Abstract
Coronavirus disease 2019 (COVID-19) can potentially affect all organs owing to the ubiquitous diffusion of the angiotensin-converting enzyme II (ACE2) receptor-binding protein. Indeed, the SARS-CoV-2 virus is capable of causing heart disease. This systematic review can offer a new perspective on the potential consequences of COVID-19 through an analysis of the current literature on cardiac involvement. This systematic review, conducted from March 2020 to July 2021, searched the current literature for postmortem findings in patients who were positive for SARS-CoV-2 by combining and meshing the terms “COVID-19”, “postmortem”, “autopsy”, and “heart” in titles, abstracts, and keywords. The PubMed database was searched following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Sixteen papers met the inclusion criteria (case reports and series, original research, only English-written). A total of 209 patients were found (mean age (interquartile range (IQR)), 60.17 years (IQR, 54.75–70.75 years); 122 men (58.37%, ratio of men to women of 1:0.7%)). Each patient tested positive for SARS-CoV-2. Death was mainly the result of respiratory failure. The second most common cause of death was acute heart failure. Few patients specifically died of myocarditis. Variables such as pathological findings, immunohistochemical data, and previous clinical assessments were analyzed. Main cardiac pathological findings were cardiac dilatation, necrosis, lymphocytic infiltration of the myocardium, and small coronary vessel microthrombosis. Immunohistochemical analyses revealed an inflammatory state dominated by the constant presence of CD3+ and CD8+ cytotoxic lymphocytes and CD68+ macrophages. COVID-19 leads to a systemic inflammatory response and a constant prothrombotic state. The results of our systematic review suggest that SARS-CoV-2 was able to cause irreversible changes in several organs, including the heart; this is reflected by the increased cardiac risk in patients who survive COVID-19. Postmortem analysis (including autopsy, histologic, and immunohistochemical examination) is an indispensable tool to better understand pathological changes caused by emerging diseases such as COVID-19. Our results may provide more information on the involvement of the heart in COVID-19 patients.
Keywords: COVID-19, SARS-CoV-2, postmortem, autopsy, heart, heart failure, myocarditis
1. Introduction
Coronaviruses have always coexisted with humans; however, in 2020, a new severe acute respiratory syndrome coronavirus (SARS-CoV) developed and caused a worldwide pandemic, as the World Health Organization (WHO) declared in March 2020 [1]. SARS-CoV-2 caused a new infectious disease called coronavirus disease 2019 (COVID-19). SARS-CoV-2 is an ssRNA virus belonging to the family of Coronaviridae. The viral genome consists of 27 open reading frames (ORFs) and codes for 27 proteins [2], 4 of which are major proteins and 8 accessory proteins [3]. The most important one, the spike protein, is functionally divided into the S1 and S2 domain; the first domain is crucial for the binding of angiotensin-converting enzyme II (ACE2) on the endothelial cells of the lung. The S2 domain is important for cell membrane fusion [4]. The main feature of SARS-CoV-2 is its high airborne transmission capability [5].
The natural course of severe COVID-19 consists of a primordial phase of airborne transmission, a variable period of viral replication or flulike symptoms, and then an inflammatory dominant phase [6]. The last phase leads to an aberrant host immune response mediated by natural killer (NK) cells, macrophages, and T lymphocytes. This systemic hyperinflammatory response involves the endothelial cells of the lungs and acute respiratory distress syndrome (ARDS), eventually leading to death from respiratory failure [7]. Considering that ACE2 is diffusely expressed among various organs [8], it is commonly accepted that SARS-CoV-2 can and does affect different organs. Therefore, COVID-19 is considered a systemic disease that involves the lungs, heart, kidneys, and liver. During clinical evaluations of patients with COVID-19, it is usual to find evidence of acute respiratory disease syndrome (71%), cardiac injury (33%), acute kidney injury (20%), and liver dysfunction (15%) [9].
Heart involvement correlates to a high mortality rate [10,11]. Specifically, high blood troponin levels strictly relate to higher mortality rates, as confirmed by prospective observational cohort studies [12].
Autopsy data and histopathological analysis of cardiac involvement may help to understand COVID-19 pathophysiology and its clinical features [13]. The aim of the present study was to evaluate the current knowledge about postmortem cardiac findings in patients who died of COVID-19 and to provide a systematic review of existing literature.
2. Materials and Methods
This systematic review was conducted from March 2020 to July 2021 and followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [14]. A systematic literature search of the PubMed database and a critical review of the collected studies were conducted. The database was searched by combining and meshing the terms “COVID-19”, “postmortem”, “autopsy”, and “heart” in titles, abstracts, and keywords. The references of all identified articles were examined and cross-referenced to further isolate the relevant literature. A methodologic appraisal of each study was conducted including an evaluation of bias. The data collection process included study selection and data extraction. This study was exempt from an institutional review board approval as it did not involve human subjects. A waiver of patient consent was provided due to the use of deidentified patient data.
Inclusion criteria included case reports, research articles, and autopsy series reports, and papers written in the English language. In Figure 1, a flowchart of our review method is shown.
Figure 1.
Research results from 70 screened articles. Inclusion criteria included case reports, research articles, autopsy series reports, and papers written in the English language.
Regarding immunohistochemistry considerations, the presence or absence of immune cells in the myocardium and myocardial vessels was highlighted. Myocardial SARS-CoV-2 presence was considered positive when viral RNA was detected in the myocardium through RNAscope® (Advanced Cell Diagnostics Inc. Leica, Nussloch, Germany) or alternative RNA in situ hybridization (RNA-ISH) methods, immunohistochemical stains, or when electron microscopy provided evidence of viral particles.
