Abstract
An acute respiratory illness caused by a novel coronavirus, namely, severe acute respiratory syndrome coronavirus 2, the virus that causes coronavirus disease 2019 (COVID-19), began spreading across China in late December 2019. The disease gained global attention as it spread worldwide. Since the COVID-19 pandemic began, many studies have focused on the impact of the disease on conditions such as diabetes, cardiovascular disease, pulmonary disorders, and renal malfunction. However, few studies have focused on musculoskeletal disorders related to COVID-19 infection. In this review, we update the current knowledge on the coronavirus with special reference to its effects during and after the pandemic on musculoskeletal aliments, which may inform clinical practice.
Keywords: Coronaviruses, COVID-19, Musculoskeletal, Infection, Pandemic, Orthopaedics
Core Tip: Severe acute respiratory syndrome coronavirus 2, the virus that causes coronavirus disease 2019 (COVID-19), began spreading across China in late December 2019 and became a pandemic. This review focuses on musculoskeletal signs and symptoms of COVID-19 infection. Furthermore, a hypothetical pathway showing factor-induced hypoxic conditions and their downflow changes in the musculoskeletal system during severe COVID-19 infection are discussed.
INTRODUCTION
Coronaviruses, RNA-positive sensory viruses, are 60–140 nm diameter spheres with spiked projections that make them appear crown-like under an electron microscope[1]. Subsequent to β-coronavirus and pneumonia outbreaks occurring in 2019 in Wuhan, China, the World Health Organization (WHO) named the novel coronavirus 2019-nCoV on January 12, 2020. The WHO formally identified coronavirus disease 2019 as COVID-19, and the Coronaviridae Study Group of the International Committee on Taxonomy of Viruses designated the severe acute respiratory syndrome coronavirus as SARS-CoV-2 on February 11, 2020[2]. The WHO declared that the COVID-19 outbreak was a global pandemic on March 11, 2020.
In more than 210 countries and territories, the virus spread within a brief period. Almost 58699047 cases of COVID-19 have been reported to date, of which 40639477 people have recovered and 1389562 people have died. The numbers are increasing, so these figures are changing every day[3].
The enormous potential of infection and low-to-moderate death rates due to COVID-19 have presented a significant challenge to health care systems worldwide. Furthermore, according to the current information, the novel coronavirus is predominately transmitted through aerosols and direct contact with infected surfaces. Therefore, for any specific prophylactic measures, both of these types of transmission need to be considered.
In this pandemic situation, it is a misconception that COVID-19 affects only specific health care specialties/systems. In fact, all body systems are affected by COVID-19. It has been observed that most developed countries, even those with well-established health care systems, have suffered greatly due to COVID-19. Therefore, it is not difficult to understand the disastrous effect of COVID-19 in low-resource countries with very limited health care . In the present review, we provide information about coronavirus with special reference to musculoskeletal aliments that have been found in COVID-19 patients during the pandemic and may continue to appear after the pandemic is over, thereby affecting clinical practice.
CORONAVIRUSES
Severe acute respiratory syndrome coronavirus (SARS-CoV) first appeared in 2003, and later, in 2012, the Middle East Respiratory Syndrome coronavirus (MERS-CoV) emerged, although the first coronavirus was reported even earlier, in 1965[4]. The diverse family of viruses infects mammalian and avian hosts' respiratory and gastrointestinal tracts, and the bat is a natural reservoir for these viruses[5]. The virus belongs to the Nidovirales order, which includes four families: Coronaviridae, Arteriviridae, Roniviridae and Mesoniviridae, in which Coronaviridae consists of a vast genome size of 26–32 kb; again, it has a subfamily, coronavirinae and toronavirinae with four genera, α, β, γ and Δ coronavirus[6].
SARS-CoV-2 is the causative agent of the novel COVID-19 and belongs to the β-genera coronavirus family. The virus consists of positive single-stranded RNA with a single linear RNA fragment[7]. Under an electron microscope, it appears crown-shaped, circular or oval with a diameter of 60–140 nm, and the length of the genome is 30 kb[8].
