Abstract
BNT162b2 has proven to be highly effective, but there is a paucity of data regarding immunogenicity factors and comparison between response to vaccination and natural infection. This study included 871 vaccinated healthcare workers (HCW) and 181 patients with natural infection. Immunogenicity was assessed by measuring anti-SARS-CoV-2 against the RBD domain of the spike protein (anti-RBD). Samples were collected 1–2 weeks after vaccination or 15–59 days post-onset of symptoms. Post-vaccine anti-RBD concentrations were associated with age, gender, vaccination side-effects (VSE) and prior infection (Pr-CoV). Anti-RBD median levels (95%CI) were lower by 2466 (651–5583), 6228 (3254–9203) and 7651 (4479–10,823) AU/mL in 35–44, 45–54, 55–70 yrs, respectively, compared with the 18–34 yrs group. In females, the median levels were higher by 2823 (859–4787), 5024 (3122–6926) in individuals with VSE, and 9971 (5158–14,783) AU/mL in HCWs with Pr-CoV. The ratio of anti-RBD in vaccinated individuals versus those with natural infection varied from 1.0 to 19.4. The high immunogenicity of BNT162b2 is verified, although its sustainability has yet to be elucidated. The use of comparative data from natural infection serological panels, expressing the clinical heterogeneity of natural infection, may facilitate early decisions for candidate vaccines to be evaluated in clinical trials.
Keywords: COVID-19, BNT162b2 vaccine, health care workers, immune response, anti-RBD
1. Introduction
The coronavirus disease 2019 (COVID-19) is expanding despite mitigation policies of variable levels of success. By the end of 2020, an increasing number of safe and effective vaccines were approved, and large vaccination programs are underway around the world. By 13 July 2021, more than 188,000,000 cases and 4,000,000 deaths were reported while more than 3.4 billion vaccine doses were administered.
The first approved vaccine was Pfizer-BNT162b2, a lipid-nanoparticle-formulated mRNA vaccine encoding the SARS-CoV-2 full length spike modified by two proline mutations. Preliminary findings among healthy men and women showed that two 30 mg doses elicited high SARS-CoV-2-neutralizing antibody titers and robust antigen-specific CD8+- and Th1-type CD4+ T-cell responses [1]. Moreover, in a multinational, placebo-controlled, observer-blinded efficacy trial including 43.548 healthy or stable-chronic-condition participants, the vaccine was found to be safe with an efficacy of 95% (95% credible interval 90.3–97.6%) in preventing symptomatic COVID-19 disease ≥7 days after the 2nd dose [2]. A similar vaccine efficacy (90–100%) was observed across groups defined by age, sex, race, baseline body mass index (BMI) and the presence of coexisting conditions. History of previous COVID-19 treatment, immunosuppressive therapy or diagnosis with an immunocompromising condition were exclusion criteria in this pivotal study.
A second approved mRNA vaccine, Moderna-mRNA 1273, showed similar efficacy 94.1% (95% 89.3–96.8) in preventing the COVID-19 illness [3].
The BNT162b2 was also evaluated in a mass vaccination campaign in Israel with an efficacy, 7 days from the 2nd dose, of 94%, 87% and 92% in preventing symptomatic disease, hospitalization, and severe disease, respectively [4].
The presence of neutralizing antibodies is a strong correlate of vaccine efficacy, although a protection threshold has not been established [5]. However, measuring neutralizing antibodies on a large scale is challenging. The development of binding assays directed against the spike protein of SARS-CoV-2 showed excellent correlation with neutralizing antibodies [6,7,8,9] and provides an opportunity to assess the immunogenicity of SARS-CoV-2 vaccines over time on a large scale. The assessment of vaccine immunogenicity to predict and monitor vaccine effectiveness is important in groups of individuals not included in clinical trials, such as patients with immunocompromising conditions [10].
Despite the high levels of vaccine efficacy in immunocompetent individuals, the duration of BNT162b2 protection remains unknown. Antibody titers in other coronaviruses (seasonal, SARS CoV-1, MERS) wane over time and this is the case with COVID-19 antibodies in natural infection [11,12,13].
Natural infection protects from reinfection for at least 7 months, and represents a benchmark for comparison with vaccine efficacy and immunogenicity [14].
Higher levels of neutralizing antibodies (NA) and binding antibodies have been associated with increased clinical severity of natural infection in several studies [14,15,16].
Phase I/II SARS-CoV-2 vaccine immunogenicity studies on approved or under approval vaccines included limited numbers of individuals with natural infection as a control group [1,17,18]. Moreover, the heterogeneity of natural infection was not considered and comparisons of vaccinated individuals with the former group were incomplete.
Herein, we report comparative immunogenicity data of BNT162b2 mRNA vaccine with a large cohort of individuals with natural COVID-19 infection and we provide suggestions for the use of comparative immunogenicity of candidate vaccines with natural infection for an early prediction of vaccine efficacy.
2. Materials and Methods
2.1. Vaccination for HCWs
Participants were vaccinated with 2 doses of BNT162b2 21 days apart. The vaccine was administered intramuscularly and included 30 mg of SARS-CoV-2 full-length lipid-nanoparticle-formulated mRNA.
The study was designed to assess immunogenicity at time intervals 1–2 weeks after the 2nd dose (28–35 days) and 4, 6, 8, and 12 months after the 1st dose. Immunogenicity 1–2 weeks after the 2nd dose was expected to be highest, based on the results from phase I/II studies [1].
HCWs from 2 teaching hospitals, Laiko General Hospital (Hospital 1) and Onassis Cardiac Surgery Center (Hospital 2), were informed about the study and participated after signing an informed consent.
