Abstract
The use of synthetic fungicide needs to be gradually reduced because of its adverse effect on human health and the environment. An integrated approach combining fungicides with biological control agents (BCAs) can be used to reduce the fungicide doses, thereby minimizing the risks associated with chemical fungicides. In this study, the combined application of a BCA Trichoderma and a fungicide hymexazol was used to manage the cowpea wilt disease caused by Fusarium oxysporum. The Trichoderma SC012 strain, which is resistant to hymexazol, was screened out and identified as T. asperellum. T. asperellum SC012 showed hyperparasitism to F. oxysporum and could penetrate and encircle the hyphae of pathogen on a medium amended or not with hymexazol. When combined with hymexazol, the population density in the rhizosphere soil of cowpea showed no significant difference compared with the treatment Trichoderma used alone. When the concentration of T. asperellum SC012 or hymexazol was halved, their combined application could control cowpea wilt disease more effectively than their individual use. The findings showed that the combination of Trichoderma and hymexazol could reduce the use of chemical fungicide, which is eco-friendly and may be an important part of integrated control of Fusarium wilt in cowpea.
Keywords: Trichoderma asperellum, hymexazol, Fusarium wilt, cowpea
1. Introduction
Cowpea (Vigna unguiculata (Linn.) Walp.), which is rich in proteins and some important amino acids such as tryptophan and lysine, can be used as a vegetable for human beings and forage to feed livestock [1]. It is an important leguminous crop and plays a crucial part in supporting soil conservation, and facilitating symbiotic nitrogen fixation, which is compatible with integrated farming systems [2]. According to statistics, about 14.5 million hectares of land worldwide are planted with cowpeas every year. However, many biotic and abiotic factors affect the growth of cowpea [3], among which wilt disease caused by Fusarium oxysporum poses a great threat to cowpea production and causes yield loss ranging from 30% to 100% worldwide every year [4].
F. oxysporum f. sp. tracheiphilum, the pathogen of cowpea wilt disease, enters the plant through the root system and invades vascular tissue. The infected plant shows leaf fading, wilting, vascular bundle discoloration, and final death [5]. The thick-walled chlamydospore produced by F. oxysporum can survive for several years in soil [6], which makes Fusarium wilt difficult to control. Soil sterilization with fumigants such as methyl bromide is an effective method to control Fusarium wilt. However, the use of methyl bromide causes serious pollution to the environment and may lead to the development of drug resistant pathogens, and methyl bromide has banned in different countries, including EU and China. The breeding of resistant varieties is also an effective way to control the disease, but there is always a risk that the pathogen strain will evolve to overcome resistance [7]. Using of biocontrol agents for the management of plant pathogens is an eco-friendly and safe approach. Trichoderma, one of the most widely used biocontrol fungi found in various habitats [8], has been used to control wilt disease caused by F. oxysporum on many crops [9,10,11,12]. However, little attention has been paid to the efficacy of Trichoderma in controlling cowpea wilt disease.
Trichoderma performs a variety of antagonistic mechanisms against plant pathogens, such as lytic enzymes, antifungal secondary metabolites, mycoparasitism and competition for nutrients [13,14,15,16]. Meanwhile, Trichoderma could induce plant resistance to disease, and promote root development and growth of plants [17]. Therefore, Trichoderma has a wide range of agricultural uses such as being a biofertilizer, biopesticide and bioremediation agent [14,18]. A recent research showed that Trichoderma could penetrate and encircle the hyphae of pathogens, thus degrading and killing the pathogens [19].
Several species of Trichoderma have been used for the management of plant pathogens. However, the control effect of biocontrol fungus alone to prevent disease is slow and unstable. It may be due to the fact that biocontrol fungi, as exotic microorganisms, are easily affected by biotic and abiotic factors and are not easy to propagate and function in the soil [20]. Thus, the combination of biocontrol agent with resistance inducer or even conventional fungicide can be used to control plant disease effectively and has attracted researchers’ attention [21,22,23]. Combined use of biocontrol agent and an effective fungicide is also useful in reducing the fungicide dose to control plant pathogens, thus helping in minimizing the risks associated with chemical pesticides.
Hymexazol (3-hydroxy-5-methylisoxazole, C4H5NO2), which is a systemic fungicide and soil disinfectant, can inhibit the spore germination of pathogens by combining aluminum and iron ions in the soil under acidic conditions [22], and has been used to control Fusarium wilt in many crops, such as watermelon, cucumber, soybean, and so on. Hymexazol can be absorbed directly by plant roots, transferred quickly to multiple parts of the plant, and then transformed into two glucosides (O-glucoside and N-glucoside). The O-glucoside has fungitoxicity activity as a result of interference with RNA and DNA synthesis, while the N-glucoside is associated with plant growth promoting effects [24,25].
In this study, the effectiveness of the combined application of T. asperellum SC012 and hymexazol on cowpea Fusarium wilt was explored, with the main goal of reducing fungicide dose.
2. Materials and Methods
2.1. Fungal Isolates and Pesticide
The Trichoderma strains (SC012, LS007-21, HN082102.1, HL167 and DQ1) and F. oxysporum f. sp. tracheiphilum strain FC018 used in this experiment were provided by the Key Laboratory of Green Prevention and Control of Tropical Diseases and Pests (College of plant protection, Hainan University, Haikou, China). The pesticide used in the study was the 98% hymexazol technical (3-Hydroxy-5-methylisoxazole) (Weifang Huanuo Biotechnology Co., Ltd., Weifang, China).
2.2. Sensitivity Test of Trichoderma Strains and F. oxysporum to Hymexazol
To test the sensitivity of fungi to hymexazol, EC50 (concentration for 50% of maximal effect) were calculated using the method of mycelium growth rate. Strains of Trichoderma and F. oxysporum were cultured on PDA medium mixed with different concentrations of hymexazol (50, 100, 200, 300, 400 and 600 µg mL−1) at 28 °C, and hymexazol -free PDA medium was used as control [26]. When the fungal colony in control completely covered the Petri dishes (2 days for Trichoderma and 7 days for F. oxysporum), the diameters of colonies were measured, and the inhibition rate was calculated as follows: inhibition rate (%) = ((colony diameter of control—colony diameter of experimental group)/colony diameter of control) × 100%.
2.3. Taxonomic Classification of Hymexazol-Tolerant Trichoderma
The hymexazol-tolerant Trichoderma strain was identified on a morphological and molecular basis. For morphology identification, hyphal morphology and colony growth patterns of the Trichoderma strain was observed after 3, 4, 5, 6, and 7 days cultured on PDA corn meal dextrose agar (CMD: 40 g L−1 cornmeal, 20 g L−1 dextrose, 20 g L−1 agar) and synthetic low nutrient agar (SNA: 1.0 g L−1 KH2PO4, 10.5 g L−1 KCl, 1.0 g L−1 KNO3, 0.5 g L−1 MgSO4, 0.2 g L−1 dextrose, 0.2 g L−1 sucrose, 18 g L−1 agar), respectively. The characterization of conidium, conidiophore, chlamydospores and conidial pustules of Trichoderma strain cultured on the CMD and SNA mediums were examined after 3 and 7 days under optical microscope (Olympus, BX53F) and stereoscopic microscope (Olympus, SZX16), and compared with published morphology.
