Abstract
Cold stress is a major environmental factor that detrimentally affects plant growth and development. Melatonin has been shown to confer plant tolerance to cold stress through activating the C-REPEAT BINDING FACTOR (CBF) pathway; however, the underlying modes that enable this function remain obscure. In this study, we investigated the role of H2O2 and Ca2+ signaling in the melatonin-induced CBF pathway and cold tolerance in watermelon (Citrullus lanatus L.) through pharmacological, physiological, and genetic approaches. According to the results, melatonin induced H2O2 accumulation, which was associated with the upregulation of respiratory burst oxidase homolog D (ClRBOHD) during the early response to cold stress in watermelon. Besides, melatonin and H2O2 induced the accumulation of cytoplasmic free Ca2+ ([Ca2+]cyt) in response to cold. This was associated with the upregulation of cyclic nucleotide-gated ion channel 2 (ClCNGC2) in watermelon. However, blocking of Ca2+ influx channels abolished melatonin- or H2O2-induced CBF pathway and cold tolerance. Ca2+ also induced ClRBOHD expression and H2O2 accumulation in early response to cold stress in watermelon. Inhibition of H2O2 production in watermelon by RBOH inhibitor or in Arabidopsis by AtRBOHD knockout compromised melatonin-induced [Ca2+]cyt accumulation and melatonin- or Ca2+-induced CBF pathway and cold tolerance. Overall, these findings indicate that melatonin induces RBOHD-dependent H2O2 generation in early response to cold stress. Increased H2O2 promotes [Ca2+]cyt accumulation, which in turn induces H2O2 accumulation via RBOHD, forming a reciprocal positive-regulatory loop that mediates melatonin-induced CBF pathway and subsequent cold tolerance.
Keywords: melatonin, hydrogen peroxide, calcium signal, CBF-responsive pathway, respiratory burst oxidase homolog D, cold stress
1. Introduction
Plants are sessile organisms and, therefore, must withstand multiple environmental stresses throughout their life cycle. As a major environmental constraint, cold stress causes adverse effects on plant growth and development, threatening agricultural production worldwide [1]. Based on temperature and various physiological mechanisms, cold stress in plants is classified as chilling stress (temperatures below optimum but above 0 °C) and freezing stress (<0 °C) [2]. Watermelon (Citrullus lanatus L.) is an economically important crop globally. Its origin can be traced to tropical and subtropical regions of Africa. Watermelon production is threatened by its susceptibility to low temperatures [3]. Chilling temperatures adversely affect watermelon seedlings, including reduction of photosynthetic ability, oxidative damage, membrane dysfunction, and hormonal imbalance, leading to reduced growth and development, delay in flowering and fruit set, and decrease in fruit quality and total output [4,5,6].
Plants have evolved sophisticated defense mechanisms for surviving cold stress. They can perceive temperature declines, leading to signal transduction from the plasma membrane to the nucleus, activating diverse transcriptional regulators, and inducing osmotic factors [2]. Calcium (Ca2+) signaling plays an essential function in signal transduction in plant response to cold stress. Temperature decrease activates Ca2+ channels in plants, leading to rapid induction of Ca2+ signals, which confers cold tolerance by activating downstream phosphorylation cascade and transcriptional regulation pathways, such as the C-repeat binding factors (CBFs)/Drought response element-binding factors (DREBs)-dependent signaling pathway [7,8]. CBFs/DREBs control the expression of numerous cold-responsive (COR) genes by specifically recognizing and binding to the C-repeat/dehydration responsive element (DRE) motif [9]. Overexpression of CBFs enhanced plant tolerance to cold stress, while mutation of CBFs reduced cold tolerance [10,11]. Furthermore, the CBF-response pathway is highly conserved among plants [12].
Besides Ca2+ influx, oxidative burst is one of the initial plant responses to cold exposure. It involves enhanced production and steady state levels of reactive oxygen species (ROS), such as superoxide (O2−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH·). H2O2 produced by the NADPH oxidase encoded by respiratory burst oxidase homologues (RBOHs), is an important signaling molecule that triggers downstream responses such as CBF-dependent pathway to confer cold tolerance [13,14]. Plant RBOHs have a highly conserved cytosolic N-terminal with two Ca2+-binding EF-hand motifs [15]. Cold-induced Ca2+ activates RBOH activity by direct binding to the N-terminal EF-hands to trigger H2O2 generation, which in turn elicits Ca2+ transients in plant cells [8,16,17].
In the last two decades, melatonin (N-acetyl-5-methoxytryptamine) has emerged as an essential bio-stimulant in plants because of its essential regulatory functions in growth, development, and response to various environmental agents [18]. The recent identification of the first phytomelatonin receptor (CAND2/PMTR1) in Arabidopsis validated phytomelatonin as a new plant hormone [19,20]. Several lines of evidence prove that melatonin has a protective function against cold stress in plants. Exogenous melatonin has been shown to strengthen chilling tolerance in diverse plant species by scavenging excessive ROS and improving the photosynthesis system [21,22,23]. A recent study has shown that melatonin, as a potential root-to-shoot signal, induces the increases in methy jasmonate and H2O2 in rootstock-scion communication in response to cold stress [24]. Melatonin induces the expression of CBFs and COR genes under cold stress, suggesting that the CBF pathway may participate in melatonin-induced cold tolerance [25,26,27]. Furthermore, the upregulation of COR genes induced by melatonin is in an ABA-independent manner [28]. However, the mechanism by which melatonin activates the CBF pathway and subsequently enhances plant tolerance to cold stress remains unclear.
Increasing evidence indicates that H2O2 and Ca2+ signaling facilitate melatonin-mediated regulation of multiple physiological processes, including seed germination and stomatal closure [19,29]. These findings raise a presumption that H2O2 and Ca2+ signaling may also have a critical role in the melatonin-induced CBF-responsive pathway and subsequent chilling tolerance. To verify this presumption, we examined the functions of H2O2 and Ca2+ and their interaction in melatonin-induced chilling tolerance. Our results reveal that the interaction between H2O2 and Ca2+ mediates the melatonin-induced CBF pathway and subsequent cold tolerance. This study provides new insights into understanding the regulation mechanism of melatonin-induced cold tolerance.
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
Watermelon (Citrullus lanatus cv. Nongkeda No. 5) seeds were collected from the Watermelon and Melon Research Group of Northwest A&F University, Yangling, China. Germinated seeds were planted into plastic pots filled with a mixture of peat and vermiculite (3:1). The seedlings were cultivated in a growth chamber under photoperiod of 12/12 h (day/night), temperature of 28/18 °C (day/night), and a photosynthetic photo flux density (PPFD) of 600 μmol m−2 s−1. Arabidopsis seeds of Atrbohd (SALK_120299) mutant with Columbia-0 (Col-0) genetic background were received from the Arabidopsis Biological Resource Center (https://www.arabidopsis.org/, accessed date 16 May 2019) [29]. After surface sterilization with 75% ethanol and 3% sodium hypochlorite, Arabidopsis seeds were sown on half-strength Murashige-Skoog (MS) medium containing 0.8% agar and 1.0% sucrose and cultured at 22 °C in a growth chamber under 16/8 h photoperiod.
2.2. Experimental Treatment
To evaluate the influences of melatonin, H2O2, or Ca2+ on watermelon response to cold stress, the seedlings were sprayed with 150 µM melatonin [24], H2O2 (0.04, 0.2, 1, 5 mM), 20 mM CaCl2 [30,31], or distilled water (as the control). Melatonin (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in ethanol and then diluted with distilled water at a ratio of 1:10,000 [ethanol:water; v:v]. After 12 h, the watermelon seedlings were exposed to chilling treatment at 4 °C for 48 h. The leaves were sampled at 3, 6, 12, and 24 h time points during chilling exposure to analyze the expression of CBF pathway genes and at 48 h for cold tolerance assay. After the initial experiments, 1 mM H2O2 was selected for the subsequent experiments.
To determine the role of H2O2 in melatonin- or Ca2+-induced cold tolerance, watermelon seedlings were pretreated with 100 µM diphenyleneiodonium (DPI, an inhibitor of NADPH oxidases, which catalyzes H2O2 production) [32] 2 h before melatonin or Ca2+ application. After 12 h, the seedlings were transferred to 4 °C for 48 h. To investigate the role of Ca2+ in melatonin- or H2O2-induced cold tolerance, the watermelon seedlings were sprayed with 10 mM lanthanum chloride (LaCl3, a Ca2+ channel blocker) [33] 2 h before melatonin or H2O2 treatment. After 12 h, the seedlings were transferred to 4 °C for 48 h.
