Abstract
Type 2 diabetes continues to be a serious and highly prevalent public health problem worldwide. In 2019, the highest prevalence of diabetes in the world at 12.2%, with its associated morbidity and mortality, was found in the Middle East and North Africa region. In addition to a genetic predisposition in its population, evidence suggests that obesity, physical inactivity, urbanization, and poor nutritional habits have contributed to the high prevalence of diabetes and prediabetes in the region. These risk factors have also led to an earlier onset of type 2 diabetes among children and adolescents, negatively affecting the productive years of the youth and their quality of life. Furthermore, efforts to control the rising prevalence of diabetes and its complications have been challenged and complicated by the political instability and armed conflict in some countries of the region and the recent coronavirus disease 2019. Broad strategies, coupled with targeted interventions at the regional, national, and community levels are needed to address and curb the spread of this public health crisis.
Keywords: Type 2 diabetes, Middle East and North Africa, Epidemiology, Prevalence, Prediabetes, Complications
Core Tip: The Middle East and North Africa region has the world’s highest diabetes prevalence, the second highest rate of rise, the highest adjusted mortality from noncommunicable disease, and the highest diabetes-related disability adjusted life years. This review provides an up-to-date review of the diabetes status in this dynamic region of the world and touches on new elements that affect diabetes such as the high number of refugees and the coronavirus disease 2019 pandemic. This review identifies gaps and weaknesses in type 2 diabetes in the Middle East and North Africa region and highlights areas where planning and action are highly needed.
INTRODUCTION
The worldwide prevalence of diabetes mellitus continues to grow with no sign of reversal. Data from the World Health Organization (WHO) shows that diabetes rose 80% in prevalence between 1980 and 2014[1], and the International Diabetes Federation (IDF) estimates that in 2019, 9.3% of the global adult population age 20-79 years suffered from diabetes[2]. Compared to high income countries (HIC), this increase disproportionately affects low- and middle-income countries and adds a burden of excess morbidity, mortality, and health care costs[3]. Specifically, the Middle East and North Africa region (MENA) carried the highest prevalence of diabetes in 2019 at 12.2% and is expected to witness a 96% increase in diabetes prevalence between 2019 and 2045, second only to the African region with a 143% projected rise[4]. To compare, over the same time period, the prevalence in Europe and North America/Caribbean regions is expected to increase by 15% and 33%, respectively. Moreover, 44.7% of people with type 2 diabetes (T2D) in the MENA region are unaware of their condition[4].
Despite the heterogeneity within the MENA countries in terms of culture, income, population size, and sociopolitical stability[5,6], multiple common predisposing factors for diabetes have been implicated, including aging of the population, the change in lifestyle with reduction in physical activity, and increased consumption of calories and unhealthy food items, which have led to a rise in the prevalence of overweightness and obesity[2]. Genetic and epigenetic factors may also be contributing elements[7]; in a region that has a high rate of consanguinity[8], multiple gene loci that predispose to diabetes have been identified in the Eastern Mediterranean Region (EMR) population[9]. In addition to diabetes, prediabetes has been identified in a sizable proportion of the MENA population[2], out of whom a majority is expected to progress to diabetes over time[10].
This unrelenting diabetes epidemic was an important driving factor that spurred the 2011 declaration of the United Nations general assembly, in which countries committed to work on national plans for preventing and controlling noncommunicable diseases (NCD)[11]. In addition to the risk factors behind the worldwide rise in diabetes prevalence, other factors, specific to the MENA region, are contributing to the epidemic[12]. Measures to address the high numbers in the MENA area have been ineffective partly due to inadequate funding, insufficient commitment, political instability, and armed conflict in multiple countries[13,14].
This review aims at describing the current prevalence of diabetes and prediabetes in the MENA region, the contributing risk factors, common diabetes complications, and strategies that can help curb its spread and complications.
METHODOLOGY
We searched Medline (OVID electronic database Ovid MEDLINE(R) and Epub Ahead of Print, In-Process and Other Non-Indexed Citations and Daily 1946 to December 07, 2020) for studies reporting in 20 countries of the MENA region not only diabetes mellitus and cardiovascular diseases (CVDs), but also diabetic foot or amputation, diabetic nephropathies, diabetic retinopathy (DR), the prevalence of diabetes, and the prevalence of diabetes for the pediatric and adolescent age group. Each of the latter searches was exported into Endnote X9 Software, and the library was screened for relevant literature. The search did include the Medical Subject headings (MESH) for all the concepts except for countries in which keywords were added along to MESH to ensure a wider range of results. Only articles in the English language and with studies where specific complication prevalence was evaluated were selected for our paper. We limited the prevalence studies to publications within the last decade (2010-2020). For diabetes prevalence studies, we excluded hospital and clinic-based studies.
There has been no consensus on which countries define the MENA region. For our review, we have included the following 20 countries: Afghanistan, Algeria, Bahrain, Egypt, Iran, Iraq, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Pakistan, Palestine, Qatar, Saudi Arabia, Syria, Tunisia, United Arab Emirates (UAE), and Yemen. These countries are commonly included in the definition of EMR (EMRO). Therefore, we included data from the WHO, which reports on EMRO, and IDF, which reports on MENA.
PREVALENCE OF T2D
Diagnostic criteria for diabetes
The diagnostic criteria for diabetes by the American Diabetes Association (ADA) include the following: hemoglobin A1c 6.5%, fasting plasma glucose 126 mg/dL (7.0 mmol/L), oral glucose tolerance test with 2 h plasma glucose 200 mg/dL (11.1 mmol/L), or casual plasma glucose 200 mg/dL (11.1 mmol/L) in the presence of hyperglycemic symptoms[15]. It is worth noting that the IDF follows the ADA criteria in the diagnosis of T2D[16]. On the other hand, the WHO defines diabetes as raised fasting glucose 126 mg/dL (7.0 mmol/L), history of diabetes, or using antidiabetic medication[17]. Unless stated otherwise, the studies included in the prevalence paragraph follow either ADA or WHO diagnostic criteria.
Prevalence
In 2019 and as previously stated, 9.3% of adults were living with diabetes worldwide, with a predicted rise to 10.2% and 10.9% by years 2030 and 2045, respectively. The highest age adjusted prevalence was reported in the MENA reaching 12.2%, where 1 in 8 adults was living with diabetes[2]. The WHO provides periodic country-specific rates of diabetes compiled from various studies[17]. We used WHO data to rank MENA countries by diabetes prevalence between 2000 and 2014[17], as shown in Figure 1.
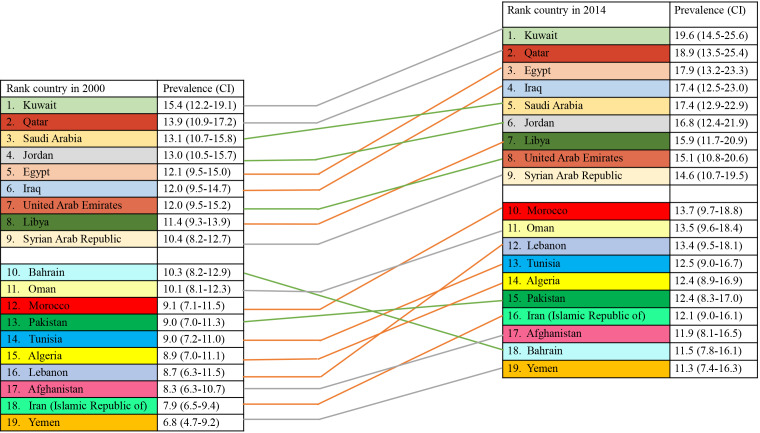
Figure 1.
Middle East and North Africa countries ranked by prevalence of type 2 diabetes in 2000 and 2014 (prevalence and confidence intervals are in %)[1]. CI: Confidence interval.
The highest age standardized diabetes prevalence in the MENA region in the year 2000 was in Kuwait with a prevalence of 15.4%, and the lowest prevalence was 6.8% in Yemen. Between 2000 and 2014, all 20 countries of the MENA region discussed in this paper experienced an increase in prevalence while Kuwait kept its first ranking among these countries, for having the highest prevalence among them at 19.6% (Figure 1). Kuwait has already exceeded its projected prevalence of diabetes for 2030, which was anticipated to be 16.9%[18]. Another high-income country, Qatar, ranked second in prevalence among MENA countries, following Kuwait, both in 2000 and 2014 (Figure 1). In Saudi Arabia, a cross-sectional study was conducted from 2007 to 2009 and included 18034 individuals older than 30 years. It found the prevalence of diabetes was 25.4% (out of which 10.2% were previously undiagnosed), and it was significantly higher in urban compared to rural areas[19].
Moving to Pakistan, in 2017, a large-scale national study including 18856 adults (above 20 years of age), found that T2D prevalence was 16.9% (n = 3201), and diabetes was significantly associated with age[20]. In 2019, the age-adjusted comparative prevalence in Pakistan rose to 19.9%, and that country was ranked first among the MENA countries for having the highest number of people (19.4 million) living with diabetes[2]. To understand the trend in diabetes prevalence in Jordan, four surveys using the same diagnostic criteria (the WHO criteria) were conducted in the years 1994, 2004, 2009, and 2017. Over the years, the age-adjusted prevalence increased from 17.1% in 2004 to 23.7% in 2017. This steep increase was attributed not only to increased incidence, but also to other factors like aging of the population and better survival of individuals with diabetes. In addition, the percentage of previously diagnosed diabetes increased as well, accounting for 82.6% of all diabetes cases in 2017, indicating improved screening and diabetes awareness[21].
Moving to North Africa, and according to the WHO, the prevalence of diabetes in Egypt in 2014 was 17.9%, the third highest prevalence in the MENA region. In 2019, Egypt occupied the second highest rank among MENA countries with respect to number of adults living with diabetes, with almost 9 million cases[2]. However, within Egypt, the prevalence was much lower in rural areas compared to the national average. As an example, a community-based cross-sectional study conducted in Qena Governorate over 2 years (2013-2015) found that 811 out of 9303 had T2D (8.7%). It is worth noting that the majority of participants (n = 7701) were younger than 40 years of age, which likely contributed to the relatively low prevalence in this governorate[22].
In a neighboring country to Egypt, Tunisia, the most recent national cross-sectional survey we could identify was conducted in 2005 and involved 7700 adults, age 35 to 70 years. It found that the prevalence of T2D, based on WHO criteria, was 15.5%. Again, within that study, the urban prevalence was twice as high as in rural areas (17.7% vs 9.7%, respectively)[23].
As for Iran, a national survey conducted in 2011 found that the prevalence of diabetes (T1D and T2D combined) was 11.4%, and the annual incidence was estimated to be 1%[24]. Two large community-based cross-sectional studies yielded the following: in the Yazd area of central Iran, out of 2269 adults above 20 years, the crude prevalence of self-reported diabetes in 2014-2015 was 14.1%, and 1 out of every 5 people over 40 years of age was living with diabetes[25]. In contrast, the Pars Cohort Study in Southern Iran conducted on 9264 adults aged 40-75 years, found a slightly lower prevalence of diabetes of 9.9% using self-report and fasting plasma glucose[26,27]. In either case, the prevalence is increasing over time, as was shown in a 5 year study in the city of Ahvaz. Out of 593 participants above 20 years of age, the prevalence of diabetes was 15.2% in 2009 and increased to 20.9% in 2014[27]. In both Southern and Southwest areas of Iran, diabetes prevalence was positively correlated with low education level, body mass index (BMI), and age.
Moving to Lebanon, a cross-sectional national survey including adults above 25 years of age found a prevalence of self-reported diabetes of 8.5%; the prevalence was higher among older age, obese, and less physically active groups[28]. A more recent community-based survey that was conducted in Beirut in 2014, found that the prevalence of diabetes, based on self-report, fasting glucose, or hemoglobin A1c, was 18.0%. Similarly, increasing age and BMI were risk factors[29]. The higher prevalence reported in this study, compared to the previously mentioned one, can be attributed to the different diagnostic criteria of diabetes and the characteristics of the participants, where they had a higher obesity rate and were residing in Beirut, the capital of Lebanon, unlike the first survey that included both an urban and a rural population. In 2019, the IDF estimated the age adjusted comparative diabetes prevalence in Lebanon at 11.2%[2]. Other countries in the MENA region did not have recent or large community-based studies to estimate the prevalence of diabetes in the population. These countries (except for Morocco and Algeria) are more likely to have political instability and/or conflict (Libya, Iraq, Yemen, Afghanistan, Syria, Palestine, and Bahrain). The most recent community-based survey from Syria was conducted in 2006 in the city of Aleppo and reported the total prevalence of T2D to be 15.6%, out of whom 5% were newly diagnosed, while the rest were self-reported cases. Like other studies, the prevalence was correlated with obesity and a positive family history[30]. In 2019, based on extrapolation from similar countries, the IDF stated that more than 1 million adults were living with diabetes in Syria[2]. Unfortunately, Palestine, like Syria, lacks recent surveys and studies on a national level; however, the IDF estimated that the prevalence of diabetes in Palestine was 7% in 2017[31] and 9.5% in 2019[2].
Prevalence of diabetes by gender
The prevalence of diabetes was higher in women than men in several of the MENA countries (Table 1). Yet, some countries showed a higher prevalence in males compared to females, as is the case for Lebanon[28]. Gender differences may vary even within regions of the same country; for example, women in Central Iran[25] and the rural area of Kurdistan province[32] had a higher prevalence than men, whereas no significant differences in prevalence by genders was observed in the Southwest of Iran[27].
Table 1.
