Abstract
Objective
Many have reported odd dreams during the pandemic. Given that dreams are associated with mental health, understanding these changes could provide crucial information about wellbeing during the pandemic. This study explored associations between COVID-19 and dream recall frequency (DRF), and related social, health, and mental health factors.
Methods
We conducted a cross-sectional web survey of 19,355 individuals in 14 countries from May to July 2020. We collected data on COVID-19, mental health, sleep and DRF during the pandemic. We performed McNemar Tests to compare low (<3 nights per week) and high DRF (≥3 nights per week) before and during COVID-19 and to evaluate changes in sleep variables segmented by DRF. Chi-square tests were conducted to compare characteristics between low and high DRF. Logistic regression analyses were conducted to examine associations between various independent variables and DRF.
Results
Reports of high DRF during the pandemic were higher than before the pandemic (P<0.001). Female gender (aOR=1.25, 95% CI 1.10–1.41), nightmares (aOR=4.22, 95% CI 3.45–5.17), sleep talking (aOR= 2.36, 1.73–3.23), sleep maintenance problems (aOR=1.34, 95% CI 1.15–1.56), symptoms of REM sleep behavior disorder (RBD; aOR=1.24, 95% CI 1.09–1.41) and repeated disturbing thoughts (posttraumatic stress disorder (PTSD) symptoms) were associated with high DRF. Age group 55–64 years (aOR=0.69, 95% CI 0.58–0.83) reported less high DRF than younger participants. Unadjusted OR showed associations between depression, anxiety, and DRF; however, in adjusted regression depression (aOR= 0.71, 0.59–0.86) and anxiety (aOR=0.79, 95% CI 0.66–0.94) were negatively associated with high DRF.
Conclusion and Relevance
DRF was higher than pre-pandemic levels across four continents. DRF was associated with gender and parasomnias like nightmares and RBD symptoms, sleep maintenance problems, PTSD symptoms and negatively associated with depression and anxiety. The results implicate that COVID-19 is reflected in our dreams as an expression of the emotional intensity of the pandemic.
Keywords: sleep, sleep disorder, mental health, parasomnia, collective threat
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader, the video abstract will appear. Or use:
Introduction
In December 2019, an outbreak of a novel respiratory virus, SARS-CoV-2, was reported. The rapid global spread and the rising number of deaths caused by coronavirus disease (COVID-19) led the World Health Organization to declare a pandemic in March 2020.1 To contain the spread of COVID-19, protective measures were imposed around the globe.
The COVID-19 crisis has touched every person in the world in some way, whether it is related to becoming infected, suffering financially, through reduced social contacts, missed opportunities, or an inability to get required supplies and materials. It has become a communal trauma that has a profound impact on people around the world. One of the most difficult aspects of the pandemic is social isolation and confinement. Solitude goes against our inborn social instincts to form and maintain relationships as human beings2,3 and live in herd- or swarm-like alignments.4,5 Recent research has shown that the pandemic has led to increased anxiety levels, panic attacks, irrational fears, post-traumatic stress, depression, fatigue, reduced sleep quality, and sleep disturbances.6,7
Given the effects it has had on our everyday lives, perhaps it is unsurprising that COVID-19 has crept into our dreams. Observations of increased psychological distress during the pandemic8 and similar changes in sleep patterns9,10 suggest possible connections between the pandemic and dream patterns.
To date, only a handful of studies have investigated how the pandemic is reflected in our dreams.11–16 Previous research indicates that experiencing collective threatening situations, such as earthquakes,17,18 hurricanes,19 and terrorist attacks,20,21 is associated with changes in dreams and sleep patterns. This would make sense as such experiences can cause immense psychological stress, and dreaming is hypothesized to be involved in emotional processing22 and emotional memory consolidation.23
Some recent studies on dreams during the COVID-19 pandemic are indeed reporting increased dream recall frequency (DRF).11,12,24 In the early stages of the COVID-19 crisis, a study in China observed a higher frequency of pandemic-related dreams, which were associated with higher levels of psychological distress.13 This finding of qualitative changes in dreams during the pandemic is in line with the continuity hypotheses, which suggests that emotional waking experiences are reflected in dreams.25 There appears to be demographic differences in dream recall. In an Italian survey 20% of the sample reported having dreams with explicit COVID-19 references, with women reporting higher DRF (50.8% of women were high recallers, 39.4% of men were high recallers), emotional intensity, and negative emotions in their dreams compared to men.11 Similarly, two other web-surveys conducted in Italy revealed that age, gender, not having children, depression and living alone were significantly related to pandemic DRF, respectively.14,15 These findings are consistent with a U.S. study, where the dreams of female participants, participants with high education level, and participants most affected by COVID-19 regarding physical health, mental health and social life, were more influenced by the pandemic compared to others.16 This raises the question of what factors may be associated with these changes beyond demographics.
DRF has previously been linked to frequent nocturnal awakenings, which often occur with sleep disorders, such as insomnia and RBD.26 Increased reports of sleep disorders during COVID-19 could be associated with heightened DRF.27 Another potential factor is change in sleep schedule. Gorgoni and colleagues showed higher DRF during the pandemic was associated with altered sleep duration and sleep quality.15 This makes sense, as sleep extended into the morning hours due to changes in work schedules, likely resulting in increased time spent in REM sleep.28–31 It has been hypothesized that during longer periods of sleep extension throughout the pandemic, sleep duration eventually relapses to its habitual length. If sleep extension is continued beyond this relapse of sleep duration, sleep becomes more fragmented resulting in more nocturnal awakenings, which can cause an increase in DRF.32 Also, DRF is closely linked to mental health and psychological wellbeing.33 Since the pandemic has reportedly led to worsened mental health and higher levels of depression, anxiety, distress and symptoms of PTSD, heightened DRF might be an expression of the current mental health status.34,35
Current research has demonstrated that DRF increased during the pandemic, and these increases were related to several demographic factors. However, there is still much we do not yet know about why dream recall increased, or its effects on mental health.
To assess the effects of the pandemic on psychological health and sleep habits, ICOSS – the International COVID-19 Sleep Study was initiated in March 2020.7 This world-wide project over four continents with 14 participating countries and over 19,000 participants provides the opportunity to examine this important question across countries and cultures. Gathering data from different countries allows us to take the varying level of restrictions and number of infections with COVID-19 into account.
Based on previous research, we hypothesized that DRF was elevated during the COVID-19 pandemic as compared to retrospective pre-pandemic levels. Additionally, we hypothesized that financial burden due to the pandemic, younger age, female gender, low level of education, irregular work schedules or work schedules that were affected by the pandemic (eg shift work, temporarily laid off), were predictors for high DRF during the pandemic. We also hypothesized that poor sleep quality, symptoms of insomnia, nightmares, symptoms of obstructive sleep apnea, REM sleep behavior disorder, anxiety symptoms, depressive symptoms, PTSD symptoms, stress, lower quality of life, and lower quality of health are associated with high DRF.
Methods
This paper is part of the International COVID-19 Sleep Study (ICOSS). The research protocol and the final standardized survey questionnaire used in the project were previously published by Partinen and colleagues.7 The project was carried out in the form of a cross-sectional survey in 14 countries including Austria, Brazil, Canada, Hong Kong, Province Jilin (China), Finland, France, Italy, Japan, Norway, Sweden, Poland, the UK, and the USA. Data used for the analysis were obtained from May to July 2020 using an online survey, which was distributed via media platforms, newsletters at universities and hospitals and through websites to different sleep societies. Web survey platforms were used, including REDcap and Qualtrics. All investigators obtained ethical approval or exemptions from their local ethics committee. Before taking part in the survey, all participants provided consent. Participants aged 18 years and older were eligible to complete the survey. The survey was anonymous, and participants did not have to provide identification information except for general sociodemographic variables. General data protection regulations were applied to ensure privacy and confidentiality. The survey was translated into the national language of each country.
The survey included questions taken from existing and validated questionnaires, as well as questions that were developed for this study. The survey included sociodemographic variables (age, gender, marital status, number of people living in the same household, residential area, ethnicity, education, work) and COVID-19 related data (infection, severity of disease, treatment, confinement, number of people infected among family and friends, worsening of financial status). To evaluate sleep problems, dream recall and the psychological impact of the pandemic, we incorporated items from the following standardized and validated questionnaires.
Basic Nordic Sleep Questionnaire
Items concerning sleep quality and the frequency of sleep onset and sleep maintenance problems, morning awakenings, use of hypnotics, sleepiness and fatigue were rated on a scale from 1 to 5, relating to how many nights per week sleep problems occurred.36 In a similar format, items on dreams, nightmares, sleep talking and singing and laughing in your sleep were added. All items were rated “during the pandemic” and “before the pandemic”. Dream recall frequency (DRF) was categorized as low DRF (< 3 nights per week) and high DRF (≥ 3 nights per week).14,37 Similarly, the occurrences of other sleep phenomena were categorized as infrequent (< 3 nights per week) and frequent (≥ 3 nights per week). Sleep quality was categorized as good (well, rather well, neither well nor badly) and poor (rather badly, badly).
Insomnia Severity Index (ISI)
A 7-item instrument to assess the perceived severity of nocturnal and daytime symptoms of insomnia rated on a scale of 0 to 4. A total score of 0–7 indicates no insomnia, 8 to 14 subthreshold clinical insomnia, 15 to 21 insomnia of moderate severity, and 22 and above indicates severe insomnia.38
STOP Questionnaire
The STOP (Snoring, Tiredness, Observed apnea, high blood Pressure) questionnaire is a screening tool for obstructive sleep apnea. The recommended cut-off scores of two or greater were classified as high-risk and total scores of one or less as low-risk of OSA.39
REM Sleep Behavior Disorder (RBD)
A single question on RBD,40 asking if the participants have ever “acted out” their dreams, while asleep (for example, punching, flailing your arms in the air, making running movement).