For molecular identification, the genomic DNAs were extracted using the CTAB method as described by Stewart and Via [27]. The partial translation elongation factor 1-alpha (tef1) gene and RNA polymerase subunit II (rpb2) gene were amplified using primer pairs EF1−728 F/TEF1LLErev and Frpb2-5f/Frpb2-7cr, respectively [28]. The PCR reaction mixture was as follows: 1× Rapid Taq Master Mix (Vazyme Biotech Co., Ltd., Nanjing, China), 0.2 μM Primer 1, 0.2 μM Primer 2, 100 ng gDNA, and the PCR conditions were: 94 °C for 5 min; followed by 30 cycles of 94 °C for 10 s, 55 °C for tef1 or 59 °C for rpb2 for 10 s, and 72 °C for 90 s; and 72 °C for 10 min. The PCR products were detected using 1.0 % (w/v) agarose gel electrophoresis, purified using ZTOPO-TA Fast Cloning Kit (Beijing Zoman Biotechnology Co., Ltd., Beijing, China, Kit No. ZC206-1), and sequenced by Guangzhou Tianyi Huiyuan Biotechnology Co., Ltd. (Guangzhou, China). The phylogenetic tree based on tef1 or rpb2 gene sequences was constructed using the neighbor-joining method in the MEGA-X program.
2.4. The Antagonistic Effect of the Combination of Hymexazol-Tolerant Trichoderma and Hymexazol to F. oxysporum
To evaluate the antagonism of the combination of hymexazol-tolerant Trichoderma and hymexazol to F. oxysporum, a dual culture of Trichoderma SC012 strain and F. oxysporum FC018 was conducted on a PDA medium mixed with different concentrations of hymexazol (0, 50, 100, 200, 300 and 400 µg mL−1) at 28 °C in darkness, and the strain F. oxysporum FC018 was cultured alone on a PDA medium with different concentrations of hymexazol used as negative control. Seven days later, the radius of F. oxysporum strain FC018 colonies was measured to calculate the inhibition rate according to the formula: inhibition rate % = ((the radius of pathogen colony of negative control—the radius of pathogen colony of treatment group)/the radius of pathogen colony of negative control) × 100% (Morton and Stroube 1955).
2.5. Analysis for Antagonistic Activity between Trichoderma and F. oxysporum
2.5.1. Trypan Blue Staining
To determine the antagonistic activity of the pathogen at the interaction zone between Trichoderma and F. oxysporum, the trypan blue staining method was conducted [29]. Trichoderma SC012 strain and F. oxysporum FC018 strain were dual cultured on PDA dishes incubated at 28 °C in darkness for 7 days. Then, 5 mL 0.1% trypan blue dye solution was added into the plate to dye for 10 min at room temperature and the residual dye was removed with sterile water. The changes in mycelium staining and the interaction zone were observed under microscope and photographed.
2.5.2. Effect of Volatile and Non-Volatile Metabolites Produced by Trichoderma SC012 Strain
The inhibitory effect of volatile and non-volatile metabolites produced by the Trichoderma SC012 strain against the F. oxysporum FC018 strain was evaluated by the method described by Dennis and Webster [30,31]. To test the effect of volatile metabolites, a cellophane sheet was sandwiched between two Petri dishes on which mycelium plugs of Trichoderma and F. oxysporum were inoculated, respectively. For non-volatile metabolites, Trichoderma was cultured on cellophane covering on the medium for two days and then F. oxysporum was inoculated on the medium after it was uncovered. The cultures were incubated at 28 °C in darkness for 7 days without the cellophane, and the colony radius of the pathogen was measured to calculate the inhibition ratio of mycelial growth. The formula of inhibition rates are as follows:
NT/N (%) = ((colony diameter of control without hymexazol − colony diameter of treatment group without hymexazol)/colony diameter of control without hymexazol) × 100%;
T/H (%) = ((colony diameter of control with hymexazol − colony diameter of treatment group with hymexazol)/colony diameter of control with hymexazol) × 100%;
T/N (%) = ((colony diameter of control without hymexazol − colony diameter of treatment group with hymexazol)/colony diameter of control without hymexazol) × 100%.
2.6. Trichoderma Population Dynamics in Rhizosphere Soil of Cowpea
The population dynamics of Trichoderma SC012 strain in the rhizosphere soil of cowpea were determined through the modified gnotobiotic tube system of Marco [32]. The soil was disinfected through dry heat sterilization at 180 °C for 6h, then autoclaved at 121 °C for 60 min for three consecutive days. The sterility of autoclaved soil was determined by the dilution plate technique. In aseptic conditions, the soil was placed into glass tubes (540 mm in length, 51 mm in inner diameter), and then the cowpea seeds which were surface-sterilized by sequential immersion in 75% (v/v) ethanol for 30 s and 8% (w/v) NaOCl for 1 min were planted in the soil, one seed per tube. The glass tubes were divided into four groups equally: the first group was irrigated with sterilized distilled water (50 mL per tube), the second group was irrigated with 100 µg mL−1 hymexazol (50 mL per tube), the third group was irrigated with 1 × 107 cfu mL−1 Trichoderma SC012 strain spore suspension (50 mL per tube) and the last group was irrigated with the mixture of 5 × 106 cfu mL−1 Trichoderma SC012 strain spore suspension and 50 µg mL−1 hymexazol (50 mL per tube). All tubes were incubated in a growth chamber (22–25 °C, 70% relative humidity, 12 h L/12 h D photoperiod) for 10 days. Trichoderma populations were re-isolated from the rhizosphere soil using the method of plate dilution [33] and incubated at 28 °C for 48 h. The colonies that appeared on the plates were counted as colony forming units (cfu g−1).
