Abstract
B-cell receptor (BCR)-induced activation of phospholipase C-γ1 (PLCγ1) and PLCγ2 is crucial for B-cell function. While several signaling molecules have been implicated in PLCγ activation, the mechanism coupling PLCγ to the BCR remains undefined. The role of PLCγ1 SH2 and SH3 domains at different steps of BCR-induced PLCγ1 activation was examined by reconstitution in a PLCγ-negative B-cell line. PLCγ1 membrane translocation required a functional SH2 N-terminal [SH2(N)] domain, was decreased by mutation of the SH3 domain, but was unaffected by mutation of the SH2(C) domain. Tyrosine phosphorylation did not require the SH2(C) or SH3 domains but depended exclusively on a functional SH2(N) domain, which mediated the association of PLCγ1 with the adapter protein, BLNK. Forcing PLCγ1 to the membrane via a myristoylation signal did not bypass the SH2(N) domain requirement for phosphorylation, indicating that the phosphorylation mediated by this domain is not due to membrane anchoring alone. Mutation of the SH2(N) or the SH2(C) domain abrogated BCR-stimulated phosphoinositide hydrolysis and signaling events, while mutation of the SH3 domain partially decreased signaling. PLCγ1 SH domains, therefore, have interrelated but distinct roles in BCR-induced PLCγ1 activation.
One of the earliest consequences of lymphocyte antigen receptor triggering is the activation of phosphoinositide-specific phospholipase C-γ (PLCγ) (67). PLCγ hydrolyzes phosphatidylinositol (4,5)-bisphosphate (PtdInsP2) to inositol (1,4,5)-trisphosphate and diacylglycerol, metabolites which control calcium mobilization and protein kinase C activation, respectively (3, 40, 41). Together these second messengers coordinate the activation of downstream signaling pathways that ultimately control the metabolic and biological response of the cell.
PLCγ is a cytoplasmic enzyme that, in order to hydrolyze PtdInsP2, needs to both translocate to the membrane where its substrate resides and undergo an increase in its intrinsic catalytic potential (2, 57). Tyrosine phosphorylation of PLCγ is an obligatory step that augments its catalytic activity (2, 21, 30) and allows PLCγ to overcome the substrate sequestration and inhibitory effect of the actin- and phosphoinositide-binding protein, profilin (11).
Two structurally related PLCγ isozymes, PLCγ1 and PLCγ2, have been identified (3, 40). Receptor tyrosine kinases, like the epidermal growth factor (EGF) receptor or the platelet-derived growth factor (PDGF) receptor, recruit PLCγ1 to their intracellular autophosphorylated tails and phosphorylate PLCγ1 by way of their intrinsic tyrosine kinase activity (31, 62, 63). The antigen receptors of T and B lymphocytes, however, have no intrinsic kinase activity. These receptors recruit protein tyrosine kinases via their immunoreceptor tyrosine-based activation motifs, leading to the activation of several signaling cascades, including the PLCγ-regulated Ca2+ pathway (68). In both T and B lymphocytes, PLCγ1 and/or PLCγ2 are tyrosine phosphorylated (4, 14, 32, 43, 67) and have been found in association with several signaling molecules, including the CD3 chains of the T-cell receptor (TCR) (6), kinases of the Src and Syk families (24, 36, 37, 49, 65), and adapter molecules such as Grb2 (48), Slp76 (19), BLNK/Slp65 (9, 10, 70), or pp36-38/LAT (48, 66, 73).
Studies using cells with altered signaling molecules have demonstrated that Lck (53), Zap70 (71), Itk (25), and the adapter, Slp76 (72), play a role in TCR-induced PLCγ1 tyrosine phosphorylation and/or activation in T lymphocytes. In B lymphocytes, both PLCγ isoforms are activated in response to B-cell receptor (BCR) engagement (4, 14, 43). Expression of Syk is necessary for PLCγ phosphorylation and activation in B lymphocytes (56). Furthermore, Syk can phosphorylate PLCγ in vitro (24). However, coexpression of a functional BCR together with Fyn and Syk in nonlymphoid cells does not induce PLCγ phosphorylation or Ca2+ mobilization (42), suggesting that additional molecules may be involved in coupling PLCγ to Syk. The recently identified adapter, BLNK/Slp65 (9, 10, 18, 70), may serve such a coupling function. An additional tyrosine kinase involved in PLCγ phosphorylation in B lymphocytes is the Tec family kinase, Btk, as shown by the defective tyrosine phosphorylation of PLCγ2 in Btk-deficient cells (55). Btk and its T-lymphocyte counterpart, Itk, may play a role in controlling the antigen receptor-induced PLCγ activation that lead to a sustained Ca2+ influx (8, 25, 55).
Despite the large number of molecules shown to interact with PLCγ isozymes, the mechanism of PLCγ activation by the lymphocyte antigen receptors remains largely undefined. The involvement of multiple molecules in PLCγ activation suggests the presence of a complex molecular network regulating PLCγ translocation, phosphorylation, and catalytic activity. These activation events, while highly interrelated, are likely to be regulated in a manner independent of one another. To gain further insights into the mechanism of PLCγ activation, we sought to explore the relationship between certain PLCγ structural features and the sequence of activation events induced by BCR engagement.
Both PLCγ1 and PLCγ2 have two Src homology 2 (SH2) domains and a single SH3 domain. SH2 domains bind tyrosine-phosphorylated proteins and may interact with certain phospholipids (34, 38). SH2 domains are highly conserved modular regions of ∼100 residues containing an Arg residue at the structurally conserved position βB5, which coordinates the interaction with the phosphorylated tyrosine (33, 64). The selectivity of binding by SH2 domains is primarily conferred by the amino acid in position βD5 (Cys in either SH2 domain of PLCγ1 or PLCγ2), which makes contact with residues at the +1 and +3 positions immediately carboxy terminal to the phosphotyrosine of the bound peptide (50). Both SH2 domains of PLCγ belong to a group that recognizes target sequences with the motif pY-hydrophobic-X-hydrophobic, although amino acid differences within this consensus may further select between the SH2 N-terminal [SH2(N)] and SH2 C-terminal [SH2(C)] domains (50).
SH3 domains bind proteins that contain proline-rich regions (34). The PLCγ1 SH3 domain has an apparent binding preference for proteins containing a PPVP motif (47, 51), with sequences surrounding the SH3 domain contributing to the stabilization of the core interaction (12). The specific function of this domain in PLCγ activation, however, remains uncertain. Because a fusion protein encompassing the SH3 domain of PLCγ1 codistributed with cytoskeletal structures (1), it has been proposed that this domain may function in targeting PLCγ1 to the cell cytoskeleton and possibly contribute to bringing the active enzyme in proximity of its substrate.
To further explore the role of PLCγ1 SH domains in activation, we have expressed wild-type PLCγ1 and PLCγ1 variants bearing functional mutations of the SH2 domains or the SH3 domain in the PLCγ-deficient B-cell line, P10-14 (54). These cells do not express PLCγ1, and PLCγ2 expression has been disrupted by gene targeting. The effect of these mutations on BCR-induced PLCγ1 translocation, phosphorylation, and activity has revealed a critical role for each domain at various points in the activation sequence.
MATERIALS AND METHODS
Cells and reagents.
The parental chicken B-cell line, DT-40, the PLCγ-deficient derivative, P10-14 (54), and stable PLCγ1 P10-14 transfectants were all maintained in RPMI 1640 containing 7.5% fetal bovine serum and 1% chicken serum. The anti-influenza virus hemagglutinin (HA) antibody, 12CA5, was a gift from Allan Weissman (National Cancer Institute, National Institutes of Health, Bethesda, Md.). The antiphosphotyrosine antibody (Ab), 4G10, was from Upstate Biotechnology (Lake Placid, N.Y.). The PLCγ1 glutathione S-transferase (GST)-SH2(N) domain and GST-SH2(C) domain fusion proteins were from Santa Cruz Biotechnology. The GST-Grb2 fusion protein construct (46) was a gift from Pier Giuseppe Pelicci (European Oncology Institute, Milan, Italy). The rabbit anti-chicken BLNK serum (amino acids 79 to 201) has been previously described (18). The NF-AT luciferase reporter gene construct was a gift from Gerald Crabtree (Stanford University, Stanford, Calif.).
DNA plasmids, transfections, and generation of stable transfectants.
The construction of the HA-tagged bovine PLCγ1 (PLCγ1-HA) in the expression vector, pCIneo (Promega, Madison, Wis.), and the site-directed mutagenesis of the SH2(N) domain (Arg to Lys at position 586) and the SH2(C) domain (Arg to Lys at positions 694 or 694 and 696) have been described previously (52). A mutation of Pro to Leu at position 842 within the SH3 domain was introduced by PCR using appropriate oligonucleotides. The amplified fragment was shuttled via BSSHII and StuI sites into a pBluescript SK− vector (Stratagene, La Jolla, Calif.) which encoded bovine PLCγ1. A fragment encompassing the SH3 domain-coding region was excised with EcoRV and SacII and ligated in the identical position of PLCγ1-HA in the pCIneo expression vector. The loss of binding by the SH2 domain mutants was validated by encoding the same mutations as GST fusion proteins (52). Loss of function of the SH3 domain mutant was demonstrated by the failure of a GST fusion protein encoding the proline-rich region of c-Cbl to precipitate PLCγ1-HA SH3P842L compared to the wild-type (WT) protein (data not shown). The amino-terminal myristoylation (myr) signal sequence, (M)GSSKKSKPKD, and the control sequence, (M)ASSKKSKPKD, were introduced by PCR using appropriate oligonucleotides and ligated directly into PLCγ1-HA in pCIneo via XbaI and Eco72I sites. All transfections were performed by electroporation as previously described (52). For the preparation of stable transfectants, P10-14 cells were electroporated with 20 μg of the indicated PLCγ1-HA construct and 2 μg of pBABEpuro (29) and selected with puromycin (Sigma, St. Louis, Mo.). Different sets of clones stably expressing closely matched levels of WT and SH domain mutants of PLCγ1 were screened by anti-HA immunoblotting and selected for further analysis.
