Abstract
Integrin-mediated interactions of cells with components of the extracellular matrix regulate cell survival, cell proliferation, cell differentiation, and cell migration. Some of these physiological responses are regulated via activation of transcription factors such as activator protein 1 (AP-1). Integrin-linked kinase (ILK) is an ankyrin repeat containing serine-threonine protein kinase whose activity is rapidly and transiently stimulated by cell-fibronectin interactions as well as by insulin stimulation. ILK activates protein kinase B and inhibits the glycogen synthase kinase 3 (GSK-3) activity in a phosphatidylinositol-3-kinase (PI 3-kinase)-dependent manner. We now show that cell adhesion to fibronectin results in a rapid and transient stimulation of AP-1 activity. At the same time, the kinase activity of ILK is stimulated whereas that of GSK-3 is inhibited. This fibronectin-dependent activation of AP-1 activity is inhibited in a dose-dependent manner if the cells are transfected with wild-type GSK-3, and also by inhibitors of PI 3-kinase. Stable or transient overexpression of ILK results in a stimulation of AP-1 activity which is inhibited by cotransfection with wild-type GSK-3 and kinase-deficient ILK. Transient transfection of ILK in HEK-293 cells stimulates complex formation between an AP-1 consensus oligonucleotide and nuclear proteins containing c-jun. The formation of this complex is inhibited by cotransfection with active GSK-3 or kinase-deficient ILK, suggesting that ILK may regulate AP-1 activation by inhibiting GSK-3, which has previously been shown to be a negative regulator of AP-1. In the presence of serum, ILK has no effect on the phosphorylation of Ser-73 in the N-terminal transactivation domain of c-jun. These results demonstrate a novel signaling pathway for the adhesion-mediated stimulation of AP-1 transcriptional activity involving ILK and GSK-3 and the subsequent regulation of the c-jun–DNA interaction.
Integrin-mediated interactions of cells with components of the extracellular matrix regulate cell survival, cell proliferation, cell differentiation, and cell migration (9, 11, 20, 44) by regulating the activity of transcription factors such as activator protein 1 (AP-1) (4). The activation of the c-jun N-terminal kinase (JNK) group of mitogen-activated protein kinases (MAPK) has been proposed for the regulation of AP-1 activity by cell-extracellular matrix interactions (20).
The AP-1 family of transcription factors consists predominantly of homodimeric or heterodimeric complexes of c-jun and c-fos proteins. These complexes bind to a specific target DNA sequence, the palindromic tetradecanoyl phorbol acetate (TPA)-responsive element TGAC/GTCA (2, 3), which is found in the promoters of many genes, including those for cell cycle regulators such as cyclin D1 (24), or vascular endothelial growth factor (25). The nuclear proto-oncogene product c-jun is a central component of all AP-1 complexes and is expressed in many cell types at low levels (29). c-jun contains a C-terminal DNA-binding/leucine zipper domain and an N-terminal transactivation domain (28, 29). AP-1 activity is tightly regulated at both the transcriptional and posttranscriptional levels. In the latter case, dephosphorylation of serine and threonine in the C-terminal domain of c-jun is necessary for binding to DNA, while phosphorylation of serine-63 and serine-73 by JNKs in the N-terminal domain promotes AP-1 transactivation (7, 8, 46). Although the activation of the N-terminal transactivation domain of c-jun is well characterized in the activation of AP-1, the contribution of the C-terminal DNA-binding domain to the regulation of AP-1 is less well understood. It has been demonstrated that glycogen synthase kinase 3 (GSK-3) can phosphorylate c-jun at C-terminal sites, resulting in the inhibition of the DNA-binding activity of c-jun (8, 21, 35, 39).
We have identified integrin-linked kinase (ILK), an ankyrin repeat containing serine-threonine protein kinase, which interacts with the cytoplasmic domains of integrin subunits (23). Overexpression of ILK in epithelial cells results in anchorage-independent cell survival and cell cycle progression via upregulation of the expression of cyclin D1 (40). When overexpressed in epithelial cells, ILK also induces nuclear translocation of β-catenin, resulting in the formation of an active lymphoid enhancer factor 1 (LEF-1) transcription factor–β-catenin complex and in the enhancement of LEF-1 transcriptional activity (37). It has recently been shown that ILK activates protein kinase B (PKB/AKT) and inhibits GSK-3 activity in a phosphatidylinositol-3-kinase (PI 3-kinase)-dependent manner (13, 15).
Here we show that AP-1 activity is induced by the interaction of cells with fibronectin (FN) and that this activation is dependent upon the activation of ILK, which, by inhibiting the activity of GSK-3, can stimulate the binding of c-jun to the AP-1-responsive element.
MATERIALS AND METHODS
Cell lines.
Rat intestinal epithelial cells (IEC-18) were cultured in α-minimal essential medium (Gibco BRL, Burlington, Ontario, Canada) supplemented with 5% fetal bovine serum (Gibco BRL), 2 mM l-glutamine (Gibco BRL) and 3.6 mg of glucose and 10 μg of insulin (Sigma, Oakville, Ontario, Canada) per ml. Stably transfected IEC-18 cells with wild-type ILK in the sense orientation (ILK-13 cells; clones A1a3 and A4a) or the antisense orientation (ILK-14 cells; clones A2C3 and A2C6) were established as described previously (23). Both ILK-13 and ILK-14 cells were grown under conditions that were the same as those for the parental IEC-18 cell line, except for the addition of 80 μg of geneticin (G418; Gibco BRL) per ml to maintain selection. Human embryonic kidney cells (HEK-293 cells; obtained from M. Moran, University of Toronto) (22) were grown in Dulbecco’s modified Eagle medium (DMEM; Gibco BRL) supplemented with 10% donor calf serum (Gibco BRL).
Adhesion assays.
Cells were serum starved for 18 h, harvested by being scraped in phosphate-buffered saline (PBS) containing 5 mM EDTA, and then seeded on plates coated with FN (10 μg per ml of PBS; Gibco BRL) or bovine serum albumin (BSA; 2.5 mg per ml of DMEM) and maintained with DMEM for an adequate length of time. For AP-1 activity experiments after adhesion, cells were transfected by calcium phosphate as described by us (23) with 50 μg of pGL3-AP-1 plasmid containing the AP-1-responsive promoter and the luciferase reporter gene and other cDNAs. Adhesion assays were performed 48 h after transfection, and the AP-1 activity was measured by luciferase assay described below.
