Abstract
Viral polymerase is an essential enzyme for the amplification of the viral genome and is one of the major targets of antiviral therapies. However, a serious concern to be solved in hepatitis B virus (HBV) infection is the difficulty of eliminating covalently closed circular (ccc) DNA. More recently, therapeutic strategies targeting various stages of the HBV lifecycle have been attempted. Although cccDNA-targeted therapies are attractive, there are still many problems to be overcome, and the development of novel polymerase inhibitors remains an important issue. Interferons and nucleos(t)ide reverse transcriptase inhibitors (NRTIs) are the only therapeutic options currently available for HBV infection. Many studies have reported that the combination of interferons and NRTI causes the loss of hepatitis B surface antigen (HBsAg), which is suggestive of seroconversion. Although NRTIs do not directly target cccDNA, they can strongly reduce the serum viral DNA load and could suppress the recycling step of cccDNA formation, improve liver fibrosis/cirrhosis, and reduce the risk of hepatocellular carcinoma. Here, we review recent studies on combination therapies using polymerase inhibitors and discuss the future directions of therapeutic strategies for HBV infection.
Keywords: hepatitis B virus, polymerase, combination therapy, NRTI, NNRTI, cccDNA
1. Introduction
As one of the main causes of liver disease, hepatitis B virus (HBV) infection is a global health problem affecting approximately 296 million individuals worldwide (https://www.who.int/news-room/fact-sheets/detail/hepatitis-b, accessed on 27 July 2021). Chronic hepatitis B (CHB) virus infection can be classified into several phases with variable levels of serum alanine aminotransferase (ALT) activity, which is a marker of liver damage, HBV antigens, including HBsAg, HBeAg, and core antigen, and HBV DNA [1]. All patients with chronic HBV infection are at high risk of developing liver fibrosis and hepatocellular carcinoma (HCC). To increase the survival rate and decrease the risk of disease progression, many research teams have been working on novel strategies to eliminate the virus.
Unfortunately, current therapies against HBV are limited to interferons and NRTIs (nucleos(t)ide reverse transcriptase inhibitors). Treatment by interferon (IFN)-α, which is an immune modulator, was the first approach developed for treatment of CHB. IFN-α was approved in 1991, and it has been replaced by its pegylated form (Peg-IFN-α), which has a significantly extended half-life and more sustained virologic response. Nucleos(t)ide analogues (NAs), also called NRTIs, are potent polymerase inhibitors directly targeting the viral polymerase elongation process via incorporation into replicated DNA, and namely function as chain terminators. Lamivudine, commonly called 3TC, was utilized at first, but because of the high rate of HBV polymerase gene mutants or variants capable of evading its activity, other NRTIs such as entecavir (ETV) and tenofovir have been introduced as further anti-HBV treatments [2,3].
Even if the replication of the viral genome is inhibited by NRTIs, the effect on HBV replication is only transient; once treatment is discontinued, mRNA expression is resumed because of the existence of cccDNA, which leads to the resumption of viral replication [4,5]. This phenomenon has led to the definition of two types of HBV “cure”: the “functional” cure and the “complete” or “virologic” cure [6]. “Functional” cure refers to the persistent disappearance of HBsAg and acquisition of anti-HBs antibodies, as well as normalization of liver enzymes after treatment. “Complete” or “virological” cure adds to these effects the loss of cccDNA from hepatocytes. For this purpose, long-term suppression of HBV replication is the main endpoint of current therapeutic strategies, with the elimination of HBsAg as an optimal endpoint. Therefore, levels of serum ALT, HBV DNA, and HBsAg are important predictors of long-term prognosis.
The HBV genome is converted to cccDNA from relaxed circular (rc) DNA after its entry into the nucleus, using cellular DNA repair systems [7,8,9,10]. cccDNA transcribes virus-related mRNAs, of which pregenomic RNA (pgRNA) is a template for reverse transcription followed by plus-strand DNA synthesis to generate rcDNA, the viral genome. Since cccDNA lacks a replication origin, it cannot replicate by semi-conservative replication; thus, cccDNA is amplified by its conversion from rcDNA during de novo infection (de novo synthesis) or through recycling steps after intracellular rcDNA amplification (intracellular amplification) [11,12,13]. Once cccDNA is formed, it is stably pooled in the nucleus via intracellular recycling of the HBV genome [14]. Chronic HBV infection is caused by the persistence of cccDNA, which is transcriptionally competent for all HBV RNAs, in the nucleus of hepatocytes [15]. A recent study suggested that the de novo synthesis and intracellular amplification of cccDNA are differentially controlled by viral and/or cellular mechanisms [16].
DNA polymerase κ and λ, which are specifically involved in translesion synthesis and nucleotide excision DNA repair [17], and in meiotic recombination and DNA repair [18], support cccDNA formation in the de novo infection pathway [8]. On the other hand, DNA polymerase α has a role in repairing the minus strand in the conversion of rcDNA to cccDNA and supports the biosynthesis of cccDNA in the intracellular amplification cycle and the viral genome recycling step [19].
The dynamics of cccDNA during long-term culture was investigated by an in vitro infection assay system using NTCP-expressing HepG2 cells [14]. After infection, the cccDNA increased to 5 to 12 copies per cell over a period of 45 days. HBV cccDNA collapsed with a half-life of 40 days under treatment by entecavir (ETV), which inhibits intracellular recycling of the HBV genome by preventing rcDNA formation. When lamivudine was used in HBV-infected woodchucks and ducks, the half-lives were 33–50 and 35–57 days, respectively [20,21].
