender essential oil.
| MBC and MFC, μg/mL | ||||||
|---|---|---|---|---|---|---|
| Sample |
Bacillus
subtilis |
Pseudomonas
fluorescens |
Xantdomonas
campestris |
Erwinia
amylovora |
Erwinia
carotovora |
Candida
utilis |
| LEO | 300 | 300 | 300 | 150 | 300 | 150 |
MBC: Minimal bactericidal concentration; MFC: Minimal fungicidal concentration.
The same method was applied for residual waters, ethanolic extracts from solid residues, and freshly dried lavender plant materials (Table 6).
Table 6.
The antimicrobial activity of residual water and ethanolic extracts from lavender plants.
|
Sample |
MIC (μg/mL) | ||||
|---|---|---|---|---|---|
| Aspergillus niger | Alternaria alternata | Penicillium chrysogenum | Bacillus sp. |
Pseudomonas
aeruginosa |
|
| Residual Water |
0.08 | 0.08 | 0.08 | 0.125 | 0.125 |
| Extract from SR | 0.50 | 0.50 | 0.50 | 4 | 4 |
| Extract from LPM | 0.75 | 0.75 | 0.75 | 6 | 6 |
| Caspofungin a | 0.24 | 0.24 | 0.24 | - | - |
| Kanamycin b | - | - | - | 3.5 | 3.5 |
RSD (μg/mL): a ±0.001 b ±0.0002.
All of the samples were preliminarily tested for their in vitro antimicrobial activity and antifungal effect against pure cultures of three species of fungi (Aspergillus niger, Alternaria alternate, Penicillium chrysogenum) and against Gram-positive (Bacillus sp.) and Gram-negative bacteria (Pseudomonas aeruginosa). Microorganisms were provided by the American Type Culture Collection (ATCC, USA). Caspofungin and Kanamycin were used as performance standards for testing the antifungal and antibacterial activities. The minimum inhibitory concentration values (MIC) for all the samples and standards are summarized in Table 6.
3. Discussion
3.1. Chemical Composition of Lavender Essential Oils
The essential oil manufactured by producer P1, destined for export, had the following physico-chemical properties: Density (20 °C)—0.8920 g/mL; refractive index (n20D)—1.4660, and optical rotation (α20D)— −7.0°.
The most multitudinous group of terpenic compounds are monoterpenes, which include C10-hidrocarbones (8.72–15.32%) and their oxygenated derivatives (69.0–83.83%). The main constituents of this group which determine the quality and genuineness of lavender essential oil, according to the International Standard [48], are (%): 1,8-cineol (eucalyptol) (<1.0), (E)-ocimene (4.0–10.0), (Z)-ocimene (1.5–6.0), linalool (25.0–38.0), camphor (<0.5), terpin-1-en-4-ol (2.0–6.0), α-terpineol (<1.0), linalyl acetate (25.0–45.0), and lavandulyl acetate (>2.0) (Table 1 and Table 2).
The content of sesquiterpene hydrocarbons and their oxygenated derivatives is reported to be within the limits of 3.09–12.83% and 0.19–1.26%, respectively. According to the same source [48], the most important sesquiterpenes are: β-caryophyllene (4.78%), (E)-β-farnesene (1.52%), and caryophyllene oxide (0.36%) (Table 1 and Table 2).
Aliphatic compounds are of lesser concentration (1.42–3.90%) and in [48] are mentioned: 1-octen-3-ol (0.33%) and octan-3-one (<2.0%) (Table 1 and Table 2).
3.2. Chemical Composition of Lavender Plant Material
For the selective extraction of ursolic and oleanolic triterpene acids from the lavender plant materials (LPM), the extraction yield varied between 8.83–9.94%, with the OA content between 13.43–19.09 mg/g and UA content between 33.28–60.82 mg/g. The content of OA and UA in dry (DW) LPM was in the range of 133.11–168.57 mg/100 g, and respectively 329.83–537.00 mg/100 g DW LPM (Table 3).
Moreover, the experimental results showed that the sum of isomeric OA and UA in LPM was about 5% of the DW, in a 1:3.7 ratio, confirming that lavender is a valuable source of natural OA and UA triterpene acids.
3.3. Chemical Composition of Lavender by-Products
The GC-MS analysis of etheric extracts of residual water (RW) proved that they contain hydrophilic monoterpenic compounds such as 1,8-cineol (eucalyptol, 6.31%), linalool oxide (3.08%), linalool (78.05%), terpin-1-en-4-ol (1.92%), and α-terpineol (10.64%).
HPLC quantification of UA and OA indicated that RWs did not contain OA and UA triterpene acids.
In the case of solid waste residues (SR), the average extraction yield was about 3.91%, with the OA content between 27.48–39.37 mg/g and UA content between 80.82–135.56 mg/g (Table 4). The isomeric OA and UA in DW SR ranged between 113.47–144.98 and 313.95–499.15 mg/100 g, respectively (Table 4), with their amount accounting to about 1% of DW, in a 1:3.1 ratio, indicating that lavender by-products are a promising source of OA and UA triterpene acids.
Our results are consistent with other literature data reporting DW of lavender SR values between 136.0–259.7 and 346.3–648.4 mg/100 g [49].