3. Results
Literature containing information on the role of SARS-CoV-2 in heart disease was limited but well represented. We detected a total of 72 articles, as reported in the PRISMA flowchart. After reading the abstracts, we screened the remaining articles through a critical revision of the entire datasheet (when available).
After the screening was complete, we selected 16 articles using additional criteria, such as excluding reviews to avoid including the same article twice; or when the article did not provide clinical and laboratory details that we considered essential for consistency with other papers. We decided to include only case reports, research articles, and autopsy series reports.
A total of 209 patients (mean age (interquartile range (IQR)), 60.17 years (IQR, 54.75–70.75 years); 122 men (58.37%)) were identified, with a ratio of men to women of 1:0.7. Each patient tested positive for SARS-CoV-2. Almost all patients had previous comorbidities including chronic obstructive pulmonary disease (COPD), diabetes, hypertension, chronic kidney disease (CKD), hypertrophic cardiomyopathy, ischemic heart disease, atrial fibrillation, cancer, and dementia. A summary of the found articles is reported in Table 1, while the Section 3.1 “Autopsy data” a brief description of the pathological findings described in the papers is provided.
Table 1.
Summary of data obtained from the 16 found articles.
| Authors | Number of Cases | Age | M:F | Cardiac Death |
Cardiac Infection |
Macroscopic | Microscopic | IHC | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight | Ventricular Dilatation/ Myocyte Hypertrophy |
Necrosis | Lymphocytic Infiltration Parenchyma |
Microthrombi/ Cardiac Vessels Involvement |
||||||||
| Lindner et al. 2020 [15] |
39 | 85 (78–89) | 16:23 | 1 Prior HF, 38 ARDS | 15 (+) interstitial cells within the cardiac tissue |
Not reported | None | None | 0 | - | Yes | |
| Fox et al. 2020 [16] |
22 | 68.5 (44–79) | 10:12 | 0 | 16 | 340 to 1010 g | 9 RVD Se R:L >1:1 Right Hypertrophy |
Individual cell dropout/necrosis/apoptosis | 0 | - | Yes | |
| Jacobs et al., 2020 [17] | 1 | 48 | 1 | 1 Fulminant Myocarditis due to COVID-19 |
1 | 605 | 1 | piecemeal necrosis | 1 | - | Yes | |
| Pellegrini et al. 2020 [19] |
40 | 74 | 29:11 | 3 HF, 37 RF | 8 | Not reported | Not reported | 14 (3 infarction, 11 focal necrosis) | 9 | 9 | No | |
| Gauchotte et al. 2021 [20] |
1 | 69 | 1 | 1 Fulminant myocarditis due to COVID-19 | 1 | 403 | none | None | 1 | - | Yes | |
| Bulfamante et al., 2020 [21] | 6 | 59.5 (54–69) | 1:5 | 6 RF | 6 | 410–750 | 1 LVH (prior), 6 RVD (lung overload) | 6 | 6 | - | Yes | |
| Del Nonno et al., 2020 [22] | 9 | 70 (35–92) | 7:2 | 9 (6 shock, 3 sudden death) | 0 | 450 | No supplementary available | 9 | 9 | 9 | No | |
| Basso et al. 2020 [23] |
21 | 69 (44–86) | 15:6 | 1 HF, 1 Sudden death, 19 RF | Not reported | Not reported | Not reported | 4 | 3 criteria /11 infiltration |
3 | Yes | |
| Hanley et al. 2020 [24] |
10 | 73 (52–79) | 6:3 | 9 RF | 2 | 450 (312–513) | 4 LVH | 1 | 0 | 6 | No | |
| Rapkiewicz et al., 2020 [25] | 7 | 57 (44–65) | 3:4 | 7 RF | Not reported | Not Reported | Not reported | 3 | 1 | 7 | No | |
| Cîrstea et al., 2020 [26] | 1 | 30 | 0:1 | 1 HF | Not reported | Not reported | Not reported | 0 | 1 | 1 | No |
|
| Bradley et al., 2020 [27] | 14 | 73.5 (42–84) | 6:8 | 1 VF, 1 LVF, prior 12 RF |
0 | Not reported | 13 CH | 1 | 1 | 0 | No | |
| Duarte-Neto et al., 2020 [30] | 10 | 69 (33–83) | 5:5 | 10 RF | Not reported | Not reported | 9 CH | 0 | 2 | 2 | No | |
| Schaller et al., 2020 [32] | 10 | 69 (64–90) | 7:4 | Not reported | Not reported | Not reported | Not reported | Not reported | 4 | 0 | No | |
| Buja et al., 2020 [33] | 3 | 48 (34–62) | 3:0 | 2RF, 1 SD | Not reported | 720 (420–1070) | 2CH | 0 | 2 | 0 | No | |
| Bois et al., 2021 [34] | 15 | 78 (71–86) | 12:3 | Not reported | 5 | 443.1 (286.3–545.0) | Not reported | 9 | 5 | 12 | Yes | |
HF—Heart Failure; RF—Respiratory failure; CH—Cardiac Hypertrophy; LVH—Left Ventricular Hypertrophy; RVD—Right Ventricular Disfunction; IHC—immunohistochemistry.
3.1. Autopsy Data
In November 2020, Lindler et al., published a cohort study of 39 patients who died of COVID-19–related ARDS [15]. No one died of fulminant myocarditis. The authors evaluated cardiac involvement at autopsy and found that only 15 hearts tested positive for SARS-CoV-2 using RNAscope. Moreover, through immunochemical analysis, they discovered the expression of multiple proinflammatory genes which led to a cytokine storm and the expression of CD3, CD45RO, and CD68. There was diffuse SARS-CoV-2 involvement (>1000 copies) in five hearts associated with a myocardial inflammatory storm. Despite data scarcity, it seems that SARS-CoV-2 infection correlates with a massive inflammatory response.
