Abstract
Schizophrenia is a severe neuropsychiatric disorder, and its etiology remains largely unknown. Environmental factors have been reported to play roles in the pathogenesis of schizophrenia, and one of the major environmental factors identified for this disorder is psychosocial stress. Several studies have suggested that stressful life events, as well as the chronic social stress associated with city life, may lead to the development of schizophrenia. The other factor is the gut–brain axis. The composition of the gut microbiome and alterations thereof may affect the brain and may lead to schizophrenia. The main interest of this review article is in overviewing the major recent findings on the effects of stress and the gut–brain axis, as well as their possible bidirectional effects, in the pathogenesis of schizophrenia.
Keywords: schizophrenia, neuropsychiatric, stress, gut–brain axis, psychosocial
1. Introduction
Around 792 million people in the world live with mental disorders, which is more than one in ten people (10.7%) globally [1]. Neuropsychiatry is a discipline that deals with mental disorders that are attributable to diseases of the nervous system; these are related to cognitive and behavioral diseases caused by direct cerebral dysfunctions or indirect extra-cerebral diseases [2]. In spite of major advances in neurobiology and neuroscience research, the prevalence of neuropsychiatric diseases has not decreased. Our knowledge in the basic and clinical pathophysiology of neuropsychiatric diseases, such as schizophrenia, is still limited. “Schizophrenia is a severe neuropsychiatric disease that is characterized by impairments in perception, cognition, and avolition, leading to positive, cognitive, and negative symptoms, respectively” [3]. The etiology of schizophrenia remains unknown [4]. As a result, the objective of this review is to assess the newly emerging paradigm of the brain–gut–microbiota axis, in which imbalances are risk factors for the pathogenesis of schizophrenia, in addition to the effects of other environmental factors (e.g., stress) on the pathogenesis of schizophrenia. Several studies focused on the risk factors for schizophrenia separately, and we mention the important findings in this review article. For example, there is increasing evidence that disorders such as schizophrenia are associated with an imbalance in the gut microbiome [5,6]. Another important factor is stress. Stress can induce changes in the gut microbiome [7] and it is well known that stress is one of the risk factors for the pathogenesis of schizophrenia [3,8,9,10,11]. Finally, several lines of evidence support the notion that gut microbiota can affect stress-related behaviors [12].
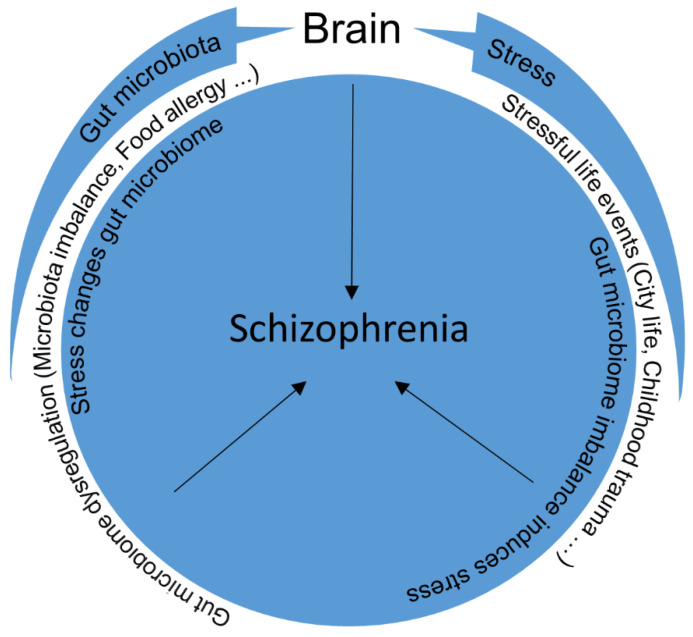
The effects of environmental factors (stress) together with those of the dysregulation of the gut–brain axis on the pathogenesis of schizophrenia will be discussed in this review (Figure 1). This can be interesting due to the fact that there are few studies that show the bidirectional effects of these factors. Some of the important studies, summarized in Table 1.
Figure 1.
Stress and dysregulation of the gut microbiome can rarely be effective alone. Stressful life events in combination with the effects of the gut–brain axis may induce bidirectional effects and lead to schizophrenia.
Table 1.
Role of stress and the gut–brain axis in the pathogenesis of schizophrenia. Important studies summary.
| Reference | Animal Model/Human | Stress/Gut Microbiome | Outcome |
|---|---|---|---|
| [39] | animal model | stress | attention impairment, decreased locomotion and hyperreactivity in the adult offspring |
| [33] | animal model | stress | role of maternal deprivation |
| [38] | human | stress | hypercortisolemia in schizophrenic patients |
| [107,108] | human | both | prefrontal cortex role in anxiety and fear processes |
| [30,31] | human | stress | children raised in dysfunctional adoptive families and institutions |
| [30] | animal model | stress | maternal stress increases locomotor behavior in the adult offspring |
| [34] | animal model | stress | maternal malnutrition model and reduced PPI |
| [29] | human | stress | childhood trauma |
| [8,9,10,11] | human | stress | stressful life events, especially city life |
| [76] | human | gut microbiome | gluten sensitivity |
| [65] | human | gut microbiome | different microbial composition in schizophrenic patients |
| [105] | human | both | important role of the microbiome in prefrontal cortex myelination |
| [105,117] | animal model | both | abnormalities in prefrontal cortex and amygdala changes were seen in germ free mice |
| [5] | human | gut microbiome | disorders such as schizophrenia are associated with an imbalance in the gut microbiome |
| [90] | animal model | both | alterations in the gut microbiota due to the early-life stress |
| [97] | animal model | both | microbiome can regulate amygdala-dependent fear memory in germ free mice |
| [62] | animal model | gut microbiome | the schizophrenic patients gut microbiome modulates the glutamate-glutamine-GABA cycle and schizophrenia like behaviors in mice |
| [3] | animal model | stress | psychosocial stress induces schizophrenia-like behavior in genetically modified Mice |
| [80] | animal model | both | stressors can change the gut microbiome composition |
| [92] | human | both | the early childhood trauma can affect the gut microbiome and this may influence the risk of schizophrenia |
| [66] | human | gut microbiome | human gut microbiota transplantation, might improve the treatment of the schizophrenia |
| [64] | animal model | gut microbiome | transplantation of Streptococcus vestibularis (schizophrenia-enriched bacterium) can cause the social behaviors deficits in mice |
2. Schizophrenia
Schizophrenia is a severe neuropsychiatric disorder that affects 20 million people worldwide [13]. Schizophrenia causes a significant burden on wellbeing and global health. The interactions of different factors that play roles in the disease’s etiology are not fully understood. There are possible reasons for the onset of schizophrenia, such as the possible increased activation of components of the immune system [14], including alterations in intestinal permeability and the gut microbiome [15], and stressful life events [8,9,10,11]. There are three major groups of symptoms recognized in schizophrenia, which are classified as positive, negative, and cognitive [16]. The etiology of schizophrenia remains largely unknown, although the roles of the genes in combination with environmental factors have been reported [17,18]. The onset of schizophrenia is typically in early adulthood, and it is associated with life time disability [19,20,21]. Dysfunctions in dopaminergic neurotransmitters may contribute to psychotic symptoms, although there is evidence that shows the involvement of other areas and circuits of the brain [22].