Types of coronaviruses
According to the U.S. Centers for Disease Control, there are seven varieties of coronaviruses: 229E, NL63 (α coronaviruses), OC43, and HKU1 are beta coronaviruses, MERS-CoV (β coronavirus), SARS-CoV (β coronaviruses), and COVID-19 (SARS-CoV-2)[9].
Differential molecular structure of COVID-19
A study on protein sequences found that there is 94.6% similarity among all seven nonstructural proteins and amino acids as well as the genomes of both COVID-19 and SARS-CoV. The spikes on the viruses consist of two linked parts; when those halves divide, the spike activates, and only then does the virus reach the host cell. This division happens in SARS-CoV with some difficulty. Nevertheless, in SARS-CoV-2, the bridge that links the two halves can be quickly broken by an enzyme named furin produced by human cells and crucially in several tissues[8]. COVID-19 shares a 79.5% identical genomic structure with SARS CoV[9].
Key virulent factor of COVID-19
According to a report by Wu et al[10], nonstructural protein 1 (Nsp1), Nsp3c, and open reading frame 7a (ORF7a) are the three main coronavirus virulence factors that interfere with the host’s innate immunity and assist in coronavirus immune escape. Nsp1 interacts with the host’s 40S ribosomal subunit, which directly causes mRNA degradation in the host and inhibits interferon development. Nsp3c has the potential to bind ADP ribose from the host to allow coronavirus escape of innate immunity. In addition, bone marrow matrix antigen 2 (BST2) may prevent host cells from releasing newly assembled coronavirus. SARS-CoV ORF7a binds directly to BST2 and inhibits its activity by preventing BST2 glycosylation[10].
What is COVID-19?
COVID-19 is an infectious disease caused by a novel coronavirus that may induce flu-like symptoms, such as fever and dry cough (the two most frequent symptoms), weakness, nausea, and nasal congestion. As the pandemic progresses globally, certain new signs have arisen, such as loss of smell and taste. COVID-19 has a fatality risk of 4.4%, which is significantly lower than that of SARS (10%) and MERS-CoV (approximately 30%). However, the potency of infection with COVID-19 is much greater than that of either SARS or MERS-CoV. In addition, it is crucially undetectable, even asymptomatic patients or patients with minor symptoms are able to transmit the infection[11].
Why is COVID-19 the most virulent form?
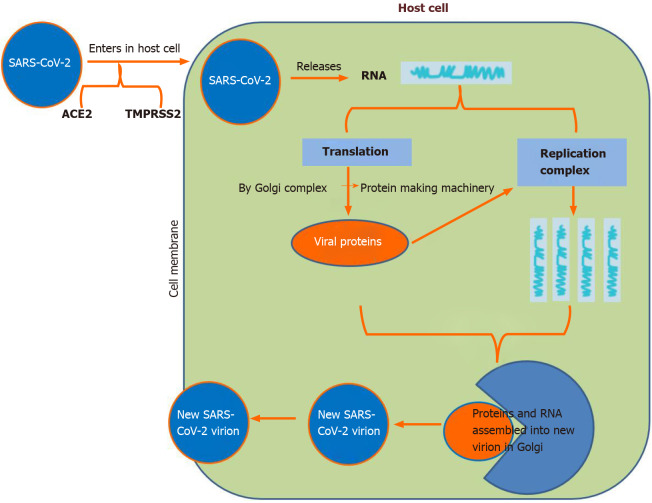
A study showed that the virus uses the human angiotensin-converting enzyme-2 (ACE-2) receptor and recognizes it with comparable affinity to SARS-CoV isolates, which indicates that it can spread effectively in humans[12]. Researchers also found that the RBD spike protein of COVID-19 binds with human ACE-2. This is why the COVID-19 spike protein with high affinity (10–20 times) to human ACE-2 is the most contagious and virulent form (Figure 1)[13,14].
Figure 1.