3.4. Antimicrobial Assessments
Phytopathogenic bacteria can cause various diseases of agricultural plants, especially the genera Erwinia and Xanthomonas. For example, Erwinia amylovora, the Gram-negative bacterium of the Enterobacteriaceae family, is the causative agent of fire blight, a devastating plant disease that affects a wide range of species of the family Rosaceae and is a major global threat to commercial apple and pear production. [50]. Another species, E. carotovora, causes bacterial soft rot in economically important crops, such as potatoes, tomatoes, and cucumbers. In the case of potatoes, the soft rot of the stem and tubers occurs even after harvest, thus considerably reducing the yield [51]. Xanthomonas campestris pv. vesicatoria is a biotrophic Gram-negative bacterium and is the agent that causes bacterial leaf scorch on tomatoes (Solanum lycopersicum L.) and peppers (Capsicum annuum), a disease that is present worldwide. Symptoms of bacterial infection include defoliation and chlorotic necrotic lesions on leaves, stems, fruits, and flowers, which subsequently lead to reduced fruit yield [52].
The species Bacillus subtilis and Pseudomonas fluorescens do not cause any disease to plants but were selected as reference bacteria from the Gram-positive and Gram-negative groups. They are also very suitable as test objects for evaluating the antibacterial activity of the lavender extract. Candida utilis and Saccharomyces cerevisiae are also non-pathogenic but were used as representatives of the yeast-fungus group for evaluating the antifungal activity of the extract.
It should be mentioned that there is a lack of information about any antimicrobial effects of lavender essential oil on E. carotovora, E. amylovora, and C. utilis.
The in vitro assessment of lavender essential oil of Moldovan origin showed good antibacterial activity against both non-pathogenic Gram-positive/Gram-negative bacteria (B. subtilis and P. fluorescens) at MBC of 300 μg/mL and good to high antifungal activity against phytopathogenic bacteria (X. campestris, E. amylovora, E. carotovora) and C. utilis fungi at MFC of 150–300 μg/mL (Table 5).
The highest antifungal and antibacterial activities were observed for residual water (RW) at 0.08 and 0.125 μg/mL, respectively. Good antifungal and antibacterial activities were ascertained for the SR extract as well (0.50 and 4 μg/mL). The LPM extract showed moderate antifungal and antibacterial activity (0.75 and 6 μg/mL).
The two techniques employed for testing both the disc diffusion and the dilution methods have been developed to yield accurate measurements of antibacterial and antifungal activities and are routinely used in antimicrobial susceptibility testing.
According to the obtained results, the antibacterial activity was similar but the antifungal activity was slightly different, thus suggesting that the activity against different microorganisms could be caused by different components of the oil.
3.5. Statistical Data Analysis
Univariate as well as multivariate statistical data analysis (SDA) represent one of the most reliable methods that permit extracting useful information and inferring different hypotheses concerning the considered set of data. Given the great diversity of organic compounds which can be found in lavender essential oil, multivariate statistical data analysis was an appropriate method allowing to group samples, in this case, according to the lavender oil producer and based on the concentrations of organic compounds (R mode), or, to classify an experimentally determined organic compound based on the concentration in samples (Q mode) [53,54].
It is worth mentioning that, to avoid any errors induced by missing data, SDA was applied only in the cases of compounds with a non-negligible variation present in all the samples (Table 1), i.e., the compounds which permitted generating the box plots in Figure 1a,b.
Figure 1.
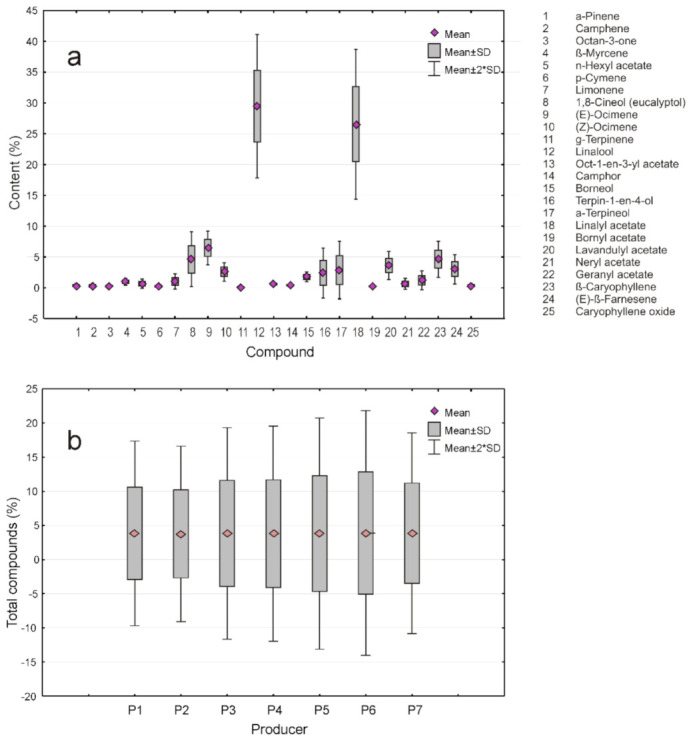
Box plots representing the distribution of (a) 25 components of lavender oil and (b) total content of compounds present in all the samples (producers).
