Highlights
-
•
We determined reference values for the dorsal sural nerve from a large cohort, aged 21-80.
-
•
Amplitude and conduction velocity depended on both age and height.
-
•
We provide formulas for reference limits.
Keywords: Diagnosis, Dorsal sural nerve, Nerve conduction studies, Polyneuropathy, Reference values
Abstract
Objectives
Dorsal sural nerve conduction studies (NCS) may increase the sensitivity for the diagnosis of polyneuropathy, but clinical use is limited by a lack of reliable normative reference values in all age-groups. The aim of our study was to develop reference values for the dorsal sural nerve, based on a large multicenter cohort of healthy subjects.
Methods
Bilateral antidromic NCS were performed using standard surface electrodes in 229 healthy subjects (aged 21–80 years; median: 54 years). We assessed the normality of data distribution for amplitudes and conduction velocity (CV) and for their logarithmic (ln) transformation. The effects of age and height were determined using linear regression analysis.
Results
Sensory potentials were present in all subjects. Logarithmically transformed data were normally distributed. Age2 and height were most significantly associated with amplitude, and age and height with CV, respectively. There was no significant side-difference. Mean amplitudes (right and left) were 4.8 and 4.9 μV and mean CV 46.7 and 46.9 m/s. Reference limits were e (3.712515 – 0.0000956 * age2 – 0.0115883 * height ± 1.96 * 0.51137) for amplitude and e (4.354374 – 0.0021081 * age – 0.0023354 * height ± 1.96 * 0.11161) for CV.
Conclusions
Dorsal sural nerve NCS are robust and have well defined normative limits.
Significance
The findings provide a basis for more sensitive NCS in clinical practice and future studies of the diagnostic accuracy of NCS in polyneuropathy.
1. Introduction
Polyneuropathy is a common neurological disorder with an overall prevalence ranging from 1 to 3%, increasing to 7% in the elderly (Hanewinckel et al., 2016). Nerve conduction studies (NCS) are essential for diagnosing large and mixed fiber polyneuropathy, which are the most common types in e.g. diabetic polyneuropathy (Itani et al., 2021). Abnormal NCS (Tankisi et al., 2019) are included in the criteria for the definite diagnosis of diabetic polyneuropathy (Tesfaye et al., 2010) and polyneuropathy in general (England et al., 2005).
Specifically, the sural nerve is considered crucial for the diagnosis of polyneuropathy (England et al., 2005) because there is no risk of focal compression. However, the sural nerve, when studied at the calf using surface electrodes, has a low sensitivity for polyneuropathy of approximately 50% (Tankisi et al., 2019). Tibial nerve F-wave latencies are more sensitive. However, F-wave abnormalities are unspecific. The sensitivity of sural NCS can be increased using the near-nerve needle technique (Krøigård and Sindrup, 2016, Kural et al., 2016, Kural et al., 2017) but this method is invasive, more painful for the test subject than surface electrode recordings, time-consuming and requires special proficiency.
Examination of the distal part of the sural nerve, the dorsal sural nerve, increases the sensitivity of NCS in mixed etiology (Killian and Foreman, 2001, Kural et al., 2017) and diabetic (Uluc et al., 2008) polyneuropathy patients. However, its implementation in clinical practice has been limited so far, due to lack of comprehensive reference values for all age-groups. The general concern with examining the sural nerve is also based on the opinions that it might be absent in the older age-group, even in normal subjects.
The correct classification of sural NCS has clinical implications for the diagnosis of polyneuropathy in general, and the distinction between mixed large and small fiber neuropathy and pure small fiber neuropathy, which in turn, guides further polyneuropathy workup including diagnosis of underlying conditions.
The aim of this study was to provide neurophysiologically useful normative reference values for the dorsal sural nerve based on a large multicenter cohort of healthy subjects, including the older age-group.
