Abstract
High-fat diet (HFD) consumption has been linked to dyslipidemia, low-grade inflammation and oxidative stress. This study investigated the effects of a mixed formulation with Limosilactobacillus fermentum 139, L. fermentum 263 and L. fermentum 296 on cardiometabolic parameters, fecal short-chain fatty acid (SCFA) contents and biomarkers of inflammation and oxidative stress in colon and heart tissues of male rats fed an HFD. Male Wistar rats were grouped into control diet (CTL, n = 6), HFD (n = 6) and HFD with L. fermentum formulation (HFD-Lf, n = 6) groups. The L. fermentum formulation (1 × 109 CFU/mL of each strain) was administered twice a day for 4 weeks. After a 4-week follow-up, biochemical parameters, fecal SCFA, cytokines and oxidative stress variables were evaluated. HFD consumption caused hyperlipidemia, hyperglycemia, low-grade inflammation, reduced fecal acetate and propionate contents and increased biomarkers of oxidative stress in colon and heart tissues when compared to the CTL group. Rats receiving the L. fermentum formulation had reduced hyperlipidemia and hyperglycemia, but similar SCFA contents in comparison with the HFD group (p < 0.05). Rats receiving the L. fermentum formulation had increased antioxidant capacity throughout the colon and heart tissues when compared with the control group. Administration of a mixed L. fermentum formulation prevented hyperlipidemia, inflammation and oxidative stress in colon and heart tissues induced by HFD consumption.
Keywords: high-fat diet, inflammation, oxidative stress, probiotic, Limosilactobacillus fermentum
1. Introduction
Impairment in gut microbiota composition, gut dysbiosis and enhanced systemic inflammation have been reported in cardiometabolic disorders, such as obesity, diabetes, stroke hypercholesterolemia and heart failure [1,2], suggesting that alterations in the “gut–heart axis” could be involved in pathogenesis of cardiometabolic disorders.
Excessive high-fat diet (HFD) consumption can trigger gut dysbiosis, a state characterized by impairment of gut microbiota diversity and increased intestinal permeability [3,4]. In addition, an HFD is likely to promote oxidative stress in the colon [5], low-grade chronic inflammation [6], cardiac oxidative stress, ventricular dysfunction [7], increased blood pressure, autonomic dysfunction and metabolic disorder [8]. Altogether, these findings suggest an association involving the gut–heart axis in HFD-induced cardiometabolic disorders [9].
Gut microbiota modulation through probiotic use has received special attention as a safe approach for the prevention and/or treatment of cardiometabolic dysfunction [10]. Probiotics have been defined as live microorganisms that confer health benefits to the host when administered in adequate doses [11]. Lactobacillus and amended genera are the most common genera of probiotics, being commonly recognized as safe and with qualified presumption of safety. The genus Lactobacillus was recently reclassified into 25 genera [12]. In the new proposed taxonomic reclassification, Lactobacillus fermentum was renamed as Limosilactobacillus fermentum and described as Gram-positive, rod- or coccoid-shaped, heterofermentative and anaerobic or aerotolerant, being found in fermented cereals and other fermented plant materials, dairy products, manure, sewage and the feces and vagina of humans [12].
It has been demonstrated that probiotics, when administered as a single strain or mixed strains, can exert health-promoting effects on the host through different mechanisms, such as the normalization of unbalanced gut microbiota, production of short-chain fatty acids (SCFAs), increased turnover of enterocytes, colonization resistance and competitive exclusion of pathogens [11]. Additionally, some probiotics have displayed strain-specific effects, such as: production of specific bioactive metabolites, enhanced activity of the immune system at the intestinal or extraintestinal level [13] and enhanced activity of antioxidant enzymes, which cause elimination of reactive oxygen species in the host intestine and alleviation of oxidative damage [14]. Thus, the identification of strain-specific qualities or mechanisms in potentially probiotic microorganisms should be relevant and required in the development and applicability of a probiotic product.
In recent years, our research group has isolated and characterized potentially probiotic fruit-derived strains. The strains of L. fermentum 139, L. fermentum 296 and L. fermentum 263 were recovered from Brazilian fruit by-products [15,16]. L. fermentum 139 was isolated from Mangifera indica L. (mango), L. fermentum 263 was isolated from Ananas comosus (pineapple) and L. fermentum 296 was isolated from Fragaria vesca L. (strawberry). All the three strains displayed potential for use as probiotics in terms of a set of functionality-related in vitro properties, such as auto-aggregation, co-aggregation, survival during exposure to simulated gastrointestinal conditions and pathogen antagonism, in addition to showing the absence of hemolytic and mucolytic activities and resistance to antibiotics [16]. Such findings indicated that these L. fermentum fruit-derived strains could be potential candidates for use as novel probiotics.
Early investigations of our laboratory verified that administration of L. fermentum 296 alone [8] or a mix with three L. fermentum strains [17] reduced blood pressure, autonomic dysfunction and dyslipidemia in rats. However, the effects of L. fermentum administration on immune and enzymatic activities, which are strain-specific functional characteristics and not reported in all probiotic strains, remain to be elucidated. Here, we have evaluated the effects of a mixed formulation with three potentially probiotic L. fermentum strains on cardiometabolic parameters, fecal SCFA contents and biomarkers of inflammation and oxidative stress in colon and heart tissues of rats fed an HFD.
2. Methods
2.1. Animals and Ethical Aspects
Male Wistar rats (Rattus norvegicus, 100 days of age) were used in this study. The rats were kept in collective polypropylene cages (3 animals/cage) with controlled temperature (22 ± 1 °C), humidity (50–55%) and light–dark cycle (12 h), receiving water and diet ad libitum. The procedures were in accordance with the National Council for Control of Animal Experimentation (CONCEA), and International Principles for Biomedical Research. The experimental protocols were approved by an Institutional Animal Care Committee (CEUA-UFPB protocol number # 6080240418).
2.2. Probiotic Strains and Preparation of Probiotic Suspension
The strains of L. fermentum 139, L. fermentum 263 and L. fermentum 296 were provided by the Laboratory of Food Microbiology, Department of Nutrition, Federal University of Paraíba (João Pessoa, Brazil). Stocks were kept at −20 °C in de Mann, Rogosa and Sharpe (MRS) broth (HiMedia, Mumbai, India) with glycerol (Sigma-Aldrich, St. Louis, MO, USA; 20 mL/100 mL). Suspensions of probiotic cells were prepared from overnight cultures grown in MRS broth under anaerobiosis (Anaerobic System Anaerogen, Oxoid Ltda., Wade Road, UK) at 37 °C [8,17]. The mixed cell suspension (counts of approximately 9 log CFU/mL of each strain) was prepared with the mixture of the suspension of each strain (ratio 1:1:1).
