Abstract
Chitosan begins its humble journey from marine food shell wastes and ends up as a versatile nutraceutical. This review focuses on briefly discussing the antioxidant activity of chitosan and retrospecting the accomplishments of chitosan nanoparticles as an anticarcinogen. The various modified/functionalized/encapsulated chitosan nanoparticles and nanoforms have been listed and their biomedical deliverables presented. The anticancer accomplishments of chitosan and its modified composites have been reviewed and presented. The future of surface modified chitosan and the lacunae in the current research focus have been discussed as future perspective. This review puts forth the urge to expand the scientific curiosity towards attempting a variety of functionalization and surface modifications to chitosan. There are few well known modifications and functionalization that benefit biomedical applications that have been proven for other systems. Being a biodegradable, biocompatible polymer, chitosan-based nanomaterials are an attractive option for medical applications. Therefore, maximizing expansion of its bioactive properties are explored. The need for applying the ideal functionalization that will significantly promote the anticancer contributions of chitosan nanomaterials has also been stressed.
Keywords: chitosan, nanochitosan, sea food waste, biomedical applications, functionalization, surface modifications
1. Introduction
The biopolysaccharide chitosan is recovered from chitin through the deacetylation process. Chitin, the primary structural polymer occurring in the exoskeletons of crustaceans, mollusks, and insects [1,2,3,4], is second to cellulose, which is the first naturally abundant polysaccharide [1]. Chitosan is derived from chitin following alkaline deacetylation, leading to a structure having 2-amino-2-dedoxy-d-glucose and 2-acetamino-dedoxy-d-glucose units linked by β-(1→4) bonds [1,2]. Amino groups are the principal functional groups of chitosan. The degree of deacetylation (DDA) is opposed to the degree of acetylation (DA), DA = 100-DDA or DDA = 100-DA. DA is also very important for solubility and in separating the terms chitin and chitosan: if DA > 50%, the biopolymer is chitin-like, if DA < 50%, the biopolymer is chitosan-like
Generally, chitosan has three types of reactive functional groups [5]. Chitosan has an amino group at the C2 position of each deacetylated unit and hydroxyl groups at the C6 and C3 positions. These play a significant role while modifying chitosan for enhancing its biological activity. The amine at the C-2″ position is the one responsible for the biological activity of chitosan [6]. Chitosan has received significant attention for accomplishing several biological activities. The degree of deacetylation (DDA) has an important say on the bioactivity of chitosan [3,4,7,8,9,10].
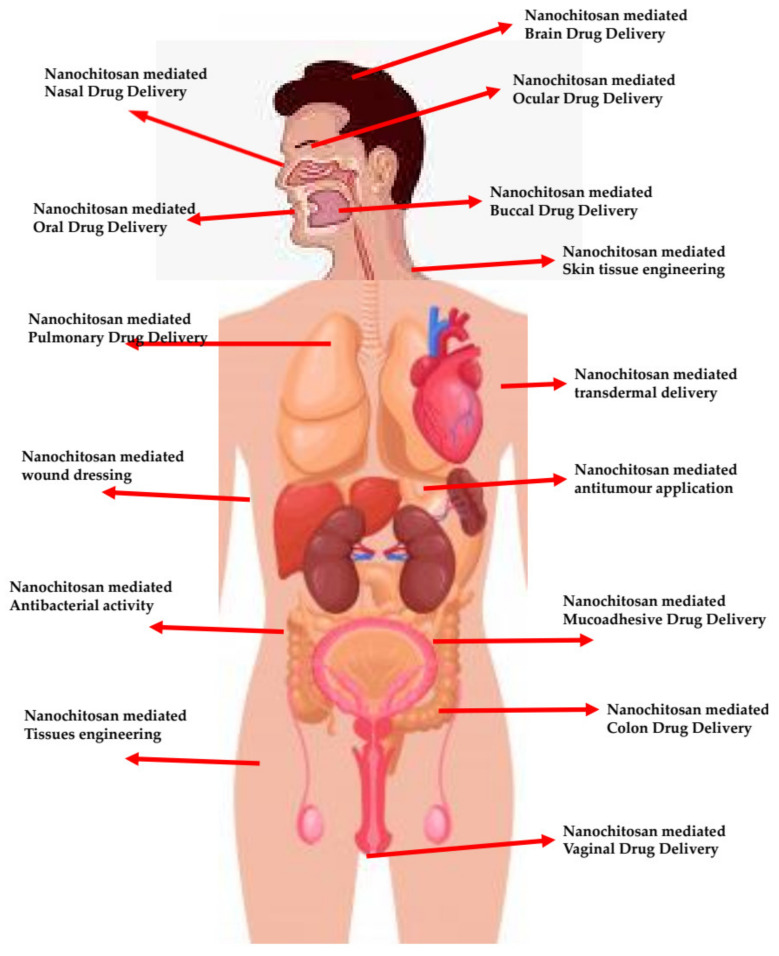
Chitosan and its derivatives have been extensively applied into medical and pharmaceutical applications (Figure 1). This is because of their highly competitive biological properties that include: biocompatibility, biodegradability, hypocholesterolemic, antimicrobial, nontoxic and antitumor, analgesic, hemostatic, and antioxidant properties [11,12]. These properties promote chitosan as an ideal candidate for biomedical applications, wound healing, tissue engineering, and for drug and gene delivery [13,14,15,16,17]. Chitosan morphologies include: films, gels, membranes, nanoparticles, coatings, suspensions, and hydrogels. Each of these unique morphologies are capable of influencing their biomedical activities and properties [18].
Figure 1.
Overview of chitosan nanomaterial-based biomedical applications.
Because of its status ‘generally recognition as safe’ (GRAS) and its excellent biodegradability, chitosan has been extensively employed for the encapsulation of bioactive compounds [19]. The ability of chitosan towards the loading and delivery of sensitive bioactive compounds [20,21], polyphenolic compounds [19,22], and vitamins [23] has been well documented. The functional amino groups and their flexibility to modifications enhance their mechanical and physical properties. Because of chitosan’s high molecular weight, it shows limited bioavailability. Depolymerization by hydrolysis of polymer chains helps acquire low molecular oligomers of chitosan [24]. The enzymatic (using lysosome, chitinase, pectinase, cellulose) degradation of chitosan is also gaining attention [23,25]. Pepsin, papain, pronase [26,27], hepatopancreas [28], and chitosanase [25] can yield chitosan of low molecular weight [3].
Aranaz et al. reported that when the DDA increases, chitosan’s solubility also increases and biological interactions also increase [3]. The cationic characteristic of chitosan promotes its interaction with proteins, phospholipids, therapeutic DNA or RNA, bile, fatty acids, and anionic polyelectrolytes [11,13,14,29]. The high viscosity and low solubility of chitosan limits its biological applications [18].
The following review summarizes the antioxidant milestones reached through engaging chitosan for biomedical applications. The importance of nanochitosan and its allies, ideally in areas of biomedical applications, has been extensively reported. The reports on the antioxidant activity of chitosan have been briefly discussed and duly acknowledged. Surface modification of nanochitosan and specific functionalization that has aided in attaining significant medical milestones have been discussed. The need for furtherance of more such nanochitosan modifications and their prospective candidature in expanding its bio-applicability has been speculated and discussed as a future perspective.
2. Antioxidant Activity of Chitosan
Much research work has been directed in the area of free radical reactions, since they have been held responsible for several specific human diseases. Reactive oxygen species (ROS) are formed within the human body, in the process of normal metabolism. ROS are highly interactive with biomolecules and they oxidize lipids, proteins, carbohydrates, and DNA, ultimately leading to oxidative stress [2]. Enzymes such as catalase, superoxide dismutase and glutathione peroxidase are the innate cellular defense systems available against ROS-mediated cellular injury [30]. On excessive generation of ROS, the defense mechanism becomes inadequate and leads to oxidative stress. Oxidative stress is now associated with hypertension, dyslipidemia aging, rheumatoid arthritis, cancer, myocardial infraction, atherosclerosis, heart failure, angina pectoris, wrinkle formation, inflammation, and neurodegenerative diseases such as Alzheimer, Parkinson, and amyotrophic lateral sclerosis [31,32,33,34].
As a remedy to the ROS issue, the search for antioxidants intensified. It is in this context, that the antioxidant activity of chitosan has become rather attractive. Chitosan is an attractive option since it is a biopolymer and it is economical, biodegradable, and versatile. Chitosan has demonstrated notable scavenging activity against different radical species. Xie et al. proposed several theories on the scavenging activity of chitosan and its derivatives against free radicals [35]: (i) The hydroxyl groups in chitosan react with hydroxyl radicals via H-abstraction reaction. (ii) OH reacts with residual-free amino NH2 to form stable macromolecules radicals. (iii) The NH2 groups absorb H+ from the solution to form ammonium groups and react with OH through other addition reactions. These are the plausible mechanisms deciphered for the antioxidant strategies of chitosan [35].
The ROS scavenging capacity of chitosan largely rests on the DDA and MW of chitosan [3]. Chitin being insoluble in water is limited from being used as an antioxidant agent. The NH2 groups of chitosan deal with the scavenging activity and they can be protonated in an acidic solution. Samar et al. assessed the antioxidant activity of different DDA and MW chitosan samples. They concluded that higher DDA and lower MW are characteristic patterns for higher antioxidant activity [9]. Hajji et al. studied antioxidant activity of chitosan from crab (Carcinus mediterraneus) shells (DDA: 83%), Tunisian marine sources shrimp (Penaeus kerathurus) waste (DDA: 88%), and cuttlefish (Sepia officinalis) bones (DDA: 95%) [36]. Chitosan from cuttlefish showed highest antioxidant potential. Kim and Thomas proved that higher antioxidant activity was obtained with low MW chitosan (30 kDa) [37].
Sun et al. evaluated the scavenging capacity of chitosan oligomers with different MW, against hydroxyl radicals and superoxide anion [38]. In both superoxide anion and hydroxyl radical, lower MW worked well. Chang et al. reported antioxidant activity of chitosan against hydrogen peroxide, 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, and chelating ferrous ion [39]. The results confirmed that low MW chitosan (~2.2 kDa) has the highest impact. Li et al. confirmed that the MW of chitosan and its concentration are major attributes [40].
Although the antioxidant activity of chitosan is strongly proven, the lack of an H-atom donor to serve as a good chain-breaking antioxidant is a key limitation [41]. The scavenging capacity of free radicals is correlated with the O–H or N–H bond dissociation energy and the stability of the radicals. Because the intramolecular and intermolecular hydrogen bonds in chitosan molecules are strong, the OH and NH2 groups are hard to dissociate [35]. This is the reason why various surface modifications and functionalization of chitosan were sought after. Chitosan molecules were modified by grafting functional groups into their molecular structure. We will briefly discuss the existing modifications of chitosan, for its antioxidant activity as well as for serving enhanced bioactivities.
3. Surface Modifications of Chitosan
Surface modifications are ways by which superior properties replace weaknesses of the core material through modifying the surfaces physically/chemically. In case of chitosan, its backbone is modified to alter properties such as solubility, mucoadhesion, and stability. Ideally, the -NH2 and -OH groups are the active modification sites. Chitosan polymers are modified through: blending, graft co-polymerization, and curing [42]. The mixing of two or more polymers involves blending, which is a form of physical modification. This is the simplest form of surface modification. Few examples of surface modification of chitosan by blending are poly (vinyl alcohol) (PVA), poly (vinyl pyrrolidone) (PVP), and poly (ethyl oxide) (PEO). PVA modification of chitosan improves the tensile strength and water vapor permeability of chitosan films [43]. PVA-chitosan also show improved mechanical properties for controlled drug delivery [44]. Amoxicillin formulated with a crosslinked chitosan/PVP blend with glutaraldehyde to form a semi-interpenetrating polymer network (semi-IPN) is an example of physical modification [45].
Graft co-polymerization is the covalent bonding of polymers. Curing converts, the polymers into a solidified mass by means of thermal, electrochemical, or ultraviolet radiation [46]. Chemical modification is achieved by altering the functional groups in a compound by chemical, radiation, photochemical, plasma-induced, and enzymatic grafting methods [42]. Chemical modification of chitosan results in quaternized chitosan, thiolated chitosan, carboxylated chitosan, amphiphilic chitosan, chitosan with chelating agents, PEGylated chitosan, and lactose-modified chitosan. The primary amine (-NH2) group is the chemical modification site, key to pharmaceutical applications [42], reacting with sulphates, citrates, and phosphates [47]. This enhances the stability and drug encapsulation efficiency of the modified chitosan [48]. Improved solubility of chitosan in intestinal media is achieved through N-trimethyl chitosan chloride (TMC), a quaternized chitosan [49]. The mucoadhesiveness of chitosan is enhanced incorporating thiolated chitosan [50]. Quaternization of chitosan forms several derivatives such as dimethylethyl (DMEC), diethylmethyl (DEMC), trimethyl (TMC), and triethyl chitosan (TEC). Quaternization of chitosan helps in increasing the permeability of insulin across Caco-2 cells [51]. Chitosan-thioglycolic acid, chitosan-cysteine, chitosan-glutathione, and chitosan-thioethylamidine are some of the thiolated chitosan derivatives presently in use. Grafting of polyphenols on chitosan has been studied [52,53,54,55]. Phenolic compounds are oxidized to o-quinones and further covalently grafted to nucleophilic amine groups (through Schiff-base and/or Michael-type addition reaction) [52,56]. Chitosan modified by grafting polyphenols, showed significantly enhanced antioxidant activity, a classic review of the above-mentioned modifications has been published [57].
4. Nanochitosan and Its Modifications
4.1. Methods Used to Prepare Nanochitosan
Nanotechnology has impacted almost every area of science and technology. Nanomaterials are now accepted to be highly versatile, and superior compared to their bulk counterparts. Hence, the nanoforms of almost all successful materials are readily attempted, since they are destined and proven to have a higher success rate. In case of chitosan, formulation of chitosan nanoparticles and nanomaterials has been attempted. Chitosan nanomaterials are synthesized using emulsification, emulsion based solvent evaporation, ionotropic gelation, microemulsion, and solvent diffusion. Drugs are loaded within the chitosan nanomaterials via electrostatic interaction, hydrogen bonding, and hydrophobic interactions. Coacervation is done by separating spherical particles by mixing electrostatically driven liquids [58,59]. In the polyelectrolyte complex (PEC) method, an anionic solution is added to the cationic polymer under mechanical stirring, to obtain nanoparticles [60,61]. In the coprecipitation method, chitosan solution is added, leading to coprecipitation of highly monodisperses chitosan nanoparticles [62]. In the microemulsion method, chitosan in acetic acid solution and glutaraldehyde are added to a surfactant in an organic solvent such as hexane for cross-linking [63]. Emulsification Solvent Diffusion Method is where an o/w emulsion is prepared under mechanical stirring followed by high pressure homogenization [64,65]. Emulsion Based Solvent Evaporation Method is a slight modification of the above method that uses no high shear forces. Reverse Micellar Method is where a surfactant is added to an organic solvent followed by the addition of chitosan, drug, and crosslinking agent [66].