Kinase assays.
Kinase assays were carried out as described by us (15). Cells were lysed in a solution containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate, 10 μg of leupeptin per ml, 2.5 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride (PMSF), 5 mM sodium fluoride, and 5 mM sodium orthovanadate. Equivalent protein concentrations of cell lysates were precleared with nonspecific immunoglobulin G (IgG) and bound to protein A-Sepharose. The supernatants were immunoprecipitated with the appropriate antibodies, and the kinase assays were performed as described previously (23). Myelin basic protein (MBP) was used as a substrate for ILK, and glycogen synthase 1 peptide (GS-1) was used for GSK-3. Phosphorylated MBP was detected after electrophoresis on sodium dodecyl sulfate–15% polyacrylamide gel, and phosphorylated GS-1 was detected after electrophoresis on a Tricine gel (43). The gels were visualized by autoradiography. The autoradiographic signals were quantified by densitometric analysis (Bio-Rad Laboratories, Canada).
Immunoblots.
Protein extracts were resolved by SDS-PAGE and electrotransferred onto Immobilon-P membranes (Millipore, Nepean, Ontario, Canada). Membranes were probed with the appropriate primary antibody. The following antibodies were used: rabbit anti-ILK antibody (0.5 μg/ml) (23), mouse anti-GSK-3 antibody (1:400; Transduction Laboratories), mouse anti-V5 antibody (1:5,000; Invitrogen, Carlsbad, Calif.), mouse anti-hemagglutinin (HA), antibody (1 μg/ml; Babco, Richmond, Calif.), rabbit anti-c-jun antibody (1 μg/ml; Santa Cruz Biotechnology), rabbit anti-phospho-c-jun (Ser-73) antibody (1 μg/ml; Upstate Biotechnology, Lake Placid, N.Y.), rabbit anti-c-fos antibody (1 μg/ml; Santa Cruz Biotechnology), mouse anti-phospho-extracellular signal-regulated kinase (Tyr-204) antibody (1 μg/ml; Santa Cruz Biotechnology). Protein detection was carried out with the appropriate horseradish peroxidase-conjugated secondary antibody (Jackson Laboratory, Bar Harbor, Maine) and an enhanced chemiluminescence detection system (Amersham Corp.).
Transient transfections.
Transient transfections were performed with Lipofectin reagent (Gibco BRL) when serum-exposed cells reached 40 to 50% confluency. Cells were transfected with AP-1, β-galactosidase, wild-type ILK-V5-tagged, ILK-kinase dead (ILK-KD), wild-type GSK-3-HA-tagged, or AKT-kinase dead (AKT-KD) HA-tagged cDNAs. The plasmids used were pGL3-AP-1 containing the AP-1-responsive promoter and the luciferase reporter gene (from Kinetek Pharmaceuticals, Inc.), pcDNA3.1-lacZ-V5 (Invitrogen), pcDNA3.1-ILK-V5, pcDNA3-ILK-KD, pcDNA3-GSK-3-HA, pcDNA-AKT-KD-HA (mutant K179A), and the corresponding empty vectors. Transfections were carried out overnight in serum-free medium. The transfection medium was then replaced by serum-containing medium, and cells were harvested 36 to 48 h later.
Luciferase assays for AP-1 activity.
Luciferase assays were performed according to the manufacturer’s instructions (Promega Corp., Madison, Wis.). All assays (except for those shown in Fig. 2F) were normalized by measuring β-galactosidase enzymatic activity (42), and the luciferase reaction was then performed by using lysates with an equivalent amount of β-galactosidase activity. For Fig. 2F, a dual-luciferase reporter assay (Promega) was performed. Two reporters were cotransfected: the experimental AP-1 reporter and a control reporter (Renilla). Both luciferase activities were evaluated, and AP-1 activity was normalized to the activity of the control. Protein concentrations were determined by a Bradford assay. Results are expressed in relative light units per microgram of protein. When testing inhibitors of AP-1 activity, cells were exposed to wortmannin, LY294002, or PD98059 (Calbiochem, La Jolla, Calif.) prior to harvesting the cells.
FIG. 2.
