Abstract
Steroid hormone receptors are distinguished from other members of the nuclear hormone receptor family through their association with heat shock proteins and immunophilins in the absence of ligands. Heat shock protein association represses steroid receptor DNA binding and protein-protein interactions with other transcription factors and facilitates hormone binding. In this study, we investigated the hormone-dependent interaction between the DNA binding domain (DBD) of the glucocorticoid receptor (GR) and the POU domains of octamer transcription factors 1 and 2 (Oct-1 and Oct-2, respectively). Our results indicate that the GR DBD binds directly, not only to the homeodomains of Oct-1 and Oct-2 but also to the homeodomains of several other homeodomain proteins. As these results suggest that the determinants for binding to the GR DBD are conserved within the homeodomain, we examined whether the ectopic expression of GR DBD peptides affected early embryonic development. The expression of GR DBD peptides in one-cell-stage zebra fish embryos severely affected their development, beginning with a delay in the epibolic movement during the blastula stage and followed by defects in convergence-extension movements during gastrulation, as revealed by the abnormal patterns of expression of several dorsal gene markers. In contrast, embryos injected with mRNA encoding a GR peptide with a point mutation that disrupted homeodomain binding or with mRNA encoding the DBD of the closely related mineralocorticoid receptor, which does not bind octamer factors, developed normally. Moreover, coinjection of mRNA encoding the homeodomain of Oct-2 completely rescued embryos from the effects of the GR DBD. These results highlight the potential of DNA-independent effects of GR in a whole-animal model and suggest that at least some of these effects may result from direct interactions with homeodomain proteins.
Nuclear hormone receptors comprise a broadly distributed class of transcriptional regulators implicated in diverse physiological processes (4, 54). Many nuclear receptors play major roles in development; these include the control of patterning and tissue differentiation. Nuclear receptors are marked by a highly conserved DNA binding domain (DBD) comprised of two Cys4 zinc fingers (48, 54). They also contain a less well conserved C-terminal region that confers ligand responsiveness to the transcriptional regulatory functions of many receptors.
Most nuclear receptors are constitutive transcriptional regulators whose transcriptional regulatory potential is altered by association with ligands. Steroid hormone receptors, however, are distinguished by their association into transcriptionally inactive heat shock protein (hsp)- and immunophilin-containing complexes in the absence of steroidal ligands (4, 63). Association into hsp complexes prevents steroid receptor DNA binding by physically masking the DBD. hsp association also alters the conformation of the steroid receptor ligand binding domain in a manner that facilitates ligand binding (62, 63). Indeed, hsp association appears to be required for ligand binding by the glucocorticoid receptor (GR).
In addition to direct regulation of transcription through DNA response elements, it is becoming increasingly apparent that many important functions of nuclear receptors are mediated through protein-protein interactions with other transcription factors in the absence of DNA binding (30, 37). The physiological importance of these activities has recently been highlighted by a report that mice with a mutation in the GR that compromised DNA binding are viable (69).
Interestingly, several protein-protein interactions between nuclear receptors and other sequence-specific transcription factors are mediated through receptor DBDs (13, 20, 55, 76). The best characterized of these interactions occurs with AP-1 (29, 38, 53). Several nuclear hormone receptors, including GR (29), androgen receptor (AR) (75), estrogen receptor α (ERα) (100), thyroid hormone receptor (108), and retinoic acid receptors and retinoid X receptors (81), form complexes with AP-1 that block the interaction of AP-1 with its normal response elements. At least for GR, the receptor–AP-1 complex is redirected to composite response elements specific for the GR–AP-1 complex. In addition, GR can bind to and repress the DNA binding of NF-κB (76). For steroid hormone receptors, these interactions are dependent upon the dissociation of hsp complexes and thus are sensitive to both steroids and steroid antagonists (29).
Recently, we determined that three steroid hormone receptors, GR, AR, and progesterone receptor (PR), associated through their DBDs with the POU DBDs of octamer transcription factors 1 and 2 (Oct-1 and Oct-2, respectively) (64, 65). In contrast, ERα, mineralocorticoid receptor (MR), and several other nuclear receptors failed to interact with Oct-1 and Oct-2 in transfected cells. These results indicated that Oct-1 and Oct-2 binding was restricted within the nuclear hormone receptor superfamily. For GR, AR, and PR, octamer factor binding was strictly dependent upon the dissociation of free receptors from hsp-immunophilin complexes that followed ligand binding. Functionally, binding to GR, AR, and PR affected the DNA targeting of the octamer factors in the cell by preferentially increasing their binding to octamer motifs in the transcriptional regulatory regions of steroid hormone-responsive reporter genes (11, 64, 65, 97). There also has been a report for GR that this interaction may simultaneously decrease the occupancy of octamer motifs in transcriptional regulatory regions lacking steroid hormone response elements (46). For rat GR, C500Y and L501P substitutions in the second zinc finger of the DBD abrogated octamer factor binding and their recruitment to DNA (65).
Like GR, POU transcription factors comprise a large family of transcriptional regulators with a highly conserved DBD (73). The POU domain is bipartite, consisting of POU-specific and POU homeodomain motifs that bind cooperatively to the 5′ and 3′ halves of DNA response elements (43). Homeobox genes encode master developmental control proteins involved in virtually all aspects of pattern formation and tissue differentiation in the developing embryo (70, 72, 73, 101).
Several homeobox genes involved in the first steps of the establishment of the vertebrate body axis during gastrulation have been characterized. Homeobox genes such as goosecoid (8, 9, 32, 92), floating head or Xnot (95, 98), GSX (51), Xlim-1 (94), Otx2 (61, 89), Xtwin (49), and Siamois (22, 39, 49, 52) are expressed in the organizer and show dorsalizing properties when ectopically expressed in the embryo. The homeobox genes Xvent-1 and Vox (also named Xvent-2) have been shown to be involved in ventral fate specification (27, 59, 78). Most of these genes are activated between the midblastula transition and the onset of gastrulation. However, some of them, such as goosecoid (92), Otx2 (61, 89), and Xtwin (49), as well as a number of POU factors (58, 60, 79, 80) are expressed as maternal factors prior to being zygotically expressed. Despite the important functions of these genes at the onset of gastrulation, little is known about the role of maternal homeodomain proteins during cleavage of the vertebrate embryo and the role of the maternal and zygotic homeodomain proteins prior to gastrulation.
Glucocorticoids are teratogenic (1). The most frequent correlate of embryonic exposure is cleft palate (26). In mammals, the expression of GR has been detected beginning at a stage equivalent to day 9.5 of mouse embryogenesis (18, 42). Further, the down regulation of GR mRNA at the final differentiation stages of tissues in which it is expressed is suggestive of a morphogenetic role (42). Indeed, insertional inactivation of the GR gene results in mice that die soon after birth due to a defect in the final stages of lung development (17). Studies with Xenopus also indicate a role for GR at the earliest stages of embryogenesis. GR mRNA is abundant in Xenopus oocytes but is rapidly degraded during the early cleavage stages of embryogenesis. GR transcripts are reexpressed prior to the completion of gastrulation and become localized to the dorsal ectoderm (25).
In this study, we report the Oct-1 homeodomain and the Oct-2 homeodomain (Oct-2HD) to be necessary and sufficient for the direct binding of Oct-1 and Oct-2 to the GR DBD. L501P-sensitive GR binding to the homeodomain was not limited to Oct-1 and Oct-2 but also was observed for several other homeodomain proteins, including zebra fish (Danio rerio) dlx2 and hoxd4. Intriguingly, the expression of GR DBD but not MR DBD peptides in one- or two-cell-stage zebra fish embryos specifically affected embryonic development at or before the time of blastoderm migration in a manner that correlated with the L501P-sensitive binding of the GR DBD to maternal and embryonic factors. Moreover, these developmental defects were rescued by the coexpression of a peptide containing Oct-2HD. These results establish the GR DBD as a molecular probe for important developmental events occurring near the time when embryonic transcription is initiated and predict an important role for homeodomain proteins that bind to the GR DBD during these events.
MATERIALS AND METHODS
Plasmids.
pGEM7Z-Oct-1 was produced by insertion of the HindIII/BamHI Oct fragment from pCGN-Oct-1 (96) into the corresponding sites of pGEM7Z (Promega). pGEX2T-GR (amino acids [aa] 407 to 568) and pGEX2TGRC500Y (aa 407 to 556) were constructed by insertion of the BamHI/EcoRI fragment from pSP64X568 (71) and the BamHI/SmaI fragment from pT7C500Y (77), respectively, in frame into the appropriate restriction sites of pGEX2T (Pharmacia). pTL2HAOct-2 was produced by first inserting the Oct-2 XbaI (blunted)/BamHI fragment from pCGN-Oct-2 (96) into pACT-2 (Clontech) to acquire a hemagglutinin epitope and then recloning with BglII/XhoI into the corresponding sites of pTL2. pTL2OCT2ΔHD was created by removing the EagI/PstI DNA fragment corresponding to the homeodomain (aa 296 to 359) and religating it in frame. pTL2-OCT2ΔPOU and pTL2-OCT2ΔSP were created through a similar deletion and religation strategy to remove aa 152 to 349 and aa 152 to 286, respectively. pGEX2TGR-PKA vectors (wild type [WT], C500Y, L501P, C460Y, and C460Y-L501P; aa 407 to 550) were constructed by insertion of an oligonucleotide encoding a protein kinase A (PKA) recognition motif, LARRASYP, into the StyI/EcoRI sites of plasmid pGEX2T-GR. pGEX2T-OCT2HD (aa 294 to 377) was constructed by insertion of a 14-bp linker into the BamHI/EagI sites of pGEX2T-OCT2POU (aa 195 to 377) (65). Expression plasmids for dlx2 (pGEX3X-dlx2 and pTL2-dlx2), hoxd4 (pTL2-hoxd4), and msxB (pGEX3X-msxB; aa 135 to 196) were as described previously (107), as were the pGEX2T-PrdHD, pGEX2T-FtzHD, and pGEX2T-OtdHD constructs (104). pGEX2T-HoxC4 (aa 148 to 227) was subcloned from a human Jurkat T-cell cDNA library. pCGNOCT-1, pCGNOCT-2, and GR plasmids pT7X556 (aa 407 to 556), pT7C460Y, pT7R489R, pT7L501P, and pT7C500Y have been described previously (71, 96). The GR double-mutant plasmid pT7C460Y/L501P was constructed by replacing the XhoI/SphI DNA fragment from pT7L501P with the corresponding fragment from pT7C460Y. pET-11a-MR-DBD and pET-11a-OCT2HD were constructed by insertion of the fragments containing the MR DBD (aa 567 to 700) and Oct-2HD (aa 294 to 377) coding sequences created by PCR from pGALO-MR-DBD (64) and pGEX2T-OCT2HD, respectively, in frame into pET-11a (Novagen; the NheI/BclI and NheI/BamHI sites, respectively). The plasmids used for making the in situ probes have been described previously as pBluescript SK for ntl (82), shh (44), flh (95), MyoD (102), gsc (92), and bmp4 (57) and pBluescript KS for axial (93).
GST pull-down and immunoprecipitation protein binding assays.
Glutathione S-transferase (GST) fusion proteins were expressed, purified, and tested in a GST pull-down assay as previously described (65). The 35S-Met-labeled proteins were produced by in vitro translation (Promega TNT coupled in vitro transcription-translation kit) with either T7 or SP6 RNA polymerase. To produce C-terminal Oct-1 protein truncations, plasmid DNAs were restricted prior to in vitro translation. To assay for binding, labeled proteins were incubated with 0.5 μg of GST fusion protein attached to glutathione-Sepharose in binding buffer (20 mM HEPES [pH 7.9], 60 mM KCl, 12% glycerol, 1.5 mM EDTA, 1 mM dithiothreitol, 0.15 mM phenylmethylsulfonyl fluoride [PMSF], 0.1% Nonidet P-40 [NP-40]) for 2 h at 4°C. Following extensive washing with binding buffer, the samples were eluted by boiling for 5 minutes in sodium dodecyl sulfate (SDS) sample buffer prior to SDS-polyacrylamide gel electrophoresis (PAGE) analysis. The gels were dried and visualized by fluorography.
For coimmunoprecipitation assays, in vitro-translated Oct-2FL, Oct-2ΔSP, Oct-2ΔHD, Oct-2ΔPOU, dlx2, and hoxd4 were incubated with c-myc-tagged GR immunoprecipitated with antibody 9E10 from nuclear extracts prepared from dexamethasone-treated, stably expressing SF-7 fibroblasts or the parental cell line as previously described (65). Following extensive washing with binding buffer, the bound proteins were resolved by SDS-PAGE and visualized by fluorography. Myc-GR loading in each experiment was confirmed by Western blot analysis with antibody 9E10 and visualized by enhanced chemiluminescence (Amersham). Phosphorimager quantification (Bio-Rad model GS-525) of the SDS-polyacrylamide gels and the immunoblots was done with background subtraction.
For the direct binding assay, GST-GR WT and mutants tagged with a PKA phosphorylation site were expressed in bacteria. The GST fusion proteins were first immobilized on glutathione-Sepharose in TEGz50 buffer (50 mM Tris [pH 7.5], 50 mM NaCl, 10% glycerol, 0.5 mM EDTA, 50 μM ZnCl2, 0.5 mM PMSF) plus 1% Triton X-100 and then labeled with [γ-32P]ATP by a kinase reaction with the catalytic subunit of PKA (Sigma) in HMK buffer (20 mM Tris [pH 7.5], 100 mM NaCl, 12 mM MgCl2, 1 mM dithiothreitol) for 30 min at 30°C. The reaction was terminated by the addition of 1 ml of stop buffer (10 mM NaPO4, 10 mM Na4O7P2, 10 mM EDTA, 2 mg of bovine serum albumin [BSA] per ml). The labeled proteins were eluted in TEGz50 buffer (23) plus 1% Triton X-100 by thrombin (Sigma) cleavage. Approximately 5 ng of the 32P-labeled peptides was incubated with immobilized GST fusion proteins in binding buffer in the presence of 2 mg of BSA per ml and 1 mM PMSF. Following extensive washing, the bound proteins were eluted in SDS sample buffer, resolved by SDS-PAGE, and visualized by autoradiography. The input GST-homeodomain proteins and BSA were resolved by SDS-PAGE and visualized by Coomassie blue staining.
