Abstract
Oncolytic virus (OV) as a promising therapeutic agent can selectively infect and kill tumor cells with naturally inherited or engineered properties. Considering the limitations of OVs monotherapy, combination therapy has been widely explored. MEK inhibitor (MEKi) Trametinib is an FDA-approved kinase inhibitor indicated for the treatment of tumors with BRAF V600E or V600K mutations. In this study, the oncolytic activity in vitro and anti-tumor therapeutic efficacy in vivo when combined with oHSV and MEKi Trametinib were investigated. We found: (1) Treatment with MEKi Trametinib augmented oHSV oncolytic activity in BRAF V600E-mutated tumor cells. (2) Combination treatment with oHSV and MEKi Trametinib enhanced virus replication mediated by down-regulation of STAT1 and PKR expression or phosphorylation in BRAF V600E-mutated tumor cells as well as BRAF wt/KRAS-mutated tumor cells. (3) A remarkably synergistic therapeutic efficacy was shown in vivo for BRAF wt/KRAS-mutated tumor models, when a combination of oHSV including PD-1 blockade and MEK inhibition. Collectively, these data provide some new insights for clinical development of combination therapy with oncolytic virus, MEK inhibition, and checkpoint blockade for BRAF or KRAS-mutated tumors.
Keywords: oHSV, MEKi trametinib, BRAF, KRAS, antitumor
1. Introduction
Oncolytic virus (OV) as a promising therapeutic agent can selectively infect and destroy tumor cells with naturally inherited or engineered properties [1]. However, therapeutic responses with OVs monotherapy remains limited to a few patients, and the limitations include physical and immunological barriers that impede OV delivery, replication, and spread within tumor tissues [2]. Thus, development of combination therapy with therapeutic modalities has been widely explored in antitumor research and clinical trials. For example, the combination of OVs with other immunotherapies, especially immune checkpoint blockade (ICB) can exert synergistic effect to increase therapeutic effects against tumors [1,3]. The pharmacoviral approaches combining OVs with molecular therapeutics targeting kinases commonly linked to cancer development also showed remarkably synergistic responses during tumor treatment [4].
The mitogen-activated protein kinase (MAPK) cascade (RAS/RAF/MEK/ERK), which is one of the most dysregulated pathways in human cancers, regulates multiple critical cellular functions including proliferation, growth, survival and motility [5,6]. In the RAS/RAF/MEK/ERK signaling cascade, MEK is activated through phosphorylation by its upstream MAPK kinase kinase (known as RAF) and RAF is activated when the small GTP-binding protein, RAS, is in its GTP-bound form [7]. The mutations involving the GTP-ase RAS protein family and its downstream BRAF lead to loss of cell cycle regulation at key checkpoints, which are the main driver mutations for several tumor types, e.g., colorectal carcinogenesis [8].
Pre-clinical studies have shown that MAPK pathway inhibition synergizes with oHSV infection in melanoma cells. Bommareddy et al. reported that the T-VEC/ MEK inhibitor (MEKi) regime increased viral replication and apoptosis within melanoma cells in vitro, furthermore, reduced tumor growth in vivo [9]. Yoo et al. demonstrated that pre-treatment with the MEKi can enhance oHSV replication within glioma cells by suppressing macrophage-mediated apoptosis of tumor cells [10]. The MEKi Trametinib used in these studies, is an FDA-approved kinase inhibitor indicated for the treatment of melanoma, non-small cell lung cancer and thyroid cancer with BRAF V600E or V600K mutations [11].
Inhibition of MEK was presumed to be particularly efficacious in cancers harboring a RAS or RAF mutation, leading to overactivation of the MAPK pathway [12]. In BRAF V600E–mutant melanoma, MEK inhibition was shown to activate caspase-3 through BIM-EL and BMF-mediated mitochondrial depolarization, which leads to apoptosis in the tumor cells [13]. In addition, MEK inhibition can sensitize unresponsive KRAS-mutated tumors to immunotherapy [14]. Here, studies in this manuscript tested whether the combination with oHSV and MEKi Trametinib can enhance oncolytic virus replication and oncolytic activity in BRAF-mutated or RAS-mutated tumor in vitro as well as the antitumor efficacy in vivo.
2. Materials and Methods
2.1. Cell Lines and Virus
Vero cells and Widr cells were obtained from the American Type Culture Collection and were routinely cultured in DMEM (Life Technologies) supplemented with 5% (vol/vol) newborn bovine serum (NBCS) or 10% (vol/vol) fetal bovine serum (FBS, Gibco), respectively. Caco-2 cells were purchased from ATCC and maintained in MEM-EBSS (HYCLONE) supplemented with 20% (vol/vol) FBS. HT29 cells were obtained from the Cell Bank of Sun Yat-sen University Experimental Animal Center and were cultured in McCoy’s 5A Medium Modified (ATCC) supplemented with 10% (vol/vol) FBS. 4T1(Mouse breast cancer cell), CT26 (Mouse colorectal carcinoma cell) and LLC (Mouse lung adenocarcinoma cell) were kindly provided by Stem Cell Bank, Chinese Academy of Sciences. 4T1 and CT26 were maintained in RPMI-1640 (Life Technologies), containing 10% (vol/vol) FBS and LLC was routinely cultured in DMEM (Life Technologies) supplemented with 10% (vol/vol) FBS (Gibco). All culture media were supplemented with 100 U/mL penicillin and 100 μg/mL streptomycin.
Oncolytic herpes virus (oHSV) T1012G and T3855 encoding murine IL-12 and PD-1 antibody was reported elsewhere [15].
2.2. Antibodies
Antibodies used in this study included p-ERK (Cat. #4370T, Cell Signaling Technology), ERK (Cat. #4695T, Cell Signaling Technology), p-STAT1 (Cat. #7649S, Cell Signaling Technology), STAT1 (Cat. #10144-2-AP, Proteintech), p-PKR (Cat. #ab32036, Abcam), Anti-phospho-PKR (Thr451) (07-886, Merck), PKR (Cat. #ab32506, Abcam), and anti-GAPDH (Cat. #2118S, Cell Signaling Technology).
