Abstract
Interest in integrating Magnetic Resonance Imaging (MRI) in radiation therapy (RT) practice has increased dramatically in recent years due to its unique advantages such as excellent soft tissue contrast and capability of measuring biological properties. Continuous real-time imaging for intra-fractional motion tracking without ionizing radiation serves as a particularly attractive feature for applications in RT. Despite its many advantages, the integration of MRI in RT workflows is not straightforward with many unmet needs. MR safety remains one of the key challenges and concerns in the clinical implementation of MR simulators and MR-guided radiotherapy systems in radiation oncology. Most RT staff are not accustomed to working in an environment with strong magnetic field. There are specific requirements in RT that are different from diagnostic applications. A large variety of implants and devices used in routine RT practice do not have clear MR safety labels. RT specific imaging pulse sequences focusing on fast acquisition, high spatial integrity and continuous real-time acquisition require additional MR safety testing and evaluation. This article provides an overview of MR safety tailored toward RT staff, followed by discussions on specific requirements and challenges associated with MR safety in the RT environment. Strategies and techniques for developing a MR safety program specific to RT are presented and discussed.
Introduction:
Recent technological advances in Magnetic Resonance Imaging (MRI) simulators and MR-guided radiotherapy (MRgRT) systems have led to a strong interest in incorporating MRI into radiation therapy (RT) workflow due to its unique imaging advantages for accurate planning and delivery of radiation treatment 1-6. The superior soft-tissue contrast of MR images enables better localization of the tumor and patient anatomy; therefore, it has the potential to improve patient setup accuracy and reduce treatment planning margins. In addition, MRI can measure physiological and functional information including but not limited to tissue perfusion, diffusion, blood volume, cellularity, and pH 7-11. Therefore, it is possible to monitor the treatment response of tumor and surrounding critical organs using daily MRI and adapt the treatment to account for these variations for better tumor control and reduced toxicity 12-14. Another advantage of MRI is that it does not subject patients to ionizing radiation, which is ideal for continuous real-time imaging for tumor and organ motion tracking 15-18.
Despite its many advantages, integrating MRI into RT workflow is not straightforward and requires collaborative efforts and strong cross-training between the radiation oncology and diagnostic radiology community 19-21. MR safety is one of the major concerns in the clinical integration of MRI in radiation oncology since most RT staff are not yet accustomed to working in an environment with strong magnetic fields. In addition, there are many RT-specific requirements that are beyond the scope of diagnostic applications. For example, many immobilization devices and QA equipment used in radiotherapy lack clear MR safety labels. Guidelines on MRI safety developed by current regulatory and professional societies have established standards for safe practices in clinical and research MR environments based on knowledge and expertise from diagnostic settings 22,23. The principles behind these practice guidelines can be used as a basis for developing MR safety programs in RT. However, special considerations and adaptations are required when tailoring these guidelines to meet the focus and specific requirements of MR applications in the RT environment. This article provides an overview of MR safety geared toward RT staff including radiation oncologists, therapeutic medical physicists, and radiation therapists. We outline specific requirements and challenges associated with MR safety in the RT environment and discuss strategies to overcome these challenges.
Magnetic field induced hazards
A MRI system usually consists of several major components: a magnet that produces a uniform static magnetic field for the polarization of the tissue magnetization, gradient coils that introduce changes in field strength to spatially encode the MRI signal, and radiofrequency (RF) coils that transmit and receive signals from the imaged tissue. Each of these systems can be potentially hazardous and result in adverse effects on patient and professional personnel. A brief summary is provided below and in-depth discussions can be found in references 22,24-26.
Force and torque generated by static magnetic field
Translational and rotational forces are generated when a metallic object is placed in a magnetic field. The magnitude of the force exerted on a ferromagnetic object depends on the strength of the magnetic field, the size of the object, its distance to the magnet and its material composition. Clinical MRI systems usually use high magnetic field ranging from 0.2 Tesla (T) to 3T, compared to the earth’s magnetic field of 0.5 Gauss (10−4T). Significant attraction force and torque between ferromagnetic materials within medical equipment or implanted devices and the static magnetic field can induce dangerous and damaging projectile effects. Additionally, the magnetic field can also potentially impact the equipment or treatment machines in the neighboring area.
Time-varying gradient magnetic field induced issues
The static magnet of an MRI system produces a homogenous magnetic field across the patient’s body. During the imaging acquisition, a time-varying magnetic field, namely a gradient, is generated by a set of coils to produce a spatially varying magnetic field inside the patient’s body so that spatial encoding of the MR signal can be performed. The rapid switching of the field gradient can produce several safety concerns. First, electrical currents i.e., eddy currents can be induced in nearby conductors by the changing magnetic field. The current induced in a loop of tissue depends on the rate of field change (dB/dt), the electrical conductivity of the tissue, and the cross-sectional area of the loop 24. The induced electrical voltage and current can lead to adverse effects including heating and tissue burns. Eddy currents can interfere with the normal function or damage the electronic components in active implanted devices and other medical equipment. The induced electrical currents can also produce neurostimulation in patents. Mild sensation or painful response can be felt at the patient's surface and extremities, often referred to as peripheral nerve and muscle stimulation (PNST). The stimulation effect depends on the rate of field change (dB/dt) and the maximum strength of the gradient field. The U.S. Food and Drug Administration (FDA) has established guidelines that require the dB/dt rate to be at a factor of three below the mean threshold required to cause palpable peripheral nerve stimulation 24. Another potential hazard to patients is the strong acoustic noise originating from the gradient system due to rapid gradient field switching. Acceptable acoustic noise levels and hearing protection guidelines have also been established by the FDA 22.
Time-varying radiofrequency magnetic field induced issues
Radiofrequency (RF) coils, functioning as “antennae” of the MRI system, are used to transmit and receive signals from tissue under magnetization, excitation, and relaxation. The time-varying electromagnetic (EM) field induced by the RF coils deposits energy into the patient’s tissue, resulting in localized heating. The thermal effect depends on the amount of energy absorbed and can be quantified by Specific Absorption Rate (SAR), defined as the energy dissipated in tissue per kilogram of tissue mass (W/kg). The SAR is proportional to the tissue conductivity, patient size and the square of the electric field strength generated by the RF pulse, which is spatially nonuniform. It is also pulse sequence dependent and thus can be mitigated by using lower flip angles and longer repetition times. Both the International Electrotechnical Commission (IEC) and FDA has established limits on the maximum SAR averaged over the whole-body and local regions such as head and extremity 27,28. Most modern MR scanners can provide an estimation of the whole-body SAR using the information of the total emitted RF power and the patient’s weight. It is vital to review imaging protocols to ensure that SAR does not exceed the allowed limits. However, one must note that this estimation is very rudimentary and can be inaccurate. The final temperature increase in a patient’s tissue depends on many other factors including blood perfusion, local tissue conductivity, and patient anatomy and positioning. Focal hotspot with excessive heating can be generated due to the presence of implanted devices using electrically conductive materials such as wires and leads or when there is direct contact between the patient’s skin and conductive materials in the surface coils, cables, immobilization devices and clothing with metallic materials. In addition, a conductive loop can form when a patient crosses legs or clasps hands, leading to heating at the high-resistance skin-to-skin contact point 29,30. Thermal burns are one of the often-overlooked MR safety issues 31,32. Therefore, care must be taken to prevent excessive heating and possible burn injuries associated with MRI.