Cell activation.
BCR stimulation was routinely accomplished by treating cells with a polyclonal goat anti-chicken immunoglobulin M (IgM) purified antiserum (Bethel Laboratories, Montgomery, Tex.) for 1 min at 37°C. In some experiments, cells were coated on ice with the goat anti-chicken IgM Ab, washed, and stimulated for 1 min at 37°C with a rabbit anti-goat IgG purified antiserum (Jackson Immunoresearch, West Grove, Pa.). For induction of phosphoinositide hydrolysis, the anti-chicken IgM was immobilized onto 3 μM polystyrene latex beads (Polysciences, Warrington, Pa.) at 100 μg/109 beads.
Immunofluorescence.
Stable PLCγ1-HA transfectants were activated, fixed with 3.7% formaldehyde, and permeabilized by immersion in methanol 70%. Slides were incubated with the anti-HA monoclonal Ab (diluted 1:10,000 in phosphate-buffered saline containing human immunoglobulins [4 mg/ml] and goat immunoglobulins [4 mg/ml]) and then reacted with a fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse Ab (DAKO Sp.A., Milan, Italy) diluted 1:30. Analysis was carried out with a TCS 4D (Leica) mounted on a Leitz DMRB microscope equipped with a 100×/1.3 NA oil immersion objective. High-resolution fluorescence images were obtained by exciting fluorescein at 488 nm. Serial optical sections, acquired with an averaging function line by line, top down, with a scanning mode format of 512 by 512 pixels, were elaborated by a three-dimensional image processing system providing an extended focus image.
Cell fractionation.
Cells (30 × 106 cells) were resuspended in hypotonic buffer (10 mM Tris-HCl [pH 7.5] containing 0.5 mM MgCl2 plus protease and phosphatase inhibitors). After 10 min on ice, cells were subjected to Dounce homogenization, NaCl was added to restore tonicity along with EDTA, and samples were centrifuged (550 × g, 5 min) to remove nuclei and unbroken cells. The particulate (membrane) fraction was separated from the cytosol by ultracentrifugation at 100,000 × g for 60 min. Membrane-containing pellets were washed extensively and resuspended in lysis buffer (50 mM Tris-HCl [pH 7.5], 300 mM NaCl, 0.5% Triton X-100, protease and phosphatase inhibitors), and the solubilized material was recovered by centrifugation (10,000 × g for 15 min). After determination of the protein concentration in the cytosol and solubilized particulate fractions, identical amounts of proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblotted as described below.
Precipitation and Western blot analysis.
Cells were lysed in a buffer comprised of 60 mM Tris-HCl (pH 7.8) containing 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Triton X-100, and phosphatase and protease inhibitors as previously described (52). Postnuclear fractions were precipitated with GST fusion proteins bound to glutathione-Sepharose beads (Pharmacia, Piscataway, N.J.) or specific Ab prebound to protein A-Trisacryl beads (Pierce, Rockford, Ill.). Proteins were eluted with sample buffer, resolved by SDS-PAGE under reducing conditions, and transferred to nitrocellulose membranes (Hybond-C Super; Amersham, Arlington Heights, Ill.). Protein detection was via primary Ab with or without second Ab (rabbit anti-mouse IgG; Cappel, Aurora, Ohio) followed by [125I]protein A (ICN, Costa Mesa, Calif.). For certain experiments, immunoblots were stripped according to the membrane manufacturer’s instructions and reprobed with other Abs followed by detection with [125I]protein A or the Amersham ECL system. Radioimmunoblots were scanned on a PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.) to produce the images shown (ImageQuant software; Molecular Dynamics) with no manipulation, except for the adjustment of the exposure range. All experiments shown were repeated at least three times.
Phosphoinositide hydrolysis.
Cells were labeled with [myo-3H]inositol (7.5 μCi/0.5 × 107 cells/ml; Amersham) for 4 h at 37°C. Activation was accomplished by mixing cells with anti-chicken IgM-coated polystyrene beads and incubating in the presence of 10 mM LiCl at 37°C for 45 min. Reactions were terminated by the addition of trichloroacetic acid. The soluble material was applied to an AG1-X8 column, and total inositol phosphates were eluted with 1 M ammonium formate containing 0.1 M formic acid. Precipitable material was dissolved in 10% Triton X-100 and used to determine the amount of [3H]inositol incorporated into the phospholipid pool. Data were normalized as the percentage of the total cellular radioactivity.
Measurement of NF-AT transcriptional activity.
Cells were transfected with 10 μg of a plasmid containing a luciferase reporter under the control of the interleukin 2 minimal promoter and three optimized NF-AT enhancer elements (pNF-AT-luc) together with 10 μg of pADβ (Clontech, Palo Alto, Calif.), a control vector for transfection efficiency in which the expression of β-galactosidase is regulated by the adenovirus major late promoter. After 24 h, 2 × 105 cells were incubated for additional 6 h in 100 μl of medium containing 10 μg of anti-chicken IgM. Cells were washed twice, disrupted in lysis buffer (Promega), and assayed using luciferin (Promega). Data were normalized by assaying for β-galactosidase activity using Galacton Plus and Emerald Enhance (Tropix, Bedford, Mass.).
PLC assay.
Cells (2.5 × 107 cells/sample) stably expressing the various PLCγ1-HA constructs were treated with medium alone or activated with anti-chicken IgM antiserum. Lysates were clarified by centrifugation, and postnuclear fractions were subjected to immunoprecipitation with an anti-HA Ab as described above. Samples from untransfected P10-14 cells were always included as negative controls. The immunoprecipitates were washed extensively and assayed for PLC activity by using a modification of the method described by Wahl et al. (60). For this assay, 1 mg of PtdInsP2 (Roche Diagnostic) and 2 μCi of [3H]PtdInsP2 (New England Nuclear) were combined, dried, resuspended in 180 μl of 50 mM NaH2PO4 (pH 6.8), and 100 mM KCl, and sonicated. β-Octylglucoside was then added to a final concentration of 50 mM in a total volume of 360 μl. The assay mix (50 μl) included 100 mM NaH2PO4 (pH 6.8), 175 mM KCl, 2 mM EGTA, 2 mM CaCl2, 0.07% Triton X-100 (final concentration, 1.2 mM), and 5 μl of the micellar suspension of PtdInsP2 (final concentration, ∼250 μM). The assay was conducted at 35°C and started by the addition of the substrate. The assay was terminated at the indicated times with the addition of 10% ice-cold trichloroacetic acid and serum albumin as a carrier. Samples were centrifuged, and the acid-soluble radioactivity was determined by β-scintillation counting.
RESULTS
The SH2(N) and SH3 domains of PLCγ1 are involved in BCR-induced membrane translocation of PLCγ1.
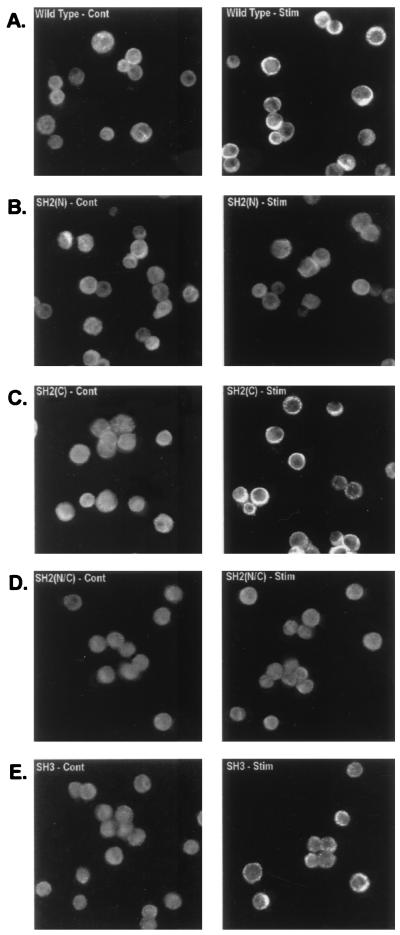
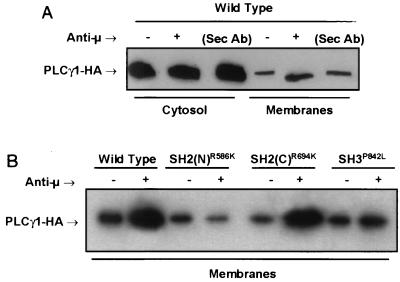
The P10-14 cell line was derived from the chicken B-cell line, DT40, and expresses no detectable PLCγ isozymes (54). P10-14 cells were transfected with expression vectors encoding a HA-tagged PLCγ1 WT protein or PLCγ1 proteins bearing mutations of the SH domains. Stable cell lines with similar levels of PLCγ1-HA expression (data not shown) were established. The role of PLCγ1 SH domains in BCR-induced membrane translocation was explored first by immunofluorescence studies of stable PLCγ1-HA transfectants activated via Ab-mediated BCR aggregation and probed with an anti-HA-FITC. In unstimulated B cells, WT PLCγ1-HA was present as a diffuse cytoplasmic fluorescence (Fig. 1A). Within 1 min of treatment with anti-BCR stimulation, a portion of WT PLCγ1 localized to the cell membrane and the juxtamembrane areas, as indicated by a strong reinforcement of the fluorescence signal at and immediately below the cell rim. No membrane redistribution was observed in cells expressing the SH2(N) domain PLCγ1-HA mutant (Fig. 1B). Mutation of PLCγ1 SH2(C) domain (double mutation of Arg at positions 694 and 696 to Lys) had no effect on BCR-induced PLCγ1 membrane association (Fig. 1C). A reduction in the stimulated redistribution of the SH3 domain mutant PLCγ1-HA was also observed (Fig. 1E), with the edge of the cells expressing the SH3 domain mutant showing a distinct fluorescent granularity rather than a continuous pattern of reinforcement in response to BCR stimulation. A PLCγ1 construct bearing a double mutation of both the SH2(N) and SH2(C) domains failed to translocate to the membrane and was indistinguishable from the single PLCγ1 SH2(N) domain mutant (Fig. 1D). Results obtained by fluorescence microscopy were confirmed by cell fractionation experiments. Western blots of membranes from stable transfectants expressing WT PLCγ1 and the SH2(C) domain mutant showed an increase in PLCγ1-HA compared to unactivated control cells (Fig. 2). No stimulated increase was observed in the membrane preparation from the PLCγ1 SH2(N) domain mutant, while only a partial increase was detected in that of the PLCγ1 SH3 domain mutant. These data suggest that an intact SH2(N) domain is absolutely required for BCR-induced PLCγ1 membrane translocation, whereas the SH3 domain contributes some additional redistribution function. The SH2(C) domain plays no apparent role in receptor-induced translocation of PLCγ1.
