Abstract
Although it has been over 20 years since Neural Cell Adhesion Molecule 2 (NCAM2) was identified as the second member of the NCAM family with a high expression in the nervous system, the knowledge of NCAM2 is still eclipsed by NCAM1. The first studies with NCAM2 focused on the olfactory bulb, where this protein has a key role in axonal projection and axonal/dendritic compartmentalization. In contrast to NCAM1, NCAM2’s functions and partners in the brain during development and adulthood have remained largely unknown until not long ago. Recent studies have revealed the importance of NCAM2 in nervous system development. NCAM2 governs neuronal morphogenesis and axodendritic architecture, and controls important neuron-specific processes such as neuronal differentiation, synaptogenesis and memory formation. In the adult brain, NCAM2 is highly expressed in dendritic spines, and it regulates synaptic plasticity and learning processes. NCAM2’s functions are related to its ability to adapt to the external inputs of the cell and to modify the cytoskeleton accordingly. Different studies show that NCAM2 interacts with proteins involved in cytoskeleton stability and proteins that regulate calcium influx, which could also modify the cytoskeleton. In this review, we examine the evidence that points to NCAM2 as a crucial cytoskeleton regulation protein during brain development and adulthood. This key function of NCAM2 may offer promising new therapeutic approaches for the treatment of neurodevelopmental diseases and neurodegenerative disorders.
Keywords: NCAM2, cytoskeleton, Actin, microtubule, MAP2, CaMKII, Autism Spectrum Disorder, Alzheimer’s disease
1. Introduction
Neurons express a large variety of membrane proteins involved in multiple processes. These proteins act as a link between the extracellular environment and the intracellular compartment, and mediate different processes. Cell adhesion molecules (CAMs) include more than 200 proteins that are classified into four families [1]: cadherins, immunoglobulin superfamily (IgSF), integrins and neurexins/neuroligins. CAMs display a large structural variety, which reflects the functional diversity of these proteins. Typically, CAMs are involved in cell signaling [2,3], cytoskeleton remodeling [4] and the regulation of gene expression [5]. Several studies and hypotheses relate particular combinations of CAMs to a specific molecular identity of each neuron. This identity can be crucial for the processes that take place during brain development and adult maintenance, such as axon guidance, membrane recognition and neuronal network formation [6,7,8,9,10,11]. In Drosophila this molecular identity is conferred by dscam, which has multiple splicing sites that produce different isoforms of Down Syndrome Cell Adhesion Molecule, DSCAM. The particular pattern of isoforms expressed in neurons allows a correct membrane recognition and neuronal network formation [11,12]. Flies without dscam present multiple dysfunctions in axon guidance and dendritic tree development [13]. In mammals, according to the current hypotheses, neuronal molecular identity is determined by the expression of different CAMs. Although mammalian CAMs have parallel functions and their distribution is similar, they confer a specific molecular identity and are involved in several processes during brain development and synaptic plasticity, such as the proper formation of visual and hippocampal circuits [6,7].
Several functions of CAMs are correlated with their ability to transduce the external inputs to the cytoskeleton structure. During brain development, a proper regulation of cytoskeleton stability and dynamics are crucial for neuronal migration, cell differentiation and synapse formation [14]. In the adult brain, the cytoskeleton participates in synaptic maintenance and plasticity, and regulates the composition of presynaptic and postsynaptic compartments. There are various genomic studies that link adhesion proteins with mental retardation and psychiatric disorders, such as autism and schizophrenia [15,16,17]. These pathologies show altered neuronal development and connectivity [18]. The different functions performed by CAMs make them target molecules of interest in these pathologies [7], Table 1.
Table 1.
Involvement of Cell Adhesion Molecules in disease.
| Autism Spectrum Disorder | |||
|---|---|---|---|
| Molecule | Type of Study | Implications | References |
| CDHs | Genetic in humans | CNVs and SNPs in CDHs genes found in ASD patients. | [19,20] |
| PDCH | Genetic in humans | Alterations in PCDH9, PCDH10 and PCDH19 genes in patients with autism. | [21,22] |
| FAT1 | Genetic in humans | Genetic modifications in FAT1 in Autism Spectrum Disorder patients. | [23,24,25,26] |
| NRXN | Genetic in humans | Mutations and CNVs in NRXN1-3 genes are associated with ASD. | [27,28] |
| Experimental in mouse | NRXN1 deletion causes electrophysiological and behavioral changes consistent with cognitive impairments. | [29] | |
| NLGN | Genetic in humans | Genetic modifications in NLGN1-4 genes found in ASD patients. | [30,31] |
| Experimental in mice models | Ngln3 and Ngln4 KO mice displayed reduced social interaction | [32] | |
| CNTNAP2 | Genetic in humans | Genetic alterations in ASD patients | [33,34] |
| Experimental in mice models | CNTNAP2 deficient mice present deficits in communication and social interaction; and repetitive behaviors. | [35,36,37] | |
| CNTN | Genetic in humans | CNTN3–6 are considered as gene risk for ASD. | [21,30] |
| Schizophrenia | |||
| Molecule | Type of Study | Implications | References |
| NCAM1 | Genetic in humans | SNPs in NCAM1 in schizophrenia and bipolar patients. | [38,39] |
| NLGN1 | GWAS study | NLGN1 contributed to schizophrenia susceptibility in Han Chinese population. | [17,40] |
| Selectin | Proteomic in humans | Reduced levels in plasma from adolescents with early-onset psycosis. | [41] |
| VCAM1 | Proteomic in humans | Reduced levels in plasma from adolescents with early-onset psycosis. | [41] |
| Epilepsy | |||
| Molecule | Type of Study | Implications | References |
| N-cadherin | Mouse model | N-cadherin reduction changes mature excitatory and inhibitory circuits and contribute to significant impairment in spatial memory | [42] |
| Mouse model | N-cadherin antibody alleviate brain pathology | [43] | |
| DSCAML1 | Genetic in humans | single nucleotide substitution resulting in its loss of function of DSCAML1. | [44,45] |
| Rat model | GABAergic neurons were reduced and neurons’ excitability was enhanced. | [46] | |
| NCAM1 | Proteomics in CSF | NCAM1 concentration in CSF is lower in epilepsy patients. | [47] |
| β-integrin | Mouse model | Seizure activity and nervous system hyperexcitability. | [48] |
| Fragile X Syndrome | |||
| Molecule | Type of Study | Implications | References |
| DSCAML1 | Mouse model | Dosage variations of DSCAML1 disrupt cell–cell and cell–environment interactions crucial for neuronal migration and brain formation | [49] |
| NLGNs | Mouse model | FMRP controls the synaptic level of NLGNs | [50] |
| Down Syndrome | |||
| Molecule | Type of Study | Implications | References |
| DSCAML1 | Mouse model | Overexpression of DSCAM led to the inhibition of dendritic branching, a phenotype observed in DS patients. | [51] |
| IPSC from DS patients | DSCAM/PAK1 pathway suppression reverses neurogenesis deficits. | [52] | |
Among CAMs, the immunoglobulin superfamily (IgSF) represents one of the oldest and most diverse families. IgSFs have an extracellular part with different immunoglobulin and fibronectin domains, a transmembrane domain and a cytosolic tail through which they interact with the cytosol. Different families exist in the IgSF superfamily: L1 Cell Adhesion Molecule (L1CAM); Contactins; DSCAM and Neural Cell Adhesion Molecule (NCAM).
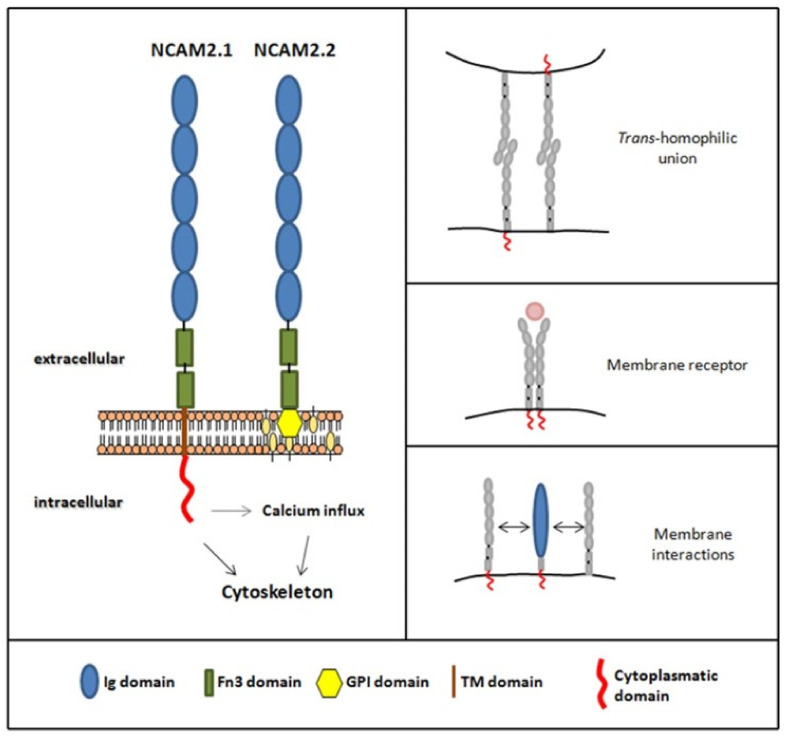
The mammalian NCAM family has two members: NCAM1 and NCAM2 [53,54]. The two proteins have similar ectodomains, each of them containing five immunoglobulin domains and two fibronectin type III domains [55], Figure 1. NCAM1 was the first cell adhesion molecule identified in the nervous system and has been extensively studied. The different isoforms of NCAM1 (180KDa, 140KDa and 120KDa) have the same extracellular domain, but different transmembrane and cytosolic domains as a result of alternative splicing of the gene. The NCAM180 isoform is mostly expressed in neurons in the adult brain; NCAM140, in neurons in both embryonic and adult stages; and NCAM120, mostly in glia in adult stages.
Figure 1.
Schematic representation of NCAM2 protein and isoforms. Both isoforms have an extracellular part with five immunoglobulin domains (blue) and two fibronectin type III domains (green). NCAM2.1 isoform bears a transmembrane domain (brown) and a cytoplasmic domain (red), whereas NCAM2.2 isoform is glycosylphosphatidylinositol, GPI, anchored (yellow). It has been described that NCAM2 protein could do different type of interactions, such as trans-homophilic unions, membrane interactions and could also act as a membrane receptor.
NCAM1 participates in multiple processes in the brain. NCAM1 controls neurite growth, axonal and dendritic elongation, neuronal migration and cell positioning. Those functions are regulated through homophilic and heterophilic interactions of NCAM1 that activate different pathways ranging from calcium signaling to cytoskeleton modifications or transcription activation. Some of the functions undergone by NCAM1 are dependent on the polysialylation of NCAM1 and the ability of NCAM1 to interact with the cytoskeleton [56]. The polysialylation levels of NCAM1 increase during embryonic stages to progressively decline in postnatal stages [57] and it has been described that mouse models deficient in the enzymes responsible for polysialylation present an altered expression of transcription factors and show defects in migration. The functions of this cell adhesion molecule are also relevant in the processes of synaptogenesis and synapse maintenance. NCAM1 is one of the first molecules to accumulate at forming synapsis, promoting membrane binding between neurons and the stabilization of the connection. Indeed, the overexpression of the protein led to a stimulation of the formation of synapses while a deficiency results in a reduction in the total volume of dendritic spines [58,59,60]. NCAM1 is important not only for synapse formation but also for the regulation of synapse dynamics due to its interaction with different cytosolic (e.g., actin and microtubules cytoskeleton) and membrane components from the presynaptic and postsynaptic compartments [56,57,58,59,60,61,62]. Overall, NCAM1 is a key protein in neuronal network formation in the excitatory system but also in the inhibitory connections as it is important for the maturation of GABAergic synapses [63,64]. Moreover, modifications in NCAM1 protein levels due to genetic or environmental factors are associated with different neuronal pathologies [61,62].
In contrast to the wealth of knowledge currently available for NCAM1, NCAM2 has been less extensively studied and therefore its functions are still poorly understood, relegating this member of the protein family to a dark area for many years. However, over the last few years, a number of studies have revealed the importance of NCAM2 in neuronal morphogenesis, synapse formation and synaptic plasticity, with some of these functions being related to calcium dynamics and the ability of NCAM2 to modulate the cytoskeleton. In this review, we aim to shed light on the dark side of the NCAM family by focusing on the function of NCAM2 as a key molecule in the organization of the cytoskeleton.
2. NCAM2 Expression and Interactors
The NCAM2 gene is believed to have been originated by genetic duplication of ncam1, as they are paralog genes [53,54]. In humans, the NCAM2 gene is located on chromosome 21 (region 21q21.1) and contains 25 exons. In mice, it is located on chromosome 16 (16 C3.3) and contains 19 exons. In both species, and due to an alternative splicing process, two isoforms are transcribed: ncam2.1 and ncam2.2. Compared to the mRNA of the ncam2.1 isoform, which contains a stability element localized to the 3’-UTR region [65], the mRNA of the ncam2.2 isoform is more unstable, there is less expression and it is more temporarily restricted [66]. In addition to the isoform differences at the transcript level, the two resulting proteins are also different: the NCAM2.1 isoform bears transmembrane and cytoplasmic domains, whereas the shorter NCAM2.2 isoform is glycosylphosphatidylinositol, GPI, anchored [65,66], Figure 1.
