Abstract
Hepatitis B is one of the major burdens for health services and is the leading cause of morbidity and mortality from cirrhosis of liver and hepatocellular carcinoma. Current treatment strategies using nucleos(t)ide analogue reverse-transcriptase inhibitors or interferons are targeted for the long-term suppression of hepatitis B DNA. However, functional cure of hepatitis B infection (HBsAg clearance) was difficult to attain with such treatments. Therefore, new treatment strategies or innovative treatments are urgently needed. The new treatments should focus on the potential therapeutic targets such as covalently closed circular DNA which may be important for the HBsAg clearance. Plant based medicines have been used in different traditional medicine practices and these natural products/compounds serve as a good source of information or clues for use in drug discovery and design. Many natural products were found to be effective against hepatitis B virus and some even have better therapeutic activities than currently used compounds. This review summarizes the current evidence of Myanmar medicinal plants in basic and clinical research which shows promising potential for the development of novel therapeutic agents for the treatment of hepatitis B.
Keywords: Hepatitis B, Medicinal plants, Natural products, Drug discovery
Introduction
Hepatitis B is one of the most common causes of chronic liver disease, and hepatocellular carcinoma (HCC) worldwide. According to the World Health Organization, 257 million people were living with chronic hepatitis B infection and it was responsible for an estimated 887,000 deaths due to cirrhosis and (HCC) in 2015 [67]. Myanmar had a 6.5% of hepatitis B sero-prevalence in the general population according to a national survey done in 2015. Yangon region, an economic hub of Myanmar, had the highest prevalence of 12.3% [37].
The current treatment strategies are targeted to the long-term suppression of hepatitis B virus (HBV) replication as the main endpoint. The long-term administration of a nucleos(t)ide analogue reverse-transcriptase inhibitors such as entecavir (ETV), tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide (TAF), are regarded as the treatment of choice because they have a low chance of resistance. Pegylated interferon-alfa can be considered in mild to moderate chronic hepatitis B cases. Although Hepatitis B surface antigen (HBsAg) loss is regarded as an optimal endpoint or functional cure, the current treatments (ETV or TDF) could only achieve that endpoint in approximately 1% of the chronic hepatitis B patients [29]. One of the reasons is that HBV DNA is integrated into the host genome, and viral covalently closed circular DNA (cccDNA) were detectable in the liver even after the patients recovered from acute hepatitis B. Therefore, future treatment strategies focused on the clearance of HBsAg in a significant proportion of patients were urgently needed. The researches are being carried out with direct-acting antivirals including HBV entry inhibitors, drugs targeting cccDNA or viral transcription or translation, nucleocapsid assembly modulators and approaches to decrease HBsAg release in serum [8, 29].
Phytocompounds have been a good source of drugs with favourable safety profile, and a majority of the world population living in the rural area have to depend on the plant-based medicines for their health from time immemorial. Therefore, it would be wise to explore them for their anti-HBV activities. The variety of researches (in vitro, in vivo, and clinical trials) have been made and reported in the international literature. This review was aimed to summarize the anti-HBV activities of plants which are grown, available or being used in Myanmar.
According to the literature search in PubMed, ScienceDirect and Google scholar with Keywords such as anti-hepatitis B viral infection, natural products/medicines, traditional medicine, phytocompounds, medicinal plants, it was found that a total of 162 plant species from 72 families were tested for their anti-HBV activity. These plants were described in the Table 1. From these 162 plants, those that may be available in Myanmar were identified by their inclusion in the Medicinal Plant List of Myanmar and A Checklist of the Trees, Shrubs, Herbs, and Climbers of Myanmar [27, 36]. Then, 35 plants were identified and their anti-hepatitis B activities were as follows:
Table 1.
The plants used in the anti-hepatitis B studies
| Family | Genus and Species | Family | Genus and Species | Family | Genus and Species |
|---|---|---|---|---|---|
| Acanthaceae |
Acanthus ilicifolius Andrographis paniculata |
Geraniaceae | Geranium carolinianum | Pinaceae | Pinus massoniana |
| Alisamataceae | Alisma orientalis | Ginkgoaceae | Ginkgo biloba | Piperaceae | Piper longum |
| Aloaceae | Aloe vera | Hypericaceae |
Cratoxylum formosum Hypericum perforatum |
Plantaginaceae | Plantago asiatica |
| Amaranthaceae | Alternanthera philoxeroides | Hypoxidaceae | Curculigo orchioides | Pleosporaceae | Alternaria brassicae |
| Annonaceae | Cananga odorata | Illiciaceae | Illicium henryi | Polygonaceae |
Polygonum cuspidatum Rheum palmatum |
| Apiaceae | Hydrocotyle sibthorpioides | Iridaceae | Iris confusa | Polypodiaceae | Microsorium fortunei |
| Apiceae |
Bupleuri radix (Bupleurum chinense, Bupleurum scorzonerifolium) Bupleurum sp. |
Lamiaceae |
Ocimum basilicum Perovskia atriplicifolia Scutellariae radix (Scutellaria baicalensis) |
Rhizophoraceae | Bruguiera gymnorrhiza |
| Arecaceae |
Areca catechu Euterpe precatoria |
Lauraceae | Machilus thunderbergii | Rhubiaceae | Uncaria rhynchophylla |
| Asteraceae |
Artemisia capillaris Artemisia morrisonensis Artemisia scoparia Aster tataricus Lactuca indica Pulicaria crispa Saussurea laniceps Saussurea lappa Senecio tsoongianus Taraxacum mongolicum |
Libiatae | Rabdosia japonica | Rosaceae |
Agrimonia coreana pilosella Agrimonia eupatoria Agrimonia pilosa Chaenomeles japonica Crataegus pinnatifida Potentilla anserine Rubus coreanus Sanguisorba officinalis |
| Bignoniaceae |
Jacaranda copaia Jacaranda obtusifolia |
Lilaceae | Liriope platyphylla | Rubiaceae |
Rubia cordifolia Warscewiczia coccinea |
| Bombacaceae | Bombyx mori | Linderniaceae | Lindernia ruellioides | Saxifragaceae | Saniculiphyllum guangxiense |
| Caesalpiniceae |
Caesalpinia sappan Cassia fistula |
Malvaceae |
Abelmoschus manihot Abutilon figarianum |
Schisandraceae |
Kadsura heteroclita Kadsura induta Kadsura japonica Schisandra wilsoniana |
| Capparaceae | Capparis decidua | Melastomataceae | Melastoma malabathricum | Scrophylariaceae | Lindera strychnifolia |
| Caprifoliaceae | Patrinia villosa | Menispermaceae | Pericampylus glaucus | Selaginellaceae | Selaginella moellendorffii |
| Caryophyllaceae |
Dianthus superbusn Stellaria media |
Mimosaceae |
Acacia mellifera Acacia oerfota Pithecellobium clypearia |
Sterculiaceae | Helicteres angustifolia |
| Clavicipitaceae | Metarhizium anisopliae | Moraceae |
Morus alba Streblus asper |
Thymelaeaceae | Stellera chamaejasme |
| Combretaceae |
Guiera senegalensis Terminalia chebula |
Moringaceae | Moringa oleifera | Urticaceae | Boehmeria nivea |
| Cornaceae | Cornus officinalis | Myrsinoideae | Ardisia chinensis | Violaceae | Viola diffusa |
| Cucurbitaceae |
Coccinia grandis Corallocarpus epigeus Herpetospermum caudigerum Momordica charantia Platycladus Orientalis Radix Trichosanthis (Trichosanthes kirilowii or Trichosanthes rosthornii) |
Myrtaceae |
Eucalyptus Maidenii Eugenia caryophyllate |
Viscaceae | Viscum coloratum |
| Cynomoriaceae | Cynomorium songaricum | Oleaceae | Jasminum officinale L. var. grandiflorum | Vitaceae | Vitis vinifer |
| Ephedraceae | Epedra sinica | Onagraceae | Oenanthe javanica | Vochysiaceae | Vochysia glaberrima |
| Euphorbiaceae |
Euphorbia fischeriana Euphorbia humifusa Phyllanthus acidus Phyllanthus amarus Phyllanthus emblica Phyllanthus gasstroemii Phyllanthus gunnii Phyllanthus hirtellus Phyllanthus multiflorus, Phyllanthus niruri Phyllanthus reticulatus Phyllanthus rheedei Phyllanthus similis Phyllanthus tenellus Phyllanthus urinaria Phyllanthus urinaria koreanis Phyllanthus urinaria Phyllanthus virgatus |
Ophioglossaceae | Ophioglossum pedunculosum | Zingiberaceae |
Alpinia officinarum Curcuma longa |
| Fabaceae |
Indigofera caerulea Senna silvestris Sophora alopecuroides Sophora flavescens Fucus vesiculosus (Fucoidan) |
Paeoniaceae | Paeonia lactiflora | NF | Rubusanus miqua |
| Gentianaceae |
Halenia elliptica Swertia chirayita Swertia cincta Swertia Delavayi Swertia macrosperma Swertia mileensis Swertia mussotii Swertia yunnanensis |
Papaveraceae |
Corydalis caucasica Corydalis rutifolia ssp. erdelii Corydalis rutifolia ssp. Kurdica Corydalis saxicola Corydalis solida ssp. Brachyloba Corydalis solida ssp. Solida Corydalis solida ssp. Tauricola Fumaria asepala Fumaria bracteosa Fumaria capreolata Fumaria cilicica Fumaria densiflora Fumaria flabellata Fumaria gaillardotii Fumaria judaica Fumaria kralikii subsp. Thuretii Fumaria macrocarpa Fumaria microcarpa Fumaria officinalis Fumaria parviflora Fumaria petteri Fumaria vaillantii |
NF = not found
Abelmoschus manihot (Sunset muskmallow or edible hibiscus)
According to an in vitro and in vivo study, the hyperoxide obtained from the ethanolic extract of Abelmoschus manihot was found to decrease HBsAg and hepatitis B e antigen (HBeAg) secretion in Hep G2.2.15 cells and was also found to inhibit the duck HBV DNA in concentration dependent manner in day 5, 10 and 13. This activity was compared with lamivudine (3TC), and the hyperoxide was inferior to 3TC except at day 13. The protective effect was confirmed by histopathological examination and it showed a significant improvement of the hepatocellular architecture in the ducklings [68].
Aloe vera (True aloe)
In HepG2.2.15 cell cultures, ethanolic extract of gel from the leaves of Aloe vera (AV) and anthraquinones from AV extract (aloe‐emodin, chrysophanol, and aloin B) were found to inhibit HBsAg and HBeAg synthesis by time dependent manner. The results showed that aloe‐emodin showed the maximal inhibition of viral antigens (aloe‐emodin > chrysophanol > aloin B > AV extract). Among the anthraquinones, the effect of aloe‐emodin was comparable with 3TC. There may also be synergistic interaction when aloe-emodin was given sequentially after 3TC and this combination increased the efficacy of aloe-emodin monotherapy by ~ 12%. These actions may occur possibly via inhibition of HBV polymerase activity according to the molecular docking study. Aloe‐emodin was also found to have the CYP3A4 activating property [48].
