Abstract
Diabetic cardiomyopathy is associated with an increase in oxidative stress. However, antioxidant therapy has shown a limited capacity to mitigate disease pathology. The molecular mechanisms responsible for the modulation of reactive oxygen species (ROS) production and clearance must be better defined. The objective of this study was to determine how insulin affects superoxide radical (O2•–) levels. O2•– production was evaluated in adult cardiomyocytes isolated from control and Akita (type 1 diabetic) mice by spin-trapping electron paramagnetic resonance spectroscopy. We found that the basal rates of O2•– production were comparable in control and Akita cardiomyocytes. However, culturing cardiomyocytes without insulin resulted in a significant increase in O2•– production only in the Akita group. In contrast, O2•– production was unaffected by high glucose and/or fatty acid supplementation. The increase in O2•– was due in part to a decrease in superoxide dismutase (SOD) activity. The PI3K inhibitor, LY294002, decreased Akita SOD activity when insulin was present, indicating that the modulation of antioxidant activity is through insulin signaling. The effect of insulin on mitochondrial O2•– production was evaluated in Akita mice that underwent a 1-week treatment of insulin. Mitochondria isolated from insulin-treated Akita mice produced less O2•– than vehicle-treated diabetic mice. Quantitative proteomics was performed on whole heart homogenates to determine how insulin affects antioxidant protein expression. Of 29 antioxidant enzymes quantified, thioredoxin 1 was the only one that was significantly enhanced by insulin treatment. In vitro analysis of thioredoxin 1 revealed a previously undescribed capacity of the enzyme to directly scavenge O2•–. These findings demonstrate that insulin has a role in mitigating cardiac oxidative stress in diabetes via regulation of endogenous antioxidant activity.
Keywords: Diabetes, Heart, Cardiomyocytes, EPR, Thioredoxin, Insulin
Graphical abstract
Highlights
-
•
Insulin decreases ROS production in T1D Akita cardiomyocytes.
-
•
Insulin signaling downstream of PI3K is required for this effect.
-
•
Insulin increases the antioxidant capacity in the Akita heart.
-
•
Trx1 is upregulated by insulin in the Akita heart in vivo.
1. Introduction
Heart disease and heart failure are prevalent in type 1 and type 2 diabetic patients. This is due in part to the additive effects of diabetes with other underlying conditions, such as hypertension and atherosclerosis. However, diabetes can also lead to alterations in cardiac function in the absence of other risk factors and this is termed diabetic cardiomyopathy [1]. Although the manner by which diabetes affects the heart is multifold and complex, it is now widely accepted that oxidative stress is a major contributor [1,2].
Oxidative stress occurs when the production of reactive oxygen species (ROS) exceeds cellular antioxidant defenses. Superoxide anions (O2•–) is the dominant ROS in cardiomyocytes and its primary sources are NADPH oxidase and the mitochondrial electron transport chain [2]. The expression of specific NADPH oxidase isoforms, as well as its activators, are increased with diabetes [2,3]. The mitochondrial electron transport chain generates O2•– at low levels under normal conditions [4]. Diabetes-induced deficits in mitochondrial function and electron transport chain activity promote an increase in O2•– production [5]. Antioxidant defenses, including both enzymatic and nonenzymatic small molecule scavengers, normally are sufficient to prevent oxidative damage. However, this balance shifts under diabetic conditions as the amount of O2•– production exceeds the scavenging capacity.
While ROS are widely implicated in the pathology of both diabetic heart disease and cardiomyopathy, there is surprisingly sparse information regarding how diabetes affects O2•– production in cardiomyocytes. In an earlier study, ROS production was measured in adult cardiomyocytes isolated from OVE26 type 1 diabetic mice by a redox sensitive fluorescent probe [6]. Cardiomyocytes from diabetic mice produced similar amounts of ROS to controls under basal culture conditions. However, when cardiomyocytes were cultured with high glucose media or with angiotensin II, the OVE26 cardiomyocytes produced significantly more ROS. Additionally, oxidative stress was blunted in diabetic mice overexpressing the antioxidant enzyme, metallothionein [7]. This suggested that diabetes alone may not increase basal cardiomyocyte ROS/O2•– production, but when combined with a secondary stressor, ROS production exceeds antioxidant capacity.
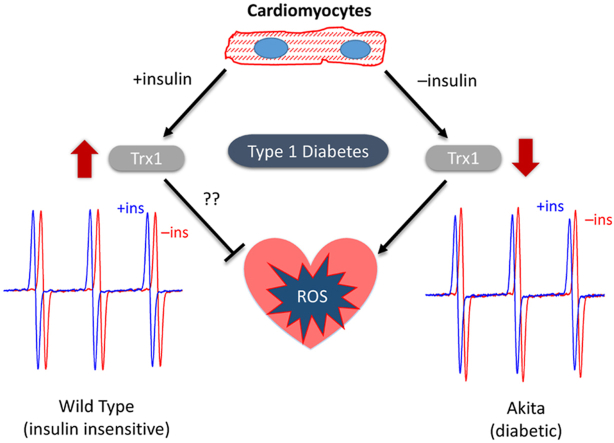
In this study, we sought to examine factors that could enhance O2•– production in the diabetic heart. Adult cardiomyocytes were isolated from control and Akita mice. Akita mice are a well characterized type 1 diabetes (T1D) model that develops diabetic cardiomyopathy and are characterized by lipotoxicity and diastolic dysfunction [8]. Unlike other type 1 and type 2 diabetes models, Akita hearts reportedly do not have increased O2•– production [6]. Consequently, an added motive of this study was thus to determine factors that may affect Akita cardiomyocyte O2•– production. O2•– were measured by electron paramagnetic resonance (EPR) spectroscopy spin-trapping with different culture conditions that mimic diabetic stresses. Our results, like that of the previous studies in both OVE26 and Akita mice [6], support that basal O2•– production is similar in diabetic and control cardiomyocytes. However, in the absence of insulin O2•– production by Akita cardiomyocytes is significantly enhanced. Proteomic analysis of insulin-treated Akita mice revealed thioredoxin as the most upregulated antioxidant enzyme. Our results support that the lack of insulin signaling is a determinant of oxidative stress in cardiomyocytes.
2. Methods
Adult mouse cardiomyocyte isolation: Adult cardiomyocytes were isolated from 5-month C57BL/6J or C57BL/6J-Ins2Akita/J male mice (Akita, The Jackson Laboratory 003548) and cultured as previously described [9,10]. Akita mice are a well-established model of hypoinsulinemia and hyperglycemia [8,11]. Blood glucose was measured by a glucose test strip (Contour) at the time of sacrifice to confirm hyperglycemia. All Akita mice had blood glucose levels of at least 400 mg/dL. Briefly, after isoflurane administration the heart was excised, the aorta was cannulated, and it was then perfused with type II collagenase (Worthington #LS004176). Calcium was reintroduced to the subsequent single cell suspension and cells were plated on laminin (Corning 354232) coated plates. Media was switched to serum-free culture media (minimal essential medium with Hanks’ balanced salt solution, Gibco (11575-032) supplemented with 0.2 mg/mL sodium bicarbonate, penicillin-G, 0.1%BSA, glutamine, 10 mM butanedione monoxime, and 10 μg/mL insulin as indicated. Cells were cultured 18 h at 37 °C and 5%CO2 with or without 2.5 μM LY294002 (BioGems 1543664) as described in the figure legends. All procedures were approved by the Oklahoma Medical Research Foundation Animal Care and Use Committee.