A brief questionnaire was administered to HCWs with information about age, gender, education, position within hospital, BMI, history of risk factors for severe COVID-19 (RFS-CoV), previous COVID-19 (Pr-CoV) and history of self-reported adverse reactions after vaccination (VSE). The VSE were grouped according to the major symptom as local, allergic reactions, fever, fatigue and systematic. Combinations of VSE were counted in each one of the individuals’ groups.
2.2. Natural Infection Group
A group of 315 patients with natural SARS-CoV-2 infection diagnosed with RT-PCR testing was included in the study. Participants provided informed consent. Age, gender, diagnostic tests, symptoms, hospitalization, disease severity [19] and admission in intensive care unit (ICU) were recorded. The present analysis includes in total 180 patients, 157 hospitalized and 23 non-hospitalized, 171 symptomatic and 9 asymptomatic individuals, 155 patients with available time from symptom onset (PSO) and 163 with estimation of severity.
The study was approved by the IRB committees of Laiko General Hospital (Athens, Greece) and Onasis Cardiac Surgery Center (Kallithea, Greece).
2.3. Serological Tests
Serum samples collected after venipuncture were tested for SARS-CoV-2 IgG binding antibodies to nucleocapsid protein (anti-N) and anti-SARS-CoV-2 receptor binding domain (RBD) spike protein IgG (anti-RBD).
The first assay is a qualitative one with an index [sample/calibrator (s/c)] cutoff of 1.4. Samples with an index ≥ 1.4 are considered positive and <1.4 negative. The clinical sensitivity of the anti-N assay in samples collected ≥15 days after onset of symptoms is 100% (95% CI 95.9–100%) and the clinical specificity 99.63% (95% CI 99.05–99.90%), according to the manufacturer [20].
The second assay (Abbott SARS-CoV-2 IgG II Quant) or anti-RBD was used to quantify IgG antibodies against the receptor binding domain (RBD) of the S1 subunit of the spike protein. The linear range is between 21 and 40,000 AU/mL. The lower limit of detection was 6.8 AU/mL and the reportable interval 6.8–80,000 AU/mL. The clinical sensitivity was 98.81% (95% CI 93.56–99.94%) in samples collected ≥ 15 days after the positive PCR and the clinical specificity 99.55% (95% CI 99.15–99.76%), at a cutoff value 50 AU/mL [20].
Both assays are based on chemiluminescent microparticle immune assay (CLIA) [20].
The correlation coefficient in weighted linear regression of WHO standard with the Abbott anti-RBD is 0.999, and transformation of Abbott anti-RBD AU/mL to WHO BAU/mL is feasible using the equation BAU/mL = 0.142 × AU/mL [20].
2.4. Statistical Analysis
Median values, 25th and 75th percentiles, were used to describe anti-RBD levels. We compared levels between vaccinated health care workers and individuals with a prior SARS-CoV-2 infection diagnosis or other groups using the non-parametric Mann–Whitney U test or Kruskal–Wallis test. Multiple linear regression was used to identify factors associated with anti-RBD levels. Ratios of the median anti-RBD levels among vaccinated people versus the median levels among persons with natural immunity (asymptomatic, mild symptoms and moderate/severe symptoms) were also calculated.
We conducted all statistical analyses using STATA 13.1. Figures were created in R (v 4.1.0).
3. Results
3.1. Vaccinated HCWs
Eight hundred seventy-one HCWs participated in the study. Their sociodemographic characteristics are shown in Table 1.
Table 1.
Sociodemographic and clinical characteristics of health care workers participating in immunogenicity studies.
| N (%) | |
|---|---|
| Total | 871 (100.0) |
| Gender | |
| Male | 318 (36.5) |
| Female | 553 (63.5) |
| Age (y) | |
| Mean (SD) | 47.8 (10.3) |
| 18–34 | 113 (13.0) |
| 35–44 | 215 (24.7) |
| 45–54 | 315 (36.2) |
| 55–70 | 228 (26.2) |
| Country of Birth | |
| Greece | 804 (92.3) |
| Other | 67 (7.7) |
| Hospital | |
| 1 | 514 (59.0) |
| 2 | 357 (41.0) |
| Risk Factors for Severe COVID-19 | |
| Yes | 134 (15.7) |
| No | 721 (84.3) |
| Job title | |
| HCWs involved with the patient care | 709 (81.4) |
| HCWs not involved with the patient care | 162 (18.6) |
| History of SARS-CoV-2 infection | |
| Yes | 32 (3.7) |
| No | 839 (96.3) |
The prevalence of the SARS-CoV-2 anti-N IgG was 3.7% (32 out of 871) (95% CI 2.5–5.2%) and anti-RBD IgG was 99.7% (868 out of 871) (95% CI 99.0–99.3%). The concentrations of anti-RBD ranged from <6.8 up to higher than 80.000 AU/mL. The 2.5th, 50th and 97.5th percentiles of anti-RBD were 1680, 15,877 and 55,309 AU/mL, respectively (Figure S1A,B).
The median (IQR) anti-RBD levels by age, gender, country of birth, BMI, risk factors for severe COVID-19, side effects of vaccination and previous SARS-CoV-2 infection are shown in Table 2. Gender, age, previous SARS-CoV-2 infection, side effects of vaccination and risk factors for COVID-19 showed statistically significant association with concentrations of anti-RBD. However, in a multivariable linear regression analysis only gender, age, side effects of vaccination and previous SARS-CoV-2 infection showed statistically significant associations with concentrations of anti-RBD (Table 2). More specifically, females had, on average, a 2823 (95% CI 859–4787) AU/mL concentration higher than male HCWs (p = 0.05). Participants aged 55–70 yrs had, on average, a 7651 (95% CI 4479–10,823) AU/mL lower concentration than HCWs 18–34 yrs (p < 0.001).
