Abstract
Background
Perioperative care for total knee arthroplasty (TKA) has improved over time. We present an analysis of inpatient safety after TKA.
Methods
14,057 primary TKAs captured by the Medicare Patient Safety Monitoring System between 2010 and 2017 were retrospectively reviewed. We calculated changes in demographics, comorbidities, and adverse events (AEs) over time. Risk factors for AEs were also assessed.
Results
Between 2010 and 2017, there was an increased prevalence of obesity (35.1% to 57.6%), tobacco smoking (12.5% to 17.8%), and renal disease (5.2% to 8.9%). There were reductions in coronary artery disease (17.3% to 13.4%) and chronic warfarin use (6.7% to 3.1%). Inpatient AEs decreased from 4.9% to 2.5%, (P < .01), primarily driven by reductions in anticoagulant-associated AEs, including major bleeding and hematomas (from 2.8% to 1.0%, P < .001), catheter-associated urinary tract infections (1.1% to 0.2%, P < .001), pressure ulcers (0.8% to 0.2%, P < .001), and venous thromboembolism (0.3% to 0.1%, P = .04). The adjusted annual decline in the risk of developing any in-hospital AE was 14% (95% confidence interval [CI] 10%-17%). Factors associated with developing an AE were advanced age (odds ratio [OR] = 1.01, 95% CI 1.00-1.01), male sex (OR = 1.21, 95% CI 1.02-1.44), coronary artery disease (OR = 1.35, 95% CI 1.07-1.70), heart failure (OR = 1.70, 95% CI 1.20-2.41), and renal disease (OR = 1.71, 95% CI 1.23-2.37).
Conclusions
Despite increasing prevalence of obesity, tobacco smoking, and renal disease, inpatient AEs after primary TKA have decreased over the past several years. This improvement is despite the increasing complexity of the inpatient TKA population over time.
Keywords: Adverse events, Knee arthroplasty, Patient safety, Risk factors, Time trends
Introduction
Total knee arthroplasty (TKA) is one of the most commonly performed and successful surgical procedures in the United States [1]. Over the past decade, the growing demand for TKA has been met with improved patient optimization, minimally invasive surgical approaches, and efficient perioperative care pathways [2,3]. These refinements have been associated with reduced recovery times and overall costs of care [4]. As short hospital stays for TKA become more common [[5], [6], [7]], there is a greater need to monitor the longitudinal trends in safety with an emphasis on preoperative risk stratification.
Amid the advances in the perioperative management of patients undergoing TKA over the past few years, it remains unclear how the rates of in-hospital adverse events (AEs) have evolved during this time. In addition, as efforts to maximize patient safety continue to be a top priority, surgeons need to be able to accurately assess and mitigate patients’ risks for developing postoperative complications. Risk stratification is essential to guide preoperative optimization, patient counseling, monitoring for AEs, and clinical decision-making.
The objective of this study was to report on the temporal trends in comorbidity profiles, rates of inpatient AEs, and risk factors for those AEs in a recent national sample of patients undergoing primary TKA at a hospital setting.
Material and methods
The institutional review board was waived based on the deidentified nature of the data. The Medicare Patient Safety Monitoring System (MPSMS), which includes only hospital stays and is detailed in previous publications [[8], [9], [10], [11], [12], [13]], was queried for all patients who underwent primary, elective TKA from 2010 to 2017. The MPSMS is derived from chart abstraction; hence, compared to large administrative database analyses, identification of AEs is more sophisticated, includes more clinical details, and is potentially less prone to errors. Medical record abstraction was conducted by the Centers for Medicare and Medicaid Services’ (CMS) Clinical Data Abstraction Center. Medical records in the MPSMS are randomly selected from the CMS “validation sample” for process-of-care measures required for the Hospital Inpatient Quality Reporting Program. Randomly selected hospitals contributed approximately equal numbers of randomly selected medical records to the MPSMS, regardless of their size. This sampling method is used to represent common in-hospital AEs at the national level.
Patient demographic factors that were assessed included age, sex, race (white, black, other), documentation of obesity, and tobacco smoking within 1 year of the surgery. In addition, a number of comorbidities including congestive heart failure (CHF), coronary artery disease (CAD), renal disease, cerebrovascular disease, chronic obstructive pulmonary disease, history of cancer, diabetes, and warfarin use in the week before admission were assessed.