Patient Health Questionnaire-2 (PHQ-2)
A 2-item abbreviated version of the PHQ-9 as a screening tool for depression. The two items are rated on scales of 0 to 3. We used the recommended cut-off of three or more to screen for clinically relevant symptoms of depression.41
Generalized Anxiety Disorder-2 (GAD-2)
A 2-item abbreviated version of the GAD-7 as a screening tool for anxiety rated on scales of 0 to 3. A total score of three or more was used as a cut-off for identifying clinically relevant symptoms of anxiety.41
Well-Being Index (WHO-5)
Five items were rated on 0–5 scales to measure overall psychological wellbeing. The raw score is multiplied by four resulting in a total score from 0 to 100, with a higher score indicating higher quality of life.42
Post-Traumatic Stress Disorder
A two-item self-report derived from the PTSD Checklist as a measure of key symptoms of PTSD was rated on scales of 1 (“Not at all”) to 5 (“Extremely”).43
Stress
We used a single item (1–5 rating) to evaluate the current stress from “not at all” to “very much”.44
Quality of Life and Health
Two single items using 0–100 linear visual analog scales were used to measure quality of life and quality of health, with higher scores indicating better quality of life and health.45
Statistical Analysis
The statistical procedures were carried out using Stata/SE 16.1.46 The original data included 25,484 participants. We excluded data from the USA from the analyses, as the data collection method differed from the other countries (eg paid mTurk nationwide vs convenience sample, which overemphasized the US with stratification). Additionally, data from Sweden were excluded from the analysis as there was no lockdown in Sweden at the time of data collection. After excluding data from USA and Sweden, the sample size was n= 23,539. After excluding 4184 participants with incomplete data on DRF, a total of 19,355 participants (82.2%) were included in the analyses.
We used McNemar tests and Wilcoxon matched-pairs signed-rank test to analyze DRF before and during the pandemic. Changes in sleep quality, nightmares, sleep problems, sleep talking and singing, laughing in your sleep segmented by DRF were analyzed with McNemar tests. The results were described as proportions (percentages). We compared differences in sociodemographic data of participants between low and high DRF using independent sample t-test or chi-square test. The participants’ characteristics were described using mean ± standard deviation or frequencies (percentages). Logistic regression analyses were conducted to examine associations between financial burden due to COVID-19, age, gender, education, work, sleep quality, sleep problems, nightmare frequency, insomnia, obstructive sleep apnea (OSA), REM sleep behavior disorder (RBD), posttraumatic stress disorder (PTSD), anxiety, depression, stress, quality of life, quality of health, psychological wellbeing (independent variables), and high DRF during the pandemic (dependent variable). The logistic regression analyses were first run as an unadjusted (univariate) model, followed by an adjusted model where all independent variables were entered simultaneously to control for other independent variables, additionally entering country, ethnicity, residential area (urban vs rural), confinement and COVID-19 as covariates. The analyses were stratified by country and weighted by the number of inhabitants in the country/area of interest and by the number of responders in that country/area. We presented the results as odds ratio (OR) with 95% confidence interval (95% CI).
Multicollinearity between the independent variables was assessed before running the logistic regression by calculating Variance Inflation Factors (VIF). The VIF statistics for all variables included in the regression model were under 5. In order to adjust the α-values for the regression models predicting DRF, we applied the false discovery rate (FDR) correction (adjusted critical P= 0.032 for unadjusted model, adjusted critical P= 0.010 for adjusted model).47
Results
The prevalence of high DRF before and during the pandemic is summarized in Table 1. The proportion of participants reporting a high DRF increased by 9.2% during the pandemic (P<0.001). The proportion of both participants with low and high DRF reporting poor sleep quality, nightmares, and frequent sleep problems was significantly higher during the pandemic, which is summarized in Table 2. The increase in reports was notably higher for participants with high DRF during the pandemic, especially for sleep quality (worsened for 24.4% of high DRF; P<0.001) and nightmares (worsened for 11.3% of high DRF; P<0.001).
Table 1.
High DRF Before and During the Pandemic
| High DRFa | Never or Less Than Monthly | Less Than Weekly | 1–2 Days per Week | 3–5 Days per Week | Every Night or Almost Every Night | |
|---|---|---|---|---|---|---|
| % Before | 27.8 | 21.0 | 24.2 | 27.0 | 14.2 | 13.6 |
| % During | 33.6 | 20.0 | 20.7 | 25.7 | 17.5 | 16.1 |
| Change | +5.8 | −1.0 | −3.5 | −1.3 | +3.3 | +2.5 |
| Decreased | 3.4 | |||||
| Unchanged | 87.4 | |||||
| Increased | 9.2 |
Notes: Mc Nemar test were conducted to compare high DRF before and during pandemic; aProportion reporting high DRF (≥ 3 nights per week); Wilcoxon matched-pairs signed-rank test was conducted to compare DRF as categorical outcome before and during pandemic; The difference in dream recall frequency was significant (p<0.001); The results were not weighted.
Abbreviation: %, percentage.
Table 2.
Changes in Sleep Problems/Behavior Before and During the Pandemic (Low DRF vs High DRF)
| Sleep Qualitya | Night-Maresb | Sleep Onset Problemsb | Sleep Maintenance Problemsb | Early Morning Awakeningsb | Sleep Talkingb | Singing, Laughing in Sleepb | |
|---|---|---|---|---|---|---|---|
| Low DRF | |||||||
| % Before | 12.5 | 1.3 | 10.6 | 14.8 | 8.8 | 2.9 | 1.8 |
| % During | 23.4* | 2.3* | 18.5* | 20.9* | 14.4* | 2.8* | 1.9* |
| Change | +10.9 | +1.0 | +7.9 | +6.1 | +5.6 | −0.1 | +0.1 |
| Improved | 4.2 | 0.6 | 2.3 | 2.6 | 2.0 | 0.6 | 0.3 |
| Unchanged | 80.7 | 97.8 | 87.4 | 88.7 | 90.3 | 98.9 | 99.3 |
| Worsened | 15.1 | 1.6 | 10.3 | 8.7 | 7.7 | 0.5 | 0.4 |
| High DRF | |||||||
| % Before | 19.5 | 8.4 | 17.6 | 23.8 | 13.5 | 8.5 | 4.5 |
| % During | 37.6* | 18.3* | 31.0* | 36.2* | 23.2* | 10.5* | 5.9* |
| Change | +18.1 | +9.9 | +13.4 | +12.4 | +9.7 | +2.0 | +1.4 |
| Improved | 6.3 | 1.5 | 4.0 | 3.7 | 3.1 | 0.7 | 0.2 |
| Unchanged | 69.2 | 87.2 | 78.6 | 80.2 | 84.1 | 96.6 | 98.2 |
| Worsened | 24.4 | 11.3 | 17.4 | 16.1 | 12.8 | 2.7 | 1.6 |
Notes: Mc Nemar tests were conducted to compare sleep problems before and during pandemic segmented by low DRF (< 3 nights per week) and high DRF (≥ 3 nights per week); aProportion reporting sleep quality “rather badly” or “badly”; bProportion reporting sleep problems/behavior ≥ 3 nights per week; Significant differences from before pandemic (p<0.001) are marked with asterisks*; The results were not weighted.
Abbreviation: %, percentage.
Characteristics Low and High DRF
For the evaluation of characteristics of participants with low and high DRF sample size varied for each variable. Overall, 12,856 participants (66.4%) reported a low DRF during the pandemic and 6499 participants (33.6%) reported high DRF. The characteristics of participants with low DRF and high DRF are shown in Table 3.
Table 3.