The terminology for positive and negative symptoms has been evolved for around 150 years [23].
Clinically, schizophrenia is characterized by its diverse psychopathology; the core features are positive symptoms (thought disorders, delusions and hallucinations, and psychotic symptoms in which the person loses contact with reality), negative symptoms (restricted affect, poverty of speech, impaired motivation, and social withdrawal), and cognitive impairments (impairments in cognitive processes, such as memory dysfunction, poor performance in a wide range of cognitive functions, working memory deficits, executive dysfunction, and attentional impairments) [3,24,25,26,27]. Importantly, a patient with cognitive symptoms may show a cognitive disability; however, a patient with negative symptoms may just show either anhedonia or unsociability without other signs. Furthermore, each of those symptoms can be individually scaled [27]. The heterogeneity of the symptoms of the disease makes it very difficult to diagnose and cure patients. As a result, the etiology of schizophrenia is not yet fully understood. The disorder’s etiology is multifactorial; as a result, investigation of the different risk factors (e.g., gut microbiome dysregulation and stress) alone or in combination can be very helpful.
3. Stress
Psychological stress can lead to several neuropsychiatric disorders, such as depression and schizophrenia [28]. Nowadays, it is suggested that environmental factors may play a major role in the pathogenesis of schizophrenia. Epidemiological results have demonstrated specific circumstances in which the risk for schizophrenia is increased. These circumstances include migration, adversity in early life, growing up in an urban environment and urban residence, and position in a minority group [11], can be interpreted as stressful situations.
Interestingly, several studies have suggested that stressful life events, especially city life, may lead to schizophrenia [8,9,10,11]. Furthermore, the risk for schizophrenia is increased with urban birth and/or upbringing [9]. Childhood trauma is another important environmental factor that can increase the risk for the development of schizophrenia in adulthood [29]. It is known that schizophrenic patients are exposed more to early life trauma than the controls; for example, children who were raised in dysfunctional adoptive families and institutions [30,31]. Stress animal models can be proper models to study the neurodevelopmental factors in the pathogenesis of schizophrenia, as well [32]. There are animal studies that have shown the role of stress in the pathogenesis of schizophrenia. For instance, ref. [33] showed the role of maternal deprivation in animal models of schizophrenia. In another study, ref. [34] used a maternal malnutrition model and a Prepulse inhibition test (PPI) in rat models. The PPI was reduced in females with age, which is a clinical symptom of schizophrenia [35]. Ref. [3] used adolescent matrix metalloproteinase-9 (Mmp-9) heterozygous mice (after weaning) that were chronically subjected to psychosocial resident–intruder stress and then examined by using behavioral tests. In those mice, negative symptoms were manifested after exposure to stress. Interestingly, after clozapine (atypical antipsychotic) administration, the negative symptoms were ameliorated.
Stress can rarely be effective alone, and it usually acts in parallel with one or more other factors. One example is immune dysregulation caused by stress. Stress can trigger the hypothalamic–pituitary–adrenal axis (HPA), which is an important part of the neuroendocrine system and has a role in regulating the immune system and mood [36]. Evidence of HPA dysfunction also exists in schizophrenia [30]. It was also revealed in animal models that maternal separation is an early-life stressor that can lead to long-term increases in HPA activity [37]. Stressful situations can lead to hypothalamic activation, which causes the secretion of corticoids by the adrenal cortex. Several reports showed hypercortisolemia in schizophrenic patients [38]. Maternal stress increases locomotor behavior (amphetamine induced) in the adult offspring (model of psychosis) [30] and in primates, prenatal stress can cause attention impairment, and decreased locomotion as well as hyperreactivity in the adult offspring [39]. The rhesus monkeys, which were under prenatal stress, showed more disturbance behavior as well as dysregulation of the HPA axis [40].
These findings show the fact that chronic stress can contribute to vulnerability to schizophrenia [41].
4. Gut–Brain Axis
It is well known that the gut microbiome is the most important regulator of the gut–brain axis. This effect is so prominent that some studies have called human gut microflora the “second brain” [42]. The gut is a niche for microbes—especially bacteria, but also other microorganisms [12]. The gut microbiome mainly consists of two major phylotypes—Bacteroidetes and Firmicutes, and the rest, mostly including Proteobacteria, Actinobacteria, Fusobacteria, and Verrucomicrobia phyla [43].
The bilateral effects of the gastrointestinal tract on brain function have been recognized since the nineteenth century [44]. Our knowledge about the gut microbiome and its effect on brain function has been expanded over the last decade. The microbiome has been introduced as an important cause for neurological disorders according to clinical and preclinical reports [45]. The importance of this bilateral homeostatic communicational route that uses neural, hormonal, and immunological routes—dysfunctions of which can lead to pathophysiological consequences—is still gaining recognition [46]. Dysfunctions in brain–gut interactions can be associated with eating disorders and gut inflammation. It is also clear that the modulation of gut–brain interaction can be associated with stressful behaviors. Stress-related psychiatric symptoms, such as stressful behaviors, and gastrointestinal disorders, such as irritable bowel syndrome, show the significant importance of the pathophysiology of the brain–gut axis [44,46,47].
Communication between gut microbes and the brain is possible through the gut–brain connection, and this may cause microbiota to be modulators of the brain and behavior [47,48]. There is communication between gut microbes and centrally or peripherally mediated behavior. This communication can be facilitated by the vagus nerve. A central Lactobacillus rhamnosus deletion was shown through a full truncal vagotomy. Similarly, patients with peptic ulcers showed a lower risk for neurological disorders, such as Parkinsonism, in old age after a full truncal vagotomy [49,50]. Apart from nerves, gut microbes can regulate neurotransmitters by changing the precursor levels (e.g., serotonin). Serotonin (5-HT) is a key neurotransmitter in the gut–brain axis communication system, and it functions between the central nervous system and the gastrointestinal tract [51]. The precursor of 5-HT is tryptophan, however, it has limited storage in the brain. As a result, intestinal refilling is crucial. This can be performed with Bifidobacterium infantis. There are also bacteria that can produce and release different neurotransmitters. For instance, acetylcholine, dopamine, 5-HT, and norepinephrine can be produced and released by the Lactobacillus, Bacillus, and Enterococcus species, the Candida, Streptococcus, Escheridia, and Saccharomyces species, and the Escheridia and Bacillus species, respectively [52,53,54]. These interactions are also very important for mental health and sociability [5,55]. It was revealed through oral microbiome analyses that people with schizophrenia had different levels of Lactobacillus phage phiadh. The presence of immunological conditions was correlated with different levels of Lactobacillus phage phiadh [56].