EPR Spin trapping: Cellular O2•– production was quantified by an EPR spin-trapping technique utilizing hydroxylamine spin-trapping agents [12], 1-Hydroxy-3-methoxycarbonyl-2,2,5,5-tetramethylpyrrolidine (CMH; Enzo Lifescience ALX-430-117) or 1-Hydroxy-4-phosphono-oxy-2,2,6,6-tetramethylpiperidine (PPH; Enzo Lifescience ALX-430-080). After overnight treatment, cardiomyocytes from wild-type or Akita mouse were washed twice with PBS and then incubated for 15 min at 37 °C in PBS containing 1 mM EDTA, 50 μM DTPA, and 500 μM CMH/PPH. The supernatant was then removed, quickly centrifuged (10s) to remove debris and detached cells, and then snap frozen and stored in LN2 until EPR measurement. After the supernatant was removed, cardiomyocytes were digested with RIPA buffer for BCA protein determination that was used for standardization.
The thawed PBS containing cellular O2•–-mediated CMH/PPH product were transferred to a quartz flat cell for EPR analysis. For each sample, the EPR spectrum was measured exactly 3min after being completely thawed. To validate there was minimal auto-oxidation product formed from the debris in the solution, another spectrum was measured at a 9min time point. The EPR spectra were obtained using a Bruker (Billerica, MA) EMX spectrometer operating at X-band (∼9.78 GHz) with a 100 kHz modulation frequency and an ER 41225SHQ high-sensitivity cavity. Typical settings for the spectrometer are as follows: microwave power, 6.325 mW; modulation amplitude, 1.5 G; scan range, 50 G; time constant, 82 ms. The peak signal intensity for each spectrum was quantified by the WinEPR software (Bruker). EPR signal intensity was calibrated with TEMPOL (Enzo Lifescience ALX-430-081) standards.
SOD activity: Superoxide dismutase (SOD) activity in cardiomyocytes was measured using an SOD assay kit according to the manufacturer's protocol (Cayman 706002). Briefly, SOD activity was quantified by measuring the absorbance upon incubation of lysates with tetrazolium salt, hypoxanthine, and xanthine oxidase. A decrease in absorbance, relative to the no-lysate condition, is indicative of SOD activity. To generate cardiomyocyte lysates, after the described culture conditions (overnight ± insulin, in the presence or absence of PI3K inhibitor), the culture media was removed, cardiomyocytes were washed with PBS, and cells were scraped in PBS and snap-frozen. Whole-cell lysates (18.2 μg/mL) from wild-type and Akita cardiomyocytes were compared to SOD standards. The end-point absorption in a 96-well plate was measured by TECAN Sunrise with Magellan 6 acquisition software. Activities were measured in the presence or absence of the SOD1 inhibitor 5 mM KCN. Superoxide scavenging was decreased by less than 5% with KCN, indicating the assay primarily measured SOD2 and other superoxide scavenging activities. Total SOD activity is shown.
Mitochondrial isolation and O2•–production measurements: Subsarcolemmal mitochondria were isolated from hearts as previously described [5,13]. After 8 days-total treatment of insulin (5U/kg) or vehicle (saline) IP injected, hearts were excised, and immersed and rinsed in ice-cold isolation buffer containing 210 mM mannitol, 70 mM sucrose, 10 mM MOPS, and 1.0 mM EDTA (pH 7.4). The hearts were then minced and homogenized in 5 mL of isolation buffer with a pestle homogenizer (5 × 2 s passes). The homogenate was then centrifuged at 500g for 5.0 min, and the supernatant was collected. The supernatant was then filtered through cheese cloth and centrifuged at 10000g for 10 min. The resulting mitochondrial pellets were washed, resuspended in 25 mM MOPS (pH 7.4), and immediately snap-frozen in liquid N2 for analysis of O2•– production.
The NADH-supported rate of O2•– production was measured as described previously [5,12]. Briefly, the oxidation of hydroethidine by O2•– to the fluorescent product 2-hydroxyethidium was measured utilizing a Horiba Jobin Yvon Fluoromax-4 spectrofluorometer. The fluorescent signals were recorded (excitation, 480 nm; emission, 567 nm) over time utilizing 10 μM hydroethidine and 500 μM NADH. Exogenous SOD1 (8.0 units/mL) was used to test the specificity of the measurement for O2•– anion. It should be noted that oxidation of hydroethidine by O2•– resulted in a product with properties (reverse phase high-performance liquid chromatography elution profile and fluorescence properties) consistent with 2-hydroxyethidium and not ethidium as reported previously. Nevertheless, the structurally related compound ethidium was used as a standard to estimate the relative rate of O2•– production because of the absence of a commercially available 2-hydroxyethidium standard.
Quantitative proteomic analysis: Selected reaction monitoring (SRM) MS analysis was used as a quantitative measure of specific protein quantities and performed as reported previously [14,15]. Briefly, 20 μg of total heart homogenate samples (n = 4 biological replicates/group) were run on a 12.5% SDS-polyacrylamide gel (Bio-Rad Criterion). The gel was then fixed and stained with GelCode Blue (Pierce). Protein lanes were cut into ∼1 mm3 pieces which were then washed, reduced with DTT, alkylated with iodoacetamide, and digested with trypsin. Peptides were extracted with 70% methanol and 5% acetic acid, and then dried and reconstituted in 1% acetic acid. The samples were then analyzed using SRM with a triple quadrupole mass spectrometer (Thermo Scientific TSQ Vantage) configured with a splitless capillary column HPLC system (Eksigent) as described in [15]. The data were processed as in [15] using the Skyline software application [16]. Protein abundance was determined by normalization to BSA used as a nonendogenous internal standard. Housekeeping proteins were also used for normalization.
Western blot analysis: After culture media was removed, primary cardiomyocytes cultured in 12 well plates overnight in the absence or presence of insulin were washed with 0.5 mL PBS, and 75 μL of 1× sample buffer containing 25 mM DTT and 1× Halt Protease/Phosphatase Inhibitor Cocktail (Thermo Fisher 78442) was added per well. For isolated mitochondria, samples were diluted to 1 mg/mL protein in 1× sample buffer and 15 μL used for analysis. Samples were heated at 95 °C for 5 min, resolved by SDS-PAGE (Invitrogen NP0321), transferred to nitrocellulose membranes, and blocked for 30 min with Odyssey TBS blocking buffer (LI-COR). Antibodies used in this study are: thioredoxin 2 (14907S) and TXNIP (14715S) from Cell Signaling Technology, SOD2 (FL-222, Santa Cruz Biotechnology), and acetylated SOD2 K68 (ab137037, Abcam). Primary antibodies were diluted in block buffer and added to blots overnight at 4 °C, subsequently washed, and the secondary antibody (LI-COR IRDye 800CW) was incubated for 1 h. Following additional washing, blots were imaged on an Odyssey CLx system and analyzed using the Image Studio software (LI-COR).
Glutathione Measurements: Total and oxidized glutathione content in cardiomyocytes was measured using a glutathione colorimetric detection kit according to the manufacturer's protocol (Invitrogen EIAGSHC). Pulverized tissue obtained from snap-frozen whole Akita mouse hearts that underwent the 8-day insulin treatment were used for this assay. Samples were normalized based on the pulverized tissue weights. The end-point absorption in a 96-well plate was measured by TECAN Sunrise with Magellan 6 acquisition software.