The primary analyses were the temporal trends in patient characteristics and rates of in-hospital AEs over the study period. A complete list of postoperative AEs captured by the MPSMS database is provided in the Appendix A. A secondary outcome was to identify risk factors associated with the development of AEs.
A descriptive analysis to compare temporal differences in patient characteristics and in-hospital AEs was performed using the Mantel-Haenszel χ2 test for categorical variables and the Kruskal-Wallis test for continuous variables. Linear mixed effects models were fitted with a logit link function to evaluate the temporal trends, adjusting for patient characteristics described previously. An ordinal time variable, ranging from 0 to 7, corresponding to years 2010 (time = 0) to 2017 (time = 7), was included in the models to represent the annual trend in AE rates. The models were also fitted with state-specific random intercepts to account for within-state and between-state variations. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC). The study followed the guidelines for cohort studies, described in the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies [14].
Results
There were 14,057 patients included in the study. The mean (standard deviation) age was 65.7 (10.0), 37.5% were male, and 55.9% were older than 65 years. The mean annual patient sample size was 1757 patients. Over the study period, there was a significant increase in the prevalence of obesity (35.17% to 57.6%, P < .001), tobacco smoking (12.5% to 17.8%, P < .001), and renal disease (5.2% to 8.9%, P < .001). This was accompanied by a significant decrease in the rates of CAD (17.3% to 13.4%, P < .001) and warfarin use during the week before surgery (6.7% to 3.1%, P < .001). Table 1 summarizes the demographic and comorbidity profiles of the study sample.
Table 1.
Baseline characteristics of the study sample from 2010 to 2017.
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P value |
|---|---|---|---|---|---|---|---|---|---|
| N | 2133 | 2545 | 2145 | 1247 | 1520 | 969 | 2159 | 1339 | – |
| Age (y) | 65.72 ± 10.46 | 65.68 ± 10.22 | 65.90 ± 9.82 | 65.45 ± 10.07 | 65.38 ± 9.62 | 65.42 ± 9.73 | 65.71 ± 9.92 | 65.81 ± 9.61 | .6612 |
| Sex | .1235 | ||||||||
| Male | 777 (36%) | 948 (37%) | 798 (37%) | 465 (37%) | 562 (37%) | 376 (39%) | 840 (39%) | 505 (38%) | |
| Female | 1356 (64%) | 1597 (63%) | 1347 (63%) | 782 (63%) | 958 (63%) | 593 (61%) | 1319 (61%) | 834 (62%) | |
| Insurance type | .3296 | ||||||||
| Medicare | 1170 (55%) | 1310 (51%) | 1166 (54%) | 659 (53%) | 750 (49%) | 506 (52%) | 1128 (52%) | 722 (54%) | |
| Other | 963 (45%) | 1235 (49%) | 979 (46%) | 588 (47%) | 770 (51%) | 463 (48%) | 1031 (48%) | 617 (46%) | |
| Race | .6845 | ||||||||
| White | 1837 (86%) | 2201 (87%) | 1892 (88%) | 1086 (87%) | 1314 (86.5%) | 841 (87%) | 1870 (87%) | 1154 (86%) | |
| Black | 167 (8%) | 187 (7%) | 146 (7%) | 98 (8%) | 114 (7.5%) | 74 (8%) | 169 (8%) | 105 (8%) | |
| Other | 129 (6%) | 157 (6%) | 107 (5%) | 63 (5%) | 92 (6%) | 54 (5%) | 120 (5%) | 80 (6%) | |
| Diabetes | 566 (26.5%) | 638 (25.1%) | 559 (26.1%) | 308 (24.7%) | 347 (22.8%) | 235 (24.2%) | 544 (25.2%) | 351 (26.2%) | .4169 |
| Obesity | 748 (35.07%) | 1023 (40.2%) | 938 (43.7%) | 608 (48.8%) | 762 (50.1%) | 549 (56.7%) | 1239 (57.4%) | 772 (57.6%) | <.0001 |
| Current smoker | 267 (12.5%) | 323 (12.7%) | 282 (13.1%) | 187 (15%) | 230 (15.1%) | 152 (15.7%) | 374 (17.3%) | 239 (17.8%) | <.0001 |
| Cancer | 261 (12.2%) | 287 (11.3%) | 251 (11.7%) | 145 (11.6%) | 181 (11.9%) | 119 (12.3%) | 286 (13.2%) | 168 (12.5%) | .1208 |
| CVD | 127 (5.9%) | 145 (5.7%) | 118 (5.5%) | 78 (6.3%) | 81 (5.3%) | 60 (6.2%) | 122 (5.6%) | 82 (6.1%) | .8758 |
| CHF/pulmonary edema | 109 (5.1%) | 121 (4.7%) | 97 (4.5%) | 54 (4.3%) | 58 (3.8%) | 40 (4.1%) | 104 (4.8%) | 62 (4.6%) | .4499 |
| COPD | 192 (9%) | 262 (10.3%) | 181 (8.4%) | 103 (8.3%) | 13 (8.6%) | 104 (10.7%) | 189 (8.7%) | 121 (9%) | .5985 |
| CAD | 370 (17.3%) | 449 (17.6%) | 365 (17%) | 193 (15.5%) | 202 (13.3%) | 138 (14.2%) | 292 (13.5%) | 180 (13.4%) | <.0001 |
| Renal disease | 112 (5.2%) | 151 (5.9%) | 146 (6.8%) | 84 (6.7%) | 96 (6.3%) | 75 (7.7%) | 164 (7.6%) | 119 (8.9%) | <.0001 |
| Warfarin in week before surgery | 142 (6.7%) | 136 (5.3%) | 154 (7.2%) | 76 (6.1%) | 63 (4.1%) | 40 (4.1%) | 85 (3.9%) | 42 (3.1%) | <.0001 |
COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease.
