Abstract
Purpose
To evaluate the rate and predictors of non-adherence to imatinib in gastrointestinal stromal tumor (GIST) patients, as well as to compare the difference in health-related quality of life (HRQOL) between adherent and non-adherent patients.
Patients and Methods
A cross-sectional study at the Oncology Clinic, Hospital Kuala Lumpur was conducted from March to August 2018. All patients with metastatic and/or unresectable GIST aged ≥18 years old and on at least 3 months of imatinib were included. Adherence to imatinib was assessed using the 10-item validated Medication Compliance Questionnaire, with a score of <100% indicating non-adherence. Non-adherence predictors were determined by multiple logistic regressions. HRQOL was evaluated by the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30). The difference in the mean HRQOL scores between adherent and non-adherent groups was determined by multivariate analysis of variance.
Results
A total of 89 patients were enrolled, of which 49 (55.1%) were considered non-adherent. The significant predictors of non-adherence were age (adjusted odds ratio [OR] 0.93; CI 0.89–0.98; P = 0.007), presence of nausea and vomiting (OR 5.63; CI 1.25–25.27; P = 0.024), and presence of comorbidities (OR 4.56; CI 1.44–14.40; P = 0.010). Patients who were in the adherent group showed significantly better score in overall HRQOL, F (15, 73) = 2.09, P < 0.02; Pillai’s trace = 0.3, partial eta squared = 0.30.
Conclusion
Non-adherence to long-term treatment with imatinib among patients with GIST should not be underestimated. Significant predictors of non-adherence among this population are younger age, presence of nausea and vomiting, as well as comorbidities. Patients with good adherence portrayed better HRQOL.
Keywords: compliance, imatinib, gastrointestinal stromal tumor, quality of life
Introduction
The treatment landscape of gastrointestinal stromal tumor (GIST) has changed dramatically with the introduction of tyrosine kinase inhibitor (TKI). Prior to TKI availability, chemotherapy was the primary treatment option for metastatic and/or unresectable GIST; however, it was associated with poor response rate and low overall survival.1 Imatinib, being indicated as a first-line oral TKI treatment for patients with unresectable and/or metastatic GIST, has been shown to significantly improve the survival from about 1 year to 5 years.1
As the management of GIST requires long-term oral therapy, studies have shown that continuous and adequate dosing of imatinib is pivotal in achieving good therapeutic outcomes.2,3 A randomized Phase 3 trial found that interruption of imatinib after 3 years resulted in significantly lower progression-free survival compared with the continuation group.2 Similarly, in another randomized phase 3 study, imatinib interruption after 1 year resulted in rapid progression of disease in most patients with advanced GIST.3 Based on these findings, it is evident that long-term and continuous dosing of imatinib is essential to achieve good clinical outcomes. Hence, patient adherence, ie, the extent to which a patient’s behavior in taking medication corresponds with the agreed recommendations from a healthcare provider, is therefore critical.4
Adherence to imatinib has been extensively studied in patients with chronic myeloid leukemia (CML),5–8 however, limited research has been carried out among GIST patients across the world. A cross-sectional study on adherence to imatinib found that 92 of 158 GIST patients (58.2%) were considered non-adherent.9 In another retrospective study that analyzed pharmacy records over 24 months showed an overall compliance rate of 73% among GIST patients.10 In the prospective Adherence Assessment with Glivec: Indicators and Outcomes (ADAGIO) study, the non-adherence rates assessed with Basel Assessment of Adherence Scale (BAAS) were 29% and 24% in the 4 weeks prior to baseline and follow-up, respectively.11
Various factors have been associated with non-adherence to imatinib. In a cross-sectional study among Chinese GIST patients, the predominant indicators of non-adherence were female gender, living in rural areas and having low global health status scores.9 Another study, based on data from a United States (US) health plan, found that non-adherence was associated with age (>51 years old), gender (female), a higher number of other medications and more cancer complications.12
Non-adherence to long-term therapies has been associated with poor health outcomes in patients with chronic diseases and may eventually affect patients’ quality of life.4 In Malaysia, most studies on adherence to imatinib are only limited to CML patients.13,14 Malaysia is a multiracial country with diverse cultures; hence, factors affecting adherence to oral anticancer may be unique and different from study findings from other countries. Therefore, this study aims to determine the rate of non-adherence to imatinib in GIST patients and to explore factors associated with non-adherence. Besides that, it also aims to compare the difference in health-related quality of life (HRQOL) between adherent and non-adherent patients.