Univariate SDA was useful in establishing the extent to which the samples of lavender oil by the seven producers were similar. This information was obtained by analyzing the box plot shown in Figure 1a. It was observed that all the samples were quite similar. To confirm this, we used more univariate tests, such as one-way ANOVA, Tukey’s pairwise test, Kruskal-Wallis test of equal medians, as well as Mann-Whitney U tests. All of them confirmed that between the lavender oil samples there are no statistically significant differences. For this reason, we have proceeded with multivariate SDA.
Within multivariate SDA, each sample (case) is characterized by independent parameters (variables), so that the final analysis can be performed in R mode (to study relations between samples based on variables) or Q mode (to study the interrelations between variables based on samples). As both methods were based on the same set of samples and variables, R and Q modes could be considered complementary, which significantly enhanced the analysis.
Depending on the situation, cases/variables can be grouped by a multitude of procedures among which covariance and correlation are frequently utilized.
In the case of lavender samples, the best results were obtained by the principal component analysis (PCA) applied in both R and Q modes. With respect to the other two SDA methods, cluster analysis and K mean clustering, PCA permitted evidencing the association of samples, i.e., seven producers of lavender oil in R mode, as well as 25 lavender oil compounds in Q mode. Moreover, in R mode, a tree diagram corresponding to the cluster analysis (Euclidean distances) is, concerning the number and structure of clusters, similar to PCA based on correlation. For this reason, we restrained our SDA to both R and Q mode PCA.
The results, represented by the principal component (PC) 2 vs. PC 1 bi-plots, are illustrated in Figure 2a,b, respectively. In both cases, the PCA was based on correlations between variables (organic compounds, R mode) or samples (lavender oil producer, Q mode). Moreover, the loadings of each variable or sample were represented by Factor 2 vs. Factor 1 bi-plots in the corresponding insets: Variables in Figure 2a and samples in Figure 2b.
Figure 2.
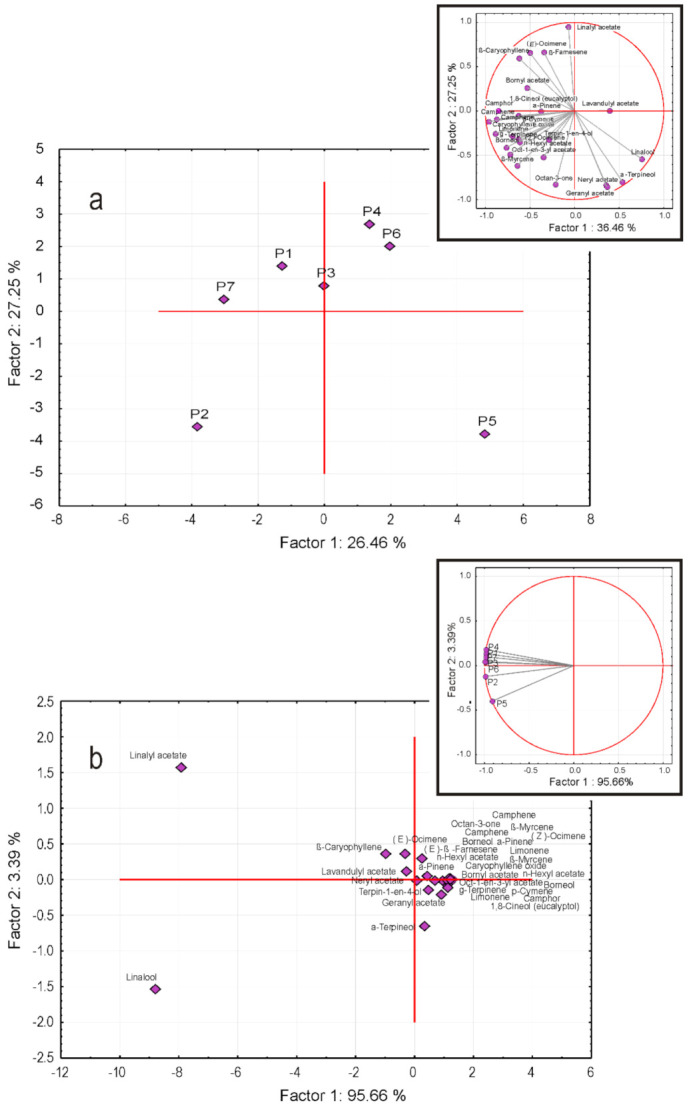
The results of R (a) and Q (b) mode PCA. The insets illustrate the contribution of the corresponding principal component (PC) analysis.
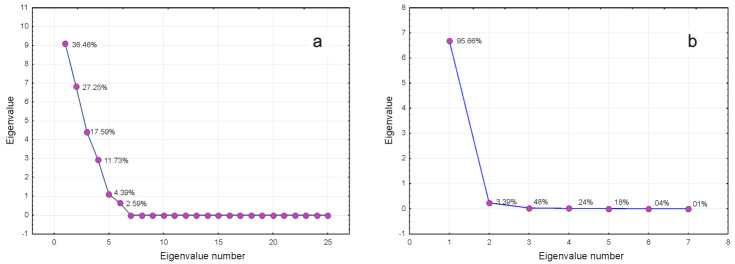
Accordingly, the result of PCA in R mode is illustrated by the bi-plot in Figure 1a. The existence of at least three clusters can be remarked, two of which consist of only one member, i.e., producers P2 and P5, and a third one, grouping the rest of the producers. The bi-plot illustrating the contribution of each compound to the PC1 and PC2 showed a relatively balanced situation, as both Factors 1 and 2 had similar contributions to PC, consisting of 36.46 and 27.25%, respectively. It is worth mentioning that a similar result was obtained by considering the PC3 vs. PC2, which most probably could be explained by their contribution to the total variance, 25.25 and 17.57%, respectively. The corresponding screen plot in Figure 3a illustrated this finding.