2.3. Cell Counting Kit 8 Assay
The tumor cell killing activity was assessed using a CCK8 assay. Briefly, Widr, HT29 or Caco-2 cells were plated in 96-well plates at 5–8 × 103 cells/well. MEK inhibitor (MEKi) Trametinib (MCE) was added after the cells had adhered overnight at a final concentration of 15 nM for 2 h. Then, cells were infected with T1012G for 48 h after pretreatment with MEKi. Cell viability was evaluated using Cell Counting Kit-8 (MCE) according to manufacturer’s instructions. The cell viability rate was calculated according to the following formula: Cell viability (100%) = ((A value of experimental wells)-(A value of the blank wells)/(A value of target cell wells)-(A value of the blank wells)) × 100%. The assay was performed in triplicate.
2.4. RNA Interference
The knockdown of STAT1 was achieved using siRNAs (GenePharma). The sequences of the siRNA were as follows:
si-STAT1: 5′-GAACCUGACUUCCAUGCGGTT-3′;
The None Target (NT) siRNA: 5′-UUCUCCGAACGUGUCACGUTT-3′.
The siRNA transfections were carried out using Lipofectamine 2000 (Invitrogen) according to manufacturer’s instructions.
2.5. RNA Extraction and Real-Time PCR
CT26 and 4T1 cell pellets after treatment were collected and total RNAs from cells were isolated using TRIzol reagent (Invitrogen). A total amount of 0.5 µg of RNA was reverse transcribed to cDNA using the PCR RT kit (TOYOBO) according to manufacturer’s instructions. The real-time PCR were performed using the StepOnePlus ™ Real-Time PCR System (Applied Biosystems) and the amplifications were performed using the SYBR Premix (TaKaRa). The mRNA expression levels were normalized by18S rRNA and analyzed using the 2 −ΔΔCT method.
Primers used for amplifications were as follows:
Mouse PKR-F 5′-CCTGAGCACAGCATGAGTGA-3′
Mouse PKR-R 5′-TACTCCACTCCGGTCACGAT-3′
Mouse STAT1-F 5′-GGAAGGGGCCATCACATTCA-3′
Mouse STAT1-R 5′-TACTTCCCAAAGGCGTGGTC-3′
18S-F 5′- CTCAACACGGGAAACCTCAC -3′
18S-R 5′- CGCTCCACCAACTAAGAACG -3′
2.6. Immunoblotting Assays
Caco-2, HT29, and Widr cell pellets were collected at the indicated time points post treatment. Total protein was extracted by RIPA Lysis Buffer (Beyotime Biotechnology) supplemented with 1 mM phenylmethylsulfonyl fluoride (PMSF). The isolated protein concentration was determined using BCA Protein Assay Kit (Beyotime Biotechnology). Twenty micrograms of the proteins were separated on a 10% SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride membranes (Millipore). The proteins were detected by incubation with indicated primary antibody, followed by horseradish peroxidase (HRP)-conjugated secondary antibody (Invitrogen) and the ECL reagent (Merck Millipore). Images were captured using a ChemiDoc Touch Imaging System (Bio-Rad) and processed using ImageLab software. The densities of corresponding bands were quantified using the ImageJ software.
2.7. Virus Infection and Titration
A total of 1 × 106 cells were seeded onto six-well plates. After 24 h, the adherent cells were pretreated with DMSO or MEKi for 2 h and then exposed to 0.1 PFU T1012G or T3855 per cell. The cells were harvested at 48 h or 72 h after infection. Viral progeny was titrated on Vero cells after three freeze–thaw cycles.
2.8. Syngeneic Mouse Model and Tumor Treatment
Five-week-old female Balb/c mice and C57BL/6 mice were purchased from Charles River Laboratories (Beijing, China) and housed under specific pathogen-free (SPF) conditions. The syngeneic mice were Balb/c for 4T1 and CT26, C57BL/6 for LLC. The 4T1 and CT26 tumor models were generated by implantation of 1 × 106 cells and 5 × 105 cells subcutaneously into Balb/c mouse flanks, respectively. The LLC tumor model was generated by implantation of 1 × 106 cells subcutaneously into C57BL/6 mouse flanks. In tumor treatment studies, BALB/c or C57BL/6 mice (n = 8 per group) were treated with oHSV T3855 (1 × 107 PFU/mouse for 4T1 or LLC; 5 × 106 PFU/mouse for CT26) or vehicle control via intratumoral injection on days 1, 8, 15, 22; MEKi (1.0 mg/kg) or vehicle control via oral gavage from days 1 to day 14. In addition, the combined group received equivalent doses of combination treatment with oHSV and MEKi. Tumor growth was measured in two dimensions, recording the greatest length and width using digital calipers. Tumor sizes were plotted as average size for each group. All institutional and national guidelines for the care and use of laboratory animals were followed.
2.9. Statistical Analysis
Data were expressed as mean ± standard deviation. Differences between datasets were assessed by student’s t-test (two-tailed) using the GraphPad Prism software. N.S p > 0.05, * p < 0.05, ** p < 0.01, *** p < 0.001.
3. Result
3.1. Treatment with MEK Inhibitor (MEKi) Trametinib Enhanced oHSV Replication and Tumor Cell Killing in BRAF V600E-Mutated Tumor Cells
As resistance to oHSV therapy occurred in many tumors, combination with other antitumor agents or therapies becomes a good strategy for augment of the oHSV antitumor therapeutic efficacy. In this study, MEKi Trametinib, an FDA-approved kinase inhibitor indicated for tumor treatment with BRAF V600E or V600K mutations was used as an antitumor agent to combine with oHSV T1012G. Then, the oHSV replication and tumor cell killing were investigated in both BRAF wild type (wt) (Caco-2 cell) and V600E-mutated tumor cells (Widr and HT29 cells) (Table 1). It is noted that the viral yields increased significantly in BRAF V600E-mutated tumor cells, about 8.6-fold for Widr cell and 6.7-fold for HT29 cell, respectively, when combination of oHSV and Trametinib at the concentration of 15 nM compared to oHSV alone at 72 hpi. In contrast, for BRAF wt Caco-2 cell, there is no increase but an around 5-fold decrease of the oHSV yields when combined treatment with Trametinib at 72 hpi (Figure 1A). At 48hpi, the oHSV replication displayed the similar pattern as 72 hpi, though without significant difference (Figure 1A). Compared with BRAF wt tumor cells, the oHSV infected tumor cells harboring BRAF V600E mutations exhibited increased cytotoxicity when pretreated with Trametinib (Figure 1B). Collectively, it suggested that pretreatment with MEKi Trametinib in BRAF V600E-mutated tumor cells facilitated oHSV replication and augmented virus oncolytic activity.
