Abstract
Despite the progressive advances, current standards of treatments for peripheral nerve injury do not guarantee complete recovery. Thus, alternative therapeutic interventions should be considered. Complementary and alternative medicines (CAMs) are widely explored for their therapeutic value, but their potential use in peripheral nerve regeneration is underappreciated. The present systematic review, designed according to guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols, aims to present and discuss the current literature on the neuroregenerative potential of CAMs, focusing on plants or herbs, mushrooms, decoctions, and their respective natural products. The available literature on CAMs associated with peripheral nerve regeneration published up to 2020 were retrieved from PubMed, Scopus, and Web of Science. According to current literature, the neuroregenerative potential of Achyranthes bidentata, Astragalus membranaceus, Curcuma longa, Panax ginseng, and Hericium erinaceus are the most widely studied. Various CAMs enhanced proliferation and migration of Schwann cells in vitro, primarily through activation of MAPK pathway and FGF-2 signaling, respectively. Animal studies demonstrated the ability of CAMs to promote peripheral nerve regeneration and functional recovery, which are partially associated with modulations of neurotrophic factors, pro-inflammatory cytokines, and anti-apoptotic signaling. This systematic review provides evidence for the potential use of CAMs in the management of peripheral nerve injury.
Keywords: complementary and alternative medicines, natural products, peripheral nerve injury, nerve repair, nerve regeneration, functional recovery
1. Introduction
Peripheral nerve injury (PNI) can result in partial or total loss of motor, sensory and autonomic functions at denervated regions, leading to temporary or life-long disability [1]. In addition to reduced quality of life, functional deficits from PNI have a substantial economic impact on the affected individuals [2]. A recent study found that, over nine years (from 2009 to 2018), more than 550,000 individuals were afflicted by PNI in the United States. Moreover, the incidence rate has more than doubled throughout that period of time [3]. Such injuries are primarily due to vehicular and traumatic accidents, lacerations, and iatrogenic causes [4,5,6].
Despite progressive advances in our understanding of the processes and mechanisms of nerve injury, effective nerve repair and regeneration approaches that ensure complete functional recovery remain scarce [7]. Nerve autograft is considered the gold standard for repairing peripheral nerve defects [8]. However, this method is restricted by limited donor nerves and donor site morbidity, while successful recovery rates remain unsatisfactory [9]. Consequently, alternative strategies for enhancing nerve repairs have been proposed, including the application of nerve conduits and the addition of growth factors [10,11]. Likewise, the exploration of novel therapeutics, even combinatorial therapies, capable of enhancing axonal regeneration and promoting functional recovery, are of great interest.
PNI often results in neuropathic pain, and when conventional treatments are inadequate in providing relief, patients may turn to complementary and alternative medicines (CAMs), such as herbal medicines and nutritional supplements [12]. Indeed, medicinal plants, including the Acorus calamus [13], Curcuma longa [14], and Ginkgo biloba [15], have displayed ameliorating effects in animal models of neuropathic pain. Research on the potential of medicinal plants in the treatment of PNI is prompted by the notion that plants are great sources of natural products (NPs), which are small molecules produced by living organisms. Many NPs are the focus of drug development, as it is generally believed that they are largely devoid of adverse effects compared to synthetic drugs [16,17]. NPs also have the advantage of being evolutionary-driven, thus they are more likely to possess tremendous chemical and structural diversity that facilitates efficient engagement with biologically relevant targets and receptors, making them more biologically active [18]. In fact, many small-molecule drugs that have been approved by regulatory agencies were derived from natural sources [19], including Taxol from Taxus brevifolia [20] and Vinblastine from Catharanthus roseus [21].
However, compared to the extensive research on naturally derived products for other non-communicable and infectious diseases, NPs remain largely unexplored in the field of nerve repair and regeneration. A review published nearly half a decade ago has shed light on the neuroprotective effects of NPs in PNI models [22]. This review presents current research findings and evaluates the role of CAMs, focusing on plants or herbs, mushrooms, and decoctions, as well as their NPs, in peripheral nerve regeneration, to highlight their therapeutic potential for the management of PNI.
2. Materials and Methods
This systematic review was designed according to guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) [23].
2.1. Search Strategy and Data Extraction
A literature search was performed to find all relevant publications up to 25 October 2020 across three electronic databases, PubMed, Scopus, and Web of Science. The following keywords were used to search each respective database: ((“peripheral* nerve* regenera*” OR “peripheral* nerve* repair*” OR “neuroregenera*”) AND (“alga*” OR “seaweed*” OR “plant” OR “natural product*” OR “mushroom” OR “Basidiomycete*” OR “herb*” OR “Traditional Chinese Medicine*” OR “alternative medicine” OR “complementary medicine*”)).
2.2. Eligibility Criteria
Research articles describing the use of plants or herbs, mushrooms, algae, decoction, and their natural products in peripheral nerve repair and regeneration, written in English, and having full-text availability were considered. Articles not representing original research studies and NPs derived from sources other than plants, herbs, algae, and mushrooms were excluded (e.g., Lumbricus rubellus—earthworm). Retrieved articles were screened based on their title, abstract, and full-text to determine their eligibility for inclusion in this review.
3. Results
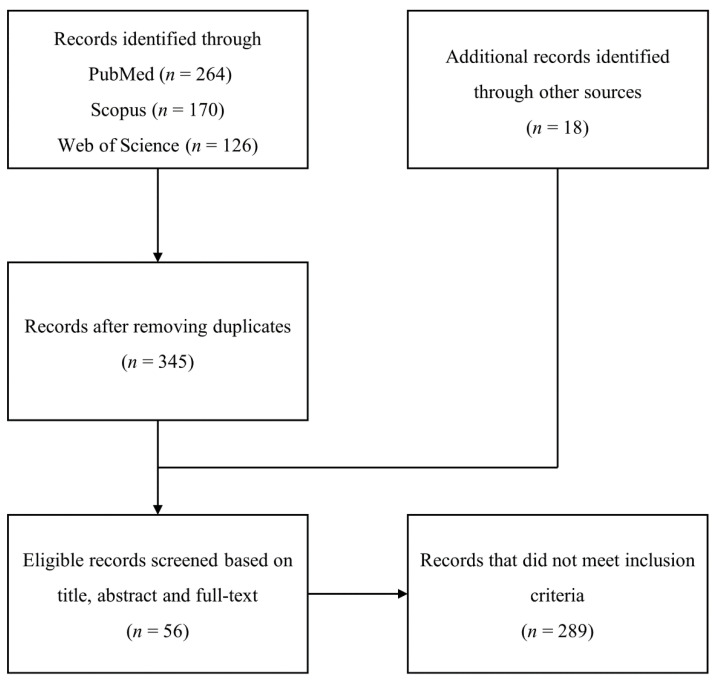
A preliminary search across the three databases yielded 560 records, of which 215 were duplicates (Figure 1). Together with 18 other records identified by other means, the remaining articles were screened based on the eligibility criteria, resulting in 289 additional records being excluded, leaving 56 records remaining and their findings being included in the qualitative synthesis (Figure 1). The studies investigated the neuroregenerative potential of 25 species of plants, three different mushrooms, and four traditional Chinese medicine decoctions, of which 18 known NPs were characterized. None of the studies investigated the potential of algae in peripheral nerve regeneration.
Figure 1.
Flow diagram of the literature search procedure for the selection of studies up to 25 October 2020 on the use of plants, mushrooms, algae, decoctions, and their natural products (NPs) in peripheral nerve repair and regeneration. Only articles written in English, and having full-text availability were included. Articles not representing original research studies and NPs derived from sources other than plants, herbs, algae, and mushrooms were excluded.
Among the 58 records, the majority of the reported findings were from in vivo studies (38 records) that used mainly histological and electrophysiological evaluation to examine peripheral nerve regeneration in rat models of sciatic nerve injury (SNI). In contrast, 11 records were in vitro studies, which included reports of the promoting effects of plants, mushrooms, decoctions, and their natural products on the proliferation and migration of Schwann cells (SCs), and on neurite outgrowth in dorsal root ganglion (DRG) explants and neurons. Additionally, nine records included both in vitro and in vivo studies. In terms of the mechanisms of the biological effects, regulation of the mitogen-activated protein kinase (MAPK) pathway was reported to be highly involved across these studies.
4. Discussion
4.1. Current Therapeutic Approaches against Peripheral Nerve Injuries
Peripheral nerves are prone to injury because of their delicate structures and superficial location throughout the human body. The prevalence of PNI together with its societal impact poses a health concern that needs to be addressed properly. Current treatment strategies for PNI are divided into surgical and non-surgical approaches that can be effective when applied appropriately [24]. Surgical techniques, including suturing of severed nerves and nerve grafting, do yield successful outcomes but are sometimes not feasible due to limitations such as the timing of surgery, size of nerve gaps, and donor site morbidity [25,26]. Consequently, other promising alternatives have emerged in recent years and have been receiving increasing attention, such as the utilization of different nerve conduits capable of housing and delivering biological cues whilst enhancing and guiding nerve regeneration 11, growth factor treatments [27], and cell-based therapies [28]. In contrast, non-surgical options for the management of PNI are far more limited, including approved medications on the market, electrical nerve stimulation [29], and the application of phytochemicals and secondary metabolites. The latter is widespread in other areas of research including cancer [30] and neurological disorders [31], but are far less prevalent in the field of peripheral nerve regeneration.
4.2. Mechanisms of Peripheral Nerve Injury and Regeneration
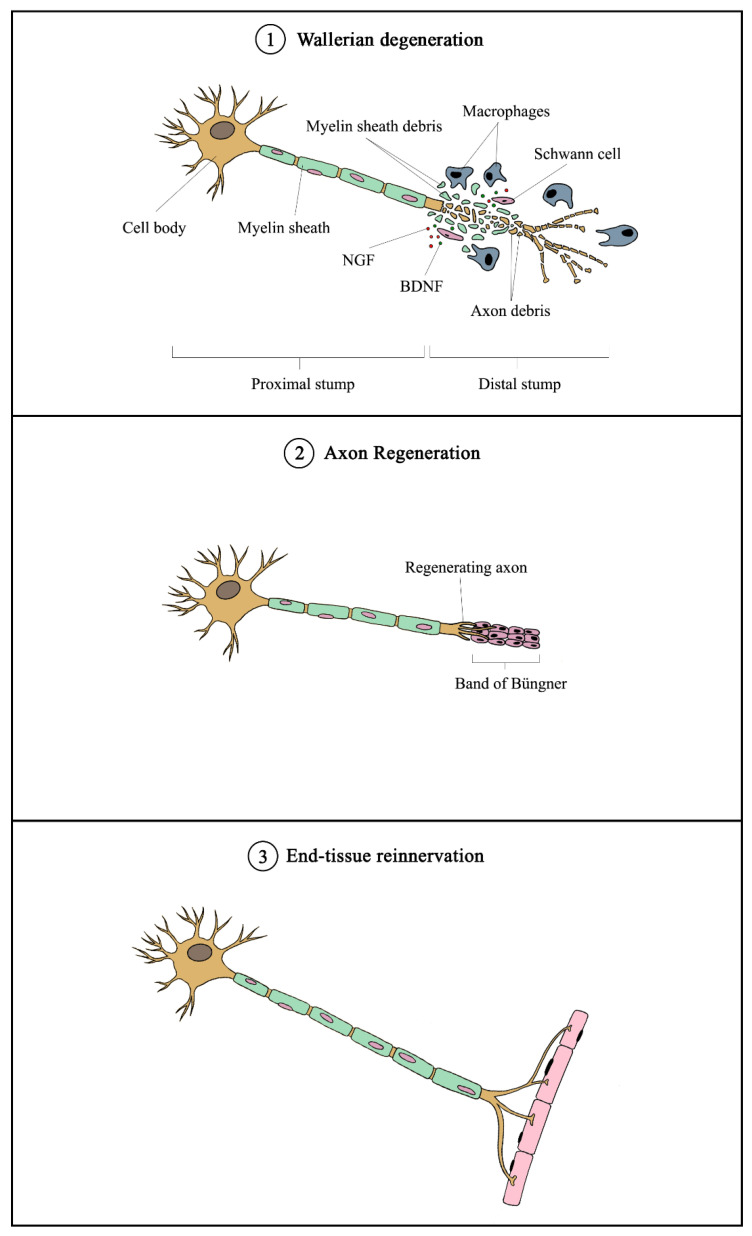
Nerve bundles are primarily composed of axons covered with myelin sheaths produced by Schwann cells with fibroblasts scattered in between the nerve fibers. During peripheral nerve injury, instantaneous tissue damage occurs at the site of the lesion together with the accumulation of galectin-3 macrophages, whereas nerve stumps that are distally located undergo cellular variation despite not being directly affected [32]. After an axonal injury, Wallerian degeneration occurs, followed by axonal regeneration, and eventually end-organ reinnervation (see Figure 2) [33]. Wallerian degeneration takes place 24 to 48 h following nerve injury. Axons begin to disintegrate and growth factors such as nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF) are released by SCs in the segment distal to the injured site. Galectin-3 macrophages are then recruited to the distal end, which contributes to myelin degradation and removal of remaining debris [34]. Growth factors are also retrogradely transported proximally toward the cell body. Subsequent removal of deteriorated myelin and axonal matter leads to the proliferation and alignment of SCs, forming the bands of Büngner that further guide the regenerating axons from the proximal to the distal site [35]. Axonal regeneration in humans is known to occur at a rate of approximately 1 mm per day [36], which would require months or even years for severe nerve injuries to fully recover. Moreover, poor functional recovery can occur due to a number of reasons, including progressive failure of axonal regeneration, disruption of SC function in providing a growth-supportive environment, and misdirection of regenerating axons [36].
Figure 2.
Overview of mechanism of peripheral nerve injury and regeneration. Following nerve injury, Wallerian degeneration occurs, in which axons begin to disintegrate at the distal end, and growth factors (such as NGF and BDNF) are released by Schwann cells. Galectin-3 macrophages are recruited to remove axonal debris and degrade myelin sheaths. Subsequently, SCs align to form the Band of Büngner, which guides the regenerating axons from the proximal to distal sites. Eventually, the regenerated axons innervate the end tissue to complete the recovery process. NGF—nerve growth factor; BDNF—brain-derived neurotrophic factor.
4.3. Role of Schwann Cells in Nerve Regeneration
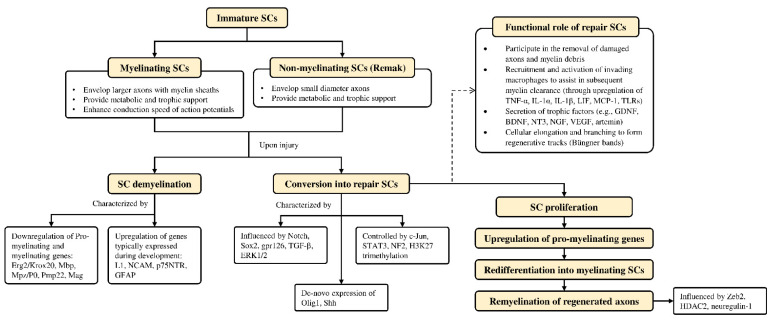
Schwann cells are supportive glial cells that are known to play a pivotal role in the proper functioning and maintenance of peripheral nerves. They are responsible for producing the basal lamina that determines the polarity of SCs and myelinating axons [37]. The myelin sheaths on axons allow the conduction of action potentials at high velocity via the formation of specialized nodes of Ranvier [38]. The high plasticity of SCs allows them to further develop into repair phenotypes in response to nerve injury (Figure 3). Following nerve injury, SCs can re-differentiate into repair SCs that align themselves to form bands of Büngner. This in turn allows axons to emerge from growth cones proximal to the injured site, which then elongate along the bands until the target organ is reinnervated. The repair SCs also participate in the removal of axon and myelin debris, and they can recruit macrophages to assist in the process [39]. In addition, repair SCs can also secrete neurotrophic factors that help promote cellular survival, proliferation, and differentiation, which are all essential for peripheral nerve repair [40]. Due to the importance of SCs in promoting peripheral nerve regeneration, it is expected that any disruption in SC proliferation, such as that caused by impairment in cyclin D1, will affect nerve regeneration following injury [41]. However, findings from past studies suggest that axonal regeneration is independent of SC proliferation [42,43]. Nevertheless, considering the association of SCs with axonal elongation and myelination, it is reasonable to hypothesize that enhanced SC proliferation may lead to greater regenerative potential. Hence, numerous studies have attempted to investigate the effects of NPs in promoting the proliferation and migration ability of SCs (Table 1).