2.3. Experimental Design
Rats were grouped into: (i) a control group (CTL, n = 6), fed with a control diet prepared according to the American Institute of Nutrition—AIN-93M [18]; (ii) an HFD group, fed with a high-fat diet (HFD, n = 6) purchased from Rhoster® Company (Araçoiaba da Serra, São Paulo, Brazil) and receiving a placebo; and (iii) an HFD group receiving the formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 296 (HFD-Lf, n = 6). Compositions of the CTL diet and HFD are shown in Table 1.
Table 1.
Composition of control and high-fat diet (HFD) offered.
| Ingredients (g/100 g) | Diets | |
|---|---|---|
| Control (AIN-93M) * | HFD ** | |
| Corn starch | 39.75 | 33.09 |
| Dextrinized corn starch | 13.20 | 15.50 |
| Casein # | 20.00 | 19.86 |
| Sucrose | 10.00 | 6.00 |
| Soybean oil | 7.00 | 3.00 |
| Animal fat (lard) | 0.00- | 6.00 |
| Non-hydrolyzed vegetable fat | 0.00 | 5.00 |
| Sigma cholesterol | 0.00 | 1.00 |
| Sigma colic acid | 0.00 | 0.50 |
| Cellulose | 5.00 | 5.00 |
| Mineral mix 93M | 3.50 | 3.50 |
| Vitamin mix | 1.00 | 1.00 |
| L-cystine | 0.30 | 0.30 |
| Choline bitartrate | 0.25 | 0.25 |
| t-BHQ *** | 0.014 | 0.014 |
| Nutritional composition | ||
| Calories (Kj/100 g) | 16.46 | 18.05 |
| Carbohydrate (%) | 63.8 | 50.5 |
| Protein (%) | 20.3 | 18.3 |
| Lipids (%) | 15.9 | 31.2 |
* Adapted from Reeves et al. (1993). ** Rhoster—Industry and Trade Ltd. *** t-BHQ: tert-Butylhydroquinone. # Casein showed 85% purity (85 g protein for each 100 g casein).
Phosphate-buffered saline (PBS) was given as a placebo for 4 weeks in the CTL and HFD groups. The L. fermentum formulation in a PBS solution of approximately 3 × 109 CFU/mL was administered twice a day for 4 weeks to the HFD-Lf group. Administration of placebo or L. fermentum formulation was carried out with oral gavage. Body weight was measured every 3 days during the experimental period with an appropriate scale (model AS-1000; Marte, Santa Rita MG, Brazil). After 4 weeks, rats were euthanized by decapitation and biochemical parameters and cytokines were measured in serum; acetic and propionic acids were measured in feces; and oxidative stress parameters were measured in colon and heart tissues.
2.4. Quantification of Organic Acids in Colonic Contents
Acetic, butyric and propionic acids were measured with a high-performance liquid chromatography (HPLC) technique using an LC 1260 Infinity system (Agilent Technologies, St. Clara, CA, USA) coupled to a photo diode detector array (PDA) detector (G1315D; Agilent Technologies) as previously described [19].
2.5. Biochemical Analysis and Atherogenic Indices
Measurements of levels of total cholesterol, high-density lipoprotein cholesterol (HDL-c), triglycerides and glucose in serum were taken using commercial kits and a HumaLyzer 3500 semi-automatic photometer (HUMAN Gesellschaft für Biochemica und Diagnostica mbH, Wiesbaden, Germany). Levels of low-density lipoprotein cholesterol (LDL-c) were calculated with the Friedewald equation: LDL-c (mg/dL) = [TC − HDL-c − TG]/5 [20].
Atherogenic indices were calculated as follows: cardiac risk ratio (CRR) = total cholesterol/HDL-c [21]; atherogenic index of plasma (AIP) = triglycerides/HDL-c [22] and Castelli’s risk index II (CRI-II) = LDL-c/HDL-c [23].
Levels of cytokines (IL-6, IL-10, IL1β and TNF-α) were measured with a Millipore 7-plex kit (Millipore Corp., Billerica, MA, USA) following the manufacturer’s instructions. The estimation of the levels of cytokines was carried out from a standard curve using a third order polynomial equation, expressed as pg/mL. Samples with levels of cytokines below the limit of detection were recorded as zero, while samples with levels of cytokines above the upper limit of quantification of standard curves were assigned the highest value of the curve.
2.6. Measurement of Oxidative Stress in Colon and Heart
Heart and colon tissues were homogenized using a cold buffer solution (50 mM Tris and 1 mM EDTA (pH 7.4), 1 mM sodium orthogonadate and 200 μg/mL of phenylmethanesulfonyl fluoride) with an IKA RW 20 digital homogenizer, a Potter–Elvehjem pestle and glass tubes on ice. Homogenates were centrifuged (1.180 g, 10 min, 4 °C) [24] and levels of proteins were determined using the Bradford protocol [25]. The homogenates of heart and colon tissues (0.3 mg/mL) were used to measure the lipid peroxidation, enzymatic activities and total thiol contents.
Lipid peroxidation was quantified by the production of malondialdehyde (MDA) in reaction with thiobarbituric acid (TBA) at 100 °C. Sequential additions of trichloroacetic acid and Tris-HCl (3 mM) were carried out, followed by centrifugation (2.500 g, 10 min, 0.8% (v/v)). Afterwards, TBA was added to the resultant supernatant, mixed and boiled for 15 min. After cooling, the reaction was read at 535 nm on a spectrophotometer.
Enzymatic activity of total superoxide dismutase (SOD) was determined following the Misra and Fridovich method. Tissue homogenates were incubated with sodium carbonate buffer (0.05% (p/v), pH 10.2, 0.1 mmol/L EDTA) at 37 °C. Next, 30 mM/L of epinephrine (in 0.05% acetic acid) was added and SOD activity was measured by the kinetics of epinephrine auto-oxidation inhibition for 1.5 min at 480 nm [26].
Catalase activity was measured by decomposition of H2O2 into O2 and H2O. Tissue homogenates were incubated with 50 mM phosphate buffer (pH 7.0). Next, 0.3 M of H2O2 was added and absorbance was read at 240 nm for 1.5 min [27].
For measurement of glutathione S-transferase (GST) activity [28], tissue homogenates were added to phosphate buffer (0.1 M, pH 6.5 with 1 mM EDTA), 1 mM 1-chloro-2,4-dinitrobenzene (CDNB) and 1 mM of reduced glutathione (GSH). Absorbance was read at 340 nm for 1.5 min.
