Abstract
The epidermis is a stratified squamous epithelium composed primarily of keratinocytes that become postmitotic and undergo sequential changes in gene expression during terminal differentiation. The expression of the transcription factor CCAAT/enhancer binding protein β (C/EBPβ) within mouse epidermis and primary keratinocytes has recently been described; however, the function of C/EBPβ within the epidermal keratinocyte is unknown. We report here that transient transfection of mouse primary keratinocytes with a C/EBP-responsive promoter-reporter construct resulted in a sevenfold increase in luciferase activity when keratinocytes were switched to culture conditions that induce growth arrest and differentiation. Forced expression of C/EBPβ in BALB/MK2 keratinocytes inhibited growth, induced morphological changes consistent with a more differentiated phenotype, and upregulated two early markers of differentiation, keratin 1 (K1) and keratin 10 (K10) but had a minimal effect on the expression of late-stage markers, loricrin and involucrin. Analysis of the epidermis of C/EBPβ-deficient mice revealed a mild epidermal hyperplasia and decreased expression of K1 and K10 but not of involucrin and loricrin. C/EBPβ-deficient primary keratinocytes were partially resistant to calcium-induced growth arrest. Analysis of terminally differentiated spontaneously detached keratinocytes or those induced to differentiate by suspension culture revealed that C/EBPβ-deficient keratinocytes displayed striking decreases in K1 and K10, while expression of later-stage markers was only minimally altered. Our results demonstrate that C/EBPβ plays an important role in the early events of stratified squamous differentiation in keratinocytes involving growth arrest and K1 and K10 expression.
The epidermis is a stratified squamous epithelium composed primarily of keratinocytes that form four distinct morphological layers. Each epidermal layer or compartment represents a different phenotypic stage in the terminal differentiation program of the keratinocyte. This program begins when the basal keratinocyte becomes postmitotic and initiates its migration upward through the spinous and granular layers to eventually form the nonviable cornified stratum corneum (for reviews, see references 18 and 52). The process of stratified squamous differentiation is a dynamic one involving a highly coordinated program of gene expression that includes both induction and repression. For example, the transition of the basal keratinocyte from the basal layer to the spinous layer is accompanied by the repression of basal keratinocyte transcripts keratin 5 (K5), keratin 14 (K14) (17, 56), and α4β6 integrin (49) and the upregulation of the early-stage differentiation markers, keratin 1 (K1) and keratin 10 (K10) (34, 37, 41). The transition from the spinous to granular layer is accompanied by the suppression of K1 and K10 transcripts and the upregulation of transcripts for the cornified envelope precursor proteins such as involucrin, loricrin, and filaggrin (13–15, 26, 39). Epidermal transglutaminase cross-links these and other proteins to form the cornified envelope, and subsequent to the digestion of the intracellular organelles, the mature nonviable squame is formed. While the stages of squamous differentiation with their concomitant changes in gene expression are well characterized, the transcription factors that regulate the induction and repression of differentiation-specific genes remain largely uncharacterized.
The C/EBP family of transcription factors is composed of at least five distinct members [C/EBPα, C/EBPβ, C/EBPδ, C/EBPɛ, and Ig/EBP(C/EBPγ)] (6, 55) (for a review, see reference 53) belonging to the basic leucine zipper (bZIP) class of transcription factors. C/EBPα and C/EBPβ are expressed in human and mouse primary keratinocytes (31, 51) as well as in the human, mouse, and rat interfollicular epidermis (25, 31, 47). Within the mouse interfollicular epidermis, C/EBPα is expressed in the nuclei and cytoplasm of suprabasal keratinocytes and weakly expressed in a perinuclear manner in some basal keratinocytes (31). C/EBPβ expression is highly compartmentalized and is exclusive to the nuclei of a three-cell cluster of suprabasal keratinocytes which is morphologically consistent with the differentiative column of the epidermal proliferative unit. In primary mouse keratinocytes, C/EBPβ expression is upregulated during calcium-induced growth arrest and squamous differentiation (31). Thus, C/EBPβ appears to have a role in the regulation of genes involved in or specifically expressed during squamous differentiation of the epidermis. Additional indirect evidence for a role for C/EBPβ in squamous differentiation comes from the observation that C/EBPβ expression is greatly diminished in squamous cell carcinomas (31), as is the expression of K1, K10, loricrin, and filaggrin (59).
C/EBPβ (also known as NF-IL6, IL-6DBP, NF-M, CRP2, or LAP) is involved in the regulation of the expression of a number of cytokine genes, and C/EBPβ binding motifs are found in the regulatory regions of interleukin-1β (IL-1β), IL-6, IL-8, tumor necrosis factor alpha, and granulocyte colony-stimulating factor (1, 11, 28, 29, 60). C/EBPβ also plays a role in the early stages of preadipocyte differentiation (6, 57) and differentiation of certain cells of the myeloid lineage (29, 42). C/EBPβ-deficient mice display immune defects including lymphoproliferative disorder; distorted humoral, innate, and cellular immunity; imbalanced T-helper cell response (43); and impaired tumor cytotoxicity and bactericidal activity of macrophages (48). Female mice lacking C/EBPβ are infertile due to the failure of ovarian granulosa cells to differentiate into luteal cells (46), and these mice also demonstrate defects in the proliferation and differentiation of mammary epithelial cells (36, 44).
In the present study, we have evaluated the role of C/EBPβ in epidermal keratinocyte proliferation and squamous differentiation. We have examined the transactivation activity of endogenous C/EBP in primary keratinocytes under both proliferative and differentiative conditions and have evaluated the effect of the forced expression of C/EBPβ on keratinocyte growth and differentiation. In addition, we have analyzed the epidermis of C/EBPβ-deficient mice in vivo, have isolated primary keratinocytes from these mice, and have examined their ability to undergo growth arrest and terminal differentiation. Our results demonstrate that C/EBPβ plays an important role in the early stages of squamous differentiation involving growth arrest and K1 and K10 expression.
MATERIALS AND METHODS
Materials.
Fetal bovine serum, trypsin, antibiotics-antimycotics, and protein molecular weight markers were purchased from GIBCO BRL (Gaithersburg, Md.). Eagle minimal essential medium (EMEM) (Ca2+ free) was purchased from BioWhittaker (Walkersville, Md.). Human recombinant epidermal growth factor (hEGF) was purchased from United States Biochemical (Cleveland, Ohio). pcDNA3 expression vector and the PerFect lipid (pFx-3) were purchased from Invitrogen (San Diego, Calif.). Rabbit polyclonal antibodies for C/EBPα, C/EBPβ, and p21Cip1/WAF1 and mouse monoclonal antibody to C/EBPβ were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). K1, K10, K5, and involucrin rabbit polyclonal antibodies were purchased from Berkeley Antibody Company (Richmond, Calif.). Rabbit polyclonal antibody for loricrin was a kind gift from G. Paolo Dotto, Harvard Medical School, Charlestown, Mass. Mouse monoclonal bromodeoxyuridine (BrdU) antibody was purchased from Becton Dickinson (San Jose, Calif.). Goat anti-rabbit immunoglobulin G (IgG) Texas Red and goat anti-mouse IgG fluorescein isothiocyanate (FITC) were purchased from Southern Biotechnology Associates, Inc. (Birmingham, Ala.). Horseradish peroxidase-linked donkey anti-rabbit IgG and the ECL kit were purchased from Amersham (Arlington Heights, Ill.). Biotinylated secondary goat anti-rabbit IgG was purchased from Boehringer Mannheim (Indianapolis, Ind.). Peroxidase-conjugated streptavidin and 5,5′-diaminobenzidine were purchased from BioGenex (San Ramon, Calif.). [3H-methyl]thymidine (20 Ci/mmol) was purchased from DuPont-New England Nuclear Research Products (Boston, Mass.). BrdU, methylcellulose (4,000 cP), and calcium chloride were purchased from Sigma (St. Louis, Mo.). Tris-glycine precast gels were from Novex (San Diego, Calif.). Bio-Rad DC protein assay reagent was purchased from Bio-Rad (Richmond, Calif.).
Animals.
CD-1 mice were purchased from Charles River Laboratory (Raleigh, N.C.). C/EBPβ-deficient mice generated by homologous recombination have been described previously (46). C/EBPβ-deficient male mice were mated with heterozygous female mice to produce greater yields of C/EBPβ-deficient mice. C/EBPβ+/+ mice were mated to generate control subjects. Both mutants and controls represented F2 × F4 crosses of C57BL/6 and 129/SV strains. Mice were genotyped by Southern blot analysis of tail DNA as described previously (46). The mice were fed no. 5001 rodent chow (Purina Mills, Inc., Richmond, Ind.) and water ad libitum. The mice were kept on corncob bedding and placed on a 12-h light-dark cycle until they were used.
Isolation and cultivation of primary epidermal keratinocytes.
Primary keratinocytes were isolated from newborn CD-1, C/EBPβ wild-type, or C/EBPβ-deficient mice (less than 3 days old) by overnight trypsin flotation at 4°C (10, 19). C/EBPβ-deficient newborn mice were genotyped by Western blot analysis with whole-liver homogenates. Isolated keratinocytes (pooled from 5 to 10 newborn mice) were plated at 6 × 106 cells/60-mm-diameter plate or at 0.75 × 106 cells/well in 24-well culture dishes in Ca2+-free EMEM supplemented with 10% non-Chelex-treated fetal bovine serum and 4 ng of hEGF per ml for 4 h to enhance cell attachment. Cultures were then gently washed with Mg2+- and Ca2+-free phosphate-buffered saline (PBS) to remove any remaining calcium and unattached cells and then refed with low-calcium medium (Ca2+-free EMEM supplemented with 4% Chelex-treated fetal bovine serum, 10 ng of hEGF per ml, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 250 ng of amphotericin B [Fungizone]/ml, with added calcium chloride to a final concentration of 0.05 mM). Medium was changed daily.