Sociodemographic Characteristics of Participants for Low DRF (< 3 Nights per Week) and High DRF (≥ 3 Nights per Week)
| Low DRF (n=12,856) N(%)/M(SD) |
High DRF (n=6499) N(%)/M(SD) |
P-value | Effect Size V/Cohen‘s d |
|
|---|---|---|---|---|
| Gender (n=19,319) | <0.001 | 0.080 | ||
| Male | 4676 (71.8) | 1840 (28.2) | ||
| Female | 8160 (63.7) | 4643 (36.3) | ||
| Age, years (n= 19,282) | <0.001 | 0.056 | ||
| <25 | 1859 (61.2) | 1178 (38.8) | ||
| 25–34 | 3055 (65.2) | 1628 (34.8) | ||
| 35–44 | 2342 (67.4) | 1132 (32.6) | ||
| 45–54 | 2216 (68.7) | 1022 (31.3) | ||
| 55–64 | 1816 (68.7) | 829 (31.3) | ||
| 65+ | 1527 (69.3) | 678 (30.7) | ||
| Ethnicity (n=19,058) | <0.001 | 0.144 | ||
| Caucasian | 4536 (60.1) | 3008 (39.9) | ||
| Asian | 6559 (73.8) | 2333 (26.2) | ||
| African | 107 (56.3) | 83 (43.7) | ||
| Hispanic | 403 (58.4) | 287 (41.6) | ||
| Other | 1082 (62.1) | 660 (37.9) | ||
| Residential area (n= 19,287) | <0.001 | −0.034 | ||
| Rural | 1373 (62.0) | 841 (38.0) | ||
| Urban | 11,448 (67.0) | 5625 (33.0) | ||
| Marital status (n= 19,301) | <0.001 | 0.038 | ||
| Single | 4728 (64.4) | 2617 (35.6) | ||
| Married/cohabiting | 7124 (68.1) | 3344 (31.9) | ||
| Divorced/separated | 754 (65.1) | 405 (34.9) | ||
| Widowed | 224 (68.1) | 105 (31.9) | ||
| Living Alone (n= 19,339) | <0.001 | −0.037 | ||
| Yes | 346 (56.7) | 264 (43.3) | ||
| No | 12,495 (66.7) | 6234 (33.3) | ||
| Education (n= 19,078) | 0.039 | |||
| Primary | 223 (64.1) | 125 (35.9) | ||
| Secondary | 2798 (66.8) | 1388 (33.2) | ||
| Vocational | 1353 (65.4) | 717 (34.6) | ||
| Bachelor | 5306 (68.0) | 2492 (32.0) | ||
| Master | 2164 (63.7) | 1236 (36.3) | ||
| Doctor | 805 (63.1) | 471 (36.9) | ||
| Work (n= 18,838) | <0.001 | 0.080 | ||
| Regular day work | 6030 (68.5) | 2771 (31.5) | ||
| Irregular day work | 1049 (66.7) | 523 (33.3) | ||
| Student | 1653 (59.7) | 1117 (40.3) | ||
| Shift/night work | 684 (71.5) | 273 (28.5) | ||
| At home, no salary | 1206 (70.3) | 509 (29.7) | ||
| Temporary laid off | 209 (61.1) | 133 (38.9) | ||
| Unemployed | 465 (59.1) | 322 (40.9) | ||
| Retired | 1108 (65.9) | 574 (34.1) | ||
| Lost job due to pandemic | 132 (62.3) | 80 (37.7) | ||
| COVID-19 (n= 19,355) | 0.175 | 0.010 | ||
| No | 12,590 (66.5) | 6345 (33.5) | ||
| Yes | 266 (63.3) | 154 (36.7) | ||
| Confinement (n= 18,431) | <0.001 | 0.078 | ||
| Yes | 4484 (62.0) | 2751 (38.0) | ||
| No | 7781 (69.5) | 3415 (30.5) | ||
| Financial burden (n=19,336) | <0.001 | 0.030 | ||
| Not at all | 5437 (65.7) | 2843 (34.3) | ||
| A little | 3911 (67.6) | 1878 (32.4) | ||
| Somewhat | 1924 (68.1) | 899 (31.9) | ||
| Much | 1089 (66.2) | 555 (33.8) | ||
| Very much | 483 (60.4) | 317 (39.6) | ||
| Sleep qualitya (n= 19,347) | 0.150 | |||
| Good | 9849 (70.8) | 4053 (29.2) | ||
| Poor | 3001 (55.1) | 2444 (44.9) | ||
| Nightmaresb (n= 19,329) | <0.001 | 0.285 | ||
| Infrequent | 12,545 (70.3) | 5308 (29.7) | ||
| Frequent | 289 (19.6) | 1187 (80.4) | ||
| Sleeptalkingb (n= 19,263) | <0.001 | 0.161 | ||
| Infrequent | 12,433 (68.3) | 5787 (31.7) | ||
| Frequent | 356 (34.5) | 677 (65.5) | ||
| Singing, laughing during sleepb (n= 19,266) | <0.001 | 0.107 | ||
| Infrequent | 12,562 (67.4) | 6086 (32.6) | ||
| Frequent | 239 (38.7) | 379 (61.3) | ||
| Sleep onset problemsb (n= 19,332) | <0.001 | 0.141 | ||
| Infrequent | 10,463 (70.0) | 4480 (30.0) | ||
| Frequent | 2378 (54.2) | 2011 (45.8) | ||
| Sleep maintenance problemsb (n= 19,322) | <0.001 | 0.164 | ||
| Infrequent | 10,152 (71.0) | 4139 (29.0) | ||
| Frequent | 2685 (53.4) | 2346 (46.6) | ||
| Early morning awakeningsb (n= 19,317) | <0.001 | 0.110 | ||
| Infrequent | 10,978 (68.8) | 4980 (31.2) | ||
| Frequent | 1852 (55.1) | 1507 (44.9) | ||
| ISI (n= 18,498) | <0.001 | 0.173 | ||
| No insomnia | 7215 (73.4) | 2618 (26.6) | ||
| Subthreshold Insomnia | 3595 (63.2) | 2096 (36.8) | ||
| Clinical Insomnia | 1227 (51.9) | 1137 (48.1) | ||
| Severe Insomnia | 294 (48.2) | 316 (51.8) | ||
| OSA (n= 18,545) | <0.001 | 0.060 | ||
| Low risk | 11,488 (67.4) | 5556 (32.6) | ||
| High risk | 857 (57.1) | 644 (42.9) | ||
| RBD (n= 19,299) | <0.001 | |||
| No | 10,517 (69.5) | 4624 (30.5) | 0.122 | |
| Yes | 2305 (55.4) | 1853 (44.6) | ||
| PTSD: repeated disturbing thoughts/memories (n= 19,005) | <0.001 | 0.196 | ||
| Not at all | 6845 (74.2) | 2384 (25.8) | ||
| A little bit | 3571 (65.4) | 1889 (34.6) | ||
| Moderately | 1207 (56.2) | 940 (43.8) | ||
| Quite A Bit | 662 (48.5) | 702 (51.5) | ||
| Extremely | 352 (43.7) | 453 (56.3) | ||
| PTSD: feeling very upset about past (n= 18,982) | <0.001 | 0.186 | ||
| Not at all | 6825 (73.5) | 2455 (26.5) | ||
| A little bit | 3455 (65.8) | 1798 (34.2) | ||
| Moderately | 1279 (57.9) | 930 (42.1) | ||
| Quite A Bit | 673 (49.0) | 700 (51.0) | ||
| Extremely | 388 (44.8) | 479 (55.2) | ||
| Anxiety (n= 19,025) | <0.001 | 0.121 | ||
| No | 10,832 (69.1) | 4835 (30.9) | ||
| Yes | 1820 (54.2) | 1538 (45.8) | ||
| Depression (n= 19,014) | <0.001 | 0.110 | ||
| No | 11,126 (68.7) | 5082 (31.3) | ||
| Yes | 1517 (54.1) | 1289 (45.9) | ||
| Stress (n= 18,809) | 0.136 | |||
| Mild | 10,316 (69.8) | 4454 (30.2) | ||
| Severe | 2191 (54.3) | 1848 (45.7) | ||
| Quality of Life (n= 18,824) | 65.8 ± 23.0 | 63.3 ± 23.7 | <0.001 | 0.107 |
| Quality of Health (n=18,806) | 70.9 ± 21.7 | 68.8 ± 22.5 | <0.001 | 0.095 |
| Wellbeing (WHO-5) (n= 18,748) | 58.5 ± 23.5 | 51.5 ± 23.3 | <0.001 | 0.303 |
Notes: Independent sample t-test or chi-square was conducted to test the difference between low DRF (< 3 nights per week) and high DRF (≥ 3 nights per week); Results are shown as frequencies (percentages); Anxiety= GAD2≥ 3, Depression= PHQ2≥ 3; aSleep quality was categorized as good (well, rather well, neither well nor badly) and poor (rather badly, badly); bSleep problems/behavior were categorized as infrequent (< 3 nights per week) and frequent (≥ 3 nights per week); Results were not weighted; Total number of participants was 19,355 but sample size varied for each variable.
Abbreviations: N, number of participants; %, percentage; M, mean; SD, standard deviation; ISI, Insomnia Severity Index; OSA, obstructive sleep apnea; RBD, REM sleep behavior disorder; PTSD, posttraumatic stress disorder; WHO, World Health Organization.
In short, 36.3% of the female participants reported a high DRF during the pandemic, whereas 28.2% of the male participants reported a high DRF (P<0.001). There were also meaningful differences by age with prevalence of high DRF decreasing with age: 38.8% of participants younger than 25 years and 30.7% of 65 years and older reported high DRF (P<0.001). With regard to the level of education, people with a doctorate had the highest proportion of high DRF (36.9%), whereas people with secondary education had the lowest proportion of high DRF (33.2%; P<0.001). There were significant differences concerning DRF between countries, which are shown in Figure 1.
Figure 1.
Percentage of low DRF and high DRF between countries.
Notes: Chi-square was conducted to test the difference between low DRF (< 3 nights per week) and high DRF (≥ 3 nights per week) across countries. DRF differed significantly (P<0.001, V=0.178).
Abbreviations: %, percentage; n, number of participants.
Within the sample, 36.7% of participants infected with Covid-19 (P=0.175) and 30.5% of participants experiencing confinement (P<0.001) had frequent dreams during the pandemic. Additionally, 39.6% of the participants whose financial status suffered severely from Covid-19 and 34.3% whose financial status was not affected at all reported high DRF (P<0.001).
Sleep quality notably differed for low and high DRF, 29.2% of participants with good sleep quality had high DRF, whereas 44.9% of participants with poor sleep quality had high DRF (P<0.001). Nightmares, unsurprisingly, were strongly associated with DRF (P<0.001). Prevalence of insomnia symptoms (ISI-score) also varied, with 51.8% of the participants with symptoms of severe insomnia symptoms reporting high DRF compared to only 26.6% of the participants with no symptoms of insomnia (P<0.001).