Spike protein on the virion binds to angiotensin-converting enzyme-2 (cell surface protein), transmembrane protease serine-2, an enzyme that helps the virion enter and release virion RNA. Some RNA is translated into proteins by the cell machinery; some of these proteins form replication complexes to make more RNA. The proteins and RNA assemble into a new virion in the Golgi and are finally released. ACE-2: Angiotensin-converting enzyme-2; SARS-CoV-2: Severe acute respiratory syndrome coronavirus-2; TMPRSS-2: Transmembrane protease serine-2.
IMPACT OF RISK FACTORS AND COMORBIDITIES ON THE CLINICAL OUTCOME OF COVID-19-INFECTED PATIENTS
Previous studies[15-29] have indicated and correlated the risk and severity of COVID-19 illness in patients with comorbidities and compared these patients to those without comorbidities. Therefore, triage is carried out by carefully reviewing the medical history of COVID-19 patients, as this will help to distinguish patients according to their prognoses. Patients with COVID-19 who have any associated comorbidities are at high risk and should be treated with extra caution in an intensive care unit (ICU) facility if needed.
There is an urgent need to consider severe clinical comorbidities to improve risk stratification and strategic planning for COVID-19 patients. Based on the knowledge and clinical experience currently available, older people and people of all ages with severe underlying medical conditions, such as diabetes, cardiovascular disease, pulmonary disorders, and renal malfunction, could be at higher risk of acute disease following COVID-19[15].
In a retrospective study, Zhou et al[16] found that advanced age, higher rates of sepsis-related organ failure assessment score, and high d-dimer at enrolment were risk factors for the deaths of COVID-19 adult patients[16]. In the sex-based comparison, there were more male than female patients. This inequality indicates that women have more robust innate and adaptive immune responses[17,18]. However, this observation may also be due to occupational risk factors for men in Huanan’s wet market[19].
According to some studies[20-23], the most prominent comorbidity with COVID-19 infections was hypertension (14%-30%) and diabetes (6%-19%), followed by cardiovascular diseases such as acute cardiac injury or failure (4%-8%) and respiratory system diseases such as pulmonary hypertension and chronic obstructive pulmonary disease (COPD) (1%-3%)[20-23]. Among these comorbidities, hypertension is associated with an almost 2.5-fold higher risk of severe illness or death with SARS-CoV-2 infections. According to Schiffrin et al[24], there is no evidence to date that hypertension is related to COVID-19 outcomes. In COVID-19 patients, people with diabetes present poor outcomes; however, the susceptibility to COVID-19 infection in diabetic patients may not be more significant[25]. Because of a lack of clinical evidence, current guidelines from the European Cardiology Society also strongly recommend that patients use their typical diabetic/antihypertensive medications as usual in the COVID-19 pandemic situation[26]. In a comprehensive meta-analysis, Lippi and Henry[27] showed that COPD is associated with more than a fivefold elevated risk and severity of COVID-19 infection; thus, patients with COPD must be advised to take more effective measures to prevent from becoming infected with COVID-19. Patients with chronic cardiovascular disease are among the individuals at highest risk for severe COVID-19 disease and death from acute cardiac injury or failure[20]. According to Li et al[28], patients with prior cardiovascular and metabolic disorders may face a higher risk of COVID-19 infection with a poor prognosis due to sudden morphological and hemodynamic damage to heart tissues.
Similarly, patients with chronic musculoskeletal disorders may also be at high risk of COVID-19 infection. This might be due to impaired immunity because of prolonged use of corticosteroids or nonsteroidal anti-inflammatory drugs (NSAIDs) in daily life.
However, the main limitation to properly assessing the comorbid risk with COVID-19 is self-reporting on admission, mainly due to lack of awareness. Moreover, underreporting of comorbidities might be a significant confounding factor affecting the strength of association with poor prognoses. Thus, more controlled and well-designed studies with large sample sizes are needed to explore their associations in a more reliable way.
MUSCULOSKELETAL ANOMALY DURING SEVERE COVID-19 INFECTION
The number of COVID-19 patients is increasing dramatically worldwide. It is necessary to discriminate between patients with mild and severe cases of COVID-19 to prevent overburdening the ICU and to timely triage severely ill patients. In severely ill patients with COVID-19, due to prolonged pulmonary malfunction, chronic hypoxic conditions develop.