Patients and Methods
A cross-sectional study was conducted from March 2018 to August 2018, at the Oncology Clinic in a tertiary hospital, Kuala Lumpur Hospital. In Malaysia, there were only 6 existing Radiotherapy and Oncology Centers within the Ministry of Health at the time of the study.15 Kuala Lumpur Hospital is located in the central region of the Peninsular Malaysia and serves as one of the six Radiotherapy and Oncology Centers.15 Participants in this study consisted of patients with unresectable and/or metastatic malignant GIST receiving imatinib treatment. Patients who were aged ≥18 years old, on at least 3 months of imatinib and able to understand and communicate in Malay and/or English were included. Patients with known hypersensitivity to imatinib, documented psychiatric/psychological disorders, as well as patients who were too ill to be interviewed or unable to respond to questions were excluded. This study was conducted in accordance with the Declaration of Helsinki. Approval from the Medical Research and Ethics Committee (MREC), Ministry of Health, Malaysia was obtained before the commencement of the study (Approval Reference: NMRR-18-65-39693). Written informed consent was obtained from all participants who agreed to participate.
The primary outcome of this study was the rate of non-adherence to imatinib. Secondary outcomes were non-adherent predictors, as well as differences in the mean HRQOL scores between adherent and non-adherent groups. Sample size was calculated based on the following formula:16
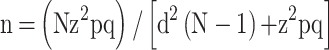 |
where n is the sample size with finite population correction, N is the population size (N = 102 patients), z is the z statistic for a level of confidence (Z = 1.96), p is the expected proportion of non-adherence to imatinib among GIST patients (p = 0.3),11 q is (1 – p), d is precision or margin of error (d = 0.05). The sample size of 78 patients was calculated. In this study, total population sampling was employed, whereby all patients who were diagnosed with unresectable and/or metastatic GIST receiving imatinib from the Oncology Clinic were screened for eligibility. Face-to-face interviews were conducted with all eligible patients by trained researchers. A set of questionnaires in both English and Malay languages were used to collect information on demographic data, assessment of adherence to imatinib, and HRQOL. Each interview session took approximately 20 minutes. Other relevant clinical data were obtained through a review of the medical records.
Demographics and Clinical Data
Information on age, gender, race, marital status, highest educational level, social support and payment status for oncology treatment service were collected during the interview. In addition, clinical data, such as presence of comorbidities, concomitant use of medications, duration of imatinib, pill burden of imatinib, dosing frequency of imatinib, as well as presence and type of adverse drug reaction (ADR), were obtained from medical records.
Assessment of Adherence
Adherence to imatinib was assessed using the 10-item validated Medication Compliance Questionnaire (MCQ).17 Permission to use the questionnaire was obtained from the questionnaire developers. The questionnaire was initially developed to assess adherence to antihypertensive medication but has since been used in other chronic conditions including colorectal and breast carcinomas.18 The questionnaire consists of two domains, ie a drug-taking behavior domain (7 items) and a drug-stopping behavior domain (3 items). It has been validated with internal consistency of Cronbach’s alpha of 0.67 and 0.84 for the respective domains, with test–retest single measure intraclass correlation coefficients (ICC), were 0.78 and 0.93.17
The 10-item MCQ has possible scores on the Likert scale that range from 1 (never) to 5 (very frequent). The codes of the negatively worded items (items 2 to 10) were reversed, and the item scores were converted to a 0 to 100 scale. The final adherence score is reported as the mean of the 10-items on a percentage scale ranging from 0% to 100%. Higher scores indicated better adherence to imatinib. The reliability of the MCQ was also tested with Cronbach’s alpha.
In the management of cancer with oral anticancer, there is no consensus or agreement regarding a definition for “adequate adherence”, with previous investigators using ranges between 80% and 95%.19 Similar to a study on adherence rate of imatinib in GIST patients, this study considered patients with a score of <100% as non-adherent.9
Assessment of HRQOL
HRQOL was evaluated by the validated European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30).20 Both the English and Malay versions of EORTC QLQ-C30 were validated in cancer patients.20,21 Permission to use the questionnaires was obtained from the European Organization for Research and Treatment of Cancer (EORTC) Group, Brussels, Belgium. EORTC QLQ-C30 consists of 30 statements, which are categorized into five functional scales (physical, role, cognitive, emotional, social), three symptom scales (fatigue, pain, nausea/vomiting), six single symptom items (dyspnea, insomnia, appetite loss, constipation, diarrhea, financial difficulties), and a global quality of life scale. Higher scores for the functional scales indicate better functioning and quality of life; higher scores for the symptom scales reflect more severe symptoms. All scores were transformed linearly into a range of 0 to 100.
Statistical Analysis
Data were analyzed using the IBM® Statistical Package for Social Sciences (SPSS) Desktop version 22. Categorical data were expressed as frequencies and percentages, while numerical data were expressed as means and standard deviations. Chi-squared test was used to look for association between categorical variables. In contrast, the independent t-test or the Mann–Whitney test was employed to identify differences in continuous variables between groups. Covariates with P < 0.25 were included in multiple logistic regression. Non-adherence predictors were determined by multiple logistic regressions. The difference in the mean HRQOL scores between adherent and non-adherent groups was determined by multivariate analysis of variance (MANOVA). A value of P < 0.05 is considered statistically significant. Bonferroni analyses were also performed to compare each of the quality of life scales separately.