Of the participants with probable depression, 45.9% had high DRF whereas 31.3% of those without depressive symptoms had high DRF (P<0.001). Corresponding prevalence for participants with or without symptoms of anxiety were 45.8% and 30.9% (P<0.001), respectively. Of the participants with extreme PTSD symptoms (feeling very upset about past), 56.3% had high DRF whereas 25.8% had high DRF among individuals with no PTSD symptoms (P<0.001).
Associations with Pandemic Dream Recall Frequency
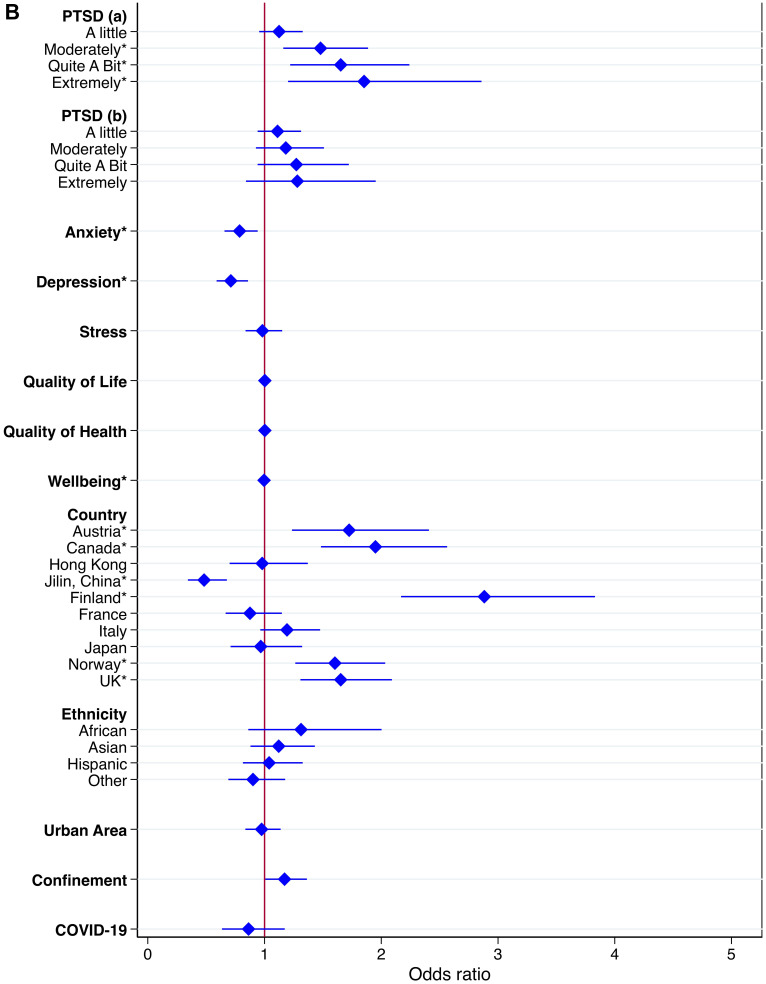
Logistic regressions were conducted to examine associations between independent variables and high DRF during the pandemic. We excluded all missing values within the independent variables from the analysis, and data from Poland were excluded completely due to a large number of missing values within the independent variables. In total, we included 15,899 participants with complete data in the regression. The results of the logistic regression are shown as OR in Table 4 and as adjusted OR (aOR) in Figure 2.
Table 4.
Associations with High DRF (≥ 3 Nights per Week) During the Pandemic
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Gender | ||
| Male | Reference | |
| Female | 1.54 (1.39–1.70)* | 1.25 (1.10–1.41)* |
| Age, years | ||
| 25–34 | Reference | |
| <25 | 1.24 (1.07–1.43)* | 1.04 (0.86–1.26) |
| 35–44 | 0.78 (0.67–0.91)* | 0.86 (0.72–1.01) |
| 45–54 | 0.74 (0.64–0.86)* | 0.81 (0.68–0.97) |
| 55–64 | 0.65 (0.56–0.76)* | 0.69 (0.58–0.83)* |
| 65+ | 0.67 (0.57–0.78)* | 0.87 (0.70–1.09) |
| Marital Status | ||
| Single | Reference | |
| Married/Cohabiting | 0.70 (0.64–0.78)* | 0.97 (0.84–1.12) |
| Divorced/Separated | 0.72 (0.58–0.88)* | 0.95 (0.74–1.21) |
| Widowed | 0.75 (0.53–1.07) | 1.04 (0.69–1.55) |
| Living Alone | ||
| Yes | Reference | |
| No | 1.02 (0.78–1.35) | 1.09 (0.78–1.51) |
| Education | ||
| Primary | Reference | |
| Secondary | 1.35 (0.97–1.87) | 1.10 (0.75–1.62) |
| Vocational | 1.28 (0.91–1.80) | 1.00 (0.67–1.49) |
| Bachelor | 1.13 (0.82–1.56) | 1.01 (0.69–1.47) |
| Master | 1.24 (0.89–1.73) | 1.02 (0.69–1.52) |
| Doctor | 1.28 (0.89–1.82) | 1.36 (0.89–2.10) |
| Work | ||
| Regular day work | Reference | |
| Irregular day work | 1.02 (0.85–1.22) | 0.91 (0.74–1.11) |
| Student | 1.64 (1.43–1.88)* | 1.14 (0.93–1.39) |
| Shift/night work | 0.94 (0.75–1.17) | 1.11 (0.87–1.41) |
| At home, no salary | 0.96 (0.81–1.13) | 0.94 (0.77–1.14) |
| Temporary laid off | 1.01 (0.71–1.45) | 0.71 (0.46–1.08) |
| Unemployed | 1.44 (1.13–1.81)* | 1.22 (0.95–1.57) |
| Retired | 0.93 (0.78–1.09) | 0.99 (0.79–1.24) |
| Lost job due to pandemic | 1.60 (1.07–2.41)* | 1.34 (0.83–2.15) |
| Financial burden | ||
| Not at all | Reference | |
| A little | 1.05 (0.94–1.18) | 1.01 (0.89–1.15) |
| Somewhat | 0.94 (0.81–1.09) | 0.92 (0.78–1.09) |
| Much | 0.98 (0.83–1.16) | 0.92 (0.75–1.13) |
| Very much | 1.22 (0.98–1.52) | 1.07 (0.81–1.42) |
| Sleep qualitya | ||
| Good | Reference | |
| Poor | 1.65 (1.50–1.83) | 0.93 (0.80–1.08) |
| Nightmaresb | ||
| Infrequent | Reference | |
| Frequent | 6.06 (5.04–7.28)* | 4.22 (3.45–5.17)* |
| Sleeptalkingb | ||
| Infrequent | Reference | |
| Frequent | 3.05 (2.56–3.64)* | 2.36 (1.73–3.23)* |
| Singing, laughing during sleepb | ||
| Infrequent | Reference | |
| Frequent | 2.50 (2.04–3.06)* | 0.63 (0.43–0.93) |
| Sleep onset problemsb | ||
| Infrequent | Reference | |
| Frequent | 1.84 (1.66–2.05)* | 1.05 (0.91–1.22) |
| Sleep maintenance problemsb | ||
| Infrequent | Reference | |
| Frequent | 1.83 (1.65–2.03)* | 1.34 (1.15–1.56)* |
| Early morning awakeningsb | ||
| Infrequent | Reference | |
| Frequent | 1.50 (1.33–1.68)* | 0.89 (0.75–1.05) |
| ISI | ||
| No insomnia | Reference | |
| Subthreshold Insomnia | 1.56 (1.40–1.73)* | 1.05 (0.91–1.20) |
| Clinical Insomnia | 2.38 (2.08–2.73)* | 1.16 (0.94–1.44) |
| Severe Insomnia | 2.44 (1.92–3.11)* | 0.90 (0.63–1.27) |
| OSA | ||
| Low risk | Reference | |
| High risk | 1.28 (1.09–1.52)* | 1.22 (1.01–1.48) |
| RBD | ||
| No | Reference | |
| Yes | 1.73 (1.55–1.93)* | 1.24 (1.09–1.41)* |
| PTSD: repeated disturbing thoughts/memories | ||
| Not at all | Reference | |
| A little bit | 1.41 (1.26–1.58)* | 1.13 (0.95–1.33) |
| Moderately | 2.16 (1.85–2.51)* | 1.48 (1.16–1.89)* |
| Quite A Bit | 2.84 (2.40–3.36)* | 1.65 (1.22–2.24)* |
| Extremely | 3.62 (2.92–4.50)* | 1.85 (1.20–2.86)* |
| PTSD: feeling very upset about past | ||
| Not at all | Reference | |
| A little bit | 1.42 (1.27–1.59)* | 1.11 (0.94–1.31) |
| Moderately | 1.94 (1.67–2.25)* | 1.18 (0.93–1.51) |
| Quite A Bit | 2.80 (2.36–3.32)* | 1.27 (0.94–1.72) |
| Extremely | 3.43 (2.79–4.21)* | 1.28 (0.84–1.95) |
| Anxiety | ||
| No | Reference | |
| Yes | 1.61 (1.44–1.80)* | 0.79 (0.66–0.94)* |
| Depression | ||
| No | Reference | |
| Yes | 1.59 (1.41–1.79)* | 0.71 (0.59–0.86)* |
| Stress | ||
| Mild | Reference | |
| Severe | 1.69 (1.51–1.88)* | 0.98 (0.84–1.15) |
| Quality of Life | 1.00 (0.99–1.00)* | 1.00 (1.00–1.01) |
| Quality of Health | 1.00 (0.99–1.00)* | 1.00 (1.00–1.01) |
| Wellbeing (WHO-5) | 0.99 (0.99–1.00)* | 1.00 (0.99–1.00)* |
| Country | ||
| Brazil | Reference | |
| Austria | 1.08 (0.82–1.43) | 1.72 (1.24–2.41)* |
| Canada | 1.21 (1.04–1.41)* | 1.95 (1.48–2.56)* |
| Hong Kong | 0.58 (0.49–0.69)* | 0.98 (0.70–1.37) |
| Jilin, China | 0.30 (0.25–0.37)* | 0.48 (0.34–0.68)* |
| Finland | 1.54 (1.26–1.88)* | 2.88 (2.17–3.83)* |
| France | 0.59 (0.48–0.73)* | 0.88 (0.67–1.15) |
| Italy | 0.96 (0.82–1.11) | 1.19 (0.96–1.48) |
| Japan | 0.54 (0.48–0.61)* | 0.97 (0.71–1.32) |
| Norway | 0.93 (0.78–1.13) | 1.60 (1.26–2.03)* |
| UK | 1.27 (1.08–1.50)* | 1.65 (1.31–2.09)* |
| Ethnicity | ||
| Caucasian | Reference | |
| Asian | 0.53 (0.49–0.58)* | 1.12 (0.88–1.43) |
| African | 1.30 (0.91–1.87) | 1.31 (0.86–2.00) |
| Hispanic | 1.10 (0.91–1.34) | 1.04 (0.81–1.33) |
| Other | 0.90 (0.77–1.05) | 0.90 (0.69–1.18) |
| Residential area | ||
| Rural | Reference | |
| Urban | 0.87 (0.76–0.99)* | 0.97 (0.84–1.14) |
| Confinement | ||
| No | Reference | |
| Yes | 1.50 (1.37–1.63)* | 1.17 (1.01–1.36) |
| COVID-19 | ||
| No | Reference | |
| Yes | 1.06 (0.79–1.41) | 0.86 (0.64–1.17) |
Notes: Table shows unadjusted OR, and adjusted OR additionally controlling for country, ethnicity, residential area (urban vs rural), confinement and COVID-19; Results were weighted and stratified by countries; Significant independent variables are marked with asterisks* (p<0.032 for unadjusted model, p< 0.010 for adjusted model); Anxiety= GAD2≥ 3, Depression= PHQ2≥ 3; aSleep quality was categorized as good (well, rather well, neither well nor badly) and poor (rather badly, badly); bSleep problems were categorized as infrequent (< 3 nights per week) and frequent (≥ 3 nights per week); Due to missing values, Poland was excluded from the analysis.