Alpinia officinarum (Lesser galangal)
In an in vitro and in vivo study from Korea, the aqueous extract of Alpinia officinarum showed the binding potency to the HBsAg in concentration dependent manners. It did not show hepatoprotective effects in galactosamine induced hepatotoxicity in rats [7].
Andrographis paniculata (Creat/Green Chirayta/King of bitters)
Chen et al., 2014, found that dehydroandrographolide and andrographolide isolated from Andrographis paniculata have anti-HBV DNA replication activity with IC50 values of 22.58 and 54.07 µM and low SI (selectivity index values of 8.7 and 3.7). To find out the structure–activity relationship (SAR), 48 derivatives of dehydroandrographolide and andrographolide were synthesized and evaluated for their anti-HBV activities. Among these derivatives, 14 derivatives were found to inhibit HBsAg secretion, 19 derivatives for HBeAg secretion and 38 derivatives for HBV DNA replication. Compound 4e (19-O-2’-thenoyl derivative) could inhibit not only HBsAg and HBeAg secretions but also HBV DNA replication with IC50 and SI values of 121 µM & 20.3, 19.7 µM & 125.0 and 23.5 µM & 104.9. The most active compound inhibiting HBV DNA replication was compound 2c (with 3,4,5-trimethoxycinnamic group at C-19) which has IC50 and SI values of 10.3 µM and > 165.1. In that study, tenofovir (TDF) was used as a positive control, and most of the compounds active against HBsAg and HBeAg were found to be more potent than TDF. However, for the HBV DNA, TDF was more potent with higher SI than the tested compounds [5].
Areca catechu (Betel-nut-palm)
An in vitro and in vivo study done in Korea reported that aqueous extract of Areca catechu (AC) showed the binding potency to the HBsAg and inhibitory activity of HBV DNA polymerase in concentration dependent manners. It can also inhibit the in vitro production of tumour necrosis factor (TNF) by macrophage. In rats, this extract did not exert hepatoprotective activity in galactosamine induced hepatotoxicity [7]. We should be cautious with the translation of anti-HBV activity of AC because AC was found to increase the risks of cirrhosis and HCC and, these risks were synergistic to the risk imposed by hepatitis B/C infections [69]. Another study found that betel quid chewing was found to be related to HCC, and it could be aggravated by chronic hepatitis B and hepatitis C [21].
Caesalpinia sappan (Sappan Wood)
A study from Korea reported that aqueous extract of Caesalpinia sappan was found to have the binding potency to the HBsAg, HBV DNA polymerase inhibition activity, and inhibitory activity on in vitro production of TNF by macrophage. But it did not have the hepatoprotective activity in galactosamine induced hepatotoxicity in rats [7].
Camellia sinensis (Green tea)
Epigallocatechin gallate (EGCG), a flavonoid that belongs to the subclass of catechin is the major constituent of Camellia sinensis. EGCG was found to inhibit HBV infection (HBV relaxed circular DNA (RC DNA) and HBsAg mRNA) and replication in HuS-E/2 cells. The same effect was seen in in vivo experiment in which EGCG injection can decrease HBV DNA and HBsAg levels in serum and HBcAg expression in livers of human chimeric mice [28].
Two studies which tested anti-HBV activities at transcriptional level with cccDNA and mRNA were reported by Xu et al., 2008 and He et al., 2011. According to Xu et al., 2008, green tea extract (GTE) and its principal component EGCG were found to inhibit the HBsAg, HBeAg, extracellular HBV DNA, intracellular replicative intermediates, intracellular HBV cccDNA, HBV mRNAs, and HBV x proteins. The inhibitory effect of both GTE and EGCG on HBsAg and HBeAg were more pronounced than the standard drug 3TC. Regarding extracellular DNA, intracellular replicative intermediates, and intracellular HBV cccDNA, the effects of GTE and EGCG were less than those of 3TC. One point to note is that 40 µg/mL of GTE could decrease the cccDNA level by more than 90%. GTE also had activity against HBV mRNAs and HBV x protein. Base on the findings, EGCG has a relatively weaker efficacy compared to GTE [71]. Another study done by He et al., 2011, confirmed the inhibitory activity of EGCG on HBeAg, HBV precore mRNA level, HBV cccDNA and replicative intermediates of DNA. According to the results, EGCG could not inhibit the HBsAg, transcription of precore mRNA and transcription of HBV pgRNA from chromosome-integrated HBV genome. These findings indicated that that EGCG might target only to the replicative intermediates of DNA synthesis [16].
Huang and colleagues also reported that EGCG could inhibit the HBV mRNA and HBsAg secretion in vitro but no effect on HBV genome replication or virion secretion. They also reported that EGCG has the ability to inhibit the entry of HBV into hepatocytes by inhibiting the sodium taurocholate cotransporting polypeptide (NTCP) which is responsible for high affinity attachment of HBV to hepatocytes [18].
An in vitro study done in HepG2 2.2.15 cells found that EGCG can inhibit the secretion of HBsAg and HBeAg, and this effect is more potent than the positive control 3TC. EGCG could also inhibit the intracellular and extracellular HBV DNA levels but its less potent than 3TC [47].
Another study done by Xu et al., 2016, also reported that EGCG could decrease the secretion of HBsAg and HBeAg, and HBV mRNA levels. This anti-HBV activity may be achieved by interacting with farnesoid X receptor alpha (FXRα) in liver cells which is required for the synthesis of HBV mRNA [72].
One study from China found that EGCG can effectively decrease the intracellular and extracellular HBV DNA replication. The underlying mechanism may be the EGCG enhance the lysosomal acidification, thereby opposing HBV-induced incomplete autophagy [79].
Green tea polyphenols suppressed HBsAg and strongly suppressed the secretion of HBeAg in a dose-dependent and time-dependent manner with IC50 value (for HBeAg) of 7.34 μg/mL. It could also significantly decrease the expression of HBV DNA in a dose-dependent fashion with IC50 of 2.54 μg/mL [74].
Another in vitro study found the anti-HBV activity of Pu-erh Tea, which is the fermented tea traditionally produced in Yunnan Province, China. Pu-erh Tea Extract and tea polyphenols, theaflavins, and theanine were found to inhibit the HBeAg secretion, HBV mRNA expression, HBV DNA levels, encapsidated DNA in intracellular core particles, and HBV x gene. Pu-erh Tea Extract could also significantly reduced intracellular reactive oxygen species level [49].
Capparis decidua (Bare caper/Caper berry/Leafless caper-bush)
According to a study done by Arbab et al., 2017, the ethanolic and aqueous extracts of the stem of Capparis decidua was found to inhibit the HBsAg secretion in vitro. The ethanolic extract has IC50—76.85 µg/mL with therapeutic index (TI) of 4.77, and aqueous extract has IC50—66.82 µg/mL with TI of 7.78. The qualitative phytochemical screening revealed alkaloids, flavonoids, tannins, and saponins were present in the active extracts [1].
Cassia fistula (Golden shower tree)
An in vitro study done in Japan reported that methanolic crude extract of the bark of Cassia fistula (CF) was found to reduce the HBV DNA and HBsAg levels in vitro. The HBV DNA level of HepG2-NTCP cells treated with CF extract (100 µg/mL) was 42.3 ± 6.8% with cell viability of 98.8 ± 3.6%. That effect was inferior to the 3TC (5 μM) which showed 0.7 ± 0.3% HBV DNA level with cell viability of 95.3 ± 4.7%. Regrading anti-HBsAg activity, HBsAg level for CF extract was 51.2 ± 2.6% with cell viability 91.9 ± 2.1% [19].
Curculigo orchioides (Golden eye-grass)
An in vitro study done by Zuo and colleagues found that three new phenolic glycosides (Curculigosides F–H) from the rhizome of Curculigo orchioides had anti-HBsAg activity but no anti-HBeAg activity. Among three compounds, compound 1 (Curculigosides F) had a potent anti-HBsAg activity with IC50 – 2.08 mM with SI of 0.65 compared to the positive control 3TC which has IC50 – 41.27 mM with SI of 1.30 [81].
Curcuma longa (Turmeric)
Kim et al., 2009 reported that aqueous extract of Curcuma longa (CL) rhizome decreased the secretion of HBsAg, HBV DNAs, and intracellular HBV RNAs in HepG 2.2.15 cells and this activity was mediated through enhancing the cellular accumulation of p53 protein. The CL extract did not show any cytotoxic effects on liver cells [26]. The other study done in Thailand found that buffer (Tris–HCl) and hydroalcoholic extracts of the bulb of CL significantly decreased the level of HBV cccDNA in the HepG2 cells (hydroalcoholic > buffer) compared to the hot water extract (which was used as a positive control in that study). These extracts did not show any cytotoxic effects on COS-7 and HepG2 cells [62].
Curcumin, the main bioactive ingredient of Curcuma species was tested for its anti-HBV activities in literature. Curcumin inhibited HBV replication by reducing the secretion of HBsAg (up to 73% reduction as compared to control) and by decreasing HBV Core protein level (up to 45% from baseline) in stably-transfected HepG2215 cells. Curcumin was also found to reduce the HBV mRNA levels (62%) more effectively than the 3TC (35%), and combination of these two treatments act synergistically and able to achieve up to 75% suppression of HBV expression. These inhibitory actions may be due to the down-regulation of Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) that initiates the gluconeogenesis cascade, and that has been shown to coactivate HBV transcription robustly [41]. Another study found that curcumin showed time and dose dependent reductions in HBsAg and HBeAg expression. It can also reduce the intracellular HBV DNA replication intermediates and cccDNA significantly in HepG2.2.15 cells. That study also determined the underlying mechanism and the results showed that curcumin inhibits HBV gene replication via downregulation of cccDNA-bound histone acetylation [66].
Epedra sinica (Ma huang/yellow horse/sea grape)
The aqueous extract of Epedra sinica had the binding potency to the HBsAg with the relatively weak inhibition on HBV DNA polymerase. It can also inhibit the in vitro production of TNF by macrophage. This extract did not show any hepatoprotective activity in galactosamine induced hepatotoxicity in rats [7].
Ginkgo biloba (Maidenhair tree)
Wang et al., 2014 found that nanoemulsion of polyprenols from Ginkgo biloba leaves had the ability to inhibit HBsAg, HBeAg and HBV DNA in HepG 2215 cells. Its anti-HBV activities were lower than the control drug 3TC except for HBeAg. It was also found to be non-toxic to normal cells and HepG2215 cells [64].
Glycyrrhiza glabra (Licorice)
In Japan, glycyrrhizin both in oral or parenteral form has been used for the treatment of chronic hepatitis B. According to the research done in Japan, glycyrrhizin, a component of liquorice (Glycyrrhiza glabra) roots, was found to increase the interferon γ production from lymphocyte-macrophage cultures derived from an asymptomatic carrier of hepatitis B virus in response to concanavalin A and to surface Antigen of Hepatitis B virus [55].
