Abstract
The corpus luteum is an endocrine gland that synthesizes the steroid hormone progesterone. luteinizing hormone (LH) is a key luteotropic hormone that stimulates ovulation, luteal development, progesterone biosynthesis, and maintenance of the corpus luteum. Luteotropic and luteolytic factors precisely regulate luteal structure and function; yet, despite recent scientific progress within the past few years, the exact mechanisms remain largely unknown. In the present review, we summarize the recent progress towards understanding cellular changes induced by LH in steroidogenic luteal cells. Herein, we will focus on the effects of LH on inter-organelle communication and steroid biosynthesis, and how LH regulates key protein kinases (i.e., AMPK and MTOR) responsible for controlling steroidogenesis and autophagy in luteal cells.
Keywords: corpus luteum, LH, mitochondria, lipid droplets, autophagy, cholesterol, progesterone
1. Introduction
The ovary is a highly dynamic organ that undergoes some of the most dramatic structural and functional changes of any adult tissue. Reproductive potential and reproductive life span are determined by the ovarian follicles, which are the major endocrine and reproductive compartments of the ovary. The follicles are comprised of three essential cells: theca cells, granulosa cells, and the oocyte. Together, these cells interact in a synergistic manner to secrete sex steroids and protein hormones that contribute to oocyte maturation for successful fertilization.
Gonadotropin-releasing hormone (GnRH) is released in a pulsatile fashion from the hypothalamus, inducing the release of the gonadotropins, follicle stimulating hormone (FSH) and luteinizing hormone (LH), from the anterior pituitary gland. FSH acts directly on granulosa cells of the developing ovarian follicle to stimulate growth of the follicle, secretion of estrogen and expression of the LH receptor (LHCGR) [1,2,3]. LH stimulates androgen production by the theca cells of developing follicles and triggers ovulation when follicular maturation is complete [4]. During the ovulatory process, LH causes the final maturation and release of the ovum from preovulatory follicles and stimulates the differentiation of the theca and granulosa cells of the preovulatory follicle into the small and large steroidogenic cells of the corpus luteum, respectively [5,6]. Corpus luteum formation involves interactions between steroidogenic cells, microvascular cells, and immune cells, leading to remodeling of the follicle and intense angiogenesis required for luteal formation [4,7]. As a result, the newly formed corpus luteum prepares and sustains the reproductive tract for conception, implantation, and early pregnancy [8,9,10].
The corpus luteum is a transient endocrine gland that synthesizes peptide and steroid hormones and serves as the primary source of progesterone during estrous/menstrual cycles and early pregnancy [10,11]. Progesterone acting via nuclear receptors is important for ovulation, embryo development, and preparation of the uterus for implantation [12,13,14]. Removal of the corpus luteum during the first trimester of pregnancy leads to miscarriage, whereas inappropriate production of progesterone is one of the main causes of preterm pregnancy losses in human [15]. Moreover, in some species such as dog, sheep, pig, and cow the corpus luteum persists and is necessary for most of pregnancy [16,17,18]. Therefore, appropriate function of the corpus luteum is crucial for pregnancy establishment and maintenance. Luteotropic and luteolytic factors precisely regulate luteal structure and function; yet, despite recent scientific progress within the past few years, the exact intracellular mechanisms controlling luteal function remain largely unknown. LH is the key luteotropic hormone that stimulates ovulation, luteal development, and maintenance of the corpus luteum. This pituitary gonadotropin induces not only the differentiation of the follicular cells into steroidogenic luteal cells, but also further stimulates progesterone biosynthesis in luteal cells. LH binds to LHCGR, a G-protein coupled, transmembrane receptor, present mainly on the steroidogenic small luteal cells. Canonical signaling by the LHCGR involves stimulation of adenylyl cyclase leading to increases in intracellular cyclic adenosine monophosphate (cAMP), activation of protein kinase A (PKA) and phosphorylation of PKA substrates, and ultimately stimulation of steroidogenesis. LH can also activate phospholipase C, leading to an increase in intracellular calcium and activation of mitogen-activated protein kinases (MAPK) [19,20]. Another luteotrophic and/or antiluteolytic factor is prostaglandin E2 (PGE2) that acts through four distinct G protein–coupled receptors (GPCRs), termed EP1–4, which activate signaling mechanisms similar to those produced in response to LH. PGE2 exerts a luteotrophic functions in multiple species, including the cow, pig, mare, rabbit, dog, monkey, and human [21,22,23,24]. In a reproductive cycle the corpus luteum terminates progesterone production and regresses in the absence of fertilization. Regression is triggered by a lack of luteotropic support and prostaglandin F2α (PGF2α), a key luteolytic factor that is produced within the gland or by the endometrium in domestic farm animals [16,25,26]. Notably, the early corpus luteum is insensitive to the luteolytic actions of PGF2α, but acquires luteolytic sensitivity later in the luteal phase in a species-specific manner [27,28,29]. Recent reviews provide excellent coverage of additional molecules that contribute to the formation, maintenance and regression of the corpus luteum [30,31,32,33].
Progesterone plays a vital role in fertility. Expression and activation of the progesterone receptor (PGR) is required for ovulation [14]. Furthermore, progesterone action is crucial for preparation of the uterine environment for implantation, embryo development and regulation of the estrous cycle [10,11]. The steroidogenic acute regulatory protein (STAR) mediates the rapid steroidogenic response to hormones by facilitating the transport of cholesterol across the outer mitochondria membrane to the inner mitochondria membrane (IMM). The cholesterol side-chain cleavage enzyme (CYP11A1) located within the inner mitochondria membrane, mediates the initial and rate-limiting step in steroidogenesis, converting cholesterol to pregnenolone [34]. Pregnenolone exits the mitochondria and is converted to progesterone by the enzyme 3β-hydroxysteroid dehydrogenase (HSD3B) located in the endoplasmic reticulum (ER). In the male, progesterone in further metabolized into testosterone by the enzymes cytochrome P450 Family 17 subfamily A member 1 (CYP17A1), and 17β-hydroxysteroid dehydrogenases (HSD17B) in the smooth ER [35]. Although steroidogenic luteal cells can synthesize cholesterol de novo [36], the majority of the cholesterol in luteal cells comes from the blood in the form of lipoproteins (LDL and/or HDL) [36]. Lipoproteins are internalized either through receptor-mediated endocytic or selective cellular uptake, where cholesterol is sorted from lipoproteins within endosomes. Transport of dietary cholesterol from endocytic organelles to the ER is essential for cholesterol homoeostasis, but the mechanism and regulation of this transport in luteal cells remains poorly defined [34,37,38].
In the present review, we summarize the recent progress in LH-triggered metabolic events in luteal cells. We review the contribution of various organelles and inter-organelle communication as a fundamental platform for the maintenance of basic luteal cell functions. The review also focuses on the role of LH on the activity of two main kinases (i.e., AMPK and MTOR) responsible for regulating metabolism, as well as metabolic events triggered downstream of 5′-AMP-activated protein kinase (AMPK) and mechanistic target of rapamycin (MTOR) in luteal cells. Due to the narrow focus of this review, the authors apologize for the inability to include all of the excellent contributions by other scientists studying the formation and regression of the corpus luteum.
2. Molecular Changes Occurring during Corpus Luteum Formation
Luteinization is a complex process characterized with rapid proliferation of endothelial cells, and cessation of proliferation and hypertrophy of the steroidogenic cells. In studies using a rat model, ovulatory doses of LH inhibited proliferation of granulosa cells [39]. Increased staining for a marker of proliferation (Ki67) was found among endothelial cells but not steroidogenic cells in the newly developed primate corpus luteum [40]. Similar observations were reported during luteal formation in cows, pigs, and sheep [41,42,43]. LH/PKA signaling triggers post-translational modifications or induction of early response transcription factors including phosphorylation of cAMP regulatory element binding protein (CREB), reproductive homeobox 5 (RHOX5) and early growth response-1 (EGR1), which cause multiple morphological and molecular changes [44,45,46,47,48]. Among the most prominent molecular changes triggered during luteinization are increased expression of mRNA transcripts for proteases (e.g., PTX3; ADAMTS1; CTS; VNN2; MMP9), as well as angiogenic (e.g., VEGFA; ANGPT; NOS3), or proinflammatory factors (e.g., PTGS2), which are crucial for tissue remodeling and formation of new vessels [23,48,49,50,51,52]. Higher expression of gene transcripts for lipoprotein uptake and cholesterol mobilization as well as steroidogenesis and progesterone signaling (e.g., LDLR, SCARB1, NPC-1, STAR, PGR) was also determined among LH triggered changes during luteinization [23]. In contrast, expression of factors involved in estradiol synthesis (e.g., CYP19A1), gonadotropin receptors (FSHR, LHCGR) or cell cycle (e.g., CDK2, CDK1, CDKN2, MCM3, MCM5, MCM6, CCNA2) was found to be downregulated during corpus luteum formation. Interestingly, proteomic analysis of preovulatory follicles of pigs revealed abundance of proteins involved in cellular infiltration, endoplasmic stress responses and protein ubiquitination. In addition, most of proteins in the newly formed corpus luteum were associated with steroid metabolism, cell death and survival, free radical scavenging, and protein ubiquitination pathways [53]. More cell specific transcriptomic characteristics were provided by Romereim et al., who determined expression of genes in the ovarian follicle cells and luteal cells [54]. Transcripts enriched in small and large luteal cells indicated increased metabolism/synthesis of lipids and steroids (cholesterol, eicosanoid, sterol, terpenoid, fatty acids andlipid membranes), cellular proliferation and survival, cell maturation, inflammatory and immune response, angiogenesis and migration of endothelial cells, and cell-to-cell signaling [54]. Among the predicted functions that were exclusive to the small luteal cells were metabolism of phospholipids, peptides, and sterols as well as regulation of the concentration of adenosine 5′-triphosphate (ATP). In large luteal cells, specific functions were related to adhesion (binding of cells, growth of epithelial tissue, and quantity of connective tissue) and cytoskeletal dynamics (microtubules and cell branching), molecular transport, development of blood cells, production of reactive oxygen species, and cellular homeostasis [54].
3. Morphological Characteristic of Small and Large Luteal Cells
In domestic farm animals like the cows, sheep and pigs, the small and large luteal cells are highly steroidogenic and differ based on the size, morphology, and function [55,56,57]. In the cow, the small luteal cells are 10–20 µm in diameter, possess a low cytoplasmic/nuclear ratio, and cytoplasm with mitochondria and numerous large lipid droplets. Large luteal cells are 30–40 µm in diameter, have a higher cytoplasmic/nuclear ratio, contain a well-developed, dense network of mitochondria and copious small lipid droplets dispersed through the cytoplasm (Figure 1 and Figure 2). Both small and large luteal cells are abundant in Golgi elements, granules, and ER or lysosomes [57]. Highly condensed chromatin is found at the nuclear periphery and in the central region of small luteal cells. However, in large luteal cells dispersed chromatin is found throughout the nucleus with condensed chromatin at the nuclear periphery [56,58,59]. The main functional differences between small and large luteal cells are that large luteal cells possess PGF2α receptor and produce 10- to 30-fold more progesterone than small luteal cells, whereas small luteal cells are highly responsive to treatment with LH and PKA activators [16,26,60,61].
Figure 1.
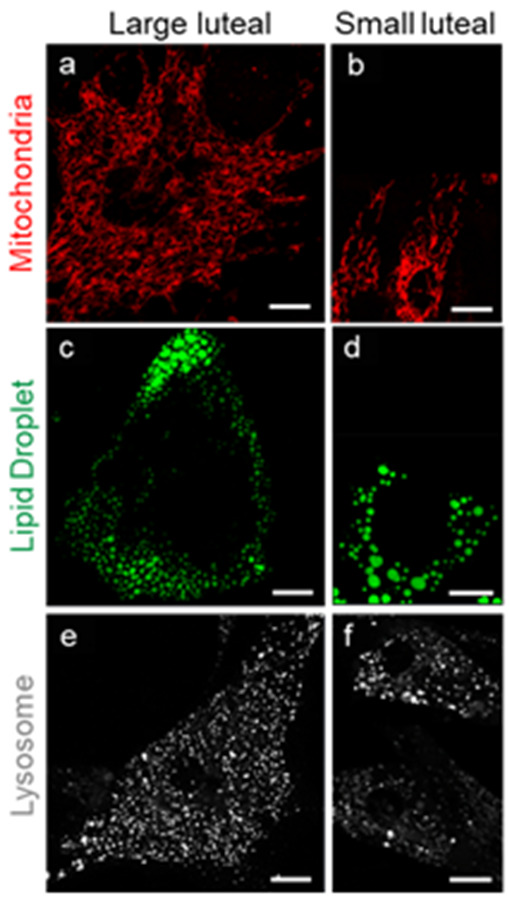
Organelle differences between steroidogenic large and small bovine luteal cells. Confocal microscopy was employed to visualize intracellular organelles in cultured bovine luteal cells. Representative micrograms of mitochondria ((a,b) MitoTracker); lipid droplets ((c,d) TopFluor Cholesterol);and lysosomes ((e,f) LysoTracker) in large and small luteal cells (left to right). Micron bar represents 10 µm.
Figure 2.
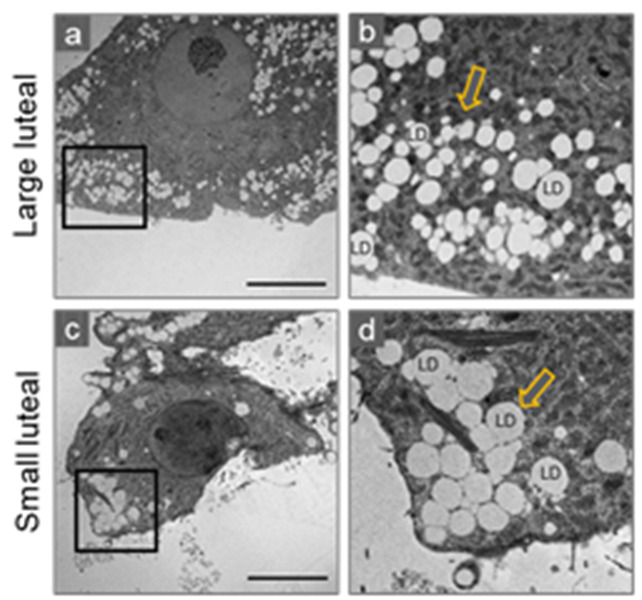
Distribution of lipid droplets in steroidogenic large and small bovine luteal cells. Transmission electron microscopy was employed to visualize intracellular lipid droplets (LD) in cultured bovine luteal cells. Representative micrograms of large (a,b) and small (c,d) luteal cells. Black box represents enlarged area (b,d). Arrows indicating LDs. Micron bar represents 10 µm.
4. Organelle Communication: Perspectives on Mitochondria, Endoplasmic Reticulum, Lipid Droplets, and Lysosomes in the Maintenance of Luteal Function
Intracellular organelles were once thought to function as individual units. However, recent progress in the understanding of membrane dynamics has revealed intracellular membrane compartments engage in extensive communication, either indirectly, or directly through membrane contacts, orchestrating dynamic communication between organelles that can be modulated to serve cellular requirements. Inter-organelle communication is fundamental for cell and tissue homeostasis as it regulates vital processes including calcium ions homeostasis, lipid and protein synthesis, metabolism, and cell death [62,63,64,65,66]. Inter-organelle communication also serves as platform for the control of signaling [67]. Here, we provide an overview of the mitochondria, ER, lipid droplets, and lysosomes in luteal cells and current information on inter-organelle communication and the functional roles of membrane contacts in ovarian steroidogenic cells.