In vitro antioxidant analysis: The superoxide scavenging activities of thioredoxin 1 (Trx1), glutathione (GSH), and n-acetyl cysteine (NAC) were assessed by using a xanthine/xanthine oxidase-supported enzymatic O2•– generation system in the presence of the hydroxyl radical scavenger, dimethyl thiourea (DMTU). The decrease in the rate of O2•– production was quantified as the decrease in the slope of the O2•–-dependent 2-hydroxyethidium fluorescence formation by added Trx1, GSH, or NAC. The fluorescent signals were recorded (excitation, 480 nm; emission, 567 nm) over time utilizing 25mU/mL xanthine oxidase, 500 μM xanthine, and 10 μM hydroethidine in 25 mM Tris buffer (pH 7.5) supplemented with 50 mM DMTU. For quantification of O2•– scavenging activity by Trx1, exogenous thioredoxin reductase (0.05U/mL) and 1.0 mM NADPH were included in addition to the thioredoxin (0, 0.01–0.5U/mL).
Statistical analysis: Data were analyzed using GraphPad Prism 8.4.3. Data are presented as means. Pairwise comparison between groups was performed using a paired or unpaired two-tailed Student's t-test, as specified. Multiple comparison was performed using two-way ANOVA with Tukey post hoc analysis, unless otherwise noted. p < .05 was considered statistically significant.
3. Results
3.1. Characterization of cardiomyocyte superoxide production by EPR spin-trapping
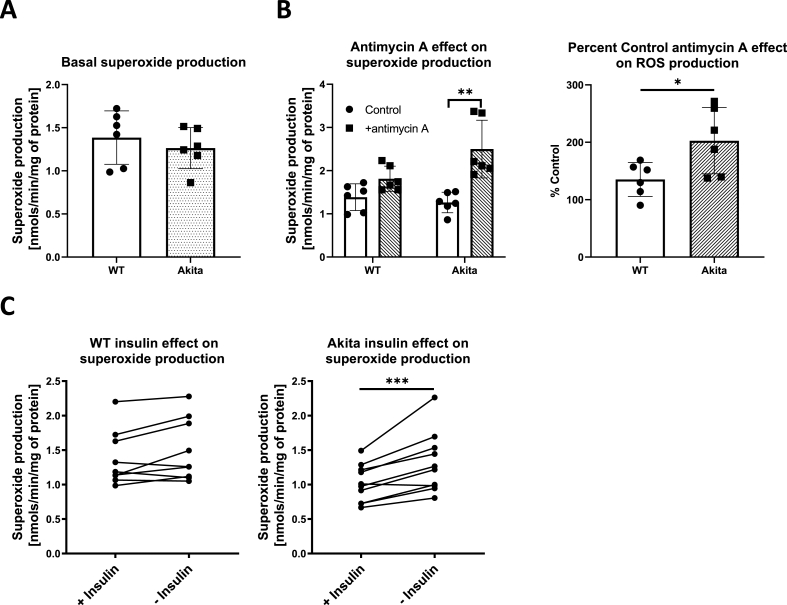
Our initial goal was to implement the EPR spin-trapping technique as a means of quantifying overall cellular superoxide (O2•–) production in adult C57/BL6 control and Akita type 1 diabetic mouse cardiomyocytes. The membrane-permeable cyclic hydroxylamine EPR spin-trapping agent, CMH [12,17], was added to freshly isolated cardiomyocytes and incubated for 15min. Media was then collected and analyzed by EPR spectroscopy. As shown in Fig. 1A, wild type and Akita cardiomyocytes produced similar amounts of cellular O2•– under these experimental conditions. Similar results were also found when using the related cyclic hydroxylamine spin-trap, PPH (data not shown). We next sought to determine whether a difference in O2•– production was observable when compounded with an added stress. The complex III inhibitor, antimycin A, is a well-known O2•– stimulating agent [18]. Addition of antimycin A had minimal effect on O2•– production in wild type cardiomyocytes. In contrast, antimycin A induce a 2-fold increase in the O2•– signal in Akita cardiomyocytes (Fig. 1B). This result supports that Akita cardiomyocytes have either an increased propensity for complex III mediated O2•– production or a decreased endogenous antioxidant defense capacity.
Fig. 1.
Akita cardiomyocytes have enhanced ROS production in response to antimycin A and to the lack of insulin. Cardiomyocytes were isolated from wild-type and Akita mice and ROS production was measured by EPR spin trapping with CMH. (A) ROS production was measured basally in cardiomyocytes cultured in standard culture media. (B) ROS production was measured in the presence or absence of 1.0 μM antimycin A and shown as the raw intensities (left) and the % change (right). Antimycin A significantly increased ROS production in Akita cardiomyocytes (*p < .05; **p < .01; n = 6 biological replicates). (C) ROS production was compared when cardiomyocytes isolated from wild-type (left) and Akita (right) mice were cultured for 24 h in the presence or absence of 10 μg/mL insulin. Insulin treatment significantly decreased ROS in Akita cardiomyocytes (***p < .005; n = 9 or 10 biological replicates for WT and Akita, respectively). Statistics were performed by Student's t-test.
3.2. Insulin regulates antioxidant defenses in Akita cardiomyocytes
We next sought to identify physiological stresses that may affect cellular O2•– production in the diabetic heart. Because of their relevance to diabetes, we examined the effects of glucose, lipids, and insulin. Superoxide production by wild type and Akita cardiomyocytes was unaffected by 24 h culture in media containing high glucose (25 mM glucose) and/or fatty acids (100 μM palmitate/oleate with BSA; data not shown). In contrast, O2•– production in Akita cardiomyocytes cultured in the absence of insulin was 29% higher than in the presence of insulin (Fig. 1C). This inhibitory effect of insulin on O2•– production was not observed in wild type cardiomyocytes.
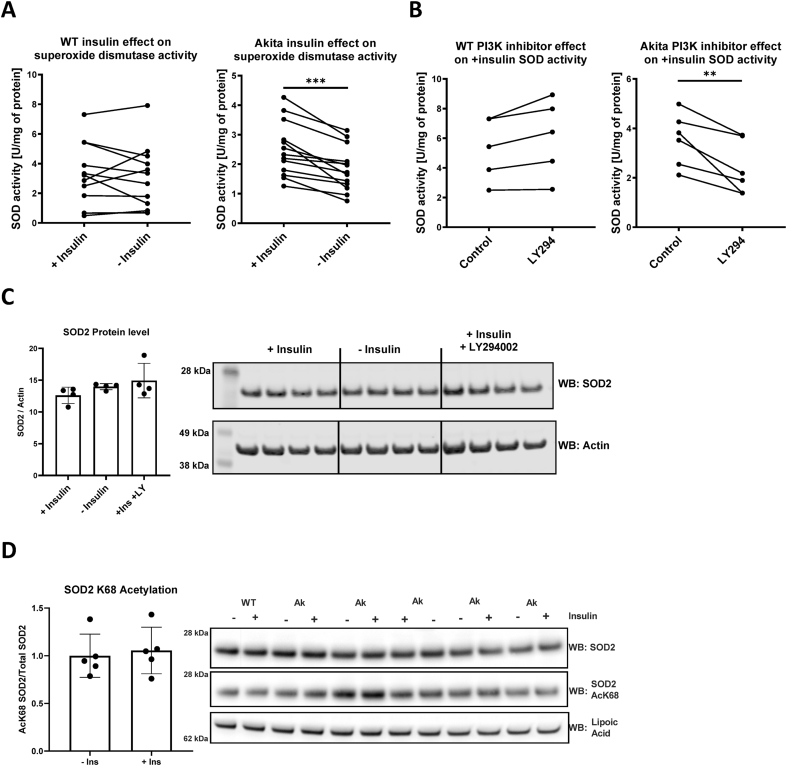
The observed increase in O2•– production may be mediated in part by reduced endogenous antioxidant capacity. To test this, we assayed superoxide scavenging activity in wild type and Akita cardiomyocytes cultured in the presence or absence of insulin. This activity was largely insensitive to the SOD1 inhibitor, KCN (5% inhibition), and thus represents primarily SOD2 and nonenzymatic scavenging capacity. As shown in Fig. 2A, SOD activity was significantly reduced in Akita cardiomyocytes cultured in the absence of insulin. In contrast, wild type cardiomyocytes had similar SOD activity regardless of whether they were cultured with insulin. We next sought to determine whether downstream insulin signaling is required for this antioxidant effect. Upon insulin binding, the insulin receptor interacts with IRS proteins to activate phosphatidylinositol-3-kinase (PI3K) and Akt [19]. SOD activity in Akita cardiomyocytes cultured with insulin and the PI3K inhibitor, LY294002, was decreased by 33% (Fig. 2B). Interestingly, this effect was specific to Akita cardiomyocytes, as SOD activity in wild type cardiomyocytes trended toward an increase when cultured in the presence of insulin and LY294002. These results support that blocking downstream insulin signaling is sufficient to increase O2•– production in Akita cardiomyocytes.