Tissue cultures and transient transfections.
CHO-K1 cells (American Type Culture Collection [ATCC]) were maintained in α-minimal essential medium supplemented with 10% fetal bovine serum (Life Technologies). SF-7 and clonal cell lines (65) were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum.
For the mammalian two-hybrid assay, bait plasmids pCGN-Oct-2, pTL2-hoxd4, pTL2-dlx2, and pSG5CREB were cotransfected with pGAL4-GR-DBD, pGAL4-GRL501P, or pGALO and pG5E1BCAT into CHO cells (ATCC) with Lipofectamine (10 μl per 60-mm dish; 50 ng of the chloramphenicol acetyltransferase [CAT] reporter, 50 ng of the GAL4 expression plasmid, and 25 ng of the bait plasmid). Cells were harvested 48 h posttransfection. The CAT activities of cytoplasmic extracts were determined by standard protocols and are expressed as fold induction of GAL4-GR DBD or GAL4-GRL501P over GALO. pRSV-βGAL (50 ng) was cotransfected to provide an internal control for transfection efficiency. All transfections and CAT assays were performed in duplicate in at least three independent assays. Error bars represent the standard errors of the mean.
Fish and embryos.
Zebra fish eggs were obtained by natural spawning from a colony of fish derived from stock from a local fish supplier. Embryos were raised at a constant 28°C temperature in system water or in embryo medium by standard methods (103). Developmental stages are given in hours postfertilization (p.f.).
In vitro transcription and microinjection.
mRNAs of the GR DBD were produced in vitro by linearizing the pT7 plasmids with EcoRV, pET-11a-OCT2HD with BamHI, and pET-11a-MR-DBD with HindIII and transcribing with T7 polymerase by use of an mRNA capping kit (Stratagene, La Jolla, Calif.). Following ethanol precipitation, the RNAs were resuspended in filtered distilled water. The purified mRNAs were quantified on a Bio-Rad Gel Doc system following agarose gel electrophoresis. Injections of 0.2 to 0.4 nl of synthetic, capped RNA at concentrations of 100 to 600 μg/ml were given to one- or two-cell-stage zebra fish embryos by use of backfilled capillaries (Flaming/Brown micropipette puller; Sutter Instrument Co., Novato, Calif.) and a pressure-pulsed microinjector (PV830 Pneumatic Picopump; WPI, Sarasota, Fla.). Injected embryos were raised in embryo medium and monitored at 2-h intervals and at other times as specificallly indicated. Death and developmental abnormalities were recorded to 24 h p.f. Embryos were photographed with a Leica stereomicroscope. Embryos stored for in situ analysis were fixed in phosphate-buffered saline (pH 7.4) containing 4% paraformaldehyde overnight at 4°C, the chorion was manually removed, and the samples were stored in methanol at −20°C.
In situ hybridizations.
In situ hybridization with whole-mount zebra fish embryos was performed as described previously (2, 3) by use of nonhydrolyzed antisense RNA probes. Enzymes used for linearization and for transcription for probe synthesis were as follows: ntl (82), XhoI and T7 RNA polymerase (T7); shh (44), BglII and T7; flh (95), EcoRI and T7; axial (93), DraI and T3; MyoD (102), EcoRI and T7; gsc (92), BamHI and T7; and bmp4 (57), EcoRI and T7.
Far-Western analysis of proteins binding to the GR DBD in embryonic extracts.
Embryo lysates were prepared from zebra fish embryos collected between cell cycles 8 and 10 (early blastula stages) and from embryos at 50% epiboly (early gastrula stage). The dissected embryos were placed in cell lysis buffer (25 mM HEPES [pH 7.9], 100 mM KCl, 20% glycerol, 0.2 mM PMSF, 2 mM EDTA, 2 mM DTT, 0.01% NP-40) (1 μl per embryo) at 4°C, and the cells were dispersed by vortexing. An equal volume of 2× gel loading buffer (100 mM Tris-HCl, [pH 6.8], 200 mM DTT, 4% SDS, 0.2% bromophenol blue, 20% glycerol) was added. Lysate (25 μg) from the early blastula or the early gastrula stage was loaded and resolved by SDS–12% PAGE. Following electrophoresis, the proteins were transferred to polyvinylidene difluoride membranes (Millipore). Denaturation and renaturation of filter-bound proteins were accomplished by immersing the membranes in 6 M guanidine HCl, gently agitating the mixture at room temperature for 10 min, and renaturing the samples by 1:1 serial dilutions with binding buffer (5, 7). The membrane was washed in binding buffer for 10 min, and the filter was blocked with 3% BSA (wt/vol) for a minimum 1 h at 20°C. The filter was then incubated with 32P-labeled GRC460Y (GR carrying a C460Y substitution) or GRC460Y/L501P (GR carrying C460Y and L501P substitutions) (in binding buffer containing 0.3% BSA; the final specific activity was about 106 cpm per ml) overnight at 4°C. After five 15-min washes with binding buffer, the filter was exposed to X-AR film (Kodak).
RESULTS
The octamer factor homeodomain is sufficient for C500Y- and L501P-sensitive GR DBD binding in vitro.
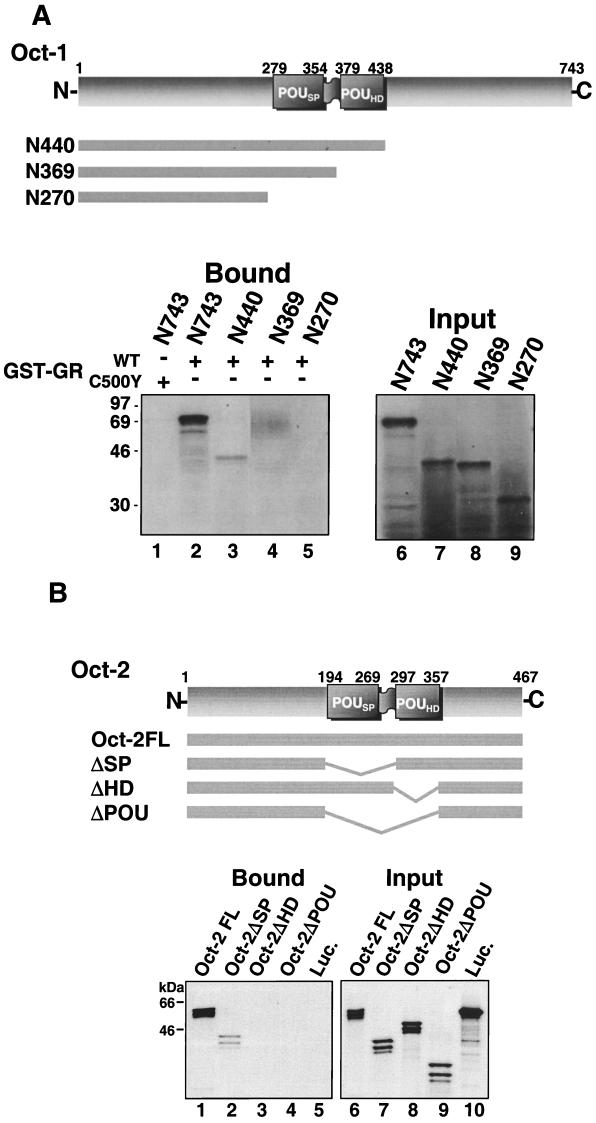
To begin to delimit the requirements within full-length Oct-1 and Oct-2 for binding to steroid receptors, we examined the binding of C-terminally truncated forms of Oct-1 to the GR DBD (aa 407 to 568) by using a GST pull-down assay (Fig. 1A). In vitro-translated full-length Oct-1 bound specifically to the WT GR DBD fused to GST but did not interact with GST-GRC500Y (Fig. 1A, lanes 1 and 2). Deletion of the Oct-1 C terminus up to the homeodomain had only a slight effect on the interaction with GR (Fig. 1A, lane 3). Further truncation into the homeodomain, however, abrogated binding (Fig. 1A, lanes 4 and 5). In an additional experiment, it was confirmed that binding of the GR DBD to the Oct-1 homeodomain is distinct from the binding previously reported for herpes simplex virus protein 16 (VP16), as substitutions in the Oct-1 homeodomain that have been shown previously to disrupt VP16 binding (47) had no effect on GR binding in this assay (66).
FIG. 1.
The association of Oct-1 and Oct-2 with GR is lost upon deletion of the Oct homeodomains. (A) Binding of in vitro-translated, 35S-Met-labeled Oct-1 peptides to the WT GR DBD (aa 407 to 568; lanes 2 to 5) or the GR DBD with a C500Y substitution (lane 1), expressed as GST fusion proteins. Lanes 6 to 9 show the signal obtained with 10% of the labeled proteins added to the binding assay. (B) Binding of full-length, 35S-labeled, in vitro-translated Oct-2 (lane 1), Oct-2 deletion constructs lacking the POU-specific domain (ΔSP) (lane 2), the POU homeodomain (ΔHD) (lane 3), and the complete POU domain (ΔPOU) (lane 4), and firefly luciferase (Luc; lane 5) to full-length GR with a c-myc epitope tag. Samples were immunoprecipitated with c-myc antibody 9E10 from whole-cell extracts prepared from dexamethasone-treated, stably transfected murine SF-7 fibroblasts. Lanes 6 to 10 show the signal obtained with 10% of the labeled proteins added to the binding assay. In both panels A and B, bound peptides were resolved by SDS-PAGE (12% gels) and visualized by fluorography.
Similarly, immunoprecipitation assays performed with in vitro-translated Oct-2 constructs containing internal deletions and full-length GR with an N-terminal c-myc tag in crude nuclear extracts prepared from murine SF-7 cells indicated that deletion of Oct-2HD was sufficient to eliminate GR binding (Fig. 1B). While full-length Oct-2 bound strongly to immunoprecipitated GR (Fig. 1B, lane 1), deletion of the entire POU domain or the POU homeodomain alone from full-length Oct-2 eliminated binding (Fig. 1B, lanes 3 and 4). Interestingly, deletion of the POU-specific domain alone decreased binding to the GR DBD somewhat (Fig. 1B, lane 2). This result may suggest a role for the POU-specific domain in GR binding. However, as the result was not supported in subsequent experiments, it would seem more likely that it reflects a decrease in the accessibility of the homeodomain to GR when the adjacent POU-specific domain was deleted from Oct-2.
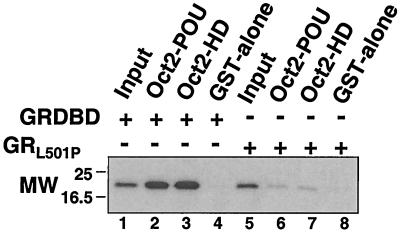
To resolve whether the octamer factor homeodomain was sufficient for GR binding and to determine whether binding was direct, we performed a binding assay with purified components that had been expressed in bacteria. WT and L501P-substituted GR DBDs (aa 407 to 550) containing PKA recognition sequences were expressed as GST fusion proteins, labeled with 32P by use of PKA, and then separated from the GST moiety by cleavage with thrombin. The purified GR DBD peptides were tested for binding to the complete Oct-2 POU domain (aa 194 to 377) or to Oct-2HD (aa 294 to 377) expressed as GST fusion proteins and bound to glutathione-Sepharose (Fig. 2). The WT GR DBD bound strongly to both the complete POU domain and the POU homeodomain alone (Fig. 2, lanes 2 and 3), but no binding was detected to GST alone (Fig. 2, lane 4). Further, the L501P substitution in the GR DBD prevented binding (Fig. 2, lanes 6 to 8). Thus, Oct-2HD was sufficient for direct binding to the GR DBD in vitro.
FIG. 2.
The GR DBD binds directly to Oct-2HD. GST-tagged WT GR DBD and GRL501P DBD (aa 407 to 550) containing PKA consensus phosphorylation sites were expressed in and purified to homogeneity from Escherichia coli and labeled with 32P by use of PKA. Signals obtained with 10% of the 32P-labeled GR and GRL501P DBDs added to the binding assay are shown in lanes 1 and 5. The 32P-labeled GR peptides were tested for binding to the POU domain and POU homeodomain of Oct-2 or to GST alone (lanes 2 to 4 and 6 to 8, respectively). MW, molecular weight (in thousands).
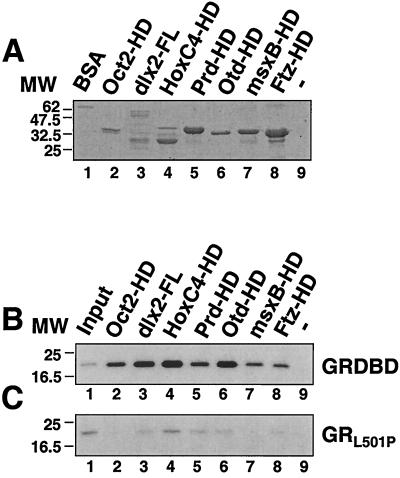
As the homeodomain is remarkably well conserved, we next became interested in determining whether GR might also bind directly to the homeodomains of other proteins. To first test this possibility in vitro, we expressed the homeodomains of several proteins as GST fusion proteins (Fig. 3A) and tested them for binding to 32P-labeled GR DBD peptides (Fig. 3B and C). Remarkably, all of the homeodomains tested bound to the WT GR DBD peptides (Fig. 3B, lanes 2 to 8), but none interacted significantly with the GR DBD peptide containing the L501P substitution (Fig. 3C, lanes 2 to 8). By comparison with the input GST-homeodomain proteins, binding was strongest to the HoxC4 homeodomain (Fig. 3B, lane 4) and to full-length dlx2 (Fig. 3B, lane 3) and was weakest to the Prd homeodomain.