For measurement of total thiol groups, tissue homogenates were incubated (30 min) in phosphate buffer with 10 mM of 5,5′-dithiobis (2-nitrobenzoic acid) in the dark. The absorbance was read at 412 nm [29].
2.7. Statistical Analysis
The results were described as mean ± standard deviation for parametric data or median (maximum–minimum) for non-parametric data. A Kolmogorov–Smirnov test was used to assess data normality. Parametric variables were analyzed with one-way ANOVA and a Tukey post hoc test. Non-parametric variables were compared with a Kruskal–Wallis test with Dunn’s post hoc test. A Pearson’s or Spearman correlation coefficient (r) was used to evaluate the relationships among biochemical and inflammatory parameters and SCFA contents. The correlations were classified as bad (r ≤ 0.20), weak (0.21–0.40), moderate (0.41–0.60), good (0.61–0.80) and excellent (0.81–1.00). Statistical analysis was carried out with Prism 6 software (GraphPad Software 6, San Diego, CA, USA). A p-value of <0.05 was considered significant.
3. Results
3.1. Body Weight, Biochemical Parameters and Cytokine Serum Levels
The percentage of weight gain at the end of the protocol was similar among groups (Table 2). Rats fed an HFD had higher serum levels of glucose, total cholesterol, LDL-c and triglycerides, as well as higher atherogenic indices in comparison with the CTL group (Table 2). Additionally, rats fed an HFD had higher serum levels of proinflammatory cytokines TNF-α and IL-1β and lower serum levels of IL-6 and IL-10 in comparison with the CTL group (Table 2). Administration of a mixed L. fermentum formulation effectively reduced serum levels of glucose, triglycerides, total cholesterol, LDL-c, proinflammatory cytokine IL1β and atherogenic indices, as well as increased serum levels of HDL-c and anti-inflammatory cytokine IL-10 in rats fed an HFD (Table 2). These results show that supplementation of a mixed L. fermentum formulation had ameliorative effects on dyslipidemia, atherogenic indices and low-grade inflammation in rats fed an HFD.
Table 2.
Body weight, serum levels of biochemical parameters, atherogenic indices and cytokines in rats fed a control (CTL), high-fat diet with a placebo (HFD) and HFD with a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 269 (HFD-Lf) twice a day for 4 weeks.
| CTL (n = 6) | HFD (n = 6) | HFD-Lf (n = 6) | F | p-Value | |
|---|---|---|---|---|---|
| % Weight gain | 12.1 ± 2.9 | 6.6 ± 3.6 | 7.6 ± 4.4 | 2.9 | 0.09 |
| Biochemical parameters | |||||
| Glucose (mmol/L) | 5.8 ± 0.5 | 11.2 ± 2.0 * | 8.2 ± 0.9 * # | 25.45 | <0.0001 |
| Triglycerides (mmol/L) | 0.9 ± 0.03 | 1.6 ± 0.12 * | 1.1 ± 0.06 * # | 69.05 | <0.0001 |
| Total cholesterol (mmol/L) | 36.1 ± 1.4 | 113.5 ± 7.6 * | 43.6 ± 5.6 * # | 360.3 | <0.0001 |
| LDL-cholesterol (mmol/L) | 17.4 ± 1.4 | 62.5 ± 7.3 * | 27.3 ± 5.0 * # | 128.4 | <0.0001 |
| HDL-cholesterol (mmol/L) | 14.5 ± 1.0 | 10.6 ± 2.7 * | 17.5 ± 1.6 * # | 17.47 | 0.0001 |
| Atherogenic indices | |||||
| CRR | 2.5 ± 0.2 | 11.3 ± 2.9 * | 2.5 ± 0.5 # | 53.11 | <0.0001 |
| AIP | 1.5 ± 0.1 | 3.5 ± 0.7 * | 1.4 ± 0.2 # | 54.86 | <0.0001 |
| CRI-II | 1.2 ± 0.2 | 6.2 ± 1.5 * | 1.6 ± 0.3 # | 63.93 | <0.0001 |
| Cytokine levels | |||||
| TNF-α (pg/mL) † | 78.4 (70.7–80.6) | 139.3 (134.8–179.7) * | 137.2 (98.8–158.0) * | 11.79 | 0.0003 |
| IL-6 (pg/mL) | 63.8 ± 3.9 | 43.3 ± 5.8 * | 49.3 ± 5.4 * | 25.65 | <0.0001 |
| IL-1β (pg/mL) | 52.6 ± 1.7 | 139.3 ± 4.4 * | 96.7 ± 9.2 * # | 313.0 | <0.0001 |
| IL-10 (pg/mL) | 66.4 ± 2.5 | 25.2 ± 3.6 * | 49.4 ± 11.4 * # | 51.23 | <0.0001 |
CRR: cardiac risk ratio. AIP: atherogenic index of plasma. CRI-II: Castelli’s risk index II. (*) indicates significant difference (p < 0.05) in comparison with CTL. (#) indicates significant difference (p < 0.05) in comparison with HFHC group. † Non-parametric data.
3.2. Short-Chain Fatty Acids, Fructose and Raffinose in Feces
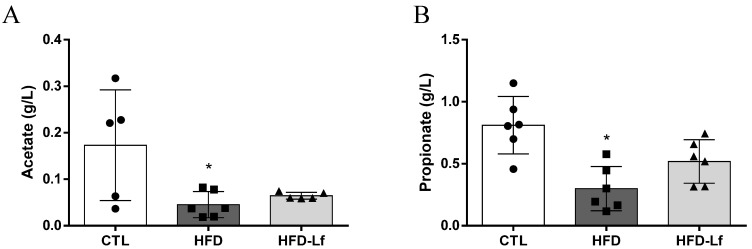
HFD consumption decreased fecal acetic (0.05 ± 0.02 vs. 0.17 ± 0.11 g/L, p < 0.05) and propionic acid contents (0.30 ± 0.17 vs. 0.81 ± 0.23 g/L, p < 0.05) in comparison with the CTL group (Figure 1A,B). Butyric acid contents were below the limit of detection. Administration of a mixed L. fermentum formulation did not change fecal contents of SCFAs (Figure 1A,B).
Figure 1.
Effects of a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 269 on short-chain fatty acid concentration in fecal samples of rats fed an HFD. Assessment of acetic (A) and propionic acids (B) in fecal samples. Groups: control (CTL, n = 6), high-fat diet (HFD, n = 6) and HFD with L. fermentum formulation (HFD-Lf, n = 6). Data are displayed as mean ± standard deviation, and were analyzed by ANOVA one-way test with Tukey post hoc test. * p < 0.05 indicates significant difference between HFD and CTL groups. During HPLC experiments, acetate contents of 2 rats (01 of CTL group and 01 of HFD-Lf group) were below the analytical detection limit.
