Abstract
Proper nutrition is a modifiable factor in preventing frailty. This study was conducted to identify the association between dietary patterns and frailty in the older adult population. The cross-sectional analysis was performed on 4632 subjects aged ≥65 years enrolled in the Korea National Health and Nutrition Examination Survey from 2014–2018. Food variety score (FVS) was defined as the number of foods items consumed over a day. Three dietary patterns were identified using factor analysis: “white rice and salted vegetables,” “vegetables, oils, and fish,” and “noodles and meat.” The higher “white rice and salted vegetables” pattern score was related to significantly lower FVS, whereas higher “vegetables, oils, and fish” and “noodles and meat” pattern scores were associated with a higher FVS. Participants with higher FVS showed a low risk of frailty (odds ratio (OR) (95% confidence interval, CI) = 0.44 (0.31–0.61), p-trend = 0.0001) than those with lower FVS. Moreover, the “vegetables, oils, and fish” pattern score was significantly associated with a low risk of frailty (OR (95% CI) = 0.55 (0.40–0.75), p-trend = 0.0002). These results suggested that consuming a dietary pattern based on vegetables, oils, and fish with high FVS might ameliorate frailty in older adults.
Keywords: frailty, dietary pattern, food variety score
1. Introduction
Given the rapid aging of the world’s population, the prevention of frailty is becoming more important than ever. Frailty is a geriatric syndrome characterized by a reduced physical and psychological function and a decline in the ability to maintain homeostasis [1]. Frailty in older adults is a risk factor for falls, morbidity, disability, and even mortality [2] and is often viewed as a major challenge for medical and health care services [3]. Thus, preventing frailty can help reduce medical and related costs and address the challenges of successful aging.
Nutritional status is considered one of the modifiable risk factors for frailty. It is well known that inadequate protein and micronutrients can contribute to frailty [4]. However, understanding the nutrition–health interface requires shifting the focus from individual nutrients toward food-based approaches. In this regard, investigating the impact of dietary patterns on frailty may be more useful than analyzing the effects of a single nutrient in establishing a frailty prevention strategy. In Western countries, adherence to a Mediterranean diet has been associated with a reduced incidence of frailty among older people [4,5,6], but whether there is an association between frailty and dietary patterns other than the Mediterranean diet is still unknown [7]. It is difficult to compare the results of dietary patterns between populations because dietary patterns are strongly related to the study population’s diet.
Therefore, it would be interesting to understand the relationship between dietary patterns and frailty and the impact of dietary quality to identify the most appropriate diets for decreasing frailty prevalence. The food variety score (FVS) is a simple count of food items and has been proven to be a useful indicator of the nutritional adequacy of the diet [8]. Consuming a wide variety of food groups increases the chances of providing the various nutrients and phytochemicals needed for optimal health, which could reduce the risk of frailty [9]. In contrast, inadequate nutrient intake that accompanies poor dietary variety causes oxidative stress [10] and inflammatory reactions [11], contributing to the risk of frailty. In some studies, frailty decreased as a result of high overall diet quality [12], characterized by increased consumption of fruits and vegetables [13] and optimal intakes of antioxidant nutrients [14].
Dietary variation is important for health maintenance and disease prevention in older adults. Many prior studies have reported the association between healthy dietary patterns and frailty, but most of them have been focused on Western populations [5,6,7]. Very few studies have been conducted with Asian population [8,10,15], and the results are not consistent. Thus, in-depth research on the association between dietary factors and frailty is highly needed. Therefore, this study aimed to identify dietary patterns related to frailty in a larger sample of older Korean adults. Moreover, we investigate not only dietary patterns but also dietary variety that is comprehensively available in various food culture as an indicator of dietary factors related to frailty.
2. Materials and Methods
2.1. Data Collection
This study used the data from the 2014–2018 Korea National Health and Nutrition Examination Survey (KNHANES), which included KNHANES VI (2013–2015) and KNHANES VII (2016–2018), conducted by the Korea Centers for Disease Control and Prevention (KCDC). The KNHANES is an ongoing cross-sectional survey designed to use complex, multistage, stratified, and probability cluster sampling to obtain nationally representative estimates [16]. The investigation included a health questionnaire, health examination, and nutrition surveys. The Institutional Review Board (IRB) of the KCDC approved this study (2013-07CON-03-4C, 2013-12EXP-03-5C, 2018-01-03-P-A). Detailed information about the data and survey is available on the KNHANES website (http://knhanes.cdc.go.kr accessed on 9 September 2021).
2.2. Subjects
The participants in the 2014–2018 survey totaled 39,199. The present analysis was limited to adults aged 65 or older who completed the survey (n = 7166). Participants with incomplete data on frailty classification were excluded (n = 1229). Those with missing dietary intake data, having energy intakes below 500 kcal and over 5000 kcal, and unusual intake on the previous day were also excluded (n = 1232). In addition, we excluded subjects with missing data on other covariates, such as sociodemographic information, smoking, and alcohol consumption (n = 73). Thus, 4632 subjects were included in the study.
2.3. Frailty Classification
Frailty was measured using a slight modification of the five criteria for the frailty phenotype developed by Fried et al. [17]: (1) unintentional weight loss (self-reported unintentional weight loss in the last year of >3 kg) [18], (2) exhaustion (if self-perception of stress is extremely high, it is considered to be emotional/physical exhaustion) [19], (3) weakness (handgrip strength <26 kg for men and <18 kg for women based on the Asian Working Group criteria for sarcopenia) [20], (4) walking difficulties (if the subjects responded to the mobility question of the European Quality of Life 5-Dimensions (EuroQoL-5D) questionnaire that walking was difficult, it was classified as walking difficulties) [21], and (5) low physical activity (physical activity was measured using the Global Physical Activity Questionnaire (GPAQ) developed by the World Health Organization (WHO) and was classified as low physical activity when recreational activity was <2 h per week) [22]. Participants were classified as robust if they fulfilled none of the criteria, pre-frail if they fulfilled one or two criteria, and frail if they fulfilled three or more criteria.