Table 2.
Median (25th, 75th) concentration of anti-SARS-CoV-2 IgG-II antibodies after the second dose of BNT162b2 vaccine and coefficients (β) along with 95% Confidence Intervals from multiple linear regression.
| Covariate | N (%) | Median (25th, 75th) (AU/mL) | p | β (95% CI) | p |
|---|---|---|---|---|---|
| Overall | 871 (100.0) | 15,877 (8854–27,355) | – | ||
| Gender | <0.001 | ||||
| Male | 318 (36.5) | 13,661 (7780–23,245) | Ref. | ||
| Female | 553 (63.5) | 17,711 (9678–29,726) | 2823 (859–4787) | 0.005 | |
| Age (y) | <0.001 | ||||
| 18–34 | 113 (13.0) | 23,248 (14,447–33,403) | Ref. | ||
| 35–44 | 215 (24.7) | 19,669 (12,210–29,683) | −2466 (−5583–651) | 0.121 | |
| 45–54 | 315 (36.2) | 14,748 (7636–25,363) | −6228 (−9203–−3254) | <0.001 | |
| 55–70 | 228 (26.2) | 11,977 (5993–21,101) | −7651 (−10,823–−4479) | <0.001 | |
| Country of birth | 0.524 | ||||
| Greece | 804 (92.3) | 15,612 (8785–26,994) | – | ||
| Other | 67 (7.7) | 17,293 (9569–28,664) | – | ||
| Risk factors for COVID-19 | 0.066 | ||||
| No | 721 (84.3) | 16,289 (9348–27,506) | Ref. | ||
| Yes | 134 (15.7) | 13,374 (7422–25,044) | −246 (−2800–2309) | 0.850 | |
| BMI (kg/m2) | 0.125 | ||||
| Under/Normal weight: <25 | 383 (44.0) | 16,692 (9597–29,375) | – | ||
| Overweight: 25–30 | 323 (37.1) | 14,823 (7931–24,804) | – | ||
| Obesity: ≥30 | 165 (18.9) | 15,525 (8326–25,606) | – | ||
| Side effects of vaccination | |||||
| No | 375 (43.1) | 12,210 (6848–21,298) | Ref. | Ref. | |
| Yes | 496 (56.9) | 19,196 (11,334–30,841) | <0.001 a | 5024 (3122–6926) | <0.001 |
| Fever | 128 (25.81) | 28,687 (16,207–39,100) | <0.0001 b | ||
| Fatigue | 287 (57.9) | 19,616 (12,137–31,255) | <0.0001 c | ||
| Allergic reactions | 14 (2.8) | 16,163 (9947–27,997) | 0.2792 d | ||
| Local | 198 (39.9) | 18,425 (10,086–30,300) | <0.0001 e | ||
| Other systematic | 248 (50.0) | 21,692 (12,229–33,381) | <0.0001 f | ||
| Previous SARS-COV-2 | <0.001 | ||||
| No | 839 (96.3) | 15,520 (8710–26,480) | Ref. | ||
| Yes | 32 (3.7) | 29,324 (17,751–41,821) g | 9971 (5158–14,783) | <0.001 | |
| PCR Positive (+) | 19 (59.4) | 26,986 (19,212–33,866) h | |||
| No history of PCR testing | 13 (40.6) | 33,950 (9947–49,915) i |
p-values for pairwise comparisons to the reference value are presented: a p < 0.001, b p < 0.001, c p < 0.000, d p = 2792, e p < 0.000, f p < 0.000, g p < 0.00, h p = 0.0017, i p = 0.0167.
HCWs reporting VSE had a concentration of 5024 (95% CI 3122–6926) AU/mL higher than those not reporting side effects (p < 0.001).
Among HCWs, 496 individuals reported 988 VSE, ranging from 1 to 6 per HCW. HCWs reporting fever, fatigue, local or other systematic reactions had statistically significantly higher concentrations of anti-RBD. Fever was associated with 2.3 times higher levels of anti-RBD compared with no VSE (Table 2).
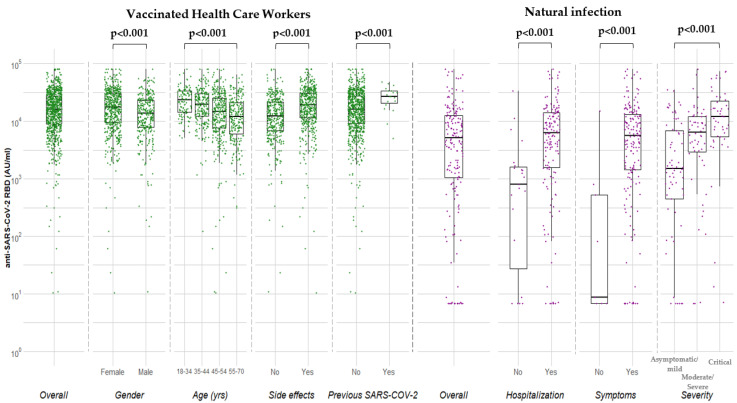
HCWs with previous SARS-CoV-2 had higher levels by 9971 (95% 5158–14,783) AU/mL compared to COVID-19 naïve individuals (p < 0.001). Figure 1 depicts the median levels of anti-RBD overall and by gender, age, side effects and previous SARS-CoV2 in vaccinated HCWs.
Figure 1.
Median concentrations of anti-SARS-CoV-2 RBD (AU/mL) in vaccinated health care workers 7–15 days after the 2nd dose of BNT162b2 and individuals with natural infection.
