Abstract
Purpose
In this study, we aimed to determine the value of hypoxic liver injury (HLI) in the emergency room (ER) for predicting hypoxic hepatitis (HH) and in-hospital mortality in ST elevation myocardial infarction (STEMI) patients.
Materials and Methods
1537 consecutive STEMI patients were enrolled. HLI in the ER was defined as a ≥2-fold increase in serum aspartate transaminase (AST). HH was defined as a ≥20-fold increase in peak serum transaminase. Patients were divided into four groups according to HLI and HH status (group 1, no HLI or HH; group 2, HLI, but no HH; group 3, no HLI, but HH; group 4, both HLI and HH).
Results
The incidences of HLI and HH in the ER were 22% and 2%, respectively. In-hospital mortality rates were 3.1%, 11.8%, 28.6%, and 47.1% for groups 1, 2, 3, and 4, respectively. Patients with HLI and/or HH had worse Killip class, higher cardiac biomarker elevations, and lower left ventricular ejection fraction. Multivariate logistic regression analysis showed that HLI in the ER was an independent predictor of HH [odds ratio 2.572, 95% confidence interval (CI) 1.166–5.675, p=0.019]. The predictive value of HLI in the ER for the development of HH during hospitalization was favorable [area under the curve (AUC) 0.737, 95% CI 0.643–0.830, sensitivity 0.548, specificity 0.805, for cut-off value AST >80]. Furthermore, in terms of in-hospital mortality, predictive values of HLI in the ER and HH during hospitalization were comparable (AUC 0.701 for HLI at ER and AUC 0.674 for HH).
Conclusion
Among STEMI patients, HLI in the ER is a significant predictor for the development of HH and mortality during hospitalization (INTERSTELLAR ClinicalTrials.gov number, NCT02800421).
Keywords: STEMI, hypoxic liver injury, hypoxic hepatitis, in-hospital mortality
INTRODUCTION
Coronary intervention has evolved drastically in recent years, and consequentially, the survival of ST elevation myocardial infarction (STEMI) patients has improved.1 However, despite an abundance of studies and reports on treatment strategies for STEMI, mortality rates remain relatively high,1 and methods for reaching an accurate prognosis for STEMI patients remain elusive, despite several proposed parameters and risk factors validated for use in risk-scoring systems.2 While left ventricular ejection fraction (LVEF), Killip class, and presence of multivessel disease are also known risk factors contributing to the survival of STEMI patients,3,4 convincing biochemical markers predictive of prognoses in STEMI patients have not been developed.
Since the pathophysiological nature of STEMI is directly associated with LVEF,3,4 one of the organs vulnerable to injury is the liver. Hypoxic liver injury (HLI) is caused by an acute cardiovascular event resulting in decreased hepatic blood flow or hepatic congestion due to increased central venous pressure.5 Hypoxic hepatitis (HH) is another term used to describe liver damage, and the mechanism of insult is equivalent to that of HLI. While the predictive value of HLI in STEMI patients and the development of HH as a prognostic factor in critically ill patients have been reported, the effects that they have upon each other and their prognostic value in STEMI patients has not been addressed.6,7 Therefore, we sought to determine the value of HLI in the emergency room (ER) for predicting HH and inhospital mortality in STEMI patients undergoing primary percutaneous coronary intervention (PCI).
MATERIALS AND METHODS
Study design and patient selection
The study protocol was approved by the Institutional Review Board of Inha University Hospital, Inha University College of Medicine (IRB No. 2016-05-015), and written consent was obtained from each patient. We collected data from four different hospitals (Inha University Hospital, Gachon University Gil Hospital, Sejong General Hospital, Soon Chun Hyang University Bucheon Hospital) located in the Incheon-Bucheon province. The four hospitals previously formed a registry on STEMI patients, called INcheon-Bucheon cohorT of patients undERwent primary PCI for acute ST-Elevation myocardial infARction (INTERSTELLAR). A total of 1537 consecutive STEMI patients (79.2% male, mean age 60.5±13.2 years) who had undergone primary PCI between 2007 and 2014 were enrolled. Patients with a prior history of chronic hepatitis, viral hepatitis, alcoholic liver disease, or toxic hepatitis were excluded. Coronary intervention was performed in accordance with current guidelines for myocardial revascularization.8 Pharmacological treatment and mechanical support related to primary PCI were performed at the operator's discretion.
Patients were divided into four groups according to their HLI and HH states: group 1 had no HLI or HH; group 2 had HLI, but no HH; group 3 had no HLI, but HH; and group 4 had both HLI and HH. Baseline characteristics, risk factors, echocardiographic and coronary angiographic findings and clinical features, such as Killip class, were recorded and analyzed.