NCAM2 is mostly expressed in numerous central nervous system areas, including the cerebral cortex, the hippocampus and the olfactory system. Several studies showed expression of the murine NCAM2 gene in cortical areas, from embryonic day (E) 14 onwards, with the protein localizing to both dendritic and fiber compartments [65,67]. At adult stages, there is a switch in NCAM2 cell localization that results in higher levels of the protein being present in synaptic contacts [68,69].
The NCAM2 protein undergoes different post-translational modifications including palmitoylation, phosphorylation, proteolysis and glycosylation. Firstly, the cytosolic domain of NCAM2.1 contains four cysteine residues that may undergo palmitoylation: C723, C729, C734 and C740 [70]. Palmitoylation is an important modification for proteins to cluster into lipid raft domains [4,71,72]. Secondly, three phosphorylated residues in the cytoplasmatic tail have been described in murine samples from developing brains: S765, T780, and S786 [73] and the cytosolic tail of NCAM2 is a PEST region—that is, a peptide sequence that is rich in phosphorylatable residues: proline (P), glutamic acid (E), serine (S), and threonine (T) [74]. Besides, the cytosolic tail of NCAM2.1 presents SH2 and SH3 domains. Many proteins with these domains are involved in cell signaling through the activation of tyrosine kinases, such us Tyrosine-protein kinase Fyn or focal adhesion kinase, FAK. Thirdly, both NCAM2 isoforms present a proteolytic site between amino acids 682 and 701; proteolysis at this site causes the extracellular fragment to be released [75,76]. Fourthly, the extracellular region has eight N-glycosylation sites which are HNK-1 sequences. It has been reported that HNK-1 sequences and glycosylation present in L1CAM and myelin-associated glycoprotein, MAG, could facilitate migration of neural-crest-derived cells [77]. Noteworthy, and despite the high structural similarity of NCAM2 and NCAM1, polysialylation modifications have not been reported in NCAM2 proteins.
Few studies have addressed to NCAM2 interactions; these are summarized in Table 2. NCAM2 establishes homophilic bonds through its first immunoglobulin domain. Such bonds facilitate interactions in cis, which lead to dimerization of the protein, and in trans, which facilitate binding between different neurons [78]. Various studies have described interactions of the extracellular part of NCAM2 with prion protein (Prp), beta-amyloid peptide, fibroblast growth factor receptor (FGFR), epidermal growth factor (EGFR), Beta-site APP cleaving enzyme 1 (BACE1), Nogo and granulin (GRN) [70,78,79,80,81,82]. Other studies have shown interactions of the cytosolic tail of NCAM2.1 with members of the 14-3-3 protein family; Proto-oncogene tyrosine-protein kinase, c-Src; microtubule associated proteins, MAPs; neurofilaments; NFs, Calcium/calmodulin-dependent protein kinase type II, CaMKII; and F-actin-capping protein, CAPZ [69,70,76,78,81,82,83], Figure 1.
Table 2.
NCAM2 interactions and post-translational modifications.
| Extracellular Region | |
|---|---|
| Interaction | References |
| Fibroblast Growth Factor Receptor (FGFR) | [80] |
| Epidermal Growth Factor Receptor (EGFR) | [79] |
| Nogo | [70] |
| Granulin | [70] |
| Prion protein (Prp) | [81,82] |
| Beta-site APP cleaving enzyme 1 (BACE1) | [75] |
| Post-translational modification | |
| N-glycosylation | [78] |
| Proteolytic cleavage | [75,76] |
| Intracellular region | |
| Interaction | References |
| Proto-oncogene tyrosine-protein kinase Src | [83] |
| Calcium/calmodulin-dependent protein kinase type II (CaMKII) | [69,83] |
| Microtubule-associated protein 2 (MAP2) | [69] |
| Actin | [70] |
| Tubulin | [69] |
| 14-3-3 family proteins | [69,81,82] |
| Microtubule-associated protein 1B (MAP1B) | [70] |
| F-actin-capping complex (CAPZ) | [70] |
| Heat shock cognate 71 protein (HSC70) | [70] |
| Postranslational modification | |
| Palmitoylation | [70,74] |
| Phosphorylation | [73] |
3. NCAM2 in Neuronal Cell Fate Determination and Differentiation
During neuronal development, the differentiation of a neuron from a postmitotic cell encompasses different processes [84]. Imbalances in the homeostasis of the cytoskeleton dynamics of the postmitotic cell give rise to neuronal polarization, which results in the formation of dendrites and an axonal terminal [85,86,87]. This polarization process is intrinsic to the differentiating neuron but modulated through external signals. Different CAMs are involved in neuronal polarization, act as transducers with the outside, and are key in the overall neuronal development and differentiation processes both in vivo and in vitro [14,88]. Studies with NCAM2 revealed that this protein is essential in the process of neuronal differentiation [69,83]. The distribution of NCAM2 inside the neuron and its interactions with proteins that regulate the cytoskeleton dynamics are key to NCAM2 participating in the establishment of both the dendritic tree and the axonal process.
3.1. NCAM2 Role in Neuronal Migration and Corticogenesis
Brain formation involves a myriad of mechanisms, including temporal and spatial regulations during neuronal development that only occur in vivo [14]. The study of the mechanisms that regulate the polarization and development of neurons in vitro made it possible to identify proteins that have an implication at the physiological level [89]. Not only that, but the complexity of in vivo models has demonstrated the relevance of the extracellular matrix and cell adhesion molecules in the overall development of the brain. In this sense, adhesion proteins participate in different processes of neuronal development, such as neuronal migration, positioning, morphogenesis, development of dendritic and axonal compartments, and synaptogenesis [58,88,90,91]. In vivo, NCAM2 expression progressively increases during neuronal development; NCAM2 is involved in neuronal migration, cell positioning during corticogenesis, neuronal differentiation and synaptogenesis in the olfactory bulb, neocortex and hippocampus. In these regions, NCAM2 participates in the development of dendrites and axons, the establishment of connections, and the regulation of synaptic plasticity [66,68,92], see Figure 2.
Figure 2.
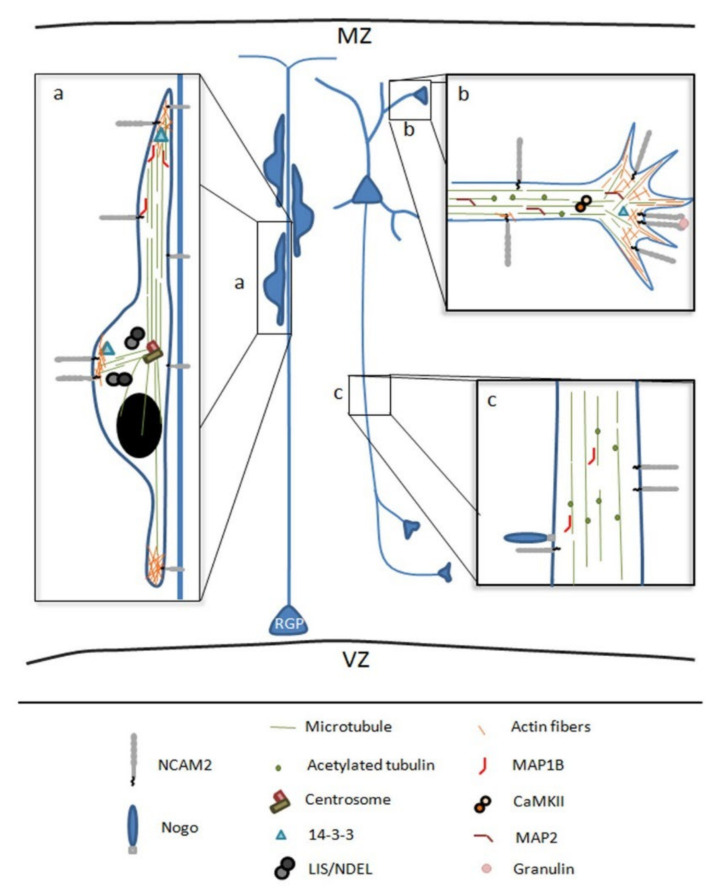
Schematic representation of NCAM2 functions and interactions during brain development. (a) NCAM2 is involved in neuronal migration, NCAM2 protein levels have been associated with an aberrant neuronal migration and layering positioning in the cortex. NCAM2 interacts with different cytoskeletal associated proteins which control cytoskeleton dynamics during neuronal migration. (b,c) NCAM2 has a crucial role during neuronal differentiation interacting with different proteins that are necessary for dendritic tree development and axon elongation. NCAM2 depletion alters the growth cone mobility and microtubules stability. Microtubule associated protein 1B, MAP1B; Microtubule associated protein 2, MAP2; and Calcium/calmodulin-dependent protein kinase type II, CaMKII. Lissencephaly-1/Nuclear distribution protein nudE-like1, LIS/NDEL; radial glia progenitors, RGP; marginal zone, MZ; and ventricular zone, VZ.
In mice embryonic stages, NCAM2 is expressed in different brain regions. In the neocortex, NCAM2 expression is detected from the area of neuronal neurogenesis, ventricular zone and subventricular zone, to the cortical plate. In the olfactory bulb, NCAM2 has been shown to be important for establishing synaptic connections in the glomerulus. In animals constitutively deficient in NCAM2, defects have been described in the formation of olfactory bulb connections, leading to abnormal electrophysiological patterns [93,94]. However, no alterations have been reported in the formation of cortex in animals constitutively deficient in NCAM2 [94]. In contrast, using an in utero electroporation approach, changes in NCAM2 protein levels have been associated with an aberrant neuronal migration and layering positioning in cortex [69] (Ortega-Gascó 2021, data unpublished). The discrepancy between results obtained in mice constitutively deficient in NCAM2 and mice where NCAM2 was manipulated through in utero electroporation could be explained because in utero electroporation allows researchers to cause changes in NCAM2 levels sharply and specifically, thus avoiding any possible compensatory effects.
Neuronal migration and brain layering are crucial processes for the formation a well-structured brain. During neuronal migration, a proper regulation and functioning of cytoskeleton dynamics is involved in the leading process, the nucleokinesis and the tailing process [95]. The downregulation of NCAM2 in vivo causes an alteration of cortical migration, which in turn leads to a mislocalization of layer II-III fated neurons and an altered morphology [69]. NCAM2 overexpression results in a delay in neuronal migration during cortical development (Ortega-Gascó 2021, data unpublished). The molecular mechanism through which NCAM2 is affecting migration is unknown, but there are indications that it could involve radial migration or terminal somal translocation. NCAM2 interacts with several proteins that are involved in the regulation of migration through the Lissencephaly-1/Nuclear distribution protein nudE-like1 (LIS/NDEL) complex, such as 14-3-3ε or the light chains of the dyneins, Dyll1 and Dyll2. The LIS1/NDEL protein complex is key to the process of nucleokinesis in radial migration through the control of microtubule dynamics and centrosome positioning [96,97,98,99]. Heterogeneous deletions of the LIS1 gene have been observed to induce lissencephaly and Miller–Dieker syndrome. In these pathologies, defects in neuronal migration occur that prevent the correct positioning of neurons and the formation of folds in the cortex [100]. The LIS/NDEL complex interacts with different proteins that control its positioning and activity: dyneins, cell division kinesin 5, Aurora A and 14-3-3ε [101,102,103,104]. The interaction of the complex with cytoplasmic dyneins is key to the positioning of the complex during nucleokinesis. The interaction of 14-3-3ε with the complex is essential for the maintenance of NDEL phosphorylation, which controls the complex activity [103]. Thus, 14-3-3ε-deficient animals have erroneous neuronal migration, leading to aberrant brain development that resembles the phenotype of the LIS1-deficient animal [96,103,105]. NCAM2 interacts with MAP1B, which also participates in the regulation of neuronal migration. Changes in MAP1B protein levels disrupt the migration process. In particular, MAP1B controls microtubule stability and reorientation [95,106]. So, NCAM2 interacts with proteins involved in the positioning and functioning of the LIS/NDEL complex and in the correct arrangement of the microtubule cytoskeleton. The results obtained about NCAM2.1 and NCAM2.2 overexpression point to their role in transition from multipolar to bipolar fate due to overactivation of intracellular signaling, resulting in cell retention in the subventricular and intermediate zones (unpublished data). During the transition from multipolar to bipolar fate, there is a retraction of neurites and a rearrangement of the cytoskeleton [88]. The increase of NCAM2 levels produces more protein interaction in the membrane domain (Ortega-Gascó 2021, unpublished data). In the same way, TAG-1 is also an extracellular cell adhesion molecule anchored to the membrane with a GPI domain, and its deficiency in neurons prevents the transition from multipolar to bipolar and the axon specification [107,108]. The mechanism by which TAG-1 produces this phenotype is based on cis interactions with Src family proteins present in the raft lipid domains [107,108].
3.2. Neuronal Differentiation
NCAM2 is expressed in dendritic and axonal compartments and several studies showed its crucial role in neurite outgrowth, dendrite development and axon elongation.
3.2.1. NCAM2 in Dendritic Tree Development
The activation of NCAM2 produces an increase in the number of filopodia and the length of neurites due to an increase in the intracellular calcium levels, which activate the CaMKII complex [83]. Conversely, the knockdown of ncam2 expression during dendrites’ formation causes the retraction of existing neurites and the appearance of new processes from the soma [69]. These phenomena produce a significant increase in the number of primary dendrites; however, these dendrites are shorter, have more branching points, and (at a qualitative level) they are thinner compared to the control situation.