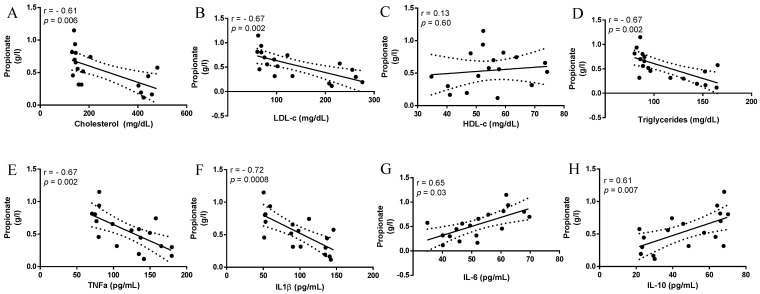
Fecal propionic acid contents correlated negatively with serum levels of cholesterol (r = −0.61, p = 0.006), LDL-c (r = −0.67, p = 0.002) and triglycerides (r = −0.67, p = 0.002), but did not correlate with HDL-c serum levels (r = 0.13, p = 0.60) (Figure 2A–D). Fecal propionic acid contents correlated negatively with serum levels of TNF-α (r = −0.67, p = 0.002, Figure 2E) and IL-1β (r = −0.72, p = 0.0008, Figure 2F), and correlated positively with serum levels of IL-6 (r = 0.65, p = 0.03, Figure 2G), and IL-10 (r = 0.61, p = 0.007, Figure 2H).
Figure 2.
Assessment of correlation coefficients between propionic acid concentration, biochemical and cytokine variables. High-density lipoprotein cholesterol (HDL-c); low-density lipoprotein cholesterol (LDL-c) levels; tumor necrosis factor-α (TNF-α); interleukin 1-beta (IL1β); interleukin 6 (IL-6); interleukin 10 (IL-10).
3.3. Oxidative Stress Biomarkers in Colon Tissues
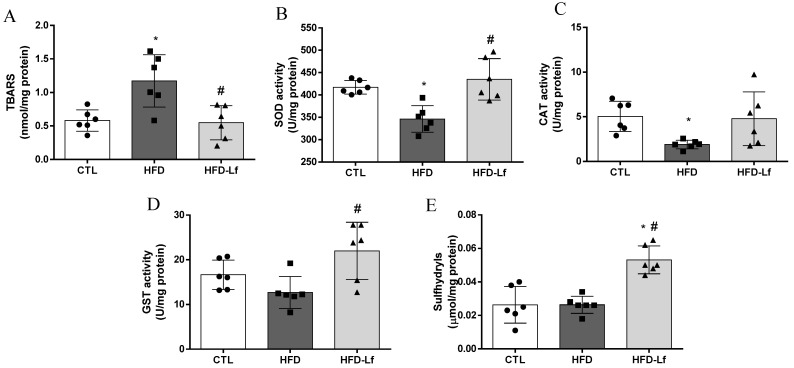
Rats fed an HFD had increased MDA levels (1.2 ± 0.4 vs. 0.6 ± 0.1 nmol/mg protein, p < 0.05), and decreased SOD (346.2 ± 29.8 vs. 417.2 ± 15.1 U/mg protein, p < 0.05) and CAT activities (1.9 ± 0.5 vs. 5.0 ± 1.7 U/mg protein, p < 0.05) in colon tissues in comparison with the CTL group (Figure 3A–C). GST activity and sulfhydryl contents in colon tissues were similar between CTL and HFD groups (p > 0.05, Figure 3D,E). In comparison to the HFD group, supplementation of a mixed L. fermentum formulation, despite not showing improved CAT activity (p > 0.05), reduced the MDA levels (0.5 ± 0.2 vs. 1.2 ± 0.4 nmol/mg protein, p < 0.05) and increased SOD (435 ± 46 vs. 346.2 ± 29.8 U/mg protein, p < 0.05) and GST activities (22.0 ± 6.4 vs. 12.7 ± 3.6 U/mg protein, p < 0.05) in colon tissues (Figure 3A–D). In addition, administration of a mixed L. fermentum formulation increased the sulfhydryl content in the colon in comparison with HFD and CTL groups (p < 0.05, Figure 3E).
Figure 3.
Effects of a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 269 on oxidative stress parameters in colon mucosa of rats fed an HFD. Measurement of malondialdehyde levels (MDA, A), superoxide dismutase activity (SOD, B), catalase activity (CAT, C), glutathione S-transferase activity (GST, D) and total sulfhydryl content (E) in colon mucosa. Groups: control (CTL, n = 6), high-fat diet (HFD, n = 6) and HFD with L. fermentum formulation (HFD-Lf, n = 6). Data are displayed as mean ± standard deviation, and were analyzed by ANOVA one-way test with Tukey post hoc test. * p < 0.05 indicates significant difference between HFD or HFD-Lf and CTL groups; # p < 0.05 indicates significant difference between HFD-Lf and HFD groups.
3.4. Oxidative Stress Biomarkers in Heart Tissues
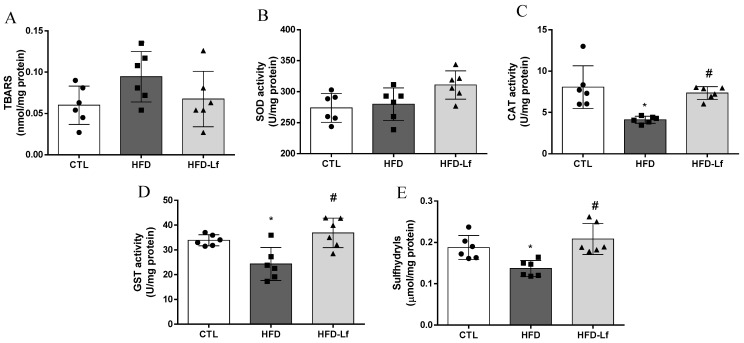
Levels of MDA and SOD activity in heart tissues were similar among groups (p > 0.05, Figure 4A,B). Rats fed an HFD had reduced CAT (4.1 ± 0.4 vs. 8.1 ± 2.5 U/mg protein, p < 0.05) and GST activities (24.3 ± 6.7 vs. 33.9 ± 2.2 U/mg protein, p < 0.05) in heart tissues in comparison with the CTL group (Figure 4C,D). Total sulfhydryl content was decreased in heart tissue of the HFD group in comparison with the CTL group (0.13 ± 0.02 vs. 0.19 ± 0.03 mmol/mg protein, p < 0.05, Figure 4E). Administration of a mixed L. fermentum formulation restored CAT (7.3 ± 0.8 vs. 4.1 ± 0.4 U/mg protein, p < 0.05) and GST activities (36.9 ± 5.9 vs. 24.3 ± 6.7 U/mg protein, p < 0.05), as well as sulfhydryl content (0.21 ± 0.04 vs. 0.13 ± 0.02 mmol/mg protein, p < 0.05) in heart tissues in comparison with the HFD group (Figure 4C–E).