FIG. 3.
The GR DBD binds directly to the homeodomains of several proteins. (A) Coomassie blue-stained SDS-polyacrylamide gel of 0.5 μg of BSA (lane 1) and GST fusion proteins containing human Oct-2HD (lane 2), full-length zebra fish dlx2 (lane 3), and the homeodomains of human HoxC4 (lane 4), Drosophila paired (Prd, lane 5), Drosophila orthodenticle (Otd, lane 6), zebra fish msxB (lane 7), and Drosophila fushi tarazu (Ftz, lane 8). MW, molecular weight (in thousands). (B and C) Autoradiographs of SDS-polyacrylamide gels showing the binding of 32P-labeled GR DBD (B) and GRL501P DBD (C) peptides to the GST fusion proteins shown in panel A. Lanes 1 show the signal from 10% of the 32P-labeled peptides added to each incubation, while lane 9 shows binding to a glutathione-Sepharose extract from mock-transformed bacterial cells.
L501P-sensitive binding of GR to homeodomain proteins in vivo.
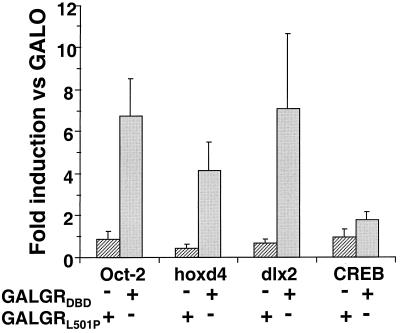
To determine whether GR DBD binding to full-length homeodomain proteins could be detected in the cell, we selected two zebra fish homeodomain proteins, hoxd4 and dlx2, which are unrelated outside the homeodomain and only distantly related to Oct-1 and Oct-2 within the homeodomain family, for further study. hoxd4 is a close zebra fish relative of human HoxC4, whose homeodomain bound to the GR DBD (see above). First, we compared the interaction of the GR DBD with hoxd4 and dlx2 in a one-hybrid assay performed with CHO cells (Fig. 4). In these experiments, the activity of the CAT reporter gene used was dependent upon a specific association of the GAL4-GR DBD fusion proteins and the full-length homeodomain proteins. Transactivation was mediated by the natural transcriptional activation domains within Oct-2, hoxd4, and dlx2. Expression of the GAL4 DBD alone or fused to the GR DBD peptides had no significant effect on transcription of the CAT reporter gene (66). However, coexpression of full-length Oct-2 with the WT GAL4-GR DBD fusion protein potentiated CAT activity six- to eightfold above the level obtained with the GAL4 DBD alone. This induction in activity was completely sensitive to the L501P mutation in the GR DBD, as coexpression of Oct-2 with the GAL4-GRL501P fusion protein had no effect on reporter gene expression.
FIG. 4.
One-hybrid analysis of the L501P-sensitive interaction of the GR DBD with full-length Oct-2, hoxd4, and dlx2 in mammalian cells. The GR DBD, the DBD with an L501P substitution fused to the GAL4 DBD, or the GAL4 DBD alone was coexpressed with Oct-2, hoxd4, dlx2, or CREB in CHO cells. At 48 h after transfection, transcription from a reporter gene with an E1B minimal promoter and five GAL4 binding sites was assessed by a CAT assay. The data are expressed as the fold induction of CAT activity in the presence of GAL-GR DBDL501P (hatched bars) and GAL-GR DBD (solid bars) versus in the presence of GAL4 DBD (GALO). The error bars represent the standard error of the mean of three to five independent experiments performed in duplicate (P < 0.02).
Coexpression of full-length hoxd4 and dlx2 with the GAL4-GR DBD fusion proteins resulted in similar GRL501P-sensitive activation of transcription from the E1B promoter. In contrast, coexpression of the bZip transcription factor CREB had no significant effect on reporter gene activity under these conditions. These results indicated that specific binding to the GR DBD in the cell occurred for at least two homeodomain proteins outside the octamer transcription factor subfamily.
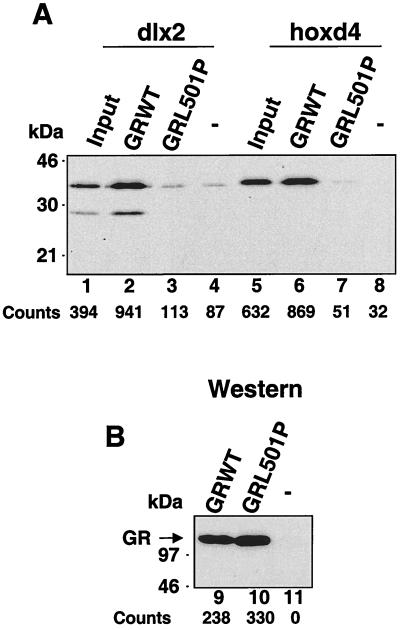
To confirm further the potential for interactions between full-length GR and full-length homeodomain proteins, we examined the ability of in vitro-translated dlx2 and hoxd4 to bind to full-length, ligand-activated GRs extracted from the nucleus of murine fibroblasts (Fig. 5). Previously, we had demonstrated that full-length GR and both Oct-1 and Oct-2 associated in this assay in a manner that was completely sensitive to the GRL501P mutation (64). Similarly, both dlx2 and hoxd4 were observed to bind WT GR from extracts prepared from dexamethasone-treated cells (Fig. 5A, lanes 2 and 6). In contrast, no binding was observed with extracts prepared from cells expressing GRL501P (Fig. 5A, lanes 3 and 7) or with extracts prepared from control parental cells lacking a stably expressed GR (Fig. 5A, lanes 4 and 8).
FIG. 5.
The binding of full-length GR in nuclear extracts to full-length zebrafish dlx2 and hoxd4 is sensitive to GRL501P. (A) Binding of full-length, 35S-labeled, in vitro-translated dlx2 (lanes 2 and 3) and hoxd4 (lanes 6 and 7) to GR and GRL501P immunoprecipitated with c-myc antibody 9E10 from whole-cell extracts prepared from dexamethasone-treated, stably transfected murine SF-7 fibroblasts. Lanes 4 and 8 show binding to extracts prepared from untransfected SF-7 cells, while lanes 1 and 5 show 10% of the in vitro-translated homeodomain proteins added to the binding assay. (B) Western blot of the GRs immunoprecipitated from stably transfected SF-7 cells expressing WT GR and GRL501P and from untransfected SF-7 cells (lanes 9 to 11). Below each lane in panels A and B are the counts obtained following phosphorimager analysis.
Together, these results indicated, through three independent assays, that a peptide encompassing the GR DBD could bind directly to the homeodomains of distantly related homeodomain proteins. Further, in all these assays, binding was sensitive to an L501P substitution in the DNA-contacting α-helix of the second zinc finger.
L501P-sensitive, DNA-independent effects of the GR DBD on early embryogenesis in zebra fish.
The homeodomain mediates DNA binding and protein-protein interactions that determine a large component of homeodomain protein function in the cell (73). Recent gene replacement experiments have suggested that DNA-independent functions of GR are important components of the steroid response (69). The GR DBD contains several interfaces for protein-protein interactions that paradoxically may mediate at least some of these DNA-independent functions of GR (37). The diverse nature of GR DBD-homeodomain binding observed in our molecular assays suggested that ectopic expression of the GR-DBD in whole animals might affect the normal activities of at least some of the proteins that can be contacted by the GR DBD. Further, parallel experiments with the L501P substitution would be expected to reveal effects specific to the surface involved in homeodomain binding.
To test our hypothesis, we examined the consequences for development of introducing mRNAs encoding GR DBD peptides into one- or two-cell-stage zebra fish embryos. Zebra fish are particularly well suited for studies examining the developmental consequences of the ectopic expression of peptides during embryogenesis (19, 41). Eggs are fertilized externally, and proteins can be reliably expressed by microinjection of mRNA immediately following fertilization. Analysis of developmental abnormalities that may be induced is facilitated by the optical clarity and rapid development of the embryos (40). Further, preliminary microinjection experiments with mRNA encoding green fluorescent protein (GFP) established that the proteins encoded from the microinjected mRNAs in our experiments were expressed uniformly through 24 h of development in almost all cells of the embryos (99).
All of the GR constructs expressed in the embryos in our experiments contained the primary GR nuclear localization sequence to ensure that the peptides expressed would be concentrated in the embryonic nuclei. Further, to exclude effects on development that might result from the binding of GR DBD to DNA, the GR DBD peptides expressed also contained amino acid substitutions C460Y, K489R, and/or L501P, which compromise the DNA binding of GR (77). Although C460Y and K489R interfere with GR DNA binding, they were not observed to affect binding to the POU domain of Oct-1 (65).
In the first experiments, the status of the microinjected embryos was examined 24 h after injection, at the end of the segmentation period. The results of these experiments are presented in Fig. 6 and 7 and in Table 1.
FIG. 6.
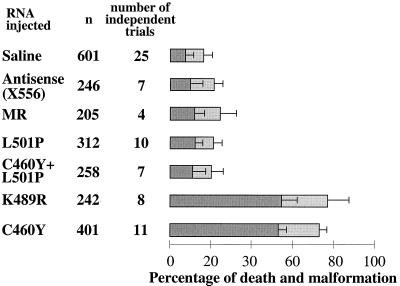
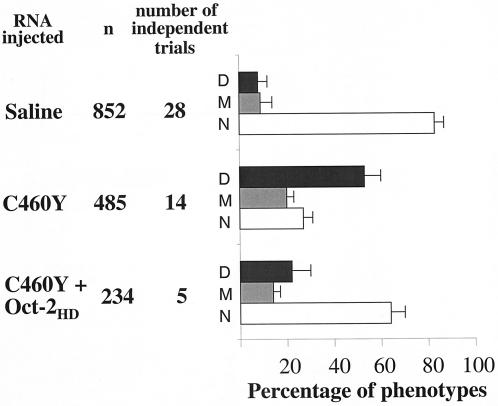
Microinjection of mRNA encoding a GR peptide with the homeodomain binding surface into one- or two-cell-stage zebra fish embryos interferes with embryogenesis in an L501P-sensitive manner. The outcome at 24 h is displayed for one- or two-cell-stage embryos injected with 0.2 to 0.4 nl of RNAs (600 μg/ml) encoding GR mutant peptides with a GR DBD backbone (aa 407 to 556), an antisense transcript of the WT GR, or the MR DBD (aa 567 to 700) or with saline alone. The peptides encoded by the RNAs injected are listed at the left, followed by the total number of embryos injected (n) and the total number of independent injection series performed with each sample. To the right, the percentage of embryos failing to survive for 24 h following injection is indicated by dark gray bars, while the percentage of embryos with malformations visible under the stereomicroscope is indicated by light gray bars. The error bars indicate the standard deviations for independent trials (for GRC460Y and GRK489R compared to the controls, the P value was <0.001).
FIG. 7.
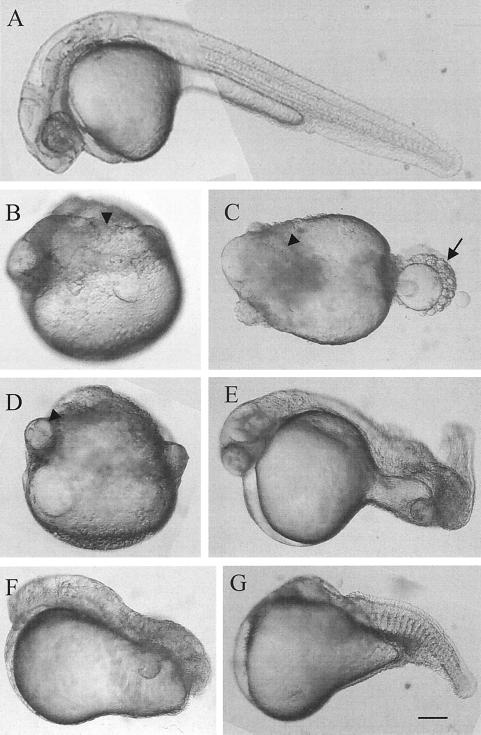
Examples of the four major classes of visible defects in zebra fish embryos 24 h following injection with GRC460Y. From microinjected embryos, the chorion was manually removed, and the samples were photographed under a stereomicroscope at a magnification of ×63. Anterior is to the left; dorsal is to the top. (A) A normally developed control embryo injected with saline. (B to D) Examples of embryos lacking a distinct axis. (E) Embryo with a curved tail. (F) Embryo lacking both a head and a tail. (G) Embryo missing a head and with a truncated tail. The arrowheads indicate the axial mesoderm (B to D), and the arrow indicates the somites (C). Scale bar: A, 130 μm; B to G, 106 μm.
TABLE 1.
Dose response of the microinjection of GR DBD-encoding mRNA into zebra fish embryos
| pg of GRC460Y injected/embryo | %a of embryos at 24 h showing:
|
|
|---|---|---|
| Failure to survive | Visible malformations | |
| 180 | 53 ± 4.2 | 24 ± 3.4 |
| 30 | 30.3 ± 4.6 | 43 ± 6.8 |
Mean ± standard deviation from at least four independent injections.