Results
Socio-Demographic and Clinical Characteristics
All patients who were on imatinib treatment were screened for eligibility. Out of the 102 screened patients, 90 patients were eligible, of which 89 patients gave written consent to participate in the study. Socio-demographic and clinical characteristics of patients are summarized in Table 1. The median age was 56 years old. There were more Chinese (48.3%), followed by Malays (46.1%), and Indians (5.6%). All patients were diagnosed with metastatic and/or unresectable GIST; 35% of them had primary tumors in the stomach, 21% in the small bowel, 9% in the rectum and duodenum, respectively, while the remaining 26% in other sites. More than half of the patients experienced at least 1 or more side effects from imatinib, of which edema (27.0%) and nausea and vomiting (23.6%) being most frequently reported. In terms of nausea and vomiting, no grade 3, 4 or 5 adverse effects were observed. More than half of the patients had known comorbidities (50.6%). Hypertension was the most common comorbidity (38.2%), followed by diabetes mellitus type 2 (22.5%), dyslipidemia (10.1%), ischemic heart disease (4.5%), stroke (4.5%) and chronic kidney disease (3.4%).
Table 1.
Socio-Demographic and Clinical Characteristics of Patients (n=89)
| Characteristics | Total Patients N=89, (%) | Adherence to Imatinib | ||
|---|---|---|---|---|
| Adherent N=40 (%) | Non-Adherent N=49 (%) | p-Value | ||
| Age in years, mean (SD) | 60.6 (12.5) | 62.5 (10.9) | 59.0 (13.6) | 0.183 |
| Gender | 0.321 | |||
| Male | 46 (52.7) | 23 (25.8) | 23 (25.8) | |
| Female | 43 (48.3) | 17 (19.1) | 26 (29.2) | |
| Race | 0.345 | |||
| Malay | 41 (46.1) | 17 (19.1) | 24 (27.0) | |
| Chinese | 43 (48.3) | 22 (24.7) | 21 (23.6) | |
| Indian | 5 (5.6) | 1 (1.1) | 4 (4.5) | |
| Marital status | 0.460 | |||
| Single/divorced/widow | 9 (10.1) | 3 (3.4) | 6 (6.7) | |
| Married | 80 (89.9) | 37 (41.6) | 43 (48.3) | |
| Highest educational level | 0.849 | |||
| Primary school or less | 28 (31.5) | 13 (14.6) | 15 (16.9) | |
| Secondary school or more | 61 (68.5) | 27 (30.3) | 34 (38.2) | |
| Social support | 0.142 | |||
| Living alone | 7 (7.9) | 5 (5.6) | 2 (2.2) | |
| Living with family | 82 (92.1) | 35 (39.3) | 47 (52.8) | |
| Payment status for oncology treatment service | 0.872 | |||
| Government-referred charge | 50 (56.2) | 23 (25.8) | 27 (30.3) | |
| Private-referred charge | 10 (11.2) | 5 (5.6) | 5 (5.6) | |
| Free of charge | 29 (32.6) | 12 (13.5) | 17 (19.1) | |
| Presence of comorbidities | 0.169 | |||
| No | 44 (49.4) | 23 (25.8) | 21 (23.6) | |
| Yes | 45 (50.6) | 17 (19.1) | 28 (31.5) | |
| Concomitant medications | 0.774 | |||
| None | 43 (48.3) | 20 (22.5) | 23 (25.8) | |
| ≥1 type of medication(s) | 46 (51.7) | 20 (22.5) | 26 (29.2) | |
| Duration of imatinib therapy | 0.391 | |||
| ≤1 year | 24 (27.0) | 9 (10.1) | 15 (16.9) | |
| >1 year | 65 (73.0) | 31 (34.8) | 34 (38.2) | |
| Presence of ADR | 0.003 | |||
| No | 46 (51.7) | 23 (25.8) | 13 (14.6) | |
| Yes | 53 (59.6) | 17 (19.1) | 36 (40.4) | |
| Presence of edema | 0.706 | |||
| No | 65 (73.0) | 30 (33.7) | 35 (39.3) | |
| Yes | 24 (27.0) | 10 (11.2) | 14 (15.7) | |
| Presence of nausea and vomiting | 0.006 | |||
| No | 68 (76.4) | 36 (40.4) | 32 (36.0) | |
| Yes | 21 (23.6) | 4 (4.5) | 17 (19.1) | |
| Presence of diarrhea | 0.806 | |||
| No | 77 (86.5) | 35 (39.3) | 42 (47.2) | |
| Yes | 12 (13.5) | 5 (5.6) | 7 (7.9) | |
| Presence of skin rash | 0.089 | |||
| No | 82 (92.1) | 39 (43.8) | 43 (48.3) | |
| Yes | 7 (7.9) | 1 (1.1) | 6 (6.7) | |
| Pill burden of imatinib | 0.677 | |||
| 1 pill | 58 (65.2) | 27 (30.3) | 31 (34.8) | |
| ≥2 pills | 31 (34.8) | 13 (14.6) | 18 (20.2) | |
| Dosing frequency of imatinib | 0.819 | |||
| Once daily | 84 (92.1) | 38 (42.7) | 46 (51.7) | |
| Twice daily | 5 (5.6) | 2 (2.2) | 3 (3.4) | |
Abbreviations: SD, standard deviation; ADR, adverse drug reaction.
