Abstract
BACKGROUND
Pneumonia is the main manifestation of coronavirus disease 2019 (COVID-19) infection. Chest computed tomography is recommended for the initial evaluation of the disease; this technique can also be helpful to monitor the disease progression and evaluate the therapeutic efficacy.
AIM
To review the currently available literature regarding the radiological follow-up of COVID-19-related lung alterations using the computed tomography scan, to describe the evidence about the dynamic evolution of COVID-19 pneumonia and verify the potential usefulness of the radiological follow-up.
METHODS
We used pertinent keywords on PubMed to select relevant studies; the articles we considered were published until October 30, 2020. Through this selection, 69 studies were identified, and 16 were finally included in the review.
RESULTS
Summarizing the included works’ findings, we identified well-defined stages in the short follow-up time frame. A radiographic deterioration reaching a peak roughly within the first 2 wk; after the peak, an absorption process and repairing signs are observed. At later radiological follow-up, with the limitation of little evidence available, the lesions usually did not recover completely.
CONCLUSION
Following computed tomography scan evolution over time could help physicians better understand the clinical impact of COVID-19 pneumonia and manage the possible sequelae; a longer follow-up is advisable to verify the complete resolution or the presence of long-term damage.
Keywords: COVID-19, Computed tomography, Pneumonia, Radiological evolution, Follow-up, Long-term consequences, Lung damage, SARS-CoV-2
Core Tip: Given the recent discovery and study of severe acute respiratory syndrome coronavirus 2 infection, the evolution of coronavirus disease 2019 pneumonia has not been entirely defined yet. Chest computed tomography is an effective method to identify and follow coronavirus disease 2019 pneumonia over time. In this review, we considered the radiological changes on computed tomography scan and described the possible clinical pulmonary sequelae in order to understand the long-term outcome of coronavirus disease 2019 pneumonia better.
INTRODUCTION
SARS-CoV-2, which stands for severe acute respiratory syndrome coronavirus 2, was first identified in December 2019 in Wuhan, China. The coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 has rapidly spread from China to all around the world within a few months, leading the World Health Organization to declare it a pandemic on March 11, 2020[1].
The transmission of SARS-CoV-2 happens through direct, indirect or close contact with infected people through infected secretions, such as saliva and respiratory secretions or their respiratory droplets. The main organ affected is the lung, with pneumonia being the major manifestation of the infection[2].
The gold standard for SARS-CoV-2 diagnosis is real-time reverse transcription-polymerase chain reaction. However, computed tomography (CT) is recommended for initial evaluation and diagnosis, and it is also useful in monitoring the disease progression and evaluating the therapeutic efficacy[3,4].
Until now, many reports have focused on CT scan features at diagnosis[5-7]. On the other hand, there are relatively few studies evaluating serial temporal changes in patients who underwent repeated CT examinations and, particularly, in the late follow-up.
Our aim is to review the literature currently available on the radiological follow-up of COVID-19-related lung alterations using the CT scan to describe the evidence about the dynamic evolution of COVID-19 pneumonia.
MATERIALS AND METHODS
We conducted this systematic review according to the Preferred Reporting Items guidelines for Systematic Reviews and Meta-Analysis (PRISMA) Statement[8]. The primary aim was to collect, describe and discuss the dynamic radiological evolution of COVID-19 pneumonia.
Search strategy
Two authors (Casartelli C and Perrone F) carried out a comprehensive systematic search for published articles on the MEDLINE/PubMed library until October 31, 2020. Given the absence of articles on this topic before December 2019, when the first COVID-19 outbreak started, no upper limit for the search was chosen.
The following search keywords were used: “COVID-19” [all fields] AND “computed tomography” [all fields] AND “evolution” [all fields]. The reference lists of the included articles and reviews/meta-analyses on our research topic were also reviewed to identify additional relevant papers.
Study selection and eligibility criteria
Retrospective studies, prospective studies and case reports describing the evolution of COVID-19 pneumonia on CT scan were included. Only English language articles were considered eligible. Studies with insufficient radiological data were excluded. We planned qualitative analysis only, forecasting a high heterogeneity between the eligible studies, likely preventing quantitative analyses.
Data extraction and synthesis
The study characteristics (first author, year of publication, type of study, number of patients included, CT scan follow-up, dynamic evolution and main CT manifestations) were extracted from the included articles by a single author (Casartelli C). Two reviewers (Perrone F and Casartelli C) initially performed the data extraction, and then it was independently reviewed by an additional reviewer (Bersanelli M).
Any doubt or disagreement was discussed with a fourth investigator (Buti S) and resolved with all investigators’ consensus.
RESULTS
General description
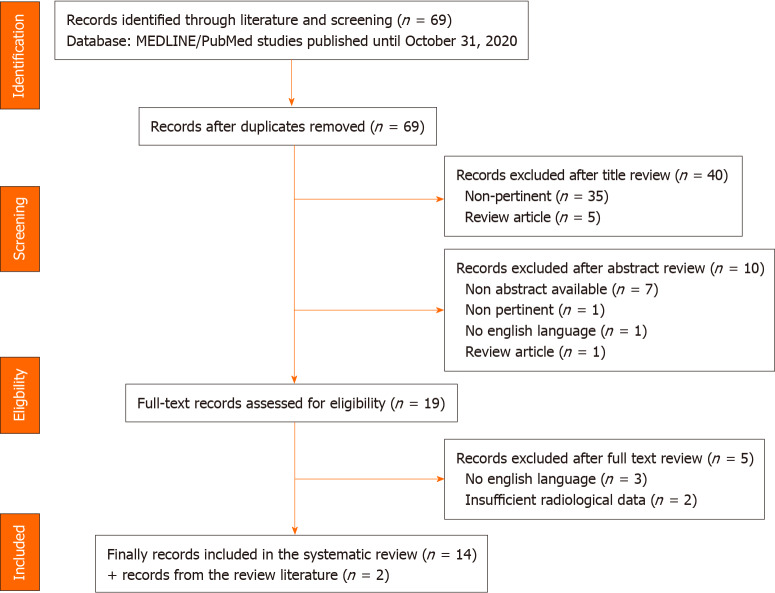
The study selection led to the inclusion of 16 reports: 13 retrospective studies[9-21], 1 prospective study[22] and 2 case series[23,24]. The outline of the search is reported in Figure 1.
Figure 1.
PRISMA flow diagram.
These reports (more specifically, 15 from China[9-23], 1 from Italy[24]) have analyzed several cases of pneumonia caused by SARS-CoV-2 diagnosed through CT without contrast (Table 1).
Table 1.
