Abstract
Swine leukocyte antigen (SLA) plays a central role in controlling the immune response by discriminating self and foreign antigens and initiating an immune response. Studies on SLA polymorphism have demonstrated associations between SLA allelic variants, immune response, and disease resistance. The SLA polymorphism is due to host-pathogen co-evolution resulting in improved adaptation to diverse environments making SLA a crucial genomic region for comparative diversity studies. Although locally-adapted African pigs have small body sizes, they possess increased resilience under harsh environmental conditions and robust immune systems with reported tolerance to some diseases, including African swine fever. However, data on the SLA diversity in these pigs are not available. We characterized the SLA of unrelated locally-adapted domestic pigs from Homa Bay, Kenya, alongside exotic pigs and warthogs. We undertook SLA comparative diversity of the functionally expressed SLA class I (SLA-1, SLA-2) and II (DQB1) repertoires in these three suids using the reverse transcription polymerase chain reaction (RT-PCR) sequence-based typing (SBT) method. Our data revealed higher genetic diversity in the locally-adapted pigs and warthogs compared to the exotic pigs. The nucleotide substitution rates were higher in the peptide-binding regions of the SLA-1, SLA-2, and DQB1 loci, indicative of adaptive evolution. We obtained high allele frequencies in the three SLA loci, including some breed-specific private alleles, which could guide breeders to increase their frequency through selection if confirmed to be associated with enhanced resilience. Our study contributes to the growing body of knowledge on genetic diversity in free-ranging animal populations in their natural environment, availing the first DQB1 gene data from locally-adapted Kenyan pigs.
Keywords: SLA, diversity, domestic pigs, warthogs, MHC, locally-adapted pigs, DQB1, PBR
1. Introduction
The major histocompatibility complex (MHC) proteins play a vital role in binding and presenting endogenous and exogenous epitopes derived from the host or pathogen to the circulating T-cells, initiating a T-cell-mediated immune response [1,2,3,4,5]. MHC structure and functions have been widely studied in different species, including comparative MHC genetic diversity analyses in humans and the Suidae family that comprises Sus scrofa (wild boars), Sus scrofa domesticus (domestic pig), and warthogs (Phacochoerus africanus) [2,6,7,8]. However, no comparative MHC data are available from locally-adapted Kenyan pigs.
In swine, MHC encodes the swine leukocyte antigen (SLA) mapped to the pig’s chromosome 7 [9,10]. SLA consists of three major gene families known as SLA class I, II, and III [9,11]. SLA class I genes comprise three classes: Classical (or class Ia) genes, non-classical (or class Ib), and MHC class I chain-related (MIC) genes (or class Ic). Classical MHC I molecules are highly polymorphic and present antigenic peptide ligands on infected cells to CD8+ T cells, whereas the non-classical MHC I molecules facilitate repressively or activate stimuli in natural killer cells [12]. Seven classical SLA class, I loci are known: SLA-1, SLA-2, SLA-3, SLA-4, SLA-5, SLA-9, and SLA-11, of which only SLA-1, SLA-2, and SLA-3 are highly polymorphic and functional, with a classical MHC class I molecular structure consisting of a leader sequence, three exons encoding the extracellular α1, α2, and α3 domains, a transmembrane region, and three cytoplasmic exons [13,14]. The α1 and α2 domains form an 8- to 10-mer peptide-binding region (PBR). In pigs, a cluster of three non-classical class Ib genes consists of SLA-6, SLA-7, and SLA-8 [9,13,14]. Conversely, SLA class II consists of DMA, DMB, DOA, DOB1, DQA, DQB1, DRA, DRB1, DRB2, DRB3, DRB4, and DRB5 genes of which DRB1 and DQB1 are highly polymorphic [2]. SLA class II is a heterodimer consisting of an alpha (α) and a beta (β) chain, both with an intracellular, a transmembrane, and two extracellular domains [13,15]. Pairing of the α1 and β1 domains from MHC class II form the PBR with open ends, allowing the apportionment for a larger peptide of 14-mer or more to extend out of both sides of the groove. The PBRs are highly polymorphic and diverse regions of the SLA, thereby ensuring greater diversity for binding and presenting peptides [13,16,17,18].
Genetic diversity in the host and pathogen populations is thought to be maintained by antagonistic co-evolution between interacting hosts and pathogens [19,20,21,22,23,24]. Comparative studies in the model and non-model organisms have shown that the high levels of genetic diversity observed at the MHC of vertebrate hosts are consistent with the hypothesis of pathogen-driven balancing selection through one of two mechanisms: Frequency-dependent selection and heterozygote advantage [18,25,26,27]. Consequently, high variations at the MHC loci reflect the level of fitness that can be exploited to genetically characterize different individuals in a population [18,28,29,30,31,32]. Thus, studying the variable sites of SLA molecules in as many suid breeds as possible is important to obtain crucial information on the SLA structure and the associations between SLA alleles and valuable traits.
Pigs (Sus scrofa domesticus) are a source of high-quality protein, are easy to manage with minimal space and feed requirements [33,34,35], providing a livelihood source for smallholder farmers [36,37]. Pigs are also used as experimental models in biomedical research [38,39]. To this end, very few functional SLA class I and II diversity studies have been conducted on the domestic pigs and warthogs in East Africa [6,40]. The locally-adapted pig breeds are characterized by a rich genetic reservoir that has made them adaptive to harsh environmental conditions and disease challenges [41,42,43]. These pigs, found in diverse parts of Africa, show resilience under harsh climatic conditions [43] and tolerance to diseases [44,45,46] such as the lethal African swine fever (ASF) while remaining asymptomatic despite harboring the virus in their tissues [47,48,49,50]. The locally-adapted pigs are preferred by smallholder farmers in Africa who manage them in free-range systems (Figure 1B), exposing them to various pathogens and parasites. However, these locally-adapted pigs have a slower growth rate, resulting in smaller body sizes yielding lesser carcass weight than the exotic pigs such as Large white and Duroc breeds [51]. Despite this, these pigs are preferred by farmers who keep them for their resilience under harsh environments and disease challenges. We included exotic pigs (Landrace x Large white) that rapidly grow into big body sizes that yield higher carcass weight but are highly susceptible to many diseases, including ASF. Comparative genetic diversity was achieved by including the east African warthogs (Phacochoerus africanus) that live in the savannah areas at the livestock-wildlife interphase, where they play a vital role in the disease transmission cycle by acting as the reservoir for many pathogens, including ASF virus [52,53,54,55]. Warthogs grow at a slow rate and have small body sizes. The locally-adapted pigs, exotic pigs, and warthogs are closely related suid species but of different demographic histories, disease susceptibilities, and varying growth rates that make them a perfect combination to study the genetic diversity of SLA.
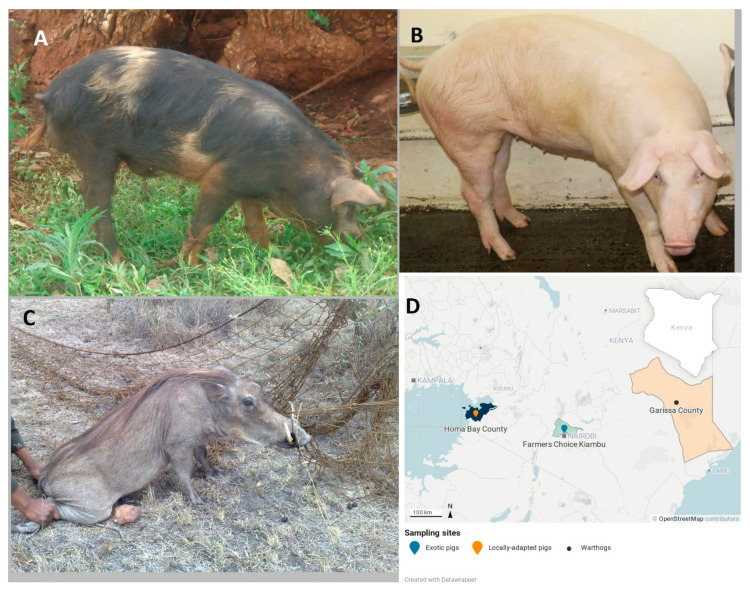
Figure 1.
(A) A locally-adapted pig feeding at a homestead in Homa Bay County. Photograph: Edward Okoth/2016. (B) The exotic pig in a pen. Photograph: Eunice Machuka/2019. (C) An adult warthog being restrained in a net by a Kenya Wildlife Service Officer. Photograph: Edward Okoth/2018. (D) Map of Kenya showing the sampling areas in Homa Bay, Farmers Choice in Kiambu County, and Garissa county (Map was created using Datawrapper. https://www.datawrapper.de; accessed on 1 May 2021).
We analyzed the highly polymorphic and functionally expressed MHC class I (SLA-1 and SLA-2) and class II (DQB1) loci of unrelated locally-adapted pigs from Homa Bay County in Kenya that have been shown to tolerate ASF for more extended periods against the natural ASF-resistant swine relatives, the warthogs [54,55,56], and a highly ASF-susceptible exotic domestic pig breed. This study supplements our understanding of the SLA diversity among the domestic pigs and their wild suid relatives, the warthogs.
2. Materials and Methods
2.1. Sampling Sites
The locally-adapted pigs (Sus scrofa domesticus) (Figure 1A) were randomly selected from smallholder farms in Homa Bay County, south-western Kenya. Lake Victoria borders Homa Bay County on the west, and the Ruma National Park is in the central part of this county (formerly Nyanza Province). The climatic conditions are warm and overcast most of the year, with temperatures ranging between 18–29 °C. The exotic pigs (Sus scrofa domesticus) (Figure 1B) were crosses of Large white and Landrace pigs randomly selected from a commercial pig farm called Farmer’s Choice Limited, located in Limuru Municipality, Kiambu County in central Kenya. The climatic conditions in Limuru are mostly cool and overcast most of the year, with temperatures ranging between 8–25 °C. The warthogs (Phacochoerus africanus) (Figure 1C) represent the wild suid population, collected from a national park in Garissa County, Kenya, at the far north-eastern side bordering the Tana River (Figure 1A). Garissa is an arid area characterized by mostly dry weather and temperatures ranging between 22–38 °C and rainfall in two seasons in February to April and October to December. The distance between Homa Bay and Limuru is about 273.35 km, Garissa to Limuru is 345.4 km, while Garissa and Homa Bay are 590.4 km apart (Figure 1D). Homa Bay County is an area endemic for ASF, while Limuru in Kiambu County experiences sporadic outbreaks of ASF.
2.2. Sample Collection
Between 2014 and 2019, we collected blood from locally-adapted pigs, exotic pigs, and warthogs. Porcine (Sus scrofa domestica) blood was collected from the jugular vein into Vacutainers® (Becton, Dickinson and Company, Franklin Lakes, NJ, USA) from 27 locally-adapted pigs (Figure 1A) from select farms in Homa Bay County, Kenya. Additionally sampled were 16 Landrace x Large White crossed exotic pigs, maintained by the Farmer’s Choice in Limuru, Kiambu County, Kenya. In addition, the study included 16 warthogs (Phacochoerus africanus) blood samples representing the wild suid population, collected from Garissa County, Kenya. Blood samples were collected from immobilized free-range warthogs. Blood samples were stored in RNALater® (Ambion, Austin, TX, United States) for RNA extraction.
2.3. RNA Extraction
Total RNA was extracted either from 200 µL of whole blood using an in-house protocol that combined lysis and phase separation with 1 mL of TRIzol™ (Thermo Fisher Scientific, Waltham, MA, USA) and 200 µL Chloroform (Sigma-Aldrich, St. Louis, MO, USA) followed by on-column purification using the RNeasy® Mini Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. The RNA was eluted from the silica columns in 40 μL of RNase-free water. The RNA integrity was checked on a 1.2% (w/v) agarose gel electrophoresis at 4 V/cm2, and the RNA quantity was determined using an ssRNA assay kit on the Qubit® 2.0 fluorometer (Thermo Fisher Scientific, USA) system. The RNA purity was determined on a Nanodrop™ spectrophotometer (Thermo Fisher Scientific, USA), considering the 260/280 ratio close to 2.0 as highly pure RNA. The extracted RNA was stored at −80 °C until required for downstream applications.