Table 1.
Tumor cells with different BRAF and KRAS mutations.
| Cell Line | BRAF | KRAS | |
|---|---|---|---|
| Caco-2 | Human Colon Carcinoma | wt | wt |
| Widr | Human Colon Carcinoma | V600E | wt |
| HT29 | Human Colon Carcinoma | V600E | wt |
| 4T1 | Mouse Breast Carcinoma | wt | wt |
| Pan02 | Mouse Pancreatic Carcinoma | wt | wt |
| LLC | Mouse Lung Carcinoma | wt | G12C |
| CT26 | Mouse Colon Carcinoma | wt | G12D |
Figure 1.
MEK inhibitor (MEKi) Trametinib treatment promoted oHSV replication and tumor cell killing in BRAF V600E-mutated tumor cells. (A) Caco-2, Widr, and HT29 cells were pretreated with 15 nM of Trametinib (MEKi) for 2 h and then infected with T1012G at 0.01 PFU/cell. The infected cell pellets were harvested at 48 and 72 h post-infection, respectively. The titration was measured by conventional plaque assay on Vero cells after three freeze–thaw cycles. (B) The tumor cell killing activity was assessed using a CCK8 assay. Caco-2, Widr, and HT29 cells were mock treated or pretreated with 15 nM of Trametinib (MEKi) for 2 h and then infected with T1012G at an indicated MOI or MEKi only treated. Cell viability of infected cells was measured with a CCK8 assay (mean ± SD) 48 h after infection. The assay was performed in triplicate. N.S. (not significant); p > 0.05, * p < 0.05.
3.2. Treatment with Trametinib Induced Decreased Phosphorylation of STAT1 and PKR in BRAF V600E-Mutated Tumor Cells
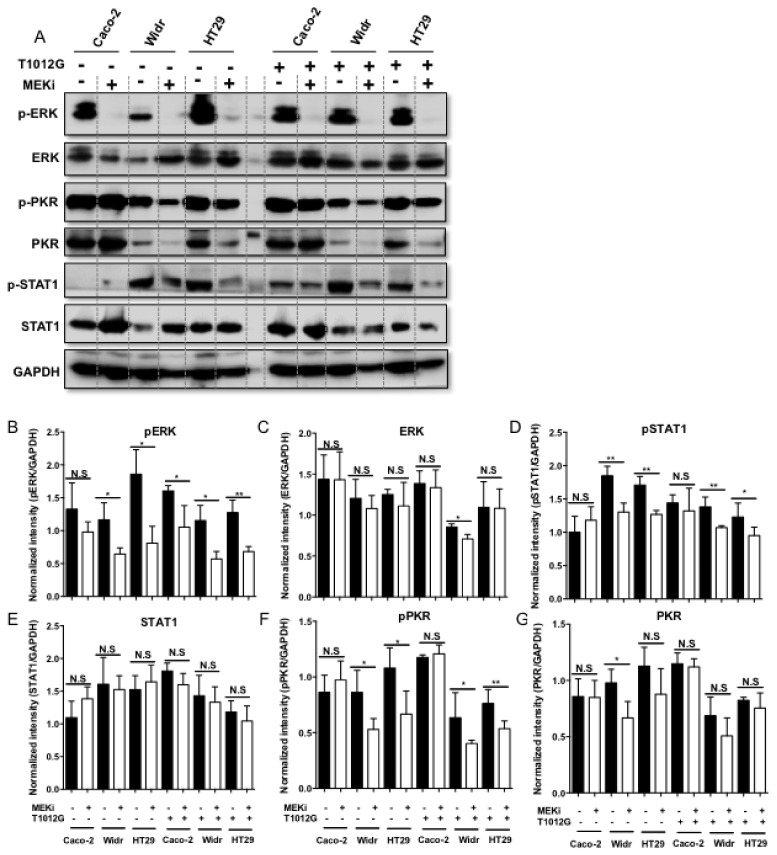
Next, investigation into the molecular basis behind how combination of MEKi and oHSV to enhance virus replication was performed. It has been reported that PKR as a host antiviral kinase can limit γ34.5 deleted oHSV late-gene expression and replication [16]. In addition to PKR, cells also express a diverse set of pattern recognition receptors (PRR) that detect pathogen-associated molecular patterns such as viral nucleic acids. Among them, IFN/STAT1 signaling modulators contributes to promote amplification of the antiviral response [17]. Thus, we sought to determine whether the expression and phosphorylation of PKR and STAT1 were down-regulated when treated with Trametinib alone or combined with oHSV. Firstly, we confirmed that MEKi alone induced significantly decreased levels of phosphorylated ERK (p-ERK) as well as ERK in both BRAF wt Caco-2 cell and V600E-mutated Widr and HT29 cells (Figure 2A–C). Similar decreased expression pattern of p-ERK and ERK was also observed in combination treatment with oHSV (Figure 2A–C). Then, we observed that the phosphorylation of PKR and STAT1 was significantly down-regulated with both MEKi alone and combination treatment in Widr and HT29 cells compared to Caco-2 cell (Figure 2D,F). For the total PKR and STAT1 expression, there was no significant difference either with MEKi alone or combination treatment in BRAF V600E-mutated tumor cells, with an exception that there is a remarkable decrease of the total PKR expression in Widr cells (Figure 2E,G). Taken together, it suggested that down-regulation of p-PKR and p-STAT1 induced by MEKi Trametinib may contribute to enhance oHSV replication in BRAF V600E-mutated cells.
Figure 2.
Down-regulation of STAT1 and PKR phosphorylation when combination of oHSV and MEKi in BRAF mutant cancer cells. (A) Caco-2, Widr, and HT29 cells were pretreated with 0.25 μM of Trametinib (MEKi) for 2 h and then mock infected or infected with 0.1 PFU/cell T1012G. The cell pellets were harvested at 24 h post-infection, respectively, and lysates were analyzed by Western blotting for the indicated proteins. (B–G). Quantification of the protein level of pERK, ERK, pSTAT1, STAT1, pPKR, and PKR. N.S. (not significant); p > 0.05, * p < 0.05, ** p < 0.01.