Gender specific prevalence of type 2 diabetes in % (CI) in 20 Middle East and North Africa countries in 2000 and 2014 as reported by the World Health Organization[1] and for Palestine[144]
|
Country
|
2000
|
2014
|
||
|
Men
|
Women
|
Men
|
Women
|
|
| Afghanistan | 8.1 (5.3-11.5) | 8.5 (5.7-11.9) | 11.6 (6.4-18.2) | 12.2 (6.8-18.8) |
| Algeria | 8.6 (6.0-11.8) | 9.2 (6.5-12.3) | 12.3 (7.4-18.8) | 12.6 (7.7-18.9) |
| Bahrain | 10.6 (7.6-14.2) | 9.9 (7.1-13.5) | 12.0 (7.0-18.5) | 10.6 (6.1-16.7) |
| Egypt | 10.8 (7.5-14.7) | 13.3 (9.6-17.5) | 16.0 (10.0-23.6) | 19.8 (12.9-28.2) |
| Iran | 7.4 (5.5-9.4) | 8.5 (6.4-10.7) | 11.4 (7.2-17.2) | 12.9 (8.4-18.8) |
| Iraq | 11.5 (8.2-15.4) | 12.4 (9.1-16.2) | 17.2 (10.7-25.3) | 17.5 (11.1-25.4) |
| Jordan | 12.0 (8.8-15.9) | 14.0 (10.5-18.0) | 16.5 (10.5-24.0) | 17.2 (11.3-24.6) |
| Kuwait | 15.3 (11.2-20.3) | 15.6 (11.4-20.4) | 19.7 (12.8-28.1) | 19.6 (12.9-27.7) |
| Lebanon | 9.0 (5.7-13.1) | 8.4 (5.3-12.2) | 14.5 (8.7-21.8) | 12.2 (7.4-18.5) |
| Libya | 10.7 (7.9-14.1) | 12.2 (9.1-15.8) | 15.2 (9.5-22.5) | 16.6 (10.7-23.8) |
| Morocco | 9.0 (6.2-12.4) | 9.2 (6.4-12.6} | 14.0 (8.4-21.5} | 13.4 (8.1-20.5} |
| Oman | 10.2 (7.6-13.6) | 9.9 (7.3-13.1) | 14.3 (8.6-21.7) | 12.3 (7.4-18.4) |
| Pakistan | 9.1 (6.3-12.2) | 9.0 (6.3-12.2) | 12.6 (7.0-19.5) | 12.1 (7.0-18.6) |
| Palestine | 10.6 (7.8-14.0) | 11.8 (8.9-15.2) | 16.5 (10.3-24.3) | 17.5 (11.4-24.9) |
| Qatar | 13.7 (9.9-18.1) | 14.2 (10.4-18.5) | 18.9 (12.0-27.0) | 18.8 (12.2-26.8) |
| Saudi Arabia | 13.1 (9.8-17.1) | 13.1 (9.8-17.0) | 17.6 (11.5-25.4) | 17.0 (11.1-24.4) |
| Syria | 9.8 (7.0-13.2) | 10.9 (8.1-14.2) | 14.0 (8.5-21.0) | 15.3 (9.6-22.4) |
| Tunisia | 8.3 (6.0-11.1) | 9.7 (7.2-12.7) | 12.1 (7.4-18.3) | 12.9 (7.9-19.0) |
| United Arab Emirates | 11.8 (8.6-16.0) | 12.4 (9.1-16.5) | 15.0 (9.2-22.5) | 15.4 (9.7-22.6) |
| Yemen | 7.4 (4.5-11.3) | 6.2 (3.6-9.4) | 12.6 (6.7-20.6) | 10.1 (5.3-17.0) |
Given that other global studies do not show a higher predisposition to diabetes among females, it is likely that it is the gender factor, and not the physiologic sex factor, that contributes to the risk. This is supported by the higher prevalence of obesity and sedentary lifestyle among women, as described in the risk factors section. The prevalence by gender is shown in Table 1 and Figure 2.
Figure 2.
Prevalence of type 2 diabetes by gender in years 2000 and 2014 from the World Health Organization[1] and for Palestine[144].
Urban vs rural
Studies mentioned previously for Egypt[21], Tunisia[22], and Lebanon[28,29] have shown a higher T2D prevalence in urban compared to rural areas. Similarly, a study including 9149 participants aged 7-80 years from Riyadh, the capital of Saudi Arabia, found that the prevalence of diabetes, based on the WHO definition, was 31.6%[33], which is higher than the overall reported national prevalence[17].
The higher urban compared to rural prevalence is not consistent across or within studies. For example, a national diabetes survey in Pakistan conducted in the years 2016-2017 found a significantly higher prevalence in urban vs rural areas among males above 60 years of age and among females; however, younger males showed a higher prevalence in rural areas[34].
Similarly, the prevalence of diabetes in Iran was higher in rural compared to urban areas; it was noted that in the rural population of Kurdistan province, the prevalence of T2D in 2011-2017 was 19.6%. The prevalence was significantly associated with age and lower level of education. In this specific population, genetic polymorphisms were more common among rural populations, predisposing them to a higher risk of diabetes[32].
It is likely that the difference in prevalence in urban vs rural areas is attributed to different nutritional habits, activity level, and possibly a more health-promoting environment.
Unknown vs known diabetes
The rate of unknown or undiagnosed diabetes can be detected by population-based studies that collect blood samples and measure hemoglobin A1c or glucose levels. The proportion of undiagnosed diabetes in the MENA region was 44.7% in 2019[16]. In Kuwait, a cross-sectional survey in 2007 found that 23 subjects out of 120 diabetic adults were previously undiagnosed (19%)[35]. Similarly, in Pakistan, the rate of unknown diabetes was 27%[34].
In central Iran, undiagnosed diabetes was found to be more common in men (4%) than in women (3.7%), and it was significantly associated with older age; the prevalence of undiagnosed diabetes was 4.8 times higher in the age group 60-69 years compared to the youngest age group 20-29 years, indicating a higher level of diabetes unawareness in the older population[25]. In Beirut (Lebanon), 26 subjects out of 90 were unaware that they had diabetes (29%)[29]. In Jordan, the percentage of newly diagnosed cases compared to all diabetic cases was 25.5% in 2004, and it dropped to 17.4% in 2017[21]. In Qena, Egypt, around 35% of all the diabetes cases (both types) were newly diagnosed[22], and Tunisia showed one of the highest rates of undiagnosed diabetes at 51.1%[23].
PREDIABETES
The development of diabetes tends to be a gradual process with rising glucose levels from the normoglycemic range to the diabetic range. This process is driven by a combination of metabolic disorders that include both insulin resistance and a progressive decline in insulin secretion[10]; the term prediabetes has been used to characterize the intermediate state between normoglycemia and the glucose levels that define diabetes[2,15]. Patients with prediabetes comprise those with impaired fasting glucose (IFG) and/or impaired glucose tolerance (IGT)[15]. Like diabetes, patients with prediabetes are at an increased risk of cardiovascular events but to a lesser extent. Previous studies have suggested that up to 70% of patients with prediabetes will eventually progress to diabetes[10], however implementation of lifestyle changes or pharmacologic therapy may prevent or delay that progression[36]. It is estimated that 25%-50% of patients with prediabetes may progress to diabetes within 5 years of diagnosis[37].
In addition to the high prevalence of diabetes, countries in the MENA region also suffer from a high prevalence of prediabetes. The IDF estimates that in 2019, the worldwide age adjusted comparative prevalence of IGT in adults aged 20-79 years was 8.6%, with the European region being least affected at a prevalence of 4.4%, and the Western Pacific region having the highest prevalence at 10.4%[2]. The estimated MENA prevalence of IGT was intermediate at 9.2% and is projected to increase to 9.9% by 2045. Even though the projected prevalence goes up only by 0.7%, it translates into near doubling of the number from 35.5 million to 64.5 million adults with IGT mostly due to population growth.
Within the past 20 years, a relatively small number of studies have reported on the prevalence of prediabetes in the MENA countries, however there was a wide variability among them in the definition of prediabetes, the sample size and sampling technique, and the age group sampled (Table 2). Overall, there did not appear to be a major difference between gender subgroups. A relatively high prevalence of prediabetes was reported in Iraq[38], Saudi Arabia[19], the UAE[39], and Kuwait[40], ranging between 19.3% and 28.6%. An intermediate prevalence was found in the countries of Iran[41], Pakistan[34], and Qatar[42] with a prevalence ranging from 13.8% to 14.6%. The countries of Yemen[43], Syria[30], Oman[44], and Tunisia[45] boasted a relatively low prevalence with a range of 4.6%-9.0%. A Lebanese study limited to the greater Beirut area reported an unusually high prevalence of prediabetes of 40.3%[29]; the way prediabetes was defined could have been a factor affecting the results, since either an elevated fasting glucose or a high A1c were acceptable parameters, while most of the other studies limited the prediabetes definition to IFG.
Table 2.
Prevalence of prediabetes (%) in some Middle East and North Africa countries
|
Ref.
|
Country, yr
|
Age group (n)
|
Definition of prediabetes
|
Sampling technique
|
Prevalence of prediabetes % (95%CI)
|
||
|
Male
|
Female
|
Total
|
|||||
| Nasrallah et al[29] | Lebanon (Beirut area), 2014 | ≥ 18 yr (501) | IFG: FPG 5.6-6.9 mmol/L or A1c 5.8%-6.49% | Probability multistage random sampling | 48.0 (40.6-55.4) | 36.0 (30.7-41.3) | 40.3 (36.0-44.6) |
| Mansour et al[38] | Iraq, 2011-2012 | 19-94 yr (5445) | IFG: FPG 5.7-6.9 mmol/L or A1c: 5.7%-6.4% | Population-based random sample | 28.6 | 29.5 | 29.1 |
| Al-Rubeaan et al[19] | Saudi Arabia, 2007-2009 | ≥ 30 yr (18034) | IFG: FPG 5.6-6.9 mmol/L | Random household national sample | 26.4 | 24.7 | 25.5 |
| Saadi et al[39] | United Arab Emirates (Al-Ain), 2005-2006 | > 18 yr (2455) | IFG: FPG 5.6-6.9 mmol/l or IGT | Simple random sample | 19.7 | 22.8 | 22.8 |
| Alkandari et al[40] | Kuwait, 2014 | 18-69 yr (2561) | IFG: FPG 6.1-6.9 mmol/L | Random sample | 19.3 (16.9-22.0) | 19.5 (17.6-21.5) | 19.4 (17.9-21.0) |
| Esteghamati et al[41] | Iran, 2011 | 25-70 yr (11867) | IFG: FPG 5.6-6.9 mmol/L | Randomized multistage cluster sample | 15.45 (12.71-18.18) | 13.74 (11.55-15.94) | 14.6 (12.41-16.78) |
| Basit et al[34] | Pakistan, 2016-2017 | ≥ 20 yr (10834) | IFG: FPG 6.1-6.9 mmol/L orIGT | Multistage clustering technique | NA | NA | 14.4 |
| Bener et al[42] | Qatar, 2007-2008 | > 20 yr (1117) | IFG: FPG 5.6-6.9 mmol/L or IGT | Multistage stratified cluster sampling | NA | NA | 13.8 |
| Gunaid et al[43] | Yemen, 2000 | ≥ 35 yr (250) | IFG: FPG 5.6-6.1 mmol/L or IGT | Multistage random sampling | 5.7 (2.8-8.6) | 10.9 (7.1-14.7) | 9.0 (6.0-12.0) |
| Albache et al[30] | Syria (Aleppo), 2006 | ≥ 25 yr (806) | IFG: FPG 6.1-6.9 mmol/L | Random sampling | 10.4 (4.7-21.0) | 6.8 (2.9-15.1) | 8.6 (3.8-18.1) |
| Al-Lawati et al[44] | Oman, 2000 | ≥ 20 yr (5838) | IFG: FPG 6.1-6.9 mmol/L | Multistage stratified probability sampling | 7.1 (6.2-8.1) | 5.1 (4.4-6.0) | 6.1 (5.5-6.8) |
| Bouguerra et al[45] | Tunisia, 1996-1997 | ≥ 19 yr (7860) | IFG: FPG 6.1-6.9 mmol/L | National cross-sectional sample | 4.58 | 4.91 | NA |
IFG: Impaired fasting glucose according to specified glucose range; FPG: Fasting plasma glucose; IGT: Impaired glucose tolerance (using World Health Organization definition, glucose ≥ 7.8 but < 11.1 mmol/L, 2 h after 75 gm oral glucose load); CI: Confidence interval; NA: Not available; A1c: Hemoglobin A1c.
The values estimated by IDF for the prevalence of IGT in 2019 are overall lower compared to those reported for individual countries in the table, possibly because IDF limited their data collection to studies of IGT and excluded those that had also included isolated IFG in the definition of prediabetes. In addition, for the IDF data, only Algeria, Jordan, Oman, Pakistan, Saudi Arabia, Palestine, and the UAE had estimates based on oral glucose tolerance test. Diabetes prevalence for the remaining countries were extrapolated using values from countries deemed to be similar (geographic location, World Bank income group, ethnicity, language, and IDF region) and may be under-estimated[4].
RISK FACTORS
Multiple risk factors have been implicated in the increase in T2D prevalence. The change to a more sedentary lifestyle and the westernization of dietary habits with a shift to fast food and items rich in refined sugar and animal fat play a major role. Additional factors may also contribute to the rising prevalence, including cigarette and waterpipe smoking[46], pollution of the environment[47], and a high prevalence of hepatitis C in some countries (mainly Egypt and Pakistan)[48,49]. Moreover, there is evidence that people in lower socioeconomic groups are at increased risk of T2D[50].
Genetics
In addition to the well-known contributing effect of aging, sedentary lifestyle, unhealthy diets, and obesity in the development of diabetes, genetics also appear to play a role. A family history of diabetes in first degree relatives has long been known to increase the diabetes risk by up to 3-fold[51].