Compared to other body organs, the musculoskeletal system is more adaptive to hypoxic situations due to special muscle fibers (intermediate muscle fibers). However, the WHO recently declared that some musculoskeletal-associated symptoms (14.8%) are related to severe COVID-19 infection, including myalgia or arthralgia[29]. Hypoxia-inducible factor (HIF) studies in skeletal muscles are complicated due to various energy metabolism mechanisms, including various O2 supplies and homeostasis and varying proportions of oxidative, glycolytic and intermediate fibers[30]. This section discusses the downflow changes of severe COVID-19-infected patients with the initiation of musculoskeletal-associated symptoms.
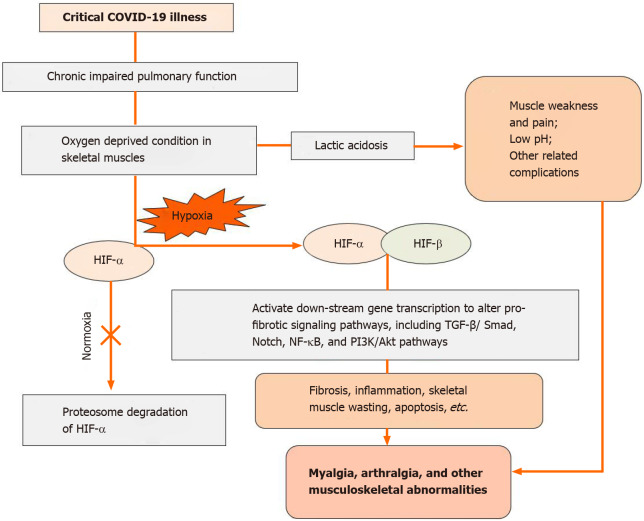
In severe COVID-19 patients, the low oxygen level in skeletal muscles may lead to the formation of excess lactic acid because of muscle pain (myalgia), a low pH level (cramps) or other related complications. Furthermore, in chronic hypoxic conditions, muscle tissue shows a significant alteration in gene regulation[29]. The HIF family plays a crucial role in the hypoxic response of the musculoskeletal system, similar to other tissues[29]. HIFs are heterodimeric transcriptional regulatory factors comprised of unstable HIF-α and HIF-β subunits. HIF signaling contributes to an adaptive pathway to minimize the oxygen requirement and increase the oxygen supply to achieve a new equilibrium. The cellular level of HIF-α is oxygen-dependent and conditionally balanced by proteasomal degradation (normoxia)[31-34].
As seen in severe COVID-19 patients in chronic hypoxic conditions, the low oxygen level inactivates the prolyl hydroxylase action and constrains HIF-α hydroxylation. Subsequently, stabilized HIF-α binds with HIF-β to form a stable dimer. This dimer enters the nucleus and transactivates target genes by directly stimulating the expression of fibrogenic factors. It affects signaling pathways, including the transforming growth factor-β/Smad, Notch, phosphoinositide 3-kinase/Akt, and nuclear factor kappa B pathways, to further affect various biological and pathological processes, ranging from fibrosis and skeletal muscle wasting angiogenesis, erythropoiesis, cell proliferation, inflammation, and apoptosis[30,32-38]. These hypoxic conditions induce altered gene regulation that may lead to more pronounced initial musculoskeletal symptoms, such as myalgia (which may be due to excess lactic acid production), arthralgia (which may be due to inflammation), and other musculoskeletal abnormalities (such as lower back pain and cervical pain), that ultimately indicate the severity of COVID-19 in patients (Figure 2). Apart from associated symptoms and inadequate peripheral oxygen saturation levels, several laboratory parameters such as low lymphocyte count and elevated C-reactive protein, D-dimers, interleukin 6, ferritin and cardiac troponin may also indicate the severity or poor prognosis of COVID-19 patients. The clinician should consider these parameters in prioritizing risk stratification and admittance to the ICUs of COVID-19 patients[38]. Additionally, these alterations in blood parameters might directly or indirectly affect musculoskeletal physiology. However, further research needs to delineate the possible musculoskeletal pathophysiology mechanism.