Figure 3.
The screen plots corresponding to R-mode (a) and Q-mode (b) PCA.
Complementary to the R-mode, a Q mode PC2 vs. PC1 bi-plot, shown in Figure 2b, consisted of three clusters, two of which contained a single organic compound, i.e., linalyl acetate and linalool, while the third one included all other 23 compounds. This result was in good agreement with the composition of the investigated samples, according to which, both linalyl acetate and linalool were characterized by the highest concentrations and variances.
On the contrary, Factor 2 vs. Factor 1 (Figure 2b, inset), except for Producers 2 (P2) and 5 (P5), were nearly coincident and negatively oriented along the first axis, which suggested an almost equivalent contribution to the total variance. This finding may explain the fact that PC1 contributed about 96% to the total variance, as shown in the corresponding screen plot (Figure 3b). In this regard, it is of interest to remark, as mentioned before, that P2 and P5 formed two different uni-component clusters (Figure 2b).
4. Materials and Methods
4.1. Samples Collection
The samples of L. angustifolia vegetal raw material, by-products, as well as the main product—lavender essential oil (LEO), were provided between 2016 and 2018 by seven producers (P 1-7) from different regions of the Republic of Moldova (Northern, Central, and Southern): P1—Causeni district; P2—Donduseni district; P3 and P6—Rezina district; P4—Falesti district; P5—Dubasari district; and P7—Ungheni district.
For OA and UA characterization, fresh lavender inflorescences were collected directly from the lavender fields near the Pervomaisc village, Causeni district (46°42′04″ N 29°05′21″ E). The inflorescences were dried in shaded places to obtain lavender plant material samples (LPM) (n = 3) which were subjected to HPLC characterization. The by-products which resulted after hydrodistillation (solid residue—SR (n = 3) and residual water—RW (n = 1)), were collected from the factories, dried, and bottled.
4.2. Chemicals
All of the used solvents, reagents, and standards were of analytical grade. Anhydrous sodium carbonate, aluminium chloride, sodium acetate, 96% ethanol, methanol, diethyl ether, and petroleum ether were obtained from Merck (Darmstadt, Germany). Deionized water produced by a Milli-Q Millipore system (Bedford, MA, USA) was used for the preparation of aqueous solutions and UHPLC mobile phases.
The standards used for HPLC-PDA analysis (ursolic and oleanolic acids) were HPLC purity and purchased from Sigma-Aldrich (Steinheim, Germany). Stock solutions of all the standards were prepared in methanol. Working standards were made by diluting the stock solutions in the same solvent. Both stock and working standards were stored at 4 °C until further use.
4.3. Extracts Preparation
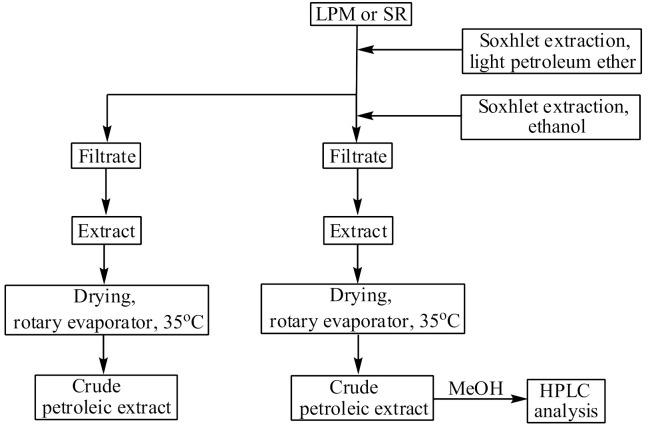
The selective extraction of triterpenic ursolic and oleanolic acids with ethanol from the LPM and SR was performed using a Soxhlet type extractor after degreasing with light petroleum ether (b.p. 40 °C) (Figure 4.). The ethanolic extracts were evaporated to dryness at 35 °C under reduced pressure using a rotary evaporator. For HPLC analysis, aliquots of each crude extract were dissolved in methanol using ultrasonication and filtered through a 0.45 μm micro-filter. The extraction and HPLC analysis were performed in duplicate for each plant material and the results were expressed as a mean value. For GC-MS analysis, the industrially produced essential oil samples were dissolved in hexane. The RWs were extracted with diethyl ether and the obtained extracts were subjected to GC chromatographic analysis.
Figure 4.
Flowchart of the UA and OA extract preparation.
4.4. Analytical GC-MS Analysis
The GC-MS analysis was performed using an Agilent Technologies 7890A gas chromatograph coupled with a 5975C Mass-Selective Detector (MSD) equipped with a split/splitless injector (1 µL). The analysis was carried out on an HP-5MS fused silica capillary calibrated column (30 m × 0.25 mm i.d.; film thickness 0.25 µm). The injector and detector temperatures were kept at 250 °C. Helium was used as carrier gas at a flow rate of 1.1 mL/min; oven temperature program was 70 °C/2 min, which was then programmed to 200 °C at the rate of 5 °C/min, and finally to 300 °C at the rate of 20 °C/min. The split ratio was 1:50, the MSD ionization energy was 70 eV, scan time was 1 s, the acquisition mass was in the range from 30 to 450 amu, and the solvent delay was 3 min.