Reports from Palestine, Iran, and Lebanon showed that a positive family history of T2D raised the risk by 1.6, 1.8, and 3.4 times, respectively[28,52,53]. Moreover, despite a higher prevalence of obesity in North America and Europe, MENA has a comparatively higher prevalence of diabetes, suggesting the presence of a genetic predisposition to glucose intolerance.
Genome wide association studies have yielded several single nucleotide polymorphisms that appear to be associated with the development of diabetes. A recent meta-analysis reported that, for people in the MENA area, 71 single nucleotide polymorphisms in 32 genes increased the risk of T2D by 24%-69%[7]. There was a strong association with single nucleotide polymorphisms in the TCF7L2 (in 9 countries) and CDKAL1 genes (in 4 countries), in addition to a variety of other loci, including ADIPOQ, FTO, MC4R. COL8A1, KCNQ1, ALX4, and HNF1. TCF7L2 was the most widely reported gene in the region, in countries that include Palestine[54], Lebanon[55], UAE[56], Egypt[57], Qatar[58], and Tunisia[59]. In the Lebanese population, associations with T2D have been found with variants of the COL8A1, KCNQ1, ALX4, and HNF1 genes[60]. The high rate of consanguinity reported for the MENA region, varying from 30% and up to 60%[8], likely further enhances the genetic susceptibility observed.
Transition in nutrition
In addition to the genetic predisposition, a worldwide transition to unhealthy diets and reduction in physical activity[61] plays a role in the development of obesity and diabetes. Diets have shifted to a higher consumption of calories, processed food, and animal fat, and a lower intake of fiber, fruits, and vegetables. In particular, the MENA area has experienced a rapid rate of modernization and urbanization over the past decades. An analysis of food availability and consumption by Mehio Sibai et al[62] showed a gradual and significant rise in daily caloric, protein, and fat intake between 1969-1971 and 2002-2004. It is estimated that the energy supply during that period rose from 2200 up to 2930 kilocalorie per day. In parallel, there was an increase in sugar intake and a reduction in the intake of fruits and vegetables. In Saudi Arabia, a recent study of people aged 35-70 years showed that 34% of participants reported an unhealthy diet, with a higher rate in younger individuals and those living in urban areas[63]. Similarly, a review paper from Lebanon found a rising trend of increased energy consumption from fat and animal product in the population, with a reduction in carbohydrate and cereal intake[64]. Another study from Lebanon found that consuming minimally processed food such as fruits, vegetables, legumes, breads, and cheeses was less likely to be associated with the metabolic syndrome (odds ratio = 0.18, 95%CI: 0.04-0.77) and hyperglycemia (odds ratio = 0.25, 95%CI: 0.07-0.98) compared to the consumption of highly processed food such as fast foods, snacks, meat, nuts, sweets, and liquor[65].
Physical inactivity
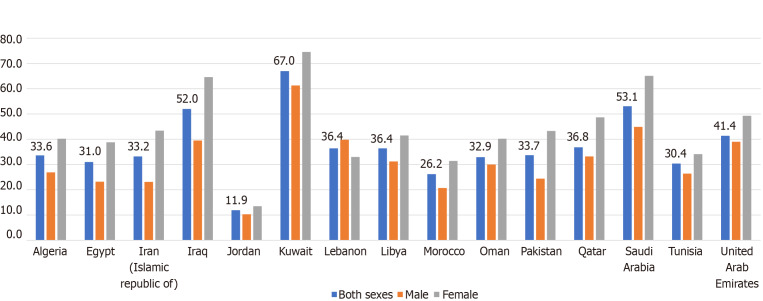
With the rapid worldwide modernization and advancement in technology, a reduction in the rate of physical activity has occurred. In the MENA region, all countries have demonstrated an increase in physical inactivity, with a higher prevalence among females compared to males. Figure 3 shows the prevalence of physical inactivity in MENA countries subdivided by gender. Insufficient physical activity was defined as the percentage of the population aged above 18 years who are not performing at least 150 min/wk of moderate-intensity physical activity or its equivalent[66]. There was a very high prevalence of insufficient physical activity in the high income Gulf countries with a prevalence ranging from 33%-67%, possibly because of a shift from manual labor/high physical activity jobs to occupations that are more sedentary in the services sector[67]. A study in Saudi Arabia found that more than 90% of surveyed individuals had an inadequate level of physical activity[68]. In contrast, that prevalence was under 30% for Jordan, Morocco, and Tunisia. Females were consistently less active compared to males, possibly due to prevailing local customs in conservative countries where women may not spend as much time outside the home or in public places and may not frequent exercise facilities.
Figure 3.
Prevalence (%) of insufficient physical activity among adults aged 18+ year in 2016 (age-standardized estimate)[1].
Obesity
Because of the adoption of unhealthy dietary habits and food choices, and the significant reduction in physical activity, the prevalence of obesity has been steadily increasing worldwide including in the MENA region. It is well-known that obesity is a significant risk factor for diabetes, with many studies showing a correlation between BMI and the incidence of diabetes[69].
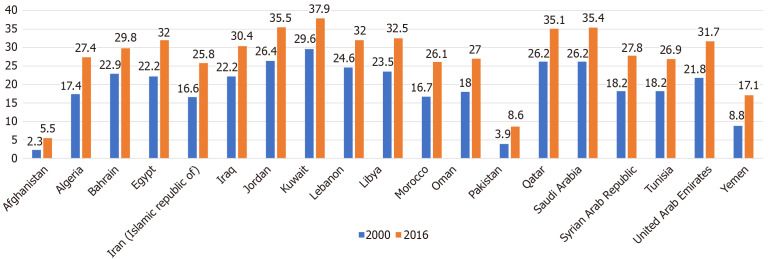
Data from the WHO risk surveillance program has shown a significant rise in the rate of obesity across all countries. The countries most affected are the high-income Gulf countries, in addition to Egypt, Libya, Lebanon, and Iraq with rates exceeding 30%. In contrast, Afghanistan, Pakistan, and Yemen boasted a relatively low prevalence, ranging from 5% to 17%. However, all countries suffered a rise in obesity prevalence between 2000 and 2016, ranging from 30%-100% (Figure 4). Interestingly, females were disproportionately more affected than males, with a prevalence that is comparatively 1.5-fold to 2.0-fold higher. Specifically, the prevalence of female obesity exceeded 40% in some Gulf countries (Saudi Arabia, Kuwait, Qatar, and UAE), in addition to Egypt and Jordan[70] (Figure 5).
Figure 4.
Prevalence (%) of obesity by country among adults aged 18 years or older, defined as body mass index ≥ 30 kg/m2 (age-standardized estimate) in 2000 and 2016[70].
Figure 5.
Prevalence (%) of obesity by country among male and female adults aged 18 years or older. Obesity defined as body mass index ≥ 30 kg/m2 (age-standardized estimate) in 2000 and 2016[70].
COMPLICATIONS OF DIABETES
Mortality
NCDs account for around 70% of deaths worldwide[70]. In 2019, diabetes was the ninth leading cause of death with around 1.8 million directly attributed to hyperglycemia[71]. In addition, CVD, which is the leading cause of death and commonly a chronic complication of diabetes[72], claimed during the same year around 9 million lives[71]. Since 2019 and up until January 2021, the coronavirus disease 2019 (COVID-19) pandemic had already surpassed world diabetes mortality with over 2 million deaths; however, diabetes was again an important risk factor for mortality or the development of severe COVID-19 infection[73].
Regionally, the MENA region scores the worst in terms of hyperglycemia-related mortality with an age-standardized mortality rate per 100000 (ASM) of 139.6 in 2016, followed by Southeast Asia, which has an ASM of 115.3 and in contrast to Europe with an ASM of 55.7 and the Americas with an ASM of 72.6[74].
Focusing on the MENA region, there seems to be a pattern of lower ASM mortality from NCDs (diabetes, CVD, cancer, and chronic obstructive pulmonary disease) for some HIC of the Gulf and for the North African countries. Thus, in 2016, the ASMs for Oman, Qatar, Bahrain, and UAE were 404.6, 425.5, 430.1, and 460, respectively; similarly, for the North African countries of Algeria, Tunisia, and Morocco, they were 430.7, 460.6, and 483.9, respectively. In contrast, in low/middle low-income countries, ASM was 681.0 for Pakistan, 805.3 for Afghanistan, and 819.7 for Yemen, per 100000[75]. The ASM from NCDs for the MENA countries as well as the absolute number of deaths from diabetes or from CVD are presented Table 3. In addition to the apparently higher mortality in middle-income countries and low-income countries, the largest proportion of diabetes-related deaths occur among individuals under 60 years of age, with loss of productive years, adding to the socioeconomic burden of diabetes[2]. The finding of lower mortality for the HIC is consistent with recent world data[76,77].
Table 3.
Mortality from high glucose and cardiovascular disease extracted from the World Health Organization country profile data site for 2016[75]
|
2016
|
Total population (million)
|
Age-standardized mortality rate for NCD per 100000
|
Diabetes deaths (n)
|
Diabetes mortality per 10000
|
CVD deaths (n)
|
CVD mortality per 10000
|
| Afghanistan (LIC) | 32527000 | 805.3 | 7056 | 2.17 | 51244 | 15.75 |
| Algeria (MIC) | 39667000 | 430.7 | 8390 | 2.12 | 69173 | 17.44 |
| Bahrain (HIC) | 1377000 | 430.1 | 404 | 2.93 | 775 | 5.63 |
| Egypt (MIC) | 91508000 | 711.8 | 17851 | 1.95 | 245904 | 26.87 |
| Iran (MIC) | 79109000 | 532.5 | 14842 | 1.88 | 160823 | 20.33 |
| Iraq (MIC) | 36423000 | 604.5 | 7279 | 2.00 | 51593 | 14.16 |
| Jordan (MIC) | 7595000 | 542.4 | 2347 | 3.09 | 13384 | 17.62 |
| Kuwait (HIC) | 3892000 | 541.4 | 326 | 0.84 | 4552 | 17.62 |
| Lebanon (MIC) | 5851000 | 516.4 | 1886 | 3.22 | 17814 | 30.45 |
| Libya (MIC) | 6278000 | 567.0 | 1292 | 2.06 | 11638 | 18.54 |
| Morocco (MIC) | 34378000 | 483.9 | 10645 | 3.10 | 69457 | 20.20 |
| Oman (HIC) | 4491000 | 404.6 | 903 | 2.01 | 4047 | 9.01 |
| Pakistan (MIC) | 189000000 | 681 | 44666 | 2.36 | 411569 | 21.78 |
| Qatar (HIC) | 2235000 | 425.5 | 359 | 1.60 | 1054 | 4.72 |
| Saudi Arabia (HIC) | 31540000 | 508.5 | 3737 | 1.18 | 42440 | 13.46 |
| Syria (MIC) | 18502000 | 594.7 | 1322 | 0.71 | 37885 | 20.48 |
| Tunisia (MIC) | 11254000 | 460.6 | 3523 | 3.13 | 31987 | 28.42 |
| UAE (HIC) | 9157000 | 460 | 707 | 0.77 | 5970 | 6.52 |
| Yemen (MIC) | 26832000 | 819.7 | 3854 | 1.44 | 56793 | 21.17 |
NCD: Noncommunicable diseases; CVD: Cardiovascular disease; LIC: Low income countries; MIC: Middle income countries; HIC: High income countries.
Even if not leading to premature death, diabetes causes significant morbidity with disability, lower productivity, and quality of life. The MENA region again has the highest rate of disability-adjusted life years caused by diabetes[77].
Macrovascular complications
Having diabetes essentially increases the risk of having a major adverse vascular event defined as nonfatal myocardial infarction, stroke, heart failure, and/or cardiovascular death by 2-3-fold after adjusting for age, sex, and smoking status[72,76,78]. We could not find any large cohort studies nor national data on the above hard outcomes even from countries with a high prevalence of T2D who have a national diabetes registry and/or have a relatively well-funded health care system[79]. The heterogeneity of the MENA population, the presence or absence of risk factors, and the inconsistent definition of outcomes makes comparisons between individual regions challenging. However, a recent study of 143567 adults with diabetes aged 35-70 years from 21 countries around the world, including 5 countries from the MENA (Saudi Arabia, UAE, Iran, Palestine, and Pakistan) and followed for 9 years found an absolute incidence of major CVD among people with diabetes of 8.3; 9.2; and 10.3 per 1000 person-years in HIC, middle-income countries, and low-income countries, respectively, as compared to 3.4; 4.9; and 5.3 per 1000 person-years in people without diabetes, respectively[76].
Specific to the MENA, the Tehran Lipid Study sampled 1198 adults aged ≥ 30 years with T2D and followed them for a median of 10 years. It reported a 23.4% and 14.3% cardiovascular and all-cause mortality, respectively. More than half of the mortality was due to cardiovascular events. Risk factors for death were male gender, smoking, and hypertension[80]. In a sample of 1308 adults with T2D recruited from primary care centers in Palestine, the prevalence of self-reported CVD was 12.2%[81]. The prevalence for CVD for Iran[4,80] and Palestine[82] is shown in Table 4.
Table 4.