Fox et al., performed a cohort study of 22 COVID-19-related deaths and published their findings in September 2020 [16]. Macroscopic analysis in 9 of 22 hearts showed a severe right ventricular dilatation due to pulmonary hypertension caused by COVID-19 pneumonia. Furthermore, transmission electron microscopy (TEM) examination of six hearts revealed a large and diffuse viral invasion, in association with CD8+ lymphocytes near the endothelial cells of small vessels.
In September 2020, Jacobs et al., described the case of a man aged 48 years who died of fulminant myocarditis [17]. The aim of the study was to demonstrate the role of ferroptosis (programmed cell death dependent on iron and the accumulation of lipid peroxide) induced by COVID-19. The patient had a progressive multiorgan failure (MOF). In particular, the authors stated that the patient had kidney failure secondary to heart failure. This patient case fulfilled the Dallas criteria for a myocarditis diagnosis, demonstrated by a diffuse muscular and vascular CD3+ lymphocytic invasion [18].
Then, in January 2021, Pellegrini et al., reported a series of 40 autopsies in which the subjects died primarily of respiratory failure (90%) and secondarily of vascular ischemic disease (10%) [19]. The authors sampled the myocardium for histologic processing and analyzed the coronary artery aspirates. Microscopy revealed focal necrosis in 35% of cases, with a significant relationship (p < 0.001) between myocardial necrosis and concomitant microthrombi.
In January 2020, Gauchotte et al., described the postmortem heart findings of a 69-year-old man who died of heart failure without lung involvement [20]. Histology findings demonstrated inflammatory infiltration of the cardiac muscle along with positivity for SARS-CoV-2 using RNAscope. Noteworthy, RNAscope analysis of the lung tissue was negative for COVID-19.
Further investigation of infectious myocardial changes was performed by Bulfamante et al., in December 2020 [21]. The authors studied myocardial sections, which had tested positive for the presence of SARS-CoV-2, to investigate changes in the ultrastructure of the myocardium. The main features found were cardiac edema, loss of regular morphology, and cardiomyocyte swelling. Interestingly, SARS-CoV-2 viral particles and sarcomere ruptures were co-localized.
Del Nonno et al., published a report in November 2020 that investigated patients who died of COVID-19 but had negative heart samples [22]. They examined a small cohort of nine hospitalized patients who died of cardiogenic shock [6] or sudden death [13]. They were all receiving pharmacologic treatment for SARS-CoV-2-related pneumonia. The aim of this study was to evaluate direct and indirect involvement of COVID-19-related cardiac damage through a series of autopsies. The lymphomononuclear infiltrates CD68- and CD45Ro+ were found, surrounded by focal necrosis of adjacent myocytes; these were indicative of myocarditis. Six patients who died of acute heart failure had necrosis of the vessel walls. Inflammatory infiltrates were found in the subepicardial ganglia of three patients who died of sudden cardiac death. Molecular tests ruled out the presence of viral infections—including SARS-CoV-2—as the cause of myocarditis. The authors concluded that the inflammatory component was a consequence of the initial damage from the viral infection; the virus may have triggered a secondary and independent phase of inflammation.
In September 2020, Basso et al., examined a total of 22 hearts, 3 of which were affected by myocarditis and 19 by lymphocytic infiltration [23]. This study revealed an abundant number of CD68+ macrophages in the hearts of patients who died of COVID-19. As a matter of fact, CD68+ macrophages and CD3+ lymphocytes were more abundant in the myocarditis group than in the control group. The presence of CD68+ cells may suggest a systemic elevation of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), during COVID-19 infection; this could explain the recruitment of macrophages.
Hanley et al., reported an autopsy series in August 2020 that focused on cardiac findings: five hearts of patients who died of COVID-19 had thrombi in the microcirculation, and one revealed macroscopic evidence of acute right coronary artery thrombosis [24]. Necrosis associated with high viral load was found in two myocardial samples.
Rapkiewicz et al., performed seven autopsies of patients who tested positive for SARS-CoV-2 [25] in June 2020. There was only one patient with evidence of predominant CD4+ lymphocytic infiltration. However, the main finding was the presence of fibrin microthrombi regardless of anticoagulation therapy.
In July 2020, Cîrstea et al., published a case of a woman aged 30 years who died of COVID-19-related pneumonia at Drobeta-Turnu Severin hospital in Romania [26]. The patient had presented with breathing difficulties, high fever, chest pain, and loss of appetite. At autopsy, the forensic pathologist found bilateral pneumonia and right ventricular dilatation, which lead to respiratory failure. Heart analysis showed leukostasis with thrombi formation and massive interstitial edema that obliterated the intercalated discs. There was no evidence of lymphocyte or macrophage infiltration.
In August 2020, Bradley et al., described a cohort of older patients with multiple comorbidities [27]. According to recent literature which asserts the high mortality rate of patients with comorbidities, the authors did not describe specific pathological findings [28,29]. They only reported nonspecific findings, such as fibrosis and hypertrophy. In eight patients, the authors found lymphocytes surrounding necrotic myocytes. No immunochemistry analysis was performed.