Characteristics and findings of the studies included in the systematic review
| Ref. | Type of study | Patients included | Mean age in yr, range | CT scan follow-up | CT evaluation, scoring system |
| Han et al[9], 2020 | Retrospective | 17 surviving and discharged patients with COVID-19 pneumonia | 40 ± 6 | 4 wk (4 weekly CT scan during hospitalization) | Semi-quantitative |
| Wang et al[10], 2020 | Retrospective | 63 patients with asymptomatic/mild, 378 with moderate, 43 with severe/critically COVID-19 pneumonia | 47 (33-57) | From symptoms onset to beyond day 15 | Quantitative |
| Liang et al[11], 2020 | Retrospective | 88 patients with mild COVID-19 pneumonia | 42.7 (4-82) | 3 wk after disease onset | Semi-quantitative |
| Sun et al[23], 2021 | Case series | 11 patients with severe COVID-19 pneumonia | 52 (33-75) | CT scan during hospitalization (not well defined, at least 3 wk during hospitalization) | Qualitative |
| Zhou et al[12], 2020 | Retrospective | 100 patients with COVID-19 pneumonia (without ARDS) | 52.3 ± 13.1 (27-80) | CT during hospitalization (from symptoms onset to beyond day 21) | Semi-quantitative |
| Wang et al[13], 2020 | Retrospective | 126 patients with COVID-19 pneumonia, (severe and critical cases excluded) | 41.2 ± 10.8 | CT scan during hospitalization (mean days of hospitalization 22 ± 5 d (12-40) | Qualitative |
| Wang et al[14], 2020 | Retrospective | 79 patients with non-severe (mild/common) COVID-19 pneumonia, 27 with severe pneumonia | 48.0 ± 15.4 | CT scan during hospitalization (mean days of hospitalization 25) + CT scan at 2-4 wk after discharge | Semi-quantitative |
| Zhang et al[15], 2020 | Retrospective | 33 patients with moderate COVID-19 pneumonia | 49.0 ± 15.5 | CT scan during hospitalization (mean days of hospitalization 20.8, range 18-37) | Semi-quantitative |
| Feng et al[16], 2020 | Retrospective | 19 patients with COVID-19 pneumonia | 43.6 ± 15.5 (10-67) | 0-34 d after symptoms onset | Quantitative |
| Liu et al[17], 2020 | Retrospective | 149 discharged patients with COVID-19 pneumonia (142 pneumonia, 7 severe pneumonia, no critical patients included) | 43 (36-56) | Basal CT scan at discharge and at 1st, 2nd and 3rd week after discharge | Semi-quantitative |
| Pan et al[18], 2020 | Retrospective | 105 patients with COVID-19 pneumonia (severe pneumonia excluded) | 48.6 ± 13.1 (23-72) | 1-47 d after symptoms onset | Semi-quantitative |
| Zhuang et al[19], 2021 | Retrospective | 22 patients with COVID-19 pneumonia with solitary pulmonary lesion | 40.7 ± 10.3 (23-54) | CT scan during hospitalization (mean days of hospitalization 19 d, range: 11-44) + first CT scan after discharge | Semi-quantitative |
| Urciuoli and Guerriero[24], 2020 | Case series | 6 patients with mild COVID-19 pneumonia | 59.5 | First CT on admission and 4 mo after symptoms onset | Qualitative |
| Zhang et al[20], 2020 | Retrospective | 53 patients with common COVID-19 pneumonia, 20 patients with severe COVID-19 pneumonia | 45 ± 14 common pneumonia, 50 ± 15 severe pneumonia | 0-30 d after symptoms onset | Quantitative |
| Pan et al[21], 2020 | Retrospective | 21 patients with COVID-19 pneumonia (severe pneumonia excluded) | 40 ± 9 (25-63) | 0-26 d after symptoms onset | Semi-quantitative |
| Wang et al[22], 2020 | Prospective | 90 patients with COVID-19 pneumonia | 45 ± 14 (5-43) | 0-24 d after symptoms onset | Semi-quantitative |
ARDS: Acute respiratory distress syndrome; COVID-19: Coronavirus disease 2019; CT: Computed tomography.
Most of the reports have considered moderate/common pneumonia; if pneumonia was not explicitly classified, most of the articles included patients with a good and defined prognosis, who were ultimately discharged from the hospital, while patients with severe/critical pneumonia were generally excluded.
Four studies have also included a minority group of patients showing severe/critical pneumonia[10,14,17,20]; the 11 patients described by Sun Q et al[23] case series had severe pneumonia[23].
Scoring system
The most common score used to evaluate dynamic CT evolution was a semi-quantitative scoring system, which considered the total area of involvement of the lesions. The nature of the semi-quantitative scoring system was similar in the studies considered, even with some adjustments and discrepancies among them.
For example, Liang et al[11] assigned a 0-4 score based on the percentage of each lung lobe involvement; in agreement with this, the overall lung total severity score was reached by summing up the five lobe scores, with a possible range from 0 to 20.
Zhou et al[12] divided each lung into six zones, and the total score, given by the sum of the different lung regions, could reach a maximum of 48.
Zhang et al[15] used yet another adaptation of the system based on the lung segments involved, assigning a score based on the percentage of ground glass opacities (GGOs) and consolidation, with a possible range from 0 to 36.
The study from Liu et al[17], analyzing the CT of discharged patients, focused the score on non-GGO lesions since extended GGO areas were defined as a basic manifestation of convalescence, which could lead to an overestimation of the CT score.
Other authors, considering the limited accuracy and sensitivity of the semi-quantitative score based mainly on visual evaluation, proposed evaluating dynamic evolution by quantitative techniques.
For example, Feng et al[16] measured the total volume (VT) and mean CT value (CT), and from these, they calculated the mass (m): VT × (CT + 1000)[16].
In the report from Wang et al[10], quantitative CT measurements of pulmonary opacities, including volume, density and location, were extracted through deep learning algorithms.
In another report, quantitative CT features were automatically calculated using intelligent artificial algorithms, giving back the percentage of GGO volume, consolidation volume and total lesion volume[15].
Other reports described the evolution of lung lesions qualitatively[13,23,24].
Radiological dynamic evolution: Severity and timing
Almost all the reports present a short-term radiological follow-up, focusing on the first few weeks from the symptoms appearance and studying serial CT scan approximately in the first 4 wk during hospitalization (Table 1).
It has been observed that the initial CT features and dynamic evolution of COVID-19 pneumonia have specific characteristics and regularity.
Several reports identify well-defined stages, from the onset of the symptoms to radiological recovery.
The most common pattern of radiographic evolution found is as follows. First, there is a progressive rapid radiographic deterioration, during which the lesions keep growing until they reach a peak; once this peak is reached, the lesions stop growing and are gradually reabsorbed and repairing signs appear. Almost all the studies found that the peak was reached roughly within 2 wk after the symptoms appearance, and after that lung abnormalities started to decrease.
