Abstract
Simple Summary
There is no doubt that immunotherapeutic approaches will change the current treatment landscape of multiple myeloma in the near future; in particular, a wave of BCMA-targeted therapies is currently entering clinical routine. Although the increasing availability of different therapeutic approaches is highly welcome, it also increases the daily challenges in clinical decision making if they all use the same target. Here, we provide a comprehensive summary of BCMA-targeted approaches in myeloma and aim to share some basic concepts in clinical decision making.
Abstract
Since the introduction of first-generation proteasome inhibitors and immunomodulatory agents, the multiple myeloma (MM) treatment landscape has undergone a remarkable development. Most recently, immunotherapeutic strategies targeting the B cell maturation antigen (BCMA) entered the clinical stage providing access to highly anticipated novel treatment strategies. At present, numerous different approaches investigate BCMA as an effective multi-modal target. Currently, BCMA-directed antibody–drug conjugates, bispecific and trispecific antibodies, autologous and allogeneic CAR-T cell as well as CAR-NK cell constructs are either approved or in different stages of clinical and preclinical development for the treatment of MM. This armamentarium of treatment choices raises several challenges for clinical decision making, particularly in the absence of head-to-head comparisons. In this review, we provide a comprehensive overview of BCMA-targeting therapeutics, deliver latest updates on clinical trial data, and focus on potential patient selection criteria for different BCMA-targeting immunotherapeutic strategies.
Keywords: multiple myeloma, BCMA, CAR-T, bispecific antibodies, BiTEs®, antibody-drug conjugates (ADC), treatment selection
1. Introduction
Multiple myeloma (MM) is a heterogenous disease, characterized by a malignant proliferation of clonal plasma cells. In 2020, the incidence of MM was 176,404 and the overall mortality was 117,077 worldwide (absolute numbers) [1]. Despite a markedly improved survival of patients throughout recent decades due to the development of new anti-myeloma agents, prognosis for patients with refractory disease is poor. Moreover, patients who are refractory to a proteasome inhibitor (PI), an immunomodulatory agent (IMiD), and a monoclonal antibody have a median overall survival (OS) of less than a year [2,3]. Therefore, new treatment strategies using alternative targets are urgently needed.
B cell maturation antigen (BCMA), a transmembrane glycoprotein member of the tumor necrosis factor receptor superfamily, is expressed on the surface of normal and malignant plasma cells and mature B cells, with minimal expression on other tissues. BCMA has a crucial role in the survival of malignant plasma cells through the regulation of maturation, differentiation and activation of survival and proliferation pathways [4,5,6]. Thus, BCMA seems to be an ideal target in the treatment of multiple myeloma and is therefore extensively studied. The most clinically advanced BCMA-targeting treatment modalities are bispecific antibody constructs, antibody–drug conjugates (ADCs) and chimeric antigen receptor (CAR) T cells. Figure 1 shows a schematic illustration of BCMA-targeted immunotherapeutic constructs.
Figure 1.
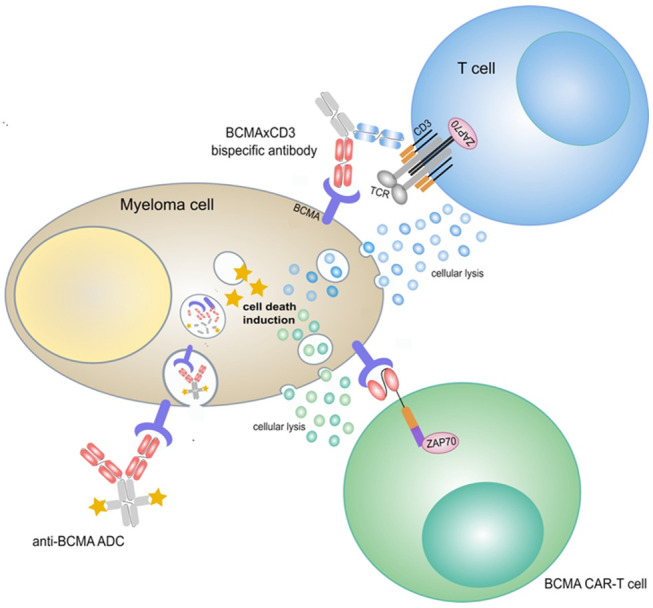
Schematic illustration of BCMA-targeted immunotherapeutic constructs. ADC, antibody drug conjugate; BCMA, B cell maturation antigen; CAR, chimeric antigen receptor; TCR, T cell receptor.
In this review, we give an overview of novel immunotherapeutic approaches in MM, with particular focus on BCMA-targeted therapies. Moreover, we aim to share our thoughts on key selection criteria for the future stratification of MM patients to different BCMA-targeting treatment options.
2. CAR-T Cells
Chimeric antigen-receptor T cells are immune cells genetically modified to target antigen-expressing tumor cells. Chimeric antigen receptors are fusion proteins containing an antigen-recognition moiety, a T cell activation domain, and a costimulatory domain. The costimulatory domain in addition to the CD3ζ intracellular signaling domain leads to better clinical activity through an enhanced likelihood of T cell proliferation [7,8].
CAR-T cells targeting CD19 showed impressive responses in different hematologic malignancies and are approved for the treatment of relapsed and refractory (r/r) acute lymphoblastic leukemia, r/r diffuse large B cell lymphoma, and r/r mantle cell lymphoma [9,10,11,12]. Through the promising results of targeting CD19, other targets are also intensively investigated.
The first anti-BCMA CAR-T cells were synthesized in 2013 and showed activity in multiple myeloma cell lines [6]. Since then, several BCMA-targeting CAR-T cell constructs have been developed and are currently explored in clinical trials. In 2016, the first clinical results of anti-BCMA CAR-T cells were published with promising responses in individual patients with refractory disease and high tumor burden [13]. To date, idecabtagene vicleucel (ide-cel, bb2121) is the only approved CAR-T cell product for the treatment of relapsed and refractory multiple myeloma (RRMM), but other products will presumably follow within the next year. Furthermore, trials with anti-BCMA CAR-T cells in earlier stages of the disease are ongoing and CAR-T cells targeting other multiple myeloma antigens such as CD38, CD138, and SLAMF7 are also being explored.
In general, CAR-T cells are typically generated from T cells collected from the patient via leukapheresis and then modified and expanded ex vivo. As the manufacturing process usually lasts several weeks, patients may receive bridging therapy to maintain disease control before CAR-T cell infusion. Most patients also undergo a conditioning lymphodepletion chemotherapy to reduce endogenous levels of lymphocytes before reinfusion of the CAR-T cell product. The most common side effects across all CAR-T cell therapies are cytokine release syndrome (CRS), neurotoxicity, cytopenia, and infections. CRS is initiated by T-cell activation and results from a massive release of inflammatory cytokines, particularly interleukin 6 (IL-6) and interferon gamma (IFN-γ) [14,15,16]. CRS can cause a wide variety of clinical symptoms, usually beginning with fever, followed by hypotension, tachycardia, or respiratory insufficiency [14]. Neurotoxicity can also manifest itself in several ways, up to seizures and brain edema in rare cases. A typical early sign of neurotoxicity is altered handwriting, which is why a handwriting sample is included in daily cognitive testing [17]. BCMA-targeting CAR-T cells showed no surprising off-target toxicity in clinical studies so far [7].
2.1. Idecabtagene Vicleucel
Ide-cel or bb2121 is the first FDA- and EMA-approved BCMA-targeting CAR-T cell construct for the treatment of relapsed and refractory multiple myeloma. The first in-human phase I/II CRB-401 study demonstrated good tolerability and promising efficacy in patients with RRMM [18]. The updated results showed deep and durable responses with ide-cel in triple-class exposed patients with a median PFS of 8.8 months and a median OS of 34.2 months across all treated patients (n = 62). Half of the ongoing responders achieved a duration of response (DOR) of more than two years. All patients with at least a CR who had a qualified assessment achieved MRD negativity by NGS (sensitivity ≤10−4 nucleated cells). Efficacy and safety are consistent with prior reports and highlight a favorable clinical benefit–risk profile for ide-cel at target dose levels ≥150 × 106. The CRS rate was 75.8% with 6.5% grade 3 CRS, and all others were grade 1 or 2. Time to first onset was 2 days and the median duration was 5 days. Neurotoxicity was less common, with 35.5% overall and mostly grade 1 [19]. The pivotal phase 2 KarMMa study demonstrated similar results. A total of 128 patients were treated with ide-cel and included a high proportion of high-risk patients, namely 35% with high-risk cytogenetics and 39% with extramedullary disease. The longest responses were achieved at the highest dose level of 450 × 106 with a PFS of 12.2 months [20]. At a median follow-up time of 13.3 months, 33% of the patients had a complete response or better. In this case, of heavily pretreated patients, the most exciting fact was that an MRD negativity of <10−5 nucleated cells were confirmed in 26% of all patients treated and 79% of 42 patients with a CR or better [21]. In subgroup analyses of the KarMMa, study responses of high-risk patients were promising, the median DOR was 9.2 months, and the median PFS 7.5 months in this difficult-to-treat population. Additionally, older patients ≥70 years (16% of all treated patients) responded well, and no new safety signals were observed [22,23].
To obtain longer persistence and function of CAR-T cells, bb21217 was developed. bb21217 uses the same CAR molecule as bb2121 but is cultured with a PI3K inhibitor to enrich for T cells displaying a memory-like phenotype. The last data update of the phase 1 CRB-402 study showed long-term CAR-T cell persistence in 6 of 11 evaluable patients. ORR at the recommended phase 2 dose (RP2D) was 84% [24].
There are several ongoing trials addressing different types of patients and different lines of therapy, such as KarMMa-2, a phase 2 trial studying high-risk MM with progression within 18 months after first line therapy or inadequate response to ASCT (NCT03601078). Another study, KarMMa-3, is comparing standard of care (SOC) in triple class refractory patients with ide-cel (NCT03651128). The KarMMa-4 trial is attempting to demonstrate efficacy in newly diagnosed multiple myeloma (NDMM) with R-ISS stage III disease per IMWG criteria (NCT04196491). Finally, KarMMa-7 is a phase 1/2 study to determine the safety, tolerability, and efficacy of bb2121 in combination with other therapies in patients with RRMM (NCT04855136).
2.2. Ciltacabtagene Autoleucel
The next CAR-T cell product on the horizon is Ciltacabtagene autoleucel (cilta-cel, also known as JNJ-4528 or LCAR-B38M in China). Cilta-cel contains two BCMA-targeting single heavy chain domains and a 4-1BB costimulatory domain besides the typical T cell activating domain [25].
The first clinical results of this CAR-T cell construct were presented from the LEGEND-2 study from China. The ORR was 88% and responses were deep and durable with low CAR-T cell doses. Overall, a median PFS of 20 months was reached (n = 57); OS at 18 months was 68% [26]. The CARTITUDE-1 trial also investigated the efficacy and safety of cilta-cel in RRMM. Patients were eligible after at least three prior regimens including a PI, an IMiD, and an anti-CD38 antibody. In this heavily pretreated patient cohort, 87.6% were triple-class refractory and 42.3% penta-class refractory. Patients received a median of six prior lines. At the latest update at EHA 2021 for 97 treated patients, ORR was 97.9% with an impressive sCR rate of 80.4%. Remarkably, the sCR rate increased from 67% to 80.4% in the six months since the last data update at ASH 2020. Almost all evaluable patients were MRD negative (91.8%; MRD at 10−5). The 18-month PFS was 66% and 18-month OS resulted in 80.9%. The most common AEs were cytopenia and CRS in 95% of patients each. CRS was mostly grade 1 and 2; 5% of patients developed higher-grade CRS and one patient died. The median time to CRS onset was 7 days, which is longer than in other products and is attributed to the lower administered cell dose with later peak expansion of CAR-T cells. Tocilizumab was used in 69.1% and corticosteroids in 21.6% of patients. CRS resolved in 98.9% of patients within 14 days of onset. Higher-grade neurotoxicity (grade ≥3) occurred in 10.3%. Unlike in other CAR-T cell trials, neurotoxic events were divided in ICANS (2.1% grade ≥3) and other neurotoxicities (9.3% grade ≥3), which were defined as events with later onset after a period of recovery from ICANS. These quite atypical neurological symptoms could be prevented by a potent prior salvage therapy regimen and extended monitoring with early therapy of CRS and ICANS [27,28,29].
Recently, the first results from cohort A of the CARTITUDE-2 study were presented (NCT04133636). In this phase 2 study, cilta-cel is investigated in patients in different MM settings. Cohort A is eligible for patients with progressive disease after 1–3 lines of therapy and who are lenalidomide refractory. ORR and CR rates were similar to CARTITUDE-1. No progression of disease was observed at a median follow up of 5.8 months [30]. Furthermore, cilta-cel is being investigated in the phase 3 CARTITUDE-4 study comparing CAR-T cell therapy versus Pomalidomide, Bortezomib and Dexamethasone (PVd) or Daratumumab, Pomalidomide and Dexamethasone (DPd) in patients with relapsed and Lenalidomide-refractory MM (NCT04181827). The CARTITUDE-5 study will be the first study exploring cilta-cel in newly diagnosed elderly multiple myeloma patients (NCT04923893).
2.3. ALLO-715
Another interesting but preliminary approach in adoptive T cell transfer are allogeneic CAR-T cells: ALLO-715 is a genetically modified allogeneic anti-BCMA CAR-T cell product in which the TCR alpha constant gene is disrupted to reduce the risk of graft versus host disease (GvHD). This CAR-T cell construct will be concomitantly administered with an anti-CD52 monoclonal antibody (ALLO-647) for selective and prolonged host lymphodepletion. The greatest advantage in this technique is the rapid availability of the transgenic CAR-T cells. The first presented result of a phase 1 dose escalation trial showed dose-dependent activity in heavily pretreated patients. Altogether, about 60% (six patients) achieved ORR and 40% a VGPR+ (sCR, CR or VGPR). ALLO-715 was well-tolerated at all dose levels. A very remarkable aspect was that no GvHD and no neurotoxicity were described. The safety data refer to CRS up to grade 2 as a maximum grading. All other side effects were comparable to other CAR-T cell constructs. A total of 90% of study patients were treated within 5 days of study enrollment, which underscores the fast availability of an allogeneic construct compared to autologous CAR-T cells [31].