4.2. Chitosan/Functionalized Chitosan Nanocarriers—A Snap Shot of Biomedical Achievements
Chitosan has been widely used to produce nanoparticles as standalone materials or in combination with others. In addition to this, chitosan nanomaterial has been functionalized with various bioactive moieties in order to enhance its biological properties and overcome the limitations of the source material. Surface modification is the act of modifying the surface of a material by altering the surface of the material to impact their original physical, chemical, or biological characteristics. Surface functionalization introduces chemical functional groups to a surface. The onset of nanotechnology has brought about key advances in nanomaterial-based applications. Functionalization of nanoparticles has overcome default properties of the nanomaterial and brought about improvisation. This is believed to hold high promises towards pharmaceutical and biomedical sciences. Functionalization of nanoparticles has emerged as a highly promising routine to yield multifunctional nanoparticles that have overcome inherent weakness.
Functionalization of chitosan nanoparticles and their surface modification especially to suit their therapeutic applications has been successfully demonstrated. We present a brief overview of the reports on the diverse modified/functionalized chitosan nanoparticles and their applications. Chitosan has been formulated as polymeric nanoparticles for oral drug delivery by conjugating antioxidants, catechin and epigallocatechin (flavonoids from green tea) with chitosan nanoparticles. Usually, these catechins are poorly absorbed across intestinal membranes and undergo degradation in intestinal fluid. This is overcome by encapsulating these catechins inside chitosan nanoparticles [67]. Tamoxifen, a water soluble anti-cancer drug, is useful for oral cancer. Tamoxifen encapsulated in lecithin-chitosan nanoparticles led to the successful movement of tamoxifen across the intestinal epithelium [68]. Nanoparticles of doxorubicin hydrochloride (DOX)/chitosan/carboxymethyl chitosan, increased the intestinal absorption of DOX [69]. Alendronate sodium (drug for osteoporosis) suffers from gastrointestinal side effects and low oral bioavailability. Encapsulation of alendronate sodium in chitosan nanoparticles overcame the above mentioned limitations. Sustained drug release of sunitinib (a tyrosine kinase inhibitor) was achieved by encapsulating the drug in chitosan nanoparticles [70]. Insulin-loaded chitosan nanoparticles were functionalized with tripolyphosphate (TPP), which enhanced the NP uptake by the stomach epithelium [71]. Bay 41-4109, an active hepatitis B virus inhibitor, was formulated into chitosan nanoparticles improving drug solubility and oral bioavailability [72].
One of the advantages of using chitosan is its mucoadhesive property, this makes chitosan a successful candidate for nasal and intestinal drug delivery [73]. Carboxymethyl chitosan nanoparticles of carbamazepine, enhanced their bioavailability and brain targeting through the nasal passage [74]. Thiolated-chitosan nanoparticles of the drug leuprolide showed significant enhancement in the transportation of the leuprolide drug across porcine nasal mucosa. A nanoparticle based dry powder inhalation (DPI) of rifampicin (antitubercular drug) was formulated with chitosan as the polymer, achieving sustained drug release upto 24 h and zero toxicity [75].
Itraconazole, an anti-fungal drug suffers low solubility during oral adminstration. The drug in spray-dried chitosan nanoparticles alongwith lactose, mannitol, and leucine, increased deposition of itraconazole in the lungs [76]. Giovino et al. developed chitosan buccal films of insulin loaded poly (ethylene glycol) methyl ether-block-polylactide (PEG-b-PLA) nanoparticles [77], that exhibited excellent mucoadhesive properties and sustained insulin release. Curcumin prepared as polycaprolactone nanoparticles coated with chitosan have been used for buccal delivery. Nanochitosan encapsulated enriched flavonoid fraction (EFF-Cg) loaded PLGA nanoparticles were used for buccal delivery as chitosan films. The bioavailability of EFF-Cg was improved with no cytotoxicity [78].
Nayak et al. prepared chitosan nanoformulations using ascorbic acid (Vitamin C), α-tochopherol (Vitamin E), and catechol, along with green synthesized AgNPs for targeted drug delivery to breast cancer cells. These nanoformulations possessed higher antioxidant activity and also were hemocompatible [79]. Curcumin encapsulated by chitosan-tripolyphosphate (CS-TPP) nanoparticles were demonstrated to show high radical scavenging activity [80]. In another study, Kaur et al. [81] formulated catechin hydrate (CH)-loaded nanoparticles functionalized with TPP. These catechin loaded nanoparticles showed higher antioxidant activity. Chlorogenic acid (CGA), a polyphenolic antioxidant, was encapsulated into chitosan nanoparticles and demonstrated for their improved antioxidant activity [82]. Chitosan/DNA co-assemblies were used for the encapsulation of astaxanthin, a carotenoid that possesses strong antioxidant properties [77,83].
Chitosan-vancomycin nanoparticles for colon delivery were prepared for better drug release [84]. Coco et al. have studied the comparative ability of chitosan nanoparticles against other polymers, for inflamed colon drug delivery [85]. Trimethyl nanochitosan entrapping ovalbumin (OVA) showed high permeability of OVA. Chitosan-carboxymethyl starch nanoparticles of 5-aminosalicylic acid were demonstrated for inflammatory bowel disease, showing controlled drug release [85]. Rosmarinic acid loaded chitosan nanoparticles have been used for non-cytotoxic ocular delivery. Imiquimod was prepared as chitosan coated PCL nanocapsules embedded in hydroethylcellulose gel and also as PCL nanocapsules embedded in chitosan hydrogel for vaginal delivery for treating human papillomavirus infection [86]. The former showed higher mucoadhesion, and the latter higher drug permeation.
Chitosan-based material have been used as bone substitutes, due to their excellent biocompatibility and biodegradability. However, the hydrophobic surface of chitosan films inhibits osteogenesis mineralization process during bone regeneration. To resolve this issue, a novel polydopamine-modified chitosan film with good hydrophilicity has been developed for bone tissue engineering applications. These films showed enhanced growth rate of apatite on the modified chitosan film. Due to the method being capable of generating large quantities in bulk, this sure has a huge potential in the area of bone tissue engineering. Table 1 lists the various modified chitosan nanomaterials with their biomedical applications.
Table 1.
Biomedical applications of modified chitosan nanomaterials.
| Functionalized/Surface Modified/Encapsulated Chitosan Nanomaterial |
Purpose | Application | Reference |
|---|---|---|---|
| Chitosan, soybean lecithin nanoparticles | Oral drug delivery | Intestinal permeation of tamoxifen through the rat intestinal wall | [68] |
| Chitosan nanoparticles | Oral drug delivery | Sunitinib drug delivery | [70] |
| LMW chitosan NP | Oral drug delivery | Solubility and bioavailability of Hydrophobic Bay41-4109 in rats | [72] |
| Chitosan, TPP | Oral delivery of insulin | Decreased glycaemia in diabetic rats after administering insulin-chitosan nanoparticles, in vivo | [71] |
| Chitosan HCl, Poloxamer 188, sodium glycolate, gelatin, soya lecithin |
Oral delivery of Cyclosporin-A | Beagle dogs showed relative bioavailability of Cy-A was significantly increased by chitosan nanoparticles, in vivo. |
[64] |
| Chitosan carboxymethyl chitosan | oral antigen delivery in fish vaccination | Extra cellular products (ECPs) of Vibrio anguillarum | [87] |
| Chitosan LMW, sodium tripolyphosphate (TPP), fluorenyl-methyloxycarbonyl chloride (FMOC) |
Oral drug delivery | Chitosan nanoparticles released Alendronate sodium faster in 0.1 N HCl compared to PBS | [88] |
| Chitosan LMW, sodium tripolyphos-phate, tris[2-carboxyethyl] phosphine hydrochloride (TCEP) |
Oral drug delivery | Enhanced intestinal absorption of catechins | [67] |
| Chitosan, STPP, sodium alginate | Oral drug delivery | Alginate coated chitosan nanoparticles containing enoxaparin for oral controlled release | [89] |
| Chitosan, deoxycholic acid, vitamin B12 |
Oral drug delivery | Enhancement of scutellarin oral delivery | [90] |
| Chitosan, Tc-methylene di-phosphonate |
Oral drug delivery | Chitosan nanoparticles/F nanoparticles stable in the stomach and decompose in the intestine |
[91] |
| Chitosan, PLGA, streptozotocin | Oral drug delivery of Tolbutamide | PLGA nanoparticles modified with chitosan to form TOL-CS-PLGA NPs to improve bioavailability and reduce dose frequency | [92] |
| Chitosan LMW, penta sodium tripolyphos-phate | Oral drug release | Gemcitabine-loaded chitosan nanoparticles (Gem- Chitosan nanoparticles) for oral bio-availability enhancement | [93] |
| Sodium alginate, chitosan, streptozotocin |
Naringenin nanoparticles have better efficacy in lowering blood glucose levels compare to free drug | Alginate coated chitosan core shell nanoparticles for effective oral delivery | [94] |
|
N-carboxymethyl chitosan, chitosan hydrochloride |
Oral drug delivery | EGCG-chitosan/β-Lg NPs to achieve prolonged release during oral administration in gastrointestinal tract | [95] |
| Chitosan, sodium alginate, sodium pyruvate, l-glutamine |
Oral drug delivery | Quercetin-chitosan/alginate nanoparticles high antioxidant property no systemic toxicity | [96] |
| Chitosan nanoparticles -TPP, lactose, Tween 80 | Oral drug delivery | 90% release of RFM from Chitosan nanoparticles within 24 h, in vitro | [75] |
| Hydroxypropyl-beta-cyclodextrin (HPβCD), mannitol, lactose, TPP, l-leucine | Pulmonary delivery | Chitosan nanoparticles for pulmonary delivery of itraconazole as a dry powder formulation | [76] |
| N,N,N-tri-methyl chitosan, TPP | Pulmonary drug delivery | Cellular uptake of Bac-TMC3/TPP/siRNA nananoparticles greatly enhanced by clathrin -mediated cellular uptake pathway | [97] |
| Chitosan, lipoid S100, glycol chitosan |
Pulmonary drug delivery | LMWH chitosan and glycol Chitosan nanoparticles for enhancing pulmonary absorption of LMW heparin | [98] |
| Chitosan thioglycolic acid, TPP | Pulmonary drug delivery | Theophylline-thiolated Chitosan nanoparticles enhances theophylline’s capacity to alleviate allergic asthma | [99] |
| Thiolated chitosan | Pulmonary drug delivery | In vitro slow and sustained release of leuprolide from thiolated chitosan about 43% in 2 h | [100] |
| Chitosan, methylated β-cyclodextrin, TPP |
Intranasal administration | Estradiol-chitosan nanoparticles for improving nasal absorption and brain targeting | [101] |
| LMW Chitosan, TPP, trehalose | Intranasal immunization | Tetanus toxoid chitosan nanoparticles (TT-CS NPs) as a new long-term nasal vaccine delivery vehicle | [102] |
| Chitosan, 4-CBS, TPP, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide HCl (EDAC) |
mucoadhesive drug delivery | In vitro drug release of DOX loaded 4-CBS-chitosan/PLA nanoparticles showed sustained release up to 26 days | [103] |
| Chitosan (MW = 600 kDa), methane-sulfonic acid, oleoyl chloride, sodium bicarbonate, glycidyl-trimethyl ammonium chloride |
oral administration with enhanced mucoadhesion | In vivo toxicology study was performed in zebrafish embryos | [104] |
| Chitosan, 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC. HCl), N-hydroxyl succinimide | mucoadhesive drug delivery | Mucosal adhesion and drug release of cetirizine-chitosan |
[105] |
| Chitosan, lactic acid | mucoadhesive drug delivery | Chitosan-based 5-ALA mucoadhesive film to enhance its retention in oral mucosa | [106] |
| Chitosan Low, polycaprolactone, glycerol nanoparticle | mucoadhesive drug delivery | Mucoadhesive films containing curcumin-loaded nanoparticles to prolong the residence time in the oral cavity and to increase drug absorption through the buccal mucosa | [107] |
| Chitosan, TPP, Carbopol 940, poloxamer 407 | Drug delivery | Propranolol-chitosan nanoparticles of transdermal gels to improve the systemic bioavailability of the drug | [108] |
| Resomer PLGA, ploxamer 188, sorbitan monoaleate, chitosan | flavonoid enriched cytotoxic film | EFF-Cg nanocomposites chitosan film containing PLGA NPs, showed low toxicity | [109] |
| Chitosan, TPP, Triton X-100 | Oral drug delivery of alginate and pectin | Preparation of alginate and pectin chitosan nanoparticles for oral drug delivery | [110] |
| Chitosan MMW, PEG, PVP, trehalose | Insulin release | Chitosan films with insulin loaded PEG-b-PLA nanoparticles with sustained release | [111] |
| Chitosan buccal films of insulin loaded poly (ethylene glycol) methyl ether-block-polylactide (PEG-b-PLA) NP |
Insulin release | Excellent mucoadhesive properties and insulin release | [77] |
| Polycaprolactone nanoparticles coated with chitosan | Buccal delivery | Delivery of curcumin | [57,78] |
| EFF-Cg loaded PLGA nanoparticles as chitosan films. | Buccal delivery | The bioavailability of EFF-Cg was improved and no signs of cytotoxicity were seen | [78] |
| Conjugating C2-N position of chitosan with aromatic sulfonamide, 4-carboxybenzenesulfonamide-chitosan (4-CBS-chitosan) |
drug release in small intestine | Mucoadhesive property of chitosan in stomach acidic environment increased | [57] |
| Entrapping ovalbumin (OVA) into Eudragit S 100, trimethylchitosan, PLGA, PEG-PLGA and PEG-PCL | inflamed colon drug delivery | Nanoparticles with trimethyl chitosan have shown the highest permeability of OVA. And high permeability | [84] |