Several animal studies have shown the role of the gut–brain axis in psychiatric illnesses [57]. In one study, it was shown that adolescence and early adulthood can be critical periods in which the dysregulation of the communication along the microbiota–gut–brain axis can significantly impact brain development and behavior. This can lead to alterations in cognitive and anxious phenotypes in adulthood. Additionally, chronic antibiotic treatment is a useful model for revealing the importance of gut microbiota during early stages of life in brain development and behavior [58]. The gut–brain axis and microbiome have potential roles implicated in schizophrenia pathogenesis [59,60]. Additionally, gut microbiome changes may contribute to schizophrenia pathophysiology and behavioral symptoms development [61]. Increasing evidence has shown that disorders such as schizophrenia are associated with gut microbiome imbalances [5,6]. For example, ref. [62] showed that the schizophrenic patients gut microbiome can modulate the glutamate-glutamine-GABA cycle and schizophrenia-like behaviors in mice. Similarly, ref. [63] revealed that drug free schizophrenic patients microbiota transplantation can lead to the mice schizophrenia-like behaviors by tryptophan-kynurenine metabolism dysregulation. Additionally, transplantation of Streptococcus vestibularis (schizophrenia-enriched bacterium) can cause social behavior deficits and change the peripheral tissues neurotransmitters levels in mice [64]. In another study, ref. [65] reported that patients with schizophrenia had decreased oral microbial biodiversity. In this study, they used MaAsLin to detect the effect of schizophrenia on the composition of microbiome species while considering the effects of other variables (e.g., medication) in the studied population. They found that the overall microbial composition in schizophrenic patients was significantly different from that in the non-schizophrenic subjects [65]. Finally, ref. [66] showed that human gut microbiota transplantation might improve the treatment of the schizophrenia.
The composition of gut microbiota may affect the gastrointestinal barrier, immune regulation, and metabolism associated with schizophrenia [60]. Schizophrenia is a brain disorder; therefore, to assess the gastrointestinal system’s role in the etiology of schizophrenia, mechanisms that can affect the brain should be included. Toxic products can exit the gastrointestinal tract, which may cause an immune response by entering the brain; thus, these products need to penetrate the barriers in the gastrointestinal tract, as well as the barriers of the blood and central nervous system [67]. We know that the microbiome can modulate the immune response [68]. A microbial imbalance can cause an inflammatory response that is followed by immune reactions and vice versa. In one study, ref. [69] used the maternal immune activation by poly I:C injection. Afterwards, they studied the behavioral changes relevant to the gut–brain axis in the offspring of an outbred NIH Swiss and an inbred C57BL6/J mouse strain. They revealed that this can cause social deficits in both strains.
Alterations in the gut microbiome have been linked to the pathogenesis of allergic, neurodevelopmental, psychiatric, and neurodegenerative diseases [70].
Environmental factors, such as microbial exposure, have been introduced as possible factors that cause allergic diseases [71]. The reason is still unknown, however, a possible cause can be the microbiota that colonize the gut of infants. Recent studies have shown that there are imbalances in the intestinal microbial flora of children and infants with food allergies [72]. A typical food that can lead to different psychological states is bread. Bread can make the gut permeable and, therefore, cause the migration of food allergens to areas in which they should not be. This causes the immune system to attack these allergens, as well as brain-related substances [73]. Similarly, the availability of wheat has been correlated with hospitalization rates for schizophrenia [74]. It is known that gluten can be broken into bioactive opioid receptor peptides, which can enter the gastrointestinal tract, as well as brain barriers [75]. Another condition called gluten sensitivity has also been shown to have an association with schizophrenia [76]. Increases in other food antigens, such as milk caseins, have also been reported in schizophrenic patients [67]. Autopsies of schizophrenic patients revealed that 50% of them had gastritis, 88% had enteritis, and 92% had colitis [77,78]. Additionally, there is a well-known association of schizophrenia with celiac disease [67].
5. Stress and Gut Microbiome
The stress and gut microbiome interaction is an important topic. Stress and diet, together or independently, can influence the gut microbiome. On the other hand, the gut microbiome can modulate stress and mood. Finally, the stress and microbiome interactions can affect health [79]. It is known that the gut microbiome composition can be changed by stressors. The microbiome role in stress response regulation was also shown [80]. There are several effects of stress on gut function: alterations in gastrointestinal motility, increases in visceral perception, alterations in gastrointestinal secretion, changes in intestinal permeability, and dysregulation of intestinal microbiota. Gastrointestinal physiology can be affected by pro-inflammatory cytokines and different kinds of neurotransmitters. These can be released by stress signals translated by mast cells [7]. For example, in rhesus monkeys, maternal separation led to a decrease in fecal lactobacilli [81]. In adult rats, stress in early life may induce long-term effects on the composition of gut microbiota by causing the elevation of the circulating levels of interleukin-6 and the chemokine CCL2. These have been correlated with changes that are induced by stressors in the levels of three bacterial genera: Pseudobutyrivibrio, Coprococcus, and Dorea. This may show that repeated stress affects the populations of gut bacteria, and these changes are correlated with alterations in the levels of pro-inflammatory cytokines [44,82]. Microbiota can also influence the priming and recovery of the innate immune system with respect to an acute stressor [83]. Ref. [84] showed an association of the human infant gut microbiome with fearful behavior and the microbiome’s relationship with fear-related brain structures. On the other hand, the effects of the gut microbiome on the brain and stress have been reported in animal studies. According to these studies, the gut microbiome may affect stress-related behaviors, such as those related to depression and anxiety. For example, germ-free mice experiments showed a link between the microbiome and anxiety-like behavior [85,86,87,88]. Similarly, ref. [89] investigated if penicillin administration in early postnatal life induces long-term effects on the offspring of mice. They showed that there were changes in gut microbiota, increases in cytokine expression in the frontal cortex, anxiety-like behaviors, and impaired social behaviors [89]. The fearful behaviors and related neurocircuitry was changed in animal models, which their gut microbes manipulated [84]. On the other hand, early life stress can also lead to the gut–brain axis alterations that may contribute to stress-related and psychiatric disorders development in adulthood [37]. It is also known, as well, that gut microbiome chronic change (i.e., being under long time stress) may lead to neuroplastic alterations [32].
6. Stress, the Gut, and Schizophrenia
Research on animal models has been used to discuss the possible effects of the gut microbiome on immune changes in the central nervous system and behavior, as well as their possible contributions to the pathophysiology of psychiatric disorders. Early life events may change the gut microbiome and can cause psychosis development later [61]. Early life stress can cause long term gut microbiome alterations, and may lead to neuronal malfunction and behavioral changes which have a role in psychiatric disorders. One study was about behavioral changes after weaning under social isolation in rats. The composition of the microbiota of these rats was altered with an elevation of Actinobacteria and decrease in Clostridia. The positive correlations were reported for open-field exploration, conditioned freezing, microbiota, and hippocampal IL-6 and IL-10 [90]. This may show alterations in the gut microbiota due to early-life stress. The production of different cytokines in the hippocampus can contribute to the abnormal neuronal functions and behavior development, which may lead to the onset of psychiatric illnesses [90].