Figure 3.
Overview of Schwann cell plasticity and their roles following peripheral nerve injury. Immature SCs develop into either myelinated or non-myelinated forms depending on the type of axon association. Upon nerve injury, SCs are capable of converting into a repair phenotype alongside the demyelination process that is mediated by different genes and transcriptional mechanisms. These events promote neuronal survival and enhance axonal regeneration following injury. Subsequently, repair SCs can be reprogrammed back to remyelinate regenerated axons. Further details on SC plasticity are presented in the reviews by Jessen & Mirsky [39] and Nocera & Jacob [100]. BDNF—brain-derived neurotrophic factor; Erg2/Krox20—early growth response 2; ERK—extracellular signal-regulated protein kinase; GDNF—glial cell-derived neurotrophic factor; GFAP—glial fibrillary acidic protein; gpr126—adhesion G protein-coupled receptor G6; H3K27—methylation of histone H3 on lysine 27; HDAC2—histone deacetylase 2; IL—interleukin; L1—L1 cell adhesion molecule; LIF—leukemia inhibitory factor; Mag—myelin associated glycoprotein; Mbp—myelin basic protein; MCP-1—monocyte chemotactic protein 1; Mpz/P0—myelin protein zero; NCAM—neural cell adhesion molecule; NF2—neurofibromatosis 2; NGF—nerve growth factor; NT3—neurotrophin-3; Olig1—oligodendrocyte transcription factor 1; p75NTR—p75 neurotrophin receptor; Pmp22—peripheral myelin protein 22; SCs—Schwann cells; Shh—Sonic Hedgehog; Sox2—(sex determining region Y)-box 2; STAT3—signal transducer and activator of transcription 3; TGF-β—transforming growth factor-β; TLRs—Toll-like receptors; TNF-α—tumor necrosis factor-α; VEGF—vascular endothelial growth factor; Zeb2—zinc finger E-box-binding homeobox 2.
Table 1.
Summary of plants, mushrooms, and decoctions their natural products relating to peripheral nerve regeneration.
| Source | Molecule(s)/Ingredients | Experimental Model | Effective Concentration | Application Method | Biological Effect | Mechanism | Reference |
|---|---|---|---|---|---|---|---|
| PLANT | |||||||
| Achyranthes bidentata | Polypeptides |
In vitro (SCs isolated from the sciatic nerves of 1-day old SD rats) |
0.1 µg/mL | Incubation | Promoted migration of SCs | Upregulation of NOX4/DUOX2-derived ROS production | [44] |
| Polypeptides |
In vitro
(DRG explants harvested from spinal and peripheral roots of postnatal day 1 SD rats) |
0.01, 0.1, 1 µg/mL (dose-dependent manner) | Incubation | Promoted neurite outgrowth from cultured DRG explants/neurons | Activation of ERK1/2 | [45] | |
|
In vivo (Adult New Zealand rabbits) |
6.0 mg/kg | Intravenous injection | Enhanced nerve regeneration and functional restoration after crush injury to rabbit common peroneal nerve (increased CMAP, density, diameter and thickness of myelinated fibers, and number of motor neurons in anterior horn) | N/A | |||
| Polypeptides (Fraction K) |
In vitro (DRG explants harvested from spinal and peripheral roots of postnatal day 1 SD rats) |
50, 250 ng/mL (dose-dependent manner) | Incubation | Promoted neurite outgrowth in DRG explant and neurons | Activation of ERK1/2 | [46] | |
|
In vivo (ICR mice) |
10 mg/kg | Intravenous injection | Promoted peripheral nerve regeneration in mice after SNI (increased diameter and thickness of myelinated fibers, CSA of gastrocnemius muscle fibers, SFI, and CMAP) | N/A | |||
| Polypeptides |
In vivo (SD rats) |
2 mg in 0.2 mL saline | Intraperitoneal injection | Promoted functional and histological recovery after rat sciatic nerve crush (increased SFI, CMAP, MNCV, myelin thickness, lamellae number, CSA of gastrocnemius muscle fibers) | Modulation of mRNA expression of GAP-43, neurotrophic factors (NGF, BDNF, CNTF), and neurotrophic factor receptors (TrkA, TrkB) | [47] | |
| Polypeptides |
In vivo (ICR mice) |
1, 4, 16 mg/kg (dose-independent manner) | Tail vein injection | Promoted functional and histological recovery after rat sciatic nerve crush (increased SFI, CMAP, MNCV, number, and diameter of myelinated fibers, axon diameter, myelin thickness, lamellae number, CSA of gastrocnemius muscle fibers) | N/A | [48] | |
| Aqueous extract |
In vivo (Adult New Zealand rabbits) |
10, 20 mg/kg (dose-dependent manner) | Intravenous injection | Promoted peripheral nerve regeneration in the crushed common peroneal nerve in rabbits (increased CMAP, CSA of tibialis posterior muscle, number of regenerated myelinated nerve fibers, and motoneurons in anterior horn of the spinal cord) | N/A | [49] | |
| Alpinate Oxyphyllae Fructus (Alpinia oxyphylla Miq) | Protocatechuic acid |
In vitro (RSC96 SCs) |
1 mM | Incubation | Promoted proliferation and survival of RSC96 SCs | Upregulation of IGF-1 and activation of PI3K/Akt signaling | [50] |
| Aqueous extract |
In vitro (RSC96 SCs) |
Proliferation: 20, 60, 200 µg/mL (dose-independent manner Migration: 20–200 µg/mL (dose-dependent manner |
Incubation | Promoted proliferation and migration of RSC96 SCs | Upregulation of PAs (uPA, tPA) and MMP2/9 mediated through the activation of MAPK pathway (ERK1/2, JNK, p38) | [51] | |
|
In vivo (SD rats) |
30, 60, 100, 150, 200 µg/mL (dose-independent manner) | Injection into a silicone rubber tube bridging a 15mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI | ||||
| Astragalus membranaceus | Astragaloside IV |
In vivo (BALB/c mice) |
2.5, 5, 10 mg/kg (dose-dependent manner) | Intraperitoneal injection | Promoted sciatic nerve regeneration and functional recovery in mice (increased number and diameter of myelinated nerve fibers, MNCV, CMAP) | Upregulation of GAP-43 expression | [52] |
| Astragaloside IV |
In vivo (SD rats) |
50 µM | Injection into a silicone rubber tube bridging a 15mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased number of myelinated axons and CMAP) | N/A | [53] | |
| Extract |
In vivo (SD rats) |
3 g/kg in 0.01 M of PBS | Intragastric gavage | Promoted peripheral nerve regeneration in rats with SNI (increased MNCV and latency, fluorogold labeling in the DRG, mean axonal density, percentage of CGRP area ratio, and macrophage density) | Modulation of local growth factors (FGF, NGF, PDGF, TGF-β) and immunoregulatory factors (IL-1, IFN-γ) | [54] | |
| Aqueous extract |
In vitro (RSC96 SCs) |
Proliferation: 12.5, 125, 250, 500 µg/mL (optimal at 12.5 µg/mL) Migration: 1.25, 12.5, 125, 250, 500 µg/mL (optimal at 1.25 µg/mL) |
Incubation | Promoted proliferation and migration of RSC96 SCs | Proliferation: Increased cyclin protein A, D1, and E via ERK and p38 signaling pathways Migration: Activation of FGF-2 signaling, leading to upregulation of uPA and downregulation of PAI-1 |
[55] | |
| Centella asiatica | Hydro-ethanolic extract |
In vivo (SD rats) |
400 µg/mL | Nerve conduit developed using decellularized artery seeded with C. asiatca-neurodifferentiated mesenchymal stem cells bridging a 15mm sciatic nerve defect | Promoted nerve regeneration and functional restoration in rats with SNI (increased CMAP, latency, MNCV, confirmation of angiogenesis, increased MBP expression, and number of myelinated axons) | N/A | [56] |
| Citrus medica var. sarcodactylis | Aqueous extract |
In vitro (RSC96 SCs) |
0.85, 1.7, 2.55, 3.4, 4.25 µg/mL (dose-dependent manner) | Incubation | Promoted proliferation and migration of RSC96 SCs | Proliferation: Upregulation of cyclin A and B1 Migration: Activation of FGF-2 signaling, leading to the upregulation of uPA and MMP-9 |
[57] |
| Codonopsis pilosula | Aqueous extract |
In vitro (RSC96 SCs) |
20, 40, 60, 80, 100 µg/mL (dose-independent manner) | Incubation | Promoted proliferation and migration of RSC96 SCs | Proliferation: Enhanced IGF-I signaling pathway, cell cycle controlling protein expressions (cyclin A, D1, E) and MAPK pathway (ERK, p38) Migration: Stimulated FGF-2-uPA-MMP9 migration pathway |
[58] |
| Crocus sativus | Crocin |
In vivo (Wistar rats) |
20, 80 mg/kg | Intraperitoneal injection | Promoted functional recovery in rats with SNI (Increased SFI, reduced plasma MDA levels, alleviated histological changes due to a crushing injury) | N/A | [59] |
| Curcuma longa | Alcoholic extract |
In vivo (Wistar rats) |
100 mg/kg (3, 6, or 9 times across 28 days) | Intraperitoneal injection | Protected against peripheral nerve degeneration in rats with SNI (Increased number of intact neurons in the right ventral horn of spinal cord region) | N/A | [60] |
| Curcumin |
In vivo (SD rats) |
100 mg/kg (dissolved in olive oil) | Oral gavage | Promoted peripheral nerve regeneration in rats with SNI (increased mean cell volume, total volume and surface of DRG cells, total number, diameter, and area of myelinated nerve fibers) | N/A | [61] | |
| Curcumin |
In vivo (SD rats) |
100 mg/kg (dissolved in olive oil) | Oral gavage | Promoted functional recovery (improved SFI) and protective effect on DRG (increased volume and number of A- and B- cells, number of satellite cells) in rats with SNI | N/A | [62] | |
| Curcumin |
In vivo (SD rats) |
50, 100, 300 mg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased number of motoneurons, number and diameter of myelinated axons, SFI, MNCV, amplitude of CMAP, muscle fiber area and reduced latency of CMAP, mechanical withdrawal threshold, thermal withdrawal latency) | N/A | [63] | |
| Curcumin |
In vitro (SCs isolated from S100β-DsRed transgenic mice) |
0.04-1 µM (0.1 µM having the highest proliferative effect) | Incubation | Promoted proliferation and migration of SCs | Proliferation: Modulated by ERK and p38 kinase pathways | [64] | |
| Curcuma longa (curcumin); from honeybees (propolis) | Curcumin, propolis |
In vivo (Wistar rats) |
Curcumin (100 mg/kg) Propolis (200 mg/kg) |
Administration through a nasogastric tube | Promoted functional recovery in rats with SNI (Increased SFI and amplitude of CMAP, reduced latency time) | N/A | [65] |
| Dioscoreae rhizoma | Aqueous extract |
In vivo (SD rats) |
10 mg/mL | Applied directly into the crush site | Promoted peripheral nerve regeneration in rats with SNI (increased number of DRG sensory neurons and motor neurons in the spinal cord) | Increasing protein levels of GAP-43 and Cdc2 | [66] |
| Epimedium | Icariin |
In vivo (SD rats) |
20 mg in 5 mL | Injection into a poly(lactic-co-glycolic acid) biological conduit sleeve bridging a 5mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased sciatic nerve conduction velocity and number of myelinated fibers) | N/A | [67] |
| Epimedium extract, icariin |
In vivo (SD rats) |
4.873 mg/mL | Intragastric administration | Promoted peripheral nerve regeneration in rats with SNI (Increased SFI, nerve regeneration based on nerve pinch test, MNCV, muscle wet weight) | N/A | [68] | |
| Gardenia jasminoides Ellis | Genipin |
In vivo (SD rats) |
3% aqueous gelatin solution fixed with 3% genipin | Injection into a silicone rubber tube bridging a 10mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI | N/A | [69] |
| Gastrodia elata Blume | Gastrodin |
In vitro (RSC96 SCs) |
50, 100, 200 µM (dose-dependent manner) | Incubation | Promoted proliferation of RSC96 SCs in a dose- and time-dependent manner | Inhibition of ERK1/2 phosphorylation and activation of Akt phosphorylation | [70] |
| Ginkgo biloba | Ginkgo biloba extract (EGb 761) |
In vivo (SD rats) |
50 mg/kg | Intraperitoneal injection paired with an 18mm acellular nerve allograft bridging a 15mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased density of regenerated axons, muscle mass, axon number and diameter, expression of CD34 and NF200) | Increasing expression of angiogenesis-related genes (Vegf, Sox18, Prom1, IL-6) | [71] |
| Ginkgo biloba extract (EGb 761) |
In vitro (SCs isolated from spinal nerves of 1-day old SD rats) |
1, 10, 20, 50, 100 µg/mL (dose-dependent manner) | Incubation | Promoted cell attachment and survival of SCs | N/A | [72] | |
|
In vivo (SD rats) |
10, 50 µg/mL | Injection into poly(DL-lactic acid-co-glycolic acid) conduit seeded with Schwann cells bridging a 12mm sciatic nerve defect |
Promoted histological and functional recovery in rats with SNI (increased number and area of myelinated axons, increased CMAP) |
||||
| Ginseng | Ginsenoside Rg1 |
In vitro (RSC96 SCs) |
Ginseng: 100, 200, 300, 400, 500 µg/mL Ginsenoside: 5, 10, 15, 20, 25 µg/mL) (Dose-dependent manner for both) |
Incubation | Promoted proliferation and migration of RSC96 SCs | Proliferation: Enhancing protein expression of IGF-I pathway regulators (IGF-IR, PI3K, p-Akt, p-Bad, Bcl-2), cell cycle controlling proteins (cyclin D1, E, A), and MAPK signaling pathway (ERK, JNK, p38) Migration: Stimulating the FGF-2-uPA-MMP9 migrating pathway |
