Abstract
Multiple system atrophy (MSA) is a rapidly progressive, fatal neurodegenerative disease of uncertain aetiology that belongs to the family of α-synucleinopathies. It clinically presents with parkinsonism, cerebellar, autonomic, and motor impairment in variable combinations. Pathological hallmarks are fibrillary α-synuclein (αSyn)-rich glial cytoplasmic inclusions (GCIs) mainly involving oligodendroglia and to a lesser extent neurons, inducing a multisystem neurodegeneration, glial activation, and widespread demyelinization. The neuronal αSyn pathology of MSA has molecular properties different from Lewy bodies in Parkinson’s disease (PD), both of which could serve as a pool of αSyn (prion) seeds that could initiate and drive the pathogenesis of synucleinopathies. The molecular cascade leading to the “prion-like” transfer of “strains” of aggregated αSyn contributing to the progression of the disease is poorly understood, while some presented evidence that MSA is a prion disease. However, this hypothesis is difficult to reconcile with postmortem analysis of human brains and the fact that MSA-like pathology was induced by intracerebral inoculation of human MSA brain homogenates only in homozygous mutant 53T mice, without production of disease-specific GCIs, or with replication of MSA prions in primary astrocyte cultures from transgenic mice expressing human αSyn. Whereas recent intrastriatal injection of Lewy body-derived or synthetic human αSyn fibrils induced PD-like pathology including neuronal αSyn aggregates in macaques, no such transmission of αSyn pathology in non-human primates by MSA brain lysate has been reported until now. Given the similarities between αSyn and prions, there is a considerable debate whether they should be referred to as “prions”, “prion-like”, “prionoids”, or something else. Here, the findings supporting the proposed nature of αSyn as a prion and its self-propagation through seeding as well as the transmissibility of neurodegenerative disorders are discussed. The proof of disease causation rests on the concordance of scientific evidence, none of which has provided convincing evidence for the classification of MSA as a prion disease or its human transmission until now.
Keywords: multiple system atrophy, α-synuclein, prion-like spreading, neurodegeneration
1. Introduction
Multiple system atrophy (MSA) is a fatal adult-onset neurodegenerative disorder of uncertain aetiology with a mean incidence of 0.6–0.7 cases per 100,000 person-years. It is clinically featured by various combinations of parkinsonism, cerebellar impairment, autonomic and motor dysfunction due to the degeneration of striatonigral, olivopontocerebellar, and autonomic nervous systems [1,2,3,4] caused by the self-templated misfolding of the protein α-synuclein (αSyn). The core pathological features are fibrillary αSyn-rich glial cytoplasmic inclusions (GCI) mainly involving oligodendroglia [5]. Misfolded αSyn aggregates are also present in Parkinson’s disease (PD) and Lewy body dementia (DLB), which are summarized as α-synucleinopathies [6]. In contrast to PD and DLB, where aggregated αSyn predominantly accumulates within astrocytes and neurons, in MSA, it mainly accumulates within oligodendroglia and to a lesser extent in neurons [7,8,9]. The pathogenic cascade leading to αSyn aggregation and the neurodegeneration of this oligodendroglioneuronal proteinopathy is poorly understood [10,11], but recent studies elucidated the early cellular dysfunction in MSA indicating both increased susceptibility to oxidative stress and disease-related translocation of αSyn to the cell nucleus [12], while others demonstrated mislocalization of myelin-associated oligodendrocyte basic protein (MOBP) and huntingtin protein 1 (HIP1) due to DNA methylation interacting with αSyn in the oligodendrocyte as a pathogenic way of MSA [13]. Converging evidence suggests a “prion-like” spreading of misfolded αSyn “strains” as a pathogenic key event [14,15,16,17,18,19,20,21,22,23,24,25], while others suggested that MSA is a prion disease [26,27,28,29]. The prion hypothesis of human synucleinopathies and the question of whether αSyn is a prion or prion-like are a matter of continuous discussion [15,30,31,32,33,34,35,36,37,38,39,40,41,42]. In both PD and MSA, the debate for and against considering them as prion diseases simply from a prionoid perspective—is ongoing [22,43,44,45,46,47]. This is a critical review of the current data about the prion hypothesis of MSA and other synucleinopathies.
2. Self-Propagation of Prionoids
In a series of articles, Prusiner et al. assessed whether αSyn aggregates may act as a prion disease [26,27,29,46,48,49]. A prion was originally described as “a small proteinaceous infectious particle which is resistant to interaction by most procedures that modify nucleic acids” [50]. Later the definition was updated to “proteins that acquire alternative conformations that become self-propagating”, leaving out the requirement for infectivity [51], while, according to others, prions are composed of self-propagating assemblies of misfolded cellular proteins that encode information, generate neurotoxicity, and evolve and adapt in vivo [52]. The prion hypothesis was initially suggested to be incompatible with the observation that the disease agent was capable of inducing multiple disorders in the absence of nucleic acid [53]. This question was resolved with the introduction of the strain hypothesis, which suggests that a disorder is determined by the conversion of the cellular prion protein (PrPC) into the pathologic isoform PrPSc rather than by maturation in a real viral genome [54,55]. In prion diseases, biochemically different species may be responsible for propagation and toxicity. Recent research has shown that in MSA and other neurodegenerative diseases, the protein misfolding is not exclusive to the conversion of PrPC into PrPSc [56,57]. The recognition that strains have a profound impact across neurodegenerative diseases has provided further insight into their pathogenesis [58,59]. Many observations strengthened the notion that proteins like β-amyloid (Aβ), tau, and αSyn behave like prions. EM structures of recombinant fibrils or patient-derived aggregates containing these proteins show templated misfolding implying that the same mechanisms that induce PrPSc aggregation also enable the self-templating of these pathologic proteins [56,60]. Similarities are present with the αSyn protein responsible for MSA and other synucleinopathies, and mounting genetic, structural, and biological data support the hypothesis that MSA αSyn prions are distinct from those found in LB diseases, including PD [60]. Carboxy truncations of αSyn promote both its aggregation and toxicity [61]. Specific carboxy truncated forms of αSyn have been detected by immunostaining with antibodies that specifically react with their precise forms showing their specific distribution [41]. In DLB, neuronal inclusions in the SN and amygdala were positive for αSyn cleaved after residues 103, 119, 122, and 125, whereas in MSA GCIs αSyn truncated at residues 103, 115, 119, and 125 were present. In the pontine nuclei, MSA NCIs were reactive to the αSyn x-122 neo-epitope but negative for 103 cleavage. These data demonstrate significant disease-, region-, and cell type-specific differences in carboxy-terminal αSyn in pathological inclusions that may contribute to their distinct strain-like prion properties in the different synucleinopathies, but they also may be related to different prion-like conformational species with various sequences responsible for cleavage [40]. Proteinase K digestion is used to demonstrate the signature of different prion-like protein strains. Altered cleavage profiles due to structural differences are consistent with MSA αSyn profiles structurally different from those in DLB [62]. The differences of the αSyn strains are responsible for the heterogeneity of pathological features and disease processes among synucleinopathies [63] and have been recently proposed to contribute to the more aggressive progression of MSA [64].
Using this modern definition, αSyn aggregates can be classified as prions as they are able to self-multiply during disease, leading to cellular transmission and the spreading of protein aggregates [65,66]. Endogenous αSyn can aggregate through a homotypic (self-seeding) or a heterotypic seeding [67,68], the latter referring to the involvement of other proteins in the initiation of αSyn aggregation [69,70]. The essential point of the prion hypothesis is the notion that self-propagating αSyn aggregates are able to escape from a cell, enter a neighboring one, and then act as a seed to introduce the aggregation of αSyn in the recipient cell [71]. The internalization of the pathogenic conformation of αSyn facilitates its spreading from neuron to neuron [72,73]. αSyn oligomers are internalized primarily through endocytosis [74]. The seeding activity of pathological αSyn species is thought to mediate a process of mutation-dependent aggregation, self-templated propagation of the pathological protein state [32,75]. Intraneuronal αSyn aggregates are triggered by internalized small fibers that do not contact membranes directly, suggesting that this mechanism is relevant to the spreading of aggregated pathologies [76]. Although the molecular mechanisms responsible for spreading pathologic αSyn are poorly understood, a growing body of evidence indicates that de novo misfolding and/or neuronal internalization of aggregated αSyn facilitates conformational templating of endogenous αSyn monomers in a prion-like manner [77]. Recent studies demonstrating that cellular prion protein (PrPC) mediates αSyn uptake, localization, and toxicity in vitro and in vivo confirmed previous results which showed that PrPC internalizes soluble misfolded αSyn, indicating its important role in its internalization required for the intercellular spread of αSyn [78]. Cell intrinsic features also may play a critical role in the formation of pathologic αSyn, such as mechanisms that increase endogenous αSyn levels, selective expression profiles in distinct neuron types, altered function of proteins involved in αSyn synthesis and degradation, and oxidative stress. The cell-intrinsic mechanisms that trigger αSyn aggregation and facilitate the conversion of αSyn to a fibrillar pathway to assemble into Lewy bodies, and others, may be important for its self-propagation [79]. Aggregation and propagation in the brain and peripheral organs suggested that αSyn as a prionoid is transmitted from the periphery to the brain via specific pathways [47]. This non-cell-autonomous mechanism was suggested to be similar to that in prion diseases such as Creutzfeldt-Jakob disease, where the prion protein (PrP) in its misfolded form catalyzes the conformational conversion of normal PrP into additional copies of the misfolded PrP (PrPSc) [80]. The ability of prions to self-propagate allows them to spread within host tissues and underlies the transmissible nature of the prion disorders with the capacity to spread between individuals or species [81]. Various proteins have been shown to spread between cells and tissues of the host (for reviews see [82,83,84]), but there is no clear evidence of transmission between individuals, at least by artificial or natural routes [85,86,87]. As the risk of clinical transmission of proteinopathies between humans is critically evaluated, the scenarios for PrP-based prion diseases and their many experimental models must be considered. Not all prion diseases can “spread” horizontally between individuals. Thus, human prion diseases are not contagious in this manner, in contrast to scrapie and others. PrP-based diseases can represent deadly examples of transmissible proteinopathies. However, the ability of an ordered protein assembly to propagate in vitro or in vivo does not necessarily mean that they are transmissible by any casual contacts or medical procedures, or even if they are, that a disease may definitely result from that transmission [86]. There is currently no evidence that αSyn-dependent PD or MSA can be transmitted from person to person [37,85,88]. Many scientists described such intermediate self-propagating protein states as “prion-like”, while others prefer to simply call them all prions [49,89]. The proposed prion-like mechanisms (prionoid) would be restricted to proteins with or among adjacent cells [90], whereas others commented on certain similarities between prion and prionoid and the possibility of prion-like transmissibility of some prionoid strains [91]. Their role in the pathogenesis of neurodegenerative diseases has been critically reviewed [23,92].
