Highlights
-
•
Use of pineapple and sacha inchi wastes in biotechnological processes.
-
•
Valorization of agroindustrial waste in the context of circular economy.
-
•
Use of alternative fermentation substrates (SFS) in the production of probiotics (Weissella cibaria), in order to substitute conventional substrates.
-
•
Optimal conditions of the fermentation process for the reproduction and viability of W. cibaria.
Keywords: Weissella cibaria, Biomass, Ananas comosus, Plukenetia volubilis, Viability
Abstract
Agroindustrial wastes contain macronutrients and micronutrients essential for the reproduction of lactic acid bacteria. In this research, the reproduction of Weissella cibaria was experimentally optimized in a supplemented fermentation substrate (SFS) formulated from pineapple and sacha inchi wastes. Response surface methodology was used to evaluate the influence of the following independent variables: temperature (32–40 °C), pH (5.0–6.0), and stirring speed (SS) (100–150 rpm) on the following dependent variables: viability (Log10 CFU mL−1), biomass production (BWc), lactic acid production (LA), biomass yield (YBwc/S), biomass volumetric productivity (VPWc), LA volumetric productivity (VPLA), carbon source consumption (CSC), N2 consumption (N2C), and specific growth rate (µ). The experimental optimization of multiple responses presented a desirability of 76.8%, thus defining the independent variables of the process: temperature = 35.1 °C, pH = 5.0, and SS = 139.3 rpm; and the dependent variables: viability = 10.01 Log10 CFU mL−1, BWc = 2.9 g L−1, LA = 19.4 g mL−1, YBwc/S = 43.9 g biomass/g CSC, VPWc = 0.49 g L−1h − 1, VPLA = 3.2 g L−1 h−1, CSC = 17.2%, N2C = 63.6% and µ = 0.28 h−1. From these, viability, YBwc/S, CSC, N2C, and LA presented significant statistical differences, while the independent variable with the least important effect on the process was pH. Under optimal conditions of temperature, pH and SS; SFS favors the reproduction and viability of W. cibaria. This provides evidence of a sustainable alternative for the production of probiotics in the context of circular economy.
Abreviations
- Name
Abbreviature
- Biomass W. cibaria
BWc
- Biomass Yield
YX/S
- Colony Forming Units
CFU
- Carbon Sourse Consumption
CSC
- Fermentation time
t
- Free Amino Nitrogen
FAN
- Lactic acid
LA
- Lactic Acid Bacteria
LAB
- Man Rogosa Sharpe
MRS
- Maximum biomass production
MBWc
- Nitrogen Consumption
N2C
- Reducing sugar concentrations
S0 - S
- Regression coefficient - linear model
R2order1
- Regression coefficient - logistic equation
R2LE
- Relative mean error
RME
- Specific growth Speed
µ
- Specific growth rate– linear model
μ1 (h−1)
- Specific growth rate– logistic equation
μ2 (h−1)
- Stirring Speed
SS
- Supplemented fermentation substrate
SFS
- Temperature
T
- Volumetric Productivity of lactic acid
VPLA
- Volumetric Productivity of W. cibaria
VPWc
- Working volume in the reactor
VT
1. Introduction
Agroindustrial wastes are complex and easily decomposed structures; therefore, its accumulation generates environmental problems [1,2]. In 2017, global waste from the food industry exceeded 1300 million tons [3]. However, these residues are important sources of micro and macronutrients (minerals, vitamins, carbohydrates, lipids, and proteins) [2,4] with great potential for recovery of bioactive compounds through biotechnological processes (hydrolysis, fermentation, extraction, concentration, purification, biocatalysis, among others) [5], [6], [7]. In agroindustrial waste, the high concentration of C and N2 stands out, which determines its potential use as a substrate for fermentation. Elements such as C and N2 are essential nutrients in the production of organic acids, bioactive peptides, bacteriocins, and probiotic reproduction [6,[8], [9], [10]].
In fermentation processes, commercial substrates represent between 40 and 70% of the costs of the process, and some research highlights the challenges of more profitable and sustainable processes using agroindustrial waste [11]. In addition, the composition of the substrate and the process conditions are factors that favor a greater or lesser production of cellular biomass or other secondary metabolites with wide application in the food industry (organic acids, bacteriocins, exopolysaccharides) [12], [13], [14].
Agroindustrial wastes have been evaluated as fermentation substrates mainly for the production of lactic acid (LA) because it is widely used in the food, chemical, medical and pharmaceutical industries [9,15]. In terms of LA productivity, some authors report favorable results when conventional fermentation substrates are replaced by substrates formulated with organic C and N2 sources. In their review, Ahmad et al. [11], highlighted the use of various organic substrates and food residues for the production of LA, such as cassava bagasse (2.74 g L−1 h−1), apple pulp (5.41 g L−1 h−1), and xylose fermented corn liquor (6.15 g L−1 h−1). For the production of bacterial biomass, the commercial substrate (MRS) has been principally used [16]. This type of substrate favors the metabolic efficiency represented by the specific growth rate, a property that determines the speed of reproduction and increase of cellular biomass in the fermentation kinetics. However, the high cost of commercial substrates reduces the profitability of the biotechnological process [17].
On the other hand, the circular economy is a production and consumption model that involves minimizing the waste generated in production, generating value-added products with said waste [3]. Therefore, the use of agroindustrial waste as fermentation substrates has been relevant in the context of circular economy [15,18,19].
One of the largest waste-generating agrochains is pineapple because most of the primary production is destined to the generation of products such as juices, concentrates, jams, salads, preserves, jellies, cakes, among others [20,21]. The aforementioned process generates production residues that represent 50% of the total weight: crown, epicarp, and cores [21,22]. These wastes are characterized by their high fiber and sugar content, as well as their minerals and to a lesser contain vitamins [23]. Similarly, the sacha inchi seed oil extraction industry generates a cake byproduct that represents between 60 and 75% of the total weight of the seed. It is also characterized as an important source of protein (> 50%) [24], in addition to carbohydrates, fats, fibers, and minerals [25,26]. In this sense, pineapple and sacha inchi wastes could be used as fermentation substrates, and they would have environmental impacts and positive contributions to the circular economy model in the pineapple production chain [3,18,27].
Lactic acid bacteria (LAB) are mostly represented within the genera Lactobacillus, Bifidobacterium, Streptococcus, and Weissella [28]. One of the most recent genera is the genus Weissella, highlighting the Weissella cibaria species. This LAB is of great interest for the following reasons: (i) its high capacity to produce exopolysaccharides with applications in the food industry [14,16]; (ii) its high potential to reduce cholesterol [29]; (iii) its antimicrobial activity against Gram positive bacteria (Staphylococcus aureus, Streptococcus agalactiae, Listeria monocytogenes) and Gram negative bacteria (Escherichia coli and Klebsiella pneumoniae) [30,31]; (iv) its excellent adaptation to low pH environments, due to its ability to survive in the gastrointestinal tract [14,16]; and (v) its high adhesion capacity to intestinal epithelial cells [14,32].