It is predicted that the mutation rates of essential genes such as polymerase are lower than those of non-essential genes [22,23,24]. However, the possibility of the emergence of drug-resistant mutants cannot be eliminated if the HBV DNA levels are not controlled by long-term administration of NRTIs. The final treatment goal of therapy for HBV-infected patients is to inhibit cccDNA amplification and finally eliminate cccDNA. Lately, numerous researchers have been trying to develop novel technologies such as genome editing for the elimination of cccDNA [25,26,27]. However, there are many issues to be solved before clinical trials begin: for instance, off-target effects, un-expected chromosomal DNA recombinations and cleavage of integrated HBV DNA. Unfortunately, we do not know the details of the biochemical processes of cccDNA formation and amplification, or the adverse effects of new technologies. A better understanding of the mechanisms of these events should be obtained before devoting extensive resources into the development of new therapies targeting cccDNA. At present, polymerase inhibitors are still the most feasible and powerful therapeutic agents for HBV therapy. In this review, we summarize recent studies of combination therapy using polymerase inhibitors and discuss the importance of non-nucleos(t)ide reverse transcriptase inhibitor (NNRTI) therapies for future treatment of HBV infection.
2. Potential of Polymerase Inhibitors
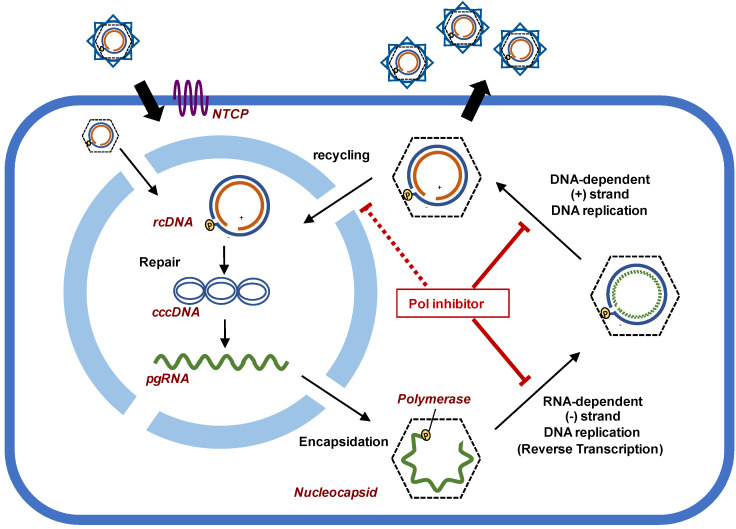
HBV polymerase is one of the most appropriate therapeutic targets because it has multiple and essential roles in the viral replication cycle (Figure 1). pgRNA is transcribed from cccDNA by cellular transcription machineries, and is transported to the cytoplasm, where it is packaged into a nucleocapsid by interacting with the polymerase through the “ε” (“epsilon” for encapsidation) packaging signal. Protein priming starts at position tyrosine 63 (Y63) of the HBV polymerase after RNA packaging, followed by RNA-dependent minus-strand DNA replication, namely, reverse transcription. While pgRNA is degraded by the RNase H activity of polymerase, rcDNA is produced by a DNA-dependent plus strand DNA synthesis, the details of which are described elsewhere [28,29]. Polymerase inhibitors strongly block rcDNA formation and, thereby, inhibit intracellular cccDNA amplification. To avoid further amplification of cccDNA after infection, development of novel NRTIs and NNRTIs is still essential. Until now, NRTIs are the only available direct-acting antivirals (DAAs) for HBV therapy; there are no NNRTIs against HBV polymerase, in contrast to the case for human immunodeficiency virus (HIV) reverse transcriptase (RT).
Figure 1.
The lifecycle of hepatitis B virus (HBV) focusing on viral genome replication. HBV polymerase has several crucial roles in viral replication. Polymerase inhibitors block the reverse transcription pathway, namely RNA-dependent minus-strand DNA synthesis; they also block DNA-dependent plus-strand DNA synthesis, thereby suppressing the recycling step for cccDNA amplification.
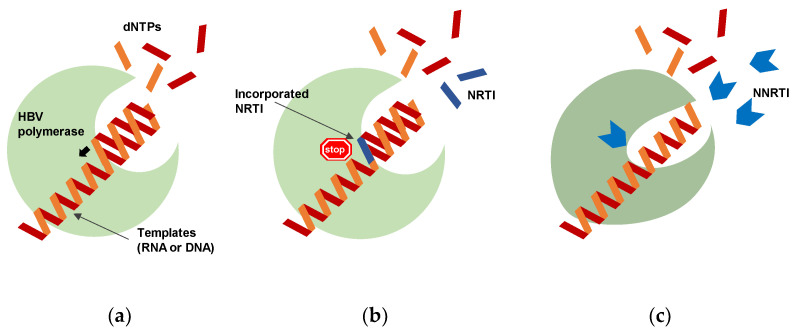
Deoxyribonucleotide triphosphates (dNTPs) are incorporated into nascent DNA by the enzymatic activity of HBV polymerase (Figure 2a), where NRTIs are converted to their triphosphate form in the cell, and are incorporated into nascent DNA by HBV polymerase similarly to natural dNTPs. These NRTIs compete with the natural dNTPs and stop DNA elongation due to their lack of a 3′-hydroxyl group, thereby terminating the incorporation of subsequent incoming nucleotides (Figure 2b) [30,31,32].
Figure 2.
Different roles between nucleos(t)ide RT inhibitors (NRTIs) and non-nucleos(t)ide RT inhibitors (NNRTIs). (a) Deoxyribonucleotide triphosphates (dNTPs) are incorporated into nascent DNA during reverse transcription (minus-strand DNA synthesis) and plus-strand DNA synthesis. (b) NRTIs block DNA synthesis via chain termination by incorporating themselves into the nascent DNA. (c) NNRTIs block DNA synthesis via direct binding to the polymerase and causing enzyme conformational changes that disrupt active-site function, leading to impairment of polymerization activity.
