Abstract
Whipple’s disease is a rare disease, which can be fatal if not treated. It is often diagnosed at a late stage because of the varied disease presentation and its rare incidence. Classic Whipple’s disease presents with arthralgia, abdominal pain, diarrhea and weight loss. CT abdomen may show mesenteric lymphadenopathy with hypodense centers. These CT findings along with clinical presentation should prompt early diagnosis of Whipple’s disease. We present a case of classic Whipple’s disease with interesting CT abdomen and PET scan findings.
Keywords: Whipple’s disease, Tropheryma whipplei, PET-CT, CT abdomen, Mesenteric and retroperitoneal lymphadenopathy
Introduction
Whipple's disease is a rare systemic disease caused by gram positive bacillus Tropheryma whipplei. Whipple's disease has a varied clinical spectrum, ranging from a classic form that predominantly involves the GI tract causing abdominal pain and diarrhea; to localized form involving extraintestinal organs like brain, eye and heart; and acute self-limiting infections like acute gastroenteritis. It is often diagnosed at a late stage because of nonspecific chronic symptoms. Diagnosis is confirmed by histological detection with positive PAS staining in small bowel biopsies. Immunohistochemistry and PCR can also help in specific diagnosis. Role of imaging studies in diagnosis is unclear. It can present as mesenteric lymphadenitis on imaging like CT abdomen and PET/CT. Just like other signs and symptoms of Whipple’s disease, mesenteric lymphadenitis is also a nonspecific finding and can be caused by a myriad of neoplastic, inflammatory and infectious conditions. The appearance and enhancement pattern of the lymph nodes can give a clue to the underlying condition. We present a case where a patient had a CT abdomen following a motor vehicle accident and incidentally showed mesenteric lymphadenitis. As a work up of mesenteric lymphadenitis, the patient also underwent PET/CT that showed enlarged low attenuation lymph nodes in the root of mesentery and retroperitoneum. A few months later, the patient started having symptoms like diarrhea, abdominal pain, and weight loss. Diagnosis of Whipple's disease was made months later with small bowel histology confirmation. We believe that mesenteric lymphadenitis on CT abdomen and PET/CT along with other clinical features could have aided an earlier diagnosis.
Case presentation
53-year-old male truck driver with past medical history of benign prostatic hypertrophy and dyslipidemia, presented with diarrhea, weight loss, abdominal pain and fatigue for 4‐5 months. He had multiple ER visits and hospitalizations for diarrhea, weight loss, dehydration, and abdominal pain in a four-month period. He reported fatigue, shortness of breath and dysphagia. No fevers or skin rash. He reported diffuse joint pains which he related to arthritis. He emigrated from Mexico in 1979, the last trip back to Mexico was approximately 7 years ago. He reported no recent travel or any exposure to tuberculosis. Physical exam showed no fever, normal vital signs, no peripheral lymphadenopathy, benign abdominal exam, no organomegaly, no neurological deficits, no joint swellings or skin rash. Lab data showed normal WBC count, mild microcytic anemia (ranging between 9 and 11 mg/dl), mildly elevated ESR, and normal LFTs. GI pathogen panel negative, endomysial antibody negative, tissue transglutaminase (tTG) IgA and IgG negative, antimitochondrial antibody negative, rheumatoid factor negative. CT abdomen pelvis showed low attenuation mesenteric lymphadenopathy, similar to that noted on CT abdomen done a year ago.