FIG. 1.
BCR-induced translocation of PLCγ1 to the cell membrane is affected by mutation of the SH2(N) domain or SH3 domain. Stable transfectants, expressing similar levels of PLCγ1-HA by Western blot analysis, were treated with medium (left panels, Cont) or anti-chicken IgM (right panels, Stim) for 1 min, fixed with formaldehyde, permeabilized, reacted with anti-HA Ab, and stained with a secondary FITC-conjugated antiserum as described in Materials and Methods. (A) PLCγ1-HA WT; (B) PLCγ1-HA SH2(N)R586K; (C) PLCγ1-HA SH2(C)R694/6K; (D) PLCγ1-HA SH2(N/C)R586/694/6K; (E) PLCγ1-HA SH3P842L.
FIG. 2.
BCR-induced translocation of PLCγ1 to the membrane fraction is affected by mutation of the SH2(N) domain and SH3 domain. (A) Stable transfectants expressing WT PLCγ1-HA were precoated with a murine anti-chicken IgM monoclonal Ab and stimulated for 1 min at 37°C with a rabbit anti-mouse IgG Ab. Medium-treated (uncoated) cells or cells treated only with the rabbit anti-murine IgG serum (Sec Ab) were included as controls. Cytosol and particulate (membrane) fractions were resolved by SDS-PAGE and immunoblotted with anti-HA. (B) Resolubilized membrane fractions from control- or BCR-activated (1 min at 37°C) stable transfectants expressing identical levels of the indicated PLCγ1-HA WT and mutant proteins were resolved by SDS-PAGE and immunoblotted with anti-HA.
BCR-induced tyrosine phosphorylation of PLCγ1 requires a functionally intact SH2(N) domain capable of interacting with the adapter molecule, BLNK.
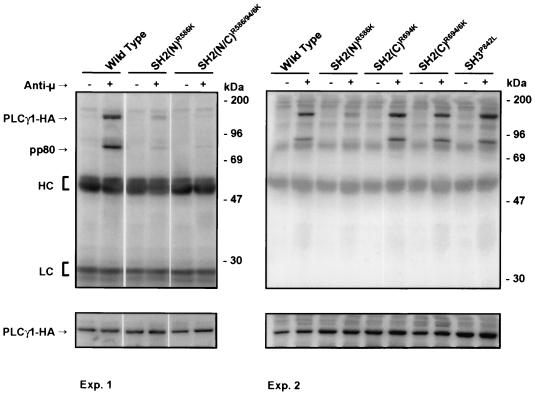
BCR aggregation induces rapid tyrosine phosphorylation of PLCγ1 and PLCγ2 in B cells (4, 14, 43), an event which is required for PLCγ activation. We therefore performed anti-HA immunoprecipitation experiments from resting and activated P10-14 cells expressing PLCγ1-HA proteins followed by antiphosphotyrosine immunoblotting. WT PLCγ1-HA was tyrosine phosphorylated in P10-14 cells following BCR engagement (Fig. 3). The level of tyrosine phosphorylation of the SH2(N) domain mutant, however, was significantly less than that of the WT protein. Mutation of a single or both critical Arg residues to Lys in the SH2(C) domain had no discernible effect on BCR-induced tyrosine phosphorylation of PLCγ1-HA. Similarly, mutation of the SH3 domain did not decrease the stimulated level of tyrosine phosphorylation of PLCγ1. Identical results were obtained in several stable transfectants as well as in transient transfection experiments (data not shown), suggesting that the differences observed cannot be attributed to clonal variations. Therefore, BCR-induced PLCγ1 tyrosine phosphorylation was exclusively dependent on the function of the SH2(N) domain.
FIG. 3.
BCR-induced tyrosine phosphorylation of PLCγ1 requires an intact SH2(N) domain. Stable WT or SH mutant PLCγ1-HA transfectants were stimulated with anti-chicken IgM for 1 min at 37°C. Anti-HA immunoprecipitates were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine (upper panels). The same blots were stripped and reprobed with anti-HA (lower panels) for comparison of the relative amounts of PLCγ1-HA expressed. HC, heavy chain; LC, light chain.
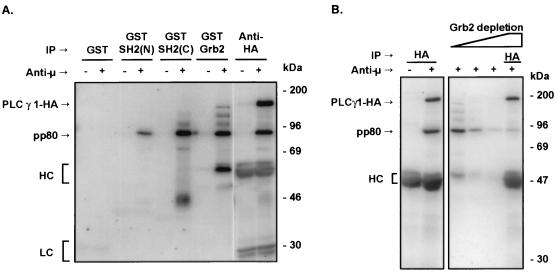
In activated B cells, PLCγ1-HA WT coprecipitated with a prominent 80-kDa phosphoprotein (Fig. 3). This phosphoprotein was absent from anti-HA immunoprecipitates of stimulated cells transfected with the SH2(N) domain mutant. Mutation of the SH2(C) or the SH3 domains of PLCγ1 resulted in no detectable difference in coprecipitating pp80 levels. A protein with identical electrophoretic mobility on SDS-PAGE was precipitated from lysates of activated P10-14 cells by GST fusion proteins encompassing the PLCγ1 SH2(N) or SH2(C) domain or the whole Grb2 molecule (Fig. 4A). Note that PLCγ1 GST-SH2(N) domain bound exclusively to this 80-kDa phosphoprotein, while the SH2(C) domain or Grb2 bound this phosphoprotein as well as a differential spectrum of additional phosphoproteins. Thus, the SH2(N) domain of PLCγ1 demonstrates greater selectivity of binding than that of the SH2(C) domain or Grb2. The 80-kDa phosphoprotein observed in GST-Grb2 precipitates was the same as that coprecipitating with PLCγ1-HA, as shown by GST-Grb2 depletion followed by precipitation with anti-HA (Fig. 4B). In addition, preclearing with GST-Grb2 depleted the 80-kDa phosphoprotein precipitated by PLCγ1 GST-SH2(N) or SH2(C) domains (data not shown), further confirming the shared identity of the Grb2- and PLCγ1-bound pp80.
FIG. 4.
pp80 binds the SH2 domains of PLCγ1 or Grb2. (A) Untransfected P10-14 cells were treated with medium or anti-chicken IgM for 1 min. Lysates were precipitated with GST alone or with the GST-SH2(N) or GST-SH2(C) domain fusion protein of PLCγ1 or a GST-Grb2 fusion protein immobilized onto glutathione-Sepharose beads. Lysates from stable P10-14 transfectants expressing WT PLCγ1-HA were subjected to immunoprecipitation (IP) with anti-HA. Proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. (B) Lysates from stable P10-14 transfectants expressing WT PLCγ1-HA were immunoprecipitated with anti-HA or were sequentially precipitated with three rounds of GST-Grb2 fusion protein followed by anti-HA immunoprecipitation. Proteins were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. HC, heavy chain; LC, light chain.
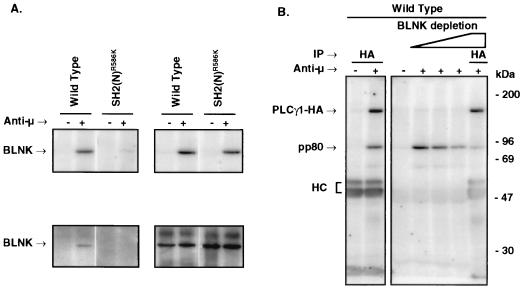
The chicken homologue of the mammalian adapter, BLNK/Slp-65, is an 80-kDa phosphoprotein recently identified by two of us (18). BLNK is a molecule capable of interacting with PLCγ and Grb2 (9, 70). Immunoblot analysis with an antiserum raised against chicken BLNK showed that BCR ligation induced the association of BLNK with WT PLCγ1-HA, but this interaction was lost in cells expressing the SH2(N) domain mutant of PLCγ1 (Fig. 5A). The presence of phosphorylated BLNK in activated P10-14 cells expressing the SH2(N) domain PLCγ1-HA mutant rules out a phosphorylation defect of this protein associated with the expression of this PLCγ1 construct. Furthermore, sequential precipitation with anti-BLNK followed by anti-HA precipitation showed greatly reduced amounts of pp80 coprecipitated with PLCγ1-HA (Fig. 5B), confirming that the 80-kDa phosphoprotein is BLNK. These data demonstrate that PLCγ1 binds BLNK via its SH2(N) domain.