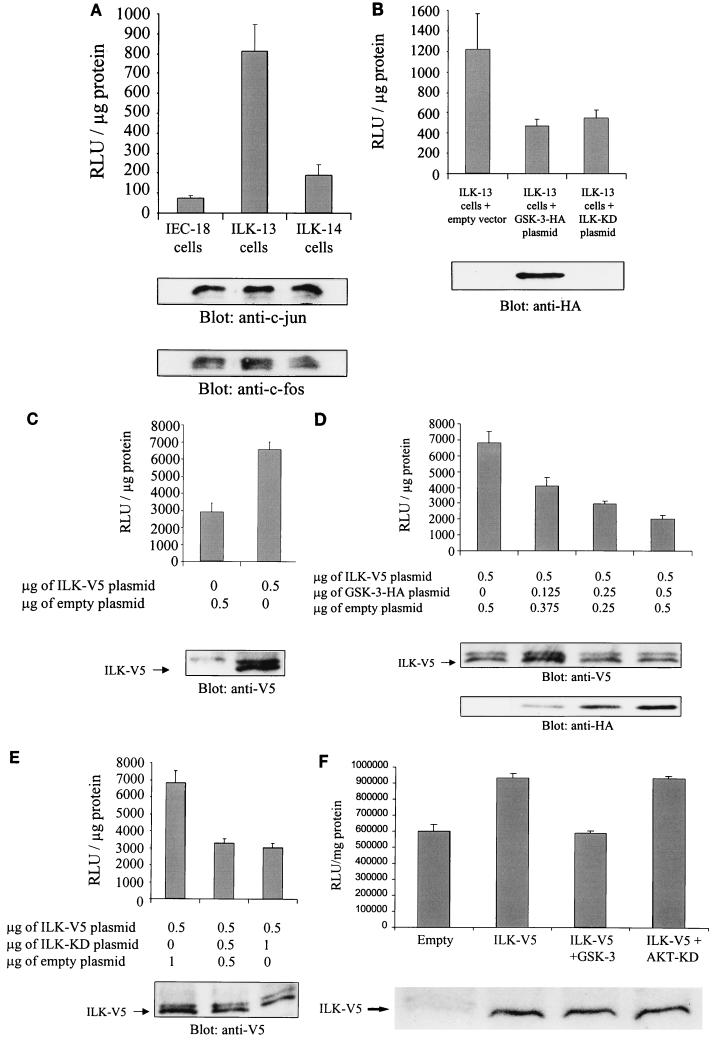
ILK regulates AP-1 activity in a GSK-3-dependent manner. (A) AP-1 activity in IEC-18 cells and in IEC-18 cells stably transfected with wild-type ILK (ILK-13; clone A1a3 (37, 40) and anti-sense ILK (ILK-14; clone A2C3) was measured. After transfection of 0.5 μg of pGL3-AP-1 by Lipofectin, AP-1 activity was determined by luciferase assay. c-jun and c-fos protein expression levels in each cell line were estimated by Western blotting. RLu, relative light units. (B) Overexpression of GSK-3 decreases AP-1 activity in ILK-13 cells. Luciferase assays were performed with ILK-13 cells transfected with 0.5 μg of pGL3-AP-1 and 0.5 μg of pcDNA-GSK-3-HA or 0.5 μg of pcDNA-ILK-KD. Transfection and expression of GSK-3 were established by Western blot analysis using an anti-HA antibody. (C) AP-1 activity in HEK-293 cells transiently transfected with ILK. HEK-293 cells were transiently cotransfected with 0.5 μg of pGL3-AP-1 and 0.5 μg of pcDNA3.1-ILK-V5 or empty vector. ILK expression in the transfected cells was detected by immunoblotting with an anti-V5 antibody. (D) Dose-dependent effect of GSK-3 on ILK-induced AP-1 activity. Increasing amounts of pcDNA-GSK-3-HA (0.125, 0.25, and 0.5 μg) were cotransfected with 0.5 μg of pGL3-AP-1 and 0.5 μg of pcDNA3.1-ILK-V5, and AP-1 activity was evaluated. Expression of ILK and GSK-3 in the transfected cells is shown by Western blotting with anti-V5 and anti-HA antibodies, respectively. (E) Effect of ILK-KD on ILK-induced AP-1 activity. The same experiment as that shown in panel D was performed with 0.5 μg and 1 μg of pcDNA-ILK-KD. Expression of ILK in the transfected cells was established by Western blot analysis with an anti-V5 antibody. (F) Effect of AKT-KD on ILK-induced AP-1 activity. HEK-293 cells were cotransfected with 0.5 μg pGL3-AP-1, 0.5 μg of pcDNA3.1-ILK-V5, and 0.5 μg of pcDNA-GSK-3-HA or 0.5 μg of pcDNA-AKT-KD-HA. Expression of ILK in the transfected cells was established by Western blot analysis with an anti-V5 antibody.
Nuclear extracts and gel shift assays.
Nuclear extracts were prepared by the miniextraction method as previously described (1). Cells were washed with ice-cold PBS and harvested by being scraped in 1.5 ml of PBS. Cells were pelleted and resuspended in 400 μl of 10 mM HEPES-potassium hydroxide (pH 7.9)–1.5 mM magnesium chloride–10 mM potassium chloride–0.5 mM dithiothreitol–0.2 mM PMSF. After 10 min of incubation on ice, nuclei were pelleted by being spun for 10 s and resuspended in 50 μl of 20 mM HEPES-potassium hydroxide (pH 7.9)–25% glycerol–420 mM sodium chloride–1.5 mM magnesium chloride–0.2 mM EDTA–0.5 mM dithiothreitol–0.2 mM PMSF. Tubes were incubated for 20 min on ice and then centrifuged to clear the cellular debris. Nuclear extracts were stored at −70°C. Gel shift assays were performed by incubating 2 μg of the nuclear extracts for 20 min at room temperature with a 32P-end-labeled DNA fragment containing the putative protein binding site (for AP-1, 5′CGC TTG ATG AGT CAG CCG GAA3′; Promega; [γ-32P]ATP was from Amersham Life Science). Reaction products were analyzed on a nondenaturing 5% polyacrylamide gel (0.5% Tris-borate-EDTA, 3.5% glycerol). The specificity of the DNA-protein interaction was established by competition experiments using 10× cold AP-1 oligonucleotide as the competitor. For the supershift assay, 10 μg of rabbit anti-c-jun antibody (Santa Cruz Biotechnology) or nonspecific IgG was added to the reaction mixture, subsequent to the addition of the 32P-labeled oligonucleotide probe, and the mixture was incubated for 45 min at room temperature. Complexes were resolved by electrophoresis as described for the gel shift assay.
RESULTS
Regulation of ILK, GSK-3, and AP-1 by adhesion to FN.