2. Methods
2.1. Study population
Healthy subjects were examined at four clinical neurophysiology laboratories in Denmark (Aarhus University Hospital, Odense University Hospital, the Danish Epilepsy Centre, and Aleris-Hamlet Hospital). In Aarhus and Odense, subjects had served as a control group in a large study of diabetic neuropathy (Gylfadottir et al., 2020) or a study of polyneuropathy (Kural et al., 2017). They had been recruited from within the patients’ social or work circle or by social media or a specialized web site (forsøgsperson.dk). A detailed neurological examination of lower extremities including standard sensory examination of light brush stroking, pinprick, and cold (20 °C) and warm (40 °C) thermal rolls was performed by study physicians. The upper thigh or chest was used as the control area. Tendon stretch reflexes and muscle strength was assessed. Vibration sense was determined on the dorsum of the great toe. At the Danish Epilepsy Centre and Aleris-Hamlet Hospital, patients examined due to suspicion of carpal tunnel syndrome without any other previous or present neurological symptoms were included. Subjects had normal muscle strength, tendon stretch reflexes and sensitivity to light touch and pin prick in the lower extremities. Further, they had no comorbidities or medication known to affect peripheral nerves and no neurological symptoms or signs in the lower extremities. Approval had been obtained from the Regional Research Ethics Committee of Central Denmark Region (file number 1‐10‐72‐130‐16) (Aarhus and Odense sites).
2.2. Nerve conduction studies
Examinations were performed using Keypoint.Net EMG equipment (Dantec, Skovlunde, Denmark). Disposable, pre-gelled surface electrodes (Ag/AgCL) with a recording area of 15 mm × 20 mm were used (9013S0212 Dantec/Natus). The skin temperature was maintained at 32–36 °C by a heating lamp. Averaging of at least 20 stimuli was performed. A low pass filter of 10.000 Hz was used.
Examinations were performed by experienced neurophysiologists or technicians supervised by neurophysiologists. Cursors were set automatically if the sensory potential was identified by the Keypoint.Net EMG equipment. Otherwise, they were set manually.
Dorsal sural nerves (Fig. 1) were stimulated antidromically using a surface bar stimulator (Dantec 13L36). The surface recording electrode was placed at the mid-portion of the fifth metatarsal bone just lateral to the extensor digitorum longus tendon of the fifth toe with the reference electrode 2 cm distally. The stimulation site was posterior to the lateral malleolus with the cathode placed 12 cm proximal to the recording electrode. A ground electrode was placed between the recording and the stimulating electrodes.
Fig. 1.
Antidromic nerve conduction study of the dorsal sural nerve. Stimulation: fixed bar electrode. Recording electrode: stick on electrodes with a 2 cm distance (E1 black cable, E2 red cable). Ground electrode green. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Latencies were calculated from the stimulus onset to the first positive peak for the determination of CV, and amplitudes were measured peak-to-peak (lowest positive peak to the negative peak).
2.3. Data analysis
Normal distribution of amplitude, conduction velocity (CV), ln(amplitude), and ln(CV) was examined using a Shapiro-Wilk test. Data with a normal distribution were used in further analyses. Side-to-side differences were examined using paired t-tests. As there were no significant side-to-side differences, the right side was used for further analysis. Univariate regression analysis was performed for age, age2, and for height and height2. Based on this analysis, the variable age vs. age2 and height vs. height2 with the most significant correlation with amplitude or CV was selected for the final regression model.
The normative reference limits were calculated based on linear regression analysis ± 1.96 standard deviation.
3. Results
3.1. Subjects
Two hundred twenty-nine subjects were examined. Physicians examined 98 subjects, and experienced clinical neurophysiology technicians examined 131 subjects. The median age was 54 years, ranging from 21 to 80 years. Eighty-four subjects (37%) were 60 years or older. Median height was 170 cm, ranging from 152 to 194 cm. The results of a subgroup of 37 healthy controls was published previously (Kural et al., 2017).