2.4. Reverse Transcription and PCR Amplification of SLA-1, SLA-2, and DQB1 RNA
The cDNA was prepared from 1 μg of the purified total RNA using the RevertAid First Strand cDNA Synthesis Kit (Catalog No. K1622, Thermo Fisher Scientific, USA), with random hexamers contained in the kit and following the manufacturer’s instructions. A GAPDH control was prepared alongside the cDNA reactions. Each cDNA sample was prepared in duplicate and used as a template for the SLA genotyping PCR. The full-length locus-specific primers for the SLA-2 and DQB1 loci were designed to include the untranslated regions to obtain the complete coding sequence [3]. For SLA-1, we designed primers to include only the alpha-1 region (exon 2) of the SLA-1 gene. The SLA loci-specific PCR reactions were conducted under optimized conditions with 2U of Phusion™ Taq DNA polymerase (Thermo Fisher Scientific, USA) in 30 µL reaction volumes containing GC buffer, 2 mM of MgCl2, 0.2 mM of each dNTPs, 0.3 µM of each primer, and cDNA (prediluted 1:100 with nuclease-free water) as a template. The PCR thermal profile included an initial denaturation at 98 °C for 2 min, 35 cycles of 98 °C for 30 s, annealing at loci-specific temperatures (see Table S1) for 30 s, and 72 °C for 30 s, with a final extension at 72 °C for 30 s. The amplicons were analyzed on a 1.5% (w/v) agarose gel pre-stained with 2.5 × GelRed® (Biotium, Fremont, CA, USA) in 1 × TAE buffer and electrophoresed at 4 V/cm2 to confirm the amplicon size before purification with the QIAquick® PCR Purification Kit (Qiagen, Germany). The cleaned SLA amplicons were sequenced in forward and reverse directions using the BigDye® Terminator v3.1 cycle sequencing kit (Thermo Fisher Scientific, USA) on a 3730xl DNA Analyzer (Applied Biosystems, Waltham, MA, USA) at Macrogen Inc., Amsterdam, The Netherlands. The same SLA gene-specific primers for PCR amplification were used for sequencing, except SLA-1 primers had universal T7 and SP6 tags for sequencing (Table S1). We obtained clear amplicons of the expected sizes on agarose gels for the three loci studied. It was not possible to obtain a clear amplicon for sequencing in some loci, probably due to similarities in the primer-binding sites between the SLA class I genes in different pig breeds [40].
2.5. SLA Sanger Sequence Data Analysis and Construction of Phylogenetic Trees
The SLA class I and II gene-specific Sanger sequences were trimmed and assembled using the CLC Genomics Workbench v8.03 (Qiagen, Germany) with the default settings. Secondary peaks were detected to discover heterozygous mutations using the secondary peak calling plugin in CLC set at a 50% peak height threshold, a process augmented by visual inspection of the double peaks and manual editing. We disentangled the mixed signal represented by secondary peaks in Sanger chromatograms by reconstructing haplotype phases from the unphased sequence data containing ambiguous codes representing heterogeneous positions in PHASE v2.1.1 [57] at 100 burn-in steps followed by 100 actual iterations, each with a thinning interval of 1. The unfolded consensus sequences were used for sequence similarity search in BLASTn [58] in the NCBI database. The unfolded SLA-1, SLA-2, and DQB1 nucleotide sequences were compared with the published SLA alleles in the IPD-MHC sequence database (http://www.ebi.ac.uk/ipd/mhc/sla/, accessed on 12 July 2021) to obtain the matching alleles [59]. Then, we constructed phylogenetic trees for two purposes: (i) To assign corresponding SLA alleles, and (ii) to infer orthologous relationships of the SLA-1, SLA-2, and DQB1 genes within the MHC suids studied. Maximum likelihood (ML) phylogenetic trees were constructed in RAxML-NG v9.0 at https://github.com/amkozlov/raxml-ng (accessed on 12 July 2021) [60] with the GTR+G substitution model and edited in iToL [61]. The unfolded DNA sequences were further translated into amino acids using the Translate tool at Expasy Swiss Bioinformatics Resource Portal [62].
2.6. MHC-SLA Diversity Assessment
We used hierfstat package v0.5–7 under R v4.0.4 to estimate these genetic diversity parameters: Mean observed heterozygosity (Ho), mean gene diversity within the population (expected heterozygosity), total gene diversity (Ht), gene diversity among samples (Dst), heterozygosity corrected (Dstp), Fis = inbreeding coefficient per overall loci (Fis: 1-Ho / Hs); Dest = measure of population differentiation. Comparisons were made for each suid and all combined into one population for each of the three loci.
The multiple sequence alignments generated above were used to assess the variation between the unfolded SLA-specific sequences in DnaSP v6.0 [63]. The nucleotide diversity (π), haplotype diversity (Hd) Ewenns-Watterson neutrality test, and the total number of mutations per nucleotide site were also calculated for each individual in DnaSP v6.0 [63]. The PBR sequence diversity of each SLA loci-specific DNA sequence was analyzed in DnaSP v6.0 using default settings.
2.7. Evaluating Signals of Selection Acting on the SLA-1, SLA-2, and DQB1 loci
To determine the diversity, conservation, and evolutionary forces acting within the selected MHC genes between domestic pigs and warthogs, we tested for selection in (i) the entire SLA-1, SLA-2, and DQB1 codons and (ii) exons 2 to 3, the codons for the PBR in SLA-2 and exon 2, the codons for the PBR in DQB1. Due to the high numbers of heterozygous positions within the loci studied, phased data were used for the selection analysis. The non-synonymous (dN) and synonymous (dS) substitutions rates were tested within each SLA-1, SLA-2, and DQB1 loci’s coding regions using CodeML [64], a package within PAML v4.10.0, over the entire coding sequence and exclusively on the concatenated codons for the PBR, in the three suid populations (warthogs, local, and exotic pigs). We performed a Likelihood-Ratio Test (LRT) to compare three models: M0, M7 (beta), and M8 (beta and ω), a model including at least one category of sites under positive selection (ω = dN/dS > 1). The value obtained was used in the LRT at 2 degrees of freedom and allowed the rejection of the null model (p < 0.05). Amino acids detected to be under positive selection in M8 were identified using the Bayes Empirical Bayes approach (BEB), with posterior probabilities of 95% and 99% [64]. The amino acid positions were numbered according to the published sequences in GenBank. For SLA-1, we assigned amino acid positions by comparing the sequences against CAB63936.1, the genomic DNA translation of exon 2 of AJ251829 (SLA-1*01:01). For SLA-2, we used AJ251829 that corresponds to the SLA-2*03:01 allele, and for DQB1, we compared against NM_001113694.1, the mRNA sequence encoding SLA-DQB1*03:01 allele.
2.8. SLA Functional Cluster Analysis
Identifying the peptides that bind to the MHC is critical to understanding the T-cell immune responses. We performed the MHC cluster analysis to assess the clustering of the functional SLA peptide-binding motifs against known peptides. The phased SLA-1, SLA-2, and DQB1 nucleotide sequences were first translated to proteins. The longest open reading frames (ORFs) alongside protein sequences from the IPD-MHC database were selected and submitted for the MHC cluster analysis on MHCcluster 2.0 (http://www.cbs.dtu.dk/services/MHCcluster/; accessed on 20 September 2020) [65] by comparing the peptide-binding function of 50,000 random natural 10 amino acid long peptides of known SLA alleles. Default parameters were applied, and each SLA locus was analyzed separately.
2.9. Statistical Analyses
The allele frequencies were determined by direct counting, along with their respective standard errors. We applied the likelihood-ratio test (LRT) to compare three substitution rate models: M0, M7 (beta), and M8. The value obtained was used in the LRT at 2 degrees of freedom and rejected the null model (p < 0.05). Amino acids under positive selection in M8 were identified using the BEB approach, with posterior probabilities of 95% and 99% [66]. For the MHC cluster analysis, we used 100 bootstrap samples, and 50,000 peptides in the functional correlation analysis, at a threshold of 10 peptides. The estimated accuracy of the predicted sequence motifs was estimated from the distance to the nearest MHC molecules included in the training of the peptide binding prediction method. Correlations > 0.7 were considered accurate.
3. Results
3.1. SLA Sequence Analysis of Domestic Pigs and Warthogs
RT-PCR amplification was performed on blood samples from 59 animals (43 domestic pigs and 16 warthogs) using the locus-specific MHC SLA primers (Table S1). The average sequence sizes for SLA-1, SLA-2, and DQB1 loci were 240, 1056, and 917 bp, respectively, after assembly of the forward and the reverse sequences and trimming off the T7 and SP6 tags on SLA-1 primers (Table S1). The secondary signals in the Sanger sequences with assigned IUPAC ambiguous codes were resolved by reconstructing haplotype phases in PHASE v2.1.1 yielding at least two haplotypes per heterozygous sample. A BLAST search showed similarity to known Sus scrofa MHC class I antigens (SLA-1 and SLA-2) and MHC class II antigens (DQB1) mRNA (Table S2). Using the standard genetic code, the longest ORF for all the SLA-1, SLA-2, and DQB1 nucleotide sequences yielded, on average, 80, 365, and 271 (amino acids) aa, respectively (Table S3).
3.2. Phylogenetic Analysis
Phylogenetic relationships were determined using ML trees from SLA-1, SLA-2, and DQB1 phased nucleotide sequences (Figure 2, Figure 3 and Figure 4). The ML trees showed clustering based on allele nomenclature, verifying further that correct allele names were assigned. Allele sharing among suid breeds was detected, with some alleles clustering separate from the others due to differences in the length of the nucleotide sequences used to generate the phylogenetic trees.
Figure 2.
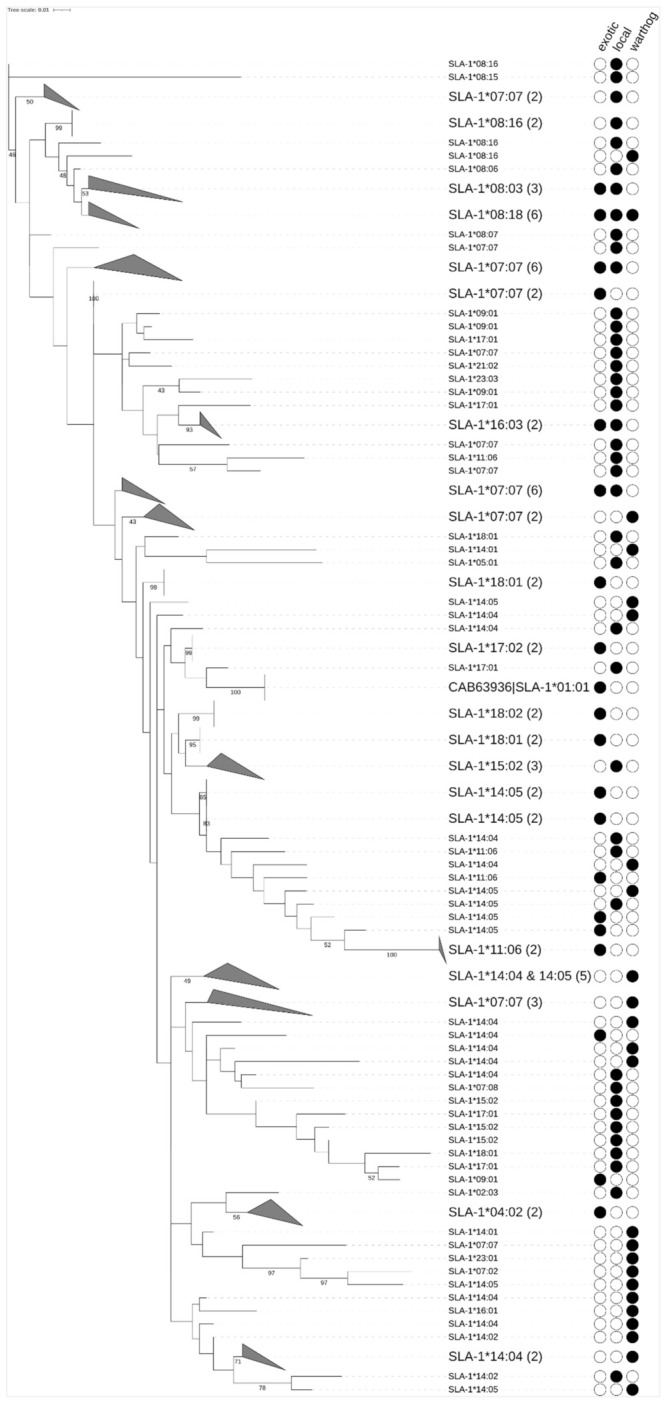
Phylogenetic tree of SLA-1 alleles with leaf names, the closest match in IPD from a Blast search. Clades are collapsed (gray triangle) where the assignment to IPD alleles is the same (or very similar). Filled circles indicate the allele detected in the suid breeds studied. In parentheses are the number of phased haplotypes included in a collapsed node. Bootstrap support values were calculated on 100 bootstrap trees and values lower than 40 are not shown. CAB63936 is an SLA-1 allele reference sequence obtained from GenBank.