4.1. Mitochondria
Mitochondria play a diverse role in cellular physiology from cell survival and metabolism, to initiating cell death mechanisms by producing free radicals and releasing apoptotic proteins [68]. These highly specialized organelles are bounded by two membranes, the outer and inner membranes, which function uniquely. The outer membrane contains protein-based pores regulating the passage of ions and molecules into the mitochondria, whereas the inner mitochondrial membrane is the active site for the electron transport chain and ATP production. Membrane integrity is crucial for mitochondrial function and depends on protein and phospholipid composition. Mitochondria are structurally dynamic organelles, continuously undergoing coordinated fission and fusion to sustain biological function [69]. In healthy cells, mitochondria maintain a highly regulated equilibrium between fusion and fission, forming either individual units or interconnected mitochondrial networks within the cell. The balance between mitochondrial fission and fusion often controls the overall fate of the cell [69,70]. In healthy cells, changes in mitochondrial morphology are under the control of fusion proteins [optic atrophy 1 (OPA1), mitofusin 1 (MFN1), and mitofusin 2 (MFN2)]; and fission proteins [dynamin 1 (DNM1), also known as dynamin related protein 1 (DRP1); dynamin 2 (DNM2); dynamin 1-like protein (DNM1L; commonly referred to as DRP1), mitochondrial fission factor (MFF), and fission 1 protein (FIS1)] [71,72,73,74]. These structural mitochondrial regulators are vital for proper development and tissue function. In vivo, Mfn1 or Mfn2 [75] or Opa1 [76] knockout mice are embryonic lethal as a result of insufficient mitochondrial fusion. Knockout of genes encoding Dnm2 [77] and Dnm1 [78] are also embryonic lethal; and DNM1L/DRP1 knockout mice die within two weeks of birth [79].
The dynamin family member DRP1 is a key mediator of outer mitochondrial fission. DRP1, a large dynamin GTPase, is recognized as the major player in mitochondrial fission in mammals [80], but the mechanism(s) by which DRP1 is regulated in steroidogenic tissues has only recently received attention. During mitochondrial fission, DRP1 is recruited to the mitochondria where it binds to its outer mitochondrial receptor, MFF, forming oligomeric complexes that surround to constrict and divide mitochondria. The regulation of DRP1 by post-translational modifications and differential phosphorylation is important for DRP1 translocation to mitochondria and induction of mitochondrial fission [81]. Phosphorylation of DRP1 at Ser616 by protein kinase C (PKC) [82] or cyclin dependent kinase (CDK) 1/cyclin B [83] results in activation and translocation of DRP1 from cytoplasmic compartment to mitochondrial membrane receptors. Moreover, phosphorylation of DRP1 on Ser616 mediates DRP1 interactions with other proteins and mitochondrial receptors to activate fission [84]. In contrast, phosphorylation of DRP1 at Ser637 within the GTPase effector domain (GED) domain inhibits DRP1 GTPase activity and translocation to mitochondria, thereby preventing mitochondrial fission [85,86,87]. The inactivation site of DRP1, Ser637, has a PKA consensus sequence and is widely accepted as a site for phosphorylation by PKA [86]. In the ovary, LH via PKA regulates the phosphorylation of DRP1 Ser637 in bovine luteal cells promoting optimal progesterone biosynthesis [88].
Genetic knockdown of DRP1 and MFF, but not fission protein FIS1, disrupts exogenous stimuli–induced mitochondrial fission and apoptosis in HeLa and MEF cells [89]. Recently, dynamin proteins have become novel targets to treat cardiovascular [90,91,92,93] and neurodegenerative diseases [94], as well as, cancer [95]. In small luteal cells genetic knockdown of MFF, the DRP1 mitochondria receptor, and a small molecule inhibitor of DRP1, Mdivi-1, increased both basal and LH-induced progesterone production [88]. These findings imply that LH may stabilize luteal mitochondria and promote cholesterol trafficking for steroidogenesis in bovine luteal cells via modulating the phosphorylation and activity of the mitochondrial fission protein DRP1.
Mitochondrial dynamin like GTPase (OPA1, also referred to as optic atrophy protein 1) is a mitochondrial dynamin related GTPase that is characterized as an inner mitochondria membrane fusion protein [96]. OPA1 is localized in the inner mitochondrial membrane and interacts with MFN1 and MFN2 to promote elongation by fusion of mitochondrial membranes. While previously described as a fusion protein, recent studies have emerged demonstrating that OPA1 has several additional important functions, such as stabilizing mitochondrial cristae, maintenance of mtDNA, and regulating the response of multiple tissues to apoptotic, necrotic, and atrophic stimuli [97].
Constitutive proteolytic processing of OPA1 by YME1 like 1 ATPase (YME1L) and/or OMA1 (zinc metallopeptidase) generates short (S-OPA1) and long (l-OPA1) isoforms that are released into the intermembrane space or tethered to the inner mitochondria membrane, respectively [98]. l-OPA1 contains a transmembrane domain and mediates mitochondrial fusion, while S-OPA1 takes on a soluble form and is involved in modulating mitochondrial energetics. Alterations to mitochondrial physiology, such as loss of mitochondrial membrane potential, ATP depletion or induction of apoptosis, leads to further proteolytically processing of l-OPA1 by OMA1 to generate the S-OPA1 isoform that is released into the intermembrane space. Regulation of OPA1 processing allows for alterations in the mitochondrial network through post-translational mechanisms that are more rapid than changes in gene expression [99]. In addition to sustaining mitochondrial homeostasis, this unique dynamin GTPase also serves as an A-kinase anchoring protein (AKAP). AKAPs act as scaffold proteins tethered to PKA and other signaling proteins such as phosphodiesterase, phosphatases and other protein kinases [100,101]. OPA1 has been shown to associate with lipid droplets, which are abundant in steroidogenic luteal cells [102]. In adipocytes, OPA1 associates with lipid droplets, serving as an AKAP, anchoring PKA for lipid hydrolysis. While the interaction between OPA1 and PKA on lipid droplets has been studied in adipocytes and adrenal cells [103,104], little is known about the role of OPA1 in ovary, specifically the corpus luteum. In small luteal cells, S-OPA1 is present on lipid droplets and interacts with PKA [105]. Interestingly, this interaction is amplified following acute LH-stimulation. Moreover, genetic knockdown of both, OPA1 and OMA1, using siRNA inhibits LH-induced progesterone synthesis, suggesting a critical role in steroid biosynthesis [106]. Additional studies are required to understand the processing, intra-cellular location, and role of OPA1 isoforms in steroidogenic cells.
4.2. Mitochondrial Associated Membranes
The mitochondria and ER are dynamic organelles that play a vital role in maintaining cellular homeostasis. Mitochondria are responsible for synthesizing ATP, maintaining intracellular of calcium ion (Ca2+) homeostasis and regulating apoptosis; while the ER is involved in protein folding, lipid metabolism and Ca2+ homeostasis [107,108,109,110]. Mitochondria and the ER continuously relay messages to maintain intracellular homeostasis and this communication is achieved by a physical association between the two organelles, forming a specialized microdomain termed mitochondria-associated membranes (MAMs) [111]. Tethering of mitochondria-ER membranes serves as a platform for cell signaling and scaffold for other proteins and regulatory factors [112,113,114].
Mitochondria-ER communication has been reported to increase upon hormone stimulation. Cholesterol synthesis occurs in the cytosol and ER through a multistep pathway beginning with acetyl-CoA. Newly synthesized cholesterol in the ER is rapidly transported to the plasma membrane by the secretory pathway and other organelles including the mitochondria and lipid droplets [111,115,116]. Mitochondria, however, are not part of the secretory pathway and, therefore, rely on lipid transport proteins to shuttle cholesterol from the ER. The AAA domain-containing protein 3 (ATAD3) is an essential mitochondrial protein that governs mitochondrial dynamics through the functional regulations of mitochondrial fission and fusion GTPases, DRP1 and OPA1, respectively. Of interest to luteal cells, ATAD3 has been identified as an essential component of the mitochondrial cholesterol transfer complex [117]. ATAD3A has the ability to assist with the transportation and metabolism of cholesterol by interacting with mitochondrial voltage-dependent anion channel (VDAC) and CYP11A1. The 67-kDa ATAD3 is anchored in the inner mitochondrial membrane and is enriched in at mitochondrial membrane contact sites [117]. Interestingly, in progesterone synthesizing cells, the 67-kDa long isoform of the ATPase family is also enriched in MAMs [118]. Although expression of ATAD3 is not hormonally regulated, ATAD3 localization participates in hormone-dependent steroidogenesis. Depletion of ATAD3 in mouse Leydig cells inhibits hormone-stimulated acute steroid biosynthesis, and this inhibition is recovered by treatment with 22R-hydroxycholesterol, a cell permeable metabolic cholesterol derivative [118]. This suggests ATAD3 plays a key role in the delivery of the substrate cholesterol into the mitochondria. A similar mechanism may occur in luteal cells, allowing direct delivery of substrate to the mitochondria for immediate progesterone production.
In steroidogenic cells, cholesterol is transported to the mitochondria by STAR, a transport protein that regulates cholesterol transfer within the mitochondria [37,119]. STAR is synthesized as a 37-kDa pre-protein that is further processed to a mature 30-kDa form. This is achieved by cleavage of an N-terminal mitochondrial import sequence. Recent studies have revealed voltage-dependent anion channel 2 (VDAC2) and translocase of outer membrane 22 (TOM22) are both involved in the regulation of STAR activity at the outer mitochondria membrane. In steroidogenic cells, STAR interacts with VDAC2 prior to its translocation to the mitochondrial matrix [117]. This interaction has been reported to occur at MAM sites and is a central location for initiating mitochondrial steroidogenesis [120]. In the absence of VDAC2, STAR is unable to interact with MAM-associated proteins and translocate into the mitochondria, halting steroid biosynthesis [120].
4.3. Mitochondria-Actin
Mitochondrial dynamics are dependent on interactions with microtubules and actin filaments, both components of the cellular cytoskeleton. Mitochondrial-cytoskeletal interactions have a well-established role in mitochondrial motility [121]. Actin cytoskeletal dynamics are regulated by the Rho GTPase family of proteins that consist of Rho, Rac, and Cdc42. During cell migration, Rho activation leads to the formation of stress fibers and retraction of the lagging edge of the cell during migration, whereas activation of Rac causes the formation of lamellipodia and membrane protrusions at the leading edge of the cell [122]. Members of the ADF/cofilin family of actin depolymerizing proteins are key regulators of actin dynamics and are considered essential for fundamental cellular processes [123]. In mammals, the ADF/cofilin family comprises three members: actin-depolymerizing factor (ADF, aka destrin), cofilin1, and cofilin2. Cofilin is regulated by cofilin binding molecules and differential phosphorylation and dephosphorylation. Phosphorylation of cofilin at Ser3 by LIM-kinases (LIMKs) and testicular protein kinases (TESKs) leads to inactivation whereas, dephosphorylation of cofilin by slingshot protein phosphatases (SSHs) leads to reactivation [123,124,125]. The signaling pathway initiated by hCG binding to the LHGCR leads to accumulation of intracellular cAMP and activation of PKA. In preovulatory granulosa cells, LH-induced PKA activation promotes rapid dephosphorylation of Ser3 of the actin-depolymerizing factor cofilin [126]. This dephosphorylation leads to rearrangement of the actin cytoskeleton and is required for progesterone biosynthesis [126,127].
4.4. Endoplasmic Reticulum (ER)
The ER is a large, dynamic organelle essential for maintaining cellular functions including Ca2+ storage, protein synthesis and lipid metabolism [128]. ER serves as a key site for protein synthesis of both secreted and integral membrane proteins, as well as a subpopulation of cytosolic proteins. Regulation of ER homeostasis plays vital roles in ovarian folliculogenesis, cumulus cells survival, cumulus–oocyte complex interactions and oocyte quality, luteal function, as well as pre-implantation embryo development [129,130,131,132].
ER homeostasis is sustained by ER chaperone proteins, glucose-regulated protein 78 (GRP78), heat shock protein 90 kDa beta member 1 (GRP94), calreticulin (CRT) and protein disulfide isomerase (PDI). ER stress occurs when the capacity of the ER to fold proteins becomes saturated, often as a result of impair protein glycosylation or disulfide bond formation, or by overexpression/mutations in proteins entering the secretory pathway [133,134]. In granulosa cells, increased follicular lipid accumulation has been reported to compromise ER function by activating ER stress pathways [135]. Activation of ER stress pathways attenuate hCG-stimulated PKA activation, and the expression and enzymatic activity of steroidogenic proteins, STAR and HSD3B, without affecting hCG-stimulated intracellular cAMP accumulation [136]. Moreover, induced ER stress reduces phosphorylation of PKA substrates responsible for cholesterol mobilization to the mitochondria leading to inhibition of progesterone biosynthesis. This increased activation of ER stress pathways has been shown to contribute to progesterone deficiency often associated with obesity [135,137]. ER stress has also been reported to contribute to the induction of profibrotic growth factors during ovarian fibrosis in polycystic ovary syndrome (PCOS) patients [138,139].
Recent studies demonstrated connection between metabolic hormones such as Visceral-adipose-tissue derived serine protease inhibitor (Vaspin) and ER homeostasis. Vaspin, also known as Serpin A12, is an adipokine that plays important roles in glucose metabolism and inflammation. Vaspin is a member of the serine protease family and binds to its receptor 78-kDa GRP78 ER chaperone. Like adiponectin and leptin, the level of vaspin are affected by gender, with significantly higher expression in females [140]. Recently, vaspin has been proposed to play a role in ovarian and luteal function. In the corpus luteum, vaspin and GRP78 expression are low during early luteal development and upregulated during the middle and late stages of the estrous cycles [141]. Interestingly, Kurowska et al. using immunohistochemistry, determined that vaspin and GRP78 were localized within the cytoplasm of both steroidogenic small and large luteal cells [141]. In vitro experiments reveal vaspin increases the expression of angiogenic and prolific genes, such as vascular endothelial growth factor (VEGFA), fibroblast growth factor 2 (FGF2), angiopoietin 1 (ANGPT1), VEGFA receptors (VEGFR1, VEGFR2), proliferating cells nuclear antigen (PCNA), and cyclin A, and decreases pro-apoptotic genes like caspase 3 and bcl-2-like protein 4 (BAX) in porcine luteal cells [141]. More research is required to determine how hormones and stress inducers impact the ER and ER-mitochondrial interactions in order to better understand the cellular control of steroidogenesis and luteal cell fate.
Lipid Droplets
Lipid droplets are unique organelles that serve as lipid reservoirs storing neutral lipids such as cholesterol esters and triglycerides [142,143,144]. Lipid droplets are coated with lipid droplet-associated proteins that embed within the surrounding phospholipid monolayer. These lipid droplet-associated proteins stabilize the droplet, interact with additional proteins that incorporate or remove lipids from the lipid droplet core, enable lipid droplets trafficking, and mediate association of lipid droplets with other organelles [145]. The perilipin (PLIN) proteins, designated PLIN1-5, are a family of lipid droplet coat proteins that are important for stabilizing lipid droplets structure and provide a platform for protein assembly on the lipid droplet surface. Although lipid droplets have been observed in nearly all tissues, lipid droplets have been most extensively studied in adipose cells, where they form large unilocular droplets [145]. Moreover, size, distribution, protein, and lipid content of lipid droplets can differ among various tissues [145]. Unraveling the dynamics and distinct characteristics of ovarian lipid droplets is key to fully understanding the role these lipid reservoirs in steroidogenesis and cell metabolism.
Ovarian luteal tissue is easily distinguished from other tissues due to the abundant lipid content and cytoplasmic lipid droplet [59]. Luteal droplets exist in all species examined to date including mice, rats, sheep, cattle, pigs, buffalo, rabbits, bats, non-human primates, and humans [146,147,148,149,150,151,152,153,154,155,156], and are abundant in both small and large luteal cells [59,157]. Bovine luteal cells contain higher levels of triglycerides than other tissues (cardiac, hepatic, & pulmonary) with the unsurprising exception of adipose tissue [59]. Additionally, the lipid droplets present in luteal cells are enriched with cholesterol esters and triglycerides that can be used to synthesize steroids in both humans and cattle [59,150,151,153]. The high triglyceride content of luteal tissue has unknown importance but could be the result of re-esterification of fatty acids liberated from cholesteryl esters during steroidogenesis to prevent fatty acid induced lipotoxicity. Interestingly, luteal lipid droplets are also enriched in key steroidogenic proteins required for optimal progesterone biosynthesis including STAR, vimentin, CYP11A1, and HSD3B. Moreover, acute stimulation with LH induces mobilization and/or increased expression of these key proteins to lipid droplets, suggesting a role of lipid droplets as signaling platforms for steroidogenesis [105].
