Abstract
Pseudomonas aeruginosa is a significant mortality factor due to nosocomial infections in humans. P. aeruginosa has been known with severe infections, high incidence, and multiple drug resistance. The present study aims to rapidly diagnose and biotype the isolates of P. aeruginosa isolated from human infections in Shiraz hospitals and health centers. Ninety six different isolates were collected from skin, urine, sputum, blood, wound, central vein blood, body fluids and burn wounds between January 2016 and February 2017. After phenotypic confirmation, isolates were examined by PCR for molecular confirmation. Ninety three isolates were verified as P. aeruginosa in molecular analysis. Enterobacterial Repetitive Intergenic Consensus (ERIC) PCR and Random Amplified Polymorphic DNA (RAPD) were done for 67 isolates. In ERIC-PCR, the patterns obtained included 2–11 bands. The RAPD patterns obtained with primers 272 and 208 consisted of 3–11 and 1–12 bands respectively. Based on dice similarity coefficient of greater than 80%, 38, 45 and 38 groups were identified in ERIC, RAPD 272 and RAPD 208 respectively. The results showed that the isolates of P. aeruginosa have a high polymorphism apparently because of the high genetic variation.
Keywords: ERIC-PCR, RAPD, Human infections, Pseudomonas aeruginosa, Iran
ERIC-PCR, RAPD, Human infections, Pseudomonas aeruginosa, Iran.
1. Introduction
Pseudomonas aeruginosa is a ubiquitous, gram-negative, motile and non-fermenting bacterium. This opportunistic bacterium belongs to the family Pseudomonadaceae. P. aeruginosa is an opportunistic pathogen causing several human infections via biofilm formation. It is known as a common hospital-acquired pathogen and responsible for bacteremia, dermatitis, respiratory, bone and joint, soft tissue, gastrointestinal infections, urinary tract infections (UTIs), and a variety of generalized infections [1, 2]. The susceptibility of P. aeruginosa to antimicrobial agents has been limited. The agent is known as an emergence of antimicrobial resistance, and is usually difficult to treat [3]. According to the reports, mortality rate associated with P. aeruginosa can range from 18% to 61% in hospital-acquired infections in Iran [1].
Typing strains of this bacterium is of particular importance specially for antibiotic selection, detection of non-usual phenotypes, identification of some specific characters of the isolates, and also identification of a potential cluster in patients with single clone [4, 5]. Today, different typing methods (e.g. serotyping, biotyping, and pyocin typing) have been suggested to identify common P. aeruginosa clones [6]. Nevertheless, the equitable power and constancy are lower in the molecular typing systems. We can use molecular typing techniques to identify transmission routes and control cross-infections in human societies. Various gene-typing methods including enterobacterial repetitive intergenic consensus polymerase chain reaction (ERIC-PCR), random amplification of polymorphic DNA (RAPD), pulsed field gel electrophoresis (PFGE) and multilocus sequence typing (MLST) have been utilized to study the epidemiology of P. aeruginosa [4, 5, 7, 8]. The use of PFGE and MLST methods despite the high reproducibility and good discriminatory power are limited because of technical complexity, cost, and long turnaround times for results [6].
Repetitive extragenic palindromic PCR (rep-PCR) is one of the most widely used, that uses primers targeting highly conserved repetitive sequence elements known as ERIC sequences common to Gram-negative enteric bacteria [9]. In several studies ERIC-PCR was a better choice among other PCR-based methods for the subtyping of P. aeruginosa isolates [4, 10]. ERIC-PCR makes it possible to clearly distinguish between different bacterial species and strains which contain these repetitive elements [11].
Primer is single and short in RAPD method, and specific sequence is not followed. The primer finds its complementary on the target DNA [12]. RAPD and ERIC-PCR have high levels of reproducibility, low complexity, low expense and exhibit swift result turnaround times.
The purpose of this study was rapid diagnosis and biotyping of isolates of P. aeruginosa. In this study, ERIC-PCR and RAPD were compared using isolates obtained from human infections in hospitals and health centers in Shiraz (Iran). The results of this study will probably guide researchers to select the suitable typing method for epidemiological purposes.
2. Materials and methods
2.1. Sample collection
In a prospective study, 96 bacterial isolates of P. aeruginosa were collected between January 2016 and February 2017. Isolates were collected from patients attending medical care services in hospitals and health centers in Shiraz (Iran). Ten isolates were from skin, 25 from urine, 21 from sputum, 11 from blood, 13 from wounds, 2 from central vein blood, 8 from body fluids and 6 from burn wounds. P. aeruginosa isolates were recognized based on the morphological, microscopic, and biochemical tests. All strains were stored at –70 °C in 10% glycerol (v/v) until use. This study was approved by the Ethical and Research Committee (95GCU5M1304) of the School of Veterinary Medicine, Shiraz University, Iran. Informed consent was obtained from all patients.
