Abstract
Plants and insect herbivores are in a relentless battle to outwit each other. Plants have evolved various strategies to detect herbivores and mount an effective defense system against them. These defenses include physical and structural barriers such as spines, trichomes, cuticle, or chemical compounds, including secondary metabolites such as phenolics and terpenes. Plants perceive herbivory by both mechanical and chemical means. Mechanical sensing can occur through the perception of insect biting, piercing, or chewing, while chemical signaling occurs through the perception of various herbivore-derived compounds such as oral secretions (OS) or regurgitant, insect excreta (frass), or oviposition fluids. Interestingly, ion channels or transporters are the first responders for the perception of these mechanical and chemical cues. These transmembrane pore proteins can play an important role in plant defense through the induction of early signaling components such as plasma transmembrane potential (Vm) fluctuation, intracellular calcium (Ca2+), and reactive oxygen species (ROS) generation, followed by defense gene expression, and, ultimately, plant defense responses. In recent years, studies on early plant defense signaling in response to herbivory have been gaining momentum with the application of genetically encoded GFP-based sensors for real-time monitoring of early signaling events and genetic tools to manipulate ion channels involved in plant-herbivore interactions. In this review, we provide an update on recent developments and advances on early signaling events in plant-herbivore interactions, with an emphasis on the role of ion channels in early plant defense signaling.
Keywords: reactive oxygen species, herbivory, membrane potential, ion channel
1. Introduction
Plants regularly encounter a wide range of abiotic and biotic stresses in nature. Abiotic stress includes drought, salinity, extreme temperatures, radiation, floods, and heavy metals, whereas biotic stressors include insect, animal herbivores, and microbial pathogens. Plant and insect-herbivore interactions are among the most significant species interactions found in nature [1,2], and it is estimated that, annually, herbivory causes a 20% loss in the total productivity of agricultural crops [3]. However, plants are not totally defenseless against herbivory and are able to perceive and respond to this onslaught. They can perceive the insect attack through both mechanical and chemical cues. Mechanical signals are elicited through the damage caused by herbivores by piercing, chewing, or biting of plant tissues, and chemical signals are relayed via herbivore-associated elicitors (HAEs) such as oral secretions (OS) or regurgitant, insect excreta (frass), or oviposition fluids, to name a few [4,5]. Plants not only actively respond to herbivory, but also initiate a series of biochemical responses following the perception of herbivory. These biochemical cascades are initiated through ion channels that control the changes in the plasma membrane potential (Vm), generation of reactive oxygen species (ROS), cytosolic calcium fluxes, and ultimately induce plant defense genes to mount a multi-layered defense response that can act at both local and systemic levels [4,6,7,8,9,10]. In recent years, there have been several reviews on plant-herbivore interactions [4,5,7,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26]. Here we complement these existing reviews with current research and recent discoveries on plant-herbivore interactions, focusing on early plant defense signaling, with a particular emphasis on ion channels involved in early plant defense signaling.
2. Long-Distance Communication in Plant Defense
During herbivory, the damaged areas of the plant need to inform the rest of the plant to keep them ready for the imminent herbivory threat. Therefore, plants need to alert their unaffected parts by sending long-distance signals from the site of damage to various parts of the plant to appraise the threat. Plants respond to diverse stimuli by communicating amongst cells from distinct tissues or organs, a process called systemic signaling [27]. Studies have revealed the existence of complex regulatory mechanisms that allow the plant to activate resistance in systemic tissues, commonly referred to as systemic acquired resistance (SAR) [28]. SAR is characterized by a more potent and faster response to future encounters with microbes, insects, or abiotic stress.
Considerable progress has been made in understanding this intricate relationship between plants and herbivores with a plethora of field and lab studies. These include studies that have dissected pairwise interactions between a specific herbivore and its host; interactions at species, genus, and community levels with multiple hosts and herbivores; and studies examining plant defense signaling networks through molecular genetics genomics, to name a few [29,30]. However, our knowledge of how plants perceive these cues and how that leads to specific and tightly regulated defense responses is still in its infancy. It has been proposed that following the insect attack, the foremost event is the recognition of the cue and its perception by specific membrane receptors and the transduction of these signals into the plant cell. These cues are termed as “early defense signaling molecules” such as the depolarization of plasma membrane along with the generation of secondary messengers such as cytosolic Ca2+ [31], reactive oxygen species (ROS), and reactive nitrogen species (RNS) [32,33,34,35] that contribute to plant defense signal transduction events.
Long-distance communication in plants has been linked with ion channels or membrane transporters. These are transmembrane pore proteins involved in the movement of ions across the cell membrane. In recent years, with electrophysiological tools, the research on ion channels in plants has been gaining momentum. Studies have reported that ion channels facilitate long-distance communication via Vm, Ca2+, and ROS (Figure 1). Ion channels have been shown to mediate systemic signaling by modulating the influx of ions into different plant tissues [36]. They sense signals from the functional cells at the site of herbivory to activate other cells, which in turn relay this signal to induce defense responses. For example, a recent study [37] identified glutamate receptor-like channels (GLRs) in Arabidopsis thaliana that are related to mammalian ionotropic glutamate receptors, play a role in Ca2+ signaling during herbivory, nutrient transport, root gravitropism, and plant defense [38,39]. However, in mammals, these channels are involved in neurotransmission, and their openings are stimulated by glutamate binding to the postsynaptic neuron, resulting in Ca2+ and other cations influx. The signal is transmitted because of voltage changes caused by ion flux [40]. Remarkably, these GLRs are also responsible for long-distance Ca2+ transmission in plants in response to herbivory or mechanical injury, efficiently communicating herbivore attacks to surrounding cells.
Figure 1.
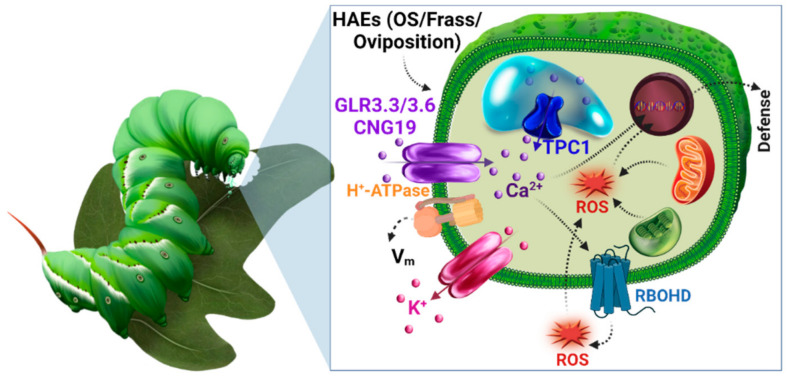
Initiation of early defense signaling mechanisms in response to insect herbivore attack. Schematic diagram showing herbivore M. sexta feeding induced signaling events, which include the perception of HAEs such as OS, frass, and oviposition by specialized receptors on the outer plasma membrane, which trigger modulation of Vm via H+-ATPase and Ca2+ ion influx into the cell via Ca2+ channels, GLR3.3/3.6 and/or CNGC19. The increase in cytosolic Ca2+ may trigger the further release of vacuolar Ca2+ via the TPC1 channel. The subsequent release of Ca2+ may activate nicotinamide adenine dinucleotide phosphate (NADPH oxidase) and respiratory burst oxidase homologues (RBOHDs), leading to ROS generation, and induction of plant defense responses. Illustration by Annette Diaz.
There has been considerable research on identifying the factors that are involved in long-distance signaling. Plants can appraise their unaffected parts by extensive network of intracellular regulators, Vm, Ca2+, and ROS [18,41]. The transmission rate of all these waves ranges from ~100 to >1000 µm/sec [41,42]. The process starts with the propagation of long-distance electrical signals as a result of variation in membrane potential due to potassium (K+) and Ca2+ flux. Variation in Vm is critical for plant wounding responses [43]. Finally, Ca2+ and ROS, versatile secondary messenger, were generated that plants use to sense and transform environmental stimuli into an adaptive intracellular response [44]. Insect feeding and OS can lead to changes to the cytosolic Ca2+ concentration, and these spatiotemporal variations have been shown to yield Ca2+ signatures [45,46,47,48]. On the other hand, ROS are extremely reactive and hazardous chemicals formed from oxygen. Among them are O2, H2O2, and OH−. ROS which has been demonstrated to act as a self-propagating long-distance and fast wound signal [49]. Throughout this review, we will focus on the role of ion channels, Vm, Ca2+, and ROS in plant response to herbivory and provide an overview of what is currently known about the role of ion channels in plant-herbivore interactions.
3. Membrane Potential (Vm)
The Vm is an electrical potential of the cell membrane that is maintained via the balance of ion fluxes across the plasma membrane. Vm indicates whether a cell is excited or not. It is responsible for generating action potentials in tissues, muscles, and nerves in animals and plays a crucial role in diverse biological functions such as biological sensing, hearing, cell cycle, proliferation, contractility, and circadian rhythm, to name a few [50]. Unlike animals, plants use Vm to regulate plant cellular functions such as maintaining turgor pressure, osmotic balance, and stomatal closure. There is no net flux of ions through the membrane when in equilibrium, called the resting membrane potential. Changes in the resting membrane potential will occur due to an unbalanced movement of ions, thus leading to Vm being more positive (depolarization) or more negative (hyperpolarization). In general, plants maintain a negative resting membrane potential in the order of −110 to −150 mV [51,52]. It has been reported that the signal transduction mechanism of plants to respond to minor changes in Vm leads to plant defense responses. The way plants sense insect cues and initiate defense responses has been a point of interest for many years. One hypothesis that has evolved by studying cellular responses following herbivory suggests that the first event following herbivory generates the fluctuation in Vm [53]. Maffei et al. [43] has also demonstrated that both mechanical wounding and OS of cotton leafworm (Spodoptera littoralis) alter Vm in lima bean (Phaseolus lunatus L.) at increasing distances of 5, 30, and 60 mm from the bite zone. Vm depolarization was observed within the first 15 min of feeding by S. littoralis in the palisade cells. The effect of S. littoralis regurgitant and its components were also tested on Vm in P. lunatus leaf and the results showed that Vm alterations were independent of regurgitate concentration. In addition, they also examined changes in Vm in response to the application of various H2O2 concentrations to mechanically damaged and herbivore-wounded P. lunatus leaves. H2O2 treatment induced a robust Vm that was significantly greater in herbivory-wounded plants than in mechanically injured leaves [54].
Bricchi et al. [55] studied Vm alterations in wild-type and plasmodesmata mutated A. thaliana pdko3 lines; plasmodesmata are channels within the plant cell that allow chemicals to pass through, establishing a pathway for cell-to-cell communication. A strong Vm depolarization occurred in wild-type A. thaliana plants within 7 to 8 min after herbivory, but the pdko3 mutant did not exhibit Vm depolarization in response to herbivory or application of OS from S. littoralis. However, Ca2+ elevation was observed in both wild types as well as in pdko3 mutant. This observation ruled out the possibility of Ca2+ channels being involved in Vm depolarization. To dissect the dependence of Vm depolarization on potassium (K+) channels, the K+ channel activity was measured using fluorescent indicator FluxORTM. A significant increase in K+ channel activity was observed in wild-type plants, whereas a complete loss of K+ channel activity was observed in pdko3 plants. This finding also suggests that K+ channels are involved in Vm depolarization and supports the hypothesis that plant cells respond to OS by a Vm-mediated signal transduction pathway.
The fluctuation in Vm has been known to be induced by the binding of specific components from herbivore OS with the receptors present at the plasma membrane [56]. These components can alter ion channel activities, causing an imbalance in ion movement, which influences the membrane potential of the plasma membrane [43]. A study by Mohanta et al. [57] showed that Kew tree (Ginkgo biloba), a living fossil plant, responds to S. littoralis herbivory by inducing Vm depolarization, which was evident up to 6 h. Another study using A. thaliana also showed that the extent of Vm depolarization was the same for S. littoralis, green peach aphid (Myzus persicae), and the plant pathogenic bacteria Pseudomonas syringe, but the timing of the occurrence of Vm depolarization was different for each of these biotrophs. Moreover, the magnitude of early defense response depends upon the amount of tissue damage by the biotroph. Vm depolarization was rapid upon the attack of chewing herbivore, S. littoralis (30 min to 2 h), as it caused substantial tissue loss, since it consumed large amounts of leaf tissue. On the other hand, less damage was observed by a phloem feeder, M. persicae (4 to 6 h), that delayed the plant defense response since phloem feeders with sucking mouthparts feed on vascular tissues without visible tissue damage as observed with chewing herbivores [58]. It is apparent that Ca2+ and ROS generation are directly tied to Vm when herbivores interact with plants, and Vm is essential for plant defense responses.
4. Calcium (Ca2+)
Ca2+ is a ubiquitous signaling molecule in plants. It functions as a secondary messenger in cellular pathways that regulate plant growth and development, cell polarity, cytoskeleton organization, ion transport across membranes, stomatal regulation, root growth, fertilization, nutrient signaling, and plant immunity [59]. Consequently, each of these processes has its own “Ca2+ signature,” linked with distinct fluctuations in Ca2+ concentration in the cytosol and sometimes in a particular intracellular compartment. Therefore, Ca2+ fluxes, especially oscillations between calcium stores and the cytosol, are important for cell signaling [60,61,62].
In plants, the cytosolic Ca2+ concentration is maintained at or below 100 nM; however, the majority of Ca2+ is stored in the apoplast, vacuole, endoplasmic reticulum (ER), and Golgi apparatus. The apoplast serves as the first Ca2+ reservoir of a cell that can store 0.33 mM free resting Ca2+ and the first area that responds to stimuli, while the vacuole serves as the largest Ca2+ pool of a cell that can store up to 0.2–5 mM free resting Ca2+ [60,63].