Abbreviations: OR, odds ratio; %, percentage; CI, confidence interval; ISI, Insomnia Severity Index; OSA, obstructive sleep apnea; RBD, REM sleep behavior disorder; PTSD, posttraumatic stress disorder; WHO, World Health Organization.
Figure 2.
Continued.
Figure 2.
Adjusted OR with high DRF as dependent variable.
Notes: Results of adjusted logistic regression with high DRF as dependent variable, controlling for country, ethnicity, residential area, confinement and COVID-19. Graphic representation of odds ratio and relative 95% confidence intervals for each independent variable. The figure is split in two. (A) Independent variables: gender (reference: male), age (reference: 25–34 years), marital status (reference: single), living alone (reference: yes), education (reference: primary), work (reference: regular day work), financial burden (reference: not at all), sleep quality (reference: good), nightmares, sleep talking, singing and laughing in your sleep, sleep onset problems, sleep maintenance problems, early morning awakenings (all as reference: infrequent), insomnia (reference: no insomnia), obstructive sleep apnea (reference: low risk), REM sleep behavior disorder (reference: no). Significant independent variables are marked with asterisks*. (B) Independent variables: PTSD symptoms (reference: not at all), anxiety (reference: no), depression (reference: no), stress (reference: mild), Quality of Life, Quality of Health, Wellbeing, country (reference: Brazil), ethnicity (reference: Caucasian), residential area (reference: rural), confinement (reference: no), COVID-19 (reference: no). Significant independent variables are marked with asterisks*.
Abbreviations: OSA, obstructive sleep apnea; RBD, REM sleep behavior disorder; PTSD, posttraumatic stress disorder.
Female gender was associated with high DRF (aOR=1.25, 95% CI 1.10–1.41), compared to males. Age group 55–64 years (aOR=0.69, 95% CI 0.58–0.83) reported less high DRF than younger participants. Other characteristics such as marital status and living alone were not related to high DRF after adjusting for other independent variables.
Frequent nightmares (aOR=4.22, 95% CI 3.45–5.17), talking in sleep (aOR= 2.36, 95% CI 1.73–3.23), sleep maintenance problems (aOR=1.34, 95% CI 1.15–1.56) and symptoms of RBD (aOR=1.24, 95% CI 1.09–1.41) were associated with high DRF. Sleep quality and other sleep problems were not related to high DRF.
Repeated disturbing thoughts (PTSD symptoms) were significantly associated with high DRF (“Moderately”: aOR=1.48, 95% CI 1.16–1.89; “Quite A Bit”: aOR=1.65, 95% CI 1.22–2.24; “Extremely”: aOR=1.85, 95% CI 1.20–2.86). Unadjusted OR showed that depression and anxiety were significantly associated with high DRF; however, in the fully adjusted model, depressive symptoms (aOR= 0.71, 0.59–0.86) and anxiety symptoms (aOR=0.79, 95% CI 0.66–0.94) were negatively associated with high DRF. For overall psychological wellbeing (aOR=1.00, 95% CI 0.99–1.00), the p-value showed significant association with DRF, but the odds ratio indicated no clinical significance. Feeling upset about the past as a symptom of PTSD, stress and quality of life and health were not associated with high DRF during the pandemic.
Discussion
We found that there was a significant increase in DRF during the pandemic, with 9.2% reporting heightened DRF, which is in line with other reports.11,12,24 Participants with high DRF during the pandemic experienced more pronounced changes in sleep behavior as a consequence of the pandemic, such as worsened sleep quality and more sleep problems.
Participants reporting high DRF and low DRF during the pandemic differed significantly concerning sociodemographic characteristics, for the most part with very small effect sizes. There were also significant differences concerning COVID-related data, such as confinement and financial burden, but with small effect sizes. However, there were no differences concerning DRF between people who reported having had COVID-19 and people who had not been infected. That said, it is important to note that only a small number of participants reported COVID-19 at all in our sample (n= 420; 2.2%), so the lack of an effect could be due to insufficient power. Our data revealed variations within sleep-related variables between low and high DRF, including subjective sleep quality, nightmares, insomnia symptoms, obstructive sleep apnea, and REM sleep behavior disorder, with moderate to very strong effect sizes. Furthermore, the analysis revealed significant differences between participants with low and high DRF in mental wellbeing, including symptoms of PTSD, anxiety, depression, stress, quality of life and health, and overall subjective wellbeing.
DRF differed significantly between countries, with Finland reporting the highest percentage of high DRF and Jilin (China) reporting the lowest percentage of high DRF. To our knowledge, this is the first study to compare DRF between a large number of countries. However, a previous study comparing dream recall between German and Japanese students reported significantly lower dream recall in the Japanese sample.48 Another study investigating a Chinese sample showed lower dream recall in the Chinese population compared to a German sample.49 Similar trends were seen in our data, with the province Jilin showing the overall lowest percentage of high DRF (17.2%) and Japan also reporting relatively low percentage of high DRF (26.8%). Besides already observed cross-cultural differences in DRF, we propose that COVID-related aspects such as varying degrees of lockdown and number of Covid-19 cases people at the time of data sampling between countries substantiate the variation of DRF across countries.
Further analysis with logistic regression showed that female gender was related to higher DRF. This is in line with other studies that examined DRF during the pandemic.14–16 The differences in dream patterns between males and females have been well established.50 This may result from gender-related differences in prevalence of sleep complaints, cognitive functions, such as better memory for emotional stimuli in women,51 and women may be reporting their dreams more easily. Additionally, women seem to be more affected by the pandemic, in terms of poorer sleep quality and worse mental health.52 Mental health is often reflected in DRF,33 which would further explain the observed dream recall in women. Similarly, a study by Wang and colleagues revealed that psychological distress was related to epidemic-related dreams during the COVID-19 crisis.13
Our data revealed that older age (55–64 years) was related to lower DRF. This finding is consistent with other COVID-19 related studies that showed younger age was a significant predictor of high DRF.14,15 The observed effect of age on dream recall in our data diminishes with ages older than 65 years. This trend has been reported in previous studies: Herman and Shows demonstrated that DRF decreased with advancing age but only until the age of 59.53 Another study obtained similar results with DRF decreasing with age, but only up to the age of 56.54 Age-related changes in DRF could be associated with a decrease in REM sleep and changes in sleep physiology.50
The descriptive data and unadjusted regression analyses indicated that symptoms of depression and anxiety were related to high DRF during the pandemic. However, the adjusted regression model yielded different results, with symptoms of depression and anxiety negatively predicting high DRF. The results are in conflict with a study by Gorgoni et al and Schredl et al, where higher scores of depressive symptoms were related to higher DRF during COVID-19.15,16
The change in direction of effects occurred by including PTSD variables in the regression model. This suggests that symptoms of PTSD and depression and anxiety are interrelated, raising questions of multicollinearity. Even though the VIF statistics were <5, correlations between the PTSD variables, depression, and anxiety were all significant, as shown in Table 5. Indeed, high comorbidity rates of PTSD in depression and anxiety have been well established in previous studies.55,56 PTSD symptoms that are also common symptoms of depression are dysphoria, sleep disturbances, and concentration difficulties,57 whereas an overlapping symptom of PTSD and anxiety is worry.58 Worry as a symptom of anxiety and feeling depressed and hopeless as a symptom of depression were both included in the questionnaires used to collect data. Therefore, we believe an overlap between symptoms of PTSD, depression, and anxiety in the sample could explain why anxiety and depression were negatively associated with high DRF in our adjusted analyses. Additionally, to our knowledge, we are one of the first studies controlling for this number of variables in the context of pandemic DRF, which could further explain the conflicting findings.