2.4. Other BCMA-Targeting CAR-T Cell Constructs
Of the several other anti-BCMA CAR-T cell constructs, P-BCMA-101 is of special interest as it is the first BCMA-targeting construct that uses a gene transfer system without a viral vector (piggyBac® DNA Modification System). This CAR-T cell construct comprises a safety switch for rapid CAR-T cell elimination in case of severe CRS. The ongoing phase 1/2 PRIME study includes multiple exploratory cohorts with different drug combinations. During phase 1, the manufacturing process was modified with Nanoplasmid to improve transposition. P-BCMA-1010 with Nanoplasmid demonstrated an ORR of 66.7% (n = 6). CRS occurred in 17% (n = 53), all grade 1 and 2. The safety switch was not needed so far [32].
Table 1 lists clinically investigated CAR-T cell constructs for the treatment of multiple myeloma.
Table 1.
Characteristics, efficacy and safety data from selected clinical trials of BCMA-targeting CAR-T cell constructs.
| CAR-T Cell Construct (Name) | Study Name and/or Phase |
Number of Patients | Triple-Class Refractory, % | High-Risk Cytogenetics/EMD, % | Median PFS, Months (95% CI) | Median OS, Months (95% CI) |
≥CR, % | CRS, All Grades, % |
Neurotoxicity, All Grades, % | NCT Number | References |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ide-cel (bb2121) | CRB-401, Phase 1 |
62 | 69 | 27/37 | 8.8 (5.9–11.9) | 34.2 (19.2–NE) | 39 | 76 | 36 | NCT02658929 | [18,19] |
| Ide-cel (bb2121) | KarMMa, Phase 2 |
128 | 84 | 35/39 | 8.8 (5.6–11.6) | 19.4 (18.2–NE) | 33 | 84 | 18 | NCT03361748 | [20,21] |
| Cilta-cel | LEGEND-2, Phase 1/2 |
57 | NR | NR | 20 (10–28) | Not reached, 18-month OS 68% (54–79%) | 74 | 90 | 1 | NCT03090659 | [26] |
| Cilta-cel | CARTITUDE-1, Phase 1b/2 |
97 | 88 | 24/13 | 22.8 (22.8–NE) | Not reached, 18-month OS 80.9% (71.4–87.6%) | 80 | 95 | 21 | NCT03548207 | [27,29] |
| Orva-cel | EVOLVE, Phase 1/2 |
62 | 94 | 41/23 | 9.3 in the 300 × 106 group (n = 19), not reached in the other groups | NR | 36 | 89 | 13 | NCT03430011 | [33] |
| bb21217 | CRB-402, Phase 1 |
69 | 64 | 33/NR | NR, mDOR 17.0 (9.4–NE) |
NR | 29 | 70 | 16 | NCT03274219 | [24] |
| NCI CAR-BCMA | Phase 1 | 24 | NR | 46/NR | NR, mEFS 31 weeks |
NR | 8 | 71 | NR | NCT02215967 | [13,34] |
| UPenn CART-BCMA | Phase 1 | 25 | 72 | 96/28 | 65/57/125 days in cohort 1/2/3 | NR | 8 | 88 | 32 | NCT02546167 | [35] |
| P-BCMA-101 | PRIME, Phase 1/2 |
55 | 60 | NR | NR | NR | NR, ≥VGPR: 50 (n = 6) * |
17 (n = 53) |
4 (n = 53) |
NCT03288493 | [32] |
| CT053 | LUMMICAR STUDY 2, Phase 1 |
20 | 85 | 55/25 | NR | NR | 25 | 79 | 16 | NCT03915184 | [36] |
| ALLO-715 | UNIVERSAL, Phase 1 |
31 | NR | 48/23 | NR | NR | ≥VGPR: 40 | 45 | 0 | NCT04093596 | [31] |
| C-CAR088 | Phase 1 | 23 | NR | 81/NR | Not reached, 6-month PFS 65.1% (47–90) |
NR | 44 | 91 | 4 |
NCT03751293 NCT03815383 NCT04322292 NCT04295018 |
[37] |
CAR = chimeric antigen receptor; CI = confidence interval; CR = complete remission; CRS = cytokine release syndrome; EMD = extramedullary disease; mDOR = median duration of response; mEFS = median event free survival; NE = not estimable: NR = not reported; OS = overall survival; PFS = progression free survival; VGPR = very good partial remission. * with modified manufacturing process. Only data of studies with results for at least 20 patients are reported.
3. Bispecific Antibodies
Redirecting T cells to tumor cells by means of bispecific antibodies (BsAbs) is an attractive strategy because these antibody constructs are available off-the-shelf and are relatively easy to administer. The T cell-recruiting BsAbs activate T cells by binding CD3ε in the T-cell receptor complex, which leads to T cell activation independent of major histocompatibility complex (MHC) restriction. Furthermore, BsAbs have the ability of T cell activation in the absence of co-stimulation [38,39]. Because formation of the cytolytic synapse is independent of standard antigen recognition and co-stimulation, immune escape mechanisms of tumor cells are evaded [8]. T cell activation finally leads to tumor cell lysis through the release of perforins and granzymes which induce apoptosis [40,41].
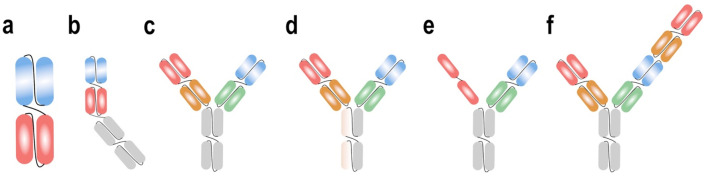
In general, two distinct groups of BsAbs can be distinguished based on the presence or absence of an Fc domain. Apart from the longer half-life, the Fc region induces antibody-dependent cell-mediated cytotoxicity (ADCC) and mediates complement-dependent cytotoxicity, if not silenced. Constructs without an Fc domain are known as bispecific T cell engagers (BiTEs®) and consist of two different single-chain variable fragments [42,43]. The final antibody can be made of various known fragments and leads to a great diversity of antibody constructs (Figure 2).
Figure 2.
Selected bispecific T cell engaging constructs targeting BCMA and CD3. BCMA-targeting regions are colored red, CD3-targeting regions are blue. Fc regions are colored grey. (a) Bispecific T cell engager (BiTE®, AMG420). (b) Half-life extended bispecific T cell engager (HLE BiTE®, AMG701). (c) Bispecific antibody, IgG4 Fc region (REGN5458, Elranatamab). (d) Bispecific antibody, IgG4 Fc region (DuoBody®, Teclistamab). (e) Bispecific antibody, IgG4 Fc region, dual BCMA binding domains (TNB-383B). (f) Bispecific antibody, IgG1 Fc region, bivalent anti-BCMA arm (CC-93269).
The main toxicities of BsAbs are CRS, neurotoxicity, cytopenia and infections. Usually, CRS occurs only during step up dosing or after the first administration of a BsAb [44]. Another challenging fact is tumor cell resistance caused by T cell exhaustion, antigen escape, or an immunosuppressive microenvironment. Different strategies to overcome these problems have been developed and are currently being investigated, for example combined targeting of several antigens or combination of BsAbs with other immunomodulatory therapeutics [38,45,46].
BsAbs-targeting multiple myeloma cells have demonstrated encouraging results in preclinical studies in vitro, in mouse xenograft models, and in cynomolgus monkeys [47,48,49,50,51,52]. To date, more than 10 different BsAbs-targeting MM antigens have been clinically investigated, most of them directed to BCMA and CD3 with promising clinical data from phase 1/2 studies (Table 2) [45].
Table 2.
Characteristics, efficacy and safety data from selected clinical trials of bispecific T-cell engaging constructs targeting BCMA and CD3.
| Agent | Drug Design | Trial Phase | Drug Status | Best ORR % | CRS % (n) |
NCT Number | References |
|---|---|---|---|---|---|---|---|
| AMG420 | BiTE | 1 | No further development | 70% (at MTD, n = 10) |
38% (16/42) | NCT03836053 | [53] |
| AMG701 | Half-life extended BiTE | 1/2 | Phase 1/2 study ongoing | 83% (last evaluated dose expansion cohort, n = 6) | 61% (46/75) | NCT03287908 | [54] |
| Teclistamab (JNJ-64007957) | BsAb, IgG4 Fc region (DuoBody) | 1/2 | Several phase 1/2 studies ongoing, monotherapy and combinations | 69% (most active IV and SC doses, n = 68) |
55% (82/149) |
NCT04557098 NCT03145181 |
[55,56,57,58] |
| REGN5458 | BsAb, IgG4 Fc region (VelociBi) | 1/2 | Phase 1/2 study ongoing | 62.5% (highest tested dose level, n = 8) |
39% (19/49) | NCT03761108 | [59] |
| TNB-383B | BsAb, IgG4 Fc region, dual BCMA binding domains | 1 | Phase 1 study ongoing | 80% (highest tested dose levels, n = 15) | 45% (26/58) | NCT03933735 | [60] |
| Elranatamab (PF-06863135) | BsAb, IgG2a Fc region | 2 | Phase 2 study ongoing (MAGNETISMM-3) | 83.3% (RP2D SC, n = 6) |
73% (22/30) | NCT04649359 | [61] |
| CC-93269 | BsAb, IgG1 Fc region, bivalent anti-BCMA arm | 1 | Phase 1 study ongoing | 89% (highest tested dose level, n = 9) |
77% (23/30) | NCT03486067 | [62] |
CRS = cytokine release syndrome; IV = intravenous; MTD = maximum tolerated dose; ORR = overall response rate; RP2D = recommended phase 2 dose; SC = subcutaneous.
3.1. AMG420 and AMG701
The BCMA/CD3 BiTE® AMG420 is comprised of two single-chain variable fragments (scFvs) and was the first-in-class bispecific construct in MM [47]. The first-in-human study showed an overall response rate (ORR) of 70% (n = 7 of 10) at the maximum tolerated dose (MTD) of 400 μg/d. Five patients achieved an MRD-negative complete remission (CR) (MRD at 10−4). Serious adverse events (SAE) included infections, polyneuropathy and edema. CRS rate was 38% and mostly grade 1 or 2; only one patient experienced a CRS grade 3. There were no higher-grade central nervous system (CNS) toxicities. Long-term follow-up data have been presented for 23 of the 42 enrolled patients. Ten of the 23 patients responded. The PFS of the responders was 23.5 months. Because of logistical challenges due to continuous intravenous infusion, further development has been halted [53,63].
AMG701 is a half-life extended BiTE® comprised of the same two scFv regions as AMG420 and an additional Fc region which enables once-weekly dosing [48]. Initial results of an ongoing phase 1/2 study showed promising response rates at higher doses of AMG701. At data cutoff, 75 patients received AMG701 as weekly intravenous infusion in 4-week cycles. Patients were heavily pretreated with a median of six prior lines of therapy; 68% were triple refractory to a PI, an IMiD, and an anti-CD38 antibody, and 27% of patients had extramedullary disease. As expected, cytopenia was common; 43% of patients had anemia, 23% neutropenia and 20% thrombocytopenia. The most common non-hematological AE was CRS (61%); 7% of patients experienced grade 3 CRS, and all others were grade 1 or 2. All CRS were reversible with corticosteroids and tocilizumab, with a median duration of two days. Other AEs included diarrhea (31%), fatigue (25%), and fever (25%). The response rate was 36% (16/45) at doses of 3–12 mg. With further dose escalation, the response rate in the last evaluated cohort (n = 6) was 83%. Across all patients, responses included four stringent CRs (3/3 tested patients MRD-negative). Responses occurred fast, the data for the duration of response were not mature, and maximum duration of response was 23 months [54]. In addition, the ongoing study will include a sequential dose escalation part to identify the recommended phase 2 dose (RP2D) of AMG701 in combination with pomalidomide, with and without dexamethasone (NCT03287908). The rationale for combination therapy with an IMiD is based on preclinical studies, which showed enhanced anti-tumor activity with combination therapy in vitro and in xenograft mouse models [64].
3.2. Teclistamab
Teclistamab (formerly known as JNJ-64007957 or JNJ-7957) is a humanized IgG-4-bispecific DuoBody® antibody targeting BCMA and CD3 [51]. The phase 1 first-in-human study consisted of two parts with weekly intravenous or subcutaneous administration of Teclistamab to assess RP2D. The first presented results for intravenous dosing showed a 67% ORR at the highest tested dose of 270 μg/kg (n = 12) [55]. Overall, 157 patients were enrolled; 84 of them received intravenous and 73 subcutaneous Teclistamab in different dosing groups. Eligible patients had to be relapsed/refractory (RR) or intolerant to established MM therapies. The median number of prior lines was six, 33% of patients had high-risk cytogenetics, 82% were triple-class refractory, 39% were penta-drug refractory, and 90% were refractory to the last line of therapy. In addition to hematological AEs, the most common treatment-related AE was CRS in 57% of patients with a maximum grade 2. Tocilizumab was administered in 24%, steroids in 15%. Of note, CRS occurred later and lasted longer with subcutaneous administration with a median time to onset of two days and a duration of two days versus one day each with intravenous dosing. Neurotoxicity was observed in 4% of patients, including two higher-grade events with intravenous dosing. Other common AEs included infections, pyrexia, diarrhea, cough, fatigue, back pain, and headache [56]. Most active doses were 270–720 μg/kg IV and 720–3000 μg/kg SC. ORR in this dosing groups was 69% (47/68) with at least VGPR in 59% and at least CR in 26% [57]. Forty patients received the RP2D of 1500 μg/kg SC. The ORR in this group was 65%, with 40% achieving at least a CR. The median duration of follow-up for all 40 patients treated at the RP2D was 6.1 months. Of the 26 evaluable patients across all doses and cohorts, 18 had MRD-negative CR/sCR (69%). All evaluable patients in the RP2D cohort (n = 6) achieved MRD negativity (<10−5) [56]. Responses were durable and deepened over time, and 85% (22/26) of responders at the RP2D of 1500 μg/kg SC remained on therapy after a median follow up of 7.1 months [58]. The efficacy of Teclistamab in the RP2D of 270 μg/kg IV and 1500 μg/kg SC is currently evaluated in a phase 2 study (NCT04557098). Furthermore, another study investigates subcutaneous Daratumumab in combination with either Teclistamab or Talquetamab with or without pomalidomide (NCT04108195). The rationale of a combination of Daratumumab with Teclistamab is based on preclinical data, which showed enhanced MM cell lysis with the direct combination. Furthermore, the activity of Teclistamab was significantly enhanced in bone marrow samples of Daratumumab-pretreated patients and tumor cell lysis was superior when T cells obtained from patients treated with Daratumumab were used [65].