2.4. Dietary Assessment
Dietary intake information was obtained from a nutrition survey of the KNHANES using the 24 h recall method [16]. Skilled and well-trained dietary interviewers conducted the 24 h recall by face-to-face interview. The participants reported all the food and beverage that was consumed the previous day, including food name, types of ingredients, and amount of food intake per meal. For the analysis of dietary patterns, food items from the 24 h recall data were integrated into 18 food groups based on similarities. The grains and grain products group accounted for nearly half of the daily energy intake, so this food group was further divided into white rice, grains, noodles and dumplings, flour, bread and rice cakes, and pizza and hamburgers. Salted vegetables, including kimchi, were separated from other raw vegetables because, as a traditional fermented food in Korea, it has a high frequency of consumption and contains high sodium. Beverages were divided into alcohol, coffee and tea, and sugar-sweetened beverages. Our final analysis included a total of 24 food groups. The difference in weight between solid and liquid foods was corrected by representing the food groups as a percentage of energy.
To assess diet quality, the overall FVS was adopted. FVS was calculated by the simple count of the number of food items consumed by each subject during the last 24 h [23]. If the main ingredients were the same, they were classified as the same food items even if prepared by different cooking methods. The amount of food consumed and the frequency of consumption were not taken into account.
2.5. Assessment of Other Variables
Information on demographic and socioeconomic characteristics, including age, body mass index (BMI; kg/m2), living status (living alone, living with others), residential area (urban, rural), education level (≤elementary school, ≥middle school), household income level (≤the lowest quartile, ≥middle–low), smoking status (current smoker or non-current smoker), high-risk alcohol consumption (yes or no), and comorbidity (whether subjects suffered from three or more simultaneous diseases diagnosed by a doctor), was obtained using a general questionnaire and health interview questionnaire.
2.6. Statistical Analysis
All statistical analyses were performed using SAS software version 9.4 (SAS Institute, Inc., Cary, NC, USA). Due to the complex sampling design of the KNHANES study, sample weights, stratifying variables (k strata), and primary sampling units were included in our analysis. The dietary pattern was derived using factor analysis with the FACTOR procedure and VARIMAX rotation function that maintains uncorrelated factors and increases interpretability. Eigenvalues, scree plot, and interpretability ability were considered in deciding the number of factors. Significance was given to the food group whose factor load value exceeded 0.25 or −0.25. Scores of the individual dietary patterns of the whole population were categorized into tertiles and used for comparison of FVS, nutrient intake, and other general characteristics. Differences in the distribution of characteristics between tertiles of dietary pattern scores were analyzed using the SURVEY FREQ procedure for categorical variables or the SURVEY MEAN procedure for continuous variables. Significant differences between tertiles of dietary patterns were determined using the χ2 test or a general linear model (Scheffe’s test of multiple comparisons). Multinomial SURVEYLOGISTIC analysis was performed to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for frailty (robust vs. pre-frail vs. frail, with robust as the reference) across tertiles of dietary pattern scores and FVS. Adjustments were performed for potential confounding variables, selected based on the prior knowledge from the scientific literature and whether they are related to the independent and dependent variables. Confounders included age, gender, BMI, residential area, family income, education level, smoking status, high-risk alcohol consumption, total energy intake, and comorbidity and there was no significant multicollinearity among these variables. All reported probability tests were two-sided, with a p-value < 0.05 considered statistically significant.
3. Results
3.1. General Characteristics of Study Subjects
The general characteristics of the study population are presented in Table 1. Of the 2184 males (48.8%) and 2448 females (51.2%) included in the study, 17.5% lived alone, 21.6% were rural residents, and 78.4% were urban residents. The average age of the study subjects was 72.5 ± 0.1 years. More than half of the subjects had education levels below elementary school (56.2%), and 44.9% had income levels below the lowest quartile. Comorbidity, defined as having more than three diseases simultaneously, was seen in 19.7% of the subjects.
Table 1.
General characteristics of the study subjects.
| Variables | Total (n = 4632) |
|---|---|
| Age (years, mean ± SE) | 72.5 ± 0.1 |
| Age range | |
| 65–69 | 1538 (33.6) |
| 70–79 | 2411 (51.9) |
| ≥80 | 683 (14.5) |
| Gender | |
| Male | 2184 (48.8) |
| Female | 2448 (51.2) |
| Living status | |
| Living alone | 990 (17.5) |
| Living with others | 3642 (82.5) |
| Residence | |
| Rural | 1300 (21.6) |
| Urban | 3332 (78.4) |
| Education | |
| ≤Elementary school | 2708 (56.2 |
| ≥Middle school | 1924 (43.8) |
| Family income | |
| ≤The lowest quartile | 2164 (44.9) |
| ≥Middle–low | 2468 (55.1) |
| Current smoking status | |
| Current smoker | 439 (9.6) |
| Non-current smoker | 4193 (90.4) |
| High-risk alcohol consumption | |
| Yes | 187 (4.1) |
| No | 4444 (95.9) |
| Body mass index (kg/m2, mean ± SE) | 24.0 ± 0.1 |
| Body mass index range | |
| <18.5 | 120 (2.6) |
| 18.5–24.9 | 2833 (61.7) |
| ≥25.0 | 1679 (35.7) |
| Comorbidity | |
| Yes | 925 (19.7) |
| No | 3707 (80.3) |
Values are presented as mean ± standard error (SE) or n (%).
3.2. Dietary Patterns in the Study Population
Table 2 gives the three dietary patterns identified by factor analysis. Pattern 1 showed the highest factor loadings for white rice and kimchi and salted vegetables and negative loadings for flour, pizza, snacks, and fruits. We named Pattern 1 “white rice and salted vegetables.” Pattern 2 had the highest factor loadings for non-salted vegetables, seasonings, oils, and fish and shellfish, so we described Pattern 2 as “vegetables, oils, and fish.” Pattern 3 had high factor loadings for noodles and dumplings, meat, alcohol, and coffee and tea, and negative loadings for fruits and non-salted vegetables. We named Pattern 3 “noodles and meat.” These three patterns accounted for 19.5% of the total variance in food intakes.
Table 2.