Assessment of Adherence to Imatinib
The overall mean and standard deviation (SD) of imatinib adherence score from MCQ was 94.4% (7.4%). In this study, based on the definition of non-adherence, 49 (55.1%) were considered non-adherent. The mean scores of each item are shown in Table 2. Among these responses, item 3 (“you find it difficult to take the medication everyday”) was reported with the lowest mean adherence score of 87.2%. Reliability of the MCQ used in this study (Cronbach’s alpha) were 0.701 and 0.744 for drug-taking and drug-stopping behavior domains, respectively.
Table 2.
MCQ Score for Each Item (n=89)
| Number | Items | Mean, % (SD) |
|---|---|---|
| 1 | You take your medication as agreed with your doctor | 95.3 (10.5) |
| 2 | You only take your medication when you are not feeling well | 89.9 (21.3) |
| 3 | You find it difficult to take your medication everyday | 87.2 (20.1) |
| 4 | You forget to take your medication | 89.2 (16.5) |
| 5 | When you forget to take your medication, you take the following dose twice as much as directed by your doctor | 99.8 (2.1) |
| 6 | You change the time to take the medication without consulting your doctor | 94.6 (12.3) |
| 7 | You reduce the dose of medication when you are feeling well | 96.6 (12.1) |
| 8 | You stop taking the medication when you feel that it is not effective | 98.4 (6.9) |
| 9 | You stop taking the medication when you experience adverse effect from the medication | 94.4 (12.8) |
| 10 | You stop taking the medication when you feel healthy | 98.0 (8.0) |
Abbreviation: SD, standard deviation.
The results from multiple logistic regression analysis shown in Table 3 indicated that younger age, presence of comorbidities, and presence of nausea and vomiting were the associated factors of non-adherence to imatinib. In this study, younger patients were reported to be more likely to be non-adherent compared with older age (adjusted odds ratio [OR] 0.93, P=0.007). Patients with comorbidities were also more likely to be non-adherent compared with patients with no known comorbidities (OR 4.56, P=0.010). In addition, patients who experienced the presence of nausea and vomiting were more likely to be non-adherent compared with patients who did not experience that side effect (OR 5.63, P=0.024). This model fits reasonably well with no significant interaction among independent variables being detected. The area under the curve of Receiver Operating Characteristics (ROC) is 0.746 (95% CI: 0.65–0.85), indicating the model can accurately discriminate 74.6% of the cases.
Table 3.
Factors Predicting the Non-Adherence to Imatinib (n=89)
| Variables | Univariate Analysis | Multiple Logistic Regression | ||||||
|---|---|---|---|---|---|---|---|---|
| b | Crude OR | 95% CI | p-Value | b | Adj OR | 95% CI | p-Value | |
| Age | −0.02 | 0.98 | 0.94–1.01 | 0.184 | −0.07 | 0.93 | 0.89–0.98 | 0.007 |
| Living with family | 1.21 | 3.36 | 0.62–18.33 | 0.162 | ||||
| Presence of comorbidities | 0.59 | 1.80 | 0.78–4.20 | 0.171 | 1.52 | 4.56 | 1.44–14.40 | 0.010 |
| Presence of ADR | 1.32 | 3.75 | 1.54–9.14 | 0.004 | ||||
| Presence of nausea and vomiting | 1.57 | 4.78 | 1.46–15.70 | 0.010 | 1.73 | 5.63 | 1.25–25.27 | 0.024 |
| Presence of skin rash | 1.69 | 5.44 | 0.63–47.23 | 0.124 | ||||
Abbreviations: OR, odds ratio; CI, confidence interval; Adj OR, adjusted odds ratio; ADR, adverse drug reaction.
