Abstract
The ternary complex factors (TCFs) are targets for Ras/mitogen-activated protein kinase signalling pathways. They integrate the transcriptional response at the level of serum response elements in early-response genes, such as the c-fos proto-oncogene. An important aim is to understand the individual roles played by the three TCFs, Net, Elk1, and Sap1a. Net, in contrast to Elk1 and Sap1a, is a strong repressor of transcription. We now show that Net is regulated by nuclear-cytoplasmic shuttling in response to specific signalling pathways. Net is mainly nuclear under both normal and basal serum conditions. Net contains two nuclear localization signals (NLSs); one is located in the Ets domain, and the other corresponds to the D box. Net also has a nuclear export signal (NES) in the conserved Ets DNA binding domain. Net is apparently unique among Ets proteins in that a particular leucine in helix 1, a structural element, generates a NES. Anisomycin, UV, and heat shock induce active nuclear exclusion of Net through a pathway that involves c-Jun N-terminal kinase kinase and is inhibited by leptomycin B. Nuclear exclusion relieves transcriptional repression by Net. The specific induction of nuclear exclusion of Net by particular signalling pathways shows that nuclear-cytoplasmic transport of transcription factors can add to the specificity of the response to signalling cascades.
The Ets proteins form a large family of transcription factors with diverse functions (reviewed in references 5, 19, 67, 76, and 77). They regulate gene expression by direct or assisted DNA binding by the ets domain to sequences containing the trinucleotide GGA. One of the subfamilies of Ets proteins involved in Ras signal transduction is composed of the ternary complex factors (TCFs) Elk1, Sap1a, and Net (also called ERP [41] or SAP-2 [61]). The TCFs have three similar domains, A (ets), B, and C, involved, respectively, in DNA binding, interaction with serum response factor (SRF), and activation of transcription when phosphorylated by mitogen-activated protein (MAP) kinases (23, 27, 28, 31, 61, 74, 81). The TCFs are coexpressed in many cell types and are highly conserved from mouse to human (16, 61). Net is different from the other TCFs in its ability to strongly repress transcription (16, 44).
The TCFs bind with SRF to the serum response element and mediate the response to ERK (extracellular signal-regulated kinases), JNK (c-Jun N-terminal kinase), and p38 MAP kinases, through phosphorylation of their C domains. Apparently Elk1 is activated by the three types of MAP kinase, whereas Sap1a is activated only by ERK and p38 (29, 30, 60, 70, 81, 82, 87, 88). Net can be activated by the ERK pathway and is phosphorylated by p38 (24, 60, 61). The TCFs integrate the response to the MAP kinase signalling pathways, and the interplay between these factors contributes to precise regulation of gene expression.
The ERK, JNK, and p38 MAP kinases are downstream components of kinase cascades (for reviews, see references 74, 76, and 77). ERKs generally respond to growth signals, whereas JNKs and p38 (also known as SAPKs [stress-activated protein kinases]), are induced by stress signals (anisomycin, UV, and heat shock) and cytokines (20, 34, 35, 38, 65). MAP kinases are activated by dual phosphorylation on tyrosine and threonine residues by MAP kinase kinases (MKKs) (for reviews, see references 26, 34, and 74). p38 is activated by MKK3 and MKK6 (4, 20, 53, 62, 69), whereas SAPK/JNK is induced by JNKK (also called MKK4/SEK1) and MKK7 (4, 25, 39, 42, 54, 72, 80). The pathways may not be so simple. For example, there could be other JNK kinases (JNKKs) on the JNK pathway (47, 52, 59). In turn, an upstream activator of JNKK1 is MEKK1 (39, 50, 86), although there are MEKK1-independent pathways for activation of JNKK (89). The pathways are complex and cell type dependent and are not completely understood.
Substrate selection by MAP kinases is regulated at different levels, including nuclear-cytoplasmic shuttling of both the kinases and transcription factors (11, 71). The complexities of nuclear-cytoplasmic transport have been unravelled in recent years (for reviews, see references 17, 55, 57, and 85). Proteins enter the nucleus through nuclear pore complexes in conjunction with receptors that recognize nuclear localization signals (NLSs) composed of basic amino acids. Some of the factors involved include importins α and β, which form the NLS receptor, and the GTPase Ran/TC4. Nuclear export signals (NESs) are rich in leucines and are necessary and sufficient for the export of carrier proteins. Among the proteins that contain NESs are human immunodeficiency virus (HIV) Rev, cyclic AMP (cAMP)-dependent protein kinase inhibitor, MAPKK, and cyclin B1. NES binding to CRM1 is required for export, and inhibition of the interaction by leptomycin B blocks exit (33, 84). The activities of several transcription factors are regulated by nuclear-cytoplasmic shuttling. The localization of E2F-4 changes during the cell cycle (reviewed in references 21 and 32). NF-κB translocates to the nucleus after phosphorylation and degradation of its inhibitor IκBα (reviewed in references 12, 45, 46, 68, and 75). Nuclear import of NFAT4 is mediated by the opposing actions of phosphatases and kinases (90).
In this report, we show that Net has a NES and two regions involved in nuclear localization, one located in the ets domain, as reported for Elk1 (31) and Sap1a (27), and another corresponding to the D box. Stress conditions such as anisomycin, UV, and heat shock induce nuclear exclusion of Net by a NES-dependent mechanism. We discuss how regulation of nuclear exclusion of the Net repressor by specific MAP kinase pathways represents a new mechanism that contributes to signal integration at the level of the TCFs.
MATERIALS AND METHODS
Construction of plasmids.
To make pGFP-N1 to -N19, pGFP-N5 Dm, and pGFP-C7 to -C16, appropriate KpnI fragments (40) were inserted in the KpnI site of pEGFP-C1 (Clontech).
pGFP-Net/NES, pGFP-Net/NES mP, pGFP-Net/NES mA1, and pGFP-Net/NES mA2 were generated by cloning KpnI site-flanked oligonucleotides in the corresponding site of pEGFP-C1 as shown in Table 1.
TABLE 1.
Oligonucleotides used to generate plasmids
| Plasmid genes | Oligonucleotide used | Nucleotides and amino acids sequences |
|---|---|---|
| pGFP | Net/NES | 5′ C ACG CTG TGG CAG TTC CTC TTG CAC TTG CTG CTG GAC GGTAC 3′ |
| 3′ CATGG TGC GAC ACC GTC AAG GAG AAC GTG AAC GAC GAC CTG C5′ | ||
| Thr Leu Trp Gln Phe Leu Leu His Leu Leu Leu Asp | ||
| Net/NES mP | 5′ C ACG CTG TGG CAG AAA CCA CCG GTG CCG CCT TCT GAC GGTAC 3′ | |
| 3′ CATGG TGC GAC ACC GTC TTT GGT GGC CAC GGC GGA AGA CTG C5′ | ||
| Thr Leu Trp Gln Lys Pro Pro Val Pro Pro Ser Asp | ||
| Net/NES mA1 | 5′ C ACG CTG TGG CAG TTC GCA GCT CAC TTG CTG CTG GAC GGTAC 3′ | |
| 3′ CATGG TGC GAC ACC GTC AAG CGT CGA GTG AAC GAC GAC CTG C5′ | ||
| Thr Leu Trp Gln Phe Ala Ala His Leu Leu Leu Asp | ||
| Net/NES mA2 | 5′ C ACG CTG TGG CAG TTC CTC TTG CAC GCA GCT GCG GAC GGTAC 3′ | |
| 3′ CATGG TGC GAC ACC GTC AAG GAG AAC GTG CGT CGA CGC CTG C5′ | ||
| Thr Leu Trp Gln Phe Leu Leu His Ala Ala Ala Asp | ||
| pGEX | Net/NES | 5′ GATCC ACG CTG TGG CAG TTC CTC TTG CAC TTG CTG CTG GAC G3′ |
| 3′ G TGC GAC ACC GTC AAG GAG AAC GTG AAC GAC GAC CTG CCTAG 5′ | ||
| Thr Leu Trp Gln Phe Leu Leu His Leu Leu Leu Asp | ||
| Rev/NES | 5′ GATCC CTT CAG TTG CCT CCC TTA GAG CGT TTA ACT CTA GAC G 3′ | |
| 3′ G GAA GTC AAC GGA GGG AAT CTC GCA AAT TGA GAT CTG CCTAG 5′ | ||
| Leu Gln Leu Pro Pro Leu Glu Arg Leu Thr Leu Asp |
pGEX-Net/NES and pGEX-Rev/NES were generated by cloning BamHI site-flanked oligonucleotides in the corresponding site of pGEX-4T-3 (Pharmacia) as shown in Table 1.
Recombinants were screened for the correct orientation and sequenced.
pGFP-Net(L16A), pTL2-Net(L16A), pGFP-N5Dm, pGFP-NetDm, and pGFP-NetDelk were constructed by double-round PCR mutagenesis and sequenced.
pTL2-Net encodes mouse Net (16). pTL1-Elk1 encodes human Elk1 (16). pKOZ1-Sap1a encodes human Sap1a (44). pRasCTBx2 is an Ha-Ras expression vector (78). pCMV5-MEKK1 and pCMV5-MEKK1(KM) are described in reference 50. pSRα-mJNKK and pSRα-JNKK(KR) are described in reference 39. pRc/RSV-Flag-MKK3(Glu), pRc/RSV-Flag-MKK3(Ala), pCDNA3-Flag-MKK6(Glu), and pCDNA3-Flag-MKK6(KA) are described in reference 62.
To make the reporter Palx8-TK-LUC, eight copies of the palindromic ets site inserted upstream from the thymidine kinase promoter of pGL2-basic (Promega).