The Ca2+ signature plays an important role in long-distance signal transduction during herbivore attack through which HAEs such as OS, oviposition, and frass is sensed by the cell membrane, and then, a Ca2+ is rapidly propagated in the cytosol and travels throughout the plant to induce defense responses. The shaping of this “Ca2+ signature” during plant-herbivore interactions is achieved through the amplification and integration of Ca2+ signals. The amplification step is mediated via specific ion channels or transporter proteins and enhances Ca2+ fluxes at sites of herbivore attack, whereas the integration step is mediated via Ca2+ sensor proteins, which allow efficient transmission of Ca2+ signals from one cell to another in a tissue or organ. Herbivory induces Ca2+ entry from the apoplast to the cytosol via plasma membrane Ca2+ channels which stimulates Ca2+ signals in the cytosol leading to the amplification of Ca2+ signals. The localized Ca2+ signals from the cytosol are distributed throughout the whole plant. In this way, amplification, and integration of Ca2+ signals constitute two important ways by which “Ca2+ signature” contributes as a signaling molecule during plant-herbivore interactions [64].
The amplification of intracellular Ca2+ signal requires selective Ca2+ sensor proteins that respond to changes in cytosolic Ca2+ levels and encipher the frequency, amplitude, and signal localization of Ca2+ signatures. It is estimated that A. thaliana contains around 250 Ca2+ sensor proteins [65]. These can be classified into three main categories: (1) the calcineurin B-like proteins (CBLs) [66]; (2) the calmodulin (CaM), and calmodulin-like proteins (CMLs) [67]; and (3) the Ca2+ dependent protein kinases (CPKs) and the Ca2+ and calmodulin-dependent protein kinase (CCPK) [68]. All of these sensors contain EF-hand motifs, which enable Ca2+ binding and cause conformational changes in their structure [69].
CaM functions as a sensor relay protein since it lacks an enzymatic function. The Arabidopsis genome has seven calmodulin genes encoding four different isoforms (CaM1/4; CaM2/3/5; CaM6; and CaM7) [70]. CaM/CaM-like proteins (CML) regulate a variety of transcription factors, protein kinases, phosphatases, metabolic enzymes, ion pumps, and ion exchangers [71]. A. thaliana signal responsive (AtSR1) proteins [67], also known as CaM-binding transcription activators (AtCAMTAs) [72], have been shown to participate in wound-mediated defense responses. Atsr1 mutants of A. thaliana were sensitive to attack by dark winged fungus gnats (Bradysia impatiens), suggesting the role of CaM as an important sensor in the early stages of the insect-plant attack [73]. Along with CaM, the plant has CML that undergo secondary structural changes in response to Ca2+ binding and act as Ca2+ relays/sensors [74]. CML and CAM share a 16% amino acid sequence similarity and include two to six EF-hand motif [70]. CML42 gene expression was shown to be increased in A. thaliana upon S. littoralis OS treatment, implying a function in early defense plant signaling [75]. CPKs have been classified as sensor responders because they combine a Ca2+ binding domain and a serine/threonine kinase domain into a single protein that performs the fundamental function of converting Ca2+ signals to phosphorylation events [76,77]. A. thaliana contains 34 CPK family genes that play a role in plant defense responses. CPK 3 and CPK 13 both participate in signaling after Ca2+ influx upon S. littoralis attack through regulation of plant defensin gene (PDF1.2) by phosphorylation of the transcription factor, HsfB2a [78]. The cpk3 and cpk13 mutants had much lower transcript levels of the plant defensin gene PDF1.2 in comparison to wild-type plants.
Tools Used to Monitor Ca2+ Signaling in Plant-Herbivore Interactions
In recent years, the research on Ca2+ signaling has gained momentum with the advance in Ca2+ imaging techniques. Therefore, it is important to discuss different plant Ca2+ imaging methods, which are widely used in the context of plant-herbivore interactions to observe and record cytosolic Ca2+ concentration in herbivore-infested plants. These techniques include the use of Ca2+ sensing fluorescent dyes and genetically encoded Ca2+ indicators. Various fluorescent Ca2+ sensing dyes, such as Fluo-3, Calcium Orange, etc., have been used to investigate the dynamics of cytosolic Ca2+ signals in plant- herbivore interaction [33,43,55,57,58,79,80,81]. For example, the Ca2+ indicator Ca2+ orange was utilized to identify changes in cytosolic Ca2+ concentrations in P. lunatus following S. littoralis herbivory. The changes in Ca2+ concentration were compared in response to a single wounding (MD) event, continual mechanical damage caused by a robotic worm (MecWorm, MW), and herbivory. After 30 min, a considerable increase in Ca2+ fluorescence was observed due to herbivory in the wounding zone, which persisted for 4 h, but in MD and MW plants, just a faint fluorescence was noticed [33]. Even though these dye-based markers have been demonstrated to be quite effective, these Ca2+ sensing dyes have some limitations, including toxicity, fragility, low fluorescence signals, and they cannot be imaged in living plants without permeabilization. To overcome these limitations, researchers have initiated research on the use of genetically encoded Ca2+ indicators. The most widely used Ca2+ imaging method includes genetically encoded Ca2+ indicators, such as GCaMP, Yellow Cameleon (YC) Ca2+-sensors. The Ca2+ sensors were developed from GFP by combining them with calmodulin. These Ca2+ sensors can be expressed in the whole plant and are functional throughout the entire plant. Therefore, it can be used to monitor cytosolic Ca2+ in plants subjected to various herbivore attack conditions [37,42,82,83,84]. For example, Toyota et al. [37] showed that the P. rapae caterpillars induced cytosolic Ca2+ responses in the leaves of A. thaliana can be monitored with GCaMP3. This study reported that the increases in cytosolic Ca2+ concentration were associated with ion influx through plasma membrane Ca2+ channels such as GLR3.3/GLR3.6. Another example is Verrillo et al. [83], who showed that Ca2+ induction could be monitored with YC3.60, a YC-based Ca2+ sensor, following application of S. littoralis OS on mechanically damaged A. thaliana leaves. By using these tools, it is now possible to study the dynamics of Ca2+ signaling in plant-herbivore interactions at single-leaf, whole-plant, and whole-plant-insect herbivore attack conditions.
Intracellular Ca2+ level is controlled by the influx of Ca2+ ions from extracellular through apoplastic and vacuolar membranes. Therefore, plant ion channels play an important role in regulating plant development and the perception of many stimuli, including herbivory.
5. Plant Ion Channels
Ion channels are macromolecular pores in the membrane that regulate the influx and efflux of ions across the membrane at a rate of 106 ions per second. Ion channels can control ion fluxes in their target compartment and, thus, modify cellular homeostasis, and are vital in osmoregulation, development, signaling, mobility, and uptake of nutrients by the root and long-distance communication [85,86]. The first plant ion channel discovered, in 1984, is a K+ channel, Stelar K+ outward rectifier (SKOR) [87]. The last two to three decades have seen a dramatic increase in the number of ion channel subfamilies and their diverse functions. A large proportion of plant ion channel families have an analogous expression in animals. Ion channels are arranged into large families and are generally classified as cation, anion, or ligand-gated channels. Cation channels include voltage-gated K+ channels such as the shaker family (AKT1, AKT2, AKT6, KAT1, KAT2, KAT3, GORK, and SKOR; K+ transport), tandem pore, and two-pore K+ channels (TPK1, TPK4; K+ transport and TPC1; Ca2+ and other cation transport), are responsible for permeation of K+ ion across the plasma membrane and tonoplast membrane. Anion channels include slowly activating anion channels (SLAC1, SLAH1, SLAH2, SLAH3; Cl−/NO3− transport), aluminum-activated malate transporters (ALMT1, ALMT6, ALMT9, ALMT12; Malate, Cl− transport), chloride channels/transporters (CLCc, CLCg, CLCe; Cl− transport), and detoxification efflux carrier (DTX33, DTX35; Cl− transport). Ligand-gated channels include cyclic nucleotide-gated channel (CNGC2, CNGC4, CNGC5, CNGC14, CNGC15, CNGC18, CNGC19, CNGC20; Ca2+/Ba2+ transport) and glutamate receptor-like channels (GLR3.1, GLR3.3, GLR3.4, GLR3.5, GLR3.6; Ca2+ and other cations transport) [88] (Figure 2). These channels are responsible for setting up membrane potential, signal transduction, water, and solute transport [89], stomatal opening and closure [90,91], pollination [92], salt tolerance [93], and plant defense [94], to name a few. However, four distinct families of Ca2+-transporting ion channels have been shown to play a role in plant-herbivore interactions, including cyclic nucleotide-gated channels (CNGC19) [95,96], glutamate receptor-like channels (GLR3.3, GLR3.6) [37,42,97], two-pore channel 1 (TPC1) [59,84,98], and annexins (ANNEXIN 1) [99,100].
Figure 2.
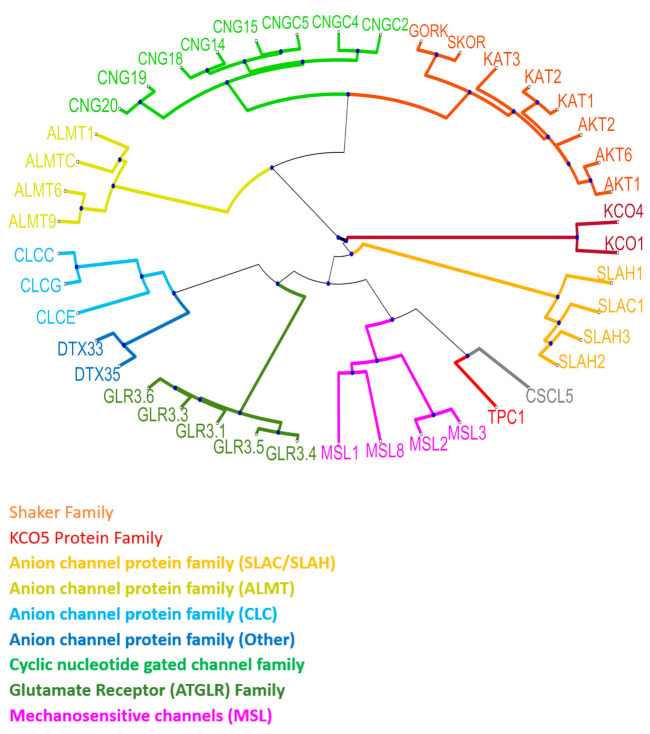
Phylogeny of plant ion channels. Representation of the phylogenetic tree of plant ion channels listed in Pantoja, 2020 [88], based on the analysis of protein homologs extracted from Uniprot.org. Progressive alignment and BLOSUM30 scoring method were used for multiple sequence alignment. The distance between the aligned sequences was calculated using Jukes-Cantor method. The phylogenetic tree was created by using the distance matrix. Unweighted pair group method average (UPGMA) was used to calculate group distance in the tree. Different colors represent different families of ion channels.
5.1. Cyclic Nucleotide Gated Channels (CNGC)
The cyclic nucleotide-gated channels (CNGCs) are ligand-gated Ca2+ channels, first discovered in retinal photoreceptors and olfactory neurons [101]. They play a role in signal transduction in animals and are also present in other non-neuronal tissues [102]. These ion transport proteins have also been identified in plants [74,103,104] and have been known to be involved in a variety of biological processes, ranging from plant development and stress tolerance, disease resistance [105,106], thermal tolerance [107], and salt stress [108]. These channels are typically localized at the plasma membrane and in the model plant A. thaliana, which consists of 20 family members [109].
CNGC channel is composed of four subunits, and each of these subunits consists of six membrane-spanning regions and a pore domain [110]. There is a cyclic-nucleotide binding (CNB) and a calmodulin-binding domain (CaMB) present at the C-termini of the channel (Figure 3) [111]. In contrast, the animal system has a CaMB domain at the N-termini [112,113]. The plant and the animal CNGC differ in their pore amino acid sequence as well as the selectivity for various cations [105,114]. The amino acids that form the CaM binding domain overlap with the polypeptide region that includes the CNBD [115]. This overlapping affects the channel activation as the binding of CaM at the C termini hinders cyclic nucleotide-binding, suggesting variability in plant and animal CNGC channel regulation [116,117]. These channels are activated by the binding of cyclic nucleotides such as cAMP (cyclic adenosine monophosphate) and cGMP (cyclic guanosine monophosphate) [118,119,120], and inhibited by calmodulin binding [121]. These channels also show similarity with shaker-like K+ channels [105]. Patch-clamp recordings on plant cell protoplasts membrane directly show that CNGC activation can be achieved by the application of hyperpolarizing potentials (more negative than −120 MV), which allow Ca2+ entry into the cell [111,121].
Figure 3.