3.3. Down-Regulation of STAT1 Supressed the Expression of PKR in BRAF V600E-Mutated Tumor Cells
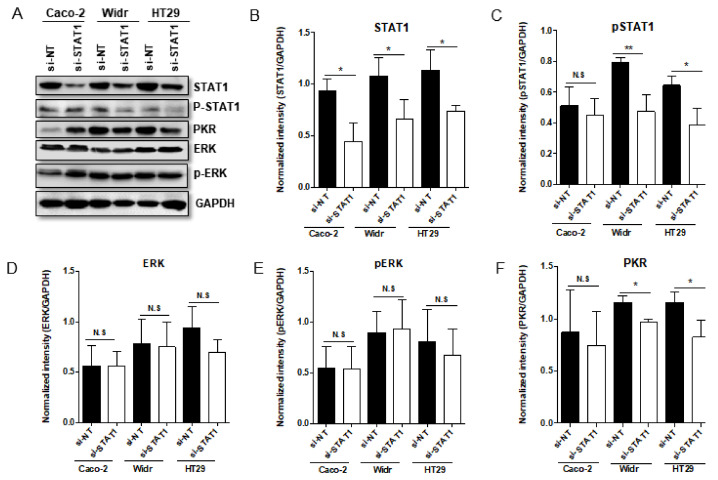
To further confirm the possible relevance between STAT1 and PKR, knockdown of STAT1 by siRNA was performed in Caco-2, Widr, and HT29 cell. The results indicated that siRNA targeting STAT1 (si-STAT1) can efficiently down-regulate STAT1 protein expression compared to non-targeting siRNA (si-NT) transfection in all the cells tested (Figure 3A,B), whereas the efficient down-regulation of p-STAT1 was only shown in BRAF V600E-mutated Widr and HT29 cells (Figure 3A,C). Strikingly, the PKR levels were decreased under the condition with lower abundance of STAT1 in BRAF V600E-mutated Widr and HT29 cells while in BRAF wt Caco-2 cell, there is no significant decrease for PKR expression (Figure 3A,F). The difference of PKR expression induced by down-regulation of STAT1 between BRAF wt and V600E-mutated cells may explain the diversity of oHSV replication on these cells. Additional studies showed that down-regulation of STAT1 had no impact on ERK expression and ERK phosphorylation (Figure 3A,D,E).
Figure 3.
Knockdown of STAT1 in Caco-2, Widr and HT29 cells by siRNA. Panel (A) Caco-2, Widr, and HT29 cells were either transfected with 100 nM STAT1 siRNA or nontarget siRNA (siNT). The cells were harvested at 72 h after transfection. The proteins were electrophoretically separated in a 10% denaturing gel and reacted with indicated antibodies. Panel (B–F) Quantification of the protein level of STAT1, pSTAT1, ERK, pERK, and PKR in Caco-2, Widr, and HT29 cells when Knockdown of STAT1 by siRNA. N.S. (not significant); p > 0.05, * p < 0.05, ** p < 0.01.
3.4. MEKi Trametinib Treatment Promoted oHSV Replication by Down-Regulation of PKR and STAT1 mRNA Expression in KRAS Mutant Cancer Cells
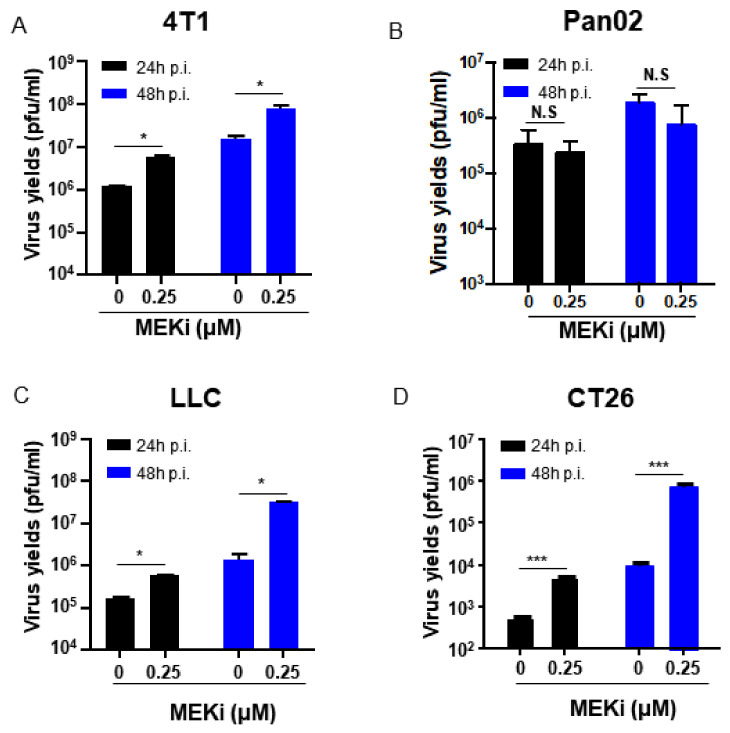
BRAF and KRAS are two key oncogenes in the RAS/RAF/MEK/ERK signaling pathway [18]. Single mutation or concomitant mutations in both KRAS and BRAF genes have been identified in different type of cancers [19,20]. All the three cell lines (Caco-2, Widr, and HT29 cells) with different BRAF mutations mentioned in the above studies, are KRAS wild type cells (Table 1). Then, we sought to determine whether the MEKi treatment can enhance the oHSV replication in the tumor cells with BRAF wt and the upstream KRAS mutations. Thus, the tumor cells with KRAS wt 4T1 cell, Pan02 cell, KRAS G12C-mutated LLC cell, and KRAS G12D-mutated CT26 cells were used (Table 1). The oHSV T3855 containing murine IL-12 and PD-1 antibody, which will be used in the following in vivo study, was used in the combined treatment. As shown in Figure 4C,D, oHSV replication remarkably increased when treated with Trametinib at the concentration of 0.25 μM in KRAS mutated LLC cell (p < 0.05) and CT26 cell (p < 0.001), while KRAS wt Pan02 cells showed slight decrease of viral replication. In KRAS wt 4T1 cell, the viral replication increased significantly with the same treatment, which may be due to the ERK phosphorylation in 4T1 cells [21] (Figure 4A). It suggested that MEKi Trametinib treatment preferred to enhance oHSV replication in KRAS mutated tumor cells compared with KRAS wt.
Figure 4.