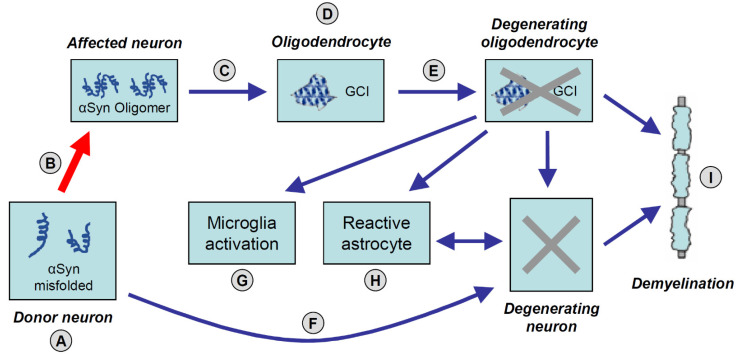
This mechanism would include the misfolding (from α-helix to β-sheet) of native proteins that aggregate into “seeds” that structurally have the capacity to corrupt proteins in their physiological conformation and induce their misfolding. Aggregated αSyn moreover can disrupt glial function, thus contributing to neurodegeneration through various pathways [93]. This process would spread in a chain reaction of misfolding and aggregation ranging from oligomers to large masses of pathologic proteins, leading to neurodegeneration, glial activation, and demyelination [94,95] (Figure 1).
Figure 1.
Suggested accumulation and spreading mechanisms of pathological α-synuclein (αSyn) leading to neurodegeneration in MSA. (A) Aggregation of αSyn as tetramers and oligomers in neurons. (B) “Prion-like” cell-to-cell spreading of abnormally folded αSyn via exosomes (red arrow). (C) αSyn aggregates and oligomers released from affected neurons into the extracellular space through exocytosis. (D) αSyn aggregation in oligodendroglial cytoplasm. (E) Formation of characteristic glial cytoplasmic inclusions (GCIs) in oligodendroglia containing αSyn and p25α. (F) Prionoid material can be transmitted directly across synapses causing neurodegeneration. (G) Activated micro-/astroglial cells by cytokines released from damaged oligodendrocytes and degenerating neurons. (H) Aggregation of αSyn (or other prionoid material) in astrocytes that are also activated by neuronal loss. (I) Demyelination caused by damaged oligodendroglia and related to degenerating neurons.
It has been suggested to change the definition of prions to “proteinaceous nucleating particle”, to avoid the infectious implication and to highlight the molecular action of the agents [83]. The term “propagon” has also been suggested to denominate these proteins that act like prions [96]. Both evidence of self-templating propagation in cellular cultures, animal models, and in humans are required to define MSA as a prion disease [97].
3. In Vivo and In Vitro Data
The theory that the prion-like mechanisms may underlie neurodegenerative disorders was supported by the demonstration of Lewy pathology after therapeutic transplantation in people with PD, which was discovered not only in the host neurons but also in grafted dopamine neurons about two decades after transplantation. It was hypothesized that prion-like transfer of αSyn might underlie the unexpected pathology in these patients [98,99,100]. Earlier work demonstrating the presence of extracellular αSyn in human plasma and cerebrospinal fluid suggested that αSyn could enter cells from extracellular space [101]. These results as well as many studies using cell culture and animal models gave support to the prion-like hypothesis of the intercellular transfer of αSyn [20,27,102]. In PD brains, αSyn accumulates within neurons and propagates from cell to cell in a prion-like manner [15]. Transmission of pathological αSyn (and tau) between anatomically connected brain regions underlies the stereotypical spread of these and other pathological proteins [59], but it is modulated by the selective vulnerability of cell types and predicted by networks analysis [103]. The spread of αSyn pathology from one cell to another not only within the central nervous system (CNS) and even from one nervous system structure to another in vivo as well as from peripheral locations (such as the gut or olfactory networks into the CNS [47]) has already been convincingly demonstrated [14,20,27,98,104,105,106,107]. Prion-like αSyn propagation has been widely studied in PD [18], where there is evidence of misfolded αSyn in neural cell cultures [108,109]. This spreading pattern resembling that of prions has led to the concept of prion-like propagation of αSyn and tau [52,98,110,111,112], and the Aβ-induced acceleration of tau pathology spreading and its association with prion protein [113,114] (for rev. see [23,39]). This hypothesis in humans is well documented in AD [115], although it is still a hypothesis, like the amyloid cascade [52].
The inoculation of wild-type (WT) mice with synthetic mouse αSyn pre-formed fibrils (PFFs) into striatum induced widespread αSyn pathology and dopaminergic neuronal loss in the substantia nigra compacta, whereas αSyn knockout mice inoculated with PFFs did not develop αSyn pathology [68]. This suggested that endogenous αSyn was necessary to propagate αSyn pathology. Intracerebral injection of sarkosyl-insoluble αSyn homogenates from DLB brain induced αSyn pathology in WT mice [116]. While exogenous human αSyn disappeared a week after inoculation, endogenous mouse αSyn was converted into a pathological form and accumulated in neurons through a prion-like mechanism at three months post-inoculation [117]. Propagation of hyperphosphorylated αSyn occurred along neuronal circuits and involved trans-synaptic transport mechanisms, suggesting that exogenous pathological αSyn can propagate in a neuron-to-neuron manner, but was not associated with motor deficits. A recent review described modeling αSyn propagation with PFF injections and the outcome of these models [118]. The self-propagation of αSyn oligomers in vitro, however, is not sufficient to define them as prions, because they show “seeding” activity rather than infectivity of αSyn [37]. Disease-associated αSyn adopts a conformation that induces it to form oligomers and fibrils with reduced solubility. They become hyperphosphorylated, and contribute to the spatiotemporal spreading of pathology in the CNS, but how the uptake of αSyn varies with the size of oligomers is less clear [74]. The binding of αSyn oligomers to the gap junction protein connexin-32 (Cx32) has been shown to facilitate protein uptake and transfer in neurons and oligodendrocytes [119]. Oligodendroglial p25α is suggested to be responsible for generating a highly pathological αSyn strain in MSA [120]. Oral or intravenous transmission of αSyn fibrils to TgM83+/− mice induces a progressive synucleinopathy associated with overt motor dysfunction and pathological deposition of αSyn aggregates within the brain [121]. Hemizygous TgM83 mice do not develop α-synucleinopathy spontaneously, allowing them to detect disease transmission following extended incubation periods of more than one year post-inoculation. Inoculation studies using homogenates from MSA brain regions lacking detectable αSyn pathology also transmitted neurological lesions to mice indicating that αSyn prion formation precedes neuropathology in the brain, suggesting that the lesions are not limited to affected brain regions [25]. This clearly indicates that αSyn fibrils, like prions, can neuroinvade the CNS after a single oral or intravenous challenge and cause neuropathology. These findings are in agreement with studies demonstrating a cerebral synucleinopathy in transgenic (tg) mice following a peripheral inoculation with αSyn aggregates [122,123,124,125]. Peripheral application of αSyn fibrils that can induce cerebral αSyn pathology suggests that they possess the innate ability to propagate from the periphery to the brain [47,126]. This appears important for understanding the pathogenesis of PD, as it has been speculated that synucleinopathies may originate in the gut before propagating to the brain in a prion-like manner [127].
The intrastriatal injection of PFF αSyn in rodent brains induced a PD-like propagation of Lewy body (LB) pathology together with significant nigrostriatal neurodegeneration. The injection of exogenous αSyn PFFs into the putamen of non-human primates (cynomolgus monkeys) resulted in a significant reduction in dopaminergic neurons in the ipsilateral substantia nigra (−29.3%), downregulation of the dopamine markers tyrosine hydroxylase and Nurr1, associated with LB-like intraneuronal αSyn-positive inclusions; all taken together indicative of early PD [67]. Previous studies using αSyn containing LB-enriched fractions from PD patients into the striatum of macaques showed similar results with the accumulation of αSyn pathology within host neurons and neurodegeneration beginning in dopaminergic terminals, over the course of 12–14 months involving related regions in a PD-like distribution [128]. The injection of synthetic human αSyn fibrils in the striatum of macaque monkeys (Macaca fuscata) showed into the left striatum of Macaca fuscata induced LBs, massive αSyn + NVIs and neurites in the left striatum, some NCIs and neurites in the left SN and bilateral frontal cortex associated with mild neuronal loss and gliosis, while other brain areas were not affected. These results indicated that abnormal αSyn fibrils propagate throughout the brain via projection, association, and commissural fibers, though the progression of αSyn pathology was limited [129]. The intracerebral injection of synthetic αSyn fibrils into adult WT marmoset brains caused abundant αSyn pathologies within only three months post-injection. Robust LB-like inclusions were formed in tyrosine hydroxylase-positive neurons associated with a significant decrease in these neurons suggesting the retrograde spreading of abnormal αSyn and its neurotoxicity [130]. These studies indicate that exogenous αSyn is internalized by dopaminergic terminals, spread to the substantia nigra, and induces PD-like pathology including αSyn aggregation and nigral neuronal degeneration. This supports the ability of abnormal αSyn to propagate to distant brain regions and to trigger neurodegeneration.