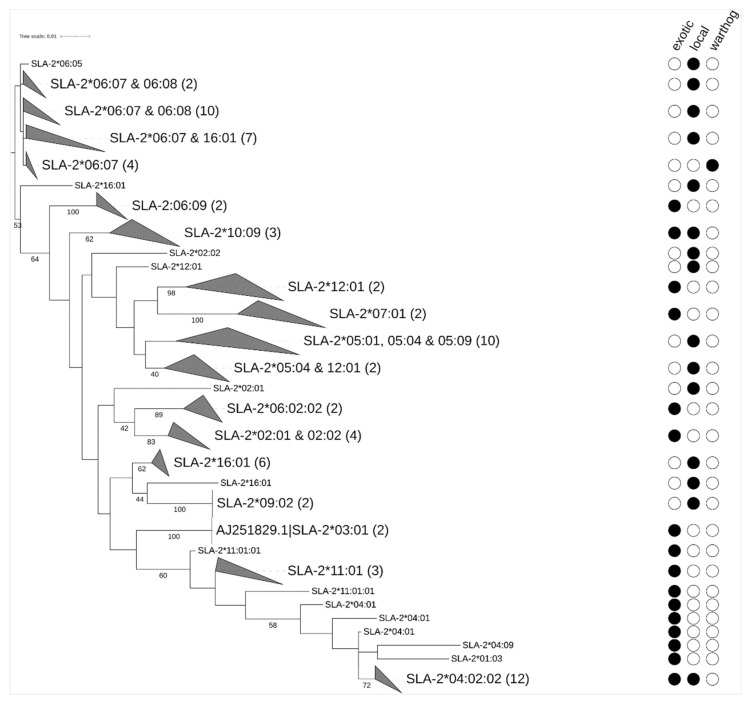
Figure 3.
Phylogenetic tree of SLA-2 alleles with leaf names, the closest match in IPD from a Blast search. Clades are collapsed (gray triangle) where the assignment to IPD alleles is the same (or very similar). Filled circles indicate the allele detected in the suid breeds studied. In parentheses are the number of phased haplotypes included in a collapsed node. Bootstrap support values were calculated on 100 bootstrap trees and values lower than 40 are not shown. AJ251829 is an SLA-2 allele reference sequence obtained from GenBank.
Figure 4.
Phylogenetic tree of DQB1 alleles with leaf names, the closest match in IPD from a Blast search. Clades are collapsed (gray triangle) where the assignment to IPD alleles is the same (or very similar). Filled circles indicate the allele detected in the suid breeds studied. In parentheses are the number of phased haplotypes included in a collapsed node. Bootstrap support values were calculated on 100 bootstrap trees and values lower than 40 are not shown. NM_001113694.1 is a DQB1 allele reference sequence obtained from GenBank.
3.3. SLA-1, SLA-2, and DQB1 Allele Assignment
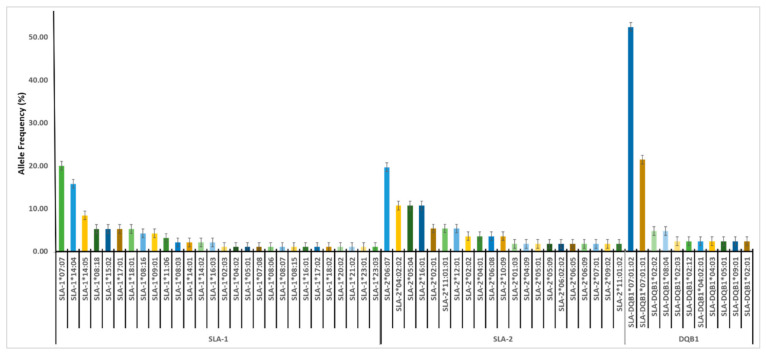
We analyzed the SLA-1, SLA-2, and DQB1 phased nucleotide sequences from 60 individuals (locally-adapted pigs n = 27, exotic pigs n = 16, and warthogs n = 16) on SLA-IPD BLAST and selected allele names with >95% match (Table S2). We obtained 28 unique SLA-1 alleles in this study (Figure 5; Table S4). The most frequently detected SLA-1 allele was SLA1*07:07 (20%) in all three suids studied. The second most frequent SLA-1 allele was SLA1*14:04 (15.79%) and the third being SLA1*14:05 (8.42%) detected in all three suids. The rest of the SLA-1 alleles occurred at frequencies of less than 5.6%. Minor alleles were detected at frequencies less than 1% (Figure 5; Table S4). A total of 17 private SLA-1 alleles were detected among the three suids. Of these private alleles, 11, 3, and 3 were for locally-adapted pigs, exotic pigs, and warthogs, respectively. The high frequency (>5%) alleles were shared by all three suids detected namely SLA-1*07:07, SLA-1*08:18, SLA-1*14:04, SLA-1*14:05, and SLA-1*18:01. The local and exotic pigs shared alleles SLA-1*09:01, SLA-1*11:06, and SLA-1*16:03. The locally-adapted pigs and warthogs shared alleles SLA-1*08:16 and SLA-1*14:02. The exotic pigs did not share any SLA-1 alleles with the warthogs (Table S4).
Figure 5.
SLA allele frequencies grouped by SLA loci studied. Error bars indicate the standard error.
A total of 21 unique SLA-2 alleles were obtained in this study (Figure 5; Table S4). The most frequently detected SLA-2 allele was SLA-2*06:07 (19.64%) in all three suids studied. Other high-frequency SLA-2 alleles were SLA-2*05:04 and SLA-2*04:02:02, occurring at a frequency of 10.71%. The rest of the SLA-2 alleles occurred at frequencies of less than 5%. Minor alleles were detected with frequencies of 1.79% (Figure 5; Table S4). A total of 15 private SLA-2 alleles were detected among the domestic pigs only. Of these private alleles, seven belonged to locally-adapted pigs and eight were from exotic pigs. The local and exotic pigs shared five alleles namely SLA-2*02:02, SLA-2*04:02:02, SLA-2*12:01, SLA-2*02:01, and SLA-2*10:09. The locally-adapted pigs and warthogs shared only one allele SLA-2*06:07. The exotic pigs did not share any SLA-2 alleles with the warthogs (Table S4).
There were 11 unique SLA-DQB1 alleles obtained in this study (Figure 4; Table S4). The high-frequency DQB1 allele was SLA-DQB1*07:01:02 (52.38%) detected in all three suids, followed by SLA-DQB1*07:01:01 (21.43%). While SLA-DQB1*02:02 and SLA-DQB1*08:04 were detected at a frequency of 4.76%. Minor alleles unique to each suid breed were detected with frequencies of 2.38% each, comprising the nine private DQB1 alleles detected in the domestic pigs (Figure 4; Table S4). Of these DQB1 private alleles, three belonged to locally-adapted pigs and six were from exotic pigs. The local and exotic pigs shared one allele with the warthogs, the SLA-DQB1*07:01:02 (Table S4).
3.4. Selection Signals Acting on SLA-1, SLA-2, and DQB1 Loci
Following the selection analyses, the estimated ω value under the M8 model for only the sites under positive selection in the locally-adapted pigs and warthogs was higher (ω > 1; p < 0.05) in the loci studied in the three suids. The ω = dN/dS value under the M8 model was significantly higher (ω > 1; p < 0.05) in all the PBR regions of SLA-1, SLA-2, and DQB1 in all the three suids studied (Table 1, Table 2 and Table 3). When the (overall) global M0 model was considered, our results showed that the ω value was significantly less than 1 (ω < 1; p < 0.05) when all coding sequences and individual coding regions (exons) for SLA-1, SLA-2, and DQB1 were considered. Moreover, the DQB1 loci ω value was significantly higher (ω > 1; p < 0.05) in the locally-adapted pigs when whole sequences of DQB1 were considered (Table 3). However, other non-PBR exons also had higher ω values, but were not significant, namely: DQB1 exon 4, DQB1 exon 5 (in warthogs and exotic pigs only), SLA-2 exon 6 in all sequences and exotic pigs, and lastly, SLA-2 exon 7 among locally-adapted pigs had ω = 2 though not significant (Table 2). The warthog sequences available for the SLA-2 and DQB1 analysis were all identical.
Table 1.
The estimated dN/dS for the coding sequences of SLA-1 loci and the least-likelihood ratios for the three suid breeds studied.
| Region | Population | Overall dN/dS Value (M0) | Log Likelihood Model 1 (M7, Neutral) | Log Likelihood Model 2 (M8, Selection) | Prop. of Sites under Positive Selection (p1) Estimated by M8 | Estimated dN/dS Value under M8 for the Sites under Selection | LRT Statistic = −2 Delta (lnl) | Critical Value for the Chi-Square Test (df = 2) at 5% Alpha | p-Value (Selection vs. Neutral) | Significant at Alpha Level = 5% |
|---|---|---|---|---|---|---|---|---|---|---|
|
SLA-1 exon 2 (PBR, 80 codons) |
All | 0.59 | −3799.06 | −3709.20 | 0.01 | 3.36 | 179.71 | 5.99 | 0 | Yes |
| Exotic | 0.43 | −1234.51 | −1227.72 | 0.04 | 2.52 | 13.58 | 5.99 | 0.0011 | Yes | |
| Local | 0.54 | −2228.69 | −2190.62 | 0.02 | 3.13 | 76.13 | 5.99 | 0 | Yes | |
| Warthog | 0.69 | −1612.83 | −1581.76 | 0.01 | 4.55 | 62.12 | 5.99 | 0.000001 | Yes |
Table 2.
The estimated dN/dS for the coding sequences of SLA-2 loci and the least-likelihood ratios for the three suid breeds studied.
| SLA−2 Loci | Population | Overall dN/dS Value (M0) | Log Likelihood Model 1 (M7, Neutral) | Log Likelihood Model 2 (M8, Selection) | Prop. of Sites under Positive Selection Estimated under M8 | dN/dS Value under m8 for Sites under Selection | LRT Statistic = −2 delta (lnl) | p-Value (Selection vs. Neutral) | Significant at Alpha Level = 5% |
|---|---|---|---|---|---|---|---|---|---|
| All SLA−2 exons | All | 0.54 | −7631.46 | −7381.62 | 0.02 | 5.37 | 499.68 | 0 | Yes |
| Exotic | 0.47 | −4594.24 | −4485.75 | 0.02 | 5.52 | 216.97 | 0 | Yes | |
| Local | 0.63 | −4727.60 | −4584.63 | 0.02 | 7.72 | 285.93 | 0 | Yes | |
| Exon 1 (non−PBR, 10 codons) |
All | 0.63 | −83.33 | −83.33 | 0.00 | 1.00 | 0.00 | 1 | No |
| Exotic | 0.62 | −50.61 | −50.61 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Local | 0.13 | −59.18 | −59.18 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Exon 2 (PBR, 91 codons) |
All | 0.39 | −2613.44 | −2585.88 | 0.03 | 2.14 | 55.13 | 0.00001 | Yes |
| Exotic | 0.34 | −1460.17 | −1450.36 | 0.03 | 2.46 | 19.63 | 0.00001 | Yes | |
| Local | 0.43 | −1717.28 | −1697.02 | 0.04 | 2.67 | 40.51 | 0.00001 | Yes | |
| Exon 3 (PBR, 92 codons) |
All | 0.63 | −1837.16 | −1760.56 | 0.01 | 6.80 | 153.19 | 0 | Yes |
| Exotic | 0.54 | −1152.89 | −1111.09 | 0.02 | 8.59 | 83.59 | 0 | Yes | |
| Local | 0.94 | −1205.34 | −1163.72 | 0.03 | 9.07 | 83.25 | 0 | Yes | |
| Exon 4 (non−PBR, 92 codons) |
All | 0.17 | −774.71 | −774.52 | 0.05 | 1.36 | 0.38 | 0.825 | No |
| Exotic | 0.12 | −584.31 | −584.31 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Local | 0.16 | −598.96 | −598.95 | 0.09 | 1.00 | 0.00 | 0.998 | No | |
| Exon 5 (non−PBR, 37 codons) |
All | 0.79 | −583.16 | −581.87 | 0.34 | 1.65 | 2.57 | 0.276 | No |
| Exotic | 0.87 | −462.28 | −461.19 | 0.28 | 1.96 | 2.18 | 0.336 | No | |
| Local | 0.81 | −291.38 | −290.44 | 0.26 | 2.64 | 1.89 | 0.387 | No | |
| Exon 6 (non−PBR, 11 codons) | All | 4.02 | −139.79 | −129.77 | 0.20 | 12.09 | 20.04 | 0.0001 | Yes |
| Exotic | 1.38 | −122.15 | −117.88 | 0.21 | 5.01 | 8.54 | 0.014 | Yes | |
| Local | N/A | −71.28 | −68.99 | 0.46 | N/A | 4.59 | 0.101 | No | |
| Exon 7 (non−PBR, 17 codons) | All | 0.72 | −244.99 | −243.59 | 0.14 | 2.61 | 2.79 | 0.248 | No |
| Exotic | 0.62 | −180.57 | −180.52 | 0.49 | 1.17 | 0.09 | 0.956 | No | |
| Local | 2.09 | −130.49 | −130.10 | 0.76 | 2.99 | 0.78 | 0.675 | No |
Table 3.