Definitions and measurements
Patients with systolic blood pressure above 140 mm Hg, diastolic blood pressure above 90 mm Hg, or prior use of antihypertensive medication were defined as having hypertension. Diabetes mellitus was defined as 1) prior use of hypoglycemic agents or insulin, 2) fasting plasma glucose above 126 mg/dL or glycosylated hemoglobin above 6.5%, or 3) previously diagnosed, but untreated hyperglycemia. The definition of dyslipidemia was total cholesterol ≥240 mg/dL, low-density lipoprotein cholesterol ≥130 mg/dL, high-density lipoprotein cholesterol <40 mg/dL, triglycerides ≥200 mg/dL, and prior use of lipid-lowering agents. A patient who was currently smoking or had smoked until 1 month prior to primary PCI was considered as a smoker. STEMI was diagnosed upon an electrocardiogram showing a ST elevation of >1 mm in at least two consecutive leads or new-onset left bundle branch block, two-fold elevation of serum levels of troponin-I or creatine kinase-MB (CK-MB) above the upper normal limit, and typical anginal chest pain lasting for more than 30 min. Coronary artery disease (CAD) was defined as luminal narrowing of more than 50% in any coronary artery.9 HLI was defined as ≥2-fold increase in serum aspartate transaminase (AST) and/or alanine transaminase (ALT) above the upper normal limit at admission.10 HH was defined as a ≥20-fold increase in peak AST and/or ALT above the upper normal limit.11
Endpoint determination and follow-up data acquisition
Our primary endpoint was all-cause in-hospital mortality with respect to the presence of HLI at ER admission and HH development during hospitalization. Patient follow-up data were collected through either electronic medical record review or standardized telephone interviews.
Data analysis and statistical methods
Continuous data are presented as means±standard deviations. Categorical data are presented as a percentage or absolute number. Analyses of continuous data were performed using analysis of variance, and analyses of categorical data were performed using the chi-square test to assess differences among the four study groups. Receiver operating characteristic (ROC) analysis was applied to evaluate the predictive value of HLI in the ER and HH during hospitalization on in-hospital mortality and the predictive value of HLI upon developing HH during hospitalization.12 Binary logistic regression analyses were performed to identify risk factors associated with developing HH during hospitalization; potential factors included clinical characteristics, laboratory findings, Killip classification, LVEF, and HLI. Hazard ratios (HR) were calculated as an estimate of the risk associated with a particular variable with 95% confidence intervals (CI). All analyses were performed using SPSS version 19.0 (IBM Corp., Armonk, NY, USA) and SAS version 9.3 (SAS Institute, Cary, NC, USA). A p value of less than 0.05 was considered statistically significant.
RESULTS
Baseline characteristics of the study population and comparison among patients according to their HLI state in the ER and development of HH
In total, 1537 patients were enrolled. 1185 patients (77.1%) were allocated to group 1, 321 patients (20.9%) to group 2; 14 patients (0.9%) to group 3, and 17 patients (1.1%) to group 4. A summary of the baseline, laboratory, and angiographic characteristics according to HLI at ER and HH state is provided in Table 1. Groups 2, 3, and 4 had lower systolic and diastolic blood pressure (p=0.024 and p=0.048, respectively), higher heart rate (p<0.001), worse Killip class (p<0.001), higher cardiac biomarker elevation (p<0.001), lower LVEF (p<0.001), less frequent right coronary artery infarct (p=0.008), and more intra-aortic balloon pump usage (p=0.002), compared to group 1. The prevalences of commonly known CAD risk factors, such as diabetes mellitus, hypertension, and dyslipidemia, were not significantly different among the four groups, and the extent of CAD did not differ among these groups. Liver enzyme (AST/ALT) elevations were significantly higher in groups 2, 3, and 4 (p<0.001, respectively). Peak CK-MB levels were significantly higher in groups 2, 3, and 4 (p<0.001). The left anterior descending artery was the most common infarct-related artery in all four groups (p=0.008). Univariate logistic regression analysis showed that presence of Killip class 4, elevated heart rate, LVEF ≤35%, and HLI were predictive factors of HH. Multivariate analysis adjusted for the predictive risk factors mentioned above showed that HLI was an independent predictive factor for developing HH (odds ratio=2.572, 95% CI 1.166–5.675, p=0.019) (Table 2).