MEKi treatment increased oHSV virus yields in KRAS mutated cancer cells. 4T1 (A), Pan02 (B), LLC (C), and CT26 (D) cells were pretreated with 0.25 μM of Trametinib (MEKi) for 2 h and then infected with 0.1 PFU/cell T3855. The virus-containing samples were harvested at 24 and 48 h post-infection, respectively. Additionally, then the titration was measured by conventional plaque assay on Vero cells after three freeze–thaw cycles. N.S. (not significant); p > 0.05, * p < 0.05, *** p < 0.001.
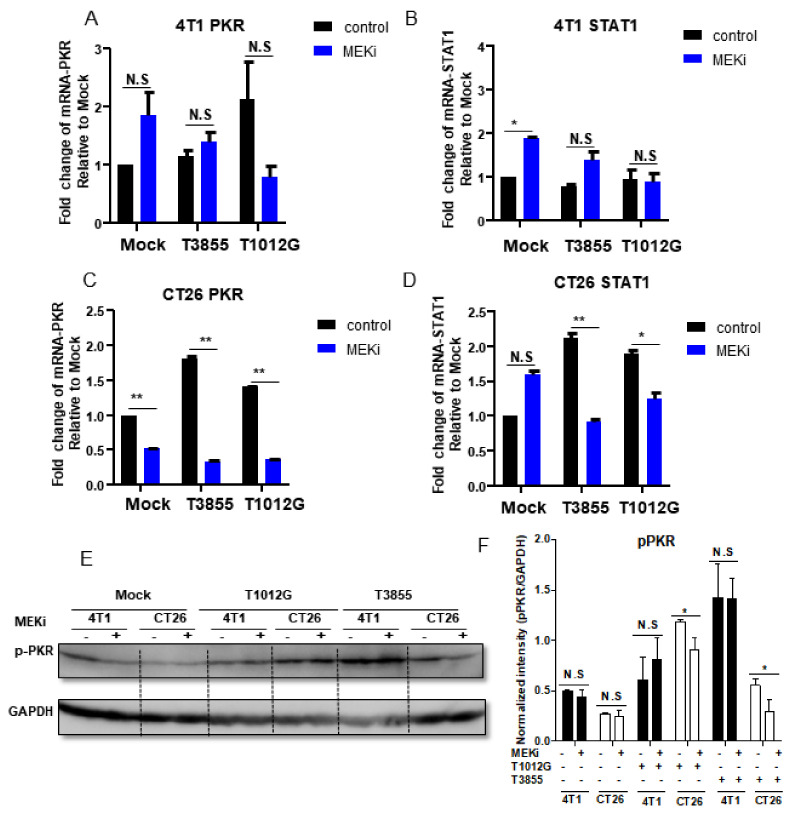
Next, we sought to investigate whether the augment of oHSV replication was induced by down-regulation of PKR and STAT1 expression when combined treatment with Trametinib. For both oHSVs, T1012G and T3855, we observed similar mRNA expression pattern of PKR and STAT1 during the combined treatment (Figure 5). The result shown in Figure 5A,E,F indicated that the mRNA and phosphorylation level of PKR decreased significantly in MEKi alone and combined treatment in CT26 cells, whereas there was no obvious difference of PKR mRNA and phosphorylation in 4T1 cells with the same treatment (Figure 5C,E,F). Additional studies on the expression of STAT1 showed that combined treatment suppressed STAT1 mRNA expression in CT26 cells but not in 4T1 cells (Figure 5B,D). In addition, there was no STAT1 phosphorylation detected, which may be due to the level of STAT1 phosphorylation in these two murine tumor cell lines. In summary, MEKi Trametinib treatment facilitated oHSV replication, which may be mediated by down-regulation of PKR phosphorylation in KRAS mutant cancer cells.
Figure 5.
Decreased STAT1 and PKR expression in MEKi treated KRAS mutated cancer cells. 4T1 and CT26 cells were mock treated or pretreated with 0.25 μM of Trametinib (MEKi) for 2 h and then mock infected or infected with 0.1 PFU/cell T1012G or T3855. The cell pellet was harvested at 24 h post-infection, respectively. The total RNAs were extracted and 0.5 μg of RNAs were reverse transcribed to cDNA as described in Materials and Methods. The PKR and STAT1 mRNAs were quantified and normalized with respect to 18S rRNA and shown as fold change compared with mRNA from mock treated and infected cells (Panel (A–D)). With the same treatment, cell pellets were harvested and the proteins were electrophoretically separated in a 10% denaturing gel and reacted with indicated antibodies (Panel (E)). Quantification of the protein level of pERK in 4T1 and CT26 cells (Panel (F)). N.S. (not significant); p > 0.05, * p < 0.05, ** p < 0.01.
3.5. Combined Treatment with MEKi Trametinib and oHSV T3855 Further Enhanced Anti-Tumor Therapeutic Activity
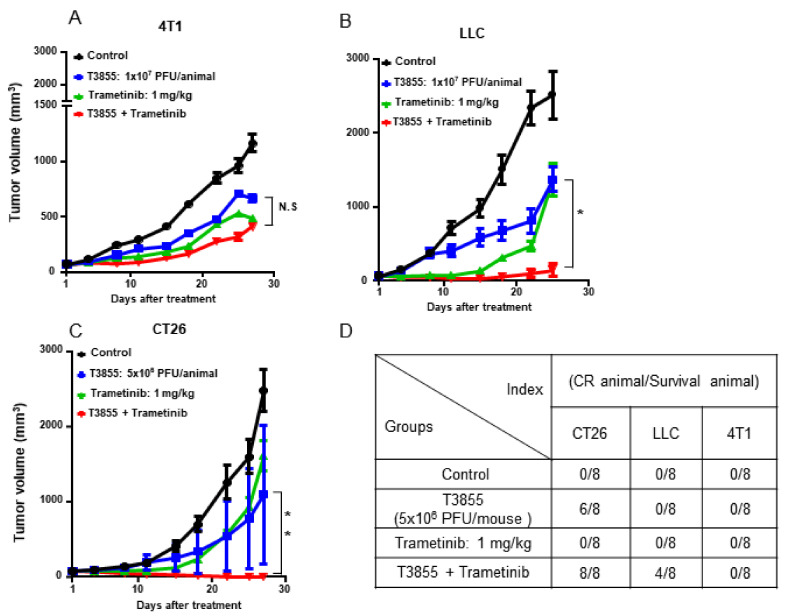
Last, whether MEKi Trametinib and oHSV T3855 combination therapy had an enhanced anti-tumor therapeutic efficacy in vivo was investigated. Syngeneic tumor mice were used to implant with murine tumors 4T1, LLC, and CT26, respectively. As shown in Figure 6B,C, delayed tumor growth was observed with Trametinib alone and oHSV T3855 alone, but combined treatment was associated with a significant decrease in tumor growth compared with oHSV alone in LLC model (p < 0.05) and CT26 model (p < 0.01). Furthermore, 8 of 8 mice were shown complete tumor eradication in the group with combined treatment with CT26 model whereas in LLC model, 4 of 8 mice were shown complete response after 4 weeks treatment (Figure 6D). In addition, we also observed the reductions in tumor volumes in 4T1 model with different treatments compared with control, but there was no significant difference between combined treatment and Trametinib alone (Figure 6A; p > 0.05). There were no signs of toxicity or weight loss in any of these animals (data not shown).