There are some exceptions. Zhang et al[15] found an earlier peak, 8 d after symptoms onset, and lung lesions improved after 11 d. Wang et al[22] discovered a similar peak at around 6-11 d; in this case, though, a significant extent of lung lesions was found for longer times after the peak, showing a slower recovery.
Specific patterns of temporal evolution and relative peaks are shown in Table 2.
Table 2.
Computed tomography scan features of lung lesions according to the follow-up timing of coronavirus disease 2019 pneumonia
| Ref. | Short-term follow-up, dynamic evolution during hospitalization period: Severity and timing | Main CT features at short-term follow-up | Late follow- up, dynamic evolution after hospital discharge | Main CT features at late follow- up |
| Han et al[9], 2020 | Initial deterioration to a peak at the 2nd week followed by improvement in the 3rd and 4th week | GGO decreased from 1st week to 2nd week, then increased in 3 and 4. Consolidation and a mixed pattern noted in 2 wk. Crazy paving pattern had the highest frequency in 2nd week | N/A | N/A |
| Wang et al[10], 2020 | Severe/critically ill group: Opacity volume continued to increase beyond 15 d. Moderate group: Peak on days 13-15 (the opacity density began to drop from day 10 to day 12). Asymptomatic/mild group: Highest opacity volume on days 1-3 and almost resolved after 15 d | GGO in the early stages, followed by appearance of consolidations. In the severe/critically ill group: Decreasing trend of GGO, increasing trend of consolidation over time | N/A | N/A |
| Liang et al[11], 2020 | Total severity score showed an increasing trend in the first 2 wk, followed by a slight decrease in the 3rd week | GGO was the most common finding over time, consolidation decreased 2 wk after symptom onset. Reticulations and linear opacities and fibrosis became increasing prevalent later in the disease course | N/A | N/A |
| Sun et al[23], 2021 | Improvement in the first 3 wk after hospitalization | Decrease in consolidation and GGO overtime and appearance of fibrous-like stripes | N/A | N/A |
| Zhou et al[12], 2020 | 3 stages: Early rapid progressive stage (1-7 d from symptom onset); > advanced stage with peak levels of abnormalities on CT at 8-14 d; > improvement after 14 d (particularly, after 21 d the absorption was more obvious) | GGO, GGO + reticular pattern/consolidation in the rapid progressive stage. ↑ GGO + reticular pattern and consolidation in the advanced stage. ↓ GGO + reticular pattern and consolidation and ↑ subpleural line, bronchus distortion, and fibrotic strips in the absorption stage | N/A | N/A |
| Wang et al[13], 2020 | 3 stages: Progression process; > absorption process; > stage of discharge | ↑ GGO with consolidation (↑ crazy paving pattern, ↑ vascular thickening sign ↑ air bronchogram sign) in the progression process. Absorption of consolidation displayed as inhomogeneous partial GGOs with fibrosis shadows, occurrence of the fishing net on trees sign, ↑ fibrosis sign, ↑ subpleural line sign in the absorption process. Further absorption of GGOs, consolidation and fibrosis shadows and no appearance of new lesions in the stage of discharge | N/A | N/A |
| Wang et al[14], 2020 | Radiological aggravation (< 2 wk) and improvement (> 2 wk) | GGO decreased while mixed GGO and consolidation increased from 1 wk to 2 wk after onset; linear opacity increased from 2 wk to 3 wk after onset | 1-2 mo after symptom onset (median day 38): In 1/3 of cases complete absorption of lesions. Patients with more severe lesions at day 8-14 (> consolidations, CT score > 4, > 3 lobes involved) were more prone to have pulmonary residuals | Mainly linear opacities |
| Zhang et al[15], 2020 | 4 stages: Early stage (0-5 d); > peak stage (6-10 d); > absorption stage (11-15 d); > recovery stage (≥ 16 d) | Mainly GGO, (vascular thickening, bronchial wall thickening, and consolidation were also noted) in the early stage. ↑ GGO, vascular and bronchial thickening, and consolidation (mean peak at 8 d) in the peak stage. GGO and consolidation were predominantly present, with ↑ bronchial wall thickening and vascular thickening in the absorption stage. GGO and consolidation were partially absorbed, and bronchial wall thickening and vascular thickening ↓ (residual GGO and subpleural parenchymal bands) in the recovery stage | N/A | N/A |
| Feng et al[16], 2020 | 3 stages: Progressive stage (0-5 d); > peak stage (5-15 d). The greatest severity showed approximately 7-8 d from onset; > absorption stage (15-30 d) | GGO and interlobular/intralobular septal thickening were the most frequent CT manifestation | N/A | N/A |
| Liu et al[17], 2020 | N/A | N/A | At 3 wk follow up CT scan: Complete absorption of lesions in more than half of the patients | Gradually decrease of GGO and fibrous stripe (GGO during the first and fibrous stripe the 3rd week after discharge). “Tinted” sign and bronchovascular bundle distortion |
| Pan et al[18], 2020 | 5 stages: 0-3, 4-7, 8-14, 15-21, and > 21 d from symptoms onset (stages A-E, respectively). The total CT score of lung involvement was significantly higher in Stage C. The lung lesions in most patients improved after 14 d since initial symptom onset | Proportion of GGO was similar in each stage, consolidation gradually ↑ from Stage A to C and gradually ↓ from Stage C to E | N/A | N/A |
| Zhuang et al[19], 2021 | Lung involvement peak at approximately 11 d, then lung lesions improved significantly | Mainly GGO in the first scan (0-4 d), crazy-paving pattern and consolidation in scan-2 (4-22 d), lesions were gradually absorbed and tended to be stable and linear opacities were noted in the scan-3 (before discharge, 6-41 d) | 1st CT scan after discharge (22-51 d): Further absorption of lung lesions | Various presentations: negative CT scan, GGO, consolidation, linear opacities |
| Urciuoli and Guerriero[24], 2020 | N/A | N/A | Persistence of lung abnormalities in 5/6 cases even if all the patients completely asymptomatic | Various presentations: 1 negative CT scan; in 2 patients, persistence of mixed pattern (GGO and fibrous streaks); in 1 patient fibrotic stripes, in 1 patient mixed pattern (interlobular septal thickening and patchy GGO); in 1 patient fibrotic pattern |
| Zhang et al[20], 2020 | 5 stages: Stage 1 (0-3 d), stage 2 (4-7 d), stage 3 (8-14 d), stage 4 (15-21 d), and stage 5 (22-30 d). PTV peaks at 12 d in common pneumonia, at 17 d in severe pneumonia | Common pneumonia: No significant differences in the PTV, PGV and PCV between stages 1-4 (percent of lesions was reduced in stage 5 compared with stage 4). Severe pneumonia PTV, PGV and PCV ↑ from stage 2 to stage 4 and ↓ in stage 5 | N/A | N/A |
| Pan et al[21], 2020 | 4 stages: Early stage (0-4 d); progressive stage (5-8 d); peak stage (10-13 d); and absorption stage (≥ 14 d). Peak at 10 d after symptoms onset. CT signs improvement at approximately 14 d | GGO in the early stage, ↑ crazy-paving pattern and consolidation in the progressive stage, consolidation in the peak stage, progressive resolution of consolidation in the absorption stage | N/A | N/A |
| Wang et al[22], 2020 | Lung abnormalities increased quickly after the onset of symptoms, peaked around 6-11 d, and were followed by persistence of high levels in extent for a long duration (slow absorption of the lesions) | GGOs trend: “first falling then rising”. Consolidation was the second most common feature seen in the first 11 d. Mixed pattern: The second most predominant pattern since illness days 12-17 | N/A | N/A |
CT: Computer tomography; GGO: Ground glass opacity; N/A: Not applicable; PCV: Percentage of consolidation volume; PGV: Percentage of ground glass opacity volume; PTV: Percentage of total lesion volume.