In previous studies by Micanquer-Carlosama et al. [22], the use of both pineapple epicarps and cores and the sacha inchi cake is reported as sources of C and N2 in the formulation of a fermentation substrate used for the reproduction of Weissella cibaria. In this context, the objective of this research was to optimize the reproduction of probiotic (W. cibaria) as a function of the independent variables pH, temperature (T) and stirring speed (SS), using the substrate formulated in previous studies from the pineapple wastes and the sacha inchi cake.
2. Materials and methods
2.1. Fermentation substrate
A supplemented fermentation substrate (SFS) was formulated from a mixture of powdered residues. The total formulation included epicarps and cores of gold honey variety pineapple (Ananas comosus) (41.33%), the by-product of the sacha inchi oil extraction process (Plukenetia volubilis) (57.95%), and mineral salts (C2H3NaO2 = 5.0%, C6H5O7×2NH3 = 2.0%, K2HPO4 = 2.0% and MgSO4 = 0.2%), which were obtained from previous research by Micanquer-Carlosama et al. [22].
2.2. Activation and reproduction of W. cibaria in the SFS
A W. cibaria strain from the Strain Bank of the Biotechnology Institute of the National University of Colombia (IBUN Strain and Genes Bank 090 – 03,684; AN: KU132362) was used. The purity of W. cibaria in the strain bank is confirmed by the following tests: ability to assimilate aniline blue, morphology by Gram stain, biochemical confirmation by API 50 CHL kit and effectiveness in antimicrobial activity. The bacteria was activated following the methodology described by Micanquer-Carlosama et al. [22] and its reproduction in the SFS was optimized by evaluating the process conditions (pH, T, and SS). The optimization was performed through discontinuous fermentation in 600 mL batches, using a 1 L capacity reactor (BioFlo® / CelliGen® 115, Germany).
2.3. Fermentation kinetic parameters with W. cibaria
20 mL of fermentation product were taken at 0, 2, 4, 6, 8, 10, 12, and 24 h (0 corresponded to the initial conditions of the substrate). Viability (CFU mL−1) was measured by plating (36 °C for 48 h) [31] 1 mL of the fermentation product. The remaining volume was centrifuged at 4508 g for 10 min at 4 °C (Penendorf centrifuge 5804 R, Germany), and the supernatant was separated from the precipitate. The supernatant was filtered (0.45 μm cellulose filter) and used to measure: concentration of reducing sugars (g L−1) by the 3,5-dinitrosalicylic acid methodology (Miller, 1959). The N2 concentration and LA production (g L−1) were measured by the method of free amino nitrogen (FAN) (g L−1) Eq. (1)) and by reflectometry (Reflectoquant Merck - RQflex Plus 10, Germany), respectively [33]. The precipitate was used to determine biomass production of W. cibaria (BWc), which was expressed as g of dry biomass (105 °C, time 5 h) per L of fermented sample (g L−1). Biomass yield (YBwc/S) (g dry biomass / g CSC), volumetric biomass productivity (VPWc) (g L−1 h−1), carbon source consumption (CSC) (%), N2 source consumption (N2C) (%), and volumetric productivity of LA (VPLA) (g L−1 h−1) were calculated using Eqs. (2), ((3), (4), (5), and (6), respectively.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
| (6) |
Where, BWc0 and BWc are the dry biomasses, S0 and S are the reducing sugar concentrations, and FAN0 and FAN are the free nitrogen concentrations at the initial time and at each sampling time, respectively. MBWc: maximum biomass production. VT: working volume in the reactor. t: fermentation time.
2.4. Experimental design and statistical analysis
Response surface methodology was used with a central composite design – centered face (α = 1) (15 experiments), depending on the following independent variables: pH (5.0–6.0), T (32–40 °C), and SS (100–150 rpm); and the dependent variables: viability, BWc, YBwc/S, VPWc, CSC, N2C, µ, LA, and VPLA. The results were analyzed using the analysis of variance (ANOVA) with a significance level of 5% obtained from the Statgraphics software (version XVII. II). The experimental data of the dependent variables are reported as the mean value ± standard deviation. These were obtained from triplicate measurements for each experiment and adjusted to a 2nd order polynomial model (Eq. (7)); where, Y are the dependent variables, β0 is a constant; βi βii βij, correspond to the regression coefficients, and Xi and Xj represent the independent variables.
| (7) |
An experimental optimization of multiple responses to the fermentation process was carried out. The analysis considered the results of the ANOVA and criteria, weights, and impacts of the dependent variables. These optimum parameters favored the production and viability of W. cibaria biomass, and the relative mean error (RME) was used to assess the accuracy of the mathematical model.
2.5. Specific growth rate of W. cibaria
The specific growth rate (μ) (h−1) was calculated for each of the design experiments, considering the exponential phase (time: 0 → 10 h). This parameter was determined using a model of order 1 (Eq. (8)) (Specific growth rate– linear model → μ1 h−1) and the model adjusted by the logistic equation (Eq. (9)) (Specific growth rate– logistic equation → μ2 h−1) [17]. These values were compared with the experimental value obtained at the optimal condition.
| (8) |
| (9) |
2.6. Morphology of W. cibaria biomass
The morphological characterization of W. cibaria biomass was performed by means optical microscopy (Leica ICC50 W, Switzerland) for wet biomass with Gram staining and in Scanning Electron Microscope (JSM-5910LV. JEOL) for dry biomass [34]. The biomass was obtained as a product of the fermentation process with the optimal experimental condition, using a supplemented fermentation substrate (SFS).
3. Results and discussion
3.1. Fermentation kinetic parameters with W. cibaria
Table 1 shows the dependent variables (viability, BWc, YBwc/S, VPWc, CSC, N2C, LA and VPLA) as a function of the independent variables (pH, T, and SS) during the fermentation kinetics of W. cibaria. Table 2 presents the p-values of the dependent variables produced by the response surface methodology. Fig. 1, Fig. 2, Fig. 3, Fig. 4 show the surface and volume response graphs of viability, BWc, YBwc/S, CSC, N2C and LA respectively with respect to the effects of the independent variables.
Table 1.