Microinjection of mRNAs encoding aa 407 to 556 of GR with a C460Y or K489R substitution severely affected embryonic survival (Fig. 6). Fewer than 50% of the embryos injected with either mRNA survived to 24 h. In contrast, only approximately 10% of embryos microinjected with saline or an antisense GR DBD-encoding mRNA failed to develop to 24 h. This value is well within the percentage of growth arrest that is expected to result from the mechanical manipulation and puncture of the embryos in this procedure (6, 24). Moreover, the mean time to arrest for the embryos injected with the test mRNAs was 8 to 10 h, during late gastrulation, while the mean time to arrest for the control embryos was approximately 12 h (99). Remarkably, the development of embryos microinjected with mRNA encoding GR DBD with an L501P mutation or a combination of C460Y and L501P mutations was indistinguishable from that of the control embryos (Fig. 6). Moreover, the effects on development were highly specific to expression of the GR DBD. In contrast to the severe effects of the ectopic expression of the GR DBD peptides, microinjection of mRNA encoding comparable DBD peptide from MR, which differs from GR by only 4 aa within the core of the DBD but which does not interact with the octamer factor homeodomains (65), resulted in completely normal embryonic development that was indistinguishable from that of all of the controls (Fig. 6).
In addition, over 50% of the embryos that were microinjected with the GRC460Y- and GRK489R-encoding mRNAs and that survived to 24 h were afflicted with axial abnormalities that were readily visible under the stereomicroscope. In contrast, malformations of embryos microinjected with L501P-substituted and C460Y- and L501P-substituted GR DBDs and with the MR DBD occurred at the same low frequency as that observed for embryos microinjected with antisense mRNA or saline.
Several examples of the developmental abnormalities observed at 24 h p.f. for embryos injected with the GRC460Y construct are shown in Fig. 7. The predominant phenotype observed (46% ± 7%) was dramatic underdevelopment, with embryos lacking a unitary body axis as well as differentiated anteroposterior structures (Fig. 7B to D). These embryos generally exhibited distinct notochord-like regions that were separated by lobes of yolk. Many embryos (25% ± 5%) contained a nearly normal axis in the trunk region but lacked head and tail structures (Fig. 7F and G), while the rest exhibited defects primarily in the development of the tail (29% ± 8%) (Fig. 7E).
The severity of the phenotypes observed correlated directly with the quantity of GR DBD mRNA microinjected into the embryos (Table 1). A decrease in the concentration of the GRC460Y peptide injected from 600 to 100 μg/ml led to a 40% decrease in the number of embryos whose development was arrested prior to 24 h. In addition, at 100 μg/ml there was a concomitant increase in the percentage of severely malformed embryos that survived to 24 h.
Together, these results provided a strong indication that the region of the GR DBD including the homeodomain binding interface specifically interfered with some early events important for the axial development of the embryos.
Expression of the GRC460Y peptide perturbs the formation of the axial mesoderm.
The phenotype of the malformed embryos uniformly suggested that the defects induced by the GR DBD peptides most likely resulted from defects in the formation of the axial mesoderm, in particular, notochord formation. To examine in greater detail the nature of the effect of the GR peptides on the early development of the embryo, we analyzed the expression of several markers for the early development of the axial mesoderm and notochord by in situ hybridization with microinjected whole-mount embryos.
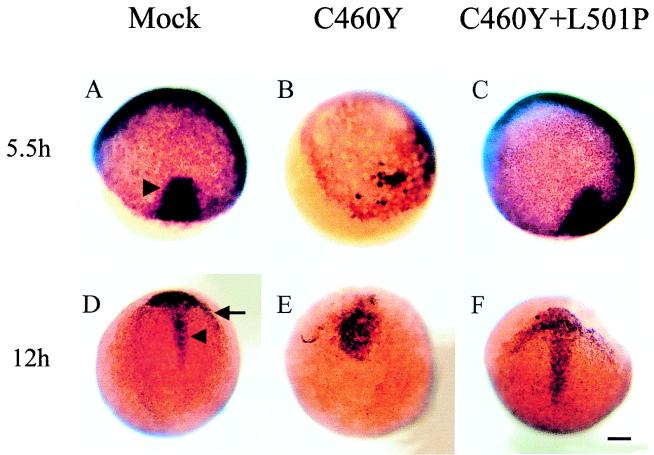
The zebra fish no tail (ntl) gene is a member of the T-box gene family (82–84) and is likely the orthologue of the mammalian Brachyury gene, a developmental control gene which encodes a transcription factor directly implicated in the formation of the primitive streak (68, 105). In zebra fish, ntl is required for notochord and tail formation (28, 82).
In wild-type embryos, ntl expression is first detected at the late blastula stage (4 h p.f.) in a few dispersed cells at the dorsal side of the blastula (84). Later, ntl is expressed in cells of the presumptive mesoderm of the germ ring (or marginal zone) and at the early gastrula stage (5.5 h p.f.). It is also activated in cells of the embryonic shield at the dorsal midline (Fig. 8A). The expression of ntl is specifically maintained in the axial mesoderm as cells migrate away from the blastoderm margin (Fig. 8F). By the end of gastrulation, ntl expression is confined to the developing notochord cells in the axial mesoderm and to both axial and nonaxial cells in the developing tail bud (Fig. 8K). After 24 h of development, ntl expression is localized to the notochord cells of the tail (Fig. 8P).
FIG. 8.
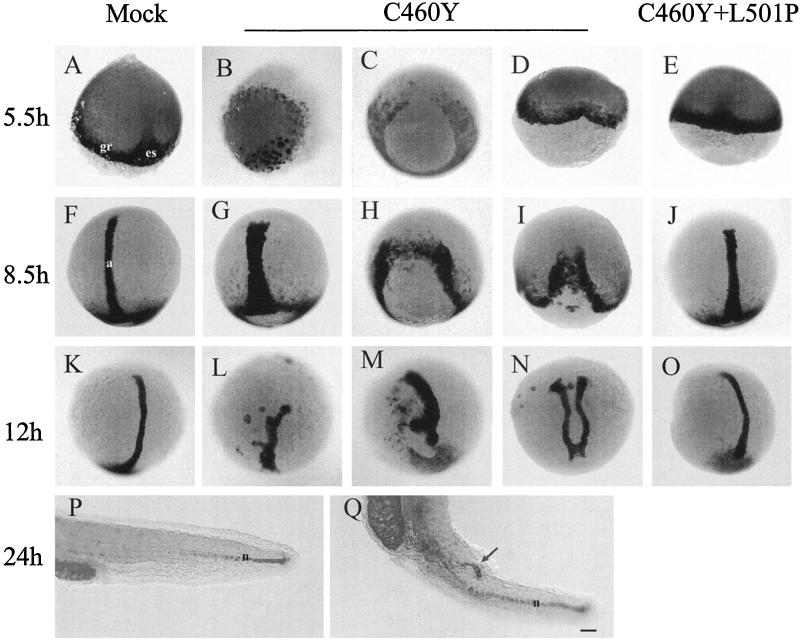
The expression of the GRC460Y peptide in zebra fish embryos disrupts the pattern of no tail expression during embryogenesis. In situ hybridization of whole-mount zebra fish embryos with a no tail antisense RNA probe at the indicated times after fertilization is shown. Embryos were injected at the one- or two-cell stage with saline (mock) (A, F, K, and P) or mRNAs encoding the GR DBD (aa 407 to 556) with C460Y (B to D, G to I, L to N, and Q) or C460Y and L501P (E, J, and O) mutations. Three examples of the hybridization patterns observed upon expression of the GRC460Y peptide at each time point are shown. Dorsal views are shown in panels A and C to O, while an animal pole view is shown in panel B and a lateral view of the tail is shown in panels P and Q. The arrow in panel Q indicates the secondary notochord axis. a, axial mesoderm; gr, germ ring; es, embryonic shield; n, notochord. Scale bar: A to O, 100 μm; P and Q, 70 μm.
The pattern of expression of ntl in embryos injected with RNAs encoding GR DBDs with the C460Y and L501P mutations was similar to that observed in control embryos at all developmental stages (Fig. 8E, J, and O). In contrast, microinjection of RNA encoding GR DBD with the C460Y mutation severely affected ntl expression from the earliest stages analyzed. For the majority of the embryos, blastoderm migration over the yolk cell had not yet reached 50% epiboly, as expected at 5.5 h p.f. (Fig. 8B to D). In a large proportion of the GRC460Y-injected embryos, ntl transcripts were found in a few cells at the dorsal margin of the blastoderm, a pattern similar to that observed in normal embryos at 4 h p.f. (84), suggesting a developmental delay in these embryos (Fig. 8B). Strikingly, in the rest of the embryos, ntl expression was either interrupted in the marginal zone (Fig. 8C) or was seen as a small indentation (Fig. 8D) at the dorsal side of the embryos. These observations were suggestive of defects in the formation of the embryonic shield.
At 8.5 h p.f., while ntl expression in GRC460Y-injected embryos appeared essentially normal in cells of the germ ring, expression in the notochord precursors was affected to various degrees (Fig. 8G to I). In the less affected embryos, the domain of ntl-expressing cells in the axial mesoderm was wider and less anteriorly extended than in the control embryos, a finding which may have reflected defects in the convergence and extension movements of the cells during gastrulation (compare Fig. 8F and G). In more affected embryos, in addition to a delay in development, it seemed that the shield area was devoid of ntl-expressing cells (Fig. 8H and I). Nevertheless, involution movement probably occurred, since rudiments of stripes of cells extending anteriorly were visible starting from the extremities of the germ ring surrounding the normal position of the shield (Fig. 8H and I). These kind of patterns would have presumably led to embryos (if viable) with partially duplicated axes.
At later stages (12 h), ntl expression revealed that most surviving embryos had a short and kinked notochord axis (Fig. 8L and M), suggesting that extension movements toward the anterior part of the embryos may have been affected. Some embryos at the tail bud stage had two distinct short duplicated axes as a possible consequence of convergence defects during the gastrula stage (Fig. 8N). Finally, among the embryos surviving to 24 h p.f., we observed the presence of a short secondary notochord axis expressing ntl and developing from the tail region of the embryos, a finding which may have been the result of a late duplication of the notochord axis during the elongation period of the embryos (Fig. 8Q).
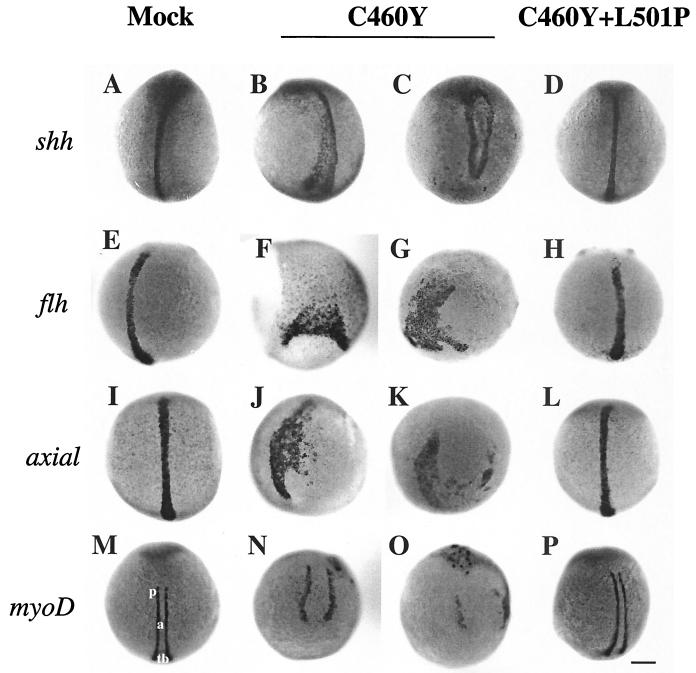
To confirm and expand on the observations obtained with ntl, we examined the expression of other markers of the axial mesoderm in the microinjected embryos (Fig. 9). These markers included sonic hedgehog (shh), which encodes a signaling protein involved in the differentiation and patterning of various embryonic tissues, including the notochord and the floor plate cells of the neural tube (44); the homeobox gene floating head (flh), the zebra fish ortholog of the Xenopus gene Xnot, which is involved in notochord development (95); and axial, the ortholog of the mouse HNF-3β gene, which encodes a transcription factor of the winged-helix family and which has been shown to be essential for the development of the axial mesoderm (93) (Fig. 9A, E, and I). Injection of zebra fish embryos with RNAs encoding GR DBDs carrying the C460Y and L501P mutations did not significantly alter the expression of these markers (Fig. 9D, H, and L). In contrast, the expression of the GRC460Y peptide severely affected the pattern of expression of shh, flh, and axial in the developing axial mesoderm in ways that were strikingly similar to those observed for ntl (Fig. 9B, C, F, G, J, and K). Together, these results strongly support our conclusion that the GR DBD peptides interfered with early embryonic developmental processes required for the normal formation of the axial mesoderm.
FIG. 9.
Whole-mount in situ hybridization showing the expression of developmental markers for axial mesoderm (a) (A to L) and paraxial mesoderm (p) (M to P) formation in 12-h embryos. Embryos were injected at the one- or two-cell stage with saline (mock) (A, E, I, and M) or mRNAs encoding the GR DBD (aa 407 to 556) with C460Y (B, C, F, G, J, K, N, and O) or C460Y and L501P (D, H, L, and P) mutations. Dorsal views of the patterns of expression of sonic hedgehog (shh, A to D), floating head (flh, E to H), axial (I to L), and MyoD (M to P) in 12-h embryos are shown. In GRC460Y/L501P-injected embryos (D, H, L, and P), the patterns of expression of shh, flh, and axial in the developing notochord and of MyoD in the paraxial mesoderm do not differ significantly from those observed in control embryos (A, E, I, and M). In contrast, in GRC460Y-injected embryos, the shh, flh, axial, and MyoD patterns of expression are highly perturbed (B, C, F, G, J, K, N, and O). tb, tail bud. Scale bar: A to P, 140 μm.