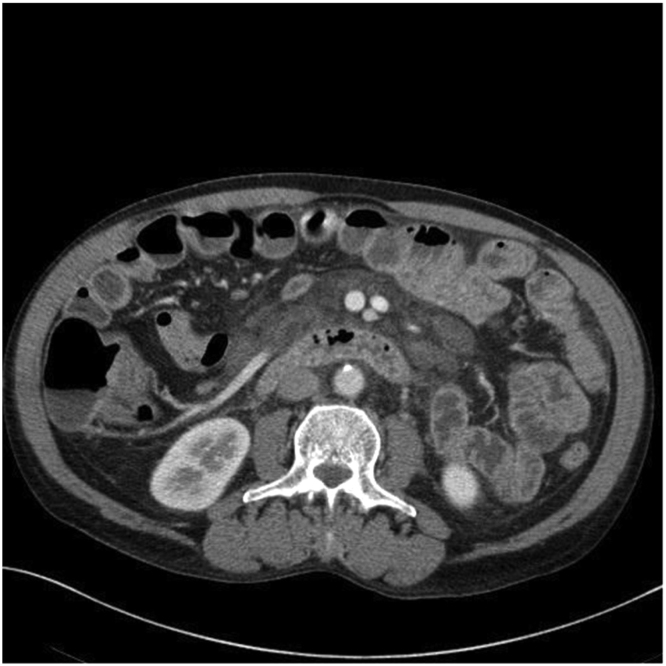
CT abdomen/pelvis done 1 year ago (after he was involved in a motor vehicle accident) showed enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum with surrounding infiltrative changes (Figs. 1 and 2). Low attenuation mediastinal lymphadenopathy was also noted on chest CT. He underwent extensive workup following that for this lymphadenopathy. HIV, hepatitis B, hepatitis C, LDH, CEA, CA 19–9, peripheral flow cytometry was negative. PET-CT demonstrated enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum without significant FDG uptake (Figs. 3 and 4). Subsequently, he underwent EGD and colonoscopy. Antral biopsies showed mild inflammation of the gastric mucosa and moderate duodenitis, well preserved villous architecture and no evidence of gluten enteropathy. H. pylori was negative. Colonoscopy did not show any significant finding. Bone marrow biopsy was inconclusive and showed possible low-frequency B cell lymphoproliferative disorder with monoclonal B-cell lymphocytes. Cytogenetics and the fluorescence in situ hybridization (FISH) panel were negative. Repeat PET scan done 3 months later, showed improved findings.
Fig. 1.
CT abdomen and pelvis with IV contrast showing enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum.
Fig. 2.
CT abdomen and pelvis with IV contrast showing enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum.
Fig. 3.
PET CT showing enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum.
Fig. 4.
PET CT showing enlarged low attenuation lymph nodes in the root of the mesentery and retroperitoneum.
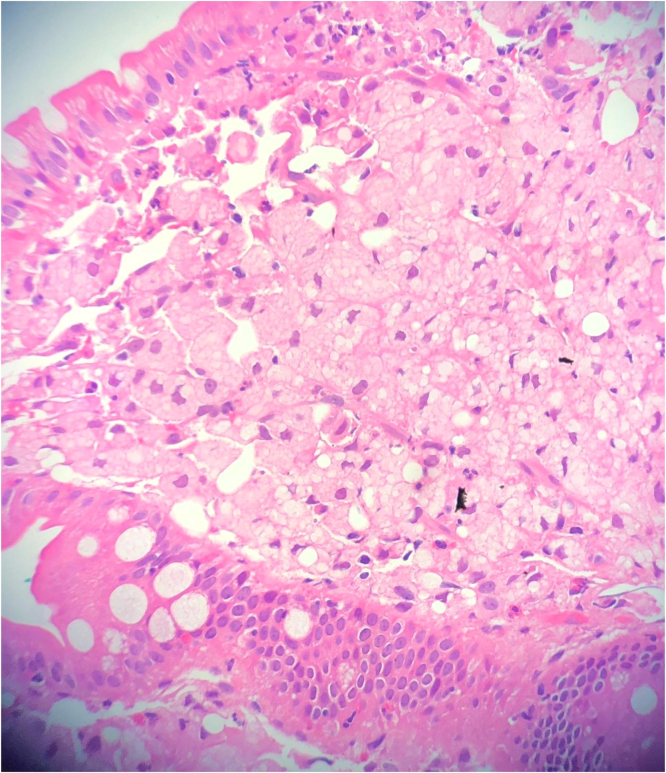
He was referred to Gastroenterology and underwent small bowel biopsy that showed extensive infiltration of lamina propria by foamy macrophages with resultant villous blunting, PAS positive diastase resistance within macrophages, negative GMS and acid-fast stain (Figs. 5 and 6). Blood PCR came positive for Tropheryma whipplei, confirming the diagnosis of Whipple’s disease. He then had transesophageal echo which was unremarkable and spinal tap was negative for PCR Whipple disease. He was treated with ceftriaxone 2 g IV every 12 h for 2 weeks and then switched to sulfamethoxazole/trimethoprim 800 mg/160 mg twice daily for 1 year. A month later on follow-up outpatient visit, he was feeling much better, abdominal pain and diarrhea resolved, and he had gained more than 10 pounds. He still had some intermittent arthralgia.