FIG. 5.
The PLCγ1 SH2(N) domain-bound pp80 is BLNK. (A) Stable P10-14 transfectants expressing WT PLCγ1-HA or PLCγ1-HA SH2(N)R586K domain mutant were treated for 1 min at 37°C with medium alone or anti-chicken IgM. Lysates were immunoprecipitated with anti-HA and proteins resolved by SDS-PAGE. Blots were probed with antiphosphotyrosine (upper panels) and stripped and reprobed with anti-chicken BLNK (lower panels). (B) Lysates from activated WT PLCγ1-HA transfectants were immunoprecipitated (IP) with anti-HA or were sequentially immunoprecipitated with anti-chicken BLNK followed by anti-HA immunoprecipitation. Samples were resolved by SDS-PAGE and immunoblotted with antiphosphotyrosine. HC, heavy chain.
Forced membrane localization of PLCγ1 does not overcome the SH2(N) requirement for tyrosine phosphorylation.
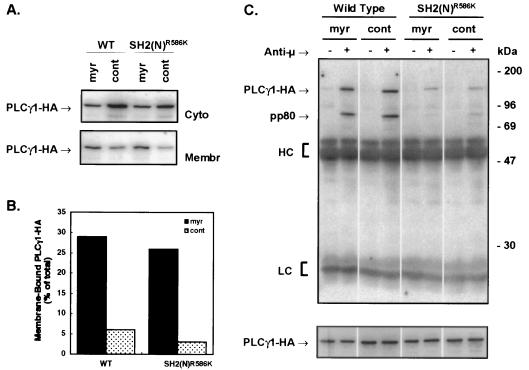
The SH2(N) domain is involved in both BCR-induced membrane translocation and tyrosine phosphorylation of PLCγ1. To determine if the inability of the SH2(N) domain mutant of PLCγ1 to be phosphorylated could be primarily attributed to its defect in membrane translocation, PLCγ1-HA was forced to the membrane by the addition of a myr sequence (39). Cell fractionation experiments confirmed that a greater percentage of the myr-PLCγ1-HA constructs repartitioned to the membrane fraction compared to control constructs in which the amino-terminal myristoyl acceptor Gly was mutated to Ala (G→A) (Fig. 6A and B) or with the unmodified WT protein (data not shown). The amount of transiently expressed myr-PLCγ1-HA constitutively associated to the membrane fraction (∼25%) exceeded the amount of stably expressed WT PLCγ1-HA repartitioned to this fraction upon BCR engagement (∼10% [data not shown]). The presence of cytosolic myr-PLCγ1-HA was likely due to the limited ability of transiently transfected cells to modify the overexpressed protein. The resting level of phosphorylation of myr-PLCγ1-HA constructs transiently expressed in P10-14 cells was comparable to that of the G→A controls (Fig. 6C). BCR-stimulated tyrosine phosphorylation of myr-PLCγ1-HA WT was also similar to that of the G→A PLCγ1-HA WT control. BCR-induced tyrosine phosphorylation, however, was abrogated by mutation of the SH2(N) domain regardless of its myristoylation (Fig. 6C). Addition of a myr signal to the SH2(C) or SH3 domain mutants did not affect the resting or stimulated phosphorylation status of these proteins (data not shown). These data indicate that targeting PLCγ1 to the membrane via a myr signal sequence does not bypass the need for the SH2(N) domain to interact with a protein that promotes PLCγ1 tyrosine phosphorylation. They further suggest that PLCγ1 tyrosine phosphorylation is not controlled by simple membrane/cytosol compartmentalization.
FIG. 6.
Membrane targeting of PLCγ1 via myristoylation does not bypass the SH2(N) domain requirement for BCR-induced tyrosine phosphorylation. (A) P10-14 cells were transiently transfected with 20 μg of pCIneo PLCγ1-HA WT or pCIneo PLCγ1-HA SH2(N)R586K containing an amino-terminal Src myr myr sequence or a control sequence (cont). After 24 h, cells were lysed by Dounce homogenization and fractionated into cytosol (Cyto) and membrane (Membr) components. Twenty-five micrograms of protein was resolved by SDS-PAGE and immunoblotted with anti-HA. (B) Densitometric analysis of the blot shown in panel A. (C) Transiently transfected P10-14 cells were treated for 1 min with medium or anti-chicken IgM. Lysates were immunoprecipitated with anti-HA, resolved by SDS-PAGE, and probed with antiphosphotyrosine (upper panel). The same blot was stripped and reprobed with anti-HA (lower panel) for comparison of the relative amounts of PLCγ1-HA expressed. HC, heavy chain; LC, light chain.
Both PLCγ1 SH2(N) and SH2(C) domains are required for BCR-induced phosphoinositide hydrolysis and NF-AT signaling.
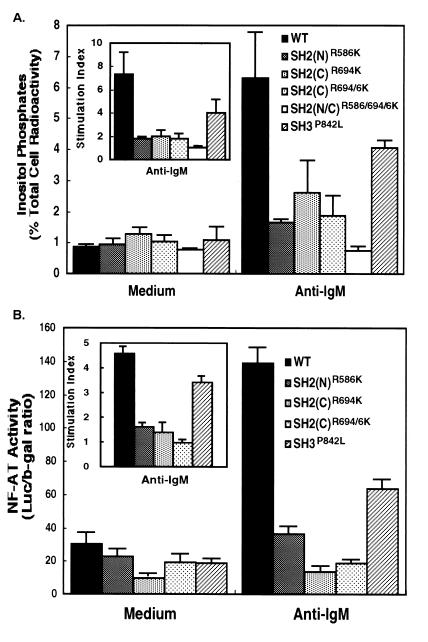
PLCγ1 catalyzes the hydrolysis of inositol phospholipids resulting in the generation of diacylglycerol, which activates protein kinase C and several inositol phosphates, including inositol (1,4,5)-trisphosphate (40). To establish the role of PLCγ1 SH domains in BCR-induced enzyme activation, P10-14 transfectants expressing PLCγ1-HA WT or mutant constructs were labeled with [myo-3H]inositol and stimulated via BCR ligation in the presence of lithium chloride to block inositol phosphate phosphatases. The accumulated inositol phosphates were separated from free inositol by ion-exchange chromatography. Whereas untransfected P10-14 cells showed no stimulated generation of inositol phosphates, activity was restored by expressing the PLCγ1-HA WT (Fig. 7A), with inositol phosphate accumulation levels similar to that of the parental DT-40 cells (data not shown). BCR engagement, however, did not stimulate inositol phosphate production in cells expressing the SH2(N) mutant PLCγ1-HA, consistent with its lack of membrane translocation and phosphorylation. Furthermore, inositol phospholipid hydrolysis was also abrogated in cells expressing PLCγ1-HA bearing mutations of the SH2(C) domain and partially reduced in cells expressing a PLCγ1-HA with a mutated SH3 domain. These results indicate that all SH domains must be functional for full PLCγ1 enzymatic activity in response to BCR ligation and that steps in addition to tyrosine phosphorylation and membrane translocation control PLCγ1 activation.
FIG. 7.
PLCγ1 SH domains are required for BCR-induced phosphoinositide hydrolysis and NF-AT induction. (A) Cells (2 × 106) expressing PLCγ1-HA WT and mutant constructs were labeled with [3H]inositol, washed, and incubated with 5 × 107 3-μm-diameter polystyrene beads precoated with bovine albumin or anti-chicken IgM. Inositol phosphates were allowed to accumulated for 45 min at 37°C in the presence of 5 mM LiCl and separated from free inositol by ion-exchange chromatography. Data are expressed as the percentage of total cell-associated radioactivity (mean ± standard deviation of three separate experiments). The inset represents the data normalized per the resting, unstimulated levels of inositol phosphates (stimulation index). (B) Stable PLCγ1-HA transfectants were transiently transfected with pNF-AT-Luc and pADβ for 24 h. Cells were stimulated for 6 h with plate-immobilized anti-chicken IgM, harvested, lysed, and assayed for luciferase and β-galactosidase activities. Specific NF-AT activity is expressed as the luciferase/β-galactosidase (b-gal) ratio (mean ± standard deviation of three separate experiments). The inset represents the data normalized per the resting, unstimulated NF-AT activity (stimulation index).