When severe pneumonia was considered separately, the disease seemed to have a slightly longer evolution, showing the peak later than for moderate pneumonia cases.
In the report from Zhang et al[20], severe pneumonia exhibited a peak approximately 17 d after symptoms onset (compared to moderate pneumonia, which peaked at 12 d in the same study). In the report from Wang et al[10], the opacity volume kept increasing even after 15 d in the severe/critical group. Four reports had taken into account a longer CT follow-up, considering CT scan after discharge[14,17,19,24].
Zhuang et al[19] considered both CT during hospitalization and the first CT after discharge (22-51 d after symptoms onset). During the latter phase, further absorption of the lung lesions compared with the previous radiological exam was observed, but not all patients showed a complete resolution.
Liu et al[17] studied the radiological evolution during the first few weeks after discharge, in particular 1, 2 and 3 wk after discharge. The aim was to determine the cumulative percentage of complete radiological resolution at each time point. They discovered that lung lesions could be entirely absorbed with no sequelae, and they suggested that the optimal time point for an early radiological estimation might be 2 wk after discharge. In their analysis, the cumulative percentage of the complete radiological resolution was 8%, 42%, 50% and 53% at discharge and during the 1st, 2nd and 3rd week after discharge, respectively[17].
Wang et al[14] conducted a study including both common and severe pneumonia, showing that approximately 1/3 of cases had complete absorption of lesions in the first 1-2 mo after symptom onset (median day 38). In their study, patients with more severe lung involvement at days 8-14 (peak) were more prone to have pulmonary residuals.
Urciuoli and Guerriero[24] considered a longer follow-up, with the study of CT up to 4 mo after the onset of the symptoms; the sample of this report was relatively small, as it considered only 6 patients with mild pneumonia. Interestingly, the follow-up CT scan revealed the persistence of lung abnormalities in 5 cases out of 6, even if all patients were completely asymptomatic at that point[24].
CT scan features of lung lesions at follow-up
The main features of lung lesions in the retrieved reports were multiple, bilateral, with a peripheral subpleural distribution.
In the short-term follow-up some features recurred. Consolidations and GGOs were always described, and often a mixed pattern was noted. Consolidations were more frequent during the peak, sometimes with accompanying signs such as a “crazy paving pattern” or “vascular thickening sign;” after the peak, they were gradually absorbed.
GGOs were described mainly in the early phase, but they could be observed also in later stages. In fact, in the report from Pan et al[18] the proportion of GGOs was similar in each stage. In those from Wang et al[22], the observed trend of GGOs was described as “first falling then rising” as they were present both in the first phase and in the last CT scan.
After the peak, besides GGOs, repairing CT signs, such as linear opacities, fibrous stripes, subpleural line sign and fibrosis shadows, were noted. Wang et al[13] proposed, in the absorption process, a particular sign called “fishing net on trees.” This sign “indicated that the pulmonary lesions were in the stage of obvious absorption but not complete absorption. CT showed that the large area of consolidation was reduced, the density was reduced, the edge had shrunk, and there were significantly more bands and incomplete absorption of fibrosis shadows. The area was similar to a fishing net hanging on a branch that was not fully spread under the background of the increased bronchovascular bundle”[13].
In the longer-term follow-up, CT scans showed various presentations. Zhuang et al[19] observed in the first CT scan after discharge further absorption of the lung lesions. Also, GGOs, consolidations and linear opacities were still found in some patients. In the case series of Urciuoli and Guerriero[24], 2 patients presented persistence of a mixed pattern with GGO and fibrous streaks, 1 patient fibrotic stripes, 1 patient a mixed pattern with interlobular septal thickening and patchy GGOs and 1 patient fibrotic pattern[24].
Wang et al[22], who followed the CT scan until 4 wk after discharge, found mainly linear opacities. Liu et al[17] still observed in some patients GGOs and fibrous stripes even at the 3 wk radiological follow-up, even with a decreasing trend (GGO during the 1st week and fibrous stripes during the 3rd wk). Two additional signs were found during the evolution: “tinted” sign and bronchovascular bundle distortion. The “tinted” sign was demonstrated to coincide with an extension of the GGO area and a decrease in its density. According to the authors, the appearance of this pattern probably implied the gradual resolution of inflammation with re-expansion of alveoli. The bronchovascular bundle may be caused by inflammatory distraction or subsegmental atelectasis[17].
DISCUSSION
Current evidence of the temporal evolution of COVID-19 pneumonia derives from studies evaluating a relatively short follow-up period, and data about long-term radiological (and clinical) sequelae are still awaited[17,22,25,26]. The hallmark of early COVID-19 pneumonia includes bilateral, peripheral GGOs and consolidation often showing features resembling organizing pneumonia, such as a perilobular distribution and “reversed halo” sign (i.e. a focal, rounded area of ground-glass surrounded by a ring or arc of denser consolidation)[27,28]. These findings are non-specific and variably comprise foci of edema, organization and diffuse alveolar damage that are not too far removed from patients with other acute injuries, even noninfectious[29,30]. Notably, up to 56% of patients have been reported to demonstrate no abnormalities in the first 3 d after onset of symptoms, while conversely patients with no symptoms may show abnormal CT findings[31]. Moreover, still in the initial phase of the disease, pulmonary opacities may be unilateral and lack the characteristic peripheral distribution, possibly reducing diagnostic confidence in differentiating COVID-19 from potential mimickers such as heart failure and other infections[21,32].