Takahara and colleagues found the mechanism of action of glycyrrhizin on HBsAg processing, intracellular transport, and secretion. In that study, glycyrrhizin was found to suppress the secretion of HBsAg, resulting in its accumulation in the cytoplasmic vacuoles in the Golgi apparatus area. Glycyrrhizin also suppressed the intracellular transport of HBsAg at the trans-Golgi area [57].
Authors from Japan reported that glycyrrhizin suppressed the secretion of HBsAg in PLC/PRF/5 cells. When glycyrrhizin, glycyrrhetic acid 3-O-monoglucuronide and glycyrrhetic acid were administered intravenously (IV) to guinea pigs, only glycyrrhizin, glycyrrhetic acid 3-O-monoglucuronide were detected in the livers. It indicates that glycyrrhizin given intravenously might bind to the hepatocytes to suppress the HBsAg secretion [52].
In the study reported by Chiang et al., 2005, glycyrrhizin was used as a positive control together with 3TC. According to the results, glycyrrhizin had IC50 of 119.3 ± 0.7 mg/L (SI – 1.4) for HBsAg and 150.1 ± 1.3 mg/L (SI – 1.1) for HBeAg, but it was less potent compared to 3TC (IC50 – 74.2 mg/L with SI – 3.7 for HBsAg and IC50 – 69.3 mg/L with SI – 3.9 for HBeAg) [6].
Glycyrrhetinic acid (GA) is the metabolite and pharmacologically active form of glycyrrhizin. In an in vitro study done in China, GA and its 57 derivatives were synthesized, and their anti-HBV activity was determined in HepG 2.2.15 cells, and SAR was also done. Among them, sixteen compounds showed greater anti-HBV activity than GA. Compounds 29, 32, 35, 41 could significantly inhibit HBV DNA replication with IC50 values of 5.71, 5.36, 8.90 and 9.08 µM respectively and all these compounds were found to be less potent than the positive control drug TDF. GA and compounds 1–3, 5, 13, 14, 17–22, 24, 25, 27, 34, 35, 39–42, 45, 46, 55, and 57 have the activity against HBsAg, and all these compounds were more potent than TDF. For HBeAg, GA and compound 2–4, 24 and 55 were found to be active and more potent than TDF [63].
In 2001, Matsuo and colleagues reported a case of chronic HBV carrier with non-Hodgkin lymphoma, who developed HBV hepatitis following conventional dose chemotherapy. The patient had elevated serum levels of transaminase and HBV-DNA due to HBV reactivation and was treated with 3TC (100 mg/day). HBV-DNA level was suppressed shortly after the treatment, and after additional treatment with glycyrrhizin (200 mg/day, IV), transaminase levels reached normal [38].
A pilot study done in India investigated the effects of 3TC and IV glycyrrhizin in subacute hepatitis B patients (n = 7). IV glycyrrhizin (60 mL/day for 7–10 days followed by three times a week) and ursodeoxycholic acid were given. After one week, oral 3TC (100 mg daily) was added. Patients’ ALT, AST and bilirubin normalized between 14 and 28 weeks after the onset of illness and 7 and 21 weeks after initiation of treatment. HBeAg to HBeAb seroconversion occurred in all patients by 21 weeks of therapy, and five patients became HBsAg negative during this period. HBsAg of the remaining two patients became negative 13 months and 18 months later [58].
In 1992, Eisenburg from Germany tested the therapeutic efficacy of Remefa S, a pharmaceutical comprising glycyrrhizinic acid, in chronic HBV patients. In that study, seven patients were treated with Remefa S (three times a week, short infusions) for 12 months. During the treatment and 10 months follow up, biochemical disease activity regressed in 4 patients, and HBeAg seroconversion occurred in 2 of them. One initial HBeAg negative patient became HBeAb positive during treatment and none of the patients experienced HBsAg seroconversion. Histological improvements were seen in 2 of these responders [12].
A randomized clinical trial was reported by the Zhang et al., 2002 and this study tested the efficacy and safety of Stronger Neo-Minophagen C (SNMC) (an IV drug with glycyrrhizin as the principal ingredient) in 194 patients with chronic HBV infection. Two doses of SNMC 100 mL/day and 40 mL/day (equivalent to 200 and 80 mg of glycyrrhizin) were given to group A and B of patients for four weeks. After that, all patients from group A and B received oral glycyrrhizin (GLYCYRON Tab) 9 tablets (equivalent to 225 mg of glycyrrhizin) a day for an additional four weeks. ALT levels became normal in 58 (58%) and 54 (57%) patients in Groups A and B, and Aspartate transaminase (AST) levels normalized in 57 (59%) and 58 (62%) respectively. Total bilirubin and γ-glutamyl transpeptidase were also found to be reduced. Only four patients were withdrawn from the study due to mild and reversible side effects such as chest distress and hypertension. Hypokalaemia occurred in two patients in group A and five in group B, and these patients did not leave the study. According to the results, glycyrrhizin administration for eight weeks was found to reduce liver enzymes in Chinese chronic HBV patients [76].
Hypericum perforatum (St. John's wort)
Hypericum perforatum (HP), commonly known as St John's Wort, was found to have anti-HBV activity in vitro. Ethanolic extract of HP was able to inhibit the secretion of HBsAg, HBeAg, and HBV DNA replication and transcription in HepG2 2.2.15 cells. For HBeAg, all tested concentrations of HP extract were found to be more potent than 3TC at 200 µg/mL, and for HBsAg, only 40 µg/mL of HP extract was more potent than 3TC. HP extract was found to be less potent than 3TC in the suppression of HBV DNA replication. According to that study, the HP extract may exert anti-HBV effects via inhibition of HBV transcription [46].
Hypericin prepared from St John's Wort was tested for its virucidal activity against duck hepatitis B virus (DHBV) in vivo. Hypericin was found to be slightly virucidal against DHBV, and it did not block the initiation of infection [40].
Jasminum officinale L. var. grandiflorum (Spanish Jasmine/French Jasmine)
Zhao and colleagues studied the anti-HBV activities of the two compounds oleuropein and 8-epi-kingiside from Jasminum officinale var. grandiflorum. Oleuropein was derived from flowers, and 8-epi-kingiside was from buds. Oleuropein was able to block HBsAg secretion in HepG2 2.2.15 cells in a dose-dependent manner with IC50 = 23.2 µg/mL whereas it had no effect on HBeAg. Oleuropein at 80 mg/kg, given intraperitoneally, twice daily was able to reduce DHBV DNA level in ducks, but oleuropein at all concentrations was less effective than 3TC at 50 mg/kg [77]. In another study, 8-epi-kingiside was found to be effectively blocked HBsAg secretion in HepG2 2.2.15 cells in a dose-dependent manner with IC50 = 19.4 ± 1.04 μg/mL. For HBeAg secretion, there was only a minimal inhibitory action on day 8. It was also found to reduce DHBV DNA level when it was given intraperitoneally at 40 or 80 mg/kg, twice daily in ducks but 8-epi-kingiside at all tested concentrations was less potent than 3TC at 50 mg/kg [78].
Melastoma malabathricum (Banks melastoma/Malabar melastome)
An in vitro study done by Indrasetiawan et al., 2019 reported that methanolic extract of the leaves of Melastoma malabathricum (MM) was found to reduce the HBV DNA and HBsAg levels in vitro. The HBV DNA level after treated with MM extract (100 µg/mL) was 90.5 ± 44.1% with cell viability 110.9 ± 3.3%, and this effect was weak compared to the 3TC (5 μM) which showed 0.7 ± 0.3% HBV DNA level with cell viability of 95.3 ± 4.7%. HBsAg level after treatment with MM extract was 40.1 ± 2.7% with cell viability 91.9 ± 2.4% [19].
Momordica charantia (Bitter melon/Bitter gourd)
Momordica charantia (MC), an edible plant, was found to have anti-HBV activities in HepG2 cells. The hydroalcoholic extracts of leaves and fruits of MC showed inhibitory activity on the level of HBV cccDNA (leaves > fruits). Buffer (Tris–HCl) extract of the leaves of MC found to have a mild cytotoxicity effect on the HepG2 cells [62]. An in vitro study done in HepG2.2.15 cells reported that recombinant MAP30 protein from bitter melon was found to inhibit the secretion of HBsAg and HBeAg in concentration and time dependent manners. MAP30 could inhibit extracellular HBV DNA in concentration dependent manner. It was also found to be capable of inhibiting cccDNA molecules [13].
In another study, α-momorcharin, type I ribosome-inactivating protein from bitter melon seeds, was found to inhibit the HBV DNA effectively (up to 58% on the 6th day) in dose and time dependent manners [73].
Moringa oleifera (Drumstick tree)
Hydroalcoholic and buffer (Tris–HCl) extracts of leaves and fruits of an edible plant Moringa oleifera (MO) was found to have a drastic inhibitory effect on cccDNA in HepG2 cells. Buffer extract of the leaves of MO had mild cytotoxicity on the HepG2 cells [62].
When the aqueous extract of MO leaves was tested in Huh7 cells expressing either HBV genotypes C or H, MO leaves extract could reduce the secretion of HBsAg with no cytotoxic effects. However, regarding replication, MO leaves extract can slightly reduce HBV DNA only for genotype C. It was also found to have antifibrotic, antioxidative, and anti-IL-6 activities [14].
Morus alba (Mulberry)
A naturally occurring aminosugar- 1-deoxynojirimycin derived from the Morus alba was found to inhibit the HBV maturation in a dose and time dependent manner. According to that study, the possible mechanism of antiviral action may be the inhibiting glycosylation of the viral envelope glycoproteins [20].
Mulberrofuran G and Isomulberrofuran G isolated from the ethanolic extract of root bark of Morus alba was found to reduce HBsAg, HBeAg and HBV DNA. In comparison with the positive control TDF, the two compounds were more potent in reducing HBs Ag and HBeAg but this potential therapeutic effect was limited by the low SI of < 1 with IC50 values of 14.34 and 33.77 µM for HBsAg and 61.55 and 183.10 µM for HBeAg. Regarding anti-HBV DNA activity, the two tested compounds were less effective than TDF with low SI of 2 (IC50 – 3.99 µM) and < 1 (IC50 – 9.1 µM) [15].
Ocimum basilicum (Sweet basil)
According to a study done by Chiang et al., 2005, the aqueous extract of Ocimum basilicum and selected purified components (apigenin, linalool and ursolic acid) were found to inhibit HBsAg and HBeAg. The ethanolic extract did not have such activities. For HBsAg inhibition activity, apigenin and ursolic acid were more potent than 3TC (linalool < ursolic acid < apigenin) and among them, apigenin had the strongest activity with half maximal effective concentration (EC50) of 7.1 ± 1.5 mg/L. Regarding HBeAg inhibition activity, aqueous extract, apigenin and ursolic acid had stronger activity than 3TC (Aqueous extract < ursolic acid < apigenin) and EC50 of apigenin was 12.8 ± 0.9 mg/L [6].