3.3. REGN5458
REGN5458 is a fully human BsAb that binds to BCMA and CD3 [50]. The safety and efficacy data of 49 patients from an ongoing phase 1 study were recently presented. Patients were eligible after at least three prior lines of therapy, including a PI, an IMiD, and an anti-CD38 antibody. The BsAb was administered weekly, followed by a maintenance phase with biweekly doses. All included patients were triple-refractory, 57% were penta-refractory. CRS occurred in 39%, and no grade 3 or higher events were observed. As in other studies with BsAbs, hematologic AEs, infections, fatigue, and myalgias were common. Mild neurotoxicity appeared in 12% of patients. No new safety signals were seen. Responses were dose-dependent and occurred early. In the highest tested dose level of 96 mg, ORR was 62.5% (n = 8), all VGPR. Overall, 95% (18/19) of responders achieved VGPR or better, and 42% (8/19) had CR or sCR. MRD status was evaluated in seven patients; 57% were MRD-negative (<10−5) [59]. Another phase 1 trial was initiated for REGN5459, a similar BCMA/CD3 BsAb, which has different binding characteristics (NCT04083534).
3.4. TNB-383B
TNB-383B is a fully human triple-chain BCMA × CD3 BsAb with a unique anti-CD3 moiety for target lysis with minimal cytokine release and two anti-BCMA moieties [52]. Data of 58 patients from the ongoing first-in-human study are available. TNB-383B was administered intravenously every 3 weeks without step-up dosing. Patients enrolled had received a median of six prior lines of therapy, 64% were triple-class refractory and 34% penta-drug refractory. Safety data were comparable to the other described studies. No higher-grade CRS was observed. One patient developed grade 3 neurotoxicity (confusion). At the highest evaluated doses of 40–60 mg, ORR was 80% (n = 15), 73% achieved VGPR or better. A total of 81% (22/27) of responding subjects have ongoing responses [60].
3.5. Elranatamab
Elranatamab (formerly known as PF-06863135) is a humanized anti-BCMA/CD3 BsAb with an IgG2a backbone [49]. Early clinical data for intravenous and subcutaneous administration showed activity in patients with RRMM. With weekly intravenous dosing, the best response in the early-dose escalation phase (n = 17) was minimal response in 6% and stable disease in 35%. A total of 29% of patients had received another BCMA-targeted therapy prior to study enrollment [66]. Subcutaneous dose escalation was subsequently initiated to achieve a more favorable therapeutic window. Among 30 patients in the SC dose escalation cohort, responses were seen at doses ≥215 μg/kg in 20 patients. ORR in these heavily pretreated patients was 70% with a CR/sCR rate of 30% (n = 20). Six patients received the RP2D of 1000 μg/kg SC; the confirmed ORR was 83.3%. Three out of four patients with prior BCMA-directed therapy responded, two of them achieved VGPR and one CR. The probability of responders being event-free at 6 months was 92.3%. CRS occurred in 73.3% of patients, with a maximum grade of 2. CRS onset was observed early, after a median of 1 day, and lasted for a median of 2 days. Tocilizumab was administered in 30% and steroids in 10% of patients. No higher-grade neurotoxicity was observed in this cohort [61].
3.6. CC-93269
CC-93269 is an asymmetric dual-arm, human IgG1-based BsAb with one CD3 and two BCMA binding sites [67]. Patients had received at least three prior regimens. CC-93269 was administered by IV weekly until cycle 3, biweekly from cycle 4 to 6, and every 4 weeks thereafter. In the most recent study update, data from 30 patients were presented. Overall, the safety profile was comparable with other published data for bispecifics. CRS was reported in 77% of patients, in 27% at least grade 3. One patient died during the study in the setting of CRS. At the highest dose of 10 mg (n = 9), ORR was 89%, including 44% CR/sCR. 92% of responding patients (n = 13) achieved MRD negativity (<10−5) [62].
3.7. Other Constructs
HPN-217 is a BCMA-targeting trispecific T cell activating construct (TriTAC) containing three humanized antibody-derived binding domains, targeting BCMA, CD3, and albumin (for half-life extension). The phase 1 study is recruiting, and the first clinical data are awaited [68].
Other BCMA-targeting constructs in preclinical development are bispecific or trispecific NK cell-based antibodies. In comparison to T cell-based antibodies, NK cell-based constructs show less inflammatory cytokine secretion in preclinical models. Examples for NK-cell BsAbs are AFM26 and CTX-8573, which showed preclinical activity in vitro and in vivo [38,39].
4. Antibody Drug Conjugates
Antibody-drug conjugates (ADCs) represent a novel class of immunotherapeutics which are increasingly used in the treatment of hematologic malignancies and solid cancers [69,70,71,72]. They consist of a monoclonal antibody that is linked to a cytotoxic drug and is internalized upon binding to its target antigen on the cell surface, thus delivering the toxic payload directly to the tumor cell (Table 3).
Table 3.
Characteristics and drug status of BCMA-targeting antibody drug conjugates.
| Agent | Cytotoxic Conjugate | Trial Phase | Drug Status/Published Results | NCT Number | References |
|---|---|---|---|---|---|
| Belantamab mafodotin (GSK2857916) | Monomethyl auristatin F (MMAF) | 2, 3 | First-in-class approval 2020; several studies with different drug combinations ongoing | NCT02064387 | [73] |
| MEDI2228 | Pyrrolobenzodiazepine (PBD) | 1 | Early phase 1 results published | NCT03489525 | [74] |
| HDP-101 | Amanitin | 1/2 | Phase 1 not yet recruiting | NCT04879043 | [75] |
| AMG-224 | Mertansine (DM1) | 1 | Early phase 1 results published; deprioritized in favor of the development of bispecific antibody constructs by the company | NCT02561962 | [76] |
| CC99712 | Undisclosed | 1 | Recruiting, no results available | NCT04036461 |
4.1. Belantamab Mafodotin
Belantamab mafodotin (GSK2857916, belamaf) is a first-in-class ADC for the treatment of multiple myeloma featuring the humanized anti-BCMA antibody J6M0 with a defucosylated Fc portion and monomethyl auristatin F (MMAF, mafodotin) as its effector molecule [73]. In preclinical models, the compound competes for binding to BCMA with BAFF and APRIL and inhibits ligand induced NFkB signaling. Once internalized, MMAF is cleaved and retained inside target cells where it causes dose- and time-dependent G2/M cell cycle arrest followed by apoptosis [73,77]. In addition, the compound induces enhanced antibody-dependent cell lysis due to its modified Fc moiety as well as macrophage-mediated phagocytosis of MM cells [73].
The first-in-human, open-label phase 1 study DREAMM-1 enrolled 73 patients with refractory MM and previous exposure to alkylators (including an autologous stem cell transplant, if eligible), IMiDs, and PIs. The dose-escalation phase (part 1, n = 38) reported corneal events (53%), nausea (49%), fatigue (47%), and thrombocytopenia (42%) as the most common side effects [78]. No dose-limiting AEs were observed and a formal MTD was not reached, but ocular toxicities, which are frequently observed in association with the administration of ADCs [79], tended to be more severe at higher dose levels. Based on these observations and a lack of clinical activity in patients treated at 2.5 mg/kg, a recommended phase 2 dose of 3.4 mg/kg was determined. In the dose expansion phase (part 2), 35 patients with heavily pretreated MM (40% with >5 prior therapies) received belamaf at 3.4 mg/kg as intravenous infusion every three weeks for a maximum of 16 cycles [78]. Totals of 94% and 97% of patients were refractory to PIs and IMiDs, respectively, and 40% had daratumumab refractory disease. Again, keratopathy resulting in blurred vision, dry eyes, and photophobia was the single most common adverse event, affecting 69% of patients. Infusion-related reactions occurred with the first dose in 29% of patients and were mainly grade 1 and 2, and no new toxicities emerged. Dose reductions and dose delays or interruptions were required in 66% and 71% of patients, respectively. AEs leading to permanent discontinuation of the drug occurred in only four cases (11%). The overall response rate in this cohort was 60% and five patients achieved a complete remission. Responses were rapid with a median time to first response of 1.2 months. Progression-free survival was 12.0 months overall, 7.9 months in double refractory patients, and 6.8 months in those with prior daratumumab.
DREAMM-2 was designed as a two-arm, randomized, open-label phase 2 study to evaluate the efficacy and safety of belamaf administered every 3 weeks both at the recommended phase 2 dose of 3.4 mg/kg and a lower dose of 2.5 mg/kg due to the frequent dose modifications in the phase 1 trial [80]. The study enrolled 196 patients with relapsed or refractory MM after three or more lines of therapy who were refractory to PIs and IMiDs and refractory or intolerant to an anti-CD38 monoclonal antibody. A total of 83% of patients had received >four prior therapies; high-risk cytogenetics and extramedullary lesions were present in 45% and 20%, respectively. The median number of treatment cycles was three in both treatment arms (n = 97 in the 2.5 mg/kg group, n = 99 in the 3.4 mg/kg group). An overall response was achieved in 30/97 patients (31%) in the 2.5 mg/kg cohort and 34/99 patients (34%) in the 3.4 mg/kg cohort. The proportion of patients reaching a very good partial response or better was identical at both dose levels (19% vs. 20%), as was the complete response rate (three patients in each cohort). The median duration of response (DoR) and overall survival were not reached at 6 months of follow-up in the primary analysis. With an extended follow-up of 13 months, the median DoR and OS estimate was 11 months and 13.7 months, respectively, in the 2.5 mg/kg cohort compared to 6.2 months and 13.8 months, respectively, in the 3.4 mg/kg cohort [81]. Among patients treated at a dose of 2.5 mg/kg, there was no apparent difference in efficacy and occurrence of adverse events between subgroups with 3–6 versus ≥ 7 prior lines of therapy [82]. In prespecified subgroups of patients with moderate renal insufficiency or high-risk cytogenetics, depth and durability of responses were comparable to those seen in the overall population [83]. However, belamaf seemed to confer less benefits in patients with extramedullary disease. In terms of safety, the most frequent AEs were corneal events seen in 71% and 77%, respectively, in both treatment arms. The internalization of belamaf by corneal epithelial cells through macropinocytosis and subsequent apoptosis is thought to induce the typical microcyst-like epithelial changes (MECs) that appear as bilateral, diffuse, microcyst-like lesions on slit-lamp photography [84]. Keratopathy was the most frequent cause for dose reductions (23% and 27%) and delays (47% and 48%, starting at week 4), but was a rare cause for permanent discontinuation (one and three patients). Twenty-two patients in each treatment arm had definite worsening of best-corrected visual acuity (BCVA) at the end of treatment, with no reports of complete permanent vision loss. Most patients recovered from their first keratopathy event (77%) or from clinically meaningful BCVA deterioration (82%) [85]. In an ocular substudy of DREAMM-2 (n = 30), corticosteroid eyedrops failed to mitigate eye toxicity [80]. Preservative-free lubricating eyedrops administered at least four times daily prior to and throughout the treatment period remain the only recommended pharmacotherapy along with frequent eye examinations and dose modification in the event of worsening symptoms. Other adverse events in DREAMM-2 were more common in the 3.4 mg/kg group, such as thrombocytopenia (grade 3/4 in 33% vs. 20%) and pneumonia grade 3 or worse (11% vs. 4%). Dose modifications were more frequent and median dose intensity was significantly lower in than the intended dose for the 3.4 mg/kg group. Based on these data, belamaf at a dose of 2.5 mg/kg administered every three weeks was approved by both the FDA and EMA in 2020. A lyophilized formulation of the drug is intended for future clinical use instead of the liquid–frozen preparation, both of which showed comparable efficacy and safety data [86].
Combinations with both standard and novel treatments are currently being explored. A phase 1 trial reports a high efficacy of belamaf in combination with pomalidomide/dexamethasone in 35 patients, but frequent dose holds due to keratopathy at a dose of 2.5 mg/kg [87]. Alternative dosing schedules are under investigation and DREAMM-8 will compare the above-mentioned combination with bortezomib/pomalidomide/dexamethasone in patients with ≥1 prior therapies in a randomized phase 3 design [88]. Belamaf plus bortezomib/dexamethasone has a high response rate of 78% in heavily pretreated patients [89]. DREAMM-7 is currently enrolling patients with relapsed/refractory MM after ≥ 1 prior line of treatment to receive bortezomib/dexamethasone either with belamaf or daratumumab in a randomized phase 3 study [90]. Finally, DREAMM-5 is a phase 1/2 platform to explore the efficacy and safety of belamaf combined with an OX40 agonist, a gamma-secretase inhibitor, and a PD-1 blocker, among others [91].