Practical safety considerations for MR in RT environment
To address the critical safety issues described above, the American Colleague of Radiology (ACR) has established de facto industry standards for safe and responsible practices in the clinical and research MR environments. The ACR MR Safety Practice Guidelines describe site planning and access restrictions, patient and personnel screening, device labeling and screening, personnel training and MR safety policies and procedures 22. In the rest of this section, special considerations for implementing these guidelines in the RT environment and strategies to overcome the specific needs and challenges in RT are elaborated, as a supplement to the ACR MR Safety Practice Guidance.
Site planning and access restriction
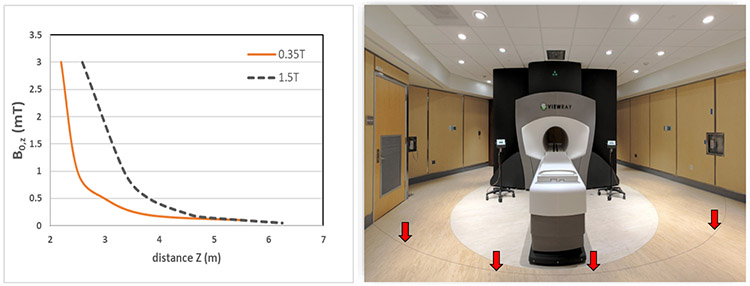
MR safety should be considered as early as possible in site planning to address issues related to cryogen safety, siting consideration and area access restrictions. The ACR MR Safety Guideline provides detailed recommendations on MR facility safety design in its Appendix. There are specific safety considerations when siting a MRgRT system or MR simulator. Since the magnetic force and torque is proportional to the field strength, which varies as a function of the distance from the center of the magnet, it is important to map and survey the fringe field of the system. The 5 Gauss line should be clearly marked on the floor as being potentially hazardous. Figure 1 compares the fringe fields in the transverse direction as a function of the distance from the isocenter for a 0.35T and 1.5T MRI. A picture of a 0.35T MRgRT system is also shown in Figure 1 demonstrating the 5 Gauss line marked on the floor. For systems with high strength magnetic fields, especially in a compact siting environment, the impact of the fringe field on neighboring equipment needs to be evaluated. Perik et al. investigated the radiation beam performance of three clinical medical accelerators surrounding a 1.5T MR Linac 33. It was observed that beam flatness and symmetry as a function of gantry angle changed up to 4% after the magnet was ramped up. Active beam steering had to be performed to account for the deviation of beam performance due to the magnetic field. A similar observation was reported in another study evaluating the impact of a 1.5T MR Linac34. A maximum increase of 1.5 Gauss was measured at 8m distance to the magnet and the neighboring Linac had to be recalibrated in order to operate within clinical tolerance.
Figure 1.
(a) Fringe field strength of 0.35T and 1.5T MRI along the B0 direction as a function of the distance from isocenter (b) picture of a MRgRT vault with the 5 Gauss line clearly marked on the floor as being potentially hazard
The four-zone concept recommended by ACR can be followed when designing the site access restrictions for MR Linac or simulator installed in RT facilities. In general, zone I includes areas that are freely accessible to the general public, for instance, the patient waiting area before check-in. Zone II is the area between the open free-accessible area and more strictly controlled zones. Examples of Zone II include the nursing area and patient waiting area after check-in. Zone III is the post-screening area which should be physically restricted from public access, while zone IV is essentially the room where the MR scanner is installed. A unique challenge in the RT environment is that patients receiving MR guided radiotherapy or simulation are often mingled with other patients treated on regular clinical Linac. A designated patient holding area is desirable in Zone III for patients receiving MRI after proper screening procedures. In practice, it could be challenging to assign a dedicated private MR patient waiting area physically restricted from other patients. An alternative solution is to share the control room and perform patient screening right before the patient enters the treatment room at the cost of throughput. Once the zoning is determined, MRI safety zone signage should be posted in each area. Adequate posting space should be designed so that both the MRI safety zone and radiation safety warning signs can be posted together.
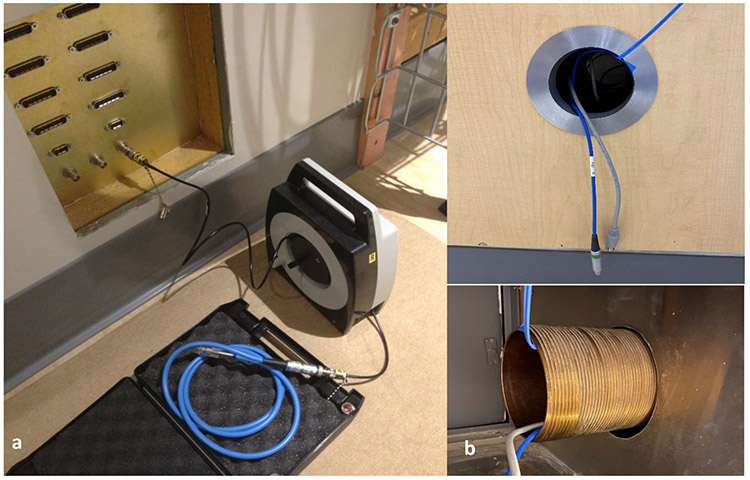
Another special consideration in the design of an MR suite in RT is equipment storage. Various equipment such as physics quality assurance (QA) equipment and patient immobilization devices are used in daily RT practices. Dedicated equipment storage space for MR safe equipment in the MR suite can effectively reduce the risk of misuse of unsafe equipment designed for conventional Linac treatments. In addition, many physics QA devices require power and data cable connection between the treatment vault and control room. To prevent interference to the MR performance by the RF noise transmitted through the cables, appropriate RF filter connectors should be considered ahead of time during site planning to provide clean power and data into the RF shielded room. For example, RF filters for commonly used tri- and co-axial cables should be provided for radiation dose measurement devices as shown in Figure 2. A conduit should be built for other cables that do not need RF filters to allow a direct connection. However, waveguides should be used to prevent the conduit from compromising the room’s RF shielding as shown in Figure 235.
Figure 2.