2.2. DNA extraction and PCR confirmation
All chemicals were purchased from Cinnagen (Iran). Simple boiling method was selected for DNA extraction from colonies of all isolates as described elsewhere [13]. In summary, a few colonies were dissolved in sterile distilled water and placed in dry bath at 95 °C for 15 minutes. Then the isolates were placed at -20 °C for 10 minutes and then centrifuged at 13000 rpm for 10 min. The supernatant was used as template. The extracted DNA was kept at -20 °C until use. The bacteria were verified via the PCR method for 16S rRNA gene of the P. aeruginosa [13]. The targeted DNA was amplified in 25 μl reaction volumes, each containing 1 μL of each primer (F: 5′-GGGGGATCTTCGGACCTCA-3′ and R: 5′-TCCTTAGAGTGCCCACCCG-3′), 2.5 μL 10x PCR buffer, 1 μL of MgCl2, 1 μL of deoxyribonucleoside triphosphates, 0.2 μL Taq DNA polymerase, 3 μL template DNA and adjusted to 25 μl by the addition of high-performance liquid chromatography-grade H2O.The following protocol was used for DNA amplification: initial denaturation (94 °C for 2 min), followed by 25 cycles of denaturation (94 °C for 20 s), annealing (58 °C for 20 s) and extension (72 °C for 40 s), with a single final extension of 1 min at 72 °C [13]. P. aeruginosa ATCC 27853 was our positive control.
2.3. ERIC-PCR
Two primers, ERIC1 (5′-ATGTAAGCTCCTGGGGATTCAC-3′) and ERIC2 (5′-AAGTAAGTACTGGGGTGAGCG-3′) were applied in PCR experiments [14]. Final volume of PCR mixture was 25 μl. The components of the mixture were as follows: 2.5 μl of 10x PCR buffer, 1 μl MgCl2, 1 μl dNTP mix, 1 μl of each primer, 0.2 μl of Taq DNA polymerase and 50 ng of template DNA. Cycling conditions for ERIC-PCR are as follows: 4 min at 94 °C, and then 35 cycles of PCR consisting denaturation at 94 °C for 1min, annealing at 52 °C for 1 min, and extension at 72 °C for 4 min and a final extension at 72 °C for 16 min [14]. P. aeruginosa ATCC 27853 was our positive control.
2.4. RAPD
After the extraction, genomic DNA was amplified using RAPD in accordance with the modified method of Halfiane and Ravaoarinoro [5] and Tosin, et al [15]. via arbitrary sequences 272 (5′-AGCGGGCCAA-3′) and 208 (5′-ACGGCCGACC-3′). PCR was performed in a volume of 25 μl under optimal conditions and contained 2.5 μl of 10x PCR buffer, 1.5 μl MgCl2, 0.75 μl dNTP mix, 2 μl of primer, 0.2 μl of Taq DNA polymerase and 50 ng of template DNA. Cycling conditions for RAPD were as follows: 2 min at 94 °C, and then 35 cycles of PCR consisting of denaturation at 94 °C for 30 s, annealing at 35 °C for 30 s, extension at 72 °C for 2 min and a final extension at 72 °C for 10 min [5, 15]. P. aeruginosa ATCC 27853 was our positive control.
2.5. Analyses and dendrogram construction
The amplified products of ERIC-PCR and RAPD were exposed to electrophoresis on 1.5% agarose gel with Sybr Green and analyzed under UV light in a gel documentation system (Proteinsimp, USA). DNA banding patterns of images were entered into a database in BioNumerics 7.1 software (Applied Maths, Sint-Martens-Latem, Belgium). The interpretation and comparison of the patterns were carried out according to previous research [16]. The similarity index was calculated via the Dice coefficient and the unweighted pair group average (UPGMA) for cluster analyses. Related clones were classified as the same type when the banding pattern similarity was 80% or higher.