To examine whether the GR DBD peptides also affected development beyond the axial mesoderm, we analyzed the expression of MyoD, a gene encoding a basic helix-loop-helix protein expressed in presomitic mesoderm during gastrulation and then in somites (Fig. 9M to P) (21, 102). We observed that the expression of MyoD in 10-h zebra fish embryos injected with RNA encoding the GRC460Y/L501P peptide did not significantly differ from that in control embryos (Fig. 9M and P). At this stage, MyoD transcripts are normally found in two elongating rows of cells, the adaxial cells, adjacent to the axial mesoderm. These cells differentiate into the slow muscle fibers of the zebra fish myotome (21). In contrast, the pattern of expression of MyoD in GRC460Y-injected embryos showed various degrees of disruption, ranging from more widely separated stripes of adaxial cells (Fig. 9N) to a complete disruption of MyoD expression (Fig. 9O), reflecting the defects observed in the patterns of the axial markers.
As our analysis of axial markers indicated defects in the formation of the embryonic shield, we extended our analysis further to examine the expression of goosecoid (gsc), a homeobox gene that has been proposed to participate in the establishment and maintenance of the organizer and shield (16, 84, 92). Furthermore, gsc is expressed very early during zebra fish embryogenesis; it is first maternally and ubiquitously expressed in the embryo, and then zygotic transcripts are detected at 4 h p.f., shortly after the midblastula transition (84, 92). Therefore, it is a valuable marker for analyzing the developmental effects of GR peptides prior to gastrulation.
At the late blastula stage, gsc is expressed as a patch of cells at the margin of the blastoderm (84, 92). At the onset of gastrulation, the domain of gsc expression is limited to the shield region (Fig. 10A). As was observed with the other axial markers, only embryos injected with RNA encoding the GRC460Y/L501P peptide reproduced the normal pattern of expression of gsc at that stage (Fig. 10C).
FIG. 10.
Whole-mount in situ hybridization showing the expression of goosecoid in microinjected embryos. Embryos were injected at the one- or two-cell stage with saline (mock) (A and D) or mRNAs encoding the GR DBD (aa 407 to 556) with C460Y (B and E) or C460Y and L501P (C and F) mutations. Patterns of expression of goosecoid at 5.5 h (A to C) and 12 h (D to F) are shown. Dorsal views are shown in panels A, B, and D to F, while an animal pole view is shown in panel C. Injection of GR DBDC460Y/L501P failed to modify gsc expression at all developmental stages (compare panels A, D, C, and F). (A and C) At the onset of gastrulation, gsc is expressed in the embryonic shield on the dorsal part of the embryo (indicated by an arrowhead in panel A). (B) Blastoderm migration in most GRC460Y-injected embryos is delayed compared to that in control embryos and GRC460Y/L501P-injected embryos, and gsc expression is greatly reduced. (D and F) gsc expression is confined to the prechordal plate (indicated by an arrow in panel D) and cells of the anterior part of the dorsal midline (indicated by an arrowhead in panel D). (E) In GRC460Y-injected embryos, gsc is expressed in a cluster of cells without any distinct pattern; in particular, no rostral crescent corresponding to the prechordal mesoderm is visible. Scale bar: A to F, 106 μm.
Even taking into account the developmental delay of these embryos, the pattern of expression of gsc differed from the normal expression of gsc observed in noninjected embryos at the late blastula stage. Indeed, at 5.5 h p.f., a time at which embryos injected with the GRC460Y-encoding RNA had an appearance normally associated with 4.7 h p.f. (30% epiboly), gsc was expressed only in a very small subset of cells in the dorsal area of the embryo but was not localized at the embryonic margin (Fig. 10B), as expected for WT embryos at 30% epiboly. This observation lends further credence to the suggestion that the misexpression of the GRC460Y peptide affected events occurring very early during development.
As gastrulation proceeds, gsc transcripts are restricted to the anterior edge of the progressing shield and start to be expressed in a rostral crescent of cells of the prechordal plate and some cells of the anterior part of the dorsal midline (Fig. 10D). While embryos injected with GRC460Y/L501P-encoding RNA had a gsc expression pattern similar to that of control embryos, GRC460Y-injected embryos always had a wider gsc domain of expression in the dorsal midline and rarely showed the characteristic crescent-shaped expression in the prechordal plate cells (Fig. 10E and F).
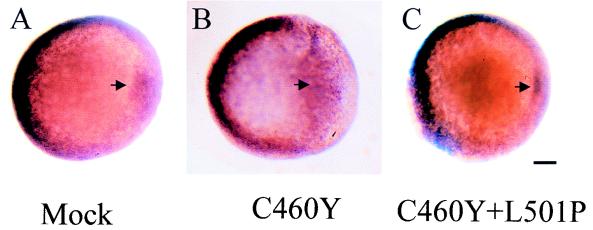
To determine whether, in addition to the observed dorsal defects, the expression of the GRC460Y peptide also generated ventral defects in the embryos, we analyzed the expression of the bone morphogenetic gene, bmp4 (41, 57), which is involved in the ventral patterning of zebra fish embryos (Fig. 11). At the beginning of gastrulation (shield stage, 6 h p.f.), bmp4 is strongly expressed on the ventral region of embryos, especially in the marginal zone (Fig. 11A). In addition, bmp4 transcripts are also found on the lateral areas and in the inner cells of the embryonic shield (41, 57). We observed, at the shield stage, a lateral expansion of the domain of bmp4 expression on the dorsal region of GRC460Y-injected embryos (Fig. 11B). In contrast, bmp4 expression on the ventral part of these embryos appeared unchanged. Embryos injected with saline or with GRC460Y/L501P-encoding RNA showed the normal bmp4 pattern of expression (Fig. 11C). Similarly, the expression of bmp2, another member of the bone morphogenetic gene family, and eve-1, a homeobox gene related to the Drosophila even-skipped gene, whose patterns of expression on the ventrolateral areas of embryos at the early gastrula stage overlap extensively with that of bmp4 (33, 57), remained unchanged in GRC460Y-injected embryos (99). These results suggest that the developmental effects of GR DBD peptide expression may be restricted to the dorsal region of zebra fish embryos.
FIG. 11.
Whole-mount in situ hybridization showing the expression of bmp4 in microinjected embryos at the shield stage. Embryos were injected at the one- or two-cell stage with saline (mock) (A) or mRNAs encoding the GR DBD (aa 407 to 556) with C460Y (B) or C460Y and L501P (C) mutations. Animal pole views of embryos are oriented with their ventral area to the left and their dorsal area to the right. Note that the bmp4 pattern of expression is affected only in the dorsal region of GRC460Y-injected embryos. The arrow indicates the position of the embryonic shield. Scale bar: A to C, 105 μm.
Developmental defects induced by ectopic expression of the GR DBD are rescued by coexpression of Oct-2HD.
To begin to assess the specificity of the developmental defects induced by the GR DBD peptides, we tested whether the phenotypes induced by the GRC460Y peptide could be influenced by the coexpression of a homeodomain peptide. For this experiment, we injected the mRNA encoding GRC460Y alone (180 pg) or together with a twofold excess of the mRNA encoding Oct-2HD (360 pg). Oct-2HD was selected for these experiments as it binds poorly to DNA in the absence of the POU-specific domain but binds avidly to the GR DBD. The results were striking (Fig. 12). The coexpression of Oct-2HD almost completely rescued the embryos from the effects of GRC460Y. Fully 65% of the coinjected embryos developed without visible defects to 24 h p.f., compared to the nearly 75% of embryos injected with the GRC460Y-encoding mRNA alone that failed to develop or displayed the severely malformed phenotypes illustrated in Fig. 7.
FIG. 12.
Microinjection of mRNA encoding Oct-2HD into one- or two-cell-stage zebra fish embryos rescues the developmental defects induced by GRC460Y. The outcome at 24 h is displayed for one- or two-cell-stage embryos injected with 0.2 to 0.4 nl of RNA encoding GRC460Y (600 μg/ml), saline alone, or RNAs encoding GRC460Y and Oct-2HD (1,200 μg/ml). The peptides encoded by the RNAs injected are listed at the left, followed by the total number of embryos injected (n) and the total number of independent injection series performed with each sample. To the right, the percentage of embryos failing to survive for 24 h following injection is indicated by dark gray bars (D), the percentage of embryos with malformations visible under the stereomicroscope is indicated by light gray bars (M), and the percentage of normal embryos is indicated by white bars (N). The error bars indicate the standard deviations for independent trials (for GRC460Y and GRC460Y plus Oct-2 compared to the controls, the P value was <0.001).
The effect of the Oct-2HD-encoding mRNA appeared to occur in direct opposition to the effect of the GRC460Y peptide rather than by nonspecifically influencing GRC460Y production. Coinjection of the same amounts of Oct-2HD-encoding mRNA and GFP-encoding mRNA had no effect on the level of expression of GFP compared to injection of GFP-encoding mRNA alone, and coinjection of GFP-encoding mRNA and GRC460Y-encoding RNA had no effect on the phenotypes obtained (99). Further, as the concentration of the Oct-2HD-encoding mRNA was decreased within the same total mRNA concentration, the effects of GRC460Y reappeared proportionally (99). These results do not in any way prove that the developmental effects of the GR peptides were mediated through binding to embryonic homeodomain proteins. However, they do strongly suggest that the defects resulting from the embryonic expression of the GR DBD arise from GR DBD-mediated protein-protein interactions that overlap with the binding of the GR to a homeodomain.
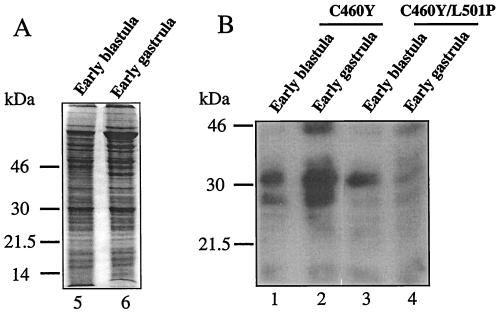
Last, our molecular analysis suggested that specific binding to a protein or proteins expressed in the early embryo interfered with their normal function at a time coincident with the onset of embryonic transcription. To obtain direct evidence in support of this hypothesis, cell lysates prepared from early-blastula- and early-gastrula-stage zebra fish embryos were examined for specific binding to WT and GRL501P DBD peptides by a far-Western approach (Fig. 13). Extracts prepared from early-blastula-stage embryos collected between zygotic cell cycles 8 and 11 (approximately between 2.25 and 2.75 h p.f.) were composed entirely of proteins derived from maternal mRNAs (35). In contrast, in extracts prepared from early-gastrula-stage embryos (collected at 50% epiboly), there was also a strong representation of proteins derived from mRNA transcribed from the embryo genome (35, 36, 40).
FIG. 13.
Far-Western analysis of the binding of GRC460Y and GRC460Y/L501P peptides to proteins extracted from early zebra fish embryos. (A) Protein lysates were prepared from early-blastula-stage and early-gastrula-stage dissected zebra fish embryos from which the chorion had been removed. A Coomassie blue-stained SDS–12% polyacrylamide gel analysis of 25 μg of each extract is shown. (B) Following transfer to nylon membranes, the extracts were denatured and renatured and the membranes were hybridized with either GRC460Y (lanes 1 and 2) or GRC460Y/L501P (lanes 3 and 4) peptides labeled with 32P to the same specific activity at a PKA phosphorylation site added to the C termini of the peptides. Incubation, washing, and exposure to autoradiographic film of the membranes were performed identically for each probe.
Following electrophoresis in duplicate on a single gel, transfer to a nylon membrane, and renaturation, duplicate samples were incubated with equal numbers of counts of 32P-labeled GRC460Y and GRC460Y/L501P peptides labeled to the same specific activity. Exposure of the membrane revealed the presence of several nuclear factors that were preferentially recognized by the GRC460Y peptide in several independent experiments. In the extract from the early blastula, two bands at 31 and 28 kDa bound the GRC460Y peptide (Fig. 13B, lane 1). While binding to the 31-kDa band was only modestly affected by the L501P mutation, the signal at 28 kDa decreased markedly (Fig. 13B, lane 3). Thus, the GR DBD bound specifically to at least one maternally expressed factor in a manner that was sensitive to the L501P substitution.
In the extract from the gastrula, the intensity of the signals obtained with the GRC460Y peptide at 31 and 28 kDa increased severalfold (Fig. 13B, lane 2). However, although equal amounts of proteins from blastula and gastrula lysates were examined, we cannot rule out the possibility that the increase in signal intensity at 31 and 28 kDa reflected an increase in the number of cells expressing these proteins at the gastrula stage rather than an increase in protein synthesis per cell. In addition to the 28- and 31-kDa signals, a third L501P-sensitive signal, at 45 kDa, was also detected. Interestingly, in this extract, the 31-kDa signal was highly sensitive to the L501P substitution in the GR DBD peptide (Fig. 13B, lane 4), suggesting that the signal originated from a factor different from that in the extract from the early blastula. Thus, it appears that the L501P-sensitive developmental defects induced by the expression of the GR DBD may arise from specific interactions with proteins encoded by either or both maternal or zygotically transcribed RNAs.