(a) RF filter panel designed specifically for tri-axial and co-axial cable used for dosimeters such as ion chambers (b) Waveguide conduit for cables that do not need RF filter
Patient and personnel safety screening
Patient and personnel safety screening is one of the most effective approaches to prevent hazardous conditions and adverse events in a MR unit. The detailed practice guidelines and examples of safety screening procedures and forms provided in the ACR MR Safety Guideline can be used as references to develop screening procedures and policies for an RT institution. For RT practice, it is recommended that the screening process start as early as possible. If the MR questionnaire can be completed during initial patient consultation, it will provide additional time for the care team to investigate questionable devices before patient simulation and treatment. Many RT patients may already undergo various diagnostic MRIs before visiting the RT department. Existing medical records and diagnostic MR images may be helpful in assisting in the evaluation and decision-making process. A major challenge of RT patient safety screening is that most patients receive multiple treatments and need to visit the facility daily. It is important to perform daily screening before each treatment fraction to monitor any potential changes in the MR safety status of the patient. A simplified questionnaire can be used to identify potential safety status changes between fractions. Physical screening using a hand-held magnet and ferromagnetic detection system is highly recommended to be performed every time the patient enters the room. Despite these screening efforts, the challenge and need for comprehensive patient safety screening for RT patients was demonstrated in a case report in which significant image artifacts that compromised accurate delivery of MRgRT treatment were observed in some fractions, as shown in Figure 3. It was found that these metal artifacts were actually caused by iron-rich food and vitamin pills taken shortly before the treatment 36. This example clearly signifies the importance of careful and consistent patient screening and education to minimize potential safety events throughout the long course of radiation treatments that can range over weeks or months.
Figure 3.
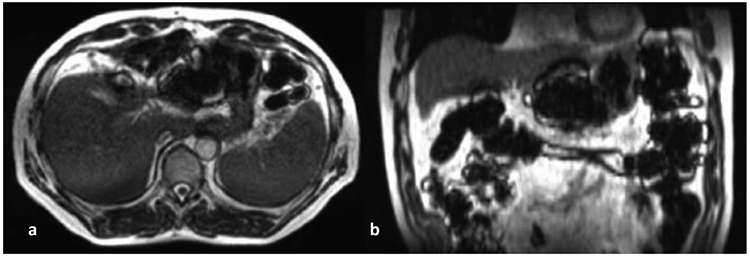
Transverse (left) and coronal (right) views of a patient's abdomen a few hours after ingesting iron-fortified breakfast cereal. The susceptibility artifact was present throughout the bowel and prevented safe treatment for that fraction. Reprint from publication [34]
Device and equipment safety management
Patient implants and portable devices brought into the MR environment can present significant safety risks to the patient and other individuals. In general, these devices can be divided into two major groups, active devices that require a power supply and passive devices that do not contain powered electronic components. For passive devices, major safety concerns include magnetically induced displacement force and torque and RF-induced heating. Furthermore, device malfunction due to circuitry interference by eddy currents and EM fields need to be carefully evaluated for active devices. In addition to these safety concerns, the presence of these implants and devices may also lead to image artifacts and geometric distortions on the MR images. For all the portable equipment or objects to be brought into MR safety zone IV, appropriate MR safe labelling should be determined based on current FDA labeling criteria which are outlined in America Society for Testing and Materials (ASTM) Standards F2503 37,38. In general, a device composed of nonconductive, nonmetallic and non-magnetic materials that poses no known hazards in MR environments can be labeled as “MR Safe”. In comparison, devices that pose unacceptable risks to patients and other personnel within the MR environment must be labeled as “MR Unsafe”. “MR Conditional” labeling is used to identify items or devices with demonstrated safety in the MR environment under specifically defined conditions. MRI safety information should be provided for MR conditional devices specifying the conditions under which the device can be safely used. Associated labels with distinct color and shape were developed for these different safety terms and can be affixed to devices for easy identification 22. One should note that the prior term of “MR compatible” defined in 1990s was deemed confusing and obsolete and should thus be avoided in device labeling 39. Further details and recommendations on item labeling can be found in FDA regulatory documents and the ACR MR Safety Guideline 22,38,40. In the rest of this section, MR safety concerns and strategies to manage specific implants and equipment used in the RT environment are discussed, respectively.
Patient implants
An abundance of literature is available on MR safety practices for various passive patient implants such as orthopedic, interventional, cardiovascular and neurovascular devices 31,41-45. The presence of metallic implants can cause significant artifacts including the signal void and geometric distortion in MR images. The basic principles and strategies to correct the artifacts have been discussed as well 46,47. For an active device such as cardiac implantable electronic devices (CIEDs), recent expert consensus statements by the Heart Rhythm Society (HRS) provides comprehensive review and guidelines on the safe management of MRI of patients with CIEDs 48. In addition to these commonly seen patient implants, radiopaque implanted fiducial markers are commonly used in RT for target localization and respiration motion tracking. They exist in a variety of shapes, sizes, and materials including gold, nitinol and platinum. Due to its small size, the magnetic field induced displacement and MR related heating were found to be negligible even at 3T field strength49. However, these fiducial markers can introduce strong susceptibility related image artifacts impacting their visibility and localization accuracy. The magnitude of artifacts depends on the MRI sequence and the type and orientation of the markers50. The apparent positions of gold markers determined from the images were found to deviate from the actual positions by up to 1mm, adding additional uncertainty in localization of the target by using these markers 51. Electromagnetic Positioning Transponders are a new type of implanted fiducial that can provide real-time tumor tracking. These transponders consist of a coil circuit with a ferromagnetic inductor which may interact with MR and cause both image artifacts and the possibility of migration. Phantom studies indicated that the migration is relatively small (<1mm) for both 1.5 and 3T MRI and the heating effect is also minimal 52,53. However, substantial null signal artifacts (up to a few cm) around the transponders were observed in both phantom and patient MR images.