Transfection of primary CD-1 keratinocytes and luciferase assays.
Primary CD-1 keratinocytes (3 days after plating) were transfected in triplicate with the following construct: pXP1, pMGF40, pMGF65, or pMGF82 (45). Two micrograms of vector DNA and 12 μg of lipid transfection reagent, pFx-3, were incubated for 20 min at room temperature to form complexes and overlaid onto primary keratinocyte culture in the serum-free EMEM containing 4 ng of hEGF per ml and 0.05 mM calcium chloride. Cultures were incubated at 37°C and 5% CO2 for 4 h and then washed with PBS and refed with low-calcium medium. After 15 h, cultures were either switched to high-calcium medium (0.12 mM) or refed with low-calcium medium (0.05 mM). Forty-eight hours later, cells were harvested and the luciferase activity was determined by using the luciferase assay kit (Promega). Protein concentrations were determined by the Bio-Rad DC protein assay.
Construction of C/EBPβ vector and its transfection in BALB/MK2 keratinocytes.
The C/EBPβ coding region (∼0.8 kb) containing a Kozak translation initiation sequence was released from pMEX-C/EBPβ vector (55) by BamHI and KpnI digestion and ligated to linearized pcDNA3 (by BamHI and EcoRI) at the BamHI site. This ligated vector was recut by KpnI (pcDNA3 contains a KpnI site 5′ to the BamHI site) to release the coding sequence of C/EBPβ with KpnI sites on both ends, which was used as the insert DNA in a final ligation reaction with KpnI-linearized pcDNA3. The resulting ligated vector was transformed into One-Shot Top 10 F′-competent cells (Invitrogen, Carlsbad, Calif.), and vector DNA was prepared from expanded individual colonies. The recombinant vector containing a single copy of the C/EBPβ insert (determined by restriction enzyme mapping analysis) in the sense orientation (determined by PCR analysis) was designated pcDNA3-C/EBPβ.
BALB/MK2 keratinocytes were a gift from B. Weissman (University of North Carolina, Chapel Hill). BALB/MK2 keratinocytes were transfected when they reached 30 to 40% confluence in 60-mm-diameter dishes with 2 μg of vector DNA (pcDNA3 or pcDNA3-C/EBPβ) and 12 μg of Lipofectin reagent, pFx-3. Transfection was performed in serum-free EMEM (containing 0.05 mM calcium and 4 ng of hEGF per ml) at 37°C and 5% CO2 for 15 h, after which time the cells were refed with low-calcium medium (Ca2+-free EMEM supplemented with 8% Chelex-treated fetal bovine serum, 4 ng of hEGF per ml, and calcium chloride to a final concentration of 0.05 mM). Twenty-four hours later, the cultures were split (1:5) and replated in the above medium. Twenty-four hours after replating, G418 was added to the medium at a concentration of 500 μg/ml, and this selection medium was changed every other day. On days 3, 5, 7, and 10 after G418 selection, the total number of colonies in 50 random grid squares was counted and then converted to colonies per dish (550 grid squares/plate). The number of cells per colony was scored directly from 50 randomly chosen colonies.
Immunochemical staining of C/EBPβ, involucrin, loricrin, K1, and K10 in pcDNA3- and pcDNA3-C/EBPβ-transfected BALB/MK2 keratinocytes.
BALB/MK2 cells were transfected by pcDNA3 and pcDNA3-C/EBPβ vector as described in the previous section. Forty-eight hours after transfection, cultures were rinsed three times with PBS and fixed in cold methanol for 10 min. The endogenous peroxidase activity was quenched by incubation in 0.1% H2O2 in PBS for 10 min at room temperature. After three washings with PBS, the cultures were blocked with 1.5% normal goat serum (NGS) in PBS for 30 min at room temperature and then incubated with the primary rabbit polyclonal antibodies against C/EBPβ, K1, K10, involucrin, or loricrin (all 1:2,000) in 1.5% NGS in PBS at 4°C overnight. After three washings with PBS, the samples were incubated with a biotinylated goat anti-rabbit IgG for 30 min at room temperature followed by a 30-min incubation with peroxidase-conjugated streptavidin. The avidin-biotin-peroxidase complexes were visualized by incubation with 5,5′-diaminobenzidine according to the manufacturer’s protocol. Cultures incubated with the secondary antibody alone (biotinylated goat anti-rabbit IgG) did not develop any positive immunostaining. Cultures were observed at an ×100 magnification, and single dark-brown-stained positive cells were counted in 25 fields per sample. Results are expressed as the number of positive cells per field. Observations from 10 fields/sample showed that there was no significant difference in the total number of cells per field between pcDNA3 vector control-transfected cultures (2,280 ± 190) and pcDNA3-C/EBPβ-transfected cultures (2,250 ± 210). For immunofluorescence detection of C/EBPβ and K1, BALB/MK2 cells were plated onto coverslips (0.5-in. diameter) in 60-mm-diameter dishes and transfected as described above. Forty-eight hours after transfection, the cultures were fixed in methanol at −20°C for 10 min, and coverslips were mounted on a microscope slide. Coverslip cultures were treated as described above and then incubated with rabbit K1 polyclonal antibody (1:2,000) and mouse C/EBPβ monoclonal antibody (1:2,000) in 1.5% NGS at 4°C overnight. After washing, the samples were incubated with secondary antibodies (FITC-conjugated goat anti-mouse IgG and Texas Red-conjugated goat anti-rabbit IgG) at room temperature for 30 min. After rinsing, glass coverslips were mounted over the samples with Vector Mounting medium and cells were examined with a Nikon microscope equipped with filter cubes for the detection of FITC and Texas Red fluorescence.
Western blot analysis of C/EBPs and various differentiation-associated marker proteins.
Pooled primary keratinocytes isolated from newborn wild-type and C/EBPβ-deficient mice were grown in low-calcium medium with medium change daily. On day 5, one set of the cultures was detached from the plates by trypsinization and inoculated into a suspension culture medium (low-calcium medium plus 1.4% methylcellulose) at a density of 2 × 106 cells/ml and incubated at 37°C and 5% CO2 for 16 h. On day 6, attached cells, spontaneously detached cells, and suspension-cultured cells were harvested separately and placed in a lysis buffer (10 mM Tris HCl [pH 7.5] containing 5% sodium dodecyl sulfate and 20% β-mercaptoethanol). Cell lysates were sonicated for 5 s and boiled for 5 min. For the in vivo study, protein samples were prepared from epidermis of both wild-type and C/EBPβ-deficient female mice (16 to 18 weeks old). Dorsal hair was clipped with electric clippers, dorsal skin was removed, and epidermal cells were isolated by trypsin flotation (10, 19). The isolated cells were placed directly into the above-described lysis buffer, sonicated, centrifuged to remove hair fibers, and then boiled for 5 min. In order to determine the protein concentration, a portion of each sample was first precipitated in 6% trichloroacetic acid in the presence of 125 μg of Na-deoxycholate (4) per ml and then quantitated by the Lowry assay (23). Equal amounts of each protein sample were loaded on 10 or 12% polyacrylamide Tris-glycine gels (Novex) and separated by electrophoresis. The separated proteins were transferred to an Immobilon P membrane (Millipore, Bedford, Mass.). Following incubation in blocking buffer (PBS with 1% bovine serum albumin, 5% milk, and 0.1% Tween) for 1 h at room temperature, the membranes were probed overnight at 4°C with rabbit polyclonal IgG raised against C/EBPα (1:2,000), C/EBPβ (1:2,000), K1 (1:2,000), K10 (1:2,000), K5 (1:2,000), involucrin (1:2,000), loricrin (1:2,000), or p21Cip1/WAF1 (1:1,000). The membranes were washed and then probed with a secondary antibody (1:2,500-diluted horseradish peroxidase-linked donkey anti-rabbit immunoglobulin from Amersham) for 1 h at room temperature. Detection was made with an enhanced chemiluminescence reagent followed by exposure film. The densitometric quantitation of the bands of interest was conducted with a Zeineh laser scanning densitometer (model SLR-1D/2D; Fullerton, Calif.).
Northern blot analysis of K1 expression.
Primary keratinocytes isolated from wild-type and C/EBPβ-deficient newborn mice were cultured in low-calcium medium for 7 days, the attached proliferative population of cells was collected, and RNA was isolated. In addition, differentiation was induced in attached keratinocytes by placing these cells in suspension culture (12) for 16 h, and these cells were also collected on day 7. RNA was isolated and Northern blot analysis was conducted as previously described (31) with a 32P-labeled 400-bp K1 probe (kindly provided by Stuart Yuspa, National Cancer Institute, Bethesda, Md.).
Analysis of epidermal keratinocyte proliferation in C/EBPβ-deficient mice in vivo and in BALB/MK2 keratinocytes.