2.7. Greenhouse Experiments
The experiments were carried out in the Experimental Farm, Agricultural Science Base of College of Plant Protection, Hainan University (20°3′ N, 110°19′ E). The seeds of American non-bracket cowpea were surface-sterilized with 8% (w/v) NaOCl for 1 min, washed using distilled water three times, placed in plates with distilled water for germination and then the germinated seeds were sown in pots with 2 kg composite soil (soil: sand = 7: 3, 3 plants/pot). After 10 days, seedlings with uniform size were selected and equally divided into five groups. One group was irrigated with 30 mL distilled water as “blank control”, and the other four groups were irrigated with a 30 mL spore suspension of F. oxysporum (1 × 107 cfu mL−1). Two days later, among the four groups irrigated with the spore suspension of F. oxysporum, one group was irrigated with sterilized distilled water (100 mL per pot) as “negative control”, the second group was irrigated with 100 µg mL−1 hymexazol (100 mL per pot) as “hymexazol treatment”, the third group was irrigated with 1 × 107 cfu mL−1 spore suspension of Trichoderma SC012 strain (100 mL per pot) as “Trichoderma treatment” and the last group was irrigated with the mixture of 5 × 106 cfu mL−1 Trichoderma SC012 strain spore suspension and 50 µg mL−1 hymexazol (100 mL per pot) as “combine treatment”. Before inoculated by spore suspension of pathogen, the cowpea roots were injured by inserting transplanting shovel into soil near the plant. Then, all groups were irrigated with water when needed for the next 20 days. All treatments were arranged in a completely randomized design (CRD) with at least 40 cowpea seedlings for each treatment. Twenty days after inoculation, the disease index and control effect were investigated according to the grading standard of Rigert and Foster [34]. Disease index = Ʃ (number of diseased plants × representative series)/(total number of plants × highest representative level value) × 100. Control effect (%) = (infected control disease index − treatment disease index)/infected control disease index × 100%.
2.8. Field Experiments
The field experiments were conducted at the Experimental Farm, Danzhou Campus, Hainan University (19°30′ N, 110°29′ E) for two independent times in June 2020 and June 2021. Field plots (10 m × 19.9 m) comprising 17 rows and 30 holes per row were arranged in a completely randomized block design and three cowpea seeds were sown in each hole. The treatments of the field experiment were the same as that of the greenhouse experiment, with three rows and at least 230 cowpea seedlings for each treatment, and the outside two rows serving as guard rows. All plants were harvested after 20 days and used to evaluate control effect.
2.9. Statistical Analyses
Statistical analyses of quantification data were carried out using IBM SPSS Statistics 21 (SPSS Inc, Chicago, IL, USA) software, and were subject to one-way analysis of variance (ANOVA) analysis. Means were analyzed using Tukey’s test and Dunnett’s t-test at p < 0.05. All experiments were repeated at least three times.
3. Results
3.1. The Sensitivity of Trichoderma SC012 Strain and F. oxysporum FC018 to Hymexazol
Among all the Trichoderma strains tested, the SC012 strain displayed the strongest tolerance to hymexazol (EC50 = 263.68 µg mL−1) and was chosen for further analysis (Table 1). The results also showed that hymexazol significantly suppressed the mycelial growth of F. oxysporum FC018 with an EC50 value being 62.15 µg mL−1.
Table 1.
Inhibition activities of hymexazol on Trichoderma and pathogen F. oxysporum.
| Taxonomic Status | Strains | EC50 (µg mL−1) |
|---|---|---|
| Trichoderma | SC012 | 263.68 ± 4.01 a |
| LS007-21 | 192.02 ± 0.98 c | |
| HN082102.1 | 128.53 ± 3.16 d | |
| HL167 | 153.55 ± 3.51 e | |
| DQ1 | 226.01 ± 2.65 b | |
| F. oxysporum | FC018 | 62.15 ± 1.78 f |
Note: Data presented are the means ± SE. The lowercase letters indicate significant difference (p < 0.05) among the treatments.
3.2. Taxonomic Classification of Trichoderma SC012 Strain
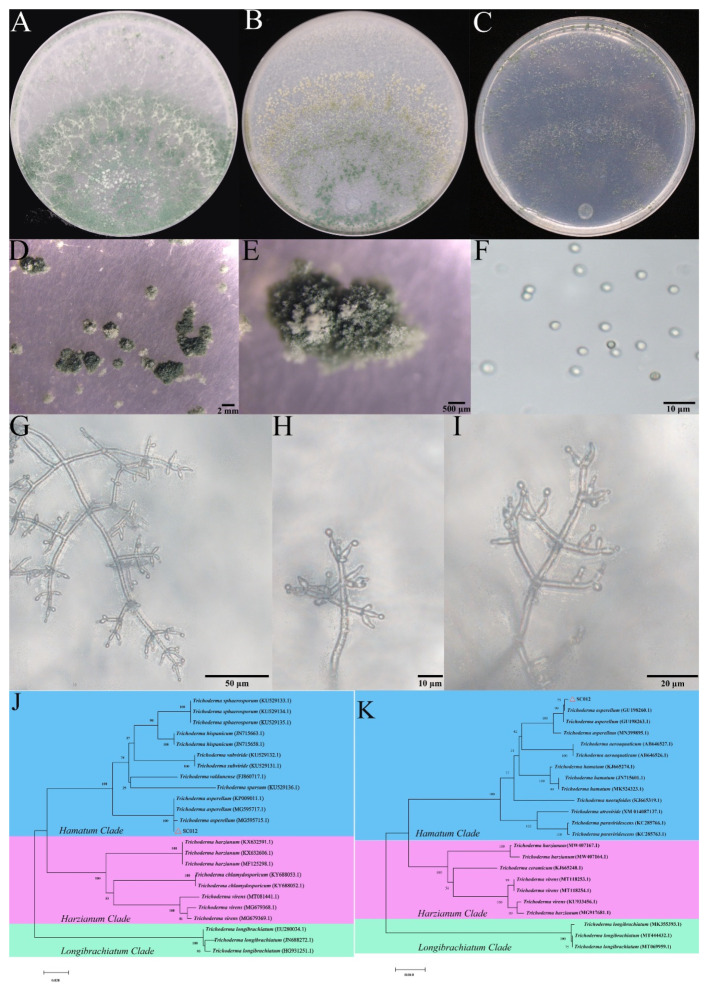
Seven day old culture of Trichoderma SC012 strain showed two or more concentric rings on PDA, CMD and SNA medium (Figure 1A–C) and there was no pigment or distinctive odor detected on PDA medium. On the CMD medium, loosely organized and dark green conidial pustules with a diameter of 1–2 mm were observed (Figure 1B,D,E). The branches of conidiophore were pyramidal with verticillate and paired lateral branches arising from main axis and rebranching, and all branches aroused at an angle of nearly 90° with the subtending branch (Figure 1G–I). The conidia were smooth and 2.5–3.0 × 3.0–4.0 μm in size, showing sub globose to ovoidal shape (Figure 1F). The morphology of SC012 was the same as T. asperellum described in Samuels et al. [35]. The partial tef1 and rpb2 genes amplified from Trichoderma SC012 strain were sequenced and deposited in GenBank receiving the accession numbers MW197094 and MZ753814. The phylogenetic tree based on tef1 gene sequences showed that Trichoderma SC012 strain was closely related to KP009011.1, MG595717.1 and MG595715.1 (T. asperellum) (Figure 1J), and the phylogenetic tree based on rpb2 gene sequences indicated that Trichoderma SC012 strain was intimately related to GU198260.1, GU198263.1 and MN399895.1 (T. asperellum) (Figure 1K). Therefore, the SC012 strain was identified as T. asperellum according to the morphological characterization and molecular analysis.