[73] |
| Ginsenoside Rg1 | In vivo(SD rats) | 1.5 mg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased number of motoneurons, number, and diameter of myelinated axons, SFI, MNCV, improved CMAP latency and amplitude, the increased average percentage of muscle fiber) | N/A | [74] | |
| Ginsenoside Re |
In vitro (SCs isolated from sciatic nerves of 3-day old SD rats) |
0.5 mg/mL | Incubation | Promoted proliferation and migration of SCs | Phosphorylation of ERK1/2 and JNK 1/2 | [75] | |
|
In vivo (SD rats) |
2.0 mg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased SFI, TSI, PCNA expression level, improved pathological changes due to crushing injury, GAP43, and S-100 expression) | ||||
| Green tea | (-)-Epigallocatechin-3-gallate (EGCG) |
In vivo (Wistar rats) |
50 mg/kg | Intraperitoneal injection | Promoted functional recovery (improved outcomes of foot position, toe spreading, extensor postural thrust, hopping reflex, von Frey hair, Randall–Sellito, hotplate, and tail-flick tests), improved morphological recovery in skeletal muscle tissues muscles, and protection towards muscle fibers in rats with SNI | Protection of muscle fibers from cellular death through activation of an anti-apoptotic signaling pathway (modulation of Bax, Bcl-2, and p53 expression) | [76] |
| (-)-Epigallocatechin-3-gallate (EGCG) |
In vivo (Wistar rats) |
50 mg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (improved nerve morphology and functional recovery assessed by foot position, extensor postural thrust test, and withdrawal reflex threshold) | Reversal of Bax, Bcl-2, and survivin mRNA expression induced by sciatic nerve injury | [77] | |
| Can be found in a wide variety of plants | Syringic acid |
In vitro (RSC96 SCs) |
600 µM | Incubation | Promoted proliferation and migration of RSC96 SCs | Downregulation of miR-451-5p | [78] |
| Can be found in a wide variety of plants | Ursolic acid |
In vivo (BALB/c mice) |
2.5, 5, 10 mg/kg (dose-dependent manner) | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased number and diameter of myelinated nerve fibers and soleus muscle mass) | Increasing S100 protein expression levels | [79] |
| Lycium barbarum | Polysaccharide |
In vitro (1) PC12 cells (2) Rat SCs (3) DRG neurons isolated from the embryo of 14-day pregnant rat |
10, 30, 50 mg/mL (optimal at 30 mg/mL) | Incorporated into core-shell structured nanofibrous scaffolds by coaxial electrospinning | (1) Promoted proliferation and neuronal differentiation of PC12 cells (2) Promoted proliferation and myelination of SCs (3) Promoted neurite outgrowth of DRG neurons |
N/A | [80] |
| Can be found in a wide variety of plants | Quercetin |
In vitro (RSC96 SCs) |
0.1, 1, 10 µg/mL | Incubation | Promoted proliferation of RSC96 SCs | N/A | [81] |
|
In vivo (SD rats) |
0.1, 1, 10 µg/mL | Injection into a silicone rubber tube bridging a 15mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased count and density of myelinated axons, and resulted in larger area and amplitude of CMAP) | ||||
| Morus sp. | Cortex Mori Radicis (aqueous extract) |
In vivo (SD rats) |
100 mg/kg | Gastrointestinal administration | Reduced blood glucose levels, improved nerve functions (thermal latency and mechanical threshold), reversed the loss of Nissl bodies and induced neurite outgrowth in DRG neurons, and restored the response of growth cones to NGF in diabetic rats | Neurite outgrowth: Increased expression of TRPC1, reduced Ca2+ influx, and activation of PI3K/Akt signaling | [82] |
| Pueraria lobata | Puerarin |
In vitro (RSC96 SCs) |
1, 10, 100 µM (dose-independent manner) | Incubation | Promoted growth of SCs | N/A | [83] |
|
In vivo (SD rats) |
1, 10, 100 µM (dose-independent manner) | Injection into a silicone rubber tube bridging a 15mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased density of myelinated axons, CMAP, and MNCV) | ||||
| Serum metabolites (obtained from rats fed with Pueraria lobata extract) |
In vitro (PC12 cells) |
0.01, 0.1, 1 unit | Incubation | Enhanced NGF-mediated neurite outgrowth and expression of synapsin I in PC12 cells | N/A | [84] | |
|
In vivo (SD rats) |
0.01, 0.1, 1 unit | Injection into silicone rubber chamber bridging a 10mm sciatic nerve defect |
Promoted peripheral nerve regeneration in rats with SNI (increased mean values of myelinated axon number, endoneurial area, and total nerve area) | ||||
| Radix Hedysari | Aqueous extract |
In vivo (SD rats) |
1 g/mL | Oral gavage paired with biodegradable chitin conduit bridging a 2mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with SNI (increased MNCV, fiber and axon diameter, g-ratio) | N/A | [85] |
| Polysaccharides |
In vivo (SD rats) |
0.25 g/mL | Oral gavage | Promoted peripheral nerve regeneration in rats with sciatic nerve defect (increased SFI, TFI, PFI values, MNCV, and number of regenerated myelinated nerve fibers) | N/A | [86] | |
| Rhodiola rosea L. | Salidroside |
In vivo (SD rats) |
5, 10 mg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased number and diameter of myelinated axons, number of motoneurons, SFI, amplitude of CMAP, MNCV) | N/A | [87] |
| Scutellaria baicalensis Georgi | Baicalin |
In vitro (RSC96 SCs) |
5, 10, 20 µM (dose-dependent manner) | Incubation | Promoted proliferation of RSC96 SCs | Modulation of neurotrophic factors (GDNF, BDNF, CNTF) and S100β | [88] |
| Trigonella foenum-graecum (fenugreek) | IND01 (Fenugreek seed extract) |
In vivo (Wistar rats) |
50, 100, 200 mg/kg | Oral administration | Promoted peripheral nerve regeneration in rats with: (1) partial sciatic nerve ligation (ameliorated thermal hyperalgesia, improved motor function test scores) (2) SNI (ameliorated thermal hyperalgesia, improved motor function test scores, increased MNCV) |
N/A | [89] |
| Tripterygium wilfordii Hook. F. | Triptolide |
In vivo (SD rats) |
100 µg/kg | Intraperitoneal injection | Promoted peripheral nerve regeneration in rats with SNI (increased number of motoneurons, number of myelinated axons, diameter of nerve fibers, SFI, CMAP amplitude, MNCV, muscle fiber area) | Reduction of TNF-α, IL-β, and IL-6 expression | [90] |
| MUSHROOM | |||||||
| Amanita muscaria | Muscimol |
In vivo (SD rats) |
400 μg/mL | Applied directly to the right L5 DRG | Promoted peripheral nerve regeneration in rats with SNI (prevented the development of thermal and mechanical hypersensitivity and mechanical allodynia, improved basal membrane integrity, and increased nerve fibers) | Normalization of PMP22 protein expression level by GABAergic modulation in the ipsilateral DRG | [91] |
| Hericium erinaceus | Aqueous extract |
In vivo (SD rats) |
10 mL/kg | Oral administration | Promoted peripheral nerve regeneration in rats following peroneal nerve crush | Activation of signaling pathways (Akt, MAPK, c-Jun, c-Fos) and protein synthesis | [92] |
| Polysaccharide |
In vivo (SD rats) |
30 mg/mL/kg | Oral administration | Promoted sensory functional recovery following peroneal nerve crush in rats (reduced withdrawal reflex latency) | Activation of Akt and p38 MAPK signaling and increased expression of RECA-1 | [93] | |
| Aqueous extract |
In vivo (SD rats) |
10, 20 mL/kg | Oral administration | Promoted peripheral nerve regeneration in rats following peroneal nerve crush (increased PFI, improved axon morphology, and development of neuromuscular junction) | N/A | [94] | |
| Lignosus rhinocerotis | Aqueous extract |
In vivo (SD rats) |
500, 1000 mg/kg | Oral administration | Promoted motor and sensory functional recovery in rats with SNI (improved WRL and toe-spreading reflex) | N/A | [95] |
| DECOCTION | |||||||
| Bogijetong | (1) Bogijietong decoction (18 ingredients) (2) A reconstituted formulation of BGJTD (BeD) with 4 ingredients (3) Angelica gigas (an ingredient in BeD) |
In vitro (Primary neurons isolated from DRG at lumbar levels 4 and 5 in adult rats) |
400 mg/kg | Incubation | Promoted neurite outgrowth of DRG neurons | (1) BGJTD and BeD: Downregulation of TNF-α and p38, upregulation of p-ERK1/2; (2) Angelica gigas: Regulation of ERK1/2 activity and TNF-α production | [96] |
|
In vivo (SD rats and BALB/c mice) |
400 mg/kg | Oral administration | Reduced latency time in rats | ||||
| Buyang Huanwu | Buyang Huanwu decoction (16 ingredients: Modified formulation) |
In vivo (SD rats) |
1800 mg/kg | Oral administration paired with silicone rubber tube bridging a 10mm sciatic nerve defect | Promoted peripheral nerve regeneration in rats with sciatic nerve defect (increased nerve formation, myelinated axons, and endoneurial area) | N/A | [97] |
| Jiaweibugan | Jiaweibugan decoction (9 ingredients) |
In vivo (Wistar rats) |
28.6 g/kg | Intragastric administration | Protective effect on peripheral nerve injury by playing an anti-oxidative role in a diabetic rat model (increased MNCV and serum levels of glutathione, decreased serum levels of MDA) | Downregulation of NF-kB p65 and p38 MAPK mRNA expression | [98] |
| Qian-Zheng-San | Qian-Zheng-San (3 ingredients: Typhonii rhizoma, Bombyx batryticatus, Scorpio) |
In vivo (SD rats) |
1.75 g/mL | Oral gavage | Promoted peripheral nerve regeneration in rats with sciatic nerve defect (Increased SFI, MNCV, muscle wet weight, number of regenerated axons, axon diameter, nerve fiber diameter, myelin thickness, number of motor neurons in the lumbar spinal cord anterior horn) | N/A | [99] |
Akt—protein kinase B; Bad—Bcl-2 associated agonist of cell death; Bax—Bcl-2-associated X protein; Bcl-2—B-cell lymphoma 2; BDNF—brain-derived neurotrophic factor; BGJTD—Bogijetong decoction; Cdc2—cell division cycle protein 2 homolog; CGRP—calcitonin gene-related peptide; CMAP—compound muscle action potential; CNTF—ciliary neurotrophic factor; CSA—cross-sectional area; DRG—dorsal root ganglion; DUOX2—dual oxidase 2; ERK—extracellular signal-regulated kinase; FGF—fibroblast growth factor; GABA—γ-aminobutyric acid; GAP-43—growth associated protein 43; GDNF—glial cell-derived neurotrophic factor; ICR mice—Institute of Cancer Research mice; IFN—interferon; IGF-1—insulin-like growth factor 1; IGF-IR—insulin-like growth factor 1 receptor; IL—interleukin; JNK—c-Jun N-terminal kinase; MAPK—mitogen-activated protein kinase; MBP—myelin basic protein; MDA—malondialdehyde; MMP—matrix metallopeptidase; MNCV—motor nerve conduction velocity; NF-κB—nuclear factor kappa B; NGF—nerve growth factor; NOX4—nicotinamide adenine dinucleotide phosphate oxidase 4; PAI-1—plasminogen activator inhibitor-1; PBS—phosphate buffered saline; PC12—pheochromocytoma cells; PCNA—proliferating cell nuclear antigen; PDGF—platelet-derived growth factor; PFI—peroneal nerve function index; PI3K—phosphoinositide 3-kinase; PMP22—peripheral myelin protein 22; Prom1—prominin 1; RECA-1—mouse monoclonal endothelial cell antibody; ROS—reactive oxygen species; RSC96 SC—RSC96 Schwann cell; SCs—Schwann cells; SD rats—Sprague-Dawley rats; SFI—sciatic function index; SNI—sciatic nerve injury; Sox18—sex-determining region Y-box transcription factor 18; TGF-β—transforming growth factor beta; TNF-α—tumor necrosis factor alpha; tPA—tissue plasminogen activator; Trk—tropomyosin receptor kinase; TRPC1—classical transient receptor potential 1; TSI—toe spread index; uPA—urokinase plasminogen activator; Vegf—vascular endothelial growth factor; WRL—withdrawal reflex latency.
4.4. Experimental Strategies and Neuroprotective Effects of Complementary and Alternative Medicines (CAMs) against Peripheral Nerve Injury
4.4.1. CAMs with Neuroregenerative Potential
Due to the limitations of conventional therapies for PNIs, much attention has been dedicated to finding alternative approaches in treating PNIs. To date, studies have explored the potential of 20 species of plants, three species of mushrooms, and four types of decoctions in promoting peripheral nerve regeneration (Table 1). Notably, the neuroregenerative potential of Achyranthes bidentata [44,45,46,47,48,49], Astragalus membranaceus [52,53,54,55], Curcuma longa [60,61,62,63,64,65], Panax ginseng [73,74,75], and Hericium erinaceus [92,93,94] have been most studied. A total of 18 natural products have been identified across the studies, and their chemical structures are shown in Table 2. Among those, ursolic acid, syringic acid, and quercetin are the NPs that can be found across a variety of plant species [78,79,81,101,102,103]. Decoctions are usually made according to traditional formulae. However, among the decoctions discussed in this study, the Bogijetong decoction is a relatively modern formulation that was specifically developed to treat neuropathic pain [96].