Fermentation kinetic parameters of W. cibaria in substrate formulated with agroindustrial wastes (parameters measured at 10 h of fermentation).
|
Dependent Variables |
Independent Variables | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Weissella cibaria | LA | ||||||||||
| Run | pH | T ( °C) | SS (rpm) | Viability (Log10 CFU mL−1) | BWc (g L−1) | YBwc/S | VPWc (g L−1 h−1) | CSC (%) | N2C (%) | LA (g L−1) | VPLA (g L−1 h−1) |
| 1 | 5.0 | 32 | 100 | 9.49 ± 0.04 | 1.99 ± 0.09 | 66.52 | 0.33 | 2.20 ± 0.14 | 72.97 ± 1.35 | 14.40 ± 0.56 | 2.40 |
| 2 | 6.0 | 32 | 150 | 9.88 ± 0.01 | 2.10 ± 0.07 | 26.11 | 0.35 | 9.77 ± 0.16 | 51.35 ± 0.84 | 22.80 ± 0.56 | 3.80 |
| 3 | 6.0 | 40 | 100 | 9.71 ± 0.04 | 2.25 ± 0.05 | 57.50 | 0.37 | 3.31 ± 0.05 | 61.11 ± 1.39 | 23.40 ± 0.46 | 3.90 |
| 4 | 5.5 | 40 | 125 | 9.54 ± 0.03 | 2.65 ± 0.07 | 27.40 | 0.44 | 7.28 ± 0.18 | 67.57 ± 0.77 | 20.60 ± 0.98 | 3.43 |
| 5 | 5.5 | 36 | 125 | 9.72 ± 0.05 | 2.80 ± 0.04 | 17.24 | 0.47 | 14.32 ± 0.57 | 70.27 ± 0.70 | 18.30 ± 0.40 | 3.05 |
| 6 | 5.0 | 36 | 125 | 9.82 ± 0.05 | 3.31 ± 0.04 | 52.90 | 0.55 | 5.59 ± 0.35 | 66.67 ± 0.44 | 25.40 ± 0.70 | 4.23 |
| 7 | 5.5 | 36 | 125 | 9.99 ± 0.02 | 2.86 ± 0.05 | 14.11 | 0.48 | 18.53 ± 0.46 | 67.57 ± 1.07 | 24.20 ± 0.46 | 4.03 |
| 8 | 5.5 | 36 | 125 | 10.08 ± 0.02 | 2.83 ± 0.04 | 26.87 | 0.47 | 8.22 ± 0.16 | 68.42 ± 0.61 | 20.30 ± 0.46 | 3.38 |
| 9 | 5.5 | 36 | 150 | 9.90 ± 0.02 | 2.68 ± 0.03 | 6.78 | 0.45 | 25.37 ± 2.51 | 65.79 ± 1.36 | 19.10 ± 0.66 | 3.18 |
| 10 | 5.5 | 36 | 125 | 10.02 ± 0.2 | 2.91 ± 0.01 | 20.32 | 0.48 | 15.26 ± 0.55 | 67.57 ± 0.76 | 19.30 ± 0.62 | 3.22 |
| 11 | 6.0 | 36 | 125 | 9.68 ± 0.04 | 3.20 ± 0.10 | 71.38 | 0.53 | 4.39 ± 0.09 | 70.59 ± 0.96 | 23.40 ± 0.56 | 3.90 |
| 12 | 5.5 | 36 | 100 | 9.87 ± 0.01 | 3.47 ± 0.08 | 16.40 | 0,58 | 16.73 ± 0.34 | 68.57 ± 0.87 | 44.50 ± 1.50 | 7.42 |
| 13 | 5.0 | 40 | 150 | 9.43 ± 0.04 | 2.69 ± 0.05 | 5.87 | 0.45 | 38.20 ± 1.82 | 60.53 ± 2.79 | 19.50 ± 0.66 | 3.25 |
| 14 | 5.5 | 32 | 125 | 9.98 ± 0.02 | 3.26 ± 0.05 | 50.43 | 0.54 | 15.26 ± 0.65 | 68.42 ± 1.34 | 37.50 ± 0.52 | 6.25 |
| 15 | 5.5 | 36 | 125 | 9.99 ± 0.02 | 2.79 ± 0.08 | 11.48 | 0.46 | 18.40 ± 0.77 | 67.57 ± 0.97 | 20.30 ± 0.50 | 3.38 |
Table 2.
P values of the ANOVA corresponding to the kinetic parameters of fermentation of W. cibaria by effects of temperature (T), pH, and stirring speed (SS).
| Dependent variables | Log10 CFU mL−1 | BWc (g L−1) | YBwc/S | VPWc (g L−1 h−1) | CSC (%) | N2C (%) | LA (g L−1) |
|---|---|---|---|---|---|---|---|
| pH | 0.396 | 0.840 | 0.319 | 0.842 | 0.845 | 0.209 | 0.834 |
| T | 0.026* | 0.304 | 0.227 | 0.306 | 0.228 | 0.766 | 0.114 |
| SS | 0.617 | 0.205 | 0.590 | 0.207 | 0.197 | 0.353 | 0.032* |
| pH2 | 0.044* | 0.803 | 0.008* | 0.808 | 0.027* | 0.216 | 0.268 |
| pH - T | — | 0.170 | 0.136 | 0.171 | 0.137 | 0.055 | 0.049* |
| pH - SS | 0.132 | 0.176 | 0.698 | 0.177 | 0.024* | 0.889 | 0.128 |
| T2 | 0.053 | 0.189 | 0.344 | 0.189 | 0.563 | 0.109 | — |
| T - SS | 0.043* | 0.942 | 0.557 | 0.938 | 0.140 | 0.008* | 0.494 |
| SS2 | — | 0.358 | 0.043* | 0.357 | 0.023* | 0.046* | 0.496 |
*Significant for p < 0.05.
Fig. 1.
Response surface and volume plots of viability of W. cibaria (Log10 UFC mL−1) as a function of the independent variables (T, pH, and SS) during the fermentation process.
Fig. 2.
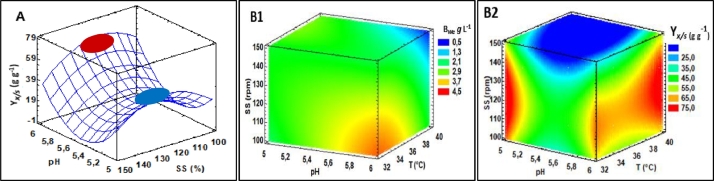
Response surface graph: A: biomass yield (YBwc/S). Response volume graphs: B1: Biomass production (BWc) and B2: Biomass yield (YBwc/S) of W. cibaria as a function of the independent variables (T, pH, and SS) during the fermentation process.
Fig. 3.
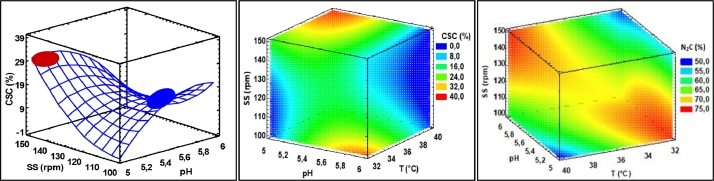
Response surface and volume plots: C source consumption (CSC) and N2 consumption (N2C) of W. cibaria as a function of the independent variables (T, pH, and SS) during the fermentation process by effects of T, pH, and SS (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.).