In May 2020, Duarte-Neto et al., presented the histopathological features of 10 minimally invasive autopsies (MIA-US) [30,31]. Histologic observation of the cardiac tissue revealed cardiomyocyte hypertrophy, edema, and myocardial fibrosis in nine patients and previous myocardial infarction in four patients. Further, the authors found microthrombi in the arterioles of two patients and evidence of myocarditis. They concluded that MIA-US was a safe, rapid, and effective procedure for obtaining tissue samples for the study of severe COVID-19.
Schaller et al., wrote a letter in June 2020 regarding the features of 10 COVID-19 autopsies [32]. Cardiac involvement was minimal, as they reported only mild lymphocytic myocarditis in four cases. The main characteristics included diffuse alveolar damage (DAD), which was found in both ventilated and nonventilated patients; this suggests the idea that DAD is not only a consequence of noninvasive ventilation.
In April 2020, Buja et al., described three autopsies conducted at Houston hospital [33]. They reported the findings of patients who were treated earlier during the onset of the pandemic. Immunochemistry was performed in each case. The authors found a large lymphocytic infiltration in the myocardium and pericardium. Specifically, tissue staining revealed CD8+–predominant inflammation, with diffuse CD68+ macrophages. The cardiac conduction system was not involved.
Bois et al., recently described histopathological, immunohistochemical, ultrastructural, and molecular findings of the heart in patients who died of active or cured SARS-CoV-2 infection [34]. One-third of the patients with COVID-19 showed myocarditis but this, due to its limited extent, could not account for the clinical picture. The finding of cardiac fibrin microthrombi in the absence of acute ischaemic lesions was the most significant finding of this study. No viral particles were identified within cardiac myocytes by transmission electron microscopy.
Immunohistochemistry results and the comorbidities of the patients are summarized in Table 2 and Table 3, respectively. Figure 2 shows the percentage of comorbidities observed among all the subjects (209).
Table 2.
Immunohistochemistry results as reported by authors.
| Authors | Number of Cases | Tissue RNA Scope or TEM | CD3 | CD4 | CD8 | CD4/CD8 | CD68 |
|---|---|---|---|---|---|---|---|
| Lindner et al., 2020 [15] | 39 | 24 | 21 | - | - | 1 | 19 |
| Fox et al., 2020 [16] | 22 | 6 | 6 | 0 | 6 | <1 | - |
| Jacobs et al., 2020 [17] | 1 | 1 | 1 | 1 | 1 | 1:7 | 1 |
| Pellegrini et al., 2020 [19] | 40 | 40 | 8 | - | - | - | - |
| Gauchotte et al., 2021 [20] | 1 | 1 | 1 | 1 | 1 | 1:9 | 1 |
| Bulfamante et al., 2020 [21] | 6 | 6 | 6 | - | - | - | - |
| Del Nonno et al., 2020 [22] | 9 | - | - | - | - | - | - |
| Basso et al., 2020 [23] | 21 | 21 | 21 | 2 | 19 | <1 | 18 |
| Hanley et al., 2020 [24] |
10 | 9 | - | - | - | - | - |
| Rapkiewicz et al., 2020 [25] | 7 | 7 | 1 | 1 | 1 | <1 | 0 |
| Cîrstea et al., 2020 [26] | 1 | 1 | - | - | - | - | - |
| Bradley et al., 2020 [27] | 14 | - | - | - | - | - | - |
| Duarte-Neto et al., 2020 [30] |
10 | - | - | - | - | - | - |
| Schaller et al., 2020 [32] | 10 | 10 | - | - | - | - | - |
| Buja et al., 2020 [33] | 3 | 2 | 2 | 2 | 2 | 2:1 | 1 |
| Bois et al., 2021 [34] | 15 | 15 | - | - | - | - | - |
Table 3.
Anamnesis findings of patient comorbidities. COPD indicates chronic obstructive pulmonary disease.
| Number of Cases | Diabetes | Overweight | Hypertension | Previous Ischemic Disease |
Cardiomyopathy | Atrial Fibrillation | Heart Failure | Chronic Kidney Disease |
Cancer | Dementia | COPD | No Comorbidities |
|
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lindner et al., 2020 [15] | 39 | 17 | - | 17 | 17 | - | - | 1 | - | - | - | 0 | - |
| Fox et al., 2020 [16] | 22 | 22 | 11 | 9 | 18 | 1 | 9 | 2 | 2 | 4 | 0 | 0 | 0 |
| Jacobs et al., 2020 [17] | 1 | - | 1 | 1 | - | 1 | - | 0 | 0 | 0 | 0 | 0 | 0 |
| Pellegrini et al., 2020 [19] | 40 | 11 | 40 | 29 | 8 | 0 | 3 | 0 | 7 | 5 | 0 | 0 | 0 |
| Gauchotte et al., 2021 [20] | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 |
| Bulfamante et al., 2020 [21] | 6 | 0 | 0 | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Del Nonno et al., 2020 [22] | 9 | - | - | - | - | 2 | 0 | 6 | 0 | 1 | 1 | 0 | 3 |
| Basso et al., 2020 [23] | 21 | 7 | - | 16 | 3 | 7 | 4 | 7 | 2 | 4 | - | 1 | 0 |
| Hanley et al., 2020 [24] | 10 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 4 |
| Rapkiewicz et al., 2020 [25] | 7 | 5 | 5 | 6 | 1 | 1 | 0 | 0 | 1 | 2 | 0 | 1 | 1 |
| Cîrstea et al., 2020 [26] | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Bradley et al., 2020 [27] | 14 | 5 | 4 | 6 | 4 | 13 | 8 | 8 | 5 | 2 | 2 | 3 | 0 |
| Duarte-Neto et al., 2020 [30] | 10 | 5 | - | 5 | 4 | 5 | - | - | 1 | 1 | - | 3 | 0 |
| Schaller et al., 2020 [32] | 10 | 2 | 2 | 7 | 1 | 3 | - | - | 3 | 3 | 1 | 2 | 0 |
| Buja et al., 2020 [33] | 3 | 1 | 3 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 |
| Bois et al., 2021 [34] | 15 | - | - | - | - | - | - | - | - | - | - | - | 3 |
| Total | 209 | 76 | 66 | 103 | 58 | 36 | 26 | 25 | 22 | 22 | 4 | 14 | 15 |
Figure 2.