3. Results
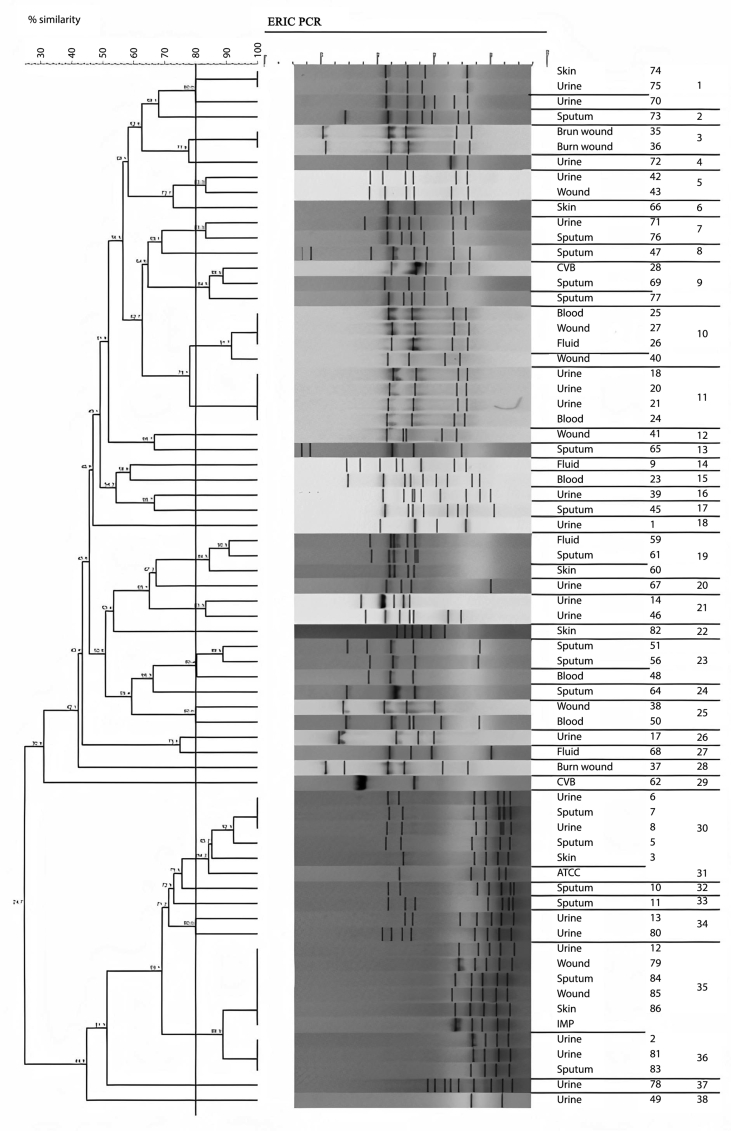
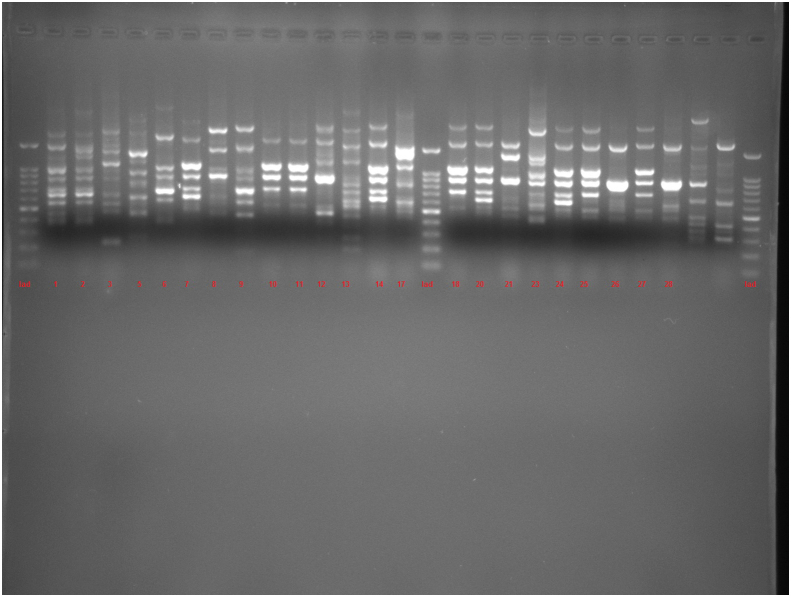
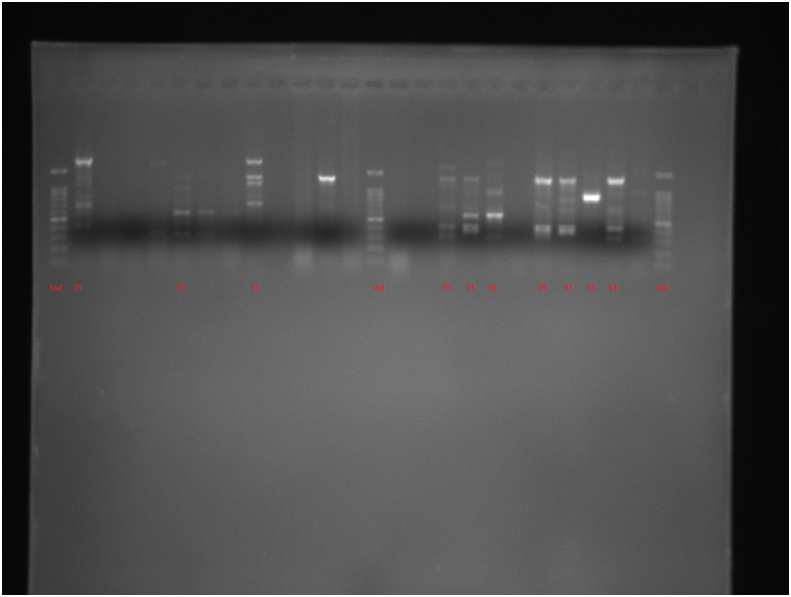
This study used PCR-based techniques to examine 96 isolates previously characterized phenotypically as P. aeruginosa. According to PCR confirmation, 93 (96.87%) strains were positive for P. aeruginosa. Of these 67 isolates with P. aeruginosa ATCC 27853 (as positive control) were examined by ERIC and RAPD. According to the ERIC amplification of P. aeruginosa isolates, all isolates showed amplification bands ranging in number from 2 to 11, with the length of 150 bp to ≥1500 bp. Genetic analysis of P. aeruginosa strains based on ≥ 80% showed 38 divergent ERIC fingerprints, 22 of which contained only one strain. Twenty three of the isolates had 100% similarity with at least one isolate (Figure 1; See Figure S1 for uncropped image).