Paeonia lactiflora (Common garden peony)
Lee and colleagues from Korea reported an in vitro study about the anti-HBV activity of Paeonia lactiflora tested in HepG2.2.15 cell culture system. The ethyl acetate fraction of the methanolic extract of Paeonia lactiflora roots was shown to have over 70% suppression of HBV DNA replication with an IC50 value of 8.1 µg/mL. From that fraction, the active anti-HBV principle was isolated and identified as 1,2,3,4,6-Penta-O-galloyl-β-D-glucose (PGG) which had an IC50 value of 1.0 µg/mL, but it was less potent than that of 3TC (IC50 – 0.06 µg/mL). PGG could also suppress the HBsAg secretion with concentration dependent manner [31].
Perovskia atriplicifolia (Russian sage)
Jiang and colleagues from China identified the icetexane diterpenoids from ethanolic extract of Perovskia atriplicifolia and tested for their anti-HBV activities in HepG 2.2.15 cells. All identified compounds (1–8) were found to decrease HBsAg and HBeAg, and their IC50 values and SI values are more favourable than those of the control drug 3TC. Only compound 1 and 2 had the ability to suppress the HBV DNA levels, but their activities were less than that of 3TC (IC50 – 13.8 and 20.7 µM vs. 1.12 µM, SI – 154.3 and 137.7 vs. 26,750) [23].
Phyllanthus acidus (Gooseberry Tree/Star Gooseberry)
The roots and stems of Phyllanthus acidus were extracted with methanol, and 21 norbisabolane sesquiterpenoids were identified. The methanolic extract, sesquiterpenoids fraction and compounds 1–4, 7–10 and 14 were found to be active against HBeAg secretion. For HBsAg, only methanolic extract, sesquiterpenoids fraction and compounds 1 and 7–9 were found to be active. These anti-HBV activities were found to be more potent than 3TC. According to the SAR, the presence of 5-ketal group and sugar moieties may contribute to the selectivity of HBsAg and HBeAg [35].
Phyllanthus emblica (Indian gooseberry/Amla)
A polyphenolic compound, 1,2,4,6-tetra-O-galloyl-β-D-glucose (1246TGG), was isolated from the fresh leaves and branches of Phyllanthus emblica. 1246TGG at concentrations 3.13 and 6.25 µg/mL was found to inhibit the HBsAg and HBeAg in HepG2.2.15 cell culture, but the inhibitory effects tend to decline with time [70]. Another study done by same investigators reported that sesquiterpenoid glycosides were identified from the ethanolic extract of the roots of Phyllanthus emblica among them compound 6–10 had the ability to decrease HBsAg and HBeAg secretion and all except compound 10 were found to be more potent than 3TC [34].
Phyllanthus niruri/amarus (Gale of the wind)
In Myanmar traditional medicine practice, Phyllanthus amarus (PA) and its synonym, Phyllanthus niruri (PN) are locally known as Taung-zee-phyu and they were widely used for hepatitis and believed to have hepatoprotective activity [10, 11]. According to a study done in the USA, the aqueous extract PN of was found to inhibit HBV DNA polymerase and bind to HBsAg in vitro. The extract also showed inhibitory activity to woodchuck hepatitis virus DNA polymerase and binding to the surface antigen of that virus. In comparing the liver biopsies of woodchucks, more favourable histological findings were found in animals treated with PN extract [60]. An in vitro study done in China reported that the lignans isolated from methanolic extract of PN had anti-HBV activities in HepG 2.2.15 cells. Among the isolated compounds, nirtetralin A and nirtetralin B could effectively suppress the secretion of HBsAg and HBeAg with IC50 of 9.5 and 16.7 mM for HBsAg and 17.4 and 69.3 mM for HBeAg respectively [65]. Another recent study done in Indonesia found that dichloromethane and ethanolic extracts of PN could reduce HBsAg production from HepG2-NTCP cells with an IC50 value of 170.48 μg/mL. In HepAD38.7-Tet cells, PN extract could reduce the extracellular HBV DNA levels by 70% with no cytotoxicity. However, this activity was less effective than the positive control 3TC [61].
Aqueous extract of PA was found to suppress the HBsAg secretion but not HBeAg secretion in HepA2 cell lines and this effect occurred at mRNA level in a time-dependent manner [75]. This finding was confirmed by the other 2 studies which PA was found to inihibit HBV mRNA transcription in HUH-7 cells and was able to decrease the HBsAg mRNA transcription and HBV DNA polymerase activity in HepG2 2.2.15 cells [30, 45]. As the in vivo experiments, PA was tested with duck HBV in two studies from India. When PA was given orally or intraperitoneally, it could not reduce the HBV DNA in serum as well as liver of ducks and it only had minor effect on duck HBsAg production [44]. When the aqueous, butanol, and alcoholic extracts of PA were given intraperitoneally to Pekin ducks, they have no activity against duck HBV replicative intermediates in the liver and no definite antiviral property was observed in that study [42]. When PA was tested as preliminary study in 60 Indian HBV carriers, HBsAg loss was occurred in 59% of patients in treatment group compared to 4% in placebo group [59]. However, PA failed to show therapeutic activities in another 2 randomize placebo-controlled studies done in Netherland and Thailand. In these studies, PA oral treatment given orally for 28 and 30 days showed no significant difference in HBsAg, HBeAg, HBV DNA and liver enzymes compared to placebo [2, 32].
Phyllanthus reticulatus (Black-Honey Shrub/black-berried featherfoil)
According to an in vitro study done by Das et al., two semi-purified organic fractions of the fat free ethanolic extract of Phyllanthus reticulatus was shown to have anti-HBsAg activity especially at the higher concentration of 40 mg/mL [9].
Phyllanthus rheedei (Kozhikode Leaf-flower)
Suresh et al., 2014 screened the anti-HBV activity of different extracts (aquous, ethanol and hexane) of Phyllanthus rheedei on PLC/PRF, Hep3B, FLCII10 and HepG2215 cell lines. Among them, the ethanolic extract had the maximum activity in lowering HBsAg, HBV Core and HBV x protein and whole virions in concentration dependent manner. However, the inhibitory percentage of ethanolic extracts at all concentrations were found to be less effective than 3TC. Then, the ethanolic extract was divided into fractions, and the fractions with significant activity were found to have a significant quantity of phyllanthin, hypophyllanthin and ellagic acid [56].
Phyllanthus urinaria (Shatterstone/Leafflower/Chamber Bitter)
According to a study done by Shin et al., 2005, aqueous extract of Phyllanthus urinaria (PU) was tested for anti-HBV activity in HepG2 2.2.15 cells, and the active compound was identified as ellagic acid. Although ellagic acid could block HBeAg secretion effectively (IC50 = 0.07 µg/mL), it did not inhibit HBV polymerase activity, HBV replication and HBsAg secretion [54]. Aqueous extract of Phyllanthus urinaria koreanis was also found to inhibit HBsAg and HBcAg secretion, and DNA synthesis in 3TC resistant HBV. The extract produced these effects by inducing the expression of IFN-β, COX-2, and IL-6. Corilagin and gallic acid may be responsible for these anti-HBV activities [24].
Although PU was shown to have anti-HBV activity in vitro, it has no demonstrable antiviral effect in chronic hepatitis B patients. A double-blinded placebo-controlled study was done to investigate the antiviral effect of taking PU ethanolic extract for six months. All three doses of PU extract (1, 2 and 3 g) could not result in the significant difference in HBV DNA reduction, HBeAg seroconversion and Alanine transaminase (ALT) normalization compared to placebo. At 24 weeks after the cessation of treatment, no delayed virological or biochemical response was reported [3].
Piper longum (Long pepper)
An in vitro study was done in China to study the anti-HBV activity of the ethanolic extract of Piper longum in Hep G 2.2.15 cell line. Bioassay-guided fractionation with repeated purification resulted in the isolation of compound 1–11. Compounds 3, 4, 7, 9 and 10 were found to inhibit both HBsAg and HBeAg, and compound 8 inhibit only HBsAg. All these compounds were found to be more potent than the positive control 3TC. Among them, compound 7 (piperine) had remarkable inhibitory activity against HBsAg and HBeAg secretion with the SI values of 15.7 (IC50 – 0.15 mM) and 16.8 (IC50 – 0.14 mM), respectively [22].
Polygonum cuspidatum (Japanese Knotweed)
Aqueous and ethanolic extract of Polygonum cuspidatum were tested against HBV in HepG2 2.2.15 cells. The ethanolic extract could inhibit the production of HBV dose-dependently with the minimal effective dosage of 10 µg/mL. At higher doses of 30 µg/mL, the aqueous extract inhibited the production of HBV and HBeAg. Interestingly, the expression of HBsAg was significantly increased by both ethanol and water extracts in dose and time dependent manner [4].
Rubia cordifolia (Common madder)
According to the study done by Ho and colleagues from Taiwan, three naphthohydroquinones were isolated from the methanolic extract from the Rubia cordifolia roots. Among them, compound 1 and 2 (furomollugin and mollugin) strongly suppressed the secretion of HBsAg both with IC50 = 2.0 µg/mL, in human hepatoma Hep3B cells with little cytotoxicity. SAR study on 9 structurally related derivatives of compound 1 and 2 revealed that a 6-hydroxy group and a pyran or furan ring contribute to this therapeutic effect [17].
Swertia chirayita (Chirayita)
Although seven different species of Swertia were tested for their anti-HBV activity, only the Swertia chirayita was known to be available in Myanmar. An in vitro study was done in HepG 2.2.15 cells line to investigate the anti-HBV activity of Swertia chirayita. Four new compounds and 26 known compounds were identified from ethanol–water extract of the whole plant. Among them, compounds 14 and 19 could inhibit the HBsAg secretion with IC50 values of 0.31 mM (SI—4.29) and 1.49 mM (SI – 1.23) respectively. In comparison with the TDF (with IC50—1.25, SI > 1.39), compound 14 (( +)-cycloolivil-4′-O-β-D-glucopyranoside) was found to be more potent in anti-HBsAg activity with favourable SI. For HBeAg, compounds 14 and 28 had the ability to suppress the HBeAg secretion with IC50 values of 0.77 mM (SI – 1.75) and 5.92 mM (SI—> 1.21) respectively and again, compound 14 was found to be more potent than TDF with IC50—1.21, SI > 1.44. Eight compounds (8,9,13,14,24–26,29) had activity against HBV DNA replication with IC50 values ranging between 0.07 and 0.33 mM, but all these compounds were found to be less potent than TDF [80].
Terminalia chebula (Chebulic Myrobalan/Gall nut)
According to a study done in Korea, aqueous extract of Terminalia chebula (TC) (leaves, nuts and roots) had the ability to bind to HBsAg and inhibit HBV DNA polymerase in vitro. It was also found to inhibit the in vitro production of TNF by macrophage. It did not have hepatoprotective activity in galactosamine induced hepatotoxicity in rats [7].