Comorbidities’ frequency in the subjects described in the articles included in this review (total n = 209). COPD indicates chronic obstructive pulmonary disease.
4. Discussion
Post-mortem studies on COVID-19 deaths revealed various organs alterations. The lungs were extensively described. They appeared edematous and their weight was generally increased. Microscopically, the main characteristic was exudative and proliferative DAD, less frequently with fibrotic features [13]. The other organs manifested unspecific findings, often related to lymphocyte infiltration, small vessels inflammation, and microthrombi [13]. Concerning heart involvement, it may be interesting to take into consideration the alterations caused by other human coronaviruses. Myocardial damage in SARS-CoV infection consists of edema in both the myocardial stroma and vascular walls, in addition to atrophy of the cardiac muscle fibers [35]. In Middle East respiratory syndrome (MERS), the other well-known betacoronavirus disease, cardiac involvement is secondary to lung inflammation that occurs in bronchiolitis obliterans organizing pneumonia [36]. In contrast, in this study, we found substantial cardiac involvement in SARS-CoV-2 infections.
In this systematic review, we evaluated the cardiac features of 209 hearts, the majority of which were not analyzed with immunostaining.
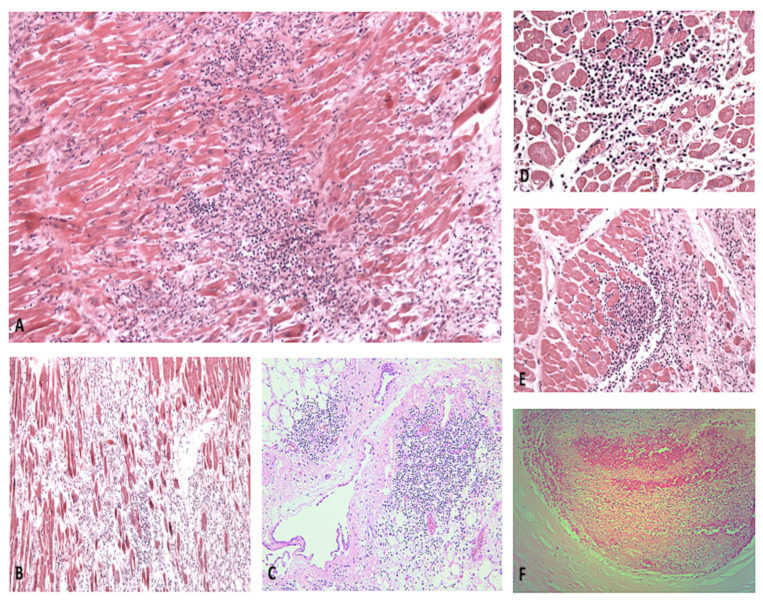
According to our results, most patients died of respiratory failure, and less than 10% died of cardiac failure. One of the main features was right heart dilatation due to pulmonary overload. Recurrent microscopic findings included necrosis, lymphocytic infiltration of the myocardium, and microthrombosis of the small cardiac vessels (Figure 3), as it was described in other organs [13].
Figure 3.
(A,B) Myocarditis with extensive myocyte necrosis, at different developmental stages (H&E, 60× and 20×, respectively). (C) Massive lymphocytic infiltration of the pericardium and perivascular area (H&E, 40×). Foci of acute myocyte necrosis (D) and regions undergoing tissue repair (E) (H&E, 100× and 80×, respectively). (F) Acute coronary thrombosis (H&E, 200×).
When histologic infiltrates were studied, they revealed a large CD3+ and CD8+ cytotoxic lymphocytic infiltration and the presence of CD68+ macrophages (Figure 4).
Figure 4.
Myocarditis: immunohistochemistry demonstrating CD68 macrophage population (A) and CD8 expression (B).
Cellular immunity has been demonstrated to play a fundamental role in COVID-19 host response, so the relative abundance of CD3 + cells in the tissues is not surprising [37]. In our results, the ratio of CD4 to CD8 was generally less than 1, as it happens in other viral infections [38]. On the contrary, other studies performed on COVID-19 patients showed that the CD4:CD8 ratio was not reduced, whereas a reduction of the total number of both CD4+ and CD8+ lymphocytes in the blood was observed [7,39]. It may depend on the different localization of CD8+ T cells, which may be mainly located within the tissues showing a higher positivity at immunohistochemical staining. It must be taken into account also that in some studies the CD4:CD8 ratio was increased and correlated with the severity of the disease [40,41]. Various hypotheses may be advanced to explain these differences among the studies. At first, the CD4:CD8 ratio may depend on the different stages of the disease, with a progressive decrease in CD8+ T cells. For sure, more studies are needed to deepen the immunological pattern in COVID-19 patients and our review does not include enough cases to allow further speculations.