Figure 6.
Combined treatment with MEKi Trametinib and oHSV T3855 enhanced antitumor therapeutic activity in LLC and CT26 tumor models. 4T1 (Panel (A)) or CT26 (Panel (C)) tumor cells were injected s.c. into the right flanks of Balb/c mice, respectively. LLC (Panel (B)) tumor cells were injected s.c. into the right flanks of C57BL/6 mice. Different Tumor models averaging 80 mm3 (n = 8 per group) were treated via intratumoral injection with PBS or T3855 (1 × 107 PFU/animal for 4T1 or LLC; 5 × 106 PFU /animal for CT26) on days 1, 8, 15, and 22 and MEKi (trametinib; 1 mg/kg) or vehicle was given from day 1 to 14 via oral gavage. Tumor volumes are shown as mean ± SEM of 8 animals in each group. The complete tumor eradication (Panel (D)) was summarized at the end of the experiment. N.S. (not significant); p > 0.05, * p < 0.05, ** p < 0.01.
4. Discussion
Considering the limitation of OVs monotherapy in some tumors, combining OVs with molecular therapeutics targeting kinases associated with cancer development is becoming a promising anti-tumor therapy [4]. In this study, MEK inhibitor Trametinib, was used to combine with oHSV and we provide evidence that pretreatment with Trametinib enhanced oHSV replication mediated by down-regulation of STAT1 and PKR phosphorylation in vitro. Previous studies have described the effects of MAPK pathway inhibition on several oncolytic viruses [22,23]. MEKi CI1040 was found to increase oncolytic adenovirus replication possibly by up-regulation of the expression of coxsackievirus and adenovirus receptor (CAR) in colorectal carcinoma cell [24]. In contrast, MEKi PD98059 increased cell killing, without increasing oncolytic adenovirus replication in glioma cells [25]. For oHSV, combination of T-VEC and MEKi Trametinib increased viral replication in melanoma cell whereas MEK inhibition with PD98059 reduced oHSV-1 R3616 replication by about 15-fold in vitro [26]. It suggested that there is a diverse impact of MEK inhibition on different oncolytic viruses. Furthermore, we have confirmed that the increase of virus replication when pretreated with MEKi Trametinib is correlated with down-regulation of STAT1 and PKR phosphorylation, which likely explains the combination of oHSV-1 and MEKi Trametinib facilitated virus replication.
The first STAT family member, STAT1, primarily mediates cytokine- and growth factor-induced signals that are activated by diverse biological responses including proliferation, differentiation and apoptosis [27]. It has been reported that STAT1 contains highly conserved ERK phosphorylation sites; therefore, STAT protein can be activated by ERK [28,29,30,31]. Here, we further demonstrated that p-STAT1 was significantly down-regulated when decreased levels of p-ERK was induced by MEKi alone and combined. Additionally, the down-regulation of p-PKR was observed with the same treatment. Then, we investigated the possible relevance between STAT1 and PKR when MEKi treated. We found that down-regulation of STAT1 by siRNA can suppress the expression of PKR. Based on these results, we hypothesized that combination of oHSV and MEKi enhanced virus replication possibly mediated by down-regulation of PKR, which is induced by low level of p-STAT1. Interestingly, when mentioned enhancement of oHSV replication with MEKi, it only functions with BRAF V600E-mutated tumor cells, and BRAF wt/RAS-mutated tumor cells, not for BRAF wt/RAS wt tumor cells.
BRAF is a serine-threonine protein kinase involved in the RAS/RAF/MEK/ERK signaling pathway. The most common missense mutation of BRAF (mainly V600E) is known to constitutively activate the MAPK pathway and contributes to the incidence of various cancers [32]. Therapies targeting this pathway, such as those involving a BRAF inhibitor (BRAFi), as well as BRAFi with MEKi have improved antitumor outcomes, although resistance has not been abolished [33]. The combined treatment of oHSV and MEKi performed in this study indicated that combination of MEKi and oHSV increased tumor cell killing by facilitating viral replication, only for BRAF V600E-mutated tumor cells.
In our studies, therapeutic effectiveness was seen in syngeneic tumor mice with BRAF wt/KRAS-mutations. We found that in CT26 (KRAS-G12D) mouse model, the combined treatment of oHSV T3855 with MEKi showed 100% complete tumor eradication while LLC (KRAS-G12C) showed 50% complete response at 4 weeks post treatment. The remarkably therapeutic efficacy may be induced by increased virus replication as we have determined that MEKi treatment enhanced oHSV replication in BRAF wt/KRAS mutated tumor cells in vitro. On the other hand, the oHSV T3855 used in vivo contains murine IL-12 and murine scFV (single-chain variable fragment) antibody against PD-1. The combination of oHSV T3855 with MEKi is equal to a triple-drug regimen, including oncolytic effect of oHSV, PD-1 blockade, and MEK inhibition. It is consistent with the augmented antitumor responses with the combination of T-VEC, MEK inhibition, and anti-PD-1 antibody [9]. In addition, down-regulation of PKR and STAT1 mRNA levels was also observed in KRAS mutant cancer cells when combined treatment performed. It has been reported that in in human KRAS-G12D colon tumor cells, STAT1 can act in a cell-autonomous manner to promote tumor growth [34], which indicated that inhibition of STAT1 will suppress the KRAS-G12D colon tumor growth.