Lipid droplets have a unique architecture enclosed by a phospholipid monolayer that is decorated by a specific set of proteins which forms the boundary between the aqueous cytosol and the hydrophobic inner lipid core. The PLIN family of lipid droplets coat proteins that are important for stabilizing lipid droplets structure and provide a platform for protein assembly on the lipid droplets surface. PLIN1 localizes to the surface of adipose tissue lipid droplets, however it is not clear whether PLIN1 binds at the surface or is embedded within the phospholipid monolayer. The activity of PLIN1 is regulated in a PKA-phosphorylation dependent manner, and its phosphorylation is known to rapidly initiate lipolysis. Adipocytes highly express PLIN1, a major lipid droplet binding protein, whereas PLIN1 is undetectable in lipid droplets of the bovine corpus luteum [59]. Luteal lipid droplets express PLIN2, PLIN3, PLIN5, hormone sensitive lipase, and 1-acylglycerol-3-phosphate O-acyltransferase, alpha beta hydrolase domain containing 5 and lower levels of adipose triglyceride lipase, and sterol O-acyltransferase proteins [59]. The presence of PLIN2, PLIN3, and PLIN5 suggest that luteal lipid droplets are metabolically active, oxidative, and mitochondrial-associated [145]. However, there appears to be a delicate balance between PLIN expression and luteal function. Preliminary studies from our laboratory indicate that overexpression of PLINs, 1, 2, or 3 change the size and distribution of luteal lipid droplets, detrimentally influencing acute LH-stimulated steroidogenesis [105].
Luteal lipid droplets are known to be regulated by luteal trophic hormones, such as LH, which mobilize lipid droplet cholesterol, and luteolytic hormones, such as PGF2α, which increase lipid droplet triglyceride content [156,158,159]. Hormone sensitive lipase (HSL) is an intracellular neutral lipase that hydrolyzes a variety of intracellular esters. It plays an essential role in lipid metabolism and is responsible for mediating the hydrolysis of di- and triacylglycerol, as well as cholesterol esters [160,161]. Hydrolysis of cholesterol esters by HSL in steroidogenic tissues provides substrate for the immediate synthesis of steroid hormones in adrenal glands, ovaries, and testis [58,162,163,164]. One of the unique features of HSL that differentiates it from other lipases is that its activity against triacylglycerol and cholesteryl ester substrates is regulated by reversible phosphorylation [160]. Structural studies have identified several amino acids and regions on HSL that are critical for regulating enzymatic activity. This has led to important insights into its function, including the hormone stimulated interactions with other key intracellular proteins, such as STAR [162] and lipid droplet-associated proteins, PLINs [165]. In luteal cells, LH/PKA signaling increases the hydrolytic activity of HSL by phosphorylation of a single site on Ser563, located within the regulatory module. Following cAMP/PKA dependent phosphorylation, HSL translocates to lipid droplets where it docks and interacts with other lipid droplet associated proteins. In adipocytes, the translocation of HSL to the lipid droplets occurs by virtue of PLIN1 localization to the surface of lipid droplets, physically interacting with HSL within the N-terminal region of PLIN1 to initiate the hydrolysis of cholesterol esters. This mechanism is not prominent in bovine luteal cells that express little PLIN1 [58,59]. Exactly how HSL associates with luteal lipid droplets is presently unknown.
Although HSL has broad substrate specificity, the kinetics of hydrolysis of neutral lipids and cholesterol esters vary based on fatty acid size and degree of unsaturation. Structurally, fatty acids consist of a carboxylic acid cap, a long aliphatic carbon chain, and hydrogen atoms along the length of the chain. In steroidogenic tissue, HSL more readily mobilizes fatty acids by hydrolysis as their chain length decrease (between 12–24 carbons) and degree of unsaturation increases [166,167]. The cholesteryl esters in bovine luteal lipid droplets are primarily (60%) mono- and polyunsaturated fatty acids evenly distributed among oleic acid (18:1), and the omega-3 and omega-6 20:4, 22:4 and 22:5 fatty acids [59]. This differs from ovine luteal cholesterol ester fatty acids which are predominantly palmitic acid [(16:0), 30.7%], oleic acid [(18:1) 22.3%], and linoleic acid [(18:2) 17.5%] [156]. Cholesteryl ester fatty acid content of rat luteal tissue had many similarities to those in bovine luteal lipid droplets, but contained more palmitic acid (16:0), and less oleic and arachidonic acids (18:1 and 20:4) content, and a reversed ratio of 18:0 to 18:1 fatty acids. The differences in fatty acid composition among these species could reflect species or diet effects [59,155,156,157]. Additional studies are needed to determine whether the fatty acids released upon HSL-induced cleavage of cholesterol esters enter luteal metabolic pathways or return to lipid droplets to be esterified into triglycerides.
4.5. Lipid Droplets and Mitochondria: Peri-Droplet Mitochondria
The notion that lipid droplets and mitochondria interact is well-supported. Luteal mitochondria also interact with lipid droplets at specific contact sites [58,59]. Proteomic analysis suggests that the luteal lipid droplets also interact with other key intracellular organelles involved in steroidogenesis and energy homeostasis, such as the ER, endosomes, and lysosomes [105]. Recently, a new subpopulation of lipid droplets interacting mitochondria have been described and may play a role in luteal steroidogenesis. Peri-droplet mitochondria have been reported in brown adipose tissue [168] and represent a segregated subpopulation of mitochondria that exhibit distinct composition, bioenergetics, and dynamics to support triglyceride synthesis. Peri-droplet mitochondria have higher levels of cytochrome c oxidase, ATP synthase, and super complex I + III assembly. Following stimulation of bovine luteal cells with analog of cAMP (8-BrcAMP), we observed an increase in proteins composing complex I and III (NDUA8, NDUA9, NDUBA, NDUS3, and NNRD), and ATP Synthase (ATPA, ATPB, ATPG, and ATP5H) [105] in isolated lipid droplets. Moreover, we reported an increased abundance of HADHA and HADHB, a mitochondrial trifunctional enzyme that catalyzes 75% of the reactions involved in the mitochondrial beta-oxidation pathway. This suggests following PKA activation, interactions between lipid droplets and mitochondria shift, promoting an intracellular environment for optimal steroidogenesis. One explanation for this rearrangement may be to prevent lipotoxicity. Fatty acids cleaved from cholesterol esters stored in lipid droplets may be quickly transported to a subpopulation of mitochondria capable of beta-oxidation during steroidogenesis.
4.6. Lysosomes
Lysosomes are the terminal degradative compartment of endocytosis, phagocytosis and autophagy [169]. Lysosomes regulate lipid metabolism through transcriptional as well as post-translational mechanisms. Lysosomes, once considered simple acidic organelles, are a hub for metabolic signaling regulated by different nutrient classes including amino acids and lipids. These dynamic organelles can integrate cellular signals and producing signal outputs. The lysosomal surface serves as a platform to assemble major signaling hubs, which relays multiple nutrient cues to the master growth regulators, MTOR, AMPK, and glycogen synthase kinase 3 beta (GSK3B) as reviewed further in Section 5.
Lysosomes play a dual role in both lipid transport and lipid biogenesis and integration of these two processes may play important roles in progesterone biosynthesis and luteal function [170,171]. One key role of lysosomes in steroidogenesis is mobilization of cholesterol from the lipoprotein complex. Cholesterol substrate for steroid biosynthesis in vivo is derived from internalized and degraded LDL and HDL. Drug inhibition of lysosomal functions using chloroquine, results in parallel inhibition of LDL degradation and progesterone production [172]. Cholesterol, the main substrate for progesterone synthesis, can also be provided by de novo synthesis in a multistep pathway from acetyl-CoA [37]. The reactions in the pathway occur in both the cytosol and ER. Newly synthesized cholesterol is rapidly transported from the ER to the plasma membrane and other organelles such as lipid droplets for storage or mitochondria for immediate steroid biosynthesis.
Lysosomes have been implicated in multiple events in the ovary, such as follicular and oocyte atresia, ovulation, steroidogenesis, and luteal regression [173,174,175]. Follicular atresia is an important process to ensure successful ovulation. Follicular atresia begins with the death of granulosa cells and finalizes with the degeneration of the oocyte. The pathway of granulosa cell death during follicular atresia depends on the state of energy metabolism related to follicular size. Small follicles result in changes in mitochondrial dynamics, whereas, lysosome function destabilizes in large follicles, resulting in major lysosomal rupture-induced necrosis.
Changes in lysosomes number and size, as well as enzyme activity have been implicated in late luteal regression in the porcine and bovine [176,177]. Lysosomes are important for luteal cell survival in addition to their well-established role in luteal regression. Mutations in TRPML1/Mcoln1, a lysosomal counter ion channel, reduced fertility in young and abolished fertility in old mice, despite normal mating activities [178]. Interestingly, corpus luteum formation was not impaired in old TRPML1/Mcoln1 mutant females; yet, progesterone biosynthesis was attenuated. This decrease in progesterone was accompanied by reduced expression of mitochondrial proteins, HSP60, and StAR, indicating impaired mitochondrial functions.
5. Metabolic Events Induced by LH in the Corpus Luteum: The Crosstalk of PKA/MTOR and AMPK Signaling in the Regulation of Metabolic Events in Luteal Cells
The maintenance of cell function requires utilization and metabolism of nutrients, which are an important source of proteins, lipids, amino acids, nucleotides, and energy. This is achieved through a network of well-synchronized cellular metabolic pathways that direct the utilization of various nutrients. Characterizing the principles underlying these cellular pathways can provide important insights into the understanding of cell function. Cellular metabolism is regulated by two main protein kinases i.e., AMPK and MTOR, which act in an antagonist manner. Both protein kinases are expressed in the granulosa and theca cells, oocytes and luteal cells [179,180,181,182,183]. For instance, AMPK activates catabolic processes such as autophagy, glucose transport, glycolysis, glycogen synthesis, or gluconeogenesis, whereas MTOR triggers anabolic processes including ribosome biogenesis and protein, nucleotide, fatty acid, and lipid synthesis [184,185]. The present section reviews how these key metabolic chaperones are regulated and the role of LH/PKA signaling on regulation of lipolysis, translation, and autophagy in luteal cells.
5.1. AMPK Attenuates Progesterone Production via Post-Translational Modifications of Enzymes Involved in Lipolysis in the Luteal Cells
AMPK is a trimeric protein composed of catalytic α subunit, scaffolding β subunit, and a regulatory γ subunit and is activated in response to reduced levels of ATP (elevated AMP/ATP ratio) [184]. Intracellular AMP/ADP directly bind to the regulatory γ subunit, inducing a conformational change that exposes an active phosphorylation site (Thr172) on the catalytic α subunit of AMPK (AMPKα) [184]. Phosphorylation of AMPKα at Thr172 is necessary for AMPK activation and can be triggered by upstream kinases, such as liver kinase B1 (LKB1) or in response to calcium flux via calcium/calmodulin dependent kinase kinase 2 (CAMKK2) [184,186]. Protein phosphatases (protein phosphatases 1 and 2) dephosphorylate AMPKα Thr172 leading to its inactivation. Moreover, PKA-dependent phosphorylation of AMPKα at Ser485 inhibits phosphorylation at Thr172, which limits AMPK activity [184].
AMPK regulates diverse metabolic events including inhibition of lipolysis and synthesis of fatty acids by phosphorylating HSL and acetyl-coA carboxylase alpha (ACACA) [184]. HSL interacts with lipid droplets and mediates the hydrolysis of cholesterol esters providing cholesterol substrate for immediate synthesis of steroid hormones [58,187]. ACACA catalyzes the carboxylation of acetyl-CoA to malonyl-CoA and is the rate-limiting enzyme in fatty acid synthesis. Phosphorylation of HSL at Ser565, a site specific for AMPK, prevents PKA-dependent phosphorylation and HSL activation [184,188]. Studies using bovine luteal cells showed that AMPK activity can be evoked by activating the PGF2α/CAMKK2 pathway leading to decreased progesterone production [179]. Moreover, activation of AMPK by AICAR (5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside) inhibits LH-stimulated progesterone production in bovine luteal cells [180,181]. Furthermore, AICAR-induced activation of AMPK triggers phosphorylation and inhibition of both ACACA (Ser79) and HSL (Ser565) in bovine luteal cells. Interestingly, the inhibitory effect of AICAR on LH-stimulated progesterone synthesis was ameliorated by incubating cells in the presence of 22-OH-cholesterol [181]. These findings suggest that AMPK acutely inhibits hormone-stimulated cholesterol mobilization in luteal cells, depriving cells of cholesterol the precursor for progesterone synthesis.
In contrast, treatment with LH increases the phosphorylation of HSL at Ser563 (activation site), a site specific for PKA, and decreases HSL phosphorylation at Ser565 (inactivation site). Additionally, LH prominently reduces phosphorylation of AMPKα at Thr172 (activation site), while simultaneously increasing the phosphorylation of AMPKα at Ser485 (inactivation site) [181]. These findings indicate that in luteal cells, AMPK, by virtue of its ability to inhibit HSL, acutely limits cholesterol mobilization from lipid droplets and inhibits steroid biosynthesis.
Metformin, an oral medication commonly used to treat type 2 diabetes, is a known activator of AMPK. Metformin is thought to activate AMPK by the inhibition of complex I of the electron transport chain leading to reductions in ATP production and increased content of AMP. The cellular bioavailability of metformin is controlled by membrane transporter proteins belonging to the solute carrier family (SLC) and multidrug resistant transporters of the superfamily of ATP-binding cassette (ABC) transporters [189]. In ovarian granulosa cells from multiple species, enhanced activity of AMPK in response to metformin decreased progesterone production by inhibiting transcription of steroidogenic genes, thus reducing the content of proteins involved in steroidogenesis [183,190,191]. In contrast, metformin did not affect progesterone production by luteal cells. Although, AICAR activation of AMPK prominently inhibited LH-stimulated progesterone production, it did not affect content of STAR, CYP11A1, and HSD3B in the luteal cells, pointing the importance of acute post-translational modifications mediated by AMPK in luteal steroidogenesis [181]. Interestingly, comparison of ovarian follicular cells to small and large luteal cells revealed reduced levels of transporters responsible for metformin uptake (SLC22A3) and increased transporters responsible for metformin secretion (ABCB1) in large and small luteal cells [54]. Thus, changes occurring during luteinization likely reduce the accumulation of metformin in luteal cells limiting its ability to activate AMPK. This may be beneficial for pregnant woman with diabetes who are treated with metformin.
5.2. MTOR Regulates Translation and Autophagy in the Luteal Cells
The MTOR is a conserved serine/threonine kinase, which forms two structurally and functionally distinct complexes i.e., MTORC1 containing MTOR, RPTOR (regulatory associated protein of MTOR complex 1 or raptor), MLST8 (MTOR associated protein, LST8 homolog), and PRAS40 (proline-rich AKT substrate of 40 kDa); and MTORC2 consisting of MTOR, RICTOR (RPTOR independent companion of MTOR complex 2), mLST8, and mSIN1 (also known as MAPK associated protein 1) [192,193]. MTORC1 regulates cell growth through modulating translation, in part by stimulating the phosphorylation of ribosomal protein S6 kinase beta-1 (S6K1) and eukaryotic translation initiation factor 4E binding protein (4EBP1), whereas MTORC2 regulates the phosphorylation of AKT on Ser473 and changes in the cytoskeletal that are involve in actin polymerization [194,195,196,197]. Both MTORC1 and MTORC2 are regulators of cellular metabolism. MTORC1 and S6K regulate metabolic enzymes directly, whereas mTORC2 evokes metabolic changes via AKT. S6K and AKT can also activate key metabolic transcriptional factors such as MYC proto-oncogene (MYC), hypoxia-inducible factor-1 (HIF-1), forkhead box (FOXO) transcription factors and sterol regulatory element binding transcription factor 1 (SREBF1) [185]. MTORC1 evokes various metabolic pathways including glucose metabolism via glycolysis and pentose phosphate pathway (PPP) as well as glutaminolysis and de novo lipogenesis in adipocytes, lymphocytes or cancer cells [185,193,198,199].