3.2. Data distribution, side-to-side, age and height differences
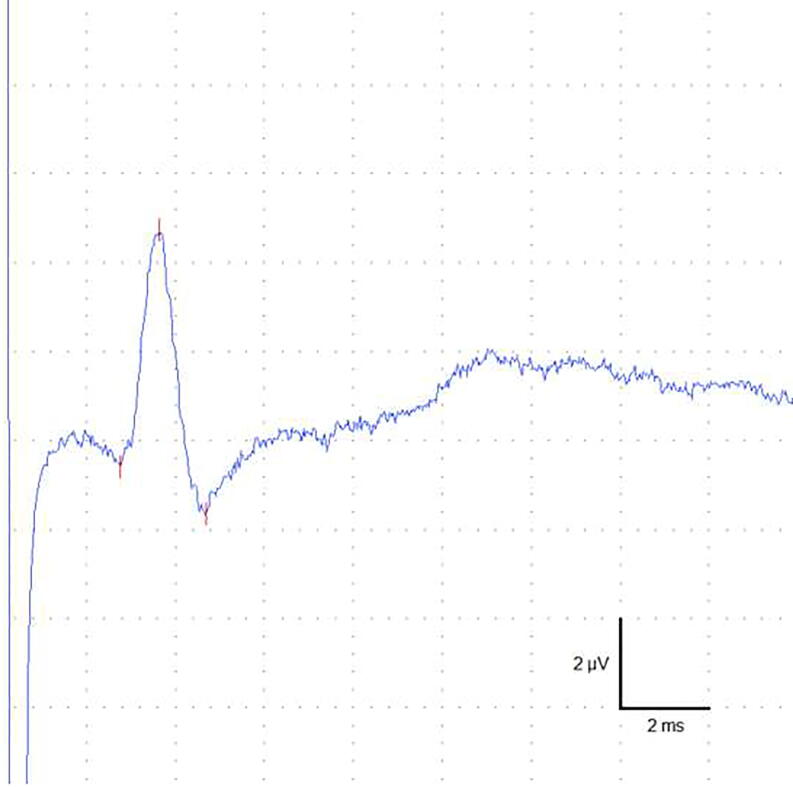
Sensory potentials (Fig. 2) were successfully recorded in all subjects and all examined nerves/sides.
Fig. 2.
Typical action potential from the dorsal sural nerve with an amplitude of 6.4 µV and a conduction velocity of 43.5 m/s.
There was no significant side-to-side difference for neither amplitude (p = 0.61) nor conduction velocity (p = 0.32). Mean side to side difference (absolute difference divided by greatest) was 21.5% (standard deviation 17.4% and 95th percentile 56.7%) for amplitude and 5.53% (standard deviation 5.77% and 95th percentile 18.4%) for CV.
Amplitude (Prob > z 0.00000) and CV (Prob > z 0.01561) were not normally distributed. Ln(amplitude) (Prob > z 0.53154) and ln(CV) (Prob > z 0.51312) were normally distributed. Univariate linear regression analysis showed significant correlations between both age (p = 0.000; t = −3.89), height (p = 0.005; t = −2.86), age2 (p = 0.000; t = −4.09) and height2 (p = 0.005; t = −2.83) and ln(amplitude). For ln(CV) there were significant correlations with both age (p = 0.000; t = −4.04), height (p = 0.009; t = −2.62), age2 (p = 0.000; t = −4.03) and height2 (p = 0.009; t = −2.62).
Based on these findings, ln(amplitude), age2, and height were included in the model for amplitude reference limits, whereas ln(CV), age and height were included in the model for CV reference limits.
The results of individual subjects are presented in Fig. 3.
Fig. 3.
Amplitude (A) and conduction velocity (B) vs age and vs height (C and D) in healthy subjects in dorsal sural nerve NCS. Y axes are in logarithmic (ln) scale. Lines represent linear regression.
3.3. Linear regression model for normative reference values
Normative reference values for amplitude: Amplitude = e (3.712515 – 0.0000956 * age2 – 0.0115883 * height ± 1.96 * 0.51137).
Normative reference values for CV: CV = e (4.354374 – 0.0021081 * age – 0.0023354 * height ± 1.96 * 0.11161).