Fig. 2.
Insulin increases superoxide dismutase activity selectively in Akita cardiomyocytes. Cardiomyocytes were isolated from wild-type and Akita mice and cultured for 24 h in the presence or absence of insulin. (A) Superoxide dismutase activity was measured in cardiomyocyte lysates from wild-type (left) and Akita (right) mice after being cultured for 24 h in the presence or absence of insulin. Insulin treatment significantly increased superoxide dismutase activity in Akita cardiomyocytes (***p < .0005; n = 12 or 13 biological replicates for WT and Akita, respectively). (B) Superoxide dismutase activity was measured in cardiomyocyte lysates from wild-type (left) and Akita (right) mice after being cultured for 24 h with insulin and the presence or absence of LY294002. LY294002 treatment significantly decreased superoxide dismutase activity in Akita cardiomyocytes (**p < .01; n = 5 or 6 biological replicates for WT and Akita, respectively). Less than 5% of SOD activity was inhibited by 5 mM KCN, indicating the data primarily represent SOD2 and/or other nonenzymatic scavenging activity. Statistics were performed by Student's t-test. (C) Change in superoxide dismutase 2 (SOD2) levels in Akita primary cardiomyocytes in the presence or absence of insulin overnight were assessed by western blotting. Quantitative SOD2 protein levels in the lack of insulin were statistically insignificant as compared to basal (+insulin) or + insulin with LY294002 supplemented conditions. (D) SOD2 K68 acetylation levels were measured by Western blot in Akita cardiomyocytes cultured either in the presence or absence of insulin overnight treatment. Quantification indicated no statistical significance (n = 5).
The insulin mediated effect on SOD activity may be due to changes in enzyme content. However, the expression of SOD2, as measured by Western blot, was unaffected by either insulin or LY294002 (Fig. 2C). This supports that insulin increases superoxide scavenging activity selectively in Akita cardiomyocytes but is independent of SOD content. SOD2 activity is also regulated by the posttranslational modification, acetylation [20,21], and we have previously reported Akita heart mitochondria exhibit hyperacetylation [13]. We therefore examined the acetylation status of SOD2 using a site-specific antibody against acetyl-lysine 68. As shown in Fig. 2D, the acetylation status of SOD2 at this known regulatory site was unaffected by insulin treatment in Akita cardiomyocytes.
3.3. Insulin-mediated change in endogenous antioxidants in vivo
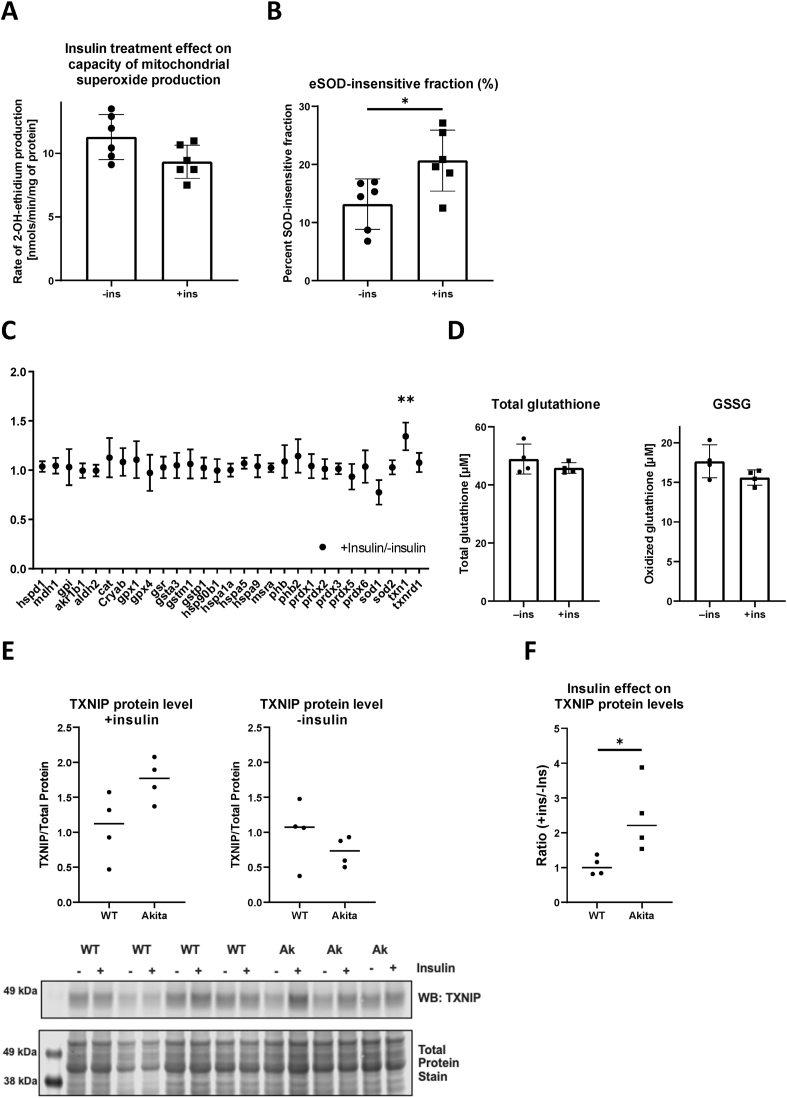
Mitochondria are a primary source of ROS. We therefore sought to determine whether insulin can decrease mitochondrial free radical production in Akita hearts. Akita mice were treated with insulin for 1 week, after which heart mitochondria were isolated and superoxide production was measured by fluorescence. Heart mitochondria isolated from Akita mice that underwent a 1-week treatment of insulin trended towards a decrease in O2•– production as compared to heart mitochondria isolated from vehicle treated Akita mice (Fig. 3A). We also measured O2•– production in the presence of SOD as a means to estimate endogenous antioxidant capacity. Insensitivity to SOD indicates that endogenous antioxidants are elevated and thus less responsive to the addition of an exogenous scavenger. Reciprocally, sensitivity to exogenously added SOD indicates that endogenous antioxidants are overwhelmed. As shown in Fig. 3B, the rate of O2•– production from insulin-treated Akita mice was significantly less SOD-sensitive, supporting the notion that there is an insulin-mediated increase in endogenous antioxidant capacity.