Figure 4.
Effects of a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 269 on oxidative stress parameters in heart tissue of rats fed an HFD. Measurement of levels of malondialdehyde (MDA, A), superoxide dismutase activity (SOD, B), catalase activity (CAT, C), glutathione S-transferase activity (GST, D) and total sulfhydryl content (E) in heart tissue. Groups: control (CTL, n = 6), high-fat diet (HFD, n = 6) and HFD with L. fermentum formulation (HFD-Lf, n = 6). Data are displayed as mean ± standard deviation, and were analyzed by ANOVA one-way test with Tukey post hoc test. * p < 0.05 indicates significant difference between HFD or HFD-Lf and CTL groups; # p < 0.05 indicates significant difference between HFD-Lf and HFD groups.
4. Discussion
The results of this study showed that administration of a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 296 twice a day for 4 weeks caused improvements in cardiometabolic parameters, and decreased systemic low-grade inflammation and biomarkers of oxidative stress in colon and heart tissues induced by excessive HFD consumption in rats.
It was previously demonstrated that administration of a single strain, L. fermentum 296, for 4 weeks reduced cholesterol and triglyceride serum levels in rats fed an HFD, but did not increase the HDL-c levels, and did not improve glucose tolerance and insulin sensitivity [8]. In this study, the administration of a mixed L. fermentum formulation effectively decreased the serum levels of glucose, cholesterol, LDL-c, triglycerides and atherogenic indices, and increased HDL-c serum levels, demonstrating superior hypolipidemic and hypoglycemic effects in rats fed an HFD. Similarly, early investigations have demonstrated that administration of L. fermentum FTDC 8312, L. fermentum MJM60397, L. fermentum ME-3 and L. fermentum MTCC: 5898 had hypolipidemic effects in rodents [30,31] and humans [32].
Some lactobacilli and amended genera strains may bind to cholesterol in the intestine, causing increased fecal cholesterol excretion. In addition, colonic bacteria may produce SCFAs from carbohydrate or protein fermentation, which has been linked to inhibition of hepatic and gut cholesterol synthesis, an energy source for colonocytes, maintenance of gut barrier integrity and anti-inflammatory effects [10,33,34]. Acetic, propionic and butyric acids are the most predominant SCFAs in the colon, accounting for 90–95% of total colonic SCFAs [35]. In the present study, the administration of a mixed L. fermentum formulation did not increase SCFA fecal contents in rats fed an HFD. However, fecal propionic acid contents were correlated negatively with serum levels of cholesterol, LDL-c, triglycerides, TNF-α and IL-1β, and correlated positively with IL-10 and IL-6, indicating that dyslipidemia and pro-inflammatory conditions should be associated with reduced fecal propionic acid contents. These results are in accordance with early findings demonstrating that fecal and circulating propionate could exert inhibitory effects on “de novo” lipogenesis and cholesterogenesis in the liver [36,37]. Colonic propionate may also inhibit NF-κB signaling and reduce expression of pro-inflammatory factors, such as TNF-α and IL-1β in colon tissues [38], as well as induce IL10-producing Treg cells [39], which are related as a novel candidate to improve metabolism and inflammation [40].
The gut microbiota is greatly responsive to dietary modifications, with it being verified that HFD consumption can cause gut dysbiosis through oxidative stress in gut mucosa, decrease the population of gut barrier-protecting bacteria and increase the population of endotoxin-producing bacteria [41,42]. The impairment in the gut antioxidant system provoked by HFD consumption can lead to disruption of intestinal epithelial tight junctions, damage to intestinal mucosal integrity, translocation of indigenous intestinal bacteria, systemic low-grade inflammation through the activation of nuclear factor kappa B (NF-κB) pathways and upregulation of TNF-α and IL-1, with increased risk of peripheral organ damage and systemic metabolic dysfunction [43,44,45]. The results of this study have demonstrated that HFD consumption for 4 weeks reduced the activities of antioxidant enzymes and increased the MDA levels in colon tissues, as well as induced low-grade inflammation and cardiometabolic disorders, in rats.
Oxidative stress tolerance and antioxidant capacity have been reported in probiotic strains [14,46]. Early studies have reported antioxidant effects in several L. fermentum strains, such as L. fermentum MTCC: 5898 [31], L. fermentum ME-3 [47], L. fermentum CQPC07 [46], L. fermentum CECT5716 [48] and L. fermentum I5007 [49]. Here, we have demonstrated that administration of a mixed formulation with L. fermentum 139, L. fermentum 263 and L. fermentum 296 effectively reduced MDA levels and increased SOD and GST activity and sulfhydryl content (for non-enzymatic antioxidant defense) in colonic mucosa of rats fed an HFD, indicating an antioxidant capacity of the tested strains.
The mechanism involved in the antioxidant capacity of some probiotic strains is still under investigation. However, it has been suggested that antioxidant capacity of L. fermentum strains could be associated with a fully functional GSH system formed by GSH peroxidase and GSH reductase, which acts to protect cells against oxidative stress [14]. In fact, this information corroborates the results of our study, which have shown an increase in total thiol content. The improvement in antioxidant capacity in colonic mucosa promoted by administration of a mixed L. fermentum formulation was associated with a downregulation in systemic low-grade inflammation, and alleviation of oxidative stress in heart tissues of rats fed an HFD.
An early study demonstrated that daily supplementation of L. fermentum KBL374 and L. fermentum KBL375 (1 × 109 CFU, for 8 days) alleviated inflammation in the gut through regulation of immune responses, and alteration of gut microbiota in a dextran sulfate sodium-induced colitis model [50]. Similarly, daily administration of L. fermentum MTCC: 5898 (2 × 109 CFU, for 12 weeks) reduced gene expression of cytokines TNF-α and IL-6 in the liver of rats fed a cholesterol-enriched diet [31].