Antibodies.
Anti-Net rabbit polyclonal antibodies (PAb) 375 and PAb 376 (16) were raised, respectively, against amino acids 385 to 409 and 151 to 176 of mouse Net. Others antibodies were as follows: anti-Elk1, rabbit polyclonal PAb 512 (16) raised against amino acids 411 to 427 of human Elk1; anti-Sap1a, rabbit polyclonal PAb 643 (44), raised against amino acids 131 to 153 of murine Sap1a; and anti-glutathione S-transferase (GST), monoclonal antibody 1D10 (42a).
Western blotting.
Cells extracts, prepared by lysis with loading buffer (0.1 M Tris [pH 7.9], 6% sodium dodecyl sulfate [SDS], 15% glycerol, 10% β-mercaptoethanol, 0.1% bromophenol blue), were boiled for 10 min; 40-μg samples were analyzed by SDS-polyacrylamide gel electrophoresis (PAGE) on 10% gels and transferred to 0.45-μm-pore-size nitrocellulose membranes (Schleicher & Schuell), which were then blocked in PBSTM (phosphate-buffered saline [PBS] with 0.1% Tween 20 and 5% milk) for 30 min at room temperature, incubated in PBSTM with anti-Net primary antibodies (PAb 375; diluted 1:1,000) for 2 h at room temperature, washed three times in PBS–0.1% Tween 20, incubated with goat anti-rabbit secondary antibodies coupled to peroxidase (diluted 1:5,000; Jackson) in PBSTM for 1 h at room temperature, washed three times, and revealed with an enhanced chemiluminescence detection kit (Amersham product no. RPN 2106).
Cell culture and transfections.
NIH 3T3 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) containing 10% fetal calf serum (FCS) and transfected by the BBS calcium phosphate method (3) in 36-mm-diameter plates (six-well clusters; Costar 3516) with 4 μg of DNA. After 20 h, the cells were washed twice with DMEM, incubated in DMEM containing 0.05% FCS for 24 h, and scraped for luciferase assays or fixed for immunofluorescent detection of subcellular localization.
Subcellular localization. (i) Endogenous Net and exogenous Net, Elk1, and Sap1a.
Cells were fixed in 1:1 acetone-methanol for 40 min at −20°C. Nonspecific sites were blocked with 3% bovine serum albumin in PBS for 40 min at room temperature. Cells were incubated with primary antibody (PAb 376 for Net, PAb 512 for Elk1, or PAb 643 for Sap1a; diluted 1:250) for 2 h at room temperature, washed three times with PBS, exposed to a Texas red or a Cy3-conjugated anti-rabbit antibody (diluted 1:250; Jackson), washed three times with PBS, and visualized by confocal microscopy.
(ii) Green fluorescent protein (GFP) fusion proteins.
Cells were fixed in freshly made 4% paraformaldehyde in PBS for 40 min at room temperature, washed three times with PBS, and visualized by confocal microscopy.
(iii) Determination of localization.
Multiple fields containing at least 100 positive cells were examined, and localizations were classified as nuclear, cytoplasmic, or both when at least 70% of the cells displayed the corresponding localization.
Expression and purification of GST fusion proteins for microinjection.
Expression of GST fusion proteins was induced in Escherichia coli with 0.1 mM isopropyl β-d-thiogalactopyranoside for 5 h. Bacteria were sonicated in lysis buffer (1% Triton X-100, 1% NP-40, and 10 mM dithiothreitol in PBS) and centrifuged at 12,000 rpm for 15 min at 4°C. The supernatants were incubated for 2 h at 4°C with glutathione-Sepharose beads (Pharmacia). The beads were washed extensively with PBS, and the GST fusion proteins were eluted with glutathione (10 mM reduced glutathione in 50 mM Tris-HCl [pH 8.3]). After centrifugation (500 rpm, 5 min; Sorvall GSA rotor), the supernatants contained the majority of the GST fusion proteins and were dialyzed against PBS.
Microinjection.
NIH 3T3 cells were cultured in DMEM supplemented with 10% FCS. Purified proteins were adjusted to a final concentration of 2 mg/ml in PBS and microinjected with a modified Eppendorf apparatus. Six hours after injection, the cells were rinsed in PBS, fixed for 10 min at room temperature in a freshly made solution of 4% formaldehyde in PBS, rinsed in PBS, and permeabilized with 0.2% Triton X-100 in PBS at room temperature for 5 min. After reduction of free aldehyde groups with sodium borohydride (0.5 mg/ml) in PBS for 10 min, the cells were incubated with a monoclonal antibody against GST (diluted 1:200 in PBS) for 90 min at room temperature, rinsed three times for 10 min each with PBS, incubated with a Texas red-conjugated anti-mouse antibody (diluted 1:500 in PBS; Jackson), and visualized by confocal microscopy.
RESULTS
Net contains a NES.
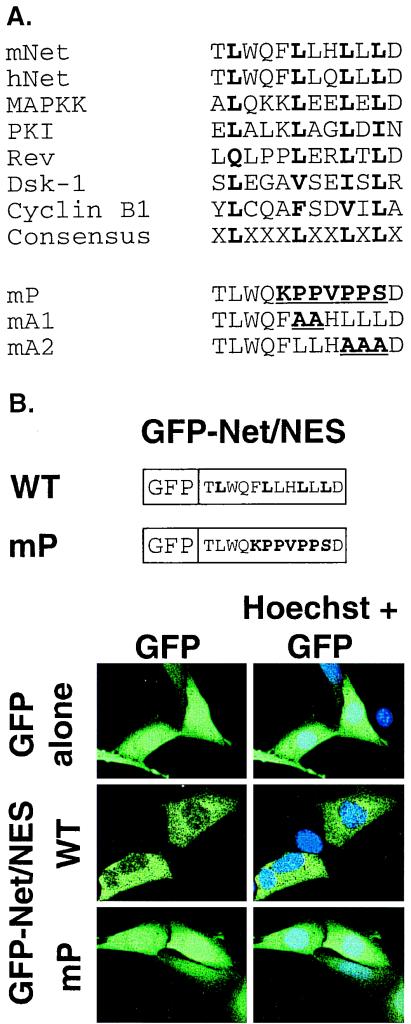
The ets domain of Net has a leucine-rich sequence that resembles NES found in a number of proteins (Fig. 1A). To test whether this sequence could affect the subcellular localization of proteins, it was fused to GFP and expressed in NIH 3T3 cells. GFP alone was distributed diffusely throughout the cell, whereas GFP fused to the putative NES of Net (GFP-Net/NES) was clearly cytoplasmic (Fig. 1B). The Net NES was altered to contain either prolines (mP [Fig. 1A]) or substitutions of leucines by alanines that by analogy should inactivate Net NES (mA1 and mA2 [Fig. 1A and references 7, 18, 55, 73, and 79]). The GFP-Net/NES mutants were distributed throughout the cell, similar to GFP alone (mP in GFP-Net/NES [Fig. 1B]; mA1 and mA2 [data not shown]). These results show that the NES-like sequence of Net can localize GFP to the cytoplasm.
FIG. 1.
The NES of Net. (A) Alignment of NES sequences. The NES-like sequence in the ets domain of Net (residues 6 to 18 in mice and humans) is aligned with known NESes. The functionally important hydrophobic residues are shown in boldface. Sequences used for comparison: MAPKK (10), cAMP-dependent protein kinase inhibitor (PKI) (79), HIV-1 Rev (7), Dsk-1 (9), and cyclin B1 (73). The Net NES mutants mP (F10K, L11P, L12P, H13V, L14P, L15P, L16S), mA1 (L11A, L12A), and mA2 (L14A, L15A, L16A) have the amino acids changes shown in parentheses. (B) Cellular localization of GFP, GFP-Net/NES, and GFP-Net/NES mP. Drawings representing GFP fused to NES wild-type (WT) and mP sequences are shown. NIH 3T3 cells transiently expressing GFP, GFP-Net/Nes, and GFP-Net/NES mP, were analyzed by confocal microscopy. Hoechst was used to stain nuclei.
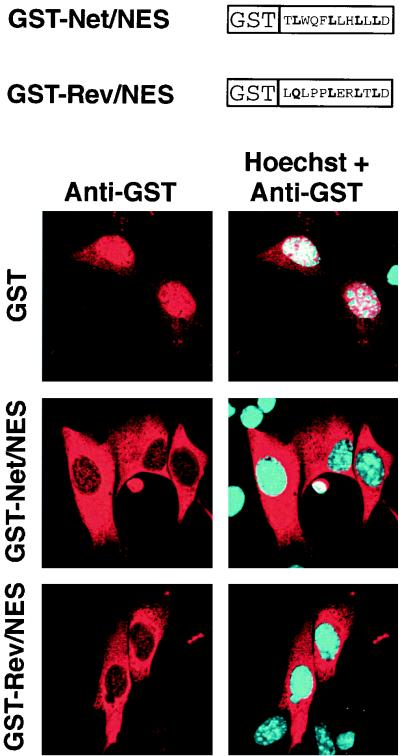
We then tested whether Net NES can drive the export of a heterologous protein from the nucleus. Net NES fused the the C terminus of GST, a similar fusion protein containing a known NES (HIV Rev NES), and GST alone were purified from E. coli and microinjected into the nuclei of NIH 3T3 cells. Six hours later, the cells were fixed and stained with an antibody against GST followed by a Texas red-coupled secondary antibody. GST mainly remained in the nucleus, with a small amount in the cytoplasm (Fig. 2), most likely due to passive diffusion (18). In contrast, GST-Rev/NES relocalized virtually completely to the cytoplasm, as shown previously (48). GST-Net/NES was exported to a similar extent as GST-Rev/NES, providing evidence that Net NES functions as a NES.