Skin histological sections were prepared from both wild-type and C/EBPβ-deficient mice (24 weeks old) according to our previous methods (30). In vivo BrdU labeling was conducted by a single-dose intraperitoneal injection of BrdU (100 mg/kg of body weight) 1 h before the animals were sacrificed. Immunochemical staining of BrdU-positive cells was performed as described before (30). The BrdU labeling index (quantitated in 1,000 interfollicular basal keratinocytes per section), the thickness of epidermis, and the number of nucleated cell layers (determined in 20 locations per section) were determined. For BrdU labeling studies in BALB/MK2 cells, BALB/MK2 keratinocytes were transfected with C/EBPβ or empty vector control. Transfected keratinocytes were selected for 24 h with G418, and the number of S-phase BrdU-positive cells was determined at 0, 24, and 48 h post-G418 removal. BrdU (10 μg/ml) was added to the culture medium 4 h before each time point, and the cultures were fixed in ethanol-acetic acid (49:1) at −20°C for 20 min. Immunochemical staining for BrdU was performed as described for the in vivo samples.
Primary keratinocyte proliferation determination.
Pooled primary keratinocytes from newborn wild-type and C/EBPβ-deficient mice were plated in 24-well culture plates as described above. The number of attached cells as well as the number of spontaneous detached cells per well was determined in triplicate cultures on days 1 to 8 after plating. DNA synthesis was also measured every day in triplicate samples. Briefly, cultures were pulse-labeled with [3H-methyl]thymidine (3 μCi/ml) for 1 h. After three washings with PBS, cells were collected by trypsinization, resuspended in 1 mM EDTA buffer, and sonicated for 10 s, and aliquot samples were collected onto glass fiber filters with a manifold sample collector. After sequential washings with cold 4% perchloric acid and 70, 95, and 100% ethanol, the filters were counted for radioactivity in a liquid scintillation counter. DNA quantitation was conducted by Hoechst 33258 fluorometry (5). An aliquot of each sample and 5 μl of Hoechst 33258 solution (0.1 mg/ml in distilled water) were mixed in 2 ml of 0.01 M Tris (pH 7.0)–0.1 M NaCl–0.01 M EDTA buffer and incubated at room temperature for 5 min. The fluorescent units were determined with a fluorimeter (excitation at 365 nm and emission at 450 nm). Sample DNA concentrations were determined by use of the calf thymus DNA standard curve, and results were expressed as disintegrations per minute per microgram of DNA. In separate experiments, the response of wild-type and C/EBPβ-deficient keratinocytes to the growth-inhibitory effects of calcium chloride was also studied. Primary cultures were maintained for 6 to 7 days in low-calcium medium and switched to medium containing 0.12 mM Ca2+ or refed low-Ca2+ medium. [3H-methyl]thymidine incorporation was determined as described above.
RESULTS
Endogenous C/EBP transactivation activity is increased under conditions that induce growth arrest and differentiation in primary keratinocytes.
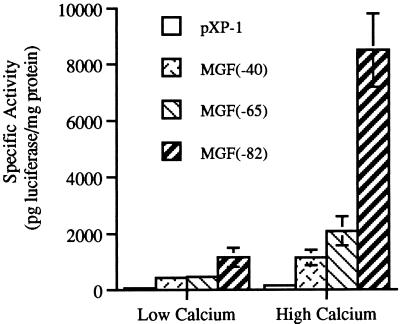
Primary mouse keratinocytes can be shifted from a proliferative state to a growth-arrested state by increasing the calcium concentration in the medium from low (0.05 mM) to high (0.12 mM) (19). Following growth arrest, some keratinocytes undergo differentiation as indicated by the expression of K1, K10, loricrin, and filaggrin (58). Previously, we have demonstrated that when primary keratinocyte cultures from CD-1 mice are switched from low to high calcium, C/EBPα protein levels are modestly increased by 20% while the C/EBPβ protein level is increased 400 to 800% by 16 h post-calcium switch (31). In the present study, we evaluated the trans-activating activity of endogenous C/EBP proteins during this process by utilizing a luciferase reporter gene under the regulation of different lengths of the C/EBP-dependent myelomonocytic growth factor (MGF) promoter (45). The following constructs were employed: pXP1, a promoterless construct; pMGF40, a 40-bp portion of the MGF promoter that lacks C/EBP sites; and pMGF65 and pMGF82, which contain one and two C/EBP binding sites, respectively. Two C/EBP binding sites are necessary for maximal C/EBP transactivation of the MGF promoter (45). As shown in Fig. 1, primary CD-1 mouse keratinocytes transfected with pXP1 or pMGF40 demonstrated low luciferase activity in low-calcium medium with a minimal twofold or less increase in luciferase expression in high-calcium medium. In contrast, keratinocytes transfected with pMGF65 and pMGF82 exhibited four- and sevenfold induction, respectively, of luciferase activity when switched from low- to high-calcium medium. In low-calcium medium, pMGF82 exhibited approximately twofold-greater luciferase activity than pMGF40 while in high-calcium medium pMGF82 exhibited eightfold-greater activity than pMGF40. Thus, the increase in luciferase activity is dependent upon C/EBP binding sites in the MGF promoter, indicating that the endogenous trans-activating activity of C/EBP is increased in primary mouse keratinocytes in high-calcium medium.
FIG. 1.
Transactivation potential of endogenous C/EBPs in primary keratinocytes. Three days after plating, primary CD-1 keratinocytes were transfected with the indicated promoter-luciferase reporter constructs. Keratinocyte cultures were shifted to high-calcium medium or maintained in low-calcium medium for 48 h. Cells were then harvested, and the luciferase activity was determined. Results are expressed as the mean ± standard deviation of a representative experiment with three plates/group.
C/EBPβ inhibits the growth and alters the cell morphology of BALB/MK2 keratinocytes.
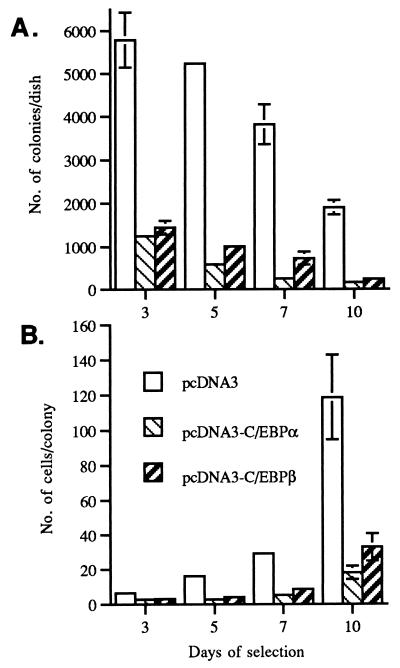
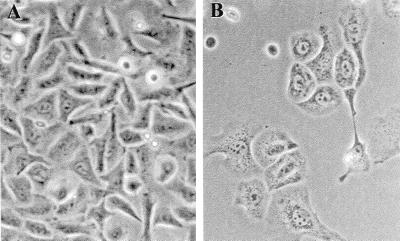
To determine whether C/EBPβ can influence keratinocyte growth, we examined the effect of the forced expression of C/EBPβ on BALB/MK2 keratinocytes when cultured under proliferative conditions (low-calcium medium). BALB/MK2 keratinocytes were employed, as mouse primary keratinocytes cannot be passaged in serum-containing medium and they require high cell densities for growth. BALB/MK2 keratinocytes are a nontransformed immortalized cell line that retains responsiveness to the modulation of growth arrest and terminal differentiation induced by increased calcium concentrations (54). An expression vector, pcDNA3-C/EBPβ, which placed the C/EBPβ cDNA under the regulation of the cytomegalovirus promoter was constructed. The pcDNA3 vector also contains a neomycin resistance gene under the regulation of the simian virus 40 promoter. Empty pcDNA3 or pcDNA3-C/EBPβ constructs were transfected into BALB/MK2 keratinocytes, and some plates from both groups were immunostained with a C/EBPβ-specific antibody. At 24 and 48 h posttransfection, keratinocytes transfected with pcDNA3-C/EBPβ demonstrated a five- and a ninefold increase, respectively, in the number of cells staining positive for C/EBPβ compared to cells transfected with empty pcDNA3, confirming that C/EBPβ was expressed from the construct. At 24 h after transfection, the cells were cultured in low-calcium medium in the presence of G418, and as shown in Fig. 2A, after 3 days of G418 selection there were 75% fewer colonies per dish in cultures transfected with pcDNA3-C/EBPβ than in cultures transfected with empty pcDNA3. The number of colonies per dish continued to decrease, and by 10 days, the cultures transfected with pcDNA3-C/EBPβ demonstrated approximately 90% fewer colonies per dish than did cultures transfected with empty pcDNA3. In addition to the decrease in the number of colonies per dish, the number of cells per colony in the cultures transfected with pcDNA3-C/EBPβ was also decreased. As shown in Fig. 2B, there were 70 to 80% fewer cells per colony in the pcDNA3-C/EBPβ-transfected cultures than in the cultures transfected with the pcDNA3 vector control after 5, 7, and 10 days of G418 selection. As shown in Fig. 3, cells transfected with pcDNA3-C/EBPβ exhibited an enlarged and flattened morphology similar to the cell morphology observed in BALB/MK2 cells switched to high-calcium medium. These results indicate that forced expression of C/EBPβ inhibits the growth and alters the cell morphology of BALB/MK2 keratinocytes. The above experiments were also conducted with C/EBPα, and as shown in Fig. 2, C/EBPα also reduced the number of colonies per dish and cells per colony, and these cells also demonstrated an enlarged and flattened morphology. These results indicate that there is functional overlap with regard to the ability of C/EBPα and C/EBPβ to inhibit keratinocyte growth and induce alterations in cell morphology.