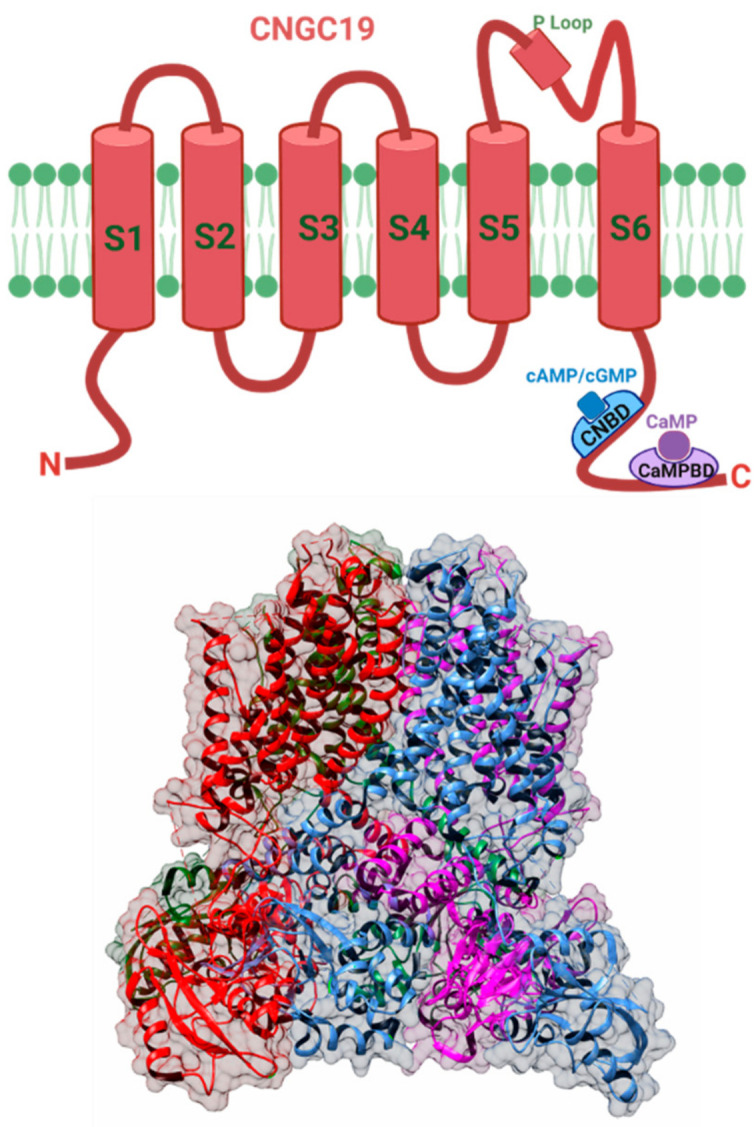
Putative structure of CNGC19 channel. (Top) Schematic cartoon representation of CNGC19 channel subunit showing six membrane-spanning regions (S1–S6) and a large pore domain (S5–S6). Functionally relevant sites in the C-terminus consist of a CNB, cyclic nucleotide-binding domain which can bind cAMP/cGMP, and a CaMBD, calmodulin-binding domain which can bind calmodulin. The functional channel is formed by four subunits. (Bottom) The structure of CNGC19 has not been solved to date but is likely to show similarities with the animal CNG family of channels. Therefore, the structure shown in the figure is an approximation based on homology to other channels. The predicted CNGC19 secondary 3D structure model, showing four subunits in transparent surface view, was developed from the closest homolog PDB structure, 5VA1 (human ether-a-go-go related K+ channel) using PHYRE 2.0 program. The image was prepared using Chimera software [122]. Created with BioRender.com (accessed on 30 August 2021).
It has been demonstrated that CNGC channels are important in modulating biotic stress responses such as Ca2+ influx in plant responses mediated by insect herbivore feeding [95]. A recent study by Meena et al. [96] has shown that the A. thaliana CNGC19 is responsible for generating and transmitting Ca2+ signals in local and systemic leaves mediated by the herbivore S. litura. A loss-of-function CNGC19 mutant in which the Ca2+ signals were attenuated was found to be more susceptible to attack by S. litura. In addition, jasmonic acid, a key signaling molecule in plant defense, was also observed in lower amounts in the CNGC19 mutant. These results suggest that CNGCs are involved in modulating plant resistance to insect herbivores, thus playing a role in the modulation of plant-herbivore interactions.
5.2. Glutamate Receptor-Like Channels
Glutamate receptor-like (GLR) is a non-selective ion channel responsible for permeating Ca2+ ions across the plasma membrane of animals and plants. Plant glutamate receptor-like (GLR) channels are ionotropic glutamate receptor homologs in mammals (iGluRs). The iGluRs have been extensively studied for their central nervous system and have been known to play a vital role in synaptic transmission, learning, and memory [123,124]. It is intriguing that GLRs also exist in plants despite the absence of the central nervous system [125]. In plants, GLRs play a crucial role in carbon and nitrogen metabolism [126], gravitropism [127], pollen tube growth [128,129], immune defense reactions [38,130,131,132,133], and wound-induced intracellular signaling [97]. Arabidopsis consists of 20 GLR genes; each subunit hosts a N-terminal domain, two extracellular ligand-binding sites (L1, L2), and transmembrane domains (S1–S4), including a pore region (P) and the C-terminal domain [134] (Figure 4). In mammals, iGluRs are divided into three groups according to their sequence diversity and ligand specificities [124]. These include N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA), and Kainate receptors. Plant GluRs share a high degree of similarity with the NMDA receptors that range from 16 to 63% in the ligand-binding domains and the transmembrane domains [135]. These channels are not only present at the plasma membrane but can also be found in chloroplasts, mitochondria [136], and vacuolar membranes [129]. Unlike their mammalian counterparts, the plant GLRs have much broader ligand selectivity. The major difference in plant and animal iGLR is the pore region. These non-selective cation channels are activated by amino acid glutamate, which acts as a metabolite, energy source, and neurotransmitter in animals [137,138].
Figure 4.
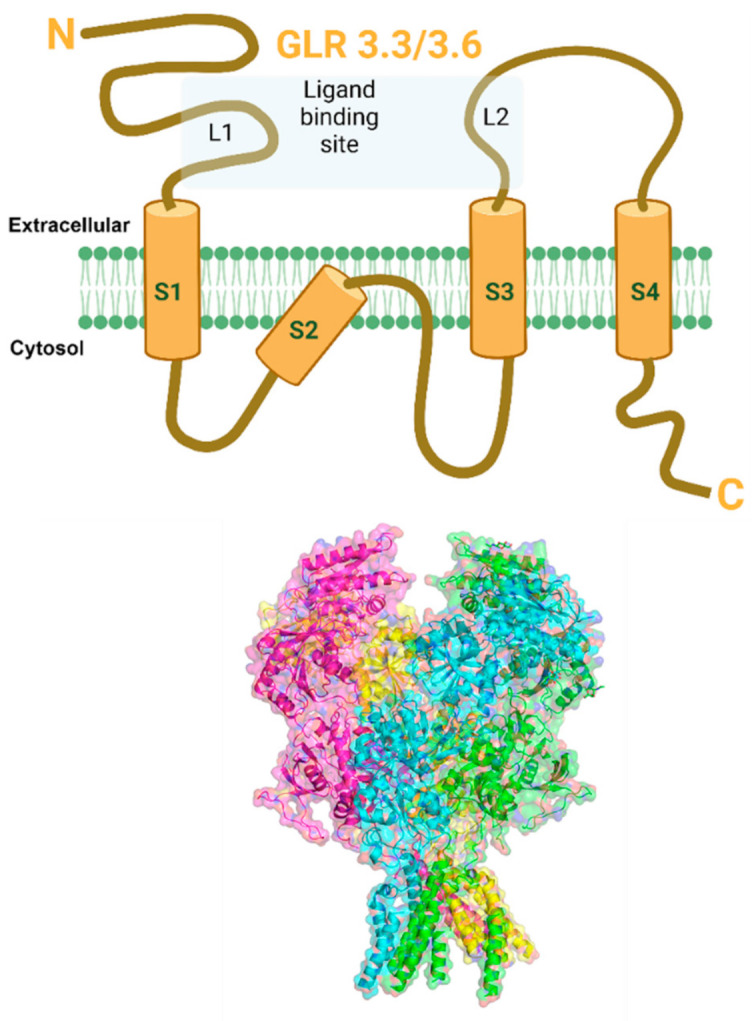
Putative structure of GLR3.3/3.6 channel. (Top) Schematic cartoon representation of GLR3.3/3.6 channel subunit showing extracellular N-terminus, four membrane-spanning regions (S1–S4), 2 extracellular ligand-binding sites (L1, L2), and intracellular C-terminus. (Bottom) The structure of GLR3.3/3.6 has not been solved to date but is likely to show similarities with the animal NMDA receptor family of channels. Therefore, the structure shown in the figure is an approximation based on homology to other channels. The predicted GLR3.3/3.6 secondary 3D structure model showing four subunits in transparent surface view was developed from closest homolog PDB structure 4TLL (Xenopus laevis GluN1/GluN2B NMDA receptor), using PHYRE 2.0 program. The image was prepared using PyMol software (PyMOL Molecular Graphics System, Version 2.4, Schrödinger, LLC, New York, NY, USA). Created with BioRender.com (accessed on 30 August 2021).
Electrophysiological studies have shown the involvement of GLRs in inducing a Ca2+ influx in plants that leads to the modulation of plant defense signaling to insect herbivores [139,140]. A study by Vasta et al. [140] showed that the application of GLR agonists such as glutamate induced a strong and rapid cytosolic Ca2+ increase in tobacco (Nicotiana tabacum) var xanthi while the application of lanthanum and Ca2+ chelator, BAPTA, inhibited glutamate-induced Ca2+ increase. This observation suggests that the plant GLR has a role in the modulation of Ca2+ influx that ensures plant defense responses against insect herbivores.
GLR3.3 has been implicated in the transmission of signals in the form of Ca2+ waves from wounded to unwounded sections of the plant. When S. littoralis larvae were allowed to feed on A. thaliana wild-type plants, wound-induced surface potential alterations were detected. However, wounding reduced the surface potential alterations in the four GLR mutants GLR3.1, GLR3.2, GLR3.3, and GLR 3.6. [97]. This suggests that GLR3.3 plays an important role in the modulation of plant defense signaling to insect herbivores. Recently, Toyota et al. [37] showed that GLRs are activated by wounding and upon herbivory by cabbage butterfly (Pieris rapae) caterpillars in A. thaliana leaf expressing genetically encoded Ca2+ sensor GCaMP3. The cytosolic Ca2+ elevation and subsequent defense gene expression were observed after the application of glutamate and not with other amino acids such as sorbitol. Furthermore, the Ca2+ signals were completely abolished in the GLR3.3/GLR3.6 double mutant in A. thaliana, suggesting that GLR3.3 and GLR3.6 are essential for transmitting Ca2+ signals induced by wounding and herbivory. Another recent study by Shao et al. [42] demonstrated that wounding of the main root at a distance of 2 cm from the root-shoot junction increased the Ca2+ elevation and surface wave potential (SWP) in A. thaliana expressing calcium sensor GCaMP6. Additionally, the application of glutamate to the wound site in the root induced an increase in Ca2+ and SWP in all leaves. Interestingly, in the GLR3.3/GLR3.6 double mutant, this wound and glutamate-induced rise in root to shoot Ca2+ was attenuated. This finding suggests that GLR3.3 and GLR3.6 are involved in propagating systemic Ca2+ signaling from leaf to leaf and root to shoot. These results provide evidence for the role of plant GLRs in the modulation of Ca2+ signaling during plant defense responses against insect herbivores.
5.3. ANNEXIN1
Annexins are the phospholipid-binding proteins and are considered novel mechanosensitive Ca2+ channels [141,142]. In animal cells, annexins are present in the cytoplasm and cellular membranes [143]. They are involved in vital cellular processes such as membrane trafficking, ion flux, mitotic signaling, and cytoskeleton rearrangement [143,144]. Eight annexin genes have been identified in A. thaliana by genome sequencing [145]. Plant annexins are structurally different from their animal homologs but have a conserved primary sequence. These 32–42 kDa proteins have two major domains: a N-terminal head and a C-terminal annexin core [143] (Figure 5). The annexin core is composed of four annexin domains (I–IV), each of which is 70 amino acids in length and contains five short helices and a conserved endonexin fold (G-X-G-T-{38-40}-D/E). Ca2+ binding activity occurs in type II and III binding sites of annexin proteins [141,143]. Plant annexins have a shorter N-terminal region than their animal counterparts [146] and are crucial for actin binding, inhibition of callose synthase, and oxidative stress responses [147,148,149,150]. The functional diversity of annexins is due to the variable N-terminal region that interacts with other proteins.
Figure 5.
Putative structure of ANNEXIN1 channel. ANNEXIN1 secondary 3D structure model showing two subunits (homodimer) in transparent surface view was developed from PDB structure 1YCN (Arabidopsis thaliana ANNEXIN). The presence of Ca2+ or H2O2 appears to be required for homodimerization. The image was prepared using Chimera software [122].
A recent study by Malabarba et al. [100] reported the role of ANNEXIN1 (ANN-1) in initiating systemic defense in A. thaliana in response to Egyptian cotton leafworm (S. littoralis) herbivory. The study found that annexin 1 was responsible for inducing cytosolic free Ca2+ elevation upon wounding and simulated herbivory in A. thaliana. ANN-1 knock-out and ANN-1 overexpressing lines were employed in this work to evaluate their role in herbivory-mediated Ca2+ signaling. The result showed that in the ANN-1 deletion line, the increase in cytosolic Ca2+ upon herbivory by S. littoralis was impaired, and the larvae gained more weight than those fed on wild-type plants. On the other hand, weight increase was significantly lower in larvae that fed on the ANN-1 overexpressed line compared to the wild type. Additionally, jasmonate accumulation and defense responses were diminished in ANN-1 systemic leaves, demonstrating that ANN-1 is involved in systemic cytosolic Ca2+-dependent jasmonate induction. This finding suggests that ANN-1 modulates plant defenses against herbivore damage through the Ca2+-dependent jasmonate signaling pathway and is required for systemic rather than local defense activation in plants attacked by herbivorous insects.