Fig. 4.
Response surface and volume plots of the production of LA (g L−1) as a function of the independent variables (T, pH, and SS) during the fermentation process with W. cibaria.
The viability of W. cibaria varied between 9.43 and 10.08 Log10 (CFU mL−1), and the ANOVA showed significant differences (p < 0.05) with respect to the T and the linear T-SS interaction. A decrease in viability (blue zone) of the order of 0.6 Log10 (CFU mL−1) is observed in the response volume graph when T increases (mainly between 38 and 40 °C), which is attributed to the effect of heat stress on the bacteria [35].
In general, W. cibaria presented better viability when the system was operated at low temperatures (32–36 °C), higher SS (130 - 150 rpm), and pH between 5.0–5.8. In relation to the T-SS interaction, two areas of importance were identified in the response surface graph when the system was operated at higher SS (140–150 rpm): 1) higher viability at low T (32–34 °C); and 2) higher lethality at high T (38–40 °C). This occurred due to the lower or higher thermal stress generated. Zone 1 favored the reproduction of W. cibaria, and this is attributed to mechanical stress caused by agitation and low T. These conditions induce greater metabolic activity of bacterial cells and, consequently, greater resistance to lethality. The above is explained because W. cibaria is a facultative anaerobic bacterium that adapts to the presence of O2 without altering its reproduction [35,36]. Furthermore, this behavior is affected by the quadratic interaction of the pH of the system; since, it presents curvilinear behaviors that provide both less and greater favorability in different conditions of T and SS.
This is attributed to the fact that LAB are characterized by their easy adaptation to acidic conditions and wide versatility in diverse temperatures (25–50 °C) [13,16]. Similar results were reported by both Lakra et al. [16], using Weissella confusa MD1 and MD2 (10.34 - 10.39 Log10 CFU mL−1) with commercial MRS substrate, and by Zannini et al. [37], using W. cibaria MG1 (1.31 × 10 9 UFC mL−1) with whole quinoa milk substrate. Lower viability was reported by López et al. [29], when using W. cibaria 3LA (7.50–7.75 Log UFC mL−1), W. confusa L9 (7.69–8.66 Log UFC mL−1), W. confusa L17 (7.33–7.84 Log UFC mL−1), and W. confusa Snc40 k (7.34–7.94 Log UFC mL−1) with commercial MRS fermentation as the substrate added with xylan as a source of carbon.
The production of BWc presented average values that fluctuated between 1.99 and 3.47 g L−1. The ANOVA did not show significant differences (p < 0.05) in the production of BWc with respect to the independent variables (T, SS, and pH) or their interactions, showing a homogeneous behavior (green and yellow-green areas) mainly in the ranges between 2.0 and 3.0 g L−1. This situation indicates that W. cibaria adapted and effectively assimilated the nutrients from the SFS based on pineapple, sacha inchi, and minerals (in the evaluated ranges of the independent variables). The availability of nutrients in SFS favors the cell growth of W. cibaria [7,8,26] and the generation of secondary metabolites [9]. In addition, the enzymatic hydrolysis process applied to the substrate allows for increasing the content of disaccharides and monosaccharides [22], compounds easily metabolized by LAB during fermentation [15,18]. LAB such as W. cibaria are nutritionally demanding microorganisms. Therefore, the substrates used for their growth must be a source of the main macronutrients (C and N2) and micronutrients (vitamins and minerals), which are essential in fermentation processes [18,27]. The results obtained in the present investigation were superior to those reported by Ma et al. [38], using W. paramesentedamientos JT13 and commercial fermentation substrate (MRS) (1.17 g L−1), as well as those obtained by Serna et al. [39], when using W. confusa and a worm meal-based substrate as a source of N2. (1.47 g L−1). The YBwc/S presented significant differences (p < 0.05) with respect to the pH-pH and SS-SS interactions, and high variability fluctuating between 5.87 and 66.52g−1. The quadratic interactions show the curvature of the response surface graphs, obtaining the highest YBwc/S at extreme pH (5.0 and 6.0) values and intermediate SS (120 rpm) values (Fig. 2).
CSC presented statistical differences (p < 0.05) with the pH-SS, pH-pH and SS-SS interactions, and the CSC fluctuated between 2.2–38.2%. These interactions show a curvature of the response surface graphs, where the lowest CSC consumption was found at pH (5.0–5.2) and SS (100–110 rpm), and the highest consumption was at pH (5.0–5.2) and SS (140–150 rpm) (Fig. 3). The low CSC is attributed to the fact that it takes two routes during the process. In one case, it is a nutrient or energy source for cell construction during the reproduction of W. cibaria [31], and in another, it goes to the production of LA [4,18]. Generally, CSC is progressive during fermentation kinetics [40], and it could even run out at the end of the process. However, the low CSC in this research is attributed, firstly, to the greater depletion of N2 that limits the continuity of cellular reproduction, and, secondly, to the complex molecular structure of SFS. This is rationalized give that pineapple wastes and sacha inchi residues have high fiber and protein contents, respectively [23,26]. Indeed, a greater use of the lignocellulosic material can be achieved through the application of pretreatments (homogenization or thermal and enzymatic hydrolysis) [22].
Serna et al. [41], and Serna et al. [19], evaluated the CSC in W. cibaria and found that the lower or higher CSC depends on the type of substrate used. Therefore, the presence of macro and micronutrients favors its metabolic capacity. On the other hand, various researchers have shown the progressive consumption of the C source in different applications: W. cibaria in commercial MRS substrate [22]; W. cibaria MG1 in maltose and sucrose substrate [37]; B. coagulans on glucose and fructose substrate [42]; L. crustorum W19 and L. sanfranciscensis MR29 on wheat straw hydrolyzate substrate [18], among others.
On the other hand, the T-SS interaction and the quadratic S-S interaction affected the N2C variable. The response surface plot shows a large area where N2C becomes higher (blue area) (70–73%) (Fig. 3). This is consistent with the behavior of the high viability of the microorganism, which varied only by 1 Log unit. These results confirm that the bacterium W. cibaria easily adapts to the evaluated process conditions. It efficiently assimilates and metabolizes the N2 source, which is an essential macronutrient for its cellular structure [5].
The supply of essential nutrients in the fermentation substrate is important for cell reproduction. The cell cycle of bacteria depends on the composition of the substrate, concentration of available nutrients, process conditions, and physical conditions such as pH, T and SS, among others [13,35,36]. The limiting nutrient source of the fermentation process was N2, since this macronutrient was depleted first (73.0%) in relation to the C source (38.2%). This greater depletion of N2 compared to C could have occurred for two reasons: (1) high demand for N2 in the bacterial cell metabolism because LAB use the protein content for the formation of the cell membrane and for cell growth [5,13]; and (2) W. cibaria adapted to a new substrate with specific compositional characteristics for its reproduction [7,26]. In the SFS, the protein content was provided by the sacha inchi, and the sugar content was provided by the two residues, both pineapple and sacha inchi.