Assessment of HRQOL
Table 4 shows the results of HRQOL as reported by both adherent and non-adherent patients. There was a statistically significant difference in the overall quality of life between adherent and non-adherent groups, F (15, 73) = 2.09, p < 0.02; Pillai’s trace = 0.3, partial eta squared = 0.30. When the results for each of the quality of life scale were considered separately, the only difference to reach statistical significance, using a Bonferroni adjusted alpha level of 0.003, was physical functioning, F (1,87) = 13.50, p < 0.001, partial eta squared = 0.13; emotional functioning, F (1,87) = 17.0, p < 0.001, partial eta squared = 0.16; cognitive functioning, F (1,87) = 11.6, p=0.001, partial eta squared = 0.12; fatigue, F (1,87) = 10.2, p=0.002, partial eta squared = 0.11; nausea and vomiting, F (1,87) = 12.7, p=0.001, partial eta squared =0.13; and insomnia, F (1,87) = 9.9, p=0.002, partial eta squared = 0.10 (Table 4).
Table 4.
Results of the General Linear Model Multivariate Analysis of Variance Testing the Association Between Adherence to Imatinib and the Individual EORTC QLQ-C30 Item
| EORTC QLQ-C30 Item | All Patients | Adherence to Imatinib | p-Value | Partial η2 | |
|---|---|---|---|---|---|
| Adherent | Non-Adherent | ||||
| N=89 Mean (SD) | N=40 Mean (SD) | N=49 Mean (SD) | |||
| Global quality of life | 70.6 (22.3) | 76.7 (23.6) | 65.7 (20.0) | 0.019 | 0.06 |
| Functioning scale | |||||
| Physical functioning | 81.0 (20.3) | 89.2 (15.7) | 74.3 (21.3) | <0.001 | 0.13 |
| Role functioning | 80.9 (27.8) | 87.1 (25.2) | 75.9 (29.1) | 0.058 | 0.04 |
| Emotional functioning | 82.0 (20.8) | 91.3 (13.6) | 74.5 (22.6) | <0.001 | 0.16 |
| Cognitive functioning | 82.4 (19.0) | 89.6 (16.7) | 76.5 (18.9) | 0.001 | 0.12 |
| Social functioning | 85.0 (24.2) | 92.5 (18.5) | 78.9 (26.7) | 0.008 | 0.08 |
| Symptom scale | |||||
| Fatigue | 35.5 (27.8) | 25.6 (28.6) | 43.5 (24.5) | 0.002 | 0.11 |
| Nausea and vomiting | 32.2 (37.4) | 17.5 (29.2) | 44.2 (39.3) | 0.001 | 0.13 |
| Pain | 18.0 (23.9) | 11.7 (19.7) | 23.1 (25.9) | 0.023 | 0.06 |
| Dyspnea | 8.24 (18.3) | 4.2 (11.2) | 11.6 (22.1) | 0.058 | 0.04 |
| Insomnia | 23.6 (31.5) | 12.5 (26.9) | 32.7 (32.3) | 0.002 | 0.10 |
| Appetite loss | 27.0 (32.1) | 22.5 (33.2) | 30.6 (31.1) | 0.238 | 0.02 |
| Constipation | 13.9 (23.5) | 11.7 (25.7) | 15.7 (20.5) | 0.429 | 0.01 |
| Diarrhea | 16.1 (24.7) | 11.7 (25.7) | 19.7 (23.5) | 0.126 | 0.03 |
| Financial difficulty | 20.6 (24.9) | 16.7 (24.7) | 23.8 (24.8) | 0.179 | 0.02 |
Abbreviations: EORTC QLQ-C30, European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; SD, standard deviation; partial η2, partial eta square.
Discussion
The study was conducted to assess the rate of non-adherence to imatinib among patients with metastatic and/or unresectable GIST. More than half of these patients (55.1%) were found to be non-adherent in this study. A previous cross-sectional study reported a similar rate, whereby 58.2% of GIST patients were considered non-adherent to imatinib based on self-reported adherence questionnaire.9 Meanwhile, other studies using BAAS, pharmacy records and medication possession ratio for assessment of adherence reported imatinib non-adherence rate ranging from 24% to 29% among GIST patients.10–12 These differences may be attributed to variations in methodology, definition of level of non-adherence, tools of adherence assessment, and different disease stages of the recruited population.
In the present study, MCQ scores <100% were considered non-adherent. Hence, it is unsurprising that the rate of non-adherence reported in this study was high, which is comparable to previous studies that employed similar definitions of non-adherence.8,9,22 In addition, this study was conducted on patients with metastatic and/or unresectable GIST, whereby adherence to imatinib may be further limited since a clinical response following initiation of imatinib is often achieved quickly in this setting.23 Given the paucity of data in the GIST literature, the current study provides additional evidence that suboptimal adherence to imatinib remains a significant problem in this population.