Concerning the pathophysiology of the cardiac involvement, we hypothesize both direct organ damage mediated by SARS-CoV-2 and an indirect insult due to the subsequent inflammatory response. The inflammatory cascade leads to multiple proinflammatory agents, such as cytokines and CD8 cytotoxic cells, that induce tissue damage [42]. Halushka and Vander Heide described a low incidence of histopathologic myocarditis interpreting cardiac symptoms in COVID-19 patients as consequences of other stressors such as endothelial cell activation, cytokine storms, or electrolyte imbalances [43]. According to the current model of SARS-CoV-2 tropism for the ACE2 receptor, the primary cause of COVID-19–related death is respiratory failure due to atypical severe ARDS [7,44]. ACE2 is expressed also in the heart, and it also may be the target of SARS-CoV-2 in this organ, inducing cardiac injury [45,46,47]. Regarding the cardiac alterations in clinical studies, many Authors described troponin levels elevation and echocardiographic abnormalities [45,48]. It is not clear if these findings should be interpreted as direct myocardial damage since, as our results also show, usually COVID-19 patients have cardiovascular comorbidities that may complicate the clinical picture [49]. Lassen et al., performed a prospective multicentre cohort study, collecting heart ultrasound data from 214 hospitalized COVID-19 patients. They found that the left and right ventricular systolic function was significantly decreased, even correcting their results for previous cardiac comorbidities [50]. However, echocardiography remains a crucial investigation in monitoring COVID-19 patients and detecting cardiac involvement [51].
Postmortem findings, in particular histological and immunohistochemical analysis, are fundamental to better understand the pathophysiological mechanism of cardiac involvement in COVID-19, and consequently to develop future disease management strategies [52,53,54].
5. Conclusions
Currently, there is minimal standardized and validated literature available about the cardiac implications of COVID-19. Our study results suggest a high rate of cardiac involvement in such cases. In the future, COVID-19 outcomes should be further studied to better individualize therapeutic strategies. Postmortem examination and histologic and immunohistochemical analysis of patients who died of COVID-19 are pivotal to the development of future methods of disease management and to provide useful tools with the goal of controlling viral spread in a pandemic.
As our results demonstrated, cardiac inflammation, mostly represented by lymphocytic infiltration and perivascular aggregates, is not an unusual finding in COVID-19 deaths. Besides, further studies are needed to disclose the possible therapeutic applications of these findings. The prevention or attenuation of the cytokine storm and endothelial cell activation may be helpful to mitigate cardiac involvement [55]. Indeed, a better understanding of cardiac involvement pathophysiology and the pathological pattern is needed and autoptic studies on COVID-19 deaths should be further promoted [13,56].
Further studies, specifically performed on forensic casuistry, are needed for the thorough evaluation of the etiology of death of patients infected with COVID-19 and for the identification of useful pathognomonic signs of cardiac involvement.
Author Contributions
Conceptualization, A.M. and V.F.; methodology, P.F.; validation, E.T.; formal analysis, M.D.P. and R.L.R.; investigation, A.C.M.; writing—original draft preparation, F.D.D. and P.S.; writing—review and editing, A.M. and A.C.M.; supervision, V.F.; funding acquisition, E.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization WHO Characterizes COVID-19 as a Pandemic. 2020. [(accessed on 14 August 2021)]. Available online: https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020.
- 2.Wang H., Li X., Li T., Zhang S., Wang L., Wu X., Liu J. The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:1629–1635. doi: 10.1007/s10096-020-03899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Z., Zhang Z., Chen W., Cai Z., Ge X., Zhu H., Jiang T., Tan W., Peng Y. Predicting the receptor-binding domain usage of the coronavirus based on kmer frequency on spike protein. Infect. Genet. Evol. 2018;61:183–184. doi: 10.1016/j.meegid.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y., Zhou Y., Liu S., Kou Z., Li W., Farzan M., Jiang S. Receptor-binding domain of SARS-CoV spike protein induces highly potent neutralizing antibodies: Implication for developing subunit vaccine. Biochem. Biophys. Res. Commun. 2004;324:773–781. doi: 10.1016/j.bbrc.2004.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Doremalen N., Bushmaker T., Morris D.H., Holbrook M.G., Gamble A., Williamson B.N., Tamin A., Harcourt J.L., Thornburg N.J., Gerber S.I., et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gandhi R.T., Lynch J.B., Del Rio C. Mild or Moderate Covid-19. N. Engl. J. Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 7.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiese A., Passaro G., Matteis A., Fazio V., Raffaele R., Paolo M.D. Thromboinflammatory response in SARS-CoV-2 sepsis. Med.-Leg. J. 2020;88:78–80. doi: 10.1177/0025817220926915. [DOI] [PubMed] [Google Scholar]