Factor loading matrix for the three dietary patterns of older Korean adults.
| Food Group | Pattern 1 | Pattern 2 | Pattern 3 |
|---|---|---|---|
| White Rice and Kimchi | Vegetables, Oils, and Fish | Noodles and Meat | |
| White rice | 0.83902 | −0.27383 | |
| Grains | |||
| Noodles and dumplings | −0.2601 | 0.54391 | |
| Flour, bread, and rice cakes | −0.41547 | ||
| Hamburgers, pizza, and snacks | |||
| Potatoes | −0.30472 | ||
| Sweets | |||
| Beans | |||
| Nuts | −0.3071 | −0.33496 | |
| Non-salted vegetables | 0.61885 | ||
| Kimchi and salted vegetables | 0.32283 | ||
| Mushrooms | |||
| Fruits | −0.40244 | −0.34568 | |
| Meats | 0.42253 | ||
| Processed meats | |||
| Eggs | −0.32273 | ||
| Fish and shellfish | 0.47125 | ||
| Seaweed | |||
| Milk and dairy products | −0.38096 | ||
| Oils | 0.51915 | ||
| Alcohol | 0.27234 | 0.38311 | |
| Coffee and tea | 0.37365 | ||
| Sugar-sweetened beverages | |||
| Seasonings | 0.53593 | ||
| Variance explained (%) | 8.15% | 5.85% | 5.53% |
Factor loading values < |0.25| were excluded for simplicity. The patterns were derived based on the energy contribution ratio of food groups by factor analysis.
3.3. Comparison of General Characteristics by Tertiles of Dietary Pattern Scores
The subjects’ general characteristics across the tertiles of the dietary pattern scores are summarized in Table 3. Subjects in the highest tertile of the “white rice and salted vegetables” dietary pattern tended to be male, older, rural residents of high-risk alcohol consumption with a lower education level and lower family income level than those in the lowest tertile. Meanwhile, subjects in the highest tertile of the “vegetables, oils, and fish” pattern tended to be younger, living alone, urban residents, and highly educated, with higher family income and lower comorbidity than their lowest tertile counterparts. Lastly, the highest tertile of the “noodles and meat” pattern was associated with subjects with high-risk alcohol consumption that were less educated and more likely to smoke than those in the lowest tertile of this dietary pattern (Table 3).
Table 3.
General characteristics across tertiles of dietary pattern scores.
| Variables | Dietary Pattern Score | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern 1 White Rice and Salted Vegetables |
Pattern 2 Vegetables, Oils, and Fish |
Pattern 3 Noodles and Meat |
||||||||||
| Tertile 1 (Lowest) (n = 1544) |
Tertile 2 (Middle) (n = 1544) |
Tertile 3 (Highest) (n = 1544) |
p-Value | Tertile 1 (Lowest) (n = 1544) |
Tertile 2 (Middle) (n = 1544) |
Tertile 3 (Highest) (n = 1544) |
p-Value | Tertile 1 (Lowest) (n = 1544) |
Tertile 2 (Middle) (n = 1544) |
Tertile 3 (Highest) (n = 1544) |
p-Value | |
| Age (years, mean ± SE) | 71.6 ± 0.2 | 72.5 ± 0.2 | 73.6 ± 0.1 | <0.0001 | 73.1 ± 0.1 | 72.7 ± 0.2 | 71.8 ± 0.2 | <0.0001 | 72.2 ± 0.2 | 72.9 ± 0.1 | 72.5 ± 0.2 | 0.0037 |
| Age range (years) | ||||||||||||
| 65–69 | 622 (41.3) | 518 (33.6) | 398 (25.6) | <0.0001 | 415 (27.9) | 516 (32.7) | 607 (39.9) | <0.0001 | 548 (36.3) | 487 (30.8) | 503 (33.7) | 0.0448 |
| 70–79 | 745 (47.1) | 811 (53.1) | 855 (55.7) | 850 (55.4) | 807 (52.3) | 754 (48.2) | 796 (51.0) | 809 (53.8) | 806 (50.9) | |||
| 80≤ | 177 (11.5) | 215 (13.3) | 291 (18.7) | 279 (16.7) | 221 (15.0) | 183 (11.9) | 200 (12.7) | 248 (15.4) | 235 (15.4) | |||
| Gender (male, %) | 612 (41.2) | 782 (52.5) | 790 (52.8) | <0.0001 | 630 (41.0) | 736 (50.4) | 818 (54.7) | <0.0001 | 581 (39.6) | 710 (47.5) | 893 (59.2) | <0.0001 |
| Living status (living alone, %) | 317 (17.3) | 314 (16.7) | 359 (18.5) | 0.4475 | 414 (21.9) | 276 (14.8) | 300 (16.1) | <0.0001 | 343 (18.0) | 326 (17.3) | 321 (17.3) | 0.8580 |
| Residence (rural, %) | 302 (15.0) | 442 (21.4) | 556 (28.7) | <0.0001 | 495 (24.2) | 419 (21.7) | 386 (19.2) | 0.0147 | 393 (19.8) | 453 (22.4) | 454 (22.7) | 0.1996 |
| Education (≤elementary school, %) | 707 (43.5) | 910 (56.5) | 1091 (68.9) | <0.0001 | 1042 (64.9) | 896 (57.1) | 770 (46.8) | <0.0001 | 895 (54.4) | 951 (60.3) | 862 (53.7) | 0.0031 |
| Family income level (≤low, %) | 549 (34.9) | 715 (44.9) | 900 (55.3) | <0.0001 | 851 (52.0) | 695 (43.1) | 618 (39.9) | <0.0001 | 721 (44.0) | 750 (47.5) | 693 (43.2) | 0.0974 |
| Current smoker (%) | 102 (2.3) | 145 (9.8) | 192 (12.3) | <0.0001 | 135 (9.0) | 138 (8.8) | 166 (11.0) | 0.1539 | 94 (6.0) | 127 (8.8) | 218 (14.0) | <0.0001 |
| High-risk alcohol consumption (%) | 42 (2.6) | 61 (3.8) | 84 (5.7) | 0.0002 | 38 (2.4) | 47 (3.1) | 102 (6.6) | <0.0001 | 38 (2.3) | 43 (2.8) | 106 (7.0) | <0.0001 |
| Body mass index (kg/m2, mean ± SE) | 24.2 ± 0.1 | 24.1 ± 0.1 | 23.9 ± 0.1 | 0.1164 | 24.0 ± 0.1 | 24.0 ± 0.1 | 24.1 ± 0.1 | 0.5946 | 24.0 ± 0.1 | 24.0 ± 0.1 | 24.1 ± 0.1 | 0.6819 |
| Body mass index range (kg/m2) | ||||||||||||
| <18.5 | 28 (1.8) | 35 (2.2) | 57 (3.9) | 0.0157 | 53 (3.8) | 37 (2.4) | 30 (1.7) | 0.0222 | 35 (2.1) | 43 (3.1) | 42 (2.7) | 0.5585 |
| 18.5–24.9 | 934 (60.9) | 949 (61.9) | 950 (62.2) | 923 (59.7) | 963 (63.2) | 947 (62.1) | 963 (63.1) | 930 (60.7) | 940 (61.3) | |||
| ≥25.0 | 582 (37.3) | 560 (35.8) | 537 (33.9) | 568 (36.5) | 544 (34.4) | 567 (36.2) | 546 (34.8) | 571 (36.2) | 562 (36.0) | |||
| Comorbidity (%) | 334 (21.2) | 307 (20.1) | 284 (17.6) | 0.0692 | 324 (20.6) | 328 (21.4) | 273 (17.1) | 0.0192 | 340 (21.2) | 296 (18.9) | 289 (19.0) | 0.2871 |
Values are presented as mean ± standard error (SE) or n (%). All p-values were determined by the chi-square test and general linear model.