To explore the function of RBOHD in melatonin- or Ca2+-induced freezing tolerance in Arabidopsis, three-week-old wild-type or Atrbohd mutant Arabidopsis plants were pretreated with 10 μM melatonin [34] or 1 mM CaCl2 [35]. After 12 h, the seedlings were subjected to freezing at −10 °C for 1 h and then recovered at 22 °C for 5 days [36]. The proportion of plants with green leaves was recorded to determine the survival rate. To determine the role of RBOHD in melatonin- or Ca2+-induced CBF pathway in response to cold, the Arabidopsis seedlings pretreated with melatonin or CaCl2 were exposed to 4 °C for 24 h. Leaves were sampled at 3, 6, 12, and 24 h after 4 °C treatment.
Protoplasts from watermelon or Arabidopsis leaves were extracted and used to examine the effects of melatonin or H2O2 on the accumulation of cytosolic free calcium ([Ca2+]cyt) in response to chilling stress. The protoplasts were incubated with Fluo-4 acetoxymethyl (AM) ester (a Ca2+-sensitive fluorescent dye) at 37 °C in the dark. After 30 min, the protoplasts loaded with Fluo-4/AM were treated with melatonin (10 µM), H2O2 (100 µM), or a combination of melatonin and DPI (10 µM) and then placed at 4 °C for 5 min.
2.3. Cold Tolerance Assay
The maximum photosystem II quantum yield (Fv/Fm) was determined on the upper second fully expanded leaves of watermelon seedlings after 30 min of dark adaptation using a FluorCam fluorescence imaging system (SN-FC800-240; Photon Systems Instruments; Brno, Czech Republic) [37]. The relative electrical conductivity (REC) was determined as described by Zhou and Leul [38]. The level of lipid peroxidation in plant cells was assessed by determining malondialdehyde (MDA) contents using a 2-thiobarbituric acid (TBA) reaction [39].
2.4. Hydrogen Peroxide Content Assay
Hydrogen peroxide (H2O2) content was determined as described by Willekens et al. [40]. In brief, leaf samples (0.5 g each) were ground in 5 mL of ice-cold 1 M HClO4. Next, the homogenate was centrifuged at 6000× g for 5 min at 4 °C and neutralized to pH 6.0–7.0 with 4 M KOH. After that, the homogenate was further centrifuged at 12,000× g for 5 min at 4 °C, and the supernatant was loaded on an AG1-X8 prepacked column (Bio-Red, Hercules, CA, USA) and eluted with 4 mL double-distilled water. The sample extract (800 µL) was added to the reaction mixture of 400 µL 100 mM potassium acetate buffer (pH 4.4) containing 4 mM 2,2′-azino-di (3-ethylbenzthiazoline-6-sulfonic acid), 400 µL deionized water, and 0.25 U of horseradish peroxidase (HRP). The absorbance at OD412 was recorded to calculate H2O2 content.
2.5. Protoplast Isolation and Measurement of [Ca2+]cyt
Protoplasts were isolated as described previously with modifications [41,42]. Briefly, leaves from three-week-old watermelon or four-week-old Arabidopsis seedlings were cut into 0.5 mm wide strips. Watermelon leaf strips were digested with 10 mL enzyme solution containing 1.5% cellulose R10 and 0.3% macerozyme R10, whereas Arabidopsis leaf strips were digested with enzyme solution containing 1.0% cellulose R10 and 0.2% macerozyme R10. The digestion was performed for 3–4 h in the dark, after which the enzyme solutions were diluted with 10 mL W5 solution (pH 5.7) containing 2 mM MES, 154 mM NaCl, 125 mM CaCl2, and 5 mM KCl, then filtered through a 75 µm nylon mesh. The protoplasts were collected after centrifugation at 100× g for 5 min at 4 °C and re-suspended in 10 mL W5 solution.
The [Ca2+]cyt in the protoplasts was measured using Fluo-4 AM [43]. The protoplasts were incubated with 4 µM Fluo-4 AM and 0.02% Pluronic F-127 (Shanghai Yeasen Science & Technology Co., Ltd., Shanghai, China) at 37 °C in the dark for 30 min. Fluorescence from the protoplasts loaded with Fluo-4/AM was observed under a confocal microscope (TCS SP8 SR, Leica, German), at excitation of 488 ± 10 nm and emission of 520 ± 10 nm. The fluorescence intensity was calculated using Image J v1.8.0 software (National Institutes of Health, Bethesda, MD, USA) to reflect [Ca2+]cyt accumulation levels.
2.6. RNA Extraction and qRT-PCR Assay
Total RNA was extracted from watermelon or Arabidopsis leaves using an RNA simple Total RNA kit (TIANGEN, Beijing, China). After extraction, the total RNA samples were treated with gDNase, then reverse-transcribed (1 µg per sample) to cDNA using a FastKing RT kit (TIANGEN, Beijing, China). The qRT-PCR assay was performed on a StepOnePlusTM Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA) using SYBR® Premix ExTaqTM II (2×) kit (Takara, Tokyo, Japan). The gene-specific primers used for the qRT-PCR are listed in Table S1. The qRT-PCR amplification was conducted under the conditions reported by Li et al. [29]. β-actin or AtActin2 served as the internal control genes for the normalization of gene expression [11,44]. Relative gene expression was calculated as described by Livak and Schmittgen [45].
2.7. Phylogenetic Analysis
Arabidopsis AtRBOH and watermelon ClRBOHD protein sequences were downloaded from Arabidopsis Information Resource (https://www.arabidopsis.org/, accessed date 16 May 2019) and Cucurbit Genomics Database (CuGenDB, http://cucurbitgenomics.org/, accessed date 23 July 2020), respectively. Multiple sequence alignment of these RBOH protein sequences was performed via ClustalW with default parameters [46]. Phylogenetic analysis was conducted using the MEGA7.0.21 software. Based on the result of multiple sequence alignment, a phylogenetic tree was constructed using the Neighbor-Joining method and the parameters were Jones–Taylor–Thornton (JTT) matrix-based model and 1000 bootstraps [47].
2.8. Statistical Analysis
The experiments were performed in a completely randomized design. Each experiment was repeated thrice, and each replicate included at least 18 plants. The differences among treatments were determined via one-way ANOVA using SPSS statistics 19 (SPSS Inc., Chicago, IL, USA), followed by Tukey’s test at p < 0.05.
3. Results
3.1. The Requirement of H2O2 for Melatonin-Induced CBF-Responsive Pathway and Chilling Tolerance in Watermelon
The effect of melatonin on H2O2 accumulation was first examined to determine whether H2O2 is necessary for melatonin function in watermelon response to chilling stress. The results showed no significant differences in H2O2 accumulation between melatonin-pretreated and control plants under optimum growth conditions (Figure 1A). Chilling exposure induced an H2O2 burst from 6 h; however, such induction was accelerated by melatonin. Pretreatment with melatonin triggered an H2O2 burst from 1 h after chilling treatment. For instance, after chilling exposure at 3 and 6 h, H2O2 contents in melatonin-pretreated plants increased by 15.9% and 19.0%, respectively, compared to the control plants. However, H2O2 contents in melatonin-pretreated plants were less than that in the control plants at 12 and 48 h after chilling treatment. The changes in ClRBOHD transcript levels showed similar trends with H2O2 in response to melatonin or/and chilling stress. For example, the transcript levels of ClRBOHD in melatonin-pretreated plants were 0.5 and 5.2 fold higher than that in the control plants at 3 and 6 h, respectively, after chilling exposure.
Figure 1.
Involvement of H2O2 in melatonin-induced C-REPEAT BINDING FACTOR (CBF) transcriptional regulatory cascade and chilling tolerance. (A) Dynamic changes in H2O2 accumulation and the expression of respiratory burst oxidase homolog D (ClRBOHD). Watermelon seedlings at three-leaf stage were sprayed with 150 µM melatonin (MT), and after 12 h, they were subjected to cold stress (CS) at 4 °C for 48 h. Seedlings sprayed with distilled water under temperatures of 28/18 °C (day/night) were set as control (CK). (B) The relative electrical conductivity (REC) and malondialdehyde (MDA) content. The seedlings were sprayed with 0.04, 0.2, 1, or 5 mM H2O2, and after 12 h, they were exposed to 4 °C for 48 h. (C) Chilling phenotypes. (D) Images showing the maximum PSII quantum yield (Fv/Fm). The false-color code depicted at the left of the image ranges from 0 (black) to 1 (purple). (E) The average values of Fv/Fm and MDA contents. (F) The expression of ClCBF1 and its regulons, including cold-responsive gene 47 (ClCOR47), early responsive to dehydration 10 (ClERD10), and cold induced gene 1 (ClKIN1). For (C–F), the seedlings were sprayed with 100 µM diphenyleneiodonium (DPI, an inhibitor of H2O2 production) 2 h before MT treatment. After 12 h, the seedlings were subjected to 4 °C. Relative expression of genes in CK plants was set as 1.0. Data show the means of three replicates ± standard deviation (SD). The different letters denote significant difference at p < 0.05 according to Turkey’s test.