FIG. 2.
Net NES mediates nuclear export. GST and GST fused to NES sequences from either Net or HIV Rev (see diagrams) were purified from E. coli and injected into the nuclei of NIH 3T3 cells. Six hours later, injected cells were stained with an antibody against GST and a Texas red-coupled secondary antibody. Hoechst was used to stain nuclei.
The Net NES is located in a domain that is conserved amongst Ets proteins. A comparison of the distribution of leucine residues in the NES and Ets consensus sequences (Table 2) suggests that the fourth leucine at position 16 in Net NES could be critical for nuclear export. Mutating this residue to arginine, alanine, or proline abolished the preferential cytoplasmic localization of GFP-Net/NES fusion proteins (Table 2). In contrast, mutating aspartate 16 to glutamate, which is found in Elk1, did not inactivate the NES [NES(D17E)].
TABLE 2.
Disruption of NES activitya
| NES | Sequence | Localization
|
||
|---|---|---|---|---|
| N | C | N/C | ||
| Wild-type NES | TLWQFLLHLLLDQ | − | + | − |
| NES (L16R) | TLWQFLLHLLRDQ | − | − | + |
| NES (L16A) | TLWQFLLHLLADQ | − | − | + |
| NES (L16P) | TLWQFLLHLLPDQ | − | − | + |
| NES (D17E) | TLWQFLLHLLLEQ | − | + | − |
| NES consensus | XLXXXLXXLXLXX | |||
| Ets consensus | XLXXXLLXLLXXX | |||
The Net NES mutations (underlined) were leucine 16 to either arginine [Net(L16R)], alanine [Net(L16A)] or proline [Net(L16P)] and aspartate 17 to glutamate [Net(D17E)]. Wild-type and mutant NESs were fused to GFP, and the cellular localization of each fusion protein was determined. N, nuclear; C, cytoplasmic; N/C, both nuclear and cytoplasmic.
UV, anisomycin, and heat shock induce NES-dependent nuclear exclusion of Net.
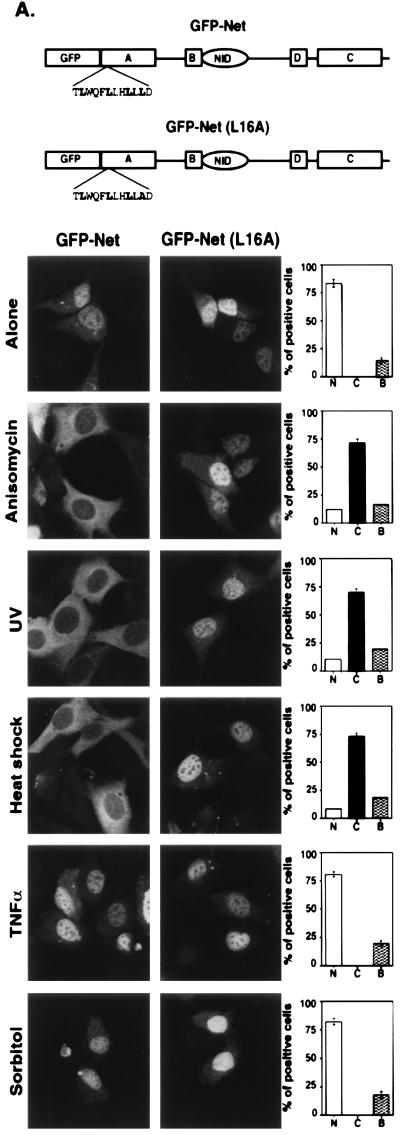
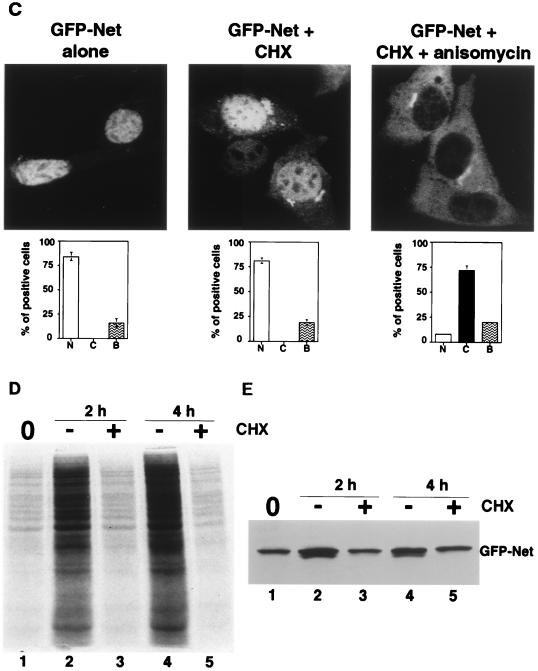
The presence of a NES in Net suggested that the subcellular localization of Net may be regulated. To study the control of Net localization, we expressed GFP-Net fusion proteins in NIH 3T3 cells and treated the cells with various stimuli (Fig. 3A). GFP-Net was nuclear in low serum. Strikingly, GFP-Net was predominantly cytoplasmic 30 min after induction with anisomycin (50 ng/ml), UV (40J/m2), and heat shock (42°C) but was nuclear after treatment with tumor necrosis factor alpha, (TNF-α) (20 ng/ml), sorbitol (600 mM), 10% serum, and Ha-Ras expression (data not shown). We studied endogenous Net, to establish that the endogenous and exogenous proteins were regulated in the same manner (Fig. 3B). NIH 3T3 cells stained with an antibody against Net (PAb 376) gave a predominantly nuclear signal (Fig. 3B). Treatment with anisomycin, UV, and heat shock resulted after 30 min in predominantly cytoplasmic staining, whereas TNF-α and sorbitol did not alter the localization. Cytoplasmic accumulation of Net could result from alterations in the kinetics of cycling between the compartments or from specific degradation in the nucleus coupled with cytoplasmic accumulation of newly synthesized Net. To study this possibility, cells were treated with anisomycin after 2 h of pretreatment with an inhibitor of protein synthesis, cycloheximide. Cycloheximide treatment did not inhibit anisomycin-induced cytoplasmic accumulation of GFP-Net (Fig. 3C). Cycloheximide treatment alone did not induce cytoplasmic accumulation. The cycloheximide treatment was efficient, since it blocked [35S]methionine incorporation into total cellular proteins, as shown by SDS-PAGE and autoradiography (Fig. 3D; compare lanes 1, 3, and 5 with lanes 2 and 4). Furthermore, it blocked GFP-Net synthesis, detected by Western blotting with anti-GFP antibodies (Fig. 3E). These results show that cytoplasmic accumulation was due to relocalization rather than specific nuclear degradation of Net.
FIG. 3.
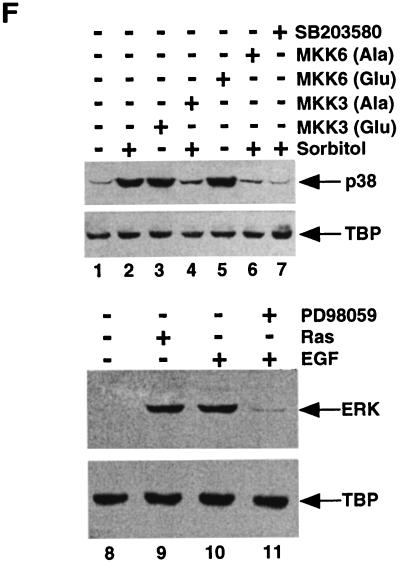
Anisomycin, UV, and heat shock induce nuclear export of Net. (A) Cellular localization of GFP-Net and GFP-Net(L16A) exposed to stress conditions. NIH 3T3 cells transiently transfected with GFP-Net or GFP-Net(L16A) (see diagrams for structures) were treated with anisomycin (50 ng/ml), UV (40 J/m2), heat shock (42°C), sorbitol (600 mM), and TNF-α (20 ng/ml) and, as a control, were left untreated (Alone). After 30 min, cells were fixed and visualized by confocal microscopy. About 100 positive cells were analyzed to calculate the percentages with GFP-Net in the nucleus (N), cytoplasm (C), or both (B). (B) Nuclear exclusion of endogenous Net. NIH 3T3 cells were treated with anisomycin, UV, heat shock, TNF-α and sorbitol. The cells were fixed and stained with an antibody raised against Net (PAb 376), as shown for the anisomycin treatment. C, cytoplasmic; N, nuclear. Hoechst was used to stain nuclei. (C to E) Cellular localization of GFP-Net in the presence of cycloheximide and anisomycin. (C) Cells transfected with the GFP-Net expression vector were either left untreated (left) or pretreated with cycloheximide (CHX; 15 μg/ml) and then either mock treated (middle) or incubated with anisomycin (right). The middle panels show percentages of cells with GFP-Net in the nucleus (N), cytoplasm (C), and both (B). (D) Protein synthesis was measured by labelling with [35S]methionine followed by SDS-PAGE and autoradiography. The cells were preincubated with [35S]methionine for 15 min, and then at time zero (lane 1) incubation was continued in the absence (−) or presence (+) of cycloheximide for 2 or 4 h. (E) GFP-Net levels were determined by Western blotting of whole-cell extracts with anti-GFP antibodies before (lane 1) or after 2 h (lanes 2 and 3) or 4 h (lanes 4 and 5) of incubation in the absence (−) or presence (+) of cycloheximide. (F) Activation of endogenous p38 was monitored by Western blotting of whole-cell extracts with antibodies specific for dual-phosphorylated activated p38 (lanes 1 to 7) or ERK (lanes 8 to 11). The cells were either untreated (lanes 1 and 8), transfected with a vector expressing MKK3(Glu) (lane 3) or MKK6(Glu) (lane 5), treated with sorbitol (600 mM) after transfection with a vector expressing MKK3(Ala) (lane 4) or MKK6(Ala) (lane 6), treated with sorbitol (600 mM) without (lane 2) or with SB203580 (20 μM), transfected with a vector that expresses Ha-Ras (lane 9), and treated with EGF (0.1 μg/ml) in the absence (lane 10) or presence (lane 11) of PD98059 (20 μM).