The effects of dysfunction in the gut microbiome on the brain can be caused by stressful situations; otherwise, inflammation can be a strong activation mechanism that affects the brain and makes it more susceptible to psychiatric conditions, such as depression [91] and schizophrenia [60]. We know that stress and schizophrenia can be influenced by the gut microbiome. The HPA axis and the brain in kids can be affected by several factors such as stress and infection. During the development, several stressors such as physical and psychic trauma, inflammations, and metabolic dysregulations may lead to microbiome changes. These interactions of microbial and neurological pathways can affect each other during the development. Early childhood trauma can affect the gut microbiome and this may influence the risk of schizophrenia [92]. There are studies which show the possible connection between stress, the gut, and schizophrenia. It was shown in animal models that several types of stressors can change the gut microbiome composition. On the other hand, the important role of the microbiome in regulating the stress response was also shown in animal models [80]. For example, ref. [93] showed that gut microbiome nurturing with prebiotics such as galacto-oligosaccharides and fructo-oligosaccharides can reduce chronic stress as well as depression-like behavior in mice. Similarly, several reports showed gut microbiome alterations in major depressive disorder animal models and patients [94]. Interestingly, depressive symptoms can be common clinical features of schizophrenic patients [94,95].
Other studies, which can show the connections between gut microbiome, stress, and schizophrenia, are about effects of the gut microbiome on brain structures and neural circuits which can be implicated in schizophrenia pathogenesis. Ref. [96] showed associations between some brain structures and the gut microbiome in schizophrenia patients. Furthermore, microbial deficiencies can change stress-related neurotransmitters in the related brain regions [12]. One of these regions is the amygdala, which is the key brain region to process anxiety [97] and can modulate fear responses [98]. Altered processes in the amygdala are associated with both stress [99] and neuropsychiatric disorders, such as schizophrenia [100,101]. Ref. [97] showed that the microbiome can regulate amygdala-dependent fear memory in germ free mice. In another study, ref. [102] investigated the gut microbiome and brain functional connectivity, focusing on the amygdala. They also demonstrated the associations between the gut microbiome and neural circuits integrity, the circuits which are important for cognition and fear processing. These two can be associated with future psychopathology vulnerability. Another brain region which dysfunction is deeply implicated in neuropsychiatric disorders such as schizophrenia is the prefrontal cortex [19,103,104,105,106]. The prefrontal cortex has an important role in anxiety and fear processes. It has been implicated in the HPA axis regulation as well [107,108]. Ref. [105] showed that the microbiome has an important role in the prefrontal cortex myelination in germ free mice and can be used as a therapeutic target for psychiatric disorders. In another study, it was shown that the gut microbiome can modify the key metabolites synthesis which are affecting the prefrontal cortex gene expression [109]. Furthermore, gut microbiome absence can influence the mice prefrontal cortex lipid metabolism [110].
As it was described earlier, both the amygdala and prefrontal cortex are regions, which are implicated in schizophrenia pathogenesis [111,112,113,114]. These are also two regions involved in controlling anxiety and fear [115,116] and the abnormalities (prefrontal cortex hypermyelination and amygdala changes) of these regions were seen in germ free mice [105,117]. Finally, it is known that the miRNA expression regulation in the prefrontal cortex and amygdala can be affected by microbiome activity [116]. Anyhow, the precise role of the gut microbiome together with environmental stressors has not yet been investigated in schizophrenia [118]. The role of the microbiome, which is affected by the environmental factors, such as stress, can be a very interesting topic for researchers. The combination of the microbiome with life stressors is a new topic that can explain features of schizophrenia [92].
7. Conclusions
In this review, the author aimed to bring together some recent studies that showed the effects of stress as well as dysregulation of the gut–brain axis on the pathogenesis of schizophrenia. There are not many studies that have shown the precise mechanisms underlying schizophrenic symptoms in human patients or in animal models of schizophrenia. Anyhow, there are several reports showing the symptoms of schizophrenia in patients or animal models after being exposed to environmental factors, such as stress, or models with dysregulations in the gut–brain axis. Each of these factors can play a role in schizophrenia pathogenesis and they may have bidirectional effects. These factors can also have bidirectional effects on the brain. Few studies have shown the bidirectional effects of stress together with the gut microbiome and the possible roles of both effects in the pathogenesis of schizophrenia. Nevertheless, it is important to recognize different symptoms of schizophrenia and the possible reasons that may lead to each symptom. The roles of stress and the gut microbiome in the pathogenesis of schizophrenia can be an interesting topic to investigate in the future, which may shed light on some of the unanswered questions regarding the etiology of schizophrenia.
Acknowledgments
The author would like to acknowledge the support from the Open Access Publication Funds of Göttingen University. The author would like to thank Swen Hülsmann for support and suggestions.
Funding
The APC was funded by the Open Access Publication Funds of the Göttingen University.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roser H.R.A.M. Mental Health. [(accessed on 5 July 2021)]. Available online: https://ourworldindata.org/mental-health.
- 2.Mervi L.S., Pitkanen T.S., Kopelman M.D. Neuropsychiatric disorders. In: David A., Warrell T.M.C., John D., editors. Oxford Textbook of Medicine. 5th ed. Oxford University Press; Oxford, UK: 2010. [Google Scholar]
- 3.Vafadari B., Mitra S., Stefaniuk M., Kaczmarek L. Psychosocial Stress Induces Schizophrenia-Like Behavior in Mice with Reduced MMP-9 Activity. Front. Behav. Neurosci. 2019;13:195. doi: 10.3389/fnbeh.2019.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vafadari B., Salamian A., Kaczmarek L. MMP-9 in translation: From molecule to brain physiology, pathology, and therapy. J. Neurochem. 2016;139((Suppl. 2)):91–114. doi: 10.1111/jnc.13415. [DOI] [PubMed] [Google Scholar]