Application of H2O2 at optimum concentrations (0.04–5 mM) alleviated chilling-induced increases in REC and MDA. The most effective H2O2 concentration was 1 mM (Figure 1B). Notably, H2O2 concentrations higher or lower than 1 mM attenuated the positive effect of H2O2 on chilling tolerance. Similarly, melatonin application enhanced watermelon defense against chilling stress, as exhibited by the alleviation of leaf wilting, an increase in Fv/Fm, and a decrease in MDA content (Figure 1C–E). However, pretreatment with DPI (an inhibitor of H2O2 production, 100 μM) completely abolished the melatonin-induced chilling tolerance. The CBF-responsive pathway plays a central role in plant defense against cold stress [9]. Chilling stress induced the expression of ClCBF1 and its regulons, including cold-responsive gene 47 (COR47), early responsive to dehydration 10 (ERD10), and cold induced gene 1 (KIN1). Importantly, transcript levels of the genes mentioned above were significantly higher in the melatonin-pretreated plants than in the control plants after chilling treatment. However, pretreatment with DPI prevented the melatonin-induced increases in the transcripts of CBF-responsive pathway genes.
3.2. Involvement of Ca2+ Signal in Melatonin- and H2O2-Induced Chilling Tolerance in Watermelon
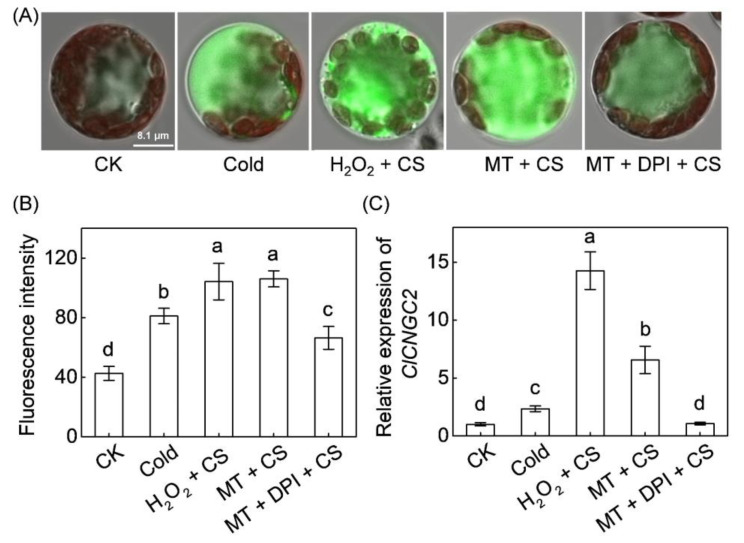
To investigate whether Ca2+ signal is involved in melatonin- or H2O2-mediated cold tolerance, the [Ca2+]cyt accumulation in watermelon protoplasts was first measured using the Fluo-4 AM ester as a fluorescent indicator of Ca2+. According to the results, chilling exposure induced the accumulation of [Ca2+]cyt in watermelon protoplasts (Figure 2). Notably, both melatonin and H2O2 pretreatment significantly increased the cold-induced accumulation of [Ca2+]cyt. For example, the fluorescence intensity of [Ca2+]cyt in protoplasts pretreated with melatonin and H2O2 increased by 46% and 32%, respectively, compared with the control protoplasts after chilling stress. However, DPI application prevented the melatonin-induced [Ca2+]cyt accumulation under chilling stress. Cyclic nucleotide-gated channel 2 (CNGC2) encodes a plasma membrane cation channel that directs extracellular Ca2+ into the cytosol [48,49]. In line with [Ca2+]cyt accumulation, both melatonin and H2O2 promoted the cold-induced ClCNGC2 upregulation; however, the effect was blocked by DPI pretreatment. These results demonstrate that H2O2 participates in melatonin-induced [Ca2+]cyt accumulation under chilling stress.
Figure 2.
The involvement of H2O2 in melatonin-induced accumulation of cytoplasmic free Ca2+ ([Ca2+]cyt) in response to chilling stress. (A) [Ca2+]cyt accumulation in protoplasts from watermelon leaves. (B) Fluorescence intensity. The protoplasts were incubated with Fluo-4 acetoxymethyl (AM) ester at 37 °C in the dark. After 30 min, the protoplasts were treated with melatonin (10 µM), H2O2 (100 µM), or a combination of melatonin and DPI (10 µM) and then placed at 4 °C for 5 min (cold stress, CS). Data in (B) are reported as means ± standard deviation (SD, n ≥ 7). (C) The relative expression of cyclic nucleotide-gated channel 2 (ClCNGC2) at 6 h after chilling exposure. The seedling leaves were sprayed with 150 µM melatonin (MT) or 1 mM H2O2. After 12 h, the seedlings were transferred to 4 °C. To inhibit H2O2 production, the seedlings were sprayed with DPI at 100 µM 2 h before melatonin application. Data are reported as means ± standard deviation (SD, n = 3). The different letters denote significant difference at p < 0.05 according to Turkey’s test. CK, control.
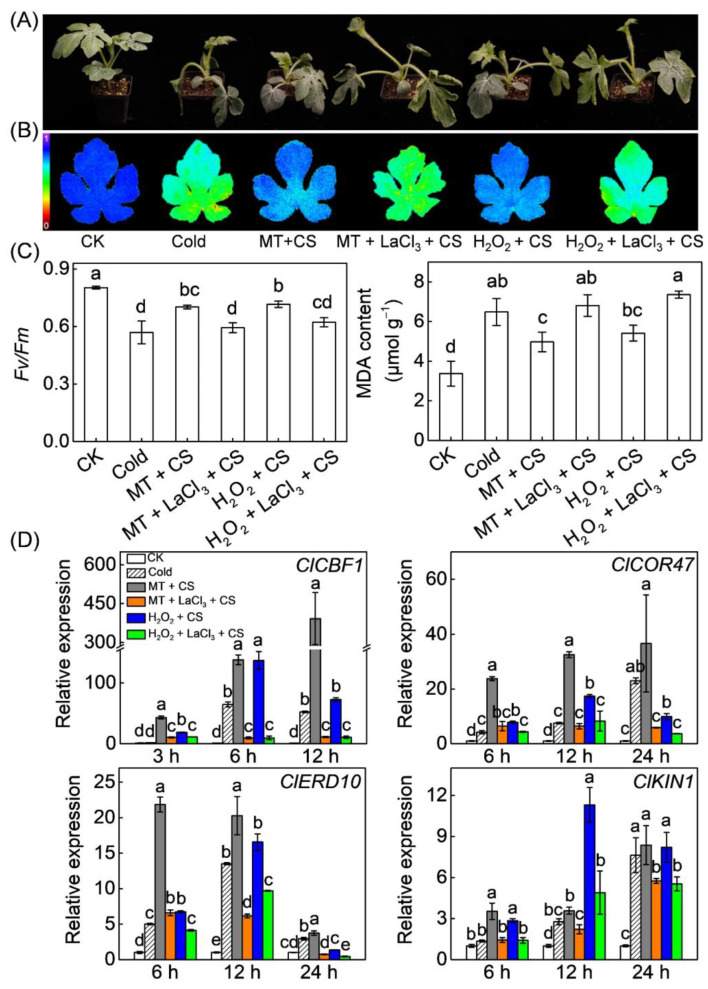
LaCl3 is an inhibitor of Ca2+ influx channels [50]. In this study, pretreatment with LaCl3 completely abolished melatonin- and H2O2-induced chilling tolerance, characterized by increased leaf wilting, reduced Fv/Fm, and high MDA content (Figure 3A–C). Moreover, application of H2O2 significantly upregulated the expression of ClCBF1 and its regulons, including ClCOR47, ClERD10, and ClKIN1 after chilling exposure (Figure 3D). Specifically, the expression of ClCBF1, ClCOR47, ClERD10, and ClKIN1 were upregulated 137.1, 7.8, 6.7, and 2.9 fold, respectively, in H2O2-pretreated plants, far higher than that (64.6, 4.2, 5.0, and 1.3 fold, respectively) in control plants, at 6 h after chilling exposure. However, LaCl3 pretreatment prevented the melatonin- or H2O2-induced upregulation of ClCBF1 and its regulons.