Time from 2nd dose: The median levels of anti-RBD were calculated according to the time from the 2nd dose. The maximum levels were reached 11 days after the 2nd dose while a sharp reduction was observed 15–17 days after the 2nd dose (Kruskal–Wallis, p = 0.007) (Table S1). Multivariable analysis showed that reduction was independent of age, gender, side effects of vaccination and previous SARS-CoV-2 infection (data not shown).
3.2. Natural Infection
The early convalescent samples post-symptoms onset (PSO) ≥15–59 days of symptomatic (n = 155), asymptomatic individuals (n = 9), hospitalized (n = 157) and non-hospitalized (n = 23) individuals were included in this analysis. The sociodemographic and clinical characteristics are shown in Table 3.
Table 3.
Sociodemographic and clinical characteristics of individuals with COVID-19 infection participating in immunogenicity studies.
| Variable | N (%) |
|---|---|
| Total | 180 (100.0) |
| Gender | |
| Male | 126 (70.0) |
| Female | 54 (30.0) |
| Age (y) | |
| Mean (SD) | 59.6 (16.7) |
| ≤54 | 63 (36.4) |
| 55–64 | 42 (24.3) |
| ≥65 | 68 (39.3) |
| Hospitalization | |
| No | 23 (12.8) |
| Yes | 157 (87.2) |
| Symptoms | |
| Symptomatic | 171 (95.0) |
| Asymptomatic | 9 (5.0) |
| Severity of Symptoms | |
| Mild | 60 (36.8) |
| Moderate | 39 (23.9) |
| Severe | 17 (10.4) |
| Critical | 47 (28.8) |
The prevalence (95% CI) of anti-N and anti-RBD was 88.3% (82.7–92.6%) and 90.6% (85.3–94.4%), respectively.
The median (IQR) anti-RBD levels by age, gender, symptomatic/asymptomatic, severity of clinical disease and time POS are shown in Table 4. The median (IQR) anti-RBD concentrations were 9 (<6.8–520) and 5547 (1415–13,325) AU/mL in asymptomatic and symptomatic individuals, respectively (p < 0.001), 6271 (1583–14,121) and 808 (9-1668) hospitalized and non-hospitalized, respectively.
Table 4.
Median (25th, 75th) concentration of anti-SARS-CoV-2 IgG-II antibodies in symptomatic SARS-CoV-2, 15–59 days after infection and asymptomatic individuals.
| Covariate | N (%) | Median (25th–75th) (AU/mL) | p |
|---|---|---|---|
| Overall | 180 (100.0) | 5088 (1050–12,620) | |
| Gender | 0.2691 | ||
| Male | 126 (70.0) | 5632 (1273–13,837) | |
| Female | 54 (30.0) | 3743 (883–11,187) | |
| Age (y) a | 0.0603 | ||
| 18–44 | 33 (19.1) | 1297 (296–7519) | |
| 45–54 | 30 (17.3) | 6577 (1601–16,623) | |
| 55–64 | 42 (24.3) | 6868 (2433–11,975) | |
| 65+ | 68 (39.3) | 5814 (1553–13,110) | |
| Hospitalization | <0.001 | ||
| No | 23 (12.8) | 808 (9–1668) | |
| Yes | 157 (87.2) | 6271 (1583–14,121) | |
| Asymptomatic | 0.0005 | ||
| No | 171 (95.0) | 5547 (1415–13,325) | |
| Yes | 9 (5.0) | 9 (<6.8–520) | |
| Severity b | 0.0001 | ||
| Mild | 60 (36.8) | 1634 (751–7868) | |
| Moderate | 39 (23.9) | 6082 (2433–9579) | |
| Severe | 17 (10.4) | 6638 (3053–13,837) | |
| Critical | 47 (28.8) | 11,975 (5318–23,351) | |
| Time from symptoms onset (days) c | 0.8887 | ||
| 15–29 | 82 (52.9) | 6980 (1060–14,968) | |
| 30–44 | 48 (31.0) | 6122 (2169–13,925) | |
| 45–59 | 25 (16.1) | 5452 (3306–9579) |
a Seven missing values, b Eight missing values, c Sixteen missing values. When grouped differently: Asymptomatic/mild: 1512 (452–6862) AU/mL and Moderate/Severe: 6360 (2895–12,137) AU/mL.
The median (IQR) anti-RBD levels were highly associated with increased severity: mild, 1634 (751–7868); moderate, 6082 (2433–12,224); severe, 6638 (3053–13,837); and critical, 11,975 (5138–23,351) AU/mL (p < 0.001 for between group comparisons).
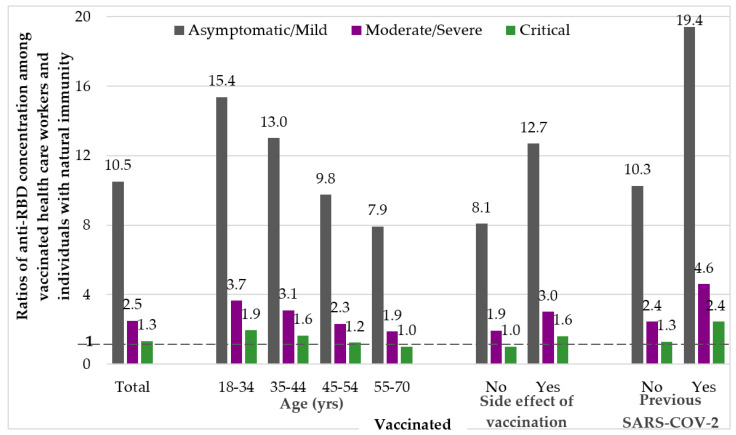
Hospitalized individuals had 7.8-fold higher median anti-RBD levels than those who were not hospitalized. Specifically, patients with moderate, severe, and critical disease had a 4.0-, 4.4-, 7.9-fold higher anti-RBD level than those with asymptomatic/mild infection, respectively (Figure 2).