Table 1. Baseline, Laboratory, and Angiographic Characteristics according to HLI at ER (ER AST >80) and HH (Peak AST and/or ALT >800).
| Variable | Total (n=1537) |
HLI at ER (−) HH (−) (n=1185) |
HLI at ER (+) HH (−) (n=321) |
HLI at ER (−) HH (+) (n=14) |
HLI at ER (+) HH (+) (n=17) |
p value | |
|---|---|---|---|---|---|---|---|
| Age (yr) | 60.5±13.2 | 60.5±13.0 | 60.8±13.8 | 59.4±14.9 | 63.9±14.5 | 0.714 | |
| Male sex | 79.2 | 79.5 | 77.9 | 78.6 | 88.2 | 0.742 | |
| Diabetes | 27.1 | 27.7 | 24.0 | 28.6 | 41.2 | 0.321 | |
| Hypertension | 48.6 | 49.4 | 44.9 | 57.1 | 58.8 | 0.362 | |
| Dyslipidemia | 19.6 | 20.5 | 17.1 | 21.4 | 5.9 | 0.272 | |
| SBP (mm Hg) | 124.0±30.1 | 125.0±29.8 | 121.7±30.7 | 116.4±31.7 | 106.9±28.7 | 0.024 | |
| DBP (mm Hg) | 75.9±19.1 | 76.3±18.6 | 75.2±20.6 | 73.9±23.3 | 63.8±15.4 | 0.048 | |
| Heart rate (bpm) | 77.7±21.4 | 76.4±20.5 | 81.6±23.6 | 82.3±19.5 | 94.0±26.8 | <0.001 | |
| Killip class | <0.001 | ||||||
| 1 | 77.6 | 80.3 | 70.8 | 64.3 | 35.3 | ||
| 2 | 7.1 | 7.3 | 6.6 | 14.3 | 0 | ||
| 3 | 6.8 | 5.8 | 10.4 | 7.1 | 11.8 | ||
| 4 | 7.8 | 6.1 | 11.3 | 14.3 | 52.9 | ||
| AST (mg/dL) | 70.2±133.3 | 31.8±14.8 | 183.3±135.6 | 33.9±9.7 | 646.2±775.8 | <0.001 | |
| ALT (mg/dL) | 39.1±56.4 | 26.4±14.1 | 74.4±57.5 | 28.6±20.0 | 266.5±363.3 | <0.001 | |
| Peak AST (mg/dL) | 176.2±533.1 | 106.6±136.9 | 242.8±165.9 | 1102.0±595.2 | 3012.3±3913.0 | <0.001 | |
| Peak ALT (mg/dL) | 71.0±315.5 | 40.4±35.5 | 100.8±272.6 | 344.6±365.3 | 1417.8±2406.0 | <0.001 | |
| Initial CK (U/L) | 495.8±1059.3 | 227.5±386.9 | 1421.2±1850.8 | 212.2±185.0 | 1872.7±1797.6 | <0.001 | |
| Initial CK-MB (μg/mL) | 46.9±187.5 | 23.5±134.4 | 129.4±302.9 | 23.9±52.5 | 141.7±121.1 | <0.001 | |
| Initial TnI (ng/mL) | 11.27±51.22 | 5.22±24.20 | 26.28±56.41 | 4.72±14.28 | 135.10±366.92 | <0.001 | |
| Peak CK (U/L) | 1881.8±2729.3 | 1460.7±2152.5 | 2872.6±3168.4 | 4737.4±5859.4 | 7814.9±7804.0 | <0.001 | |
| Peak CK-MB (μg/mL) | 214.9±264.2 | 190.6±210.7 | 283.4±383.7 | 457.7±265.0 | 421.0±435.7 | <0.001 | |
| Peak TnI (ng/mL) | 60.91±111.35 | 56.23±109.43 | 69.55±80.92 | 64.05±71.77 | 184.90±397.07 | 0.802 | |
| LVEF (%) | 48.4±12.1 | 49.6±11.6 | 45.9±11.9 | 30.7±11.6 | 28.7±18.3 | <0.001 | |
| CAD extent | 0.946 | ||||||
| One-vessel disease | 39.8 | 40.3 | 37.9 | 35.7 | 41.2 | ||
| Two-vessel disease | 33.4 | 32.9 | 35.7 | 28.6 | 29.4 | ||
| Three-vessel disease | 26.8 | 26.8 | 26.3 | 35.7 | 29.4 | ||
| Multi-vessel disease | 38.0 | 37.5 | 40.1 | 35.7 | 35.3 | 0.846 | |
| Infarct-related artery | 0.008 | ||||||
| LAD | 50.9 | 50.6 | 51.1 | 64.3 | 52.9 | ||
| LCX | 10.6 | 10.7 | 10.3 | 14.3 | 5.9 | ||
| RCA | 37.4 | 38.0 | 36.7 | 14.3 | 29.4 | ||
| LMCA | 1.2 | 0.8 | 1.9 | 7.1 | 11.8 | ||
| IABP | 3.7 | 3.0 | 7.1 | 7.1 | 18.8 | 0.002 | |
| STB (min) | 430.3±1545.3 | 368.0±1593.7 | 675.2±1404.2 | 162.7±136.2 | 346.5±517.2 | 0.016 | |
| Temporary pacemaker | 6.7 | 6.9 | 6.6 | 0 | 6.3 | 0.789 | |
| In-hospital mortality | 5.7 | 3.1 | 11.8 | 28.6 | 47.1 | <0.001 | |
AST, aspartate transaminase; ALT, alanine transaminase; CAD, coronary artery disease; CK, creatine kinase; CK-MB, creatine kinase-myocardial band; DBP, diastolic blood pressure; ER, emergency room; HH, hypoxic hepatitis; HLI, hypoxic liver injury; IABP, intra-aortic balloon pump; LAD, left anterior descending artery; LCX, left circumflex artery; LMCA, left main coronary artery; LVEF, left ventricular ejection fraction; RCA, right coronary artery; SBP, systolic blood pressure; TnI, troponin I; STB, symptom to balloon time.