| chitosan-carboxymethyl starch nanoparticles of 5-aminosalicylic acid |
Drug delivery for inflammatory bowel disease | Controlled drug release | [85] |
| Rosmarinic acid loaded chitosan nanoparticles |
ocular delivery | The nanoparticles showed no cytotoxicity against the retinal pigment epithelium nor the human cornea cell line. | [112] |
| Chitosan coated PCL nanocapsules embedded in hydroethylcellulose gel |
vaginal delivery to treat human papillomavirus infection | Imiquimod formulated chitosan coated PCL nanocapsules embedded in hydroethylcellulose gel | [86] |
| PCL nanocapsules embedded in chitosan hydrogel | vaginal delivery to treat human papillomavirus infection | Imiquimod delivery to vagina | [113] |
| Limonene coated in chitosan | Enhancing antioxidant activity | Limonene-chitosan encapsulation has antioxidant activity with IC50 value of 116 ppm | [114] |
| Carboxymethyl chitosan nanofibres PEO and PVA-Ag | Biomedical application | Antibacterial | [115] |
| Carboxymethyl chitosan nanofibres—PVA\PVA\silk fibroin | Biomedical application | Wound dressing | [116] |
| Quaternized chitosan nanofibres-coPLA/DOX/PLA | Biomedical application | Antitumor | [117] |
| Quaternized chitosan nanofibres—PVA/PVP | Biomedical application | Antibacterial | [118,119,120] |
| Quaternized chitosan nanofibres—graphene | Biomedical application | Virus removal | [121] |
| Quaternized chitosan nanofibres—PLA | Biomedical application | Wound dressing | [122] |
| Poly-3-caprolactonegra chitosan nanofibres | Biomedical application | Skin tissue engineering | [123] |
| Chitosan/Albumin Nanoparticles | Drug delivery | Used as a hydrophobic drug nanocarrier in pharmaceutical and medical applications |
[124] |
| Chitosan/Curcumin nanoparticles | Drug delivery | Transdermal delivery | [125] |
| Chitosan/Sodium Nitrate nanoparticle | Drug delivery | Delivery of DOX | [103] |
| Chitosan/HA nanoparticle | Drug encapsulation | Used to encapsulate a chemotherapeutic drug | [126] |
| Chitosan/Paromomycin nanoparticle | Anti leishmaniasis | Treatment of leishmaniasis, especially when the current drugs are impaired by resistance | [127] |
| Chitosan/Lipid Hybrid nanoparticles |
Drug delivery | Controlled delivery of cisplatin | [128] |
| Chitosan/Human serum albumin nanoparticle |
Drug delivery | Nose-to-brain drug delivery | [129] |
| Chitosan/Polylactide nanoparticle | Drug delivery | Delivery of therapeutics for triple-negative breast cancer treatment | [130] |
| Chitosan/Cadmium Quantum Dots |
Drug delivery | Drug delivery of Sesamol | [131] |
| Chitosan/Silica Nanoparticles Thin Film | Drug delivery | DOX delivery | [132] |
| Chitosan/PVA nanoparticle | Oral delivery | Sustained release of the immunosuppressant drug mycophenolate mofetil | [133] |
| Chitosan-carbon dot hybrid nanogel |
Anticancer activity | Photothermal therapy-chemo | [134] |
| PEGylated and fluorinated chitosan nanogel | Drug delivery | Targeted drug delivery | [135] |
| Chitosan grafted MPEG-PCL micelles | Drug delivery | Ocular delivery of hydrophobic drug |
[136] |
| Arginine-modified nanostructured lipid carriers | Drug delivery | Anticancer drug delivery | [137] |
| Glycosaminoglycan modified chitosan liposome | Drug delivery | Antimalarial | [138] |
| Gold nanoshell-coated liposomes | Anticancer | Photothermal and chemotherapy |
[139] |
| Glycol chitosan-coated liposomes | Drug delivery | pH-responsive drug-delivery |
[140] |
| Chitosan nanoparticles-doped cellulose films | Antibacterial activity | Inhibition of Escherichia coli | [141] |
5. Anticarcinogenic/Antitumour Activity of Chitosan Nanomaterials
5.1. Chitosan Nanocarriers—Anticancer Impacts
Chitosan nanocarriers have also been reported for their accomplishments in cancer research. Nanochitosan have raised the impact of the chitosan polymer further through nanostructurization. We summarize the available reports confirming the use of nanochitosan as nanocarriers for cancer therapy. Mifepristone (MIF) is an anticancer drug used against various cancers, Zhang et al. [142] developed MIF-loaded chitosan nanoparticles (MCNs) that exhibited increased anticancer activity in several cancer cell lines. Pharmacokinetic studies in male rats orally administered with MCNs showed a 3.2-fold increase compared to free MIF. Wang et al. [143] designed chitosan nanoparticles for co-delivery of 5-fluororacil and aspirin and induced synergistic antitumor activity through playing around with nuclear factor kappa B (NF-κB)/cyclooxygenase-2 (COX-2) signaling pathways. The designed chitosan nanocarrier operated via aspirin-induced suppression of NF-κB and inhibition of COX-2.
The anti-metabolic compounds pyrazolopyrimidine and pyrazolopyridine thioglycosides were synthesized and encapsulated by chitosan nanoparticles. Their cytotoxicity against Huh-7 and Mcf-7 cells, related to liver and breast cancer cells, was successfully demonstrated [144]. Deepa et al. demonstrated the successful release of cytarabine against solid tumors, using nanochitosan formulations [145]. Cavalli et al. prepared chitosan nanospheres with 5-FU, which were effective in reducing tumor cell proliferation and were able to inhibit both HT29 and PC-3 adhesion to HUVEC [146]. Sahu et al. prepared 5-FU loaded biocompatible chitosan nanogels (FCNGL) that released 5-FU in an acidic environment, resulting in selective drug delivery, leading to sustained delivery of 5-FU for chemotherapy. This enabled high efficacy, patient compliance, and safety [147]. Keerthikumarc et al. synthesized chitosan encapsulated curcumin nanoparticles showing sustained release of the drug and high anticancer efficacy in human oral cancer cell lines [148]. Shahiwala et al. synthesized chitosan nanoparticles in an alcoholic extract of Indigofera intricate and demonstrated 500-fold reduction in the extract concentration, when the chitosan nanocarriers laden with plant extracts were used [149]. Alipour et al. demonstrated the sustained release of silibinin-loaded chitosan nanoparticles (SCNP) against C6 glioma cells [150]. In another study [151], the effects of nanochitosan on tumor growth were investigated using nude mice xenografted with human hepatocellular carcinoma (HCC) (BEL-7402) cells. The results demonstrated that the treatment of these nude mice with nanochitosan significantly inhibited tumor growth and induced tumor necrosis.
5.2. Surface Modified/Functionalized Chitosan Nanocarriers—Anticarcinogenic Impacts
Chitosan modifications and functionalization have also been demonstrated for their anticancer activity. Chitosan based nanoparticles of Bay 41-4109 showed prolonged circulation in the blood and enhanced intestinal absorption [72]. Enoxaparin has less oral bioavailability; this was overcome by enoxaparin-loaded alginate-coated chitosan NPs (Enx-Alg-CS-NPs) and demonstrated using rat models. Novel hepatocyte-targeted delivery system with glycyrrhizin (GL) modified N-caproyl chitosan (CCS) was demonstrated in rats. These CCS-NPs-GL were demonstrated in these rate models to be able to bring about effective drug delivery for hepatocyte targeting. Sharifi-Rad et al. have elaborately published a review on chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment [152].
Chitosan modified mesoporous silica nanoparticles (MSN) offer high surface area and pore volume, including stability of chitosan at different pH values. Controlled release profile of the curcumin drug molecule has been demonstrated [153]. Amphiphilic chitosan derivatives (N-octyl-N-mPEG-chitosan, mPEG = poly(ethyl-ene glycol) monomethyl ether; OPEGC) showing good water solubility and low cytotoxicity were successfully synthesized via the Schiff base reduction reaction of chitosan [154]. Copper-loaded nanochitosan were prepared for the effective treatment of osteosarcoma [155]. The copper-loaded chitosan nanoparticles (CuCNPs) exhibited remarkable anticancer activity. The superior anticancer effect of CuCNPs is attributed to the generation of a higher mitochondrial ROS level compared to that of the control. Overall, the anticancer effect of copper has been enhanced by delivering it within biocompatible nanochitosan.
Methotrexate (MTX) has poor water solubility, low bioavailability, and leading to resistance in cancer cells. Novel folate redox-responsive chitosan (FTC) nanoparticles for intracellular MTX delivery helped confer redox responsiveness and active targeting of folate receptors (FRs) [156]. These possess tumor specificity and controlled drug release due to the overexpression of FRs and high concentration of reductive agents in the cancer microenvironment. FTC-NPs showed better inhibitory effects on HeLa cancer cells compared to non-target chitosan-based NPs. Methotrexate (MTX) and mitomycin C (MMC) loaded PEGylated chitosan nanoparticles (CS-NPs) were developed as drug delivery systems [157]. MTX, as a folic acid analogue, was employed as a tumor-targeting ligand. Effective uptake via FA receptor-mediated endocytosis and codelivery of MTX and MMC at the tumor site have been reported. (MTX + MMC)-PEG-CS-NPs as targeted drug codelivery systems can have clinical implications for combinational cancer chemotherapy. Irinotecan nanoparticles (NPs) using folate–chitosan conjugate (FCC) for more effective delivery of Irinotecan for killing breast cancer cells was developed [158]. Since breast cancer cells express folate receptors on their surface, these irinotecan-loaded folic acid–chitosan conjugated nanocarriers could be used for targeted delivery against metastatic breast cancer with some modifications.
Liu et al. prepared chitosan grafted halloysite nanotubes (HNTs-g-CS) as potential nanocarriers for drug delivery in cancer therapy, as curcumin loaded HNTs-g-CS increased apoptosis on EJ cells [159]. Abbas et al. introduced a chitosan (CS) and CS magnetic nanoparticles (MNPs) encapsulating polyvinylpyrrolidone (PVP)/maltodextrin (MD)-based microparticles (MPs) system that was inhalable delivering the drug to deep lung tissues [160]. Almutairi et al. prepared raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles that showed cytotoxicity against human lung A549 cancer cell lines [161]. Bae et al. prepared self-aggregates from deoxycholic acid-modified chitosan for use as delivery vehicles of anticancer drugs [162]. Wu et al. synthesized 10-hydroxycamptothecine nanoneedles integrated with an exterior thin layer of the methotrexate-chitosan conjugates, for enhanced therapeutic performances in cancer treatment [163]. Li et al. synthesized the drug carrier (Fe3O4/carboxymethyl-chitosan nanoparticles) and demonstrated with the antitumour drug rapamycin (Fe3O4/CMCS-Rapa NPs) [164]. Roy et al. encapsulated Fe3O4-bLf (Fe3O4-saturated lactoferrin) in alginate enclosed chitosan-coated calcium phosphate (AEC-CP) nanocarriers (NCs) to be usesd against tumor in mice [165]. Arunkumar et al. synthesized composite injectable chitosan gel (DZ-CGs) comprising of doxorubicin-loaded zein nanoparticles (DOX-SC ZNPs), which could bring about successful in vitro drug release in a controlled manner. The composite DZ-CGs were more effective in killing cancer cells [166]. Hwang et al. synthesized the hydrophobically modified glycol chitosan (HGC) nanoparticles loaded with the anticancer drug docetaxel (DTX), leading to reduced tumor volume of A549 lung cancer cells [167].
Gomathi et al. prepared letrozole with chitosan nanoparticles using sodium tripolyphosphate as the crosslinking agent for anticancer treatment. This was biocompatible and possessed hemocompatible properties, which makes it an efficient nanocarrier for the anticancer drug letrozole [168]. Wang and Zhao optimized the preparation of anticancer drug—gefitinib with chitosan protamine nanoparticles [169]. Koo et al. reported the preparation of water-insoluble paclitaxel encapsulated into glycol chitosan nanoparticles with hydrotropic oligomers (HO-CNPs), these paclitaxel-HO-CNPs showed higher therapeutic efficacy than the commercial Abraxane® formulation [170]. Maya et al. prepared O-carboxymethyl chitosan (O-CMC) nanoparticles, surface-conjugated with cetuximab (Cet) for targeted delivery of paclitaxel. These can be used for targeted therapy of epidermal growth factor receptor (EGFR) in overexpressing cancers [171]. Al-Musawi et al. synthesized chitosan-covered superparamagnetic iron oxide nanoparticles (CS-SPION) and applied them as a nano-carrier for loading of (5-FU) (CS-5-FU-SPION) [172]. Anitha et al. prepared a nanoformulation of curcumin using dextran sulphate and chitosan, leading to preferential killing of cancer cells compared to normal cells by the curcumin-loaded drug [173]. Baghbani et al. prepared curcumin-loaded chitosan/perfiuorohexane nanodroplets using a nanoemulsion process [174]. Rajan et al. synthesized curcumin nanoparticles loaded in chitosan biopolymer and bovine serum albumin, which resulted in selective drug targeting of colorectal carcinoma cells [175]. George et al. reported the preparation of functionalized nanohybrid hydrogel using l-histidine (HIS) conjugated chitosan, phyto-synthesised zinc oxide nanoparticles (ZNPs) and dialdehyde cellulose (DAC) for sustained drug delivery of naringenin, quercetin, and curcumin. Anticancer studies towards A431 cells (epidermoid carcinoma) exhibited excellent cytotoxicity, compared to the free polyphenol drugs [176]. Chaichanasak et al. prepared chitosan-based nanoparticles with damnacanthal (DAM), leading to improved anticancer effects [177]. Oh et al. have elaborated on the various medical and drug delivery applications of chitosan and green synthesized chitosan nanomaterials in previously published reviews [178,179].