Figure 3.
The role of Ca2+ in melatonin- or H2O2-induced C-REPEAT BINDING FACTOR (CBF) pathway and chilling tolerance in watermelon. (A) Chilling phenotypes. (B) Images showing the maximum PSII quantum yield (Fv/Fm). The false-color code depicted at the left of the image ranges from 0 (black) to 1 (purple). (C) The average values of Fv/Fm and malondialdehyde (MDA) contents. (D) The expression of ClCBF1 and its regulons, including cold-responsive gene 47 (ClCOR47), early responsive to dehydration 10 (ClERD10), and cold induced gene 1 (ClKIN1). The plants were treated with melatonin (MT, 150 µM) or H2O2 (1 mM) for 12 h and then transferred to cold stress (CS) at 4 °C. To block Ca2+ influx, the plants were sprayed with 10 mM lanthanum chloride (LaCl3) 2 h before MT or H2O2 application. Seedlings sprayed with distilled water under temperatures of 28/18 °C (day/night) were set as control (CK). Relative expression of genes in CK plants was set as 1.0. Data show the means of three replicates ± standard deviation (SD). The different letters denote significant difference at p < 0.05 according to Turkey’s test.
3.3. Role of H2O2 in Ca2+ Signal-Induced Chilling Tolerance in Watermelon
To investigate the function of Ca2+ in melatonin-induced H2O2 accumulation in response to chilling stress, the effects of CaCl2 and LaCl3 on H2O2 accumulation and ClRBOHD expression were analyzed. Like melatonin, CaCl2 application accelerated the cold-induced upregulation of ClRBOHD and subsequent H2O2 burst (Figure 4). After chilling exposure, the levels of H2O2 and ClRBOHD transcripts in CaCl2-pretreated plants increased by 0.5 and 10.3 fold, respectively, at 6 h, while those in control plants were induced at 12 h. However, LaCl3 pretreatment completely blocked melatonin-induced H2O2 accumulation and ClRBOHD upregulation under chilling stress, suggesting that Ca2+ signal mediates melatonin-induced ClRBOHD expression and H2O2 accumulation in response to chilling stress.
Figure 4.
Ca2+ mediates melatonin-induced (A) H2O2 accumulation and (B) expression of respiratory burst oxidase homolog D (ClRBOHD) in watermelon leaves under chilling stress. The leaves of watermelon seedlings were sprayed with 150 µM melatonin (MT) or 20 mM CaCl2 for 12 h, and then were subjected to cold stress (CS) at 4 °C. To block Ca2+ influx, the leaves were pretreated with LaCl3 at 10 mM 2 h before melatonin application. Seedlings sprayed with distilled water under temperatures of 28/18 °C (day/night) were set as control (CK). Data show the means of three replicates ± standard deviation (SD). The different letters denote significant difference at p < 0.05 according to Turkey’s test.
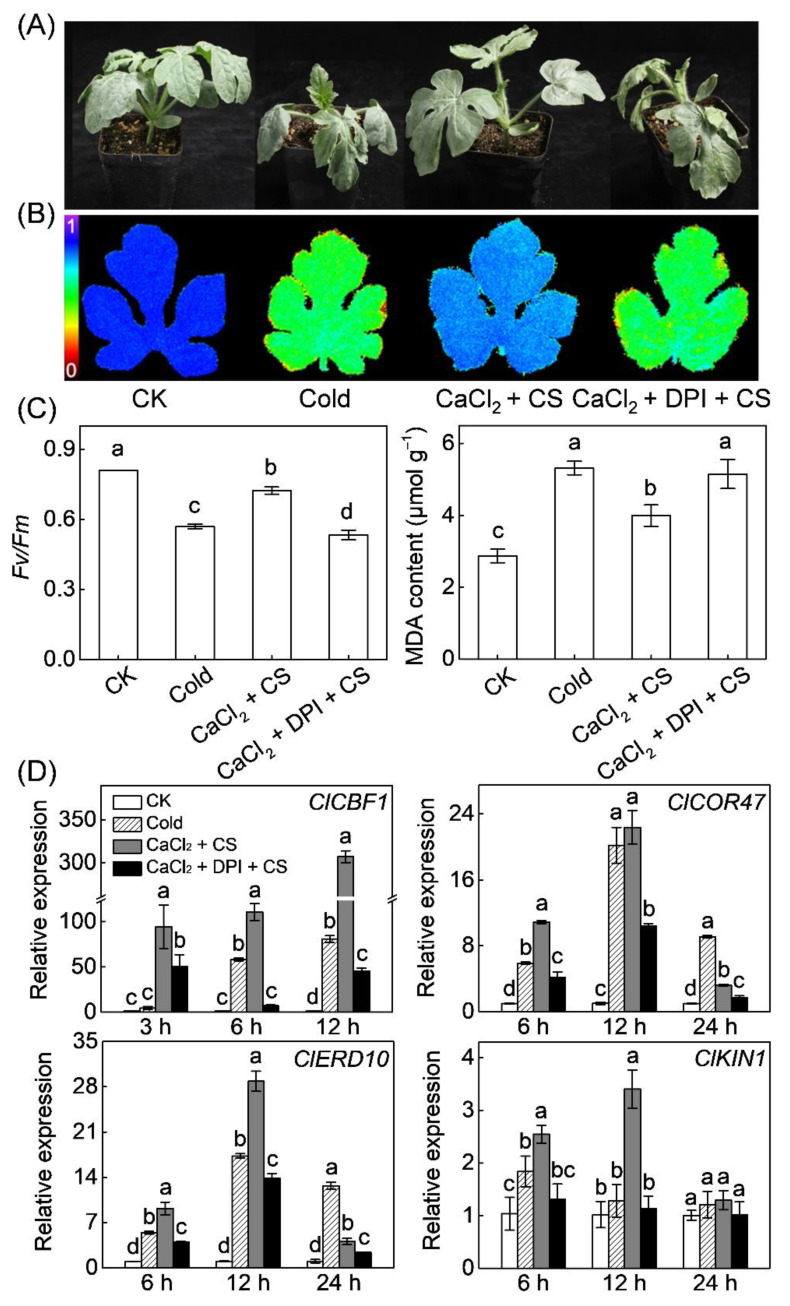
Like melatonin and H2O2, CaCl2 alleviated cold-induced leaf wilting, decreased Fv/Fm, and increased MDA content; however, these effects were abolished by DPI treatment (Figure 5A–C). Moreover, CaCl2 promoted cold-induced upregulation of CBF pathway genes, including ClCBF1, ClCOR47, ClERD10, and ClKIN1, but these effects were blocked by DPI treatment (Figure 5D). These findings suggest that H2O2 plays a vital function in Ca2+ signal-induced CBF-responsive pathway and subsequent chilling tolerance.
Figure 5.
The role of H2O2 in CaCl2-induced C-REPEAT BINDING FACTOR (CBF) pathway and chilling tolerance in watermelon. (A) Chilling phenotypes. (B) Images showing the maximum PSII quantum yield (Fv/Fm). The false-color code depicted at the left of the image ranges from 0 (black) to 1 (purple). (C) The average values of Fv/Fm and malondialdehyde (MDA) contents. (D) The expression of ClCBF1 and its regulons, including cold-responsive gene 47 (ClCOR47), early responsive to dehydration 10 (ClERD10), and cold induced gene 1 (ClKIN1). The plant leaves were sprayed with 20 mM CaCl2 for 12 h and then exposed to cold stress (CS) at 4 °C. To inhibit H2O2 production, the seedlings were pretreated with 100 µM DPI 2 h before CaCl2 application. Seedlings sprayed with distilled water under temperatures of 28/18 °C (day/night) were set as control (CK). Relative expression of genes in CK plants was set as 1.0. Data show the means of three replicates ± standard deviation (SD). The different letters denote significant difference at p < 0.05 according to Turkey’s test.