Data are expressed as percentage or means±standard deviations.
Table 2. Univariate and Multivariate Binary Logistic Regression Analyses for Predicting HH.
| Variable | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p value | OR | 95% CI | p value | |
| Age (yr) | 1.008 | 0.981–1.035 | 0.580 | |||
| Male sex | 1.370 | 0.522–3.596 | 0.523 | |||
| Smoking | 1.130 | 0.652–1.957 | 0.663 | |||
| Diabetes | 1.495 | 0.710–3.148 | 0.290 | |||
| Hypertension | 1.476 | 0.718–3.033 | 0.290 | |||
| Dyslipidemia | 0.601 | 0.209–1.729 | 0.345 | |||
| Multi-vessel disease | 0.895 | 0.425–1.881 | 0.769 | |||
| Killip class 4 | 7.033 | 3.285–15.058 | <0.001 | 4.691 | 1.949–11.288 | 0.001 |
| Heart rate | 1.021 | 1.007–1.035 | 0.003 | 1.006 | 0.991–1.022 | 0.428 |
| LVEF≤35% | 10.021 | 4.624–21.719 | <0.001 | 6.802 | 2.957–15.645 | <0.001 |
| HLI | 4.483 | 2.186–9.191 | <0.001 | 2.572 | 1.166–5.675 | 0.019 |
OR, odds ratio; CI, confidence interval; LVEF, left ventricular ejection fraction; HH, hypoxic hepatitis; HLI, hypoxic liver injury.
Predictive value of HLI in the ER and developing HH during hospitalization
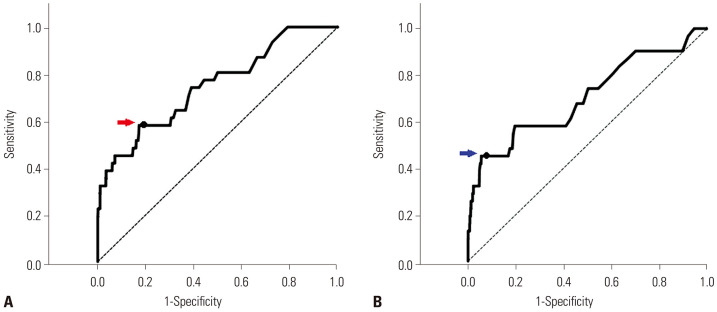
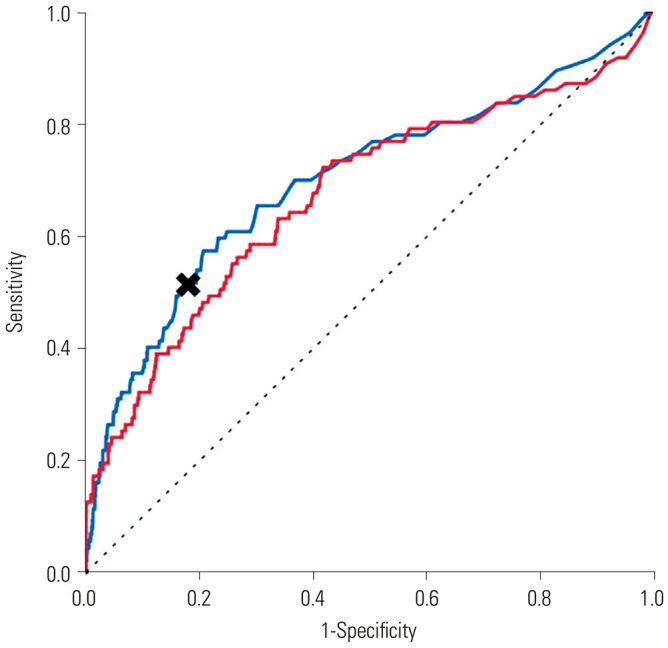
In ROC analysis, the predictive value of the presence of HLI utilizing AST at the ER for the development of HH during hospitalization was more favorable than that using ALT [cut-off value for AST>80: area under the curve (AUC) 0.737, 95% CI 0.643–0.830, sensitivity 0.548, specificity 0.805] (cut-off value for ALT >80: AUC 0.704, 95% CI 0.594–0.813) (Fig. 1). Therefore, a cut-off value of AST >80 was designated as the definition of HLI. In terms of in-hospital mortality, the predictive value of HLI at the ER and HH during hospitalization in STEMI patients who had undergone primary PCI were acceptable (AUC 0.701, 95% CI 0.635–0.767, sensitivity 0.517, specificity 0.817 for a cut-off value of AST >80 for HLI at ER and AUC 0.674, 95% CI 0.606–0.741, sensitivity 0.138, specificity 0.989 for a cut-off value of AST >800 for HH) (Fig. 2).