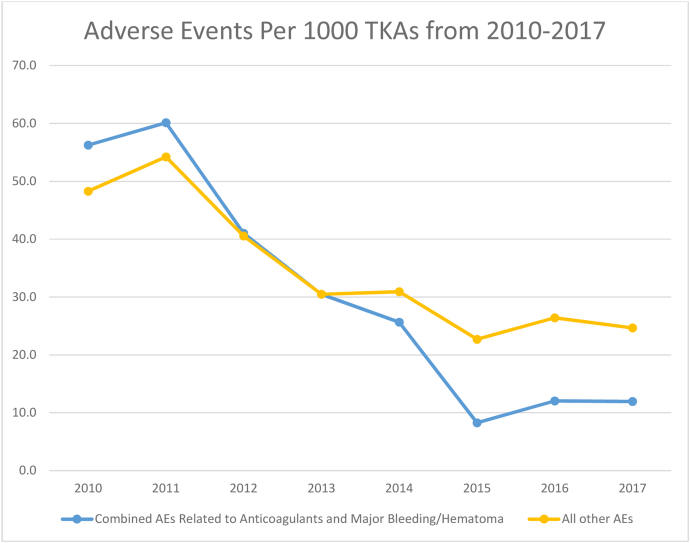
The percentage of patients experiencing in-hospital AEs decreased from 2010 to 2017 (4.9% to 2.5%, P < .001). Specifically, there were significant reductions in catheter-associated urinary tract infections (CAUTIs) (1.1% to 0.1%, P < .001), pressure ulcers (0.8% to 0.2%, P < .001), major bleeding or hematomas (2.8% to 1.0%, P < .001), and venous thromboembolism (VTE) (0.3% to 0.1%, P = .035). There were also significant reductions in drug-related AEs, including those related to low-molecular-weight heparin and factor Xa inhibitors (1.7% to 0.1%, P < .001), warfarin (0.8% to 0.1%, P < .01), and hypoglycemic agents (1.0% to 0.1%, P < .001). Collectively, AEs related to anticoagulants and major bleeding/hematomas showed the greatest decline (Fig. 1). There were no changes in the rates of inpatient falls, pneumonia, wound dehiscence, nonmajor hematoma, cardiovascular or cardiac events, deep infection, or mortality. Table 2 summarizes the annual rates of inpatient AEs. The adjusted annual decline in the risk of developing an AE was 14% (95% CI 10% to 17%).
Figure 1.
Temporal trends in adverse events related to anticoagulants (other than aspirin) and major bleeding/hematoma for primary total knee arthroplasty.
Table 2.