4.5. Analytical RP-HPLC Analysis
The ursolic and oleanolic acids were quantified by an HPLC-PDA method previously reported [55], using a Thermo Finnigan Surveyor Plus HPLC System (Thermo Fisher Scientific Inc., San Jose, CA, USA). The OA and UA from extracts were identified by their retention time and spectral data by comparison with standards. To confirm the peak identity among possible interference peaks, the technique of standard addition to the sample was applied. Moreover, the peak purity for the interest peaks was satisfactory.
Calibration curves of the standards covered the range of 1–400 mg/L for both OA and UA and revealed good linearity, with correlation coefficients higher than 0.995 (0.9989 for OA and 0.9991 for UA) [56]. The accuracy of the method (%) was evaluated for spiked samples at 50 mg/L concentration and the obtained average values were 4.31% for OA and 3.65% for UA.
4.6. Antimicrobial Activity Assessment
The in vitro antimicrobial activity tests of methanolic extracts from the SR and RW against three species of fungi (Aspergillus niger, Alternaria alternata, and Penicillium chrysogenum, ATCC 53346, 8741, and 20044) and two species of bacteria (Pseudomonas aeroginosa and Bacillus sp., ATCC 27813 and 15970) were performed using a previously reported method [57].
Antimicrobial activity assessment of the industrially obtained lavender essential oil samples was performed in vitro on the following microorganisms: Non-pathogenic Gram-positive and Gram-negative strains of Bacillus subtilis NCNM BB-01 (ATCC 33608) and Pseudomonas fluorescens NCNM-PFB-01 (ATCC 25323), phytopathogenic strains of Xanthomonas campestris NCNM BX-01 (ATCC 53196), Erwinia amylovora NCNM BE-01 (ATCC 29780), E. carotovora NCNM BE-03 (ATCC 15713), and fungus strains of Candida utilis NCNM Y-22 (ATCC 44638) and Saccharomyces cerevisiae NCNM Y-20 (ATCC 4117) following a method described elsewhere [58].
The compounds Caspofungin and Kanamycin, both from Liofilchem (Roseto degli Abruzzi, Italy), were used as standards for antifungal and antibacterial activity tests.
4.7. Statistical Analysis
All statistical data analyses were performed using the StatSoft Statistica 10 software.
5. Conclusions
More than 40 main constituents of lavender essential oil from seven Moldavian producers were quantified by means of chromatographic and statistical analyses. The experimental data for lavender plant material and solid waste residue proved the possibility of their use as sources of biologically active compounds, such as OA and UA triterpene acids. All of the subjects in the present study, essential oil, residual distillation waste water, and extracts from the solid waste residues have shown high antimicrobial activity against 11 strains of bacteria and fungi, including phytopathogenic ones.
Acknowledgments
O.G.D. and I.Z. wish to acknowledge that their contribution was done within cooperation protocols no. 4920-4-20/22 between the University of Bucharest and the Joint Institute for Nuclear Research, Dubna, Russian Federation, represented by the Frank Laboratory of Neutron Physics. L.L. is grateful to the National Collection of Non-Pathogenic Microorganisms at the Institute of Microbiology and Biotechnology as well as the Laboratory of the Phytopathology and Biotechnology at the Institute of Genetics, Physiology, and Plant Protection for kindly providing the bacterial strains.
Abbreviations
| ATCC | American type culture collection |
| DW | Dry weight |
| DW PLM | Dry weight lavender plant material |
| GC-MS | Gas chromatography-mass spectrometry |
| HPLC | High-performance liquid chromatography |
| HPLC-PDA | High-performance chromatography-photodiode array detection |
| LEO | Lavender essential oil |
| LPM | Lavender plant material |
| MBC | Minimum bactericidal concentration |
| MFC | Minimum fungicidal concentration |
| MIC | Minimum inhibitory concentration |
| NCNM | National Collection of non-pathogenic microorganisms |
| OA | Oleanolic acid |
| RP-HPLC | Reversed-phase high-performance chromatography |
| RSD | Relative standard deviation |
| RW | Residual water |
| SDA | Statistical data analysis |
| SR | Solid residue |
| UA | Ursolic acid |
Author Contributions