Table 2.
Chemical structures of natural products and their respective sources.
| Sources | Natural Product | Chemical Structure |
|---|---|---|
| Alpinate Oxyphyllae Fructus (Alpinia oxyphylla Miq) | Protocatechuic acid |
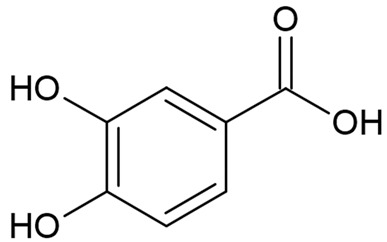
|
| Astragalus membranaceus | Astragaloside IV |
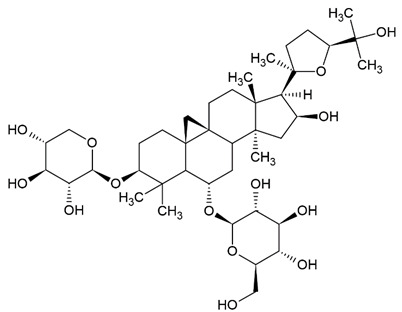
|
| Crocus sativus | Crocin |
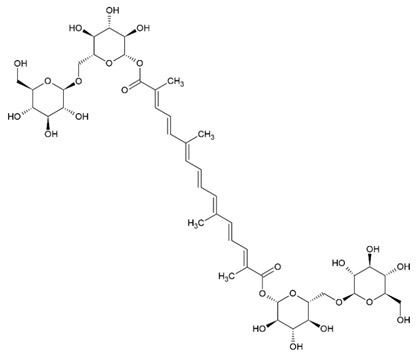
|
| Curcuma longa | Curcumin |
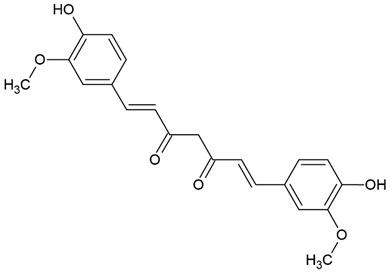
|
| Epimedium | Icariin |
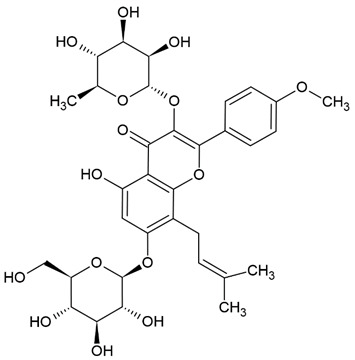
|
| Gardenia jasminoides Ellis | Genipin |
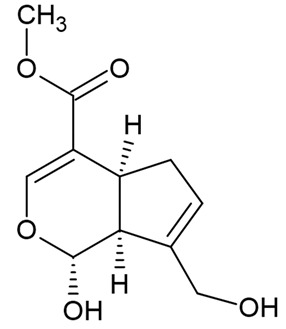
|
| Gastrodia elata Blume | Gastrodin |
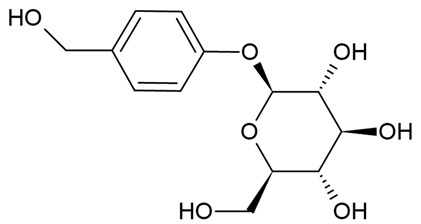
|
| Ginseng | Ginsenoside Rg1 |
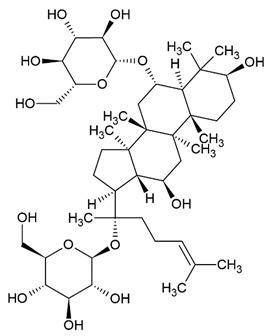
|
| Ginsenoside Re |
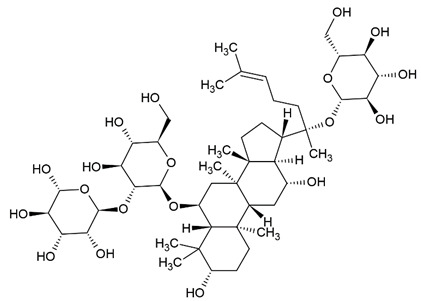
|
|
| Green tea | (-)-Epigallocatechin-3-gallate (EGCG) |
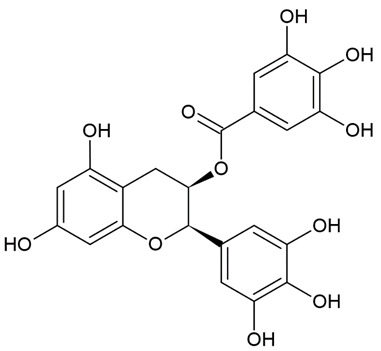
|
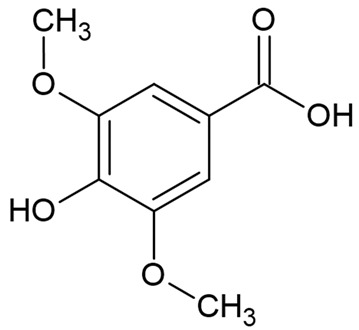
| Isolated from a variety of plants (e.g., grapes, Vitis vinifera; olive, Olea europaea; radish, Raphanus sativus; pumpkin, Cucurbita pepo [101]) | Syringic acid |
|
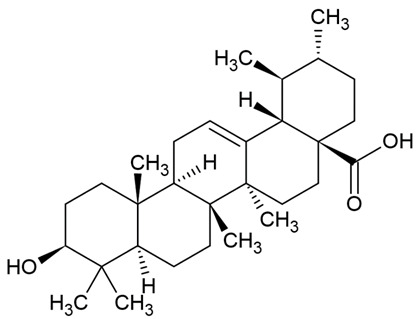
| Isolated from a variety of plants (e.g., apple, Malus domestica; cranberry, Vaccinium oxycoccus; peppermint, Mentha piperita; and thyme, Thymus vulgaris [102]) | Ursolic acid |
|
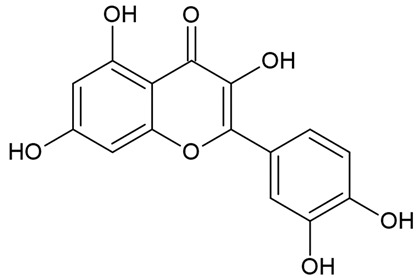
| Isolated from a variety of plants (e.g., apple, Malus domestica; caper, Capparis spinosa; onion, Allium cepa; tomato, Solanum lycopersicum; and grapes, Vitis vinifera [103]) | Quercetin |
|
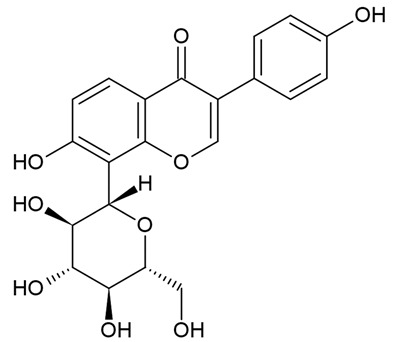
| Pueraria lobata | Puerarin |
|
| Rhodiola rosea L. | Salidroside |
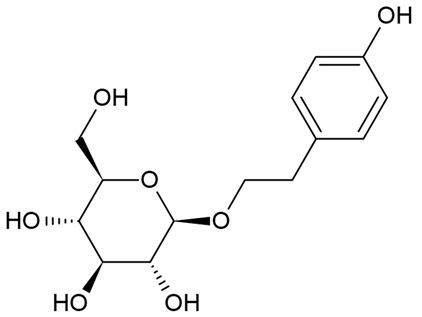
|
| Scutellaria baicalensis Georgi | Baicalin |
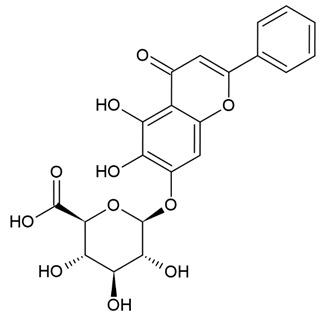
|
| Tripterygium wilfordii Hook.F. | Triptolide |
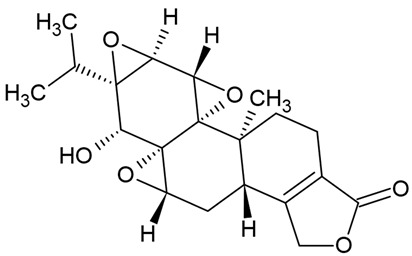
|
| Amanita muscaria | Muscimol |
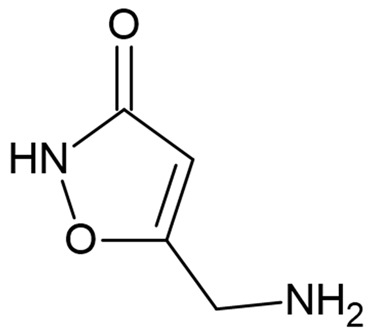
|
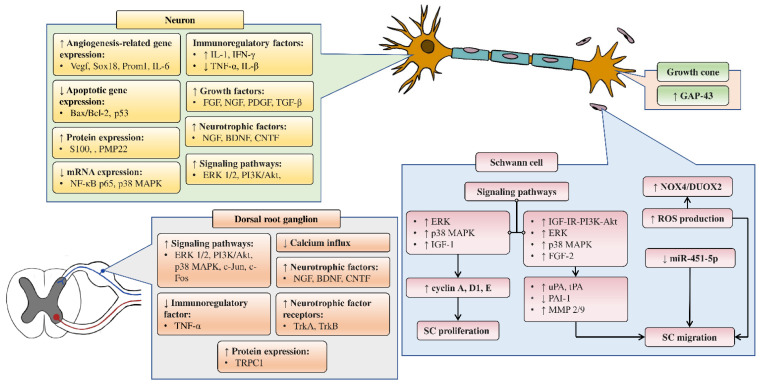
4.4.2. In Vitro Studies on Neuroregenerative Potential of CAMs
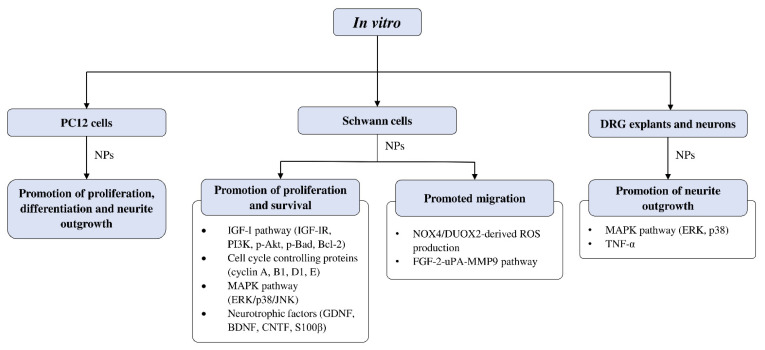
Figure 4 summarizes the in vitro studies on neuroregenerative properties of complementary and alternative medicines. Most of the studies were in Schwann cells, with a few using DRG explants, neurons, and PC12 cells (rat pheochromocytoma). Some CAMs were reported to induce proliferation, differentiation, and neurite outgrowth in PC12 cells. Similarly, neurite outgrowth was also promoted in DRG neurons through modulation of the extracellular signal-regulated kinase (ERK), p38, and tumor necrosis factor-α (TNF-α). Polypeptides isolated from Achyranthes bidentata have demonstrated the ability to promote neurite outgrowth in DRG neurons through the activation of ERK1/2 [45,46]. These findings resemble an earlier study that also reported neurite growth in DRG neurons induced by CD95 through ERK activation [104]. The Bogijetong decoction and its reconstituted formulation BeD elicited similar neuroprotective effects through downregulation of p38 and TNF-α [96] It was previously shown that TNF-α could inhibit neurite outgrowth in cultured DRG neurons [105,106], whereas the application of a TNF-α antagonist supported axonal regeneration following nerve injury [107].
Figure 4.
Overview of in vitro studies that demonstrated the effects of natural products relating to peripheral nerve regeneration across different cell types with associated mechanisms. Akt—protein kinase B; Bad—Bcl-2 associated agonist of cell death; Bcl-2—B-cell lymphoma 2; BDNF—brain-derived neurotrophic factor; CNTF—ciliary neurotrophic factor; DRG—dorsal root ganglion; DUOX2—dual oxidase 2; ERK—extracellular signal-regulated kinase; FGF—fibroblast growth factor; GDNF—glial cell-derived neurotrophic factor; IGF-I—insulin-like growth factor 1; IGF-IR—insulin-like growth factor 1 receptor; JNK—c-Jun N-terminal kinase; MAPK—mitogen-activated protein kinase; MMP9—matrix metallopeptidase 9; NOX4—nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 4; NPs—natural products; PC12—pheochromocytoma cells; PI3K—phosphoinositide 3-kinase; ROS—reactive oxygen species; S100β—S100 calcium-binding protein β; TNF-α—tumor necrosis factor-α; uPA—urokinase plasminogen activator.
Effects of CAMs on Schwann Cell Activity In Vitro
The studies examining the effects of complementary and alternative medicines and their related natural products on Schwann cells are primarily focused on promoting their proliferation and survival. The molecular mechanisms that were investigated in these studies include signaling pathways such as IGF-I and MAPK, as well as cell cycle controlling proteins and various neurotrophic factors (Figure 4). Past studies have demonstrated that ERK is required for proper myelination of SCs during development [108,109], and ERK signaling was rapidly activated following nerve injury, contributing to SC differentiation [110]. Moreover, evidence suggests that nerve regeneration following injury is closely associated with ERK [111,112], and ERK inhibition leads to impaired regenerative capability [111,113]. On the other hand, inhibition of p38 MAPK prevented SC demyelination and dedifferentiation, indicating its role in promoting the breakdown of myelin following nerve injury [114]. It is not unexpected that cyclins are associated with SC proliferation, as these proteins control cell cycle progression through the interaction of cyclin-dependent kinases. For instance, cyclin D is associated with Cdk4 or Cdk6 in the G1 phase, cyclin A participates with Cdk1 or Cdk2 in the S phase, cyclin E is involved with Cdk2 in G1 and S phases, cyclin B and Cdk1 regulates M phase [115,116].
Protocatechuic acid isolated from Alpinia oxyphylla Miq [50] and the aqueous extract of Codonopsis pilosula [58] were found to promote SCs proliferation by further enhancing IGF-I (insulin-like growth factor 1) signaling. The IGF-I growth factor is known to play a crucial role in neuromuscular recovery following injury. It is reported to be involved in promoting G1/S cell cycle progression [117] and survival of SCs [118] in vitro, and to facilitate peripheral nerve regeneration in vivo [119,120,121,122]. One study reported baicalin, a flavonoid that possesses various neuroprotective effects [123], induced proliferation of SCs through the modulation of neurotrophic factors including glial cell-derived neurotrophic factor (GDNF), BDNF, and ciliary neurotrophic factor (CTNF) [88]. These neurotrophic factors are integral to many aspects of nerve regeneration, as evident in past studies that showed their roles in myelin formation [124,125] and axonal regeneration [126,127].
In addition to promoting the proliferation of SCs, some NPs may promote the migratory ability of SCs, which is essential for regeneration and remyelination following nerve injury. Aqueous extracts of Alpinia oxyphylla Miq [51], Astragalus membranaceus [55], Citrus medica var. sarcodactylis [57], Codonopsis pilosula [58], and ginsenoside Rg1 isolated from ginseng [73] enhanced SC migration through the activation of FGF-2 signaling. The role of FGF-2 in the repair and regeneration of tissues [128] and its involvement in cell migration [129,130] is widely documented. A recently published study reported that another subfamily member, FGF5, is also involved in regulating SC migration and adhesion [131]. Besides FGF-2 signaling, another study investigating polypeptides of A. bidentata revealed that the upregulation of NOX4/DUOX2-derived reactive oxygen species (ROS) production was responsible for promoting SC migration [44]. Excessive accumulation of ROS production has been linked to neurodegenerative disorders [132] and peripheral neuropathy [133], but moderate levels of ROS may prove beneficial by acting as signal messengers in regulating biological processes, including cell adhesion and migration [134,135]. Syringic acid was shown to promote the proliferation and migration of SCs in vitro. Although the expression of several microRNAs was affected by syringic acid, further analysis suggested that the plant polyphenol promoted SC proliferation and migration mainly by suppressing the microRNA miR-451-5p [78].
4.4.3. In Vivo Studies on Neuroregenerative Potential of CAMs
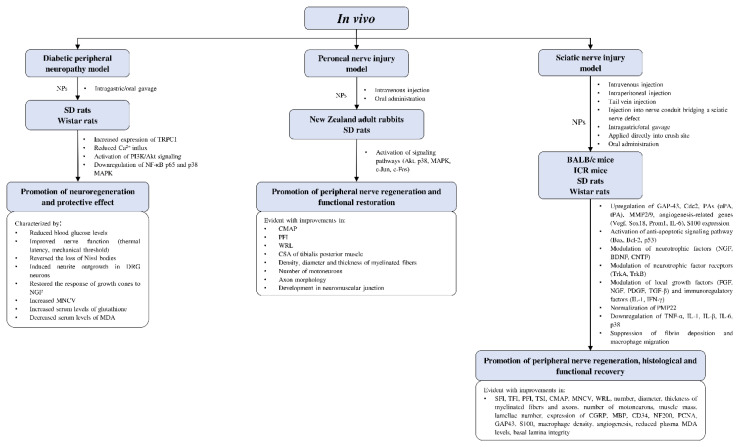
Current in vivo studies on the potential of complementary and alternative medicines in peripheral nerve regeneration are limited to rodent models (Figure 5 and Table 1). Most of the studies involved different strains of rats and mice, with only two studies using rabbits as their animal models. Models of peripheral nerve injury used in the studies include diabetic peripheral neuropathy, peroneal nerve injury, and sciatic nerve injury. The effects of CAMs on peripheral nerve regeneration were evaluated by functional recovery indexes (e.g., PFI; sciatic function index, SFI; tibial function index, TFI; CMAP; MNCV; and WRL) and histological examinations (e.g., number, diameter, the thickness of myelinated fibers and regenerated axons; the number of motoneurons; and muscle mass).
Figure 5.
Overview of in vivo studies that demonstrated the effects of natural products relating to peripheral nerve regeneration across different experimental models with associated mechanisms. Akt—protein kinase B; Bax—Bcl-2-associated X protein; Bcl-2—B-cell lymphoma 2; BDNF—brain-derived neurotrophic factor; Cdc2—cell division control protein; CGRP—calcitonin gene-related peptide; CMAP—compound muscle action potential; CNTF—ciliary neurotrophic factor; CSA—cross-sectional area; DRG—dorsal root ganglion; FGF—fibroblast growth factor; GAP-43—growth associated protein 43; ICR—Institute of Cancer Research; IFN-γ—interferon-γ; IL—interleukin; MAPK—mitogen-activated protein kinase; MBP—myelin basic protein; MDA—malondialdehyde; MMP2/9—matrix-metalloproteinase-2/9; MNCV—motor nerve conduction velocity; NF-κB—nuclear factor kappa B; NGF—nerve growth factor; NPs—natural products; PAs—plasminogen activators; PCNA—proliferating cell nuclear antigen; PDGF—platelet-derived growth factor; PFI—peroneal function index; PI3K—phosphoinositide 3-kinase; PMP22—peripheral myelin protein 22; Prom1—prominin 1; SD—Sprague-Dawley; SFI—sciatic function index; Sox18—sex-determining region Y-box transcription factor 18; TFI—tibial function index; TGF-β—transforming growth factor-β; TNF-α—tumor necrosis factor-α; tPA—tissue plasminogen activator; Trk—tropomyosin receptor kinase; TRPC1—transient receptor potential cation channel subfamily C member 1; TSI—toe spread index; uPA—urokinase plasminogen activator; Vegf—vascular endothelial growth factor; WRL—withdrawal reflex latency.
Diabetic Peripheral Neuropathy Model
In the diabetic neuropathy (DPN) model, aqueous extract of Cortex Mori Radicis had anti-diabetic and neuroregenerative effects, as evidenced by reduced blood glucose levels, induced neurite outgrowth, restoration of the loss of Nissl bodies, and a response in the growth cones of DRG neurons [82]. The authors identified that the observed effects were mediated by the activation of the PI3K/Akt pathway and increased expression of TRPC1, which in turn reduced Ca2+ influx. The PI3K/Akt pathway is a crucial intracellular signaling pathway that governs cell proliferation, survival, and metabolism [136], its protective role against DPN has been previously hinted at [137,138]. The transient receptor potential (TRPC) is a family of Ca2+-permeable channels that participates in axonal regeneration [139]. In particular, TRPC1 and TRPC4 were shown to induce neurite outgrowth in PC12 cells and DRG neurons [140,141]. In another study, administration of Jiaweibugan decoction in a DPN model ameliorated changes in motor nerve conduction velocity (MNCV), and malondialdehyde (MDA), and glutathione levels through an anti-oxidative pathway via downregulating NF-κB p65 and p38 MAPK [98]. The activation of p38 MAPK, which belongs to a family of kinases that are responsive to stress stimuli, further activates NF-κB leading to inflammation, a driving factor of DPN [142,143].