Another study from Korea reported that aqueous extract of TC plants was found to decrease extracellular HBV DNA levels and inhibit the secretion of HBsAg in HepG2 2.2.15 cells. The significant inhibition was seen in 128, 256 and 512 µg/mL concentrations and TC extract was found to be more potent than the positive control dideoxycytidine (10 µM). Among the tested plants in that study, TC has the most prominent anti-HBV activities compared to Sanguisorba officinalis, Rubus coreanus and Rheum palmatum [25].
The study done by Mohan and colleagues from India reported that the ethanolic extract of TC fruits inhibited HBsAg and HBV DNA polymerase. TC fruit extract showed 62.8% inhibition of HBV DNA polymerase, but it was inferior to 3TC with 81.66% inhibition. It also induced a significant level of IFN-γ and IL-2 in peripheral blood mononuclear cell culture [39].
An in vitro study done by Rajarajan from India found that the lyophilized aqueous and ethanolic extracts of TC had the ability to inhibit HBsAg binding and HBV DNA polymerase. In comparison with 3TC, the aqueous extract of TC was more active in inhibiting HBV DNA polymerase, and ethanolic extract has equal activity in inhibiting HBsAg binding [50].
Vitis vinifer (Grapevine/Grape)
Total triterpene (VTT), total flavonoids (VTF) and total polysaccharides (VTP) from Suosuo grapes (Vitis vinifer) were found to suppress the secretion of HBsAg, HBeAg, HBV DNA in HepG2.2.15 cells. The VTT and VTP were more potent in inhibiting HBsAg secretion with IC50 values of 62.54 µg/mL (TI – 2.57) and 112.69 µg/mL (TI – 12.54) compared with 3TC (IC50 = 315.37 µg/mL (TI – 0.78). For HBeAg, VTT, VTP and VTF were more potent than 3TC (89.98, 204.46, 215.34 vs 9051.01 µg/mL) with more favourable TI. Regarding HBV DNA inhibition, all preparations were found to be inferior to 3TC. The combination of VTT 20 μg/mL, VTF 50 μg/mL and VTP 50 μg/mL had the best inhibitory effects on HBeAg secretion [33]. However, we should interpret these results cautiously because resveratrol, a polyphenol found in a variety of plants, including grapes, was found to enhance the HBV virus replication in vitro and in vivo [53].
The anti-HBV acitivities of each plant species and their local Myanmar names were summarized in Table 2.
Table 2.
Summary of anti-HBV activities of Myanmar medicinal plants
| No | Species and local Myanmar names | Anti-HBV activities | Ref: |
|---|---|---|---|
| 1 |
Abelmoschus manihot (Kon-kado, Okra) |
In vitro and in vivo study (hyperoxide from ethanolic extract) HBsAg & HBeAg duck hepatitis B DNA < Lamivudine histological improvement |
[68] |
| 2 |
Aloe vera (Shar-saung-lat-pat-pin) |
In vitro study (extract & anthraquinones) HBsAg, HBeAg synthesis (aloe‐emodin > chrysophanol > aloin B > ethanol extract) (aloe-emodin = lamivudine) synergistic interaction with lamivudine Activate CYP3A4 activation |
[48] |
| 3 |
Alpinia officinarum (Pa-tal-kaw-lay) |
In vitro and in vivo study (Aqueous extract) binding potency to the HBsAg no hepatoprotective action |
[7] |
| 4 |
Andrographis paniculata (Say-khar-gyi) |
In vitro study (dehydroandrographolide, andrographolide and their 48 derivatives) - HBsAg & HBeAg secretions > Tenofovir DNA replication < Tenofovir |
[5] |
| 5 |
Areca catechu (Kun-thee) |
In vitro and in vivo study (Aqueous extract) binding potency to the HBsAg HBV DNA polymerase, TNF production by macrophage no hepatoprotective action |
[7] |
| 6 |
Caesalpinia sappan (Tane-nyet) |
In vitro and in vivo study (Aqueous extract) binding potency to the HBsAg HBV DNA polymerase, TNF production by macrophage no hepatoprotective action |
[7] |
| 7 |
Camellia sinensis (Green tea) (Lat-phat-sane) |
In vitro and in vivo studies (Green tea extract & EGCG) HBV RC DNA, HBsAg mRNA, HBV DNA replication, HBsAg, HBcAg (HBsAg, HBeAg > Lamivudine), (extracellular HBV DNA, intracellular replicative intermediates, cccDNA < Lamivudine), HBV mRNAs, HBV x (EGCG < extract) HBeAg, HBV precore mRNA level, HBV cccDNA and replicative intermediates of DNA HBsAg, HBV mRNA, entry (HBsAg, HBeAg > Lamivudine), (intracellular/extracellular HBV DNA < Lamivudine) HBsAg, HBeAg, HBV mRNA intracellular/extracellular HBV DNA HBsAg, HBeAg, HBV DNA (Pu-erh Tea extract) HBeAg, HBV mRNA, HBV DNA, encapsidated DNA in intracellular core particles, HBV x, reduce ROS |
[13–21] |
| 8 |
Capparis decidua (Kari-la-pin) |
In vitro study (ethonolic& aqueous extract, stem) HBsAg |
[1] |
| 9 |
Cassia fistula (Ngu-pin) |
In vitro study (methanolic crude extract, bark) HBsAg, HBV DNA < Lamivudine |
[19] |
| 10 |
Curculigo orchioides (Ka-nyut-net) |
In vitro study (phenolic glycosides (Curculigosides F–H) from rhizome) HBsAg, Curculigosides F > Lamivudine |
[81] |
| 11 |
Curcuma longa (Na-nwin) |
In vitro studies (extracts and curcumin) HBsAg, HBeAg, intracellular HBV RNA cccDNA HBV mRNA > Lamivudine (synergistic with Lamivudine) HBsAg, HBeAg, intracellular HBV DNA replication intermediates, cccDNA |
[25–28] |
| 12 |
Epedra sinica (Pan-nar-yin-kyat-say-pin) |
In vitro and in vivo study (Aqueous extract) Binding potency to the HBsAg HBV DNA polymerase, TNF production by macrophage No hepatoprotective action |
[7] |
| 13 |
Ginkgo biloba (Kabar-oo-pin) |
In vitro study (polyprenols from leaves) HBsAg, HBV DNA < Lamivudine, HBeAg > Lamivudine |
[64] |
| 14 |
Glycyrrhiza glabra (Nwe-cho) |
In vitro studies (Glycyrrhizin) Increase IFN γ production from macrophage HBsAg (processing, intracellular transport, secretion) HBsAg, HBeAg < Lamivudine (Glycyrrhetinic acid) (HBsAg & HBeAg > Tenofovir), (HBV DNA < Tenofovir) In vivo study (Glycyrrhizin) HBsAg (bind to the hepatocytes) Clinical studies (Glycyrrhizin) Liver enzymes (case report) HBsAg, HBeAg, liver enzymes (pilot study) (Glycyrrhizinic acid) Liver enzymes (randomized controlled trial) |
[30–38] |
| 15 |
Hypericum perforatum (St John's Wort) (Taung-yaychan-yar-pin) |
In vitro studies (ethonolic extract) (HBsAg & HBeAg > Lamivudine), (HBV DNA replication and transcription < Lamivudine) In vivo studies (Hypericin) duck HBV (slight) |
[46, 40] |
| 16 |
Jasminum officinale L. var. grandiflorum (Myat-lay-pan (White)) |
In vitro & in vivo studies (oleuropein from flowers) HBsAg, Duck HBV DNA < Lamivudine (8-epi-kingiside from buds) HBsAg, HBeAg (minimal), Duck HBV DNA < Lamivudine |
[77, 78] |
| 17 |
Melastoma malabathricum (Nyaung-yayoh-pan-pin/Say-oh-pote-pin) |
In vitro study (methanolic extract, leaves) HBsAg, HBV DNA < Lamivudine |
[19] |
| 18 |
Momordica charantia (Kyet-hin-khar) |
In vitro studies (hydroalcoholic extracts, leaves & fruits) cccDNA (Leaves > Fruits) (Recombinant MAP30) HBsAg, HBeAg, extracellular HBV DNA, cccDNA (α-momorcharin from seeds) HBV DNA |
[62, 13, 73] |
| 19 |
Moringa oleifera (Dant-tha-lun-pin) |
In vitro studies (Hydroalcoholic and buffer extracts of leaves and fruits) cccDNA (significant) (leaves and fruits) HBsAg, DNA (slightly), antifibrotic, antioxidative, and anti-IL-6 activities (leaves) |
[62, 14] |
| 20 |
Morus alba (Poe-sar-pin (White)) |
In vitro studies (1-deoxynojirimycin) HBV maturation (Mulberrofuran G and Isomulberrofuran G from root bark) (HBsAg & HBeAg > Tenofovir), HBV DNA < Tenofovir |
[20, 15] |
| 21 |
Ocimum basilicum (Pin-sane-a-yine) |
In vitro study (Aqueous extract and apigenin, linalool and ursolic acid) HBsAg (linalool < Aqueous extract < lamivudine < ursolic acid < apigenin), HBeAg (linolool < lamivudine < Aqueous extract < ursolic acid < apigenin) |
[6] |
| 22 |
Paeonia lactiflora (Thila-sandara) |
In vitro study (ethyl acetate fraction of methanolic extract) HBV DNA (1,2,3,4,6-Penta-O-galloyl-β-D-glucose) HBV DNA < Lamivudine, HBsAg |
[31] |
| 23 |
Perovskia atriplicifolia (Ma-ya-thein) |
In vitro study (icetexane diterpenoids from ethanolic extract) HBsAg & HBeAg > Lamivudine, HBV DNA < Lamivudine |
[23] |
| 24 |
Phyllanthus acidus (Thinbaw-zee-phyu) |
In vitro study (methanolic extract, sesquiterpenoids fraction and 21 norbisabolane sesquiterpenoids, roots and stems) HBsAg & HBeAg > Lamivudine |
[35] |
| 25 |
Phyllanthus emblica (Zee-phyu) |
In vitro studies (1,2,4,6-tetra-O-galloyl-β-D-glucose from leaves & branches) HBsAg, HBeAg (sesquiterpenoid glycosides from the ethanolic extract of the roots) HBsAg & HBeAg > Lamivudine |
[70, 34] |
| 26 |
Phyllanthus niruri/amarus (Taung-zee-phyu) |
In vitro & in vivo studies HBV mRNA transcription, HBsAg mRNA transcription, HBV DNA polymerase activity duck HBsAg production (aqueous extract) Bind to HBsAg, HBV DNA polymerase, DNA polymerase and surface antigen of woodchuck hepatitis virus, histological improvement HBsAg (mRNA level) (nirtetralin A & nirtetralin B from methanolic extract) HBsAg, HBeAg (dichloromethane and ethanolic extracts) HBsAg, (HBV DNA < Lamivudine) (aqueous, butanol, and alcoholic extracts) no activity against duck HBV replicative intermediates Clinical studies HBsAg loss (study from India) No significant difference in HBsAg, HBeAg, HBV DNA and liver enzymes (studies from Netherland and Thailand) |