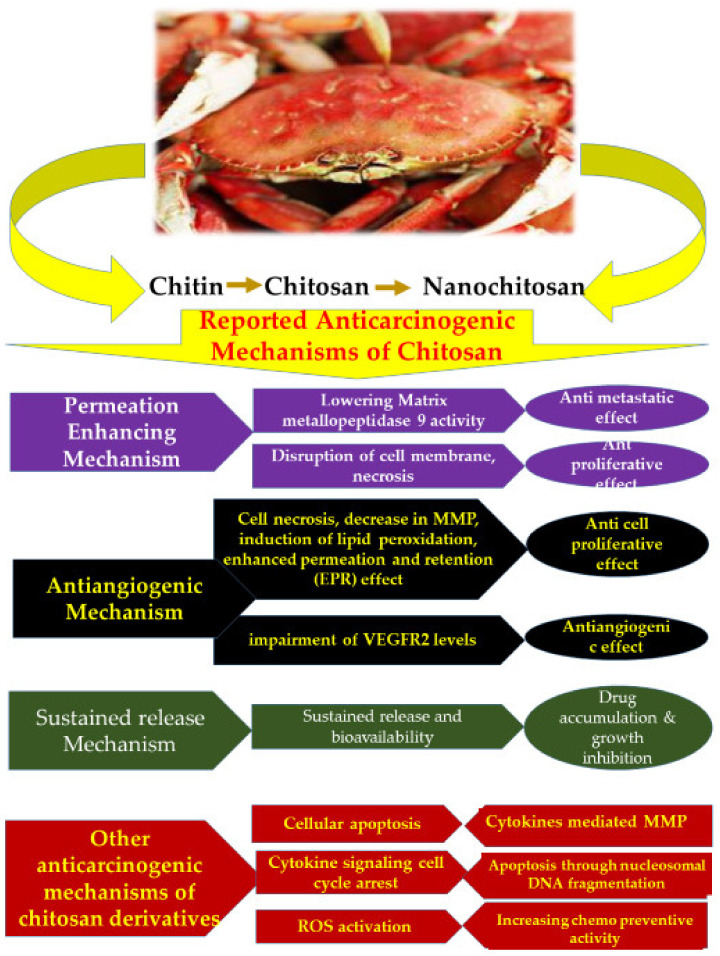
Although the exact mechanism behind the anticancer activity of chitosan remains elusive, Adhikari and Yadav (2018) have elucidated few plausible mechanisms that may explain the mechanism involved in the anticancer activity of chitosan. Table 2 presents a consolidated list of the mechanisms suggested for chitosan and nanochitosan mode of anticarcinogenic activity. Those included are: (i) permeation enhancing mechanism, (ii) antiangiogenic mechanism, and (iii) sustained release mechanism [180] (Figure 2). Chitosan has been demonstrated for its anticarcinogenic effect on MDA-MB-231 [181,182] and antiproliferative effect on T24 urinary bladder cell lines [183,184], while chitosan nanoparticles have been proven for their antiangiogenic effect on human hepatocarcinoma [151,185] and cell proliferation inhibition on BEL7402, HT-29 cell lines [186,187,188,189,190,191,192]. Mifepristone (MIF) loaded chitosan nanoparticles have demonstrated an enhanced anticarcinogenic effect through sustained drug release as well as enhanced bioavailability of MIF [142].
Table 2.
Established mechanisms for anticarcinogenic activity of chitosan and nanochitosan.
| Test | Chitosan Form | Target Cell Line | Mode of Action | Reference |
|---|---|---|---|---|
| In vitro and in vivo | Chitosan | MDA-MB-231 | Permeation enhancement, lowering of matrix metallopeptidase 9 activity leading to antimetastatic effect | [180,181] |
| In vitro | Chitosan | T24 urinary bladder cell lines | Disruption of cell membrane, necrosis resulting in antiproliferative effect | [182,183] |
| In vitro | Chitosan nano particles | Human hepato carcinoma | Antiangiogenic effect through, antiangiogenic action of chitosan nanoparticles and impairment of vascular endothelial growth factor (VEGFR) 2 levels | [184,185] |
| In vitro | Chitosan nano particles | BEL7402, HT-29 | Cell necrosis, lipid peroxidation, decrease in MMP, enhanced permeation and retention (EPR) effect, resulting in inhibition of cellular proliferation | [155,186,187,188,189] |
| In vivo | Mifepristone (MIF) loaded chitosan nano particles | Solid tumor | Sustained release and enhancement of bioavailability of drug. Drug accumulation and growth inhibition | [190] |
Figure 2.
Overview of the anticarcinogenic mechanisms of chitosan and its forms that have been reported in literature.
6. Limitations and Future Endeavors
6.1. Toxicity Aspects of Chitosan
Chitin has its own list of biomedical applications; chitosan has its own credentials far surpassing those of chitin. Nanochitosan has also made significant progress. Chitosan nanoparticles effectively deliver drugs to the specific sites by retaining the drug longer, allowing extended time for drug absorption. As in the case of any material used for biomedical applications, the toxicity aspects are quiet a concern. Chitosan is biodegradable and its degradation is dependent on the degree of deacetylation and the availability of amino groups. The toxicity of chitosan increases as charge density increases and degree of deacetylation increases too [191]. As of now, no human toxicity reports on chitosan-based formulations exist, but there are several animal toxicity-reports on its safety in vivo and in vitro. Aluani et al. have studied and confirmed the in vivo toxicity of two types of quercetin-loaded chitosan NPs (QR-NP1, QR-NP2) on male Wistar albino rats [192]. Their data concluded that chitosan nanoparticle are safe carriers for quercetin in oxidative stress associated injuries. Death and malformation of zebrafish embryos occurred with increasing chitosan nanoparticle concentrations. Almost 100% mortality was observed at a concentration of 40 mg/L for the 200 nm chitosan nanoparticles. Therefore, chitosan toxicity appears to be dose-dependent and needs to be considered at high concentrations [193]. Toxicity studies of chitosan and chitosan nanoparticles is lacking; it is necessary that chitosan should not be taken for granted in terms of its toxicity. When it specially comes to chitosan nanoforms, it is essential that well defined toxicity assessment is made for each and every study, with respect to specific cell lines and their relevant experimental set ups. Nanomaterial properties are very different from their bulk and so toxicity aspects need to be studied for each respective system. In fact, it is necessary that any application reporting the use of chitosan nanocarriers for medical purposes should culminate in toxicity assessment and toxicity validation of their nanocarrier system. This review points out to this gap and the need to fill in.
Clinical use of oral or mucoadhesive drug formulations containing chitosan are yet to be activated, however, human vaccines have been formulated that use chitosan as an adjuvant [194,195]. Novel chitosan-modified polylactic-co-glycolicacid nanoparticles (CS@PLGA nanoparticles) were formulated for improving the bioavailability of tolbutamide (TOL) [92]. Using mice models, Bronchial Calu-3 and alveolar 549 cells were used to study the effect of chitosan-based drugs targeted for drug delivery to lungs [196]. Chitosan-coated PLGA nanoparticles showed better biodistribution and lower toxicity compared to those without the chitosan coating [197]. Grenha et al. 2007 reported absence of toxicity in vitro using Calu-3 cells and A549 epithelial cells at specific concentrations [198]. In vitro cytotoxicity of chitosan nanoparticles against buccal cells (TR146) was evaluated by Pistone et al. [110]. Chitosan nanoparticles were less cytotoxic than alginate and pectin nanoparticles. Moreover, bulk chitosan was more cytotoxic than nanochitosan, because of the linker attached to chitosan nanoparticles. It was also observed that the cytotoxicity of chitosan nanoparticles was shown to be further reduced by increasing the concentration of the linker (TPP) or using chitosan with lesser degree of deacetylation. To date, there are almost no toxicity reports in animal models and no reports of major adverse effects in healthy human volunteers and clinical data are lacking. Even though chitosan is approved in dietary use, wound dressing applications and cartilage formulations, yet a chitosan-based drug formulations have not been approved for mass marketing [199].
6.2. Inadequate Clinical Testings
In terms of clinical studies, except for scattered one or two reports, no progress has been made. Without putting the formulations to clinical trials, we will not get a clear picture of its performance. This is a huge lacuna that this review points out, and one that needs to be addressed specifically in order to see progress in this area of research. A chitosan-based nasal formulation of morphine (RylomineTM) is currently in Phase 2 clinical trials (UK and EU) and Phase 3 clinical trials in the U.S. When it reaches the market, it will pave way for similar products in the near future, as well as assist in discerning any unanticipated effects in humans [200]. A need for promoting the use of chitosan nanoparticles, in targeted cancer theranostics, dermatologic applications, and targeted parenteral drug delivery systems is also stressed [201,202,203]; apart from a few reports no concrete progress has been made in this direction. We hope that future work on chitosan nanoparticles will also focus on toxicity studies in humans.
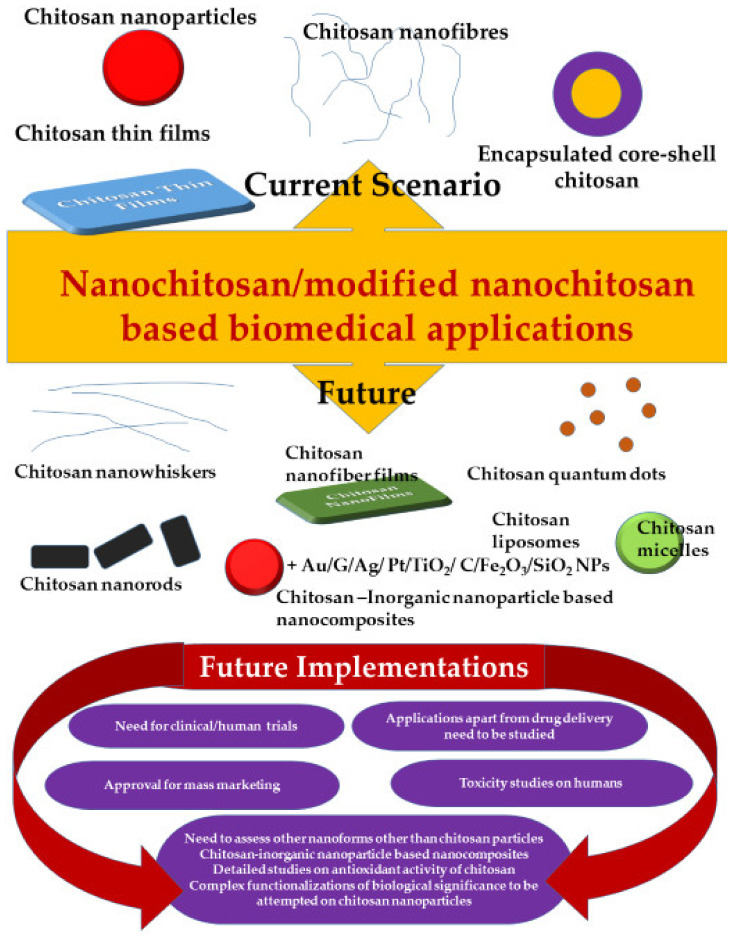
6.3. Unexplored Arenas
In addition to the above concerns, this review points out the fact that almost 99% of the nanochitosan work is surrounding chitosan nanoparticles alone. While chitosan nanomaterial includes quantum dots, micelles, nanogels, nanofibers, nanowhiskers, and nanospheres, none of these advanced nanoforms have been involved as much as chitosan nanoparticles have been involved. As is well known, morphological impacts of nanomaterial could be distinct and unique. Given this fact, it is rather intriguing why none of these have been studied stand-alone or conjugated with the diverse modifications and functionalizations that chitosan nanoparticles have been studied with. This is an area worth addressing and applying for achieving more prominent deliverables. This review prompts progress and inputs in this direction. Moreover, not much has been evaluated with respect to enhancement of antioxidant properties of chitosan nanoparticles as yet. Antioxidant activity of chitosan is one of the outstanding features of chitosan that validates its use in biomedical applications. With this being the case, nanoantioxidant chitosan should have hit the headlines by overcoming limitations. Compared to bulk materials, nanomaterials have always outshined and broken barriers. In this case too, such positive reports were expected and not much evidenced. We hope this review will trigger enthusiasm in this direction too. Extensive reviews pertaining to drug delivery applications of chitosan nanoparticles have been published by Li et al., 2018 [204] and Mohammed et al., 2017 [153], a lot less has been achieved in other areas of biomedical applications, such as tissue engineering, bone/skin grafts, antitumor applications, and the like. This review encourages expansion under these themes.
As much as encapsulation, surface modifications of chitosan are reported, a lot less on actual functionalization with usual functional moieties that are prevalently used for biological functions, have been reported. PLA, PVA, and PEG are repetitively used, when there is a school of other groups that could prove worthy of assessment. Moreover, a lot fewer less reports on functionalization of well-known biocompatible inorganic nanomaterial such as Au, iron oxides, TiO2, Ag, C, Pt, Pd, etc., with chitosan exist. Nanoantioxidants that are reputed for their remarkable antioxidant features have been extensively reviewed [205] in a recent review by Khalil et al., 2020. These include SiO2 nanoparticles, Au NPs, Ag NPs, and Fe2O3 NPs, ceria nanoparticles that have proven to show enhanced antioxidant activity when functionalized with 2′,3,4′,5,7-pentahydroxyflavone, PEG, Polly tannic acid, salvianic acid, trolox, lignin, dextran, curcumin, and the like [205]. Such nanocomposites have been proven to be highly effective in various other fields, nanoantioxidants such as these should be combined with chitosan, to harness the full potential of such a nanocomposite. This is one area worth probing and expanding. Figure 3 projects the areas that need to be explored as future perspective in this subject area.
Figure 3.
Future of chitosan nanomaterial based biomedical applications.
More focus on application of modified chitosan nanocarriers as well as chitosan composites towards anticancer research is essential. Not much has been achieved in this direction. This review expects expansion in these applications, via synthesis of such composite materials as well as their application into anticancer research. Not many clinical studies have been reported from the existing chitosan nanoformulations; this deserves research inputs.
7. Conclusions
This review highlights the importance of various modifications and functionalization on chitosan and nanochitosan. Given the fact that a biodegradable material, obtained from food waste, can be put to effective biomedical use, there is certainly plenty of room to improvise and expand the horizons. When resources are already running out, such recycled resources are essentially the future.
