Abstract
This single-center retrospective study of invasive fungal disease (IFD) enrolled 251 adult patients undergoing induction chemotherapy for newly diagnosed acute myeloid leukemia (AML) from 2014–2019. Patients had primary AML (n = 148, 59%); antecedent myelodysplastic syndrome (n = 76, 30%), or secondary AML (n = 27, 11%). Seventy-five patients (30%) received an allogeneic hematopoietic cell transplant within the first year after induction chemotherapy. Proven/probable IFD occurred in 17 patients (7%). Twelve of the 17 (71%) were mold infections, including aspergillosis (n = 6), fusariosis (n = 3), and mucomycosis (n = 3). Eight breakthrough IFD (B-IFD), seven of which were due to molds, occurred in patients taking antifungal prophylaxis. Patients with proven/probable IFD had a significantly greater number of cumulative neutropenic days than those without an IFD, HR = 1.038 (95% CI 1.018–1.059), p = 0.0001. By cause-specific proportional hazards regression, the risk for IFD increased by 3.8% for each day of neutropenia per 100 days of follow up. Relapsed/refractory AML significantly increased the risk for IFD, HR = 7.562 (2.585–22.123), p = 0.0002, and Kaplan-Meier analysis showed significantly higher mortality at 1 year in patients who developed a proven/probable IFD, p = 0.02. IFD remains an important problem among patients with AML despite the use of antifungal prophylaxis, and development of IFD is associated with increased mortality in these patients.
Keywords: invasive fungal disease, mold infection, yeast infection, acute myeloid leukemia, breakthrough fungal disease
1. Introduction
Invasive fungal disease (IFD) is a highly morbid complication in patients with hematologic malignancies, including acute myeloid leukemia (AML) [1]. Prior studies have demonstrated a benefit of mold-active antifungal agents for IFD prophylaxis in patients at high risk [2,3]. With widespread use of prophylaxis, breakthrough IFD (B-IFD) have become an increasing problem and have been reported in up to 18% of patients with AML [4,5,6,7,8,9,10]. Early studies suggested that the risk factors predisposing AML patients for B-IFD were similar to those for IFD, in general, and included underlying severe myeloid immunosuppression from leukemia, prolonged neutropenia, use of central venous catheters, mucositis from chemotherapy or from graft versus host disease (GVHD) after hematopoietic cell transplantation (HCT), and use of broad-spectrum antibiotics [10,11]. However, heterogenous populations were included and no standardized definition of B-IFD was available when those studies were performed, calling into question whether the results are generalizable [12].
The recent development of new antifungals and improved formulations of existing agents has prompted changes in antifungal prophylaxis strategies for patients with AML. Additionally, revised definitions for IFD have been developed by the European Organization of Research and Treatment of Cancer and Mycoses Study Group Education and Research Consortium (EORTC/MSGERC) [13], and a consensus definition for B-IFD has been published by the MSGERC and the European Confederation of Medical Mycology (ECMM) [14]. This new definition for B-IFD incorporates the newer antifungal agents and better defines antifungal exposure by including pharmacokinetic parameters of the prophylactic agents.
We sought to determine the effectiveness of newer strategies for the prevention of IFD in patients with AML and to better define the occurrence of B-IFD at our institution.
2. Materials and Methods
2.1. Patients and Setting
This retrospective cohort study was conducted at the University of Michigan Medical Center, a 1000 bed tertiary care center. Approval for this study was granted by the institutional review board. All adult patients at least 18 years of age who had newly diagnosed AML and who were admitted for induction chemotherapy between June 2014 and January 2019 were screened for eligibility. Patients who expired prior to initiation of induction chemotherapy and those who received umbilical cord blood HCT or more than one allogeneic HCT within 1 year after first induction chemotherapy were excluded. Study patients were followed for one year from the first day of induction chemotherapy unless death occurred before that time.
The electronic medical record was reviewed to collect data on patient demographics and comorbidities, AML status at baseline, at time of IFD diagnosis, and at the last follow up, chemotherapy regimens, HCT, graft versus host disease (GVHD), cumulative duration of neutropenia, cumulative prophylactic antifungal exposure, serum trough concentrations of prophylactic antifungals when available, outcome of IFD and B-IFD at 12 weeks from the date of diagnosis, and overall mortality at 1 year after first induction chemotherapy.
2.2. Definitions
AML was defined by WHO 2016 revised guidelines [15]. Induction chemotherapy regimens were determined by the primary hematology team in accordance with National Comprehensive Cancer Network guidelines and recommendations [16]. Cumulative neutropenia was defined as the total duration, in days, of an absolute neutrophil count (ANC) of <500 cells/µL for the year following first induction chemotherapy. Cumulative neutropenic days were normalized per 100 days of follow up to account for the variable follow up times for each patient. Cumulative exposure to antifungals was defined as the total number of days that prophylactic antifungal agents were given to each patient over the follow up period and was normalized per 100 days of follow up.