Currently, ETV, tenofovir disoproxil fumarate (TDF), and tenofovir alafenamide (TAF) are the first-line agents for the antiviral treatment of CHB in the clinical guidelines because of the high antiviral effect and low emergence rate of drug-resistant viruses [1,33,34]. Serum hepatitis B surface antigen (HBsAg) is used as a diagnostic marker of HBV infection and reflects the content of intrahepatic HBV cccDNA [35,36]. NRTIs efficiently suppress the viral DNA replication steps by inhibiting reverse transcription and DNA-dependent DNA replication, but they are not involved in the direct inhibition of cccDNA. However, recent studies have reported that NRTI treatment increased IFN-λ3 levels in both in vivo and in vitro experiments, inducing IFN-stimulated genes (ISGs), and then inhibited production of HBsAg in hepatoma cells, suggesting hitherto unknown, synergistic pharmacological effects of NRTIs [37,38].
In addition to NRTIs, pegylated interferon (peg-IFN) has been used in combination therapy, and although the cure rate (diagnosed by loss of HBsAg) of each alone is often very low, combined treatment with peg-IFN and NRTIs has shown a dramatic increase in the cure rate as described in Section 3.2. To overcome HBV infection by combination therapy, it is important not only to develop therapeutic drugs targeting various life cycles, but also to develop drugs targeting the polymerase itself, including NNRTIs. NNRTIs directly bind to the polymerase and cause a conformational change that disrupts the active site function, thereby leading to the loss of polymerization activity (Figure 2c). NNRTIs are one of the essential drugs in the combination therapies for HIV because of their strong antiviral activity, high specificity, and low toxicity.
Combinations of NRTIs and NNRTIs are widely used in HIV therapies. The mode of action of NNRTIs has been investigated by crystallography, structural analysis, and a docking model of HIV RT complexed with NNRTIs. NNRTIs bind to RT and inhibit polymerization through conformational changes of some residues of RT, and NNRTIs are not necessary for intracellular metabolism like NRTIs. Das et al. revealed the molecular mechanism of inhibition by nevirapine using structural analysis [39]. Binding of nevirapine opens the NNRTI-binding pocket, and the formation of this pocket causes the 3′ end of the DNA primer to move away from the position of the polymerase active site, which reduces nucleic acid interaction and strains the dNTP-binding pocket, resulting in inhibition of DNA synthesis. Unfortunately, efficient NNRTIs for HBV therapies have not yet been developed.
Structural information is always helpful for the design of drugs targeting specific molecules; unfortunately, the structural analysis of HBV polymerase has not progressed due to its highly insoluble protein character. This is one of the reasons why novel anti-HBV polymerase drugs have not been developed yet. Recent studies have reported the successful development of an advanced purification system for HBV polymerase using the partial domains having nucleotide- and template/primer-binding activity [40,41]. Highly advanced technologies for purification of polymerase will provide a new avenue for further development of novel NNRTIs.
3. Combination Therapy Is a Prerequisite for the Elimination of Virus
Long-term monotherapy with NRTI might induce the emergence of drug-resistant viruses. Therefore, combination therapy to inactivate viruses from multiple angles is an attractive strategy as a treatment against viral infections because of its additive and possibly synergistic inhibitory effects against even drug-resistant mutants.
3.1. Combination Therapies against Human Immunodeficiency Virus (HIV) and Hepatitis C Virus (HCV)
Highly active antiretroviral therapy (HAART) has significantly reduced the incidence of HIV infection and the mortality of HIV-infected patients [42]. HAART targets multiple viral replication cycles using a combination of drugs including NRTI, NNRTI, protease inhibitor (PI), and/or integrase inhibitor types [43,44]. HIV reverse transcriptase (RT) has an essential role for converting ssRNA to ssDNA, i.e., reverse transcription, and is one of the important targets of HAART [45]. NNRTIs bind to an allosteric region to form a complex with HIV RT, changing its conformation and hence impeding its function [46,47,48]. A recent study demonstrated that a new NNRTI and NRTI combination therapy showed a strong synergistic inhibitory effect even on mutant strains that are resistant to an NRTI or NNRTI when used alone [49].
The development of new combination therapies has led to a dramatic therapeutic outcome for hepatitis C virus (HCV) infection as well. Drugs targeting the viral polymerase significantly increased the cure rate [50,51,52]. Sofosbuvir, which is a potent nucleotide analog against HCV polymerase NS5B, and ledipasvir, which is a polymerase associate factor NS5A inhibitor, have been used in combination for the treatment of chronic HCV since 2014, and dramatic therapeutic effects have been shown [53,54,55]. Sofosbuvir suppresses ledipasvir-resistant mutants with the combination of sofosbuvir and ledipasvir in an HCV replicon cell line [56]. These studies indicated that the combination of NRTIs and NNRTIs in the treatment of either HIV or HCV can provide a drastic inhibitory effect.
3.2. Combination with Interferon (IFN) and Nucleos(t)ide RT Inhibitors (NRTIs) for Hepatitis B Virus (HBV) Therapy
In case of HBV, it was reported that combination therapy using interferon and NRTIs effectively increased the cure rate. Hagiwara et al. reported the effectiveness of a simultaneous combination of ETV and peg-IFNα. CHB patients treated with ETV and peg-IFNα for 48 weeks showed reduced HBsAg levels, regardless of their HBeAg-positivity; the loss rate of HBsAg was 3.8% at 1 year, 8.4% at 3 years, and 15% at 5 years after treatment [57,58]. Another study showed that after 48 weeks of treatment with both TDF and IFN, 13% of the HBV patients (both HBeAg-positive and -negative) lost HBsAg, whereas only 3% of the patients treated with only Peg-IFNα lost HBsAg [59].
Sequential “switch to combination”, which starts with one therapy followed by another therapy, has been shown to be more effective than simultaneous-combination treatment. Several reports have shown that loss rate of HBsAg increased from 32 to 36% in CHB patients by the sequential administration of a 1- or 2-year course of NRTI (predominantly ETV) followed by interferon for 48 or 60 weeks. On the other hand, only 0 or 4.3% of patients showed HBsAg loss by administration of an NRTI alone [60,61].