A consequence of inositol phosphate-mediated increase in intracellular Ca2+ is the activation of calcineurin (5). This phosphatase binds to and dephosphorylates the cytoplasmic transcription factor, NF-ATp, thereby enabling it to enter the nucleus (5). Further information regarding the role of the SH domains in the regulation of PLCγ1 was obtained by investigating BCR-induced transcriptional activation of NF-AT in P10-14 cells expressing WT and mutant proteins. Stable PLCγ1-HA transfectants were transiently transfected with a NF-AT reporter gene construct whose promoter/enhancer sequences control the expression of luciferase. A vector encoding for β-galactosidase under the control of the constitutive adenovirus promoter was used as an internal control for transfection efficiency. BCR engagement in cells expressing PLCγ1-HA WT produced a four- to sixfold increase in NF-AT-driven transcription, while cells expressing the SH2(N) or SH2(C) domain mutant did not induce NF-AT activation (Fig. 7B), consistent with the inability of these constructs to mediate phosphoinositide hydrolysis. Cells expressing a PLCγ1-HA bearing a mutated SH3 domain showed intermediate NF-AT reporter activity. These data further confirm that each SH domain plays a role in coupling the BCR to PLCγ1 and in regulating its function.
The SH2(N) and SH2(C) domains affect the in vitro enzymatic activity of PLCγ1.
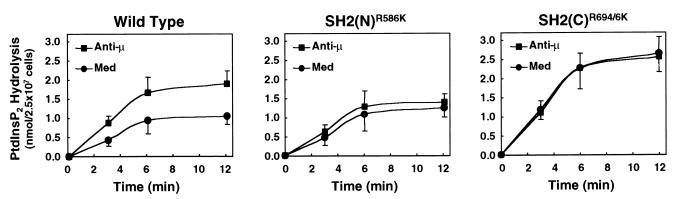
To further investigate the function of the SH2 domains, the enzymatic activity of PLCγ1 was tested in anti-HA immunoprecipitates from resting and activated P10-14 cells expressing WT PLCγ1 or the SH2 domain mutants. The in vitro assay was performed at pH 6.8 and used a suspension of the substrate, PtdInsP2, with Triton X-100 to yield a PtdInsP2/Triton X-100 micellar molar ratio of 1:3.8 (assuming a nominal critical micellar concentration for Triton X-100 of 0.24 mM). These conditions were experimentally determined to give an optimal differential between nonactivated and BCR-stimulated PLC activity (data not shown). Under these experimental conditions, WT PLCγ1-HA from medium-treated cells demonstrated PtdInsP2 hydrolytic activity that was linear for up to 6 min upon addition of the substrate (Fig. 8). Such activity was kinetically increased in PLCγ1 recovered from BCR-activated cells, consistent with the BCR induction of PLCγ1 phosphorylation and phosphoinositide hydrolysis observed in intact cells. The SH2(N) domain mutant of PLCγ1 demonstrated only basal levels of in vitro activity, irrespective of BCR aggregation. These levels were identical to those observed with the WT protein from unstimulated cells. This finding is consistent with that lack of BCR-induced membrane translocation, tyrosine phosphorylation, and activation observed with the PLCγ1 SH2(N) domain mutant in vivo.
FIG. 8.
Influence of the SH2 domains on the in vitro enzymatic activity of PLCγ1 from resting and BCR-activated cells. Cells stably expressing PLCγ1-HA WT, the PLCγ1-HA SH2(N)R586K domain mutant, or the PLCγ1-HA SH2(C)R694/6K domain mutant were treated with medium alone or with anti-BCR Ab for 2 min at 37°C. Anti-HA immunoprecipitates from 2.5 × 107 cells were assayed for PLC activity by using a mixed micellar suspension of [3H]PtdInsP2 and Triton X-100 as the substrate. The activity was measured as the formation of trichloroacetic acid-soluble [3H]inositol phosphates. No acid-soluble radioactivity accumulated over time in samples precipitated from untransfected P10-14 cells (not shown), and this background radioactivity was subtracted from the experimental samples. Shown is the mean ± standard error of the mean of five separate experiments.
In contrast to the SH2(N) domain mutant, the SH2(C) domain mutant of PLCγ1 showed in vitro activity levels that slightly exceeded those of the activated WT protein. These activity levels were also independent of BCR ligation. Thus, despite its inability to function in intact cells, the PLCγ1 SH2(C) domain mutant protein is constitutively active in vitro, suggesting that the SH2(C) domain plays a role in coupling the enzyme to the receptor and as an intrinsic regulator of the protein’s enzymatic activity. Results identical to those shown in Fig. 8 were obtained with a different set of stable transfectants that expressed lower levels of PLCγ1 (data not shown), ruling out artifacts due to clonal variation. These data also indicate that the defect in BCR-induced phosphoinositide hydrolysis of cells reconstituted with the SH2 domain mutants of PLCγ1 cannot be attributed to an intrinsic enzymatic defect of the mutant proteins.
DISCUSSION
Our data indicate that the SH domains of PLCγ1 perform autonomous yet overlapping functions in BCR-induced activation. The SH2(N) domain is essential, having a role in membrane translocation, phosphorylation, and activation of the enzyme, while the SH2(C) domain is required for its activity but dispensable for translocation and phosphorylation. The SH3 domain contributes to BCR-induced PLCγ1 membrane translocation and activity but has no apparent role in phosphorylation.
Similar to its role in BCR-induced PLCγ1 activation, the SH2(N) domain is also essential for TCR-induced PLCγ1 membrane translocation (6a) and tyrosine phosphorylation (52) in Jurkat cells. The utilization of the SH2(N) domain by PLCγ1 for coupling to the T- or B-cell receptor contrasts with the reported preference of its SH2(C) domain for activation by the EGF or PDGF receptor (26, 44, 58, 59). This apparent preference is supported by the observation that sequences found in either the EGF or PDGF receptor (Y992LIP and Y1021IIP, respectively) match the motif selected by the isolated SH2(C) domain. The SH2(C) domain favors Pro at the +3 position, while the SH2(N) prefers Leu (50). Studies using recombinant fragments of PLCγ1, however, have also implicated the SH2(N) domain in binding to the intracellular tail of the phosphorylated EGF receptor (45). A sequence in the tail of the basic fibroblast growth factor (FGF) receptor (Y766LDL), on the other hand, matches the specificity of the SH2(N) domain (50), and inhibition of FGF signaling by competition with membrane-permeable tyrosine-phosphorylated peptides revealed a preference for SH2(N) domain-binding sequences (13). However, a GST fusion protein encompassing the SH2(C) domain but not the SH2(N) domain of PLCγ1 bound pTyr766 in a recombinant fragment of the basic FGF receptor (28). Therefore, PLCγ1 SH2 domains may cross-react with each other’s preferred ligand. Recently, Ji and coworkers (20) have shown that the SH2(N) domain is sufficient for PLCγ1 to bind the phosphorylated tail of the PDGF receptor in Plcγ1−/− mouse embryonic fibroblasts. PDGF-induced PLCγ1 phosphorylation, as well as phosphoinositide hydrolysis and Ca2+ mobilization, however, required both SH2 domains (20). The exquisite requirement for the SH2(N) domain demonstrated by the T- or B-cell receptor not only for translocation but also for PLCγ1 phosphorylation suggests a coupling mechanism different from that used primarily by the PDGF or EGF receptor. This observation suggests that PLCγ SH2 domains may be utilized differently by different receptors.
PLCγ1 binds through its SH2(N) domain to the chicken homologue (18) of the recently identified adapter alternatively termed BLNK (10) or Slp65 (70). BLNK/Slp65 shares sequence homology with the T-cell-specific adapter, Slp76, a phosphoprotein required for optimal tyrosine phosphorylation and activation of PLCγ1 by the TCR (72). BLNK/Slp65 binds Grb2 (9, 10, 70) and can associate with either PLCγ1 or PLCγ2 in Daudi cells (10). Furthermore, coexpression of BLNK/Slp65 with PLCγ1 and Syk in insect cells led to increased tyrosine phosphorylation of PLCγ1 (10) and B cells deficient in BLNK fail to activate PLCγ2 (18). BLNK proteins show several conserved binding sites for PLCγ1 SH2 domains, albeit none displayed the motif preferred by the SH2(N) domain. The ability of both GST-SH2(N) and SH2(C) domain fusion proteins to recognize BLNK, however, suggests that PLCγ1 interaction with phosphorylated proteins is not exclusively regulated by the SH2 domain selectivity.
Of the SH2(N) domain-dependent functions, PLCγ1 membrane translocation may be the primary event which in itself could be sufficient to induce phosphorylation by promoting proximity to an active kinase. Sequestration of regulatory proteins from their targets by membrane/cytoplasmic compartmentalization is a well-known control mechanism, which includes Ras regulation by Sos (15). However, membrane targeting of PLCγ1-HA via an amino-terminal myr signal sequence did not result in elevated resting levels of tyrosine phosphorylation and still required a functional SH2(N) domain for BCR-stimulated PLCγ1 phosphorylation. These data suggest that the ability of the SH2(N) domain to mediate phosphorylation of PLCγ1 is not simply due to membrane translocation and that the primary function of this domain is to interact with an activation complex. Because BLNK, a cytoplasmic protein, is translocated to the cell membrane in a signal-dependent fashion (10), this molecule may nucleate an activation complex involving PLCγ1 and promote both its membrane translocation and phosphorylation.
The precise function of the SH2(C) domain in BCR-induced PLCγ1 activation remains unclear. Our data rule out a gross defect in tyrosine phosphorylation of the SH2(C) domain mutant, although a requirement for this domain in the phosphorylation of a specific tyrosine residue critical for enzyme activity cannot be completely eliminated. Three tyrosine phosphorylation sites (Tyr771, Tyr783, and Tyr1254 of the bovine PLCγ1 sequence) are critical for PDGF-induced enzymatic activation of PLCγ1 (21). A fourth additional site (Tyr472), whose role in PLCγ1 activation has not been established, is also phosphorylated in response to EGF (22, 61). While the tyrosine residues phosphorylated in response to BCR engagement are not known, a decreased overall PLCγ1 phosphorylation should have been observed if the SH2(C) domain mutation would have affected any of the major phosphorylation sites.