4. Prion-Like Properties of αSyn
αSyn has a prion-like property, the propensity to aggregate, that converts its physiological protein conformation into a pathogenic one, forming disease-causing fibrils. The aggregation of these fibrils and subsequent inclusion formations are suggested to interfere with vesicular trafficking and organelle functions in neurons [131]. However, the applicability of the prion hypothesis in α-synucleinopathies remains controversial [45,97]. Although some research groups showed the “infectious” activity of LB extracts from PD brains in mice and also in monkeys [128], demonstrating the prion propensities of αSyn assemblies [44], so far there is no evidence of pathologic αSyn aggregate transmission between individuals leading to the prion-like definition to make a distinction between this protein and actively infectious prions [132]. Several studies demonstrated that pathological αSyn aggregates of GCIs have distinct conformational and biological activities both in vitro and in vivo from those of LBs [26,133,134]. This indicates that αSyn from MSA has a different conformation and a much higher spreading potential than that from PD [133,134]. Injections of MSA brain lysates failed to replicate the oligodendroglial αSyn pathology, raising questions about the pathogenesis of oligodendroglial αSyn aggregates in MSA [72]. WT mice injected with mouse αSyn PFFs developed neuronal αSyn pathology after short post-injection (PI) intervals on the scale of weeks, while oligodendroglial αSyn pathology emerged after longer PI intervals of several months [135]. The protease activity profiles of oligodendrocytes may be distinctive from that of neurons, resulting in the differential αSyn cleavage products responsible for their higher pathogenicity of oligodendroglial αSyn prion-like strains. This would be consistent with a low αSyn expression in oligodendrocytes [136], but that αSyn pathology predominantly spreads in these cells in MSA. It is preferentially propagated in oligodendrocytes despite their lower αSyn expression levels due to a favorable cleavage environment that produces the more potent strains. Aberrant protease activities in MSA could exacerbate this process, but this should be confirmed by future experiments [41]. Both soluble and insoluble fractions of MSA extracts have robust seeding activity, while only the insoluble fraction of PD extracts displayed seeding activity. MSA-seeded inclusions differed from PD-seeded inclusions persisting upon propagation of aggregation to second-generation biosensor cells. It was concluded that PD and MSA feature αSyn conformers with distinct biochemical properties that can be transmitted to αSyn monomers in a cell system. These findings are consistent with the assumption that distinct αSyn strains underlie PD and MSA [137]. The observation that familial Parkinson’s point mutation abolishes MSA prion replication also established that MSA αSyn “prions” are conformationally distinct from the misfolded αSyn in PD [48].
The intracerebral injection of insoluble αSyn into WT mice induced prion-like propagation of phosphorylated αSyn pathology even one month after injection, while injection into αSyn-knockout mice failed to induce any pathology [71]. Abundant oligodendroglial αSyn pathology in white matter developing later was reminiscent of that in MSA. Comparison between young and aged mice injected with mouse αSyn PFFs revealed that PI intervals rather than aging corresponded with oligodendroglia αSyn aggregation in MSA [135]. While these studies indicate that oligodendroglial αSyn pathology can be replicated in WT mice and provide novel insights into the pathological mechanisms of oligodendroglial αSyn aggregations in MSA, the seeded assembly of recombinant human αSyn in vitro did not replicate the structures of αSyn filaments from MSA. This suggests that additional, as yet unknown factors may be essential for the prion-like spreading of αSyn proteinopathies [138]. In MSA, intracerebral inoculation studies in non-human primates, to the best of our knowledge, have not been performed yet.
Homozygous tg mice expressing human αSyn with A53T mutation, termed TgM83+/+ mice, spontaneously develop motor deficits at about one year of age, along with widespread αSyn pathology [139]. Inoculations of young asymptomatic TgM83+/+ mice with brain homogenates from old TgM83+/+ mice induced motor dysfunction [29]. Both intracerebral and peripheral inoculation of brain homogenates from MSA but not from PD patients, were able to produce αSyn pathology in TgM83+/− (hemizygous for the transgene), but not TgM83−/− WT mice, suggesting that different αSyn strains may have different seeding characteristics [27,29,48]. This suggests that αSyn strains different from those observed in PD may be the causative mechanism of MSA [73,137]. It further means that TgM83 mice probably cannot be considered a valid animal model for MSA. This was supported by the fact that Lewy pathology but not MSA-typical GCI pathology was induced by inoculation in αSyn TgM83+/− hemizygous mice, but not in WT mice, suggesting that the A53T αSyn mutation in the SNCA gene plays a critical role in αSyn spreading and self-propagation in this model system. A53T mutation is probably not involved in MSA, but the model is nevertheless responsive to MSA homogenates and, therefore, can be used to study how MSA αSyn adapts, which could be a valid research question, comparable to studies in the prion field where, e.g., hamster prions have been inoculated into mice. Homogenate from diseased MSA patients readily transmits neurodegeneration to TgM83+/− mice [29]. This provided the opportunity to determine if αSyn adopts an alternative confirmation that undergoes self-propagation, which was suggested to become a prion. Nineteen MSA patient samples from three continents transmitted disease to TgM83+/− mice, while those from PD patients did not [26]. Using human embryonic kidney (HEK293T) cells expressing mutated αSyn fused to a yellow fluorescent protein (αSyn140*A53T-YFP). They infected the cells with αSyn isolated from MSA brains but not from PD samples [26]. Studies showing conformational and biological differences between aggregated αSyn species in PD and MSA indicated that the cellular environment affects the aggregation process of αSyn [26,133,134]. The existence of two distinct strains in MSA and PD patients is consistent with the findings that αSyn misfolding into distinct conformations produces differing pathophysiological effects [140,141]. These and other findings suggest that specific strains of αSyn aggregates are responsible for each disease, and may underlie the pathological and clinical diversity of α-synucleinopathies [142,143]. The inoculation of tg mice with different strains of recombinant or brain-derived αSyn aggregates produced clinically and pathologically distinct diseases [56], suggesting that both prion-like spreading and selective vulnerability contribute to the temporal and spatial evolution of αSyn pathology within the brain. Thus, pathogenic αSyn exhibits key hallmarks of prion strains, which provides evidence that disease heterogeneity among the synucleinopathies is caused by distinct αSyn strains [39,63].
The mechanisms of the seeding of αSyn pathology in the nervous system are defined by several factors that can differently influence the pathology among strains, thereby causing distinct disease entities [60,64,144,145]. Therefore, it may be necessary to use disease-specific aggregates in such experiments. In prion disorders, approaches targeting PrPC oligomers are developed based on the observation that only oligomers, not monomers, are infectious [22].
5. Multiple System Atrophy: A “Prion” Disease?
There are several challenges to the hypothesis that MSA is a prion-disease. First, endogenous WT αSyn is insufficient to propagate αSyn pathology and mutated αSyn is needed as a template. Tg Nbm mice inoculated with the PFFs of brain homogenates from MSA brains developed “prions”, while the control sample did not. Transmission of αSyn “prions” to a second synucleinopathy model and their ability to propagate between two distinct mouse lines while retaining strain-specific properties were suggested to provide compelling evidence that MSA is a prion disease [28]. Another recent study showed that MSA brain lysates contain sufficient seeding activity to induce αSyn inclusion pathology following neonatal injection in TgM83+/− mice, probably inducing several mechanisms besides conformational templating such as the disruption of normal protein homeostasis and neuroinflammatory reactions. Tg(SNCA*A53T(+/+))Nbm mice developed αSyn pathology in neurons and astrocytes throughout the limbic system, which is in contrast to MSA-inoculated TgM83(+/−) mice, which developed exclusively neuronal αSyn aggregations in the hindbrain that caused motor deficits with advanced disease. In crossover experiments, TgM83(+/−) mice inoculated with mouse-passaged control samples had no effect. The same was seen in that mouse-passaged MSA samples induced αSyn formation in Tg(SNCA*A53T(+/+))Nbm mice, but not in controls. The confirmed transmission of αSyn “prions” to a second synucleinopathy model and the ability to propagate prions between two distinct mouse lines while retaining strain-specific properties were suggested to provide compelling evidence that MSA is a prion disease [28]. However, these and other transmission studies could not explain why in MSA αSyn pathology predominantly accumulates in oligodendroglia as MSA-derived αSyn does not have the ability to induce strain-like cell-specific αSyn aggregation. The intrinsic properties of the A53T αSyn in the TgM83 mouse model have been shown to dominate over any strain features harbored by misfolded αSyn in MSA brains [146]. Previous studies indicated that the “prion-like” progression of synucleinopathy in TgM83 mice depends on the mouse genotype (levels of αSyn expression by the mouse) and type of inoculum [147]. Replication of MSA “prions” in primary astrocyte cultures from TG mice expressing human αSyn showed that human αSyn forms distinct inclusion morphologies and propagates within cultured Tg astrocytes exposed to MSA αSyn. This indicates that αSyn expression dominates the tropism of inclusion formation in certain cells, elucidating the role of astrocytes in the pathogenic mechanism of MSA neurodegeneration [148].