Rates of in-hospital adverse events from 2010 to 2017.
| Year | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | P value |
|---|---|---|---|---|---|---|---|---|---|
| Total patients, N | 2133 | 2545 | 2145 | 1247 | 1520 | 969 | 2159 | 1339 | |
| N (%) | |||||||||
| Any adverse event (AE) | 105 (4.92) | 121 (4.75) | 78 (3.64) | 28 (2.25) | 40 (2.63) | 20 (2.06) | 44 (2.04) | 33 (2.46) | <.0001 |
| Mortality | 1 (0.05) | 7 (0.28) | 1 (0.05) | 2 (0.16) | 1 (0.07) | 0 (0.00) | 6 (0.28) | 2 (0.15) | .5269 |
| Return to operating room | 3 (0.14) | 3 (0.12) | 2 (0.09) | 0 (0.00) | 0 (0.00) | 1 (0.10) | 2 (0.09) | 2 (0.15) | .7649 |
| AEs associated with hypoglycemic agents | 21 (0.98) | 18 (0.71) | 9 (0.42) | 2 (0.16) | 5 (0.33) | 4 (0.41) | 5 (0.23) | 1 (0.07) | <.0001 |
| AEs associated with intravenous heparin | 1 (0.05) | 1 (0.04) | 2 (0.09) | 0 (0.00) | 1 (0.07) | 0 (0.00) | 0 (0.00) | 0 (0.00) | .2096 |
| AEs associated with low-molecular-weight heparin and factor Xa inhibitor | 36 (1.69) | 57 (2.24) | 32 (1.49) | 15 (1.20) | 13 (0.86) | 2 (0.21) | 3 (0.14) | 1 (0.07) | <.0001 |
| AEs associated with warfarin | 18 (0.84) | 27 (1.06) | 13 (0.61) | 8 (0.64) | 2 (0.13) | 0 (0.00) | 1 (0.05) | 0 (0.00) | <.0001 |
| Catheter-associated urinary tract infections | 23 (1.08) | 29 (1.14) | 15 (0.7) | 9 (0.72) | 6 (0.39) | 4 (0.41) | 2 (0.09) | 2 (0.15) | <.0001 |
| Pressure ulcers | 18 (0.84) | 23 (0.90) | 19 (0.89) | 6 (0.48) | 4 (0.26) | 2 (0.21) | 3 (0.14) | 2 (0.15) | <.0001 |
| Falls | 17 (0.80) | 29 (1.14) | 21 (0.98) | 15 (1.20) | 19 (1.25) | 3 (0.31) | 17 (0.79) | 5 (0.37) | .0628 |
| Cardiac events | 2 (0.09) | 6 (0.24) | 4 (0.19) | 1 (0.08) | 3 (0.20) | 0 (0.00) | 4 (0.19) | 4 (0.30) | .5915 |
| Pneumonia | 10 (0.47) | 11 (0.43) | 8 (0.37) | 1 (0.08) | 7 (0.46) | 1 (0.10) | 5 (0.23) | 3 (0.22) | .0644 |
| Venous thromboembolic events | 6 (0.28) | 9 (0.35) | 10 (0.47) | 4 (0.32) | 3 (0.20) | 2 (0.21) | 3 (0.14) | 1 (0.07) | .0351 |
| Deep infection | 1 (0.05) | 1 (0.04) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 2 (0.09) | 0 (0.00) | .9104 |
| Wound dehiscence | 1 (0.05) | 3 (0.12) | 1 (0.05) | 0 (0.00) | 1 (0.07) | 0 (0.00) | 1 (0.05) | 1 (0.07) | .6577 |
| Hematoma | 6 (0.28) | 4 (0.16) | 6 (0.28) | 1 (0.08) | 4 (0.26) | 1 (0.1) | 3 (0.14) | 2 (0.15) | .3178 |
| Major bleeding/hematoma | 59 (2.77) | 64 (2.51) | 35 (1.63) | 14 (1.12) | 19 (1.25) | 5 (0.52) | 19 (0.88) | 13 (0.97) | <.0001 |
| Cardiovascular AEs | 6 (0.28) | 7 (0.28) | 7 (0.33) | 2 (0.16) | 0 (0.00) | 1 (0.10) | 3 (0.14) | 4 (0.30) | .2268 |
| Revision surgery during index hospitalization | 0 (0.00) | 1 (0.04) | 0 (0.00) | 0 (0.00) | 1 (0.07) | 6 (0.62) | 7 (0.32) | 7 (0.52) | <.0001 |
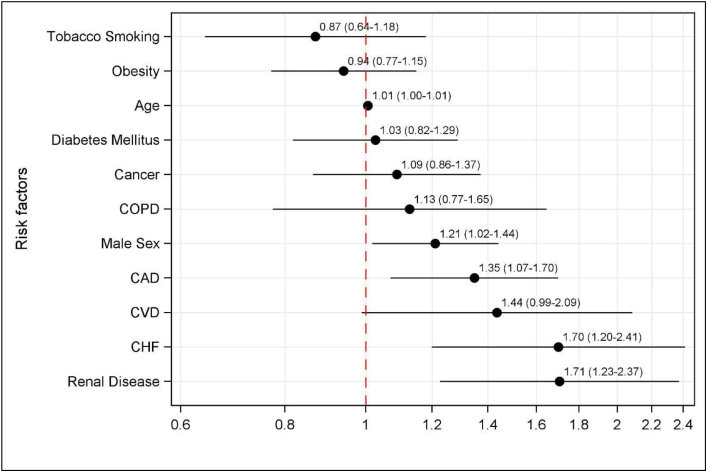
Mixed models identified five patient factors that were associated with developing any inpatient AEs (Fig. 2): age (OR = 1.01, 95% CI 1.00-1.01 for each year of increased age), male sex (OR = 1.21, 95% CI 1.02-1.44), CAD (OR = 1.35, 95% CI 1.07-1.70), CHF (OR = 1.85, 95% CI 1.70 (1.20-2.41), and renal disease (OR = 1.71, 95% CI 1.23-2.37). Figure 2 presents a forest plot of the risk factors for developing a postoperative inpatient AE based on the mixed model.