- 9.Maccio U., Zinkernagel A.S., Shambat S.M., Zeng X., Cathomas G., Ruschitzka F., Schuepbach R.A., Moch H., Varga Z. SARS-CoV-2 leads to a small vessel endotheliitis in the heart. EBioMedicine. 2021;63:103182. doi: 10.1016/j.ebiom.2020.103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F., Gong W., Liu X., Liang J., Zhao Q., et al. Association of Cardiac Injury with Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T., Wang H., Wan J., Wang X., Lu Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendel Garcia P.D., Fumeaux T., Guerci P., Heuberger D.M., Montomoli J., Roche-Campo F., Schuepbach R.A., Hilty M.P., RISC-19-ICU Investigators Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maiese A., Manetti A.C., La Russa R., Di Paolo M., Turillazzi E., Frati P., Fineschi V. Autopsy findings in COVID-19-related deaths: A literature review. Forensic. Sci. Med. Pathol. 2020;7:279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., GÃtzsche P.C., Ioannidis J.P.A., Clarke M., Devereaux P.J., Kleijnen J., Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindner D., Fitzek A., Bräuninger H., Aleshcheva G., Edler C., Meissner K., Scherschel K., Kirchhof P., Escher F., Schultheiss H.P., et al. Association of Cardiac Infection With SARS-CoV-2 in Confirmed COVID-19 Autopsy Cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fox S.E., Li G., Akmatbekov A., Harbert J.L., Lameira F.S., Brown J.Q., Vander Heide R.S. Unexpected Features of Cardiac Pathology in COVID-19 Infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 17.Jacobs W., Lammens M., Kerckhofs A., Voets E., Van San E., Van Coillie S., Peleman C., Mergeay M., Sirimsi S., Matheeussen V., et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): Autopsy reveals a ferroptosis signature. ESC Heart Fail. 2020;7:3772–3781. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aretz H.T., Billingham M.E., Edwards W.D., Factor S.M., Fallon J.T., Fenoglio J.J., Olsen E.G., Jr., Schoen F.J. Myocarditis. A histopathologic definition and classification. Am. J. Cardiovasc. Pathol. 1987;1:3–14. [PubMed] [Google Scholar]
- 19.Pellegrini D., Kawakami R., Guagliumi G., Sakamoto A., Kawai K., Gianatti A., Nasr A., Kutys R., Guo L., Cornelissen A., et al. Microthrombi as a major cause of cardiac injury in COVID-19: A pathologic study. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 20.Gauchotte G., Venard V., Segondy M., Cadoz C., Esposito-Fava A., Barraud D., Louis G. SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study. Int. J. Legal. Med. 2021;135:577–581. doi: 10.1007/s00414-020-02500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bulfamante G.P., Perrucci G.L., Falleni M., Sommariva E., Tosi D., Martinelli C., Songia P., Poggio P., Carugo S., Pompilio G. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. medRxiv. 2020;8:626. doi: 10.1101/2020.08.24.20170175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Del Nonno F., Frustaci A., Verardo R., Chimenti C., Nicastri E., Antinori A., Petrosillo N., Lalle E., Agrati C., Ippolito G., et al. Virus-Negative Myopericarditis in Human Coronavirus Infection: Report from an Autopsy Series. Circ. Heart Fail. 2020;13:e007636. doi: 10.1161/CIRCHEARTFAILURE.120.007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Basso C., Leone O., Rizzo S., De Gaspari M., van der Wal A.C., Aubry M.C., Bois M.C., Lin P.T., Maleszewski J.J., Stone J.R. Pathological features of COVID-19-associated myocardial injury: A multicentre cardiovascular pathology study. Eur. Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hanley B., Naresh K.N., Roufosse C., Nicholson A.G., Weir J., Cooke G.S., Thursz M., Manousou P., Corbett R., Goldin R., et al. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: A post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rapkiewicz A.V., Mai X., Carsons S.E., Pittaluga S., Kleiner D.E., Berger J.S., Thomas S., Adler N.M., Charytan D.M., Gasmi B., et al. Megakaryocytes and platelet-fibrin thrombi characterize multi-organ thrombosis at autopsy in COVID-19: A case series. EClinicalMedicine. 2020;24:100434. doi: 10.1016/j.eclinm.2020.100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cîrstea A.E., Buzulică R.L., Pirici D., Ceauşu M.C., Iman R.V., Gheorghe O.M., Neamţu S.D., Stanca L., Ene R., Kumar-Singh S., et al. Histopathological findings in the advanced natural evolution of the SARS-CoV-2 infection. Rom. J. Morphol. Embryol. 2020;61:209–218. doi: 10.47162/RJME.61.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bradley B.T., Maioli H., Johnston R., Chaudhry I., Fink S.L., Xu H., Najafian B., Deutsch G., Lacy J.M., Williams T., et al. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: A case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gautret P., Million M., Jarrot P.A., Camoin-Jau L., Colson P., Fenollar F., Leone M., La Scola B., Devaux C., Gaubert J.Y., et al. Natural history of COVID-19 and therapeutic options. Expert Rev. Clin. Immunol. 2020;16:1159–1184. doi: 10.1080/1744666X.2021.1847640. [DOI] [PubMed] [Google Scholar]
- 30.Duarte-Neto A.N., Monteiro R.A.A., da Silva L.F.F., Malheiros D.M.A.C., de Oliveira E.P., Theodoro-Filho J., Pinho J., Gomes-Gouvêa M.S., Salles A., de Oliveira I., et al. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186–197. doi: 10.1111/his.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D’Onofrio V., Donders E., Vanden Abeele M.E., Dubois J., Cartuyvels R., Achten R., Lammens M., Dendooven A., Driessen A., Augsburg L., et al. The clinical value of minimal invasive autopsy in COVID-19 patients. PLoS ONE. 2020;15:e0242300. doi: 10.1371/journal.pone.0242300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller T., Hirschbühl K., Burkhardt K., Braun G., Trepel M., Märkl B., Claus R. Postmortem Examination of Patients With COVID-19. JAMA. 2020;323:2518–2520. doi: 10.1001/jama.2020.8907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buja L.M., Wolf D.A., Zhao B., Akkanti B., McDonald M., Lelenwa L., Reilly N., Ottaviani G., Elghetany M.T., Trujillo D.O., et al. The emerging spectrum of cardiopulmonary pathology of the coronavirus disease 2019 (COVID-19): Report of 3 autopsies from Houston, Texas, and review of autopsy findings from other United States cities. Cardiovasc. Pathol. 