Figure 2.
Ratio of median concentrations of anti-SARS-CoV-2 RBD in vaccinated groups versus naturally infected individuals with asymptomatic/mild, moderate/severe and critical infection.
3.3. Comparison of Anti-RBD Levels in Vaccinated HCWs and in Individuals with Natural Infection
Figure 1 depicts the median anti-RBD levels in naturally infected individuals overall and according to hospitalization, symptoms and severity. The ratio of median anti-RBD levels in vaccinated after the 2nd dose versus the median levels of those with natural infection in the early convalescent period 15–59 days POS is shown in Figure 2. Anti-RBD concentrations of natural infection were used as denominators (asymptomatic/mild, moderate/severe and critical infection).
We observed several-fold differences in the anti-RBD ratio for each vaccinated group, e.g., across different age groups, i.e., 18–34, 35–44, 45–54, 55–70 years old, and the ratio of median anti-RBD levels for vaccinated over natural infection was 1.9–15.4, 1.6–13.0, 1.2–9.8 and 1.0–7.9, respectively. In the group with VSE the ratio was 1.6–12.7 and in the group with Pr-CoV 2.4–19.4 (Figure 2). For the whole group of vaccinated individuals, the ratio was 1.3, 2.5, 10.5 based on critical, moderate/severe and asymptomatic/mild patients with natural infection, respectively.
4. Discussion
Several lines of evidence suggest that neutralizing antibodies are correlates of protection (CoP) against SARS-CoV-2 infection.
Studies on macaques infected by SARS-CoV-2 demonstrated the pivotal role of NA and S specific CD4+ and CD8+ T-Cell responses to provide near complete protection in rechallenge experiments [21].
Studies in non-human primates vaccinated by SARS-CoV-2 vaccines demonstrated NA threshold for complete protection [22,23].
A study of natural infection outbreak in a fishery vessel where, of the 117 individuals who were seronegative to NA and binding antibodies prior to departure, 104 were (88.9%) infected by RT-PCR while, of the three crew members with presence of NA, anti-RBD and antibodies to full-length spike, none were infected [24].
A prospective study in 3.168 marine recruits revealed aggregate infection of 48% during a 6-week training period. Among 189 anti-spike and anti-RBD-positive cases, 19 (10%) were infected by RT-PCR during training. Lower levels of neutralizing and binding antibodies were associated with a higher incidence of infection [25].
A higher AZD1222 vaccine efficacy was demonstrated with higher levels of NA and anti-spike IgG in a vaccination trial [26]. Moreover, anti-RBD and anti-Spike binding assays were equivalent to NA in predicting vaccine efficacy for predicting symptomatic infection [26]. An excellent correlation of anti-RBD and NA was observed in vaccination trials [27,28,29,30] or other studies [6,7,9].
In a recent study including non-human primates, purified spike antibodies elicited by mRNA-1273 vaccine were associated with a dose-dependent protection in a highly pathogenic hamster model [31].
A remarkable finding of mRNA vaccines immunogenicity studies is that levels of anti-RBD and neutralizing antibodies titers do not change after the 2nd dose in individuals previously infected, suggesting that the 2nd dose of BNT122b2 or other vaccines may not be necessary in previously infected immunocompetent individuals [30,31,32]. In our study, we tested vaccinated HCWs by anti-N and anti-RBD 1–2 weeks after the 2nd dose. Thirty-two HCWs were found to be positive for anti-N, which is a prevalence of 3.2% (32/871) (95% CI 2.5–5.2%). Only 19/32 (59.4%) had a history of previous COVID-19 diagnosis by RT-PCR, suggesting that anti-N is a useful test to assess previous COVID-19 infection during vaccination. Both groups of anti-N-positive subjects had significantly higher anti-RBD levels compared with HCWs without previous SARS-CoV-2 infection.
Studies of BNT162b2 immunogenicity after the 1st and 2nd dose found various associations with age, gender, obesity, vaccination side effects and previous COVID-19. However, confounding effects were not controlled by multivariable analysis [33,34,35]. In our study, anti-RBD levels significantly decreased with older age, male gender, presence of risk-factors for COVID-19 and increased with side-effects and previous SARS-CoV-2 infection. After multivariable analysis, a significant association with age, gender, side-effects of vaccination and previous COVID-19 remained.
We further studied a large group of individuals with natural infection including 175 symptomatic and 26 asymptomatic people diagnosed with RT-PCR and available demographic and clinical information. Sera were collected 15–59 days POS in symptomatic individuals. To our knowledge, this is the largest and more comprehensive natural infection panel included in SARS-CoV-2 vaccine immunogenicity studies. The immunogenicity difference of moderate/severe and asymptomatic infection exceeds 1 log10 and it may bias immunogenicity comparisons in vaccine studies. By using asymptomatic infection as the baseline group, the anti-RBD concentration was several-fold higher in vaccinated individuals compared with natural infection. In contrast, by using moderate/severe infection, the vaccinated individuals had slightly elevated concentrations of anti-RBD compared to patients with natural infection.
In a phase 1 study assessing the immunogenicity of a candidate mRNA vaccine, the ratio of anti-RBD and NA in vaccinated vs. hospitalized or non-hospitalized patients with natural infection was 0.15 (anti-RBD, hospitalized), 0.76 (anti-RBD, non-hospitalized), 0.18 (NA, hospitalized) and 1.0 (NA, non-hospitalized), respectively [36]. Indeed, vaccine manufacturers recently released data which revealed that vaccine efficacy was 47% [37], verifying that a suboptimal immunogenicity was associated with lower efficacy of the candidate vaccine. These data strongly support that vaccine efficacy can be predicted by comparisons of immunogenicity in few vaccinated individuals with serological panels from natural infection, including patient sera from all COVID-19 stages.