Conceptualization, A.A. and I.Z.; microbiological assessments, L.L. and N.V.; GC-MC analysis, I.D.; sample preparation, V.P.; HPLC analysis, E.-I.G.; statistical analysis, O.G.D.; data curation, O.G.D.; writing—original draft preparation, A.C.; writing—review and editing, R.E.I., G.H. and I.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Agency for Research and Development (ANCD), project PLANTERAS 20.80009.8007.03.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Basch E., Foppa I., Liebowitz R., Nelson J., Smith M., Sollars D., Ulbricht C. Monograph from National Standard: Lavender (Lavandula angustifolia Miller) J. Herb. Pharmacother. 2004;4:63–78. [PubMed] [Google Scholar]
- 2.Denner S.S. Lavandula angustifolia Miller. Holist. Nurs. Pract. 2009;23:57–64. doi: 10.1097/01.HNP.0000343210.56710.fc. [DOI] [PubMed] [Google Scholar]
- 3.Da Porto C., Decorti D., Kikic I. Flavour compounds of Lavandula angustifolia Mill. To use in food manufacturing. Comparison of three different extraction methods. Food Chem. 2009;112:1072–1078. doi: 10.1016/j.foodchem.2008.07.015. [DOI] [Google Scholar]
- 4.Erland L.A.E., Mahmoud S.S. Lavender (Lavandula angustifolia) Oils. In: Preedy V.E., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; Amsterdam, The Netherlands: pp. 501–507. [Google Scholar]
- 5.Smigielski K., Sikora M., Majewska M., Raj A. The application of essentials oils to natural and organic cosmetics. Pol. J. Cosmet. 2008;11:89–107. [Google Scholar]
- 6.Prusinowska R., Smigielski K. Composition, biological activity and therapeutic effects of lavander (Lavandula angustifolia L.): A review. Herba Pol. 2014;60:56–66. doi: 10.2478/hepo-2014-0010. [DOI] [Google Scholar]
- 7.Hajhashemi V., Ghannadi A., Sharif B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharm. 2003;89:67–81. doi: 10.1016/S0378-8741(03)00234-4. [DOI] [PubMed] [Google Scholar]
- 8.Hamad K.J., Al-Shaheen S.J.A., Kaskoos R.A., Ahanad J., Jameel M., Mir S.R. Essential oil composition and antioxidant activity of Lavandula angustifolia from Irak. Int. Res. J. Pharm. 2013;4:117–120. [Google Scholar]
- 9.Djenane D., Aıder M., Yanguela J., Idir L., Gomez D., Roncales P. Antioxidant and antibacterial effects of Lavandula and Mentha essential oils in minced beef inoculated with E. coli O157:H7 and S. aureus during storage at abuse refrigeration temperature. Meat Sci. 2012;92:667–674. doi: 10.1016/j.meatsci.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 10.Adaszynska-Skwirzynska M., Swarcewicz M., Dobrowolska A. The potential of use lavender from vegetable waste as effective antibacterial and sedative agents. Med. Chem. 2014;4:734–737. [Google Scholar]
- 11.Mantovani A.L.L., Vieira G.P.G., Cunha W.R., Groppo M., Santos R.A., Rodrigues V., Magalhaes L.G., Crotti A.E.M. Chemical composition, antischistosomal and cytotoxic effects of the essential oil of Lavandula angustifolia grown in South-eastern Brazil. Rev. Braz. Farmacogn. 2013;23:877–884. doi: 10.1590/S0102-695X2013000600004. [DOI] [Google Scholar]
- 12.Shou-Dong S., Chang-Xu C., Ji-Shu Q., Ming-Hua S. Study on antitumor effect of Lavender angustifolia extract. Food Sci. Technol. 2009;2:213–215. [Google Scholar]
- 13.Martucci J.F., Gende L.B., Neira L.M., Ruseckaite R.A. Oregano and lavender essential oils as antioxidant and antimicrobial additives of biogenic gelatine films. Ind. Crop. Prod. 2015;71:205–213. doi: 10.1016/j.indcrop.2015.03.079. [DOI] [Google Scholar]
- 14.Tarek N., Hassan H.M., AbdelGhani S.M.M., Radwan I.A., Hammouda O., El-Gendy A.O. Comparative chemical and antimicrobial study of nine essential oils obtained from medicinal plants growing in Egypt. Beni-Seuf Univ. J. Appl. Sci. 2014;3:149–156. doi: 10.1016/j.bjbas.2014.05.009. [DOI] [Google Scholar]
- 15.Yamada K., Mimaki Y., Sashida Y. Anticonvulsive effects of inhaling lavender oil vapour. Biol. Pharm. Bull. 1994;17:359–360. doi: 10.1248/bpb.17.359. [DOI] [PubMed] [Google Scholar]
- 16.Aprotosoaie A.C., Gille E., Trifan A., Luca V.S., Miron A. Essential oil of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017;16:761–799. doi: 10.1007/s11101-017-9517-1. [DOI] [Google Scholar]
- 17.Smigielski K., Raj A., Krosowiak K., Grusca R. Chemical composition of the essential oil of Lavandula angustifolia cultivated in Poland. J. Essent. Oil-Bear. Plants. 2009;12:338–347. doi: 10.1080/0972060X.2009.10643729. [DOI] [Google Scholar]
- 18.Hassanpouraghdam M.B., Hassani A., Vojodi L., Hajisamadi A.B., Rostami A. Essential oil constituents of Lavandula angustifolia Chaix from Northwest Iran. Chemija. 2011;22:167–171. [Google Scholar]
- 19.Belhadj M.M., Kabouche A., Abaza I., Aburjai T., Tauzani R., Kabouche Z. Chemotypes investigation of Lavandula essential oils growing at different North African soils. J. Mater. Environ. Sci. 2014;5:1896–1901. [Google Scholar]