Figure 1.
Dendrogram of Pseudomonas aeruginosa isolates based on ERIC-PCR (see Figure S1 for uncropped image). The genetic distance between the isolates has been shown by the scale at the top. The scale indicates the percentage of genetic similarity. Columns (left to right) indicate list of isolates, isolates number and ERIC-PCR types.
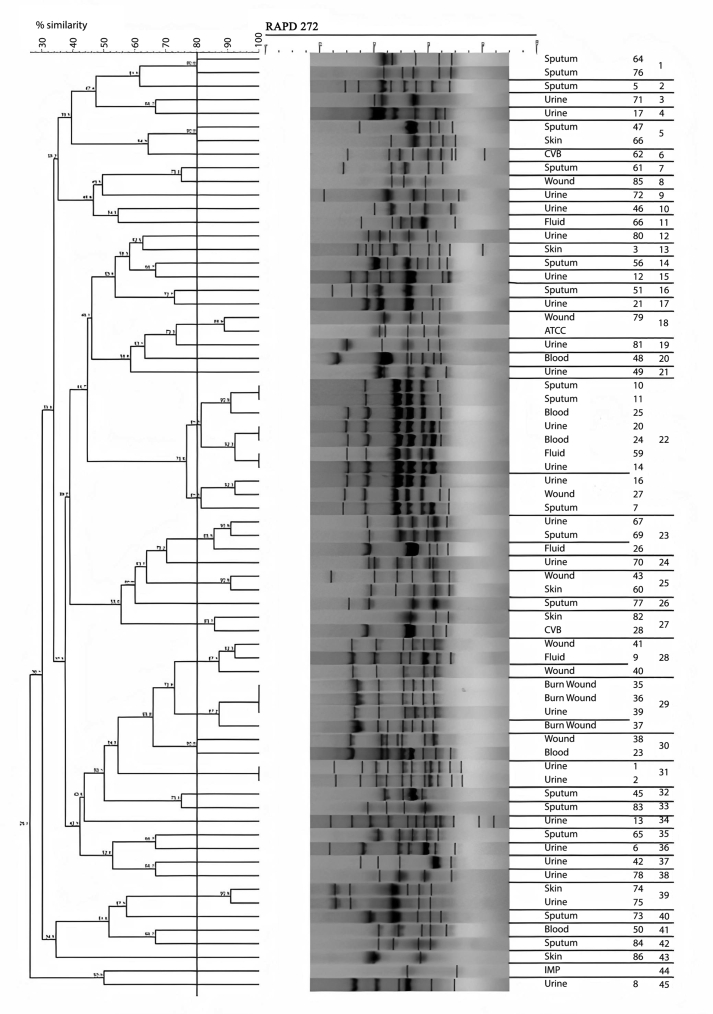
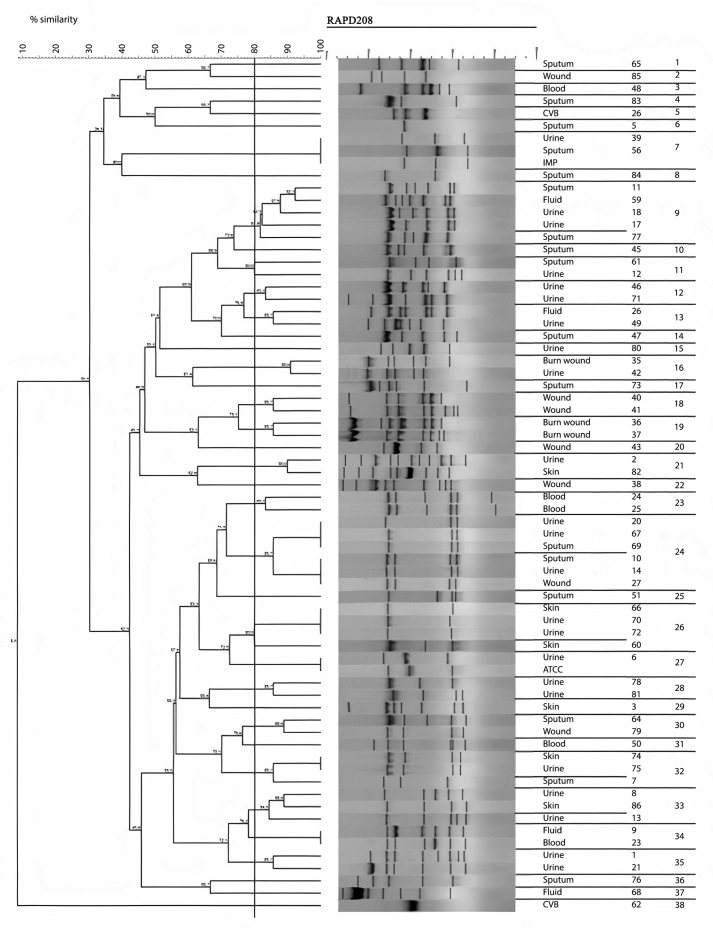
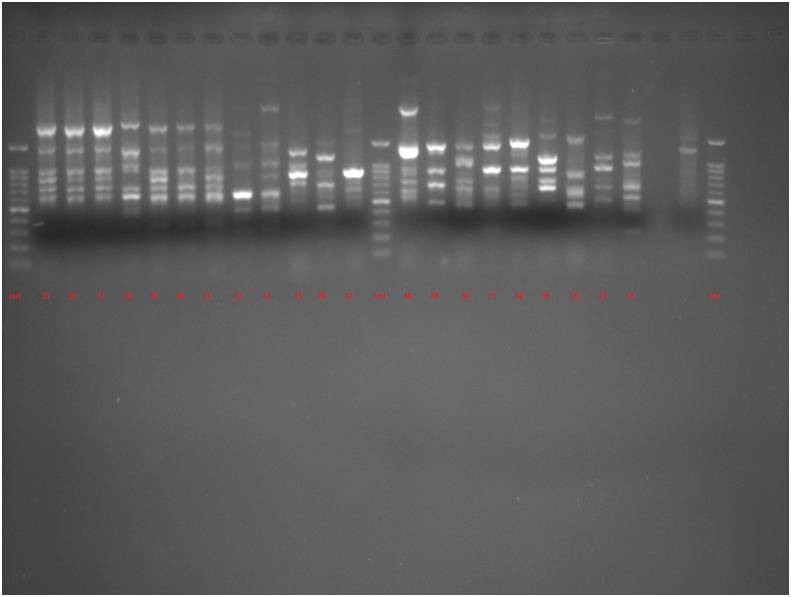
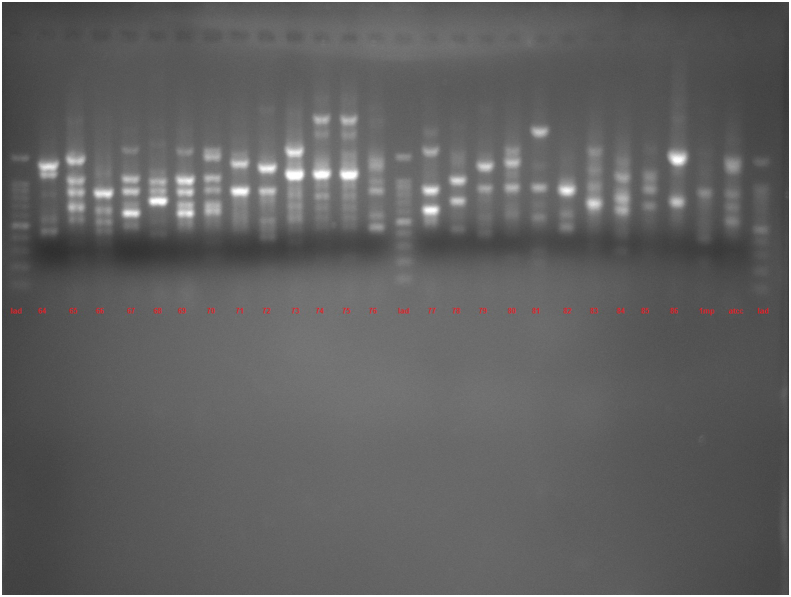
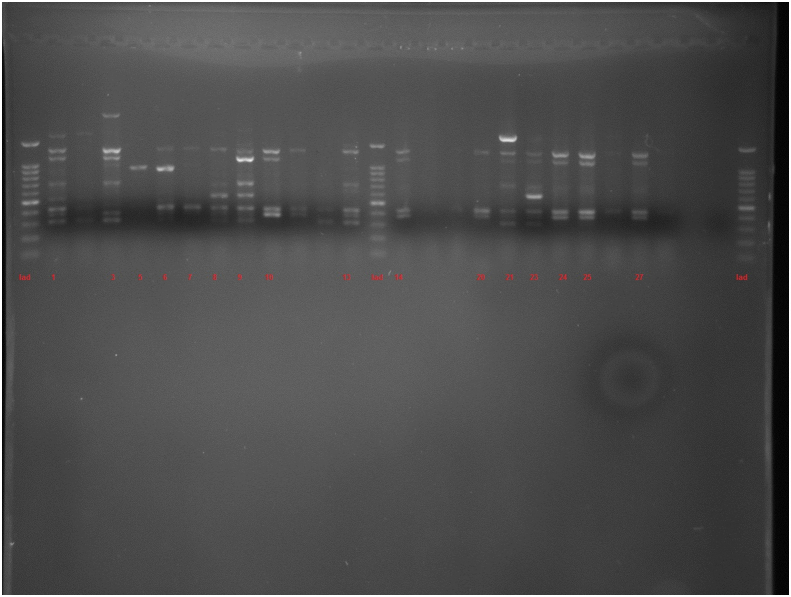