Author Contributions
Conceptualization, writing—original draft preparation, writing—review and editing, M.M., J.G. and review, J.S., S.C. and funding acquisition and supervision, J.-W.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Islam S., Bhuiyan M.A.R., Islam M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016;25:854–866. doi: 10.1007/s10924-016-0865-5. [DOI] [Google Scholar]
- 2.Park B.K., Kim M.-M. Applications of Chitin and Its Derivatives in Biological Medicine. Int. J. Mol. Sci. 2010;11:5152–5164. doi: 10.3390/ijms11125152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aranaz I., Mengíbar M., Harris R., Panos I., Miralles B., Acosta N., Galed G., Heras A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009;3:203–230. [Google Scholar]
- 4.Younes I., Rinaudo M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs. 2015;13:1133–1174. doi: 10.3390/md13031133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Venkatesan J., Lowe B., Anil S., Manivasagan P., Al Kheraif A.A., Kang K.-H., Kim S.-K. Seaweed polysaccharides and their potential biomedical applications. Starch-Stärke. 2015;67:381–390. doi: 10.1002/star.201400127. [DOI] [Google Scholar]
- 6.Aranaz I., Harris R., Heras A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010;14:308–330. doi: 10.2174/138527210790231919. [DOI] [Google Scholar]
- 7.Grenha A., Al-Qadi S., Seijo B., Remuñán-López C. The potential of chitosan for pulmonary drug delivery. J. Drug Deliv. Sci. Technol. 2010;20:33–43. doi: 10.1016/S1773-2247(10)50004-2. [DOI] [Google Scholar]
- 8.Kim S., Requejo K.I., Nakamatsu J., Gonzales K.N., Torres F.G., Cavaco-Paulo A. Modulating antioxidant activity and the controlled release capability of laccase mediated catechin grafting of chitosan. Process. Biochem. 2017;59:65–76. doi: 10.1016/j.procbio.2016.12.002. [DOI] [Google Scholar]
- 9.Samar M.M., El-Kalyoubi M., Khalaf M., El-Razik M.A. Physicochemical, functional, antioxidant and antibacterial properties of chitosan extracted from shrimp wastes by microwave technique. Ann. Agric. Sci. 2013;58:33–41. doi: 10.1016/j.aoas.2013.01.006. [DOI] [Google Scholar]
- 10.Qinna N.A., Karwi Q.G., Al-Jbour N., Al-Remawi M.A., Alhussainy T.M., Al-So’Ud K.A., Al Omari M.M.H., Badwan A.A. Influence of Molecular Weight and Degree of Deacetylation of Low Molecular Weight Chitosan on the Bioactivity of Oral Insulin Preparations. Mar. Drugs. 2015;13:1710–1725. doi: 10.3390/md13041710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ways T.M.M., Lau W.M., Khutoryanskiy V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers. 2018;10:267. doi: 10.3390/polym10030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan Y., Chesnutt B.M., Haggard W.O., Bumgardner J.D. Deacetylation of Chitosan: Material Characterization and in vitro Evaluation via Albumin Adsorption and Pre-Osteoblastic Cell Cultures. Materials. 2011;4:1399–1416. doi: 10.3390/ma4081399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheung R.C.F., Ng T.B., Wong J.H., Chan W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs. 2015;13:5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang M., Li X., Gong Y., Zhao N., Zhang X. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials. 2001;23:2641–2648. doi: 10.1016/S0142-9612(01)00403-3. [DOI] [PubMed] [Google Scholar]
- 15.Ahmed S., Ikram S. Chitosan Based Scaffolds and Their Applications in Wound Healing. Achiev. Life Sci. 2016;10:27–37. doi: 10.1016/j.als.2016.04.001. [DOI] [Google Scholar]
- 16.Zhang Y., Sun T., Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm. Sin. B. 2017;8:34–50. doi: 10.1016/j.apsb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakar L.M., Abdullah M.Z., Doolaanea A.A., Ichwan S.J.A. PLGA-Chitosan nanoparticle-mediated gene delivery for oral cancer treatment: A brief review. J. Phys. Conf. Ser. 2017;884:12117. doi: 10.1088/1742-6596/884/1/012117. [DOI] [Google Scholar]
- 18.Kumirska J., Weinhold M.X., Thöming J., Stepnowski P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers. 2011;3:1875–1901. doi: 10.3390/polym3041875. [DOI] [Google Scholar]
- 19.Keawchaoon L., Yoksan R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf. B Biointerfaces. 2011;84:163–171. doi: 10.1016/j.colsurfb.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 20.Ajun W., Yan S., Li G., Huili L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009;75:566–574. doi: 10.1016/j.carbpol.2008.08.019. [DOI] [Google Scholar]
- 21.Arya N., Chakraborty S., Dube N., Katti D.S. Electrospraying: A facile technique for synthesis of chitosan-based mi-cro/nanospheres for drug delivery applications. J. Biomed. Mater. Res. Part B. 2009;88:17–31. doi: 10.1002/jbm.b.31085. [DOI] [PubMed] [Google Scholar]
- 22.Hu B., Pan C., Sun Y., Hou Z., Ye H., Hu B., Zeng X. Optimization of fabrication parameters to produce chitosan− tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food Chem. 2008;56:7451–7458. doi: 10.1021/jf801111c. [DOI] [PubMed] [Google Scholar]
- 23.Luo Y., Zhang B., Whent M., Yu L.L., Wang Q. Preparation and characterization of zein/chitosan complex for encap-sulation of α-tocopherol, and its in vitro controlled release. Colloids Surf. B Biointerfaces. 2011;85:145–152. doi: 10.1016/j.colsurfb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Tsao C.T., Chang C.-H., Lin Y.Y., Wu M.F., Han J.L., Hsieh K.H. Kinetic study of acid depolymerization of chitosan and effects of low molecular weight chitosan on erythrocyte rouleaux formation. Carbohydr. Res. 2011;346:94–102. doi: 10.1016/j.carres.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Yao D.R., Zhou M.Q., Wu S.J., Pan S.K. Depolymerization of chitosan by enzymes from the digestive tract of sea cucumber Stichopus japonicus. Afr. J. Biotechnol. 2012;11:423–428. [Google Scholar]
- 26.Kumar A.B., Tharanathan R.N. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym. 2004;58:275–283. [Google Scholar]
- 27.Novikov V.Y., Mukhin V. Chitosan Depolymerization by Enzymes from the Hepatopancreas of the Crab Paralithodes camtschaticus. Appl. Biochem. Microbiol. 2003;39:464–468. doi: 10.1023/A:1025440501320. [DOI] [PubMed] [Google Scholar]
- 28.Muanprasat C., Chatsudthipong V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017;170:80–97. doi: 10.1016/j.pharmthera.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 29.Enescu D., Olteanu C.E. Functionalized chitosan and its use in pharmaceutical, biomedical, and biotechnological research. Chem. Eng. Commun. 2008;195:1269–1291. doi: 10.1080/00986440801958808. [DOI] [Google Scholar]
- 30.Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative Stress: An Essential Factor in the Pathogenesis of Gastrointestinal Mucosal Diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pittayapruek P., Meephansan J., Prapapan O., Komine M., Ohtsuki M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int. J. Mol. Sci. 2016;17:868. doi: 10.3390/ijms17060868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoo S.-J., Go E., Kim Y.-E., Lee S., Kwon J. Roles of Reactive Oxygen Species in Rheumatoid Arthritis Pathogenesis. J. Rheum. Dis. 2016;23 doi: 10.4078/jrd.2016.23.6.340. [DOI] [Google Scholar]
- 33.Abbas M., Monireh M. The role of reactive oxygen species in immunopathogenesis of rheumatoid arthritis. Iran. J. Allergy Asthma Immunol. 2008;7:195–202. [PubMed] [Google Scholar]
- 34.Rahman T., Hosen I., Islam M.M.T., Shekhar H.U. Oxidative stress and human health. Adv. Biosci. Biotechnol. 2012;3:997–1019. doi: 10.4236/abb.2012.327123. [DOI] [Google Scholar]
- 35.Xie W., Xu P., Liu Q. Antioxidant activity of water-soluble chitosan derivatives. Bioorg. Med. Chem. Lett. 2001;11:1699–1701. doi: 10.1016/S0960-894X(01)00285-2. [DOI] [PubMed] [Google Scholar]
- 36.Hajji S., Younes I., Rinaudo M., Jellouli K., Nasri M. Characterization and in vitro evaluation of cytotoxicity, antimi-crobial and antioxidant activities of chitosans extracted from three different marine sources. Appl. Biochem. Biotechnol. 2015;177:18–35. doi: 10.1007/s12010-015-1724-x. [DOI] [PubMed] [Google Scholar]
- 37.Kim K.W., Thomas R. Antioxidative activity of chitosans with varying molecular weights. Food Chem. 2007;101:308–313. doi: 10.1016/j.foodchem.2006.01.038. [DOI] [Google Scholar]
- 38.Sun T., Zhou D., Xie J., Mao F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2006;225:451–456. doi: 10.1007/s00217-006-0439-1. [DOI] [Google Scholar]
- 39.Chang S.-H., Wu C.-H., Tsai G.-J. Effects of chitosan molecular weight on its antioxidant and antimutagenic properties. Carbohydr. Polym. 2018;181:1026–1032. doi: 10.1016/j.carbpol.2017.11.047. [DOI] [PubMed] [Google Scholar]
- 40.Li H., Xu Q., Chen Y., Wan A. Effect of concentration and molecular weight of chitosan and its derivative on the free radical scavenging ability. J. Biomed. Mater. Res. Part A. 2013;102:911–916. doi: 10.1002/jbm.a.34749. [DOI] [PubMed] [Google Scholar]
- 41.Božič M., Gorgieva S., Kokol V. Laccase-mediated functionalization of chitosan by caffeic and gallic acids for mod-ulating antioxidant and antimicrobial properties. Carbohydr. Polym. 2012;87:2388–2398. doi: 10.1016/j.carbpol.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 42.Shukla S.K., Mishra A.K., Arotiba O., Mamba B. Chitosan-based nanomaterials: A state-of-the-art review. Int. J. Biol. Macromol. 2013;59:46–58. doi: 10.1016/j.ijbiomac.2013.04.043. [DOI] [PubMed] [Google Scholar]
- 43.Park S., Jun S., Marsh K. Physical properties of PVOH/chitosan-blended films cast from different solvents. Food Hydrocoll. 2001;15:499–502. doi: 10.1016/S0268-005X(01)00055-8. [DOI] [Google Scholar]
- 44.Mima S., Miya M., Iwamoto R., Yoshikawa S. Highly deacetylated chitosan and its properties. J. Appl. Polym. Sci. 1983;28:1909–1917. doi: 10.1002/app.1983.070280607. [DOI] [Google Scholar]
- 45.Risbud M.V., Hardikar A.A., Bhat S.V., Bhonde R.R. pH-sensitive freeze-dried chitosan–polyvinyl pyrrolidone hydrogels as controlled release system for antibiotic delivery. J. Control. Release. 2000;68:23–30. doi: 10.1016/S0168-3659(00)00208-X. [DOI] [PubMed] [Google Scholar]
- 46.Strobl G.R. The Physics of Polymers. Springer; Berlin/Heidelberg, Germany: 2007. [Google Scholar]
- 47.Dambies L., Vincent .T., Domard .A.A., Guibal .E. Preparation of Chitosan Gel Beads by Ionotropic Molybdate Gelation. Biomacromolecules. 2001;2:1198–1205. doi: 10.1021/bm010083r. [DOI] [PubMed] [Google Scholar]
- 48.Al-Qadi S., Grenha A., Carrión-Recio D., Seijo B., Remuñán-López C. Microencapsulated chitosan nanoparticles for pulmonary protein delivery: In vivo evaluation of insulin-loaded formulations. J. Control. Release. 2011;157:383–390. doi: 10.1016/j.jconrel.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Thanou M., Kotzé A., Scharringhausen T., Lueßen H., de Boer A., Verhoef J., Junginger H. Effect of degree of quaternization of N-trimethyl chitosan chloride for enhanced transport of hydrophilic compounds across intestinal Caco-2 cell monolayers. J. Control. Release. 2000;64:15–25. doi: 10.1016/S0168-3659(99)00131-5. [DOI] [PubMed] [Google Scholar]
- 50.Bernkop-Schnürch A., Hornof M., Zoidl T. Thiolated polymers—Thiomers: Synthesis and in vitro evaluation of chitosan–2-iminothiolane conjugates. Int. J. Pharm. 2003;260:229–237. doi: 10.1016/S0378-5173(03)00271-0. [DOI] [PubMed] [Google Scholar]
- 51.Sadeghi A., Dorkoosh F., Avadi M., Weinhold M., Bayat A., Delie F., Gurny R., Larijani B., Rafieetehrani M., Junginger H. Permeation enhancer effect of chitosan and chitosan derivatives: Comparison of formulations as soluble polymers and nanoparticulate systems on insulin absorption in Caco-2 cells. Eur. J. Pharm. Biopharm. 2008;70:270–278. doi: 10.1016/j.ejpb.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Sousa F., Guebitz G.M., Kokol V. Antimicrobial and antioxidant properties of chitosan enzymatically functionalized with flavonoids. Process. Biochem. 2009;44:749–756. doi: 10.1016/j.procbio.2009.03.009. [DOI] [Google Scholar]
- 53.Aytekin A.., Morimura S., Kida K. Synthesis of chitosan–caffeic acid derivatives and evaluation of their antioxidant activities. J. Biosci. Bioeng. 2011;111:212–216. doi: 10.1016/j.jbiosc.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 54.Božič M., Štrancar J., Kokol V. Laccase-initiated reaction between phenolic acids and chitosan. React. Funct. Polym. 2013;73:1377–1383. doi: 10.1016/j.reactfunctpolym.2013.01.005. [DOI] [Google Scholar]
- 55.Aljawish A., Chevalot I., Piffaut B., Rondeau-Mouro C., Girardin M., Jasniewski J., Scher J., Muniglia L. Functionalization of chitosan by laccase-catalyzed oxidation of ferulic acid and ethyl ferulate under heterogeneous reaction conditions. Carbohydr. Polym. 2012;87:537–544. doi: 10.1016/j.carbpol.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 56.Silva C., Matamá T., Kim S., Padrão J., Prasetyo E.N., Kudanga T., Nyanhongo G.S., Guebitz G.M., Casal M., Cavaco-Paulo A. Antimicrobial and antioxidant linen via laccase-assisted grafting. React. Funct. Polym. 2011;71:713–720. doi: 10.1016/j.reactfunctpolym.2011.03.011. [DOI] [Google Scholar]
- 57.Munawar A.M., Syeda J.T.M., Wasan K.M., Wasan E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics. 2017;9:53. doi: 10.3390/pharmaceutics9040053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao K., Shi X., Zhao Y., Wei H., Sun Q., Huang T., Zhang X., Wang Y. Preparation and immunological effectiveness of a swine influenza DNA vaccine encapsulated in chitosan nanoparticles. Vaccine. 2011;29:8549–8556. doi: 10.1016/j.vaccine.2011.09.029. [DOI] [PubMed] [Google Scholar]