Peroneal Nerve Injury Model
In the peroneal nerve injury model, aqueous extract and polypeptides of A. bidentata were shown to enhance nerve regeneration [45,49], as indicated by increased density and diameter of myelinated fibers, and numbers of motor neurons. Although behavioral analyses were not performed in the studies, improvements in compound muscle action potential (CAMP) demonstrated the ability of A. bidentata aqueous extract and polypeptides to promote functional recovery. Aqueous extract and polysaccharides from Hericium erinaceus also exhibited nerve regeneration and functional recovery following peroneal nerve crush [92,93,94], as evidenced by the improvements in the peroneal function index (PFI), withdrawal reflex latency (WRL) and axon morphology, and the development of neuromuscular junction. These findings were supported by the activation of Akt, p38, c-Jun, and c-Fos, which is in line with other studies that showed the importance of these signaling events for axonal regeneration [144,145,146].
Sciatic Nerve Injury Model
The sciatic nerve injury (SNI) model is the most commonly used model in the study of the effects of complementary and alternative medicines on peripheral nerve regeneration, and many studies have investigated the underlying mechanisms or molecular pathways through which CAMs elicit their neuroregenerative properties. For instance, polypeptides of A. bidentata [47], astragaloside IV isolated from A. membranaceus [52], and aqueous extract of Dioscoreae rhizoma [66] promoted nerve regeneration via upregulation of GAP-43 expression. The GAP-43 protein is highly associated with the development and plasticity of the nervous system [147]. Its expression is known to be elevated following nerve injury [148] and is involved in the neurite outgrowth of hippocampal neurons [149]. Similarly, modulation of other neurotrophic factors such as NGF, BDNF, CNTF [47,54], and pro-inflammatory cytokines including IL-1, IL-6, IL-β, and TNF-α [54,90] were also involved in promoting nerve regeneration as well. Although an inflammatory response following injury is necessary for the regenerative process [150], prolonged inflammation can impede recovery and may even lead to the development of neuropathic pain [151], which reflects the double-edged property of inflammation and the importance of proper regulation. Additionally, a study on Ginkgo biloba extract showed that it promoted axonal angiogenesis through the modulation of related genes, including Vegf, Sox18, Prom1, and IL-6 [71]. Studies have also demonstrated the participation of Vegf [152,153], Prom1 [154], and another subfamily gene, Sox11 in sciatic nerve regeneration [155], and the restorative role of IL-6 has also been implied in DPN and central nervous system models [156,157]. Muscimol prevented hyperalgesia through the modulation of PMP22 [91], a key component of the basal lamina. The expression of PMP22 is correlated with myelin formation and nerve regeneration [158,159]. Studies investigating EGCG in an SNI model showed that it had neuroprotective and regenerative effects, partly due to the modulation of the apoptotic machinery, including Bax, Bcl-2, p53, and survivin [76,77]. The subsequent loss of neurons after PNI is known to be closely related to apoptosis [160] which is partly influenced by p53 and Bax [161], while the association of survivin in nerve injury has also been documented [162].
4.4.4. Involvement of CAMs in Combinatorial Approaches for the Treatment of PNI
There is increasing evidence that the successful repair and regeneration of nerves will require not just a single treatment strategy, but a multifaceted strategy involving different disciplines. Studies adopting combinatorial approaches have yielded interesting findings. For example, Lycium barbarum polysaccharide incorporated into core-shell structured nanofibrous scaffolds by coaxial electrospinning showed proliferative effects in PC12, SCs, and DRG neurons [80]. In two separate studies, puerarin, the active component extracted from Pueraria lobata roots, as well as rat serum metabolites of P. lobata enhanced the neuroregenerative effects of silicone rubber nerve chambers. Increases in myelinated axons and structurally mature regenerated axons were observed, while muscle reinnervation led to functional recovery, as indicated by an increase in action potential and nerve conduction [83,84]. Similar results were obtained with Buyang Huanwu decoction being administered as a co-treatment alongside silicone rubber nerve chambers, which led to more prominent axonal regeneration [97]. In an SNI model, a magnetic nanocomposite scaffold produced from using magnetic nanoparticles and biodegradable chitosan-glycerophosphate polymer enhanced SC viability, nerve regeneration, and functional recovery when paired with an applied magnetic field [163]. The use of nerve guiding conduits gained popularity over the years. They have been used to isolate regenerating axons from fibrotic tissues, to protect them from mechanical forces, and to guide new-forming tissue as well as condensing growth factors secreted by SCs [164]. The concept was initiated with a simple hollow design but has since advanced to innovative ways of redesigning nerve conduits to further extend their original capabilities 11. The attractive characteristics of modern nerve conduits offer tremendous potentials. These nerve conduits are occasionally paired with other strategies for improving nerve outcomes. For instance, Chang et al. [165] developed a natural biodegradable multi-channeled scaffold with aligned electrospun nanofibers and a neurotrophic gradient, which resulted in superior nerve recovery and less muscle atrophy compared with nerve autografts. Hussin et al. [56] used Centella asiatica (L.) to neurodifferentiate mesenchymal stem cells. This was subsequently developed with decellularized artery as a nerve conduit, which demonstrated functional restoration in an SNI model similar to that of reversed autograft.
4.5. Limitations and Future Prospects
As mentioned earlier, PNI represents a significant health issue while the effectiveness of current treatment approaches is highly subjective. Hence, substantial effort is required to discover and establish proper methods for the management of PNI. Present studies have shown promising findings in utilizing various applications including nerve conduits [166], stem cell therapy [167], phytochemicals [22], and electrical stimulation [168] for treating PNI, and their potential may subsequently be improved when paired together. Evidence from in vitro and in vivo studies have delineated the neuroregenerative properties of various CAMs, and the underlying mechanisms have been investigated (as summarized in Figure 6), although they still remain incompletely understood and require further elucidation. Subsequently, pre-clinical and clinical studies on existing potential candidates and approaches should be supported to drive the development of future therapeutics.
Figure 6.
Summary of the molecular mechanisms associated with the neuroregenerative effects of CAMs. Vegf—vascular endothelial growth factor; Sox18—sex-determining region Y-box transcription factor 18; Prom1—prominin 1; IL—interleukin; IFN-γ—interferon-γ; Bax—Bcl-2-associated X protein; Bcl-2—B-cell lymphoma 2; Trk—tropomyosin receptor kinase; PMP22—peripheral myelin protein 22; FGF—fibroblast growth factor; NGF—nerve growth factor; PDGF—platelet-derived growth factor; TGF-β—transforming growth factor-β; NF-κB—nuclear factor kappa B; MAPK—mitogen-activated protein kinase; PI3K—phosphoinositide 3-kinase; Akt—protein kinase B; BDNF—brain-derived neurotrophic factor; CNTF—ciliary neurotrophic factor; TNF-α—tumor necrosis factor-α; TRPC1—transient receptor potential cation channel subfamily C member 1; GAP-43—growth-associated protein 43; PAI-1—plasminogen activator inhibitor-1; MMP2/9—matrix-metalloproteinase-2/9; tPA—tissue plasminogen activator; uPA—urokinase plasminogen activator.
Existing studies on the effect of complementary medicines in treating PNI are preliminary findings with limited information (Table 1). The majority of studies investigated crude extracts or specific fractions of extracts, with only 24 out of the 56 studies managing to identify the exact NPs responsible for the observed effects. Additionally, 25 studies did not report the underlying mechanisms for the resultant effects of NPs, especially at the in vivo stage. This situation highlights the need for greater efforts among the scientific community to fully investigate the purported effects of NPs. Another issue is the route and method of administration in vivo. It is known that oral administration is generally economical and relatively safe, but the resultant efficacy may not be reliable due to uncontrollable animal habits and behaviors [169]. In contrast, gavage or injection routes typically require some form of restraint, which may result in animal stress that may influence study outcomes. The administration routine also varied across studies, with the treatments lasting from a few days to months. Moreover, treatment frequency also influences experimental outcomes. Although it is difficult to standardize animal handling procedures, these factors should be taken into account with carefully designed studies.
In this review, the majority of studies on NPs as a treatment for PNI were based on plants and herbs, with a few studies on mushrooms such as Amanita muscaria, Hericium erinaceus, and Lignosus rhinocerotis, as well as some decoctions. This is unsurprising, considering that phytochemicals are highly studied for drug development, which should shed more light on this area of research [170,171,172]. However, the use of NPs for peripheral nerve repair and regeneration is still largely overlooked and could be an untapped potential source for promising drug candidates. For instance, a previous study demonstrated that various mushrooms including Agaricus blazei Murrill, Antrodia cinnamomea, Ganoderma lucidum, and Hirsutella sinensis could activate intracellular signaling kinases ERK, JNK, and p38, which are associated with peripheral nerve regeneration [173]. Another study showed that G. lucidum, Ganoderma neo-japonicum, and Grifola frondosa promoted neuritogenesis via the involvement of the MAPK signaling pathway [174]. Aside from exploring untapped sources of NPs, future research may also simultaneously examine the efficiency of CAMs or NPs with known neuroregenerative properties to compare their ability to promote regeneration of peripheral nerves.
The use of algae in peripheral nerve regeneration merits attention. Algae are well-known for their diverse applications in food nutrition [175], biofuels [176], cosmetics [177], and pharmaceuticals [178,179]. Recent studies have also demonstrated that algae could have potential in the treatment of neurological disorders, including Parkinson’s and Alzheimer’s disease [180,181]. However, the potential uses of algae in peripheral nerve regeneration have yet to be explored, despite evidence showing the ability of macroalgae to promote neurite outgrowth in hippocampal neurons [182,183,184]. More recently, a study showed that Gracilaria manilaensis induced the proliferation of neurite-bearing cells in the rat pheochromocytoma cell line, which is believed to mimic the neuroactivity of NGF [185]. Thus, investigation on the nerve regenerative potential of other NPs holds much promise.
5. Conclusions
Peripheral nerve injury remains a challenge, while future prospects are leaning towards multi-combinatorial approaches. Natural products are highly appreciated for their therapeutic value, and there is a growing body of evidence in their potential for peripheral nerve regeneration. The present findings showed that various NPs promote the proliferation and migration of SCs, most commonly through the activation of MAPK and FGF-2 signaling pathways, respectively. Promotion of peripheral nerve regeneration was also observed in rodent models, partly through the modulation of neurotrophic factors, pro-inflammatory cytokines, and anti-apoptotic signaling. Hence, NPs could play key roles in nerve repair and regeneration in the near future, especially when paired with other innovative approaches such as modern nerve conduits.
Acknowledgments
The authors are thankful to Sunway University for providing the necessary internet and library facilities for literature searching.
Author Contributions
Conceptualization, Y.-Y.Y., K.-H.W. and L.-W.L.; data curation, Y.-Y.Y., T.-K.G. and writing—original draft preparation, Y.-Y.Y., T.-K.G. and K.-Y.N.; review and editing, Y.-Y.Y., K.-Y.N., K.-H.W., L.-W.L., S.-M.P., S.-H.L. and S.R.; supervision, Y.-Y.Y., K.-H.W. and L.-W.L.; project administration, Y.-Y.Y. and K.-H.W.; funding acquisition, Y.-Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by Fundamental Research Grant Scheme FRGS/1/2019/STG05/SYUC/02/1 from the Ministry of Higher Education Malaysia.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Navarro X. Functional evaluation of peripheral nerve regeneration and target reinnervation in animal models: A critical overview. Eur. J. Neurosci. 2016;43:271–286. doi: 10.1111/ejn.13033. [DOI] [PubMed] [Google Scholar]
- 2.Wojtkiewicz D.M., Saunders J., Domeshek L., Novak C.B., Kaskutas V., Mackinnon S.E. Social impact of peripheral nerve injuries. Hand. 2015;10:161–167. doi: 10.1007/s11552-014-9692-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li N.Y., Onor G.I., Lemme N.J., Gil J.A. Epidemiology of peripheral nerve injuries in sports, exercise, and recreation in the United States, 2009–2018. Phys. Sportsmed. 2020;49:1–8. doi: 10.1080/00913847.2020.1850151. [DOI] [PubMed] [Google Scholar]
- 4.Scholz T., Krichevsky A., Sumarto A., Jaffurs D., Wirth G.A., Paydar K., Evans G.R.D. Peripheral nerve injuries: An international survey of current treatments and future perspectives. J. Reconstr. Microsurg. 2009;25:339–344. doi: 10.1055/s-0029-1215529. [DOI] [PubMed] [Google Scholar]
- 5.Antoniadis G., Kretschmer T., Pedro M.T., König R.W., Heinen C.P.G., Richter H.P. Iatrogenic nerve injuries - prevalence, diagnosis and treatment. Dtsch. Arztebl. Int. 2014;111:273–279. doi: 10.3238/arztebl.2014.0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ciaramitaro P., Mondelli M., Logullo F., Grimaldi S., Battiston B., Sard A., Scarinzi C., Migliaretti G., Faccani G., Cocito D. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst. 2010;15:120–127. doi: 10.1111/j.1529-8027.2010.00260.x. [DOI] [PubMed] [Google Scholar]
- 7.Grinsell D., Keating C.P. Peripheral nerve reconstruction after injury: A review of clinical and experimental therapies. BioMed Res. Int. 2014;2014:698256. doi: 10.1155/2014/698256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray W.Z., Mackinnon S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010;223:77–85. doi: 10.1016/j.expneurol.2009.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang E.W., Zhang J., Huang J.H. Repairing peripheral nerve injury using tissue engineering techniques. Neural Regen. Res. 2015;10:1393–1394. doi: 10.4103/1673-5374.165501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Houschyar K.S., Momeni A., Pyles M.N., Cha J.Y., Maan Z.N., Duscher D., Jew O.S., Siemers F., van Schoonhoven J. The role of current techniques and concepts in peripheral nerve repair. Plast. Surg. Int. 2016;2016:4175293. doi: 10.1155/2016/4175293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carvalho C.R., Oliveira J.M., Reis R.L. Modern trends for peripheral nerve repair and regeneration: Beyond the hollow nerve guidance conduit. Front. Bioeng. Biotechnol. 2019;7:337. doi: 10.3389/fbioe.2019.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunelli B., Gorson K.C. The use of complementary and alternative medicines by patients with peripheral neuropathy. J. Neurol. Sci. 2004;218:59–66. doi: 10.1016/j.jns.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 13.Muthuraman A., Singh N., Jaggi A.S. Effect of hydroalcoholic extract of Acorus calamus on tibial and sural nerve transection-induced painful neuropathy in rats. J. Nat. Med. 2011;65:282–292. doi: 10.1007/s11418-010-0486-6. [DOI] [PubMed] [Google Scholar]