- 5.Dinan T.G., Cryan J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017;79:920–926. doi: 10.1097/PSY.0000000000000519. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen T.T., Kosciolek T., Eyler L.T., Knight R., Jeste D.V. Overview and systematic review of studies of microbiome in schizophrenia and bipolar disorder. J. Psychiatr. Res. 2018;99:50–61. doi: 10.1016/j.jpsychires.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Konturek P.C., Brzozowski T., Konturek S.J. Stress and the gut: Pathophysiology, clinical consequences, diagnostic approach and treatment options. J. Physiol. Pharm. 2011;62:591–599. [PubMed] [Google Scholar]
- 8.Haddad L., Schafer A., Streit F., Lederbogen F., Grimm O., Wust S., Deuschle M., Kirsch P., Tost H., Meyer-Lindenberg A. Brain structure correlates of urban upbringing, an environmental risk factor for schizophrenia. Schizophr. Bull. 2015;41:115–122. doi: 10.1093/schbul/sbu072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kelly B.D., O’Callaghan E., Waddington J.L., Feeney L., Browne S., Scully P.J., Clarke M., Quinn J.F., McTigue O., Morgan M.G., et al. Schizophrenia and the city: A review of literature and prospective study of psychosis and urbanicity in Ireland. Schizophr. Res. 2010;116:75–89. doi: 10.1016/j.schres.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 10.Lederbogen F., Haddad L., Meyer-Lindenberg A. Urban social stress--risk factor for mental disorders. The case of schizophrenia. Env. Pollut. 2013;183:2–6. doi: 10.1016/j.envpol.2013.05.046. [DOI] [PubMed] [Google Scholar]
- 11.Van Os J., Kenis G., Rutten B.P. The environment and schizophrenia. Nature. 2010;468:203–212. doi: 10.1038/nature09563. [DOI] [PubMed] [Google Scholar]
- 12.Foster J.A., Rinaman L., Cryan J.F. Stress & the gut-brain axis: Regulation by the microbiome. Neurobiol. Stress. 2017;7:124–136. doi: 10.1016/j.ynstr.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disease G.B.D., Injury I., Prevalence C. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haddad F.L., Patel S.V., Schmid S. Maternal Immune Activation by Poly I:C as a preclinical Model for Neurodevelopmental Disorders: A focus on Autism and Schizophrenia. Neurosci. Biobehav. Rev. 2020;113:546–567. doi: 10.1016/j.neubiorev.2020.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Genedi M., Janmaat I.E., Haarman B., Sommer I.E.C. Dysregulation of the gut-brain axis in schizophrenia and bipolar disorder: Probiotic supplementation as a supportive treatment in psychiatric disorders. Curr. Opin. Psychiatry. 2019;32:185–195. doi: 10.1097/YCO.0000000000000499. [DOI] [PubMed] [Google Scholar]
- 16.Health, Topics. Schizophrenia. [(accessed on 5 July 2021)]; Available online: https://www.nimh.nih.gov/health/topics/schizophrenia/
- 17.Akdeniz C., Tost H., Streit F., Haddad L., Wust S., Schafer A., Schneider M., Rietschel M., Kirsch P., Meyer-Lindenberg A. Neuroimaging evidence for a role of neural social stress processing in ethnic minority-associated environmental risk. JAMA Psychiatry. 2014;71:672–680. doi: 10.1001/jamapsychiatry.2014.35. [DOI] [PubMed] [Google Scholar]
- 18.Meyer-Lindenberg A., Tost H. Neural mechanisms of social risk for psychiatric disorders. Nat. Neurosci. 2012;15:663–668. doi: 10.1038/nn.3083. [DOI] [PubMed] [Google Scholar]
- 19.Flores G., Morales-Medina J.C., Diaz A. Neuronal and brain morphological changes in animal models of schizophrenia. Behav. Brain Res. 2016;301:190–203. doi: 10.1016/j.bbr.2015.12.034. [DOI] [PubMed] [Google Scholar]
- 20.Lewis D.A., Lieberman J.A. Catching up on schizophrenia: Natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/S0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt A., Bertsch T., Tost H., Bergmann A., Henning U., Klimke A., Falkai P. Increased serum interleukin-1beta and interleukin-6 in elderly, chronic schizophrenic patients on stable antipsychotic medication. Neuropsychiatr. Dis. Treat. 2005;1:171–177. doi: 10.2147/nedt.1.2.171.61048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen M.J., Craddock N., O’Donovan M.C. Schizophrenia: Genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Foussias G., Remington G. Negative symptoms in schizophrenia: Avolition and Occam’s razor. Schizophr. Bull. 2010;36:359–369. doi: 10.1093/schbul/sbn094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elvevag B., Goldberg T.E. Cognitive impairment in schizophrenia is the core of the disorder. Crit. Rev. Neurobiol. 2000;14:1–21. doi: 10.1615/CritRevNeurobiol.v14.i1.10. [DOI] [PubMed] [Google Scholar]
- 25.Joyce E.M., Roiser J.P. Cognitive heterogeneity in schizophrenia. Curr. Opin. Psychiatry. 2007;20:268–272. doi: 10.1097/YCO.0b013e3280ba4975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis D.A., Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigues-Amorim D., Rivera-Baltanas T., Lopez M., Spuch C., Olivares J.M., Agis-Balboa R.C. Schizophrenia: A review of potential biomarkers. J. Psychiatr. Res. 2017;93:37–49. doi: 10.1016/j.jpsychires.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 28.Lodge D.J., Grace A.A. Developmental pathology, dopamine, stress and schizophrenia. Int. J. Dev. Neurosci. 2011;29:207–213. doi: 10.1016/j.ijdevneu.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgan C., Fisher H. Environment and schizophrenia: Environmental factors in schizophrenia: Childhood trauma—A critical review. Schizophr. Bull. 2007;33:3–10. doi: 10.1093/schbul/sbl053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Corcoran C., Walker E., Huot R., Mittal V., Tessner K., Kestler L., Malaspina D. The stress cascade and schizophrenia: Etiology and onset. Schizophr. Bull. 2003;29:671–692. doi: 10.1093/oxfordjournals.schbul.a007038. [DOI] [PubMed] [Google Scholar]
- 31.Tienari P., Wynne L.C., Moring J., Lahti I., Naarala M., Sorri A., Wahlberg K.E., Saarento O., Seitamaa M., Kaleva M., et al. The Finnish adoptive family study of schizophrenia. Implications for family research. Br. J. Psychiatry Suppl. 1994;164:20–26. doi: 10.1192/S0007125000292696. [DOI] [PubMed] [Google Scholar]
- 32.Patrono E., Svoboda J., Stuchlik A. Schizophrenia, the gut microbiota, and new opportunities from optogenetic manipulations of the gut-brain axis. Behav. Brain Funct. 2021;17:7. doi: 10.1186/s12993-021-00180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ellenbroek B.A., Derks N., Park H.J. Early maternal deprivation retards neurodevelopment in Wistar rats. Stress. 2005;8:247–257. doi: 10.1080/10253890500404634. [DOI] [PubMed] [Google Scholar]
- 34.Palmer A.A., Printz D.J., Butler P.D., Dulawa S.C., Printz M.P. Prenatal protein deprivation in rats induces changes in prepulse inhibition and NMDA receptor binding. Brain Res. 2004;996:193–201. doi: 10.1016/j.brainres.2003.09.077. [DOI] [PubMed] [Google Scholar]
- 35.Ratajczak P., Wozniak A., Nowakowska E. Animal models of schizophrenia: Developmental preparation in rats. Acta. Neurobiol. Exp. 2013;73:472–484. doi: 10.55782/ane-2013-1953. [DOI] [PubMed] [Google Scholar]