We investigated whether stress-induced Net exclusion depends on the NES. Mutation of the critical leucine 16 to an alanine in the full-length protein fused to GFP [GFP-Net(L16A)] abolished nuclear exclusion induced by UV, anisomycin, and heat shock (Fig. 3A). Leptomycin B, a specific inhibitor of NES-dependent intracellular transport that which blocks the interaction of the NES receptor (CRM1) with NES (8, 9, 56), prevented nuclear exclusion of GFP-Net induced by anisomycin and resulted in the same subcellular localization as for GFP-Net (L16A) under the same conditions (Table 3, conditions C). These results show that the NES is required for stress-induced nuclear exclusion of Net.
TABLE 3.
Net nuclear depletion depends on JNKK
| Conditions | Localizationb
|
|
|---|---|---|
| GFP-Net | GFP-Net(L16A) | |
| A (transfections) | ||
| Ras | N | N |
| MEKK1 | C | N |
| JNKK | C | N |
| MKK3(Glu) | N | N |
| MKK6(Glu) | N | N |
| B (anisomycin) | ||
| MEKK1 (KM) | C | N |
| JNKK (KR) | N | N |
| MKK3 (Ala) | C | N |
| MKK6 (KA) | C | N |
| C (anisomycin) | ||
| SB203580 | C | N |
| PD98059 | C | N |
| Leptomycin B | N | N |
A, MEKK1 and JNKK induce Net export. NIH 3T3 cells were cotransfected with GFP-Net or GFP-Net(L16A) and pRasCTBx2 (constitutively active Ras), pCMV5-MEKK1 (constitutively activated MEKK1), pSRα-mJNKK (mouse Jun kinase kinase), pRc/RSV-Flag-MKK3(Glu) (constitutively active MKK3), or pCDNA3-Flag-MKK6(Glu) (constitutively active MKK6). B, NIH 3T3 cells were cotransfected with GFP-Net or GFP-Net(L16A) and pCMV5-MEKK1 (KM) (dominant negative MEKK1), pSRα-JNKK(KR) (dominant negative JNKK), pRc/RSV-Flag-MKK3(Ala) (dominant negative MKK3), or pCDNA3-Flag-MKK6(KA) (dominant negative MKK6) and treated with anisomycin (50 ng/ml) for 30 min. C, Cells transiently expressing GFP-Net and GFP-Net(L16A) were stimulated by treatment with anisomycin (50 ng/ml) for 30 min in the presence of SB203580 (p38 cascade inhibitor; 20 μM), PD98059 (ERK cascade inhibitor); 20 μM), or leptomycin B (NES-dependent nuclear export inhibitor; 2 ng/ml).
Analyzed by confocal microscopy. N, nuclear; C, cytoplasmic.
Stress-induced nuclear exclusion requires JNKK.
Cellular stresses and inflammatory cytokines lead to the activation of at least two types of MAP kinase pathways, involving SAPK/JNK and p38/MPK2/CSAID binding protein (CSBP) (20, 34, 35, 38, 65). We studied the pathways involved in stress-induced relocalization of Net by expression of activators or transdominant inhibitors of the pathways, or chemical inhibitors. Previous studies have shown that ERK1/2 is activated by Ras (34); SAPK/JNK is activated by both MEKK1 (49) and JNKK/SEK1/MKK4 (4, 39); and p38 (4, 20, 53, 62, 69) is activated by both MKK3 and MKK6. Net relocalization to the cytoplasm was induced by MEKK1 and JNKK but not Ras, MKK3(Glu), or MKK6(Glu) (Table 3, conditions A). Anisomycin-stimulated relocalization of Net was blocked by dominant negative JNKK(KR) but not MEKK(KM), MKK3(Ala), or MKK6(KA) (Table 3, conditions B). Furthermore, it was not blocked by SB203580 and PD98059, specific inhibitors of the p38 and ERK pathways, respectively (Table 3, conditions C). We verified that the inhibitors and activators that had no effect were indeed active. The activity of the ERK and p38 pathways was monitored by Western blotting of cell extracts with antibodies that specifically recognize the activated Tyr/Thr dual-phosphorylated forms of the kinases (26, 34, 74). As expected, sorbitol-stimulated phosphorylation of p38 (Fig. 3F, lanes 1 and 2) was inhibited by the expression of the transdominant inhibitors MKK3(Ala) (lane 4) and MKK6(Ala) (lane 6) and by treatment with SB203580 (lane 7). Constitutively active MKK3(Glu) and MKK6(Glu) stimulated p38 phosphorylation (lanes 3 and 5). Ras expression and epidermal growth factor (EGF) treatment stimulated ERK phosphorylation (lanes 8 to 10), and PD98059 inhibited ERK phosphorylation induced by EGF (lane 11). The TATA binding protein control showed that the lanes contained equal amounts of protein. Net relocalization always required the NES, since Net(L16A), with a mutated NES, was always nuclear (see especially MEKK1 and JNKK in Table 3, condition A). Taken together, these results suggest that stress-induced Net relocalization is dependent on the JNK (SAPK1) pathway and the NES sequence.
Sequences related to Net NES in other Ets proteins do not mediate nuclear exclusion.
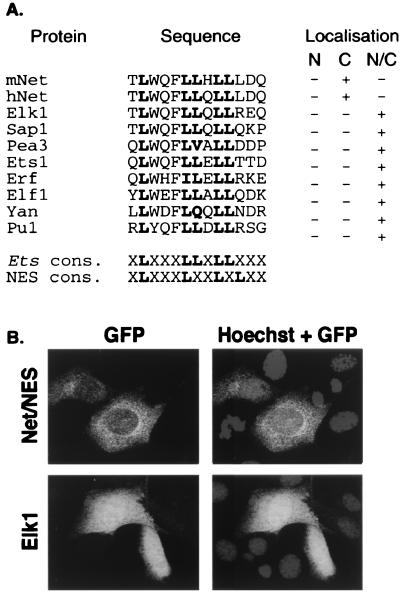
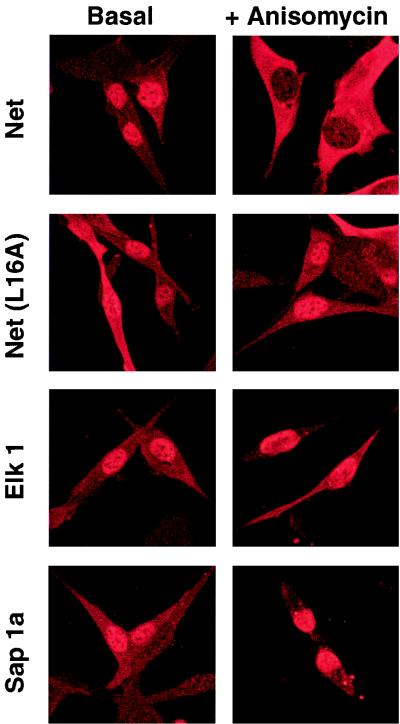
Net NES is located in the ets domain, which is related in sequence to a structural domain found in a large group of Ets proteins from humans, mice, flies, and nematodes. Most of the leucine residues found in Net NES are conserved (Fig. 4A), raising the possibility that other Ets proteins contain nuclear export signals. To test whether these sequences mediate export, we grouped the sequences by homology and then selected one representative from each group and fused it to GFP (Fig. 4A). In contrast to GFP-Net/NES, which was cytoplasmic (Fig. 4B), all the other hybrid proteins were distributed throughout the cell (Elk1 in Fig. 4B and summary in Fig. 4A), suggesting that the Net NES sequence is unique among Ets proteins. Elk1 is closely related to Net. The Elk1 sequence differs from Net NES at two positions, leucine instead of arginine at position 16 and glutamate instead of aspartate at 17. Mutating leucine 16 of Net NES to arginine resulted in GFP fusion proteins that were found throughout the cell (Table 3). In contrast, the aspartate-to-glutamate change did not inactivate the NES [NES(D17E)]. These results show that the presence of a NES in the Ets domain is quite unusual in the Ets family and is apparently unique to Net. It is possible that other Ets proteins, and especially those related to Net, may contain cryptic NES sequences that are active only under stress conditions. To test this possibility, Net, Net(L16A), Elk1, and Sap1a were expressed by transient transfection, and after anisomycin treatment, their subcellular localization was studied by using specific antibodies. Thirty minutes after induction only Net was cytoplasmic (Fig. 5), indicating that stress-induced nuclear exclusion is specific for Net among the TCFs.
FIG. 4.
NES-like sequences in other ets domains do not mediate nuclear export. (A) Comparison of mouse and human Net (mNet and hNet) NES with corresponding sequences in other Ets family members. The Ets proteins used for the alignment are human Elk1, human Sap1a, mouse Pea3, mouse Ets1, human Erf, human Elf1, Drosophila Yan (Pok), and human Pu1. Cons., consensus. Cellular localization of the corresponding GFP-NES fusion proteins is summarized on the right. N, nuclear; C, cytoplasmic; N/C, both nuclear and cytoplasmic. (B) Cellular localization of GFP fusion proteins. Proteins with GFP linked to the sequences listed in panel A were transiently expressed in NIH 3T3 cells and localized by confocal microscopy. Only GFP-Net/NES and GFP-Elk1 are shown. The localization of the other GFP-Ets proteins was similar to that of GFP-Elk1, as summarized in panel A.