The severity of acute COVID-19 manifestations is likely to peak within 2 wk from the disease onset, though reported temporal evolution varies depending on the studied population[12,13,18,21,31]. In this phase, patients may show an increasing extent of pulmonary consolidation, which parallels lung injury evolution. With the awareness of the heterogeneous studies included in the present analysis and intrinsic individual variation of the disease course, patients have been found to enter the so-called absorption stage roughly 14 d from the disease onset[12,13,18,21]. During this period, consolidation tends to wane, while other findings such as linear opacities, parenchymal bands and reticulation possibly emerge, sometimes leading to a “fibrotic-like” appearance[26]. Even in this last case, it remains unclear whether residual abnormalities truly represent irreversible disease or will solve over time as no studies with a follow-up period greater than 6 mo have been performed so far[26,33]. Remarkably, most studies examined CT patterns in isolation at various time points rather than temporal changes of each pulmonary finding, providing valuable information about the overall disease evolution but missing the opportunity to examine regional linkages between patterns. Future studies are needed to explore how underlying pathogenetic pathways such as diffuse alveolar damage and an auto-inflammatory response would determine imaging features of COVID-19. In this regard, the role of baseline risk factors such as vascular thrombosis and interstitial lung abnormalities remains poorly investigated.
Besides providing clues to assess COVID-19 morphological changes, CT has been used to enrich clinical and laboratory findings to quantify disease severity in the acute setting and longitudinal evolution[12,18,21]. Various methods have been employed to assess CT lung involvement in COVID-19, including qualitative, semi-quantitative and software-based quantitative scoring systems[12,18,21,34-37]. In the included works, most CT scores were based on semi-quantitative methods, while only two studies used artificial intelligence techniques. Several parameters such as symptoms, oxygenation status and laboratory measures of infection and inflammation have been found to correlate with parenchymal involvement at CT, highlighting the potential role of imaging in predicting the clinical course of COVID-19 and optimizing patient care[38-40]. However, further evidence is needed to demonstrate CT scoring usefulness to manage COVID-19 and its actual impact on clinical decision-making in the acute and follow-up setting.
Clinical compendium: Pulmonary sequelae of COVID-19
The clinical counterpart of long-term radiological outcomes of COVID-19 pneumonia is a topic of growing interest. After the first wave of COVID-19, the awareness of patients suffering from residual symptoms, persistent beyond the acute phase of the disease, became very common, leading to the description of a post-COVID syndrome or Long-COVID[41]. However, the type and severity of respiratory impairment or functional sequelae are still unknown.
The current knowledge gained from the previous coronavirus outbreaks (SARS-CoV-1 in 2002-2004 and Middle East respiratory syndrome coronavirus in 2012) and the general understanding about outcomes in the acute distress respiratory syndrome suggest that some COVID-19 survivors might experience impaired lung function and exercise limitation, and some of them develop interstitial lung disease in the mid-long term[42-44].
Up until recently, only a few retrospective studies, including small samples, showed that patients might experience a reduction of forced vital capacity (13 patients at 6 wk)[45] and of forced vital capacity, forced expiratory volume in the first second, total lung capacity (TLC) and diffusion lung carbon monoxide (DLCO) (55 patients at 3 mo)[46].
In one of the largest cohorts studied to date describing the medium-term consequences of the infection (767 patients, follow-up at median time of 81 d after discharge), 51.4% of the patients reported being still symptomatic, with fatigue (55.0%), exertional dyspnea (45.8%) and post-traumatic psychological consequences (30.5%) as the most reported symptoms. Impaired lung function was found in 19% of the patients (reduced DLCO with or without restrictive pattern)[47].
Anastasio et al[48] recently published a study on 379 patients evaluated 4 mo after the diagnosis of COVID-19. Almost 69% of the patients reported almost one residual symptom. Patients who had pneumonia showed lower SpO2 at rest and during the six-minute walking test and TLC compared with patients without prior pneumonia. Furthermore, the authors found an association between SpO2/FiO2 ratio and the pneumonia severity index during the acute phase, and mid-term alteration in SpO2 at rest and during six-minute walking test, TLC, residual volume and forced vital capacity[48].
In an Italian study with 238 patients enrolled, DLCO was reduced less than 80% of the predicted value in more than half of the patients at 4 mo follow-up, and in 15.5% of the cases were less than 60%. More than 50% of the patients showed functional impairment assessed with Short Physical Performance Battery and 2-minute walk test[49].
In another large cohort of 647 patients evaluated at 3 mo follow-up, patients reported ongoing symptoms, in particularly fatigue (13%), palpitation (10%) and dyspnea (9%). Those symptoms were significantly higher in patients who experienced severe COVID-19 compared to non-severe patients. In this cohort, only 81 patients were assessed with lung function test. More than half of the patients showed reduced DLCO. Similarly to symptoms, an impaired DLCO was more frequently associated with severe cases than non-severe (68% vs 42%). On a multivariate analysis, a CT total severity score > 10.5 and acute distress respiratory syndrome were significantly associate with impaired DLCO[50].
Similar results were found in a smaller cohort of 22 patients at 3 mo follow-up. Furthermore, on multivariate analysis, low TLC was associated with the need for mechanical ventilation and low forced expiratory volume in the first second with a high APACHE II score[51].
In a cohort of 119 patients who survived severe COVID-19 evaluated at 2 mo after discharge, respiratory symptoms (breathlessness 32%, cough 7%) were less frequent than persistent fatigue (68%), sleep disturbance (57%), anxiety and depression (22% and 18%, respectively) and post-traumatic stress disorder (25%). Despite radiological resolution in 87% of the patients, 41% reported persistent limitations in everyday life, and 44% had a Modified British Medical Research Council Questionnaire grade above the pre-COVID19 baseline[52]. A similar study on 134 patients found breathlessness as the most commonly reported symptoms (68%) followed by myalgia (51.5%), extreme fatigue (39.6%), low mood (37.3%) and sleep disturbance (35.1%)[53].
Long-term follow-up will help understand the impact of COVID-19 pneumonia on lung pathophysiology. Therefore, it is advisable to schedule serial follow-up in patients that still present lung function impairment or exercise limitation.
CONCLUSION
At present, the available literature focus on the acute phase of radiological follow-up of COVID-19 pneumonia and describes well-defined stages in the first few weeks after the onset of the symptoms.
The most common finding seems to be a peak of lung involvement reached roughly within the first 2 wk, characterized mainly by the growth of GGOs and consolidations. After that peak, these manifestations are gradually absorbed, and repairing signs, such as linear opacities, fibrous stripes, subpleural line sign and fibrosis shadows, tend to appear.