4.2. Other BCMA-Targeting ADCs
Recently, novel ADCs targeting BCMA have entered clinical development. MEDI2228 uses an antibody domain that preferentially targets membrane-bound versus soluble BCMA, a cleavable linker and a DNA cross-linking pyrrolobenzodizepine (PBD) dimer as its payload [74]. As of 16 October 2020, 82 patients with refractory MM after treatment with PIs, IMiDs, and moAbs have been enrolled in the first-in-human, open-label phase 1 trial (NCT03489525) [92]. In the dose-escalation phase (n = 41), dose-limiting thrombocytopenia occurred in two patients at 0.20 mg/kg. An additional 41 patients, 56% of whom had triple-class refractory disease, were included in the MTD expansion cohort, and treated with MEDI2228 at 0.14 mg/kg every 3 weeks. Early onset photophobia was seen in 58.5% of patients (17.1% grade 3) after approximately 2–3 cycles which was not associated with keratopathy and was a frequent cause for drug discontinuation. Symptoms improved in 9/24 patients (37%) and completely resolved in four patients, but follow-up was limited. Further PBD class toxicities included a grade 1/2 rash (31.7%), thrombocytopenia (31.7%, grade 3/4 24.4%), pleural effusions (24.4%, grade 1/2 in all but one patient) and increased GGT (24.4%, grade 3/4 19.5%). MEDI2228 demonstrated clinical efficacy across all dose levels with an overall response rate of 66% at 0.14 mg/kg and a median time to response of 2.1 months. The median number of treatment cycles was three and the duration of response was 5.9 months, but this analysis was impacted by loss of patients to follow-up. Further expansions cohorts at 0.14 mg/kg with alternative dose and schedule to mitigate eye toxicities are currently being explored.
The RNA-polymerase inhibitor amanitin serves as the cytotoxic moiety in HDP-101, another BCMA-targeting ADC with potent activity in preclinical models [75]. A first-in-human phase 1/2a trial is expected to open recruitment in 2021 (NCT04879043) [93].
5. Drug Targets beyond BCMA in Clinical Development
5.1. FcRH5
The FcRH gene family members are Ig superfamily type I membrane proteins and are expressed only in the B-cell compartment. Fc receptor-homolog 5 (FcRH5) is found on naïve and memory B cells and on plasma cells [94]. As compared to normal plasma cells, FcRH5 expression is higher in multiple myeloma cells, and therefore FcRH5 seems to be a reliable target for the treatment of multiple myeloma [95,96]. The first drug in clinical development targeting FcRH5 was DFRF4539A, an antibody–drug conjugate linking a humanized IgG1 monoclonal antibody against FcRH5 with monomethyl auristatin E. As the phase I study was unsuccessful due to limited activity and adverse events, further development was stopped [97]. A more promising research approach is bispecific antibodies. An anti-FcRH5/CD3 bispecific antibody demonstrated anti-myeloma activity in vitro and in a model with cynomolgus monkeys [96]. The first results of a phase I trial of the humanized IgG-based T-cell engaging bispecific antibody Cevostamab (formerly known as BFCR4350A) were presented by the end of 2020. Cevostamab was administered by IV infusion in 21-day cycles. At data cut-off, 53 patients had been enrolled with a median number of six prior treatment lines, including prior anti-BCMA therapy in 21%. Responses were observed at doses ≥3.6/20 mg and ORR was 53% (18/34) in this patient cohort, with 18% of patients achieving CR or better. So far, eight patients had a duration of response longer than 6 months. CRS was the most common adverse event in 76% of patients; one patient had grade 3 CRS, and all other cases were grade 1 or 2 [98].
5.2. GPRC5D
Another potential target to circumvent antigen escape through reduced surface expression of BCMA is G-protein-coupled receptor family C group 5 member D (GPRC5D). GPRC5D is overexpressed in poor-risk myeloma, whereas only low expression is detected in normal tissues, except in hair follicles [99,100].
A model that investigated CAR-T cells targeting BCMA and GPRC5D simultaneously showed that targeting GPRC5D can prevent a BCMA escape-mediated relapse [101]. A phase I clinical trial currently investigates GPRC5D-directed CAR-T cell therapy (MCARH109) in relapsed/refractory multiple myeloma, including patients who have received prior BCMA-targeted therapies (NCT04555551). Dual-targeted CAR-T cell constructs are also in development, and a combination of BCMA and GPRC5D showed promising activity in preclinical models [102].
Talquetamab is a first-in-class humanized DuoBody® with an IgG4 backbone that binds to GPRC5D and CD3 to redirect T-cells to myeloma cells and induces killing of GPRC5D positive cells [103]. The first-in-human MonumenTAL-1 study evaluates the efficacy and safety of Talquetamab in patients with multiple myeloma, relapsed/refractory, or intolerance to established therapies. So far, data from 184 patients were presented; 102 in the IV cohorts, and 82 in the SC cohorts. The median number of prior therapies was six, most patients were triple-class refractory and refractory to the last line of therapy. In addition to hematologic AEs, the most common non-hematologic AE was CRS with 73% at the RP2D in the SC cohort (n = 30), including one grade 3 CRS, two grade 2 and all others grade 1. Tocilizumab was used in 60% of these patients. Neurotoxicity was observed in four patients with SC dosing (all grade 1/2). Skin-related AEs were seen in 67% of SC treated patients, nail disorders in 21%, and infections in 37%. ORR at most active IV doses was 67% (12/18), and ORR at the RP2D of 405 μg/kg SC once weekly was 70.0% (21/30). Responses were durable and deepened over time, and 81% of responders were continuing on treatment after a median follow up of 6.3 months [104,105]. A phase 2 expansion study of Talquetamab at the RP2D is recruiting (NCT04634552).
5.3. SLAMF7
Signaling lymphocytic activation molecule F7 (SLAMF7, also known as CS-1 or CD319) is a well-known target in multiple myeloma. SLAMF7 is highly expressed on myeloma cells with minimal expression on healthy tissue [106]. The phase III ELOQUENT-2 and ELOQUENT-3 trials resulted in the approval of the fully humanized monoclonal antibody Elotuzumab in combination with lenalidomide or pomalidomide and dexamethasone [107,108].
Several new constructs targeting SLAMF7 have been investigated, most of them in preclinical or in early clinical development. While an ADC failed to show significant efficacy [109] and redirecting T-cells against a self-antigen may appear difficult, preclinical models demonstrated promising efficacy of SLAMF7-directed CAR-T cells [110] and combinatorial targeting with hemibodies addressing CD38 and SLAMF7 [46]. SLAMF7 or CS-1 CAR-T-cells are currently being tested in several phase I trials (NCT03710421, NCT04541368, NCTO04499339, NCT04142619).
5.4. CD38
CD38 is a transmembrane glycoprotein and is expressed on plasma cells, but also on other hematopoietic cells. Anti-CD38 monoclonal antibodies have changed the treatment landscape of multiple myeloma in the last years. Daratumumab and Isatuximab have been approved in different combinations and lines of therapy for multiple myeloma and are broadly used [111]. Preclinical studies demonstrated efficacy of CD38-directed CAR-T cells, ADCs, and bispecific antibodies, but to date no clinical data are available [112,113,114]. Data from a phase I study of a bispecific CAR-T cell construct targeting CD38 and BCMA showed an ORR of 87.5%. Toxicities were manageable and responses were ongoing up to 51 weeks [115].
5.5. Dual Targeted CAR-T Cells
Several studies are currently evaluating the simultaneous targeting of two different antigens to improve responses and avoid acquired resistance. These strategies combine mostly BCMA with another antigen such as CD19, SLAMF7, or CD38, either through co-infusion of two different CAR-T cell products or with dual-targeted CAR-T cells [115,116,117].
An ongoing first-in-human study is investigating a dual FasT CAR-T cell therapy targeting BCMA and CD19 simultaneously. The data of 16 heavily pretreated patients with multiple myeloma were presented. The median number of prior lines was five. All patients developed CRS; two patients had grade 3 CRS, while all others were grade 1 or 2. No neurotoxicity was observed. Patients in all dose levels responded, and the ORR was 93.8%. At data cut-off, the best response was MRD-negative CR/sCR in 9/16 patients at a median follow up of 7.3 months [116].
5.6. Other Targets
Other potential targets for the treatment of multiple myeloma are evaluated in preclinical and very early clinical settings. These include CD138, CD229, CD44v6, and CD46, among others [118,119,120,121].
CD138 (or syndecan-1) is expressed on normal and malignant plasma cells and therefore seems to be a reliable target for multiple myeloma. CD138-specific CAR-T cells were able to eliminate myeloma cells in vitro and in vivo [118]. The first clinical results showed a response in 4 of 5 treated patients, but no durable responses were observed [122].
CD229 CAR-T cells efficiently eliminated differentiated MM plasma cells and also memory B cells, a potential reservoir for MM-propagating cells, in myeloma cell lines and xenograft mouse models [119].
Clinical data of these alternative targets have to be awaited.
6. Who Is Who—How to Find the Right BCMA-Targeted Drug for the Right Patient? Potential Key Patient Selection Criteria for ADCs vs. Bispecific Antibodies vs. CAR-T Cells
Since the introduction of numerous new anti-myeloma drugs and treatment concepts such as cellular therapies in recent years, it has become extremely challenging for physicians to address the most appropriate therapy for each individual patient with multiple myeloma.
BCMA is extensively studied and has been approved as a promising target for clonal-directed MM therapies [123]. The three most promising treatment modalities targeting BCMA are bispecific antibodies, antibody–drug conjugates, and CAR-T cell constructs [124,125,126]. To date, Belantamab mafodotin is the only approved BCMA-targeting drug for RRMM. However, there are multiple other targets comprising novel ADCs, CAR-T cells, and bispecific antibodies. A major challenge for the future is to define the most effective therapy combinations and the best sequence of the different substances for the individual patient.
To opt for the best treatment regimen, clinicians have to consider plenty of disease-related and patient-related factors, such as disease morbidity (e.g., refractory disease, renal impairment, extramedullary disease, or aggressiveness of disease in general), age and frailty (performance status, disability), and co-morbidities. Further important tools for decision making are risk assessment (cytogenetics and R-ISS), treatment history (previous therapies), and patient’s preference and social support (family and/or travel support) [127,128]. All these factors should be incorporated into the decision-making process aiming for the most effective treatment regimen which is also safe and well-tolerated and supports good quality of life.
6.1. Disease Based Decision Factors
For difficult-to-treat patient subsets, such as extramedullary disease (EMD), plasma cell leukemia, high-risk (HR) cytogenetics or high tumor burden, two CAR-T cell trials have shown efficacy, and moreover, the efficacy was not negatively influenced by these adverse prognostic parameters [22,27,129]. Early-phase trials with bispecific antibody constructs also included patients with high-risk features (such as EMD, HR, or high tumor burden). No large subgroup analyses have been available until now, but preliminary results suggest that these adverse disease-specific factors may not significantly influence efficacy in patients treated with BsAbs [54,57,98,104]. If these findings can be confirmed by extended subgroup analyses, CAR-T cells and bispecific monoclonal antibodies will become the highest priority for high-risk disease.
Another important disease-specific high-risk factor will directly influence the choice of therapy: the intensity of disease aggressiveness. It is important to know that CAR-T cell therapy is not available immediately like an emergency drug. The creation and production of CAR-T cells is highly personalized, patient-specific, and time consuming. Highly specialized multi-disciplinary teams are needed. Leukapheresis and lymphodepletion, and genetic editing are the mainstays of the CAR-T cell manufacturing process. In contrast, bispecific monoclonal antibodies are off the shelf products and probably the preferable strategy in the case of rapidly progressing and aggressive disease. Taken together, the vein-to-vein time may significantly influence the selection of the regimen.
Renal impairment is a frequent problem in multiple myeloma and represents an important factor in treatment-decision making. Most clinical trials including those investigating CAR-T cells and BsAbs only enroll patients with normal renal function or a moderately reduced glomerular filtration rate (creatinine clearance >40–45 mL/min). The Belantamab mafodotin-based DREAMM trials are less restrictive regarding patients with renal dysfunction. The results suggest that this ADC may be suitable for patients with renal impairment.
Very special high-risk situations within the MM entity are plasma cell leukemia (PCL) and CNS involvement. These conditions are also listed as clear exclusion criteria in most of the clinical studies. Therefore, only very limited data are available for these patient subgroups. The use of CAR-T cells in PCL is obvious, considering the good efficacy of this therapy in other aggressive lymphomas and acute lymphoblastic leukemia [9,10,11,12]. In addition, currently available therapeutic options in PCL can often achieve only a short response, making CAR-T cell therapy a potentially attractive alternative. However, generating valid data for this particular entity is difficult due to its rarity.
Having demonstrated promising response rates of BCMA-targeting therapeutics in RRMM, studies are now ongoing or planned in earlier lines of therapy in a wide variety of combinations, including first line combinations, consolidation, and maintenance. It may be beneficial to use T-cell-based or T-cell-interacting agents in earlier lines of therapy to take advantage of better T-cell function. T-cell exhaustion often occurs after multiple therapies, which is a possible explanation for the limited efficacy of these therapeutic approaches in late lines of therapy. Taking this consideration into account, it might be reasonable to collect and store T cells at initial diagnosis. Regardless of an expected good efficacy of a CAR-T cell construct in the setting of first-line therapy in MM, the treatment planning and administration of such a therapy is associated with considerable logistical and financial effort, which makes the use of CAR-T cells for the majority of MM patients probably an unfeasible task. Therefore, it will be interesting to see if similar success can be achieved with BCMA-targeting therapies that are available off-the-shelf.
The data from studies using anti-BCMA therapeutics in early lines of therapy are eagerly awaited. If durable responses can be achieved with the formation of a plateau, then there may also be a possibility to use BCMA-targeting agents in newly diagnosed MM or even in Smoldering MM in the future.