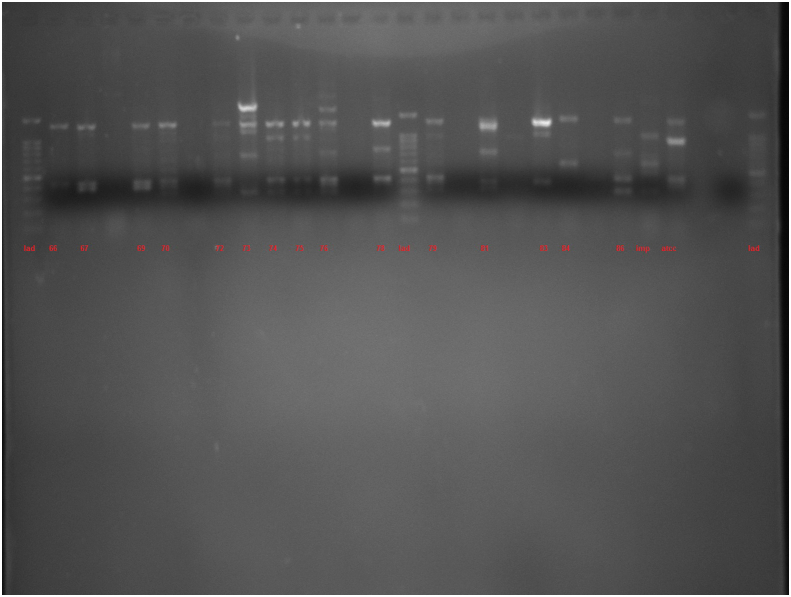
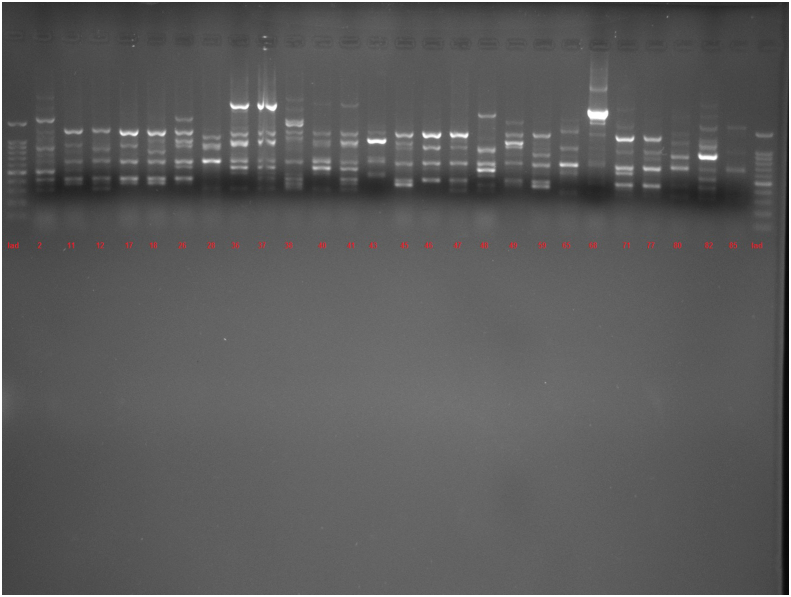
The amplification bands of the results of all RAPD experiments were in the range of 3–11 in number. with the length of 200 bp to ≥1500 bp. With primer 208, amplification of P. aeruginosa isolates revealed bands ranging from 1 to 12, with lengths of 100 bp to ≥1500 bp. Different genotypes were demonstrated with the use of primers 272 and 208 in RAPD fingerprints. RAPD 272 and 208 dendrograms of clinical P. aeruginosa isolates showed 45 and 38 different clusters respectively. Although the majority of isolates had a unique fingerprint, 11 and 18 isolates had 100% similarity with at least one isolate, based on RAPD 272 and 208 respectively (Figures 2 and 3; See Figures S2A-S2C, and S3A-S3D for uncropped images).
Figure 2.
Dendrogram of Pseudomonas aeruginosa based on RAPD 272 (See Figures S2A-S2C for uncropped images). The genetic distance between the isolates has been shown by the scale at the top. The scale indicates the percentage of genetic similarity. Columns (left to right) indicate list of isolates, isolates number and RAPD 272 types.