5.4. Two Pore Channel 1 (TPC1)
Two pore channels (TPCs) are organellar cation channels that are widely expressed in animals and plants. In animals, they are localized in the endolysosomal membrane, while in plants they reside in the tonoplast of plant vacuoles [151,152,153,154]. They are members of the voltage-gated ion channel superfamily. The vacuolar TPC1 channel, also known as the slowly activating vacuole (SV) channel, has been implicated in a variety of processes in plants, including nutrient sensing, pH homeostasis, and modulation of the membrane potential. The first plant TPC1 gene was cloned in A. thaliana (AtTPC1), with 733 amino acids identical to the rat TPC1 sequence [152].
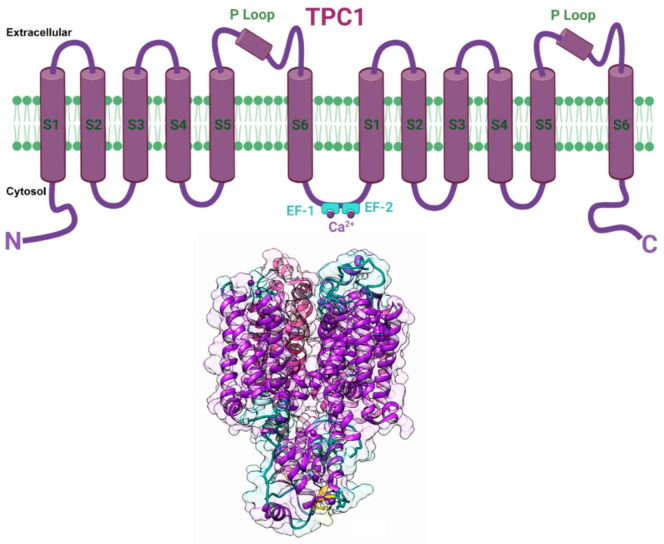
Plant and animal TPCs are similar in sequence to voltage-gated Ca2+ and Na+ channels and feature two shaker-like units with six transmembrane domains (S1–S6), each joined by a cytosolic linker containing two Ca2+-binding EF-hands (EF1 and EF2). (Figure 6). Voltage and an increase in the cytosolic Ca2+ level both influence the activity of plant TPCs. Ca2+ binding to the cytosolic EF-hand domain induces conformational changes in the pair of pore-lining inner helices from the first 6-TM domains, whereas membrane potential activates the second voltage-sensing domain, which undergoes conformational changes and facilitates pore opening [155]. The SV channel transports Ca2+ in addition to Na+ and K+ and has a permeability ratio of 3:1 for Ca2+ to K+ [156,157]. Ca2+ release is substantially dependent on the concentration of cytosolic free Ca2+, indicating that this channel is involved in Ca2+-induced Ca2+ release [156,158]. The plant TPC1 has been implicated in insect-plant interactions. A study by Kiep et al. [98] has shown that an increase in local cytosolic Ca2+ and systemic Ca2+ response was induced in response to S. littoralis feeding on A. thaliana. By using real-time imaging in A. thaliana expressing the Ca2+ reporter aequorin to monitor the induction of local and systemic cytosolic Ca2+ signals, this study showed that simulated herbivory by wounding inhibited the systemic Ca2+ signal in the tpc1 knockout mutant. These results indicated that the TPC1 channel plays a key role in the systemic [Ca2+] cyt signal induced by insect herbivory in A. thaliana. Another study by Vincent et al. [84] employed A. thaliana plants expressing the GFP-based Ca2+ indicator GCaMP3 to visualize Ca2+ accumulation in response to aphid M. persicae feeding. Within 95 s of the aphids settling, a robust fluorescence burst was seen, indicating cytosolic Ca2+ elvation. The rise in cytosolic Ca2+ was strongly dependent on Brassinosteroid Insensitive Associated Kinase I (BAK1), the plasma membrane Ca2+ permeable ion channels glutamate receptor-like 3.3 and 3.6 (GLR3.3 and GLR3.6), which are critical regulators of extracellular Ca2+ import into the cytoplasm of plant cells. In addition, this study also revealed that the increase in cytosolic Ca2+ induced TPC1 mediated vacuolar Ca2+ release in response to aphid feeding, suggesting that the TPC1 channel operates in conjunction with the plasma membrane Ca2+ permeable ion channels GLR3.3 and GLR3.6 in mediating cytosolic Ca2+ increase during insect herbivory [84].
Figure 6.
Putative structure of TPC1 channel. (Top) Schematic cartoon representation of individual plant TPC1 channel subunit comprising two repeated domains showing six membrane-spanning regions (S1–S6), two pore loops (P), and joined via a cytosolic linker containing two Ca2+ binding EF-hands (EF1 and EF2). (Bottom) TPC1 secondary 3D structure model showing two subunits in transparent surface view was developed from PDB structure 5DQQ (Arabidopsis thaliana TPC1). The image was prepared using Chimera software [122]. Created with BioRender.com (accessed on 30 August 2021).
5.5. H+-ATPase
The proton-pumping ATPases (H+-ATPases) are the primary pumps responsible for the generation of a proton gradient across cellular membranes. This electrogenic transporter uses energy from ATP hydrolysis to drive the translocation of protons against their concentration gradient from the cytosol to the external aqueous environment [159]. The H+-ATPase is located in the plasma membrane (PM) of plant cells. It has been demonstrated that the activation and suppression of the H+-ATPase activity in the plant plasma membrane modulate Vm, resulting in the alteration of PM ion channels and transporters functions [160]. The PM H+-ATPase is a single 100 kDa polypeptide and a member of the large family of phosphorylation (P)-type ATPases. It is composed of six transmembrane helices (M1–M6) and a cytoplasmic domain containing phosphorylation (P), nucleotide-binding (N), and actuator (A) domains involved in ATP hydrolysis. The PM H+-ATPase has been implicated in various physiological processes, including cell development, intracellular pH regulation, food uptake, stomatal opening, salt tolerance, and cellular expansion [161,162,163,164,165].
Plant PM H+-ATPase has been shown to contribute in the propagation of the intracellular defense signaling cascade by modifying Vm in response to herbivore feeding [166]. A study by Camoni et al. [167] demonstrated that S. littoralis oral secretions effectively inhibited Phaseolus lunatus PM H+-ATPase, resulting in decreased H+ extrusion from the cytosol and modification of the Vm. This observation implied that H+ extrusion by the plant H+-ATPase was involved in Vm regulation and might initiate a plant defensive response to herbivory. Another recent study by Kumari et al. [168] has revealed that Arabidopsis H+-ATPase 1 (AHA1) is involved in the formation of slow wave potentials (SWPs), which are required for long-distance electrical transmission during herbivore-induced plant defense. Fusicoccin, a PM H+-ATPase activator, prolonged the SWP repolarization phase in leaves distal to wounds. The repolarization phase was significantly prolonged in reduced function aha1 mutants, whereas the duration of SWP repolarization was reduced in the presence of a gain-of-function mutant ost2-2D. Additionally, S littoralis larvae performed better on aha1-7 mutants than on wild-type plants. Overall, these observations suggest that the PM H+-ATPase is required for the regulation of the Vm and electrical signal propagation between different parts of a plant during insect herbivory.
6. Reactive Oxygen Species (ROS)
Reactive oxygen species (ROS) are highly reactive molecules generated by redox reactions. They are part of several biological processes, such as photorespiration, oxidative phosphorylation, the electron transport chain (ETC), as well as a plant defense against pathogens and herbivores. ROS is produced in the mitochondria, chloroplast, and peroxisomes. There are several forms of ROS like superoxide anion (O2−•−), hydrogen peroxide (H2O2•), hydroxyl radical (HO•), peroxynitrite (ONOO), and singlet oxygen (1O2) [169]. ROS is typically produced by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase complex, which catalyzes the reduction of molecular oxygen to superoxide anion, which is then converted to H2O2. In plants, respiratory burst oxidase homologs (RBOHs) were found to be the key enzymes that catalyze the formation of ROS, which is a key step in plant protection against herbivores [170,171,172]. The respiratory burst oxidase homolog D (RBOHD) has been found to be essential for the propagation of ROS waves [173]. The significance of RBOHs in organizing responses against chewing insect herbivores was verified in N. attenuate where tobacco hornworm (Manduca sexta) OS enhanced NaRBOHD (N. attenuata NADPH oxidase homolog) on damaged leaves. ROS accumulation was diminished in M. sexta OS treated NaRBOHD-silenced N. attenuata plants without affecting OS-induced gene expression of defense-related genes [174].
The production of ROS is an inevitable by-product of metabolism in many cell types. Previously, it was assumed that ROS are toxic molecules that cause cellular damage to macromolecules [175]. Still, the role of ROS in plant defense has only recently emerged. It is well established that ROS can act as early defense signaling molecules that promote plant defense responses against a variety of pathogens and herbivores [54,176]. ROS act as secondary messengers that can penetrate up to 8.4 cm/min in A. thaliana [177]. Plants use ROS to alert the non-injured tissue about a plant attack by either releasing small quantities, which activates certain defense responses or prevent cell death by limiting the production of ROS [178]. ROS production has also been suggested to be involved in plant-microbe interactions as ROS can activate or repress the expression of defense-related genes [179,180]. The role of ROS in plant resistance to herbivores has been demonstrated in resistant and near-isogenic susceptible wheat after the attack of Russian wheat aphid (Diuraphis noxia). A strong burst of H2O2, as well as NADPH oxidase activity, was observed in resistant plants 3 h after infestation in comparison to susceptible plants. Treatments of plants with diphenyleneiodonium (DPI), an inhibitor of NADPH oxidase, suppressed the H2O2 production. Elevation in H2O2 levels (47%) was observed by treating resistant wheat plants with a mixture of glucose and glucose oxidase [181], suggesting that H2O2 plays a role in the defense response against D. noxia infestation.
Studies have shown that ROS serve as early defense signaling molecules in response to herbivore-induced wounding and secretions such as OS and oviposition. Imbiscuso et al. [182] investigated the effect of brake fern (Pteris vittata) response to herbivory by S. littoralis. The P. vittata plants responded to the attack of S. littoralis by activating peroxidases which produced H2O2. The concentration of H2O2 in leaves was lower in mechanically wounded young leaves than herbivory wounded leaves, suggesting that P. vittata can distinguish between mechanical and herbivory wounding by modulating the amount of ROS production. A study by Shinya et al. [183] demonstrated that the application of OS isolated from generalist herbivore, nightfeeding rice armyworm, (Mythimna loreyi), caused a strong intracellular ROS generation on rice cells, and a similar effect was obtained upon application of synthetically prepared N-linolenoyl-L-Glu, the most abundant FAC present in OS of M. loreyi, indicating that FAC from M. loreyi OS promoted ROS production in rice cells.
Recently, our group Gandhi et al. [184] demonstrated that M. sexta oral secretions (OS) induced ROS generation in isolated tomato protoplasts. Interestingly, our study showed that the application of tomato plant-fed (PF) M. sexta OS enhanced ROS generation while artificial diet-fed (DF) OS could not induce ROS in tomato protoplasts, suggesting that the oral secretions of M. sexta play an indispensable role in inducing ROS generation in tomato protoplasts. Our study also showed that the M. sexta PF-OS induced ROS increase was diminished in the presence of a Ca2+ chelator, BAPTA-AM, suggesting that there is a link between Ca2+ and ROS signaling. Several lines of evidence have indicated the existence of a positive feedback mechanism between ROS and Ca2+ production. In a heterologous expression system, treatment with ionomycin, an ionophore that leads to Ca2+ influx into cells, resulting in activation of RHD2 NADPH oxidase (root hair defective 2 reduced nicotinamide adenine dinucleotide phosphate) in root tips of A. thaliana confirming Ca2+ triggered RHD2 NADPH oxidase activity. These observations suggest that Ca2+ acts upstream of ROS production [185].
Compelling evidence indicates that ROS production by RBOHD is dependent on the Ca2+ binding [186,187]. RBOHD carries 2 EF-hands which are known to participate in Ca2+ dependent modulation [188]. Abscisic acid (ABA) signaling in guard cells involves both Ca2+ and ROS. A. thaliana mutants lacking certain NADPH oxidases (AtRBOHD and AtRBOHF) do not close their stomata and produce ROS, Ca2+, and Ca2+ channel activation when they are exposed to ABA. Supplementation of H2O2 to guard cells rescues the mutant phenotype, implying that Ca2+ entry proceeds downstream of ROS generation in ABA signaling [189,190].
In A. thaliana, the production of H2O2 was observed in leaves 72 h after oviposition by cabbage moth (Pieris brassicae) and was recognized by the formation of a reddish-brown precipitate. This result indicates that oviposition can trigger a localized response that resembles the hypersensitive response induced by pathogens [191]. A recent study by Stahl et al. [192] showed that eggs of P. brassicae induced generation of H2O2, salicylic acid and defense gene expression in A. thaliana. This study also revealed phosphatidylcholines (PCs) released from eggs is the key signaling molecule that activates gene expression and triggers various defenses in the plants.