- 14.Zhao X., Xu Y., Zhao Q., Chen C.R., Liu A.M., Huang Z.L. Curcumin exerts antinociceptive effects in a mouse model of neuropathic pain: Descending monoamine system and opioid receptors are differentially involved. Neuropharmacology. 2012;62:843–854. doi: 10.1016/j.neuropharm.2011.08.050. [DOI] [PubMed] [Google Scholar]
- 15.Kim Y.S., Park H.J., Kim T.K., Moon D.E., Lee H.J. The effects of Ginkgo biloba extract EGB 761 on mechanical and cold allodynia in a rat model of neuropathic pain. Anesth. Analg. 2009;108:1958–1963. doi: 10.1213/ane.0b013e31819f1972. [DOI] [PubMed] [Google Scholar]
- 16.Calixto J.B. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz. J. Med. Biol. Res. 2000;33:179–189. doi: 10.1590/S0100-879X2000000200004. [DOI] [PubMed] [Google Scholar]
- 17.Karimi A., Majlesi M., Rafieian-Kopaei M. Herbal versus synthetic drugs; beliefs and facts. J. Nephropharmacology. 2015;4:27–30. [PMC free article] [PubMed] [Google Scholar]
- 18.Lahlou M. The success of natural products in drug discovery. Pharmacol. Pharm. 2013;04:17–31. doi: 10.4236/pp.2013.43a003. [DOI] [Google Scholar]
- 19.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 20.Weaver B.A. How taxol/paclitaxel kills cancer cells. Mol. Biol. Cell. 2014;25:2677–2681. doi: 10.1091/mbc.e14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Noble R.L. The discovery of the vinca alkaloids—Chemotherapeutic agents against cancer. Biochem. Cell Biol. 1990;68:1344–1351. doi: 10.1139/o90-197. [DOI] [PubMed] [Google Scholar]
- 22.Araújo-Filho H.G., Quintans-Júnior L.J., Barreto A.S., Almeida J.R.G.S., Barreto R.S.S., Quintans J.S.S. Neuroprotective effect of natural products on peripheral nerve degeneration: A systematic review. Neurochem. Res. 2016;41:647–658. doi: 10.1007/s11064-015-1771-2. [DOI] [PubMed] [Google Scholar]
- 23.Moher D., Shamseer L., Clarke M., Ghersi D., Liberati A., Petticrew M., Shekelle P., Stewart L.A., Group P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015;4:1–19. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hussain G., Wang J., Rasul A., Anwar H., Qasim M., Zafar S., Aziz N., Razzaq A., Hussain R., de Aguilar J.L.G., et al. Current status of therapeutic approaches against peripheral nerve injuries: A detailed story from injury to recovery. Int. J. Biol. Sci. 2020;16:116–134. doi: 10.7150/ijbs.35653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millesi H. Bridging defects: Autologous nerve grafts. Acta Neurochir. Suppl. 2007;100:37–38. doi: 10.1007/978-3-211-72958-8_8. [DOI] [PubMed] [Google Scholar]
- 26.Griffin J.W., Hogan M.C.V., Chhabra A.B., Deal D.N. Peripheral nerve repair and reconstruction. J. Bone Joint Surg. Am. 2013;95:2144–2151. doi: 10.2106/JBJS.L.00704. [DOI] [PubMed] [Google Scholar]
- 27.Li R., Li D.H., Zhang H.Y., Wang J., Li X.K., Xiao J. Growth factors-based therapeutic strategies and their underlying signaling mechanisms for peripheral nerve regeneration. Acta Pharmacol. Sin. 2020;41:1289–1300. doi: 10.1038/s41401-019-0338-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kubiak C.A., Grochmal J., Kung T.A., Cederna P.S., Midha R., Kemp S.W.P. Stem-cell–based therapies to enhance peripheral nerve regeneration. Muscle Nerve. 2020;61:449–459. doi: 10.1002/mus.26760. [DOI] [PubMed] [Google Scholar]
- 29.Gordon T., English A.W. Strategies to promote peripheral nerve regeneration: Electrical stimulation and/or exercise. Eur. J. Neurosci. 2016;43:336–350. doi: 10.1111/ejn.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choudhari A.S., Mandave P.C., Deshpande M., Ranjekar P., Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Front. Pharmacol. 2020;10:1614. doi: 10.3389/fphar.2019.01614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar G.P., Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev. 2012;6:81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rotshenker S. Wallerian degeneration: The innate-immune response to traumatic nerve injury. J. Neuroinflammation. 2011;8:1–14. doi: 10.1186/1742-2094-8-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menorca R.M.G., Fussell T.S., Elfar J.C. Peripheral nerve trauma: Mechanisms of injury and recovery. Hand Clin. 2013;29:317–330. doi: 10.1016/j.hcl.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perry V.H., Brown M.C., Gordon S. The macrophage response to central and peripheral nerve injury: A possible role for macrophages in regeneration. J. Exp. Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tetzlaff W. Tight junction contact events and temporary gap junctions in the sciatic nerve fibres of the chicken during Wallerian degeneration and subsequent regeneration. J. Neurocytol. 1982;11:839–858. doi: 10.1007/BF01153522. [DOI] [PubMed] [Google Scholar]
- 36.Sulaiman W., Gordon T. Neurobiology of peripheral nerve injury, regeneration, and functional recovery: From bench top research to bedside application. Ochsner J. 2013;13:100–108. [PMC free article] [PubMed] [Google Scholar]
- 37.Simons M., Trotter J. Wrapping it up: The cell biology of myelination. Curr. Opin. Neurobiol. 2007;17:533–540. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 38.Arancibia-Carcamo I.L., Attwell D. The node of Ranvier in CNS pathology. Acta Neuropathol. 2014;128:161–175. doi: 10.1007/s00401-014-1305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jessen K.R., Mirsky R. The success and failure of the Schwann cell response to nerve injury. Front. Cell. Neurosci. 2019;13:1–33. doi: 10.3389/fncel.2019.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Arthur-Farraj P.J., Latouche M., Wilton D.K., Quintes S., Chabrol E., Banerjee A., Woodhoo A., Jenkins B., Rahman M., Turmaine M., et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Atanasoski S., Shumas S., Dickson C., Scherer S.S., Suter U. Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol. Cell. Neurosci. 2001;18:581–592. doi: 10.1006/mcne.2001.1055. [DOI] [PubMed] [Google Scholar]
- 42.Kim H.A., Pomeroy S.L., Whoriskey W., Pawlitzky I., Benowitz L.I., Sicinski P., Stiles C.D., Roberts T.M. A developmentally regulated switch regenerative growth of Schwann cells through cyclin D1. Neuron. 2000;26:405–416. doi: 10.1016/S0896-6273(00)81173-3. [DOI] [PubMed] [Google Scholar]
- 43.Yang D.P., Zhang D.P., Mak K.S., Bonder D.E., Scott L., Kim H.A. Schwann cell proliferation during Wallerian degeneration is not necessary for regeneration and remyelination of the peripheral nerves: Axon-dependent removal of newly generated Schwann cells by apoptosis. Mol. Cell. Neurosci. 2008;38:80–88. doi: 10.1016/j.mcn.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song H., Zhao H., Yang L., Li L., Zhang T., Pan J., Meng Y., Shen W., Yuan Y. Achyranthes bidentata polypeptides promotes migration of Schwann cells via NOX4/DUOX2-dependent ROS production in rats. Neurosci. Lett. 2019;696:99–107. doi: 10.1016/j.neulet.2018.12.023. [DOI] [PubMed] [Google Scholar]
- 45.Cheng Q., Yuan Y., Sun C., Gu X., Cao Z., Ding F. Neurotrophic and neuroprotective actions of Achyranthes bidentata polypeptides on cultured dorsal root ganglia of rats and on crushed common peroneal nerve of rabbits. Neurosci. Lett. 2014;562:7–12. doi: 10.1016/j.neulet.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 46.Cheng Q., Jiang C., Wang C., Yu S., Zhang Q., Gu X., Ding F. The Achyranthes bidentata polypeptide k fraction enhances neuronal growth in vitro and promotes peripheral nerve regeneration after crush injury in vivo. Neural Regen. Res. 2014;9:2142–2150. doi: 10.4103/1673-5374.147948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y., Shen W., Yang L., Zhao H., Gu W., Yuan Y. The protective effects of Achyranthes bidentata polypeptides on rat sciatic nerve crush injury causes modulation of neurotrophic factors. Neurochem. Res. 2012;38:538–546. doi: 10.1007/s11064-012-0946-3. [DOI] [PubMed] [Google Scholar]
- 48.Yuan Y., Shen H., Yao J., Hu N., Ding F., Gu X. The protective effects of Achyranthes bidentata polypeptides in an experimental model of mouse sciatic nerve crush injury. Brain Res. Bull. 2010;81:25–32. doi: 10.1016/j.brainresbull.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Ding F., Cheng Q., Gu X. The repair effects of Achyranthes bidentata extract on the crushed common peroneal nerve of rabbits. Fitoterapia. 2008;79:161–167. doi: 10.1016/j.fitote.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 50.Ju D.T., Liao H.E., Shibu M.A., Ho T.J., Padma V.V., Tsai F.J., Chung L.C., Day C.H., Lin C.C., Huang C.Y. Nerve Regeneration potential of protocatechuic acid in RSC96 Schwann cells by induction of cellular proliferation and migration through IGF-IR-PI3K-Akt signaling. Chin. J. Physiol. 2015;58:412–419. doi: 10.4077/CJP.2015.BAD340. [DOI] [PubMed] [Google Scholar]
- 51.Chang Y.M., Ye C.X., Ho T.J., Tsai T.N., Chiu P.L., Tsai C.C., Lin Y.M., Kuo C.H., Tsai F.J., Tsai C.H., et al. Alpinia oxyphylla Miquel fruit extract activates MAPK-mediated signaling of PAs and MMP2/9 to induce Schwann cell migration and nerve regeneration. Int. J. Artif. Organs. 2014;37:402–413. doi: 10.5301/ijao.5000313. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X.H., Chen J.J. The mechanism of astragaloside IV promoting sciatic nerve regeneration. Neural Regen. Res. 2013;8:2256–2265. doi: 10.3969/j.issn.1673-5374.2013.24.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng C.Y., Yao C.H., Liu B.S., Liu C.J., Chen G.W., Chen Y.S. The role of astragaloside in regeneration of the peripheral nerve system. J. Biomed. Mater. Res. A. 2006;76:463–469. doi: 10.1002/jbm.a.30249. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y.S., Chen C.C., Chang L.C., Yao C.H., Hsu Y.M., Lin J.H., Yang T.Y., Chen Y.H. Increased calcitonin gene-related peptide and macrophages are involved in Astragalus membranaceus-mediated peripheral nerve regeneration in rats. Am. J. Chin. Med. 2018;46:69–86. doi: 10.1142/S0192415X18500040. [DOI] [PubMed] [Google Scholar]
- 55.Fang W.K., Ko F.Y., Wang H.L., Kuo C.H., Chen L.M., Tsai F.J., Tsai C.H., Chen Y.S., Kuo W.W., Huang C.Y. The proliferation and migration effects of huangqi on RSC96 Schwann cells. Am. J. Chin. Med. 2009;37:945–959. doi: 10.1142/S0192415X09007363. [DOI] [PubMed] [Google Scholar]
- 56.Hussin H.M., Lawi M.M., Haflah N.H.M., Kassim A.Y.M., Idrus R.B.H., Lokanathan Y. Centella asiatica (L.)-neurodifferentiated mesenchymal stem cells promote the regeneration of peripheral nerve. Tissue Eng. Regen. Med. 2020;17:237–251. doi: 10.1007/s13770-019-00235-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang C.Y., Kuo W.W., Shibu M.A., Hsueh M.F., Chen Y.S., Tsai F.J., Yao C.H., Lin C.C., Pan L.F., Ju D.T. Citrus medica var. sarcodactylis (foshou) activates fibroblast growth factor-2 signaling to induce migration of RSC96 Schwann cells. Am. J. Chin. Med. 2014;42:443–452. doi: 10.1142/S0192415x14500293. [DOI] [PubMed] [Google Scholar]
- 58.Chen H.T., Tsai Y.L., Chen Y.S., Jong G.P., Chen W.K., Wang H.L., Tsai F.J., Tsai C.H., Lai T.Y., Tzang B.S., et al. Dangshen (Codonopsis pilosula) activates IGF-I and FGF-2 pathways to induce proliferation and migration effects in RSC96 Schwann cells. Am. J. Chin. Med. 2010;38:359–372. doi: 10.1142/S0192415X10007907. [DOI] [PubMed] [Google Scholar]
- 59.Tamaddonfard E., Farshid A.A., Ahmadian E., Hamidhoseyni A. Crocin enhanced functional recovery after sciatic nerve crush injury in rats. Iran. J. Basic Med. Sci. 2013;16:83–90. doi: 10.22038/ijbms.2013.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tehranipour M., Javaheri R. Neuroprotective effect of Curcuma longa alcoholic extract on peripheral nerves degeneration after sciatic nerve compression in rats. J. Biol. Sci. 2009;9:889–893. doi: 10.3923/jbs.2009.889.893. [DOI] [Google Scholar]
- 61.Noorafshan A., Omidi A., Karbalay-Doust S. Curcumin protects the dorsal root ganglion and sciatic nerve after crush in rat. Pathol. Res. Pract. 2011;207:577–582. doi: 10.1016/j.prp.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 62.Noorafshan A., Omidi A., Karbalay-Doust S., Aliabadi E., Dehghani F. Effects of curcumin on the dorsal root ganglion structure and functional recovery after sciatic nerve crush in rat. Micron. 2011;42:449–455. doi: 10.1016/j.micron.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 63.Ma J., Liu J., Yu H., Wang Q., Chen Y., Xiang L. Curcumin promotes nerve regeneration and functional recovery in rat model of nerve crush injury. Neurosci. Lett. 2013;547:26–31. doi: 10.1016/j.neulet.2013.04.054. [DOI] [PubMed] [Google Scholar]
- 64.Tello Velasquez J., Nazareth L., Quinn R.J., Ekberg J.A.K., St John J.A. Stimulating the proliferation, migration and lamellipodia of Schwann cells using low-dose curcumin. Neuroscience. 2016;324:140–150. doi: 10.1016/j.neuroscience.2016.02.073. [DOI] [PubMed] [Google Scholar]
- 65.Yüce S., Cemal Gökçe E., Işkdemir A., Koç E.R., Cemil D.B., Gökçe A., Sargon M.F. An experimental comparison of the effects of propolis, curcumin, and methylprednisolone on crush injuries of the sciatic nerve. Ann. Plast. Surg. 2015;74:684–692. doi: 10.1097/SAP.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 66.Lee J.M., Namgung U.K., Hong K.E. Growth-promoting activity of sanyak (Dioscoreae rhizoma) extract on injured sciatic nerve in rats. JAMS J. Acupunct. Meridian Stud. 2009;2:228–235. doi: 10.1016/S2005-2901(09)60059-5. [DOI] [PubMed] [Google Scholar]
- 67.Chen B., Niu S.P., Wang Z.Y., Wang Z.W., Deng J.X., Zhang P.X., Yin X.F., Han N., Kou Y.H., Jiang B.G. Local administration of icariin contributes to peripheral nerve regeneration and functional recovery. Neural Regen. Res. 2015;10:84–89. doi: 10.4103/1673-5374.150711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kou Y., Wang Z., Wu Z., Zhang P., Zhang Y., Yin X., Wong X., Qiu G., Jiang B. Epimedium extract promotes peripheral nerve regeneration in rats. Evid. Based Complement. Altern. Med. 2013;2013:954798. doi: 10.1155/2013/954798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu B.S., Yao C.H., Hsu S.H., Yeh T.S., Chen Y.S., Kao S.T. A novel use of genipin-fixed gelatin as extracellular matrix for peripheral nerve regeneration. J. Biomater. Appl. 2004;19:21–34. doi: 10.1177/0885328204042544. [DOI] [PubMed] [Google Scholar]
- 70.Zuo W., Xu F., Zhang K., Zheng L., Zhao J. Proliferation-enhancing effects of gastrodin on RSC96 Schwann cells by regulating ERK1/2 and PI3K signaling pathways. Biomed. Pharmacother. 2016;84:747–753. doi: 10.1016/j.biopha.2016.09.106. [DOI] [PubMed] [Google Scholar]