Furthermore, GCIs, the morphological hallmarks of MSA, have never been identified in WT mouse brains inoculated with MSA-derived αSyn [29]. In fact, αSyn aggregates (“prions”) derived from MSA patients generated a neurodegenerative pattern that is atypical for MSA [37]. These, and other findings, indicate that αSyn in MSA may be different from that in PD in “prion-like” properties. The fact that the in vivo phenotype has been observed only from inoculating MSA samples into TgM83+/− mice, but not into WT mice [26] needs further investigation in order to better elucidate this particular issue. However, recent studies have shown that MSA “prions” retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Moreover, the mouse-passaged MSA samples induced αSyn motor deficits despite showing αSyn pathology [68,116]. Tg mice expressing WT αSyn developed αSyn deposition at six months of age but no motor deficits [72]. Second, phosphorylated αSyn aggregates, the morphological hallmarks of MSA, were not detected in oligodendrocytes in MSA-inoculated TgM83+/− mice [26,72]. Third, the intracerebral injection of homogenates from spinal cord tissue from naive motor-impaired TgM83+/+ mice induced robust αSyn pathology that mimics the prion-like pathological changes that occur in TgM83 mice when purified αSyn PFFs are injected to accelerate disease onset, suggesting non-prion-type transmission in A53/αSyn tg mice [149]. Fourth, while αSyn strains may exist, no study has definitely propagated patient-derived seeds from cell-to-cell or mouse-to-mouse, nor fully characterized αSyn strains from MSA vs. PD [137]. Furthermore, they have failed to propagate distinct αSyn conformers in clonal lines as has been shown for tau [150], because aggregate-containing clones lose their aggregation state over time. The variety of seeds, animal models, and methodologies currently prevents clear conclusions regarding αSyn-related spreading and toxicity, as well as translation of preclinical findings to human disease [38]. In recent years, many studies have shown that prion-like proteins share not only the prion replication paradigm but also the specific ability to aggregate in different conformations, i.e., strains, with relevant clinical, pathological, diagnostic, and therapeutic implications, related to the molecular basis of the strain phenomenon in prion and prion-like proteins [39]. To confirm the existence of bona fide αSyn prion strains, it will be necessary to test whether distinct structures propagate through living systems and produce consistent pathology as do tau and PFFs. Further studies will be necessary to define the species that induce αSyn aggregation.
Another problem is the relation between PrPC and αSyn. PrPC knockdown in neuroblastoma cells was shown to attenuate the uptake of recombinant αSyn oligomers with a similar effect observed when comparing αSyn uptake in mouse primary hippocampal neurons, prepared from WT or PrPC knockout mice. The latter developed lower levels of αSyn aggregates in the cortex, striatum, thalamus, and hippocampus, suggesting that PrPC may facilitate the uptake and aggregation of αSyn oligomers [151]. They further showed that the replication of scrapie prions was blocked by αSyn oligomers [152], providing a possible explanation for the observation that Creutzfeldt-Jakob disease patients have a more protracted disease course when there is concomitant synucleinopathy [153]. However, another study found no evidence of binding between PrPC and αSyn oligomers and noted that PrPC neither binds to αSyn oligomers nor mediates their detrimental effects [154]. It could be suggested that there may be different species of αSyn oligomers, which have a different binding capacity with PrPC, and it is possible that future studies could demonstrate that both PrPC-dependent and -independent pathways could play a role in the pathogenesis of synucleinopathies [155]. Accordingly, aggregated αSyn may be potent in the cross-seeding of prion protein misfolding and aggregation in vitro, producing self-replicating states that can lead to prion diseases upon serial passaging in WT animals [156]. On the other hand, the presence of PrPSc was required to promote the efficient internalization and spreading of abnormal αSyn between cells. However, recent studies showed that PrPSc was able to efficiently propagate in the brain of animals even in the absence of αSyn, suggesting that this protein may not act as a key modulator of prion propagation. This suggests that αSyn may take part in this process of self-propagation but is not specifically required for sustaining prion conversion and propagation [157]. Prion strains can interfere with each other, influencing the emergence of a dominant strain, and both environmental and host factors may influence the evolution and distribution of prion strains within a population [158]. Moreover, gene analyses have shown that the homozygous state of positions 129 in the PRNP gene is not a risk factor for MSA and no other variants in the PRNP gene were associated with increased risk for MSA [159]. A review of the clinical histories of patients, who had died of MSA or PD, showed no evidence of neurosurgical transmission [160]. Furthermore, studies of couples whose spouses had autopsy-confirmed PD, PSP, or MSA, did not suggest an increased risk of α-synucleinopathy development in the other spouses [161,162]. Up to the present, there is no evidence of iatrogenic transmission of autopsy-confirmed MSA cases. The current absence of evidence, however, is not evidence of the absence of human transmission of misfolded proteins other than prions and Aβ. In view of the importance of this question, the potential for non-invasive human transmission of proteinopathic neurodegenerative disorders needs further research [163] before any conclusion can be drawn [23].
In conclusion, one may postulate that even if the prion-like spreading of αSyn in experimental model systems may justify the view that the progression of neurodegeneration in MSA reflects the cell-to-cell spread of pathological αSyn, this is not sufficient to define MSA as a classical prion disease. After the injection of αSyn fibrils into the olfactory bulb of WT mice, despite the transneuronal spreading of αSyn aggregates to over 40 other brain regions, even at 18 months PI, there was no loss of mitral cells in the olfactory bulb. The lack of progression of αSyn pathology may be due to the compromise of the neuronal circuitry, and the activation of proteolytic mechanisms in resilient neurons may counterbalance the spread of pathogenic αSyn [164]. This underlines the interpretation that prion-like particles and prions are different entities, and that a more precise definition of both, which is capable of differentiating them from one another, is necessary [37]. Initial work on many prion studies has been hampered by incomplete or atypical transmissions, which were then refined with the advent of better animal models, often as tg mouse models. However, in view of the limited availability of human brain material, it is indispensable to develop new methodologies that enable the production of sufficient amounts of disease-specific aggregates for research in order to enable a deeper understanding of the molecular mechanisms underlying the pathogenesis of MSA—and other synucleinopathies—and to develop novel therapeutic strategies to target αSyn aggregation and disease progression in MSA. Therefore, future work may lead to better transmissions for MSA-causing αSyn conformers once a suitable animal model has been found.
Acknowledgments
The author thanks E. Mitter-Ferstl, for secretarial and graphical work.
Abbreviations
| Aβ | β-amyloid |
| αSyn | α-synuclein |
| CNS | central nervous system |
| DLB | Lewy body dementia |
| GCIs | glial cytoplasmic inclusions |
| LB | Lewy body |
| LB | Lewy body |
| MSA | Multiple system atrophy |
| PD | Parkinson’s disease |
| PFFs | pre-formed fibrils |
| PI | post-injection |
| PrPC | cellular prion protein |
| PrPSc | pathologic PrP isoform |
| tg | transgenic |
| WT | wild-type |
Author Contributions
K.A.J. wrote the manuscript; G.K.W. contributed ideas and supervised the manuscript; N.S. contributed comments and suggestions for the manuscript revision. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jellinger K.A. Neuropathological findings in multiple system atrophy with cognitive impairment. J. Neural Transm. 2020;127:1031–1039. doi: 10.1007/s00702-020-02201-2. [DOI] [PubMed] [Google Scholar]
- 2.Krismer F., Wenning G.K. Multiple system atrophy: Insights into a rare and debilitating movement disorder. Nat. Rev. Neurol. 2017;13:232–243. doi: 10.1038/nrneurol.2017.26. [DOI] [PubMed] [Google Scholar]
- 3.Gilman S., Wenning G.K., Low P.A., Brooks D.J., Mathias C.J., Trojanowski J.Q., Wood N.W., Colosimo C., Durr A., Fowler C.J., et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71:670–676. doi: 10.1212/01.wnl.0000324625.00404.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campese N., Fanciulli A., Stefanova N., Haybaeck J., Kiechl S., Wenning G.K. Neuropathology of multiple system atrophy: Kurt Jellinger’s legacy. J. Neural Transm. 2021 doi: 10.1007/s00702-00021-02383-00703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trojanowski J.Q., Revesz T. Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol. Appl. Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 6.Spillantini M.G., Goedert M. Synucleinopathies: Past, present and future. Neuropathol. Appl. Neurobiol. 2016;42:3–5. doi: 10.1111/nan.12311. [DOI] [PubMed] [Google Scholar]
- 7.Koga S., Dickson D.W. Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J. Neurol. Neurosurg. Psychiatry. 2018;89:175–184. doi: 10.1136/jnnp-2017-315813. [DOI] [PubMed] [Google Scholar]