Proven and probable IFD, including Pneumocystis pneumonia, were defined by the EORTC/MSGERC 2019 revised consensus criteria [13]. The day of diagnosis of IFD was defined as the date when the diagnosis was first suspected based on clinical, radiological, and microbiological findings. Breakthrough IFD was defined based on the MSGERC/ECMM consensus definitions and included fungal infections that occurred at least 7 days after initiation of any antifungal agent or that occurred less than one day after discontinuing any antifungal agent [14].
2.3. Prophylaxis and Isolation Strategies
Antifungal prophylaxis for all AML patients undergoing chemotherapy was voriconazole (with therapeutic drug monitoring and a goal trough level of 1 to 5.5 µg/mL) when ANC fell to ≤1500/µL; this was continued until neutrophils were >500/µL for at least 3 consecutive days. Alternative regimens, including posaconazole, isavuconazole, fluconazole, or micafungin, were used at the discretion of the hematology team if intolerance or drug-drug interactions were present. Pneumocystis prophylaxis was reserved for patients receiving purine analogues; either inhaled pentamidine 300 mg monthly or double-strength trimethoprim-sulfamethoxazole (TMP-SMX) tablet three times a week was given throughout chemotherapy and for six months after the last dose of a purine analogue agent. Acyclovir was given throughout all chemotherapy cycles for antiviral prophylaxis. Bacterial prophylaxis with a fluoroquinolone was given only to patients with relapsed or refractory AML.
After allogeneic HCT, fluconazole was given for antifungal prophylaxis unless the patient met one of the following criteria: prolonged neutropenia (>21 days) after or preceding transplant; corticosteroid treatment for GVHD, engraftment syndrome or idiopathic pneumonia syndrome; calcineurin inhibitor therapy in combination with any other immunosuppressive agent; use of etanercept, alemtuzumab, or anti-thymocyte globulin for conditioning; or a history of IFD prior to transplant. Patients who met one or more of these criteria received voriconazole rather than fluconazole. Alternative regimens included posaconazole and micafungin and were used at the discretion of the transplant physician in settings of drug intolerance or drug-drug interactions. Antifungal prophylaxis continued until day +100 and until the specific risk factor for IFD was no longer present, whichever occurred later. Pneumocystis prophylaxis was a single-strength TMP-SMX tablet daily starting at day +30 and through day +180 or until immunosuppression was stopped, provided cell counts had recovered. Alternative agents in cases of persistent cytopenia included atovaquone or inhaled pentamidine. Acyclovir was given for antiviral prophylaxis for one year. Antibacterial prophylaxis was with a fluoroquinolone from day +1 until either engraftment or development of febrile neutropenia.
A protective environment consisting of a positive pressure room with 12 air exchanges per hour and HEPA filtration was used for allogeneic HCT patients who are in the peri-transplant period and the first 100 days post-HCT, those who have an ANC < 1000 cells/mm3, and those who have acute GVHD. AML patients undergoing induction therapy with an ANC < 1000/mm3 also are placed in this type of room.
2.4. Statistical Analysis
Univariable analysis accounting for competing risks (deaths) was conducted using cause-specific proportional hazards regression to evaluate the impact of cumulative days of neutropenia per 100 days of follow up, cumulative prophylactic antifungal exposure per 100 days of follow up, AML status and other patient characteristics at time of IFD. Hazard ratios and 95% confidence intervals were calculated. Further univariable analyses were performed using the Wilcoxon rank sums test. Kaplan-Meier survival analysis with a log-rank test was conducted to evaluate the impact of IFD on survival. SAS version 9.4 statistical software (SAS Inc., Cary, NC, USA) was used for all analyses.
3. Results
3.1. Patient Characteristics
Altogether, 264 patients were screened and 251 patients were entered into the study. Excluded were eight patients who died before induction therapy was accomplished, four who received a cord blood HCT, and one who received a second HCT within the year after induction therapy. Of the 251 patients, 113 were women (45%), and the average age was 62 ± 14 years (Table 1). Most patients had primary AML (n = 148, 59%); AML related to antecedent myelodysplastic syndrome (MDS) was present in 76 (30%) patients, and 27 (11%) had secondary AML related to treatment for a prior malignancy. The most common comorbidities were diabetes in 37 patients (15%) and coronary artery disease in 31 (12%) (Table 1).
Table 1.