Select studies reporting on diabetic foot ulcer and macrovascular complications of diabetes for the Middle East and North Africa region
|
Complication
|
Ref.
|
Country
|
Sample size (% male)
|
Setting
|
Duration of diabetes (yr)
|
Method of assessment
|
Prevalence %
|
| Diabetic foot ulcer | Assaad-Khalil et al[88], 2015 | Egypt (Alexandria) | 2000 (50.0) | Diabetes Foot Clinic | 11.7 ± 8.3 | Physical exam | 8.7 |
| Al-Rubeaan et al[89], 2015 | Saudi Arabia | 62681 (52.4) | Saudi National Diabetes Registry | 13.3 ± 8.1 | Chart review | 2.1 | |
| Yazdanpanah et al[92], 2018 | Iran (Ahfaz) | 605 (42.8) | Diabetes Clinic | 9.2 ± 7.1 | Physical exam | 6.4 | |
| AlAyed et al[93], 2017 | Jordan | 1000 (48.2) | Diabetes Clinic | 57.1% ≥ 5 | Physical exam | 5.3 | |
| Peripheral vascular disease | Akram et al[90], 2011 | Pakistan | 830 (49.0) | Outpatient Clinic | 8.1 ± 6.2 low ABI; .4 ± 6.4 normal ABI | ABI below 0.9 | 31.6 |
| Coronary artery disease | Saeedi et al[4], 2020 | Iran (Kurdistan) | 400 (18.0) | Diabetes Clinic | 14.6 ± 4.1 | Angiography or physician | 21.7 (5.75 CABG, 3.75 angioplasty) |
| Abu Al-Halaweh et al[81], 2017 | Palestine | 1308 (35.9) | Primary Care Centers | 7.1 ± 6.3 | Questionnaire | 12.2 (myocardial infarction) | |
| Afsharian et al[80], 2016 | Iran (Tehran) | 1198 (42.1) | Community-based | NA | Physician assessment | 23.4 |
ABI: Ankle brachial index; NA: Not available; CABG: Coronary artery bypass grafting.
On the other hand, risk factors for CVD, such as hypertension, dyslipidemia, smoking, physical inactivity, and obesity, are well documented through the implementation of the STEPS program by the WHO for most countries[17]. As an example, the prevalence of raised blood pressure (systolic blood pressure ≥ 140 and diastolic blood pressure ≥ 90 mmHg) was 26.3% for the EMR, which is the second highest in the world, with no gender predilection. It ranged from a low of 16.2% in Oman to as high as 25.0% in Pakistan. Similarly, the habit of smoking was high in this population, with a large gender difference, with 36.3% of men being active smokers (third highest in the world) vs 2.9% of women[17]. Of more concern is that a substantial proportion of youth (aged 13-15 years) smoke cigarettes, reaching 10% in Qatar and 35% in the West Bank (Palestine)[82]. Additionally, the habit of waterpipe smoking is highly prevalent among youth in predominantly Arabic-speaking countries of the MENA region, with waterpipe exceeding cigarette smoking[82]. The prevalence of hypercholesterolemia (total cholesterol level ≥ 5.2 mmol/L) was 36.8% in the EMR[70], which is comparable to the world prevalence. However, there is a higher predisposition to the atherogenic dyslipidemias in this region, with an elevated prevalence of low high-density lipoprotein-cholesterol and familial hypercholesterolemia[83,84].
Given that the prevalence of CVD risk factors in the MENA region is high, it would be desirable to develop a predictive model adapted to its population. One such CVD predictive tool used 1314 Omani adults to develop it and another 405 individuals for validating it; that population sample was followed prospectively for 6 years and confirmed a 9% incidence of CV events[85].
Diabetic foot ulcers
Diabetic foot ulcers (DFU) are a consequence of neuropathy and/or peripheral vascular disease. The lifetime risk for a person with diabetes to develop a DFU is 15%-25%, and the global prevalence is 3%-8%[86]. In a systematic review of DFU involving Arabic speaking countries, a total of 9 studies were reviewed, representing 16512 participants recruited from outpatient clinics. The prevalence of DFU was available for the following countries: Saudi Arabia (8.5%), Egypt (3.6%), Jordan (4.6%), Bahrain (5.9%), and Iraq (2.7%)[87]. A clinic-based study of 2000 adults with T2D in Alexandria (Egypt) found that 8.7% had DFU[88]. In a retrospective review of 62675 patients in Saudi Arabia, the prevalence of DFU was 2.05%, with an additional 1.06% suffering amputations[89].
Risk factors for DFU were a longer diabetes duration, male gender, higher BMI, the presence of an abnormal ankle-brachial index[90], and sensory neuropathy[88,91,92]. Prevalence of amputations varied from 1% to 2%[91,93]. Among 840 patients with diabetes in the Saudi National Diabetes Registry, the risk of mortality rose with DFU and increased further in the presence of lower extremity amputation, with a standardized mortality ratio of 4.39 and 7.21, respectively[94].
Microvascular complications
Most studies reporting on microvascular complications from the MENA region are either clinic or hospital based. However, some countries, like Saudi Arabia, possess a national diabetes registry, and others such as Iran have large cohorts followed longitudinally, like the Tehran Lipid Cohort. Among the three microvascular complications of diabetes namely nephropathy, neuropathy, and retinopathy, the latter is the most documented and studied.
Retinopathy
The overall global prevalence of DR is 34.6%, including 7.0% proliferative DR and 6.8% clinically significant macular edema[95]. It is the most common cause of blindness in adults worldwide including the MENA region. The overall prevalence of DR in the MENA countries ranges from 12.6% to 37.8%, with proliferative DR ranging from 2.3% to 10.6%[96-99].
In a nationwide study of 50464 Saudi adults with T2D from the Saudi National Diabetes Registry, the prevalence of DR was 19.7%, with 10.6% proliferative and 5.7% clinically significant macular edema[100]. In a hospital-based study of 1325 adults with T2D from Egypt, the prevalence of DR was 20.5%[101]. In a clinic-based survey of 1308 Palestinian adults with T2D, the prevalence of DR using nonmydriatic images was 21.8%[81]. In a cross-sectional Tunisian clinic-based study of 2320 adults, the prevalence of DR was 26.3%, out of whom 3.4% had proliferative DR and 4.2% had clinically significant macular edema[102]. In a systematic review in Iran which included 17079 individuals, the overall prevalence of DR was 37.8%, with wide variability among regions[103]. The prevalence of DR for the various countries is shown in Table 5.
Table 5.
Select studies reporting on microvascular complications of diabetes in the Middle East and North Africa region
|
Complication
|
Ref.
|
Country
|
Sample size (% male)
|
Setting
|
Duration of diabetes (yr)
|
Method of assessment
|
Prevalence %
|
| Retinopathy | Al-Rubeaan et al[89], 2015 | Saudi Arabia | 50464 (56.0) | Saudi National Diabetes Registry | 13.4 ± 8.2 | Chart review | 19.7 (10.6 PDR) |
| Macky et al[101], 2011 | Egypt | 1325 (28.5) | Hospital-based | 48% for 5-15 | Slit lamp | 20.5 (2.3 PDR) | |
| Jammal et al[104], 2013 | Jordan | 127 (63.8) | Clinic-based | Newly diagnosed | Slit lamp | 7.9 | |
| Uddin et al[99], 2018 | Pakistan | 958 (56.0) | Multi-Clinics | Newly diagnosed | Slit lamp | 15.9 | |
| Abu Al-Halaweh et al[81], 2017 | Palestine | 1308 (35.9) | Primary Care Centers | 7.1 ± 6.3 | Digital retinal photo | 21.8 | |
| Elshafei et al[97], 2011 | Qatar | 540 (360/540) | Community-based | 12.9 ± 9.1 | Slit lamp | 23.5 | |
| Heydari et al[98], 2012 | Iran | 1022 (40.2) | Clinic-based | 11.2 ± 8.2 DR; 5.8 ± 5.4 no DR | Slit lamp | 23.6 | |
| Arej et al[96], 2019 | Lebanon | 2205 | Community-based | 9.1 ± 7.1 | Digital retinal photo | 12.6 | |
| Kahloun et al[102], 2014 | Tunis | 2320 (39.8) | Hospital-based | 7.6 | Slit lamp | 26.3 (3.4 PDR) | |
| Nephropathy | Al-Rubeaan et al[107], 2018 | Saudi Arabia | 54670 (51.2) | Saudi National Diabetes Registry | 13.6 ± 8.1 | ACR and GFR | 10.8 (1.2 micro; 8.1 macro; 1.5 ESRD) |
| Uddin et al[99], 2018 | Pakistan | 958 (56.0) | Multi-Clinics | Newly diagnosed | ACR | 24.0 | |
| Zakkerkish et al[110], 2013 | Iran | 350 (32.0) | Diabetes Clinic | 4.6 ± 5.5 | ACR | 20.6 (5.1 macro) | |
| Shahwan et al[108], 2019 | Palestine (Ramallah) | 550 (54.7); Age above 35 yr | Diabetes Clinic | 8.9 ± 6.8 | ACR | 34.6 (5.8 macro) | |
| Ali and Al Lami[109], 2016 | Iraq | 224 (58.9) | Diabetes Clinic | 23.2 % ≥ 9 | ACR (2 out of 3) | 16.1 | |
| Neuropathy | Khedr et al[22], 2016 | Egypt (Qena) | 9303 (51.1); 837 with diabetes | Community | NR | MNSI, then ENG | 18.5 |
| Ghandour et al[114], 2018 | Palestine (Ramallah) | 517 (32.0) | Primary Health Clinic | 9.0 ± 7.5 | Monofilament test | 38.2 | |
| Chahbi et al[115], 2018 | Morocco | 300 (50.7) | Diabetes Clinic | 10.6 ± 7.4 | Diabetic Neuropathy Score | 15.4 (DN4 painful) | |
| Garoushi et al[112], 2019 | Libya | 450 (50.2) | Diabetes Clinic | 15.1± 7.1 | Diabetic Neuropathy Score | 42.2 (s-LANSS ≥ 12 pain) | |
| Kiani et al[113], 2013 | Iran | 521 (NR) | Diabetes Clinic | 9.2 ± 7.4 | NSS and NDS | 49.3 |
NR: Not recorded; MNSI: Michigan neuropathy screening instrument, ENG: Electroneurogram; PDR: Proliferative diabetic retinopathy; ACR: Albumin to creatinine ratio; S-LANSS: Leeds assessment of neuropathic symptoms and signs; NSS: Neuropathy symptom score; NDS: Neuropathy disability score; DR: Diabetic retinopathy; GFR: Glomerular filtration rate; ESRD: End-stage renal disease.
Importantly, retinopathy was found even among newly diagnosed adults with T2D. In Pakistan, DR was present at diagnosis in 15.9% of 958 adults with T2D[99]. Similarly, in Jordan, DR was documented in 7.9% of 127 adults with T2D within 6 mo of diagnosis[104]. Finally, in a retrospective chart review from Lebanon of 484 adults with T2D, DR was present in 26.6% at first ophthalmologic examination[105].
The elevated prevalence of DR at diagnosis is in line with the high proportion (44.7%) of undiagnosed diabetes for the MENA region[2]. It is likely that there is a latency period from onset of diabetes to time of its diagnosis, during which damage to the retina is taking place. In support of this theory is that duration of diabetes has been consistently reported as a risk factor for DR across most studies[96,100,101]. Even after being diagnosed, regular ophthalmologic check-ups were uncommon. This was evidenced in a community-based screening campaign of 2205 adults with mostly T2D, for whom only one third had regular retinal exams by an ophthalmologist[96]. Similarly, in Egypt, out of 1325 adults with long-standing diabetes, 82% were not aware of the need to do retinal checks[101]. Other risk factors for DR were poor glycemic control, older age, higher BMI, hypertension, smoking, the use of insulin, and the presence of other microvascular complications[97,98,100].
Nephropathy
Studies assessing nephropathy are more heterogeneous, with some reporting on albuminuria, others on glomerular filtration rate, and very few on end-stage renal disease. A global study evaluating the impact of diabetes on disability-adjusted life years in the EMR reported more than doubling of diabetes-related chronic kidney disease between 1990 and 2005[77]. This doubling was not only due to the increased prevalence of diabetes or aging but also due to more obesity, salt intake, and uncontrolled blood pressure[77]. In a national study from Libya, the estimated prevalence of end-stage renal disease was 624 per million. The major cause was diabetes, followed by hypertension[106].
The prevalence of microalbuminuria ranged from 10.8% in Saudi Arabia[107] to 34.6% in Palestine[108], with Iraq[109], Iran[110], and Pakistan[99] at 16.1%, 20.6%, and 24.0%, respectively. Macroalbuminuria constitutes about 15% of the reported albuminuria. The prevalence of nephropathy from selected studies is shown in Table 5.
Risk factors for albuminuria were elevated blood pressure, high BMI, duration of disease, hyperglycemia, and the presence of diabetes complications[107,109,110].
Neuropathy
Diabetic peripheral neuropathy is the most common complication of diabetes; at least 50% of individuals with diabetes will develop it to some extent[111]. Neuropathy negatively affects quality of life and constitutes a major risk for DFUs. Despite its high prevalence, studies on neuropathy are heterogeneous in terms of the populations studied, the setting (clinic or community-based), and the testing procedure used (questionnaire, monofilament test, or electroneurogram). Similarly, the prevalence in the MENA region varies from 18.5% (Egypt)[22] to 42.2% (Libya)[112] and 49% (Iran)[113], using a standardized scoring system. Using a simple monofilament test, the prevalence was 38% in an outpatient clinic in Palestine[114]. Painful neuropathy is less common and was 15.4% in a cross-sectional study of 300 adults in Morocco[115]. Increasing age, duration of disease, high BMI, poor glycemic control, smoking, and low educational level were predisposing factors[112,113]. Peripheral neuropathy is overall more common in men, whereas painful neuropathy was more common in women[115].
DIABETES IN CHILDREN AND ADOLESCENTS
Whereas diabetes in children and adolescents used to be mostly limited to T1D, there has been, over the last two decades, an increasing proportion developing T2D globally[15]. Moreover, in some countries, such as Japan, T2D in children and adolescents has become more prevalent than T1D[2]. Because of the earlier onset of the disease, affected children are expected to have a higher prevalence of complications in adulthood compared to others with adult-onset T2D within the same age group[17]. Furthermore, T2D starting at an early age would adversely affect productivity at its peak, raise the healthcare costs, and increase morbidity and mortality[15].