FIG. 5.
Anisomycin induces export of Net but not Elk1 or Sap1a. Cells transiently transfected with pTL2-Net, pTL2-Net(L16A), pTL1-Elk1, or pKOZ1-Sap1a were left untreated (Basal) or exposed to anisomycin (50 ng/ml) for 30 min (+ Anisomycin), fixed, and stained with an antibody against Net (PAb 376), Elk1 (PAb 512), or Sap1a (PAb 643) followed by a Texas red-conjugated anti-mouse antibody.
Nuclear localization of Net.
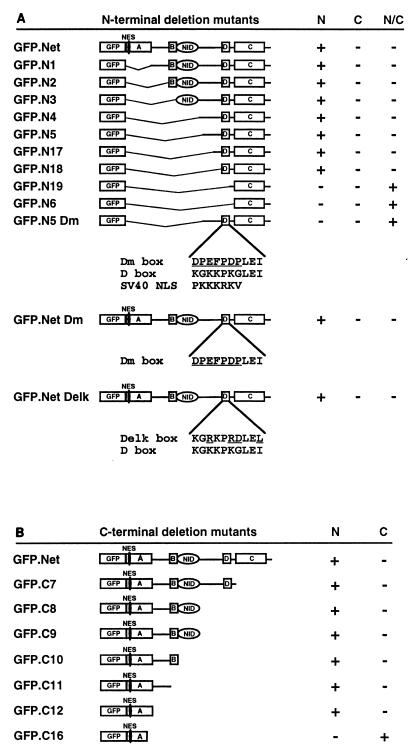
Exogenous (Fig. 3A and 5) or endogenous (Fig. 3B) Net is mainly nuclear, indicating that it has NLSs. We localized the NLS initially by using N-terminal deletion mutants (Fig. 6A). GFP.N1 to GFP.N18 were found to be nuclear, whereas GFP.N18 and GFP.N19 were located in both compartments (Fig. 6A), indicating that the D box is important for nuclear localisation. The D box is a lysine-rich sequence that is homologous to the simian virus 40 (SV40) NLS. A mutant based on N5 with point mutations in this lysine-rich sequence (GFP.N5Dm [Fig. 6A]) lost nuclear targeting, showing that the D box can function as an NLS. To test whether this sequence is uniquely responsible for the nuclear localization of Net, we replaced the D box by the inactive Dm box or by the D box of Elk1 (Delk) in the context of the full-length protein (Fig. 6A). Both Net mutants remained in the nucleus (Fig. 6A), suggesting that there is another NLS. With C-terminal deletion mutants, we found that deletions that removed the D box and extended to the ets domain gave nuclear proteins (GFP.Net to GFP.C12 [Fig. 6B]), whereas a further deletion of 20 amino acids prevented nuclear targeting (GFP.C16). These results suggest that for Net, as for Elk1 (31) and Sap1a (27), the ets domain plays a role in nuclear localization.
FIG. 6.
The D box and the Ets domain of Net are required for nuclear import. N-terminal (A) and C-terminal (B) deletion mutants of Net fused to GFP were expressed in NIH 3T3 cells and analyzed for cellular location. The sequences of the D box, the D box mutants (Dm and Delk), and the SV40 NLS are shown. Localizations: N, nuclear; C, cytoplasmic; N/C, nuclear and cytoplasmic. A (ets domain), B, C, D, and NID represent domains of Net.
JNKK relieves inhibition by Net.
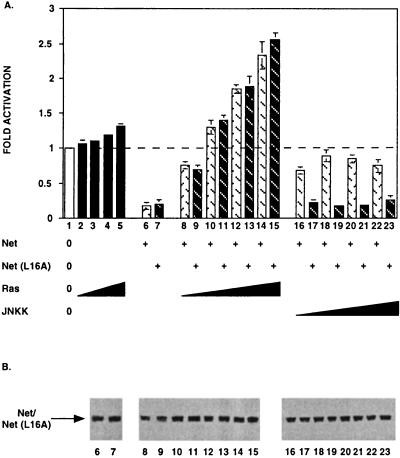
We have shown that Net nuclear exclusion is dependent on JNKK (Table 3). Nuclear export induced by JNKK would be expected to relieve repression of transcription by Net. This possibility was investigated in transfection assays using NIH 3T3 cells with a luciferase reporter containing eight copies of the ets motif. Net and Net(L16A) expression repressed transcription (Fig. 7A, lanes 6 and 7). Coexpression of increasing quantities of Ras progressively relieved repression by Net and then activated transcription (Fig. 7A, lanes 8, 10, 12, and 14), as reported previously (16). Ras expression has no effect on the activity of the reporter in absence of coexpressed Net (Fig. 7A, lanes 2 and 5). In contrast, increasing quantities of JNKK relieved repression but never led to activation of transcription (Fig. 7A, lanes 16, 18, 20, and 22). To study whether the lack of activation was due to translocation to the cytoplasm, we investigated the transcriptional activity of the mutant, Net (L16A), which is not exported in response to JNKK (Table 3). The point mutation did not affect the response of Net to Ras (Fig. 7A, lanes 9, 11, 13, and 15) but did prevent release from repression by JNKK (Fig. 7A, lanes 17, 19, 21, and 23). The differences do not result from effects on the level of expression of Net and Net (L16A), as shown by Western blotting (Fig. 7B). The similar functional responses of Net and Net(L16A) to Ras indicate that the L16A mutation does not affect vital functions involved in transcription activation. The function of stress-induced nuclear exclusion could be to prevent transcriptional repression by Net.
FIG. 7.
Net and Net(L16A) exhibit different transcriptional responses to JNKK and Ras. (A) NIH 3T3 cells were cotransfected with the reporter (1 μg of Palx8-TK-LUC; all lanes), the empty vector lacking Net coding sequences (lanes 2 to 5), or expression vectors for Net [250 ng of pTL2Net; lanes 6, 8, 10, 12, 14, 16, 18, 20, and 22], Net(L16A) [250 ng of pTL2Net(L16A); lanes 7, 9, 11, 13, 15, 17, 19, 21, and 23], Ha-Ras (pRasCTBx2; 100 ng [lanes 2, 8, and 9], 250 ng [lanes 3, 10, and 11], 500 ng [lanes 4, 12, and 13], 1,000 ng [lanes 5, 14, and 15]), or JNKK (pSRα-mJNKK; 100 ng [lanes 16 and 17], 250 ng [lanes 18 and 19], 500 ng [lanes 20 and 21], 1,000 ng [lanes 22 and 23]). Fold activation was calculated relative to the basal activity of the reporter in the presence of empty vector (pSG5). (B) Expression levels of Net and Net(L16A) were assessed by Western blotting using an antibody against Net (PAb 375). The specific band is indicated on the left.
DISCUSSION
A balance between nuclear import and export of Net regulated by stress stimuli.
Net has a functional NES with the consensus sequence LXXXLXXLXL. Altering the sequence to alanines or prolines abolishes the cytoplasmic localization of the isolated NES fused to GFP. The NES from Net can mediate the export of a carrier protein injected into the nucleus. However, full-length Net remains in the nucleus under basal conditions, even though it contains a NES. Net contains two nuclear localization signals: an NLS that corresponds to the D box, and another in the ets domain. The ets domains of Elk1 and Sap1a have also been shown to direct nuclear localization (27, 31). It remains to be established whether there is a short sequence motif in the ets domain that signals nuclear localization or whether DNA binding itself can keep the proteins in the nucleus.
The presence of both NES and nuclear localization sequences in Net suggests that there is a balance between import and export that is regulated. Relocalization could result from changes in the rates of either import, export, or both. We have shown that the balance can be tipped by stress signals, induced by anisomycin, UV, and heat shock. The redistribution of Net is an active process, since it occurs rapidly within 30 min, is prevented by point mutation of Net NES, and is sensitive to leptomycin B, a specific inhibitor of NES-dependent transport (33, 84). Anisomycin induced redistribution still occurs in the absence of protein synthesis, ruling out the possibility that Net is specifically degraded in the nucleus and newly made Net accumulates in the cytoplasm. The JNK pathway is apparently specifically involved in the stress-induced nuclear exclusion. JNK activators such as MEKK and JNKK induce relocalization, and transdominant JNKK blocks stress-induced redistribution. In contrast, activators and inhibitors of the ERK and p38 pathways do not affect the process (Table 3). MEKK1 expression stimulates nuclear exclusion whereas the transdominant kinase does not block stress-induced relocalization, suggesting that MEKK1 and the stress stimuli used (anisomycin, UV, and heat shock) stimulate JNK through different mechanisms, as has been shown recently (89). Net transcriptional activity is affected in different ways by Ras and JNKK. Ras switches Net from a repressor to an activator, whereas JNKK blocks repression by inducing nuclear exclusion of Net. Release from repression is prevented by mutating Net NES.
The presence of a NES is specific for Net among the TCFs and other Ets proteins.
The three TCFs, Net, Elk1, and Sap1a, have related functions mediated by three homologous regions: DNA binding by region A, ternary complex formation with SRF by region B, and MAP kinase-dependent transcription activation by region C. The TCFs also have different properties. First, Net does not bind DNA or form ternary complexes as efficiently as Elk1 or Sap1a (61). Second, under basal conditions, Net inhibits promoter activity whereas Sap1a has no effect and Elk1 activates (44). We show here that Net can be actively transferred to the cytoplasm due to a NES located in the ets domain, whereas the corresponding sequences of Elk1 and Sap1a are inactive in the same assays. Net relocalization is induced by anisomycin, whereas Elk1 and Sap1a remain nuclear. Interestingly, the difference stems from a single amino acid change in the Ets DNA binding domain.