DISCUSSION
Many studies have demonstrated the ability of nuclear hormone receptor DBDs to enter into productive protein-protein interactions with other transcription factors. Elsewhere, we have demonstrated that three steroid hormone receptors, GR, AR, and PR, can interact productively with the Oct-1 and Oct-2 proteins through their DBDs to recruit them to DNA but that MR is unable to interact with Oct-1 and Oct-2 under the same conditions (64). In the present work, we determined that, when ectopically expressed following mRNA microinjection into one- or two-cell-stage zebra fish embryos, GR but not MR DBD peptides severely perturbed the development of the axial mesoderm. These effects correlated directly with the L501P-sensitive direct binding of GR to the homeodomains of several homeodomain proteins in vitro and in transfected cells and were rescued by the coexpression of Oct-2HD in embryos. While the full extent of the influence of GR-homeodomain binding on homeodomain protein activity in vivo remains to be proven, the sensitivity in our experiments of the developmental defects to the same L501P substitution that abrogated GR-homeodomain binding in tissue culture cells is highly suggestive of a causal linkage to the developmental effects.
DBD-mediated nuclear receptor-homeodomain protein binding influences homeodomain protein function.
The homeodomain contains a highly conserved DNA binding domain. Homeodomain proteins generally bind to DNA sequences containing a highly redundant core motif. However, alone, most homeodomain proteins bind DNA only with a relatively low affinity. In many instances, therefore, homeodomain protein targeting to specific DNA response elements in the cell has been shown to be dependent upon protein-protein interactions with other transcription factors that promote the localization of the homeodomain proteins to specific transcriptional regulatory regions. For example, Hox homeodomain proteins from Hox gene complexes gain DNA binding specificity and affinity through cooperative binding with the divergent homeodomain protein Pbx1 (87), and abdB-like Hox proteins stabilize DNA binding via the homeodomain protein Meis1 (88). It has also been demonstrated that Pbx1 and Meis1 dimerize and display distinctive DNA binding specificities (14). In another example, the human homeodomain protein Phox1 has been shown to impart serum-responsive transcriptional activity to the c-fos serum response element by interacting with the serum response factor (90). Another homeodomain protein, the cardiogenic Nkx-2.5 factor, is recruited by serum response factor to activate cardiac alpha-actin gene transcription in murine fibroblasts (15). Nkx-2.5 also cooperates with GATA-4, a zinc finger transcription factor, to activate the transcription of the cardiac alpha-actin and atrial natriuretic factor genes (50, 85); in both cases, the protein interaction region has been mapped to the Nkx-2.5 homeodomain (15, 50, 85).
Protein-protein interactions with factors other than GR have also been shown to influence the DNA targeting of POU domain proteins beyond the increase in DNA binding specificity provided by the POU-specific domain. For example, the formation of a ternary complex among Oct-1, host cell factor, and herpes simplex virus protein 16 redirects Oct-1 from octamer motifs to TAATGARAT sequences (45). Additionally, the transcription factor Ets-1, a nuclear phosphoprotein involved in cell proliferation, functionally and physically interacts with GHF-1/Pit-1 to direct transcription from the prolactin promoter (10).
While it is noteworthy that all of the homeodomain proteins tested in vitro in our experiments bound directly to the GR DBD in an L501P-sensitive manner and that the phenotype derived from the ectopic expression of the GR DBD was rescued by the coexpression of Oct-2HD, the extent of GR-homeodomain binding in vivo remains to be established. In particular, the degree to which the GR DBD binds productively to homeodomain proteins in the cell under physiological conditions will need to be evaluated for each potential interaction. While the high degree of conservation within the homeodomain may permit broad-based binding to the GR DBD in vitro and in transient overexpression assays, it would seem more probable that productive interactions in vivo would be tightly restricted within glucocorticoid-responsive tissues to the subset of homeodomain proteins with a physiologically relevant affinity for GR. The first example of such a physiologically relevant effect appears to be the interaction among GR, Oct-1, and Oct-2, which seems to be crucial for the responsiveness of mouse mammary tumor virus to steroid hormones (11, 64, 65).
AR and PR also bind to the POU domains of Oct-1 and Oct-2 and promote their binding to the mouse mammary tumor virus promoter in transfected cells (64). Therefore, it may seem tempting to speculate more broadly that nuclear receptors generally bind to and affect homeodomain protein function. However, other experiments reinforce the expectation that nuclear receptor-homeodomain protein binding in situ will be found to occur at the individual, rather than at the family, level. For example, although GR, AR, and PR appear to interact productively with Oct-1 and Oct-2, several other nuclear receptors, including ERα, MR, retinoid X receptor α retinoic acid receptor α, and FTZ-F1α, were unable to interact with Oct-1 and Oct-2 in two hybrid experiments with transfected cells (64).
Nevertheless, we suggest that the apparently general nature of the potential for direct GR DBD-homeodomain binding highlighted in our experiments may reflect a broadly conserved ability of nuclear hormone receptors to enter, on an individual basis, into productive interactions with specific homeodomain proteins. Further, a conserved basic mechanism of protein-protein binding may underlie specific interactions between individual proteins from each family. In this regard, we note a recent report that the Drosophila FTZ-F1 nuclear receptor binds through its DBD to the homeodomain of fushi tarazu (FTZ) in a way that promotes the cooperative binding of FTZ to transcriptional regulatory regions also containing DNA binding sites for FTZ-F1 (106). Similarly, the POU domains of Brn-3a and Brn-3b interact with the DBD of ERα to differentially modulate transcription from estrogen response elements (12).
Ectopic expression of the GR DBD disrupts critical events that control the earliest stages of embryogenesis.
The expression of GR DBD peptides compromised for DNA binding by C460Y or K489R point mutations after microinjection of mRNA into one- or two-cell-stage zebra fish embryos affected development prior to the completion of the blastula stage in a manner that predominantly led to embryonic death during gastrulation. Remarkably, these effects were completely sensitive to the L501P substitution that abrogated GR-homeodomain binding. Further, the coexpression of Oct-2HD counteracted the effects of the GR DBD to allow normal embryonic development.
Our analysis of the affected embryos suggests that the phenotypes and the defects in gene expression observed between 5.5 and 8.5 h p.f. are most likely to have arisen from an L501P-sensitive, GR DBD-mediated defect in the normal cell movements that occur through the blastula and gastrula stages and that are at the origin of the formation of the embryonic axis. At the blastula stage, during epibolic movement, cells of the blastoderm migrate toward the vegetal pole of the embryo. These movements occurred normally in embryos microinjected with control RNA and RNAs encoding GR DBDs with L501P and with C460Y and L501P substitutions. In contrast, the spreading of the blastoderm toward the vegetal pole was clearly delayed following the microinjection of a GR DBD-encoding RNA lacking the L501P substitution.
This observation suggested that the defects were initiated at or before the early phase of epiboly. The blastula stage is a crucial developmental stage during which, concomitant with the acquisition of cell motility, zygotic transcription is activated during the midblastula transition period that initiates at cell cycle 10 of zebra fish embryogenesis (35). However, the molecular events underlying the onset of transcription in the zygote are not presently understood. Our results raise the interesting possibility that the GRC460Y peptide affected the function of key maternal proteins required for the initiation of zygotic transcription and/or interacted with and affected the developmental function of one or more of the earliest zygotic proteins. Further, they also may reflect the specific interference of the GR DBD with the transcriptional regulatory activity of homeodomain proteins at the onset of zygotic transcription.
However, it is also possible that the defects observed during epiboly were mediated at a level other than transcription. They may reflect the consequences of GR DBD peptide effects on proteins acting at an even earlier developmental stage, such as during the early phase of the blastula stage or during the cleavage period (0 to 2.25 h). Indeed, far-Western analysis of potential GR DBD binding factors indicated the potential for both maternally and zygotically expressed proteins to be recognized by the GR DBD in an L501P-sensitive manner. Further experiments, including analyses of cell death and cell proliferation at these early times of development, are being pursued to more clearly localize the onset of the effects of the GR DBD peptides.
Interestingly, the GRC460Y-injected embryos share some early phenotypic characteristics with some zebra fish epiboly mutants isolated in zebra fish mutagenesis screens (34, 56) which illustrated that both maternal and embryonic contributions are essential for controlling early embryonic cell movements. Indeed, Kane et al. described the characterization of four recessive epiboly mutations which, when homozygous, result in a slowdown and arrest of epiboly around midepiboly; these mutations are lethal during or shortly after gastrulation (34). The similarity of the phenotypes of some of these mutants with the defects observed in the GRC460Y-injected embryos suggests that the ectopic expression of GR DBD peptides may have interfered with proteins involved in the same pathways as those affected in these mutants. Thus, GR DBD peptides may offer a means to identify key components of the machinery responsible for early morphogenetic movements.
The consequences of the early L501P-sensitive events induced by the GR DBD peptides became more prominent as embryogenesis progressed. In zebra fish, gastrulation is characterized by several morphogenetic movements, including involution of the cells at the embryonic margin and convergence and extension movements that reshape the blastula embryo. Our present analysis does not allow us to comment on potential defects in the involution movement, which marks the onset of gastrulation. To obtain this information, a detailed analysis of cell movement at the margin of the embryo by use of Nomarski optics and time lapse will be necessary.
However, the localization of the expression of axial mesodermal markers surrounding but not within the normal field of the embryonic shield is suggestive of L501P-sensitive inhibition of cell migration or convergence to the organizer field in affected embryos. Moreover, the blunting of the anterior extension and the widening of the axial mesoderm in GRC460Y-expressing embryos, which in extreme cases led to axial duplication, are also suggestive of defects in convergence and/or extension movements. Alternatively, it is also possible that the widening of the domains of expression of the axial markers observed in the GRC460Y-injected embryos originated from an increased number of dorsal cells. However, the induction of axial duplication in many embryos is more likely to be consistent with the hypothesis of incomplete convergence of laterally and ventrally located cells toward the dorsal midline, as an increase in cell number would be expected to enlarge the width of only a single axis.
The combination of defects in convergence and extension movements during gastrulation could account for the phenotype of the surviving 24-h embryos lacking anterior and posterior structures. Interestingly, this phenotype resembles that which has been observed for a number of gastrulation mutants that remain to be molecularly characterized, including mutants showing defects in convergence and extension movements (56, 91).
Finally, we note with interest that the defects that we have observed in the development of zebra fish embryos upon expression of the GR DBD with a C460Y or K489R substitution resemble the effects observed upon the overexpression of full-length nuclear receptors in Xenopus embryos (25, 26, 67, 86). In preliminary studies, the overexpression of full-length GR in Xenopus embryos resulted in developmental defects starting during the early blastula stage (25, 26). Interestingly, while these defects were also lethal prior to the completion of the gastrula stage, they were entirely dependent upon steroid treatment.
The defects observed in our experiments also overlapped extensively with the defects observed upon the overexpression of retinoid X receptor and thyroid hormone receptor in Xenopus embryos (67). In these experiments, the amounts of receptors required to generate the severe phenotypes decreased markedly when the embryos were treated with triiodothyronine (67). Moreover, the occurrence of phenotypic defects in these experiments and other, related transgenic experiments in which the role of the DBD was investigated was dependent on the presence of an intact receptor DBD (31, 67, 74, 86). Our results suggest that, rather than resulting strictly from the DNA-dependent properties of the nuclear receptors used in these studies, these phenotypes also may be determined by the DNA-independent actions of the receptor DBDs.
Our far-Western and one-hybrid analysis results suggest that the identity of the key factors controlling early morphogenetic events that are targeted by GR may be expected to be revealed by expression library screening approaches that detect specific protein binding. Experiments to further localize the onset of the developmental defects and to identify the L501P-sensitive molecular targets of the GR DBD are ongoing.
ACKNOWLEDGMENTS
We thank the many people who provided the plasmids used in this work including, in particular, Q. Long, T. Zerucha, and M. Ekker but also K. Yamamoto, W. Herr, C. Schild-Poulter, D. Grunwald, M. Halpern, V. Korzh, U. Strähle, M. Tada, D. Wilson, and E. Weinberg. We are grateful to the members of the Haché laboratory and to Y. Lefebvre and M. Ekker for critical comments on the manuscript.
This work was supported by an operating grant from the Medical Research Council of Canada. J.M.W. has been funded by an L. Siminovitch postdoctoral fellowship from The Loeb Health Research Institute at the Ottawa Hospital and a fellowship from the Natural Sciences and Engineering Research Council of Canada. G.G.P. is the recipient of an MRC studentship. M.E.L. holds a junior postdoctoral fellowship from the National Cancer Institute of Canada. R.J.G.H. is a Medical Research Council of Canada scientist.