Overall, these data document the high immunogenicity of BNT162b2 vaccine in comparison with natural infection. Both mRNA BNT1162b2 and mRNA 1273 vaccines are highly immunogenic although emergence of SARS-CoV-2 variants deserve careful consideration.
Novel SARS-CoV-2 variants harboring mutations can either enhance transmissibility or reduce neutralization activity of vaccine sera. Variants of concern and include the B.1.1.7, B.1.351, P.1 and B.1.617.2 documented first in the UK, South Africa, Brazil and India, respectively. The effectiveness of the BNT162b2 vaccine was assessed under real-world conditions in Qatar, where B.1.1.7 and B.1.351 variants were co-circulating [38]. Specifically, the effectiveness of the vaccine against the B.1.1.7 and B.1.351 documented infections was 89.5% (95% CI, 85.9 to 92.3) and 75.0% (95% CI, 70.5 to 78.9), respectively, suggesting that the mRNA vaccine can protect against immune escape variants of concerns, such as the B.1.351. Recent data suggest effectiveness of BNT162b2 against B.1.167.2 variant [39]. Overall, despite the BNT162b2 effectiveness in the presence of variants, it is wise to maintain high immunogenicity levels to secure adequate vaccine efficacy [40].
The present analysis assumes according to findings from previous reports that NA and anti-RBD are correlates of protection against SARS-CoV-2 [5,21,22,23,24,25,26,31]. Although the mechanism of protection is complex including several aspects of humoral and cell-mediated immunity, the role of neutralizing antibodies seems profound. The use of multiple NA assays, many of them requiring increased biosafety laboratories is a barrier for large clinical studies. The use of anti-RBD or other antibodies against spike protein may accelerate the study of pathogenesis of SARS-CoV-2 and provide useful tools for assessing vaccine efficacy and their effectiveness over time. Expensive laboratory testing (functional NAs, cellular immunity testing) may be limited in phase 1/2 studies, and vaccine safety evaluation should be assessed in large observational studies without conducting expensive and time-consuming randomized phase 3 clinical trials [41].
This study has some strengths including the large number of vaccinated individuals evaluated, the use of a large natural infection panel and the use of a validated, commercially available assay for anti-RBD testing. The major limitation is the lack of cell-mediated immunity tests to capture aspects of T and B cell immunity. The study is also limited in assessing anti-RBD as correlate of protection without further assessment of other antibody targets or functional aspects of humoral immune response.
5. Conclusions
Prospective evaluation of vaccine immunogenicity in large, vaccinated cohorts is underway and the comparison, with prospective data from natural infection potentially clarifying important questions of COVID-19 pathogenesis and vaccine effectiveness over time.
Acknowledgments
HCWs from Laiko General Hospital and Onassis Cardiac Surgery Center; P. Minogiannis, I. Papaparaskevas, I. Boletis-Onassis Cardiac Surgery Center; Z. Moschidis, E. Kokolesis, M. Katsimicha–Hellenic Scientific Society for the Study of AIDS, Sexually Transmitted and Emerging Diseases.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vaccines9091017/s1, Supplementary Figure S1A: Cumulative distribution of anti-SARS-CoV-2 RBD AU/mL in vaccinated health care workers 5–17 days after the 2nd dose of BNT162b2, Supplementary Figure S1B: Frequency distribution of anti-SARS-CoV-2 RBD AU/mL in vaccinated health care workers 5–17 days after the 2nd dose of BNT162b2, Supplementary Table S1: Median (25–75th) levels of anti-SARS-CoV-2 RBD IgG by time (days) from 2nd dose of BNT162b2 vaccine.
Author Contributions
Conceptualization, M.P. (Mina Psichogiou) and A.H.; Data curation, S.R. and K.P. (Konstantinos Petsios); Formal analysis, S.R., V.S. and A.H.; Funding acquisition, M.P. (Mina Psichogiou), A.K. (Andreas Karabinis), A.K. (Anastasia Kotanidou), D.D. and A.H.; Investigation, M.P. (Mina Psichogiou), G.P., A.A., A.K. (Anastasia Kotanidou), D.D., A.C., E.M., I.E., D.B., K.P. (Konstantinos Petsios), K.L., E.K., K.P. (Konstantinos Protopapas), E.J., M.P. (Maria Pratikaki), K.N.S. and H.G.; Methodology, M.P. (Mina Psichogiou), A.K. (Andreas Karabinis), A.K. (Anastasia Kotanidou), I.D.P., P.L., S.T., G.M., D.P., V.S. and A.H.; Project administration, K.P. (Konstantinos Petsios); Resources, M.P. (Mina Psichogiou), A.K. (Anastasia Kotanidou), D.D. and A.H.; Software, S.R. and V.S.; Supervision, M.P. (Mina Psichogiou), A.K. (Andreas Karabinis), G.P., A.A., A.K. (Anastasia Kotanidou) and A.H.; Validation, S.R. and V.S.; Visualization, S.R.; Writing—original draft, M.P. (Mina Psichogiou) and A.H.; Writing—review and editing, A.K. (Andreas Karabinis), G.P., A.A., A.K. (Anastasia Kotanidou), D.D., I.D.P., A.C., S.R., E.M., I.E., D.B., K.P. (Konstantinos Petsios), K.L., E.K., K.P. (Konstantinos Protopapas), E.J., M.P. (Maria Pratikaki), K.N.S., H.G., S.T., G.M., D.P., V.S. and A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by SYN-ENOSIS (protocol number 55/13-10-2020) and donations from the Onassis Cardiac Surgery Center; SB Bioanalytica S.A.; Viatris Hellas and the Hellenic Scientific Society for the Study of AIDS, Sexually Transmitted and Emerging Diseases.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Laiko General Hospital Scientific and Ethics Review Board (protocol number: 291/02-04-2020) and the Onassis Cardiac Surgery Center Scientific and Ethics Review Board (protocol number: 681/06-04-2020).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data are available at the Pergamos Institutional Repository of the National and Kapodistrian University of Athens, Greece. Link available upon review of manuscript.