Figure 1.
Morphology and molecular analysis of Trichoderma SC012 strain. (A–C) The morphology of colony grown on PDA, CMD and SNA medium, respectively; (D,E) the morphology of a conidial pustule grown on CMD plates; (F) morphology of the conidia and chlamydospores; (G–I) the morphology of conidiophores; (J) neighbor-joining tree based on translation elongation factor 1 (tef1) gene sequences and evaluated by 1000 bootstrap replications; (K) neighbor-joining tree based on RNA polymerase subunit II (rpb2) gene sequences and evaluated by 1000 bootstrap replications.
3.3. Antagonism of the Combination of Trichoderma and Hymexazol to F. oxysporum
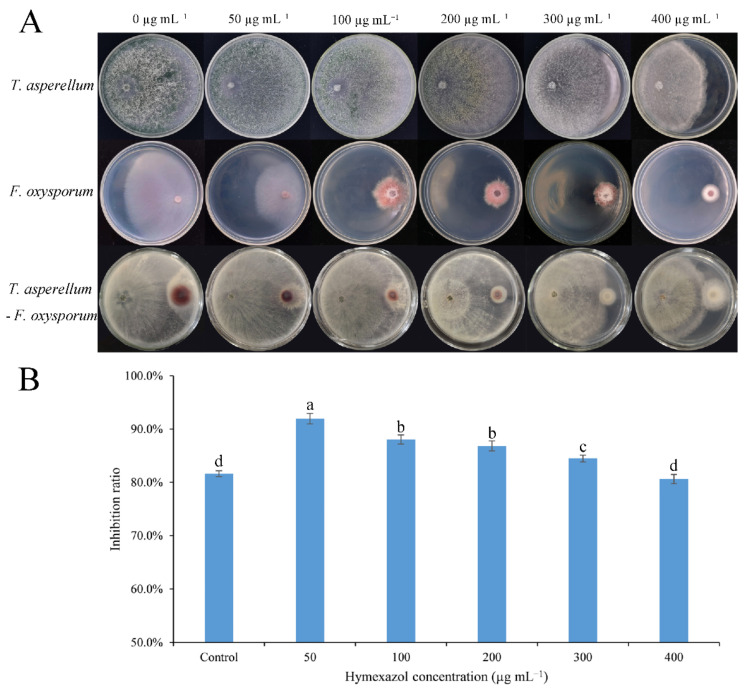
The inhibition rate of T. asperellum SC012 against F. oxysporum FC018 was evaluated at different concentrations (0, 50, 100, 200, 300 and 400 µg mL−1) of hymexazol. Results showed that at 50 µg mL−1 of hymexazol, the inhibition rate of T. asperellum SC012 to F. oxysporum FC018 was the highest compared with control and other treatments (Figure 2). Therefore, 50 µg mL−1 of hymexazol was considered to have a better inhibition effect to F. oxysporum when combined with T. asperellum SC012 and was chosen for further analysis.
Figure 2.
The antagonism effect of the combination of Trichoderma SC012 and hymexazol to F. oxysporum FC018. (A) Dual culture of T. asperellum SC012 and F. oxysporum FC018 at different concentrations of hymexazol; (B) inhibition rate of Trichoderma strain to F. oxysporum at different concentrations of hymexazol. Data presented are the means ± SE. The different lowercase letters indicate significant difference (p < 0.05) among the treatments.
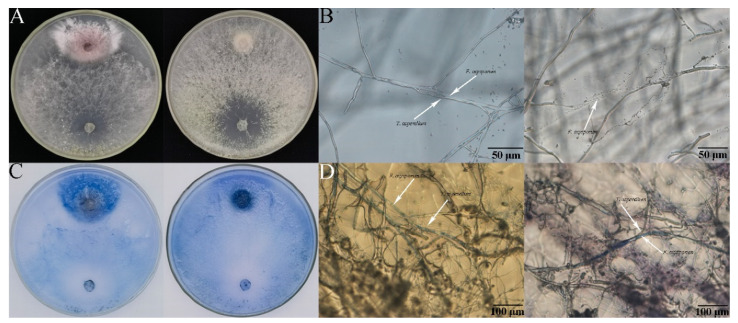
The hyphae of F. oxysporum FC018 were encircled and disintegrated by hyphae of T. asperellum SC012 strain at the interaction zone between these two fungi (Figure 3B). In the trypan blue staining experiment, the hyphae of F. oxysporum at the interaction zone were clearly stained blue, indicating that these hyphae had died (Figure 3C). Microscopic observation showed that unstained T. asperellum hyphae penetrated and encircled the stained F. oxysporum hyphae (Figure 3D).
Figure 3.
Observation of the interaction zone between T. asperellum SC012 and F. oxysporum FC018. (A), Dual culture of T. asperellum SC012 and F. oxysporum FC018 at the presence (right) or absence (left) of 50 µg mL−1 hymexazol. (B), Hyphae of F. oxysporum FC018 encircled (left) and disintegrated (right) by T. asperellum SC012. (C), Trypan blue staining of T. asperellum and F. oxysporum at the presence (right) or absence (left) of 50 µg mL−1 hymexazol. (D), Stained hyphae of F. oxysporum.
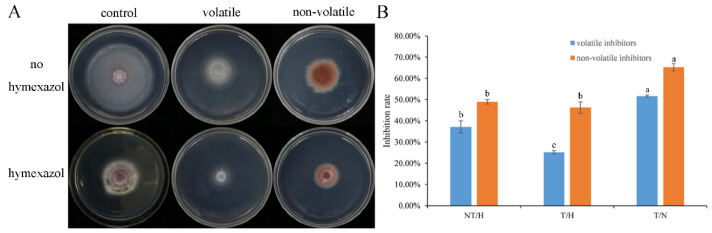
The volatile and non-volatile compounds produced by T. asperellum SC012 suppressed the growth of hyphae and the inhibition rates were higher than 40% (Figure 4). The inhibition rates of volatile and non-volatile compounds (“T/H”) decreased by 11.94% and 2.98% and “T/N” increased by 14.53% and 16.33% when compared with “NT/N”, respectively (Figure 4B). It seems that the combined application has greater effect on F. oxysporum growth.
Figure 4.
Effect of volatile and non-volatile metabolites produced by T. asperellum SC012 to F. oxysporum FC018 at the presence or absence of 50 µg mL−1 hymexazol. (A) F. oxysporum FC018 grown on PDA medium affected by volatile and non-volatile metabolites; (B) the inhibition rates of volatile and non-volatile metabolites to the growth of F. oxysporum FC018. Data presented are the means ± SE. The lowercase letters indicate significant difference (p < 0.05) among the treatments.