REFERENCES
- 1.Abbott B D, McNabb F M, Lau C. Glucocorticoid receptor expression during the development of the embryonic mouse secondary palate. J Craniofac Genet Dev Biol. 1994;14:87–96. [PubMed] [Google Scholar]
- 2.Akimenko M-A, Ekker M. Anterior duplication of the Sonic hedgehog expression pattern in the pectoral fin buds of zebrafish treated with retinoic acid. Dev Biol. 1995;170:243–247. doi: 10.1006/dbio.1995.1211. [DOI] [PubMed] [Google Scholar]
- 3.Akimenko M-A, Ekker M, Wegner J, Lin W, Westerfield M. Combinatorial expression of three zebrafish genes related to distal-less: part of a homeobox gene code for the head. J Neurosci. 1994;14:3475–3486. doi: 10.1523/JNEUROSCI.14-06-03475.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beato M, Herrlich P, Schutz G. Steroid receptors: many actors in search of a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- 5.Blackwood E M, Eisenman R N. Identification of protein-protein interactions by lambda gt11 expression cloning. Methods Enzymol. 1995;254:229–240. doi: 10.1016/0076-6879(95)54017-2. [DOI] [PubMed] [Google Scholar]
- 6.Blagden C S, Currie P D, Ingham P W, Hughes S M. Notochord induction of zebrafish slow muscle mediated by Sonic hedgehog. Genes Dev. 1997;11:2163–2175. doi: 10.1101/gad.11.17.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blanar M A, Rutter W J. Interaction cloning: identification of a helix-loop-helix zipper protein that interacts with c-Fos. Science. 1992;256:1014–1018. doi: 10.1126/science.1589769. [DOI] [PubMed] [Google Scholar]
- 8.Blum M, Gaunt J, Cho K W Y, Steinbeisser H, Blumberg B, Bittner D, De Robertis E M. Gastrulation of the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg B, Wright V E, De Robertis E M, Cho K W Y. Organizer-specific homeobox genes in Xenopus laevis embryos. Science. 1991;253:194–196. doi: 10.1126/science.1677215. [DOI] [PubMed] [Google Scholar]
- 10.Bradford A P, Wasylyk C, Wasylyk B, Gutierrez-Hartmann A. Interaction of Ets-1 and the POU-homeodomain protein GHF-1/Pit-1 reconstitutes pituitary-specific gene expression. Mol Cell Biol. 1997;17:1065–1074. doi: 10.1128/mcb.17.3.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruggemeier U, Kalff M, Franke S, Scheidereit C, Beato M. Ubiquitous transcription factor OTF-1 mediates induction of the MMTV promoter through synergistic interaction with hormone receptors. Cell. 1991;64:565–572. doi: 10.1016/0092-8674(91)90240-y. [DOI] [PubMed] [Google Scholar]
- 12.Budhram-Mahadeo V, Parker M, Latchman D S. POU transcription factors Brn-3a and Brn-3b interact with the estrogen receptor and differentially regulate transcriptional activity via an estrogen response element. Mol Cell Biol. 1998;18:1029–1041. doi: 10.1128/mcb.18.2.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns K, Duggan B, Atkinson E A, Famulski K S, Nemer M, Bleackley R C, Michalak M. Modulation of gene expression by calreticulin binding to the glucocorticoid receptor. Nature. 1994;367:476–480. doi: 10.1038/367476a0. [DOI] [PubMed] [Google Scholar]
- 14.Chang C P, Jacobs Y, Nakamura T, Jenkins N A, Copeland N G, Cleary M L. Meis proteins are major in vivo DNA binding partners for wild-type but not chimeric Pbx proteins. Mol Cell Biol. 1997;17:5679–5687. doi: 10.1128/mcb.17.10.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C Y, Schwartz R J. Recruitment of the tinman homolog Nkx-2.5 by serum response factor activates cardiac alpha-actin gene transcription. Mol Cell Biol. 1996;16:6372–6384. doi: 10.1128/mcb.16.11.6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho K H Y, Blumberg B, Steinbeisser H, De Robertis E M. Molecular nature of Spemann’s organizer: the role of the Xenopus homeobox gene goosecoid. Cell. 1991;67:1111–1120. doi: 10.1016/0092-8674(91)90288-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole T J, Blendy J A, Monaghan A P, Krieglstein K, Schmid W, Aguzzi A, Fantuzzi G, Hummler E, Unsicker K, Schutz G. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 18.Cole T J, Blendy J A, Schmid W, Strahle U, Schutz G. Expression of the mouse glucocorticoid receptor and its role during development. J Steroid Biochem Mol Biol. 1993;47:49–53. doi: 10.1016/0960-0760(93)90056-3. [DOI] [PubMed] [Google Scholar]
- 19.Concordet J-P, Lewis K E, Moore J W, Goodrich L V, Johnson R L, Scott M P, Ingham P W. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development. 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- 20.Dedhar S, Rennie P S, Shago M, Hagesteijn C Y, Yang H, Filmus J, Hawley R G, Bruchovsky N, Cheng H, Matusik R J, Giguère V. Inhibition of nuclear hormone receptor activity by calreticulin. Nature. 1994;367:480–483. doi: 10.1038/367480a0. [DOI] [PubMed] [Google Scholar]
- 21.Devoto S H, Melancon E, Eisen J S, Westerfield M. Identification of separate slow and fast muscle precursor cells in vivo, prior to somite formation. Development. 1996;122:3371–3380. doi: 10.1242/dev.122.11.3371. [DOI] [PubMed] [Google Scholar]
- 22.Fan M J, Sokol S Y. A role for Siamois in Spemann organizer formation. Development. 1997;124:2581–2589. doi: 10.1242/dev.124.13.2581. [DOI] [PubMed] [Google Scholar]
- 23.Freedman L P, Luisi B F, Korszun Z R, Basavappa R, Sigler P B, Yamamoto K R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988;334:543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- 24.Furthauer M, Thisse C, Thisse B. A role for FGF-8 in the dorsoventral patterning of the zebrafish gastrula. Development. 1997;124:4253–4264. doi: 10.1242/dev.124.21.4253. [DOI] [PubMed] [Google Scholar]
- 25.Gao X, Kalkhoven E, Peterson-Maduro J, van der Burg B, Destree O H. Expression of the glucocorticoid receptor gene is regulated during early embryogenesis of Xenopus laevis. Biochim Biophys Acta. 1994;1218:194–198. doi: 10.1016/0167-4781(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 26.Gao X, Stegeman B I, Lanser P, Koster J G, Destree O H. GR transcripts are localized during early Xenopus laevis embryogenesis and overexpression of GR inhibits differentiation after dexamethasone treatment. Biochem Biophys Res Commun. 1994;199:734–741. doi: 10.1006/bbrc.1994.1290. [DOI] [PubMed] [Google Scholar]
- 27.Gawantka V, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. Antagonizing the Spemann organizer: role of the homeobox gene Xvent-1. EMBO J. 1995;14:6268–6279. doi: 10.1002/j.1460-2075.1995.tb00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Halpern M E, Ho R K, Walker C, Kimmel C B. Induction of muscle pioneers and floor plate is distinguished by the zebrafish no tail mutation. Cell. 1993;75:99–111. [PubMed] [Google Scholar]
- 29.Heck S, Kullmann M, Gast A, Ponta H, Rahmsdorf H J, Herrlich P, Cato A C. A distinct modulating domain in glucocorticoid receptor monomers in the repression of activity of the transcription factor AP-1. EMBO J. 1994;13:4087–4095. doi: 10.1002/j.1460-2075.1994.tb06726.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Horwitz K B, Jackson T A, Bain D L, Richer J K, Takimoto G S, Tung L. Nuclear receptor coactivators and corepressors. Mol Endocrinol. 1996;10:1167–1177. doi: 10.1210/mend.10.10.9121485. [DOI] [PubMed] [Google Scholar]
- 31.Imakado S, Bickenbach J R, Bundman D S, Rothnagel J A, Attar P S, Wang X J, Walczak V R, Wisniewski S, Pote J, Gordon J S, Heyman R A, Evans R M, Roop D R. Targeting expression of a dominant-negative retinoic acid receptor mutant in the epidermis of transgenic mice results in loss of barrier function. Genes Dev. 1995;9:317–329. doi: 10.1101/gad.9.3.317. [DOI] [PubMed] [Google Scholar]
- 32.Izpisua-Belmonte J C, De Robertis E M, Storey K G, Stern C D. The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell. 1993;74:645–659. doi: 10.1016/0092-8674(93)90512-o. [DOI] [PubMed] [Google Scholar]
- 33.Joly J S, Joly C, Schulte-Merker S, Boulekbache H, Condamine H. The ventral and posterior expression of the zebrafish homeobox gene eve1 is perturbed in dorsalized and mutant embryos. Development. 1993;119:1261–1275. doi: 10.1242/dev.119.4.1261. [DOI] [PubMed] [Google Scholar]
- 34.Kane D A, Hammerschmidt M, Mullins M C, Maischein H-M, Brand M, van Eeden F J M, Furutani-Seiki M, Granato M, Haffter P, Heisenberg C-P, Jiang Y-J, Kelsh R N, Odenthal J, Warga R M, Nüsslein-Volhard C. The zebrafish epiboly mutants. Development. 1996;123:47–55. doi: 10.1242/dev.123.1.47. [DOI] [PubMed] [Google Scholar]
- 35.Kane D A, Kimmel C B. The zebrafish midblastula transition. Development. 1993;119:447–456. doi: 10.1242/dev.119.2.447. [DOI] [PubMed] [Google Scholar]
- 36.Kane D A, Maischein H-M, Brand M, van Eeden F J M, Furutani-Seiki M, Granato M, Haffter P, Hammerschmidt M, Heisenberg C-P, Jiang Y-J, Kelsh R N, Mullins M C, Odenthal J, Warga R M, Nüsslein-Volhard C. The zebrafish early arrest mutants. Development. 1996;123:57–66. doi: 10.1242/dev.123.1.57. [DOI] [PubMed] [Google Scholar]
- 37.Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? Cell. 1998;93:487–490. doi: 10.1016/s0092-8674(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 38.Kerppola T K, Luk D, Curran T. Fos is a preferential target of glucocorticoid receptor inhibition of AP-1 activity in vitro. Mol Cell Biol. 1993;13:3782–3791. doi: 10.1128/mcb.13.6.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kessler D S. Siamois is required for formation of Spemann’s organizer. Proc Natl Acad Sci USA. 1997;94:13017–13022. doi: 10.1073/pnas.94.24.13017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimmel C B, Ballard W W, Kimmel S R, Ullmann B, Schilling T F. Stages of embryonic development of the zebrafish. Dev Dynamics. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto Y, Lee K H, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- 42.Kitraki E, Kittas C, Stylianopoulou F. Glucocorticoid receptor gene expression during rat embryogenesis. An in situ hybridization study. Differentiation. 1997;62:21–31. doi: 10.1046/j.1432-0436.1997.6210021.x. [DOI] [PubMed] [Google Scholar]
- 43.Klemm J D, Rould M A, Aurora R, Herr W, Pabo C O. Crystal structure of the Oct-1 POU domain bound to an octamer site: DNA recognition with tethered DNA-binding modules. Cell. 1994;77:21–32. doi: 10.1016/0092-8674(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 44.Krauss S, Concordet J-P, Ingham P W. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- 45.Kristie T M, Sharp P A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990;4:2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- 46.Kutoh E, Stromstedt P-E, Poellinger L. Functional interference between the ubiquitous and constitutive octamer transcription factor 1 (OTF-1) and the glucocorticoid receptor by direct protein-protein interaction involving the homeo subdomain of OTF-1. Mol Cell Biol. 1992;12:4960–4969. doi: 10.1128/mcb.12.11.4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai J-S, Cleary M A, Herr W. A single amino acid exchange transfers VP16-induced positive control from the Oct-1 to the Oct-2 homeo domain. Genes Dev. 1992;6:2058–2065. doi: 10.1101/gad.6.11.2058. [DOI] [PubMed] [Google Scholar]
- 48.Laudet V, Hanni C, Coll J, Catzeflis F, Stehelin D. Evolution of the nuclear receptor gene superfamily. EMBO J. 1992;11:1003–1013. doi: 10.1002/j.1460-2075.1992.tb05139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Laurent M N, Blitz I L, Hashimoto C, Rothbächer U, Cho K W-Y. The Xenopus homeobox gene Twin mediates Wnt induction of Goosecoid in establishment of Spemann’s organizer. Development. 1997;124:4905–4916. doi: 10.1242/dev.124.23.4905. [DOI] [PubMed] [Google Scholar]
- 50.Lee Y, Shioi T, Kasahara H, Jobe S M, Wiese R J, Markham B E, Izumo S. The cardiac tissue-restricted homeobox protein Csx/Nkx2.5 physically associates with the zinc finger protein GATA4 and cooperatively activates atrial natriuretic factor gene expression. Mol Cell Biol. 1998;18:3120–3129. doi: 10.1128/mcb.18.6.3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemaire L, Roeser T, Izpisúa-Belmonte J C, Kessel M. Segregating expression domains of two goosecoid genes during the transition from gastrulation to neurulation in chick embryos. Development. 1997;124:1443–1452. doi: 10.1242/dev.124.8.1443. [DOI] [PubMed] [Google Scholar]
- 52.Lemaire P, Garrett N, Gurdon J B. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 53.Liu W, Hillmann A G, Harmon J M. Hormone-independent repression of AP-1-inducible collagenase promoter activity by glucocorticoid receptors. Mol Cell Biol. 1995;15:1005–1013. doi: 10.1128/mcb.15.2.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Overview: the nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McEwan I J, Wright A P, Gustafsson J A. Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. Bioessays. 1997;19:153–160. doi: 10.1002/bies.950190210. [DOI] [PubMed] [Google Scholar]
- 56.Mullins M C, Hammerschmidt M, Kane D A, Odenthal J, Brand M, van Eeden F J M, Furutani-Seiki M, Granato M, Haffter P, Heisenberg C-P, Jiang Y-J, Kelsh R N, Nüsslein-Volhard C. Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- 57.Nikaido M, Tada M, Saji T, Ueno N. Conservation of BMP signaling in zebrafish mesoderm patterning. Mech Dev. 1997;61:75–88. doi: 10.1016/s0925-4773(96)00625-9. [DOI] [PubMed] [Google Scholar]
- 58.Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H. A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell. 1990;60:461–472. doi: 10.1016/0092-8674(90)90597-8. [DOI] [PubMed] [Google Scholar]
- 59.Onichtchouk D, Gawantka V, Dosch R, Delius H, Hirschfeld K, Blumenstock C, Niehrs C. The Xvent-2 homeobox gene is part of the BMP-4 signalling pathway controlling dorsoventral patterning of Xenopus mesoderm. Development. 1996;122:3045–3053. doi: 10.1242/dev.122.10.3045. [DOI] [PubMed] [Google Scholar]
- 60.Palmieri S L, Peter W, Hess H, Scholer H R. Oct-4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages involved in implantation. Dev Biol. 1994;166:259–267. doi: 10.1006/dbio.1994.1312. [DOI] [PubMed] [Google Scholar]
- 61.Pannese M, Polo C, Andreazzoli M, Vignali R, Kablar B, Barsacchi G, Boncinelli E. The Xenopus homologue of Otx2 is a maternal homeobox gene that demarcates and specifies anterior body regions. Development. 1995;121:707–720. doi: 10.1242/dev.121.3.707. [DOI] [PubMed] [Google Scholar]
- 62.Pratt W B. The role of heat shock proteins in regulating the function, folding, and trafficking of the glucocorticoid receptor. J Biol Chem. 1993;268:21455–21458. [PubMed] [Google Scholar]
- 63.Pratt W B, Toft D O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocrinol Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- 64.Préfontaine, G. G., W. Giffin, M. E. Lemieux, L. Pope, and R. J. G. Haché. Selective binding of steroid hormone receptors to octamer transcription factors determines transcriptional synergism at the MMTV promoter. J. Biol. Chem., in press. [DOI] [PubMed]
- 65.Préfontaine G G, Lemieux M E, Giffin W, Schild-Poulter C, Pope L, LaCasse E, Walker P, Haché R J G. Recruitment of octamer transcription factors to DNA by glucocorticoid receptor. Mol Cell Biol. 1998;18:3416–3430. doi: 10.1128/mcb.18.6.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Préfontaine, G. G., M. E. Lemieux, and R. J. G. Haché. Unpublished observation.