3.4. FVS and Nutrient Intakes across Tertiles of Dietary Pattern Scores
Table 4 lists the age- and sex-adjusted mean values for FVS and nutrient intakes across the tertiles of the dietary pattern scores. Both the “vegetables, oils, and fish” and the “noodles and meat” patterns showed a significant positive trend of FVS. On the contrary, the “white rice and salted vegetables” pattern showed a significant negative tendency for FVS. The “white rice and salted vegetables” pattern showed a significant negative association with energy, energy from protein, energy from fat, and intake of nutrients, such as fiber, calcium, phosphorus, potassium, thiamin, riboflavin, vitamin C, ω-3/-6 polyunsaturated fatty acids (PUFA), flavonoids, and carotenoids. Conformability to the “vegetables, oils, and fish” pattern was significantly positively related to energy, energy from protein, energy from fat, and intake of nutrients, such as fiber, calcium, iron, sodium, potassium, thiamin, riboflavin, niacin, vitamin C, ω-3/-6 PUFA, flavonoids, and carotenoids. In the “noodles and meat” pattern, there was a significantly positive tendency to consume energy and iron but a negative tendency for other nutrients.
Table 4.
Age- and sex-adjusted mean daily energy and nutrient intake across tertiles of food variety scores and dietary pattern scores.
| Dietary Pattern Score | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pattern 1 White Rice and Salted Vegetables |
Pattern 2 Vegetables, Oils, and Fish |
Pattern 3 Noodles and Meat |
||||||||||
| Tertile 1 | Tertile 2 | Tertile 3 | p-Trend | Tertile 1 | Tertile 2 | Tertile 3 | p-Trend | Tertile 1 | Tertile 2 | Tertile 3 | p-Trend | |
| Food variety score | 34.7 ± 0.4 | 32.9 ± 0.4 | 25.5 ± 0.4 | <0.0001 | 24.4 ± 0.4 | 32.7 ± 0.4 | 36.0 ± 0.5 | <0.0001 | 29.9 ± 0.4 | 31.4 ± 0.4 | 32.0 ± 0.4 | 0.0077 |
| Total energy (kcal) | 1803.4 ± 21.0 | 1722.2 ± 18.4 | 1548.4 ± 19.3 | <0.0001 | 1655.0 ± 19.7 | 1701.7 ± 19.2 | 1719.9 ± 20.1 | <0.0001 | 1659.0 ± 21.5 | 1648.4 ± 17.8 | 1771.2 ± 20.9 | <0.0001 |
| percentage from energy | ||||||||||||
| Carbohydrates (%) | 68.7 ± 0.3 | 71.1 ± 0.3 | 74.8 ± 0.3 | <0.0001 | 76.3 ± 0.3 | 72.2 ± 0.3 | 66.1 ± 0.3 | <0.0001 | 74.5 ± 0.3 | 73.4 ± 0.3 | 66.6 ± 0.3 | <0.0001 |
| Protein (%) | 13.4 ± 0.1 | 13.2 ± 0.1 | 12.1 ± 0.1 | <0.0001 | 11.2 ± 0.1 | 13.1 ± 0.1 | 14.4 ± 0.1 | <0.0001 | 13.1 ± 0.1 | 12.7 ± 0.1 | 13.0 ± 0.1 | 0.7579 |
| Fat (%) | 17.3 ± 0.2 | 13.4 ± 0.2 | 8.8 ± 0.2 | <0.0001 | 10.6 ± 0.2 | 12.9 ± 0.2 | 16.0 ± 0.3 | <0.0001 | 12.2 ± 0.2 | 11.8 ± 0.2 | 15.6 ± 0.2 | <0.0001 |
| Fiber (g) | 29.7 ± 0.5 | 25.8 ± 0.4 | 21.0 ± 0.4 | <0.0001 | 22.3 ± 0.5 | 26.2 ± 0.5 | 28.1 ± 0.5 | <0.0001 | 31.2 ± 0.5 | 23.6 ± 0.4 | 21.9 ± 0.4 | <0.0001 |
| Calcium (mg) | 507.7 ± 9.7 | 444.7 ± 9.3 | 365.4 ± 8.9 | <0.0001 | 364.0 ± 6.8 | 433.1 ± 8.1 | 520.8 ± 11.7 | <0.0001 | 515.4 ± 12.3 | 416.8 ± 7.7 | 387.6 ± 6.7 | <0.0001 |
| Phosphorus (mg) | 1003.6 ± 13.1 | 934.9 ± 11.7 | 779.2 ± 11.8 | <0.0001 | 780.2 ± 10.7 | 916.9 ± 11.7 | 1020.6 ± 14.1 | <0.0001 | 985.4 ± 14.8 | 877.7 ± 10.9 | 857.9 ± 11.6 | <0.0001 |