- 71.Zhu Z., Zhou X., He B., Dai T., Zheng C., Yang C., Zhu S., Zhu J., Zhu Q., Liu X. Ginkgo biloba extract (EGb 761) promotes peripheral nerve regeneration and neovascularization after acellular nerve allografts in a rat model. Cell. Mol. Neurobiol. 2015;35:273–282. doi: 10.1007/s10571-014-0122-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hsu S.H., Chang C.J., Tang C.M., Lin F.T. In vitro and in vivo effects of Ginkgo biloba extract EGb 761 on seeded Schwann cells within poly (DL-lactic acid-co-glycolic acid) conduits for peripheral nerve regeneration. J. Biomater. Appl. 2004;19:163–182. doi: 10.1177/0885328204045580. [DOI] [PubMed] [Google Scholar]
- 73.Lu M.C., Lai T.Y., Hwang J.M., Chen H.T., Chang S.H., Tsai F.J., Wang H.L., Lin C.C., Kuo W.W., Huang C.Y. Proliferation- and migration-enhancing effects of ginseng and ginsenoside Rg1 through IGF-I- and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem. Funct. 2009;27:186–192. doi: 10.1002/cbf.1554. [DOI] [PubMed] [Google Scholar]
- 74.Ma J., Li W., Tian R., Lei W. Ginsenoside Rg1 promotes peripheral nerve regeneration in rat model of nerve crush injury. Neurosci. Lett. 2010;478:66–71. doi: 10.1016/j.neulet.2010.04.064. [DOI] [PubMed] [Google Scholar]
- 75.Wang L., Yuan D., Zhang D., Zhang W., Liu C., Cheng H., Song Y., Tan Q. Ginsenoside Re promotes nerve regeneration by facilitating the proliferation, differentiation and migration of Schwann cells via the ERK- and JNK-dependent pathway in rat model of sciatic nerve crush injury. Cell. Mol. Neurobiol. 2015;35:827–840. doi: 10.1007/s10571-015-0177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Renno W.M., Al-Maghrebi M., Al-Banaw A. (-)-Epigallocatechin-3-gallate (EGCG) attenuates functional deficits and morphological alterations by diminishing apoptotic gene overexpression in skeletal muscles after sciatic nerve crush injury. Naunyn. Schmiedebergs. Arch. Pharmacol. 2012;385:807–822. doi: 10.1007/s00210-012-0758-7. [DOI] [PubMed] [Google Scholar]
- 77.Renno W.M., Al-Maghrebi M., Alshammari A., George P. (-)-Epigallocatechin-3-gallate (EGCG) attenuates peripheral nerve degeneration in rat sciatic nerve crush injury. Neurochem. Int. 2013;62:221–231. doi: 10.1016/j.neuint.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 78.Lin Y., Jiang X., Yin G., Lin H. Syringic acid promotes proliferation and migration of Schwann cells via down-regulating MiR-451-5p. Acta Biochim. Biophys. Sin. (Shanghai) 2019;51:1198–1207. doi: 10.1093/abbs/gmz118. [DOI] [PubMed] [Google Scholar]
- 79.Liu B., Liu Y., Yang G., Xu Z., Chen J. Ursolic acid induces neural regeneration after sciatic nerve injury. Neural Regen. Res. 2013;8:2510–2519. doi: 10.3969/j.issn.1673-5374.2013.27.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang J., Tian L., He L., Chen N., Ramakrishna S., So K.F., Mo X. Lycium barbarum polysaccharide encapsulated poly lactic-co-glycolic acid nanofibers: Cost effective herbal medicine for potential application in peripheral nerve tissue engineering. Sci. Rep. 2018;8:8669. doi: 10.1038/s41598-018-26837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang W., Huang C.Y., Tsai F.J., Tsai C.C., Yao C.H., Chen Y.S. Growth-promoting effects of quercetin on peripheral nerves in rats. Int. J. Artif. Organs. 2011;34:1095–1105. doi: 10.5301/ijao.5000064. [DOI] [PubMed] [Google Scholar]
- 82.Lu M., Yi T., Xiong Y., Wang Q., Yin N. Cortex Mori Radicis extract promotes neurite outgrowth in diabetic rats by activating PI3K/AKT signaling and inhibiting Ca2+ influx associated with the upregulation of transient receptor potential canonical channel 1. Mol. Med. Rep. 2020;21:320–328. doi: 10.3892/mmr.2020.11008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hsiang S.W., Lee H.C., Tsai F.J., Tsai C.C., Yao C.H., Chen Y.S. Puerarin accelerates peripheral nerve regeneration. Am. J. Chin. Med. 2011;39:1207–1217. doi: 10.1142/S0192415X11009500. [DOI] [PubMed] [Google Scholar]
- 84.Chen H.-T., Yao C.-H., Chao P.-D.L., Hou Y.-C., Chiang H.-M., Hsieh C.-C., Ke C.-J., Chen Y.-S. Effect of serum metabolites of Pueraria lobata in rats on peripheral nerve regeneration: In vitro and in vivo studies. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007;84B:256–262. doi: 10.1002/jbm.b.30868. [DOI] [PubMed] [Google Scholar]
- 85.Wang Z., Zhang P., Kou Y., Yin X., Han N., Jiang B. Hedysari extract improves regeneration after peripheral nerve injury by enhancing the amplification effect. PLoS ONE. 2013;8:e67921. doi: 10.1371/journal.pone.0067921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei S.Y., Zhang P.X., Han N., Dang Y., Zhang H.B., Zhang D.Y., Fu Z.G., Jiang B.G. Effects of Hedysari polysaccharides on regeneration and function recovery following peripheral nerve injury in rats. Am. J. Chin. Med. 2009;37:57–67. doi: 10.1142/S0192415X09006618. [DOI] [PubMed] [Google Scholar]
- 87.Sheng Q.S., Wang Z.J., Zhang J., Zhang Y.G. Salidroside promotes peripheral nerve regeneration following crush injury to the sciatic nerve in rats. Neuroreport. 2013;24:217–223. doi: 10.1097/WNR.0b013e32835eb867. [DOI] [PubMed] [Google Scholar]
- 88.Zuo W., Wu H., Zhang K., Lv P., Xu F., Jiang W., Zheng L., Zhao J. Baicalin promotes the viability of Schwann cells in vitro by regulating neurotrophic factors. Exp. Ther. Med. 2017;14:507–514. doi: 10.3892/etm.2017.4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morani A.S., Bodhankar S.L., Mohan V., Thakurdesai P.A. Ameliorative effects of standardized extract from Trigonella foenum-graecum L. seeds on painful peripheral neuropathy in rats. Asian Pac. J. Trop. Med. 2012;5:385–390. doi: 10.1016/S1995-7645(12)60064-9. [DOI] [PubMed] [Google Scholar]
- 90.Zhang Y.G., Sheng Q.S., Wang H.K., Lv L., Zhang J., Chen J.M., Xu H. Triptolide improves nerve regeneration and functional recovery following crush injury to rat sciatic nerve. Neurosci. Lett. 2014;561:198–202. doi: 10.1016/j.neulet.2013.12.068. [DOI] [PubMed] [Google Scholar]
- 91.Naik A.K., Latham J.R., Obradovic A., Jevtovic-Todorovic V. Dorsal root ganglion application of muscimol prevents hyperalgesia and stimulates myelin protein expression after sciatic nerve injury in rats. Anesth. Analg. 2012;114:674–682. doi: 10.1213/ANE.0b013e31823fad7e. [DOI] [PubMed] [Google Scholar]
- 92.Wong K.H., Kanagasabapathy G., Naidu M., David P., Sabaratnam V. Hericium erinaceus (Bull.: Fr.) Pers., a medicinal mushroom, activates peripheral nerve regeneration. Chin. J. Integr. Med. 2016;22:759–767. doi: 10.1007/s11655-014-1624-2. [DOI] [PubMed] [Google Scholar]
- 93.Wong K.H., Kanagasabapathy G., Bakar R., Phan C.W., Sabaratnam V. Restoration of sensory dysfunction following peripheral nerve injury by the polysaccharide from culinary and medicinal mushroom, Hericium erinaceus (Bull.: Fr.) Pers. through its neuroregenerative action. Food Sci. Technol. 2015;35:712–721. doi: 10.1590/1678-457X.6838. [DOI] [Google Scholar]
- 94.Wong K.H., Naidu M., David P., Abdulla M.A., Abdullah N., Kuppusamy U.R., Sabaratnam V. Peripheral nerve regeneration following crush injury to rat peroneal nerve by aqueous extract of medicinal mushroom Hericium erinaceus (Bull.: Fr) Pers. (Aphyllophoromycetideae) Evid. Based Complement. Altern. Med. 2011;2011:580752. doi: 10.1093/ecam/neq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Farha M., Parkianathan L., Amir N.A.I.A., Sabaratnam V., Wong K.H. Functional recovery enhancement by tiger milk mushroom, Lignosus rhinocerotis in a sciatic nerve crush injury model and morphological study of its neurotoxicity. J. Anim. Plant Sci. 2019;29:930–942. [Google Scholar]
- 96.Kim K.J., Namgung U., Cho C.S. Protective effects of Bogijetong decoction and its selected formula on neuropathic insults in streptozotocin-induced diabetic animals. Evid. Based Complement. Altern. Med. 2017;2017:4296318. doi: 10.1155/2017/4296318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chen Y.S., Yao C.H., Chen T.H., Hsieh C.L., Lao C.J., Tsai C.C. Effect of Buyang Huanwu decoction on peripheral nerve regeneration using silicone rubber chambers. Am. J. Chin. Med. 2001;29:423–432. doi: 10.1142/S0192415X01000447. [DOI] [PubMed] [Google Scholar]
- 98.Wang Y., Chen Z., Ye R., He Y., Li Y., Qiu X. Protective effect of Jiaweibugan decoction against diabetic peripheral neuropathy. Neural Regen. Res. 2013;8:1113–1121. doi: 10.3969/j.issn.1673-5374.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang Z.Y., Qin L.H., Zhang W.G., Zhang P.X., Jiang B.G. Qian-Zheng-San promotes regeneration after sciatic nerve crush injury in rats. Neural Regen. Res. 2019;14:683–691. doi: 10.4103/1673-5374.247472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nocera G., Jacob C. Mechanisms of Schwann cell plasticity involved in peripheral nerve repair after injury. Cell Mol. Life Sci. 2020;77:3977–3989. doi: 10.1007/s00018-020-03516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Srinivasulu C., Ramgopal M., Ramanjaneyulu G., Anuradha C.M., Suresh Kumar C. Syringic acid (SA) ‒ A review of its occurrence, biosynthesis, pharmacological and industrial importance. Biomed. Pharmacother. 2018;108:547–557. doi: 10.1016/j.biopha.2018.09.069. [DOI] [PubMed] [Google Scholar]
- 102.Baliga M.S., Shivashankara A.R., Venkatesh S., Bhat H.P., Palatty P.L., Bhandari G., Rao S. Phytochemicals in the prevention of ethanol-induced hepatotoxicity: A revisit. In: Watson R.R., Preedy V.R., editors. Dietary Interventions in Liver Disease: Foods, Nutrients, and Dietary Supplements. Academic Press; Cambridge, MA, USA: 2019. pp. 79–89. [Google Scholar]
- 103.Li Y., Yao J., Han C., Yang J., Chaudhry M.T., Wang S., Liu H., Yin Y. Quercetin, inflammation and immunity. Nutrients. 2016;8:167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desbarats J., Birge R.B., Mimouni-Rongy M., Weinstein D.E., Palerme J.S., Newell M.K. Fas engagement induces neurite growth through ERK activation and P35 upregulation. Nat. Cell Biol. 2003;5:118–125. doi: 10.1038/ncb916. [DOI] [PubMed] [Google Scholar]
- 105.Schneider-Schaulies J., Kirchhoff F., Archelos J., Schachner M. Down-regulation of myelin-associated glycoprotein on Schwann cells by interferon-γ and tumor necrosis factor-α affects neurite outgrowth. Neuron. 1991;7:995–1005. doi: 10.1016/0896-6273(91)90344-Y. [DOI] [PubMed] [Google Scholar]
- 106.Larsson K., Rydevik B., Olmarker K. Disc related cytokines inhibit axonal outgrowth from dorsal root ganglion cells in vitro. Spine. 2005;30:621–624. doi: 10.1097/01.brs.0000155410.48700.9e. [DOI] [PubMed] [Google Scholar]
- 107.Kato K., Liu H., Kikuchi S.I., Myers R.R., Shubayev V.I. Immediate anti-tumor necrosis factor-α (etanercept) therapy enhances axonal regeneration after sciatic nerve crush. J. Neurosci. Res. 2010;88:360–368. doi: 10.1002/jnr.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newbern J.M., Li X., Shoemaker S.E., Zhou J., Zhong J., Wu Y., Bonder D., Hollenback S., Coppola G., Geschwind D.H., et al. Specific functions for ERK/MAPK signaling during PNS development. Neuron. 2011;69:91–105. doi: 10.1016/j.neuron.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ishii A., Furusho M., Bansal R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and Schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci. 2013;33:175–186. doi: 10.1523/JNEUROSCI.4403-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Harrisingh M.C., Perez-Nadales E., Parkinson D.B., Malcolm D.S., Mudge A.W., Lloyd A.C. The Ras/Raf/ERK signalling pathway drives Schwann cell dedifferentiation. EMBO J. 2004;23:3061–3071. doi: 10.1038/sj.emboj.7600309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agthong S., Kaewsema A., Tanomsridejchai N., Chentanez V. Activation of MAPK ERK in peripheral nerve after injury. BMC Neurosci. 2006;7:45. doi: 10.1186/1471-2202-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yamazaki T., Sabit H., Oya T., Ishii Y., Hamashima T., Tokunaga A., Ishizawa S., Jie S., Kurashige Y., Matsushima T., et al. Activation of MAP kinases, Akt and PDGF receptors in injured peripheral nerves. J. Peripher. Nerv. Syst. 2009;14:165–176. doi: 10.1111/j.1529-8027.2009.00228.x. [DOI] [PubMed] [Google Scholar]
- 113.Huang H., Sun Z., Liu H., Ma J., Hu M. ERK/MAPK and PI3K/AKT signal channels simultaneously activated in nerve cell and axon after facial nerve injury. Saudi J. Biol. Sci. 2017;24:1853–1858. doi: 10.1016/j.sjbs.2017.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yang D.P., Kim J., Syed N., Tung Y.J., Bhaskaran A., Mindos T., Mirsky R., Jessen K.R., Maurel P., Parkinson D.B., et al. P38 MAPK activation promotes denervated Schwann cell phenotype and functions as a negative regulator of Schwann cell differentiation and myelination. J. Neurosci. 2012;32:7158–7168. doi: 10.1523/JNEUROSCI.5812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pines J. Four-dimensional control of the cell cycle. Nat. Cell Biol. 1999;153:E73. doi: 10.1038/11041. [DOI] [PubMed] [Google Scholar]
- 116.Lim S., Kaldis P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development. 2013;140:3079–3093. doi: 10.1242/dev.091744. [DOI] [PubMed] [Google Scholar]
- 117.Mairet-Coello G., Tury A., DiCicco-Bloom E. Insulin-like growth factor-1 promotes G1/S cell cycle progression through bidirectional regulation of cyclins and cyclin-dependent kinase inhibitors via the phosphatidylinositol 3-kinase/Akt pathway in developing rat cerebral cortex. J. Neurosci. 2009;29:775–788. doi: 10.1523/JNEUROSCI.1700-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Syroid D.E., Zorick T.S., Arbet-Engels C., Kilpatrick T.J., Eckhart W., Lemke G. A role for insulin-like growth factor-I in the regulation of Schwann cell survival. J. Neurosci. 1999;19:2059–2068. doi: 10.1523/JNEUROSCI.19-06-02059.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hansson H.A., Dahlin L.B., Danielsen N., Fryklund L., Nachemson A.K., Polleryd P., Rozell B., Skottner A., Stemme S., Lundborg G. Evidence indicating trophic importance of IGF-I in regenerating peripheral nerves. Acta Physiol. Scand. 1986;126:609–614. doi: 10.1111/j.1748-1716.1986.tb07862.x. [DOI] [PubMed] [Google Scholar]
- 120.Cheng H.L., Randolph A., Yee D., Delafontaine P., Tennekoon G., Feldman E.L. Characterization of insulin-like growth factor-I and its receptor and binding proteins in transected nerves and cultured Schwann cells. J. Neurochem. 1996;66:525–536. doi: 10.1046/j.1471-4159.1996.66020525.x. [DOI] [PubMed] [Google Scholar]
- 121.Apel P.J., Ma J., Callahan M., Northam C.N., Alton T.B., Sonntag W.E., Li Z. Effect of locally delivered IGF-1 on nerve regeneration during aging: An experimental study in rats. Muscle Nerve. 2010;41:335–341. doi: 10.1002/mus.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu H., Xue C., Yao M., Wang H., Zhang P., Qian T., Zhou S., Li S., Yu B., Wang Y., et al. MIR-129 controls axonal regeneration via regulating insulin-like growth factor-1 in peripheral nerve injury. Cell Death Dis. 2018;9:720. doi: 10.1038/s41419-018-0760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sowndhararajan K., Deepa P., Kim M., Park S.J., Kim S. Neuroprotective and cognitive enhancement potentials of baicalin: A review. Brain Sci. 2018;8:104. doi: 10.3390/brainsci8060104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stankoff B., Aigrot M.-S., Noël F., Wattilliaux A., Zalc B., Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: A novel role for CNTF and CNTF-related molecules. J. Neurosci. 2002;22:9221–9227. doi: 10.1523/JNEUROSCI.22-21-09221.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xiao J., Hughes R.A., Lim J.Y., Wong A.W., Ivanusic J.J., Ferner A.H., Kilpatrick T.J., Murray S.S. A small peptide mimetic of brain-derived neurotrophic factor promotes peripheral myelination. J. Neurochem. 2013;125:386–398. doi: 10.1111/jnc.12168. [DOI] [PubMed] [Google Scholar]