Figure 2.
Tentative representation of musculoskeletal anomalies during severe coronavirus disease 2019 infection. COVID-19: Coronavirus disease 2019; HIF: Hypoxia-inducible factors; NF-κβ: Nuclear factor-kappa beta; PI3K: Phosphoinositide 3-kinase; TGF-β: Transforming growth factor β.
COVID-19 IN MUSCULOSKELETAL MANIFESTATIONS
As already discussed, the effect of comorbidities on the outcome of COVID-19 is well observed, but information that highlights the chronic musculoskeletal comorbid condition is not currently available. Many questions remain, such as “Do musculoskeletal comorbidities worsen the prognosis of COVID-19 patients?” Whether these comorbidities are a prominent risk factor for COVID-19 infections remains a dilemma to date. This may alter the natural course of the disease.
It is also a matter of concern that most musculoskeletal patients are either middle-aged or elderly persons with a history of taking corticosteroids or NSAIDs for a long time to control pain and relieve localized inflammation[39]. A long-term history of taking these medications may result in a weaker immune system and make these individuals more susceptible to any infection , including COVID-19 infections. Thus, corticosteroids or NSAIDs may alter the clinical picture of these patients. The use of methylprednisolone causes delayed viral shedding in SARS-CoV2 and MERS-CoV and avascular necrosis and psychosis related to SARS-CoV. It was also observed that using methylprednisolone can increase mortality in influenza infections[40]. Thus, the WHO currently does not advise the use of any corticosteroids during COVID-19 infection unless they are associated with acute respiratory distress[41]. A previous human study showed that intraarticular steroid application significantly diminished the effectiveness of the influenza vaccine, and patients became more susceptible to viral streaming[42]. However, to date, no research paper explicitly focusing on intraarticular steroid administration in the COVID-19 pandemic situation is available, but the WHO advises not using steroids/NSAIDs unless the patient is in acute respiratory distress syndrome[29].
The current advice is to use mild analgesics and antipyretics such as paracetamol to treat symptoms such as fever and pain[43]. Corticosteroids or NSAIDs may cause significant release of specific cytokines, termed “cytokine storms,” and could lead to multiple organ failure. Taking these drugs at the initial stages of COVID-19 may lead to more severe respiratory or cardiac complications with altered disease outcomes.
Again, the problem is not limited to the current COVID-19 pandemic. It is assumed that musculoskeletal disorders will worsen during the post-pandemic situation. According to the WHO[30], musculoskeletal disorders are the world's most significant contributor to disabilities, with low back pain being the single most significant global cause of disabilities. Severe COVID-19 infection affects muscle tissue (proposed pathway given in the previous section), leading to pain, muscle weakness, myalgia, arthralgia and other musculoskeletal issues. In recovered COVID-19 patients, these musculoskeletal disorders may persist for a longer duration and significantly affect their mental health and socioeconomic loss, as well as place an extra burden on treatment centers. The altered musculoskeletal physiology induced due to severe COVID-19 infection may also increase the chance of many bony pathologies or fractures. Therefore, it is clear that in post-pandemic circumstances, apart from patients with regular musculoskeletal disorders, recovered COVID-19 patients with musculoskeletal anomalies will dramatically increase.
CONCLUSION
This COVID-19 outbreak has challenged the health care infrastructure and socioeconomic balance worldwide. In addition, it was assumed that the severity of COVID-19 infection significantly affects many associated musculoskeletal disorders. Therefore, it is necessary to improve the overall health care system and revise related comprehensive guidelines for every specialty to better prepare providers, especially those in orthopedics, to cope with the effects of post-pandemic COVID-19 and future pandemics.