We could not find recent data about the overall prevalence of T2D in children and adolescents in the MENA region; however, data from specific countries reflect an increasing incidence and prevalence of T2D in this age group. In Kuwait, for instance, the incidence rate of T2D between 2011 and 2013 among children and adolescents, aged ≤ 14 years, was 2.56 per 100000 per year[116]. In Qatar, there were no registered cases of T2D among children and adolescents before 2008. Afterwards, the incidence of T2D increased from 1.16 per 100000 in 2012 to 2.72 per 100000 in 2016[117]. In Saudi Arabia, a nationwide survey conducted over years 2007-2009 found that 0.45% of the adolescents aged less than 18 years were known to have diabetes; out of which, 0.07% was T2D (n = 17). Moreover, the prevalence of newly diagnosed IFG and diabetes (both types) were 6.1% and 4.3%, respectively[118]. In Riyadh, T2D in the age group 7-17 years was found to be more common than T1D with a prevalence of 4.5%[33].
Moving to Iran, from 2001 to 2011, 1% of adolescents developed prediabetes and T2D each year[119]. The study found that males were 1.28 times more likely to develop prediabetes and T2D than females, which differs from an American study for diabetes in youth, the SEARCH study, that found the prevalence to be higher among females. This lower prevalence among Iranian females could be attributed to the ethnic differences and to how prediabetes was defined. This study included IFG only in its definition of prediabetes, which is, in contrast to IGT, a more common phenotype in males than females, thus contributing to the higher prediabetes prevalence among Iranian males[119]. As for Lebanon, a cross-sectional study was conducted in 2007 among students aged 11-18 years at three private schools in an urban area. It found that 10.5% of the students had IFG, and 3.5% had diabetes. Among overweight and obese individuals, the risk of developing prediabetes or diabetes was 4.93 and 2.85 times higher, respectively. The diagnostic criteria used did not differentiate between T1D and T2D; however, since the majority of those diagnosed with prediabetes and diabetes were overweight or obese, they were likely insulin resistant. Therefore, T2D is on the differential and should be confirmed for such a patient profile[120].
Modifiable risk factors
Obesity: One of the most important risk factors for T2D in adolescents is obesity. In the UAE, the prevalence of prediabetes and T2D among overweight and obese public school students, aged 11-17 years, were 5.4% and 0.87%, respectively[121]. Furthermore, 100% of all the children and adolescents with T2D in Qatar were either overweight or obese[117], and 23.35% of Saudi adolescents with diagnosed diabetes were obese[118].
Obesity presents a challenging health problem in the MENA region, where the rates of obesity have been increasing rapidly among children and adolescents at a faster rate than adults[122]. According to the WHO, the crude prevalence of obesity among children and adolescents in the region ranged from 2.4% in Afghanistan to 22.8% in Kuwait in 2016[123].
In Lebanon, two cross-sectional studies were conducted in 1997 and 2009 using the WHO definition for obesity in children and adolescents as age and sex specific scores + 2. The prevalence of obesity in the age group 6-19 years increased from 7.3% in 1997 to 10.9% in 2009[124]. Moreover, obesity was more common in boys (10.1%) than in girls (4.2%) in private schools, whereas there was no gender difference in public schools (6.7% and 6.0% for boys and girls, respectively)[120]. In Pakistan, recent data from Hazara city showed that the prevalence of obesity among school students was 4.78%, and similarly to Lebanon the prevalence was higher in private compared to public schools[125].
Physical inactivity: The Global School-based Student Health Surveys found that physical inactivity, defined as less than 60 min activity per day on 5 or more days in the past 7 d, was very high among children and adolescents in MENA countries and was higher in girls compared to boys. The prevalence was 65.4% in Lebanon, 72.5% in the UAE, 80% in Iraq, and more than 80% in many MENA countries, reaching as high as 90.6% in Egypt. The high sedentary lifestyle rates can be partly due to lack of encouragement of physical activities from parents, teachers, and friends, while favoring spending more time on other tasks such as their education[126].
Non modifiable risk factors
Family history: Along with obesity and physical inactivity, the genetic predisposition and family history present significant risk factors for T2D in adolescents[127]. The risk of abnormal glycemic status among Emirati school students was 1.9 times higher among those who have first degree relatives with diabetes; moreover, it was found that more than half of Emirati students with prediabetes and all those with T2D had a positive family history of diabetes[121]; the same applies to Qatari adolescents with T2D[117].
Other non-modifiable risk factors are low birth weight, maternal diabetes during pregnancy, and the postmenarchal phase in females[126]. During puberty, there is a physiological 30% increase in insulin resistance that, in the presence of other risk factors, can precipitate T2D; thus, the onset of T2D in youth is earlier in girls than boys and manifests itself usually during the second decade of life.
In summary, the youth of the MENA region are particularly vulnerable to developing T2D because of a culmination of risk factors. Multilevel interventions are needed to be able to effectively reverse this wave.
The management of T2D in children and adolescents presents a real challenge due to the poor adherence to the management recommendations of this age group and the difficulty in reversing obesity[128]; moreover, many of the young T2D patients are asymptomatic. Thus, it is important to screen those at risk. The ADA guidelines recommend screening children starting age 10 years or earlier (in case of an earlier onset of puberty) for all obese or overweight children if they have one additional risk factor[128]. The management for T2D in children and adolescents should include reduction of 7%-10% of the excess weight, physical activity for at least 60 min/d, and a healthy balanced diet rich in nutrients. Moreover, optimal blood pressure control and annual screening for neuropathy and retinopathy are crucial to prevent and manage complications[128]. All of these should be accompanied by educating patients and their parents about self-management including self-monitoring of blood glucose. We could not identify any studies from the MENA on the management of T2D in this age group.
CHALLENGES
In brief, the MENA region has the highest prevalence of T2D, and the rates continue to rise steeply. Furthermore, it has one of the highest diabetes related disability-adjusted life years and mortality rates from NCDs. Modifiable risk factors for both diabetes and its complications are highly prevalent in this population and extend to the pediatric and adolescent age group.
To address and reverse this situation is a daunting yet unavoidable task. Emerging factors add to the challenge, such as the increasing pollution level and environmental degradation, the high level of geopolitical instability, and most recently the COVID-19 pandemic. The latter two have had a direct impact on diabetes care.
Refugees and displaced people
In 2021, the United Nations High Commissioner Report indicated that there are 17.4 million refugees, internally displaced or physically constrained people in the MENA region. These are predominantly from Syria, Iraq, Yemen, and Libya[129]. They constitute about 3% of the MENA population, and 18% of the global population of concern. During the year 2000, 24 million Yemeni people depended on assistance. In parallel, the rates of extreme poverty have doubled in the region, from 3.8% to 7.2% between 2015 and 2018, and are likely to rise further due to the financial strain of the COVID-19 pandemic[130]. Furthermore, in 2020 the MENA region was deemed the ‘least peaceful’ area in the world with its many armed conflicts and/or political instability[131].
The above factors negatively influence NCDs in general and diabetes in particular. They also weaken the public healthcare system. The optimal management of diabetes requires a patient-centered approach with continuity of care[132]. Instead, in an unstable setting, the care is typically fragmented and interrupted, and the health of refugees and of displaced civilians is very likely to suffer. Unfortunately, very few national studies address the health status of refugees, so the prevalence of diabetes complications may be underestimated.
One such study was conducted in Lebanon on both the Lebanese population and Syrian refugees, as part of the STEPwise approach[133]. Out of 1899 Lebanese and 2134 Syrian adults aged 18-69 years, 51.2% of Lebanese had three or more cardiovascular risk factors vs 59.8% of Syrians. A 2014 survey by the United Nations High Commissioner Report found that close to 15% of adult Syrian refugees had at least one chronic condition, with 16% of those having diabetes, half of whom reported difficulties accessing health services[13]. With social instability and lack of security in parts of other MENA countries, it is expected that similar conditions may be prevalent[14]. The situation is likely to be complicated by poverty, food insecurity, poor nutrition, and inability to access health care. A concerted effort by the international community and governmental health agencies is needed to assist vulnerable people and provide them with access to medical care.
COVID-19 and diabetes
The COVID-19 pandemic has stressed the healthcare system and highlighted the need for a stronger infrastructure. Resources of all countries, even those that are relatively affluent, have been strained. National health systems should invest in interventions that aim at improving diabetes care. This is of paramount importance, in view of the data that shows a worse prognosis and an increased risk of complications and mortality for patients with diabetes hospitalized with COVID-19 infections[134]. A recent report from Qatar suggested that patients with diabetes hospitalized with COVID-19 infection had more severe clinical manifestations of pneumonia and acute respiratory distress syndrome and longer duration of hospitalization, intensive care stay, and mechanical ventilation[135]. Another study from Saudi Arabia reported that during the COVID-19 lockdown, patients with diabetes had reduced compliance with their medications and with healthy lifestyle habits[136]. Moreover, it is likely that many patients with diabetes have had a disrupted outpatient follow-up care due to visit cancellations during the pandemic, a situation that may adversely impact their overall diabetes control.
CONCLUSION
Call for action
There is ample evidence that diabetes can be prevented or delayed and its complications significantly reduced[137]. A recent editorial emphasized that worldwide implementation of diabetes prevention measures is an urgent matter[138]. Our review of T2D in the MENA region provides an opportunity to identify gaps and potential remedies for diabetes-related public health problems.
More accurate and complete data collection is needed for individual countries because available data is frequently scarce, outdated, relies on historical estimates, or is extrapolated from other similar countries. Without reliable data, the magnitude of the problem can be under/overestimated, and areas of need cannot be properly identified.
In addition, the socioeconomic disparities in the care of diabetes need to be addressed because a lower socioeconomic background frequently emerged as a risk factor for diabetes and for complications. Strategies need to be put in place to address this issue, with interventions that are evidence-based, cost effective, and designed to improve access to care.
Another worrisome finding is the gender difference manifesting as a higher prevalence of obesity and physical inactivity among women compared to men. It calls for an investigation of potential reasons and for implementation of remedial solutions, especially that gestational diabetes carries the risk of generational transmission of metabolic syndrome[139].
Furthermore, there is an urgent need to introduce programs that educate about healthy nutrition choices and promote healthy habits. Proper monitoring of implementation of planned interventions followed by evaluation of their impact is mandatory. Strategies to promote and encourage physical activity are needed, whether through urban planning with introduction of sidewalks and walking trails or through building exercise facilities that are accessible and affordable.
Policies are needed to label food items with their nutritional and caloric content. Educational programs need to be introduced at the school, community, and workplace levels, with emphasis on adapting them to the local cultures and norms. To be effective, educational programs need to be culturally sensitive and tailored to the functional health literacy of the local population[140]. The national media and advertising industry need to be involved in educating the public, in partnership with the national health system. Telehealth may play an important role in terms of raising awareness and providing better access to care[141], especially in view of the geopolitical instability and the COVID-19 pandemic.
In addition, there is a need to promote a public health awareness at the level of primary health care providers for screening and managing diabetes and associated metabolic comorbidities. There is evidence to suggest that quality of care is not optimal[142]. Educational activities need not only address risk factors for diabetes such as obesity and unhealthy lifestyles but also how to manage diabetes and its potential complications and how to implement preventive measures to avoid complications, such as regular eye and foot exams and kidney function tests.
The prevention and management of diabetes should be the perfect prototype of ‘health into all policies.’ In 2013, the WHO announced the Global Action Plan for the Prevention and Control of Non-Communicable Diseases 2013-2020 aimed at curbing the rise in obesity and diabetes. However, it is evident that not enough has been done so far to achieve this goal, and efforts have fallen short of the magnitude of the problem. Therefore, national strategies need to be put in place to address noncommunicable diseases in general and diabetes in particular, with an emphasis not only on planning but also on implementation. In 2021, the WHO is launching the WHO Global Diabetes Compact, which would enable countries (especially low and middle income) to prevent diabetes by addressing risk factors and help these countries develop their capabilities in identifying and treating people with diabetes[143]. Such programs are urgently needed in the MENA region.
ACKNOWLEDGEMENTS
The authors would like to thank Dr Amy Zenger for her kind and valuable assistance in English revisions.
Footnotes
Conflict-of-interest statement: All authors declare no relationships/conditions/circumstances that present a potential conflict of interest.
Manuscript source: Invited manuscript
Peer-review started: January 29, 2021
First decision: June 5, 2021
Article in press: August 2, 2021
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Lebanon
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cojocariu C, Tavan H S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT
Contributor Information
Imad M El-Kebbi, Department of Internal Medicine, Division of Endocrinology, American University of Beirut Medical Center, Faculty of Medicine, Beirut 11072020, Lebanon; Department of Internal Medicine, Sheikh Shakhbout Medical City, Abou Dhabi 11001, United Arab Emirates.
Nayda H Bidikian, School of Medicine, American University of Beirut, Faculty of Medicine, Beirut 11072020, Lebanon.
Layal Hneiny, University Libraries, Saab Medical Library, American University of Beirut, Beirut 11072020, Lebanon.
Mona Philippe Nasrallah, Department of Internal Medicine, Division of Endocrinology, American University of Beirut Medical Center, Faculty of Medicine, Beirut 11072020, Lebanon. mn36@aub.edu.lb.
References
- 1.World Health Organization. Diabetes: Key Facts 2020. [cited 27 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/diabetes .
- 2.International Diabetes Federation. IDF Diabetes Atlas. 9th ed. [cited 27 Jan 2021]. In: International Diabetes Federation [Internet]. Available from: https://www.diabetesatlas.org .