The estimated dN/dS analyses for the coding sequences of DQB1 loci and the least-likelihood ratios for the three suid breeds studied.
| Region | Population | Overall dN/dS Value (M0) | Log Likelihood Model 1 (M7, Neutral) | Log Likelihood Model 2 (M8, Selection) | Prop. Of Sites under Positive Selection (p1) as Estimated under M8 | Estimated dN/dS Value under M8 for the Sites under Selection | LRT Statistic = −2 Delta (lnl) | p-Value (Selection vs. Neutral) | Significant at Alpha Level = 5% |
|---|---|---|---|---|---|---|---|---|---|
| All DQB1 exons | All | 0.79 | −2065.05 | −1999.56 | 0.02 | 11.72 | 130.99 | 0 | Yes |
| Exotic | 0.74 | −1611.00 | −1567.22 | 0.03 | 12.28 | 87.55 | 0 | Yes | |
| Local | 1.06 | −1395.52 | −1384.42 | 0.06 | 10.39 | 22.20 | 0.0001 | Yes | |
| Warthog | 0.43 | −1055.07 | −1055.07 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Exon 1 (non−PBR, 26 codons) |
All | 0.22 | −115.78 | −115.78 | 0.00 | 1.00 | 0.00 | 1 | No |
| Exotic | 0.45 | −110.01 | −110.01 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Local | 0.44 | −108.57 | −108.57 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Warthog | 0.00 | −102.68 | −102.68 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Exon 2 (PBR, 90 codons) |
All | 0.62 | −1092.77 | −1069.99 | 0.01 | 7.13 | 45.57 | 0.0001 | Yes |
| Exotic | 0.65 | −778.98 | −768.58 | 0.06 | 4.12 | 20.80 | 0.0001 | Yes | |
| Local | 0.71 | −608.18 | −606.12 | 0.24 | 2.53 | 4.13 | 0.1269 | No | |
| Warthog | 0.78 | −366.08 | −366.08 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Exon 3 (non−PBR, 94 codons) |
All | 0.14 | −479.76 | −479.76 | 0.00 | 1.00 | 0.00 | 1 | No |
| Exotic | 0.04 | −427.40 | −427.40 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Local | 0.43 | −414.48 | −414.48 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Warthog | 0.13 | −393.72 | −393.72 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Exon 4 (non−PBR, 37 codons) |
All | 0.56 | −162.40 | −162.40 | 0.00 | 1.00 | 0.00 | 1 | No |
| Exotic | N/A | −147.19 | −146.87 | N/A | N/A | 0.63 | 0.7308 | No | |
| Local | 0.56 | −162.39 | −162.39 | 0.00 | 1.00 | 0.00 | 1 | No | |
| Warthog | 1.00 | −140.47 | −140.47 | 0.00 | 1.04 | 0.00 | 1 | No | |
| Exon 5 (non−PBR, 6 codons) |
All | N/A | −16.31 | −16.01 | N/A | N/A | 0.62 | 0.7339 | No |
| Exotic | 1.00 | −12.01 | −12.01 | 0.10 | 1.05 | 0.00 | 1 | No | |
| Local | N/A | −16.31 | −16.00 | N/A | N/A | 0.62 | 0.7340 | No | |
| Warthog | 1.00 | −12.00 | −12.00 | 0.00 | 1.04 | 0.00 | 1 | No |
We detected 10 positively selected codons in SLA-1 loci when all suid breeds were considered, with eight being significantly positively selected sites at 0.95 and 0.99 probabilities. The exotic pigs alone had 22 sites, and the locally-adapted pigs had 19 sites (Table S5).
In all SLA-2 codons, the total number of significantly positively selected sites at 0.95 and 0.99 posterior probabilities were 18, 30, and 31 in all suids, exotic pigs, and locally-adapted pigs, respectively (Table S5). The available warthog sequences were strictly identical, so we did not perform within-group analyses for warthogs.
In DQB1 loci, all codons combined, we detected a total of 27 positively selected sites when all suid breeds were considered. The exotic pigs alone had 27 sites, and the locally-adapted pigs had 34 sites, while the warthog sequences were not considered since the comparison between M7-M8 was not significant because they were strictly identical. In the DQB1 loci, the number of significantly positively selected sites at 0.95 and 0.99 probabilities were 18, 18, and 11 in all suids, exotic pigs, and locally-adapted pigs, respectively (Table 4). Most of the positively selected codon sites within DQB1 were common across the three suid breeds studied, with a high proportion of positive selection occurring in the PBR-coding regions (Table 4; Table S5).
Table 4.
Determination of SLA-1 codons under positive selection by the Bayes empirical Bayes (BEB) approach.
| Loci | Population | Model | dN/dS Value | Significance a | Codons Predicted to Be under Positive Selection BEB Inference b |
|---|---|---|---|---|---|
| SLA-1 (exon 2) | All | M7 vs. M8 | 3.36 | p < 0 | 6 **, 15 **, 53 **, 57 **, 58 **, 61 **, 64 **, 68 ** |
| Exotic | M7 vs. M8 | 2.52 | p < 0.001 | 6 *, 15 *, 36 *, 53 **, 57 **, 58 **, 65 **, 68 ** | |
| Local | M7 vs. M8 | 3.13 | p < 0 | 15 **, 36 **, 53 **, 56 **, 57 **, 58 **, 59 **, 61 *, 68 ** | |
| Warthog | M7 vs. M8 | 4.55 | p < 0 | 15 **, 53 **, 58 *, 64 **, 65 ** | |
| SLA-2 | All | M7 vs. M8 | 5.37 | p < 0 |
11 *, 17 **, 20 **, 28 **, 34 **, 35 **, 56 **, 64 **, 69 *, 77 **, 78 **, 79 *, 80 **, 81 *, 84 *, 88 **, 90 **, 91 *, 92 **, 106 **, 125 **, 127 **, 128 **, 154 **, 158 **, 162 **, 163 **, 166 **, 167 **, 174 **, 178 **, 180 **, 181 *, 286 *, 288 **, 312 **, 322 **, 345 *, 350 *, 351 ** |
| Exotic | M7 vs. M8 | 5.52 | p < 0 |
17 **, 20 **, 34 *, 35 **, 56* *, 74 **, 78 **, 79 *, 80 **, 81 **, 84 **, 88 **, 90 **, 92 *, 106 **, 108 **, 125 **, 127 *, 158 **, 162 *, 163 **, 166 **, 167 **, 174 **, 178 **, 181 **, 312 *, 330 *, 350 *, 351 ** |
|
| Local | M7 vs. M8 | 7.72 | p < 0 |
11 *, 17 **, 20 **, 28 **, 34 **, 35 **, 55 *, 56 **, 64 **, 73 **, 77 **, 78 **, 80 **, 88 **, 90 **, 91 **, 92 **, 106 *, 113 **, 125 **, 154 **, 158 **, 162 **, 163 **, 166 **, 167 **, 174 **, 178**, 180**, 288 *, 322 ** |
|
| DQB1 | All | M7 vs. M8 | 11.72 | p < 0 | 8 *, 31 **, 35 **, 40**, 47 *, 48 **, 50 **, 52 *, 59 **, 60 **, 65 *, 79 **, 85 *, 88 **, 93 **, 99 *, 204 *, 242 ** |
| Exotic | M7 vs. M8 | 12.28 | p < 0 | 8 **, 31 **, 35 **, 40 *, 47 **, 48 **, 49 *, 50 **, 52 **, 59 **, 60 *, 65 *, 79 **, 85 *, 88 **, 89 *, 93 *, 204 ** | |
| Local | M7 vs. M8 | 10.39 | p < 0.00001 | 31 **, 35 **, 48 *, 52 *, 59 *, 60 *, 79 *, 88 *, 93 *, 113 *, 242 * |
a Significance was calculated by comparing the difference in the log likelihoods corresponding to the M7-M8 model. b Underlined are residues on the peptide-binding region (PBRs). * Posterior probability ≥ 0.95. ** Posterior probability ≥ 0.99.
3.5. SLA-1, SLA-2, and DQB1 Genetic Polymorphism and Differentiation
For the SLA-1 loci, a total of 60 individuals (16 exotic pigs, 27 local pigs, and 16 warthogs), each with 240 nucleotide sites were used to analyze the genetic diversity. Genetic diversity parameters and the results of neutrality tests are shown in Table 5. The observed heterozygosity (Ho) ranged between 0.0341 to 0.0778, while the expected heterozygosity was between 0.0887 to 0.1637, whereby the locally-adapted pigs and warthogs had higher Ho, Hs, and Ht values compared to the exotic pigs. The Fst values between populations ranged from 0 to 0.0485, and the Dst value was 0.0058 when all the individuals were combined into one. The inbreeding coefficient was highest among the exotic breeds (Fis = 0.6154). The measure of population differentiation (Dest) was 0.0087 when all the individuals were combined.
Table 5.
Genetic diversity within SLA-1, SLA-2, and DQB1 loci among three suid breeds. N: Number of individuals; Ho: Mean observed heterozygosity; Hs: Mean gene diversity within population (expected heterozygosity); Ht: Total gene diversity; Dst: Gene diversity among samples; Dstp: Heterozygosity corrected Dst; Fis: Inbreeding coefficient per overall loci (Fis: 1-Ho/Hs); Dest: Measure of population differentiation.
| Loci | Population | N | Ho | Hs | Ht | Htp | Dst | Dstp | Fst | Fstp | Fis | Dest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SLA-1 | Exotic pigs | 16 | 0.0341 | 0.0887 | 0.0887 | NaN | 0 | NaN | 0 | NaN | 0.6154 | NaN |
| Locally-adapted pigs | 27 | 0.0778 | 0.1116 | 0.1116 | NaN | 0 | NaN | 0 | NaN | 0.3029 | NaN | |
| Warthogs | 16 | 0.0766 | 0.1637 | 0.1637 | NaN | 0 | NaN | 0 | NaN | 0.5323 | NaN | |
| All included | 59 | 0.0471 | 0.1133 | 0.1191 | 0.121 | 0.0058 | 0.0077 | 0.0485 | 0.0636 | 0.5843 | 0.0087 | |
| SLA-2 | Exotic pigs | 16 | 0.0203 | 0.0585 | 0.0585 | NaN | 0 | NaN | 0 | NaN | 0.653 | NaN |
| Locally-adapted pigs | 25 | 0.0167 | 0.0633 | 0.0633 | NaN | 0 | NaN | 0 | NaN | 0.7367 | NaN | |
| Warthogs | 2 | 0.0038 | 0.0019 | 0.0019 | NaN | 0 | NaN | 0 | NaN | −1 | NaN | |
| All included | 43 | 0.0102 | 0.046 | 0.0556 | 0.0588 | 0.0096 | 0.0128 | 0.173 | 0.2181 | 0.7786 | 0.0135 | |
| DQB1 | Exotic pigs | 16 | 0.0021 | 0.0197 | 0.0197 | NaN | 0 | NaN | 0 | NaN | 0.8911 | NaN |
| Locally-adapted pigs | 21 | 0.0006 | 0.0099 | 0.0099 | NaN | 0 | NaN | 0 | NaN | 0.9428 | NaN | |
| Warthogs | 2 | 0.002 | 0.0152 | 0.0152 | NaN | 0 | NaN | 0 | NaN | 0.8696 | NaN | |
| All included | 39 | 0.0012 | 0.0147 | 0.0206 | 0.0225 | 0.0059 | 0.0079 | 0.2867 | 0.3489 | 0.9202 | 0.008 |
In SLA-2 loci, a total of 43 individuals (16 exotic pigs, 25 local pigs, and two warthogs), each with 1056 nucleotide sites were used to analyze the genetic diversity (Table 5). The observed heterozygosity (Ho) ranged between 0.0038 to 0.0167, while the expected heterozygosity was between 0.0019 to 0.0.0633, whereby the locally-adapted pigs and exotic pigs had higher Ho, Hs, and Ht values compared to the warthogs in which the power of numbers were limiting. The Fst values between populations ranged from 0 to 0.173, and the Dst value was 0.0096 when all the individuals were combined into one. The inbreeding coefficient was higher among the locally-adapted pigs (Fis = 0.7367) than the exotic pig breeds, which had an inbreeding value of 0.653. The measure of population differentiation (Dest) was 0.0135 when all the individuals were combined.