Figure 2.
Risk factors associated with developing a postoperative in-hospital adverse event. COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease.
Discussion
In this study, we used a nationwide, chart-abstracted patient safety monitoring database to examine the trends in comorbidities profiles and in-hospital AEs after primary TKA. We found significant upward trends in the rates of obesity, tobacco smoking, and renal disease but lower rates of CAD and warfarin use. Because MPSMS captures only the course of care during hospitalization, an increase in the prevalence of some comorbidities in our patient population could be related to the increasing tendency for TKA to be performed on an ambulatory basis, leaving the higher risk patients to more likely be included. There was a persistent decrease in the observed incidence of in-hospital AEs (a relative 14% per year), which was mainly due to reductions in major bleeding/hematoma, VTEs, pressure ulcers, CAUTIs, and adverse drug events related to hypoglycemics and anticoagulants. Advanced age, male sex, history of CAD, CHF, and renal disease were associated with in-hospital AEs.
Our study updates previous reports on the incidence of inpatient AEs after primary TKA. In a retrospective review of the MPSMS database between 2002 and 2004, Huddleston et al. [15] reported that the rates of major bleeding/hematoma, CAUTI, and VTE were 1.7%, 2.4%, and 1.1%, respectively. Ten years since that study, we found that the rates of those complications have decreased further, especially for CAUTI and VTE. In a more recent retrospective review using the American College of Surgeons National Surgical Quality Improvement Program (ACS-NSQIP), between 2006 and 2016, Sarpong et al. [16] found the rates of deep vein thrombosis and CAUTI to be 0.79% and 0.74%, respectively. The decline in CAUTIs is likely due to orthopedic surgeons increasingly abandoning the practice of routine indwelling catheters and widespread guidelines regarding appropriate use of urinary catheters [17,18]. The decline in the rates of major bleeding and hematoma is likely multifactorial. Since 2013, there has been widespread use of tranexamic acid administered intraoperatively, which has been shown to reduce blood loss and transfusion risk for TKA [19]. In addition, the past few years have witnessed a greater shift toward the use of aspirin for deep vein thrombosis prophylaxis as an alternative to warfarin and heparin-based anticoagulants [[20], [21], [22]]. In our study, in 2010, 95.1% of patients received warfarin, low-molecular-weight heparin, or a factor Xa inhibitor, while in 2017, only 40.5% of patients received these agents. This factor likely played a role in the marked decrease in the rate of AEs attributed to nonaspirin anticoagulants. MPSMS does not abstract aspirin use, so we were unable to detect the rates of bleeding events specifically related to aspirin; nonetheless, the overall rate of major bleeding/hematomas, whether or not associated with a specific agent, declined significantly.
Although we observed relatively low rates of in-hospital mortality ranging from 0.1% to 0.3%, these rates have remained stagnant during our study period and even when compared to older reports. In a retrospective review of the MPSMS database between 2002 and 2004, Huddleston et al. [15] found a 0.3% rate of inpatient mortality among patients undergoing primary TKA. In a systematic review of the literature, Berstock et al. [23] estimated the 30- and 90-day mortality after TKA at 0.2% and 0.4%, respectively, with cardiovascular complications such as myocardial infarction being the primary cause for mortality.