- 3.Moradi-Lakeh M, Forouzanfar MH, El Bcheraoui C, Daoud F, Afshin A, Hanson SW, Vos T, Naghavi M, Murray CJ, Mokdad AH Global Burden of Disease Collaborators on Eastern Mediterranean Region and Diabetes. High Fasting Plasma Glucose, Diabetes, and Its Risk Factors in the Eastern Mediterranean Region, 1990-2013: Findings From the Global Burden of Disease Study 2013. Diabetes Care. 2017;40:22–29. doi: 10.2337/dc16-1075. [DOI] [PubMed] [Google Scholar]
- 4.Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, Unwin N, Wild SH, Williams R. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2020;162:108086. doi: 10.1016/j.diabres.2020.108086. [DOI] [PubMed] [Google Scholar]
- 5.Azizi F, Hadaegh F, Hosseinpanah F, Mirmiran P, Amouzegar A, Abdi H, Asghari G, Parizadeh D, Montazeri SA, Lotfaliany M, Takyar F, Khalili D. Metabolic health in the Middle East and north Africa. Lancet Diabetes Endocrinol. 2019;7:866–879. doi: 10.1016/S2213-8587(19)30179-2. [DOI] [PubMed] [Google Scholar]
- 6.MENARA Project. European policy brief. [cited 27 Jan 2021]. In: Istituto Affari Internazionali [Internet]. Available from: https://www.iai.it/sites/default/files/menara_pb_2.pdf .
- 7.Abuhendi N, Qush A, Naji F, Abunada H, Al Buainain R, Shi Z, Zayed H. Genetic polymorphisms associated with type 2 diabetes in the Arab world: A systematic review and meta-analysis. Diabetes Res Clin Pract. 2019;151:198–208. doi: 10.1016/j.diabres.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Bener A, Mohammad RR. Global distribution of consanguinity and their impact on complex diseases: Genetic disorders from an endogamous population. Egypt J Med Human Genet . 2017;18:315–320. [Google Scholar]
- 9.Musambil M, Siddiqui K. Genetics and genomics studies in type 2 diabetes: A brief review of the current scenario in the Arab region. Diabetes Metab Syndr. 2019;13:1629–1632. doi: 10.1016/j.dsx.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Davidson MB, DeFronzo RA, Heine RJ, Henry RR, Pratley R, Zinman B American Diabetes Association. Impaired fasting glucose and impaired glucose tolerance: implications for care. Diabetes Care. 2007;30:753–759. doi: 10.2337/dc07-9920. [DOI] [PubMed] [Google Scholar]
- 11.United Nations. Leaders Gather at UN Headquarters for a High-Level Meeting on Non-communicable Diseases (NCDs). [cited 26 Jan 2021]. In: United Nations [Internet]. Available from: https://www.un.org/en/ga/ncdmeeting2011 .
- 12.World Health Organization. Diabetes Programme: Diabetes Action Now 2021. [cited 26 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/diabetes/actionnow/en/
- 13.Khan Y, Albache N, Almasri I, Gabbay RA. The Management of Diabetes in Conflict Settings: Focus on the Syrian Crisis. Diabetes Spectr. 2019;32:264–269. doi: 10.2337/ds18-0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qirbi N, Ismail SA. Health system functionality in a low-income country in the midst of conflict: the case of Yemen. Health Policy Plan. 2017;32:911–922. doi: 10.1093/heapol/czx031. [DOI] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Understanding A1C. [cited 27 Jan 2021]. In: American Diabetes Association [Internet]. Available from: https://www.diabetes.org/a1c/diagnosis .
- 16.International Diabetes Federation. Insulin at 100. [cited 27 Jan 2021]. In: International Diabetes Federation [Internet]. Available from: https://idf.org/
- 17.World Health Organization. Raised fasting blood glucose (>=7.0 mmol/L or on medication)(age-standardized estimate). [cited 27 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/data/gho/data/indicators/indicator-details/GHO/raised-fasting-blood-glucose-(-=7-0-mmol-l-or-on-medication)(age-standardized-estimate. )
- 18.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010;87:4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rubeaan K, Al-Manaa HA, Khoja TA, Ahmad NA, Al-Sharqawi AH, Siddiqui K, Alnaqeb D, Aburisheh KH, Youssef AM, Al-Batel A, Alotaibi MS, Al-Gamdi AA. Epidemiology of abnormal glucose metabolism in a country facing its epidemic: SAUDI-DM study. J Diabetes. 2015;7:622–632. doi: 10.1111/1753-0407.12224. [DOI] [PubMed] [Google Scholar]
- 20.Aamir AH, Ul-Haq Z, Mahar SA, Qureshi FM, Ahmad I, Jawa A, Sheikh A, Raza A, Fazid S, Jadoon Z, Ishtiaq O, Safdar N, Afridi H, Heald AH. Diabetes Prevalence Survey of Pakistan (DPS-PAK): prevalence of type 2 diabetes mellitus and prediabetes using HbA1c: a population-based survey from Pakistan. BMJ Open. 2019;9:e025300. doi: 10.1136/bmjopen-2018-025300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ajlouni K, Batieha A, Jaddou H, Khader Y, Abdo N, El-Khateeb M, Hyassat D, Al-Louzi D. Time trends in diabetes mellitus in Jordan between 1994 and 2017. Diabet Med. 2019;36:1176–1182. doi: 10.1111/dme.13894. [DOI] [PubMed] [Google Scholar]
- 22.Khedr EM, Fawi G, Allah Abbas MA, El-Fetoh NA, Al Attar G, Zaki AF, Gamea A. Prevalence of Diabetes and Diabetic Neuropathy in Qena Governorate: Population-Based Survey. Neuroepidemiology. 2016;46:173–181. doi: 10.1159/000444056. [DOI] [PubMed] [Google Scholar]
- 23.Ben Romdhane H, Ben Ali S, Aissi W, Traissac P, Aounallah-Skhiri H, Bougatef S, Maire B, Delpeuch F, Achour N. Prevalence of diabetes in Northern African countries: the case of Tunisia. BMC Public Health. 2014;14:86. doi: 10.1186/1471-2458-14-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Esteghamati A, Larijani B, Aghajani MH, Ghaemi F, Kermanchi J, Shahrami A, Saadat M, Esfahani EN, Ganji M, Noshad S, Khajeh E, Ghajar A, Heidari B, Afarideh M, Mechanick JI, Ismail-Beigi F. Diabetes in Iran: Prospective Analysis from First Nationwide Diabetes Report of National Program for Prevention and Control of Diabetes (NPPCD-2016) Sci Rep. 2017;7:13461. doi: 10.1038/s41598-017-13379-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirzaei M, Rahmaninan M, Mirzaei M, Nadjarzadeh A, Dehghani Tafti AA. Epidemiology of diabetes mellitus, pre-diabetes, undiagnosed and uncontrolled diabetes in Central Iran: results from Yazd health study. BMC Public Health. 2020;20:166. doi: 10.1186/s12889-020-8267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbarzadeh A, Salehi A, Molavi Vardanjani H, Poustchi H, Gandomkar A, Fattahi MR, Malekzadeh R. Epidemiology of Adult Diabetes Mellitus and its Correlates in Pars Cohort Study in Southern Iran. Arch Iran Med. 2019;22:633–639. [PubMed] [Google Scholar]
- 27.Latifi SM, Karandish M, Shahbazian H, Hardani Pasand L. Incidence of Prediabetes and Type 2 Diabetes among People Aged over 20 Years in Ahvaz: A 5-Year Perspective Study (2009-2014) J Diabetes Res. 2016;2016:4908647. doi: 10.1155/2016/4908647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costanian C, Bennett K, Hwalla N, Assaad S, Sibai AM. Prevalence, correlates and management of type 2 diabetes mellitus in Lebanon: findings from a national population-based study. Diabetes Res Clin Pract. 2014;105:408–415. doi: 10.1016/j.diabres.2014.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Nasrallah MP, Nakhoul NF, Nasreddine L, Mouneimne Y, Abiad MG, Ismaeel H, Tamim H. PREVALENCE OF DIABETES IN GREATER BEIRUT AREA: WORSENING OVER TIME. Endocr Pract. 2017;23:1091–1100. doi: 10.4158/EP171876.OR. [DOI] [PubMed] [Google Scholar]
- 30.Albache N, Al Ali R, Rastam S, Fouad FM, Mzayek F, Maziak W. Epidemiology of Type 2 diabetes mellitus in Aleppo, Syria. J Diabetes. 2010;2:85–91. doi: 10.1111/j.1753-0407.2009.00063.x. [DOI] [PubMed] [Google Scholar]
- 31.International Diabetes Federation. IDF Diabetes Atlas 8th ed. [cited 27 Jan 2021]. In: International Diabetes Federation [Internet]. Available from: https://www.diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf .
- 32.Ghafuri S, Ghaderi E, Fahami Y, Rajabnia M, Naleini SN. Epidemiologic study of type 2 diabetes mellitus and metabolic syndrome in rural population of kurdistan province, Iran, in 2011-2017. Diabetes Metab Syndr. 2019;13:1689–1697. doi: 10.1016/j.dsx.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 33.Al-Daghri NM, Al-Attas OS, Alokail MS, Alkharfy KM, Yousef M, Sabico SL, Chrousos GP. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. BMC Med. 2011;9:76. doi: 10.1186/1741-7015-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basit A, Fawwad A, Qureshi H, Shera AS NDSP Members. Prevalence of diabetes, pre-diabetes and associated risk factors: second National Diabetes Survey of Pakistan (NDSP), 2016-2017. BMJ Open. 2018;8:e020961. doi: 10.1136/bmjopen-2017-020961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al Khalaf MM, Eid MM, Najjar HA, Alhajry KM, Doi SA, Thalib L. Screening for diabetes in Kuwait and evaluation of risk scores. East Mediterr Health J. 2010;16:725–731. [PubMed] [Google Scholar]
- 36.Tuomilehto J, Lindström J, Eriksson JG, Valle TT, Hämäläinen H, Ilanne-Parikka P, Keinänen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M Finnish Diabetes Prevention Study Group. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 37.Richter B, Hemmingsen B, Metzendorf MI, Takwoingi Y. Development of type 2 diabetes mellitus in people with intermediate hyperglycaemia. Cochrane Database Syst Rev. 2018;10:CD012661. doi: 10.1002/14651858.CD012661.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansour AA, Al-Maliky AA, Kasem B, Jabar A, Mosbeh KA. Prevalence of diagnosed and undiagnosed diabetes mellitus in adults aged 19 years and older in Basrah, Iraq. Diabetes Metab Syndr Obes. 2014;7:139–144. doi: 10.2147/DMSO.S59652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saadi H, Carruthers SG, Nagelkerke N, Al-Maskari F, Afandi B, Reed R, Lukic M, Nicholls MG, Kazam E, Algawi K, Al-Kaabi J, Leduc C, Sabri S, El-Sadig M, Elkhumaidi S, Agarwal M, Benedict S. Prevalence of diabetes mellitus and its complications in a population-based sample in Al Ain, United Arab Emirates. Diabetes Res Clin Pract. 2007;78:369–377. doi: 10.1016/j.diabres.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Alkandari A, Longenecker JC, Barengo NC, Alkhatib A, Weiderpass E, Al-Wotayan R, Al Duwairi Q, Tuomilehto J. The prevalence of pre-diabetes and diabetes in the Kuwaiti adult population in 2014. Diabetes Res Clin Pract. 2018;144:213–223. doi: 10.1016/j.diabres.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Esteghamati A, Etemad K, Koohpayehzadeh J, Abbasi M, Meysamie A, Noshad S, Asgari F, Mousavizadeh M, Rafei A, Khajeh E, Neishaboury M, Sheikhbahaei S, Nakhjavani M. Trends in the prevalence of diabetes and impaired fasting glucose in association with obesity in Iran: 2005-2011. Diabetes Res Clin Pract. 2014;103:319–327. doi: 10.1016/j.diabres.2013.12.034. [DOI] [PubMed] [Google Scholar]
- 42.Bener A, Zirie M, Janahi IM, Al-Hamaq AO, Musallam M, Wareham NJ. Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar. Diabetes Res Clin Pract. 2009;84:99–106. doi: 10.1016/j.diabres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Gunaid AA, Assabri AM. Prevalence of type 2 diabetes and other cardiovascular risk factors in a semirural area in Yemen. East Mediterr Health J. 2008;14:42–56. [PubMed] [Google Scholar]
- 44.Al-Lawati JA, Al Riyami AM, Mohammed AJ, Jousilahti P. Increasing prevalence of diabetes mellitus in Oman. Diabet Med. 2002;19:954–957. doi: 10.1046/j.1464-5491.2002.00818.x. [DOI] [PubMed] [Google Scholar]
- 45.Bouguerra R, Alberti H, Salem LB, Rayana CB, Atti JE, Gaigi S, Slama CB, Zouari B, Alberti K. The global diabetes pandemic: the Tunisian experience. Eur J Clin Nutr. 2007;61:160–165. doi: 10.1038/sj.ejcn.1602478. [DOI] [PubMed] [Google Scholar]
- 46.Khattab A, Javaid A, Iraqi G, Alzaabi A, Ben Kheder A, Koniski ML, Shahrour N, Taright S, Idrees M, Polatli M, Rashid N, El Hasnaoui A BREATHE Study Group. Smoking habits in the Middle East and North Africa: results of the BREATHE study. Respir Med. 2012;106 Suppl 2:S16–S24. doi: 10.1016/S0954-6111(12)70011-2. [DOI] [PubMed] [Google Scholar]
- 47.Meo SA, Memon AN, Sheikh SA, Rouq FA, Usmani AM, Hassan A, Arian SA. Effect of environmental air pollution on type 2 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2015;19:123–128. [PubMed] [Google Scholar]
- 48.Lonardo A, Adinolfi LE, Petta S, Craxì A, Loria P. Hepatitis C and diabetes: the inevitable coincidence? Expert Rev Anti Infect Ther. 2009;7:293–308. doi: 10.1586/eri.09.3. [DOI] [PubMed] [Google Scholar]