3.4. Colonization of Trichoderma SC012 Strain in the Rhizosphere Soil of Cowpea
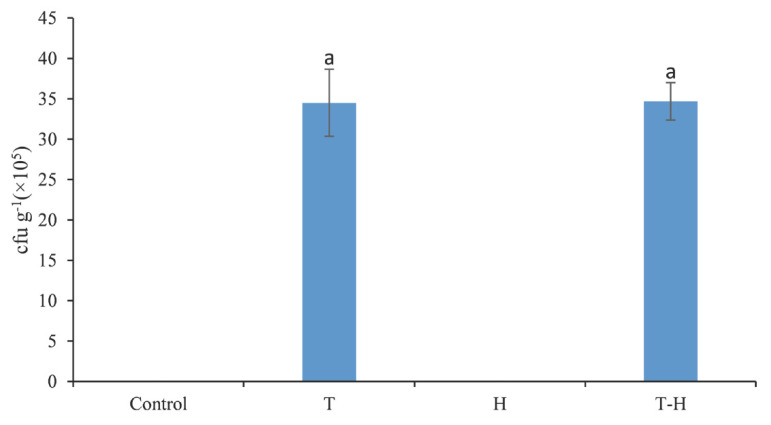
T. asperellum SC012 strain could colonize well the rhizosphere soil of cowpea both in the presence or absence of hymexazol and no significant difference was found between these two treatments (Figure 5). The population density of T. asperellum SC012 strain was 3.45 × 106 cfu g−1 when inoculated by Trichoderma alone and was 3.47 × 106 cfu g−1 when the combination of Trichoderma and hymexazol was used (Figure 5). This showed that the combination of Trichoderma and hymexazol had no significant effect on the colonization of T. asperellum SC012 strain in the rhizosphere soil of cowpea.
Figure 5.
Colonization of T. asperellum SC012 strain in rhizosphere soil of cowpea. “Control”, “T”, “H” and “T-H” stand for “negative control”, “Trichoderma treatment”, “hymexazol treatment” and “combination treatment”, respectively. Data presented are the means ± SE (n = 3). Different letters indicate significant difference (p < 0.05) among the treatments.
3.5. The Combination of Trichoderma and Hymexazol Can Effectively Control Cowpea Fusarium Wilt in Greenhouse
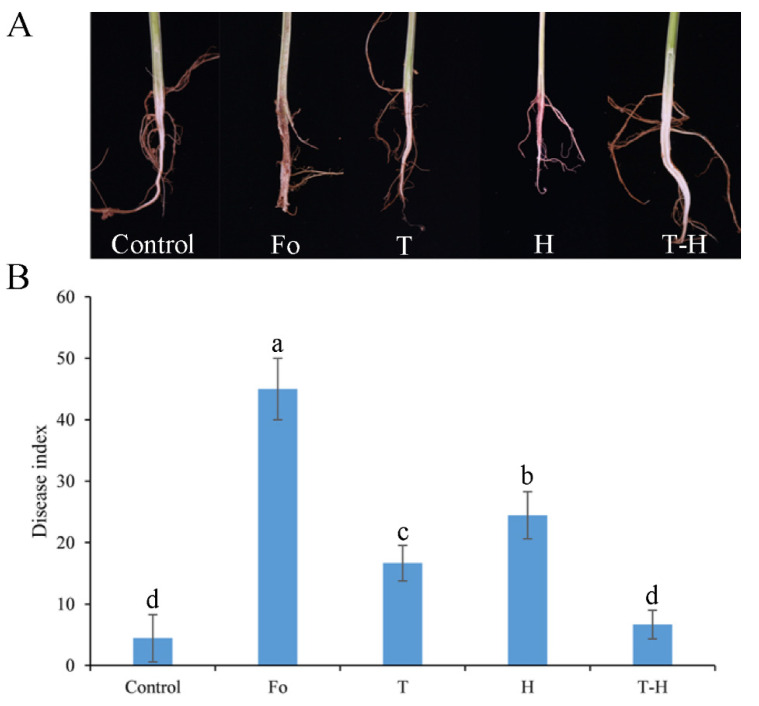
The severity of symptoms of cowpea Fusarium wilt disease were significantly reduced when seedlings were treated separately with the Trichoderma, hymexazol and their combination preparation, which could be concluded from the different incidence of rot and browning of vascular bundles in cowpea roots under different treatments (Figure 6A). Additionally, also, the disease index of the group treated with the combination of Trichoderma and hymexazol was reduced significantly compared to other treatments (Figure 6B). The control effect of the group treated with T. asperellum SC012 alone was 62.69% and was 45.80% in the treatment with hymexazol alone. Conversely, for the treatment with the combination of Trichoderma and hymexazol, the control effect was 84.74%, which was significantly higher than other treatments (Figure 6B). The results indicated that this combination preparation had distinctive inhibitory effect on cowpea Fusarium wilt in greenhouse.
Figure 6.
The effect of the combination of T. asperellum SC012 and hymexazol to control cowpea Fusarium wilt in greenhouse. (A) The symptoms of cowpea root; (B) the disease index of cowpea Fusarium wilt. “Control”, “Fo”, “T”, “H” and “T-H” stand for “blank control”, “negative control”, “Trichoderma treatment”, “hymexazol treatment” and “combination treatment”, respectively. Data presented are the means ± SE. Different letters indicate significant difference (p < 0.05) among the treatments.