[55–65] |
| 27 |
Phyllanthus reticulatus (Ye-chin-yar) |
In vitro study (2 semi-purified organic fractions of the fat free ethanolic extract) HBsAg |
[9] |
| 28 |
Phyllanthus rheedei (Ma-shaw, Ma-shaw-se) |
In vitro study (aqueous, ethanol & hexane extracts) HBsAg, HBV Core, HBV x protein and whole virions Ethanolic extract – most active but < Lamivudine |
[56] |
| 29 |
Phyllanthus urinaria (Myay-zee-phyu) |
In vitro study (aqueous extract) HBeAg (ellagic acid) HBsAg, HBcAg, DNA synthesis in lamivudine resistant HBV (Corilagin and gallic acid) Clinical study No demonstrable antiviral effect in chronic hepatitis B patients (randomized controlled trial) |
[68–70] |
| 30 |
Piper longum (Pate-chin) |
In vitro study (11 compounds from ethanolic extract) HBsAg & HBeAg > Lamivudine |
[22] |
| 31 |
Polygonum cuspidatum (Japan-bote-taung-pin) |
In vitro study (Aqueous & ethanolic extract) HBV production & HBeAg HBsAg—increase* |
[4] |
| 32 |
Rubia cordifolia (Htan-kyint, Pal-saint-ni) |
In vitro study (3 naphthohydroquinones from the methanolic extract, roots) HBsAg (furomollugin & mollugin) |
[17] |
| 33 |
Swertia chirayita (Thinbaw-say-khar-gyi) |
In vitro study (compounds from ethanol–water extract, whole plant) HBsAg & HBeAg > Tenofovir HBV DNA < Tenofovir |
[80] |
| 34 |
Terminalia chebula (Phan-khar-pin) |
In vitro & in vivo studies (aqueous extract from leaves, nuts & roots) HBsAg, DNA polymerase, reduce TNF from macrophage, no hepatoprotective action HBsAg, HBV DNA > Dideoxycytidine (ethanolic extract of fruits) HBsAg, DNA polymerase < Lamivudine, induce IFN-γ and IL-2 (lyophilized aqueous & ethanolic extracts) HBsAg binding (Ethanolic extract = Lamivudine), DNA polymerase (Aqueous > Lamivudine) |
[7, 75–77] |
| 35 |
Vitis vinifera (Sa-pyit-pin) |
In vitro study (Total triterpene, total flavonoids & total polysaccharides) HBsAg & HBeAg < Lamivudine HBV DNA < Lamivudine |
[33] |
> Superior to, < Inferior to, = comparable to
HBsAg—Hepatitis B surface antigen, HBeAg—Hepatitis B envelope antigen, DNA—Deoxyribonucleic acid, CYP3A4—Cytochrome P450 3A4, TNF—Tumour necrosis factor, EGCG—Epigallocatechin gallate, RC DNA- relaxed circular DNA, RNA—Ribonucleic acid, mRNA—Messenger RNA, cccDNA—covalently closed circular DNA, ROS—Reactive oxygen species, IFN γ—Interferon gamma, IL2—interleukin-2
Anti-HBV mechanisms of the selected plants
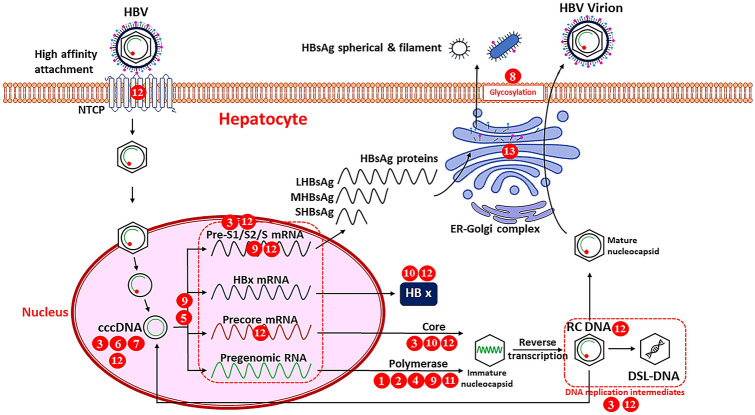
The mechanism of actions of the aforementioned selected medicinal plants with respect to the life cycle of HBV was summarized in Fig. 1. The NTCP serves as a high affinity attachment site for large HBsAg protein (LHBsAg) when HBV enter the hepatocytes. EGCG had the ability to inhibit NTCP thereby inhibiting HBV entry into hepatocytes [18]. The role of cccDNA in cure of HBV infection is emerging and it may be the potential target for the therapeutic cure of chronic HBV infection. Extracts of Curcuma longa bulb, MAP30 protein and leaves and fruits extract of Momordica charantia, extracts of Moringa oleifera leaves and fruits, and green tea extract and EGCG showed the inhibitory activities against HBV cccDNA [13, 16, 62, 66, 72]. Hypericum perforatum and Phyllanthus amarus extracts may inhibit the HBV transcription into mRNA [30, 45, 46]. Curcuma longa extract/curcumin and green tea/Pu-erh tea extracts and tea polyphenols, and EGCG acts by reducing the HBV RNA levels [18, 26, 41, 49, 71, 72]. EGCG was found to inhibit the precore mRNA and both EGCG and Phyllanthus amarus extract were found to reduce HBsAg mRNA levels [16, 28, 75]. Phyllanthus rheedei extract, green tea/Pu-erh tea extract and tea polyphenols, and EGCG were found to inhibit the HBV x protein which may play a role in HCC development, proliferation and metastasis [49, 56, 71]. Curcumin, Phyllanthus rheedei extract and Pu-erh tea extract and tea polyphenols could inhibit HBV core protein levels and encapsidated DNA in intracellular core particles in vitro [41, 49, 71]. HBV DNA polymerase is the key enzyme in the replication of HBV and most of numerous plants extracts such as Aloe vera, Caesalpinia sappan, Epedra sinica, Phyllanthus niruri/amarus, and Terminalia chebula were found to inhibit HBV DNA polymerase [7, 39, 48, 50, 60]. Curcumin, green tea extract and EGCG had the ability to lower the HBV DNA replicative intermediates within the cells and specifically, EGCG inhibit the HBV RC DNA thereby inhibiting HBV infection [13–15, 71]. The spherical HBsAg were secreted mainly via the ER-Golgi complex and glycyrrhizin was found to suppress the intracellular transport of HBsAg at the trans-Golgi area thereby reducing the HBsAg secretion [57]. N-linked glycosylation is essential for the maturation of enveloped viruses including HBV, and 1-deoxynojirimycin from Morus alba was found to inhibit the glycosylation of the viral envelope glycoproteins thereby inhibiting the secretion of HBV and HBsAg [20].
Fig. 1.
The anti-HBV mechanisms of selected plants and compounds in the life cycle of the Hepatitis B virus. 1—Aloe vera; 2—Caesalpinia sappan; 3—Curcuma longa; 4—Epedra sinica; 5—Hypericum perforatum; 6—Momordica charantia; 7—Moringa oleifera; 8—Morus alba; 9—Phyllanthus niruri/amarus; 10—Phyllanthus rheedei; 11—Terminalia chebula; 12—EGCG; 13—Glycyrrhizin. (HBV – hepatitis B virus; NTCP—sodium taurocholate cotransporting polypeptide; cccDNA—covalently closed circular DNA; mRNA—messenger RNA; RC DNA—relaxed circular DNA; DSL-DNA—double-stranded linear DNA; L/M/SHBsAg—large/middle/small HBsAg, ER—endoplasmic reticulum) (Fig. 1 was adapted from (4))
Discussion
Most of the identified studies were in vitro ones. In vivo and clinical studies were relatively rare. Therefore, potential clinical benefits, dosage consideration and drug interaction information could not be deducted from the current evidence.
Among the aforementioned plants, the genus Phyllanthus species were tested in many studies, including human trials. Although anti-HBV activities were shown in in vitro experiments, the in vivo and clinical trials failed to show therapeutic benefits. This may be due to the formulation or pharmacokinetic issues which may be solved in the future studies with new compounds or formulations from these species.
Regarding the methodologies in testing the anti-HBV activities, most of the in vitro tests were done in human hepatoma cell lines such as HepG2 cells. These cell lines were transfected with HBV and activities were tested by measuring their secretions of HBV antigens and viral DNA etc. For in vivo tests, other members of hepadnaviruses family such as woodchuck, ground squirrel, and duck hepatitis viruses were used instead of HBV due to the limited host range of HBV, i.e. humans [43].
The treatment guidelines for hepatitis B was changed from time to time. Therefore, the studies done in couple of decades ago used lamivudine as a positive control drug with which the tested compounds/extracts are compared for their potency and efficacy. Currently, tenofovir and entecavir were regarded as the treatment of choice for chronic hepatitis B [29] and the studies that used tenofovir or entecavir as a control drug were relatively scarce up to now. In interpreting the results of the studies that compare with lamivudine, we should be cautious because lamivudine has one major concern of antiviral-resistant mutations with long-term treatment [51].
According to the results of the included studies, plant-based preparations/compounds are generally more potent in inhibiting the HBsAg and HBeAg in comparison to the control drugs such as 3TC or tenofovir. However, they become less potent than those control drugs when comparing their inhibitory activity against HBV DNA. Therefore, the future treatment strategies would be the combination of plant-based medicines with nucleos(t)ide analogue reverse-transcriptase inhibitors to achieve the better therapeutic outcomes.
Most of the studies mainly focused on the HBsAg, HBeAg and HBV DNA. Clinical studies also tested the HBeAg seroconversion, HBsAg clearance, transaminases levels and imaging. Recent clinical guidelines redefined the role of HBsAg quantitative levels and HBV cccDNA in defining the optimal endpoint [8, 29]. Although the evidence is accumulating, studies that determined those parameters were not very common in international literature. This review may provide some summarized information about the anti-HBV activities of Myanmar medicinal plants and compounds. Future researches should be carried out to identify the novel compounds and for better understanding of the underlying mechanisms in treating HBV infection.
Acknowledgements
We wish to thank the staffs of Research and Development Department and Executive Office of FAME Pharmaceuticals Industry Co., Ltd. for their kind help and cooperation.