Characteristics of 251 patients undergoing first induction chemotherapy for acute myeloid leukemia.
| Variable | n (%) |
|---|---|
| Male | 138 (55) |
| Female | 113 (45) |
| Age, years (mean ± std dev) | 61.8 ± 14 |
| Hematologic malignancy | |
| Primary AML | 148 (59) |
| MDS with transformation to AML | 76 (30) |
| Therapy-related AML | 27 (11) |
| Comorbid conditions | |
| Diabetes mellitus | 37 (15) |
| Coronary artery disease | 31 (12) |
| Chronic obstructive pulmonary disease | 24 (10) |
| Congestive heart failure | 22 (9) |
| Rheumatologic disease | 11 (4) |
| Interstitial lung disease | 7 (3) |
| Heart transplant | 2 (1) |
| Induction chemotherapy regimens (no.) | |
| 1 | 157 (63) |
| ≥2 | 94 (37) |
| Allogeneic hematopoietic cell transplant | 75 (30) |
| Matched related | 27 |
| Matched unrelated | 43 |
| Haploidentical | 4 |
| Mismatched | 1 |
| Graft-versus-host disease treatment | 52 |
| Corticosteroids * | 37 |
| Tacrolimus | 16 |
| Anti-thymocyte globulin | 4 |
| Methotrexate | 3 |
AML: acute myeloid leukemia, MDS: myelodysplastic syndrome; * defined as ≥ 0.3 mg/kg/day of prednisone equivalent.
Of the 251 patients, 157 (63%) received only one cycle of induction chemotherapy for active disease within one year, and 94 (37%) received ≥ 2 cycles, including 15 patients who received three cycles and one patient who received four cycles. A total of 75 (30%) patients received an allogeneic HCT, and 52 of the 75 (69%) developed either acute or chronic GVHD. The mean length of follow up from the onset of induction chemotherapy was 236 ± 140 days.
3.2. Invasive Fungal Disease (IFD)
Among the 251 patients, 17 patients (7%) had a proven (n = 4) or probable (n = 13) IFD. Sixteen episodes of possible IFD occurred in 14 patients for whom the mycological criteria of proven or probable IFD were not met. With the exception of 3 patients who had pulmonary consolidation or cavitation, patients with possible IFD had only pulmonary nodules on CT scan. Five patients were too ill to undergo further diagnostic studies and died within 11 days from the diagnosis of possible IFD. Patients with possible IFD were excluded from further analysis.
Among the 17 cases of proven and probable IFD, 12 (71%) were caused by molds. The most common IFD was invasive pulmonary aspergillosis (n = 6), followed by mucormycosis (n = 3), fusariosis (n = 3), Pneumocystis pneumonia (n = 3), and invasive candidiasis (n = 2) (Table 2).
Table 2.
Proven or probable invasive fungal disease in 17 patients with acute myeloid leukemia.
| Organism | Type of IFD | Site of IFD | Prophylaxis Agent | Timing of IFD Diagnosis (Days) 1 | Status of AML at Time of IFD Diagnosis | Outcome at 12 Weeks |
|---|---|---|---|---|---|---|
| Breakthrough IFD | ||||||
| Aspergillus (n = 2) | Proven | Pleural space 2 | Fluconazole | 81 | Primary consolidation | Died |
| Probable | Pulmonary 3 | Isavuconazole | 321 | Post-allo HCT | Died | |
| Fusarium (n = 3) | Proven | Disseminated | Fluconazole | 242 | Relapsed refractory | Died |
| Probable | Pulmonary | Fluconazole | 358 | Relapsed after allo HCT | Survived | |
| Probable | Pulmonary | Posaconazole | 320 | Relapsed after allo HCT | Died | |
| Mucorales (n = 2) | Proven | Disseminated | Voriconazole | 150 | Relapsed refractory | Died |
| Probable | Pulmonary | Voriconazole | 29 | Primary induction | Died | |
| Pneumocystis (n = 1) | Probable | Pulmonary | Pentamidine (inh) | 15 | Primary induction | Survived |
| Non-Breakthrough IFD | ||||||
| Aspergillus (n = 4) | Probable | Pulmonary 3 | -- | 262 | Relapsed refractory | Survived |
| Probable | Pulmonary 3 | -- | 48 | Primary induction | Survived | |
| Probable | Pulmonary 3 | -- | 256 | Relapsed refractory | Died | |
| Probable | Pulmonary 3 | -- | 137 | Primary consolidation | Died | |
| Candida (n = 2) | Probable | Invasive candidiasis | -- | 261 | Remission | Died |
| Proven | Invasive candidiasis | -- | 340 | Initial relapse | Died | |
| Pneumocystis (n = 2) | Probable | Pulmonary | -- | 22 | Primary refractory | Died |
| Probable | Pulmonary | -- | 197 | Post-allo HCT | Died | |
| Mucorales (n = 1) | Probable | Pulmonary | -- | 365 | Relapsed refractory | Died |
AML: acute myeloid leukemia; Allo HCT: allogeneic hematopoietic cell transplant; IFD: invasive fungal disease; inh: inhaled. 1 Timing of IFD diagnosis from study entry date (in days). 2 positive pleural fluid culture for Aspergillus flavus. 3 Probable invasive pulmonary aspergillosis determined on basis of positive serum galactomannan in 3 patients (range 1.2 –3.75 ODI), positive BAL galactomannan in 2 (range 0.9–2.3 ODI).