| Iron (mg) | 13.3 ± 0.3 | 13.7 ± 0.3 | 11.9 ± 0.2 | 0.2458 | 11.4 ± 0.2 | 13.0 ± 0.3 | 14.6 ± 0.3 | <0.0001 | 14.6 ± 0.3 | 12.7 ± 0.2 | 11.7 ± 0.2 | <0.0001 |
| Sodium (mg) | 3179.9 ± 61.9 | 3097.1 ± 60.8 | 2725.9 ± 59.1 | 0.4835 | 2465.6 ± 51.9 | 2913.5 ± 55.1 | 3615.2 ± 71.7 | <0.0001 | 2782.3 ± 62.9 | 2818.5 ± 51.9 | 3413.0 ± 64.5 | <0.0001 |
| Potassium | 3069.1 ± 47.7 | 2715.1 ± 34.8 | 2197.7 ± 34.8 | <0.0001 | 2302.9 ± 37.6 | 2680.9 ± 38.5 | 3001.7 ± 46.8 | <0.0001 | 3028.9 ± 49.3 | 2555.7 ± 36.4 | 2409.7 ± 37.2 | <0.0001 |
| Vitamin A (μg RAE) | 341.0 ± 9.9 | 318.3 ± 10.8 | 293.2 ± 22.8 | 0.9197 | 204.6 ± 11.1 | 294.7 ± 8.7 | 450.8 ± 20.6 | <0.0001 | 391.5 ± 21.7 | 292.6 ± 9.4 | 269.0 ± 10.6 | <0.0001 |
| Thiamin (mg) | 1.3 ± 0.0 | 1.5 ± 0.0 | 1.3 ± 0.0 | <0.0001 | 1.2 ± 0.0 | 1.4 ± 0.0 | 1.5 ± 0.0 | <0.0001 | 1.4 ± 0.0 | 1.4 ± 0.0 | 1.3 ± 0.0 | <0.0001 |
| Riboflavin (mg) | 1.4 ± 0.0 | 1.1 ± 0.0 | 0.8 ± 0.0 | <0.0001 | 0.9 ± 0.0 | 1.1 ± 0.0 | 1.3 ± 0.0 | <0.0001 | 1.2 ± 0.0 | 1.0 ± 0.0 | 1.1 ± 0.0 | <0.0001 |
| Niacin (mg) | 11.9 ± 0.1 | 11.8 ± 0.2 | 10.1 ± 0.2 | 0.6320 | 9.6 ± 0.2 | 11.8 ± 0.2 | 13.0 ± 0.2 | <0.0001 | 11.9 ± 0.2 | 10.8 ± 0.2 | 11.2 ± 0.2 | <0.0001 |
| Vitamin C (mg) | 91.4 ± 3.4 | 75.9 ± 3.6 | 48.7 ± 1.8 | <0.0001 | 63.2 ± 3.9 | 68.9 ± 2.3 | 84.3 ± 2.7 | <0.0001 | 97.0 ± 4.1 | 64.0 ± 2.0 | 55.6 ± 2.3 | <0.0001 |
| ω-3 PUFA (g) | 1.8 ± 0.1 | 1.5 ± 0.0 | 1.1 ± 0.1 | <0.0001 | 0.8 ± 0.0 | 1.4 ± 0.0 | 2.2 ± 0.1 | <0.0001 | 1.8 ± 0.1 | 1.4 ± 0.1 | 1.3 ± 0.0 | <0.0001 |
| ω-6 PUFA (g) | 8.6 ± 0.2 | 6.2 ± 0.1 | 3.9 ± 0.1 | <0.0001 | 4.4 ± 0.1 | 6.0 ± 0.1 | 8.3 ± 0.2 | <0.0001 | 6.5 ± 0.2 | 5.4 ± 0.1 | 6.9 ± 0.2 | 0.8982 |
| Flavonoids (mg) | 102.3 ± 2.6 | 91.2 ± 2.3 | 75.9 ± 2.3 | <0.0001 | 79.6 ± 2.6 | 91.6 ± 2.4 | 98.4 ± 2.7 | <0.0001 | 114.9 ± 3.0 | 82.0 ± 2.0 | 73.0 ± 2.1 | <0.0001 |
| Carotenoids (mg) | 11.6 ± 0.4 | 9.4 ± 0.2 | 6.8 ± 0.2 | <0.0001 | 7.3 ± 0.3 | 98.4 ± 2.7 | 11.5 ± 0.4 | <0.0001 | 11.8 ± 0.4 | 6.9 ± 0.2 | 7.0 ± 0.2 | <0.0001 |
Values are presented as means (least-square means) ± standard error adjusted for gender and age. PUFA, polyunsaturated fatty acid.
3.5. Association of Frailty with Dietary Pattern Scores Considering FVS
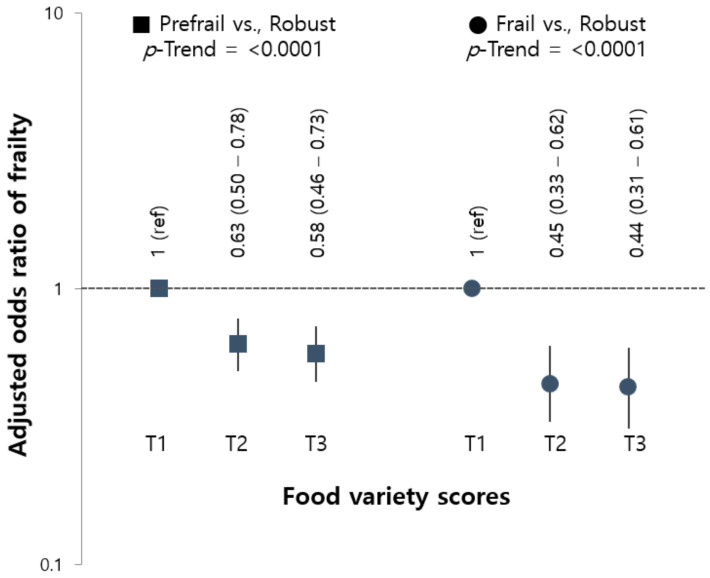
The prevalence of frail and pre-frail in this study was 11.9% (n = 572) and 62.5% (n = 2945), respectively. From the results of the multinomial logistic analysis for the association of frailty with the tertile of FVS (Figure 1), frailty was inversely associated with each tertile of FVS. The OR of pre-frail and frail was significantly lower in the highest tertile of FVS (OR (95% CI) = 0.44 (0.31–0.61), p-trend < 0.0001) compared to the lowest tertile.
Figure 1.
Adjusted ORs (95% CI) for pre-frailty and frailty according to the tertile of food variety scores. Ref.: reference category; OR: odds ratio; CI: confidence interval. Data were calculated using the multinomial SURVEYLOGISTIC model. ORs (95% CIs) were adjusted for gender, age, BMI, living status, residential area, family income, education level, smoking status, high-risk alcohol consumption, comorbidity, and energy intake.