Net NES fits the consensus sequence of helix 1 of ets domains. Helix 1 is an integral part of the structure of the DNA binding domain which does not directly contact DNA. However, helix 1 has additional roles, in regulation of DNA binding activity. In Ets1, it interacts intramolecularly with sequences N and C terminal to the ets domain, leading to conformation changes that inhibit DNA binding (6, 58). In GABPα, interactions of helix 1 with another protein, GABPβ, facilitates DNA binding (1). By analogy, interactions of Net NES with other parts of Net or other proteins may regulate its functions. Our results raise the possibility that one of these roles is to export Net in response to JNK signalling. It is possible that JNK signalling induces a structure in Net that allows interactions between helix 1 and the export machinery. Interestingly, the critical leucine required for nuclear export (L16) is predicted to be exposed, if it is placed in the equivalent position of the recently determined structure of the Sap1a DNA binding domain (51). Leucine 16 appears to be available for interactions with other proteins, such as the nuclear export machinery. The other leucines of helix 1 are buried in the hydrophobic core of the Ets structural motif. These considerations raise the possibility that JNKK signalling somehow activates the export function of helix 1.
Mechanism of nuclear exclusion of Net.
One of the nuclear localization signals of Net is located in the D box, which is related to sequences in Elk1 and Sap1a (41). The other signal is located in the ets domain, as described for Elk1 and Sap1a (27, 31). Elk1 has a second NLS (31) in a C-terminal part of the protein that is distinct from the D box. The D box resembles the SV40 NLS, suggesting that it mediates nuclear import. However, the nuclear localization of Net may also result from nuclear retention, due to interactions with DNA or MAP kinases. The D domain of Elk1 interacts with MAP kinases (87, 88), raising the possibility that the Net D domain has a similar property. However, the D boxes may have distinct functions in different TCFs.
There is a certain similarity between the TCFs and E2F, a family of transcription factors that regulate cell cycle control genes. The cellular localization of one of the E2F factors is regulated. E2F-4 is located in the cytoplasm in a cell cycle-dependent manner, whereas E2F-1, -2, and -3 are always nuclear (40). E2F-4 can be recruited to the nucleus by interactions with DP and certain pocket proteins (43). The distinct cellular localizations likely result from the absence of an NLS in E2F-4.
The TCFs respond differently to the MAP kinase pathways. JNK, ERK, and p38 phosphorylate Elk1 (2, 13–15, 28, 62, 81, 91), whereas only ERK and p38 efficiently phosphorylate Sap1a (27, 61, 81, 82). The ERK and p38 pathways lead to phosphorylation of Net (24, 60, 61). It remains to be seen if stress-induced JNK signalling leads to phosphorylation of Net on the C domain or another sequence and subsequently nuclear exclusion.
Phosphorylation regulates the subcellular localization of NF-κB and NFAT. NF-κB controls genes involved in immune and inflammatory responses. In unstimulated cells, NF-κB is sequestered in a cytoplasmic inactive complex by IκBα or related proteins. MEKK1 induces phosphorylation and proteolytic degradation of IκBα (37), freeing NF-κB to translocate to the nucleus (22). NFAT4 regulates cytokine genes in response to T-cell activation (63). NFAT4 is cytoplasmic in unstimulated cells and rapidly translocates to the nucleus in response to various stimuli. It is maintained in the cytoplasm by NLS masking due to MEKK1 and casein kinase Iα (90). By analogy with NF-κB and NFAT4, the regulation of nuclear export of Net may involve phosphorylation of Net or associated factors, ultimately resulting in alterations in the balance between import and export signals.
The JNK pathway induces nuclear exclusion of Net, thereby removing a strong transcriptional repressor. Nuclear exclusion is the target for regulation of other transcription factors. Yan, another repressor belonging to the Ets family, prevents inappropriate neural differentiation during Drosophila eye development (36). Phosphorylation of Yan through the Ras1/MAPK pathway leads to rapid inactivation of the factor by nuclear export and proteolytic degradation (64). Neither Yan nor Erf, another negative Ets factor regulated by MAPK phosphorylation (66), has a functional NES equivalent to Net, indicating that the two are regulated by distinct mechanisms. Pap1, an AP1 homologue in fission yeast, is cytoplasmic in the absence of stress due to active export through a NES and Crm1-dependent mechanism. Oxidative stress apparently induces nuclear localization by inhibition of nuclear export (71, 83).
A model for the regulation of Net.
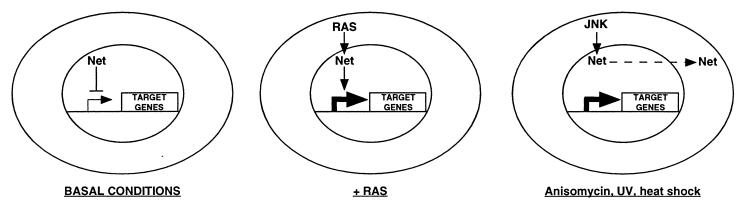
Our results lead to a working model for the regulation of Net by different signalling pathways (Fig. 8). Under basal conditions, Net is located in the nucleus and represses transcription of target genes. Ras-mediated signals result in phosphorylation of the C terminus of Net without affecting cellular localization, leading to an overall switch in the activity of Net from negative to positive. Stress signals such as anisomycin, UV, and heat shock induce nuclear exclusion of Net, thereby freeing genes from its negative influence and allowing activation by positive factors, such as Elk1 or Sap1a. Ras signalling pathways are associated with growth control, predicting that Net has positive as well as negative effects on growth control genes but only negative effects on stress-responsive genes. Identification of Net target genes will help to validate these predictions. The novel aspects of the regulation of Net presented here help shed light on the mechanisms of integration of Ras and other regulatory pathways at the level of transcription.
FIG. 8.
A model for two signalling pathways regulating Net. Under basal conditions, Net is nuclear and inhibits the transcription of target genes. Ras-mediated signals switch Net from a negative to a positive factor in the nucleus, leading to activation of transcription by Net. Stress signal activation of the JNK signalling cascade induces nuclear export of Net, releasing genes from Net repression.
ACKNOWLEDGMENTS
We thank the following: for generous gifts of reagents and cells, B. Wolff and Novartis (leptomycin B), A. Bradford and A. Gutierrez-Hartmann (UAS-TK-Luc), R. Davis [expression vectors for MKK3(Glu), MKK6(Glu), MKK3 (Ala), and MKK6(Ala)], M. Karin [expression vectors for MEKK1, JNKK, MEKK1(KM), and JNKK (KR)]; for confocal microscopy, Jean-Luc Vonesch; and for invaluable help, the IGBMC facilities staff.
For fellowships, we thank the MRT (C.D.) and the MRT, the Ligue, and BioAvenir (S.-M.M.). For financial assistance, we thank BioAvenir, the Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Hôpital Universitaire de Strasbourg, the Association pour la Recherche sur le Cancer, Fondation pour la Recherche Médicale, Ligue Nationale Française contre le Cancer, Ligue Régionale (Haut-Rhin) contre le Cancer, and Ligue Régionale (Bas-Rhin) contre le Cancer (the Legs Meyer).