- 8.Kaji S., Maki T., Kinoshita H., Uemura N., Ayaki T., Kawamoto Y., Furuta T., Urushitani M., Hasegawa M., Kinoshita Y., et al. Pathological endogenous alpha-synuclein accumulation in oligodendrocyte precursor cells potentially induces inclusions in multiple system atrophy. Stem Cell Rep. 2018;10:356–365. doi: 10.1016/j.stemcr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cykowski M.D., Coon E.A., Powell S.Z., Jenkins S.M., Benarroch E.E., Low P.A., Schmeichel A.M., Parisi J.E. Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain. 2015;138:2293–2309. doi: 10.1093/brain/awv114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monzio Compagnoni G., Di Fonzo A. Understanding the pathogenesis of multiple system atrophy: State of the art and future perspectives. Acta Neuropathol. Commun. 2019;7:113. doi: 10.1186/s40478-019-0730-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellinger K.A. Multiple system atrophy: An oligodendroglioneural synucleinopathy. J. Alzheimers Dis. 2018;62:1141–1179. doi: 10.3233/JAD-170397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herrera-Vaquero M., Heras-Garvin A., Krismer F., Deleanu R., Boesch S., Wenning G.K., Stefanova N. Signs of early cellular dysfunction in multiple system atrophy. Neuropathol. Appl. Neurobiol. 2021;47:268–282. doi: 10.1111/nan.12661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bettencourt C., Miki Y., Piras I.S., de Silva R., Foti S.C., Talboom J.S., Revesz T., Lashley T., Balazs R., Viré E., et al. MOBP and HIP1 in multiple system atrophy: New alpha-synuclein partners in glial cytoplasmic inclusions implicated in the disease pathogenesis. Neuropathol. Appl. Neurobiol. 2021;47:640–652. doi: 10.1111/nan.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peelaerts W., Bousset L., Baekelandt V., Melki R. Alpha-synuclein strains and seeding in Parkinson’s disease, incidental Lewy body disease, dementia with Lewy bodies and multiple system atrophy: Similarities and differences. Cell Tissue Res. 2018;373:195–212. doi: 10.1007/s00441-018-2839-5. [DOI] [PubMed] [Google Scholar]
- 15.Vargas J.Y., Grudina C., Zurzolo C. The prion-like spreading of alpha-synuclein: From in vitro to in vivo models of Parkinson’s disease. Ageing Res. Rev. 2019;50:89–101. doi: 10.1016/j.arr.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Vasili E., Dominguez-Meijide A., Outeiro T.F. Spreading of alpha-synuclein and tau: A systematic comparison of the mechanisms involved. Front. Mol. Neurosci. 2019;12:107. doi: 10.3389/fnmol.2019.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tarutani A., Arai T., Murayama S., Hisanaga S.I., Hasegawa M. Potent prion-like behaviors of pathogenic alpha-synuclein and evaluation of inactivation methods. Acta Neuropathol. Commun. 2018;6:29. doi: 10.1186/s40478-018-0532-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visanji N.P., Brooks P.L., Hazrati L.N., Lang A.E. The prion hypothesis in Parkinson’s disease: Braak to the future. Acta Neuropathol. Commun. 2013;1:2. doi: 10.1186/2051-5960-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dhillon J.S., Trejo-Lopez J.A., Riffe C., McFarland N.R., Hiser W.M., Giasson B.I., Yachnis A.T. Dissecting alpha-synuclein inclusion pathology diversity in multiple system atrophy: Implications for the prion-like transmission hypothesis. Lab. Investig. 2019;99:982–992. doi: 10.1038/s41374-019-0198-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steiner J.A., Quansah E., Brundin P. The concept of alpha-synuclein as a prion-like protein: Ten years after. Cell Tissue Res. 2018;373:161–173. doi: 10.1007/s00441-018-2814-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karpowicz R.J., Jr., Trojanowski J.Q., Lee V.M. Transmission of alpha-synuclein seeds in neurodegenerative disease: Recent developments. Lab. Investig. 2019;99:971–981. doi: 10.1038/s41374-019-0195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brás I.C., Outeiro T.F. Alpha-synuclein: Mechanisms of release and pathology progression in synucleinopathies. Cells. 2021;10:375. doi: 10.3390/cells10020375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaunmuktane Z., Brandner S. The role of prion-like mechanisms in neurodegenerative diseases. Neuropathol. Appl. Neurobiol. 2020;46:522–545. doi: 10.1111/nan.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veys L., Van Houcke J., Aerts J., Van Pottelberge S., Mahieu M., Coens A., Melki R., Moechars D., De Muynck L., De Groef L. Absence of uptake and prion-like spreading of alpha-synuclein and tau after intravitreal injection of preformed fibrils. Front. Aging Neurosci. 2021;12:614587. doi: 10.3389/fnagi.2020.614587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woerman A.L., Patel S., Kazmi S.A., Oehler A., Lee J., Mordes D.A., Olson S.H., Prusiner S.B. Kinetics of alpha-synuclein prions preceding neuropathological inclusions in multiple system atrophy. PLoS Pathog. 2020;16:e1008222. doi: 10.1371/journal.ppat.1008222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prusiner S.B., Woerman A.L., Mordes D.A., Watts J.C., Rampersaud R., Berry D.B., Patel S., Oehler A., Lowe J.K., Kravitz S.N., et al. Evidence for alpha-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. USA. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woerman A.L., Watts J.C., Aoyagi A., Giles K., Middleton L.T., Prusiner S.B. Alpha-synuclein: Multiple system atrophy prions. Cold Spring Harb. Perspect. Med. 2018;8:a024588. doi: 10.1101/cshperspect.a024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woerman A.L., Oehler A., Kazmi S.A., Lee J., Halliday G.M., Middleton L.T., Gentleman S.M., Mordes D.A., Spina S., Grinberg L.T., et al. Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol. 2019;137:437–454. doi: 10.1007/s00401-019-01959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watts J.C., Giles K., Oehler A., Middleton L., Dexter D.T., Gentleman S.M., DeArmond S.J., Prusiner S.B. Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA. 2013;110:19555–19560. doi: 10.1073/pnas.1318268110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chu Y., Kordower J.H. The prion hypothesis of Parkinson’s disease. Curr. Neurol. Neurosci. Rep. 2015;15:28. doi: 10.1007/s11910-015-0549-x. [DOI] [PubMed] [Google Scholar]
- 31.Ma J., Gao J., Wang J., Xie A. Prion-like mechanisms in Parkinson’s disease. Front. Neurosci. 2019;13:552. doi: 10.3389/fnins.2019.00552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melki R. Alpha-synuclein and the prion hypothesis in Parkinson’s disease. Rev. Neurol. 2018;174:644–652. doi: 10.1016/j.neurol.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Olanow C.W. Do prions cause Parkinson disease? The evidence accumulates. Ann. Neurol. 2014;75:331–333. doi: 10.1002/ana.24098. [DOI] [PubMed] [Google Scholar]
- 34.Rey N.L., George S., Brundin P. Review: Spreading the word: Precise animal models and validated methods are vital when evaluating prion-like behaviour of alpha-synuclein. Neuropathol. Appl. Neurobiol. 2016;42:51–76. doi: 10.1111/nan.12299. [DOI] [PubMed] [Google Scholar]
- 35.Tamguney G., Korczyn A.D. A critical review of the prion hypothesis of human synucleinopathies. Cell Tissue Res. 2018;373:213–220. doi: 10.1007/s00441-017-2712-y. [DOI] [PubMed] [Google Scholar]
- 36.Leak R.K., Frosch M.P., Beach T.G., Halliday G.M. Alpha-synuclein: Prion or prion-like? Acta Neuropathol. 2019;138:509–514. doi: 10.1007/s00401-019-02057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wenning G., Trojanowski J.Q., Kaufmann H., Wisniewski T., Rocca W.A., Low P.A. Is multiple system atrophy an infectious disease? Ann. Neurol. 2018;83:10–12. doi: 10.1002/ana.25132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meissner W.G., Fernagut P.O., Dehay B., Peran P., Traon A.P., Foubert-Samier A., Lopez Cuina M., Bezard E., Tison F., Rascol O. Multiple system atrophy: Recent developments and future perspectives. Mov. Disord. 2019;34:1629–1642. doi: 10.1002/mds.27894. [DOI] [PubMed] [Google Scholar]
- 39.Scialò C., De Cecco E., Manganotti P., Legname G. Prion and prion-like protein strains: Deciphering the molecular basis of heterogeneity in neurodegeneration. Viruses. 2019;11:261. doi: 10.3390/v11030261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hass E.W., Sorrentino Z.A., Lloyd G.M., McFarland N.R., Prokop S., Giasson B.I. Robust a-synuclein pathology in select brainstem neuronal populations is a potential instigator of multiple system atrophy. Acta Neuropathol. Commun. 2021;9:80. doi: 10.1186/s40478-021-01173-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hass E.W., Sorrentino Z.A., Xia Y., Lloyd G.M., Trojanowski J.Q., Prokop S., Giasson B.I. Disease-, region- and cell type specific diversity of alpha-synuclein carboxy terminal truncations in synucleinopathies. Acta Neuropathol. Commun. 2021;9:146. doi: 10.1186/s40478-021-01242-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uchihara T., Giasson B.I. Propagation of alpha-synuclein pathology: Hypotheses, discoveries, and yet unresolved questions from experimental and human brain studies. Acta Neuropathol. 2016;131:49–73. doi: 10.1007/s00401-015-1485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Makin S. Pathology: The prion principle. Nature. 2016;538:S13–S16. doi: 10.1038/538S13a. [DOI] [PubMed] [Google Scholar]
- 44.Brundin P., Melki R. Prying into the prion hypothesis for Parkinson’s disease. J. Neurosci. 2017;37:9808–9818. doi: 10.1523/JNEUROSCI.1788-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Surmeier D.J., Obeso J.A., Halliday G.M. Parkinson’s disease is not simply a prion disorder. J. Neurosci. 2017;37:9799–9807. doi: 10.1523/JNEUROSCI.1787-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Siddique Y.H. Does human alpha synuclein behave like prions? CNS Neurol. Disord. Drug Targets. 2021 doi: 10.2174/1871527320666210420140123. [DOI] [PubMed] [Google Scholar]
- 47.Zheng H., Shi C., Luo H., Fan L., Yang Z., Hu X., Zhang Z., Zhang S., Hu Z., Fan Y., et al. Alpha-synuclein in Parkinson’s disease: Does a prion-like mechanism of propagation from periphery to the brain play a role? Neuroscientist. 2021;27:367–387. doi: 10.1177/1073858420943180. [DOI] [PubMed] [Google Scholar]