- 126.Wilhelm J.C., Xu M., Cucoranu D., Chmielewski S., Holmes T., Lau K.S., Bassell G.J., English A.W. Cooperative roles of BDNF expression in neurons and Schwann cells are modulated by exercise to facilitate nerve regeneration. J. Neurosci. 2012;32:5002–5009. doi: 10.1523/JNEUROSCI.1411-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Tannemaat M.R., Eggers R., Hendriks W.T., De Ruiter G.C.W., Van Heerikhuize J.J., Pool C.W., Malessy M.J.A., Boer G.J., Verhaagen J. Differential effects of lentiviral vector-mediated overexpression of nerve growth factor and glial cell line-derived neurotrophic factor on regenerating sensory and motor axons in the transected peripheral nerve. Eur. J. Neurosci. 2008;28:1467–1479. doi: 10.1111/j.1460-9568.2008.06452.x. [DOI] [PubMed] [Google Scholar]
- 128.Yun Y.R., Won J.E., Jeon E., Lee S., Kang W., Jo H., Jang J.H., Shin U.S., Kim H.W. Fibroblast growth factors: Biology, function, and application for tissue regeneration. J. Tissue Eng. 2010;1:218142. doi: 10.4061/2010/218142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Holland E.C., Varmus H.E. Basic fibroblast growth factor induces cell migration and proliferation after glia-specific gene transfer in mice. Proc. Natl. Acad. Sci. USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hossain W.A., Morest D.K. Fibroblast growth factors (FGF-1, FGF-2) promote migration and neurite growth of mouse cochlear ganglion cells in vitro: Immunohistochemistry and antibody perturbation. J. Neurosci. Res. 2000;62:40–55. doi: 10.1002/1097-4547(20001001)62:1<40::AID-JNR5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 131.Chen B., Hu R., Min Q., Li Y., Parkinson D.B., Dun X. FGF5 regulates Schwann cell migration and adhesion. Front. Cell. Neurosci. 2020;14:1–12. doi: 10.3389/fncel.2020.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li J., Wuliji O., Li W., Jiang Z.G., Ghanbari H.A. Oxidative stress and neurodegenerative disorders. Int. J. Mol. Sci. 2013;14:24438–24475. doi: 10.3390/ijms141224438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Areti A., Yerra V.G., Naidu V.G.M., Kumar A. Oxidative stress and nerve damage: Role in chemotherapy induced peripheral neuropathy. Redox Biol. 2014;2:289–295. doi: 10.1016/j.redox.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Li Y., Shi Z., Yu X., Feng P., Wang X.-J. The effects of urotensin II on migration and invasion are mediated by NADPH oxidase-derived reactive oxygen species through the c-Jun N-terminal kinase pathway in human hepatoma cells. Peptides. 2017;88:106–114. doi: 10.1016/j.peptides.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 135.Ma S., Fu A., Lim S., Chiew G.G.Y., Luo K.Q. MnSOD mediates shear stress-promoted tumor cell migration and adhesion. Free Radic. Biol. Med. 2018;129:46–58. doi: 10.1016/j.freeradbiomed.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 136.Yu J.S.L., Cui W. Proliferation, survival and metabolism: The role of PI3K/AKT/MTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 137.Anitha M., Gondha C., Sutliff R., Parsadanian A., Mwangi S., Sitaraman S.V., Srinivasan S. GDNF rescues hyperglycemia-induced diabetic enteric neuropathy through activation of the PI3K/Akt pathway. J. Clin. Invest. 2006;116:344–356. doi: 10.1172/JCI26295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chen L., Gong H.Y., Xu L. PVT1 protects diabetic peripheral neuropathy via PI3K/AKT pathway. Eur. Rev. Med. Pharmacol. Sci. 2018;22:6905–6911. doi: 10.26355/eurrev_201810_16160. [DOI] [PubMed] [Google Scholar]
- 139.Shim S., Ming G.L. Roles of channels and receptors in the growth cone during PNS axonal regeneration. Exp. Neurol. 2010;223:38–44. doi: 10.1016/j.expneurol.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu D., Huang W., Richardson P.M., Priestley J.V., Liu M. TRPC4 in rat dorsal root ganglion neurons is increased after nerve injury and is necessary for neurite outgrowth. J. Biol. Chem. 2008;283:416–426. doi: 10.1074/jbc.M703177200. [DOI] [PubMed] [Google Scholar]
- 141.Heo D.K., Chung W.Y., Park H.W., Yuan J.P., Lee M.G., Kim J.Y. Opposite regulatory effects of TRPC1 and TRPC5 on neurite outgrowth in PC12 cells. Cell. Signal. 2012;24:899–906. doi: 10.1016/j.cellsig.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 142.Du Y., Tang J., Li G., Berti-Mattera L., Lee C.A., Bartkowski D., Gale D., Monahan J., Niesman M.R., Alton G., et al. Effects of P38 MAPK inhibition on early stages of diabetic retinopathy and sensory nerve function. Investig. Ophthalmol. Vis. Sci. 2010;51:2158–2164. doi: 10.1167/iovs.09-3674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Suryavanshi S.V., Kulkarni Y.A. NF-Κβ: A potential target in the management of vascular complications of diabetes. Front. Pharmacol. 2017;8:798. doi: 10.3389/fphar.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ruff C.A., Staak N., Patodia S., Kaswich M., Rocha-Ferreira E., Da Costa C., Brecht S., Makwana M., Fontana X., Hristova M., et al. Neuronal c-Jun is required for successful axonal regeneration, but the effects of phosphorylation of its N-terminus are moderate. J. Neurochem. 2012;121:607–618. doi: 10.1111/j.1471-4159.2012.07706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kato N., Matsumoto M., Kogawa M., Atkins G.J., Findlay D.M., Fujikawa T., Oda H., Ogata M. Critical role of P38 MAPK for regeneration of the sciatic nerve following crush injury in vivo. J. Neuroinflammation. 2013;10:757. doi: 10.1186/1742-2094-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sang Q., Sun D., Chen Z., Zhao W. NGF and PI3K/Akt signaling participate in the ventral motor neuronal protection of curcumin in sciatic nerve injury rat models. Biomed. Pharmacother. 2018;103:1146–1153. doi: 10.1016/j.biopha.2018.04.116. [DOI] [PubMed] [Google Scholar]
- 147.Holahan M.R. A shift from a pivotal to supporting role for the growth-associated protein (GAP-43) in the coordination of axonal structural and functional plasticity. Front. Cell. Neurosci. 2017;11:266. doi: 10.3389/fncel.2017.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Benowitz L.I., Routtenberg A. GAP-43: An intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 1997;20:84–91. doi: 10.1016/S0166-2236(96)10072-2. [DOI] [PubMed] [Google Scholar]
- 149.Tang X., Chen Y., Gu X., Ding F. Achyranthes bidentata Blume extract promotes neuronal growth in cultured embryonic rat hippocampal neurons. Prog. Nat. Sci. 2009;19:549–555. doi: 10.1016/j.pnsc.2008.08.008. [DOI] [Google Scholar]
- 150.Fregnan F., Muratori L., Simões A.R., Giacobini-Robecchi M.G., Raimondo S. Role of inflammatory cytokines in peripheral nerve injury. Neural Regen. Res. 2012;7:2259–2266. doi: 10.3969/j.issn.1673-5374.2012.29.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Li J., Wei G.H., Huang H., Lan Y.P., Liu B., Liu H., Zhang W., Zuo Y.X. Nerve injury-related autoimmunity activation leads to chronic inflammation and chronic neuropathic pain. Anesthesiology. 2013;118:416–429. doi: 10.1097/ALN.0b013e31827d4b82. [DOI] [PubMed] [Google Scholar]
- 152.Hobson M.I., Gree C.J., Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J. Anat. 2000;197:591–605. doi: 10.1046/j.1469-7580.2000.19740591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Fang Z., Ge X., Chen X., Xu Y., Yuan W.E., Ouyang Y. Enhancement of sciatic nerve regeneration with dual delivery of vascular endothelial growth factor and nerve growth factor genes. J. Nanobiotechnol. 2020;18:46. doi: 10.1186/s12951-020-00606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee J., Shin J.E., Lee B., Kim H., Jeon Y., Ahn S.H., Chi S.W., Cho Y. The stem cell marker Prom1 promotes axon regeneration by down-regulating cholesterol synthesis via Smad signaling. Proc. Natl. Acad. Sci. USA. 2020;117:15955–15966. doi: 10.1073/pnas.1920829117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jankowski M.P., McIlwrath S.L., Jing X., Cornuet P.K., Salerno K.M., Koerber H.R., Albers K.M. Sox11 Transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Leibinger M., Müller A., Gobrecht P., Diekmann H., Andreadaki A., Fischer D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013;4:e609. doi: 10.1038/cddis.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cox A.A., Sagot Y., Hedou G., Grek C., Wilkes T., Vinik A.I., Ghatnekar G. Low-dose pulsatile interleukin-6 as a treatment option for diabetic peripheral neuropathy. Front. Endocrinol. (Lausanne) 2017;8:89. doi: 10.3389/fendo.2017.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Snipes G.J., Suter U., Welcher A.A., Shooter E.M. Characterization of a novel peripheral nervous system myelin protein (PMP- 22/SR13) J. Cell Biol. 1992;117:225–238. doi: 10.1083/jcb.117.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kuhn G., Lie A., Wilms S., Müller H.W. Coexpression of PMP22 gene with MBP and P0 during de novo myelination and nerve repair. Glia. 1993;8:256–264. doi: 10.1002/glia.440080406. [DOI] [PubMed] [Google Scholar]
- 160.Martin L.J., Kaiser A., Price A.C. Motor neuron degeneration after sciatic nerve avulsion in adult rat evolves with oxidative stress and is apoptosis. J. Neurobiol. 1999;40:185–201. doi: 10.1002/(SICI)1097-4695(199908)40:2<185::AID-NEU5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 161.Liu Y., Wang H. Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration. Neural Regen. Res. 2020;15:189–198. doi: 10.4103/1673-5374.265540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Amiri S., Movahedin M., Mowla S.J., Hajebrahimi Z., Tavallaei M. Differential gene expression and alternative splicing of survivin following mouse sciatic nerve injury. Spinal Cord. 2009;47:739–744. doi: 10.1038/sc.2009.26. [DOI] [PubMed] [Google Scholar]
- 163.Liu Z., Zhu S., Liu L., Ge J., Huang L., Sun Z., Zeng W., Huang J., Luo Z. A magnetically responsive nanocomposite scaffold combined with Schwann cells promotes sciatic nerve regeneration upon exposure to magnetic field. Int. J. Nanomedicine. 2017;12:7815–7832. doi: 10.2147/IJN.S144715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lundborg G., Dahlin L.B., Danielsen N., Gelberman R.H., Longo F.M., Powell H.C., Varon S. Nerve regeneration in silicone chambers: Influence of gap length and of distal stump components. Exp. Neurol. 1982;76:361–375. doi: 10.1016/0014-4886(82)90215-1. [DOI] [PubMed] [Google Scholar]
- 165.Chang Y.C., Chen M.H., Liao S.Y., Wu H.C., Kuan C.H., Sun J.S., Wang T.W. Multichanneled nerve guidance conduit with spatial gradients of neurotrophic factors and oriented nanotopography for repairing the peripheral nervous system. ACS Appl. Mater. Interfaces. 2017;9:37623–37636. doi: 10.1021/acsami.7b12567. [DOI] [PubMed] [Google Scholar]
- 166.Vijayavenkataraman S. Nerve guide conduits for peripheral nerve injury repair: A review on design, materials and fabrication methods. Acta Biomater. 2020;106:54–69. doi: 10.1016/j.actbio.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 167.Fathi S.S., Zaminy A. Stem cell therapy for nerve injury. World J. Stem Cells. 2017;9:144–151. doi: 10.4252/wjsc.v9.i9.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Willand M.P., Nguyen M.A., Borschel G.H., Gordon T. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil. Neural Repair. 2016;30:490–496. doi: 10.1177/1545968315604399. [DOI] [PubMed] [Google Scholar]
- 169.Turner P.V., Brabb T., Pekow C., Vasbinder M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011;50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 170.Katiyar C., Kanjilal S., Gupta A., Katiyar S. Drug discovery from plant sources: An integrated approach. AYU (An. Int. Q. J. Res. Ayurveda) 2012;33:10–19. doi: 10.4103/0974-8520.100295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Atanasov A.G., Waltenberger B., Pferschy-Wenzig E.M., Linder T., Wawrosch C., Uhrin P., Temml V., Wang L., Schwaiger S., Heiss E.H., et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015;33:1582–1614. doi: 10.1016/j.biotechadv.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Singh S., Sharma B., Kanwar S.S., Kumar A. Lead phytochemicals for anticancer drug development. Front. Plant Sci. 2016;7:1667. doi: 10.3389/fpls.2016.01667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Lu C.C., Hsu Y.J., Chang C.J., Lin C.S., Martel J., Ojcius D.M., Ko Y.F., Lai H.C., Young J.D. Immunomodulatory properties of medicinal mushrooms: Differential effects of water and ethanol extracts on NK cell-mediated cytotoxicity. Innate Immun. 2016;22:522–533. doi: 10.1177/1753425916661402. [DOI] [PubMed] [Google Scholar]
- 174.Seow S.L.-S., Naidu M., David P., Wong K.H., Sabaratnam V. Potentiation of neuritogenic activity of medicinal mushrooms in rat pheochromocytoma cells. BMC Complement. Altern. Med. 2013;13:157. doi: 10.1186/1472-6882-13-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Wells M.L., Potin P., Craigie J.S., Raven J.A., Merchant S.S., Helliwell K.E., Smith A.G., Camire M.E., Brawley S.H. Algae as nutritional and functional food sources: Revisiting our understanding. J. Appl. Phycol. 2017;29:949–982. doi: 10.1007/s10811-016-0974-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Khan M.I., Shin J.H., Kim J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018;17:1–21. doi: 10.1186/s12934-018-0879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ariede M.B., Candido T.M., Jacome A.L.M., Velasco M.V.R., de Carvalho J.C.M., Baby A.R. Cosmetic attributes of algae—A review. Algal Res. 2017;25:483–487. doi: 10.1016/j.algal.2017.05.019. [DOI] [Google Scholar]
- 178.Borowitzka M.A. Microalgae as sources of pharmaceuticals and other biologically active compounds. J. Appl. Phycol. 1995;7:3–15. doi: 10.1007/BF00003544. [DOI] [Google Scholar]
- 179.Shannon E., Abu-Ghannam N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs. 2016;14:81. doi: 10.3390/md14040081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Barbosa M., Valentão P., Andrade P.B. Bioactive compounds from macroalgae in the new millennium: Implications for neurodegenerative diseases. Mar. Drugs. 2014;12:4934–4972. doi: 10.3390/md12094934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Olasehinde T.A., Olaniran A.O., Okoh A.I. Macroalgae as a valuable source of naturally occurring bioactive compounds for the treatment of Alzheimer’s disease. Mar. Drugs. 2019;17:609. doi: 10.3390/md17110609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hannan M.A., Mohibbullah M., Hwang S.Y., Lee K., Kim Y.C., Hong Y.K., Moon I.S. Differential neuritogenic activities of two edible brown macroalgae, Undaria pinnatifida and Saccharina japonica. Am. J. Chin. Med. 2014;42:1371–1384. doi: 10.1142/S0192415X14500864. [DOI] [PubMed] [Google Scholar]
- 183.Mohibbullah M., Bhuiyan M.M.H., Hannan M.A., Getachew P., Hong Y.K., Choi J.S., Choi I.S., Moon I.S. The edible red alga Porphyra yezoensis promotes neuronal survival and cytoarchitecture in primary hippocampal neurons. Cell. Mol. Neurobiol. 2016;36:669–682. doi: 10.1007/s10571-015-0247-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Tirtawijaya G., Mohibbullah M., Meinita M.D.N., Moon I.S., Hong Y.K. The ethanol extract of the rhodophyte Kappaphycus alvarezii promotes neurite outgrowth in hippocampal neurons. J. Appl. Phycol. 2016;28:2515–2522. doi: 10.1007/s10811-016-0795-6. [DOI] [Google Scholar]
- 185.Pang J.R., Goh V.M.J., Tan C.Y., Phang S.M., Wong K.H., Yow Y.Y. Neuritogenic and in vitro antioxidant activities of Malaysian Gracilaria manilaensis Yamamoto & Trono. J. Appl. Phycol. 2018;30:3253–3260. doi: 10.1007/s10811-018-1438-x. [DOI] [Google Scholar]