Table 5.
Associations Between PTSD, Anxiety and Depression
| GAD2<3 | GAD2≥ 3 | PHQ2<3 | PHQ2≥ 3 | |
|---|---|---|---|---|
| PTSD: repeated disturbing thoughts/memories | ||||
| Not at all | 8735 (94.7) | 487 (5.3) | 8857 (96.1) | 359 (3.9) |
| A little bit | 4704 (86.3) | 748 (13.7) | 4897 (89.9) | 553 (10.1) |
| Moderately | 1422 (66.3) | 724 (33.7) | 1551 (72.4) | 592 (27.6) |
| Quite A Bit | 592 (43.5) | 769 (56.5) | 667 (48.9) | 697 (51.1) |
| Extremely | 179 (22.2) | 626 (77.8) | 202 (25.2) | 600 (74.81) |
| PTSD: feeling very upset about past | ||||
| Not at all | 8830 (95.2) | 447 (4.8) | 8957 (96.6) | 313 (3.4) |
| A little bit | 4502 (85.9) | 741 (14.1) | 4699 (89.6) | 544 (10.4) |
| Moderately | 1482 (67.2) | 725 (32.8) | 1593 (72.2) | 612 (27.8) |
| Quite A Bit | 606 (44.2) | 765 (55.8) | 673 (49.0) | 699 (51.0) |
| Extremely | 192 (22.2) | 675 (77.8) | 233 (27.0) | 631 (73.0) |
Notes: Chi-square tests were conducted to analyze associations between PTSD, depression and anxiety; Results are shown as frequencies (percentages); Anxiety= GAD2≥ 3, Depression= PHQ2≥ 3; The associations were all significant (P<0.001).
Abbreviations: PTSD, posttraumatic stress disorder; GAD, generalized anxiety disorder; PHQ, Patient Health Questionnaire.
While some studies indicate a positive association between anxiety and DRF, there have also been reports of a decrease in DRF in anxiety disorders.59 Similarly, the relationship between depression and DRF is still unclear. In fact, a review indicated that depressed individuals had fewer and less detailed dream recall compared with healthy subjects.60 Patients with mood alterations reported shorter duration of dreams than controls,61 and qualitative and quantitative dream features may reflect the effect of antidepressant treatment.62 Unfortunately, in our investigation we did not collect information about pharmacological treatments; hence, we cannot disentangle this issue.
The association between PTSD symptoms and DRF is not surprising. People experiencing major trauma tend to have disturbing and repetitive trauma-related dreams,17,63 as well as other sleep problems like insomnia and restless leg syndrome.64 Dream-related disorders often appear as a symptom of trauma, especially in the form of nightmares. There is also evidence of traumatic situations leading to an increase in dream recall, for example observed in children living under military violence in Gaza65 and children exposed to war.63 This is consistent with previously mentioned results concerning the influence of collective threatening situations on dream pattern.17,18,20,21 These findings are consistent with the assumption that dreams and nightmares function to process stressful events and to help us deal with overwhelming experiences.66,67 At this point, further research on the COVID-19 pandemic as a potential collective trauma would be of interest, as the pandemic is characterized by a constant threat and uncertainty,68 that might in itself lead to symptoms of PTSD and in consequence to further disruptions in sleep and dream patterns.
Also, sleep phenomena including nightmares, sleep talking, sleep maintenance problems, and symptoms of RBD were associated with high DRF. This is in general consistent with other studies reporting that altered sleep patterns and nocturnal disruptive behaviors are associated with greater dream recall during the pandemic,15 and the idea of the arousal-retrieval model,69 that frequent nocturnal arousals facilitate the long-term storage of dream content.70 Higher DRF has previously already been observed in RBD patients compared to healthy participants.71,72 RBD is a parasomnia, where dreams occur with simple or complex motor behaviors, which reflect dream contents and often lead to sleep disruptions.73–75 Frequent nocturnal awakenings have previously been reported with increased dream recall.26
Experiences from waking life, particularly emotionally intense events,76,77 are often incorporated into dreams,78 as suggested by the continuity hypothesis of dreaming. Although the relationship between emotion regulation and dreaming remains unclear, there is growing evidence of similar neurobiological patterns that indicate dreams could have a crucial role in emotional processing.67 Even though reliving distressing and threatening events from the waking life in the course of a dream can have negative effects on waking mood, it is postulated to facilitate the processing of current negative emotions,22 hence leading to better adapted affective responses to real-life events.79 The notion that dreams are a response to threatening waking life experiences is rooted in evolutionary theories, where dreaming is hypothesized to increase threat-avoidance skills.80
Limitations
There are methodological limitations to be addressed. The data collection via online survey might have introduced a bias in the final sample, since participation was limited to individuals with internet access and self-selected for accepting to participate, which could lead to that people who were more interested in the topic of dreams and sleep were more likely to take part in the survey. DRF has been linked to attitudes toward dreams, so people who are more interested in the topic of sleep and dreams might be more likely to overestimate their dream recall.82 Additionally, we had an uneven distribution of characteristics, such as gender and ethnicity within the sample, which limits the generalizability of the results. There were large variations concerning the sample sizes from each country. However, this issue was addressed by weighting the data accordingly in the logistic regression. There were local differences concerning the level of restrictions applied to contain the spread of COVID-19 in each country at the time of the survey, which could have influenced the data collected. Another limitation of our study concerns the self-reported data. Self-reported retrospective data in general are prone to inaccuracy and can be distorted by memory bias compared to prospective sleep and dreams diaries.83 It is therefore important to emphasize that our study evaluated the subjective perception of dream recall frequency before and during the pandemic. Lastly, our measures of depression, anxiety and PTSD were very short versions of well-validated measures and correlated highly with each other. We also used short versions of screening tools for other disorders, like OSA and RBD, which provide information about the presence of symptoms but do not allow a diagnosis.
Conclusion
In conclusion, we found that reports of high DRF increased during the pandemic. Participants with high DRF during the pandemic experienced more pronounced changes in sleep behavior, such as worsened sleep quality and more sleep problems. Participants with low and high DRF differed significantly concerning sociodemographic characteristics as gender and age, but also concerning mental health and various aspects of sleep. The adjusted analysis revealed that female gender, parasomnias, and psychological symptoms were associated with high DRF and older age was negatively associated with high DRF.
Dreams and dream activity are an often forgotten expression of the existential situation of individuals. Based on our results, we assume that disruption in sleep patterns due to changes caused by the pandemic explains the increase in dream recall. Additionally, we propose that the observed increased DRF in our sample is an expression of the emotional intense and demanding experience of the current situation and could be an indicator that the pandemic is indeed turning into a previously mentioned collective trauma.81 This also corresponds to reports of increased psychological distress8 and prevalence of mental health problems during COVID-19.34 Therefore, dreams and dream recall deserve more attention as potential support for coping with crisis situations, such as the COVID-19 pandemic and overall in supporting psychological wellbeing. Dreams and dream recall need to be accepted more and integrated into approaches for improving mental health and health in general.
Acknowledgments
We would like to acknowledge all ICOSS group collaborators.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data Sharing Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available.
Ethics Approval
All investigators obtained ethical approval or exemptions from their local ethics committee. Participants voluntarily and anonymously agreed to take part in the survey. General data protection regulations were applied to ensure privacy and confidentiality.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval for the version to be published, agreed to the submitted journal, and agreed to be accountable for all aspects of the work.
Disclosure
Dr. Chung reports Grants from the University Health Network Foundation and personal fees from Takeda Pharma, outside the submitted work. Yun Kwok Wing reports grants from the Research Grant Council of University Grants Committee, Health Medical Research Fund, Hong Kong SAR, received personal fees for delivering a lecture from Eisai Co., Ltd. and sponsorship from Lundbeck HK Limited outside the currently submitted work. Dr. Matsui has received speaker’s honoraria from Eisai, Meiji Seika Pharma, Mochida, MSD, Otsuka, Takeda and Yoshitomi. Colin Espie is co-founder of and a shareholder in Big Health, the developer that created Sleepio which is a digital CBT programme for insomnia. Eirin Fränkl, Serena Scarpelli, Michael R Nadorff, Bjørn Bjorvatn, Courtney Bolstad, Ngan Yin Chan, Yves Dauvilliers, Yuichi Inoue, Damien Leger, Tainá Macêdo, Ilona Merikanto, Charles M. Morin, Sérgio Mota-Rolim, Markku Partinen, Thomas Penzel, Giuseppe Plazzi, Mariusz Sieminski, Luigi De Gennaro and Brigitte Holzinger have nothing to disclose. The authors report no other conflicts of interest in this work.