When considering later follow-up, up to 4 mo, lesions are usually not completely absorbed. A longer follow-up is definitely needed, especially to check whether the later signs are reversible and how they affect patients’ conditions. Following CT scan evolution over time could help physicians better understand the clinical impact of COVID-19 pneumonia and manage the possible sequelae.
ARTICLE HIGHLIGHTS
Research background
Pneumonia is the main manifestation of severe acute respiratory syndrome coronavirus 2 infection. Chest computed tomography is an effective way to detect and keep track of coronavirus disease 2019 pneumonia cases over time.
Research motivation
As of now, few studies evaluated serial computed tomography scan temporal changes during the course of severe acute respiratory syndrome coronavirus 2 pneumonia.
Research objectives
This systematic review describes the dynamic evolution of coronavirus disease 2019 pneumonia, considering the available literature on this topic.
Research methods
A systematic review according to PRISMA guidelines was performed. Pertinent keywords on PubMed were used.
Research results
Different and well-defined stages characterized the first few weeks after the onset of the symptoms.
Research conclusions
A peak of lung involvement within the first 2 wk, followed by the gradual absorption of the lesions and the advent of repairing signs was observed. Later follow-up showed that lesions were usually not completely absorbed, at least up to 4 mo.
Research perspectives
Longer follow-up is needed to check whether the later signs are reversible and how they affect patients’ conditions.
Footnotes
Conflict-of-interest statement: Bersanelli M received honoraria as a speaker at scientific events by Bristol-Myers Squibb (BMS), Novartis, Astra Zeneca, Pierre Fabre, and Pfizer and as a consultant for advisory role by Novartis, BMS, IPSEN, and Pfizer; she also received fees for copyright transfer by Sciclone Pharmaceuticals and research funding by Roche S.p.A., Seqirus UK, Pfizer, Novartis, BMS, Astra Zeneca, and Sanofi Genzyme. Buti S received honoraria as a speaker at scientific events and advisory role by Bristol-Myers Squibb (BMS), Pfizer; MSD, Ipsen, Roche, Eli-Lilly, AstraZeneca and Novartis; he also received research funding from Novartis. All the other authors declare they have no conflicts of interest to disclose.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised according to the PRISMA 2009 Checklist.
Manuscript source: Invited manuscript
Peer-review started: February 26, 2021
First decision: July 18, 2021
Article in press: August 13, 2021
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Braga MB S-Editor: Gao CC L-Editor: Filipodia P-Editor: Liu JH
Contributor Information
Chiara Casartelli, Medical Oncology Unit, University Hospital of Parma, Parma 43126, Italy; Department of Medicine and Surgery, University of Parma, Parma 43126, Italy.
Fabiana Perrone, Medical Oncology Unit, University Hospital of Parma, Parma 43126, Italy; Department of Medicine and Surgery, University of Parma, Parma 43126, Italy. fabiana.perrone89@libero.it.
Maurizio Balbi, Division of Radiology, University of Parma, Parma 43126, Italy.
Veronica Alfieri, Department of Medicine and Surgery, Respiratory Disease and Lung Function Unit, University of Parma, Parma 43126, Italy.
Gianluca Milanese, Division of Radiology, University of Parma, Parma 43126, Italy.
Sebastiano Buti, Medical Oncology Unit, University Hospital of Parma, Parma 43126, Italy.
Mario Silva, Division of Radiology, University of Parma, Parma 43126, Italy.
Nicola Sverzellati, Department of Medicine and Surgery, University of Parma, Parma 43126, Italy; Division of Radiology, University of Parma, Parma 43126, Italy.
Melissa Bersanelli, Medical Oncology Unit, University Hospital of Parma, Parma 43126, Italy; Department of Medicine and Surgery, University of Parma, Parma 43126, Italy.
References
- 1.World Health Organization. WHO Director‐General's remarks at the media briefing on 2019-nCoV on 11 February 2020. [cited 11 February 2020] In: World Health Organization [Internet]. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020 .
- 2.World Health Organization. Transmission of SARS-CoV-2: implications for infection prevention precautions. [cited 11 February 2020] In: World Health Organization [Internet]. Available from: https://www.who.int/news-room/commentaries/detail/transmission-of-sars-cov-2-implications-for-infection-prevention-precautions .
- 3.World Health Organization. Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). [cited 11 February 2020] In: World Health Organization [Internet]. Available from: https://www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf .
- 4.Qiu T, Liang S, Dabbous M, Wang Y, Han R, Toumi M. Chinese guidelines related to novel coronavirus pneumonia. J Mark Access Health Policy. 2020;8:1818446. doi: 10.1080/20016689.2020.1818446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, Fan Y, Zheng C. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu X, Yu C, Qu J, Zhang L, Jiang S, Huang D, Chen B, Zhang Z, Guan W, Ling Z, Jiang R, Hu T, Ding Y, Lin L, Gan Q, Luo L, Tang X, Liu J. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2. Eur J Nucl Med Mol Imaging. 2020;47:1275–1280. doi: 10.1007/s00259-020-04735-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C. The Clinical and Chest CT Features Associated With Severe and Critical COVID-19 Pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kristjansson M, Bieluch VM, Byeff PD. Mycobacterium haemophilum infection in immunocompromised patients: case report and review of the literature. Rev Infect Dis. 1991;13:906–910. doi: 10.1093/clinids/13.5.906. [DOI] [PubMed] [Google Scholar]