Fig. 3.
Insulin increases thioredoxin level in Akita cardiomyocytes. (A)(B) Mitochondrial NADH-supported superoxide production was measured fluorometrically using mitoplast isolated from Akita mice hearts underwent 1-week either vehicle (-ins) or 5U/kg insulin (+ins) treatment. Mitochondria isolated from insulin injected animals exhibited decreased capacity of superoxide production, and that was in part due to increased endogenous antioxidant (*p < .05; n = 6 biological replicates). Statistics were performed by Student's t-test. (C) Quantitative proteomic analysis was performed by selective reactive monitoring mass-spectrometry utilizing whole heart homogenates acquired from Akita hearts underwent 1-week of vehicle or insulin treatment. Among the 29 proteins reported in the antioxidant panel, thioredoxin 1 (txn1) was the only protein exhibiting statistically significant insulin sensitivity (**p < .02; n = 4 biological replicates). (D) Total and oxidized glutathione levels in those homogenates were unchanged. (E)(F) Change in thioredoxin interacting-protein (TXNIP) levels in WT and Akita primary cardiomyocytes by insulin overnight incubation were assessed by western blotting. Quantitative TXNIP protein levels in WT and Akita under basal (+insulin) and the lack of insulin were statistically insignificant, however, the Akita cardiomyocytes showed significantly higher sensitivity toward insulin (*p < .05; n = 4 biological replicates). Statistics were performed by Student's t-test.
Insulin may be affecting the relative levels of antioxidant defenses in vivo. To examine this possibility, quantitative proteomics analysis of antioxidant enzymes in Akita mice, with or without insulin treatment, was performed. As shown in Fig. 3C, SOD1, SOD2, and other major antioxidant enzymes were expressed at similar levels in mice regardless of insulin treatment. The only measured antioxidant enzyme that was significantly increased by insulin treatment was thioredoxin 1 (Trx1; 34% increase). Mitochondrial thioredoxin (Trx2) was not included in the proteomic analysis but its levels, as determined by Western blot, revealed Trx2 were unchanged (data not shown). We also investigated whether insulin had an effect on glutathione (GSH), the primary thiol-containing small-molecule antioxidant. As shown in Fig. 3D, there was no significant difference in both total (GSH and GSSG) and oxidized GSH levels between vehicle- and insulin-treated Akita mice, further supporting that the insulin effect on antioxidants is specific to thioredoxin.
Thioredoxin interacting protein (TXNIP) is a well-described inhibitor of thioredoxin that is important for redox homeostasis in the heart [22], and is decreased by insulin signaling in most cell types [23,24]. Thus, insulin may be decreasing TXNIP as an antioxidant response to enhance thioredoxin activity. We therefore examined TXNIP protein content in wild type and Akita cardiomyocytes cultured either in the presence or absence of insulin. In the presence of insulin, Akita cardiomyocytes had significantly more TXNIP than wild type (Fig. 3E). However, when cultured in the absence of insulin TXNIP levels in Akita and wild type cardiomyocytes were comparable. The insulin-induced changes in TXNIP levels were also plotted as the ratio of content between±insulin conditions for each cardiomyocyte preparation (Fig. 3F). This further revealed that TXNIP content is highly influenced by insulin in Akita, but not wild type, cardiomyocytes. These results demonstrate that insulin increases TXNIP levels specifically in Akita cardiomyocytes. It also suggests that inhibition by TXNIP is not the direct mechanism of reduced antioxidant capacity by thioredoxin.
3.4. Superoxide scavenging capacity of thioredoxin
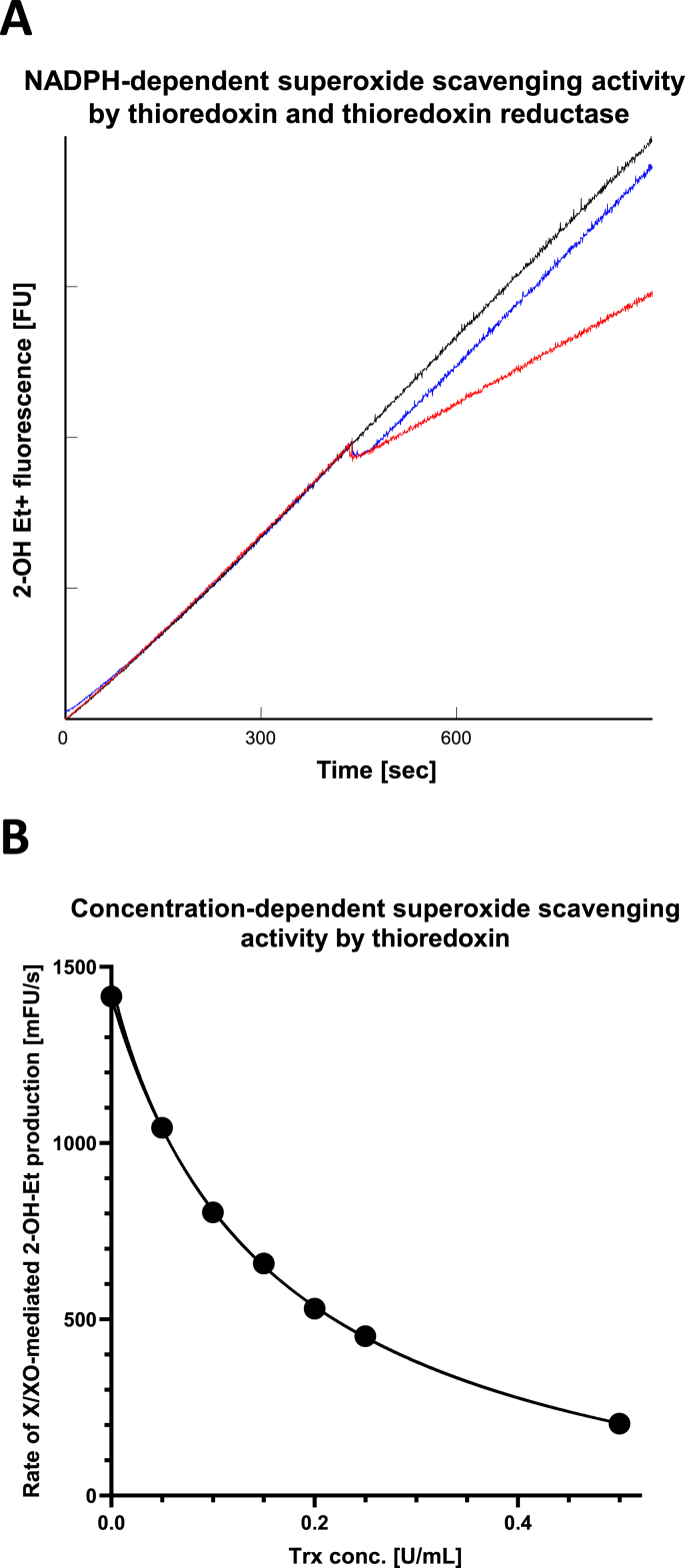
Small molecular thiols, such as GSH and n-acetyl cysteine (NAC), are well documented for their capacity to react with O2•– and other ROS [25]. Thioredoxin regulates redox homeostasis by acting as an electron donor to peroxiredoxins and other antioxidant enzymes and by modulating the activity of numerous transcriptional factor substrates in response to stress [26]. However, the reactivity of thioredoxin, a member of the H2O2 detoxification system, toward O2•– is largely unknown. We therefore evaluated enzymatic superoxide scavenging activity of thioredoxin in vitro using a xanthine/xanthine oxidase O2•– generation system. The reaction was carried out in the presence of dimethyl thiourea (DMTU) to scavenge any generated hydroxyl radical [27]. As shown in Fig. 4A, thioredoxin significantly decreased O2•– and this effect was dependent upon the presence of thioredoxin reductase and NADPH. Furthermore, the scavenging activity was found to be protein concentration dependent (Fig. 4B). The EC50 for the experimental condition from non-linear regression fitting was calculated as 0.139 U/mL for O2•– production by 25mU/mL xanthine oxidase. Under the same experimental conditions, the Km's for NAC and reduced GSH were 0.758 and 1.08 mM, respectively.