6.2. Patient Based Decision Factors
Additional factors with a high impact on the course of disease are the individual patient-based conditions. The most important features are age and frailty. Although there is no age cut-off in cell therapy studies, most patients are fit and do not meet frailty criteria (median age around 61 years in CAR-T cell trials and 64 years in studies with BsAbs) [33,55,62,130,131]. However, in a subgroup analysis with ide-cel patients >65 and >70 years of age, both subgroups had comparable DoR and PFS to the ITT population [23]. These results imply that this patient subgroup of older MM patients achieves the same efficacy and the same safety results, and that CAR-T cells can be applied principally to older but fit patients. For elderly, frail patients with more co-morbidities, presumably T cell engagers (TCE)/bispecific antibodies are more appropriate. More data on this topic with larger patient cohorts have to be analyzed. Other patient-based factors focus on organ function in general, e.g., normal organ function is required for CAR-T cells and TCE, but CAR-T cell therapy requires a more stringent evaluation. In fact, a cardiac and neurologic assessment is required as well as functional respiratory tests and vein access evaluation has to be performed. By contrast, belantamab mafodotin does not require a stringent evaluation except continuous ophthalmological assessment and monitoring.
The familiar and social support has to be considered also [16]: since CAR-T cell therapy requires hospitalization from lymphodepletion for up to 14 days; most protocols require that patients have to stay within a 60-min drive around the hospital after discharge, at least for the first month after CAR-T cell infusion. By contrast, TCEs require hospitalization only at priming doses and until full dosing because of potential CRS development. The continuous treatment can be performed in an outpatient setting. Moreover, ADCs such as Belantamab mafodotin are off-the-shelf and outpatient administration can be performed from the beginning.
Quality of life is a particularly important aspect of any therapy for the patient. Evaluations during and after CAR-T cell therapy with ide-cel and cilta-cel have shown significant improvements in quality of life [132,133].
7. Conclusions
One of the most important drivers for treatment selection is efficacy. Although specific data are rapidly increasing, most of them are still preliminary up to now. Nevertheless, the new cell-based immune therapies are already included in the recent myeloma treatment guidelines. At present, these very new options are allocated in the later course of disease and most of the patients have already been exposed to PIs, IMIDs and anti-CD38 antibodies. Treatment efficacy may be increased by bringing the new options into the earlier lines of therapy. Answering this question will be one of the most important issues for the near future.
Taken together, patients with MM will be exposed to IMIDS, PIs and CD38 antibodies within the first or second line of therapy in the near future. The trend towards using all these drugs during the first line/induction therapy will lead to a large cohort of triple-class refractory MM patients early in the course of their disease. Therefore, BCMA-targeted therapies will be indicated at an early time point. For these patients, as well as for patients with later relapses, we have to consider patient and disease-related factors in order to offer the most appropriate therapy to each individual MM patient.
Author Contributions
Conceptualization, M.T.K. and I.S.; writing—original draft preparation, I.S., M.S., N.S., J.R., H.A., T.K., N.M., W.W., A.P., P.N., M.T.K.; writing—review and editing, I.S., J.R., M.T.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
I.S. received honoraria from Janssen, BMS/CELGENE, and Takeda. M.S. received honoraria from Janssen, BMS/CELGENE, and GSK. H.A. received honoraria from Amgen, BMS/Celgene, Janssen, Takeda, Sanofi, Novartis, and GSK. W.W. received honoraria from Amgen, BMS/Celgene, GSK, Incyte, Gilead, Janssen, Novartis, Morphosys, Merck, Pfizer, Roche, Sandoz, Sanofi, and Takeda. A.P. received honoraria from Amgen, Janssen, BMS/Celgene, Novartis, Takeda, Sanofi, and Gilead. P.N. received honoraria from Janssen, BMS/Celgene, Sanofi, Amgen, and Roche. M.T.K. received honoraria from Amgen, BMS/Celgene, Janssen, Takeda, Sanofi, Novartis, and GSK. All other authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Ervik M., Lam F., Colombet M., Mery L., Piñeros M., Znaor A., Soerjomataram I., Bray F. Global Cancer Observatory: Cancer Today. Lyon, France: International Agency for Research on Cancer. [(accessed on 11 July 2021)]. Available online: https://gco.iarc.fr/today.
- 2.Usmani S., Ahmadi T., Ng Y., Lam A., Desai A., Potluri R., Mehra M. Analysis of Real-World Data on Overall Survival in Multiple Myeloma Patients With ≥3 Prior Lines of Therapy Including a Proteasome Inhibitor (PI) and an Immunomodulatory Drug (IMiD), or Double Refractory to a PI and an IMiD. Oncologist. 2016;21:1355–1361. doi: 10.1634/theoncologist.2016-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi U.H., Cornell R.F., Lakshman A., Gahvari Z.J., McGehee E., Jagosky M.H., Gupta R., Varnado W., Fiala M.A., Chhabra S., et al. Outcomes of patients with multiple mye-loma refractory to CD38-targeted monoclonal antibody therapy. Leukemia. 2019;33:2266–2275. doi: 10.1038/s41375-019-0435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Connor B.P., Raman V.S., Erickson L.D., Cook W.J., Weaver L., Ahonen C., Lin L.-L., Mantchev G.T., Bram R.J., Noelle R.J. BCMA Is Essential for the Survival of Long-lived Bone Marrow Plasma Cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai Y.-T., Acharya C., Xiaoyan F., Moschetta M., Zhong M.Y., Feng X., Cea M., Cagnetta A., Wen K., Van Eenennaam H., et al. APRIL and BCMA promote human multiple myeloma growth and immunosuppression in the bone marrow microenvironment. Blood. 2016;127:3225–3236. doi: 10.1182/blood-2016-01-691162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter R.O., Evbuomwan M.O., Pittaluga S., Rose J.J., Raffeld M., Yang S., Gress R.E., Hakim F.T., Kochenderfer J.N. B-cell Maturation Antigen Is a Promising Target for Adoptive T-cell Therapy of Multiple Myeloma. Clin. Cancer Res. 2013;19:2048–2060. doi: 10.1158/1078-0432.CCR-12-2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van de Donk N., Usmani S.Z., Yong K. CAR-T-cell therapy for multiple myeloma: State of the art and prospects. Lancet Haematol. 2021;8:e446–e461. doi: 10.1016/S2352-3026(21)00057-0. [DOI] [PubMed] [Google Scholar]
- 8.Shah N., Chari A., Scott E., Mezzi K., Usmani S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for target-ing and current therapeutic approaches. Leukemia. 2020;34:985–1005. doi: 10.1038/s41375-020-0734-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neelapu S.S., Locke F.L., Bartlett N., Lekakis L.J., Miklos D.B., Jacobson C.A., Braunschweig I., Oluwole O.O., Siddiqi T., Lin Y., et al. Axicabtagene Ciloleucel CAR T-Cell Therapy in Refractory Large B-Cell Lymphoma. N. Engl. J. Med. 2017;377:2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuster S.J., Bishop M.R., Tam C.S., Waller E.K., Borchmann P., McGuirk J.P., Jäger U., Jaglowski S., Andreadis C., Westin J.R., et al. Tisagenlecleucel in Adult Relapsed or Refrac-tory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019;380:45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 11.Wang M., Munoz J., Goy A., Locke F.L., Jacobson C.A., Hill B.T., Timmerman J.M., Holmes H., Jaglowski S., Flinn I.W., et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020;382:1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude S.L., Laetsch T.W., Buechner J., Rives S., Boyer M., Bittencourt H., Bader P., Verneris M.R., Stefanski H.E., Myers G.D., et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018;378:439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ali S.A., Shi V., Maric I., Wang M., Stroncek D.F., Rose J.J., Brudno J.N., Stetler-Stevenson M., Feldman S.A., Hansen B.G., et al. T cells expressing an anti-B-cell maturation antigen chimeric an-tigen receptor cause remissions of multiple myeloma. Blood. 2016;128:1688–1700. doi: 10.1182/blood-2016-04-711903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert M.L., Schmitt M., Wang L., Ramos C.A., Jordan K., Müller-Tidow C., Dreger P. Side-effect management of chimeric antigen receptor (CAR) T-cell therapy. Ann. Oncol. 2021;32:34–48. doi: 10.1016/j.annonc.2020.10.478. [DOI] [PubMed] [Google Scholar]
- 15.Lee D.W., Santomasso B.D., Locke F.L., Ghobadi A., Turtle C.J., Brudno J.N., Maus M.V., Park J.H., Mead E., Pavletic S., et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2018;25:625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 16.Yakoub-Agha I., Chabannon C., Bader P., Basak G.W., Bonig H., Ciceri F., Corbacioglu S., Duarte R.F., Einsele H., Hudecek M., et al. Management of adults and children undergoing chimeric antigen receptor T-cell therapy: Best practice recommendations of the European Society for Blood and Marrow Transplantation (EBMT) and the Joint Accreditation Committee of ISCT and EBMT (JACIE) Haematologica. 2019;105:297–316. doi: 10.3324/haematol.2019.229781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neelapu S.S., Tummala S., Kebriaei P., Wierda W., Gutierrez C., Locke F.L., Komanduri K.V., Lin Y., Jain N., Daver N., et al. Chimeric antigen receptor T-cell therapy–assessment and management of toxicities. Nat. Rev. Clin. Oncol. 2018;15:47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raje N., Berdeja J., Lin Y., Siegel D., Jagannath S., Madduri D., Liedtke M., Rosenblatt J., Maus M.V., Turka A., et al. Anti-BCMA CAR T-Cell Therapy bb2121 in Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2019;380:1726–1737. doi: 10.1056/NEJMoa1817226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin Y., Raje N.S., Berdeja J.G., Siegel D.S., Jagannath S., Madduri D., Liedtke M., Rosenblatt J., Maus M.V., Massaro M., et al. Idecabtagene Vicleucel (ide-cel, bb2121), a BCMA-Directed CAR T Cell Therapy, in Patients with Relapsed and Refractory Multiple Myeloma: Updated Results from Phase 1 CRB-401 Study. Blood. 2020;136:26–27. doi: 10.1182/blood-2020-134324. [DOI] [Google Scholar]
- 20.Larry D., Anderson J., Munshi N.C., Shah N., Jagannath S., Berdeja J.G., Lonial S., Raje N.S., Siegel D.S.D., Lin Y., et al. Idecabtagene vicleucel (ide-cel, bb2121), a BCMA-directed CAR T cell therapy, in relapsed and refractory multiple myeloma: Updated KarMMa results. J. Clin. Oncol. 2021;39:8016. doi: 10.1200/JCO.2021.39.15_suppl.8016. [DOI] [Google Scholar]
- 21.Munshi N.C., Anderson L.D. J., Shah N., Madduri D., Berdeja J., Lonial S., Raje N., Lin Y., Siegel D., Oriol A., et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021;384:705–716. doi: 10.1056/NEJMoa2024850. [DOI] [PubMed] [Google Scholar]
- 22.Raje N.S., Siegel D.S., Jagannath S., Lonial F.S., Munshi N.C., Moreau P., Goldschmidt H., Cavo M., Truppel-Hartmann A., Rowe E., et al. Idecabtagene Vicleucel (ide-cel, bb2121) in Relapsed and Refractory Multiple Myeloma: Analyses of High-Risk Subgroups in the KarMMa Study. Blood. 2020;136:37–38. doi: 10.1182/blood-2020-134319. [DOI] [Google Scholar]
- 23.Berdeja J.G., Raje N.S., Siegel D.S., Lin Y., Anderson J.L.D., Rodriguez-Otero P., Manier S., Einsele H., Cavo M., Truppel-Hartmann A., et al. Efficacy and Safety of Idecabtagene Vicleucel (ide-cel, bb2121) in Elderly Patients with Relapsed and Refractory Multiple Myeloma: KarMMa Subgroup Analysis. Blood. 2020;136:16–17. doi: 10.1182/blood-2020-134322. [DOI] [Google Scholar]
- 24.Alsina M., Shah N., Raje N.S., Jagannath S., Madduri D., Kaufman J.L., Siegel D.S., Munshi N.C., Rosenblatt J., Lin Y., et al. Updated Results from the Phase I CRB-402 Study of Anti-Bcma CAR-T Cell Therapy bb21217 in Patients with Relapsed and Refractory Multiple Myeloma: Correlation of Expansion and Duration of Response with T Cell Phenotypes. Blood. 2020;136:25–26. doi: 10.1182/blood-2020-140410. [DOI] [Google Scholar]