The fundamental strategy of HBV therapy is long-term NRTI treatment, because potent NRTI treatment results in excellent viral suppression (more than 95% at 5 years) [1]. Sequential “add-on” combination therapy, in which interferon is added to ongoing NRTI treatment, has also been studied. Campenhout et al. reported that the peg-IFN add-on treatment resulted in twice as many patients achieving a decrease in HBsAg levels of more than 1 log compared to ETV monotherapy (add-on therapy, 59%; ETV monotherapy, 28%), and the loss of HBsAg was observed in 2.1% patients with add-on treatment [62]. Bourlière et al. reported that at 144 weeks, 10% of patients with add-on combination treatment had lost HBsAg compared to 4% of patients with the NRTI monotherapy [63].
As described above, many studies have reported that simultaneous, sequential, or add-on combination therapy using ETV or TDF with peg-IFNα provides more effective therapeutic results. It has been noted that the choice of therapy should be carefully determined for each patient, because adverse effects to interferon vary among individuals.
3.3. Combination Treatment with NRTIs and Other Agents
Several in vitro and in vivo studies have suggested the potent inhibitory effects of combination treatment of NRTI with chemical agents. Zhu et al. reported that the combination of tenofovir with either emtricitabine (FTC), lamivudine (3TC), ETV, telbivudine (LdT) or adefovir (AFV) showed additive or synergistic inhibition effects, both in vitro and in a mouse model [64]. Zhen et al. reported that the combination of an NRTI and an anti-PD-1 antibody resulted in the inhibition of viral gene expression and improvement of the survival of HBV transgenic mice [65]. Additionally, the combination treatment enhanced the production of interferons from T cells, and increased the expression of Th1-related immunostimulatory genes, resulting in reduction of the transcription of regulatory and inhibitory immune genes. These results suggested that a combination targeting HBV and blocking the PD-1 immune checkpoint should have a strong synergistic effect. Moreover, synergistic anti-HBV activity and anti-HBV replication activity has been shown by the combination of LdT with saikosaponin c isolated from the herbal drug, Radix Bupleuri [66]. In addition, saikosaponin c inhibits pgRNA synthesis by stimulating IL-6 expression, resulting in the attenuation of HNF1α and HNF4α expression [67]. ABI-H0731, an HBV core protein inhibitor, has exhibited significant antiviral activity in phase 1b clinical trial in CHB patients [68]. ABI-H0731 directly targets HBV core protein, preventing HBV pgRNA encapsidation, thereby inhibiting HBV DNA replication. The combination of ABI-H0731 with ETV shows an additive to moderately synergistic effect.
4. Discussion and Perspectives
Silencing or depleting the cccDNA pool in infected hepatocytes is the goal for new approaches of the treatment. Direct targeting of cccDNA is a strong therapeutic strategy, and polymerase inhibitors play an essential role for preventing further cccDNA accumulation via prevention of the intracellular recycling steps and of viral DNA replication itself. The active development of new therapeutic options to inhibit various life-cycle steps, including cccDNA formation, is ongoing.
Nevertheless, HBV polymerase is absolutely the most effective therapeutic target to drastically reduce viral replication. Although genome editing to directly eliminate cccDNA would provide an innovative therapy, problems such as off-target effects, unexpected chromosomal DNA recombination, and cleavage of integrated HBV DNA must be solved, as mentioned above.
NNRTIs against HIV are available for the current therapies, but in the case of HBV, there have been no available NNRTIs until now. The development of NNRTIs against HBV based on the nature of the HBV polymerase is one of the most important issues to be addressed in order to achieve dramatic therapeutic effects with a combination therapy and to increase treatment options for resistant viruses.
Failure to control viral replication during prolonged monotherapy increases the risk of the emergence of drug-resistant viruses. The emergence of drug-resistant mutant strains of HBV occurs frequently due to the use of monotherapy with antivirals that are less potent and have a lower genetic barrier to resistance [69]. Previously, long-term monotherapy with ADV and 3TC resulted in a high incidence of drug-resistant mutations, but with the advent of ETV, TDF and TAF, the incidence of resistant mutations has decreased [1]. In addition, recent HIV study has reported that NRTIs in combination with NNRTIs also have a synergistic inhibitory effect on NRTI-resistant mutants [49], suggesting that combination therapy may be effective in inhibiting the growth and emergence of drug-resistant strains. Therefore, if rapid and sustainable control of viral replication can be achieved by combination therapy using multiple targeted therapeutic agents including polymerase, the risk of the emergence of resistant viruses can be reduced. For this purpose, the development of combination strategies targeting different viral life stages including viral genome replication is needed to improve the cure rate of chronic hepatitis B.
Taking into consideration the successful treatment of HCV and HIV by combination therapy and the strict control of HBV DNA levels by NRTIs, combination therapy of NRTIs with interferons or other inhibitors can be expected to provide improved therapeutic responses in HBV infection.