3.6. The Combination of Trichoderma and Hymexazol Can Effectively Control Cowpea Fusarium Wilt in Field
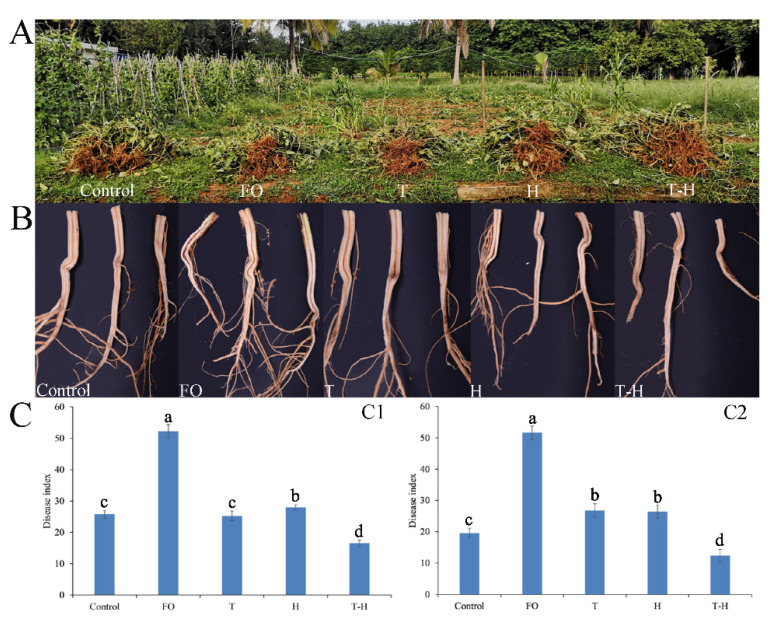
The field experiments were conducted for two seasons in June 2020 and June 2021, respectively, and we found that the combination of T. asperellum SC012 and hymexazol reduced the cowpea wilt disease caused by F. oxysporum (Figure 7). The disease index of the groups treated with the combination of hymexazol and Trichoderma was reduced significantly compared to other treatments in the two field experiments (Figure 7B,C). The control effects of the groups treated with the combination of hymexazol and Trichoderma were 68.39% and 75.69%, respectively, and were significantly higher than the groups treated with T. asperellum SC012 or hymexazol. The disease severity was reduced by 51.62% (Trichoderma treatment) and 46.38% (hymexazol treatment) in the first field experiment and by 47.45% (Trichoderma treatment) and 48.24% (hymexazol treatment) in the second field experiment. The results showed that the combination of hymexazol and T. asperellum SC012 could inhibit cowpea Fusarium wilt effectively under field conditions.
Figure 7.
The effect of the combination of T. asperellum SC012 and hymexazol to control cowpea Fusarium wilt in fields. (A) The growth status of cowpea; (B) the symptoms of cowpea root; (C) the disease index of cowpea Fusarium wilt. (C1) and (C2) represent two field experiments conducted in June of 2020 and June of 2021, respectively. “Control”, “FO”, “T”, “H” and “T-H” stand for “blank control”, “negative control”, “Trichoderma treatment”, “hymexazol treatment” and “combination treatment”, respectively. Data presented are the means ± SE. Different letters indicate significant difference (p < 0.05) among the treatments.
4. Discussion
Previous studies have reported that fungicides combined with biocontrol agents can inhibit pathogens or improve disease resistance of plants [36,37]. Hymexazol can effectively inhibit the normal growth of fungal mycelium or directly kill pathogens of plants, and can promote the growth of plant roots, such as through rooting and seedling strengthening [38,39]. Myresiotis et al. [22] reported that the combination of plant-growth-promoting rhizobacteria and hymexazol could effectively control Fusarium crown and root rot on tomatoes. Abo-Elyousr et al. [21] demonstrated that Trichoderma combined with resistance inducers could control cotton root rot. However, few studies showed that the combination of Trichoderma and fungicide can prevent plant diseases [21,40]. In this study, the combined use of T. asperellum SC012 and hymexazol against cowpea wilt disease caused by F. oxysporum was investigated. Biocontrol fungus can be affected by chemical pesticides and the impact should be considered when they are applied together. In the present study, a hymexazol-resistant T. asperellum strain was selected and when hymexazol was combined at the concentration of 50 µg mL−1, the combination of Trichoderma and hymexazol enhanced antagonistic effects towards F. oxysporum.
Studies have shown that T. asperellum is particularly effective in controlling Fusarium wilt [41,42]. Our results demonstrated that the selected hymexazol-resistant T. asperellum SC012 strain showed high antagonistic activities (77.36%), inhibiting the mycelial growth of F. oxysporum FC018. The hyphae of F. oxysporum FC018 were penetrated, encircled and degraded by T. asperellum SC012, indicating that Trichoderma could parasitize the cowpea pathogenic fungi F. oxysporum. Previous studies have illustrated that Trichoderma species produce attachment and infection structures and kill parasitized fungi by cell wall-degrading enzymes (CWDEs), often in combination with the secretion of antimicrobial secondary metabolites which rely on G-protein signaling, the cAMP pathway, and MAPK cascades [13,14,18,43].
The colonization of rhizosphere microorganisms in plant roots plays an important role in plant nutrition, competition and disease resistance [44]. This symbiotic relationship could mildly and effectively activate the plant immune response locally or systemically, thus plants respond more effectively to the stresses of potential biotic and abiotic factors [45,46]. Moreover, the relatively poor biocontrol effect observed in previous studies was usually associated with the failure of the colonization of inoculated biocontrol bacteria in the rhizosphere [47]. In this study, the results indicated that the T. asperellum SC012 strain could successfully colonize the rhizosphere of cowpea even in the presence of hymexazol. Additionally, when the concentration of the T. asperellum SC012 spore was half in the mixture Trichoderma/hymexazol, the population density of Trichoderma in the rhizosphere soil did not differ significantly from the full-dose treatment with T. asperellum SC012 alone. We guessed that in this range of spore concentrations the reduction in the amount of inoculum of T. asperellum SC012 did not affect disease control effectiveness of Trichoderma. Therefore, further experiments are needed in order to investigate the colonizing ability of T. asperellum SC012 in the cowpea root system in natural soil.
In the greenhouse and field experiments, when T. asperellum SC012 and hymexazol were applied together they controlled cowpea wilt disease more effectively than when they were applied singularly, even when their dose was halved. In conclusion, the combination of Trichoderma and hymexazol can reduce the use of chemical fungicide, which offers the opportunity of more eco-friendly management strategies for the integrated control of Fusarium wilt of cowpea and the possibly of soil-borne diseases of other crops.