- 36.Chida Y., Sudo N., Sonoda J., Hiramoto T., Kubo C. Early-life psychological stress exacerbates adult mouse asthma via the hypothalamus-pituitary-adrenal axis. Am. J. Respir. Crit. Care Med. 2007;175:316–322. doi: 10.1164/rccm.200607-898OC. [DOI] [PubMed] [Google Scholar]
- 37.O’Mahony S.M., Hyland N.P., Dinan T.G., Cryan J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- 38.Gispen-de Wied C.C. Stress in schizophrenia: An integrative view. Eur. J. Pharm. 2000;405:375–384. doi: 10.1016/S0014-2999(00)00567-7. [DOI] [PubMed] [Google Scholar]
- 39.Schneider M.L., Clarke A.S., Kraemer G.W., Roughton E.C., Lubach G.R., Rimm-Kaufman S., Schmidt D., Ebert M. Prenatal stress alters brain biogenic amine levels in primates. Dev. Psychopathol. 1998;10:427–440. doi: 10.1017/S0954579498001679. [DOI] [PubMed] [Google Scholar]
- 40.Schneider M.L., Moore C.F., Roberts A.D., Dejesus O. Prenatal stress alters early neurobehavior, stress reactivity and learning in non-human primates: A brief review. Stress. 2001;4:183–193. doi: 10.3109/10253890109035017. [DOI] [PubMed] [Google Scholar]
- 41.Walker E., Mittal V., Tessner K. Stress and the hypothalamic pituitary adrenal axis in the developmental course of schizophrenia. Annu. Rev. Clin. Psychol. 2008;4:189–216. doi: 10.1146/annurev.clinpsy.4.022007.141248. [DOI] [PubMed] [Google Scholar]
- 42.Sochocka M., Donskow-Lysoniewska K., Diniz B.S., Kurpas D., Brzozowska E., Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease-a Critical Review. Mol. Neurobiol. 2019;56:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F., McClain C.J., Feng W. Microbiome dysbiosis and alcoholic liver disease. Liver Res. 2019;3:218–226. doi: 10.1016/j.livres.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cryan J.F., Dinan T.G. Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 2012;13:701–712. doi: 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- 45.Cryan J.F., O’Riordan K.J., Sandhu K., Peterson V., Dinan T.G. The gut microbiome in neurological disorders. Lancet Neurol. 2020;19:179–194. doi: 10.1016/S1474-4422(19)30356-4. [DOI] [PubMed] [Google Scholar]
- 46.Mayer E.A. Gut feelings: The emerging biology of gut-brain communication. Nat. Rev. Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rhee S.H., Pothoulakis C., Mayer E.A. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat. Rev. Gastroenterol. Hepatol. 2009;6:306–314. doi: 10.1038/nrgastro.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cowan C.S.M., Dinan T.G., Cryan J.F. Annual Research Review: Critical windows—The microbiota-gut-brain axis in neurocognitive development. J. Child Psychol. Psychiatry. 2020;61:353–371. doi: 10.1111/jcpp.13156. [DOI] [PubMed] [Google Scholar]
- 49.Bravo J.A., Forsythe P., Chew M.V., Escaravage E., Savignac H.M., Dinan T.G., Bienenstock J., Cryan J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Svensson E., Horvath-Puho E., Thomsen R.W., Djurhuus J.C., Pedersen L., Borghammer P., Sorensen H.T. Vagotomy and subsequent risk of Parkinson’s disease. Ann. Neurol. 2015;78:522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 51.Gheorghe C.E., Martin J.A., Manriquez F.V., Dinan T.G., Cryan J.F., Clarke G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharm. 2019;48:137–145. doi: 10.1016/j.coph.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 52.Desbonnet L., Garrett L., Clarke G., Kiely B., Cryan J.F., Dinan T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170:1179–1188. doi: 10.1016/j.neuroscience.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Lyte M. Microbial endocrinology in the microbiome-gut-brain axis: How bacterial production and utilization of neurochemicals influence behavior. PLoS Pathog. 2013;9:e1003726. doi: 10.1371/journal.ppat.1003726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. Adv. Exp. Med. Biol. 2014;817:3–24. doi: 10.1007/978-1-4939-0897-4_1. [DOI] [PubMed] [Google Scholar]
- 55.Sherwin E., Bordenstein S.R., Quinn J.L., Dinan T.G., Cryan J.F. Microbiota and the social brain. Science. 2019;366:6465. doi: 10.1126/science.aar2016. [DOI] [PubMed] [Google Scholar]
- 56.Yolken R.H., Severance E.G., Sabunciyan S., Gressitt K.L., Chen O., Stallings C., Origoni A., Katsafanas E., Schweinfurth L.A., Savage C.L., et al. Metagenomic Sequencing Indicates That the Oropharyngeal Phageome of Individuals With Schizophrenia Differs From That of Controls. Schizophr. Bull. 2015;41:1153–1161. doi: 10.1093/schbul/sbu197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scheepers I.M., Cryan J.F., Bastiaanssen T.F.S., Rea K., Clarke G., Jaspan H.B., Harvey B.H., Hemmings S.M.J., Santana L., van der Sluis R., et al. Natural compulsive-like behaviour in the deer mouse (Peromyscus maniculatus bairdii) is associated with altered gut microbiota composition. Eur. J. Neurosci. 2020;51:1419–1427. doi: 10.1111/ejn.14610. [DOI] [PubMed] [Google Scholar]
- 58.Desbonnet L., Clarke G., O’Sullivan O., Cotter P.D., Dinan T.G., Cryan J.F. Re: Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain Behav. Immun. 2015;50:335–336. doi: 10.1016/j.bbi.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 59.Ng Q.X., Soh A.Y.S., Venkatanarayanan N., Ho C.Y.X., Lim D.Y., Yeo W.S. A Systematic Review of the Effect of Probiotic Supplementation on Schizophrenia Symptoms. Neuropsychobiology. 2019;78:1–6. doi: 10.1159/000498862. [DOI] [PubMed] [Google Scholar]
- 60.Nemani K., Hosseini Ghomi R., McCormick B., Fan X. Schizophrenia and the gut-brain axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;56:155–160. doi: 10.1016/j.pnpbp.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 61.Kraeuter A.K., Phillips R., Sarnyai Z. The Gut Microbiome in Psychosis from Mice to Men: A Systematic Review of Preclinical and Clinical Studies. Front. Psychiatry. 2020;11:799. doi: 10.3389/fpsyt.2020.00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng P., Zeng B., Liu M., Chen J., Pan J., Han Y., Liu Y., Cheng K., Zhou C., Wang H., et al. The gut microbiome from patients with schizophrenia modulates the glutamate-glutamine-GABA cycle and schizophrenia-relevant behaviors in mice. Sci. Adv. 2019;5:8317. doi: 10.1126/sciadv.aau8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu F., Guo R., Wang W., Ju Y., Wang Q., Ma Q., Sun Q., Fan Y., Xie Y., Yang Z., et al. Transplantation of microbiota from drug-free patients with schizophrenia causes schizophrenia-like abnormal behaviors and dysregulated kynurenine metabolism in mice. Mol. Psychiatry. 2020;25:2905–2918. doi: 10.1038/s41380-019-0475-4. [DOI] [PubMed] [Google Scholar]