- 48.Woerman A.L., Kazmi S.A., Patel S., Aoyagi A., Oehler A., Widjaja K., Mordes D.A., Olson S.H., Prusiner S.B. Familial Parkinson’s point mutation abolishes multiple system atrophy prion replication. Proc. Natl. Acad. Sci. USA. 2018;115:409–414. doi: 10.1073/pnas.1719369115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Watts J.C. Calling alpha-synuclein a prion is scientifically justifiable. Acta Neuropathol. 2019;138:505–508. doi: 10.1007/s00401-019-02058-0. [DOI] [PubMed] [Google Scholar]
- 50.Prusiner S.B. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 51.Prusiner S.B. Biology and genetics of prions causing neurodegeneration. Annu. Rev. Genet. 2013;47:601–623. doi: 10.1146/annurev-genet-110711-155524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Colin M., Dujardin S., Schraen-Maschke S., Meno-Tetang G., Duyckaerts C., Courade J.P., Buee L. From the prion-like propagation hypothesis to therapeutic strategies of anti-tau immunotherapy. Acta Neuropathol. 2020;139:3–25. doi: 10.1007/s00401-019-02087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ghaemmaghami S. Biology and genetics of PrP prion strains. In: Prusiner S.B., editor. Prion Diseases. Cold Spring Harbor Laboratory Press; Long Island, NY, USA: 2017. pp. 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka M., Chien P., Naber N., Cooke R., Weissman J.S. Conformational variations in an infectious protein determine prion strain differences. Nature. 2004;428:323–328. doi: 10.1038/nature02392. [DOI] [PubMed] [Google Scholar]
- 55.Telling G.C., Parchi P., DeArmond S.J., Cortelli P., Montagna P., Gabizon R., Mastrianni J., Lugaresi E., Gambetti P., Prusiner S.B. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274:2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 56.Lau A., So R.W., Lau H.H., Sang J.C., Ruiz-Riquelme A., Fleck S.C., Stuart E., Menon S., Visanji N.P., Meisl G., et al. Alpha-synuclein strains target distinct brain regions and cell types. Nat. Neurosci. 2020;23:21–31. doi: 10.1038/s41593-019-0541-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prusiner S.B. An introduction to prion diseases. In: Prusiner S.B., editor. Prion Diseases. Cold Spring Harbor Laboratory Press; Long Island, NY, USA: 2017. pp. 1–29. [Google Scholar]
- 58.Woerman A.L. Strain diversity in neurodegenerative disease: An argument for a personalized medicine approach to diagnosis and treatment. Acta Neuropathol. 2021;142:1–3. doi: 10.1007/s00401-021-02311-5. [DOI] [PubMed] [Google Scholar]
- 59.Uemura N., Uemura M.T., Luk K.C., Lee V.M., Trojanowski J.Q. Cell-to-cell transmission of tau and alpha-synuclein. Trends Mol. Med. 2020;26:936–952. doi: 10.1016/j.molmed.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Holec S.A., Woerman A.L. Evidence of distinct alpha-synuclein strains underlying disease heterogeneity. Acta Neuropathol. 2020;142:73–86. doi: 10.1007/s00401-020-02163-5. [DOI] [PubMed] [Google Scholar]
- 61.Sorrentino Z.A., Giasson B.I. The emerging role of alpha-synuclein truncation in aggregation and disease. J. Biol. Chem. 2020;295:10224–10244. doi: 10.1074/jbc.REV120.011743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schweighauser M., Shi Y., Tarutani A., Kametani F., Murzin A.G., Ghetti B., Matsubara T., Tomita T., Ando T., Hasegawa K., et al. Structures of alpha-synuclein filaments from multiple system atrophy. Nature. 2020;585:464–469. doi: 10.1038/s41586-020-2317-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu D., Guo J.J., Su J.H., Svanbergsson A., Yuan L., Haikal C., Li W., Gouras G., Li J.Y. Differential seeding and propagating efficiency of a-synuclein strains generated in different conditions. Transl. Neurodegener. 2021;10:20. doi: 10.1186/s40035-021-00242-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van der Perren A., Gelders G., Fenyi A., Bousset L., Brito F., Peelaerts W., Van den Haute C., Gentleman S., Melki R., Baekelandt V. The structural differences between patient-derived alpha-synuclein strains dictate characteristics of Parkinson’s disease, multiple system atrophy and dementia with Lewy bodies. Acta Neuropathol. 2020;139:977–1000. doi: 10.1007/s00401-020-02157-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo J.L., Lee V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ugalde C.L., Finkelstein D.I., Lawson V.A., Hill A.F. Pathogenic mechanisms of prion protein, amyloid-beta and alpha-synuclein misfolding: The prion concept and neurotoxicity of protein oligomers. J. Neurochem. 2016;139:162–180. doi: 10.1111/jnc.13772. [DOI] [PubMed] [Google Scholar]
- 67.Chu Y., Muller S., Tavares A., Barret O., Alagille D., Seibyl J., Tamagnan G., Marek K., Luk K.C., Trojanowski J.Q., et al. Intrastriatal alpha-synuclein fibrils in monkeys: Spreading, imaging and neuropathological changes. Brain. 2019;142:3565–3579. doi: 10.1093/brain/awz296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luk K.C., Kehm V., Carroll J., Zhang B., O’Brien P., Trojanowski J.Q., Lee V.M. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bassil F., Brown H.J., Pattabhiraman S., Iwasyk J.E., Maghames C.M., Meymand E.S., Cox T.O., Riddle D.M., Zhang B., Trojanowski J.Q., et al. Amyloid-beta (Abeta) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Abeta pathology. Neuron. 2020;105:260–275.e6. doi: 10.1016/j.neuron.2019.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Badiola N., de Oliveira R.M., Herrera F., Guardia-Laguarta C., Gonçalves S.A., Pera M., Suárez-Calvet M., Clarimon J., Outeiro T.F., Lleó A. Tau enhances alpha-synuclein aggregation and toxicity in cellular models of synucleinopathy. PLoS ONE. 2011;6:e26609. doi: 10.1371/journal.pone.0026609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Masuda-Suzukake M., Hasegawa M. Prion-like propagation of pathological alpha-synuclein in vivo. Yakugaku Zasshi. 2019;139:1007–1013. doi: 10.1248/yakushi.18-00165-4. [DOI] [PubMed] [Google Scholar]
- 72.Bernis M.E., Babila J.T., Breid S., Wusten K.A., Wullner U., Tamguney G. Prion-like propagation of human brain-derived alpha-synuclein in transgenic mice expressing human wild-type alpha-synuclein. Acta Neuropathol. Commun. 2015;3:75. doi: 10.1186/s40478-015-0254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Candelise N., Schmitz M., Llorens F., Villar-Pique A., Cramm M., Thom T., da Silva Correia S.M., da Cunha J.E., Mobius W., Outeiro T.F., et al. Seeding variability of different alpha synuclein strains in synucleinopathies. Ann. Neurol. 2019;85:691–703. doi: 10.1002/ana.25446. [DOI] [PubMed] [Google Scholar]
- 74.Shearer L.J., Petersen N.O., Woodside M.T. Internalization of alpha-synuclein oligomers into SH-SY5Y cells. Biophys. J. 2021;120:877–885. doi: 10.1016/j.bpj.2020.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oueslati A., Ximerakis M., Vekrellis K. Protein transmission, seeding and degradation: Key steps for a-synuclein prion-like propagation. Exp. Neurobiol. 2014;23:324–336. doi: 10.5607/en.2014.23.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Trinkaus V.A., Riera-Tur I., Martínez-Sánchez A., Bäuerlein F.J., Guo Q., Arzberger T., Baumeister W., Dudanova I., Hipp M.S., Hartl F.U., et al. In situ architecture of neuronal alpha-synuclein inclusions. Nat. Commun. 2021;12:2110. doi: 10.1038/s41467-021-22108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jan A., Gonçalves N.P., Vaegter C.B., Jensen P.H., Ferreira N. The prion-like spreading of alpha-synuclein in Parkinson’s disease: Update on models and hypotheses. Int. J. Mol. Sci. 2021;22:8338. doi: 10.3390/ijms22158338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thom T., Schmitz M., Fischer A.L., Correia A., Correia S., Llorens F., Pique A.V., Möbius W., Domingues R., Zafar S., et al. Cellular prion protein mediates alpha-synuclein uptake, localization, and toxicity in vitro and in vivo. Mov. Disord. 2021 doi: 10.1002/mds.28774. [DOI] [PubMed] [Google Scholar]
- 79.Hijaz B.A., Volpicelli-Daley L.A. Initiation and propagation of alpha-synuclein aggregation in the nervous system. Mol. Neurodegener. 2020;15:19. doi: 10.1186/s13024-020-00368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ayers J.I., Paras N.A., Prusiner S.B. Expanding spectrum of prion diseases. Emerg. Top. Life Sci. 2020;4:155–167. doi: 10.1042/ETLS20200037. [DOI] [PubMed] [Google Scholar]
- 81.Scheckel C., Aguzzi A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 2018;19:405–418. doi: 10.1038/s41576-018-0011-4. [DOI] [PubMed] [Google Scholar]
- 82.Kraus A., Groveman B.R., Caughey B. Prions and the potential transmissibility of protein misfolding diseases. Annu. Rev. Microbiol. 2013;67:543–564. doi: 10.1146/annurev-micro-092412-155735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Walker L.C., Jucker M. Neurodegenerative diseases: Expanding the prion concept. Annu. Rev. Neurosci. 2015;38:87–103. doi: 10.1146/annurev-neuro-071714-033828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Collinge J. Mammalian prions and their wider relevance in neurodegenerative diseases. Nature. 2016;539:217–226. doi: 10.1038/nature20415. [DOI] [PubMed] [Google Scholar]
- 85.Irwin D.J., Abrams J.Y., Schonberger L.B., Leschek E.W., Mills J.L., Lee V.M., Trojanowski J.Q. Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol. 2013;70:462–468. doi: 10.1001/jamaneurol.2013.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Caughey B., Kraus A. Transmissibility versus Pathogenicity of Self-Propagating Protein Aggregates. Viruses. 2019;11:1044. doi: 10.3390/v11111044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ritchie D.L., Barria M.A. Prion diseases: A unique transmissible agent or a model for neurodegenerative diseases? Biomolecules. 2021;11:207. doi: 10.3390/biom11020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schwarzman A.L., Senkevich K.A., Emelyanov A.K., Pchelina S.N. Prion properties of alpha-synuclein. Mol. Biol. 2019;53:335–341. doi: 10.1134/S002689331903018X. [DOI] [PubMed] [Google Scholar]