- 49.Gomaa A, Allam N, Elsharkawy A, El Kassas M, Waked I. Hepatitis C infection in Egypt: prevalence, impact and management strategies. Hepat Med. 2017;9:17–25. doi: 10.2147/HMER.S113681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Agardh E, Allebeck P, Hallqvist J, Moradi T, Sidorchuk A. Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. Int J Epidemiol. 2011;40:804–818. doi: 10.1093/ije/dyr029. [DOI] [PubMed] [Google Scholar]
- 51.Florez JC, Hirschhorn J, Altshuler D. The inherited basis of diabetes mellitus: implications for the genetic analysis of complex traits. Annu Rev Genomics Hum Genet. 2003;4:257–291. doi: 10.1146/annurev.genom.4.070802.110436. [DOI] [PubMed] [Google Scholar]
- 52.Mousa HS, Yousef S, Riccardo F, Zeidan W, Sabatinelli G. Hyperglycaemia, hypertension and their risk factors among Palestine refugees served by UNRWA. East Mediterr Health J. 2010;16:609–614. [PubMed] [Google Scholar]
- 53.Harati H, Hadaegh F, Saadat N, Azizi F. Population-based incidence of Type 2 diabetes and its associated risk factors: results from a six-year cohort study in Iran. BMC Public Health. 2009;9:186. doi: 10.1186/1471-2458-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ereqat S, Nasereddin A, Cauchi S, Azmi K, Abdeen Z, Amin R. Association of a common variant in TCF7L2 gene with type 2 diabetes mellitus in the Palestinian population. Acta Diabetol. 2010;47 Suppl 1:195–198. doi: 10.1007/s00592-009-0161-0. [DOI] [PubMed] [Google Scholar]
- 55.Ghassibe-Sabbagh M, Haber M, Salloum AK, Al-Sarraj Y, Akle Y, Hirbli K, Romanos J, Mouzaya F, Gauguier D, Platt DE, El-Shanti H, Zalloua PA. T2DM GWAS in the Lebanese population confirms the role of TCF7L2 and CDKAL1 in disease susceptibility. Sci Rep. 2014;4:7351. doi: 10.1038/srep07351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osman W, Tay GK, Alsafar H. Multiple genetic variations confer risks for obesity and type 2 diabetes mellitus in arab descendants from UAE. Int J Obes (Lond) 2018;42:1345–1353. doi: 10.1038/s41366-018-0057-6. [DOI] [PubMed] [Google Scholar]
- 57.El-Lebedy D, Ashmawy I. Common variants in TCF7L2 and CDKAL1 genes and risk of type 2 diabetes mellitus in Egyptians. J Genet Eng Biotechnol. 2016;14:247–251. doi: 10.1016/j.jgeb.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Beirne SL, Salit J, Rodriguez-Flores JL, Staudt MR, Abi Khalil C, Fakhro KA, Robay A, Ramstetter MD, Al-Azwani IK, Malek JA, Zirie M, Jayyousi A, Badii R, Al-Nabet Al-Marri A, Chiuchiolo MJ, Al-Shakaki A, Chidiac O, Gharbiah M, Bener A, Stadler D, Hackett NR, Mezey JG, Crystal RG. Type 2 Diabetes Risk Allele Loci in the Qatari Population. PLoS One. 2016;11:e0156834. doi: 10.1371/journal.pone.0156834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mtiraoui N, Turki A, Nemr R, Echtay A, Izzidi I, Al-Zaben GS, Irani-Hakime N, Keleshian SH, Mahjoub T, Almawi WY. Contribution of common variants of ENPP1, IGF2BP2, KCNJ11, MLXIPL, PPARγ, SLC30A8 and TCF7L2 to the risk of type 2 diabetes in Lebanese and Tunisian Arabs. Diabetes Metab. 2012;38:444–449. doi: 10.1016/j.diabet.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 60.Almawi WY, Nemr R, Keleshian SH, Echtay A, Saldanha FL, AlDoseri FA, Racoubian E. A replication study of 19 GWAS-validated type 2 diabetes at-risk variants in the Lebanese population. Diabetes Res Clin Pract. 2013;102:117–122. doi: 10.1016/j.diabres.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 61.Popkin BM. Global nutrition dynamics: the world is shifting rapidly toward a diet linked with noncommunicable diseases. Am J Clin Nutr. 2006;84:289–298. doi: 10.1093/ajcn/84.1.289. [DOI] [PubMed] [Google Scholar]
- 62.Mehio Sibai A, Nasreddine L, Mokdad AH, Adra N, Tabet M, Hwalla N. Nutrition transition and cardiovascular disease risk factors in Middle East and North Africa countries: reviewing the evidence. Ann Nutr Metab. 2010;57:193–203. doi: 10.1159/000321527. [DOI] [PubMed] [Google Scholar]
- 63.Alhabib KF, Batais MA, Almigbal TH, Alshamiri MQ, Altaradi H, Rangarajan S, Yusuf S. Demographic, behavioral, and cardiovascular disease risk factors in the Saudi population: results from the Prospective Urban Rural Epidemiology study (PURE-Saudi) BMC Public Health. 2020;20:1213. doi: 10.1186/s12889-020-09298-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nasreddine L, Naja FA, Sibai AM, Helou K, Adra N, Hwalla N. Trends in nutritional intakes and nutrition-related cardiovascular disease risk factors in Lebanon: the need for immediate action. J Med Liban. 2014;62:83–91. doi: 10.12816/0004102. [DOI] [PubMed] [Google Scholar]
- 65.Nasreddine L, Tamim H, Itani L, Nasrallah MP, Isma'eel H, Nakhoul NF, Abou-Rizk J, Naja F. A minimally processed dietary pattern is associated with lower odds of metabolic syndrome among Lebanese adults. Public Health Nutr. 2018;21:160–171. doi: 10.1017/S1368980017002130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.World Health Organization. Global Recommendations on Physical Activity for Health. [cited 25 Jan 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/44399/9789241599979_eng.pdf .
- 67.Sherif S, Sumpio BE. Economic development and diabetes prevalence in MENA countries: Egypt and Saudi Arabia comparison. World J Diabetes. 2015;6:304–311. doi: 10.4239/wjd.v6.i2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Al-Nozha MM, Al-Hazzaa HM, Arafah MR, Al-Khadra A, Al-Mazrou YY, Al-Maatouq MA, Khan NB, Al-Marzouki K, Al-Harthi SS, Abdullah M, Al-Shahid MS. Prevalence of physical activity and inactivity among Saudis aged 30-70 years. A population-based cross-sectional study. Saudi Med J. 2007;28:559–568. [PubMed] [Google Scholar]
- 69.Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, fat distribution, and weight gain as risk factors for clinical diabetes in men. Diabetes Care. 1994;17:961–969. doi: 10.2337/diacare.17.9.961. [DOI] [PubMed] [Google Scholar]
- 70.World Health Organization. Noncommunicable Diseases. [cited 26 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/health-topics/noncommunicable-diseases .
- 71.World Health Organization. The top 10 causes of death. [cited 26 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death .
- 72.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L INTERHEART Study Investigators. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364:937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 73.Selvin E, Juraschek SP. Diabetes Epidemiology in the COVID-19 Pandemic. Diabetes Care. 2020;43:1690–1694. doi: 10.2337/dc20-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.World Health Organization. Global Report on Diabetes. [cited 25 Jan 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf .
- 75.World Health Organization. Noncommunicable diseases: Mortality. [cited 25 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-mortality .
- 76.Anjana RM, Mohan V, Rangarajan S, Gerstein HC, Venkatesan U, Sheridan P, Dagenais GR, Lear SA, Teo K, Karsidag K, Alhabib KF, Yusoff K, Ismail N, Mony PK, Lopez-Jaramillo P, Chifamba J, Palileo-Villanueva LM, Iqbal R, Yusufali A, Kruger IM, Rosengren A, Bahonar A, Zatonska K, Yeates K, Gupta R, Li W, Hu L, Rahman MO, Lakshmi PVM, Iype T, Avezum A, Diaz R, Lanas F, Yusuf S. Contrasting Associations Between Diabetes and Cardiovascular Mortality Rates in Low-, Middle-, and High-Income Countries: Cohort Study Data From 143,567 Individuals in 21 Countries in the PURE Study. Diabetes Care. 2020;43:3094–3101. doi: 10.2337/dc20-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.GBD 2015 Eastern Mediterranean Region Diabetes and Chronic Kidney Disease Collaborators. Diabetes mellitus and chronic kidney disease in the Eastern Mediterranean Region: findings from the Global Burden of Disease 2015 study. Int J Public Health. 2018;63:177–186. doi: 10.1007/s00038-017-1014-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robert AA, Al Dawish MA. Cardiovascular Disease among Patients with Diabetes: The Current Scenario in Saudi Arabia. Curr Diabetes Rev. 2021;17:180–185. doi: 10.2174/1573399816666200527135512. [DOI] [PubMed] [Google Scholar]
- 80.Afsharian S, Akbarpour S, Abdi H, Sheikholeslami F, Moeini AS, Khalili D, Momenan AA, Azizi F, Hadaegh F. Risk factors for cardiovascular disease and mortality events in adults with type 2 diabetes - a 10-year follow-up: Tehran Lipid and Glucose Study. Diabetes Metab Res Rev. 2016;32:596–606. doi: 10.1002/dmrr.2776. [DOI] [PubMed] [Google Scholar]
- 81.Abu Al-Halaweh A, Davidovitch N, Almdal TP, Cowan A, Khatib S, Nasser-Eddin L, Baradia Z. Prevalence of type 2 diabetes mellitus complications among palestinians with T2DM. Diabetes Metab Syndr. 2017;11 Suppl 2:S783–S787. doi: 10.1016/j.dsx.2017.05.017. [DOI] [PubMed] [Google Scholar]
- 82.Maziak W, Nakkash R, Bahelah R, Husseini A, Fanous N, Eissenberg T. Tobacco in the Arab world: old and new epidemics amidst policy paralysis. Health Policy Plan. 2014;29:784–794. doi: 10.1093/heapol/czt055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jelwan YA, Asbeutah AAA, Welty FK. Comprehensive Review of Cardiovascular Diseases, Diabetes, and Hypercholesterolemia in Lebanon. Cardiol Rev. 2020;28:73–83. doi: 10.1097/CRD.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 84.Al Rasadi K, Almahmeed W, AlHabib KF, Abifadel M, Farhan HA, AlSifri S, Jambart S, Zubaid M, Awan Z, Al-Waili K, Barter P. Dyslipidaemia in the Middle East: Current status and a call for action. Atherosclerosis. 2016;252:182–187. doi: 10.1016/j.atherosclerosis.2016.07.925. [DOI] [PubMed] [Google Scholar]
- 85.Alrawahi AH, Lee P. Validation of the cardiovascular risk model developed for Omanis with type 2 diabetes. Diabetes Metab Syndr. 2018;12:387–391. doi: 10.1016/j.dsx.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 86.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet. 2005;366:1719–1724. doi: 10.1016/S0140-6736(05)67698-2. [DOI] [PubMed] [Google Scholar]
- 87.Mairghani M, Elmusharaf K, Patton D, Burns J, Eltahir O, Jassim G, Moore Z. The prevalence and incidence of diabetic foot ulcers among five countries in the Arab world: a systematic review. J Wound Care. 2017;26:S27–S34. doi: 10.12968/jowc.2017.26.Sup9.S27. [DOI] [PubMed] [Google Scholar]
- 88.Assaad-Khalil SH, Zaki A, Abdel Rehim A, Megallaa MH, Gaber N, Gamal H, Rohoma KH. Prevalence of diabetic foot disorders and related risk factors among Egyptian subjects with diabetes. Prim Care Diabetes. 2015;9:297–303. doi: 10.1016/j.pcd.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 89.Al-Rubeaan K, Al Derwish M, Ouizi S, Youssef AM, Subhani SN, Ibrahim HM, Alamri BN. Diabetic foot complications and their risk factors from a large retrospective cohort study. PLoS One. 2015;10:e0124446. doi: 10.1371/journal.pone.0124446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Akram J, Aamir AU, Basit A, Qureshi MS, Mehmood T, Shahid SK, Khoso IA, Ebrahim MA, Omair A. Prevalence of peripheral arterial disease in type 2 diabetics in Pakistan. J Pak Med Assoc. 2011;61:644–648. [PubMed] [Google Scholar]
- 91.Ababneh A, Bakri FG, Khader Y, Lazzarini P, Ajlouni K. Prevalence and Associates of Foot Deformities among Patients with Diabetes in Jordan. Curr Diabetes Rev. 2020;16:471–482. doi: 10.2174/1573399815666191001101910. [DOI] [PubMed] [Google Scholar]
- 92.Yazdanpanah L, Shahbazian H, Nazari I, Arti HR, Ahmadi F, Mohammadianinejad SE, Cheraghian B, Latifi SM. Prevalence and related risk factors of diabetic foot ulcer in Ahvaz, south west of Iran. Diabetes Metab Syndr. 2018;12:519–524. doi: 10.1016/j.dsx.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 93.AlAyed MY, Younes N, Al-Smady M, Khader YS, Robert AA, Ajlouni K. Prevalence of Foot Ulcers, Foot at Risk and Associated Risk Factors Among Jordanian Diabetics. Curr Diabetes Rev. 2017;13:182–191. doi: 10.2174/1573399812666151210143140. [DOI] [PubMed] [Google Scholar]
- 94.Al-Rubeaan K, Almashouq MK, Youssef AM, Al-Qumaidi H, Al Derwish M, Ouizi S, Al-Shehri K, Masoodi SN. All-cause mortality among diabetic foot patients and related risk factors in Saudi Arabia. PLoS One. 2017;12:e0188097. doi: 10.1371/journal.pone.0188097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY Meta-Analysis for Eye Disease (META-EYE) Study Group. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Arej N, Antoun J, Waked R, Saab C, Saleh M, Waked N. [Screening for diabetic retinopathy by non-mydriatic fundus photography: First national campaign in Lebanon] J Fr Ophtalmol. 2019;42:288–294. doi: 10.1016/j.jfo.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 97.Elshafei M, Gamra H, Khandekar R, Al Hashimi M, Pai A, Ahmed MF. Prevalence and determinants of diabetic retinopathy among persons ≥ 40 years of age with diabetes in Qatar: a community-based survey. Eur J Ophthalmol. 2011;21:39–47. doi: 10.5301/ejo.2010.2699. [DOI] [PubMed] [Google Scholar]