- 64.Zhu F., Ju Y., Wang W., Wang Q., Guo R., Ma Q., Sun Q., Fan Y., Xie Y., Yang Z., et al. Metagenome-wide association of gut microbiome features for schizophrenia. Nat. Commun. 2020;11:1612. doi: 10.1038/s41467-020-15457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Castro-Nallar E., Bendall M.L., Perez-Losada M., Sabuncyan S., Severance E.G., Dickerson F.B., Schroeder J.R., Yolken R.H., Crandall K.A. Composition, taxonomy and functional diversity of the oropharynx microbiome in individuals with schizophrenia and controls. Peer J. 2015;3:e1140. doi: 10.7717/peerj.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fond G.B., Lagier J.C., Honore S., Lancon C., Korchia T., Sunhary De Verville P.L., Llorca P.M., Auquier P., Guedj E., Boyer L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients. 2020;12:1024. doi: 10.3390/nu12041024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Severance E.G., Prandovszky E., Castiglione J., Yolken R.H. Gastroenterology issues in schizophrenia: Why the gut matters. Curr. Psychiatry Rep. 2015;17:27. doi: 10.1007/s11920-015-0574-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pascal M., Perez-Gordo M., Caballero T., Escribese M.M., Lopez Longo M.N., Luengo O., Manso L., Matheu V., Seoane E., Zamorano M., et al. Microbiome and Allergic Diseases. Front. Immunol. 2018;9:1584. doi: 10.3389/fimmu.2018.01584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morais L.H., Felice D., Golubeva A.V., Moloney G., Dinan T.G., Cryan J.F. Strain differences in the susceptibility to the gut-brain axis and neurobehavioural alterations induced by maternal immune activation in mice. Behav. Pharm. 2018;29:181–198. doi: 10.1097/FBP.0000000000000374. [DOI] [PubMed] [Google Scholar]
- 70.Polkowska-Pruszynska B., Gerkowicz A., Krasowska D. The gut microbiome alterations in allergic and inflammatory skin diseases—An update. J. Eur. Acad. Derm. Venereol. 2020;34:455–464. doi: 10.1111/jdv.15951. [DOI] [PubMed] [Google Scholar]
- 71.Prince B.T., Mandel M.J., Nadeau K., Singh A.M. Gut Microbiome and the Development of Food Allergy and Allergic Disease. Pediatr. Clin. North Am. 2015;62:1479–1492. doi: 10.1016/j.pcl.2015.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhuang L., Chen H., Zhang S., Zhuang J., Li Q., Feng Z. Intestinal Microbiota in Early Life and Its Implications on Childhood Health. Genom. Proteom. Bioinform. 2019;17:13–25. doi: 10.1016/j.gpb.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bressan P., Kramer P. Bread and Other Edible Agents of Mental Disease. Front Hum. Neurosci. 2016;10:130. doi: 10.3389/fnhum.2016.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dohan F.C. Wheat “consumption” and hospital admissions for schizophrenia during World War II. A preliminary report. Am. J. Clin. Nutr. 1966;18:7–10. doi: 10.1093/ajcn/18.1.7. [DOI] [PubMed] [Google Scholar]
- 75.Reichelt K.L., Seim A.R., Reichelt W.H. Could schizophrenia be reasonably explained by Dohan’s hypothesis on genetic interaction with a dietary peptide overload? Prog. Neuropsychopharmacol Biol. Psychiatry. 1996;20:1083–1114. doi: 10.1016/S0278-5846(96)00099-1. [DOI] [PubMed] [Google Scholar]
- 76.Lachance L.R., McKenzie K. Biomarkers of gluten sensitivity in patients with non-affective psychosis: A meta-analysis. Schizophr. Res. 2014;152:521–527. doi: 10.1016/j.schres.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Buscaino V.M. Patologia Extraneurale della Schizofrenia: Fegato, Tubo Digerente, Sistema Reticolo-Endoteliale. Volume 4 Policlinico; Napoli, Italy: 1953. [Google Scholar]
- 78.Hemmings G. Schizophrenia. Lancet. 2004;364:1312–1313. doi: 10.1016/S0140-6736(04)17181-X. [DOI] [PubMed] [Google Scholar]
- 79.Madison A., Kiecolt-Glaser J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019;28:105–110. doi: 10.1016/j.cobeha.2019.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rea K., Dinan T.G., Cryan J.F. Gut Microbiota: A Perspective for Psychiatrists. Neuropsychobiology. 2020;79:50–62. doi: 10.1159/000504495. [DOI] [PubMed] [Google Scholar]
- 81.Bailey M.T., Coe C.L. Maternal separation disrupts the integrity of the intestinal microflora in infant rhesus monkeys. Dev. Psychobiol. 1999;35:146–155. doi: 10.1002/(SICI)1098-2302(199909)35:2<146::AID-DEV7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 82.Bailey M.T., Dowd S.E., Galley J.D., Hufnagle A.R., Allen R.G., Lyte M. Exposure to a social stressor alters the structure of the intestinal microbiota: Implications for stressor-induced immunomodulation. Brain Behav. Immun. 2011;25:397–407. doi: 10.1016/j.bbi.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Van de Wouw M., Lyte J.M., Boehme M., Sichetti M., Moloney G., Goodson M.S., Kelley-Loughnane N., Dinan T.G., Clarke G., Cryan J.F. The role of the microbiota in acute stress-induced myeloid immune cell trafficking. Brain Behav. Immun. 2020;84:209–217. doi: 10.1016/j.bbi.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 84.Carlson A.L., Xia K., Azcarate-Peril M.A., Rosin S.P., Fine J.P., Mu W., Zopp J.B., Kimmel M.C., Styner M.A., Thompson A.L., et al. Infant gut microbiome composition is associated with non-social fear behavior in a pilot study. Nat. Commun. 2021;12:3294. doi: 10.1038/s41467-021-23281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Clarke G., Grenham S., Scully P., Fitzgerald P., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F. The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Mol. Psychiatry. 2013;18:666–673. doi: 10.1038/mp.2012.77. [DOI] [PubMed] [Google Scholar]
- 86.Diaz Heijtz R., Wang S., Anuar F., Qian Y., Bjorkholm B., Samuelsson A., Hibberd M.L., Forssberg H., Pettersson S. Normal gut microbiota modulates brain development and behavior. Proc. Natl. Acad. Sci. USA. 2011;108:3047–3052. doi: 10.1073/pnas.1010529108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luczynski P., McVey Neufeld K.A., Oriach C.S., Clarke G., Dinan T.G., Cryan J.F. Growing up in a Bubble: Using Germ-Free Animals to Assess the Influence of the Gut Microbiota on Brain and Behavior. Int. J. Neuropsychopharmacol. 2016;19:8. doi: 10.1093/ijnp/pyw020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Neufeld K.M., Kang N., Bienenstock J., Foster J.A. Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterol. Motil. 2011;23:255–264.e119. doi: 10.1111/j.1365-2982.2010.01620.x. [DOI] [PubMed] [Google Scholar]