Fig. 1. HLI at ER for predicting HH utilizing AST and ALT. (A) ROC analysis utilizing AST for predicting the development of HH (AUC 0.737, 95% CI 0.643–0.830, red arrow: cut-off value AST>80 sensitivity 0.548 specificity 1–0.195=0.805). (B) ROC analysis utilizing ALT for predicting the development of HH (AUC 0.704, 95% CI 0.594–0.813, blue arrow: cut-off value ALT>80 sensitivity 0.452 specificity 1–0.071=0.929). ROC, receiver operating characteristics; AUC, area under the curve; AST, aspartate transaminase; ALT, alanine transaminase; CI, confidence interval; ER, emergency room; HH, hypoxic hepatitis; HLI, hypoxic liver injury.
Fig. 2. HLI at ER and HH for predicting in-hospital mortality (AUC 0.701, 95% CI 0.635–0.767, black cross: cut-off value AST>80 sensitivity 0.517 specificity 1–0.183=0.817 for HLI blue line) (AUC 0.674, 95% CI 0.606–0.741, cut-off value AST>800 sensitivity 0.138 specificity 1–0.011=0.989 for HH red line) (AST 400 sensitivity 0.287 specificity 1–0.086=0.914). HLI, hypoxic liver injury; ER, emergency room; HH, hypoxic hepatitis; AUC, area under the curve; AST, aspartate transaminase; CI, confidence interval.
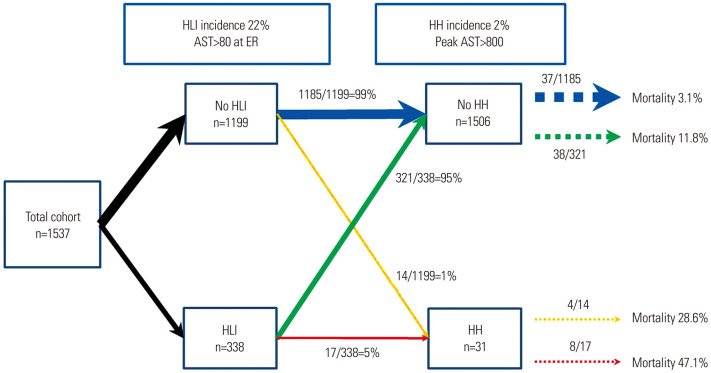
In-hospital mortality according to development of HH and HLI state
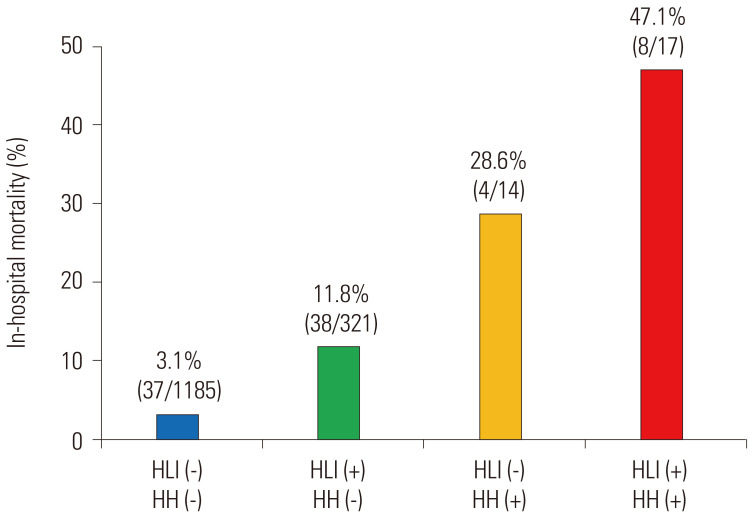
Fig. 3 depicts a schematic summary of our study. Of all 1537 patients, 338 (22%) had HLI, and 31 (2%) developed HH during hospitalization. Fourteen patients from the non HLI group developed HH, whereas the development of HH occurred in 17 patients in the HLI group (incidence rates of 1% and 5%, respectively). The overall in-hospital mortality in the study population was 5.7% (87/1537). The mortality rate for patients who had developed HH was higher than that for patients who did not develop HH (39% and 5%, respectively). Among patients without HLI and no HH, the mortality was 3.1% (37/1185). and for patients without HLI who developed HH, the mortality was 28.6% (4/14). Among patients with HLI and no HH, the mortality rate reached 11.8% (3/321), while that for patients with HLI who developed HH was 47.1% (8/17) (Figs. 3 and 4).