Immobilization and accessory devices
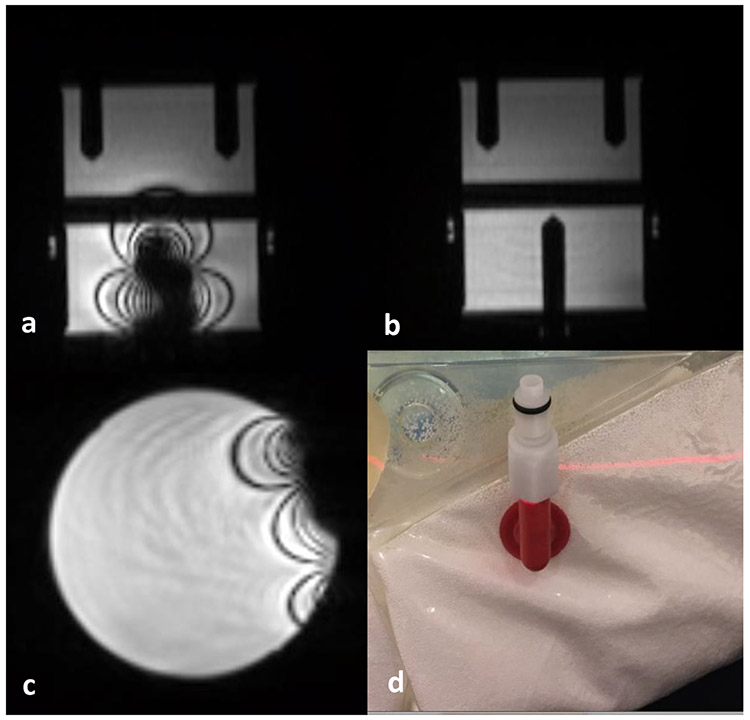
Unlike diagnostic MR scanning, a large variety of immobilization and accessory devices are routinely used to maintain a reproducible patient position throughout the entire RT treatment course. The MR safety profiles of these RT specific devices have not been well established, potentially posing safety risks to patients under MRI. Vacuum compressed cushions are one of the most popular immobilization devices to mold and maintain a consistent patient treatment position. In general, they can be considered as safe with regard to the projectile effect since they consist of mostly non-ferromagnetic materials. Its heating effect is also minimal; however, care must be taken to avoid thermal insulation, direct skin to conductor contact and potential conducting loops when using the vacuum cushions to mold patient treatment position. Some of the vacuum cushions contain a small metal spring in the valve which can cause significant imaging artifact as shown in Figure 4. The artifacts can be mitigated by placing the valve away from the region of interest during image acquisition. Carbon fiber is a commonly used material for immobilization device and treatment couch top due to its combination of strong mechanical strength, rigidity, light weight, and low radiation attenuation 54. However, carbon fiber is electrically conductive and therefore can pose potential MR safety concerns due to RF thermal heating effect and image susceptibility artifacts. As shown in Figure 5, strong shading artifacts are evident on a uniform sphere water phantom placed on a conventional head and neck immobilization board made of carbon fiber. The RF-induced heating effect of an in-house carbon fiber flatbed for a 3T MRI scanner was evaluated by using two different thermometry techniques 55. Minimal temperature increases (<0.2°C) were observed as demonstrated by the temperature-time profiles measured for three different imaging sequences. However, severe shading image artifacts were evident on both phantom and patient images caused by the magnetic susceptibility difference of the carbon fiber couch top. Other non-conducting composite materials, for example, composites made of polypropylene and fiberglass, were investigated and found to be a good replacement for carbon fiber with similar mechanical and dosimetric properties but less impact on image quality 56. The effect of the magnetic susceptibility of various thermoset and thermoplastic materials was evaluated and it was found that glass-cloth resin reinforced thermosets, specifically G-8 fiberglass, produced the smallest susceptibility changes, making it an optimal material for immobilization device in an MR environment 57.
Figure 4.
(a) Metal image artifacts caused by a regular ionization chamber with metal components (b) the same phantom image with an MR-safe chamber inserted. (c) Image artifacts caused by a metal spring in the valve of vacuum cushion used for patient immobilization as shown in (d)
Figure 5.
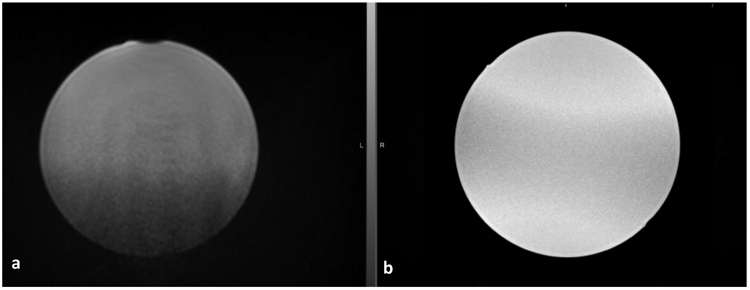
(a) Shading artifacts caused by a conventional carbon-fiber head and neck immobilization board placed underneath a uniform spherical phantom (b) image of the same phantom placed on an immobilization board made of plastic acrylic
Quality assurance (QA) equipment
A large variety of physics QA equipment is widely used in RT for dosimetry data collection and routine QA measurements. Most QA equipment used in radiotherapy was developed specifically for RT applications before the advent of MR guided technologies and consequently do not have clear MR safety labeling information, posing one of the major challenges in the integration of MR in RT workflow. It is important to establish a safety screening process to evaluate and label the equipment before it can be brought into the MR room. There are four general aspects to consider when evaluating QA equipment for the MR environment: 1) projectile hazard effect due to ferromagnetic components, 2) electronic components that can be damaged by the magnet or interfered by the time-varying RF and gradient fields, 3) the impact on the measurement accuracy by the magnet and 4) image artifacts and distortion caused by the device. A handheld magnet (>1000 Gauss) can be used to discover grossly detectable ferromagnetic components in the device 22. Efforts should be made to contact the vendor to obtain detailed information on the internal components of the device. For equipment with electronic components operated with a power supply, additional evaluation should be made for potential malfunction of the device under the strong magnetic field. For example, the stepper motor in many QA devices contains a small electric permanent magnet, which may become saturated by the fringe field and lead to erroneous motor operation or an increase in operating current that could damage the motor. Alternative solutions are available for motors intended for the MR environment, including special actuators such as piezoelectric and ultrasonic motors, EM actuators, and pneumatic and hydraulic actuators 58. Both electric and EM actuator requires special shielding from the magnetic field while the pneumatic and hydraulic actuators are considered as intrinsically MR safe. The advantages and limitations of these actuators are compared in detail 58,59. Other electronic components such as transformers, ferrite cores, relays, and switches may also lose their functionality due to ferromagnetic saturation 60. The presence of a strong magnetic field can influence the detector response and impair the measurement accuracy of conventional dosimeters. For instance, it was found that the dose response initially increases up to approximately 8.3% at 1 T and slowly decreases thereafter when a Farmer ion chamber is placed perpendicular to both the incident beam and the magnetic field 61. It was also reported that the dose response of radiochromic film can decrease by up to 15% under a 0.35T magnet due to the changes in crystal orientation within the active layer under the magnetic field 62. On the other hand, the presence of a QA device in the MR environment can also adversely interact with the MRI system through distortion of the homogeneity of the magnetic field or generation of RF noise. Due to the complicated MR safety, imaging, and measurement performance concerns described above, many of the conventional QA devices used in RT need to be altered in order to be used in MR environment. Basic correction strategies include active shielding against the magnetic field, distance away from magnet and replacement of sensitive metallic and electronic components, which can be illustrated by the adaption of a few commonly used RT QA devices as described below.