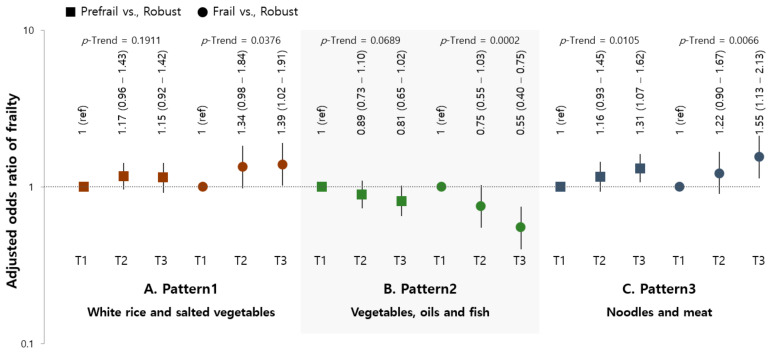
The results of the multinomial logistic analysis for the association between frailty and dietary pattern score are presented in Figure 2. The highest tertile of the “vegetables, oils, and fish” pattern was significantly inversely associated with frailty (OR (95% CI) = 0.55 (0.40–0.75), p-trend = 0.0002). The ORs (95% CI) of frailty for those in the highest tertile compared to the lowest tertile of pattern scores were 1.39 (1.02–1.91) for the “white rice and salted vegetables” pattern (p-trend = 0.0376) and 1.55 (1.13–2.13) for the “noodles and meat” pattern (p-trend = 0.0066).
Figure 2.
Adjusted ORs (95% CI) for pre-frailty and frailty according to the tertile of dietary pattern scores. (A) “White rice and salted vegetables”; (B) “Vegetables, oils, and fish”; (C) “Noodles and meat.” Ref.: reference category; OR: odds ratio; CI: confidence interval. Data were calculated using the multinomial SURVEYLOGISTIC model. ORs (95% CIs) were adjusted for gender, age, BMI, living status, residential area, family income, education level, smoking status, high-risk alcohol consumption, comorbidity, and energy intake.
4. Discussion
This study was conducted to identify the relationship of dietary patterns with frailty considering food variety. A greater food variety was significantly associated with lower odds of frailty. Three major dietary patterns were identified in this study of older Korean adults: “white rice and salted vegetables,” “vegetables, oils, and fish,” and “noodles and meat.” Among these patterns, “vegetables, oils, and fish” was associated positively with FVS and showed an inverse relationship with the risk of frailty.
Assessing the relationship between individual nutrients and frailty may not take into consideration the interactions between nutrients. An increasing number of investigations in recent years has evaluated the association between dietary patterns and frailty. As mentioned above, some studies in Western countries have reported that adherence to Mediterranean dietary patterns protects against frailty [4,5]. In this current study of the specific dietary pattern of Koreans, we found that the highest tertile of the “vegetables, oils, and fish” pattern was associated with a low prevalence of frailty. A prospective study of older Spanish adults [6] found that a “prudent” dietary pattern (characterized by a high intake of olive oil and vegetables) was inversely associated with frailty incidence. Furthermore, in a study of Taiwanese older adults, a reduced prevalence of frailty was observed in those with a dietary pattern high in ω-3-rich deep-sea fish, phytonutrient-rich plant foods, and other protein-rich foods, such as shellfish and milk [24]. In another study involving a large cohort of older European subjects, high consumption of fruits and vegetables was associated with a reduced frailty risk [5]. A sufficient intake of fish or oils rich in ω-3 and vegetables rich in antioxidants and phytonutrients could have the effect of preventing frailty through various mechanisms, including anti-oxidative, anti-inflammatory, and muscle decomposition prevention. Similar to our research, one study of the Korean population reported that dietary patterns with high consumption of meat, fish, and vegetables lower the risk of pre-frailty. It suggests a potentially protective effect against frailty of a protein-rich and vegetable-rich food pattern [15]. Dietary patterns are analyzed based on the diet of the study reference population, so the dietary patterns extracted from each study may be different. In the present study, a dietary pattern of “white rice and salted vegetables” or “noodles and meat” was associated with an increased risk of frailty. This outcome might also be linked to oxidative stress and inflammation because high intakes of carbohydrates, sodium, red meat, and N-nitroso compounds found in processed meat products are related to oxidative stress and inflammation [25,26].
In a prospective investigation of older Chinese people there was no association between a dietary pattern including a “vegetable–fruit” pattern and the incidence of frailty [7]. The FVS refers to the total number of different food items consumed individually over a particular time. It is the main measure used to assess the overall diet and has been associated with the nutrient adequacy ratio of the nutrients and diet quality [8]. A previous study reported that the FVS could reflect the overall dietary quality and is related to the health status of Korean adults [27]. In this current study, considering both dietary pattern and food diversity, the higher the compliance with the “vegetable, oils, and fish” pattern, the higher the FVS and the lower the prevalence of frailty. On the contrary, the “white rice and salted vegetables” pattern was inversely associated with FVS and an increased risk of frailty. Furthermore, we found that a lower diet diversity, indicated by a significantly low FVS, was also associated with frailty. This finding is consistent with another study that found frailty was intimately related to low dietary diversity in older adults [9]. Low food variety and a narrow range of food choices may result in an inadequate intake of micronutrients and phytochemicals [28]. Antioxidant nutrients could reduce the risk of frailty through different biological pathways, such as oxidative stress [10] and inflammation [11]. Antioxidant nutrients have been shown to protect against oxidative stresses that may cause muscle atrophy and loss of muscle fibers [10]. Inflammation is an inevitable reaction to the aging process and plays an important role in frailty pathogenesis by influencing key components of the frailty syndrome. Moreover, increased pro-inflammatory cytokines and interleukin-6 have been associated with slow walking speed and reduced muscle strength [29]. High levels of C-reactive protein have also been associated with frailty [30].
In our study, we found no detail confirming whether frailty was related to individual macronutrients, such as carbohydrates, proteins, and fats. Protein intake has been a major focus of literature studies evaluating specific nutrients related to frailty because of the progressive loss of muscle mass and strength with aging. Amino acids stimulate muscle protein synthesis. However, previous studies on protein intake and frailty showed some-what contradictory results [31,32,33,34]. Bartali et al. [31] reported an association of low protein intake and frailty after adjusting for energy intake. Kobayashi et al. [32] showed that increased total protein intake was associated with a decreased prevalence of frailty among older Japanese women. However, our research did not follow these results. As with our findings, Schoufour et al. [33] and Shikany et al. [34] did not observe an association between frailty and energy-adjusted protein intake. Although the overall quality of meals and dietary patterns are more important than single nutrients, the importance of protein intake in older adults should not be overlooked in relation to muscle strength, the main cause of aging. In our study, the positive association of the “vegetables, oils, and fish” pattern, which was inversely related to frailty, and the protein-energy intake, might provide some evidence to support the relationship between frailty and protein. Detailed research on frailty and proteins is needed, considering not only amount of protein intake but also the source of protein.