Fig. 3. Flow chart of HLI at ER and HH incidence and outcomes in STEMI patients undergoing primary PCI. HLI, hypoxic liver injury; ER, emergency room; HH, hypoxic hepatitis; AST, aspartate transaminase; STEMI, ST elevation myocardial infarction; PCI, percutaneous coronary intervention.
Fig. 4. In-hospital mortality of STEMI patients according to HLI at ER and HH. STEMI, ST elevation myocardial infarction; HLI, hypoxic liver injury; ER, emergency room; HH, hypoxic hepatitis.
DISCUSSION
In our study, more than one-fifth of the total study population had HLI, whereas only 2% developed HH. In-hospital mortality was significantly higher for patients who had either HLI at ER or developed HH during hospitalization. Patients who had HLI in the ER were more susceptible to developing HH during hospitalization, and both HLI at ER and HH development during hospitalization were predictive factors for in-hospital mortality. While it is evident that HH is a poor prognosticator in STEMI patients, it seems that HLI is a better prognostic factor, since the overall incidence of HH development is quite low.
Recent developments in interventional cardiology have provided STEMI patients who undergo PCI with higher survival rates.13 The prognosis of each individual, however, can vary from patient to patient, and there have been many studies reporting on prognostic factors related to coronary intervention. Tsai, et al.14 reported that acute kidney injury in patients who have undergone PCI was an independent predictor of in-hospital mortality. Others showed that dysglycemia was another indicator of poor outcomes: dysregulation of hormones in stressful situation precipitates insulin resistance causing elevated serum glucose that leads to organ damage.15
Many endeavors from recent years have discovered that serum aminotransferase, a biomarker broadly used to assess liver function, is a novel indicator of myocardial injury.16,17,18 The notion of serum aminotransferase as indicative marker for prognosis in STEMI has been reiterated several times in recent studies. One study showed that liver function enzymes (AST/ALT) were independent predictors of in-hospital mortality in STEMI patients, even after adjusting for CK-MB.7 Meanwhile, another indicated that HLI (even mild to modest serum aminotransferase elevation) results in poor prognosis in STEMI patients.10 Moreover, HLI in conjunction with acute kidney injury and dysglycemia maintained clinical significance as a prognosticator.19,20,21
Up until recently, the term HLI was used synonymously with HH. Hepatic function impairment is usually derived from either hypotensive state or passive congestion due to heart failure that causes centrilobular necrosis and ultimately elevates liver enzymes.22,23 Despite its clinical significance, the terminology used describing hepatic hypoxic insult is ambiguous. We acknowledged HLI and HH as independent syndromes and sought to verify the effect of each condition on the prognosis of STEMI patients. To minimize controversy regarding the type of liver enzyme and the cut-off value selected to define HLI, ROC analysis with both AST and ALT was performed. The AUC for AST with respect to a cut-off value above 80 was not only larger, but also had higher sensitivity than ALT in predicting the development of HH and in-hospital mortality, supporting our selection of AST as an HLI defining biomarker. Our study showed that patients presenting with HLI in the ER were at higher risk of mortality than patients without hepatic dysfunction. This result is consistent with a previous study suggesting that HLI could be a useful predictor of prognosis in STEMI patients.10 Patients developing HH during hospitalization were also related with higher death rates. In support thereof, a previous study highlighting the clinical implication of HH in ICU patients showed that HH has clinical significance in predicting mortality in critically ill patients.24
In our study, patients who had HLI presenting in the ER were more prone to developing HH during hospitalization than patients without HLI (5% vs. 1%). Univariate and multivariate logistic analyses also indicated that HLI was a predictive factor for developing HH. Indeed, patients who were absent of HLI were likely not to develop HH or experience in-hospital mortality. However, for patients with HLI, the rate of HH development was five-fold higher than that for patients without HLI, and for individuals who did develop HH, in-hospital mortality was highest. Even for patients who did not develop HH, those with HLI in the ER were at higher risk of death, which suggests that both the presence of HLI and HH development are associated with poor prognosis. Interestingly, conventional risk factors for CAD were not associated with the development of HH, whereas clinical factors (Killip class, heart rate) and hemodynamic status (LVEF ≤35%) were factors indicative of developing HH, supporting the hypothesis that hepatic hypoxic injury is usually due to a hypotensive condition and dysregulation of hormones.22,23
Numerous studies have reported the clinical manifestation of hepatic dysfunction in STEMI patients. However, the interpretation of the terms HLI and HH have been arbitrary, causing misconception of the two distinctly different syndromes. Our study differentiated HLI from HH according to degrees of hepatic dysfunction and managed to investigate their prognostic relevance and clinical relationships between the two syndromes.