Declaration
Conflict of interest
Authors declare that they have no conflict of interest. We declared that this manuscript/data has not been published or currently under review for publication elsewhere.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arbab AH, Parvez MK, Al-Dosari MS, et al. In vitro evaluation of novel antiviral activities of 60 medicinal plants extracts against hepatitis B virus. Exp Ther Med. 2017;14(1):626–634. doi: 10.3892/etm.2017.4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berk L, de Man RA, Schalm SW, et al. Beneficial effects of Phyllanthus amarus for chronic hepatitis B, not confirmed. J Hepatol. 1991;12(3):405–406. doi: 10.1016/0168-8278(91)90850-B. [DOI] [PubMed] [Google Scholar]
- 3.Chan HLY, Sung JJY, Fong WF, et al. Double-blinded placebo-controlled study of Phyllanthus urinaris for the treatment of chronic hepatitis B. Aliment Pharmacol Ther. 2003;18(3):339–345. doi: 10.1046/j.1365-2036.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 4.Chang J, Liu H, Wang K, et al. Ethanol extract of Polygonum cuspidatum inhibits hepatitis B virus in a stable HBV-producing cell line. Antivir Res. 2005;66(1):29–34. doi: 10.1016/j.antiviral.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Chen H, Ma YB, Huang XY, et al. Synthesis, structure–activity relationships and biological evaluation of dehydroandrographolide and andrographolide derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett. 2014;24(10):2353–2359. doi: 10.1016/j.bmcl.2014.03.060. [DOI] [PubMed] [Google Scholar]
- 6.Chiang LC, Ng LT, Cheng PW, et al. Antiviral activities of extracts and selected pure constituents of Ocimum basilicum. Clin Exp Pharmacol Physiol. 2005;32(10):811–816. doi: 10.1111/j.1440-1681.2005.04270.x. [DOI] [PubMed] [Google Scholar]
- 7.Chung TH, Kim JC, Kim MK, et al. Investigation of Korean plant extracts for potential phytotherapeutic agents against B-virus hepatitis. Phytother Res. 1995;9(6):429–434. doi: 10.1002/ptr.2650090609. [DOI] [Google Scholar]
- 8.Cornberg M, Wong VWS, Locarnini S, et al. The role of quantitative hepatitis B surface antigen revisited. J Hepatol. 2017;66(2):398–411. doi: 10.1016/j.jhep.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Das BK, Shohel M, Pavel AM, et al. Anti hepatitis B viral activity of Phyllanthus reticulatus. Bangladesh Pharm J. 2011;14(1):4. [Google Scholar]
- 10.Department of Traditional Medicine. Medicinal Plants of Myanmar (Vol. 2). Ministry of Health. Naypyitaw; 2007.
- 11.Department of Traditional Medicine. Myanmar Herbal Pharmacopoeia (Vol. 2). Ministry of Health and Sports. Naypyitaw; 2018.
- 12.Eisenburg J. Treatment of chronic hepatitis B. Part 2: effect of glycyrrhizic acid on the course of illness. Fortschr Med. 1992;110(21):395–398. [PubMed] [Google Scholar]
- 13.Fan JM, Zhang Q, Xu J, et al. Inhibition on Hepatitis B virus in vitro of recombinant MAP30 from bitter melon. Mol Biol Rep. 2009;36(2):381–388. doi: 10.1007/s11033-007-9191-2. [DOI] [PubMed] [Google Scholar]
- 14.Feustel S, Ayón-Pérez F, Sandoval-Rodriguez A, et al. Protective effects of Moringa oleifera on HBV Genotypes C and H transiently transfected Huh7 cells. J Immunol Res. 2017;2017:1–9. doi: 10.1155/2017/6063850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng CA, Ma YB, Zhang XM, et al. Mulberrofuran G and Isomulberrofuran G from Morus alba L.: Anti-hepatitis B virus activity and mass spectrometric fragmentation. J Agric Food Chem. 2012;60(33):8197–8202. doi: 10.1021/jf302639b. [DOI] [PubMed] [Google Scholar]
- 16.He W, Li LX, Liao QJ, et al. Epigallocatechin gallate inhibits HBV DNA synthesis in a viral replication—inducible cell line. World J Gastroenterol. 2011;17(11):1507–1514. doi: 10.3748/wjg.v17.i11.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho LK, Don MJ, Chen HC, et al. Inhibition of hepatitis b surface antigen secretion on human Hepatoma Cells. Components from Rubia cordifolia. J Nat Prod. 1996;59(3):330–333. doi: 10.1021/np960200h. [DOI] [PubMed] [Google Scholar]
- 18.Huang HC, Tao MH, Hung TM, et al. (−)-Epigallocatechin-3-gallate inhibits entry of hepatitis B virus into hepatocytes. Antivir Res. 2014;111:100–111. doi: 10.1016/j.antiviral.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 19.Indrasetiawan P, Aoki-Utsubo C, Hanafi M, et al. Antiviral activity of Cananga odorata against hepatitis B virus. Kobe J Med Sci. 2019;65(2):E71–79. [PMC free article] [PubMed] [Google Scholar]
- 20.Jacob JR, Mansfield K, You JE, et al. Natural iminosugar derivatives of 1-Deoxynojirimycin inhibit glycosylation of hepatitis viral envelope proteins. J Microbiol. 2007;45(5):431–440. [PubMed] [Google Scholar]
- 21.Jeng JE, Tsai MF, Tsai HR, et al. Impact of Chronic Hepatitis B and Hepatitis C on adverse hepatic fibrosis in hepatocellular carcinoma related to betel quid chewing. Asian Pac J Cancer Prev. 2014;15(2):637–642. doi: 10.7314/APJCP.2014.15.2.637. [DOI] [PubMed] [Google Scholar]
- 22.Jiang ZY, Liu WF, Zhang XM, et al. Anti-HBV active constituents from Piper longum. Bioorg Med Chem Lett. 2013;23(7):2123–2127. doi: 10.1016/j.bmcl.2013.01.118. [DOI] [PubMed] [Google Scholar]
- 23.Jiang ZY, Yu YJ, Huang CG, et al. Icetexane Diterpenoids from Perovskia atriplicifolia. Planta Med. 2015;81(03):241–246. doi: 10.1055/s-0034-1396151. [DOI] [PubMed] [Google Scholar]
- 24.Jung J, Kim NK, Park S, et al. Inhibitory effect of Phyllanthus urinaria L. extract on the replication of lamivudine-resistant hepatitis virus in vitro. BMC Complement Altern Med. 2015;15(1):255. doi: 10.1186/s12906-015-0792-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim TG, Kang SY, Jung KK, et al. Antiviral activities of extracts isolated from Terminalis chebula retz, Sanguisorba officinalis L, Rubus coreanus miq and Rheum palmatum L against hepatitis B virus. Phytother Res. 2001;15(8):718–720. doi: 10.1002/ptr.832. [DOI] [PubMed] [Google Scholar]
- 26.Kim HJ, Yoo HS, Kim JC, et al. Antiviral effect of Curcuma longa Linn extract against hepatitis B virus replication. J Ethnopharmacol. 2009;124(2):189–196. doi: 10.1016/j.jep.2009.04.046. [DOI] [PubMed] [Google Scholar]
- 27.Kress WJ. A checklist of the trees, shrubs, herbs, and climbers of Myanmar: Vol. Contributions from the United States National Herbarium. Department of Systematic Biology—Botany, National Museum of Natural History; 2003.
- 28.Lai YH, Sun CP, Huang HC, et al. Epigallocatechin gallate inhibits hepatitis B virus infection in human liver chimeric mice. BMC Complement Altern Med. 2018;18(1):248. doi: 10.1186/s12906-018-2316-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lampertico P, Agarwal K, Berg T, et al. EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol. 2017;67(2):370–398. doi: 10.1016/j.jhep.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Lee CD, Ott M, Thyagarajan SP, et al. Phyllanthus amarus down-regulates hepatitis B virus mRNA transcription and replication. Eur J Clin Invest. 1996;26(12):1069–1076. doi: 10.1046/j.1365-2362.1996.410595.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee SJ, Lee HK, Jung MK, et al. In vitro antiviral activity of 1,2,3,4,6-Penta-O-galloyl-b-D-glucose against hepatitis B virus. Biol Pharm Bull. 2006;29(10):2131–2134. doi: 10.1248/bpb.29.2131. [DOI] [PubMed] [Google Scholar]
- 32.Leelarasamee A, Trakulsomboon S, Maunwongyathi P, et al. Failure of Phyllanthus amarus to eradicate hepatitis B surface antigen from symptomless carriers. Lancet. 1990;335(8705):1600–1601. doi: 10.1016/0140-6736(90)91436-E. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Zhao J, Li H, et al. Evaluation on anti-hepatitis viral activity of Vitis vinifer L. Molecules. 2010;15(10):7415–7422. doi: 10.3390/molecules15107415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lv JJ, Wang YF, Zhang JM, et al. Anti-hepatitis B virus activities and absolute configurations of sesquiterpenoid glycosides from Phyllanthus emblica. Org Biomol Chem. 2014;12(43):8764–8774. doi: 10.1039/C4OB01196A. [DOI] [PubMed] [Google Scholar]
- 35.Lv JJ, Yu S, Wang YF, et al. Anti-Hepatitis B virus Norbisabolane Sesquiterpenoids from Phyllanthus acidus and the establishment of their absolute configurations using theoretical calculations. J Org Chem. 2014;79(12):5432–5447. doi: 10.1021/jo5004604. [DOI] [PubMed] [Google Scholar]
- 36.Lwin KM, Lwin MKT. Medicinal plant list of Myanmar. 2. Yangon: FAME Publishing House; 2015. [Google Scholar]
- 37.Lwin AA, Aye KS, Htun MM, et al. Sero-prevalence of Hepatitis B and C viral infections in Myanmar: national and regional survey in 2015. Myanmar Health Sci Res J. 2017;29(3):167–175. [Google Scholar]
- 38.Matsuo K, Takenaka K, Shimomura H, et al. Lamivudine and Glycyrrhizin for treatment of chemotherapy-induced hepatitis B virus (HBV) hepatitis in a chronic HBV carrier with Non-Hodgkin Lymphoma. Leuk Lymphoma. 2001;41(1–2):191–195. doi: 10.3109/10428190109057970. [DOI] [PubMed] [Google Scholar]
- 39.Mohan K, Paramasivam R, Chandran P, et al. Inhibition of hepatitis B virus DNA polymerase and modulation of TH1 & TH2 cytokine secretion by three Indian medicinal plants and its correlation with antiviral properties. J Pharm Res. 2011;4(4):1044–1046. [Google Scholar]
- 40.Moraleda G, Wu TT, Jilbert AR, et al. Inhibition of duck hepatitis B virus replication by hypericin. Antivir Res. 1993;20(3):235–247. doi: 10.1016/0166-3542(93)90023-C. [DOI] [PubMed] [Google Scholar]
- 41.Mouler Rechtman M, Har-Noy O, Bar-Yishay I, et al. Curcumin inhibits hepatitis B virus via down-regulation of the metabolic coactivator PGC-1α. FEBS Lett. 2010;584(11):2485–2490. doi: 10.1016/j.febslet.2010.04.067. [DOI] [PubMed] [Google Scholar]