Alternative presentations of these expressions are:
Ln(amplitude) = 3.712515–0.0000956 * age2 − 0.0115883 * height ± 1.96 * 0.51137.
Ln(CV) = 4.354374–0.0021081 * age − 0.0023354 * height ± 1.96 * 0.11161.
An Excel file, where the age and height of the subject can be entered to calculate the corresponding normative values, is presented as online Supplementary Material.
4. Discussion
We present reference normal limits for the dorsal sural nerve NCS, based on a large multicenter cohort. We were able to identify sensory potentials in all subjects, including the group of elderly patients. This finding confirms that sensory NCS of the dorsal sural nerve are suitable for clinical implementation, and suggests a high specificity of absent potentials in patients with a clinical suspicion of polyneuropathy.
In contrast, not all healthy subjects have a recordable potential from the superficial peroneal nerve (Saffarian et al., 2017), which in another study had been shown to increase the sensitivity of NCS in mild polyneuropathy (Kushnir et al., 2005). For comparison, potentials were recorded from the medial plantar nerve, another distal nerve with the potential to increase sensitivity of NCS (Uluc et al., 2008), in all but two of 81 healthy elderly subjects (Keskin et al., 2015).
We cannot exclude that our population of healthy subjects is not entirely representative of the general population, as we do not have information regarding the socio-economic status and potential causes of focal peripheral nerve lesions e.g. football or skiing activities. Also, it is possible that the use of alcohol is lower than in the general population. Finally, our subjects did not use any medications known to affect peripheral nerves.
Normative values for the dorsal sural nerve were presented in one previous large study, including 294 healthy subjects (Frigeni et al., 2012). However, height, which in the present study was significantly correlated with both amplitude and CV, was not analyzed in the previous study by Frigeni et al. In comparison, mean CV was significantly higher and mean amplitude was slightly lower in the present study. The difference in amplitudes may be explained by the different distances between stimulation and recording sites, which varied between 9 and 12 cm in the previous study and was fixed at 12 cm in the present study. We cannot explain differences in CV as temperature was comparable in the two studies.
A retrospective study reported that dorsal sural nerve potentials were not found in 26% of patients who did not have polyneuropathy based on a clinical evaluation (Vrancken et al., 2008), which is surprising considering our findings. A possible explanation could be the presence of subclinical polyneuropathy in these patients. Further studies in patients examined due to a clinical suspicion of polyneuropathy, are needed to establish the real diagnostic value of dorsal sural nerve NCS.
In conclusion, normative values for the dorsal sural nerve were dependent on age and height. Potentials were recorded from all healthy subjects and the presented reference limits are applicable to clinical diagnosis and further studies of the diagnostic accuracy of NCS in polyneuropathy.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
We thank technicians Anne Mette Kappel Overby, Kitt Marstrand, Mette Stenaa Servé, Ma Grace De Vera Eilertsen, Bendte Erenskjold Madsen, Majbritt Smollerup and Mia Dyhr Thomsen, for performing nerve conduction studies.