3.4. Involvement of RBOHD in Melatonin-Induced [Ca2+]cyt Accumulation and Freezing Tolerance in Arabidopsis
Phylogenetic analysis showed that watermelon ClRBOHD has high homology to Arabidopsis AtRBOHD with 79.3% similarity (Figure 6A). ClRBOHD and AtRBOHD contain the same critical conserved domains, including NADPH_Ox domain (PF08414), EFh (IPR002048), FAD_binding_8 (PF08022), and NAD_binding_6 (PF08030) (Figure 6B). The NADPH_Ox domain, which is found in respiratory burst NADPH oxidase proteins, shares 75.5% similarity to AtRBOHD and ClRBOHD. Thus, the Arabidopsis loss-of-function mutant Atrbohd was used to study the role of RBOHD in melatonin-induced [Ca2+]cyt accumulation and cold tolerance.
Figure 6.
(A) Phylogenetic analysis of ClRBOHD and 10 AtRBOH proteins and (B) protein sequence alignment and domain structure of ClRBOHD and AtRBOHD. In (B), the NADPH_Ox (PF08414) domain is shown in the red box. The EF-hand (IPR002048), FAD_binding_8 (PF08022), and NAD_binding_6 (PF08030) domains are underlined with solid, dashed, and dotted lines, respectively. RBOH, respiratory burst oxidase homolog; Cl, Citrullus lanatus; At, Arabidopsis thaliana.
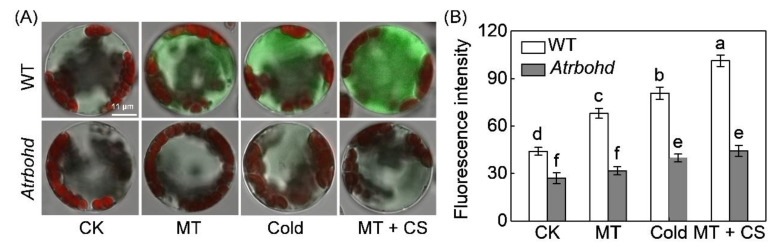
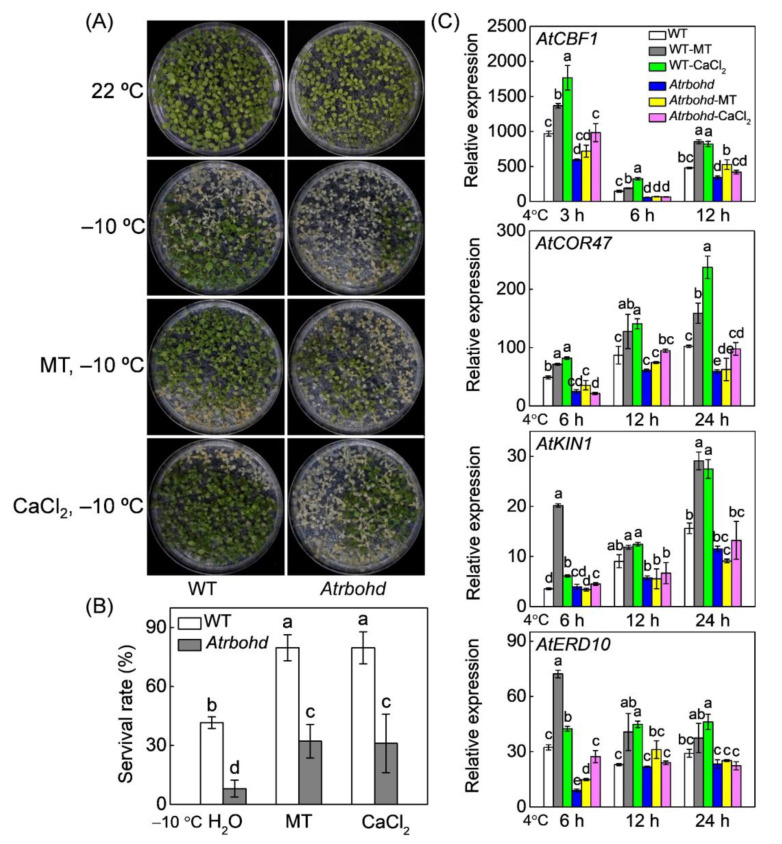
Atrbohd mutant protoplasts showed 38.6% and 50.8% less accumulation of [Ca2+]cyt than wild-type (WT) protoplasts under normal and chilling conditions, respectively (Figure 7). Similar to watermelon protoplasts, melatonin significantly induced [Ca2+]cyt accumulation in WT protoplasts under normal and chilling conditions. However, these effects were abolished by AtRBOHD mutation. Moreover, AtRBOHD knockout significantly increased Arabidopsis sensitivity to freezing at −10 °C and downregulated the expression of AtCBF1 and its targets, including AtCOR47, AtERD10, and AtKIN1 under chilling stress of 4 °C (Figure 8). After freezing exposure, the survival rate of Atrbohd mutant was 80.7% lower than the WT plants, suggesting that AtRBOHD plays an essential role in Arabidopsis response to freezing. Both melatonin and Ca2+ application dramatically induced the freezing tolerance and enhanced the expression of CBF pathway genes in WT Arabidopsis; however, such effects were attenuated in Atrbohd mutants. Overall, these results indicate AtRBOHD mediates melatonin-induced [Ca2+]cyt accumulation and melatonin/Ca2+-induced freezing tolerance.
Figure 7.
Respiratory burst oxidase homolog D (AtRBOHD) mediates melatonin-induced accumulation of cytoplasmic free Ca2+ ([Ca2+]cyt) in Arabidopsis response to cold stress. (A) Fluorescence images. (B) Fluorescence intensity. The protoplasts from leaves of wild-type (WT) Arabidopsis or Atrbohd mutant were incubated with Fluo-4 acetoxymethyl (AM) ester at 37 °C in the dark. After 30 min, the protoplasts were treated with melatonin (10 µM) and then placed at 4 °C for 5 min (cold stress, CS). Data in (B) are expressed as means ± standard deviation (SD, n ≥ 7). The different letters mean significant difference at p < 0.05 according to Turkey’s test. CK, control.
Figure 8.
Respiratory burst oxidase homolog D (AtRBOHD) mediates melatonin- or Ca2+-induce freezing tolerance in Arabidopsis. (A) Freezing phenotypes. (B) Survival rates. (C) The relative expression of C-repeat binding factor 1 (AtCBF1) and its regulons, including cold-responsive gene 47 (AtCOR47), early responsive to dehydration 10 (AtERD10), and cold induced gene 1 (AtKIN1) under cold stress at 4 °C. Three-week-old wild-type (WT) and Atrbohd mutant seedlings grown on the ½ MS medium were treated with 10 µM melatonin (MT) or 1 mM CaCl2 (Ca2+). After 12 h, the seedlings were subjected to freezing at −10 °C for 1 h and then recovered at 22 °C for 5 days or exposed to chilling at 4 °C for 24 h. Relative expression of genes in untreated WT plants was set as 1.0. Data show the means of three replicates ± standard deviation (SD). The different letters denote significant difference at p < 0.05 according to Turkey’s test.
4. Discussion
The CBF transcriptional regulatory cascade plays a central role in the regulation of cold stress response in plants. Consistent with the previous studies [25,27,34], melatonin application enhanced watermelon tolerance to chilling stress, and its action was closely correlated with the activated CBF-responsive pathway in this study (Figure 1). Furthermore, our results verified that the interaction between H2O2 and Ca2+ signals mediates melatonin-induced CBF-responsive pathway and subsequent cold tolerance.
4.1. RBOHD-Dependent H2O2 Is Required for Melatonin-Induced CBF-Responsive Pathway and Cold Tolerance
As an essential signaling molecule, RBOH-generated H2O2 plays a primary role in regulating plant response to various abiotic stimuli, such as cold stress [51,52,53]. For instance, cold acclimation enhanced RBOH1 transcript levels and apoplastic H2O2 accumulation in tomato, while RBOH1 silencing suppressed acclimation-induced cold tolerance [54]. Arabidopsis has 10 RBOH genes, AtRBOHA to AtRBOHJ. Among them, AtRBOHD encoded protein potentially regulates ROS-derived responses and plays a fundamental role in stress tolerance [51,55,56,57]. Plant RBOH genes are highly conserved [58]. Phylogenetic analysis and protein sequence alignment performed herein revealed that Arabidopsis AtRBOHD and watermelon ClRBOHD are homologous genes and their proteins share 79.3% similarity (Figure 6). Thus, we speculated that ClRBOHD might exhibit a similar function of producing H2O2 like AtRBOHD in Arabidopsis. In this study, chilling exposure induced H2O2 accumulation accompanied by ClRBOHD upregulation. Furthermore, exogenous application of H2O2 induced the expression of CBF-responsive pathway genes and chilling tolerance in watermelon. However, AtRBOHD knockout in Arabidopsis reduced the expression of CBF-responsive pathway genes and freezing tolerance. These results demonstrate that RBOHD-dependent H2O2 regulates the CBF-responsive pathway and cold tolerance in plants.