The above dendritic tree alterations could be explained by the interaction of NCAM2 with MAP2. Different studies showed interaction and colocalization of NCAM2 and MAP2 in in vitro neuronal cultures and cingulate cortex [68,69,92]. MAP2, a microtubule-binding protein that promotes microtubule formation and stabilization [109], is key to dendritic formation [86]. In in vitro cultures, a decrease in MAP2 levels impedes the formation and differentiation of dendrites [110]. Not only that, but MAP2-deficient mice exhibit alterations and a reduction in dendritic length [111,112]. Several researchers have hypothesized that NCAM2 could contribute to dendritic tree development and corticogenesis in mouse models. The NCAM2-MAP2 interaction reveals the mechanism through which a loss of NCAM2 produces a reduction in MAP2 levels in dendrites, which in turn alters dendrite formation [69]. In line with this, a microtubule stabilization drug such as taxol reverts the effects of NCAM2 depletion in MAP2 protein levels and dendritic tree formation [69].
Aside from interacting with MAP2, NCAM2 interacts with the proteins of the CaMKII and 14-3-3 families [69,83]. CaMKIIα and CaMKIIβ, two subunits of the CaMKII complex, are involved in neurite formation and dendritic tree development. The CaMKII complex activates different signaling pathways that cause cytoskeletal modifications and activate transcription factors necessary for neuronal differentiation [113,114]. It has been shown that activation of CaMKII through different adhesion molecules, such as NCAM2, is required for neuronal differentiation [83,115]. The 14-3-3 proteins regulate the dynamics of the actin cytoskeleton and the microtubules [116,117]. In particular, 14-3-3ζ controls neurite growth through Protein kinase A, PKA [118] or GSK3β activation [119].
MAP2 transcription is controlled by 14-3-3ζ and L1CAM. 14-3-3ζ controls MAP2 transcription via the PI3K/Akt/NF-κB signaling pathway [120], while L1CAM controls the transcription of MAP2 via Src and the MAPK signaling pathway [121]. The effect of NCAM2 activation on gene transcription is still unknown, although NCAM2 activates Src [83]. NCAM2 interactions and calcium flux could activate different transcription factors that enhance the expression of proteins involved in neuronal morphogenesis, such as MAP2, thus producing a loop that would facilitate dendritic development.
3.2.2. NCAM2 in Axon Formation and Development
Different mechanisms are involved in the branching and elongation of the axonal terminal, some of which required a certain level of cytoskeleton stability [122]. In this sense, excessive instability of the microtubule cytoskeleton results in a higher number of branching points [123]. NCAM2 deficiency increases the number of branches and reduces the maximum length of the main axon, without altering the total length of the axonal shaft [69]. Moreover, 20% of NCAM2-depleted neurons exhibit two or more axons. At the cytoskeleton level, NCAM2 deficiency reduces the signal of acetylated tubulin in axons. Tubulin acetylation is a posttranslational modification that occurs only when microtubules are polymerized and thus is an indirect measure of microtubule cytoskeleton stability [124]. Regarding axon elongation, NCAM2 interacts with MAP1B, which binds to the microtubules and regulates their dynamics. The regulation of MAP1B binding to microtubules is crucial during the axon branching process and changes according to the degree of phosphorylation [125]. In addition, NCAM2 interactions with the light- and medium subunits of neurofilaments have been identified. During development and neuritogenesis, these two subunits form heterodimers and participate in the cytoskeleton dynamics required for axonal growth and transport [126]. Through direct interaction with these neurofilament subunits, with MAP1B, or through signaling pathways that modulate said stability, NCAM2 has a role in stabilizing microtubule cytoskeleton to ensure a proper axon development in terms of length and branching points.
A key structure during neuronal differentiation is the growth cone. Growth cones are located at the end of both dendrites and axons, and explore the extracellular environment [127]. NCAM2 has been shown to modify the dynamics of the growth cone: a NCAM2 deficiency causes the protrusions coming out of the soma to be more dynamic and to present an aberrant morphology, as compared with the control situation [69]. Growth cone dynamics require a proper structure of the actin and microtubules cytoskeletons [127]. The fact that NCAM2 interacts with actin and the CAPZ complex (CAPZ2α and CAPZβ) is relevant since these proteins are involved in growth cone morphology and function. Specifically, the loss of a CAPZ subunit produces an aberrant growth cone with an ectopic position of the microtubules due to a failure in CAPZ binding capacity to tubulin [128]. At the same time, the NCAM2 interactors MAP2, MAP1B and proteins of the 14-3-3 family interact with the actin and microtubule cytoskeleton facilitating the organization and function of the growth cone [118,129].
In mature neurons, as opposed to other cell types, most microtubules do not depend on the centrosome and are polymerized by non-centrosomal mechanisms [130,131]. The mechanisms by which these phenomena occur are not known in detail; several mechanisms are believed to be involved. In relation to the polymerization process, it has been observed that a reduction in NCAM2 levels reduces the non-centrosomal polymerization in axons and dendrites. This finding, together with a reduction in acetylated tubulin levels upon a reduction in NCAM2 levels, indicates a greater presence of tubulin not bound to microtubules. So, when NCAM2 is depleted the equilibrium in the ratio of polymerized versus free tubulin is displaced towards free tubulin [69].
Overall, NCAM2 is essential in the process of neuronal differentiation. Its distribution throughout the neuron and the interactions with proteins that regulate the dynamics of the cytoskeleton enable it to participate in the development of both the dendritic tree and the axonal terminal. NCAM2 is crucial for the microtubule stabilization and the proper neuronal cytoskeleton organization; both processes are required for the maturation of neurites that became dendrites and axons.
3.2.3. NCAM2 in Synaptogenesis and Synaptic Plasticity
During brain development, neuronal polarization and synaptogenesis occur in parallel. The process of stabilization of synaptic contacts is dependent on cytoskeleton rearrangement and neuronal activity. Synaptogenesis is highly regulated by cell adhesion molecules, which control the recognition of membranes, allow the formation of synaptic structures and modulate the neuronal maturation through calcium signaling [132,133,134,135].
In vivo, NCAM2 is detected in presynaptic and postsynaptic compartments and presents trans-homophilic binding between both compartments [68]—see Figure 3. During synapse formation, NCAM2 could participate in the molecular recognition of presynaptic and postsynaptic compartments, facilitate the rearrangement of the cytoskeleton through direct or indirect interaction and modulate calcium flow that facilitates neuronal stabilization. In developing cultured cortical neurons, changes in the expression of NCAM2 can cause abnormalities in synapse formation and function. The activation of NCAM2 increases calcium levels via activation of L-type voltage-dependent calcium channels (VDCCs); these channels are highly expressed in synapses and along the dendrites and axons of mature neurons, and they play a role in regulating dendritic spine morphology [128,131,132,136]. An increase in the frequency of propagating submembrane calcium spikes in neurons with elevated levels of NCAM2 results in a reduction in dendritic spine stability and in a reduced number of mature synapses [137]. Moreover, Ca2+ levels via VDCCs control CaMKII complex, a key protein in the process of synapse formation and plasticity. CaMKII interacts with actin filaments [138,139]; its activation due to the increase of the intracellular calcium causes the disruption of actin filaments and the remodeling of the spine structure through multiple mechanisms, such as the translocation of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, AMPA, receptors to the membrane, the activation of different members of the small GTPases family and the effect on different signaling pathways [140].
Figure 3.
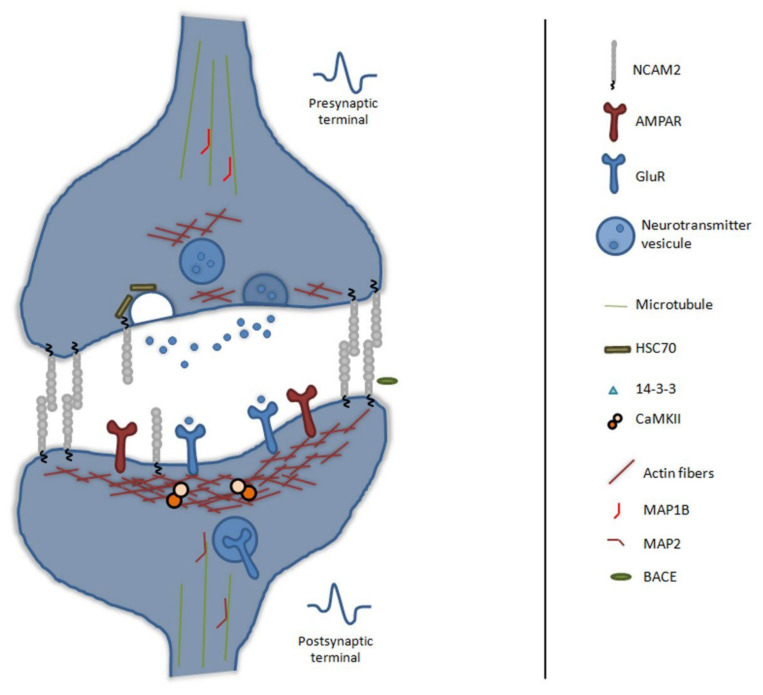
Schematic representation of NCAM2 functions and interactions in synapse maintenance and plasticity. NCAM2 is involved in synapses formation, it is detected in the presynaptic and postsynaptic compartments and undergoes trans-homophilic binding between both compartments. NCAM2 interacts with different scaffold proteins or complexes, which control the shape and dynamics of synapses; such as CaMKII, Actin, 14-3-3 or CAPZ. NCAM2 is involved in synaptic transmission through the neurotransmitters vesicles recycling pathway and the amount of glutamate receptors. Proteolytic cleavage of NCAM2 by BACE1 is important for synapse plasticity and remodeling. Beta-site APP cleaving enzyme 1, BACE1; Microtubule associated protein 1B, MAP1B; Microtubule associated protein 2, MAP2; Calcium/calmodulin-dependent protein kinase type II, CaMKII; F-actin-capping protein, CAPZ; α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, AMPAR; glutamate receptor, GluR and Heat shock cognate 71 kDa, HSC70.
In the adult brain, synaptic plasticity is crucial for learning processes and memory tasks, and it causes a restructuration of presynaptic and postsynaptic compartments. In these processes, cell adhesion molecules interact with neurotransmitter receptors and transduce the synaptic inputs to the cytoskeleton structure. NCAM2 is located at the excitatory synapses and is important for glutamate receptor positioning [76]. The disruption of NCAM2 functions at the cell surface results in the disassembly of glutamatergic synapses and reduces the amount of actin filaments in the spine [76]. Dendritic spines have an actin cytoskeleton core, which plays a key role in maintaining the shape and plasticity of the spine [141,142]. NCAM2 interacts with actin, CAPZ complex and 14-3-3. It is known that CAPZ stabilizes actin fibers [143], is localized in the postsynaptic density [144], and neuronal activity causes its clustering in the spines, which facilitates the remodeling of the structure [145]. The loss of a subunit of the CAPZ complex is associated with alterations in the formation of spines and defects in the specification of presynaptic and postsynaptic compartments, all of which leads to errors in neurotransmission [146]. 14-3-3 is a protein involved in the regulation of cofilin phosphorylation and stabilizes the actin filaments. These functions are related to the phenotype of 14-3-3-deficient animals: a reduction in dendritic tree complexity and number of spines, along with multiple behavioral problems linked to susceptibility to schizophrenia [147,148,149]. The microtubule cytoskeleton is also present in the structure of the spine. Changes in calcium levels produce a cascade of events that modify microtubule cytoskeleton. This results in the entry of microtubules into the spine and a remodeling and stabilization of the structure [150]. In this way, all proteins that interact with the actin cytoskeleton and the microtubule cytoskeleton inside the dendritic spine are crucial for its stabilization [59,98,99], such as MAP2 or MAP1B. These MAPs are associated with maintaining the number and shape of spines [151,152,153]. A reduction in either of these proteins causes spine defects and loss of synaptic transmission [120,121]. So, the function of NCAM2 in maintenance and remodeling of the spine structure would occur through the calcium influx and cytoskeleton, where NCAM2 would interact, directly or indirectly, with proteins associated with actin cytoskeleton dynamics (such as CAPZ, cofilin, 14-3-3 or CaMKII) or with the microtubule cytoskeleton (such as MAP1 or MAP2).
All mechanisms that are involved in the release of synaptic vesicles are relevant for proper synaptic functioning. NCAM2 is detected in presynaptic compartment and interacts with Heat shock cognate 71 kDa, HSC70, and Nogo proteins [70]. On the one hand, the HSC70 protein is a key chaperone in vesicle recycling in presynaptic structures [154]. Aberrant functioning of HSC70 is associated with neurodegenerative diseases, as HSC70 prevents the aggregation of poorly folded proteins [155]. At the functional level, CHL1 is an adhesion protein of the immunoglobulin superfamily that interacts with HSC70 [156]. Through this interaction, CHL1 also controls the vesicle recycling process, and its deficiency produces errors in vesicle recycling. In a similar way to CHL1, NCAM2 could also be involved in vesicle recycling through its interaction with HSC70. On the other hand, Nogo is a protein detected in presynaptic and postsynaptic membranes [157] that controls the number of synapses, modulates plasticity processes [158,159] and is involved in axonal growth and post-traumatic plasticity processes [160]. In addition, Nogo, MAG and other proteins are structural components of myelin present in axonal tracts [70]. The detection of NCAM2 in axonal tracts supports an interaction of NCAM2 with myelin-associated proteins, such as Nogo. Overall, these data would indicate a function of NCAM2 in the presynaptic membrane, affecting vesicle recycling, and an involvement in the functioning of the presynaptic terminal.