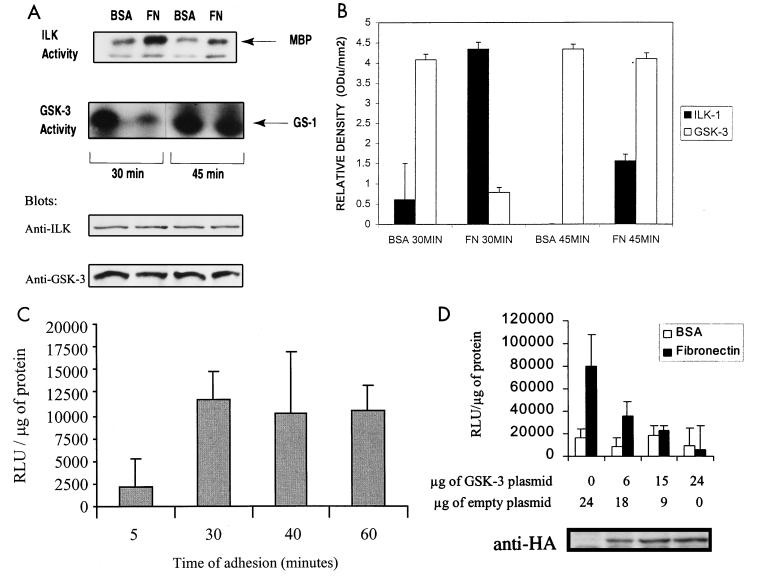
It has recently been demonstrated that the attachment of rat intestinal epithelial IEC-18 cells to FN stimulates ILK activity (15). Since ILK directly phosphorylates GSK-3 in vitro and inhibits GSK-3 activity when overexpressed in cells (15), we examined whether adhesion to FN results in an inhibition of GSK-3 activity. Attachment of IEC-18 cells to FN led to increased ILK kinase activity, concomitant with decreased GSK-3 kinase activity, compared to attachment to BSA as a control (Fig. 1A). Maximal stimulation of ILK activity occurred at 30 min after plating, and this corresponds to the maximal inhibition of GSK-3 activity. As shown previously (15), ILK activity declines after 30 min and is substantially lower at 45 min after plating. At this time point, the GSK-3 activity rebounds but is still lower than that for cells plated on BSA. The autoradiographic signals were quantified by densitometric analysis, and the data are shown in Fig. 1B. The expression levels of ILK and GSK-3 did not change during the course of cell adhesion to FN or BSA (Fig. 1A).
FIG. 1.
Regulation of ILK, GSK-3, and AP-1 activities by adhesion to FN. (A) ILK and GSK-3 kinase activities were measured following an adhesion assay. IEC-18 cells were serum starved for 18 h, harvested, and seeded on FN or BSA for 30 or 45 min. ILK and GSK-3 activities were determined with MBP and glycogen synthase 1 peptide as the substrates, respectively. Immunoblots with anti-ILK and anti-GSK-3 antibodies show equivalent amounts of ILK and GSK-3 in each extract. (B) Quantification of ILK and GSK-3 kinase activities by densitometric analysis. ODu, optical density units. (C) Time course of adhesion-induced stimulation of AP-1 activity. HEK-293 cells were transfected by calcium phosphate with 50 μg of pGL3-AP-1 containing the AP-1-responsive promoter and luciferase reporter gene. Cells were then seeded on BSA or FN for the indicated times and lysed, and a luciferase assay was performed. Results represent the difference between adhesion to FN and to BSA. RLu, relative light units. (D) Effect of GSK-3 on FN-induced AP-1 activity. HEK-293 cells were cotransfected with 50 μg of pGL3-AP-1 and the indicated amounts of pcDNA-GSK-3-HA-tagged and empty vector. Cells were harvested, seeded on FN or BSA for 30 min, lysed, and assessed for AP-1 activity by luciferase assay. GSK-3 expression in HEK-293 cells is shown by Western blot analysis using an anti-HA antibody.
We next wanted to determine whether cell attachment to FN resulted in the stimulation of AP-1 activity and whether ILK and GSK-3 were upstream of this activation. To demonstrate this, we switched to HEK-293 (human embryonic kidney) cells, because these cells have a higher efficiency of transient transfection than IEC-18 cells. We measured AP-1 activity by a luciferase assay of transiently transfected HEK-293 cells with a plasmid containing the AP-1-responsive promoter and the luciferase reporter gene at various time points of attachment to FN or BSA. AP-1 activity was maximally stimulated 30 min after attachment to FN (Fig. 1C). Cotransfection of the AP-1 reporter gene with increasing amounts of wild-type HA-tagged GSK-3 cDNA in HEK-293 cells resulted in an inhibition of AP-1 activity induced by adhesion to FN for 30 min (Fig. 1D).
These results demonstrate that attachment of cells to FN increases ILK kinase activity, resulting in the inhibition of GSK-3 activity. Furthermore, adhesion-dependent stimulation of AP-1 activity is dependent on the inhibition of GSK-3 activity.
ILK regulates AP-1 in a GSK-3-dependent manner.
To further understand the link between ILK and AP-1, we measured AP-1 activity in intestinal epithelial cells (IEC-18) and in IEC-18 cells stably transfected with ILK in the sense orientation (ILK-13; clones A1a3 and A4a) and in the antisense orientation (ILK-14) as controls (23). ILK-13 clones have been shown to overexpress ILK (23) and to have higher constitutive ILK activity (15). AP-1 activity was severalfold higher in ILK-13 (A1a3) adherent and nonadherent (data not shown) cells than in IEC-18 and ILK-14 cells (Fig. 2A). All independently derived ILK-overexpressing IEC-18 clones have previously been shown to behave identically (23, 37, 40), and, indeed, identical results were obtained for both independently derived ILK-13 clones, A1a3 and A4a. The data shown in Fig. 2A are from the clone A1a3. Thus overexpression of ILK in epithelial cells appears to mimic the effects of adhesion on AP-1 activity, which is adhesion independent in these cells. In addition, we also examined the protein expression levels of c-jun and c-fos in these three cell lines by Western blotting (Fig. 2A). As both c-jun and c-fos expression levels are equivalent, we concluded that the increased AP-1 activity is not due to an elevated expression of c-jun and c-fos in cells stably transfected with ILK.
We next explored the role of GSK-3 in the regulation of AP-1 activity. To investigate if ILK regulates AP-1 activity through GSK-3, we measured AP-1 activity in ILK-13 cells after transfection of these cells with wild-type GSK-3 or ILK-KD cDNAs. In the cells stably transfected with ILK, ILK-induced AP-1 activity is inhibited both by GSK-3 and by ILK-KD (Fig. 2B). Transfection with the empty vector, as a control, did not alter AP-1 activity (data not shown).
To further understand the effects of GSK-3 and ILK-KD on AP-1 activity, we carried out transient transfections in HEK-293 cells. Transient transfection of ILK cDNA resulted in an approximately twofold stimulation of AP-1 activity compared to transfection with the empty vector (Fig. 2C). Cotransfection of ILK cDNA with increasing amounts of wild-type GSK-3 or ILK-KD cDNAs resulted in a dose-dependent inhibition of ILK-stimulated AP-1 activity (Fig. 2D and E, respectively). Overexpressed inactive ILK-KD protein appears to function as a dominant-negative molecule in this context. These data show that ILK regulates AP-1 activity via GSK-3.