Fig. 5.
small bowel biopsy showing villi infiltrated with macrophages.
Fig. 6.
small bowel biopsy showing PAS positive macrophages.
Of interest, recently his brother also started reporting similar symptoms and is undergoing more work up.
Discussion
Whipple's disease is a rare infectious disease, caused by bacteria, Tropheryma whipplei. It can be fatal if not treated. The annual incidence of classic Whipple's disease is approximately 12 new cases per year worldwide. With the introduction of PCR testing, the incidence rate of Whipple’s disease has gone up and is now estimated to be between 1 and 6 new cases per year per 10,000,000 people worldwide (1). It is more common in Caucasian males, with the mean age of onset around 50 years [1], [2]. There is evidence for genetic predisposition and some familial cases have been reported [1]. Most common symptoms in classic Whipple's disease include intermittent arthralgia (reported in 70–90% patients), weight loss (80–90%), diarrhea (70–85%) and abdominal pain (50–90%) [2]. Almost all organ systems including eyes, heart, lungs, skeletal muscle and CNS can be affected. Whipple's disease is frequently diagnosed at a late stage, because it is an uncommon disease and has varied nonspecific clinical presentations that can be observed in several other diseases. Histopathology and PCR are the most common diagnostic methods. Cultivation of T. whipplei is difficult [1]. Diagnosis is mostly made by proximal small bowel biopsy that demonstrates diastase-resistant, non-acid-fast, PAS-positive inclusions in macrophages in the lamina propria. PAS-positive macrophages may be found in other affected organs like lymph nodes, heart, lungs, CNS, eyes, liver and bone marrow [2]. Immunohistochemical staining for antibodies against T. whipplei can be used to detect the organism in a variety of tissues [3]. PCR based assay is also a popular method for diagnosing Whipple’s disease and can be used as a confirmatory test if performed on intestinal samples. It can also help in diagnosing extra intestinal involvement if positive in blood, vitreous fluid, synovial fluid, heart valves, or cerebrospinal fluid [3]. Treatment with antibiotics usually results in rapid improvement but prolonged treatment is required for eradication [1].
CT abdomen findings suggestive of Whipple’s disease include thickening of intestinal wall, mesenteric fat infiltration, and mesenteric and/or retroperitoneal lymphadenopathy. Normal CT abdomen does not exclude the diagnosis. The most specific criterion for Whipple’s disease on CT imaging is lymphadenopathy with hypodense centers, corresponding to fat infiltration of the lymph node sinus [4]. There has been a case described where mesenteric lymphadenitis was the presenting feature [5]. A retrospective study done in France reported the prevalence of mesenteric lymphadenitis in Whipple’s disease to be 17% [6]. With the frequent use of multidetector CT these days, mesenteric lymphadenopathy is being identified more commonly in the adult population. It is a nonspecific finding which can be seen in a variety of neoplastic, infectious and inflammatory conditions like lymphoma, GI tract malignancies, metastatic malignancies, inflammatory bowel disease, connective tissue disorders like SLE and systemic sclerosis, tuberculosis, Mycobacterium avium cellulare( MAC) infection, yersinia infection and HIV [7]. The distribution, size, attenuation and enhancement pattern of the lymph nodes can aid narrowing the differential diagnosis. Mesenteric lymphadenopathy seen in Whipple’s disease characteristically have central low CT attenuation due to their high fat content. Lymphadenopathy responds to antibiotic therapy and serial CT’s can be used to assess response to treatment. Celiac disease can cause low attenuation but cavitating lymph nodes. Mesenteric lymphadenopathy from malignancy typically has large lymph nodes with soft tissue attenuation and homogenous enhancement with IV contrast. Malignant lymph nodes with central necrosis can have lower attenuation with peripheral enhancement. Tuberculous lymph nodes typically have low attenuation with peripheral enhancement [7].