- 25.Zhao W.-H., Liu J., Wang B.-Y., Chen Y.-X., Cao X.-M., Yang Y., Zhang Y.-L., Wang F.-X., Zhang P.-Y., Lei B., et al. A phase 1, open-label study of LCAR-B38M, a chimeric antigen receptor T cell therapy directed against B cell maturation antigen, in patients with relapsed or refractory multiple myeloma. J. Hematol. Oncol. 2018;11:141. doi: 10.1186/s13045-018-0681-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang B.-Y., Zhao W.-H., Liu J., Chen Y.-X., Cao X.-M., Yang Y., Zhang Y.-L., Wang F.-X., Zhang P.-Y., Lei B., et al. Long-Term Follow-up of a Phase 1, First-in-Human Open-Label Study of LCAR-B38M, a Structurally Differentiated Chimeric Antigen Receptor T (CAR-T) Cell Therapy Targeting B-Cell Maturation Antigen (BCMA), in Patients (pts) with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2019;134:579. doi: 10.1182/blood-2019-124953. [DOI] [Google Scholar]
- 27.Berdeja J.G., Madduri D., Usmani S.Z., Jakubowiak A., Agha M., Cohen A.D., Stewart A.K., Hari P., Htut M., Lesokhin A., et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTI-TUDE-1): A phase 1b/2 open-label study. Lancet. 2021;398:P314–P324. doi: 10.1016/S0140-6736(21)00933-8. [DOI] [PubMed] [Google Scholar]
- 28.Lin Y., Martin T., Cohen A.D., Jakubowiak A., Jasielec J., Usmani S.Z., Madduri D., Agha M., Stewart A.K., Singh I., et al. Cytokine Release Syndrome in Patients with Relapsed/Refractory Multiple Myeloma Treated with Ciltacabtagene Autoleucel in the Phase 1b/2 CARTITUDE-1 Study. Blood. 2020;136:45–46. doi: 10.1182/blood-2020-136360. [DOI] [Google Scholar]
- 29.Usmani S.Z., Berdeja J.G., Madduri D., Jakubowiak A.J., Agha M.E., Cohen A.D., Hari P., Yeh T.-M., Olyslager Y., Banerjee A., et al. Ciltacabtagene autoleucel, a B-cell maturation antigen (BCMA)-directed chimeric antigen receptor T-cell (CAR-T) therapy, in relapsed/refractory multiple myeloma (R/R MM): Updated results from CARTITUDE-1. J. Clin. Oncol. 2021;39:8005. doi: 10.1200/JCO.2021.39.15_suppl.8005. [DOI] [Google Scholar]
- 30.Agha M.E., Cohen A.D., Madduri D., Cohen Y.C., Delforge M., Hillengass J., Goldschmidt H., Weisel K., Raab M.-S., Scheid C., et al. CARTITUDE-2: Efficacy and safety of ciltacabtagene autoleucel (cilta-cel), a BCMA-directed CAR T-cell therapy, in patients with progressive multiple myeloma (MM) after one to three prior lines of therapy. J. Clin. Oncol. 2021;39:8013. doi: 10.1200/JCO.2021.39.15_suppl.8013. [DOI] [Google Scholar]
- 31.Mailankody S., Matous J.V., Liedtke M., Sidana S., Malik S., Nath R., Oluwole O.O., Karski E.E., Lovelace W., Zhou X., et al. Universal: An Allogeneic First-in-Human Study of the Anti-Bcma ALLO-715 and the Anti-CD52 ALLO-647 in Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:24–25. doi: 10.1182/blood-2020-140641. [DOI] [Google Scholar]
- 32.Costello C.L., Cohen A.D., Patel K.K., Ali S.S., Berdeja J.G., Shah N., Ganguly S., Kocoglu M.H., Abedi M., Ostertag E.M., et al. Phase 1/2 Study of the Safety and Response of P-BCMA-101 CAR-T Cells in Patients with Relapsed/Refractory (r/r) Multiple Myeloma (MM) (PRIME) with Novel Therapeutic Strategies. Blood. 2020;136:29–30. doi: 10.1182/blood-2020-142695. [DOI] [Google Scholar]
- 33.Mailankody S., Jakubowiak A.J., Htut M., Costa L.J., Lee K., Ganguly S., Kaufman J.L., Siegel D.S.D., Bensinger W., Cota M., et al. Orvacabtagene autoleucel (orva-cel), a B-cell matu-ration antigen (BCMA)-directed CAR-T cell therapy for patients (pts) with relapsed/refractory multiple myeloma (RRMM): Update of the phase 1/2 EVOLVE study ( NCT03430011) J. Clin. Oncol. 2020;38:8504. doi: 10.1200/JCO.2020.38.15_suppl.8504. [DOI] [Google Scholar]
- 34.Brudno J.N., Maric I., Hartman S.D., Rose J.J., Wang M., Lam N., Stetler-Stevenson M., Salem D., Yuan C., Pavletic S., et al. T Cells Genetically Modified to Express an Anti–B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J. Clin. Oncol. 2018;36:2267–2280. doi: 10.1200/JCO.2018.77.8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cohen A., Garfall A.L., Stadtmauer E.A., Melenhorst J.J., Lacey S.F., Lancaster E., Vogl D.T., Weiss B.M., Dengel K., Nelson A., et al. B cell maturation antigen–specific CAR T cells are clinically active in multiple myeloma. J. Clin. Investig. 2019;129:2210–2221. doi: 10.1172/JCI126397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kumar S.K., Baz R.C., Orlowski R.Z., Anderson L.D., Jr., Ma H., Shrewsbury A., Croghan K.A., Bilgi M., Kansagra A., Kapoor P., et al. Results from Lummicar-2: A Phase 1b/2 Study of Fully Human B-Cell Maturation Antigen-Specific CAR-T Cells (CT053) in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood. 2020;136:28–29. doi: 10.1182/blood-2020-139802. [DOI] [Google Scholar]
- 37.An G., Sui W., Wang T., Qu X., Zhang X., Yang J., Zhang Y., Zhang L., Zhu J., Zheng C., et al. An Anti-Bcma CAR T-Cell Therapy (C-CAR088) Shows Promising Safety and Efficacy Profile in Relapsed or Refractory Multiple Myeloma. Blood. 2020;136:29–30. doi: 10.1182/blood-2020-138734. [DOI] [Google Scholar]
- 38.Lejeune M., Köse M.C., Duray E., Einsele H., Beguin Y., Caers J. Bispecific, T-Cell-Recruiting Antibodies in B-Cell Malignancies. Front. Immunol. 2020;11:762. doi: 10.3389/fimmu.2020.00762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caraccio C., Krishna S., Phillips D., Schürch C.M. Bispecific Antibodies for Multiple Myeloma: A Review of Targets, Drugs, Clinical Trials, and Future Directions. Front. Immunol. 2020;11:501. doi: 10.3389/fimmu.2020.00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kumar S., Rajkumar S.V. BiTEing the Tumor. J. Clin. Oncol. 2020;38:2077–2079. doi: 10.1200/JCO.20.00223. [DOI] [PubMed] [Google Scholar]
- 41.Verkleij C.P., Frerichs K.A., Broekmans M., Absalah S., Maas-Bosman P.W., Kruyswijk S., Nijhof I.S., Mutis T., Zweegman S., Van De Donk N.W. T-cell redirecting bispecific antibodies targeting BCMA for the treatment of multiple myeloma. Oncotarget. 2020;11:4076–4081. doi: 10.18632/oncotarget.27792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kontermann R.E., Brinkmann U. Bispecific antibodies. Drug Discov. Today. 2015;20:838–847. doi: 10.1016/j.drudis.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 43.Velasquez M.P., Bonifant C.L., Gottschalk S. Redirecting T cells to hematological malignancies with bispecific antibodies. Blood. 2018;131:30–38. doi: 10.1182/blood-2017-06-741058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez L., Dardac A., Madduri D., Richard S., Richter J. B-cell maturation antigen (BCMA) in multiple myeloma: The new frontier of targeted therapies. Ther. Adv. Hematol. 2021;12:2040620721989585. doi: 10.1177/2040620721989585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou X., Einsele H., Danhof S. Bispecific Antibodies: A New Era of Treatment for Multiple Myeloma. J. Clin. Med. 2020;9:2166. doi: 10.3390/jcm9072166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geis M., Nowotny B., Bohn M.-D., Kouhestani D., Einsele H., Bumm T., Stuhler G. Combinatorial targeting of multiple myeloma by complementing T cell engaging antibody fragments. Commun. Biol. 2021;4:44. doi: 10.1038/s42003-020-01558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hipp S., Tai Y.T., Blanset D., Deegen P., Wahl J., Thomas O., Rattel B., Adam P.J., Adam P.J., Friedrich M. A novel BCMA/CD3 bispecific T-cell engager for the treatment of multiple myeloma induces selective lysis in vitro and in vivo. Leukemia. 2017;31:1743–1751. doi: 10.1038/leu.2016.388. [DOI] [PubMed] [Google Scholar]
- 48.Goldstein R.L., Goyos A., Li C.-M., Deegen P., Bogner P., Sternjak A., Thomas O., Klinger M., Wahl J., Friedrich M., et al. AMG 701 induces cytotoxicity of multiple myeloma cells and depletes plasma cells in cynomolgus monkeys. Blood Adv. 2020;4:4180–4194. doi: 10.1182/bloodadvances.2020002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panowski S.H., Kuo T.C., Zhang Y., Chen A., Geng T., Aschenbrenner L., Kamperschroer C., Pascua E., Chen W., Delaria K., et al. Preclinical Efficacy and Safety Comparison of CD3 Bispecific and ADC Modalities Targeting BCMA for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2019;18:2008–2020. doi: 10.1158/1535-7163.MCT-19-0007. [DOI] [PubMed] [Google Scholar]
- 50.DiLillo D.J., Olson K., Mohrs K., Meagher T.C., Bray K., Sineshchekova O., Startz T., Kuhnert J., Retter M.W., Godin S., et al. A BCMAxCD3 bispecific T cell-engaging anti-body demonstrates robust antitumor efficacy similar to that of anti-BCMA CAR-T cells. Blood Adv. 2021;5:1291–1304. doi: 10.1182/bloodadvances.2020002736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pillarisetti K., Powers G., Luistro L., Babich A., Baldwin E., Li Y., Zhang X., Mendonça M., Majewski N., Nanjunda R., et al. Teclistamab is an active T cell-redirecting bispecific anti-body against B-cell maturation antigen for multiple myeloma. Blood Adv. 2020;4:4538–4549. doi: 10.1182/bloodadvances.2020002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trinklein N.D., Pham D., Schellenberger U., Buelow B., Boudreau A., Choudhry P., Clarke S.C., Dang K., Harris K.E., Iyer S., et al. Efficient tumor killing and minimal cytokine release with novel T-cell agonist bispecific antibodies. mAbs. 2019;11:639–652. doi: 10.1080/19420862.2019.1574521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Topp M.S., Duell J., Zugmaier G., Attal M., Moreau P., Langer C., Krönke J., Facon T., Salnikov A.V., Lesley R., et al. Anti–B-Cell Maturation Antigen BiTE Molecule AMG 420 Induces Responses in Multiple Myeloma. J. Clin. Oncol. 2020;38:775–783. doi: 10.1200/JCO.19.02657. [DOI] [PubMed] [Google Scholar]
- 54.Harrison S.J., Minnema M.C., Lee H.C., Spencer A., Kapoor P., Madduri D., Larsen J., Ailawadhi S., Kaufman J.L., Raab M.S., et al. A Phase 1 First in Human (FIH) Study of AMG 701, an Anti-B-Cell Maturation Antigen (BCMA) Half-Life Extended (HLE) BiTE® (bispecific T-cell engager) Molecule, in Relapsed/Refractory (RR) Multiple Myeloma (MM) Blood. 2020;136:28–29. doi: 10.1182/blood-2020-134063. [DOI] [Google Scholar]
- 55.Usmani S.Z., Mateos M.-V., Nahi H., Krishnan A.Y., Van De Donk N.W., Miguel J.S., Oriol A., Rosiñol L., Chari A., Adams H., et al. Phase I study of teclistamab, a humanized B-cell maturation antigen (BCMA) x CD3 bispecific antibody, in relapsed/refractory multiple myeloma (R/R MM) J. Clin. Oncol. 2020;38:100. doi: 10.1200/JCO.2020.38.15_suppl.100. [DOI] [Google Scholar]
- 56.Usmani S.Z., Garfall A.L., van de Donk N.W.C.J., Nahi H., San-Miguel J.F., Oriol A., Rosinol L., Chari A., Bhutani M., Karlin L., et al. Teclistamab, a B-cell maturation antigen × CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MajesTEC-1): A multicentre, open-label, single-arm, phase 1 study. Lancet. 2021;398:665–674. doi: 10.1016/S0140-6736(21)01338-6. [DOI] [PubMed] [Google Scholar]
- 57.Garfall A.L., Usmani S.Z., Mateos M.-V., Nahi H., Van De Donk N.W., San-Miguel J.F., Rocafiguera A.O., Rosinol L., Chari A., Bhutani M., et al. Updated Phase 1 Results of Teclistamab, a B-Cell Maturation Antigen (BCMA) × CD3 Bispecific Antibody, in Relapsed and/or Refractory Multiple Myeloma (RRMM) Blood. 2020;136:27. doi: 10.1182/blood-2020-138831. [DOI] [Google Scholar]
- 58.Krishnan A.Y., Garfall A.L., Mateos M.-V., van de Donk N.W.C.J., Nahi H., San-Miguel J.F., Oriol A., Rosiñol L., Chari A., Bhutani M., et al. Updated phase 1 results of teclistamab, a B-cell maturation antigen (BCMA) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM) J. Clin. Oncol. 2021;39:8007. doi: 10.1200/JCO.2021.39.15_suppl.8007. [DOI] [Google Scholar]
- 59.Madduri D., Rosko A., Brayer J., Zonder J., Bensinger W.I., Li J., Xu L., Adriaens L., Chokshi D., Zhang W., et al. REGN5458, a BCMA x CD3 Bispecific Monoclonal Antibody, Induces Deep and Durable Responses in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2020;136:41–42. doi: 10.1182/blood-2020-139192. [DOI] [Google Scholar]
- 60.Rodriguez C., D’Souza A., Shah N., Voorhees P.M., Buelow B., Vij R., Kumar S.K. Initial Results of a Phase I Study of TNB-383B, a BCMA × CD3 Bispecific T-Cell Redirecting Antibody, in Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:43–44. doi: 10.1182/blood-2020-139893. [DOI] [Google Scholar]