Author Contributions
Conceptualization, E.O.; writing—original draft preparation, E.O.; writing—review and editing, Y.S. and K.U.; supervision, K.U.; project administration, E.O.; funding acquisition, E.O. and K.U. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by JSPS KAKENHI (grant number 19K05734) and by Japan Agency for Medical Research and Development (AMED) (grant number 21fk0310105h0005).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lampertico P., Agarwal K., Berg T., Buti M., Janssen H.L.A., Papatheodoridis G., Zoulim F., Tacke F. EASL 2017 Clinical Practice Guidelines on the Management of Hepatitis B Virus Infection. J. Hepatol. 2017;67:370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 2.Boucher C.A., Cammack N., Schipper P., Schuurman R., Rouse P., Wainberg M.A., Cameron J.M. High-Level Resistance to (−) Enantiomeric 2′-Deoxy-3′-Thiacytidine In Vitro Is due to One Amino Acid Substitution in the Catalytic Site of Human Immunodeficiency Virus Type 1 Reverse Transcriptase. Antimicrob. Agents Chemother. 1993;37:2231–2234. doi: 10.1128/AAC.37.10.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cane P.A., Mutimer D., Ratcliffe D., Cook P., Beards G., Elias E., Pillay D. Analysis of Hepatitis B Virus Quasispecies Changes during Emergence and Reversion of Lamivudine Resistance in Liver Transplantation. Antivir. Ther. 1999;4:7–14. [PubMed] [Google Scholar]
- 4.Dienstag J.L., Perrillo R.P., Schiff E.R., Bartholomew M., Vicary C., Rubin M. A Preliminary Trial of Lamivudine for Chronic Hepatitis B Infection. N. Engl. J. Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 5.Nowak M.A., Bonhoeffer S., Hill A.M., Boehme R., Thomas H.C., McDade H. Viral Dynamics in Hepatitis B Virus Infection. Proc. Natl. Acad. Sci. USA. 1996;93:4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Block T.M., Locarnini S., McMahon B.J., Rehermann B., Peters M.G. Use of Current and New Endpoints in the Evaluation of Experimental Hepatitis B Therapeutics. Clin. Infect. Dis. 2017;64:1283–1288. doi: 10.1093/cid/cix129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Königer C., Wingert I., Marsmann M., Rösler C., Beck J., Nassal M. Involvement of the Host DNA-Repair Enzyme TDP2 in Formation of the Covalently Closed Circular DNA Persistence Reservoir of Hepatitis B Viruses. Proc. Natl. Acad. Sci. USA. 2014;111:E4244–E4253. doi: 10.1073/pnas.1409986111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi Y., Gao Z., Xu G., Peng B., Liu C., Yan H., Yao Q., Sun G., Liu Y., Tang D., et al. DNA Polymerase κ Is a Key Cellular Factor for the Formation of Covalently Closed Circular DNA of Hepatitis B Virus. PLoS Pathog. 2016;12:e1005893. doi: 10.1371/journal.ppat.1005893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sheraz M., Cheng J., Tang L., Chang J., Guo J.-T. Cellular DNA Topoisomerases Are Required for the Synthesis of Hepatitis B Virus Covalently Closed Circular DNA. J. Virol. 2019;93:e02230-18. doi: 10.1128/JVI.02230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitamura K., Que L., Shimadu M., Koura M., Ishihara Y., Wakae K., Nakamura T., Watashi K., Wakita T., Muramatsu M. Flap Endonuclease 1 Is Involved in CccDNA Formation in the Hepatitis B Virus. PLoS Pathog. 2018;14:e1007124. doi: 10.1371/journal.ppat.1007124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason W.S., Halpern M.S., England J.M., Seal G., Egan J., Coates L., Aldrich C., Summers J. Experimental Transmission of Duck Hepatitis B Virus. Virology. 1983;131:375–384. doi: 10.1016/0042-6822(83)90505-6. [DOI] [PubMed] [Google Scholar]
- 12.Pourcel C., Summers J. Formation of the Pool of Covalently Closed Circular Viral DNA in Hepadnavirus-Infected Cells. Cell. 1986;47:451–460. doi: 10.1016/0092-8674(86)90602-1. [DOI] [PubMed] [Google Scholar]
- 13.Wu T.-T., Coates L., Aldrics C.E., Summers J., Mason W.S. In Hepatocytes Infected with Duck Hepatitis B Virus, the Template for Viral RNA Synthesis Is Amplified by an Intracellular Pathway. Virology. 1990;175:255–261. doi: 10.1016/0042-6822(90)90206-7. [DOI] [PubMed] [Google Scholar]
- 14.Ko C., Chakraborty A., Chou W.-M., Hasreiter J., Wettengel J.M., Stadler D., Bester R., Asen T., Zhang K., Wisskirchen K., et al. Hepatitis B Virus Genome Recycling and de Novo Secondary Infection Events Maintain Stable CccDNA Levels. J. Hepatol. 2018;69:1231–1241. doi: 10.1016/j.jhep.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong D.K.-H., Seto W.-K., Cheung K.-S., Chong C.-K., Huang F.-Y., Fung J., Lai C.-L., Yuen M.-F. Hepatitis B Virus Core-Related Antigen as a Surrogate Marker for Covalently Closed Circular DNA. Liver Int. 2017;37:995–1001. doi: 10.1111/liv.13346. [DOI] [PubMed] [Google Scholar]
- 16.Guo F., Zhao Q., Sheraz M., Cheng J., Qi Y., Su Q., Cuconati A., Wei L., Du Y., Li W., et al. HBV Core Protein Allosteric Modulators Differentially Alter CccDNA Biosynthesis from De Novo Infection and Intracellular Amplification Pathways. PLoS Pathog. 2017;13:e1006658. doi: 10.1371/journal.ppat.1006658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerlach V.L., Feaver W.J., Fischhaber P.L., Friedberg E.C. Purification and Characterization of Polκ, a DNA Polymerase Encoded by the Human DINB1 Gene. J. Biol. Chem. 2001;276:92–98. doi: 10.1074/jbc.M004413200. [DOI] [PubMed] [Google Scholar]