Ionization chambers are widely utilized for absolute and reference dosimetry in RT. For this purpose, their accuracy and reproducibility of the measurement are extremely critical. Ionization chamber is primarily made of tissue or air equivalent materials such as acrylic and graphite, although aluminum, stainless steel, and other low Z metallic material can be used for the central electrode, guard ring, or stem. The magnetic projectile attraction effect on the ionization chamber is negligible because of its minimal amount of metallic component and non-ferromagnetic property. Therefore, conventional ionization chambers can be considered safe for dose measurement in the MR environment. However, metallic components in ionization chamber can cause image artifacts, making it challenging to perform QA tests requiring both image acquisition and dose measurement, as demonstrated in Figure 4. Ionization chambers made of non-metallic materials are commercially available from several vendors that mitigate this issue. Nevertheless, the most significant issue of using ionization chamber in an MR environment is the impact of the magnetic field on the measurement accuracy due to the electron return effect, particularly near interfaces between materials of significantly different densities – a defining characteristic of ionization chambers. Several studies have investigated the dose measurement performance of the ionization chamber under the influence of a magnetic field and indicated that ionization chambers could be reliably used for standard dose calibration procedures with appropriate correction factors, but care must be taken with the choice of beam quality specifier and chamber orientation 63,64. Hand-held ion chamber survey meters are commonly used for radiation safety related measurement in various RT applications. These devices usually consist of a step-up transformer of the high voltage supply circuit and may contain other ferromagnetic components that can lead to a strong attractive force. The ion chamber itself is a capacitor, and when it moves along the magnetic field, the induced voltage can lead to substantial changes in chamber response with magnitude depending on the device’s orientation and the direction of motion65.
Scanning water phantoms are very important QA devices for beam measurement and dose calibration. Most of these water phantoms cannot be directly used for MRgRT because they are typically equipped with electric stepper-motors and parts containing ferromagnetic materials. Simple water phantoms with mechanical manual motion controls can be used for dose calibration. In combination with treatment couch motion these water phantoms can also be used for beam scanning, but at the cost of a very time-consuming process. A prototypical automatic scanning water phantom was shown to achieve safe and accurate beam measurements for MR Linac 66. It was modified from a commercial conventional water tank by replacing the stepper-motors with ultrasonic motors and using non-ferromagnetic materials such as aluminum for components such as the driving spindles.
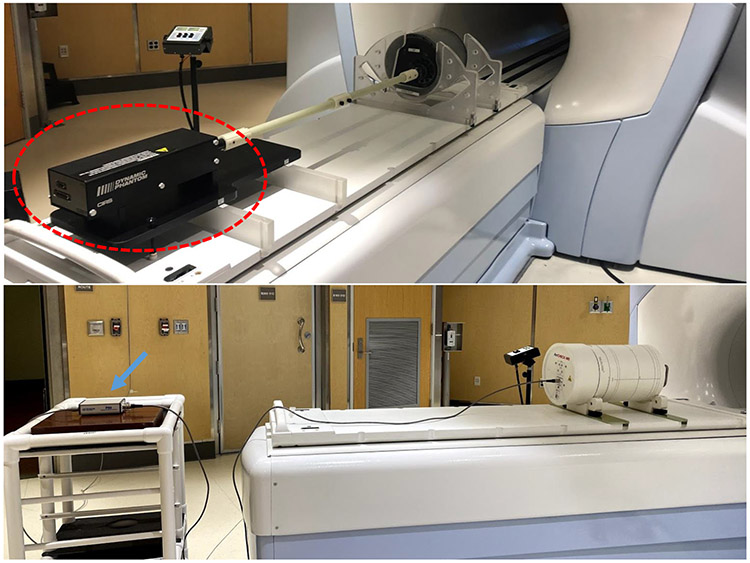
Devices consisting of ion chamber and diode arrays are commonly used for beam performance and patient-specific QA measurements. These devices usually contain complex electronic components and power supplies that can be damaged by the presence of the magnet field. A cost-effective solution is to use the distance principle, i.e. the device can be modified by relocating the sensitive components outside the 5 Gauss line. Figure 6 shows an example of a patient-specific QA device with sensitive electronic components in an extension box placed outside the magnetic field. Satisfying performance of these modified devices has been reported for different MRgRT systems 67,68.
Figure 6.
Examples of QA devices of which sensitive electronic components are placed away from region with high magnetic field. Top: a motion QA phantom where the controller (circled in red) is placed at the end of couch. Bottom: an array dosimetric QA phantom with electronic components placed in an extension box (blue arrow) placed outside the 5-guass line.
One of the major advantages of MRgRT is its real-time imaging for motion management and gating, which necessitates rigorous QA testing with motion phantoms. It is challenging to modify existing motion phantoms for an MR environment because of the requirement of simultaneous high-quality image acquisition and continuous complex phantom motion. It can be achieved by keeping the controller and electronics at a safe distance from the magnet as shown in Figure 6. Other solutions include replacing the electric motor with pneumatic or piezoelectric motors 69.
Policy, Procedure and Personnel Training
MR guided radiotherapy and simulation introduce new paradigms into the RT community. Many RT staff, including therapeutic medical physicists may be insufficiently trained to work in the MR environment. For successful integration of MR in the RT workflow, it is critical to develop an MR safety program consisting of well-established policies, procedures and personnel training. Written MR safety policies and procedures must be developed, enforced, reviewed and updated frequently to keep up with changes in the technology and workflows. The two-level MR personnel concept developed by the ACR can be followed to develop personnel training in RT 22. The level 1 MR personnel include those who have passed minimal safety training to work safely within zone III, while extensively training is provided to level 2 MR personnel to ensure safe practice in zone III and IV. Efforts should be made to ensure all RT staff obtain minimal safety education to qualify as level 1 MR personnel. As recommended by ACR guidelines, MR safety training lectures or presentations should be provided to all involved staff at least annually and appropriate record of such training should be maintained to confirm the training efforts23. It is also a good practice to incorporate the training into the new employee onboarding process to ensure timely training for new hires and trainees. Although currently there are no regulations or established guidelines on the credentialing and certification requirements of MR training for radiation therapists who work with MR simulators or MRgRT machines, it is highly recommended that radiation therapists and medical physicists working in zone III and IV should be considered as level 2 personnel and receive extensive MR safety training. Radiation oncologists who enter zones III and IV on a regular basis may need to be trained as level 2 personnel as well. As the use of dedicated MR simulators and MRgRT systems continue to increase, recommendations and guidelines specific to MR safety training and credentialing in radiation oncology from regulatory agencies or international scientific and professional associations are still in need.