- 20.Cong Y., Abulizi P., Zhi L., Wang X. Chemical composition of the essential oil of Lavandula angustifolia from Xinjiang, China. Chem. Nat. Compd. 2008;44:810. doi: 10.1007/s10600-009-9210-8. [DOI] [Google Scholar]
- 21.Renaud E.N.C., Denys C.J., Simon J.E. Essential oil quantity and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001;13:269–273. doi: 10.1080/10412905.2001.9699691. [DOI] [Google Scholar]
- 22.Jianu C., Pop G., Gruia A.T., Horhat F.G. Chemical composition and antimicrobial activity of essential oils of lavender (Lavandula angustifolia) and lavandin (Lavandula x intermedia) grown in western Romania. Int. J. Agric. Biol. 2013;15:772–776. [Google Scholar]
- 23.Zagorcheva T., Stanev S., Rusanov K., Atanassov I. Comparative GC/MS analysis of lavender (Lavandula angustifolia Mill.) inflorescence and essential oil volatiles. Agric. Sci. Technol. 2013;5:459–462. [Google Scholar]
- 24.Chatzopolou P.S., Goliaris A.H., Katsiotis T. Contribution to the analysis of the volatile constituents from some lavender and lavandin cultivars grown in Greece. Sci. Pharm. 2003;71:229–234. [Google Scholar]
- 25.Raina A.P., Negi K.S. Comparative essential oil composition of Lavandula species from India. J. Herb Spice Med. Plants. 2012;18:268–273. doi: 10.1080/10496475.2012.690142. [DOI] [Google Scholar]
- 26.Verma R.S., Rahman L.U., Chanotiya C.S., Verma R.K., Chauhan A., Singh A., Yadav A.K. Essential oil composition of Lavandula angustifolia Mill. cultivated in the mid hills of Uttarakhand. India. J. Serbian Chem. Soc. 2010;75:343–348. doi: 10.2298/JSC090616015V. [DOI] [Google Scholar]
- 27.Jaeger S., Trojan H., Kopp T., Laszczyk M.N., Scheffler A. Pentacyclic triterpene distribution in various plants-rich sources for a new group of multi-potent plant extracts. Molecules. 2009;14:2016–2031. doi: 10.3390/molecules14062016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janicsak G., Veres K., Zoltan Kakasy A., Mathe I. Study of the oleanolic and ursolic acid contents of some species of the Lamiaceae. Biochem. Syst. Ecol. 2006;34:392–396. doi: 10.1016/j.bse.2005.12.004. [DOI] [Google Scholar]
- 29.Duke J.A. Handbook of Biologically Active Phytochemicals and Their Activities. 1st ed. CRC Press; Boca Raton, FL, USA: 1992. p. 208. [Google Scholar]
- 30.Liu J. Pharmacology of oleanolic acid and ursolic acid. J. Ethnopharmacol. 1995;49:57–68. doi: 10.1016/0378-8741(95)90032-2. [DOI] [PubMed] [Google Scholar]
- 31.Wolska K., Grudniak A., Fiecek B., Kraczkiewicz-Dowjat A., Kurek A. Antibacterial activity of oleanolic and ursolic acids and their derivatives. Open Life Sci. 2010;5:543–553. doi: 10.2478/s11535-010-0045-x. [DOI] [Google Scholar]
- 32.Hsu Y.L., Kuo P.L., Lin C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004;75:2303–2316. doi: 10.1016/j.lfs.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 33.Chiang L.C., Chiang W., Chang M.Y., Ng L.T., Lin C.C. Antileukemic activity of selected natural products in Taiwan. Am. J. Chin. Med. 2003;31:37–46. doi: 10.1142/S0192415X03000825. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W., Hong D., Zhou Y., Zhang Y., Shen Q., Li J., Hu L., Li J. Ursolic acid and its derivative inhibit protein tyrosine phosphatase 1B, enhancing insulin receptor phosphorylation and stimulating glucose uptake. Biochim. Biophys. Acta. 2006;1760:1505–1512. doi: 10.1016/j.bbagen.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Tian Z., Lin G., Zheng R.X., Huang F., Yang M.S., Xiao P.G. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J. Gastroenterol. 2006;12:874–879. doi: 10.3748/wjg.v12.i6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim Y.K., Yoon S.K., Ryu S.Y. Cytotoxic Triterpenes from Stem Bark of Physocarpus intermedius. Planta Med. 2000;66:485–486. doi: 10.1055/s-2000-8585. [DOI] [PubMed] [Google Scholar]
- 37.Kashiwada Y., Nagao T., Hashimoto A., Ikeshiro Y., Okabe H., Cosentino L.M., Lee K.H. Anti-AIDS agents 38. Anti-HIV activity of 3-O-acyl ursolic acid derivatives. J. Nat. Prod. 2000;63:1619–1622. doi: 10.1021/np990633v. [DOI] [PubMed] [Google Scholar]
- 38.Yan S., Huang C., Wu S., Yin M. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol. In Vitro. 2010;24:842–848. doi: 10.1016/j.tiv.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Kowalski R. Studies of selected plant raw materials as alternative sources of triterpenes of oleanolic and ursolic acid types. J. Agric. Food Chem. 2007;55:656–662. doi: 10.1021/jf0625858. [DOI] [PubMed] [Google Scholar]