- 89.Watts J.C., Prusiner S.B. Beta-Amyloid prions and the pathobiology of Alzheimer’s disease. Cold Spring Harb. Perspect. Med. 2018;8:a023507. doi: 10.1101/cshperspect.a023507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aguzzi A. Cell biology: Beyond the prion principle. Nature. 2009;459:924–925. doi: 10.1038/459924a. [DOI] [PubMed] [Google Scholar]
- 91.Verma A. Prions, prion-like prionoids, and neurodegenerative disorders. Ann. Indian Acad Neurol. 2016;19:169–174. doi: 10.4103/0972-2327.179979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wells C., Brennan S.E., Keon M., Saksena N.K. Prionoid proteins in the pathogenesis of neurodegenerative diseases. Front. Mol. Neurosci. 2019;12:271. doi: 10.3389/fnmol.2019.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mavroeidi P., Xilouri M. Neurons and glia interplay in alpha-synucleinopathies. Int. J. Mol. Sci. 2021;22:4994. doi: 10.3390/ijms22094994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jucker M., Walker L.C. Self-propagation of pathogenic protein aggregates in neurodegenerative diseases. Nature. 2013;501:45–51. doi: 10.1038/nature12481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Prusiner S.B. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Duyckaerts C., Clavaguera F., Potier M.C. The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr. Opin. Neurol. 2019;32:266–271. doi: 10.1097/WCO.0000000000000672. [DOI] [PubMed] [Google Scholar]
- 97.Heras-Garvin A., Stefanova N. From synaptic protein to prion: The long and controversial journey of alpha-synuclein. Front. Synaptic Neurosci. 2020;12:584536. doi: 10.3389/fnsyn.2020.584536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goedert M., Masuda-Suzukake M., Falcon B. Like prions: The propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain. 2017;140:266–278. doi: 10.1093/brain/aww230. [DOI] [PubMed] [Google Scholar]
- 99.Kordower J.H., Chu Y., Hauser R.A., Freeman T.B., Olanow C.W. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 100.Li J.Y., Englund E., Holton J.L., Soulet D., Hagell P., Lees A.J., Lashley T., Quinn N.P., Rehncrona S., Bjorklund A., et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 101.El-Agnaf O.M., Salem S.A., Paleologou K.E., Cooper L.J., Fullwood N.J., Gibson M.J., Curran M.D., Court J.A., Mann D.M., Ikeda S., et al. Alpha-synuclein implicated in Parkinson’s disease is present in extracellular biological fluids, including human plasma. FASEB J. 2003;17:1945–1947. doi: 10.1096/fj.03-0098fje. [DOI] [PubMed] [Google Scholar]
- 102.Hasegawa M., Nonaka T., Masuda-Suzukake M. Alpha-synuclein: Experimental pathology. Cold Spring Harb. Perspect. Med. 2016;6:a024273. doi: 10.1101/cshperspect.a024273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henderson M.X., Cornblath E.J., Darwich A., Zhang B., Brown H., Gathagan R.J., Sandler R.M., Bassett D.S., Trojanowski J.Q., Lee V.M. Spread of alpha-synuclein pathology through the brain connectome is modulated by selective vulnerability and predicted by network analysis. Nat. Neurosci. 2019;22:1248–1257. doi: 10.1038/s41593-019-0457-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dehay B., Vila M., Bezard E., Brundin P., Kordower J.H. Alpha-synuclein propagation: New insights from animal models. Mov. Disord. 2016;31:161–168. doi: 10.1002/mds.26370. [DOI] [PubMed] [Google Scholar]
- 105.Hasegawa M., Nonaka T., Masuda-Suzukake M. Prion-like mechanisms and potential therapeutic targets in neurodegenerative disorders. Pharmacol. Ther. 2017;172:22–33. doi: 10.1016/j.pharmthera.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 106.Stopschinski B.E., Diamond M.I. The prion model for progression and diversity of neurodegenerative diseases. Lancet Neurol. 2017;16:323–332. doi: 10.1016/S1474-4422(17)30037-6. [DOI] [PubMed] [Google Scholar]
- 107.Valdinocci D., Radford R.A., Siow S.M., Chung R.S., Pountney D.L. Potential modes of intercellular alpha-synuclein transmission. Int. J. Mol. Sci. 2017;18:469. doi: 10.3390/ijms18020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Desplats P., Lee H.J., Bae E.J., Patrick C., Rockenstein E., Crews L., Spencer B., Masliah E., Lee S.J. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc. Natl. Acad. Sci. USA. 2009;106:13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Woerman A.L., Stohr J., Aoyagi A., Rampersaud R., Krejciova Z., Watts J.C., Ohyama T., Patel S., Widjaja K., Oehler A., et al. Propagation of prions causing synucleinopathies in cultured cells. Proc. Natl. Acad. Sci. USA. 2015;112:E4949–E4958. doi: 10.1073/pnas.1513426112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dujardin S., Lécolle K., Caillierez R., Bégard S., Zommer N., Lachaud C., Carrier S., Dufour N., Aurégan G., Winderickx J., et al. Neuron-to-neuron wild-type tau protein transfer through a trans-synaptic mechanism: Relevance to sporadic tauopathies. Acta Neuropathol. Commun. 2014;2:14. doi: 10.1186/2051-5960-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Clavaguera F., Akatsu H., Fraser G., Crowther R.A., Frank S., Hench J., Probst A., Winkler D.T., Reichwald J., Staufenbiel M., et al. Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc. Natl. Acad. Sci. USA. 2013;110:9535–9540. doi: 10.1073/pnas.1301175110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Clavaguera F., Lavenir I., Falcon B., Frank S., Goedert M., Tolnay M. “Prion-like” templated misfolding in tauopathies. Brain Pathol. 2013;23:342–349. doi: 10.1111/bpa.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Olsson T.T., Klementieva O., Gouras G.K. Prion-like seeding and nucleation of intracellular amyloid-beta. Neurobiol. Dis. 2018;113:1–10. doi: 10.1016/j.nbd.2018.01.015. [DOI] [PubMed] [Google Scholar]
- 114.Gomes L.A., Hipp S.A., Rijal Upadhaya A., Balakrishnan K., Ospitalieri S., Koper M.J., Largo-Barrientos P., Uytterhoeven V., Reichwald J., Rabe S., et al. Abeta-induced acceleration of Alzheimer-related tau-pathology spreading and its association with prion protein. Acta Neuropathol. 2019;138:913–941. doi: 10.1007/s00401-019-02053-5. [DOI] [PubMed] [Google Scholar]
- 115.Fornari S., Schäfer A., Kuhl E., Goriely A. Spatially-extended nucleation-aggregation-fragmentation models for the dynamics of prion-like neurodegenerative protein-spreading in the brain and its connectome. J. Theor. Biol. 2020;486:110102. doi: 10.1016/j.jtbi.2019.110102. [DOI] [PubMed] [Google Scholar]
- 116.Masuda-Suzukake M., Nonaka T., Hosokawa M., Oikawa T., Arai T., Akiyama H., Mann D.M., Hasegawa M. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–1138. doi: 10.1093/brain/awt037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Masuda-Suzukake M., Nonaka T., Hosokawa M., Kubo M., Shimozawa A., Akiyama H., Hasegawa M. Pathological alpha-synuclein propagates through neural networks. Acta Neuropathol. Commun. 2014;2:88. doi: 10.1186/s40478-014-0088-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chung H.K., Ho H.A., Pérez-Acuña D., Lee S.J. Modeling a-synuclein propagation with preformed fibril injections. J. Mov. Disord. 2019;12:139–151. doi: 10.14802/jmd.19046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reyes J.F., Sackmann C., Hoffmann A., Svenningsson P., Winkler J., Ingelsson M., Hallbeck M. Binding of a-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes. Acta Neuropathol. 2019;138:23–47. doi: 10.1007/s00401-019-02007-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ferreira N., Gram H., Sorrentino Z.A., Gregersen E., Schmidt S.I., Reimer L., Betzer C., Perez-Gozalbo C., Beltoja M., Nagaraj M., et al. Multiple system atrophy-associated oligodendroglial protein p25α stimulates formation of novel α-synuclein strain with enhanced neurodegenerative potential. Acta Neuropathol. 2021;142:87–115. doi: 10.1007/s00401-021-02316-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lohmann S., Bernis M.E., Tachu B.J., Ziemski A., Grigoletto J., Tamguney G. Oral and intravenous transmission of alpha-synuclein fibrils to mice. Acta Neuropathol. 2019;138:515–533. doi: 10.1007/s00401-019-02037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sacino A.N., Brooks M., Thomas M.A., McKinney A.B., Lee S., Regenhardt R.W., McGarvey N.H., Ayers J.I., Notterpek L., Borchelt D.R., et al. Intramuscular injection of alpha-synuclein induces CNS alpha-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA. 2014;111:10732–10737. doi: 10.1073/pnas.1321785111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Breid S., Bernis M.E., Babila J.T., Garza M.C., Wille H., Tamguney G. Neuroinvasion of alpha-synuclein prionoids after intraperitoneal and intraglossal inoculation. J. Virol. 2016;90:9182–9193. doi: 10.1128/JVI.01399-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ayers J.I., Brooks M.M., Rutherford N.J., Howard J.K., Sorrentino Z.A., Riffe C.J., Giasson B.I. Robust central nervous system pathology in transgenic mice following peripheral injection of alpha-synuclein fibrils. J. Virol. 2017;91:e02095-16. doi: 10.1128/JVI.02095-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Van Den Berge N., Ferreira N., Gram H., Mikkelsen T.W., Alstrup A.K., Casadei N., Tsung-Pin P., Riess O., Nyengaard J.R., Tamguney G., et al. Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol. 2019;138:535–550. doi: 10.1007/s00401-019-02040-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim S., Kwon S.H., Kam T.I., Panicker N., Karuppagounder S.S., Lee S., Lee J.H., Kim W.R., Kook M., Foss C.A., et al. Transneuronal propagation of pathologic alpha-synuclein from the gut to the brain models Parkinson’s disease. Neuron. 2019;103:627–641.e7. doi: 10.1016/j.neuron.2019.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Breen D.P., Halliday G.M., Lang A.E. Gut-brain axis and the spread of alpha-synuclein pathology: Vagal highway or dead end? Mov. Disord. 2019;34:307–316. doi: 10.1002/mds.27556. [DOI] [PubMed] [Google Scholar]