Conclusions
The use of MRI in the radiotherapy workflow for simulation and delivery guidance is experiencing rapid growth due to its unique imaging features of superior soft-tissue contrast, functional information as well as real-time imaging without radiation dose. However, MR safety remains one of the key challenges in incorporating MRI in the RT environment. Most RT staff are not accustomed to working in a high magnetic field environment. A large variety of implants and devices are used in routine RT practice and do not have clear MR safety labeling information. RT specific imaging pulse sequences focusing on fast image acquisition, high geometric integrity and continuous real-time acquisition throughout treatment require additional MR safety testing and evaluation. It is paramount to develop an MR safety program that fits specific RT needs. Extensive knowledge and expertise on MR safety are available from the diagnostic imaging community, and close collaboration and cross-training should be formed to achieve this goal.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: Dr. Sheng is a co-founder of Celestial Oncology.
Data Sharing Statement: Research data are not available at this time.
References:
- 1.Glide-Hurst CK, Wen N, Hearshen D, et al. Initial clinical experience with a radiation oncology dedicated open 1.0T MR-simulation. J Appl Clin Med Phys. 2015;16(2):218–240. doi: 10.1120/jacmp.v16i2.5201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kupelian P, Sonke J-J. Magnetic resonance-guided adaptive radiotherapy: a solution to the future. Semin Radiat Oncol. 2014;24(3):227–232. doi: 10.1016/j.semradonc.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 3.Chandarana H, Wang H, Tijssen RHN, Das IJ. Emerging Role of MRI in Radiation Therapy. J Magn Reson Imaging JMRI. September2018. doi: 10.1002/jmri.26271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KM, Michel KA, Bankson JA, Fuller CD, Klopp AH, Venkatesan AM. Emerging Magnetic Resonance Imaging Technologies for Radiation Therapy Planning and Response Assessment. Int J Radiat Oncol. 2018;101(5):1046–1056. doi: 10.1016/j.ijrobp.2018.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollard JM, Wen Z, Sadagopan R, Wang J, Ibbott GS. The future of image-guided radiotherapy will be MR guided. Br J Radiol. 2017;90(1073):20160667. doi: 10.1259/bjr.20160667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagendijk JJW, Raaymakers BW, Van den Berg CAT, Moerland MA, Philippens ME, van Vulpen M. MR guidance in radiotherapy. Phys Med Biol. 2014;59(21):R349–369. doi: 10.1088/0031-9155/59/21/R349 [DOI] [PubMed] [Google Scholar]
- 7.Matsuo M, Matsumoto S, Mitchell JB, Krishna MC, Camphausen K. Magnetic Resonance Imaging of the Tumor Microenvironment in Radiotherapy: Perfusion, Hypoxia, and Metabolism. Semin Radiat Oncol. 2014;24(3):210–217. doi: 10.1016/j.semradonc.2014.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao Y, Popovtzer A, Li D, et al. Early Prediction of Outcome in Advanced Head-and-Neck Cancer Based on Tumor Blood Volume Alterations During Therapy: A Prospective Study. Int J Radiat Oncol. 2008;72(5):1287–1290. doi: 10.1016/j.ijrobp.2008.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thoeny HC, Ross BD. Predicting and monitoring cancer treatment response with diffusion-weighted MRI. J Magn Reson Imaging. 2010;32(1):2–16. doi: 10.1002/jmri.22167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang Y, Cao M, Sheng K, et al. Longitudinal diffusion MRI for treatment response assessment: Preliminary experience using an MRI-guided tri-cobalt 60 radiotherapy system: Longitudinal diffusion MRI using an MRI-guided radiotherapy system. Med Phys. 2016;43(3):1369–1373. doi: 10.1118/1.4942381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tyagi N, Riaz N, Hunt M, et al. Weekly response assessment of involved lymph nodes to radiotherapy using diffusion-weighted MRI in oropharynx squamous cell carcinoma: Weekly assessment of lymph nodes using diffusion-weighted MRI. Med Phys. 2015;43(1):137–147. doi: 10.1118/1.4937791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pathmanathan AU, van As NJ, Kerkmeijer LGW, et al. Magnetic Resonance Imaging-Guided Adaptive Radiation Therapy: A “Game Changer” for Prostate Treatment? Int J Radiat Oncol Biol Phys. 2018;100(2):361–373. doi: 10.1016/j.ijrobp.2017.10.020 [DOI] [PubMed] [Google Scholar]
- 13.Acharya S, Fischer-Valuck BW, Kashani R, et al. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int J Radiat Oncol. 2016;94(2):394–403. doi: 10.1016/j.ijrobp.2015.10.015 [DOI] [PubMed] [Google Scholar]
- 14.Kashani R, Olsen JR. Magnetic Resonance Imaging for Target Delineation and Daily Treatment Modification. Semin Radiat Oncol. 2018;28(3):178–184. doi: 10.1016/j.semradonc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 15.Stemkens B, Tijssen RHN, de Senneville BD, Lagendijk JJW, van den Berg CAT. Image-driven, model-based 3D abdominal motion estimation for MR-guided radiotherapy. Phys Med Biol. 2016;61(14):5335–5355. doi: 10.1088/0031-9155/61/14/5335 [DOI] [PubMed] [Google Scholar]
- 16.Han F, Zhou Z, Du D, et al. Respiratory motion-resolved, self-gated 4D-MRI using Rotating Cartesian K-space (ROCK): Initial clinical experience on an MRI-guided radiotherapy system. Radiother Oncol. 2018;127(3):467–473. doi: 10.1016/j.radonc.2018.04.029 [DOI] [PubMed] [Google Scholar]
- 17.Mickevicius NJ, Chen X, Boyd Z, Lee HJ, Ibbott GS, Paulson ES. Simultaneous motion monitoring and truth-in-delivery analysis imaging framework for MR-guided radiotherapy. Phys Med Biol. 2018;63(23):235014. doi: 10.1088/1361-6560/aaec91 [DOI] [PubMed] [Google Scholar]
- 18.Mostafaei F, Tai A, Omari E, et al. Variations of MRI-assessed peristaltic motions during radiation therapy. Zhang Q, ed. PLOS ONE. 2018;13(10):e0205917. doi: 10.1371/journal.pone.0205917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McGee KP, Hu Y, Tryggestad E, et al. MRI in radiation oncology: Underserved needs. Magn Reson Med. 2016;75(1):11–14. doi: 10.1002/mrm.25826 [DOI] [PubMed] [Google Scholar]