Author Contributions
Conceptualization, C.Z., M.X. (Ming Xue) and T.L.; methodology, C.Z., W.W. and J.H.; software, Z.L.; validation, C.Z., M.X. (Mengyu Xing) and T.L.; formal analysis, C.Z. and M.X. (Ming Xue); investigation, Q.Z.; resources, W.W.; data curation, R.W., C.Z., Z.L. and T.L.; writing—original draft preparation, C.Z.; writing—review and editing, W.W. and T.L.; visualization, W.W.; supervision, W.W. and T.L.; project administration, T.L.; funding acquisition, T.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by the National Natural Science Foundation of China (Grant Nos. 31760510), Hainan Provincial Natural Science Foundation of China (Grant Nos. 319MS015) and National Undergraduate Training Programs for Innovation and Entrepreneurship of China (201910589025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh S., Nag S.K., Kundu S.S., Maity S.B. Relative intake, eating pattern, nutrient digestibility, nitrogen metabolism, fermentation pattern and growth performance of lambs fed organically and inorganically produced cowpea hay-barley grain diets. Trop. Grassl. 2010;44:55–61. doi: 10.1007/s11250-009-9397-5. [DOI] [Google Scholar]
- 2.Reddy L.J., Upadhyaya H.D., Gowda C., Singh S. Development of Core Collection in Pigeonpea [Cajanus cajan (L.) Millspaugh] using Geographic and Qualitative Morphological Descriptors. Genet. Resour. Crop Evol. 2005;52:1049–1056. doi: 10.1007/s10722-004-6152-7. [DOI] [Google Scholar]
- 3.Singh B.B. Cowpea [Vigna unguiculata (L.)] Walp. In: Singh R.J., Jauhar P.P., editors. Genetic Resources, Chromosome Engineering and Crop Improvemen. Volume 1. CRC Press; Boca Raton, FL, USA: 2005. pp. 117–162. [DOI] [Google Scholar]
- 4.Reddy M.V., Sharma S.B., Nene Y.L. Pigeonpea: Disease management. In: Nene Y.L., Hall S.D., Sheila V.K., editors. The Pigeonpea. CAB International; Wallingford, UK: 1990. pp. 303–347. [Google Scholar]
- 5.Beckman C.H. The Nature of Wilt Diseases of Plants. APS Press; St. Paul, MN, USA: 1987. p. 176. [Google Scholar]
- 6.Nelson P.E. Life Cycle and Epidemiology of Fusarium oxysporum—ScienceDirect. Fungal Wilt Dis. Plants. 1981:51–80. doi: 10.1016/B978-0-12-464450-2.50008-5. [DOI] [Google Scholar]
- 7.Rowe R.C. Strategies for Controlling Fusarium Crown and Root Rot in Greenhouse Tomatoes. Plant Dis. 1981;65:107. doi: 10.1094/PD-65-107. [DOI] [Google Scholar]
- 8.Kumar K., Amaresan N., Bhagat S., Madhuri K., Srivastava R.C. Isolation and Characterization of Trichoderma spp. for Antagonistic Activity Against Root Rot and Foliar Pathogens. Indian J. Microbiol. 2012;52:137–144. doi: 10.1007/s12088-011-0205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Castillo A.G., Puig C.G., Cumagun C.J.R. Non-Synergistic Effect of Trichoderma harzianum and Glomus spp. in Reducing Infection of Fusarium Wilt in Banana. Pathogens. 2019;8:43. doi: 10.3390/pathogens8020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hewedy O.A., Abdel-lateif K.S., Bakr R.A. Genetic diversity and biocontrol efficacy of indigenous Trichoderma isolates against Fusarium wilt of pepper. J. Basic Microb. 2019;60:126–135. doi: 10.1002/jobm.201900493. [DOI] [PubMed] [Google Scholar]
- 11.Yang X.M., Chen L.H., Yong X.Y., Shen Q.R. Formulations can affect rhizosphere colonization and biocontrol efficiency of Trichoderma harzianum SQR-T037 against Fusarium wilt of cucumbers. Biol. Fert. Soils. 2011;47:239–248. doi: 10.1007/s00374-010-0527-z. [DOI] [Google Scholar]
- 12.Zhang Y., Tian C., Xiao J.L., Wei L., Tian Y., Liang Z.H. Soil inoculation of Trichoderma asperellum M45a regulates rhizosphere microbes and triggers watermelon resistance to Fusarium wilt. AMB Express. 2020;10:1–13. doi: 10.1186/s13568-020-01126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harman G.E. Overview of Mechanisms and Uses of Trichoderma spp. Phytopathology. 2006;96:190–194. doi: 10.1094/PHYTO-96-0190. [DOI] [PubMed] [Google Scholar]
- 14.Mukherjee M., Mukherjee P.K., Horwitz B.A., Zachow C., Berg G., Zeilinger S. Trichoderma-Plant-Pathogen Interactions: Advances in Genetics of Biological Control. Indian J. Microbiol. 2012;52:522–529. doi: 10.1007/s12088-012-0308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stracquadanio C., Quiles J.M., Meca G., Cacciola S.O. Antifungal Activity of Bioactive Metabolites Produced by Trichoderma asperellum and Trichoderma atroviride in Liquid Medium. J. Fungi. 2020;6:263. doi: 10.3390/jof6040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stracquadanio C., Luz C., La Spada F., Meca G., Cacciola S.O. Inhibition of Mycotoxigenic Fungi in Different Vegetable Matrices by Extracts of Trichoderma Species. J. Fungi. 2021;7:445. doi: 10.3390/jof7060445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorito M., Woo S.L., Harman G.E., Monte E. Translational Research on Trichoderma: From ‘Omics to the Field. Annu. Rev. Phytopathol. 2010;48:395–417. doi: 10.1146/annurev-phyto-073009-114314. [DOI] [PubMed] [Google Scholar]
- 18.Harman G.E., Howell C.R., Viterbo A., Chet I., Lorito M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004;2:43–56. doi: 10.1038/nrmicro797. [DOI] [PubMed] [Google Scholar]
- 19.Tomah A.A., Abd Alamer I.S., Li B., Zhang J.Z. A new species of Trichoderma and gliotoxin role: A new observation in enhancing biocontrol potential of T. virens against Phytophthora capsici on chili pepper. Biol. Control. 2020;145:104261. doi: 10.1016/j.biocontrol.2020.104261. [DOI] [Google Scholar]
- 20.Getha K., Vikineswary S., Wong W.H., Seki T., Ward A., Goodfellow M. Evaluation of Streptomyces sp. strain g10 for suppression of Fusarium wilt and rhizosphere colonization in pot-grown banana plantlets. J. Ind. Microbiol. Biotechnol. 2005;32:24–32. doi: 10.1007/s10295-004-0199-5. [DOI] [PubMed] [Google Scholar]
- 21.Abo-Elyousr K.A.M., Hashem M., Ali E.H. Integrated control of cotton root rot disease by mixing fungal biocontrol agents and resistance inducers. Crop Prot. 2009;28:295–301. doi: 10.1016/j.cropro.2008.11.004. [DOI] [Google Scholar]
- 22.Myresiotis C.K., Karaoglanidis G.S., Vryzas Z., Papadopoulou-Mourkidou E. Evaluation of plant-growth-promoting rhizobacteria, acibenzolar-S-methyl and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manag. Sci. 2012;68:404–411. doi: 10.1002/ps.2277. [DOI] [PubMed] [Google Scholar]