Melatonin plays primary functions in reducing ROS accumulation and alleviating stress-induced oxidative stress in plants [59]. Consistently, our results showed that melatonin alleviated chilling-induced H2O2 accumulation and lipid peroxidation after watermelon seedlings were exposed to 4 °C for 48 h (Figure 1). Many studies have shown that H2O2 plays an essential signaling role in melatonin-mediated regulation of various physiological processes, including stomatal closure, seed germination, lateral root formation, and response to environmental stimulus [19,29,60,61]. A recent report revealed that H2O2 mediates melatonin-induced cold tolerance in grafted watermelon plants [24]. In this study, melatonin induced H2O2 accumulation and ClRBOHD expression in early response (within 6 h) to chilling stress in watermelon (Figure 1A). Furthermore, exogenous application of H2O2 enhanced watermelon tolerance to chilling stress. However, DPI application in watermelon or AtRBOHD knockout in Arabidopsis inhibited H2O2 accumulation, suppressing the melatonin-induced expression of CBF pathway genes and subsequent cold stress tolerance (Figure 1, Figure 3 and Figure 8). These findings demonstrate that RBOHD-dependent H2O2 is required for melatonin-induced CBF-responsive pathway and the subsequent cold stress tolerance.
4.2. Ca2+ Mediates Melatonin-Induced CBF-Responsive Pathway and Cold Tolerance
Like H2O2, Ca2+, as an important second messenger, plays a critical role in regulation of responses to various abiotic stimulus in plants [62]. Low temperature induces a rapid and transient Ca2+ influx in plant cells by activating Ca2+ channels, such as cyclic nucleotide-gated ion channels (CNGCs) [8,63,64]. Various Ca2+ sensors decode the Ca2+ signal evoked by Ca2+ transient changes, subsequently regulating the expression of CBFs and cold responsive (COR) genes [8,65]. Several studies have confirmed that Ca2+ signaling is involved in melatonin-induced stomatal closure, seed germination, and salt tolerance in plants [19,29,66]. However, the relationship between Ca2+ and melatonin in response to cold stress remains unclear. In this study, melatonin promoted [Ca2+]cyt accumulation under cold stress, accompanied by ClCNGC2 upregulation (Figure 2). Arabidopsis CNGC2, a homologous gene of ClCNGC2, plays a critical role in contributing to Ca2+ entry into cytosol [48,49]. Here, exogenous CaCl2 induced the expression of CBF-responsive pathway genes and chilling tolerance in both watermelon and Arabidopsis. Meanwhile, blocking Ca2+ influx channels by LaCl3 counteracted melatonin-induced expression of CBF-responsive pathway genes and chilling tolerance in watermelon (Figure 3 and Figure 8). These results reveal that Ca2+ signaling is essential for melatonin-mediated CBF-responsive pathway and subsequent cold tolerance.
4.3. H2O2 and Ca2+ Function Together in a Self-Amplifying Feedback Loop in Melatonin-Induced CBF-Responsive Pathway and Cold Tolerance
As two crucial signal molecules, the interactions between H2O2 and Ca2+ signal have been well documented in various physiological actions, especially in defense against abiotic stressors [53,67]. For instance, stress-triggered Ca2+ signal can phosphorylate and activate RBOHD via Ca2+-dependent protein kinase to generate H2O2, which in turn elicits a Ca2+ signal through receptor-like kinases, such as GUARD CELL HYDROGEN PEROXIDE RESISTANT1, forming a self-propagating mutual activation loop between Ca2+ and H2O2 signals [62,67]. Consistent with the previous findings, we found that H2O2 and Ca2+ interact, forming a reciprocal positive-regulatory loop that mediates melatonin-induced CBF pathway and cold tolerance (Figure 9). The following evidence supports this conclusion: (1) H2O2 promoted [Ca2+]cyt accumulation, while H2O2 deficiency in watermelon and Arabidopsis prevented melatonin-induced [Ca2+]cyt accumulation in response to cold stress (Figure 2 and Figure 7); (2) CaCl2 stimulated H2O2 accumulation and upregulated ClRBOHD expression, while the blocking of Ca2+ influx by LaCl3 inhibited melatonin-induced increases in H2O2 accumulation and ClRBOHD transcripts (Figure 4); (3) LaCl3 counteracted H2O2-induced expression of CBF-responsive pathway genes and chilling tolerance in watermelon (Figure 3); (4) DPI treatment in watermelon or AtRBOHD knockout in Arabidopsis prevented Ca2+-induced expression of CBF pathway genes and cold tolerance (Figure 5 and Figure 8).
Figure 9.
Schematic illustration of the proposed model of melatonin action on cold stress tolerance. During the early response to cold stress, melatonin increases the accumulation of H2O2 and cytoplasmic free Ca2+ ([Ca2+]cyt) by upregulating the expressions of respiratory burst oxidase homolog (RBOH) D and cyclic nucleotide-gated channel (CNGC) 2, respectively. Increased H2O2 further promotes the accumulation of [Ca2+]cyt, which in turn elevates H2O2 accumulation, forming a reciprocal positive-regulatory loop that mediates melatonin-induced CBF pathway and subsequent cold tolerance.
5. Conclusions
At present, the signaling mechanisms underlying melatonin-induced cold tolerance in watermelon are still elusive. This study reveals an intricate signaling cascade of melatonin-induced cold stress tolerance in watermelon. Melatonin induces H2O2 accumulation by upregulating ClRBOHD expression in early response to cold stress. Increased H2O2 induces ClCNGC2 expression and [Ca2+]cyt accumulation, which boosts H2O2 accumulation by triggering the ClRBOHD expression, forming a reciprocal positive-regulatory loop that induces the expression of CBF pathway genes and subsequent cold tolerance. This is the first study to investigate the interplay between H2O2 and Ca2+ signaling in melatonin-mediated cold tolerance to the best of our knowledge. However, other components of melatonin signaling in plant response to cold stress need to be further explored in the future.
Abbreviations
CBF, c-repeat binding factor; COR, cold-responsive gene; ERD10, early responsive to dehydration 10; KIN1, cold induced gene 1; H2O2, hydrogen peroxide; RBOH, respiratory burst oxidase homolog; DPI, diphenyleneiodonium; Ca2+, calcium ion; CNGC, cyclic nucleotide-gated ion channel; LaCl3, lanthanum chloride; Fv/Fm, maximum photosystem II quantum yield; REC, relative electrical conductivity; MDA, malondialdehyde; CK, control; MT, melatonin; CS, cold stress; WT, wild-type.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antiox10091457/s1, Table S1: Gene-specific primers used for qRT-PCR analysis.
Author Contributions
Conceptualization, H.L. and X.Z.; data curation, J.C., H.L. and Y.G.; formal analysis, J.C. and H.L.; funding acquisition, X.Z. and H.L.; investigation, J.C., Y.G., J.L., Z.S., C.W. (Chunxia Wang), R.Z. and C.W. (Chunhua Wei); project administration, H.L.; resources, J.M. and X.Z.; supervision, X.Z.; writing—original draft, J.C. and H.L.; writing—review and editing, H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China, grant number 2018YFD1000800; the National Natural Science Foundation of China, grant number 31801884 and 31972479; the China Agriculture Research System of MOF and MARA, grant number CARS-25; the funding for Tang Scholar of Northwest A&F University, grant number A1190021003.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All datasets generated for this study are included in the article or Supplementary File.