PLC assays based on substrate dispersion with surface modifiers allow discrimination of the activity from activated cells compared to that of resting cells (60). Under these assay conditions, tyrosine phosphorylation of PLCγ1 results in increased in vitro activity by primarily shifting the equilibrium in favor of the association of PLCγ1 with the micelles containing the substrate. When assayed under these conditions, PLCγ1 bearing a nonfunctional SH2(N) domain, which results in a protein with deficient phosphorylation in response to BCR ligation, was unable to be activated in vitro. The presence of a nonfunctional SH2(C) domain, however, resulted in a protein with constitutive in vitro activity and no longer responsive to BCR-induced activation. Several pieces of evidence indicate that the SH2-SH2-SH3 region of PLCγ isozymes contains regulatory elements that affect its enzymatic activity. Homma and Takenawa (16) identified an amino acid sequence immediately carboxy terminal to the SH2(C) domain that is capable of inhibiting the activity of PLCγ as well as that of PLCβ and -δ. Therefore, this PLC inhibitory (PCI) region may directly interact with the catalytic site, which is conserved among different phosphoinositide-specific PLC family members. While peptides limited to the PCI region display inhibitory activity with a Ki of 15 μM, the whole SH2-SH2-SH3 region shows much increased inhibitory activity (Ki = 25 nM [16]). Therefore, other elements within this region contribute to the inhibition. Furthermore, expression and assembly of the catalytic domains in the absence of the SH2-SH2-SH3 region resulted in a protein complex with increased PLC activity in vitro compared to that of the holoenzyme (7, 17). Finally, tyrosine-phosphorylated peptides capable of binding PLCγ1 SH2 domains can act as allosteric activators of PLCγ1 activity in vitro (23). Taken together with these findings, our data suggest that the SH2(C) domain of PLCγ1 exerts a negative control on the enzymatic activity of PLCγ1, possibly by an intramolecular mechanism. Consistent with this possibility, a recent study of pH dependence of PLCγ1 catalytic machinery presented a model whereby at neutral pH the SH2-SH2-SH3 region forms a “lid” that closes the active sites (74). Our data suggest that such a putative lid depends primarily upon an intact SH2(C) domain. It is possible that optimal positioning of the PCI peptide, which is near the SH2(C) domain, is favored by an intramolecular interaction mediated by the SH2(C) domain. Such an interaction can then be displaced in vitro either by SH2 domain ligands, whose binding is known to results in a conformational change of PLCγ1 (23), by decreasing the pH, resulting in protonation of residues critical for the intramolecular interaction (74), or by tyrosine phosphorylation. Therefore, the binding of SH2 domain ligands and tyrosine phosphorylation appear to be two physiologic mechanisms that can lead to a similar kinetic activation of PLCγ1. It remains to be determined if, under physiologic conditions, tyrosine phosphorylation occurs first and allows the SH2(C) domain to be displaced from its intramolecular interaction, whether the two events are independently regulated or are regulated with an opposite interdependency. Because tyrosine phosphorylation is necessary for enzyme activation in vivo (21), the first mechanism, which depends exclusively on the SH2(N) domain in the case of the BCR or TCR (52), appears more plausible.
In contrast with its constitutive in vitro activity, the SH2(C) domain mutant of PLCγ1 was unable to reconstitute the BCR-induced phosphoinositide hydrolysis in P10-14 cells. This was observed with cells stably or transiently expressing the SH2(C) domain mutant, ruling out clonal artifacts or other concerns (e.g., substrate exhaustion) due to stable expression of a potentially deregulated protein. Therefore, an interaction of the SH2(C) domain with a potential target appears necessary for BCR-induced PLCγ1 activation in vivo. The SH2(C) domain mutant of PLCγ1 did not display a pattern of coprecipitating phosphoproteins different from that of WT PLCγ1, although the SH2(C) domain, when expressed as a GST fusion protein, associated with several phosphoproteins (reference 52 and Fig. 4). This is consistent with structural constraints within PLCγ1 that prevent accessibility to the SH2(C) domain, whereas a posttranslational modification or the engagement of other domains might influence the domain’s availability for interaction with other molecules. It is possible that the interaction with these molecules is weak, occurs at low stoichiometry, or is lost under our conditions of lysis, or that its phosphorylation is transient. Another possibility is that the fraction of PLCγ1 that is involved in SH2(C) domain-mediated interactions is relocated to a detergent-insoluble compartment (35) and selectively lost during extraction. Replacement of Triton X-100 with 60 mM β-octylglucoside, a detergent capable of solubilizing membrane rafts (27), during cell solubilization failed to reveal additional coimmunoprecipitating tyrosine-phosphorylated proteins (data not shown), suggesting that within the sensitivity of our assay, the target protein is not present in this compartment. Last, the SH2(C) domain may play a role in the ability of PLCγ1 to bind regulatory phospholipids, such as PtdInsP3 (38). These alternate possibilities are currently under investigation.
The SH3 domain, while dispensable for tyrosine phosphorylation, has a role in PLCγ1 anchoring to the membrane. Although not as critical in this function as the SH2(N) domain, it may provide a secondary level of membrane attachment. The SH3 domains of PLCγ1 and Src can bind cytoskeletal components (1, 69), and compartmentalization of activated PLCγ1 molecules to the detergent-insoluble cell cytoskeleton has been documented (35). The PLCγ1 SH3 domain may therefore be important for stabilizing the association of PLCγ1 with the membrane through the interaction with cytoskeletal elements and possibly contribute to bringing the active enzyme in proximity of its substrate.
These data are consistent with a sequential model of BCR-induced PLCγ1 activation whereby the different SH domains have interrelated but distinct roles. In this model, the earliest event is the engagement of the SH2(N) domain with a phosphoprotein that recruits PLCγ1 in an activation complex and translocates it to the membrane. PLCγ1 phosphorylation ensues at this stage as a consequence of the interaction of PLCγ1 with one or more kinases, an event mediated primarily or exclusively by the SH2(N) domain. The SH2(C) domain plays no apparent role in these events, whereas the SH3 domain contributes additional membrane anchoring, possibly by associating with cytoskeletal components. Up-regulation of PLCγ1 enzymatic activity depends on translocation and phosphorylation and is therefore completely abrogated by disruption of the SH2(N) domain function. The SH3 domain partially contributes to PLCγ1 activity, consistent with its role in membrane translocation. The SH2(C) domain, which plays a negative role in regulating the intrinsic catalytic activity of the enzyme, is absolutely required for BCR-induced PLCγ1 activation, possibly by binding a yet unidentified phosphoprotein or by interacting with other regulatory membrane phospholipids.
ACKNOWLEDGMENTS
We thank G. Crabtree, P. G. Pelicci, and A. Weissman for the generous gift of reagents, and we thank M. Brunswick, S. Kozlowski, M. Shapiro, E. W. Shores, and R. Wange for helpful discussion and critical review of the manuscript.
The first two authors contributed equally to this work.
REFERENCES
- 1.Bar-Sagi D, Rotin D, Batzer A, Mandiyan V, Schlessinger J. SH3 domains direct cellular localization of signaling molecules. Cell. 1993;74:83–91. doi: 10.1016/0092-8674(93)90296-3. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter G, Hernandez-Sotomayor S M, Nishibe S, Todderud G, Mumby M, Wahl M. Growth factor phosphorylation of PLC-gamma 1. Ciba Found Symp. 1992;164:223–233. [PubMed] [Google Scholar]
- 3.Cockcroft S, Thomas G M H. Inositol-lipid-specific phospholipase C isoenzymes and their differential regulation by receptors. Biochem J. 1992;288:1–14. doi: 10.1042/bj2880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coggeshall K M, McHugh J C, Altman A. Predominant expression and activation-induced tyrosine phosphorylation of phospholipase C-gamma 2 in B lymphocytes. Proc Natl Acad Sci USA. 1992;89:5660–5664. doi: 10.1073/pnas.89.12.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree G R, Clipstone N A. Signal transmission between the plasma membrane and nucleus of T lymphocytes. Annu Rev Biochem. 1994;63:1045–1083. doi: 10.1146/annurev.bi.63.070194.005145. [DOI] [PubMed] [Google Scholar]
- 6.Dasgupta J D, Granja C, Druker B, Lin L L, Yunis E J, Relias V. Phospholipase C-gamma 1 association with CD3 structure in T cells. J Exp Med. 1992;175:285–288. doi: 10.1084/jem.175.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.DeBell, K. E., A. DiBaldassarre, S. Miscia, and E. Bonvini. Unpublished observation.