- 23.Obradovic A., Jones J.B., Momol M.T., Olson S.M., Jackson L.E., Balogh B., Guven K., Iriarte F.B. Integration of Biological Control Agents and Systemic Acquired Resistance Inducers Against Bacterial Spot on Tomato. Plant Dis. 2005;89:712–716. doi: 10.1094/PD-89-0712. [DOI] [PubMed] [Google Scholar]
- 24.Roberts T.R., Hutson D.H. Metabolic pathways of agrochemicals. Part 2: Insecticides and fungicides. R. Soc. Chem. 1999 doi: 10.1039/9781847551375. [DOI] [Google Scholar]
- 25.Fan Y., Gao R.H., Xiao X., Tao Z. Inclusion Complexes of Hymexazol with Three Different Cucurbit[n]uril: Preparation, and Physicochemical and Antifungal Characterization. Israel J. Chem. 2018;58:466–471. doi: 10.1002/ijch.201700070. [DOI] [Google Scholar]
- 26.Lu H., Zou W.X., Meng J.C., Hu J., Tan R.X. New bioactive metabolites produced by Colletotrichum sp., an endophytic fungus in Artemisia annua. Plant Sci. 2000;151:67–73. doi: 10.1016/S0168-9452(99)00199-5. [DOI] [Google Scholar]
- 27.Stewart C.N., Jr., Via L.E. A rapid CTAB DNA isolation technique useful for RAPD fingerprinting and other PCR applications. BioTechniques. 1993;14:748–750. [PubMed] [Google Scholar]
- 28.Druzhinina I.S., Kopchinskiy A.G., Komon M., Bissett J., Szakacs G., Kubicek C.P. An oligonucleotide barcode for species identification in Trichoderma and Hypocrea. Fungal Genet. Biol. 2005;42:813–828. doi: 10.1016/j.fgb.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 29.Strober W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015;111:A3.B.1–A3.B.3. doi: 10.1002/0471142735.ima03bs111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dennis C., Webster J. Antagonistic properties of species-groups of Trichoderma: I. Production of non-volatile antibiotics. Trans. Br. Mycol. Soc. 1971;57 doi: 10.1016/S0007-1536(71)80077-3. [DOI] [Google Scholar]
- 31.Dennis C., Webster J. Antagonistic properties of species-groups of Trichoderma: II. Production of volatile antibiotics. Trans. Br. Mycol. Soc. 1971;57:1. doi: 10.1016/S0007-1536(71)80077-3. [DOI] [Google Scholar]
- 32.Marco S. Gnotobiotic System for Studying Rhizosphere Colonization by Plant Growth-Promoting Pseudomonas Bacteria Gnotobiotic System for Studying Rhizosphere. Mol. Plant-Microbe Interact. 1996;9:600–607. doi: 10.1094/mpmi-9-0600. [DOI] [PubMed] [Google Scholar]
- 33.Pai S.G., Riley M.B., Camper N.D. Microbial degradation of mefenoxam in rhizosphere of Zinnia angustifolia. Chemosphere. 2001;44:577–582. doi: 10.1016/S0045-6535(00)00368-4. [DOI] [PubMed] [Google Scholar]
- 34.Rigert K.S., Foster K.W. Inheritance of Resistance to Two Races of Fusarium Wilt in Three Cowpea Cultivars. Crop Sci. 1987;27:220–224. doi: 10.2135/cropsci1987.0011183X002700020018x. [DOI] [Google Scholar]
- 35.Samuels G.J., Lieckfeldt E., Nirenberg H.I. Trichoderma asperellum, a new species with waited conidia, and redescription of T. viride. Sydowia. 1999;51:71–88. doi: 10.2135/cropsci1987.0011183X002700020018x. [DOI] [Google Scholar]
- 36.Meyer S.L.F., Roberts D.P. Combinations of biocontrol agents for management of plant-parasitic nematodes and soilborne plant-pathogenic fungi. J. Nematol. 2002;34:1–8. [PMC free article] [PubMed] [Google Scholar]
- 37.Raupach G.S., Kloepper J.W. Mixtures of plant growth-promoting rhizobacteria enhance biological control of multiple cucumber pathogens. Phytopathology. 1998;88:1158–1164. doi: 10.1094/PHYTO.1998.88.11.1158. [DOI] [PubMed] [Google Scholar]
- 38.Payne P.A., Williams G.E. Hymexazol treatment of sugar-beet seed to control seedling disease caused by Pythium spp. and Aphanomyces cochlioides. Crop Prot. 1990;9:371–377. doi: 10.1016/0261-2194(90)90010-5. [DOI] [Google Scholar]
- 39.Xu H., Jian K.-Z., Guan Q., Ye F., Lv M. Antifungal activity of some diaryl ethers. Chem. Farm. Bull. 2007;55:1755–1757. doi: 10.1248/cpb.55.1755. [DOI] [PubMed] [Google Scholar]
- 40.Wang S.Q., Ma J., Wang M., Wang X.H., Li Y.Q., Chen J. Combined application of Trichoderma harzianum SH2303 and difenoconazole-propiconazolein controlling Southern corn leaf blight disease caused by Cochliobolus heterostrophus in maize. J. Integr. Agr. 2019;18:2063–2071. doi: 10.1016/S2095-3119(19)62603-1. [DOI] [Google Scholar]
- 41.El_Komy M.H., Saleh A.A., Ibrahim Y.E., Hamad Y.K., Molan Y.Y. Trichoderma asperellum strains confer tomato protection and induce its defense-related genes against the Fusarium wilt pathogen. Trop. Plant Pathol. 2016;41:277–287. doi: 10.1007/s40858-016-0098-0. [DOI] [Google Scholar]
- 42.Thangavelu R., Gopi M. Combined application of native Trichoderma isolates possessing multiple functions for the control of Fusarium wilt disease in banana cv. Grand Naine. Biocontrol Sci. Technol. 2015;25:1147–1164. doi: 10.1080/09583157.2015.1036727. [DOI] [Google Scholar]
- 43.Karlsson M., Atanasova L., Dan F.J., Zeilinger S. Necrotrophic Mycoparasites and Their Genomes. Microbiol. Spectr. 2017;5 doi: 10.1128/microbiolspec.FUNK-0016-2016. [DOI] [PubMed] [Google Scholar]
- 44.Kloepper J.W., Beauchamp C.J. A review of issues related to measuring colonization of plant roots by bacteria. Can. J. Microbiol. 1992;38:1219–1232. doi: 10.1139/m92-202. [DOI] [Google Scholar]
- 45.Jung S.C., Martinez-Medina A., Lopez-Raez J.A., Pozo M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012;38:651–664. doi: 10.1007/s10886-012-0134-6. [DOI] [PubMed] [Google Scholar]
- 46.La Spada F., Stracquadanio C., Riolo M., Pane A., Cacciola S.O. Trichoderma Counteracts the Challenge of Phytophthora nicotianae Infections on Tomato by Modulating Plant Defense Mechanisms and the Expression of Crinkler, Necrosis-Inducing Phytophthora Protein 1, and Cellulose-Binding Elicitor Lectin Pathogenic Effectors. Front. Plant Sci. 2020;11:583539. doi: 10.3389/fpls.2020.583539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benizri E., Baudoin E., Guckert A. Root Colonization by Inoculated Plant Growth-Promoting Rhizobacteria. Biocontrol Sci. Technol. 2001;11:557–574. doi: 10.1080/09583150120076120. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.