The activation of MTORC1 is dependent on nutrients and growth factors [185]. In the presence of nutrients such as amino acids (e.g., glutamine, arginine, leucine) MTORC1 translocates from the cytoplasm to the surface of lysosomes [198,200]. Activation of MTOR signaling is inhibited by the tuberous sclerosis complex (TSC), which consists of hamartin (TSC1) and tuberin (TSC2). TSC1 maintains the stability of the complex, whereas TSC2 functions as a GTPase and prevents the activation of MTOR by inhibition of the small G protein Ras homolog enriched in brain (RHEB) [201]. The activity of TSC2 is suppressed by growth factors or insulin via (PI3K)/AKT and/or MAPK1/3 signaling pathways leading to enhanced MTOR activity. In contrast, the activity of TSC2 can be evoked by AMPK and GSK3B resulting in a reduction in MTORC1 activity [202,203]. Studies on bovine luteal cells demonstrated that LH stimulates the phosphorylation and inactivation of GSK3B [180,204]. Additionally, LH/PKA pathway activates MTORC1 signaling reflected by increased activity of S6K1 and 4EBP1, proteins required for translation. LH mediated effects were independent on PI3K/AKT signaling since inhibition of PI3K/AKT signaling using wortmannin did not prevent the actions of LH on S6K1 phosphorylation. In contrast, treatment of luteal cells with insulin-like growth factor 1 (IGF-I) increased phosphorylation of the MTORC1 substrates S6K1 and 4EBP confirming that PI3K/AKT signaling is involved in S6K1 activity under basal conditions and in response to IGF-I but not to LH [180]. In rat granulosa cells, FSH stimulated PKA/MAPK signaling resulted in TSC2 phosphorylation leading to enhanced MTOR activity. In addition, inhibition of MTOR by treatment of granulosa cells with rapamycin decreased expression of cyclin D2, a critical factor regulating cell proliferation [205]. In human luteinized granulosa cells, rapamycin inhibited hCG-induced expression of steroidogenic genes (CYP11A1, HSD3B1 and STAR) as well as acute progesterone production [206]. Based on studies by Hou et al. [180] LH/PKA signaling stimulated MTOR activity in luteal cells enhancing phosphorylation of proteins related with translation but incubation with rapamycin did not affect basal or LH-stimulated progesterone production in bovine luteal cells. Thus, it seems that MTORC1 may have various roles in ovarian cells based on the state of cellular differentiation.
5.3. Autophagy
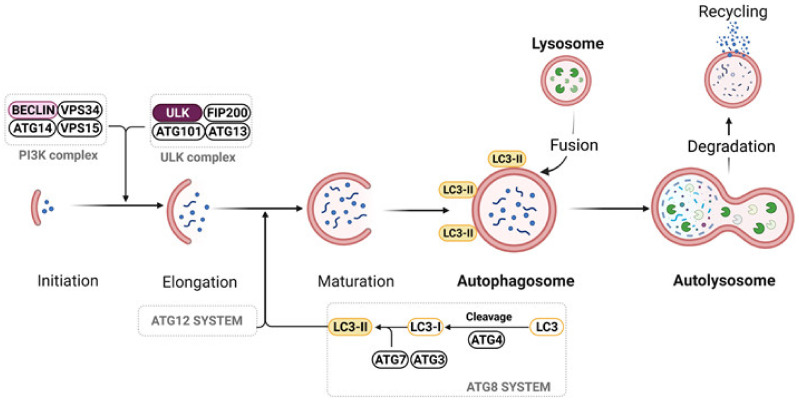
Autophagy, so called ‘self-eating’, is a process during which cells destroy damage or used organelles to recycle inter-cellular content and building blocks required to maintain basic cellular functions [207]. Autophagy occurs at a basal level in normal functioning cells and can be also induced under certain conditions, such as starvation, low oxygen levels, and growth factor withdrawal, among others. Due to differences in cargo, autophagy is divided into microautophagy, chaperone-mediated autophagy, and macroautophagy. During macroautophagy targeted cytoplasmic constituents are isolated from the rest of the cell within a double-membraned vesicle known as an autophagosome. The content of the autophagosome is degraded when the autophagosome fuses with lysosomes (Figure 3). Various subtypes of macroautophagy have been identified based on the content of the autophagosome, such as mitophagy, ribophagy, lipophagy, and ER-phagy. In microautophagy, the lysosomal surface directly engulfs the cytoplasm, which is further degraded, whereas in chaperon mediated autophagy the material designated for degradation fuses with lysosomal membrane directly [207,208].
Figure 3.
Process of autophagy. Autophagy is initiated by post-translational modifications in ULK1 or phosphatidylinositol-3-kinase (PI3K) complex. Autophagosomes elongation and maturation are catalyzed by ATG12 and ATG8 system, respectively. Formation of LC3-II occurs by proteolytic cleavage of pro-LC3 to LC3-I by ATG4, and then conjugation of LC3 family members to phosphatidylethanolamine (PE) on the surface of autophagosomes catalyzed by ATG7 and ATG3. Formed LC3B-II is a marker of autophagosomes. Mature autophagosomes fuse with lysosomes to form autolysosomes, which degrade cargo to amino acids, fatty acids or nucleotides that can be reused by cells. Created with BioRender.com. Adapted from “Autophagy In Cancer Pathways”, by BioRender.com (2021) (accessed on 1 September 2021). Retrieved from https://app.biorender.com/biorender-templates. accessed on 1 September 2021) [209].
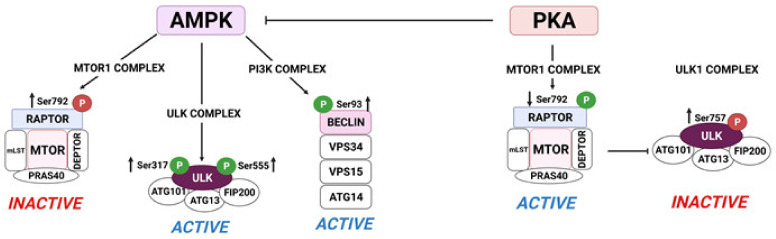
Autophagy is inversely regulated by the metabolic regulators, AMPK and MTOR. Activation of AMPK regulates autophagy at different levels including inactivation of MTOR via phosphorylation of RPTOR (Ser772 or Ser792) or TSC1/2 (Thr1227 or Ser1345) as well as direct activation of proteins related to autophagy induction and autophagosomes formation such as Unc-51-like kinase 1 (ULK1 Ser555 and Ser317) or (Ser93 or Ser15) (Figure 4). In addition, AMPK can also regulate the activity of transcription factor EB (TFEB) or FOXO3, nuclear transcription factors governing the expression of lysosomal and autophagic genes [208]. In contrast, MTOR inhibits autophagy by phosphorylation of ULK1 at Ser757, which disrupts the interaction between AMPK and ULK1 [210,211], thus preventing autophagosome formation [212,213].
Figure 4.
AMPK and PKA signaling oppositely regulate proteins crucial for autophagy initiation in bovine luteal cells. AMPK signaling stimulates autophagy initiation by (1) phosphorylation ULK1 at Ser317 and Ser555 leading to ULK1 activation; and (2) phosphorylation of RPTOR at Ser792 leading to MTOR inactivation. PKA signaling phosphorylates inhibits autophagy initiation by (1) phosphorylation of ULK1 at Ser757 and dephosphorylation of RPTOR at Ser792 allowing maintenance of MTOR activity. 5′-AMP-activated protein kinase (AMPK); protein kinase A (PKA); Unc-51-like kinase 1 (ULK1); regulatory associated protein of MTOR complex 1 (Raptor); MTOR associated protein, LST8 homolog (mLST8); proline-rich AKT substrate of 40 kDa (PRAS40); DEP domain containing MTOR interacting protein (DEPTOR); autophagy related 101 (ATG101); autophagy related 13 (ATG13); FAK family-interacting protein of 200 kDa (FIP200); autophagy related 14 (ATG14). Created with BioRender.com (accessed on 1 September 2021) [209]. “P” in the red circle represents phosphorylation site required for inactivation; “P” in the green circle means phosphorylation required for activation.
In the ovary, autophagy has been associated with follicle development and atresia as well as luteal formation and regression [174,176,214,215,216,217,218,219]. Recently, Tang et al. (2020) found that HIF1α enhances autophagy in the rat luteinized granulosa cells [217]. HIF1α is highly expressed in the granulosa cells of preovulatory follicles in various species [220]. HIF1α upregulated BNIP3 (BCL2 interacting protein 3) expression in rat luteinized granulosa cells, which contributed to autophagy initiation by displacing Beclin1 from the Bcl2/Beclin complex and at the same time protecting cells from apoptosis [217]. In the rat corpus luteum, expression of autophagy related proteins increases simultaneously with decreases in Akt/MTOR signaling [218]. Moreover, treatment with 3-methyl adenine (3-MA), an inhibitor of PI3K, inhibited progesterone synthesis and decreased the number of lipid droplets in luteal cells. On the other hand, Beclin1 flox/flox conditional knockout mice had corpora lutea at pregnancy day (P) 8.5 suggesting that Beclin1 is not required for luteal formation. In pregnant dams, pregnancy began 4 days earlier than in wild type animals and had lower progesterone level after P13.5. Interestingly, females with one floxed allele and one null allele did not give birth, and resorption occurred between P5.5 and P14.5. In addition, Beclin1 conditional knockout corpora lutea have reduced lipid stores compared to wild-type luteal cells [221]. Therefore, it is possible that autophagy can be related with formation of lipid droplets in the luteal cells and therefore affecting progesterone synthesis.
Many independent studies demonstrate the occurrence of autophagy during luteal regression [176]. In cows, elevated expression of genes associated with autophagy (LC3α, LC3β, ATG3, and ATG7) and protein LC3B-II was observed in corpora lutea during the late luteal phase in comparison to corpora lutea of mid-luteal phase. These findings are correlated with studies showing enhanced activity of lysosomes and decreased expression of MTOR in the late corpus luteum [176]. In the pig, the expression of Beclin1 and LAMP1, proteins specific for autophagosomes and lysosomes, respectively, was highest in the corpus luteum during late luteal phase [215]. Our recent data showed elevated content of LC3B in the luteal tissue during induced luteolysis [222]. Moreover, Choi et al. demonstrated that after 24 h of PGF2α treatment, both LC3B and caspase 3 are increased in cultured rat luteal cells suggesting that autophagy can be associated with cell death during regression [214]. It is known that autophagy may lead to apoptosis and necroptosis [223] and it is possible that ‘autophagy mediated cell-death’ occurs during structural regression of the corpus luteum. A common element between autophagy and apoptosis is the interaction among Beclin1 and proteins of the BCL2 family. For example, Bcl-2 can bind with Beclin1 and this interaction can be regulated by posttranslational modifications of both proteins by phosphorylation, ubiquitination, or caspase-mediated cleavage [224,225]. Phosphorylation of Bcl-2 or Beclin1 disrupts the interaction between Beclin1 and Bcl-2 leading to autophagy [226,227]. This protein complex can be also disrupted by pro-apoptotic proteins such as BNIPS, Bad, Noxa, Puma or anti-apoptotic proteins such as tBid and Bad leading to autophagy initiation [226]. Recently, Beclin1 was found to be involved in the regulation of ferroptosis, a form of cell death triggered by lipid peroxidation after inhibition of the cystine/glutamate antiporter system xc-. This antiporter system is formed by two proteins: SLC7A11 (solute carrier family 7 member 11; the catalytic subunit) and SLC3A2 (solute carrier family 3 member 2; an anchoring protein) and plays a role in maintaining an intracellular redox homeostasis by importing cystine, which is used to synthesize the major antioxidant glutathione (GSH). Impairment of the system xc–-dependent antioxidant defense system results in oxidative injury and cell death. Phosphorylation of Beclin1 at S90/93/96 by AMPK increases binding to SLC7A11, while AMPK inhibition and mutation of S90/93/96 compromised the pro-ferroptotic function of Beclin1 in human cancer cell lines [228]. Therefore, understanding the factors that regulate the luteal Beclin1 interactome can be helpful to elucidate the role of Beclin1 and Beclin1 mediated autophagy in the maintenance of luteal function.
Recent studies demonstrate that AMPK and MTOR1 differentially regulate autophagic signaling pathways in bovine luteal cells [211,213]. In bovine small luteal cells stimulation of AMPK, a known negative regulator of luteal steroidogenesis, increased phosphorylation of RAPTOR (Ser792), which generates a docking site for inhibitory 14-3-3 proteins and is required for AMPK-mediated inhibition of MTOR1 [229]. In addition, treatment of luteal cells with an AMPK activator enhanced phosphorylation of Beclin1 (Ser93) and ULK1 (Ser317), two proteins necessary for autophagy initiation [222]. The effects of AMPK on phosphorylation of RPTOR, ULK1, and Beclin1 were abolished after pretreatment of luteal cells with inhibitor of AMPK (compound C) (Figure 4). Moreover, incubation with the AMPK activator AICAR increased the luteal content of LC3B-II, staining for autophagosomes and colocalization of lysosomes with autophagosomes confirming that activation of AMPK enhances autophagy in the luteal cells. In contrast, incubation of luteal cells with LH decreased activation of AMPKα which was correlated with reduced phosphorylation of RPTOR (Ser792). LH also increased phosphorylation of MTOR (Ser2448) and the MTOR substrates S6K (Thr389) and ULK (Ser757). Similar effects were observed after incubation of luteal cells with forskolin, an activator of adenylyl cyclase, whereas pretreatment with an inhibitor of PKA abolished LH-mediated effects on phosphorylation of AMPKα, ULK1 (Ser757), and RAPTOR (Ser792) [222] (Figure 4). Treatment with LH also rapidly decreased the content of LC3B-II and staining of autophagosomes thus confirming that LH inhibits signaling related with autophagy induction and autophagosome formation. Therefore, it is plausible that LH/PKA/MTOR and AMPK signaling differentially regulate autophagy in the luteal cells.
6. Summary
The cyclical development and regression of ovarian follicles and corpora lutea in the ovary represent one of the most fascinating endocrine-controlled, developmental biology systems in the adult. Proper systemic and local controls of these processes ensure fertility and fetal development, or resumption of the ovarian cycle in the absence of pregnancy. To understand the mechanisms of action of hormones and other factors that control ovarian steroidogenesis and cell fate it is imperative to appreciate the contribution of various intracellular organelles and potential inter-organelle communication as a fundamental platform for regulation and maintenance of basic luteal cell functions.
Understanding the intracellular mechanism of action of hormones and other factors requires knowledge of the contributions of two vital proteins kinases (i.e., AMPK and MTOR) to ovarian cellular metabolism, inter-organelle communication and steroidogenesis.
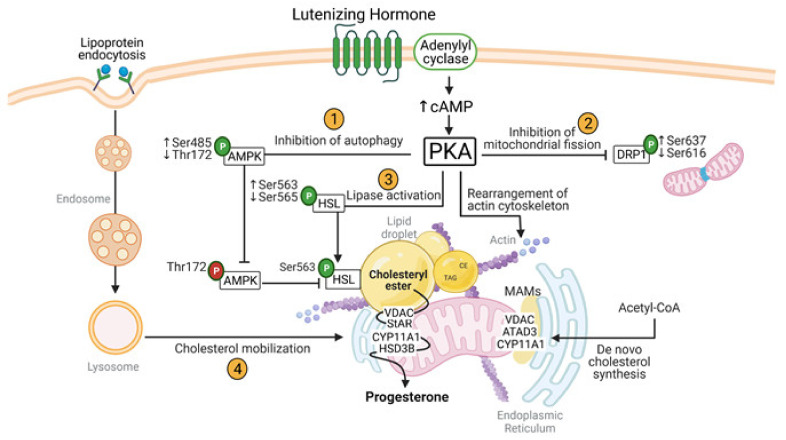
LH is a key luteotropic hormone that stimulates ovulation, luteal development, progesterone biosynthesis and maintenance of the corpus luteum. A summary of cellular and metabolic changes induced by LH in steroidogenic luteal cells is shown at Figure 5. LH via activation of its cognate cell surface receptor activates adenylyl cyclase, elevating cAMP and activating PKA. Intracellular signaling triggered by LH acutely (1) suppress autophagy, (2) stabilizes mitochondria, (3) activates lipolysis, and (4) stimulates cholesterol mobilization. Each of these events leading to steroidogenesis involves carefully orchestrated interactions among mitochondria, ER, lipid droplets, cytoskeletal fibers, lysosomes, and autophagosomes.