It should come as no surprise that our multivariate analyses identified preoperative CAD as an independent risk factor for AEs along with advanced age, male sex, renal disease, and CHF. These findings are consistent with some previous reports. In a recent retrospective review using the ACS-NSQIP database, Robinson et al. [24] found that male gender was associated with higher risk for sepsis and cardiovascular complications. In another ACS-NSQIP study, Curtis et al. [25] demonstrated increased risk for wound dehiscence and myocardial infarction in patients with heart failure.
One of the interesting findings of this study is the reduction in overall rates of AEs despite the increasing prevalence of obesity, tobacco smoking, and renal disease in our sample and the abundant evidence demonstrating the negative postsurgical impact of those factors [26,27]. A plausible explanation that may be contributing to the overall reduction in AEs is the increased awareness and screening for those risk factors in the preoperative period. Value-based incentive payment programs measuring risk-standardized complication rates for elective primary THA and TKA procedures, such as the CMS’ Hospital Value-Based Purchasing program, may further incentivize documentation of certain risk factors. Furthermore, changes in specific processes of care may have played a role for some types of AEs, for example, declining usage of perioperative bladder catheterization might contribute to decreasing CAUTI rates. Preoperative optimization has received heightened attention in the arthroplasty community during the study period. Springer [27] and Thomsen et al. [28] showed that preoperative smoking cessation resulted in lower risk of postsurgical AEs and need for revision surgery. Similarly, more patients are now undergoing weight loss programs including bariatric surgery before joint arthroplasty, potentially optimizing associated comorbidities and leading to improved outcomes [29,30]. Growing efforts by orthopedic surgeons to address modifiable risk factors before surgery may, therefore, be helpful in mitigating their detrimental effects.
This study should be interpreted in the context of some limitations. First, given the retrospective methodology, we can only measure AEs that are detected and documented. Second, MPSMS does not collect all potential AEs. Furthermore, the declining length of stay (from a mean of 3.4 ± 1.5 days in 2010 to 2.4 ± 1.2 days in 2017) during the period of our study could have reduced the chance of detecting late AEs, such as VTE, wound dehiscence, or CAUTI. However, our findings corroborate recent reports of THA and TKA 90-day complication rates calculated using administrative claims data, supporting the validity of our study results [31]. Third, the lack of significant trends for certain AEs and mortality may be due to insufficient power to detect an association with these very rare AEs. Fourth, no conclusions can be drawn regarding complications occurring after hospital discharge. Fifth, it is possible that the decreased in-hospital complication rates could be related because of improved documentation of chronic conditions present on admission. Finally, as a primarily safety monitoring database, it was not possible to pinpoint the specific reasons for the observed decline in complication rates.
Conclusions
The safety of inpatient TKA has continued to improve over the past decade despite worsening trends in the prevalence of obesity, tobacco smoking, and renal disease in our sample. Elderly male patients, especially those with CAD, chronic heart failure, and/or renal disease appear to be at the highest risk for experiencing in-hospital AEs.
Acknowledgments and funding sources:
The authors thank all previous and current MPSMS team members for their contributions to this work, with a special thanks to Shih-Yieh Ho (Senior Analyst at Yale New Haven Health Services Corporation/Center for Outcomes Research and Evaluation) and Deron H. Galusha (Statistician at the Yale School of Medicine) for their contributions to the statistical analysis and valuable comments.
This project was funded under contract no. HHSA290201800005C from the Agency for Healthcare Research and Quality, U.S. Department of Health and Human Services. The content of the publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. The authors assume full responsibility for the accuracy and completeness of the ideas presented. Dr. Wang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to https://doi.org/10.1016/j.artd.2021.08.010.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.artd.2014.12.004.
Conflicts of interest
M.J.H. is in the editorial or governing board of Journal of Bone and Joint Surgery and Arthroplasty Today and is a board member for American Orthopaedic Association. L.G.S. receives $5000 for consultancy on NIH grant on knee OA (PI, E. Losina) through Brigham & Women’s Hospital, receives salary support from Centers for Medicare and Medicaid services through contract to YNHHS to develop, maintain, and implement quality measures for federal payment programs, and is a board member for Quality Measures Subcommittee Chair for American College of Rheumatology.