REFERENCES
- 1.Batchelor A H, Piper D E, de la Brousse F C, McKnight S L, Wolberger C. The structure of GABPα/β: an ETS domain-ankyrin repeat heterodimer bound to DNA. Science. 1998;279:1037–1041. doi: 10.1126/science.279.5353.1037. [DOI] [PubMed] [Google Scholar]
- 2.Cavigelli M, Dolfi F, Claret F X, Karin M. Induction of c-fos expression through JNK-mediated TCF/Elk-1 phosphorylation. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Derijard B, Raingeaud J, Barrett T, Wu I H, Han J, Ulevitch R J, Davis R J. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267:682–685. doi: 10.1126/science.7839144. . (Erratum, 269:17.) [DOI] [PubMed] [Google Scholar]
- 5.Dittmer J, Nordheim A. Ets transcription factors and human disease. Biochim Biophys Acta. 1998;1377:F1–F11. doi: 10.1016/s0304-419x(97)00039-5. [DOI] [PubMed] [Google Scholar]
- 6.Donaldson L W, Petersen J M, Graves B J, McIntosh L P. Solution structure of the ETS domain from murine Ets-1: a winged helix-turn-helix DNA binding motif. EMBO J. 1996;15:125–134. [PMC free article] [PubMed] [Google Scholar]
- 7.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 8.Fornerod M, Ohno M, Yoshida M, Mattaj I W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- 9.Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- 10.Fukuda M, Gotoh I, Gotoh Y, Nishida E. Cytoplasmic localization of mitogen-activated protein kinase kinase directed by its NH2-terminal, leucine-rich short amino acid sequence, which acts as a nuclear export signal. J Biol Chem. 1996;271:20024–20028. doi: 10.1074/jbc.271.33.20024. [DOI] [PubMed] [Google Scholar]
- 11.Gaits F, Degols G, Shiozaki K, Russell P. Phosphorylation and association with the transcription factor Atf1 regulate localization of Spc1/Sty1 stress-activated kinase in fission yeast. Genes Dev. 1998;12:1464–1473. doi: 10.1101/gad.12.10.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghosh S, May M J, Kopp E B. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 13.Gille H, Kortenjann M, Thomae O, Moomaw C, Slaughter C, Cobb M H, Shaw P E. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 1995;14:951–962. doi: 10.1002/j.1460-2075.1995.tb07076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gille H, Strahl T, Shaw P E. Activation of ternary complex factor Elk-1 by stress-activated protein kinases. Curr Biol. 1995;5:1191–1200. doi: 10.1016/s0960-9822(95)00235-1. [DOI] [PubMed] [Google Scholar]
- 15.Gille H G, Sharrocks A D, Shaw P E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at the c-fos promotor. Nature. 1992;358:414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- 16.Giovane A, Pintzas A, Maira S M, Sobieszczuk P, Wasylyk B. Net, a new ets transcription factor that is activated by Ras. Genes Dev. 1994;8:1502–1513. doi: 10.1101/gad.8.13.1502. [DOI] [PubMed] [Google Scholar]
- 17.Gorlich D. Transport into and out of the cell nucleus. EMBO J. 1998;17:2721–2727. doi: 10.1093/emboj/17.10.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorlich D, Mattaj I W. Nucleocytoplasmic transport. Science. 1996;271:1513–1518. doi: 10.1126/science.271.5255.1513. [DOI] [PubMed] [Google Scholar]
- 19.Graves B J, Petersen J M. Specificity within the ets family of transcription factors. Adv Cancer Res. 1998;75:1–55. doi: 10.1016/s0065-230x(08)60738-1. [DOI] [PubMed] [Google Scholar]
- 20.Han J, Lee J D, Jiang Y, Li Z, Feng L, Ulevitch R J. Characterization of the structure and function of a novel MAP kinase kinase (MKK6) J Biol Chem. 1996;271:2886–2891. doi: 10.1074/jbc.271.6.2886. [DOI] [PubMed] [Google Scholar]
- 21.Helin K. Regulation of cell proliferation by the E2F transcription factors. Curr Opin Genet Dev. 1998;8:28–35. doi: 10.1016/s0959-437x(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 22.Henkel T, Machleidt T, Alkalay I, Kronke M, Ben-Neriah Y, Baeuerle P A. Rapid proteolysis of I kappa B-alpha is necessary for activation of transcription factor NF-kappa B. Nature. 1993;365:182–185. doi: 10.1038/365182a0. [DOI] [PubMed] [Google Scholar]
- 23.Hill C S, Marais R, John S, Wynne J, Dalton S, Treisman R. Functional analysis of a growth factor-responsive transcription factor complex. Cell. 1993;73:395–406. doi: 10.1016/0092-8674(93)90238-l. [DOI] [PubMed] [Google Scholar]
- 24.Hill C S, Wynne J, Treisman R. The Rho family GTPases RhoA, Rac1, and CDC42Hs regulate transcriptional activation by SRF. Cell. 1995;81:1159–1170. doi: 10.1016/s0092-8674(05)80020-0. [DOI] [PubMed] [Google Scholar]
- 25.Holland P M, Suzanne M, Campbell J S, Noselli S, Cooper J A. MKK7 is A stress-activated mitogen-activated protein kinase kinase functionally related to hemipterous. J Biol Chem. 1997;272:24994–24998. doi: 10.1074/jbc.272.40.24994. [DOI] [PubMed] [Google Scholar]
- 26.Ip Y T, Davis R J. Signal transduction by the c-Jun N-terminal kinase (JNK)—from inflammation to development. Curr Opin Cell Biol. 1998;10:205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- 27.Janknecht R, Ernst W H, Nordheim A. SAP1a is a nuclear target of signaling cascades involving ERKs. Oncogene. 1995;10:1209–1216. [PubMed] [Google Scholar]
- 28.Janknecht R, Ernst W H, Pingoud V, Nordheim A. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 1993;12:5097–5104. doi: 10.1002/j.1460-2075.1993.tb06204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janknecht R, Hunter T. Activation of the Sap-1a transcription factor by the c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase. J Biol Chem. 1997;272:4219–4224. doi: 10.1074/jbc.272.7.4219. [DOI] [PubMed] [Google Scholar]
- 30.Janknecht R, Hunter T. Convergence of MAP kinase pathways on the ternary complex factor Sap-1a. EMBO J. 1997;16:1620–1627. doi: 10.1093/emboj/16.7.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janknecht R, Zinck R, Ernst W H, Nordheim A. Functional dissection of the transcription factor Elk-1. Oncogene. 1994;9:1273–1278. [PubMed] [Google Scholar]
- 32.Johnson, D. G., and R. Schneider-Broussard. 1998. Role of E2F in cell cycle control and cancer. Front. Biosci. 3:d447–d448. [DOI] [PubMed]
- 33.Kudo N, Wolff B, Sekimoto T, Schreiner E P, Yoneda Y, Yanagida M, Horinouchi S, Yoshida M. Leptomycin B inhibition of signal-mediated nuclear export by direct binding to CRM1. Exp Cell Res. 1998;242:540–547. doi: 10.1006/excr.1998.4136. [DOI] [PubMed] [Google Scholar]
- 34.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 35.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodgett J R. The stress-activated protein kinase subfamily of c-Jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 36.Lai Z C, Rubin G M. Negative control of photoreceptor development in Drosophila by the product of the yan gene, an ETS domain protein. Cell. 1992;70:609–620. doi: 10.1016/0092-8674(92)90430-k. [DOI] [PubMed] [Google Scholar]
- 37.Lee F S, Hagler J, Chen Z J, Maniatis T. Activation of the IkappaB alpha kinase complex by MEKK1, a kinase of the JNK pathway. Cell. 1997;88:213–222. doi: 10.1016/s0092-8674(00)81842-5. [DOI] [PubMed] [Google Scholar]
- 38.Lee J C, Laydon J T, McDonnell P C, Gallagher T F, Kumar S, Green D, McNulty D, Blumenthal M J, Heys J R, Landvatter S W. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- 39.Lin A, Minden A, Martinetto H, Claret F X, Lange-Carter C, Mercurio F, Johnson G L, Karin M. Identification of a dual specificity kinase that activates the Jun kinases and p38-Mpk2. Science. 1995;268:286–290. doi: 10.1126/science.7716521. [DOI] [PubMed] [Google Scholar]
- 40.Lindeman G J, Gaubatz S, Livingston D M, Ginsberg D. The subcellular localization of E2F-4 is cell-cycle dependent. Proc Natl Acad Sci USA. 1997;94:5095–5100. doi: 10.1073/pnas.94.10.5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lopez M, Oettgen P, Akbarali Y, Dendorfer U, Libermann T A. ERP, a new member of the ets transcription factor/oncoprotein family: cloning, characterization, and differential expression during B-lymphocyte development. Mol Cell Biol. 1994;14:3292–3309. doi: 10.1128/mcb.14.5.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X, Nemoto S, Lin A. Identification of c-Jun NH2-terminal protein kinase (JNK)-activating kinase 2 as an activator of JNK but not p38. J Biol Chem. 1997;272:24751–24754. doi: 10.1074/jbc.272.40.24751. [DOI] [PubMed] [Google Scholar]
- 42a.Lutz, Y. Unpublished data.