Proteolytic cleavage of adhesion molecules is another mechanism for remodeling synapses. In particular, different metalloproteases cleave adhesion molecules in order to remove the physical bond between presynaptic and postsynaptic membranes and thus reshape synapses. Cleavage has been described in several proteins of the immunoglobulin superfamily, such as NCAM1, L1CAM and NCAM2 [75,161]. In vivo, NCAM2 is cleaved by ADAM10 and BACE-1 and could participate in synapses plasticity and remodeling. The amount of NCAM2 in the membrane could regulate the potentiation or depression in these plasticity processes. In detail, it has been noted that the amount of adhesion molecules present in the presynaptic and postsynaptic membranes, such as neurexins and neuroligins [162] or cadherins [163], regulate neuronal plasticity.
In summary, NCAM2 participates in the maintenance of presynaptic and postsynaptic compartments. This function explains the high expression of NCAM2 in cortex and hippocampus during late postnatal and adult stages. The trans-homophilic binding of NCAM2 between the membranes of presynaptic and postsynaptic compartments controls the structure of the synapse. Changes in the structure of these compartments are regulated by intracellular scaffolds, which are controlled by both the calcium influx and NCAM2 interactions with the cytoskeleton, directly or indirectly by cytoskeleton associated proteins. These interactions and the cleavage of NCAM2 could participate in plasticity processes by modifying the structure of synapses.
3.3. NCAM2 in Calcium Signaling and Homeostasis
Relevant functions of CAMs members regarding neuronal development or synaptic plasticity are vehiculated through the calcium signaling [132]. The activation of different members of CAMs induces changes in calcium concentration that are transduced by different proteins to operate in survival mechanisms, modulate cytoskeleton dynamics and activate gene transcription [132]. In the case of NCAM2, there are some studies that analyze the relation between the protein and the calcium signaling during neurite growth and synapse formation [83,137]. In detail, it has been shown that the activation of NCAM2 increases calcium concentration through Scr and VDCC channels. More studies are required to explore other mechanisms, for example, the possibility that NCAM2 increases calcium concentration through FGFR or endoplasmic reticulum (ER) as there is evidence that some NCAM2 partners also interact with the ER. Moreover, the interaction of NCAM2 with FGFR has been previously observed [132] and it is known that the interaction of different CAMs with FGFR could increase intracellular calcium concentration, promoting neurite outgrowth and dendritic development.
4. NCAM2 in Neuronal Diseases
NCAM2 is expressed during brain development as well as in adulthood and its functions are crucial for the proper cognitive process. Genetic and molecular studies show that changes in NCAM2 expression could be the cause of different pathologies both during brain development and in the adult stages—see Table 3.
Table 3.
NCAM2 implications in neurodevelopmental disorders and neurodegenerative diseases.
| Neurodevelopmental Disorder | |||
|---|---|---|---|
| Disorder | Type of Study | Implications | References |
| Autism Spectrum Disorders | Genetic in humans | Genetic studies associate alterations and deletions in Ncam2 with ASD. | [23,164,165] |
| Down Syndrome | Genetic in humans | Increased expression in DS patients due to the location of Ncam2 in the 21 chromosome. | [67,166] |
| Other neurodevelopmental disorders | Genetic in humans | Deletions Ncam2 are found in patients with neurodevelpmental disorders. | [167] |
| Neurodegenerative diseases | |||
| Disorder | Implications | References | |
| Alzheimer’s Disease | Genetic in humans | Alterations in Ncam2 found in AD patients. | [168,169] |
| Experimental with human and mouse samples. | β-amyloid induces proteolysis of synaptic NCAM2. | [76] | |
| Frontotemporal dementia | Experimental with mouse tissue samples | NCAM2 proposed as a candidate receptor for GRN | [70] |
4.1. Neurodevelopment Diseases
NCAM2 is necessary both for the correct neuronal migration and for the appropriate development of dendritic trees and axonal compartments in cortical neurons and olfactory bulb neurons. Upon NCAM2 knockdown, cortical neurons lacked a clear main apical dendrite and showed numerous primary dendrites arising from any position in the cell body [69]. This phenotype could explain the implication of NCAM2 in neurodevelopment disorders. Alterations in NCAM2 have been associated with different disorders, such as Down Syndrome or Autism Spectrum Disorders [23,67,164,165,166]. Due to its location in human chromosome 21, NCAM2 has been proposed as a candidate for the intellectual disability phenotype observed in Down Syndrome [67,166]. Even though Ncam2 is located outside the critical region of chromosome 21, it is believed that an increased expression of NCAM2 could negatively affect the nervous system development due to dosage-related effects. In addition, genetic analyses revealed single nucleotide polymorphisms in NCAM2 gene in patients with abnormal neurodevelopment [167], Marden–Walker syndrome patients [170] and Autism Spectrum Disorders [23,164,165]. Not only does NCAM2 regulate neuronal polarization and formation of axo-dendritic compartments, but it also interacts with different cytoskeleton components that are linked to autism disorder [69,70,171,172,173]. Genetic variations in the NCAM2 gene are less known but could be the cause of intellectual disability. Therefore, one hypothesis is that structural alterations observed in patients with aberrant neurodevelopment and autism could be triggered by cytoskeleton dynamics modifications produced by variations in NCAM2 gene expression.
4.2. Neurodegenerative Diseases
Changes in the density and structure of the spines have been associated with the early stages of neurodegenerative diseases. At a molecular level, changes in the expression and cellular localization of adhesion proteins have been described in these diseases. Genetic studies established a relation between NCAM2 and Alzheimer’s Disease [168,169]. It has been proposed that the early loss of synapses detected in patients with Alzheimer’s Disease could be the result of the β-amyloid-induced proteolysis of synaptic NCAM2. Due to the pivotal role of NCAM2 in synaptic structures and the mentioned interactions of NCAM2 with the cytoskeleton, the ablation of NCAM2 in synapses could lead to major changes in cytoskeleton structures, thus compromising synaptic viability in Alzheimer’s Disease [76,174,175,176,177]. The interaction of NCAM2 with Granulin, GRN, one of the principal proteins associated with familial frontotemporal dementia, suggest a plausible role of NCAM2 as a receptor of the protein and a possible implication in frontotemporal dementia [70,178]. A decrease in NCAM2 levels and a change in NCAM2 localization in the spine could be associated with loss of structure of synapses in the early stages of neurodegenerative diseases.
5. Conclusions
With all the data and observations presented in this review, we can hypothesize that NCAM2 contributes to the neuronal molecular identity and is crucial for the neuronal functions—see Figure 4. In the development of the nervous system, NCAM2 regulates the molecular recognition that allows the formation of contacts between axonal and dendritic compartments leading to the formation of synapses and neural circuits. In particular, NCAM2 is present in both axonal and dendritic compartments and establishes homophilic junctions and intracellular interactions. These interactions regulate the modification of the cytoskeleton and affect the structure of the dendrite and axon. Different mechanisms are modulated by NCAM2 interactions but these can be divided in two groups: calcium signaling mechanisms and structuring of the cytoskeleton. NCAM2 is essential for the proper development of dendritic and axonal structures; its depletion causes aberrant neuron migration and differentiation and is detected in neurodevelopmental diseases. With these results, we can affirm that changes in NCAM2 alter the neuronal molecular identity, produces alterations in neuronal development and conduces to an aberrant neuronal network formation. Moreover, different CAMs modulate the neuronal differentiation, structure pruning and synaptic stabilization through the activation of gene expression. It is known that calcium influx and CaMKII modulate gene expression. More studies are necessary to identify the possible role of NCAM2 in gene expression.
Figure 4.
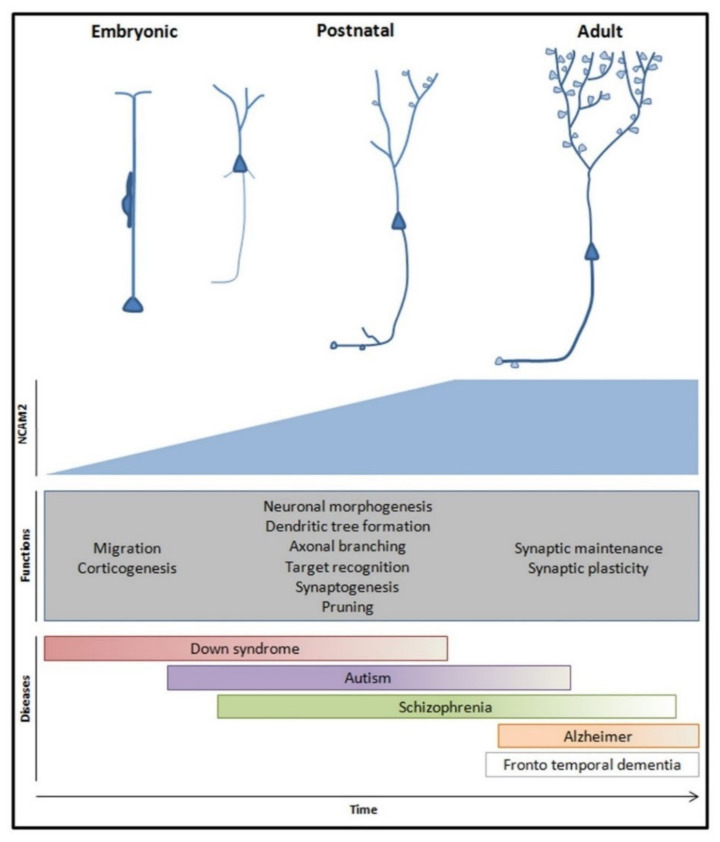
Global representation of NCAM2 physiological functions and pathological implications during brain development and in adult stages. NCAM2 expression increases during embryonic and early postnatal stages and it is maintained in adulthood. NCAM2 participates in the establishment of the neuronal molecular identity which is essential for an amount of processes during neuron development and neuronal network morphogenesis; e.g., neuronal migration, dendritic tree development, synaptogenesis and synaptic plasticity. These functions and genetic studies of neuronal pathologies showed that NCAM2 could be an important target for new therapeutic strategies.
In the adult brain, the neuronal network is less plastic, the dendrites and axons are stable structures and neuronal plasticity is restricted to synapses and adult neurogenesis. For synaptic plasticity to take place, neuronal molecular identity and a proper balance between stabilization of membranes and remodeling of contacts are necessary. NCAM2 is present in membranes in both synaptic compartments: presynaptic and postsynaptic. NCAM2 participates in plasticity processes through the diverse interactions in the membrane and with both presynaptic and postsynaptic scaffold structures. The amount of NCAM2 in the membrane could regulate the synaptic plasticity processes. NCAM2 controls the cytoskeleton structure and dynamics, which proves crucial for the maintenance of the synaptic scaffold and neuronal synapses. Moreover, NCAM2 could participate in local transcription, as it interacts with different transcription complexes. NCAM2 would be controlling the specific transcription of proteins in synapses, a necessary mechanism for synapse remodeling and potentiation. Besides, NCAM2 cleavage by metalloproteinases is another mechanism for synapse remodeling. Some processes controlled by NCAM2 are altered at the early stages of neurodegenerative diseases, such as Alzheimer’s disease. So, NCAM2 could represent a therapeutic target for a new strategy to preserve the synapse structure.