Our study has several limitations. First, the design of our current study was observational, and the study population only comprised Koreans. Second, histopathological confirmation of centrilobular necrosis of hepatocytes, consistent with HH was not undertaken. Third, elevated AST levels could have been attributed to the sizes of myocardial infarctions, along with symptom to balloon time, in these patients. Patients with large myocardial infarctions and long symptom to balloon times tend to have higher incidences of HLI and HH and could have obscured the results of our study.
In conclusion, our study was able to show that STEMI patients presenting with HLI are at higher risk of developing HH during hospitalization. Both the presence of HLI in the ER and development of HH during hospitalization were independent predictors of in-hospital mortality. Therefore, HLI in the ER and development of HH during hospitalization could emerge as significant predictors for mortality during hospitalization in STEMI patients undergoing primary PCI.
ACKNOWLEDGEMENTS
This work was supported by an Inha University Hospital Research Grant.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: Seong Huan Choi, Ho-Jun Jang, Kyounghoon Lee, and Sung Woo Kwon.
- Data curation: Seong Huan Choi, Ho-Jun Jang, Sang-Don Park, and Sung Woo Kwon.
- Formal analysis: Seong Huan Choi, Ho-Jun Jang, Kyounghoon Lee, Sung Woo Kwon, and Young Ju Suh.
- Funding acquisition: Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Investigation: Seong Huan Choi, Ho-Jun Jang, Kyounghoon Lee, Sung Woo Kwon, and Tae-Hoon Kim.
- Methodology: Seong Huan Choi, Ho-Jun Jang, Sang-Don Park, and Sung Woo Kwon.
- Project administration: Seong Huan Choi, Ho-Jun Jang, Sung Woo Kwon, and Tae-Hoon Kim.
- Resources: Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Software: Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Supervision: Pyung Chun Oh, Jon Suh, Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Validation: Pyung Chun Oh, Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Visualization: Seong Huan Choi, Ho-Jun Jang, Sung Woo Kwon, and Jon Suh.
- Writing—original draft: Seong Huan Choi, Ho-Jun Jang, and Sung Woo Kwon.
- Writing—review & editing: Seong Huan Choi, Ho-Jun Jang, Sung Woo Kwon, and WoongChol Kang.
- Approval of final manuscript: all authors.
References
- 1.Kostis WJ, Deng Y, Pantazopoulos JS, Moreyra AE, Kostis JB Myocardial Infarction Data Acquisition System (MIDAS14) Study Group. Trends in mortality of acute myocardial infarction after discharge from the hospital. Circ Cardiovasc Qual Outcomes. 2010;3:581–589. doi: 10.1161/CIRCOUTCOMES.110.957803. [DOI] [PubMed] [Google Scholar]
- 2.Morrow DA, Antman EM, Charlesworth A, Cairns R, Murphy SA, de Lemos JA, et al. TIMI risk score for ST-elevation myocardial infarction: a convenient, bedside, clinical score for risk assessment at presentation: an intravenous nPA for treatment of infarcting myocardium early II trial substudy. Circulation. 2000;102:2031–2037. doi: 10.1161/01.cir.102.17.2031. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg RJ, Spencer FA, Gore JM, Lessard D, Yarzebski J. Thirty-year trends (1975 to 2005) in the magnitude of, management of, and hospital death rates associated with cardiogenic shock in patients with acute myocardial infarction: a population-based perspective. Circulation. 2009;119:1211–1219. doi: 10.1161/CIRCULATIONAHA.108.814947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mamas MA, Anderson SG, O'Kane PD, Keavney B, Nolan J, Oldroyd KG, et al. Impact of left ventricular function in relation to procedural outcomes following percutaneous coronary intervention: insights from the British Cardiovascular Intervention Society. Eur Heart J. 2014;35:3004–3012. doi: 10.1093/eurheartj/ehu303. [DOI] [PubMed] [Google Scholar]
- 5.Rashed KA, McNabb WR, Lewis RR. Ischaemic hepatitis in the elderly. Gerontology. 2002;48:245–249. doi: 10.1159/000058358. [DOI] [PubMed] [Google Scholar]
- 6.Chávez-Tapia NC, Balderas-Garces BV, Meza-Meneses P, Herrera-Gomar M, García-López S, Gónzalez-Chon O, et al. Hypoxic hepatitis in cardiac intensive care unit: a study of cardiovascular risk factors, clinical course, and outcomes. Ther Clin Risk Manag. 2014;10:139–145. doi: 10.2147/TCRM.S59312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lofthus DM, Stevens SR, Armstrong PW, Granger CB, Mahaffey KW. Pattern of liver enzyme elevations in acute ST-elevation myocardial infarction. Coron Artery Dis. 2012;23:22–30. doi: 10.1097/MCA.0b013e32834e4ef1. [DOI] [PubMed] [Google Scholar]