In this study, younger age was found to be a significant factor associated with non-adherence to imatinib. Existing data on the association between age and adherence to imatinib has been conflicting. In a study investigating the adherence level of imatinib among patients with CML using a microelectronic monitoring device, it was concluded that younger patients were more likely to have lower adherence rate.7 Similarly, another study found that younger patients were found to have lower adherence rate to imatinib.24 In contrast, another study found that increasing age (>51 years old) was significantly associated with non-adherence to imatinib.12 Although the reasons for non-adherence to oral anticancer drug in younger individuals are unclear, previous studies have found that younger patients may not adjust as well as older patients to their cancer condition, affecting their medication adherence.25
The current study also found that the presence of comorbidities was a significant predictor of non-adherence to imatinib, in keeping with results from previous study.26 This was further supported by a systematic review, which found that the presence of comorbidities was associated with non-adherence in patients taking oral anticancer therapy.25 This finding may be explained by the fact that patients with multiple comorbid conditions may have a lower adherence due to adverse effects, drug–drug interaction or polypharmacy.27 Hence, it is important that clinicians should pay more attention to patients with comorbidities by identifying polypharmacy, possible drug–drug interaction, and addressing other barriers to medication adherence.
In agreement with findings from previous studies, the presence of adverse reaction nausea and vomiting was also found to be significantly associated with non-adherence to imatinib.23,28,29 Nausea and vomiting are unpleasant adverse reactions and have been reported to have disrupted patients’ daily activities, hence leading to intentional non-adherence to TKIs, as illustrated in a qualitative study conducted among CML patients in Malaysia.14 This finding indicates that adverse reactions to imatinib should be recognized as soon as possible and managed effectively. The effects of nausea and vomiting from imatinib may be alleviated by administering the medication after meal, splitting the dose, taking the dose at bedtime or administering effective antiemetic therapy.30
As formerly reported, non-adherence to imatinib has been associated with higher cost sharing of the treatment.23,31,32 In contrast, the present study did not find any socioeconomic factors being associated with adherence to imatinib. A plausible explanation was that all the participants in the current study were enrolled in the Malaysian Patient Assistance Program (MYPAP) which helped to reduce the financial burden on the treatment cost. Similar findings were also reported in other countries in which similar patient assistance programs were available.8,9
Apart from that, other socio-demographic data including race, marital status, educational level and social support did not appear to be significantly associated with adherence to imatinib. Although previous local qualitative studies on adherence to TKIs in CML patients have pointed out that religious and social issues may affect patients’ adherence,14,33 the present study did not find a significant difference in adherence levels across the different races.
Pertaining to the HRQOL scores in the functional dimension, only physical, emotional and cognitive functionings were significantly better among adherent patients. However, it is also important to note that there were non-significant trends for higher scores of adherent patients on all other scales in the functional dimension, similar to a finding in a previous study.8 Apart from the functional dimension, the scores of the symptom dimensions were also reported to be higher in the non-adherent group, indicating a worse symptom burden in this group of patients. Undoubtedly, management of associated side effects is an important key in ensuring continuous adherence to imatinib among GIST patients.30 In cancer management, health-related quality of life has been increasingly emphasized as an important part of patient care.34 In this study, it is shown that adherence to imatinib has a significant association with overall HRQOL, although no causality is indicated. It is therefore vital that optimal medication adherence among these patients should be achieved to ensure patients receive the best possible outcomes.
The limitations of this study include the small sample size of a single center. GIST is a relatively rare type of cancer; hence, total population sampling was used in this study to recruit the maximum number of patients possible during the study duration. In addition, another limitation of the study is adherence that was measured using a self-reported questionnaire. In this study, adherence to imatinib was assessed using MCQ, a validated self-reported questionnaire that was previously used among hypertensive and cancer patients in Malaysia.17,18 It was not specifically validated in a GIST population in view of the rarity of the disease and is a limitation. Another limitation of this study is that it did not investigate the impact of adherence on clinical response or outcomes of GIST. Despite these limitations, the findings in this study are valuable and provide additional data on what is scarcely known about adherence to imatinib in patients with GIST in this region. Further study should be warranted by having a larger sample size from a multicenter study. Besides that, further study should also be carried out to investigate the relationship between adherence and clinical response.
The main implication of this study includes providing an insight and awareness among the clinicians on the importance of promoting adherence to imatinib among GIST patients. Adherence should be assessed during every follow-up visit, and barriers to adherence should be identified and addressed accordingly. Adverse events from imatinib, particularly nausea and vomiting, should be managed proactively, while any potential drug–drug interaction or polypharmacy should be intervened appropriately. Adherence can be promoted by enhancing patient education at multidisciplinary levels, such as clinicians, nurses and pharmacists. Patients can be provided with a diary to write down their daily medication intake, as well as to document their concerns and side effects. Besides that, home visits may be conducted to monitor patients in terms of their adherence and the occurrence of adverse events from the therapy. Last but not least, when clinicians are exploring reasons for treatment failure in GIST, poor adherence must always be ruled out before switching to second-line therapy.