Footnotes
Conflict-of-interest statement: There is no conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: November 30, 2020
First decision: December 31, 2020
Article in press: April 22, 2021
Specialty type: Orthopedics
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Farshadpour F S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Sabir Ali, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Ajai Singh, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India. ajaipaedortho62@gmail.com.
Nayeem Sharief, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Manish Yadav, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Salma Siddiqui, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Vaishnavi Pandey, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Archana Raikwar, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
Anamika Singh, Department of Paediatric Orthopaedics, King George’s Medical University, Lucknow 226003, Uttar Pradesh, India.
References
- 1.Richman DD, Whitley RJ, Hayden FG. Clinical Virology, 4th ed. Washington: ASM Press; 2016. [Google Scholar]
- 2.Liu Y, Gayle AA, Wilder-Smith A, Rocklöv J. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J Travel Med. 2020;27 doi: 10.1093/jtm/taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Worldometer COVID-19 Coronavirus pandemic. [cited 22 November 2020]. In: Worldometer [Internet]. Available from: https://www.worldometers.info/coronavirus/#countries .
- 4.Chatterjee P, Nagi N, Agarwal A, Das B, Banerjee S, Sarkar S, Gupta N, Gangakhedkar RR. The 2019 novel coronavirus disease (COVID-19) pandemic: A review of the current evidence. Indian J Med Res. 2020;151:147–159. doi: 10.4103/ijmr.IJMR_519_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valitutto MT, Aung O, Tun KYN, Vodzak ME, Zimmerman D, Yu JH, Win YT, Maw MT, Thein WZ, Win HH, Dhanota J, Ontiveros V, Smith B, Tremeau-Brevard A, Goldstein T, Johnson CK, Murray S, Mazet J. Detection of novel coronaviruses in bats in Myanmar. PLoS One. 2020;15:e0230802. doi: 10.1371/journal.pone.0230802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tatar G, Tok TT. Structures and Functions of Coronavirus Proteins: Molecular Modeling of Viral Nucleoprotein. Int J Virol Infect Dis. 2017 [Google Scholar]
- 7.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang P, Wang X. COVID-19: a new challenge for human beings. Cell Mol Immunol. 2020;17:555–557. doi: 10.1038/s41423-020-0407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo YR, Cao QD, Hong ZS, Tan YY, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak - an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, Xu Y, Li M, Li X, Zheng M, Chen L, Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.The Vaccine Alliance. What is COVID-19 and how is it spread? 27 Mar 2020 [cited 28 April 2020]. In: Gavi [Internet]. Available from: https://www.gavi.org/vaccineswork/what-is-covid-19-and-how-does-it-spread .
- 12.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell. 2020;181:281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bullock HA, Tamin A. Coronavirus Evolved naturally and is Not a Laboratory construct genetic Study Shows. 19 March 200 [cited 26 April 2020]. In: Genetic Engineering & Biotechnology News [Internet]. Available from: https://www.genengnews.com/news/coronavirus-evolved-naturally-and-is-not-a-laboratory-construct-genetic-study-shows/
- 14.Xia S, Liu M, Wang C, Xu W, Lan Q, Feng S, Qi F, Bao L, Du L, Liu S, Qin C, Sun F, Shi Z, Zhu Y, Jiang S, Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shahid Z, Kalayanamitra R, McClafferty B, Kepko D, Ramgobin D, Patel R, Aggarwal CS, Vunnam R, Sahu N, Bhatt D, Jones K, Golamari R, Jain R. COVID-19 and Older Adults: What We Know. J Am Geriatr Soc. 2020;68:926–929. doi: 10.1111/jgs.16472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): a systematic review and meta-analysis. Int J Infect Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-Based Differences in Susceptibility to Severe Acute Respiratory Syndrome Coronavirus Infection. J Immunol. 2017;198:4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaillon S, Berthenet K, Garlanda C. Sexual Dimorphism in Innate Immunity. Clin Rev Allergy Immunol. 2019;56:308–321. doi: 10.1007/s12016-017-8648-x. [DOI] [PubMed] [Google Scholar]
- 20.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, Ji R, Wang H, Wang Y, Zhou Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lippi G, Wong J, Henry BM. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130:304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 24.Schiffrin EL, Flack JM, Ito S, Muntner P, Webb RC. Hypertension and COVID-19. Am J Hypertens. 2020;33:373–374. doi: 10.1093/ajh/hpaa057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferlita S, Yegiazaryan A, Noori N, Lal G, Nguyen T, To K, Venketaraman V. Type 2 Diabetes Mellitus and Altered Immune System Leading to Susceptibility to Pathogens, Especially Mycobacterium tuberculosis. J Clin Med. 2019;8 doi: 10.3390/jcm8122219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Simone G. Position Statement of the ESC Council on Hypertension on ACE‐Inhibitors and Angiotensin Receptor Blockers. 13 March 2020 [cited 22 August 2020]. In: European Society of Cardiology [Internet]. Available from: https://www.escardio.org/Councils/Council‐on‐Hypertension‐(CHT)/News/position‐statement‐of‐the‐esc‐council‐on‐hypertension‐on‐ace‐inhibitors‐and‐ang .