Author Contributions
E.S., L.P., A.O.-G. and A.P. participated in the creation of the items and overarching manuscript organization. A.P. and A.O.-G. participated in the information search. All authors wrote the initial draft. A.P. and A.O.-G. drew the figures of the manuscript. E.S., L.P. and A.P. reviewed and commented on the different versions of the manuscript and prepared the final version. E.S supervised and controlled the manuscript execution. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by grants from the Spanish MINECO (SAF2016-76340R and PID2019-106764RB-C21) to E.S. and L.P., CIBERNED (ISCIII), Spanish MECD (FPU14/02156), Excellence Unit María de Maeztu/Institute of Neurosciences to E.S, and BES-2017-080570 to A.O.-G. and a grant from Secretary of Universities and Research of the Department of Economy and Knowledge of the Generalitat de Catalunya to A.P.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shapiro L., Love J., Colman D.R. Adhesion Molecules in the Nervous System: Structural Insights into Function and Diversity. Annu. Rev. Neurosci. 2007;30:451–474. doi: 10.1146/annurev.neuro.29.051605.113034. [DOI] [PubMed] [Google Scholar]
- 2.Hansen S.M., Berezin V., Bock E. Signaling mechanisms of neurite outgrowth induced by the cell adhesion molecules NCAM and N-Cadherin. Cell. Mol. Life Sci. 2008;65:3809–3821. doi: 10.1007/s00018-008-8290-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shima Y., Kawaguchi S.-Y., Kosaka K., Nakayama M., Hoshino M., Nabeshima Y., Hirano T., Uemura T. Opposing roles in neurite growth control by two seven-pass transmembrane cadherins. Nat. Neurosci. 2007;10:963–969. doi: 10.1038/nn1933. [DOI] [PubMed] [Google Scholar]
- 4.Maness P.F., Schachner M. Neural recognition molecules of the immunoglobulin superfamily: Signaling transducers of axon guidance and neuronal migration. Nat. Neurosci. 2006;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 5.Kleene R., Mzoughi M., Joshi G., Kalus I., Bormann U., Schulze C., Xiao M.-F., Dityatev A., Schachner M. NCAM-Induced Neurite Outgrowth Depends on Binding of Calmodulin to NCAM and on Nuclear Import of NCAM and fak Fragments. J. Neurosci. 2010;30:10784–10798. doi: 10.1523/JNEUROSCI.0297-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S.-Y., Mo J., Han S.B., Choi S., Moon B., Rhyu I., Sun W., Kim H. The expression of non-clustered protocadherins in adult rat hippocampal formation and the connecting brain regions. Neuroscience. 2010;170:189–199. doi: 10.1016/j.neuroscience.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 7.Missaire M., Hindges R. The role of cell adhesion molecules in visual circuit formation: From neurite outgrowth to maps and synaptic specificity. Dev. Neurobiol. 2015;75:569–583. doi: 10.1002/dneu.22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gennarini G., Bizzoca A., Picocci S., Puzzo D., Corsi P., Furley A.J.W. The role of Gpi-anchored axonal glycoproteins in neural development and neurological disorders. Mol. Cell. Neurosci. 2017;81:49–63. doi: 10.1016/j.mcn.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai T. The role of cell adhesion molecules in brain wiring and neuropsychiatric disorders. Mol. Cell. Neurosci. 2017;81:4–11. doi: 10.1016/j.mcn.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 10.Jin Y., Li H. Revisiting Dscam diversity: Lessons from clustered protocadherins. Experientia. 2019;76:667–680. doi: 10.1007/s00018-018-2951-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jan Y.-N., Jan L. Branching out: Mechanisms of dendritic arborization. Nat. Rev. Neurosci. 2010;11:316–328. doi: 10.1038/nrn2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu K., Xu Y., Liu J., Xu Q., Ye H. Down syndrome cell adhesion molecule and its functions in neural development. Neurosci. Bull. 2011;27:45–52. doi: 10.1007/s12264-011-1045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson K.M., Vonhoff F., Duch C. Dscam1 Is Required for Normal Dendrite Growth and Branching But Not for Dendritic Spacing in Drosophila Motoneurons. J. Neurosci. 2014;34:1924–1931. doi: 10.1523/JNEUROSCI.3448-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Namba T., Funahashi Y., Nakamuta S., Xu C., Takano T., Kaibuchi K. Extracellular and Intracellular Signaling for Neuronal Polarity. Physiol. Rev. 2015;95:995–1024. doi: 10.1152/physrev.00025.2014. [DOI] [PubMed] [Google Scholar]
- 15.Baig D.N., Yanagawa T., Tabuchi K. Distortion of the normal function of synaptic cell adhesion molecules by genetic variants as a risk for autism spectrum disorders. Brain Res. Bull. 2017;129:82–90. doi: 10.1016/j.brainresbull.2016.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Schmid R., Maness P.F. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr. Opin. Neurobiol. 2008;18:245–250. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Yu H., Jiang S., Liao J., Lu T., Wang L., Zhang D., Yue W. Evidence for Association of Cell Adhesion Molecules Pathway and NLGN1 Polymorphisms with Schizophrenia in Chinese Han Population. PLoS ONE. 2015;10:e0144719. doi: 10.1371/journal.pone.0144719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tost H., Bilek E., Meyer-Lindenberg A. Brain connectivity in psychiatric imaging genetics. NeuroImage. 2012;62:2250–2260. doi: 10.1016/j.neuroimage.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Wang K.S., Liu X.F., Aragam N. A genome-wide meta-analysis identifies novel loci associated with schizophrenia and bipolar disorder. Schizophr. Res. 2010;124:192–199. doi: 10.1016/j.schres.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Chapman N.H., Estes A., Munson J., Bernier R., Webb S.J., Rothstein J.H., Minshew N.J., Dawson G., Schellenberg G.D., Wijsman E.M. Genome-scan for IQ discrepancy in autism: Evidence for loci on chromosomes 10 and 16. Qual. Life Res. 2010;129:59–70. doi: 10.1007/s00439-010-0899-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow E., Yoo S., Flavell S., Kim T.K., Lin Y., Hill R.S., Mukaddes N.M., Balkhy S., Gascon G., Hashmi A., et al. Identifying autism loci and genes by tracing recent shared ancestry. Science. 2008;321:218–223. doi: 10.1126/science.1157657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Depienne C., Bouteiller D., Keren B., Cheuret E., Poirier K., Trouillard O., Benyahia B., Quelin C., Carpentier W., Julia S., et al. Sporadic Infantile Epileptic Encephalopathy Caused by Mutations in PCDH19 Resembles Dravet Syndrome but Mainly Affects Females. PLoS Genet. 2009;5:e1000381. doi: 10.1371/annotation/314060d5-06da-46e0-b9e4-57194e8ece3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussman J.P., Chung R.-H., Griswold A.J., Jaworski J.M., Salyakina D., Ma D., Konidari I., Whitehead P.L., Vance J.M., Martin E.R., et al. A noise-reduction GWAS analysis implicates altered regulation of neurite outgrowth and guidance in autism. Mol. Autism. 2011;2:1. doi: 10.1186/2040-2392-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neale B.M., Kou Y., Liu L., Ma’Ayan A., Samocha K.E., Sabo A., Lin C.F., Stevens S., Wang L.-S., Makarov V., et al. Patterns and rates of exonic de novo mutations in autism spectrum disorders. Nature. 2012;485:242–245. doi: 10.1038/nature11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cukier H.N., Lee J.M., Ma D., Young J.I., Mayo V., Butler B.L., Ramsook S.S., Rantus J.A., Abrams A.J., Whitehead P.L., et al. The Expanding Role of MBD Genes in Autism: Identification of aMECP2Duplication and Novel Alterations inMBD5,MBD6, andSETDB1. Autism Res. 2012;5:385–397. doi: 10.1002/aur.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenny E.M., Cormican P., Furlong S., Heron E., Kenny G., Fahey C., Kelleher E., Ennis S., Tropea D., Morris D.W., et al. Excess of rare novel loss-of-function variants in synaptic genes in schizophrenia and autism spectrum disorders. Mol. Psychiatry. 2014;19:872–879. doi: 10.1038/mp.2013.127. [DOI] [PubMed] [Google Scholar]
- 27.Feng J., Schroer R., Yan J., Song W., Yang C., Bockholt A., Cook E.H., Jr., Charles C.S., Steve E.S., Sommer S.S., et al. High frequency of neurexin 1β signal peptide structural variants in patients with autism. Neurosci. Lett. 2006;409:10–13. doi: 10.1016/j.neulet.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Szatmari P., Paterson A., Zwaigenbaum L., Roberts W., Brian J., Liu X.Q., Vincent J., Skaug J., Thompson A., Meyer K., et al. Mapping autism risk loci using genetic linkage and chromosomal rearrangements. Nat. Genet. 2007;39:319. doi: 10.1038/ng1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zweier C., de Jong E.K., Zweier M., Orrico A., Ousager L.B., Collins A.L., Bijlsma E.K., Oortveld M.A., Ekici A.B., Reis A., et al. CNTNAP2 and NRXN1 Are Mutated in Autosomal-Recessive Pitt-Hopkins-like Mental Retardation and Determine the Level of a Common Synaptic Protein in Drosophila. Am. J. Hum. Genet. 2009;85:655–666. doi: 10.1016/j.ajhg.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Glessner J.T., Wang K., Cai G., Korvatska O., Kim C.E., Wood S., Zhang H., Estes A., Brune C.W., Hakonarson H., et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu X., Xiong Z., Zhang L., Liu Y., Lu L., Peng Y., Guo H., Zhao J., Xia K., Hu Z. Variations analysis of NLGN3 and NLGN4X gene in Chinese autism patients. Mol. Biol. Rep. 2014;41:4133–4140. doi: 10.1007/s11033-014-3284-5. [DOI] [PubMed] [Google Scholar]
- 32.Jamain S., Radyushkin K., Hammerschmidt K., Granon S., Boretius S., Varoqueaux F., Ramanantsoa N., Gallego J., Ronnenberg A., Winter D., et al. Reduced social interaction and ultrasonic communication in a mouse model of monogenic heritable autism. Proc. Natl. Acad. Sci. USA. 2008;105:1710–1715. doi: 10.1073/pnas.0711555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li X., Hu Z., He Y., Xiong Z., Long Z., Peng Y., Fengxiao B., Jie L., Guanglei X., Xia K., et al. Association analysis of CNTNAP2 poly-morphisms with autism in the Chinese Han population. Psychiatr. Genet. 2010;20:113–117. doi: 10.1097/YPG.0b013e32833a216f. [DOI] [PubMed] [Google Scholar]
- 34.Whitehouse A.J.O., Bishop D.V.M., Ang Q.W., Pennell C.E., Fisher S. CNTNAP2variants affect early language development in the general population. Genes Brain Behav. 2011;10:451–456. doi: 10.1111/j.1601-183X.2011.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peñagarikano O., Abrahams B.S., Herman E.I., Winden K.D., Gdalyahu A., Dong H., Sonnenblick L.I., Gruver R., Almajano J., Geschwind D.H. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell. 2011;147:235–246. doi: 10.1016/j.cell.2011.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gdalyahu A., Lázaro M., Penagarikano O., Golshani P., Trachtenberg J.T., Gescwind D.H. The Autism Related Protein Contactin-Associated Protein-Like 2 (CNTNAP2) Stabilizes New Spines: An In Vivo Mouse Study. PLoS ONE. 2015;10:e0125633. doi: 10.1371/journal.pone.0125633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Varea O., Martin-De-Saavedra M.D., Kopeikina K.J., Schürmann B., Fleming H.J., Fawcett-Patel J.M., Bach A., Jang S., Peles E., Kim E., et al. Synaptic abnormalities and cytoplasmic glutamate receptor aggregates in contactin associated protein-like 2/Caspr2 knockout neurons. Proc. Natl. Acad. Sci. USA. 2015;112:6176–6181. doi: 10.1073/pnas.1423205112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atz M.E., Rollins B., Vawter M.P. NCAM1 association study of bipolar disorder and schizophrenia: Polymorphisms and alternatively spliced isoforms lead to similarities and differences. Psychiatr. Genet. 2007;17:55–67. doi: 10.1097/YPG.0b013e328012d850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan P.F., Keefe R.S., Lange L.A., Lange E.M., Stroup T.S., Lieberman J., Maness P.F. NCAM1 and Neurocognition in Schizophrenia. Biol. Psychiatry. 2007;61:902–910. doi: 10.1016/j.biopsych.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 40.Deng S.-P., Hu W., Calhoun V.D., Wang Y.-P. Integrating Imaging Genomic Data in the Quest for Biomarkers of Schizophrenia Disease. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018;15:1480–1491. doi: 10.1109/TCBB.2017.2748944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wedervang-Resell K., Ueland T., Aukrust P., Friis S., Holven K.B., Johannessen C.H., Lekva T., Lonning V., Smelror R.E., Szabo A., et al. Reduced levels of circulating adhesion molecules in adolescents with early-onset psychosis. NPJ Schizophr. 2020;6:1–8. doi: 10.1038/s41537-020-00112-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nikitczuk J.S., Patil S.B., Matikainen-Ankney B.A., Scarpa J., Shapiro M.L., Benson D.L., Huntley G.W. N-cadherin regulates molecular organization of excitatory and inhibitory synaptic circuits in adult hippocampus in vivo. Hippocampus. 2014;24:943–962. doi: 10.1002/hipo.22282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avdic U., Ahl M., Andersson M., Ekdahl C.T. Levetiracetam and N-Cadherin Antibody Alleviate Brain Pathology Without Reducing Early Epilepsy Development After Focal Non-convulsive Status Epilepticus in Rats. Front. Neurol. 2021;12:630154. doi: 10.3389/fneur.2021.630154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takano K., Nakagawa E., Inoue K., Kamada F., Kure S., Goto Y.-I. A loss-of-function mutation in theFTSJ1 gene causes nonsyndromic X-linked mental retardation in a japanese family. Am. J. Med Genet. Part B Neuropsychiatr. Genet. 2008;147B:479–484. doi: 10.1002/ajmg.b.30638. [DOI] [PubMed] [Google Scholar]