FIG. 2.
Forced expression of C/EBPβ and C/EBPα inhibits BALB/MK2 keratinocyte growth. BALB/MK2 keratinocytes were transfected with empty pcDNA3, pcDNA3-C/EBPβ, or pcDNA3-C/EBPα and subsequently subcultured in low-calcium medium in the presence of 500 μg of G418 per ml. The number of colonies per dish and the number of cells per colony were determined at days 3, 5, 7, and 10 of G418 selection. Data are expressed as the mean ± standard deviation of a representative experiment done in triplicate for each group. (A) Number of colonies per dish. (B) Number of cells per colony.
FIG. 3.
Forced expression of C/EBPβ alters BALB/MK2 keratinocyte morphology. BALB/MK2 keratinocytes were transfected with empty pcDNA3 or pcDNA3-C/EBPβ and subsequently subcultured in low-calcium medium in the presence of 500 μg of G418 per ml. At day 7 of G418 selection, photographs of colonies (×200) were taken of BALB/MK2 keratinocytes transfected with empty pcDNA3 (A) and BALB/MK2 keratinocytes transfected with pcDNA3-C/EBPβ (B).
After 10 days of G418 selection, some colonies in the plates transfected with pcDNA3-C/EBPβ survived and continued to proliferate. At least six colonies from each group were isolated and expanded in the presence of G418. Western blot analysis of whole-cell lysates prepared from these pcDNA3-C/EBPβ colonies showed no increase in C/EBPβ protein expression compared with that from pcDNA3 colonies (data not shown). These results suggest that the surviving and proliferating G418-resistant colonies originating from cultures transfected with pcDNA3-C/EBPβ have retained the neomycin resistance gene but have lost the ability to express C/EBPβ from the pcDNA3-C/EBPβ construct.
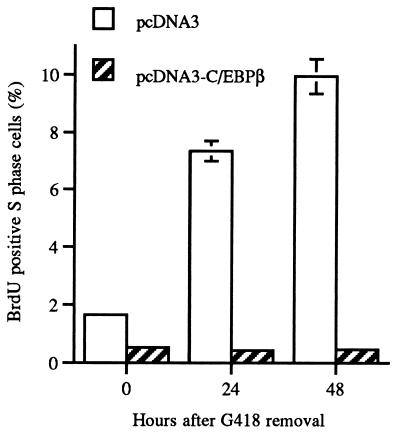
To provide additional evidence that C/EBPβ has the capacity to inhibit growth independent of the presence of G418 and long-term G418 selection, we conducted BrdU labeling studies. Twenty-four hours following transfection of BALB/MK2 keratinocytes with C/EBPβ or empty vector control, transfected keratinocytes were selected for 24 h with G418 and the number of S-phase BrdU-positive cells was determined at 0, 24, and 48 h post-G418 removal. As shown in Fig. 4, C/EBPβ-transfected keratinocytes were growth inhibited and did not display any increase in the number of S-phase cells in the absence of G418 while empty vector-transfected cells displayed a four- to fivefold increase in the number of S-phase BrdU-positive cells. Keratinocytes that remained in the presence of G418 for 48 h continued to be growth inhibited and displayed BrdU S-phase labeling indices similar to that observed at 0 h after G418 removal. These results indicate that C/EBPβ induces growth inhibition independent of the presence of G418 and long-term G418 selection.
FIG. 4.
Transient transfection of BALB/MK2 keratinocytes with pcDNA3-C/EBPβ inhibits growth as indicated by the number of BrdU-positive S-phase cells. Twenty-four hours following transfection of BALB/MK2 keratinocytes with C/EBPβ or empty vector control, transfected keratinocytes were selected for 24 h with G418, and the number of S-phase BrdU-positive cells was determined at 0, 24, and 48 h post-G418 removal. The number of S-phase BrdU-positive cells was determined per 1,000 cells, and the results are expressed as the number of S-phase BrdU-positive cells per 1,000 cells × 100.
C/EBPβ induces K1 and K10 expression in BALB/MK2 keratinocytes.
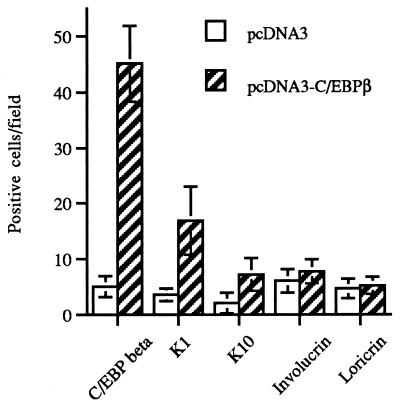
BALB/MK2 keratinocytes were transfected with empty pcDNA3 or pcDNA3-C/EBPβ to determine if C/EBPβ can alter the expression of differentiation-specific genes. We examined the expression of K1 and K10, two early markers of keratinocyte differentiation, and involucrin and loricrin, two markers which are expressed later in the differentiation program. Forty-eight hours after transfection, keratinocytes were immunostained for C/EBPβ, K1, K10, involucrin, and loricrin. As shown in Fig. 5, there was a ninefold increase in the number of C/EBPβ-positive cells in the keratinocytes transfected with pcDNA3-C/EBPβ vector compared to that in the keratinocyte cultures transfected with the empty pcDNA3 vector. In addition, there was a five- and a threefold increase in the number of K1- and K10-positive cells, respectively, in the keratinocytes transfected with pcDNA3-C/EBPβ. The number of cells staining positive for involucrin and loricrin was only minimally increased in the keratinocytes transfected with pcDNA3-C/EBPβ. Similar results were obtained when the cells were immunostained 72 h after transfection (data not shown). The cells that stained positive for C/EBPβ, K1, and K10 were detected as isolated single cells despite the fact that the BALB/MK2 keratinocytes were transfected when the cells were 30% confluent and immunostained 48 h later when they were 100% confluent. These data indicate that increased expression of C/EBPβ is associated with increases in the expression of K1 and K10 proteins and further support our notion that C/EBPβ modulates keratinocyte growth and the early events in differentiation.
FIG. 5.
Transient transfection of BALB/MK2 keratinocytes with pcDNA3-C/EBPβ increases K1 and K10 expression. BALB/MK2 keratinocytes were cultured in low-calcium medium and transfected with empty pcDNA3 or pcDNA3-C/EBPβ. Immunochemical staining for C/EBPβ, involucrin, loricrin, K1, and K10 was conducted at 48 h after transfection. Single dark-brown-stained positive cells were quantitated in 10 fields (×100 magnification) per dish. Data were expressed as number of positive cells per field (means ± standard deviations). The number of C/EBPβ-, K1-, and K10-positive cells in the pcDNA3-C/EBPβ-transfected cultures was statistically significantly different from that in pcDNA3-transfected cultures (P < 0.01, two-tailed Student’s t test).
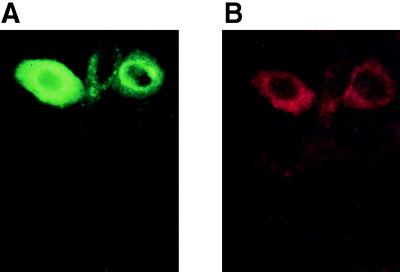
To ensure that K1 expression occurs in the C/EBPβ-transfected cell populations, double-immunofluorescence detection studies were conducted. As shown in Fig. 6A, C/EBPβ-transfected positive cells demonstrated bright green fluorescence nuclear staining and these same cells coexpressed K1 as indicated by intense red fluorescence cytoplasmic staining (Fig. 6B). Double immunofluorescence staining showed that 26% of the C/EBPβ-positive cells coexpressed K1, and this result is similar to the value reported in Fig. 5. It was observed that the brightest C/EBPβ-transfected cells generally did not display the strongest K1 signal but that rather the medium- to lower-intensity C/EBPβ-transfected cells produced the greatest K1 signal, suggesting that very high levels of C/EBPβ may be inhibitory to K1 expression. Empty vector-transfected cells demonstrated very few C/EBPβ- or K1-positive cells.
FIG. 6.
Immunofluorescence detection of the coexpression of C/EBPβ and K1 in pcDNA3-C/EBPβ-transfected BALB/MK2 keratinocytes. BALB/MK2 keratinocytes were transiently transfected with pcDNA3-C/EBPβ and processed for detection of C/EBPβ and K1 coexpression as described in Materials and Methods. (A) C/EBPβ staining (FITC). (B) K1 staining (Texas Red).
C/EBPβ-deficient mice demonstrate abnormalities in keratinocyte proliferation and differentiation.