- 42.Munshi A, Mehrotra R, Ramesh R, et al. Evaluation of anti-hepadnavirus activity of Phyllanthus amarus and Phyllanthus maderaspatensis in duck hepatitis b virus carrier Pekin ducks. J Med Virol. 1993;41(4):275–281. doi: 10.1002/jmv.1890410404. [DOI] [PubMed] [Google Scholar]
- 43.Murray PR, Rosenthal KS, Pfaller MA. Medical microbiology (8th edition) Amsterdam: Elsevier; 2016. [Google Scholar]
- 44.Niu J, Wang Y, Qiao M, et al. Effect of Phyllanthus amarus on duck hepatitis B virus replication in vivo. J Med Virol. 1990;32(4):212–218. doi: 10.1002/jmv.1890320404. [DOI] [PubMed] [Google Scholar]
- 45.Ott M, Thyagarajan SP, Gupta S. Phyllanthus amarus suppresses hepatitis B virus by interrupting interactions between HBV enhancer I and cellular transcription factors. Eur J Clin Invest. 1997;27(11):908–915. doi: 10.1046/j.1365-2362.1997.2020749.x. [DOI] [PubMed] [Google Scholar]
- 46.Pang R, Tao J, Zhang S, et al. In vitro anti-hepatitis B virus effect of Hypericum perforatum L. J Huazhong Univ Sci Technol Med Sci. 2010;30(1):98–102. doi: 10.1007/s11596-010-0118-0. [DOI] [PubMed] [Google Scholar]
- 47.Pang J, Zhao K, Wang J, et al. Green tea polyphenol, epigallocatechin-3-gallate, possesses the antiviral activity necessary to fight against the hepatitis B virus replication in vitro. J Zhejiang Univ Sci B. 2014;15(6):533–539. doi: 10.1631/jzus.B1300307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parvez MK, Al-Dosari MS, Alam P, et al. The anti-hepatitis B virus therapeutic potential of anthraquinones derived from Aloe vera. Phytother Res. 2019;33(11):2960–2970. doi: 10.1002/ptr.6471. [DOI] [PubMed] [Google Scholar]
- 49.Pei S, Zhang Y, Xu H, et al. Inhibition of the Replication of Hepatitis B Virus in Vitro by Pu-erh Tea Extracts. J Agric Food Chem. 2011;59(18):9927–9934. doi: 10.1021/jf202376u. [DOI] [PubMed] [Google Scholar]
- 50.Rajarajan S. Antiviral activity of lyophilized aqueous and ethanolic extracts of Terminalia chebula Retz on hepatitis B virus. Paripex Indian J Res. 2019;8(7):10–12. [Google Scholar]
- 51.Ralston SH, Penman ID, Strachan MWJ, et al. Davidson’s principles and practice of medicine. 23. Amsterdam: Elsevier; 2018. [Google Scholar]
- 52.Sato H, Goto W, Yamamura J, et al. Therapeutic basis of glycyrrhizin on chronic hepatitis B. Antivir Res. 1996;30(2–3):171–177. doi: 10.1016/0166-3542(96)00942-4. [DOI] [PubMed] [Google Scholar]
- 53.Shi Y, Li Y, Huang C, et al. Resveratrol enhances HBV replication through activating Sirt1-PGC-1α-PPARα pathway. Sci Rep. 2016;6(1):24744. doi: 10.1038/srep24744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin M, Kang E, Lee Y. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir Res. 2005;67(3):163–168. doi: 10.1016/j.antiviral.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 55.Shinada M, Azuma M, Kawai H, et al. Enhancement of Interferon-γ production in Glycyrrhizin-treated human peripheral lymphocytes in response to Concanavalin A and to surface antigen of hepatitis B virus. Proc Soc Exp Biol Med. 1986;181(2):205–210. doi: 10.3181/00379727-181-42241. [DOI] [PubMed] [Google Scholar]
- 56.Suresh V, Sojan J, Krishna Radhika N, et al. Anti-HBV activity of the different extracts from Phyllanthus rheedei Wight in cell culture based assay systems. J Ethnopharmacol. 2014;156:309–315. doi: 10.1016/j.jep.2014.08.028. [DOI] [PubMed] [Google Scholar]
- 57.Takahara T, Watanabe A, Shiraki K. Effects of glycyrrhizin on hepatitis B surface antigen: a biochemical and morphological study. J Hepatol. 1994;21(4):601–609. doi: 10.1016/S0168-8278(94)80108-8. [DOI] [PubMed] [Google Scholar]
- 58.Tandon A. Treatment of subacute hepatitis with Lamivudine and intravenous Glycyrrhizin: a pilot study. Hepatol Res. 2001;20(1):1–8. doi: 10.1016/S1386-6346(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 59.Thyagarajan S, Subramanian S, Thirunalasundari T, et al. Effect of Phyllanthus amarus on chronic carriers of hepatitis B virus. Lancet. 1988 doi: 10.1016/s0140-6736(88)92416-6. [DOI] [PubMed] [Google Scholar]
- 60.Venkateswaran PS, Millman I, Blumberg BS. Effects of an extract from Phyllanthus niruri on hepatitis B and woodchuck hepatitis viruses In vitro and in vivo studies. Proc Natl Acad Sci. 1987;84(1):274–278. doi: 10.1073/pnas.84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahyuni TS, Permanasari AA, Aty Widyawaruyanti W, et al. Antiviral activity of Indonesian medicinal plants against Hepatitis B Virus. Pharmacogn J. 2020;12(5):1108–1114. doi: 10.5530/pj.2020.12.157. [DOI] [Google Scholar]
- 62.Waiyaput W, Payungporn S, Issara-Amphorn J, et al. Inhibitory effects of crude extracts from some edible Thai plants against replication of hepatitis B virus and human liver cancer cells. BMC Complement Altern Med. 2012;12(1):1233. doi: 10.1186/1472-6882-12-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang LJ, Geng CA, Ma YB, et al. Synthesis, biological evaluation and structure–activity relationships of glycyrrhetinic acid derivatives as novel anti-hepatitis B virus agents. Bioorg Med Chem Lett. 2012;22(10):3473–3479. doi: 10.1016/j.bmcl.2012.03.081. [DOI] [PubMed] [Google Scholar]
- 64.Wang CZ, Li WJ, Tao R, et al. Antiviral Activity of a Nanoemulsion of polyprenols from ginkgo leaves against influenza A H3N2 and hepatitis B virus in vitro. Molecules. 2015;20(3):5137–5151. doi: 10.3390/molecules20035137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wei W, Li X, Wang K, et al. Lignans with Anti-Hepatitis B Virus activities from Phyllanthus niruri L. Phytother Res. 2012;26(7):964–968. doi: 10.1002/ptr.3663. [DOI] [PubMed] [Google Scholar]
- 66.Wei ZQ, Zhang YH, Ke CZ, et al. Curcumin inhibits hepatitis B virus infection by down-regulating cccDNA-bound histone acetylation. World J Gastroenterol. 2017;23(34):6252. doi: 10.3748/wjg.v23.i34.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.World Health Organization. Hepatitis B Fact sheets. 2020. https://www.who.int/news-room/fact-sheets/detail/hepatitis-b Accessed 15 Oct 2020.
- 68.Wu L, Yang X, Huang Z, et al. In vivo and in vitro antiviral activity of hyperoside extracted from Abelmoschus manihot (L) medik. Acta Pharmacol Sin. 2007;28(3):404–409. doi: 10.1111/j.1745-7254.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 69.Wu GHM, Boucher BJ, Chiu YH, et al. Impact of chewing betel-nut (Areca catechu) on liver cirrhosis and hepatocellular carcinoma: a population-based study from an area with a high prevalence of hepatitis B and C infections. Public Health Nutr. 2009;12(1):129–135. doi: 10.1017/S1368980008002073. [DOI] [PubMed] [Google Scholar]
- 70.Xiang Y, Ju H, Li S, et al. Effects of 1,2,4,6-tetra-O-galloyl-β-D-glucose from P. emblica on HBsAg and HBeAg secretion in HepG2215 cell culture. Virol Sin. 2010;25(5):375–380. doi: 10.1007/s12250-010-3144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu J, Wang J, Deng F, et al. Green tea extract and its major component epigallocatechin gallate inhibits hepatitis B virus in vitro. Antivir Research. 2008;78(3):242–249. doi: 10.1016/j.antiviral.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 72.Xu J, Gu W, Li C, et al. Epigallocatechin gallate inhibits hepatitis B virus via farnesoid X receptor alpha. J Nat Med. 2016;70(3):584–591. doi: 10.1007/s11418-016-0980-6. [DOI] [PubMed] [Google Scholar]
- 73.Yao X, Li J, Deng N, et al. Immunoaffinity purification of α-momorcharin from bitter melon seeds (Momordica charantia) J Sep Science. 2011;34(21):3092–3098. doi: 10.1002/jssc.201100235. [DOI] [PubMed] [Google Scholar]
- 74.Ye P, Zhang S, Zhao L, et al. Tea polyphenols exerts anti-hepatitis B virus effects in a stably HBV-transfected cell line. J Huazhong Univ Sci Technol Med Sci. 2009;29(2):169–172. doi: 10.1007/s11596-009-0206-1. [DOI] [PubMed] [Google Scholar]
- 75.Yeh SF, Hong CY, Huang YL, et al. Effect of an extract from Phyllanthus amarus on hepatitis B surface antigen gene expression in human hepatoma cells. Antivir Res. 1993;20(3):185–192. doi: 10.1016/0166-3542(93)90019-F. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L. Randomized clinical trial with two doses (100 and 40 ml) of Stronger Neo-Minophagen C in Chinese patients with chronic hepatitis B. Hepatol Res. 2002;24(3):220–227. doi: 10.1016/S1386-6346(02)00086-4. [DOI] [PubMed] [Google Scholar]
- 77.Zhao G, Yin Z, Dong J. Antiviral efficacy against hepatitis B virus replication of oleuropein isolated from Jasminum officinale L var grandiflorum. J Ethnopharmacol. 2009;125(2):265–268. doi: 10.7501/j.issn.1674-6384.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 78.Zhao G, Yin ZF, Liu LY, et al. Anti-hepatitis B Virus Activity of 8-epi-Kingiside in Jasminum officinale var. grandiflorum. Chinese Herb Med. 2013;5(1):53–57. doi: 10.7501/j.issn.1674-6384.2013.01.006. [DOI] [Google Scholar]
- 79.Zhong L, Hu J, Shu W, et al. Epigallocatechin-3-gallate opposes HBV-induced incomplete autophagy by enhancing lysosomal acidification, which is unfavorable for HBV replication. Cell Death Dis. 2015;6(5):e1770–e1770. doi: 10.1038/cddis.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou NJ, Geng CA, Huang XY, et al. Anti-hepatitis B virus active constituents from Swertia chirayita. Fitoterapia. 2015;100:27–34. doi: 10.1016/j.fitote.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 81.Zuo AX, Shen Y, Jiang ZY, et al. Three new phenolic glycosides from Curculigo orchioides G. Fitoterapia. 2010;81(7):910–913. doi: 10.1016/j.fitote.2010.06.003. [DOI] [PubMed] [Google Scholar]