Pro- and anti-inflammatory properties, as well as regulation of metabolic, regenerative and neural processes have, been reported as potential functions of IL-6 [51]. Administration of a mixed L. fermentum formulation did not change the IL-6 serum level, but reduced the IL-1β level and upregulated the serum level of anti-inflammatory cytokine IL-10 in rats fed an HFD. These results suggest that the L. fermentum formulation may alleviate low-grade inflammation provoked by HFD consumption.
The IL-10 is a cytokine exerting anti-inflammatory properties with a key role for the limitation of host immune responses to pathogens, which could prevent impairment in the host, and maintaining tissue homeostasis [52]. It has been reported that L. fermentum CECT5716 can increase regulatory T cells (Treg1) and IL-10-producing cells [53]. In addition, probiotic strains can reduce inflammation via downregulation of NF-κB pathways in in vitro and in vivo conditions [54]. The underlying mechanism involved in immune modulation caused by the examined L. fermentum formulation remains to be elucidated.
Previously, we demonstrated that HFD consumption for 4 weeks caused dysautonomia, cardiac baroreflex control impairment and increased blood pressure in male rats [8]. Systemic inflammation and a high level of oxidative stress can induce autonomic dysfunction [55,56], and some evidence has suggested that cardiac impairment may be linked to gut–heart axis damage [57,58]. The lack of gut microbiota composition analysis could be described as a main limitation of this study, although we have previously documented enhanced Lactobacillus counts in feces from rats treated with L. fermentum strains with claimed probiotic properties [8,17]. Another possible limitation of this study could be the lack of a well-established probiotic strain as a comparative reference. In fact, this was not conducted in earlier studies due to: (i) initial metabolic characterization of a potentially probiotic strain and (ii) different Lactobacillus (or amended genera) species analyzed. For example, regarding L. fermentum strains, there are several strains from different countries and isolated from diverse sources exhibiting antioxidant and anti-inflammatory properties. However, there has been no consensus on the strain of L. fermentum that should be used as a comparative reference for other studies. Indeed, this is an important aspect to be solved in the coming years.
In this study, we have shown for the first time that administration of a mixed L. fermentum formulation concomitantly increased GST activity and sulfhydryl content in colonic mucosa and heart tissue of rats fed an HFD, indicating that probiotic administration may impact directly on heart metabolism, probably via the gut–heart axis.
5. Conclusions
Administration of a formulation containing a mix of L. fermentum 139, L. fermentum 263 and L. fermentum 296 with claimed probiotic properties twice a day, for 4 weeks, caused hypocholesterolemia and hypoglycemic effects, increased HDL-c serum levels and alleviated loss of fecal SCFAs induced by HFD consumption. In addition, this study has shown that administration of the L. fermentum formulation may effectively decrease low-grade inflammation and biomarkers of oxidative stress in colon and heart tissues in rats fed an HFD. Finally, it may be reasonable to suggest that the examined L. fermentum formulation has great potential to act as a novel antidyslipidemia product due to its ability to attenuate lipid metabolism disorders, inflammation and oxidative stress.
Acknowledgments
The authors are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil—Finance code 001) for the scholarships awarded to M.O.d.L.F. The authors are also grateful to the CAPES for the scholarships awarded to K.Á.R.d.O. The authors are grateful to the Fundação de Apoio à Pesquisa do Estado da Paraíba (FAPESQ, Brazil) for the scholarships awarded to L.C.P.d.N. Additionally, the authors give thanks for the research productivity fellowship granted by the Brazilian National Council for Scientific and Technological Development (CNPq) to J.L.d.B.A.
Author Contributions
Conceptualization, J.L.d.B.A.; methodology, M.O.d.L.F., L.C.P.d.N. and K.Á.R.d.O.; formal analysis, J.L.d.B.A.; investigation, M.O.d.L.F., L.C.P.d.N., K.Á.R.d.O., A.M.d.O. and M.d.S.L.; resources, J.L.d.B.A., T.H.N., C.J.L. and E.L.d.S.; data curation, J.L.d.B.A. and M.O.d.L.F.; writing—original draft preparation, J.L.d.B.A. and M.O.d.L.F.; writing—review and editing, C.J.L. and E.L.d.S.; visualization, J.L.d.B.A.; supervision, J.L.d.B.A.; project administration, J.L.d.B.A.; funding acquisition, J.L.d.B.A., E.L.d.S. and C.J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Federal University of Paraíba (PROPESQ/UFPB Nº03/2021).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Ethics Committee of Federal University of Paraíba (protocol code # 6080240418).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Madan S., Mehra M.R. The heart-gut microbiome axis in advanced heart failure. J. Heart Lung Transpl. 2020;39:891–893. doi: 10.1016/j.healun.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Liu Y.W., Liong M.T., Tsai Y.C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018;56:601–613. doi: 10.1007/s12275-018-8079-2. [DOI] [PubMed] [Google Scholar]
- 3.Murphy E.A., Velazquez K.T., Herbert K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:515–520. doi: 10.1097/MCO.0000000000000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schulz M.D., Atay C., Heringer J., Romrig F.K., Schwitalla S., Aydin B., Ziegler P.K., Varga J., Reindl W., Pommerenke C., et al. High-fat-diet-mediated dysbiosis promotes intestinal carcinogenesis independently of obesity. Nature. 2014;514:508–512. doi: 10.1038/nature13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X., Wei X., Sun Y., Du J., Li X., Xun Z., Li Y.C. High-fat diet promotes experimental colitis by inducing oxidative stress in the colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;317:G453–G462. doi: 10.1152/ajpgi.00103.2019. [DOI] [PubMed] [Google Scholar]