References
- 1.Timeline: WHO’s COVID-19 reponse. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/interactive-timeline#!. Accessed July7, 2021.
- 2.Baumeister R, Leary M. The need to belong: desire for interpersonal attachments as a fundamental human motivation. Psychol Bull. 1995;117:497–529. doi: 10.1037/0033-2909.117.3.497 [DOI] [PubMed] [Google Scholar]
- 3.Cacioppo JT, Cacioppo S. Social relationships and health: the toxic effects of perceived social isolation. Soc Personal Psychol Compass. 2014;8(2):58–72. doi: 10.1111/spc3.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turner RH, Killian LM. Collective Behavior. Englewood Cliffs, NJ: Prentice-Hall; 1957. [Google Scholar]
- 5.Raafat R, Chater N, Frith C. Herding in humans. Trends Cogn Sci. 2009;13:420–428. doi: 10.1016/j.tics.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Zhao N. Generalized anxiety disorder, depressive symptoms and sleep quality during COVID-19 outbreak in China: a web-based cross-sectional survey. Psychiatry Res. 2020;288:112954. doi: 10.1016/j.psychres.2020.112954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Partinen M, Bjorvatn B, Holzinger B, et al. Sleep and circadian problems during the coronavirus disease 2019 (COVID-19) pandemic: the International COVID-19 Sleep Study (ICOSS). J Sleep Res. 2021;30(1):e13206. doi: 10.1111/jsr.13206 [DOI] [PubMed] [Google Scholar]
- 8.Chirico A, Lucidi F, Galli F, et al. COVID-19 outbreak and physical activity in the Italian population: a cross-sectional analysis of the underlying psychosocial mechanisms. Front Psychol. 2020;11:2100. doi: 10.3389/fpsyg.2020.02100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck F, Léger D, Fressard L, Peretti-Watel P, Verger P; Coconel Group. Covid-19 health crisis and lockdown associated with high level of sleep complaints and hypnotic uptake at the population level. J Sleep Res. 2021;30(1):e13119. doi: 10.1111/jsr.13119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franceschini C, Musetti A, Zenesini C, et al. Poor sleep quality and its consequences on mental health during the COVID-19 lockdown in Italy. Front Psychol. 2020;11:3072. doi: 10.3389/fpsyg.2020.574475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iorio I, Sommantico M, Parrello S. Dreaming in the time of COVID-19: a quali-quantitative Italian study. Dreaming. 2020;30(3):199–215. doi: 10.1037/drm0000142 [DOI] [Google Scholar]
- 12.Pesonen A-K, Lipsanen J, Halonen R, et al. Pandemic dreams: network analysis of dream content during the COVID-19 lockdown. Front Psychol. 2020;11:2569. doi: 10.3389/fpsyg.2020.573961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Xia Q, Xiong Z, et al. The psychological distress and coping styles in the early stages of the 2019 coronavirus disease (COVID-19) epidemic in the general mainland Chinese population: a web-based survey. PLoS One. 2020;15(5):e0233410. doi: 10.1371/journal.pone.0233410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scarpelli S, Alfonsi V, Mangiaruga A, et al. Pandemic nightmares: effects on dream activity of the COVID-19 lockdown in Italy. J Sleep Res. 2021;e13300. doi: 10.1111/jsr.13300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorgoni M, Scarpelli S, Alfonsi V, et al. Pandemic dreams: quantitative and qualitative features of the oneiric activity during the lockdown due to COVID-19 in Italy. Sleep Med. 2021;81:20–32. doi: 10.1016/j.sleep.2021.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schredl M, Bulkeley K. Dreaming and the COVID-19 pandemic: a survey in a U.S. sample. Dreaming. 2020;30(3):189–198. doi: 10.1037/drm0000146 [DOI] [Google Scholar]
- 17.Wood JM, Bootzin RR, Rosenhan D, Nolen-Hoeksema S, Jourden F. Effects of the 1989 San Francisco earthquake on frequency and content of nightmares. J Abnorm Psychol. 1992;101(2):219–224. doi: 10.1037/0021-843X.101.2.219 [DOI] [PubMed] [Google Scholar]
- 18.Tempesta D, Curcio G, De Gennaro L, Ferrara M. Long-term impact of earthquakes on sleep quality. PLoS One. 2013;8(2):e55936. doi: 10.1371/journal.pone.0055936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pagel JF, Vann BH, Altomare CA. Reported association of stress and dreaming: community background levels and changes with disaster (Hurricane Iniki). Dreaming. 1995;5(1):43–50. doi: 10.1037/h0094422 [DOI] [Google Scholar]
- 20.Nielsen TA, Stenstrom P, Levin R. Nightmare frequency as a function of age, gender, and September 11, 2001: findings from an internet questionnaire. Dreaming. 2006;16(3):145–158. doi: 10.1037/1053-0797.16.3.145 [DOI] [Google Scholar]
- 21.Hartmann E, Brezler T. A systematic change in dreams after 9/11/01. Sleep. 2008;31(2):213–218. doi: 10.1093/sleep/31.2.213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen T, Levin R. Nightmares: a new neurocognitive model. Sleep Med Rev. 2007;11(4):295–310. doi: 10.1016/j.smrv.2007.03.004 [DOI] [PubMed] [Google Scholar]
- 23.Payne JD. Memory consolidation, the diurnal rhythm of cortisol, and the nature of dreams: a new hypothesis. In: Clow A, McNamara P, editors. International Review of Neurobiology. Vol. 92. Academic Press; 2010:101–134. [DOI] [PubMed] [Google Scholar]
- 24.Nielsen T. Infectious dreams. How the COVID-19 pandemic is changing our sleeping lives. Sci Am. 2020;323:31–35. [DOI] [PubMed] [Google Scholar]
- 25.Pesant N, Zadra A. Dream content and psychological well-being: a longitudinal study of the continuity hypothesis. J Clin Psychol. 2006;62(1):111–121. doi: 10.1002/jclp.20212 [DOI] [PubMed] [Google Scholar]
- 26.Siclari F, Valli K, Arnulf I. Dreams and nightmares in healthy adults and in patients with sleep and neurological disorders. Lancet Neurol. 2020;19(10):849–859. doi: 10.1016/S1474-4422(20)30275-1 [DOI] [PubMed] [Google Scholar]
- 27.Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi: 10.5664/jcsm.8930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Florea C, Topalidis P, Hauser T, et al. Sleep during COVID-19 lockdown: a cross-cultural study investigating job system relevance. Biochem Pharmacol. 2021:191:114463. doi: 10.1016/j.bcp.2021.114463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Korman M, Tkachev V, Reis C, et al. COVID-19-mandated social restrictions unveil the impact of social time pressure on sleep and body clock. Sci Rep. 2020;10(1):22225. doi: 10.1038/s41598-020-79299-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha M, Pande B, Sinha R. Impact of COVID-19 lockdown on sleep-wake schedule and associated lifestyle related behavior: a national survey. J Public Health Res. 2020;9(3):1826. doi: 10.4081/jphr.2020.1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staller N, Randler C. Changes in sleep schedule and chronotype due to COVID-19 restrictions and home office. Somnologie. 2021:25:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bottary R, Simonelli G, Cunningham TJ, Kensinger EA, Mantua J. Sleep extension: an explanation for increased pandemic dream recall? Sleep. 2020;43(11):zsaa131. doi: 10.1093/sleep/zsaa131 [DOI] [PubMed] [Google Scholar]
- 33.Schredl M, Doll E. Dream recall, attitude towards dreams and mental health. Sleep Hypnosis. 2001;3:135–143. [Google Scholar]
- 34.Wu T, Jia X, Shi H, et al. Prevalence of mental health problems during the COVID-19 pandemic: a systematic review and meta-analysis. J Affect Disord. 2021;281:91–98. doi: 10.1016/j.jad.2020.11.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang L, Pan R, Cai Y, Pan J. The prevalence of post-traumatic stress disorder in the general population during the COVID-19 pandemic: a systematic review and single-arm meta-analysis. Psychiatry Investig. 2021;18(5):426–433. doi: 10.30773/pi.2020.0458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4(S1):150–155. doi: 10.1111/j.1365-2869.1995.tb00205.x [DOI] [PubMed] [Google Scholar]
- 37.Vallat R, Eichenlaub J-B, Nicolas A, Ruby P. Dream recall frequency is associated with medial prefrontal cortex white-matter density. Front Psychol. 2018;9:1856. doi: 10.3389/fpsyg.2018.01856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi: 10.1093/sleep/34.5.601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung F, Yegneswaran B, Liao P, et al. STOP questionnaire: a tool to screen patients for obstructive sleep apnea. Anesthesiology. 2008;108(5):812–821. doi: 10.1097/ALN.0b013e31816d83e4 [DOI] [PubMed] [Google Scholar]
- 40.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord. 2012;27(7):913–916. doi: 10.1002/mds.25037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroenke K, Spitzer RL, Williams JB, Löwe B. An ultra-brief screening scale for anxiety and depression: the PHQ-4. Psychosomatics. 2009;50(6):613–621. [DOI] [PubMed] [Google Scholar]