- 27.Lippi G, Henry BM. Chronic obstructive pulmonary disease is associated with severe coronavirus disease 2019 (COVID-19) Respir Med. 2020;167:105941. doi: 10.1016/j.rmed.2020.105941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li B, Yang J, Zhao F, Zhi L, Wang X, Liu L, Bi Z, Zhao Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.World Health Organization. Coronavirus disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update: Situation reports. [cited 27 April 2020]. In: World Health Organization [Internet]. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/
- 30.Pisani DF, Dechesne CA. Skeletal muscle HIF-1alpha expression is dependent on muscle fiber type. J Gen Physiol. 2005;126:173–178. doi: 10.1085/jgp.200509265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol. 2013;591:2027–2042. doi: 10.1113/jphysiol.2013.251470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Semenza GL. Oxygen sensing, homeostasis, and disease. N Engl J Med. 2011;365:537–547. doi: 10.1056/NEJMra1011165. [DOI] [PubMed] [Google Scholar]
- 33.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 34.Semenza GL. Hydroxylation of HIF-1: oxygen sensing at the molecular level. Physiology (Bethesda) 2004;19:176–182. doi: 10.1152/physiol.00001.2004. [DOI] [PubMed] [Google Scholar]
- 35.Yamaguchi J, Tanaka T, Eto N, Nangaku M. Inflammation and hypoxia linked to renal injury by CCAAT/enhancer-binding protein δ. Kidney Int. 2015;88:262–275. doi: 10.1038/ki.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaelin WG Jr. The von Hippel-Lindau tumour suppressor protein: O2 sensing and cancer. Nat Rev Cancer. 2008;8:865–873. doi: 10.1038/nrc2502. [DOI] [PubMed] [Google Scholar]
- 37.Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG Jr. HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- 38.Velavan TP, Meyer CG. Mild vs severe COVID-19: Laboratory markers. Int J Infect Dis. 2020;95:304–307. doi: 10.1016/j.ijid.2020.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griffin MR. Epidemiology of nonsteroidal anti-inflammatory drug-associated gastrointestinal injury. Am J Med. 1998;104:23S–29S; discussion 41S. doi: 10.1016/s0002-9343(97)00207-6. [DOI] [PubMed] [Google Scholar]
- 40.Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395:473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vetter P, Eckerle I, Kaiser L. Covid-19: a puzzle with many missing pieces. BMJ. 2020;368:m627. doi: 10.1136/bmj.m627. [DOI] [PubMed] [Google Scholar]
- 42.Sytsma TT, Greenlund LK, Greenlund LS. Joint Corticosteroid Injection Associated With Increased Influenza Risk. Mayo Clin Proc Innov Qual Outcomes. 2018;2:194–198. doi: 10.1016/j.mayocpiqo.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Day M. Covid-19: ibuprofen should not be used for managing symptoms, say doctors and scientists. BMJ. 2020;368:m1086. doi: 10.1136/bmj.m1086. [DOI] [PubMed] [Google Scholar]