- 43.Magae J, Wu C L, Illenye S, Harlow E, Heintz N H. Nuclear localization of DP and E2F transcription factors by heterodimeric partners and retinoblastoma protein family members. J Cell Sci. 1996;109:1717–1726. doi: 10.1242/jcs.109.7.1717. [DOI] [PubMed] [Google Scholar]
- 44.Maira S M, Wurtz J M, Wasylyk B. Net (ERP/SAP2) one of the Ras-inducible TCFs, has a novel inhibitory domain with resemblance to the helix-loop-helix motif. EMBO J. 1996;15:5849–5865. [PMC free article] [PubMed] [Google Scholar]
- 45.May M J, Ghosh S. Rel/NF-kappa B and I kappa B proteins: an overview. Semin Cancer Biol. 1997;8:63–73. doi: 10.1006/scbi.1997.0057. [DOI] [PubMed] [Google Scholar]
- 46.May M J, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- 47.Meier R, Rouse J, Cuenda A, Nebreda A R, Cohen P. Cellular stresses and cytokines activate multiple mitogen-activated-protein kinase kinase homologues in PC12 and KB cells. Eur J Biochem. 1996;236:796–805. doi: 10.1111/j.1432-1033.1996.00796.x. [DOI] [PubMed] [Google Scholar]
- 48.Meyer B E, Meinkoth J L, Malim M H. Nuclear transport of human immunodeficiency virus type 1, visna virus, and equine infectious anemia virus Rev proteins: identification of a family of transferable nuclear export signals. J Virol. 1996;70:2350–2359. doi: 10.1128/jvi.70.4.2350-2359.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Minden A, Lin A, Claret F X, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 50.Minden A, Lin A, McMahon M, Lange-Carter C, Derijard B, Davis R J, Johnson G L, Karin M. Differential activation of ERK and JNK mitogen-activated protein kinases by Raf-1 and MEKK. Science. 1994;266:1719–1723. doi: 10.1126/science.7992057. [DOI] [PubMed] [Google Scholar]
- 51.Mo Y, Vaessen B, Johnston K, Marmorstein R. Structures of SAP-1 bound to DNA targets from the E74 and c-fos promoters: insights into DNA sequence discrimination by Ets proteins. Mol Cell. 1998;2:201–212. doi: 10.1016/s1097-2765(00)80130-6. [DOI] [PubMed] [Google Scholar]
- 52.Moriguchi T, Kawasaki H, Matsuda S, Gotoh Y, Nishida E. Evidence for multiple activators for stress-activated protein kinase/c-Jun amino-terminal kinases. Existence of novel activators. J Biol Chem. 1995;270:12969–12972. doi: 10.1074/jbc.270.22.12969. [DOI] [PubMed] [Google Scholar]
- 53.Moriguchi T, Kuroyanagi N, Yamaguchi K, Gotoh Y, Irie K, Kano T, Shirakabe K, Muro Y, Shibuya H, Matsumoto K, Nishida E, Hagiwara M. A novel kinase cascade mediated by mitogen-activated protein kinase kinase 6 and MKK3. J Biol Chem. 1996;271:13675–13679. doi: 10.1074/jbc.271.23.13675. [DOI] [PubMed] [Google Scholar]
- 54.Moriguchi T, Toyoshima F, Masuyama N, Hanafusa H, Gotoh Y, Nishida E. A novel SAPK/JNK kinase, MKK7, stimulated by TNFalpha and cellular stresses. EMBO J. 1997;16:7045–7053. doi: 10.1093/emboj/16.23.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nigg E A. Nucleocytoplasmic transport: signals, mechanisms and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- 56.Ossareh-Nazari B, Bachelerie F, Dargemont C. Evidence for a role of CRM1 in signal-mediated nuclear protein export. Science. 1997;278:141–144. doi: 10.1126/science.278.5335.141. [DOI] [PubMed] [Google Scholar]
- 57.Pemberton L F, Blobel G, Rosenblum J S. Transport routes through the nuclear pore complex. Curr Opin Cell Biol. 1998;10:392–399. doi: 10.1016/s0955-0674(98)80016-1. [DOI] [PubMed] [Google Scholar]
- 58.Petersen J M, Skalicky J J, Donaldson L W, McIntosh L P, Alber T, Graves B J. Modulation of transcription factor Ets-1 DNA binding: DNA- induced unfolding of an alpha helix. Science. 1995;269:1866–1869. doi: 10.1126/science.7569926. [DOI] [PubMed] [Google Scholar]
- 59.Pribyl L J, Watson D K, McWilliams M J, Ascione R, Papas T S. The Drosophila ets-2 gene: molecular structure, chromosomal localization, and developmental expression. Dev Biol. 1988;127:45–53. doi: 10.1016/0012-1606(88)90187-x. [DOI] [PubMed] [Google Scholar]
- 60.Price M A, Cruzalegui F H, Treisman R. The p38 and ERK MAP kinase pathways cooperate to activate ternary complex factors and c-fos transcription in response to UV light. EMBO J. 1996;15:6552–6563. [PMC free article] [PubMed] [Google Scholar]
- 61.Price M A, Rogers A E, Treisman R. Comparative analysis of the ternary complex factors Elk-1, SAP-1a and SAP-2 (ERP/NET) EMBO J. 1995;14:2589–2601. doi: 10.1002/j.1460-2075.1995.tb07257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raingeaud J, Whitmarsh A J, Barrett T, Derijard B, Davis R J. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16:1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rao A, Luo C, Hogan P G. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 64.Rebay I, Rubin G M. Yan functions as a general inhibitor of differentiation and is negatively regulated by activation of the Ras1/MAPK pathway. Cell. 1995;81:857–866. doi: 10.1016/0092-8674(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 65.Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares A, Zamanillo D, Hunt T, Nebreda A R. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 66.Sgouras D N, Athanasiou M A, Beal G J, Fisher R J, Blair D G, Mavrothalassitis G J. ERF: an ETS domain protein with strong transcriptional repressor activity, can suppress ets-associated tumorigenesis and is regulated by phosphorylation during cell cycle and mitogenic stimulation. EMBO J. 1995;14:4781–4793. doi: 10.1002/j.1460-2075.1995.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharrocks A D, Brown A L, Ling Y, Yates P R. The ETS-domain transcription factor family. Int J Biochem Cell Biol. 1997;29:1371–1387. doi: 10.1016/s1357-2725(97)00086-1. [DOI] [PubMed] [Google Scholar]
- 68.Stancovski I, Baltimore D. NF-kappaB activation: the I kappaB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 69.Stein B, Brady H, Yang M X, Young D B, Barbosa M S. Cloning and characterization of MEK6, a novel member of the mitogen-activated protein kinase kinase cascade. J Biol Chem. 1996;271:11427–11433. doi: 10.1074/jbc.271.19.11427. [DOI] [PubMed] [Google Scholar]
- 70.Strahl T, Gille H, Shaw P E. Selective response of ternary complex factor Sap1a to different mitogen-activated protein kinase subgroups. Proc Natl Acad Sci USA. 1996;93:11563–11568. doi: 10.1073/pnas.93.21.11563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Toone W M, Kuge S, Samuels M, Morgan B A, Toda T, Jones N. Regulation of the fission yeast transcription factor Pap1 by oxidative stress: requirement for the nuclear export factor Crm1 (exportin) and the stress-activated MAP kinase Sty1/Spc1. Genes Dev. 1998;12:1453–1463. doi: 10.1101/gad.12.10.1453. . (Erratum, 12:2650.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tournier C, Whitmarsh A J, Cavanagh J, Barrett T, Davis R J. Mitogen-activated protein kinase kinase 7 is an activator of the c-Jun NH2-terminal kinase. Proc Natl Acad Sci USA. 1997;94:7337–7342. doi: 10.1073/pnas.94.14.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Toyoshima F, Moriguchi T, Wada A, Fukuda M, Nishida E. Nuclear export of cyclin B1 and its possible role in the DNA damage-induced G2 checkpoint. EMBO J. 1998;17:2728–2735. doi: 10.1093/emboj/17.10.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Treisman R. Regulation of transcription by MAP kinase cascades. Curr Opin Cell Biol. 1996;8:205–215. doi: 10.1016/s0955-0674(96)80067-6. [DOI] [PubMed] [Google Scholar]
- 75.Verma I M, Stevenson J. IkappaB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wasylyk B, Hagman J, Gutierrez-Hartmann A. Ets transcription factors: nuclear effectors of the Ras-MAP-kinase signaling pathway. Trends Biochem Sci. 1998;23:213–216. doi: 10.1016/s0968-0004(98)01211-0. [DOI] [PubMed] [Google Scholar]
- 77.Wasylyk B, Nordheim A. Ets transcription factors: partners in the integration of signal responses. In: Papavassiliou A G, editor. Transcription factors in eucaryotes. Austin, Tex: Landes Bioscience; 1997. pp. 253–286. [Google Scholar]
- 78.Wasylyk C, Imler J L, Perez-Mutul J, Wasylyk B. The c-Ha-ras oncogene and a tumor promoter activate the polyoma virus enhancer. Cell. 1987;48:525–534. doi: 10.1016/0092-8674(87)90203-0. [DOI] [PubMed] [Google Scholar]
- 79.Wen W, Meinkoth J L, Tsien R Y, Taylor S S. Identification of a signal for rapid export of proteins from the nucleus. Cell. 1995;82:463–473. doi: 10.1016/0092-8674(95)90435-2. [DOI] [PubMed] [Google Scholar]
- 80.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 81.Whitmarsh A J, Shore P, Sharrocks A D, Davis R J. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269:403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 82.Whitmarsh A J, Yang S H, Su M S, Sharrocks A D, Davis R J. Role of p38 and JNK mitogen-activated protein kinases in the activation of ternary complex factors. Mol Cell Biol. 1997;17:2360–2371. doi: 10.1128/mcb.17.5.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wilkinson M G, Millar J B. SAPKs and transcription factors do the nucleocytoplasmic tango. Genes Dev. 1998;12:1391–1397. doi: 10.1101/gad.12.10.1391. . (Comment.) [DOI] [PubMed] [Google Scholar]
- 84.Wolff B, Sanglier J J, Wang Y. Leptomycin B is an inhibitor of nuclear export: inhibition of nucleo- cytoplasmic translocation of the human immunodeficiency virus type 1 (HIV-1) Rev protein and Rev-dependent mRNA. Chem Biol. 1997;4:139–147. doi: 10.1016/s1074-5521(97)90257-x. [DOI] [PubMed] [Google Scholar]
- 85.Wozniak R W, Rout M P, Aitchison J D. Karyopherins and kissing cousins. Trends Cell Biol. 1998;8:184–188. doi: 10.1016/s0962-8924(98)01248-3. [DOI] [PubMed] [Google Scholar]
- 86.Yan M, Dai T, Deak J C, Kyriakis J M, Zon L I, Woodgett J R, Templeton D J. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 87.Yang S H, Whitmarsh A J, Davis R J, Sharrocks A D. Differential targeting of MAP kinases to the ETS-domain transcription factor Elk-1. EMBO J. 1998;17:1740–1749. doi: 10.1093/emboj/17.6.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yang S H, Yates P R, Whitmarsh A J, Davis R J, Sharrocks A D. The Elk-1 ETS-domain transcription factor contains a mitogen-activated protein kinase targeting motif. Mol Cell Biol. 1998;18:710–720. doi: 10.1128/mcb.18.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yujiri T, Sather S, Fanger G R, Johnson G L. Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science. 1998;282:1911–1914. doi: 10.1126/science.282.5395.1911. [DOI] [PubMed] [Google Scholar]
- 90.Zhu J, Shibasaki F, Price R, Guillemot J C, Yano T, Dotsch V, Wagner G, Ferrara P, McKeon F. Intramolecular masking of nuclear import signal on NF-AT4 by casein kinase I and MEKK1. Cell. 1998;93:851–861. doi: 10.1016/s0092-8674(00)81445-2. [DOI] [PubMed] [Google Scholar]
- 91.Zinck R, Cahill M A, Kracht M, Sachsenmaier C, Hipskind R A, Nordheim A. Protein synthesis inhibitors reveal differential regulation of mitogen-activated protein kinase and stress-activated protein kinase pathways that converge on Elk-1. Mol Cell Biol. 1995;15:4930–4938. doi: 10.1128/mcb.15.9.4930. [DOI] [PMC free article] [PubMed] [Google Scholar]