- 61.Bahlis N.J., Raje N.S., Costello C., Dholaria B.R., Solh M.M., Levy M.Y., Tomasson M.H., Dube H., Liu F., Liao K.H., et al. Efficacy and safety of elranatamab (PF-06863135), a B-cell maturation antigen (BCMA)-CD3 bispecific antibody, in patients with relapsed or refractory multiple myeloma (MM) J. Clin. Oncol. 2021;39:8006. doi: 10.1200/JCO.2021.39.15_suppl.8006. [DOI] [Google Scholar]
- 62.Costa L.J., Wong S.W., Bermúdez A., De La Rubia J., Mateos M.-V., Ocio E.M., Rodríguez-Otero P., San-Miguel J., Li S., Sarmiento R., et al. First Clinical Study of the B-Cell Maturation Antigen (BCMA) 2+1 T Cell Engager (TCE) CC-93269 in Patients (Pts) with Relapsed/Refractory Multiple Myeloma (RRMM): Interim Results of a Phase 1 Multicenter Trial. Blood. 2019;134:143. doi: 10.1182/blood-2019-122895. [DOI] [Google Scholar]
- 63.Topp M.S., Duell J., Mauser M., Einsele H. Outcome of BCMA Bite (AMG420) Therapy in Relapse and Refractory Multiple Myeloma (RRMM) Patients. Blood. 2020;136:25–26. doi: 10.1182/blood-2020-143470. [DOI] [Google Scholar]
- 64.Cho S.-F., Lin L., Xing L., Li Y., Wen K., Yu T., Hsieh P.A., Munshi N., Wahl J., Matthes K., et al. The immunomodulatory drugs lenalidomide and pomalidomide enhance the potency of AMG 701 in multiple myeloma preclinical models. Blood Adv. 2020;4:4195–4207. doi: 10.1182/bloodadvances.2020002524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Frerichs K.A., Broekmans M.E.C., Marin Soto J.A., van Kessel B., Heymans M.W., Holthof L.C., Verkleij C.P.M., Boominathan R., Vaidya B., Sendecki J., et al. Preclinical Activity of JNJ-7957, a Novel BCMA×CD3 Bispecific Antibody for the Treatment of Multiple Myeloma, Is Potentiated by Daratumumab. Clin. Cancer Res. 2020;26:2203–2215. doi: 10.1158/1078-0432.CCR-19-2299. [DOI] [PubMed] [Google Scholar]
- 66.Raje N., Jakubowiak A., Gasparetto C., Cornell R., Krupka H., Navarro D., Forgie A.J., Udata C., Basu C., Chou J., et al. Safety, Clinical Activity, Pharmacokinetics, and Pharmacodynamics from a Phase I Study of PF-06863135, a B-Cell Maturation Antigen (BCMA)-CD3 Bispecific Antibody, in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2019;134:1869. doi: 10.1182/blood-2019-121805. [DOI] [Google Scholar]
- 67.Cho S.-F., Anderson K.C., Tai Y.-T. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front. Immunol. 2018;9:1821. doi: 10.3389/fimmu.2018.01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schade H., Madan S., Medvedova E., Nath R., Knapp L., Lemon B., Sun L.L. HPN217-3001: A Phase 1/2 Open-Label, Multicenter, Dose Escalation and Dose Expansion Study of the Safety, Tolerability, and Pharmacokinetics of HPN217, a Bcma-Targeting T-Cell Engager, in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:10. doi: 10.1182/blood-2020-136012. [DOI] [Google Scholar]
- 69.Younes A., Bartlett N., Leonard J.P., Kennedy D.A., Lynch C.M., Sievers E., Forero-Torres A. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N. Engl. J. Med. 2010;363:1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 70.Sievers E., Larson R., Stadtmauer E.A., Estey E., Löwenberg B., Dombret H., Karanes C., Theobald M., Bennett J.M., Sherman M.L., et al. Efficacy and Safety of Gemtuzumab Ozogamicin in Patients With CD33-Positive Acute Myeloid Leukemia in First Relapse. J. Clin. Oncol. 2001;19:3244–3254. doi: 10.1200/JCO.2001.19.13.3244. [DOI] [PubMed] [Google Scholar]
- 71.Burris H.A., Rugo H.S., Vukelja S.J., Vogel C.L., Borson R.A., Limentani S., Tan-Chiu E., Krop I.E., Michaelson R.A., Girish S., et al. Phase II Study of the Antibody Drug Conjugate Trastuzumab-DM1 for the Treatment of Human Epidermal Growth Factor Receptor 2 (HER2) –Positive Breast Cancer After Prior HER2-Directed Therapy. J. Clin. Oncol. 2011;29:398–405. doi: 10.1200/JCO.2010.29.5865. [DOI] [PubMed] [Google Scholar]
- 72.Powles T., Rosenberg J.E., Sonpavde G.P., Loriot Y., Durán I., Lee J.-L., Matsubara N., Vulsteke C., Castellano D., Wu C., et al. Enfortumab Vedotin in Previously Treated Advanced Urothelial Carcinoma. N. Engl. J. Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tai Y.-T., Mayes P.A., Acharya C., Zhong M.Y., Cea M., Cagnetta A., Craigen J., Yates J.R.W., Gliddon L., Fieles W., et al. Novel anti–B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123:3128–3138. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kinneer K., Flynn M., Thomas S.B., Meekin J., Varkey R., Xiao X., Zhong H., Breen S., Hynes P.G., Fleming R., et al. Preclinical assessment of an antibody–PBD conjugate that targets BCMA on multiple myeloma and myeloma progenitor cells. Leukemia. 2018;33:766–771. doi: 10.1038/s41375-018-0278-7. [DOI] [PubMed] [Google Scholar]
- 75.Singh R.K., Jones R.J., Hong S., Shirazi F., Wang H., Kuiatse I., Pahl A., Orlowski R.Z. HDP101, a Novel B-Cell Maturation Antigen (BCMA)-Targeted Antibody Conjugated to α-Amanitin, Is Active Against Myeloma with Preferential Efficacy Against Pre-Clinical Models of Deletion 17p. Blood. 2018;132:593. doi: 10.1182/blood-2018-99-118412. [DOI] [Google Scholar]
- 76.Lee H.C., Raje N.S., Landgren O., Upreti V.V., Wang J., Avilion A.A., Hu X., Rasmussen E., Ngarmchamnanrith G., Fujii H., et al. Phase 1 study of the anti-BCMA antibody-drug conjugate AMG 224 in patients with relapsed/refractory multiple myeloma. Leukemia. 2021;35:255–258. doi: 10.1038/s41375-020-0834-9. [DOI] [PubMed] [Google Scholar]
- 77.Lee L., Bounds D., Paterson J., Herledan G., Sully K., Seestaller-Wehr L.M., Fieles W.E., Tunstead J., McCahon L., Germaschewski F.M., et al. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. Br. J. Haematol. 2016;174:911–922. doi: 10.1111/bjh.14145. [DOI] [PubMed] [Google Scholar]
- 78.Trudel S., Lendvai N., Popat R., Voorhees P.M., Reeves B., Libby E.N., Richardson P.G., Hoos A., Gupta I., Bragulat V., et al. Antibody–drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: An update on safety and efficacy from dose expansion phase I study. Blood Cancer J. 2019;9:37. doi: 10.1038/s41408-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eaton J.S., Miller P.E., Mannis M.J., Murphy C.J. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015;31:589–604. doi: 10.1089/jop.2015.0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lonial S., Lee H.C., Badros A., Trudel S., Nooka A.K., Chari A., Abdallah A.-O., Callander N., Lendvai N., Sborov D., et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): A two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2019;21:207–221. doi: 10.1016/S1470-2045(19)30788-0. [DOI] [PubMed] [Google Scholar]
- 81.Lonial S., Lee H.C., Badros A., Trudel S., Nooka A.K., Chari A., Abdallah A.-O.A., Callander N.S., Sborov D.W., Suvannasankha A., et al. Pivotal DREAMM-2 study: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) refractory to proteasome inhibitors (PIs), immunomodulatory agents, and refractory and/or intolerant to anti-CD38 monoclonal antibodies (mAbs) J. Clin. Oncol. 2020;38:8536. doi: 10.1200/jco.2020.38.15_suppl.8536. [DOI] [Google Scholar]
- 82.Lonial S., Lee H.C., Badros A., Trudel S., Nooka A., Chari A., Abdallah A.-O., Callander N.S., Sborov D., Suvannasankha A., et al. DREAMM-2: Single-Agent Belantamab Mafodotin (Belamaf) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) – 1-Year Outcomes By Prior Therapies. Blood. 2020;136:1417. doi: 10.1182/blood-2020-140078. [DOI] [Google Scholar]
- 83.Cohen A.D., Trudel S., Lonial S., Libby E.N., Lee H.C., Besemer B., Facon T., Nooka A.K., Callander N.S., Chari A., et al. DREAMM-2: Single-agent belantamab mafodotin (GSK2857916) in patients with relapsed/refractory multiple myeloma (RRMM) and high-risk (HR) cytogenetics. J. Clin. Oncol. 2020;38:8541. doi: 10.1200/JCO.2020.38.15_suppl.8541. [DOI] [Google Scholar]
- 84.Farooq A.V., Degli Esposti S., Popat R., Thulasi P., Lonial S., Nooka A.K., Jakubowiak A., Sborov D., Zaugg B.E., Badros A.Z., et al. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody-Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020;9:889–911. doi: 10.1007/s40123-020-00280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lonial F.S., Nooka M.A., Thulasi P., Badros A.Z., Jeng B.H., Callander N.S., Sborov M.D., Zaugg B.E., Popat R., Degli Esposti S., et al. Recovery of Ocular Events with Longer-Term Follow-up in the DREAMMM-2 Study of Single-Agent Belantamab Mafodotin (Belamaf) in Patients with Relapsed or Refractory Multiple Myeloma (RRMM) Blood. 2020;136:26–27. doi: 10.1182/blood-2020-140078. [DOI] [Google Scholar]
- 86.Richardson P.G., Lee H.C., Abdallah A.-O., Cohen A.D., Kapoor P., Voorhees P.M., Hoos A., Wang K., Baron J., Piontek T., et al. Single-agent belantamab mafodotin for relapsed/refractory multiple myeloma: Analysis of the lyophilised presentation cohort from the pivotal DREAMM-2 study. Blood Cancer J. 2020;10:106. doi: 10.1038/s41408-020-00369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Trudel S., McCurdy A., Sutherland H. Part 1 Results of a Dose Finding Study of Belantamab Mafodotin (GSK2857916) in Combination with Pomalidomide (POM) and Dexamethasone (DEX) for the Treatment of Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2021;136:25. [Google Scholar]
- 88.Trudel S., Davis R., Lewis N.M., Bakshi K.K., Chopra B., De Oca R.M., Ferron-Brady G., Eliason L., Kremer B.E., Gupta I., et al. DREAMM-8: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin with Pomalidomide and Dexamethasone (B-Pd) Vs Pomalidomide Plus Bortezomib and Dexamethasone (PVd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2020;136:4. doi: 10.1182/blood-2020-139785. [DOI] [Google Scholar]
- 89.Popat R., Nooka M.A., Stockerl-Goldstein K., Abonour R., Ramaekers R., Khot A., Forbes A., Lee C., Augustson M.F.F.B., Spencer A., et al. DREAMM-6: Safety, Tolerability and Clinical Activity of Belantamab Mafodotin (Belamaf) in Combination with Bortezomib/Dexamethasone (BorDex) in Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2020;136:19–20. doi: 10.1182/blood-2020-139332. [DOI] [Google Scholar]
- 90.Rifkin R.M., Boyd M.B.K., Grosicki S., Kim K., Di Raimondo F., A Dimopoulos M., Weisel K., Arnulf B., Hajek R., Hungria V.T.M., et al. DREAMM-7: A Phase III Study of the Efficacy and Safety of Belantamab Mafodotin (Belamaf) with Bortezomib, and Dexamethasone (B-Vd) in Patients with Relapsed/Refractory Multiple Myeloma (RRMM) Blood. 2020;136:53–54. doi: 10.1182/blood-2020-139181. [DOI] [Google Scholar]
- 91.Nooka A.K., Weisel K., van de Donk N.W., Routledge D., Otero P.R., Song K., Quach H., Callander N., Minnema M.C., Trudel S., et al. Belantamab mafodotin in combination with novel agents in relapsed/refractory multiple myeloma: DREAMM-5 study design. Futur. Oncol. 2021;17:1987–2003. doi: 10.2217/fon-2020-1269. [DOI] [PubMed] [Google Scholar]
- 92.Kumar S.K., Migkou M., Bhutani M., Spencer A., Ailawadhi S., Kalff A., Walcott F., Pore N., Gibson D., Wang F., et al. Phase 1, First-in-Human Study of MEDI2228, a BCMA-Targeted ADC in Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:26–27. doi: 10.1182/blood-2020-136375. [DOI] [Google Scholar]
- 93.Strassz A., Raab M.S., Orlowski M.R.Z., Kulke M., Schiedner G., Pahl A. A First in Human Study Planned to Evaluate Hdp-101, an Anti-BCMA Amanitin Antibody-Drug Conjugate with a New Payload and a New Mode of Action, in Multiple Myeloma. Blood. 2020;136:34. doi: 10.1182/blood-2020-142285. [DOI] [Google Scholar]
- 94.Polson A.G., Zheng B., Elkins K., Chang W., Du C., Dowd P., Yen L., Tan C., Hongo J.-A., Koeppen H., et al. Expression pattern of the human FcRH/IRTA receptors in normal tissue and in B-chronic lymphocytic leukemia. Int. Immunol. 2006;18:1363–1373. doi: 10.1093/intimm/dxl069. [DOI] [PubMed] [Google Scholar]
- 95.Elkins K., Zheng B., Go M., Slaga D., Du C., Scales S.J., Yu S.-F., McBride J., De Tute R., Rawstron A., et al. FcRL5 as a Target of Antibody–Drug Conjugates for the Treatment of Multiple Myeloma. Mol. Cancer Ther. 2012;11:2222–2232. doi: 10.1158/1535-7163.MCT-12-0087. [DOI] [PubMed] [Google Scholar]