- 6.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 7.Apaijai N., Arinno A., Palee S., Pratchayasakul W., Kerdphoo S., Jaiwongkam T., Chunchai T., Chattipakorn S.C., Chattipakorn N. High-Saturated Fat High-Sugar Diet Accelerates Left-Ventricular Dysfunction Faster than High-Saturated Fat Diet Alone via Increasing Oxidative Stress and Apoptosis in Obese-Insulin Resistant Rats. Mol. Nutr. Food Res. 2019;63:e1800729. doi: 10.1002/mnfr.201800729. [DOI] [PubMed] [Google Scholar]
- 8.Cavalcante R.G.S., de Albuquerque T.M.R., de Luna Freire M.O., Ferreira G.A.H., Carneiro Dos Santos L.A., Magnani M., Cruz J.C., Braga V.A., de Souza E.L., de Brito Alves J.L. The probiotic Lactobacillus fermentum 296 attenuates cardiometabolic disorders in high fat diet-treated rats. Nutr. Metab. Cardiovasc. Dis. 2019;29:1408–1417. doi: 10.1016/j.numecd.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Nagatomo Y., Tang W.H. Intersections Between Microbiome and Heart Failure: Revisiting the Gut Hypothesis. J. Card Fail. 2015;21:973–980. doi: 10.1016/j.cardfail.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalcanti Neto M.P., Aquino J.S., Romao da Silva L.F., de Oliveira Silva R., Guimaraes K.S.L., de Oliveira Y., de Souza E.L., Magnani M., Vidal H., de Brito Alves J.L. Gut microbiota and probiotics intervention: A potential therapeutic target for management of cardiometabolic disorders and chronic kidney disease? Pharmacol. Res. 2018;130:152–163. doi: 10.1016/j.phrs.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 11.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 12.Zheng J., Wittouck S., Salvetti E., Franz C., Harris H.M.B., Mattarelli P., O'Toole P.W., Pot B., Vandamme P., Walter J., et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020;70:2782–2858. doi: 10.1099/ijsem.0.004107. [DOI] [PubMed] [Google Scholar]
- 13.Lomax A.R., Calder P.C. Probiotics, immune function, infection and inflammation: A review of the evidence from studies conducted in humans. Curr. Pharm. Des. 2009;15:1428–1518. doi: 10.2174/138161209788168155. [DOI] [PubMed] [Google Scholar]
- 14.Feng T., Wang J. Oxidative stress tolerance and antioxidant capacity of lactic acid bacteria as probiotic: A systematic review. Gut Microbes. 2020;12:1801944. doi: 10.1080/19490976.2020.1801944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia E.F., Luciano W.A., Xavier D.E., da Costa W.C., de Sousa Oliveira K., Franco O.L., de Morais Junior M.A., Lucena B.T., Picao R.C., Magnani M., et al. Identification of Lactic Acid Bacteria in Fruit Pulp Processing Byproducts and Potential Probiotic Properties of Selected Lactobacillus Strains. Front. Microbiol. 2016;7:1371. doi: 10.3389/fmicb.2016.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Albuquerque T.M.R., Garcia E.F., de Oliveira Araujo A., Magnani M., Saarela M., de Souza E.L. In Vitro Characterization of Lactobacillus Strains Isolated from Fruit Processing By-Products as Potential Probiotics. Probiotics Antimicrob. Proteins. 2018;10:704–716. doi: 10.1007/s12602-017-9318-2. [DOI] [PubMed] [Google Scholar]
- 17.de Oliveira Y., Cavalcante R.G.S., Cavalcanti Neto M.P., Magnani M., Braga V.A., de Souza E.L., de Brito Alves J.L. Oral administration of Lactobacillus fermentum post-weaning improves the lipid profile and autonomic dysfunction in rat offspring exposed to maternal dyslipidemia. Food Funct. 2020;11:5581–5594. doi: 10.1039/D0FO00514B. [DOI] [PubMed] [Google Scholar]
- 18.Reeves P.G., Nielsen F.H., Fahey G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 19.Bezerra M.L.R., de Souza E.L., de Sousa J.M.B., Lima M.D.S., Alves A.F., Almeida M.D.G., Coutinho Alves R., Verissimo de Araujo E., Soares N.L., da Silva G.A., et al. Effects of honey from Mimosa quadrivalvis L. (malicia) produced by the Melipona subnitida D. (jandaira) stingless bee on dyslipidaemic rats. Food Funct. 2018;9:4480–4492. doi: 10.1039/C8FO01044G. [DOI] [PubMed] [Google Scholar]
- 20.Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 21.Martirosyan D.M., Miroshnichenko L.A., Kulakova S.N., Pogojeva A.V., Zoloedov V.I. Amaranth oil application for coronary heart disease and hypertension. Lipids Health Dis. 2007;6:1. doi: 10.1186/1476-511X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dobiasova M. Atherogenic index of plasma [log(triglycerides/HDL-cholesterol)]: Theoretical and practical implications. Clin. Chem. 2004;50:1113–1115. doi: 10.1373/clinchem.2004.033175. [DOI] [PubMed] [Google Scholar]
- 23.Castelli W.P., Abbott R.D., McNamara P.M. Summary estimates of cholesterol used to predict coronary heart disease. Circulation. 1983;67:730–734. doi: 10.1161/01.CIR.67.4.730. [DOI] [PubMed] [Google Scholar]
- 24.Pedroza A., Ferreira D.S., Santana D.F., da Silva P.T., de Aguiar Junior F.C.A., Sellitti D.F., Lagranha C.J. A maternal low-protein diet and neonatal overnutrition result in similar changes to glomerular morphology and renal cortical oxidative stress measures in male Wistar rats. Appl. Physiol. Nutr. Metab. 2019;44:164–171. doi: 10.1139/apnm-2018-0288. [DOI] [PubMed] [Google Scholar]
- 25.Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Misra H.P., Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. doi: 10.1016/S0021-9258(19)45228-9. [DOI] [PubMed] [Google Scholar]
- 27.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 28.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249:7130–7139. doi: 10.1016/S0021-9258(19)42083-8. [DOI] [PubMed] [Google Scholar]
- 29.Ellman G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 30.Palaniyandi S.A., Damodharan K., Suh J.W., Yang S.H. Probiotic Characterization of Cholesterol-Lowering Lactobacillus fermentum MJM60397. Probiotics Antimicrob. Proteins. 2020;12:1161–1172. doi: 10.1007/s12602-019-09585-y. [DOI] [PubMed] [Google Scholar]
- 31.Yadav R., Khan S.H., Mada S.B., Meena S., Kapila R., Kapila S. Consumption of Probiotic Lactobacillus fermentum MTCC: 5898-Fermented Milk Attenuates Dyslipidemia, Oxidative Stress, and Inflammation in Male Rats Fed on Cholesterol-Enriched Diet. Probiotics Antimicrob. Proteins. 2019;11:509–518. doi: 10.1007/s12602-018-9429-4. [DOI] [PubMed] [Google Scholar]