Fig. 4.
Thioredoxin scavenges enzymatically generated superoxide in vitro. The capacity of superoxide scavenging activity of thioredoxin (Trx) and thiol-containing small molecules were quantified using xanthine and xanthine oxidase-generated superoxide and pure proteins. (A) The superoxide scavenging activity required cycling reduced Trx. Representative kinetics traces are from control (no addition; black), with the addition of Trx and thioredoxin reductase (TR) at middle time point (blue), and with the addition of Trx and TR in the presence of NADPH (red). (B) Quantification of the superoxide scavenging activity was assessed with varied Trx concentration, while all other variables were fixed. The capacity of superoxide scavenging were found to be Trx concentration-dependent and can be fit to a classical Hill equation (see text for EC50 value). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Diabetes is associated with increased oxidative stress mediated by the overproduction of pro-oxidants in multiple organs. However, the specific mechanisms leading to the sustained increase of O2•– production are not completely understood. The present study demonstrates that insulin affects endogenous antioxidant activity in Akita cardiomyocytes. This complements previous work identifying that insulin provides neuroprotection via a Nrf2-dependent increase in antioxidant defenses [28]. In the heart, it has been presumed that insulin mitigates oxidative stress by decreasing hyperglycemia and minimizing glucose-mediated ROS production [29]. However, our results demonstrate that insulin treatment increases antioxidant capacity in cardiomyocytes isolated from diabetic animals and removed from a hyperglycemic environment. Additionally, we have shown that there is enhanced antioxidant capacity in isolated mitochondria of Akita mice that had been treated with insulin.
We utilized EPR spin-trapping technique on isolated adult primary cardiomyocytes in this study. EPR remains the gold standard for measuring O2•– production because of its sensitivity and specificity. EPR spin-trapping has been used in neonatal primary cardiomyocytes [30], but to the best of our knowledge it has not been applied to adult mouse cardiomyocytes and the Akita mouse model. Prior ROS studies have been performed with adult cardiomyocytes using fluorescent indicators [6,31], while EPR spin-trapping has been more widely applied to measuring O2•– in cardiac perfusates [32,33]. There have been limitations with the application of EPR spin-trapping to cardiomyocytes because of the sensitivity of commercially available spin-traps which require they be used at high concentrations, heightening the concern for toxic and off target effects. The hydroxylamine spin-trap used here, CMH, has the advantage of having higher sensitivity and cell permeability. Our approach also has the advantage of examining primary cardiomyocytes isolated from hearts exposed to long-term diabetes which were then manipulated by in vitro culture conditions. Primary cardiomyocytes, unlike immortalized cell lines, have abundant mitochondria that are adapted for oxidative phosphorylation. Nevertheless, limitations of this methodology are that the isolated cardiomyocytes are not contracting and are removed from their in vivo environment. In addition, the CMH spin-trap probe can react with other ROS and thus does not offer complete specificity.
We have found that steady-state cellular O2•– production is comparable in cardiomyocytes isolated from wild type and Akita mice. Similarly, an earlier study examining hydrogen peroxide production in mitochondria isolated from Akita hearts also found rates comparable to controls [34]. The metabolic perturbations of diabetes that lead to increased ROS are well described, and this paradox could be related to an increase in antioxidant programming that counters the persistent diabetic state [35]. More recently, engulfment and cell motility protein 1 (ELMO1), a guanine nucleotide exchange factor, was investigated for its role in ROS production in Akita mouse hearts [36]. Increasing ELMO1 subsequently increased Rac activity and ROS production via activation of NADPH oxidase.
NADPH oxidase is a major contributor of increased cellular O2•– production in response to diabetes and in vitro high-glucose/high-fat treatment. Both Nox2 and Nox4 have been identified as sources of insulin signaling-dependent cellular O2•– production sites [37]. In this study, though, NADPH oxidase likely has a minimal role in the cellular superoxide production observed in isolated primary cardiomyocytes. We assessed extracellular O2•– levels by utilizing a derivative of hydroxylamine spin-traps that is cell impermeable (CAT-1H). The O2•– levels were comparable in Akita and wild type cardiomyocytes and were insensitive to high-glucose and/or palmitate/oleate culture conditions. Akita cardiomyocytes showed higher O2•– production only when presented with the additional stress of insulin deprivation, suggesting that the observed increase in O2•– production is driven by a compromised antioxidant defense.
We provide additional evidence that insulin treatment of Akita mice affects the antioxidant capacity of mitochondria. Furthermore, using a targeted proteomics approach, we identified that thioredoxin 1 (Trx1) expression is uniquely insulin-signaling dependent. A previous study has shown that Trx1 expression is decreased in an STZ-induced T1D rat model and reported that adenoviral expression of Trx1 reduced fibrosis, oxidative stress, and apoptosis [38]. The capacity of thioredoxin, a well-known H2O2 detoxification system in conjunction with peroxiredoxin, to scavenge reactive oxygen and carbon species through thiol redox is not well understood. Here we report an NADPH-dependent O2•– scavenging activity of thioredoxin. Small molecular thiols, such as NAC and glutathione, react robustly with hydroxyl, alkyl, and alkoxyl radicals (107–1010 M−1 s−1) [[39], [40], [41]], and to lesser extent toward superoxide and peroxyl radicals (102–103 M−1 s−1). The Km for GSH under our experimental conditions was 1.08 mM, which is within the documented values and validates our approach for assessing antioxidant capacity. Thioredoxin is essential for maintaining a proper redox environment and regulating thiol-containing oxidative stress-sensing enzymes through thiol-disulfide exchange [26,42], and our results suggest a further direct detoxifying role. Nevertheless, it is plausible that the classically defined mechanism of thiol-based H2O2 scavenging activity might confer ancillary superoxide suppressing activity. Alternatively, increasing cytoplasmic Trx activity could have downstream effects on O2•– production. For example, a previous study in endothelial cells showed that H2O2 can regulate cellular O2•– production via a feed-forward cycle in which increased H2O2 scavenging activity also decreases O2•– [43].