Figure 3.
Dendrogram of Pseudomonas aeruginosa based on RAPD 208 (See Figures S3A-S3D for uncropped images). The genetic distance between the isolates has been shown by the scale at the top. The scale indicates the percentage of genetic similarity. Columns (left to right) indicate list of isolates, isolates number and RAPD 208 types.
4. Discussion
Biotyping P. aeruginosa strains based on mucoid strains, hemolysis production or associated with loss of pigmentation made it possible to classify several biotypes. Biotyping did not demonstrate any significant correlation between the biotypes. It seems that the method has limited ability and poor discriminatory power for differentiation between the isolates. Genotyping P. aeruginosa strains made it possible to show the restrictions of each phenotyping method. The RAPD method used 10-base primers, of high guanine-cytosine content (60–70%) [17]. Hafiane et al. showed that RAPD with primers 272 and 208 were able to type P. aeruginosa strains successfully [5].
In this study, all 67 isolates were typed by RAPD and ERIC PCR. Although both ERIC-PCR and RAPD analyses have been explained as equally effective in characterizing clinical isolates of P. aeruginosa, our study found that RAPD was more discriminatory than ERIC-PCR. RAPD with primer 272 was able to distinguish isolates that were not differentiated by ERIC. So that, RAPD is probably a better PCR-based method for the subtyping of P. aeruginosa isolates.
Based on dice similarity coefficient of greater than 80%, 38, 45 and 38 groups were identified in ERIC, RAPD 272 and RAPD 208 respectively. Different distribution of genotypes has been shown in several studies in Iran. For example, Salimi et al. had 29 isolates with 8 different groups via RAPD typing [18]. Nanvazadeh et al. observed 9 groups among 50 clinical isolates of P. aeruginosa [19]. Also Vaez et al. observed 39 different groups from 54 clinical isolates [20].
The isolates in our study showed considerable genetic diversity. This may be due to the different sources of P. aeruginosa that can lead to host colonization. In this study 23, 11 and 18 of the isolates had 100% similarity (based on ERIC, RAPD 272 and 208 respectively), forming 7, 5 and 7 real clones. This similarity shows that hospital environments have common sources of infections, although the virulence and isolation source may be different. P. aeruginosa can spread clonal strains from one patient to another in hospitals. In conclusion, our results could help us to get distinct patterns of bands from ERIC and RAPD methods. Both methods characterized the isolates effectively. However, it should be noticed that the RAPD method especially with primer 272 could differentiate the isolates were detected as non-differentiable by ERIC method.
However, next generation of sequencing allows the high through put typing of the complete genome of the bacterium. This would of course provide the ultimate resolution of typing. Nevertheless, this might not be available/feasible under many conditions.
Declarations
Author contribution statement
Aida Hematzadeh: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Masoud Haghkhah: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Shiraz University Grant Number 95GCU5M1304.
Data availability statement
Data included in article/supp. material/referenced in article.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Thanks to Miss Maryam Sadat Moezzi for helping in data analysis and Dr. Maryam Motevasel for sampling and laboratory techniques.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Figure S1.
Figure S2A.
Figure S2B.
Figure S2C.
Figure S3A.
Figure S3B.
Figure S3C.
Figure S3D.
References
- 1.Fazeli N., Momtaz H. Virulence gene profiles of multidrug-resistant Pseudomonas aeruginosa isolated from Iranian hospital infections. Iran Red. Crescent Med. 2014;J16 doi: 10.5812/ircmj.15722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon S. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2003, issued August 2003. Am. J. Infect. Contr. 2003;31:481–498. doi: 10.1016/j.ajic.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Carmeli Y., Troillet N., Eliopoulos G.M., Samore M.H. Emergence of antibiotic-resistant Pseudomonas aeruginosa: comparison of risks associated with different antipseudomonal agents. Antimicrob. Agents Chemother. 1999;43(6):1379–1382. doi: 10.1128/aac.43.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ojeniyi B., Høiby N. Pseudomonas aeruginosa in Human Diseases. Karger Publishers; 1991. Comparison of different typing methods of Pseudomonas aeruginosa; pp. 13–22. [DOI] [PubMed] [Google Scholar]