- 32.Kullisaar T., Zilmer K., Salum T., Rehema A., Zilmer M. The use of probiotic L. fermentum ME-3 containing Reg'Activ Cholesterol supplement for 4 weeks has a positive influence on blood lipoprotein profiles and inflammatory cytokines: An open-label preliminary study. Nutr. J. 2016;15:93. doi: 10.1186/s12937-016-0213-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar M., Nagpal R., Kumar R., Hemalatha R., Verma V., Kumar A., Chakraborty C., Singh B., Marotta F., Jain S., et al. Cholesterol-lowering probiotics as potential biotherapeutics for metabolic diseases. Exp. Diabetes Res. 2012;2012:902917. doi: 10.1155/2012/902917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parada Venegas D., De la Fuente M.K., Landskron G., Gonzalez M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cummings J.H., Pomare E.W., Branch W.J., Naylor C.P., Macfarlane G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Granado-Serrano A.B., Martin-Gari M., Sanchez V., Riart Solans M., Berdun R., Ludwig I.A., Rubio L., Vilaprinyo E., Portero-Otin M., Serrano J.C.E. Faecal bacterial and short-chain fatty acids signature in hypercholesterolemia. Sci. Rep. 2019;9:1772. doi: 10.1038/s41598-019-38874-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weitkunat K., Schumann S., Nickel D., Kappo K.A., Petzke K.J., Kipp A.P., Blaut M., Klaus S. Importance of propionate for the repression of hepatic lipogenesis and improvement of insulin sensitivity in high-fat diet-induced obesity. Mol. Nutr. Food Res. 2016;60:2611–2621. doi: 10.1002/mnfr.201600305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Filippone A., Lanza M., Campolo M., Casili G., Paterniti I., Cuzzocrea S., Esposito E. The Anti-Inflammatory and Antioxidant Effects of Sodium Propionate. Int. J. Mol. Sci. 2020;21:3026. doi: 10.3390/ijms21083026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smith P.M., Howitt M.R., Panikov N., Michaud M., Gallini C.A., Bohlooly Y.M., Glickman J.N., Garrett W.S. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cani P.D. Is colonic propionate delivery a novel solution to improve metabolism and inflammation in overweight or obese subjects? Gut. 2019;68:1352–1353. doi: 10.1136/gutjnl-2019-318776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao Y., Sun J., Ding Y., Le G., Shi Y. Alterations of the gut microbiota in high-fat diet mice is strongly linked to oxidative stress. Appl. Microbiol. Biotechnol. 2013;97:1689–1697. doi: 10.1007/s00253-012-4323-6. [DOI] [PubMed] [Google Scholar]
- 42.Cani P.D., Neyrinck A.M., Fava F., Knauf C., Burcelin R.G., Tuohy K.M., Gibson G.R., Delzenne N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 43.Creely S.J., McTernan P.G., Kusminski C.M., Fisher F.M., Da Silva N.F., Khanolkar M., Evans M., Harte A.L., Kumar S. Lipopolysaccharide activates an innate immune system response in human adipose tissue in obesity and type 2 diabetes. Am. J. Physiol. Endocrinol. Metab. 2007;292:E740–E747. doi: 10.1152/ajpendo.00302.2006. [DOI] [PubMed] [Google Scholar]
- 44.Pussinen P.J., Havulinna A.S., Lehto M., Sundvall J., Salomaa V. Endotoxemia is associated with an increased risk of incident diabetes. Diabetes Care. 2011;34:392–397. doi: 10.2337/dc10-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu Y.C., Yeh W.C., Ohashi P.S. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–151. doi: 10.1016/j.cyto.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y., Li X., Tan F., Zhou X., Mu J., Zhao X. Lactobacillus fermentum CQPC07 attenuates obesity, inflammation and dyslipidemia by modulating the antioxidant capacity and lipid metabolism in high-fat diet induced obese mice. J. Inflamm. Lond. 2021;18:5. doi: 10.1186/s12950-021-00272-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mikelsaar M., Zilmer M. Lactobacillus fermentum ME-3—An antimicrobial and antioxidative probiotic. Microb. Ecol. Health Dis. 2009;21:1–27. doi: 10.1080/08910600902815561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robles-Vera I., Toral M., de la Visitacion N., Sanchez M., Romero M., Olivares M., Jimenez R., Duarte J. The Probiotic Lactobacillus fermentum Prevents Dysbiosis and Vascular Oxidative Stress in Rats with Hypertension Induced by Chronic Nitric Oxide Blockade. Mol. Nutr. Food Res. 2018;62:e1800298. doi: 10.1002/mnfr.201800298. [DOI] [PubMed] [Google Scholar]
- 49.Wang A.N., Cai C.J., Zeng X.F., Zhang F.R., Zhang G.L., Thacker P.A., Wang J.J., Qiao S.Y. Dietary supplementation with Lactobacillus fermentum I5007 improves the anti-oxidative activity of weanling piglets challenged with diquat. J. Appl. Microbiol. 2013;114:1582–1591. doi: 10.1111/jam.12188. [DOI] [PubMed] [Google Scholar]
- 50.Jang Y.J., Kim W.K., Han D.H., Lee K., Ko G. Lactobacillus fermentum species ameliorate dextran sulfate sodium-induced colitis by regulating the immune response and altering gut microbiota. Gut Microbes. 2019;10:696–711. doi: 10.1080/19490976.2019.1589281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta. 2011;1813:878–888. doi: 10.1016/j.bbamcr.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012;32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Perez-Cano F.J., Dong H., Yaqoob P. In vitro immunomodulatory activity of Lactobacillus fermentum CECT5716 and Lactobacillus salivarius CECT5713: Two probiotic strains isolated from human breast milk. Immunobiology. 2010;215:996–1004. doi: 10.1016/j.imbio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 54.Bhardwaj R., Singh B.P., Sandhu N., Singh N., Kaur R., Rokana N., Singh K.S., Chaudhary V., Panwar H. Probiotic mediated NF-kappaB regulation for prospective management of type 2 diabetes. Mol. Biol. Rep. 2020;47:2301–2313. doi: 10.1007/s11033-020-05254-4. [DOI] [PubMed] [Google Scholar]
- 55.Campese V.M., Shaohua Y., Huiquin Z. Oxidative stress mediates angiotensin II-dependent stimulation of sympathetic nerve activity. Hypertension. 2005;46:533–539. doi: 10.1161/01.HYP.0000179088.57586.26. [DOI] [PubMed] [Google Scholar]
- 56.Levick S.P., Murray D.B., Janicki J.S., Brower G.L. Sympathetic nervous system modulation of inflammation and remodeling in the hypertensive heart. Hypertension. 2010;55:270–276. doi: 10.1161/HYPERTENSIONAHA.109.142042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen W., Zhang S., Wu J., Ye T., Wang S., Wang P., Xing D. Butyrate-producing bacteria and the gut-heart axis in atherosclerosis. Clin. Chim. Acta. 2020;507:236–241. doi: 10.1016/j.cca.2020.04.037. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Wang Y., Ke B., Du J. TMAO: How gut microbiota contributes to heart failure. Transl. Res. 2021;228:109–125. doi: 10.1016/j.trsl.2020.08.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.