- 7.Fernald A W, Jones G A, Carpenter G. Limited proteolysis of phospholipase C-gamma 1 indicates stable association of X and Y domains with enhanced catalytic activity. Biochem J. 1994;302:503–509. doi: 10.1042/bj3020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fluckiger A C, Li Z, Kato R M, Wahl M I, Ochs H D, Longnecker R, Kinet J P, Witte O N, Scharenberg A M, Rawlings D J. Btk/Tec kinases regulate sustained increases in intracellular Ca2+ following B-cell receptor activation. EMBO J. 1998;17:1973–1985. doi: 10.1093/emboj/17.7.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu C, Chan A C. Identification of two tyrosine phosphoproteins, pp70 and pp68, which interact with phospholipase Cgamma, Grb2, and Vav after B cell antigen receptor activation. J Biol Chem. 1997;272:27362–27368. doi: 10.1074/jbc.272.43.27362. [DOI] [PubMed] [Google Scholar]
- 10.Fu C, Turck C W, Kurosaki T, Chan A C. BLNK: a central linker protein in B cell activation. Immunity. 1998;9:93–103. doi: 10.1016/s1074-7613(00)80591-9. [DOI] [PubMed] [Google Scholar]
- 11.Goldschmidt-Clermont P J, Kim J W, Machesky L M, Rhee S G, Pollard T D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991;251:1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- 12.Graham L J, Stoica B A, Shapiro M, DeBell K E, Rellahan B, Laborda J, Bonvini E. Sequences surrounding the Src-homology 3 domain of phospholipase Cgamma-1 increase the domain’s association with Cbl. Biochem Biophys Res Commun. 1998;249:537–541. doi: 10.1006/bbrc.1998.9177. [DOI] [PubMed] [Google Scholar]
- 13.Hall H, Williams E J, Moore S E, Walsh F S, Prochiantz A, Doherty P. Inhibition of FGF-stimulated phosphatidylinositol hydrolysis and neurite outgrowth by a cell-membrane permeable phosphopeptide. Curr Biol. 1996;6:580–587. doi: 10.1016/s0960-9822(02)00544-4. [DOI] [PubMed] [Google Scholar]
- 14.Hempel W M, Schatzman R C, DeFranco A L. Tyrosine phosphorylation of phospholipase C-gamma 2 upon cross-linking of membrane Ig on murine B lymphocytes. J Immunol. 1992;148:3021–3027. [PubMed] [Google Scholar]
- 15.Holsinger L J, Spencer D M, Austin D J, Schreiber S L, Crabtree G R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci USA. 1995;92:9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Homma Y, Takenawa T. Inhibitory effect of src homology (SH) 2/SH3 fragments of phospholipase C-gamma on the catalytic activity of phospholipase C isoforms. Identification of a novel phospholipase C inhibitor region. J Biol Chem. 1992;267:21844–21847. [PubMed] [Google Scholar]
- 17.Horstman D A, DeStefano K, Carpenter G. Enhanced phospholipase C-gamma1 activity produced by association of independently expressed X and Y domain polypeptides. Proc Natl Acad Sci USA. 1996;93:7518–7521. doi: 10.1073/pnas.93.15.7518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishiai M, Kurosaki M, Pappu R, Okawa K, Ronko I, Fu C, Shibata M, Iwamatsu A, Chan A C, Kurosaki T. BLNK required for coupling Syk to PLCγ2 and Rac1-JNK in B cells. Immunity. 1999;10:117–125. doi: 10.1016/s1074-7613(00)80012-6. [DOI] [PubMed] [Google Scholar]
- 19.Jackman J K, Motto D G, Sun Q, Tanemoto M, Turck C W, Peltz G A, Koretzky G A, Findell P R. Molecular cloning of SLP-76, a 76-kDa tyrosine phosphoprotein associated with Grb2 in T cells. J Biol Chem. 1995;270:7029–7032. doi: 10.1074/jbc.270.13.7029. [DOI] [PubMed] [Google Scholar]
- 20.Ji Q, Chattopadhyay A, Vecchi M, Carpenter G. Physiological requirement for both SH2 domains for phospholipase C-γ1 function and interaction with platelet-derived growth factor receptors. Mol Cell Biol. 1999;19:4961–4970. doi: 10.1128/mcb.19.7.4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H K, Kim J W, Zilberstein A, Margolis B, Kim J G, Schlessinger J, Rhee S G. PDGF stimulation of inositol phospholipid hydrolysis requires PLC-gamma 1 phosphorylation on tyrosine residues 783 and 1254. Cell. 1991;65:435–441. doi: 10.1016/0092-8674(91)90461-7. [DOI] [PubMed] [Google Scholar]
- 22.Kim J W, Sim S S, Kim U H, Nishibe S, Wahl M I, Carpenter G, Rhee S G. Tyrosine residues in bovine phospholipase C-gamma phosphorylated by the epidermal growth factor receptor in vitro. J Biol Chem. 1990;265:3940–3943. [PubMed] [Google Scholar]
- 23.Koblan K S, Schaber M D, Edwards G, Gibbs J B, Pompliano D L. src-homology 2 (SH2) domain ligation as an allosteric regulator: modulation of phosphoinositide-specific phospholipase C gamma 1 structure and activity. Biochem J. 1995;305:745–751. doi: 10.1042/bj3050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Law C L, Chandran K A, Sidorenko S P, Clark E A. Phospholipase C-γ1 interacts with conserved phosphotyrosyl residues in the linker region of Syk and is a substrate for Syk. Mol Cell Biol. 1996;16:1305–1315. doi: 10.1128/mcb.16.4.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu K Q, Bunnell S C, Gurniak C B, Berg L J. T cell receptor-initiated calcium release is uncoupled from capacitative calcium entry in Itk-deficient T cells. J Exp Med. 1998;187:1721–1727. doi: 10.1084/jem.187.10.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Margolis B, Li N, Koch A, Mohammadi M, Hurwitz D R, Zilberstein A, Ullrich A, Pawson T, Schlessinger J. The tyrosine phosphorylated carboxyterminus of the EGF receptor is a binding site for GAP and PLC-gamma. EMBO J. 1990;9:4375–4380. doi: 10.1002/j.1460-2075.1990.tb07887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melkonian K A, Chu T, Tortorella L B, Brown D A. Characterization of proteins in detergent-resistant membrane complexes from Madin-Darby canine kidney epithelial cells. Biochemistry. 1995;34:16161–16170. doi: 10.1021/bi00049a031. [DOI] [PubMed] [Google Scholar]
- 28.Mohammadi M, Honegger A M, Rotin D, Fischer R, Bellot F, Li W, Dionne C A, Jaye M, Rubinstein M, Schlessinger J. A tyrosine-phosphorylated carboxy-terminal peptide of the fibroblast growth factor receptor (Flg) is a binding site for the SH2 domain of phospholipase C-γ 1. Mol Cell Biol. 1991;11:5068–5078. doi: 10.1128/mcb.11.10.5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morgenstern J P, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–3596. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishibe S, Wahl M I, Hernandez-Sotomayor S M, Tonks N K, Rhee S G, Carpenter G. Increase of the catalytic activity of phospholipase C-gamma 1 by tyrosine phosphorylation. Science. 1990;250:1253–1256. doi: 10.1126/science.1700866. [DOI] [PubMed] [Google Scholar]
- 31.Nishibe S, Wahl M I, Rhee S G, Carpenter G. Tyrosine phosphorylation of phospholipase C-II in vitro by the epidermal growth factor receptor. J Biol Chem. 1989;264:10335–10338. [PubMed] [Google Scholar]
- 32.Park D J, Rho H V, Rhee S G. CD3 Stimulation causes phosphorylation of phospholipase C gamma 1 on serine and tyrosine residues in a human T cell line. Proc Natl Acad Sci USA. 1991;88:5453–5457. doi: 10.1073/pnas.88.12.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pascal S M, Singer A U, Gish G, Yamazaka T, Shoelson S E, Pawson T, Kay L E, Forman-Kay J D. Nuclear magnetic resonance structure of an SH2 domain of phospholipase C-gamma1 complexed with a high affinity binding peptide. Cell. 1994;77:461–472. doi: 10.1016/0092-8674(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 34.Pawson T. Protein modules and signalling networks. Nature. 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 35.Pei Z, Yang L, Williamson J R. Phospholipase C-gamma 1 binds to actin-cytoskeleton via its C-terminal SH2 domain in vitro. Biochem Biophys Res Commun. 1996;228:802–806. doi: 10.1006/bbrc.1996.1735. [DOI] [PubMed] [Google Scholar]
- 36.Peri K G, Gervais F G, Weil R, Davidson D, Gish G D, Veillette A. Interactions of the SH2 domain of lymphocyte-specific tyrosine protein kinase p56lck with phosphotyrosine-containing proteins. Oncogene. 1993;8:2764–2772. [PubMed] [Google Scholar]
- 37.Pleiman C M, Clark M R, Gauen L K, Winitz S, Coggeshall K M, Johnson G L, Shaw A S, Cambier J C. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fvn, and p56lyn which interact with the effector molecules phospholipase C-γ2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993;13:5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantley L G. Phosphoinositide 3-kinase regulates phospholipase Cgamma-mediated calcium signaling. J Biol Chem. 1998;273:23750–23758. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- 39.Resh M D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 40.Rhee S G, Bae Y S. Regulation of phosphoinositide-specific phospholipase C isozymes. J Biol Chem. 1997;272:15045–15048. doi: 10.1074/jbc.272.24.15045. [DOI] [PubMed] [Google Scholar]
- 41.Rhee S G, Suh P G, Ryu S H, Lee S Y. Studies of inositol phospholipid specific phospholipase C. Science. 1989;244:546–550. doi: 10.1126/science.2541501. [DOI] [PubMed] [Google Scholar]