Figure 5.
Cellular and metabolic changes induced by LH in steroidogenic luteal cells. Illustration of essential intracellular signals regulating inter-organelle communication provoked by LH/PKA signaling for optimal progesterone biosynthesis. Cyclic adenosine monophosphate (cAMP); protein kinase A (PKA); hormone sensitive lipase (HSL); steroidogenic acute regulatory protein (STAR); cholesterol side-chain cleavage enzyme (CYP11A1); 3β-hydroxysteroid dehydrogenase (HSD3B); voltage-dependent anion channel (VDAC); AAA domain-containing protein 3 (ATAD3); 5′-AMP-activated protein kinase (AMPK); mitochondrial associated membranes (MAMs); triglyceride (TAG); cholesteryl ester (CE). Created with BioRender.com [209]. “P” in the green circle means phosphorylation site regulated by LH; “P” in the red circle means phosphorylation site required for AMPK activation. Up and down arrows mean increased and decreased phosphorylation, respectively.
Over the last several years, considerable new research by many laboratories has identified several key factors and signaling mechanisms that play crucial roles in the cyclical development and regression of ovarian follicles and corpora lutea. These studies have contributed to significant enhancement of the basic knowledge underlying the mechanisms of hormone action, aiding the development of novel clinical approaches to improve female fertility or manage infertility-associated disorders. However, many questions remain unanswered:
What is the repertoire of intracellular signaling events triggered by luteotropic and luteolytic agents? Where are these signals located in the cell and how do they affect cellular function?
Can advanced cellular and organelle imaging techniques be used to improve our understanding of inter-organelle communication? Can this information be used to better understand cellular metabolism and control of steroidogenesis?
Will analysis of the follicular and luteal proteome reveal actionable targets for improvement or control of fertility? Can we identify specific hormone-responsive protein kinases and their substrates in follicular and luteal cell types?
How do hormones control post-translational changes other than phosphorylation (methylation, acetylation, ubiquitylation, etc.) and what is their contribution to ovarian function?
What important cellular metabolic changes occur during the follicular to luteal transition? Can these be targeted to rescue or terminate luteal function?
What are the intracellular metabolic changes induced by luteotropic and luteolytic agents? Can metabolites be identified that can regulate steroidogenesis and cellular fate?
Author Contributions
E.P., M.R.P. and J.S.D. wrote and edited the review and approved the final version of the manuscript. Figures were conceived by E.P. and M.R.P. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by NIH grants R01 HD087402, R01 HD092263, P01 AG029531, USDA NIFA grants 2017-67015-26450 to J.S.D. and 2018-08068 to M.R.P., the Department of Veterans Affairs I01 BX004272 to J.S.D. and IK2 BX004911 to M.R.P., and the Olson Center for Women’s Health. J.S.D. is the recipient of the VA Senior Research Career Scientific Award IK6 BX005797.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Casarini L., Crépieux P. Molecular Mechanisms of Action of FSH. Front. Endocrinol. 2019;10:305. doi: 10.3389/fendo.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez C.G., Campbell B.K., Webb R. Development of a long-term bovine granulosa cell culture system: Induction and maintenance of estradiol production, response to follicle-stimulating hormone, and morphological characteristics. Biol. Reprod. 1997;56:608–616. doi: 10.1095/biolreprod56.3.608. [DOI] [PubMed] [Google Scholar]
- 3.Riccetti L., Sperduti S., Lazzaretti C., Casarini L., Simoni M. The cAMP/PKA pathway: Steroidogenesis of the antral follicular stage. Minerva Ginecol. 2018;70:516–524. doi: 10.23736/S0026-4784.18.04282-X. [DOI] [PubMed] [Google Scholar]
- 4.Abedel-Majed M.A., Romereim S.M., Davis J.S., Cupp A.S. Perturbations in Lineage Specification of Granulosa and Theca Cells May Alter Corpus Luteum Formation and Function. Front. Endocrinol. 2019;10:832. doi: 10.3389/fendo.2019.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meidan R., Girsh E., Blum O., Aberdam E. In vitro differentiation of bovine theca and granulosa cells into small and large luteal-like cells: Morphological and functional characteristics. Biol. Reprod. 1990;43:913–921. doi: 10.1095/biolreprod43.6.913. [DOI] [PubMed] [Google Scholar]
- 6.Sanders S.L., Stouffer R.L. Localization of steroidogenic enzymes in macaque luteal tissue during the menstrual cycle and simulated early pregnancy: Immunohistochemical evidence supporting the two-cell model for estrogen production in the primate corpus luteum. Biol. Reprod. 1997;56:1077–1087. doi: 10.1095/biolreprod56.5.1077. [DOI] [PubMed] [Google Scholar]
- 7.Shrestha K., Rodler D., Sinowatz F., Meidan R. Chapter 16–Corpus Luteum Formation. In: Leung P.C.K., Adashi E.Y., editors. The Ovary. 3rd ed. Academic Press; Cambridge, MA, USA: 2019. pp. 255–267. [Google Scholar]
- 8.Diskin M.G., Morris D.G. Embryonic and early foetal losses in cattle and other ruminants. Reprod. Domest. Anim. 2008;43:260–267. doi: 10.1111/j.1439-0531.2008.01171.x. [DOI] [PubMed] [Google Scholar]
- 9.Lonergan P. Influence of progesterone on oocyte quality and embryo development in cows. Theriogenology. 2011;76:1594–1601. doi: 10.1016/j.theriogenology.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 10.Pereira M.M., Mainigi M., Strauss J.F. Secretory products of the corpus luteum and preeclampsia. Hum. Reprod. Update. 2021;27:651–672. doi: 10.1093/humupd/dmab003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis J.S., Rueda B.R. The corpus luteum: An ovarian structure with maternal instincts and suicidal tendencies. Front. Biosci. 2002;7:d1949–d1978. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- 12.Daya S. Luteal support: Progestogens for pregnancy protection. Maturitas. 2009;65((Suppl. 1)):S29–S34. doi: 10.1016/j.maturitas.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Nardo L.G., Sallam H.N. Progesterone supplementation to prevent recurrent miscarriage and to reduce implantation failure in assisted reproduction cycles. Reprod. Biomed. Online. 2006;13:47–57. doi: 10.1016/S1472-6483(10)62015-9. [DOI] [PubMed] [Google Scholar]
- 14.Park C.J., Lin P.C., Zhou S., Barakat R., Bashir S.T., Choi J.M., Cacioppo J.A., Oakley O.R., Duffy D.M., Lydon J.P., et al. Progesterone Receptor Serves the Ovary as a Trigger of Ovulation and a Terminator of Inflammation. Cell Rep. 2020;31:107496. doi: 10.1016/j.celrep.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Csapo A.I., Pulkkinen M.O., Ruttner B., Sauvage J.P., Wiest W.G. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am. J. Obstet. Gynecol. 1972;112:1061–1067. doi: 10.1016/0002-9378(72)90181-0. [DOI] [PubMed] [Google Scholar]
- 16.Niswender G.D., Juengel J.L., Silva P.J., Rollyson M.K., McIntush E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000;80:1–29. doi: 10.1152/physrev.2000.80.1.1. [DOI] [PubMed] [Google Scholar]
- 17.Papa P.C., Kowalewski M.P. Factors affecting the fate of the canine corpus luteum: Potential contributors to pregnancy and non-pregnancy. Theriogenology. 2020;150:339–346. doi: 10.1016/j.theriogenology.2020.01.081. [DOI] [PubMed] [Google Scholar]
- 18.Stouffer R.L., Hennebold J.D. Chapter 23–Structure, Function, and Regulation of the Corpus Luteum. In: Plant T.M., Zeleznik A.J., editors. Knobil and Neill’s Physiology of Reproduction. 4th ed. Academic Press; San Diego, CA, USA: 2015. pp. 1023–1076. [Google Scholar]
- 19.Davis J.S., Weakland L.L., Farese R.V., West L.A. Luteinizing hormone increases inositol trisphosphate and cytosolic free Ca2+ in isolated bovine luteal cells. J. Biol. Chem. 1987;262:8515–8521. doi: 10.1016/S0021-9258(18)47444-3. [DOI] [PubMed] [Google Scholar]
- 20.Davis J.S., Weakland L.L., West L.A., Farese R.V. Luteinizing hormone stimulates the formation of inositol trisphosphate and cyclic AMP in rat granulosa cells. Evidence for phospholipase C generated second messengers in the action of luteinizing hormone. Biochem. J. 1986;238:597–604. doi: 10.1042/bj2380597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Janowski T., Fingerhut J., Kowalewski M.P., Zduńczyk S., Domosławska A., Jurczak A., Boos A., Schuler G., Hoffmann B. In vivo investigations on luteotropic activity of prostaglandins during early diestrus in nonpregnant bitches. Theriogenology. 2014;82:915–920. doi: 10.1016/j.theriogenology.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Gregoraszczuk E.L., Michas N. Progesterone and estradiol secretion by porcine luteal cells is influenced by individual and combined treatment with prostaglandins E2 and F2alpha throughout the estrus cycle. Prostaglandins Other Lipid Mediat. 1999;57:231–241. doi: 10.1016/S0090-6980(99)00009-X. [DOI] [PubMed] [Google Scholar]
- 23.Przygrodzka E., Kaczmarek M.M., Kaczynski P., Ziecik A.J. Steroid hormones, prostanoids, and angiogenic systems during rescue of the corpus luteum in pigs. Reproduction. 2016;151:135–147. doi: 10.1530/REP-15-0332. [DOI] [PubMed] [Google Scholar]
- 24.Arosh J.A., Banu S.K., McCracken J.A. Novel concepts on the role of prostaglandins on luteal maintenance and maternal recognition and establishment of pregnancy in ruminants. J. Dairy Sci. 2016;99:5926–5940. doi: 10.3168/jds.2015-10335. [DOI] [PubMed] [Google Scholar]
- 25.Skarzynski D.J., Okuda K. Inter- and intra-cellular mechanisms of prostaglandin F2alpha action during corpus luteum regression in cattle. Soc. Reprod. Fertil. Suppl. 2010;67:305–324. doi: 10.7313/upo9781907284991.025. [DOI] [PubMed] [Google Scholar]
- 26.Wiltbank M.C., Salih S.M., Atli M.O., Luo W., Bormann C.L., Ottobre J.S., Vezina C.M., Mehta V., Diaz F.J., Tsai S.J., et al. Comparison of endocrine and cellular mechanisms regulating the corpus luteum of primates and ruminants. Anim. Reprod. 2012;9:242–259. [PMC free article] [PubMed] [Google Scholar]
- 27.Boiti C., Canali C., Zerani M., Gobbetti A. Changes in refractoriness of rabbit corpora lutea to a prostaglandin F2 alpha analogue, alfaprostol, during pseudopregnancy. Prostaglandins Other Lipid Mediat. 1998;56:255–264. doi: 10.1016/S0090-6980(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 28.Diaz F.J., Crenshaw T.D., Wiltbank M.C. Prostaglandin f(2alpha) induces distinct physiological responses in porcine corpora lutea after acquisition of luteolytic capacity. Biol. Reprod. 2000;63:1504–1512. doi: 10.1095/biolreprod63.5.1504. [DOI] [PubMed] [Google Scholar]
- 29.Wiltbank M.C., Shiao T.F., Bergfelt D.R., Ginther O.J. Prostaglandin F2 alpha receptors in the early bovine corpus luteum. Biol. Reprod. 1995;52:74–78. doi: 10.1095/biolreprod52.1.74. [DOI] [PubMed] [Google Scholar]
- 30.Pate J.L. Roadmap to pregnancy during the period of maternal recognition in the cow: Changes within the corpus luteum associated with luteal rescue. Theriogenology. 2020;150:294–301. doi: 10.1016/j.theriogenology.2020.01.074. [DOI] [PubMed] [Google Scholar]
- 31.Stocco C., Telleria C., Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007;28:117–149. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 32.Zerani M., Polisca A., Boiti C., Maranesi M. Current Knowledge on the Multifactorial Regulation of Corpora Lutea Lifespan: The Rabbit Model. Animals. 2021;11:296. doi: 10.3390/ani11020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Galvão A.M., Skarzynski D., Ferreira-Dias G. Chapter Eleven–Luteolysis and the Auto-, Paracrine Role of Cytokines From Tumor Necrosis Factor α and Transforming Growth Factor β Superfamilies. In: Litwack G., editor. Vitamins and Hormones. Volume 107. Academic Press; Cambridge, MA, USA: 2018. pp. 287–315. [DOI] [PubMed] [Google Scholar]
- 34.Miller W.L. Steroidogenesis: Unanswered Questions. Trends Endocrinol. Metab. 2017;28:771–793. doi: 10.1016/j.tem.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Conley A.J., Bird I.M. The role of cytochrome P450 17 alpha-hydroxylase and 3 beta-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the delta 5 and delta 4 pathways of steroidogenesis in mammals. Biol. Reprod. 1997;56:789–799. doi: 10.1095/biolreprod56.4.789. [DOI] [PubMed] [Google Scholar]
- 36.Gwynne J.T., Strauss J.F., 3rd The role of lipoproteins in steroidogenesis and cholesterol metabolism in steroidogenic glands. Endocr. Rev. 1982;3:299–329. doi: 10.1210/edrv-3-3-299. [DOI] [PubMed] [Google Scholar]
- 37.Christenson L.K., Devoto L. Cholesterol transport and steroidogenesis by the corpus luteum. Reprod. Biol. Endocrinol. 2003;1:90. doi: 10.1186/1477-7827-1-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller W.L. Disorders in the initial steps of steroid hormone synthesis. J. Steroid Biochem. Mol. Biol. 2017;165:18–37. doi: 10.1016/j.jsbmb.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Rao M.C., Midgley A.R., Jr., Richards J.S. Hormonal regulation of ovarian cellular proliferation. Cell. 1978;14:71–78. doi: 10.1016/0092-8674(78)90302-1. [DOI] [PubMed] [Google Scholar]
- 40.Christenson L.K., Stouffer R.L. Proliferation of microvascular endothelial cells in the primate corpus luteum during the menstrual cycle and simulated early pregnancy. Endocrinology. 1996;137:367–374. doi: 10.1210/endo.137.1.8536637. [DOI] [PubMed] [Google Scholar]
- 41.Murphy B.D., Gévry N., Ruiz-Cortés T., Coté F., Downey B.R., Sirois J. Formation and early development of the corpus luteum in pigs. Reprod. Suppl. 2001;58:47–63. doi: 10.1530/biosciprocs.16.0004. [DOI] [PubMed] [Google Scholar]
- 42.Farin C.E., Moeller C.L., Sawyer H.R., Gamboni F., Niswender G.D. Morphometric analysis of cell types in the ovine corpus luteum throughout the estrous cycle. Biol. Reprod. 1986;35:1299–1308. doi: 10.1095/biolreprod35.5.1299. [DOI] [PubMed] [Google Scholar]
- 43.Zheng J., Fricke P.M., Reynolds L.P., Redmer D.A. Evaluation of growth, cell proliferation, and cell death in bovine corpora lutea throughout the estrous cycle. Biol. Reprod. 1994;51:623–632. doi: 10.1095/biolreprod51.4.623. [DOI] [PubMed] [Google Scholar]
- 44.Espey L.L., Ujioka T., Russell D.L., Skelsey M., Vladu B., Robker R.L., Okamura H., Richards J.S. Induction of early growth response protein-1 gene expression in the rat ovary in response to an ovulatory dose of human chorionic gonadotropin. Endocrinology. 2000;141:2385–2391. doi: 10.1210/endo.141.7.7582. [DOI] [PubMed] [Google Scholar]
- 45.MacLean J.A., 2nd, Rao M.K., Doyle K.M., Richards J.S., Wilkinson M.F. Regulation of the Rhox5 homeobox gene in primary granulosa cells: Preovulatory expression and dependence on SP1/SP3 and GABP. Biol. Reprod. 2005;73:1126–1134. doi: 10.1095/biolreprod.105.042747. [DOI] [PubMed] [Google Scholar]
- 46.Richards J.S., Russell D.L., Robker R.L., Dajee M., Alliston T.N. Molecular mechanisms of ovulation and luteinization. Mol. Cell Endocrinol. 1998;145:47–54. doi: 10.1016/S0303-7207(98)00168-3. [DOI] [PubMed] [Google Scholar]