Conflicts of Interest
None of the authors have relevant conflict of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and poly-specific T cells in humans. Nature. 2021;595:572–577. doi: 10.1038/s41586-021-03653-6. [DOI] [PubMed] [Google Scholar]
- 2.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Marc G.P., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernan M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Mass Vaccination Setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 6.Wajnberg A., Amanat F., Firpo A., Altman D.R., Bailey M.J., Mansour M., McMahon M., Meade P., Mendu D.R., Muellers K., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson M., Wagstaffe H.R., Gilmour K.C., Mai A.L., Lewis J., Hunt A., Sirr J., Bengt C., Grandjean L., Goldblatt D. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J. Clin. Virol. 2020;130:104572. doi: 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perkmann T., Perkmann-Nagele N., Koller T., Mucher P., Radakovics A., Marculescu R., Wolzt M., Wagner O.F., Binder C.J., Haslacher H. Anti-Spike protein assays to determine post-vaccination antibody levels: A head-to-head comparison of five quantitative assays. medRxiv. 2021:preprint. doi: 10.1101/2021.03.05.21252977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Irsara C., Egger A.E., Prokop W., Nairz M., Loacker L., Sahanic S., Pizzini A., Sonnweber T., Holzer B., Mayer W., et al. Clinical validation of the quantitative Siemens SARS-CoV-2 spike IgG assay (sCOVG) reveals improved sensitivity and a good correlation with virus neutralization titers. medRxiv. 2021:preprint. doi: 10.1101/2021.02.17.21251907. [DOI] [PubMed] [Google Scholar]
- 10.Marinaki S., Adamopoulos S., Degiannis D., Roussos S., Pavlopoulou I.D., Hatzakis A., Boletis I.N. Immunogenicity of SARS-CoV-2 BNT162b2 vaccine in solid organ transplant recipients. Am. J. Transplant. 2021;21:2913–2915. doi: 10.1111/ajt.16607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang A.T., Garcia-Carreras B., Hitchings M.D.T., Yang B., Katzelnick L.C., Rattigan S.M., Borgert B.A., Moreno C.A., Solomon B.D., Trimmer-Smith L., et al. A systematic review of antibody mediated immunity to coronaviruses: Kinetics, correlates of protection, and association with severity. Nat. Commun. 2020;11:4704. doi: 10.1038/s41467-020-18450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Post N., Eddy D., Huntley C., van Schalkwyk M.C.I., Shrotri M., Leeman D., Rigby S.I., Williams S.V., Bermingham W.H., Kellam P., et al. Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS ONE. 2020;15:e0244126. doi: 10.1371/journal.pone.0244126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lau E.H.Y., Tsang O.T.Y., Hui D.S.C., Kwan M.Y.W., Chan W., Chiu S.S., Ko R.L.W., Chan K.H., Cheng S.M.S., Perera R.A.P.M., et al. Neutralizing antibody titres in SARS-CoV-2 infections. Nat. Commun. 2021;12:63. doi: 10.1038/s41467-020-20247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall V.J., Foulkes S., Charlett A., Atti A., Monk E.J.M., Simmons R., Wellington E., Cole M.J., Saei A., Oguti B., et al. SARS-CoV-2 infection rates of antibody-positive compared with antibody-negative health-care workers in England: A large, multicentre, prospective cohort study (SIREN) Lancet. 2021;397:1459–1469. doi: 10.1016/S0140-6736(21)00675-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lucas C., Klein J., Sundaram M., Liu F., Wong P., Silva J., Mao T., Tokuyama M., Lu P., Yale IMPACT Research Team Kinetics of antibody responses dictate COVID-19 outcome. medRxiv. 2020 doi: 10.1101/2020.12.18.20248331. preprint. [DOI] [Google Scholar]
- 16.Lynch K.L., Whitman J.D., Lacanienta N.P., Beckerdite E.W., Kastner S.A., Shy B.R., Goldgof G.M., Levine A.G., Bapat S.P., Stramer S.L., et al. Magnitude and Kinetics of Anti–Severe Acute Respiratory Syndrome Coronavirus 2 Antibody Responses and Their Relationship to Disease Severity. Clin. Infect. Dis. 2020;72:301–308. doi: 10.1093/cid/ciaa979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keech C., Albert G., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO; Jan 25, 2021. [(accessed on 15 June 2021)]. COVID-19 Clinical Management: Living Guidance. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1. [Google Scholar]
- 20.SARS-CoV-2 Immunoassay. [(accessed on 15 June 2021)]. Available online: https://www.corelaboratory.abbott/int/en/offerings/segments/infectious-disease/sars-cov-2.