In the DQB-1 loci, a total of 39 individuals (16 exotic pigs, 21 local pigs, and two warthogs), each with 759 nucleotide sites were used to analyze the genetic diversity (Table 5). The observed heterozygosity (Ho) ranged between 0.0006 to 0.002, while the expected heterozygosity was between 0.0099 to 0.0197, whereby the exotic pigs and warthogs had higher Ho, Hs, and Ht values compared to the locally-adapted pigs in which the power of numbers were limiting. The Fst values between populations ranged from 0 to 0.287, and the Dst value was 0.0059 when all the individuals were combined into one. The inbreeding coefficient was higher among the locally-adapted pigs (Fis = 0.9428) than the exotic pig breeds, which had an inbreeding value of 0.8911 and warthogs at 0.8696. The measure of population differentiation (Dest) was 0.008 when all the individuals were combined.
The SLA-1 sequences from the locally-adapted pigs and warthogs showed greater nucleotide diversity (π = 0.10083 ± 0.0053 and 0.11654 ± 0.00952) compared to those from the exotic pigs (0.085 ± 0.00796). The locally-adapted pigs had the lowest haplotype diversity at the PBR of DQB1 loci (Table 6). The warthogs had a larger θW at exon 2 of the SLA-1 loci among the three study groups. Theta per site from Eta (θEta) was considerably higher in warthog samples (θEta = 0.166), followed closely by the locally-adapted pigs (θEta = 0.13146) at the SLA-1 loci. The exon 3 of the SLA-2 loci nucleotide sequences from the exotic pigs showed greater nucleotide diversity (π = 0.06599 ± 0.00461) compared to those from the locally-adapted pigs (π = 0.05030 ± 0.00434). The warthogs had the lowest nucleotide diversity at the SLA-2 exon 2 (Table 6). The locally-adapted pigs had higher θW values at the exon 2 of SLA-2 loci while theta (θEta) was relatively higher in exotic pigs, followed closely by the locally-adapted pigs’ exon 3 of SLA-2 loci. At exon 2 of the DQB1 loci, the exotic pigs showed greater nucleotide diversity while Theta (θEta) was considerably higher in exon 2 of the DQB1 loci exotic pigs than in the locally-adapted pigs (Table 5).
Table 6.
The DNA polymorphism measures for PBRs in SLA-1, SLA-2, and DQB1. Where N: The sample size; π: Nucleotide diversity; Hd: Haplotype (gene) diversity; θW: Watterson mutation estimator from variable sites, Eta: Total number of mutations; and θEta: Theta per site from Eta.
| Loci | MHC | Pop | N | Π | Hd | θW | θEta | Eta |
|---|---|---|---|---|---|---|---|---|
| SLA-1 | PBR (Exon 2) |
All samples | 60 | 0.12178 ± 0.00472 | 0.9973 ± 0.0015 | 0.13415 ± 0.03321 | 0.20751 | 198 |
| Locally-adapted pigs | 27 | 0.10083 ± 0.00539 | 0.997 ± 0.004 | 0.0973 ± 0.02795 | 0.13146 | 127 | ||
| Exotic pigs | 18 | 0.085 ± 0.00796 | 0.98 ± 0.010 | 0.08 ± 0.02747 | 0.11017 | 100 | ||
| Warthogs | 16 | 0.11654 ± 0.00952 | 0.998 ± 0.08 | 0.12834 ± 0.04066 | 0.166 | 119 | ||
| SLA-2 | PBR (Exon 2) |
All samples | 44 | 0.1098 ± 0.00396 | 0.968 ± 0.011 | 0.09404 ± 0.02462 | 0.13149 | 158 |
| Locally-adapted pigs | 25 | 0.09812 ± 0.00793 | 0.953 ± 0.021 | 0.08442 ± 0.02464 | 0.1135 | 121 | ||
| Exotic pigs | 16 | 0.09582 ± 0.00464 | 0.944 ± 0.035 | 0.07725 ± 0.02459 | 0.09564 | 104 | ||
| Warthogs * | 2 | 0.00494 ± 0.00151 | 0.667 ± 0.204 | 0.00404 ± 0.00324 | 0.00404 | 2 | ||
| PBR (Exon 3) |
All samples | 44 | 0.05975 ± 0.00311 | 0.925 ± 0.021 | 0.05956 ± 0.01593 | 0.07822 | 109 | |
| Locally-adapted pigs | 25 | 0.05030 ± 0.00434 | 0.878 ± 0.042 | 0.05015 ± 0.000406 | 0.06471 | 80 | ||
| Exotic pigs | 16 | 0.06599 ± 0.00461 | 0.935 ± 0.035 | 0.05488 ± 0.01781 | 0.06658 | 74 | ||
| Warthogs * | 2 | 0 | 0 | 0 | 0 | 0 | ||
| DQB1 | PBR (Exon 2) |
All samples | 40 | 0.03332 ± 0.00423 | 0.735 ± 0.055 | 0.04636 ± 0.01291 | 0.05085 | 68 |
| Locally-adapted pigs | 21 | 0.02152 ± 0.00663 | 0.455 ± 0.095 | 0.03357 ± 0.01083 | 0.03529 | 41 | ||
| Exotic pigs | 16 | 0,04146 ± 0.00442 | 0.905 ± 0.039 | 0.03863 ± 0.01293 | 0.04047 | 44 | ||
| Warthogs | 2 | 0.01914 ± 0.00557 | 0.833 ± 0.222 | 0.01616 ± 0.00993 | 0.01616 | 8 |
* Warthog samples were almost identical and fewer in number.
3.6. MHC Functional Cluster Analysis
To determine MHC peptides function-based clustering, we selected the representatives of the SLA-1, SLA-2, and DQB1 alleles from among the domestic pigs and warthogs for analysis using MHCcluster 2.0. The longest ORF for each SLA gene-specific loci was submitted for analysis on MHCcluster 2.0 [65] to allow for the comparison of the peptide-binding function of 50,000 random natural 10 amino acid long peptides of known SLA alleles and predict the binding motifs for the SLA Class I and II proteins [65]. We selected 29, 10, and 10 of the SLA-1, SLA-2, and DQB1 as sequence representatives and added the corresponding MHC alleles in the MHCcluster 2.0 server. The peptide-binding motifs were visualized in the form of an unrooted tree and a heatmap for similarities of predicted binding to a set of predefined natural peptides. The analysis of SLA-2 peptides generated 12 significant clusters (Figure 6A,B). The intensity of the color on the heatmaps correlates with the behavior of the epitopes’ predictive ability between different MHCs (Figure 6A,C). The red sections represent the regions where the alleles of MHC with a similar high affinity anchor to the epitopes, the yellow sections represent the regions where the similarity of affinity with epitopes is distant. The warthog polypeptide sequences included showed functional similarity to those of the locally-adapted pigs. The SLA-2*05:01, SLA-2*05:02, and SLA-2*12:01 alleles clustered together on the heatmap and tree generated, while SLA-2*03:02 and SLA-2*10:02 also clustered together, indicating functional homologies, with accuracy levels of 1.00 (100% match), and that the molecules were characterized with high precision by the peptide-binding data.
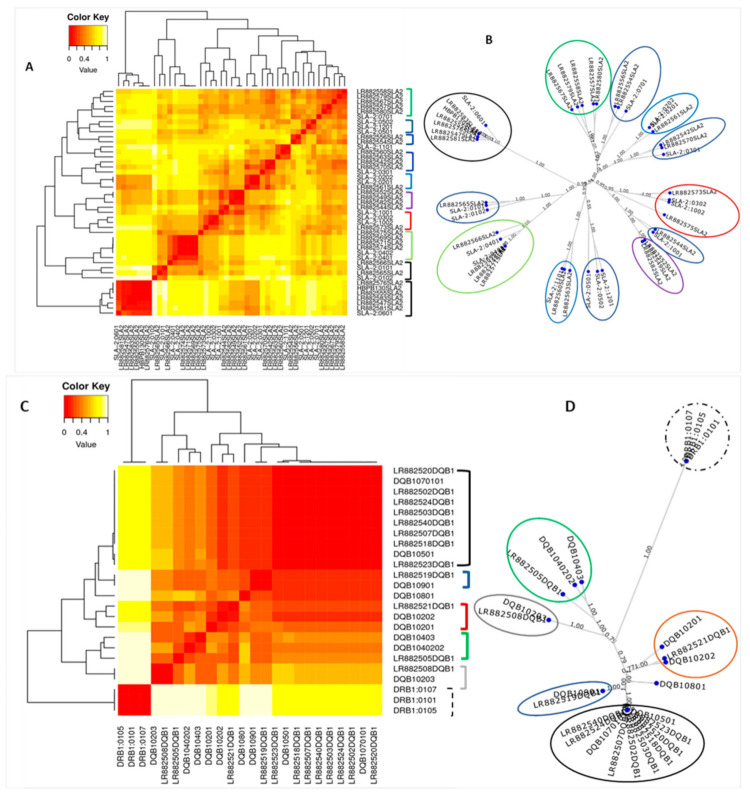
Figure 6.
MHC functional cluster analysis of the peptide sequences of entire amino acid sequences of the SLA-2 and DQB1 representative alleles identified in this study from locally-adapted pigs, exotic pigs, and warthogs: (A) A heatmap of the SLA-2 MHCcluster. (C) A heatmap of the DQB-1 clustering. (B,D) Unrooted phylogenetic trees for MHC alleles with visualization of the MHC motifs showing the clustering of the SLA-2 and DQB1 alleles, respectively, with those of known SLA-2 and DQB1 alleles. The clusters obtained are circled (B,D).
The analysis of DQB1 peptides generated seven major clusters (Figure 6C,D). The DQB1 warthog polypeptide sequences included in MHCcluster analysis showed functional similarity to those of the locally-adapted pigs and exotic pigs. The SLA-DQB1*07:01 and SLA-DQB1*05:01 alleles clustered together, and SLA-DQB1 *09:01 and SLA-DQB1*08:01 alleles also clustered together, indicating functional homologies between alleles. The clustering was supported by accuracy levels of 1.00 (100%), indicating that the molecules in each cluster obtained were highly similar to the known peptide-binding motifs. The DRB1 sequences included in the DQB1 analysis formed a distinct cluster (Figure 6C,D; dotted black lines) and were thus functionally unique. The SLA-1 polypeptides generated in this study did not show similar or strong peptide-binding motifs (accuracy level < 0.5) with known SLA-1 obtained from IPD and the MHCcluster server (Figure S1).
4. Discussion
Analyzing genetic diversity within MHC genes is useful in understanding their adaptive significance in host immune function towards improving pig health and guiding pig breeding strategies. This study aimed at comparing SLA class I (SLA-1 and SLA-2) and class II (DQB1) genetic diversity for the first time in locally-adapted pigs and their wild relatives, warthogs from Kenya. Our results show greater MHC diversity in the locally-adapted pigs and warthogs previously reported to be more resilient under harsh climatic conditions with some levels of tolerance or resistance to disease [43,48] than the exotic pigs. The factors driving this MHC diversity are likely to be interactions between differential fitness and pathogenic pressures.
Our study identified a high number of SLA class I (SLA-1, SLA-2) and class II (DQB1) sequence variants in the three suid populations. However, we refrain from categorizing these sequence variants as novel alleles since these MHC sequence variants were inferred using phased sequence data (see the Methods Section). Ultimately, the validation of alleles by resequencing of clones or re-genotyping of individuals with rare haplotypes is imperative.