- 47.Wissing M.L., Kristensen S.G., Andersen C.Y., Mikkelsen A.L., Høst T., Borup R., Grøndahl M.L. Identification of new ovulation-related genes in humans by comparing the transcriptome of granulosa cells before and after ovulation triggering in the same controlled ovarian stimulation cycle. Hum. Reprod. 2014;29:997–1010. doi: 10.1093/humrep/deu008. [DOI] [PubMed] [Google Scholar]
- 48.Xu J., Stouffer R.L., Searles R.P., Hennebold J.D. Discovery of LH-regulated genes in the primate corpus luteum. Mol. Hum. Reprod. 2005;11:151–159. doi: 10.1093/molehr/gah157. [DOI] [PubMed] [Google Scholar]
- 49.Espey L.L., Richards J.S. Temporal and spatial patterns of ovarian gene transcription following an ovulatory dose of gonadotropin in the rat. Biol. Reprod. 2002;67:1662–1670. doi: 10.1095/biolreprod.102.005173. [DOI] [PubMed] [Google Scholar]
- 50.Kfir S., Basavaraja R., Wigoda N., Ben-Dor S., Orr I., Meidan R. Genomic profiling of bovine corpus luteum maturation. PLoS ONE. 2018;13:e0194456. doi: 10.1371/journal.pone.0194456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Agca C., Ries J.E., Kolath S.J., Kim J.H., Forrester L.J., Antoniou E., Whitworth K.M., Mathialagan N., Springer G.K., Prather R.S., et al. Luteinization of porcine preovulatory follicles leads to systematic changes in follicular gene expression. Reproduction. 2006;132:133–145. doi: 10.1530/rep.1.01163. [DOI] [PubMed] [Google Scholar]
- 52.Christenson L.K., Gunewardena S., Hong X., Spitschak M., Baufeld A., Vanselow J. Research resource: Preovulatory LH surge effects on follicular theca and granulosa transcriptomes. Mol. Endocrinol. 2013;27:1153–1171. doi: 10.1210/me.2013-1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Likszo P., Skarzynski D.J., Moza Jalali B. Proteomic Analysis of Porcine Pre-ovulatory Follicle Differentiation Into Corpus Luteum. Front. Endocrinol. 2019;10:774. doi: 10.3389/fendo.2019.00774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romereim S.M., Summers A.F., Pohlmeier W.E., Zhang P., Hou X., Talbott H.A., Cushman R.A., Wood J.R., Davis J.S., Cupp A.S. Transcriptomes of bovine ovarian follicular and luteal cells. Data Brief. 2017;10:335–339. doi: 10.1016/j.dib.2016.11.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fitz T.A., Mayan M.H., Sawyer H.R., Niswender G.D. Characterization of two steroidogenic cell types in the ovine corpus luteum. Biol. Reprod. 1982;27:703–711. doi: 10.1095/biolreprod27.3.703. [DOI] [PubMed] [Google Scholar]
- 56.Chegini N., Ramani N., Rao C.V. Morphological and biochemical characterization of small and large bovine luteal cells during pregnancy. Mol. Cell Endocrinol. 1984;37:89–102. doi: 10.1016/0303-7207(84)90131-X. [DOI] [PubMed] [Google Scholar]
- 57.Fields M.J., Dubois W., Fields P.A. Dynamic features of luteal secretory granules: Ultrastructural changes during the course of pregnancy in the cow. Endocrinology. 1985;117:1675–1682. doi: 10.1210/endo-117-4-1675. [DOI] [PubMed] [Google Scholar]
- 58.Plewes M.R., Krause C., Talbott H.A., Przygrodzka E., Wood J.R., Cupp A.S., Davis J.S. Trafficking of cholesterol from lipid droplets to mitochondria in bovine luteal cells: Acute control of progesterone synthesis. FASEB J. 2020;34:10731–10750. doi: 10.1096/fj.202000671R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Talbott H.A., Plewes M.R., Krause C., Hou X., Zhang P., Rizzo W.B., Wood J.R., Cupp A.S., Davis J.S. Formation and characterization of lipid droplets of the bovine corpus luteum. Sci. Rep. 2020;10:11287. doi: 10.1038/s41598-020-68091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Alila H.W., Dowd J.P., Corradino R.A., Harris W.V., Hansel W. Control of progesterone production in small and large bovine luteal cells separated by flow cytometry. J. Reprod. Fertil. 1988;82:645–655. doi: 10.1530/jrf.0.0820645. [DOI] [PubMed] [Google Scholar]
- 61.Mamluk R., Chen D., Greber Y., Davis J.S., Meidan R. Characterization of messenger ribonucleic acid expression for prostaglandin F2 alpha and luteinizing hormone receptors in various bovine luteal cell types. Biol. Reprod. 1998;58:849–856. doi: 10.1095/biolreprod58.3.849. [DOI] [PubMed] [Google Scholar]
- 62.Vallese F., Barazzuol L., Maso L., Brini M., Calì T. ER-Mitochondria Calcium Transfer, Organelle Contacts and Neurodegenerative Diseases. Adv. Exp. Med. Biol. 2020;1131:719–746. doi: 10.1007/978-3-030-12457-1_29. [DOI] [PubMed] [Google Scholar]
- 63.Rimessi A., Pedriali G., Vezzani B., Tarocco A., Marchi S., Wieckowski M.R., Giorgi C., Pinton P. Interorganellar calcium signaling in the regulation of cell metabolism: A cancer perspective. Semin. Cell Dev. Biol. 2020;98:167–180. doi: 10.1016/j.semcdb.2019.05.015. [DOI] [PubMed] [Google Scholar]
- 64.Ikonen E., Zhou X. Cholesterol transport between cellular membranes: A balancing act between interconnected lipid fluxes. Dev. Cell. 2021;56:1430–1436. doi: 10.1016/j.devcel.2021.04.025. [DOI] [PubMed] [Google Scholar]
- 65.Hariri H., Ugrankar R., Liu Y., Henne W.M. Inter-organelle ER-endolysosomal contact sites in metabolism and disease across evolution. Commun. Integr. Biol. 2016;9:e1156278. doi: 10.1080/19420889.2016.1156278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Grimm S. The ER-mitochondria interface: The social network of cell death. Biochim. Biophys. Acta. 2012;1823:327–334. doi: 10.1016/j.bbamcr.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 67.Pesaresi P., Schneider A., Kleine T., Leister D. Interorganellar communication. Curr. Opin. Plant Biol. 2007;10:600–606. doi: 10.1016/j.pbi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 68.Bock F.J., Tait S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020;21:85–100. doi: 10.1038/s41580-019-0173-8. [DOI] [PubMed] [Google Scholar]
- 69.Dhingra R., Kirshenbaum L.A. Regulation of mitochondrial dynamics and cell fate. Circ. J. 2014;78:803–810. doi: 10.1253/circj.CJ-14-0240. [DOI] [PubMed] [Google Scholar]
- 70.Yu R., Lendahl U., Nistér M., Zhao J. Regulation of Mammalian Mitochondrial Dynamics: Opportunities and Challenges. Front. Endocrinol. 2020;11:374. doi: 10.3389/fendo.2020.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 72.Roy M., Reddy P.H., Iijima M., Sesaki H. Mitochondrial division and fusion in metabolism. Curr. Opin. Cell Biol. 2015;33:111–118. doi: 10.1016/j.ceb.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramachandran R. Mitochondrial dynamics: The dynamin superfamily and execution by collusion. Semin. Cell Dev. Biol. 2018;76:201–212. doi: 10.1016/j.semcdb.2017.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schrepfer E., Scorrano L. Mitofusins, from Mitochondria to Metabolism. Mol. Cell. 2016;61:683–694. doi: 10.1016/j.molcel.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 75.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen L., Liu T., Tran A., Lu X., Tomilov A.A., Davies V., Cortopassi G., Chiamvimonvat N., Bers D.M., Votruba M., et al. OPA1 mutation and late-onset cardiomyopathy: Mitochondrial dysfunction and mtDNA instability. J. Am. Heart Assoc. 2012;1:e003012. doi: 10.1161/JAHA.112.003012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ferguson S., Raimondi A., Paradise S., Shen H., Mesaki K., Ferguson A., Destaing O., Ko G., Takasaki J., Cremona O., et al. Coordinated Actions of Actin and BAR Proteins Upstream of Dynamin at Endocytic Clathrin-Coated Pits. Dev. Cell. 2009;17:811–822. doi: 10.1016/j.devcel.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wakabayashi J., Zhang Z., Wakabayashi N., Tamura Y., Fukaya M., Kensler T.W., Iijima M., Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J. Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ferguson S.M., Brasnjo G., Hayashi M., Wolfel M., Collesi C., Giovedi S., Raimondi A., Gong L.W., Ariel P., Paradise S., et al. A selective activity-dependent requirement for dynamin 1 in synaptic vesicle endocytosis. Science. 2007;316:570–574. doi: 10.1126/science.1140621. [DOI] [PubMed] [Google Scholar]
- 80.Smirnova E., Shurland D.L., Ryazantsev S.N., van der Bliek A.M. A human dynamin-related protein controls the distribution of mitochondria. J. Cell Biol. 1998;143:351–358. doi: 10.1083/jcb.143.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santel A., Frank S. Shaping mitochondria: The complex posttranslational regulation of the mitochondrial fission protein DRP1. IUBMB Life. 2008;60:448–455. doi: 10.1002/iub.71. [DOI] [PubMed] [Google Scholar]
- 82.Zaja I., Bai X., Liu Y., Kikuchi C., Dosenovic S., Yan Y., Canfield S.G., Bosnjak Z.J. Cdk1, PKCδ and calcineurin-mediated Drp1 pathway contributes to mitochondrial fission-induced cardiomyocyte death. Biochem. Biophys. Res. Commun. 2014;453:710–721. doi: 10.1016/j.bbrc.2014.09.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Taguchi N., Ishihara N., Jofuku A., Oka T., Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J. Biol. Chem. 2007;282:11521–11529. doi: 10.1074/jbc.M607279200. [DOI] [PubMed] [Google Scholar]
- 84.Wu Q., Luo C.L., Tao L.Y. Dynamin-related protein 1 (Drp1) mediating mitophagy contributes to the pathophysiology of nervous system diseases and brain injury. Histol. Histopathol. 2017;32:551–559. doi: 10.14670/HH-11-841. [DOI] [PubMed] [Google Scholar]
- 85.Nakamura T., Cieplak P., Cho D.-H., Godzik A., Lipton S.A. S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration. Mitochondrion. 2010;10:573–578. doi: 10.1016/j.mito.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang C.-R., Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J. Biol. Chem. 2007;282:21583–21587. doi: 10.1074/jbc.C700083200. [DOI] [PubMed] [Google Scholar]
- 87.Chang C.R., Blackstone C. Drp1 phosphorylation and mitochondrial regulation. EMBO Rep. 2007;8:1088–1089. doi: 10.1038/sj.embor.7401118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Plewes M.R., Hou X., Talbott H.A., Zhang P., Wood J.R., Cupp A.S., Davis J.S. Luteinizing hormone regulates the phosphorylation and localization of the mitochondrial effector dynamin-related protein-1 (DRP1) and steroidogenesis in the bovine corpus luteum. FASEB J. 2020;34:5299–5316. doi: 10.1096/fj.201902958R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ong S.B., Kalkhoran S.B., Cabrera-Fuentes H.A., Hausenloy D.J. Mitochondrial fusion and fission proteins as novel therapeutic targets for treating cardiovascular disease. Eur. J. Pharmacol. 2015;763:104–114. doi: 10.1016/j.ejphar.2015.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hall A.R., Burke N., Dongworth R.K., Hausenloy D.J. Mitochondrial fusion and fission proteins: Novel therapeutic targets for combating cardiovascular disease. Br. J. Pharmacol. 2014;171:1890–1906. doi: 10.1111/bph.12516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marsboom G., Toth P.T., Ryan J.J., Hong Z., Wu X., Fang Y.H., Thenappan T., Piao L., Zhang H.J., Pogoriler J., et al. Dynamin-related protein 1-mediated mitochondrial mitotic fission permits hyperproliferation of vascular smooth muscle cells and offers a novel therapeutic target in pulmonary hypertension. Circ. Res. 2012;110:1484–1497. doi: 10.1161/CIRCRESAHA.111.263848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sharp W.W., Fang Y.H., Han M., Zhang H.J., Hong Z., Banathy A., Morrow E., Ryan J.J., Archer S.L. Dynamin-related protein 1 (Drp1)-mediated diastolic dysfunction in myocardial ischemia-reperfusion injury: Therapeutic benefits of Drp1 inhibition to reduce mitochondrial fission. FASEB J. 2014;28:316–326. doi: 10.1096/fj.12-226225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nakamura T., Lipton S.A. Protein S-Nitrosylation as a Therapeutic Target for Neurodegenerative Diseases. Trends Pharmacol. Sci. 2016;37:73–84. doi: 10.1016/j.tips.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou J., Li G., Zheng Y., Shen H.-M., Hu X., Ming Q.-L., Huang C., Li P., Gao N. A novel autophagy/mitophagy inhibitor liensinine sensitizes breast cancer cells to chemotherapy through DNM1L-mediated mitochondrial fission. Autophagy. 2015;11:1259–1279. doi: 10.1080/15548627.2015.1056970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Olichon A., Emorine L.J., Descoins E., Pelloquin L., Brichese L., Gas N., Guillou E., Delettre C., Valette A., Hamel C.P., et al. The human dynamin-related protein OPA1 is anchored to the mitochondrial inner membrane facing the inter-membrane space. FEBS Lett. 2002;523:171–176. doi: 10.1016/S0014-5793(02)02985-X. [DOI] [PubMed] [Google Scholar]
- 97.Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G.V., Rudka T., Bartoli D., Polishuck R.S., Danial N.N., De Strooper B., et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 98.McBride H., Soubannier V. Mitochondrial function: OMA1 and OPA1, the grandmasters of mitochondrial health. Curr. Biol. 2010;20:R274–R276. doi: 10.1016/j.cub.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 99.MacVicar T., Langer T. OPA1 processing in cell death and disease–the long and short of it. J. Cell Sci. 2016;129:2297–2306. doi: 10.1242/jcs.159186. [DOI] [PubMed] [Google Scholar]
- 100.Wong W., Scott J.D. AKAP signalling complexes: Focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 101.Dodge-Kafka K.L., Langeberg L., Scott J.D. Compartmentation of cyclic nucleotide signaling in the heart: The role of A-kinase anchoring proteins. Circ. Res. 2006;98:993–1001. doi: 10.1161/01.RES.0000218273.91741.30. [DOI] [PubMed] [Google Scholar]
- 102.Pidoux G., Witczak O., Jarnaess E., Myrvold L., Urlaub H., Stokka A.J., Kuntziger T., Tasken K. Optic atrophy 1 is an A-kinase anchoring protein on lipid droplets that mediates adrenergic control of lipolysis. EMBO J. 2011;30:4371–4386. doi: 10.1038/emboj.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogne M., Chu D.T., Küntziger T.M., Mylonakou M.N., Collas P., Tasken K. OPA1-anchored PKA phosphorylates perilipin 1 on S522 and S497 in adipocytes differentiated from human adipose stem cells. Mol. Biol. Cell. 2018;29:1487–1501. doi: 10.1091/mbc.E17-09-0538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fülöp L., Rajki A., Katona D., Szanda G., Spät A. Extramitochondrial OPA1 and adrenocortical function. Mol. Cell Endocrinol. 2013;381:70–79. doi: 10.1016/j.mce.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 105.Plewes M.R., Davis J.S. University of Nebraska Medical Center; Omaha, NE, USA: 2021. Unpublished work. to be submitted. [Google Scholar]
- 106.Plewes M.R., Zhang P., Hou X., Davis J.S. Effect of Mitochondrial Dynamin Like GTPase on Steroidogenesis in the Corpus Luteum; Proceedings of the 52nd Annual Meeting of the Society for the Study of Reproduction; San Jose, CA, USA. 18–21 July 2019. [Google Scholar]