- 8.Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS); European Association for Percutaneous Cardiovascular Interventions (EAPCI) Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501–2555. doi: 10.1093/eurheartj/ehu278. [DOI] [PubMed] [Google Scholar]
- 9.Kim TH, Lee HJ, Jang HJ, Kim JS, Park JS, Choi RK, et al. Impact of final kissing balloon inflation after simple stent implantation for the treatment of non-left main true coronary bifurcation lesions in patients with acute coronary syndrome. Int J Cardiol. 2014;177:907–911. doi: 10.1016/j.ijcard.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Moon J, Kang W, Oh PC, Seo SY, Lee K, Han SH, et al. Serum transaminase determined in the emergency room predicts outcomes in patients with acute ST-segment elevation myocardial infarction who undergo primary percutaneous coronary intervention. Int J Cardiol. 2014;177:442–447. doi: 10.1016/j.ijcard.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Henrion J, Descamps O, Luwaert R, Schapira M, Parfonry A, Heller F. Hypoxic hepatitis in patients with cardiac failure: incidence in a coronary care unit and measurement of hepatic blood flow. J Hepatol. 1994;21:696–703. doi: 10.1016/s0168-8278(94)80226-2. [DOI] [PubMed] [Google Scholar]
- 12.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 13.Lim HS, Farouque O, Andrianopoulos N, Yan BP, Lim CC, Brennan AL, et al. Survival of elderly patients undergoing percutaneous coronary intervention for acute myocardial infarction complicated by cardiogenic shock. JACC Cardiovasc Interv. 2009;2:146–152. doi: 10.1016/j.jcin.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 14.Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, et al. Contemporary incidence, predictors, and outcomes of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the NCDR Cath-PCI registry. JACC Cardiovasc Interv. 2014;7:1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, et al. Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation. 2008;117:1018–1027. doi: 10.1161/CIRCULATIONAHA.107.740498. [DOI] [PubMed] [Google Scholar]
- 16.Wróblewski F, Ruegsegger P, LaDue JS. Serum lactic dehydrogenase activity in acute transmural myocardial infarction. Science. 1956;123:1122–1123. doi: 10.1126/science.123.3208.1122. [DOI] [PubMed] [Google Scholar]
- 17.Naschitz JE, Slobodin G, Lewis RJ, Zuckerman E, Yeshurun D. Heart diseases affecting the liver and liver diseases affecting the heart. Am Heart J. 2000;140:111–120. doi: 10.1067/mhj.2000.107177. [DOI] [PubMed] [Google Scholar]
- 18.Kavoliuniene A, Vaitiekiene A, Cesnaite G. Congestive hepatopathy and hypoxic hepatitis in heart failure: a cardiologist's point of view. Int J Cardiol. 2013;166:554–558. doi: 10.1016/j.ijcard.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Park SD, Moon J, Kwon SW, Suh YJ, Kim TH, Jang HJ, et al. Prognostic impact of combined contrast-induced acute kidney injury and hypoxic liver injury in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention: results from INTERSTELLAR registry. PLoS One. 2016;11:e0159416. doi: 10.1371/journal.pone.0159416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang HJ, Oh PC, Moon J, Suh J, Park HW, Park SD, et al. Prognostic impact of combined dysglycemia and hypoxic liver injury on admission in patients with ST-segment elevation myocardial infarction who underwent primary percutaneous coronary intervention (from the INTERSTELLAR cohort) Am J Cardiol. 2017;119:1179–1185. doi: 10.1016/j.amjcard.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Oh PC, Eom YS, Moon J, Jang HJ, Kim TH, Suh J, et al. Prognostic impact of the combination of serum transaminase and alkaline phosphatase determined in the emergency room in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. PLoS One. 2020;15:e0233286. doi: 10.1371/journal.pone.0233286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birrer R, Takuda Y, Takara T. Hypoxic hepatopathy: pathophysiology and prognosis. Intern Med. 2007;46:1063–1070. doi: 10.2169/internalmedicine.46.0059. [DOI] [PubMed] [Google Scholar]
- 23.Arcidi JM, Jr, Moore GW, Hutchins GM. Hepatic morphology in cardiac dysfunction: a clinicopathologic study of 1000 subjects at autopsy. Am J Pathol. 1981;104:159–166. [PMC free article] [PubMed] [Google Scholar]
- 24.Ebert EC. Hypoxic liver injury. Mayo Clin Proc. 2006;81:1232–1236. doi: 10.4065/81.9.1232. [DOI] [PubMed] [Google Scholar]