- 18.García-Díaz M., Bebenek K., Sabariegos R., Domínguez O., Rodríguez J., Kirchhoff T., García-Palomero E., Picher A.J., Juárez R., Ruiz J.F., et al. DNA Polymerase λ, a Novel DNA Repair Enzyme in Human Cells. J. Biol. Chem. 2002;277:13184–13191. doi: 10.1074/jbc.M111601200. [DOI] [PubMed] [Google Scholar]
- 19.Tang L., Sheraz M., McGrane M., Chang J., Guo J.-T. DNA Polymerase Alpha Is Essential for Intracellular Amplification of Hepatitis B Virus Covalently Closed Circular DNA. PLoS Pathog. 2019;15:e1007742. doi: 10.1371/journal.ppat.1007742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y., Yamamoto T., Cullen J., Saputelli J., Aldrich C.E., Miller D.S., Litwin S., Furman P.A., Jilbert A.R., Mason W.S. Kinetics of Hepadnavirus Loss from the Liver during Inhibition of Viral DNA Synthesis. J. Virol. 2001;75:311–322. doi: 10.1128/JVI.75.1.311-322.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Addison W.R., Walters K.-A., Wong W.W.S., Wilson J.S., Madej D., Jewell L.D., Tyrrell D.L.J. Half-Life of the Duck Hepatitis B Virus Covalently Closed Circular DNA Pool In Vivo Following Inhibition of Viral Replication. J. Virol. 2002;76:6356–6363. doi: 10.1128/JVI.76.12.6356-6363.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox E.C. On the Organization of Higher Chromosomes. Nat. New Biol. 1972;239:133–134. doi: 10.1038/newbio239133a0. [DOI] [PubMed] [Google Scholar]
- 23.McVean G.T., Hurst L.D. Evidence for a Selectively Favourable Reduction in the Mutation Rate of the X Chromosome. Nature. 1997;386:388–392. doi: 10.1038/386388a0. [DOI] [PubMed] [Google Scholar]
- 24.Smith N.G.C., Hurst L.D. The Causes of Synonymous Rate Variation in the Rodent Genome: Can Substitution Rates Be Used to Estimate the Sex Bias in Mutation Rate? Genetics. 1999;152:661–673. doi: 10.1093/genetics/152.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maepa M.B., Roelofse I., Ely A., Arbuthnot P. Progress and Prospects of Anti-HBV Gene Therapy Development. Int. J. Mol. Sci. 2015;16:17589–17610. doi: 10.3390/ijms160817589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seeger C. Complete Spectrum of CRISPR/Cas9-Induced Mutations on HBV CccDNA. Cell Ther. 2016;24:9. doi: 10.1038/mt.2016.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bloom K., Maepa M.B., Ely A., Arbuthnot P. Gene Therapy for Chronic HBV—Can We Eliminate CccDNA? Genes. 2018;9:207. doi: 10.3390/genes9040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanning G.C., Zoulim F., Hou J., Bertoletti A. Therapeutic Strategies for Hepatitis B Virus Infection: Towards a Cure. Nat. Rev. Drug Discov. 2019;18:827–844. doi: 10.1038/s41573-019-0037-0. [DOI] [PubMed] [Google Scholar]
- 29.Iannacone M., Guidotti L.G. Immunobiology and Pathogenesis of Hepatitis B Virus Infection. Nat. Rev. Immunol. 2021 doi: 10.1038/s41577-021-00549-4. [DOI] [PubMed] [Google Scholar]
- 30.Gallois-Montbrun S., Schneider B., Chen Y., Giacomoni-Fernandes V., Mulard L., Morera S., Janin J., Deville-Bonne D., Veron M. Improving Nucleoside Diphosphate Kinase for Antiviral Nucleotide Analogs Activation. J. Biol. Chem. 2002;277:39953–39959. doi: 10.1074/jbc.M206360200. [DOI] [PubMed] [Google Scholar]
- 31.Sluis-Cremer N., Tachedjian G. Mechanisms of Inhibition of HIV Replication by Non-Nucleoside Reverse Transcriptase Inhibitors. Virus Res. 2008;134:147–156. doi: 10.1016/j.virusres.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deval J. Antimicrobial Strategies: Inhibition of viral polymerase by 3′-hydroxyl nucleosides. Drugs. 2009;69:151–166. doi: 10.2165/00003495-200969020-00002. [DOI] [PubMed] [Google Scholar]
- 33.Terrault N.A., Lok A.S.F., McMahon B.J., Chang K.-M., Hwang J.P., Jonas M.M., Brown R.S., Bzowej N.H., Wong J.B. Update on Prevention, Diagnosis, and Treatment of Chronic Hepatitis B: AASLD 2018 Hepatitis B Guidance. Hepatology. 2018;67:1560–1599. doi: 10.1002/hep.29800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarin S.K., Kumar M., Lau G.K., Abbas Z., Chan H.L.Y., Chen C.J., Chen D.S., Chen H.L., Chen P.J., Chien R.N., et al. Asian-Pacific Clinical Practice Guidelines on the Management of Hepatitis B: A 2015 Update. Hepatol. Int. 2016;10:1–98. doi: 10.1007/s12072-015-9675-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Werle–Lapostolle B., Bowden S., Locarnini S., Wursthorn K., Petersen J., Lau G., Trepo C., Marcellin P., Goodman Z., Delaney IV W.E. Persistence of CccDNA during the Natural History of Chronic Hepatitis B and Decline during Adefovir Dipivoxil Therapy. Gastroenterology. 2004;126:1750–1758. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 36.Wursthorn K., Lutgehetmann M., Dandri M., Volz T., Buggisch P., Zollner B., Longerich T., Schirmacher P., Metzler F., Zankel M., et al. Peginterferon Alpha-2b plus Adefovir Induce Strong CccDNA Decline and HBsAg Reduction in Patients with Chronic Hepatitis B. Hepatology. 2006;44:675–684. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- 37.Murata K., Asano M., Matsumoto A., Sugiyama M., Nishida N., Tanaka E., Inoue T., Sakamoto M., Enomoto N., Shirasaki T., et al. Induction of IFN-Λ3 as an Additional Effect of Nucleotide, Not Nucleoside, Analogues: A New Potential Target for HBV Infection. Gut. 2018;67:362–371. doi: 10.1136/gutjnl-2016-312653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamada N., Murayama A., Shiina M., Aly H.H., Iwamoto M., Tsukuda S., Watashi K., Tanaka T., Moriishi K., Nishitsuji H., et al. Anti-viral Effects of Interferon-λ3 on Hepatitis B Virus Infection in Cell Culture. Hepatol. Res. 2020;50:283–291. doi: 10.1111/hepr.13449. [DOI] [PubMed] [Google Scholar]