- 107.Skulachev V.P. Functions of mitochondria: From intracellular power stations to mediators of a senescence program. Cell Mol. Life Sci. 2009;66:1785–1793. doi: 10.1007/s00018-009-9183-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kleizen B., Braakman I. Protein folding and quality control in the endoplasmic reticulum. Curr. Opin. Cell Biol. 2004;16:343–349. doi: 10.1016/j.ceb.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 109.Fagone P., Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J. Lipid Res. 2009;50:S311–S316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Di Mattia T., Tomasetto C., Alpy F. Faraway, so close! Functions of Endoplasmic reticulum-Endosome contacts. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2020;1865:158490. doi: 10.1016/j.bbalip.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 111.Hayashi T., Rizzuto R., Hajnoczky G., Su T.P. MAM: More than just a housekeeper. Trends Cell Biol. 2009;19:81–88. doi: 10.1016/j.tcb.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Van Vliet A.R., Verfaillie T., Agostinis P. New functions of mitochondria associated membranes in cellular signaling. Biochim. Biophys. Acta. 2014;1843:2253–2262. doi: 10.1016/j.bbamcr.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 113.Annunziata I., d’Azzo A. Interorganellar membrane microdomains: Dynamic platforms in the control of calcium signaling and apoptosis. Cells. 2013;2:574–590. doi: 10.3390/cells2030574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sassano M.L., van Vliet A.R., Agostinis P. Mitochondria-Associated Membranes As Networking Platforms and Regulators of Cancer Cell Fate. Front. Oncol. 2017;7:174. doi: 10.3389/fonc.2017.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hugenroth M., Bohnert M. Come a little bit closer! Lipid droplet-ER contact sites are getting crowded. Biochim. Biophys. Acta Mol. Cell Res. 2020;1867:118603. doi: 10.1016/j.bbamcr.2019.118603. [DOI] [PubMed] [Google Scholar]
- 116.Issop L., Rone M.B., Papadopoulos V. Organelle plasticity and interactions in cholesterol transport and steroid biosynthesis. Mol. Cell Endocrinol. 2013;371:34–46. doi: 10.1016/j.mce.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 117.Rone M.B., Midzak A.S., Issop L., Rammouz G., Jagannathan S., Fan J., Ye X., Blonder J., Veenstra T., Papadopoulos V. Identification of a dynamic mitochondrial protein complex driving cholesterol import, trafficking, and metabolism to steroid hormones. Mol. Endocrinol. 2012;26:1868–1882. doi: 10.1210/me.2012-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Issop L., Fan J., Lee S., Rone M.B., Basu K., Mui J., Papadopoulos V. Mitochondria-associated membrane formation in hormone-stimulated Leydig cell steroidogenesis: Role of ATAD3. Endocrinology. 2015;156:334–345. doi: 10.1210/en.2014-1503. [DOI] [PubMed] [Google Scholar]
- 119.Strauss J.F. Chapter 5–Organization of Ovarian Steroidogenic Cells and Cholesterol Metabolism. In: Leung P.C.K., Adashi E.Y., editors. The Ovary. 3rd ed. Academic Press; Cambridge, MA, USA: 2019. pp. 83–94. [Google Scholar]
- 120.Prasad M., Kaur J., Pawlak K.J., Bose M., Whittal R.M., Bose H.S. Mitochondria-associated endoplasmic reticulum membrane (MAM) regulates steroidogenic activity via steroidogenic acute regulatory protein (StAR)-voltage-dependent anion channel 2 (VDAC2) interaction. J. Biol. Chem. 2015;290:2604–2616. doi: 10.1074/jbc.M114.605808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moore A.S., Holzbaur E.L.F. Mitochondrial-cytoskeletal interactions: Dynamic associations that facilitate network function and remodeling. Curr. Opin. Physiol. 2018;3:94–100. doi: 10.1016/j.cophys.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nobes C.D., Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J. Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bamburg J.R. Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Dev. Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- 124.Niwa R., Nagata-Ohashi K., Takeichi M., Mizuno K., Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/S0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- 125.Ohta Y., Kousaka K., Nagata-Ohashi K., Ohashi K., Muramoto A., Shima Y., Niwa R., Uemura T., Mizuno K. Differential activities, subcellular distribution and tissue expression patterns of three members of Slingshot family phosphatases that dephosphorylate cofilin. Genes Cells. 2003;8:811–824. doi: 10.1046/j.1365-2443.2003.00678.x. [DOI] [PubMed] [Google Scholar]
- 126.Karlsson A.B., Maizels E.T., Flynn M.P., Jones J.C., Shelden E.A., Bamburg J.R., Hunzicker-Dunn M. Luteinizing hormone receptor-stimulated progesterone production by preovulatory granulosa cells requires protein kinase A-dependent activation/dephosphorylation of the actin dynamizing protein cofilin. Mol. Endocrinol. 2010;24:1765–1781. doi: 10.1210/me.2009-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nagy L., Freeman D.A. Effect of cholesterol transport inhibitors on steroidogenesis and plasma membrane cholesterol transport in cultured MA-10 Leydig tumor cells. Endocrinology. 1990;126:2267–2276. doi: 10.1210/endo-126-5-2267. [DOI] [PubMed] [Google Scholar]
- 128.Schwarz D.S., Blower M.D. The endoplasmic reticulum: Structure, function and response to cellular signaling. Cell Mol. Life Sci. 2016;73:79–94. doi: 10.1007/s00018-015-2052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Guzel E., Arlier S., Guzeloglu-Kayisli O., Tabak M.S., Ekiz T., Semerci N., Larsen K., Schatz F., Lockwood C.J., Kayisli U.A. Endoplasmic Reticulum Stress and Homeostasis in Reproductive Physiology and Pathology. Int. J. Mol. Sci. 2017;18:792. doi: 10.3390/ijms18040792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Harada M., Nose E., Takahashi N., Hirota Y., Hirata T., Yoshino O., Koga K., Fujii T., Osuga Y. Evidence of the activation of unfolded protein response in granulosa and cumulus cells during follicular growth and maturation. Gynecol. Endocrinol. 2015;31:783–787. doi: 10.3109/09513590.2015.1062862. [DOI] [PubMed] [Google Scholar]
- 131.Lin P., Yang Y., Li X., Chen F., Cui C., Hu L., Li Q., Liu W., Jin Y. Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol. Reprod. Dev. 2012;79:423–432. doi: 10.1002/mrd.22045. [DOI] [PubMed] [Google Scholar]
- 132.Park H.J., Park S.J., Koo D.B., Kong I.K., Kim M.K., Kim J.M., Choi M.S., Park Y.H., Kim S.U., Chang K.T., et al. Unfolding protein response signaling is involved in development, maintenance, and regression of the corpus luteum during the bovine estrous cycle. Biochem. Biophys. Res. Commun. 2013;441:344–350. doi: 10.1016/j.bbrc.2013.10.056. [DOI] [PubMed] [Google Scholar]
- 133.Braakman I., Bulleid N.J. Protein folding and modification in the mammalian endoplasmic reticulum. Annu. Rev. Biochem. 2011;80:71–99. doi: 10.1146/annurev-biochem-062209-093836. [DOI] [PubMed] [Google Scholar]
- 134.Chambers J.E., Marciniak S.J. Cellular mechanisms of endoplasmic reticulum stress signaling in health and disease. 2. Protein misfolding and ER stress 2. Protein misfolding and ER stress. Am. J. Physiol. Cell Physiol. 2014;307:C657–C670. doi: 10.1152/ajpcell.00183.2014. [DOI] [PubMed] [Google Scholar]
- 135.Takahashi N., Harada M., Hirota Y., Zhao L., Azhary J.M., Yoshino O., Izumi G., Hirata T., Koga K., Wada-Hiraike O., et al. A Potential Role for Endoplasmic Reticulum Stress in Progesterone Deficiency in Obese Women. Endocrinology. 2017;158:84–97. doi: 10.1210/en.2016-1511. [DOI] [PubMed] [Google Scholar]
- 136.Park S.J., Kim T.S., Park C.K., Lee S.H., Kim J.M., Lee K.S., Lee I.K., Park J.W., Lawson M.A., Lee D.S. hCG-induced endoplasmic reticulum stress triggers apoptosis and reduces steroidogenic enzyme expression through activating transcription factor 6 in Leydig cells of the testis. J. Mol. Endocrinol. 2013;50:151–166. doi: 10.1530/JME-12-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ullah S., Zhang M., Yu H., Mustafa S., Shafiq M., Wei Q., Wang W., Jan M., Mao D. Heat exposure affected the reproductive performance of pregnant mice: Enhancement of autophagy and alteration of subcellular structure in the corpus luteum. Reprod. Biol. 2019;19:261–269. doi: 10.1016/j.repbio.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 138.Azhary J.M.K., Harada M., Kunitomi C., Kusamoto A., Takahashi N., Nose E., Oi N., Wada-Hiraike O., Urata Y., Hirata T., et al. Androgens Increase Accumulation of Advanced Glycation End Products in Granulosa Cells by Activating ER Stress in PCOS. Endocrinology. 2020;161:bqaa015. doi: 10.1210/endocr/bqaa015. [DOI] [PubMed] [Google Scholar]
- 139.Takahashi N., Harada M., Hirota Y., Nose E., Azhary J.M., Koike H., Kunitomi C., Yoshino O., Izumi G., Hirata T., et al. Activation of Endoplasmic Reticulum Stress in Granulosa Cells from Patients with Polycystic Ovary Syndrome Contributes to Ovarian Fibrosis. Sci. Rep. 2017;7:10824. doi: 10.1038/s41598-017-11252-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dimova R., Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed. Res. Int. 2015;2015:823481. doi: 10.1155/2015/823481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Kurowska P., Mlyczyńska E., Dupont J., Rak A. Novel Insights on the Corpus Luteum Function: Role of Vaspin on Porcine Luteal Cell Angiogenesis, Proliferation and Apoptosis by Activation of GRP78 Receptor and MAP3/1 Kinase Pathways. Int. J. Mol. Sci. 2020;21:6823. doi: 10.3390/ijms21186823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barbosa A.D., Siniossoglou S. Function of lipid droplet-organelle interactions in lipid homeostasis. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864:1459–1468. doi: 10.1016/j.bbamcr.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 143.Sztalryd C., Brasaemle D.L. The perilipin family of lipid droplet proteins: Gatekeepers of intracellular lipolysis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1221–1232. doi: 10.1016/j.bbalip.2017.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Welte M.A., Gould A.P. Lipid droplet functions beyond energy storage. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:1260–1272. doi: 10.1016/j.bbalip.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fujimoto T., Parton R.G. Not just fat: The structure and function of the lipid droplet. Cold Spring Harb. Perspect. Biol. 2011;3 doi: 10.1101/cshperspect.a004838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bloor W.R., Okey R., Corner G. The relation of the lipids to physiological activity i. The changes in the lipid content of the corpus luteum of the sow. J. Biol. Chem. 1930;86:291–306. doi: 10.1016/S0021-9258(18)76927-5. [DOI] [Google Scholar]
- 147.Claesson L. Quantitative relationship between gonadotrophic stimulation and lipid changes in the interstitial gland of the rabbit ovary. Acta Physiol. Scand. Suppl. 1954;31:23–51. [PubMed] [Google Scholar]
- 148.Deane H.W., Andrews J.S. A comparison of the staining of lipid droplets in the mouse ovary by the Schiff reaction and by the Ashbel-Seligman carbonyl reaction. J. Histochem. Cytochem. 1953;1:283–295. doi: 10.1177/1.5.283. [DOI] [PubMed] [Google Scholar]
- 149.Dorlikar A., Dhamani A., Charde P., Mohite A. Ultrastructure of ovarian preantral follicles and corpus luteum in Indian flying fox Pteropus giganteus (Brunnich) Edorium J. Cell Biol. 2014;1:1–11. doi: 10.5348/c06-2014-1-OA-1. [DOI] [Google Scholar]
- 150.Green J.A., Maqueo M. Ultrastructure of the human ovary. I. The luteal cell during the menstrual cycle. Am. J. Obstet. Gynecol. 1965;92:946–957. doi: 10.1016/0002-9378(65)90728-3. [DOI] [PubMed] [Google Scholar]
- 151.Guraya S.S. Histochemical study of granulosa and theca interna during follicular development, ovulation, and corpus luteum formation and regression in the human ovary. Am. J. Obset. Gynecol. 1968;101:448–457. doi: 10.1016/0002-9378(68)90552-8. [DOI] [PubMed] [Google Scholar]
- 152.Kapoor K. Lipid Distribution Variations in Different Stages of Cyclic Corpus Luteum of Indian Buffalo. J. Anim. Res. 2018;8:379–385. doi: 10.30954/2277-940X.06.2018.7. [DOI] [Google Scholar]
- 153.Parry D.M., Willcox D.L., Thorburn G.D. Ultrastructural and cytochemical study of the bovine corpus luteum. J. Reprod. Fertil. 1980;60:349–357. doi: 10.1530/jrf.0.0600349. [DOI] [PubMed] [Google Scholar]
- 154.Seachord C.L., VandeVoort C.A., Duffy D.M. Adipose differentiation-related protein: A gonadotropin- and prostaglandin-regulated protein in primate periovulatory follicles. Biol. Reprod. 2005;72:1305–1314. doi: 10.1095/biolreprod.104.037523. [DOI] [PubMed] [Google Scholar]
- 155.Strauss J.F., 3rd, Seifter E., Lien E.L., Goodman D.B., Stambaugh R.L. Lipid metabolism in regressing rat corpora lutea of pregnancy. J. Lipid Res. 1977;18:246–258. doi: 10.1016/S0022-2275(20)41704-3. [DOI] [PubMed] [Google Scholar]
- 156.Waterman R.A. Changes in lipid contents and fatty acid compositions in ovine corpora lutea during the estrous cycle and early pregnancy. Biol. Reprod. 1988;38:605–615. doi: 10.1095/biolreprod38.3.605. [DOI] [PubMed] [Google Scholar]
- 157.Khanthusaeng V., Thammasiri J., Bass C.S., Navanukraw C., Borowicz P., Redmer D.A., Grazul-Bilska A.T. Lipid droplets in cultured luteal cells in non-pregnant sheep fed different planes of nutrition. Acta Histochem. 2016;118:553–559. doi: 10.1016/j.acthis.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 158.Armstrong D.T., Flint A.P. Isolation and properties of cholesterol esterstorage granules from ovarian tissues. Biochem. J. 1973;134:399–406. doi: 10.1042/bj1340399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Heath E., Weinstein P., Merritt B., Shanks R., Hixon J. Effects of prostaglandins on the bovine corpus luteum: Granules, lipid inclusions and progesterone secretion. Biol. Reprod. 1983;29:977–985. doi: 10.1095/biolreprod29.4.977. [DOI] [PubMed] [Google Scholar]
- 160.Kraemer F.B., Shen W.J. Hormone-sensitive lipase: Control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.R200009-JLR200. [DOI] [PubMed] [Google Scholar]
- 161.Zechner R., Zimmermann R., Eichmann T.O., Kohlwein S.D., Haemmerle G., Lass A., Madeo F. FAT SIGNALS--lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shen W.J., Patel S., Natu V., Hong R., Wang J., Azhar S., Kraemer F.B. Interaction of hormone-sensitive lipase with steroidogenic acute regulatory protein: Facilitation of cholesterol transfer in adrenal. J. Biol. Chem. 2003;278:43870–43876. doi: 10.1074/jbc.M303934200. [DOI] [PubMed] [Google Scholar]
- 163.Talbott H., Davis J.S. Lipid Droplets and Metabolic Pathways Regulate Steroidogenesis in the Corpus Luteum. In: Meidan R., editor. The Life Cycle of the Corpus Luteum. Springer International Publishing; Cham, Switzerland: 2017. pp. 57–78. [Google Scholar]
- 164.Manna P.R., Cohen-Tannoudji J., Counis R., Garner C.W., Huhtaniemi I., Kraemer F.B., Stocco D.M. Mechanisms of action of hormone-sensitive lipase in mouse Leydig cells: Its role in the regulation of the steroidogenic acute regulatory protein. J. Biol. Chem. 2013;288:8505–8518. doi: 10.1074/jbc.M112.417873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Macpherson R.E., Vandenboom R., Roy B.D., Peters S.J. Skeletal muscle PLIN3 and PLIN5 are serine phosphorylated at rest and following lipolysis during adrenergic or contractile stimulation. Physiol. Rep. 2013;1:e00084. doi: 10.1002/phy2.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raclot T., Holm C., Langin D. Fatty acid specificity of hormone-sensitive lipase. Implication in the selective hydrolysis of triacylglycerols. J. Lipid Res. 2001;42:2049–2057. doi: 10.1016/S0022-2275(20)31534-0. [DOI] [PubMed] [Google Scholar]