Regarding LA, the pH-T and SS interaction significantly influenced LA, fluctuating their mean values between 14.40 and 44.50 g L−1, which corresponds to a VPLA of 2.4 and 7.42 g L−1 h−1, respectively.
The response volume graph identifies the process conditions with the highest production of LA: T (32–36 °C), SS (100–110 rpm), and pH (5.4–6.0) (Fig. 4). This area corresponds to the conditions with the highest production of BWc, showing that cell growth is proportional to the production of LA [18]. The above is explained because W. cibaria is a heterofermentative LAB, a characteristic that favors the production of various secondary metabolites (LA, propionate, butyrate, acetate, among others [14]. In the present investigation and under the process conditions evaluated, LA production was favored (44.5 g L−1). The results obtained are higher than the LA concentration reported by several authors: Serna et al. [39], using W. confusa in substrates based on worm meal and commercial substrate MRS (4.79 and 4.33 g L−1, respectively); Zannini et al. [37], reporting LA = 7.46 g L−1 with W. cibaria MG1 using a substrate based on quinoa flour; and Cizeikiene et al. [18], who obtained 0.96–4.94 g L−1 for different LAB using hydrolyzed wheat straw as substrate. Other authors reported 0.89 g L−1 for W. cibaria WC018 and 10.79 g L−1 with mixed culture of W. cibaria WC018 + L. plantarum LP067 using commercial MRS [43]. Other investigations obtained higher concentrations of LA (54.97 g L−1) using L. delbrueckii and pineapple residues supplemented with N2 as fermentation substrates [8].
3.2. Experimental optimization of multiple responses
Table 3 presents the regression coefficients of the 2nd order polynomial models of the dependent variables (viability, BWc, YBwc/S, VPWc, CSC, N2C and LA) and their respective R2 values. Table 4 presents the criteria, weights, and impacts established in the experimental optimization of multiple responses during the fermentation process of W. cibaria. In addition, the theoretical values were predicted by the mathematical and experimental models obtained from 3 replicates at the optimal conditions of the independent variables. The RME was determined in order to validate the results of the mathematical models.
Table 3.
Regression coefficients of the mathematical model and R2 of the dependent variables (viability, BWc, YBwc/S, VPWc, CSC, N2C, and LA) in the reproduction of W. cibaria.
| Regression coefficient | Log10 CFU mL−1 | BWc (g L−1) | YBwc/S | VPWc (g L−1 h−1) | CSC (%) | N2C (%) | LA (g L−1) |
|---|---|---|---|---|---|---|---|
| Constant | −39.48 | −114.49 | 5768.34 | −19.04 | −1806.63 | 339.37 | −2413.11 |
| pH | 8.68 | 17.43 | −1624.57 | 2.89 | 571.51 | 0.59 | 549.86 |
| T | 0.94 | 2.97 | −95.28 | 0.50 | 15.62 | −10.15 | 41.00 |
| SS | 0.16 | 0.30 | 7.22 | 0.05 | −0.35 | −1.51 | 4.20 |
| pH2 | −0.65 | −0.25 | 123.41 | −0.04 | −31.42 | −6.73 | −18.57 |
| pH - T | — | −0.27 | 9.10 | −0.04 | −3.15 | 2.08 | −6.91 |
| pH - SS | −0.01 | −0.04 | −0.34 | −0.01 | −0.91 | 0.02 | −0.79 |
| T2 | −0.01 | −0.02 | 0.48 | 0.00 | −0.10 | −0.14 | — |
| T - SS | 0.00 | 0.00 | 0.06 | 0.00 | 0.06 | 0.07 | −0.04 |
| SS2 | — | 0.00 | −0.03 | 0.00 | 0.01 | −0.01 | 0.00 |
| R2 | 84.94 | 71.91 | 89.89 | 71.86 | 93.38 | 95.18 | 69.89 |
Table 4.
Experimental optimization of multiple responses in the fermentation process with W. cibaria.
| Dependent variables | Criteria | Weight | Impact | Theoretical Optimum | Experimental Optimal | RME (%) |
|---|---|---|---|---|---|---|
| Viability (Log10 CFU mL−1) | Maximize | 1.0 | 5 | 10.03 | 10.07 ± 0.05 | 0.39 |
| BWc (g L−1) | Maximize | 1.0 | 5 | 2.96 | 3.16 ± 0.19 | 6.33 |
| YBwc/S | 38,63 | 0.3 | 1 | 45,16 | 25.38 ± 0.11 | 77.93 |
| VPWc (g L−1 h−1) | Maximize | 0.9 | 4 | 0.49 | 0.53 ± 0.03 | 7.55 |
| CSC (%) | Minimize | 0.6 | 3.0 | 16.95 | 45.60 ± 0.04 | 62.83 |
| N2C (%) | Minimize | 0.6 | 3.0 | 63.57 | 63.46 ± 1.82 | 0.17 |
| LA (g L−1) | Minimize | 0.8 | 4.0 | 19.23 | 20.66 ± 1.27 | 6.92 |
| VPLA (g L−1 h−1) | Minimize | 0.8 | 4.0 | 3.20 | 3.44 ± 0.21 | 6.98 |
The R2 values showed a good acceptable fit of the mathematical models (R2 ≥ 85%) for the dependent variables Log10 CFU, YBwc/S, CSC, and N2C, while the variables BWc, VPWc, LA, and VPLA presented acceptable regression adjustments (R2 ≈ 70–72%). This lack of adjustment is due to the low influence of the values evaluated in pH, T, and SS on the variables BWc, VPWc, LA, and VPLA. On the other hand, it is highlighted that the models are adequate to describe the behavior of the results found. All the variables presented a random distribution of the residuals, which ensures that the data can be parameterized according to a normal distribution.
The experimental optimization defined the independent variables as follows: pH = 5.0, T = 35.1 °C, and SS = 139.3 rpm, which reached a desirability value of 76.8% according to the results obtained in the design and the criteria defined. For most of the dependent variables, the RME values were less than 7.5%, which revalidates the values obtained at the optimal process conditions. The degree of deviation presented in the CSC is highlighted (62.83%) (experimental value > value predicted by the model), and a lower YBwc/S (RME = 77.93%) was attributed to the curvilinear behavior of the surface responses (significant effects with the quadratic interactions of pH and SS) that affected the RME. Despite the specific difference, the experimental value obtained in the CSC contributed to a better production and viability of W. cibaria produced in the SFS.