Conflicts of Interest
The authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Allen D.J., Ort D.R. Impacts of chilling temperatures on photosynthesis in warm-climate plants. Trends Plant Sci. 2001;6:36–42. doi: 10.1016/S1360-1385(00)01808-2. [DOI] [PubMed] [Google Scholar]
- 2.Guo X., Liu D., Kang C. Cold signaling in plants: Insights into mechanisms and regulation. J. Int. Plant Biol. 2018;9:745–756. doi: 10.1111/jipb.12706. [DOI] [PubMed] [Google Scholar]
- 3.Bates M.D., Robinson R.W. Cucumber, melon and watermelon. In: Smart J., Simmonds N.W., editors. Evolution of Crop Plants. Longman Scientific & Tech; Essex, UK: 1995. pp. 89–97. [Google Scholar]
- 4.Korkmaz A., Dufault R.J. Developmental consequences of cold temperature stress at transplanting on seedling and field growth and yield. I. watermelon. J. Am. Soc. Hort. Sci. 2001;126:404–409. doi: 10.21273/JASHS.126.4.404. [DOI] [Google Scholar]
- 5.Sheikh S., Noh J., Seong M.H., Jung G.T., Kim J.M. Consequences of chilling stress on watermelon [Citrullus lanatus (Thunb.) Matsum. and Nakai] germplasm lines at seedling stage. Hort. Environ. Biotechnol. 2015;56:79–88. doi: 10.1007/s13580-015-0174-2. [DOI] [Google Scholar]
- 6.Hou W., Sun A.H., Chen H.L., Yang F.S., Pan J.L., Guan M.Y. Effects of chilling and high temperatures on photosynthesis and chlorophyll fluorescence in leaves of watermelon seedlings. Biol. Plant. 2016;60:148–154. doi: 10.1007/s10535-015-0575-1. [DOI] [Google Scholar]
- 7.Pareek A., Khurana A., Sharma A.K., Kumar R. An overview of signaling regulons during cold stress tolerance in plants. Curr. Genom. 2017;18:498–511. doi: 10.2174/1389202918666170228141345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yuan P., Yang T., Poovaiah B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018;19:3896. doi: 10.3390/ijms19123896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J.Y., Shi Y.T., Yang S.H. Insights into the regulation of C-repeat binding factors in plant cold signaling. J. Int. Plant Biol. 2018;60:780–795. doi: 10.1111/jipb.12657. [DOI] [PubMed] [Google Scholar]
- 10.Jaglo-Ottosen K.R., Gilmour S.J., Zarka D.G., Schabenberger O., Thomashow M.F. Arabidopsis CBF1 overexpression induces COR genes and enhances freezing tolerance. Science. 1998;280:104–106. doi: 10.1126/science.280.5360.104. [DOI] [PubMed] [Google Scholar]
- 11.Jia Y.X., Ding Y.L., Shi Y.L., Zhang X.Y., Gong Z.Z., Yang S.H. The cbfs triple mutants reveal the essential functions of CBFs in cold acclimation and allow the definition of CBF regulons in Arabidopsis. New Phytol. 2016;212:345–353. doi: 10.1111/nph.14088. [DOI] [PubMed] [Google Scholar]
- 12.Jaglo K.R., Kleff S., Amundsen K.L., Zhang X., Haake V., Zhang J.Z., Deits T., Thomashow M.F. Components of the Arabidopsis C-repeat/dehydration-responsive element binding factor cold-response pathway are conserved in Brassica napus and other plant species. Plant Physiol. 2001;127:910–917. doi: 10.1104/pp.010548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena I., Srikanth S., Chen Z. Cross talk between H2O2 and interacting signal molecules under plant stress response. Front. Plant Sci. 2016;7:570. doi: 10.3389/fpls.2016.00570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang P., Wang Y., Wang M., Wang F., Chi C., Zhou Y., Zhou J., Shi K., Xia X., Foyer C.H., et al. Crosstalk between brassinosteroid and redox signaling contributes to the activation of cbf expression during cold responses in tomato. Antioxidants. 2021;10:509. doi: 10.3390/antiox10040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Drerup M.M., Schlücking K., Hashimoto K., Manishankar P., Steinhorst L., Kuchitsu K., Kudl J. The Calcineurin B-Like Calcium Sensors CBL1 and CBL9 together with their interacting protein kinase CIPK26 regulate the Arabidopsis NADPH oxidase RBOHF. Mol. Plant. 2013;6:559–569. doi: 10.1093/mp/sst009. [DOI] [PubMed] [Google Scholar]
- 16.Baxter A., Mittler R., Suzuki N. ROS as key players in plant stress signalling. J. Exp. Bot. 2013;65:1229–1240. doi: 10.1093/jxb/ert375. [DOI] [PubMed] [Google Scholar]
- 17.Kawarazaki T., Kimura S., Iizuka A., Hanamata S., Nibori H., Michikawa M., Imai A., Abe M., Kaya H., Kuchitsu K. A low temperature-inducible protein AtSRC2 enhances the ROS-producing activity of NADPH oxidase AtRbohF. Biochim. Biophys. Acta. 2013;1833:2775–2780. doi: 10.1016/j.bbamcr.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y., Reiter R.J., Chan Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018;69:963–974. doi: 10.1093/jxb/erx473. [DOI] [PubMed] [Google Scholar]
- 19.Wei J., Li D.X., Zhang J.R., Shan C., Rengel Z., Song Z.B., Chen Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018;65:e12500. doi: 10.1111/jpi.12500. [DOI] [PubMed] [Google Scholar]
- 20.Arnao M.B., Hernández-Ruiz J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2018;24:38–48. doi: 10.1016/j.tplants.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y.P., Xu S., Yang J., Chen Y.Y. Melatonin alleviates cold-induced oxidative damage by regulation of ascorbate–glutathione and proline metabolism in melon seedlings (Cucumis melo L.) J. Hortic. Sci. Biotechnol. 2017;92:313–324. doi: 10.1080/14620316.2016.1266915. [DOI] [Google Scholar]
- 22.Li H., Chang J., Zheng J.X., Dong Y.C., Liu Q.Y., Yang X.Z., Wei C., Zhang Y., Ma J., Zhang X. Local melatonin application induces cold tolerance in distant organs of Citrullus lanatus L. via long distance transport. Sci. Rep. 2017;7:40858. doi: 10.1038/srep40858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang X.L., Xu H., Li D., Gao X., Li T.L., Wang R. Effect of melatonin priming on photosynthetic capacity of tomato leaves under low-temperature stress. Photosynthetica. 2018;56:884–892. doi: 10.1007/s11099-017-0748-6. [DOI] [Google Scholar]
- 24.Li H., Guo Y., Lan Z., Xu K., Chang J., Ahammed G.J., Ma J., Wei C., Zhang X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021;8:57. doi: 10.1038/s41438-021-00496-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H., Qian Y., Tan D.X., Reiter R.J., He C. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015;59:334–342. doi: 10.1111/jpi.12262. [DOI] [PubMed] [Google Scholar]
- 26.Ding F., Liu B., Zhang S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017;219:264–271. doi: 10.1016/j.scienta.2017.03.029. [DOI] [Google Scholar]
- 27.Chang J., Guo Y., Zhang Z., Wei C., Zhang Y., Ma J., Yang J., Zhang X., Li H. CBF-responsive pathway and phytohormones are involved in melatonin-improved photosynthesis and redox homeostasis under aerial cold stress in watermelon. Acta Physiol. Plant. 2020;42:159. doi: 10.1007/s11738-020-03147-4. [DOI] [Google Scholar]
- 28.Fu J., Wu Y., Miao Y., Xu Y., Zhao E., Wang J., Sun H., Liu Q., Xue Y., Xu Y., et al. Improved cold tolerance in Elymus nutans by exogenous application of melatonin may involve ABA-dependent and ABA-independent pathways. Sci. Rep. 2017;7:e39865. doi: 10.1038/srep39865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li H., Guo Y., Lan Z., Zhang Z., Ahammed G.J., Chang J., Zhang Y., Wei C., Zhang X. Melatonin antagonizes ABA action to promote seed germination by regulating Ca2+ efflux and H2O2 accumulation. Plant Sci. 2021;303:110761. doi: 10.1016/j.plantsci.2020.110761. [DOI] [PubMed] [Google Scholar]
- 30.Carvajal M., Cerd A., Martínez V. Does calcium ameliorate the negative effect of NaCl on melon root water transport by regulating aquaporin activity? New Phytol. 2010;145:439–447. doi: 10.1046/j.1469-8137.2000.00593.x. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y.F., Zhang G.X., Qi M.F., Li T.L. Effects of calcium on photosynthesis, antioxidant system, and chloroplast ultrastructure in tomato leaves under low night temperature stress. J. Plant Growth Regul. 2015;34:263–273. doi: 10.1007/s00344-014-9462-9. [DOI] [Google Scholar]