- 5.Hafiane A., Ravaoarinoro M. Characterization of Pseudomonas aeruginosa strains isolated from cystic fibrosis patients by different typing methods. Pathol. Biol. 2011;59:e109–e114. doi: 10.1016/j.patbio.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 6.Stehling E.G., Leite D.S., Silveira W.D. Molecular typing and biological characteristics of Pseudomonas aeruginosa isolated from cystic fibrosis patients in Brazil. Braz. J. Infect. Dis. 2010;14:462–467. [PubMed] [Google Scholar]
- 7.Khosravi A.D., Hoveizavi H., Mohammadian A., Farahani A., Jenabi A. Genotyping of multidrug-resistant strains of Pseudomonas aeruginosa isolated from burn and wound infections by ERIC-PCR. Acta Cir. Bras. 2016;31:206–211. doi: 10.1590/S0102-865020160030000009. [DOI] [PubMed] [Google Scholar]
- 8.Auda I.G., Al-Kadmy I.M.S., Kareem S.M., Lafta A.K., A'Affus M.H.O., Khit I.A.A., Al Kheraif A.A., Divakar D.D., Ramakrishnaiah R. RAPD-and ERIC-based typing of clinical and environmental Pseudomonas aeruginosa isolates. J. AOAC Int. 2017;100:532–536. doi: 10.5740/jaoacint.16-0267. [DOI] [PubMed] [Google Scholar]
- 9.Kidd T.J., Gibson J.S., Moss M., Greer R.M., Cobbold R.N., Wright J.D., Ramsay K.A., Grimwood K., Bell S.C. Clonal complex Pseudomonas aeruginosa in horses. Vet. Microbiol. 2011;149:508–512. doi: 10.1016/j.vetmic.2010.11.030. [DOI] [PubMed] [Google Scholar]
- 10.Speert D.P. Molecular epidemiology of Pseudomonas aeruginosa. Front. Biosci. 2002;7:e354–361. doi: 10.2741/A929. [DOI] [PubMed] [Google Scholar]
- 11.Syrmis M.W., O'Carroll M.R., Sloots T.P., Coulter C., Wainwright C.E., Bell S.C., Nissen M.D. Rapid genotyping of Pseudomonas aeruginosa isolates harboured by adult and paediatric patients with cystic fibrosis using repetitive-element-based PCR assays. J. Med. Microbiol. 2004;53:1089–1096. doi: 10.1099/jmm.0.45611-0. [DOI] [PubMed] [Google Scholar]
- 12.Lim K.T., Yasin R.M., Yeo C.C., Puthucheary S.D., Balan G., Maning N., Wahab Z.A., Ismail N., Tan E.A., Mustaffa A., Thong K.L. Genetic fingerprinting and antimicrobial susceptibility profiles of Pseudomonas aeruginosa hospital isolates in Malaysia. J. Microbiol. Immunol. Infect. 2009;42:197–209. [PubMed] [Google Scholar]
- 13.Spilker T., Coenye T., Vandamme P., LiPuma J.J. PCR-based assay for differentiation of Pseudomonas aeruginosa from other Pseudomonas species recovered from cystic fibrosis patients. J. Clin. Microbiol. 2004;42:2074–2079. doi: 10.1128/JCM.42.5.2074-2079.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarei O., Shokoohizadeh L., Hossainpour H., Alikhani M.Y. Molecular analysis of Pseudomonas aeruginosa isolated from clinical, environmental and cockroach sources by ERIC-PCR. BMC Res. Notes. 2018;11:668. doi: 10.1186/s13104-018-3765-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tosin I., Silbert S., Sader H.S. The use of molecular typing to evaluate the dissemination of antimicrobial resistance among Gram-negative rods in Brazilian hospitals. Braz. J. Infect. Dis. 2003;7(6):360–369. doi: 10.1590/s1413-86702003000600002. [DOI] [PubMed] [Google Scholar]
- 16.Ghazi M., Khanbabaee G., Fallah F., Kazemi B., Mahmoudi S., Navidnia M., Pourakbari B., Bakhshi B., Goudarzi H. Emergence of Pseudomonas aeruginosa cross-infection in children with cystic fibrosis attending an Iranian referral pediatric center. Iran. J. Microbiol. 2012;4:124–129. [PMC free article] [PubMed] [Google Scholar]
- 17.Mahenthiralingam E., Campbell M.E., Foster J., Lam J.S., Speert D.P. Random amplified polymorphic DNA typing of Pseudomonas aeruginosa isolates recovered from patients with cystic fibrosis. J. Clin. Microbiol. 1996;34:1129–1135. doi: 10.1128/jcm.34.5.1129-1135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salimi H., Owlia P., Yakhchali B., Lari A.R. Drug susceptibility and molecular epidemiology of Pseudomonas aeruginosa isolated in a burn unit. Am. J. Infect. Dis. 2009;5(4):301–306. [Google Scholar]
- 19.Nanvazadeh F., Khosravi A.D., Zolfaghari M.R., Parhizgari N. Genotyping of Pseudomonas aeruginosa strains isolated from burn patients by RAPD-PCR. Burns. 2013;39:1409–1413. doi: 10.1016/j.burns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Vaez H., Faghri J., Nasr Esfahani B., Moghim S., Fazeli H., Sedighi M., Ghasemian Safaei H. Antibiotic resistance patterns and genetic diversity in clinical isolates of Pseudomonas aeruginosa isolated from patients of a referral hospital, Isfahan, Iran. Jundishapur J. Microbiol. 2015;8 doi: 10.5812/jjm.20130v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.