- 128.Recasens A., Dehay B., Bove J., Carballo-Carbajal I., Dovero S., Perez-Villalba A., Fernagut P.O., Blesa J., Parent A., Perier C., et al. Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann. Neurol. 2014;75:351–362. doi: 10.1002/ana.24066. [DOI] [PubMed] [Google Scholar]
- 129.Kawakami I., Motoda A., Hashimoto M., Shimozawa A., Masuda-Suzukake M., Ohtani R., Takase M., Kumashiro M., Samejima K., Hasegawa M. Progression of phosphorylated a-synuclein in Macaca fuscata. Brain Pathol. 2021;31:e12952. doi: 10.1111/bpa.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shimozawa A., Ono M., Takahara D., Tarutani A., Imura S., Masuda-Suzukake M., Higuchi M., Yanai K., Hisanaga S.I., Hasegawa M. Propagation of pathological alpha-synuclein in marmoset brain. Acta Neuropathol. Commun. 2017;5:12. doi: 10.1186/s40478-017-0413-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ogata J., Takemoto D., Shimonaka S., Imai Y., Hattori N. Alpha-synuclein seeding assay using cultured cells. Methods Mol. Biol. 2021;2322:27–39. doi: 10.1007/978-1-0716-1495-2_3. [DOI] [PubMed] [Google Scholar]
- 132.Aguzzi A., Lakkaraju A.K. Cell biology of prions and prionoids: A status report. Trends Cell Biol. 2016;26:40–51. doi: 10.1016/j.tcb.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 133.Peng C., Gathagan R.J., Covell D.J., Medellin C., Stieber A., Robinson J.L., Zhang B., Pitkin R.M., Olufemi M.F., Luk K.C., et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature. 2018;557:558–563. doi: 10.1038/s41586-018-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Peng C., Gathagan R.J., Lee V.M. Distinct alpha-synuclein strains and implications for heterogeneity among alpha-synucleinopathies. Neurobiol. Dis. 2018;109:209–218. doi: 10.1016/j.nbd.2017.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Uemura N., Uemura M.T., Lo A., Bassil F., Zhang B., Luk K.C., Lee V.M., Takahashi R., Trojanowski J.Q. Slow progressive accumulation of oligodendroglial alpha-synuclein (alpha-syn) pathology in synthetic alpha-syn fibril-induced mouse models of synucleinopathy. J. Neuropathol. Exp. Neurol. 2019;78:877–890. doi: 10.1093/jnen/nlz070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miller D.W., Johnson J.M., Solano S.M., Hollingsworth Z.R., Standaert D.G., Young A.B. Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J. Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- 137.Yamasaki T.R., Holmes B.B., Furman J.L., Dhavale D.D., Su B.W., Song E.S., Cairns N.J., Kotzbauer P.T., Diamond M.I. Parkinson’s disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J. Biol. Chem. 2019;294:1045–1058. doi: 10.1074/jbc.RA118.004471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lövestam S., Schweighauser M., Matsubara T., Murayama S., Tomita T., Ando T., Hasegawa K., Yoshida M., Tarutani A., Hasegawa M., et al. Seeded assembly in vitro does not replicate the structures of a-synuclein filaments from multiple system atrophy. FEBS Openbio. 2021;11:999–1013. doi: 10.1002/2211-5463.13110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 140.Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Van den Haute C., Melki R., Baekelandt V. Alpha-synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 141.Bousset L., Melki R. Infectious properties of protein aggregates involved in neurodegenerative diseases. Biol. Aujourdhui. 2013;207:55–59. doi: 10.1051/jbio/2013004. [DOI] [PubMed] [Google Scholar]
- 142.Peelaerts W., Baekelandt V. Alpha-synuclein strains and the variable pathologies of synucleinopathies. J. Neurochem. 2016;139((Suppl. S1)):256–274. doi: 10.1111/jnc.13595. [DOI] [PubMed] [Google Scholar]
- 143.Tarutani A., Hasegawa M. Prion-like propagation of alpha-synuclein in neurodegenerative diseases. Prog. Mol. Biol. Transl. Sci. 2019;168:323–348. doi: 10.1016/bs.pmbts.2019.07.005. [DOI] [PubMed] [Google Scholar]
- 144.Fearon C., Farrell M.A. Disease-specific strains of alpha-synuclein in multiple system atrophy and Parkinson’s disease: But why? Mov. Disord. 2020;35:756–757. doi: 10.1002/mds.28035. [DOI] [PubMed] [Google Scholar]
- 145.Shahnawaz M., Mukherjee A., Pritzkow S., Mendez N., Rabadia P., Liu X., Hu B., Schmeichel A., Singer W., Wu G., et al. Discriminating alpha-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–277. doi: 10.1038/s41586-020-1984-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dhillon J.K., Trejo-Lopez J.A., Riffe C., Levites Y., Sacino A.N., Borchelt D.R., Yachnis A.Y., Giasson B.I. Comparative analyses of the in vivo induction and transmission of alpha-synuclein pathology in transgenic mice by MSA brain lysate and recombinant alpha-synuclein fibrils. Acta Neuropathol. Commun. 2019;7:80. doi: 10.1186/s40478-019-0733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sargent D., Verchere J., Lazizzera C., Gaillard D., Lakhdar L., Streichenberger N., Morignat E., Betemps D., Baron T. ‘Prion-like’ propagation of the synucleinopathy of M83 transgenic mice depends on the mouse genotype and type of inoculum. J. Neurochem. 2017;143:126–135. doi: 10.1111/jnc.14139. [DOI] [PubMed] [Google Scholar]
- 148.Krejciova Z., Carlson G.A., Giles K., Prusiner S.B. Replication of multiple system atrophy prions in primary astrocyte cultures from transgenic mice expressing human alpha-synuclein. Acta Neuropathol. Commun. 2019;7:81. doi: 10.1186/s40478-019-0703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sacino A.N., Ayers J.I., Brooks M.M., Chakrabarty P., Hudson V.J., 3rd, Howard J.K., Golde T.E., Giasson B.I., Borchelt D.R. Non-prion-type transmission in A53T alpha-synuclein transgenic mice: A normal component of spinal homogenates from naive non-transgenic mice induces robust alpha-synuclein pathology. Acta Neuropathol. 2016;131:151–154. doi: 10.1007/s00401-015-1505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sanders D.W., Kaufman S.K., DeVos S.L., Sharma A.M., Mirbaha H., Li A., Barker S.J., Foley A.C., Thorpe J.R., Serpell L.C., et al. Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82:1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aulic S., Masperone L., Narkiewicz J., Isopi E., Bistaffa E., Ambrosetti E., Pastore B., De Cecco E., Scaini D., Zago P., et al. Alpha-Synuclein amyloids hijack prion protein to gain cell entry, facilitate cell-to-cell spreading and block prion replication. Sci. Rep. 2017;7:10050. doi: 10.1038/s41598-017-10236-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.De Cecco E., Legname G. The role of the prion protein in the internalization of alpha-synuclein amyloids. Prion. 2018;12:23–27. doi: 10.1080/19336896.2017.1423186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Haik S., Privat N., Adjou K.T., Sazdovitch V., Dormont D., Duyckaerts C., Hauw J.J. Alpha-synuclein-immunoreactive deposits in human and animal prion diseases. Acta Neuropathol. 2002;103:516–520. doi: 10.1007/s00401-001-0499-z. [DOI] [PubMed] [Google Scholar]
- 154.La Vitola P., Beeg M., Balducci C., Santamaria G., Restelli E., Colombo L., Caldinelli L., Pollegioni L., Gobbi M., Chiesa R., et al. Cellular prion protein neither binds to alpha-synuclein oligomers nor mediates their detrimental effects. Brain. 2019;142:249–254. doi: 10.1093/brain/awy318. [DOI] [PubMed] [Google Scholar]
- 155.Gelpi E., Colom-Cadena M. Oligomers: A hot topic for neurodegeneration and a note of caution for experimental models. Brain. 2019;142:228–230. doi: 10.1093/brain/awy342. [DOI] [PubMed] [Google Scholar]
- 156.Visanji N.P., Lang A.E., Kovacs G.G. Beyond the synucleinopathies: Alpha synuclein as a driving force in neurodegenerative comorbidities. Transl. Neurodegener. 2019;8:28. doi: 10.1186/s40035-019-0172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bistaffa E., Rossi M., De Luca C.M.G., Cazzaniga F., Carletta O., Campagnani I., Tagliavini F., Legname G., Giaccone G., Moda F. Prion efficiently replicates in alpha-synuclein knockout mice. Mol. Neurobiol. 2019;56:7448–7457. doi: 10.1007/s12035-019-1602-6. [DOI] [PubMed] [Google Scholar]
- 158.Bartz J.C. Environmental and host factors that contribute to prion strain evolution. Acta Neuropathol. 2021;142:5–16. doi: 10.1007/s00401-021-02310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chelban V., Manole A., Pihlstrom L., Schottlaender L., Efthymiou S., O’Conner E., Meissner W.G., Holton J.L., Houlden H. Analysis of the prion protein gene in multiple system atrophy. Neurobiol. Aging. 2017;49:216.e15–216.e18. doi: 10.1016/j.neurobiolaging.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.De Pablo-Fernandez E., Cerdan Santacruz D., Warner T.T., Holton J.L. No evidence of iatrogenic human transmission in autopsy confirmed multiple system atrophy. Mov. Disord. 2018;33:1183–1184. doi: 10.1002/mds.27370. [DOI] [PubMed] [Google Scholar]
- 161.Rajput A.H., Ferguson L.W., Robinson C.A., Guella I., Farrer M.J., Rajput A. Conjugal parkinsonism—Clinical, pathology and genetic study. No evidence of person-to-person transmission. Parkinsonism Relat. Disord. 2016;31:87–90. doi: 10.1016/j.parkreldis.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 162.Coon E.A., Rocca W., Melson C.S., Ahlskog J.E., Matsumoto J.Y., Low P.A., Singer W. Conjugal multiple system atrophy: Chance, shared risk factors, or evidence of transmissibility? Parkinsonism Relat. Disord. 2019;67:10–13. doi: 10.1016/j.parkreldis.2019.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Asher D.M., Belay E., Bigio E., Brandner S., Brubaker S.A., Caughey B., Clark B., Damon I., Diamond M., Freund M., et al. Risk of transmissibility from neurodegenerative disease-associated proteins: Experimental knowns and unknowns. J. Neuropathol. Exp. Neurol. 2020;79:1141–1146. doi: 10.1093/jnen/nlaa109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Rey N.L., George S., Steiner J.A., Madaj Z., Luk K.C., Trojanowski J.Q., Lee V.M., Brundin P. Spread of aggregates after olfactory bulb injection of alpha-synuclein fibrils is associated with early neuronal loss and is reduced long term. Acta Neuropathol. 2018;135:65–83. doi: 10.1007/s00401-017-1792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.