- 39.Das K., Martinez S.E., Bauman J.D., Arnold E. HIV-1 Reverse Transcriptase Complex with DNA and Nevirapine Reveals Non-Nucleoside Inhibition Mechanism. Nat. Struct. Mol. Biol. 2012;19:253–259. doi: 10.1038/nsmb.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ohsaki E., Ueda K. Screening and Evaluation of Novel Compounds against Hepatitis B Virus Polymerase Using Highly Purified Reverse Transcriptase Domain. Viruses. 2020;12:840. doi: 10.3390/v12080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu X., Ohsaki E., Ueda K. Establishment of a System for Finding Inhibitors of ε RNA Binding with the HBV Polymerase. Genes Cells. 2020;25:523–537. doi: 10.1111/gtc.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chong P., Sebahar P., Youngman M., Garrido D., Zhang H., Stewart E.L., Nolte R.T., Wang L., Ferris R.G., Edelstein M., et al. Rational Design of Potent Non-Nucleoside Inhibitors of HIV-1 Reverse Transcriptase. J. Med. Chem. 2012;55:10601–10609. doi: 10.1021/jm301294g. [DOI] [PubMed] [Google Scholar]
- 43.Shafer R.W., Vuitton D.A. Highly Active Antiretroviral Therapy (Haart) for the Treatment of Infection with Human Immunodeficiency Virus Type 1. Biomed. Pharmacother. 1999;53:73–86. doi: 10.1016/S0753-3322(99)80063-8. [DOI] [PubMed] [Google Scholar]
- 44.Brechtl J.R., Breitbart W., Galietta M., Krivo S., Rosenfeld B. The Use of Highly Active Antiretroviral Therapy (HAART) in Patients with Advanced HIV Infection: Impact on Medical, Palliative Care, and Quality of Life Outcomes. J. Pain Symptom Manag. 2001;21:11. doi: 10.1016/S0885-3924(00)00245-1. [DOI] [PubMed] [Google Scholar]
- 45.Esposito F., Corona A., Tramontano E. HIV-1 Reverse Transcriptase Still Remains a New Drug Target: Structure, Function, Classical Inhibitors, and New Inhibitors with Innovative Mechanisms of Actions. Mol. Biol. Int. 2012;2012:1–23. doi: 10.1155/2012/586401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohlstaedt L.A., Wang J., Friedman J.M., Rice P.A., Steitz T.A. Crystal Structure at 3.5Å Resolution of HIV-1 Reverse Transcriptase Complexed with an Inhibitor. Science. 1992;256:9. doi: 10.1126/science.1377403. [DOI] [PubMed] [Google Scholar]
- 47.Rittinger K., Divita G., Goody R.S. Human Immunodeficiency Virus Reverse Transcriptase Substrate-Induced Conformational Changes and the Mechanism of Inhibition by Nonnucleoside Inhibitors. Proc. Natl. Acad. Sci. USA. 1995;92:8046–8049. doi: 10.1073/pnas.92.17.8046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esnouf R., Ren J., Ross C., Jones Y., Stammers D., Stuart D. Mechanism of Inhibition of HIV-1 Reverse Transcriptase by Non-Nucleoside Inhibitors. Nat. Struct. Mol. Biol. 1995;2:303–308. doi: 10.1038/nsb0495-303. [DOI] [PubMed] [Google Scholar]
- 49.Yu F., Li W., Wang L., Dai Y., Lu X., Wang Q., Xie L., Jiang S. Combining New Non-Nucleoside Reverse Transcriptase Inhibitors (RTIs) with AZT Results in Strong Synergism against Multi-RTI-Resistant HIV-1 Strains. Molecules. 2018;23:1599. doi: 10.3390/molecules23071599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benítez-Gutiérrez L., Barreiro P., Labarga P., de Mendoza C., Fernandez-Montero J.V., Arias A., Peña J.M., Soriano V. Prevention and Management of Treatment Failure to New Oral Hepatitis C Drugs. Expert Opin. Pharmacother. 2016;17:1215–1223. doi: 10.1080/14656566.2016.1182156. [DOI] [PubMed] [Google Scholar]
- 51.Buti M., Riveiro-Barciela M., Esteban R. Management of Direct-Acting Antiviral Agent Failures. J. Hepatol. 2015;63:1511–1522. doi: 10.1016/j.jhep.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Jadoul M., Martin P. Hepatitis C Treatment in Chronic Kidney Disease Patients: The Kidney Disease Improving Global Outcomes Perspective. Blood Purif. 2017;43:206–209. doi: 10.1159/000452730. [DOI] [PubMed] [Google Scholar]
- 53.Lawitz E., Poordad F.F., Pang P.S., Hyland R.H., Ding X., Mo H., Symonds W.T., McHutchison J.G., Membreno F.E. Sofosbuvir and Ledipasvir Fixed-Dose Combination with and without Ribavirin in Treatment-Naive and Previously Treated Patients with Genotype 1 Hepatitis C Virus Infection (LONESTAR): An Open-Label, Randomised, Phase 2 Trial. Lancet. 2014;383:515–523. doi: 10.1016/S0140-6736(13)62121-2. [DOI] [PubMed] [Google Scholar]
- 54.Bourlière M., Bronowicki J.-P., de Ledinghen V., Hézode C., Zoulim F., Mathurin P., Tran A., Larrey D.G., Ratziu V., Alric L., et al. Ledipasvir-Sofosbuvir with or without Ribavirin to Treat Patients with HCV Genotype 1 Infection and Cirrhosis Non-Responsive to Previous Protease-Inhibitor Therapy: A Randomised, Double-Blind, Phase 2 Trial (SIRIUS) Lancet Infect. Dis. 2015;15:397–404. doi: 10.1016/S1473-3099(15)70050-2. [DOI] [PubMed] [Google Scholar]