- 20.Rai R, Kumar S, Batumalai V, et al. The integration of MRI in radiation therapy: collaboration of radiographers and radiation therapists. J Med Radiat Sci. 2017;64(1):61–68. doi: 10.1002/jmrs.225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao M, Padgett KR, Rong Y. Are in-house diagnostic MR physicists necessary for clinical implementation of MRI guided radiotherapy? J Appl Clin Med Phys. 2017;18(5):6–9. doi: 10.1002/acm2.12171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Expert Panel on MR Safety:, Kanal E, Barkovich AJ, et al. ACR guidance document on MR safe practices: 2013. J Magn Reson Imaging. 2013;37(3):501–530. doi: 10.1002/jmri.24011 [DOI] [PubMed] [Google Scholar]
- 23.ACR Committee on MR Safety:, Greenberg TD, Hoff MN, et al. ACR guidance document on MR safe practices: Updates and critical information 2019. J Magn Reson Imaging. 2020;51(2):331–338. doi: 10.1002/jmri.26880 [DOI] [PubMed] [Google Scholar]
- 24.Price RR. The AAPM/RSNA Physics Tutorial for Residents: MR Imaging Safety Consideration. RadioGraphics. 1999;19(6):11. [DOI] [PubMed] [Google Scholar]
- 25.Tsai LL, Grant AK, Mortele KJ, Kung JW, Smith MP. A Practical Guide to MR Imaging Safety: What Radiologists Need to Know. RadioGraphics. 2015;35(6):1722–1737. doi: 10.1148/rg.2015150108 [DOI] [PubMed] [Google Scholar]
- 26.Panych LP, Madore B. The physics of MRI safety: Physics of MRI Safety. J Magn Reson Imaging. 2018;47(1):28–43. doi: 10.1002/jmri.25761 [DOI] [PubMed] [Google Scholar]
- 27.Bottomley PA. Turning Up the Heat on MRI. J Am Coll Radiol. 2008;5(7):853–855. doi: 10.1016/j.jacr.2008.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration. Guidance for Industry and FDA Staff – Criteria for Significant Risk Investigations of Magnetic Resonance Diagnostic Devices. June2014. https://www.fda.gov/files/medical%20devices/published/Criteria-for-Significant-Risk-Investigations-of-Magnetic-Resonance-Diagnostic-Devices---Guidance-for-Industry-and-Food-and-Drug-Administration-Staff-%28PDF%29.pdf. [Google Scholar]
- 29.Eising EG, Hughes J, Nolte F, Jentzen W, Bockisch A. Burn injury by nuclear magnetic resonance imaging. Clin Imaging. 2010;34(4):293–297. doi: 10.1016/j.clinimag.2009.06.025 [DOI] [PubMed] [Google Scholar]
- 30.Friedstat JS, Moore ME, Goverman J, Fagan SP. An Unusual Burn During Routine Magnetic Resonance Imaging. J Burn Care Res. 2013;34(2):e110–e111. doi: 10.1097/BCR.0b013e3182642a40 [DOI] [PubMed] [Google Scholar]
- 31.Mosher ZA, Sawyer JR, Kelly DM. MRI Safety with Orthopedic Implants. Orthop Clin North Am. 2018;49(4):455–463. doi: 10.1016/j.ocl.2018.05.010 [DOI] [PubMed] [Google Scholar]
- 32.Health C for D and R. Benefits and Risks. FDA. February2019. http://www.fda.gov/radiation-emitting-products/mri-magnetic-resonance-imaging/benefits-and-risks.Accessed February 22, 2020. [Google Scholar]
- 33.Perik T, Kaas J, Wittkämper F. The impact of a 1.5 T MRI linac fringe field on neighbouring linear accelerators. Phys Imaging Radiat Oncol. 2017;4:12–16. doi: 10.1016/j.phro.2017.10.002 [DOI] [Google Scholar]
- 34.Kok JGM, Raaymakers BW, Lagendijk JJW, Overweg J, Graaff CHW de, Brown KJ Installation of the 1.5 T MRI accelerator next to clinical accelerators: impact of the fringe field. Phys Med Biol. 2009;54(18):N409–N415. doi: 10.1088/0031-9155/54/18/N02 [DOI] [PubMed] [Google Scholar]
- 35.Bronskill Michael J., Carson Paul L., Einstein Steve, Koshine Michael, Lassen Margit, Seong Ki Mun William Pavlicek, Price Ronald R., Wrightn Ann. SITE PLANNING FOR MAGNETIC RESONANCE IMAGING SYSTEMS. American Association of Physicists in Medicine AAPM Report No. 20; 1986. [Google Scholar]
- 36.Green O, Henke LE, Parikh P, Roach MC, Michalski JM, Gach HMM. Practical Implications of Ferromagnetic Artifacts in Low-field MRI-guided Radiotherapy. Cureus. March2018. doi: 10.7759/cureus.2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ASTM International F2503-13. Standard Practice for Marking Medical Devices and Other Items for Safety in the Magnetic Resonance Environment. June2013. https://www.astm.org/DATABASE.CART/HISTORICAL/F2503-13.htm. [DOI] [PubMed] [Google Scholar]
- 38.U.S. Food and Drug Administration. Guidance for Industry and Food and Drug Administration Staff: Establishing Safety and Compatibility of Passive Implants in the Magnetic Resonance (MR) Environment. December2014. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/establishing-safety-and-compatibility-passive-implants-magnetic-resonance-mr-environment. [Google Scholar]
- 39.Shellock FG, Woods TO, Crues JV. MR Labeling Information for Implants and Devices: Explanation of Terminology. Radiology. 2009;253(1):26–30. doi: 10.1148/radiol.2531091030 [DOI] [PubMed] [Google Scholar]
- 40.Woods TO. Standards for medical devices in MRI: Present and future. J Magn Reson Imaging. 2007;26(5):1186–1189. doi: 10.1002/jmri.21140 [DOI] [PubMed] [Google Scholar]
- 41.Kotze DJ, De Vries C. A quick guide to safety and compatibility of passive implants and devices in an MR environment. South Afr J Radiol. 2004;8(2):6. doi: 10.4102/sajr.v8i2.126 [DOI] [Google Scholar]
- 42.Milby JN, Bible JE, Mosher TJ, Garner MR. External Orthopaedic Implants in the Magnetic Resonance Environment: Current Concepts and Controversies. JAAOS - J Am Acad Orthop Surg. 2020;28(4):e139. doi: 10.5435/JAAOS-D-19-00178 [DOI] [PubMed] [Google Scholar]
- 43.Song T, Xu Z, Iacono MI, Angelone LM, Rajan S. Retrospective analysis of RF heating measurements of passive medical implants. Magn Reson Med. 2018;80(6):2726–2730. doi: 10.1002/mrm.27346 [DOI] [PubMed] [Google Scholar]