- 167.Raclot T., Holm C., Langin D. A role for hormone-sensitive lipase in the selective mobilization of adipose tissue fatty acids. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids. 2001;1532:88–96. doi: 10.1016/S1388-1981(01)00119-6. [DOI] [PubMed] [Google Scholar]
- 168.Benador I.Y., Veliova M., Mahdaviani K., Petcherski A., Wikstrom J.D., Assali E.A., Acín-Pérez R., Shum M., Oliveira M.F., Cinti S., et al. Mitochondria Bound to Lipid Droplets Have Unique Bioenergetics, Composition, and Dynamics that Support Lipid Droplet Expansion. Cell Metab. 2018;27:869–885.e866. doi: 10.1016/j.cmet.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Inpanathan S., Botelho R.J. The Lysosome Signaling Platform: Adapting With the Times. Front. Cell Dev. Biol. 2019;7:113. doi: 10.3389/fcell.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Settembre C., Ballabio A. Lysosome: Regulator of lipid degradation pathways. Trends Cell Biol. 2014;24:743–750. doi: 10.1016/j.tcb.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Thelen A.M., Zoncu R. Emerging Roles for the Lysosome in Lipid Metabolism. Trends Cell Biol. 2017;27:833–850. doi: 10.1016/j.tcb.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Liu Q., Gao H., Yang F., Zhang H., Zeng S. FSH Promotes Progesterone Synthesis by Enhancing Autophagy to Accelerate Lipid Droplet Degradation in Porcine Granulosa Cells. Front. Cell Dev. Biol. 2021;9:626927. doi: 10.3389/fcell.2021.626927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Alonso-Pozos I., Rosales-Torres A.M., Avalos-Rodríguez A., Vergara-Onofre M., Rosado-García A. Mechanism of granulosa cell death during follicular atresia depends on follicular size. Theriogenology. 2003;60:1071–1081. doi: 10.1016/S0093-691X(03)00123-7. [DOI] [PubMed] [Google Scholar]
- 174.Escobar M.L., Echeverría O.M., Ortíz R., Vázquez-Nin G.H. Combined apoptosis and autophagy, the process that eliminates the oocytes of atretic follicles in immature rats. Apoptosis. 2008;13:1253–1266. doi: 10.1007/s10495-008-0248-z. [DOI] [PubMed] [Google Scholar]
- 175.Zhang J.Y., Wu Y., Zhao S., Liu Z.X., Zeng S.M., Zhang G.X. Lysosomes are involved in induction of steroidogenic acute regulatory protein (StAR) gene expression and progesterone synthesis through low-density lipoprotein in cultured bovine granulosa cells. Theriogenology. 2015;84:811–817. doi: 10.1016/j.theriogenology.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 176.Aboelenain M., Kawahara M., Balboula A.Z., Montasser Ael M., Zaabel S.M., Okuda K., Takahashi M. Status of autophagy, lysosome activity and apoptosis during corpus luteum regression in cattle. J. Reprod. Dev. 2015;61:229–236. doi: 10.1262/jrd.2014-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gregoraszczuk E.L., Sadowska J. Lysosomal acid phosphatase activity and progesterone secretion by porcine corpora lutea at various periods of the luteal phase. Folia. Histochem. Cytobiol. 1997;35:35–39. [PubMed] [Google Scholar]
- 178.Wang Z., El Zowalaty A.E., Li Y., Andersen C.L., Ye X. Association of luteal cell degeneration and progesterone deficiency with lysosomal storage disorder mucolipidosis type IV in Mcoln1-/- mouse model. Biol. Reprod. 2019;101:782–790. doi: 10.1093/biolre/ioz126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bowdridge E.C., Goravanahally M.P., Inskeep E.K., Flores J.A. Activation of Adenosine Monophosphate-Activated Protein Kinase Is an Additional Mechanism That Participates in Mediating Inhibitory Actions of Prostaglandin F2Alpha in Mature, but Not Developing, Bovine Corpora Lutea. Biol. Reprod. 2015;93:7. doi: 10.1095/biolreprod.115.129411. [DOI] [PubMed] [Google Scholar]
- 180.Hou X., Arvisais E.W., Davis J.S. Luteinizing hormone stimulates mammalian target of rapamycin signaling in bovine luteal cells via pathways independent of AKT and mitogen-activated protein kinase: Modulation of glycogen synthase kinase 3 and AMP-activated protein kinase. Endocrinology. 2010;151:2846–2857. doi: 10.1210/en.2009-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Przygrodzka E., Hou X., Zhang P., Plewes M.R., Franco R., Davis J.S. PKA and AMPK Signaling Pathways Differentially Regulate Luteal Steroidogenesis. Endocrinology. 2021;162:bqab015. doi: 10.1210/endocr/bqab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Thomas R.E., Armstrong D.T., Gilchrist R.B. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol. Reprod. 2004;70:548–556. doi: 10.1095/biolreprod.103.021204. [DOI] [PubMed] [Google Scholar]
- 183.Tosca L., Chabrolle C., Uzbekova S., Dupont J. Effects of metformin on bovine granulosa cells steroidogenesis: Possible involvement of adenosine 5′ monophosphate-activated protein kinase (AMPK) Biol. Reprod. 2007;76:368–378. doi: 10.1095/biolreprod.106.055749. [DOI] [PubMed] [Google Scholar]
- 184.Herzig S., Shaw R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018;19:121–135. doi: 10.1038/nrm.2017.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Saxton R.A., Sabatini D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell. 2017;168:960–976. doi: 10.1016/j.cell.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Willows R., Sanders M.J., Xiao B., Patel B.R., Martin S.R., Read J., Wilson J.R., Hubbard J., Gamblin S.J., Carling D. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem. J. 2017;474:3059–3073. doi: 10.1042/BCJ20170458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Shen W.J., Azhar S., Kraemer F.B. Lipid droplets and steroidogenic cells. Exp. Cell Res. 2016;340:209–214. doi: 10.1016/j.yexcr.2015.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kim S.J., Tang T., Abbott M., Viscarra J.A., Wang Y., Sul H.S. AMPK Phosphorylates Desnutrin/ATGL and Hormone-Sensitive Lipase To Regulate Lipolysis and Fatty Acid Oxidation within Adipose Tissue. Mol. Cell Biol. 2016;36:1961–1976. doi: 10.1128/MCB.00244-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pernicova I., Korbonits M. Metformin—mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014;10:143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 190.Tosca L., Crochet S., Ferré P., Foufelle F., Tesseraud S., Dupont J. AMP-activated protein kinase activation modulates progesterone secretion in granulosa cells from hen preovulatory follicles. J. Endocrinol. 2006;190:85–97. doi: 10.1677/joe.1.06828. [DOI] [PubMed] [Google Scholar]
- 191.Tosca L., Solnais P., Ferré P., Foufelle F., Dupont J. Metformin-induced stimulation of adenosine 5′ monophosphate-activated protein kinase (PRKA) impairs progesterone secretion in rat granulosa cells. Biol. Reprod. 2006;75:342–351. doi: 10.1095/biolreprod.106.050831. [DOI] [PubMed] [Google Scholar]
- 192.Martin D.E., Hall M.N. The expanding TOR signaling network. Curr. Opin. Cell Biol. 2005;17:158–166. doi: 10.1016/j.ceb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 193.Wullschleger S., Loewith R., Hall M.N. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 194.Guertin D.A., Sabatini D.M. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 195.Yang Q., Guan K.L. Expanding mTOR signaling. Cell Res. 2007;17:666–681. doi: 10.1038/cr.2007.64. [DOI] [PubMed] [Google Scholar]
- 196.Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M.A., Hall A., Hall M.N. Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 2004;6:1122–1128. doi: 10.1038/ncb1183. [DOI] [PubMed] [Google Scholar]
- 197.Sarbassov D.D., Ali S.M., Kim D.H., Guertin D.A., Latek R.R., Erdjument-Bromage H., Tempst P., Sabatini D.M. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 2004;14:1296–1302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 198.González A., Hall M.N. Nutrient sensing and TOR signaling in yeast and mammals. Embo. J. 2017;36:397–408. doi: 10.15252/embj.201696010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Grolleau A., Bowman J., Pradet-Balade B., Puravs E., Hanash S., Garcia-Sanz J.A., Beretta L. Global and specific translational control by rapamycin in T cells uncovered by microarrays and proteomics. J. Biol. Chem. 2002;277:22175–22184. doi: 10.1074/jbc.M202014200. [DOI] [PubMed] [Google Scholar]
- 200.Alessi D.R., James S.R., Downes C.P., Holmes A.B., Gaffney P.R., Reese C.B., Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 1997;7:261–269. doi: 10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 201.Li Y., Corradetti M.N., Inoki K., Guan K.L. TSC2: Filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 2004;29:32–38. doi: 10.1016/j.tibs.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 202.Inoki K., Zhu T., Guan K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 203.Inoki K., Ouyang H., Zhu T., Lindvall C., Wang Y., Zhang X., Yang Q., Bennett C., Harada Y., Stankunas K., et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–968. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 204.Roy L., McDonald C.A., Jiang C., Maroni D., Zeleznik A.J., Wyatt T.A., Hou X., Davis J.S. Convergence of 3′,5′-cyclic adenosine 5′-monophosphate/protein kinase A and glycogen synthase kinase-3beta/beta-catenin signaling in corpus luteum progesterone synthesis. Endocrinology. 2009;150:5036–5045. doi: 10.1210/en.2009-0771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kayampilly P.P., Menon K.M. Follicle-stimulating hormone increases tuberin phosphorylation and mammalian target of rapamycin signaling through an extracellular signal-regulated kinase-dependent pathway in rat granulosa cells. Endocrinology. 2007;148:3950–3957. doi: 10.1210/en.2007-0202. [DOI] [PubMed] [Google Scholar]
- 206.Moravek M.B., Shang M., Menon B., Menon K. HCG-mediated activation of mTORC1 signaling plays a crucial role in steroidogenesis in human granulosa lutein cells. Endocrine. 2016;54:217–224. doi: 10.1007/s12020-016-1065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Parzych K.R., Klionsky D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014;20:460–473. doi: 10.1089/ars.2013.5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yang Z., Klionsky D.J. An overview of the molecular mechanism of autophagy. Curr. Top. Microbiol. Immunol. 2009;335:1–32. doi: 10.1007/978-3-642-00302-8_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.How Do I Cite BioRender? [(accessed on 1 September 2021)]. Available online: https://help.biorender.com/en/articles/3619405-how-do-i-cite-biorender.
- 210.Wang Y., Zhang H. Regulation of Autophagy by mTOR Signaling Pathway. In: Qin Z.-H., editor. Autophagy: Biology and Diseases: Basic Science. Springer Singapore; Singapore: 2019. pp. 67–83. [DOI] [PubMed] [Google Scholar]
- 211.Li Y., Chen Y. AMPK and Autophagy. Adv. Exp. Med. Biol. 2019;1206:85–108. doi: 10.1007/978-981-15-0602-4_4. [DOI] [PubMed] [Google Scholar]
- 212.Egan D., Kim J., Shaw R.J., Guan K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy. 2011;7:643–644. doi: 10.4161/auto.7.6.15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Choi J., Jo M., Lee E., Choi D. The role of autophagy in corpus luteum regression in the rat. Biol. Reprod. 2011;85:465–472. doi: 10.1095/biolreprod.111.091314. [DOI] [PubMed] [Google Scholar]
- 215.Grzesiak M., Michalik A., Rak A., Knapczyk-Stwora K., Pieczonka A. The expression of autophagy-related proteins within the corpus luteum lifespan in pigs. Domest. Anim. Endocrinol. 2018;64:9–16. doi: 10.1016/j.domaniend.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 216.Meng L., Jan S.Z., Hamer G., van Pelt A.M., van der Stelt I., Keijer J., Teerds K.J. Preantral follicular atresia occurs mainly through autophagy, while antral follicles degenerate mostly through apoptosis. Biol. Reprod. 2018;99:853–863. doi: 10.1093/biolre/ioy116. [DOI] [PubMed] [Google Scholar]
- 217.Tang Z., Zhang Z., Lin Q., Xu R., Chen J., Wang Y., Zhang Y., Tang Y., Shi C., Liu Y., et al. HIF-1α/BNIP3-Mediated Autophagy Contributes to the Luteinization of Granulosa Cells During the Formation of Corpus Luteum. Front. Cell. Dev. Biol. 2020;8:619924. doi: 10.3389/fcell.2020.619924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Tang Z., Zhang Z., Zhang H., Wang Y., Zhang Y., Zhao J., Yang H., Wang Z. Autophagy Attenuation Hampers Progesterone Synthesis during the Development of Pregnant Corpus Luteum. Cells. 2019;9:71. doi: 10.3390/cells9010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Wen X., Liu L., Li S., Lin P., Chen H., Zhou D., Tang K., Wang A., Jin Y. Prostaglandin F2α Induces Goat Corpus Luteum Regression via Endoplasmic Reticulum Stress and Autophagy. Front. Physiol. 2020;11:868. doi: 10.3389/fphys.2020.00868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baddela V.S., Sharma A., Michaelis M., Vanselow J. HIF1 driven transcriptional activity regulates steroidogenesis and proliferation of bovine granulosa cells. Sci. Rep. 2020;10:3906. doi: 10.1038/s41598-020-60935-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gawriluk T.R., Rucker E.B. BECN1, corpus luteum function, and preterm labor. Autophagy. 2015;11:183–184. doi: 10.4161/15548627.2014.984269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Przygrodzka E., Monaco C., Plewes M.R., Li G., Wood J.R., Cupp A.S., Davis J.S. Protein Kinase A and 5′ AMP-ActivatedProtein Kinase Signaling Pathways Exert Opposite Effects on Induction ofAutophagy in Luteal Cells. 2021. to be submitted. [DOI] [PMC free article] [PubMed]
- 223.Doherty J., Baehrecke E.H. Life, death and autophagy. Nat. Cell Biol. 2018;20:1110–1117. doi: 10.1038/s41556-018-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Djavaheri-Mergny M., Maiuri M.C., Kroemer G. Cross talk between apoptosis and autophagy by caspase-mediated cleavage of Beclin 1. Oncogene. 2010;29:1717–1719. doi: 10.1038/onc.2009.519. [DOI] [PubMed] [Google Scholar]
- 225.Shi C.S., Kehrl J.H. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci. Signal. 2010;3:ra42. doi: 10.1126/scisignal.2000751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Zalckvar E., Berissi H., Mizrachy L., Idelchuk Y., Koren I., Eisenstein M., Sabanay H., Pinkas-Kramarski R., Kimchi A. DAP-kinase-mediated phosphorylation on the BH3 domain of beclin 1 promotes dissociation of beclin 1 from Bcl-XL and induction of autophagy. EMBO Rep. 2009;10:285–292. doi: 10.1038/embor.2008.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Wei Y., Pattingre S., Sinha S., Bassik M., Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol. Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Song X., Zhu S., Chen P., Hou W., Wen Q., Liu J., Xie Y., Liu J., Klionsky D.J., Kroemer G., et al. AMPK-Mediated BECN1 Phosphorylation Promotes Ferroptosis by Directly Blocking System Xc- Activity. Curr. Biol. 2018;28:2388–2399.e5. doi: 10.1016/j.cub.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]