Research reported in this publication is part of the International Diabetic Neuropathy Consortium (IDNC), which is supported by a Novo Nordisk Foundation Challenge program grant (grant number NNF14OC0011633).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cnp.2021.08.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- England J.D., Gronseth G.S., Franklin G., Miller R.G., Asbury A.K., Carter G.T. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64:199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- Frigeni B., Cacciavillani M., Ermani M., Briani C., Alberti P., Ferrarese C. Neurophysiological examination of dorsal sural nerve. Muscle Nerve. 2012;46:891–894. doi: 10.1002/mus.23454. [DOI] [PubMed] [Google Scholar]
- Gylfadottir S.S., Itani M., Krøigård T., Kristensen A.G., Christensen D.H., Nicolaisen S.K. Diagnosis and prevalence of diabetic polyneuropathy: a cross-sectional study of Danish patients with type 2 diabetes. Eur. J. Neurol. 2020;27:2575–2585. doi: 10.1111/ene.14469. [DOI] [PubMed] [Google Scholar]
- Hanewinckel R., van Oijen M., Ikram M.A., van Doorn P.A. The epidemiology and risk factors of chronic polyneuropathy. Eur. J. Epidemiol. 2016;31:5–20. doi: 10.1007/s10654-015-0094-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itani M., Gylfadottir S.S., Krøigård T., Kristensen A.G., Christensen D.H., Karlsson P. Small and large fiber sensory polyneuropathy in type 2 diabetes: Influence of diagnostic criteria on neuropathy subtypes. J. Peripher. Nerv. Syst. 2021;26:55–65. doi: 10.1111/jns.12424. [DOI] [PubMed] [Google Scholar]
- Keskin G., Kahraman Koytak P., Bastan B., Tanridag T., Us O., Uluc K. The reliability of medial and lateral plantar nerve recordings in healthy elderly individuals. Neurol. Sci. 2015;36:883–888. doi: 10.1007/s10072-014-2056-2. [DOI] [PubMed] [Google Scholar]
- Killian J.M., Foreman P.J. Clinical utility of dorsal sural nerve conduction studies. Muscle Nerve. 2001;24:817–820. doi: 10.1002/mus.1074. [DOI] [PubMed] [Google Scholar]
- Krøigård T., Sindrup S.H. Diagnostic value of near-nerve recordings of the sural nerve in polyneuropathy patients. Clin. Neurophysiol. 2016;127:1741–1743. doi: 10.1016/j.clinph.2015.07.028. [DOI] [PubMed] [Google Scholar]
- Kural M.A., Pugdahl K., Fuglsang-Frederiksen A., Andersen H., Tankisi H. Near-nerve needle technique versus surface electrode recordings in electrodiagnosis of diabetic polyneuropathy. J. Clin. Neurophysiol. 2016;33:346–349. doi: 10.1097/WNP.0000000000000244. [DOI] [PubMed] [Google Scholar]
- Kural M.A., Karlsson P., Pugdahl K., Isak B., Fuglsang-Frederiksen A., Tankisi H. Diagnostic utility of distal nerve conduction studies and sural near-nerve needle recording in polyneuropathy. Clin. Neurophysiol. 2017;128:1590–1595. doi: 10.1016/j.clinph.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Kushnir M., Klein C., Kimiagar Y., Pollak L., Rabey J.M. Medial dorsal superficial peroneal nerve studies in patients with polyneuropathy and normal sural responses. Muscle Nerve. 2005;31:386–389. doi: 10.1002/mus.20183. [DOI] [PubMed] [Google Scholar]
- Saffarian M.R., Condie N.C., Austin E.A., Mccausland K.E., Andary M.T., Sylvain J.R. Comparison of four different nerve conduction techniques of the superficial fibular sensory nerve. Muscle Nerve. 2017;56:458–462. doi: 10.1002/mus.25543. [DOI] [PubMed] [Google Scholar]
- Tankisi H., Pugdahl K., Beniczky S., Andersen H., Fuglsang-Frederiksen A. Evidence-based recommendations for examination and diagnostic strategies of polyneuropathy electrodiagnosis. Clin. Neurophysiol. Pract. 2019;4:214–222. doi: 10.1016/j.cnp.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesfaye S., Boulton A.J.M., Dyck P.J., Freeman R., Horowitz M., Kempler P. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–2293. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uluc K., Isak B., Borucu D., Temucin C.M., Cetinkaya Y., Koytak P.K. Medial plantar and dorsal sural nerve conduction studies increase the sensitivity in the detection of neuropathy in diabetic patients. Clin. Neurophysiol. 2008;119:880–885. doi: 10.1016/j.clinph.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Vrancken A.F., Notermans N.C., Wokke J.H., Franssen H. The realistic yield of lower leg SNAP amplitudes and SRAR in the routine evaluation of chronic axonal polyneuropathies. J. Neurol. 2008;255:1127–1135. doi: 10.1007/s00415-008-0817-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.