- 40.Zheljazkov V.D., Astatkie T. Effect of residual distillation water of 15 plants and three plant hormones on Scotch spearmint (Mentha × gracilis Sole) Ind. Crop. Prod. 2011;33:704–709. doi: 10.1016/j.indcrop.2011.01.011. [DOI] [Google Scholar]
- 41.Tiliacos C., Gaydou E.M., Bessiere J.-M., Agnel R. Distilled lavandin (Lavandula intermedia Emeric ex. Loise l) wastes: A rich source of coumarin and herniarin. J. Essent. Oil Res. 2008;20:412–413. doi: 10.1080/10412905.2008.9700043. [DOI] [Google Scholar]
- 42.Torras-Claveria L., Jauregui O., Bastida J., Codina C., Viladomat F. Antioxidant Activity and Phenolic Composition of Lavandin (Lavandula x intermedia Emeric ex. Loiseleur) J. Agric. Food Chem. 2007;55:8436–8443. doi: 10.1021/jf070236n. [DOI] [PubMed] [Google Scholar]
- 43.Khadzhieva P., Aleksiev K., Topalova I. Di-and triterpenoids in essential oil waste-from pinus, lavender, and salvia. Int. Conf. Chem. Biotechnol. Biol. Act. Nat. Prod. 1987;5:519–524. [Google Scholar]
- 44.Amorese V., Donadu M., Usai D., Sanna A., Milia F., Pisanu F., Molicotti P., Zanetti S., Doria C. In vitro activity of essential oils against Pseudomonas aeruginosa isolated from infected hip implants. J. Infect. Dev. Ctries. 2018;12:996–1001. doi: 10.3855/jidc.10988. [DOI] [PubMed] [Google Scholar]
- 45.Yap P.S.X., Krishnan T., Yiap B.C., Hu C.P., Chan K.-G., Lim S.H.E. Membrane disruption and anti-quorum sensing effects of synergistic interaction between Lavandula angustifolia (lavender oil) in combination with antibiotic against plasmid-conferred multi-drug-resistant Escherichia coli. J. Appl. Microbiol. 2014;116:1119–1128. doi: 10.1111/jam.12444. [DOI] [PubMed] [Google Scholar]
- 46.Yap P.S.X., Lim S.H.E., Hu C.P., Yiap B.C. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20:710–713. doi: 10.1016/j.phymed.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 47.Kunicka-Styczyńska A., Śmigielski K., Prusinowska R., Rajkowska K., Kuśmider B., Sikora M. Preservative activity of lavender hydrosols in moisturizing body gels. Lett. Appl. Microbiol. 2015;60:27–32. doi: 10.1111/lam.12346. [DOI] [PubMed] [Google Scholar]
- 48.ISO 3515:2002 Oil of Lavender (Lavandula Angustifolia Mill.) ISO; Geneva, Switzerland: 2002. [Google Scholar]
- 49.Ivanov I., Petkova N., Tumbarski Y., Vrancheva R., Stoyanova M. Lavender waste–promising source of triterpenoids and polyphenols with antioxidant and antimicrobial activity. Ind. Technol. 2018;5:26–32. [Google Scholar]
- 50.Piqué N., Miñana-Galbis D., Merino S.M., Tomás J. Virulence Factors of Erwinia amylovora: A Review. Int. J. Mol. Sci. 2015;16:12836–12854. doi: 10.3390/ijms160612836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Benada M., Boumaaza B., Boudalia S., Khaladi O., Guessas B. Variability of aggressiveness and virulence of Erwinia carotovora subsp. carotovorum causing the soft rot on potato tubers in the western of Algeria. Int. J. Plant Biol. 2018;9:52–56. doi: 10.4081/pb.2018.7568. [DOI] [Google Scholar]
- 52.Tamir-Ariel D., Navon N., Burdman S. Identification of Genes in Xanthomonas campestris pv. vesicatoria induced during its interaction with tomato. J. Bacteriol. 2007;189:6359–6371. doi: 10.1128/JB.00320-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Davies J.C. Statistics and Data Analysis in Geology. Elsevier; Amsterdam, The Netherlands: 2002. [Google Scholar]
- 54.Shi G. Cluster Analysis. In: Shi G., editor. Data Mining and Knowledge Discovery for Geoscientists. Elsevier; Amsterdam, The Netherlands: 2014. [Google Scholar]
- 55.Ciocarlan A., Aricu A., Geană E.-I. Investigating the therapeutic potential of some medicinal plant and wild fruits based on terpenic ursolic and oleanolic acids. Cient Period. Nutr. 2019;3:1–12. [Google Scholar]
- 56.Geană E.I., Ionete R.E., Ciocarlan A., Aricu A., Fulga A., Ungur N., Podogova M., Nikolaeva D. HPLC determination of oleanolic and ursolic acids in apples and by-products. Prog. Cryog. Isot. Separation. 2014;17:53–62. [Google Scholar]
- 57.Lungu L., Ciocarlan A., Barba A., Shova S., Pogrebnoi S., Mangalagiu I., Moldoveanu C., Vornicu N., D’Ambrosio M., Babak M.V., et al. Synthesis and evaluation of biological activity of homodrimane sesquiterpenoids bearing hydrazinecarbothioamide or 1,2,4-triazole unit. Chem. Heter. Comp. 2019;55:716–724. doi: 10.1007/s10593-019-02526-1. [DOI] [Google Scholar]
- 58.Lupașcu G., Ciocarlan A., Dragalin I., Lupașcu L. Antimicrobial activity of the Coriander oil (Coriandrum sativum L.) Rom. J. Biol. 2019;64:31–42. [Google Scholar]