- 45.Takeshita E., Nakagawa E., Nakatani K., Sasaki M., Goto Y.-I. Novel AGTR2 missense mutation in a Japanese boy with severe mental retardation, pervasive developmental disorder, and epilepsy. Brain Dev. 2012;34:776–779. doi: 10.1016/j.braindev.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 46.Hayase Y., Amano S., Hashizume K., Tominaga T., Miyamoto H., Kanno Y., Ueno-Inoue Y., Inoue T., Yamada M., Ogata S., et al. Down syndrome cell adhesion molecule like-1 (DSCAML1) links the GABA system and seizure susceptibility. Acta Neuropathol. Commun. 2020;8:1–17. doi: 10.1186/s40478-020-01082-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W., Wang L., Luo J., Xi Z., Wang X., Chen G., Chu L. Role of a Neural Cell Adhesion Molecule Found in Cerebrospinal Fluid as a Potential Biomarker for Epilepsy. Neurochem. Res. 2012;37:819–825. doi: 10.1007/s11064-011-0677-x. [DOI] [PubMed] [Google Scholar]
- 48.Cho S., Muthukumar A.K., Stork T., Coutinho-Budd J.C., Freeman M.R. Focal adhesion molecules regulate astrocyte morphology and glutamate transporters to suppress seizure-like behavior. Proc. Natl. Acad. Sci. USA. 2018;115:11316–11321. doi: 10.1073/pnas.1800830115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mitsogiannis M.D., Pancho A., Aerts T., Sachse S.M., Vanlaer R., Noterdaeme L., Schmucker D., Seuntjens E. Subtle Roles of Down Syndrome Cell Adhesion Molecules in Embryonic Forebrain Development and Neuronal Migration. Front. Cell Dev. Biol. 2021;8:1859. doi: 10.3389/fcell.2020.624181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chmielewska J.J., Kuzniewska B., Milek J., Urbanska K., Dziembowska M. Neuroligin 1, 2, and 3 Regulation at the Synapse: FMRP-Dependent Translation and Activity-Induced Proteolytic Cleavage. Mol. Neurobiol. 2018;56:2741–2759. doi: 10.1007/s12035-018-1243-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alves-Sampaio A., Troca-Marín J.A., Montesinos M.L. NMDA-Mediated Regulation of DSCAM Dendritic Local Translation Is Lost in a Mouse Model of Down’s Syndrome. J. Neurosci. 2010;30:13537–13548. doi: 10.1523/JNEUROSCI.3457-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang X.-Y., Xu L., Wang J., Hong Y., Wang Y., Zhu Q., Wang D., Zhang X.-Y., Liu C.-Y., Fang K.-H., et al. Suppressing the DSCAM/PAK1 pathway reverses neurogenesis deficits in Down Syndrome patient iPSC-derived cerebral organoids. J. Clin. Investig. 2021;131:e135763. doi: 10.1172/JCI135763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pebusque M.J., Coulier F., Birnbaum D., Pontarotti P. Ancient large-scale genome duplications: Phylogenetic and linkage analyses shed light on chordate genome evolution. Mol. Biol. Evol. 1998;15:1145–1159. doi: 10.1093/oxfordjournals.molbev.a026022. [DOI] [PubMed] [Google Scholar]
- 54.Makino T., McLysaght A. Ohnologs in the human genome are dosage balanced and frequently associated with disease. Proc. Natl. Acad. Sci. USA. 2010;107:9270–9274. doi: 10.1073/pnas.0914697107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kiselyov V.V., Skladchikova G., Hinsby A.M., Jensen P.H., Kulahin N., Soroka V., Pedersen N., Tsetlin V., Poulsen F.M., Berezin V., et al. Structural Basis for a Direct Interaction between FGFR1 and NCAM and Evidence for a Regulatory Role of ATP. Structure. 2003;11:691–701. doi: 10.1016/S0969-2126(03)00096-0. [DOI] [PubMed] [Google Scholar]
- 56.Mehrabian M., Hildebrandt H., Schmitt-Ulms G. NCAM1 Polysialylation: The Prion Protein’s Elusive Reason for Being? ASN Neuro. 2016;8 doi: 10.1177/1759091416679074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Angata K., Huckaby V., Ranscht B., Terskikh A., Marth J.D., Fukuda M. Polysialic Acid-Directed Migration and Differentiation of Neural Precursors Are Essential for Mouse Brain Development. Mol. Cell. Biol. 2007;27:6659–6668. doi: 10.1128/MCB.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sytnyk V., Leshchyns’Ka I., Schachner M. Neural Cell Adhesion Molecules of the Immunoglobulin Superfamily Regulate Synapse Formation, Maintenance, and Function. Trends Neurosci. 2017;40:295–308. doi: 10.1016/j.tins.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Dityatev A., Dityateva G., Sytnyk V., Delling M., Toni N., Nikonenko I., Muller D., Schachner M. Polysialylated Neural Cell Adhesion Molecule Promotes Remodeling and Formation of Hippocampal Synapses. J. Neurosci. 2004;24:9372–9382. doi: 10.1523/JNEUROSCI.1702-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muller D., Mendez P., DeRoo M., Klauser P., Steen S., Poglia L. Role of NCAM in Spine Dynamics and Synaptogenesis. Adv. Exp. Med. Biol. 2010;663:245–256. doi: 10.1007/978-1-4419-1170-4_16. [DOI] [PubMed] [Google Scholar]
- 61.Shaw A.D., Tiwari Y., Kaplan W., Heath A., Mitchell P.B., Schofield P.R., Fullerton J.M. Characterisation of Genetic Variation in ST8SIA2 and Its Interaction Region in NCAM1 in Patients with Bipolar Disorder. PLoS ONE. 2014;9:e92556. doi: 10.1371/journal.pone.0092556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Xiao M., Ji S., Tang J., Xu L., Li X., Li M., Wang H.-Z., Jiang H.-Y., Zhang D.-F., et al. Promoter variant rs2301228 on the neural cell adhesion molecule 1 gene confers risk of schizophrenia in Han Chinese. Schizophr. Res. 2014;160:88–96. doi: 10.1016/j.schres.2014.09.036. [DOI] [PubMed] [Google Scholar]
- 63.Di Cristo G., Chattopadhyaya B., Kuhlman S., Fu Y., Bélanger M.-C., Wu C.Z., Rutishauser U., Maffei L., Huang Z.J. Activity-dependent PSA expression regulates inhibitory maturation and onset of critical period plasticity. Nat. Neurosci. 2007;10:1569–1577. doi: 10.1038/nn2008. [DOI] [PubMed] [Google Scholar]
- 64.Chattopadhyaya B., Baho E., Huang Z.J., Schachner M., Di Cristo G. Neural Cell Adhesion Molecule-Mediated Fyn Activation Promotes GABAergic Synapse Maturation in Postnatal Mouse Cortex. J. Neurosci. 2013;33:5957–5968. doi: 10.1523/JNEUROSCI.1306-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alenius M., Bohm S. Identification of a Novel Neural Cell Adhesion Molecule-related Gene with a Potential Role in Selective Axonal Projection. J. Biol. Chem. 1997;272:26083–26086. doi: 10.1074/jbc.272.42.26083. [DOI] [PubMed] [Google Scholar]
- 66.Yoshihara Y., Kawasaki M., Tamada A., Fujita H., Hayashi H., Kagamiyama H., Mori K. OCAM: A New Member of the Neural Cell Adhesion Molecule Family Related to Zone-to-Zone Projection of Olfactory and Vomeronasal Axons. J. Neurosci. 1997;17:5830–5842. doi: 10.1523/JNEUROSCI.17-15-05830.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paoloni-Giacobino A., Chen H., Antonarakis S.E. Cloning of a Novel Human Neural Cell Adhesion Molecule Gene (NCAM2) That Maps to Chromosome Region 21q21 and Is Potentially Involved in Down Syndrome. Genomics. 1997;43:43–51. doi: 10.1006/geno.1997.4782. [DOI] [PubMed] [Google Scholar]
- 68.Ichinohe N., Yoshihara Y., Hashikawa T., Rockland K.S. Developmental study of dendritic bundles in layer 1 of the rat granular retrosplenial cortex with special reference to a cell adhesion molecule, OCAM. Eur. J. Neurosci. 2003;18:1764–1774. doi: 10.1046/j.1460-9568.2003.02900.x. [DOI] [PubMed] [Google Scholar]
- 69.Parcerisas A., Pujadas L., Ortega-Gascó A., Perelló-Amorós B., Viais R., Hino K., Figueiro-Silva J., La Torre A., Trullás R., Simó S., et al. NCAM2 Regulates Dendritic and Axonal Differentiation through the Cytoskeletal Proteins MAP2 and 14-3-3. Cereb. Cortex. 2020;30:3781–3799. doi: 10.1093/cercor/bhz342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Parcerisas A., Ortega-Gascó A., Hernaiz-Llorens M., Odena M.A., Ulloa F., de Oliveira E., Bosch M., Pujadas L., Soriano E. New Partners Identified by Mass Spectrometry Assay Reveal Functions of NCAM2 in Neural Cytoskeleton Organization. Int. J. Mol. Sci. 2021;22:7404. doi: 10.3390/ijms22147404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Niethammer P., Delling M., Sytnyk V., Dityatev A., Fukami K., Schachner M. Cosignaling of NCAM via lipid rafts and the FGF receptor is required for neuritogenesis. J. Cell Biol. 2002;157:521–532. doi: 10.1083/jcb.200109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kamiguchi H. The region-specific activities of lipid rafts during axon growth and guidance. J. Neurochem. 2006;98:330–335. doi: 10.1111/j.1471-4159.2006.03888.x. [DOI] [PubMed] [Google Scholar]
- 73.Huttlin E.L., Jedrychowski M.P., Elias J.E., Goswami T., Rad R., Beausoleil S.A., Villen J., Haas W., Sowa M.E., Gygi S.P. A Tissue-Specific Atlas of Mouse Protein Phosphorylation and Expression. Cell. 2010;143:1174–1189. doi: 10.1016/j.cell.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulahin N., Walmod P.S. Structure and Function of the Neural Cell Adhesion Molecule NCAM. Volume 663. Springer; New York, NY, USA: 2009. The Neural Cell Adhesion Molecule NCAM2/OCAM/RNCAM, a Close Relative to NCAM; pp. 403–420. [DOI] [PubMed] [Google Scholar]
- 75.Kim W.H., Watanabe H., Lomoio S., Tesco G. Spatiotemporal processing of neural cell adhesion molecules 1 and 2 by BACE1 in vivo. J. Biol. Chem. 2021;296:100372. doi: 10.1016/j.jbc.2021.100372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leshchyns’Ka I., Liew H.T., Shepherd C., Halliday G.M., Stevens C.H., Ke Y.D., Ittner L.M., Sytnyk V. Aβ-dependent reduction of NCAM2-mediated synaptic adhesion contributes to synapse loss in Alzheimer’s disease. Nat. Commun. 2015;6:8836. doi: 10.1038/ncomms9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu R.K., Yanagisawa M. Glycobiology of neural stem cells. CNS Neurol. Disord. Drug Targets. 2006;5:415–423. doi: 10.2174/187152706777950675. [DOI] [PubMed] [Google Scholar]
- 78.Kulahin N., Kristensen O., Rasmussen K.K., Olsen L., Rydberg P., Vestergaard B., Kastrup J.S., Berezin V., Bock E., Walmod P.S., et al. Structural Model and trans-Interaction of the Entire Ectodomain of the Olfactory Cell Adhesion Molecule. Structure. 2011;19:203–211. doi: 10.1016/j.str.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 79.Deleyrolle L., Sabourin J.-C., Rothhut B., Fujita H., Guichet P.-O., Teigell M., Ripoll C., Chauvet N., Perrin F., Mamaeva D., et al. OCAM Regulates Embryonic Spinal Cord Stem Cell Proliferation by Modulating ErbB2 Receptor. PLoS ONE. 2015;10:e0122337. doi: 10.1371/journal.pone.0122337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rasmussen K.K., Falkesgaard M.H., Winther M., Roed N.K., Quistgaard C.L., Teisen M.N., Edslev S.M., Petersen D.L., Aljubouri A., Christensen C., et al. NCAM2 Fibronectin type-III domains form a rigid structure that binds and activates the Fibroblast Growth Factor Receptor. Sci. Rep. 2018;8:8957. doi: 10.1038/s41598-018-27089-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collins M., Husi H., Yu L., Brandon J.M., Anderson C.N.G., Blackstock W.P., Choudhary J., Grant S. Molecular characterization and comparison of the components and multiprotein complexes in the postsynaptic proteome. J. Neurochem. 2006;97:16–23. doi: 10.1111/j.1471-4159.2005.03507.x. [DOI] [PubMed] [Google Scholar]