4.1. Comparative Analysis of SLA Alleles and Sequence Diversity
Genetic diversity is fundamental in biodiversity and adaptation of species and a high genetic diversity is an indicator of stronger viability and adaptability of populations to a given environment [67]. Our study found low Fst values in all three loci, indicating low genetic differentiation between populations, confirming frequent gene flow between populations, which increases the species’ adaptability to changes in the environment. We found higher genetic diversity amongst the locally-adapted pigs and warthogs at the SLA-1 loci than the exotic pigs that undergo intensive inbreeding to maintain important reproductive traits for commercial purposes (Table 5). The higher diversity may enhance the tolerance of the locally-adapted pigs and warthogs populations to changes in the environment resulting in higher adaptability to novel environmental pressures [8,67].
In this study, we observed differences in MHC allele distribution between the exotic, locally-adapted pigs, and warthogs which could be attributed to their fitness level resulting from a natural or artificial selection over time. Our study identified 49 class I (SLA-1, SLA-2) and 11 class II (DQB1) alleles in the samples of the three suid populations studied. The locally-adapted pigs had a high number of alleles, although the allele distributions in the three loci were highly skewed, whereby at least one allele occurred at a higher proportion than others, consistent with previous MHC studies [16]. The skewed occurrence of alleles could be due to the adaptive evolution driven by natural selection to increase the frequencies of beneficial alleles and decrease frequencies of deleterious alleles [18,68].
Similar to previous studies, we reported a high proportion of SLA-1, SLA-2, and DQB1 breed-specific alleles among the locally-adapted pigs and warthogs compared to the exotic pigs [6,8]. The alleles occurring at high frequency were detected amongst the locally-adapted pigs and warthogs. We found several SLA-1 and DQB1 alleles shared by the domestic pigs and warthogs, which occurred at high frequencies (Table S4). The alleles shared by all three suids indicate the early origin of these alleles in suids and their possible importance in antigen recognition [8] as it is generally recognized that a high diversity of MHC alleles confers protection to a range of pathogens [6,22]. A large number of breed-specific private alleles (26, 29, and 11 for SLA-1, SLA-2, and DQB1, respectively) observed in this study indicate the impact of recent selective breeding or possible barriers to gene transfer among the sample of suids populations studied [3,8].
High allele sharing was observed at the SLA-1 locus among the suid breeds, indicating convergence evolution at this locus that likely confers a fitness advantage to these suid breeds. SLA-2 had low allele sharing among the three suid breeds studied, with a large number being shared between the domestic pigs only. At the SLA-2 locus, only one allele, SLA-2*06:07, was shared between local pigs and the warthogs, occurring at a high frequency (19.64%) indicative of convergence evolution and maintenance of this allele during breed diversification [8]. We observed a different pattern in SLA class II (DQB1) genes, whereby a lower rate of breed-specific alleles was identified than the SLA class I genes, probably due to the fact that the SLA class II genes are less polymorphic than the SLA class I genes [8]. Thus, the SLA class I genes are better suited markers to study the history of the suid species than the SLA class II genes.
Our study reported several private SLA alleles in all three suid breeds studied (Table S4). Private alleles are indicators of gene flow, where they can be correlated with the mean number of migrants exchanged per generation between populations. Private alleles unique to each pig breed are essential in screening potential T-cell epitopes for diagnostics and vaccine development [69,70,71]. In addition, the unique or private breed-specific alleles may guide pig breeders to selectively increase the frequency of the specific SLA alleles, primarily if shown to be associated with advantageous agronomic traits and disease tolerance or resistance.
PBRs directly influence the peptides that the SLA allele can bind, and this information is essential in peptide-histocompatibility for peptide-based vaccines [69,70,71]. We compared the PBR-encoding sequences across the three suid breeds for each locus studied to infer orthologous relationships despite inherent phenotypic differences. We observed high SLA sequence polymorphisms within the PBR, as reported in previous studies [8,18,72,73]. PBR-encoding sequences of the east African warthogs grouped with locally-adapted pigs indicated high similarity between the SLA motifs. This could imply that the local pigs and warthogs have undergone similar host-pathogen co-evolutionary pressures [6,74,75]. The observed homology between the locally-adapted pigs and warthogs contradicts a previous study that showed that the locally-adapted Homa Bay pig populations did not share genetic similarities with wild pigs using the porcine SNP60/80 chip to genotype the pigs [43]. Our findings affirm SLA as a superior adaptive marker, capable of deciphering genetic diversity relevant to conservation compared to commonly used markers outside the MHC regions [18,76]. The SLA class II DQB1 exon 3 PBR-encoding sequences did not yield any significant clustering pattern since the DQB1 genes are more conserved than SLA-1 and SLA-2, and therefore, intragenic variations would be difficult to decipher [77].
4.2. MHC Functional Cluster Analysis
The MHC peptide-binding groove contains polymorphic residues responsible for the different peptide specificities of the different alleles, ensuring that some individuals within a population can recognize protein antigens produced by virtually any microbe, reducing the likelihood that a single pathogen can evade host defenses in all individuals in a given species. When we compared the SLA sequences of alleles of the polymorphic class I and class II loci, we found that the nucleotide substitutions were concentrated in the exons that encode the peptide-binding groove, the site of interaction with the T-cell receptor [15,78]. It is crucial to characterize the peptide-binding motifs to identify peptides that respond directly to viral antigens to establish their adaptive immunity roles. The clustering of SLA-2 alleles confirmed the presence of 12 specificity supertypes or specificity groups (Figure 6A,B) in our study cohort. The warthog SLA-2 polypeptide sequences included in this cluster analysis showed functional similarity to those of the locally-adapted pigs (Figure 6A,B). We observed shared functional homology between the different SLA-2 alleles illustrating the immunological phenotypic similarities between pig breeds, particularly those heterozygous at SLA class I loci [79]. Functional peptide clustering of the most frequent DQB1 alleles in the study cohort yielded seven specificity supertypes or specificity groups (Figure 6C,D). The SLA-DQB1 *07:01 and SLA-DQB1 *05:01 alleles clustered together, and SLA-DQB1 *09:01 and SLA-DQB1 *08:01 alleles also clustered together, indicating functional homologies between these alleles. In this same cluster, the warthog DQB1 peptide sequences (LR882540) showed functional similarity to peptide sequences from both the exotic and locally-adapted pigs (Figure 6A,B). Four unique clusters that contained peptide sequences from exotic pigs (LR882519 and LR882521) and two from locally-adapted pigs (LR882505 and LR882508) were obtained, demonstrating that these four pigs contained peptides unique at the DQB1 loci.
Due to the smaller size of the predicted SLA-1 protein in this study, we were not able to perform a cluster similarity analysis on the MHCcluster method, which has been shown to perform functional similarities at best between large sets of MHC molecules [65,79]. These peptides can be synthesized to induce and screen for the T-cell response, thus broadening the SLA repertoires and consequently attaining increased chances of matching animals in terms of SLA expression in future peptide–epitope studies [2,17,79].
4.3. Differences in SLA Genetic Diversity and Selection Analysis
MHC diversity is beneficial to the host when faced with emerging diseases due to its role in recognizing new pathogens and survival during infection. We observed differences in MHC diversity between the exotic pigs, locally-adapted pigs, and warthogs, which could be attributed to differences in exposure to pathogen communities. Pathogens have evolved to escape recognition by the most frequent SLA molecule, resulting in several MHC variants expressed by the host so that the pathogens do not totally evade detection [73]. These high levels of polymorphism of the MHC genes are maintained by balancing selection to increase the repertoire of antigen-presenting molecules on the cell surface [23]. The genetic signature of this positive selection results in an increased ratio between non-synonymous and synonymous substitution, particularly within the PBR-encoding regions but not outside the PBR region. An invaluable statistic to measure the strength and mode of natural selection acting on the protein-coding genes is (omega) ω = dN/ dS [80]. The ω value summarizes the evolutionary rates of genes and identifies conserved genes and those that have undergone periods of adaptive evolution. Our study obtained higher ω values (ω > 1) in all the loci studied and in the PBR-encoding regions, where the ω value was statistically significant (ω > 1; p < 0.05). The locally-adapted pigs and warthogs consistently had higher ω values (ω > 1) except in DQB1, where the exotic pigs had higher values than the locally-adapted pigs and warthogs, indicating that DQB1 had undergone adaptive evolution among the exotic pigs studied. Thus, our analysis confirms a strong positive selection within the PBR-encoding regions of SLA-1, SLA-2, and DQB1 involved in binding pathogen-derived epitopes consistent with previous MHC diversity studies [18,29,72,73,81]. However, the dN/dS ratio analyses are limited in explaining events that occur at shorter time scales, such as during a high pathogen load [18].
There were more site-specific codons being under positive selection in PBR regions or close to PBR codons, consistent with previous MHC studies [72,73], confirming that these residues have a critical role maintained by balancing selection in the suid immune function. We found that MHC class I, specifically SLA-2 PBR-encoding regions, had a higher proportion of positively selected codons than DQB1 (Table 4), indicative of exposure to epitopes of exogenic origin. Since the analysis of site-specific selection in warthogs was constrained by the small number of SLA-2 and DQB1 sequences obtained in our study, only SLA-1 (exon 2; PBR) yielded site-specific selection data from warthogs in which five out of the 12 sites identified were significantly positively selected at posterior probabilities of 0.95 and 0.99 (Table 6). We found several similar positively selected codons among the three suid species studied (Table 5, Table S5 and Table S6), indicating similar selective pressures acting on these codons. Likewise, the presence of several independent positively selected codons (Table 5) in these different suid species indicates that they may have functional implications on peptide binding and further contribute to the observed polymorphism [72,73].
Overall, our analysis showed positive balancing selection for the SLA regions studied, a distinctive feature of MHC genes that elevates the repertoire of antigen-presenting molecules on the cell surfaces, increasing the diversity of the antigen-presenting peptides, thus providing an advantage to the host in combating different infectious diseases [6,18,72]. Our findings support the hypothesis that pathogen-driven selection is the primary driver of MHC diversity [16]. The differences observed in diversity between the locally-adapted and exotic pigs can be attributed to the differential fitness of the swine hosts and differences in exposure to pathogens and parasites. Thus, the lower SLA diversity observed in the exotic pigs could be due to high selection and resultant inbreeding to maintain the desirable production traits in modern pig production [18]. However, the extent of SLA diversity reported in this study may not fully reveal that of the domestic Kenyan pigs and warthog populations due to the relatively small sample size, the possibility of founder effects in this population, the breeding schemes, and management systems that were not considered.
5. Conclusions
We undertook a comparative SLA genetic diversity analysis of three closely related suids: Exotic and locally-adapted Kenyan pigs and warthogs, reporting differences in the distribution of alleles among the suid breeds as well as breed-specific private alleles. Our study provides substantial evidence of positive selection acting on the PBR-coding region of the SLA-1, SLA-2, and DQB1 genes in domestic pigs and warthogs. In addition, we identified several positively selected codons in the domestic pigs and warthogs that can be targeted for association studies with disease resistance or susceptibility traits. The findings from our study contribute to the body of knowledge on genetic diversity in free-ranging animal populations in their natural environment, availing the first DQB1 gene data from locally-adapted Kenyan pigs. Therefore, we recommend extensively and carefully designed population-level and experimental challenge studies to determine the association between SLA diversity and valuable agronomic traits among the locally-adapted pigs and warthogs, which are crucial in pig genetic improvement programs.
Acknowledgments
The authors thank the pig farmers in Homa Bay County and at Farmers Choice, Limuru, Kenya. In addition, we express gratitude to the ILRI ASF team members (years 2014–2021) who collected some of the samples used in this study. Finally, special gratitude goes to the Kenya Wildlife Service for granting permission to sample warthogs for research purposes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vetsci8090180/s1. Figure S1: MHC functional cluster analysis of the peptide sequences of SLA-1 representative alleles identified in this study from locally-adapted pigs, exotic pigs, and warthogs: (A) A heatmap of the SLA-2 MHCcluster. (B) Unrooted phylogenetic trees for MHC alleles with visualization of the MHC motifs showing the clustering of the SLA-1 alleles, respectively, with those of known SLA-1 alleles. Table S1: The locus-specific primers for reverse transcription-polymerase chain reaction (RT-PCR) based method for DNA sequence-based typing (SBT) of the three SLA genes. Table S2: SLA-IPD BLAST results for allele matching. Table S3: The polypeptide predictions for the SLA-2 sequences. Table S4: SLA allele frequencies and distribution amongst locally-adapted pigs and warthogs. Table S5: SLA-1, SLA-2, and DQB-1 codons under positive selection by employing a Bayes empirical Bayes approach. Table S6: SLA-1, SLA-2, and DQB-1 peptide-binding codons under positive selection using a Bayes empirical Bayes approach.