We have recently shown that ILK activates PKB/AKT in a PI 3-kinase-dependent manner by phosphorylating the Ser-473 residue (15). It has also been reported that PKB/AKT inactivates GSK-3 (47). To determine whether PKB/AKT is involved in the ILK-mediated regulation of AP-1 activity, we evaluated AP-1 activity in transiently transfected HEK-293 cells with active ILK and two dominant-negative forms of PKB/AKT. Both mutants (K179A-PKB and AAA-PKB) have previously been shown to behave as dominant negative molecules and to inhibit endogenous PKB/AKT activity (10, 18, 49). As shown in Fig. 2F, the dominant-negative mutant K179A-PKB had no effect on ILK-induced AP-1 activity, whereas wild-type GSK-3 clearly inhibited this activity. Identical results were obtained with the AAA-PKB dominant-negative mutant (data not shown). These data argue against the role of PKB/AKT in the ILK- and GSK-3-dependent stimulation of AP-1 activity and suggest that ILK may be regulating GSK-3 activity by direct phosphorylation. We have previously shown that ILK can directly phosphorylate GSK-3 in vitro (15).
FN- and ILK-mediated stimulation of AP-1 is dependent on PI 3-kinase.
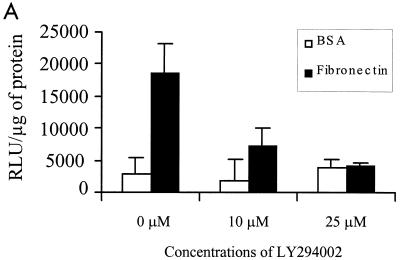
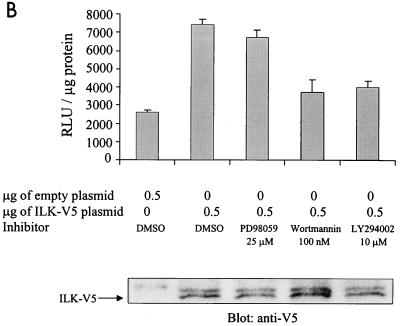
We have also recently shown that ILK inhibits GSK-3 activity in a PI 3-kinase-dependent manner (15). Several studies have implicated PI 3-kinase in AP-1 transactivation. Specifically, TPA (27), epidermal growth factor, and insulin (26) have all been shown to activate AP-1 in a PI 3-kinase-dependent manner. We therefore studied the effect of PI 3-kinase-specific inhibitors wortmannin and LY294002 (5, 48) on FN adhesion-dependent and ILK-induced AP-1 activity via GSK-3. We also used mitogen-activated protein kinase kinase (MEK) inhibitor PD98059 (19) to study a potential role of the MAPK pathway in this activation. FN adhesion-stimulated AP-1 activity was inhibited by LY294002 (Fig. 3A), whereas both wortmannin and LY294002 inhibited the stimulation of AP-1 activity by ILK in transfected HEK-293 cells (Fig. 3B). The MEK inhibitor PD98059 had a minimal effect on the ILK-induced AP-1 activity (Fig. 3B). These data suggest that PI 3-kinase, ILK, and GSK-3 are involved in FN-mediated cell adhesion stimulation of AP-1.
FIG. 3.
FN- and ILK-mediated stimulation of AP-1 is dependent on PI 3-kinase. (A) FN-induced AP-1 activity is sensitive to LY294002. HEK-293 cells transfected by calcium phosphate with 50 μg of pGL3-AP-1 were plated on BSA or FN for 30 min in the presence of the indicated concentrations of LY294002, and AP-1 activity was evaluated by luciferase assay. (B) ILK-induced AP-1 activity is sensitive to PI 3-kinase inhibitors. AP-1 activity in HEK-293 cells cotransfected by Lipofectin with 0.5 μg of pGL3-AP-1 and 0.5 μg of pcDNA3.1-ILK-V5 was measured. PD98059 (25 μM), wortmannin (100 nM), LY294002 (10 μM), or dimethyl sulfoxide (DMSO) was added to the cell culture medium 30 min prior to harvesting the cells. AP-1 activity was then evaluated by a luciferase assay. ILK expression is shown by Western blotting with anti-V5 antibody.
ILK promotes complex formation between c-jun and an AP-1 consensus oligonucleotide.
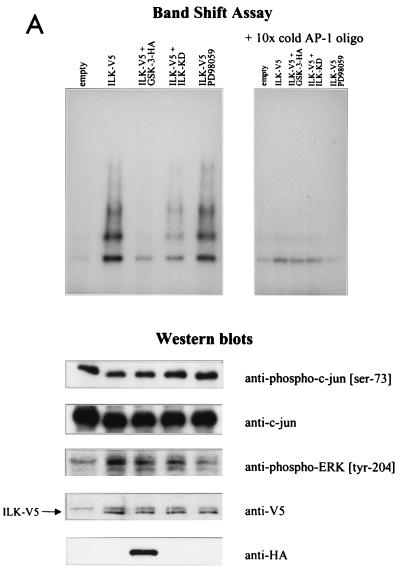
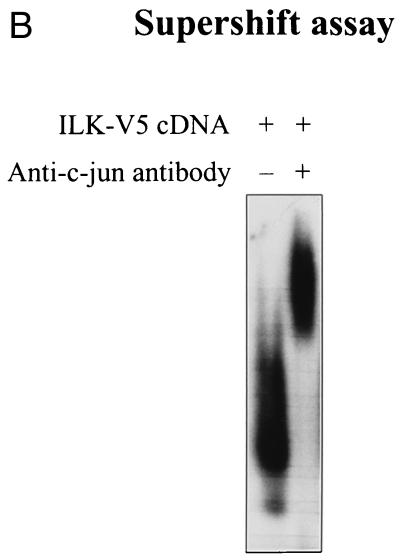
The AP-1 complex consists of dimeric transcription factors composed of c-jun–c-jun or c-jun–c-fos, which bind to the palindromic TPA-responsive element sequence TGA(C/G)TCA and enhance gene expression (2, 3). It is known that one of the components of AP-1, c-jun, is phosphorylated by GSK-3 in a region proximal to the DNA-binding domain (amino acids 247 to 263), resulting in decreased DNA binding (35). We therefore analyzed the ability of c-jun to form a complex with an AP-1 consensus oligonucleotide (14) in the presence of ILK and GSK-3. HEK-293 cells were transfected with AP-1 reporter gene and ILK cDNAs, without or with GSK-3 or ILK-KD cDNAs. Cell lysates for luciferase assays and nuclear extracts for gel mobility shift assays were prepared. As expected, the expression of ILK in HEK-293 cells increased AP-1 activity and the cotransfection of GSK-3 or ILK-KD decreased this activity (data not shown). Gel mobility shift analysis (Fig. 4A) showed that a protein-DNA complex was induced in HEK-293 cells transfected with ILK cDNA (lane 2 [lanes are numbered from left to right]) compared with HEK-293 cells transfected with the empty vector (lane 1). Cotransfection of