Conclusion
Non-adherence to long-term treatment with imatinib is common and should not be underestimated among patients with metastatic and/or unresectable GIST. Significant predictors of non-adherence among this population are younger age, presence of nausea and vomiting, as well as comorbidities. These findings suggest that more emphasis should be placed on these patients, particularly in identifying and overcoming barriers to non-adherence. Patients with good adherence portrayed better HRQOL.
Acknowledgments
We would like to thank the Director-General of Health Malaysia for his permission to publish this article. We would also like to thank the Director and the Head of Pharmacy Department of Kuala Lumpur Hospital for providing administrative support for this study. We would also like to acknowledge Madam Malathi a/p Sriraman and Madam Nurfarahana Hafzan Mahmud for their involvement in the proposal development. We would like to thank Madam Hannah Abdul Halim for the useful discussion during data analysis. This study was not funded by any organization.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.von Mehren M, Joensuu H. Gastrointestinal stromal tumors. J Clin Oncol. 2018;36(2):136–143. doi: 10.1200/JCO.2017.74.9705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Le Cesne A, Ray-Coquard I, Bui BN, et al. Discontinuation of imatinib in patients with advanced gastrointestinal stromal tumours after 3 years of treatment: an open-label multicentre randomised phase 3 trial. Lancet Oncol. 2010;11(10):942–949. doi: 10.1016/S1470-2045(10)70222-9 [DOI] [PubMed] [Google Scholar]
- 3.Blay JY, Le Cesne A, Ray-Coquard I, et al. Prospective multicentric randomized Phase III study of imatinib in patients with advanced gastrointestinal stromal tumors comparing interruption versus continuation of treatment beyond 1 year: the French sarcoma group. J Clin Oncol. 2007;25(9):1107–1113. doi: 10.1200/JCO.2006.09.0183 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Adherence to Long-Term Therapies: evidence for Action; 2003. Available from: https://www.who.int/chp/knowledge/publications/adherence_full_report.pdf?ua=1. Accessed May31, 2021.
- 5.Noens L, Van Lierde MA, De Bock R, et al. Prevalence, determinants, and outcomes of nonadherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. doi: 10.1182/blood-2008-12-196543 [DOI] [PubMed] [Google Scholar]
- 6.Geissler J, Sharf G, Bombaci F, et al. Factors influencing adherence in CML and ways to improvement: results of a patient-driven survey of 2546 patients in 63 countries. J Cancer Res Clin Oncol. 2017;143(7):1167–1176. doi: 10.1007/s00432-017-2372-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marin D, Bazeos A, Mahon FX, et al. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. J Clin Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Unnikrishnan R, Veeraiah S, Mani S, et al. Comprehensive evaluation of adherence to therapy, its associations, and its implications in patients with chronic myeloid leukemia receiving imatinib. Clin Lymphoma Myeloma Leuk. 2016;16(6):366–371. doi: 10.1016/j.clml.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Zhang P, Han Y, et al. Adherence to adjuvant imatinib therapy in patients with gastrointestinal stromal tumor in clinical practice: a Cross-Sectional Study. Chemotherapy. 2019;64(4):197–204. doi: 10.1159/000505177 [DOI] [PubMed] [Google Scholar]
- 10.Tsang J, Rudychev I, Pescatore SL. Prescription compliance and persistency in chronic myelogenous leukemia (CML) and gastrointestinal stromal tumor (GIST) patients (pts) on imatinib (IM) [abstract]. J Clin Oncol. 2006;24(18 suppl):6119. doi: 10.1200/jco.2006.24.18_suppl.6119 [DOI] [Google Scholar]
- 11.Mazzeo F, Duck L, Joosens E, et al. Nonadherence to imatinib treatment in patients with gastrointestinal stromal tumors: the ADAGIO study. Anticancer Res. 2011;31(4):1407–1409. [PubMed] [Google Scholar]
- 12.Feng W, Henk H, Thomas S, et al. Compliance and persistency with imatinib [abstract]. J Clin Oncol. 2006;24(18 suppl):6038. doi: 10.1200/jco.2006.24.18_suppl.6038 [DOI] [Google Scholar]
- 13.Lee PM, Chang CT, Yusoff ZM. Adherence to tyrosine kinase inhibitors among adult chronic myeloid leukemia patients in a Malaysia hospital. Int J Clin Pharm. 2021;43(1):46–54. doi: 10.1007/s11096-020-01070-9 [DOI] [PubMed] [Google Scholar]
- 14.Tan BK, Tan SB, Chen LC, et al. Medication-related issues associated with adherence to long-term tyrosine kinase inhibitors for controlling chronic myeloid leukemia: a qualitative study. Patient Prefer Adherence. 2017;11:1027–1034. doi: 10.2147/PPA.S132894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministry of Health Malaysia. National Strategic Plan for Cancer Control Programme 2016–2020. 1st ed; 2017. Available from: https://www.moh.gov.my/index.php/dl/554756755a584a6961585268626938794d4445334c314a31616e567259573476546d46306157397559577866553352795958526c5a326c6a5831427359573566516d39766131396d61573568624541794e564e46554651794d4445334c6e426b5a673d3d. Accessed May31, 2021. [Google Scholar]