Author Contributions
E.M.M., E.O.A., A.W.T.M., I.L., and R.P. conceived and designed the experiments; E.M.M. performed the experiments; E.M.M., J.-B.D.E. and R.P. analyzed the data; E.M.M. and J.O.A. wrote the paper; J.O.A., E.O.A., J.-B.D.E., A.W.T.M., I.L. and R.P. edited and reviewed the paper. All authors read and approved the final version of the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The sample collection and Sanger sequencing were funded by the Defense Threat Reduction Agency (DTRA), grant number HDTRA1-16-1-0050. The African Union, through the Pan African University Institute of Basic Sciences Technology and Innovation (PAUISTI), and Japan International Corporation Agency (JICA) under the Africa-ai-Japan Project, provided funds for reagents and sequencing. The funding agencies had no role in the project design, data collection, analysis, decision to publish, or manuscript preparation.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki approved by the Institutional Animal Care and Use Committee (IACUC), International Livestock Research Institute, Kenya (Ref: VN_IACUC-2011-ASF in vivo infection, Ref: 2011-04). The warthog samples were collected with permission from the Kenya Wildlife Service (Ref: KWS/VET/5806).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed for this study can be found in the European Nucleotide Archives (ENA) under the Project ID: PRJEB39824 with the following accession numbers: LR882502-LR882643.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hurt P., Walter L., Sudbrak R., Klages S., Müller I., Shiina T., Inoko H., Lehrach H., Günther E., Reinhardt R., et al. The Genomic Sequence and Comparative Analysis of the Rat Major Histocompatibility Complex. Genome Res. 2004;14:631–639. doi: 10.1101/gr.1987704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen L.E., Jungersen G., Sørensen M.R., Ho C.-S., Vadekær D.F. Swine Leukocyte Antigen (SLA) class I allele typing of Danish swine herds and identification of commonly occurring haplotypes using sequence specific low and high resolution primers. Veter. Immunol. Immunopathol. 2014;162:108–116. doi: 10.1016/j.vetimm.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Gao C., Jiang Q., Guo D., Liu J., Han L., Qu L. Characterization of swine leukocyte antigen (SLA) polymorphism by sequence-based and PCR-SSP methods in Chinese Bama miniature pigs. Dev. Comp. Immunol. 2014;45:87–96. doi: 10.1016/j.dci.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Tennant L.M., Renard C., Chardon P., Powell P.P. Regulation of porcine classical and nonclassical MHC class I expression. Immunogenetics. 2007;59:377–389. doi: 10.1007/s00251-007-0206-x. [DOI] [PubMed] [Google Scholar]
- 5.Janeway C.A., Jr., Travers P., Walport M. Immunobiology: The Immune System in Health and Disease. Garland Science; New York, NY, USA: 2001. The major histocompatibility complex and its functions. [Google Scholar]
- 6.Lee C., Moroldo M., Perdomo-Sabogal A., Mach N., Marthey S., Lecardonnel J., Wahlberg P., Chong A.Y., Estellé J., Ho S.Y.W., et al. Inferring the evolution of the major histocompatibility complex of wild pigs and peccaries using hybridisation DNA capture-based sequencing. Immunogenetics. 2017;70:401–417. doi: 10.1007/s00251-017-1048-9. [DOI] [PubMed] [Google Scholar]
- 7.Chenais E., Ståhl K., Guberti V., Depner K. Identification of wild boar-habitat epidemiologic cycle in African swine fever epizootic. Emerg. Infect. Dis. 2018;24:810–812. doi: 10.3201/eid2404.172127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le M.T., Choi H., Lee H., Le V.C.Q., Ahn B., Ho C.-S., Hong K., Song H., Kim J.-H., Park C. SLA-1 Genetic Diversity in Pigs: Extensive Analysis of Copy Number Variation, Heterozygosity, Expression, and Breed Specificity. Sci. Rep. 2020;10:743. doi: 10.1038/s41598-020-57712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renard C., Hart E., Sehra H., Beasley H., Coggill P., Howe K., Harrow J., Gilbert J., Sims S., Rogers J., et al. The genomic sequence and analysis of the swine major histocompatibility complex. Genomics. 2006;88:96–110. doi: 10.1016/j.ygeno.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Renard C., Vaiman M., Chiannilkulchai N., Cattolico L., Robert C., Chardon P. Sequence of the pig major histocompatibility region containing the classical class I genes. Immunogenetics. 2001;53:490–500. doi: 10.1007/s002510100348. [DOI] [PubMed] [Google Scholar]
- 11.Chardon P., Renard C., Vaiman M. The major histocompatibility complex in swine. Immunol. Rev. 1999;167:179–192. doi: 10.1111/j.1600-065X.1999.tb01391.x. [DOI] [PubMed] [Google Scholar]
- 12.Halenius A., Gerke C., Hengel H. Classical and non-classical MHC i molecule manipulation by human cytomegalovirus: So many targets—But how many arrows in the quiver? Cell. Mol. Immunol. 2015;12:139–153. doi: 10.1038/cmi.2014.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hammer S.E., Ho C.S., Ando A., Rogel-Gaillard C., Charles M., Tector M., Tector A.J., Lunney J.K. Importance of the Major Histocompatibility Complex (Swine Leukocyte Antigen) in Swine Health and Biomedical Research. Annu. Rev. Anim. Biosci. 2020;8:171–198. doi: 10.1146/annurev-animal-020518-115014. [DOI] [PubMed] [Google Scholar]
- 14.Ando A., Chardon P. Gene organization and polymorphism of the swine major histocompatibility complex. Anim. Sci. J. 2006;77:127–137. doi: 10.1111/j.1740-0929.2006.00331.x. [DOI] [Google Scholar]
- 15.Gutiérrez S.E., Esteban E.N., Lützelschwab C.M., Juliarena M.A. Major Histocompatibility Complex-Associated Resistance to Infectious Diseases: The Case of Bovine Leukemia Virus Infection. Trends Adv. Vet. Genet. 2017:103–105. [Google Scholar]
- 16.Spurgin L.G., Richardson D.S. How pathogens drive genetic diversity: MHC, mechanisms and misunderstandings. Proc. R. Soc. B Biol. Sci. 2010;277:979–988. doi: 10.1098/rspb.2009.2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fan S., Wang Y., Wang S., Wang X., Wu Y., Li Z., Zhang N., Xia C. Polymorphism and peptide-binding specificities of porcine major histocompatibility complex (MHC) class I molecules. Mol. Immunol. 2018;93:236–245. doi: 10.1016/j.molimm.2017.06.024. [DOI] [PubMed] [Google Scholar]
- 18.Manlik O., Krützen M., Kopps A.M., Mann J., Bejder L., Allen S.J., Frère C., Connor R.C., Sherwin W.B. Is MHC diversity a better marker for conservation than neutral genetic diversity? A case study of two contrasting dolphin populations. Ecol. Evol. 2019;9:6986–6998. doi: 10.1002/ece3.5265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paterson S., Wilson K., Pemberton J.M. Major histocompatibility complex variation associated with juvenile survival and parasite resistance in a large unmanaged ungulate population (Ovis aries L.) Proc. Natl. Acad. Sci. USA. 1998;95:3714–3719. doi: 10.1073/pnas.95.7.3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bérénos C., Schmid-Hempel P., Wegner K.M. Antagonistic Coevolution Accelerates the Evolution of Reproductive Isolation in Tribolium castaneum. Am. Nat. 2012;180:520–528. doi: 10.1086/667589. [DOI] [PubMed] [Google Scholar]
- 21.Borghans J.A.M., Beltman J.B., De Boer R.J. MHC polymorphism under host-pathogen coevolution. Immunogenetics. 2004;55:732–739. doi: 10.1007/s00251-003-0630-5. [DOI] [PubMed] [Google Scholar]
- 22.Phillips K.P., Cable J., Mohammed R.S., Herdegen-Radwan M., Raubic J., Przesmycka K.J., van Oosterhout C., Radwan J. Immunogenetic novelty confers a selective advantage in host–pathogen coevolution. Proc. Natl. Acad. Sci. USA. 2018;115:1552–1557. doi: 10.1073/pnas.1708597115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan M.A.W., Stephens W.Z., Mohammed A.D., Round J.L., Kubinak J.L. Does MHC heterozygosity influence microbiota form and function? PLoS ONE. 2019;14:e0215946. doi: 10.1371/journal.pone.0215946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterson S., Vogwill T., Buckling A., Benmayor R., Spiers A., Thomson N., Quail M., Smith F., Walker D., Libberton B., et al. Antagonistic coevolution accelerates molecular evolution. Nature. 2010;464:275–278. doi: 10.1038/nature08798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Worley K., Collet J., Spurgin L., Cornwallis C., Pizzari T., Richardson D.S. MHC heterozygosity and survival in red junglefowl. Mol. Ecol. 2010;19:3064–3075. doi: 10.1111/j.1365-294X.2010.04724.x. [DOI] [PubMed] [Google Scholar]
- 26.Niskanen A.K., Kennedy L.J., Ruokonen M., Kojola I., Lohi H., Isomursu M., Jansson E., Pyhäjärvi T., Aspi J. Balancing selection and heterozygote advantage in major histocompatibility complex loci of the bottlenecked Finnish wolf population. Mol. Ecol. 2014;23:875–889. doi: 10.1111/mec.12647. [DOI] [PubMed] [Google Scholar]
- 27.De Boer R.J., Borghans J.A.M., Van Boven M., Kesmir C., Weissing F.J. Heterozygote advantage fails to explain the high degree of polymorphism of the MHC. Immunogenetics. 2004;55:725–731. doi: 10.1007/s00251-003-0629-y. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q., Wu X., Yan P., Zheng S. Sequence variability analysis on major histocompatibility complex class II DRB alleles in three felines. Front. Biol. China. 2008;3:55–62. doi: 10.1007/s11515-008-0004-3. [DOI] [Google Scholar]
- 29.Pokorny I., Sharma R., Goyal S.P., Mishra S., Tiedemann R. MHC class I and MHC class II DRB gene variability in wild and captive Bengal tigers (Panthera tigris tigris) Immunogenetics. 2010;62:667–679. doi: 10.1007/s00251-010-0475-7. [DOI] [PubMed] [Google Scholar]
- 30.Hendrickson S.L., Mayer G.C., Wallen E.P., Quigley K. Genetic variability and geographic structure of three subspecies of tigers (Panthera tigris) based on MHC class I variation. Anim. Conserv. 2000;3:135–143. doi: 10.1111/j.1469-1795.2000.tb00238.x. [DOI] [Google Scholar]
- 31.Bos D.H., DeWoody J.A. Molecular characterization of major histocompatibility complex class II alleles in wild tiger salamanders (Ambystoma tigrinum) Immunogenetics. 2005;57:775–781. doi: 10.1007/s00251-005-0038-5. [DOI] [PubMed] [Google Scholar]
- 32.Piertney S.B., Oliver M.K. The evolutionary ecology of the major histocompatibility complex. Heredity. 2006;96:7–21. doi: 10.1038/sj.hdy.6800724. [DOI] [PubMed] [Google Scholar]
- 33.Bastos A.D.S., Penrith M.-L., Crucière C., Edrich J.L., Hutchings G., Roger F., Couacy-Hymann E., Thomson G.R. Genotyping field strains of African swine fever virus by partial p72 gene characterisation. Arch. Virol. 2003;148:693–706. doi: 10.1007/s00705-002-0946-8. [DOI] [PubMed] [Google Scholar]
- 34.Dixon L.K., Takamatsu H. African swine fever virus: Current situation and prospects for control. Pig J. 2011;67:11–17. [Google Scholar]
- 35.FAO . World Livestock 2011: Livestock in Food Security World. FAO; Rome, Italy: 2011. [Google Scholar]
- 36.Ouma E., Dione M., Lule P., Rosel K., Pezo D. Characterization of smallholder pig production systems in Uganda: Constraints and opportunities for engaging with market systems. Livest. Res. Rural. Dev. 2014 doi: 10.22004/ag.econ.160677. [DOI] [Google Scholar]
- 37.Chauhan A., Patel B.H.M., Maurya R., Kumar S., Shukla S., Kumar S. Pig Production System as a Source of Livelihood in Indian Scenario: An Overview. Int. J. Sci. Technol. 2016;5:2089–2096. [Google Scholar]