TXNIP is a well described inhibitor of thioredoxin that increases in content in response to hyperglycemia. Indeed, TXNIP gene expression and protein content have been shown to increase in the human diabetic heart with a commensurate decrease in thioredoxin activity [44]. In the same study, it was also shown that TXNIP protein levels were increased in rodent type 1 and type 2 diabetic models concurrently with decreased thioredoxin activity and increased ROS. This increase in TXNIP was attributed to hyperglycemia. In our primary cardiomyocyte culture experiments, we only observed increased O2•– production when cells were insulin starved. Unexpectedly, TXNIP protein expression increased when insulin was present, potentially because of an influx of glucose. In contrast, TXNIP expression in control cardiomyocytes was unaffected by insulin. Together, our results reveal a unique regulatory mechanism of TXNIP expression in Akita cardiomyocytes and its role in regulating thioredoxin activity.
Hyperglycemia is well established as a propagator of oxidative stress in the diabetic heart. Our findings suggest that insulin may mitigate oxidative stress through a mechanism that involves signaling directly at the level of cardiomyocytes in addition to its role in normalizing glucose levels. Future studies will aim at determining if the effects of insulin elucidated here are held in other type 1 and type 2 models of diabetes.
Funding
This work was supported by National Institutes of Health Grants R01HL125625 (to K. M. H.), P30AG050911 (to M. K.), P20GM103447 (to M. K.), R24GM137786 (to M.K.); by Grant HR17-094 (to K. M. H.) from the Oklahoma Center for the Advancement of Science; and by National Science Foundation Graduate Research Fellowship Program Grant 1849507 (to M. F. N.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Declaration of competing interest
The authors declare that they have no conflicts of interest with the contents of this article.
References
- 1.Ritchie R.H., Abel E.D. Basic mechanisms of diabetic heart disease. Circ. Res. 2020;126:1501–1525. doi: 10.1161/CIRCRESAHA.120.315913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayama Y., Raaz U., Jagger A., Adam M., Schellinger I.N., Sakamoto M., Suzuki H., Toyama K., Spin J.M., Tsao P.S. Diabetic cardiovascular disease induced by oxidative stress. Int. J. Mol. Sci. 2015;16:25234–25263. doi: 10.3390/ijms161025234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huynh K., Kiriazis H., Du X.J., Love J.E., Gray S.P., Jandeleit-Dahm K.A., McMullen J.R., Ritchie R.H. Targeting the upregulation of reactive oxygen species subsequent to hyperglycemia prevents type 1 diabetic cardiomyopathy in mice. Free Radic. Biol. Med. 2013;60:307–317. doi: 10.1016/j.freeradbiomed.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 4.Boveris A., Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem. J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vadvalkar S.S., Baily C.N., Matsuzaki S., West M., Tesiram Y.A., Humphries K.M. Metabolic inflexibility and protein lysine acetylation in heart mitochondria of a chronic model of type 1 diabetes. Biochem. J. 2013;449:253–261. doi: 10.1042/BJ20121038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye G., Metreveli N.S., Ren J., Epstein P.N. Metallothionein prevents diabetes-induced deficits in cardiomyocytes by inhibiting reactive oxygen species production. Diabetes. 2003;52:777–783. doi: 10.2337/diabetes.52.3.777. [DOI] [PubMed] [Google Scholar]
- 7.Liang Q., Carlson E.C., Donthi R.V., Kralik P.M., Shen X., Epstein P.N. Overexpression of metallothionein reduces diabetic cardiomyopathy. Diabetes. 2002;51:174–181. doi: 10.2337/diabetes.51.1.174. [DOI] [PubMed] [Google Scholar]
- 8.Basu R., Oudit G.Y., Wang X., Zhang L., Ussher J.R., Lopaschuk G.D., Kassiri Z. Type 1 diabetic cardiomyopathy in the Akita (Ins2WT/C96Y) mouse model is characterized by lipotoxicity and diastolic dysfunction with preserved systolic function. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H2096–H2108. doi: 10.1152/ajpheart.00452.2009. [DOI] [PubMed] [Google Scholar]
- 9.O'Connell T.D., Rodrigo M.C., Simpson P.C. Isolation and culture of adult mouse cardiac myocytes. Methods Mol. Biol. 2007;357:271–296. doi: 10.1385/1-59745-214-9:271. [DOI] [PubMed] [Google Scholar]
- 10.Bockus L.B., Humphries K.M. cAMP-dependent protein kinase (PKA) signaling is impaired in the diabetic heart. J. Biol. Chem. 2015;290:29250–29258. doi: 10.1074/jbc.M115.681767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoshioka M., Kayo T., Ikeda T., Koizumi A. A novel locus, Mody4, distal to D7Mit189 on chromosome 7 determines early-onset NIDDM in nonobese C57BL/6 (Akita) mutant mice. Diabetes. 1997;46:887–894. doi: 10.2337/diab.46.5.887. [DOI] [PubMed] [Google Scholar]
- 12.Matsuzaki S., Humphries K.M. Selective inhibition of deactivated mitochondrial complex I by biguanides. Biochemistry. 2015;54:2011–2021. doi: 10.1021/bi501473h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vadvalkar S.S., Matsuzaki S., Eyster C.A., Giorgione J.R., Bockus L.B., Kinter C.S., Kinter M., Humphries K.M. Decreased mitochondrial pyruvate transport activity in the diabetic heart: role OF mitochondrial pyruvate carrier 2 (MPC2) acetylation. J. Biol. Chem. 2017;292:4423–4433. doi: 10.1074/jbc.M116.753509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes J., Weddle A., Kinter C.S., Humphries K.M., Mather T., Szweda L.I., Kinter M. Lysine acetylation activates mitochondrial aconitase in the heart. Biochemistry. 2015;54:4008–4018. doi: 10.1021/acs.biochem.5b00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newhardt M.F., Batushansky A., Matsuzaki S., Young Z.T., West M., Chin N.C., Szweda L.I., Kinter M., Humphries K.M. Enhancing cardiac glycolysis causes an increase in PDK4 content in response to short-term high-fat diet. J. Biol. Chem. 2019;294:16831–16845. doi: 10.1074/jbc.RA119.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.MacLean B., Tomazela D.M., Shulman N., Chambers M., Finney G.L., Frewen B., Kern R., Tabb D.L., Liebler D.C., MacCoss M.J. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26:966–968. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dikalov S.I., Kirilyuk I.A., Voinov M., Grigor'ev I.A. EPR detection of cellular and mitochondrial superoxide using cyclic hydroxylamines. Free Radic. Res. 2011;45:417–430. doi: 10.3109/10715762.2010.540242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Quinlan C.L., Gerencser A.A., Treberg J.R., Brand M.D. The mechanism of superoxide production by the antimycin-inhibited mitochondrial Q-cycle. J. Biol. Chem. 2011;286:31361–31372. doi: 10.1074/jbc.M111.267898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riehle C., Abel E.D. Insulin signaling and heart failure. Circ. Res. 2016;118:1151–1169. doi: 10.1161/CIRCRESAHA.116.306206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metabol. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S., Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakayama Y., Mukai N., Wang B.F., Yang K., Patwari P., Kitsis R.N., Yoshioka J. Txnip C247S mutation protects the heart against acute myocardial infarction. J. Mol. Cell. Cardiol. 2021;155:36–49. doi: 10.1016/j.yjmcc.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minn A.H., Hafele C., Shalev A. Thioredoxin-interacting protein is stimulated by glucose through a carbohydrate response element and induces β-cell apoptosis. Endocrinology. 2005;146:2397–2405. doi: 10.1210/en.2004-1378. [DOI] [PubMed] [Google Scholar]