- 98.Heydari B, Yaghoubi G, Yaghoubi MA, Miri MR. Prevalence and risk factors for diabetic retinopathy: an Iranian eye study. Eur J Ophthalmol. 2012;22:393–397. doi: 10.5301/ejo.5000044. [DOI] [PubMed] [Google Scholar]
- 99.Uddin F, Ali B, Junaid N. Prevalence Of Diabetic Complications In Newly Diagnosed Type 2 Diabetes Patients In Pakistan: Findings From National Registry. J Ayub Med Coll Abbottabad. 2018;30(Suppl 1):S652–S658. [PubMed] [Google Scholar]
- 100.Al-Rubeaan K, Abu El-Asrar AM, Youssef AM, Subhani SN, Ahmad NA, Al-Sharqawi AH, Alguwaihes A, Alotaibi MS, Al-Ghamdi A, Ibrahim HM. Diabetic retinopathy and its risk factors in a society with a type 2 diabetes epidemic: a Saudi National Diabetes Registry-based study. Acta Ophthalmol. 2015;93:e140–e147. doi: 10.1111/aos.12532. [DOI] [PubMed] [Google Scholar]
- 101.Macky TA, Khater N, Al-Zamil MA, El Fishawy H, Soliman MM. Epidemiology of diabetic retinopathy in Egypt: a hospital-based study. Ophthalmic Res. 2011;45:73–78. doi: 10.1159/000314876. [DOI] [PubMed] [Google Scholar]
- 102.Kahloun R, Jelliti B, Zaouali S, Attia S, Ben Yahia S, Resnikoff S, Khairallah M. Prevalence and causes of visual impairment in diabetic patients in Tunisia, North Africa. Eye (Lond) 2014;28:986–991. doi: 10.1038/eye.2014.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mohammadi M, Raiegani AAV, Jalali R, Ghobadi A, Salari N. The prevalence of retinopathy among type 2 diabetic patients in Iran: A systematic review and meta-analysis. Rev Endocr Metab Disord. 2019;20:79–88. doi: 10.1007/s11154-019-09490-3. [DOI] [PubMed] [Google Scholar]
- 104.Jammal H, Khader Y, Alkhatib S, Abujbara M, Alomari M, Ajlouni K. Diabetic retinopathy in patients with newly diagnosed type 2 diabetes mellitus in Jordan: prevalence and associated factors. J Diabetes. 2013;5:172–179. doi: 10.1111/1753-0407.12015. [DOI] [PubMed] [Google Scholar]
- 105.Harb W, Harb G, Chamoun N, Kanbar A, Harb M, Chanbour W. Severity of diabetic retinopathy at the first ophthalmological examination in the Lebanese population. Ther Adv Ophthalmol. 2018;10:2515841418791950. doi: 10.1177/2515841418791950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alashek WA, McIntyre CW, Taal MW. Epidemiology and aetiology of dialysis-treated end-stage kidney disease in Libya. BMC Nephrol. 2012;13:33. doi: 10.1186/1471-2369-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Al-Rubeaan K, Siddiqui K, Alghonaim M, Youssef AM, AlNaqeb D. The Saudi Diabetic Kidney Disease study (Saudi-DKD): clinical characteristics and biochemical parameters. Ann Saudi Med. 2018;38:46–56. doi: 10.5144/0256-4947.2018.03.01.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shahwan MJ, Gacem SA, Zaidi SK. Prevalence of Diabetic Nephropathy and associated risk factors among type 2 diabetes mellitus patients in Ramallah, Palestine. Diabetes Metab Syndr. 2019;13:1491–1496. doi: 10.1016/j.dsx.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 109.Ali AA, Al Lami FH. Prevalence and determinants of microalbuminurea among type 2 diabetes mellitus patients, Baghdad, Iraq, 2013. Saudi J Kidney Dis Transpl. 2016;27:348–355. doi: 10.4103/1319-2442.178561. [DOI] [PubMed] [Google Scholar]
- 110.Zakkerkish M, Shahbazian HB, Shahbazian H, Latifi SM, Moravej Aleali A. Albuminuria and its correlates in type 2 diabetic patients. Iran J Kidney Dis. 2013;7:268–276. [PubMed] [Google Scholar]
- 111.neuropathy. Nat Rev Dis Primers. 2019;5:42. doi: 10.1038/s41572-019-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Garoushi S, Johnson MI, Tashani OA. A cross-sectional study to estimate the point prevalence of painful diabetic neuropathy in Eastern Libya. BMC Public Health. 2019;19:78. doi: 10.1186/s12889-018-6374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kiani J, Moghimbeigi A, Azizkhani H, Kosarifard S. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch Iran Med. 2013;16:17–19. [PubMed] [Google Scholar]
- 114.Ghandour R, Mikki N, Abu Rmeileh NME, Jerdén L, Norberg M, Eriksson JW, Husseini A. Complications of type 2 diabetes mellitus in Ramallah and al-Bireh: The Palestinian Diabetes Complications and Control Study (PDCCS) Prim Care Diabetes. 2018;12:547–557. doi: 10.1016/j.pcd.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 115.Chahbi Z, Lahmar B, Hadri SE, Abainou L, Kaddouri S, Qacif H, Baizri H, Zyani M. The prevalence of painful diabetic neuropathy in 300 Moroccan diabetics. Pan Afr Med J. 2018;31:158. doi: 10.11604/pamj.2018.31.158.14687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Al-Kandari H, Al-Abdulrazzaq D, Davidsson L, Sharma P, Al-Tararwa A, Mandani F, Al-Shawaf F, Al-Hussaini F, Qabazard M, Haddad D, Al-Mahdi M, Al-Jasser F, Al-Anzi A, Al-Sanea H, Al-Basari I, Al-Adsani A, Shaltout A, AbdulRasoul M. Incidence of Type 2 Diabetes in Kuwaiti Children and Adolescents: Results From the Childhood-Onset Diabetes Electronic Registry (CODeR) Front Endocrinol (Lausanne) 2019;10:836. doi: 10.3389/fendo.2019.00836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Alyafei F, Soliman A, Alkhalaf F, Sabt A, De Sanctis V, Waseef R, Elsayed N. Incidence of type 1 and type 2 diabetes, between 2012-2016, among children and adolescents in Qatar. Acta Biomed. 2018;89:7–10. doi: 10.23750/abm.v89i5.7360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Al-Rubeaan K. National surveillance for type 1, type 2 diabetes and prediabetes among children and adolescents: a population-based study (SAUDI-DM) J Epidemiol Community Health. 2015;69:1045–1051. doi: 10.1136/jech-2015-205710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mirbolouk M, Derakhshan A, Charkhchi P, Guity K, Azizi F, Hadaegh F. Incidence and predictors of early adulthood pre-diabetes/type 2 diabetes, among Iranian adolescents: the Tehran Lipid and Glucose Study. Pediatr Diabetes. 2016;17:608–616. doi: 10.1111/pedi.12343. [DOI] [PubMed] [Google Scholar]
- 120.Salameh P, Barbour B. Pattern of obesity and associated diabetes in Lebanese adolescents: a pilot study. East Mediterr Health J. 2011;17:226–230. [PubMed] [Google Scholar]
- 121.Al Amiri E, Abdullatif M, Abdulle A, Al Bitar N, Afandi EZ, Parish M, Darwiche G. The prevalence, risk factors, and screening measure for prediabetes and diabetes among Emirati overweight/obese children and adolescents. BMC Public Health. 2015;15:1298. doi: 10.1186/s12889-015-2649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Farrag NS, Cheskin LJ, Farag MK. A systematic review of childhood obesity in the Middle East and North Africa (MENA) region: Health impact and management. Adv Pediatr Res. 2017;4 doi: 10.12715/apr.2017.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.World Health Organization. Prevalence of obesity among children and adolescents, BMI>+2 standard deviation above the median, crude Estimates by country, among children aged 5-19 years. [cited 27 Jan 2021]. In: World Health Organization [Internet]. Available from: https://apps.who.int/gho/data/view.main.BMIPLUS2C05-19v .
- 124.Nasreddine L, Naja F, Chamieh MC, Adra N, Sibai AM, Hwalla N. Trends in overweight and obesity in Lebanon: evidence from two national cross-sectional surveys (1997 and 2009) BMC Public Health. 2012;12:798. doi: 10.1186/1471-2458-12-798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Afzal W. Type 2 diabetes and weight dynamics in young: current view. J Pak Med Assoc. 2019;69:1234–1236. [PubMed] [Google Scholar]
- 126.Sharara E, Akik C, Ghattas H, Makhlouf Obermeyer C. Physical inactivity, gender and culture in Arab countries: a systematic assessment of the literature. BMC Public Health. 2018;18:639. doi: 10.1186/s12889-018-5472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Temneanu OR, Trandafir LM, Purcarea MR. Type 2 diabetes mellitus in children and adolescents: a relatively new clinical problem within pediatric practice. J Med Life. 2016;9:235–239. [PMC free article] [PubMed] [Google Scholar]
- 128.American Diabetes Association. 13. Children and Adolescents: Standards of Medical Care in Diabetes-2020. Diabetes Care. 2020;43:S163–S182. doi: 10.2337/dc20-S013. [DOI] [PubMed] [Google Scholar]
- 129.United Nations High Commissioner for Refugees. UNHCR Global appeal 2021 update. [cited 26 Jan 2021]. In: United Nations High Commissioner for Refugees [Internet]. Available from: https://reporting.unhcr.org/sites/default/files/ga2021/pdf/Chapter_MENA.pdf .
- 130.The World Bank. MENA: Global Action is Urgently Needed to Reverse Damaging Jumps in Extreme Poverty. [cited 26 Jan 2021]. In: The World Bank [Internet]. Available from: https://www.worldbank.org/en/news/press-release/2020/10/09/mena-global-action-is-urgently-needed-to-reverse-damaging-jumps-in-extreme-poverty .
- 131.Global Peace Index. Global Peace Index 2018. [cited 26 Jan 2021]. In: Wikipedia [Internet]. Available from: https://en.wikipedia.org/wiki/Global_Peace_Index#/media/File:Global_Peace_Index.svg .
- 132.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Aff (Millwood) 2001;20:64–78. doi: 10.1377/hlthaff.20.6.64. [DOI] [PubMed] [Google Scholar]
- 133.World Health Organization. WHO stepwise approach for non-communicable diseases risk factor surveillance: Lebanon, 2016-2017. [cited 26 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/ncds/surveillance/steps/Lebanon_STEPS_report_2016-2017.pdf .
- 134.Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Soliman AT, Prabhakaran Nair A, Al Masalamani MS, De Sanctis V, Abu Khattab MA, Alsaud AE, Sasi S, Ali EA, Ola A H, Iqbal FM, Nashwan AJ, Fahad J, El Madhoun I, Yassin MA. Prevalence, clinical manifestations, and biochemical data of type 2 diabetes mellitus vs nondiabetic symptomatic patients with COVID-19: A comparative study. Acta Biomed. 2020;91:e2020010. doi: 10.23750/abm.v91i3.10214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Alshareef R, Al Zahrani A, Alzahrani A, Ghandoura L. Impact of the COVID-19 Lockdown on diabetes patients in Jeddah, Saudi Arabia. Diabetes Metab Syndr. 2020;14:1583–1587. doi: 10.1016/j.dsx.2020.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rawshani A, Rawshani A, Franzén S, Sattar N, Eliasson B, Svensson AM, Zethelius B, Miftaraj M, McGuire DK, Rosengren A, Gudbjörnsdottir S. Risk Factors, Mortality, and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 2018;379:633–644. doi: 10.1056/NEJMoa1800256. [DOI] [PubMed] [Google Scholar]
- 138.The Lancet. Turning evidence into action on diabetes. Lancet. 2020;396:1535. doi: 10.1016/S0140-6736(20)32412-0. [DOI] [PubMed] [Google Scholar]
- 139.Al-Rifai RH, Majeed M, Qambar MA, Ibrahim A, AlYammahi KM, Aziz F. Type 2 diabetes and pre-diabetes mellitus: a systematic review and meta-analysis of prevalence studies in women of childbearing age in the Middle East and North Africa, 2000-2018. Syst Rev. 2019;8:268. doi: 10.1186/s13643-019-1187-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Baker DW, Parker RM, Williams MV, Clark WS, Nurss J. The relationship of patient reading ability to self-reported health and use of health services. Am J Public Health. 1997;87:1027–1030. doi: 10.2105/ajph.87.6.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Al Alawi E, Ahmed AA. Screening for diabetic retinopathy: the first telemedicine approach in a primary care setting in Bahrain. Middle East Afr J Ophthalmol. 2012;19:295–298. doi: 10.4103/0974-9233.97928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shivashankar R, Kirk K, Kim WC, Rouse C, Tandon N, Narayan KM, Ali MK. Quality of diabetes care in low- and middle-income Asian and Middle Eastern countries (1993-2012): 20-year systematic review. Diabetes Res Clin Pract. 2015;107:203–223. doi: 10.1016/j.diabres.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 143.World Health Organization. WHO announces the Global Diabetes Compact. [cited 26 Jan 2021]. In: World Health Organization [Internet]. Available from: https://www.who.int/news/item/17-11-2020-who-announces-the-global-diabetes-compact .
- 144.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]