3.3. Specific growth rate (μ)
Table 5 presents the experimental and theoretical μ values of W. cibaria obtained from the production kinetics of BWc for each evaluated experiment. In addition, it presents the adjusted R2 coefficients according to the order 1 and logistic models.
Table 5.
Results of experimental and theoretical µ, and the real and adjusted R2 coefficients of the growth kinetics of W. cibaria.
| Variable | Run | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| μ1 (h−1) | 0.23 | 0.23 | 0.25 | 0.26 | 0.28 | 0.29 | 0.27 | 0.27 | 0.27 | 0.27 | 0.30 | 0.29 | 0.27 | 0.28 | 0.26 |
| μ2 (h−1) | 0.16 | 0.16 | 0.18 | 0.19 | 0.21 | 0.22 | 0.20 | 0.20 | 0.20 | 0.21 | 0.22 | 0.22 | 0.20 | 0.21 | 0.20 |
| R2order1 | 0.90 | 0.85 | 0.95 | 0.93 | 0.92 | 0.95 | 0.91 | 0.88 | 0.94 | 0.90 | 0.91 | 0.92 | 0.94 | 0.91 | 0.86 |
| R2LE | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 | 0.99 |
Note: Experiments 5, 7, 8, 10 and 15 are replicates obtained under the same fermentation process conditions. R2order1: Regression coefficient - linear model and R2LE: Regression coefficient - logistic equation.
The μ1 values of W. cibaria in the SFS were higher in all cases (0.23 - 0.30 h−1) compared to μ2 (0.16 - 0.22 h−1). However, the logistic model presented a better regression fit for the estimate of µ for W. cibaria reproduced in the SFS, where the R2 values were 0.99 in all cases. In the model of order 1, the estimate of μ1 fluctuated between 0.85 and 0.95. Similar results of μ have been reported by Serna et al. [39], (0.28 h−1) and by Micanquer-Carlosama et al. [22], (0.27 h−1), using the same bacteria and worm meal supplemented with yeast extract and MRS respectively. In another investigation, Serna et al. [19], reported higher values of μ (0.32 and 0.35 h−1) with W. confusa growing on glucose supplemented with guava seed flour and MRS, respectively.
Fig. 5 presents the kinetic behavior of BWc production (t = 0 → 48 h) at the optimal process conditions. In general, the growth kinetics behavior defines the adaptation, exponential, stationary, and cell death phases, allowing for determining the highest production intervals of BWc and obtaining the primary product or other secondary metabolites as a result of bacteria fermentation metabolism [35,36].
Fig. 5.
Kinetics of biomass production (BWc) obtained at the optimal experimental condition, using a supplemented fermentation substrate (SFS) (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.).
The behavior of the growth kinetics of W. cibaria obtained under the optimal process conditions was similar to the kinetics obtained when the commercial substrate (MRS) was used [22]. Although, the absence of an adaptation phase was observed (Fig. 5), which indicates the easy and rapid adaptation of W. cibaria in the SFS. This is attributed to the previous adaptation that the bacteria had in the preparation phase for the fermentation inoculum. Furthermore, the optimal process conditions allowed for improved cell production (3.16 ± 0.19 g L−1) compared when SFS was used in reference conditions (T = 36 °C, pH = 6, SS = 100 rpm) (2.93 ± 0.03 g L−1) [22]. This highest cell growth occurred during the exponential phase (10 h). Next, an asymptotic behavior was identified, and this defined the stationary phase, as well as a BWc corresponding to 3.13 ± 0.05 (g L−1). The easy adaptation of W. cibaria in SFS is attributed to the fact that the substrate was specifically formulated to meet the nutritional requirements (macronutrients and micronutrients of W. cibaria).
On the other hand, the evaluation of the μ obtained to the optimal experimental condition and using the order 1 and logistic models, presented the following results: μ1 = 0.24 h−1 and μ2 = 0.28 h−1, behavior similar to that described for all experiments (μ1 > μ2). Additionally, the assessment of the R2 for the two models reported values of 0.90 and 0.99, respectively. These values were similar to those achieved for the 15 treatments in the statistical design, which guarantees an acceptable prediction of the model of order 1 and excellent in the logistic model.
3.4. Morphology of W. cibaria biomass
The product of the fermentation process consists of W. cibaria biomass and SFS (Fig. 5). Before using the strain, its purity is checked by the following tests: (1) ability to assimilate aniline blue. (2) Morphology of the cells by Gram staining under optical microscope. (3) Biochemical tests by API 50 CHL Kit (positive values for Amygdalin, Arbutin, Esculin, Salicin, Cellobiose, Maltose and Sucrose, and Catalase negative). (4) LA production (range 11 to 12 g l-1 during 12 h) and (5) Antimicrobial activity [19,39,41]. Micrograph (A) shows Gram positive stained bacteria and micrograph (B) shows a large amount of agglomerated biomass and in its characteristic bacilli form (Fig. 6).
Fig. 6.
(A) Micrograph in wet biomass of W. cibaria using optical microscopy at 100X magnification. (B) Micrograph in dry biomass of W. cibaria using scanning electron microscopy.
4. Conclusions
The research allowed for experimental optimization of W. cibaria reproduction and viability, showing that the temperature and stirring speed independent variables had a greater influence on the fermentation process. The non-extreme conditions of temperature and higher stirring speed allowed for greater use of the substrate and metabolic efficiency of W. cibaria, which was reflected in the results obtained in yield and volumetric productivity. It was evidenced that the SFS formulated based on pineapple and sacha inchi wastes provided the macronutrients (C and N2) and micronutrients (Na, NH3, K2, and Mg) necessary for effective cell reproduction. Therefore, it is inferred that the use of agroindustrial waste as fermentation substrates is an excellent alternative for optimizing probiotic production processes, contributing positively to environmental impacts and, in effect, to the circular economy.
Funding statement
The authors thank Minscience (Development and industrial scaling of innovative, practical, and functional foods from pineapple pulp, peel, and leaf as a strategy to improve the competitiveness of the agrocadena-808) for financing this research project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.btre.2021.e00671.