- 24.Nishiyama A., Matsui M., Iwata S., Hirota K., Masutani H., Nakamura H., Takagi Y., Sono H., Gon Y., Yodoi J. Identification of thioredoxin-binding protein-2/vitamin D3 up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999;274:21645–21650. doi: 10.1074/jbc.274.31.21645. [DOI] [PubMed] [Google Scholar]
- 25.Winterbourn C.C. Revisiting the reactions of superoxide with glutathione and other thiols. Arch. Biochem. Biophys. 2016;595:68–71. doi: 10.1016/j.abb.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 26.Andreadou I., Efentakis P., Frenis K., Daiber A., Schulz R. Thiol-based redox-active proteins as cardioprotective therapeutic agents in cardiovascular diseases. Basic Res. Cardiol. 2021;116:44. doi: 10.1007/s00395-021-00885-5. [DOI] [PubMed] [Google Scholar]
- 27.Matsuzaki S., Kotake Y., Humphries K.M. Identification of mitochondrial electron transport chain-mediated NADH radical formation by EPR spin-trapping techniques. Biochemistry. 2011;50:10792–10803. doi: 10.1021/bi201714w. [DOI] [PubMed] [Google Scholar]
- 28.Song Y., Ding W., Bei Y., Xiao Y., Tong H.-D., Wang L.-B., Ai L.-Y. Insulin is a potential antioxidant for diabetes-associated cognitive decline via regulating Nrf2 dependent antioxidant enzymes. Biomed. Pharmacother. 2018;104:474–484. doi: 10.1016/j.biopha.2018.04.097. [DOI] [PubMed] [Google Scholar]
- 29.Ng K.W., Allen M.L., Desai A., Macrae D., Pathan N. Cardioprotective effects of insulin: how intensive insulin therapy may benefit cardiac surgery patients. Circulation. 2012;125:721–728. doi: 10.1161/CIRCULATIONAHA.111.063784. [DOI] [PubMed] [Google Scholar]
- 30.Durot I., Maupoil V., Ponsard B., Cordelet C., Vergely-Vandriesse C., Rochette L., Athias P. Oxidative injury of isolated cardiomyocytes: dependence on free radical species. Free Radic. Biol. Med. 2000;29:846–857. doi: 10.1016/s0891-5849(00)00382-8. [DOI] [PubMed] [Google Scholar]
- 31.Obal D., Dai S., Keith R., Dimova N., Kingery J., Zheng Y.T., Zweier J., Velayutham M., Prabhu S.D., Li Q., Conklin D., Yang D., Bhatnagar A., Bolli R., Rokosh G. Cardiomyocyte-restricted overexpression of extracellular superoxide dismutase increases nitric oxide bioavailability and reduces infarct size after ischemia/reperfusion. Basic Res. Cardiol. 2012;107:305. doi: 10.1007/s00395-012-0305-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traverse J.H., Nesmelov Y.E., Crampton M., Lindstrom P., Thomas D.D., Bache R.J. Measurement of myocardial free radical production during exercise using EPR spectroscopy. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H2453–H2458. doi: 10.1152/ajpheart.00412.2005. [DOI] [PubMed] [Google Scholar]
- 33.Zweier J.L., Kuppusamy P. Electron paramagnetic resonance measurements of free radicals in the intact beating heart: a technique for detection and characterization of free radicals in whole biological tissues. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5703–5707. doi: 10.1073/pnas.85.15.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bugger H., Boudina S., Hu X.X., Tuinei J., Zaha V.G., Theobald H.A., Yun U.J., McQueen A.P., Wayment B., Litwin S.E., Abel E.D. Type 1 diabetic akita mouse hearts are insulin sensitive but manifest structurally abnormal mitochondria that remain coupled despite increased uncoupling protein 3. Diabetes. 2008;57:2924–2932. doi: 10.2337/db08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dewey S., Lai X., Witzmann F.A., Sohal M., Gomes A.V. Proteomic analysis of hearts from Akita mice suggests that increases in soluble epoxide hydrolase and antioxidative programming are key changes in early stages of diabetic cardiomyopathy. J. Proteome Res. 2013;12:3920–3933. doi: 10.1021/pr4004739. [DOI] [PubMed] [Google Scholar]
- 36.Kakoki M., Bahnson E.M., Hagaman J.R., Siletzky R.M., Grant R., Kayashima Y., Li F., Lee E.Y., Sun M.T., Taylor J.M., Rice J.C., Almeida M.F., Bahr B.A., Jennette J.C., Smithies O., Maeda-Smithies N. Engulfment and cell motility protein 1 potentiates diabetic cardiomyopathy via Rac-dependent and Rac-independent ROS production. JCI Insight. 2019;4 doi: 10.1172/jci.insight.127660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinhorn B., Sartoretto J.L., Sorrentino A., Romero N., Kalwa H., Abel E.D., Michel T. Insulin-dependent metabolic and inotropic responses in the heart are modulated by hydrogen peroxide from NADPH-oxidase isoforms NOX2 and NOX4. Free Radic. Biol. Med. 2017;113:16–25. doi: 10.1016/j.freeradbiomed.2017.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Samuel S.M., Thirunavukkarasu M., Penumathsa S.V., Koneru S., Zhan L., Maulik G., Sudhakaran P.R., Maulik N. Thioredoxin-1 gene therapy enhances angiogenic signaling and reduces ventricular remodeling in infarcted myocardium of diabetic rats. Circulation. 2010;121:1244–1255. doi: 10.1161/CIRCULATIONAHA.109.872481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benrahmoune M., Thérond P., Abedinzadeh Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic. Biol. Med. 2000;29:775–782. doi: 10.1016/s0891-5849(00)00380-4. [DOI] [PubMed] [Google Scholar]
- 40.Jones C.M., Lawrence A., Wardman P., Burkitt M.J. Electron paramagnetic resonance spin trapping investigation into the kinetics of glutathione oxidation by the superoxide radical: re-evaluation of the rate constant. Free Radic. Biol. Med. 2002;32:982–990. doi: 10.1016/s0891-5849(02)00791-8. [DOI] [PubMed] [Google Scholar]
- 41.Winterbourn C.C. In: Oxidative Stress and Redox Regulation. Jakob U., Reichmann D., editors. Springer Netherlands; Dordrecht: 2013. Radical scavenging by thiols and the fate of thiyl radicals; pp. 43–58. [Google Scholar]
- 42.Shao D., Oka S.-i., Liu T., Zhai P., Ago T., Sciarretta S., Li H., Sadoshima J. A redox-dependent mechanism for regulation of AMPK activation by Thioredoxin1 during energy starvation. Cell Metabol. 2014;19:232–245. doi: 10.1016/j.cmet.2013.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dikalov S.I., Nazarewicz R.R., Bikineyeva A., Hilenski L., Lassègue B., Griendling K.K., Harrison D.G., Dikalova A.E. Nox2-Induced production of mitochondrial superoxide in angiotensin II-mediated endothelial oxidative stress and hypertension. Antioxidants Redox Signal. 2013;20:281–294. doi: 10.1089/ars.2012.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Connelly K.A., Advani A., Advani S.L., Zhang Y., Kim Y.M., Shen V., Thai K., Kelly D.J., Gilbert R.E. Impaired cardiac anti-oxidant activity in diabetes: human and correlative experimental studies. Acta Diabetol. 2014;51:771–782. doi: 10.1007/s00592-014-0608-9. [DOI] [PubMed] [Google Scholar]