2020;48:107233. doi: 10.1016/j.carpath.2020.107233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bois M.C., Boire N.A., Layman A.J., Aubry M.C., Alexander M.P., Roden A.C., Hagen C.E., Quinton R.A., Larsen C., Erben Y., et al. COVID-19-Associated Nonocclusive Fibrin Microthrombi in the Heart. Circulation. 2021;143:230–243. doi: 10.1161/CIRCULATIONAHA.120.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gu J., Korteweg C. Pathology and pathogenesis of severe acute respiratory syndrome. Am. J. Pathol. 2007;170:1136–1147. doi: 10.2353/ajpath.2007.061088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van den Brand J.M., Smits S.L., Haagmans B.L. Pathogenesis of Middle East respiratory syndrome coronavirus. J. Pathol. 2015;235:175–184. doi: 10.1002/path.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganji A., Farahani I., Khansarinejad B., Ghazavi A., Mosayebi G. Increased expression of CD8 marker on T-cells in COVID-19 patients. Blood Cells Mol. Dis. 2020;83:102437. doi: 10.1016/j.bcmd.2020.102437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McBride J.A., Striker R. Imbalance in the game of T cells: What can the CD4/CD8 T-cell ratio tell us about HIV and health? PLoS Pathog. 2017;13:e1006624. doi: 10.1371/journal.ppat.1006624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallotto C., Suardi L.R., Esperti S., Tarquini R., Grifoni E., Meini S., Valoriani A., Di Martino S., Cei F., Sisti E., et al. Increased CD4/CD8 ratio as a risk factor for critical illness in coronavirus disease 2019 (COVID-19): A retrospective multicentre study. Infect. Dis. 2020;52:675–677. doi: 10.1080/23744235.2020.1778178. [DOI] [PubMed] [Google Scholar]
- 41.Wang F., Nie J., Wang H., Zhao Q., Xiong Y., Deng L., Song S., Ma Z., Mo P., Zhang Y. Characteristics of Peripheral Lymphocyte Subset Alteration in COVID-19 Pneumonia. J. Infect. Dis. 2020;221:1762–1769. doi: 10.1093/infdis/jiaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wenzel P., Kopp S., Göbel S., Jansen T., Geyer M., Hahn F., Kreitner K.F., Escher F., Schultheiss H.P., Münzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc. Res. 2020;116:1661–1663. doi: 10.1093/cvr/cvaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halushka M.K., Vander Heide R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021;50:107300. doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanza C., Racca F., Longhitano Y., Piccioni A., Franceschi F., Artico M., Abenavoli L., Maiese A., Passaro G., Volonnino G., et al. Risk Management and Treatment of Coagulation Disorders Related to COVID-19 Infection. Int. J. Environ. Res. Public Health. 2021;18:1268. doi: 10.3390/ijerph18031268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ng A.C.T., Delgado V., Bax J.J. An international, multicentre survey of echocardiographic abnormalities in COVID-19 patients. Eur. Heart J. Cardiovasc. Imaging. 2020;21:959–960. doi: 10.1093/ehjci/jeaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turner A.J., Hiscox J.A., Hooper N.M. ACE2: From vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:294–297. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahmoud-Elsayed H.M., Moody W.E., Bradlow W.M., Khan-Kheil A.M., Senior J., Hudsmith L.E., Steeds R.P. Echocardiographic Findings in Patients With COVID-19 Pneumonia. Can. J. Cardiol. 2020;36:1203–1207. doi: 10.1016/j.cjca.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deng Q., Hu B., Zhang Y., Wang H., Zhou X., Hu W., Cheng Y., Yan J., Ping H., Zhou Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020;311:116–121. doi: 10.1016/j.ijcard.2020.03.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lassen M.C.H., Skaarup K.G., Lind J.N., Alhakak A.S., Sengeløv M., Nielsen A.B., Espersen C., Ravnkilde K., Hauser R., Schöps L.B., et al. Echocardiographic abnormalities and predictors of mortality in hospitalized COVID-19 patients: The ECHOVID-19 study. ESC Heart Fail. 2020;7:4189–4197. doi: 10.1002/ehf2.13044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cau R., Bassareo P., Saba L. Cardiac Involvement in COVID-19-Assessment with Echocardiography and Cardiac Magnetic Resonance Imaging. SN Compr. Clin. Med. 2020;2:845–851. doi: 10.1007/s42399-020-00344-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maiese A., Manetti A.C., Bosetti C., Del Duca F., La Russa R., Frati P., Di Paolo M., Turillazzi E., Fineschi V. SARS-CoV-2 and the brain: A review of the current knowledge on neuropathology in COVID-19. Brain Pathol. 2021;13:e13013. doi: 10.1111/bpa.13013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofman P., Copin M.C., Tauziede-Espariat A., Adle-Biassette H., Fortarezza F., Passeron T., Salmon I., Calabrese F. Les lésions histologiques associées à l’infection par le SARS-CoV-2 [Histopathological features due to the SARS-CoV-2] Ann. Pathol. 2021;41:9–22. doi: 10.1016/j.annpat.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Santurro A., Scopetti M., D’Errico S., Fineschi V. A technical report from the Italian SARS-CoV-2 outbreak. Postmortem sampling and autopsy investigation in cases of suspected or probable COVID-19. Forensic Sci. Med. Pathol. 2020;16:471–476. doi: 10.1007/s12024-020-00258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li A., Garcia-Bengochea Y., Stechel R., Azari B.M. Management of COVID-19 myopericarditis with reversal of cardiac dysfunction after blunting of cytokine storm: A case report. Eur. Heart J. Case Rep. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frisoni P., Neri M., D’Errico S., Alfieri L., Bonuccelli D., Cingolani M., Di Paolo M., Gaudio R.M., Lestani M., Marti M., et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: From viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci. Med. Pathol. 2021:1–15. doi: 10.1007/s12024-021-00414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.