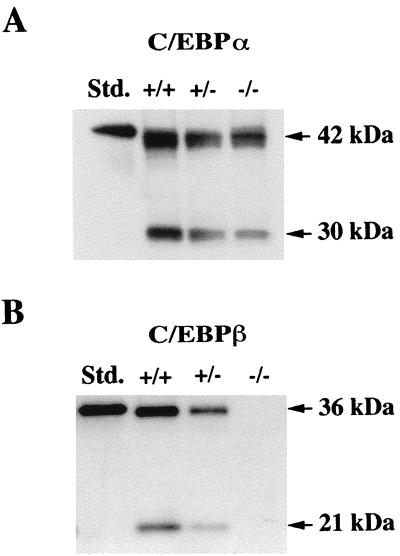
To gain further insight into the functional role of C/EBPβ in epidermal keratinocytes, we analyzed the epidermis of mice which carry a targeted deletion of C/EBPβ. Since both C/EBPα and C/EBPβ are expressed in mouse epidermis, it was of interest to first determine whether the absence of the C/EBPβ protein influenced the level of expression of the C/EBPα protein. Whole-cell epidermal lysates were prepared from three C/EBPβ-null, three heterozygous, and three wild-type mice. Representative Western blot analyses are shown in Fig. 7. As shown in Fig. 7A, C/EBPα protein levels (42 kDa) were similar in all three genotypes. The 30-kDa C/EBPα truncated protein level appears to be decreased in C/EBPβ-null mice; however, other Western blot analyses did not demonstrate such a decrease, suggesting that the observed decrease may be an artifact due to poor transfer or poor wetting of the membrane with the chemiluminescence solutions. As expected, C/EBPβ (36 and 21 kDa) proteins could not be detected in epidermal lysates isolated from C/EBPβ-null mice, while their levels in the C/EBPβ heterozygous mice were intermediate between those of the C/EBPβ-deficient mice and those of the wild-type mice (Fig. 7B). Thus, the absence of the C/EBPβ protein in the epidermis has little or no effect on epidermal C/EBPα protein levels.
FIG. 7.
C/EBPα and C/EBPβ expression in the epidermis of wild-type, C/EBPβ-heterozygous, and C/EBPβ-deficient adult mice. Whole-cell epidermal lysates were prepared from the epidermis of adult female mice, and Western blot analysis was conducted. (A) C/EBPα protein in wild-type (+/+), C/EBPβ-heterozygous (+/−), and C/EBPβ-deficient (−/−) mice. (B) C/EBPβ protein in wild-type (+/+), C/EBPβ-heterozygous (+/−), and C/EBPβ-deficient (−/−) mice. C/EBPα and C/EBPβ standards (Std.) are histidine tagged and migrate more slowly than the native protein.
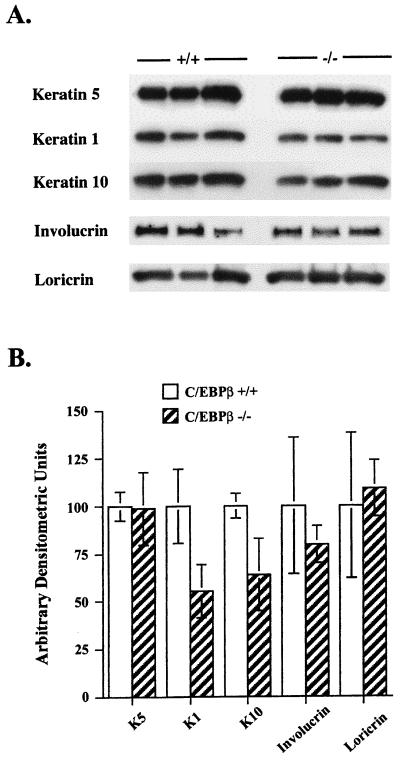
As shown in Table 1, C/EBPβ-deficient mice demonstrated a mild epidermal hyperplasia. There were statistically significant (P < 0.05) increases in epidermal thickness and the number of nucleated cell layers, as well as the number of S-phase BrdU-positive keratinocytes in the interfollicular epidermis of C/EBPβ-deficient mice compared with that of wild-type mice. To determine if the observed abnormalities in keratinocyte proliferation are accompanied by alterations in keratinocyte differentiation, the epidermis was isolated from the wild-type and C/EBPβ-deficient mice and Western blot analysis was conducted to determine whether the expression of K5, K1, K10, and the cornified envelope proteins loricrin and involucrin was altered. We chose to examine K5, as it is expressed in the basal layer, while K1 and K10 are expressed upon transition from the basal to the spinous layer of the epidermis. Involucrin and loricrin are expressed later in the differentiation program in the granular layers of the epidermis. As shown in Fig. 8, there were modest but consistent decreases in K1 and K10 levels (45 and 35%, respectively; P < 0.05) in the epidermis of C/EBPβ-deficient mice compared to that of wild-type mice as determined by laser densitometric analysis. In contrast, the levels of K5, loricrin, and involucrin in epidermal preparations isolated from C/EBPβ-deficient mice were similar to those in wild-type mice (P > 0.05). These results indicate that C/EBPβ-deficient mice display abnormalities in keratinocyte growth and K1 and K10 expression in the interfollicular epidermis and that these abnormalities occur in the absence of alterations in the level of C/EBPα.
TABLE 1.
Altered epidermal keratinocyte proliferation in C/EBPβ-deficient micea
| Genotype | Epidermal thickness (μm) | Nucleated cell layers | Labeling index (% S phase) |
|---|---|---|---|
| C/EBPβ+/+ | 13.1 ± 0.7 | 2.0 ± 0.2 | 4.2 ± 1.5 |
| C/EBPβ−/− | 21.3 ± 2.7b | 3.0 ± 0.4b | 7.6 ± 2.8c |
Each value represents the mean ± standard deviation from five mice/group.
Value is significantly different from corresponding wild-type value (P < 0.001) as determined by two-tailed Student’s t test.
Value is significantly different from corresponding wild-type value (P < 0.05).
FIG. 8.
C/EBPβ-deficient mice demonstrate alterations in epidermal K1 and K10 expression. Whole-cell epidermal lysates were prepared from the epidermis of adult wild-type and C/EBPβ-deficient female mice. (A) Western blot analysis was conducted with specific antisera as indicated. Epidermal lysates from wild-type and C/EBPβ-deficient mice are represented by +/+ and −/−, respectively, and each lane contains protein from a different mouse. (B) Densitometric analysis was conducted on Western blot autoradiographs in which wild-type and C/EBPβ-deficient extracts were run on the same gel. Results are expressed as the mean ± standard deviation of six mice/group. K1 and K10 levels in the C/EBPβ-deficient mice were significantly different from the wild-type levels (P < 0.05, two-tailed Student’s t test).
Primary keratinocytes isolated from C/EBPβ-deficient mice display decreases in K1 and K10 expression.
In low-calcium medium, attached keratinocytes resemble the basal keratinocytes of the epidermis. The attached keratinocytes are a proliferative population, and when an attached keratinocyte terminally differentiates, it spontaneously detaches from the plate and is replaced by the attached proliferative keratinocytes. Therefore, two distinct populations of keratinocytes, the spontaneously detached terminally differentiated cells and the attached proliferative undifferentiated cells, can be evaluated. Keratinocytes from wild-type and C/EBPβ-deficient newborn mice were isolated, and the ability of these primary keratinocytes to undergo growth arrest and differentiation was examined. All experiments used pooled keratinocytes of a single genotype and were repeated at least three times. In low-calcium medium, C/EBPβ-deficient keratinocytes grew to 50% higher saturation density than wild-type keratinocytes, and at confluence, C/EBPβ-deficient keratinocytes were smaller and more polygonal in shape with more highly distinct intercellular spaces. Confluent cultures of C/EBPβ-deficient keratinocytes exhibited a 45 to 50% decrease in DNA synthesis as determined by [3H]thymidine incorporation into DNA and a concomitant 30% decrease in the number of spontaneously detached differentiated cells compared to the wild-type keratinocytes. Based on the decreased number of spontaneously detached cells and the increased number of attached cells at confluence, we speculated that C/EBPβ-deficient keratinocytes may have an attenuated ability to initiate or execute early events in the process of keratinocyte differentiation.
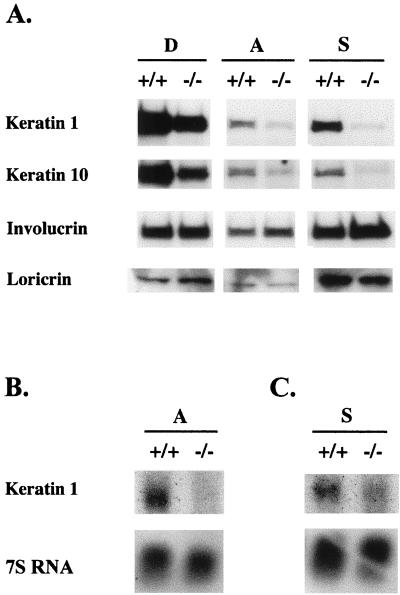
To characterize defects in differentiation at the molecular level, C/EBPβ-deficient and wild-type primary keratinocytes were cultured in low-calcium medium for 6 days, and then the spontaneously detached and attached proliferative populations of cells were collected and lysates were prepared for Western blot analysis. In addition, we induced differentiation in attached keratinocytes by placing these cells in suspension culture for 16 h and collected the cells for Western blot analysis on day six (12). A comparison of the expression of K1, K10, involucrin, and loricrin in C/EBPβ-deficient keratinocytes with that in wild-type keratinocytes revealed striking differences in the expression of K1 and K10 (Fig. 9A). The spontaneously detached, attached, and suspension-cultured C/EBPβ-deficient keratinocytes expressed 40, 70, and 95% less K1 than the wild-type counterparts, respectively. Likewise, K10 expression was dramatically decreased. Compared to that in their wild-type keratinocyte counterparts, K10 expression was decreased by 50, 30, and 95% in spontaneously detached, attached, and suspension-cultured C/EBPβ-deficient keratinocytes, respectively. Overexposure of the K1 and K10 signals in the detached keratinocytes was necessary to produce detectable signals for K1 and K10 in attached and suspension-cultured cells. Densitometric analysis of films produced from shorter exposures revealed that K1 and K10 in detached C/EBPβ-deficient keratinocytes were decreased by 70 and 80%, respectively, compared to detached wild-type keratinocytes (data not shown). As shown in Fig. 9A, involucrin and loricrin protein expression was similar in the C/EBPβ-deficient keratinocytes compared to the wild-type counterpart, indicating that the lack of C/EBPβ does not cause a general decrease in all markers of differentiation. Based on these results, the early differentiation-specific events involving K1 and K10 expression that occur upon transition from the basal to the spinous layer of the epidermis are preferentially altered by the deletion of the C/EBPβ gene. Since keratin expression is predominately regulated at the level of transcription (17, 38), we conducted Northern blot analysis for K1 mRNA on RNA isolated from the attached and suspension-cultured keratinocytes. As shown in Fig. 9B and C, C/EBPβ-deficient keratinocytes display significantly decreased K1 mRNA levels compared to the wild-type keratinocytes. These results were consistently observed in experiments with different preparations of isolated primary newborn keratinocytes. These results support a role for C/EBPβ in the regulation of K1 mRNA levels.