In summary, combination treatment with oHSV and MEKi Trametinib enhanced virus replication mediated by down-regulation of STAT1 and PKR phosphorylation in BRAF V600E-mutated tumor cells as well as BRAF wt/KRAS-mutated tumor cells. In vivo, there is a synergistic therapeutic efficacy shown in the combination of oHSV including PD-1 blockade and MEK inhibition, only for BRAF wt/KRAS-mutated tumors. Collectively, these data provide some new insights for clinical development of combination therapy with oncolytic virus, MEK inhibition, and checkpoint blockade for BRAF V600E-mutated or KRAS-mutated tumors.
5. Conclusions
Treatment with MEKi Trametinib augmented oHSV oncolytic activity in BRAF V600E-mutated tumor cells. Combination treatment with oHSV and MEKi Trametinib enhanced virus replication mediated by down-regulation of STAT1 and PKR expression or phosphorylation in BRAF V600E-mutated tumor cells as well as BRAF wt/KRAS-mutated tumor cells. A remarkably synergistic therapeutic efficacy was shown in vivo for BRAF wt/KRAS-mutated tumor models, when a combination of oHSV including PD-1 blockade and MEK inhibition.
Acknowledgments
We thank Weixuan Zou for her support during the animal experiment.
Author Contributions
Conceptualization, X.Z., X.C., and G.G.Z.; methodology, X.Z. and X.C.; validation, X.Z., X.C., and G.G.Z.; formal analysis, J.Z.; investigation, X.Z., Y.W., and L.W.; writing—original draft preparation, J.Z.; writing—review and editing, J.Z., X.Z., X.C., G.G.Z., D.J., and J.V.Z.; supervision, X.C., G.G.Z., and J.V.Z.; project administration, J.Z., X.C., and G.G.Z.; funding acquisition, X.C. and G.G.Z. All authors have read and agreed to the published version of the manuscript.
Funding
These studies were funded by grants from Shenzhen Science and Innovation Commission Project Grants J20180306173333907 and KCXFZ202002011010232 to Shenzhen International Institute for Biomedical Research.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data related to this study will be made available upon request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chiu M., Armstrong E.J.L., Jennings V., Foo S., Crespo-Rodriguez E., Bozhanova G., Patin E.C., McLaughlin M., Mansfield D., Baker G., et al. Combination therapy with oncolytic viruses and immune checkpoint inhibitors. Expert Opin. Biol. Ther. 2020;20:635–652. doi: 10.1080/14712598.2020.1729351. [DOI] [PubMed] [Google Scholar]
- 2.Zheng M., Huang J., Tong A., Yang H. Oncolytic Viruses for Cancer Therapy: Barriers and Recent Advances. Mol. Ther. Oncolytics. 2019;15:234–247. doi: 10.1016/j.omto.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lan Q., Xia S., Wang Q., Xu W., Huang H., Jiang S., Lu L. Development of oncolytic virotherapy: From genetic modification to combination therapy. Front. Med. 2020;14:160–184. doi: 10.1007/s11684-020-0750-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gilchrist V.H., Jémus-Gonzalez E., Said A., Alain T. Kinase inhibitors with viral oncolysis: Unmasking pharmacoviral approaches for cancer therapy. Cytokine Growth Factor Rev. 2020;56:83–93. doi: 10.1016/j.cytogfr.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y., Pan W., Liu S., Shen Z., Xu Y., Hu L. ERK/MAPK signalling pathway and tumorigenesis (Review) Exp. Ther. Med. 2020;19:1997–2007. doi: 10.3892/etm.2020.8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aberrant T., Raf R.A.S., Erk M.E.K. Targeting Aberrant RAS/RAF/MEK/ERK Signaling for Cancer Therapy. Cells. 2020;9:198. doi: 10.3390/cells9010198. [DOI] [Google Scholar]
- 7.Tibbles L.A., Woodgett J.R. The stress-activated protein kinase pathways. Cell. Mol. Life Sci. 1999;55:1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Midthun L., Shaheen S., Deisch J., Senthil M., Tsai J., Hsueh C.T. Concomitant KRAS and BRAF mutations in colorectal cancer. J. Gastrointest. Oncol. 2019;10:577–581. doi: 10.21037/jgo.2019.01.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bommareddy P.K., Aspromonte S., Zloza A., Rabkin S.D., Kaufman H.L. MEK inhibition enhances oncolytic virus immunotherapy through increased tumor cell killing and T cell activation. Sci. Transl. Med. 2018;10 doi: 10.1126/scitranslmed.aau0417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoo J.Y., Swanner J., Otani Y., Nair M., Park F., Banasavadi-Siddegowda Y., Liu J., Jaime-Ramirez A.C., Hong B., Geng F., et al. Oncolytic HSV therapy increases trametinib access to brain tumors and sensitizes them in vivo. Neuro-Oncology. 2019;21:1131–1140. doi: 10.1093/neuonc/noz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roskoski R. Properties of FDA-approved small molecule protein kinase inhibitors. Pharmacol. Res. 2019;144:19–50. doi: 10.1016/j.phrs.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Mahapatra D.K., Asati V., Bharti S.K. MEK inhibitors in oncology: A patent review (2015-Present) Expert Opin. Ther. Pat. 2017;27:887–906. doi: 10.1080/13543776.2017.1339688. [DOI] [PubMed] [Google Scholar]
- 13.Beck D., Niessner H., Smalley K.S.M., Flaherty K., Paraiso K.H.T., Busch C., Sinnberg T., Vasseur S., Iovanna J.L., Drießen S., et al. Vemurafenib potently induces endoplasmic reticulum stress-mediated apoptosis in BRAFV600E melanoma cells. Sci. Signal. 2013;6:ra7. doi: 10.1126/scisignal.2003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kun E., Tsang Y.T.M., Ng C.W., Gershenson D.M., Wong K.K. MEK inhibitor resistance mechanisms and recent developments in combination trials. Cancer Treat. Rev. 2021;92 doi: 10.1016/j.ctrv.2020.102137. [DOI] [PubMed] [Google Scholar]