- 42.Topp CW, Østergaard SD, Søndergaard S, Bech P. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom. 2015;84(3):167–176. doi: 10.1159/000376585 [DOI] [PubMed] [Google Scholar]
- 43.Lang AJ, Wilkins K, Roy-Byrne PP, et al. Abbreviated PTSD Checklist (PCL) as a guide to clinical response. Gen Hosp Psychiatry. 2012;34(4):332–338. doi: 10.1016/j.genhosppsych.2012.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elo AL, Leppänen A, Jahkola A. Validity of a single-item measure of stress symptoms. Scand J Work Environ Health. 2003;29(6):444–451. doi: 10.5271/sjweh.752 [DOI] [PubMed] [Google Scholar]
- 45.de Boer AG, van Lanschot JJ, Stalmeier PF, et al. Is a single-item visual analogue scale as valid, reliable and responsive as multi-item scales in measuring quality of life? Qual Life Res. 2004;13(2):311–320. doi: 10.1023/B:QURE.0000018499.64574.1f [DOI] [PubMed] [Google Scholar]
- 46.StataCorp. Stata Statistical Software: Release 16 [Computer Program]. College Station, TX: StataCorp LLC; 2019. [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57(1):289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 48.Erlacher D, Schredl M, Watanabe T, Yamana J, Gantzert F. The incidence of lucid dreaming within a Japanese university student sample. Int J Dream Res. 2008;1(2):39–43. [Google Scholar]
- 49.Yu CKC. Dream intensity inventory and Chinese people’s dream experience frequencies. Dreaming. 2008;18(2):94–111. doi: 10.1037/1053-0797.18.2.94 [DOI] [Google Scholar]
- 50.Nielsen T. Variations in dream recall frequency and dream theme diversity by age and sex. Front Neurol. 2012;3:106. doi: 10.3389/fneur.2012.00106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canli T, Desmond JE, Zhao Z, Gabrieli JDE. Sex differences in the neural basis of emotional memories. Proc Natl Acad Sci. 2002;99(16):10789–10794. doi: 10.1073/pnas.162356599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guadagni V, Umilta A, Iaria G. Sleep quality, empathy, and mood during the isolation period of the COVID-19 pandemic in the Canadian population: females and women suffered the most. Front Glob Women’s Health. 2020;1:585938. doi: 10.3389/fgwh.2020.585938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Herman S, Shows WD. How often do adults recall their dreams? Int J Aging Hum Dev. 1983;18(4):243–254. doi: 10.2190/A3R8-C69H-13X5-P5V0 [DOI] [PubMed] [Google Scholar]
- 54.Giambra LM, Jung RE, Grodsky A. Age changes in dream recall in adulthood. Dreaming. 1996;6(1):17–31. doi: 10.1037/h0094443 [DOI] [Google Scholar]
- 55.Angelakis S, Nixon R. The comorbidity of PTSD and MDD: implications for clinical practice and future research. Behav Change. 2015;32:1–25. doi: 10.1017/bec.2014.26 [DOI] [Google Scholar]
- 56.Spinhoven P, Penninx BW, van Hemert AM, de Rooij M, Elzinga BM. Comorbidity of PTSD in anxiety and depressive disorders: prevalence and shared risk factors. Child Abuse Negl. 2014;38(8):1320–1330. doi: 10.1016/j.chiabu.2014.01.017 [DOI] [PubMed] [Google Scholar]
- 57.Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations. Dialogues Clin Neurosci. 2015;17(2):141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Przeworski A, Dunbeck K. Generalized anxiety disorder: how it compares to PTSD. 2016:193–204.
- 59.DeCicco TL, Zanasi M, Dale A, Murkar A, Longo G, Testoni F. A cultural comparison of dream content, mood and waking day anxiety between Italians and Canadians. Int J Dream Res. 2013;6(1):8–12. [Google Scholar]
- 60.Palagini L, Rosenlicht N. Sleep, dreaming, and mental health: a review of historical and neurobiological perspectives. Sleep Med Rev. 2011;15(3):179–186. doi: 10.1016/j.smrv.2010.07.003 [DOI] [PubMed] [Google Scholar]
- 61.Barrett D, Loeffler M. Comparison of dream content of depressed vs nondepressed dreamers. Psychol Rep. 1992;70(2):403–406. doi: 10.2466/pr0.1992.70.2.403 [DOI] [PubMed] [Google Scholar]
- 62.Schredl M, Berger M, Riemann D. The effect of trimipramine on dream recall and dream emotions in depressive outpatients. Psychiatry Res. 2009;167(3):279–286. doi: 10.1016/j.psychres.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 63.Valli K, Revonsuo A, Pälkäs O, Ismail KH, Ali KJ, Punamäki RL. The threat simulation theory of the evolutionary function of dreaming: evidence from dreams of traumatized children. Conscious Cogn. 2005;14(1):188–218. doi: 10.1016/S1053-8100(03)00019-9 [DOI] [PubMed] [Google Scholar]
- 64.Pillar G, Malhotra A, Lavie P. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med Rev. 2000;4(2):183–200. doi: 10.1053/smrv.1999.0095 [DOI] [PubMed] [Google Scholar]
- 65.Helminen E, Punamäki RL. Contextualized emotional images in children’s dreams: psychological adjustment in conditions of military trauma. Int J Behav Dev. 2008;32(3):177–187. doi: 10.1177/0165025408089267 [DOI] [Google Scholar]
- 66.Holzinger B. Nightmares - What They Tell Us and How We Can Change Them. Langen Mueller Herbig; 2016. [Google Scholar]
- 67.Scarpelli S, Bartolacci C, D’Atri A, Gorgoni M, De Gennaro L. The functional role of dreaming in emotional processes. Front Psychol. 2019;10:459. doi: 10.3389/fpsyg.2019.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Woods ET, Schertzer R, Greenfeld L, Hughes C, Miller-Idriss C. COVID-19, nationalism, and the politics of crisis: a scholarly exchange. Nations Nationalism. 2020;26(4):807–825. doi: 10.1111/nana.12644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Koulack D, Goodenough DR. Dream recall and dream recall failure: an arousal-retrieval model. Psychol Bull. 1976;83(5):975–984. doi: 10.1037/0033-2909.83.5.975 [DOI] [Google Scholar]
- 70.van Wyk M, Solms M, Lipinska G. Increased awakenings from non-rapid eye movement sleep explain differences in dream recall frequency in healthy individuals. Front Hum Neurosci. 2019;13:370. doi: 10.3389/fnhum.2019.00370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fantini ML, Corona A, Clerici S, Ferini-Strambi L. Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder. Neurology. 2005;65(7):1010–1015. doi: 10.1212/01.wnl.0000179346.39655.e0 [DOI] [PubMed] [Google Scholar]
- 72.Seong MJ, Jung A, Park HR, Choi SJ, Joo EY. Dream recall frequency and sleep in patients with rapid eye movement sleep behavior disorder. J Sleep Med. 2017;14(2):55–60. doi: 10.13078/jsm.17008 [DOI] [Google Scholar]
- 73.Boeve BF. REM sleep behavior disorder: updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions. Ann NY Acad Sci. 2010;1184:15–54. doi: 10.1111/j.1749-6632.2009.05115.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Högl B, Stefani A, Videnovic A. Idiopathic REM sleep behaviour disorder and neurodegeneration — an update. Nat Rev Neurol. 2018;14(1):40–55. doi: 10.1038/nrneurol.2017.157 [DOI] [PubMed] [Google Scholar]
- 75.Wang Y, Wang ZW, Yang YC, Wu HJ, Zhao HY, Zhao ZX. Validation of the rapid eye movement sleep behavior disorder screening questionnaire in China. J Clin Neurosci. 2015;22(9):1420–1424. doi: 10.1016/j.jocn.2015.03.008 [DOI] [PubMed] [Google Scholar]
- 76.Malinowski J, Horton CL. Evidence for the preferential incorporation of emotional waking-life experiences into dreams. Dreaming. 2014;24(1):18–31. doi: 10.1037/a0036017 [DOI] [Google Scholar]
- 77.Eichenlaub J-B, van Rijn E, Gaskell MG, et al. Incorporation of recent waking-life experiences in dreams correlates with frontal theta activity in REM sleep. Soc Cogn Affect Neurosci. 2018;13(6):637–647. doi: 10.1093/scan/nsy041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eichenlaub JB, Cash SS, Blagrove M. Daily life experiences in dreams and sleep-dependent memory consolidation. In: Cognitive Neuroscience of Memory Consolidation. Cham, Switzerland: Springer International Publishing; 2017:161–172. [Google Scholar]
- 79.Sterpenich V, Perogamvros L, Tononi G, Schwartz S. Fear in dreams and in wakefulness: evidence for day/night affective homeostasis. Hum Brain Mapp. 2020;41(3):840–850. doi: 10.1002/hbm.24843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Revonsuo A. The reinterpretation of dreams: an evolutionary hypothesis of the function of dreaming. Behav Brain Sci. 2000;23(6):877–901. doi: 10.1017/S0140525X00004015 [DOI] [PubMed] [Google Scholar]
- 81.Watson MF, Bacigalupe G, Daneshpour M, Han WJ, Parra-Cardona R. COVID-19 Interconnectedness: health inequity, the climate crisis, and collective trauma. Fam Process. 2020;59(3):832–846. doi: 10.1111/famp.12572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beaulieu-Prévost D, Zadra A. Dream recall frequency and attitude towards dreams: a reinterpretation of the relation. Pers Indiv Differ. 2005;38:919–927. doi: 10.1016/j.paid.2004.06.017 [DOI] [Google Scholar]
- 83.Ribeiro N, Gounden Y, Quaglino V. Measure of dream recall frequency: comparing retrospective and logbook evaluations. Int J Dream Res. 2018;11(1):66–68. [Google Scholar]