There have not been many reports on PET scan findings in Whipple’s disease. Our literature search showed that two cases have been described where PET scan helped in diagnosing Whipple’s disease [8], [9]. In one of these cases, classic Whipple’s disease was suspected in patients with a history of Whipple’s endocarditis when PET scan showed moderate intestinal fixation with hypermetabolic inflammatory activity [9]. In our case, PET scan showed retroperitoneal and mesenteric lymphadenopathy with no significant FDG uptake. Low FDG uptake on PET scan is likely due to high fatty content of the lymph nodes in Whipple's disease. Repeat PET scan done three months later showed some improvement, even before diagnosis was established and treatment initiated. One possible explanation for this could be that the patient received some antibiotic courses for suspected bronchitis; infectious diarrhea on his multiple ER visits, and that could have led to some improvement in PET scan findings. More studies are needed to establish the definite role of PET scan in diagnosing Whipple’s disease.
Although CT abdomen and PET/CT findings of mesenteric and retroperitoneal lymphadenopathy are nonspecific and can be seen in other infections like tuberculosis, inflammatory conditions and malignancies but these findings along with symptoms such as arthralgia, abdominal pain, weight loss and chronic diarrhea should prompt early testing for Whipple’s disease with PCR and histopathology.
Consent
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of written consent is available for review by the Editor-in-Chief of this journal on request.
Ethics approval and consent to participate
None required.
Funding
This research did not receive any specific grant from funding agencies in public, commercial, or not-for-profit sectors.
Declarations of interest
None, for all authors.
Acknowledgments
None.
References
- 1.Dolmans R.A.V., Boel C.H.E., Lacle M.M., Kusters J.G. Clinical manifestations, treatment, and diagnosis of Tropheryma whipplei infections. Clin Microbiol Rev. 2017;30:529–555. doi: 10.1128/CMR.00033-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dutly F., Altwegg M. Whipple’s disease and “Tropheryma whippelii”. Clin Microbiol Rev. 2001;14(3):561–583. doi: 10.1128/CMR.14.3.561-583.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elkholy S., Khaled Y., Aziz A.A., Helmy D.O., Alkady M.N. Whipple’s disease: a case report and review of literature. MOJ Clin Med Case Rep. 2017;7(3):00202. [Google Scholar]
- 4.Gervaise A., Corberan D., Naulet P., Pernin Y., PortronY, Lapierre-Combes M. Whipple’s disease with gastrointestinal involvement and multiple abdominal adenopathies. Diagn Interv Imaging. 2013;Volume 94(Issue 11):1145–1147. doi: 10.1016/j.diii.2013.04.007. -11-01. [DOI] [PubMed] [Google Scholar]
- 5.Chizinga M., Schiliro D., Mullin B., Barrie R. Mesenteric lymphadenitis as a presenting feature of Whipple’s disease. IDCasesVolume. 2017;9:50–52. doi: 10.1016/j.idcr.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagier J.C., Lepidi H., Raoult D., Fenollar F. Systemic Tropheryma whipplei: clinical presentation of 142 patients with infections diagnosed or confirmed in a reference center. Med (Baltim) 2010;89(5):337–345. doi: 10.1097/MD.0b013e3181f204a8. [DOI] [PubMed] [Google Scholar]
- 7.Brian C.L., Joshua W.S., Jorge A.S. Mesenteric Lymph Nodes seen at Imaging: causes and significance. RadioGraphics. 2005;25(2):351–365. doi: 10.1148/rg.252045108. [DOI] [PubMed] [Google Scholar]
- 8.Jos S., Angelakis E., Caus T., Raouly D. Positron emission tomography in the diagnosis of Whipple’s endocarditis: a case report. BMC Res Notes. 2015;8:56. doi: 10.1186/s13104-015-1022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.J.C. Lagier et al., Classic Whipple's disease diagnosed by 18F-fluorodeoxyglucose PET. The Lancet Infectious Diseases, Volume 16, Issue 1, 130. [DOI] [PubMed]