- 16.Daniel WW, Cross CL. Biostatistics: A Foundation for Analysis in the Health Sciences. 10th ed. New York: John Wiley & Sons; 2013. [Google Scholar]
- 17.Hassan NB, Hasanah CI, Foong K, et al. Identification of psychosocial factors of noncompliance in hypertensive patients. J Hum Hypertens. 2006;20(1):23–29. doi: 10.1038/sj.jhh.1001930 [DOI] [PubMed] [Google Scholar]
- 18.Zahrina AK, Norsa’adah B, Hassan NB, et al. Adherence to capecitabine treatment and contributing factors among cancer patients in Malaysia. Asian Pac J Cancer Prev. 2014;15(21):9225–9232. doi: 10.7314/APJCP.2014.15.21.9225 [DOI] [PubMed] [Google Scholar]
- 19.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. doi: 10.3322/caac.20004 [DOI] [PubMed] [Google Scholar]
- 20.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organisation for research and treatment of cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85:365–376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 21.Yusoff N, Low W, Ch Y. The Malay version of the European Organization for research and treatment of cancer quality of life questionnaire (EORTC-QLQ C30): Reliability and Validity Study. Int Med J Malays. 2010;9(2):45–50. [Google Scholar]
- 22.Efficace F, Baccarani M, Rosti G, et al. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: an observational patient-centered outcome study. Br J Cancer. 2012;107(6):904–909. doi: 10.1038/bjc.2012.348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blay JY, Rutkowski P. Adherence to imatinib therapy in patients with gastrointestinal stromal tumors. Cancer Treat Rev. 2014;40(2):242–247. doi: 10.1016/j.ctrv.2013.07.005 [DOI] [PubMed] [Google Scholar]
- 24.Anderson KR, Chambers CR, Lam N, et al. Medication adherence among adults prescribed imatinib, dasatinib, or nilotinib for the treatment of chronic myeloid leukemia. J Oncol Pharm Pract. 2015;21(1):19–25. doi: 10.1177/1078155213520261 [DOI] [PubMed] [Google Scholar]
- 25.Verbrugghe M, Verhaeghe S, Lauwaert K, Beeckman D, Van Hecke A. Determinants and associated factors influencing medication adherence and persistence to oral anticancer drugs: a systematic review. Cancer Treat Rev. 2013;39(6):610–621. doi: 10.1016/j.ctrv.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 26.Fentie AM, Tadesse F, Engidawork E, Gebremedhin A. Prevalence and determinants of non-adherence to imatinib in the first 3-months treatment among newly diagnosed Ethiopian’s with chronic myeloid leukemia. PLoS One. 2019;14(3):e0213557. doi: 10.1371/journal.pone.0213557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen LC, Chen TC, Huang Y-B, Chang CS. Disease acceptance and adherence to imatinib in Taiwanese chronic myeloid leukaemia outpatients. Int J Clin Pharm. 2014;36(1):120–127. doi: 10.1007/s11096-013-9867-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eliasson L, Clifford S, Barber N, Marin D. Exploring chronic myeloid leukemia patients’ reasons for not adhering to the oral anticancer drug imatinib as prescribed. Leuk Res. 2011;35(5):626–630. doi: 10.1016/j.leukres.2010.10.017 [DOI] [PubMed] [Google Scholar]
- 29.Efficace F, Rosti G, Cottone F, et al. Profiling chronic myeloid leukemia patients reporting intentional and unintentional non-adherence to lifelong therapy with tyrosine kinase inhibitors. Leuk Res. 2014;38(3):294–298. doi: 10.1016/j.leukres.2013.07.003 [DOI] [PubMed] [Google Scholar]
- 30.Joensuu H, Trent JC, Reichardt P. Practical management of tyrosine kinase inhibitor-associated side effects in GIST. Cancer Treat Rev. 2011;37(1):75–88. doi: 10.1016/j.ctrv.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 31.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL. Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol. 2014;32(4):306–311. doi: 10.1200/JCO.2013.52.9123 [DOI] [PubMed] [Google Scholar]
- 32.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract. 2011;7(3 Suppl):46s–51s. doi: 10.1200/JOP.2011.000316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim YM, Eng WL, Chan HK. Understanding and challenges in taking tyrosine kinase inhibitors among Malaysian chronic myeloid leukemia patients: a qualitative study. Asian Pac J Cancer Prev. 2017;18(7):1925–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitlinger A, Zafar SY. Health-related quality of life: the impact on morbidity and mortality. Surg Oncol Clin N Am. 2018;27(4):675–684. doi: 10.1016/j.soc.2018.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]