- 42.Richards J D, Gold M R, Hourihane S L, DeFranco A L, Matsuuchi L. Reconstitution of B cell antigen receptor-induced signaling events in a nonlymphoid cell line by expressing the Syk protein-tyrosine kinase. J Biol Chem. 1996;271:6458–6466. doi: 10.1074/jbc.271.11.6458. [DOI] [PubMed] [Google Scholar]
- 43.Roifman C M, Wang G. Phospholipase C-gamma 1 and phospholipase C-gamma 2 are substrates of the B cell antigen receptor associated protein tyrosine kinase. Biochem Biophys Res Commun. 1992;183:411–416. doi: 10.1016/0006-291x(92)90496-8. [DOI] [PubMed] [Google Scholar]
- 44.Rotin D, Honegger A M, Margolis B L, Ullrich A, Schlessinger J. Presence of SH2 domains of phospholipase C gamma 1 enhances substrate phosphorylation by increasing the affinity toward the epidermal growth factor receptor. J Biol Chem. 1992;267:9678–9683. [PubMed] [Google Scholar]
- 45.Rotin D, Margolis B, Mohammadi M, Daly R J, Daum G, Li N, Fischer E H, Burgess W H, Ullrich A, Schlessinger J. SH2 domains prevent tyrosine dephosphorylation of the EGF receptor: identification of Tyr992 as the high-affinity binding site for SH2 domains of phospholipase C gamma. EMBO J. 1992;11:559–567. doi: 10.1002/j.1460-2075.1992.tb05087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sastry L, Lin W, Wong W T, Di Fiore P P, Scoppa C A, King C R. Quantitative analysis of Grb2-Sos1 interaction: the N-terminal SH3 domain of Grb2 mediates affinity. Oncogene. 1995;11:1107–1112. [PubMed] [Google Scholar]
- 47.Seedorf K, Kostka G, Lammers R, Bashkin P, Daly R, Burgess W H, van der Bliek A M, Schlessinger J, Ullrich A. Dynamin binds to SH3 domains of phospholipase C gamma and GRB-2. J Biol Chem. 1994;269:16009–16014. [PubMed] [Google Scholar]
- 48.Sieh M, Batzer A, Schlessinger J, Weiss A. GRB2 and phospholipase C-gamma 1 associate with a 36- to 38-kilodalton phosphotyrosine protein after T-cell receptor stimulation. Mol Cell Biol. 1994;14:4435–4442. doi: 10.1128/mcb.14.7.4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sillman A L, Monroe J G. Association of p72syk with the src homology-2 (SH2) domains of PLC gamma 1 in B lymphocytes. J Biol Chem. 1995;270:11806–11811. doi: 10.1074/jbc.270.20.11806. [DOI] [PubMed] [Google Scholar]
- 50.Songyang Z, Shoelson S E, Chaudhuri M, Gish G, Pawson T, Haser W G, King F, Roberts T, Ratnofsky S, Lechleider R J, Neel B G, Birge R B, Fajardo J E, Chou M M, Hanafusa H, Schaffhausen B, Cantley L C. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 51.Sparks A B, Rider J E, Hoffman N G, Fowlkes D M, Quilliam L A, Kay B K. Distinct ligand preferences of Src homology 3 domains from Src, Yes, Abl, Cortactin, p53bp2,PLCgamma, Crk, and Grb2. Proc Natl Acad Sci USA. 1996;93:1540–1544. doi: 10.1073/pnas.93.4.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stoica B, DeBell K E, Graham L, Rellahan B L, Alava M A, Laborda J, Bonvini E. The amino-terminal Src homology 2 domain of phospholipase C gamma 1 is essential for TCR-induced tyrosine phosphorylation of phospholipase C gamma 1. J Immunol. 1998;160:1059–1066. [PubMed] [Google Scholar]
- 53.Straus D B, Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 54.Takata M, Homma Y, Kurosaki T. Requirement of phospholipase C-gamma 2 activation in surface immunoglobulin M-induced B cell apoptosis. J Exp Med. 1995;182:907–914. doi: 10.1084/jem.182.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Takata M, Kurosaki T. A role for Bruton’s tyrosine kinase in B cell antigen receptor-mediated activation of phospholipase C-gamma 2. J Exp Med. 1996;184:31–40. doi: 10.1084/jem.184.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takata M, Sabe H, Hata A, Inazu T, Homma Y, Nukada T, Yamamura H, Kurosaki T. Tyrosine kinases Lyn and Syk regulate B cell receptor-coupled Ca2+ mobilization through distinct pathways. EMBO J. 1994;13:1341–1349. doi: 10.1002/j.1460-2075.1994.tb06387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Todderud G, Wahl M I, Rhee S G, Carpenter G. Stimulation of phospholipase C-gamma 1 membrane association by epidermal growth factor. Science. 1990;249:296–298. doi: 10.1126/science.2374928. [DOI] [PubMed] [Google Scholar]
- 58.Valius M, Bazenet C, Kazlauskas A. Tyrosines 1021 and 1009 are phosphorylation sites in the carboxy terminus of the platelet-derived growth factor receptor beta subunit and are required for binding of phospholipase C gamma and a 64-kilodalton protein, respectively. Mol Cell Biol. 1993;13:133–143. doi: 10.1128/mcb.13.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vega Q C, Cochet C, Filhol O, Chang C P, Rhee S G, Gill G N. A site of tyrosine phosphorylation in the C terminus of the epidermal growth factor receptor is required to activate phospholipase C. Mol Cell Biol. 1992;12:128–135. doi: 10.1128/mcb.12.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wahl M I, Jones G A, Nishibe S, Rhee S G, Carpenter G. Growth factor stimulation of phospholipase C-gamma 1 activity. Comparative properties of control and activated enzymes. J Biol Chem. 1992;267:10447–10456. [PubMed] [Google Scholar]
- 61.Wahl M I, Nishibe S, Kim J W, Kim H, Rhee S G, Carpenter G. Identification of two epidermal growth factor-sensitive tyrosine phosphorylation sites of phospholipase C-gamma in intact HSC-1 cells. J Biol Chem. 1990;265:3944–3948. [PubMed] [Google Scholar]
- 62.Wahl M I, Nishibe S, Suh P G, Rhee S G, Carpenter G. Epidermal growth factor stimulates tyrosine phosphorylation of phospholipase C-II independently of receptor internalization and extracellular calcium. Proc Natl Acad Sci USA. 1989;86:1568–1572. doi: 10.1073/pnas.86.5.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wahl M I, Olashaw N E, Nishibe S, Rhee S G, Pledger W J, Carpenter G. Platelet-derived growth factor induces rapid and sustained tyrosine phosphorylation of phospholipase C-γ in quiescent BALB/c 3T3 cells. Mol Cell Biol. 1989;9:2934–2943. doi: 10.1128/mcb.9.7.2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Waksman G, Kominos D, Robertson S C, Pant N, Baltimore D, Birge R B, Cowburn D, Hanafusa H, Mayer B J, Overduin M, Resh M D, Rios C B, Silverman L, Kuriyan J. Crystal structure of the phosphortyrosine recognition domain SH2 of v-src complexed with tyrosine-phosphorylated peptides. Nature. 1992;358:646–653. doi: 10.1038/358646a0. [DOI] [PubMed] [Google Scholar]
- 65.Weber J R, Bell G M, Han M Y, Pawson T, Imboden J B. Association of the tyrosine kinase LCK with phospholipase C-gamma 1 after stimulation of the T cell antigen receptor. J Exp Med. 1992;176:373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weber J R, Orstavik S, Torgersen K M, Danbolt N C, Berg S F, Ryan J C, Tasken K, Imboden J B, Vaage J T. Molecular cloning of the cDNA encoding pp36, a tyrosine-phosphorylated adaptor protein selectively expressed by T cells and natural killer cells. J Exp Med. 1998;187:1157–1161. doi: 10.1084/jem.187.7.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Weiss A, Koretzky G, Schatzman R C, Kadlecek T. Functional activation of the T-cell antigen receptor induces tyrosine phosphorylation of phospholipase C-gamma 1. Proc Natl Acad Sci USA. 1991;88:5484–5488. doi: 10.1073/pnas.88.13.5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Weiss A, Littman D R. Signal transduction by lymphocyte antigen receptors. Cell. 1994;76:263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- 69.Weng Z, Taylor J A, Turner C E, Brugge J S, Seidel-Dugan C. Detection of Src homology 3-binding proteins, including paxillin, in normal and v-Src-transformed Balb/c 3T3 cells. J Biol Chem. 1993;268:14956–14963. [PubMed] [Google Scholar]
- 70.Wienands J, Schweikert J, Wollscheid B, Jumaa H, Nielsen P J, Reth M. SLP-65: a new signaling component in B lymphocytes which requires expression of the antigen receptor for phosphorylation. J Exp Med. 1998;188:791–795. doi: 10.1084/jem.188.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams B L, Schreiber K L, Zhang W, Wange R L, Samelson L E, Leibson P J, Abraham R T. Genetic evidence for differential coupling of Syk family kinases to the T-cell receptor: reconstitution studies in a ZAP-70-deficient Jurkat T-cell line. Mol Cell Biol. 1998;18:1388–1399. doi: 10.1128/mcb.18.3.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yablonski D, Kuhne M R, Kadlecek T, Weiss A. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 1998;281:413–416. doi: 10.1126/science.281.5375.413. [DOI] [PubMed] [Google Scholar]
- 73.Zhang W, Sloan-Lancaster J, Kitchen J, Trible R P, Samelson L E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhou C, Horstman D, Carpenter G, Roberts M F. Action of phosphatidylinositol-specific phospholipase C-γ1 on soluble and micellar substrates. Separating effects on catalysis from modulation of the surface. J Biol Chem. 1999;274:2786–2793. doi: 10.1074/jbc.274.5.2786. [DOI] [PubMed] [Google Scholar]