GSK-3 cDNA inhibited this ILK-induced complex formation (lane 3), and cotransfection of ILK-KD cDNA reduced the amount of the complex (lane 4). To determine whether the ILK-induced complex contains c-jun, we carried out a supershift assay using an anti-c-jun antibody to highlight the presence of c-jun in the complexes (Fig. 4B). As can be seen, anti-c-jun antibody induced a protein-DNA mobility shift in the complex, indicating the massive presence of c-jun. The results show that ILK activates the binding of c-jun to its DNA response element and that this stimulation requires the inhibition of GSK-3, since the presence of wild-type GSK-3 suppresses the ILK-induced activation. The MEK inhibitor PD98059 did not have a significant effect on the ILK-induced complex formation (Fig. 4A, lane 5). As shown by Western blot analyses in Fig. 4, the effects of ILK on c-jun-DNA complex formation and AP-1 activation appear to be independent of the N-terminal phosphorylation of c-jun, because ILK had no effect on the further stimulation, over and above that of serum, of Ser-73 phosphorylation by JNK. This phosphorylation was also not modified by the coexpression of GSK-3 or ILK-KD. Furthermore, although ILK stimulates the phosphorylation of ERK-1 (Fig. 4A) but does not affect ERK-1 expression (not shown), inhibition of this phosphorylation by PD98059 did not significantly affect the c-jun–DNA complex formation, demonstrating that the ILK- and GSK-3-dependent activation of AP-1 is mediated by the regulation of the DNA-binding domain of c-jun.
FIG. 4.
ILK-induced AP-1 activity is mediated by c-jun. (A) In this band shift assay, serum-exposed HEK-293 cells were transfected by Lipofectin with 0.5 μg of each of the indicated cDNAs. PD98059 (25 μM) was added to the medium 12 h prior to harvesting the cells. Nuclear extracts (2 μg) from the transfected cells were incubated with 32P-end-labeled AP-1 consensus oligonucleotide containing the protein binding site. Reaction products were analyzed on a nondenaturing 5% polyacrylamide gel (left gel). The specificity of complex formation was established by a competition experiment using cold AP-1 oligonucleotide as the competitor (right gel). For immunoblot studies, 10 μg of protein was resolved by SDS–10% PAGE. c-jun (Ser-73) phosphorylation, c-jun protein expression level, ERK phosphorylation (Tyr-204), and ERK protein expression level (not shown) were determined by Western blot analysis. ILK and GSK-3 expression levels in the transfected cells were evaluated by Western blot analysis using anti-V5 and anti-HA antibodies, respectively. (B) Anti-c-jun antibody shifts ILK-induced AP-1 complex. Nuclear extracts (2 μg) from HEK-293 cells transfected with ILK-V5 cDNA were incubated with 32P-AP-1 oligonucleotide in the absence or presence of anti-c-jun antibody or nonspecific IgG (10 μg). The latter did not induce a mobility shift of the complex (not shown).
DISCUSSION
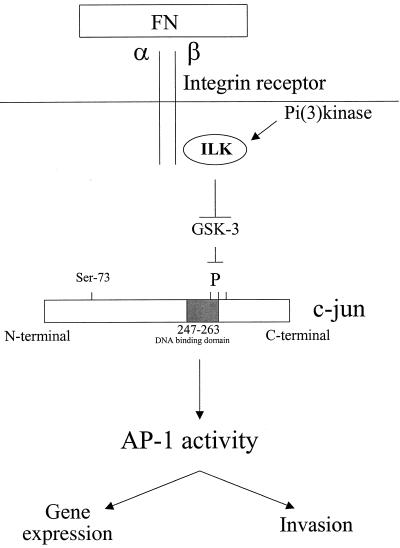
The data presented in this paper demonstrate a novel signaling pathway for the activation of the AP-1 transcription factor via cell adhesion to FN. In this pathway, ILK regulates AP-1 activity through GSK-3. We have shown that adhesion of cells to FN stimulates ILK activity. Since the regulation of ILK activity is PI 3-kinase dependent (15), we have shown, with the use of pharmacologic PI 3-kinase inhibitors wortmannin and LY294002, that adhesion and ILK-mediated AP-1 activation are PI 3-kinase dependent. ILK, by inhibiting GSK-3, may prevent the GSK-3 dependent C-terminal phosphorylation of one or more sites located near the DNA-binding domain of c-jun. This would then result in the promotion of the formation of a DNA-protein complex containing c-jun, enhancing AP-1 activity (Fig. 5). Our data demonstrate further that although ILK regulates the activities of both PKB/AKT and GSK-3 in a PI 3-kinase-dependent manner (15), it is the PKB-independent regulation of GSK-3 by ILK that is involved in AP-1 stimulation.
FIG. 5.
Schematic model for ILK-induced AP-1 activity. ILK regulates AP-1 activity through the GSK-3 pathway. Stimulation of ILK activity by adhesion of cells to FN results in an inhibition of GSK-3 activity, likely via direct phosphorylation of GSK-3. Inhibition of GSK-3 could prevent phosphorylation of the C terminus DNA-binding domain of c-jun, resulting in the formation of a protein-DNA complex and consequently an enhancement of AP-1 activity.