The present study has some limitations. First, because of the cross-sectional nature of KNHANES, we could not define the causality of frailty and dietary factors. Further investigation is warranted to identify the causal relationship. Second, as a retrospective study, we developed a modified frailty index using the variables investigated in KNHANES to analyze the association between frailty with nutritional factors. In addition to the phenotype of frailty, there may be several other operations and definitions of frailty. Third, our dietary data were derived from a single 24 h dietary recall, which may not be sufficient to estimate usual dietary intake. However, only minor variations were observed between a single-day (24 h) dietary recall and data obtained over 2–10 days (3.9% for energy, within 10% for macronutrients and micronutrients) in the 2009 KNHANES [35]. Nevertheless, to the best of our knowledge, this current study is the first to report that a dietary pattern of “vegetables, oils, and fish” that includes diverse food items might be a therapeutic approach to decreasing frailty among older adults, based on a nationally representative population.
5. Conclusions
Our findings revealed that dietary pattern characterized by a high intake of vegetables, oils, and fish with a wide variety of foods might decrease frailty among older adults. To prevent frailty in older adults, encouraging the consumption of various kinds of food based on an increased intake of vegetables, oils, and fish may be one of the most straightforward and effective public nutrition strategies for the aging population. Future longitudinal study may be promising to confirm the causal association between dietary pattern and the risk of frailty.
Author Contributions
Y.K. and W.J. conceived and designed the study; W.J. performed the statistical analysis; W.J. and Y.S. wrote the paper; Y.K. contributed to the critical review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the academic -research cooperation program in the Korea Maritime Institute (KMI) (No.2021-0010-1002).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki. The Institutional Review Board (IRB) of the KCDC approved this study (2013-07CON-03-4C, 2013-12EXP-03-5C, 2018-01-03-P-A).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available from the Korea National Health and Nutrition Examination Survey (KNHANES VI and VII), conducted by the Korea Centers for Disease Control and Prevention (KCDCP), and are freely available from KCDCP (https://knhanes.cdc.go.kr, accessed on 9 September 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Morley J.E., Haren M.T., Rolland Y., Kim M.J. Frailty. Med. Clin. 2006;90:837–847. doi: 10.1016/j.mcna.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 2.Sieber C.C. Frailty–from concept to clinical practice. Exp. Gerontol. 2017;87:160–167. doi: 10.1016/j.exger.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Lacas A., Rockwood K. Frailty in primary care: A review of its conceptualization and implications for practice. BMC Med. 2012;10:4. doi: 10.1186/1741-7015-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veronese N., Stubbs B., Noale M., Solmi M., Rizzoli R., Vaona A., Demurtas J., Crepaldi G., Maggi S. Adherence to a Mediterranean diet is associated with lower incidence of frailty: A longitudinal cohort study. Clin. Nutr. 2018;37:1492–1497. doi: 10.1016/j.clnu.2017.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rahi B., Ajana S., Tabue-Teguo M., Dartigues J.-F., Peres K., Feart C. High adherence to a Mediterranean diet and lower risk of frailty among French older adults community-dwellers: Results from the Three-City-Bordeaux Study. Clin. Nutr. 2018;37:1293–1298. doi: 10.1016/j.clnu.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 6.León-Muñoz L.M., García-Esquinas E., López-García E., Banegas J.R., Rodríguez-Artalejo F. Major dietary patterns and risk of frailty in older adults: A prospective cohort study. BMC Med. 2015;13:11. doi: 10.1186/s12916-014-0255-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan R., Leung J., Woo J. Dietary Patterns and Risk of Frailty in Chinese Community-Dwelling Older People in Hong Kong: A Prospective Cohort Study. Nutrients. 2015;7:7070–7084. doi: 10.3390/nu7085326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foote J.A., Murphy S.P., Wilkens L.R., Basiotis P.P., Carlson A. Dietary Variety Increases the Probability of Nutrient Adequacy among Adults. J. Nutr. 2004;134:1779–1785. doi: 10.1093/jn/134.7.1779. [DOI] [PubMed] [Google Scholar]
- 9.Motokawa K., Watanabe Y., Edahiro A., Shirobe M., Murakami M., Kera T., Kawai H., Obuchi S., Fujiwara Y., Ihara K. Frailty severity and dietary variety in Japanese older persons: A Cross-sectional study. J. Nutr. Health Aging. 2018;22:451–456. doi: 10.1007/s12603-018-1000-1. [DOI] [PubMed] [Google Scholar]
- 10.Soysal P., Isik A.T., Carvalho A.F., Fernandes B.S., Solmi M., Schofield P., Veronese N., Stubbs B. Oxidative stress and frailty: A systematic review and synthesis of the best evidence. Maturitas. 2017;99:66–72. doi: 10.1016/j.maturitas.2017.01.006. [DOI] [PubMed] [Google Scholar]
- 11.De Martinis M., Franceschi C., Monti D., Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp. Mol. Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 12.Bollwein J., Diekmann R., Kaiser M.J., Bauer J.M., Uter W., Sieber C.C., Volkert D. Dietary quality is related to frailty in community-dwelling older adults. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2012;68:483–489. doi: 10.1093/gerona/gls204. [DOI] [PubMed] [Google Scholar]
- 13.García-Esquinas E., Rahi B., Peres K., Colpo M., Dartigues J.-F., Bandinelli S., Feart C., Rodríguez-Artalejo F. Consumption of fruit and vegetables and risk of frailty: A dose-response analysis of 3 prospective cohorts of community-dwelling older adults. Am. J. Clin. Nutr. 2016;104:132–142. doi: 10.3945/ajcn.115.125781. [DOI] [PubMed] [Google Scholar]
- 14.Balboa-Castillo T., Struijk E.A., Lopez-Garcia E., Banegas J.R., Rodríguez-Artalejo F., Guallar-Castillon P. Low vitamin intake is associated with risk of frailty in older adults. Age Ageing. 2018;47:872–879. doi: 10.1093/ageing/afy105. [DOI] [PubMed] [Google Scholar]