- 59.Zhuo Y., Han J., Tang L., Liao N., Gui G.-F., Chai Y.-Q., Yuan R. Quenching of the emission of peroxydisulfate system by ferrocene functionalized chitosan nanoparticles: A sensitive “signal off” electrochemiluminescence immunosensor. Sens. Actuators B Chem. 2013;192:791–795. doi: 10.1016/j.snb.2013.11.032. [DOI] [Google Scholar]
- 60.Nagpal K., Singh S., Mishra D.N. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010;58:1423–1430. doi: 10.1248/cpb.58.1423. [DOI] [PubMed] [Google Scholar]
- 61.Hembram K.C., Prabha S., Chandra R., Ahmed B., Nimesh S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2014;44:305–314. doi: 10.3109/21691401.2014.948548. [DOI] [PubMed] [Google Scholar]
- 62.Tiyaboonchai W. Chitosan Nanoparticles: A Promising System for Drug Delivery. Naresuan Univ. J. 2003;11:51–66. [Google Scholar]
- 63.Wang Y., Wang X., Luo G., Dai Y. Adsorption of bovin serum albumin (BSA) onto the magnetic chitosan nanoparticles prepared by a microemulsion system. Bioresour. Technol. 2008;99:3881–3884. doi: 10.1016/j.biortech.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 64.El-Shabouri M. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002;249:101–108. doi: 10.1016/S0378-5173(02)00461-1. [DOI] [PubMed] [Google Scholar]
- 65.Niwa T., Takeuchi H., Hino T., Kunou N., Kawashima Y. Preparations of biodegradable nanospheres of water-soluble and insoluble drugs with D,L-lactide/glycolide copolymer by a novel spontaneous emulsification solvent diffusion method and the drug release behavior. J. Control Release. 1993;25:89–98. doi: 10.1016/0168-3659(93)90097-O. [DOI] [Google Scholar]
- 66.Banerjee T., Mitra S., Singh A.K., Sharma R.K., Maitra A. Preparation, characterization and biodistribution of ultrafine chitosan nanoparticles. Int. J. Pharm. 2002;243:93–105. doi: 10.1016/S0378-5173(02)00267-3. [DOI] [PubMed] [Google Scholar]
- 67.Dube A., Nicolazzo J.A., Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (−)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010;41:219–225. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 68.Barbieri S., Buttini F., Rossi A., Bettini R., Colombo G., Ponchel G., Sonvico F. Ex vivo permeation of tamoxifen and its 4-OH metabolite through rat intestine from lecithin/chitosan nanoparticles. Int. J. Pharm. 2015;491:99–104. doi: 10.1016/j.ijpharm.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 69.Feng C., Wang Z., Jiang C., Kong M., Zhou X., Li Y., Cheng X., Chen X. Chitosan/o-carboxymethyl chitosan nanoparticles for efficient and safe oral anticancer drug delivery: In vitro and in vivo evaluation. Int. J. Pharm. 2013;457:158–167. doi: 10.1016/j.ijpharm.2013.07.079. [DOI] [PubMed] [Google Scholar]
- 70.Joseph J.J., Sangeetha D., Gomathi T. Sunitinib loaded chitosan nanoparticles formulation and its evaluation. Int. J. Biol. Macromol. 2016;82:952–958. doi: 10.1016/j.ijbiomac.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 71.Diop M., Auberval N., Viciglio A., Langlois A., Bietiger W., Mura C., Peronet C., Bekel A., David D.J., Zhao M., et al. Design, characterisation, and bioefficiency of insulin–chitosan nanoparticles after stabilisation by freeze-drying or cross-linking. Int. J. Pharm. 2015;491:402–408. doi: 10.1016/j.ijpharm.2015.05.065. [DOI] [PubMed] [Google Scholar]
- 72.Xue M., Hu S., Lu Y., Zhang Y., Jiang X., An S., Guo Y., Zhou X., Hou H., Jiang C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015;495:771–782. doi: 10.1016/j.ijpharm.2015.08.056. [DOI] [PubMed] [Google Scholar]
- 73.Saboktakin M.R., Tabatabaie R.M., Maharramov A., Ramazanov M. Synthesis and in vitro evaluation of carboxymethyl starch–chitosan nanoparticles as drug delivery system to the colon. Int. J. Biol. Macromol. 2011;48:381–385. doi: 10.1016/j.ijbiomac.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 74.Liu S., Yang S., Ho P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2017;13:72–81. doi: 10.1016/j.ajps.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rawal T., Parmar R., Tyagi R.K., Butani S. Rifampicin loaded chitosan nanoparticle dry powder presents an improved therapeutic approach for alveolar tuberculosis. Colloids Surf. B Biointerfaces. 2017;154:321–330. doi: 10.1016/j.colsurfb.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 76.Jafarinejad S., Gilani K., Moazeni E., Ghazi-Khansari M., Najafabadi A.R., Mohajel N. Development of chitosan-based nanoparticles for pulmonary delivery of itraconazole as dry powder formulation. Powder Technol. 2012;222:65–70. doi: 10.1016/j.powtec.2012.01.045. [DOI] [Google Scholar]
- 77.Giovino C., Ayensu I., Tetteh J., Boateng J. An integrated buccal delivery system combining chitosan films impregnated with peptide loaded PEG-b-PLA nanoparticles. Colloids Surf. B Biointerfaces. 2013;112:9–15. doi: 10.1016/j.colsurfb.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 78.Mazzarino L., Travelet C., Ortega-Murillo S., Otsuka I., Pignot-Paintrand I., Lemos-Senna E., Borsali R. Elaboration of chitosan-coated nanoparticles loaded with curcumin for mucoadhesive applications. J. Colloid Interface Sci. 2012;370:58–66. doi: 10.1016/j.jcis.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 79.Nayak D., Minz A.P., Ashe S., Rauta P.R., Kumari M., Chopra P., Nayak B. Synergistic combination of antioxidants, silver nanoparticles and chitosan in a nanoparticle based formulation: Characterization and cytotoxic effect on MCF-7 breast cancer cell lines. J. Colloid Interface Sci. 2016;470:142–152. doi: 10.1016/j.jcis.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 80.Shah B.R., Zhang C., Li Y., Li B. Bioaccessibility and antioxidant activity of curcumin after encapsulated by nano and Pickering emulsion based on chitosan-tripolyphosphate nanoparticles. Food Res. Int. 2016;89:399–407. doi: 10.1016/j.foodres.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 81.Kaur R., Rajput R., Nag P., Kumar S., Rachana, Singh M. Synthesis, characterization and evaluation of antioxidant properties of catechin hydrate nanoparticles. J. Drug Deliv. Sci. Technol. 2017;39:398–407. doi: 10.1016/j.jddst.2017.04.030. [DOI] [Google Scholar]
- 82.Nallamuthu I., Devi A., Khanum F. Chlorogenic acid loaded chitosan nanoparticles with sustained release property, retained antioxidant activity and enhanced bioavailability. Asian J. Pharm. Sci. 2015;10:203–211. doi: 10.1016/j.ajps.2014.09.005. [DOI] [Google Scholar]
- 83.Wang Q., Zhao Y., Guan L., Zhang Y., Dang Q., Dong P., Li J., Liang X. Preparation of astaxanthin-loaded DNA/chitosan nanoparticles for improved cellular uptake and antioxidation capability. Food Chem. 2017;227:9–15. doi: 10.1016/j.foodchem.2017.01.081. [DOI] [PubMed] [Google Scholar]
- 84.Cerchiara T., Abruzzo A., di Cagno M., Bigucci F., Bauer-Brandl A., Parolin C., Vitali B., Gallucci M., Luppi B. Chitosan based micro- and nanoparticles for colon-targeted delivery of vancomycin prepared by alternative processing methods. Eur. J. Pharm. Biopharm. 2015;92:112–119. doi: 10.1016/j.ejpb.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 85.Coco R., Plapied L., Pourcelle V., Jérôme C., Brayden D., Schneider Y.-J., Préat V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013;440:3–12. doi: 10.1016/j.ijpharm.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 86.Frank L.A., Chaves P.S., D’Amore C.M., Contri R.V., Frank A.G., Beck R., Pohlmann A.R., Buffon A., Guterres S.S. The use of chitosan as cationic coating or gel vehicle for polymeric nanocapsules: Increasing penetration and adhesion of imiquimod in vaginal tissue. Eur. J. Pharm. Biopharm. 2017;114:202–212. doi: 10.1016/j.ejpb.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 87.Gao P., Xia G., Bao Z., Feng C., Cheng X., Kong M., Liu Y., Chen X. Chitosan based nanoparticles as protein carriers for efficient oral antigen delivery. Int. J. Biol. Macromol. 2016;91:716–723. doi: 10.1016/j.ijbiomac.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 88.Miladi K., Sfar S., Fessi H., Elaissari A. Enhancement of alendronate encapsulation in chitosan nanoparticles. J. Drug Deliv. Sci. Technol. 2015;30:391–396. doi: 10.1016/j.jddst.2015.04.007. [DOI] [Google Scholar]
- 89.Bagre A.P., Jain K., Jain N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013;456:31–40. doi: 10.1016/j.ijpharm.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 90.Wang J., Tan J., Luo J., Huang P., Zhou W., Chen L., Long L., Zhang L.-M., Zhu B., Yang L., et al. Enhancement of scutellarin oral delivery efficacy by vitamin B12-modified amphiphilic chitosan derivatives to treat type II diabetes induced-retinopathy. J. Nanobiotechnol. 2017;15:1–17. doi: 10.1186/s12951-017-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Huang Y.-C., Chen J.-K., Lam U.-I., Chen S.-Y. Preparing, characterizing, and evaluating chitosan/fucoidan nanoparticles as oral delivery carriers. J. Polym. Res. 2014;21:1–9. doi: 10.1007/s10965-014-0415-6. [DOI] [Google Scholar]
- 92.Shi Y., Xue J., Jia L., Du Q., Niu J., Zhang D. Surface-modified PLGA nanoparticles with chitosan for oral delivery of tol-butamide. Colloids Surf. B Biointerfaces. 2018;161:67–72. doi: 10.1016/j.colsurfb.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 93.Derakhshandeh K., Fathi S. Role of chitosan nanoparticles in the oral absorption of Gemcitabine. Int. J. Pharm. 2012;437:172–177. doi: 10.1016/j.ijpharm.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 94.Maity S., Mukhopadhyay P., Kundu P.P., Chakraborti A.S. Alginate coated chitosan core-shell nanoparticles for efficient oral delivery of naringenin in diabetic animals—An in vitro and in vivo approach. Carbohydr. Polym. 2017;170:124–132. doi: 10.1016/j.carbpol.2017.04.066. [DOI] [PubMed] [Google Scholar]
- 95.Liang J., Yan H., Yang H.-J., Kim H.W., Wan X., Lee J., Ko S. Synthesis and controlled-release properties of chi-tosan/β-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016;25:1583–1590. doi: 10.1007/s10068-016-0244-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Aluani D., Tzankova V., Kondeva-Burdina M., Yordanov Y., Nikolova E., Odzhakov F., Apostolov A., Markova T., Yoncheva K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quer-cetin. Int. J. Biol. Macromol. 2017;103:771–782. doi: 10.1016/j.ijbiomac.2017.05.062. [DOI] [PubMed] [Google Scholar]
- 97.Ni S., Liu Y., Tang Y., Chen J., Li S., Pu J., Han L. GABA B receptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2017;179:135–144. doi: 10.1016/j.carbpol.2017.09.075. [DOI] [PubMed] [Google Scholar]
- 98.Trapani A., Di Gioia S., Ditaranto N., Cioffi N., Goycoolea F.M., Carbone A., Garcia-Fuentes M., Conese M., Alonso M.J. Systemic heparin delivery by the pulmonary route using chitosan and glycol chitosan nanoparticles. Int. J. Pharm. 2013;447:115–123. doi: 10.1016/j.ijpharm.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 99.Lee D.-W., Shirley S.A., Lockey R.F., Mohapatra S.S. Thiolated chitosan nanoparticles enhance anti-inflammatory effects of intranasally delivered theophylline. Respir. Res. 2006;7:112. doi: 10.1186/1465-9921-7-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lytting E., Nguyen J., Wang X., Kissel T. Biodegradable polymeric nanocarriers for pulmonary drug delivery Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008;56:629–639. doi: 10.1517/17425247.5.6.629. [DOI] [PubMed] [Google Scholar]
- 101.Wang X., Chi N., Tang X. Preparation of estradiol chitosan nanoparticles for improving nasal absorption and brain targeting. Eur. J. Pharm. Biopharm. 2008;70:735–740. doi: 10.1016/j.ejpb.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 102.Vila A., Sánchez A., Janes K., Behrens I., Kissel T., Jato J.L.V., Alonso M.J. Low molecular weight chitosan nanoparticles as new carriers for nasal vaccine delivery in mice. Eur. J. Pharm. Biopharm. 2004;57:123–131. doi: 10.1016/j.ejpb.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 103.Soares P.I.P., Isabel A., Carvalho J., Ferreira I.M.M., Novo C.M.M., Paulo J. Chitosan-based nanoparticles as drug delivery systems for doxorubicin: Optimization and modelling. Carbohydr. Polym. 2016;147:304–312. doi: 10.1016/j.carbpol.2016.03.028. [DOI] [PubMed] [Google Scholar]
- 104.Yostawonkul J., Surassmo S., Iempridee T., Pimtong W., Suktham K., Sajomsang W., Gonil P., Ruktanonchai U.R. Surface modification of nanostructure lipid carrier (NLC) by oleoyl-quaternized-chitosan as a mucoadhesive nanocarrier. Colloids Surf. B Biointerfaces. 2016;149:301–311. doi: 10.1016/j.colsurfb.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 105.Yu X., Mu Y., Xu M., Xia G., Wang J., Liu Y., Chen X. Preparation and characterization of mucosal adhesive and two-step drug releasing cetirizine-chitosan nanoparticle. Carbohydr. Polym. 2017;173:600–609. doi: 10.1016/j.carbpol.2017.05.067. [DOI] [PubMed] [Google Scholar]
- 106.Costa I.D.S.M., Abranches R.P., Garcia M.T.J., Pierre M.B.R. Chitosan-based mucoadhesive films containing 5-aminolevulinic acid for buccal cancer’s treatment. J. Photochem. Photobiol. B Biol. 2014;140:266–275. doi: 10.1016/j.jphotobiol.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Mazzarino L., Borsali R., Lemos-Senna E. Mucoadhesive Films Containing Chitosan-Coated Nanoparticles: A New Strategy for Buccal Curcumin Release. J. Pharm. Sci. 2014;103:3764–3771. doi: 10.1002/jps.24142. [DOI] [PubMed] [Google Scholar]