- 38.Swindle M.M., Makin A., Herron A.J., Clubb F.J., Frazier K.S. Swine as Models in Biomedical Research and Toxicology Testing. Veter. Pathol. 2011;49:344–356. doi: 10.1177/0300985811402846. [DOI] [PubMed] [Google Scholar]
- 39.Rajao D.D.S., Vincent A.L. Swine as a Model for Influenza A Virus Infection and Immunity. ILAR J. 2015;56:44–52. doi: 10.1093/ilar/ilv002. [DOI] [PubMed] [Google Scholar]
- 40.Sørensen M.R., Ilsøe M., Strube M.L., Bishop R., Erbs G., Hartmann S.B., Jungersen G. Sequence-Based Genotyping of Expressed Swine Leukocyte Antigen Class I Alleles by Next-Generation Sequencing Reveal Novel Swine Leukocyte Antigen Class I Haplotypes and Alleles in Belgian, Danish, and Kenyan Fattening Pigs and Göttingen Minipigs. Front. Immunol. 2017;8:701. doi: 10.3389/fimmu.2017.00701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amills M., Ramírez O., Galman-Omitogun O., Clop A. Domestic Pigs in Africa. Afr. Archaeol. Review. 2013;30:73–82. doi: 10.1007/s10437-012-9111-2. [DOI] [Google Scholar]
- 42.Mazur-Panasiuk N., Żmudzki J., Woźniakowski G. African swine fever virus—Persistence in different environmental conditions and the possibility of its indirect transmission. J. Veter. Res. 2019;63:303–310. doi: 10.2478/jvetres-2019-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mujibi F.D., Okoth E., Cheruiyot E.K., Onzere C., Bishop R.P., Fevre E., Thomas L., Masembe C., Plastow G., Rothschild M. Genetic diversity, breed composition and admixture of Kenyan domestic pigs. PLoS ONE. 2018;13:e0190080. doi: 10.1371/journal.pone.0190080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutua F.K., Dewey C.E., Arimi S.M., Ogara W.O., Githigia S.M., Levy M., Schelling E. Indigenous pig management practices in rural villages of Western Kenya. Livest. Res. Rural. Dev. 2011;23:144. [Google Scholar]
- 45.Kagira J.M., Kanyari P.W.N., Maingi N., Githigia S.M., Ng’Ang’A J.C., Karuga J.W. Characteristics of the smallholder free-range pig production system in western Kenya. Trop. Anim. Heal. Prod. 2009;42:865–873. doi: 10.1007/s11250-009-9500-y. [DOI] [PubMed] [Google Scholar]
- 46.Obonyo F.O., Maingi N., Githigia S.M., Ng’ang’a C.J. Farming practices and risk factors for transmission of helminths of free range pigs in Homabay District, Kenya. Livest. Res. Rural. Dev. 2013;25 [Google Scholar]
- 47.Patrick B.N., Machuka E.M., Githae D., Banswe G., Amimo J.O., Ongus J.R., Masembe C., Bishop R.P., Steinaa L., Djikeng A., et al. Evidence for the presence of African swine fever virus in apparently healthy pigs in South-Kivu Province of the Democratic Republic of Congo. Veter. Microbiol. 2019;240:108521. doi: 10.1016/j.vetmic.2019.108521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abworo E.O., Onzere C., Amimo J.O., Riitho V., Mwangi W., Davies J., Blome S., Bishop R.P. Detection of African swine fever virus in the tissues of asymptomatic pigs in smallholder farming systems along the Kenya–Uganda border: Implications for transmission in endemic areas and ASF surveillance in East Africa. J. Gen. Virol. 2017;98:1806–1814. doi: 10.1099/jgv.0.000848. [DOI] [PubMed] [Google Scholar]
- 49.Atuhaire D.K., Afayoa M., Ochwo S., Mwesigwa S., Mwiine F.N., Okuni J.B., Olaho-Mukani W., Ojok L. Prevalence of African swine fever virus in apparently healthy domestic pigs in Uganda. BMC Veter. Res. 2013;9:263. doi: 10.1186/1746-6148-9-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chambaro H.M., Sasaki M., Sinkala Y., Gonzalez G., Squarre D., Fandamu P., Lubaba C., Mataa L., Shawa M., Mwape K.E., et al. Evidence for exposure of asymptomatic domestic pigs to African swine fever virus during an inter-epidemic period in Zambia. Transbound. Emerg. Dis. 2020;67:2741–2752. doi: 10.1111/tbed.13630. [DOI] [PubMed] [Google Scholar]
- 51.Herold P., Roessler R., Willam A., Momm H., Zárate A.V. Breeding and supply chain systems incorporating local pig breeds for small-scale pig producers in Northwest Vietnam. Livest. Sci. 2010;129:63–72. doi: 10.1016/j.livsci.2010.01.004. [DOI] [Google Scholar]
- 52.Gallardo C., Okoth E., Pelayo V., Anchuelo R., Martín E., Simón A., Llorente A., Nieto R., Soler A., Martín R., et al. African swine fever viruses with two different genotypes, both of which occur in domestic pigs, are associated with ticks and adult warthogs, respectively, at a single geographical site. J. Gen. Virol. 2010;92:432–444. doi: 10.1099/vir.0.025874-0. [DOI] [PubMed] [Google Scholar]
- 53.Powell P.P., Dixon L.K., Parkhouse R.M. An IkappaB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J. Virology. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oura C.A., Parkhouse R.M., Powell P.P., Anderson E. The pathogenesis of African swine fever in the resistant bushpig. J. Gen. Virol. 1998;79:1439–1443. doi: 10.1099/0022-1317-79-6-1439. [DOI] [PubMed] [Google Scholar]
- 55.Dixon L.K., Chapman D. African Swine Fever Virus. Encycl. Virol. 2008:43–51. doi: 10.1099/vir.0.83343-0. [DOI] [PubMed] [Google Scholar]
- 56.Arias M., De La Torre A., Dixon L., Gallardo C., Jori F., Laddomada A., Martins C., Parkhouse R.M., Revilla Y., Rodriguez F.A.J.-M., et al. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines. 2017;5:35. doi: 10.3390/vaccines5040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stephens M., Donnelly P. A Comparison of Bayesian Methods for Haplotype Reconstruction from Population Genotype Data. Am. J. Hum. Genet. 2003;73:1162–1169. doi: 10.1086/379378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J.S., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinform. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson J., Halliwell J.A., McWilliam H., López R., Marsh S.G.E. IPD—The Immuno Polymorphism Database. Nucleic Acids Res. 2012;41:D1234–D1240. doi: 10.1093/nar/gks1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kozlov A.M., Darriba D., Flouri T., Morel B., Stamatakis A. RAxML-NG: A fast, scalable and user-friendly tool for maximum likelihood phylogenetic inference. Bioinformatics. 2019;35:4453–4455. doi: 10.1093/bioinformatics/btz305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Letunic I., Bork P. Interactive Tree Of Life (iTOL) v5: An online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 2021;49:W293–W296. doi: 10.1093/nar/gkab301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Artimo P., Jonnalagedda M., Arnold K., Baratin D., Csardi G., de Castro E., Duvaud S., Flegel V., Fortier A., Gasteiger E., et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–W603. doi: 10.1093/nar/gks400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S., Sánchez-Gracia A. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 64.Zhang C., Wang J., Xie W., Zhou G., Long M., Zhang Q. Dynamic programming procedure for searching optimal models to estimate substitution rates based on the maximum-likelihood method. Proc. Natl. Acad. Sci. USA. 2011;108:7860–7865. doi: 10.1073/pnas.1018621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thomsen M., Lundegaard C., Buus S., Lund O., Nielsen M. MHCcluster, a method for functional clustering of MHC molecules. Immunogenetics. 2013;65:655–665. doi: 10.1007/s00251-013-0714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang Z., Wong W.S., Nielsen R. Bayes Empirical Bayes Inference of Amino Acid Sites Under Positive Selection. Mol. Biol. Evol. 2005;22:1107–1118. doi: 10.1093/molbev/msi097. [DOI] [PubMed] [Google Scholar]
- 67.Nonić M., Šijačić-Nikolić M. Genetic Diversity: Sources, Threats, and Conservation. Springer; Cham, Switzerland: 2021. [Google Scholar]
- 68.Radwan J., Babik W., Kaufman J., Lenz T.L., Winternitz J. Advances in the Evolutionary Understanding of MHC Polymorphism. Trends Genet. 2020;36:298–311. doi: 10.1016/j.tig.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 69.Holzer B., Martini V., Edmans M., Tchilian E. T and B Cell Immune Responses to influenza viruses in pigs. Front. Immunol. 2019;10:98. doi: 10.3389/fimmu.2019.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Q.V.C., Le T.M., Cho H.-S., Kim W.-I., Hong K., Song H., Kim J.-H., Park C. Analysis of peptide-SLA binding by establishing immortalized porcine alveolar macrophage cells with different SLA class II haplotypes. Veter. Res. 2018;49:96. doi: 10.1186/s13567-018-0590-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gutierrez A., Loving C., Moise L., Terry F.E., Brockmeier S.L., Hughes H.R., Martin W.D., De Groot A.S. In Vivo Validation of Predicted and Conserved T Cell Epitopes in a Swine Influenza Model. PLoS ONE. 2016;11:e0159237. doi: 10.1371/journal.pone.0159237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amills M., Ramirez O., Tomàs A., Obexer-Ruff G., Vidal O. Positive selection on mammalian MHC-DQ genes revisited from a multispecies perspective. Genes Immun. 2008;9:651–658. doi: 10.1038/gene.2008.62. [DOI] [PubMed] [Google Scholar]
- 73.Cortázar-Chinarro M., Meyer-Lucht Y., Laurila A., Höglund J. Signatures of historical selection on MHC reveal different selection patterns in the moor frog (Rana arvalis) Immunogenetics. 2018;70:477–484. doi: 10.1007/s00251-017-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Evans M.L., Neff B.D. Major histocompatibility complex heterozygote advantage and widespread bacterial infections in populations of Chinook salmon (Oncorhynchus tshawytscha) Mol. Ecol. 2009;18:4716–4729. doi: 10.1111/j.1365-294X.2009.04374.x. [DOI] [PubMed] [Google Scholar]
- 75.Ebert D., Fields P.D. Host-parasite co-evolution and its genomic signature. Nat. Rev. Genetics. 2020;21:754–768. doi: 10.1038/s41576-020-0269-1. [DOI] [PubMed] [Google Scholar]
- 76.Mwambene P.L., Kyallo M., Machuka E., Githae D., Pelle R. Genetic diversity of 10 indigenous chicken ecotypes from Southern Highlands of Tanzania based on Major Histocompatibility Complex-linked microsatellite LEI0258 marker typing. Poult. Sci. 2019;98:2734–2746. doi: 10.3382/ps/pez076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arbanasić H., Huber D., Kusak J., Gomerčić T., Hrenović J., Galov A. Extensive polymorphism and evidence of selection pressure on major histocompatibility complex DLA-DRB1, DQA1 and DQB1 class II genes in Croatian grey wolves. Tissue Antigens. 2012;81:19–27. doi: 10.1111/tan.12029. [DOI] [PubMed] [Google Scholar]
- 78.Cruz-Tapias P., Castiblanco J., Correa N.E., Montoya-Ortíz G. Autoimmunity: From Bench to Bedside. El Rosario University Press; Bogota, Colombia: 2013. [PubMed] [Google Scholar]
- 79.Gao C., Quan J., Jiang X., Li C., Lu X., Chen H. Swine Leukocyte Antigen Diversity in Canadian Specific Pathogen-Free Yorkshire and Landrace Pigs. Front. Immunol. 2017;8:282. doi: 10.3389/fimmu.2017.00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sydykova D., Wilke C.O. Calculating site-specific evolutionary rates at the amino-acid or codon level yields similar rate estimates. PeerJ. 2017;5:e3391. doi: 10.7717/peerj.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kubinak J.L., Ruff J., Hyzer C.W., Slev P.R., Potts W. Experimental viral evolution to specific host MHC genotypes reveals fitness and virulence trade-offs in alternative MHC types. Proc. Natl. Acad. Sci. USA. 2012;109:3422–3427. doi: 10.1073/pnas.1112633109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed for this study can be found in the European Nucleotide Archives (ENA) under the Project ID: PRJEB39824 with the following accession numbers: LR882502-LR882643.