Supplementary data
References
- 1.Kim Y., Flamm A., ElSohly M.A. Poison Ivy, Oak, and sumac Dermatitis: what is known and what is new? Dermatitis. 2019;30(3):183. doi: 10.1097/DER.0000000000000472. [DOI] [PubMed] [Google Scholar]
- 2.Peters C.L., Shirley B., Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty. 2006;21(6 Suppl 2):132. doi: 10.1016/j.arth.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Kim S., Losina E., Solomon D.H., Wright J., Katz J.N. Effectiveness of clinical pathways for total knee and total hip arthroplasty: literature review. J Arthroplasty. 2003;18(1):69. doi: 10.1054/arth.2003.50030. [DOI] [PubMed] [Google Scholar]
- 4.Molloy I.B., Martin B.I., Moschetti W.E., Jevsevar D.S. Effects of the length of stay on the cost of total knee and total hip arthroplasty from 2002 to 2013. J Bone Joint Surg Am. 2017;99(5):402. doi: 10.2106/JBJS.16.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Backstein D., Thiagarajah S., Halawi M.J., Mont M.A. Outpatient total knee arthroplasty-the new Reality and how can it Be Achieved? J Arthroplasty. 2018;33(12):3595. doi: 10.1016/j.arth.2018.09.042. [DOI] [PubMed] [Google Scholar]
- 6.Sutton J.C., 3rd, Antoniou J., Epure L.M., Huk O.L., Zukor D.J., Bergeron S.G. Hospital discharge within 2 Days following total hip or knee arthroplasty does not increase major-complication and Readmission rates. J Bone Joint Surg Am. 2016;98(17):1419. doi: 10.2106/JBJS.15.01109. [DOI] [PubMed] [Google Scholar]
- 7.Healy W.L., Iorio R., Ko J., Appleby D., Lemos D.W. Impact of cost reduction programs on short-term patient outcome and hospital cost of total knee arthroplasty. J Bone Joint Surg Am. 2002;84(3):348. doi: 10.2106/00004623-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Metersky M.L., Wang Y., Klompas M., Eckenrode S., Bakullari A., Eldridge N. Trend in Ventilator-associated pneumonia rates between 2005 and 2013. JAMA. 2016;316(22):2427. doi: 10.1001/jama.2016.16226. [DOI] [PubMed] [Google Scholar]
- 9.Metersky M.L., Eldridge N., Wang Y., Mortensen E.M., Meddings J. National trends in the frequency of bladder catheterization and physician-diagnosed catheter-associated urinary tract infections: results from the Medicare Patient Safety Monitoring System. Am J Infect Control. 2017;45(8):901. doi: 10.1016/j.ajic.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 10.Vorhies J.S., Wang Y., Herndon J., Maloney W.J., Huddleston J.I. Readmission and length of stay after total hip arthroplasty in a national Medicare sample. J Arthroplasty. 2011;26(6 Suppl):119. doi: 10.1016/j.arth.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y., Eldridge N., Metersky M.L. Association between hospital performance on patient safety and 30-day mortality and Unplanned Readmission for Medicare Fee-for-Service patients with Acute myocardial infarction. J Am Heart Assoc. 2016;5(7):e003731. doi: 10.1161/JAHA.116.003731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eckenrode S., Bakullari A., Metersky M.L. The association between age, sex, and hospital-acquired infection rates: results from the 2009-2011 National Medicare Patient Safety Monitoring System. Infect Control Hosp Epidemiol. 2014;35(Suppl 3):S3. doi: 10.1086/677831. [DOI] [PubMed] [Google Scholar]
- 13.Metersky M.L., Eldridge N., Wang Y. Predictors of warfarin-associated adverse events in hospitalized patients: Opportunities to prevent patient harm. J Hosp Med. 2016;11(4):276. doi: 10.1002/jhm.2528. [DOI] [PubMed] [Google Scholar]
- 14.von Elm E., Altman D.G., Egger M. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 15.Huddleston J.I., Maloney W.J., Wang Y., Verzier N., Hunt D.R., Herndon J.H. Adverse events after total knee arthroplasty: a national Medicare study. J Arthroplasty. 2009;24(6 Suppl):95. doi: 10.1016/j.arth.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Sarpong N.O., Boddapati V., Herndon C.L., Shah R.P., Cooper H.J., Geller J.A. Trends in length of stay and 30-day complications after total knee arthroplasty: an analysis from 2006 to 2016. J Arthroplasty. 2019;34(8):1575. doi: 10.1016/j.arth.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 17.Seyhan Ak E., Ozbas A. The effect of education of nurses on preventing catheter-associated urinary tract infections in patients who undergo hip fracture surgery. J Clin Nurs. 2018;27(5-6):e1078. doi: 10.1111/jocn.14160. [DOI] [PubMed] [Google Scholar]