- 108.Al-Kassas R., Wen J., Cheng A.E.-M., Kim A.M.-J., Liu S.S.M., Yu J. Transdermal delivery of propranolol hydrochloride through chitosan nanoparticles dispersed in mucoadhesive gel. Carbohydr. Polym. 2016;153:176–186. doi: 10.1016/j.carbpol.2016.06.096. [DOI] [PubMed] [Google Scholar]
- 109.dos Santos T.C., Rescignano N., Boff L., Reginatto F.H., Simões C.M.O., de Campos A.M., Mijangos C.U. Manufacture and characterization of chitosan/PLGA nanoparticles nanocomposite buccal films. Carbohydr. Polym. 2017;173:638–644. doi: 10.1016/j.carbpol.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 110.Pistone S., Goycoolea F.M., Young A., Smistad G., Hiorth M. Formulation of polysaccharide-based nanoparticles for local administration into the oral cavity. Eur. J. Pharm. Sci. 2017;96:381–389. doi: 10.1016/j.ejps.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 111.Giovino C., Ayensu I., Tetteh J., Boateng J. Development and characterisation of chitosan films impregnated with insulin loaded PEG-b-PLA nanoparticles (NPs): A potential approach for buccal delivery of macromolecules. Int. J. Pharm. 2012;428:143–151. doi: 10.1016/j.ijpharm.2012.02.035. [DOI] [PubMed] [Google Scholar]
- 112.Suvannasara P., Juntapram K., Praphairaksit N., Siralertmukul K., Muangsin N. Mucoadhesive 4-carboxybenzenesulfonamide-chitosan with antibacterial properties. Carbohydr. Polym. 2013;94:244–252. doi: 10.1016/j.carbpol.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 113.Baptista da Silva S., Ferreira D., Pintado M., Sarmento B. Chitosan-based nanoparticles for rosmarinic acid ocular delivery—In vitro tests. Int. J. Biol. Macromol. 2016;84:112–120. doi: 10.1016/j.ijbiomac.2015.11.070. [DOI] [PubMed] [Google Scholar]
- 114.Sarjono P.R., Putri L.D., Budiarti C.E., Mulyani N.S., Kusrini D., Prasetya N.B. Antioxidant and antibacterial activities of secondary metabolite endophytic bacteria from papaya leaf (Carica papaya L.) IOP Conf. Ser. Mater. Sci. Eng. 2019;509:012112. doi: 10.1088/1757-899X/509/1/012112. [DOI] [Google Scholar]
- 115.Zhou Y.S., Yang D.Z., Chen X.M., Xu Q., Lu F.M., Nie J. Electrospun water-soluble carboxyethyl chitosan/poly(vinyl al-cohol) nanofibrous membrane as potential wound dressing for skin regeneration. Biomacromolecules. 2008;9:349–354. doi: 10.1021/bm7009015. [DOI] [PubMed] [Google Scholar]
- 116.Zhou Y., Yang H., Liu X., Mao J., Gu S., Xu W. Electrospinning of carboxyethyl chitosan/poly(vinyl alcohol)/silk fibroin nanoparticles for wound dressings. Int. J. Biol. Macromol. 2012;53:88–92. doi: 10.1016/j.ijbiomac.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 117.Toshkova R., Manolova N., Gardeva E., Ignatova M., Yossifova L., Rashkov I., Alexandrov M. Antitumor activity of quaternized chitosan-based electrospun implants against Graffi myeloid tumor. Int. J. Pharm. 2010;400:221–233. doi: 10.1016/j.ijpharm.2010.08.039. [DOI] [PubMed] [Google Scholar]
- 118.Alipour S.M., Nouri M., Mokhtari J., Bahrami S.H. Electrospinning of poly(vinyl alcohol)–water-soluble quaternized chitosan derivative blend. Carbohydr. Res. 2009;344:2496–2501. doi: 10.1016/j.carres.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Ignatova M., Starbova K., Markova N., Manolova N., Rashkov I. Electrospun nano-fibre mats with antibacterial properties from quaternised chitosan and poly(vinyl alcohol) Carbohydr. Res. 2006;341:2098–2107. doi: 10.1016/j.carres.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 120.Ignatova M., Manolova N., Rashkov I. Novel antibacterial fibers of quaternized chitosan and poly(vinyl pyrrolidone) pre-pared by electrospinning. Eur. Polym. J. 2007;43:1112–1122. doi: 10.1016/j.eurpolymj.2007.01.012. [DOI] [Google Scholar]
- 121.Bai B., Mi X., Xiang X., Heiden P.A., Heldt C.L. Non-enveloped virus reduction with quaternized chitosan nanofibers containing graphene. Carbohydr. Res. 2013;380:137–142. doi: 10.1016/j.carres.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 122.Ignatova M., Manolova N., Markova N., Rashkov I. Electrospun Non-Woven Nanofibrous Hybrid Mats Based on Chitosan and PLA for Wound-Dressing Applications. Macromol. Biosci. 2009;9:102–111. doi: 10.1002/mabi.200800189. [DOI] [PubMed] [Google Scholar]
- 123.Chen H.L., Huang J., Yu J.H., Liu S.Y., Gu P. Electrospun chitosan-graft-poly (epsilon-caprolactone)/poly (epsi-lon-caprolactone) cationic nanofibrous mats as potential scaffolds for skin tissue engineering. Int. J. Biol. Macromol. 2011;48:13–19. doi: 10.1016/j.ijbiomac.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 124.Razi M.A., Wakabayashi R., Goto M., Kamiya N. Self-Assembled Reduced Albumin and Glycol Chitosan Nanoparticles for Paclitaxel Delivery. Langmuir. 2019;35:2610–2618. doi: 10.1021/acs.langmuir.8b02809. [DOI] [PubMed] [Google Scholar]
- 125.Nair R.S., Morris A., Billa N., Leong C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech. 2019;20:69. doi: 10.1208/s12249-018-1279-6. [DOI] [PubMed] [Google Scholar]
- 126.Wang T., Hou J., Su C., Zhao L., Shi Y. Hyaluronic acid-coated chitosan nanoparticles induce ROS-mediated tumor cell apoptosis and enhance antitumor efficiency by targeted drug delivery via CD44. J. Nanobiotechnol. 2017;15:1–12. doi: 10.1186/s12951-016-0245-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Esfandiari F., Motazedian M., Asgari Q., Morowvat M.H., Molaei M., Heli H. Paromomycin-loaded mannosylated chitosan nanoparticles: Synthesis, characterization and targeted drug delivery against leishmaniasis. Acta Trop. 2019;197:105045. doi: 10.1016/j.actatropica.2019.105045. [DOI] [PubMed] [Google Scholar]
- 128.Khan M.M., Madni A., Torchilin V., Filipczak N., Pan J., Tahir N., Shah H. Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Deliv. 2019;26:765–772. doi: 10.1080/10717544.2019.1642420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Piazzini V., Landucci E., D’Ambrosio M., Fasiolo L.T., Cinci L., Colombo G., Pellegrini-Giampietro D.E., Bilia A.R., Luceri C., Bergonzi M.C. Chitosan coated human serum albumin nanoparticles: A promising strategy for nose-to-brain drug delivery. Int. J. Biol. Macromol. 2019;129:267–280. doi: 10.1016/j.ijbiomac.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 130.Gomillion C.T. Assessing the potential of chitosan/polylactide nanoparticles for delivery of therapeutics for tri-ple-negative breast cancer treatment. Regener. Eng. Transl. Med. 2019;5:61–73. [Google Scholar]
- 131.Abdelhamid H.N., El-Bery H.M., Metwally A.A., Elshazly M., Hathout R.M. Synthesis of CdS-modified chitosan quantum dots for the drug delivery of Sesamol. Carbohydr. Polym. 2019;214:90–99. doi: 10.1016/j.carbpol.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 132.Chen C., Yao W., Sun W., Guo T., Lv H., Wang X., Ying H., Wang Y., Wang P. A self-targeting and controllable drug delivery system constituting mesoporous silica nanoparticles fabricated with a multi-stimuli responsive chitosan-based thin film layer. Int. J. Biol. Macromol. 2018;122:1090–1099. doi: 10.1016/j.ijbiomac.2018.09.058. [DOI] [PubMed] [Google Scholar]
- 133.Mohammed M., Mansell H., Shoker A., Wasan K.M., Wasan E.K. Development and in vitro characterization of chi-tosan-coated polymeric nanoparticles for oral delivery and sustained release of the immunosuppressant drug mycophenolate mofetil. Drug Dev. Ind. Pharm. 2019;45:76–87. doi: 10.1080/03639045.2018.1518455. [DOI] [PubMed] [Google Scholar]
- 134.Wang H., Mukherjee S., Yi J., Banerjee P., Chen Q., Zhou S. Biocompatible Chitosan–Carbon Dot Hybrid Nanogels for NIR-Imaging-Guided Synergistic Photothermal–Chemo Therapy. ACS Appl. Mater. Interfaces. 2017;9:18639–18649. doi: 10.1021/acsami.7b06062. [DOI] [PubMed] [Google Scholar]
- 135.Belabassi Y., Moreau J., Gheran V., Henoumont C., Robert A., Callewaert M., Rigaux G., Cadiou C., Elst L.V., Laurent S., et al. Synthesis and Characterization of PEGylated and Fluorinated Chitosans: Application to the Synthesis of Targeted Nanoparticles for Drug Delivery. Biomacromolecules. 2017;18:2756–2766. doi: 10.1021/acs.biomac.7b00668. [DOI] [PubMed] [Google Scholar]
- 136.Shi S., Zhang Z., Luo Z., Yu J., Liang R., Li X., Chen H. Chitosan grafted methoxy poly(ethylene gly-col)-poly(epsilon-caprolactone) nanosuspension for ocular delivery of hydrophobic diclofenac. Sci. Rep. 2015;5:11337–11348. doi: 10.1038/srep11337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sun M., Li J., Zhang C., Xie Y., Qiao H., Su Z., Oupicky D., Ping Q. Arginine-Modified Nanostructured Lipid Carriers with Charge-Reversal and pH-Sensitive Membranolytic Properties for Anticancer Drug Delivery. Adv. Healthc. Mater. 2017;6:1600693–1600705. doi: 10.1002/adhm.201600693. [DOI] [PubMed] [Google Scholar]
- 138.Marques J., Valle-Delgado J.J., Urban P., Baró E., Prohens R., Mayor A., Cisteró P., Delves M., Sinden R.E., Grandfils C., et al. Adaptation of targeted nanocarriers to changing requirements in antimalarial drug delivery. Nanomed. Nanotechnol. Biol. Med. 2017;13:515–525. doi: 10.1016/j.nano.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Luo L., Bian Y., Liu Y., Zhang X., Wang M., Xing S., Li L., Gao D. Combined Near Infrared Photothermal Therapy and Chemotherapy Using Gold Nanoshells Coated Liposomes to Enhance Antitumor Effect. Small. 2016;12:4103–4112. doi: 10.1002/smll.201503961. [DOI] [PubMed] [Google Scholar]
- 140.Yan L., Crayton S.H., Thawani J.P., Amirshaghaghi A., Tsourkas A., Cheng Z. A pH-Responsive Drug-Delivery Platform Based on Glycol Chitosan-Coated Liposomes. Small. 2015;11:4870–4874. doi: 10.1002/smll.201501412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Romainor A.N.B., Chin S.F., Pang S.C., Bilung L.M. Preparation and Characterization of Chitosan Nanoparticles-Doped Cellulose Films with Antimicrobial Property. J. Nanomater. 2014;2014:710459. doi: 10.1155/2014/710459. [DOI] [Google Scholar]
- 142.Zhang H., Wu F., Li Y., Yang X., Huang J., Lv T., Zhang Y., Chen J., Chen H., Gao Y., et al. Chitosan-based nanoparticles for improved anticancer efficacy and bioavailability of mifepristone. Beilstein J. Nanotechnol. 2016;7:1861–1870. doi: 10.3762/bjnano.7.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang P., Shen Y., Zhao L. Chitosan nanoparticles loaded with aspirin and 5-fluororacil enable synergistic antitumour activity through the modulation of NF-κB/COX-2 signalling pathway. IET Nanobiotechnol. 2020;14:479–484. doi: 10.1049/iet-nbt.2020.0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Abu-Zaied M.A., Loutfy S.A., Hassan A.E., Elgemeie G.H. Novel purine thioglycoside analogs: Synthesis, nanoformulation and biological evaluation in in vitro human liver and breast cancer models. Drug Des. Dev. Ther. 2019;13:2437–2457. doi: 10.2147/DDDT.S201249. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145.Deepa G., Sivakumar K.C., Sajeevan T.P. Molecular simulation and in vitro evaluation of chitosan nanoparticles as drug delivery systems for the controlled release of anticancer drug cytarabine against solid tumours. 3 Biotech. 2018;8:493. doi: 10.1007/s13205-018-1510-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cavalli R., Leone F., Minelli R., Fantozzi R., Dianzani C. New Chitosan Nanospheres for the Delivery of 5-Fluorouracil: Preparation, Characterization and in vitro Studies. Curr. Drug Deliv. 2014;11:270–278. doi: 10.2174/1567201811666140206103609. [DOI] [PubMed] [Google Scholar]
- 147.Sahu P., Kashaw S.K., Sau S., Kushwah V., Jain S., Agrawal R.K., Iyer A.K. pH Responsive 5-Fluorouracil Loaded Biocompatible Nanogels For Topical Chemotherapy of Aggressive Melanoma. Colloids Surf. B Biointerfaces. 2018;174:232–245. doi: 10.1016/j.colsurfb.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Keerthikumarc W., Jalalpure S., Mallashwara Rao P.V.S. Chitosan encapsulated Curcumin nanoparticles as an effective drug delivery system for oral cancer treatment. Indian Drugs. 2015;52:40–48. [Google Scholar]
- 149.Shahiwala A., Shehab N., Khider M., Khan R. Chitosan nanoparticles as a carrier for indigofera intricata plant extract: Prepa-ration, characterization and anticancer activity. Curr. Cancer Ther. Rev. 2019;15:162–169. doi: 10.2174/1573394714666181008112804. [DOI] [Google Scholar]
- 150.Alipour M., Bigdeli M., Aligholi H., Rasoulian B., Khaksarian M. Sustained release of silibinin-loaded chitosan nanoparticle in-duced apoptosis in glioma cells. J. Biomed. Mater. Res. A. 2020;3:458–469. doi: 10.1002/jbm.a.36827. [DOI] [PubMed] [Google Scholar]
- 151.Xu Y., Wen Z., Xu Z. Chitosan Nanoparticles Inhibit the Growth of Human Hepatocellular Carcinoma Xenografts through an Antiangiogenic Mechanism. Anticancer Res. 2009;29:5103–5109. [PubMed] [Google Scholar]
- 152.Sharifi-Rad J., Quispe C., Butnariu M., Rotariu L.S., Sytar O., Sestito S., Rapposelli S., Akram M., Iqbal M., Krishna A., et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021;21:1–21. doi: 10.1186/s12935-021-02025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Santoso A.V., Susanto A., Irawaty W., Hadisoewignyo L., Hartono S.B. Chitosan modified mesoporous silica nanoparticles as a versatile drug carrier with pH dependent properties. AIP Conf. Proc. 2019;2114:20011. doi: 10.1063/1.5112395. [DOI] [Google Scholar]
- 154.Lv Y., Li K., Li Y. Surface modification of quantum dots and magnetic nanoparticles with PEG-conjugated chi-tosan derivatives for biological applications. Chem. Pap. 2013;67:1404–1413. doi: 10.2478/s11696-013-0401-1. [DOI] [Google Scholar]