Tools Used to Monitor ROS Signaling in Plant-Herbivore Interactions
While ROS relevance in plant-herbivore interaction is gaining momentum, the detection and characterization of ROS are still a significant bottleneck in this field. The early detection and quantification of ROS can be carried out by either utilizing genetically encoded fluorescent ROS sensors such as redox-sensitive green fluorescence protein (Ro-GFP), or synthetic fluorescent probes, such as 3,3′-diaminobenzidine (DAB) and 2′,7′-dichlorofluorescein diacetate (H2DCFDA). Genetically encoded ROS sensors “Ro-GFP” can monitor the cellular redox status at a high spatiotemporal resolution [193,194,195,196,197,198,199]. A recent study by Hipsch et al. [200] measured the whole plant ROS generation in response to high light, cold, and drought by using a chloroplast-targeted redox-sensitive green fluorescence protein 2 (RoGFP2). This finding suggests that whole-plant redox imaging using genetically encoded ROS sensors can be applied in a wide range of abiotic and biotic stress conditions, including plant-herbivore interaction. Despite the promising findings, the application of genetically encoded ROS sensors in plant-herbivore interactions is still limited due to the laborious and time-consuming method of its application. In contrast, synthetic fluorescent probes such as DAB and H2DCFDA are easier to use and can measure ROS in real-time with high sensitivity [201]. DAB has been used in many studies on plants as a reliable biomarker for reactive oxygen species (ROS) production [202,203,204]. However, in recent years, H2DCFDA has gained attention for its potential to measure the ROS levels in real-time on whole plants and as well as plant protoplasts [184,205,206]. Fichman et al. [205] measured the effect of light stress, injury, and pathogen, P. syringae pv. tomato DC 3000 on ROS signaling in H2DCFDA dye sprayed A. thaliana by using whole plant-live imaging. This study suggests that the combination of live-cell imaging and the use of H2DCFDA enables real-time monitoring of ROS in plants in response to various stress and pathogen treatments. This study also utilized an RBOHD (rbohD) knockout, and upon treatment with different stimuli, less ROS generation was observed. In contrast, another cytosolic ascorbate peroxidase 1 (apx) knockout produced more local as well as systemic ROS upon wounding or light stress treatments implying that this mutant had less ROS quenching capacity.
7. Conclusions
Recent years have witnessed immense progress in identifying the early defense signaling components in plant defense against herbivores, but studies on the molecular identification and characterization of these components are still a work in progress. However, with the advent of state-of-the-art imaging techniques, physiological and biochemical assays, and genomics may help us to understand the early defense signaling events by coordinating the plasma membrane potential changes, ion channels modulation, intracellular Ca2+ and ROS generation, gene expression, and, ultimately, the host plant defense response against herbivores. Transforming plants with biosensors such as GCaMP-Ca2+ and Ro-GFP-ROS sensors can help in the early identification of the plant defense responses. HAEs such as OS, frass, and oviposition could be used to develop strategies for early detection of the impending herbivory. So far, only a handful of Ca2+ permeable channels have been identified that plays a role in plant-herbivore interactions. Further studies are needed to unravel other ion channels that may be contributing to the modulation of Vm, Ca2+, and ROS, the downstream signaling cascade, and, more importantly, the role of these ion channels in triggering a rapid defense response. A deeper understanding of these early signaling events will eventually help us to minimize herbivory by developing pest management strategies based on plant-herbivore monitoring systems. Such knowledge can be instrumental in the design of plants with improved resistance against herbivores. As such, in the future, it will be important to develop effective small-molecule modulators that can inhibit or enhance the early signaling events in plant-herbivore interactions. Such an approach would not only facilitate research on early plant signaling events but also help in developing novel strategies for the development of herbivore-resistant crops.
Author Contributions
N.S. conceptualized and wrote the manuscript with A.G.; N.S. designed the final figures and edited the final manuscript draft with A.G. and R.K.; A.G., A.H., M.A., A.B. and N.S. contributed to the preparation of figures. A.G., R.K. and N.S. proofread and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the College of Sciences, University of Rio Grande Valley startup fund and the University of Texas System Rising STARs Award to N.S. and College of Sciences Seed grant to R.K.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Johnson M.T. Evolutionary ecology of plant defences against herbivores. Funct. Ecol. 2011;25:305–311. doi: 10.1111/j.1365-2435.2011.01838.x. [DOI] [Google Scholar]
- 2.Schäfer M., Fischer C., Meldau S., Seebald E., Oelmüller R., Baldwin I.T. Lipase activity in insect oral secretions mediates defense responses in Arabidopsis. Plant Physiol. 2011;156:1520–1534. doi: 10.1104/pp.111.173567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Meijden E. Herbivorous Insects—A Threat for Crop Production. Springer; Cham, Switzerland: 2014. pp. 103–114. [Google Scholar]
- 4.Felton G.W., Tumlinson J.H. Plant–insect dialogs: Complex interactions at the plant–insect interface. Curr. Opin. Plant Biol. 2008;11:457–463. doi: 10.1016/j.pbi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Wu J., Baldwin I.T. Herbivory-induced signalling in plants: Perception and action. Plant Cell Environ. 2009;32:1161–1174. doi: 10.1111/j.1365-3040.2009.01943.x. [DOI] [PubMed] [Google Scholar]
- 6.Kessler A. Defensive function of herbivore-induced plant volatile emissions in nature. Science. 2001;291:2141–2144. doi: 10.1126/science.291.5511.2141. [DOI] [PubMed] [Google Scholar]
- 7.Mithofer A., Boland W. Recognition of herbivory associated molecular patterns. Plant Physiol. 2008;146:825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonaventure G., VanDoorn A., Baldwin I.T. Herbivore-associated elicitors: FAC signaling and metabolism. Trends Plant Sci. 2011;16:294–299. doi: 10.1016/j.tplants.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Felton G.W., Chung S.H., Hernandez M.G., Louis J., Peiffer M., Tian D. Herbivore oral secretions are the first line of protection against plant-induced defences. Annu. Plant Rev. 2014;47:37–76. [Google Scholar]
- 10.Acevedo F.E., Rivera-Vega L.J., Chung S.H., Ray S., Felton G.W. Cues from chewing insects—The intersection of DAMPs, HAMPs, MAMPs and effectors. Curr. Opin. Plant Biol. 2015;26:80–86. doi: 10.1016/j.pbi.2015.05.029. [DOI] [PubMed] [Google Scholar]
- 11.Howe G.A., Jander G. Plant Immunity to Insect Herbivores. Annu. Rev. Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 12.Walling L.L. The myriad plant responses to herbivores. J. Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 13.Gatehouse J.A. Plant resistance towards insect herbivores: A dynamic interaction. New Phytol. 2002;156:145–169. doi: 10.1046/j.1469-8137.2002.00519.x. [DOI] [PubMed] [Google Scholar]
- 14.Peng J.Y., Huang Y.P. The signaling pathways of plant defense response and their interaction. Zhi WU sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao = J. Plant Physiol. Mol. Biol. 2005;31:347–353. [PubMed] [Google Scholar]
- 15.Hogenhout S.A., Bos J.I. Effector proteins that modulate plant--insect interactions. Curr. Opin. Plant Biol. 2011;14:422–428. doi: 10.1016/j.pbi.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 16.Fürstenberg-Hägg J., Zagrobelny M., Bak S. Plant defense against insect herbivores. Int. J. Mol. Sci. 2013;14:10242–10297. doi: 10.3390/ijms140510242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zebelo S.A., Maffei M.E. Role of early signaling events in plant-insect interactions. J. Exp. Bot. 2015;66:435–448. doi: 10.1093/jxb/eru480. [DOI] [PubMed] [Google Scholar]
- 18.Gilroy S., Białasek M., Suzuki N., Górecka M., Devireddy A.R., Karpiński S., Mittler R. ROS, calcium, and electric signals: Key mediators of rapid systemic signaling in plants. Plant Physiol. 2016;171:1606–1615. doi: 10.1104/pp.16.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo S., Zhang X., Wang J., Jiao C., Chen Y., Shen Y. Plant ion channels and transporters in herbivory-induced signalling. Funct. Plant Biol. 2017;45:111–131. doi: 10.1071/FP16318. [DOI] [PubMed] [Google Scholar]
- 20.Demidchik V., Maathuis F., Voitsekhovskaja O. Unravelling the plant signalling machinery: An update on the cellular and genetic basis of plant signal transduction. Funct. Plant Biol. 2018;45:1–8. doi: 10.1071/FP17085. [DOI] [PubMed] [Google Scholar]
- 21.Farmer E.E., Gao Y.Q., Lenzoni G., Wolfender J.L., Wu Q. Wound- and mechanostimulated electrical signals control hormone responses. New Phytol. 2020;227:1037–1050. doi: 10.1111/nph.16646. [DOI] [PubMed] [Google Scholar]
- 22.Tian W., Wang C., Gao Q., Li L., Luan S. Calcium spikes, waves and oscillations in plant development and biotic interactions. Nat. Plants. 2020;6:750–759. doi: 10.1038/s41477-020-0667-6. [DOI] [PubMed] [Google Scholar]
- 23.Vega-Muñoz I., Duran-Flores D., Fernández-Fernández Á.D., Heyman J., Ritter A., Stael S. Breaking bad news: Dynamic molecular mechanisms of wound response in plants. Front. Plant Sci. 2020;11:1959. doi: 10.3389/fpls.2020.610445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oelmüller R. Threat at one end of the plant: What travels to inform the other parts? Int. J. Mol. Sci. 2021;22:3152. doi: 10.3390/ijms22063152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Johns S., Hagihara T., Toyota M., Gilroy S. The fast and the furious: Rapid long-range signaling in plants. Plant Physiol. 2021;185:694–706. doi: 10.1093/plphys/kiaa098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh S., Kaur I., Kariyat R. The multifunctional roles of polyphenols in plant-herbivore interactions. Int. J. Mol. Sci. 2021;22:1442. doi: 10.3390/ijms22031442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mittler R., Blumwald E. The roles of ROS and ABA in systemic acquired acclimation. Plant Cell. 2015;27:64–70. doi: 10.1105/tpc.114.133090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spoel S.H., Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat. Rev. Immunol. 2012;12:89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- 29.Agrawal A.A. Induced responses to herbivory and increased plant performance. Science. 1998;279:1201–1202. doi: 10.1126/science.279.5354.1201. [DOI] [PubMed] [Google Scholar]
- 30.Kariyat R.R., Hardison S.B., Ryan A.B., Stephenson A.G., De Moraes C.M., Mescher M.C. Leaf trichomes affect caterpillar feeding in an instar-specific manner. Commun. Integr. Biol. 2018;11:1–6. doi: 10.1080/19420889.2018.1486653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reddy A.S.N., Ali G.S., Celesnik H., Day I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell. 2011;23:2010–2032. doi: 10.1105/tpc.111.084988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller G.A.D., Mittler R.O.N. Could heat shock transcription factors function as hydrogen peroxide sensors in plants? Ann. Bot. 2006;98:279–288. doi: 10.1093/aob/mcl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bricchi I., Leitner M., Foti M., Mithöfer A., Boland W., Maffei M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in Lima bean (Phaseolus lunatus L.) Planta. 2010;232:719–729. doi: 10.1007/s00425-010-1203-0. [DOI] [PubMed] [Google Scholar]
- 34.Arimura G.-I., Ozawa R., Maffei M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011;12:3723–3739. doi: 10.3390/ijms12063723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marino D., Dunand C., Puppo A., Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17:9–15. doi: 10.1016/j.tplants.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 36.Danilova M.N., Kudryakova N.V., Andreeva A.A., Doroshenko A.S., Pojidaeva E.S., Kusnetsov V.V. Differential impact of heat stress on the expression of chloroplast-encoded genes. Plant Physiol. Biochem. 2018;129:90–100. doi: 10.1016/j.plaphy.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 37.Toyota M., Spencer D., Sawai-Toyota S., Jiaqi W., Zhang T., Koo A.J., Howe G.A., Gilroy S. Glutamate triggers long-distance, calcium-based plant defense signaling. Science. 2018;361:1112–1115. doi: 10.1126/science.aat7744. [DOI] [PubMed] [Google Scholar]
- 38.Manzoor H., Kelloniemi J., Chiltz A., Wendehenne D., Pugin A., Poinssot B., Garcia-Brugger A. Involvement of the glutamate receptor AtGLR3.3 in plant defense signaling and resistance to Hyaloperonospora arabidopsis. Plant J. 2013;76:466–480. doi: 10.1111/tpj.12311. [DOI] [PubMed] [Google Scholar]