Patients with proven or probable IFD were significantly older and had a greater number of cumulative neutropenic days when compared with those without IFD, HR 1.046 (95% CI 1.002- 1.093), p = 0.04 and HR 1.038 (1.018–1.059), p = 0.0001, respectively (Table 3).
Table 3.
Univariable analysis of risk factors for the development of proven/probable invasive fungal disease (IFD) in patients with acute myeloid leukemia 1.
| Risk Factor | No IFD n = 220 |
IFD n = 17 |
Hazard Ratio (95%CI) | p Value |
|---|---|---|---|---|
| Age, years (mean ± std dev) | 61 ± 15 | 66 ± 12 | 1.046 (1.002–1.093) | 0.04 |
| Gender | ||||
| Male | 119 | 11 | 0.588 (0.218–1.591) | 0.30 |
| Female | 101 | 6 | ||
| Hematological disease | ||||
| Primary AML | 132 | 12 | 1.062 (0.37–3.05) | 0.91 |
| MDS with transformation to AML | 62 | 5 | 0.52 (0.18–1.499) | 0.23 |
| Therapy-related AML | 26 | 0 | ||
| Cycles of induction chemotherapy | ||||
| 1 | 143 | 10 | 0.885 (0.337–2.326) | 0.80 |
| ≥2 | 77 | 7 | ||
| AML status (relapse/refractory) | 99 | 12 | 7.562 (2.585–22.123) | 0.0002 |
| Allogeneic hematopoietic cell transplant | 68 | 4 | 2.638 (0.854–8.149) | 0.09 |
| Graft vs. host disease | 47 | 3 | 2.047 (0.586–7.15) | 0.26 |
| Cumulative days of neutropenia (mean ± std dev) |
28 ± 25 | 34 ± 20 | 1.038 (1.018–1.059) | 0.0001 |
AML: acute myeloid leukemia, MDS: myelodysplastic syndrome. 1 Patients with possible IFD were excluded from the analysis.
By cause-specific proportional hazards regression, the risk for IFD increased by 3.8% for each day of neutropenia per 100 days of follow up. The risk for development of IFD was increased significantly in patients who had relapsed/refractory AML, HR = 7.562 (2.585–22.123), p = 0.0002.
Among the 17 patients with proven or probable IFD, five were undergoing primary induction or consolidation therapy, five had relapsed/refractory leukemia, and only one was in remission (Table 2). Four patients had received an HCT, and two of these had relapsed leukemia after the HCT.
Nine of the 17 (53%) patients with proven and probable IFD were not receiving antifungal prophylaxis at the time of the IFD diagnosis. Two patients did not receive Pneumocystis prophylaxis despite having an indication for this. Two patients were involved in clinical trials of experimental agents for the treatment of AML and did not have antifungal prophylaxis prescribed because the trial protocol prohibited the use of these agents. One patient had numerous drug-drug interactions and was unable to tolerate any antifungal prophylaxis. Three patients were no longer neutropenic, and another patient was no longer receiving chemotherapy. Infections in these nine patients included invasive aspergillosis (n = 4), invasive candidiasis (n = 2), mucormycosis (n = 1), and Pneumocystis pneumonia (n = 2) (Table 2).
Eight of the 17 (47%) proven and probable IFD were B-IFD, including aspergillosis (n = 2), fusariosis (n = 3), mucormycosis (n = 2), and Pneumocystis pneumonia (n = 1) (Table 2). Three patients receiving fluconazole prophylaxis developed invasive aspergillosis (n = 1) and fusariosis (n = 2), two patients who developed mucormycosis were taking voriconazole. The serum trough concentration of posaconazole (1580 ng/mL) and isavuconazole (3 µg/mL) demonstrated appropriate exposure when measured prior to occurrence of Fusarium pneumonia and invasive pulmonary aspergillosis, respectively. Exploratory proportional hazards regression analysis of cumulative antifungal exposure and development of IFD showed no significant differences among patients with IFD when compared with those without IFD, HR = 0.999 (0.983–1.015), p = 0.9. Using the Wilcoxon rank sums test, cumulative exposure to any specific antifungal agent was not associated with the development of B-IFD, p = 0.26, but cumulative exposure to non-mold active fungal prophylaxis (fluconazole) was associated with development of B-IFD (p = 0.012).