- 15.Yan R., Zhou X., Chen X., Liu X., Tang Y., Ma J., Wang L., Liu Z., Zhan B., Chen H., et al. Enhancement of Oncolytic Activity of oHSV Expressing IL-12 and Anti PD-1 Antibody by Concurrent Administration of Exosomes Carrying CTLA-4 miRNA. Immunother. Open Access. 2019;5:1–10. doi: 10.35248/2471-9552.19.5.154. [DOI] [Google Scholar]
- 16.Cassady K.A. Human Cytomegalovirus TRS1 and IRS1 Gene Products Block the Double-Stranded-RNA-Activated Host Protein Shutoff Response Induced by Herpes Simplex Virus Type 1 Infection. J. Virol. 2005;79:8707–8715. doi: 10.1128/JVI.79.14.8707-8715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jackson J.D., Markert J.M., Li L., Carroll S.L., Cassady K.A. STAT1 and NF-κB inhibitors diminish basal interferon-stimulated gene expression and improve the productive infection of oncolytic HSV in MPNST Cells. Mol. Cancer Res. 2016;14:482–492. doi: 10.1158/1541-7786.MCR-15-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santarpia L.L., Lippman S., El-Naggar A. Targeting the Mitogen-Activated Protein Kinase RAS-RAF Signaling Pathway in Cancer Therapy. Expert Opin. Ther. Targets. 2012;16:103–119. doi: 10.1517/14728222.2011.645805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 20.McCubrey J.A., Steelman L.S., Chappell W.H., Abrams S.L., Wong E.W.T., Chang F., Lehmann B., Terrian D.M., Milella M., Tafuri A., et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta Mol. Cell Res. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phan V.T., Wu X., Cheng J.H., Sheng R.X., Chung A.S., Zhuang G., Tran C., Song Q., Kowanetz M., Sambrone A., et al. Oncogenic RAS pathway activation promotes resistance to anti-VEGF therapy through G-CSF-induced neutrophil recruitment. Proc. Natl. Acad. Sci. USA. 2013;110:6079–6084. doi: 10.1073/pnas.1303302110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gholami S., Chen C.H., Gao S., Lou E., Fujisawa S., Carson J., Nnoli J.E., Chou T.C., Bromberg J., Fong Y. Role of MAPK in oncolytic herpes viral therapy in triple-negative breast cancer. Cancer Gene Ther. 2014;21:283–289. doi: 10.1038/cgt.2014.28. [DOI] [PubMed] [Google Scholar]
- 23.Roulstone V., Pedersen M., Kyula J., Mansfield D., Khan A.A., Mcentee G., Wilkinson M., Karapanagiotou E., Coffey M., Marais R., et al. BRAF-and MEK-targeted small molecule inhibitors exert enhanced antimelanoma effects in combination with oncolytic reovirus through ER stress. Mol. Ther. 2015;23:931–942. doi: 10.1038/mt.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bagheri N., Shiina M., Lauffenburger D.A., Korn W.M. A dynamical systems model for combinatorial cancer therapy enhances oncolytic adenovirus efficacy by MEK-inhibition. PLoS Comput. Biol. 2011;7:e1001085. doi: 10.1371/journal.pcbi.1001085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Botta G., Passaro C., Libertini S., Abagnale A., Barbato S., Maione A.S., Hallden G., Beguinot F., Formisano P., Portella G. Inhibition of autophagy enhances the effects of E1A-defective oncolytic adenovirus dl922-947 against glioma cells in vitro and in vivo. Hum. Gene Ther. 2012;23:623–634. doi: 10.1089/hum.2011.120. [DOI] [PubMed] [Google Scholar]
- 26.Smith K.D., Mezhir J.J., Bickenbach K., Veerapong J., Charron J., Posner M.C., Roizman B., Weichselbaum R.R. Activated MEK Suppresses Activation of PKR and Enables Efficient Replication and In Vivo Oncolysis by Δγ134.5 Mutants of Herpes Simplex Virus 1. J. Virol. 2006;80:1110–1120. doi: 10.1128/JVI.80.3.1110-1120.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ihle J.N. The Stat family in cytokine signaling. Curr. Opin. Cell Biol. 2001;13:211–217. doi: 10.1016/S0955-0674(00)00199-X. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Zhang Y., Yun H., Chen S., Chen Y., Liu Z. ERK expression and its correlation with STAT1 in esophageal squamous cell carcinoma. Oncotarget. 2017;8:45249–45258. doi: 10.18632/oncotarget.16902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Athié-M V., Flotow H., Hilyard K.L., Cantrell D.A. IL-12 selectively regulates STAT4 via phosphatidylinositol 3-kinase and Ras-independent signal transduction pathways. Eur. J. Immunol. 2000;30:1425–1434. doi: 10.1002/(SICI)1521-4141(200005)30:5<1425::AID-IMMU1425>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Fehrenbach H., Kasper M., Tschernig T., Pan T., Schuh D., Shannon J.M., Müller M., Masor R.J. Keratinocyte growth factor-induced hyperplasia of rat alveolar type II cells in vivo is resolved by differentiation into type I cells and by apoptosis. Eur. Respir. J. 1999;14:534–544. doi: 10.1034/j.1399-3003.1999.14c10.x. [DOI] [PubMed] [Google Scholar]
- 31.Jiang Q.S., Huang X.N., Yang G.Z., Jiang X.Y., Zhou Q.X. Inhibitory effect of ginsenoside Rb1 on calcineurin signal pathway in cardiomyocyte hypertrophy induced by prostaglandin F 2α. Acta Pharmacol. Sin. 2007;28:1149–1154. doi: 10.1111/j.1745-7254.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- 32.Taieb J., Lapeyre-Prost A., Laurent Puig P., Zaanan A. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br. J. Cancer. 2019;121:434–442. doi: 10.1038/s41416-019-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ros J., Baraibar I., Sardo E., Mulet N., Salvà F., Argilés G., Martini G., Ciardiello D., Cuadra J.L., Tabernero J., et al. BRAF, MEK and EGFR inhibition as treatment strategies in BRAF V600E metastatic colorectal cancer. Ther. Adv. Med. Oncol. 2021;13 doi: 10.1177/1758835921992974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Darini C., Désaubry L., Koromilas A.E. STAT1 promotes KRAS colon tumor growth and susceptibility to pharmacological inhibition of translation initiation factor eIF4A. Mol. Cancer Ther. 2016;15:3055–3063. doi: 10.1158/1535-7163.MCT-16-0416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data related to this study will be made available upon request.