- 67.Puzianowska-Kuznicka M, Damjanovski S, Shi Y B. Both thyroid hormone and 9-cis retinoic acid receptors are required to efficiently mediate the effects of thyroid hormone on embryonic development and specific gene regulation in Xenopus laevis. Mol Cell Biol. 1997;17:4738–4749. doi: 10.1128/mcb.17.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rashbass P, Cooke L A, Herrmann B G, Beddington R S. A cell autonomous function of Brachyury in T/T embryonic stem cell chimaeras. Nature. 1991;353:348–351. doi: 10.1038/353348a0. [DOI] [PubMed] [Google Scholar]
- 69.Reichardt H M, Kaestner K H, Tuckermann J, Kretz O, Wessely O, Bock R, Gass P, Schmid W, Herrlich P, Angel P, Schutz G. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 70.Rosenfeld M G. POU-domain transcription factors: pou-er-ful developmental regulators. Genes Dev. 1991;5:897–907. doi: 10.1101/gad.5.6.897. [DOI] [PubMed] [Google Scholar]
- 71.Rusconi S, Yamamoto K R. Functional dissection of the hormone and DNA binding activities of the glucocorticoid receptor. EMBO J. 1987;6:1309–1315. doi: 10.1002/j.1460-2075.1987.tb02369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ruvkun G, Finney M. Regulation of transcription and cell identity by POU domain proteins. Cell. 1991;64:475–478. doi: 10.1016/0092-8674(91)90227-p. [DOI] [PubMed] [Google Scholar]
- 73.Ryan A K, Rosenfeld M G. POU domain family values: flexibility, partnerships, and developmental codes. Genes Dev. 1997;11:1207–1225. doi: 10.1101/gad.11.10.1207. [DOI] [PubMed] [Google Scholar]
- 74.Saitou M, Sugai S, Tanaka T, Shimouchi K, Fuchs E, Narumiya S, Kakizuka A. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature. 1995;374:159–162. doi: 10.1038/374159a0. [DOI] [PubMed] [Google Scholar]
- 75.Sato N, Sadar M D, Bruchovsky N, Saatcioglu F, Rennie P S, Sato S, Lange P H, Gleave M E. Androgenic induction of prostate-specific antigen gene is repressed by protein-protein interaction between the androgen receptor and AP-1/c-Jun in the human prostate cancer cell line LNCaP. J Biol Chem. 1997;272:17485–17494. doi: 10.1074/jbc.272.28.17485. [DOI] [PubMed] [Google Scholar]
- 76.Scheinman R I, Gualberto A, Jewell C M, Cidlowski J A, Baldwin A S J. Characterization of mechanisms involved in transrepression of NF-kappa B by activated glucocorticoid receptors. Mol Cell Biol. 1995;15:943–953. doi: 10.1128/mcb.15.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schena M, Freedman L P, Yamamoto K R. Mutations in the glucocorticoid receptor zinc finger region that distinguish interdigitated DNA binding and transcriptional enhancement activities. Genes Dev. 1989;3:1590–1601. doi: 10.1101/gad.3.10.1590. [DOI] [PubMed] [Google Scholar]
- 78.Schmidt J E, Von Dassow G, Kimelman D. Regulation of dorso-ventral patterning: the ventralising effects of the novel Xenopus homeobox gene Vox. Development. 1996;122:1711–1721. doi: 10.1242/dev.122.6.1711. [DOI] [PubMed] [Google Scholar]
- 79.Scholer H R, Dressler G R, Balling R, Rohdewohld H, Gruss P. Oct-4: a germline-specific transcription factor mapping to the mouse t-complex. EMBO J. 1990;9:2185–2195. doi: 10.1002/j.1460-2075.1990.tb07388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scholer H R, Ruppert S, Suzuki N, Chowdhury K, Gruss P. New type of POU domain in germ line-specific protein Oct-4. Nature. 1990;344:435–439. doi: 10.1038/344435a0. [DOI] [PubMed] [Google Scholar]
- 81.Schroen D J, Brinckerhoff C E. Inhibition of rabbit collagenase (matrix metalloproteinase-1; MMP-1) transcription by retinoid receptors: evidence for binding of RARs/RXRs to the −77 AP-1 site through interactions with c-Jun. J Cell Physiol. 1996;169:320–332. doi: 10.1002/(SICI)1097-4652(199611)169:2<320::AID-JCP11>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 82.Schulte-Merker S, Hammerschmidt M, Beuchle D, Cho K W, De Robertis E M, Nüsslein-Volhard C. Expression of zebrafish goosecoid and no tail gene products in wild-type and mutant no tail embryos. Development. 1994;120:843–852. doi: 10.1242/dev.120.4.843. [DOI] [PubMed] [Google Scholar]
- 83.Schulte-Merker S, Ho R K, Herrmann B G, Nüsslein-Volhard C. The protein product of the zebrafish homologue of the mouse T gene is expressed in nuclei of the germ ring and the notochord of the early embryo. Development. 1992;116:1012–1032. doi: 10.1242/dev.116.4.1021. [DOI] [PubMed] [Google Scholar]
- 84.Schulte-Merker S, van Eeden F J M, Halpern M E, Kimmel C B, Nüsslein-Volhard C. no tail (ntl) is the zebrafish homologue of the mouse T (Brachyury) gene. Development. 1994;120:1009–1015. doi: 10.1242/dev.120.4.1009. [DOI] [PubMed] [Google Scholar]
- 85.Sepulveda J L, Belaguli N, Nigam V, Chen C Y, Nemer M, Schwartz R J. GATA-4 and Nkx-2.5 coactivate Nkx-2 DNA binding targets: role for regulating early cardiac gene expression. Mol Cell Biol. 1998;18:3405–3415. doi: 10.1128/mcb.18.6.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sharpe C R, Goldstone K. Retinoid receptors promote primary neurogenesis in Xenopus. Development. 1997;124:515–523. doi: 10.1242/dev.124.2.515. [DOI] [PubMed] [Google Scholar]
- 87.Shen W F, Montgomery J C, Rozenfeld S, Moskow J J, Lawrence H J, Buchberg A M, Largman C. AbdB-like Hox proteins stabilize DNA binding by the Meis1 homeodomain proteins. Mol Cell Biol. 1997;17:6448–6458. doi: 10.1128/mcb.17.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shen W F, Rozenfeld S, Lawrence H J, Largman C. The Abd-B-like Hox homeodomain proteins can be subdivided by the ability to form complexes with Pbx1a on a novel DNA target. J Biol Chem. 1997;272:8198–8206. doi: 10.1074/jbc.272.13.8198. [DOI] [PubMed] [Google Scholar]
- 89.Simeone A, Acampora D, Mallamaci A, Stornaiuolo A, D’Apice M R, Nigro V, Boncinelli E. A vertebrate gene related to orthodenticle contains a homeodomain of the bicoid class and demarcates anterior neuroectoderm in the gastrulating mouse embryo. EMBO J. 1993;12:2735–2747. doi: 10.1002/j.1460-2075.1993.tb05935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Simon K J, Grueneberg D A, Gilman M. Protein and DNA contact surfaces that mediate the selective action of the Phox1 homeodomain at the c-fos serum response element. Mol Cell Biol. 1997;17:6653–6662. doi: 10.1128/mcb.17.11.6653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Solnica-Krezel L, Stemple D L, Mountcastle-Shah E, Rangini Z, Neuhauss S C F, Malicki J, Schier A F, Stainier D Y R, Zwartkruis F, Abdelilah S, Driever W. Mutations affecting cell fates and cellular rearrangements during gastrulation in zebrafish. Development. 1996;123:67–80. doi: 10.1242/dev.123.1.67. [DOI] [PubMed] [Google Scholar]
- 92.Stachel S E, Grunwald D J, Myers P Z. Lithium perturbation and goosecoid expression identify a dorsal specification pathway in the pregastrula zebrafish. Development. 1993;117:1261–1274. doi: 10.1242/dev.117.4.1261. [DOI] [PubMed] [Google Scholar]
- 93.Strähle U, Blader P, Henrique D, Ingham P W. Axial, a zebrafish gene expressed along the developing body axis, shows altered expression in cyclops mutant embryos. Genes Dev. 1993;7:1436–1446. doi: 10.1101/gad.7.7b.1436. [DOI] [PubMed] [Google Scholar]
- 94.Taira M, Jamrich M, Good P J, Dawid I B. The LIM domain-containing homeobox gene Xlim-1 is expressed specifically in the organizer region of Xenopus gastrula embryos. Genes Dev. 1992;6:356–366. doi: 10.1101/gad.6.3.356. [DOI] [PubMed] [Google Scholar]
- 95.Talbot W S, Trevbarrow B, Halpern M E, Melby A E, Farr G, Postlethwait J H, Jowett T, Kimmel C B, Kimelman D. A homeobox gene essential for zebrafish notochord development. Nature. 1995;378:150–157. doi: 10.1038/378150a0. [DOI] [PubMed] [Google Scholar]
- 96.Tanaka M, Herr W. Differential transcriptional activation by Oct-1 and Oct-2: interdependent activation domains induce Oct-2 phosphorylation. Cell. 1990;60:375–386. doi: 10.1016/0092-8674(90)90589-7. [DOI] [PubMed] [Google Scholar]
- 97.Truss M, Bartsch J, Schelbert A, Haché R J G, Beato M. Hormone induces binding of receptors and transcription factors to a rearranged nucleosome on the MMTV promoter in vivo. EMBO J. 1995;14:1737–1751. doi: 10.1002/j.1460-2075.1995.tb07163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.von Dassow G, Schmidt J E, Kimelman D. Induction of the Xenopus organizer: expression and regulation of Xnot, a novel FGF and activin-regulated homeo box gene. Genes Dev. 1993;7:355–366. doi: 10.1101/gad.7.3.355. [DOI] [PubMed] [Google Scholar]
- 99.Wang, J. M., M.-A. Akimenko, and R. J. G. Haché. Unpublished observation.
- 100.Webb P, Lopez G N, Uht R M, Kushner P J. Tamoxifen activation of the estrogen receptor/AP-1 pathway: potential origin for the cell-specific estrogen-like effects of antiestrogens. Mol Endocrinol. 1995;9:443–456. doi: 10.1210/mend.9.4.7659088. [DOI] [PubMed] [Google Scholar]
- 101.Wegner M, Drolet D W, Rosenfeld M G. POU-domain proteins: structure and function of developmental regulators. Curr Opin Cell Biol. 1993;5:488–498. doi: 10.1016/0955-0674(93)90015-i. [DOI] [PubMed] [Google Scholar]
- 102.Weinberg E S, Allende M L, Kelly C S, Abdelhamid A, Murakami T, Andermann P, Doerre O G, Grunwald D J, Riggleman B. Developmental regulation of zebrafish MyoD in wild-type, no tail and spadetail embryos. Development. 1996;122:271–280. doi: 10.1242/dev.122.1.271. [DOI] [PubMed] [Google Scholar]
- 103.Westerfield M. The zebrafish book. University of Oregon Press; 1995. , Eugene. [Google Scholar]
- 104.Wilson D, Sheng G, Lecuit T, Dostatni N, Desplan C. Cooperative dimerization of paired class homeo domains on DNA. Genes Dev. 1993;7:2120–2134. doi: 10.1101/gad.7.11.2120. [DOI] [PubMed] [Google Scholar]
- 105.Wilson V, Rashbass P, Beddington R S. Chimeric analysis of T (Brachyury) gene function. Development. 1993;117:1321–1331. doi: 10.1242/dev.117.4.1321. [DOI] [PubMed] [Google Scholar]
- 106.Yu Y, Li W, Su K, Yussa M, Han W, Perrimon N, Pick L. The nuclear hormone receptor Ftz-F1 is a cofactor for the Drosophila homeodomain protein Ftz. Nature. 1997;385:552–555. doi: 10.1038/385552a0. [DOI] [PubMed] [Google Scholar]
- 107.Zerucha T. Ph.D. thesis. Ottawa, Ontario, Canada: University of Ottawa; 1999. [Google Scholar]
- 108.Zhang X K, Wills K N, Husmann M, Hermann T, Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991;11:6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]