3.3. Outcomes
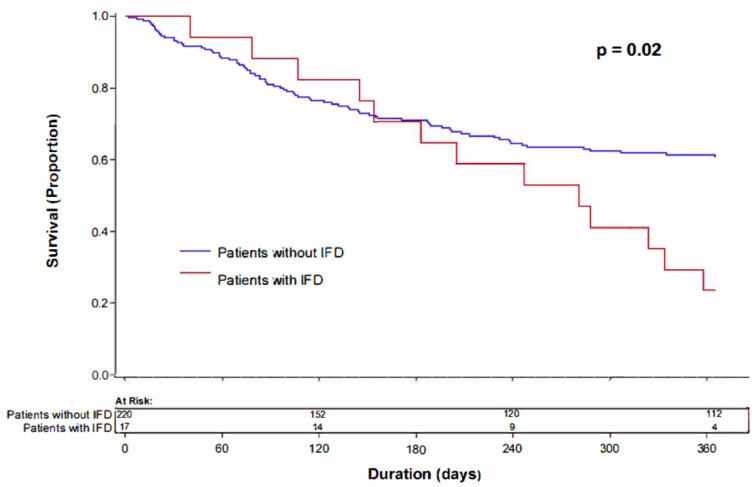
Overall mortality in our cohort of 251 patients was 37% (n = 92) within the first year after receiving first induction chemotherapy for the treatment of AML. The mortality rate in patients who had relapsed/refractory AML was 70% (n = 77) compared with 12% (n = 15) in those without relapse, p < 0.001. Kaplan-Meier survival analysis showed significantly higher mortality at 1 year from first induction in patients who developed proven or probable IFD (13/17, 76%) when compared with those who did not develop IFD (79/220, 36%) (log-rank test, p = 0.02) (Figure 1).
Figure 1.
Kaplan-Meier analysis showing survival at one year after the date of first induction therapy for acute myeloid leukemia comparing patients who did or did not have invasive fungal disease.
Among the small number of patients who did not have relapsed/refractory AML, development of IFD was significantly associated with a higher mortality rate, p < 0.001. In those patients who had relapsed or refractory AML, no differences were noted among patients who developed IFD and those who did not, p = 0.26. All 13 patients with IFD who died did so within 12 weeks from the date of IFD diagnosis. Seven of nine patients (78%) with non-B-IFD and six of eight (75%) who had B-IFD died.
4. Discussion
Within a 1-year period from the time of initiation of induction therapy, 12% of patients with AML developed proven, probable or possible IFD. Of 17 patients (7%) who had proven or probable IFD, 8 (3%) were B-IFD in patients receiving antifungal prophylaxis. These rates are comparable with those reported from other centers [7,8,9,17,18] and slightly lower than we noted at our institution in the years 2010–2013 [10]. Mold infections accounted for 71% of proven and probable IFD, with Aspergillus the predominant organism, similar to prior reports [1,10]. Increasingly, however, non-Aspergillus molds are responsible for infections in patients with acute leukemia [19,20,21]. These more difficult-to-treat mold infections, including fusariosis and mucormycosis, have been reported more often in patients who were receiving antifungal prophylaxis, as we observed in our cohort. However, we did not isolate unusual and rare molds, such as Scopulariopsis, Lomentospora, Lichtheimia, and Trichosporon, as has been noted in other recent reports [8,19,20,21]. It is likely that geographic and environmental factors are as important as the specific antifungal agent used for prophylaxis to explain differences in the molds that predominate in various different institutions.
Cumulative duration of profound neutropenia was a significant risk factor for development of IFD among patients receiving chemotherapy for the treatment of newly diagnosed AML. We used a proportional hazard regression model and long-term follow up to determine the contribution of neutropenia to risk for IFD. We found a 3.8% increased risk of developing IFD by each additional day of neutropenia per 100 days follow up. Other studies have utilized the D-index, based on the area over the neutrophil curve which is defined by the duration and severity of neutropenia, as a predictor of risk factor for invasive mold infections in leukemia patients [22,23,24]. Our calculation differs in that it includes the cumulative duration of neutropenia over the first year, rather than depth and persistence of neutropenia during a single neutropenic episode prior to the diagnosis of IFD. Our study confirms findings from prior studies that neutropenia is a key risk factor for IFD and breakthrough IFD in patients with AML [5,6,10,11,25].
Developing an IFD had a significant impact on 1-year-survival, and this was most notable among patients whose leukemia was controlled. Aggressive chemotherapy allowing control of the leukemia likely contributed to the net state of immunosuppression and increased the risk for development of an IFD. For those patients who had relapsed/refractory disease, it appeared that both IFD and uncontrolled AML contributed to their poor outcomes.