Appendix. Supplementary materials
References
- 1.Margallo M., Ziegler-Rodriguez K., Vázquez-Rowe I., Aldaco R., Irabien Á., Kahhat R. Enhancing waste management strategies in Latin America under a holistic environmental assessment perspective: a review for policy support. Sci. Total Environ. 2019;689:1255–1275. doi: 10.1016/j.scitotenv.2019.06.393. [DOI] [PubMed] [Google Scholar]
- 2.Singh R.S., Kaur N., Kennedy J.F. Pullulan production from agro-industrial waste and its applications in food industry: a review. Carbohydr. Polym. 2019;217:46–57. doi: 10.1016/j.carbpol.2019.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Greses S., Tomás-Pejó E., Gónzalez C. Agroindustrial waste as a resource for volatile fatty acids production via anaerobic fermentation. Bioresour. Technol. 2020;297 doi: 10.1016/j.biortech.2019.122486. [DOI] [PubMed] [Google Scholar]
- 4.Carpinelli J.V., de Barros Ranke F.F., Escaramboni B., Campioni T.S., Fernández Núñez E.G., de Oliva Neto P. Cost-effective lactic acid production by fermentation of agro-industrial residues. Biocatal. Agric. Biotechnol. 2020;27 doi: 10.1016/j.bcab.2020.101706. [DOI] [Google Scholar]
- 5.Boulay M., Al Haddad M., Rul F. Streptococcus thermophilus growth in soya milk: sucrose consumption, nitrogen metabolism, soya protein hydrolysis and role of the cell-wall protease PrtS. Int. J. Food Microbiol. 2020;335 doi: 10.1016/j.ijfoodmicro.2020.108903. [DOI] [PubMed] [Google Scholar]
- 6.Girelli A.M., Astolfi M.L., Scuto F.R. Agro-industrial wastes as potential carriers for enzyme immobilization: a review. Chemosphere. 2020;244 doi: 10.1016/j.chemosphere.2019.125368. [DOI] [PubMed] [Google Scholar]
- 7.Yusof A.H., Dailin D.J., Low L.Z.M.I., Abg Zaidel D.N., El Enshasy H. Potential application of pineapple waste as a fermentation substrate in yeast production. Int. J. Sci. Technol. Res. 2020;9:1933–1937. [Google Scholar]
- 8.Abdullah A., Winaningsih I. AIP Conf. Proc. American Institute of Physics Inc.; 2020. Effect of some parameter on lactic acid fermentation from pineapple waste by Lactobacillus delbrueckii. [DOI] [Google Scholar]
- 9.Arrioja-Bretón D., Mani-López E., Palou E., López-Malo A. Antimicrobial activity and storage stability of cell-free supernatants from lactic acid bacteria and their applications with fresh beef. Food Control. 2020;115 doi: 10.1016/j.foodcont.2020.107286. [DOI] [Google Scholar]
- 10.Sepúlveda L., Laredo E., Buenrostro J.J., Ascacio J.A., Genisheva Z., Aguilar C., Teixeira J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020;43:1–7. doi: 10.1016/j.ejbt.2019.11.002. [DOI] [Google Scholar]
- 11.Ahmad A., Banat F., Taher H. A review on the lactic acid fermentation from low-cost renewable materials: recent developments and challenges. Environ. Technol. Innov. 2020;20 doi: 10.1016/j.eti.2020.101138. [DOI] [Google Scholar]
- 12.Rolim P.M., Hu Y., Gänzle M.G. Sensory analysis of juice blend containing isomalto-oligosaccharides produced by fermentation with Weissella cibaria. Food Res. Int. 2019;124:86–92. doi: 10.1016/j.foodres.2018.08.089. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz S., Gonçalves M.T.P., Galván A.I., Merchán A.V., González E., de G. Córdoba M., Benito M.J. Screening of autochthonous lactic acid bacteria strains from artisanal soft cheese: probiotic characteristics and prebiotic metabolism. LWT. 2019;114 doi: 10.1016/j.lwt.2019.108388. [DOI] [Google Scholar]
- 14.Yu H.S., Jang H.J., Lee N.K., Paik H.D. Evaluation of the probiotic characteristics and prophylactic potential of Weissella cibaria strains isolated from kimchi. LWT. 2019;112 doi: 10.1016/j.lwt.2019.05.127. [DOI] [Google Scholar]
- 15.Hassan S.E.D., Abdel-Rahman M.A., Roushdy M.M., Azab M.S., Gaber M.A. Effective biorefinery approach for lactic acid production based on co-fermentation of mixed organic wastes by Enterococcus durans BP130. Biocatal. Agric. Biotechnol. 2019;20 doi: 10.1016/j.bcab.2019.101203. [DOI] [Google Scholar]
- 16.Lakra A.K., Domdi L., Hanjon G., Tilwani Y.M., Arul V. Some probiotic potential of weissella confusa MD1 and Weissella cibaria MD2 isolated from fermented batter. LWT. 2020;125 doi: 10.1016/j.lwt.2020.109261. [DOI] [Google Scholar]
- 17.Vázquez J.A., Docasal S.F., Prieto M.A., González M.P., Murado M.A. Growth and metabolic features of lactic acid bacteria in media with hydrolysed fish viscera. an approach to bio-silage of fishing by-products. Bioresour. Technol. 2008;99:6246–6257. doi: 10.1016/j.biortech.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Cizeikiene D., Juodeikiene G., Damasius J. Use of wheat straw biomass in production of l-lactic acid applying biocatalysis and combined lactic acid bacteria strains belonging to the genus Lactobacillus. Biocatal. Agric. Biotechnol. 2018;15:185–191. doi: 10.1016/j.bcab.2018.06.015. [DOI] [Google Scholar]
- 19.Serna L., Mera J.D., Angulo J.E. Guava Psidium guajava seed flour and dry aspergillus niger mycelium as nitrogen sources for the production of biomass and antimicrobial compounds produced by Weissella confusa. Electron. J. Biotechnol. 2013;16:17. doi: 10.2225/vol16-issue6-fulltext-1. [DOI] [Google Scholar]
- 20.Selani M.M., Brazaca S.G.C., Dos Santos Dias C.T., Ratnayake W.S., Flores R.A., Bianchini A. Characterisation and potential application of pineapple pomace in an extruded product for fibre enhancement. Food Chem. 2014;163:23–30. doi: 10.1016/j.foodchem.2014.04.076. [DOI] [PubMed] [Google Scholar]
- 21.Sepúlveda L., Romaní A., Aguilar C.N., Teixeira J. Valorization of pineapple waste for the extraction of bioactive compounds and glycosides using autohydrolysis. Innov. Food Sci. Emerg. Technol. 2018;47:38–45. doi: 10.1016/j.ifset.2018.01.012. [DOI] [Google Scholar]