- 96.Li J., Stagg N., Johnston J., Harris M., Menzies S., DiCara D., Clark V., Hristopoulos M., Cook R., Slaga D., et al. Membrane-Proximal Epitope Facilitates Efficient T Cell Synpase Formation by Anti-FcRH5/CD3 and Is a Requirement for Myeloma Cell Killing. Cancer Cell. 2017;31:383–395. doi: 10.1016/j.ccell.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stewart A.K., Krishnan A.Y., Singhal S., Boccia R.V., Patel M.R., Niesvizky R., Chanan-Khan A.A., Ailawadhi S., Brumm J., Mundt K.E., et al. Phase I study of the anti-FcRH5 antibody-drug conjugate DFRF4539A in relapsed or refractory multiple myeloma. Blood Cancer J. 2019;9:17. doi: 10.1038/s41408-019-0178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen A.D., Harrison S.J., Krishnan A., Fonseca R., Forsberg P.A., Spencer A., Berdeja J.G., Laubach J.P., Li M., Choeurng V., et al. Initial Clinical Activity and Safety of BFCR4350A, a FcRH5/CD3 T-Cell-Engaging Bispecific Antibody, in Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:42–43. doi: 10.1182/blood-2020-136985. [DOI] [Google Scholar]
- 99.Atamaniuk J., Gleiss A., Porpaczy E., Kainz B., Grunt T.W., Raderer M., Hilgarth B., Drach J., Ludwig H., Gisslinger H., et al. Overexpression of G protein-coupled receptor 5D in the bone marrow is associated with poor prognosis in patients with multiple myeloma. Eur. J. Clin. Investig. 2012;42:953–960. doi: 10.1111/j.1365-2362.2012.02679.x. [DOI] [PubMed] [Google Scholar]
- 100.Inoue S., Nambu T., Shimomura T. The RAIG Family Member, GPRC5D, Is Associated with Hard-Keratinized Structures. J. Investig. Dermatol. 2004;122:565–573. doi: 10.1046/j.0022-202X.2004.12628.x. [DOI] [PubMed] [Google Scholar]
- 101.Smith E.L., Harrington K., Staehr M., Masakayan R., Jones J., Long T.J., Ng K.Y., Ghoddusi M., Purdon T.J., Wang X., et al. GPRC5D is a target for the immunotherapy of multiple myeloma with rationally designed CAR T cells. Sci. Transl. Med. 2019;11:eaau7746. doi: 10.1126/scitranslmed.aau7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Fernández de Larrea C., Staehr M., Lopez A., Ng K., Chen Y., Godfrey W., Purdon T.J., Ponomarev V., Wendel H.G., Brentjens R.J., et al. Defining an Optimal Dual-Targeted CAR-T-cell Therapy Approach Simultaneously Targeting BCMA and GPRC5D to Prevent BCMA Escape-Driven Relapse in Multiple Myeloma. Blood Cancer Discov. 2020;1:146–154. doi: 10.1158/2643-3230.BCD-20-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pillarisetti K., Edavettal S., Mendonça M., Li Y., Tornetta M., Babich A., Majewski N., Husovsky M., Reeves D., Walsh E., et al. A T-cell-redirecting bispecific G-protein-coupled receptor class 5 member D x CD3 antibody to treat multiple myeloma. Blood. 2020;135:1232–1243. doi: 10.1182/blood.2019003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chari A., Berdeja J., Oriol A., Van de Donk N., Rodriguez P., Askari E., Mateos M.-V., Minnema M.C., Verona R., Girgis C., et al. A Phase 1, First-in-Human Study of Talquetamab, a G Protein-Coupled Recetor Family C Group 5 Member D (GPRC5D) × CD3 Bispecific Antibody, in Patients with Relapsed and/or Refractory Multiple Myeloma (RRMM) Blood Adv. 2020;136:40–41. doi: 10.1182/blood-2020-133873. [DOI] [Google Scholar]
- 105.Berdeja J.G., Krishnan A.Y., Oriol A., Van De Donk N.W.C.J., Rodríguez-Otero P., Askari E., Mateos M.-V., Minnema M.C., Costa L.J., Verona R., et al. Updated results of a phase 1, first-in-human study of talquetamab, a G protein-coupled receptor family C group 5 member D (GPRC5D) × CD3 bispecific antibody, in relapsed/refractory multiple myeloma (MM) J. Clin. Oncol. 2021;39:8008. doi: 10.1200/JCO.2021.39.15_suppl.8008. [DOI] [Google Scholar]
- 106.His E.D., Steinle R., Balasa B., Szmania S., Draksharapu A., Shum B.P., Huseni M., Powers D., Nanisetti A., Zhang Y., et al. CS1, a potential new therapeutic antibody target for the treatment of multiple myeloma. Clin. Cancer Res. 2008;14:2775–2784. doi: 10.1158/1078-0432.CCR-07-4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lonial S., Dimopoulos M., Palumbo A., White D., Grosicki S., Spicka I., Walter-Croneck A., Moreau P., Mateos M.V., Magen H., et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N. Engl. J. Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 108.Dimopoulos M.A., Dytfeld D., Grosicki S., Moreau P., Takezako N., Hori M., Leleu X., Leblanc R., Suzuki K., Raab M.S., et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018;379:1811–1822. doi: 10.1056/NEJMoa1805762. [DOI] [PubMed] [Google Scholar]
- 109.Vij R., Nath R., Afar D.E.H., Mateos M.V., Berdeja J.G., Raab M.S., Guenther A., Martínez-López J., Jakubowiak A.J., Leleu X., et al. First-in-Human Phase I Study of ABBV-838, an Anti-body-Drug Conjugate Targeting SLAMF7/CS1 in Patients with Relapsed and Refractory Multiple Myeloma. Clin. cancer Res. 2020;26:2308–2317. doi: 10.1158/1078-0432.CCR-19-1431. [DOI] [PubMed] [Google Scholar]
- 110.Gogishvili T., Danhof S., Prommersberger S., Rydzek J., Schreder M., Brede C., Einsele H., Hudecek M. SLAMF7-CAR T cells eliminate myeloma and confer selective fratricide of SLAMF7+ normal lymphocytes. Blood. 2017;130:2838–2847. doi: 10.1182/blood-2017-04-778423. [DOI] [PubMed] [Google Scholar]
- 111.Radocha J., van de Donk N., Weisel K. Monoclonal Antibodies and Antibody Drug Conjugates in Multiple Myeloma. Cancers. 2021;13:1571. doi: 10.3390/cancers13071571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Drent E., Groen R.W., Noort W.A., Themeli M., Van Bueren J.J.L., Parren P.W., Kuball J., Sebestyen Z., Yuan H., De Bruijn J., et al. Pre-clinical evaluation of CD38 chimeric antigen receptor engineered T cells for the treatment of multiple myeloma. Haematologica. 2016;101:616–625. doi: 10.3324/haematol.2015.137620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bruins M.W.S., Zheng M.W., Higgins J.P., Willert E.K., Newcomb J., Dash A.B., Van De Donk N.W., Zweegman M.S., Mutis T. TAK-169, a Novel Recombinant Immunotoxin Specific for CD38, Induces Powerful Preclinical Activity Against Patient-Derived Multiple Myeloma Cells. Blood. 2020;136:11–12. doi: 10.1182/blood-2020-136928. [DOI] [Google Scholar]
- 114.Doucey M.-A., Pouleau B., Estoppey C., Stutz C., Croset A., Laurendon A., Monney T., Pluess M., Ries-Fecourt C., Macoin J., et al. ISB 1342: A first-in-class CD38 T cell engager for the treatment of relapsed refractory multiple myeloma. J. Clin. Oncol. 2021;39:8044. doi: 10.1200/JCO.2021.39.15_suppl.8044. [DOI] [Google Scholar]
- 115.Li C., Mei H., Hu Y., Guo T., Liu L., Jiang H., Tang L., Wu Y.H., Ai L., Deng L., et al. A Bispecific CAR-T Cell Therapy Targeting Bcma and CD38 for Relapsed/Refractory Multiple Myeloma: Updated Results from a Phase 1 Dose-Climbing Trial. Blood. 2019;134:930. doi: 10.1182/blood-2019-130340. [DOI] [Google Scholar]
- 116.Jiang H., Dong B., Gao L., Liu L., Ge J., He A., Du J.J., Li L., Lu J., Chen X., et al. Clinical Results of a Multicenter Study of the First-in-Human Dual BCMA and CD19 Targeted Novel Platform Fast CAR-T Cell Therapy for Patients with Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:25–26. doi: 10.1182/blood-2020-138614. [DOI] [Google Scholar]
- 117.Yan L., Qu S., Shang J., Shi X., Kang L., Xu N., Zhu M., Zhou J., Jin S., Yao W., et al. Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med. 2020;10:563–574. doi: 10.1002/cam4.3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sun C., Mahendravada A., Ballard B., Kale B., Ramos C., West J., Maguire T., McKay K., Lichtman E., Tuchman S., et al. Safety and efficacy of targeting CD138 with a chimeric antigen receptor for the treatment of multiple myeloma. Oncotarget. 2019;10:2369–2383. doi: 10.18632/oncotarget.26792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Radhakrishnan S., Leutkens T., Scherer S., Davis P., Erica V.M., Olson M. CD229 CAR-T cells eliminate multiple myeloma and tumor propagating cells without fratricide. Nat. Commun. 2020;11:798. doi: 10.1038/s41467-020-14619-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Casucci M., Di Robilant B.N., Falcone L., Camisa B., Norelli M., Genovese P., Gentner B., Gullotta F., Ponzoni M., Bernardi M., et al. CD44v6-targeted T cells mediate potent antitumor effects against acute myeloid leukemia and multiple myeloma. Blood. 2013;122:3461–3472. doi: 10.1182/blood-2013-04-493361. [DOI] [PubMed] [Google Scholar]
- 121.Sherbenou D., Aftab B.T., Su Y., Behrens C.R., Wiita A., Logan A.C., Acosta-Alvear D., Hann B.C., Walter P., Shuman M.A., et al. Antibody-drug conjugate targeting CD46 eliminates multiple myeloma cells. J. Clin. Investig. 2016;126:4640–4653. doi: 10.1172/JCI85856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Guo B., Chen M., Han Q., Hui F., Dai H., Zhang W. CD138-directed adoptive immunotherapy of chimeric antigen recep-tor (CAR)-modified T cells for multiple myeloma. Transplant Cell Ther. 2016;2:28–35. [Google Scholar]
- 123.Yu B., Jiang T., Liu D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020;13:125. doi: 10.1186/s13045-020-00962-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Timmers M., Roex G., Wang Y., Campillo-Davo D., Van Tendeloo V.F.I., Chu Y., Berneman Z., Luo F., Van Acker H.H., Anguille S. Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front. Immunol. 2019;10:1613. doi: 10.3389/fimmu.2019.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dahlén E., Veitonmäki N., Norlén P. Bispecific antibodies in cancer immunotherapy. Ther. Adv. Vaccines Immunother. 2018;6:3–17. doi: 10.1177/2515135518763280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yu B., Liu D. Antibody-drug conjugates in clinical trials for lymphoid malignancies and multiple myeloma. J. Hematol. Oncol. 2019;12:94. doi: 10.1186/s13045-019-0786-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Palumbo A., Bringhen S., Mateos M.V., Larocca A., Facon T., Kumar S.K., Offidani M., McCarthy P., Evangelista A., Lonial S., et al. Geriatric assessment predicts survival and toxicities in elderly myeloma patients: An International Myeloma Working Group report. Blood. 2015;125:2068–2074. doi: 10.1182/blood-2014-12-615187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chng W.J., Chung T.H., Kumar S., Usmani S., Munshi N., Avet-Loiseau H., Goldschmidt H., Durie B., Durie B. Gene signature combinations improve prognos-tic stratification of multiple myeloma patients. Leukemia. 2016;30:1071–1078. doi: 10.1038/leu.2015.341. [DOI] [PubMed] [Google Scholar]
- 129.Samur M.K., Aktas Samur A., Fulciniti M., Szalat R., Han T., Shammas M., Richardson P., Magrangeas F., Minvielle S., Corre J., et al. Genome-Wide Somatic Alterations in Multiple Myeloma Reveal a Superior Outcome Group. J. Clin. Oncol. 2020;38:3107–3118. doi: 10.1200/JCO.20.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berdeja J.G., Madduri D., Usmani S.Z., Singh I., Zudaire E., Yeh T.-M., Allred A.J., Olyslager Y., Banerjee A., Goldberg J.D., et al. Update of CARTITUDE-1: A phase Ib/II study of JNJ-4528, a B-cell maturation antigen (BCMA)-directed CAR-T-cell therapy, in relapsed/refractory multiple myeloma. J. Clin. Oncol. 2020;38:8505. doi: 10.1200/JCO.2020.38.15_suppl.8505. [DOI] [Google Scholar]
- 131.Munshi N.C., Larry D., Anderson J., Shah N., Jagannath S., Berdeja J.G., Lonial S., Raje N.S., Siegel D.S.D., Lin Y., et al. Idecabtagene vicleucel (ide-cel; bb2121), a BCMA-targeted CAR-T-cell therapy, in patients with relapsed and refractory multiple myeloma (RRMM): Initial KarMMa results. J. Clin. Oncol. 2020;38:8503. doi: 10.1200/JCO.2020.38.15_suppl.8503. [DOI] [Google Scholar]
- 132.Cohen A.D., Hari M.P., Htut M., Berdeja J.G., Madduri D., Usmani M.S.Z., Allred A.J., Olyslager M.Y., Banerjee A., Goldberg J.D., et al. Patient Expectations and Perceptions of Treatment in CARTITUDE-1: Phase 1b/2 Study of Ciltacabtagene Autoleucel in Relapsed/Refractory Multiple Myeloma. Blood. 2020;136:13–15. doi: 10.1182/blood-2020-136383. [DOI] [Google Scholar]
- 133.Shah N., Delforge M., San-Miguel J.F., Bertin K.B., Tahir M.J., Lewis H.B., Wang J., Braverman J., Campbell T.B., Dhanda D., et al. Secondary Quality-of-Life Domains in Patients with Relapsed and Refractory Multiple Myeloma Treated with the Bcma-Directed CAR T Cell Therapy Idecabtagene Vicleucel (ide-cel; bb2121): Results from the Karmma Clinical Trial. Blood. 2020;136:28–29. doi: 10.1182/blood-2020-136665. [DOI] [Google Scholar]