- 15.Kim J., Lee Y., Won C., Kim M., Kye S., Shim J.-S., Ki S., Yun J.-H. Dietary Patterns and Frailty in Older Korean Adults: Results from the Korean Frailty and Aging Cohort Study. Nutrients. 2021;13:601. doi: 10.3390/nu13020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kweon S., Kim Y., Jang M.-J., Kim Y., Kim K., Choi S., Chun C., Khang Y.-H., Oh K. Data Resource Profile: The Korea National Health and Nutrition Examination Survey (KNHANES) Int. J. Epidemiol. 2014;43:69–77. doi: 10.1093/ije/dyt228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fried L.P., Tangen C.M., Walston J., Newman A.B., Hirsch C., Gottdiener J., Seeman T., Tracy R., Kop W.J., Burke G., et al. Frailty in Older Adults: Evidence for a Phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001;56:M146–M157. doi: 10.1093/gerona/56.3.M146. [DOI] [PubMed] [Google Scholar]
- 18.Jönsson A., Lindgren I., Norrving B., Lindgren A. Weight loss after stroke: A population-based study from the Lund Stroke Register. Stroke. 2008;39:918–923. doi: 10.1161/STROKEAHA.107.497602. [DOI] [PubMed] [Google Scholar]
- 19.Grossi G., Perski A., Osika W., Savic I. Stress-related exhaustion disorder–clinical manifestation of burnout? A review of as-sessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scand. J. Psychol. 2015;56:626–636. doi: 10.1111/sjop.12251. [DOI] [PubMed] [Google Scholar]
- 20.Chen L.-K., Liu L.-K., Woo J., Assantachai P., Auyeung T.-W., Bahyah K.S., Chou M.-Y., Chen L.-Y., Hsu P.-S., Krairit O., et al. Sarcopenia in Asia: Consensus Report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 21.Kim S.-H., Ahn J., Ock M., Shin S., Park J., Luo N., Jo M.-W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016;25:1845–1852. doi: 10.1007/s11136-015-1205-2. [DOI] [PubMed] [Google Scholar]
- 22.Savela S.L., Koistinen P., Stenholm S., Tilvis R.S., Strandberg A.Y., Pitkälä K.H., Salomaa V.V., Strandberg T.E. Leisure-time physical activity in midlife is related to old age frailty. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013;68:1433–1438. doi: 10.1093/gerona/glt029. [DOI] [PubMed] [Google Scholar]
- 23.Krebs-Smith S.M., Smiciklas-Wright H., Guthrie H.A., Krebs-Smith J. The effects of variety in food choices on dietary quality. J. Am. Diet. Assoc. 1987;87:897–903. doi: 10.1016/S0002-8223(21)03212-0. [DOI] [PubMed] [Google Scholar]
- 24.Lo Y., Hsieh Y., Hsu L., Chuang S., Chang H., Hsu C., Chen C., Pan W. Dietary pattern associated with frailty: Results from nutrition and health survey in Taiwan. J. Am. Geriatr. Soc. 2017;65:2009–2015. doi: 10.1111/jgs.14972. [DOI] [PubMed] [Google Scholar]
- 25.Chai W., Morimoto Y., Cooney R.V., Franke A.A., Shvetsov Y.B., Le Marchand L., Haiman C.A., Kolonel L.N., Goodman M.T., Maskarinec G. Dietary Red and Processed Meat Intake and Markers of Adiposity and Inflammation: The Multiethnic Cohort Study. J. Am. Coll. Nutr. 2017;36:378–385. doi: 10.1080/07315724.2017.1318317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tan B.L., Norhaizan M.E., Liew W.-P.-P. Nutrients and Oxidative Stress: Friend or Foe? Oxidative Med. Cell. Longev. 2018;2018:9719584. doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S.H., Kim J.Y., Ryu K.A., Sohn C.M. Evaluation of the dietary diversity and nutrient intakes in obese adults. Korean J. Community Nutr. 2007;12:583. [Google Scholar]
- 28.Bernstein M.A., Tucker K.L., Ryan N.D., O’Neill E.F., Clements K.M., Nelson M.E., Evans W.J., Singh M.A.F. Higher dietary variety is associated with better nutritional status in frail elderly people. J. Am. Diet. Assoc. 2002;102:1096–1104. doi: 10.1016/S0002-8223(02)90246-4. [DOI] [PubMed] [Google Scholar]
- 29.Ferrucci L., Penninx B.W., Volpato S., Harris T.B., Bandeen-Roche K., Balfour J., Leveille S.G., Fried L.P., Md J.M.G. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002;50:1947–1954. doi: 10.1046/j.1532-5415.2002.50605.x. [DOI] [PubMed] [Google Scholar]
- 30.Walston J., McBurnie M.A., Newman A., Tracy R.P., Kop W.J., Hirsch C.H., Gottdiener J., Fried L.P. Frailty and activation of the in-flammation and coagulation systems with and without clinical comorbidities: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 31.Bartali B., Frongillo E.A., Bandinelli S., Lauretani F., Semba R.D., Fried L.P., Ferrucci L. Low nutrient intake is an essential compo-nent of frailty in older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006;61:589–593. doi: 10.1093/gerona/61.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kobayashi S., Asakura K., Suga H., Sasaki S. High protein intake is associated with low prevalence of frailty among old Japa-nese women: A multicenter cross-sectional study. Nutr. J. 2013;12:164. doi: 10.1186/1475-2891-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoufour J.D., Franco O.H., Kiefte-de Jong J.C., Trajanoska K., Stricker B., Brusselle G., Rivadeneira F., LaHousse L., Voortman T. The association between dietary protein intake, energy intake and physical frailty: Results from the Rotterdam Study. Br. J. Nutr. 2019;121:393–401. doi: 10.1017/S0007114518003367. [DOI] [PubMed] [Google Scholar]
- 34.Shikany J.M., Barrett-Connor E., Ensrud K.E., Cawthon P.M., Lewis C.E., Dam T.L., Shannon J., Redden D.T., Osteoporotic Fractures in Men (MrOS) Research Group Macronutrients, diet quality, and frailty in older men. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013;69:695–701. doi: 10.1093/gerona/glt196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korea Health Industry Development Institute . National Food & Nutrition Statistics: Based on 2009 Korea National Health and Nutrition Examination Survey. Korea Health Industry Development Institute; Osong, Korea: 2011. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from the Korea National Health and Nutrition Examination Survey (KNHANES VI and VII), conducted by the Korea Centers for Disease Control and Prevention (KCDCP), and are freely available from KCDCP (https://knhanes.cdc.go.kr, accessed on 9 September 2021).