- 155.Ai J.-w., Liao W., Ren Z.-L. Enhanced anticancer effect of copper-loaded chitosan nanoparticles against osteosarcoma. RSC Adv. 2017;7:15971–15977. doi: 10.1039/C6RA21648J. [DOI] [Google Scholar]
- 156.Mazzotta E., De Benedittis S., Qualtieri A., Muzzalupo R. Actively Targeted and Redox Responsive Delivery of Anticancer Drug by Chitosan. Nanoparticles Pharm. 2020;12:26. doi: 10.3390/pharmaceutics12010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jia M., Li Y., Yang X., Huang Y., Wu H., Huang Y., Lin J., Li Y., Hou Z., Zhang Q. Development of Both Methotrexate and Mitomycin C Loaded PEGylated Chitosan Nanoparticles for Targeted Drug Codelivery and Synergistic Anticancer Effect. ACS Appl. Mater. Interfaces. 2014;6:11413–11423. doi: 10.1021/am501932s. [DOI] [PubMed] [Google Scholar]
- 158.Singh A.V. Anti-breast cancer activity of folate-chitosan nanoparticles loaded with irinotecan. Ann. Oncol. 2017;28:x2–x3. doi: 10.1093/annonc/mdx652.006. [DOI] [Google Scholar]
- 159.Liu M., Chang Y., Yang J., You Y., He R., Chen T., Zhou C. Functionalized halloysite nanotube by chitosan grafting for drug de-livery of curcumin to achieve enhanced anticancer efficac. J. Mater. Chem. B. 2016;4:2253–2263. doi: 10.1039/C5TB02725J. [DOI] [PubMed] [Google Scholar]
- 160.Abbas Y., Azzazy H.M., Tammam S., Lamprecht A., Ali M.E., Schmidt A., Sollazzo S., Mathur S. Development of an inhalable, stimuli-responsive particulate system for delivery to deep lung tissue. Colloids Surf. B Biointerfaces. 2016;146:19–30. doi: 10.1016/j.colsurfb.2016.05.031. [DOI] [PubMed] [Google Scholar]
- 161.Almutairi F., Abd-Rabou A., Mohamed M.S. Raloxifene-encapsulated hyaluronic acid-decorated chitosan nanoparticles selec-tively induce apoptosis in lung cancer cells. Bioorg. Med. Chem. 2019;27:1629–1638. doi: 10.1016/j.bmc.2019.03.004. [DOI] [PubMed] [Google Scholar]
- 162.Bae M.S., Kwon I.C., Jeong S.Y., Lee K.Y. Nano-structured chitosan self-aggregates as a drug delivery carrier; Proceedings of the NSTI Nanotechnology Conference and Trade Show—NSTI Nanotech 2005; Anaheim, CA, USA. 8–12 May 2005. [Google Scholar]
- 163.Wu S., Yang X., Lu Y., Fan Z., Li Y., Jiang Y., Hou Z. A green approach to dual-drug nanoformulations with targeting and syn-ergistic effects for cancer therapy. Drug Deliv. 2017;24:51–60. doi: 10.1080/10717544.2016.1228716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Li Z., Yang F., Yang R. Synthesis and characterization of chitosan derivatives with dual-antibacterial functional groups. Int. J. Biol. Macromol. 2015;75:378–387. doi: 10.1016/j.ijbiomac.2015.01.056. [DOI] [PubMed] [Google Scholar]
- 165.Roy K., Kanwar R.K., Kanwar J.R. Corrigendum to “LNA aptamer based multi-modal, Fe3O4-saturated lactoferrin (Fe3O4-bLf) nanocarriers for triple positive (EpCAM, CD133, CD44) colon tumour targeting and NIR, MRI and CT imaging” [Biomaterials 71C (2015) 84–99] Biomaterials. 2017;138:118–120. doi: 10.1016/j.biomaterials.2017.05.041. [DOI] [PubMed] [Google Scholar]
- 166.Arunkumar P., Indulekha S., Vijayalakshmi S., Srivastava R. In vitro comparative studies of Zein nanoparticles and composite Chitosan thermogels based injectable formulation of Doxorubicin. J. Drug Deliv. Sci. Technol. 2017;40:116–124. doi: 10.1016/j.jddst.2017.05.015. [DOI] [Google Scholar]
- 167.Hwang H.-Y., Kim I.-S., Kwon I.C., Kim Y.-H. Tumor targetability and antitumor effect of docetaxel-loaded hydrophobically modified glycol chitosan nanoparticles. J. Control. Release. 2008;128:23–31. doi: 10.1016/j.jconrel.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 168.Gomathi T., Sudha P., Florence J.A.K., Venkatesan J., Anil S. Fabrication of letrozole formulation using chitosan nanoparticles through ionic gelation method. Int. J. Biol. Macromol. 2017;104:1820–1832. doi: 10.1016/j.ijbiomac.2017.01.147. [DOI] [PubMed] [Google Scholar]
- 169.Wang Z., Zhao W. Optimized preparation of gefitinib chitosan protamine nanoparticles by central composite design-response surface method. Chin. J. New Drugs. 2016;25:807–812. [Google Scholar]
- 170.Koo H., Min K., Lee S.C., Park J., Park K., Jeong S., Choi K., Kwon I.C., Kim K. Enhanced drug-loading and therapeutic efficacy of hydrotropic oligomer-conjugated glycol chitosan nanoparticles for tumor-targeted paclitaxel deliver. J. Control Release. 2013;172:823–831. doi: 10.1016/j.jconrel.2013.08.297. [DOI] [PubMed] [Google Scholar]
- 171.Maya S., Kumar L.G., Sarmento B., Rejinold N.S., Menon D., Nair S.V., Jayakumar R. Cetuximab conjugated O-carboxymethyl chitosan nanoparticles for targeting EGFR overexpressing cancer cells. Carbohydr. Polym. 2013;93:661–669. doi: 10.1016/j.carbpol.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 172.Al-Musawi S., Jawad A., Hadi S., Hindi N. Preparation and characterization of folated chitosan/magnetic nanocarrier for 5-fluorouracil drug delivery and studying its effect in bladder cancer therapy. J. Glob. Pharm. Technol. 2019;11:628–637. [Google Scholar]
- 173.Anitha A., Gopal D., Rani V.V., Menon D. Preparation, characterization, in vitro drug release and biological studies of curcumin loaded dextran sulphate-chitosan nanoparticles. Carbohydr. Polym. 2011;84:1158–1164. doi: 10.1016/j.carbpol.2011.01.005. [DOI] [Google Scholar]
- 174.Baghbani F., Chegeni M., Moztarzadeh F., Hadian-Ghazvini S., Raz M. Novel ultrasound-responsive chitosan/perfluorohexane nanodroplets for image-guided smart delivery of an anticancer agent: Curcumin. Mater. Sci. Eng. C. 2017;74:186–193. doi: 10.1016/j.msec.2016.11.107. [DOI] [PubMed] [Google Scholar]
- 175.Rajan S.S., Pandian A., Palaniappan T. Curcumin loaded in bovine serum albumin–chitosan derived nanoparticles for targeted drug delivery. Bull. Mater. Sci. 2016;39:811–817. doi: 10.1007/s12034-016-1213-z. [DOI] [Google Scholar]
- 176.George D., Maheswari P.U., Begum K.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020;236:116101. doi: 10.1016/j.carbpol.2020.116101. [DOI] [PubMed] [Google Scholar]
- 177.Chaichanasak N., Rojanapanthu P., Yoon Y., Gritsanapan W., Chirachanchai S., Sathirakul K., Nualsanit T., Seong J.K., Baek S.J. Chitosan-based nanoparticles with damnacanthal suppress CRM1 expression. Oncol. Lett. 2018;16:7029–7034. doi: 10.3892/ol.2018.9507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sivanesan I., Muthu M., Gopal J., Hasan N., Ali S.K., Shin J., Oh J.-W. Nanochitosan: Commemorating the Metamorphosis of an ExoSkeletal Waste to a Versatile Nutraceutical. Nanomaterials. 2021;11:821. doi: 10.3390/nano11030821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sivanesan I., Gopal J., Muthu M., Shin J., Mari S., Oh J. Green Synthesized Chitosan/Chitosan Nanoforms/Nanocomposites for Drug Delivery Applications. Polymers. 2021;13:2256. doi: 10.3390/polym13142256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Adhikari H.S., Yadav P.S. Anticancer Activity of Chitosan, Chitosan Derivatives, and Their Mechanism of Action. Int. J. Biomater. 2018;2018:2952085. doi: 10.1155/2018/2952085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gavhane Y.N., Gurav A.S., Yadav A.V. Chitosan and Its Applications: A Review of Literature. Int. J. Res. Pharm. Biomed. Sci. 2013;4:312–331. [Google Scholar]
- 182.Nam K.-S., Shon Y.-H. Suppression of metastasis of human breast cancer cells by chitosan oligosaccharides. J. Microbiol. Biotechnol. 2009;19:629–633. doi: 10.4014/jmb.0811.603. [DOI] [PubMed] [Google Scholar]
- 183.Kuppusamy S., Karuppaiah J. Screening of Antiproliferative Effect of Chitosan on Tumor Growth and Metastasis in T24 Urinary Bladder Cancer Cell Line. Austrl-Asian J. Cancer. 2013;12:145–149. [Google Scholar]
- 184.Hasegawa M., Yagi K., Iwakawa S., Hirai M. Chitosan Induces Apoptosis via Caspase-3 Activation in Bladder Tumor Cells. Jpn. J. Cancer Res. 2001;92:459–466. doi: 10.1111/j.1349-7006.2001.tb01116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Jiang Z., Han B., Li H., Yang Y., Liu W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015;129:1–8. doi: 10.1016/j.carbpol.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 186.Hosseinzadeh H., Atyabi F., Dinarvand R., Ostad S.N. Chitosan-Pluronic nanoparticles as oral delivery of anticancer gemcitabine: Preparation and in vitro study. Int. J. Nanomed. 2012;7:1851–1863. doi: 10.2147/IJN.S26365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Qi L., Xu Z., Chen M. In vitro and in vivo suppression of hepatocellular carcinoma growth by chitosan nanoparticles. Eur. J. Cancer. 2007;43:184–193. doi: 10.1016/j.ejca.2006.08.029. [DOI] [PubMed] [Google Scholar]
- 188.Ramasamy T., Ruttala H.B., Chitrapriya N., Poudal B.K., Choi J.Y., Kim S.T., Youn Y.S., Ku S.K., Choi H.-G., Yong C.S., et al. Engineering of cell microenvironment-responsive polypeptide nanovehicle co-encapsulating a synergistic combination of small molecules for effective chemotherapy in solid tumors. Acta Biomater. 2017;48:131–143. doi: 10.1016/j.actbio.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 189.Zeng Z.W., Wang J.J., Xiao R.Z., Xie T., Zhou G.L., Zhan X.R., Wang S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011;6:765–774. doi: 10.2147/IJN.S17296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Casettari L., Illum L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release. 2014;190:189–200. doi: 10.1016/j.jconrel.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 191.Gao J.-Q., Hu Y.-L., Wang Q., Han F., Shao J.Z. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011;6:3351–3359. doi: 10.2147/IJN.S25853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mills K.H.G., Cosgrove C., McNeela E.A., Sexton A., Giemza R., Jabbal-Gill I., Church A., Lin W., Illum L., Podda A., et al. Protective Levels of Diphtheria-Neutralizing Antibody Induced in Healthy Volunteers by Unilateral Priming-Boosting Intranasal Immunization Associated with Restricted Ipsilateral Mucosal Secretory Immunoglobulin A. Infect. Immun. 2003;71:726–732. doi: 10.1128/IAI.71.2.726-732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.El-Kamary S.S., Pasetti M.F., Mendelman P.M., Frey S.E., Bernstein D.I., Treanor J.J., Ferreira J., Chen W.H., Sublett R., Richardson C., et al. Adjuvanted Intranasal Norwalk Virus-Like Particle Vaccine Elicits Antibodies and Antibody-Secreting Cells That Express Homing Receptors for Mucosal and Peripheral Lymphoid Tissues. J. Infect. Dis. 2010;202:1649–1658. doi: 10.1086/657087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ramirez K., Wahid R., Richardson C., Bargatze R.F., El-Kamary S.S., Sztein M.B., Pasetti M.F. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin. Immunol. 2012;144:98–108. doi: 10.1016/j.clim.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 195.Islam N., Ferro V. Recent advances in chitosan-based nanoparticulate pulmonary drug delivery. Nanoscale. 2016;8:14341–14358. doi: 10.1039/C6NR03256G. [DOI] [PubMed] [Google Scholar]
- 196.Aragao-Santiago L., Hillaireau H., Grabowski N., Mura S., Nascimento T.L., Dufort S., Coll J.-L., Tsapis N., Fattal E. Compared in vivo toxicity in mice of lung delivered biodegradable andnon-biodegradable nanoparticles. Nanotoxicology. 2016;10:292–302. doi: 10.3109/17435390.2015.1054908. [DOI] [PubMed] [Google Scholar]
- 197.Hua S., Marks E., Schneider J.J., Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in in-flammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomedicine. 2015;11:1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 198.Grenha A., Grainger C.I., Dailey L.A., Seijo B., Martin G.P., Remuñán-López C., Forbes B. Chitosan nanoparticles are compatible with respiratory epithelial cells in vitro. Eur. J. Pharm. Sci. 2007;31:73–84. doi: 10.1016/j.ejps.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 199.Bellich B., D’Agostino I., Semeraro S., Gamini A., Cesàro A. “The good, the bad and the ugly” of chitosans. Mar. Drugs. 2016;14:99. doi: 10.3390/md14050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Key J., Park K. Multicomponent, Tumor-Homing Chitosan Nanoparticles for Cancer Imaging and Therapy. Int. J. Mol. Sci. 2017;18:594. doi: 10.3390/ijms18030594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang X., Yang X., Ji J., Liu A., Zhai G. Tumor targeting strategies for chitosan-based nanoparticles. Colloids Surfaces B: Biointerfaces. 2016;148:460–473. doi: 10.1016/j.colsurfb.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 202.Ahmed T.A., Aljaeid B.M. Preparation, characterization and potential application of chitosan, chitosan derivatives and chitosan metal nanoparticles in pharmaceutical drug delivery. Drug Des. Devel. Ther. 2016;10:483–507. doi: 10.2147/DDDT.S99651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Swierczewska M., Han H., Kim K., Park J., Lee S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2015;99:70–84. doi: 10.1016/j.addr.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Li J., Cai C., Li J., Li J., Li J., Sun T., Wang L., Wu H., Yu G. Chitosan-Based Nanomaterials for Drug Delivery. Molecules. 2018;23:2661. doi: 10.3390/molecules23102661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Khalil I., Yehye W.A., Etxeberria A.E., Alhadi A.A., Dezfooli S.M., Julkapli N.B.M., Basirun W.J., Seyfoddin A. Nanoantioxidants: Recent Trends in Antioxidant Delivery Applications. Antioxidants. 2019;9:24. doi: 10.3390/antiox9010024. [DOI] [PMC free article] [PubMed] [Google Scholar]