FIG. 9.
Altered expression of K1 and K10 in attached, spontaneously detached, and suspension-cultured primary keratinocytes from C/EBPβ-deficient mice. (A) Primary newborn keratinocytes were maintained in low-calcium medium. At day 6 after plating, attached cells (A), spontaneously detached cells (D), and 16-h suspension-cultured cells (S) (see text) were collected; whole-cell lysates were prepared; and Western blot analysis was conducted as indicated. Lysates from wild-type and C/EBPβ-deficient samples are represented by +/+ and −/−, respectively. (B and C) Primary newborn keratinocytes were maintained in low-calcium medium. At day 7 after plating, attached cells (B) and 16-h suspension-cultured cells (C) (see text) were collected; RNA was isolated; and Northern blot analysis for K1 was conducted.
Primary keratinocytes isolated from C/EBPβ-deficient mice are resistant to calcium-induced growth arrest.
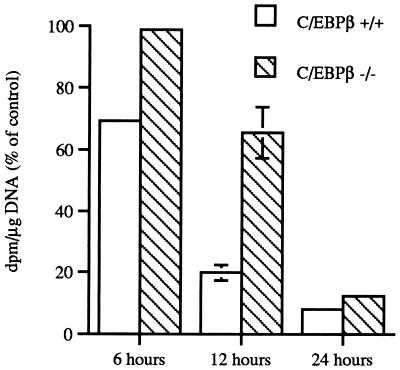
Since C/EBPβ appeared to influence the early events in keratinocyte differentiation and C/EBPβ-deficient mice displayed an epidermal hyperplasia, we examined whether C/EBPβ-deficient keratinocytes displayed defects in their ability to undergo calcium-induced growth arrest. Growth arrest of primary keratinocytes is an early event in the process of keratinocyte differentiation, occurring prior to the expression of early markers of differentiation, such as K1 and K10. Keratinocytes isolated from the epidermis of wild-type and C/EBPβ-deficient mice were shifted from medium containing 0.05 mM calcium to medium containing 0.12 mM calcium. The cells were harvested at 6, 12, and 24 h, and growth arrest was monitored by 1-h pulse-labeling with [3H]thymidine during the last hour prior to harvest. As shown in Fig. 10, when wild-type keratinocytes were switched to medium containing 0.12 mM calcium, growth arrest occurred very rapidly; by 6 h there was a 35% decrease in DNA synthesis and by 12 h DNA synthesis was decreased by greater than 80%. In contrast, C/EBPβ-deficient keratinocytes were resistant to calcium-induced growth arrest. As shown in Fig. 10, there was no decrease in DNA synthesis in C/EBPβ-deficient keratinocytes at 6 h after the switch to medium containing 0.12 mM calcium and only a 35% decrease at 12 h. However, by 24 h DNA synthesis was decreased to a level similar to that observed in the wild-type keratinocytes. C/EBPβ-deficient keratinocytes shifted to medium containing 2.0 mM calcium demonstrated a similar resistance to the growth arrest effects of calcium (data not shown). To determine if C/EBPα underwent a compensatory upregulation in the C/EBPβ-deficient keratinocytes, Western blot analysis was conducted on cell extracts isolated from wild-type and C/EBPβ-deficient keratinocytes at 0, 6, 12, and 24 h post-high-calcium shift. No differences in C/EBPα levels were observed between wild-type and C/EBPβ-deficient keratinocytes (data not shown). Recent evidence indicates that p21Cip1/WAF1 plays an important role in regulating both keratinocyte growth and differentiation (9). Following the addition of a high level of calcium to the medium, it has been shown that p21Cip1/WAF1 is rapidly induced and produces a block in cell cycle progression at the G1 phase. However, keratinocyte differentiation is also blocked by p21Cip1/WAF1 and does not ensue until p21Cip1/WAF1 levels return to basal levels. Thus, it has been proposed that p21Cip1/WAF1 couples growth arrest and differentiation in keratinocytes (9). Western blot analysis of whole-cell lysates from wild-type and C/EBPβ-deficient keratinocytes for p21Cip1/WAF1 protein levels revealed that p21Cip1/WAF1 levels increased two- to threefold at 6 h after the switch to 0.12 mM calcium in both groups and subsequently decreased within 24 h to levels lower than that observed before the calcium switch (data not shown), indicating that p21Cip1/WAF1 expression is not altered in the C/EBPβ-deficient keratinocytes.
FIG. 10.
C/EBPβ-deficient epidermal keratinocytes are resistant to calcium-induced growth arrest in vitro. Primary keratinocytes were cultured in low-calcium medium for 6 to 7 days and then either switched to high-calcium medium or refed with low-calcium medium. Cultures were pulse-labeled with [3H-methyl]thymidine for 1 h prior to harvest. Disintegrations per minute per microgram of DNA were determined from triplicate plates per group, and the data are presented as percentages of control [3H-methyl]thymidine incorporation in keratinocytes cultured in low-calcium medium at each time point.
DISCUSSION
Within the mouse epidermis, C/EBPβ is exclusively detected in the nuclei of suprabasal keratinocytes (31). This highly compartmentalized location of C/EBPβ suggested that C/EBPβ plays a role in the regulation of genes involved in or specifically expressed during the process of squamous differentiation (31). Our current results provide the first evidence that C/EBPβ can directly modulate the program of squamous differentiation in the epidermis and in isolated keratinocytes. We propose that C/EBPβ is involved in the regulation of the early stages of squamous differentiation of epidermal keratinocytes based on the following experimental evidence: (i) forced expression of C/EBPβ inhibits growth, induces K1 and K10 in BALB/MK2 keratinocytes, and has minimal effects on later-stage differentiation markers; (ii) differentiated C/EBPβ-deficient primary keratinocytes, both spontaneous detached and suspension culture-induced, demonstrate striking decreases in K1 and K10 expression with minimal alterations in later-stage differentiation markers; (iii) C/EBPβ-deficient primary keratinocytes display resistance to calcium-induced growth arrest; and (iv) direct analysis of C/EBPβ-deficient mouse skin revealed a hyperplastic epidermis and decreases in K1 and K10 expression with minimal differences in involucrin, loricrin, or K5 expression. Thus, results derived from both mutating and overexpressing C/EBPβ support a functional role for the protein in the regulation of growth arrest and K1 and K10 expression in keratinocytes.
While our findings identify a functional role for C/EBPβ in the regulation of K1 and K10 levels, it is not known if this is a direct effect of C/EBPβ within the promoter regions of K1 and K10 or if C/EBPβ is indirectly modulating K1 and K10 levels. However, the levels of both K1 and K10 are largely regulated at the level of transcription (17, 38). Sequence analysis of the K1 and K10 promoters (22, 35) revealed that both promoters contain several potential C/EBP binding sites. Utilizing the C/EBP-dependent MGF promoter, we demonstrated that the transactivation activity of endogenous C/EBP in keratinocytes increases under conditions known to induce growth arrest and differentiation. In addition, K1 mRNA levels are dramatically decreased in C/EBPβ-deficient keratinocytes. Taken together, these findings suggest that C/EBPβ may directly modulate the transcription of K1 and K10. Regardless, it is clear that C/EBPβ influences K1 and K10 levels and that additional factors also contribute to the regulation of K1 and K10, as their expression was not completely abolished in C/EBPβ-deficient epidermis or primary keratinocytes. Skn-1a, a member of the POU domain family of transcription factors, is expressed in the suprabasal layers of the epidermis and has been shown previously to activate the K10 promoter in HeLa cells (2); however, keratinocytes from Skn-1a-deficient mice do not demonstrate alterations in K10 levels (3). With regard to K1, an AP-1 and/or steroid site has been identified in the 3′ flanking region of the K1 gene, and this element imparts some responsiveness to calcium-induced differentiation (21, 24, 40). c-Fos, a component of AP-1 that has been proposed to function in the terminal stages of epidermal differentiation, also exhibits exclusive expression in the three cells of the epidermal proliferative unit (16). Fos and C/EBP can form an association in vitro (20) which could impart another level of complexity to the regulation of K1. Further work will be required to determine whether bona fide C/EBP binding sites exist in the K1 and K10 promoters and whether C/EBP interacts with other transcription factors.