- 9.Han X, Cao Y, Jiang N, Chen Y, Alwalid O, Zhang X, Gu J, Dai M, Liu J, Zhu W, Zheng C, Shi H. Novel Coronavirus Disease 2019 (COVID-19) Pneumonia Progression Course in 17 Discharged Patients: Comparison of Clinical and Thin-Section Computed Tomography Features During Recovery. Clin Infect Dis. 2020;71:723–731. doi: 10.1093/cid/ciaa271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang YC, Luo H, Liu S, Huang S, Zhou Z, Yu Q, Zhang S, Zhao Z, Yu Y, Yang Y, Wang D, Ju S. Dynamic evolution of COVID-19 on chest computed tomography: experience from Jiangsu Province of China. Eur Radiol. 2020;30:6194–6203. doi: 10.1007/s00330-020-06976-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang T, Liu Z, Wu CC, Jin C, Zhao H, Wang Y, Wang Z, Li F, Zhou J, Cai S, Liang Y, Zhou H, Wang X, Ren Z, Yang J. Evolution of CT findings in patients with mild COVID-19 pneumonia. Eur Radiol. 2020;30:4865–4873. doi: 10.1007/s00330-020-06823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou S, Zhu T, Wang Y, Xia L. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan, China. Eur Radiol. 2020;30:5446–5454. doi: 10.1007/s00330-020-06879-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang C, Shi B, Wei C, Ding H, Gu J, Dong J. Initial CT features and dynamic evolution of early-stage patients with COVID-19. Radiol Infect Dis. 2020;7:195–203. doi: 10.1016/j.jrid.2020.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Jin C, Wu CC, Zhao H, Liang T, Liu Z, Jian Z, Li R, Wang Z, Li F, Zhou J, Cai S, Liu Y, Li H, Liang Y, Tian C, Yang J. Organizing pneumonia of COVID-19: Time-dependent evolution and outcome in CT findings. PLoS One. 2020;15:e0240347. doi: 10.1371/journal.pone.0240347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Liu X, Yu P, Cheng M, Wang W, Sun Y, Zeng B, Fan B. Dynamic CT assessment of disease change and prognosis of patients with moderate COVID-19 pneumonia. J Xray Sci Technol. 2020;28:851–861. doi: 10.3233/XST-200711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feng X, Ding X, Zhang F. Dynamic evolution of lung abnormalities evaluated by quantitative CT techniques in patients with COVID-19 infection. Epidemiol Infect. 2020;148:e136. doi: 10.1017/S0950268820001508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu D, Zhang W, Pan F, Li L, Yang L, Zheng D, Wang J, Liang B. The pulmonary sequalae in discharged patients with COVID-19: a short-term observational study. Respir Res. 2020;21:125. doi: 10.1186/s12931-020-01385-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pan Y, Xia L, Wang Y, Guan H. Dynamic changes in computed tomography manifestations of 105 patients with novel coronavirus pneumonia in Wuhan, China. J Int Med Res. 2020;48:300060520972913. doi: 10.1177/0300060520972913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhuang Y, Lin L, Xu X, Xia T, Yu H, Fu G, Yang Y, Wang M, Sun H. Dynamic changes on chest CT of COVID-19 patients with solitary pulmonary lesion in initial CT. Jpn J Radiol. 2021;39:32–39. doi: 10.1007/s11604-020-01037-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Y, Liu Y, Gong H, Wu L. Quantitative lung lesion features and temporal changes on chest CT in patients with common and severe SARS-CoV-2 pneumonia. PLoS One. 2020;15:e0236858. doi: 10.1371/journal.pone.0236858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19) Radiology. 2020;295:715–721. doi: 10.1148/radiol.2020200370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Dong C, Hu Y, Li C, Ren Q, Zhang X, Shi H, Zhou M. Temporal Changes of CT Findings in 90 Patients with COVID-19 Pneumonia: A Longitudinal Study. Radiology. 2020;296:E55–E64. doi: 10.1148/radiol.2020200843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun Q, Li Q, Gao F, Li J, Xu X, Huang X. Evolution of computed tomography manifestations of eleven patients with severe coronavirus disease 2019 (COVID-19) pneumonia. Int J Clin Pract. 2021;75:e13654. doi: 10.1111/ijcp.13654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Urciuoli L, Guerriero E. Chest CT Findings after 4 Months from the Onset of COVID-19 Pneumonia: A Case Series. Diagnostics (Basel) 2020;10 doi: 10.3390/diagnostics10110899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guler SA, Ebner L, Aubry-Beigelman C, Bridevaux PO, Brutsche M, Clarenbach C, Garzoni C, Geiser TK, Lenoir A, Mancinetti M, Naccini B, Ott SR, Piquilloud L, Prella M, Que YA, Soccal PM, von Garnier C, Funke-Chambour M. Pulmonary function and radiological features 4 mo after COVID-19: first results from the national prospective observational Swiss COVID-19 Lung study. Eur Respir J. 2021;57 doi: 10.1183/13993003.03690-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, Li Y, Cao Y, Gu J, Wu H, Shi H. Six-month Follow-up Chest CT Findings after Severe COVID-19 Pneumonia. Radiology. 2021;299:E177–E186. doi: 10.1148/radiol.2021203153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, Cui J, Xu W, Yang Y, Fayad ZA, Jacobi A, Li K, Li S, Shan H. CT Imaging Features of 2019 Novel Coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological Society of North America Expert Consensus Statement on Reporting Chest CT Findings Related to COVID-19. Endorsed by the Society of Thoracic Radiology, the American College of Radiology, and RSNA - Secondary Publication. J Thorac Imaging. 2020;35:219–227. doi: 10.1097/RTI.0000000000000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, Rech R, Colombo R, Antinori S, Corbellino M, Galli M, Catena E, Tosoni A, Gianatti A, Nebuloni M. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis. 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vasquez-Bonilla WO, Orozco R, Argueta V, Sierra M, Zambrano LI, Muñoz-Lara F, López-Molina DS, Arteaga-Livias K, Grimes Z, Bryce C, Paniz-Mondolfi A, Rodríguez-Morales AJ. A review of the main histopathological findings in coronavirus disease 2019. Hum Pathol. 2020;105:74–83. doi: 10.1016/j.humpath.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. doi: 10.1148/radiol.2020200463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parekh M, Donuru A, Balasubramanya R, Kapur S. Review of the Chest CT Differential Diagnosis of Ground-Glass Opacities in the COVID Era. Radiology. 2020;297:E289–E302. doi: 10.1148/radiol.2020202504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wells AU, Devaraj A, Desai SR. Interstitial Lung Disease after COVID-19 Infection: A Catalog of Uncertainties. Radiology. 2021;299:E216–E218. doi: 10.1148/radiol.2021204482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li K, Fang Y, Li W, Pan C, Qin P, Zhong Y, Liu X, Huang M, Liao Y, Li S. CT image visual quantitative evaluation and clinical classification of coronavirus disease (COVID-19) Eur Radiol. 2020;30:4407–4416. doi: 10.1007/s00330-020-06817-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin X, Min X, Nan Y, Feng Z, Li B, Cai W, Xi X, Wang L. Assessment of the Severity of Coronavirus Disease: Quantitative Computed Tomography Parameters vs Semiquantitative Visual Score. Korean J Radiol. 