- 82.Kislinger T., Cox B., Kannan A., Chung C., Hu P., Ignatchenko A., Scott M.S., Gramolini A.O., Morris Q., Hallett M.T., et al. Global Survey of Organ and Organelle Protein Expression in Mouse: Combined Proteomic and Transcriptomic Profiling. Cell. 2006;125:173–186. doi: 10.1016/j.cell.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 83.Sheng L., Leshchyns’Ka I., Sytnyk V. Neural Cell Adhesion Molecule 2 Promotes the Formation of Filopodia and Neurite Branching by Inducing Submembrane Increases in Ca2+ Levels. J. Neurosci. 2015;35:1739–1752. doi: 10.1523/JNEUROSCI.1714-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Urbanska M., Blazejczyk M., Jaworski J. Molecular Basis of Dendritic Arborization. [(accessed on 3 August 2021)]; doi: 10.55782/ane-2008-1695. Available online: https://pubmed.ncbi.nlm.nih.gov/18511961/ [DOI] [PubMed]
- 85.Da Silva J.S., Dotti C.G. Breaking the neuronal sphere: Regulation of the actin cytoskeleton in neuritogenesis. Nat. Rev. Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- 86.Cheng P.-L., Poo M.-M. Early Events in Axon/Dendrite Polarization. Annu. Rev. Neurosci. 2012;35:181–201. doi: 10.1146/annurev-neuro-061010-113618. [DOI] [PubMed] [Google Scholar]
- 87.Takano T., Xu C., Funahashi Y., Namba T., Kaibuchi K. Neuronal polarization. Development. 2015;142:2088–2093. doi: 10.1242/dev.114454. [DOI] [PubMed] [Google Scholar]
- 88.Kawauchi T. Cell Adhesion and Its Endocytic Regulation in Cell Migration during Neural Development and Cancer Metastasis. Int. J. Mol. Sci. 2012;13:4564–4590. doi: 10.3390/ijms13044564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Farah C.A., Leclerc N. HMWMAP2: New perspectives on a pathway to dendritic identity. Cell Motil. Cytoskelet. 2008;65:515–527. doi: 10.1002/cm.20284. [DOI] [PubMed] [Google Scholar]
- 90.Dalva M.B., McClelland A.C., Kayser M.S. Cell adhesion molecules: Signalling functions at the synapse. Nat. Rev. Neurosci. 2007;8:206–220. doi: 10.1038/nrn2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seong E., Yuan L., Arikkath J. Cadherins and catenins in dendrite and synapse morphogenesis. Cell Adhes. Migr. 2015;9:202–213. doi: 10.4161/19336918.2014.994919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ichinohe N., Knight A., Ogawa M., Ohshima T., Mikoshiba K., Yoshihara Y., Terashima T., Rockland K. Unusual Patch-Matrix Organization in the Retrosplenial Cortex of the reeler Mouse and Shaking Rat Kawasaki. Cereb. Cortex. 2008;18:1125–1138. doi: 10.1093/cercor/bhm148. [DOI] [PubMed] [Google Scholar]
- 93.Borisovska M., McGinley M.J., Bensen A., Westbrook G.L. Loss of olfactory cell adhesion molecule reduces the synchrony of mitral cell activity in olfactory glomeruli. J. Physiol. 2011;589:1927–1941. doi: 10.1113/jphysiol.2011.206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Walz A., Mombaerts P., Greer C.A., Treloar H.B. Disrupted compartmental organization of axons and dendrites within olfactory glomeruli of mice deficient in the olfactory cell adhesion molecule, OCAM. Mol. Cell. Neurosci. 2006;32:1–14. doi: 10.1016/j.mcn.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 95.Kawauchi T., Hoshino M. Molecular Pathways Regulating Cytoskeletal Organization and Morphological Changes in Migrating Neurons. Dev. Neurosci. 2008;30:36–46. doi: 10.1159/000109850. [DOI] [PubMed] [Google Scholar]
- 96.Hippenmeyer S. Molecular Pathways Controlling the Sequential Steps of Cortical Projection Neuron Migration. Cell. Mol. Control. Neuronal Migr. 2014;800:1–24. doi: 10.1007/978-94-007-7687-6_1. [DOI] [PubMed] [Google Scholar]
- 97.Reiner O., Sapir T. LIS1 functions in normal development and disease. Curr. Opin. Neurobiol. 2013;23:951–956. doi: 10.1016/j.conb.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 98.Tsai L.-H., Gleeson J.G. Nucleokinesis in Neuronal Migration. Neuron. 2005;46:383–388. doi: 10.1016/j.neuron.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 99.Vallee R.B. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 100.Dobyns W.B. Lissencephaly: A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. JAMA. 1993;270:2838–2842. doi: 10.1001/jama.1993.03510230076039. [DOI] [PubMed] [Google Scholar]
- 101.Niethammer M., Smith D.S., Ayala R., Peng J., Ko J., Lee M.-S., Morabito M., Tsai L.-H. NUDEL Is a Novel Cdk5 Substrate that Associates with LIS1 and Cytoplasmic Dynein. Neuron. 2000;28:697–711. doi: 10.1016/S0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 102.Sasaki S., Shionoya A., Ishida M., Gambello M.J., Yingling J., Wynshaw-Boris A., Hirotsune S. A LIS1/NUDEL/Cytoplasmic Dynein Heavy Chain Complex in the Developing and Adult Nervous System. Neuron. 2000;28:681–696. doi: 10.1016/S0896-6273(00)00146-X. [DOI] [PubMed] [Google Scholar]
- 103.Toyooka K., Shionoya A., Gambello M.J., Cardoso C., Leventer R., Ward H.L., Ayala R., Tsai L.-H., Dobyns W., Ledbetter D., et al. 14-3-3ε is important for neuronal migration by binding to NUDEL: A molecular explanation for Miller–Dieker syndrome. Nat. Genet. 2003;34:274–285. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- 104.Yamada M., Hirotsune S., Wynshaw-Boris A. The essential role of LIS1, NDEL1 and Aurora—A in polarity formation and microtubule organization during neurogensis. Cell Adhes. Migr. 2010;4:180–184. doi: 10.4161/cam.4.2.10715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gambello M.J., Darling D.L., Yingling J., Tanaka T., Gleeson J.G., Wynshaw-Boris A. Multiple Dose-Dependent Effects of Lis1 on Cerebral Cortical Development. J. Neurosci. 2003;23:1719–1729. doi: 10.1523/JNEUROSCI.23-05-01719.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.González-Billault C., Del Rio J.A., Ureña J.M., Jiménez-Mateos E.M., Barallobre M.J., Pascual M., Pujadas L., Simó S., La Torre A., Gavin R., et al. A role of MAP1B in Reelin-dependent Neuronal Migration. Cereb. Cortex. 2005;15:1134–1145. doi: 10.1093/cercor/bhh213. [DOI] [PubMed] [Google Scholar]
- 107.Kasahara K., Watanabe K., Kozutsumi Y., Oohira A., Yamamoto T., Sanai Y. Association of GPI-anchored protein TAG-1 with src-family kinase Lyn in lipid rafts of cerebellar granule cells. Neurochem. Res. 2002;27:823–829. doi: 10.1023/A:1020265225916. [DOI] [PubMed] [Google Scholar]
- 108.Kasahara K., Watanabe K., Takeuchi K., Kaneko H., Oohira A., Yamamoto T., Sanai Y. Involvement of Gangliosides in Glycosylphosphatidylinositol-anchored Neuronal Cell Adhesion Molecule TAG-1 Signaling in Lipid Rafts. J. Biol. Chem. 2000;275:34701–34709. doi: 10.1074/jbc.M003163200. [DOI] [PubMed] [Google Scholar]
- 109.Al-Bassam J., Ozer R.S., Safer D., Halpain S., Milligan R.A. MAP2 and tau bind longitudinally along the outer ridges of microtubule protofilaments. J. Cell Biol. 2002;157:1187–1196. doi: 10.1083/jcb.200201048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Caceres A., Mautino J., Kosik K.S. Suppression of MAP2 in cultured cerebeller macroneurons inhibits minor neurite formation. Neuron. 1992;9:607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- 111.Harada A., Teng J., Takei Y., Oguchi K., Hirokawa N. MAP2 is required for dendrite elongation, PKA anchoring in dendrites, and proper PKA signal transduction. J. Cell Biol. 2002;158:541–549. doi: 10.1083/jcb.200110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Khuchua Z., Wozniak D., Bardgett M., Yue Z., McDonald M., Boero J., Hartman R., Sims H., Strauss A. Deletion of the n-terminus of murine map2 by gene targeting disrupts hippocampal ca1 neuron architecture and alters contextual memory. Neuroscience. 2003;119:101–111. doi: 10.1016/S0306-4522(03)00094-0. [DOI] [PubMed] [Google Scholar]
- 113.Fink C.C., Bayer K.-U., Myers J.W., Ferrell J.E., Schulman H., Meyer T. Selective Regulation of Neurite Extension and Synapse Formation by the β but not the α Isoform of CaMKII. Neuron. 2003;39:283–297. doi: 10.1016/S0896-6273(03)00428-8. [DOI] [PubMed] [Google Scholar]
- 114.Sogawa Y., Yoshimura Y., Yamauchi T. Investigation of the Ca2+-independent form of Ca2+/calmodulin-dependent protein kinase II in neurite outgrowth. Brain Res. Protoc. 2001;8:159–169. doi: 10.1016/S1385-299X(01)00106-4. [DOI] [PubMed] [Google Scholar]
- 115.Bodrikov V., Sytnyk V., Leshchyns’Ka I., Hertog J.D., Schachner M. NCAM induces CaMKIIα-mediated RPTPα phosphorylation to enhance its catalytic activity and neurite outgrowth. J. Cell Biol. 2008;182:1185–1200. doi: 10.1083/jcb.200803045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jansen S., Melková K., Trosanova Z., Hanakova K., Zachrdla M., Novacek J., Župa E., Zdrahal Z., Hritz J., Žídek L. Quantitative mapping of microtubule-associated protein 2c (MAP2c) phosphorylation and regulatory protein 14-3-3ζ-binding sites reveals key differences between MAP2c and its homolog Tau. J. Biol. Chem. 2017;292:6715–6727. doi: 10.1074/jbc.M116.771097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sluchanko N., Gusev N.B. 14-3-3 Proteins and regulation of cytoskeleton. Biochemistry. 2010;75:1528–1546. doi: 10.1134/S0006297910130031. [DOI] [PubMed] [Google Scholar]
- 118.Kent C.B., Shimada T., Ferraro G.B., Ritter B., Yam P.T., McPherson P.S., Charron F., Kennedy T.E., Fournier A.E. 14-3-3 Proteins Regulate Protein Kinase A Activity to Modulate Growth Cone Turning Responses. J. Neurosci. 2010;30:14059–14067. doi: 10.1523/JNEUROSCI.3883-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Agarwal-Mawal A., Qureshi H.Y., Cafferty P.W., Yuan Z., Han D., Lin R., Paudel H.K. 14-3-3 Connects Glycogen Synthase Kinase-3β to Tau within a Brain Microtubule-associated Tau Phosphorylation Complex. J. Biol. Chem. 2003;278:12722–12728. doi: 10.1074/jbc.M211491200. [DOI] [PubMed] [Google Scholar]
- 120.Tang Y., Lv P., Sun Z., Han L., Luo B., Zhou W. 14-3-3ζ up-regulates hypoxia-inducible factor-1α in hepatocellular carcinoma via activation of PI3K/Akt/NF-кB signal transduction pathway. Int. J. Clin. Exp. Pathol. 2015;8:15845–15853. [PMC free article] [PubMed] [Google Scholar]
- 121.Poplawski G., Tranziska A.-K., Leshchyns’Ka I., Meier I.D., Streichert T., Sytnyk V., Schachner M. L1CAM increases MAP2 expression via the MAPK pathway to promote neurite outgrowth. Mol. Cell. Neurosci. 2012;50:169–178. doi: 10.1016/j.mcn.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 122.Katherine K., Dent W.E. Developing vertebrate CNS. Nat. Rev. Neurosci. 2014;15:7–18. doi: 10.1038/nrn3650.Branch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Homma N., Takei Y., Tanaka Y., Nakata T., Terada S., Kikkawa M., Noda Y., Hirokawa N. Kinesin superfamily protein 2A (KIF2A) functions in suppression of collateral branch extension. Cell. 2003;114:229–239. doi: 10.1016/S0092-8674(03)00522-1. [DOI] [PubMed] [Google Scholar]
- 124.Garnham C.P., Roll-Mecak A. The chemical complexity of cellular microtubules: Tubulin post-translational modification enzymes and their roles in tuning microtubule functions. Cytoskeleton. 2012;69:442–463. doi: 10.1002/cm.21027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Halpain S., Dehmelt L. The MAP1 family of microtubule-associated proteins. Genome Biol. 2006;7:1–7. doi: 10.1186/gb-2006-7-6-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee M.K., Cleveland D. Neuronal Intermediate Filaments. Annu. Rev. Neurosci. 1996;19:187–217. doi: 10.1146/annurev.ne.19.030196.001155. [DOI] [PubMed] [Google Scholar]
- 127.Dent E.W., Gupton S.L., Gertler F.B. The Growth Cone Cytoskeleton in Axon Outgrowth and Guidance. Cold Spring Harb. Perspect. Biol. 2010;3:a001800. doi: 10.1101/cshperspect.a001800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Davis D.A., Wilson M.H., Giraud J., Xie Z., Tseng H.-C., England C., Herscovitz H., Tsai L.-H., Delalle I. Capzb2 Interacts with β-Tubulin to Regulate Growth Cone Morphology and Neurite Outgrowth. PLoS Biol. 2009;7:e1000208. doi: 10.1371/journal.pbio.1000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mohan R., John A. Microtubule-associated proteins as direct crosslinkers of actin filaments and microtubules. IUBMB Life. 2015;67:395–403. doi: 10.1002/iub.1384. [DOI] [PubMed] [Google Scholar]