- 39.Salvador-Recatalà V. New roles for the glutamate receptor-like 3.3, 3.5, and 3.6 genes as on/off switches of wound-induced systemic electrical signals. Plant Signal. Behav. 2016;11:e1161879. doi: 10.1080/15592324.2016.1161879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muday G.K., Brown-Harding H. Nervous system-like signaling in plant defense. Science. 2018;361:1068–1069. doi: 10.1126/science.aau9813. [DOI] [PubMed] [Google Scholar]
- 41.Choi W.-G., Hilleary R., Swanson S.J., Kim S.-H., Gilroy S. Rapid, long-distance electrical and calcium signaling in plants. Annu. Rev. Plant Biol. 2016;67:287–307. doi: 10.1146/annurev-arplant-043015-112130. [DOI] [PubMed] [Google Scholar]
- 42.Shao Q., Gao Q., Lhamo D., Zhang H., Luan S. Two glutamate- and pH-regulated Ca2+ channels are required for systemic wound signaling in Arabidopsis. Sci. Signal. 2020;13:1453. doi: 10.1126/scisignal.aba1453. [DOI] [PubMed] [Google Scholar]
- 43.Maffei M., Bossi S., Spiteller D., Mithöfer A., Boland W. Effects of feeding Spodoptera littoralis on lima bean leaves. I. membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 2004;134:1752–1762. doi: 10.1104/pp.103.034165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jammes F., Hu H.-C., Villiers F., Bouten R., Kwak J.M. Calcium-permeable channels in plant cells. FEBS J. 2011;278:4262–4276. doi: 10.1111/j.1742-4658.2011.08369.x. [DOI] [PubMed] [Google Scholar]
- 45.McAinsh M.R., Hetherington A.M. Encoding specificity in Ca2+ signalling systems. Trends Plant Sci. 1998;3:32–36. doi: 10.1016/S1360-1385(97)01150-3. [DOI] [Google Scholar]
- 46.Reddy A.S.N. Calcium: Silver bullet in signaling. Plant Sci. 2001;160:381–404. doi: 10.1016/S0168-9452(00)00386-1. [DOI] [PubMed] [Google Scholar]
- 47.Moore C.A., Bowen H.C., Scrase-Field S., Knight M.R., White P.J. The deposition of suberin lamellae determines the magnitude of cytosolic Ca2+ elevations in root endodermal cells subjected to cooling. Plant J. 2002;30:457–465. doi: 10.1046/j.1365-313X.2002.01306.x. [DOI] [PubMed] [Google Scholar]
- 48.Hetherington A.M., Brownlee C. The generation of signals in plants. Annu. Rev. Plant Biol. 2004;55:401–427. doi: 10.1146/annurev.arplant.55.031903.141624. [DOI] [PubMed] [Google Scholar]
- 49.Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009;2:ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 50.Abdul Kadir L., Stacey M., Barrett-Jolley R. Emerging roles of the membrane potential: Action beyond the action potential. Front. Physiol. 2018;9:1661. doi: 10.3389/fphys.2018.01661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thiel G., MacRobbie E.A.C., Blatt M.R. Membrane transport in stomatal guard cells: The importance of voltage control. J. Membr. Biol. 1992;126:1–18. doi: 10.1007/BF00233456. [DOI] [PubMed] [Google Scholar]
- 52.Roelfsema M.R., Steinmeyer R., Staal M., Hedrich R. Single guard cell recordings in intact plants: Light-induced hyperpolarization of the plasma membrane. Plant J. 2001;26:1–13. doi: 10.1046/j.1365-313x.2001.01000.x. [DOI] [PubMed] [Google Scholar]
- 53.Maffei M.E., Arimura G.-I., Mithöfer A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012;29:1288. doi: 10.1039/c2np20053h. [DOI] [PubMed] [Google Scholar]
- 54.Maffei M.E., Mithöfer A., Arimura G.-I., Uchtenhagen H., Bossi S., Bertea C.M., Cucuzza L.S., Novero M., Volpe V., Quadro S., et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006;140:1022–1035. doi: 10.1104/pp.105.071993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bricchi I., Occhipinti A., Bertea C.M., Zebelo S.A., Brillada C., Verrillo F., De Castro C., Molinaro A., Faulkner C., Maule A.J., et al. Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J. 2012;73:14–25. doi: 10.1111/j.1365-313X.2012.05103.x. [DOI] [PubMed] [Google Scholar]
- 56.Zebelo S.A., Maffei M.E. Signal Transduction in Plant–Insect Interactions: From Membrane Potential Variations to Metabolomics. Springer; Berlin, Germany: 2012. pp. 143–172. [Google Scholar]
- 57.Mohanta T.K., Occhipinti A., Atsbaha Zebelo S., Foti M., Fliegmann J., Bossi S., Maffei M.E., Bertea C.M. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE. 2012;7:e32822. doi: 10.1371/journal.pone.0032822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bricchi I., Bertea C.M., Occhipinti A., Paponov I.A., Maffei M.E. Dynamics of membrane potential variation and gene expression induced by Spodoptera littoralis, Myzus persicae, and Pseudomonas syringae in Arabidopsis. PLoS ONE. 2012;7:e46673. doi: 10.1371/journal.pone.0046673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edel K.H., Marchadier E., Brownlee C., Kudla J., Hetherington A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017;27:R667–R679. doi: 10.1016/j.cub.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 60.Costa A., Navazio L., Szabo I. The contribution of organelles to plant intracellular calcium signalling. J. Exp. Bot. 2018;69:4175–4193. doi: 10.1093/jxb/ery185. [DOI] [PubMed] [Google Scholar]
- 61.Monshausen G.B. Visualizing Ca2+ signatures in plants. Curr. Opin. Plant Biol. 2012;15:677–682. doi: 10.1016/j.pbi.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 62.Whalley H.J., Knight M.R. Calcium signatures are decoded by plants to give specific gene responses. New Phytol. 2012;197:690–693. doi: 10.1111/nph.12087. [DOI] [PubMed] [Google Scholar]
- 63.Stael S., Wurzinger B., Mair A., Mehlmer N., Vothknecht U.C., Teige M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2011;63:1525–1542. doi: 10.1093/jxb/err394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tuteja N. Integrated Calcium Signaling in Plants. Springer; Berlin, Germany: 2009. pp. 29–49. [Google Scholar]
- 65.Day I.S., Reddy V.S., Shad Ali G., Reddy A.S.N. Analysis of EF-hand-containing proteins in Arabidopsis. Genome Biol. 2002;3:1–24. doi: 10.1186/gb-2002-3-10-research0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luan S. The CBL–CIPK network in plant calcium signaling. Trends Plant Sci. 2009;14:37–42. doi: 10.1016/j.tplants.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 67.Yang T., Poovaiah B.W. A calmodulin-binding/CGCG Box DNA-binding protein family involved in multiple signaling pathways in plants. J. Biol. Chem. 2002;277:45049–45058. doi: 10.1074/jbc.M207941200. [DOI] [PubMed] [Google Scholar]
- 68.Cheng S.-H., Willmann M.R., Chen H.-C., Sheen J. Calcium signaling through protein kinases. The Arabidopsis Calcium-dependent protein kinase gene family. Plant Physiol. 2002;129:469–485. doi: 10.1104/pp.005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Batistič O., Kudla J. Analysis of calcium signaling pathways in plants. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012;1820:1283–1293. doi: 10.1016/j.bbagen.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 70.McCormack E., Braam J. Calmodulins and related potential calcium sensors of Arabidopsis. New Phytol. 2003;159:585–598. doi: 10.1046/j.1469-8137.2003.00845.x. [DOI] [PubMed] [Google Scholar]
- 71.Bouché N., Yellin A., Snedden W.A., Fromm H. Plant-specific calmodulin-binding proteins. Annu. Rev. Plant Biol. 2005;56:435–466. doi: 10.1146/annurev.arplant.56.032604.144224. [DOI] [PubMed] [Google Scholar]
- 72.Bouché N., Scharlat A., Snedden W., Bouchez D., Fromm H. A novel family of calmodulin-binding transcription activators in multicellular organisms. J. Biol. Chem. 2002;277:21851–21861. doi: 10.1074/jbc.M200268200. [DOI] [PubMed] [Google Scholar]
- 73.Qiu Y., Xi J., Du L., Suttle J.C., Poovaiah B.W. Coupling calcium/calmodulin-mediated signaling and herbivore-induced plant response through calmodulin-binding transcription factor AtSR1/CAMTA3. Plant Mol. Biol. 2012;79:89–99. doi: 10.1007/s11103-012-9896-z. [DOI] [PubMed] [Google Scholar]
- 74.Kohler C., Merkle T., Neuhaus G. Characterisation of a novel gene family of putative cyclic nucleotide- and calmodulin-regulated ion channels in Arabidopsis thaliana. Plant J. 1999;18:97–104. doi: 10.1046/j.1365-313X.1999.00422.x. [DOI] [PubMed] [Google Scholar]
- 75.Vadassery J., Reichelt M., Hause B., Gershenzon J., Boland W., Mithöfer A. CML42-mediated calcium signaling coordinates responses to Spodoptera herbivory and abiotic stresses in Arabidopsis. Plant Physiol. 2012;159:1159–1175. doi: 10.1104/pp.112.198150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tena G., Boudsocq M., Sheen J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 2011;14:519–529. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Boudsocq M., Sheen J. CDPKs in immune and stress signaling. Trends Plant Sci. 2013;18:30–40. doi: 10.1016/j.tplants.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kanchiswamy C., Takahashi H., Quadro S., Maffei M.E., Bossi S., Bertea C., Zebelo S., Muroi A., Ishihama N., Yoshioka H., et al. Regulation of Arabidopsis defense responses against Spodoptera Littoralis by CPK-mediated calcium signaling. BMC Plant Biol. 2010;10:97. doi: 10.1186/1471-2229-10-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mithöfer A., Mazars C., Maffei M.E. Plant Signal Transduction. Humana Press; Totowa, NJ, USA: 2009. Probing spatio-temporal intracellular calcium variations in plants; pp. 79–92. [DOI] [PubMed] [Google Scholar]
- 80.Kanchiswamy C.N., Mohanta T., Capuzzo A., Occhipinti A., Verrillo F., Maffei M.E., Malnoy M. Differential expression of CPKs and cytosolic Ca2+ variation in resistant and susceptible apple cultivars (Malus x domestica) in response to the pathogen Erwinia amylovora and mechanical wounding. BMC Genom. 2013;14:760. doi: 10.1186/1471-2164-14-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kanchiswamy C., Malnoy M., Occhipinti A., Maffei M. Calcium Imaging Perspectives in Plants. Int. J. Mol. Sci. 2014;15:3842–3859. doi: 10.3390/ijms15033842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Russell J.T. Imaging calcium signals in vivo: A powerful tool in physiology and pharmacology. Br. J. Pharmacol. 2011;163:1605–1625. doi: 10.1111/j.1476-5381.2010.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Verrillo F., Occhipinti A., Kanchiswamy C.N., Maffei M.E. Quantitative analysis of herbivore-induced cytosolic calcium by using a Cameleon (YC 3.6) calcium sensor in Arabidopsis thaliana. J. Plant Physiol. 2014;171:136–139. doi: 10.1016/j.jplph.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Vincent T.R., Avramova M., Canham J., Higgins P., Bilkey N., Mugford S.T., Pitino M., Toyota M., Gilroy S., Miller A.J., et al. Interplay of plasma membrane and vacuolar ion channels, together with BAK1, elicits rapid cytosolic calcium elevations in Arabidopsis during aphid feeding. Plant Cell. 2017;29:1460–1479. doi: 10.1105/tpc.17.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hedrich R. Ion channels in plants. Physiol. Rev. 2012;92:1777–1811. doi: 10.1152/physrev.00038.2011. [DOI] [PubMed] [Google Scholar]
- 86.Ward J.M., Mäser P., Schroeder J.I. Plant ion channels: Gene families, physiology, and functional genomics analyses. Annu. Rev. Physiol. 2009;71:59–82. doi: 10.1146/annurev.physiol.010908.163204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaymard F., Pilot G., Lacombe B., Bouchez D., Bruneau D., Boucherez J., Michaux-Ferrière N., Thibaud J.-B., Sentenac H. Identification and disruption of a plant shaker-like outward channel involved in K+ release into the xylem sap. Cell. 1998;94:647–655. doi: 10.1016/S0092-8674(00)81606-2. [DOI] [PubMed] [Google Scholar]
- 88.Pantoja O. Recent advances in the physiology of ion channels in plants. Annu. Rev. Plant Biol. 2021;72:463–495. doi: 10.1146/annurev-arplant-081519-035925. [DOI] [PubMed] [Google Scholar]
- 89.Johansson I., Larsson C., Ek B., Kjellbom P. The major integral proteins of spinach leaf plasma membranes are putative aquaporins and are phosphorylated in response to Ca2+ and apoplastic water potential. Plant Cell. 1996;8:1181–1191. doi: 10.1105/tpc.8.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suh S.J., Park J.G., Lee Y. Possible involvement of phospholipase A2 in light signal transduction of guard cells of Commelina communis. Physiol. Plant. 1998;104:306–310. doi: 10.1034/j.1399-3054.1998.1040303.x. [DOI] [Google Scholar]
- 91.Armstrong F., Leung J., Grabov A., Brearley J., Giraudat J., Blatt M.R. Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc. Natl. Acad. Sci. USA. 1995;92:9520–9524. doi: 10.1073/pnas.92.21.9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holdaway-Clarke T.L., Feijo J.A., Hackett G.R., Kunkel J.G., Hepler P.K. Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell. 1997;9:1999–2010. doi: 10.2307/3870560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Suzuki K., Costa A., Nakayama H., Katsuhara M., Shinmyo A., Horie T. OsHKT2;2/1-mediated Na+ influx over K+ uptake in roots potentially increases toxic Na+ accumulation in a salt-tolerant landrace of rice Nona Bokra upon salinity stress. J. Plant Res. 2015;129:67–77. doi: 10.1007/s10265-015-0764-1. [DOI] [PubMed] [Google Scholar]