- 44.Walsh EG, Brott BC, Johnson VY, Venugopalan R, Anayiotos A. Assessment of passive cardiovascular implant devices for MRI compatibility. Technol Health Care. 2008;16(4):233–245. doi: 10.3233/THC-2008-16401 [DOI] [PubMed] [Google Scholar]
- 45.Dill T Contraindications to magnetic resonance imaging. Heart. 2008;94(7):943–948. doi: 10.1136/hrt.2007.125039 [DOI] [PubMed] [Google Scholar]
- 46.Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-Induced Artifacts in MRI. Am J Roentgenol. 2011;197(3):547–555. doi: 10.2214/AJR.11.7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jungmann P, Agten C, Pfirrmann C, Sutter R. Advances in MRI around metal - Jungmann - 2017 - Journal of Magnetic Resonance Imaging - Wiley Online Library. J Magn Reson Imaging. 2017;46:972–991. doi: 10.1002/jrmri.25708 [DOI] [PubMed] [Google Scholar]
- 48.Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14(7):e97–e153. doi: 10.1016/j.hrthm.2017.04.025 [DOI] [PubMed] [Google Scholar]
- 49.Karacozoff AM, Shellock FG. In Vitro Assessment of a Fiducial Marker for Lung Lesions: MRI Issues at 3 T. Am J Roentgenol. 2013;200(6):1234–1237. doi: 10.2214/AJR.12.9120 [DOI] [PubMed] [Google Scholar]
- 50.Gurney-Champion OJ, Lens E, van der Horst A, et al. Visibility and artifacts of gold fiducial markers used for image guided radiation therapy of pancreatic cancer on MRI: Visibility and artifacts of fiducial markers on MRI. Med Phys. 2015;42(5):2638–2647. doi: 10.1118/1.4918753 [DOI] [PubMed] [Google Scholar]
- 51.Jonsson JH, Garpebring A, Karlsson MG, Nyholm T. Internal Fiducial Markers and Susceptibility Effects in MRI—Simulation and Measurement of Spatial Accuracy. Int J Radiat Oncol. 2012;82(5):1612–1618. doi: 10.1016/j.ijrobp.2011.01.046 [DOI] [PubMed] [Google Scholar]
- 52.Magnetic Resonance Compatibility of a Transponder Aimed for Radiotherapy Positioning – A Phantom Study. Anticancer Res. 2017;37(9). doi: 10.21873/anticanres.11911 [DOI] [PubMed] [Google Scholar]
- 53.Zhu X, Bourland JD, Yuan Y, et al. Tradeoffs of integrating real-time tracking into IGRT for prostate cancer treatment. Phys Med Biol. 2009;54(17):N393–N401. doi: 10.1088/0031-9155/54/17/N03 [DOI] [PubMed] [Google Scholar]
- 54.Olch AJ, Gerig L, Li H, Mihaylov I, Morgan A. Dosimetric effects caused by couch tops and immobilization devices: Report of AAPM Task Group 176. Med Phys. 2014;41(6Part1):061501. doi: 10.1118/1.4876299 [DOI] [PubMed] [Google Scholar]
- 55.Jafar MM, Reeves J, Ruthven MA, et al. Assessment of a carbon fibre MRI flatbed insert for radiotherapy treatment planning. Br J Radiol. 2016;89(1062):20160108. doi: 10.1259/bjr.20160108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langmack KA. The use of an advanced composite material as an alternative to carbon fibre in radiotherapy. Radiography. 2012;18(2):74–77. doi: 10.1016/j.radi.2012.02.001 [DOI] [Google Scholar]
- 57.Paulson ES, Jesmanowicz A. Evaluation of Common Material Magnetic Susceptibility Effects for Immobilization Devices Used in MRI-Guided Therapies. Med Phys. 2014;41(6):92. doi: 10.1118/1.4889702 [DOI] [Google Scholar]
- 58.Boland BL, Xu S, Wood B, Tse ZTH. High Speed Pneumatic Stepper Motor for MRI Applications. Ann Biomed Eng. 2019;47(3):826–835. doi: 10.1007/s10439-018-02174-0 [DOI] [PubMed] [Google Scholar]
- 59.Mangeot C, Eriksen RS. Design and testing of non-magnetic motors for MRI application. Proc Actuator. 2014:4. [Google Scholar]
- 60.Keeler EK, Casey FX, Engels H, et al. Accessory equipment considerations with respect to MRI compatibility. J Magn Reson Imaging. 1998;8(1):12–18. doi: 10.1002/jmri.1880080107 [DOI] [PubMed] [Google Scholar]
- 61.Meijsing I, Raaymakers BW, Raaijmakers AJE, et al. Dosimetry for the MRI accelerator: the impact of a magnetic field on the response of a Farmer NE2571 ionization chamber. Phys Med Biol. 2009;54(10):2993–3002. doi: 10.1088/0031-9155/54/10/002 [DOI] [PubMed] [Google Scholar]
- 62.Reynoso FJ, Curcuru A, Green O, Mutic S, Das IJ, Santanam L. Technical Note: Magnetic field effects on Gafchromic-film response in MR-IGRT: Effects of magnetic field on Gafchromic films. Med Phys. 2016;43(12):6552–6556. doi: 10.1118/1.4967486 [DOI] [PubMed] [Google Scholar]
- 63.O’Brien DJ, Roberts DA, Ibbott GS, Sawakuchi GO. Reference dosimetry in magnetic fields: formalism and ionization chamber correction factors: Reference dosimetry in B-fields: Formalism and correction factors. Med Phys. 2016;43(8Part1):4915–4927. doi: 10.1118/1.4959785 [DOI] [PubMed] [Google Scholar]
- 64.Malkov VN, Rogers DWO. Monte Carlo study of ionization chamber magnetic field correction factors as a function of angle and beam quality. Med Phys. 2018;45(2):908–925. doi: 10.1002/mp.12716 [DOI] [PubMed] [Google Scholar]
- 65.Liu JC, Mao S, McCall RC, Donahue R. The effect of the static magnetic field on the response of radiation survey instruments. Health Phys. 1993;64(1):59–63. doi: 10.1097/00004032-199301000-00007 [DOI] [PubMed] [Google Scholar]
- 66.Smit K, Sjöholm J, Kok JGM, Lagendijk JJW, Raaymakers BW. Relative dosimetry in a 1.5 T magnetic field: an MR-linac compatible prototype scanning water phantom. Phys Med Biol. 2014;59(15):4099–4109. doi: 10.1088/0031-9155/59/15/4099 [DOI] [PubMed] [Google Scholar]
- 67.Li HH, Rodriguez VL, Green OL, et al. Patient-Specific Quality Assurance for the Delivery of 60Co Intensity Modulated Radiation Therapy Subject to a 0.35-T Lateral Magnetic Field. Int J Radiat Oncol. 2015;91(1):65–72. doi: 10.1016/j.ijrobp.2014.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Houweling AC, Vries JHW de, Wolthaus J, et al. Performance of a cylindrical diode array for use in a 1.5 T MR-linac. Phys Med Biol. 2016;61(3):N80–N89. doi: 10.1088/0031-9155/61/3/N80 [DOI] [PubMed] [Google Scholar]
- 69.Steinmann A, Alvarez P, Lee H, et al. MRIgRT dynamic lung motion thorax anthropomorphic QA phantom: Design, development, reproducibility, and feasibility study. Med Phys. 2019;46(11):5124–5133. doi: 10.1002/mp.13757 [DOI] [PubMed] [Google Scholar]