- 89.Leclercq S., Mian F.M., Stanisz A.M., Bindels L.B., Cambier E., Ben-Amram H., Koren O., Forsythe P., Bienenstock J. Low-dose penicillin in early life induces long-term changes in murine gut microbiota, brain cytokines and behavior. Nat. Commun. 2017;8:15062. doi: 10.1038/ncomms15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dunphy-Doherty F., O’Mahony S.M., Peterson V.L., O’Sullivan O., Crispie F., Cotter P.D., Wigmore P., King M.V., Cryan J.F., Fone K.C.F. Post-weaning social isolation of rats leads to long-term disruption of the gut microbiota-immune-brain axis. Brain Behav. Immun. 2018;68:261–273. doi: 10.1016/j.bbi.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 91.Bastiaanssen T.F.S., Cussotto S., Claesson M.J., Clarke G., Dinan T.G., Cryan J.F. Gutted! Unraveling the Role of the Microbiome in Major Depressive Disorder. Harv. Rev. Psychiatry. 2020;28:26–39. doi: 10.1097/HRP.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hoffman K.W., Lee J.J., Corcoran C.M., Kimhy D., Kranz T.M., Malaspina D. Considering the Microbiome in Stress-Related and Neurodevelopmental Trajectories to Schizophrenia. Front. Psychiatry. 2020;11:629. doi: 10.3389/fpsyt.2020.00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 94.Liu J.C.W., Gorbovskaya I., Hahn M.K., Muller D.J. The Gut Microbiome in Schizophrenia and the Potential Benefits of Prebiotic and Probiotic Treatment. Nutrients. 2021;13:1152. doi: 10.3390/nu13041152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Upthegrove R., Marwaha S., Birchwood M. Depression and Schizophrenia: Cause, Consequence, or Trans-diagnostic Issue? Schizophr. Bull. 2017;43:240–244. doi: 10.1093/schbul/sbw097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li S., Song J., Ke P., Kong L., Lei B., Zhou J., Huang Y., Li H., Li G., Chen J., et al. The gut microbiome is associated with brain structure and function in schizophrenia. Sci. Rep. 2021;11:9743. doi: 10.1038/s41598-021-89166-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hoban A.E., Stilling R.M., Moloney G., Shanahan F., Dinan T.G., Clarke G., Cryan J.F. The microbiome regulates amygdala-dependent fear recall. Mol. Psychiatry. 2018;23:1134–1144. doi: 10.1038/mp.2017.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ressler K.J. Amygdala activity, fear, and anxiety: Modulation by stress. Biol. Psychiatry. 2010;67:1117–1119. doi: 10.1016/j.biopsych.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Qiao H., Li M.X., Xu C., Chen H.B., An S.C., Ma X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural. Plast. 2016;2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tebartz Van Elst L., Baeumer D., Lemieux L., Woermann F.G., Koepp M., Krishnamoorthy S., Thompson P.J., Ebert D., Trimble M.R. Amygdala pathology in psychosis of epilepsy: A magnetic resonance imaging study in patients with temporal lobe epilepsy. Brain. 2002;125:140–149. doi: 10.1093/brain/awf008. [DOI] [PubMed] [Google Scholar]
- 101.Zhang M., Yang F., Fan F., Wang Z., Hong X., Tan Y., Tan S., Hong L.E. Abnormal amygdala subregional-sensorimotor connectivity correlates with positive symptom in schizophrenia. Neuroimage Clin. 2020;26:102218. doi: 10.1016/j.nicl.2020.102218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao W., Salzwedel A.P., Carlson A.L., Xia K., Azcarate-Peril M.A., Styner M.A., Thompson A.L., Geng X., Goldman B.D., Gilmore J.H., et al. Gut microbiome and brain functional connectivity in infants-a preliminary study focusing on the amygdala. Psychopharmacology. 2019;236:1641–1651. doi: 10.1007/s00213-018-5161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buchanan R.W., Vladar K., Barta P.E., Pearlson G.D. Structural evaluation of the prefrontal cortex in schizophrenia. Am. J. Psychiatry. 1998;155:1049–1055. doi: 10.1176/ajp.155.8.1049. [DOI] [PubMed] [Google Scholar]
- 104.Goto Y., Yang C.R., Otani S. Functional and dysfunctional synaptic plasticity in prefrontal cortex: Roles in psychiatric disorders. Biol. Psychiatry. 2010;67:199–207. doi: 10.1016/j.biopsych.2009.08.026. [DOI] [PubMed] [Google Scholar]
- 105.Hoban A.E., Stilling R.M., Ryan F.J., Shanahan F., Dinan T.G., Claesson M.J., Clarke G., Cryan J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry. 2016;6:e774. doi: 10.1038/tp.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wible C.G., Anderson J., Shenton M.E., Kricun A., Hirayasu Y., Tanaka S., Levitt J.J., O’Donnell B.F., Kikinis R., Jolesz F.A., et al. Prefrontal cortex, negative symptoms, and schizophrenia: An MRI study. Psychiatry Res. 2001;108:65–78. doi: 10.1016/S0925-4927(01)00109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sullivan R.M., Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/S0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- 108.Morgan M.A., Romanski L.M., LeDoux J.E. Extinction of emotional learning: Contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-C. [DOI] [PubMed] [Google Scholar]
- 109.Gacias M., Gaspari S., Santos P.M., Tamburini S., Andrade M., Zhang F., Shen N., Tolstikov V., Kiebish M.A., Dupree J.L., et al. Microbiota-driven transcriptional changes in prefrontal cortex override genetic differences in social behavior. Elife. 2016;5:e13442. doi: 10.7554/eLife.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen J.J., Xie J., Zeng B.H., Li W.W., Bai S.J., Zhou C., Chen W., Wei H., Xie P. Absence of gut microbiota affects lipid metabolism in the prefrontal cortex of mice. Neurol. Res. 2019;41:1104–1112. doi: 10.1080/01616412.2019.1675021. [DOI] [PubMed] [Google Scholar]
- 111.Brunet-Gouet E., Decety J. Social brain dysfunctions in schizophrenia: A review of neuroimaging studies. Psychiatry Res. 2006;148:75–92. doi: 10.1016/j.pscychresns.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 112.Ikegame T., Bundo M., Okada N., Murata Y., Koike S., Sugawara H., Saito T., Ikeda M., Owada K., Fukunaga M., et al. Promoter Activity-Based Case-Control Association Study on SLC6A4 Highlighting Hypermethylation and Altered Amygdala Volume in Male Patients with Schizophrenia. Schizophr. Bull. 2020;46:1577–1586. doi: 10.1093/schbul/sbaa075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joyal C.C., Laakso M.P., Tiihonen J., Syvalahti E., Vilkman H., Laakso A., Alakare B., Rakkolainen V., Salokangas R.K., Hietala J. The amygdala and schizophrenia: A volumetric magnetic resonance imaging study in first-episode, neuroleptic-naive patients. Biol. Psychiatry. 2003;54:1302–1304. doi: 10.1016/S0006-3223(03)00597-3. [DOI] [PubMed] [Google Scholar]
- 114.Maas D.A., Valles A., Martens G.J.M. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. Transl. Psychiatry. 2017;7:e1171. doi: 10.1038/tp.2017.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Calhoon G.G., Tye K.M. Resolving the neural circuits of anxiety. Nat. Neurosci. 2015;18:1394–1404. doi: 10.1038/nn.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hoban A.E., Stilling R.M., Moloney G.M., Moloney R.D., Shanahan F., Dinan T.G., Cryan J.F., Clarke G. Microbial regulation of microRNA expression in the amygdala and prefrontal cortex. Microbiome. 2017;5:102. doi: 10.1186/s40168-017-0321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Luczynski P., Whelan S.O., O’Sullivan C., Clarke G., Shanahan F., Dinan T.G., Cryan J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016;44:2654–2666. doi: 10.1111/ejn.13291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kelly J.R., Minuto C., Cryan J.F., Clarke G., Dinan T.G. The role of the gut microbiome in the development of schizophrenia. Schizophr. Res. 2020;234:4–23. doi: 10.1016/j.schres.2020.02.010. [DOI] [PubMed] [Google Scholar]