GVHD is a known risk factor for the development of IFD. However, in our cohort, GVHD was not associated with an increased risk for IFD. This finding could be explained by the small number of patients who underwent HCT and developed GVHD (14%) and by the use of antimould prophylaxis in patients with GVHD.
Typically, antifungal prophylaxis for AML patients undergoing chemotherapy is initiated on the first day of neutropenia and continued until neutrophils are >500/µL. In our study, cumulative days of neutropenia and chemotherapy failure significantly increased the risk of IFD. These findings suggest that factors contributing to the net state of immunosuppression, rather than only the absolute neutrophil count, play a central role in the development of IFD. Novel approaches to antifungal prophylaxis perhaps should be devised to include alternative endpoints given that recovery of neutropenia alone is not wholly adequate to define decreased risk for IFD.
Current evidence supports the use of mould active prophylaxis for patients at high risk for IFD, such as those receiving induction chemotherapy for AML. Results from a large open label trial support the use of posaconazole to prevent IFD, and this drug is licensed for this indication [2]. There are not large studies of voriconazole prophylaxis in AML populations and the drug is not licensed for this indication, but effectiveness of this agent can be inferred from studies of the pre-engraftment neutropenic phase in HCT patients [26]. Furthermore, decisions about specific agents for antifungal prophylaxis in this patient population must take into account factors, such as the local epidemiology, especially the local incidence of Mucorales and other resistant moulds, drug interactions with chemotherapy agents, and costs.
In our study, 14 patients had 16 possible IFD and were excluded from further analysis; this constitutes almost 50% of all episodes of IFD, and 87.5% occurred despite antifungal prophylaxis. Similarly, in a prior study, almost 70% of episodes were designated as possible and were excluded from further analysis [10]. Mortality among patients with possible IFD was similar to that observed in patients with proven/probable IFD (73% and 76%, respectively). The lack of mycological evidence to support the diagnosis of IFD in this patient population may be secondary to the low yield of cultures, especially in the setting of widespread use of prophylaxis, limitations of non-culture methods, and an inability to use invasive methods in these patients to obtain tissue for culture and histopathology [27,28,29]. Possible IFD pose a dilemma for both clinicians and researchers. In clinical practice, patients with possible IFD typically receive empiric antifungal therapy, but treatment endpoints are unclear. Excluding this group of patients from analysis in clinical trials results in a decreased number of evaluable patients. Further understanding the impact and outcomes of possible IFD could lead to an improvement in the management of these patients.
The strengths of this study are related to the relatively large sample size, the homogenous patient population, and the use of recently updated definitions of IFD and B-IFD.
Our study has several limitations, including its retrospective and single-center design. We have excluded patients with episodes of possible IFD, which accounted for almost half of all episodes of IFD within a year of AML diagnosis, and excluding those episodes might have resulted in an underrepresentation of the real impact of IFD in this patient population. Finally, while the overall rate of IFD was similar to that reported in other studies, the actual number of patients with B-IFD was low, precluding further analysis or meaningful conclusions regarding risk factors for specific IFD in this population.
In conclusion, IFD remains an important problem among patients with AML despite the use of antifungal prophylaxis. Increasing cumulative days of neutropenia, as well as relapsed/refractory AML, correlate with increased risk of developing IFD, and development of IFD significantly increases mortality among patients with AML.
Author Contributions
Conceptualization, A.I.W. and M.H.M.; Data curation, A.I.W., K.A.L., S.M.M. and V.M.S.; Formal analysis, B.J.R. and M.H.M.; Investigation, C.A.K., A.I.W., S.M.M., V.M.S. and M.H.M.; Methodology, C.A.K., K.A.L., B.J.R., L.B.C. and M.H.M.; Project administration, C.A.K.; Resources, C.A.K.; Supervision, C.A.K., K.A.L. and M.H.M.; Validation, B.J.R. and L.B.C.; Writing—original draft, A.I.W. and K.A.L.; Writing—review & editing, C.A.K., K.A.L., B.J.R., S.M.M., V.M.S., L.B.C. and M.H.M. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by grant number UL1TR002240 from the National Center for Advancing Translational Sciences (NCATS) and by the Veterans Education and Research Association of Michigan.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Medical School Institutional Review Board of the University of Michigan.
Informed Consent Statement
Patient consent was waived due to the fact this was a retrospective chart review.