Integrin-mediated cell adhesion is known to regulate gene expression via transcription factors such as NF-κB and AP-1 (6). A number of signal transduction pathways, normally associated with the binding of soluble growth factors to their receptors, are also activated by integrin engagement (44). There is evidence that the growth factor-mediated activation of PI 3-kinase and downstream elements, such as PKB/AKT, depends on cell adhesion (31). Cell anchorage via integrins can activate the PI 3-kinase PKB/AKT pathway, leading to the suppression of cell detachment-induced apoptosis or anoikis (30). Integrin engagement also triggers activation of the Raf-1/MEK/MAPK pathway (33, 41). However, in this case, integrin-mediated adhesion itself does not result in mitogenesis; soluble growth factors are also required. Three groups of MAPK have been identified: ERK, p38 MAPK, and JNK (12). JNK can be activated upon integrin clustering (33) and by a variety of environmental signals (16, 32, 45). Targets of the JNK signal transduction pathway include the transcription factor c-jun (50). The fact that JNK enhances AP-1 activity by binding to the N-terminal region of c-jun and phosphorylating serine-63 and -73 within the activation domain has been well characterized (29, 50).
Several lines of evidence indicate that the C-terminal DNA-binding domain of c-jun also regulates AP-1 activity. It has been shown that in resting cells, in which AP-1 activity is low, c-jun is phosphorylated on three amino acid residues located near the DNA-binding domain (8). Activation of these cells by TPA leads to an increase in the DNA binding of AP-1. This is independent of any protein synthesis and results in the specific dephosphorylation of c-jun in the C-terminal domain. These authors showed that, in vitro, GSK-3 can efficiently phosphorylate c-jun on these sites, resulting in decreased DNA-protein interaction. Later, these in vitro results were confirmed, and it has been shown by cotransfection experiments that GSK-3 can inhibit AP-1 activity in intact cells (35). These results support the hypothesis that GSK-3 is an important regulator of AP-1 in vivo.
We have demonstrated here that adhesion to FN activates ILK and consecutively inhibits GSK-3, leading to higher AP-1 activity. The PI 3-kinase-dependent activation of AP-1 by ILK via GSK-3 could explain why the overexpression of ILK in epithelial cells suppresses suspension-induced apoptosis and stimulates anchorage-independent cell cycle progression (40). This is concomitant with the higher AP-1 activity observed in ILK-13 cells, although c-jun protein expression is not increased. Overexpression of ILK leads to increased cyclin D1 and cyclin A, to activated cyclin D1-cdk4 and cyclin E-cdk2 kinases resulting in the hyperphosphorylation of the retinoblastoma protein (pRB) (40), and to the promotion of anchorage-independent cell growth. It has recently been shown that interactions between c-jun and pRB, two components regulated by ILK, may represent an important mechanism for controlling transcription, cell growth, and differentiation (36). GSK-3 is an important molecule in the regulation of cell proliferation and cell survival. Active GSK-3 can induce apoptosis (38), and it is also implicated in the regulation of β-catenin and cyclin D1, resulting in their degradation (17, 34).
Our work emphasizes that although growth factors and the extracellular matrix can regulate AP-1 via JNK activation, the involvement of ILK and GSK-3 also appears to be required for extracellular matrix stimulation of AP-1 activity.
ACKNOWLEDGMENTS
We thank Jim Woodgett and Jasbinder Sanghera for valuable discussions. We also thank Jim Woodgett for providing the GSK-3 and AKT-KD plasmids.
This work was supported by grants from the National Cancer Institute of Canada and Kinetek Pharmaceuticals, Inc., to S.D.
REFERENCES
- 1.Andrews N C, Faller D V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angel P, Baumann I, Stein B, Delius H, Rahmsdorf H J, Herrlich P. 12-O-tetradecanoylphorbol-13-acetate induction of the human collagenase gene is mediated by an inducible enhancer element located in the 5′-flanking region. Mol Cell Biol. 1987;7:2256–2266. doi: 10.1128/mcb.7.6.2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Rahmsdorf H J, Jonat C, Herrlich P, Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 4.Angel P, Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 5.Arcaro A, Wymann M P. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-trisphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldwin A S., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 7.Binetruy B, Smeal T, Karin M. Ha-Ras augments c-Jun activity and stimulates phosphorylation of its activation domain. Nature. 1991;351:122–127. doi: 10.1038/351122a0. [DOI] [PubMed] [Google Scholar]
- 8.Boyle W J, Smeal T, Defize L H K, Angel P, Woodgett J R, Karin M, Hunter T. Activation of protein kinase C decreases phosphorylation of c-jun at sites that negatively regulate its DNA-binding domain. Cell. 1991;64:573–584. doi: 10.1016/0092-8674(91)90241-p. [DOI] [PubMed] [Google Scholar]
- 9.Clark E A, Brugge J S. Integrins and signal transduction pathways: the road taken. Science. 1995;268:223–239. doi: 10.1126/science.7716514. [DOI] [PubMed] [Google Scholar]
- 10.Cong L N, Chen H, Li Y, Zhou L, McGibbon M A, Taylor S I, Quon M J. Physiological role of Akt in insulin-stimulated translocation of GLUT4 in transfected rat adipose cells. Mol Endocrinol. 1997;11:1881–1890. doi: 10.1210/mend.11.13.0027. [DOI] [PubMed] [Google Scholar]
- 11.Damsky C H, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Opin Cell Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 12.Davis R J. MAPKS: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 13.Dedhar S. Integrins and signal transduction. Curr Opin Hematol. 1999;6:37–43. doi: 10.1097/00062752-199901000-00007. [DOI] [PubMed] [Google Scholar]
- 14.de Groot R P, Auwerx J, Bourouis M, Sassone-Corsi P. Negative regulation of Jun/AP-1: conserved function of glycogen synthase kinase 3 and the Drosophila kinase shaggy. Oncogene. 1993;8:841–847. [PubMed] [Google Scholar]