The alterations in K1 and K10 expression observed in isolated C/EBPβ-deficient primary keratinocytes were more striking than the more modest changes observed in the epidermis of C/EBPβ-deficient mice. It is possible that the disruption of epidermal homeostatic mechanisms that tend to attenuate the expression of genetic defects in intact skin may allow for a fuller expression of the defect in keratinocytes in primary culture. Phenotypic differences between intact skin and primary keratinocytes have been reported elsewhere for other null mice (27).
Growth arrest of primary keratinocytes is an early event in the process of keratinocyte differentiation occurring prior to the expression of early markers of differentiation, such as K1 and K10. Our results indicate that C/EBPβ-deficient keratinocytes exhibit growth abnormalities in intact skin as well as in primary culture. In addition, we have found that the forced expression of C/EBPα also inhibits keratinocyte growth. Since C/EBPα is expressed in the basal keratinocytes of the epidermis, it may initiate growth inhibition, and then C/EBPβ maintains growth arrest and also induces the expression of K1 and K10 upon upward movement of the basal keratinocyte to the suprabasal layers of the epidermis. Since both C/EBPα and C/EBPβ are expressed in suprabasal keratinocytes, it is possible that they form heterodimers and cooperate to induce growth arrest. Further studies are necessary to determine whether C/EBPα and C/EBPβ induce growth arrest through different mechanisms. p21Cip1/WAF1 is considered to be a factor in keratinocyte growth arrest and differentiation (9). In colorectal cancer cells, p21Cip1/WAF1 is induced via a pathway involving C/EBPβ (8). While we did observe the normal characteristic increase in p21Cip1/WAF1 levels followed by a decrease to below control levels in keratinocytes in high-calcium medium, we did not observe any major differences between wild-type and C/EBPβ-deficient mice. These results suggest that the deletion of the C/EBPβ gene does not interfere with the expression of p21Cip1/WAF1 in primary keratinocytes and provide evidence that keratinocyte growth can be regulated by multiple molecular mechanisms. The retinoblastoma (Rb) family of proteins are important regulators of the cell cycle, and recently, Rb has been shown to influence adipocyte differentiation through its physical interaction with C/EBP (7). Rb family members can influence epidermal differentiation and keratinocyte proliferation (32) and as such may represent potential target proteins for C/EBPβ interactions and growth inhibition. A recent paper by Paramio et al. (33) demonstrated that K10 expression but not K12, K14, and K16 expression inhibits keratinocyte proliferation through an Rb pathway. These authors suggest that the complex differential expression of cytokeratins that occurs during squamous differentiation may be important in cell cycle regulation. The altered regulation of K10 in C/EBPβ-deficient keratinocytes may contribute to the altered growth characteristics of these cells. Recently, it has been demonstrated that C/EBPα can interact with Rb family member p107, and this interaction results in the disruption of E2F/p107 S-phase complexes (50). The disruption of these complexes is associated with C/EBPα-induced growth arrest in hepatocytes of newborn mice. Whether C/EBPα and C/EBPβ can interact with p107 and alter cell cycle progression in keratinocytes is an area of future study. While further studies are required to discern the downstream pathway through which C/EBPβ regulates K1, K10, and growth arrest, our study provides novel fundamental insights into the function of C/EBPβ in the early events of squamous differentiation.
REFERENCES
- 1.Akira S, Issiki H, Sugita T, Tanabe O, Kinoshita S, Nishio Y, Nakajima T, Hirano T, Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990;9:1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson B, Schonemann M D, Flynn S E, Pearse II R V, Singh H, Rosenfeld M G. Skn-1a and Skn-1i: two functionally distinct Oct-2-related factors expressed in epidermis. Science. 1993;260:78–82. doi: 10.1126/science.7682011. [DOI] [PubMed] [Google Scholar]
- 3.Anderson B, Weinberg W C, Rennekampff O, McEvilly R J, Bermingham J R, Jr, Hooshmand F, Vasilyev V, Hansbrough J F, Pittelkow M R, Yuspa S H, Rosenfeld M G. Functions of the POU domain genes SKN-1a/i and Tst-1/Oct-6/SCIP in epidermal differentiation. Genes Dev. 1997;11:1873–1884. doi: 10.1101/gad.11.14.1873. [DOI] [PubMed] [Google Scholar]
- 4.Bensadoun A, Weinstein D. Assay of protein in the presence of interfering materials. Anal Biochem. 1976;70:241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- 5.Brunk C F. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979;92:497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- 6.Cao Z, Umek R M, McKnight S L. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes Dev. 1991;5:1538–1552. doi: 10.1101/gad.5.9.1538. [DOI] [PubMed] [Google Scholar]
- 7.Chen P L, Riley D J, Lee W H. Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev. 1996;10:2794–2804. doi: 10.1101/gad.10.21.2794. [DOI] [PubMed] [Google Scholar]
- 8.Chinery R, Brockman J A, Peeler M O, Shyer S Y, Beauchamp R D, Coffey R J. Antioxidants enhance the cytotoxicity of chemotherapeutic agents in colorectal cancer: a p53-independent induction of p21 WAF1/CIPI via C/EBPβ. Nat Med. 1997;11:1233–1241. doi: 10.1038/nm1197-1233. [DOI] [PubMed] [Google Scholar]
- 9.Di Cunto F, Topley G, Calautti E, Hsiao J, Ong L, Seth P K, Dotto G P. Inhibitory function of p21/Cip1/WAF1 in differentiation of primary mouse keratinocytes independent of cell cycle control. Science. 1998;280:1069–1072. doi: 10.1126/science.280.5366.1069. [DOI] [PubMed] [Google Scholar]
- 10.Dlugosz A A, Glick A B, Tennenbaum T, Weinberg W C, Yuspa S H. Isolation and utilization of epidermal keratinocytes for oncogene research. Methods Enzymol. 1995;254:3–20. doi: 10.1016/0076-6879(95)54003-2. [DOI] [PubMed] [Google Scholar]
- 11.Drouet C, Shakhov A N, Jongeneel C V. Enhancers and transcription factors controlling the inducibility of the tumor necrosis factor-α promoter in primary macrophages. J Immunol. 1991;147:1694–1700. [PubMed] [Google Scholar]
- 12.Drozdoff V, Pledger W J. Commitment to differentiation and expression of early differentiation markers in murine keratinocytes in vitro are regulated independently of extracellular calcium concentrations. J Cell Biol. 1993;123:909–919. doi: 10.1083/jcb.123.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eckert R L. Structure, function and differentiation of the keratinocyte. Physiol Rev. 1989;69:1316–1346. doi: 10.1152/physrev.1989.69.4.1316. [DOI] [PubMed] [Google Scholar]
- 14.Eckert R L, Yaffe M B, Crish J F, Murthy S, Rorke E A, Welter J F. Involucrin—structure and role in envelope assembly. J Investig Dermatol. 1993;100:613–617. doi: 10.1111/1523-1747.ep12472288. [DOI] [PubMed] [Google Scholar]
- 15.Fisher C, Haydock P V, Dale B A. Localization of profilaggrin mRNA in newborn rat skin by in situ hybridization. J Investig Dermatol. 1987;88:661–664. doi: 10.1111/1523-1747.ep12470281. [DOI] [PubMed] [Google Scholar]
- 16.Fisher C, Byers M R, Iadarola M J, Powers E A. Patterns of epidermal expression of Fos protein suggest important role in the transition from viable to cornified cell during keratinization. Development. 1991;111:253–258. doi: 10.1242/dev.111.2.253. [DOI] [PubMed] [Google Scholar]
- 17.Fuchs E, Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980;19:1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990;111:2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hennings H, Michael D, Cheng C, Steinert P, Holbrook K, Yuspa S H. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell. 1980;19:245–254. doi: 10.1016/0092-8674(80)90406-7. [DOI] [PubMed] [Google Scholar]
- 20.Hsu W, Kerppola T K, Chen P L, Curran T, Chen-Kiang S. Fos and Jun repress transcription activation by NF-IL6 through association at the basic zipper region. Mol Cell Biol. 1994;14:268–276. doi: 10.1128/mcb.14.1.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huff C A, Yuspa S H, Rosenthal D. Identification of control elements 3′ to the human keratin 1 gene that regulate cell type and differentiation-specific expression. J Biol Chem. 1993;268:377–384. [PubMed] [Google Scholar]
- 22.Johnson L D, Idler W W, Zhou X, Roop D R, Steinert P M. Structure of a gene for human epidermal 67-kDa keratin. Proc Natl Acad Sci USA. 1985;82:1896–1900. doi: 10.1073/pnas.82.7.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein determination with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 24.Lu B, Rothnagel J A, Longley M A, Tsai S Y, Roop D R. Differentiation-specific expression of human keratin 1 is mediated by a composite AP-1/steroid hormone element. J Biol Chem. 1994;269:7443–7449. [PubMed] [Google Scholar]