- 21.Chandrashekar A., Liu J., Martinot A.J., McMahan K., Mercado N.B., Peter L., Tostanoski L.H., Yu J., Maliga Z., Nekorchuk M., et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science. 2020;369:812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O’Connell S., Bock K.W., Minai M., et al. Evaluation of the mRNA-1273 Vaccine against SARS-CoV-2 in Nonhuman Primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mercado N.B., Zahn R., Wegmann F., Loos C., Chandrashekar A., Yu J., Liu J., Peter L., McMahan K., Tostanoski L.H., et al. Single-shot AD26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature. 2020;586:583–588. doi: 10.1038/s41586-020-2607-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Addetia A., Crawford K.H.D., Dingens A., Zhue H., Roychoudhury P., Huang M.L., Jerome K.R., Bloom J.D., Greninger A.L. Neutralizing Antibodies Correlate with Protection from SARS-CoV-2 in Humans during a Fishery Vessel Outbreak with a High Attack Rate. J. Clin. Microbiol. 2020;58:e02107-20. doi: 10.1128/JCM.02107-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Letizia A.G., Ge Y., Vangeti S., Goforth C., Weir D.L., Kuzmira N.A., Balinsky C.A., Chen H.W., Ewing D., Soares-Schanoski A., et al. SARS-CoV-2 seropositivity and subsequent infection risk in healthy young adults: A prospective cohort study. Lancet Respir. Med. 2021;9:712–720. doi: 10.1016/S2213-2600(21)00158-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng S., Phillips D.J., White T., Sayal H., Aley P.K., Bibi S., Dold C., Fuskova M., Gilbert S.C., Hirsch I., et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. medRxiv. 2021:preprint. doi: 10.1101/2021.06.21.21258528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Han W., Chen Z., Tang R., Yin W., Chen X., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020;21:181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Z., Schmidt F., Weisblum Y., Muecksch F., Barnes C.O., Finkin S., Schaefer-Babajew D., Cipolla M., Gaebler C., Lieberman J.A., et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. Nature. 2021;592:616–622. doi: 10.1038/s41586-021-03324-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anderson E.J., Rouphael N.G., Widge A.T., Jackson L.A., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020;383:2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebinger J.E., Fert-Bober J., Printsev I., Wu M., Sun N., Prostko J.C., Frias E.C., Stewart J.L., Van Eyk J.E., Braun J.G., et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat. Med. 2021;27:981–984. doi: 10.1038/s41591-021-01325-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Corbett K.S., Nason M.C., Flach B., Gagne M., O’Connell S., Johnston T.S., Shah S.N., Edara V.V., Floyd K., McDanal C., et al. Immune correlates of protection by mRNA -1273 immunization against SARS-CoV-2 Infection in nonhuman primates. Science. 2021 doi: 10.1126/science.abj0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabal K.A., Ben-Amram H., Beiruti K., Batheesh Y., Sussan C., Zarka S., Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162b2 mRNA COVID-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveill. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collier D.A., Ferreira I.A.T.M., Kotagiri P., Datir R., Lim E., Touzier E., Meng B., Abdullahi A., The CITIID-NIHR BioResource COVID-19 Collaboration. Elmer A., et al. Age-related heterogeneity in immune responses to SARS-CoV-2 vaccine BNT162b2. medRxiv. 2021 doi: 10.1101/2021.02.03.21251054. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nabeer P., Jürjenson V., Adamson A., Sepp E., Tserel L., Kisand K., Peterson P. Antibody response after COVID-19 mRNA vaccination in relation to age, sex, and side effects. medRxiv. 2021:preprint. doi: 10.1101/2021.04.19.21255714. [DOI] [Google Scholar]
- 35.Pellini R., Venuti A., Pimpinelli F., Abril E., Blandino G., Campo F., Conti L., De Virgilio A., De Marco F., Di Domenico E.G., et al. Obesity may hamper SARS-CoV-2 vaccine immunogenicity. medRxiv. 2021:preprint. doi: 10.1101/2021.02.24.21251664. [DOI] [Google Scholar]
- 36.Kremsner P., Mann P., Bosch J., Fendel R., Gabor J.J., Kreidenweiss A., Kroidl A., Leroux-Roels G., Schindler C., Schunk M., et al. Phase 1 Assessment of the Safety and Immunogenicity of an mRNA-Lipid Nanoparticle Vaccine Candidate Against SARS-CoV-2 in Human Volunteers. medRxiv. 2020 doi: 10.1101/2020.11.09.20228551. preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dolgin E. CureVac COVID vaccine let-down spotlights mRNA design challenges. Nature. 2021;594:483. doi: 10.1038/d41586-021-01661-0. [DOI] [PubMed] [Google Scholar]
- 38.Abu-Raddad L.J., Chemaitelly H., Butt A.A. Effectiveness of the BNT162b2 Covid-19 Vaccine against the B.1.1.7 and B.1.351 Variants. N. Engl. J. Med. 2021;385:187–189. doi: 10.1056/NEJMc2104974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bernal J.L., Andrews N., Gower C., Gallagher E., Simmons R., Thelwall S., Stowe J., Tessier E., Groves N., Dabrera G., et al. Effectiveness of COVID-19 vaccines against the B.1.617.2 variant. medRxiv. 2021:preprint. doi: 10.1101/2021.05.22.21257658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Planas D., Veyer D., Baidaliuk A., Staropoli I., Guivel-Berhassine F., Rajah M.M., Planchais C., Porrot F., Robillard N., Puech J., et al. Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature. 2021;596:276–280. doi: 10.1038/s41586-021-03777-9. [DOI] [PubMed] [Google Scholar]
- 41.Jin P., Li J., Pan H., Wu Y., Zhu F. Immunological surrogate endpoints of COVID-2019 vaccines: The evidence we have versus the evidence we need. Signal Transduct. Target Ther. 2021;6:48. doi: 10.1038/s41392-021-00481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are available at the Pergamos Institutional Repository of the National and Kapodistrian University of Athens, Greece. Link available upon review of manuscript.