- 15.Delcommenne M, Tan C, Gray V, Ruel L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase-3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Derijard B, Hibi M, Wu I H, Barrett T, Su B, Deng T, Karin M, Davis R J. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 17.Diehl J A, Cheng M, Roussel M F, Sherr C J. Glycogen synthase kinase-3 beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 1998;12:3499–3511. doi: 10.1101/gad.12.22.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudek H, Datta S R, Franke T F, Birnbaum M J, Yao R, Cooper G M, Segal R A, Kaplan D R, Greenberg M E. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275:661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 19.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch S M, Ruoslahti E. Integrins and anoikis. Curr Opin Cell Biol. 1997;9:701–706. doi: 10.1016/s0955-0674(97)80124-x. [DOI] [PubMed] [Google Scholar]
- 21.Goode N, Hughes K, Woodgett J R, Parker P J. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267:6878–6882. [PubMed] [Google Scholar]
- 22.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 23.Hannigan G E, Leung-Hagesteijn C, Fitz-Gibbon L, Coppolino M G, Radeva G, Filmus J, Bell J C, Dedhar S. Regulation of cell adhesion and anchorage-dependent growth by a new β1-integrin-linked protein kinase. Nature. 1996;379:91–96. doi: 10.1038/379091a0. [DOI] [PubMed] [Google Scholar]
- 24.Herber B, Truss M, Beato M, Muller R. Inducible regulatory elements in the human cyclin D1 promoter. Oncogene. 1994;9:2105–2107. [PubMed] [Google Scholar]
- 25.Hu E, Mueller E, Oliviero S, Papaioannou V E, Johnson R, Spiegelman B M. Targeted disruption of the c-fos gene demonstrates c-fos-dependent and -independent pathways for gene expression stimulated by growth factors or oncogenes. EMBO J. 1994;13:3094–3103. doi: 10.1002/j.1460-2075.1994.tb06608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C, Ma W-Y, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P+ cells. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C, Schmid P C, Ma W Y, Schmid H H O, Dong Z. Phosphatidylinositol 3-kinase is necessary for 12-O-tetradecanoylphorbol-13-acetate-induced cell transformation and activated protein 1 activation. J Biol Chem. 1997;272:4187–4194. doi: 10.1074/jbc.272.7.4187. [DOI] [PubMed] [Google Scholar]
- 28.Karin M. The regulation of AP-1 activity by mitogen-activated protein kinases. J Biol Chem. 1995;270:16483–16486. doi: 10.1074/jbc.270.28.16483. [DOI] [PubMed] [Google Scholar]
- 29.Karin M, Lieu Z G, Zandi E. AP-1 function and regulation. Curr Opin Cell Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- 30.Khwaja A, Rodriguez-Viciana P, Wennstrom S, Warne P H, Downward J. Matrix adhesion and Ras transformation both activate a phosphoinositide 3-OH kinase and protein kinase B/Akt cellular survival pathway. EMBO J. 1997;16:2783–2793. doi: 10.1093/emboj/16.10.2783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.King W G, Mattaliano M D, Chan T O, Tsichlis P N, Brugge J S. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyriakis J M, Avruch J. Protein kinase cascades activated by stress and inflammatory cytokines. Bioessays. 1996;18:567–577. doi: 10.1002/bies.950180708. [DOI] [PubMed] [Google Scholar]
- 33.Lin A, Frost J, Deng T, Smeal T, Al-Alawi N, Kikkawa U, Hunter T, Brenner D, Karin M. Casein kinase II is a negative regulator of c-jun DNA binding and AP-1 activity. Cell. 1992;70:777–789. doi: 10.1016/0092-8674(92)90311-y. [DOI] [PubMed] [Google Scholar]
- 34.Miller J R, Moon R T. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- 35.Nikolakaki E, Coffer P J, Hemelsoet R, Woodgett J R, Defize L H. Glycogen synthase kinase 3 phosphorylates Jun family members in vitro and negatively regulates their transactivating potential in intact cells. Oncogene. 1993;8:833–840. [PubMed] [Google Scholar]
- 36.Nishitani J, Nishinaka T, Cheng C-H, Rong W, Yokoyama K K, Chiu R. Recruitment of the retinoblastoma protein to c-jun enhances transcription activity mediated through the AP-1 binding site. J Biol Chem. 1999;274:5454–5461. doi: 10.1074/jbc.274.9.5454. [DOI] [PubMed] [Google Scholar]
- 37.Novak A, Hsu S C, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, Roskelley C, Grosschedl R, Dedhar S. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pap M, Cooper G M. Role of glycogen synthase kinase-3 in the phosphatidylinositol 3-kinase/Akt cell survival pathway. J Biol Chem. 1998;273:19929–19932. doi: 10.1074/jbc.273.32.19929. [DOI] [PubMed] [Google Scholar]
- 39.Papavassiliou A G, Chavrier C, Bohmann D. Phosphorylation state and DNA-binding activity of c-Jun depend on the intracellular concentration of binding sites. Proc Natl Acad Sci USA. 1992;89:11562–11565. doi: 10.1073/pnas.89.23.11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radeva G, Petrocelli T, Behrend E, Leung-Hagesteijn C, Filmus J, Slingerland J, Dedhar S. Overexpression of the integrin-linked kinase promotes anchorage-independent cell cycle progression. J Biol Chem. 1997;272:13937–13944. doi: 10.1074/jbc.272.21.13937. [DOI] [PubMed] [Google Scholar]
- 41.Renshaw M W, Ren X D, Schwartz M A. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Schagger H, von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- 44.Schwartz M A. Integrins, oncogenes, and anchorage independence. J Cell Biol. 1997;139:575–578. doi: 10.1083/jcb.139.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 46.Smeal T, Binetruy B, Mercola D A, Birrer M, Karin M. Oncogenic and transcriptional cooperation with Ha-Ras requires phosphorylation of c-Jun on serines 63 and 73. Nature. 1991;354:494–496. doi: 10.1038/354494a0. [DOI] [PubMed] [Google Scholar]
- 47.van Weeren P C, de Bruyn K M, de Vries-Smits A M, van Lint J, Burgering B M. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–13156. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 48.Vlahos C J, Matter W F, Hui K Y, Brown R F. A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- 49.Wang Q, Somwar R, Bilan P J, Liu Z, Jin J, Woodgett J R, Klip A. Protein kinase B/Akt participates in GLUT4 translocation by insulin in L6 myoblasts. Mol Cell Biol. 1999;19:4008–4018. doi: 10.1128/mcb.19.6.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]