- 18.Thakker A., Briggs N., Maeda A., Byrne J., Davey J.R., Jackson T.D. Reducing the rate of post-surgical urinary tract infections in orthopedic patients. BMJ Open Qual. 2018;7(2):e000177. doi: 10.1136/bmjoq-2017-000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fillingham Y.A., Ramkumar D.B., Jevsevar D.S. The Efficacy of tranexamic acid in total knee arthroplasty: a Network meta-analysis. J Arthroplasty. 2018;33(10):3090. doi: 10.1016/j.arth.2018.04.043. [DOI] [PubMed] [Google Scholar]
- 20.Daines B.K., Dennis D.A., Amann S. Infection prevention in total knee arthroplasty. J Am Acad Orthop Surg. 2015;23(6):356. doi: 10.5435/JAAOS-D-12-00170. [DOI] [PubMed] [Google Scholar]
- 21.Xia Z.N., Zhou Q., Zhu W., Weng X.S. Low molecular weight heparin for the prevention of deep venous thrombosis after total knee arthroplasty: a systematic review and meta-analysis. Int J Surg. 2018;54(Pt A):265. doi: 10.1016/j.ijsu.2018.04.059. [DOI] [PubMed] [Google Scholar]
- 22.Petersen P.B., Kehlet H., Jorgensen C.C., Lundbeck H. Foundation Centre for Fast-track, and G. Knee Replacement Collaborative, Safety of in-hospital only Thromboprophylaxis after Fast-Track total Hip and knee arthroplasty: a Prospective Follow-Up Study in 17,582 procedures. Thromb Haemost. 2018;118(12):2152. doi: 10.1055/s-0038-1675641. [DOI] [PubMed] [Google Scholar]
- 23.Berstock J.R., Beswick A.D., Lopez-Lopez J.A., Whitehouse M.R., Blom A.W. Mortality after total knee arthroplasty: a systematic review of incidence, temporal trends, and risk factors. J Bone Joint Surg Am. 2018;100(12):1064. doi: 10.2106/JBJS.17.00249. [DOI] [PubMed] [Google Scholar]
- 24.Robinson J., Shin J.I., Dowdell J.E., Moucha C.S., Chen D.D. Impact of gender on 30-day complications after primary total joint arthroplasty. J Arthroplasty. 2017;32(8):2370. doi: 10.1016/j.arth.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Curtis G.L., Newman J.M., George J., Klika A.K., Barsoum W.K., Higuera C.A. Perioperative outcomes and complications in patients with heart failure following total knee arthroplasty. J Arthroplasty. 2018;33(1):36. doi: 10.1016/j.arth.2017.07.043. [DOI] [PubMed] [Google Scholar]
- 26.AbdelSalam H., Restrepo C., Tarity T.D., Sangster W., Parvizi J. Predictors of intensive care unit admission after total joint arthroplasty. J Arthroplasty. 2012;27(5):720. doi: 10.1016/j.arth.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Springer B.D. Modifying risk factors for total joint arthroplasty: Strategies that work Nicotine. J Arthroplasty. 2016;31(8):1628. doi: 10.1016/j.arth.2016.01.071. [DOI] [PubMed] [Google Scholar]
- 28.Thomsen T., Tonnesen H., Moller A.M. Effect of preoperative smoking cessation interventions on postoperative complications and smoking cessation. Br J Surg. 2009;96(5):451. doi: 10.1002/bjs.6591. [DOI] [PubMed] [Google Scholar]
- 29.Fournier M.N., Hallock J., Mihalko W.M. Preoperative optimization of total joint arthroplasty surgical risk: obesity. J Arthroplasty. 2016;31(8):1620. doi: 10.1016/j.arth.2016.02.085. [DOI] [PubMed] [Google Scholar]
- 30.Martin J.R., Jennings J.M., Dennis D.A. Morbid obesity and total knee arthroplasty: a growing Problem. J Am Acad Orthop Surg. 2017;25(3):188. doi: 10.5435/JAAOS-D-15-00684. [DOI] [PubMed] [Google Scholar]
- 31.Bozic K., Yu H., Zywiel M.G. Quality measure public reporting is associated with improved outcomes following hip and knee Replacement. J Bone Joint Surg Am. 2020;102(20):1799. doi: 10.2106/JBJS.19.00964. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.