- 55.Charlton M., Everson G.T., Flamm S.L., Kumar P., Landis C., Brown R.S., Fried M.W., Terrault N.A., O’Leary J.G., Vargas H.E., et al. Ledipasvir and Sofosbuvir plus Ribavirin for Treatment of HCV Infection in Patients with Advanced Liver Disease. Gastroenterology. 2015;149:649–659. doi: 10.1053/j.gastro.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 56.Brown A.N., Liu L., Rodriquez J.L., Zhao L., Schuster L., Li E., Wang G.P., Neely M.N., Yamada W., Drusano G.L. Sofosbuvir (SOF) Suppresses Ledipasvir (LDV)-Resistant Mutants during SOF/LDV Combination Therapy against Genotype 1b Hepatitis C Virus (HCV) Sci. Rep. 2017;7:14421. doi: 10.1038/s41598-017-15007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hagiwara S., Kudo M., Osaki Y., Matsuo H., Inuzuka T., Matsumoto A., Tanaka E., Sakurai T., Ueshima K., Inoue T., et al. Impact of Peginterferon Alpha-2b and Entecavir Hydrate Combination Therapy on Persistent Viral Suppression in Patients with Chronic Hepatitis B. J. Med. Virol. 2013;85:987–995. doi: 10.1002/jmv.23564. [DOI] [PubMed] [Google Scholar]
- 58.Hagiwara S., Nishida N., Watanabe T., Ida H., Sakurai T., Ueshima K., Takita M., Komeda Y., Nishijima N., Osaki Y., et al. Sustained Antiviral Effects and Clearance of Hepatitis Surface Antigen after Combination Therapy with Entecavir and Pegylated Interferon in Chronic Hepatitis B. Antivir. Ther. 2018;23:513–521. doi: 10.3851/IMP3225. [DOI] [PubMed] [Google Scholar]
- 59.Zheng C., Yan H., Zeng J., Cai S., Wu X. Comparison of Pegylated Interferon Monotherapy and de Novo Pegylated Interferon plus Tenofovir Combination Therapy in Patients with Chronic Hepatitis B. Infect. Drug Resist. 2019;12:845–854. doi: 10.2147/IDR.S195144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Y., Yan R., Ru G.Q., Yu L.L., Yao J., Wang H. Pegylated-Interferon Consolidation Treatment versus Nucleos(t)Ide Analogue Consolidation Treatment in Non-Cirrhotic Hepatitis B Patients with Hepatitis B e Antigen Seroconversion: An Open-Label Pilot Trial. Hepatol. Int. 2019;13:422–430. doi: 10.1007/s12072-019-09957-0. [DOI] [PubMed] [Google Scholar]
- 61.Huang J., Zhang K., Chen W., Liao J., Luo X., Chen R. Switching to PegIFNα-2b Leads to HBsAg Loss in Patients with Low HBsAg Levels and HBV DNA Suppressed by NAs. Sci. Rep. 2017;7:13383. doi: 10.1038/s41598-017-13747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Campenhout M.J.H., Brouwer W.P., Xie Q., Guo S., Chi H., Qi X., Tabak F., Streinu-Cercel A., Wang J.-Y., Zhang N.-P., et al. Long-Term Follow-up of Patients Treated with Entecavir and Peginterferon Add-on Therapy for HBeAg-Positive Chronic Hepatitis B Infection: ARES Long-Term Follow-Up. J. Viral Hepat. 2019;26:109–117. doi: 10.1111/jvh.12997. [DOI] [PubMed] [Google Scholar]
- 63.Bourlière M., Rabiega P., Ganne-Carrie N., Serfaty L., Marcellin P., Barthe Y., Thabut D., Guyader D., Hezode C., Picon M., et al. Effect on HBs Antigen Clearance of Addition of Pegylated Interferon Alfa-2a to Nucleos(t)Ide Analogue Therapy versus Nucleos(t)Ide Analogue Therapy Alone in Patients with HBe Antigen-Negative Chronic Hepatitis B and Sustained Undetectable Plasma Hepatitis B Virus DNA: A Randomised, Controlled, Open-Label Trial. Lancet Gastroenterol. Hepatol. 2017;2:177–188. doi: 10.1016/S2468-1253(16)30189-3. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y., Curtis M., Qi X., Miller M.D., Borroto-Esoda K. Anti-Hepatitis B Virus Activity in Vitro of Combinations of Tenofovir with Nucleoside/Nucleotide Analogues. Antivir. Chem. Chemother. 2009;19:165–176. doi: 10.1177/095632020901900404. [DOI] [PubMed] [Google Scholar]
- 65.Zhen S., Qiang R., Lu J., Tuo X., Yang X., Li X. Enhanced Antiviral Benefit of Combination Therapy with Anti-HBV and Anti-PD1 GRNA/Cas9 Produces a Synergistic Antiviral Effect in HBV Infection. Mol. Immunol. 2021;130:7–13. doi: 10.1016/j.molimm.2020.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Li X., Ke Z., Lian D., Yuan J., Pan Y. Combination of Saikosaponin c and Telbivudine Synergistically Enhances the Anti-HBV Activity. Inflamm. Res. 2020;69:545–547. doi: 10.1007/s00011-020-01336-y. [DOI] [PubMed] [Google Scholar]
- 67.Pan Y., Ke Z., Ye H., Sun L., Ding X., Shen Y., Zhang R., Yuan J. Saikosaponin C Exerts Anti-HBV Effects by Attenuating HNF1α and HNF4α Expression to Suppress HBV PgRNA Synthesis. Inflamm. Res. 2019;68:1025–1034. doi: 10.1007/s00011-019-01284-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang Q., Cai D., Yan R., Li L., Zong Y., Guo L., Mercier A., Zhou Y., Tang A., Henne K., et al. Preclinical Profile and Characterization of the Hepatitis B Virus Core Protein Inhibitor ABI-H0731. Antimicrob. Agents Chemother. 2020;64:e01463-20. doi: 10.1128/AAC.01463-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang H., Liu L., Ye C., Chen C., Hang S., Zhu Z., Shen H., Huang Z., Chen W., Xue Y. Evolution of Drug-Resistant Mutations in HBV Genomes in Patients with Treatment Failure during the Past Seven Years (2010–2016) Virus Genes. 2018;54:41–47. doi: 10.1007/s11262-017-1518-z. [DOI] [PubMed] [Google Scholar]