- 94.Nguyen C.T., Kurenda A., Stolz S., Chételat A., Farmer E.E. Identification of cell populations necessary for leaf-to-leaf electrical signaling in a wounded plant. Proc. Natl. Acad. Sci. USA. 2018;115:10178–10183. doi: 10.1073/pnas.1807049115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma Y., Walker R.K., Zhao Y., Berkowitz G.A. Linking ligand perception by PEPR pattern recognition receptors to cytosolic Ca2+ elevation and downstream immune signaling in plants. Proc. Natl. Acad. Sci. USA. 2012;109:19852–19857. doi: 10.1073/pnas.1205448109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Meena M.K., Prajapati R., Krishna D., Divakaran K., Pandey Y., Reichelt M., Mathew M.K., Boland W., Mithöfer A., Vadassery J. The Ca2+ channel CNGC19 regulates Arabidopsis defense against spodoptera herbivory. Plant Cell. 2019;31:1539–1562. doi: 10.1105/tpc.19.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mousavi S.A., Chauvin A., Pascaud F., Kellenberger S., Farmer E.E. Glutamate-receptor like genes mediate leaf-to-leaf wound signalling. Nature. 2013;500:422–426. doi: 10.1038/nature12478. [DOI] [PubMed] [Google Scholar]
- 98.Kiep V., Vadassery J., Lattke J., Maaß J.P., Boland W., Peiter E., Mithöfer A. Systemic cytosolic Ca2+ elevation is activated upon wounding and herbivory in Arabidopsis. New Phytol. 2015;207:996–1004. doi: 10.1111/nph.13493. [DOI] [PubMed] [Google Scholar]
- 99.Dodd A.N., Kudla J., Sanders D. The language of calcium signaling. Annu. Rev. Plant Biol. 2010;61:593–620. doi: 10.1146/annurev-arplant-070109-104628. [DOI] [PubMed] [Google Scholar]
- 100.Malabarba J., Meents A.K., Reichelt M., Scholz S.S., Peiter E., Rachowka J., Konopka-Postupolska D., Wilkins K.A., Davies J.M., Oelmüller R., et al. ANNEXIN1 mediates calcium-dependent defense in Arabidopsis plants upon herbivory and wounding. New Phytol. 2021;231:243–254. doi: 10.1111/nph.17277. [DOI] [PubMed] [Google Scholar]
- 101.Zagotta W.N., Siegelbaum S.A. Structure and function of cyclic nucleotide-gated channels. Annu. Rev. Neurosci. 1996;19:235–263. doi: 10.1146/annurev.ne.19.030196.001315. [DOI] [PubMed] [Google Scholar]
- 102.Kaupp U.B., Seifert R. Cyclic nucleotide-gated ion channels. Physiol. Rev. 2002;82:769–824. doi: 10.1152/physrev.00008.2002. [DOI] [PubMed] [Google Scholar]
- 103.Schuurink R.C., Shartzer S.F., Fath A., Jones R.L. Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc. Natl. Acad. Sci. USA. 1998;95:1944–1949. doi: 10.1073/pnas.95.4.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Leng Q., Mercier R.W., Yao W., Berkowitz G.A. Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol. 1999;121:753–761. doi: 10.1104/pp.121.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kaplan B., Sherman T., Fromm H. Cyclic nucleotide-gated channels in plants. FEBS Lett. 2007;581:2237–2246. doi: 10.1016/j.febslet.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 106.Abdel-Hamid H., Chin K., Shahinas D., Moeder W., Yoshioka K. Calmodulin binding to Arabidopsis cyclic nucleotide gated ion channels. Plant Signal. Behav. 2010;5:1147–1149. doi: 10.4161/psb.5.9.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finka A., Cuendet A.F., Maathuis F.J.M., Saidi Y., Goloubinoff P. Plasma membrane cyclic nucleotide gated calcium channels control land plant thermal sensing and acquired thermotolerance. Plant Cell. 2012;24:3333–3348. doi: 10.1105/tpc.112.095844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kugler A., Köhler B., Palme K., Wolff P., Dietrich P. Salt-dependent regulation of a CNG channel subfamily in Arabidopsis. BMC Plant Biol. 2009;9:140. doi: 10.1186/1471-2229-9-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moon J., Belloeil C., Ianna M., Shin R. Arabidopsis CNGC family members contribute to heavy metal ion uptake in plants. Int. J. Mol. Sci. 2019;20:413. doi: 10.3390/ijms20020413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Duszyn M., Świeżawska B., Szmidt-Jaworska A., Jaworski K. Cyclic nucleotide gated channels (CNGCs) in plant signalling—Current knowledge and perspectives. J. Plant Physiol. 2019;241:153035. doi: 10.1016/j.jplph.2019.153035. [DOI] [PubMed] [Google Scholar]
- 111.Dietrich P., Anschütz U., Kugler A., Becker D. Physiology and biophysics of plant ligand-gated ion channels. Plant Biol. 2010;12:80–93. doi: 10.1111/j.1438-8677.2010.00362.x. [DOI] [PubMed] [Google Scholar]
- 112.Liu M., Chen T.-Y., Ahamed B., Li J., Yau K.-W. Calcium-calmodulin modulation of the olfactory cyclic nucleotide-gated cation channel. Science. 1994;266:1348–1354. doi: 10.1126/science.266.5189.1348. [DOI] [PubMed] [Google Scholar]
- 113.Grunwald M.E., Yu W.-P., Yu H.-H., Yau K.-W. Identification of a domain on the β-Subunit of the rod cGMP-gated cation channel that mediates inhibition by calcium-calmodulin. J. Biol. Chem. 1998;273:9148–9157. doi: 10.1074/jbc.273.15.9148. [DOI] [PubMed] [Google Scholar]
- 114.Chin K., Moeder W., Yoshioka K. Biological roles of cyclic-nucleotide-gated ion channels in plants: What we know and don’t know about this 20-member ion channel. Botany. 2009;87:668–677. doi: 10.1139/B08-147. [DOI] [Google Scholar]
- 115.Jha S.K., Sharma M., Pandey G.K. Role of cyclic nucleotide gated channels in stress management in plants. Curr. Genom. 2016;17:315–329. doi: 10.2174/1389202917666160331202125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Varnum M.D., Zagotta W.N. Interdomain interactions underlying activation of cyclic nucleotide-gated channels. Science. 1997;278:110–113. doi: 10.1126/science.278.5335.110. [DOI] [PubMed] [Google Scholar]
- 117.Trudeau M.C., Zagotta W.N. Mechanism of calcium/calmodulin inhibition of rod cyclic nucleotide-gated channels. Proc. Natl. Acad. Sci. USA. 2002;99:8424–8429. doi: 10.1073/pnas.122015999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Swarbreck S.M., Colaço R., Davies J.M. Plant Calcium-Permeable Channels. Plant Physiol. 2013;163:514–522. doi: 10.1104/pp.113.220855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Talke I. CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci. 2003;8:286–293. doi: 10.1016/S1360-1385(03)00099-2. [DOI] [PubMed] [Google Scholar]
- 120.Kudla J., Batistič O., Hashimoto K. Calcium signals: The lead currency of plant information processing. Plant Cell. 2010;22:541–563. doi: 10.1105/tpc.109.072686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Y.-F., Munemasa S., Nishimura N., Ren H.-M., Robert N., Han M., Puzõrjova I., Kollist H., Lee S., Mori I., et al. Identification of Cyclic Gmp-activated Nonselective Ca2+-permeable cation channels and Associated cngc5 and cngc 6 genes in arabidopsis Guard Cells. Plant Physiol. 2013;163:578–590. doi: 10.1104/pp.113.225045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF chimera? A visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 123.Dingledine R., Borges K., Bowie D., Traynelis S.F. The glutamate receptor ion channels. Pharmacol. Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 124.Traynelis S.F., Wollmuth L.P., McBain C.J., Menniti F.S., Vance K.M., Ogden K.K., Hansen K.B., Yuan H., Myers S.J., Dingledine R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vezzani A., Fujinami R.S., White H.S., Preux P.-M., Blümcke I., Sander J.W., Löscher W. Infections, inflammation and epilepsy. Acta Neuropathol. 2015;131:211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang J., Turano F.J. The putative glutamate receptor 1.1 (AtGLR1.1) functions as a regulator of carbon and nitrogen metabolism in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 2003;100:6872–6877. doi: 10.1073/pnas.1030961100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Miller N.D., Durham Brooks T.L., Assadi A.H., Spalding E.P. Detection of a gravitropism phenotype in glutamate receptor-like 3.3 mutants of Arabidopsis thaliana using machine vision and computation. Genetics. 2010;186:585–593. doi: 10.1534/genetics.110.118711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Michard E., Lima P.T., Borges F., Silva A.C., Portes M.T., Carvalho J.E., Gilliham M., Liu L.-H., Obermeyer G., Feijo J.A. Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil D-serine. Science. 2011;332:434–437. doi: 10.1126/science.1201101. [DOI] [PubMed] [Google Scholar]
- 129.Wudick M.M., Portes M.T., Michard E., Rosas-Santiago P., Lizzio M.A., Nunes C.O., Campos C., Santa Cruz Damineli D., Carvalho J.C., Lima P.T., et al. Cornichon sorting and regulation of GLR channels underlie pollen tube Ca2+ homeostasis. Science. 2018;360:533–536. doi: 10.1126/science.aar6464. [DOI] [PubMed] [Google Scholar]
- 130.Li F., Wang J., Ma C., Zhao Y., Wang Y., Hasi A., Qi Z. Glutamate Receptor-Like channel3.3 is involved in Mediating glutathione-triggered cytosolic Calcium Transients, Transcriptional changes, and innate Immunity responses in Arabidopsis. Plant Physiol. 2013;162:1497–1509. doi: 10.1104/pp.113.217208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kwaaitaal M., Huisman R., Maintz J., Reinstädler A., Panstruga R. Ionotropic glutamate receptor (iGluR)-like channels mediate MAMP-induced calcium influx in Arabidopsis thaliana. Biochem. J. 2011;440:355–373. doi: 10.1042/BJ20111112. [DOI] [PubMed] [Google Scholar]
- 132.Weiland M., Mancuso S., Baluska F. Signalling via glutamate and GLRs in Arabidopsis thaliana. Funct. Plant Biol. 2016;43:1–25. doi: 10.1071/FP15109. [DOI] [PubMed] [Google Scholar]
- 133.Forde B.G., Roberts M.R. Glutamate receptor-like channels in plants: A role as amino acid sensors in plants. F1000Prime Rep. 2014;6:37. doi: 10.12703/P6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Davenport R. Glutamate receptors in plants. Ann. Bot. 2002;90:549–557. doi: 10.1093/aob/mcf228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lam H.-M., Chiu J., Hsieh M.-H., Meisel L., Oliveira I.C., Shin M., Coruzzi G. Glutamate-receptor genes in plants. Nature. 1998;396:125–126. doi: 10.1038/24066. [DOI] [PubMed] [Google Scholar]
- 136.Teardo E., Carraretto L., De Bortoli S., Costa A., Behera S., Wagner R., Lo Schiavo F., Formentin E., Szabo I. Alternative splicing-mediated targeting of the Arabidopsis glutamate receptor 3.5 to mitochondria affects organelle morphology. Plant Physiol. 2014;167:216–227. doi: 10.1104/pp.114.242602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Young V.R., Ajami A.M. Glutamate: An amino acid of particular distinction. J. Nutr. 2000;130:892S–900S. doi: 10.1093/jn/130.4.892S. [DOI] [PubMed] [Google Scholar]
- 138.Forde B.G., Lea P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007;58:2339–2358. doi: 10.1093/jxb/erm121. [DOI] [PubMed] [Google Scholar]
- 139.Dennison K.L., Spalding E.P. Glutamate-gated calcium fluxes in Arabidopsis. Plant Physiol. 2000;124:1511–1514. doi: 10.1104/pp.124.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vatsa P., Chiltz A., Bourque S., Wendehenne D., Garcia-Brugger A., Pugin A. Involvement of putative glutamate receptors in plant defence signaling and NO production. Biochimie. 2011;93:2095–2101. doi: 10.1016/j.biochi.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 141.Mortimer J.C., Laohavisit A., Macpherson N., Webb A., Brownlee C., Battey N.H., Davies J.M. Annexins: Multifunctional components of growth and adaptation. J. Exp. Bot. 2008;59:533–544. doi: 10.1093/jxb/erm344. [DOI] [PubMed] [Google Scholar]
- 142.Clark G.B., Morgan R.O., Fernandez M.P., Roux S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012;196:695–712. doi: 10.1111/j.1469-8137.2012.04308.x. [DOI] [PubMed] [Google Scholar]
- 143.Gerke V., Moss S.E. Annexins: From structure to function. Physiol. Rev. 2002;82:331–371. doi: 10.1152/physrev.00030.2001. [DOI] [PubMed] [Google Scholar]
- 144.Seaton B.A., Dedman J.R. Annexins. Biometals. 1998;11:399–404. doi: 10.1023/A:1009205925714. [DOI] [PubMed] [Google Scholar]
- 145.Cantero A., Barthakur S., Bushart T.J., Chou S., Morgan R.O., Fernandez M.P., Clark G.B., Roux S.J. Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol. Biochem. 2006;44:13–24. doi: 10.1016/j.plaphy.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 146.Hofmann A., Proust J., Dorowski A., Schantz R., Huber R. Annexin 24 from capsicum annuum. J. Biol. Chem. 2000;275:8072–8082. doi: 10.1074/jbc.275.11.8072. [DOI] [PubMed] [Google Scholar]
- 147.Calvert C.M., Gant S.J., Bowles D.J. Tomato annexins p34 and p35 bind to F-actin and display nucleotide phosphodiesterase activity inhibited by phospholipid binding. Plant Cell. 1996;8:333. doi: 10.1105/tpc.8.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McClung A.D., Carroll A.D., Battey N.H. Identification and characterization of ATPase activity associated with maize (Zea mays) annexins. Biochem. J. 1994;303:709–712. doi: 10.1042/bj3030709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Andrawis A., Solomon M., Delmer D.P. Cotton fiber annexins: A potential role in the regulation of callose synthase. Plant J. 1993;3:763–772. doi: 10.1111/j.1365-313X.1993.00763.x. [DOI] [PubMed] [Google Scholar]
- 150.Gidrol X., Sabelli P.A., Fern Y.S., Kush A.K. Annexin-like protein from Arabidopsis thaliana rescues delta oxyR mutant of Escherichia coli from H2O2 stress. Proc. Natl. Acad. Sci. USA. 1996;93:11268–11273. doi: 10.1073/pnas.93.20.11268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ishibashi K., Suzuki M., Imai M. Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 2000;270:370–376. doi: 10.1006/bbrc.2000.2435. [DOI] [PubMed] [Google Scholar]
- 152.Furuichi T., Cunningham K.W., Muto S. A putative two pore channel AtTPC1 mediates Ca2+ flux in arabidopsis leaf cells. Plant Cell Physiol. 2001;42:900–905. doi: 10.1093/pcp/pce145. [DOI] [PubMed] [Google Scholar]