Conflicts of Interest
All authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bhatt V.R., Viola G.M., Ferrajoli A. Invasive fungal infections in acute leukemia. Ther. Adv. Hematol. 2011;2:231–247. doi: 10.1177/2040620711410098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornely O.A., Maertens J., Winston D.J., Perfect J., Ullmann A.J., Walsh T.J., Helfgott D., Holowiecki J., Stockelberg D., Goh Y.-T., et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 2007;356:348–359. doi: 10.1056/NEJMoa061094. [DOI] [PubMed] [Google Scholar]
- 3.Ananda-Rajah M.R., Grigg A., Downey M.T., Bajel A., Spelman T., Cheng A., Thusky K.T., Vincent J., Slavin M.A. Comparative clinical effectiveness of prophylactic voriconazole/posaconazole to fluconazole/itraconazole in patients with acute myeloid leukemia/myelodysplastic syndrome undergoing cytotoxic chemotherapy over a 12-year period. Haematologia. 2012;97:459–463. doi: 10.3324/haematol.2011.051995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rausch C.R., DiPippo A.J., Bose P., Kontoyiannis D.P. Breakthrough fungal infections in patients with leukemia receiving isavuconazole. Clin. Infect. Dis. 2018;67:1610–1612. doi: 10.1093/cid/ciy406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim S.-H., Choi J.-K., Cho S.-Y., Lee H.-J., Park S.H., Choi S.-M., Lee D.-G., Choi J.-H., Yoo J.-H., Lee J.-W. Risk factors and clinical outcomes of breakthrough yeast bloodstream infections in patients with hematological malignancies in the era of newer antifungal agents. Med. Mycol. 2018;56:197–206. doi: 10.1093/mmy/myx038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Breda G.L., Tuon F.F., Meis J.F., Herkert P.F., Hagen F., deOliveira L.Z., de Carvalho Dia V., da Cunha C.A., Queiroz-Telles F. Breakthrough candidemia after the introduction of broad spectrum antifungal agents: A 5-year retrospective study. Med. Mycol. 2018;56:406–415. doi: 10.1093/mmy/myx077. [DOI] [PubMed] [Google Scholar]
- 7.Lionakis M.S., Lewis R.E., Kontoyiannis D.P. Breakthrough invasive mold infections in the hematology patient: Current concepts and future directions. Clin. Infect. Dis. 2018;67:1621–1630. doi: 10.1093/cid/ciy473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auberger J., Lass-Florl C., Aigner M., Clausen J., Gastl G., Nachbaur D. Invasive breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 2012;67:2268–2273. doi: 10.1093/jac/dks189. [DOI] [PubMed] [Google Scholar]
- 9.Biehl L.M., Vehreschild J.J., Liss B., Franke B., Markiefka B., Persigehl T., Bucker V., Wisplinghoff H., Scheid C., Cornely O.A., et al. A cohort study on breakthrough invasive fungal infections in high-risk patients receiving antifungal prophylaxis. J. Antimicrob. Chemother. 2016;71:2634–2641. doi: 10.1093/jac/dkw199. [DOI] [PubMed] [Google Scholar]
- 10.Wasylyshyn A., Linder K.A., Castillo C.G., Zhou S., Kauffman C.A., Miceli M.H. Breakthrough invasive fungal infections in patients with acute myeloid leukemia. Mycopathologia. 2020;185:299–306. doi: 10.1007/s11046-019-00418-8. [DOI] [PubMed] [Google Scholar]
- 11.Miceli M.H., Churay T., Braun T., Kauffman C.A., Couriel D.R. Risk factors and outcomes of invasive fungal infections in allogeneic hematopoietic cell transplant recipients. Mycopathologia. 2017;182:495–504. doi: 10.1007/s11046-017-0115-y. [DOI] [PubMed] [Google Scholar]
- 12.Jenks J.D., Cornely O.A., Chen S.C., Thompson G.R., 3rd, Hoenigl M. Breakthrough invasive fungal infections: Who is at risk? Mycoses. 2020;63:1021–1032. doi: 10.1111/myc.13148. [DOI] [PubMed] [Google Scholar]
- 13.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., Clancy C.J., Wingard J.R., Lockhart S.R., Groll A.H., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cornely O.A., Hoenigl M., Lass-Flörl C., Chen S.C., Kontoyiannis D.P., Morrissey C.O., Thompson G.R. Defining breakthrough invasive fungal infection-Position paper of the Mycoses Study Group Education and Research Consortium and the European Confederation of Medical Mycology. Mycoses. 2019;62:716–729. doi: 10.1111/myc.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arber D.A., Orazi A., Hasserjian R., Thiele J., Borowitz M.J., Le Beau M.M., Bloomfield C.D., Cazzola M., Vardiman J.W. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405. doi: 10.1182/blood-2016-03-643544. [DOI] [PubMed] [Google Scholar]
- 16.National Comprehensive Cancer Network Acute Myeloid Leukemia. [(accessed on 22 December 2020)]. (Version 2.2021) Available online: http://www.nccn.org/professionals/physician_gls/pdf/aml.pdf.