- 22.Micanquer-Carlosama A., Cortés-Rodríguez M., Serna-Cock L. Formulation of a fermentation substrate from pineapple and sacha inchi wastes to grow Weissella cibaria. Heliyon. 2020;6:e03790. doi: 10.1016/j.heliyon.2020.e03790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seguí L., Maupoey P.Fito. An integrated approach for pineapple waste valorisation. bioethanol production and bromelain extraction from pineapple residues. J. Clean. Prod. 2018;172:1224–1231. doi: 10.1016/j.jclepro.2017.10.284. [DOI] [Google Scholar]
- 24.Chirinos R., Aquino M., Pedreschi R., Campos D. Optimized methodology for alkaline and enzyme-assisted extraction of protein from sacha Inchi (Plukenetia volubilis) kernel cake. J. Food Process Eng. 2017;40:e12412. doi: 10.1111/jfpe.12412. [DOI] [Google Scholar]
- 25.Vásquez Osorio D., Hincapié Llanos G.A., Cardona M., Jaramillo D.I., Vélez Acosta L. Formulación de una colada empleando harina de sacha inchi (Plukenetia volubilis L.) proveniente del proceso de obtención de aceite. Perspect. En Nutr. Humana. 2017;19:167–179. doi: 10.17533/udea.penh.v19n2a04. [DOI] [Google Scholar]
- 26.Wang S., Zhu F., Kakuda Y. sacha inchi (Plukenetia volubilis L.): nutritional composition, biological activity, and uses. Food Chem. 2018;265:316–328. doi: 10.1016/j.foodchem.2018.05.055. [DOI] [PubMed] [Google Scholar]
- 27.Khedkar M.A., Nimbalkar P.R., Gaikwad S.G., Chavan P.V., Bankar S.B. Sustainable biobutanol production from pineapple waste by using Clostridium acetobutylicum B 527: drying kinetics study. Bioresour. Technol. 2017;225:359–366. doi: 10.1016/j.biortech.2016.11.058. [DOI] [PubMed] [Google Scholar]
- 28.Batista A.L.D., Silva R., Cappato L.P., Almada C.N., Garcia R.K.A., Silva M.C., Raices R.S.L., Arellano D.B., Sant'Ana A.S., Conte Junior C.A., Freitas M.Q., Cruz A.G. Quality parameters of probiotic yogurt added to glucose oxidase compared to commercial products through microbiological, physical-chemical and metabolic activity analyses. Food Res. Int. 2015;77:627–635. doi: 10.1016/j.foodres.2015.08.017. [DOI] [Google Scholar]
- 29.López M., Rodríguez M.E., López A., Wacher C. Evaluation of xylan as carbon source for Weissella spp., a predominant strain in pozol fermentation. LWT Food Sci. Technol. 2018;89:192–197. doi: 10.1016/j.lwt.2017.10.030. [DOI] [Google Scholar]
- 30.García-Gonzalez E., García Salazar A.P., Rojas Dorado M.C., Ordoñez Artunduaga D.A., Serna Cock L. Formulación mixta de bacterias lácticas para el control de Listeria monocytogenes. Rev. Colomb. Biotecnol. 2017;19:38–41. doi: 10.15446/rev.colomb.biote.v19n1.55879. [DOI] [Google Scholar]
- 31.Serna L., Pabón O.V. Development of a teat bio-sealant and evaluation of its technological and functional properties. Prob. Antimicrob. Proteins. 2016;8:111–119. doi: 10.1007/s12602-016-9210-5. [DOI] [PubMed] [Google Scholar]
- 32.Serna L., Pabón O.V., Giraldo G.I. Adhesion capacity of Weissella cibaria to bovine mammary tissue and the effect of bio-sealant topical application on physicochemical properties of milk. Prob. Antimicrob. Proteins. 2019;11:1293–1299. doi: 10.1007/s12602-018-9481-0. [DOI] [PubMed] [Google Scholar]
- 33.Valencia L.J., López K., Gómez E.D., Serna L., Aguilar C.N. In-vitro assessment for the control of Fusarium species using a lactic acid bacterium isolated from yellow pitahaya (Selenicereus megalanthus (K. Schum. Ex Vaupel Moran)) J. Integr. Agric. 2021;20:159–167. doi: 10.1016/S2095-3119(20)63284-1. [DOI] [Google Scholar]
- 34.da Silva T.M., de Deus C., de Souza Fonseca B., Lopes E.J., Cichoski A.J., Esmerino E.A., de Bona da Silva C., Muller E.I., Moraes Flores E.M., de Menezes C.R. The effect of enzymatic crosslinking on the viability of probiotic bacteria (Lactobacillus acidophilus) encapsulated by complex coacervation. Food Res. Int. 2019;125 doi: 10.1016/j.foodres.2019.108577. [DOI] [PubMed] [Google Scholar]
- 35.Suo X., Huang S., Wang J., Fu N., Jeantet R., Chen X.D. Effect of culturing lactic acid bacteria with varying skim milk concentration on bacteria survival during heat treatment. J. Food Eng. 2021;294 doi: 10.1016/j.jfoodeng.2020.110396. [DOI] [Google Scholar]
- 36.Wang Y., Hao F., Lu W., Suo X., Bellenger E., Fu N., Jeantet R., Chen X.D. Enhanced thermal stability of lactic acid bacteria during spray drying by intracellular accumulation of calcium. J. Food Eng. 2020;279 doi: 10.1016/j.jfoodeng.2020.109975. [DOI] [Google Scholar]
- 37.Zannini E., Jeske S., Lynch K., Arendt E.K. Development of novel quinoa-based yoghurt fermented with dextran producer Weissella cibaria MG1. Int. J. Food Microbiol. 2018;268:19–26. doi: 10.1016/j.ijfoodmicro.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 38.Ma J., Hong Y., Deng L., Yi L., Zeng K. Screening and characterization of lactic acid bacteria with antifungal activity against Penicillium digitatum on citrus. Biol. Control. 2019;138 doi: 10.1016/j.biocontrol.2019.104044. [DOI] [Google Scholar]
- 39.Serna L., Rengifo C.A., Rojas M.A. The use of earthworm flour for lactic acid biomass production. Afr. J. Biotechnol. 2013;12:5962–5967. doi: 10.5897/ajb2012.2924. [DOI] [Google Scholar]
- 40.Hu J., Lin Y., Zhang Z., Xiang T., Mei Y., Zhao S., Liang Y., Peng N. High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour. Technol. 2016;214:74–80. doi: 10.1016/j.biortech.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 41.Serna L., Valencia L.J., Campos R. Kinetic of fermentation and antimicrobial activity of Weissella confusa against Staphylococcus aureus and Streptococcus agalactiae. Rev. Fac. Ing. 2010;1:55–65. [Google Scholar]
- 42.Alexandri M., Blanco-Catalá J., Schneider R., Turon X., Venus J. High L(+)-lactic acid productivity in continuous fermentations using bakery waste and lucerne green juice as renewable substrates. Bioresour. Technol. 2020;316 doi: 10.1016/j.biortech.2020.123949. [DOI] [PubMed] [Google Scholar]
- 43.Xiang W.L., Di Zhang N., Lu Y., Zhao Q.H., Xu Q., Rao Y., Liu L., Zhang Q. Effect of Weissella cibaria co-inoculation on the quality of Sichuan Pickle fermented by Lactobacillus plantarum. LWT. 2020;121 doi: 10.1016/j.lwt.2019.108975. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.