- 25.Maytin E V, Habener J F. Transcription factors C/EBPα, C/EBPβ, and CHOP (Gadd153) expressed during the differentiation program of keratinocytes in vivo and in vitro. J Investig Dermatol. 1998;110:238–246. doi: 10.1046/j.1523-1747.1998.00123.x. [DOI] [PubMed] [Google Scholar]
- 26.Mehrel T, Hohl D, Rothnagel J A, Longley M A, Bunoman D, Cheng C, Lichti U, Bisher M E, Steven A C, Steiner P M, Yuspa S H, Roop D R. Identification of a major keratinocyte cell envelope protein, loricrin. Cell. 1990;61:1103–1112. doi: 10.1016/0092-8674(90)90073-n. [DOI] [PubMed] [Google Scholar]
- 27.Missero C, Cunto F D, Kiyokawa H, Koff A, Dotto G P. The absence of p21Cip1/WAF1 alters keratinocyte growth and differentiation and promotes ras-tumor progression. Genes Dev. 1996;10:3065–3075. doi: 10.1101/gad.10.23.3065. [DOI] [PubMed] [Google Scholar]
- 28.Mukaida N, Mahe Y, Matsushima K. Cooperative interaction of NF-κB- and C/EBP-like factor binding elements in activating the interleukin-8 gene by proinflammatory cytokines. J Biol Chem. 1990;265:21128–21133. [PubMed] [Google Scholar]
- 29.Natsuka S, Akira S, Nishio Y, Hashimoto S, Sugita T, Isshiki H, Kishimoto T. Macrophage differentiation specific expression of NF-IL6, a transcription factor for IL-6. Blood. 1992;79:460–466. [PubMed] [Google Scholar]
- 30.Oh H, Smart R C. An estrogen receptor pathway regulates the telogen-anagen hair follicle transition and influences epidermal cell proliferation. Proc Natl Acad Sci USA. 1996;95:12525–12530. doi: 10.1073/pnas.93.22.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oh H, Smart R C. Expression of CCAAT/enhancer binding protein (C/EBP) is associated with squamous differentiation in epidermis and isolated primary keratinocytes and is altered in skin neoplasms. J Investig Dermatol. 1998;110:939–945. doi: 10.1046/j.1523-1747.1998.00199.x. [DOI] [PubMed] [Google Scholar]
- 32.Paramio J M, Lain S, Segrelles C, Lane E B, Jorcano J L. Differential expression and functionally co-operative roles for the retinoblastoma family of proteins in epidermal differentiation. Oncogene. 1998;17:949–957. doi: 10.1038/sj.onc.1202031. [DOI] [PubMed] [Google Scholar]
- 33.Paramio J M, Casanova M L, Segrelles C, Mittnacht S, Lane E B, Jorcano J L. Modulation of cell proliferation by cytokeratins K10 and K16. Mol Cell Biol. 1999;19:3086–3094. doi: 10.1128/mcb.19.4.3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Regnier M, Vaigot P, Darmon M, Prunieras M. Onset of epidermal differentiation in rapidly proliferating basal keratinocytes. J Investig Dermatol. 1986;87:472–476. doi: 10.1111/1523-1747.ep12455517. [DOI] [PubMed] [Google Scholar]
- 35.Rieger M, Franke W W. Identification of an orthologous mammalian cytokeratin gene. High degree of intron sequence conservation during evolution of human cytokeratin 10. J Mol Biol. 1988;204:841–856. doi: 10.1016/0022-2836(88)90045-9. [DOI] [PubMed] [Google Scholar]
- 36.Robinson G W, Johnson P F, Hennighausen L, Sterneck E. The C/EBP β transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland. Genes Dev. 1988;12:1907–1916. doi: 10.1101/gad.12.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roop D R, Hawley-Nelson P, Cheng C K, Yuspa S H. Keratin gene expression in mouse epidermis and cultured epidermal cells. Proc Natl Acad Sci USA. 1983;80:716–720. doi: 10.1073/pnas.80.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roop D R, Krieg T M, Mehrel T, Yuspa S H. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 1988;48:3245–3252. [PubMed] [Google Scholar]
- 39.Rothnagel J A, Mehrel T, Idler W W, Roop D R, Steinert P M. The gene for mouse epidermal filaggrin precursor. Its partial characterization, expression, and sequence of a repeating filaggrin unit. J Biol Chem. 1987;262:15643–15648. [PubMed] [Google Scholar]
- 40.Rothnagel J A, Greenhalgh D A, Gagne T A, Longley M A, Roop D R. Identification of a calcium-inducible, epidermal-specific regulatory element in the 3′-flanking region of the human keratin 1 gene. J Investig Dermatol. 1993;101:506–513. doi: 10.1111/1523-1747.ep12365886. [DOI] [PubMed] [Google Scholar]
- 41.Schweizer J, Kinjo M, Furstenberger G, Winter H. Sequential expression of mRNA-encoded keratin sets in neonatal mouse epidermis: basal cells with properties of terminally differentiating cells. Cell. 1984;37:159–170. doi: 10.1016/0092-8674(84)90311-8. [DOI] [PubMed] [Google Scholar]
- 42.Scott L M, Civin C I, Roth P, Friedman A D. A novel temporal expression pattern of three C/EBP family members in differentiating myelomonocytic cells. Blood. 1992;80:1725–1735. [PubMed] [Google Scholar]
- 43.Screpanti I, Romani L, Musiani P, Modesti A, Fattori E, Lazzaro D, Sellitto C, Scarpa S, Bellavia D, Lattanzio G, et al. Lymphoproliferative disorder and imbalanced T-helper response in C/EBP β-deficient mice. EMBO J. 1995;14:1932–1941. doi: 10.1002/j.1460-2075.1995.tb07185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seagroves T N, Krnacik S, Raught B, Gay J, Burgess-Beusse B, Darlington G J, Rosen J M. C/EBP β, but not C/EBP α, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland. Genes Dev. 1998;12:1917–1928. doi: 10.1101/gad.12.12.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sterneck E, Muller C, Katz S, Leuz A. Autocrine growth induced by kinase type oncogenes in myeloid cells requires AP-1 and NF-M, a myeloid specific, C/EBP-like factor. EMBO J. 1992;11:115–126. doi: 10.1002/j.1460-2075.1992.tb05034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterneck E, Tessarollo L, Johnson P F. An essential role for C/EBPβ in female reproduction. Genes Dev. 1997;11:2153–2162. doi: 10.1101/gad.11.17.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swart G W M, van Groningen J J M, van Ruissen F, Bergers M, Schalkwilk J. Transcription factor C/EBPα: novel sites of expression and cloning of the human gene. Biol Chem. 1997;378:373–379. doi: 10.1515/bchm.1997.378.5.373. [DOI] [PubMed] [Google Scholar]
- 48.Tanaka T, Akira S, Yoshida K, Umemoto M, Yoneda Y, Shirafuji N, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T. Targeted disruption of the NF-IL6 gene discloses its essential role in bacteria killing and tumor cytotoxicity by macrophages. Cell. 1995;80:353–361. doi: 10.1016/0092-8674(95)90418-2. [DOI] [PubMed] [Google Scholar]
- 49.Tennenbaun T, Weiner A K, Belanger A J, Glick A B, Hennings H, Yuspa S H. The suprabasal expression of α6β4 integrin is associated with a high risk for malignant progression in mouse skin carcinogenesis. Cancer Res. 1993;53:4803–4810. [PubMed] [Google Scholar]
- 50.Timchenko N A, Wilde M, Darlington G J. C/EBPα regulates formation of S-phase-specific E2F-p107 complexes in livers of newborn mice. Mol Cell Biol. 1999;19:2936–2945. doi: 10.1128/mcb.19.4.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H, Liu K, Yuan F, Berdichevsky L, Taichman L B, Auborn K. C/EBPβ is a negative regulator of human papillomavirus type II in keratinocytes. J Virol. 1996;70:4839–4844. doi: 10.1128/jvi.70.7.4839-4844.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Watt F M. Terminal differentiation of epidermal keratinocytes. Curr Opin Cell Biol. 1989;1:1107–1115. doi: 10.1016/s0955-0674(89)80058-4. [DOI] [PubMed] [Google Scholar]
- 53.Wedel A, Löms Zieglere-Heitbrock H W. The C/EBP family of transcription factors. Immunobiology. 1995;193:171–185. doi: 10.1016/s0171-2985(11)80541-3. [DOI] [PubMed] [Google Scholar]
- 54.Weissman B E, Aaronson S A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent BALB/c mouse epidermal keratinocyte lines. Cell. 1983;32:599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- 55.Williams S C, Cantwell C A, Johnson P F. A family of C/EBP-related proteins capable of forming covalently linked leucine zipper dimers in vitro. Genes Dev. 1991;5:1553–1567. doi: 10.1101/gad.5.9.1553. [DOI] [PubMed] [Google Scholar]
- 56.Woodcock-Mitchell J, Eichner R, Nelson W G, Sun T T. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J Cell Biol. 1982;95:580–588. doi: 10.1083/jcb.95.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeh W-C, Cao Z, Classon M, McKnight S L. Cascade regulation of terminal adipocyte differentiation by three members of the C/EBP family of leucine zipper proteins. Genes Dev. 1995;9:168–181. doi: 10.1101/gad.9.2.168. [DOI] [PubMed] [Google Scholar]
- 58.Yuspa S H, Kilkenny A E, Steinert P M, Roop D R. Expression of murine epidermal differentiation markers is tightly regulated by restricted extracellular calcium concentrations in vitro. J Cell Biol. 1989;109:1207–1217. doi: 10.1083/jcb.109.3.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yuspa S H, Kilkenny A E, Cheng C. Alterations in epidermal biochemistry as a consequence of stage-specific genetic changes in skin carcinogenesis. Environ Health Perspect. 1991;93:3–10. doi: 10.1289/ehp.91933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang Y, Rom W N. Regulation of the interleukin-1β (IL-1β) gene by mycobacterial components and lipopolysaccharide is mediated by two nuclear factor-IL6 motifs. Mol Cell Biol. 1993;13:3831–3837. doi: 10.1128/mcb.13.6.3831. [DOI] [PMC free article] [PubMed] [Google Scholar]