- 17.Rodríguez-Veiga R., Montesinos P., Boluda B., Lorenzo I., Martínez-Cuadrón D., Salavert M., Pemán J., Calvillo P., Cano I., Acuña E., et al. Incidence and outcome of invasive fungal disease after front-line intensive chemotherapy in patients with acute myeloid leukemia: Impact of antifungal prophylaxis. Ann. Hematol. 2019;98:2081–2088. doi: 10.1007/s00277-019-03744-5. [DOI] [PubMed] [Google Scholar]
- 18.Cornely O.A., Böhme A.l., Reichert D., Reuter S., Maschmeyer G., Maertens J., Buchheidt D., Paluszewska M., Arenz D., Bethe U., et al. Multinational Case Registry of the Infectious Diseases Working Party of the German Society for Hematology and Oncology. Risk factors for breakthrough invasive fungal infection during secondary prophylaxis. J. Antimicrob. Chemother. 2008;61:939–946. doi: 10.1093/jac/dkn027. [DOI] [PubMed] [Google Scholar]
- 19.Lamoth F., Chung S.J., Damonti L., Alexander B.D. Changing epidemiology of invasive mold infections in patients receiving azole prophylaxis. Clin. Infect. Dis. 2017;64:1619–1621. doi: 10.1093/cid/cix130. [DOI] [PubMed] [Google Scholar]
- 20.Ramírez I., Moncada D. Fatal disseminated infection by Trichosporon asahii under voriconazole therapy in a patient with acute myeloid leukemia: A review of breakthrough infections by Trichosporon spp. Mycopathologia. 2020;185:377–388. doi: 10.1007/s11046-019-00416-w. [DOI] [PubMed] [Google Scholar]
- 21.Salmanton-García J., Koehler P., Kindo A., Falces-Romero I., García-Rodríguez J., Ráčil Z., Chen S.C., Klimko N., Desoubeaux G., Thompson G.R., III, et al. ECMM/ISHAM working group. Needles in a haystack: Extremely rare invasive fungal infections reported in FungiScope-Global Registry for Emerging Fungal Infections. J. Infect. 2020;8:802–815. doi: 10.1016/j.jinf.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 22.Kimura S., Oshima K., Sato K., Sato M., Terasako K., Nakasone H., Kikuchi M., Okuda S., Kako S., Yamazaki R., et al. Retrospective evaluation of the area over the neutrophil curve index to predict early infection in hematopoietic stem cell transplantation recipients. Biol. Blood Marrow Transpl. 2010;16:1355–1361. doi: 10.1016/j.bbmt.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 23.Portugal R.D., Garnica M., Nucci M. Index to predict invasive mold infection in high-risk neutropenic patients based on the area over the neutrophil curve. J. Clin. Oncol. 2009;27:3849–3854. doi: 10.1200/JCO.2008.21.0856. [DOI] [PubMed] [Google Scholar]
- 24.Yılmaz G., Coşkun B., Elhan A., Azap A., Akan H. D-index: A new scoring system in febrile neutropenic patients for predicting invasive fungal infections. Turk. J. Haematol. 2016;33:102–106. doi: 10.4274/tjh.2014.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Almyroudis N., Segal B. Prevention and treatment of invasive fungal diseases in neutropenic patients. Curr. Opin. Infect. Dis. 2009;22:385–393. doi: 10.1097/QCO.0b013e32832e074d. [DOI] [PubMed] [Google Scholar]
- 26.Wingard J.R., Carter S.T., Walsh T.J., Kurtzberg J., Small T.N., Baden L.R., Gersten I.D., Mendizabal A.M., Leather H.L., Confer D.L., et al. Blood and Marrow Transplant Clinical Trials Network. Randomized, double-blind trial of fluconazole versus voriconazole for prevention of invasive fungal infection after allogeneic hematopoietic cell transplantation. Blood. 2010;116:5111–5118. doi: 10.1182/blood-2010-02-268151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkelman M.A. Specificity influences in (1→3)-β-d-glucan-supported diagnosis of invasive fungal disease. J. Fungi. 2020;7:14. doi: 10.3390/jof7010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duarte R.F., Sánchez-Ortega I., Cuesta I., Arnan M., Patiño B., Fernández de Sevilla A., Gudiol C., Ayats J., Cuenca-Estrella M. Serum galactomannan-based early detection of invasive aspergillosis in hematology patients receiving effective antimold prophylaxis. Clin. Infect. Dis. 2014;59:1696–1702. doi: 10.1093/cid/ciu673. [DOI] [PubMed] [Google Scholar]
- 29.Miceli M.H., Maertens J. Role of non-culture-based tests, with an emphasis on galactomannan testing for the diagnosis of invasive aspergillosis. Semin. Respir. Crit. Care Med. 2015;36:650–661. doi: 10.1055/s-0035-1562892. [DOI] [PubMed] [Google Scholar]