- 153.Dadacz-Narloch B., Beyhl D., Larisch C., López-Sanjurjo E.J., Reski R., Kuchitsu K., Müller T.D., Becker D., Schönknecht G., Hedrich R. A novel calcium binding site in the slow vacuolar cation channel TPC1 senses luminal calcium levels. Plant Cell. 2011;23:2696–2707. doi: 10.1105/tpc.111.086751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cang C., Bekele B., Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014;10:463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 155.Guo J., Zeng W., Chen Q., Lee C., Chen L., Yang Y., Cang C., Ren D., Jiang Y. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2015;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ward J.M., Schroeder J.I. Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell. 1994:669–683. doi: 10.1105/tpc.6.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pitt S.J., Funnell T.M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G.C., Zhu M.X., et al. TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+ J. Biol. Chem. 2010;285:35039–35046. doi: 10.1074/jbc.M110.156927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bewell M.A., Maathuis F.J.M., Allen G.J., Sanders D. Calcium-induced calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet. FEBS Lett. 1999;458:41–44. doi: 10.1016/S0014-5793(99)01109-6. [DOI] [PubMed] [Google Scholar]
- 159.Sondergaard T.E., Schulz A., Palmgren M.G. Energization of transport processes in plants. roles of the plasma membrane H+-ATPase. Plant Physiol. 2004;136:2475–2482. doi: 10.1104/pp.104.048231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Haruta M., Gray W.M., Sussman M.R. Regulation of the plasma membrane proton pump (H(+)-ATPase) by phosphorylation. Curr. Opin. Plant Biol. 2015;28:68–75. doi: 10.1016/j.pbi.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Briskin D.P., Hanson J.B. How does the plant plasma membrane H+-ATPase pump protons? J. Exp. Bot. 1992;43:269–289. doi: 10.1093/jxb/43.3.269. [DOI] [Google Scholar]
- 162.Sussman M.R. Molecular analysis of proteins in the plant plasma membrane. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994;45:211–234. doi: 10.1146/annurev.pp.45.060194.001235. [DOI] [Google Scholar]
- 163.Morsomme P., Boutry M. The plant plasma membrane H+-ATPase: Structure, function and regulation. Biochim. Biophys. Acta (BBA)—Biomembr. 2000;1465:1–16. doi: 10.1016/S0005-2736(00)00128-0. [DOI] [PubMed] [Google Scholar]
- 164.Palmgren M.G. Plant plasma membrane h+-atpases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 165.Lutsenko S., Kaplan J.H. Organization of p-type atpases: Significance of structural diversity. Biochemistry. 1995;34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 166.Zimmermann M.R., Maischak H., Mithöfer A., Boland W., Felle H.H. System potentials, a novel electrical long-distance apoplastic signal in plants, induced by wounding. Plant Physiol. 2009;149:1593–1600. doi: 10.1104/pp.108.133884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Camoni L., Barbero F., Aducci P., Maffei M.E. Spodoptera littoralis oral secretions inhibit the activity Of Phaseolus lunatus plasma membrane H+-ATPase. PLoS ONE. 2018;13:e0202142. doi: 10.1371/journal.pone.0202142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kumari A., Chételat A., Nguyen C.T., Farmer E.E. Arabidopsis h+-atpase aha1 controls slow wave potential duration and wound response Jasmonate pathway activation. Proc. Natl. Acad. Sci. USA. 2019;116:20226–20231. doi: 10.1073/pnas.1907379116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Miller G., Shulaev V., Mittler R. Reactive oxygen signaling and abiotic stress. Physiol. Plant. 2008;133:481–489. doi: 10.1111/j.1399-3054.2008.01090.x. [DOI] [PubMed] [Google Scholar]
- 170.Torres M.A., Dangl J.L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 2005;8:397–403. doi: 10.1016/j.pbi.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 171.Suzuki N., Miller G., Morales J., Shulaev V., Torres M.A., Mittler R. Respiratory burst oxidases: The engines of ROS signaling. Curr. Opin. Plant Biol. 2011;14:691–699. doi: 10.1016/j.pbi.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 172.Baxter A., Mittler R., Suzuki N. ROS as key players in plant stress signalling. J. Exp. Bot. 2013;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 173.Mittler R., Vanderauwera S., Suzuki N., Miller G., Tognetti V.B., Vandepoele K., Gollery M., Shulaev V., Van Breusegem F. ROS signaling: The new wave? Trends Plant Sci. 2011;16:300–309. doi: 10.1016/j.tplants.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 174.Wu J., Wang L., Wünsche H., Baldwin I.T. Narboh D, a Respiratory Burst Oxidase Homolog in Nicotiana attenuata, is required for Late Defense responses after Herbivore attack. J. Integr. Plant Biol. 2013;55:187–198. doi: 10.1111/j.1744-7909.2012.01182.x. [DOI] [PubMed] [Google Scholar]
- 175.Asada K., Takahashi M. Production and scavenging of active oxygen in photosynthesis. In: Kyle D.J., Osmond B., Arntzen C.J., editors. Photoinhibition. Elsevier; Amsterdam, The Netherlands: 1987. pp. 227–287. [Google Scholar]
- 176.Lamb C., Dixon R.A. The oxidative burst in plant resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:251–275. doi: 10.1146/annurev.arplant.48.1.251. [DOI] [PubMed] [Google Scholar]
- 177.Gilroy S., Suzuki N., Miller G., Choi W.-G., Toyota M., Devireddy A.R., Mittler R. A tidal wave of signals: Calcium and ROS at the forefront of rapid systemic signaling. Trends Plant Sci. 2014;19:623–630. doi: 10.1016/j.tplants.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 178.Sharma P., Jha A.B., Dubey R.S., Pessarakli M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012;2012:217037. doi: 10.1155/2012/217037. [DOI] [Google Scholar]
- 179.Thordal-Christensen H., Zhang Z., Wei Y., Collinge D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J. 1997;11:1187–1194. doi: 10.1046/j.1365-313X.1997.11061187.x. [DOI] [Google Scholar]
- 180.Jacks T.J., Davidonis G.H. Superoxide, hydrogen peroxide, and the respiratory burst of fungally infected plant cells. Mol. Cell. Biochem. 1979;158:77–79. doi: 10.1007/BF00225885. [DOI] [PubMed] [Google Scholar]
- 181.Moloi M.J., van der Westhuizen A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006;163:1118–1125. doi: 10.1016/j.jplph.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 182.Imbiscuso G., Trotta A., Maffei M., Bossi S. Herbivory induces a ROS burst and the release of volatile organic compounds in the fern Pteris vittate L. J. Plant Interact. 2009;4:15–22. doi: 10.1080/17429140802387739. [DOI] [Google Scholar]
- 183.Shinya T., Hojo Y., Desaki Y., Christeller J.T., Okada K., Shibuya N., Galis I. Modulation of plant defense responses to herbivores by simultaneous recognition of different herbivore-associated elicitors in rice. Sci. Rep. 2016;6:32537. doi: 10.1038/srep32537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Gandhi A., Kariyat R.R., Chappa C., Tayal M., Sahoo N. Tobacco Hornworm (Manduca sexta) Oral Secretion Elicits Reactive Oxygen Species in Isolated Tomato Protoplasts. Int. J. Mol. Sci. 2020;21:8297. doi: 10.3390/ijms21218297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. Local positive feedback regulation determines cell shape in root hair cells. Science. 2008;319:1241–1244. doi: 10.1126/science.1152505. [DOI] [PubMed] [Google Scholar]
- 186.Ogasawara Y., Kaya H., Hiraoka G., Yumoto F., Kimura S., Kadota Y., Hishinuma H., Senzaki E., Yamagoe S., Nagata K., et al. Synergistic Activation of the Arabidopsis NADPH oxidase AtrbohD by Ca2+ and phosphorylation. J. Biol. Chem. 2008;283:8885–8892. doi: 10.1074/jbc.M708106200. [DOI] [PubMed] [Google Scholar]
- 187.Kimura S., Kaya H., Kawarazaki T., Hiraoka G., Senzaki E., Michikawa M., Kuchitsu K. Protein phosphorylation is a prerequisite for the Ca2+-dependent activation of Arabidopsis NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+ and reactive oxygen species. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012;1823:398–405. doi: 10.1016/j.bbamcr.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 188.Kadota Y., Shirasu K., Zipfel C. Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol. 2015;56:1472–1480. doi: 10.1093/pcp/pcv063. [DOI] [PubMed] [Google Scholar]
- 189.Pei Z.-M., Murata Y., Benning G., Thomine S., Klüsener B., Allen G.J., Grill E., Schroeder J.I. Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 190.Kwak J.M. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Little D., Gouhier-Darimont C., Bruessow F., Reymond P. Oviposition by pierid butterflies triggers defense responses in Arabidopsis. Plant Physiol. 2006;143:784–800. doi: 10.1104/pp.106.090837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Stahl E., Brillatz T., Ferreira Queiroz E., Marcourt L., Schmiesing A., Hilfiker O., Riezman I., Riezman H., Wolfender J.-L., Reymond P. Phosphatidylcholines from Pieris brassicae eggs activate an immune response in Arabidopsis. eLife. 2020;9:e60293. doi: 10.7554/eLife.60293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dooley C.T., Dore T.M., Hanson G.T., Jackson W.C., Remington S.J., Tsien R.Y. Imaging dynamic redox changes in mammalian cells with green fluorescent protein indicators. J. Biol. Chem. 2004;279:22284–22293. doi: 10.1074/jbc.M312847200. [DOI] [PubMed] [Google Scholar]
- 194.Hanson G.T., Aggeler R., Oglesbee D., Cannon M., Capaldi R.A., Tsien R.Y., Remington S.J. Investigating mitochondrial redox potential with redox-sensitive green fluorescent protein indicators. J. Biol. Chem. 2004;279:13044–13053. doi: 10.1074/jbc.M312846200. [DOI] [PubMed] [Google Scholar]
- 195.Jiang K., Schwarzer C., Lally E., Zhang S., Ruzin S., Machen T., Remington S.J., Feldman L. Expression and characterization of a redox-sensing green fluorescent protein (reduction-oxidation-sensitive green fluorescent protein) in Arabidopsis. Plant Physiol. 2006;141:397–403. doi: 10.1104/pp.106.078246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Meyer A.J., Brach T., Marty L., Kreye S., Rouhier N., Jacquot J.-P., Hell R. Redox-sensitive GFP in Arabidopsis thaliana is a quantitative biosensor for the redox potential of the cellular glutathione redox buffer. Plant J. 2007;52:973–986. doi: 10.1111/j.1365-313X.2007.03280.x. [DOI] [PubMed] [Google Scholar]
- 197.Gutscher M., Sobotta M.C., Wabnitz G.H., Ballikaya S., Meyer A.J., Samstag Y., Dick T.P. Proximity-based protein thiol oxidation by H2O2-scavenging peroxidases. J. Biol. Chem. 2009;284:31532–31540. doi: 10.1074/jbc.M109.059246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Swanson S.J., Choi W.-G., Chanoca A., Gilroy S. In vivo imaging of Ca2+, pH, and reactive oxygen species using fluorescent probes in plants. Annu. Rev. Plant Biol. 2011;62:273–297. doi: 10.1146/annurev-arplant-042110-103832. [DOI] [PubMed] [Google Scholar]
- 199.Nietzel T., Elsässer M., Ruberti C., Steinbeck J., Ugalde J.M., Fuchs P., Wagner S., Ostermann L., Moseler A., Lemke P., et al. The fluorescent protein sensor roGFP2-Orp1 monitors in vivo H2O2 and thiol redox integration and elucidates intracellular H2O2 dynamics during elicitor-induced oxidative burst in Arabidopsis. New Phytol. 2018;221:1649–1664. doi: 10.1111/nph.15550. [DOI] [PubMed] [Google Scholar]
- 200.Hipsch M., Lampl N., Zelinger E., Barda O., Waiger D., Rosenwasser S. Sensing stress responses in potato with whole-plant redox imaging. bioRxiv. 2021;6:kiab159. doi: 10.1093/plphys/kiab159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Janků M., Luhová L., Petřivalský M. On the origin and fate of reactive oxygen species in plant cell compartments. Antioxidants. 2019;8:105. doi: 10.3390/antiox8040105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li X., Liu Y., He Q., Li S., Liu W., Lin C., Miao W. A candidate secreted effector protein of rubber tree powdery mildew fungus contributes to infection by regulating plant ABA biosynthesis. Front. Microbiol. 2020;11:2788. doi: 10.3389/fmicb.2020.591387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Chen K., Guo Y., Song M., Liu L., Xue H., Dai H., Zhang Z. Dual role of MdSND1 in the biosynthesis of lignin and in signal transduction in response to salt and osmotic stress in apple. Hortic. Res. 2020;7:1–13. doi: 10.1038/s41438-020-00433-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fu H., Zhao M., Xu J., Tan L., Han J., Li D., Wang M., Xiao S., Ma X., Deng Z. Citron C-05 inhibits both the penetration and colonization of Xanthomonas citri subsp. citri to achieve resistance to citrus canker disease. Hortic. Res. 2020;7I:1–12. doi: 10.1038/s41438-020-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Fichman Y., Mittler R. Rapid systemic signaling during abiotic and biotic stresses: Is the ROS wave master of all trades? Plant J. 2020;102:887–896. doi: 10.1111/tpj.14685. [DOI] [PubMed] [Google Scholar]
- 206.Bissoli G., Muñoz-Bertomeu J., Bueso E., Sayas E., Vilcara E.A., Felipo A., Niñoles R., Rubio L., Fernández J.A., Serrano R. An Arabidopsis mutant over-expressing subtilase SBT4.13 uncovers the role of oxidative stress in the inhibition of growth by intracellular acidification. Int. J. Mol. Sci. 2020;21:1173. doi: 10.3390/ijms21031173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.