- 32.Li H., He J., Yang X., Li X., Luo D., Wei C., Ma J., Zhang Y., Yang J., Zhang X. Glutathione-dependent induction of local and systemic defense against oxidative stress by exogenous melatonin in cucumber (Cucumis sativus L.) J. Pineal Res. 2016;60:206–216. doi: 10.1111/jpi.12304. [DOI] [PubMed] [Google Scholar]
- 33.Yan C., Fan M., Yang M., Zhao J., Zhang W., Su Y., Xiao L., Deng H., Xie X. Injury activates Ca2+/Calmodulin-dependent phosphorylation of JAV1-JAZ8-WRKY51 complex for jasmonate biosynthesis. Mol. Cell. 2018;70:136–149. doi: 10.1016/j.molcel.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Bajwa V.S., Shukla M.R., Sherif S.M., Murch S.J., Saxena P. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014;56:238–245. doi: 10.1111/jpi.12115. [DOI] [PubMed] [Google Scholar]
- 35.Qin Y.Z., Li X., Guo M., Deng K.Q., Lin J.Z., Tang D.Y., Guo X.H., Liu X.M. Regulation of salt and ABA responses by CIPK14, a calcium sensor interacting protein kinase in Arabidopsis. Sci. China. 2008;5:391–401. doi: 10.1007/s11427-008-0059-z. [DOI] [PubMed] [Google Scholar]
- 36.Xie Y., Chen P., Yan Y., Bao C., Li X., Wang L., Shen X., Li H., Liu X., Niu C., et al. An atypical R2R3 MYB transcription factor increases cold hardiness by CBF-dependent and CBF-independent pathways in apple. New Phytol. 2017;218:201–218. doi: 10.1111/nph.14952. [DOI] [PubMed] [Google Scholar]
- 37.Baker N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo, Neil R. Annu. Rev. Plant Biol. 2008;59:89–113. doi: 10.1146/annurev.arplant.59.032607.092759. [DOI] [PubMed] [Google Scholar]
- 38.Zhou W.J., Leul M. Uniconazole-induced alleviation of freezing injury in relation to changes in hormonal balance, enzyme activities and lipid peroxidation in winter rape. J. Plant Growth Regul. 1998;26:4–47. [Google Scholar]
- 39.Hodges D.M., DeLong J.M., Forney C.F., Prange R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta. 1999;207:604–611. doi: 10.1007/s004250050524. [DOI] [PubMed] [Google Scholar]
- 40.Willekens H., Chamnongpol S., Davey M., Schraudner M., Langebartels C., Montagu M.V., Inzé D., Camp W.V. Catalase is a sink for H2O2 and is indispensable for stress defence in C3 plants. EMBO J. 1997;16:4806–4816. doi: 10.1093/emboj/16.16.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 42.Tian S., Jiang L., Gao Q., Zhang J., Zong M., Zhang H., Ren Y., Guo S., Gong G., Liu F., et al. Efficient CRISPR/Cas9-based gene knockout in watermelon. Plant Cell Rep. 2016;36:399–406. doi: 10.1007/s00299-016-2089-5. [DOI] [PubMed] [Google Scholar]
- 43.Wang J., Ren Y., Liu X., Luo S., Zhang X., Liu X., Lin Q., Zhu S., Wan H., Yang Y., et al. Transcriptional activation and phosphorylation of OsCNGC9 confer enhanced chilling tolerance in rice. Mol. Plant. 2020;14:315–329. doi: 10.1016/j.molp.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 44.Kong Q.S., Yuan J., Gao L., Zhao S., Jiang W., Huang Y., Bie Z. Identification of suitable reference genes for gene expression normalization in qRT-PCR analysis in watermelon. PLoS ONE. 2014;9:e90612. doi: 10.1371/journal.pone.0090612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 46.Larkin M.A., Blackshields G., Brown N.P., Chenna R.M., Higgins D.G. ClustalW and ClustalX version 2. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chin K., DeFalco T.A., Moeder W., Yoshioka K. The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol. 2013;163:611–624. doi: 10.1104/pp.113.225680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lu M., Zhang Y., Tang S., Pan J., Yu Y., Han J., Li Y., Du X., Nan Z., Sun Q. AtCNGC2 is involved in jasmonic acid-induced calcium mobilization. J. Exp. Bot. 2015;67:809–819. doi: 10.1093/jxb/erv500. [DOI] [PubMed] [Google Scholar]
- 50.White P.J. Calcium channels in higher plants. Biochim. Biophys. Acta. 2000;1465:171–189. doi: 10.1016/S0005-2736(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 51.Miller G., Schlauch K., Tam R., Cortes D., Torres M.A., Shulaev V., Dangl J.L., Mittler R. The plant NADPH oxidase RBOHD mediates rapid systemic signaling in response to diverse stimuli. Sci. Signal. 2009;2:ra45. doi: 10.1126/scisignal.2000448. [DOI] [PubMed] [Google Scholar]
- 52.Zhou J., Wang J., Shi K., Xia X.J., Zhou Y.H., Yu J.Q. Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol. Biochem. 2012;60:141–149. doi: 10.1016/j.plaphy.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 53.Mohanta T.K., Bashir T., Hashem A., Abd_Allah E.F., Khan A.L., Al-Harrasi A.S. Early events in plant abiotic stress signaling: Interplay between calcium, reactive oxygen species and phytohormones. J. Plant Growth Regul. 2018;37:1033–1049. doi: 10.1007/s00344-018-9833-8. [DOI] [Google Scholar]
- 54.Zhou J., Xia X.J., Zhou Y.H., Shi K., Chen Z., Yu J.Q. RBOH1-dependent H2O2 production and subsequent activation of MPK1/2 play an important role in acclimation-induced cross-tolerance in tomato. J. Exp. Bot. 2014;65:595–607. doi: 10.1093/jxb/ert404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma L., Zhang H., Sun L., Jiao Y., Zhang G., Miao C., Hao F. NADPH oxidase AtrbohD and AtrbohF function in ROS-dependent regulation of Na+/K+ homeostasis in Arabidopsis under salt stress. J. Exp. Bot. 2012;63:305–317. doi: 10.1093/jxb/err280. [DOI] [PubMed] [Google Scholar]
- 56.Marino D., Dunand C., Puppo A., Pauly N. A burst of plant NADPH oxidases. Trends Plant Sci. 2012;17:9–15. doi: 10.1016/j.tplants.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 57.Sun L., Ma L., He S., Hao F. AtrbohD functions downstream of ROP2 and positively regulates waterlogging response in Arabidopsis. Plant Signal Behav. 2018;13:e1513300. doi: 10.1080/15592324.2018.1513300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cheng C., Xu X., Gao M., Li J., Guo C., Song J., Wang X. Genome-wide analysis of respiratory burst oxidase homologs in grape (Vitis vinifera L.) Int. J. Mol. Sci. 2013;14:24169–24186. doi: 10.3390/ijms141224169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang H.M., Zhang Y.Q. Melatonin: A well-documented antioxidant with conditional pro-oxidant actions. J. Pineal Res. 2014;57:131–146. doi: 10.1111/jpi.12162. [DOI] [PubMed] [Google Scholar]
- 60.Gong B., Yan Y., Wen D., Shi Q. Hydrogen peroxide produced by NADPH oxidase: A novel downstream signaling pathway in melatonin-induced stress tolerance in Solanum lycopersicum. Physiol. Plant. 2017;160:396–409. doi: 10.1111/ppl.12581. [DOI] [PubMed] [Google Scholar]
- 61.Chen Z., Gu Q., Yu X., Huang L., Xu S., Wang R., Shen W., Shen W. Hydrogen peroxide acts downstream of melatonin to induce lateral root formation. Ann. Bot. 2018;121:1127–1136. doi: 10.1093/aob/mcx207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeon J., Kim J. Cold stress signaling networks in Arabidopsis. J. Plant Biol. 2013;56:69–76. doi: 10.1007/s12374-013-0903-y. [DOI] [Google Scholar]
- 64.Cui Y., Lu S., Li Z., Cheng J., Hu P., Zhu T., Wang X., Jin M., Wang X., Li L., et al. CYCLIC NUCLEOTIDE-GATED ION CHANNELs 14 and 16 promote tolerance to heat and chilling in rice. Plant Physiol. 2020;183:1794–1808. doi: 10.1104/pp.20.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Miura K., Furumoto T. Cold signaling and cold response in plants. Int. J. Mol. Sci. 2013;14:5312–5337. doi: 10.3390/ijms14035312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vafadar F., Amooaghaie R., Ehsanzadeh P., Ghanati F., Sajedi R.H. Crosstalk between melatonin and Ca2+/CaM evokes systemic salt tolerance in Dracocephalum kotschyi. J. Plant Physiol. 2020;252:153237. doi: 10.1016/j.jplph.2020.153237. [DOI] [PubMed] [Google Scholar]
- 67.Pottosin I., Zepeda-Jazo I. Powering the plasma membrane Ca2+-ROS self-amplifying loop. J. Exp. Bot. 2018;69:3317–3320. doi: 10.1093/jxb/ery179. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the article or Supplementary File.