2020;21:998–1006. doi: 10.3348/kjr.2020.0423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pu J, Leader JK, Bandos A, Ke S, Wang J, Shi J, Du P, Guo Y, Wenzel SE, Fuhrman CR, Wilson DO, Sciurba FC, Jin C. Automated quantification of COVID-19 severity and progression using chest CT images. Eur Radiol. 2021;31:436–446. doi: 10.1007/s00330-020-07156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lassau N, Ammari S, Chouzenoux E, Gortais H, Herent P, Devilder M, Soliman S, Meyrignac O, Talabard MP, Lamarque JP, Dubois R, Loiseau N, Trichelair P, Bendjebbar E, Garcia G, Balleyguier C, Merad M, Stoclin A, Jegou S, Griscelli F, Tetelboum N, Li Y, Verma S, Terris M, Dardouri T, Gupta K, Neacsu A, Chemouni F, Sefta M, Jehanno P, Bousaid I, Boursin Y, Planchet E, Azoulay M, Dachary J, Brulport F, Gonzalez A, Dehaene O, Schiratti JB, Schutte K, Pesquet JC, Talbot H, Pronier E, Wainrib G, Clozel T, Barlesi F, Bellin MF, Blum MGB. Integrating deep learning CT-scan model, biological and clinical variables to predict severity of COVID-19 patients. Nat Commun. 2021;12:634. doi: 10.1038/s41467-020-20657-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214:1072–1077. doi: 10.2214/AJR.20.22976. [DOI] [PubMed] [Google Scholar]
- 39.Leonardi A, Scipione R, Alfieri G, Petrillo R, Dolciami M, Ciccarelli F, Perotti S, Cartocci G, Scala A, Imperiale C, Iafrate F, Francone M, Catalano C, Ricci P. Role of computed tomography in predicting critical disease in patients with covid-19 pneumonia: A retrospective study using a semiautomatic quantitative method. Eur J Radiol. 2020;130:109202. doi: 10.1016/j.ejrad.2020.109202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanza E, Muglia R, Bolengo I, Santonocito OG, Lisi C, Angelotti G, Morandini P, Savevski V, Politi LS, Balzarini L. Quantitative chest CT analysis in COVID-19 to predict the need for oxygenation support and intubation. Eur Radiol. 2020;30:6770–6778. doi: 10.1007/s00330-020-07013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mahase E. Covid-19: What do we know about "long covid"? BMJ. 2020;370:m2815. doi: 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- 42.Hui DS, Joynt GM, Wong KT, Gomersall CD, Li TS, Antonio G, Ko FW, Chan MC, Chan DP, Tong MW, Rainer TH, Ahuja AT, Cockram CS, Sung JJ. Impact of severe acute respiratory syndrome (SARS) on pulmonary function, functional capacity and quality of life in a cohort of survivors. Thorax. 2005;60:401–409. doi: 10.1136/thx.2004.030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, Leung CB, Rainer TH, Cameron P, Chung SS, Sung JJ, Ahuja AT. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. 2003;228:810–815. doi: 10.1148/radiol.2283030726. [DOI] [PubMed] [Google Scholar]
- 44.Herridge MS, Tansey CM, Matté A, Tomlinson G, Diaz-Granados N, Cooper A, Guest CB, Mazer CD, Mehta S, Stewart TE, Kudlow P, Cook D, Slutsky AS, Cheung AM Canadian Critical Care Trials Group. Functional disability 5 years after acute respiratory distress syndrome. N Engl J Med. 2011;364:1293–1304. doi: 10.1056/NEJMoa1011802. [DOI] [PubMed] [Google Scholar]
- 45.Fumagalli A, Misuraca C, Bianchi A, Borsa N, Limonta S, Maggiolini S, Bonardi DR, Corsonello A, Di Rosa M, Soraci L, Lattanzio F, Colombo D. Pulmonary function in patients surviving to COVID-19 pneumonia. Infection. 2021;49:153–157. doi: 10.1007/s15010-020-01474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao YM, Shang YM, Song WB, Li QQ, Xie H, Xu QF, Jia JL, Li LM, Mao HL, Zhou XM, Luo H, Gao YF, Xu AG. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Venturelli S, Benatti SV, Casati M, Binda F, Zuglian G, Imeri G, Conti C, Biffi AM, Spada MS, Bondi E, Camera G, Severgnini R, Giammarresi A, Marinaro C, Rossini A, Bonaffini PA, Guerra G, Bellasi A, Cesa S, Rizzi M. Surviving COVID-19 in Bergamo province: a post-acute outpatient re-evaluation. Epidemiol Infect. 2021;149:e32. doi: 10.1017/S0950268821000145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anastasio F, Barbuto S, Scarnecchia E, Cosma P, Fugagnoli A, Rossi G, Parravicini M, Parravicini P. Medium-term impact of COVID-19 on pulmonary function, functional capacity and quality of life. Eur Respir J. 2021 doi: 10.1183/13993003.04015-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, Baldon G, Bartolomei G, Battaglia M, Battistini S, Binda V, Borg M, Cantaluppi V, Castello LM, Clivati E, Cisari C, Costanzo M, Croce A, Cuneo D, De Benedittis C, De Vecchi S, Feggi A, Gai M, Gambaro E, Gattoni E, Gramaglia C, Grisafi L, Guerriero C, Hayden E, Jona A, Invernizzi M, Lorenzini L, Loreti L, Martelli M, Marzullo P, Matino E, Panero A, Parachini E, Patrucco F, Patti G, Pirovano A, Prosperini P, Quaglino R, Rigamonti C, Sainaghi PP, Vecchi C, Zecca E, Pirisi M. Respiratory and Psychophysical Sequelae Among Patients With COVID-19 Four Months After Hospital Discharge. JAMA Netw Open. 2021;4:e2036142. doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qin W, Chen S, Zhang Y, Dong F, Zhang Z, Hu B, Zhu Z, Li F, Wang X, Wang Y, Zhen K, Wang J, Wan Y, Li H, Elalamy I, Li C, Zhai Z, Wang C. Diffusion capacity abnormalities for carbon monoxide in patients with COVID-19 at 3-month follow-up. Eur Respir J. 2021;58 doi: 10.1183/13993003.03677-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Truffaut L, Demey L, Bruyneel AV, Roman A, Alard S, De Vos N, Bruyneel M. Post-discharge critical COVID-19 Lung function related to severity of radiologic lung involvement at admission. Respir Res. 2021;22:29. doi: 10.1186/s12931-021-01625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.D'Cruz RF, Waller MD, Perrin F, Periselneris J, Norton S, Smith LJ, Patrick T, Walder D, Heitmann A, Lee K, Madula R, McNulty W, Macedo P, Lyall R, Warwick G, Galloway JB, Birring SS, Patel A, Patel I, Jolley CJ. Chest radiography is a poor predictor of respiratory symptoms and functional impairment in survivors of severe COVID-19 pneumonia. ERJ Open Res. 2021;7 doi: 10.1183/23120541.00655-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sykes DL, Holdsworth L, Jawad N, Gunasekera P, Morice AH, Crooks MG. Post-COVID-19 Symptom Burden: What is Long-COVID and How Should We Manage It? Lung. 2021;199:113–119. doi: 10.1007/s00408-021-00423-z. [DOI] [PMC free article] [PubMed] [Google Scholar]