- 130.Sánchez-Huertas C., Lüders J. The Augmin Connection in the Geometry of Microtubule Networks. Curr. Biol. 2015;25:R294–R299. doi: 10.1016/j.cub.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 131.Kuijpers M., Hoogenraad C.C. Centrosomes, microtubules and neuronal development. Mol. Cell. Neurosci. 2011;48:349–358. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 132.Sheng L., Leshchyns’Ka I., Sytnyk V. Cell adhesion and intracellular calcium signaling in neurons. Cell Commun. Signal. 2013;11:94. doi: 10.1186/1478-811X-11-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leshchyns’Ka I., Sytnyk V. Reciprocal Interactions between Cell Adhesion Molecules of the Immunoglobulin Superfamily and the Cytoskeleton in Neurons. Front. Cell Dev. Biol. 2016;4:9. doi: 10.3389/fcell.2016.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Waites C.L., Craig A.M., Garner C. Mechanisms of Vertebrate Synaptogenesis. Annu. Rev. Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 135.Stanika R., Campiglio M., Pinggera A., Lee A., Striessnig J., Flucher B.E., Obermair G.J. Splice variants of the CaV1.3 L-type calcium channel regulate dendritic spine morphology. Sci. Rep. 2016;6:34528. doi: 10.1038/srep34528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leitch B., Szostek A., Lin R., Shevtsova O. Subcellular distribution of L-type calcium channel subtypes in rat hippocampal neurons. Neuroscience. 2009;164:641–657. doi: 10.1016/j.neuroscience.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 137.Sheng L., Leshchyns’Ka I., Sytnyk V. Neural Cell Adhesion Molecule 2 (NCAM2)-Induced c-Src-Dependent Propagation of Submembrane Ca2+ Spikes Along Dendrites Inhibits Synapse Maturation. Cereb. Cortex. 2019;29:1439–1459. doi: 10.1093/cercor/bhy041. [DOI] [PubMed] [Google Scholar]
- 138.Okamoto K., Bosch M., Hayashi Y. The Roles of CaMKII and F-Actin in the Structural Plasticity of Dendritic Spines: A Potential Molecular Identity of a Synaptic Tag? Physiology. 2009;24:357–366. doi: 10.1152/physiol.00029.2009. [DOI] [PubMed] [Google Scholar]
- 139.Okamoto K.-I., Narayanan R., Lee S.H., Murata K., Hayashi Y. The role of CaMKII as an F-actin-bundling protein crucial for maintenance of dendritic spine structure. Proc. Natl. Acad. Sci. USA. 2007;104:6418–6423. doi: 10.1073/pnas.0701656104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hell J.W. CaMKII: Claiming Center Stage in Postsynaptic Function and Organization. Neuron. 2014;81:249–265. doi: 10.1016/j.neuron.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dillon C., Goda Y. The Actin Cytoskeleton: Integrating Form and Function at the Synapse. Annu. Rev. Neurosci. 2005;28:25–55. doi: 10.1146/annurev.neuro.28.061604.135757. [DOI] [PubMed] [Google Scholar]
- 142.Eyang Y., Ecalakos N. Presynaptic long-term plasticity. Front. Synaptic Neurosci. 2013;5:8. doi: 10.3389/fnsyn.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Edwards M., Zwolak A., Schafer D.A., Sept D., Dominguez R., Cooper J.A. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yoshimura Y., Yamauchi Y., Shinkawa T., Taoka M., Donai H., Takahashi N., Isobe T., Yamauchi T. Molecular constituents of the postsynaptic density fraction revealed by proteomic analysis using multidimensional liquid chromatography-tandem mass spectrometry. J. Neurochem. 2003;88:759–768. doi: 10.1046/j.1471-4159.2003.02136.x. [DOI] [PubMed] [Google Scholar]
- 145.Kitanishi T., Sakai J., Kojima S., Saitoh Y., Inokuchi K., Fukaya M., Watanabe M., Matsuki N., Yamada M.K. Activity-dependent localization in spines of the F-actin capping protein CapZ screened in a rat model of dementia. Genes Cells. 2010;15:737–747. doi: 10.1111/j.1365-2443.2010.01411.x. [DOI] [PubMed] [Google Scholar]
- 146.Fan Y., Tang X., Vitriol E., Chen G., Zheng J.Q. Actin Capping Protein Is Required for Dendritic Spine Development and Synapse Formation. J. Neurosci. 2011;31:10228–10233. doi: 10.1523/JNEUROSCI.0115-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ikeda M., Hikita T., Taya S., Uraguchi-Asaki J., Toyooka K., Wynshaw-Boris A., Ujike H., Inada T., Takao K., Miyakawa T., et al. Identification of YWHAE, a gene encoding 14-3-3epsilon, as a possible susceptibility gene for schizophrenia. Hum. Mol. Genet. 2008;17:3212–3222. doi: 10.1093/hmg/ddn217. [DOI] [PubMed] [Google Scholar]
- 148.Wachi T., Cornell B., Toyooka K. Complete ablation of the 14-3-3epsilon protein results in multiple defects in neuropsychiatric behaviors. Behav. Brain Res. 2017;319:31–36. doi: 10.1016/j.bbr.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Foote M., Qiao H., Graham K., Wu Y., Zhou Y. Inhibition of 14-3-3 Proteins Leads to Schizophrenia-Related Behavioral Phenotypes and Synaptic Defects in Mice. Biol. Psychiatry. 2015;78:386–395. doi: 10.1016/j.biopsych.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Dent E.W. Of microtubules and memory: Implications for microtubule dynamics in dendrites and spines. Mol. Biol. Cell. 2017;28:1–8. doi: 10.1091/mbc.e15-11-0769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Caceres A., Payne M.R., Binder L.I., Steward O. Immunocytochemical localization of actin and microtubule-associated protein MAP2 in dendritic spines. Proc. Natl. Acad. Sci. USA. 1983;80:1738–1742. doi: 10.1073/pnas.80.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Conde C., Cáceres A. Microtubule assembly, organization and dynamics in axons and dendrites. Nat. Rev. Neurosci. 2009;10:319–332. doi: 10.1038/nrn2631. [DOI] [PubMed] [Google Scholar]
- 153.Tortosa E., Montenegro-Venegas C., Benoist M., Härtel S., González-Billault C., Esteban J.A., Avila J. Microtubule-associated Protein 1B (MAP1B) Is Required for Dendritic Spine Development and Synaptic Maturation. J. Biol. Chem. 2011;286:40638–40648. doi: 10.1074/jbc.M111.271320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Sousa R., Lafer E.M. The role of molecular chaperones in clathrin mediated vesicular trafficking. Front. Mol. Biosci. 2015;2:26. doi: 10.3389/fmolb.2015.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Fontaine S.N., Martin M.D., Akoury E., Assimon V.A., Borysov S., Nordhues B.A., Sabbagh J.J., Cockman M., Gestwicki J.E., Zweckstetter M., et al. The active Hsc70/tau complex can be exploited to enhance tau turnover without damaging microtubule dynamics. Hum. Mol. Genet. 2015;24:3971–3981. doi: 10.1093/hmg/ddv135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leshchyns’Ka I., Sytnyk V., Richter M., Andreyeva A., Puchkov D., Schachner M. The Adhesion Molecule CHL1 Regulates Uncoating of Clathrin-Coated Synaptic Vesicles. Neuron. 2006;52:1011–1025. doi: 10.1016/j.neuron.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 157.Lee H., Raiker S.J., Venkatesh K., Geary R., Robak L.A., Zhang Y., Yeh H.H., Shrager P., Giger R.J. Synaptic Function for the Nogo-66 Receptor NgR1: Regulation of Dendritic Spine Morphology and Activity-Dependent Synaptic Strength. J. Neurosci. 2008;28:2753–2765. doi: 10.1523/JNEUROSCI.5586-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Raiker S.J., Lee H., Baldwin K., Duan Y., Shrager P., Giger R.J. Oligodendrocyte-Myelin Glycoprotein and Nogo Negatively Regulate Activity-Dependent Synaptic Plasticity. J. Neurosci. 2010;30:12432–12445. doi: 10.1523/JNEUROSCI.0895-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zagrebelsky M., Schweigreiter R., Bandtlow C.E., Schwab M.E., Korte M. Nogo-A Stabilizes the Architecture of Hippocampal Neurons. J. Neurosci. 2010;30:13220–13234. doi: 10.1523/JNEUROSCI.1044-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Pernet V., Schwab M.E. The role of Nogo—A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012;349:97–104. doi: 10.1007/s00441-012-1432-6. [DOI] [PubMed] [Google Scholar]
- 161.Bajor M., Kaczmarek L. Proteolytic Remodeling of the Synaptic Cell Adhesion Molecules (CAMs) by Metzincins in Synaptic Plasticity. Neurochem. Res. 2012;38:1113–1121. doi: 10.1007/s11064-012-0919-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Krueger D.D., Tuffy L.P., Papadopoulos T., Brose N. The role of neurexins and neuroligins in the formation, maturation, and function of vertebrate synapses. Curr. Opin. Neurobiol. 2012;22:412–422. doi: 10.1016/j.conb.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 163.Hirano S., Takeichi M. Cadherins in Brain Morphogenesis and Wiring. Physiol. Rev. 2012;92:597–634. doi: 10.1152/physrev.00014.2011. [DOI] [PubMed] [Google Scholar]
- 164.Molloy C.A., Keddache M., Martin L.J. Evidence for linkage on 21q and 7q in a subset of autism characterized by developmental regression. Mol. Psychiatry. 2005;10:741–746. doi: 10.1038/sj.mp.4001691. [DOI] [PubMed] [Google Scholar]
- 165.Scholz C., Steinemann D., Mälzer M., Roy M., Arslan-Kirchner M., Illig T., Schmidtke J., Stuhrmann M. NCAM2 deletion in a boy with macrocephaly and autism: Cause, association or predisposition? Eur. J. Med. Genet. 2016;59:493–498. doi: 10.1016/j.ejmg.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 166.Winther M., Berezin V., Walmod P.S. NCAM2/OCAM/RNCAM: Cell adhesion molecule with a role in neuronal com-partmentalization. Int. J. Biochem. Cell Biol. 2012;44:441–446. doi: 10.1016/j.biocel.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 167.Petit F., Plessis G., DeCamp M., Cuisset J.-M., Blyth M., Pendlebury M., Andrieux J. 21q21 deletion involving NCAM2: Report of 3 cases with neurodevelopmental disorders. Eur. J. Med. Genet. 2015;58:44–46. doi: 10.1016/j.ejmg.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 168.Han M.-R., Schellenberg G.D., Wang L.-S., Initiative T.A.D.N. Genome-wide association reveals genetic effects on human Aβ 42 and τ protein levels in cerebrospinal fluids: A case control study. BMC Neurol. 2010;10:90. doi: 10.1186/1471-2377-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kimura R., Kamino K., Yamamoto M., Nuripa A., Kida T., Kazui H., Hashimoto R., Tanaka T., Kudo T., Yamagata H., et al. The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between β-amyloid production and tau phosphorylation in Alzheimer disease. Hum. Mol. Genet. 2007;16:15–23. doi: 10.1093/hmg/ddl437. [DOI] [PubMed] [Google Scholar]
- 170.Niederhoffer K.Y., Fahiminiya S., Eydoux P., Mawson J., Nishimura G., Jerome-Majewska L.A., Patel M.S. Diagnosis of Van den Ende-Gupta syndrome: Approach to the Marden-Walker-like spectrum of disorders. Am. J. Med. Genet. Part A. 2016;170:2310–2321. doi: 10.1002/ajmg.a.37831. [DOI] [PubMed] [Google Scholar]
- 171.Griesi-Oliveira K., Suzuki A.M., Alves A.Y., Mafra A.C.C.N., Yamamoto G.L., Ezquina S., Magalhães Y.T., Forti F.L., Sertie A.L., Zachi E.C., et al. Actin cytoskeleton dynamics in stem cells from autistic individuals. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-29309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Torrico B., Antón-Galindo E., Fernàndez-Castillo N., Rojo-Francàs E., Ghorbani S., Pineda-Cirera L., Hervás A., Rueda I., Moreno E., Fullerton J.M., et al. Involvement of the 14-3-3 Gene Family in Autism Spectrum Disorder and Schizophrenia: Genetics, Transcriptomics and Functional Analyses. J. Clin. Med. 2020;9:1851. doi: 10.3390/jcm9061851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mukaetova-Ladinska E.B., Arnold H., Jaros E., Perry R., Perry E. Depletion of MAP2 expression and laminar cytoarchitectonic changes in dorsolateral prefrontal cortex in adult autistic individuals. Neuropathol. Appl. Neurobiol. 2004;30:615–623. doi: 10.1111/j.1365-2990.2004.00574.x. [DOI] [PubMed] [Google Scholar]
- 174.Pham E., Crews L., Ubhi K., Hansen L., Adame A., Cartier A., Salmon D., Galasko D., Michael S., Savas J.N., et al. Progressive accumulation of amyloid-β oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J. 2010;277:3051–3067. doi: 10.1111/j.1742-4658.2010.07719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Penzes P., VanLeeuwen J.-E. Impaired regulation of synaptic actin cytoskeleton in Alzheimer’s disease. Brain Res. Rev. 2011;67:184–192. doi: 10.1016/j.brainresrev.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Scheff S.W., Price D.A. Synaptic pathology in Alzheimer’s disease: A review of ultrastructural studies. Neurobiol. Aging. 2003;24:1029–1046. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 177.Selkoe D.J. Alzheimer’s Disease Is a Synaptic Failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 178.Smith K.R., Damiano J., Franceschetti S., Carpenter S., Canafoglia L., Morbin M., Rossi G., Pareyson D., Mole S., Staropoli J.F., et al. Strikingly Different Clinicopathological Phenotypes Determined by Progranulin-Mutation Dosage. Am. J. Hum. Genet. 2012;90:1102–1107. doi: 10.1016/j.ajhg.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]